Guest Post by Willis Eschenbach
Short answer? Of course not, that would violate the Second Law of Thermodynamics … BUT it can leave the hot object warmer than it would be if the cold object weren’t there. Let me explain why this is so.
Let me start by introducing the ideas of individual flows and net flows. Suppose I owe you twenty-five dollars. I run into you, but all I have is a hundred dollar bill. You say no problem, you have seventy-five in cash. I give you the hundred, you give me the seventy-five, and the debt is paid.
Now, there are two equally valid ways to describe that transaction. One way looks at both of the individual flows, and the other way just looks at the net flow. Here they are:

Figure 1. Net flows and individual flows. The individual flows are from me to you, $100, and from you to me, $75. The net flow is from me to you, $25.
What does this have to do with cold and warm objects? It points out a very important distinction, that of the difference between individual flows of energy and the net flow of energy, and it relates to the definition of heat.
Looking at Figure 1, instead of exchanging dollars, think of it as two bodies exchanging energy by means of radiation. This is what happens in the world around us all the time. Every solid object gives off its own individual flow of thermal radiation, just as in the upper half of Figure 1. We constantly radiate energy that is then being absorbed by everything around us, and in turn, we constantly absorb energy that is being radiated by the individual objects around us.
“Heat”, on the other hand, is not those individual flows of energy. Heat is the net flow of energy, as represented in the bottom half of Figure 1. Specifically, a heat flux is the net flow of energy that occurs spontaneously as a result of temperature differences.
Now, the Second Law of Thermodynamics is only about net flows. It states that the net flow of thermal energy which we call “heat” goes from hot to cold each and every time without exception. However, the Second Law says nothing about the individual flows of energy, only the net flow. Heat can’t flow from cold to hot, but radiated energy absolutely can.
When an object emits radiation, that radiation goes on until it hits something that absorbs it, whereupon it is converted to thermal energy. The individual temperatures of the emitting and absorbing objects are not significant because these are individual energy flows, and not the net energy flow called “heat”. So there is no violation of the Second Law.
Here’s the thing that keeps it all in balance. If I can see you, you can see me, so there are no one-way energy flows.
Which means that if I am absorbing radiation from you, then you are absorbing radiation from me. If you are warmer than me, then the net flow of energy will always be from you to me. But that says nothing about the individual flows of energy. Those individual flows only have to do with the temperature of the object that is radiating.
So how do we calculate this net energy flow that we call “heat”? Simple. Gains minus losses. Energy is conserved, which means we can add and subtract flows of energy in exactly the same way that we can add and subtract flows of dollars. So to figure out the net flow of energy, it’s the same as in Figure 1. It’s the larger flow minus the smaller flow.
With all of that as prologue, let me return to the question that involves thermal radiation. Can a cold object leave a warm object warmer than it would be without the cold object?
While the answer is generally no, it can do so in the special case when the cold object is hiding an even colder object from view.
For example, if a person walks between you and a small campfire, they hide the fire from you. As soon as the fire is hidden, you can feel the immediate loss of the radiated energy. At that moment, you are no longer absorbing the radiated energy of the fire. Instead, you are absorbing the radiated energy of the person between you and the fire.
And the same thing can happen with a cold object. If there is a block of wood between you and a block of ice, if you remove the wood, you’ll get colder because you will be absorbing less radiation from the ice than you were from the wood. You no longer have the wood to shield you from the ice.
Why is all of this important? Let me offer up another graphic, which shows a simple global energy budget.

Figure 2. Greatly simplified global energy budget, patterned after the Kiehl/Trenberth budget. Unlike the Kiehl/Trenberth budget, this one is balanced, with the same amount of energy entering and leaving the surface and each of the atmospheric layers. Note that the arrows show ENERGY flows and not HEAT flows.
These ideas of individual flows, net flows, and being shielded from radiation are important because people keep repeating over and over that a cold atmosphere cannot warm the earth … and they are right. The temperature and the radiation are related to each other by the Stefan-Boltzmann equation. When we apply the S-B equation to the 321 W/m2 of downwelling “back radiation” shown in the graphic above, it tells us that the effective radiating level is somewhere around freezing, much colder than the surface.
BUT a cold atmosphere can leave the earth warmer than it would be without the atmosphere because it is hiding something even colder from view, the cosmic microwave background radiation that is only a paltry 3 W/m2 …
And as a result, with the cold atmosphere shielding us from the nearly infinite heat sink of outer space, the earth ends up much warmer than it would be without the cold atmosphere.
To summarize …
• Heat cannot flow from cold to hot, but radiated energy sure can.
• A cold atmosphere radiates about 300-plus W/m2 of downwelling radiation measured at the surface. This 300-plus W/m2 of radiated energy leaves the surface warmer than it would be if we were exposed to the 3 W/m2 of outer space.
My best regards to all,
w.
My Usual Request: When you comment, please QUOTE THE EXACT WORDS THAT YOU ARE DISCUSSING, so that we can all understand the nature of your objections.
My Second Request: Please keep it civil. Speculation about the other person’s motives and cranial horsepower are greatly discouraged.
Further Reading: My post entitled “The Steel Greenhouse” looks at how the poorly-named “greenhouse effect” work, based on the principles discussed above.
Math Notes: There’s an excellent online calculator for net energy flow between two radiating bodies here. It also has the general equation used by the calculator, viz:

with the following variables:

and Q-dot (left-hand side of the equation) being the net flow.
Now, when the first object is totally enclosed by the second object, then area A2 is set to a very large number (I used a million) and the view factor F12 is set to 1. This is the condition of the earth completely surrounded by the atmosphere. For the general case, I’ve set area A1 to 1 square metre. Finally, I’ve made the usual simplifying assumption that thermal IR emissivity is 1.0 for the surface and the atmosphere. The emissivity values are greater than 0.9 in both cases, so the error is small. With those usual assumptions, the equation above simplifies as follows, courtesy of Mathematica:

But sigma T ^ 4 is simply the Stefan-Boltzmann radiation for the given temperature. That is why, in the energy budget above, we can simply add and subtract the energy flows to produce the budget and check to see if it is balanced.
“If I can see you, you can see me…”
I think that assumes we are both “looking” at the same EM wavelength (or overlapping EM bands).
In talking science and making definitive statements, it is always is good practice to acknowledge what assumptions are being used.
If people are still unclear on this, Rabbit has a great explanation of this here, like only the Rabbit could do it: http://rabett.blogspot.com/2017/10/an-evergreen-of-denial-is-that-colder.html
If the front plate is at equilibrium with its input and surroundings, the first effect of adding a cooler plate (green) very close behind the first (blue) will be to cool the blue plate while warming the green plate. Both plates will aim to reach the same temperature. Then they will both warm back up to the original equilibrium temperature, assuming the input stays the same.
At no point with the temperature of the original plate get higher than its original temperature.
REALITY , not some bungled explanation from a rabbit. !
“the first effect of adding a cooler plate (green) very close behind the first (blue) will be to cool the blue plate “
You seem to be confused about radiation vs conduction. If the two plates were physically placed into contact (basically making a single, thicker plate), then your description is pretty accurate. But that is not the scenario under consideration. Willis’ post clearly and thoroughly explains what happens with radiation.
You seem to be confused about energy transfer.
Where did I say they were touching??
If its 2 miles away it will have no affect whatsoever.
Energy transfer rate is related to the temperature difference and distance between objects.
Andy says: “If the front plate is at equilibrium … “
First, to be precise we need to say the front plate is in a “steady-state” condition, where Qin (the total heat in) equals Qout (the total heat out). In this case, the temperature of the warmer (blue) plate will be constant. (For a true ‘equilibrium’ situation, every part would be the same temperature and there would be no heat flows).
“adding a cooler plate (green) very close behind the first (blue) will be to cool the blue plate while warming the green plate. ”
Why? How does the energy loss from the warm plate INCREASE when the cool (green) plate is added? Certainly not by conduction, since you said they are not touching. Certainly not by radiation either. Radiation loss from the blue plate DECREASES since the blue plate is radiating to a warmer region than it had been (the cool green plate vs the much colder background).
As for Eli’s thought experiment, here’s a reasonable comment to start from:
https://climateofsophistry.com/2017/10/06/slayers-vindicated-by-additional-independent-researchers/#comment-31140
Though for full context you could read from a little earlier. It’s gone through overall in quite some detail from thereabouts onwards. And is of course thoroughly refuted.
Not up for discussion here, obviously. Dusted and done, etc…
Tony, It continues to be mildly bemusing that you treat a discussion at a third-rate blog that disagrees with every thermodynamics text ever written to be the only authoritative source.
It’s a thought experiment from one blog discussed on another. Don’t get carried away. Other perspectives are not forbidden here, thankfully.
And people at this blog usually see through such tactics as poisoning the well, so you might want to give that sort of thing a miss.
Suppose you have two bodies (A and B), and the warmer one (B) has a parabolic mirror around it directing incoming radiation from A onto B. In this case, is it possible for cooler A to warm B (B not counting the mass of the mirror)? (As with many things, I have no clue, but it seemed like it might be an interesting question.)
Bob Shapiro November 28, 2017 at 11:46 am
Sorry, but if the mirror focuses the rays of A onto B, it will focus the rays of B onto A, so no change in temperature.
TANSTAAFL …
w.
Your comment is both true and surprising.
There’s very few observations that satisfy both of those criteria.
I sat down to type an explanation as to why you were wrong; reconsidered, then re-reconsidered.
The word ‘visible’ is mildly ambiguous in this context. We sometimes think of a brighter object as being more visible than a darker object. One side might well be outputting more photons at a particular wavelength than the other, ie be brighter, and thus in a sense be more ‘visible’, while at the same time at a different wavelength the converse is true. However, that’s not the sense of visibility under discussion. The alternate nuance of the word, the one under discussion, is what my old instructors referred to as inter-visibility, or ‘line of sight’.
I have heard it alleged that the earth puts out more energy at radio wavelengths than the sun does.For the sake of discussion, let’s assume that claim is true.That would be an example in which two objects are brighter than each other at different wavelengths, and therefore in one sense ‘more visible’ than the other at that wavelength. However, at each wavelength, if one object can be ‘seen’ by another at that wavelength, then the converse is true, and it can be seen from the first at that wavelength.
Whatever the wavelength, whatever the obstructions, the same line of sight applies to both sides. (A statement which I think is a fair paraphrase of Willis’s point.)
As an aside, I’m grateful to Willis for addressing this issue. Some of our fellow sceptics do misunderstand energy flows. Their ignorance is an embarrassment to all sceptics. Even worse, it’s often ascribed to all of us as a group.
If I understand correctly, what you are saying is 9in effect)…if I am made of air but have eyes that can somehow see visible light nonetheless…I can see you, but you cannot see me.
Is that it?
Relax. Catastrophic Anthropogenic
Global WarmingerrClimate ChangeerrClimate DisruptionerrClimate-Related ShockerrCarbonerr “Cannot settle the name” Apocalypse a.k.a CACA is 97% political.As long as freedom of conscience and thought remain in the universal declaration of human rights, there is no need to be embarrassed about non-compliant thoughts of individuals. The concept of “consensus science” is an oxymoron anyway.
If we focus on the remaining 3%, any concept starting with Average Global exits internationally established metrology standards de facto and, thus, also the modern scientific methods. Irrespective of subsequent clarifications, such as, “energy flows”, “energy budget”, “air temperature”, “air composition”, “sea level”, “skeptic psychoanalysis”, “social cost of carbon” etc. They may be helpful for general understanding similarly to philosophy, as long as they are acknowledged as such.
Willis, what the article ignores is that there must also be a GHG effect due to conduction if there is one due to radiation. And the GHG effect due to conduction is much larger than then one due to radiation, because conduction affects all gasses, not just GHG.
The key to understanding is to realize that conduction involves the transfer of virtual photons, which in all respect except 1 are identical to real photons. Virtual photons cannot act at a distance.
Thus a molecule of N2 accepts a virtual photon from the surface which can then be conducted upwards or back-conducted to the surface warming the surface. The one difference between N2 and CO2 is that CO2 can radaite to space while N2 cannot. Thus the CO2 GHG due to radiation provides a net cooling as compared to N2 GHG effect due to conduction.
“Can A Cold Object Warm A Hot Object?”
The answer is yes. Electromagnetic radiation flys through the 0°K outer space, once that radiation hits a molecule that absorbs that wavelength, it is thermalized. A match in a freezer is very cold, but striking it on sandpaper will ignite it to a very hot temperature. When energy is changed in form, yes, cold objects can warm a hot object. The GHG theory takes EM IR radiation and thermalizes it, changing a cold EM wave, into a hot vibrating molecule. That is why the CO2 IR signature is identified way up in the atmosphere where it is about -80°C.
Of course this is correct, but watch out for one problem in communication. Some parts of the world know only “safety matches”, so they have no experience of a match which will ignite by striking on sandpaper. I was once talking to a Swedish person and recounting a trick we used to play at school in England. We would dig a hole in the end of a stick of chalk, put a broken off head of a match in that hole and cover it up with chalk dust, then wait for the teacher to write on the blackboard and have the end of the chalk explode into flames. The Swede couldn’t understand how this could work, then I realized the Swede had never encountered a non-safety match and I explained that at that time in England non-safety matches were the norm.
David Ramsay Steele
As were blackboards, now a non PC term. But whiteboard is OK, it’s non discriminatory, apparently.
LOL, great story
well you can’t say that….. thermalization implies a lot of molecules…it is a collective concept… but well you re right..
can a cold object warm a hotter objet ..by heat flux..no.
the heat goes from cold to hot..
Yes, that is true, but my comment was about the GHG effect. People often use the cold can’t warm a hot object, which is true for heat flux, but not the conversion of energy from one form to another. That is the relevant issue when discussing the GHG effect. The GHG effect is converting cold EM radiation to warm kinetic energy.
co2islife
Agreed, it can ‘warm’ a ‘hot object’. The problem with Willis’ piece is that it has many problems with the definitions. “Flow” has a normal meaning different from the way the term is used above. For someone coming to the issue afresh, a set of definitions is needed and they have to conform to certain norms. The reason is that as soon as one takes an argument outside the text, the terms have to match. Radiated energy does not “flow”. That’s the point of giving it another term with its own definition. One can speak of radiative balance and net gain, but not flow. There is no flow going on.
There are multiple issues with the piece: EM travelling through space ‘hitting’ or ‘encountering’ an object does not mean it will be ‘absorbed’. It might be reflected or refracted. It is not always true that adding energy to a molecule raise its temperature, it may in some cases and not in others. In some cases the net effect is it lowers the temperature by inducing emission of a photon of greater energy than the one striking it. That is how extraordinarily low temperatures are achieved – by hitting the atoms with additional energy.
And so on and on. The presentation is generally true: that the surface cooling can be reduced by GHG’s through the mechanism of preventing radiation from the surface proceeding directly to space. This will prevent some of the cooling of the surface. It does not guarantee (at all) that it will prevent cooling of the atmosphere. The atmosphere (heated by the surface or by radiation) cools by radiation from GHG’s and without them, it could not.
There is in all these arguments about back-radiation, confusion abounding. The root problem is the BIG FAT ERROR which is the assumption that the appropriate comparison is a planetary body with no atmosphere at all receiving insolation from a star, and a planet with an atmosphere with GHG’s in it. That is a completely inappropriate comparison when one is speaking of the influence of the relative concentration of GHG’s in an atmosphere. It should be obvious that the appropriate comparison is between an atmosphere without GHG’s and one with them.
If one were to suggest that an atmosphere with no GHG’s at all was going to be cold, I would reply that it a stunning and obvious error. The surface would be warmed freely by incoming radiation and the surface would warm the atmosphere, which could not cool by radiation, only convection of heat to the surface when the surface was colder than the atmosphere (at night). The atmosphere would just get hotter and hotter until it warmed the radiating night side surface enough to dispose of a quantum of heat sufficient to balance that received by the atmosphere on the sun-side. A no-GHG atmosphere would be hot as Hades because it cannot cool by radiation. Adding GHG’s to it does shield the surface from directly cooling to space, but it also dramatically cools the atmosphere which can now radiate freely in all directions. This would in turn greatly reduce the surface heating at night from the hot atmosphere. Remember that the temperature of our atmosphere at a high elevation is very high because those widely dispersed molecules cannot cool by radiation or convection.
In short, the cartoon with the GHG’s and back radiation is fundamentally flawed because it is based on the assumption that without GHG’s the Earth and its atmosphere would be much colder. How many times have you seen the comparison and ’33 degrees C of warming’ by GHG’s? That is stuff and nonsense. It is always a comparison of a body without any atmosphere and one with both an atmosphere+GHG’s in it. Never is it the appropriate comparison in which one can evaluate the effect of GHG’s themselves.
Don’t confuse the sun-side surface temperature with the average surface temperature, and the sun-side atmosphere’s temperature with its average, nor with the surface temperature on the day or night side.
A planet with no atmosphere at all (like the moon) would have a surface temperature on average 33C colder than our surface.
A planet like Earth with an atmosphere with no GHG’s at all would have an atmosphere that is much warmer than ours is presently.
The claim for 33C of warming by GHG’s is false.
A planet like Earth with an atmosphere and some GHG’s would have an atmosphere that is as warm as it is now.
The temperature of a no-GHG atmosphere will be strongly influenced by the period of rotation because that affects the temperature of the surface that heats it.
thank you. I thought I was the only one 🙂
I’m so glad it’s not just me! The terms make no sense….”a cold object hiding an even colder object from view?” Flow? Net flow of heat or net flow of energy? Can low energy electromagnetic waves being emitted by a cold object cause an increase in temperature of an object that can only be heated by high energy electromagnetic waves?
The temperature of an object is determined by how much energy it absorbs vs how much it emits. Absorbs the same amount it emits? Temp is stable. Absorbs more than it emits? Temperature increases. Absorbs less than it emits-temperature drops. It doesn’t matter if the absorbed energy comes from a colder object, a hotter one, or a hundred objects of differing temperatures…it’s still about the ratio of energy absorbed to energy emitted.
Our atmosphere might be a “cold object” between Earth and the “even colder object” of space, but that colder object of space ALSO holds the “hotter object of the Sun”. The moon has almost no atmosphere and on the daylight side it’s temperature reaches 127 C and on the dark side it’s temp plunges to -173 C. Our “cold” atmosphere buffers us from both the intense cold of space AND the intense heat of the Sun. Using Willis’s terms, it “hides something colder from view AND something hotter from view”. But all of the heat warming the surface AND the atmosphere comes from the Sun. If it wasn’t there, not only would the Earth get colder than it currently does, but it would also get warmer than it currently does.
The 2nd law of thermodynamics is about the SPONTANEOUS flow of heat between objects always from a warmer to a colder. You CAN change the flow of heat but only with an additional/outside source providing additional work…but that’s not what the 2nd LOT refers to.
Uggggg…
Crispin, generally look forward to your comments from Waterloo, Ulan Batar, etc., but in this one, you are in error when you say a non GHG atmosphere cannot cool by radiation. A pure diatomic oxygen and nitrogen atmosphere is certainly not a GHG, but would be completely transparent to infrared radiation. Therefore the planetary surface directly “sees” outer space at 3 degrees Kelvin and radiates energy to it quite nicely.
DMacKenzie
Because there is no chance of us finding a true non-GHG atmosphere (in my view anyway) please treat the discussion as being about an ideal atmosphere with and without GHG’s. I agree that O2 has some ability to radiate, but it is inconsequential (kills some lovely logical tricks!) but the importance of the lesson is, I hope, evident.
The discussion everywhere I look is about the moon (no atmosphere) and the Earth with an atmosphere and GHG’s, with the difference in temperature attributed to the GHG’s alone. That is a fundamental error far worse than comparing apples and oranges.
Is it obvious that the entire edifice of CAGW is based on a comparison so logically baseless? If no one can point out another source for my analysis, I think I will claim priority. I am going to bring up the idea of modeling it next week at the Chinese Academy of Sciences and see if they will devote some time to a simple calculation of the equilibrium temperature of an Earth-like planet with no GHG’s, say, an argon atmosphere of 1 bar and a sandy desert surface with an emissivity of 0.93. They have a monster of a computer just down the road.
Crispin, I think you don’t need CAS. Manabe and Strickler covered the basics in their classic 1964 paper referenced by Spencer (search drroyspencer Why 33 deg), also Lindzen (search Some Coolness Concerning Global Warming). Your stated result is correct, but I find Spencer and Lindzen explain it in terms more relatable to the average audience.
Sorry Crispin, that read quite a little ruder than I intended…
DMacKenzie
I checked the paper by Spencer and he does not get the point at all. He repeats the error (as I understand it). The error is he says a planet with no atmosphere (case 1) and a GHG atmosphere (Case 3) are 33 degrees different but does not consider the atmospheric temperature of Case 2: a planet with a full atmosphere without any GHG’s. If he wants to calculate the effect of adding CO2, then he has to compare it with having none (Case 2), not having “no atmosphere at all” (Case 1). Case 1 v.s. Case 3 is a silly comparison.
I tried to come up with a suitable analogy today but it is difficult because the claim and the example (repeated by Spencer) is so inappropriate.
Considered this:
I want to determine the effect of the mass of yeast added to a loaf of bread baking in a bread pan, and its influence on the surface temperature of the pan measured at the centre of one side.
I propose to alter the amount of yeast added to the dough, which will alter the porosity of the bread, which will affect the heat conducting rate through the pan into the dough, which will affect the pans surface temperature. I want to see the effect of doubling the mass of yeast on the pan temperature, (which will be influenced by the porosity of the bread).
According to the CAGW method, to get a baseline I measure the temperature of an empty pan and compare it with a pan containing 1 kg of dough and 1 g of yeast, and then claim that the temperature difference is caused by the yeast! This is a reasonable analogy of their mental experiment underlying the claim for the GH effect.
They are not conducting an experiment I described at all which was to assess the effect of increasing the mass of yeast (CO2). They are testing two completely different things and claiming that the net effect is caused by one tiny portion of the dough.
People have swallowed this misconception hook, line and sinker. I could show you a thousand copies of the error. The greenhouse cartoon embeds their conceptual error. The correct comparison is an atmosphere with a pressure of 1 bar, and no GHG’s and one with GHG’s. First, get the equilibrated temperature without, then add CO2 and recalculate.
The assumption inherent in the error is that without GHG’s the atmosphere would be as cold as the average surface of a planet with no atmosphere at all. Nonsense! Ever heard of heat gain by radiation and heat disposal by conduction to a gas? There is an example in every house in Canada with a forced air furnace. All the heat from burning fuel is passed through a hot metal plate to a gas on the other side. A surface of a planet with an atmosphere without GHG’s would be heated even better than with them. That how surface would heat the atmosphere, which has no way to dump the heat save back to the surface when the planet turns away from the sun. Adding CO2 might change the temperature, up or down, we don’t know, but my silly calculator says it will be hotter just above the ground to have no GHG’s at all.
1360 W/m^2 daytime at the equator. Emissivity of sand, 0.93. What is the temperature reached when it is in radiative balance? It would heat up to about 170 C. At present sand does not get much above 70 C in a desert with 50 C air. The temperature of the atmosphere next to the ground would be in the region of 140 C. I have no idea what the nighttime air temperature minimum would be, but it would be a heck of a lot higher than 15 C because gases do not cool effectively downwards to a surface by conduction (REF: Bejan, A, 2005 “Convection heat Transfer”).
Because that atmosphere could not cool by radiation (by definition) it would simply stay hot and get even hotter the next day, if it managed to cool a bit at night, until it was eventually in equilibrium. Adding CO2 to such an atmosphere turns the atmosphere itself into a radiating body. It introduces back radiation but it introduces a shade at the same frequency. The net effect of turning the atmosphere into a gigantic radiator would be to cool it, which would draw down the surface temperature. In radiative balance, the surface would no longer be the only emitter so it would be cooler, by more than 100 C.
So where is this putative 33 degrees of heating? There is no 33 degrees for GHG’s. Without an GHG’s it would be a heck of a lot hotter because …..physics! Ask Bejan. The bottom line is that water provides evaporative cooling and is a powerful GHG. CO2 has to be a very minor player because there is so little of it (and all the usual asides). 20 ppm CO2 does not provide “6 degrees of warming” as the chart often shows.
Summary:
Case 1 No atmosphere
Case 2 Atmosphere
Case 3 Atmosphere with GHG’s
Which has the warmest average surface temp; which has the highest atmospheric temperature 1.5 m above the ground?
Case 2.
Crispin in Waterloo:
I’m sorry, but that is demonstrably and provably not true, as I showed in A Matter Of Some Gravity. Here’s the short version.
You say that a planet with an atmosphere which neither radiates nor emits thermal IR will be warmer than the S-B temperature with no atmosphere. IF that were the case, then the surface, which is the only part of the system capable of radiating heat, must be radiating more energy than it is receiving. Of course this violates the laws of thermodynamics …
Read the post for the long version.
w.
The planet viewed from space will be at S-B with a non radiative atmosphere but the surface beneath the atmosphere will be above S-B in order to supply the necessary kinetic energy to fuel the inevitable convective overturning.
co2islife
I am posting this separately – sorry for duplication of my long single comment appears later.
Agreed, it can ‘warm’ a ‘hot object’. The problem with Willis’ piece is that it has many problems with the definitions. “Flow” has a normal meaning different from the way the term is used above. For someone coming to the issue afresh, a set of definitions is needed and they have to conform to certain norms. The reason is that as soon as one takes an argument outside the text, the terms have to match. Radiated energy does not “flow”. That’s the point of giving it another term with its own definition. One can speak of radiative balance and net gain, but not flow. There is no flow going on.
There are multiple issues with the piece: EM travelling through space ‘hitting’ or ‘encountering’ an object does not mean it will be ‘absorbed’. It might be reflected or refracted. It is not always true that adding energy to a molecule raise its temperature, it may in some cases and not in others. In some cases the net effect is it lowers the temperature by inducing emission of a photon of greater energy than the one striking it. That is how extraordinarily low temperatures are achieved – by hitting the atoms with additional energy.
And so on and on. The presentation is generally true: that the surface cooling can be reduced by GHG’s through the mechanism of preventing radiation from the surface proceeding directly to space. This will prevent some of the cooling of the surface. It does not guarantee (at all) that it will prevent cooling of the atmosphere. The atmosphere (heated by the surface or by radiation) cools by radiation from GHG’s and without them, it could not.
I think the temperature of “space” at any point is reasonably considered the temperature corresponding to the the total radiant energy flux impinging on it , or equivalently , dividing by a lightsecond , the energy density at the point . That gives the temperature of a gray , ie : flat spectrum , object at that point , eg : the ~ 278.6 +- 2.3 from peri- to ap- helion on our orbit .
That the “cold can’t heat warm” issue is still in need of discussion given the simple classic energy flow law shows its stagnation . One of the first steps forward would be to recognize that those emissivities are not really scalars ; they are averages over spectra . Simply getting agreement on the experimentally testable computations at http://cosy.com/Science/warm.htm#EqTempEq seems nigh on impossible — and it is only with that full spectral computation that the radiative equilibrium of a radiantly heated object , eg : a planet , can be calculated to the 4 decimal place variation this entire fiasco is about .
Then it is found , unless someone comes up with some new physical laws , the is no way a spectral phenomenon can “trap” heat in excess of the equilibrium .
Now, I’ve been looking for an answer to this question for a long time and no-one has been able to provide one, which leads me to believe it is false: How is it possible that 3 of 4 CO2 molecules out of 10000 can warm the rest by 1 degree (for simplicity’s sake) without each of those CO2 molecules at least absorbing and transferring 2500 deg C or more of heat in the process?? If there is anyone that claims this is indeed happening, then where can we test that by measuring it? We have all the molecules available after all.
CO2 is 0.00004 or 0.04% of the atmosphere. Is it plausible that “activating” 1 out of every 2,500 molecules in the atmosphere can actually result in a material temperature change?
How to Discuss Global Warming with a “Climate Alarmist.” Scientific Talking Points to Win the Debate.
https://co2islife.wordpress.com/2017/01/16/how-to-discuss-global-warming-with-a-climate-alarmist-scientific-talking-points-to-win-the-debate/
co2islife
You really have to include water vapour. Activating 20,400 ppm of GHG’s (water and CO2) can easily move energy around. Reflecting clouds can match that, by the way.
CO2 islife:’insignificant’ 0.04% is a very poor way to couch an argument about this molecule. The reason I say this should be evident in your moniker! It is, as far as we, and the entire biosphere, atmosphere (O2) and hydrosphere (O2 and CO3) is concerned, the heaviest lifter in the atmosphere.!
Nice succinct argument.
If I take Cold Sodium and mix it with cold water, I get a very hot reaction.
https://youtu.be/NTFBXJ3Zd_4
That was a hydrogen explosion you heard.
2Na(s) + 2H2O(l) –> 2NaOH (l) + H2(g)
The “smoke” is steam, it is the water vapor that formed when the H2 combusted with atmospheric O2 to make hot H2O.
nope
Yep, my point what that energy being changed in form can in fact take a cold object and warm it. The GHG effect isn’t about heat flux, it is about converting cold EM radiation to warm kinetic energy, and that does in fact happen.
That is just a chemical reaction liberating stored chemical energy. Nothing to do with this topic.
of course, agree.
It has everything to do with this topic. The comments of cold warming hot have to be put in the context of the GHG effect, where in fact cold EM radiation is thermalized into kinetic energy and in fact does warm the atmosphere. Willis is confusing heat flux with energy being changed in form. This is a common false argument made by people trying to argue against the AGW theory. They simply don’t fully understand the concept.
J Richard
Just to quibble, it is not stored energy – not at all. It is just sodium. It is not like a canister of compressed air. When sodium makes contact with water, it makes new chemistry, releasing energy, but that energy was not stored in either the sodium nor the water.
OTOH, perhaps we can view all matter as stored energy in that matter exists and could be released upon annihilation, right? I wonder if we could calculate the total energy in the universe including all the sensible matter, all the shell of Dark Matter that surrounds it, and all the much larger shell of the Even Darker Matter that surrounds that.
If energy can be transferred between these types of matter, then it is on-topic for a discussion on GHG radiative heat transfer/obstruction.
Crispin,
It is stored energy, because making pure sodium needs a lot of energy to separate it from any other ion (OH-, CL-,…). Part of that energy is given back when Na reacts with water… Comparable with loading a battery, where you store electrical energy into chemical energy and back…
CO2, you are using the cold object’s chemical energy to accomplish this. If you destroyed the sodium completely, the E=mc^2 would warm it up even more. If a cold asteroid enters the cold atmosphere, it, too, will warm both up- friction and kinetic energy when it hits the ground. If you turn on a refrigerator, heat from the icebox will make a warm room warmer, but you have to do work to accomplish this. Hammer cold steel with a cold hammer….. I think Willis was referring to a more passive relationship between objects!
Yes he is, but all these comments have to be put in the context of the GHG effect. The GHG effect does in fact take cold EM radiation and convert it to warm kinetic energy. The GHG is about energy changing in form, not heat flux, and that doesn’t apply to the GHG effect.
This is essentially the same argument Roy Spencer made seven years ago. It is nicely rebutted here. http://principia-scientific.org/images/stories/pdfs/Pierre_commentsadded.pdf
I get so tired of hearing this stupid argument from the anti-ghg crowd. Thanks for quantifying it.
Yeah, I’ve been going through this on another thread, and I thought it deserved its own clear explanation … I suspect that there will always be people saying it is impossible.
Best to you and yours, my friend, and thanks for the endless work you’ve done to keep this site fresh and vital,
w.
Take an indoor pool. Heat it to 30C. Fill the room with 100% CO2. Will the pool’s water temp go above 30C? Nope. Impossible. CO2’s IR wavelength is very cold.
J. Richard Wakefield November 24, 2017 at 7:59 pm
Huh? Nobody said the pool’s water temp would go up in that situation, that’s a straw man.
w.
No, it’s not. The IR from the pool would get absorbed by the CO2 and be re-emitted back to the pool, and raise it’s temp. That is the EXACT argument AGW people claim happens. It’s in that flat earth graphic you posted (which is completely false of how the planet’s energy system works.)
J. Richard Wakefield November 24, 2017 at 8:09 pm Edit
Please re-read the section about how a cold object can leave a warm object warmer ONLY if the cold object is hiding something that is even colder.
And this is the case for the planet, where the atmosphere is hiding the 3 W/m2 heat sink of outer space.
But in your thought experiment, this is NOT the case. Without the CO2 you get radiation from the roof of the room. With CO2 you get radiation from the CO2, which is at the same temperature as the roof … so there is no change in downwelling radiation when you introduce the CO2.
Regards,
w.
“The IR from the pool would get absorbed by the CO2 and be re-emitted back to the pool”
No. To the pool, yes, but to the ceiling and the walls as well. The pool would still cool, but at a rate slower than without CO2.
Now if there was a constant source of energy, say an electric heating element or the sun, the temp of the pool would be higher with the CO2 than without.
“And this is the case for the planet, where the atmosphere is hiding the 3 W/m2 heat sink of outer space.”
I have no idea how the atmosphere can hide anything. What does “hide” mean?
“But in your thought experiment, this is NOT the case. Without the CO2 you get radiation from the roof of the room. With CO2 you get radiation from the CO2, which is at the same temperature as the roof … so there is no change in downwelling radiation when you introduce the CO2.”
That depends on what the roof is made of. Make the roof an absorber of IR, then none of that from the roof would go back to the pool. Or is that your “hiding”?
“No. To the pool, yes, but to the ceiling and the walls as well. The pool would still cool, but at a rate slower than without CO2.
Now if there was a constant source of energy, say an electric heating element or the sun, the temp of the pool would be higher with the CO2 than without.”
You can prove that? That would happen regardless of the gas in the room. But you are correct, insulation slows the rate of heat loss. That is NOT the same thing as making the heat source hotter.
This is one of the major flaws in that flat earth graphic. The planet is not getting a constant flow of energy from the sun on any given surface area. The planet is rotating. That acts like a thermostat. If the planet rotated more slowly, the surface would absorb more sun’s energy, and the temp would be higher, Rotate slow enough and the planet would be too hot for life during the day.
Rotate faster and the surface wouldnt be able to absorb enough energy to keep the nice tropic temps we enjoy.
This is why that flat earth graphic is a completely wrong depiction of the energy flow.
“The IR from the pool would get absorbed by the CO2”
Those are your words not mine. If the energy gets held up by CO2 and then readmitted with some of the energy going back to where it came from, of course it will preserve the pools temp.
and no one is saying the temp of the pool would heat higher. That would be stupid.
And it the sun’s energy was daily not constant, that doesn’t change the argument.
“Rotate faster and the surface wouldnt be able to absorb enough energy to keep the nice tropic temps we enjoy.”
Can you prove that?
Days are shorter, but so are the nights.
The temp would be more even, most likely. But the temps are very even in the tropics anyway.
Back radiation from the colder object is absorbed by the hotter object.
The hotter object is heated by that absorbed radiation.
Being heated, it now radiates more per unit time than it was radiating before because rate of radiation is related to temperature.
Thus this increased temperature increases its rate of cooling.
The question is, what is the net flow:
Does the increased absorbed energy result in an output increase that is equal to, less than, or greater than the back radiation absorbed?
The emissivity of the atmosphere is not 1. Actually the atmosphere is not a grey body (=surface). by the way, it has no proper surface.
“Back radiation from the colder object is absorbed by the hotter object.
The hotter object is heated by that absorbed radiation“
“Can A Cold Object Warm A Hot Object?
Willis Eschenbach / 9 hours ago November 24, 2017
Guest Post by Willis Eschenbach
Short answer? Of course not, that would violate the Second Law of Thermodynamics”
If I recall neither Willis or Anthony have a BS ( or studied Thermodynamics ) and as a result will remain in their fantasy world with these unicorn examples of science.
Bigfoot:
I have studied thermodynamics (at MIT) and have both bachelors and masters degrees. I assure you they are absolutely correct on this subject.
A lot of universities use thermodynamics as a “washout” course to quickly eliminate the people who just are not capable of this type of rigorous analysis. At first I thought it was cruel. Now when I look at many of the comments here, I see why that is so necessary to keep those people away from real-world systems where they could cause serious harm.
The issue is how the interior of a sphere can be made hotter than the radiative equilibrium temperature of the sphere by some spectral phenomenon in apparent violation of the Divergence Theorem which is another way of expressing that heat comes to equilibrium — essentially Fourier’s differential eq ( here’s a YT on it I recently watched : https://www.youtube.com/watch?v=NHucpzbD600 ) .
My brain requires simplicity . Show me the quantitative equations on a sphere . Then I can write the code and explore the parameter space .
No planets , no clouds , atmospheres . Just spherical shells of ( ae spectra ; transparency ) and power source and sink spectra . Actually it can be simplified to the 1 dimensional case something like this :
http://cosy.com/Science/1DeqDiagram.jpg
Show for what “Atmospheric Filter” and Surface ae spectrum ( b + c ) is greater than the equilibrium lumped spectrum .
So far as I know , the Schwarzschild differential ( http://www.barrettbellamyclimate.com/page47.htm ) is the definitive differential for radiant – mass heat transfer . So show under what subspace of parameter values does it “trap” a higher energy density on the side away from a source .
Thanks Willis for your very lucid explanation. There are some people who just refuse to accept this no matter how clearly it is explained. It is frustrating arguing with these people and I’ve just about given up trying to convince them. They have a false mental picture that heat flows between objects like water and are stuck on the idea that water doesn’t flow uphill.
I see they are now resorting to some sort of argument from authority by arguing you lack a PhD in science. While an argument from authority is a false argument, if they want to go down that route no problem. There are many here who do have a PhD in science, including myself, who would fully endorse your explanation. I doubt those arguing with you could come up with even one to support their position.
Ultimately a PhD is just a piece of paper. What is important is the spirit of open inquiry, curiosity and rational investigation that underlies science. You certainly have those qualities and what you write is usually both interesting and informative. I have no doubt you could have earned such a piece of paper for yourself if you had taken the time to do so; but then you would have led a much less interesting life.
JRD,
I don’t understand your boundary conditions,
Are you talking about a closed room that cannot radiate or loose any heat to the outside? i.e. a closed system?
Was the CO 2 that was added at the same temperature of the water?
If same , in a closed system then there would be no change in the temperature of the water or the gas?
If one was at a greater or lower temperature then I expect in a closed system, both would end up at the same temperature with time?
Are the boundary conditions different than I assumed?
I agree…who said the pool would get warmer if the CO2 was also at 30 degrees?
Who said the pool would get warmer if the CO2 was at -180C?
Although I’ve never used the argument, that’s a pity. But perhaps the heavenly quantifications of the pro-ghg crowd will compensate enough to maintain your balance positive also without them.
Now when “metrology”, measurement science, is no longer considered a typo in WUWT, the same recognition could perhaps now be given also to “measurand” i.e. a quantity intended to be measured, an object being measured, a physical quantity or property which is measured.
But what is the even colder object, that the cold object is hiding?
Space as such is not necessarily cold, at any rate not as we understand temperature since there are all but no molecules, thus no kinetic energy.
Richard:
“Space”, while having incredibly low density, has incredibly vast extent. The low density means there is no conductive are convective heat transfer to speak of.
However, astronomers have made incredibly detailed measurements of the background radiation from space, and it exactly (to within the precision of the measurements) matches that of a blackbody, both in magnitude and spectrum, at 2.725K (+/-0.0001).
Now, you have have semantic and philosphical arguments as to whether space “has a temperature” of ~3K, but for the purposes of radiative heat transfer calculations, it is completely correct to treat it as the equivalent (at least) of an ambient at ~3K, providing 3 microwatts/m2 (0.000003 W/m2).
On the other hand, as we sit here typing our comments, we are bathed in an ambient of ~400 W/m2 from our surroundings that are near 300K. This is what is fundamentally confusing J Richard Wakefield above with his pool example — extra CO2 over the pool under the roof is not at a much different temperature from the roof.
+1,000
This is so simple to test, and prove you wrong. Take a blow torch, measure the temp inside the flame. Then light a candle near by. Does the torches temp go up? Nope. But try it to make sure.
The downward IR from CO2 attempting to warm the warmer surface is like trying to piss into a fast flowing river.
Two problems with your contention. First I doubt you’ve ever done the experiment. The temperature change would be small and how would you mearsure it? But the big problem is that, as in another arch example above, you’re dealing with chemical reactions and it’s going to be hard to create a good experimental set up. You’d have to put the blow torch inside something, set the torch to a constant flow of gas and equilibrate the temperatue on the outside surface, then add the candle outside and see if you have to reduce the flow of gas to keep the temperature constant. I’m sure you would, but it would a difficult experiment to perform.
No, if Willis is correct, the burning hot gasses in the torch should be absorbing the IR from the candle flame, making those molecules in the torch flame hotter. If he is correct.
What you are proposing is different. You have the torch and the candle heating a third object. Of course that third object’s temp will be higher than just one heat source!
This is all academic. Willis should propose an experiment to prove his position. Conceptual analogies only work if the premise can be shown empirically to be correct.
“No, if Willis is correct, the burning hot gasses in the torch should be absorbing the IR from the candle flame, making those molecules in the torch flame hotter. If he is correct.”
My point was that you were presenting an experiment that is impossible to carry out, so I was suggesting one which could be carried out. Provide us with the actual details of how the experiment you proposed would operate and why you claimed “Nope” when both Willis and I would claim “Yes”?
J. Richard Wakefield
“No, if Willis is correct, the burning hot gasses in the torch should be absorbing the IR from the candle flame, making those molecules in the torch flame hotter.”
Good grief. The candle slightly reduces the rate at which the hot gases cool. The flame temperature is fixed by the energy released in the chemical reaction which is based on the energy needed to pull the fuel molecules apart and the energy gained by assembling them into new molecules like CO2 and H2O.
As soon as the combustion molecules form they release photons cooling the molecule and heating the gases surrounding it. Those gases in turn shed heat in all directions. At the same time they are picking up photons from all surfaces ‘in sight’, the energy of which depends on the source temperature. Suppose we have a CO flame burning to CO2. The theoretical temperature is 2200 C. Pointing two CO flames at each other will not generate a central temperature of 4400 C. Why?
Measuring a flame temperature is a good analogy for an atmosphere. Put a thermocouple into a flame. It will get very hot and emit visible photons, perhaps it will look white if it is hot enough. Whatever the temperature device says is the temperature, the flame is actually much higher because the thermocouple cools radiatively. Place a shield over the tip. This is called a “shielded thermocouple”. Place it in the flame and the temperature reading is much higher – never quite as high as the actual flame, but higher because the radiation is reflected back to the source (the metal of the thermocouple) limiting its ability to cool. The tip is like the Earth’s surface heated by the sun (flame). The reflector is like CO2 around the Earth. CO2 sends some radiation back, except that it isn’t nearly as effective as a reflective tube.
So, what’s the beef? The temperature of the thermocouple tip will never be hotter than the flame just because it is in radiative balance with the mirrored tip. The Earth’s surface (not the atmosphere) would cool faster if there were no GHG’s in the atmosphere. For an explanation of why the atmosphere would not react in the same manner, see my long post above.
Here’s an interesting link. MIT scientists have built an incandescent light bulb whose envelope passes visible light but which reflects infrared radiation back at the filament. The result is that it takes less electricity to heat the filament to the desired temperature … a lot less electricity.
There is no practical difference between the mirror and an infrared emitter. The resulting radiation hitting the filament is indistinguishable between the two.
How is this an example of a colder object causing a warm object to be warmer? The filament is emitting visible light. That requires a high temperature of around 3000 K. link The infrared reflected back at the filament would be generated by a much lower temperature of less than 800 K. That’s around the temperature where metal starts to glow red. link
The trick is not to confuse temperature (measured in degrees) with power (measured in BTU per second or calories per second). Most of the power radiated by the filament is infrared.
The cold and warm objects are not different than antennas. Antennas typically have large noise voltages and therefore radiate a significant signal at the noise frequencies. That doesn’t keep a miniscule signal from exciting the antenna and being detected. In fact it is possible that an antenna can be radiating many watts of signal while, at the same time, being used to detect microwatt signals.
Just because you can’t measure the effect of a candle on a blowtorch it doesn’t mean the effect doesn’t exist. It’s like gravity. A comet flying past the Earth will exert gravitational attraction on the planet, you just won’t be able to measure the effect. Similarly, if I pee into a fast moving river, you won’t be able to detect the greater flow. On the other hand if 100 million of me pee in the same river, the effect will be measurable.
“Similarly, if I pee into a fast moving river, you won’t be able to detect the greater flow. On the other hand if 100 million of me pee in the same river, the effect will be measurable.”
All that is doing is increasing the cold source (you peeing) into a hot source (many people peeing).
Fascinating. Someone asked for a practical example. The MIT light is an excellent example. The cold outer shell adds heat to the filament leaving it warmer than it would be otherwise.
w.
If each of us pees 100 cc in 20 seconds, the total volume is 10 million litres. There are 1000 litres in a cubic metre, so that’s 10,000 cubic metres total, in 20 seconds. That’s 30,000 cubic metres per minute. That’s about a seventh of the discharge of the Amazon river. link Impressive! We will be able to measure the effect.
Thanks be to God, there are not a hundred million of me. If we reduce the hundred million, at what point do we say that there is no effect? Why do you pick that particular number?
The effect will be there even if you can’t measure it.
You made the bold statement that Willis is wrong. Then you gave a couple of examples of experiments that would be hard to measure. That doesn’t actually prove that Willis is wrong, does it. Not only that but you have the burden of proving that your examples are apt. How about providing some numbers to back up your assertion.
Excellent. Unfortunately, 75% or so of the Earth’s surface is water and a good proportion of the remainder is covered in plants that transpire. Infrared does not heat water it is absorbed by the first molecule and raises its energy level and latent heat eventually leading to the water molecule evaporating and taking its heat with it. So 75% or more of the surface of the Earth will be cooled by ‘downwelling’ radiation. The dry portions of the rest of the Earth may be raised in temperature but following Stefan Boltzmann will increase their radiation by the 4th power (modified by emissivity) and thus radiate any increase from downwelling infrared away rapidly. As is demonstrated by the rapid increase in radiation from the bulb filament, that, absent any input electricity would cool and go dark, it cannot continually circulate the infrared.
Both you and Willis make the same mistake of an reasoning in the abstract and avoiding the fact that a volume of water will cool from infrared due to increased evaporation and that most of the Earth’s surface is water or plants transpiring water. The subsequent convection and release of latent heat are the elephant in the room that you are carefully avoiding discussing.
In effect, IR enables more visible/UV, while having correspondingly reduced exittance itself from the system, the bulb. Lovely physics, but no ghe except on the horticultural sense. Had this before, haven’t we?
Willis Eschenbach
“Fascinating. Someone asked for a practical example. The MIT light is an excellent example. The cold outer shell adds heat to the filament leaving it warmer than it would be otherwise.”
Exactly. Now the big problem: The element is the source of the emission. On planet Earth, there are two sources after the energy is put in: first, the surface and second, the atmosphere itself. They are both sources of IR.
If there were no GHG’s present in the atmosphere, there would be no second source. The surface would be the sole source of IR emission and the hot surface would warm the atmosphere because of contact with it. The atmosphere would thereby cool the surface a bit, and during the daytime, be unable to get rid of the heat gained. The remaining IR would pass through to space. Daytime after daytime, the atmosphere would warm and not be able to cool. At night the surface would radiate freely into space and be warmed by the hot atmosphere which would cool a bit depending on its circulation and the length of the night.
It is untrue, in spite of thousands of claims to the contrary, that the atmosphere would be cold because it contained no GHG’s. In the daytime it would be as hot as the surface, certainly in the vicinity of the surface on the sunny side. Unable to cool by radiation, the temperature in the atmosphere would be the same as the surface at the bottom and then cooler with altitude according to the lapse rate.
Consider how different this is from the claims made in tens of thousands of articles on ‘the GH effect”. Without GHG’s, there is no ‘reflector’ and the atmosphere cannot cool by radiation, even though it would be constantly heated by the hot surface. This special case is more like a light bulb with xenon gas in it. In that case the filament is also “hotter” (than a vacuum bulb) because the gas is heated by contact with the filament. Therefore the filament runs hotter without a reflector. Different scenario, same enhanced visible radiation effect. The xenon can only cool by contact with the quartz bulb = inefficient.
When GHG’s are added to the atmosphere, the surface cooling by contact continues, the “reflector” moves into position, and the atmosphere itself begins emitting IR to space. What will be the net effect of this additional loss? Will there be a net decrease in the system’s temperature with the addition of IR radiative gases, or will the system’s temperature as a whole increase? Speaking of the atmosphere alone, will it cool because it gained the ability to shed heat directly to space?
You see what I am emphasizing? The correct comparison is an atmosphere with and without GHG’s, not an atmosphere with GHG’s and a planet with no atmosphere at all. The light bulbs can have an increased emission in the visible range by putting in a reflector, or by adding a non GHG gas. IMAX projectors use high pressure xenon in the light source. Halogen headlights in cars uses a mix of inert gases and a reflector inside the bulb where it can be the most effective as a temperature enhancer.
“If there were no GHG’s present in the atmosphere, there would be no second source. The surface would be the sole source of IR emission”
This is incorrect. The non-greenhouse gasses are still radiating IR. Everything radiates IR unless its temperature is absolute zero. The non-GHG doesn’t absorb IR frequencies, but that doesn’t stop it from radiating IR. The atmosphere would absorb energy where it contacts the surface (conduction) and these warmer molecules/atoms would rise (convection). So the atmosphere would still radiate to space and to the planet. Thus the planet surface would be cooler with a non-GHG atmosphere than with no atmosphere because it would lose some energy to the atmosphere through conduction, and not get it all back.
Crispin in Waterloo,
The surface is heated by the sun, thus there is an indirect source of energy, as good as in the example of the flame. If that wasn’t the case, everything would would cool to space temperature, with GHGs only a little slower…
coaldust says: “The non-GHG doesn’t absorb IR frequencies, but that doesn’t stop it from radiating IR. “
It turns out that materials absorb IR exactly as well as they emit IR. “For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.” https://en.wikipedia.org/wiki/Kirchhoff%27s_law_of_thermal_radiation
If — as you claim here — the non-GHG doesn’t absorb absorb IR frequencies, then it also doesn’t emit those frequencies.
“The non-greenhouse gasses are still radiating IR. Everything radiates IR unless its temperature is absolute zero.”
While this is sort of true, it is wildly misleading. The amount of radiation emitted by N2 or O2 is minuscule compared to the IR emitted by N2. An atmosphere of pure N2 would radiate orders of magnitude less IR than a similar atmosphere with a little H20 and a little CO2. This has been confirmed by innumerable experiments and is understood on a theoretical level.
Ferdinand, you are not adding information. The electric bulb has energy from a power station. So what? It doesn’t impact the argument. We are discussing shifting the emitted spectrum using an insulator or reflector.
Crispin,
Sorry, my wrong… I am a little late in the discussion and overlooked the background in this case…
I suspect that many WUWT readers have pissed into a fast flowing river. Probably into the ocean as well.
Something commonly done in engineering combustion is to preheat the incoming gases. This can be done with something much cooler than the flame (often exhaust gas), but will increase the temperature of the flame.
Preheating is not the same as making a hot source hotter. All you are doing in the preheat is making a cold object hotter before you make it into a much hotter object.
Nick ,
Are you saying that the heat of combustion is the same but the final temperature of the gas is higher, then I agree. In most cases the fuel temperature is probably not heated, but where possible the waste heat is used to preheat the “gas or fluid” being heated, of course there are always exceptions.
“All you are doing in the preheat is making a cold object hotter before you make it into a much hotter object.”
The object is the flame. If you could make it hotter with waste heat, that would already be the supposed paradox.
It’s the same deal. You have an object which becomes hot through some source – sun for earth, enthalpy of combustion for flame. Anything that adds heat – DWLWIR for earth surface, warmer incoming gas for combustion, makes it hotter, basicallly because it has to shed more heat against the same thermal resistance. That extra heat can easily come from a cooler object, such as a preheater.
I heard a thought experiment a while back involving a light bulb inside a mirrored box…mirrored on the inside of the box.
Would it keep getting brighter and brighter inside the box?
Until the melting point of the mirror was reached, or the filament of the bulb evaporated, etc. There are practical limits to everything. When I was in the heat treating business we’d put pads of nichrome wire with ceramic beads on the outside of the wire on the pipes we were heating and high temp insulation on the outside, but where the bead coated wire had to poke through the insulation you had to be careful and let some of the heat escape or the wire would get too hot and melt the nichrome wire destroying the pad. You have to know your limits.
menicholas
Good point. In fact the brightness would change if you cannot see all the radiation. The energy in is fixed, right? Suppose for a moment it was 100W and we insulate it perfectly and see what happens. The bulb would start off with a normal spectrum – mostly IR and some visible. As the temperature rose the mix of wavelengths would change, not the amount of output energy which is fixed at 100W. As the temperature rues the frequency would keep increasing in order to shed 100W starting at a higher temperature. This would carry on until something mechanically failed.
An insulated box can reach a very high temperature inside with a constant input of electrical energy. You just have to keep the heat in.
That experiment was here on WUWT some years ago. The net result was that the filament got hotter and a hotter filament has a higher resistance. That was measured as a small drop in amperes (at a constant voltage)…
As only part was reflected and the bulb was cooled by outside air, the filament didn’t burn up, but I am pretty sure that without outside cooling that would have happened.
Every microwave oven in existence defies the above :-).
Thermal emissions are not hot or cold they aren’t anything until they are absorbed, they can be straight reflected or pass straight thru with no interaction. They are called thermal because of there origin not because they are hot. Thermal emission are not hot or cold and no different to any other RF emission.
If we look at Willis rather silly example stuff above our microwave oven breaks his law in that net heat moves from cold to hot. From a the stupid classical physics rubbish you have something at room temperature or zero (depends what temperature stupidity you try to put on the RF signal) making something very very hot AKA your food.
The key point is when working with EM waves the concept of hot and cold go out the window and EM waves aren’t hot or cold.
You seem to be somewhat confused.
The Standard Dictionary understanding of “Thermal Emission” is that it comes from a “Hot” Object.
As you say EM is neither Hot or Cold just at one or more Frequencies and only creates heat when it excites atoms in an object it strikes.
Perhaps on re-reading your post, that is what you are saying.
But a Microwave does not have energy coming from a “Cold” Object, It has Energy coming from the Energy applied the cold object which converts it to EM.
Try putting your dinner in the Microwave without switching it on to see how warm it gets.
The point you are missing is that Willis Eschenbach artcile is not even about the greenhouse effect which doesn’t remotely work like that and you can’t account for the GreenHouse Heat in that way no more than you can a microwave oven.
There are a multitude of ways to produce heat via resonance and GreenHouse Effect happens to use one of them. Greenhouse effect occurs because of resonant heating, like dielectric heating (microwave), Induction heating (Magnetic field in any conducting material) and a number of other effects they have absolutely nothing to do with heat transfer as such.
If your answer involves heat transfer it is wrong, and it is like trying to find how the heat gets into the water molecules in a microwave. The answer is dielectric effect in microwave ovens and electromagnetic resonance in GreenHouse effect it really isn’t that hard.
So is there any solar cycle induced effect that could hinder/change the apparent black sky near absolute temperature from being “seen” by earth. Thus altering the outward net flux of radiation.
We have heard that the solar cycle has a near zero impact on the energy balance of earth thus nothing to see there, we have heard about frequency changes of light through the cycle, but little definitive stuff really, or maybe just unqualified, so maybe it is a simple apparent background temperature change.
I’m not the scientist so am able to ask these questions without shame.
I didn’t read the article through, but you made a mistake in your opening analogy and chart.
You left out the “original ” flow of the $25 “loan” from me. The original $25 “flowed” from me to you, is missing in the “all flows” category and cancels out the “net flow” from you to me in the “net flows” category.
Maybe you explain the discrepancy in the article, but why use a flawed analogy at all?
Aphan November 24, 2017 at 8:03 pm
Flawed? The original reason for the $25 debt is absolutely immaterial to the concepts explained. Maybe I assumed someone else’s debt. Maybe I bought $25 worth of goods from the man. None of that matters. I’m merely trying to explain one single transaction, an exchange of money (or radiation).
w.
w.
If you want to be clear, then explain one single transaction without adding “absolutely immaterial ” details about a prior transaction.
If you want to be scientific, don’t use words like “hide” and “see” to describe things that dont/can’t perform the act of hiding or actually be seen. Don’t interchange “radiation”, “heat” and “temperature” as if they are the same thing.
The atmosphere slows down the rate at which the Earth cools. But should the Sun disappear, the Earth with the same atmosphere will eventually cool until it reaches equilibrium with space. So in the end, that colder object would NOT “leave Earth warmer”.
Here’s a link to an experiment on this topic for anyone interested:
https://climateandstuff.blogspot.com/2013/03/does-thermal-radiation-travel-from-cool.html?m=1
You’re talking about the heat death of the universe. In the end there will be no cooler objects.
commiebob,
Nope. Never mentioned the universe. I’m talking about the Sun in our universe, in our solar system, our galaxy and the fact that our atmosphere generates no “heat” of it’s own to warm something else with.
Bare planet. Add atmoaphere.
Radiating object. Add object radiating less.
In both cases we are changing the situation by adding mass. The first added mass has no surface because it is a different phase of matter., which has that characteristic. Nor can it act as a Black Body, they say. Physics is more interesting because it has many such tricks.
S-B and Planck Relation are claimed to be designed for objects in equilibrium, radiating to 0Kelvin. This has been claimed to make GH factors dodgy.
Anyone caring to comment on what effects the above have on this post, feel free.
Brett Keane November 24, 2017 at 8:05 pm Edit
I don’t understand that.
True. And … so what?
I have no idea who “they” are, but “they” are wrong. There is no reason that a gas cannot act as a black body.
Citation? I fear I don’t believe that at all … for example, the S-B equation in the math notes is specifically for two objects which are NOT in equilibrium, nor are they radiating to 0 K. That’s the equation that’s used in the online calculator … which you apparently claim is wrong, wrong, wrong …
No effects, as the statements are either untrue or immaterial.
Regards,
w.
“I have no idea who “they” are, but “they” are wrong. There is no reason that a gas cannot act as a black body.”
A gas can only radiate in its absorption bands. thus it is not a blackbody.
A couple of points- radiation does not have a temperature. It has a quantum of energy per photon based on the frequency. There is not “cold” infrared radiation from a cold object. If it is infrared it has less energy than if it is visible.
Regarding the “energy” diagram. Why is there no energy radiated from the stratosphere to space, or from the troposphere to the stratosphere? Photons aren’t radiated uniformly in one direction from a molecule.
The Earth, with or without an atmosphere will radiate the same. Assuming that all energy comes from the sun, the planet will eventually achieve equilibrium and will radiate as much energy as it receives.
Yes, there are only two ways to change the T of an object in a radiative equilibrium. Either change the input, or change the residence time of the energy entering the object. The residence time of energy in the object depends on only two factors. The W/L of the input, and the materials encountered.
“The Earth, with or without an atmosphere will radiate the same. ”
In both cases the earth will radiate with a blackbody spectrum. The Earth with its atmosphere will radiate very differently.
http://climatemodels.uchicago.edu/modtran/
Willis,
Nice condensed and simple straight forward explanation.
Thanks.
Yes, thank you very much, Willis.
I seem to remember some time ago when someone attempted to estimate the fraction of the downwelling radiation derived solely from man’s contribution to the CO2 in the atmosphere. Anybody know?
As I remember, it was a very small fraction; lost in the noise.
Interesting, but please allow me to play the devil’s advocate here.
According to the figure, the surface pays 392 W/m^2 to the atmosphere (“surface radiation”) and gets 321 W/m^2 (“back radiation”) in change.
Net radiative energy flow is therefore supposedly 71 W/m^2 from the surface to the atmosphere.
Assuming the Surface has an average temperature of 15 °C, and solving Stefan-Boltzmann for 71 W/m^2, shows that the atmosphere must have a temperature of around 1 °C. It must also be fully opaque, so that no energy radiates directly to space.
I’m using this online S-B calculator: https://www.engineeringtoolbox.com/radiation-heat-transfer-d_431.html
Problems:
1. The atmosphere is not opaque.
2. The average temperature in the troposphere (which represents 99% of the total atmospheric mass) is -15 °C.
Let the flaming begin. 🙂
Nah, no flaming. You say:
Other than the “atmospheric window”, it is very near to opaque, with almost all of the upwelling radiation absorbed more than once before it makes it to space.
The average temperature in the troposphere is not relevant. What is relevant is the temperature in the effective radiating layer. About 75% of the downwelling radiation at the surface comes from the first hundred metres of the atmosphere, which biases the effective radiating layer down in the warmer temperatures.
w.
Willis, I agree with your article 100%. One thing as an engineer, some people seem to assume this is a one step process which it is not especially when no additional outside sun radiation is added when it is night, otherwise it would not cool down overnight. There is continuous radiation and back radiation in my mind and the earth keeps cooling down after the sun goes down since the CO 2 radiates a portion of energy out to space each “step”
What am I missing?
If you are correct, then how come on a cloudless night the temperature drops much more than on a cloudy night?
Clouds are much higher up than a few hundred meters you claim is responsible for 75% of the downwelling radiation. Also clouds are visibly opaque but the clear sky is transparent.
KM November 24, 2017 at 9:35 pm
The greenhouse effect works because some of the upwelling IR is absorbed by the atmosphere. When the atmosphere subsequently radiates the heat, about half goes up, and the other half goes down to the surface. This leaves the surface warmer than it otherwise would be.
Now, obviously, the more upwelling IR is absorbed, the more downwelling IR comes back to the surface. So which one will absorb more upwelling IR?
1. Clear skies
2. Clouds
Clouds are basically black bodies for IR. If they have any thickness they don’t let any IR get through to the other side. So clouds absorb more than clear skies. As a result, they absorb more, and as a result they radiate more, both downwards and upwards. This leads to the phenomenon you describe above.
On a clear night, the IR is much freer to escape to space, so less of it comes back down to warm the ground.
w.
Willis, I understand the point you’re trying to make but it doesn’t seem to add up.
You first claim that the atmosphere is near to opaque (i.e. essentially acts as a black body).
Then you state that 75% of the back radiation happens within the lower hundred meters. If this were true then only a small part of the remaining 25% of the total outgoing IR would reach higher altitudes where the clouds are forming.
In your latest post you claim that the clear sky is transparent to IR, but clouds are not. You are contradicting your first statement that the atmosphere itself (in the absense of clouds) is near to opaque.
Along the lines of my prior comment, the downwelling radiation fraction from strictly man’s contribution to atmospheric CO2 concentrations must be minuscule.
One has to add TIME to this discussion. There is no such thing as a one-and-done with energy. What happens over time.
Then you have different places to describe. 2 feet underground, 1 inch underground, right at the surface, the atmospheric molecules that touch the surface, 2 metres high, 50 metres, 1 km, 6 km, 10 kms, 70 and 100 kms high. And you need to describe it over time as more energy is coming in and more energy is leaving over that time.
A cold object warms a warmer object through EM radiation? How much and for how long and what happens to both objects over time.
Please explain the GHE on Mars.
The Martian atmosphere, on a numerical basis, has an order of magnitude more CO2 molecules than does the atmosphere of Earth. Even taking into account water vapour, there are more molecules of so called GHGs in the Martian atmosphere, than there are in Earth’s atmosphere.
Further not only are there numerically more molecules of so called GHGs, they are much more tightly spaced together, since Mars is a smaller sphere and the atmosphere has a smaller volume.
The upshot of this is that on Mars, there is a greater prospect that an upwelling IR photon emitted from the surface will be absorbed by a so called molecule of GHG in the Martian atmosphere, and then a greater prospect that a re-radiated photon from that so called GHG molecule will then be absorbed by another molecule of so called GHGs, than is the case on planet Earth.
Put simply, it is far more difficult for an IR photon emitted from the surface of Mars to find its way out to TOA and there be radiated to space. The journey from surface to space will be much more impeded than a like journey on planet Earth, and more photons (in relative terms) will be back radiated towards the surface.
And yet there is no measurable (radiative) GHE on Mars. Why is that?
PS. Obviously Mars is further from the sun, so receives less energy from the sun than does planet Earth. Since the Martian atmosphere is less cloudy, in relative terms more of the incoming solar irradiance finds its way to the surface, compared to the position in planet Earth.
I am not suggesting that Mars ought to have a (radiative) GHE of 33 degC as claimed for Earth. There is less solar irradiance so obviously the (radiative) effect should be less, but it considered to be so close to zero that it is considered that Mars has no GHE.
Willis, Great main article and I very much appreciate you writing it. I’ve fought the same fight; very frustrating.
But your comment about the effect of clouds is misleading. You imply the atmosphere is near opaque for all infrared. That’s false.
http://clivebest.com/blog/wp-content/uploads/2010/01/ir-spectra-earth.png
In the above the first curve is the emission spectra from the sun and is mostly visible light. The earth surface blackbody emission is also shown. Note that a significant part of the earth surface’s blackbody radiation makes it to space during clear skies. It isn’t absorbed even once.
Clouds change that of course and you get significantly enhanced absorption of upwelling radiation, and a corresponding significantly enhanced downwelling radiation.
This assertion ” 75% of the back radiation happens within the lower hundred meters, is not relevant as far as I know, in that in the dense lower atmosphere all the energy, conducted, radiated and convective, acts like conducted and convective, as these conducted exchanges happen far more rapidly in the denser lower atmosphere.??
“Please explain the GHE on Mars.”
To thin an atmosphere.
It does actually have one but it amounts to just ~ 5K.
The atmospheric pressure at the surface is just 6mb
0.6% of Earth’s.
It’s atmosphere is 100x less massive.
It is much colder than Earth’s atmosphere – a min of 50K throughout it’s profile.
Therefore has a weaker LWIR radiative effect.
http://www.dinosaurtheory.com/temperature_atmosphere.jpg
Richard Veney:
GHE is weak on Mars because there is very little of the really important GHG, i e water, in the atmosphere, which is therefore much more transparent to LWIR than on Earth:
http://learningweather.psu.edu/sites/default/files/Lesson3/absorptivity.png
Making the CO2 peaks a bit taller won’t have much effect compared to taking the other four gases away. And as a matter of fact the CO2 absorption peaks will actually be a bit narrower since there is lower atmosphere pressure (=less pressure broadening) and lower temperatures (less doppler broadening) on Mars.
:
A clear and a cloudy sky both have the same amount of CO2.
However, clouds are opaque and therefore block the line of sight. The result is that fewer CO2 molecules can be reached by the surface radiation.
So we have the following:
Clear night sky, with more CO2 in the line of sight: LOWER temperature.
Cloudy night sky, with less CO2 in the line of sight: HIGHER temperature.
Looks like anti-correlation to me.
I agree that a cold opaque object (a cloud) can “warm” a hot object (the surface) by insulating it from the colder space. CO2, however, cannot.
There are some that claim it is the GHGs in Earth’s atmosphere that keep the Earth’s surface warm. However the substantial difference between Earth and Mars is that Earth has large quantities of Nitrogen, Oxygen and some Argon in its atmosphere. If one were to remove from Earth’s atmosphere all the non GHGs, then Earth would have an atmosphere of about the same mass and density to that of the Martian atmosphere..
If one were to remove all the non GHGs from Earth’s atmosphere, Mars will have slightly more molecules of GHGs. It will have a lot more CO2, and quite a bit less water vapour. Earth will have little CO2 and quite a bit of water vapour.
You state:
But that is relevant if one considers that it is the pressure of Earth’s atmosphere that keeps the planet surface warm, or if you consider that it is the thermal mass and thermal inertia of all the Nitrogen, Oxygen and Argon in the atmosphere is what keeps the surface warm by insulating and slowing down the heat loss from the surface.
Richard Verney points out simple truths here that should give one pause. Always pay attention to Richard’s posts.
You want an experiment? Don’t experiment with Mother Earth they sometimes say. But we have all these planets with atmospheres near-by that have already done the experiment for the past 4.4 billion years.
The GHG religion is disproven by all these other experiments.
Something else is going on. Rack your brain for what they might be. Think about photons and gases and electron shells absorbing photons or not and the time-scales that energy flows by and the collision rates of gaseous atmospheres and the rotation rates of planets and how long they are absorbing solar photons in a daytime period and the Stephan-Boltzmann equations/implications which is the best and most descriptive theory ever invented.
Willis
“The greenhouse effect works because some of the upwelling IR is absorbed by the atmosphere. When the atmosphere subsequently radiates the heat, about half goes up, and the other half goes down to the surface. This leaves the surface warmer than it otherwise would be.”
“Than is would be” in what condition, exactly? Than it would be if there were no greenhouse gases in the atmosphere? The assumption that the atmosphere would not be warm if there were no GHG’s is contradicted by evidence. I will give two examples:
At the top of the atmosphere there is precious little CO2 or other GHG’s. The temperature of what few molecules there are is very high. Why? Because the molecules are heated by radiation and are not emitting in the IR so they remain hot. Really hot. They can only cool by bumping into another colder molecule which can emit in IR range, but they are few and far between. This indicates that in principle a gas can be heated and the temperature will rise if it is unable to cool radiatively. An atmosphere with no GHG’s would be hotter than one with GHG’s because it can’t cool except against the ground.
Second example: The surface would be heated to a higher temperature if there were no GHG’s in the atmosphere. Agreed? The surface would cool, in part, by heating the air in contact with it (convective cooling). That heated air would rise and the heat would spread through the system. Heating would continue every day in bright sunshine (no clouds of water vapour). Unable to cool by radiation, the atmosphere would continue to warm. Radiative equilibrium would only be reached when the surface heating by the hot night air equaled the heat gained from the daytime surface heating of the air. An atmosphere with no GHG’s would be hotter than one with GHG’s.
Adding GHG’s to an atmosphere that was part of a planetary system that was already in radiative equilibrium would cool that atmosphere, becoming cooler that it would otherwise be. This is not a direct contradiction of your last sentence which mentions the surface, but the point should be evident. Let’s say the surface may or may not be warmer with GHG’s, depending on how hot it had to be at night to get rid of the heat imparted to the atmosphere during the daytime and downwelling IR. The atmosphere will definitely be cooler with GHG’s added. The fooferaw about CAGW is about the air temperature, not the temperature of the surface.
This is related to your mention of the ‘window’ The window at ground level is small. At 100m it is larger. At 1 km it is much larger again. Adding more emitters throughout the atmosphere will not cause it to get warmer than it would be with no emitters at all because with none it can’t cool except at the bottom.
I think the only way to prove my point is to model a GHG-free atmosphere using FEA and see how hot the air becomes by the time the system reaches radiative equilibrium. People are so busy claiming that a GHG-free atmosphere would be cold, while simultaneously arguing that the surface would absorb 1 kW/m2 and be hot enough to send it into space as IR. Crikey. What temperature is that? Well, the air next that heated surface would be just as hot as that surface. “Just as hot” is not “cold”.
None of this has anything to do with what the surface temperature would be if there were no atmosphere at all. How long as the IPCC and its minions been skirting around this rather large hole in their GHG explanation?
Toneb says, November 25, 2017 at 7:34 am:
No. It doesn’t “actually have one”. If anything, it’s NEGATIVE. T_e on Mars is ~211K, while T_s is ~203K.
Just a minor correction, but I think an important one.
“Heat” is NOT the “net flow of energy”. That results in radiative heat TRANSFER, from one object to another. It’s not the same as “heat”.
I don’t like to quibble, but in this particular matter, which is easily confused, precise language is called for.
Thanks, Anne.
w.
Agreed Anne.
Further:
Heat flows through objects. Radiative energy transfer is not heat flow. The term ‘flux’ can refer to heat flow or radiant energy.
From Wiki-read-with-care:
“In physics, and in particular as measured by radiometry, radiant energy is the energy of electromagnetic and gravitational radiation.[1] As energy, its SI unit is the joule (J). The quantity of radiant energy may be calculated by integrating radiant flux (or power) with respect to time. The symbol Qe is often used throughout literature to denote radiant energy (“e” for “energetic”, to avoid confusion with photometric quantities). In branches of physics other than radiometry, electromagnetic energy is referred to using E or W. The term is used particularly when electromagnetic radiation is emitted by a source into the surrounding environment. This radiation may be visible or invisible to the human eye.[2][3]”
It contains an error, however. The definition says “…radiant flux (or power) with respect to time.” Radiant flux with respect to time is power. One can’t have “radiant power with respect to time”. That would be radiant flux with respect to time with respect to time. That’s like saying “Watts per second”.
