Guest Post by Willis Eschenbach
OK, folks, for everyone who wanted to put forth your favorite theory about how downwelling radiation from the atmosphere is a fantasy, or how a cool atmosphere can’t leave the surface warmer than no atmosphere, or how pyrgeometers are fatally imprecise … this is the thread for you.
However, I’m going to ask that before you start, you understand my actual position on these questions. So I strongly request that before you comment, you read the following four posts. That way, you’ll be clear about my thoughts on the matter.
Can A Cold Object Warm A Hot Object? 2017-11-24
Short answer? Of course not, that would violate the Second Law of Thermodynamics —BUT it can leave the hot object warmer than it would be if the cold object weren’t there. Let me explain why this is so. Let me start by introducing the ideas of individual flows and ne…
Radiating the Ocean 2011-08-15
Once again, the crazy idea that downwelling longwave radiation (DLR, also called infra-red or IR, or “greenhouse radiation”) can’t heat the ocean has raised its ugly head on one of my threads. Figure 1. The question in question. There are lots of good arguments against the AGW consensus, but this…
The Steel Greenhouse 2009-11-17
There is a lot of misinformation floating around the web about the greenhouse effect works. It is variously described as a “blanket” that keeps the Earth warm, or a “mirror” that reflects part of the heat back to Earth, or “a pane of glass” that somehow keeps energy from escaping. It is none of these things.
People Living in Glass Planets 2010-11-27
Dr. Judith Curry notes in a posting at her excellent blog Climate Etc. that there are folks out there that claim the poorly named planetary “greenhouse effect” doesn’t exist. And she is right, some folks do think that. I took a shot at explaining that the “greenhouse effect” is a…
OK, now that y’all have read those four posts, and you are all clear about my position, let me offer some data to focus the discussion. Figure 1 shows the month-by-month surface shortwave (solar, “SW”) and longwave (thermal infrared, “LW”) radiant energy flows at the SURFRAD station in Goodwin Creek, Mississippi. The US maintains something called the SURFRAD (Surface Radiation Budget) Network of eight surface measuring stations. These have a variety of sensors that, as the name suggests, measure a variety of surface radiation flows. Each station has a Downwelling Pyranometer, Upwelling Pyranometer, Downwelling Pyrgeometer, Upwelling Pyrgeometer, UVB Sensor, Photosynthetically Active Radiometer, Normal Incidence Pyrheliometer, and a Shaded Pyranometer. These are calibrated annually to assure accurate measurements. They collect data on an almost continuous basis, 24/7/365. The stations have data from 1995 to the present.
So I picked a SURFRAD station at random, Goodwin Creek, Mississippi. And I picked a year at random, 2014, and downloaded the monthly average data from here. After I plotted it up I thought “I wonder how well this agrees with the CERES satellite-based dataset?” So I added the corresponding CERES data to the chart. Here is the result.

Figure 1. SURFRAD and CERES data, Goodwin Creek, Mississippi. The CERES data is for the 1° latitude by 1° longitude gridcell where the SURFRAD station is located. The background shows the Goodwin Creek SURFRAD station.
Now, folks have been questioning lately whether the CERES data is accurate enough for the type of analyses that I do, whether it is fit for the purpose … this should allay some of their concerns.
With all that as prologue, here’s the important part of this discussion.
The red|orange lines show the amount of solar energy that is absorbed by the surface. It’s the net of the downwelling solar minus the solar that is reflected back upwards from the ground. As you can see, the annual average solar energy absorbed by the surface is about 150 watts per square metre (W/m2).
The yellow|gold lines, on the other hand, show the upwelling longwave (thermal infrared) energy, energy that is radiated upwards from the surface. The annual average upwelling longwave energy is about 395 W/m2.
Now, for all of you that think that downwelling radiation from the atmosphere is a mirage, here’s the question.
If on an ongoing basis the surface is only absorbing 150 W/m2 of solar energy and is radiating 395 W/m2 of energy … why isn’t it frozen solid?
Seriously. If it is constantly radiating far more energy than it is absorbing … why isn’t it a block of ice?
To me, the obvious answer is, the surface is also absorbing downwelling radiation from the atmosphere. In Figure 1 above, the blue|cyan lines show the total of the net solar (SW, red|orange lines), plus the downwelling longwave thermal infrared (LW) from the atmosphere.
The annual average of the net downwelling radiation at the surface (SW +LW), the total energy absorbed by the surface, is about 490 W/m2. This is about a hundred W/m2 more than the energy that is lost to radiation, with the rest of the surface energy loss being in the form of the net of the sensible and latent heat lost gained and lost by the atmosphere via convection and conduction.
So there you have it. If you don’t think that downwelling LW radiation leaves the earth warmer than it would be if there was no atmosphere, you need to explain the mystery source of the additional energy necessary to keep the earth from freezing. And no, it’s not geothermal heat. We know from borehole measurements that geothermal heat, in general, is on the order of a tenth of a W/m2 or so … and we’re missing about 395 W/m2 emitted minus 150 W/m2 absorbed equals 245 W/m2 necessary to prevent freezing.
So what is the mystery source?
Let me add that the most excellent agreement between the SURFRAD and the CERES data means that it’s not instrumental error, or scientists who don’t know what they are measuring.
So where is the energy coming from?
My best to all, let the bunfight begin, and please, keep it civil … I may be wrong, but I’m not an idiot …
w.
As Usual I Politely But Loudly Request: QUOTE THE EXACT WORDS YOU ARE DISCUSSING. I can defend my own words. I can’t defend your interpretation of my words.
Can someone answer a simple question?
In what sense is a measurement of IR pointed at the sky, anything more or less than just a measurement of near surface atmospheric temperature?
Just like pointing an IR sensor at someone’s forehead measures if they have a fever?
It’s only different in the units of measurement. Downwelling IR is measured in W/m2. The IR thermometer merely converts that to temperature using the Stefan-Boltzmann equation.
w.
There is also the issue of spectral bandwidth, the IR thermometer has a much narrower bandwidth compared with the wide-bandwidth instruments used in SURFRAD (pyrgeometers).
H.E.:
When pointing an IR sensor at the sky, the IR radiation mostly comes from a significant distance (unlike from someone’s forehead). So the measurement usually comes from a noticeably different temperature level.
I have pointed my kitchen IR thermometer at a clear night sky when the near surface temperature was about +20C and it reported a temperature level less than 0C (which meant that it received radiation equivalent to an object with ~0.95 emissivity at that temperature level).
It’s easy to experiment with this, pointing it at different angles under different conditions!
The red|orange lines show the amount of solar energy that is absorbed by the surface. It’s the net of the downwelling solar minus the solar that is reflected back upwards from the ground. As you can see, the annual average solar energy absorbed by the surface is about 150 watts per square metre (W/m2).
The yellow|gold lines, on the other hand, show the upwelling longwave (thermal infrared) energy, energy that is radiated upwards from the surface. The annual average upwelling longwave energy is about 395 W/m2.
Now, for all of you that think that downwelling radiation from the atmosphere is a mirage, here’s the question.
If on an ongoing basis the surface is only absorbing 150 W/m2 of solar energy and is radiating 395 W/m2 of energy … why isn’t it frozen solid?
This question is ill-posed.
It is oversimplifies to the point of physical meaninglessness.
First we measure downward sunlight. But it is dissected twice before we get the number of 150 W/m2. It is shorn of what is “reflected back”. OK. Are we confident that we know exactly the albedo and its value at every wavelength? And all related parameters of emissivity? And what if reflected light is immediately reabsorbed locally? Does that count as absorption or reflection?
The second dissection is even more wondrous. Somehow we can separate light coming down from the sun in its first pass, from downwelled IR belched earthward from the CO2 sky dragon. Rather like a rabbit or guineapig distinguishes first time through poo – which it eats – from second time poo – which it leaves? Do we imagine the sunlight has no IR component? It does.
So our starting number of 150, sprung on us so simply, is the result of a lot of slicing and dicing.
Of course, IR from the sun won’t make it directly to the earth’s surface any more than surface IR will make it directly to space; both have a mean free path of 25 meters only. But all sunlight including IR will warm the atmosphere on its way down, causing the atmosphere to radiate IR. So the downwelling IR is caused by warming of the atmosphere by both sunlight above abd earth and sea below.
All this is glossed over in the simplistic equation, which appears to suggest that the sun’s rays pass through the atmosphere without any thermal interaction with it.
Likewise the apparent radiation of 395 W/m2 “upwelling” – when we point our IR meters downward – is simply a consequence of the earth surface being hotter than the atmosphere. Upwelling IR seems to be greater than downwelling simply because the earth’s surface is hotter than the atmosphere. All we are doing is just measuring the temperature of both.
The point is that IR simply comes from hot things. IR is downwelled because of the temperature of the atmosphere. The atmosphere is heated both directly by sunlight from above and both radiatively and convectively from the earth and sea below.
Among all the complexity of atmosphere earth and ocean heat exchange, one thing is trivially simple to the extent that it is not even a factor. That is the balance of radiation impinging on the earth from the sun and that leaving it. No anxious energy needs to be expended about this budget, it will look after itself. (Incidentally some planets like Jupiter emit significantly more energy as radiation than they receives. This is because Jupiter hasn’t finished generating heat from compression. But I digress.)
But again we have to return to the central problem of the radiative paradigm of the CO2 warming idea. The entire narrative, despite claims to the contrary, rests on the assumption that only radiation moves heat in the atmosphere.
The atmosphere is a chaotically roiling pandemonium of moving air currents, clouds and precipitation, lightning sparks, ice and even particulates. IR light is only one way out of many for heat to move around within the turbulent atmosphere. Just phase changes of water account for way more energy than CO2.
Reducing the immense complexity of atmospheric thermodynamics to just two or three terms of up or down “welling” actually simplifies it to death. No answer to anything lies along that path.
Hatter Eggburn May 29, 2021 4:52 pm
The solar energy is measured at the surface. So we don’t need to know the exact albedo. We just measure what is arriving at the surface.
Nothing “wondrous’ about it. Downwelling IR from the sun is what is called “shortwave IR”. Downwelling IR from the atmosphere is what is called “longwave IR”. When doing the measurements, a filter is used to filter out the wavelengths that we don’t want to measure.
It’s no different in concept from using say a red filter on a photo. It filters out everything but the red, and there’s nothing “wondrous” about it.
Regards,
w.
Hatter,
It would be easier to take your criticisms as coming from a position of great knowledge, if you did not seem oblivious to certain easily discovered and well known bits of information.
Personally, I am sure that the uncertainties in measurements as well as the variations over time of what is being measured, regarding all of the relevant data, is too close to any signal that is trying to be extracted, to make any huge conclusions about what is changing, and by how much, let alone why.
We have devices that can make incredibly precise measurements, and many of them may even be fairly accurate as well.
But the Earth is a big place, and as you point out it is very complicamated.
Getting back to my first point though, it seems you have not spent enough time familiarizing yourself with the subject matter regarding radiation and how it is discerned what is coming from where.
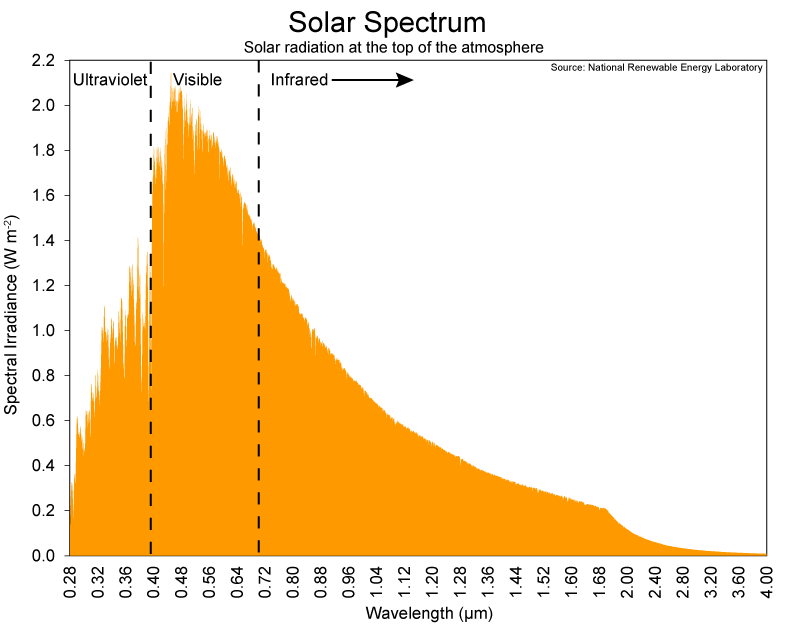
Here is what the Sun emits (and I hope these pictures post as photos and not a link…not sure why some do one and some the other):
So that is very well known…after all we have instruments in space that can measure it directly, outside of the atmosphere, or at least most of it. I think we have sent some probes far enough away for a look at the Sun that they are outside of the atmosphere, although I hear tell that the atmosphere never really ends. Instead, it is like Mike Tyson…it just fades into Bolivian.
