
Because infrared heat waves penetrates less than a millimeter into the ocean’s surface, many skeptics argued it is impossible to blame rising CO2 for ocean warming. However, several prominent skeptic scientists, people who I have great respect for, also weighed in arguing it was silly and useless to argue infrared heat can’t warm the ocean.
Transcript

Welcome everyone.
About a decade ago there was a heated and unresolved debate on whether infrared back radiation from greenhouse gases is heating the oceans. Because infrared penetrates less than a millimeter into the ocean’s surface, many skeptics argued it is impossible to blame rising CO2 for ocean warming. However, several prominent skeptic scientists, people who I have great respect for, also weighed in arguing it was silly and useless to argue infrared heat can’t warm the ocean.
After analyzing the physics detailed in this video, I’m convinced it is solar energy that drives the observed ocean heating, and any infrared ocean heating is insignificant at best. If this analysis holds, it is another significant strike against the prevailing CO2 driven global warming theory
To ensure lay people are brought up to speed, here’s a quick summary of where consensus climate science stands today.
Climate scientists construct models of the earth’s energy budget. The amount of energy absorbed by the earth or emitted back to space each second, is measured in Watts and is standardized for an area measuring one square meter. For those unfamiliar with that measurement, simply understand that more Watts signify more energy.
The energy budget illustrated here was published by Stephens 2012. Others have slightly different numbers, but this illustration is one of the best because it is one of the few that lists the range of uncertainties in their measurements.
Because the sun’s surface is so hot it emits high energy shortwave radiation. On average the earth warms as short waves add 75 Watts to the atmospheric water vapor while the earth’s surfaces absorb about 160 Watts, totaling 240 Watts that are heating the earth’s daytime climate.
According to the Stefan-Boltzman law, and remember scientific laws are undisputed, when a surface is heated it causes that surface to respond immediately by releasing an equal amount of energy from that surface.
To maintain the earth’s temperature balance, the 240 Watts of energy from the sun should cause the earth to emit 240 Watts back to space or transfer some of that energy from the surface into the oceans or soils. However, because the earth is so much cooler than the sun, it only emits that energy as longwave infrared waves, which interact very differently with the earth than the sun’s shortwaves.
While some longwaves can escape back to space unimpeded and at the speed of light, other longwaves can be absorbed by greenhouse gases like carbon dioxide and water vapor. Greenhouse gases then re-emit that absorbed energy, and redirect half back towards the earth’s surface. On average the earth’s surface also absorbs an estimated 345 Watts of re-cycled longwave energy which counteracts the rate of cooling and prevents the earth’s nighttime cooling from dropping to the point of global freezing.
However, that longwave energy is not trapped, as many media headlines suggest. Eventually nearly all the energy from the sun escapes back to space. However, the best modeled energy budgets suggest that a slightly less amount of energy radiates back to space relative to what had originally entered from the sun.
Putting aside some large uncertainties, there appears to be a radiative imbalance of 0.6 Watts less energy leaving the earth than is added by the sun. Some researchers estimate that imbalance may be as high as one Watt.
That imbalance does not violate the Stefan-Boltzmann law because that missing heat gets stored below the land surface or below the ocean surface, where the heat cannot radiate back to space in a timely manner.
There is no scientific disagreement that our oceans have been warming since the Little Ice Age ended around 1850 AD. What remains to be debated is, to what degree are oceans naturally warming due to storage of more shortwave energy from the sun, or due to storage of increased downward longwave energy emitted by rising carbon dioxide concentrations.
Some have argued, incorrectly, that the earth’s land surface heats and cools the same as the oceans.
However, in contrast to the ocean, the suns’ shortwave energy doesn’t penetrate soils much deeper than an inch. The combined heating from shortwave & longwave energy plus sensible heat transfer from warm air, increasingly heats soils at the surface reaching summertime highs. Then, primarily via conduction, surface heat slowly passes down the temperature gradient from the warm surface to cooler depths in accord with the second law of thermodynamics. Heat transfer via conduction is slow, so temperatures can remain 8ºC (15 º F) cooler just 10 inches (25 centimeters) below the surface.
During the winter, the colder surface reverses that temperature gradient, so that stored summer heat travels via conduction back to the surface. Again, because surface cooling happens quickly and conduction happens slowly, the deeper soil remains warmer than the surface soil.
Greenhouse longwave energy penetrates only a few microns into the ocean surface and even less into most soils, but the sun’s shortwave energy passes much more deeply into the ocean.
More energetic shortwaves like blue light can penetrate over 100 meters (that’s about 4000 inches) into clear ocean water, with only half its energy absorbed within the first 20 meters. In contrast 50% of less energetic red light is absorbed in just the first few meters. That’s why seaweeds in the deeper ocean cannot use red light to photosynthesize like land plants do.
Although both the heating of the land and ocean depends on surface heating, radiative and convective heating are much more important for heating the ocean. This causes important differences in the way our oceans heat and cool, thus analogies to land surface heating are misleading.
This standard, albeit overly simplistic ocean temperature profile, shows the upper layer of the ocean, often referred to as the epipelagic layer or sunlight layer, extends from the surface to 200 meters depth. Turbulence due to winds and currents mixes and homogenizes the temperature as illustrated here and globally averages 13°C or (55 °F).
Below that mixed surface layer is the thermocline layer, defined as a region of rapidly cooling temperatures, because mixing of warm surface heat into the layers below rapidly declines with depth. .
At a depth of about 1000 meters and below there is a more homogeneous temperature of just 4°C or 39°F However, the illustrated homogeneous upper sunlight layer obscures the most important dynamics of the oceans’ surface skin layer that are key to controlling ocean heating and cooling.
A 2018 paper by Wong & Minett analyzed ocean temperatures from data collected during 2 ocean cruises in warm tropical and subtropical waters of the north Atlantic. They reported important differences in heating and cooling patterns in the microns-thick surface skin layer and millimeter thick subsurface layers.
For perspective, the sharpened point of a pencil is about one millimeter wide. It takes one thousand microns to equal just one millimeter. The ocean’s surface gatekeeper is only a couple of microns thick.
Only 4.9 Watts per meter squared of solar energy was absorbed in the first 10 microns. .
In contrast, the subsurface was increasingly heated, so by 10 millimeters deep, 261 Watts of solar energy were absorbed.
Only at the surface can any ocean heat be released back to the atmosphere or space. So, this differential solar heating creates the required temperature gradient that allows the solar heated subsurface water to constantly move up towards the cooler surface.
Heating by longwave energy adds another complication that must be considered. Longwave energy only penetrates the first few microns of the skin layer. And that fact prompts some skeptics to argue CO2 back radiation cannot heat the ocean.
But on the other side of the debate, it is argued that because longwave heating can add 100 times more energy into the skin layer than solar heating, longwave heating can alter and even reverse the temperature gradient required for ocean cooling.
But if true, then how does the ocean ever lose heat.
Nonetheless, the alarmist narrative becomes that added infrared energy must alter the temperature gradient to some degree. Therefore, as more greenhouse gases add more longwave energy to the surface skin layer, it increasingly disrupts the temperature gradient enough to reduce the rate of subsurface cooling. So, rising CO2 is indirectly warming the ocean.
But measurements do not support such narratives.
Satellite measurements determined the oceans’ surface temperature by measuring the longwave radiation emitted from the skin layer. The sub-skin layer below was also measured but via emitted microwaves.
The results show the ocean’s skin layer is always cooler than subsurface layers below, despite the combined surface warming by shortwave and longwave heating plus rising heat from solar heated waters below
In the daytime, there is a deeper solar heated diurnal warm layer. At night, without solar heating, subsurface waters eventually cool and mix with the water below creating a more homogeneous upper layer temperature everywhere except in the cooler skin surface.
No matter the season, or time of day the skin layer is always cooler than the waters immediately below.
Although not intuitive, the constant cool skin surface phenomenon can be explained by the Stefan-Boltzman law. According to that law, when the skin surface layer is heated, by longwave or shortwave energy, the surface skin layer radiates an equal amount of energy back to the atmosphere immediately. Any longwave heating of the skin surface layer is so transitory there is no observable effect on the temperature gradient that’s required to cool the ocean’s solar heated sub-surface layers.
As Wong & Minett’s results illustrated, the micron thick skin layer absorbed 410 Watts of longwave and a negligible amount of shortwave, but simultaneously emitted 470 Watts out of the ocean, maintaining the observed cooler skin layer.
The 470 Watts of longwave-out vs 410 Watts of longwave-in does not violate the Stefan-Boltzman law because the skin surface heating is the combined result of warming from 67 Watts of solar heated water rising from below and the downward longwave radiation from above.

That combined heating also caused the skin surface to lose a total of 7 Watts more from sensible heat loss to the cooler air above via conduction, and more latent heat due to evaporation from the skin surface. Thus, on average the skin surface cooling balances skin surface heating, but the skin surface remains slightly cooler because it radiates heat away faster than subsurface heat can rise from below.
Still their data raises one concern. It is very unusual that their estimated heat loss via sensible and latent heat was a mere 7 Watts of cooling. That is 15 times less than globally averaged ocean cooling rates.
It is well established, that the energy needed to evaporate enough water that’s observed in the earth’s water cycle, oceans must experience over 80 Watts per meter squared of evaporative cooling.
Acknowledging the conundrum that those longwave energies do not penetrate deeper than a few microns and thus cannot warm the oceans directly, the stated intent of Wong & Minett’s analysis was to advance their hypothesis that more co2 longwave energy can still warm the ocean indirectly by reducing the temperature gradient and thus, reduce the rate of cooling of the ocean’s diurnal warm layer.
To support their claim, they argued the absorption of more longwave into the skin layer, did not result in the required increased surface temperature that would immediately increase emissions and balance the longwave energy surface budget.
To that end, they examined the increased longwave heating produced on cloudy days as an analog for the effects of increased longwave heating from rising carbon dioxide.
Their highlighted results illustrated here, show that despite an increase of 40 Watts of longwave heating from cloudy skies, there was no increased cooling via emitted longwave-out and no increased loss of sensible and latent heat so the cooling temperature gradient must have been disrupted. But that would violate the Stefan-Boltzman law, their narrative requires magical thinking.
In reality the Stefan-Boltzman law was never violated. It was simply a bad narrative. Although increased cloud cover did increase longwave heating, cloud cover simultaneously reduced the shortwave solar heating of the layers below the skin surface.
The reason 40 increased Watts of incoming longwave did not also increase outgoing longwave is due to the fact that clouds equally reduced the solar heating of subsurface waters. When long wave and shortwave heating are both considered, the balance between incoming and outgoing heat at the skin surface was maintained as predicted by the Stefan-Boltzman law.
Others have argued that warmth generated by longwave heating of the skin surface would be transported quickly downward by mixing with layers below.
However, downward mixing of the observed cooler skin layer would only cool the warmer subsurface layers. While any mixing that brings warmer subsurface water up to the surface, only enhances its cooling.
Only the mixing of deeper solar- heated subsurface waters with the cooler waters below, carries heat deeper into the ocean. The mixing of solar heated water into deeper layers, then makes solar heat less likely to resurface and cool.
Thus, it is the downward mixing of solar heated waters, not the transitory longwave heating of the skin surface layer that stores energy in the ocean and creates the estimated energy imbalance.
Taking a broader global view, analyses of heat flux into and out of the world’s oceans illustrates where the oceans are warming. Huang’s (2015) illustration of ocean heat flux contradicts claims that a thickening global blanket of CO2 is heating the world’s oceans.
Nearly half of the ocean surfaces, regions colored green, show no net heat flux into or out from the ocean.
The regions of greatest heat flux into the ocean are colored red.
There, the intense tropical heating is further amplified by the reduced cloudiness observed in the tropics, as published in Fasullo and Trenberth’s 2008 study.
Furthermore, the tropical trade winds cause greater upwelling of cold deep water in the eastern Atlantic and eastern Pacific.
Colder waters on the surface can reverse the typical heat flux so that heat flows from the warmer air above into those colder upwelled waters.
The obvious clue to the primary driver of ocean warming is that the regions of greatest solar flux into the ocean are the same regions created by pacific and Atlantic La Ninas. That solar heated water is transported westward and then poleward along ocean currents where the greatest amount heat is vented, (colored dark blue. The Holocene optimum, with temperatures warmer than today happened during perpetual La Nina conditions.
For details on how a solar heated ocean causes our current warming trend, please watch my earlier video: Global warming driven by pacific warm pool, La Nina & ITCZ: an alternative climate change theory or read its transcript.
To date there has been no provable mechanism illustrating how heating from CO2 can heat anything more than the ocean’s skin surface. In contrast the combined climate effects of solar heating, the ITCZ migrations and La Ninas are strongly supported in the peer-reviewed scientific literature.
So, I will ignore the click bait news media’s fear mongering that our oceans are “on the boil” due to rising CO2. There is simply no scientific proof to support such dishonest narratives.
And I will sleep well. There is no climate crisis.
Our democracy depends on a diverse array of good critical thinkers. So, please shun mindless group think.
Instead embrace renowned scientist, Thomas Huxley’s advice Skepticism is the highest of duties and blind faith the one unpardonable sin.
And if you appreciate the science clearly presented here, science rarely presented by mainstream media then please









“According to the Stefan-Boltzman law, and remember scientific laws are undisputed, when a surface is heated it causes that surface to respond immediately by releasing an equal amount of energy from that surface.”
If that were so then the surface would never heat up! What happens is that the heat emitted is proportional to the fourth power of the surface temperature, the surface will continue to heat up until an equilibrium is reached and an equal amount of energy is emitted.
I agree with this entirely, I can only assume that Jim meant to add “when an object is in thermal equilibrium….” This however immediately put me off reading the remainder of the article.
Skin surface will always follow Stefan Boltzmann law. It is heat carried and stored below the skin surface that causes temperatures to rise. So there will be a lag time for subsurface heat to reach the surface and cool.
Actually, the surface won’t “heats up” from absorbing a small amount of “back radiation”. It just won’t cool as fast as it would without the extra energy.
Assume a body B1 is radiating at 300 at T1 and absorbs 100 from another body B2 at T2. B1 will go right on radiating at 300 and immediately rid itself of the 100 it absorbed from B2. Now, that means B1 can only cool by an amount of 200, which is what is left over. Notice that doesn’t cause B1 to get hotter, only that it will take longer to cool.
This is all difficult to see using only the math that is generally shown for S-B. One must realize that this equation is only valid for an infinitely small point in time. These are not static quantities they are dynamic and change from instant to instant. That is why a proper analysis requires one to develop gradients and to use calculus to evaluate the processes.
Everyone should download Planck’s thesis on heat radiation and study it carefully. I have been reviewing it for over two years now and working out the math that is involved. Remember, Planck is the dude that worked out quanta (photons) absorption and how different wavelengths combine. It ain’t simple and climate scientists who discuss this using only simple math are making a mistake by not showing people how complicated it actually is.