Anyway, the common argument that cold and hot objects ‘can’t heat each other’ is rooted in the misbelief that the radiant energy “flows” which is to say, conducts from cold to hot, which is not going to happen. Terms and definitions matter.
No, radiative energy transfer CAN be heat transfer, when it’s from a warmer object to a cooler object. Heat transfer generally, by definition, is a transfer of energy from a warmer object to a cooler one, by any means (conduction, convection or radiation). Energy flows both ways, true, but heat only travels in one direction – from warmer to cooler. By definition.
There is a flow of ENERGY going from cooler to warmer even with conduction; however nobody seems to concern themselves with this “back conduction”, since more energy is always going the other way, and so HEAT of course transfers that way overall (warm to cool). Funny how everyone’s so bothered with “back radiation” and not “back conduction”…but there you go.
“When an object emits radiation, that radiation goes on until it hits something that absorbs it, whereupon it is converted to thermal energy.”
Nope. It can be re-emitted instantaneously by the much hotter molecule. Temperature is the average motion of molecules. It’s measured by the collisions of those molecules on the thermometer. Incoming colder IR frequencies will not make the motions of the molecules increase. Hence will not make the hotter object hotter. It cant even increase the energies of the hotter object increase, because the hotter object is ejecting billions of photons more than is coming in. Hence the immediate re-emission.
The NET radiative heat transfer (and I think “net” is the confusing factor here) will always be from hotter to colder. Always. Anything else violates conservation of energy.
Willis’ first diagram illustrates this. 100 one way, 75 the other, the NET transfer is 25.
Nobody is denying that it does go both ways. But it is the net that matters.
“Nobody is denying that it does go both ways.”
I’m afraid it seems that some do.
“Incoming colder IR frequencies will not make the motions of the molecules increase. Hence will not make the hotter object hotter. ”
We keep coming back to the same fundamental mistake.
No No NO the hotter object recieving the LWIR from the colder does NOT get hotter.
It just cools at a slower rate such that after a uit time it will be warmer than otherwise.
What is so difficult about the concept of cooling more slowly???
See Willis’ cartoon.
It is the NET flow that has to be considered.
I’ve used this analogy here in a recent thread…..
A water tank looses 10 gal/hr from a leak.
it is a 90 gal tank.
It would be empty in 9 hours.
But there is a feed into it of 1 gal/hour.
So 10-1 = an overall loss from the tank of 9 gal hr.
SO it takes 10 hours to empty.
At no time does the overall quantity of water in the tank go UP.
But after 9 hours it has more water in it than if there were not a 1 gal/hr feed to it.
Substitute for hotter/colder and net transfer of LWIR.
But you have added another water supply. Not just the original water.
Back radiation from the colder object is absorbed by the hotter object.
The hotter object is heated by that absorbed radiation.
Being heated, it now radiates more per unit time than it was radiating before because rate of radiation is related to temperature.
Thus this increased temperature increases its rate of cooling.
The question is, what is the net flow:
Does the increased absorbed energy result in an output increase that is equal to, less than, or greater than the back radiation absorbed?
“Back radiation from the colder object is absorbed by the hotter object.
The hotter object is heated by that absorbed radiation“
“Can A Cold Object Warm A Hot Object?
Willis Eschenbach / 9 hours ago November 24, 2017
Guest Post by Willis Eschenbach
Short answer? Of course not, that would violate the Second Law of Thermodynamics”
“But you have added another water supply. Not just the original water.”
Do you seroiusly think the result would be different if you took the 1 gal/h from the leaking water?
A C Osborn
How is this:
You are sitting in a boat that springs a leak; water is flowing into the boat at 1 gallon per second. It is going to sink in 15 minutes.
You bail furiously using a small bucket, returning 1/2 a gallon per second to the lake.
Does the boat take longer to sink when you bail or does it still sink in 15 minutes?
When I pour my hot tea into my cup , the cup dictates how hot the tea is but does not make the tea hotter than the incoming temperature.
Crispen, to do this I have to Expend Extra Energy.
Where is the Energy coming from in Back Radiation, you say CO2.
I say prove it by Measurement.
I can prove the Watts coming from the Sun by measurement.
Show me how to measure 340W/m2 of DWILR.
Dr Spencer tried it but he did not and cannot have a Control Object, so when the object got colder than Ambient he said that proved DWLIR because it would have got colder, but would it have been -340W/m2 colder.
That is an Assumption not an Observation, because without a Control he doesn’t know how cold it would have got.
Why is there no Empirical Evidence, only theory.
Probably for the first time, I disagree with Willis. I concede the concept of ‘net flow’, but there seems to be a mixing of conduction law, where thermal energy always flows to cooler regions, and radiation law, which says photons are emitted from one place and received at another. That ‘another’ place is warmed, period. If some body absorbs photons, it becomes warmer. Then, it radiates at a higher level, the old 4th power law. The sun is warmed by the earth’s radiation. It cannot be otherwise. The concept of ‘net flow’ is immaterial with radiation, because radiation ALWAYS increases thermal energy of whatever absorbs it. And that absorber will always radiate at an even higher rate – which can increase the thermal energy of the original emitter.
Just think of shining two flashlights at each other. Precision (very) thermometers on each. Turn one off and log the measurements. Reverse them, and record the measurements. Turn them both on and record the measurements. They will both increase when both are on, but radiation is the only method of transfer.
Now, place one flashlight on a piece of ice. Repeat the measurements. The readings will essentially repeat – because radiated energy has no concept of from where it was emitted, not where it is received.
“If some body absorbs photons, it becomes warmer.”
Nope, not when the object the IR is intercepting is hotter. “Hotter” (temperature) is a measure of molecular motion (vibration, rotation, translation). Cold IR wavelengths cannot add to the motion of the molecules. Either nothing happens, or more likely, it is reemitted instantaneously.
J. Richard Wakefield November 24, 2017 at 8:35 pm
I’m sorry, J. Richard, but in this case you are simply wrong. I challenge you to find one serious scientific reference that says that a photon from a colder object will NOT be absorbed by a second object that is hotter than the first.
I’ve backed up my ideas. I’ve given a website, a calculator, and an equation that says quite clearly that photons ARE absorbed regardless of temperature.
Now it’s your turn … but let me ask you politely to not repeat your incorrect statement until you provide something to back it up. We’re not into science by assertion here, you won’t do your cause any good by insisting you are right and endlessly repeating your claims.
w.
“photon from a colder object will NOT be absorbed by a second object that is hotter than the first.”
Design an experiment were we can see your claim happening. Math and numbers are just what you THINK should be happening. Prove that is what is happening.
J. Richard Wakefield November 24, 2017 at 9:00 pm
Read about the MIT lightbulb above, which uses exactly this same principle. The cooler shell around the filament makes the filament hotter than it would be without it.
In addition, pick up any college thermo textbook, and it will say what I’m saying. Or you could just look again at the web page I linked to. Seriously, it contains a calculator, a full explanation, and a complete equation for the calculations of what you claim can’t possibly happen. Play with the calculator, and consider the answers that it gives. For our situation (one object surrounded by another) set area a1 to 1, and area a2 to a million. Then put in different temperatures for the object and the surroundings, and think about what is happening.
Finally, let me repeat what I said to you above, which you have totally ignored:
It’s time to put up or shut up, J. Richard. You need to find some authorities that say that somehow photons carry information with them about the exact temperature of whatever emitted them, and that warmer objects can somehow sense that temperature information in the photon and reject the photons from colder places …
w.
“Cold IR wavelengths cannot add to the motion of the molecules. Either nothing happens, or more likely, it is reemitted instantaneously.”
The warmer body doesn’t “know” about the temperature of the other body, nor does it matter. If it’s opaque in that spectrum (which rarely depends on the temperature of a material within a given phase), it will absorb the energy. And energy absorbed is energy absorbed.
Here’s a real-world example. Water, and many metals, are pretty decent absorbers in the microwave spectrum. The microwave spectrum is “colder” than the infrared; lower-energy, but that doesn’t stop them.
Will a metal or water at room temperature, emitting in infrared, absorb microwaves? You betcha. I recommend you go try it out in your microwave for yourself.
Again: if light is hitting a body, the body does not care about where the light came from or what temperature the originating body was. It only depends on the optical properties of the absorbing body, which are generally not very sensitive to that body’s temperature.
J. Richard Wakefield
” Either nothing happens, or more likely, it is reemitted instantaneously.”
needs a little work,
If nothing happens you are denying absorption happens.
Trying to claim it is instantaneous to satisfy your argument is sophistry.
Instant re emission is basically claiming it was never absorbed at all as well.
If you accept absorption then the object has gained energy.
The science says that there is usually a delay between absorption and emission which is measurable.
The object being more energetic is capable of producing more heat.
“Design an experiment were we can see your claim happening. Math and numbers are just what you THINK should be happening. Prove that is what is happening.”
Roy Spencer has …
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
http://www.drroyspencer.com/2010/07/experiment-to-test-the-temperature-influence-of-infrared-sky-radiation/
When I read that argument from the Sky Dragon Slayers, phrased as cooler photons reflect off of hotter objects, I gave up on them. It is the only claim they can make, as the only way not to absorb the energy of the photon is to not absorb the photon. There’s plenty of data showing blackbody objects don’t work that way. If they did, my IR thermometer couldn’t measure the temperature of cooler objects.
More reading material on this:
https://wattsupwiththat.com/2013/05/27/new-wuwt-tv-segment-slaying-the-slayers-with-watts/
https://wattsupwiththat.com/2013/05/28/slaying-the-slayers-with-watts-part-2/ (real experimental data!)
https://principia-scientific.org/why-did-anthony-watts-pull-a-bait-and-switch/
That last post is referenced byt the WUWT post, but at least some of the links go to a current news URL that no longer exists.
“Nope, not when the object the IR is intercepting is hotter.”
Never wondered why a radio receiver works? The antenna is almost infinitely “hotter” than the photons it “intercepts”. Remember that the cosmic background blackbody radiation at 3 K peaks at a wavelength of about 2 mm, so even UHF radio waves are much, much colder than 3 K.
Also, consider that IR lasers can be used to heat a target to very high temperatures.
Photons are not small bullets fired by a hot body in all directions. They are interaction. The interaction will take place only if the destination can accept exactly one quantum of energy from the emitter.
Count to 10
Lasers are also used to cool gases to near absolute zero. In fact that is the standard method now to get milli-Kelvins.
J. Richard Wakefield
Nope, not when the object the IR is intercepting is hotter.
Richard, a CO2 laser may get as “warm” as about 100ºC, but the “warm” IR waves coming out of that laser can heat up steel and melt it at 1200ºC…
There are many thousands of these lasers proving daily that you are completely wrong in that point…
Hundreds of examples of the Dunning-Kruger effect.
I mock you Willis and you too Anthony for your feeble attempts to herd these cats you’ve gone out of your way to attract and cultivate.
Live by the sword, die by the sword.
Bwahahahaha.
I don’t know (much) what I’m talking about; but I seldom let that stay me from talking. It does, however slow me down! Usually.
It seems to me that the two-way flow of IR energy is exactly analogous to two waves propagating in opposite directions on a conductor. Neither wave, when launched, has any knowledge of the conditions at the far end of the line – whether the destination is hot or cold, near or far, matched or unmatched. The finite velocity of light makes this so. Assume two radiators some distance apart, with unequal energies being radiated, each toward the other. One is “bright”, the other “dim”.
S_B tells us that the energy flow is proportional to the differences in T^4 of the endpoints. This tells me that the bright source will radiate less energy toward the dim source than it would toward a dark body, because the dim source isn’t cold, it’s only dim. The consequence is that the bright source must radiate more in other directions – i.e, get hotter – because of the dim source. Else the bright source is not at zero net energy. A la the self-heating light bulb.
A bolometer inserted between the sources at some intermediate point will be heated, on one side, by the bright source, and on the “dark” side by the dim source. The two sides are shaded from each other assuming only bolometer opacity to the radiated wavelength(s). In the absence of the dim source, the bolometer assumes a temperature determined by the heating of the bright side, minus any cooling attributable to the now-elevated temperature of the dark side. Now let the “dark” side also be illuminated, such that there is energy impinging on the dim side. What is the net energy flow at the dim side? It’s determined by energy intercepted (from the dim source) minus energy radiated by the bolometer. The bolometer is a summing node. It assumes whatever temperature is necessary to achieve zero net energy. Now move the bolometer to epsilon above the bright object, and tell me what, besides relative energies, changes? I believe, by analogy, this makes the bright body a summing node also. Which is true of all situations of thermal equilibrium. The incident radiation on both sides of the bolometer is radially directed toward the other object. The radiation from the bolometer is scattered. Thus the point-to-point energy flows from the original objects is disrupted.
Radiated energy transfer is a two-way street, with the net flow from hot to cold; conductive transfer is always hot to cold. Does the presence of the bolometer affect the temperatures of the radiating objects? Almost certainly. The bolometer, or a conductor in the case of conductive transfer, is mass that was added to the original system, thus changing the system.
John
November 24, 2017 at 8:29 pm: You are measuring the temp of the measuring devices. Never that of the light. Light is EMF, not KE, and the thermometer on ice will cool in spite of your beam, which has very little power.
I have a wood heater. One log alone won’t burn (very well usually) but two logs burn on the sides facing each other where they aren’t losing heat to the colder walls of the heater.
That is so, but you are altering the draft, convection and flame front, so one would expect to see a different result.
I do not consider that that example proves the radiative issue under discussion.
If the atmosphere were to expand when it is warmed and contract when it is cooled which being a gas it can do this if not prevented by anything then there is no fixed amount of radiation returned to the surface. The ideal gas laws do not apply to a planets atmosphere because there is nothing outside of the atmosphere to stop it increasing its volume.
Convection happens. The expanding warmed air at the surface rises and cools as it expands.
Thanks, Willis.. always thought that was dumb. Math-wise it works, but sense it doesnt make. I would surely like to see someone describe how a molecule rejects photons.
JRW,, In the swimming pool example you forget one small factor. The photon never makes it past the first few molecules of water. It may heat that molecule and heated water evaporates more readily, cooling the other surface molecules.
It is the exchange that matters, and the practical effect is that water heats air, not the other way around.
Taken to the ultimate projection you would have a perpetual motion process.
And
Actually, rising air doesn’t cool at all, unless it contacts something cooler, or radiates. It apparently cools because of lowered density (pressure)…fewer air molecules are present. Remember, the ideal gas laws work for all gases, including CO2.
“The ideal gas laws do not apply to a planets atmosphere because there is nothing outside of the atmosphere to stop it increasing its volume.”
The Ideal Gas Law doesn’t say anything about fixed volume or not. It applies regardless.
The Ideal Gas Law only stops applying when your gas stops being thermodynamically ideal (e.g., close to condensation or disassociation).
The way I see it is when the atmosphere warms up it then convection occurs but what if the warm air rises further because it is warmer before it falls and cools then the atmosphere is expanding even with convection
“And as a result, with the cold atmosphere shielding us from the nearly infinite heat sink of outer space, the earth ends up much warmer than it would be without the cold atmosphere.”
I get your point. But the terminology obscures understanding. What is “cold”? If you had written “colder” atmosphere – relative to the earth – then it would make complete sense. It is all relative.
I’m sure what you are (correctly) trying to say is that the earth is warmer than it otherwise would be because it is surrounded by a relatively colder atmosphere which ‘insulates’ it – keeping it simple here – from even colder space. Sure. But you didn’t actually say that.
But here’s the rub. That “colder than Earth” thing-the atmosphere-ALSO keeps the Earth cooler than it would be would be by insulating Earth from some of the Sun’s radiation. There’s even a term and definition for it:
“Thermal insulation. 1 : the process of insulating against transmission of heat. 2 : material of relatively low heat conductivity used to shield a volume against loss or entrance of heat by radiation, convection, or conduction.”
Here’s a textbook explanation from “Physical Science Concepts for Middle School”-
https://www.ck12.org/book/CK-12-Physical-Science-Concepts-For-Middle-School/section/5.17/
“Materials that are good conductors of thermal energy are called thermal conductors. Metals are very good thermal conductors. Materials that are poor conductors of thermal energy are called thermal insulators. Gases such as air and materials such as plastic and wood are thermal insulators.”
Too bad Willis didn’t just say that or start there.
Willis,
I can see that you have a hand full on this one, but you have the persistence and patience of Job.
Or, another way of looking at it: the stubbornness and inability to admit an error of Michael Mann.
The ‘simplified’ diagram of radiation flows through the atmosphere is just that ‘simplified’.
Because MOST heat is transported through the atmosphere in the form of WATER VAPOUR ENTHALPY.
Skeptical about that?
Try looking at CIMSS tropical cyclones page and see with your own eyes the immense heat pump carrying heat in the form of EXCITED WATER VAPOUR MOLECULES….high into the atmosphere (and also to frigid winter polar regions). So just to recap these ‘radiation budgets’ are undoubtedly scientifically correct…they are also to all extents and purposes irrelevant or to put it another way…negligible.
http://tropic.ssec.wisc.edu/tropic.php
You are correct Charles in stating that most of the heat transferred off the surface is by moist convection. Of the massive (nearly 400W/m2) surface in vacuo radiative potential only about 30W/m2 is transferred to the atmosphere. THIS IS THE ELEPHANT IN THE ROOM.
The talk of ‘all the energy radiated is absorbed’ and similar are irrelevant because the atmosphere is hardly heated at all by long wave radiation. The surface never loses much to the colder atmosphere and the atmosphere receives very little energy this way. The opacity decouples because at close proximity no significant thermal gradient exists over the diurnal cycle. Hence very little heat is transferred in high opacity bands. The atmosphere’s heat content is 90% the result of direct absorption of sunlight and latent heat transfer and exhibits a thermal profile completely independent of long wave opacity.
We had a demonstration of this in a physics lesson mumble years ago with two parabolic reflectors facing each other, a thermometer at one focus, nothing at the other. Allowed to stabilise. Place a hot object at the empty focus and watch the temperature at the other focus rise. Repeat, and after things stabilise place an ice cube at the empty focus and watch the temperature fall. We had discovered cold radiation!
Except we hadn’t, of course, we had revealed the fact that when the thermometer system is in equilibrium the heat flows in and out are matched,but when you disturb the environment of the thermometer a new equilibrium is reached.
The ultimate test would be placing empty space at absolute zero at the empty focus, letting it stabilise and then replace space with an ice cube. Heating from ice…
JF
Julian Flood November 24, 2017 at 9:40 pm
Exactly. The ice is warmer than what was there before, so the other object ends up warmer as well.
Good to hear from you, Julian, long time.
w.
Hi de hi.
I accidentally got a job (saving my country in small ways) so I’ve been posting less.
Talking of warming…
There must be lakes/almost-closed bodies of water which exhibit oil/surfactant pollution warming — a large lake with a growing city on its banks should, if I’m correct, warm faster than a pristine one. Maybe we should carry out the experiment, imitating the city runoff with a few oil tankers. Finland has lots of lakes, and there’s that big empty place north of the USA, that would also be suitable. And what about the Great Lakes, they must be pretty polluted in places. More research money… err… more research is needed.
There’s a nice small lake next to UEA. I’ve been tempted.
JF
A very good example! Thanks for you, Willis and Anthony for hosting this.
The argument made sense until the leap of logic right at the end. At no time does the hotter object become even hotter. It cools slower. Therefore how does this explain the earth being 33degrees HOTTER than from the level of solar radiation alone. This persons own logic reveals how impossible the greenhouse theory is.
Nicholas:
Let’s take Willis’ monetary analogy further. You are earning $240 each week. For a long time, you are spending $240 each week (let’s say 12 $20 purchases), so your bank balance is constant from week to week.
Now, you start getting change back from each of your $20 purchases. You are still making 12 of these purchases each week. Does your bank balance increase?
By your logic, it doesn’t. By Willis’ logic it does. (If you really believe it wouldn’t, I want to be your banker!)
Now, if you lost your job and your income, the change you get from your purchases, would just slow the rate at which you deplete your bank account. But that is not analogous to what we are discussing here.
When you comment, please QUOTE THE EXACT WORDS THAT YOU ARE DISCUSSING,
“Heat”, on the other hand, is not those individual flows of energy. Heat is the net flow of energy, as represented in the bottom half of Figure 1. Specifically, a heat flux is the net flow of energy that occurs spontaneously as a result of temperature differences.-
–
Heat and heat flux are two different entities. Heat and temperature are related.
heat flux is the net flow of energy .
Heat is surely equivalent to the temperature of an object as in this object is hotter i.e. has a higher temperature than another.
Why not say this?
Yes heat can flow between them but heat is the temperature of an object surely??
Or surely not.
Heat is the transient flow of energy between two bodies of different temperature.
So I cannot ask for a hot cup of coffee?
angech: Do you not know the difference between ‘heat’ and ‘hot’? Let’s be clear, ‘heat’ is the transient flow of energy between two bodies of different temperature, whereas ‘hot’ is a non-scientific term used to describe the temperature of a body. Neither a ‘hot’ body nor a ‘cold’ body contain any ‘heat’ because ‘heat’ is a transient phenomenon.
I would disagree Philip. Heat is the (total) thermal energy in an object. The Pacific Ocean has a large heat content. This does not imply any net flow of energy. Heat transfer encompasses the various mechanisms that can transfer heat from one body to another; conduction, convection, radiation, mass flow etc.
Nuwurld you are incorrect and Phillip is correct. Heat is a energy in transit. It was part of internal energy before it left and will be internal energy again when it arrives.
“Heat transfer physics describes the kinetics of energy storage, transport, and energy transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons.[1][2][3][4][5] Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers. The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.”
Makes sense to me. Why have “heat transfer” if heat is already “transfer”
A fire ‘gives off’ heat. It is a direct loss from its ‘heat’ content. By conservation the ‘heat lost’ by the fire can be traced to ‘heat gained’ by the surroundings. All in Joules.
What is gained by specifying that heat only applies to ‘energy that can be thermal that is in transit’? Obviously this thermal energy was lost by the emitter, Joule for Joule and might never by thermal again. Could become chemical potential of gravitational potential so no heat transfer.
…might never be thermal again. Could become chemical potential or gravitational potential so no heat transfer.
Must proof read!!
Good grief you guys are frustrating to read.
Heat is form of energy. Thermalised energy.
Energy flux is a flow of energy: it could be by chemistry, gas flow, conduction, radiation or anything else you can imagine.
Energy flow is normally used to describe conduction, not radiation. Water does not flow back and forth between two connected tanks, it goes in one direction at a time. Radiated energy is not like that. To the extent it can. it emits all the time. It is not true that everything emits until there is a ground state of 3 degrees C. Some things are unable to emit IR are remain hot. (The universe is a strange place.)
Thus flux is used, not flow when discussion radiation.
Water is a thing, a form of H2O.
Water flow is a flux, not ‘water’.
So heat is not a flow or a flux.
Something can be hot (as pointed out) without heat flowing anywhere. The explanation of why the atmospheric molecules at very high elevation are so hot is they (not CO2 or H2O) gain energy from insolation and cannot radiate it so the temperature goes up and stays up! Particles in space can be very hot unless they collide with something colder. If they can emit in IR at that temp they do, whose which have the ability. Many atoms and particles do not. Their emissivity is essentially zero so they stay hot.
I’ve done my fair share of radiography. A major cause of poor quality radiographs is “back scatter”. This is where objects behind the film absorb and re-emit ionising radiation that has already passed through the object that is being radiographed and the radiographic film. The re-emited radiation travels in all directions, including back to the film and the primary source of radiation. If precautions are not taken to filter out this radiation before it reaches the film it will lead to an overall darkening or fogging of the film and a consequent loss of image quality. This effect, although it involves much shorter wavelength electromagnetic radiation, is analogous to the so-called greenhouse effect.
The USAF used blow torches to cool down the skin of SR-71 Blackbirds returning from high altitude, high speed (high Mach number) recce missions.

why?
Actually, to reduce the rate at which the skin cooled to ambient. Cool almost any very hot metal too rapidly, and you get fracturing, from microscopic ones up to spectacular explosions. (So saying that they were cooling the metal is technically accurate; its temperature was going down while they were playing the torch over it – but they were cooling it more slowly than the naked air would.)
Link, please.
With your steel greenhouse I take the outer shell down to a 1 molecule thick layer and place it adjacent to the outer layer, no gap but as molecules do not touch there is still radiation.
The outer layer still emits to space the heat of the surface.
I do not “see” the surface as being twice as hot to send back the same amount of energy as it sends out but we know it does send back the same amount.
Hence double that amount must be coming through for it to emit that amount and send it back but the temperature we measure for that energy is purely that of the outgoing energy.
Something to consider for the shell argument?
After all there is a lot of internal radiation, can we call it down welling radiation, in a conductive blackbody.
Should it be called conduction when a lot of it is radiative, just not over a very long distance and not visible.
This is simply the “net” argument all over again.
An object at a particular uniform temperature will radiate from all surfaces.
And yet: if you have (in isolation, in total dark vacuum) an inner energy source constantly radiating energy X, warming up a passive shell surrounding it, the outside of that shell will also radiate a TOTAL of X.
Always. Without fail. The universe could not exist otherwise.
Exactly. And, since the shell will always have a larger surface area than the inner energy source object, the shell must always be at a lower temperature than the inner energy source object. Same total energy radiated from larger surface area = lower flux (and also, all else being equal, lower temperature). So, a 235 W/m2 inner sphere resulting in a 470 W/m2 outer shell? Not going to happen.
Black holes are not real?
Dolphins are not real?
Thanks Anne, but as I pointed out, there is a paradox.
A paradox that is resolved once you understand that the surface of the outer shell cannot possibly come to double the temperature of the sphere in the first place, for the reasons explained. The steel greenhouse analogy is flawed in this regard from the very outset.
(since the sphere is the only energy source, the only way it could warm to a temperature producing more than its maximum radiative output of 235 W/m2, is if the outer shell was warmer; yet the outer shell must always be at a lower temperature than the inner energy source object due to its greater surface area)
Good luck. It seems that there are a lot of people who do not understand the argument. Temperature (of a region) is proportional to the average kinetic energy of the molecules (in that region). But there are always molecules with below average kinetic energy in the same region with molecules of average and above average kinetic energy.
I’ve added a note to the head post with a link to my post on the Steel Greenhouse. It goes through these questions from a different perspective.
w.
You want to try nested steel greenhouses.
YES!! Thought experiments (I am presuming no one here has actually built a steel greenhouse but if you have lets see the pictures please) should always be tested via logical extension. Nested steel greenhouse is the next step. What results do we get? What conclusions can we draw?
“Now imagine that the planet gets completely surrounded by a thin black steel shell, located a few thousand metres above the surface, as shown in a cutaway view in the picture above”
Let’s change it. Imagine if earth had this “thin black steel shell, located a few thousand metres above the surface,”
Now there are few ways to do this and it does not matter *much* which way it’s done.
I going to pick having magically strong thin black steel shell which withstand more than 1/2 atm
of outward pressure and shell is going to be at 1/2 atm pressure- 5.5 km elevation.
So other chopping off a few mountain tops, I can keep earth, earth. Though missing half it’s atmosphere mass, though has the same surface air pressure of 1 atm.
Of course Earth isn’t warmed much from it’s molten core, and has to get it’s warmth from the steel shell.
So how warm is steel shell.
Well if it was was an ideal thermally conductive blackbody it “should” be about 5 C and radiate
1367 / 4 = 341 75.
But ideal thermally conductive blackbody is magic- and you are using steel which only magic added is it’s strong enough to withstand a huge amount of force. Such magic might gotten from nanotechnology which if build something molecule by molecule you could theory make a material stronger- though we have get into to how thin is thin and unless thin is meter thick, mere nanotechnology might not enough magi- but details we will ignore.
But steel or copper, silver or even diamond doesn’t give enough thermally conductivity- one could invoke some kind magical plasma based system, perhaps. But let’s look at steel as it’s the namesake.
So steel with a good blackbody coating is going to have lower average temperature than the uniform temperature of an ideal thermal conductivity blackbody.
Or in sunlight with sun at zenith, an ideal blackbody will be about 5 C. With steel it’s going to be much hotter than this and radiate into space much more energy as compared to the ideal.
At Earth surface and where sunlight would be at zenith, the steel sky will be quite warm, and elsewhere steel sky would be cooler.
How big is the hot spot, how big is the hottest spot of hot spot,
Roughly hot spot is same as solar peak hours- 3 hours before and after noon.
An hour is 15 degree longitude. So 45 degree both east and west of the point of zenith.
Each degree is about 111 km. 111 times 45 is 4995 km. So roughly a radius of 5000 km.
So if sun over equator 45 degree north, south, east and west. So if in UK, you don’t see the hotspot. You have wait for summer to see it- and then you will see it for couple hours a day.
Now at the equator you see it for 6 hours of 24 hour day.
How does the hot spot effect someone standing a equator at noon. More than the entire sky is
hot. How hot? Well roughly the hot as it could be is about 120 C,
It takes some time for the outer surface at 120 C to heat the inner surface- and the thinner the steel the less time. Let’s assume it thin enough to do this fairly quickly.
Next, there is 1/2 atm of pressure on the inner side- how cold it would be would effect how much heat is transferred to the air. The air not going to heat well because it’s like hot air against the ceiling of room in a house.
For rough idea with current earth, if air temperature was 20 C, it’s 6.5 C cooler per 1000 meter.
So 5.5 times 6.5 C is 37.75.
So air temperature was 20 C at surface it’s 17.5 C at shell. Now can’t have air at say 100 C and a foot away have air at -17.5. Or 100 C will make gradient of heat, maybe 20 C per meter.
So like the steel it takes some time to form this gradient.
So skip some these details what the effect if entire sky is 120 where closest is 5.5 km from you?
Well, it would seem if you were only 3 km from shell the radiant heat would more than if 5.5 km from it, though air is 3 time 6.5 C cooler than 20 C if surface air was 20 C= .5 C
So could like being in winter and stand next to a fire.
Or if at surface or at 3 km elevation there would direction the heat is coming from, and if at 3 km more heat is coming directly over head but unlike being at surface more heat coming all side save the below you.
Anyway rough fairly warm- but unless the air is warm, not particularly uncomfortable- or living constant darkness would be more of problem.
And if air was 20 C, the radiant heat would warm surface and warmed surface would warm the air.
And it seems the highest air temperature would not be at surface but at the ceiling.
Well I cut it short. And to remain brief, I think if lived in such steel greenhouse, you want to live on the ceiling to remain warmer.
Steel is a thermal conductor. Air is a thermal insulator.
Steel is a thermal conductor. Air is a thermal insulator.
Yeah, but steel is also insulator.
Or steel has conductivity of 54 W/m K
Copper is 386 W/m K
Air is 0.024 W/m K
Water is 0.58 W/m K
diamond is 1000 W/m K
I guess to quantify, look at formula:
https://www.engineeringtoolbox.com/conductive-heat-transfer-d_428.html
And just using calculator there
Per square meter which is 1 meter thick
temperature difference of 120 C to 0 C
Copper can conduct:46320 watts
Steel: 6480 watts
Air: 2.88 watts
Water: 69.6 watts
Diamond: 120000
But air which 1 mm thick: 2880 watts
1 cm: 288 watts:
So steel is 120 C it warm air near, and develop heat gradient,
Likewise the steel has gradient.
So if steel is 1 cm thick [very thin]
And top is 120 and bottom is 119 C
It gives 5400 watts
2 cm is :2700 watts
What about 120 to 119.5 how much watts go thru 1 cm:
That’s also 2700 watt.
So have 2 cm steel and sunlight of 1367 watts can heat surface to 120 C
and can heat 1/2 way down to 119.5 [or more]
And 119.5 to 119 C is again 2700 watts
Where is it 119.9 C in the 2 cm of of steel?
120 to 119.9 at 1 cm depth is 540 watts
And 1/2 cm should double, and it is: 1080
and 3.9 mm it’s 1385 watts
Somewhere around .1 K per 3.9 mm
Or 10 mm is 119.7 and 20 mm is .5 C
So 20 mm or 2 cm it’s 119.5 if surface it 120 C and radiating
close to 1367 watts, but some of that 1367 watts or
119.5 C is 392.65 K is 1348 watts. some of the 1348 watts
is lost to warming the air. And the warmer air gets, the less
heat is lost. Or if air within couple mm is close to 119.5 C
it won’t conduct heat to it- but doubt it would get to that point.
But warmer the air was say 1 meter away from surface, it seems
more likely it could happen.
Of course if there convection due gravity, hotter and lighter gases
would rise. But lesson of fire safety is if in a fire, crawl out rather than
walk out, because you can get very hot air closer to ceiling.
So if there isn’t any wind [and there could be] one can trap a small amount of
hot air near the ceiling.
This assuming, less an inch of the magical steel is strong enough.
Fig 2 is complete nonsense. The surface does not absorb twice as much energy from the atmosphere as it does from the sun.
“If I can see you, you can see me, so there are no one-way energy flows. Which means that if I am absorbing radiation from you, then you are absorbing radiation from me.” More nonsense. What you are seeing is light reflected from the other person. Switch off the light and you can’t see the other person, even if he is radiating infra-red.
“More nonsense. What you are seeing is light reflected from the other person. Switch off the light and you can’t see the other person, even if he is radiating infra-red.”
So you are saying that (say) a pyrometer in a room at ambient 20C cannot see an object at the bottom of a chest freezer at -20C?
Or that when pointed at a clear sky it cannot see/measure it’s temp?
Roy Spencer shows you can here …
http://www.drroyspencer.com/2010/08/help-back-radiation-has-invaded-my-backyard/
No I didn’t say that. I was talking about people seeing each other – ie just the visible spectrum, which is what people see as a reflection off other people, not an emission from them.
That is correct. Take a totally dark room. Shine a light on you. I can see you, you cannot see me.
Oh, for chrissakes, Phiip!
He was just expressing colloquially the FACT that the path that electromagnetic radiation takes from Point A to Point B is the same as it takes from Point B to Point A. That (technically true wavelength by wavelength), combined with the fact that absorptivity and emissivity are the same for each wavelength, validates his argument.
Willis is correct. For those who think that the temperature of a body receiving radiant energy does not affect the temperature of the hotter radiating body, here is a simple experiment.
Connect an electric resistance heating element to a constant power output power source. Attach a temperature sensor (e.g. a thermocouple) to the heating element and measure its temperature in a large open area until it comes to equilibrium. Say a 1000 Watt element heats to 500 C in this situation.
Now suspend the heating element in a small sealed ceramic box under vacuum (so the only mode of heat transfer is radiation) and monitor the temperature of the box surfaces and the heating element from the time the element is energized. You will see that the temperature of both the box and the element increase until the heat loss from the box equals the heat input to the element (i.e. 1000 watts). If the box starts at 20 C and the element at 500 C and the box inside surfaces end up at 300 C then the heating element must increase in temperature to 549.43 C in order to maintain the steady 1000 watt output. And the outside of the box must transfer 1000 watts to its surroundings although this heat flux will be by a combination of radiation, convection and conduction.
Initial condition SB Temperature term = (500+273.1)^4 – (20+273.1)^4=3.49 E11
Equilibrium SB Temperature term = (549.427+273.1)^4 – (300+273.1)^4=3.49 E11
This experiment is relatively easy to do if you have access to a laboratory vacuum furnace. The results would be only slightly different in a furnace with air at ambient pressure.
In my opinion Willis has used the wrong relationship to answer the question he has posed. He should have used Planck’s Law, not the Stefan-Bolzman’s law.
The reason is that he is considering an object A emitting at wavelength X and
B an object emitting at wavelength Y
where Y is longer (cooler) than X
Has object B caused object A to emit at a wavelength shorter (warmer) than the wavelength A would emit at if B did not exist?
Or alternatively, if object B were to cease to exist, would object A emit at wavelength longer than X?
Willis need three assumptions, no heat transfer
by conduction
by convection or
by reflection.
The Stefan-Bolzman Law cannot answer this question because it is derived from Planck’s Law by integrating across all wavelengths.
This is explained on another skeptical blog, The Science of Doom,
here https://tinyurl.com/y8d7gor8
here https://tinyurl.com/y77qnqpl
The experiment proposed by Rick C PE describes a closed system. My understanding is that Willis is not imposing that condition.
Well done. I see the usual arguments above, all stemming from the completely incorrect “greenhouse” tag on the effect of atmosphere (any atmosphere). It should properly be called the “shield” effect, allowing a more rational discussion.
For instance – it is obvious that making your shield more effective – i.e., adding a gas that makes it reflect (or absorb and emit) more heat towards the surface – will make the surface warmer than it would be otherwise.
No rational “skeptic” denies that adding more shielding CO2 does increase the surface temperature. Less energy escapes directly to the far colder surroundings with that additional CO2 in the atmosphere.
The inconvenient truth, however (for alarmists) is that the net effect is very small. Two facts come into play – the effect of additional CO2 is logarithmic. Generously, humans have added approximately 150 ppm of CO2 to the atmosphere, bringing its concentration to around 400 ppm. In order to add as much CO2 shielding effect again as we already have, we would have to burn more than twice as much fuel as we have already in our entire history since the start of the industrial revolution. That actually is not an easy thing to accomplish, even if we try very hard. Which, looking at the recent history from even the rather dodgy measurement systems, we aren’t.
So the alarmists rely on the slight increase in temperature from the CO2 shield effect to vaporize a far more effective shield gas – dihydrogen monoxide (water). This is touted as the “fatal multiplier” that will roast our world.
But the alarmists just can’t win. Mother Nature (or God, or quantum physics; whatever fountain you drink from, it’s the same flavor) has arranged things so that the “fatal multiplier” is actually a “saving divisor.” The slightly higher temperature at the surface does evaporate more water into the atmosphere. Fortunately (for humanity in general, not the alarmist’s grant prospects, or long term investors in “green” companies), this temperature also creates stronger upwelling as the hot air rises, carrying the water vapor along with it. Until it reaches the upper atmosphere – where the water vapor condenses, releasing energy where there is virtually no shield between it and the far cold reaches. This natural brake on the temperature means that, at the most, the Earth can warm an average of 14 degrees (Fahrenheit – about 7 degrees Celsius). Not that we can manage to add enough CO2 to get that high – you need 7,000 ppm for that – burning forty-five times as much fuel as we have already. Their best hope is for massive vulcanism, although that just might tip us over into an ice age from aerosols before the additional CO2 can get to work.
(The honest climate researcher, of course, knows that even the above is highly simplified – but covers the most significant drivers of climate. The “fiddly bits,” such as changes in albedo, lower in the less icy northern latitudes – somewhat offset by more water surface; outgassing of CO2 from warmer water; sinking of CO2 both geologically and biologically; et cetera, et cetera, et cetera.)
“No rational “skeptic” denies…”
No true Scotsman denies…
‘Tis a bit of a difference between denying who is the rightful King, and denying a physical phenomenon. As all too many Scots with too much Scotch in them have discovered. You might escape the usurper’s men for your intemperate words, but not the ground when you trip on your feet instead of your tongue.
No, yours was a perfect example of the no true Scotsman fallacy.
Looking at your other posts, it should not surprise that you have no better idea of the “no true Scotsman fallacy” than of physics.
Not having the patience of a Job – or even a Willis – last reply here.
Sorry you were busted.
https://en.m.wikipedia.org/wiki/No_true_Scotsman
I would point you to the last few words of that definition (which is a good one, by the way). The objective facts of physics do not change – not for alarmists – not for skeptics – not for Scotsmen.
Tony, you embarrass yourself.
More clearly-
“The No True Scotsman (NTS) fallacy is a logical fallacy that occurs when a debater defines a group such that every groupmember possess some quality. ”
Writing observer did not state or imply that all skeptics are rational, so in order to be a “true” skeptic one must have that quality. By including the qualifier “rational” before the word “skeptic”, he implies that some skeptics are NOT rational.
The group was skeptics, the exclusion is to dismiss those that disagree with the GHE as irrational. Not difficult to understand.
Even disregarding the effect of H2O vapor upwelling, it seems that there is a leap of logic in alarmists’ reasoning. Are they ignoring the logarithmic characteristic of CO2 (which I assume is due to saturation effect?), or (if not ignoring that) are they assuming (or do they possibly have evidence for) that the increase in H2O vapor due to (CO2 caused) warming would not follow the CO2 increase linearly?
The narrative is that more CO2 causes more H2O, which causes more H2O, which causes even more H2O.
If the pesky molecule would only stay put on or near the surface, they’d be right.
“The narrative is that more CO2 causes more H2O, which causes more H2O, which causes even more H2O.”
Which will cause more convection of wetter air flattening the lapse rate and causing more condensation removing more heat from the surface. It isn’t even clear that the H2O feedback is positive.
It is, in the minds of climate modelers, tty. Observations do confound them, though.
“No rational “skeptic” denies that adding more shielding CO2 does increase the surface temperature. Less energy escapes directly to the far colder surroundings with that additional CO2 in the atmosphere.”
I deny that, CO2 slows the cooling, the incoming visible radiation provides the warming. Never will you see the GHG affect warm the earth at night…never (ignoring conduction and convection). CO2 is a blanket, not a heating source. The incoming radiation warms the earth, CO2 slows its cooling.
I’m leaning your way. It slows the cooling.
Yep, unless it is from a chemical reaction where energy is changed in form, you won’t heat a body above the temperature of the radiating body.
For some reason I like the word retards rather than slows. Here’s a question: Does radiant heat act like electric current? IN other words, for there to be radiant energy transfer (heat) does there have to be a difference in potential of some form? Kind of like current (analogous to heat) doesn’t flow until there is a voltage differential. Might sound like a dumb question to some people, but yano I’m just a student of the masters.
Great point, the atmosphere is like an electrical resistor. It impedes the path to outer space.
ICISIL:
My heat transfer texts, in their chapters on radiative heat transfer, are full of “electric circuit” diagrams with resistors for what they call “network analysis” of these transfers.
Remember that a “transparent” atmosphere (no GHGs) provides no resistance to radiative energy transfer.
“Slows” is a misleading term; “retards” may a little better. I prefer “reduces”, myself.
Excuse me? You just restated exactly what I said.
Less energy escapes directly to the far colder surroundings… Emphasis added!
No, CO2 does not heat the surface (nor does any other component of the atmosphere). But what both Willis and I are trying to get through heads is that the atmosphere affects the net heat flow, reducing the net flow of heat from the surface (whether day or night) to space.
Ignoring, of course, the effect of convection, which transports surface heat (in the form of latent heat in water vapor) to an altitude where there is far less atmosphere – thus increasing the net outflow of heat from the entire system.
It’s called a Thermal Insulator. From a grade school website:
“Materials that are good conductors of thermal energy are called thermal conductors. Metals are very good thermal conductors. Materials that are poor conductors of thermal energy are called thermal insulators. Gases such as air and materials such as plastic and wood are thermal insulators.”
“One way to retain your own thermal energy on a cold day is to wear clothes that trap air. That’s because air, like other gases, is a poor conductor of thermal energy. The particles of gases are relatively far apart, so they don’t bump into each other or into other things as often as the more closely spaced particles of liquids or solids. Therefore, particles of gases have fewer opportunities to transfer thermal energy. Materials that are poor thermal conductors are called thermal insulators.”
https://www.ck12.org/book/CK-12-Physical-Science-Concepts/section/5.17/
I’m sorry Writing Observer-
You state first-
“No rational “skeptic” denies that adding more shielding CO2 does increase the surface temperature.”
Then state- “No, CO2 does not heat the surface (nor does any other component of the atmosphere).”
There is a difference between something that “heats” something else-causes it’s temperature to increase-and something that slows down the rate at which something COOLS.
“But what both Willis and I are trying to get through heads is that the atmosphere affects the net heat flow, reducing the net flow of heat from the surface (whether day or night) to space.”
Affect it YES….reduces the net flow of heat from the surface to space? Nope…merely slows the rate of flow. Why? Because the atmosphere ALSO reduces the flow of heat from the SUN to the surface. It slows down the rate at which the Sun can warm the surface in a 12 hour period, and then slows down the rate at which that warmth returns to space, but it does NOT reduce the NET flow of heat from the surface or from the SUN. It does not “cool” the Sun anymore than it “warms” the Earth. It’s a SUCKY conductor of thermal energy. It’s an insulator.
If I pour hot coffee (100 F) into a plastic thermos (that contains an air pocket inside it’s walls for insulation) that is “cold” (compared to the coffee) but is at room temperature (let’s say 70 F), and then put that thermos outside in a snow bank in “colder” 10 F weather, does the “cold” previously room temperature thermos have the ability to WARM the already HOT coffee by “hiding it” from the colder snow and outdoor temps or does it simply slow down the rate at which the coffee INSIDE THE CONTAINER cools???
If I poured the original 100 F coffee onto a saucer made of metal, and placed it in the same spot outside in 10 F, the rate at which the coffee cools would be MUCH faster. Because metal is a great conductor of thermal energy. Far better than the air pocket inside the plastic thermos was. But the fact remains that the coffee began to COOL the instant the heat source (stove, coffee pot) was removed.
The net heat transfer in both scenarios is exactly the same…one just took a lot longer than the other. The net transfer of $25 dollars to “you” from “me” in Willis’s scenario would have been the EXACTLY the same whether “me” paid “you” one penny a day for 2,500 days or handed me $1.00 for 25 days, or wrote me a freaking check for $25 and handed to me in 20 seconds!!!
Words matter. Scientific principles matter. Spontaneous heat flow will ALWAYS be from a warmer object to a colder object. Net heat transfer does not END between two objects of different temperatures until both are in equilibrium and reach the same temperature.
Yes, the whole question is does a change of 100 ppm CO2, 0.0001% of the atmosphere, alter the Net heat flow, that is the whole question. Using the thermos example, you fill the Thermos with Hot Coffee, and then let it cool over night, and they you add the same amount of energy over the next day to warm the Coffee again, and then let it cool. Over time, does that does adding the same amount of energy to a thermos throughout the day result in gradual warming because the energy leakage is less than the energy added each day. The marginal difference is what would result in the warming.
Aphan, you say: “Because the atmosphere ALSO reduces the flow of heat from the SUN to the surface.”
No! The whole point of the “greenhouse” metaphor (and it is just a metaphor) is that the atmosphere is far more transparent to solar (visible and shortwave IR) radiation than it is to terrestrial longwave IR.
Yes!! The atmosphere DOES absorb incoming heat and radiation. Learn something:
https://earthobservatory.nasa.gov/Features/EnergyBalance/page4.php
“About 29 percent of the solar energy that arrives at the top of the atmosphere is reflected back to space by clouds, atmospheric particles, or bright ground surfaces like sea ice and snow. This energy plays no role in Earth’s climate system. About 23 percent of incoming solar energy is absorbed in the atmosphere by water vapor, dust, and ozone, and 48 percent passes through the atmosphere and is absorbed by the surface. Thus, about 71 percent of the total incoming solar energy is absorbed by the system.”
“and it is just a metaphor”
No, Its a FALLACY !!
Only statement above that has meaning in the real world:
So just to recap these ‘radiation budgets’ are undoubtedly scientifically correct…they are also to all extents and purposes irrelevant or to put it another way…negligible.
http://tropic.ssec.wisc.edu/tropic.php
I think that you’re dead right Willis. AFAICS, the only reason that the Second Law of Thermodynamics isn’t written in terms of net flow is that the second law was derived in the nineteenth century from the ideal gas equation pV=nRT using mental models where all heat flow was by convection/conduction. Back flows are (usually) still there with convection and conduction. But they are difficult to observe and measure.so the second law is stated in terms of heat flows without the term “net”. They are, in fact, net flows.
When one deals with energy transfer by radiation, one has to make a few adjustments to one’s mental models including recognizing that flows are net flows, and also including the property of emissivity at the source and destination. If one doesn’t do that, one will find that perpetual motion is not only possible, but easy.
I think many of us might prefer to live in a world where perpetual motion machines can be built and do useful work. But it’s clearly not the world we actually live in.
Hi Willis! Your argument is correct for two emitters with independent energy sources (e.g. two glowing light bulb filaments, two Suns, etc.). However, the Sun does not emit any significant far-infrared radiation at 667 cm^-1 which can be absorbed by CO2 in the atmosphere. The 288 K surface of the Earth, however, emits far-infrared radiation whose Planck black body curve peaks near 667 cm^-1. CO2 is such a powerful absorber that 667 cm^-1 radiation emitted from the Earth’s surface is almost 100% absorbed within metres of the surface. By Kirchhoff’s Law that a good absorber is a good emitter, CO2 will emit almost 100% at 667 cm^-1 (parabolic dish antennas are not only good radio receivers, but are also good radio emitters/radiators). But the excited CO2 molecules formed on absorption of 667 cm^-1 photons can also lose their excitation energy during radiationless collisions with the main molecules of the air (N2, O2, Ar) which cannot and do not re-emit any significant amount of infrared (IR) because their molecules do not possess any permanent electric dipole moment. The energy does not go away, but ends up as translational and rotational kinetic energy of the departing molecules. The heat capacity at constant pressure for linear molecules like N2, O2 and CO2 is 7k/2 per molecule, where k is Boltzmann’s constant. Since N2 and O2 together outnumber CO2 by 1,000,000:400 = 2500:1, almost all of the absorbed energy ends up warming N2, O2 and Ar molecules (and is stored as enthalpy, heat content, H). This is the mechanism of the greenhouse effect, and has nothing to do with back-radiation. Back-radiation does exist, but it can be thought of as energy flow that just balances an equal energy flow for two surfaces at thermal equilibrium, with no net change in the temperature of either. Some might argue that excited CO2 molecules can also be formed on collision of ground state CO2 molecules with high-energy (fast-moving) air (N2, O2, Ar ) molecules, and that powers the back-radiation. But this energy would come from the air molecules, whose average kinetic energy (temperature) must decrease; no, at thermal equilibrium there can be no net warming of the Earth’s surface by back-radiation alone. Here is an analogy that might make this more clear: suspend two flat parallel black metal plates in a blackened vacuum chamber cooled with liquid helium to 4 K, close to the 3 K of the cosmic background microwave radiation. If one metal plate contains a 100 W heating coil, and the other is a passive radiator without a heating coil, at thermal equilibrium 50 W will be emitted from the outside surfaces of the two plates, for a total of 100 W outward toward the 4 K inner walls of the enclosing vacuum chamber. But the space between the two plates will have 50 W exchanged back-and-forth, so there is no net heating or cooling of the plates. If the two plates are moved together until touching, the total output will still be 100 W, with 50 W outward from each surface. But the back-radiation will, like the forward radiation it balanced in the previous gap, disappear with no change in temperature or output. Note that when the two plates are separate, 50 W is emitted from both sides of the plate with the heating coil, and 50 W from both sides of the passive plate, for a total of 200 W emission. How can this be powered by the 100 W heating coil? The Law of Conservation of Total Energy is not violated because the 50 W back-radiation from the passive plate to the plate with the heating coil just balances 50 W in the opposite direction, so the back-radiation photons can be considered to be photons initially emitted from the powered plate and then reflected back to the power source. This is not the same as photons reflected from a mirror, because a mirror does not emit in the forward direction, so the mirror analogy is wrong for CO2 in the atmosphere which absorbs and re-emits in the forward direction.
Suppose the surface temperature of the single powered 100 W plate is T0. If another identical black 100 W plate is brought close to and parallel to the first, this time the surface temperature will rise by a factor of the 4th root of 2 = 1.1892. This time, “back-radiation” will heat up both plates because those photons come from separate power sources. This may be seen when the two plates are brought together, forming a single plate with a 200 W internal power supply, so that 100 W will be emitted from each of the two remaining surfaces to the surroundings. This is double the 50 W from each of the two surfaces of the original 100 W plate, requiring a higher surface temperature for emission by the Stefan-Boltzmann Law.
Seriously good post (From a mobile?) however, could do with some formatting, just a bit too busy for my eyes in one big chunk as it is.
Here is an observation that demonstrates ‘delayed’ cooling. Wait for a warm, calm, sunny day and note the temperature. As the sun sets below the horizon the temperature starts to fall. While the sky remains clear overhead the temperature continues to fall. Then a cloud bank arrives overhead. The temperature stops falling! In fact it will start to rise. I have noted a temperature increase of over 5C under these conditions. Remember, it is pitch dark, the sun is on the other side of the world. So what is causing the warming? It is not the wind which I said was calm, it is in fact back radiation from the cloud base. This radiation from the cloud, impacting the surface, is reducing the cooling rate of the surface. Subsurface heat from the previous day is still rising. As the surface temperature rises the net radiation increases, the back radiation from the cloud increases.
The heat capacity of the ground is considerable. To really experience this go live in a desert region for a while. Here sunlight, day after day, really does warm up the ground yet just before dawn the local temperature will be close to freezing. The outer surface of the rock is trying to shrink. Often a piece of rock spats off with a loud crack. Find that rock and feel the new exposed surface. It will be quite warm!
Eventually there will be a lot of sand. Dig into that sand on a cold dawn and you will fell a lot of warmth. Dig a little trench, lie down, and enjoy some wonderful star gazing. Mind the scorpions!
There are clearly 2 schools of thought.
1. Willis is right and ALL e-m radiation that “hits” an object is absorbed and thermalized. Temperature changes are thus based on NET radiation (in versus out).
2. Willis is not even wrong. ONLY e-m radiation from a hotter object can be thermalized in a cooler object.
This needs to be resolved before one moves on to any discussion about CAGW, atmospheric physics, etc. You need to get the FUNDAMENTAL workings of radiation and heat transfer correct FIRST. Otherwise you will be building everything else on a false foundation.
For those so inclined I invite you to invent an experiment to test which explanation is correct, or perhaps find the results of an experiment which has already been done. The experiment must clearly differentiate between the 2 fundamentally different explanations of course.
This is a useful excercise generally as you will often find arguments in science where there are 2 competing views. They are not always resolvable via reference to literature as each camp obviously has well developed arguments for its own case. In all cases it pays to carefully study BOTH sides even if you are pretty sure yourself what you believe. I recommend it in this case.
Good luck with it.
I would be interested to hear of any experiments regarding e-m radiation being thermalized always/sometimes.
If em was thermalized all the time the hottest spot in America would be the base of the antenna of a 50000 W radio station. But it isn’t.
mkelly,
If you live just under that station, you can light a fluorescent tube by only adding an antenna at one side and a ground cable at the other side…
That may be true but it does not make it hot. We used to do that with radars in the Navy. The old APS20 would if given a chance heat soup but it would not hezt air.
The Reverend,
Many thousands of CO2 lasers prove daily that point 2 is wrong. These emit IR at between 9.600 en 10.640 micrometer. That is comparable to the peak radiation of a black body at around a human’s body temperature, not really “hot”.
According to point 2, that could warm up steel to maximum the same temperature, not further up.
In the real world CO2 lasers heat up steel and melt it at 1200ºC…
Which proves that Willis is right…
http://hockeyschtick.blogspot.com/2014/11/why-cant-radiation-from-cold-body-make.html?m=1
“The radiation from the cooler source doesn’t have the high frequency light-wave energy components which would be required to fill up the higher-energy microstates that the warmer source already has filled up, let alone the higher frequency states beyond to result in an increase in temperature. You can see it a little bit in the Planck curve plot above, that the higher-temperature object has a microstate population that is “activated” at higher frequencies, i.e. shorter/smaller wavelengths, than the lower-temperature object has. (The warmer one also has a larger population in each frequency microstate as compared to the cooler one.)
The integration over all the active microstates of a system determines its macrostate, and the macrostate is the thing you actually measure to take a temperature. If you activate higher-frequency microstates, then you shift the macrostate to a higher temperature. But you can only activate the higher-frequency microstates with the frequencies required to activate them, which are obviously their frequencies.
And so because the radiation from a cooler source lacks the higher-frequency microstates that the warmer source of radiation already has, then the cooler source can not have the effect of raising the temperature of the warmer source. And obviously the same would be true of two systems with identical temperatures – they could have no effect on each other’s microstate population, hence can not heat each other.”
All the math is there too, and a link to the steel greenhouse debunking.
Aphan:
you can only activate the higher-frequency microstates with the frequencies required to activate them, which are obviously their frequencies.
A CO2 laser only sends IR at a frequency of around 10 μm. Steel at 1000 K has its peak frequency at around 5 μm, thus the CO2 laser beam can only activate some 30% of the iron atoms in the steel, if that story was right.
That means that only individual atoms with a “temperature” – or energetic level – between 0 K and about 300 K (the corresponding frequency level of the beam) can be heated by the laser beam. Or an average increase to about 1050 K for steel as a whole. Not enough to melt it. In reality steel is melted by a laser beam of only 10 μm waves up to 1500 K.
Something wrong with the theory?
Ferdinand, What is the Power of the Laser, not the Frequency?
Ordinary Cold Water can be used to make a hole through steel, how do you think that works?
It works by having enough Pressure, is not Power the same as Pressure?
A C Osborn,
A water “beam” can dig a hole in steel due to kinetic energy. That is mechanical energy, as good as drilling or using a hammer and chisel.
Power is not kinetic energy, it is electrical energy which in a laser is transformed into electromagnetic energy, which if absorbed by an object is transformed into vibrational energy of the atoms/molecules. Vibrational energy is temperature…
“BUT it can leave the hot object warmer than it would be if the cold object weren’t there. ”
No need for a large article
The cold object provides a temperature differential potential to allow heat to move from the hot object to the cold object, no colder object, no loss of heat from the warmer object to the colder object.
Done
Mark,
Well said – at last a sign of sanity in this crazy debate. Life is so simple unless one has a compulsive desire to over-complicate it. It seems that almost everyone else here is bent on grand-standing and mindless prevarication.
Simple standard physics says…
1. The surface of an object X at a temperature Tx will assert a radiative potential Px where Px is proportional to the 4th power of Tx (S-B equation).
2. The surface of an object Y at a temperature Ty will assert a radiative potential Py where Py is proportional to the fourth power of Ty (S-B equation).
3. If the surfaces of the two bodies X and Y are facing one another, and if Tx > Ty, then the rate of transfer of radiative energy between them is simply Px – Py; and the direction of energy flow is from the warmer body X towards the cooler body Y.
In the whole of physics is there anything easier to grasp than that? It applies under all situations everywhere in the universe. In particular it applies in the case of the earth’s warmer surface facing its cooler atmosphere.
In Willis’s energy budget diagram, Px = 392W/m^2 and Py = 321W/m^2. One conceptual trick that I use is never to think of these two numbers as independent energy flows, but as calculated potential flows. After all, they clearly do not exist in isolation. On the contrary they are completely bound up together by the geometry of the two facing surfaces. If one thinks of them as independent, one can easily get into the silly position that some people have done of thinking that the 321W/m^2 of ‘back radiation’ is somehow violating the 2nd law because it is larger than the incoming Sun’s radiation of 169W/m^2 absorbed at the surface. In reality it is always only the net radiation (in this case 392 – 321 = 71W/m^2) that counts.
It is also vital to appreciate that all of the above is true irrespective of any non-radiative energy transfers that may also be occurring at the same time. In Willis’s energy budget diagram, the non-radiative energy transfers from surface to atmosphere are 22W/m^2 (sensible heat) and 76W/m^2 (latent heat). Adding these on to the net radiative energy flow of 71W/m^2 makes a total of 22+76+71 = 169W/m^2. This exactly balances the amount of Solar radiation absorbed by the surface, as must be the case for steady-state temperatures. So the non-radiative and radiative energy flows coexist happily.
Bingo!
David, a simplified discussion and a question:
1) Addition of greenhouse gasses (GHG) to the system would increase back radiation, thereby reducing the net 71W/m^2 surface emissions.
2) The surface must heat in order to restore the 71W/m^2 necessary to balance input/output.
3) The net contribution of man’s production of CO2 (net of sinks) would necessarily heat the surface in order to get H2O feedbacks, positive, negative or neutral.
4) There are massive and constantly changing energy flows within the earth’s climate system. Much of this is the result of chaotic weather systems.
Is the radiative contribution of man’s net CO2 lost in the noise?
David, thank you for simply stating what should be obvious to an educated person.
It should be noted that fluxes in Trenberth’s diagram are based on averages and are presented as in a steady state. But our water world is turbulent and chaotic, with all of Trenberth’s noted fluxes changing on all time scales, except possibly the isolation of the growth of CO2 equivalent fluxes for analytical purposes.
I’m not a researcher so I keep asking simple questions: In isolation, how much of the indicated back-radiation is the result of man’s contributions to CO2 equivalent fluxes? What is the resulting theoretical warming from such contributions, in isolation. Is this theoretical warming even discernible in our turbulent and chaotic water world?
Again, it’s had a name and definition for decades. It’s called a Thermal Insulator. Thermal insulators are the opposite of thermal conductors. Air is a lousy conductor of thermal energy.
“The temperature and the radiation are related to each other by the Stefan-Boltzmann equation” Only for black bodies and by extension for the mythical gray bodies, too. For objects where absorption / emission is frequency dependent (that is, quite a LOT of them), where the radiation is not fully thermalized (which is the case for the atmosphere), it does not apply. Ex falso, quodlibet.
Pseudo sciences crap on the boundary conditions and will apply at ease for example theorems even if the assumptions they start from when proved are plain false. With the principle of explosion, such pseudo sciences can derive anything they intend to.
All the ‘extra’ radiation held within an atmosphere as a consequence of the heat capacity of the atmosphere is utilised in potential energy form ( not heat) for the purpose of holding the atmospheric gases off the surface against the force of gravity.
Thus none of that ‘extra’ energy is available for further warming the surface above S-B.
Such further warming of the surface arises not from DWIR but from the return of potential energy to kinetic energy beneath descending convective columns of air.
The cold atmosphere does heat the warmer surface but due to the gas laws that warming is a result not of DWIR but of such conversion of PE to KE
Quite – there is a whole industry of it on both sides of the debate. A great shame.
Adrian, you say: “For objects where absorption / emission is frequency dependent (that is, quite a LOT of them), where the radiation is not fully thermalized (which is the case for the atmosphere), it [the SB equation] does not apply.”
Absolutely not true! Explanations of radiative heat transfer almost always start with blackbodies, next covering graybodies, because these simplifications make it easier for the beginning students to focus on the important concepts rather than the mathematical minutiae.
If you look at the equation Willis posted for radiative transfer between graybodies, everything inside the big left parentheses is needed for graybodies and not for blackbodies (as he shows).
For “real world” bodies, the emissivity is not constant over wavelength, as it is for idealized blackbodies (e=1.0) and graybodies (e less than 1.0). So you must evaluate each wavelength band invidually. The MODTRAN database does this to moderate resolution; the HITRAN database does this to high resolution. But the physical principle is absolutely the same in all cases.
That’s a bunch of anti-logical arguments. You either don’t know what Stefan-Boltzmann law is or you only play ignorant. Stefan-Boltzmann is about total energy, not about ‘lines’. And it’s about full thermalization, not about non-equilibrium situations. For your info, energy going ‘in’ for a line can go out through other ‘lines’. Despite radiation not being fully thermalized, quite a bit of CO2 collisions will lead to non radiative transitions. The energy going ‘in’ can go ‘out’ after a sensible time, it does not need to go out instantly and there is no ‘radiative balance’. That renders Stefan-Boltzmann law useless. The energy can go ‘in’ as radiation in a place at one temperature and can go out in a different place with quite a different temperature. Stefan-Boltzmann law requires a single thermodynamic temperature, that is, equilibrium, which is not the case for Earth’s atmosphere.
MODTRAN/HITRAN is a red herring and for your info it has enough error in results (especially where H2O is more involved) to be able alone to give the ‘garbage in’ for models to be exponentially amplified in the exponentially grown pile of shit the models output.
Adrian:
You say that the SB equation applies “only for blackbodies and by extension for the mythical gray bodies, too.” It’s only slightly more of an extension to “objects where absorption / emission is frequency dependent”.
If you’re trying to be a pedantic nitpicker, you should be correct in your nitpicking. You should be talking about absorptivity and emissivity, which are different things from absorption and emission.
Besides, the graybody approximation is a VERY good approximation for most solid and liquid substances at earth ambient temperatures.
You imply that when there is frequency dependence of a and e, it is because “radiation is not fully thermalized” (although it might be awkward phrasing on your part). Two points. First, a/e frequency dependence is fundamentally independent of full thermalization. They are separate issues.
Second, the atmosphere throughout the full troposphere fully thermalizes absorbed radiation, counter to your assertion. A molecule excited by absorbed radiation is about a million times more likely to collide with another molecule while still excited than it is to re-emit before a collision. (Of course, this is even more true of solid and liquid substances.)
Besides, your arguements are fundamentally irrelevant to the qualitative argument presented.
“the graybody approximation is a VERY good approximation for most solid and liquid substances at earth ambient temperatures” It is extremely bad and it’s again a red herring, the cliamastrological religion has delusions about the atmosphere, which has a spectrum which is very far from that of a black body.
“the atmosphere throughout the full troposphere fully thermalizes absorbed radiation” As I explained, it doesn’t do that as required for a black/gray body, so your anti-logic is anti-logic and nothing else.
“your arguements are fundamentally irrelevant to the qualitative argument presented” They are fundamentally relevant, because such ‘qualitative’ anti-arguments will treat wildly non-linear systems as being linear, non-equilibrium systems as being at equilibrium, white as black and false as truth.
Ex falso, quodlibet.
Oh, and since you appear to have absolutely no idea what thermalisation means, start from here to rise yourself above the total ignorance you exhibit: https://en.wikipedia.org/wiki/Thermalisation Maybe you’ll figure out why I stressed that it’s not equilibrium.
Transfer of kinetic energy. Temperature is a misnomer. We must talk of it in terms kinetic energy
Temperature can be cumulative in directional kinetic energy application, increasing pressure but it requires an external input of kinetic energy to achieve this, it does not happen in passive kinetic energy exchange.
Passive kinetic energy exchange is decided by the difference in kinetic energy between the two objects,
Leave two blocks next to each other,
*one cooler one hotter,
*The kinetic energy transfer is passive, the warmer block loses kinetic energy to the cooler block (difference potential)
Smash the cooler block into the warmer block
* You are inputting kinetic energy into the exchange that is greater than the kinetic energy of passive transfer * The cooler block uses the kinetic enegy from momentum to warm the warmer block.
All about passive vs non passive transfer of kinetic energy
The cooler block uses the kinetic enegy from momentum to warm the warmer block *on impact
Which increases the rate of kinetic energy. KE\Time is what changes and overrides passive transfer
Ok, this is a subject I must confess to struggling with – but I very much appreciate this:
“These ideas of individual flows, net flows, and being shielded from radiation are important because people keep repeating over and over that a cold atmosphere cannot warm the earth … and they are right. The temperature and the radiation are related to each other by the Stefan-Boltzmann equation. When we apply the S-B equation to the 321 W/m2 of downwelling “back radiation” shown in the graphic above, it tells us that the effective radiating level is somewhere around freezing, much colder than the surface.”
Terms like “backradiation” and “downwelling” sort of add to the confusion by making it sound like one should be able to feel it – when it’s just shielding us from the cold of outer space – which is what we learned in grade school.
At the same time, the atmosphere as a whole functions, not just to shield us from the cold of outer space, but as a heat dispersal system as well.
It is impossible physically for a cool object to warm a warmer object unless the cooler object can make use of additional energy from outside of the two object equation.
Otherwise kinetic energy will always be passive and always be dictated by difference potential.
Exactly!! There has to be an additional source of energy to perform the work required.
I grew up in my fathers steel fabrication business. By the time I was 12 I was welding and fabricating and sometimes blacksmithing using a forge. One thing I know is that if one heats the end of a piece of a bar of carbon steel of any solid shape or compound that is a foot or a few feet long until it is cherry red and then quenches the hot end while holding the cooler end, the end that they are holding will become hotter very quickly after they have submerged the heated end in water or oil.
I love the way 5% carbon steel can be made into a chisel (Brown) or a spring (Blue) simply based on heat during hardening and colour during tempering. Now, this is a very very old memory.
You can straighten bowed or twisted “I” beams, even very heavy ones, using only a rosebud tip on an oxy-acetylene torch and some water soaked rags. One just has to know where to apply the heat.
It’s because steel is an excellent conductor of thermal energy, liquids less so, air even less than that.
The truth is, as things stand, it is impossible for us to even remotely know what earth’s energy balance is.
Impossible for us to know where all the kinetic energy goes.
Any claims otherwise are either utterly delusional or dishonest.
Logical examination is dead in science, your solution must be logical first and foremost
Too many get lost in the mathematics and lose all logical structure in their solution
See: actual physical singularties, produced from thin air by some of our “greatest minds”. There is obviously no such thing in the physical world
Relative mass is bunk, it requires transfer of kinetic energy between objects to manifest the extra kinetic energy from momentum
So an object moving at say half the speed of light has no relative mass, the mass is exactly the same as a static object, the energy that is changed is kinetic energy and that is only manifested when there is a kinetic energy transfer.
The so called extra mass that is in relative mass, does not belong to or come from that object, it is external kinetic energy provided by another source.
I can assure you that even the relatively modest speed of an electron in a CRT (like in an old-fashioned TV) will make its movement through the electric field come out significantly wrong if you don’t take the relativistic mass increase of the electrons into account. I did an experiment on this when reading freshman physics, so I know it from personal experience.
you obviously dont understand, relative mass is mass+pseudo mass ie mass and momentum
they can be calculated as one, BUT the Electron does NOT have more mass
As I clearly explained, if there is physical interaction then this kinetic energy comes into play, and in a CRT that is the case.
If the electron does not physically interact then the case is different, maybe read first then reply 😛
If you work this problem of energy balance in terms of kinetic energy, it becomes clear that we have no way to work out this problem and are left with mathematical fantasies and severe top level averaging that does not represent the problem, or provide a solution.
We’re dealing with ghosts here
Can A Cold Object Warm a Hot Object?
No. End of discussion about radiation.
Can a cold object make a warm object warmer than it would have been if the cold object wasn’t there?
Only if it insulates the warm object. If you insulate a warm object, the rate of cooling decreases.
What has this got to do with Carbon Dioxide’s contribution the ‘Greenhouse Effect’?
Hardly anything in practice. Carbon Dioxide is not an effective insulator for two reasons:
1. It is a poor insulator by itself.
2. There is not enough of it to make an appreciable difference.
To Willis’s figures:
Figure 1:
This is NOT a good analogy!
According to Willis, doubling the number of ‘Yous’ would give $150 to the ‘Me’. Hence, by implication, ‘warming’ the ‘Me’.
But RADIATION IS NOT HEAT.
Here is a counter- question: How many cold objects does it take to warm a warmer object?
Answer – it can’t be done.
In Fig 1, the ‘net’ flow (Heat) is always from ‘Me’ to ‘You’, no matter how many ‘You’s there are.
In the climate debate, doubling the ‘You’ (CO2) will have no measurable effect on the temperature of the ‘Me’.
Why?
Because the spending power of the $75 will never overcome the spending power of the $100. ‘Me’ is richer than ‘You’. ‘Me’ can afford to give all the ‘You’s their $75 back and ‘Me’ would STILL be richer than all the ‘You’s. Equate richer for warmer and you have the true picture.
Ask yourselves this:
Why does the Sun’s radiation heat the Earth? Because the energy of the radiation from the Sum is sufficient to increase the thermal energy of the Earth’s receiving molecules.
Can atmospheric CO2 not do that? NO.
Hence Figure 2 is also wrong:
The 321 W/m2 that REACHES the surface is NOT absorbed for thermal gain. The ‘absorbed by the surface’ is irrelevant in a thermal context if the Earth is warmer than the atmosphere.
This is why you can surround a hot object by billions of cold objects and the hot object will never (EVER) get hotter.
Don’t conflate insulation with insolation.
Comparing atmospheric CO2 with a blanket, thermos, whatever, is like saying that a string vest made of 99.96% air and 0.04% cotton is an effective insulator. Even that would be wrong because the atmospheric CO2 doesn’t actually prevent heat loss, so the effectiveness of the cotton is further reduced. CO2 does not trap heat. Backradiation is not heat. Remember the ONLY source of energy in this process is the Sun.
<i.["Anthony Watts: "I get so tired of hearing this stupid argument from the anti-ghg crowd. Thanks for quantifying it."]
Mr Watts, I have a lot for respect for the work you have done in making this website. However, just because you don’t understand an argument doesn’t make that argument stupid. Wilis has not ‘quantified’ your objection.
The science is far from settled!
Kind regards to all.
Arfur
“Can a cold object make a warm object warmer than it would have been if the cold object wasn’t there?
Only if it insulates the warm object. If you insulate a warm object, the rate of cooling decreases.”
And you don’t think blocking the warmer object from the coldness of space is insulation?
BTW a real definition of “insulation” might be useful here.