Then we have good measurements of what arrives at the surface, known as incoming solar radiation at sea level: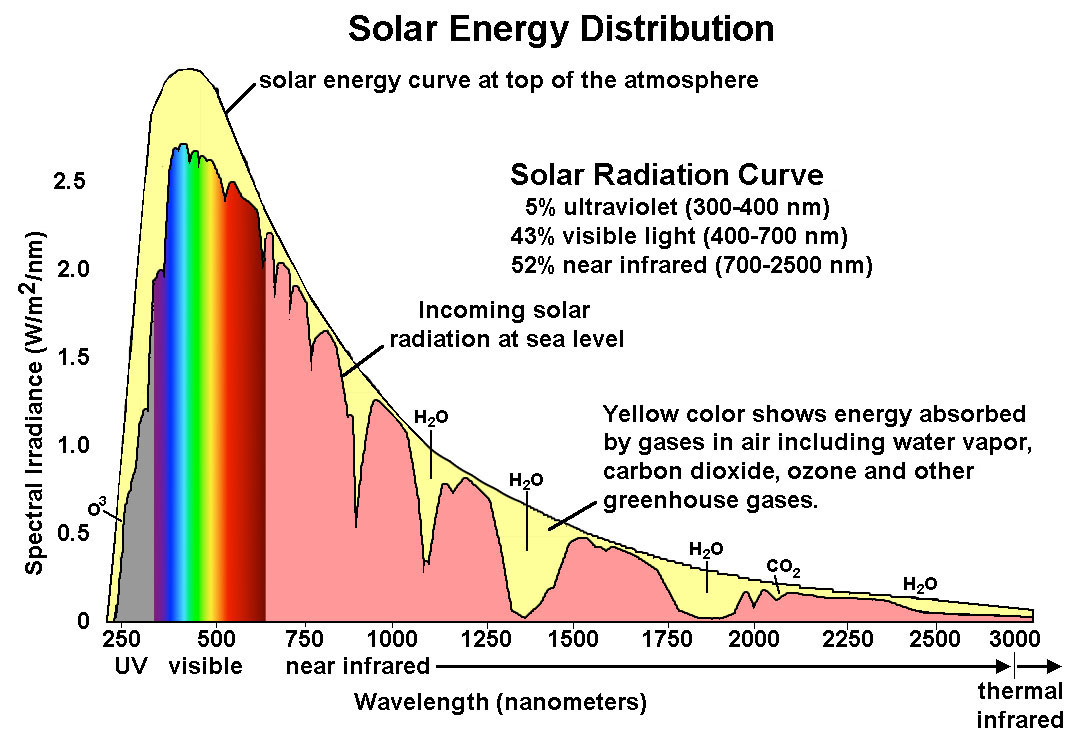
It shows very clearly where absorption has occurred, and the difference in the amount of energy making it to the surface gives a pretty good idea of how much of what is shining on the earth makes it to the surface. Although it must be noted by anyone paying close attention and wanting to be rigorous that the various measurements of incoming solar, and how they vary over time, seem to be internally consistent, there is a marked variability between what the various different instruments have recorded as an absolute value, and these differences are of the same order of magnitude as the amounts regarded as consequential re “climate change.
Here is a chart illustrating that point:

This quantity is called solar irradiance, and it can be seen to be kind of all over the map when we go to measure it carefully. Some of the newer instruments give values that form a little cluster, but even they vary between each other by a couple of watts per square meter.
However, as Willis has already relied to you, and is pointed out elsewhere in this thread, and I will now give charts to make clear…there is a big and readily discernable difference in the wavelengths of the IR emitted from the Earth and bouncing around inside the atmosphere, and the IR that comes from the Sun.
As this graph makes clear.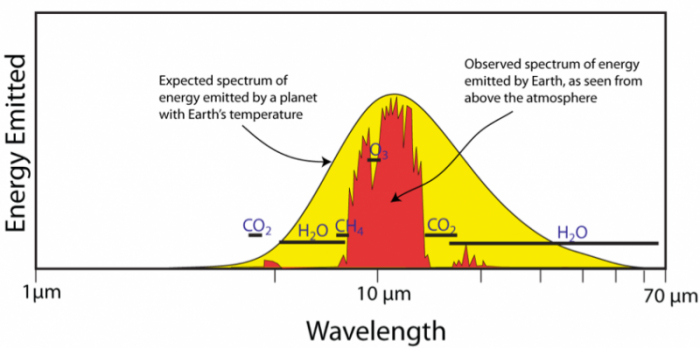
Note the wavelength values:
Here is another form of that same information. There are several very similar versions of this basic chart, and I am just picking one kind of at random. A simple search will turn up oodles of them if you want to see more:
So, yeah…it is easy to pick out what is coming from where, when it comes to wavelengths.
Because wavelengths are very specific to various materials and various temperatures, and the Sun is very hot, and the Earth is not, and for gasses it is even easier…they all have known bands of absorption and emission that are very well described.
As the charts show very clearly.
I think you are wrong when you say “No answer to anything lies along that path.”
But I would say you would be correct to say, that the idea that “All answers to everything lies along this path” is utter horsesh!t.
And that is one of the ways warmistas have gone far astray of science.
Below is a montage of various charts showing solar and terrestrial spectra on the same chart, in order to see how they relate to each other.
*Note that some charts have wavelengths in nanometers (nm) and others in micrometers (μm)
(
Nicholas
Thanks for taking the time to post those illuminating details of wavelengths and spectra, it was of course a mistake to make no reference to wavelengths in my argument. As Willis mentioned it’s simply what the instruments reliably measure, over well separated spectral bands.
I don’t dispute the data themselves, i.e. the closely agreeing CERES and ground based spectral measurements. Only what they mean.
The way it was presented implies a miracle is taking place, that energy from the sun – 150 w/m2 – is being multiplied like loaves and fishes to a 2-3 times greater downwelled flux of SW + LW.
However this is of course not the whole picture since total incident solar energy is 1380 W/m2. This shows that even inflating the 150 to 225 to allow for albedo (0.3), still the majority of incoming solar radiation is not making it to the surface to be included in that 150 W/m2 absorbed.
What happened to that energy? That’s a big part of the total picture missing. I think that Willis’ summary of the radiative energy budget would be more “transparent” if it included the total of 1380 W/m2 solar. I accept your point that electromagnetic radiation in the atmosphere is spectrally “stamped” so we know where the different measured components are coming from. But does it tell us everything about energy flow? Convection and water enthalpy are big gaps in the equation.
If 1380 W/m2 are incident at top of atmosphere but 225 make it to the surface (adding back in albedo reflection) then 1155 W/m2 have gone AWOL. Where? My guess is that a large part of it has heated the atmosphere. What else could that energy have done? This would explain the apparent multiplication of energy at the surface.
As you probably guess I’m no physicist, only a biologist, so I’m not going to make a fool of myself (any more than I already have) by diving into the minutiae of radiative physics and spectroscopy in the atmosphere. The central, obvious fact is that sunlight warms the earth’s surface. I’ll end by making only 2 comments.
1. 1155 “missing” W/m2 from top to bottom of the atmosphere show that the sun does not only heat the atmosphere from the earth’s surface. It loses most of its radiative energy on its way down through the atmosphere – heating the air.
2. In regard to the misnamed “Greenhouse” effect, what matters is how heat gets to the emission height above which the atmosphere is transparent to IR. From the surface, some will be transmitted as IR photons endlessly absorbed and re-emitted. But not all. A significant amount of heat movement upward from the surface will be convection, such as the thermal updrafts on which birds soar and glide all day long. Enthalpy of condensation with cloud formation also represents a big heat movement in the atmosphere. But as point 1 shows, not all the heat at the emission level comes from below. A lot is from above, never reaching the surface. (And atmosphere heating from above will be reduced by high atmosphere CO2 redirecting incident IR back to space.)
The spectral data give a detailed and internally consistent story of course. I don’t challenge that. Like most posters here I don’t disbelieve in downwelled IR. (Other red herrings like IR not heating the oceans are also irrelevant.)
It’s just that the intricate and beautiful radiation and spectral story is not the whole story of energy in the atmosphere.
An unspoken implication is that CO2 at one molecule in 2500 in the atmosphere, is responsible for most of the radiative heat we receive at the surface. OK let’s say half of it if the IPCC AR4 allows a greenhouse doubling by water. That effectively, the sun cannot warm the earth without CO2. This is not credible. It’s worth noting in closing that the presence of water in the atmosphere is dependent on CO2 because of plants which increase humidity. CO2 fertilisation and global plant increase are changing atmospheric humidity with some deserts shrinking from plant encroachment. (Take away all CO2 and earth’s surface becomes lifeless and arid.)
Too many other connected factors are excluded from Willis radiative formula – although internally it is entirely consistent and correct. Final pompous quote from Hamlet:
There are more things in heaven and earth, Horatio, than are dreamt of in your philosophy.
The purpose of Fig. 1 was to show the agreement between SURFRAD data and CERES data, and should not be seen as an energy balance demonstration.
Why?
First, these are monthly averages, not instantaneous values.
Second, the averages include data obtained in both daylight and nighttime. In daylight the solar irradiance is zero, but the longwave IR measured by the pyrgeometers remains above zero because the atmosphere is still warm and radiating.
So the shortwave monthly averages should be 2-3 times less, even though the solar irradiance is much higher.
The earth is a rotating sphere. That means that the Solar 1380 W/m2 must be divided by 4, IIRC.
Yes 345 is a much more credible number. Still > 225 though. Not everything is reaching the earth from space. The blue sky is evidence of that.
It is also very important to be very familiar with where all of these wavelengths fit into the big picture…the entire electromagnetic spectrum.
It can be seen that the IR band includes everything between visible light and microwaves, and microwaves blend right into the radio wave part of the spectrum, IOW photons with the lowest energy and hence the longest wavelengths…miles long at the long end.
IR itself covers several orders of magnitude of electromagnetic wavelengths:
Please elucidate:
In “Steel Greenhouse” 235 W/m^2 radiates to shell, which radiates 235 W/m^2 to space and to Earth. That is double the amount it receives, so is not a conserved quantity.
The Earth receives the additional 235 W/m^2 and now radiates at 470 W/m^2, but by the above logic this would be received back, so Earth would radiate at 940 W/m^2 and progress to infinity. So we have perpetual motion of the first kind.
Not true. Energy is conserved and there is no perpetual motion.

w.
Willis,
There Is no “conservation of energy”. Your “system”is losing energy, and will eventually reach 0 K. As to the other silliness of talking about W/m2, try making water hotter by exposing it to as much concentrated radiation from ice at 300 W/m2 as you like. Use any type of concentrator you like – lenses, parabolic mirrors – infrared is just light of longer wavelengths than visible light, after all. The laws of optics still apply,
You can’t do it, can you? Temperature is not measured in W/m2 for good reason. Your assumptions are ridiculous. Your shell is cooler than your surface, and any radiation towards that hotter surface will have precisely the same heating effect as that of 1 petawatt of radiation from ice does on a teaspoon of water. None at all.
It seems you are just making stuff up, based on refusal to accept reality.
A highly polished metal container of boiling water may be radiating 250 W/m2. Try keeping it boiling by exposing it to 300 W/m2 from ice! That’s why temperatures are measured in degrees.
“and will eventually reach 0 K”
The planet has a nuclear core producing 235 W/m2. The fluxes balance and everything is in steady state.
Thanks, Nick. I’m constantly amazed at the number of people who can misunderstand what is happening in the simple diagram above.
Ah, well, the beat goes on.
w.
Nick,
And losing an unstated amount of energy at a rate of 235 W/m2!
Everything balances?
You obviously have an imaginary nuclear core producing infinite energy – completely imaginary, of course!
You obviously have no concept of the conservation laws. Time for you to say you really meant something else, the loss is comparatively small, it’s all imaginary anyway, or any or all of the stupid excuses GHE believers come out with when anybody points out they are talking nonsense.
Maybe you could quote me exactly, and then provide facts to support any disagreement. Just making fatuous nonsensical assertions, and appealing to your own authority, is not particularly convincing.
The power generated continuously, and conveyed to space, is 235 * (planet surface area) W.
Nick,
From your infinite power source, of course. Unfortunately, Willis claimed “conservation of energy” for his imaginary fantasy.
Emitting infinite energy to space, and then claiming the system’s energy is “conserved” is just silly. Even more silly is your implication that the system will never cool, regardless of how much energy it emits.
Invoking magic in the form of an internally heated planet which has an inexhaustible power supply, maintaining a particular surface temperature until the end of time, never cooling or heating, is just fantasy.
Religion, not science, unless you can throw a few verifiable facts into the mix. Where may this magical “Greenhouse Effect” be observed and measured?
That would be a start.
Swenson
You’re the one who needs to go back to school and learn about radioactivity. No infinities or singularities involved. e=m.c^2 is a clue where the energy comes from.
Swenson, it’s called a “thought experiment”. It posits a nuclear core constantly heating the planet to 235 W/m2. You complain it won’t last until the end of time … say what?
Einstein’s famous thought experiment involved people in an elevator traveling through space. Good thing you weren’t around, you’d have whined to Einstein that “elevators don’t work in outer space” …
It’s a THOUGHT EXPERIMENT. It’s what we use when we can’t for some reason do a real experiment. It is not designed to last until the end of the universe as you claim. It’s designed to explain the reason that the poorly-named “greenhouse effect” works.
And yes, it does observe conservation of energy.
w.
Willis,
Here – your “thought experiment” is more a thoughtless experiment, not quite up to Einsteinian standards.
You wrote – “Good thing you weren’t around, you’d have whined to Einstein that “elevators don’t work in outer space”.
What happened to quoting people exactly? You know nothing about me – attempting stupid gratuitous insults achieves nothing except to make you look like a condescending and patronising idiot. I decline to feel insulted, as a rule. What about you?
Your “shell game” fails dismally. Just saying that 2 + 2 = 5 is true because it is a “thought experiment”, does not make any difference to reality.
You have a planetary surface radiating 235 W/m2. Around -9 C, say, but you need a nuclear core to maintain it. Really? Even though you don’t state it, your “shell”, starting off at 0 K, will warm to this temperature. You seem to be claiming that radiation from a “shell” of ice at -9 C, will raise the temperature of a ball of ice within it, also at -9 C, to what, precisely?
No GHE. That is why there is no experimental support for such silliness. Try again – think a bit harder next time.
Nick
Does this mean that the earth radiates more energy than it receives from the sun?
Like Jupiter does – emits significantly more radiative energy than it receives from the sun due to ongoing compressional heating.
No. As with Willis’ planet, which generates 235 W/m2, and the outer shell emits 235 W/m2. They balance.
Geothermal heat makes a very very small imbalance.
Nick,
Re Willis’ fantasy –
Balance?
You have two objects at the same temperature. One happens to be enclosed by the other. You are just being silly.
An air filled void in a block of ice radiating 275 W/m2 is receiving 275 W/m2 from all sides. In what fantasy do you believe the trapped air will become hotter?
Is that the same fantasy where a shell at the same temperature as the body it encloses magically makes the enclosed body hotter?
As to the Earth, Baron Fourier pointed out a long time ago that each night, the Earth loses all the heat it received from the Sun during the day, plus a little of the Earth’s own. Around 4×10^13 Watts, at present. No balance there, either. The Earth has cooled since the surface was molten. Lost energy. Duh!
It’s called reality. GHE believers have only a marginal attachment to reality.
Exactly: Power in must equal power out. Solar power in at TOA equals power out at TOA.