Think of it this way. There is only one point on earth at any given point in time where the sun is directly overhead, i.e., at zenith. If you are outside the tropics, that will never happen. At that one point at that certain time, the point will receive the maximum amount of radiation. Before that point in time the radiation was increasing, after that point in time, the radiation is decreasing. If there wasn’t a T^4 term in the S-B equation none of this would matter. But there is, and to scientifically address it requires some sophisticated math.
“Assume a body B1 is radiating at 300 at T1 and absorbs 100 from another body B2 at T2. B1 will go right on radiating at 300 and immediately rid itself of the 100 it absorbed from B2.”
It will not immediately rid itself of the extra energy, its surface will warm up and consequently its heat loss will increase until equilibrium is reached.
Long wave radiation does not warm the oceans, short wave energy does. However the temperature of the air determines how quickly this heat leaves the oceans. The warmer the air, the warmer the water must get in order to shed the heat it receives from the sun.
So you claim that the energy the Stefan Boltzmann law says is radiated as the 4th power of the source’s temperature actually depends on the temperature of the object to which it is radiating?
You are treating this as a static phenomenon. It is not. Think about it. What happens as the air temperature approaches the water temperature. What occurs? What happens at equilibrium? Do they both continue to cool as the sun passes zenith? Do they continue to warm as the sun approaches zenith? The traditional S-B equation assumes equilibrium is a static condition.
To truly know what happens you need to treat them mathematically as time varying. You need to know the input to the ocean and its value as it changes since it varies instant to instant. You need to know the input to the atmosphere since it varies instant to instant. They could both be warming but at different rates or they both could be cooling at different rates. Plus, radiation calculations don’t include evaporative losses.
It is all very complicated. Simple explanations may give a hint of understanding, but only a hint.
Jim,
Your “Clear sky / Cloudy Sky graph” is based on a LW out level of 470 W/m^2. However there is whole level more complexity which you allude to in your “Increasing Cloud Albedo” figure, which has cloud Albedo numbers that are incorrect, BTW. Low level clouds have a high Albedo and reflect much more SW back to outer space, thus not warming the surface to the temperature needed to emit 470 W/m^2.
The graph makes the same basic assumption as all the “Cloud Feedback =Zero” folks, which is that the surface temp is fixed and Albedo =.3 and local Albedo and is simply not true under local cloud cover, where “local” can be an afternoon thunderhead or a weather front half a continent wide.
You should number your figures so I can properly constructively criticise 😳 ! Still deserving of 8 out of 10.
The final closing sentence:
Is there more? Perhaps an object, subject or action verb?
The effect of greenhouse “back” radiation is a heat transfer issue often dealt with by heat transfer engineers in order to design solar collectors, structure thermal control systems etc.The methodology is well established and has been successful .Application to the ocean surface energy balance may be found at the website http://www.temporalpublishing.com as Item No 8 of the Supplementary Material for Instructors.After developing and perturbing the balance, issues such as the effect of CO2, and the meaning of “heating the ocean” are discussed.
Excellent post. I can’t afford the book but reading the supplement you referenced gives one a good introduction to the complicated issues involving heat transfer. One must have a good calculus background to follow what is happening and it becomes obvious that much of the simple algebra to describe what happens is way short of being true.
Is it any wonder that the climate models don’t treat the oceans and clouds in a real physical fashion? The interaction between the two is obviously non-linear and varies moment to moment. Programming this would be a nightmare, if even possible. Certainly there is no proper data to allow one to even have a hope of deciphering what occurs.
Right, please what?
My please is that policy should not be established by unsettled science but has been by arrogant fools, who are both wittingly and unwittingly destructive.
what did we do to deserve this, better, what have our unborn descendants done to deserve this?
Ay yai yai, Madre mio!
==========
LOL There is more in the video version, but the transcript stops short. However the picture should have hinted at the request, “click like, share, subscribe, etc”
How much energy is converted into LIFE?
A small amount. But life converts a hellovalot of sequestered energy into heat. That’s why we’re here. To increase the entropy of the universe.
Thoughts on this anyone?
“At the top of the atmosphere (TOA), the LWIR flux returned to space is simply a cooling flux. Conservation of energy requires a planetary average LWIR flux near 240 W m-2. This is the cumulative LWIR emission from many different levels through the atmosphere. The emission from each level is modified by the absorption and emission of the levels above. The spectral
distribution of the LWIR flux is not that of a blackbody near 255 K. The use of Stefan’s Law to create an ‘effective emission temperature’ is a mathematical construct with little physical meaning. There can be no ‘greenhouse effect temperature’ of 33 K.”
https://www.venturaphotonics.com/research-overview.html
“According to the Stefan-Boltzman law, and remember scientific laws are undisputed, when a surface is heated it causes that surface to respond immediately by releasing an equal amount of energy from that surface “.
Recall that the Stefan-Boltzmann law was established experimentally and theoretically for a completely black body. The question of how the Earth, and even more so the various parts of the Earth, corresponds to the concept of a black body is highly controversial. As for the “immediate” release of absorbed energy, there are also controversial points here: a) part of the absorbed energy is spent on the evaporation of water on the surface; b) depending on the physical properties (heat capacity, thermal conductivity) of substances in different places on the surface, these places are heated to different temperatures and radiate heat at different rates.
So we’re all convinced that dQ==0. I can attest that there are dozens of engineering equations that integrate dQ to arrive at a temperature change. Get over it. Nothing is exactly in local thermal equilibrium.
That is my point. The simple S-B assumes static equilibrium which is non-physical. It is ok for seeing what happens at an infinitely small instant of time. The problem is that for the next infinitely small instant of time, conditions have changed.
The problem with using Stephan-Boltzman to model energy transfer to the ocean from the atmosphere and vice versa is that it does not take into account all the real drivers of heat transfer and storage in the oceans, specifically:
1) S-B only deals with radiative energy transfer, and totally ignores convection which is totally dominant in atmosphere-ocean heat energy transfer, and conductive heat energy transfer. To ignore convection is quite figuratively ignoring the elephant in the room.
2) S-B ignores the effects of specific heat, which is the heat energy that must be transferred from one thing to another to cause the temperature of the first thing to change one unit of temperature per one unit mass. The specific heat of liquid water (4.182 J/kg C) is far higher than that of air (1.0035 J/kg C). And of course the mass of the world’s oceans (1.4 x 10 to the 21st kg) is vastly larger than the mass of the Earth’s atmosphere (5.15 x 10 to the 18th kg). Since is it temperature differential that drives heat energy transfer, the atmosphere has almost no effect on the temperature of the world’s oceans, while the oceans have a vast effect on the temperature of the world’s atmosphere. This is obvious to anyone who lives near an ocean coast or other large body of water – atmospheric temperatures are significantly moderated by the body of water .. not the other way around.
3) S-B ignores other key physical-chemical properties of air and especially water, which have a very large effect on heat energy transfer – primarily due to the heat of vaporization. This energy is what drives thunderstorm formation and storm cell life cycles, and formation of tropical cyclones. Everybody knows that hurricanes don’t form over continental land masses – they always form over the oceans because that is where the heat energy transfer takes place that causes the radically high cyclonal winds to form and travel until the peter out over land somewhere.
Arguing over the effects of S-B on climate is akin to arguing over how many angels can dance on the head of the pin … meanwhile a herd of elephants is trampling over the guy supposedly holding the pin doing the arguing. S-B is inconsequential.
Duane, “S-B only deals with radiative energy transfer, and totally ignores convection “
Radiative energy transfer is the only thing important for energy into earth from space and back again. The video however does not ignore other transfers of energy from conduction and convection that are ALSO critical for heat transfer WITHIN the earth’s climate. So it is quite odd that you are trying so hard to diminish the role S-B law, and its importance in radiative heating and cooling, when is central to understanding ocean heating.
I am not trying hard at all. This is easy peasy.
And you nailed it – focusing on radiative heat transfer and S-B is exactly what enables and props up the false science of the warmunists. So don’t even go there.
This kind of analysis only plays right into the warmunists hands using their model of radiative heat transfer being the be all end all to global warming. It’s what excuses their extreme narrow focus on the so-called “greenhouse effect” which completely ignores the process that actually drive climate.
Indeed the warmunistas abuse the S-B law to push a crisis, but that doesnt mean it isnt a major contributor to climate changes that have different real narratives. When you let your politics deny the S-B’s role, you have sunk to the warmunistas level of obscuring the scientific truth
Jim,
It isn’t ignoring the S-B law, but recognizing that static equilibrium doesn’t exist at the ocean/atmosphere boundary. Conduction/convection does drive a substantial heat transfer value. S-B doesn’t include this process. The temperatures in S-B are not at equilibrium and have a time varying component and because of the T^4 vastly differing conditions exist between the two temperatures. Plain algebra is ok for introducing concepts but too many people have been lured into assuming that the system is not that complicated so that predictions must be true. If they understood the intricacies involved, they would understand how far off the predictions of models are.
Of course “S-B doesn’t include” convection and conduction and it has never been argued that it does. S-B simply tells us the amount of radiation at single point in time in response to the temperature at that instance. S-B argument by no means imply a static condition that ignores other factors!
In the simplest terms, adding energy increases temperature (ignore for now the fact that different substances with different heat capacities will have different temperatures). By increasing the temperature that substance will immediately emit radiation accordingly. If evaporation removes heat, temperatures cool and according to S-B, a lower level of radiation will be emitted.
Completely agree. Once you enter the world of fairy tales with “greenhouse effect” you have descended into make believe devoid of physical reality.
many strong cyclonic winds are produced over land but their water content is somehow limited compared to those over oceans.
Until Democrats can figure out a way to regulate and tax sunshine, we will have a climate crisis.
Jim,
Give me a square mile of ocean and I could warm it for you very easily on a day when the wind is less than Force 4. For this I would need a fairly nippy boat and 640X5 ml of olive oil. No back radiation required.
Light oil on a water surface spreads about one acre per 5ml (that’s approximate, look up ‘Benjamin Franklin Mount Pond’ to check and make your own estimate). Oil on water smooths it, lowering albedo and reducing evaporation. This causes warming.
Overfed plankton in our sewage ruined oceans release lipids that do the same. You end up like the Sea of Marmara which is warming at twice the average rate.
To get some idea of the extent of the problem, look up the SeaWifs site, it’s out of date but is pretty damning.
I’ve got this written up in hand-wavy terms. You can contact me via Anthony (no point in sending it to him, he’s far too busy.) or from the TCW Defending Freedom blog. There’s a rather poor picture of a fractured smooth that I observed in 2012 which covered tens of thousands square miles.
I wish someone would listen. Anthropogenic Local Warming even explains that UEA professor’s* ‘why the blip?’ Because the Battle of the Atlantic.
*Prof Wigley?
JF
Not all the ocean surface is warming. The warming follows the sun except when then surfaces reaches 30C and it cannot get any warmer.
About the ocean surface skin and sub-skin: I wrote about this on WUWT 5 years ago.
https://wattsupwiththat.com/2017/02/18/stokes-and-the-somehow-theory-of-ocean-heat/
“the surface skin is cooler, day and night, than the subskin. What that tells you is that the net heat flow between the skin and the subskin is one-way – upwards from subskin to skin. So no matter how much the skin may affect the rate at which the subskin warms or cools, it cannot ever give it a higher temperature than its own. If the subskin’s temperature is higher, then the subskin’s other heat sources have to be capable of providing.all of its temperature.”
The article then explained how clouds, which reduce shortwave radiation from the sun and increase longwave radiation, have a net cooling effect on the ocean because shortwave radiation (mainly in the wavelengths of visible light) does penetrate into the ocean below the subskin, whereas longwave radiation does not. Note that this implies that a reduction in cloud cover leads to a warmer ocean: “the 4 percentage-point reduction in global cloud cover from 1983-2009 would see an additional 2.0 Wm-2 absorbed in top 1m [of the ocean], and 1.6 Wm-2 absorbed into the 1-15m band.”.
One other factor which I missed back then, and which is not in this excellent article by Jim Steele (or maybe I missed it) is this: The lowest fraction of atmosphere right against the ocean surface typically has a very high water vapour content and often also sea spray. These provide an additional barrier to downward longwave radiation so that some of it never even reaches the ocean surface. A higher proportion of shortwave radiation will pass through it. In other words, that is another factor reducing the ability of downward longwave radiation to heat the ocean.
How do we lose enough heat to have a glacial period?
Clouds. Probably. Probably caused by a (slightly) less active sun.
A partial answer is increasing sea ice. Once changes in the Milankovitch cycles and Atlantic Oscillations allow Arctic sea ice to form, all the heat that is transported into the Arctic that would normally ventilate under ice free conditions as happened during the warmest period of the Holocene Optimum, is now insulated and can no longer warm the atmosphere. That cooling serves as a positive feedback
If you could live another 2000 years you will understand the reason. The current cycle of glaciation began 500 years ago. That is the last time perihelion occurred before the austral summer solstice. That is when the boreal springs/summers began to get increased sunlight and the autumn/winter less sunlight.
The attached only shows the change in ToA solar EMR for the past 40 years but the process has been under way since 1500.
There is always more water in the atmosphere in December and January and more precipitation on land in those months. As boreal winters start to cool, more of the precipitation will fall as snow and will eventually accumulate.
The ice accumulation raises the average elevation and the surface remains cooler so the ice does not melt from year to year. This is observed now in Antarctica and Greenland. A large portion of Europe and North America will eventually be covered in ice mountains. THat lowers the average temperature of the northern hemisphere because more sunlight is reflected and even the tropical Atlantic will struggle to reach the 30C surface temperature limit.
The cooling trend will persist for another 9,500 years..
Another great presentation by Jim Steele.
As a layman and definitely not understanding all of the physics involved, how can we be expected to believe 0.6 +- 0.4 at TOA in the first diagram when there are so many larger ‘give or take’ values?
Taking any one of the possible values to its higher or lower bound would give different values to TOA. What if several or all were at their highest or lowest limit?
Angels and heads of pins comes to mind reading the comments
Only if the emissivity is unity over the range of wavelengths leaving the surface as predicted by the Stefan-Boltzman Law. If the emissivity is unity, then the reflectance would be zero for those wavelengths. If the emissivity is not unity, then heat will not be released as fast as it accumulates.
However, in the real world, a theoretical Black Body doesn’t exist. Real objects have a finite reflectivity at all wavelengths, typically much greater than zero. That means all impinging wavelengths will be reflected, but by different amounts. There may be some strong absorption features, but they will typically be a small fraction of the integrated total. All absorbed impinging EM radiation will be converted to heat. However, because the sun has its peak output near 550nm, visible light will contribute far more to heating than solar IR.
Therefore, we have a complex system where sunlight falling on water will vary in reflectance from a low of about 2% with normal (perpendicular) specular reflectance, with some diffuse reflectance from suspended particles, to a high of 100% specular reflectance at the Terminator. Interestingly, the spectrum of the reflected light shifts with the angle of incidence so that the spectrum of the reflected light is identical to the solar spectrum (minus atmospheric scattering and absorption) at the Terminator.