[“And you don’t think blocking the warmer object from the coldness of space is insulation?”]
A blocking material would act as an insulator but CO2 does not block anything, therefore it cannot either warm the Earth’s surface nor effectively reduce the rate of cooling. CO2 emits radiation as effectively as it absorbs. CO2 may delay the LWIR loss to space but the time delay is tiny compared to the length of time taken to increase the amount of CO2.
As to the definition, I am (obviously) referring thermal insulation, which I would define as a material capable of reducing the rate of cooling of a warm object.
Arfur: I agree mostly but I think that when an insulator is in equilibrium between the heat gain from the warm object and the heat loss to the cold surroundings it does not any more slow the cooling of the warm object. I guess that is the situation in the atmosphere.
I sort of agree but the rate of change (build up) of the insulating material is important in this context. It is not as if we have suddenly surrounded the Earth with a thermos-type shell (or a steel shell for that matter). Our situation is a very slow increase in a trace gas which has virtually no insulating properties and which exists in sparse form (each CO2 molecule is surrounded by approx 2500 non-radiative molecules).
Also, this transfer should be considered in a realistic time-frame. It takes a comparatively long time to make the measured increase in atmospheric CO2. The Mauna Loa dataset shows an increase of 90 parts per million in about 70 years. Thats just over ONE part per million each year! Even if CO2 was an effective insulator (and it’s not), how much effect could it have at that rate of increase? That is a whole different debate! 🙂
Willis, good post, and thank you for attempting to clear this up.
I suspect the effort is in vain, however. Sad as that may be.
The people that refuse to let this penetrate their dense outer bony layers of cranium have heard it before…it bounces off like rain from a duck’s back.
Now, if you could extend you laser-like focus to the subject of dowsing…
Oops, sorry…I did not see your second request on my first reading.
My bad.
Another interesting experiment. A 3mm mild steel plate about half a square metre. Lay this on a slab of polystyrene. Place these outside, a couple of feet above ground. I had a digital thermometer screwed to the plate. The plate is exposed to the sky but insulated from the ground below. Note local air temperature and temperature of plate. Needs a reasonably calm clear morning. As the sky turns blue overhead BEFORE sunrise, note the temperature of the metal plate. It will start to rise sooner than the local air temperature.
That blue sky overhead is warming stuff on the ground. Be interesting to know how blue sky warming effects the oceans, especially as that ‘blue’ colour will have good penetration into water.
It may be blue but it is not very bright.
Get a light meter, point it at the blue re-dawn sky.
Then do the same during full daylight an hour later, and again at noon.
BTW, did you do this experiment Richard?
What was the change in temp?
I did the experiment more than once. The mild steel plate weighed 11.5 kg and warmed up about 2 degrees more than the surrounding air. After that the temperatures rose together. I assume the plate was now losing heat to the air. The point being that ‘blue’ light is diffused sunlight and has energy to do some work. If the sun was allowed to shine on the plate then the temperature rocketed.
It doesn’t really matter a lot if a photon goes direct or bounces off a molecule of air before being absorbed, so the result is not very surprising. Also the air isn’t really that much warmed by the sun, but rather by re-radiated and conducted heat from the ground.
Another interesting phenomenon. In still air the ground will normally be coldest slightly after sunrise. This is because it has very high emissivity in the IR spectrum, but reflects visible light fairly well, so it takes a while before the sunlight catches up with the IR emission.
Glass has very high IR emissivity, which is the reason that you may have to scrape ice off it even when the air (and ground) temperature in the morning is slightly above freezing.
You may have to put the light meter at the bottom of a long tube.
Richard: The thermometer on the slab of polystyrene but without the steel plate. How would the temperature behave?
Esa-Matti: Have previously photographed polystyrene at night and notice it is highly reflective of the IR emitted by the camera, thus assume this is part of the insulation property therefore the polystyrene is unlikely to warm.
Richard: I was asking because I think that the slab of polystyrene insulates also the thermometer from the cooler ground. Therefore, my guess is that you would have noticed that thermometer without the steel plate would also show higher temperature than the local air temperature.
Facepalm facepalm facepalm. It hurts too much to read the whole of Willis’s self confusion on this subject.
Just take the energy budget diagram near the end. Look at the numbers on the left side versus the right side. Compare to the money example. Is the “net” flow from the earth to the atmosphere or from the atmosphere to earth? Did the Earth end up with more cash or less cash? Did the atmosphere give the earth change from $100 or did it give more cash back than was given to it?
169 from the sun into the earth where the hell do the right side numbers come from if you are not advocating cold heating hot?
There is NOTHING in physics that says the energy given off from a cooler object must be absorbed by a warmer object. That energy is NEVER added to the warmer object. The energy from an ice cube is not added to your total energy if you stand next to it, pick it up or put it in a Willis pseudoscience post. Your energy output will remain the same. It won’t jump from 169 to 392 like in the embarrassing energy budget diagram.
To raise the average surface temperature to what we observe you need something else. You can raise temperature by transferring heat from hot to cold OR you can introduce Work into your system.
The greenhouse effect does neither, so it is wrong.,
There is absolutely no difference between “NET flows from hot to cold” to “heat flows from hot to cold” the word “net” is superfluous and simply a tool of confusion. The instant that the energy from the hotter object increases in any thought experiment with a cooler one, the “net” flow is no longer from hot to cold, is it? So you are proposing a cooler object increasing the temperature of a warmer one.
“The instant that the energy from the hotter object increases in any thought experiment with a cooler one,”
Still confusion…
The energy of the hotter object is not increased!!
It is just decreasing more SLOWLY.
At no point does the energy/temp of the hotter object go UP.
Just goes down more SLOWLY.
” So you are proposing a cooler object increasing the temperature of a warmer one.”
NO NO and a zillion NOES.
“The greenhouse effect does neither, so it is wrong.,”
Then please explain why the Earth, when considering the energy it absorbs from the Sun is 33C warmer than it should be.
Why when viewed from space it does (255K).
Yet we live at 288K.
Toneb. The Earth is not 33C warmer than it should be. The calculation to derive 33C is based on modelling a flat Earth with no day and night and with the average energy received from the sun divided by four because the cross-sectional area of the earth is one quarter of the surface area. With those ridiculous assumptions you get the false result of 33C.
Phillip:
Sorry but it is. Satellites measure Earth’s temp at 255K
Phillip Bratby: “Toneb. The Earth is not 33C warmer than it should be”
Toneb: “Sorry but it is. Satellites measure Earth’s temp at 255K”
Tony: Exactly. The Earth is not 33 C warmer than it should be. Thanks Toneb.
“The energy from the hotter object is not increased. It is just decreasing more slowly”.
NO!!!!!
Look at the NUMBERS on the energy budget diagram. The sun (on that diagram) warms the surface of the earth to a value of 169W/m2. The atmosphere INCREASES that amount to 392W/m2. It has not cooled. It has WARMED.
The values on the left are what the sun provides. The values on the right are trying to explain (through bad science) what is observed.
The mechanism described CANNOT BE! Cold doesn’t INCREASE the temperature of something hotter! You need WORK to do that.
Gravity provides that work, not recycling of energy against the well established direction of heat flow.
You cannot raise the temperature of an object by “cooling it more slowly”. You can cool to a different end temperature, but that is not the same as cooling to a higher temperature than what you started with. The compete jumbled up word salad that the greenhouse effect acolytes use, is what causes the idiocracy. You can’t heat the earth up to a higher temperature than that which the sun is capable of heating it. Not without additional work.
“Tony: Exactly. The Earth is not 33 C warmer than it should be. Thanks Toneb.”
Earth absorbs solar energy such that it radiates at 255K
It does, as seen from space.
On the surface it is 288K (as an average).
33K warmer.
The GHE.
Let’s try putting my reply in the right place.
Toneb, what on Earth makes you think the calculated 255 K temperature should apply to the Earth’s surface, and not the Earth as a whole (which you agree is observed to be 255 K)?
“Toneb, what on Earth makes you think the calculated 255 K temperature should apply to the Earth’s surface, and not the Earth as a whole (which you agree is observed to be 255 K)?”
It does apply to the Earth as a whole (as satellites see the “whole Earth” while they measure an irradiance of 225K.
But actually the max radiation is being emitted at ~8km – the level the GHE has raise the effective radiance level to (on average -18C there).
“You cannot raise the temperature of an object by “cooling it more slowly”. You can cool to a different end temperature, but that is not the same as cooling to a higher temperature than what you started with. ”
You are, I think, fixated on the 288K being hotter than the 255K as though the GHE has heated the Earth (provided extra energy). It is an insulation effect, a slowing of emitted LWIR while Solar SW is still incoming (nano/micro seconds worth though it might be). The excess being mostly being stored and later (in the case of oceans – hence the thermal inertia) heating the atmosphere.
All the while the Earth is radiating. Cooling in the absence of the Sun. The GHE doesn’t (instantaneously) heat anything.
Which is hotter the surface or the atmosphere above (most generally)?
There is (nearly – some exceptions in WAA, warm air advection) always more energy being radiated from the Earth’s surface than any GHE can overcome.
It is not “new” energy. It is that that was first emitted from the surface (mainly).
The 33K higher temp has not been “switched on” – it is the result of the GHE slowed energy being stored in the oceans.
It cannot be “switched on” in the sense of switching on 33K.
An analogy:
Get into bed under a very high tog duvet.
You are going to feel warmer than a thinner one, yes?
Quite possibly you will get hot and start to sweat, yes?
Is the high tog duvet heating you?
Is it electrically powered?
You are still radiating heat (incoming solar SW) yet the duvet slows your cooling (GHE). You get hotter as there is an imbalance.
Additionally will the temp on the top of the duvet be hotter or colder?
GHE theory observed as stratospheric cooling.
To make the blanket analogy more apt you need to raise or lower the height and therefore temperature of the blanket as it moves up or down along the lapse rate slope.
Thus the presence of radiation to space from the blanket would weaken the vigour of convection beneath it and lower the blanket to a lower warmer height whereupon the blanket would radiate faster to space INSTEAD of warming the surface beneath.
The same applies to the so called steel shell concept.
The behaviour of gases as opposed to solids is quite different to the behaviour of solids such as steel or blankets.
You have to take account of convection and the lapse rate slope which are determined primarily by uneven conduction from the surface to ALL atmospheric gases.
— Toneb
November 25, 2017 at 2:15 am
Phillip:
Sorry but it is. Satellites measure Earth’s temp at 255K–
Excluding interior of earth, the warmest place on Earth is the surface.
The surface is where the sunlight is absorbed.
The satellites are obviously measuring the surface incorrectly if
they indicate the surface [which is average temperature of about
15 C [288 K] is 255 K.
Of course earth radiates 240 watts per square, and if earth was
a blackbody that would be 255 K at it’s surface where it’s absorbs
the sun’s energy. Earth of course reflects about 30% of sunlight-
anything reflecting 30% of sunlight is not a blackbody.
Blackbodies also radiate the most amount energy at a given temperature.
The earth surface has water evaporating and heat being loss due convection heat transfer
to the atmophere. Or if blackbody were losing heat by evaporation and convection heat
loss, it couldn’t radiate the most amount energy of it’s temperature. Or because
it doesn’t radiate the most at given temperature- it’s not a blackbody.
Or it would have to be in a vacuum for blackbody to be a blackbody.
You’re playing (er, comparing) Trenberth’s flat earth average cartoon with actual measured global satellite measurements? And just “how” did your source convert spherical measurements to a flat earth average square meter value for absorbed energy, reflected energy, and measured energy at the satellite altitude?
I speculate that the only reason the word “net” got included in this was because they needed it to catch all the little balls (aka photons).
So Toneb, your answer is that basically no matter what, the GHE is responsible. A temperature of 255 K (effective temperature) is calculated, you at first assume that should apply to the surface, therefore the 33 K difference is due to the GHE. When challenged on that, you switch to saying it’s because the GHE has raised the effective radiating level. No matter what, this non-existent difference has to be explained by a non-existent phenomenon. The conclusion is arrived at first – there is a GHE. Then the circumstances can be adapted to fit this conclusion. So there is no need to accept the premises unless you already accept the conclusion.
“So Toneb, your answer is that basically no matter what, the GHE is responsible. ”
For the +33K?
Yes.
Fundamentally because of the non-condensing GHG’s.
Without them than WV would comdense out as snow.
It is what the science tells us.
Do you have any that shows it isn’t?
https://pubs.giss.nasa.gov/docs/2010/2010_Lacis_la09300d.pdf

https://www.giss.nasa.gov/research/briefs/schmidt_05/
“The size of the greenhouse effect is often estimated as being the difference between the actual global surface temperature and the temperature the planet would be without any atmospheric absorption, but with exactly the same planetary albedo, around 33°C. This is more of a “thought experiment” than an observable state, but it is a useful baseline. Another way of quantifying the effect is to look at the difference between the infrared radiation emitted at the surface of the Earth, and the amount that is emitted to space at the top of the atmosphere. In the absence of the greenhouse effect, this would be zero (in other words, no difference). In actuality the surface emits about 150 Watts per square meter (W/m2) more than goes out to space.”
“The satellites are obviously measuring the surface incorrectly if
they indicate the surface [which is average temperature of about
15 C [288 K] is 255 K.”
The classic “with one bound he was free” answer.
Sorry but that level of, err, gainsaying, is beyond response – but ……
So they are measuring the temp of Venus “incorrectly” as well?.
Well yes of course!
How can a satellite measure the temp of Earth as being higher than the energy it receives from the Sun can provide?
What goes in comes out …..
However the GHE CAN keep it in via an ‘insulation’ effect.
Hidden from the view of the satellite.
What the planet cannot do is emit MORE than it receives.
Which is what you are saying it does.
Venus has an effective temp of 220K
Yet its GHE adds 510K at the surface to take it to 730K.
It is measured from space at 220 as that is what the SUN warms it to (in>>out).
The extra 510 has built up over millenia because of it’s CO2 atmosphere as it tries to get out via T^4.
This again reveals a fundamental misunderstanding of the nature of the GHE.
https://atmos.washington.edu/2002Q4/211/notes_greenhouse.html
I’m just pointing out that your logic is circular. Not making any point about why the atmosphere is warmer at the bottom than the top. You originally started out by saying:
“Then please explain why the Earth, when considering the energy it absorbs from the Sun is 33C warmer than it should be.
Why when viewed from space it does (255K).
Yet we live at 288K“
and I’m telling you that a theory that the surface is warmer than the effective temperature because of a GHE is not evidenced by the observation that the surface is warmer than the effective temperature. All that is demonstrated is that the surface is warmer than the effective temperature. But you wrote your comment as if this very observation alone was sufficient to provide evidence that the GHE was the cause.
You have received a comment further down about another possible cause.
Toneb,
The blanket analogy:
That blanket must be more of an afgan, crocheted with open holes in it, rather than a “high tog”. Only about 4% of that blanket can absorb your body heat and “return” it to you. You must also rotate beneath the blanket steadily, and place a blowing air fan under it with you.
Now how warm do you get ? Warm enough to sweat? Does the temperature of your skin even increase?
actually Willis knows exactly what he is saying and there is an article here that perfectly serves as an example of what he explains:
https://wattsupwiththat.com/climate-fail-files/gore-and-bill-nye-fail-at-doing-a-simple-co2-experiment/
think in it as the jar is the atmosphere and the infrared light is the sun.
Seems strange no one has mentioned the effect of gravity on the atmosphere and the resulting adiabatic lapse rate.
“There is NOTHING in physics that says the energy given off from a cooler object must be absorbed by a warmer object. That energy is NEVER added to the warmer object.”
And here we have the “sentient photon” myth.
Tell me, how does a photon emitted from a colder object know that it cannot be absorbed by the hotter object?
“The greenhouse effect does neither, so it is wrong.”
The D-K syndrome rages in some.
You are.
It doesn’t need to “know”. Photons from warmer objects have a different frequency and wavelength to photons from cooler objects. Nothing magical about it.
https://en.m.wikipedia.org/wiki/Photon_energy
Both object absorb/emit LWIR in the same narrow frequency band.
They are not different in that sense.
A thermalisation via atomic vibrational modes occurs.
Else Pyrometers would not work….
“Infrared radiation can have a wavelength of a fraction of a micron up to several hundred microns. Infrared thermometers measure infrared with a wavelength of between 4 and 14 microns. As it is the surface of an object that emits infrared, an infrared thermometer will not measure its internal (core) temperature.”
You’ve written some words. I’m not sure to what end, or who you were responding to, but OK.
How does the energy from a block of ice “know” not to make you warmer?
It doesn’t know squat. It just obeys the laws of physics, as does everything in the universe. You can argue over the why of it as much as you like and come up with whatever theory best fits. As long as you make your theory fit the observation and not the other way around.
So cold doesn’t transfer heat to hot. That is the UNIVERSAL observation. Whatever the theories about what photons are do or “know” needs to fit inside this basic fact.
Atoms and molecules can only absorb photons with certain discreet energy levels.
The incoming photon must be able to boost an atom or molecule into a higher energy state, otherwise it will just keep going.
Photons are not little billiard balls.
According to the theory of relativity, as an object approaches the speed of light, time slows down for that object.
At the speed of light, time would stop.
Photons are going the speed of light.
Does time stop for them?
Are they emitted at the same instant, from the perspective of the photon, as they reach their destination?
Could this explain some of the mysterious behavior we know they exhibit?
If they cannot be absorbed, could it be they are never emitted?
Also, photons are not simple particles…they behave like waves as well.
We know they have a wavelength…but do they also have breadth?
Are they getting wider in the direction of travel, perhaps?
We know photons can become entangled, exhibiting action at a distance.
And other stuff no one really understands.
because we do not have the proper way of thinking about them.
Or there would be no mysteries.
So trying to understand them in some intuitive mechanical way is never going to accurately describe their behavior properly.
“It doesn’t need to “know”. Photons from warmer objects have a different frequency and wavelength to photons from cooler objects. Nothing magical about it.”
The average frequency differs (for blackbody radiation) but to determine the temperature of the emitting object from a single photon does require magic according to present-day physics.
I have a photon with a wavelength of, say, 1.697 micron, will you please tell me the temperature of the emitter?
Toneb
November 25, 2017 at 1:28 am
“There is NOTHING in physics that says the energy given off from a cooler object must be absorbed by a warmer object. That energy is NEVER added to the warmer object.”
And here we have the “sentient photon” myth.
Tell me, how does a photon emitted from a colder object know that it cannot be absorbed by the hotter object?
Anything radiating heat is cooling, cold can’t cool to something hot.
“The average frequency differs (for blackbody radiation) but to determine the temperature of the emitting object from a single photon does require magic according to present-day physics“
Thankfully there is no requirement for the photon to do so.
“The D-K syndrome rages in some.”
And YOU are a prime example. Your baseless ego boosts you FAR beyond what you are ever capable of.
menicholas:
Atoms and molecules can only absorb photons with certain discreet energy levels.
That is true for GHGs, not for black bodies: a black body absorbs every photon of every frequency and sends out photons over a wide Gaussian range, with its peak frequency depending of only its temperature. See Wien’s displacement law:
https://en.wikipedia.org/wiki/Wien's_displacement_law
Toneb, what on Earth makes you think the calculated 255 K temperature should apply to the Earth’s surface, and not the Earth as a whole (which you agree is observed to be 255 K)?
So much anger, so much strawman, and so many errors. So little time in life. I think my children win again.
You’ll get past that anger. It will be OK.
Yes, you have to wonder what some people are on, to confabulate otherwise.
You have all forgotten Maxwell’s Demon, that could easily direct the more excited molecules continually to the warmer object for us. I nominate we call it the Al Gore’s Demon, and there must be myriads of them around doing AGW’s ( Al Gore’s Warming ) work. https://en.wikipedia.org/wiki/Maxwell%27s_demon
Willis says
“Short answer? Of course not, that would violate the Second Law of Thermodynamics … BUT it can leave the hot object warmer than it would be if the cold object weren’t there. Let me explain why this is so.”
If we are talking about TWO objects thermally isolated from the universe the answer is emphatically no.
What you have done is introduced an extra object
“it can do so in the special case when the cold object is hiding an even colder object from view.”
So now we have THREE objects.
Thermal energy in two form of EM radiation will be emitted and absorbed between three objects now instead of two.
The hottest object will also emit higher frequencies than the other two objects so a question of radiation quality is also important.
It is also helpful to consider the matter in terms of insulation
In this case radiative insulation.
If extra insulation(2nd object) is introduced the hottest object(1st object) will lose heat slower than before and so its own temperature will not drop quite so fast.
So if the 1st object is a person they will feel warmer for longer.
You are right to emphasise that heat flow will only occur spontaneously from a higher to a lower temperature.
If someone claims that the cold object heats the warmer object you can be sure they are not physicists.
Actually, it would violate the First Law of thermodynamics.
Part of the problem for a non-technical person is using temperature scales based on the freezing point of water. Sub-zero (or sub 32′) temperatures are assumed to have no “heat” which is not the case. It is sometimes quite difficult to explain that the Arctic in winter is radiating energy in the form of heat into space. The “temperature of space” is 3’K and the Arctic 250’K does not compute for many people.
I’ve probably displayed my lack of understanding here too!
Heck, most people have no understanding that coolness is not a thing.
Only lack of hotness.
Oh I don’t know. I might think your coolness is added upon by your hotness…and not a lack thereof.
Sorry…I needed the humor. 🙂
+++
Well, at least someone recognizes a joke wearing a science fact disguise.
Radiative gases within an atmosphere distort the dry adiabatic lapse rate slope to the warm side which reduces the vigour of upward convection.
The radiative gases therefore settle at a lower warmer height than would otherwise have been the case and radiation to space is greater from that lower warmer height than it otherwise would have been.
Therefore, conductive adjustments occur so as to adjust the amount of radiation to space from radiative gases which causes a neutralising of any surface warming potential.
Willis and those who agree with him would be right if radiation were the only means of energy transmission but it is not. Conduction and convection affect transmission of energy and also effect transformation of energy between KE (heat) and PE (not heat) with the net outcome being long term maintenance of hydrostatic equilibrium within planetary atmospheres.
The mass of an atmosphere (whether radiative or not) conducting and convecting provides insulation which allows the surface to rise above S-B.
The kinetic energy required to achieve such surface warming is from the reconversion of PE to KE in descending columns. That process is missing from the Trenberth diagram used by Willis which is why it has to be compensated for in the diagram by a proposed surface warming effect from DWIR.
That diagram shows sensible heat and latent heat via thermals cooling the surface in ascent but shows nothing for the warming effect in descent.
There is a simple well known observation that helps to choose between the radiative and non radiative surface warming proposals.
Observation shows that for planets with atmospheres in hydrostatic equilibrium the temperature within the atmosphere at the same pressure are similar regardless of vast composition differences adjusted only for distance from the sun.
The radiative theory cannot explain that whereas the conductive/convective theory does.
Stephen
Latent heat transport can only ever occur as part of a process of mass movement. I totally agree with you, it is not a radiative process. What I had never noticed in the Trenberth diagram, until you pointed it out just now, is that there is no downward return vector to balance the upward mass transport of air that occurred during precipitation. To balance the movement cycle downward motion must be present and clearly include both the cold rainfall as well as the falling dry air heated by adiabatic compression. And no, these two thermal processes (cold falling rain and warmed falling air) do not cancel out, there is a net heating of the atmosphere by latent heat of condensation as the phenomenon for the Chinook wind proves.
“What I had never noticed in the Trenberth diagram, until you pointed it out just now, is that there is no downward return vector to balance the upward mass transport of air that occurred during precipitation.”
It is always that way, and there are two explanations:
1. It is very difficult to measure.
2. If it was included it would make it obvious that the LWIR part of the process (including “back radiation”) is a quite small part of the whole heat exchange process in the atmosphere. Notice that the “convection arrows” are always shown as quite puny and discontinuous)
Not puny.
All the PE required to keep the entire mass of the atmosphere off the surface against gravity is involved.
That PE reservoir is the source of additional kinetic energy at the surface providing the observed surface temperature enhancement above S-B.
Philip,
You say: What I had never noticed in the Trenberth diagram, until you pointed it out just now, is that there is no downward return vector to balance the upward mass transport of air that occurred during precipitation.
That is because you were to aware that the two non-radiative energy transfers from the surface, for sensible heat (22W/m^2) and for latent heat (76W/m^2), are the net flows of energy due to these two phenomena. This is explained in the original paper that accompanied the original Trenberth diagram (I don’t know where Willis got his diagram from but the figures are in more or less the same ballpark as Trenberth’s and are definitely net figures.)
Please substantiate that.
Even Willis accepts that the net effect of convective overturning is zero so you cannot assert that the Trenberth diagram represents a net outturn.
Trenberth just erroneously includes the upward leg alone (leaving out the downward leg) and compensates for that omission by incorrectly asserting a surface warming effect from DWIR.
I have told you why and how convection changes to adjust the degree of radiative loss from GHGs to space so as to avoid destroying atmospheric hydrostatic equilibrium by warming the surface.
“Even Willis accepts that the net effect of convective overturning is zero so you cannot assert that the Trenberth diagram represents a net outturn.”
If Willis accepts that he is dead wrong. The reason is that convection carries molecules up so high that a large part of the latent heat freed by condensation/freezing radiates out into space and leaves the system. The same is true for part of the “dry” radiative cooling of the convected air. The entire mass, air, rain and snow ultimately comes back down, but it has always lost some energy on the way. The proportion lost is not large, but it is considerably larger than the energy lost by LWIR radiation.
The Earths atmosphere is a heat engine, but like all heat engines it is not perfect, it constantly loses heat to the surrounding environment.
tty
You are mixing up the radiative energy exchange with the non radiative energy exchange.
My point relates only to the adiabatic portion of the energy flow which is non radiative and fully reversible so myself and Willis are correct that convective overturning constitutes a net zero energy flow.
Where I differ from Willis is in pointing out that it was non zero during the first convective overturning cycle when the atmosphere was formed.
It is that non zero component that enables the entire mass of an atmosphere to participate in the warming of the surface above S-B.
Even if GHGs do work as proposed the mere fact that the entire mass of an atmosphere is involved renders GHGs insignificant but as I pointed out above the GHGs distort the lapse rate slope so as to radiate more effectively to space from a lower warmer level instead of warming the surface.
“You are mixing up the radiative energy exchange with the non radiative energy exchange.”
Not me, nature.
They are jumbled up in Nature but they are still discrete processes and operate as a counterpoint to each other in the way that I described.
Otherwise no long term hydrostatic equilibrium such that planets with any radiative component in their atmospheres (whether gaseous or particulate) could not retain those atmospheres.
Stephen Wilde November 25, 2017 at 2:39 am:
This may be irrelevant to your argument, Stephen, but I feel I should point out for the sake of clarity that your distinction between “heat” and “not heat” appears false to me.
“Heat” is not simply KE. In physics and thermodynamics, the heat content (a.k.a. enthalpy) of a body is defined as the sum of the KE and the PE contained by the particles that make up the body.
However, the body’s absolute temperature is simply a function of the average KE of its constituent particles.
Hence, the molecules of a block of ice at 0°C possess the same average kinetic energy as those in the equivalent amount of water at 0°C, but they contain far less heat and thus, far less potential energy too.
I don’t think the phenomenon you describe is as well-observed as you may think it has been. The data currently available to us about the temperature/pressure/chemical composition profiles of other planets’ atmospheres are still very scanty and uncertain. I think we would need to be much more certain of the reality of this alleged phenomenon than we are before we could use it as a test-criterion for the comparison of radiative versus conductive/convective theories. We might reach that degree of scientific enlightenment in a few centuries, perhaps, if we get lucky, but we’re nowhere near it now.
KE alone is heat. KE plus PE is energy so your point is wrong.
Many solar system bodies have had their surface temperatures measured and they are all very close to that predicted from the gas laws alone so that point is wrong too.
Stephen Wilde November 25, 2017 at 10:46 am:
My point is not wrong. Internal KE alone is also energy and that determines the body’s absolute temperature. PE is also energy and must be included in any calculation of the body’s total heat content.
Look, the fundamental definition of the “heat content” (a.k.a. “enthalpy” – symbol H) of a body is that of the “internal energy” (symbol – U) of its constituent particles plus the product of its pressure (symbol – p) and its volume (symbol – V). We may write this as:
H = U + pV
(See: https://www.thefreedictionary.com/enthalpy )
In this formula, the term U (internal energy) represents both the average kinetic energy (KE – determined by internal speeds of motion) and the average potential energy (PE – determined by the strengths of internal electromagnetic fields) of the constituent particles, so it is unavoidable that there is some PE in the heat content.
Then we have the pV expression, which represents the work that the body’s environment has had to do on it to bring it to the state that it’s in. That also adds directly to the PE of the body’s constituent particles (and indirectly to their average KE by increasing their average PE).
So the heat content of a body does contain both KE and PE components. QED.
Really? How many solar system bodies would that be exactly, Stephen? How accurate are the measurements of their global mean temperature and pressure profiles? And how many of those have greenhouse atmospheres? How many do not?
And finally, where are you getting your planetary data from? I hope it’s not some national, or multinational government agency like NASA or ESA, because their data is notoriously unreliable and untrustworthy for serious scientific purposes. It’s completely uncheckable, you see.
You’ve introduced one of Trenberth’s looney Earth’s Energy Budget Diagrams, Willis. According to Trenberth you are only getting an average of 169 w/sq.m of solar radiation impinging on the Earth’s surface, (you know, in certain places, melting the tar on roads in summer) ,but now you’ve also got 321 w/sq.m of this “backradiation” belting down from the atmosphere 24/7.
Is your hometown Loonyville, Willis? The people of Loonyville leave their bacon and eggs out on the porch overnight to have them cooked by the morning from the 321w/sq.m atmospheric backradiation.
I originally started to develope this issue when I read some posting purporting to prove the Stefan-Boltzmann law “wrong” based on lunar temperatures. You might find this link, regarding Newton’s law of cooling, of interest.
http://www.ugrad.math.ubc.ca/coursedoc/math100/notes/diffeqs/cool.html
The law gives this equation:
T(t) = Ta + (T0 -Ta)*1/(e^kt)
Where T(t) gives Temperature, T, as a function of time, t,
Ta is ambient background temperature, and T0 is the starting temperature of the body warming up or cooling off.
mass atmosphere = 5* 10^18 kg=5*10^21gm
temp atmosphere 255K (effective radiating temp to space- underestimates heat content)
specific heat 1.01 joules/gm C
5* 10^21*1.01*255= 1.288 * 10^24 joules
radius earth = 6400km= 6.4*10^6 meters.
area earth = 4 pi r^2 =514,718,540,364,021.76
240 watts/sq meter = 240 joules/sec per square meter
60 sec/min*60 min/hr*24hr/day=86,400 secs per day
5.147* 10^14 sq meters*240 joules/sec/sq meter *8.64*10^4 secs/day= 1.067*10^22 joules per day radiated away
1.067*10^22/1.288*10^24 = 0.83%
So the daily loss of heat of the atmosphere is less than 1% per day. That makes sense when you realized that although
temperatures may swing by 20 degrees K or more during the 24 hour day/night cycle, meteorologists are still able to make fairly accurate estimates of daily highs and lows for about a week- because of that temperature stability.
The above is to show that it is reasonable to assume a constant long wave
flux from the atmosphere over the course of a day.
We get an AVERAGE of 342 watts per day from the sun over earth’s surface. That works out to an average of 648 watts during the day and zero during the night.
You may have noticed that average temperatures do not increase to anywhere NEAR (648/390.7)^0.25 *288 K = 327 K during the day, and do not cool to anywhere NEAR 0 K during the night. That’s partly because MOST of the sun’s radiation gets absorbed by the atmosphere, which helps to moderate day/night temperatures. That 168 watts directly from the sun, as opposed from the sun to the atmosphere to earth’s surface, is entirely plausible.
“That’s partly because MOST of the sun’s radiation gets absorbed by the atmosphere”
But it is MOSTLY because of the oceans. Compare the diurnal temperature variation on Earth, Mars and the Moon. Which is the odd man out?
Or even compare the diurnal temperature swings in e. g. Central Asia and Polynesia.
True, thanks to the high specific heat of water, oceans act as a moderating influence, but my argument was that Trenberth’s hypothesis that most of the sun’s direct energy goes directly into the atmosphere still stands; else, how do you account for the ability of meteorologists to fairly accurately predict the weather for roughly a week in advance?
Mack,
You are perpetrating the common howler of failing to offset the 321W/m^2 back radiation figure against the 392W/m^2 forward radiation figure to yield a net upward flow (i.e. from surface to atmosphere) of 71W/m^2. Those two numbers cannot be cherry-picked independently.
Adding that true upward 71W/m^2 flow to the 22W/m^2 upward sensible heat flow and the 76W/m^2 upward latent heat flow gives a total upward energy flow of 169W/m^2 which EXACTLY matches the 169W/m^2 of Solar energy flowing down into the surface. As it must, for steady state temperatures to prevail!
For more details, please refer to my post of November 25, 2017 at 9:35 am.
It would be interesting if someone could resurrect the figure of how much the net 71W/m^2 is reduced by the molecules of CO2 released by man.
Well David,
You are going to have to explain this “common howler” to Dr Roy Spencer, because he is in doubt as to whether or not those energy flows even exist. He’s marked them with a big “X” here….
http://www.drroyspencer.com/2015/06/what-causes-the-greenhouse-effect/
Quoting Roy…”In the classical KT global energy budget diagram, the energy flows I have marked with an “X” would not exist without GHGs.”
I would like to say to the contrary, that, if those energy flows didn’t exist, then GHGs would not exist.
Roy goes on to say…”Now, recall I said temperature is a function of rates of energy gain and energy loss. Thus those energy flow arrows marked with an “X” in the above diagram represent huge flows of energy which can affect temperature, IF THEY REALLY EXIST”
So do they exist or do they not exist, David?
Also, I find it amazing the ever changing and extreme range variety of all these watts per sq.meters being bandied about by everybody…no actual measurements, just numbers seemingly out of thin air.
My guess is that the energy numbers marked with an “X” were pulled from the kiwi troughers ass.
Mack,
On November 25, 2017 at 10:08 pm you say: Well David,
You are going to have to explain this “common howler” to Dr Roy Spencer, because he is in doubt as to whether or not those energy flows even exist.”
I have no intention of doing anything of the sort. I put you right on an elementary conceptual error (which all too many people make) but apparently that has squashed your pride. The downward radiation must be subtracted from the upward radiation to get the net figure representing the ACTUAL flow of radiative energy, which is always from hotter to cooler body (surface to atmosphere). I even referred you to my earlier post of November 25, 2017 at 9:35 am for the rationale, which is based on standard textbook physics. And all you can do is to ignore the math and ignore the physics, rather than benefitting from the explanation.
However despite your obvious interest in grandstanding (“I would like to say to the contrary, that, if those energy flows didn’t exist, then GHGs would not exist.” etc. etc.), I will nevertheless indulge you by stating the following:
The 321W/m^2 downward radiation and the opposed 392W/m^2 upward radiation cannot be treated separately because they cannot be independently employed to do separate work. They are simultaneous and opposite – so only the net residual radiation, which is 61W/m^2 upwards, is capable of doing work. This is logically obvious when you think about it. Otherwise there would be an absurd imbalance at the earth-atmosphere interface. Which there isn’t. Otherwise the earth would have melted. Which it hasn’t.
Or, of course, you might be right and the diagram is wrong, in which case you have just discovered the most embarrassing scientific howler of all time.
Please just get over the fact that you (along with many other people, if that makes you feel better) have made an elementary mistake. If you can’t face up to that, you will for ever wander around in the wilderness of climate bloggery, blaming everyone else around you (me, Roy Spencer, Uncle Tom Cobley and all…) as you blunder on and on.
Or, for a change, you could think a bit and learn something new.
It’s your choice, mate!
“You’ve introduced one of Trenberth’s looney Earth’s Energy Budget Diagrams, Willis. According to Trenberth you are only getting an average of 169 w/sq.m of solar radiation impinging on the Earth’s surface, (you know, in certain places, melting the tar on roads in summer) ,but now you’ve also got 321 w/sq.m of this “backradiation” belting down from the atmosphere 24/7.”
It’s two-way.
Chicken and egg.
With there is 321 from GHE there is also that added to 169 solar to emit 490 from the surface >>>> which is then partially backradiated at 321 W/m2 and so on.
From Trenberth’s Energy budget diagram
Energy out:
22 + 76 + 392 = 490
Energy in:
169 + 321 = 490
The 321 is back-radiated having first being thermalised by the 169 SW absorbed AND the 392 added from the LWIR GHE.
Hi Toneb,
You cherry-picked your numbers and got the wrong answer. Don’t be embarrassed, it’s a common mistake. I have laid out the correct math, which balances perfectly with the incoming solar absorbed at the surface, a few items up-thread in my first reply to Mack (David Cosserat November 25, 2017 at 10:08 am).
Cheers,
David
Hi Toneb,
My profoundest apologies.
You are on the side of the angels. I should have read your previous post much more carefully. Of course you were quoting Mack’s misconceptions, not your own, and putting him right…
Best,
David C
Yes it can, with help. A reverse refrigerator. Air conditioning in a cold climate. All you need is a heat pump. What we will rely on for elctrical heating during the next ice age, hopefully all nuclear powered by then. Heat is removed from the colder body, making it colder, that heat is transferred to the hot body by the pump, making it warmer. First done in Glsagow taking heat out of the water supply, I don’t see that mentioned any more, just from ground or air, as in “sourced”. Performance factors are up to at round 4 as regards heat shifted to power in, Rather important given the cost of electrical energy for heating versus gas, about 4 times in the UK. Partly because gas heating is >90% efficient now but power stations energy conversion to electricity is a lot less, 60% at best for closed cycle gas turbine, 40% for the open cycle. Must dash….
brianrlcatt
Once you introduce a powered heat pump you are relying on convection and the gas laws rather than radiation so you simply confirm my point.
With a man made heat pump a separate source of electrical power is required.
For an atmosphere above an irradiated surface the necessary power is provided by gas parcels of different energy contents and densities working with and against the gravitational field as they each seek a height commensurate with their respective weights and energies.
The process nets out to zero energy consumption overall but for Earth that still requires an ‘extra’ kinetic energy worth 33C (or whatever) at the surface to drive the global pattern of convective overturning. Much more KE at the surface of Venus is required to drive convective overturning in such a heavy atmosphere.
And so it is with all planets with atmospheres.
Willis discounts convective overturning as a reason for the surface temperature enhancement on the basis that being a zero sum process it cannot add extra energy.
In fact it is only a zero sum process once hydrostatic equilibrium is achieved. The process of creating a convective overturning cycle is not a zero sum process. Rather it draws surface KE into atmospheric PE in preference to that surface KE being radiated to space and thereafter recycles that block of energy up and down indefinitely. It is an insulating process because conduction and convection transfer energy slower than radiation.
The downward leg of convection then heats the surface in addition to continuing insolation.
Even a wholly non radiative atmosphere will develop convective overturning due to uneven surface heating leading to density differentials across the surface so those who say that such an atmosphere would become isothermal are wrong.
Yes, headline should include ‘spontaneously’ but most people assumed that anyway.
A body in vacuum emits from the surface at some temperature. Add a cold fluid which gets heated by the body. Now, the same energy that was emitted by only the twodimensional surface, is shared with the volume of fluid. So, now we have both a surface emitting and a threedimensional cold fluid also emitting. But the energy supply from the source is constant, so emission from both fluid and solid body is sharing the constant limited heat flow from the source. In no way, this is a situation where the solid gets warmer.
The heat source has constant limited power(sun). Temperature is a measure of average kinetic energy per molecule. How can addition of cold fluid mass, which absorbs energy from the heat flow in a larger volume of molecules, result in higher average kinetic energy per molecule, than a smaller volume with less molecules at higher temperature?
It is not right. Just like adding water to a pot on a boiler plate makes the pot colder, adding a cold fluid to a warm solid body in space, makes the body colder.
The atmosphere is a heat sink, added to the ultimate heat sink of space.
Without the shell of the atmospheric cold fluid, the surface T would be:
(TSI/2pi*r^2)/4/3pi*r^3=510W/m^2=308K
With the shell of cold fluid:
(TSI/2pi*r^2)/(4/3pi*r^3)^2=383W/m^2=287K
Adding a heat absorbing cold fluid to a hot body cools it, only.
Prevost made the conclusion that the emission of a body depends on the internal state only. The atmosphere is definately not part of the solid surface internal state.
No.
(I don’t think I can constructively add anything to that. Just No.)
Aww shit! Good argument. I loose.
Keep denying the calculations, having faith in blankets and unicorns in greenhouses is your melody.
So Prevost was wrong then?
The emission of a body depends on the external state?
Come on, deny more of proven and applied physics. It´s fun to read.
In the history of this blog, there has never been a simpler, clearer, or more scientifically correct post; yet, we have all this irrelevant nonsense. Get a grip people.
Bless you, my son!
I agree. Everyone knows that dry ice, water and cold fluids in general, makes its heat source create more energy from nothing.
lifeisthermal:
Let’s say you’ve been outside on a cold winter’s day (255K, -18C, 0F) without heavy enough clothing. You have gotten hypothermia and your body temperature has dropped to 35K.
I offer to let you come into my house at 293K (20C, 68F), saying it will let you recover your body temperature up to 37C. By your logic, you would turn me down, because this is still colder than your body temperature, and it couldn’t make your internal “heat source create more energy from nothing.”
Seriously?
You describe a situation where a change in surrounding temperature results in less absorption of heat from my body. If I move to a surrounding environment where there is higher temperature, the surroundings will absorb less heat from my body. I will transfer less heat to the surroundings.
In what way does this support a claim where a cold atmosphere and increasing amounts of heat absorbing dry ice, causes increased temperature?
The principle of the gh-blanket is that increased heat absorption from the solid earth causes increasing output of power from earth surface. This is the exact opposite of your example.
The solid earth, is supplied with energy from solar heating. Without an atmosphere, it only has to heat itself. Adding a cold fluid which is heated exclusively by the solid body, not the sun, means that earth has to heat the atmosphere, in addition to the solid, simultaneously.
Earth provides the energy, from solar heating, for both surface emission and atmospheric emission, at the same time.
This is an inevitable consequence of continous heat emission from both bodies, cold fluid and warm solid. The sun heats the solid earth, which emits and transfers heat to the atmosphere. 4*244+383=1361W/m^2=TSI.
lifeisthermal:
You agree that moving from an ambient of 255K to 293K will result in lower thermal losses from your body, permitting the thermalization of your metabolic power to increase your body temperature. This is true even though the ambient temperature of 293K is less than your (reduced) body temperature of 308K.
By direct analogy, “moving” the earth’s surface from an ambient of 3K (which it would have with a transparent atmosphere) to one of, say, 255K, will permit the earth surface’s thermalization of solar radiation to increase its temperature. This is true even though the ambient temperature of 255K is less than the surface temperature.
The two examples are directly comparable, not opposite!
A transparent atmosphere cannot transfer energy to space, because it has no emissions. It does not require any continuous power input from the surface to maintain its temperature.
Can a current of photons travel in the opposite direction to another current of photons of greater intensity? I do not think so and infrared thermometers too.
Yes they can.
What, do you think they *collide* with each other?
And infrared thermometers too what?…
I remember helping out a presenter at public slide show. He had two projectors, and the audience was sort of in the way given how he left projector aimed at the left screen and right to right. I switched the two projectors so the beams crisscrossed which avoided hitting the audience. Worked fine, of course.
While there are photon-photon interactions, it takes an “astronomically” high flux to do anything measurable. I think it has been observed near supernovae.
As for infrared thermometers, some cold clear night this winter take yours outside and aim it directly upwards. It’s not seeing a black body, so the reading won’t reflect the true sky temperature, but whatever it sees will be a steady flux. However, it’s also seeing IR flux from the body of the thermometer and from the polyethylene lens, it uses an internal thermometer to compensate for that “noise.” The lens will cool quickly in the cold air and its flux will go down. The thermometer will reflect that by showing the sky temperature going down.
You may need an IR thermometer that will read low enough, I have a Kintrex IRT0421. Some people have found they can map the sky temperature to the water vapor content of the air column. Good tool.
An IR-thermometer works by measuring a gradient across a thermopile or thermocouple. It only measures the gradient inside itself. Then it extends that gradient into the surroundings with the s-b equation, by calculating the rate of transfer to different parts of the surroundings. It can only calculate the rate of transfer, from T1-T2. So, in the case of colder surroundings, it only measures transfer FROM the device. Because T2 is smaller, and the value is negative. Which will show as darker on the screen.
The manual will tell you this: that in the case of measuring cold atmosphere, the transfer to the device, the incoming flux, is negative. Which means: there is no incoming flux. The manual also says that the negative influx of heat, the non-existent incoming flux, is used to calculate a fictive incoming flux based on what temperature the receiver of heat transfer has, calculated by outgoing transfer of heat.
What this means is: the non-existing incoming flux, is imagined from the outgoing transfer and the device shows it as positive, even though it is not there. Because it uses the s-b equation and it can only calculate net-transfer. “Net” is the only transfer there is, because “net” is heat. And transfer of energy between bodies of different temperatures is heat. And there is no other transfer which can be calculated without breaking the 2nd law of T.
Another interesting fact: a thermocouple/IR-thermometer only have a range of some 25m into the atmosphere. So it doesn´t give much information about heat transfer in the earth system.
Optical measurement on the other hand, shows spectral distribution. But the problem is: they only show how heat flow decreases from the co2, by a very distinct “bite” which decreases intensity of emission in those wavelengths.
I’m sure the manual told me no such thing. If it did, my eyes would have bugged out, my pulse quickened, and I’d be on an epic rant.
Then my thermometer would display only one value from source colder than it.
Here’s the manual, https://www.homedepot.com/catalog/pdfImages/ef/effd4a5b-8452-4095-98ce-39265a0adde2.pdf . It’s disappointingly brief, and never mentions flux.
Willis,
I probably flatter myself if I consider that I am the gadfly the prompted this post, however as I always want to learn you will have to indulge me while I go through the following observations and resulting questions.
From first principles Science is the process of rejecting false ideas. So I will start by making statements which I hope we can easily identify as being false.
1. All forms of matter absorb (that is extinguish) all forms of light (electromagnetic radiation).
Trivially easy to prove false, because this statement denies the existence of transparent materials and also denies the existence of short frequency gamma rays and long frequency radio waves that can pass unchanged through material bodies of a specific composition. (Notice that already the caveats are creeping in), so matter in its various forms acts as a selective filter of electromagnetic radiation.
2. Absorption is the only process by which light interacts with matter. Again trivially easy to prove false because this statement denies the existence of reflection and refraction and not to mention the already discarded contention of no transmission.
3. Colour is a thermal process. Hum, now I am already getting into trouble because do I mean the emission of a specific wavelength from a hot object or the reflection of a non-absorbed wavelength from the surface of a cold body? Just for good measure what is meant here by hot and cold? Are we talking about the average kinetic property of a bulk material object or the specific translational momentum of an individual molecule, which supposedly has no temperature because temperature is a statistical measure of bulk particle motion, vibration, flexure, translation, rotation and not to even consider electron shell interactions of various kinds? (And no I don’t like the statement that an individual molecule does not have temperature because we measure the energy of translation of individual thermal particles using electron Volts).
Now for some questions:-
1. So what is colour? (Sorry dear Cos, I will spell that word the ol’ way). Let us start with the colour of an object perceived using reflected light. What is the process by which selective reflection occurs? Is it a process of absorption and immediate readmission or a boundary property of a material in which a process (perhaps similar to liquid surface iridescence?) occurs in a solid state material? Oh and just for good measure don’t forget that that liquids can also have colour, such as red-brown bromine or blue liquid oxygen, so is it that colour with reflected light is an electron shell process?
2. And what is black? We perceive carbon as being black and therefore having no colour, but this is wrong, carbon does have a colour, it is coloured infra-red, it is simply that we cannot see these reflected wavelengths and so describe carbon material as having no colour.
3. And then we come to the subject of absorption spectra. Helium was first discovered in the coronal gases of the sun because the astronomer Sir Norman Lockyer observed a unique yellow absorption band, evidence of an unknown element that selectively removed this specific wavelength from sunlight thereby dimming that wavelength when viewed from Earth. Is this not a similar dimming effect to that we observe with infra-red absorption spectra?
4. And what about the coloured light emitted by the discharge in a sodium light for example? Is this light the same process of light as I see reflected off the yellow walls of my bathroom? Well no. One colour comes from emission from a hot source and the other from selective reflection from a cold surface, so there are clearly different processes at work here that seem to me to be discounted at infra-red wavelengths.
So the key question for me is this; -How do we know that the selective infra-red frequencies that are blocked by the atmosphere are absorbed and therefore thermalise the absorbing gas and none of these infra-red wavelengths are reflected, in a perhaps recoilless process (or perhaps not i.e. radiation pressure?) that if so happened, might presumably “colour” the gas but have no thermal effect?
You Point 1, that is where the MIT Light bulb is wrongly classified, the IR screen does not absorb and then re-radiate the photon it reflects it. In other words they have found an Infra Red Mirror that allows white light to pass through, which is very clever physics indeed.
Thank you AC Osborn.
It has now occurred to me that because frequency is a conserved property, while wavelength varies with the refractive index of the transmission medium, I should not use wavelength to describe EM radiation.
Your style is very familiar. After a bit, I realised who it reminded me of – AE Van Vogt, a science fiction writer famous for making up his science off the top of his head, from ideas he got in his dreams.
To answer your question – yes, it might be reflected instead of re-emitted (although I don’t really think this is a controversy; there has been quite a bit of research done on the subject). But that makes no difference. We’re talking about back-radiation; radiation sent *back* towards the Earth from the atmosphere. Reflected, re-emitted, it still gets sent back.
And don’t tell me it’s the wrong wavelength or “colour” or whatever. It’s infra-red, and practically everything absorbs it to some extent, even objects that are slivered or painted white.
Well if “practically everything absorbs it” I wonder how it gets all the way through the atmosphere to impact the earth’s Surface from 100K away?
So you do not believe that “Wavelength” makes any difference?
OK.
I agree with Willis’s article, but I have an English quibble. The expression “than me” should be “than I.” “Than” is a subordinating conjunction, followed by a clause with its own unstated subject and verb. “He is taller than me” should really read “He is taller than I am.” The verb “am” is understood. “Than” is not a preposition, as “near” is; “near I” is obviously incorrect, and “near me” is correct, because “me” is the object of the preposition and has no following understood verb. “Than” can, indeed, be followed by “me” because “me” can be used as an object: “He likes Jenny better than [he likes] me.” On the other hand, “I” can be the correct pronoun if the sense is “He likes Jenny better than I [like her].”
John ==> eGads! that’s what my high school English teacher said after my speech in front of the whole student body accepting the office of Student Body Vice-President! She said it, of course, in front of the whole assembly — couldn’t help herself.
(I have, somewhat reluctantly, finally forgiven her after fifty some-years)
I’m faster than he. Oh no, I never could utter that. Germans might use als er, but Swedes could use än honom, ‘than him’. I’m not sure why I use than at least sometimes as a certain kind of preposition. In my own language, you can say something like I am her faster, or I am faster than (s)he. The logic probably is the same, it’s just that who said and why ‘than’ could not be a preposition requiring me/him/her.
Usually speakers are right, even if they are wrong. Than is by its etymology a conjunction, relative to when and then, so that would mean than I is historically correct. But what’s the ‘än’ though?
Color me confused.
There seems to be a general agreement on that there are 4 CO2 molecules in the atmosphere for every 10.000 other molecules.
Still, these 4 molecules are supposedly able to warm 10.000 of a different kind ?
By what mecanism, exactly ?
Sounds like a Perpetuum Mobile, to me.
They generally don’t, they generally reemit an IR photon of the same energy. However, if they collide with any other air molecule first, the vibrations between the CO2 atoms (that holds the energy from the photon) will be partially transferred as kinetic energy due to pushing both molecules away, and that means the temperature goes up. Over the next small fraction of a second subsequent collisions will heat up other molecules, restoring the bell curve shape of individual molecule’s kinetic energy.
Or so I understand, sorry I don’t have numbers and probabilities handy.
Ric, if you look up the mean time between collisions and the average emission time I think you’ll find it to be about 10,000 collisions per emission. So the small amount of CO2 in the atmosphere equilibrates with the atmospheric temperature
I defer To Dave Burton’s conversation with William Happer, https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/#comment-2676749
It says there are about 1 billion collisions per emission!
That means LWIR emitted by the Earth’s surface, winds up heating nearby air (and itself) as the mean free path of the photons is quite short. So that energy stands a good chance of going back to the ground, trees, whatever. And it implies that the radiant energy that escapes the surface is from wavelengths not blocked by CO2 or H2O. Something I get reminded of every morning I scrape frost off my car.
“By what mechanism, exactly ?”
You need to consider a photons path-length to space.
By the time it gets to ~ 8km more can escape to space than be back-radiated.
Shine a torch into a thin mist.
The beam will be attenuated at a distance.
Shine a torch in fog.
The beam will be attenuated more quickly.
Yes more fog droplets.
But also accumulated collisions because of the path.
Which is why there is no lab experiment that can duplicate the GHE in Earth’s atmosphere.
The photons come back … more fog-droplet collisions – then the brighter the back-radiated (refected – same concept) the beam.
By that mechanism.
You give an example of how radiation is reduced by mist and fog.
In what way is this a support of the gh-effect causing increasing levels of radiation?
“The photons come back”
WTF!
You are not shy at all!
Absorbed photons come back?
Lets talk about how you like to make things up.
Absorption of photons destroys the photon.
You have no clue.
For commenters who offer the argument “warm objects instantaneously reject IR from colder objects, please consider that you have defined a reflection.
The logical end point of this fallacy is that albedo is a function of energy content.
There’s a noble prize waiting for you if you can prove that one.
So Laser Directing mirrors get as hot as the Laser Beam?
Or are already hotter than the beam?
Clouds are “White Hot”, Ice is also “White Hot” and both hotter than Sunlight.
In your dreams mate.
Huh?
“The logical end point of this fallacy is that albedo is a function of energy econtent.”
First, albedo is necessarily a consequence of geometry. Irradiation on a disc, distributed over a hemisphere, absorbed in a volume, is enough to reduce the power density to 0.7i5.
Obviously, the temperature will determine the amount of ice, snow, sand, and water surfaces.
So, yes, albedo is a function of energy content. Measured as emissive power, T^4.
But geometry is the main constraint-
Thank you, Willis Eschenbach!
You’ve left out the fact that the Greenhouse Effect ALSO works during the daytime. (In fact, according to that diagram, it delivers more radiant energy to the surface than the Sun does directly, since a whopping 105 W/m2 of sunlight is reflected by the Earth and clouds.)
It’s a subject that’s insanely complicated in detail, but almost moronically simple in general terms. All you need is the ability to visualise, and and a bit of faith that scientists from more than a century ago (who never even heard of Global Warming!) are NOT trying to con you.
Love it “Faith Based Science”.
The world was flat and the Sun and stars revolved around the Earth, do you still believe it?
Ulcers were caused by stomach Acid, still believe it? Never heard of Helicobacter pylori?
Do you believe in the Standard Model, the Big Bang, Dark Matter & Dark Energy, with no proof and no actual evidence?
Because their are plenty of Scientists that don’t and have Theories just as good to prove it.
http://earthsky.org/space/erik-verlinde-gravity-theory-no-need-dark-matter
http://www.dailymail.co.uk/sciencetech/article-5109023/Dark-energy-dark-matter-NOT-exist-study-says.html
You seem to be suggesting that Scientists are never wrong.
Willis, thank you for writing this, I’ve wanted a decent post on the subject here to reference in the future. I apologize for not having written it myself years ago. You did a better job than I would have.
I guess we have nearly all the possible objections (and explanations) to what you describe. That could be a feature in its own right!
All right, I’ll take some time on this one.
I never said that scientists were never wrong, I said that these particular scientists are not trying to con us from political motives connected with the Global Warming controversy (mostly because they were all dead long before it started…).
If you think someone is trying to put one over on you, you have to pick up on every detail. Otherwise, a certain amount of faith is necessary for the maintenance of sanity. Massive objects attract each other. That’s a simple principle put forward by Sir Isaac Newton, and we trust it. It might suit us to believe instead that gravity is caused by the Flying Spaghetti Monster with His Noodly Appendages, but why should we? There’s nothing glaringly wrong with Newtonian gravitation, and even Einstein only modified it slightly with his Special Relativity.
There’s nothing glaringly wrong with the Greenhouse Effect. “It contradicts the Second Law of Thermodynamics” only works if you don’t understand radiative thermodynamics (or think that other people won’t, and hope to make a name for yourself through an invented controversy). If you’ve understood the basic underlying concepts, you can visualise the phenomenon in general terms, and it makes sense. If it doesn’t make sense to you, it’s because you’re introducing some rubbish such as “two lots of photons can’t travel in opposite directions at the same time”. (Yes, they can.)
Climate scepticism, on the other hand, is a different sort of thing entirely. When it’s not purely and simply political, it’s based on established science and attacks the *conclusions* and the *methods* of the warmers, not their scientific principles. (Their *moral* principles, now that’s another thing…)
I don’t need to start believing nonsense, just to maintain my street-cred as a sceptic.
Uncle Gus,
You say: …according to that diagram, it delivers more radiant energy to the surface than the Sun does directly
No it does NOT! The energy flow from the Sun that is absorbed by the surface is 169W/m^2. The energy flow from the surface to towards the atmosphere is exactly the same: 169W/m^2. The figures balance perfectly.
Take a closer look at the diagram…
Net upward sensible heat: 22W/m^2
Net upward latent heat: 76W/m^2
Net upward radiation: 392 – 321 = 71W/m^2
TOTAL: 169W/m^2
The mistake you have made is to look only at the 321W/m^2 downward component of radiation from the atmosphere and ignored the 392W/m^2 upward radiation from the surface. These two numbers cannot be dealt with separately. They are inextricably interlinked. You can’t have one without the other. You have cherry picked!!
For more details, please refer to my post of November 25, 2017 at 9:35 am.
“The energy flow from the Sun that is absorbed by the surface is 169W/m^2. The energy flow from the surface to towards the atmosphere is exactly the same: 169W/m^2.”
You are claiming that a surface at 288K is emitting only 169W/m^2.
Do you pee your pants on a daily basis as well?
Quoting Uncle Gus……”according to that diagram,it delivers more radiant energy to the surface than the sun does directly”.
No it does NOT! says David Cosserat..
Well, the diagram actually says this radiant energy from the “backradiation” is ABSORBED BY THE SURFACE.,David. So in Trenberth’s mind it is real and exists. It should cook bacon and eggs on the porch if left there overnight.
Incidently, Roy Spencer has doubts about the existance of these energy flows….
http://www.drroyspencer.com/2015/06/what-causes-the-greenhouse-effect/
Actually, you’re right.
I was considering only the downward radiation, and ignoring the upward. In total, they will balance exactly in the long run – they have to, or the earth would quickly burn up or freeze.
And of course, all heating (except a minute amount of geothermal) comes from the Sun.
But the diagram does seem to imply that the Earth’s surface is heated more by IR from the lower atmosphere than by combined IR and visible light directly from the Sun. That is some efficient blanket!
To be honest, I don’t know how accurate that or any of the other analyses of radiative heat transfer in the atmosphere are, or indeed can be. I suspect that without a lot more direct measurement, all we can get is a sort of rule-of-thumb sketch. I’m sort of assuming we have that much because, well, otherwise we might as well all go down the pub…
Willis, I am back with some more dumb layman questions.
You said that we can measure the LWIR coming from the CO2 in the Sky.
How do we do that?
How does the Measuring Device differentiate the CO2 from the rest of the Atmosphere between it and the CO2?
And how does it know it is LWIR?
We know it because we have tested the other atmospheric gases and know that only CO2 and Water will emit LWIR. So we measure the humidity calculate emissions from it and the rest is from CO2. As to how we know it’s LWIR you’d need to read the technical details for a given measurement device.
Actually all gases with more than two atoms in the molecule are GHG, and two-atom gases are too as a matter of fact, but only very weakly.
You miss the point completely.
You take a Sky Measurement, you get a Value, what is the value?, how do you know where it originates from?, and from what?
How do you measure the Humidity all the way up to the top of where it is with the measuring device that measures the Temperature?
The short answer, A C, is that satellites have sensors to detect whatever we want tracked.
No Satellites measure outgoing radiation, which shows more CO2 causes more atmospheric cooling.
They can’t measure Down Welling as it pointed away from the Satellite.
A C Osborn,
Some time ago there was a post at WUWT where two stations (Oklahoma and Barrow) measured the increase in backradiation caused by CO2 over the period 2000-2010, here a direct link to the article:
http://newscenter.lbl.gov/2015/02/25/co2-greenhouse-effect-increase/
Measured increase: 0.3 W/m2 for an increase of about 20 ppmv CO2.
Can a child shoot an adult?
Its not the cold object that does the warming, it is the energy it releases.
Wish we could “Like” on this page!
Willis: Very lucid explanation of a fundamental concept. When I engage in a discussion with someone, I have learned to quickly ferret out where the “bottom” is. That is, at what level is there agreement. Having a discussion at any level higher than that is generally a waste of time…if one is trying to resolve anything. At one point in my careers I was designing and making electrical power meters. I found that many of my customers, who ostensibly were trying to monitor electrical consumption, had no real understanding of the concepts of power and energy. Until that concept was understood, there was no point in engaging in any other conversation. Most of the time, I was successful in bridging that gap.
I cannot over state the importance of this general concept in communication. In the arena of AGW, I find the most fundamental/lowest level gap is the concept of the Scientific Method. In the realm of technical discussion, that’s pretty darn low (not sure you can get lower..:) )….unfortunately much lower even than the concept of radiation heat transfer.
Thanks for your excellent insights and writing skills…I always look forward to anything you write….
Regards,
Ethan Brand
Beautifully put!
Ethan
An important insight.
My work is with micro tomography systems – think hospital CT scanner but the size of a suitcase, sitting on a desk. We market these to scientists, mostly biologists. They often at first have no understanding of the nature of the technology, imagining it to have almost magical powers.
So you’re right – before any meaningful communication can occur one often needs to educate the client on the essential underlying science and principles, such as xray absorption and contrast (plus backprojection- reconstruction). Once that basis of understanding is reached, then more fruitful discussions can follow.
“…If there is a block of wood between you and a block of ice, if you remove the wood, you’ll get colder because you will be absorbing less radiation from the ice than you were from the wood. You no longer have the wood to shield you from the ice.”
That statement is wrong.
You’ll get colder because the ice will be absorbing more radiation from your body than when the wood was there.
If you were to use a sheet of aluminum instead of a block of wood, the block of ice would be absorbing more radiation from your body through the aluminum sheet than through the block of wood.
The sheet of aluminum will become warmer, this is because of the block of ice absorbing radiation from your body.
For a so called “cold body to warm a hot body”, its energy potential would have to be quantified, if you give the cold body a value of 2C and the warm body a value of 5C the differential between the two values would be 3.5C. if you consider the differential between the aluminum sheet and your body, this would be 4.25C.
3.5C>2C therefor the sheet of aluminum has warmed by 1.2C. This is in fact the block of ice absorbing radiation from your body through the aluminum sheet and not the other way around.
“The sheet of aluminum will become warmer, this is because of the block of ice absorbing radiation from your body.”
It’s sucking the heat out of your body!!!
Don’t get me wrong when I say that I absolutely love this. As the saying goes, it’s so bad, it’s not even wrong!
(And then not to make *any intelligible point at all* at the end of it all! Priceless.)
If you were to use a sheet of aluminum instead of a block of wood, the block of ice would be absorbing more radiation from your body through the aluminum sheet…
Uncle Gus
You’re giving me the impression that you believe that a persons warm body absorbs cold from a block of ice, are you for real?
I didn’t say “It’s sucking the heat out of your body!!!”
But a cold surface will draw the heat from a persons body, it’s called “heat transfer” not cold transfer.
Aluminum has less resistance than wood allowing the heat to leave the body to the block of ice more efficiently.
“This is in fact the block of ice absorbing radiation from your body through the aluminum sheet and not the other way around.”
Nevertheless, the absorption is the transfer of energy. Less transfer, higher temperature of source, increased transfer, lower temperature of the source.
It is important to locate the source of energy, by measuring the higher density of energy, to determine the direction of the flow.
Co2 increases absorption. Increased absorption means increasing rate of transfer. Increasing rate of transfer means lower temperature of the heat source.
lifeisthermal Says;
“Co2 increases absorption”
Not at saturation, Do you understand that atmospheric Carbon Dioxide is above its limit of absorption?
Do you understand that Carbon Dioxide has no further effect once saturated?
lifeisthermal Says;
“Increased absorption means increasing rate of transfer “
And
“Increasing rate of transfer means lower temperature of the heat source “
Not applicable on planet Earth.
In 1954, Hoyt C. Hottel conducted an experiment to determine the total emissivity/absorptivity of carbon dioxide and water vapor11. From his experiments, he found that the carbon dioxide has a total emissivity of almost zero below a temperature of 33 °C (306 K) in combination with a partial pressure of the carbon dioxide of 0.6096 atm cm. 17 year later, B. Leckner repeated Hottel’s experiment and corrected the graphs12 plotted by Hottel. However, the results of Hottel were verified and Leckner found the same extremely insignificant emissivity of the carbon dioxide below 33 °C (306 K) of temperature and 0.6096 atm cm of partial pressure. Hottel’s and Leckner’s graphs show a total emissivity of the carbon dioxide of zero under those conditions.
http://www.biocab.org/Overlapping_Absorption_Bands.pdf
Willis says: thermal IR emissivity is 1.0
What you are using for emissive is from water vapor not CO2. CO2 will not cause an increase in any temperature at thes concentrations or pressures.
That actually makes sense!
I think it’s wrong, and I don’t have time to read it carefully and figure out why it’s wrong, but at least it’s out of Looneyland for a change!
(If it wasn’t such a departure from the accepted science on the subject, I probably wouldn’t be so sceptical. Does anyone better qualified than I am have anything to say?)
Dr. Hottel wrote the books on radiative heat transfer in combustion chambers. His charts are still used today.
It is not wrong. At the temperatures and partial pressures we talk about emissivity of CO2 is essentially zero.
I challenge anyone to use these charts and show that CO2 has an emissivity that not essentially zero at partial pressure of .0039 ppmV and 273K.
Yes, it *does* say 33 degrees Centigrade! That’s hotter than I have my shower in the morning.
I still don’t believe it, but if true, think of the implications! CO2 effectively *transparent* to IR at the relevant temperatures. The whole Global Warming thing not a hoax or “settled science”, but based on a single unexamined (and fallacious, assumption)!
The irony!
Pity it’s BS…
mkelly,
I have read the reference, but that only talks about the influence of CO2 in the overlapping bands with water. The point is that most of the CO2 influence is in the 10 micrometer band, where water is not active at all…
feeling a little spunky, were we, Willis? 😉
thanks willis
watched these arguments for 10 years now. The cash analogy is pretty good. I may steal it someday.
the analogy, not the cash.
Why not fill a bulb with CO2 and compare (measure) the real contribution of CO2 in a “close” (sealed) word? This way we can know exactly the relation between filament and feedback temperature.
We could warm the filament at 50° and see how much at 400ppm of CO2 it will feedback.
Mariano,
If you have a bulb with a diameter of around 50 km, you may measure a difference, if you can fix all other variables…
Hello from France(so You will excuse the bad english… I hope
I love Your exemple with money, because it is the central dilemna.
Me give 100 to You
but instead off refunding 75 to me, You decide to spend 75 buying beer
the net flow is 100 to You, and I just have to fall in tears.
do’nt forget that if CO2 is a very good “interceptor of radiative wave, it is also a very good emitter
sure, the bulk of the atmosphere will be warmed by collisions with CO2,but at equilibrum, the flux will be the same.
In the dry desert, the surface of the grond will be very hot at noon, and the atmosphere surrounding also by conduction, and convection
When the sun fall, the grond can freeze, very quickly
But it all depend of the capability off the limit layer to radiate to space.
The theory is that, when it warm beneat, it cool upside and the radiative capability fall?
I doubt of that.
First, if there is more CO2near the ground, , (that the way the theory works?) more radiative flux will be intercept, and thus, it will be cooler in the upper limit.layer
But If there is more CO2 near the ground, there is also more CO2 in the limit layer, and thus a better capability to radiate to space.
If it is hotter in the ground layer, the atmophere surrounding will expanse, and the t°of the adiabatique curve will change, so any consideration of the t° at the limit layer will be modified.
Just for the fun
what if there is only CO2 in the atmosphere, no water on the ground?
nr65:
” In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.”
OK
“The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.”
You forgot to divide by 4 as 1368 is TOA.
The Earth is a rotating oblate speriod.
So that’ll be 342 W/m2 x 0.7 (albedo)
=239 W/m2 available (on average) for each m2 of the sunlit surface of Earth.
That gives a S-B temp of 255K
But we live on a planet at 288K
33K warmer.
Why?
Wrong place
Because we are a water world, Toneb. H20 not only dominates in the radiative arena, but also gives convective cooling at the surface.
Toneb, you write:
“That gives a S-B temp of 255K
But we live on a planet at 288K
33K warmer.
Why?”
As mentioned by others, Earth’s surface is not a blackbody. A blackbody is an object that only exchanges energy with its surroundings through radiative heat transfer.
The surface also exchanges heat with the troposphere via conduction (and subsequently convection).
Earth together with its troposphere, however, is a reasonably good approximation of a blackbody. The tropopause just above the troposphere ensures that there is no convective heat flow between the troposphere and the stratosphere. Conductive heat flow at this boundary is also small.
The average temperature of the troposphere is around 255K. Coincidence?
Back to the question, why is the surface 33 K warmer than 255 K?
Going further, why is the temperature at the tropopause 33 K colder than 255 K?
This is due to the lapse rate. Hot air rises and cools off. cold air drops and warms up. The end result is a temperature gradient with 33K warmer than the average near the surface, and 33K colder than the average at the tropopause. The average, 255 K stays the same.
–Just for the fun
what if there is only CO2 in the atmosphere, no water on the ground?–
I like fun.
Earth has 70% surface water and 30% land.
The average ocean surface is about 17 C and average land surface is
about 10 C.
So without water surface, roughly earth should have an average temperature
of less than 10 C
[Because the 17 C ocean surface 17 C currently increases the average land
Air surface to 10 C].
Ocean warms land and land cools ocean.
Land surface air temperature can be much higher, but basically
ocean saves money and lands spends more money that it makes.
Land dweller are in debt to the ocean, and always have been..
Interesting analogy — the atmosphere, of course, is not “cold”, nor is the surface “warm” (or “warmer”) — they are only relatively in that relationship. It only confuses the issue to think in terms of cold and warm. It is not the temperature of the material between the two objects that is the primary factor — only the case that there IS something that interacts with radiant energy between them. If the interceding object were entirely transparent to radiant energy, there would be no effect — because the object in this case, the atmosphere, is made up of gases and particles that do interact with radiant energy, the atmosphere both absorbs and radiates that energy and becomes part of the energy flow equation.
The very principle of atmospheres acting as protection from the mind-numbing coldness (there is something that can be called cold) of space itself has long been know — anyone camping on the high, dry deserts of the American Southwest has experienced the speed and efficiency of sky “sucking” the heat out into space when the atmosphere is (relatively) thin and dry, denying us that protective blanket.
The same is true in the opposite case — on the tropical islands, where altitudes are low (and the atmosphere thick) and humidity is high, less of the energy delivered by the Sun during the day escapes to space during the night — thus we lie on our boats, praying for a breeze to cool the sweltering night.
The moon (and Mars) lacking an appreciable atmosphere is hot in the direct Sun and very cold (all my our measure) in the shade or on the darkside.
A blanket itself is not “warm” — it is simply fairly efficient at slowing energy flow through it — which is what the atmosphere does. The highly efficient silvered plastic emergency “space’ blankets used by Emergency Rescue squads is prime example — the allow very little body heat to pass through and reflect back a great deal of the radiant energy (TV shows often make the error of placing these around the victim with the shiny side out which makes a better picture, but is incorrect in use).
“A blanket itself is not “warm” — it is simply fairly efficient at slowing energy flow through it”
An atmosphere is not “warm”, it simply increases the transfer of heat into it. The cause of increasing rate of transferred energy is reduced emissive power, observed as reduced T^4.
A blanket, or any other type of thermal insulation, causes reduced absorption of heat in the colder surroundings. Which is equal to reduction of the rate at which heat is transferred.