BUT: Power leaving the surface of the earth is greater than the power leaving at TOA. Willis’ steel shell toy explains why that is so.
The reason you can’t do it is because of a rather obscure principle of optics called “Conservation of Etendue.”
It’s NOT because its not value to add up W/m² from different sources, when the physics allows it.
To the contrary, you are believing silly misapplications of thermal physics that people “just made” up to try to disprove conclusions they didn’t like.
It’s fine to be skeptical. You don’t have to be committed to denying science in order to do it.
Willis is reporting accepted physics that has been well-known and extensively tested for over 150 years.
In the universe I know adding two items together at the same density does not double the density. This goes for mass and energy.
I am sorry, but the diagram clearly shows non-conservation. 235 Watts is absorbed by the steel shell, and 235 Watts is radiated into space. Simultaneously it radiates 235 Watts back to the surface. That is 470 Watts total radiated. If the steel shell absorbs 235 Watts, it cannot radiate more or conservation is broken.
Please note: I am not questioning that warming occurs or that back radiation occurs. I am merely asking for elucidation of the extra 235 Watts.
Thanks.
Robert, the diagram clearly shows 470 watts being radiated by the planet and absorbed by the steel shell, not 235. So energy is indeed conserved.
Planet receives 235 W/m2 from core, 235 W/m2 from shell, radiates 470. Steady-state.
Shell receives 470 W/m2 from planet, radiates 235 W/m2 to space, 235 W/m2 back to planet. Steady-state.
Regards,
w.
Yes, their back radiation is a “positive feedback loop” that would go to infinity amplifying its own energy and temperature , they just concocted the math to stop it when they reach their convenient temperature.
So you are as ignorant of math as you are of physics and science in general, as well as the basics of logical argumentation.
‘Kay, got it.
I for one believe you when you tell me this.
The steel shell cannot ever radiate greater than 235 W/m2 because that is the power input to the system. The energy distributions within the system don’t ultimately matter.
The shell has two sides. Radiates according to surface area and temperature.
Robert,
For one thing, the system has to be allowed to come to equilibrium before the numbers can be measured.
Yours is a failure of logic.
Another totally simple way to debunk the warmer object absorbing the radiation from a colder one is that if it did , you could easily warm up a warmer but very small object by placing it next to a very large but colder object , like placing a little warmer ball inside a a very large colder ball , the small ball would be forced to absorb many more times radiation from the large surrounding one simply because of the area difference next thing you see it would glow like a light bulb .
That obviously doesn’t happen , With this kind of fizzix the universe would turn into a total phantasmagorias.
And you have the math to show you know what you are talking about of course?
What do you think happens to photons emitted from a cooler object that impinge upon a warmer object?
Do you know what happens when a hot star and a cooler star are in a close binary orbit around one another?
What happens to the radiation from the cooler star that is falling upon the hotter star?
Note there are specific examples of the diameters of the two stars being far different from each other. It is not uncommon to have a large cool star with a small and very hot binary companion.
Besides for all of that, what is it that you suppose is innate in our intellect that allows us to think our way through your thought experiment and divine the correct result?
How many hours have you spent in labs or in various environments with thermometers and various apparatus, confirming that intuition can guide us in such matters?
Are you aware of the history of such matters going back hundreds of years? Or thousands?
Why did people back then get so many things wrong, if we can easily just think our way through such questions without referring to specific information?
Let alone disregard the findings of others who have done laborious experimentation and years long educations in the relevant subject matter, on the basis of some poorly thought out and haphazard supposing?
Nicholas,
You wrote –
“What do you think happens to photons emitted from a cooler object that impinge upon a warmer object?”
They don’t interact with it?
You may have noticed that adding energy in the form of ice cubes radiating 300 W/m2 does not raise the temperature of your soup.
You may have also noticed that visible light passes through things like glass without interaction. That’s why you can see through glass (and your eyeball, incidentally). The visible light photons do interact with cells in your retina.
Less energetic photons at radio and mobile phone frequencies pass through all sorts of material without interaction.
Thinking that matter must interact with photons of all energy just demonstrates that the thinker knows nothing about the physics involved.
Hope this helps to overcome your tendency to pose stupid gotchas, thinking it makes you appear knowledgeable.
I am knowledgeable, and they are not gotchas.
So, please tell me about this amazing insight you have regarding visible light passing through glass.
You say this is why we can see through it? (you said me but I am thinking you meant we)
Wow! I can tell you thought of that all by yourself, too.
I am impressed, no doubt about that.
So, your contention is what?
You talked a lot but said exactly nothing Swenson.
Is that what you consider communicating?
I for one never assume what other people are thinking or what they know.
I tend to ask questions if I want information.
People like you tend to tell people what they themselves think, and assume you know what other people know when you actually have not a single clue, as your comment proves.
When did I ever say or imply that “all matter must interact with all photons of all energies”?
I am quite certain that not only have I never said or thought any such thing, that if we take a look over what I have actually said (imagine that?), we will find plenty of proof that you are just making up crap.
So, you said to me, “Thinking that matter must interact with photons of all energy just demonstrates that the thinker knows nothing about the physics involved.”
And since I never thought that, what does it demonstrate that you know?
What does it say about your skill as a thinker to think you know things about people that are clearly false?
I will have to take your word for a few things, but I am not gonna assume I know what you know. I for one have never added ice cubes to soup.
Please tell us what happens?
What does happen to those 300 watts per square meter?
I’ll take it you have done some measurements on ice cube wattage, have you?
You got me there, I never did that.
Anywho, you never did explain exactly why visible light photons pass through glass, and what that has to do with anything we were discussing.
I was hoping you would, so I would have an actual reason to think you have any idea what you are talking about, instead of being, you know, just another jackass who never took an actual science class or read an actual book, but does spend lot’s of time on crank internet blogs bitching and moaning about how everyone but you knows so little about so much.
I hope this helps you overcome your urge to make a fool out of yourself in public, but…no, in fact, I am not very hopeful of that.
Nor do I care.
As for your not-quite assertion that photons from a cooler object do not interact when they impinge upon a warmer object, I have to wonder why you posed it as a question instead of a statement?
Is it because you literally have no effing clue what the hell you are talking about?
That would be my guess, although I cannot rule out the possibility that you are merely too chickensh!t to just say what you think in plain language.
That is what I do. But that’s just me, I know, I know.
Something else I do is find out things by dint of actual education.
Guesswork may be fine for you, and you keep at it, you may get something right one day, who knows?
But I like to learn things. It’s um…it is what I do.
You see, it is obvious to me that if something cannot happen because physics precludes it, it would have to be the case in all situations.
That is what happens when something is a, you know, law of nature.
Maybe you know some things by your sheer power of uninformed guesswork that people who merely dabbled in science, like Eddington, Milne, Chandrasekhar, and Schwartzschild never managed to learn in their whole life.
Like what happens when a cooler star sends out radiation that impinges on a hotter star, and vice versa, and they warm each other up.
Eddington wrote about it in 1926.
Milne in 1927.
Chandrasekhar in 1945, 1947, and 1950.
Wow…they could have saved some time talking to you first I guess, eh?
I am not sure someone who knows literally nothing, but thinks he knows everything, will be able to glean any speck of education from reading about the work of such people, but what the heck, let’s give you a shot.
I am just crazy like that, you know?
After all, I have no idea what it is you think or know unless you tell me, and even then I have to take your word for it, but I am generally the trusting type, at first anyway.
Here, I have even highlighted the parts to pay special attention to in order to quickly be disabused of unphysical ideas:
Two stars, one cooler, mutual heating, conspicuous, rather differing temperatures, physical interpretation…correctly…in terms of absorption and re-emission, Eddington, Milne, Chandrasekhar, re-radiation, radiative transfer solution, re-emitted radiation, all of the incident energy is…re-radiated, emitted spectrum of the irradiated atmosphere…different…from that if the incident light, but also from the spectrum the star would emit in…absence of incident radiation, Eddington’s results…amounts of re-radiated light…secondaries of low temperature, Changes in the emitted spectra caused by irradiation.
It is all right there, and much much more.
Here is the paper, and I even clipped and highlighted enough to show that either you are right, or else all of those guys are clueless pretenders.
It cannot be otherwise. Either you are right, or they are.
Not to mention every other astrophysicist who ever lived, then and now.
*1985Ap&SS.113..349V (harvard.edu)
One thing you will find nowhere is even a mention of any such idea that radiation from the cool star can have no effect on the warmer star because “they cannot interact”, or whatever jackassery you think you know.
Have a great day!
Oh, and BTW, I have oodles and oodles more if you are too dumb to understand it from the discussions and referenced work in this paper:
Just ask!
What he said.
Nicholas,
You posed the question “What do you think happens to photons emitted from a cooler object that impinge upon a warmer object?”
I answered with a rhetorical question (sorry about that) –
“They don’t interact with it?”
i provided a few examples, involving different wavelengths of light. You don’t want to accept reality, tough.
Try heating the smallest quantity of water using the radiation from as much ice as you like. No, Nicholas, I don’t care if every astrophysicist in the world claims you can use the radiation from ice to heat water – if you had enough!
No GHE. Back to your fantasy. Enjoy it.
You somehow think that the fact that glass is transparent to certain visible wavelengths of light has anything whatsoever to do with the relative temperatures of the glass or the object that emitted the light?
Glass is transparent to some wavelengths of EM radiation, but you obviously have no clue why.
What happens if I rub the glass surface with steel wool?
Is it still transparent?
How about if the light strikes the glass at a low angle and it acts like a mirror?
Does that happen because the glass is not transparent anymore?
When IR or UV wavelengths are absorbed by glass, what happens to that energy?
Does glass in sunshine get hot?
Why is the edge of a transparent sheet of glass green?
If you seriously think that your insightful observation that ice will not heat up hot soup has any bearing on what is being discussed here, or that this is what anyone thinks, either you do not know how to read, or you do not actually ever read anything, or you do read but are 100% unable to glean any information from having done so.
Look up the speed of light in glass, then explain how the glass is not actually interacting with the light.
Swenson – the 2.45GHz microwave frequency, as used in microwave ovens, is equivalent to a radiating hot body at around 0.042K. That’s actually pretty cold. You can however use it to heat a bowl of beans at 293K up to around 373K.
A photon is a packet of energy that carries no information about the temperature of its source, and the temperature of the object that absorbs it is also largely irrelevant (maybe some changes if you melt the surface or it becomes more-oxidised through heating).
When a photon is emitted, it can’t know the temperature of the body that will absorb it – that is beyond its event horizon. At the point it is absorbed, the emitter is also beyond its event horizon. To do otherwise would violate causality.
Thus any body that is over absolute zero will radiate as if the rest of the universe was at absolute zero, since it has no way of knowing what body will be receiving its radiation or what temperature that body will be – that’s all beyond its event horizon. Similarly it will absorb or reflect any incident photons independently of the temperature of the emitting object, since the photon carries no information about the temperature of the emitting object. All that affects absorption is the the properties of the surface.
Try a thought experiment. We have a body in space (far from the Sun) that is heated at a constant rate to 100°C (373K or so). The average radiative temperature of space is somewhere around 4K. Put a block of ice (or something else) at 273K close to our heated object. Will it cool down faster or slower than before we added that body? Since our heated object is now receiving more photons from the block of ice than it was from space, then if the heat input to the hot object remains the same, then its temperature will reach an equilibrium at a higher temperature with the ice there than if the ice was not there. Putting a colder object close to the hotter one make the hot one hotter, since we’re dealing with an object that is actively heated. If the hot object was however not being heated, it would instead cool down more slowly with the ice there than it would if the ice was removed.
The S-B equation defines how fast a body can radiate its heat energy to the environment. The hotter the object, the faster it can lose energy, to the fourth power of temperature. The only thing that stops a body cooling to absolute zero is that it also absorbs radiation from the environment. At equilibrium, a body is receiving (on average) just as much energy as it’s radiating.
It’s also maybe worth pointing out that if cold objects weren’t radiating then you couldn’t take a FLIR picture of them.
Nobody denies that cold objects radiates. The issue is the cooling rate of the hot body, or the heating rate of the cold body. A hot body at 100 (10^2)^4 = 10^8. A cold body at 10 will radiate at (10^1)^4 = 10^4. A cold body at 90 will radiate at (9×10^1)^4 = 6.561 x 10^7.
That makes the difference between 100 and 90
10×10^7 – 6.561×10^7 = 3.439
and it still hot to cold. When they are equal temps, the radiation will also be equal. There is a lot more to this than being discussed. Time and heat capacity are two of them.
Jim – the reason for the comment was that it seems that Swenson thought that the hotter body emitted only the net energy required to comply with the S-B formula, so between your bodies at 100K and 90K only the body at 100K would emit 3.439e+7 times the radiation constant and the body at 90K would not emit at all because it couldn’t send energy “up the hill” to the body at 100K. I’ve seen this assertion before from others, too. Thus I was trying to get Swenson to see that it’s a bidirectional transfer.
Interestingly, heat conduction is also a bidirectional transfer, but this is normally ignored. That gets more interesting if you can find ways to break the symmetry of the energy-transfers.
Simon,
Look through a pane of glass. The visible light photons pass straight through it.
Add energy to your pot of coffee in the form of an ice cube, and notice
that the temperature does not increase.
Try forcing water to absorb the 300 W/m2 which can be emitted by ice.
You really have no idea, have you?
Swenson – we know intuitively that heat always passes from the hotter object to the colder one. This is however a net result, and it is always a bidirectional energy transfer in practice. The hotter object emits energy at a higher rate than the colder one does, and the radiation path between them is symmetrical, and so far we have not produced a way to make that radiation path non-symmetrical (though there are some experiments using the properties of Garnet or similar that are non-symmetrical for specific wavelengths).
It appears that you think that the energy-transfers between objects are in one direction only, which is why I suggested that thought-experiment. A hot object (that is, above absolute zero) radiating energy cannot foretell the future of the photons it emits and what other object will absorb them since that absorption is beyond its event horizon.
Thus the colder objects are also emitting photons, and the hotter objects are receiving those photons and not losing net energy as fast as they would have done if those other objects were not there. Each object, no matter what temperature, emits photons as if there is nothing else in the universe. It also receives any photons that hit it, reflects some and absorbs others. The net change in energy of the object is the difference between the energy it radiates and the energy it receives.