The result is that the water temperature is the sum of the contributed energy of all the impinging solar radiation, where it is absorbed. Thus, visible light is absorbed logarithmically over the path length, with blue-green light penetrating the deepest. Most UV is absorbed very close to the surface and solar IR at the surface. What this means is that all solar radiation contributes to the heating of surface water, but it only cools by outgoing IR radiation, which is a fraction of the total energy, and at a rate dependent on the emissivity at the IR wavelengths.
To calculate the net result would require a detailed analysis of the normal spectral reflectance of water throughout the IR region, convolved with the S-B spectrum.
Interesting, Jim.
In my post, “Radiating The Ocean“, I raise 4 objections to the idea that downwelling longwave radiation (DLR) cannot heat the ocean. Let me recap them. Read the post at the link for the full details.
First, yes, IR only penetrates less than a millimeter into the ocean. But the same is true of the land, and a rock cannot mechanically circulate the heat downwards as the ocean can.
Second, if the ~ 235 W/m2 of DLR isn’t adding energy to the ocean, where is it going? Can’t be heating the air, we’d be aflame. Can’t be evaporating water, it would rain 4 meters per year on average. Can’t be destroyed. So … where is it?
Third, the ocean cools in large part via nocturnal overturning driven by surface cooling. Any additional energy added to the top surface delays the onset of the overturning, slowing energy loss and leaving the ocean warmer than it would be without the DLR.
Fourth, if the sun is the only (or the overwhelming) source of energy to the ocean, it would be an ice cube. As you point out above, only about 165 W/m2 of solar energy enter the ocean on a 24/7 average basis. That corresponds to a Stefan-Boltzmann temperature of 40 degrees below zero (curiously, it’s -40° in both Celsius and Fahrenheit—that’s where the scales cross). We know that global average ocean temps are about 16°C or so. At that temperature, water radiates at about 400 W/m2 … so if DLR isn’t adding energy to the ocean, where is the deficit of 235 W/m2 of energy coming from to keep the ocean liquid?
For your theory to be true, you need to show that all four of those objections are not valid.
My best regards to you,
w.
Willis, thanks for showing up. I had read your earlier blog post. You say “or your theory to be true, you need to show that all four of those objections are not valid.” But that is exactly what this video did.
Willis, your “deficit” of 235 W/m2 of DWLWIR is, as always, fictional. Without it, all your fabricated objections vanish into thin air.
Let me also recommend the excellent three-part series on this subject over at Science Of Doom
Part 1
Part 2
Part 3
And a final post, “The Cool Skin Of The Ocean“.
w.
These are the posts I really hate. This site is one if not the best to see these topics debated by contributors who know their stuff. Posting multiple links to other climate websites contributes nothing.
Those posts are very detailed. I had not seen them yet. It will take me some time to fully understand them. But at a cursory glace the last post “The Cool Skin of the Ocean” appears to be consistent with the Wong & Minnett 2018 publication which I suppose isn’t a surprise since Minnett’s work is referenced in the post.
Science of Doom makes the exact same arguments the video makes . The question that is not resolved is does the skin layer lose heat faster than it gains heat. The cooler skin surface suggests it loses heat faster. The fact solar heating happens more deeply clearly suggests if heat from the two sources, shortwave and longwave,it is shortwave that is far more likely to warm the deep ocean .
From SoD, “The surface is where (almost) all of absorbed ocean energy is transferred to the atmosphere.
And in general the ocean is moving heat into the atmosphere, rather than the reverse. The atmosphere is usually a few degrees cooler than the ocean surface.
Because turbulent motion is reduced the closer we get to the boundary with the atmosphere, this means that conduction is needed to transfer heat. This needs a temperature differential.
Yes. There are multiple processes taking place. Not only that, but variances in each process from moment to moment.
Looking at a small point of time is fine, but one can’t dwell on that one moment, the next one is important also.
Jim Steele recognizes that there is something dramatically wrong with the theory
that it is recycled longwave or shortwave energy which somehow is the main culprit in
increasing ocean heat. He states clearly that any heating of the oceans by
reemissions of infrared radiation from the CO2 in the air are “insignificant at best”.
And he realizes that the current global warming narrative minimizes the real cause of
the extra heating of the Earth that we have witnessed over about the last 150 years
(off and on) — solar radiation being absorbed (and retained) as it reaches the
Earth’s liquid water surface — while it wildly exaggerates the mythical warming
coming from the “infrared back-radiation from greenhouse gases”. Choosing the latter
to me is like attributing a winning home run, not to the batter who hit the ball, but to
the fan in the stadium who caught it — the atmosphere might have caught some of that
heat on the backstroke, but it wasn’t responsible for the warming coming from it.
But I do not agree that this is because of alleged CO2 longwave emissions going into
the oceans immediately coming right back out again. I believe it is true that infrared
heat does warm the ocean — of course the infrared heat (along with the visible light
heat) from the Sun directly delivered to the oceans most assuredly does accomplish
this warming and it is possible that the process can be indirect as well. As far as
those satellite measurements of the very thin skin layer being cooler than the
microwave-emitting layer just below, did you ever think, Mr. Steele, that that might
be because the very thin skin layer is somehow transferring some of its energy to just
below it, which energy could then get further mechanically subducted (liquids do flow)
into layers below even that, ultimately, therefore, allowing that longwave radiation to
indeed warm the oceans below?
But the reason why CO2 does NOT warm the oceans, just as Steele concludes, is
because any significant downwelling longwave radiation in the air reaching the oceans
came from those same oceans heating that air (ANY component of that air) right above
them, via conduction/convection, to temperatures which caused that air to then reemit
that infrared heat via blackbody radiation right back down to where it came from. CO2
had virtually nothing to do with the process from beginning to end and there has never
been any scientific evidence that any infrared radiation in the air is being
predominantly or selectively emitted by its CO2 molecules. If all the CO2
disappeared, the amount of infrared in the air would remain virtually the same (as
long as the pressures and temperatures remained the same). And no well-run experiment
under laboratory-controlled conditions has ever shown this preferential heat-emitting
effect of CO2 in our atmosphere versus all the other gases in our atmosphere (at one
atmosphere pressure).
Thus the argument of whether infrared radiation can warm the oceans or land is
completely beside the point, true or false (especially true if you include that
radiation directly emitted by the Sun as well as that radiation emitted by our
atmosphere — all of our atmosphere, not just its CO2 component), because, in either
case, that infrared radiation is not originating selectively from CO2 molecules in the
air and therefore has no relevance to the main thrust of the pronunciamentos of the
climate change wackadoodles whose intended target of demonization is that great gas
of life on Earth: carbon dioxide — at present levels in our atmosphere, the more the
better.
In any event, I do appreciate Mr. Steele’s willingness to NOT drink the
global-warming, climate-modeling Kool-Aid of CO2 warming the earth.
David Solan
David asks, “did you ever think, Mr. Steele, that that might
be because the very thin skin layer is somehow transferring some of its energy to just
below it”
Of course I thought and the video definitely addresses that possibility.
As stated, infrared doesn’t penetrate deeper than a few microns. So there are only 3 mechanisms to transport skin surface heat any deeper. Conduction , convection and turbulent mixing.
The absorption of solar heat within the first meter is negligible at the surface and increasingly warms with depth. That creates a temperature gradient so that the only way for heat to flow via conduction and convection is upwards as demanded by the 2nd Law of Thermodynamics
That leaves some form of mixing. Night time cooling at the surface can cause overturning, which means the microns that absorbed IR has emitted enough heat to cool and sink. Winds can generate turbulent mixing but the winds also increase evaporative cooling. In combination with thee skin surface radiative cooling of more IR out than in, the ral question where is the heat to be transported deeper. I think Willis’ argument that IR energy cant be released to the air otherwise we would “be aflame” is nonsense and denies the observation of more IR being emitted from the surface than it absorbs
As illustrated in W & M 2018, they reported longwave IR being emitted 470 W/M2 from the surface faster than 410 W/M2 is absorbed . As shown in the video, Nighttime cooling cools the subsurface diurnal warm layer, that subsurface heat must be transported upward to the surface far more than any heat could be transported downward causing a net cooling. And as that ~1 meter subsurface layer cooled, the the skin layer remained cooler despite being warmed from subsurface heat.
I dont see any mechanism that is carrying IR-heated skin surface warmth downward in any significant manner. I see a lot of narratives making such claims, but no mechanisms to support that claim. What I do see is narrative violating the 2nd Law of thermodynamics.
Jim: You state “I don’t see any mechanism that is carrying I.R.heated skin warmth downward manner in any significant manner”. I am sorry, but this statement indicates inadequate knowledge of the basics of the physics of heat transfer.As in a previous reply ,I recommend you visit http://www.temporalpublishing.com, and read Item 8 of the supplemental material for instructors. I offer this advice In the spirit of trying to help my team ( I am usually viewed as a “climate sceptic” whatever that may mean.)
If you are truly just trying to help, then simply explain here the mechanism that demonstrates your “more adequate knowledge”.
“I dont see any mechanism that is carrying IR-heated skin surface warmth downward in any significant manner. “
As I said in my first comment, although such a mechanism is not needed because the flux (of sun-derived heat) in the water is upward, that flux clearly shows the mechanism. It is of comparable magnitude to the IR flux, and it reaches the surface mainly by turbulent heat transfer. In the mm range near the surface, surface tension becomes important, turbulence fades, but the scale is such that molecular diffusion can carry the flux. All that really happens. And it could equally carry the IR flux the other way.
STOKES ays “a mechanism is not needed because the flux (of sun-derived heat) in the water is upward, that flux clearly shows the mechanism.”
Huh.
The question at hand is does IR heating thee microns thick skin layer then heat the deeper ocean.
As you reiterate what I and everyone says, because thee sun heats the subsurface water the heat flux is clearly upward.
So how do you magically jump to say “that is the mechanism”, carrying IR heated water downward???????
Jim: Conservation of energy requires that IR heating of the skin layer reduces the heat loss from the deeper ocean.Most people would call this a “heating” of the deep ocean, but the issue is now perhaps one of semantics.Please read the reference to the ocean surface energy balance that I have previously given you.
“Conservation of energy requires that IR heating of the skin layer reduces the heat loss from the deeper ocean”
What???? You just made that up. Conservtion of energy has no such requirement!!!
“So how do you magically jump to say “that is the mechanism”, carrying IR heated water downward???????”
I didn’t. I said
“All that really happens. And it could equally carry the IR flux the other way.”
And it could.
As originally said in the post, arguing about this is silly and useless. It is perfectly valid to see the perturbation induced by a sudden increase in down IR as the superposition of an increment solution, with the pulse increase on a zero base. In that view, the IR induces a downflux (in the sea) of heat from the IR which adds to the steady upflux of heat produced by sunlight. The net effect is a reduced upflux. There is no way of distinguishing the two views.
I Heree’s ypour words quoted exactly
“although such a mechanism is not needed because the flux (of sun-derived heat) in the water is upward, that flux clearly shows the mechanism“
My respect for you is falling rapidly
Anthony is right. If you slow heat loss by adding heat to the top layer, the bottom layers end up warmer than they would be without the additional heat.
w.
I strongly disagree that “Conservation of energy requires that IR heating of the skin layer reduces the heat loss from the deeper ocean”
What the conservation of energy only requires is that the total heating of the skin layer to be accounted for by emitted energy and stored energy. The skin surface layer has been shown to radiate more heat away, as well as evaporation and conduction losses than it absorbs.. The cool skin surface layer mantains a the needed temperature gradient
Such statements as the conservation of energy requires the bottom layers to heat is just handwaving that does not detail the mechanisms that shows how IR absorbed in the skin layer alter the temperature gradient causing a warmer bottom layer.
The temperature gradient increases during the day due to greater heating and decreases at night. The gradient is reduced at night but the bulk water below still cools and sheds its solar heat that doesnt get mixed to depth
I think that something that is being overlooked by everyone is that water molecules can only leave the surface in contact with air. Thus, the surface layer can be cooled by evaporation. However, below the surface, even a higher temperature cannot evaporate the water, thus allowing it to become even warmer during the daytime.
To follow on from that accurate description of the daytime, where increasing heat leads to stratification and warmth in the subsurface layers, we have the nocturnal overturning. This takes a curious form, the inverse of daytime overturning.
What happens is that after sufficient surface cooling, a number of distributed columns of descending colder water form. They are moving vertically downwards at a pretty good clip.
Over the rest of the ocean, the day-warmed water is rising everywhere except at the descending columns. Surface water, including the skin layer, is moving horizontally towards the nearest descending column. There, it joins the other water moving downwards.
And as a result, in between the columns, new warmer water is constantly replacing the skin layer, cooling, moving horizontally, and descending …
The net effect of this constant overturning is that every night, about the same amount of energy is lost from the subsurface layers as was gained during the day.
My best to you,
w.
Nick Stokes: “In the mm range near the surface, surface tension becomes important, turbulence fades, but the scale is such that molecular diffusion can carry the flux. All that really happens. And it could equally carry the IR flux the other way.”
WR: “Surface tension becomes important”. This means that the upper layer of molecules (from where evaporation takes place) becomes more or less fixed. Evaporation requires a lot of energy, partly because the surface tension of water is high. Insects can move on water surfaces. The energy needed for evaporation can come from below but conduction by water is very slow. Colder water of the same salinity does not flow upward because of its higher density. So where would the for evaporation necessary ‘super energizing’ energy come from? The only logical possibility is downwelling longwave radiation, in quantity double as high as downwelling solar and contrary to solar, not dispersed over tens of meters but fully concentrated in the top microns (!) at the surface. Could all this concentrated LW energy be the factor that enables evaporation to cool the surface at relatively low ocean temperatures? I think so.
I repeat: conduction in water is very slow, also downward. And warmer water never flows downward unless salinity is higher. Salinity only becomes higher after evaporation. And what is enabling evaporation in the top of the top layer? Downwelling longwave energy, absorbed in the upper microns.
Any rain diminishes salinity of the surface layer. And rain falls on the top of the ocean. Warm surface water only can go down after evaporation has been exceeding rainfall and density became higher than the density of the surrounding surface water, aside and below.
How high would evaporation be without ‘more than double solar’ (333 W/m2 of downwelling longwave) absorbed in the upper microns of water surfaces? My guess: very low, the low evaporation resulting in a strongly diminished evaporative heat loss causing rising ocean temperatures.
My conclusion: The on top of the surface absorbed downwelling longwave energy enables the deeper oceans to remain relatively cool.
And more downwelling longwave radiation, what would that do? My guess: it would be a main factor in the energizing of skin water molecules resulting in the observed 6-7% higher quantity of water vapor in the air per degree Celsius in case of warming. When highly energized water vapor escapes the ocean surface and meets colder air above, that air is warmed by collisions with water vapor and if cooled down, de-energized water vapor will fall back to the surface, once more cooling the surface.
Probably all eventual initial radiative warming by downwelling longwave will be cancelled, especially when all follow-up effects of evaporative cooling are taken into account: higher convection, higher wind speeds at the surface, higher conductive heat loss of ocean surfaces, higher convective transport of latent and sensible heat away from the surface, plus development of tropical clouds earlier on the day, reflecting more of the intense tropical solar irradiation to space before this could warm whether the surface or the atmosphere below clouds.