An atmosphere, and co2 in particular, causes increased transfer of heat, by adding heat absorbing molecules the its heat source.
This means, that an atmosphere acts exactly in the opposite way to how insulation affects temperature of a body.
Would you care to explain why your model is based on a violation of proven and applied physics?
Willis (Anthony), Thanks. This is an excellent article. Kudos to you gentlemen.
” In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.”
OK
“The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.”
You forgot to divide by 4 as 1368 is TOA.
The Earth is a rotating oblate speriod.
So that’ll be 342 W/m2 x 0.7
=239 W/m2 available (on average) for each m2 of the sunlit surface of Earth.
That gives a S-B temp of 255K
But we live on a planet at 288K
33K warmer.
Why?
Doh
The earth surface is not 33K warmer. The atmosphere is 33K colder.
This is caused by heat absorption=cooling of the source(earth surface)
Surface temp: (TSI/2pi*r^2)/(4/3pi*r^3)^2=383W/m^2=287K
In questions of science, the authority of a thousand is not worth the humble reasoning of a single individual.
Galileo Galilei
The ONLY^3 reason RGHE theory even exists is to explain how the average surface (1.5 m above ground) temperature of 288 K/15 C (K-T balance 289 K/16 C) minus 255 K/-18C , the average surface (now ground) temperature w/o an atmosphere (Which is just completely BOGUS!) equals 33 C warmer w/ than w/o atmosphere.
That Δ33 C notion is absolute rubbish and when it flies into the nearest dumpster it hauls RGHE “theory” in right behind it.
The sooner that is realized and accepted the sooner all of us will have to find something better to do with our time and the taxpayers’ money. Maybe that’s what keeps RGHE staggering down the road.
The genesis of RGHE theory is the incorrect notion that the atmosphere warms the surface (and that is NOT the ground). Explaining the mechanism behind this erroneous notion demands some truly contorted physics, thermo and heat transfer, i.e. energy out of nowhere, cold to hot w/o work, perpetual motion.
Is space cold or hot? There are no molecules in space so our common definitions of hot/cold/heat/energy don’t apply.
The temperatures of objects in space, e.g. the Earth, Moon, space station, Mars, Venus, etc. are determined by the radiation flowing past them. In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
https://science.nasa.gov/science-news/science-at-nasa/2001/ast21mar_1/
But an object’s albedo reflects away some of that energy and reduces that temperature.
The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.
https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-723
The Earth’s albedo/atmosphere doesn’t keep the Earth warm, it keeps the Earth cool.
Bring science, I did. (6,200 views and zero rebuttals.)
http://writerbeat.com/articles/14306-Greenhouse—We-don-t-need-no-stinkin-greenhouse-Warning-science-ahead-
http://writerbeat.com/articles/15582-To-be-33C-or-not-to-be-33C
http://writerbeat.com/articles/16255-Atmospheric-Layers-and-Thermodynamic-Ping-Pong
” In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.”
OK
“The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.”
You forgot to divide by 4 as 1368 is TOA.
The Earth is a rotating oblate speriod.
So that’ll be 342 W/m2 x 0.7
=239 W/m2 available (on average) for each m2 of the sunlit surface of Earth.
That gives a S-B temp of 255K
But we live on a planet at 288K
33K warmer.
Why?
Toneb
A sphere of radius r has 4 times the surface area as a disc of radius r. The incoming sees the earth as a disc the outgoing sees the earth as a sphere. In the bucket of warm poo model the incoming 1,368 discular W/m^2 is simply spread over the spherical ToA, i.e. divide by 4. Simple and dumb. K-T diagram.
The incoming is NOT actually spread evenly over spherical ToA to get an evenly heated 342 * .7 = 240. or what I refer to as the earth as a ball suspended in a warm bucket of poo.
The incoming strikes the ToA at an oblique angle so the perpendicular to ToA W/m^2 is 1,368 *cos latitude which ranges from 1,368 at the equator to zero at the poles.
The outgoing sees a sphere 24/7 with the heat leaving per Q = UAdT same as the walls of a house. Even Pierrehumbert recognized this in his 2011 paper.
Here’s an animated power point.
nr65:
“The incoming sees the earth as a disc ”
Yes, it does BUT we are calculating the energy budget for the whole Earth NOT just the sunlit side.
Hence a /4 factor is needed.
“Averaged over the entire planet, the amount of sunlight arriving at the top of Earth’s atmosphere is only one-fourth of the total solar irradiance, or approximately 340 watts per square meter.”
https://earthobservatory.nasa.gov/Features/EnergyBalance/page2.php
(I replied to this further up, but I see the same post is repeated here.)
Toneb, you write:
“That gives a S-B temp of 255K
But we live on a planet at 288K
33K warmer.
Why?”
As mentioned by others, Earth’s surface is not a blackbody. A blackbody is an object that only exchanges energy with its surroundings through radiative heat transfer.
The surface also exchanges heat with the troposphere via conduction (and subsequently convection).
Earth together with its troposphere, however, is a reasonably good approximation of a blackbody. The tropopause just above the troposphere ensures that there is no convective heat flow between the troposphere and the stratosphere. Conductive heat flow at this boundary is also small.
The average temperature of the troposphere is around 255K. Coincidence?
Back to the question, why is the surface 33 K warmer than 255 K?
Going further, why is the temperature at the tropopause 33 K colder than 255 K?
This is due to the lapse rate. Hot air rises and cools off. Cold air drops and warms up. The end result is a temperature gradient with 33K warmer than the average near the surface, and 33K colder than the average at the tropopause. The average, 255 K, stays the same.
You’ve got the Sun going round and round the Earth, Toneb . The Sun never sets in space, and space is right there, just at the TOA….where satellites measure it as 1368w/sq.m. End of story…no division by 4 or anything. Yearly global average, non directional, covering whole globe at the TOA.
Toneb November 25, 2017 at 9:35 am
As any planet with one sun only one half of the Earth is illuminated, so better to divide by 2
(no concept of heat storage for BB’s)
Without atmosphere no clouds, so most probably a more moon like albedo (~0.11)
684 W/m^2 x 0,89 = 609 W/m^2
Dark side 0K ( ~3K if you insist)
SB gives (322K + 0K)/2 = 161K
Actual moon ~197K, actual Earth ~288K.
Why?
The area perpendicular to sunshine is PI R², this flow is spread to half the surface 2 PI R² for half the time, so the factor is roughly four. Your largest mistake is to use surface temperature in S-B law. The Earth radiates and reflects a lot from colder atmosphere, putting TOA exit flow at 340W/surface m², but giving a much higher surface temperature due to the lapse rate.
By the way, what is the approximate surface temp as a function of amount of air in the atmosphere? The less air, the colder it is.
Or, if I make a hole 200m deep, how much that affects temp down there when conduction, convection and wind are not taken into consideration? I think it is about 1K. There’s a greenhouse.
What’s RGHE? At least, what’s R in R… GreenHouse Effect.
Stands for Radiative, I think.
Radiative GreenHouse Effect. As opposed to CGHE Convective GreenHouse Effect which is how it actually works.
Try this thought experiment…
Imagine a cool surface emitting, say, 3 watts per square meter of IR SMACK in the middle of the CO2 emission spectrum. Now focus this IR WITH a magical lossless 1 square meter lens into a 1 square millimeter beam and aim it at a surface emitting 300 watts per square meter.
Does the beam heat up that spot?
Paul Bahlin, this thought experiment is about the question if it is possible to concentrate black body radiation with static lenses above 100% saturation. Well, it is not possible. For example, if you concentrate sunlight on an object by using as much mirrors and lenses as you like, the temperature of that object will never exceed the surface temperature of the sun.
Interesting.
So, if you had a 1 million mile wide lens and placed it near the sun and your lens was able to focus all the radiant energy from that side of the sun onto one tiny point of a solid object, that object could never be heated up to more than the 5000 or so degree temp of the sun?
I am not doubting it, but it sounds like a surprising result.
How do you know?
I explained this before. Forget all these analogies, well, the one with the blow torch and the candle is correct, forget all the others.
The easiest way to understand this is a simple industrial boiler such as is found in power plants. From cold start-up, with pipes and walls at ambient, we light the combustors. The blue natural-gas flame is at 4,000 degrees F. After equilibration the pipes and walls come up to the max for the particular metallurgy involved, typically 1,100 or 1,200F. Guess what? Blue flame is still at 4,000F, has not varied even a tenth of a degree.
Another easy one is binary stars, ubiquitous in our Galaxy. All have orbits with apogee and perigee. Guess what? The hotter star does not change its temperature AT ALL at perigee.
Go to engineering school, pass Heat Transfer and all related pre-requisite courses, then we can talk.
Michael please see my above post about Hottel charts and CO2 emissivity.
As a matter of fact radiative interaction between close binaries is a well established phenomenon.
I think the real greenhouse effect is our rapid heating by a high-energy, small-wavelength source and the slow cooling from our low-energy, long wave emissions. The rates of energy transfer are very different and fortuitously beneficial to our life on Earth.
BTW you can not calculate electromagnetic energy budgets that way shown above.
If you have a (cold) object emitting 2W/m2 and a (warm) object emitting 5W/m2 You can not simply add both objects to get an electromagnetic energy budget of 7W/m2.
You have to work out what’s known as a differential between a (cold) object emitting 2W/m2 and a (warm) object emitting 5W/m2 which is known as potential.
This is done by adding together each object 2W/m2 and 5W/m2 and divide by 2
This will give you a differential value of 3.5W/m2 if there are other objects in the system we have to work out their differential values too, so lets say there were another objects with a potential of 4W/m2 and 6W/m2 we will get a differential value of 5W/m2
To work out our electromagnetic energy budget we then add both differential values of 5W/m2 and 3.5W/m2 and we get 8.5W/m2
The electromagnetic energy budget for this system is 8.5W/m2 with a potential of 15.5W/m2
at the molecular level depending on the angle of collision a hot molecule can cool a cold molecule and a cold molecule can heat a hot molecule.
this can easily be seen from the speed of balls on a pool table before and after a collision.
conduction is the elephant in the tea house overlooked in favor of sexy radiation.
I find this a bit frustrating that so many well educated people in here can struggle with this.
When we calculate T1-T2 we find the difference between the objects. We then can se how much warmer T2 can become.
The value T1 is the energy radiating from object 1 and since it’s already radiated away that energy has already left object 1
So we can find how much T2 can absorb but we can NOT find out how much energy that’s left in object T1. Remember once again that object 1 has already radiated away all the T1 value.
Removing T2 or adding a T3 will not change T1. T1 will always be T^4 of object 1
There will always be a T0 that any object can radiate to. (Space)
Remember the radiation window to space is not 100% closed so the surface can radiate to space.
And please don’t start counting photons. It’s not the number of photons that sets the temperature but the wavelength or frequency of the energy.
Using the money example it’s not the number of coins passed over the table, but the value of each coin that sets the balance of your account.
But I will say it’s nice to see this being discussed here at wattsupwiththat.
Not puny.
All the PE required to keep the entire mass of the atmosphere off the surface against gravity is involved.
That PE reservoir is the source of additional kinetic energy at the surface providing the observed surface temperature enhancement above S-B.
If only everyone who disagreed with Willis first went through the examples he generously provided!
This thread does show why it’s good practice to be courteous- There’s nothing wrong with being incorrect, but being incorrect and indignant really makes you look foolish.
Have done. Those examples are not adequate since they ignore conduction and convection as per my explanations above.
Ric,
Thanks, do you have a reference/ link on this ?
It just seems terribly counter-intuitive to me, that 4 molecules should be able to warm 10.000.
So, I would like to know the reasoning behind, in detail..
Willis,
How many ‘objects’ are there in the atmosphere ?
And how do they transfer energy ?
AFIK the atmosphere is mostly made of molecules and atoms.
Also, is it not true that the molecules near earth are compressed by the weight of the atmosphere, and therefore warmer
“Also, is it not true that the molecules near earth are compressed by the weight of the atmosphere, and therefore warmer”
No. There is no relation between pressure and temperature. It is only changes in pressure that causes heating and cooling.
tty,
Re the atmosphere, you say: There is no relation between pressure and temperature. It is only changes in pressure that causes heating and cooling.
Imagine that a suitable absorbing gas, initially at ambient temperature, is contained in a non-absorbing transparent container and that the gas is always at atmospheric pressure due to an escape valve in the lid.
I now aim a constant strength beam of EMR at the enclosure (e.g. from the Sun). Are you saying that the gas will not heat up to a steady-state level above ambient while remaining at the same pressure?
Your experiment only shows that a gas expands when heated and heats when absorbing EMR.
What you say is true.
Now consider this: gravity adds CONSTANT compression of the atmosphere. It is a constant force, so it adds a constant amount of energy by constant compression.
But there is. It’s called the lapse rate and it not caused by the pressure gradient only by itself.
Compression alone does not create ‘extra’ warmth at a planetary surface. You first need energy moving from an irradiated surface to the mass of an atmosphere via conduction. Then It is the surface variations in density that move energy to or from PE and KE via convection which ultimately produces that warmth once hydrostatic equilibrium has ben achieved and KE is being brought back from PE in descending columns of air.
The denser the atmosphere the more conduction can occur at a given level of insolation hence Venus having a very hot surface even after adjusting for distance from the sun.
Compression alone can generate heat on very large scales such as within stars and maybe gas giants but that is a phenomenon on a far larger scale.
Pressure allows the atmosphere to retain more heat.
The pressure/temperature gradient is a measured facet of all planets with atmosphere, up to a level of approximately 0.1 atm.
IRRESPECTIVE of atmospheric gas constituents.
Correct.
There seems to be a universal rule that at densities commensurate with pressure of less than 0.1atm allow too little conduction and convection to significantly affect radiative fluxes.
Planets with such thin atmospheres tend to closely match the S-B equation.
Excellent primer, Willis, except that I would not have said this in quite the same way: “Can a cold object leave a warm object warmer than it would be without the cold object? While the answer is generally no, it can do so in the special case when the cold object is hiding an even colder object from view…”
The answer is not “generally no.” Counterexamples abound. One such counterexample is you, if you’re wearing clothes.
Moreover, instead of “even colder” I’ve have said “even colder, or less emissive, or more reflective, or less thermally conductive.” There are many ways in which the presence of a cooler object can make a warmer object warmer than it otherwise would be.
I assume that you’re attempting to educate some confused “sky dragon slayer(s),” who stubbornly persist in the erroneous belief that GHGs cannot warm the Earth because they think that the 2nd Law of Thermodynamics forbids a cold atmospheric gas from warming an already warmer planetary surface.
Good luck with that. Been there, done that, got frustrated. Like many folks at the opposite end of the opinion spectrum, evidence is irrelevant to slayers, because don’t want to understand it. Like the White Queen, they’d rather believe as many as six impossible things before breakfast than learn something which requires admitting, even to themselves, that they were wrong.
Here are some other folks who’ve also tried their hands at educating sky dragon slayers: Dr. Roy Spencer in July 2010 & April 2014, mark4asp on April 30, 2017 at 9:44 am, Frank on May 1, 2017 at 11:58 am, Ed Bo on April 30, 2017 at 9:02 am & May 1, 2017 at 10:38 pm, commieBob on April 30, 2017 at 8:55 am, Roger Sowell on April 30, 2017 at 9:09 am & May 1, 2017 at 6:28 pm, davidmhoffer on April 30, 2017 at 1:16 pm & April 30, 2017 at 2:02 pm, matthewrmarler April 30, 2017 at 8:05 pm, and MarkW on May 1, 2017 at 8:03 am..
An example I sometimes use is space blankets. They work. It works, in part, by reflecting IR from the body being warmed, back to the same body, which helps to warm it.
Space blankets actually work two ways. The more important mechanism is by blocking air movement (and thus convective and evaporative cooling). But the other way is by reflecting IR. That’s why they’re silvered, and that’s the feature which is somewhat analogous to the misnamed greenhouse gas effect.
The space blanket, itself, can be much colder than the body which it helps to warm, and it still works quite well.
GHGs added to the atmosphere are somewhat similar. They are colorants, which tint the atmosphere in the far infrared part of the light spectrum, which causes the air to absorb more LWIR radiation than it otherwise would.
The warm surface of the Earth glows in the far infrared, and those IR emissions represent radiant energy escaping from and thus cooling the surface. GHGs in the atmosphere absorb some of that energy, preventing it from escaping to higher altitudes or outer space. That recaptured energy warms the air a bit.
Those same GHGs also emit LWIR “back-radiation,” some of which makes it back to the surface, where it is absorbed, warming the surface a bit, which is one of several mechanisms by which warmer air warms the surface.
Clouds have a similar effect. On a cloudy night, the ground temperature will cool more slowly than on a cloudless night, even if the clouds, themselves, are much colder than the ground. The reason for that is that clouds reflect and re-radiate LWIR back toward the ground.
Your characterisation of the atmospheric gravito-thermal effect is as false as the warmista’s.
What the blanket doesn´t do, is increasing absorption of heat in the colder surroundings.
Which is a proof of the flawed argument of the greenhouse-unicorn: that increasing heat absorption in the increasing amount of dry ice, cause increasing power density of its own heat source.
The blanket does the opposite of what co2 does, and what the atmosphere does. It insulates by reducing/preventing transfer of heat to the absorbing cold air.
Good example. Another one is multi-layered insulation, used in space vehicles. https://en.wikipedia.org/wiki/Multi-layer_insulation
Thanks, nate, for the interesting reference.
Interesting to think of CO2 as colourants.
I have used another example of a colourant to counter the assertion that the amount of CO2, being what it is, could not possible have much effect on the entire atmosphere.
This seems at first glance plausible, in a horse sensey kind of way, but I happen to be aware of a very real example of something completely altering the optical properties of a fluid in even tinier concentration that CO2 exists in the air.
I have worked in the lake and wetlands management industry, and an oft-used product is a type of dye which is added to lakes and ponds to block light from penetrating the water and thus it inhibits the growth of unwanted algaes and aquatic plants.
There are a lot of brands of the dye that is used, and the remarkable thing is how small of an amount will dye an entire lake so dark it blocks all but a narrow band of visible wavelengths.
One quart of a product like Blue Lagoon, for example, will block light from getting to the bottom of an entire lake one acre in area and four feet deep. It will even do twice that volume but not quite as dark.
Most of the quart is not even the pigment.
As an aside, one might wonder what happens to the light?
If the “blue lagoon” prevents the light getting to the bottom of the lake it must be either reflecting or absorbing it.
If it is absorbing it, presumably it is making the upper depths warmer than the would normally be and the bottom colder.
Has anyone done any measurements to find out?
What an interesting comment, menicholas! I had never heard of Blue Lagoon and products like it. Thank you for teaching me something.
Let’s do the arithmetic. Four acre-feet = 5,213,616 gallons. So 1 qt / 4 acre-feet = 0.1918 ppmv, blocks enough light from passing through 4 feet of water to prevent algae growth on the bottom. Impressive!
A column of the Earth’s atmosphere has about the same mass as a 30 foot column of water. So blocking the light through just four feet of water should require an even darker tint than blocking the absorbed shades of light through the Earth’s atmosphere.
A C Osborn, a four-foot-deep pond is all “upper depths.” It matters not a whit whether the light is absorbed in the top one foot or by the dirt at the bottom.
Do you really wonder “ff it [the dye] is absorbing it [the light]”? And can’t you think of any better way to find out than with a thermometer?
Quarts! Four acre-feet = 5,213,616 quarts.
and
s/ff/if/
Sigh.
daveburton, it is apparent from your condescending, dismissive and disparaging tone that you have been around Mr Eschenbach for much too long.
Or is it a prerequesite for being a CAGW adherent?
So let me tell you where I am coming from, I am just a lowly Engineer who did basic Thermodynamics 50 years ago.
But at that time I worked in a UK Metrology lab where they were measuring to 1 millionths of an inch and also in an Optics lab.
So I know about light and also heat transfer, which is a real problem when working at such fine measurements.
I was Trained to take measurements compared to Certified masters, not take anything for granted and not make assumptions and use Verification.
The absorptiuon of the light is an obvious conclusion, but I have not assumed what happens to the Light and the lake water, all I know is that the light has reduced at the bottom enough to inhibit algae and plants.
So in response to Menicholas’s muse “what happens to the light” I would test the temperature of the water at various depths before and after adding the dye to see if any areas changed temperatures.
If it is hotter at the top than before then that would strongly suggest that the dye was absorbing more of the incoming Radiation than before.
Obviously you have a superior method, presumably involving measuring the light itself, but that does not tell you “what happened to it”, just absence of it.
Please enlighten us, but without the condescension.
Oh, fer Pete’s sake, A C. It’s dye, not glitter. Dyes absorb light, they don’t reflect it.
http://maxpixel.freegreatpicture.com/static/photo/1x/Twinkle-Glimmer-Glitter-Blue-Sparkles-Pink-Party-1752823.jpg
If all warm things radiate heat, does oxygen in the atmosphere radiate at ambient temperature? If it does is it not a greenhouse gas too?
O2 radiates/absorbs in the UV and higher spectrum

Thanks Toneb
It also absorbs/emits in the microwave part of the spectrum. Satellite temperature measurements are based on this.
http://nvlpubs.nist.gov/nistpubs/jres/69D/jresv69Dn9p1201_A1b.pdf
327 posts on this & 966 posts on Radiative Heat Transfer of CO2 so far.
all top quality Q & A + discussions, learning lots & that’s what we come here for, well done Anthony for hosting.
(who said science was boring…. or settled)
Would be really useful if all posts were numbered to stop getting lost, is that possible ?
I’m not talking about changes in temperature or pressure.
I’m just talking about the fact, that the pressure at say 1 km above msl is higher than at 3 km above msl.
And that this in and of itself implies a higher temperature.
No matter how many CO2 molecules there are in the air.
Can we agree on that ?
The thicker a planets atmosphere is, (having more atmospheric mass) the higher its pressure will be. The higher the pressure, the more energy needed to move it around and the more energy that can be absorbed and stored in the system.
Venus’ atmospheric composition has nothing to do with its atmospheric pressure or temperature any more than Mars does, Their ratio of carbon dioxide may be similar, it is a thicker atmosphere on Venus absorbing more energy.
If you strip both Venus and Mars of their atmospheres, their planetary temperature will be equal, allowing for the distance of the sun.
Density is the key. Thickness can also relate to depth.
The greater the density at the surface the higher the proportion of a given level of insolation that can be transferred to the atmosphere by conduction and the hotter the surface will become once convection creates a hydrostatic equilibrium.
Density is a product of mass and gravity alone because they alone create the pressure that forces molecules closer together.
Thus the surface temperature enhancement must also be a product of mass and gravity alone at any given level of insolation.
You should finally get rid of Kiehl & Trenberth energy balance figure and fluxes because they are obsolete and based on the wrong atmosphere. The balance by Stephens et al. is much better:

Where is the component for the non radiative return of KE to the surface in descending air to offset sensible and latent heating of the atmosphere in thermals (rising air) ?
Stephens et al also miss that out and increase DWIR to offset that omission in order to achieve apparent balance.
Not only in descending air, also in descending rain and snow.
Correct. As rain and snow fall they warm up with the surrounding air as they descend the lapse rate slope. Nonetheless they still are cooler than ambient at each point in the journey.
“Nonetheless they still are cooler than ambient at each point in the journey.”
Not always, but mostly (ever heard of freezing rain?). And nevertheless they carry a lot of heat down to the surface since they are way above absolute zero,
Ok tty
There are exceptions but one should try to keep it simple. Sometimes rain falls through a freezing inversion layer on the way down but that is a local short lived event.
Radiative flux is not a conserved quantity. You cannot add 2 (or more) radiative fluxes from different sources and use the resulting algebraic sum to derive the sink temperature via S-B. It’s a complete bastardisation of physics and all diagrams, posts and comments assuming you can IN THE ENTIRETY OF THIS WHOLE BLOG SITE deserve to be thrown in the trash.
This is so fundamental I cannot believe it has not been understood properly.
Badger:
You keep repeating this completely erroneous argument. If you had taken and understood the first few weeks of a decent thermodynamics course, you would not be trying to argue that.
Energy IS a conserved quantity. Radiative fluxes transport energy. The necessary corollary to the fact that energy is a conserved quantity is that you can add the energy inputs to any defined system or subsystem, and subtract the energy outputs, to calculate the change in the energy of that system or subsystem.
A decent introductory thermo course will make you do dozens, if not hundreds, of problems like this.
This concept is no more difficult than balancing your checkbook using the principle of “conservation of money”, adding the inputs and subtracting the outputs to calculate how much your bank balance has changed.
Ed,
You need to obtain a refund from MIT. Radiative fluxes are not a conserved quantity. If you cannot grasp this simple concept, then you have no business making comments here. (hint: radiative fluxes are not measured in Joules)
SGW:
Radiative fluxes integrated over area and time are energy transfers. These most certainly can be added and subtracted, as indicated and required by the 1st LoT.
Where on Earth did you get that misconception, Badger & SGW? Seriously, what is your source?
Ed, I am impressed. You are a most patient man.
There it is, aveollila! The risible 0.6 +/- 17.
Has anyone done any better measurements; say a figure less than 28 times the measured value.
How do the Watts relate to time? Are they Watts per second as in Joules?
Thanks, Willis, for an excellent article. As usual, Willis explains things clearly and simply.
It would have been helpful if the Second Law of Thermodynamics had been clearly stated up-front, so that we all knew exactly what the article was explaining. Unfortunately, in recent years the 2nd law has been re-stated in a way which renders it almost useless for general dicussion. The law as currently used says that entropy increases. That’s about as useless as “before present” being used to mean “before 1950” – it renders normal discussion almost impossible.
So, let’s go back to a meaningful version of the 2nd law: In the absence of work, there cannot be a net transfer of energy from a cooler object to a warmer object.
What “absence of work” tells you is that things very quickly get complicated if you don’t actively keep them simple. Willis actively kept his explanation simple, and his demonstrated conclusion was therefore clear: a cool object can make a warm object warmer than it would have been if the cool object had not been there. That’s all he set out to demonstrate, and demonstrate it he did.
What this means is that the 2nd law cannot be invoked to “prove” that CO2 (or GHGs) cannot warm Earth’s surface [*]. That’s all it means. It doesn’t mean that CO2 does or doesn’t warm the surface, only that one particular argument is invalid.
[*]_Sorry about the double negative, but it’s the best I could do.
These discussions have been exceptional for me in that they’ve forced me to find out things for myself rather than accept what are often myths repeated on various blogs. The most shocking thing I’ve realised is that as sceptics we are a disorganised rabble who often disagree with each other more than we do the MMGW establishment. We know that they purvey poor science but we have no coherent response to their incorrect but coherent position. I truly believe now that the Lukewarmer position is a complete cop out like being agnostic rather than atheist. Radiation flux does not represent the transfer of heat and radiation received doesn’t always raise the energy level of a body, once this is understood everything falls into place.
“Radiation flux does not represent the transfer of heat and radiation received doesn’t always raise the energy level of a body, once this is understood everything falls into place.”
Better thus:
Radiation flux does not adequately represent the transfer of heat when there are also non radiative processes going on.
Radiation received doesn’t raise the energy level of a surface when non radiative processes can adjust to neutralise any INTERNAL radiative imbalances.
“In the absence of work, there cannot be a net transfer of energy from a cooler object to a warmer object.”
Correct, but work is done against gravity in lifting the atmospheric molecules off the surface in the first place.
The presence of that work does allow a colder atmosphere to heat a warmer surface but only by non radiative means involving conduction and convection and only after the lifting of the atmosphere has been completed and hydrostatic equilibrium achieved.
Willis completely omits non radiative processes.
Stephen
See my comment below at 1:11 pm.
Thanks ptolemy2
Convection always reorganises to eliminate internal radiative imbalances.
Otherwise no hydrostatic equilibrium and no atmosphere.
For those who ask “How does a warm body know to ignore a colder photon?”
There are lots of resources on the web documenting quantum mechanic processes where:
“The electron can be excited only if hν corresponds exactly to an allowed electron
transition in the atom. “ http://www.ntec.ac.uk/Phys/pdfs/6-Emission%20Absorption.pdf
and
“The energy levels for all physical processes at the atomic and molecular levels are quantized, and if there are no available quantized energy levels with spacings which match the quantum energy of the incident radiation, then the material will be transparent to that radiation, and it will pass through.” http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
There are some heavy duty books on the subject for those who want to delve further into this.
In a nutshell, if the IR radiation from the earths surface progressing upwards, is not at one of the precise frequencies that CO2 responds too, then those photons of that frequency just carry on towards space unchanged. (disregarding broadening/wings/shoulders for now)
The range of spectral lines (frequencies/wavelengths) transmitted from the earth’s surface will be many due to the wide range of surface material, coverings etc varying from location to location. We can assume that it is going to be very difficult come up with a value with any accuracy.
The energy contained in a photon depends upon its frequency/wavelength. If the frequency is ‘correct’ it will be absorbed into a CO2 molecule. If the frequency/wavelength is incorrect, it will pass through it.
Just to add confusion, a photon excited CO2 molecule will ‘relax’ to its ‘ground’ state within a few nanoseconds, like all other excited molecules, the decay happens at an exponentially. However, researchers are finding some molecules can exhibint ‘non-exponential decay!: http://iopscience.iop.org/article/10.1088/1742-6596/538/1/012008/pdf
So, going with the theories of a couple of years ago, decaying to ‘ground’ state within a few tens of nanoseconds, thermal IR emitted from the earths surface, some excites a CO2 molecule, say 20nS later, the photon is re-emitted from the molecule and travels elsewhere. What effect does this have on any temperature of CO2 and surrounding gas?
So much to learn.
“The electron can be excited only if hν corresponds exactly to an allowed electron
transition in the atom.“
Quite true, which is the reason gases (with relatively simple, relatively isolated molecules) have line spectra instead of continuous “black body” spectra like solids. Even so there are lots of possible transitions even in fairly simple molecules.
“disregarding broadening/wings/shoulders for now”
Which you most certainly can’t do as soon as you are dealing with more than one isolated molecule.
Also note that even in the ideal case (=isolated atom in vacuum) the lines are a bit fuzzy due to the uncertainty principle
Steve quoted, “The electron can be excited only if hν corresponds exactly to an allowed electron
transition in the atom…” and tty agreed, “Quite true.”
Not true, if we’re talking about 15 µm LWIR. Electron transitions are much too high energy. 15 µm IR photons correspond to a CO2 molecular bending mode, not an electron transition.
The word “exactly” isn’t correct, either. Other sources of energy, such as interactions with nearby molecules and the motion of the CO2 molecule as a whole, can add to and subtract from the “exact” quantum of energy, creating pressure/temperature broadening and hence the “wings” of the spectral lines.
.
Steve also wrote, “a photon excited CO2 molecule will ‘relax’ to its ‘ground’ state within a few nanoseconds…”
Actually, in the case of 15 µm LWIR absorbed by CO2, the mean time for it to give up the absorbed energy by emission of another photon is on the order of one second!!!
Are you startled by that? So was I!
Atmospheric physicist Will Happer mentioned it in a UNC Physics Colloquium three years ago, and kindly explained it to me in a subsequent email exchange.
At 1 Atm and typical temperatures, that’s more than a hundred million times longer than the average time for a CO2 molecule to lose its absorbed LWIR photon’s worth of energy by collisional transfer to another air molecule. So, regardless of how much or little LWIR the CO2 molecules in the atmosphere are absorbing, they remain at almost exactly the same temperature as the other air molecules.
It also means that this lovely animated picture, from the NSF, which even illustrates the correct vibrational mode, is wrong more than 99.999999% of the time:
It also means that when a CO2 molecule in the atmosphere emits a LWIR photon, the energy to do was almost always acquired bu collision with another air molecule, rather than by absorbing a photon.
That implies that the amount of ~15 µm LWIR emitted by atmospheric CO2 depends only on the atmosphere’s temperature (and CO2 partial pressure), not on how the air got to that temperature. Whether the ground is very cold (and emits little IR) or very warm (and emits lots of IR) will not affect the amount of IR emitted by the CO2 in the adjacent atmosphere (except by affecting the temperature of that air).
Thanks Dave for the interesting and educational email exchange!
Hi daveburton! You have raised a point that, with few exceptions like Will Happer, is not understood by the majority of CAGW scientists and commentators. The radiative lifetime of a vibrationally excited CO2 molecule is of the order of 1 second, during which time approx. 10^9 to 10^10 collisions with the air molecules (mostly N2) will occur. So most of the energy of 667 cm^-1 photons emitted from a 288 K Earth surface and absorbed by a ground state CO2 molecule will be transferred during radiationless collisions to the non-radiating molecules of the troposphere. This is the mechanism by which greenhouse gases warm the troposphere.
In a previous posting in this thread, I gave a simple analogy of a black metal plate containing a 100 W heating coil (representing the solid and liquid surface of the Earth which absorbs incoming visible radiation from the Sun) and a similar black metal plate parallel to the first, but a passive absorber/emitter (with no internal heating coil) representing a layer of CO2 greenhouse gas. The bulk of the atmosphere consisting of non-radiating N2, O2 and Ar molecules can be represented by a large insulated tank of water or antifreeze connected by a well-insulated heat pipe to the passive plate. The rise of incoming Solar radiation during the daytime can be modelled by increasing the current in the heating coil in the first plate. The IR emitted from the first plate is then absorbed by the passive plate, which also warms up, but most of the heat energy is stored in the tank of water or antifreeze. There will be a lag in the temperature rise in the passive plate/storage tank, but if the current in the heating coil is shut off, simulating nighttime, the rate of cooling of both plates is reduced as heat is now transferred from the contents of the storage tank to the passive plate, which exchanges photons with the first plate. Another analogy could be an electronic power supply converting AC to DC with a single rectifier and a humongous filter capacitor which smooths out the ripple in the half-wave rectified DC output.
Prof. Happer correctly explained that the emission of IR from CO2 to outer space depends on the temperature of the emitting layer, which at 10 km is 220 K. However, this is strictly true only for frequencies close to 667 cm^-1 which are completely saturated all the way through the troposphere and into the stratosphere. Because there is a temperature inversion from 10 to 45 km due to absorption of incoming Solar UV and visible radiation by ozone in the stratosphere, doubling CO2 will cause escape to outer space to occur at higher altitudes where the temperature actually is higher. So the central 667 cm^-1 emission increases above 20 km, requiring less emission from the Earth’s surface for energy balance. Thus doubling CO2 will mean global cooling, not warming, when only these central frequencies are considered. Jack Barrett has run MODTRAN spectral calculations to 70 km, instead of truncating at 20 km (which on average is at 220 K), showing this increased stratospheric emission [see the section “The hard bit” at http://www.barrettbellamyclimate.com/ ].
Of course, doubling CO2 does result in net global warming, not cooling, because frequencies on the far wings of the CO2 absorption ditch are not totally saturated, even in the entire 10 km path length of the troposphere. This shows up as an increase in the area of the CO2 absorption ditch in the spectrum available at https://en.wikipedia.org/wiki/Radiative_forcing . To understand this net absorption, one must go to the integrated Schwarzschild Equation [see the section “Schwarzschild’s Equation” at Jack Barrett’s website].
The radiation intensity for a frequency in the wing of the CO2 absorption ditch is the sum of a Beer-Lambert absorption term and an emission term. Because the emission at 10 km occurs at 220 K instead of at 288 K, the emission term moderates the net absorption by a factor of 0.659 [the signal I = 1 – 0.659A , where A is the Beer-Lambert absorbance, and signal = 1 – A in the absence of the emission term]. This means that the literature value for climate sensitivity is too high, since it doesn’t include the factor 0.659. It is also too high because it was derived for absorption in a cloud-free sky. Clouds are composed of liquid water droplets or ice crystals which act as miniature Planck black bodies which absorb 100% of the IR emitted from the 288 K surface, and then re-emit at the lower temperature of the cloud top. Doubling CO2 beneath and inside the clouds will not increase the absorption because it is already 100%. So only the smaller path length from the cloud top to 10 km will have CO2 molecules that will increase absorbance on doubling CO2.
The increased absorbance on doubling CO2 occurs in sidebands centered at 618 and 721 cm^-1 [see the MODTRAN spectrum referenced above]. This occurs as photons emitted from the 288 K surface are absorbed by CO2 molecules in the v=1 first vibrationally excited state [for the energy level diagram showing the transition, see Diagram 3 in the section “Spectral transitions” at Jack Barrett’s website referenced above]. At 288 K, only 3.2% of CO2 molecules are in this v=1 excited state, and the percentage goes down as temperatures decrease with increasing altitude. The bottom line is that when the 62% of the Earth’s surface which is covered with clouds is considered, the climate sensitivity (not counting feedbacks) is not 1 K, but closer to 0.6 K. And water vapor feedback has been grossly overestimated. Even if it were 50%, this would raise climate sensitivity to 0.9 K, and increased cloud cover would be expected to bring this back down closer to 0.6 or 0.7 K. The literature value of 3 K is way too high. Therefore wrecking the economy to try to keep the effect of doubling CO2 to 2 K is unnecessary, wasteful, and foolish.
I don’t want to bore others with more details in this Forum, but if you contact me at rtaguchi@rogers.com , I can send you pdf files with extended discussions. Willis, too, might be interested, and I respect his intelligence and fair-mindedness.
rogertaguchi
“This is the mechanism by which greenhouse gases warm the troposphere.”
And the surface?
Hi, RogerTaguchi. I’m glad you enjoyed the video of Prof. Happer’s colloquium. I made the video by combining his PowerPoint slides with an audio recording that I had made with my smartphone, which is far from ideal. But I think the result is serviceable.
I didn’t understand the black metal plates analogy, sorry.
I don’t think that the Effective Radiating Level (ERL) (or Effective Emission Height, or any of several similar names) is above the tropopause, except, perhaps, right at the 667 cm^-1 (15 µm) absorption peak. From what I’ve read, the ERL is within or below the tropopause over most of the 15 µm absorption/emission band. Various sources give the average ERL for CO2’s 15 µm band as being somewhere between 4 km and 12 km altitude, which is well below the ~20km altitude at which atmospheric temperature increases much with increasing height.
http://eesc.columbia.edu/courses/ees/slides/climate/atmprofile.gif
So the net effect of adding CO2 to the atmosphere and thus raising the ERL for the 15 µm absorption/emission band is to lower the average emission temperature, leading, as you noted, to net surface warming.
An interesting tidbit is the contrast between the calculated emission profile viewed at 20km, as shown in the Wikipedia article, and the actual satellite-measured emission profile.
Here’s the Wikipedia calculated profile:
Here’s a measured profile (part of Prof. Happer’s slide #16, for the Tropical western Pacific):
http://sealevel.info/slide16_excerpt2_FTIR_data_from_a_satellite_tropical_western_pacific.png
Look at the center of the CO2 “absorption ditch” — do you see the difference? The narrow spike in the middle of the measured spectrum is apparently emissions from CO2 in the warm but extremely thin stratosphere (above 20 km altitude).
http://sealevel.info/slide16_excerpt2_FTIR_data_from_a_satellite_tropical_western_pacific_annot3.png
You can also see it in Jack Barrett’s simulated spectra, looking down from 70 km (the last figure on his “hard bit” page†).
http://sealevel.info/barrett_calculated_70km_spectra.png
†Note to future readers: the links on BarrettBellamyClimate.com are numbered pages, and the numbers occasionally change. So if the link doesn’t take you to the correct page, go to the main page, ctrl-F, and search for “hard bit”.
daveburton November 26, 2017 at 1:38 am
“So the net effect of adding CO2 to the atmosphere and thus raising the ERL for the 15 µm absorption/emission band is to lower the average emission temperature, leading, as you noted, to net surface warming.”
Why do you say that when the only Actual Measurements we have show that adding CO2 Inreases the Cooling of the Atmosphere and therefore of the Earth’s Surface.
Nasa have shown that inreasing CO2 increases cooling.
see the 2013 AGU Presentation starting at about 15 minutes on this Video.
I do not see the same correlation between Raw Surface Temps and CO2 increases.
1. Sorry, for some reason Wikipedia’s calculated 20 km spectrum graph didn’t show up in my 1:38am reply to RogerTaguchi (above). It should have been:
http://sealevel.info/ModtranRadiativeForcingDoubleCO2.png
2. A C Osborn, thanks for the link.
(a) In that talk, Marty Mlynczak says that he’s discussing the thermosphere, above 100 km altitude. Cooling in the thermosphere/ionosphere does not imply cooling down in the troposphere.
(b) Here are his slides: https://fallmeeting.agu.org/2013/files/2013/12/PressConfMlynczakFinal.pdf
(c) For future reference, you can link directly to any starting point in a YouTube video using any of several syntaxes, like this (these all start that AGU video at the beginning of Dr. Mlynczak’s talk):
http://sealevel.info/youtube_starting_point_syntaxes.png
For example, here’s a direct link (using the “#t=19m49s” syntax) to the presentation immediately following M’s — which happens to have been by friend-of-WUWT Leif Svalgaard, on the topic of predicting solar cycles:
3. A C Osborn and The Reverend Badger, please note that in these “indented threads” there’s no way to tell who you’re talking to or what you’re taking about when you say things like, “Does this apply to…” or “Try talking about…”, unless you tell us. That’s why Willis always says, “When you comment, please QUOTE THE EXACT WORDS THAT YOU ARE DISCUSSING, so that we can all understand the nature of your objections.”
Pretty please!
Try talking about e-m radiation, don’t use the word “photon” and don’t say “frequency/wavelength”, just “frequency”. When you do this the argument can take on a different “flavour” which MIGHT just help in the learning.
So Badger, the last hundred years of EMR physics has been a complete mistake, and you are the only super-genius who recognizes this?
Does this apply to all materials, or just CO2?
Steve Richards November 25, 2017 at 1:04 pm
So, going with the theories of a couple of years ago, decaying to ‘ground’ state within a few tens of nanoseconds, thermal IR emitted from the earths surface, some excites a CO2 molecule, say 20nS later, the photon is re-emitted from the molecule and travels elsewhere. What effect does this have on any temperature of CO2 and surrounding gas?
So much to learn.
Indeed, the decay to the ground state in tens of nanoseconds is due to collisional deactivation and transfers energy to the surrounding molecules (about 10 collisions per nanosecond near the surface) thereby warming up the atmosphere. The radiative decay has a much longer characteristic time so doesn’t become the dominant process until higher in the atmosphere.
Energy Balance\resulting temperatures
At the fundamental level, how much kinetic energy is created from input, how much is retained after output
what happens to the KE retained, well we know, it makes the flipping weather, the oceans move, and so much more, including life, we are full of kinetic energy ourselves.
But can we master this problem? of course not.
Temperature is just an average measurement of kinetic energy, right off the bat we lose accuracy.
But sciency jargon or whatever.
An elegant clarification, thanks Willis.
Radiation is far from being the whole story of heat movement in the atmosphere. The body of theory of Ilya Prigogine concerning nonlinear thermodynamics and dissipative structures is important, though often overlooked. Changes to the thermal structure of the atmosphere may well – according to principles of self-organisation – result in rearrangement of dissipative and oscillatory structures which could establish a new equilibrium with minimal or no overall change to thermal fluxes.
The Benard or convection instability is manifest in a situation in which a fluid layer is heated from below and kept at a fixed temperature above so as to create a temperature gradient in opposition to the effects of gravitational force. At small values of this gradient heat is transported from lower to upper regions by conduction [and radiation] and macroscopic motion is absent. Random motions of the molecules and a damping of convection currents characterise the state of the fluid. However, when the gradient exceeds a critical value a convective, macroscopic motion occurs generally in the form of rolls or hexagons (for variations see Koschmeider 1977). In short, out of an initial state that is completely homogeneous there arises a well ordered spatial pattern. Moreover, with further increases in the gradient the spatial pattern becomes oscillatory.
Kugler PN, Kelso JS, Turvey MT. 1 On the Concept of Coordinative Structures as Dissipative Structures: I. Theoretical Lines of Convergence. Advances in Psychology. 1980 Dec 31;1:3-47.
Most scientists hate to talk in simplistic terms, sadly, the like to sound oh so smart
Mark
Essential chaos theory is much less complex than it sometimes looks. I’m a mere biologist. Hint – ignore the maths, just look at the pictures.
“ignore the maths, just look at the pictures”
Not recommended. You don’t necessarily have to do the mathematics but you need to understand the underlying principles and assumptions. Look at Mann and his hockey stick for a prime example of what can happen when you try to apply mathematics you don’t understand.
ptolemy2, I just taught this subject to a class of biologists this week, differential equations in a bio class! For an example of order from chaos check out the cell cycle and cyclins.
Stephen, I didn’t want to be so provocative as to say that any atmospheric model that omits the heat engine effect of the troposphere isn’t complete but you’re correct that it must.
I would also ask the following if radiation dominates atmospheric temperature:
How is it that the temperature at any point on earth can be determined by incoming solar radiation and surface emissivity with no reference to back radiation?
If radiation dominates how come the derivation of lapse rate does not include any radiation inputs?
If we have a downwelling heat source of 340 W/m2 why can’t we recover this at the surface as we can recover heat from solar radiation.
Martin Mason
I assume you are addressing me but I don’t accept that any radiation other than external radiation dominates surface temperature.
Any internal radiative imbalances are neutralised by convective adjustments.
Thus I raise the same questions as you.
As regards the apparent irrecoverability of DWIR as additional surface heat over and above S-B plus the mass induced surface temperature enhancement I suggest that any DWIR from the atmosphere is already merged into the surface temperature created by insolation, conduction and convection via the lapse rate slope.
As DWIR descends through lower levels of radiatively active material it distorts the lapse rate slope and reduces convective vigour so that warmed radiative material of any description finds itself at a lower height and warmer temperature than it otherwise would be so that it radiates more to space and the radiative capability for warming the surface is neutralised.
If there is to be a radiative balance with space all outgoing radiation must emanate either from the surface or from within the bulk of the atmosphere. If too much is going out from one source then the other adjusts accordingly. Could never maintain long term hydrostatic equilibrium otherwise.
“If we have a downwelling heat source of 340 W/m2 why can’t we recover this at the surface as we can recover heat from solar radiation.”
Very good question. The answer is simple: You need a GHG panel at 0K (-273.13°C).
Paul, it is much worse than that.
There is ZERO Empirical Data to show that there are 340 W/m2 hittng and “Warming” the surface.
There are simple tests where you can MEASURE the ACTUAL POWER of Sunlight hitting the surface.
Simple measurements with simple calculations that confirm the Satellite measurements of the Real Watts coming from the Sun.
see as an example
https://www.bing.com/videos/search?q=how+to+measure+the+power+of+sunshine&qpvt=how+to+measure+the+power+of+sunshine&view=detail&mid=1CE167A1295F871DFC441CE167A1295F871DFC44&FORM=VRDGAR
There are no such tests to show the Actual Power coming down as “Back Radiation”.
The closest they get is the sort of Experiment that Dr Spencer did and when the Target got COLDER and he said that proved that it was being warmed by the DWLIR because it would have been even colder if it wasn’t.
Does that sound like 340 Watts to you?
A C Osborn,
There are several stations on earth measuring downward IR radiation, some of them already from the 1950’s:
https://scienceofdoom.com/2010/07/17/the-amazing-case-of-back-radiation/
And specific for CO2:
http://newscenter.lbl.gov/2015/02/25/co2-greenhouse-effect-increase/
Ferdinand Engelbeen November 26, 2017 at 11:09 am
Looking forward to something similar to the Solar Challenge
https://www.worldsolarchallenge.org/dashboard/timing
but this time using cars driven by backradiation panels, and let’s make it a night race 😉
The pyrgeometers used in measuring backradiation calculate the radiation using emissivity 1.0 according a spec sheet I saw. Seems unrealistically high to me.
Ben Wouters,
The problem is converting IR to power: conventional cells transform visible light to power, with some yield, but as IR photons have a lot less energy, that doesn’t work (yet).
Read some years ago the possibility to recover low temperature waste heat by an array of micro thermocouples on a chip, where one side was heated by waste water, the other side cooled with ambient air.
Problem here is that the surface at night is loosing more energy than what is coming in as backradiation. Thus the “warm” side is the car/panels, so you need a lot of mass/weight to give some power…
Radiation is only basic knowledge (of about 50 years ago…) for me, so I can’t directly discuss your point about an emissivity of 1 (for the atmosphere?).
A C,
It’s even “worser” than you think. According to the Trenberth energy balance diagram, at the earth’s surface, backradiation from the atmosphere is over twice as powerful as the sun. Yet backradiation is powerless to heat up your pool at night as sunlight does during the day. Backradiation does not warm surfaces in the shade. Yet Trenberth assumes that 1 W/m2 of backradiation is equivalent to 1 W/m2 of solar radiation. Absurd.
The reason backradiation is powerless to perform work is simple. A colder atmosphere cannot transfer heat to a warmer earth surface.
The earth and atmosphere are purely passive. The only energy source available to heat the earth is the sun. And the sun has an average power of 341 W/m2 at the TOA. It is thermodynamic horse manure to indicate that this 341 W/m2 of solar energy gets magnified to 494 W/m2 at the earth’s surface.
Yes, they have less power, but how much less?
Why aren’t 340 LWIR watts = 340 sunshine watts?
This is the part I am trying to establish, I have seen at the bottom of this post that there is 1/40th of the energy for the radiation, so there is either 40 times as much dwi radiation or this whole back radiation thing does not work as advertised.
Everything I have seen up till now says it is not able to prove to work.
Thanks for the link to that CO2 ARM paper I remember it when it came out and it got a lot of stick at the time not least for having the last 5 years of data missing.
I may try and register with ARM to try and get some downloaded data as that paper only shows anomalies, which do not tell yo very much.
Willis, here are the five heat reservoirs in your picture.
1. sun
2. surface
3. troposphere
4. stratosphere
5. space
The sun is a tiny speck in the sky, the rest is some 186 thousand times larger. However, it is hot (5778 K), while space is cold (several kelvins), so radiative energy coming from the sun is millions of times larger than all the rest due to the fourth power in radiation law. Both heat reservoirs (1 &. 5) can be considered infinite on scales relevant to the climate system.
As for the rest, heat capacity of the surface (including oceans) is vastly larger than any one of your atmospheric layers.
About 30% of incoming solar radiation is rejected by the Earth system, it goes back to space without thermal interaction (albedo effect). That is, only 237 W/m2 gets thermalized in some part of the climate system and from then on it acts like heat (because it is heat), so “back radiation” has no meaning at all, only net heat transfer by radiation, which is an entirely different beast.
Albedo of the Earth system is strictly regulated. We know this, because annual integrated incoming solar radiation is exactly the same for the two hemispheres (due to a peculiar property of Keplerian orbits). Now, absorbed heat is also measured to be the same for the two hemispheres (for decades now, by satellites), in spite of the fact that clear sky albedo of the Southern hemisphere is much lower due to prevalence of oceans there (under clear sky conditions it reflects some 6 W/m2 less back to space than the Northern hemisphere). Not so under all sky conditions, which means there must be more clouds in the Southern hemisphere and by an amount required by an exact match.
This regulation is neither understood nor replicated by computational climate models. Therefore we have no idea what effect increasing level of atmospheric greenhouse gases may have on it, if any. However, temperature obviously depends on albedo in first approximation.
Another interesting notion is that radiative heat transfer from surface to troposphere is next to insignificant, it is 18 W/m2 compared to 98 W/m2 by convection. Therefore heat transfer between the surface and troposphere is hardly affected by greenhouse gases.
As there is no convective heat transfer to and from the other heat reservoirs, the rest of it is purely radiative.
But it is hard to tell, what effect of changing greenhouse gas levels may have on them. For example we do know the stratosphere is cooling lately (TLS: Temperature Lower Stratosphere).
http://images.remss.com/data/msu/graphics/TLS/plots/RSS_TS_channel_TLS_Global_Land_And_Sea_v03_3.png
However, it does not necessarily mean that the 147 W/m2 radiative heat transfer from here to space is decreasing, because outgoing heat radiation of a body depends not only on its temperature, but also on its emissivity. Which, for the stratosphere, is clearly increasing due to increasing greenhouse levels, because it equals to absorptivity under LTE (Local Thermodynamic Equilibrium) according to Kirchhoff’s Law, a defining feature of greenhouse gases.
Willis, here are the five heat reservoirs in your picture.
1. sun
2. surface
3. troposphere
4. stratosphere
5. space
The sun is a tiny speck in the sky, the rest is some 186 thousand times larger. However, it is hot (5778 K), while space is cold (several kelvins), so radiative energy coming from the sun is millions of times larger than all the rest due to the fourth power in radiation law. Both heat reservoirs (1 &. 5) can be considered infinite on scales relevant to the climate system.
As for the rest, heat capacity of the surface (including oceans) is vastly larger than any one of your atmospheric layers.
About 30% of incoming solar radiation is rejected by the Earth system, it goes back to space without thermal interaction (albedo effect). That is, only 237 W/m2 gets thermalized in some part of the climate system and from then on it acts like heat (because it is heat), so “back radiation” has no meaning at all, only net heat transfer by radiation, which is an entirely different beast.
Albedo of the Earth system is strictly regulated. We know this, because annual integrated incoming solar radiation is exactly the same for the two hemispheres (due to a peculiar property of Keplerian orbits). Now, absorbed heat is also measured to be the same for the two hemispheres (for decades now, by satellites), in spite of the fact that clear sky albedo of the Southern hemisphere is much lower due to prevalence of oceans there (under clear sky conditions it reflects some 6 W/m2 less back to space than the Northern hemisphere). Not so under all sky conditions, which means there must be more clouds in the Southern hemisphere and by an amount required by an exact match.
This regulation is neither understood nor replicated by computational climate models. Therefore we have no idea what effect increasing level of atmospheric greenhouse gases may have on it, if any. However, temperature obviously depends on albedo in first approximation.
Another interesting notion is that radiative heat transfer from surface to troposphere is next to insignificant, it is 18 W/m2 compared to 98 W/m2 by convection. Therefore heat transfer between the surface and troposphere is hardly affected by greenhouse gases.
As there is no convective heat transfer to and from the other heat reservoirs, the rest of it is purely radiative.
But it is hard to tell, what effect of changing greenhouse gas levels may have on them. For example we do know the stratosphere is cooling lately (TLS: Temperature Lower Stratosphere).
http://images.remss.com/data/msu/graphics/TLS/plots/RSS_TS_channel_TLS_Global_Land_And_Sea_v03_3.png
However, it does not necessarily mean that the 147 W/m2 radiative heat transfer from here to space is decreasing, because outgoing heat radiation of a body depends not only on its temperature, but also on its emissivity. Which, for the stratosphere, is clearly increasing due to increasing greenhouse levels, because it equals to absorptivity under LTE (Local Thermodynamic Equilibrium) according to Kirchhoff’s Law, a defining feature of greenhouse gases.
“annual integrated incoming solar radiation is exactly the same for the two hemispheres.” I thought that the Southern Hemisphere was getting more (we are closer to the Sun during the Southern summer). Link, please.
Journal of Climate, Volume 26, Issue 2 (January 2013)
The Observed Hemispheric Symmetry in Reflected Shortwave Irradiance
Aiko Voigt, Bjorn Stevens, Jürgen Bader and Thorsten Mauritsen
Kepler’s second law
A line segment joining a planet and the Sun sweeps out equal areas during equal intervals of time.
It means angular speed is inversely proportional to the square of distance to the Sun. On the other hand radiation flux coming from the Sun is also inversely proportional to the same quantity. Therefore incoming radiation integrated for a given angle is constant. The angle between equinoxes is 180 degrees (not counting precession, which is slow anyway and gives a negligible contribution – 25 arc seconds between equinoxes).
Between the spring and fall equinoxes the Northern hemisphere gets as much incoming solar radiation at ToA (Top of Atmosphere) as the Southern one between the fall and spring equinoxes and vice versa.
Q.E.D.
But the point is, annual reflected, consequently absorbed radiation is also the same.
Peter, thank you, you are 100% right. Let’s get to numbers. The Northern hemisphere gets as much solar radiation in 186 days as the Southern hemisphere gets in 178 days. While the total is the same, the Southern hemisphere should be a little warmer – that’s what I meant.
Annual mean temperature of the Southern hemisphere is in fact a bit lower, in spite of it is getting the same amount of energy input on annual bases.
https://data.giss.nasa.gov/gistemp/graphs/
This is why outgoing longwave radiation is not so balanced, the inter hemispheric difference is at least an order of magnitude larger (the Northern hemisphere radiates more to space).
To compensate for it, there is about 256 TW net heat transfer from the Southern hemisphere to the Northern one, mainly by oceanic currents. The difference comes out to be roughly 1 W/m^2.
Is the difference basically due to the absorption of so much more water in the SH?
@A C Osborn November 27, 2017 at 4:22 am
Yes, this is why clear sky albedo of the Southern hemisphere is 1.75% lower than that of the Northern one. Deep water (like oceans) is very dark, except for shallow incidence angles, when water surface acts like a mirror. If the light ray is perpendicular to the surface, only 2% is reflected, while the rest is absorbed at some depth. On the other hand, small water droplets (like ones in clouds) are white.
This is how albedo is regulated to the extent annual average absorbed shortwave radiation be the same for the two hemispheres.
Some say regulation is done by the exact positioning of the ITCZ (Inter Tropical Convergence Zone), which is cloudy indeed.
http://slideplayer.com/slide/7741637/25/images/8/ITCZ+JULY+ITCZ+JANUARY.jpg
However, it can’t be the whole story, because on average the ITCZ lies mostly in the Northern hemisphere, so the Southern one has to have abundant cloud cover elsewhere as well.
Yep. It is average cloudiness between July 2002 and April 2015 as measured by MODIS on NASA’s Aqua satellite.

https://www.nasa.gov/image-feature/cloudy-earth
Thank you.
This diagram appears to show the ITCZ going right through the middle of the Sahara desert in Summer.
I do not think it is accurate
Berenyi
Very illuminating and compelling.
I’ve always felt that normalising adaptation of a complex-chaotic (plus open and dissipative) system such as climate must be taking place.
Your discussion of unity of thermal budgets in both hemispheres is a strong pointer that this is indeed the case.
There is a theoretical proof, that in reproducible closed (but not isolated) non equilibrium thermodynamic systems the MEPP (Maximum Entropy Production Principle) holds.
A system is reproducible iff microstates belonging to the same macrostate can’t evolve into different macrostates, that is, the system is kinda macrostate-preserving.
If the terrestrial climate system were of this kind, Earth would be pitch black as seen from space (it would have low albedo), because most of the entropy production happens, when incoming shortwave radiation gets absorbed and thermalized.
Earth is obviously not black, its albedo is about 30%.
Therefore it can’t be reproducible, that is, it must have at least two microstates belonging to the same macrostate, that can evolve into different macrostates in a short time.
Indeed it is irreproducible, for the climate system is chaotic, because there are turbulent flows in it. True, it is not turbulent below the Kolmogorov length scale, an order-of-magnitude estimate for which is about 1 mm in the atmosphere. However, the atmosphere contains some 5×10^27 cells of this size, with 2.5×10^16 molecules in each (1.5×10^17 degrees of freedom). With that many cells for example average energy per degrees of freedom (temperature) in a cell is expected to fluctuate by one part in ten million. Which, due to the butterfly effect, contradicts reproducibility.
BTW, we would need some 10^22 times more computing power to skip turbulence in computational climate models, which is impossible. So models have to be parametrized in this respect, but, unfortunately, there is no way to validate the parametrization. An Earth sized wind channel would be way too expensive and also, impossible.
The sum of this is that the chaotic nature of the terrestrial climate system not only makes exact calculations impossible, but also determines the color of Earth (light blue, as opposed to black). Also, theory of irreproducible systems (where Jaynes entropy can’t even be defined) is one of the few uncharted territories of classical physics, so we have no idea how albedo is regulated. The only thing we know, it is.
As albedo is a major player in determining surface temperature, the end result is it’s a bit early to construct computational models, because the theoretical foundations are lacking. And building expensive policies on non existent models is beyond insanity.
If only climate scientists would make actual science instead of being mindless advocates in a policy debate, it could turn out better. For example, someone should do experiments on an irreproducible closed thermodynamic system, obviously on one which is a small enough member of this broad class to fit into a lab setup. There are plenty of such systems and we may even learn something important by studying them. Furthermore, it is not a computational exercise for sure.
JOURNAL OF PHYSICS A: MATHEMATICAL AND GENERAL 38 (2005) pp 371–381
doi: 10.1088/0305-4470/38/21/L01
Received 22 December 2004, in final form 13 April 2005
Published 10 May 2005
Maximum entropy production and the fluctuation theorem
R.C.Dewar
Peter, a great explanation, thanks.
There’s a crucial difference between the valid explanation given here in terms of the PRESENCE of a cold atmosphere, whose backradiation reduces RADIATIVE cooling of the surface, and the customary explanation of the so-called GHE, which attributes the ENTIRE increase in surface temperature solely to GHGs.
In fact, an atmosphere without any GHGs would still be warmed by thermal convection and conduction, reducing the cooling rate of the surface by those non-radiative mechanisms. And if water vapor were the sole GHG in the atmosphere, the surface temperature would not be greatly different from what is experienced now.
1sky1
The consensus view is that the reduction in cooling from the presence of GHGs accounts for the entire observed warming above S-B which is usually quantified as 33C.
You are correct that even a radiatively inert atmosphere would have conduction, convection and a surface temperature enhancement with radiation to space going out from the surface alone and the surplus surface KE being cycled up and down indefinitely and switched between KE and PE in convective overturning.
What happens if you then introduce radiative material of any kind, whether water vapour or not, is that some of the energy needed to go out to space comes from within the atmosphere so that less needs to go out from the surface.
In theory that would require a lower surface temperature but instead the lapse rate slope becomes less steep, the vigour of convection falls and energy is taken up by convection from the surface less fast so that the surface stays at the same temperature.
That is how one should integrate the thermal effects of radiative and non radiative processes.
Stephen Wilde
November 25, 2017 at 2:02 pm: Thankyou, Stephen.
Everything over 0K seems to radiate kinetically. If that has no effect, then what does that mean if anything for the spectra measured and touted by warmista?
There were only very shallow convection in an atmosphere completely transparent in IR, only to make latitudinal heat transfer possible. Otherwise temperature would be uniform above a pretty low altitude.
However, between Earth’s surface and troposphere only 15% of heat transfer happens radiatively, but there is a vigorous convection driven by radiative cooling at the top of troposphere. If radiative transfer decreased inside it, heat flux would still remain constant, because convective transfer would increase driven by a greater temperature difference.
Therefore greenhouse gases only have an effect in the upper troposphere and lower stratosphere, where convection is thwarted.
In an atmosphere completely transparent to IR all radiation to space would have to be from the surface and the vigour of convection would have to be at its fastest possible in order to get KE back to the surface in time to equalise radiation out to space with radiation in to space.
You would still have a temperature gradient along the pressure gradient due to the conversion of KE (sensible heat) to PE (non sensible heat) in uplift and the opposite in descent. Due to uneven surface heating and consequent density differentials in the horizontal plane an isothermal atmosphere would be impossible.
If you then add GHGs which provide an additional route for radiation to space from within the atmosphere then those GHGs also distort the adiabatic lapse rate slope to the warm side which reduces the vigour of convection which would otherwise be needed for convection to match surface radiation to space with radiation in from space. The GHGs are then assisting the shedding of radiation to space so that convection doesn’t work so fast.
Due to less vigorous convection the GHGs radiate to space more effectively from a lower warmer height along the lapse rate slope than would otherwise have been the case.
The net outcome is to neutralise any potential for surface warming from those GHGs.
That is why all planets with atmospheres can keep their atmospheres in hydrostatic equilibrium indefinitely regardless of the proportion of GHGs present.
Convection always adjusts to neutralise internal radiative imbalances.
The AGW radiative theory is fatally flawed.
@Stephen Wilde November 28, 2017 at 11:26 am
Why would the atmosphere get colder upwards, if only the surface is cooling?
If it is not getting colder, no convection is possible.
In fact it should be getting colder at least at the adiabatic lapse rate, otherwise a parcel rising would get colder (and denser) than its environment, so the atmosphere would be stable against convection.
FYI: I’m on a physics forum trying to get some answers. Seems Postma is banned from commenting on WUWT, but is aware of this thread. Too bad, he should be able to contribute his view of things.
I used to think all this banning on all sides of the debates was a bad thing but about 10% of the regular contributors on here are repeatedly rude / insulting in comments so I am now thinking we could have a better debate if at least 100 more were banned.
TRB,
I agree wth you. In fact, I would like to suggest to Mr Watts that the best way forward with this particular topic – with is absolutely fundamental to the ‘CO2 = CAGW’ debate – would be to have a closed debate where, for example, a selection of around 8 people only are allowed to post whilst everyone else merely views. To be fair, I would try to arrange the posters to be equally representing the Warmist, Luke-Warmist, Sceptical and Denier groups. All chosen debaters would have to agree to debate without resorting to personal attacks, ridicule, sarcasm etc.
I for one would just love to see an intellectual debate between polite, knowledgeable and respectful protagonists with maybe one other person (ideally unbiased) as referee. Finalising the chosen debaters would be challenging but worthwhile, in my opinion.
As a starting premise or proposition, I would suggest: “There is no valid physical mechanism by which a change in atmospheric Carbon Dioxide can measurably affect the Earth’s average temperature.”
J. Richard Wakefield, Mr. Postma does not “contribute” to discussions, he pollutes them. That’s why he’s unwelcome.
Anthony is very broad-minded about commenting at WUWT, and he welcomes commenters with a wide variety of viewpoints. (It’s a nice contrast to the heavy-handed censorship prevalent at most alarmist climate blogs.) But even Anthony’s great patience has its limit.
I strongly recommend that you stay away from Principia-Scientific (PSI). It is a disinformation site, run by nutjobs.
They mix truth and fiction, which just makes their web site even more dangerously deceptive than those hideous hoax/parody sites, because it makes the fiction harder to recognize.
“Falsehood is never so false as when it is very nearly true.”
– G.K. Chesterton
Much of the material on the PSI site is good articles which they have simply copied from other sources. But the material which they wrote themselves is mostly deceptive, or nuts, or both.
The four main authors at PSI are John O’Sullivan (CEO) [not to be confused with “the good John O’Sullivan,” who writes for National Review, and who the PSI John O’Sullivan occasionally impersonates], Joe Olson, Joe Postma, and Pierre Latour. In addition to the web site, they also have a book, entitled “Slaying the Sky Dragon.” So they are often referred to as “Sky Dragon Slayers,” or just “Slayers.”
Joe Olson and John O’Sullivan are the two most prolific author at Principia-Scientific, and Postma is #3. A google site search for Olson name finds 723 hits (down from 1760 in July). John O’Sullivan has 986 (down from 1030 in July), Joseph Postma has 236 (down from 453 in July), and Pierre Latour has 193 (down from 288 in July):
https://www.google.com/search?q=%28joe+OR+joseph%29+olson+site%3Aprincipia-scientific.org
They used to have a 5th prolific author, Douglas Cotton, but he had a falling out with the others:
https://www.google.com/search?q=%28doug+OR+douglas%29+cotton+site%3Aprincipia-scientific.org
When I say those guys are nuts, I don’t mean they are just a little bit odd. I mean they are stark, raving, clinically insane. For example, this is a quote from a April 17, 2015 email from Joe Olson:
> “9/11 Conspircy Solved, Names, Connections, Details” and my interview
> with Dr James Fetzer, “Unequivocal 9/11 Nukes” are both on youtube.
Yes, you read that correctly. Joe Olson, one of the top two authors at Principia-Scientific, claims to know that the 9-11-2001 attacks were an “inside job” by the Bush Administration, and that the Twin Towers at the World Trade Center were destroyed with nuclear weapons. (If you care to do so you can search for those titles and find his youtube videos; if you are a masochist you can watch them, but I would not advise it.)
For the sake of your sanity, I strongly recommend that you stay away from Principia-Scientific, their web site, and their idiotic “Sky Dragon” book — and that includes Postma.
daveburton: [“When I say those guys are nuts, I don’t mean they are just a little bit odd. I mean they are stark, raving, clinically insane.”]
I consider your post to be utterly distasteful and not worthy of this blog or this debate. I am sure J Richard Wakefield is capable of rising above your personal opinion in making his own mind up.
Moderators – what were you thinking?
[distasteful, yes, but when somebody claims 9/11 was an inside job caused by nuclear weapons, it tends to support Mr. Burton’s opinion that their views on climate aren’t to be trusted.
Mr. Postma seems to be an equal-opportunity hater. read this https://climateofsophistry.com/2017/09/13/the-walking-braindead-flat-earther-science-denier-list/ -mod]
Right Dave,
Postma believes in the stark raving mad position that heat only transfers from warm objects to cold objects per the Second Law and radiative heat flow equation.
Quite a radical belief system.
Postma has at least 7 peer-reviewed publications in legitimate science journals as well. And Dave has how many scientific publications?
Many years ago I declined an invitation to join PSI because I found a certain lack of rationality.
The mass induced surface warming effect based on atmospheric mass and the gas laws via conduction and convection seemed too simple for them since it made them look as misguided as the AGW alarmists.
Given his foul-mouthed abusive posts to anyone who disagrees with he should definitely not be allowed to post his trash on here!
Willis:
Blackbody Radiation in Optically Thick Gases?
Pierre-Marie Robitaille
http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.646.58
http://hockeyschtick.blogspot.co.nz/2014/05/new-paper-questions-basic-physics.html
EXCELLENT! Both highly relevant – well worth reading.
This reminds me of the joke
https://en.wikipedia.org/wiki/Will_Rogers_phenomenon
1. Radiative flux is not a conserved quantity. You cannot add algebraically the radiative flux intensities from two different sources and use the arithmetic result to derive the sink temperature via S-B.
2. Radiation from objects is electro-magnetic with a range of frequencies (with an upper limit) and NOT a stream of little ping pong ball like elementary particles (just stop it with the “photons”, you are confusing yourselves!).
3. If your mind is still boggling and you fancy a diversion down a different less traveled path but with the same destination try this;http://www2.ups.edu/physics/faculty/evans/Pictet%27s%20experiment.pdf
4. The ERL (Effective Radiating Level) is NOT a real physical location (level/layer) from which radiation zaps off to outer space. If you have even the slightest idea that it is please go and research the origin of the term.
Definitions:
Heat – is simply the transfer of energy from a hot object to a colder object. (hence a cold object by definition cannot transfer heat to a hotter object)
Temperature – (hot & cold) a measure of the average kinetic energy of the particles in an object.
Second Law – is about the quality of energy. It states that as energy is transferred or transformed, more and more of it is wasted. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state. In simple terms, entropy always increases.
Entropy – is defined as a state of disorder or decline into disorder.
Seems some here are thinking that hot mean heat. That IR radiation is a form of heat, which it is not when the IR is from a colder object than the warmer object interacting with the IR.
It’s the Watts wot done it! Radiative flux in WATTS/m2, people think its like their heaters, 2kW convector or WATT-eva. (Not Anthony’s fault, and probably not his parents’ either).
Superb post! Thank you.
Willis is a brave man to take this on. Willis is right, but this shows that the argument that global warming is simple physics is simply wrong. So many people do not understand this “simple” physics, so the physics is not so simple.
Figure 1 at the top and WE’s explanation of it are a very good start to explaining the Second Law of Thermodynamics. It explains that the law is about net flows. Flow is an amount of something over time, so the diagram could show the two boxes as radiators/absorbers of money over some time period like a day or year. It could even show coins going back and forth to represent photons; maybe that’s taking the analogy too far. Cash flow works to show how net flow is important. It also shows that there is no problem in a poor person sending money to a rich person; it does make the rich person richer.
However, the example does not explain the Second Law of Thermodynamics. There is nothing in the analogy which says the rich person has to radiate more money to the poor one, with a net cash flow of rich to poor.
The Second Law of Thermodynamics was formulated at first from observations, before they even knew what heat was. The theory of why it is so came later and it is not simple
WE, thanks. Unlike usual, skipped all comments to just say much appreciated post. Goes far to clarifying many here commented misconceptions. Well done, in layman’s English.
l am going back over 50 years in my memory but we had thermo course or courses first then had heat transfer courses and it all seemed simple and logical and followed in steps.
In certain circumstances, a second object can slow the cooling of a warmer object.
Only H2O has the capacity to do this in our atmosphere.
Thought about this all night. The issue is the definition of the word “Warming.” “Warming” does not mean Cooling more Slowly, it means the Hot thing actually gets Hotter.
This is impossible, as, since the Hot thing gets Hotter from the Cool thing, the Cool thing will Get Hotter too, making the Hot thing also Hotter, Perpetual Motion.