That is why you can heat your beans in a microwave oven, where the equivalent temperature of the microwave radiation is 0.042K or so, but you’ve got around a kW power in that radiation.
It’s not the temperature of the source that’s important – it’s the power level it radiates and whether the receiving object will absorb those photons. When we’re talking about heated objects and black-body, grey body, or coloured body radiation and a symmetric radiation path, then the power emitted depends on the fourth power of the temperature, and so the net energy transfer will always be from the hotter body to the colder one.
A photon carries energy from the emitting body to the receiving body. The temperature of each body makes no difference to that process, since for the photon both bodies are beyond its event horizon. This probably seems non-intuitive, but it’s what actually happens. The net energy change is just the difference between the total energy emitted and the total energy received.
Here is the thing about debunking such a question in physics: Unless you have some data in hand, you have done nothing of the sort.
If you carefully describe what you think will happen, and exactly how you have devised to test your idea, and write it down, and then do the experiment, and careful collect the data, and then find your result to be both repeatable and reproducible, you have not even met the requirement of having what is known as a hypothesis.
All you have actually done is declare you know the results of experiments without ever doing any.
Nicholas,
Here is an experiment. Look through a window. The photons interacting with your retinal cells did not appear to interact with the glass through which they passed.
Look at the stars. The photons emitted by the stars seemed to pass through hundreds of kilometers of atmosphere, your eyeball, lenses and prisms if you used binoculars, without interaction.
Good enough?
You are confusing the heat of objects with energy production.
Both the small hot ball and the larger cold ball around it are losing energy and getting colder.
Fact
The small hot ball is losing more energy per volume than the larger cold ball.
It must cool down.
It will cool down slower if surrounded by a large cold ball.
Think Eskimo’s.
The small ball does not receive all of the energy radiating from the large ball around it
Half is going off to space.
Most of the rest misses it and is absorbed by the larger colder ball.
If they are so close that they are touching it should be obvious that the amount of the cold energy going into the small hot ball is less than the energy going out of the smaller hot ball to the larger cold ball.
Simply a matter of surface areas.
Moving away does not give the surface of the larger ball any ability to give the smaller hot ball any more energy than it would get if touching.
The smaller core ball is receiving energy from a constant power source such that it maintains a temperature so as to radiate at 235 W/m2. Adding a steel outer sphere, at equilibrium, means that it must radiate outwards to balance that constantly supplied 235 W/m2 to the new total system. The outer sphere can never radiate energy at a rate that exceeds or is less than the 235W/m2 externally supplied. [Sunlight energy in at TOA equals LW energy out.]
Does anybody deny that the core is receiving an additional amount of energy radiated from the inside of the outer sphere that physically must match the radiation of outer surface of said sphere (both at the same temperature)? If it receives additional energy, it must radiate that energy away or eventually self destruct. It must heat up to radiate away that additional energy.
The energy leaving the outer sphere can never exceed nor be less than the externally supplied power source. What happens in the gap between the core and exterior sphere is of no interest in establishing the above facts. We know this is a plausible thought scenario because that is what happens between Earth’s surface and TOA.
Willis,
Why did you abandon me?
I challenge you to solve this problem, again:
https://wattsupwiththat.com/2020/02/28/the-hot-and-cold-of-space/
But this time with a PYGEOMETER instead of a block. The”bottom” faces the sun. The “top” faces space.
You can take off the dome top, since there is no point blocking shortwave radiation.
Keep the same adiabatic condition, the dimensions, and the k value – for simplicity.
Solve for Downwelling IR.
Please! As you know, I’m stupid and can’t do it myself.
Thank you.
He doesn’t love you anymore. Stalking him is not going to help.
Zoe,
It could be interesting to ask what temperatures Willis imagines (I use the term advisedly) would be recorded on a cube 1 mm on a side, front and back, with perfectly insulated “sides”.
Or on a granite block (“granitium”, perhaps, instead of unobtainium?) of 1 m x 1 m – but infinitesimal thickness (no need for perfect insulation).
It would be instructive if front and rear temperature differentials were calculated to be different depending in thickness or dimension. A magical variable thermal gradient varying from 0 to infinity, depending on the modelling ability of the user!
I’ll probably attract the wrath of the “back radiation” true believers, and a plethora of irrelevant and pointless analogies, but it seems to me that at a steady state, front and rear surfaces must be equal.
Zoe, with all respect, as I said above:
I hold that that statement is absolutely true.
Let me be clear. I do respect you and most of your work. But on certain questions, like geothermal heat and pyrgeometers, you have an idée fixe that no amount of evidence seems able to touch. For a long time I tried … and at this point, I’ve learned better.
Seriously, though, we’re not talking theory here. Your claim is that a measuring instrument based on known physical principles that has been routinely manufactured, calibrated against known IR sources, installed, re-calibrated on a regular basis, and used by scientists all over the planet for the last fifty long years ISN’T MEASURING IR AT ALL.
I have learned through bitter experience that I can’t touch that level of disconnect. As the doctor in “Macbeth” says, “This disease is beyond my practice.”
Look, I am truly impressed by lots of what you do. You have classy programming chops, and you are one of the few amateur scientists like myself who routinely does the hard yards to dig up the original data and analyze it yourself.
But on this question?
In all friendship, and I mean that seriously, I’m gonna pass.
My best to you as always,
w.
Sigh. You’ve adopted the cold view of things.
Cold – Negative Flux = Hot. See? Cold exists outside of hot.
Downwelling IR shines on top of a thermopile and most of the time comes out hotter on the other end of the thermopile.
Is this the thermopile greenhouse effect?
Sorry, but i thought you could think outside the box.
Or you can, but don’t want to be mocked by government-paid scientists?
“Sorry, but i thought you could think outside the box.”
That’s because Willis is locked in the box. Whichever way he looks he just sees box.
Or maybe he is locked in his steel greenhouse.
Yeah, it’s amazing. He can solve for a block, but not a pyrgeometer.
The idea that a heated case will emit through the thermopile, through the dome is forbidden.
Radiation to thermopile and conduction to the other end can only occur from cold to hot.
Why is the case usually warmer than the dome?
Must be cold helping warm get hotter. Couldn’t be hotter getting through the thermopile.
It’s sad.
I still believe in Downwelling IR, but only via convection from hot to cold. That’s the only case.
How can anyone defend Downwelling IR in general?
Zoe, I explained clearly why I’m passing on this question. Was there some part of “I’m gonna pass” that is hard for you to understand?
And insulting me makes me think less of you, not more. I offered you a gracious exit and complimented you on your strengths. Instead, you chose to stand on tiptoes to unsuccessfully try to bite my ankles. Why not just agree to disagree as I suggested and move on?
Making personal accusations about my mental abilities isn’t helping either you or your reputation. It just makes you look petty and vindictive, and I doubt you are either of those in real life.
In friendship,
w.
There is no such thing as Negative [IR] Flux. Above 0 K everything radiates. If such radiation hit something above zero emissivity/absorptivity, it is absorbed.
A pyrgeometer measures 2 things:
1) Temperature
2) Net IR flux, due to a difference of temperature above and below thermopile.
Net IR flux can be negative. In fact, most of the time it is .. for latitudes below ~45.
I invite you, then, to clearly state net IR flux instead of “Negative Flux.”
Last time and clearing up some misunderstandings on purpose (sad).
Anyone who thinks the thought experiment below will work and produce the 2C uplift in the way described is encouraged to do it for real and not necessarily in exactly the same proportions: all you have to do is stick to the relative temperatures which are:
Temp effect from the Sun alone at object> Temperature of local radiant heat source > Temperature effect from local radiant heat source at object alone > ambient.
The rewards will be huge – you will not only have proved the GHE, but completely destroyed the science of Thermodynamics. Your name will be in the textbooks for ever more.
Note also that these temperatures reflect the GHE relative temperatures broadly – and that’s also on purpose (Ambient in the GHE is ~4K = outer space).
To repeat: the GHE assumes that the radiation flux from the Sun is added to that of the radiation flux from GH gases and the result is an increase in temperature of the Earth’s surface from -18C to +14C.
Short summary of the GHE central process: two or more radiant heat sources can create a temperature at a target greater than any single radiant heat source can create at the same target.
If this step is not true, it doesn’t matter what else is true – the GHE fails.
This is the key mechanism.
And it is easy to see how it cannot be true; take the following example:
That cannot happen – and if it cannot be seen how it is impossible, I give up.
(However, if you manage to demonstrate this by experiment (see above) the world is your oyster.)
The conclusion is as follows:
Yes: downwelling radiation exists and can be measured (the radiation from the 35C source)
No: it does not increase the temperature of the Earth’s surface.
The GHE cannot exist.
You are wasting your breath, Zagzigger. Willis is a lukewarmer with no understanding of heat.
Have you seen the rubbish in his Steel Greenhouse claptrap?
Assume an ambient temperature of 0 C. Start a bonfire. Put a rock 2′ away. Let things settle down and measure the temperature of the rock. Start another bonfire 2′ on the other side of the rock. Let things settle down and measure the temperature of the rock. Is the rock hotter with 2 bonfires?
So, if a body (the Earth) receives a relatively fixed amount of energy from an external source (the Sun) then the body will achieve a relatively fixed temperature. But, if a second external source begins adding energy to the body (“Yes: downwelling radiation exists and can be measured …”), then the body cannot rise in temperature. Your logic is clearly beyond my intellect.
I am sorry but radiation doesn’t work that way. You need to read Planck’s treatise on heat and radiation. See the quote I placed later from his treatise.
Doing this using averages and algebra is hiding so much. Let’s put it this way. As some radiation is going out some is coming in. If the in part can’t replace or grow the part going out, the temp won’t rise.
Perhaps looking at it from the cool body will give you a better perspective. Will it continue past equilibrium and warm to a higher temp than the source originally was? If so, where did the extra energy come from for the whole system? Basically, the sun is providing the whole of the energy for the system. How did the system energy grow?
I don’t want to bring up entropy but reading Pplanck will help you understand.
Well, this thread has convinced me of two things:
• You can lead a horse to water, but teaching said equine to do the backstroke is damn nearly impossible.
• I need to choose my battles carefully, because otherwise, some of them will assuredly involve teaching the backstroke.
You are now free to continue hating on me in order to avoid discussing ideas …
w.
I’m not sure what you were expecting. The first paragraph in the post reads: “OK, folks, for everyone who wanted to put forth your favorite theory about how downwelling radiation from the atmosphere is a fantasy, or how a cool atmosphere can’t leave the surface warmer than no atmosphere, or how pyrgeometers are fatally imprecise … this is the thread for you.”
Sounds like an open call to me. I’m sure it is not surprising to you that some will not agree with your ideas. Nor is it surprising, I’m sure, that some will be unwilling to accept your teachings. There is nothing wrong with that. It takes a certain arrogance to think otherwise, and a level of blind hypocrisy to post this pyramid (based on my observations of the thread).
JCM
Aaaand JCM shows up to prove my point.
w.
It’s not clear what your point is. If one views himself as a teacher it is his responsibility to earn the respect to attract prospective students. A superiority complex does not meet that condition.
My point is that some people are so set in their ways that rather than discuss new ideas, they will immediately shift to attacking me if their ideas are threatened. So I should pick my battles.
I also posted a pyramid of disagreement, so hopefully some folks could understand where they fit in that spectrum.
Rather than discuss those thoughts, your immediate response was to accuse me of arrogance, blind hypocrisy, and a superiority complex … thus neatly proving my point.
w.
PS—I’m not looking to “attract prospective students” as you think. I’m looking to find people who actually attack my ideas rather than my style or personality, and who can expound clearly why my ideas are wrong. That’s how science progresses.
I recommend some introspection
Once again, you say nothing about the ideas in my comment. Instead, you just issue another insult by saying I’m not thinking enough about my own actions.
Hey, keep it up, you constantly re-emphasizing and re-proving my point is a good thing on my planet.
w.
Wow, I see you’ve quickly settled in on the bottom three tiers of the Pyramid. Way to be exhbit A of Willis’s post
Wow, yet another one who thinks he is above it all. All of us here are swimming in the same pile of dung whether you recognise it or not. All of us here are bottom feeders – do you see it? Nobody has any moral high ground here. It’s time to get over that sort of thing.
It’s not about “moral high ground” it’s about whether one choose to engage with the points (any of the higher tiers in that pyramid) or one chooses to be a troll (wallowing in the lower tiers), from your responses you’ve clearly chosen the later. That’s your choice, I just find it funny how butthurt you got because someone pointed out what you chose.
Another preacher without a leg to stand on. I’ve been shunned by this group days ago. I come back to see if anyone has anything interesting to say, and stop to troll along the way for entertainment value. Oh, and my butt is just fine but thanks for noticing.
It’s not a preach, it’s an observation. I must admit it is funny watching you get your knickers in a twist over the observation. Keep twisting.
I’m afraid you are retreating to the mechanics of arguments in general rather than dealing with the question at hand – which is “does the GHE exist?”.
I think we’ve seen enough doubt cast by Thorstein and Olsen’s paper – which said they can’t find the GHE through measurement. That came in for heavy criticism here, but please remember, nor has anyone else – otherwise we’d never hear the end of it.
So can we please stop the ongoing pretence / assumption that the GHE has been proven – it hasn’t.
In my small way, I’ve thrown out a thought experiment that proves that radiation fluxes cannot be added in the method demanded of the GHE – and nobody has refuted that either. I’ve also suggested that some try to actually perform my thought experiment (or a version of it) to disprove my assertion – but sadly nobody wants to do that either.
So all in all, a lot of waffle – but no confidence from anyone to either prove the GHE, or prove or disprove the flux-add mechanism. It’s a $200 experiment at most – but I think most people know the result without spending the money.
My thoughts too, Zagzigger, but if you disagree with Willis his cheerleaders will give you a negative uptick. I just moved yours back from -1 to 0.
His Steel Greenhouse hypothesis is ridiculous where in the addition of a shell, the core’s flux goes from 235W/M2 to 470W/M2 even though they are in thermal equilibrium.
Absolute rubbish which suits the majority of the posters on WUWT.
What is so hard about understanding the Steel Greenhouse. The core has an independent power source to heat it such that it radiates as a sphere at 235 W/m2 to its surroundings to keep from its total destruction by accumulated heat.