Perhaps there might happen some temporary initial warming, but ocean temperatures will remain maximized at yearly 30 degrees Celsius. And evaporative and convective cooling over the mid latitudes and cold polar oceans will be enhanced, thanks to the boost given by concentrated LW energy absorbed in the top microns of the surface.
“I repeat: conduction in water is very slow, also downward. And warmer water never flows downward unless salinity is higher. “
No, this is the same misunderstanding of turbulent heat transfer that Jim seems to have. The ocean is always turbulent. Turbulence consists of eddies at many different scales; there is ancient theory (Kolmogorov) about how the energy is partitioned between the scales. But it doesn’t involve directional flow or salinity.
Turbulence greatly enhances diffusivity. That is why the sun’s heat, thermalized at depth, can diffuse to the surface with a fairly small temperature gradient. And it would allow heat to go the other way if the temperature gradient were reversed.
As I said, turbulent transport is reduced near the surface, basically because of surface tension. That is why Jim’s diagram of the top few microns shows a steep temperature decline (going up). But it is a submillimeter range, and so molecular conduction carries the heat with a drop of a fraction of a degree.
Nick: “As I said, turbulent transport is reduced near the surface, basically because of surface tension. That is why Jim’s diagram of the top few microns shows a steep temperature decline (going up). But it is a submillimeter range, and so molecular conduction carries the heat with a drop of a fraction of a degree.”

WR: The right part of Jim’s diagram shows the situation for night or daytime well mixed. There is still that drop in temperature. DLR works day and night and in every situation.
The oceans are cooling by evaporation because evaporation takes the fastest molecules away: they have sufficient energy to continue in the gas state. The remaining molecules are the colder ones: the average temperature drops.
Strong winds mix well, as is also shown on the left of the next graphic. But still, there is that drop in skin temperature caused by evaporation. At high DLR.
Figure 1. The GHRSST diagram of the temperature at the ocean surface. The ocean conditions, especially the wind speed and daytime or nighttime can make a 2.5°C difference, or larger, in the temperature gradient from the surface to the foundation (stable portion of the mixed layer) temperature. The depth to the top of the stable portion of the mixed layer can vary from essentially zero to 10 meters. Source: GHRSST.
You are forgetting about wind and waves.
Wim, you say
While it helps explain where some of the LW energy is going, that can only account for less than a quarter of the LW energy.
We know that something on the order of 80 W/m2 of energy is lost from the surface to evaporation. But there is about 360 W/m2 of downwelling LW … what’s happening with the other 280 W/m2?
w.
An interesting question Willis, that already kept me busy some days. As usual, many thoughts came up leading to numerous side paths. One of the side paths was that we never should have started thinking about radiation in order to understand climate: too many questions will come up without ever reaching the central point in climate. We think about radiation because we are confronted with questions about “the possible role of CO2”. That question was posed by the wrong group that took the lead in the climate discussion, and was posing the wrong question and was using the wrong research method, and deliberately centered all discussion around the wrong molecule. The right molecule is H2O and that is the molecule with which we should have started climate studies, and also should have continued. (You did: as proven by your thermostat hypothesis and you were directly at the core system setting surface temperatures: convection)
So let’s look at H2O and radiation. I prefer to use the numbers for ‘Sea only’ by Martin Wild.
https://link.springer.com/article/10.1007/s10712-011-9150-2/figures/1
From the graphic, Sea only:
Solar absorbed (ocean) 170 W/m2
Net Surface cooling:
Net radiation (409 – 356 =) 53 W/m2
Net Evaporative heat loss 100 W/m2
Net Conductive heat loss 16 W/m2
In the ‘Frozen Earth’ situation (an Earth without atmosphere and without greenhouse gases), solar absorbed 170 W/m2 would have resulted in 170 W/m2 of surface radiation, and a very low surface temperature would be the result. In our present H2O-dominated climate the role of radiation in surface cooling diminished to only (net) 53 W/m2 over the average ocean. While having over oceans 409 W/m2 of surface radiation, the contribution of net radiation diminished from 170 W/m2 (frozen Earth) to just 53 W/m2 in our warm world. The warmer, the less radiation acts as a net surface cooler. An extra W/m2 of DLR coincides with a smaller share of radiative surface cooling. The more DLR, the more surface cooling becomes (and became) dependent on evaporation and connected processes.
Back to your question. If over oceans 356 W/m2 of back radiation is added to the oceanic energy, instantly 409 W/m2 is radiated away by the energy-activated surface. One could say, all back radiation is used to fuel surface emissions but that is not what happens. All back radiation is added to ocean energy and all absorbed ocean energy has to disappear through the surface. In our warm surface situation, net radiative heat loss is 53 W/m2, conduction 16 W/m2, and evaporation 100 W/m2. At least we can say that at this level of surface temperatures evaporation profits most of a huge load of DLR energy present in the [top of] the oceans, while net radiation shows negative effects.
Supposing a start by “Snowball Earth”, in that case, evaporation (sublimation) is low: but a small number of H2O molecules will disappear into the atmosphere. As a consequence, net evaporative surface cooling is very low and the role of radiation in cooling the surface still is huge. At the lowest level of evaporation back radiation (DLR) is also at its lowest point. But low DLR already coincides with a diminished net surface radiative heat loss. The share of net radiative heat loss diminished by the closing of the atmospheric window by the first greenhouse molecules absorbing surface radiation. [Note: in my point of view we can only understand climate when the surface is always the location we are starting with]
The atmospheric window shows how effective surface radiation is in cooling the surface. In our warm situation with all present water vapor in the air, only 5% of surface emissions are able to escape to space, 22 W/m2 out of 396 W/m2 surface emitted (numbers derived from Kiehl and Fasullo 2011). We can conclude that on a warmer Earth we have a very small atmospheric window, that we have massive DLR, and a diminished role for radiation as surface cooler. But in that situation of massive DLR, evaporation has largely grown in importance.
Although it is not correct to say that DLR stimulates evaporation more than radiation (it doesn’t), looking at the facts shows that high DLR coincides with high evaporation, and at present temperatures high evaporation is the dominant and most effective surface cooler, while the efficiency of surface emission has become marginal, diminishing the overall role of surface emission. The warmer, the less important surface emissions, and the more important evaporation and evaporation linked atmospheric processes: convection, solar absorption by atmospheric water vapor resulting in surface cooling, and cloud cooling.
The most interesting is the conclusion that from the very first greenhouse molecules, surface radiation gets more and more disabled as (net) surface cooler. Therefore, all attention should go to water vapor H2O: in a greenhouse situation it is not radiation that sets surface temperatures, surface temperatures are set by the strength of evaporative cooling and connected atmospheric processes. You already proved this by your thermostat hypothesis and you are right. Your TAO buoy research proved the system even works on a daily basis.
In a situation of a low greenhouse effect (low water vapor H2O), atmospheric absorption of solar will be very low (because of hardly present water vapor), cloud coverage over the tropical area will be low, and solar warming of the oceans will be strong. By the warming of the oceans, more water vapor H2O will be released which will reduce the atmospheric window, enhance DLR, warm the oceans, diminish the role of surface emission, and enhance the cooling role of evaporation and connected processes, etc., etc.
Once started, the process of continuing oceanic/atmospheric (H2O) warming is unstoppable until …. present surface temperatures are reached and massive H2O-related cooling equaled warming.
The first water vapor molecules ever started the warming of the Earth leading to the release of more water vapor and resulting in more ocean warming, and this process continued until the last water vapor molecules that were released stopped further warming.
Ocean surface temperatures set the quantity of water vapor, and the quantity of water vapor sets the level of surface temperatures. Whatever the [initial] radiative input.
Only orbital changes (redistributing solar over latitudes) and a change in the redistribution of tropical absorbed heat (and following effects) result in variations in surface temperatures.
Willis I am perplexed that you keep asking “here is about 360 W/m2 of downwelling LW … what’s happening with the other 280 W/m2?”
Several studies show greater amounts of LW leaving the skin surface every second than get absorbed. That dynamic clearly explains what is happening to the LW that doesnt increase evaporation or sensible heat loss.
So, why do you ignore that dynamic?
It is part of process that Planck calls compensation.
Max Planck. The Theory of Heat Radiation by Max Planck (p. 118). Prabhat Prakashan. Kindle Edition.
“absorption without emission, is impossible in nature.”
Great quote from Planck. Thanks so much for sharing, Jim. It is completely in line with the Stefan Boltzman Law
Thanks. We didn’t study this in thermodynamics when I went to school. I wish we had. I’ve studied Planck’s Theory of Heat Radiation over a lot hours for several years. Basically since I got into climate science and wondered how such a small amount of a trace gas could have such an outlandish effect. His book covers a lot of ground but starts at a very basic level. You want to know heat radiation, read it.
Jim Gorman: “… since I got into climate science and wondered how such a small amount of a trace gas could have such an outlandish effect”
WR: We should understand better the huge influence of greenhouse warming. Cooling by ‘radiation only’ shows how huge the theoretical temperature rise by our present greenhouse atmosphere really is. I will explain.
Of decisive importance for the right insight is the “infinitely thin shell” Planck added to Kirchoff’s theory. In combination with ‘radiation only cooling’ and the ‘atmospheric window’ the correct theoretical setting shows us the real strength of the Earth’s nearly complete greenhouse effect. Without additional cooling the Earth’s surface should have been ‘bloody hot’: more than 200 degrees Celsius – if cooled only by radiation.
With an ‘infinitely thin shell’, no storage of energy (like on Earth in oceans, soil, and a bit in the atmosphere) is permitted: at the same moment of absorption the absorbed energy needs to get lost, which happens at a certain shell (surface) temperature as calculated by the Stefan-Boltzmann (S-B) equation. As soon as there is some storage of energy, total energy rises and emission [‘surface’] temperatures have to rise in order to radiate all energy away. And that rise in temperature is huge in case the atmospheric window is nearly fully closed, as is the case with our own greenhouse atmosphere: only 22 W/m2 of 396 W/m2 of surface radiation reaches space through the remaining atmospheric window (numbers from Kiehl and Fasullo 2011). Only 5.55556% of al surface radiated energy is not absorbed and effectually cools the surface and the Earth. For the ‘radiative cooling only’ situation, the rise in temperature is much higher than expected.
Put an efficiency of 0.055556 (for ’emissivity’) in this S-B calculator and for ‘radiated power’ fill in the number 161 (= absorbed solar in W/m2), and you will see that in case the cooling of the surface is only dependent on radiation, the surface’s temperature would rise to 202.3 degrees Celsius.
Ergo, ‘something else’ is setting surface temperatures on Earth, something else than radiation. And that ‘something else’ is H2O: the evaporation of water vapor and connected processes in the atmosphere, mainly convection and cloud formation. Some more or less surface uptake of DLR energy doesn’t matter: the radiation only situation is that hot, that adding additional cooling results in a huge surface cooling if that additional cooling is by the evaporation of water, H2O, releasing water vapor.
(Water vapor is also our main greenhouse gas and produces most Downwelling Longwave Radiation DLR).
H2O will first stop its huge cooling activities (cooling the surface back from the initial 202.3 degrees Celsius as calculated) when evaporative cooling is down to the level that evaporative cooling, convection, tropical clouds, and tropical solar absorption (as permitted by the resulting quantity of tropical clouds) are such that ‘total surface heat gain’ equals ‘total surface heat loss’. The quantity of convective surface heat loss, tropical cloud cooling, and the quantity of tropical solar uptake, all depend on the quantity of water vapor evaporated from the surface. And the quantity of evaporation depends on surface temperatures. On Water Earth surface temperatures set the quantity of atmospheric water vapor and the quantity of water vapor sets surface temperatures. A closed system.
N.B. When more radiation is added by rising DLR, surface radiative emission will also rise, but this is not the case for net surface radiative heat loss to space. Extra emissivity is relatively low: no more than a 1.4% rise in emissions per 1K, while the quantity of atmospheric water vapor (and evaporative cooling) rises by nearly 7% (Clausius-Clapeyron). Further, at higher DLR the atmospheric window also closes more, diminishing the cooling efficiency of all surface radiation, and, as previously remarked here, diminishing net surface emission. And H2O? Evaporation rises by about 7% per 1K rise in surface temperature. The share of water vapor H2O rises firmly and water vapor related processes rise in concert, especially convection and the formation of tropical clouds.
Water vapor H2O sets the final [average] level of temperatures, and surface temperatures set the [average] quantity of atmospheric water vapor.
Where does actual variation in surface temperatures come from? Present temperature variations observed are caused by changes in the oceanic-atmospheric system plus by (slow but ever continuing) changes in orbit. And as described above, not by changes in the total greenhouse effect. In a greenhouse atmosphere like ours, cooling by the H2O system sets the level of surface temperatures independent of total warming by certain quantities of greenhouse gases other than water vapor. And also independent of greenhouse warming by water vapor H2O itself: surface cooling dominates. Once more, the reason is: surface cooling by H2O and related processes sets surface temperatures, not surface warming.
(More fundamental changes, the changes in topography and in ocean/continent configurations result in a different redistribution of tropical energy happening over very long periods of time and so result in different ‘Climate States’ like Hot House or Ice House States. Those changes happen at timescales we do not experience during our lifetime)
Sorry, I couldn’t edit the comment above, some corrections had to be made, sometimes important ones. Below the corrected (definite) version:
Jim Gorman: “… since I got into climate science and wondered how such a small amount of a trace gas could have such an outlandish effect”
WR: We should understand better the huge influence of greenhouse warming. Cooling by ‘radiation only’ shows how huge the theoretical temperature rise by our present greenhouse atmosphere really is. I will explain.
Of decisive importance for the right insight is the “infinitely thin shell” Planck added to Kirchoff’s theory. In combination with ‘radiation only cooling’ and the ‘atmospheric window’ the correct theoretical setting shows us the real strength of the Earth’s nearly complete greenhouse effect. Without additional cooling the Earth’s surface should have been ‘bloody hot’: more than 200 degrees Celsius – if cooled only by radiation.
With an ‘infinitely thin shell’, no storage of energy (like on Earth in oceans, soil, and a bit in the atmosphere) is permitted: at the same moment of absorption the absorbed energy needs to get lost, which happens at a certain shell (surface) temperature as calculated by the Stefan-Boltzmann (S-B) equation. As soon as there is some storage of energy, total energy rises and emission [‘surface’] temperatures have to rise in order to radiate all energy away. And that rise in temperature is huge in case the atmospheric window is nearly fully closed, as is the case with our own greenhouse atmosphere: only 22 W/m2 of 396 W/m2 of surface radiation reaches space through the remaining atmospheric window (numbers from Kiehl and Fasullo 2011). Only 5.55556% of al surface radiated energy is not absorbed and effectually cools the surface and the Earth. For the ‘radiative cooling only’ situation, the rise in temperature is much higher than expected.
Put an efficiency of 0.055556 (for ’emissivity’) in this S-B calculator and for ‘radiated power’ fill in the number 161 (= absorbed solar in W/m2), and you will see that in case the cooling of the surface is only dependent on radiation, the surface’s temperature would rise to 202.3 degrees Celsius.