This should do it for any sophisticated audience.
Micheal Moon wrote, “Thought about this all night… “Warming” does not mean Cooling more Slowly, it means the Hot thing actually gets Hotter.”
Wrong.
Instead of thinking about it all night, you should have opened the door and checked the temperature outside.
On most nights, in most places, the temperature drops as the night progresses, then rises again during the day. If, thanks to GHGs in the atmosphere, or cloud cover, or anything else that retards cooling, the rate of cooling at night is slowed, then the temperature at dawn is warmer than it otherwise would have been.
So in this context “warming” and “cooling more slowly” are equivalent: they make air temperatures warmer than they otherwise would be.
Michael Moon wrote, “This is impossible, as, since the Hot thing gets Hotter from the Cool thing, the Cool thing will Get Hotter too, making the Hot thing also Hotter, Perpetual Motion.”
Wrong.
Have you never snuggled up with a pretty girl on a chilly night (“for warmth” you told her)?
http://c8.alamy.com/comp/BYAG9P/man-and-woman-snuggling-under-blanket-BYAG9P.jpg
Or… have you never built a campfire, or a fire with charcoal briquettes?
If you separate the burning coals, they soon cool too much to sustain combustion. But if you pile several coals together, near each other, they will keep each other hot enough to continue burning much longer.
 .webp
.webp
Gonna be a wise ass and point out that the microwave (IR) background radiation at 2.75 W/m2 is somewhat stronger than the ~2 W/m2 from increased CO2.
The burning question here is does earth radiation in the atmospheric window make space warmer than it would otherwise be?
Does cosmic background radiation make the earth warmer than it would otherwise be?
And, if any of you would like to argue that Preventing Cooling, or even Slowing Cooling, is the same as Warming, then you have no place in this discussion…
Cooling more slowly is not the same thing as getting hotter. Are we clear? If not, go back to school…
Cooling more slowly involves the temperature going Down. Getting hotter involves the temperature going Up. Clear then?
One more time, going down more slowly is not the same thing as going up. Congratulate Willis on his idiocy several more times, or ruin this blog…
Michael Moon, repeating the same wrong statement over and over does not make it less erroneous. In the context of the Earth’s climate (and in many other contexts) cooling more slowly is exactly equivalent to heating.
Everything on Earth is constantly gaining energy from some sources and losing it from others. It matters not a whit whether you increase the rate of energy gain or slow the rate of energy loss, if you do either then the thing gets warmer.
What’s more, accusing Willis Eschenbach(!) of “idiocy” is an expeditious way to prove beyond a doubt that you’re as dumb as HotWhoppers’ Miriam O’Brien (a/k/a Slandering Sou from Bundangawoolarangeera), who is the only other person I can think of who is foolish enough to say something like that.
From where comes a photon/EM wave? It comes from moving charge, as in a shock from static electricity. Yes, static electricity and light are virtually the same thing. So, photons, come from charge being forced to change direction, as in moving molecules bumping into each other. If one is moving faster than the other one, the resulting EM/photons cannot necessarily catch up to the faster moving one, hence, no heat transfer from cooler to warmer.
“There are more things between Heaven and Earth Horatio than are dreamed of in your philosophy…”
Just one more! If a cool thing could heat a hotter thing, the hotter thing, being hotter now, would heat the cool thing more, which now would get hotter, and heat the hotter thing a little more, and now we have positive feedback, everything keeps getting hotter, perpetual motion, no need to dig up more coal or oil or Nat-Gas, pretty sweet! Good luck with that…
The precept here is that a cool thing can retard cooling of a hotter thing, not that it can make it hotter.
Why is that so hard for some people to understand?
RW:
Exactly.
“everything keeps getting hotter, perpetual motion”
How about you address the problem that your beliefs on the physics of the GHE lead inevitably to that conclusion.
Perhaps, just perhaps, YOUR Beliefs in the scince are therefore wrong….. and indeed the “problem”.
Just a thought you understand.
They are in denial.
Yet they all claim to have minds open to evidence.
They all see the evidence but can always construct a path to ignore…misrepresent misunderstand or reject the evidence.
That relates directly to the character of all skeptics.
Michael & Toneb, surely you are familiar with the concept of a “convergent series”. This sort of feedback may or may not lead to some infinite runaway result. Can you somehow prove the series diverges? Or are you simply picking one answer more or less at random?
“This sort of feedback may or may not lead to some infinite runaway result. ”
Err – I don’t remember saying anything about a “runaway result”.
Just that the GHE is real.
No, Steven Mosher, it relates to the character of all mankind. Well, most of mankind, anyhow — certainly most climate activists.
It is amazing to me how evidence, even overwhelming evidence, so rarely causes people to waver in their opinions. E.g., when I show climate alarmists good news, like the fact that the 30% increase in atmospheric CO2 over the last 70 years has caused no significant increase in the rate of sea-level rise, they should be glad.
http://www.sealevel.info/120-012_Warnemunde_1916-2016_smoothed.png
Wouldn’t you think that people who claim to be concerned about climate change would be relieved at such excellent news?
Strangely, they rarely are. Usually, good news upsets them. They’re apparently so emotionally wedded to their dystopian nightmare that they’ve come to want it to come to pass. No matter how conclusively it is proven, they reject the best scientific evidence, and cling doggedly to dystopian fantasies. They’d rather be right than safe. They’d rather believe their children are doomed than accept that they were wrong. So when you show them good news, they get mad, and respond with angry insults!
That’s pathological. I wonder if it is connected in some way to the strange popularity of horror movies. Maybe it’s the cats.
Steven Mosher
“That relates directly to the character of all skeptics.”
I’m skeptical of that, I’m an engineer, I have a BSC, I am qualified in electrical engineering, computer maintenance and networks, 25+ years programing experience, I owned a professional web design company and built an office/workshop servicing computer and office equipment for local business before even google existed, I have built schools, collages, homes and businesses in an engineering capacity. I have also worked for about 10 bands as a crew member, involving the production and the setting up of equipment including the programing and servicing of robotic lighting systems in hundreds of venues around the UK and Ireland, I am also qualified in horticulture I hold a hfe cert in numeracy. I have studied astronomy, solar physics and planetary mechanics, I have no criminal record, I am personally very polite, well mannered and have an awesome sense of humor and my mum says I’m good looking. Two years ago I ran into a burning building 3 times in an attempt to save a dogs life.
For the past 3 years I have worked as an electronics engineer for a security company and have been working on a project designing and building a portable security system, it’s kinda top secret…
I’m not sure that I can even begin to do myself any justice in a quick comment, but you would be the last person I would ever ask for a character reference.
Sparks, you neglected to mention that your excrement is odorless.
Sorry, Toneb. I must have misread your comment.
So, Sparks, do you have a significant other in your life?
Asking for a friend . . .
“have been working on a project designing and building a portable security system, it’s kinda top secret…”
DOH !!!!! Not any more it isn’t …
I think we can all agree that the people here commenting, at least those that agree with whatever position we hold on some issue or another, are the bestest of the bestest that humanity has to offer.
But not the ones that we disagree with, nosirree Bob…nope. They are rotten to the core…and probably dress silly, have bad hair, and stinky breath.
I can pert near guarantee it.
MM:
Apologies – In haste
I see you are on the “physics” side of the discussion.
Michael,
No perpetual motion, no more than adding insulation to my house causes that. Keep it simple. Insulation is a cool thing, it slows heat transfer from the warm house to the cold outside. Keeping all else equal (furnace heat output to the house held fixed), the house will warm.
Find the fault with this.
The Sun is the furnace.
The GHGs in the atmosphere are the insulation.
(You’ve got to be *very* specific with these bozos, Nate. And even then it rarely makes any difference…)
Uncle Gus.
Yes it is a shame that those same Insulating GHGs are between the Furnace and the Earth isn’t it?
Half the time the biggest GHG ie Water droplets and water vapour are cooling the Earth, are they not?
Or have you never noticed how much colder it is on a Cloudy day?
Or is it a shame, would we want the same daytime temperatures as the Moon?
AC,
“Or have you never noticed how much colder it is on a Cloudy day?
Beg to differ. It can go either way.
This time of year cloudy days can be warmer—indicative that warm moist air has moved in aloft. Late in the day, and at night, clouds block radiative cooling to space, keeping temps elevated.
Michael Moon November 25, 2017 at 10:00 pm
Just one more! If a cool thing could heat a hotter thing, the hotter thing, being hotter now, would heat the cool thing more, which now would get hotter, and heat the hotter thing a little more, and now we have positive feedback, everything keeps getting hotter, perpetual motion, no need to dig up more coal or oil or Nat-Gas, pretty sweet! Good luck with that…
Not if it’s a convergent series in which case a new steady state is reached.
It actually is convergent. If you do the math, it reduces to a really nice Taylor series expansion of
1/(1-h).
where h is the percentage of upwelling that is returned to the surface.
You can easily show that the energy returned to the surface is
[(1/(1-h)-1] of the incoming. So if h=0 nothing returns and if it is 1, the planet blows up.
Willis,
You are abysmally ignorant. You are not stupid, but you do not belong in the deep end of this pool, seriously. You are doing more harm than good with your profound misunderstanding of radiation and Heat Transfer. I beg you, withdraw from these questions, Mann will mock you and the entire blog..
Stop digging, please.
Michael Moon. Those are strong words, and unprettily abusive. But it is you who has missed the point. After the title Can a cold object warm a hot object?, Willis’ opening words were:
Short answer? Of course not, that would violate the Second Law of Thermodynamics … BUT it can leave the hot object warmer than it would be if the cold object weren’t there.
All of your comments have been misdirected, because they have addressed the first part of the above where you seem to have failed to notice that Willis agrees with you (“Of course not“), whereas virtually the entire article addressed the second part (“it can leave the hot object warmer than it would be if the cold object weren’t there“).
Thank you Willis, for an excellent and easy to understand explanation of a challenging subject. However, I think two conditions should be added to make the explanation more stringent.
Firstly, the question should be “Can a Cold Object Warm a Hot Object by means of radiation only
Without this condition, the answer would be “Yes, of course, just take on some clothes and you will soon notice the effect of warming from insulation”.
The second condition is that you have to take into account the effect of reflection. You describe the interaction as if all the bodies were perfect IR absorbers and perfect IR emitters. I know it is to make the description simple, but here I think you are oversimplifying.
A cold object can warm a hotter object by either shielding the warm object from an even colder object, or by being painted in a more reflective surface.
I think the situation easiest to understand is if both the cold and the warm object is rigged in a way so that they initially have a fixed temperature. Say that object A is 40 Celsius which is 20 Celsius warmer than object B because it is heated from an internal source, for instance a lightbulb.
The distance between A and B is so large that only heat exchange by radiation need to be taken in account.
Can we then do something with object B, so that the temperature in A increases?
Yes, we can for instance paint B in a more reflective paint.
Another way to increase the temperature in A is to place another object with a temperature of 30 C between A and B, but with enough distance from A to avoid any heat exchange other than radiation.
/Jan
Jan: “[A cold object can warm a hotter object by either shielding the warm object from an even colder object…”]
Seriously? When have you ever put hot coffee into a Thermos flask to find the coffee gets warmer with time?
Arfurbryant,
Many people here are giving their own examples, such as you have here, substituting them for the original post. The problem is when these examples remove key factors from the original post, that make all the difference.
Your thermos example: you need to compare the coffees temp over timme in the thermos to what it would be if the coffee was in a regular cup. Answer: it will be warmer in the thermos.
Nate,
Thanks for your input. I agree that examples used should be clear. Words are important in this debate.
Hence, Jan’s statement is just plain wrong. You are obviously aware that insulation does not make the warm object warmer. It does, however, retard the rate of cooling of that warm object. No argument with that.
But to imply that my ‘example’ somehow is not correct or even relevant is also not true.
In this context, you should be saying (as Willis did in the first paragraph of the OP) that a cold object can make an object warmer than it would have been if the cold object was not there. But instead you choose to use the phrase ‘warmer in the thermos’. You mean warmer in comparison with no thermos! To be clear, you should say that it is cooling slower than if there was no thermos. The coffee will NEVER be warmer.
Am I being pedantic? Yes. Am I wrong? No.
Does Willis answer his own question “Can a cold object warm a hot object?” Yes in the short answer but he then subtly changes the goalposts by answering a different question with a BUT in the second part of the answer.
Adding a cold object (insulation in this context) in NO WAY makes the warm object even hotter.
Cooling more slowly is not the same as warming.
CO2 is not an effective insulator and it is not in sufficient quantities to insulate, even if it was effective. Adding CO2 at the rate of just over one ppm per year should not be compared to any form of insulating material such as steel, blankets, overcoats etc.
I hope this clarifies my point.
Regards,
Arfur
Arfurbryant,
I do not think you read my example before you responded so I repeat it here:
“I think the situation easiest to understand is if both the cold and the warm object is rigged in a way so that they initially have a fixed temperature. Say that object A is 40 Celsius which is 20 Celsius warmer than object B because it is heated from an internal source, for instance a lightbulb.
The distance between A and B is so large that only heat exchange by radiation need to be taken in account.
Can we then do something with object B, so that the temperature in A increases?
Yes, we can for instance paint B in a more reflective paint. “
Do you disagree with this?
/Jan
OK, however. in the GHE, there is a continuous heat source, the sun. Again this source of heat is conveniently left out of many examples. In your coffee example that is equivalent to having an immersion heater supplying a steady power to the cup. In that case the coffee WILL BE warmer in the better insulated thermos, as compared to a regular cup.
Hi Jan,
Yes I did read your comment and the part that I objected to mostly was the phrase “A cold object can warm a hotter object by either shielding the warm object from an even colder object…”
As to your further point about object A being made warmer by either the addition of reflective paint on object B or by adding a third object C at intermediate temperature (30C) well, YES, I completely disagree with you!
In your examples, there is NO WAY that object A can be made warmer by either painting object B or by adding object C.
Kind regards,
Arfur
Arfurbryant
Imagine a poor homeless person freezing in the streets a winter night. His body temperature has fallen to 35 C and he is desperate because unless he recover his body temperature he will die. Then he finds some thick good insulating winter clothes.
Should he bother to take them on?
According to you, a colder object, the clothes, can never help him recover his body temperature.
Of course, he will be warmer because the insulation help him lose less heat and more of the energy from his body functions will then stay in his body and warm it up.
This is similar to object A, which will warm up because more of the energy from its inner energy source, the lightbulb, will stay in the box when some of the radiation is reflected back.
/Jan
Hi Jan,
You are wrong.
The homeless person will not recover his body temperature by putting more clothes on. All that would do is make him ‘feel warmer’ by reducing his rate of cooling. Unfortunately for him, as far as his body (core) temperature is concerned, all the extra clothes do is to delay his impending death from hypothermia.
By the way, this is not to say putting the clothes on is a bad idea! It would buy him (or her) more time…
Seriously, just try sleeping outside tonight with a really thick sleeping bag around you. Take your body temperature before you get into the bag and take it again in the morning. Without adding any form of external or internal heat, you will find your body temperature is colder in the morning. If you start to feel cold during the night, simply add another sleeping bag and again take your temperature in the morning. You will still be colder in the morning…
Kind regards,
Arfur
Arfurbryant
Do you really mean this?
Well, you are wrong, and I honestly think you are far off now, but let me try one more:
Imagine, as a thought experiment, that the new warm clothes are perfectly insulating. No heat escapes from his body after he takes them on.
Since he is not dead, his body functions must burn some calories, which create some heat, where do you think that heat will go?
/Jan
Hi Jan,
Yes, i really meant that. People who sleep outside will have a lower body temperature in the morning, irrespective of how much clothing they have. If you doubt me, please speak to either a physicist or a medical professional. Don’t take my word for it.
Now, to your thought experiment…
The trouble with ‘thought experiments’ is that people don’t usually think them through.
The direct answer to your question (where does the heat go?) is this:
If the insulation is truly 100% then there will be no heat loss from inside the insulation but, equally, there will be no heat gain by the body. (Can I assume there is an air gap between the body and the insulation?)
However, the person will die relatively quickly because he/she will be unable to breathe once the air inside the 100% insulation is exhausted (and the O2 is replaced by CO2). In addition, the body functions you refer to will use up stored energy, which is of a finite amount and is irreplaceable without compromising the 100% insulation. Once the stored energy is depleted he/she will die.
Either way, ‘thoughtfully’ covering a homeless person with 100% insulation is not going to do him any favours and his body core temperature will not increase at any point.
Sorry if you disagree but please ask someone else. I see no point in discussing this further but I genuinely wish you all the best in the future.
Kind regards,
Arfur
Goodbye Arfur
I had never though that I would ever meet a person who think that putting on insulating clothes in a cold day will not make you warmer.
Now I have.
All the best
Jan
Jan Kjetil Andersen, after patiently, but fruitlessly, trying to explain a little bit of basic science to arfurbryant, wrote, “I had never though that I would ever meet a person who think that putting on insulating clothes in a cold day will not make you warmer.”
In The Road Less Traveled, psychiatrist Scotty Peck defined mental health as dedication to reality. A mentally healthy person is a person who tries very hard to understand the world clearly. He is willing, even anxious, to revise his internal “map” of reality, when new information conflicts with previously held opinions.
Just like the leftists who blather on about “my truth” and “our realities,” as if reality were a matter of perspective, the slayers are not mentally healthy. Evidence, logic, and and even frequently repeated personal experience — as when they put on a coat every day before going outdoors in the winter — are irrelevant to slayers like arfurbryant, Michael Moon, skepticgonewild, “the bad John O’Sullivan,”† Joe Olson, Joe Postma, Pierre Latour, et al.
The “slayer” nonsense is such pure, refined ignorance that it inspired me to wax poetic, which is rare for me. With apologies to the late, great Ogden Nash, I give you:
† “The bad John O’Sullivan” is the Chief Slayer, i.e., CEO of PSI. He should not be confused with “the good John O’Sullivan,” who writes for National Review, and whose identity the bad John O’Sullivan has been known to borrow, when convenient.
“The bad” John O’Sullivan and his comrades cannot be trusted. Here’s a particular infuriating example of their dishonesty:
In 2015 O’Sullivan & Pierre Latour blatantly lied on the PSI web site, and also in emails, about Dr. S. Fred Singer’s views. The web site article was entitled, “Singer Concurs with Latour: CO2 Doesn’t Cause Global Warming.”
After several people objected, in email O’Sullivan wrote, “Fred Singer has now come over to PSI’s view that CO2 can only cool. I suggest you contact him.”
So I did. I forwarded that to Prof. Singer, who was then 90 years old, and asked him:
Prof. Singer replied succinctly:
That’s what I expected. I forwarded it to O’Sullivan & the other slayers, plus some of the people who had been trying to persuade PSI to remove the dishonest article, including Dr. Singer’s friend, Lord Christopher Monckton. But O’Sullivan still refused to to remove the dishonest article from the PSI web site.
Prof. Singer elaborated in a subsequent email:
Jo Nova weighed in, and appealed to O’Sullivan (and cc’d the other three), to do the right thing:
Joe Postma defended O’Sullivan’s and Latour’s dishonesty. He wrote:
Eventually, after Lord Monckton threatened legal action, PSI finally did edit and tone down the article, making it less flagrantly false. It now says “Singer Converges on ZERO Climate Carbon Forcing.”
Sorry about that. that mess is the result of typing <blockquote> when I intended to type </blockquote> Sigh.
[fixed-mod]
Thank you for the support Dave, your poetic verse is hilarious
/Jan
Thanks Dave. That was quite hilarious.
Please explain how 341 W/m2 of solar insolation at the TOA gets magically multiplied to 494 W/m2 at the earth’s surface when the sun is the ONLY source of energy to heat the earth.
Crickets………..
Arfur,
‘Seriously, just try sleeping outside tonight with a really thick sleeping bag around you. Take your body temperature before you get into the bag and take it again in the morning. Without adding any form of external or internal heat, you will find your body temperature is colder in the morning.’
Nice. Next time my toddler has a dangerously high fever, I will just put him in a sleeping bag and put him outside!
Jan, the poor homeless person (unless he is a lizard) has a self-warming body. As long as he has sugar to burn, he will be able to regain a normal body temperature after putting on the insulating clothes.
If you were to find a statue, made of bronze or stone, and put insulating clothes on it, the statue would not get warmer.
skepticgonewild wrote, “Please explain…”
Such simple stuff is left as an exercise for the reader.
Should have stuck to No … There will ALWAYS BE SOMETHING THERE … And never nothing … Unprovable and who cares … Its called insulation … Rate of cooling is NOT heating
Except for a few details, I agree with this way to represent the Earth radiative behaviour.
Indeed what really radiativeley matters is the net radiative energy transfer(~71W/m2).
It has no physical meaning, and is uninformative, to be 392-321W.
I prefer an alternative explanation. It relies on the spectral properties of the atmosphere.
The atmosphere does not behave like a black-body. Its spectrum, seen from the ground, is not a neat black-body spectrum.
Plotted in the frequency domain, it is partly opaque, with a transparent zone.
The radiation of the ground, if there were no “ghg” gasses, would be lower than it actually is, so that most of the power would fall into the opaque zone. Its energy, and temperature, increases until it radiates enough in the transparent zone to reach an energy equilibrium (not a radiative equilibrium).
If you increase the width of the opaque zone, with more water vapor or more CO2, temperatures will increase.
CO2 bands width increase very little with increased CO2 concentrations. Water vapor varies widely and thus has a far greater influence as we very well know.
On Mars there is more CO2 but less water vapor. In a cold and tenuous atmosphere CO2 absorption rays are fine. The ground is cold and emits in the transparent part of the atmosphere.
Paul Aubrin November 25, 2017 at 11:43 pm says:
Except for a few details, I agree with this way to represent the Earth radiative behaviour [Willis’s energy balance diagram]. Indeed what really radiatively matters is the net radiative energy transfer(~71W/m2). It has no physical meaning, and is uninformative, to be 392-321W.
I am open-mouthed with amazement…
You are suggesting that that, although you like the 71W/m^2 result, and 392W/m^2 upward radiation minus 321W/m^2 downward radiation exactly equals 71W/m^2, nevertheless this correct subtraction of two important measured radiation values ‘has no physical meaning’.
So you did the subtraction. You liked the 71W/m^2 result. And then you said that the result is not physically dependent on the two numbers that have generated the result.
What rubbish! Do you not understand that the reason that the upward actual transfer of energy from surface to atmosphere is 71W/m^2, rather than the 392W/m^2 asserted by the surface (due to its temperature) is because the atmosphere (being warm but not as warm as the surface) is moderating the rate of transfer of energy by an opposite assertion of 321W/m^2? This is straightforward 101 physics.
Not content with this blunder, you go one to make an even odder statement:
The radiation of the ground, if there were no “ghg” gasses, would be lower than it actually is…
On the contrary, if there were no GHG gases, the 169W/m^2 of sunlight that is absorbed at the earth’s surface energy would have to be balanced by outgoing radiation only from the surface of exactly 169W/m^2 directly to space. And last time I checked 169W/m^2 was considerably greater than 71W/m^2.
Where do you get these utterly bizarre notions from?
If the ground was at a lower temperature it would emit less, there is no discussion about this.
The 392 anW/m2) energy flux. The temperature of the ground is mostly defined by the wave number range of the “atmospheric window”. The ground must be warm enough to emit through it what is not evacuated by sensible or latent heat.
The net emission (71W/m2) is required for the energy equilibrium at the surface. The difference 382-321 is casual, 382 depends mostly on the wavelength at which H20 and CO2 molecules absorb.
The concept of shielding from a cold object is incoherent – its a misleading way of putting it. What happens, in the case of the wood and iceblock, is not that the wood shields from the iceblock, as if the iceblock were somehow emitting cold. What happens is that the wood is warmer, and radiates back to me. It also absorbs my own radiation, warms, and radiates back accordingly.
The example is right. A cold object between me and a still colder one will indeed lessen my heat loss. But not because its ‘shielding’ me from cold. Because its radiating more back to me, and also absorbing my heat radiation, warming, and then radiating that back.
The dubious concept here is “back”. Any object above 0K will radiate toward all its visible neighbours.
Yes, quite right – its a seductive misleading way of speaking!
Michel: if iceblock is not emitting cold why do you add ice cubes to your coke? Heat flows from hot to cold. It flows from you to wood and from wood to iceblock. You are cooling according to Newton’s law of cooling. The rate of your cooling depends on the temperature difference, which is smaller between you and wood than between you and iceblock. Therefore in the presence of wood between you and the iceblock you are cooling slower than in the absence of it. No backradiation.
If you sit next to a large block of Ice why do you feel the Cold coming off of it?
Why do you not feel warmer?
I have no problem with “Energy making things warmer or slowing their warming” but there are many things that do not fit the scenario.
Why does a hot object cool faster than a cooler object is a classic example.
Another that I have noticed is that the light from an LED Street Light makes the cars frost over quicker, why doesn’t it slow the cooling?
A C Osborn November 26, 2017 at 7:00 am
Another that I have noticed is that the light from an LED Street Light makes the cars frost over quicker, why doesn’t it slow the cooling?
Because for a street light emitting the same amount of visible light an incandescent light will emit even more IR whereas an LED will emit no IR, one reason why they are less expensive to operate.
Phil, I am sorry that I was not clear enough, the cars with LED shining on them do not cool quicker “Compared” to some other kind of light, they do so compared to “Not having LED light shining on them”.
ie the car directly under the light was over 2C colder compared to a car 15 feet away not directly under the light and it got there quicker.
It seems to be something to do the “frosting over” process at night. Dew or condensing moisture in the Air perhaps?
I hope the neighbors did not me sneaking around with my Electronic Thermometer.
ps I have tested an LED light shining on a very close object (a few millimetres) and it warms the object a little (0.5C)
https://en.m.wikipedia.org/wiki/Frost
So many variables. Two cars made of different metals, slight elevation differences, moisture differencs in the air moving around/between cars, length of time each car had been parked there cooling after being driven last, the directional property of LED lights rather than diffuse…etc.
Not to mention differing amounts of insulation under the hood of each car.
pps this was near midnight, I did not go back in the dead of night to see if the car not under the light got down to the same temp as the one under it.
Willis has succeeded in provoking an exceptionally illuminating thread. A number of posts are deeply informative and though-provoking. I’m thinking of posts by rogertaguchi, daveburon, Stephen Wilde and Berenyi Peter – but I haven’t read all the thread.
What seems to be emerging is the sense that the atmosphere’s thermal behaviour is adaptive, or self-regulatory.
For instance, why and how do both hemispheres have identical in-out solar heat budgets when SH albedo is much less than that of the NH (more sea). Answer – clouds compensate albedo by exactly the right amount (Berenyi Peter). No-one knows how.
Or take the surprisingly long time of one whole second between CO2 bending excitation by a photon and subsequent re-emission (following millions of thermal collisions)? This means that CO2 radiation is about temperature only, photon fluxes fall out of the picture. (daveburton).
And why are we haggling over small percentages of CO2 re-radiation anyway when IR absorption in clouds is 100% (rogertaguchi)?
In the big picture radiation may have it’s role in atmospheric heat dynamics greatly diminished by convective turbulent mixing (nonlinear dissipative structures) that may operate to effectively neutralise radiation imbalances (Stephen Wilde).
Also, if the effective radiation level – or the stratosphere as a whole – cool, but it’s emmisivity is increased by higher CO2 concentration, then in terms of emission to space, are we not back where we started and nothing has changed?
Here’s my own penny-worth of negative feedback:
CO2 makes plants 🌱.
Plants make clouds.
Clouds cool 😎 the earth 🌏 .
The atmosphere is a great washing machine.
Just how many things come out in the wash?
CO2 “back radiation warming” might very well be one of them.
Are nonlinear adaptive processes cancelling the effect of CO2, accounting for th very weak to nonexistent correlation over geological history between atmospheric CO2 and global temperature?
https://drive.google.com/file/d/0B_RXGJAF_XL5V0Y0eU1ya3E2UTA/view
Ward has good explanations covering energy vs temperature and GHG failings….http://ozonedepletiontheory.info/primary-problem-with-GG.html
Thankyou for that.
The explanation Ward gives is very clear.
The top chart explains why there is so much more Energy in Sunlight than CO2 based Radiation. So all those people on here saying the Frequency of the Radiation does not matter are talking absolute nonsense.
The missing link for me is how to get from there to the 300+ Watts/m2 attributed to CO2.
Actually, the chart explains why there is similar energy from the sun as from CO2. The vertical axis is “per steradian”. The sun covers about 6.9×10^−5 steradians, while the sky covers about 6.3 steradians — roughly 100,000x more. To calculate the TOTAL energy, the curve from the sky should first be multiplied by 6.3, while the curve from the sun should be multiplied by 6.9×10^−5. That ends up putting the two on a pretty even footing.
Ward is correct that less ozone in the stratosphere results in more energy into the oceans but that ozone change is solar induced as explained here:
http://joannenova.com.au/2015/01/is-the-sun-driving-ozone-and-changing-the-climate/
and it is correct that as a result the proportion of solar energy entering the oceans increases but the reason is changes in global cloudiness caused by changes in jet stream behaviour caused by those solar induced ozone variations.
CO2 has no effect because more GHGs change the lapse rate slope so that convection weakens and the GHGs radiate more effectively to space from a lower, warmer location which neutralises any effect on surface temperature.
“CO2 has no effect because more GHGs change the lapse rate slope so that convection weaken”
Enhanced CO2 actually decreases the specific heat of the atmosphere…
…. thus, it increases the lapse rate
Its basically immeasurable, like any other effect of CO2…… except plant growth.
Andy,
GHGs absorb radiation from the ground so as to become warmer than they ‘should’ be for their position along the lapse rate slope.
That causes temperature to decline less rapidly with height which reduces upward convection.
Temperature and heat are are two different things. A block of ice if big enough can have more heat than a thimble full of molten steel the heat flow will go from the higher temperature molten steel to the lower temperature the block of ice.
If there are two blocks of ice and you place a bag of CO2 between them, which one overheats and melts first?
Sparks,
The one without a bag will melt first, as the plastic containing the CO2 isolates better…
If it makes a difference if you use two bags, one filled with air, the other with pure CO2? Yes, if you make them 100 m thick, the difference caused by the CO2 feedback may be measurable…
Sorry, a little to fast: a bag between them, not on top, will not give much difference, as CO2 in such small radiation path has little effect…
Actually, the double glazing guys did experiments on different gases between the glazing.
CO2 allowed MORE heat through than normal atmosphere.
ie… it is a WORST insulator than normal air.
AndyG55,
You may be right, I haven’t checked conduction of CO2 vs. air at all, as the overall discussion is about radiation… Thus it may be that even a 100 m thick CO2 layer is a better conductor than backradiator, not to mention convection to make the mess complete…
Argon has a lower thermal conductivity than air (0.018W/m.K vs. 0.026W/m.K), CO2 is slightly lower (0.017W/m.K) all values at 300K.
The object isn’t always hotter. Daily land Tmin at 5cm is usually lower than Tmin at 2m.
Willis,
585 responses and counting… Heading for a new record?
Anyway, a clear overview of what is known of this point and as usual, all the objections are coming up again and again…
Having had a few discussions in the past with the Slayers, I have made an interactive Excel sheet where all initial parameters are changeable for experimenting and are recalculated momentarily.
The setup is simple: a (solar energy…) heated 1m2 plate in vacuum between two cooled walls at fixed temperature which is allowed to equilibrate (or not) in 19 seconds with each other so that incoming and outgoing energy are equal (or not). After 19 seconds a second plate is inserted at a given temperature between the first plate and one of the walls.
All radiation transfers, temperatures and energy balances are immediately calculated for the next up to 80 seconds and plotted.
Gives a good idea what the reduction in cooling is if you insert any kind of radiation hindrance, cooler or not, including GHGs:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/slayers.xlsx
Some graphs to show the possibilities:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/slayers_1.jpg
http://www.ferdinand-engelbeen.be/klimaat/klim_img/slayers_2.jpg
[Well, several threads have crossed 1000+ replies, but this one is in the top hundred …. .mod]
The key phrase here is “reduction in cooling.” If everyone clearly understood the keen physical difference between that and the usual climate-science phrasing of “heating Earth’s surface,” the entire dispute would disappear, along with any need for elementary explanations of actual heat transfer versus mere radiative exchange. There would be no endless blog discussions.
Thanks, 1sky1. If an object is at equilibrium, gaining energy at a certain rate and losing it at the same rate, and we reduce the rate of cooling, will the object at the new equilibrium be:
1. Warmer,
2. Cooler, or
3. The same as it was before
I say it will leave the object warmer than it was without the reduced cooling, which is WHAT I SAID IN THE FIRST PARAGRAPH.
I don’t know why this is so hard to understand …
w.
Indeed, the principle of the reduction of the rate of cooling is not difficult to grasp at all. The physical stumbling block lies not in the principle, however, but in the fact that Earth’s surface is cooled not by radiation alone. In fact, as careful experiments around the globe show, the Bowen ratio of sensible-to-latent heat transfer is usually well below unity, i.e., evaporation is the principal means of transfer from surface to atmosphere.
Thus the radiative GHE is by no means the dominant factor in setting the surface temperature that “climate science” makes it out to be. And the steady-state planetary temperature as seen from space is scarcely affected at all. There is LWIR absorption and isotropic emission, along with collisional transfer of thermal energy to radiationally inert constituents, but no real “trapping” of heat by the atmosphere.
Ferd,
The above does not confirm anything. It is just based on your interpretation of the science without any experimental proof.
skepticgonewild,
My “interpretation” of science is the simple application of established physical laws, even if it is in ideal circumstances. As several practical tests show that the results are similar (like the lamp at MIT), this theoretical experiment shows what happens if you change any of the intial parameters…
Ferd,
I figured as much, You have proposed a hypothesis and presented some calculations. You are only half way through the steps of the scientific method.
skepticgonewild,
I have neither the means nor the time to build that experiment, but as said before, the high yield lamp built by MIT is a clear example that reflecting IR back to the wolfram spiral heats that further up, so that you need less energy to reach the same temperature where maximum light is emitted below where the wolfram melts.
As reflecting or emitting IR from an outside source has exactly the same effect, there is no need for me to do anything further: theory and real life experiments do match…
Ferd,
You continue to make unsupported statements without providing experimental evidence. That is not how science operates. Show me the experimental data and analysis for this lamp.
skepticgonewild November 27, 2017 at 4:46 pm
Ferd,
You continue to make unsupported statements without providing experimental evidence. That is not how science operates. Show me the experimental data and analysis for this lamp.
Like Ferdinand I have posted about these lamps here in the past. You can find the patent for one of them here: http://www.google.la/patents/US3931536
Phil,
That’s just a patent. It does not provide any scientific data whatsoever. It does not confirm anything.
skepticgonewild,
Have a look at the real world experiment:
https://gizmodo.com/this-new-incandescent-bulb-uses-nano-mirrors-to-match-l-1752426237
And here directly to the Nature Nanotechnology article.
Tested and proven.
Remember the test with the reflector around the lamp from Anthony? When the mirrors were set, there was a slight decrease in amperage at a constant voltage. That is only possible if the temperature of the filament increased, then its resistance increases and the current drops…
“the high yield lamp built by MIT is a clear example that reflecting IR back to the wolfram spiral heats that further up
Ferdinand, please show me in your link where reflected IR “heats the spiral up further”.
skepticgonewild,
A few excerpts will help:
Figure 1b (theory):
The difference in energy to heat the tungsten (wolfram in Dutch and German…) to the same temperature of 3,000 K is provided by the reflected IR radiation.
Page 322 near the end (observations):
As good as Fig. 4 shows the gain in the visible spectrum and the suppression in the IR range for the same input power compared to a bare transmitter.
Ferd,
There is nothing there that indicates any temperature rise. Stop trying to force your preconceived ideas to create an outcome that is not stated in the paper You are grasping at straws.
SkepticGoneWild,
If you can reduce the electrical energy supply to 1/3 to emit the same amount of light as without the mirrors, then the reflected IR is doing 2/3 of the work to heat up the filament to the desired temperature…
SkepticGoneWild November 28, 2017 at 4:44 am
“the high yield lamp built by MIT is a clear example that reflecting IR back to the wolfram spiral heats that further up
Ferdinand, please show me in your link where reflected IR “heats the spiral up further”.
It’s in the methods section, shows a temperature increase of ~200K.
Very nice,except it doesn’t apply to ghg gases because they are not black surfaces.
The ghg effect stems from the spectral properties of “ghg” gases.
Paul Aubrin,
Agreed, but the nice thing of the above theoretical experiment is that any change in the pathway between a heated source (the earth by the sun) and a cooler recipient (outer space) that sends some energy back to the first (GHGs) will leave the first warmer than without that change…
Ferdinand Engelbeen,
Generally, it will be true. The temperature of an object will warmer in presence of another object, even if it is cold, than it would be if that the same object would be colder, unless the first object spectrally absorbs none of the emission of the second, for example if there is a shield at the common frequencies.
I’m posting here for the first time, being motivated by the desire to understand what makes planets hot, etc., and having read a lot of ideas on the internet (also I have formal training in engineering, if it comes to that).
Willis Eschenbach mentions a “further reading” post of his that I haven’t looked into, so it’s possible I am missing something. I have to say that I am encouraged by this article, first by the emphasis on thermodynamics as involving “net flows” of heat energy. When I try to understand the GHC theorists, even the more skeptical “lukewarmers”, it seems they are always saying that CO2 is the “rich guy” on the block when it comes to interrupting or reprocessing net heat flows — but there is really no good reason for this. At the same time, when it comes to discussing the effect that two parts per million of methane might have, some of them really double down and say that each part of methane has *86* times the effect (or whatever other factor), as compared to the heat trapping effect that mere CO2 has!
The last I checked, 86 times zero was still essentially zero! Maybe that’s over stating my sense of skepticism a bit — but not much.
Digging in just a bit deeper, if I am understanding Willis Eschenbach correctly, he also seems to be saying that one layer of atmosphere can effectively “hide” the heat difference involved in looking at another layer farther away. In other words, heat conductivity is primarily a locally transmitted effect, with *all* gases *in principle* regulating or resisting heat flow? For gases, the basic resistivity to heat flow is much better than you get with a pane of glass or a block of granite, say. So all the talk about IR escaping directly to space is putting emphasis on what must be just a minor effect at best. On the most basic physics level, resistivity (or it’s inverse, conductivity) has to be much more important. This is my takeaway from the writer’s talk of one layer “hiding” the layer behind it.
In essence, while it may be that not *all* heat conductivity is due to local contact, one layer to another, I’m sure that wherever the atmosphere is reasonably dense, *most* heat flow, *at least as a first principle* is going to tend to go Eschenbach’s way, by direct contact, not by radiation.
Now the real “zinger” here is that, notwithstanding all that I’ve said above, there is *another* universal or omnipresent heat moving mechanism that does get a lot of play here on WUWT, and that is *convection*. Most people seem to think that planets like the earth and Venus are generally trying to conduct heat generated by the ground ambient temperature, i.e., trying to conduct that heat flow up toward the sky. However this heat flow direction could easily be sporadic and variable, so why does this flow of heat so readily result in a steady temperature gradient or adiabatic lapse rate , with the temperature dropping from ground level going upward?
It just seems to me that the temperature lapse rate is a kind of persistent quasi-stable effect, with no prospect whatever of planets reaching anything like thermodynamic equilibrium for billions of years, if ever. My reasoning here is that any column of air that more or less matches an ideal temperature gradient is therefore going to be right on the edge of initiating convection, where any possible move toward actual convection will either try to move overly warm air upward, and/or will try to move extra cold air downward.
If convection really does tend to “teeter” on the edge of moving heat in this way, then *there’s* your basis for a persistent dry adiabatic lapse rate!
So, in other words, looking at any preconceived idea we may have about planets moving toward “thermal equilibrium” or “maximum entropy” or maybe saying that “atmospheres should move to being isothermal”, why, these things are in reality, nothing but a textbook writer’s dream.
So I’m saying that planets as such have other ideas about what to do, regardless of what *we* think is the ultimate maximum entropy endpoint.
Welcome to the discussion. A few pointers to help you get started.
H2O and CO2 do indeed absorb longwave infrared radiation, whereas N2, O2, and Ar do not. This is easily demonstrable in the lab with repeatable experiments and has been well understood for over a century. Look up some references on basic spectroscopy.
The reasons for this are that H2O, CO2, and similar molecules have polar covalent bonds that can interact with IR radiation. They also have more than two atoms, so have vibrational modes at the frequencies of some IR radiation.
Remember that radiation is the ONLY method the earth and its atmosphere have for energy transfer to and from the rest of the universe. So it is NOT a “minor effect at best”. Thermal conductivity in the atmosphere is so low that it can be ignored compared to radiation and convection, at all levels of the atmosphere.
The earth’s atmosphere is hotter at low altitudes than high because it receives energy primarily at the bottom from the surface, and loses energy primarily at the top, from radiation to space. (This would not be the case with a “transparent” atmosphere, because all radiation to space would need to come from the surface.)
The “resistance” of our atmosphere to the passage of IR radiation from surface to space is large enough that the vertical temperature gradient (lapse rate) it creates in the atmosphere would be greater than the adiabatic lapse rate. This condition is known in meteorology as an “unstable lapse rate”; when this is present, convection starts, which tends to reduce the lapse rate toward adiabatic. This is why the adiabatic lapse rate is prevalent. But remember, this is only true because of “greenhouse gases” in the atmosphere.
Many times in these discussions, people use the terms “thermal equilibrium” or “thermodynamic equilibrium” when they strictly should be talking about “steady state” conditions. This is the cause of a lot of confusion. As long as the sun is shining, the earth will never be in, or close to “thermal equilibrium” as it is technically defined.
+1
ED,
You don’t need GHGs to create a lapse rate.
Rising air would still convert KE to PE as it rises and thus would still show a cooling gradient which is fully reversible on the subsequent descent.
KE registers on sensors as heat but PE does not and that is why one sees cooling with height when PE gradually replaces KE as the rising column does work against the downward force of gravity.
Ed Bo November 26, 2017 at 4:28 pm Edit
I’m sorry, Ed, but that is simply not true. Both N2 and o2 can absorb and emit longwave IR, although only in a narrow band. Argon, like neon, helium, and zenon, are monatomic gases and neither absorb nor emit IR. Regarding O2, here’s a bog-standard diagram …
See the O2 in there, busily absorbing LWIR? I could find you a bunch more such graphs if you wish … as for N2, it absorbs more weakly, but it does absorb …
See also here, Figure 6.3, for a graphic that separately shows the absorption bands of O2 and O3 molecules
w.
Willis, first of all, that peak near 10 um is actually O3, not O2. So O2 itself is responsible for very little absorption of thermal IR.
Furthermore, this seems a lot like arguing against a statement like “aluminum conducts electricity, but glass doesn’t”. Glass has a large but finite resistivity, and hence does conduct electric current. But for any normal conversation, glass is not a conductor. Similarly, since N2 and O2 are orders of magnitude worse at absorbing/emitting IR, to a first (or second or third) approximation, we can say pretty safely ignore them.
There ARE cases where the conductivity of glass matters, just like there ARE cases where the absorption of IR by N2 is important. But global energy balances are not one of those cases.
Tim Folkerts November 27, 2017 at 9:35 am
Not only do I know that, I gave a link that specifically shows the difference between the two. So I’m not sure what your point is. Did you read the link?
Look. I was responding to a statement that O2 and N2 are unable to either emit or absorb IR. I produced a graphic and other evidence showing that they do. And no, O2 and N2 are not “orders of magnitude worse” than other absorbers.
Sez you … but the people who designed the MODTRAN calculator used for just such balances disagree. They calculate the effect of both N2 and O2 on the infrared radiation. Your claim that it is so small that it is simply ignored is not true in the slightest.
w.
Willis:
I appreciate your clarification. I was trying to keep things short and sweet.
While I agree that N2 and O2 can absorb some IR — primarily from dipoles created at the moment of collision — everything I have seen when I have looked at the magnitude of this effect has indicated that it is far, far less than that of the molecules that absorb IR even when not in the moment of collision.
Steven:
In a transparent atmosphere, what will create the lapse rate sufficient to start convection? No absorbed IR energy in the lower atmosphere, no IR energy emitted to space from the upper atmosphere.
In a day/night cycle, you are as likely to get a temperature inversion as the lower atmosphere conducts energy to the cooling surface that is radiating directly to the 3K effective ambient of space.
David
I agree with your point about convection, as di others on this thread such as Steven Wilde.
It’s all well-established theory of Benard convection:
The Benard or convection instability is manifest in a situation in which a fluid layer is heated from below and kept at a fixed temperature above so as to create a temperature gradient in opposition to the effects of gravitational force. At small values of this gradient heat is transported from lower to upper regions by conduction [and radiation] and macroscopic motion is absent. Random motions of the molecules and a damping of convection currents characterise the state of the fluid. However, when the gradient exceeds a critical value a convective, macroscopic motion occurs generally in the form of rolls or hexagons (for variations see Koschmeider 1977). In short, out of an initial state that is completely homogeneous there arises a well ordered spatial pattern. Moreover, with further increases in the gradient the spatial pattern becomes oscillatory.
Kugler PN, Kelso JS, Turvey MT. 1 On the Concept of Coordinative Structures as Dissipative Structures: I. Theoretical Lines of Convergence. Advances in Psychology. 1980 Dec 31;1:3-47.
Actually there is such a thing as a critical radius of insulation around a hot pipe where the thickness of insulation increases the exposed area of the outside diameter of the insulation whereby the heat flow thru the insulation increases as more insulation is added thus decreasing the temperature of the insulated pipe.
I have sitting on my desk at home a lava lamp that my son once gave me as a gift. It is not the greatest lava lamp – it is under-powered and I suspect it was very cheap – and it often has trouble getting up to operating temperature. On a cold day in winter it struggles to get warm enough to work at all. On a warm day however it works no problem.
Perhaps some of the “cold things cannot warm hot things” people would like to consider how it is that my cold (by comparison) office can warm the hot lava lamp. In winter, to help it get going, I sometimes put an aluminium foil cover over it for a while. Works a treat. Can they explain why is that helpful?
Ian:
No fair to use a simple real-world example. You are going to make heads explode around here!
Great example Ian
It is fascinating to what length some people go to deny this simple fact, and even deny that everyday examples from our daily life is happening.
I met one who denied that wearing insulating clothes on a cold day will make your body warmer, absolutely astonishing.
/Jan
I don’t understand why you (and others) use a completely ridiculous term like “warm”, when that is clearly not happening. Even a primary school kid could understand that the warmer air on a hot day is not warming the lamp. The heater in the lamp is warming the lava, but on a cold day that heat escapes into the atmosphere around the lamp faster than the heater can heat the lamp. That’s so simple, really. So your aluminium foil prevents the heat from warming the atmosphere, but the aluminium doesn’t warm the lamp, for goodness sake!
To me it seems that a lot of people here and in general should have paid more attention in grammar class to have been able to express themselves in way that others can understand. And possibly understand the own nonsense they spout as well, which happens far too often.
Quibble with the word “warm” all you like – I really don’t care about the word “warm”. Just notice that the lamp with foil ends up hotter than the lamp without foil. And you even correctly stated why. Just one tiny step more and you’ll be there.
The heater (sun) is warming the lamp (earth). The aluminium foil (greenhouse gases in the atmosphere) prevents the heat from warming the room (escaping into space). Consequently the lamp (planet) ends up at a higher temperature with the foil (with greenhouses gases present) than when there is no foil (without greenhouses gases present).
While the above argument is good enough to get you to the greenhouse effect (hopefully), it isn’t fully correct. Because actually (wait for it) the room REALLY DOES warm the lamp. The flow of heat is bidirectional. It is just that a lot more heat flows from the lamp to the room than is flowing from the room to the lamp. Heat isn’t water – it can flow both ways simultaneously. If we stand in a dark room and shine torches at each other then the light from my torch and the light from your torch is going through the same space in opposite directions. Replace the torches with heat lamps, it works just the same. Heat can go both directions at the same time.
There’s only one problem with the idea (which we basically agree on): The frenzied demonisation of CO2 as the big culprit can be shown to be patently false. CO2 acts as an excellent conductor, better that air in general, so it should be the last worry in the mixture that’s acting as a sort-of insulator that prevents the from earth cooling off as quickly as the moon (essentially without an atmosphere) is doing at night. But all this has absolutely nothing to do with the quest of misguided misfits that are trying to get us to part with advances in technology, money (and lots of it) and lots of other things in the name of “saving the planet”. We’re not going to fry, CO2 is good for the earth and climate change politics is founded on blatant lies and deception.
Ian
Problem with your example-
Lava lamp-“on a cold day in ,it struggles to get warm enough to work at all. On a warm day however, it works no problem.”
Cold office-struggles-a cold office on a cold winter day
Wamer office-lava lamp doesn’t struggle as much
On a cold office day, that cold air does NOT “warm” your lamp does it? The air around your lamp is the atmosphere-with greenhouse gases and everything already in it. In fact, being indoors where you breath etc, it’s probably got a higher than outside concentration of Co2 than outside air does.
When you turn up the thermostat, or the “summer temps” outside cause your office’s atmosphere to be warmer than it is on cold winter days, your lava lamp warms up faster.
Now, put the foil around your lamp without turning it on. Does the foil cause the lamp to get warmer than it would be without the foil? Nope.
Turning on the heated base of the lamp is what WARMS the lamp. Not the foil. And that base is hotter than the lamp. Now put foil on the lamp. the Foil is a CONDUCTOR. (air is an insulator) it heats up quickly and conducts the heat from the lamp (which is heated by the base) directly by conduction back into the lamp. But if you touch the foil, you will feel heat radiating out of it into the room as well. More of it is just being reflected and conducted back towards the lamp.
CO2 in our atmosphere does not work like the foil. The foil is “colder” at one point, but once it absorbs the heat from the lamp base, it becomes a “warmer/hotter” object than the glass and liquid at the surface of the lamp. It’s no longer a “cold” object warming a hot object. And the source of the warming, the entire time, is the lamp’s base.
“The heater (sun) is warming the lamp (earth). The aluminium foil (greenhouse gases in the atmosphere) prevents the heat from warming the room (escaping into space). Consequently the lamp (planet) ends up at a higher temperature with the foil (with greenhouses gases present) than when there is no foil (without greenhouses gases present).”
No it does not, because unlike the lamp the Sun is exterior to your Foil not interior to it.
Without Water and GHG the Earth would get Much Hotter during the Day and Much Colder during the night.
The Water and the GHGs reduce the variability of the Earths temperature as does the Atmosphere in general, but may allow it to end slightly warmer overall.
If you don’t believe me take a look at the Temperature of the Moon’s Surface
253F to – 243F.
Of course the moon is not rotating, but that doesn’t make that much difference.
Why do people keep referring to Occam’s Razor
instead of the simplest explanation:
the simplest explanation?
+1 I’m going to plagiarize that.
Hocus Locus November 26, 2017 at 4:54 pm
Hocus, we do it because Occam’s Razon does NOT imply that we should choose “the simplest explanation”. Instead, directly translated from the Latin that William of Occam wrote it in, Occam’s Razor says:
“Plurality should not be posited without necessity.”
Note the clause about “necessity”. It is often stated as:
“Do not multiply causes without necessity.”
Again, the “without necessity” is very important.
FOR EXAMPLE:
The simplest explanation of lightning is that it is the thunderbolts of the Gods.
The actual explanation involves the mechanics of thunderstorms, the nature of static electricity, the involvement of cosmic rays, the list is long but finite.
Should we follow your lead and take the simplest explanation for lightning?
w.
A very large rock, the size of earth with no atmosphere will equilibrate with S incoming shortwave energy and L outgoing longwave energy. S=L. If you add an atmosphere that is transparent to shortwave and translucent to longwave with factors h that tells you how much longwave does not get out and 1-h that goes back to the surface you can model a set of iterative equations to describe it.
The equations are moderately difficult power series that can be treated as Taylor series expansions of simple equations. What you get (reduced as n approaches infinity) is the surface is impinged with:
S from the sun and L/(1-h) – L from the atmosphere.
Add them up and you get S + L/(1-h) – L and since S=L the surface input energy is just:
L/(1-h)
At equilibration of the surface all of this is returned to the atmosphere and what gets out to space is:
(1-h) x L/(1-h) = L
Put some numbers on it….. Let S = L = 300 joules and let h = 0.1
You have 333.33 joules hitting (300 SW, 33.33 LW) and leaving (333.33 LW) the surface . You have 300 LW joules leaving and 300 SW entering the system. The surface is equilibrated and balanced, the system is equilibrated and balanced.
This is where intuition (and failure to do the math) fails. Yikes! You’ve created energy they say! Where did that extra 33.33 joules come from. Well it came from S, over time. See the atmosphere is not a blanket. It’s not an insulator. And it’s not adding heat.
It IS changing the equilibration point of the surface. Adding atmosphere doesn’t instantaneously get you a new equilibration. It might take thousands of years, but when you impede the outflow of energy the planet MUST heat up to create enough outbound energy radiation to equilibrate the planet-atmosphere system again. The entire system is operating at a higher energy level when you add an atmosphere. Any atmosphere. Doesn’t have to be GHG (hate the term because it has nothing to do with what is going on). Just molecules that absorb and reradiate LW energy. They get you the translucent mirror effect. Don’t ding me here. I know it’s not a mirror, just a mirror effect.
The atmosphere didn’t add energy. The sun did that over time. It doesn’t require blocks of wood or sheets of ice somewhere else in the universe. It doesn’t require a lapse rate or convection or conduction. Sure all these things exist and are important ‘add ons’ but never forget that all it takes is molecules to get some effective change to the equilibration point. As a matter of fact, it doesn’t require talking about heat or temperature at all. It’s all about energy.
Willis is not wrong. He has reduced the concept to its simplest form and the math bears it out. My feeble explanation is simply an attempt to shine a different light on it so that more might see.
Note:
Another favorite rebuttal to this model is that the energy coming from the atmosphere can’t heat the planet. It’s an admission to downwelling energy with the caveat that it doesn’t do anything because the surface is warmer than the downweilling stuff.
Well then you have defined a perfect reflection. Not only that, it is a temperature dependent reflection you propose. Think about it this way. If the surface is T and the downwelling is T-1, you are saying that it doesn’t (can’t) do anything. What does it do at T? What does it do at T+1. Finally, what does it do if the planet moves to T – 2. Does it suddenly start to absorb the previously reflected downwelling? You are hypothesizing a feature that has NEVER been observed in nature……
albedo = f(T)
Really?
Paul Bahlin
Long, nice elaborate … flat earth theory of a flat rock being illuminated on only one side at a mythical circular orbit.
Rotate away! Doesn’t change the boundary conditions one bit
One thing I’ve yet to understand is for the GHE hypothesis to be correct, with rising atmospheric CO2 levels, the tropical troposphere should be rising at a much higher rate than the surface. Yet we are observing an “upside down” GHE.
Both Dr. Roy Spencer and Dr. John Christy agree with WUWT’s official position on the subject, yet both acknowledge if that’s the case, the ubiquitous “hot spot” should be there. I’ve seen John Christy say what the theory states and that the data doesn’t support it.
This a good clear and, above all, a physically correct article. However, while the atmosphere as a receptacle of heat from the sun and to a much larger extent, the surface of the warmed earth, a far greater receptacle ifs the surface of the earth itself. Forget about carbon dioxide, forget about water vapour. The heat from the daily dose of sunshine is retained by the earth and keeps us and the atmosphere warm, either by contact between the air and the earth’s surface – with conduction which warms the air in the tropics and cools it in the higher latitudes – i.e. the air does then warm the earth by the exchanges described in this article by Wills.
For every degree centigrade (or Kelvin) that the air changes temperature in its 250 km column above us, the energy changes by 1.380,000 Joules. For every degree thet 1 metre of earth beneath us changes by 1 degree, the energy changes by 8,400,000 Joules, nearly six times as much as the whole of the atmosphere. Thus the earth is a much more significant player in stabilising our temperature. The sea, for instance, absorbs sunlight to a depth of 200 m, most of the energy being absorbed, probably in the top 10 m. But its temperature remains stable to about +- 0.5 degrees in calm conditions. In this case, the oceans, which cover 70% of the earth’s surface, absorb of emit 42,000,000 Joules for every degree change in the surface temperature. That is 30 times more efficient than the atmosphere. John Nicol
Thanks to Ed Bo in particular for his reply to my post. I agree with some of his comments about the central role of convection in maintaining a temperature vs. altitude lapse rate. In my internet searches I’ve found it difficult get a really clear or complete explanation of this. So, it’s interesting that this apparently key idea finds currency with Ed Bo.
There are a couple of things about Ed’s reply, however, that I might want to clarify or question further. First, in his reply, he says “radiation is the ONLY method the earth and its atmosphere have for energy transfer to and from the rest of the universe. So it is NOT a “minor effect at best”. In my original post, I qualified my comment about radiation being a minor effect (in my next paragraph after mentioning it) by saying that my “minor effect” idea is really only true “wherever the atmosphere is reasonably dense”. This is an important point, as I expect that our planet does have to radiate all of the heat it receives, by radiating from some quite high layer of the atmosphere. The higher, less dense layers (say, at tropopause height or even higher), are a lot more likely to be radiating the earth’s overall heat output, as compared to anything likely to be occurring much lower down (such as say, radiating right from the surface). I think I am echoing Willis Eschenbach’s ideas in thinking that there should not be too much mass of atmosphere above whatever level we might regard as a major “into deep space” radiating layer. Specifically, if the temperature of the cosmic background is roughly minus 270 Celsius for these purposes, we wouldn’t want much of an insulating layer in the way to take advantage of that, in radiating outward.
Having made that clarification, the comment that I see in Ed’s response that I *still* very much question is essentially the usual GHC or greenhouse effect perspective on things. The response here is the comment that:
“H2O and CO2 do indeed absorb longwave infrared radiation, whereas N2, O2, and Ar do not. ”
Now, truly, I can easily web search questions like “is argon a greenhouse gas?” , and get wiki entries, web pages, etc., that explain over and over again, how argon is not a greenhouse gas, how argon in particular is “transparent” to IR trying to escape earth’s surface, and how it is therefore up to greenhouse gases like CO2 or methane to trap, absorb, re-emit, or ‘downward emit’ lots of infrared. It is made to seem that, without these key minor gases, the atmosphere just could not restrict or regulate the flow of heat, since N2, O2, and Ar ” are “transparent to IR, and therefore transparent to heat flow, or so it is made to seem?
At this point, why not switch gears and think about how gases are used in home window installations these days? Double paned windows typically have a two centimeter air gap between them, as this is apparently close enough to prevent much convective heat transport. At the same time, the heat resisting effect of air, or even pure nitrogen, say, is something like *forty* times better than putting an equivalent two centimeters of glass in between! Further, I understand that the best windows use, not nitrogen, but “argon”. Argon has a sufficiently larger molecular weight compared to nitrogen that it is, again, much “better” than nitrogen in slowing the flow of heat, and no special “greenhouse” property is needed for this to work!
If I carry on a bit further on this and ask if it might be a good idea to replace argon with CO2 for this purpose (and checking a table of gas conductivities), I then find that CO2 is almost identical to argon in this regard. Conductivity (or it’s exact inverse, resistivity) has nothing to do with whether the gas in question has “greenhouse” absorption lines in its spectrum.
Going on further still, suppose I try to replace a window’s argon with methane, that greenhouse “super” gas? What I find *then* is that methane in a window would conduct heat much “faster” than nitrogen or argon ever would!
The thing to note well here, is that I am describing a practical engineering reality. The people who sell windows and the people who buy them, are *sure* that argon is a much better resistor to heat flow than nitrogen or whatever. Comparing argon to CO2, there may also be, I think, a financial reality in the sense that argon may possibly be somewhat cheaper to obtain than CO2? My thought here is that since argon is roughly twenty times more abundant in the atmosphere, it might be cheaper to get, especially if I assume the approach is to liquify and distill air as such.
So, there you have some further comments. Thinking about transparency and actual absorption of specific frequencies, I should only care about *that* if I am pointing an infrared camera through my window, trying to get the best possible image! At the same time, overall resistance to heat flow is quite a different thing! How did absorption lines ever become conflated with resistivity, anyway?
David,
I have been investigating and explaining the mass induced surface temperature enhancement since 2007.
Various relevant articles can be found here:
http://www.newclimatemodel.com/latest-articles/
David:
I have recently been playing with the thermodynamics of multi-paned windows. If you start looking at the physics in detail, you see significant differences.
In a double-paned window, the gap between panes is only about 10mm, and the temperature difference between the inside surfaces of the panes (which are virtually completely opaque to LWIR) seldom exceeds 10K.
So in this case, conductivity is the dominant mode of heat transfer between the panes. Any absorption by CO2, and there won’t be much, has very little effect in this case.
Contrast this to the atmosphere, where there are kilometers of gas between the surface and “space”, and a huge difference between surface temperatures of ~250-300K and an effective temperature of space (for radiative purposes at least) of just 3K. Here the thermal conductivity of air is not the dominant factor.
So why argon instead of CO2? Because it’s cheaper! Even 99.9999% pure argon (far purer than needed for this purpose) is only about 40% of the cost of CO2.
Jan Kjetil Andersen November 26, 2017 at 8:40 pm
Great example Ian
It is fascinating to what length some people go to deny this simple fact, and even deny that everyday examples from our daily life is happening.
I met one who denied that wearing insulating clothes on a cold day will make your body warmer, absolutely astonishing.
……………………………………………………
They do not make you warmer, they stop the cold atmosphere drawing the heat out of your skin, they insulate, thats why you take coats off indoors.
Try putting putting the clothes and coat on a rock outside on a cold day, tell us how much warmer the rock gets.
Gary, The rock does not have an internal heat source so it cannot warm up.
A living organism such as a human burn calories and therefore generate heat. This heat will make you warmer unless you can get rid of it. If the clothes are thick enough you will indeed get warmer.
Willis,
Thank you for confirming that radiation from a cool object cannot increase the temperature of an already warmer object. That is the first time I have seen you explicitly say that.
If you agree with that, then I think you then have to agree that sometimes electromagnetic emissions raise temperature and sometimes they do not.
Consider the example of a cold bar of steel. If a white hot piece of steel at 1500 c was placed next to the cold bar of steel, no doubt that the hotter bar will cause the cold bar to warm up.
Now consider starting with a white hot steel bar, at 1500 c. Then a second steel bar at 1500 c is placed next to it. Even though the second white hot bar is emitting a lot of radiation, the temperature of the first white hot bar will not increase at all. They will both stay at 1500 c. This would be the case even if 100 white hot bars were added, the temperature of the first bar would not go up. In this example, temperature would appear to have nothing to do with the massive emissions being added!
So, now you agree that sometimes electromagnetic radiation increases temperature and sometimes it does not. So I think you now must agree that although energy might always be additive (like in your money exchange example) temperatures are not. Your money flow example is perhaps too simplistic.
In your steel greenhouse essay, you assume both the surface and the shell are black bodies, emitting back and forth to each other. This means that they absorb 100% of all radiation at all wavelengths and that the energy is all converted into heat. They also emit at all frequencies causing temperatures to fall. The final temperature is determined by the net flows. The math is beyond me but I’m guessing that model does not allow for energy to be added without an increase in temperature? If so, it does not match the real world. I really don’t know if that guess is correct. If it is, your steel greenhouse example is a truism in that you prove a greenhouse effect because you build the necessary conditions for it into your model. I offer that as a genuine question to the mathematicians here to confirm or refute. Apologies in advance if my guess is incorrect.
You do say that the green house effect does not need to actually increase the temperature of the atmosphere. It would still work by reducing its cooling. I can think of a mechanism for this as follows:
Say the bottom layer of air has a temperature of 15.0 c. The next layer up has a temperature of 14.9 c and the layer above that 14.8 c etc etc. If a GHG is added that slows cooling, the 15.0 c layer could cool to 14.9 c and then be warmed by the 14.9 c layer to stop it cooling further. The 14.9 c layer could cool until it reached 14.8 c at which it would be warmed by the 14.8 c layer etc etc. In this example, the atmosphere does cool but each layer would be 0.1 c higher in temperature than if the GHG was not there, yet at no time is a cooler layer required to increase the temperature of a warmer layer.
That mechanism would work provided that the GHG did in fact slow the cooling. I am not saying if that is true or not. In fact, intuitively, it looks like the opposite might happen. If I replace a molecule of O2 with a molecule of CO2 then the CO2 will start emitting radiation that the O2 was not doing. Half of those emissions would go up and be lost to space. The CO2 would then bump into another warm air molecule, become energized again and then emit some more emissions, half of which would be lost again to space. It would seem to me that the CO2 would strip the heat out of the rest of the air and emit it to space. The fact that some of the emissions go down doesn’t change the fact that lots of new emissions go up, presumably cooling the air … even close to the surface?
In summary, I agree emissions go back and forth between warm and cold bodies; sometimes they raise temperatures and sometimes they don’t; a cool body cannot raise the temperature of a warmer body; slowing cooling would raise temperatures above what they would otherwise be; since CO2 radiates more than O2, it seems that GHG effect for CO2 would in fact be negative.
Best regards
“since CO2 radiates more than O2, it seems that GHG effect for CO2 would in fact be negative.”
Right so we have an atmosphere of O2 (say).
LWIR from the planets surface is unhindered in it’s exit to space. Yes?
How can that NOT be otherwise than maximum cooling
We have an atmosphere of CO2 (Venus).
LWIR is greatly hindered from exiting to space. Yes?
How can a substance that hinders exit to space allow more cooling as apposed to less cooling?
Thats like saying your duvet will make you colder overnight than if you didn’t have one!
This idea that emission after absorption is more efficient than none at all is bizarre.
The point is the emission takes place at a higher altitude in the atmosphere which is necessarily colder and therefor less efficient.
Net result a slowing of cooling.
It just does.
“They will both stay at 1500 c.”
Oh dear – when did anyone ever claim something would get hotter due to a colder object?
What your example does is simply confirm Willis’s article. If the bar stays at 1500c in one case, but drops to 1000c in the other case, then the 1500c case is hotter than the other case. That’s all anyone ever expected, not that it’s temperature would rise.
Bernard Lodge November 26, 2017 at 10:05 pm
I’ve said that many times. However, you appear to have missed the second part of my opening paragraph, where I said that a cold object can leave an object warmer than if the cold object were not present. Let me ask you what I asked 1sky1, viz:
Suppose a hot object is at thermal equilibrium, say with a block of ice near it. The object is heated by a heat lamp, cooled by the ice block, and reaches thermal equilibrium. We then put a block of cold foam between the ice and the hot object, shielding the hot object from the radiation coming from the ice … and guess what?
The object gets hotter! Is it warmed by the cold foam? NO! That would violate the second law.
But the rate of cooling is slowed by the cold foam, while the rate of heating stays the same, and the object ends up hotter than it would be without the cold foam.
Why does this work? Because the foam is warmer than the ice it replaces … just as the atmosphere is warmer than the infinite heat sink of outer space that it replaces.
w.
Willis, nobody with any life knowledge can argue with your example.
What you are doing is describing “Insulation” and everybody knows Insulation works.
Take away the energy source, does the object still get “hotter”? (Which by the way happens every night to the earth)
No, but you will slow the cooling and I have no arguments with that as that is also obvious every day with any weather forecast where there are clouds and also the difference in cooling between Deserts with Dry Air and ordinary earth with moist air.
My concern comes back to the previous post about the “quality” or for me the Characteristics of the CO2 induced Radiation and what Energy it can actually impart to the Surface of the Earth as the majority of it is thermalised by conduction in the Atmosphere.
It all comes back to the question of why it can’t do any kind of work other than making objects colder when concentrated?
A C Osborn November 27, 2017 at 6:11 am Edit
We’ve been through this. The reasons are:
1. Nobody knows any way to “concentrate” environmental thermal IR since it is diffuse and coming in from every angle. You cannot focus it with a mirror or a lens … how are you planning to “concentrate it?
2. Every heat engine needs a cold heat sink into which it returns the rejected heat. That heat sink must be cooler than the temperature of incoming energy. Where is the heat sink at the surface for downwelling IR from a region colder than the surface?
3. There is not enough energy in thermal IR to power the photoelectric effect.
Look, let’s take the atmosphere out of it. We get thermal IR from the objects around us. Recast your question about why we cant use that IR for useful work …
Regards,
w.
You say:
“Suppose a hot object is at thermal equilibrium, say with a block of ice near it. The object is heated by a heat lamp, cooled by the ice block, and reaches thermal equilibrium. We then put a block of cold foam between the ice and the hot object, shielding the hot object from the radiation coming from the ice … and guess what? The object gets hotter”
The thermal equilibrium is found between the cooling effects of the block of ice and the warming effects of the heat lamp. The rise in temperature is nothing to do with “shielding the hot object from the radiation coming from the ice”, it’s the opposite: you’re shielding the ice from the radiation coming from the hot object. The hot object would lose some internal energy in the form of heat, through radiation, to the ice (which would warm). However, less of that internal energy will be lost with the shield in place, so the object remains warmer. In other words, the heat lamp is then able to warm the object back up to nearer its maximum possible temperature, without the object losing so much of its internal energy to the ice, with the shield in place.
But this is not reducing the rate of cooling through addition of an object of a lower temperature inbetween. This is reducing the rate of cooling through addition of a heat (radiation) shield between objects (blocking the radiation due to the extremely low thermal conductivity through the foam). All it really shows is that if you had just the object and the heat lamp, the object would be warmer than when you add the ice near to it as well, the lower temperature object.
With the GHE, the Earth’s surface is already like the hot object warmed by the heat lamp, without the ice (or with the block in place). It would already be at or near its maximum possible temperature due to the heat from the lamp (the sun). Adding the ice (atmosphere) is just going to cool it down, if anything. NOT warm it. To warm the surface or the “ice” (atmosphere) near to that surface is going to require an additional source of energy, or work being done on it. Now, we have no (significant) additional energy source*, but we do have something that can do work…gravity!
You can point out the atmosphere is warmer than space, and it’s true that space may be thought of as having an average temperature of 3K. In a sense though (it being mostly vacuum) it doesn’t really have any temperature at all. It’s just the radiation passing through it that gives it that, and immediately outside of Earth’s atmosphere it can actually be quite “warm”, “hot” even.
Generally I’d say sun (hot lamp), Earth (warmed object) atmosphere (ice) makes more sense as the analogy.
* There is geothermal energy, but this is not generally considered to be a significant additional source of energy.
Tony November 27, 2017 at 12:26 pm
That is why we’re discussing whether the atmosphere can explain why the average surface temperature on Earth is over 90K higher than that of the moon.
see
Below the seasonal penetration depth ( ~ 10 m or so) the TEMPERATURE is completely caused by geothermal energy. The flux to the surface is very small (~65 mW/m^2 on average) and even nonexistent during the warm season, but the sun only has to increase the temperature of ~10 m of soil a little to arrive at our observed surface temperatures.
Wiilis replied this:
https://wattsupwiththat.com/2017/07/13/temperature-and-forcing/#comment-2564261
to my comments, after which the comments were closed. He seems to believe that the temperature ~10m below our feet is ~40K or so and that the sun is responsible for the increase to our ~288K average.
The atmosphere merely reduces the energy loss to space. With the average surface temperature of 288K (explained by geothermal temperature plus solar heating) and no atmosphere earth would radiate ~400 W/m^2 directly to space (and cool down quickly)
Due to the atmosphere we merely lose ~240 W/m^2 which the sun resupplies => balanced energy budget
and no heating of the surface by the atmosphere required.
Well that’s an interesting hypothesis, haven’t heard that one before. Would the depth that the solar energy can penetrate into the ground (via conduction) be determined by the rate the Earth rotates? In the lunar craters where solar energy never reaches, what is the reason you think the temperature is not at the background temperature for space?
Ben Wouters November 27, 2017 at 3:48 pm
I said NO SUCH THING. I agreed with you that if there were no sun the SURFACE of the earth would be at 40K or so. Please do not misquote me, it merely makes you look inattentive. Here’s what I said:
How you’ve twisted that to a statement about the ten metre depth is beyond me.
w.
Willis Eschenbach November 27, 2017 at 3:04 am
“Suppose a hot object is at thermal equilibrium, say with a block of ice near it. The object is heated by a heat lamp, cooled by the ice block, and reaches thermal equilibrium. We then put a block of cold foam between the ice and the hot object, shielding the hot object from the radiation coming from the ice … and guess what?
The object gets hotter! Is it warmed by the cold foam? NO! That would violate the second law.
But the rate of cooling is slowed by the cold foam, while the rate of heating stays the same, and the object ends up hotter than it would be without the cold foam.