The added outer shell’s interior surrounding the core absorbs the 235 W/m2 emitted by the sphere in all directions and gradually heats up. At equilibrium, the outer shell must radiate 235 W/m2 to its outer surroundings to keep from total destruction by accumulated heat from the internal core power supply.
The shell, no matter how thick, has two surfaces. Each surface must radiate at 235 W/m2. The inner shell radiates at 235 W/m2 to the central spherical core. The core must heat up to reradiate the additional 235 W/m2, along with radiating the original 235 W/m2 from the energy provided by the internal power source. This must equal 470 W/m2 to balance the internal power plus the radiated energy received from the interior of the shell.
The outer shell, however, can only radiate outward 235 W/m2 to match the internal power source to keep conservation of energy of the total system. The internal power source provides the only energy for the system, and that source is constant. That energy must equal the energy ejected from the outer shell. The internal distribution of radiant fluxes does not change that fundamental physical fact.
Thanks, Dave. I’m glad both that you understand it perfectly, and that you’ve given such a clear description of what’s going on.
Regards,
w.
Thank you for your kind comment, Willis.
You need to do a course in thermodynamics, Wilbur.
470W/M2 from core to shell after thermal equilibrium? What a load of bollocks.
leitmotif
The best thing about leitmotif is that when he thinks something is incorrect, he provides all of the data, logic, math, and citations to demonstrate that it is actually wrong.
w.
Do the math.
The system is in equilibrium when the power output at the outer surface of the shell equals the power input to the core sphere, which causes the core to emit at 235 W/m2. The power to the core is a constant.
The interior of the shell radiates the same energy as the exterior side. That radiation has to go to the central core (target), thus further heating it such that total sphere radiation is 470 W/m2. That extra energy can be used by the space between the core and shell for any purpose needed. Use your imagination; you could create a whole new mini-world!
Dave,
Don’t be silly. The interior of the shell is colder than the core, as it is heated by it.
You wrote –
“The system is in equilibrium when the power output at the outer surface of the shell equals the power input to the core sphere, which causes the core to emit at 235 W/m2. The power to the core is a constant.”
In equilibrium with what? Itself?
”Extra energy”? Really? I’ll have all you can spare.
You are definitely using your imagination. Have you considered accepting reality?
After reading over 500 comments, it seems to me that “The Climate Science” is not quite settled yet. 🙂
Let History be the guide. It tells the CO2 story much better, and it’s a whole lot simpler than figuring out what radiative gases are doing to the Earth’s climate. History tells us what they were doing in the past (benign), and what they will probably continue doing (benign).
Amen to that, Brother!
Look to the past.
WUWT comment software, at one time, had a feature where after you had read an article, and then revisited it later, the new posts since the last time you visited would be highlighted in a different color so they were easily picked out from the things you had already read.
With over 500 comments in this thread, something like that would come in real handy again.
I think it’s a good argument. If the only gain the surface can have is from short wave, and the upwelling long wave exceeds that, wtf? You’ve cornered them into an undefensible position. Can this be applied to the oceans? Because some people say the atmosphere cannot warm the ocean.
Can the data you highlight break out oceans only? Assume the answer is yes. Then land warming only and the SST warming only should all roughly tie out. The radiation data by surface type should tie out to the BEST temperature rises by surface type
Yes I am an accountant. Which means you want roughly the same answer using two different methods. The books balance. And people say a business degree is a waste of time.
Long Wave Radiation impinges on the ocean surfaces the same as it does on land. Radiation energy is transferred to both surfaces. Some argue that the radiation hitting the oceans simply results in more evaporation. Since that applies to the land as well, its not much of an argument. Anyway, latent heat from evaporation is handled separately from the radiative transfers between the earth and atmosphere.
[[So there you have it. If you don’t think that downwelling LW radiation leaves the earth warmer than it would be if there was no atmosphere, you need to explain the mystery source of the additional energy necessary to keep the earth from freezing]]
Ugh! Yet another example of the fake physics lie that refuses to die.
Let’s see: “If on an ongoing basis the surface is only absorbing 150 W/m2 of solar energy and is radiating 395 W/m2 of energy … why isn’t it frozen solid?”
Duh, because energy in the form of photons has wavelength, which has Planck temperature, and the solar photons are at 5500C while the surface photons are in the range of -50C to +50C. And the 150 value is bogus because it is a claimed average over a flattened globe over a whole year, while instantaneous values alone actually exist, and go way higher, to 1000 and higher.
The big killer for CO2 global warming is that out of all the photon wavelengths that Earth’s surface radiates, atmospheric CO2 only absorbs and radiates at the wavelength of 15 microns, which has a Planck radiation temperature of -80C, completely outside the surface range. Thus it actually lets all the real surface heat photons pass through it untouched, and any 15 micron radiation it emits will be absorbed and reemitted over and over by other CO2 molecules until entropy harmlessly disperses it.
Speaking of entropy. Another big misunderstanding comes from failure to account for entropy. Planck black body radiation contains the maximum amount of entropy for a given amount of energy, which explains what happens to the energy from 5500C photons after they hit the Earth’s surface, and turn into way more puny -50C to +50C photons, namely, dispersal by entropy into the Heat Death of the Universe. The Looney Tunes climate scientists who try to equate the T^4 radiation from the Sun with the T^4 radiation from Earth’s surface seem to be missing that the two T values are way different, so raising them to the 4th power makes them even more different. Everything is running down and adding to the Heat Death of the Universe. Heat isn’t cheap, it’s dear, and only the Sun’s radiation heats the Earth’s surface, while the atmosphere just cools it, CO2 included, after dispersing more energy via convection.
https://www.nature.com/articles/s41598-017-01622-6
https://www.quora.com/How-does-the-increase-of-entropy-affect-the-environment/answer/TL-Winslow
As the world increasingly becomes a madhouse, it’s all the fault of the global Marxist-run U.N. IPCC, which has hijacked physics for political purposes and created the upside-down inside-out backwards phony field of climate science that is nothing but a beehive of lies to justify extreme leftist environmentalism’s visceral hatred of the oil industry. Nothing they say can be believed. There is no compromise. One must junk all of it and start over.
My growing body of students studying my free Climate Science 101 course are becoming the first real generation of climate scientists, who one day will replace the current generation of IPCC fake climate scientists. Don’t be left behind.
http://www.historyscoper.com/climatescience101.html
I take it that you don’t agree with Willis, TL? 🙂
Take a read of his Steel Greenhouse. It’s a doozy. If you like fantasy.
Try to convince Drs. Lindzen and Pielke, Sr.
I think the steel greenhouse is the biggest piece of sophistry from Wilbur.
The 470W/M2 radiating to the shell from the core in scenario B is totally weird. The core and the shell are in thermal equilibrium. They are at the same temperature. There is no transfer of energy between the core and the shell at thermal equilibrium. The shell outputs 235W/m2 to space just like the core in scenario A. Problem solved. Add 100 shells. Is the core going to explode?
Here is a lesson for you from Joseph Postma who you have trouble remembering, Willie. This is where Postma takes us through scenarios of a plate with a point object as the source, a parallel plate as the source and finally introducing a green plate on the side of the original plate away from the source. Notice the emphasis on view factors of a point source and a plane parallel source (similar to your shell).
https://climateofsophistry.com/2021/05/19/green-plate-analyzed-and-demolished/
It’s so simple and all done without adding fluxes to create something that does not exist.
Postma even cites the Eli Rabett website where this sophistry is explained. Several posters point out where Rabett went wrong especially one called “Unknown”.
Brilliant put-down for those interested in real science and not the drivel from warmists, likewarmists and those who practice sophistry.
https://rabett.blogspot.com/2017/10/an-evergreen-of-denial-is-that-colder.html
What happens to the radiation from the interior of the shell towards the core?
Dave,
I’ll bite. What happens to it?
Please don’t say it is absorbed by the core, and the core gets hotter as a result. That would just demonstrate complete detachment from reality on your part.
Consider Willis’ example. A ball of ice surrounded by more ice. Have fun.
As long as the ball of ice has a radioactive core heating it, OK. Of course, it wouldn’t be a ball of ice very long.
Get over it — its just a thought experiment.
Dave,
You wrote –
“As long as the ball of ice has a radioactive core heating it, OK. Of course, it wouldn’t be a ball of ice very long.”
Don’t be stupid. If it’s emitting 235 W/m2, it’s around -9 C, and held at that temperature, how could it melt? Fantasy climatological GHE ice, is it?
Don’t forget, you also wrote –
“What is so hard about understanding the Steel Greenhouse. The core has an independent power source to heat it such that it radiates as a sphere at 235 W/m2 to its surroundings to keep from its total destruction by accumulated heat.” Total destruction by heating to -9 C?
Or did you just turn the nuclear reactor up to 11?
Get over it. You’re as silly as Willis,
I’m sorry I got carelessly carried away with your ball of ice. The thought experiment is a steel sphere radiating out to a 4 K environment.
Additionally, the assumption was if the sphere can’t radiate away the energy supplied by the nuclear core, it will eventually destruct.
Dave,
Thanks for the clarification. You wrote –
“I’m sorry I got carelessly carried away with your ball of ice. The thought experiment is a steel sphere radiating out to a 4 K environment.
Additionally, the assumption was if the sphere can’t radiate away the energy supplied by the nuclear core, it will eventually destruct.”
Nope. No 4 K environment that I can see. Maybe I missed it. In any case, what’s so amazing about a steel sphere cooling by radiation?
Your assumption is groundless. In lieu of stated figures, assume a sphere of 1m2, radiating 235 W/m2. Nuclear output 235 W, no more, no less. Without a perfect insulator, you are snookered. No destruction. Not enough power. Even the Earth’s core never produced enough power to self destruct, even surrounded by iron, rock, water, atmosphere.
Try again.
You can easily see from these discussions that back radiation claim warming anything is purely a mathematical concoction on a paper , no real world observation and experiment can demonstrate it , as far as climate in particular , they literally have to claim the Sun shines at night in order to fudge up some kind of low energy level that has to be made up by “something”. that something being back radiation called the greenhouse effect
Uh, the Earth’s radiation at the surface has been measured. It exceeds the radiation from TOA, which just happens to equal the net input of the Sun at TOA. How can the Earth’s surface radiate at a rate greater than the Sun’s input?
Dave,
Uh, contrary to what GHE true believers think, your comment is just nonsensical.
Instruments to measure radiation across the entire physical spectrum just don’t exist.
Fantasy instruments do not count. Except for GHE true believers, bumbling buffoons, and their ilk.
Willis:
Regarding Fig. 1, where the top curve is labeled “Net Downwelling SW + LW”—what is meant by the “net” adjective? This sounds like something is being subtracted, but
presumably this is the upward pyranometer irradiance added to the upward pyrgeometer irradiance, which would be the 0.3-50um total irradiance. Is this correct?
Good question. “Net downwelling SW” is the total downwelling solar energy minus the amount of solar energy that is reflected by the albedo of the ground.
w.
Ah, so the SURFRAD number is:
Net Downwelling SW + LW =
(upward facing pyranometer) +
(upward facing prygeometer) –
(downward facing pyranometer)
Correct?
Willis,
You wrote –
“OK, folks, for everyone who wanted to put forth your favorite theory about how downwelling radiation from the atmosphere is a fantasy, or how a cool atmosphere can’t leave the surface warmer than no atmosphere, or how pyrgeometers are fatally imprecise … this is the thread for you.”
So. You have a ball of ice emitting 235 W/m2, surrounded by a “shell” of ice at precisely the same temperature (and of course emitting 235 W/m2). Do you really expect that a ball of ice totally surrounded by ice at the same temperature will magically get hotter?
You really believe that your fantasies are superior to fact, do you?
Why would anything at a constant temperature, totally surrounded by something at the same temperature, get hotter? Are you quite mad, or just temporally deluded?
Maybe you could avoid answering, and fly off at a tangent. That might convince others that faith transcends fact. What do you think?
Swenson, the part you are missing is that the shell is heated by the planet. So it starts out receiving 235 W/m2.
But the area of the shell is ~ twice that of the planet, so the shell is only radiating half of that on each side (inside and outside), or 117.5 W/m2.

Figure 1. Building a steel greenhouse. (A) Planet without greenhouse. Surface temperature is 235 W/m2 heated from the interior. (B) Planet surrounded by steel greenhouse shell. Shell radiates the same amount to space as the planet without the shell, 235 W/m2. It radiates the same inward, which warms the planet to 470 W/m2 (29 C, 83°F, 302 K). [Clarification added] Note that the distance from the shell to the planet is greatly exaggerated. In reality, it is close enough to the planet that the difference in the areas can be neglected in practice.
And since the planet is radiating 235 W/m2 but only half of that is making it to space, the entire system has to warm up. It is gaining more heat than it is losing.
It continues to warm until the planet is receiving 235 W/m2 from the radiation on the inside of the shell. At that point the planet is radiating 470 W/m2. The shell is radiating 235 W/m2 inwards and 235 W/m2 outwards. At that point, the system is losing to space the 235 W/m2, which is what is generated by the planet, so it won’t warm any further.
Note that the planet is getting 235 W/m2 from its nuclear core, and 235 W/m2 from the inward radiation from the shell, a total of 470 W/m2. It is radiating the same amount, 470 W/m2, so it is in steady-state, neither warming nor cooling.
The shell receives 470 W/m2 from the planet. It radiates 235 W/m2, but over twice the surface area. So it also is radiating what it is receiving.
Best regards,
w.
I realise you’re not a fan of mine but it’s worth pointing out that this schematic and steel house concept is completely contrary to nature. No amount of mental gymnastic will make it true in this preposterous scenario. You’re suggesting, effectively, that adding a narrow strip of emptiness between the core and a shell causes the surface of the core to reach a higher temperature. Whether or not you’ve got this gap and a shell it’s intuitive the new surface will radiate similar to 235 W/m2 assuming the shell and core have similar material heat properties. Does it matter, in your view, how wide this strip is between the core and the shell? Seems the addition of the shell creates a new surface that will radiate happily at 235 W/m2. What if the gap between core and shell is 1mm wide, does this impact anything in your mind? Is the width of the gap any relevance? At what point does the core and the shell simply represent one unit of mass? I know it’s a tired argument but it is worth re-stating that this adding of fluxes back in from the shell and calculating a temperature does not agree with any form of radiative mathematics that I’ve ever heard of. The measured energy fluxes in real Earth atmosphere are simply the result of the atmospheric temperature. The radiative arithmetic of adding in fluxes and supposing this is causing a temperature is complete fantasy. The atmosphere heats up to whatever temperature based on its various properties, such as mass and density, in a turbulent soup of fluid with no front or back. The measured fluxes are a consequence of those properties and the resulting temperature within the Earth system.