Ergo, ‘something else’ is setting surface temperatures on Earth, something else than radiation. And that ‘something else’ is H2O: by evaporation of water vapor and connected processes in the atmosphere, mainly convection and cloud formation. Some more or less surface uptake of DLR energy doesn’t matter: the radiation-only situation is that hot, that adding additional warming results in more surface cooling by H2O.
(Water vapor is also our main greenhouse gas and produces most Downwelling Longwave Radiation DLR).
H2O will first stop its huge cooling activities (cooling the surface back from the initial 202.3 degrees Celsius as calculated) when evaporative cooling is down to the level that evaporative cooling, convection, tropical clouds, and tropical solar absorption (as permitted by the resulting quantity of tropical clouds) are such that ‘total surface heat gain’ equals ‘total surface heat loss’. The quantity of convective surface heat loss, tropical cloud cooling, and the quantity of tropical solar uptake, all depend on the quantity of water vapor evaporated from the surface. And the quantity of evaporation depends on surface temperatures. On Water Earth surface temperatures set the quantity of atmospheric water vapor and the quantity of water vapor sets surface temperatures. A closed system.
N.B. When more radiation is added by rising DLR, surface radiative emission will also rise, but this is not the case for net surface radiative heat loss to space. Extra emissivity is relatively low: no more than a 1.4% rise in emissions per 1K, while the quantity of atmospheric water vapor (and evaporative cooling) rises by nearly 7% (Clausius-Clapeyron). Further, at higher DLR the atmospheric window closes more, diminishing the cooling efficiency of all surface radiation, and, as previously remarked here, diminishing net surface emission. And H2O? Evaporation rises by about 7% per 1K rise in surface temperature. The share of water vapor H2O rises firmly and water vapor-related processes rise in concert, especially convection and the formation of tropical clouds.
Water vapor H2O sets the final [average] level of temperatures, and surface temperatures set the [average] quantity of atmospheric water vapor.
Where does actual variation in surface temperatures come from? Present temperature variations observed are caused by changes in the oceanic-atmospheric system plus by (slow but ever continuing) changes in orbit. And as described above, not by changes in the total greenhouse warming effect. In a greenhouse atmosphere like ours, cooling by the H2O system sets the level of surface temperatures independent of total warming by certain quantities of greenhouse gases other than water vapor. And also independent of greenhouse warming by water vapor H2O itself: surface cooling dominates. Once more, the reason is: surface cooling by H2O and related processes sets surface temperatures, not greenhouse surface warming.
(More fundamental changes, the changes in topography and in ocean/continent configurations result in a different redistribution of tropical energy happening over very long periods of time and so result in different ‘Climate States’ like Hot House or Ice House States. Those changes happen at timescales we do not experience during our lifetime)
I do understand what you are outlining. I was only dealing with the “radiation” portion that Planck has pretty much covered in exacting detail. He makes it pretty plain that absorption without emission is impossible. Even if all emissions are reflected and absorbed, they are instantly emitted again without a temperature increase. This only makes logical sense since the reflecting body would appear to be in equilibrium with the source.
What most don’t understand is that if you make the assumption that the sun’s insolation passes unrestricted through CO2 to the surface, then the surface becomes a proxy source of energy from the sun, at least as far as CO2 is concerned. The GHG theory must assume this or they will be required to allocate some level of absorption to insolation thus ruining their whole theory of human CO2 causing the warming.
From what I have seen, CO2 does absorb some level of near IR from the sun. I haven’t investigated how much but it will be energy that bypasses that from the surface.
When you have 450° F infrared longwave photons pouring down on one side of
the ocean surface and 55° F liquid water molecules a teensy bit below that
surface, this is not an equilibrium situation where the words “heat flow”
even apply. This is a HIGHLY “turbulent”, steady state, dynamic,
microscopic non-equilibrium process where even the concept of
“temperature” doesn’t apply. Of course, some of that low-frequency heat
will somehow (don’t ask me how — then you’re talking about
super-sophisticated physics) make its way down deeper into the ocean. My
emphasis, of course, is where that heat did NOT come from — CO2.
As a previous contributor stated, we are seeing the oceans gaining heat
content faster than the atmosphere is. So what does CO2 in the atmosphere
have to do with this warming?
David Solan
“450° F infrared “
What are you talking about? Where did you get that temperature???
So, ah: “No amount of thermal emissions in the emissive frequency of CO2 will penetrate the vapor on the surface of water in an atmosphere.” is a much much shorter explanation of part of it. This doesn’t preclude the water vapor at the surface from emissing towards the water but that input is generally going to create more water vapor because that’s how distillation works.
This whole argument can be resolved in favor of the “no ocean heating” by using a tub of water and a 2500w IR coil reflected at that surface. We can test this little hill of bullshit in a laboratory setting (or swimming pool) right off the bat.
The argument is that a warmer atmosphere do to greenhouse gases cause the ocean to shed heat slower. The problem with this has and will always be that ocean is warming before the atmosphere not the other way around.
if you have two pots of boiling water, one under a low flame and one under a high flame (more IR), does anyone really think one is hotter than the other?
increased IR doesn’t necessarily change the temperature, the outgoing heat flux can change without the temperature increasing
as to whether that’s the case for the Earth’s oceans I couldn’t say for sure, but I’m reminded of Willis’ tropical thunderstorm thermostat
Longwave IR being all absorbed in only the top couple or few microns / micrometers of water is incorrect. About half the blackbody radiation of 280 K (I think reasonably representative of back radiation) has wavelength between 7 and a little over 16 microns. About 6% has wavelength between 4 and 7 microns. The remaining 44% is mostly of wavelengths over 16 microns, with at least 13 of that 44 % having wavelengths over 30 microns. Back radiation contribution from temperatures much different from 280 K will cause the percentages to be more outside the 7-16 micron range and less within that range.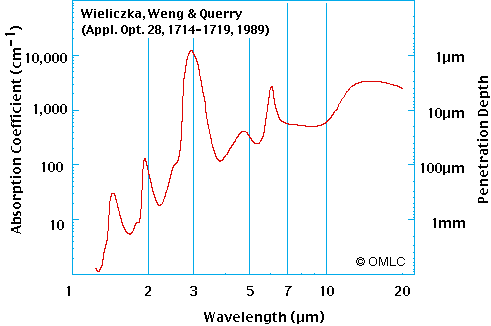 The penetration depth is the e-folding depth, and “half life” of penetration is .693 times what the graph shows. This means lots of longwave IR is being absorbed more than just a few microns below the surface.
The penetration depth is the e-folding depth, and “half life” of penetration is .693 times what the graph shows. This means lots of longwave IR is being absorbed more than just a few microns below the surface.
Meanwhile I did some Googling for graphs of spectral absorption of water. One I found is at
CO2 infrared is centered around 15 microns which on your chart penetrates only a few microns
Jim, it was my understanding that you were discussing ALL DLR, not just CO2 DLR … was I wrong?
w.
I’ve been discussing longwave radiation from greenhouse gases.
Sorry for the abbreviation, DLR is Downwelling Longwave Radiation. So your answer is, you’re discussing all DLR not just CO2?
w.
As far as I can see, nobody has answered my four questions. So I’ll ask them again:
The second and fourth questions are most important.
• If the DLR is not warming the ocean, where is it going?
• We know the ocean radiates ~ 400 W/m2 and receives ~ 165 W/m2 from the sun. If it’s not getting energy from DLR … why isn’t it frozen solid?
My best to all.
w.
PS—PLEASE don’t just say “I answered your questions”. There are 290 comments on this thread. If you think you did, please LINK to the exact location where you answered one or more of the questions.
Willis I am totally baffled that you keep asking that question when it has been shown that the skin layer is emitting most, if not all the absorbed DLR. Even Wong & Minett show that in their illustrated result. “where is it going?” It emitted out of the ocean. Where is the mystery?
You ask “If it’s not getting energy from DLR … why isn’t it frozen solid?”
That is a bogus question. FIrst I never argued it is not receiving DLR, I argue it is just emitted almost immediately before it can be trasnported to depth. As would be expected from the Stefan-Boltzan law.
Your link to SOD answers that question. “The fact solar heating happens more deeply clearly suggests if heat from the two sources, shortwave and longwave, it is shortwave that is far more likely to warm the deep ocean .”
If solar heat is trapped at depth and cant cool, the water warms. Thee ocean would only freeze if the incoming solar heat was rapidly radiated back to space.
Furthermore before the AAC froze sea ice around Antarctic, the bottom of the ocean was being fed by warm brine from evaporation in shallow seas and sinking to depth. The oceans hold heat that’s been trapped for centuries and millennia
So here is the question that no answers, If the skin surface is emitting more IR than it absorbs, how is DLR heating the ocean????
“That is a bogus question.”
It isn’t a bogus question. But to rephrase it slightly:
Given that
1. Relying on sunlight alone, the sea would be frozen solid, and
2. With DLR, the sea sits at 15°C
isn’t it reasonable to say that DLR is responsible for the warmer state?
The key point being that with more DLR, it will be warmer still.
What Nick said.
Jim, you say:
Let me ask you this.
If there were no DLR, what would the temperature of the ocean be?
I figure it like this. Without DLR, the ocean would be warmed only by the sun, at ~165 W/m2. Therefore, it would be radiating a maximum of ~165 W/m2, assuming zero sensible or latent heat loss.
And that, by Stefan-Boltzmann, is a maximum of about -40°, either F or C, take your pick. So it would be frozen solid all the way down.
Let me add that you are correct, that much of the DLR that is absorbed is radiated away on a short time scale. But without that absorbed DLR, the same amount of energy would have to come from the deeper ocean, leaving it cooler.
Basically, DLR slows the heat loss from the ocean. I mentioned above the nocturnal overturning.
Curiously, the ocean is the reverse of the atmosphere. The atmosphere overturns during the day and stratifies at night. The ocean is opposite, stratified by day and overturns at night.
The oceanic overturning starts a few hours into the night. Without the sun, the ocean is radiating much more than it is absorbing. So the surface cools. And when it cools enough, it starts sinking, and the top layer of the ocean starts to overturn.
This brings warmer water from below to the surface, where it can lose more heat than the cooler water it replaced. Remember, every night the ocean loses approximately the amount of energy that it absorbed on the previous day, and it does this via overturning.
By keeping the surface warmer than it would be otherwise, the existence of DLR delays the onset of overturning. This in turn means less energy is lost, and the overturning layer is warmer than it would be without the DLR.
So clearly, albeit curiously, the existence of DLR leaves the temperature of the whole of the ocean, not just the top 10 µm, warmer than it would be without DLR.
My best to you and yours, Jim,
w.
PS—You say:
On average, this is assuredly true. But even a light wind causes turbulence that brings warmer water to the surface and moves cool water downwards. And of course, a strong wind with waves mixes the entire sea surface vertically in the top layer with no regard for temperature.
You ask, “If there were no DLR, what would the temperature of the ocean be?
Let create a hypothetical that you first need to answer.
If the ocean is gaining strictly solar shortwave at the rate of 165 joules per second per m2 and because that heat was absorbed at depth and we assume it cannot immediatley cool like skn surface IR, then after 12 hours of daylight the ocean has accumulated 1,188,000 joules of energy.
So what is the temperature of the subsurface layer?
Then for sake of argument the assume that subsurface cools only at 90% efficiency. So at the end of day 1 the subsurface has heated by 118,800 joules. After a year the subsurface has gained 43,362,000 joules and would easily avoid freezing.
To argue the oceans would freeze without DLR you must prove the oceans do not store solar heat.
Jim Steele Reply to Willis Eschenbach
August 4, 2022 7:00 am
I’m sorry, but that question doesn’t have enough information to answer. How deep is the warming? What is the starting temperature? Without those your question is unanswerable.
I’m sorry, but that assumption is completely contradicted by observations.
The “mixed layer” is the top 30 meters or so of the ocean that is mixed by nocturnal overturning and wind action. As a result the temperature of the mixed layer doesn’t change much from the top to the bottom.
And in any given ocean location, absent currents, the temperature of the mixed layer doesn’t vary much from week to week.
Now, if something is gaining more energy than it is losing, it warms up. And if it is losing more energy that it is gaining, it cools down.
Since the mixed layer is neither warming nor cooling, this means that on average, the heat gained during the day is lost during the night.
Not “90%” of the heat gained during the day.
ALL of the heat gained during the day.
So no, we simply cannot “assume that subsurface cools only at 90% efficiency.”
Let me come at this question from a different angle. Suppose we could magically turn off the DLR. Tomorrow, would the ocean then begin to warm or cool?
Well, it’s still radiating (losing) about 400 W/m2, because it would still be at ~ the same temperature as today. But it would only be gaining 165 W/m2 from the sun. Since losses are more than twice the gains, it would cool, and cool rapidly.
How far down would it cool? Well, it would cool until losses once again equal gains, down to where on average it’s losing 165 W/m2 to balance out the 165 W/m2 gain from solar energy.
And that’s minus forty degrees … and in fact it would be even colder than that, because there still would be some loss due to sensible and latent heat loss …
Best regards,
w.
I am saddened by your replies. I hope for a more physics based answer when I sought you out for a critique. First, instead of addressing the details of the post, I we got was a copy and paste from your decades old post, in which you repeat the question “, “If there were no DLR, what would the temperature of the ocean be?”
You never stated in the starting temperature or how deep the heating happens which indeed will have an impact.
But you reply to my hypothetical with
WE says, “I’m sorry, but that question doesn’t have enough information to answer. How deep is the warming? What is the starting temperature? Without those your question is unanswerable.”
But my hypothetical clearly shows the temperature could rise simply by added 165 W/m2 of solar energy, AN exacet temprature was not demanded. It appears you avoided addressing that because it refutes your claim the oceans would freeze without DLR.
I used a completely 90% hypothetical efficiency to address the dynamics of ocean heating, but you choose to attack that 90% despite you know that was not what I stated.
Nonetheless, you stated
WE says, “Since the mixed layer is neither warming nor cooling, this means that on average, the heat gained during the day is lost during the night. Not “90%” of the heat gained during the day.
ALL of the heat gained during the day..
But since that argument applies to ALL heat, you are suggesting neither Longwave or Shortwave are heating the ocean.
For now I will close suggesting your premise is flawed that
WE says” Well, it’s still radiating (losing) about 400 W/m2, because it would still be at ~ the same temperature as today.”
But the only thing that is radiating away is the skin surface. 400 W/m2 is being immediately radiated away. SO without your hypothetical 400 W/m2 of incoming IR, the skin surface would only radiate the heat reaching the skin surface from below. Since in the W & M paper there was a net 70 W/m2 off outgoing IR (LW-in minus LW-out,) then the rising solar heated waters easily account for that radiative loss and that leaves 95 W/m2 of solar heat to warm the oceans, not freeze them, or increase evaporation or the temperature gradient to the atmosphere
You are playing fast and loose with the mathematical equations, leading you astray .
Jim, you say:
As Nick has pointed out, the amount of radiation is SOLELY a function of temperature. It doesn’t know about the “heat reaching the skin surface”. It depends ONLY on temperature.
In my hypothetical, I asked what would happen tomorrow to ocean temperatures if we turned off DLR today.