Why does this work? Because the foam is warmer than the ice it replaces … just as the atmosphere is warmer than the infinite heat sink of outer space that it replaces.”
Willis,
Thank for your response.
You often begin your comments with ‘Suppose an object is at thermal equilibrium’. In the real world, there is no such thing as thermal equilibrium as it would require a constantly varying heat source that exactly counterbalanced the movement and heat content of every other bit of matter in the universe. At first glance, it might seem I am being pedantic but I think that ‘technicality’ is important.
In your example above, when you describe a ‘hot’ object in thermal equilibrium next to a block of ice. This implies a third, hotter object that is heating the ‘hot’ object. There is also a fourth ‘object’ – outer space. So, conceptually, you have a ‘hotter’ object, a ‘hot’ object, some ice and then outer space. You then introduce a fifth object – some foam in between the ‘hot’ object and the piece of ice.
To make this scenario more visually understandable, imagine you place all five objects in declining temperature from left to right:
‘hotter’ object, ‘hot’ object, foam block, ice block, outer space
You agree that a colder object cannot INCREASE the temperature of a an already warmer object. Which to me implies that any of the five objects above can only increase the temperature of the objects to their right. They cannot increase the temperature of any object to their left. I would argue that changing the order of any of the objects on the right will still not allow them to raise the temperature of any object to the left of them. So the foam could be placed either side of the ice and it still would not make the ‘hot’ object hotter.
My ‘proof’ of this conclusion is what I said in my earlier comment. Although energy joules are additive when considering energy flows, temperature is not. My best example of this is starting with a 1 kg white hot piece of steel at 1500 c. If you then place another white hot bar next to it, the temperature of the first bar will not increase at all, even if the second white hot bar weighed 1000 kg! This seems to defy all ‘energy budget’ logic. How could you introduce so much new energy and not get an increase in temperature? The answer is because it’s against the law … the second law of thermodynamics that is! 🙂
I did not have to introduce the infamous ‘start with a body at thermal equilibrium’ to reach my conclusion. Which I think is where you are going wrong.
Tony November 27, 2017 at 5:04 pm
Yes, plus the effect of Earth’s tilt (seasons). Also the structure of the soil plays a role (dry, wet, sand, rock etc.etc.)
More interesting are the oceans. Comparable idea, different mechanism.
If not solar heating the rim from the outside, it must be the geothermal flux. The moon is supposed to have a hot core as well. And a flux comparable to the one on earth can explain these temperatures. (25K or so) very well.
Willis Eschenbach November 27, 2017 at 5:56 pm
Your own ‘simple algebra’:
The temperature at ~10 meters is ~288K on average, and COMPLETELY caused by geothermal energy.
The 40K number will only be reached if the sun shuts down AND the Earth is allowed to cool down until the flux through the crust is equal to the radiation from the surface directly to space (no atmosphere).
Ben Wouters December 1, 2017 at 4:51 am Edit
Your own diagram shows a layer of permafrost … so I doubt greatly that the earth there is at 288K (15°C). Here’s a look at a number of years of data in Deadhorse, Yukon territory …
There are two things of note. First, temperature variations extend beyond the depth shown in the graph, which is 55 metres. Second, it is cold all the way down to 55 metres and well beyond.
This is in total contradiction to your claim that the soil at 10 metres is 15°C …
It is also in contradiction to your claim in the previous thread, viz:
Clearly, that’s not true. The lack of solar energy in Deadhorse has left the ground far below zero to a depth of 55 metres.
If we remove the sun, you say the surface temperature will be -40K. So are you agreeing with me, that everything above 40K has to be from the sun? Because before, you said that somehow the base temperature was 288K, or 15°C, and the sun only needs to warm us up from there … but Deadhorse says different.
Finally, you claim that the ocean temperature at 300 m is the “base temperature” for the oceans. Typically that’s on the order of 4°C or so. But if we remove the sun, the oceans won’t stay at that temperature. They would freeze solid … which means that what is keeping them from freezing is the sun.
Truly, I don’t get your logic. Your claim is that the “base temperature” without the sun is 15°C … but that’s the average temperature of the planet already. If I follow your logic, the sun doesn’t need to heat us at all, we’re already there …
Perhaps if you gave me the elevator speech, the short few paragraphs that distill the important ideas down to a clear statement of what your claim is, it would all be clearer.
Because I still don’t get it.
Thanks for persevering,
w.
Willis Eschenbach December 1, 2017 at 5:54 am
Couldn’t find a similar clear diagram without the permafrost. The principles remain the same regardless the temperature at the surface.
Notice I wrote 288K ON AVERAGE. The temperature at the maximum penetration depth (MPD) is normally ~equal to the average surface temperature.
With Deadhorse you’ve probably found one of the cold spots created during the last glaciation:
https://en.wikipedia.org/wiki/Geothermal_gradient#Variations
Obviously not. The sun slowly warms the soil in spring and summer up to the MPD. In autumn and winter that same soil cools down again.
Incoming solar has to be thermalized and increase the temperature of soil or water.
Total solar energy during a whole day is just able to increase the temperature of 10m of water about 0,5K.
Thinking in radiative balance doesn’t make sense for a water world. The day side of our moon however is close to radiative balance with incoming solar. The night side is way above radiative balance temperatures.
The temperature INCREASE in the soil up to MPD or in the oceans in the mixed layer is caused by the sun.
Resulting surface temperature is the base temperature plus the temperature increase caused by the sun.
On a planet with a cold (0K) interior, the sun would only warm the surface beginning from this base temperature.
You have to think in amount of energy supplied by the sun warming how much material having how much heat capacity.
Ben, the logic of GHGs warming the world does not stack up when comparing the Earth to the Moon.
What GHGs and the Water (in our water world) appear to do is decrease the Amplitudes involved in solar heating and Non Solar cooling, ie it provides Stability, not extra heat.
This is also borne out by comparing Dry Desert Areas with moist tropical areas, deserts get both hotter and colder, just like the Moon.
A C Osborn December 2, 2017 at 10:00 am
This stability is due to solar penetrating (and warming) at least the upper 10-20 meters of water directly. Combined with the very high heat capacity of this water gives the temperature stability.
see eg.
Sun only influences the temperature of the upper 150-200m. The temperature of the DEEP oceans is created and maintained by geothermal warming minus cooling by sinking cold water at high latitudes.
eg. Antarctic Bottom Water.
For continents the the sun only warms the upper 10-15 m. The temperatures below that are geothermally caused;
http://www.mpoweruk.com/images/geo_temperature.jpg
To me it is obvious that the sun is very well capable of increasing the upper layers of land and ocean to the observed temperatures. The surface in turn warms the atmosphere (plus some direct heating by the sun), which merely reduces the energy loss to space. (Insulation effect, no greenhouse effect)
Thank you.
Ed Bo
November 26, 2017 at 3:31 pm: Well no, Ed.. You are just showing your willigness to deceive. Insulation cannot warm above the available input, and CO2 is the opposite of an insulator among gases (read Hottel for instance). NASA has, and we have for safekeeping from criminals, the solar system data which proves atmospheres above 0.1bar can be figured accurately for temperature by Solar distance and gravity alone. As Maxwell, who some imagine they are smarter than, figured out well before Arrhennius’ musings (refuted by Wood).
But it does not matter about the pride of bit players. Nature is in the process of showing us what it is talking about. CO2’s role may be to help plants survive extremes of drought and cold, under the Quiet sun regime.
Thanks Brett! Phew! 🙂
I’m having a hard time believing that warmists just have blind faith in the hypotheses of others without also having a hidden agenda. Surely no-one can be that stupid?
Brett Keane November 27, 2017 at 12:15 am
First off, accusing people of deception without adducing proof is a nasty habit that you should work to rid yourself of. Your accusations in this thread have totally destroyed your reputation with me. When you started, I didn’t recognize your name. Now I shudder when I see it … and I doubt that that is the effect you are striving for.
Second, your actions are particularly unpleasant because I asked you to keep it civil, viz:
Third, I’ll ask you what I asked 1sky1 and Bernard, viz:
w.
Willis Eschenbach: what is the mechanism how CO2 reduce the rate of cooling of the atmosphere? In real life insulating materials are used. But can CO2 be an insulator because it absorbs and emits the same amount of heat?
“But can CO2 be an insulator because it absorbs and emits the same amount of heat?”
Yes.
Because the emission is less efficient than the absorption. ….. the photons finally escaping to space being emitted by CO2 molecules higher in the atmosphere and therefore colder.
How does an insulator work? An ideal insulator “returns back” the same amount of heat that it receives. CO2 is not an ideal insulator; it returns back only 50% of heat received. The other 50% it radiates forward. (That all applies only to photons in the CO2 absorption window. Water has more absorption windows, therefore it is a much better insulator).
“Water has more absorption windows, therefore it is a much better insulator).”
Not really as at 15 micron which is the planets most intense radiating wavelength is significantly overlaps WV.

Yes, but the rest it emits weakly to space as it comes from a part of the atmosphere that is around -18C.
Esa-Matti Lilius November 27, 2017 at 4:49 am
Willis Eschenbach: what is the mechanism how CO2 reduce the rate of cooling of the atmosphere? In real life insulating materials are used. But can CO2 be an insulator because it absorbs and emits the same amount of heat?
It does not “absorb and emit the same amount of heat”, as can be seen from space the emissions from the center of the CO2 band are emitted from high in the atmosphere at a temperature around 220K (~50mW.m-2.sr.(cm-1)-1). If there had been no CO2 in the atmosphere then the emissions in that band would have been from the surface at 280K (~120mW.m-2.sr.(cm-1)-1, more than 50% of the emissions have been retained.
Just like a pipe carrying hot water at say 50ºC which is surrounded by insulation the surface of which is at 20ºC.
http://lasp.colorado.edu/~bagenal/3720/CLASS5/EarthBB.jpg
Brett, you say: “Insulation cannot warm above the available input”.
For this statement to make any sense at all, you must be talking purely about temperatures. The temperature of the input source to the earth (the sun) has a surface temperature of about 5770K. I agree that that “insulation cannot warm” the earth above this temperature. But that is the ONLY fundamental thermodynamic limit (there are plenty of practical limits before that).
Then you say: “CO2 is the opposite of an insulator among gases (read Hottel for instance)”.
I do read Hottel, and I was just consulting his charts on CO2 absorption of IR as a function of concentration and distance. The greater absorption (and so less direct transmission), the more effective the insulation.
Next you say: “we have for safekeeping from criminals, the solar system data which proves atmospheres above 0.1bar can be figured accurately for temperature by Solar distance and gravity alone.”
You really need to read and understand Willis’ numerous takedowns that absolutely demolish Nikolov’s and Zeller’s claims. They don’t understand either high school physics or high school statistics.
And no, Maxwell did not endorse the “gravito-thermal” effect. He was in fact the first to authoritatively prove that it could not exist.
There is also the small problem that all warmism is indeed flat Earth calculation ignoring Holders Inequality and the rotating rotundity of our home planet. Just one of the reasons to give up the magic mushrooms.
Believing in the evidence does not make one a warmest. Spewing drivel though, does make one a spinner and fabulist
Brett:
You get Holder’s Inequality backwards. What Slayers like to call the “flat earth” calculation is the limiting case of very high thermal capacitance limiting the diurnal temperature swings and very high horizontal heat transfer lessening the tropical/polar differences to get a uniform surface temperature.
Applying Holder’s Inequality to temperature variations (given the same energy input) means that any variations from this uniformity will result in lower average temperature and therefore lower total energy levels. The bigger the variation, the lower the average. This is why the average moon temperature is so much lower than the earth’s, even though it absorbs more solar radiation.
So a more precise calculation than the simplified “flat earth” calculation actually shows a bigger greenhouse effect to explain our actual surface temperatures.
“You get Holder’s Inequality backwards“
I doubt it, because
“So a more precise calculation than the simplified “flat earth” calculation actually shows a bigger greenhouse effect to explain our actual surface temperatures.“
that’s the whole point. Too big a GHE to be explained by back-radiation.
Tony:
People like Nikolov and Zeller, who take the moon as a direct analog to what the earth would be without an atmosphere and note the very low ambient temperature, fail to note (at least) three other very significant differences that lead to its very high temperature variation and therefore low average temperature:
1. Its day is ~30 times longer than earth’s resulting in plenty of time to heat up and cool down during the diurnal cycle.
2. Its surface is almost all dust (regolith) with extremely low thermal conductivity, so the thermal capacitance of its surface layer subject to the day/night cycle is tiny compared to that of the earth, accentuating temperature swings.
3. The moon has no significant horizontal heat transfer mechanisms, unlike the earth’s oceanic and atmospheric transport mechanisms that lessen the equatorial/polar differences.
So the people like N&Z who look at the moon’s temperature profile and claim that a greenhouse effect could not explain why the earth is hotter are making a fallacious argument.
That’s very interesting, Ed, but we’re not talking about the moon. You stated:
“So a more precise calculation than the simplified “flat earth” calculation actually shows a bigger greenhouse effect to explain our actual surface temperatures.”
A calculation that shows a bigger greenhouse effect than 33 K. And this is calculated for Earth, not the moon.
Tony:
The implication of Brett’s post (if it had any sense) was that because of the rotation of the earth and so time-varying insolation at any point, the greenhouse effect is not needed to explain current temperature levels. That is what I originally responded to.
You are arguing the opposite, that the greenhouse effect is not sufficient to explain current temperature levels. You don’t say what the temperature would be, or what other effect could increase the temperature levels to what we observe.
The simple fact is that the MEASURED levels of downwelling longwave infrared radiation (aka “back radiation”) provide sufficient power to make the surface energy balance work out at current temperature levels. Without this, it can’t. And there is NO other plausible power input to the surface that can explain these temperature levels.
No no, Ed, I’m not arguing anything right now. YOU said:
“So a more precise calculation than the simplified “flat earth” calculation actually shows a bigger greenhouse effect to explain our actual surface temperatures”
So YOU are stating that the ACTUAL GHE is greater than 33 K. Nothing to do with any diversions you might want to make wrt the moon, or anything like that. Because of what you have explained re Holders Inequality, the ACTUAL GHE on Earth MUST be greater than 33 K. The 33 K is a theoretical MINIMUM. Correct?
Once you have made a definitive statement in response to that, THEN I’ll be making my argument.
Tony:
You already made the argument that the variation in temperatures of the earth implies “too big a GHE to be explained by back-radiation.”
The 255K figure commonly cited is the maximum surface temperature a body that receives the insolation that the earth does (~240 W/m2 averaged over time and surface) can have if it radiates directly to deep space (effective temperature of 3K) with an emissivity of 1.0. It would reach this maximum if it could transfer thermal energy around its surface so that it is uniform at all times.
Personally, I would use a lower emissivity value — a value of 0.95 gives a temperature of 258K, but that’s a quibble.
Yes, Holder’s inequality says that any variation in temperature makes the average lower (for the same power input), with the bigger the variation the lower the average.
So the question is, what imaginary alternative Earth are you using to claim that there is “too big a GHE to be explained by back-radiation”? And once you decide the average temperature of this imaginary alternative Earth, how do you determine what is too big to be explained by back-radiation? 34K? 35K? 40K? 50K?
I’m not sure why you’re unable to respond directly. You’re very much the politician, Ed. But I guess:
“The 255K figure commonly cited is the maximum surface temperature a body…“
is the closest you are going to get to admitting that here on this real, non-imaginary, non-alternative Earth, the 33 K is the theoretical minimum amount that the GHE could be, and in reality it MUST be higher than this. Unless you DO have it in you to talk straight and put in plain and simple terms what follows logically from what you’ve been saying? Let’s see if we can get a “yes” or “no” from you on if you agree with that statement.
Tony:
I was very clear in answering your question. It looks like you are unable to comprehend anything more than a simple sentence of more than eight words. You seem to be looking for an answer that you can pounce on and twist.
I answered your question — why won’t you answer mine??? I don’t think you can!
It’s funny how you’re allowed to be as offensive as you like. Yes, I comprehend perfectly well what your earlier answers imply, I just want to make sure there’s no way you can wriggle out of it later. So I’m looking for a “yes” or “no” here, do you agree to this statement?
Here on this real, non-imaginary, non-alternative Earth, the 33 K is the theoretical minimum amount that the GHE could be, and in reality it MUST be higher than this.
Then we can get to the bit that you already know is coming (that’s what’s so ridiculous about these farcical conversations…as if this hasn’t all been discussed a hundred times before!)
“It’s the water vapor, stupid.”
You see, one reason I’m worried about you wriggling your way out of things later on IS your first question:
“So the question is, what imaginary alternative Earth are you using to claim that there is “too big a GHE to be explained by back-radiation””
The answer is, obviously, no imaginary alternative Earth. THIS Earth. The real one we’re living on at the moment. This is why I’m phrasing the statement in the way I am, which I’m asking you to state whether or not you agree with.
Tony:
You really are too much! A statement such as “there is an X-degree GHE” is comparing our real earth to some imaginary alternate earth. There is no way around that.
But with that caveat, I’ll bite. Your statement: “Here on this real, non-imaginary, non-alternative Earth, the 33 K is the theoretical minimum amount that the GHE could be, and in reality it MUST be higher than this.”
Yes, I will agree with this statement.
Now, you need to show me what the maximum the GHE could be. Please show your work.
OK, now you can re-read the intro to the Nikolov and Zeller paper.
“A recent study has revealed that the Earth’s natural atmospheric greenhouse effect is around 90 K or about 2.7 times stronger than assumed for the past 40 years. A thermal enhancement of such a magnitude cannot be explained with the observed amount of outgoing infrared long-wave radiation absorbed by the atmosphere (i.e. ≈ 158 W m-2), thus requiring a re-examination of the underlying Greenhouse theory“
And the intro to the Volokin and ReLlez paper:
“According to satellite observations, Earth’s atmosphere retains on average 155–158 W m−2 of the upwelling long-wave radiation emitted by the surface (Kiehl and Trenberth 1997; Trenberth et al. 2009; Stephens et al. 2012; Wild et al. 2013). This infrared heat absorption by greenhouse gases a.k.a. long-wave radiative forcing (Kiehl and Trenberth 1997) is presently believed to drive 100% of the near-surface ATE (Peixoto and Oort 1992; Lacis et al. 2010; Pierrehumbert 2010; Schmidt et al. 2010).”
And you have no grounds on which to dismiss this part of their papers; since even if you disagree with an estimate as high as 90 K, you have agreed that the estimate of 33 K is a minimum, and that the true difference must be greater. Therefore, something other than long-wave radiative forcing must be responsible for at least part of the true difference, as you agree it’s greater than 33 K. Please don’t bother to pretend that you’re not sure where the 155-158 Wm-2 number comes from, as I’ve seen you argue this before. I have actually seen this entire discussion before. Lol.
”…Earth’s natural atmospheric greenhouse effect is around 90 K”
Tony, N&Z calculate 90K (their ATE) above the airless moon’s median brightness temperature from orbit of ~197K (287-197=90K).
With current semi-opaque air atm., Earth’s natural atmospheric greenhouse effect (measured in the satellite era) for the past 40 years is still ~33K over ~transparent air atm. (288-255=33K). As always in climate, one has to watch the pea.
Two people to one conversation. Me and one other.
Don’t get me wrong, anyone can comment wherever they like. In this particular conversation though, unless your name is Ed Bo, your comment will not get a response, nor even be read. So it would be pointless trying.
Tony:
I don’t think you’re interested in an honest debate. You are at least five cycles of this discussion behind. And you said to me above: “That’s very interesting, Ed, but we’re not talking about the moon.”
But you are talking about the moon (as I anticipated)!
Yesterday, I pointed out three glaring mistakes in N&Z’s analysis as to why they cannot use the moon as their alternative imaginary earth. I’ve pressed Ned directly on these points, and he never responds.
And no, I don’t agree that any difference greater than 33K cannot be explained by a radiative greenhouse effect. Why do you think this would be?
I’m not talking about the moon. Try to keep up. That’s why I made you agree to the statement I made. You can’t just agree to something and then immediately go back on it. Silly Ed.
This might help “refresh your memory”:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2679299
Unless you for some reason believe that without an atmosphere, there would be no temperature variations on Earth!? Lol, I wouldn’t put anything past you at the moment, Eddie-baby…
Tony:
You are too much! You can’t even keep out of your own way!
Way back, I predicted you were using the moon as your imaginary alternative earth. You denied it.
Then you cited the N&Z paper as your evidence, which uses the moon as its imaginary alternative earth. I called you on it, and you still deny it!!! Have you no shame???
Then you present the argument that: “Therefore, something other than long-wave radiative forcing must be responsible for at least part of the true difference, as you agree it’s greater than 33 K.”
You provide absolutely no backing for this argument (because there is none).
Nobody (but you) is making the argument that 33K is the MAXIMUM increase that the GHE can provide.
You don’t even understand the issues in play here.
I’m not using the moon as an imaginary Earth. OK let’s try to go through it slowly for you. As I said:
“And you have no grounds on which to dismiss this part of their papers; since even if you disagree with an estimate as high as 90 K…”
1) THEIR 90 K estimate comes from their moon-based calculations. Agreed. Always was agreed, known, and understood.
2) However, as you’ve agreed, due to what you’ve explained Holder’s Inequality and if we accept that without an atmosphere, the Earth is still going to have some degree of temperature variation, then the true GHE on Earth is going to be higher than 33 K.
3) So even if you dispute their 90 K estimate and think it’s too high, their point still remains. It is not a necessary condition for their point to be valid that the difference actually be as high as 90 K.
It’s as simple as 1, 2, 3.
And if you want to talk about predictions, I predicted from the beginning that you would try to wriggle out of it. And here we are…
You must be aware that this logic isn’t hard for anyone reading to follow, surely!?
* due to what you’ve explained RE Holder’s Inequality *
Tony:
Finally, you admit that you are talking about the moon, although your statement that this “always was agreed, known, and understood” is plainly laughable.
There is still a huge gaping hole in your (and N&Z’s) logic.
You and they have provided absolutely no evidence, or even a real argument, for the proposition that the earth’s greenhouse effect could provide a temperature increase greater than 33K. I’ve challenged you on this “maximum” idea multiple times, and you always ignore the issue. Why?
This’ll help clear the rest up. From the Nikolov and Zeller paper:
“In a recent study Volokin et al. [1] demonstrated that the strength of Earth’s atmospheric Greenhouse Effect (GE) is about 90 K instead of 33 K as presently assumed by most researchers e.g. [2-7]. The new estimate corrected a long-standing mathematical error in the application of the Stefan–Boltzmann (SB) radiation law to a sphere pertaining to Hölder’s inequality between integrals. Since the current greenhouse theory strives to explain GE solely through a retention (trapping) of outgoing long-wavelength (LW) radiation by atmospheric gases [2,5,7- 10], a thermal enhancement of 90 K creates a logical conundrum, since satellite observations constrain the global atmospheric LW absorption to 155–158 W m-2 [11-13]. Such a flux might only explain a surface warming up to 35 K. Hence, more than 60% of Earth’s 90 K atmospheric effect appears to remain inexplicable in the context of the current theory”
So, you see, even if you disagree with their 90 K estimate, anything over 35 K presents a li’l bit of a problem for the poor ol’ GHE. So there’s no reason for your moon obsessions. Has absolutely nothing to do with it. Keep on wrigglin’
Ed, I’ve never been talking about the moon. Why are you pretending this is so difficult to follow? Your faux-indignation/confusion was amusing at first (bear in mind I’ve seen you argue this subject before so I KNOW you’re not actually confused), but it’s just getting a bit silly now.
Tony November 29, 2017 at 5:37 pm
This is hilarious. Tony, you’ve been duped. “Volokin et al.” is Volokin and Rellez … which are the aliases that Nikolov and Zeller used when they published the paper under false names. So N&Z are QUOTING THEMSELVES AND PRETENDING THEY ARE QUOTING AN INDEPENDENT AUTHORITY. Shameful, underhanded, and disgraceful. They are a blot on science.
Now, if you were actually as knowledgeable about the climate as you claim, you would have known that … but instead, N&Z suckered you in..
Fail.
The gory details are here. Short version? N&Z are a scientific joke. Anyone who quotes them approvingly immediately identifies themself as a climate noob …
w.
Tony:
Assertion is not evidence. N&Z simply say: “satellite observations constrain the global atmospheric LW absorption to 155–158 W m-2 [11-13]. Such a flux might only explain a surface warming up to 35 K.”
Nowhere do they back this up.
And your heroes don’t even understand high school physics when they assert that static pressure can provide an ongoing power transfer to the surface of the earth, which they propose as the alternative to DWLWIR radiation. Any semi-competent high school student knows that for a mechanical force (such as the weight of the atmosphere on the surface due to its pressure) to transfer energy, it must act over a distance.
For a mechanical force to provide ongoing continuous power, it must create an ongoing velocity — that is, the pressure would have to be continuous shrinking the earth to transfer this power to the surface. It’s patently ridiculous, but you buy it!
Calm down, Willis. Authors often refer to their previous papers in their work. You don’t actually have an argument. We all know about the name reversal thing, you may have mentioned it a few times before…lol.
“Nowhere do they back this up“
How could it account for more than 35 K?
Tony November 29, 2017 at 11:52 pm
Oh, please. Point me to one other scientific author who referred to a previous paper that they published under a fake name and didn’t mention that they wrote the previous paper themselves. Just one.
I’ll wait … and while I’m waiting, I’ll note that their paper was retracted because of their deception. So no, you don’t get to pretend that this is standard in science. It is not standard science, it is a cause for getting a paper yanked before final publication.
w.
PS—And no, we don’t “all know” about their skullduggery. There are a lot of folks lurking and reading this that had no idea from reading your quotation that Nikolov was referring to himself. Heck, lots of them never heard of Nikolov, he’s not exactly well-known … and for good reason, although at this point he is a bit infamous.
Not only that, but despite knowing that Nikolov was doing it, you said nothing about Nikolov citing himself as if someone else agreed with him, so you are enabling their ongoing deception … and you would have gotten away with it if I hadn’t pointed it out. So I can understand why you’re angry with me, I spoiled your attempt to deceive … but you might more profitably direct your anger elsewhere. I’m just the messenger.
It would be unusual for an author to make a special point about that author when referencing said author. Especially if said author is themselves. Perhaps they were just not so self-involved that they felt the need to buck the tradition.
I understand that you are unhappy about the name reversal. But Willis, why are you playing these grade-school games about their name change?
Tony, I asked:
In what you obviously think is a response, you say:
Tony November 30, 2017 at 3:00 am
Huh? What does that have to do with publishing under an alias? That’s not responsive to my request in the slightest. But you persevere, viz:
I’m not “unhappy” about their scientific malfeasance, which you try to pass off and minimize as a “name reversal”. I think it is despicable, but that doesn’t affect my happiness.
In any case, what you call “grade-school games about their name change” that they, not I, played got their paper unceremoniously yanked from publication and mentioned in Retraction Watch, which for any scientific author is a Very Bad Thing™ … so clearly, the scientific world thought it was far more serious than the innocent fun you are trying to convince us it represents.
More to the point, all of this wriggling on your part can’t hide the fact that you are unwilling to just come out and admit that you cannot find one other case of any scientist practicing this kind of underhanded deception, just as I suspected. It’s not standard practice as you keep claiming, nor is it trivial. It is a one-off piece of underhanded deception. The fact that you defend it so vigorously speaks volumes …
w.
You seem to be keen to argue about what you think is malfeasance. Whilst you’re welcome to your opinion, I’m only interested in discussing the point they’re making. The validity of which isn’t changed by the activity of the name change, malfeasance or otherwise. You did catch me out though, I said I wasn’t going to respond to anyone but Ed Bo in this conversation. My mistake. Won’t happen again.
Tony:
You ask: “How could it account for more than 35 K?”
Look, N&Z make a specific claim that is the keystone of their whole theory without a shred of analysis to back it up. You obviously agree with this claim. The onus is on you to support the claim.
The real earth’s surface power balance cannot come close to being in balance without the contribution of DWLWIR (the “greenhouse effect”). And here I am not comparing it to any imaginary alternative earth.
With the contribution of DWLWIR, the balance is very close (within measurement errors). No other possible physically plausible mechanism closes the gap.
Ball’s in your court.
Nah, balls in yours. They provide a list of citations, look it up. You were talking more about those citations last time, strange…seems like you just can’t be bothered this time around. Play the game properly at least. I’ve read you trying a lot harder to deceive than this.
“They provide a list of citations.”
No, they don’t. I’ve looked several times. They cite some sources on W/m2 values, but nowhere do they say how they get from that to 35K maximum.
Ball’s back in your court.
Course they cite some W/m2 values…and they come from the energy balance diagrams, and it’s the surface minus the outgoing at TOA, as in, what’s absorbed by the atmosphere, and those values correspond to temperatures of 288 K and 255 K or thereabouts ya da ya da ya da, not estimated, measured (by satellite) W/m2 values, we know the surface is 288 K on average, we know the outgoing flux at TOA would relate to a black body temp of 255 K, blah blah blah, yeah yeah yeah, you know this already…etc
Tony:
Here’s the thing. Let’s assume you are correct that they are citing the difference between blackbody radiative flux density at ~288K and that at ~255K, the average surface and emission layer temperatures.
First, it’s appalling that they don’t either reference or derive/explain the key claim in their paper. That alone is grounds for rejection from any respectable, responsible journal.
But the bigger issue is this. The bulk of the paper expresses the argument that analysis using (average) surface temperatures that are constant over the whole planet is fundamentally incorrect. But their whole argument is based on calculations that use these very same averages as constant over the planet.
So their paper arguing that using these average values is wrong is based on the very same use of these averages! It’s hilarious and pathetic at the same time. (And yes, the “problem” they cite would go away if they used consistent analysis.)
And that’s just one of the many egregious fundamental errors they make, none of which you appear to have spotted.
“The bulk of the paper expresses the argument that analysis using (average) surface temperatures that are constant over the whole planet is fundamentally incorrect.“
Not incorrect, lower than currently thought. But that applies to the calculated (effective) temperature. Obviously what is measured, is measured. And you didn’t appear to spot that this is NOT the main claim of their paper (the second one). Etc etc etc, blah blah blah, all argued before of course.
And in fact it’s not even the “bulk” of the first paper.
Tony, putting aside for the moment whether or not a CO2 molecule’s photon from an area of the Atmosphere that is around -80C can warm the surface, the one thing that has not really been discussed on here or the previous Post by Rod Gill is not just the physics, but also the Mechanics of the process.
The Trenberth Diagram talks about averages which are not real as far as the way the Radiation in and Radiation out act.
This is the part that concerns me the most, because all the focus of Climate Scientists is on CO2 and trying to control it, but the logic of it does not add up.
Tony, did you see the reference Willis made here to a previous post that supposedly “gave the gory details”.
Willis Eschenbach November 29, 2017 at 6:36 pm
The gory details are here. Short version? N&Z are a scientific joke. Anyone who quotes them approvingly immediately identifies themself as a climate noob …
So here we have 2 Scientists trying to get their work published that contradicts the whole Climate Change industry. We all know how well that goes, especially if you are known as “Deniers”.
So they try to get it published under pseudonyms and the wonderful Willis himself complains and gets the paper withdrawn.
I suggest that Mr Eschenbach takes a look at this article with links to the various Scientists & Mathematicians who have published under pseudonyms
http://bigthink.com/neurobonkers/in-defence-of-pseudonyms-in-science-defending-the-right-to-write
So not only does he insult you, the theory of the paper was not “destroyed”, just the presence of the paper.
So here we have Willis doing the Climate Change Industries job for it.
As to the Gory Details, for me the gory details are that I always new that our host and Mr Eschenbach had it in for certain individuals and thoeries but I had no idea that he would go that far and not only that but would brag about it afterwards.
Not only didn’t the post pointed to “do anything to destroy the theory”, as lot of the comments backed it up, it just attacked the individuals. Where have we heard that before.
N & Z are not the only ones pushing an alternative to current Climate Concensus, No Tricks Zone has hundreds of papers questioning the whole concept.
You’re right, AC. It’s pretty disgraceful really.
Tony:
You are still not coming to grips with the fact that the whole justification for these papers is based on an analysis that they spend a lot of time in the papers claiming is completely invalid.
AC:
I’m all for overturning conventional wisdom. But these papers are simply ridiculous. I would reject them as undergraduate student reports.
N&Z don’t even understand high school physics. Static pressure cannot provide an ongoing power source. (If you want to claim this very basic point of physics, understood for hundreds of years, you need to provide a very convincing argument as to why it is not so. N&Z don’t even try.
These kinds of shabby efforts just serve to discredit all skeptics.
“You are still not coming to grips with the fact that the whole justification for these papers is based on an analysis that they spend a lot of time in the papers claiming is completely invalid“
That isn’t a “fact”.
1) it’s not the “whole justification for the papers”. In the second, it’s a minor footnote really.
2) the claim is that the 255 K calculation for the surface temperature of an Earth without an atmosphere is too high. This is from a WUWT post that they wrote back in 2012 that you’ve no doubt read, despite pretending not to be aware of:
“Furthermore, the application of Eq. (3) to calculate the mean temperature of a sphere runs into a fundamental mathematical problem caused by Hölder’s inequality between non-linear integrals (e.g. Kuptsov 2001). What does this mean? Hölder’s inequality applies to certain non-linear functions and states that, in such functions, the use of an arithmetic average for the independent (input) variable will not produce a correct mean value of the dependent (output) variable. Hence, due to a non-linear relationship between temperature and radiative flux in the SB law (Eq. 3) and the variation of absorbed radiation with latitude on a spherical surface, one cannot correctly calculate the mean temperature of a unidirectionally illuminated planet from the amount of spatially averaged absorbed radiation defined by Eq. (2). According to Hölder’s inequality, the temperature calculated from Eq. (3) will always be significantly higher than the actual mean temperature of an airless planet”
It’s to do with the relationship between temperature and radiative flux. Calculating a mean temperature based on fluxes. So will not be a problem when simply computing an average of recorded temperatures for the actual Earth’s surface, or at the TOA. The only reason they give it as a flux difference and not a temperature difference is:
“in the scientific literature, the actual effect is measured via the amount of outgoing infrared radiation absorbed by the atmosphere (e.g. Stephens et al. 1993; Inamdar & Ramanathan 1997; Ramanathan & Inamdar 2006; Houghton 2009). It is usually calculated as a difference (occasionally a ratio) between the total average infrared flux emanating at the surface and that at the top of the atmosphere. Defined in this way, the average atmospheric GE, according to satellite observations, is between 157 and 161 W m-2 (Ramanathan & Inamdar 2006; Lin et al. 2008; Trenberth et al. 2009). In other words, the current theory uses radiative flux units instead of temperature units to quantify ATE.”
Yep, they had already explained everything you pretended to be mystified by in a post here at WUWT that you were most likely commenting under, albeit under another name. I’m guessing, perhaps a name with initials JS?
Tony:
Nothing in your lengthy comment even tries to argue against my point that they are using a calculation that assumes a linear relationship between radiative flux density and temperature to argue that using a linear relationship between radiative flux density and temperature is not valid.
And you don’t even realize it…
They’re not. And you do realise it. You’re just intellectually dishonest.
Ed Bo December 2, 2017 at 1:04 pm
Tony December 2, 2017 at 3:04 pm
Tony, you know how above I said I didn’t think you were vicious and vindictive? I said:
You just changed my mind. Ed is doing his best to point out flaws in your logic. In response, you pretend that you can read his mind, and that you know what he thinks. Based on that untenable fantasy that you can see inside his head, you call him a liar, and you are doing your best to slime him.
You are lucky that this is not my website. I’d ban you in a minute for repeated violation of the site policies.
Go away. Don’t go away mad. Just go away.
w.
Proof of Ed’s intellectual dishonesty, for those that need it:
http://notrickszone.com/2017/09/25/another-new-paper-dismantles-the-co2-greenhouse-effect-thought-experiment/#comment-1231159
Notice how in this comment from only a few months ago, he looks through the references for the LW absorption figure of 155 – 158 W/m2, and comes to the same conclusion as I do about where they must be getting that number from (and that is confirmed by their own words from the WUWT article from five years ago, anyway). Here, in this article, he pretends to have no idea about any of it.
Tony:
Projection much?
For a long time, you wouldn’t say what source you were citing. First you denied you were talking about the moon as the no-GHE comparsion body.
Then when I called you on it, you said “THEIR 90 K estimate comes from their moon-based calculations. Agreed. Always was agreed, known, and understood.”
Then you start denying it again.
And since neither N&Z or you will ever state explicitly where their 35K maximum GHE value comes from, I can only surmise its source. I challenged you to back up the claim explicitly (I wanted to see if you made the same surmise that I did) and you have never done it. Still waiting…
Ed, the arguments have already been made. Anyone following the link above can see that before you began this discussion, you’d already been through the whole thing. Your dishonesty exposed, it’s no wonder you want to redirect back further into this discussion. But I’m not here to play your games, I’m here to expose you for what you are. Mission accomplished. Have a nice day!
“And since neither N&Z or you will ever state explicitly where their 35K maximum GHE value comes from, I can only surmise its source. I challenged you to back up the claim explicitly (I wanted to see if you made the same surmise that I did) and you have never done it. Still waiting…“
The problem for you is that the comments I’ve made in the conversation above, exist. They haven’t suddenly disappeared. People can still read them. So for you to sit there and pretend you are waiting for an answer you’ve already been given, isn’t going to work. It’s just a further demonstration that you are what you are.
Tony:
Now you’re completely desperate and delusional. In that comment at NTZ, I stated clearly than Nikolov and Zeller provided no supporting analysis for their claim that the GHE was limited to 35K, and that I had to guess at how they derived that figure.
I still don’t know for sure, and I’ve been very clear on that — that this is a surmise. And you call that “intellectual dishonesty”.
You on the other hand — “I’m not comparing it to the moon” … “I’ve always been comparing it to the moon” (We have always been at war with Oceania) … “I’m not comparing it to the moon”
Keep it up, Ed. You’re doing a great job!
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2688993
“Further, there is no logical reason that a new relationship may suddenly appear, that has not appeared in the past. For example, the 1979 report by the Climate Research Board, “Charney Report,” published by the National Academy of Sciences, contained the speculation that the modest warming from CO2 would be greatly amplified by an increased warming from water vapor, the dominant greenhouse gas. The report offered no data supporting the speculation, nor are any reported. The net effect, if it exists, is likely weak as well”.
The simplest argument I have seen is the fact that a cold body can “see” a warm one. The eye absorbs radiation at certain wavelengths, this creates an electro-chemical reaction which transmits a signal to the brain.
So if a warm body cannot absorb radiation from a colder body, how can I, at 37c, possibly see the bio-luminescence from those plankton at 20c disturbed in the wake of my fishing boat? Of course a warm body can absorb radiation from a colder one – it is plain common sense!
There is a great book called “Mistakes were made (but not by me)” by social psychologists Carol Tavris and Elliot Aronson published in 2007. It deals with cognitive dissonance, confirmation bias and other cognitive biases. The classic and most extreme case is the policeman who refused to believe a “murderer” he had helped put in jail was innocent, even when the supposed victim turned up alive and well!
These biases are absolutely rampant on both sides of the AGW argument. We all need to stop trying to prove we are right and do our best to understand that we could well be wrong on some of this stuff. In an ideal world we should all read this book before trying to discuss anything on the internet. It would make us all a lot more humble and willing to listen to others.
Willis’s explanation is a good one for the scientifically naive to understand without too much detail to cloud the issue. Space is effectively 3 kelvin, the lower troposphere somewhere around 280 kelvin. I am really glad that atmosphere at 280 kelvin is between me at 310 kelvin and space at 3 kelvin!!!
Your example is a great one. I argue that the whole class of supposition that warm objects Can’t absorb energy from cold ones is the ridiculous hypothesis that albedo is a function of temperature.
That’s a Nobel for anyone who can prove that.
TLM: how much hotter your eyes became seeing the light from plankton at 20C?
Esa-Matti Lilius:
Not very much, maybe not at all. It depends what I would be looking at instead. However, they would be warmer than they would be if my eyes were looking at the darker ocean – imperceptibly of course. Both the plankton and the ocean are colder than me, yet my eyes can absorb energy from them (both). The energy delivered by a few photons sensed by my retina is next to nothing – but not nothing – otherwise I would not be able to see the plankton.
Anyway that is not really my point. The whole gist of Willis’s piece is the refusal of some to accept the patent truth that radiation from a colder body can be absorbed by a warmer body. Newton’s Second Law is not violated provided the net transfer is from warm to cold.
I think what some people get confused over is the difference between these two statements:
1. The earth goes up in temperature in the presence of a colder atmosphere (wrong)
2. In the presence of a colder atmosphere the earth cools more slowly and is not as cold as it would be if the atmosphere were not there, and it were instead exposed to the even colder depths of space (correct)
The earth is warmer than the atmosphere, so more energy is being absorbed by the atmosphere from the earth than by the earth from the atmosphere so absent any source of energy the earth would gradually cool until it, the atmosphere, and the depths of space were all at around 3K. Luckily for us we have a constant source of energy coming from the sun making sure we do not all cool to 3K.
Remember the atmosphere cannot absorb very much of the sun’s short wave radiation, so nearly all of the sun’s energy at the top of the atmosphere reaches the surface of the earth. It is the infrared energy emitted by our earth and seas that warms the atmosphere, not the sun.
Terrestrial emission of LWIR is but a minor factor in warming the atmosphere. Moist convection is the principal one
1sky1
“Moist convection” cannot “warm the atmosphere”!?! It is not a source of heat, it is a system of heat transport. It moves the warm air from the lower levels of the atmosphere to the higher levels. So how does the air at the lower levels in the atmosphere get warmer in order to convect upwards?
Your statement that LWIR is a “minor factor” suggests that the energy to warm the atmosphere seems to come (magically?) from some other source. If not upwelling LWIR what exactly? Volcanoes? Wood burning stoves?
If the air cannot absorb the sun’s energy directly it must absorb the suns energy indirectly, via the IR re-radiation from the surface of the earth. There is no other significant source of energy above the surface of the earth other than the sun.
So the water vapour, and (to a lesser extent) the CO2 and other gases capable of absorbing radiation in the infrared, that are close to the earth’s surface, absorb the IR radiation and their temperature increases. As warm gas is less dense than cold gas, they rise up through the atmosphere cooling as they go and exchanging their energy with other gas molecules. As the warm air rises it is replaced by cool air falling, which in turn is warmed by the earth’s surface. Hence your moist convection.
By that misguided line of thought, LWIR cannot warm anything, since it too is “a system of heat transport.”
Those with a modicum of scientific grasp will recognize that the sun and, to a negligible extent, the Earth’s molten core are the only natural SOURCES of heat. All natural heating of other matter is the result of heat TRANSPORT by conduction, convection or radiation. Observations of the Bowen ratio (q.v.) throughout the globe clearly indicate that, outside of the dry environments of deserts and Antarctica, the principal means of cooling the surface and heating the troposphere is by buoyant transport and condensation of water vapor, which recovers the latent heat of evaporation. That’s what’s involved in the physical concept of moist convection.
TLM, why do you say that “If the air cannot absorb the sun’s energy directly it must absorb the suns energy indirectly”
Of course it absorbs some of the Sun’s Energy, as well as the Radiation from the Surface.
https://www.ucar.edu/communications/gcip/m7sssystem/m7pdfc3.pdf
Water in the Atmosphere also Reflects some as well, clouds do not overall slow cooling in the day, they prevent heating, they only overall slow it at night.
No, not “nearly all of the Sun’s radiation at the TOA reaches the surface of the Earth.” Less than HALF of it does.
According to NASA, “29% of the solar energy that arrives at the TOA is reflected back out to space by clouds, particles and Earths albedo. 23% is absorbed by the atmosphere (water vapor, dust, ozone etc) and 48% passes through the atmosphere and is absorbed by the surface.”
+100. It’s still amazing why people don’t get this.
Willis:Your conclusion is correct but your radiation modeling is confused.You choose to assume that the atmosphere can be represented by a radiating surface A2.The extinction depth for I.R. above the earth is of the order of hundreds of meters,so A2/A1 is not much greater than unity, not nearly infinite as you have used.However, once you assume black surfaces(epsilon=1) and F12=1,the area ratio cancels out your equation to get the trivial result that you obtained.
The net heat flow Qdot is the difference between two independent photon streams, Qdot12 and Qdot21.The net heat flow is Qdot=Qdot12-Qdot21. If Qdot21 is increased due to an increase in T2 ,or emissivit for a gray surface,Qdot is decreased.This is the nature of radiation heat transport, which is quite different to that of heat conduction and convection.It is as simple as that.
Block A 270 kelvin block B 280 kelvin in a vacuum chamber, perfectly reflective with no radiation loss.
Both blocks are emitting fluxing frequencies from 1 kelvin to 270 kelvin and even if all photons are absorbed it is as if they were never emitted, as the fluxes are exchanged in a nano-second, B to A A to B.
Block B is also emitting the frequencies between 271 kelvin and 280 kelvin, block A cannot emit at those frequencies and the thermal radiation flow is one way between the diminishing temperature differential until equilibrium is achieved
Then all frequencies representing from 1 kelvin to 275 kelvin are being emitted and absorbed by both blocks in the absence of any heat being created.
And again it is as if the energy never leaves it is just swapped between the blocks at exactly the same rate nano-second by nano-second, and the blocks stay an equal steady state. 275 kelvin.
Really Doesn’t work like that. Block A emits a broad spectrum with peak centered at 270 and big tail both ways. It has IR spectra way above 280
Gary Ashe,
As Paul Bahlin already said, both blocks send IR spectra which are largely overlapping at both sides of their peaks, only the peak value shifted a little bit, see:
https://en.wikipedia.org/wiki/Wien's_displacement_law
Thus slightly less than half of the waves sent out by block A are “hotter” than the peak wavelength of block B and in your reasoning must be absorbed. The same for B to A, but then slighly more than half is “hotter” than the peak of A.
In reality in both cases, all the radiation received is absorbed both ways, so all what counts is the net difference in transferred energy, which is slightly higher for block B than for A, until equilibrium is reached.
Gary Ashe November 27, 2017 at 10:17 am
Block A 270 kelvin block B 280 kelvin in a vacuum chamber, perfectly reflective with no radiation loss.
Both blocks are emitting fluxing frequencies from 1 kelvin to 270 kelvin and even if all photons are absorbed it is as if they were never emitted, as the fluxes are exchanged in a nano-second, B to A A to B.
Block B is also emitting the frequencies between 271 kelvin and 280 kelvin, block A cannot emit at those frequencies and the thermal radiation flow is one way between the diminishing temperature differential until equilibrium is achieved
This is not correct, both blocks emit over the same range of frequencies, the block at 270K has a peak radiance of 5.9 W/m2/sr/µm at 10.7µm whereas the block at 280K peaks at 7.05 W/m2/sr/µm at 10.35µm
How do the photons know not to thermalise when being exchanged at equilibrium, thats to the Tonyb fella, and his how do cold photons know their cold sophistry.
“How do the photons know not to thermalise when being exchanged at equilibrium, thats to the Tonyb fella, and his how do cold photons know their cold sophistry.”
It doesn’t really matter how, because reality shows they do. The backward flux auto-magically cancels the correct fraction of the forward flux
So, let’s say that a jar of hot water, first, is sitting ALONE. The jar of hot water, thus, has a quantity of “warmth” that is different without an ice cube present.
Now we introduce an ice cube into close proximity to (but not touching) the jar of hot water. The ice cube, obviously, has a SMALLER quantity of “warmth” than the jar of hot water.

But the claim is that … (1) the ice cube can leave the jar of hot water warmer than the jar of hot water would be if the ice cube weren’t there.
It this is so, then the “warmth” that the jar of hot water has WITHOUT the ice cube will INCREASE, when the ice cube enters. The ice cube, thus, heats the jar of hot water. A colder object heats a warmer object.
How is Claim #1 not a blatant violation of the Second Law? … merely worded differently?
Robert Kernodle,
It this is so, then the “warmth” that the jar of hot water has WITHOUT the ice cube will INCREASE, when the ice cube enters.
That is not what Willis said.
First, the jar of hot water will cool in both cases (as there is no heat source to keep it warm).
Second, the cooling in space (at 3 K background temperature) is faster without the ice in the neighborhood.
So, there is no increase of warmth in both cases, only less loss of warmth with the ice…
If there is a constant (electrical) heat source under the jar, there will be an increase of warmth of the jar if you bring the ice near it, for the simple reason that with the same input, you reduce the heat loss to space from the jar, thus the temperature must go up to get rid of the same incoming amount of energy…
See the temperature curve of my calculation sheet…
“thus the temperature must go up to get rid of the same incoming amount of energy“
Quick question: if the heated object were sealed inside a perfectly internally reflective casing (along with its source of power), would that object keep heating itself up, by its own emitted power, indefinitely?
Bear in mind that if your answer is no, it would seem you disagree with the logic in your final paragraph.
Tony,
If every outgoing photon is reflected back, then of course the heated object would heat up further until something gets wrong. As there is only supply and no removal, the temperature would go up until eternity…
I think that this is the fundamental point where one side of the argument diverges from the other. I would say that the slayers think the frequency spectrum of the radiation the object emits can’t be increased by itself, or the fact that the object receives its own reflected energy. Only with an increase of this frequency spectrum would the object rise in temperature.
Tony,
Indeed, the Slayers think that any photon coming from a colder object can’t be absorbed by a warmer one, while every CO2 laser proves that wrong…
As steel absorbs every frequency of IR that is hitting it, the energy contained in the 10 micrometer band sent by the laser is thermalised to increasing temperatures up to the melting point of steel. While getting hotter, the steel is emitting some of that energy as photons with increasing frequency…
Well no, a laser is a completely different matter altogether.
Tony,
Well no, a laser is a completely different matter altogether.
Not at all: the receiving steel doesn’t “know” the origin of the IR photon, which may come from the human next to it (the 10 micrometer wave is at the peak frequency of around a human body temperature) or from a laser. Thus either the steel absorbs it or reject/reflect it, no matter the origin.
As the temperature of the steel increases, a lot (if not all) of the 10 micrometer waves are thermalised, no matter the origin. At the same time, the original low temperature steel emits IR with a peak frequency and intensity directly dependent of its temperature. By thermalising the laser radiation, the steel temperature increases and due to that, the frequency and intensity of its own radiation shifts to shorter – even visible – wavelengths and higher intensity.
Yes, it’s a completely different matter altogether. For the same reasons as are explained every single time a laser is brought up in these discussions. Why you people seem to just want to discuss the same exact things over and over again is beyond me.
Tony November 28, 2017 at 2:30 am
Yes, it’s a completely different matter altogether. For the same reasons as are explained every single time a laser is brought up in these discussions. Why you people seem to just want to discuss the same exact things over and over again is beyond me.
It’s certainly not a different matter, all 10.6 micron photons are the same regardless of their origin, the absorber treats them all the same.
The amount of heating depends on the intensity of the radiation; that would involve the energy in each photon (which you and Ferdinand are discussing) AND the number of photons arriving each second. Lasers used for cutting take wattages in the hundreds and focus the beam until its arriving within a point sometimes less than 0.1 mm in diameter. That translates to an incredibly high irradiance (W/m2) received by the metal at that point.
The heated object trapped inside the reflective casing can neither increase the frequency spectrum of its own emitted radiation, nor increase its own radiant exitance/irradiance (W/m2) to a higher flux.
Tony,
Back to the essence:
I would say that the slayers think the frequency spectrum of the radiation the object emits can’t be increased by itself, or the fact that the object receives its own reflected energy. Only with an increase of this frequency spectrum would the object rise in temperature.
Which is not true. Any addition of EM energy, whatever the frequency, will increase the temperature of the object and thus increase the outgoing frequency spectrum of an object.
That is a little bit for the energy input from cold sources and some more for hot sources and extremely much for a CO2 laser, even if the latter has a very small frequency band, much lower than most emitted frequencies from a heated object…
Of course you are right that the laser provides much more energy than the energy emitted by the temperature of an object. But that is not the point, the point is that steel does absorb and thermolyses low energy IR photons, whatever the source and intensity.
The heated object trapped inside the reflective casing can neither increase the frequency spectrum of its own emitted radiation, nor increase its own radiant exitance/irradiance (W/m2) to a higher flux.
That is only true for a non-heated object, as the amount of energy reflected equals the amount of energy emitted. A heated object still receives extra energy, while emitted and received energy remain equal. As there is conservation of energy, the extra heat provided is used to warm the object further, thus increasing its emission spectrum, which is reflected 100% back,… Until the whole bunch melts down…
Tony:
At a good lighting store, you can purchase special halogen incandescent bulbs that reflect back much of the infrared output of the filament. As a result, the filament runs hotter than in a standard halogen bulb, resulting in higher total radiation output, and a spectrum shifted to higher frequencies (shorter wavelengths) that standard halogen bulbs.
Ferdinand, you and Phil. should patent your perpetual motion machine right away. All you need is a heated object surrounded by internally reflective casing, and according to you, you can get more power out than you put in. The world’s energy problems are saved!
Something is not right with your idea, Ferdinand. You work it out.
Ed, the whole halogen bulb thing has been done already, under this article I think. Either this or the last one at WUWT that ran to a similar number of comments.
You people really need to stop regurgitating the same nonsense all the time. I know you’re getting desperate, now that GHE “denial” is fast becoming more mainstream, but it’s no excuse for laziness. LikeI said before, you guys will be OK.
Tony,
If you still heat (thus add energy) to an object fully enclosed in reflecting mirrors without any loss of energy to the outside world, that will increase the temperature of that object (and as a side effect shift the frequencies to shorter and more energetic).
Never heard of conservation of energy?
Yes, I’ve heard of it, that’s why I know you’re wrong. You also need to look up the basics of heat transfer.
[Remainder snipped for unwarranted nastiness. Keep it civil, Tony. -w.]
Tony:
These bulbs, with the IR-reflective coatings increasing the filament temperature, exist, and they work as advertised. They are not some thought experiment or laboratory measurement error.
They are physical, empirical proof that you have no idea what you are talking about.
Tony November 28, 2017 at 2:07 pm
Ferdinand, you and Phil. should patent your perpetual motion machine right away. All you need is a heated object surrounded by internally reflective casing, and according to you, you can get more power out than you put in. The world’s energy problems are saved!
To late it’s already patented and it works, it doesn’t do what you claim though, you do not get out more power than you put in, no one except you made that claim. What the lamps do do is achieve the desired temperature element and therefore the desired light output for less electrical input and therefore higher efficiency.
Something is not right with your idea, Ferdinand. You work it out.
No need it’s your idea that is faulty.
Ed, the whole halogen bulb thing has been done already, under this article I think. Either this or the last one at WUWT that ran to a similar number of comments.
You people really need to stop regurgitating the same nonsense all the time.
No the nonsense that is being regurgitated is the same false ‘inverse Wien’s Law’ and the bizarre idea that a photon of a wavelength that is able to be absorbed by an object is mysteriously forbidden from being absorbed if it emitted from a source at a lower temperature. Stop regurgitating that nonscientific nonsense every time the subject of radiational heat transfer comes up and we’ll get somewhere.
Tony,
You may know a lot of heat transfer, but you need to recall some elementary calculations: if you add continuously 100 W electrical energy to a lamp inside a lot of mirrors without any loss to the environment, that energy is added to the total energy content of the lamp. The only way that the added energy is conserved is by warming the lamp and its filament up and up, until the filament melts and then it is over and out.
Even if only 1% of the energy reflected by the mirrors hits the filament, that will increase its temperature until melting as long as you add energy from outside the box: all added energy is emitted and 1% of that extra energy again is reflected to the filament. The remaining 99% reflected energy of what is added from outside will heat the rest of the lamp.
If that was not the case, where does the supplied energy resides?
Nothing to do with perpetuum mobile, only conservation of energy…
Oh, Ferdinand asked me if I’d heard of conservation of energy, so I thought we were doing a whole “condescension” bit. But I see it’s only a problem if I do it.
Anyway, all that aside, you really should patent that perpetual motion idea. There is no real scientific check on patents, as is evidenced by the many perpetual motion machines patented throughout history. Of course, these machines never work when they’re built, but that’s another story. You could use this technology:
https://phys.org/news/2016-12-devices-electricity-closer-reality.html
To convert even some of the heat (the infinite temperature Ferdinand mentioned) into electricity, and you would only need the smallest input of electricity to power the object, and the runaway self-heating will take care of the rest. Infinite temperature will equal infinite electricity, so just one of these things should theoretically (according to Ferdinand) power the whole world’s energy requirements.
Ed and Phil, the bulb thing you’re talking about has already been discussed, not sure why you keep bringing it up. See the discussion on it at Rabbett Run that several people were having about it (in enormous detail). Think it was in the “Green Plate Challenge” thread, if you’re not happy with Bret Keane’s earlier comment on this thread at WUWT. It’s no use just going through the same arguments over and over, that have already been had by other people. It won’t change what has already been said.
And just for the record, Phil and Ferdinand:
“the bizarre idea that a photon of a wavelength that is able to be absorbed by an object is mysteriously forbidden from being absorbed if it emitted from a source at a lower temperature“
and
“Indeed, the Slayers think that any photon coming from a colder object can’t be absorbed by a warmer one, while every CO2 laser proves that wrong…“
You really should not put words in people’s mouths; that is something that makes some people around here very angry, and I’m sure they will be along here soon to condemn that sort of thing as I doubt they would want to be seen as one-sided in applying that criticism. I am not aware of any argument made by myself or any one of these “slayer” characters that I’ve observed, to whit that an individual photon from a cooler object CAN’T be absorbed by a warmer one. As far as I’m aware the arguments centre around the idea of LIKELIHOOD of a photon from a cooler object being absorbed and thermalised by a warmer object compared to the likelihood of a photon from a warmer object being absorbed and thermalised by a cooler one. Thinking about individual photons gets a bit silly when there’s just so many of the darn things. Especially with irradiance as high as you can get with a laser beam.
Tony,
If you continuously add 100 W to a lamp inside a set of mirrors, its temperature will go up, as you don’t loose energy to the outside world. All what you can do is using that extra heat to warm water to a maximum of 100 W if there is zero loss to the rest of the world. That is not a perpetuum mobile, that is just conservation of energy.
If there is no cooling at all, the temperature of lamp and filament goes up until the filament melts…
Again my question: where does the 100 W input go if not used to heat up the lamp/filament?
Ferdinand, the temperature the object can get to is already determined by the incoming power. Why is it that you think that the temperature will suddenly increase just because the power supply continues supplying power? Will the mirrors somehow increase the radiant exitance from the object, or the irradiance to it, in reflecting energy all around? Do the mirrors change which molecules of the object are likely to thermalise photons received?
“the temperature the object can get to is already determined by the incoming power”
No, the temperature is determined by the thermal resistance of the pathway of that heat passing to the environment. Think of an electric blanket (without thermostat). Out in the open, it is cool with the power on. Under blankets it is warm. Too many blankets, and it can catch fire.
The electrical analogy is a current source into a resistance. The voltage (temperature) is proportional to the resistance. Here the reflecting mirrors increase the resistance.
If you want in on Ferdinand and Phil’s invention, Nick, please go ahead. You guys could make a fortune from it if it works. Temperatures going up “to eternity” is not something you want to miss out on, especially since you could couple it with that new technology I linked to. But if it does all work out, don’t forget that I gave you the idea to put the two things together, so I will want in on some of that action. If it works.
By the way, does anyone else want to chip in? I’m not sure if me arguing with four other commenters at once is enough.
Tony,
Ferdinand, the temperature the object can get to is already determined by the incoming power.
Tony, you are completely mistaken on this: the temperature of an object is determined by the incoming power and the outgoing power. If these are in equilibrium, then the temperature of the object is stable. If there is a difference between the two, either the temperature of the object goes up or goes down.
In the case of the mirrors, no/less energy is leaving the system to outside the mirrors than is supplied by the electrical energy, so the whole internals of the system get hotter, or you are destroying energy…
Yes, Ferdinand, the incoming power to the object will be the (presumably electrical) power supplied to the object. The outcoming power from the object will be the thermal radiation that the object emits.
Tony:
Yes, Ferdinand, the incoming power to the object will be the (presumably electrical) power supplied to the object. The outcoming power from the object will be the thermal radiation that the object emits.
For the lamp, the energy input is not only the 100 W electric power, but also the 100 W EM emitted by the lamp which is reflected by the mirrors. That is 200 W incoming in total, 100 W outgoing, 100 W used to heat up the total lamp.
Even if only 1% of the reflected energy is absorbed by the filament, that will heat it further, thus increasing temperature and radiation, including a shift to shorter waves.
I get that you think that reflected output from an object can be double-counted as input to the object. That is necessarily the condition which leads to your idea of runaway self-heating of the object to eternity. Restating your initial position is not going to advance this conversation.
I suggest that if you believe that objects can warm themselves through their own reflected energy, you patent and build your machine forthwith. Utilising the technology I linked to as part of the design will mean that you can get an electrical output from the machine without having to compromise the internally reflective casing. If you believe that what you say is true, why would you not be doing this?
Us talking about it, back and forth, until the end of time, is not going to settle anything.
Tony, you say: “Ed and Phil, the bulb thing you’re talking about has already been discussed, not sure why you keep bringing it up.”
We keep bringing it up because these things that you say could not possibly exist do in fact exist. They work, they do what we claim, and you can buy them in stores.
They are an absolute refutation of your position.
“they do what we claim”
The temperature goes up until eternity (Ferdinand’s words)? Astonishing.
Ed, your bulbs are a distraction, do not represent what we’re talking about (energy still leaves the bulbs, for a start – that’s why they’re bulbs) and they have been discussed to death elsewhere. I get that you’re desperate, but cooler heads have prevailed. Sorry.
Tony:
I get that you think that reflected output from an object can be double-counted as input to the object.
I don’t only think that, it is proven by the MIT lamp with IR mirrors, proven bij high yield halogen lamps, proven by the lamp test by Anthony, etc…
There is a constant input of energy to the lamp. All of that energy must be conserved.
If the lamp is free standing in vacuum, all energy supplied is emitted at the temperature of the filament at 3,000 K and nothing back radiated. Per second: 100 W.s supply, 100 W.s emitted to space, no further heating of the filament and lamp.
If the lamp is covered with mirrors with 100% reflection and no ecape to space and no absorption of the mirrored energy, then in time:
Second 1: 100 W.s energy in, 100 W.s energy radiated out, bouncing around within the mirrors.
Second 2: 100 W.s energy in, 100 W.s radiated out, 200 W.s energy bouncing around.
Second 3: 100 W.s energy in, 100 W.s radiated out, 300 W.s energy bouncing around.
….
Even if only 1% of the bouncing energy is trapped again, the temperature of the lamp and/or filament goes up.
There is no place to hide for the energy supplied other than heating the lamp and its filament.
Except if you have another explanation where the supplied energy resides…
Lol, back to the lamps again…
These lamps where, we’re conclusively told that you don’t get more power out than you put in. It’s just that you get the same power out for less power in. Hilarious:
“To late it’s already patented and it works, it doesn’t do what you claim though, you do not get out more power than you put in, no one except you made that claim. What the lamps do do is achieve the desired temperature element and therefore the desired light output for less electrical input and therefore higher efficiency.“
Either you are all effectively saying that you DO get more power out than you put in (I’m afraid saying that you get the same power out for less power in is the same thing), in which case you are DISAGREEING with me, and the laws of physics, or the bulbs do NOT get more power out than you put in (you just get more in the visible light spectrum and less IR, at both lower power in AND lower total power out), in which case you AGREE with me, and Brett Keane, and the person commenting at Rabett Run, and the laws of physics, etc etc etc.
Tony,
It’s just that you get the same power out for less power in.
Please… Who said that? You get more visible light for less power in. And you get the same temperature of the filament for less power in by recycling IR back to the filament. That is what is said.
Something which is impossible by your theory: IR at a lower average energy level increases the temperature of the filament to much higher temperatures than for the power suplied…
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2679985
Tony,
We agree on:
you just get more in the visible light spectrum and less IR, at both lower power in AND lower total power out
That is what the lamps do because they reflect IR back to the filament by mirrors. So we may agree on the result, we do disagree on the reason why: the hot filament receives extra energy from the reflected IR which is at a lower frequency, still heats the filament for 2/3 of the total energy needed to reach the desired temperature.
BTW, where gets the energy input if you enclose everything in mirrors without escape to space?
So, Ferdinand, the lamps do NOT give you a higher power output from the same power input so they do NOT prove that
“reflected output from an object can be double-counted as input to the object.”
because the reflected IR is NOT shown to increase the temperature of the system. In the bulbs case the system includes that which is emitted outwards by the bulb (so it includes the room the bulb is in) and in our case (the original, frequently overlooked case which we are actually supposed to be talking about) the system does not include such output and space since everything is contained within the reflective casing. Which is the crucial difference you continue to miss.
In answer to your question, the power output from the heated object doesn’t go anywhere. It bounces around indefinitely inside the reflective casing. Unless you are making the mistake that energy is like matter, and is therefore going to keep building up and building up until something “bursts”, then you shouldn’t have a problem with that.
Tony:
they do NOT prove that
“reflected output from an object can be double-counted as input to the object.”
because the reflected IR is NOT shown to increase the temperature of the system.
Sorry? If you do reduce the power input to 1/3 of the original and the filament still shows the same temperature of 3,000 K, only by reflecting a lot of IR back to the filament, that is NOT increasing the temperature of the filament???
Is there any physical reason why reflected IR is NOT absorbed by the object that emitted it?
The reflected IR which is at a lower frequency than average what is emitted, still heats the filament for 2/3 of the total energy needed to reach the desired temperature.
The same for the mirrored case: every bit of input, even over weeks, is bounced back and forth without ever reaching – and heating – the lamp or its filament, even if it hits it directly?
Any reason why that energy is not absorbed and thus simply reflected in your opinion?
You either agree with me that you’re not getting more power out than you’re putting in, or you don’t.
If you don’t understand, keep reading through my responses until you do.
Tony,
We, that is Ed Bo, Nick Stokes, Phil. and me, do agree that you can’t get more energy out of a system than you put into that system.
Where we do disagree is that within a system, you can have accumulation of energy (and thus temperature) that is retained before it reaches the outside of the system.
That is the case for reflected IR which heats an object within the system higher than from the external input alone.
That is the case for mirrored EM which heats a lamp until the filament melts if there is only energy supply to the system and no energy loss out of the system.
If you don’t agree, give me the reasons why an already warm filament can’t thermalyse (part of) its own reflected EM energy…
Ferdinand. I’ve already made the point that you are not seeing the difference between what constitutes “the system” in the two DIFFERENT cases of the heated object within the internally reflective casing and the light bulb. You insist on referring to the heated object within the internally reflective casing as “a filament”, for instance. The filament is a part of the bulb system, which includes the outer casing and the output from that outer casing. The heated object in the internally reflective casing, on the other hand, is not a filament. It’s just an object.
With the heated object inside the internally reflective casing, that outer casing (the other side, not the reflective side) and the output from that side, are NOT parts of “the system”.
Now take this in. Walk away from the conversation for a day or so. Mull over the implications of it. Read through all the posts again. Mull it over some more. And then, and only then, bother to respond. If you keep replying before then I’m just going to refer you to previous posts.
Tony,
You are just diverting the attention from the essence…
The essence of the whole discussion is that a hot object can and does receive and thermalise radiated energy from a colder object, even if the “colder” object is its own reflected IR radiation.
It doesn’t matter if the energy is transmitted by a filament inside a bulb or from a heated globe. If a part (or all) of the outgoing radiation is reflected back, the temperature of either the filament or the globe will go up for the same initial energy input.
Except if you have proof that for any reason the objects can’t thermalise any reflected radiation…
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2680265
Plus, not to forget:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2679660
And finally, back to the beginning…
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2678573
Then just keep reading through until you get it. Give it a couple of days. I have faith in you.
Sorry, first put on the wrong place:
Tony,
No need for a few days, I am learning fast…
Back to your answer to the last reference:
I would say that the slayers think the frequency spectrum of the radiation the object emits can’t be increased by itself, or the fact that the object receives its own reflected energy.
I don’t see why that is impossible: if even only 1% of the energy contained in the reflected EM is absorbed, the temperature will go up and the total received energy increases and thus the object’s temperature and the outgoing spectrum gets more shortwave and energy intensive.
Even if, as you say, the possibility of absorbance for low energy IR is less than for high energy IR, if the full outgoing spectrum is reflected, half of it is higher in energy than the peak frequency, thus with a high possibility of absorbance.
In realistic figures:
Start conditions: object (sphere, plate,…) with external heating 100 W in thermal equilibrium with its own emissions spectrum of 100 W in vacuum, no mirrors.
Inserted within a mirrored case, no external losses; 10% of the reflected waves absorbed by the object; time delay between object and mirrors and back: 1 second (needs a box of 150,000 km diameter, but is only used to show the energetic balances).
Second 1: 100 W.s in; 100 W.s out.
Second 2: 100 W.s in; 100 W.s out; 10 W.s absorbed; 90 W.s bouncing around.
Second 3: 100 W.s in; 5 W.s heating the object; 105 W.s out; 19 W.s absorbed; 171 W.s bouncing around.
Second 4: 100 W.s in; 9 W.s heating the object; 115 W.s out; 28 W.s absorbed; 248 W.s bouncing around.
etc…
These are not exact figures, but when I have some spare time, I can make a sheet like the above where one can play with energy inputs, % reflected, % absorbance, etc…
No matter that only 10% of the reflected energy is absorbed and thermalised, the temperature of the object goes up until something goes wrong.
There is no violation of any physical law, as the extra warming is caused by energy lost from the object in the previous (nano)second and (in part) directly absorbed again. Even if that would be 100%, the absorbed energy is from its own previous emissions.
The emission spectrum plays little role, as in every case (near) half of the spectrum is higher energetic than half of the emitter’s own spectrum, thus anyway a substantial part of the bounced energy is absorbed, even if you don’t believe that every photon, whatever its energy level, is absorbed…
There’s just no point in this conversation continuing. You are not adding anything new with what you’re saying.
Perhaps that’s unfair. You are, at least, listening to what I’m saying and taking on board new information. That’s more than we see from a lot of people…I apologise.
Tony November 29, 2017 at 1:15 am
And just for the record, Phil and Ferdinand:
“the bizarre idea that a photon of a wavelength that is able to be absorbed by an object is mysteriously forbidden from being absorbed if it emitted from a source at a lower temperature“
You really should not put words in people’s mouths; that is something that makes some people around here very angry, and I’m sure they will be along here soon to condemn that sort of thing as I doubt they would want to be seen as one-sided in applying that criticism. I am not aware of any argument made by myself or any one of these “slayer” characters that I’ve observed, to whit that an individual photon from a cooler object CAN’T be absorbed by a warmer one.
I’m not putting anything in anyone’s mouth, that comment has frequently been made here and I will continue to post rebuttals to it. Yes I have on occasion referred to the dichroic bulbs as an example of recycling of IR allowing more efficient generation of visible light and will continue to do so where necessary.
Do whatever you want, Phil.
Tony:
You are, at least, listening to what I’m saying and taking on board new information. That’s more than we see from a lot of people…I apologise.
No need to apologise… I always try to understand what another’s opinion means and try to refute it (or accept it) with arguments.
The difference in opinion still seems to be that you and others in this discussion don’t accept that reflected waves of the same and less intensive wavelengths can be thermalised by a hot object. With nuances between to a lesser extent – depending of the probability – and not at all.
The other side, myself included, are of the opinion that whatever wavelength, for a black body every radiation is absorbed and thermalised, no matter the temperature of the origin and the destination.
The latter opinion is supported by lots of real life applications: CO2 and other lasers, light bulb internal reflection,…
Some heavy, but interesting literature about lasers used in metallurgy:
https://www.diva-portal.org/smash/get/diva2:999341/FULLTEXT01.pdf
Fig. 3 at page 27 shows the graph of absorbance: Iron absorbes about 6% of the energy of a CO2 laser at 10.6 micrometer. According to that study, the absorbance increases with temperature and even gets a boost in the molten metal…
Ferdinand, I can see it’s important to you that you have the last word. That’s OK with me.
But it may (or may not) be a revelation to you that not all absorbed radiation is thermalised. That’s another thing to process.
Tony:
But it may (or may not) be a revelation to you that not all absorbed radiation is thermalised. That’s another thing to process.
Radiation that is absorbed must be thermalised (or you are destroying energy). The reason why and how is in the first book of that dissertation. But a lot of the IR beam is reflected, depending of the smoothness of the surface… The best results seems to be with a small layer of rust…
It’s not up for discussion. Absorbed radiation isn’t always thermalised. Look it up.