Furthermore, given enough mass (say half the mass of the core) the addition of the shell may in fact bring the whole system temperature DOWN as the energy generated from the nuclear process is now heating 1.5x the mass.
Putting my post above another way – suppose you create the shell using the outer few metres of the core. Mine the core and create your shell using this mined material. Place this shell, which is composed of the same material as the core, at whatever separation from the core you wish (the core is now slightly smaller but we’ve only mined a few metres). What do you suppose happens to the surface temperature of the original core? Does it go way up? Or does it stay the same? We have the same total nuclear energy source and same total mass, but in a slightly different configuration.
I believe that because of using schematic diagrams with imagined “directional flux density” arrows it tempts some people to ADD those fluxes with the result of an imaginary increase in temperature. It’s fascinating to watch the endless attempts to justify this inverted logic. In reality the observed flux density is the result of system energy and material properties. The observed flux will be the same regardless of the perspective of the observer. This is why longwave flux density in the atmosphere measured with today’s instruments is basically the same whether observed from above or below. In any scenario of warming (cooling) more (less) will be observed from any perspective. The flawed conceptualisation of this has led the field astray.
Using the core-shell visualization, once in steady state, there is no slowing of radiative flux. Any calculated steady state radiative flux density temperature effects occur instantaneously. In steady state the core and shell together can be considered to behave as a solid. From the outside you would only see a steel sphere. There is no air inside to build pressure for any internal temperature increase. Conversely, with an atmosphere of air there are many possible factors, not limited to convection, that introduce a net storage lag type mechanism due to turbulent fluid mass circulation. The concept of gas convection process includes all properties of conduction and advection. The core-shell visualization and flux labels in the graphic require the sum of flux density values to propose an increase of core surface temperature inside the sphere. Density properties can not be summed in this way – similarly, if i cut a piece of wood in two then glue it back together this does not result in a doubling of mass density. Energy flux (flow) density steady state approximate temperature effects are better described by differential calculus when considering interactions between different materials. For a simple description of the importance of mass and material properties in heat transfer for the steel dome model presented here are some calculations. Obviously steady state conditions require integrating (not included). https://courses.lumenlearning.com/physics/chapter/14-2-temperature-change-and-heat-capacity/
Willis can, I am sure, reply for himself. But I can also answer a few of your questions.
“ What if the gap between core and shell is 1mm wide, does this impact anything in your mind? “
The gap doesn’t really matter. Whether it is 1 mm or 1 m or 1 km, the impact for radiation will be basically the same.
* NOTE 1: There will be a correction if the radius of the shell, r(sh) is significantly larger than the radius of the planet, r(p). Then the temperature of the shell would be
T(sh) = 254 K * ( r(p)/r(sh) ) ^(1/2).
So if the shell is 10% larger in radius, it will be 5% lower in temperature than that bare planet. Willis is assuming both are approximately the same radius, so this is not a serious issue
* NOTE 2: There could be some quantum mechanical effects if the gap gets down to the wavelength of the IR. So a gap smaller than 0.1 mm might start to be a problem, but no one is proposing a planet-sized shell with a tolerance of less than 1 mm!
“given enough mass (say half the mass of the core) the addition of the shell may in fact bring the whole system temperature DOWN as the energy generated from the nuclear process is now heating 1.5x the mass.”
More mass would just impact the TIME it takes to reach a steady state, not the final temperature.
(Other than the fact that more mass could make the radius larger, which would impact the temperatures as outlined above.)
“The observed flux will be the same regardless of the perspective of the observer.”
Yes, the flux from the outside of the shell is 235 W/m2 and the temperature is 254 K.
Then intuitively, the deeper inward you go toward the core, the higher the temperature. Just like inside the earth. The gradient will depend on the ability of each layer to impede heat flow. If the planet is solid metal, the gradient will be small. If the planet is solid rock, the gradient will be larger. These are calculated from thermal conductivities. If there is a vacuum gap, then the thermal conductivity is infinite, and there is no heat conduction. But now there is radiation, so radiation will determine the temperature differences. A gap results in a temperature difference of 302K – 254K = 46 K for this geometry.
I’ll ask the same question I’ve asked before. Just where does the energy come from so the “shell” can radiate 235 in two directions?
You make a logical leap that “the entire system has to warm up” but you never explain where the energy to accomplish this warming comes from.
The energy comes from the nuclear core by way of the surface. It is then intercepted by the shell, which redirects half of it back downwards to further increase the surface temperature. This comes to a steady state when the shell is radiating the same amount that the planet radiated without the shell.
What the shell does is to slow the energy loss from the system. This is the same thing that happens when you put on a jacket in the winter. There’s no new energy, but because the jacket slows the energy loss, you end up warmer.
Note also that the jacket is colder than your body, but it leaves you warmer than you would be without it.
w.
Thank you for starting this. A lot of people need an education on energy and heat. Although we have not really touched on heat capacity and it’s part in the climate.
I do agree with what you just posted. It is important to learn that a cold body can not make a negative gradient. It can only reduce the rate of heat loss.
Willis,
Oh I see. You have managed to heat a ball using a much hotter body. A nuclear reactor, apparently. That’s novel.
Or are you saying that you have discovered insulation? Do you think that is novel, too?
Maybe you are saying that CO2 is really just planetary insulation – lIke Raymond Pierrehumbert?
Here’s a slight indication that you are just being silly – the Earth seems to have cooled over the last four and a half billion years, despite the hot core being surrounded by rock, water, CO2 and all the rest.
Got any more silly “thought experiments’ with infinite heat sources hidden up your sleeve?
Willis,
You wrote –
“Swenson, the part you are missing is that the shell is heated by the planet. So it starts out receiving 235 W/m2.”
You really think that your unsupported assertions are superior to fact, don’t you?
This is a usual tactic employed by GHE propagandists who find themselves caught out. I am missing no part of your nonsense.
You include a presumably infinite heat source which provides enough variable amounts of heat to make your silly fantasy appear correct.
Don’t believe me? OK, Have a ball of ice (or something) emitting 235 W/m2. Save all your diversionary heating by me accepting that the shell has miraculously heated to the same temperature as your ball. Now you have a ball at around 264 K surrounded by a shell with an absolute maximum possible temperature of 264 K. Your ball cannot make the shell warmer than the ball, can it?
Now you have a ball at 264 K totally surrounded by a shell at 264 K (or less).
What idiot is going to believe your calculations that show that the ball heats beyond 264 K? Oh, I see, you are going to put an infinite heat source into your system, and heat things to any temperature you like!
Tut, tut, Willis. You are not even a good illusionist. No wonder you have to portray fantasy as fact. You can’t even tell anyone where this supposedly scientific “Greenhouse Effect” may be observed and measured, let alone describe it in any meaningful way.
You are silly enough to think that you can just add “fluxes”, without regard to temperature. Try it. Put two ice cubes facing each other – in a freezer to keep them from melting, if you like.
According to you, each is receiving a “flux” (say 235 W/m2) and also emitting a “flux” of 235 W/m2. Surprise! In spite of your breathtakingly clever calculation (235 + 235 = 470), the ice cubes don’t melt! They just keep emitting 235 W/m2.
Learn some physics. Not GHE propaganda.
Swenson,
The ball of ice in your example, at a temperature of 264K, and emitting 235 w/m2, is just like the planet in the steel greenhouse diagram if the planet did not have an internal heat source.
The planet would be getting colder with or without the shell around it. The shell would only serve to slow the rate that the planet cools. Both planet and shell would approach absolute zero. No accumulation of heat whatsoever.
But in Willis’ thought experiment the planet is continuously heated by its core, and in that way is completely different than an ice cube. Different scenario, different result.
Western Hiker,
Oh, the cunning internal heat source. Like the Earth’s, I suppose. The inner core is surrounded by a series of shells – iron, rock, water etc. Powered by radioactivity! Woohoo!
The core still cools. Just like the rest of the Earth.
Gee. Reality or silly Willis?
“You include a presumably infinite heat source “
Well ‘nearly infinite’. Nuclear reactions in the core in this case provide a hypothesized constant rate of heat (235 W/m^2) for a (nearly) infinite time = infinite heat.
“which provides enough variable amounts of heat”
No. It provides a constant 235 W/m^2 of heat.
“Save all your diversionary heating by me accepting that the shell has miraculously heated to the same temperature as your ball.”
No, the shell is always cooler than the ball.
“Now you have a ball at around 264 K…”
I assume you mean 254 K. ‘
You seem to agree that the surroundings can be 254 K. You also accept that there is a heat source inside the ball. What person would believe calculations that show that the heated ball will be the same temperature as its surroundings? If I put a 235 W electric heater into a coffee pot, would anyone think it will stay the same temperature as the room?
Tim,
You wrote –
“No. It provides a constant 235 W/m^2 of heat.”
So the surface is maintained at 264 K, or so – less if emissivity is less than 1. For real ice, 256 K or so. Even colder. Presumably the nuclear core is at a temperature of 256 K, 264 K or whatever is necessary to produce a steady state emission of 235 W/m2.
And –
”No, the shell is always cooler than the ball.”
Couldn’t agree more. And Willis claims that totally enclosing a body with a colder shell makes it hotter! Not without surreptitiously cranking up the nuclear power output! The sort of thing you would do, eh Tim?
And –
“I assume you mean 254 K. “
And why is that, Tim? Can’t be bothered reading?
Then you try pointing out the blindingly obvious – thermometers react to heat! However, you didn’t realise that your fantasy heater has been regulated to get no hotter than room temperature.
Bad luck Tim.
I assumed you meant 254 K rather than 264 K because 254 K is the temperature for a blackbody emitting 235 W/m^2 to space. Typos happen and it is easy to hit “6” instead of “5”. I was giving the the benefit of the doubt. If you have some OTHER reason for choosing 264 K, you could let us know.
“So the surface is maintained at 264 K, or so – less if emissivity is less than 1. “
Again, the calculations lead to 254 K. And a lower emissivity would lead to a higher temperature, since the surfaces would have a harder time shedding energy via thermal radiation.
” And Willis claims that totally enclosing a body with a colder shell makes it hotter! “
If “it” is “the body”, then yes, enclosing the body within a shell at any temperature above 2.7 K does make the body warmer than if the body were out in space exposed to 2.7 K thermal radiation.
It is a standard physics / engineering problem to calculate radiation heat transfer and to find resulting temperatures. Anyone here with a basic background in physics / engineering should be to calculate the temperature of a body with an internal power supply of 235 W/m^2 inside a shell of temperature T(sh).
T(sh) T(body)
2.7 K 254K
100K 255 K
200K 275 K
300K 333 K
400K 415 K
The warmer the shell, the warmer the heated body within the shell. Willis’ claim is 100% intuitive. What would be truly remarkable would be if the body stayed at 254 K regardless of the temperature of the cooler surrounding shell!
“Then you try pointing out the blindingly obvious – thermometers react to heat!”
Actually, thermometers react to temperature.
“However, you didn’t realise that your fantasy heater has been regulated to get no hotter than room temperature.”
Well, this is Willis’ ‘fantasy heater’. And it has been regulated to provide a constant power — not to reach a specific temperature. Once again, you get the science wrong.
Bad science, Swenson.
The initial gradient will be reduced so it takes longer to reach equilibrium but once the 254 deg is reached it will warm no further.
Tim,
Apologies. My memory was faulty. I read 235 as 275. Oh well, nobody’s perfect.
As to the rest of your attempt to further Willis’ illusion, I might as well point to the Earth, and reality. The Earth’s power source is insufficient even to maintain the present surface temperature. It is cooling. You may put as many overcoats on a cooling body as you wish, its temperature will not rise.
if you are too obsessed or stupid to accept reality, you are welcome to the Climate Idiots Clan.
You are merely a religious zealot. You can’t even say where the mythical GHE may be observed and measured, which means that your religious beliefs cannot be tested by following the scientific method.
But I digress. Take a sphere with a surface area of 1m2. Supply a power source generating 235 W. Result 235 W/m2.
Now surround it with a shell, steel or otherwise, at a distance which ensures the shell is radiating 235 W/m2 towards space. That is what Willis’ silly cartoon indicates, doesn’t it?
”Oh,” you say, “That’s impossible. The outer shell has a greater surface area than the sphere – and the power of the heat source is limited to 235 W.”
You did write “Well, this is Willis’ ‘fantasy heater’. And it has been regulated to provide a constant power — not to reach a specific temperature. Once again, you get the science wrong.”, didn’t you?
Tell me again, how I got the science wrong.Then tell me why anybody should take any notice of a religious zealot, who can’t even see the stupid assumptions in Willis’ nonsense.
Carry on being a GHE proselytiser Tim. I wouldn’t expect you to be able to produce your God, and I don’t expect you to be able to produce your GHE.
Have fun.
From the Earth’s perspective the energy provided by the Sun (generator) is constant for the purpose of discussing the topic under discussion. That energy, by itself, does not determine the average temperature of the Earth’s surface. We know from measurements that the Earth radiates more energy than it receives on average from the Sun, thus it is hotter than would be the case if received energy only from the Sun. Where is that extra energy coming from?
Dave Fair June 1, 2021 10:19 pm
Citation? I’ve never seen any evidence for that claim.
w.
Willis, Perhaps Dave is referring to the well-know geothermal heat flow from the interior. But this only about 0.065 W/m^2, so it is lost in the noise of all the other energy flows.
Wait, I bet he is referring to the earth’s surface receiving less energy from the sun (~161 W/m^2) than it radiates (~ 396 W/m^2). The ‘extra’ comes from backradiation.
TOA radiates the same energy as provided at TOA by the Sun. The Earth’s surface radiates more energy than that provided by the net Sun at the surface. No?