The ocean would continue to radiate at something around 400 W/m2.
Now, note that this hypothetical situation, where a large fraction of the incoming radiation is cut off, happens every night. So we know exactly what would happen. The upper layer would start overturning.
As it radiates energy, the surface would cool and sink, overturning would begin, and warmer water below would continue to rise to the surface and continue to radiate at ~ 400 W/m2.
At this point, on average the ocean is radiating energy away at 400 W/m2, and gaining solar energy at 165 W/m2.
So to see if we can reach an agreement on one basic point, let me ask a simple question.
If the ocean is losing energy at 400 W/m2 and gaining energy at 165 W/m2, will it:
a) Warm?
b) Cool?
c) Stay the same?
Once we have an agreement on this we can move forwards.
w.
PS—please dial back on the accusations that I’m acting in bad faith by “playing fast and loose”. I don’t do that kind of thing. I tell the truth as best I know it, and I explain it in any way that I think will get the points across.
Jim, a further comment. You say:
Sorry for my lack of clarity. What I said that if something is in steady-state, neither warming nor cooling, that energy in has to equal energy out. If the energy flows were not equal, the object would be either warming or cooling.
And this in turn means that to a first order, the energy gained every day is lost every night.
I say that both LW and SW heat the ocean up to the point where increased temperature-dependent radiative energy loss equals energy gain, leading to the steady-state condition discussed above.
w.
The only agreement you can hope for is that the net effect of SW is to heat the ocean and the net effect of LW is to cool the ocean.
Steele clearly has decided not to understand more ghgs will reduce the net LW (out), hence make the ocean warmer.
And you have clearly decided to not understand that the skin surface shows an immediate net loss of IR, so more GHGs are irrelevant for ocean heating. You seem to believe that despite a net loss of IR from the skin that cools the ocean, a unicorn will turn that cooling into warming.
Yes, GHGs are irrelevant for ocean heating but they are very relevant for ocean cooling and thereby very relevant for the temperature of the ocean.
I don’t know what you base the “immediate” on. There is no “time” in the S-B law, nor is there any ‘information’ on how the energy was aquired. There is only temperature and emissivity, and like you have been told several times, without DLR the temperature would drop significantly.
All published evidence shows that heat gained by solar shortwave accumulates as the combined loss of heat from Longwave, Evapoartion (LH) and conduction with the winds (SH). Skin surface always show a net loss via Longwave. Here is a table of several ocean heat budget products revieweed by Song (2013) in How much net surface heat flux should go into the Western Pacific Warm Pool?”
“and like you have been told several times,” because the skin surface always shows a net loss of heat via longwave, ocean heat budgets would not be significantly changes if DLW is removed. Because the land heats differently DLW doees have an impact.
That LW WPWP is about the same as the global average, sea only, net LW -53 https://link.springer.com/article/10.1007/s00382-014-2430-z/figures/2 (2015)
You seem to think if the 356 DLW is removed 356 of the ULW will automatically disappear, based on your “ocean heat budgets would not be significantly changes if DLW is removed”.
That’s a totally flawed assumption of course. 2/3 of the energy input lost is not significant? Read again Willis’ explanation.
There is absolutely no logic in the world that should convince anyone that if the skin surface emits more LW than it absorbs, and that LW cant penetrate any deeper than the skin surface, then LW must be warming the ocean. LOL
Read the above paragraph again over and over, lgl, until the truth sinks in.
“ocean heat budgets would not be significantly changes if DLW is removed”
Like lgl, I am mystified by this. Firstly, Song’s definition of his acronyms:
“Net heat flux (hereafter QNET) at the air-sea interface is the sum of sensible heat flux (SHF), latent heat flux (LHF), shortwave radiation (SW), and longwave radiation (LW)”
He is doing a heat balance for the Pool. Because it is a region of ocean, it doesn’t have to balance; he is trying to show what heat is carried out by currents into adjacent regions. But the whole ocean does have to balance, and then his budget is just the same as mine in my second comment
F_SW+F_LW=F_U
where my F_U=-LHF-SHF -LW(up)
The sign changes because he treats LHF and SHF as (negative) fluxes down. His LW is net LW – ie LW in (DLW) minus LW out (hence negative). My F_LW is the down component; the up component is included in F_U.
What matters is that he treats LW (DLW) as an intrinsic part of the heat balance of the WPWP. The down component of LW is adding heat, as is SW. SHF and LHF are removing it. You can’t say that any budget is not significantly changed if a major component is removed. It all has to add up.
I dont why you felt compelled to go on about your confusing acronyms vs Song’s, but that’s what you do.
And yes Song, in this paper, is talking about the warm pool heat budget and that the accumulation of heat from shortwave requires balancing by emitting heat outside the tropics, which is what I mentioned in the video.
Regard LW budgets what all budgets of the skin surface show is LW out each second is greater than LW in each second, so there is no heating of the skin surface from LW in.
“Regard LW budgets what all budgets of the skin surface show is LW out each second is greater than LW in each second, so there is no heating of the skin surface from LW in.”
That makes no sense. It is an additive budget, and LW_in adds to it. What you say is like saying that if our business are currently making a loss, revenue isn’t doing anything for us.
The key thing is that it has to balance, and the main outflux depends on temperature. If LW_in increases, the temperature has to rise to balance.
Although all the data shows LW out from the skin surface is always greater than LW into the skin, i.e a net loss of LW, Stokes denies all the published evidence to complain, “That makes no sense. It is an additive budget, and LW_in adds to it.”
That seals it Nick! I can no longer bother reading anything you write when you deny all the evidence just to push your favored narrative!
Of course LW_out exceeds LW_in. Your absurd contention is that that means LW_in is no longer heating (because just one of the other fluxes is greater).
Suppose you have a house with a 10 kW furnace, but it turns cold and heat losses are 15 kW. So you supplement with a 5 kW electric heater. 15 kW>10 kW; does that mean the furnace is no longer heating the house?
Yes, when it gets as absurd as that, it is time to sign off.
Bad analogy, Nick. If either the furnace or the supplemental heater is outside of the house’s envelope, it’s not going to work very well for the occupants. If I recall, the point of contention here is whether or not DLR can warm the ocean’s subsurface layers. You presume it does, but haven’t provided a mechanism for it.
“ whether or not DLR can warm the ocean’s subsurface layers”
Well, it does. But to the point here is that in Song’s paper (as in any proper science) it does. What Jim is asserting is that if LW in is less than LW out, it can’t be warming. That argument is just based on flux magnitudes, as is my example.
For those who advance nonsense narratives about longwave heating of the skin surface and clearly have not doe the math First consider 1,000,000 ml= 1 m3
1 ml = 1 gram of water, so 1 m3 = 106 grams
2 microns = 0.000002 meter
Thus a skin layer that is one square meter and 2 microns thick weighs 2 grams
Since 1 gram of water requires 4.18 joules to increase by 1 °C.
Then one square meter of the 2 micron thick skin layer requires 8.35 joules to rise 1 °C
Thus absorption of 400 W/m2 of Longwave would raise skin surface 47.8 °C higher in just one second
The hottest regions of the warmpool just average 30°C, so thinking that Longwavee from greenhouse gases could raise the surface temperature by another 47.8 °C requires insanity or a university degree in the humanities.
Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.
With a observed temperature gradient showing heat ocean waters can only transport heat out of the ocean, the only explanation for the expected but unobserved rapid heating of the skin surface is that warming causes the rapid emission of an equal amount of longwave according to the Stefan-Boltzman law, so that the skin surface does not warm!
“clearly have not doe the math”
Of course we have done that math. That is why the condition is flux balance at the surface. Anything else leads to impossible temperatures.
Here was my flux balance equation (similar to Song’s):
F_SW+F_LW=F_U(T)
And again the implication: if F_LW (DLR) increases, F_SW is fixed by sunlight, so F_U must increase equally, and can only do so by increasing T.
Clearly you havent done any math because you think greater energy flux out than flux in means warming LOL and that’s just plain stupid
Just read it. Flux has to balance. If F_LW increases, F_U increases to match. That isn’t “more out than in”. It stays in balance. T rises.
You are citing Wong and Minnett. They put it thus:
“The additional energy from the absorption of increasing IR radiation adjusts the curvature of the TSL such that the upward conduction of heat from the bulk of the ocean into the TSL is reduced. The additional energy absorbed within the TSL supports more of the surface heat loss. Thus, more heat beneath the TSL is retained leading to the observed increase in upper ocean heat content.”
If heat content rises, the temperature rises.
You suffer from a premature rejection that only accounts for a partial dynamic. You say “so F_U must increase equally, and can only do so by increasing T.”
Indeed the Stefan Boltzman Law requires that temperature increase. But the rest of the story is higher temperatures emit more LW when then decreases the temperature.
We do know on average more LW is emitted than absorbed by skin surface each second . Instrumental measurements at best are taken every 10 minutes and more often every hour. That is ample time for the full dynamic of skin surface heat transfer to “absorb LW-warm-emit LW-cool”. That is the reason the skin surface does not warm by the predicted 47.8 °C due to absorbing 400 W/m2 of downward LW. It emits and cools before the transitory warming is detected. The skin surface emits more LW, and loses more heat to evaporation and sensible heat, so the skin surface is measured to be cooler than subsurface waters most of the time.
You push your agenda by ignoring the whole process. Bad science!
‘Well, it does.’
That’s an assertion, not a mechanism.
‘What Jim is asserting is that if LW in is less than LW out, it can’t be warming.’
That’s the way my refrigerator works, too.
You need to get Planck’s Theory of Heat Radiation. Radiation occurs based on temperature, period, end of story. If something radiates it cools. If it is radiating more that it is absorbing it still continues to radiate at its temperature but now part of that radiation is made up of what was absorbed. What is the upshot? It still cools but at a slower rate. It’s as simple as that.
You can’t add fluxes together in a scalar fashion. itt is more akin to a vector quantity. Like velocity, it has a direction, i.e., in or out. 180 degrees difference. If it didn’t work that way, you could never have equilibrium, i.e. two objects at the same temperature.
Look at the S-B equation. It doesn’t add absorbed to emitted to get net, it subtracts them. At equilibrium, there is a net of zero, as there should be.
I am having the same argument on Twitter about this. Planck explains it very well in his Theory of Heat Radiation.
They can’t understand that if I have a hot plate at 150 deg and put a steel bar on it, it will warm to 150 deg and radiate at 150 deg. If I then place another steel bar above it (not on it) at 50 deg. The hot bar doesn’t begin to radiate at 200 deg. It keeps radiating at 150 deg but the net radiation will be reduced by half so it cools at half the rate.
And guess what? The 50 deg bar will start to warm. Guess what the warming will be based on?
IOW, look at the S-B equation!
It is additive only in terms of vectors. You want an analogy. Two cars going in one going N at 100 mph and one going S at 50 mph. What is the resultant speed and direction?
Radiation works the same way. Look at the S-B equation and you will see they are not additive. What do you get when both are at equal temps? Double the net radiation, or zero net radiation?
lgl says “I don’t know what you base the “immediate” on”
Apparently you are quite slow to see the obvious.
As exemplified in W & M’s data on average the skin surface absorbs 400 joules each second while simultaneously losing 470 joules each second. Perhaps there is an unmeasurable time lag of a microsecond, but given the one second estimates I’ll stand by using thee word immediate. Every study I’ve seen show there is a net loss of IR every second from the skin surface.
Absorption and emission are independent processes. The surface does not have to absorb IR to be able to emit IR. It will absorb IR if there is any, and it will emit IR if its temperature is above 0 K.
DUH! So what’s your point?
Only that absorption and emission are independent processes.
You seem to think the surface emits 470 because it receives 400 from the atmosphere, so that if the 400 is removed suddenly it will only emit 70. This is implied by your “no change to the budget” statements. It’s simply wrong, not much more to say about it.
You are slow on the uptake. The residual 70 W/m2 once emitted LW balances absorbed doesnt “suddenly appear” it is the result of the cooling process heat from solar heated subsurfaces waters reaching the surface where they can cool.
And you are very wrong to rant absorption and emission are independent processes. Indeed there are cases where that may be true. But heat the first few microns of the water surface with 400 W/m2 (for the W &M paper) and the water immediately warms, and warmer waters immediately emit LW according to their new higher temperature. I think you are clueless about the Stefan Boltzman Law
Stop making up things. I wrote “suddenly it will only emit 70” ie 400 of the initial 470 ULR suddenly disappears after the 400 DLR is removed, in your self-invented physics.
“according to their new higher temperature”. Yes, like water usually does, and if the 400 DLR is removed it will cool until energy out equals energy in.
you are the only one making things up! I’ve done the math!
No, I’m just pointing to the implications of your ‘math’.
Your claim is that removing 400 from one side of the equation will leave the budget unchanged. To keep the budget unchanged, net that is, you have to remove 400 from the other side of the equation as well.
I assume you know evaporation and conduction to N2/O2 will remove heat from the skin layer also. The obsession with radiation balance ignores other forms of energy leaving the skin layer. Also, there is lots of “near IR” arriving directly from the sun. Why would removing GHG’s affect this at all.
While you are at it examine the attached image. Please notice the difference in the irradiance scale (incoming insolation) on the left side versus the irradiance scale on the right side (earth’s emissions).
“Why would removing GHG’s affect this at all.”
Not much. Who says it would? Those non-radiative losses only make things worse. Even more energy must be supplied to the surface to keep it warm. And much of the latent heat comes back to the surface as LW radiation.
Yes, E=hf, so what? The LW spectrum is much wider and it’s the area below the curve that matters, represents the total energy. Note the logarithmic scale

or, λpeak =b/T, so what?
Are you just trolling or completely clueless.You completely ignore the implications of the math presented, a behavior typical of trolls and fools. As W & M reported they observed no rise in temperature YET the surface absorbed 400 W/m2 of LW. Stefan-Boltzman Law requires that energy input to increase the skin temperature by 47.8 °C. It is also observed the temperature gradient prevents any of the heat from being transported downward. The way the Stefan Boltzman Law is not violated , is that the 400 W/m2 that is absorbed is immediately emitted.
Do you not understand the Stefan Boltzman Law cannot be violated when describing the thermodynamics? Maybe you dont even understand or believe in the Stefan Boltzman Law?
It is disturbing to see how many posters here readily violate S-B just to cling to their misguided beliefs.
The argument made in the video is very simple! The 400 W/m2 of LW from greenhouse gases that get absorbed aree immediately removed by 400 W/m2 of emitted LW. The satellites detect the LW and calculate the skin temperature. However the skin temperature is not warming the ocean at any greater depths.It is the mixing of that solar heated water more deeply penetrating solar heated water that increases ocean heat content. And the residual 70 W/m2 of the 470 observed emitting from thee ocean is due to heat upwelled from the solar heated subsurface along the temperature gradient.
Actually S-B’s law does not say anything about absorption. A simplified version of Kirchhoff’s law of thermal radiation states emissivity is equal to the absorptivity, but lets not start on that one.