Your model is a straw man. The claim is that a cool atmosphere that envelops a warmer planet, reduces the planet’s cooling rate over what it would be in its absence.
Your hot water is enveloped by ambient air and the ice cube is a fart in a windstorm
You are a sophist, you cannot delay cooling without creating ”heat” you cannot create heat with a weaker resonance making a higher resonating frequency resonate even higher, to keep ”heat” in the equation, it needs to be created,
Robert:
If you took an actual formal thermodynamics course, you would learn that the first step in analysis is to define the system completely, INCLUDING the ambient conditions. Otherwise you are lost before you start.
You say: “a jar of hot water, first, is sitting ALONE.” First mistake! There is no ALONE. What is the ambient? If the ambient is the walls of your room at 23C (296K), then adding the ice nearby will result in the water being cooler than without it.
However, if the ambient is the 3K effective radiative temperature of deep space, adding the ice nearby will result in the water being warmer than without it.
In both of these cases, if there is no separate power supply for the water, the presence of the ice nearby just changes the rate at which the water cools to ambient. In the first case, it would accelerate it; in the second, it would slow it.
But if there is a constant power input to the water, the presence of the ice nearby will change the resulting steady state temperature of the water. In the first case, it will lower the SS temperature; in the second case, it will raise the SS temperature.
The situation we are talking about with the earth is this final case, where there is a separate power input (the sun), and a very cold ambient (the 3K of deep space). In this case, the presence of nearby ice, being hotter than ambient, will lead to a higher SS temperature for the water.
Ed, I have to thank you for your clear and dispassionate answers to people throughout this thread. Your description above is exactly what would happen—if you put an ice cube next to a warm object in space, the object will be warmer than it would be without the ice cube.
You are also quite clear about the steady state case—with an external power input, the object will end up at a HIGHER steady-state temperature.
w.
Ok, let’s examine the “ambient” claim.
If the water is warmer than both the walls and the ice, will either object increase the temperature of the water?
If both the wall and the ice cube had no starting energy of their own other than the background radiation received from space, would the water cool more slowly with the presence of the ice cube?
Does oxygen, nitrogen or carbon dioxide have any starting energy of their own? Or are all three dependent on what is received from the sun and earth’s surface (which is itself dependent on what it receives from the sun) to be warmed? Has there been any change in the energy received in the global warming/Greenhouse Effect claims by changing the atmospheric composition? NO! All the claims are based on the energy emitted. The amount received doesn’t change. The sun warms the earth. The earth cools to space. The earth is warmed until the energy it emits is equal to the energy it absorbs. The amount being absorbed hasn’t changed, by the conversion of some Carbon and Oxygen into Carbon Dioxide, therefore the amount being emitted hasn’t been changed by it either.
The speed at which something cools has no relevance to what something will eventually cool to. An warmer object will ALWAYS cool to the temperature of its surrounding environment if that environment is cooler. It will ALWAYS warm to the temperature of its surrounding environment if that environment is warmer. So “cooling more slowly thereby making it warmer” is just nonsense. You may create temporary changes of the objects in a system by moving those objects around, but the total energy of that system won’t change as long as the energy absorbed from the heat source remains constant.
wickedwenchfan,
You need to make a differentiation between what happens underway and the ultimate balance.
Once the whole system is in equilibrium, whatever happens in intermediate steps, the amounts of incoming and outgoing energy are the same. So far so good.
Where then is the difference? If you introduce a hindrance to the outgoing emissions, no matter if that is some insulation or something that reflects/re-emits IR, that reduces the outgoing radiation, thus introducing an energy imbalance. That energy imbalance increases the temperature of the emittor (the earth’s surface), until the amount of outgoing energy after the hindrance is the same as the incoming energy at the emittor, thus before the hindrance.
In both cases, the ultimate energy balance is zero. In the second case the earth’s surface is warmer.
In the case that there is no incoming energy, the cooling would be slower for the second case with extra insulation/backradiation.
The ice cube, obviously, has a SMALLER quantity of “warmth” than the jar of hot water.
Remember, though, both are radiating.
But the claim is that … (1) the ice cube can leave the jar of hot water warmer than the jar of hot water would be if the ice cube weren’t there.
If the hot water exist in a infinite, empty vacuum with nothing else that radiates, and you add an ice cube which does radiate, it is trivially obviously that the ice cube must warms the hot water, since an object receiving radiation must be warmer than if it does not receive said radiation. This only seems strange to you because you’re generally surrounded by radiating objects which are warmer than ice cubes, not an infinite zero-radiation vacuum.
“How is Claim #1 not a blatant violation of the Second Law? … merely worded differently?”
The 2nd Law governs the average net flow, it doesn’t speak to the elements of the flow.
Tony,
No need for a few days, I am learning fast…
Back to your answer to the last reference:
I would say that the slayers think the frequency spectrum of the radiation the object emits can’t be increased by itself, or the fact that the object receives its own reflected energy.
I don’t see why that is impossible: if even only 1% of the energy contained in the reflected EM is absorbed, the temperature will go up and the total received energy increases and thus the object’s temperature and the outgoing spectrum gets more shortwave and energy intensive.
Even if, as you say, the possibility of absorbance for low energy IR is less than for high energy IR, if the full outgoing spectrum is reflected, half of it is higher in energy than the peak frequency, thus with a high possibility of absorbance.
In realistic figures:
Start conditions: object (sphere, plate,…) with external heating 100 W in thermal equilibrium with its own emissions spectrum of 100 W in vacuum, no mirrors.
Inserted within a mirrored case, no external losses; 10% of the reflected waves absorbed by the object; time delay between object and mirrors and back: 1 second (needs a box of 150,000 km diameter, but is only used to show the energetic balances).
Second 1: 100 W.s in; 100 W.s out.
Second 2: 100 W.s in; 100 W.s out; 10 W.s absorbed; 90 W.s bouncing around.
Second 3: 100 W.s in; 5 W.s heating the object; 105 W.s out; 19 W.s absorbed; 171 W.s bouncing around.
Second 4: 100 W.s in; 9 W.s heating the object; 115 W.s out; 28 W.s absorbed; 248 W.s bouncing around.
etc…
These are not exact figures, but when I have some spare time, I can make a sheet like the above where one can play with energy inputs, % reflected, % absorbance, etc…
No matter that only 10% of the reflected energy is absorbed and thermalised, the temperature of the object goes up until something goes wrong.
There is no violation of any physical law, as the extra warming is caused by energy lost from the object in the previous (nano)second and (in part) directly absorbed again. Even if that would be 100%, the absorbed energy is from its own previous emissions.
The emission spectrum plays little role, as in every case (near) half of the spectrum is higher energetic than half of the emitter’s own spectrum, thus anyway a substantial part of the bounced energy is absorbed, even if you don’t believe that every photon, whatever its energy level, is absorbed…
Sorry, put on the wrong place…
Paul you only ever have in-flight photons in the chamber they never stop, a minute bit of free flux yet to impinge and replace, photons containing a fraction of the radiation’s resonance from the warmer block.
The thermal resonance of both blocks are near identical if you make the temperature differential 1 kelvin only, all outward emitted fluxes replace all outward fluxes it really is that simple, the blocks are virtually identical a 0.3% temperature differential the chamber back ground is 0 kelvin., [magically].
A is replacing B’s emission, and B is replacing A’s emission, at the same time, virtually instant, however B’s emission and Only B’s s radiating resonance contains some slightly more curried up photons that thermalise.
Gary Ashe,
You haven’t looked at Wien’s displacement law: every object above 0 K emits IR at a range of frequencies, as every atom/molecule in mass has its own “temperature” which may be 0 K to 500 K as kinetic energy. All what we measure as “temperature” is the average kinetic energy of a lot of atoms/molecules.
The difference between 274 K and 275 K is only a small shift in peak wavelength and both objects have largely overlapping emissions at both sides of the peak wavelength.
So, even in your -wrong- reasoning that only more energetic waves are absorbed: near half of the photons emitted by the “colder” object have frequencies which are high enough to be absorbed by the average atoms/molecules in the “warm” object and thus are thermalised…
That even very hot objects do thermalise IR waves in bands that are in the “cold” range is proven every day by CO2 lasers: these emit all their energy around the 10 micrometer band, that is appr. the peak temperature of a human’s body. Despite that, steel at 800ºC still is warmed further up to its melting point by these “cold” waves.
If the blocks are at equilibrium all outward emissions are just replacing all outward emissions from both blocks, there is no variance, none of the continued resonance exchange creates ”heat”. and they do not slow each others cooling, they simply swap the exact same resonance for eternity. at 275 kelvin….. never again will either blocks emissions or absorption resonance act thermally………….it is redundant energy, trapped for eternity.
Unless you stick your finger in.
Not if the object was full of water Ferdinand, you could plug the object directly into the sun and shield it perfectly all you like, you still wont get the h20 above 100c.
Gary,
We were discussing radiation, not boiling. Still once all water evaporated, the jar would melt in the sun…
OK, Ferdinand, let me ask you this, if it works for an Ice Cube wouldn’t it work better for 2 objects at the same Temperature, (like the reflected Energy)?
Both objects are at 60C with a very small Air Gap, will either of them get warmer when exposed to the others Radiation Energy?
If this doesn’t happen why would it happen for and object with lower temperature Radiation?
Willis, re the statement made by both yourself and Dr Spencer on his measuring DWLIR experiment post, which I have reread yet again, including all the comments.
The statement in response to my “concentrating DWLIR” is that diffuse radiation cannot be concentrated.
His very carefully designed experiment proved the opposite.
So please take off your “scientific consensus” head and put on your usual common sense logical head that gave us your thunderstorm regulator posts.
Here is the data I would like you to process and explain to me and anybody else still reading this post where My conclusion is wrong.
The very carefully designed scientific experiment created by Dr Spencer managed to obtain a reduction of 4 degrees F below ambient.
A complete layman produced a reduction of approximately 20 degrees F below ambient, (confirmed by Universities), with no special equipment and no special insulation.
This reduction of 5 times Roy’s was achieved using a Solar Oven as I explained to you before.
What I should have explained is that it is actually a Solar Collector used as an oven.
This Solar Collector, based on those results appears to also be a DWLIR collector also.
To confirm this without realizing it’s significance Roy added a Solar (Radiation) collector to his experiment and it made a difference, however Roy did not appear to update his original graph with the new data so we do not know by how much.
So in your opinion does the Solar Collector “Concentrate” DWLIR or not based on that data?
The comments mentioned quite a few times the Solar Oven’s design and that is why Roy incorporated it to improve the reduction of temperature in his experiment.
Dr Spencer also made an incorrect statement in response to someone who suggested that increased CO2 at TOA would increase Cooling and the good Dr said it would not and would decrease cooling.
This statement by Dr Roy is completely wrong, as NASA’s own satellite data has shown.
Refer to my link to the 2013 AGU meeting for confirmation.
A C:
I’m afraid you are completely missing the point. Some people observe that you can concentrate the energy in solar radiation to get a very high temperature (above ambient) at the point of concentration, and wonder why you cannot concentrate the longwave infrared “back radiation” in the same manner to obtain temperatures above ambient.
Willis (and others) correctly answer that while solar radiation is highly parallel (with only 1/2-degree spread) and so can be highly concentrated with parabolic reflectors, the LWIR radiation is completely diffuse, so cannot be concentrated.
You are talking about cooling effects producing temperatures below ambient — completely different.
Ed, without the “Collector” only -4 degrees below zero was achievable.
So how did the Collector increase the rate of cooling to achieve -20 degrees?
AC:
You don’t provide a link to the experiment you cite, so I can’t comment on the specifics. But, I repeat, Willis was answering questions about possiblly concentrating radiation to get higher temperatures. You are talking about achieving lower temperatures.
You and Willis are both good at quoting each other.
Here is the link.
http://www.drroyspencer.com/2010/07/first-results-from-the-box-investigating-the-effects-of-infrared-sky-radiation-on-air-temperature/
Please read all the comments and then get back to me and explain just how it works.
I also have the same question for you that I asked Ferdinand.
If an Ice Cubes Radiation makes an object warmer wouldn’t it work better for 2 objects at the same Temperature, (like the reflected Energy)?
Both objects are at 60C with a very small Air Gap, will either of them get warmer when exposed to the others Radiation Energy?
If this doesn’t happen why would it happen for an object with lower temperature Radiation?
A C Osborn,
If both objects are not heated from an outside source, in both cases bringing them together in space will reduce the speed of cooling (never get one of them warmer!).
If they are both equal in temperature and size, the speed of cooling would be exactly half the speed without the second one. If the second one is cooler (or smaller) the speed of cooling would be faster in ratio to the difference in mass and temperature.
Why?
Isn’t either of the Objects being bombarded and absorbing Extra Photon Energy?
If it doesn’t work for them how does it work at night for CO2, when the Surface has No Direct Source of Energy and is in the same cooling state as the 2 objects at 60C.
Of course it would work for the CO2 because it is still getting energy from the Surface.
A C Osborn,
If there was no CO2 or other GHGs, the earth at night would cool with all the IR emitted per unit of time for its temperature.
With CO2 (even much more with clouds) part of that emitted IR is sent back to the surface. That is always less than what is emitted by the surface, as not all IR is sent back. Thus all what happens is that the speed of cooling is reduced.
As clouds and water vapor are much stronger reflectors, under cloudy or wet conditions, the nights are hardly cooling, but even the small contribution of CO2 in open skies is measurable…
The message of this thread to me is that the rate of cooling and warming can be reduced by an insulating agent which in the case of the earth is the atmosphere. During the day it reduces the warming, during the night it reduces the cooling (compare to the moon). The clouds increase both effects. What role CO2 has in this? If prof Will Happer is right (and I have not seen it debunked, even Eli Rabett admits it) how much or little LWIR the CO2 molecules in the atmosphere are absorbing, they remain at almost exactly the same temperature as the other air molecules.To me this means that CO2 has no specific insulating capacity. It acts like the other gases of the atmosphere.
a radiant gas instantly (speed of light) distributes the radiation around the globe and out to space.
the word for that is dispersion, not trapping.
“they remain at almost exactly the same temperature as the other air molecules”
Yes. When they absorb energy, they rapidly transfer it to neighboring molecules. Everything gets a little bit warmer.
Because of their warmth (shared with the air) the GHG molecules also emit photons. Then everything gets a little bit cooler again. This mostly works out to a steady state.
Nick: so you agree that CO2 has no specific insulating capacity?
Well if one has two objects without atmospheres near to one another in space both being subjected to the same external radiation source and being at different temperatures then due to the net interchanges of radiation between them the warmer one would heat up the cooler one until both were at the same temperature. It is therefore the net flow between them that matters even with bi directional radiation fluxes.
How could the radiation from the cooler one to the warmer one make the warmer object even warmer when there is no net flow from the cooler to the warmer?
The issue here is whether that final temperature OF BOTH would become elevated above that which the S-B equation predicts.
I suggest not because both having equalised at the same temperature one cannot be cooler than the other and no additional reduction in the cooling rate of either can be caused by the proximity of one to the other.
Both will stabilise at the S-B predicted temperature.
So why is it proposed that a steel shell or an atmosphere around a planet would behave any differently?
Now add an atmosphere to both of the objects.
If the atmospheres were inert they would acquire the same S-B temperature as the surfaces and both surfaces and both atmospheres would be isothermal at S-B. Either via conduction or radiation fluxes the S-B equation would be satisfied.
An atmosphere is not inert, it convects. That is the critical different because calculations based on radiation fluxes alone no longer work.
Suddenly one has to consider non radiative energy transmission processes AND the fact that KE becomes PE in rising air or PE becomes KE in falling air such that an isothermal atmosphere cannot develop.
In that situation the surface temperature DOES rise above S-B and it is nothing whatever to do with radiation.
If each object in space had a different mass of atmosphere then the surface temperatures would be different but the radiation fluxes between them would both be the same as if there were no atmospheres because both objects would be radiating to space and to each other at S-B just as the Earth with its surface temperature enhancement radiates to space at S-B.
Well if one has two objects without atmospheres near to one another in space both being subjected to the same external radiation source and being at different temperatures then due to the net interchanges of radiation between them the warmer one would heat up the cooler one until both were at the same
temperature. It is therefore the net flow between them that matters even with bi directional radiation fluxes.
How could the radiation from the cooler one to the warmer one make the warmer object even warmer when there is no net flow from the cooler to the warmer?
The issue here is whether that final temperature OF BOTH would become elevated above that which the S-B equation predicts.
I suggest not because both having equalised at the same temperature one cannot be cooler than the other and no additional reduction in the cooling rate of either can be caused by the proximity of one to the other.
Both will stabilise at the S-B predicted temperature.
So why is it proposed that a steel shell or an atmosphere around a planet would behave any differently?
Now add an atmosphere to both of the objects.
If the atmospheres were inert they would acquire the same S-B temperature as the surfaces and both surfaces and both atmospheres would be isothermal at S-B. Either via conduction or radiation fluxes the S-B equation would be satisfied.
An atmosphere is not inert, it convects. That is the critical different because calculations based on radiation fluxes alone no longer work.
Suddenly one has to consider non radiative energy transmission processes AND the fact that KE becomes PE in rising air or PE becomes KE in falling air such that an isothermal atmosphere cannot develop.
In that situation the surface temperature DOES rise above S-B and it is nothing whatever to do with radiation.
If each object in space had a different mass of atmosphere then the surface temperatures would be different but the radiation fluxes between them would both be the same as if there were no atmospheres because both objects would be radiating to space and to each other at S-B just as the Earth with its surface temperature enhancement radiates to space at S-B.
The most fundamental point being that the temperature value predicted by the S-B equation is an absolute MAXIMUM that can be achieved via radiation alone from a given external energy source.
It cannot ever be increased by INTERNAL radiation that is not INDEPENDENTLY generated.
In order to exceed S-B one must add non radiative energy transmission processes and since they are inevitably slower than radiation they must raise temperatures of surfaces affected by them.
Thereafter, to maintain hydrostatic equilibrium within those atmospheres, the non radiative processes must net out to zero which is exactly what happens in adiabatic uplift and descent.
Well, I guess I am unsurprised that one more emphatic posting on this topic has failed to resolve all disagreements and put the issue in the rearview mirror once and for all.
It sure does seem to me that at least some of the highly contentious disagreements we find in this article and comment thread ought to be resolvable by one or a series of carefully designed experiments.
It seems very unlikely that these sorts of disputes can ever be resolved by hashing it out at length.
Radiant-heat trapping by freely convective gases has never been demonstrated experimentally.
Convective gases trap conductive heat which depletes the amount of radiant energy able to escape to space,
However, the effect only occurs during the initial uplift of the atmospheric mass off the surface. Once hydrostatic equilibrium is achieved there is no further transfer of radiant energy to convective energy via conduction.
Instead the energy required for hydrostatic equilibrium is simply recycled up and down within convective overturning indefinitely in a net zero loop.
Many years ago I described it as the adiabatic loop as opposed to the diabatic loop.
Overall this is true, but this is still a bad framing: “While the answer is generally no, it can do so in the special case when the cold object is hiding an even colder object from view.”
The vacuum of space is not an object, it is the absence of objects. It’s more accurate to just say “all objects radiate and warm other objects, even objects that are warmer than they are.”
talldave2
Objects in space that are cooler than an adjacent object can only use their own radiation to slow the rate at which they are warmed by radiation from the adjacent object.
They can’t make that warmer object any warmer than the S-B prediction.
In due course the cooler object warms up to a point where both objects satisfy the S-B equation.
AGW alarmists and Willis are trying to tell us that the colder object can make the warmer object reach a temperature higher than the S-B prediction from radiation alone. That must be wrong because if it were possible there would be a perpetual warming process/ loop between the two objects as the colder one continually warms the warmer one and the warmer one then continually warms the colder one a bit more.
AGW radiation theory is the true perpetuum mobile and an abuse of the S-B equation.
The S-B temperature is the maximum temperature one can get for either object at a given level of external radiation reaching both objects simultaneously and in due course both objects will reach that maximum temperature because the net flow is from the warmer object to the colder object.
Unless one adds convecting atmospheres that is but in that event the radiative only S-B equation ceases to apply.
Oh dear oh dear oh dear, I cannot believe anybody can be so slow on the uptake. This is very categorically NOT what Willis is saying. Go and read the article again.
The warmer body benefits from a constant heat source. In the Earth’s case that is short wave radiation, mainly visible and UV, from the sun, arriving at the Earth’s surface, being absorbed and warming it. That warm surface then emits that energy in the form of LWIR. However, it cannot just radiate directly into space – it has that darn pesky atmosphere in its way. That atmosphere, despite being colder than the surface, slows down the rate at which the constantly arriving energy absorbed by the earth’s surface can be dissipated back out to space.
If the rate of energy dissipation is slower than the rate of energy absorption then the amount of energy at the surface starts to build up, in which case the temperature has to rise. A body at a higher temperature emits radiation at a greater rate than a cooler body. Therefore as the temperature rises the rate of heat loss increases until a new equilibrium is reached where the rate at which energy escapes from the earth’s surface is the same as the rate as it arrives from the Sun. That new equilibrium is at a higher temperature.
It is total common effing sense! All the S-B equation does is give you the constant that allows you to calculate the temperatures of the various bodies involved. You don’t need it to understand the principle.
Both you and Willis are talking about a cooler body reducing the rate of cooling of the warmer body such as to raise its ‘equilibrium’ temperature. You both refer exclusively to radiation and so must take into account the S-B equation which is a solely radiative calculation.
In doing so you seek to avoid the implications of the S-B equation. That equation gives a temperature that inevitably follows from external radiation impacting a blackbody that absorbs all that radiation of whatever wavelength and emits it all as sensible heat in the form of the narrower IR wavelengths.
I am pointing out that in the absence of a convecting atmosphere that equilibrium temperature cannot rise above the S-B prediction because the S-B equation defines the perfectly efficient scenario for the generation of IR from multiple other wavelengths.
Simply adding a non-convecting atmosphere can only result in the atmosphere AND THE SURFACE reaching the S-B temperature. Effectively, the atmosphere would have to behave as a solid for that to be possible.
Since the atmosphere is actually a partially radiative gas and not a solid it follows that if the surface goes above the S-B prediction then the total of energy radiated directly to space BY THE SURFACE and energy radiated to space from WITHIN THE ATMOSPHERE will be more than radiation coming in from outside the system which is not possible if the atmosphere is to be held indefinitely in hydrostatic equilibrium.
To be able to go beyond the S-B temperature AT THE SURFACE when the atmosphere also has radiative capability you need the atmosphere to be convecting so that the surplus radiation that would otherwise be coming from the hotter surface cannot be radiated to space IN ADDITION TO the radiation going to space from within the atmosphere. Instead, it is going to fuel ongoing convective turnover via further conduction.
Surface kinetic energy (sensible heat) cannot BOTH radiate and conduct simultaneously.
Thus the surface temperature enhancement is a consequence of mass convecting up and down in a gravity field and not radiation fluxes going back and forth.
Only that way can conservation of energy be complied with.
You are right to say:
“That atmosphere, despite being colder than the surface, slows down the rate at which the constantly arriving energy absorbed by the earth’s surface can be dissipated back out to space.”
But it must be done by conduction and convection and not by radiation for the reasons I stated.
TLM, “The warmer body benefits from a constant heat source. In the Earth’s case that is short wave radiation, mainly visible and UV, from the sun, arriving at the Earth’s surface, being absorbed and warming it.”
No!
It is NOT CONSTANT.
Do you not Understand the difference between Day Time and Night Time.
It is total common effing sense!
So night and day is actually the sun blinking on and off then? Who knew!
Objects in space that are cooler than an adjacent object can only use their own radiation to slow the rate at which they are warmed by radiation from the adjacent object.
Again, that’s sort of true but a poor framing. It’s more accurate to simply say both objects radiate, and some of that radiation reaches the other, ergo a cold object must warm a warmer one — indeed, all object not at absolute zero will radiate and warm all other objects, irrespective of temperature.
S-B just tells you what an object radiates at a given thermodynamic temperature. It doesn’t change any of the above.
That must be wrong because if it were possible there would be a perpetual warming process/ loop between the two objects as the colder one continually warms the warmer one and the warmer one then continually warms the colder one a bit more.
No, that’s only true when the received radiation is greater than the emitted. You’re confusing “getting warmer” with “warming” If I light a fire in a forest at -40F, it will certainly warm me, but I may very well get colder anyway.
You are forgetting that both objects are being continuously irradiated by a single external source.
S-B tells us that at a given input of external irradiation falling onto an ideal blackbody a given temperature will be achieved. It is a two way equation. EITHER a given amount of irradiation will achieve a given temperature OR a given temperature will radiate at a given level.
My point is that whatever radiation is being swapped between those two bodies you cannot go higher than the temperature predicted by the S-B equation because S-B defines the thermal outcome from the fastest and most complete conversion of incoming shortwave to outgoing longwave. All that will happen is that both bodies will come to match the S-B prediction PROVIDED that there is no additional energy source.
If you suggest that for ANY reason the radiation from either body drops below the S-B prediction whilst external irradiation continues at the same rate then you have a potentially catastrophic positive feedback loop.
That is why I have proposed two separate energy loops, one radiative matching energy in from space with energy out to space and one- non radiative matching convective energy in ascending air with convective energy in descending air.
Both loops then remain in steady state as long as external irradiation continues and any internal radiative imbalances are neutralised by convective adjustments.
That is exactly what we observe.
TLM,
If the rate of energy dissipation is slower than the rate of energy absorption then the amount of energy at the surface starts to build up, in which case the temperature has to rise. A body at a higher temperature emits radiation at a greater rate than a cooler body. Therefore as the temperature rises the rate of heat loss increases until a new equilibrium is reached where the rate at which energy escapes from the earth’s surface is the same as the rate as it arrives from the Sun. That new equilibrium is at a higher temperature.
Exacfly! I don’t know why this is so hard to understand.
However, it cannot just radiate directly into space – it has that darn pesky atmosphere in its way. That atmosphere, despite being colder than the surface, slows down the rate at which the constantly arriving energy absorbed by the earth’s surface can be dissipated back out to space.
This is another instance of being basically correct but with framing problems — objects radiate exactly the same way no matter where they are. The atmosphere isn’t “in the way” so much as it is radiating back at the warmer objects (even though it’s colder than they are!), especially when there are big lumps of water vapor in it.
I propose a Skeptic mantra: It’s the water vapor, stupid.
Paul Bahlin
November 29, 2017 at 8:45 am
So night and day is actually the sun blinking on and off then? Who knew!
This hardly deserves a response.
I am sitting here in the UK at midnight and the Sun has been effectively “turned off” for 8 hours now and the temperature is around Zero C, we have another 8 hours of cooling before the Sun effectively turns back on again tomorrow.
The “Constant Average Solar Energy at TOA” is not real it is a construct, the Sun will be varying from Zero Energy as now to around 1200W/m2 tomorrow at around midday at the equator and quite a bit less at this Latitude.
So the Sky and CO2 above me is not supplying energy to my surface with a constant solar energy supply, it is currently zero on my surface, how about yours?
Talldave2 says: “The vacuum of space is not an object”
Well, you could always look at the BIG picture and say the atmosphere is hiding radiation from the ubiquitous glowing gas from when the universe was ~ 380,000 years old. 🙂
Yeah, here in the actual universe, those ancient, red-shifted photons are still getting here from our lightcone-horizon, due to inflation and expansion. But it too warms whatever it hit 🙂
Willis E says: Further Reading: My post entitled “The Steel Greenhouse” looks at how the poorly-named “greenhouse effect” work, based on the principles discussed above.
Math Notes: There’s an excellent online calculator for net energy flow between two radiating bodies here. It also has the general equation used by the calculator,
The attached link should demonstrate why your Steel Greenhouse is wrong and leads to why this post is wrong. You neglect all view factors except F1-1 and thus make bad assumptions about the what the temperature of the inner shell will be and its effect on the original surface.
https://www.scribd.com/document/15665685/Concentric-Spheres-with-Radiation
mkelly:
You cite a very different problem. In your linked problem, the inner sphere is maintained at 1000K, regardless of what is happening around it.The power to maintain the sphere at this temperature could vary depending on conditions.
Willis’ steel greenhouse problem has a fixed power input to the sphere, with the temperature varying depending on the conditions around it.
Thermodynamics professors love to throw variations like these at students to catch the unwary, in the hopes of making sure those students who eventually pass the course can spot these distinctions.
Ed Bo
November 28, 2017 at 1:01 pm: So, how do you explain the cessation of statistically-significant warming, Ed?
“Willis’ steel greenhouse problem has a fixed power input to the sphere, with the temperature varying depending on the conditions around it.”
The earth does not have a fixed power input to the sphere. Clouds ozone, dust, aerosols, and CO2 itself changes the input power.
I’m still looking for a steel greenhouse.
Heck , not even any glass up there. !!
It is FANTASY !!
Ed,
That is total horse manure. This is the same problem. You just don’t like the outcome
SGW:
You don’t understand the difference between a problem where the power input is held constant and one where the temperature is held constant.
Seriously???
Now I understand why so many universities use thermodynamics as a “washout” course to eliminate people without the technical chops from rigorous courses of study. There are those who just cannot get the basic concepts.
Brett:
There is a huge difference between the existence of the atmospheric greenhouse effect — you cannot bring the surface power balance within 200 W/m2 without it, and possible subtle changes to that effect.
No one thinks the earth’s surface is out of balance more than about 1 W/m2 averaged over all the surface and a year. The interesting arguments are whether it is closer to 1.0 W/m2 (alarmists) or 0.0 W/m2 (skeptics).
There are many factors other than increasing CO2 levels that create effects of that magnitude. There are no other factors other than downwelling longwave infrared (“back”) radiation that can close the 200+ W/m2 “power gap” at the surface.
Ed,
You must have washed out in your thermo course, since you cannot grasp the concept of the First and Second Laws of thermodynamics. The shell in Willis’s thought experiment cannot transfer heat to the sphere (Second Law), so it will not rise in temperature. If the sphere did rise in temperature, it would violate the First Law as well since it would be at a higher temperature and energy state (violation of the First Law by creating energy. The sphere being the ONLY source of energy)
The whole GHE scenario is just an example of super-bad physics. The sun is the ONLY source of energy available to heat the earth. The earth/atmosphere system is purely passive. Using Trenberth energy balance diagram data, the GHE takes 341 W/m2 of the solar radiation at the TOA, and magically multiplies it to 494 W/m2 at the earth’s surface. The GHE creates energy out of nothing..
SkepticGoneWild November 28, 2017 at 11:55 pm
When a man starts throwing mud as you are doing, it is a sure sign that he has no ammunition. Ed is correct, as any thermo text will explain in great detail.
ENERGY is not HEAT!!! Nobody said the shell was “transferring heat to the sphere”. It transfers ENERGY to the sphere, which leaves the sphere warmer than it would be without that energy.
Heat cannot flow from cold to hot … but radiant energy certainly can. If it could not, then our eyes could only see objects warmer than body temperature …
w.
“When a man starts throwing mud as you are doing, it is a sure sign that he has no ammunition“
Ah, so Ed must have been out of ammo here:
“Thermodynamics professors love to throw variations like these at students to catch the unwary, in the hopes of making sure those students who eventually pass the course can spot these distinctions.“
and especially here:
“Now I understand why so many universities use thermodynamics as a “washout” course to eliminate people without the technical chops from rigorous courses of study. There are those who just cannot get the basic concepts.“
Ed,
Well said!
Why is that so many unschooled people in these endless debates do not understand that the whole scenario under discussion hinges around a ‘steady state’ model involving, in particular, two important fixed values:
(1) constant flow of energy input from the Sun
(2) a consequent steady-state global mean surface temperature
In that model, the Sun’s input is fixed, and (consequently) so is the earth’s surface temperature. Such a system necessarily involves a constant and equal energy flow in from the Sun and out to space. So there is no warming! And no cooling!
Only if the atmospheric composition and/or surface albedo is changed does the surface temperature begin to transition to a new fixed value. During this process of transition, the system is not in steady-state and it is legitimate to describe the surface as ‘warming’ or ‘cooling’.
Perhaps Anthony should consider asking the moderators to reject the weasel words ‘warming’ and ‘cooling’ when a contributor is quite clearly addressing a steady-state condition!
SGW:
Clausius, “father of the 2nd Law”, anticipated confusions like yours, and specificially dealt with this issue. He even had a specific term for what you say cannot exist because it would be in violation of the 2nd Law. He called it the “ascending transmission of heat” (ascending in the temperature sense).
He said this transmission could occur as long as there was “compensation”, and that the ascending and descending transmissions “compensate each other”.
These days, also to try to prevent confusions like yours, we generally only refer to the net transmission as heat, as Willis does here, with the gross transmissions referred to as some form of energy transfer.
I’ve looked at dozens of college textbooks, both physics and engineering, that discuss radiative heat transfer. Every single one discusses it in terms of radiative exchange of energy. And by the way, they all discuss the two bodies simply having temperatures of T1 and T2, or Ta and Tb. None talks about “Thot” and “Tcold”.
The discussion, figures, and equations in these texts show T1 radiating power to T2 as a function of its own temperature and emissivity, and T2 radiating power to T1 as a functiona of its own temperature and emissivity. This is true whether T1 is greater than T2, they are equal, or T2 is greater than T1.
Scientists and engineers have been educated this way for over a century (nothing to do with climate), and miraculously somehow, their thermal designs have worked.
Tony:
I have taught advanced technical subjects at the university level, and I am completely serious when I say that if I had a student that could not understand a basic issue like the difference between an object with a constant power input and an object maintained at a constant temperature, I would be telling that student that he does not belong in a technical field.
SkepticGoneWild November 28, 2017 at 11:55 pm
Ed,
You must have washed out in your thermo course, since you cannot grasp the concept of the First and Second Laws of thermodynamics. The shell in Willis’s thought experiment cannot transfer heat to the sphere (Second Law), so it will not rise in temperature. If the sphere did rise in temperature, it would violate the First Law as well since it would be at a higher temperature and energy state (violation of the First Law by creating energy. The sphere being the ONLY source of energy)
It doesn’t create energy it recycles it.
The whole GHE scenario is just an example of super-bad physics. The sun is the ONLY source of energy available to heat the earth. The earth/atmosphere system is purely passive. Using Trenberth energy balance diagram data, the GHE takes 341 W/m2 of the solar radiation at the TOA, and magically multiplies it to 494 W/m2 at the earth’s surface. The GHE creates energy out of nothing..
I guess you didn’t take a heat transfer class?
Radiative heat transfer from an object: P=k(T^4-Tc^4) where T is the temperature of the object and Tc is the temperature of the surroundings.
In Steady State:
For an object with a transparent atmosphere illuminated by a star like our sun we get:
P=k(T1^4-3^4)
For an atmosphere like ours which illuminates the planet with ~300 W/m^2, T~270K we get:
P=k(T2^4-270^4)
So for the same solar irradiance T1^4-3^4=T2^4-270^4
Therefore T2^4=T1^4+270^4-3^4
So T2 is hotter than T1, if T1 was 300 then T2 would be about 340K
No violation of thermodynamic laws, energy in equals energy out.
LMAO. More hand waving from Ed.
Phil,
I guess you’ve never heard of the First Law of Thermodynamics. Energy cannot be created. Please calculate the energy output of the sphere before the shell was placed, and then after. Then think about the First Law.
skepticgonewild November 30, 2017 at 1:02 pm
Phil,
I guess you’ve never heard of the First Law of Thermodynamics. Energy cannot be created. Please calculate the energy output of the sphere before the shell was placed, and then after. Then think about the First Law.
No, explicitly same solar input and same output only the recycle back to the surface has changed.
mkelly November 28, 2017 at 11:19 am Edit
mkelly, you have missed the essential difference between your cited analysis and mine. In your analysis, the inner sphere is maintained at a certain constant temperature.
In mine, it is NOT maintained at a certain temperature. Instead, it has a certain constant energy input. This is a totally different situation, with (predictably) a totally different steady-state. As a result, your claim that
is simply not true. It is looking at a completely different thought experiment than mine.
Regards,
w.
Willis,
I am going to remove the insulation from my water heater and place multiple steel shells around it. How many shells do I need until the water in the water heater starts to boil?
Ed,
You’re so smart. How many steel shells do I need?
I have a 50 gallon cylindrical steel walled water heater maintained at 50 degrees C, How many steel shells until the water starts to boil? Come on daveburton. Help me out.
SkepticGoneWild,
False question…
You can’t reflect more than 100% of all emitted radiation from the water heater, thus at maximum you can maintain the water temperature, not heat it up. Alle what you can is reducing the heat loss, no matter if that is by insulation or reflection. The latter is used in space with multiple layers of metal sheets. You see, it works that way…
OMG Willis. I am not flinging anything but the laws of thermodynamics. Your sphere increased in temperature. That’s what happens when you transfer heat to it. You are just calling it “energy transfer”. But the result is the same. A passive object heated by the sphere, yet colder than the sphere, magically increases the energy and temperature of the sphere. And then you conveniently stop at one iteration. For the sphere at the new temperature will warm the shell more.
This type of pseudoscience begets all types of nonsense, such as the idea that the earth actually heats the sun a minute amount. Half the people at Spencer’s blog subscribe to that fiction.
Ferdinand. No. This is the same example as the Willis Steel Greenhouse. Not a false question. The temperature is not fixed. There is nothing to prevent it from heating up.
SkepticGoneWild November 29, 2017 at 12:16 am
Skeptic, you seem to have missed the part about the vacuum between the planet and the shell … if you put a steel shell around your water heater and remove the air from between the two, you’ve made a very effective thermos bottle. If you think that won’t affect your water heater, well, I fear you are beyond my poor power to add or detract.
However, the water in your tank is thermostatically controlled so that it never boils … as a result, all that putting your water heater in a thermos bottle will do is to reduce the amount of gas needed to heat it.
So the answer is, as long as it is thermostatically controlled it will NEVER boil … but then you knew that.
Or maybe you didn’t know that. But in any case, if the thermostat fails, you see that funny little valve up there at the top of the heater? That is a pressure relief valve. Why is it needed? Because if the thermostat fails, the water will boil with exactly zero steel shells around it …
Best regards, pay attention to the law of holes,
w.
“ENERGY is not HEAT!!! Nobody said the shell was “transferring heat to the sphere”. It transfers ENERGY to the sphere, which leaves the sphere warmer than it would be without that energy.”
I’m glad that you’re starting to recognise that energy is not heat. That being the case, you should now be able to see that in saying a transfer of energy from the shell leaving the sphere warmer than it would be without the energy, is exactly the same thing as saying that the shell has transferred heat to the sphere. Different semantics won’t change the meaning of what you’re saying.
Your next problem is that heat doesn’t transfer from the shell to the sphere, it only transfers in the reverse direction, up until equilibrium, when the transfer stops.
Let’s write that sentence better:
That being the case, you should now be able to see that saying a transfer of energy from the shell leaves the sphere warmer than it would be without the energy, is exactly the same thing as saying the shell has transferred heat to the sphere.
SkepticGoneWild November 29, 2017 at 1:26 am
Ferdinand. No. This is the same example as the Willis Steel Greenhouse. Not a false question. The temperature is not fixed. There is nothing to prevent it from heating up.
There certainly is, it’s called a thermostat! It is not the same as Williis’s Steel Greenhouse which had a constant energy input your example had a variable energy input and is maintained at constant temperature.
If it had constant energy input then improving the insulation would increase the temperature reached, usually what would be done then would be to reduce the energy input to maintain the desired temperature, i.e. improve the efficiency.
Willis,
Heat is defined as energy transferred due to temperature differences Your steel greenhouse is transferring energy from the shell to the sphere and raising its temp. That is heat transfer, but from a cold object to a warm one. This is where your BA in psychology is not helping.
SkepticGoneWild November 29, 2017 at 5:54 am
Willis,
Heat is defined as energy transferred due to temperature differences Your steel greenhouse is transferring energy from the shell to the sphere and raising its temp. That is heat transfer, but from a cold object to a warm one. This is where your BA in psychology is not helping.
On the contrary it’s really good engineering, see for example radiational heat transfer text by Hottel, so it would appear that the BA in psychology is no impediment, whatever your degree is in appears to be causing problems however.
SGW starts off OK with “Your steel greenhouse is transferring energy from the shell to the sphere and raising its temp. ”
But then errs with “That is heat transfer, but from a cold object to a warm one.”
No, the HEAT transfer is still from warm to cold — from the sphere to the surroundings. Its just a smaller heat transfer than before.
* If the sphere radiates to the extremely cold regions of outer space (3K), the energy out from the sphere to space(call it E(1,2) is large and the energy back from space to the sphere (E(2,1)is basically zero. The net exchange (which is called “heat”) is Q(1,2) = E(1,2) – E(2,1) is large.
* If the sphere radiates to the mildly cold shell, the energy out from the sphere to the shell (call it E(1,3) is still as large as E(1,2). However, the energy back from shell to the sphere, E(3,1) is now much larger than E(2,1). The net exchange is now Q(1,3) = E(1,3) – E(3,1) is much smaller than Q(1,2).
If the sphere had been in a steadystate condition when radiating to space, it will no longer be in steadystate if the shell is added. It will be losing less but still gains the same, meaning it must warm.
SGW:
You need to acquaint yourself with the “multi-layer insulation” commonly used to surround industrial furnaces. It has layers of reflective metal interspersed with non-conductive layers. This allows the radiated energy from the hot furnace to be reflected back, while minimizing the conductive transfer out. This permits the furnace to achieve much higher temperatures than could otherwise be realized.
According to your analysis, this is all nonsense. But people keep building furnaces this way, and it actually works!
Tim,
Replace the sphere with the actual sun. Take away the shell and substitute it with planet earth, What your fantasy physics tells us is that that reflected EM from the earth travels to the sun and heats it up a minute amount:
“If the sphere[SUN] had been in a steadystate condition when radiating to space, it will no longer be in steadystate if the shell [EARTH] is added. It will be losing less but still gains the same, meaning it must warm.”
You can find that silliness with a boatload of people over at Spencer’s site as well. I don’t think you will find such nonsense in your standard physics textbook.
The steel greenhouse is pure fantasy. A thermodynamic nightmare of a thought experiment. It is up to Willis to confirm that it might actually work with a real experiment, conducted by people who have actually studied physics, and published in a reputable science journal. That is how real science happens.
Ed,
By all means please provide a link that provides a thermodynamic analysis of these furnaces.
SGW:
Consult an engineering heat transfer textbook. I suspect you’ve never read one.
Ed,
I am still waiting for the analysis of your fantasy furnace.
When stumped, Ed punts.
SGW:
One minute (4:48pm to 4:49pm) and I’m stumped. Sheesh!
I am completely serious when I say this is the stuff of textbooks, and introductory ones at that. But if you can’t understand the difference between an object with a constant power input and one maintained at a constant temperature, it is completely obvious that you have never even cracked such a textbook.
This no more belongs in a scientific paper than does the argument that if an apple breaks free of a tree, it will fall to the earth.
The argument is very simple for anyone who understands the very basics of thermodynamics and heat transfer.
If you apply a constant thermal power to an object (such as a furnace), it will reach a steady-state temperature when it outputs as much power to ambient as it receives from the source (1st Law). Of course, the higher the object’s temperature the more power it will output to ambient.
Anything that you do to increase the thermal resistance between the object and ambient will lessen the power it transfers to ambient for a given object temperature. This means the object must increase in temperature to restore the power balance, outputting as much power to ambient as it receives from the source.
One of the modes of heat transfer is radiative (in a vacuum it is the only one). If you can inhibit radiative transfer from the object to ambient, either by reflecting some of it back, or by absorbing some of it and re-radiating from a temperature in between that of the object and ambient, increases the radiative thermal resistance, reducing the power transfer from object to ambient. This means that the object is receiving more power than it outputs, so it increases in temperature until the power balance is restored.
This is such a basic concept that I would expect students to understand it completely within a couple of weeks of starting an introductory course.
Don’t your arms get tired with all the hand waving?
SGW:
Qualitative analysis is all it takes to reject your silly argument that adding radiative insulation cannot result in a higher temperature of a separately powered object. If you had any experience whatsoever in dealing with these kinds of problems — which obviously you don’t — you would understand that you always do this type of analysis before getting to the equations, finite element modeling, etc.
One of the root-level errors I see over and over again on “Slayer” websites and comments is a rush to plug numbers into equations before doing this kind of qualitative analysis. As a result, they use the equations in completely wrong ways, leave out important effects, etc. And they have no clue where they went wrong.
In this case, I don’t even need to get to the equations to demonstrate the wrongness of your arguments.
If you want to start with some quantitative analysis, use Willis’ “steel greenhouse” example. I had many problems like this when I was studying thermo and heat transfer. I encounter many systems like this in thermal systems in my professional work. Willis’ analysis is correct, and until you understand his very simple system, you will never even begin to start to understand the issues at play.
Skeptic,
“Heat is defined as energy transferred due to temperature differences Your steel greenhouse is transferring energy from the shell to the sphere and raising its temp. That is heat transfer, but from a cold object to a warm one. This is where your BA in psychology is not helping.”
Your whole premise is wrong. No heat is being transferred from the shell to the sphere. The internal heat source of the sphere is supplying heat to it. That internal heat is what causes the sphere to rise in temperature when its outflow of heat is reduced by shell. No 2LOT violations.
Skeptic,
“Heat is defined as energy transferred due to temperature differences Your steel greenhouse is transferring energy from the shell to the sphere and raising its temp. That is heat transfer, but from a cold object to a warm one.”
Not at all. No heat is being transferred from the shell to the sphere. The internal heat source of the sphere is supplying heat to it. That internal heat is what causes the sphere to rise in temperature when its outflow of heat is reduced by shell. No 2LOT violations are involved.
Nate,
You are ignoring First Law violations as well. The sphere has doubled in energy output. Where did that extra energy come from since the sphere is the only source? The First Law says NO ENERGY CREATION. You have energy output greater than energy input. That does not happen in the real world. Only in a fantasy world of made-up physics.
And then you stop the iteration at one cycle. Why? The new higher temperature of the sphere has to warm the shell more.
Ed,
You are seriously confused.
See the following publication:
https://tinyurl.com/ybmbxwxr
Notice that it states:
“An alternative method is to use radiation shields between the heat exchange surfaces (Holman, 2009). These shields do not deliver or remove any heat from the overall system; they only place another resistance in the heat-flow path so that the overall heat transfer is retarded”
Skeptic,
“You are ignoring First Law violations as well. The sphere has doubled in energy output. Where did that extra energy come from since the sphere is the only source? The First Law says NO ENERGY CREATION.”
You have to very careful to keep track of all energy flows before claiming a 1LOT violation. First of all, there IS a source of energy-there is input to the sphere from the source. The source CAN store extra energy in the sphere if less has gone out for a time. (e.g. my salary was the same but my bills were lower this month, so I put $$ into savings)
“The sphere has doubled in energy output.” The shell has warmed up. Therefore the net flow of heat, sphere to shell, which is proportional to (Tsp^4-Tsh^4) does not double. In fact it reaches its original value in steady state.
Nate,
So the First Law is not important to you, Just your adherence to the GHE fiction. I get it. However, the First Law violation is staring you right in the face.
Furthermore the idea the that the sphere radiating at 235 W/m2 causes the shell to radiate at 470 W/m2 is absurd as well.
You never did answer the question regarding why the process was stopped at one iteration or heating cycle?
.
Let me edit the second paragraph above which had an extra “the”:
“Furthermore, the idea that the sphere radiating at 235 W/m2 causes the shell to radiate at 470 W/m2 is absurd as well.
SGW:
You simply continue to display the fact that you don’t understand the most basic concepts of thermodynamics and heat transfer.
Look at the 2nd sentence of the paper you cite: “In this study, a simplifying approach for calculating the radiant energy is achieved using the concept of net radiation heat transfer and provides an easy way for solving a variety of situations.”
Note the word “NET” in “net radiation heat transfer”. This is the whole point of Willis’ post — distinguishing between gross and net transfers — but you can’t under stand it. You can look at the transfers either way, AS LONG AS YOU ARE CONSISTENT (which you are not).
Let’s look at Willis’ steel greenhouse example in terms of the paper you cite approvingly.
The sphere, which has an input power of (235 * Area) watts, has a “net radiation heat transfer” of (235 * Area) watts to the shell. So it is in energy balance by the First Law, therefore in steady state conditions.
The shell, which receives a “net radiation heat transfer” of (235 * Area) watts from the sphere, has a “net radiation heat transfer” of (235 * Area) watts to space. So it also is in energy balance by the First Law, therefore in steady state conditions.
Note particularly that the shell is a “shield [that does] not deliver or remove any heat from the overall system; [it] only place[s] another resistance in the heat-flow path so that the overall heat transfer is retarded.”
SGW:
You say: “Furthermore, the idea that the sphere radiating at 235 W/m2 causes the shell to radiate at 470 W/m2 is absurd as well.”
False premise! In the steady state condition, the sphere is radiating at 470 W/m2 (gross). THIS causes the shell to radiate at 470 W/m2 (235 from the inside surface, 235 from the outside surface).
The 1st Law is satisfied for the sphere, as it is receiving 235 from its power source, it is outputting 470 (gross) towards the shell, and receiving 235 (gross) from the shell. If you prefer, you could say the sphere is receiving 235 from its power source, and outputting 235 (net) towards the shell.
The 1st Law is satisfied for the shell, as it is receiving 470 (gross) from the sphere, outputting 235 (gross) from its inner surface, and outputting 235 (gross) from its outer surface. Or if you prefer, it is receiving 235 (net) from the sphere, and output 235 (net) towards space.
Ed,
Your poor, poor students. How is the First Law satisfied when the sphere’s 235 W/m2 becomes 470 out of thin air? That is energy creation. The shell has NO energy of its own. OMG! Your explanation is pure theatrics, handwaving, just like your magical furnace.
I am DONE with your nonsensical fantasy physics. It’s time Willis (and you) put up or shut up. Willis came up with this monstrosity of a thought experiment, now it’s time to perform some REAL science and follow it up with a real experiment per the scientific method. Of course we know that will never happen.
Don’t respond with more theatrical handwaving.
SGW:
This very simple but definitive experimental demonstration of the effect you consider impossible was put up on WUWT years ago:
https://wattsupwiththat.com/2013/05/28/slaying-the-slayers-with-watts-part-2/
SkepticGoneWild on November 29, 2017 at 12:55 am asked, “I have a 50 gallon cylindrical steel walled water heater maintained at 50 degrees C, How many steel shells until the water starts to boil? Come on daveburton. Help me out.”
It will never boil. Water heaters have thermostats to prevent them from boiling, regardless of how much insulation you wrap around them. (Steel shells are not the best insulators, BTW.) But if you insulate them better the energy they consume to maintain water temperature goes down.
However, I posed and answered a similar question here.
Ed,
More hand waving I see.
“Water heaters have thermostats to prevent them from boiling,”
OMG Dave. A thermostat in a water heater prevents the water from cooling when set at a certain temperature. Not the opposite.
“A thermostat in a water heater prevents the water from cooling when set at a certain temperature. Not the opposite.”
Breathtakingly ignorant, SGW. It does both.
Daveburton writes,
“It will never boil. Water heaters have thermostats to prevent them from boiling, regardless of how much insulation you wrap around them. (Steel shells are not the best insulators, BTW.) But if you insulate them better the energy they consume to maintain water temperature goes down.”
Thermostats doesn’t prevent boiling since it is never set to boil the water in the first place,settings are commonly around 110-115 F.
The concern is the Pressure build up in the tank.
You need to think a little more before you start banging on those ivories.
SkepticGoneWild December 2, 2017 at 6:06 pm
Remember my rule of thumb, that whoever uses OMG in a scientific conversation is wrong? Here is another example.
Skeptic, a thermostat in a water heater does two things. When the water gets too cool it turns on the gas. And when the water gets to the desired temperature, it turns off the gas.
Now, THINK ABOUT WHAT KEEPS IT FROM BOILING. Or as Paul Bahlin said in a more genteel manner:
You’re not doing your reputation any good with these kinds of OMG!!! incorrect but passionate assertions.
w.
SGW:
So direct experimental evidence is “handwaving” to you.
We’ve already seen that standard thermodynamic analysis that any scientist or engineer learns as an undergraduate is “handwaving” to you.
It’s now obvious that anything beyond your incredibly limited capabilities you write off as “handwaving” because you cannot understand it. Truly pathetic.
OMG Willis. I was correcting Dave’s false statement:
“Water heaters have thermostats to prevent them from boiling”
If you cannot understand the error of this statement, then you should not be posting on a science blog. Sunsettommy also corrected Dave’s error as well.
My reputation? Worry about your own.
Here’s my suggestion. Go take a general physics course at your local university. Not the dumbed-down version for climatologists. The rigorous courses for physics and engineering majors usually have a prerequisite of a year of calculus, and a maybe a semester of differential equations, so you have your work cut out for you. Then maybe people will take you seriously.
Like I state earlier, it is your responsibility to attempt to confirm this bizarre thought experiment with a real experiment. But it’s been like 8 years since the original post, so we know that’s not going to happen.
I went back and read some of the original steel greenhouse post. Some of the comments were brutal:
“I’m sorry but I thought the article was tremendous nonsense, and will cause immense damage to the reputation of this website”
“This article should be removed. The author obviously knows nothing about radiative heat transfer”
“This article is utter nonsense, and a simple logical analysis reveals this without even resorting to physics (and I am a physicist).As requested by others above… please remove this article”
“Thank you Anton. It wasn’t just me who spotted that the logic is simply busted. Willis, next time contact a physicist before doing something like this.”
“I agree withn John A. (20:11:22) this load of [snip] has no place on Wuwt. Wuwt’s reputation has now been tarnished. This is not science but fantasy. The thing is so sorely messed up that it doesn’t even qualify for print Has Wuwt fallen prey to a belief in pseudoscience? I am truly disappointed!”
“Really sad to see this kind of rubbish published on this great site. The physics is completely wrong. Anthony, please do not let this kind stuff published on this site, it will take the value of the whole site down.”
I could go on, but you get the point.
“OMG Willis. I was correcting Dave’s false statement: “Water heaters have thermostats to prevent them from boiling””
Dave’s statement was not false, SGW; it just pointed out one of the modes of a thermostat. It did not assert that there was no other mode.
In thinking about your various comments and rebuttals, I’ve come to the conclusion you like to dominate others through pettifogging and personal attacks. It’s just an opinion but, like a$$holes, everyone has one.
Wait a minute Ed. I’m going to test my 1800 W hairdryer, and direct it into my concave bathroom mirror. See if I can get 3600 watts out of it. If I’m not back in a few minutes, call 911.
Without changing the subject, and without any need for models or esoteric physics nobody is talking about, answer this simple question…….
Here’s the shot:
Your claim is that IR from a 90 k source incident on a 91 k target, does nothing to the target. If true it has to then either pass through it (albedo of 0) or be reflected from it (albedo of 1). You further claim if that very same source hits the very same target but now the target has a temperature of 89 k it is absorbed, at least partially.
Here’s the chaser:
If you would grant me the liberty of putting that in an equation, it would be albedo= f(T). Do you agree that my equation correctly supports your claim?
SkepticGoneWild December 3, 2017 at 1:00 am
OK, I’ll bite … why doesn’t the water in a water heater boil? I mean, it’s got a gas flame under it … so what keeps it from boiling? Good looks? Mystical incantations? What keeps it from boiling?
Your move, SGW …
w.
Ed,
OK. I am back. The experiment went well. I only have second degree burns, and I tripped the circuit breaker. Upon further inspection, the power is off in our neighborhood as well, and I hear sirens outside my door. So yes. I was wrong. Power output CAN exceed input.
OMG Willis!!!!
Seriously, though. The question was not about why water heaters don’t boil. The issue is with how a thermostat operates. Say for example, your steel sphere was a water heater. A thermostat does not prevent the water from heating up. A thermostat only fixes the lower temperature limit. Any external energy source (like a shell) can cause the temperature to rise (per your theory). The thermostat will not cool it down. Trivial point, really.
So why not go back to school? Take a physics class. You love the stuff, so what’s stopping you?
I love this sight because every time I come here I learn something I was formerly ignorant of.
All my life I have had the good fortune to recognize the old saying; Ignorance can be fixed but stupid is forever. I also hate to speak I’ll of anyone.
In your case I must break the latter and invoke the former of these two credos.
You are stupid.
SGW, doubling down [“A thermostat does not prevent the water from heating up. A thermostat only fixes the lower temperature limit.”] is not helping with your image.
Again, a thermostat works both ways. I trust my tender body every day to the operation of my water heater thermostat, both ways.
Although, you may prefer one that allows your water heater to get as hot as its energy supply allows. Who knows or cares.
I couldn’t have asked for a better placement for that comment from Paul. Thanks, Paul.
Welcome.sorry to break your thread
Oh my goodness. Well then, SkepticGoneWild & Sunsettommy, I confess to imprecise language. I wrote:
“Water heaters have thermostats to prevent them from boiling…”
I should have said:
“Water heaters have thermostats which prevent them from boiling…”
Or:
“Water heaters have thermostats to prevent them from getting too hot or cold…”
Better?
And Sunsettommy, water heaters are typically set to 120-150 °F, not 110-115 °F. 110-115 °F is tepid. Baby bottles are commonly heated nearly that warm. When your coffee gets that cool you probably put it in the microwave oven for 20 seconds or so, to reheat it.
To prevent the growth of dangerous bacteria in your water heater, the thermostat should be set to at least 140 °F. But you need to be careful because 140°F water can scald a person in about 5-6 seconds:
http://www.accuratebuilding.com/images/services/charts/hot_water_burn_scalding_lrg.gif
SGW:
You challenged me to cite experimental evidence for my views. I did exactly that, linking to a set of experiments posted on WUWT several years back that clearly show with quantitative evidence that my views are correct.
https://wattsupwiththat.com/2013/05/28/slaying-the-slayers-with-watts-part-2/
You simply responded by saying: “Ed, More hand waving I see.”
And you have completely refused to engage with any of the results of those experiments, desperately trying to change the subject. I wonder why?
I guess I shouldn’t expect much from a guy who can’t understand the difference between a problem with an object held at constant temperature one with an object provided with a constant power input.
Thanks, Ed. You’ve made up for Paul’s comment moving upthread. Now we’ve got perfect placement again!
Skeptic,
Put my answer in wrong thread. https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2683050
Skeptic,
Sh*t, I mean here: https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2684013
Earlier in the discussion, I suggested a shell be placed around a water heater in similar fashion to the steel greenhouse thought experiment. After all, even in the absence of a vacuum, the wall of the water heater emits IR which will warm the shell, which in turn will emit IR back to the water heater just like the steel greenhouse. But Willis said:
“However, the water in your tank is thermostatically controlled so that it never boils”
The premise was that the shell could heat the water in the water heater up. Willis and the rest of you apparently don’t understand how a thermostat operates. Just because a thermostat turns off the flame when the water heater reaches a certain temperature does not mean the water does not have the ability to heat up more (from the shell in my example). If you set the thermostat at 140 F, it will not go any lower, but there is nothing to prevent it from going higher,
What the hell? Do you think the thermostat provides cooling? NO.
So let’s give the McFly award to the following people:
Dave Burton
Dave Fair (you get the dumb***, dumb****, dumb****,and **** for brains award as well)
Phil
Paul
Willis is exempt since he may not have understood the premise.
SkepticGoneWild December 3, 2017 at 8:46 pm Edit
Skeptic, do you have to work at being that unpleasant, or does it come naturally to you?
In any case, to the example. I put a shell around a water heater. As the water warms, so does the shell. It radiates some of the heat back to the water heater. The water keeps heating.
At some point the water reaches the thermostat’s set point, call it 140°F, and the gas turns off.
What has happened so far is exactly the same as if the shell were replaced by insulation. We’re slowing heat loss from the tank. We’ve taken the water to the same temperature, but it has required less gas to do so.
But contrary to your claim, when the thermostat shuts off, the entire system, water, water heater, and shell begins to cool.
Of course, the system will cool more slowly because of the shell. Again, it is slowing heat loss from the system by intercepting the radiation and returning half of it to the heater.
So while you are correct that “If you set the thermostat at 140 F, it will not go any lower, but there is nothing to prevent it from going higher”, there is also nothing making it go any higher. The shell won’t, it just slows the rate of heat loss. The gas is off. So it is unclear just what you are fantasizing will make the water become hotter once the gas is turned off.
Note that this is different from the steel greenhouse. There, unlike in the water heater, there is no thermostat so the energy supplied by the internal radioactive decay continues unabated … which is why it can get hotter than it would with no shell. But of course, that doesn’t happen when the gas shuts off in the water heater. It just cools from that point onward, there is no energy being added to the system AS THERE IS IN THE STEEL GREENHOUSE.
Do you realize how ugly, immature, and unpleasant it makes you look when you pontificate, call a bunch of people stupid and shit for brains … and then it turns out you are wrong, wrong, wrong?
I guess you must not, or you wouldn’t do it. My advice is to emulate the rooster, and wait until it is actually dawn before you start crowing …
w.
Willis,
So you are allowed to break the laws of thermodynamics, but I am not. I need a rule book to keep things straight.
Your steel greenhouse sphere magically doubles in energy output when there is no other source of energy in your system. That is a violation of the First Law. You cannot create energy out of nothing.
You are the one transferring heat from the cold shell to the warmer sphere, so don’t give me some highbrow lecture, when you routinely break the Second Law as well..
And furthermore, it was Mr. Dave Fair who called me an asshole, but you never got on his case.
Willis,
And I don’t need a science lecture from someone with a BA in Psychology and a [pruned] certificate.
You will just be moaning on and on in another 8 years about the steel greenhouse, since your thought experiment will continue to be just an unconfirmed thought experiment.
SkepticGoneWild wrote, “…wall of the water heater emits IR which will warm the shell, which in turn will emit IR back to the water heater… the shell could heat the water in the water heater up…. Just because a thermostat turns off the flame when the water heater reaches a certain temperature does not mean the water does not have the ability to heat up more (from the shell in my example). If you set the thermostat at 140 F, it will not go any lower, but there is nothing to prevent it from going higher…” and then, ironically, “you are allowed to break the laws of thermodynamics, but I am not.”
SGW, is your name Paul W. McDonald, or perhaps Justin Reese Chrivia?
SkepticGoneWild wrote, “OMG Dave. A thermostat in a water heater prevents the water from cooling when set at a certain temperature. Not the opposite.”
And Willis replied, “You’re not doing your reputation any good with these kinds of OMG!!! incorrect but passionate assertions.”
He’s not doing his reputation any harm, either, Willis, because he hides behind an anonymous alias. My guess is that, were that not the case, he might be less careless with his words.
(In contrast, if I counted correctly, five of the seven people he’s arguing with, who he thinks are all dunces, use their own names. )
SGW: the rules are, at the point you clearly show they’re in error, they:
1) Change the subject, or
2) Put their fingers in their ears and stop responding, then further down-thread, carry on making the exact same mistakes as if nothing was ever said, or
3) Respond with a comment which completely (and sometimes quite creatively) misses your point, whilst being as condescending as possible, or
4) Respond with a comment which focuses on chastising you (usually for insults or something like that) whilst ignoring the identical (or worse) behaviour from themselves and those in agreement with them, or
5) Simply lie about what has been said so far in the discussion, even though the comments are there to be read by anyone.
Or, sometimes it’s a mixture. Being pathologically incapable of conceding even the slightest point when it comes to this subject, what else can you expect from them?
Daveburton, your response at December 4,12:36 am, was a mind-blowing example of number 3), creatively missing the point. You quote SGW explaining how (relating it to the boiler example) the steel greenhouse violates the laws of thermodynamics, and in your response indicate that you agree that this is a violation of the laws of thermodynamics. So, you agree with SGW, whilst acting like you disagree. Now this has been pointed out to you, I reckon there could be a number 2) on the horizon (never a pleasant thought).
There is only one energy source (and thus only one heat source), the sphere. There would be no reason for this to double in energy output on addition of the shell unless the shell were an additional source of heat (which it isn’t, it’s a passive object). Given that the steel greenhouse example DOES regard the shell as an additional source of heat, when relating it to the boiler example, this additional heat source will be able to continue to heat the warm water further, even when the gas is switched off by the thermostat. In reality, it wouldn’t, since the passive shell is not a heat source. It provides energy, but not heat.
Yup, the steel greenhouse is THAT easy to debunk. The only reason the debunking hasn’t been accepted yet is due to endless confusion over the definitions of heat vs energy, and due to the consistent application of the aforementioned rules 1-5 by teams of dedicated professional obfuscators.
Willis, on the other hand, argues that when the gas is off, the shell around the boiler no longer functions as an additional source of heat. It miraculously changes to being just a passive object. If it were a treated as a passive object whilst the sphere was providing heat (as it does continuously – no thermostat – in the steel greenhouse thought diversion) then the sphere would never undergo that first miraculous rise in temperature in the first place. It would (and should) carry on at the same temperature it was before the shell was added, and the shell will just warm until equilibrium (which will be at a lower temperature than the sphere, due to the larger surface area of the shell).
Skeptic,
“Furthermore, the idea that the sphere radiating at 235 W/m2 causes the shell to radiate at 470 W/m2 is absurd as well.”
Nobody is saying that. Where do you get the idea shell is radiating 470?
Again, if you desire sphere and shell to be at the same temp (why I dont know), it aint gonna work. That would a violation of 0th and 1st laws of thermo. You ok with that?
In order for temperature of sphere to not explode, it MUST be outputting all of the input heat (235) to the shell (1ST LAW my friend!).
In order for heat to pass from sphere to shell, there must be a temperature difference between sphere and shell. (0TH LAW my friend!). The amount of flow is sigma(Tsph^4-Tsh^4). No way around this.
“Nobody is saying that. Where do you get the idea shell is radiating 470?“
Ed Bo says that, during this discussion. Though if you asked him he’d probably say that he didn’t say that.
Nate:
The sphere must radiate 235 W/m2 NET outward to balance the internally generated 235 W/m2 for it to be in steady state conditions (1st Law conservation of energy).
Since the sphere is receiving 235 W/m2 GROSS from the inner surface of the shell (which must radiate 235 W/m2 outward to space — this is both gross and net, because there is nothing coming back — to keep the whole sphere+shell system in 1st Law balance), the sphere must radiate a total of 470 W/m2 GROSS to output (470 – 235) = 235 NET.
Remember that this whole post is about keeping straight the difference between GROSS and NET transfers. Willis understands it completely, but there are several others here who just cannot get it…
Tony:
Were you talking about the moon as your alternate imaginary earth or not? You have been very emphatic both ways about it — have you made up your mind yet?
You’ll get over it, Ed.
Ed Bo,
Agree. Folks keep telling me that you said the SHELL is radiating 470. I did not find you saying this anywhere. As usual people see what they want to see.
Ok 470 radiated from shell in both directions. Yes. That was what Ed said. My bad thinking outward only.
Still, as he makes clear, that is not NET flow. Net flow outward from shell = net flow out from sphere = net flow from source.
So there is no ‘energy creation’ or problem with 1LOT or 2LOT, as claimed by Tony, SGW.
It’ll be OK, Nate.
Ed, your comments were no more nor less offensive to SGW than what you received in return, yet Ol’ W only chastises those on one side of this argument. My criticism was of Ol’ W, not yourself. I couldn’t care less about insults, either received or given.
Tony November 29, 2017 at 10:40 am
A couple of comments.
First, when someone starts playing grade-school games with my name, I know that they know that they are losing the debate.
Second, I snipped what you said because it made you sound both vicious and vindictive. Maybe I’m an incurable optimist, but I don’t think you are either one. I figured you were just having a bad hair day.
However, if those nasty words actually reflect your inner self, I’m happy to let you rave on … you might note, though, that such personal attacks don’t do your reputation any good.
Sadly,
w.
Your double standards are obvious to anyone rational.
Tony, “Ed Bo says that”. Nope, not that I can see.
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2683256
Phil,
The First Law states energy cannot be created or destroyed. The Willis steel greenhouse is creating energy. The sphere is the ONLY energy source. Calculate the energy leaving the sphere before the shell arrives. Now calculate the energy leaving the sphere after the shell is put in place. The energy output of the sphere has doubled! OMG the stupidity. You need to crack open a physics or thermo text and review the FIrst Law again.
Skeptic, measure the energy leaving the sphere and going to outer space before the shell arrives. For convenience, let’s say it is 240 W/m2, the amount of energy making it past the clouds in the earth system.
Now measure the energy going to outer space after the shell is put in place and steady-state is re-established … again, it is 240 watts. It has to be.
In both cases, 240 watts is entering the system, and 240 watts is leaving the system … OMG, as you say, no energy has been created or destroyed. No First Law violation.
Let me try another example.
Suppose you have a hot light bulb at steady state. Energy in = energy out. Now put an asbestos blanket around the light bulb. Will the light bulb get hotter? Sure. It will heat up until the same amount of energy is leaving the total system as before the blanket was put on. At that point energy in once again equals energy out. … but the light bulb getting hotter doesn’t mean that the First Law is violated as you incorrectly assert. It just means that the energy loss has slowed, so the bulb heats up until the steady-state is re-established.
w.
PS—another of my rules of thumb is that when someone says OMG in a scientific discussion, they’re most likely wrong … just saying …
Skeptic,
“So the First Law is not important to you, Just your adherence to the GHE fiction. I get it. However, the First Law violation is staring you right in the face.”
1LOT is important to me. Just dont see the violation that you see. You have not made it very clear where the violation is.
If you want to obey 0th and 1st law of thermodynamics, the sphere and shell cannot be at the same temp. The sphere MUST be warmer than shell in order for it to be able transfer heat to the shell (by ordinary radiative laws). It must output heat to shell at same rate as input (1LOT)
In turn, the shell must radiate at same rate as input to it (1LOT).
I have no idea what the ‘iteration’ discussion is about.
Borrow an IR camera and look at a partially cloudy night sky. You will see that the clouds are warmer than the sky. You know this because IR from the clouds is being detected by the camera sensor. Detection involves absorbing the IR, so the detector must be infinitesimally warmer due to the IR even though the cloud is -70 degrees and the detector is at room temperature..
So Stephen, you do grant that the atmosphere is a “partially radiative gas”? Yes?
If yes than do you grant that the atmosphere acts as a partially effective interceptor and reradiator of LW?
Yes but:
Since the entire atmosphere and the oceans have a similar effect via conduction and convection any effect from ghgs counts for nothing in comparison.
Ghgs simply change the lapse rate slope as I explained before so that they radiate to space more effectively from a lower warmer height instead of warming the surface.
Conduction and convection move lots of energy around, inside the earth-atmosphere system, yes. But they only effect the system’s radiation budget if they manage to move radiating gas to more favorable position for escape. Anything else is just internal churn (weather).
So without the handwaving internal porridge, show the math that makes radiation 1st law balance inside and outside the system: atmosphere, surface, and system balance.
I’m particularly interested in where to find the insignificant bits.
Radiation in from space 255k
Radiation out to space 255k
KE to PE in atmospheric uplift 33k
PE to KE in atmospheric descent 33k
Surface temperature 288k
As per observations
Odd that you would choose 255k as a radiation figure, but I assume you mean 240 w/m^2 where you use it. Same for the 33k?
If you’ll permit me to restate ….
You have 240 w/m^2 in and out. Good. The surface though, I don’t follow. You have a ke-pe exchange, equal and opposite in energy that is raising the temperature of the surface without increasing outbound radiation. How does that work?
Simply because the same unit of kinetic energy at the surface cannot both conduct away and radiate away simultaneously. That is why S-B should not be applied to a surface beneath a convecting gaseous atmosphere.
Of the 288k at the surface only 255k gets past the conductive barrier presented by the convecting mass of the atmosphere.
As regards the units I was assuming a reader would realise that the radiation intensities were ‘worth’ a 255k contribution to the 288k surface temperature.
So instead of quibbling over numbers and units let’s say we just stick to letters and energy flow (w/m^2). Also I’m just an engineer so I like to visualize things.
If I understand what you are saying we have a surface and a box. The box contains your pe-ke thingy. The surface has A incident SW and leaving the surface we have B AND C longwave. B LEAVES the system and C goes into the box. Lastly we have A=B.
Do I have that right?
OOPS. C is not longwave it is conduction. Correct?
“If I understand what you are saying we have a surface and a box. The box contains your pe-ke thingy. The surface has A incident SW and leaving the surface we have B AND C longwave. B LEAVES the system and C goes into the box. Lastly we have A=B.
Do I have that right?”
Not quite.