I can destroy this pictured greenhouse fallacy once for all and in such a simple way that everyone can understand without any science education, physix, and math, other than 1+1=2 and 1-1= 0
First stop thinking watts, energy, and flux, and what can warm up what, and simply convert watts to money, just like you pay your energy bill put a cost of one watt as one dollar , since they already call it “energy budget” it should not be hard to grasp and it will help greatly to understand the rest.
In the picture the planet produced 235 watts, that planet is now you with 235 dollars in your hand you produced at work today.
in the picture the planet radiated those 235 watts upwards, now imagine you throw your 250 dollars in to the air , how many dollars do you have now ? you have 0 dollars,
you can wait when the money falls back down and catch it , how many dollars do you have now ? you have 250 again
now look at the picture, the planet radiated its 235 watts but yet it keeps them at the same time as if they never left, because when it supposedly returns it adds it up to the original 235watts that were supposed to have left, as if they never did, and turns it into 470 watts , that is the fallacious math,
but wait , it gets worse.
Now imagine instead the shell in the picture, there is a guy hanging above you and he catches the 235 dollars you threw up, now this guy throws your 235 dollars up again all the way into the space, does he still have 235 dollars to throw back down to you ?, no he doesn’t , but in the picture the shell re-radiates the same 235 watts in two different directions at once.
So the change from 235W no shell planet to 470W with shell planet is double counting deception trick.
Do you see it now that the whole thing is a complete nonsense ?
Eben, that’s an interesting analogy. However, in my example the nuclear core is producing new heat constantly. Same in the real world, the sun adds heat constantly. So it’s as if you had a salary of say $235 per day.
Now, without a shell, it’s like you throw $235 up and it goes straight to outer space. So you get $235 per day, you lose $235 per day, steady state.
Now suppose that, just as in your example, there’s a guy hanging in the air and he is catching your money. He takes half of it, $117.50, and throws it into space, and he gives the other half back to you.
When that happens, the next day you get your salary of $235 per day, PLUS the $117.50. So now you’re throwing about $350 into the air. The man catches it again, throws half, $175 into space, and gives $175 to you.
Here’s how that works out over time:
Note that, just as with the steel greenhouse, you end up throwing $470 skyward every day at steady state. No physical laws broken, you just end up with more money in your pocket every day.
No double counting, no deception.
Regards,
w.
Willis,
You wrote –
“Eben, that’s an interesting analogy. However, in my example the nuclear core is producing new heat constantly. Same in the real world, the sun adds heat constantly.”
Don’t be silly, Willis. In the real world, the sun is outside your imaginary shell, isn’t it?
That seems to be a problem. But anyway, what happened to your conservation of energy (within your system)? Did you only throw that in to sound scientific?
You are obviously unaware that the Earth does have a nuclear core, presently converting around 20 tonnes of matter into energy every year. In your fantasy “thought experiment” you forgot to include the sun, obviously.
I don’t blame you. Your steel shell would prevent sunlight from reaching the surface, and complicate the issue, as presumably your core and shell are orbiting said sun. Or not, in which case your shell would only shade one side of your core, in which case you would need a special nuclear reactor to heat the shaded side more than the other side, to arrive at the -9 C core surface temperature.
You don’t actually know what you are talking about, do you?
The Earth has a nuclear core. It also has sunshine. The Earth has cooled over the past four and a half billion years or so.
Maybe you could come up with a “thought experiment” to explain reality?
Or just stick with your silly fantasies.
Swenson May 31, 2021 7:25 pm
You seem to not understand the concept of a “thought experiment”. See the website here for an explanation.
Or you could look at another thought experiment, one in which the sun is outside the shell … it’s here.
Finally, let me suggest that you emulate the rooster.
Wait until it’s actually dawn before you start crowing.
Regards,
w.
Willis,
Whatever happened to “As Usual I Politely But Loudly Request: QUOTE THE EXACT WORDS YOU ARE DISCUSSING. I can defend my own words. I can’t defend your interpretation of my words.”?
Why do you think I do not understand the concept of a thought experiment? You are no Einstein or Schrodinger – and I understand their thought experiments.
You have made an unsolicited suggestion –
“Finally, let me suggest that you emulate the rooster.
Wait until it’s actually dawn before you start crowing.”
Suggest what you like. I’ll ignore it if I choose. If you don’t like it, tough.
The Earth’s inner core (at around 5400 K) is totally enclosed by a shell of iron, and rock, and water, and . . . The inner core seems to be cooling. So much for your “thought experiment”. Fact has apparently triumphed over your fantasy.
Boo hoo. Bad luck. Better luck next time.
Willis:
you do realise that “Swenson” is the most active and intrangent Troll in the Climate debate.
Real name is Mike Flynn … usually confines himself to Roy’s site where similar odd-balls reside and threads such as this go into many thousands of posts of bizarre ping ponging.
Thanks, Passing.
w.
Willis, you are violating a law of radiation from a body. That is, a body at a given temperature radiates equally from all surfaces. That is one reason “surface area” is included when calculating heat transfer. Your table starts off with both bodies apparently being at equilibrium but you then divide the radiation between two surfaces of the same body. You can’t do that.
If the outer shell is at whatever temperature gives 235 watts of energy being radiated, it will radiate 235 watts from all surfaces equally. This places the inner and outer shells in equilibrium with equal amounts of radiation moving between them. At that point, there is no longer heat being transferred and no further increase in temperature.
Your table should have started and ended with the first entry.
Planck says it this way.
Contrary to my understanding of your example, the core is not losing energy to the shell which is initially at the assumed 0 K of its surroundings. Instantaneously, the energy radiated from the core is replenished by the power source alone. As the shell heats, it begins to radiate towards the core and the core, absorbing that energy, will continue to heat up until it is hot enough to radiate 470 W/m2 such that the shell can radiate outward 235 W/m2 and inward at 235 W/m2 at equilibrium, totaling the core’s 470 W/m2.
Radiation is not “split” between sides of a substance. Even Planck recognized this. If a substance is at a given temperature it radiates equally in all directions BASED on the temperature.
Energy, energy, energy. The two surfaces combined reradiate the energy the whole body receives.
No, the two surfaces EACH radiate the energy based on the total temp of the volume! It IS NOT combined. That would mean you would divide the energy by 6 for a square volume.
The result of what you are saying is that you would need to measure both sides to determine that absolute energy/temperature. Your claim is a plate at 1000 deg would radiate at 500 deg from each side so their combination would equal 1000.
Planck says bodies don’t radiate from the surface, they radiate in all directions from the interior THROUGH the surface. If you like I can provide a quote.
I’ve thoroughly enjoyed reading this post (and many like it that have led up to this) – both sides of the argument are helping me understanding this more. Despite the trolling, it’s so important to have this free exchange. Long may it continue.
However, I have a question relating to entropy and if back radiation violates it. A bit late, but if anyone has the time and energy, maybe they could help.
My grasp of entropy goes like this: The sun illuminates the earth with a high temp and low relative entropy. The Earth absorbs all this energy and through various interactions, warms up and radiates infrared energy to space which will be a higher relative entropy than the energy it got from the Sun. The outcome for that IR to space is, lower quality energy and less ability to do work. So basically, it’s a system wide cooling process using a temporary low entropy warming ‘engine’ that maintains equilibrium.
The UWIR from the surface then gets absorbed then bangs around the atmosphere until it’s free to radiate at TOA, which is in a part of the atmosphere that’s a much lower temp. All the while the entropy is going up due to the collisions and loss of energy.
So this is bit I’m struggling with. The back radiation causes the surface to warm which means there is more ‘better quality’ energy at the surface to do more work with than there was before. (assuming that the radiation is unimpeded by the air molecules it has to re-pass through) So in effect the entropy of the system goes down (and gains work ‘quality) by absorbing it’s own lower energy radiation. .
Is this not some violation of entropy? How can an object radiate energy and increase entropy in the process (as the entire system is cooling), and then receive a portion of that energy back, which causes a reduction in that entropy by the surface warming? Does it imply that the Earth has generated it’s own ability to create extra energy to do work, through what is essentially a system wide cooling process? Perhaps the process is exporting that low entropy surface to a emergent structure that is increasing entropy at a faster rate to ensure the whole system increases entropy? Is there such an emergent structure? What would it be?
I slightly relate this to a comment I made in a post a few weeks ago, where I wondered how a molecules at a higher vibrational state at the surface were able to absorb energy from a low energy source, assuming that the higher temp state (surface) has got all it’s vibrational states filled by conduction from nearby molecules. Wouldn’t the energy be scattered and not absorbed if the frequency doors were shut?
So combing my two current lines of reading, I currently think downwelling radiation exists and is measured by devices pointing upward, but it’s scattered from the surface in the opposite direction (and not detected). But my head scratching bit is that the radiation would decrease the entropy of the surface- by raising the temp – and then give itself an extra capacity to do work. Work from work…etc. A low entropy state, from a higher one. That flies in the face of what I understand about entropy and the (max entropy) heat death of the universe.
Plus, as the troposphere is supposed to warm from back radiation, there is not emergent system wide cooling process under current AGW theory that offsets this entropy reduction at the surface. That kinda suggests that something else is going on when you put it in terms of entropy? If the planet is always tending to equilibrium with CO2 rise (net zero change in entropy) how can it lower it’s own local state while not creating a suitable structural process to restore the system wide increase?
I’m probably way too late for this comment stream, but it’s been interesting. I really should be at work! Entropy…..interesting (but confusing!)
Part of the confusion is thinking information theory entropy is the same as thermodynamic entropy. The two are not the same. The obvious difference is that the units don’t match. However, Frank Lambert does a better job of explaining this.
The laws of classical thermodynamics should only be applied where they are defined. There are three basic system types in thermodynamics: open, closed, and isolated. An open system allows both energy and matter to cross the system boundary; a closed system only allows energy to cross the system boundary; and an isolated system doesn’t allow either to cross the system boundary.
The second law only applies to isolated systems. The Universe as a whole is considered to be an isolated system. Open and closed systems may obey the second law, but they aren’t required to. If they did, then nothing could cool off. An isolated system will tend to equilibrium. Entropy will increase in an isolated system until equilibrium is reached, and at equilibrium the change in entropy is zero.
The Earth’s atmosphere is an open system and is not required to obey the second law in its entirety–parts may. (It’s sometimes modeled as a closed system.)
Pompeydano, “…. Is this not some violation of entropy? How can an object radiate energy and increase entropy in the process (as the entire system is cooling), and then receive a portion of that energy back, which causes a reduction in that entropy by the surface warming? ….”
In the comment, May 31, 2021 1:32 am, Willis’ nuclear heat generating planet is radiating 235 W/m2 to space at the surface temperature 253.7 K (-19.4 °C).
Power = sigma* epsilon*(Th^4 – Tc^4) = 5.67*10^-8*1*(253.7^4 – 3^4) = 235 W/m2.
For the planet still to be able to radiate net 235 W/m2 when shielded then the planet surface temperature increases to 301.7 K (28.6 °C) because the shield is at 253.7 K and that is quite higher than the space at 3 K :
Power = 5.67*10^-8*1*(301.7^4 – 253.7^4) = 470 – 235 = 235 W/m2.
Heat is transfered from warm to cold to coldest (as also from the earth surface to the atmosphere to the space) with no violation of thermodynamic laws.
See page 31 – 34 for radiating effects with shields :
heat_4e_chap13-radiation_ht_lecture-pdf.pdf
From page 35 for absorbing and emitting gases (CO2 and H2O) in furnaces and combustion chambers as by Hottel et al based on experimental data.
Kind regards
Anders Rasmusson
Link : https://www.scribd.com/document/295204359/Heat-4e-Chap13-Radiation-HT-Lecture-PDF?language_settings_changed=English
pompeydano:
You ask an excellent question about entropy, and one that goes to the root of the confusion many people have about these issues.
The basic equation relating heat transfer and entropy for an object is:
dS = dQ / T
where dQ is the heat transfer (expressed here as an infinitesimal) in Joules, and T is the absolute temperature of the object at the moment in Kelvin, so dS is the (infinitesimal) change in entropy of the object, in Joules per Kelvin.
Note that transfers out of the system are negative values for Q.
Expressed as rate of change, we have:
dS/dt = (dQ/dt) / T
where “t” is time, dQ/dt is the rate of transfer in Watts, and dS/dt is the rate of entropy change in Watts per Kelvin.
To analyze a real system, of course, you need to integrate over area, and possibly over time. I will use simple examples here, so we can just multiply.
I will use values from Willis’ “steel greenhouse” example to illustrate, and to keep the math even simpler, use two blackbody plates each of 1 square meter area, separated by a vacuum, and close enough that edge effects are insignificant.
Plate 1 is presently at 303K, radiating 470 W/m2, and so a power output of 470W.
Plate 2 is presently at 255K, radiating 235 W/m2, and so a power output of 235W.
Let’s look at Plate 1’s output first.
dS/dt [1->] = -470W / 303K = -1.55 W/K
But wait, the entropy of Plate 1 is DECREASING as a result of that radiative output! Doesn’t that violate the 2nd Law? No, because you have to look at the full process. So we look at Plate 2’s absorption of this radiation.
dS/dt [->2] = +470W / 255K = +1.84 W/K
So the total entropy change is:
dS/dt [1->2] = 1.84 – 1.55 = +0.29 W/K
This is increasing entropy, so no 2nd Law violation here.
Now let’s look at Plate 2’s output:
dS/dt [2->] = -235W / 255K = -0.92 W/K
And Plate 1’s absorption of this radiation yields:
dS/dt [->1] = +235W / 303K = +0.78 W/K
So the total entropy change is:
dS/dt [2->1] = 0.78 – 0.92 = -0.14 W/K
But wait! The entropy of the two plates is decreasing because of this transfer. Doesn’t this violate the 2nd Law? Again, no, because you have to look at the full process. This transfer is part of the same process as the radiation from Plate 1 to 2. They are inseparable (and this is key!). So we have for the entire process of radiative exchange between the plates:
dS/dt [1<->2] = 0.29 – 0.14 = +0.15 W/K
So there is a positive entropy change as a result of the full process, so no violation of the 2nd Law.