You confuse yourself with the “immediately emitted”. The useful thought experiment is still, what will happen if the 400 DLR is removed? The correct answer is, nothing will change immediately after. The surface will keep emitting 470 W/m2, because that’s what its temperature dictates, but now there is a huge energy deficit of 400 W/m2, so the temperature will drop following the shape of a decaying exponential function until equilibrium, energy out = energy in, 70 = 70. (disregarding the non-radiative for simplicity) The temperature at that point is of course much lower than before the perturbation. If we then ‘turn on’ the DLR again, temperature will rise following a logarithm function and the higher temperature will of course eventually be restored.
Whether you choose to call the effect of DLR on the ocean heating, warming or make warmer is not that important.
“Are you just trolling or completely clueless”
lgl is neither. He just knows stuff about heat transfer.
Sure doesnt seem like he does.
If you are talking radiation physics, there must be some kind of radiation absorbed in order for there to be enough energy to emit. Is it IR, maybe, maybe not. Can the absorbed energy come from conduction? Sure. But energy must come from somewhere for an atom or molecule to emit.
For those who advance nonsense narrative about longwave heating of the skin surface and clearly have not doe the math First consider 1,000,000 ml= 1 m3
1 ml = 1 gram of water, so 1 m3 = 106 grams
2 microns = 0.000002 meter
Thus a skin layer that is one square meter and 2 microns thick weighs 2 grams
Since 1 gram of water requires 4.18 joules to increase by 1 °C.
Then one square meter of the 2 micron thick skin layer requires 8.35 joules to rise 1 °C
Thus absorption of 400 W/m2 of Longwave would raise skin surface 47.8 °C higher in just one second
The hottest regions of the warmpool just average 30°C, so thinking that Longwavee from greenhouse gases could raise the surface temperature by another 47.8 °C requires insanity or a university degree in the humanities.
Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.
With a observed temperature gradient showing heat ocean waters can only transport heat out of the ocean, the only explanation for the expected but unobserved rapid heating of the skin surface is that warming causes the rapid emission of an equal amount of longwave according to the Stefan-Boltzman law, so that the skin surface does not warm!
No, there must be som form of energy ‘absorbed’, or generated within the body. Anything that raise the temperature will do. The point here is the earth will not stop emitting LW just because it no longer absorbs LW.
For those who advance nonsense narratives about longwave heating of the skin surface and clearly have not doe the math First consider 1,000,000 ml= 1 m3
1 ml = 1 gram of water, so 1 m3 = 106 grams
2 microns = 0.000002 meter
Thus a skin layer that is one square meter and 2 microns thick weighs 2 grams
Since 1 gram of water requires 4.18 joules to increase by 1 °C.
Then one square meter of the 2 micron thick skin layer requires 8.35 joules to rise 1 °C
Thus absorption of 400 W/m2 of Longwave would raise skin surface 47.8 °C higher in just one second
The hottest regions of the warmpool just average 30°C, so thinking that Longwavee from greenhouse gases could raise the surface temperature by another 47.8 °C requires insanity or a university degree in the humanities.
Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.
With a observed temperature gradient showing heat ocean waters can only transport heat out of the ocean, the only explanation for the expected but unobserved rapid heating of the skin surface is that warming causes the rapid emission of an equal amount of longwave according to the Stefan-Boltzman law, so that the skin surface does not warm!
“Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.”
Your problem is despite absorbing less than 200 W/m2 solar the surface emits 470 W/m2. How much would it emit with only the 200 solar input?
There is no problem if you understand the laws of thermodynamics and how LW and SW penetrate the ocean differently. 400 W/m is immediately balanced by 400 W/m2 out. The residual 70 W/m2 out is part of the escaping solar added heat along with SH and LH heat loss.
How many times does this need repeating before you understand the basics laws of thermodynamics.
I asked how much would it emit with only the 200 solar input? Or 70 solar input if you prefer.
“As exemplified in W & M’s “
Here is W&M’s abstract (my bold):
“Ocean warming trends are observed and coincide with the increase in concentrations of greenhouse gases in the atmosphere resulting from human activities. At the ocean surface, most of the incoming infrared (IR) radiation is absorbed within the top micrometers of the ocean’s surface where the thermal skin layer (TSL) exists. Thus, the incident IR radiation does not directly heat the upper few meters of the ocean. This paper investigates the physical mechanism between the absorption of IR radiation and its effect on heat transfer at the air-sea boundary. The hypothesis is that given the heat lost through the air-sea interface is controlled by the TSL, the TSL adjusts in response to variations in incident IR radiation to maintain the surface heat loss. This modulates the flow of heat from below and hence controls upper ocean heat content. This hypothesis is tested using the increase in incoming longwave radiation from clouds and analyzing vertical temperature profiles in the TSL retrieved from sea-surface emission spectra. The additional energy from the absorption of increasing IR radiation adjusts the curvature of the TSL such that the upward conduction of heat from the bulk of the ocean into the TSL is reduced. The additional energy absorbed within the TSL supports more of the surface heat loss. Thus, more heat beneath the TSL is retained leading to the observed increase in upper ocean heat content.“
I know what the abstract hypothesized and the video addresses the result of their study which challenges their conclusions.
For W & M to say “the incident IR radiation does not directly heat the upper few meters of the ocean”[meaning the micron-thick skin layer where IR is only absorbed] violates the Stefan Boltzman Law. If the skin layer absorbs more IR, then temperatures rise. PERIOD.
It does not surprise me in the least Stokes that you would eagerly violate the Stefan Boltzman Law in order to push your bogus narrative. Pathetic!
You might look at the S-B equation in more detail. What are the units of radiation? What are the units of the constant? I think you will find “time” in an integral part of the equation.
Now is it a simplified version? Of course. It is assumed that the temperatures are at equilibrium. That lets you determine a unique value. If they are not, then the determination of the equation becomes much more difficult. Which do you think sun, ocean, and atmosphere exist under? Equilibrium or dynamic?
All the S-B Law states relevant to our heat budgets is 1) Higher temperatures will emit more heat and 2) absorption of more heat raises temperatures, which cause increased in heat emissions.
For the ocean dynamics affecting heat absorption are alway changing: day vs night, clear sky vs cloudy, summer vs winter, etc.
Yes, you will find time in everything. https://en.wikipedia.org/wiki/SI_base_unit#/media/File:Unit_relations_in_the_new_SI.svg
Equilibrium or dynamic?
In this thread, in some discussions equ, in others dyn.
“As Nick has pointed out, the amount of radiation is SOLELY a function of temperature. It doesn’t know about the “heat reaching the skin surface”. It depends ONLY on temperature.”
NO ONE has ever argued against that! Why is it brought up repeatedly as if you are refuting something???
WE, “The ocean would continue to radiate at something around 400 W/m2.”
No why would it? As you correctly repeated, the skin would only radiate at temperatures caused by heat reaching the surface in this hypothetical. And the skin is no longer heated by DLW.
So let me repeat my question, How does the skin layer ever heat up due to DLR when every study shows a net LOSS of LW.
The current real time dynamics of net loss is similar to your hypothetical no DLR at all.
How can you keep arguing DLR is adding anything but a transitory warming to the skin layer. From all the studies I have read, skin temperatures are at best measured every 10 minutes and more typically every 1 to 3 hours.
What the W & M study found was on average a net loss of about 70 W/m2.
And from “Air-sea heat flux climatologies in the Mediterranean Sea: Surface energy balance and its consistency with ocean heat
storage” Song (2017)
“This study provides an analysis of the Mediterranean Sea surface energy budget using nine surface heat flux climatologies. The ensemble mean estimation shows that the net downward shortwave radiation (+192 +/-19 W/m2) is balanced by latent heat flux (-98 +/-10 W/m2), followed by net longwave radiation (-78 +/-13 W/m2) [ a loss] and sensible heat flux (-13 +/- 4 W/m2). The resulting net heat budget (Qnet) is 2 +/-12 W/m2″
This study estimates the because there is a net loss of LW the ocean gained heat from solar shortwave.
Jim, in my search for agreement on the basics, and in order to cut through the fog, I asked a very simple question. If the DLR were suddenly turned off so it was only the sun heating the ocean, would the ocean:
a) Warm?
b) Cool?
c) Stay the same?
Could I get an equally simple answer, which would either be “a”, “b”, or “c”?
===========================
Next, if your answer is “b, when you turn down the heat things cool down”, then I have a second question for you:
If the DLR is turned off and we agree that the ocean would cool from that loss of incoming energy, just how far down will it cool? In other words, approximately what will the final steady-state temperature be when the ocean stops cooling from the loss of incoming energy?
w.
WE asks”Could I get an equally simple answer,” c) The answer is it wouldnt matter. All studies show the skin surface is emitting more LW than it absorbs every second. So there would be no change in your imaginary world. It is changes in solar heating that will affect ocean temperatures.
I suspect you are confusing warming and cooling of land dynamics, which would indeed cause faster cooling if DLR was removed.
Jim, that’s not clear. Are you saying the answer is c), that if there were no DLR the temperature of the ocean would be unchanged?
Please clarify, thanks.
w.
By removing your hypothetical DLR, the ocean heat content would not change. However the skin surface temperature would drop because without the balancing 400 W/m2 being emitted the satellite algorithm would calculate a cooler skin surface.
from your SOD link “ in general the ocean is moving heat into the atmosphere, rather than the reverse. The atmosphere is usually a few degrees cooler than the ocean surface.
Because turbulent motion is reduced the closer we get to the boundary with the atmosphere, this means that conduction is needed to transfer heat. This needs a temperature differential.”
(This reduced turbulent motion at the skin surface is ignored by Stokes.)
The skin surface cools at over 100% efficiency relative to the LW-in, due to conduction and evaporation. So there is always a temperature gradient that promotes a cooling effect, and that gradient is driven by how much solar energy is stored in the subsurface.
It is true that surface waves can disrupt the cooler skin surface, but that only allow subsurface water to cool faster, as the gradient is just shifted between the subsurface and cooler atmosphere. Numerous studies indicate the skin surface is almost immediately restored after such disruptions, testifying to the efficient cooling at the surface layer where IR is absorbed, contrasting with inefficient cooling of deeper solar heated water
Jim Steele: “The skin surface cools at over 100% efficiency”
WR: While the fastest (most energetic or ‘warmest’) H2O molecules evaporate, oceans cool faster by evaporation than by radiation and conduction which are strictly bound to the average temperature level. By evaporating H2O molecules more than average energy is added to the atmosphere. Therefore it is important to identify the forces that stimulate evaporation, like skin layer absorbed DLR.
Add to this the latent energy released by condensation of H2O molecules plus their functioning in the upward transport of sensible and latent heat to elevations from where energy is efficiently radiated to space, and evaporating water vapor molecules can be identified as ‘energy bombs’ changing all and everything in weather and climate.
Climate is nothing else than the average of 30 years of weather. Weather is ruled by the H2O molecule: low and high-pressure areas depend on the presence of the H2O molecule and the presence, extension and power of low and high-pressure areas determine wind direction, wind power and so the movement of currents in the oceans. High and low-pressure areas also influence the salinity of ocean surfaces and in this way influence which water masses will sink, at what temperature, and where.
It is the H2O molecule we should study.
“(This reduced turbulent motion at the skin surface is ignored by Stokes.)”
It isn’t ignored. I spelt it out
“It is of comparable magnitude to the IR flux, and it reaches the surface mainly by turbulent heat transfer. In the mm range near the surface, surface tension becomes important, turbulence fades, but the scale is such that molecular diffusion can carry the flux. All that really happens. And it could equally carry the IR flux the other way.”
What you are dismissing is the very obvious point I have made many times. There is a large existing flux, from sunlight, which rises through he water and leaves via the surface. The mechanism for heat transfer with moderate temperature gradient is established.
LOL This is why I increasingly lose respect for you .
You say “what you are dismissing is the very obvious point I have made many times. There is a large existing flux, from sunlight, which rises through he water and leaves via the surface”
But you totally ignore that that point is clearly made in my video where I detailed the depth of solar heat absorption and the gradient that it creates to cool the shallow surface waters via conduction and convection.
Willis, Nick: “Relying on sunlight alone, the sea would be frozen solid,”
WR: compare “Relying on sunlight alone, an Earth with a greenhouse atmosphere would get hot oceans”
Oceans need evaporation to cool down to actual temperatures: by actual greenhouse atmosphere radiation is nearly completely disabled as surface cooler (see my earlier comments here and here). To escape the powerful surface tension and in order to reach the highly energetic gas state, individual water molecules need to have a very high kinetic energy. They can get that high energy from hot ocean water or by a very local radiative stimulus like DLR. The last happens because the huge quantity of DLR does not immediately reaches the deeper layers like happens with solar; the top top layers instantly absorb the huge load of DLR energy as if they were locally energized by a laser, and the most active energized molecules instantly disappear into the atmosphere. (The difference with a laser apart from intensity differences is, that a laser concentrates on the top layer and on a small surface area, and DLR concentrates broadly on a very shallow layer: microns.) A short laser pulse can burn the top layers of molecules while keeping the molecules below relatively unchanged. For the thin layer of surface molecules, DLR works out more or less the same as a laser: the top top molecules get a huge energy boost, reach the energy level needed, and can disappear into the air and in doing so can cool the surface. This all happens while oceans (and so the Earth’s surface) still are relatively cold. By DLR stimulated evaporation keeps the oceans at the present (low) temperature level. The Earth’s solar warmed surface is cooled by 50% (80 W/m2) by evaporation. Without that DLR stimulated evaporation, oceans would become hot (all other things remaining the same).
Relying on sunlight alone while having a greenhouse atmosphere as we have (but without DLR) could only lead to hot oceans: hot enough to energize the top top layer of molecules equally as is done right now by DLR. First at those very high ocean temperatures surface warming again will be controlled by evaporation and associated processes (like convection and the formation of tropical clouds) as happens now.
In case I am right, double CO2 would cool the oceans. On the (long) time scales of the Earth: indeed. But other processes in the oceanic-atmospheric system of weather and climate still will dominate surface temperatures, as they do now.
P.S. Having just read this comment by Willis: some warming of the layers below will also happen and I agree the upward flow of energy will be diminished. But without DLR, evaporation will not take place in present quantities, so much of the present 50% of evaporative surface cooling will disappear and will not be replaced by something else that will cool the surface equally: oceans will warm and not a little bit.
Part of what I have a problem with is two fold.
First, if the ocean only absorbs 165 W/m^2 from the sun, then that is all it can possibly radiate. If CO2 absorbs all the 165 W/m^2 radiation from the ocean, then it would be at equilibrium with the ocean, and they would both be at the same temperature. Radiation fluxes between two bodies don’t add, they subtract as the S-B equation shows.
What is the solution to this? Water has the ability to store heat. If all the heat created during the day is not radiated away, there will be reservoir of heat available to increase temperatures. Why doesn’t the ocean continue to heat day after day. If the gradients were examined more closely suspect that you will find there is an equilibrium point where overall warming stops. Which leads to the second point.
Second, the radiation flux of 165 W/m^2 is an average. Physical phenomena generally don’t work on an overall average. Waters in the tropics are going to receive vastly more radiation than those in the Arctic. S-B and Planck tells us that the temperature and radiation are related by the 4th power. If you could erect a “force field” every 10°, what do you think the water temps would be inside each barrier. It doesn’t take a genius to know that tropical waters would be much warmer. So, how does that heat in the tropics change? Currents, waves, wind, atmospheric currents, etc. spreads it around. Looking at averages just won’t suffice and will lead everyone down paths that don’t exist.
What does CO2 do to this movement of heat? Probably very little. If the current concentration of CO2 is trapping all the radiation the surface can provide, then additional CO2 is not going to trap more and will have little effect. Assuming that more and more CO2 will add to the “back radiation” and warming the oceans just won’t happen.
Jim Gorman: “If you could erect a “force field” every 10°, what do you think the water temps would be inside each barrier.”
WR: A very interesting question that has been answered for the Earth as a whole. When you leave out all transport and storage and confine to Kirchoff’s/Planck’s model of an infinitely thin shell (that cannot store any energy), the hottest parts radiate at T4 and cool relatively fast and on the dark side of the Earth, temperatures will be about 3K, as no heat is stored. Final average temperature: some 110 degrees Celsius/K lower than in case of a perfect transport (super-conduction). Unfortunately, the publication is Dutch: https://www.clepair.net/temperatuurverevening-2.html
Some more thoughts about this subject by the same author and in English:
http://www.clepair.net/GreenhousClP-Eng.html
The bottom line: storage and transport have enormous temperature effects. We simply cannot compare Earth with an artificial model and draw simple conclusions. We need to understand what happens in reality.
I dont buy for a second the notion “if the ocean only absorbs 165 W/m^2 from the sun, then that is all it can possibly radiate” for major 2 reasons. First you ignore the differential heating at depth. The LW emitted from the ocean is in part a result of the immediate emission of absorbed IR in the skin layer and solar hat that wells up from below. The 165 of solar heat only contributes to why the skin surface emits more IR than it absorbs. That by no means proves IR is being stored at depth and warming the ocean. It is the stored solar heat.
Second, poleward of 40 degrees latitude, the earth emits more radiation than it absorbs. But that doesnt mean the earth want warmed by the sun. That would be very silly. Again it is a function of heat storage and transport. And by all accounts solar heated subsurface waters are far more likely to be stored and transported than IR heated microns-thick skin surface water where the net IR flux is out of the ocean.
Jim: Your statement “the LW emitted from the ocean is part a result of the immediate emission of absorbed IR—-” is not necessary and misleading.The LW emitted depends only on the water surface temperature, period. It does not know or care where or when the energy came from. Likewise,the heat that upwells is simply heat, not solar heat.The heat you describe has no memory, Instead of trying to write a “narrative”, I suggest you. start with an appropriate surface energy balance with proper expressions for the various surface energy fluxes following standard heat transfer analysis practice. I think you could then better communicate your ideas on this topic.
“The LW emitted depends only on the water surface temperature, period. It does not know or care where or when the energy came from. Likewise,the heat that upwells is simply heat, not solar heat.”
Important observations.
What are you talking about???? You are making up nonsense straw man arguments. I’ve said many times, absorbed IR and upwelling of solar heated watersvheats the surface and thus emits more IR
All I pointed out is the fact that all studies show there is a net loss of heat from skin surface that totally accounts for absorbed IR plus some contribution from solar heated waters.
Anthony you have offered nothing but BS narratives. Here is some math for you nonsense narrative about longwave heating of the skin surface and clearly have not doe the math First consider 1,000,000 ml= 1 m3
1 ml = 1 gram of water, so 1 m3 = 106 grams
2 microns = 0.000002 meter
Thus a skin layer that is one square meter and 2 microns thick weighs 2 grams
Since 1 gram of water requires 4.18 joules to increase by 1 °C.
Then one square meter of the 2 micron thick skin layer requires 8.35 joules to rise 1 °C
Thus absorption of 400 W/m2 of Longwave would raise skin surface 47.8 °C higher in just one second
The hottest regions of the warmpool just average 30°C, so thinking that Longwavee from greenhouse gases could raise the surface temperature by another 47.8 °C requires insanity or a university degree in the humanities.
Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.
With a observed temperature gradient showing heat ocean waters can only transport heat out of the ocean, the only explanation for the expected but unobserved rapid heating of the skin surface is that warming causes the rapid emission of an equal amount of longwave according to the Stefan-Boltzman law, so that the skin surface does not warm!
Jim Steele Reply to Jim Gorman
August 4, 2022 8:54 am
Jim Gorman’s statement only is true, but only for a steady-state condition where energy gain equals energy loss.
On the other hand, if the total energy loss (via radiation + latent/sensible loss) from the ocean is greater than the total energy gain (165 W/m2 from the sun in my example), IT WILL COOL. That’s true for time periods of days, weeks, months and centuries. Temperature is a measure of the energy content of something. If the energy loss exceeds the gain, it will cool.
How far will it cool? It will cool until energy loss equals energy gain. At that point it’s at steady-state, and there it will remain.
w.
And again, you dont apply the same physics to the skin layer where a net loss of IR causes it thee skin surface to cool. Thus it cannot warm the layers below
For those who advance nonsense narrative about longwave heating of the skin surface and clearly have not doe the math First consider 1,000,000 ml= 1 m3
1 ml = 1 gram of water, so 1 m3 = 106 grams
2 microns = 0.000002 meter
Thus a skin layer that is one square meter and 2 microns thick weighs 2 grams
Since 1 gram of water requires 4.18 joules to increase by 1 °C.
Then one square meter of the 2 micron thick skin layer requires 8.35 joules to rise 1 °C
Thus absorption of 400 W/m2 of Longwave would raise skin surface 47.8 °C higher in just one second
The hottest regions of the warmpool just average 30°C, so thinking that Longwavee from greenhouse gases could raise the surface temperature by another 47.8 °C requires insanity or a university degree in the humanities.
Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.
With a observed temperature gradient showing heat ocean waters can only transport heat out of the ocean, the only explanation for the expected but unobserved rapid heating of the skin surface is that warming causes the rapid emission of an equal amount of longwave according to the Stefan-Boltzman law, so that the skin surface does not warm!
No it is not. What heats the ocean is the storage of heat!
If IR is absorbed at the skin surface, it raises temps which quickly emits that IR, at least what evaporation and the winds have extracted. And as W & M shows the skin surface is constantly losing more heat than it absorbs, so the temperature gradient is diminished from above, but by how much the subsurface heats or cools.
If the subsurface gains heat at the rate of 165 joules/sec per m2, and doesnt completely cool, the water warms and the turbulence in the subsurface can carry the heat to depth as it mixes with the cooler the thermocline waters below the subsurface.
Apologies for some incoherent sentences, but I could not edit so here are my corrections
should read “quickly emits that [absorbed} IR, at least what IR remains after evaporation and the winds have extracted.”
&
so the temperature gradient is not diminished by added IR from above, but by how much the subsurface solar heated water heats or cools.”
‘What heats the ocean is the storage of heat!’
I wish I had a dollar for every time Dr. Roy Spencer admonished some ‘crank’ that an object that absorbs heat faster than it loses heat will get really hot. As for DLR, since we’re neither ‘aflame’ nor experiencing perpetual Noachian floods, I would surmise that a large portion of this flux is re-emitted skyward.
In the header : “….. Does Greenhouse Back-radiation Warm the Oceans?”.
The energy balance says “Yes”.
The energy balance is always fulfilled in a system during the selected period of time. If there is an energy accumulation then there is a discrepancy between all the energy transferred in to the system and all the energy transferred out from the system, in short :
Accumulation = In – Out
When considering our system as the earth surface and say ten kilometres down (water, ice, rock solids), there are a lot of different energy transfers In and Out from this system. Some energy is partially transferred Out from the surface by radiation directly to space (-270 °C). Partially the radiation Out is blocked by clouds and other components in the atmosphere which is colder than the surface but a lot warmer than the space.
Changing the concentration of those blocking components will impact the system energy balance and we will have a different surface temperature than today.
The energy transfered In from below ten kilometres is very small in comparison to the short wave radiation from the sun.
Having 340 J/m^2/s (or W/m^2) In at the top of the atmosphere, assuming only nitrogen and oxygen in the atmosphere, no green-house gases, no clouds, no Back-radiation or radiation blocking components at all, and the
open ocean albedo=0,06 (no ice), 70 % of the surface area
desert sand albedo=0,4,
the system and its surface temperature will be ((1-(0,06*0,7+0,4*0,3))*340/(0,98*5,67*10^-8))^0,25 = -6 °C, approximately 20 °C lower than today.
The Oceans will freeze to ice (albedo will approach 0,5) and the system temperature will end up at ((1-(0,5*0,7+0,4*0,3))*340/(0,98*5,67*10^-8))^0,25 = -35 °C, a lot colder than with green-house components.
Kind regards
Anders Rasmusson
Here is a table that shows various calculations of heat entering and leaving the ocean warm pool. It is accumulated solar heat that doesnt get fully vented that heats the warm pool. Energy balance depends on diffusion out of warm pool or on heat transported poleward
Jim Steel : “Here is a table that shows various calculations of heat entering and leaving the ocean warm pool. ….”
That table is valid for an atmosphere with carbon dioxide, water vapour, clouds and other thermal radiating components. The average global atmospheric temperature is 14 °C two meters above the surface.
Having only oxygen and nitrogen in the atmosphere, then all of the 340 W/m2 will reach the surface. Assuming all is absorbed then the surface temperature will be 7 °C, calculated from :
(340÷(0,98×5,67×10^−8))^0,25 = 280 K
There will in principle be no heat transfered by convection due to the lack of radiating components in the atmosphere – no atmospheric cooling to the space. The atmosphere will approximately reach the same temperature as the surface by conduction and that minimum of convection.
Sunshine is though partly reflected from the surface resulting in an even lower temperature. The ocean surface will have a bigger part of ice cover giving rice to more sunshine reflection, higher albedo, see my comment above.
Without Green-house gases (and clouds, droplets, particles) in the atmosphere there will be a surface temperature ranging from +7 to -35 °C, be it land or ocean.
Kind regards
Anders Rasmusson
For those who advance nonsense narrative about longwave heating of the skin surface and clearly have not doe the math First consider 1,000,000 ml= 1 m3
1 ml = 1 gram of water, so 1 m3 = 106 grams
2 microns = 0.000002 meter
Thus a skin layer that is one square meter and 2 microns thick weighs 2 grams
Since 1 gram of water requires 4.18 joules to increase by 1 °C.
Then one square meter of the 2 micron thick skin layer requires 8.35 joules to rise 1 °C
Thus absorption of 400 W/m2 of Longwave would raise skin surface 47.8 °C higher in just one second
The hottest regions of the warmpool just average 30°C, so thinking that Longwavee from greenhouse gases could raise the surface temperature by another 47.8 °C requires insanity or a university degree in the humanities.
Despite absorbing 400 W/m2 of greenhouse longwave there is no such observed increase in temperature.
With a observed temperature gradient showing heat ocean waters can only transport heat out of the ocean, the only explanation for the expected but unobserved rapid heating of the skin surface is that warming causes the rapid emission of an equal amount of longwave according to the Stefan-Boltzman law, so that the skin surface does not warm!
Jim: “—requires insanity or a university degree in the humanities”. I am afraid your heat transfer “analysis” would not get you a degree at any university I know.
Anthony, all you have ever done here is throw out smears without ever detailing any specifics that would be required to refute a word I’ve written. Every analysis I’ve posted is supported by the laws of thermodynamics!
However your type of obfuscating engagement, would only get you an advanced degree from the Troll Factory.
Jim: Heat transfer analysis involves a lot more than satisfying the laws of thermodynamics.For the third time I suggest you go to http://www.temporalpublishing.com and read Item 8 of the supplementary material for instructors–which deals with the heat (and mass)transfer analysis of the ocean surface energy balance.
Anthony, for the last time, simply present your arguments here, instead of trying to drive the audience to your website, and stop your stupid condescending ding remarks like “Heat transfer analysis involves a lot more than satisfying the laws of thermodynamics.” Duh! Why would anyone want to go to your link when you have yet to represent any argument or details here relevant to the discussion at hand.
The problem here is many posters offer explanations that violate the Laws and therefore are rubbish.
Jim:The “the discussion at hand” has been the subject of numerous postings on WUWT over the last few years ,to which I have contributed .But controversy and confusion has continued.The issues are complicated and perhaps difficult for those who have not had sufficient education/experience in heat transfer analysis.My link is to a twelve page treatment of the ocean surface energy balance and carefully quantifies the contribution of back-radiation to “warming” of the oceans.But if you refuse to read it,I cannot help you.
Anthony Mills is quite familiar with the laws of thermodynamics. From the author bio of his text on heat transfer:
“Anthony F. Mills is a Professor Emeritus of Mechanical and Aerospace Engineering at the University of California, Los Angeles. Prof. Mills received his Ph.D. in Mechanical Engineering from the University of California, Berkeley in 1965, and has been a distinguished author, researcher and professor at UCLA for over 4 decades. Prof. Mills is the author and co-author of several books on transfer processes, heat and mass transfer, and more than 100 journal papers and conference articles in various aspects of fundamental and applied heat and mass transfer processes.“
Assume the atmosphere is made up of only nitrogen and oxygen.
Assume 190 W/m^2 is entering the system. All that power will penetrate to the surface and also has to be radiated out to space. The surface temperature will be at -31 °C, see also my comments above.
Assume now we introduce a thin heat shield surrounding the atmosphere.
The shield is letting in all the 190 W/m^2 to the earth surface.
The shield also has to let out that power to the space by radiation and will thereby reach -31 °C.
The earth surface (ocean and land) still has to radiate the inlet 190 W/m^2, now to the shield instead of to the much colder space at 270 K.
The earth surface temperature will increase to Tsurf :
0,98*5,67*10^-8*(Tsurf^4 – (273+-31)^4) = 190 W/m^2
==>
Tsurf = ((190 + (273+-31)^4*5,67*10^-8)/(0,98*5,67*10^-8))^0,25 = ((190 +190)/(0,98*5,67*10^-8))^0,25 = 288 K
or 15 °C
The Green-house gases (aerosols, clouds, rain,,,) in the real world act in principle as a heat shield and thereby increase the earth surface temperature, land and ocean.
Kind regards
Anders Rasmusson
Jim is dead right, IR cant penetrate water to the depth required to get past the cool layer. NO where near enough.
It can raise the skin temperature though, and hence in reducing the gradient slightly, cause water to retain more energy, energy it got from visible frequencies, in order to restore the gradient.
Think of it as adding bricks to a dam. The dame can hold more volume. Volume of water, not bricks….
SO note that this effect does not take energy from the atmosphere. It does not absorb energy form global warming.
Matthew Sykes : “……IR cant penetrate water to the depth……”
That’s right but :
The components in the atmosphere (not nitrogen, not oxygen) are though blocking the ocean very thin surface layer from fully radiate to space.
The layer will thereby be warmer than if no blocking components are present in the atmosphere, hence all the layers down to depth will be warmer than if there is only nitrogen and oxygen in the atmosphere.
Kind regards
Anders Rasmusson