Of course, we could also streamline this. The net heat flow is 235 W from 303 K to 255 K.
dS/dt = -235W/303K + 235W/255K = -0.776 W/K + 0.922 W/K = 0.146 W/K
Whether we look at two-way energy flows or net heat flows, the result is the same.
pompeydano: Clifford Truesdell wrote in his book on the history of thermodynamics that: “Every physicist knows exactly what the first and second laws (of thermodynamics) mean, but it is my experience that no two physicists agree about them.” This may have been a bit of an exaggeration but the commenter evidence around here tends to agree with Cliff. Your best source to answer all your formidable questions is a beginning college text on atm. thermodynamics not the comments around here.
Ed Bo & Tim, your work has caused me a headache trying to follow. Perhaps you have a more detailed source to supply for me to do so. Headache causes:
1) The equation for change of entropy in a solid plate of steel involves steel Cv or Cp depending on the process or assumptions and neither of you have included the C term so your results are therefore at first sight questionable.
2) Y’all apparently have surmised that if entropy increases in a heating process, then entropy decreases in a cooling process. A reasonable expectation but that is not true which attests to the peculiarity of entropy. For both heating and cooling, the entropy of the universe increases which follows from the proper entropy change formula (S-So = C*ln(T/To)) in a solid like your steel plates; To being a ref. temperature which you have not supplied like you have not supplied C.
3) A hint where your thought process starts out problematically is where Q is already a heating rate and you have made Q into a rate of a rate and you never integrate over time as you say should be done since every process takes time.
That’s about as far as I got trying to parse your work & assumptions before my headache became unbearable, maybe you can provide more background detail for some aspirin.
Trick:
Good questions all. Let me see if I can give good answers.
My example rate calculations were only for an instant where the two plates had those particular temperatures. What happens next is dependent on other aspects of the problem.
In Willis’ steel greenhouse problem, the sphere and shell are in steady-state conditions, so enough power is supplied to the first plate (his sphere) and enough emitted away from the second plate (his shell) to keep the temperature constant, then the thermal capacitance C of these objects is not an issue there.
I used capital Q as the ENERGY transferred (in Joules), so dQ/dt is the rate of energy transfer (in Watts). Sorry if that wasn’t clear.
The entropy of an object DOES decrease when its temperature goes down. However, when the energy it loses during the temperature reduction is absorbed by a lower-temperature body (like Plate 2 in my example), that body’s entropy increases MORE than the cooling body’s entropy increases. So the entropy of the whole system (of the universe if you prefer) increases as a result of this total process.
The thermal capacitance C comes into play when the temperature changes. The greater C is, the smaller the temperature change for a given energy input or output.
I note that you are using the entropy equation for a single object – which DOES show the entropy increasing with temperature — and trying to apply it to the entropy of the universe as a whole.
Ed, when you switch to plates you leave out detail. Is there still a finite power source as in Willis’ example in plate 1?
“..to keep the temperature constant…”
Don’t have much time for detail so I’ll just write then in the instant you mention your solid object 1 entropy is constant since temperature is constant so its dS/dt = 0 not -1.55.
Over time, if no plate power source, then C is important for the real answer for dS/dt and your correct answer thus is not -1.55. If a power source exists as in Willis’ example to keep plate 1 steady state temperature identically constant, then its dS/dt=0 and universe entropy increases as the power source is used up.
In a solid, you cannot have Q simply as energy, Q is a rate over the time solid’s U changes, so the solid Cv or Cp is needed to get your correct answer for solid dS/dt.
Trick:
In my parallel plate example, I am only considering the two plates. My calculations are for the radiative heat transfers between those only. So the dS/dt from Plate 1’s output to Plate 2 is -1.55 W/K, and for its input from Plate 2, the dS/dt is +0.78 W/K, for a net of -0.77 W/K from the exchange.
In Willis’ example, Plate 1 is receiving 235 W from the core providing a dS/dt of +0.77 W/K, so the net dS/dt and T are constant.
But no, C is NOT important for calculating dS/dt. The equation for temperature change as a result of heat transfer is:
dT/dt = (1/C) * dQ/dt
The C in the denominator here cancels out the C in the numerator in your equation for entropy change.
The equations and terminology I have been using are straight out of introductory thermodynamics texts.
“The equations and terminology I have been using are straight out of introductory thermodynamics texts.”
Well that’s what I was interested to check in detail; please give your citation so I can do so since as it looks to me like you have misused or missed an assumption because you leave out Cv,Cp (which are so close for a solid such as steel that the difference can be assumed away) getting what appears to be the wrong answer for your “only considering the two plates.”
Because it looks like you miss Q is already a rate to start with. Starting correctly your uncited text(s) should find for the rate of change of entropy of a homogeneous solid substance undergoing a constant volume process where Q is the heating rate:
dS/dt = Q/T = 1/T * dU/dt = Cv/T * dT/dt
which is not what you apparently used to compute -1.55 as you have left out Cv (or Cp for a constant pressure process) in your plate example (which integrated is the S-So formula I noted assuming C is approximately independent of temperature over the T range of interest in our atm.).
Trick:
In one of the textbooks I used many years ago — “Engineering Thermodynamics” by Reynolds and Perkins — one of the first equations presented is:
DeltaE = Q + W
where Q and W are explicitly labeled as ENERGY inputs, and DeltaE is labeled as “increase in ENERGY storage”. In a preceding paragraph, it states “The symbol Q is usually employed to represent an amount of ENERGY transfer as heat”. I have found this usage widely used in a large variety of sources.
(In our simple example, there is no work done, and the only energy change is internal energy U, so the equation reduces to DeltaU=Q.)
Regardless, I am specifically and consistently using Q as energy (in Joules), not power (in Watts). When talking about power, I am using dQ/dt.
But to the more central point: let’s look at your equation for entropy in differential form:
dS = C (1/T) dT
so:
dS/dT = C/T
You note that this has the thermal capacitance C in it. But look at the equation for temperature change as a function of heat input:
dT = (1/C) dQ
The larger the capacitance, the smaller the temperature change per unit of heat input.
So:
dT/dQ = 1/C
Now, by the chain rule:
dS/dQ = dS/dT * dT/dQ = (C/T) * (1/C) = 1/T
So:
dS = dQ/T
As I said before, the capacitance term cancels out in the relationship between heat transfer and entropy change.
“DeltaU=Q”
For a change in U, time is required so this equation is per unit time. Thus, here Q is really Q dot despite the verbiage.
Many texts do leave out the verbiage for unit time but you should know every process takes time so Cv,Cp cannot be correctly canceled out of the relationship for a change in entropy & energy change & temperature change as you do. Q is universally a heating rate by virtue of a temperature difference not an amount.
“But to the more central point: let’s look at your equation for entropy in differential form:
dS = C (1/T) dT”
That’s not my eqn. which is for an infinitesimal change in entropy S over time increment dt:
dS/dt = C (1/T) * dT/dt
The only…ONLY way C can cancel out is if entropy is constant which cannot happen in any real process (such as your plate example) due 2LOT. It’s possible to imagine a reversible process but none exist, you need to include Cv,Cp.
I’ll look up the text in my college library, might take a few days, and look for the words you note, a page ref. would save time. In the meantime, consider the differences in eqn.s:
Your dS = dQ/T
versus textbook: dS/dt = Q/T = 1/T * dU/dt = Cv/T * dT/dt
Trick:
I’m afraid you are so confused on very basic topics that you are lost before you get started.
A change (deltaX) in a quantity is NOT equivalent to a rate of change (dX/dt). The units aren’t even the same.
The 1st Law equations I have given you are explicitly about energy, not power. They hold true regardless of the rate of change.
Every source I can find, both printed text and on-line references, uses Q as heat transfer ENERGY, not power.
The equation YOU gave for entropy — S-S0 = C*ln(T/T0) — is NOT in terms of rate either. I correctly use its differential form dS = C * (1/T) dT.
Even if you consider the rate equations, your point does not hold.
Converting YOUR equation to rate by differentiating with respect to time, we get:
dS/dt = C * (1/T) * dT/dt
But dT/dt = (1/C) * dQ/dt {using the standard thermodynamic notation of Q as heat transfer energy}
dS/dt = C * (1/T) * (1/C) * dQ/dt
dS/dt = (1/T) * dQ/dt
dS/dQ = 1/T
dS = dQ/T
As I keep saying, the thermal capacitance term C cancels out when talking about the relationship between S and Q. None of your analysis actually shows otherwise.
You have the same understanding as I do. But, when you include “c” you must also include mass. This makes sense when determining the change in T. The same energy will change the T over a given time “t” differently for varying masses.
Ed: “A change (deltaX) in a quantity is NOT equivalent to a rate of change (dX/dt). The units aren’t even the same.”
Yes Jim 11:00am, Ed confusedly wrote above trying to show an amount IS equivalent to a rate:
“So the total entropy change is: dS/dt [1->2] = 1.84 – 1.55 = +0.29 W/K”
Total entropy change is NOT a rate; total entropy change is an amount computed from & as I wrote the integral of dS/dt:
S-So= C*ln(T/To)
There is no escaping Ed must use C and a reference temperature to compute “the total entropy change” in Ed’s steel plates. I will look for Ed’s cited text and find where Ed goes wrong but it will take some time.
Remember Cliff Truesdell: “Every physicist knows exactly what the first and second laws (of thermodynamics) mean, but it is my experience that no two physicists agree about them.”
Well, I find my local college library is covid19 open only to students/staff/faculty at present so Ed’s cited book search will not be as quick as I thought. I find it interesting to track these things down as I learn stuff on the way. My experience is as Cliff Truesdell notes, even textbook authors are notorious for being sloppy & especially I have found that true in the field of Thermodynamics. If you consult 5 books on the subject, you get 5 defn.s of heat. I remember Willis posting up a comment showing like a dozen different defn.s of heat.
Ask any historian of science. Better yet, for text book author issues read Tony Rothman’s (2003) delightful “Everything’s Relative and Other Fables from Science and Technology”, John Wiley & Sons. Another good source is “Dictionary of Scientific Biography” the DSB is the first place to look if you want to know what our illustrious predecessors really did and said.
I ordered Ed’s cited text, a page number from Ed will save time:
“Your request for Engineering Thermodynamics [by] William C. Reynolds [and] Henry C. Perkins. was successful. Your request will be delivered to your Local Library when it is available.”
This process will take ~week though.
Trick:
I’m glad you were able to track down that thermodynamics text. It’s a good one.
In my copy (2nd Edition), the First Law equation is #2-18a on page 48.
The entropy-heat relationship dS = dQ/T is equation #7-43 on page 213. (No C in the equation…)
Both are part of a larger discussion which is well worth your time. In fact, the whole text is worth your time.
I remain mystified that you cannot accept that C * (1/C) = 1. I have explained it explicity both in terms of change and rate of change. I’ll try once more, easy on the math.
You keep using the equation relating entropy change to temperature change, which has C in the numerator.
But the equation relating temperature change to heat input has C in the denominator.
So the resulting equation relating entropy change to heat input (which is the product of these two) has C both in the numerator and in the denominator, which means C disappears from the equation.
It’s that simple!
Ed, C (specific heat capacity in J/kg K) cannot be made to disappear out of the text book formula for amount of your calculated “total entropy change” in each of your solid steel plates which is:
S-So= C*ln(T/To)
I do not see how Reynolds & Perkins can make C disappear for “the total entropy change” in a solid (or liquid) but I will find out in a week or so, thanks for the page ref.s.
11:00am: C is the specific heat capacity in S-So= C*ln(T/To)
Tim:
Of course you are correct here. But I explained it the way I did to demonstrate that looking at the “back radiation” in isolation can lead to erroneous conclusions.
As I have read thru all the responses I continually see a misconception about how radiation from a body works.
At a given temperature a body radiates in all directions equally. Example, you put a metal plate in a furnace until it is white hot. You don’t have to measure both sides with an infrared thermometer to get the total temperature. You don’t have to measure all six sides of an ingot and add the readings to get the real total temperature.
This applies to point sources and homogenous bodies of any shape. Radiation won’t spontaneously occur from outside the surface of the body due to heat inside the body.
Eli Rabitt’s green/blue plate suffers from this problem as do several of the models and explanations given in this thread. For those here that use a concentric shell model, remember, what you are heating with radiation is the shell. The shell will then radiate both in and out at the same level. Heating caused by incoming radiation and subsequent outgoing radiation is NOT DIVIDED between the two surfaces.
For those who like the bucket model. The model should be one where water entering the second bucket is pumped back into the first bucket. Even you pump every bit of water entering the second bucket back into the first bucket, you will never get anything but equilibrium. This meets the conservation of energy law while adding extra water into the system does not.
I also want to emphasize that none of this discussion has dealt with heat capacity and specific heat capacity. Radiation can do one of two things, it can raise the internal energy of a substance thereby raising its temperature OR it can be radiated away thereby lowering its internal energy and temperature. Radiation can not do both at the same time. What that means is that bodies with high specific heat capacity must capture more energy radiation to raise their internal temperature so they can radiate more. This changes a number of factors such as the gradient involved in heating or cooling a body. This is probably less important when equilibrium is established but with a rotating earth, one must consider this in calculating temperature changes.
“For those who like the bucket model. The model should be one where water entering the second bucket is pumped back into the first bucket. Even you pump every bit of water entering the second bucket back into the first bucket, you will never get anything but equilibrium.”
You seem to be overlooking the continued input of ‘water’ in the model. The 2nd bucket leaks evenly in both directions. So the 1st bucket gets 117.5 liters from the 2nd bucket + 235 new liters of water from the ‘input hose’. No water is ‘created by doing this — more water flows in to the system (the buckets) (235 L/s) than flows out of the system (initially 0 L/s, then 117.5 L/s, and slowly growing to 235 L/s). This imbalance allows the water to build up in the buckets without ‘creating any water’.
Willis went through the same thing upthread, only with money instead of water.
Tim,
Buckets? Money? What’s next? Overcoats? Bathtubs?
Have you not heard of the scientific method?
Show me your GHE. Describe it – tell me where I might observe it, and measure it.
Is it a bucket? Or insulation, perhaps?
If not, why keep rambling about things you claim it isn’t, and instead tell everyone what it is. What’s the matter – cat got your tongue?
Go and cry on Willis’ shoulder. You seem to share the same bizarre fantasy.
Bugger off back to Roy’s Flynn
Jim, please read Anders Rasmusson’s reply to pompeydano, above. I assume you will be able to see where the calculations came from.