The surface has A (incident SW) plus C (conduction) from the box and leaving the surface we have B (IR to space) plus C (conduction) into the box.
The two Cs net out to zero leaving incident SW (A) having the same thermal energy value as the outgoing IR to space (B).
You then have radiation in from space equal to radiation out to space and conduction up the same as conduction down.
In effect the radiation from space gets a free pass through the system once the two Cs reach the same figure which occurs when the system stabilises at hydrostatic equilibrium for the atmosphere as a whole.
You can also envisage it as two distinct energy loops.
Radiation in and out (A and B) comprises a diabatic energy loop which is solely radiative whereas Conduction up and down comprise an adiabatic energy loop which is solely non radiative.
AGW theory and all proponents of it omit the downward C from the non radiative adiabatic heating provided by descending columns which puts the energy budget out of balance so to make it balance they have to invent a proposed surface heating effect from DWIR which is physically untenable because convection adjusts to neutralise it.
I think I’ve got it now. A is SW into the surface. B is LW out of the surface (free pass to space) and A=B. Simultaneously we have atmosphere and box exchanging equal amount of C at the surface and the whole thing is in steady state.
From this I would deduce that the atmosphere and surface are exactly equal in temperature and this exchange of C contributes no net energy to the surface therefore the atmosphere is not increasing energy of surface and the surface temp would be given by Stefan-Boltzmann, the same as for an atmosphere free planet.
Correct?
No,the atmosphere does warm the surface above S-B because you have to add the kinetic energy that is being cycled up and down to the continuing incoming insolation hence 288k instead of 255k.
The thing is that kinetic energy at the surface must EITHER radiate OR conduct.The same parcel of KE cannot do both simultaneously.
So, if you look at the system as a whole from space it is at 255k as per S-B because that represents the amount of radiative energy passing straight through.
However, the surface beneath the atmosphere must be at 288k in order to sustain hydrostatic equilibrium as well as match radiation out with radiation in.
AGW theory says that a surface at 288k MUST radiate AT 288K but it cannot do so if 33k is being conducted and convected instead.
That is how confusion arises if you try to fit a radiation only equation to a mixed radiative and non radiative scenario.
In your previous reply, you had the energy exchange by conduction between surface and box net 0, correct? There is no difference to energy flow be it radiation, conduction, or convection.
So here is the energy flow into the surface…..
A+C
Out is….
B+C
Net is….
(A+C)-(B+C)=0
There is no energy available to raise surface temp above airless planet. Not for steady state flow.
I can see your conceptual difficulty but how best to address it ?
In short, the energy tied up in conduction and convection must warm the surface above S-B and maintain that warmth otherwise there would be insufficient KE remaining at the surface after radiation out to space to keep convective turnover going.
If you have 255k radiating in and 255k radiating out and the surface is at 255k how to you fuel the constant upward pressure gradient force that opposes the downward gravitational force in order to keep atmospheric mass off the surface?
Do you say that the atmosphere can be held off the surface at zero energy cost?
If so, how?
My conceptual difficulty is the 1st law of thermodynamics. You are creating energy!
Well before you get to the handwaving inside the box, you need to resolve the 1st. Every bit of pe created in the box is returned as ke, C in = C out.
Everything inside that box is transport. The only time that’s not true is the very first time you feed it some joules.
The very first time it was fed some joules was during the very first convective overturning cycle when all the energy in the atmosphere was drawn by conduction from energy that would otherwise have radiated to space but which was in fact conducted from surface to atmospheric gas molecules to be convected upwards and which created all that PE that now acts as a permanent reservoir for continuing convection and which in the process heats the surface by 33k above S-B.
So, you see, you agree with me once you realise the significance of that very first cycle of convection.
I’m not creating energy at all. I am pointing out that the system is storing energy internally and thereby creating the so called surface temperature enhancement.
Actually, I don’t agree with you at all! You are creating energy. You agreed previously to what the energy flows were at steady state. You continue to add temperatures which is totally bogus.
With the model you previously agreed to, the surface temp could never rise above 255k. It can dip while energy is used to bring the box to steady state. But this model has to approach 255k at the surface over any reasonable integration interval.
At steady state the box is all transport. Sure it can move gobs of energy away from the surface in one place but it will deliver it all back someplace else.
Read again, carefully:
“The 33 in rising columns has no effect on surface temperature because it is taken from surface KE that would otherwise have gone to space via radiation so that the surface radiates to space at 255k and not 288k.
The 33 in falling columns does affect surface temperatures because it is arriving in addition to continuing insolation at 255k
You get a surface temperature of 288k despite the S-B prediction of 255k and it is observed that only 255k is going out to space.
That squares off the whole debate perfectly.”
You need to stop with the 33 up add 33 down. You need to stop with the 255. They are nowhere in the model under discussion.
They are obfuscations to divert attention from the facts of 1st law energy accounting that you can not balance.
Stick to the A,B,C that we stipulated upfront and show me the increase in surface energy content that makes the surface temp rise.
You Can’t heat a room by lifting weights.
You can heat a room by delaying heat loss and that is what convection achieves since it works more slowly than radiation.
Even when the up and down flows reach zero balance between themselves that is only hydrostatic equilibrium and not a removal of the energy stored as PE which is constantly being returned to the surface as KE in addition to continuing insolation.
To try and keep it really simple:
The 33k in rising air is taken from the outgoing radiation rather than directly from the surface itself.
The 33k in falling air is added directly to the surface in addition to continuing insolation.
Due to the out of phase timing that developed during the first convective overturning cycle one is borrowing energy from outgoing IR and lending it to incoming shortwave for a period of time and that forces up the equilibrium temperature of the surface to 33k above S-B.
No energy being created. Just a delay in the throughput of 33k due to conduction and convection being one convective overturning cycle slower than radiation.
That raises surface temperature because one is delaying the radiative loss of 33k by the length of time it takes the first convective cycle to complete.
That delay is then repeated ad infinitum.
Have you got it yet?
In an accounting of STEADY STATE energy balance there is no phase delay allowed. That’s why it is a steady state accounting.
You are handwaving. Your atmosphere box creates energy if, as you claim, it has raised surface temp. You have failed to show an energy imbalance that would increase surface energy content
Then you cannot or will not see the obvious.
You can not increase the temperature of something unless you increase its energy content. That means you have to put more into it than what leaves it.
You can have a gazillion joules flowing in and out of the surface from lapse rate hand waving but as long as those flows are equal, the effect on the surface is nada.
Should have added that the effect is nada over a reasonable integration interval.
And I didn’t say that the value of C is zero. I said it was net zero for the up and down processes taken together. C is still a sizeable number comprised of all the PE and KE in all the molecules that are not in contact with the surface.
It is that interplay between the proportions of KE and PE at different heights and latitudes that gives us climate zones and all forms of weather.
Do you say that a convecting atmosphere has no energy content?
Take any figure for the value of C as representing all the KE and PE in the atmosphere, say 66 for simplicity
Thus A and B cancel out but you have 33 of C in rising columns and 33 of C in descending columns.
The 33 in rising columns has no effect on surface temperature because it is taken from surface KE that would otherwise have gone to space via radiation so that the surface radiates to space at 255k and not 288k.
The 33 in falling columns does affect surface temperatures because it is arriving in addition to continuing insolation at 255k
You get a surface temperature of 288k despite the S-B prediction of 255k and it is observed that only 255k is going out to space.
That squares off the whole debate perfectly.
Note that when the atmosphere first lifted off the surface outgoing radiation dropped by 33k but the surface stayed at 255k as per S-B. To drop the surface below S-B conduction and convection would have to work faster than the speed of radiation and we know that is not possible.
When upward convection reached its maximum height and the subsequent descent then reached the ground then the adiabatic loop closed and since then the system has been in steady state with the surface temperature enhancement caused by conduction and convection storing all that energy in the atmosphere and recycling it up and down indefinitely as long as external insolation continues.
Stephen — you ARE violating conservation of energy.
“when the atmosphere first lifted off the surface outgoing radiation dropped by 33k but the surface stayed at 255k as per S-B.”
No! If 240 W/m^2 are arriving as sunlight and 240 W/m^2 are leaving as thermal IR @ 255K, then there is simply no extra power available to warm the air.
If, for example, 20 W/m^2 are being siphoned off to warm the air and initiate convection, then there are only 220 W/m^2 left to radiate and the surface will only be 250K. As long as 20 W/m^2 are being stolen, the surface stays at 250K. If that circulating air eventually returns and delivers the 20 W/m^2 back, then the net lost from convection becomes zero, and the net radiation could rise to 240 W/m^2.
Tim said:
““when the atmosphere first lifted off the surface outgoing radiation dropped by 33k but the surface stayed at 255k as per S-B.”
No! If 240 W/m^2 are arriving as sunlight and 240 W/m^2 are leaving as thermal IR @ 255K, then there is simply no extra power available to warm the air.
If, for example, 20 W/m^2 are being siphoned off to warm the air and initiate convection, then there are only 220 W/m^2 left to radiate and the surface will only be 250K. As long as 20 W/m^2 are being stolen, the surface stays at 250K. If that circulating air eventually returns and delivers the 20 W/m^2 back, then the net lost from convection becomes zero, and the net radiation could rise to 240 W/m^2.”
Tim, your error is assuming that the ‘stolen’ 20 W/m2 reduces surface temperature below S-B. It doesn’t. The surface stays at 255k as per S-B and the ‘theft’ is instead from the departing radiation some of which is diverted by conduction into convection. From space one sees a drop in the entire system temperature to 250k during convective cycle one because less radiation is escaping past atmospheric mass but the ground remains at 255k.
Then, when the first convective overturning cycle completes the surface temperature rises to 260k in your example (would have been better if you had stuck with the real world number of 288k that I used) and viewed from space the system returns to 255k as per S-B.
You see, the energy held in the atmosphere after completion of the first cycle doesn’t disappear just because energy up equals energy down. That just signifies hydrostatic equilibrium has been achieved. The additional energy accumulated during cycle one. is still there in the system forever so there is extra power to warm the surface.
You have to add the KE arriving at the surface in descending columns to the continuing 240 W/m2 from insolation and the combination raises Earth’s surface to 288k instead of it settling at the S-B level of 255k.
if conduction is ‘stealing’ radiation (an entirely new form of energy transfer) then you need to show that as an additional flow, let’s call it D, with an arrow depicting a fork to B going into the box. This makes outbound LW = B-D.
You also say that the stored energy in the atmosphere is the “power” that raises surface energy. Power is not energy flux but I assume your Intent was energy. If so, then you need to show that as an additional output from the box, we’ll call this one E.
So now we have C exchanging equal and opposite energy between box and surface ( this is the ke-pe pumping) and stolen D going into the box equal to E going back to the surface by, unstated, transfer mechanism.
The only problems i see here are the radiation imbalance at the system boundary, the new radiation-Conduction transfer science, and the new science where stored energy becomes power and gets used twice to raise the energy level of something with no net energy exchange.
Oops. Guess I’ve still got a lot to learn.
Paul, that is not an accurate or coherent summary of what I am saying. Others can make their own judgments.
In particular I resent being accused by you in another comment of presenting a slayer model when I agree with Willis that a cool atmosphere heats a warmer surface. My only difference with Willis is as to the mechanism.
It is either DWIR or convecting mass that does the job and the debate is wide open on that.
A lot of commentators are now focusing on that very issue so to block debate of it here would be very odd.
”Others can make their own judgments.”
Stephen, as I judged previously, your imagination of surface uneven warming with neat little packets of rising and descending air parcels is not found in the real world. For example, clouds are simply not popping up and down all the time & everywhere, they have bases (ceilings in aviation).
Settlement of a debate on physics is in the lab not the imagination or prose. Here is a short experimental video showing you how surface convection is much messier than you imagine.
That is not an accurate summary.
I am considering the effects of convection averaged globally including the large Hadley Cells and others.
The surface stays at 255k as per S-B”
Why in the world would you think this? The surface cannot simultaneously 1) receive 240 W/m^2 and 2) radiate 240 W/m^2 and 3) transfer some, additional power to warm the atmosphere directly by conduction and 4) maintain a steady temperature of 255K.
Any three of those can happen simultaneously, bit not all four!
S-B gives the temperature achievable by a blackbody at a given level of irradiation.
That assumes all incoming and outgoing energy runs at the speed of light.
In order to make the surface temperature fall below S-B as proposed by you a method of energy removal faster than light would have to be applied.
Conduction and convection operate at a slower rate and so cannot reduce the temperature of the irradiated surface below the S-B temperature.
Instead, conduction and convection take their energy from the outgoing radiation AFTER the S-B temperature has been achieved and cannot reduce that pre existing surface temperature.
The same parcel of kinetic energy at the surface cannot both radiate and conduct simultaneously so whatever goes into conduction reduces the number of photons emitted to space. Observed from space the planet would appear to drop below S-B but at the surface it would not have done so.
That being the case the surface temperature must then rise above S-B when KE is released from PE beneath descending columns of air and observed from space the planet would return to S-B despite the surface actually being warmer.
That is exactly what we observe in the real world.
”That is not an accurate summary.”
Seems it is when Stephen writes:
“The 33 in rising columns…The 33 in falling columns..”
Not observed in tests. While this test does show a sort of rising convective column as observed with clouds and gliders, there is no falling column as the test shows much spreading laterally and does not get back to the surface as cleanly if at all.
Simple testing shows Stephen’s “The 33 in falling columns does affect surface temperatures..” is imaginative at best. Whereas sky DWLIR is a much more constant glow measured incident on the surface which is affected by atm. opacity and T.
The surface energy balances across the globe show as much up convection as down convection, no net energy added or removed from the surface so no effect on median T at the surface or TOA. As opacity changes, each convective component will change but overall the convective net will remain zero modulation of surface T observed over 4-10 years or more.
It is non zero during the process of the first convective overturning cycle and that is where the energy for the surface temperature enhancement comes from.
The rest of your post is inaccurate and irrelevant.
“KE is released from PE beneath descending columns of air..”
As the simple test shows, and observations of clouds at various ceilings, descending columns of air are simply imaginary. Although severe downdrafts are observed locally, by and large as Stephen often writes the atm. is in hydrostatic equilibrium most of the time. Sure, rising convention significantly disturbs it some days but not generally. The atm. glow is much more constant up and down.
Darned, Trick, thanks for the information; all along I thought there were deserts at certain locations on the surface of the earth. Downwelling dry air, anyone?
Wow, that’s a crazy trick.
All this air goes up in convections and updrafts……. but NONE comes back down…..
Some sort of weird mass balancing happening, wouldn’t you say ;-).
“but NONE comes back down…..”
Only in Andy’s imaginations. Not observed coming back in columns Andy, look at the test: as in the atm. the blue spreads in from lower z height higher pressure system replacing the lower pressure system created by the rising convective ~columns as shown. Stephen’s descending columns are simply imaginative.
Yes Dave deserts are regions of descending dry air on the lee side of mountain ranges and certain atm. constant circulations. Note not descending columns which are imaginary as shown in the test. Convection goes up in reasonabe columns, descent is not columnar. As the test shows. There should be more testing around here less imagination.
Most deserts occur beneath the subtropical high pressure cells in both hemispheres and they are large scale regions of primarily descending air. There are other such cells at the poles
Stephen, yes, Atacama, Death Valley: lee side. Sahara, poles: circulation.
Lee side of mountains is not sufficient on its own for deserts. Also need to be under or near the subtropical high pressure cells.
Every region of higher than average pressure is a column of descending air. That constitutes half the bulk atmosphere.
ROFLMAO.
So the air DOES come back down.
Thanks for that, 😉
”It is non zero during the process of the first convective overturning cycle..”
Simply more imagination from Stephen, not reality.
Of course, testing and observations are inaccurate if they do not agree with Stephen’s imagination. I prefer to go with testing and observations not Stephen’s imagination. Imagination does work in movies and blogs for entertainment but that’s about it. Though some science based imagination does eventually get confirmed by test.
Please specify how any uncompleted initial convective overturning cycle could fail to have a non zero energy effect at the surface
That’s easy peasy EIN=EOUT. No net change.
I don’t think anyone would dispute localized imbalances both geographic and temporal but you need to think about integrating over the planet and over reasonable time periods to get one vector in and one vector out. They MUST be equal and opposite at steady state or energy is either being created or destroyed.
That is for a completed cycle only.
Whilst incomplete it is a non zero process.
We are talking about equilibrium states, not steady states.
Right. No net energy exchange.
You have built a fantasy with no net energy exchange except for that pesky one that has ‘equal’ exchange at the surface-atmosphere boundary. Except in one direction it acts like energy transfer and in the other it moves energy without moving energy.
Answer my question.
Why does a developing atmosphere need to reduce surface temperature below that determined by insolation when it gets all the energy it needs from energy that would otherwise have radiated to space?
If it fails to reduce surface temperature during initial uplift then it must raise the temperature above S-B when KE is released in the subsequent descent.
Not going there! Discussion is about 4 billion year old system in thermal equilibrium….
No net energy flows.
A developing atmosphere is not in thermal equilibrium
“Every region of higher than average pressure is a column of descending air.”
Not in tests and observations only in Stephen’s imagination. Watch the blue dye test Stephen, it spreads in laterally underneath at the surface just like in the windy atm. as the moderator notes. Lower pressure system being replaced by higher pressure.
Not a rotating sphere, not a gas, no density gradient with height. A very silly attempt at diversion.
“Please specify how any uncompleted initial convective overturning cycle could…” do something.
Please show a physical example of a test/actual observed uncompleted initial convective overturning cycle and we can discuss what it could do. I can’t possibly determine what such a process could do in Stephen’s imagination.
Simple logic should do it.
A rising molecule takes energy away from the surface having received energy by conduction.
Is it imagination to see that as a non zero effect on surface energy until such time as the molecule falls back to the surface?
Yes
That implies that you think a developing atmosphere requires no energy to lift it off the surface.
Bizarre.
“A rising molecule takes energy away from the surface having received energy by conduction.”
Ok, then my answer is as shown in the test video, by simple logic. Your further comments should be in line with the test not just in your imagination.
What Trickie is saying is that more energy is moved in the upward direction than downwards.
ie, the atmosphere has a net COOLING effect.
Thanks Trickie. We KNEW that. 🙂
“S-B gives the temperature achievable by a blackbody at a given level of irradiation.”
More specifically, the maximum temperature achievable if the only energy input is a given level of EM radiation. As it happens, we are discussing a situation where the only input is EM radiation, so in this setting this statement works fine
“That assumes all incoming and outgoing energy runs at the speed of light.”
No — that assumes that all incoming energy leaves as thermal IR. “Speed” has nothing to do with this. It is conservation of energy at its simplest. If some energy ‘leaks’ away via conduction or convection, then there is simply less left fly off as thermal radiation.
No energy leaks away in conduction and convection.
It later returns to warm the surface beneath descending air which constitutes half the atmosphere.
“We KNEW that.”
Sure, good for you Andy. The atm. is radiating LW energy to the sink of deep space; if not the world would be a very different place.
“It later returns to warm the surface beneath descending air which constitutes half the atmosphere.”
Not as shown by test Stephen, only in your imagination. And anyway you many times write the atm. is mostly in hydrostatic equilibrium so not much descending or rising air is the norm. There is lateral fluid movement also at the same T as shown by the blue dye at the surface and red dye at the top.
Don’t you think you have discredited yourself enough yet ?
Try answering the question I put to Paul.
Why does the inception of an atmosphere need to reduce surface temperature when it gets all the energy it needs from energy that would otherwise have radiated out?
Please show a physical example of a test/actual observed “inception of an atmosphere” and we can discuss what it could do to previous energy balances & if any different than the results of the test in the video. I can’t possibly determine what such a process could do in Stephen’s imagination.
4:00pm ”Why does a developing atmosphere need to reduce surface temperature below that determined by insolation when it gets all the energy it needs from energy that would otherwise have radiated to space?”
Stephen now proposes a developing atm. at 4:00pm not inception of an atm. as at 3:43pm.
I am not really sure what you are imagining here Stephen, which is why I ask for physical example to answer any question you may have, like in the test video. I am guessing you are looking for an answer you already have imagined and any answer not in accord with your imagination will be called inaccurate, irrelevant and discredit the author.
So please, if you really want a serious question answered in comments on a post about can a cold object warm a hot object, ask the question in a way that can be tested for accuracy, relevancy, and to credit the answerer.
A C Osborn November 28, 2017 at 3:37 am
I have no clue what you are on about. Let me once again say, since you and others seem determined to ignore it:
I don’t know which of my words you are babbling about. I have no idea which expeiment of Dr. Roys you think proves something, or what it proves. Your post is meaningless to me.
Come back when you are willing to specify your objection. This is tiresome.
… added …
Also, read what Ed Bo says above. If that is what you are talking about, Ed is right and you are wrong. The use of night-time radiative cooling has a long history. People in the Southwest US used to use it to freeze water when the air and ground temperature was above freezing … but that has absolutely nothing to do with concentrating diffuse IR.
w.
PS—Your comment about “take off your ‘scientific consensus’ head” is plain nasty. I don’t have two heads, I have one. My respect for you has taken a big hit with this comment of yours …
And your response has reduced my respect for you also.
We have been having this discussion about How a Solar Oven makes things colder, you originally denied all knowledge of what I was talking about and suddenly you state “The use of night-time radiative cooling has a long history. ”
So you appear to have known about it all about it all along.
Your exact words
Willis Eschenbach November 27, 2017 at 11:06 am
1. Nobody knows any way to “concentrate” environmental thermal IR since it is diffuse and coming in from every angle. You cannot focus it with a mirror or a lens … how are you planning to “concentrate it?
In his first experiment here
http://www.drroyspencer.com/2010/07/first-results-from-the-box-investigating-the-effects-of-infrared-sky-radiation-on-air-temperature/
Roy W. Spencer, Ph. D. says:
July 29, 2010 at 2:38 PM
you cannot focus an extended source of thermal radiation.
And reference actually INCREASING the affect of DWLIR here as suggested by others because a Solar Oven does so
Roy W. Spencer, Ph. D. says:
July 29, 2010 at 3:42 PM
OK, I added a funnel made of aluminum sheeting, and I think I can see enhanced cooling right away. We have cirrus clouds right now from surrounding thunderstorms, but it typically clears during the night. If it does, we’ll see whether we get greater cooling below ambient tonight.
So I ask you yet again If you cannot Collect, Concentrate or Focus DWLIR from CO2 or Space
How does it increase the cooling affect?
As to your taking offense at a figure of speech about “putting on another head” I appologise.
Just clarify my question
How does adding the Aluminum Funnel increase the cooling affect?
Or how does a Solar Collector oven do the same thing?
AC:
I repeat: there is nothing in that thread about concentrating thermal infrared radiation. The “funnel” that was added was simply to block radiation from the side angles, which would come from warmer sources. No concentration, and especially no heating.
A C Osborn November 29, 2017 at 8:29 am
Ed is right. Adding shielding to your setup such as the aluminum funnel is common when you want maximum radiative cooling. It shields what you are cooling from the effects of the ground, the trees, the building, and the nearly horizontal radiation of the lowest, warmest layer of air. This allows the object to reach a cooler temperature than without the shielding.
However, it is NOT concentrating diffuse IR, as you seem to think. As an example of the problem, try using a magnifying glass to concentrate the light on a day which is heavily overcast. A magnifying glass only works with essentially parallel rays like those of the sun.
If you think can truly concentrate IR, I encourage you to either find a reputable source that shows how it can be done, or to do an experiment yourself. As far as I and many other knowledgeable people on this blog know, it can’t be done … but then as Feynmann said, “science is the belief in the ignorance of the experts”.
Let us know what you find.
w.
A good answer, but again not good enough.
Why would you use a Funnel of Shiny Material, why not just use any old material if it “just a mask”?
The Solar oven is Shallow and allows any and all radiation to enter it and still works.
A C Osborn November 29, 2017 at 4:22 pm
Good question. You use aluminum, not because it is shiny, but because the emissivity of aluminum is low. It typically ranges from 0.05 to 0.4, depending on the surface treatment.
This is an advantage, because at a given temperature an aluminum shield radiates less than high emissivity materials. At say 15°C, a body with an emissivity of 0.9 emits about 350 W/m2. With an emissivity of 0.4 it would emit about 155 W/m2, and with an emissivity of 0.1 it would emit only about 40 W/m2. Since the point is cooling, we want to use a low-emissivity shield to block out the low-angle radiation from trees, buildings, and the lowest (and warmest) atmosphere.
As to the “Solar oven”, I have no clue what you mean by that. As mentioned before, you can concentrate collimated radiation, but not diffuse radiation. Solar radiation is collimated, so it’s not applicable to the current question of Dr. Roy’s experiment.
Regards,
w.
A small addition to Willis’ description. If the sheet of material at 15 C with emissivity = 0.1 was in a room at 15 C, you would measure approximately … 390 W/m^2 coming from it. It will only EMIT ~ 40 W/m^2 itself (10% of 390 W/m^2), but it will REFLECT about 350 W/m^2 from the room (90% of 390 W/m^2).
If the reflective sheet is pointed at the sky that might only be producing 200 W/m^2, then the total IR from the funnel would be something like (0.1 * 390 + 0.9 * 200) = 220 W/m^2. A lot more than 40 W/m^2, but still WAY less that ~ 390 W/m^2, so it would make for an effective cooling system.
Also, I think the ‘solar oven’ is something like this: https://www.scientificsonline.com/product/solar-oven
Basically, it would serve as the ‘funnel’.
AC Osborn: The correct answer to all these mis–directors and takers of offense is to channel the Gypper – “There y” go again”. Never mind, you are doing well, all here who won’t wear the tin hat.
Well if you had accorded me the courtesy of actually reading the Dr Spencer post which I referred to, I know you are a busy person but what is the point of asking for “references” if you are not going to follow them up?
If you had read it and the comments you would have seen multiple references to Solar Ovens (Collectors) including links to photos.
Due to the suggestions of the commentors to add a similar kind of “collector feature” Dr Spencer did.
In his quote that I supplied he called it a Funnel, not a mask or shield or block and it worked.
The definition of a funnel is “A tube or pipe that is wide at the top and narrow at the bottom, used for guiding liquid or powder into a small opening.” So replace water or powder with Radiation and what do you get?
You disparigingly said in your previous post “If you think can truly concentrate IR, I encourage you to either find a reputable source that shows how it can be done, or to do an experiment yourself. As far as I and many other knowledgeable people on this blog know, it can’t be done”.
Well I don’t need go anywhere to find a “reputable Source” , that post was in 2013 and many of those knowledgeable posters obviously believed that a “collector” would have some kind of an Affect and Dr Spencer proved it.
Universities have tested and found it can reduce temps to 20C below Ambient.
With that evidence I think it is up to you to provide an alternative reason for it working which proves it is not a Funnel (DR Spencer’s description”) or some kind of Collector.
And just to avoid confusion this is what a Solar Oven of Collector types looks like.
https://www.bing.com/images/search?view=detailV2&ccid=wzMzM1Cy&id=4579BB3763543FE29CFE7C54B595DADBE1354769&thid=OIP.wzMzM1Cyms39KnwuoU9HmwEgDY&q=photos+of+solar+ovens&simid=608005888856884813&selectedIndex=31&qpvt=photos+of+solar+ovens&ajaxhist=0
https://www.bing.com/images/search?view=detailV2&ccid=WCYY9Nc2&id=1E1249F3E1D932EFF39E477B8A95AD69776DEB09&thid=OIP.WCYY9Nc2twrnJt2-eBPIpAGoCT&q=photos+of+solar+ovens&simid=608044397524550803&selectedIndex=33&qpvt=photos+of+solar+ovens&ajaxhist=0
https://www.bing.com/images/search?view=detailV2&ccid=WCYY9Nc2&id=1E1249F3E1D932EFF39E477B8A95AD69776DEB09&thid=OIP.WCYY9Nc2twrnJt2-eBPIpAGoCT&q=photos+of+solar+ovens&simid=608044397524550803&selectedIndex=33&qpvt=photos+of+solar+ovens&ajaxhist=0
https://www.bing.com/images/search?view=detailV2&ccid=422xT3Bc&id=FB7164846187C39D4B8322CB4576C9933A7DAE7C&thid=OIP.422xT3BcmBGi0d8gZhGEUQEsDh&q=photos+of+solar+ovens&simid=608029012954909515&selectedIndex=3&qpvt=photos+of+solar+ovens&ajaxhist=0
There is also this
https://johnosullivan.livejournal.com/18334.html
A C Osborn November 30, 2017 at 2:09 am Edit
If you were citing a comment you should have actually cited it. When you cite a post, I assume you are citing the post. Foolish me.
I’m sorry, but I seem to have wandered into an alternative universe. People were asking why you couldn’t concentrate IR radiation to make it hotter, so that it could do work, in the same way that solar can be concentrated. The question was, can we use IR to make things hotter, not make them cooler.
You’ve given as an example a night-sky radiator, which attempts to shield its contents from IR in order to cool them down. But as Ed Bo noted:
So no, the shielding on Dr. Roy’s experiment doesn’t “concentrate” IR. And calling it a “funnel” just means that it is funnel-shaped, not that it is actually functioning as a funnel. Nor does someone calling it a “collector” mean that it actually collects anything. It reminds me of the old joke: “How many legs does a cow have if you call a tail a leg?”
…
The answer is “Four … because calling a tail a leg doesn’t make it one”. And calling something a “funnel” doesn’t make it one either.
But the biggest problem is that you are answering a question that wasn’t asked—is it possible to cool something by shielding it from warm IR? The answer to that question, as you noted, is assuredly “yes”. Build an insulated box, point the open side at the cold sky, shield it from warm ground radiation, and it will cool … so?
The problem is, that says nothing about the original question, which was whether you can concentrate and focus IR to get it hot enough to do useful work.
Finally, can you stop with the solar ovens? I know how they work, I used to teach Peace Corp Volunteers how to build them, yes, they have a reflective funnel to focus the COLLIMATED solar rays, and I know that they have nothing, repeat nothing, to do with whether we can focus diffuse IR to bring it to higher temperatures. As far as I know we can’t, and you haven’t provided one thing that says that we can.
As to O’Sullivan’s risible claims, he says that if you point a solar oven at the sky you can freeze water when the air temp is above freezing. This is true. We used to note that they could cool as well as heat when we built our solar ovens, although we never got anything to actually freeze because Peace Corps trainings are generally in the summer.
However, this does not, as he claims, “prove” that there is no downwelling radiation from the atmosphere. He says:
Please tell me that you know that downwelling radiation is real. It is measured routinely around the planet by dozens of scientists and has been for years. And as to whether it is absorbed at the surface, if it were not absorbed it couldn’t be measured as the scientists are doing. Nor does the global energy budget balance without downwelling IR, it is way, way off without it.
The problem is that we only get about 170 W/m2 at the surface from the sun … which equates to an S-B temperature of minus forty degrees (C or F, take your pick, they’re the same). So if there is no downwelling IR as O’Sullivan foolishly claims … what is providing the energy to keep us from going into the icebox? It’s not gravity, gravity can’t provide ongoing energy unless the earth is shrinking, so what is it keeping us from freezing if not downwelling IR?
And if you’re going to claim that all those scientists measuring downwelling IR all around the planet are either lying or mistaken, if you believe O’Sullivans fantasy that downwelling IR “doesn’t exist in the real world”, then I fear we have little to discuss.
Finally, back to the original question, O’Sullivan certainly doesn’t show that you can use a solar oven to concentrate IR. Like I said, I don’t know of anyone who knows how to concentrate diffuse radiation.
w.
One of my favorite examples of IR exploitation is smudge pots in orchards. They create copious amounts of a gas (that can not be named) that converts upwelling LW energy to downwelling LW energy that keeps fruit at a toasty above freezing temp.
The gas can be we’ll below freezing when it does this.
Stupid farmers don’t know that this Can’t work
AndyG55 November 28, 2017 at 1:43 pm
No, it is what is called a THOUGHT EXPERIMENT. Einstein used them extensively. Go accuse him of FANTASY and stop exposing your ignorance here. Everyone is entitled to an opinion … but not every opinion is worth blurting out in public.
w.
More like thought bubble.
Irrelevant to anything to do with the atmosphere.
But hey…. stick to your thought bubbles if that’s what it takes to convince yourself.
Are you really saying that your steel greenhouse actually exists?
If not, then it is a fantasy. It is make-believe.
You can call it a “thought experiment” if you like, but so is me planning a trip to Tahiti.
Not based on reality.
This makes me sad. Complex systems are ALWAYS analyzed by picking away at the components in simple, constrained ‘thought’ working from known and we’ll understood fundamentals towards what we don’t know in little bitty steps. It’s called science. It’s called engineering.
Anybody who criticizes these exercises has no business here because it demonstrates complete incomprehension of what is going on.
Indeed, most of these experiment threads go down ratholes invented to obfuscate and divert attention from the unpleasant truths that conflict with the favorite meme of the day.
If you think It’s fun and snarky to point out there isn’t a steel greenhouse, duh, then you certainly have missed important learning in that classic post and likely Can’t comprehend what it intends to teach.
I know exactly what is going on. It makes me sad that you obviously don’t.
This is an imaginary thought bubble, irrelevant to anything to do with the atmosphere.
And conflating it with Einstein? Seriously !!!
So Andy … would you say that Schrodinger’s Cat was just a useless “fantasy”? How about Maxwell’s Demon? What about Einstein’s five thought experiments that reshaped physics? Just “fantasies”.
Your callous dismissal of valuable scientific thought experiments as “fantasy” is hilarious. You truly believe that? Really?
w.
Again,
conflating with Einstein.. You are getting WAY ahead of yourself., as you often do.
Some hypothetical thought processes are “useful”..
others..
just meaningless thought bubbles.
Yes Andy, we are talking to pygmies here, in terms of understanding. Good thing they are in a sidestream while the real deconstruction of the false physics continues. Particularly by defunding, you might notice. Deasperation is rising…..
In a couple of previous posts, I mentioned the persistent effect of *convection* in creating an an adiablatic lapse or adiabatic gradient in the atmosphere. As right as I still think that is, I notice that I may have been misleading myself, just a bit, by also emphasizing the low thermal conductivity (or the high molecule-to-molecule resistivity) of gases, in quite the way that I did? The thing to understand is that the molecule to molecule resistivity of all atmospheric gases, including oxygen and nitrogren is so *good*, that *other things*, like convection and radiative transfer, tend to take over, when it comes to actually moving heat energy from the ground upward. Also, it would seem that the basic dry adiabatic lapse rate is essentially what you might call an “onset of convection” condition; the lapse rate is what happens when the most basic convective effect has *stopped*. The idea is that there is a negative feedback, with convection stopping and starting, and with a temperature gradient being maintained thereby.
I’ve now read the “Steel Greenhouse” thought experiment that Willis Eschenbach had mentioned before in his current article “Can a Cold Object Warm . . . “. My initial reaction is that the thought experiment is simply trying to establish something that I myself find intuitive, that “some” gases, at least, should be expected to either absorb and/or emit infrared radiation, just like other forms of matter, e.g., steel, tend to do. Shouldn’t I expect that even the regular gases like nitrogen, those gases being a form of matter, would also engage in “warm” infrared emissivity? My intuition, at least, says that emissivity is in general a kind of universal material process. If someone shoves a warm bag of nitrogen gas out into deep space somewhere, why wouldn’t we expect the nitrogen as such to emit IR out to the cold microwave background of the universe?
Now, if I search the internet using terms like “infrared emissivity of nitrogen”, I can easily find seemingly nonsensical statements like “oxygen, nitrogen and the rare gases (such as argon) do not emit radiation in the IR range”. At the same time, if I ‘Duckduckgo’ search a more pointed question, say, “Does clear nitrogen gas emit thermal infrared radiation or doesn’t it “, I get more interesting results! Try for instance, the third hit down on the page of hits that results from *that* question, that is the web page https://chiefio.wordpress.com/2016/02/17/nitrogen-active-in-the-ir-a-ghg/ .
The blogger at the above link is quoting a “harvard.edu”/ American Astronomical Society journal article from *1944*. This is about detecting *IR radiation of approximately one micrometer*, coming from *molecular nitrogen in the night sky*. Now, if such results as were reported in that journal article are true, why, you can just *watch* my bag of nitrogen cool down. Take *that* dudes!
My challenge, now, is to all “warmistas”, “lukewarmers” and anyone else who ever thought that CO2 or other minor gases were especially important in this regard. Try asking your favorite web searcher a pointed question, such as “Does clear nitrogen gas emit thermal infrared radiation or doesn’t it “. If it turns out that GHG theory boosters can then hardly deal with any informed results they may get, shouldn’t they be rethinking their position?
David Blenkinsop November 29, 2017 at 1:39 am
I’ve now read the “Steel Greenhouse” thought experiment that Willis Eschenbach had mentioned before in his current article “Can a Cold Object Warm . . . “. My initial reaction is that the thought experiment is simply trying to establish something that I myself find intuitive, that “some” gases, at least, should be expected to either absorb and/or emit infrared radiation, just like other forms of matter, e.g., steel, tend to do. Shouldn’t I expect that even the regular gases like nitrogen, those gases being a form of matter, would also engage in “warm” infrared emissivity? My intuition, at least, says that emissivity is in general a kind of universal material process. If someone shoves a warm bag of nitrogen gas out into deep space somewhere, why wouldn’t we expect the nitrogen as such to emit IR out to the cold microwave background of the universe?
Unfortunately this is where your intuition lets you down, a college freshman class in physical chemistry would tell you that the infrared spectra of gases is the result of quantized energy transfers between discrete rotational/vibrational energy levels, and that such transfers require a dipole which homonuclear diatomic molecules such as O2 and N2 don’t possess. In your thought experiment we wouldn’t expect the N2 to emit IR into space, the bag however would emit IR into space and thus cool the N2 down via conduction.
Now, if I search the internet using terms like “infrared emissivity of nitrogen”, I can easily find seemingly nonsensical statements like “oxygen, nitrogen and the rare gases (such as argon) do not emit radiation in the IR range”. At the same time, if I ‘Duckduckgo’ search a more pointed question, say, “Does clear nitrogen gas emit thermal infrared radiation or doesn’t it “, I get more interesting results! Try for instance, the third hit down on the page of hits that results from *that* question, that is the web page https://chiefio.wordpress.com/2016/02/17/nitrogen-active-in-the-ir-a-ghg/ .
The blogger at the above link is quoting a “harvard.edu”/ American Astronomical Society journal article from *1944*. This is about detecting *IR radiation of approximately one micrometer*, coming from *molecular nitrogen in the night sky*. Now, if such results as were reported in that journal article are true, why, you can just *watch* my bag of nitrogen cool down. Take *that* dudes!
Unfortunately as indicated above you don’t understand elementary physical chemistry, if you did you would understand that N2 when exposed to very energetic UV photons can either be electronically excited to higher electronic states (does not require a dipole) or excited so much that it splits into two N atoms. Those N atoms can recombine to form an electronically excited N2 (after losing some excess energy). In the decay of those electronically excited states some IR is emitted, this is not the same as what happens in the troposphere where such highly energetic photons have been filtered out (the processes the paper refers to occur at ~200km above the earth, the thermosphere where it can be as hot as 1500K). In fact the mechanism proposed in that paper is that the N atoms recombine to form N2 molecules in the ground vibrational state of the electronically excited triplet state (B3Пg), this then decays with the emission of the observed IR to the electronically excited singlet state (A3∑+u). My guess would be that there is first an intersystem crossing to the singlet state then a vibrational transition to the v=0 level. None of these processes are accessible in the troposphere.
My challenge, now, is to all “warmistas”, “lukewarmers” and anyone else who ever thought that CO2 or other minor gases were especially important in this regard. Try asking your favorite web searcher a pointed question, such as “Does clear nitrogen gas emit thermal infrared radiation or doesn’t it “. If it turns out that GHG theory boosters can then hardly deal with any informed results they may get, shouldn’t they be rethinking their position?
To which the answer is: not in our atmosphere.
If instead you asked ‘does nitrogen in a near vacuum at 1500K irradiated with far UV emit in the IR?’, the answer would be yes.
Because gravity creates a pressure and density gradient so the higher molecules are spaced further apart which allows creation of non sensible potential energy from sensible kinetic energy.
My comment at 4.02am was directed at Berenyi Peter but was misplaced in the thread.
talldave2 said:
“This is another instance of being basically correct but with framing problems — objects radiate exactly the same way no matter where they are. The atmosphere isn’t “in the way” so much as it is radiating back at the warmer objects (even though it’s colder than they are!), especially when there are big lumps of water vapor in it.”
Please explain how conduction works if objects radiate exactly the same way no matter where they are.
Heat is just kinetic energy which is molecular movement/vibration. If a molecule at the surface is moving/vibrating at a rate commensurate with an S-B temperature of 255k and then encounters gas molecules to which it passes 33k ‘worth’ of that energy instead of emitting a photon then how can it possibly radiate at 255k with that conducted energy then missing?
You can suggest that the removed energy is immediately replaced by fresh insolation so that the temperature remains at 255k and should emit commensurately.
The trouble is that within a convecting atmosphere the conduction process is constant so that although the surface temperature remains at 255k any fresh insolation is itself whisked away into conduction and convection so that the surface will only radiate to space at 232k despite being at 255k.
That deals only with convective cycle one when the atmosphere first lifted off the ground.
As soon as convective cycle one completes with the subsequent descent to the surface then it is the case that the previously missing conducted 33k is being reintroduced to the surface in addition to continuing insolation ‘worth’ 255k which then raises surface temperature from 255k to 288k
But you still have that constant upward flow of conducted energy elsewhere so that the radiation to space
from the 288k surface temperature is still limited to 255k as per observations.
So, surfaces beneath a convecting atmosphere do not radiate exactly the same way as if there were no convecting atmosphere.
For those who keep claiming that reflected radiation cannot heat the lamp that emitted the radiation, Anthony did the experiment here. So you are not just arguing against well-established thermodynamic theory … you’re arguing against experimental results as well.
w.
PS—Do NOT trust anything published by Principia Scientific International without first running the numbers and reviewing the logic … and you’ll still have to wash your hands afterwards.
“PS—Do NOT trust anything published by Principia Scientific International without first running the numbers and reviewing the logic … and you’ll still have to wash your hands afterwards.“
How vicious and vindictive of you.
Tony November 29, 2017 at 4:26 pm
Tony, clearly you’re not familiar with the website …
In any case, you may (or may not) recall, I said specifically that I did NOT think that you are either vicious or vindictive, viz:
So now, you are using my NOT calling you either vicious or vindictive as an excuse to call me both … dude, you are a piece of work.
w.
Tony November 29, 2017 at 4:26 pm
One more thing. Tony, here’s a quote from a typical post on Principia Scientific International … it starts off as follows:
And THAT is why I advised washing your hands after you go there. Talk about vicious and vindictive … like I said above, it appears you have no idea what the site is all about.
w.
Willis, Mr Skolnick was taken down by the Canada bar association:
“The Law Society of British Columbia (LSBC) has now ruled that green activist Andrew Skolnick’s official complaint concerning Dr. Tim Ball’s libel attorney, Michael Scherr and science writer, John O’Sullivan, was baseless.
Andrew Skolnick had filed the complaint against Dr. Ball’s legal team as part of a coordinated attack stage managed by lawyer Roger McConchie, representing disgraced climatologist, Michael Mann.
Specifically, the LSBC has affirmed there is no evidence to support Skolnick’s malicious allegation that anyone “knowingly asserted something for which there is no reasonable basis in evidence.”
http://johnosullivan.livejournal.com/41331.html
Sunsettommy November 29, 2017 at 10:21 pm
Well, kinda. ONE of Mr. Skolnick’s claims was found to be baseless … but if you read the link I gave, that claim isn’t listed. Instead, you’ll find lots of other claims with good citation and documentation.
And in any case, PSI published a post calling me psychotic and drug-addled … what does the Canada Bar Association have to say about that kind of scummy sliming? Where is the noble Mr. O’Sullivan in that? And why are you defending a man who does that?
w.
Willis, I was just pointing out another of your double standards. Your article begins with a request for civility, but your own civility stops when it comes to any mention of “extreme skeptics” or their arguments. And, “well he started it” is not a defence (nor would it most likely be true)
Tony called Willis “vicious and vindictive,” because Willis said PSI is untrustworthy.
That’s absurd. Nothing Willis said was vicious and vindictive.
Even what I sad (that the PSI people are “stark, raving, clinically insane”), though harsher than what Willis said, was not vicious and vindictive.
If you want an example of real viciousness & vindictiveness, look no further than your despicable PSI pals. Accusing President Bush of staging the 9-11-2001 terrorist attack, and murdering nearly 3000 of his fellow Americans with “micro-nukes,” as PSI’s Joe Olson did, that is truly vicious and vindictive, as well as stark, raving, clinically insane.
If Tony actually cared about civility, he would apologize to Willis, and condemn his vicious, vindictive, dishonest PSI pals.
“Tony called Willis “vicious and vindictive,” because Willis said PSI is untrustworthy.“
Or it could have been because of the ongoing campaign to smear PSI that WUWT has mounted over the last x number of years…lol
Wllis,not trying to defend John, just to point out that Andrew made a serious allegation that was found to be false.
Skolnick had other legal problems too that he lost,which included getting dismissed:
“CMS lawsuit
The AMA dismissed Skolnick when Correctional Medical Services, one of the for-profit health care companies criticized in the “Death, Neglect and the Bottom Line” article, threatened JAMA and the Post-Dispatch with litigation.[21][22] [23]
Skolnick also sued CMS, claiming their responses to the articles were defamatory, but a summary judgement ruled in favor of CMS, the defendants.”
https://en.wikipedia.org/wiki/Andrew_A._Skolnick
We remember, Willis, how it was made evident that the increase was caused by a different factor. And we wonder…..
Made evident how? Evident to whom?
The responses here from knowledgeable people — including some who have taught thermodynamics — all agree with Willis’ analysis. Everything he wrote in the top post agrees with standard textbooks on the subject. It is right that you start to wonder why you can’t convince people with your ‘evidence’.
Brett Keane November 29, 2017 at 8:12 pm
Brett, I have no clue what you are talking about. What increase? What factor?
What I wonder is why you have trouble following a polite request, viz:
Why is that so hard? As it stands, you are talking to the wall, because your shorthand is impenetrable.
w.
https://wattsupwiththat.com/2017/11/29/study-no-acceleration-in-global-warming-climate-sensitivity-to-co2-too-high/
You wil not believe the empirical evidence held in NASA solar system data, so you will also disrespect the findings of Dr Christie above. Disrespecting me peronally, your problem not mine.
But the tone is like that of most warmista, when confronted by truth. That makes me wonder about the standards of this debate, and what is going on behind it. Tony above nailed it. Never mind, truth will out…..
Brett, this is unintelligible. First off, there are dozens of commenters here. So who is the “you” whom you are addressing? Because if it is me, your claims are ridiculous. I respect Dr. Christie greatly, he’s one of my scientific heroes.
Next, you don’t know what I or anyone else will or won’t believe, or will or won’t disrespect. You’d have to be a mind reader to do that, and you’re not one. You’re just pulling claims out of your fundamental orifice.
Next, you say:
This is an absurd blanket generalization. And in any case, who are the “warmista”, and what “tone” are you referring to?
Seriously, you need to QUOTE THE WORDS THAT YOU ARE DISCUSSING, because as it stands, you could be out in space talking to aliens for all the good you are doing us.
w.
SkepticGoneWild November 29, 2017 at 11:15 pm
Either it is a thought experiment, or it is pure fantasy. You can’t have it both ways.
Oh, sure, I’ll just build a planet in space and surround it with a steel shell … in any case, as I pointed out above, ANTHONY DID THE EXPERIMENT.
In addition, we have the example of the new lightbulb which uses some of the reflected IR to heat up the incandescent element … clearly there is ENERGY flowing from cold to hot in that case.
Finally, if as folks keep saying energy cannot flow from cold to hot, that a hot object won’t absorb a “cold” photon, then how can we see cold things? For me to see something means my eyes have to absorb the energy … but lots of folks say that because my eyes are warm, they can’t accept photons from something colder. So how come I can see cold things?
Skeptic, you and others don’t seem to get it. This stuff is very basic thermodynamics. I provided an online calculator so you can check my results. I provided the actual thermodynamic equation, and I’ve shown what it reduces to. You and others have totally ignored the math … poor choice. The answer is in the math.
At this point, it is up to you to FALSIFY my claims … and to date nobody’s done that. Not only that, but the people here who are experts in the field, including folks who teach this stuff for a living, say that my steel greenhouse analysis is 100% correct.
Your move …
w.
“Either it is a thought experiment, or it is pure fantasy” you say, Willis.
Well, talking about thought experiments, vis a vis, pure fantasy….what about this thought experiment which reckons that if the Earth had no atmosphere, (thought experiment), we would have an average “atmospheric?” temperature of about negative 18 deg C. Obviously, at that temperature, all the oceans would be frozen solid…..but the last time I looked at the sea….it wasn’t frozen solid, Willis.
So the only conclusion I can reach is that the thought experiment is pure fantasy.
Oh! did I just say that the atmospheric “greenhouse effect” was pure fantasy. ..wow, sorry, heretical talk.
Should read….average surface “atmospheric?” temperature…
Mack November 30, 2017 at 1:00 am Edit
Mack, one of Einstein’s famous thought experiments started out “Suppose you were chasing a ray of light …”
Now, you and I know that we can’t just chase a ray of light through space … but Einstein wasn’t just pointlessly indulging in pure fantasy. Instead, he was conducting a “thought experiment”. One of the things about thought experiments is that we do them because we cannot do the real experiment. We can’t race a ray of light or build a steel greenhouse the size of a planet.
But that doesn’t mean that we cannot consider what would happen if we did race a ray of light or build a steel greenhouse. The key that makes these “thought experiments” and not pure fantasy is simple. In a thought experiment, once the situation is set up, we don’t suspend the laws of physics.
Instead, we use those natural laws to illuminate, investigate, and hopefully understand something we didn’t understand before … as I did with the steel greenhouse.
Surely you can see the difference between Einstein’s story about chasing a ray of light, and say the story of Sleeping Beauty, which is pure fantasy set in a world where there are witches and spells and the normal laws of physics are in abeyance. As I mentioned above, Maxwell’s Demon and Schrodinger’s Cat are thought experiments which have a solid place in science … while Sleeping Beauty is a fairy tale which has no place in science at all.
I hope that clarifies the difference.
Regards,
w.
“Finally, if as folks keep saying energy cannot flow from cold to hot, that a hot object won’t absorb a “cold” photon, then how can we see cold things? For me to see something means my eyes have to absorb the energy … but lots of folks say that because my eyes are warm, they can’t accept photons from something colder. So how come I can see cold things?“
Well, I can help with this one. Objects at room temperature emit IR, Willis, not visible light, and no, your eyes don’t see IR. You see objects because of the visible light REFLECTED off the objects. Reflected off, not emitted from. The clue is in the title, really, “visible light”. As in, it’s at a wavelength of the EM spectrum that is visible to the human eye. This visible light comes from an object at higher temperature, like the sun. Be that during the day, or reflected off the moon at night. Or it could come from a light bulb; that’s also operating at sufficient temperature to produce visible light.
You see, it doesn’t all have to be a mystery…
http://www.bbc.co.uk/bitesize/ks2/science/physical_processes/how_we_see_things/read/1/
Tony November 30, 2017 at 1:46 am Edit
http://www.bbc.co.uk/bitesize/ks2/science/physical_processes/how_we_see_things/read/1/
“KS2” in your citation means that it is designed for 7- to 11-year-old children … it says nothing of interest to what we are talking about. Stop wasting our time with your grade school explanations, this is an adult discussion.
w.
Yes, I must admit that I found it a little bit funny that you needed something explained to you that most 7 to 11 year olds understand. I’m sorry. I shouldn’t laugh.
Tony, Mr Eshenbach should place an Ice cube in a completely dark room to see if his eyes can see those special photons streaming of it. ROFL.
I put up this post earlier, challenging all the people who deny established radiation. And got crickets.
Now I see that A.C. Osborne is such a smart guy he will be just
A.C. Osborne:
Answer this simple question..
Without changing the subject, and without any need for models or esoteric physics nobody is talking about, answer this simple question…….
Here’s the shot:
Your claim is that IR from a 90 k source incident on a 91 k target, does nothing to the target. If true it has to then either pass through it (albedo of 0) or be reflected from it (albedo of 1). You further claim if that very same source hits the very same target but now the target has a temperature of 89 k it is absorbed, at least partially.
Here’s the chaser:
If you would grant me the liberty of putting that in an equation, it would be albedo= f(T). Do you agree that my equation correctly supports your claim?
Come on. It’s simple question involving just three numbers and an equation with one term. Surely such a smart one as you can answer this in a jiffy….
Wating….
Plus, nobody is saying ENERGY doesn’t flow from cold to hot. HEAT doesn’t flow from cold to hot.
Tony November 30, 2017 at 2:49 am
Say what?? Of course they are saying that, that’s the whole Slayer theory. The claim is that there is no way that the atmosphere could make the ground warmer because the atmosphere is colder than the surface. Here’s Crispin on the subject:
Nickreality says:
Richard Verney thinks the atmosphere can’t leave the ground warmer …
wickedwenchfan says:
and finally, from wickedwenchfan again:
Sorry, Tony … but you’re wrong again. People are indeed claiming that radiant energy cannot flow from cold to hot.
w.
Hey Willis don’t put me in that list! What you copied is from a thread with Stephen Wilde where I was restating HIS position. Read the whole thread.
I am trying to get a 1st law energy balance from him with his slayer atmosphere model. The handwaving is epic
Do not try to get me into trouble with Willis.
I happen to agree with him that a cooler atmosphere can warm a hotter surface.
I am not advancing a Slayer model.
I am just pointing out that the job can be done by conduction and convection without invoking a surface warming effect from DWIR.
The final decision as to which option prevails is open for debate and a lot of contributors here and elsewhere are beginning see how important atmospheric mass can be.
So…you DON’T know the difference between heat and energy?
Paul Bahlin November 30, 2017 at 4:12 am
My bad, Paul, I’ve removed you from the list.
w.
No Willis,
YOU came up with this steel greenhouse proposition. The scientific method puts the responsibility on the one proposing his idea. Your next move is an experiment. You are not even half-way through the scientific process.
Secondly, you don’t even understand what falsfification is!
Third. Who is saying energy cannot flow from cold to hot? Not I. You are the one saying HEAT flows from cold to hot, because that is what is happening in your thought experiment. (The cooler shell warms the sphere)
Our move is to sit back and watch you fumble in the dark and fail.
Tony November 30, 2017 at 1:18 am
While that is true, the claim was that photons could not go from a cold object to a hot object. Are you now saying that some photons can and some photons can’t? Because both visible light and IR are just photons …
You ever see fireflies? How about a glow stick? In addition to IR, objects at room temperature can emit visible light. We are not seeing light REFLECTED from fireflies. Despite your clear claim that it is all reflection from some hotter source, in fact it is light EMITTED FROM the cold fireflies.
As to the “clue is in the title, really, visible light”, that says nothing about whether that light is reflected or emitted, or what the temperature of the emitter is.
Again I have to ask … you ever see a firefly or a glow stick or bioluminescence in the ocean? Are you claiming that that light “comes from an object at higher temperature, like the sun”???
It’s not a mystery. Once a photon is emitted it carries its tiny bit of energy until it hits and is absorbed by something. That energy is then converted into another form, be it thermal, mechanical, or chemical.
My eyes have no way of knowing whether a light of a given color comes from a glow stick (cold) or an incandescent light (hot). Doesn’t matter. When the photon hits it is absorbed regardless of the temperature of the emitter.
So … to return to the question, how do you explain that my warm eyes can see light from a cold firefly? According to you, the “cold” photons are supposed to bounce off my warm eyes or something, I’m not sure just how you explain your claims. You seem to think there’s some essential difference between photons of visible light and photons of IR, and some other difference between “hot” and “cold” photons, I don’t know how you think this works.
So here’s your chance to explain it …
w.
https://en.m.wikipedia.org/wiki/Bioluminescence
https://www.pantone.com/how-do-we-see-color
Tony’s link: “Thus, red is not “in” an apple. The surface of the apple is reflecting the wavelengths we see as red and absorbing all the rest.” I see this so often that it is almost an immutable truth. Instruments measure otherwise.
The writer of that web page obviously has never gone into a lab and actually measured the spectrum of an apple illuminated by daylight. According to his writing the resulting spectrum would show no emission from any wavelength band (“absorbing all the rest”) and a huge peak reflected around 660nm band. That is not what is measured; the actual visible spectrum from the apple shows abundant radiance measured from 300-700nm which one’s brain interprets as the color red.
The natural opaque apple absorbs & emits about 95% of the incident daylight and scatters only about 5% as measured by spectrophotometers.
OK.
https://en.m.wikipedia.org/wiki/Photon_energy
Happy reading!
This one was for your statement:
“Because both visible light and IR are just photons“
Which to me implied that you were not aware that photons of visible light and photons of IR ARE actually different.
Oh, sorry, forgot the glow stick:
https://en.m.wikipedia.org/wiki/Glow_stick
Long story short, objects at room temperature do NOT produce visible light. A glow stick produces visible light due to an exothermic reaction between the chemicals that are mixed together when you bend the stick to start with. Bioluminescence is a related phenomenon (again the visible light is produced by a chemical reaction, this time within the organism).
Since not one of those links discusses the question we’re looking at, as to whether photons can carry energy from a cold object to a warm object, I fear that perhaps you are playing some kind of joke … or perhaps not, but if not, then what are you claiming that these links show? Because they have nothing to do with what we’re talking about here.
I do note that after curiously claiming that we only see reflected light;
and after bizarrely stating that the reflected light always comes from something hotter than our eyes;
after I pointed out that neither one is true and I gave examples showing why they are not true, you’ve gone strangely radio silent … I mean, I can understand your unwillingness to admit that neither claim is even remotely valid, but posting random links won’t cut it …
w.
Oh, sorry, Willis. I assumed you would read the five links I have posted to you before responding. This is from the Pantone “how do we see color” link:
“The human eye and brain together translate light into color. Light receptors within the eye transmit messages to the brain, which produces the familiar sensations of color.
Newton observed that color is not inherent in objects. Rather, the surface of an object reflects some colors and absorbs all the others. We perceive only the reflected colors.
Thus, red is not “in” an apple. The surface of the apple is reflecting the wavelengths we see as red and absorbing all the rest. An object appears white when it reflects all wavelengths and black when it absorbs them all.
Color is made up of red, green and blue lightRed, green and blue are the additive primary colors of the color spectrum. Combining balanced amounts of red, green and blue lights also produces pure white. By varying the amount of red, green and blue light, all of the colors in the visible spectrum can be produced.”
Let me know when you have read the links. I have given you an answer on the “examples” you provided, bioluminescence and the glow stick. Gave you those answers very quickly, in fact. No “radio silence” here.
Tony November 30, 2017 at 2:30 am Edit
I read it. It waxed lyrical about things like “color is made up of red, green and blue light”. Gosh. Who knew? … well, everybody knew, that’s a beginners explanation. However, it said nothing about the subjects under discussion, which are a) can objects at or below room temperature emit light (yes), and b) can photons from a cold object flow to a warm object (yes).
I know how we see. The links you sent are all grade-school explanations of how we see that have nothing to do with this discussion.
w.
Yes, Willis. It also says:
“Newton observed that color is not inherent in objects. Rather, the surface of an object reflects some colors and absorbs all the others. We perceive only the reflected colors.“
Tony November 30, 2017 at 3:06 am
As I said, it’s a grade school explanation so it doesn’t cover everything. For example: when you look at a glowstick, what is the light “reflecting” off of? When you look at a bioluminescent fish, what is the light “reflecting” off of?
In short: if an object does NOT emit light, then your statement about Newton is 100% correct.
But when we’re talking about something that emits light, say a blue LED, that blue is NOT a reflected color. It is an emitted color. Same is true of lasers, and light bulbs, and anything else that emits light.
Sorry, but once again, you’re taking the simplified and thus ultimately incorrect path that doesn’t include all the possibilities.
w.
“In short: if an object does NOT emit light, then your statement about Newton is 100% correct.”
Yes, precisely. I have absolutely no idea why you’re acting like you’re disagreeing with me, whilst agreeing with me. Though of course it wasn’t MY statement. It was a quote from the link. And in fact nowhere have I made the statement that energy from a cold object can’t travel to a warm object, nor that a photon cannot travel from a cold object to a warm object.
A FLIR detector sees infrared.
And it is warmer than the objects it sees, at least some of the time.
Our eyes do not see infrared, but infrared eyes are not impossible.
We have the eyes we have due to evolution giving us the ability to detect the wavelengths that are the most abundant and that therefore contain the most information about our surroundings.
Tony November 30, 2017 at 2:21 am
Bioluminescent creatures produce light at well below room temperature. I see bioluminescence in the waves on the north coast where I live, and the water there is about 50°F. Not only that, but fish living in the deep, black ocean at about 4°C are bioluminescent.
So long story short, once again you are 100% wrong—objects at room temperature and well below room temperature DO produce visible light.
In addition, this question of “room temperature” is a bit of a straw man. The issue was whether light is produced from something colder than my eyes, and the answer is absolutely yes …
Having settled that, you still haven’t explained why these “cold” photons don’t just bounce off my eyes or whatever explanation you might have for your claim that radiated energy cannot flow from a cold object to a hot object.
Because when I look at bioluminescent cold north-coast waves, that’s exactly what’s happening. Photons are flowing from a cold object to a warm object, where they are absorbed. You say this is impossible. Please explain why.
w.
PS—Regarding glow sticks, your own citation says that they “generate negligible heat”, which certainly fits with my experience. They are nowhere near as warm as my eyes … but I can still see them, which you and others have claimed is impossible because you say that photons won’t flow from cold to hot …
HEAT will not and cannot flow from cold to hot … but radiant energy absolutely can. This does NOT violate the second law, because as I explained above, if I can see you, you can see me. By this I mean that if one object is able to radiate to a second object, that means the second object is able to radiate to the first. As a result, there’s no “one-way” radiation from cold to hot, that would indeed violate the second law.
Instead, since both are radiating, whichever object is warmer will radiate more, and as a result the NET flow, not the individual flows but the NET flow which we call a heat flux, always and ever goes from hot to cold.
Again, in the head post I gave the relevant equation, its simplification for the situation of the earth and the atmosphere, and a link to an online calculator so that folks like you could actually play with the numbers and see what happens … let me recommend again that you do that.
The math is well known and tested in thousands of situations, from outer space to the factory floor. It’s not cutting edge. Instead it is cut and dried, bog-standard centuries-old stuff that you will find in any introductory thermo textbook.
You’ve yet to show me where I’ve been wrong. It goes without saying that we can see visible light. As I said, the clue is in the title. We see the visible light from a light source, reflected off objects. That’s why we see the objects. Not because of what the objects themselves emit. If an object emits visible light directly, we will see that too. We can see the sun, we can see a light bulb. What makes you think I would be arguing otherwise? The glow stick itself may be at room temperature, but it’s producing light due to an exothermic reaction. So, it has a source of energy for the light. Same as we can see the light bulb, we can see the glow stick (even with the lights off). It’s a light source. This doesn’t change the fact that we will see other objects, with the light source as a glow stick, light bulb, or sun, due to light REFLECTED off them.
It’s all getting a bit desperate, Willis.
“You’ve yet to show me where I’ve been wrong.”
You are wrong about origins of color & so is the website you linked & many more which can be demonstrated by going into the optics lab or looking up an actual measured spectrum of an apple or the paragon of yellowness, a banana. Not all visible light can be picked up by the eye. Turn off the lights in your room at night and test for that. The objects are now bathed in BB radiation at room temperature and emit at visible wavelengths too dim to be perceived by the eye but not too dim for a spectrophotometer.
OK.
Golly.
The first time I read through a comment thread on this subject, I wound up with a terrible headache.
That was years ago.
It is dismaying to see the exact same conversation again, and again, and again…
Ad nauseum.
All these years later, and I still get the exact same headache.
It is very exasperating.
menicholas, it’s like Stendahl said in “The Red And The Black” about the Church. Like you regarding this issue, someone said the Catholic Church never changed. The priest replied “Oh, it changes … it just does it so slowly that no one lives long enough to see it happening”.
What keeps me going is that I’m not writing to convince Tony. He’s beyond my poor powers to add or detract. Instead, I write for the lurkers, those that read but never comment. Among them are many who are unconvinced, many who are just beginning to be interested in climate, many whose minds are not set in stone.
I know that although I can’t see it, some among those minds are changing when they read the nonsense that comes from folks who don’t think that a GHG atmosphere can leave the surface warmer than if that atmosphere didn’t exist … so I persevere.
Onwards, thanks for the comment,
w.
Yes, the lurkers.
There are many, of that I am sure.
I too make many of the comment I make for the benefit of any who might be reading, and not because I think I will change anyone’s mind. Although I do have in mind planting seeds of doubt.
I come from a large family of stubborn people, and I have seen many instances where an idea from person A is ignored by person B when first raised, but then re-emerges in the mind of the person B some time later, as person B’s own idea.
In my family, it was hard to ever win an argument, but sometimes a suggestion made and then left alone would sprout and grow.
I sometimes ask people if they ever change their mind about anything, and some people admit they never do.
A warmista woman I have known from childhood was recently engaged in a conversation about a climate issue, and she seemed to be gaining some knowledge, but at one point she blurted out ” I will never be a den!er”. I engaged her no further, nor will I ever. Just bowed out gracefully. It was not easy, but I like her.
I just want to say, you and several others here should be in the running for sainthood with the patience you have displayed in this thread.
Keep fighting the good fight!
Onward, yes…and you are very welcome. Thank you for the reply.
Stephen, I am really trying to understand your concept but we have so many places where we diverge I think it would be useful to highlight and resolve them.
First you state:
“S-B gives the temperature achievable by a blackbody at a given level of irradiation.
That assumes all incoming and outgoing energy runs at the speed of light.”
I dispute this claim. S-B says nothing at all about irradiation. It relates the energy RADIATING from a BODY at T when it has no other means to shed the energy it needs to maintain 1st law energy balance. Stated as the inverse you could say that if you observe a radiation level, say X, then you can use X to compute a surface temperature. From a first law view of a body, if it is receiving X+Y input energy then it must be shedding X+Y at steady state. If Y is being ‘stolen’ by conduction then X will come out as radiation.
Note here the important distinction that this has nothing to do with irradiation. The body can be receiving energy by little mice bringing in pails of Joules in buckets. Most importantly, it has no dt at all. Nada. It is relating two steady state properties of an object with a constant emissivity. When emissivity is 1 then it is relating properties of a black body.
Then you say:
“In order to make the surface temperature fall below S-B as proposed by you a method of energy removal faster than light would have to be applied.
Conduction and convection operate at a slower rate and so cannot reduce the temperature of the irradiated surface below the S-B temperature.
Instead, conduction and convection take their energy from the outgoing radiation AFTER the S-B temperature has been achieved and cannot reduce that pre existing surface temperature.”
All of this is totally irrelevant to a discussion of steady state energy flux. No matter the rate differences of the various transfer mechanisms, each part of a 1st law accounting will achieve steady state over reasonable integration periods. If it is not steady state then you can’t even talk about energy balance of the parts because you don’t need to obey 1st law until you reach steady state. The water entering your bathtub won’t equal the water leaving your bathtub until it’s full and that’s OK. But, and this is the important thing, you can’t put more water into the overflowing tub and claim you are still filling it and you can’t ever get more out of it than you put in.
Next you say:
“The same parcel of kinetic energy at the surface cannot both radiate and conduct simultaneously so whatever goes into conduction reduces the number of photons emitted to space. Observed from space the planet would appear to drop below S-B but at the surface it would not have done so.”
First sentence is sort of true but it’s more correct to say that surface energy removed by conduction reduces the surface energy and the remainder is then radiated to maintain balance. It doesn’t reduce photons in the strictest sense. It just reduces the amount of energy that needs to be gotten rid of by radiation. Importantly here, and this a huge area of disagreement, surface observations made before and after the introduction of a conduction loss would most definitely show a temperature drop. You have lowered the energy content of the radiator. The temperature will go down. Not debatable.
Penultimately and this is the nut:
“That being the case the surface temperature must then rise above S-B when KE is released from PE beneath descending columns of air and observed from space the planet would return to S-B despite the surface actually being warmer.”
What you are proposing here is that energy can leave the surface by conduction and that does not result in an energy loss observable by measuring the radiative output of the body. Simultaneously (remember we are talking about steady state) you allow that an equal amount of energy entering the body by conduction as a consequence of the pe-ke pump does raise the energy (and temperature) by an amount exactly equal to what would have been lost by the conduction loss except somehow magic is happening in the atmosphere that prevents the lost energy from being lost.
Lastly:
“That is exactly what we observe in the real world.”
Where has this ever been observed?
My position here is that a surface energy loss due to conduction lowers the energy and subsequently (when you reach steady state) the temperature to a new level. Surface energy gain due to conduction results in more energy and subsequently more temperature. If the conduction loss equals the conduction gain (of energies) then the surface temperature is unchanged from what it would be if there were no atmosphere at all.
If the atmosphere radiates negligibly as you propose then the energy entering your box (atmosphere) can do anything it wants it will all reach steady state returning exactly what was put there in the first place. You can have Jupiter size storms, oceans evaporating, torrential rains, all of that, and you still will get all the energy back when it is integrated over reasonable time periods.
A lot of work there but the nub is whether or not conduction and convection reduces surface temperature when it begins at the initial formation of the atmosphere.
I say it does not because it simply absorbs energy that would otherwise have gone out to space as radiation. You appear to accept that.
The temperature appears to fall when observed from space because from that observation point one can only see the radiating portion and not the conducting portion.
Why does that require a surface temperature fall?
I don’t see how adding the slower process of conduction and convection can accelerate the rate of energy loss from the surface so as to induce a temperature fall to a level lower than could be achieved by the faster process of radiation out.
If it does not produce a temperature fall then surface temperature must rise when the descending column gets back to the surface.
“If it does not produce a temperature fall then surface temperature must rise when the descending column gets back to the surface.”
This is your imagination at work Stephen & is not in accord with test. The low pressure system created by the rise in the convecting column shows high pressure system moving in laterally with surface temperature (blue) fluid just like the windy atm. and NOT your imaginary “when the descending column gets back to the surface.”
First off, it would clarify things if you stopped talking about convection. We have defined a simple system: a surface and a box. The surface is the earth outside layer. The box is the atmosphere.
Convection is inside the box and has no relevancee to this fundamental flux accounting as it never penetrates a boundary. Likewise there is no discussion of molten core or plate tectonics.
Each component must balance and the system must balance. If you Can’t get that part done properly then discussions about quantum physics and photons and typhoons and latent heat and lapse rates are so premature as to be totally irrelevant, right?
Now as to your energy questions….
If energy is removed from the surface by conduction then X joules/s leave and after y sec you have reduced the surface by x*y joules. The temperature will go down. Three days later, mice bring back all those joules and plunk em down by conduction. The temperature will go right back where it was as long as that is the only system change.
The high level model that this represents is fundamental to getting to the internal details you are anxious to delve into. But, you can not let that internal dabbling live inside a falsity of energy imbalance.
You can speed up and slow Down offers to your heart’s content only after you vet past 1st law.
The temperature need not go down because the energy taken up in conduction is immediately replaced by continuing insolation.
That energy comes from a reduction of outgoing radiation and is ADDED to system energy content as PE until it is returned to the surface as KE to reinstate the original outward radiative flow.
You really can’t handle the idea of multiple moving parts can you?
You are using flux and energy in the same argument.
Too many units for ya’?
I used flow not flux, you must be rattled.
And since you replied to Willis with a ‘no’ that makes you the Slayer.
A cool atmosphere obviously lets an irradiated surface become warmer than S-B
The only issue is whether that is caused by DWIR or by convective overturning.
I contend that DWIR is a consequence of the lapse rate structure and not a cause of surface heating but the debate on that is ongoing.
Goodnight.
Yikes! Better check that Stephen. If the ke comes back to restore the original flow then your scenario just blew up because the temperature is right back where you started from.
Oh wait…. That’s what I’ve been saying all along
Only if it dropped in the first place and you haven’t
showed that.
The temperature need not go down because the energy taken up in conduction is immediately replaced by continuing insolation.
That energy comes from a reduction of outgoing radiation and is ADDED to system energy content as PE until it is returned to the surface as KE to reinstate the original outward radiative flow.
You really can’t handle the idea of multiple moving parts can you?
Sorry. Last paragraph is an auto correct nightmare.
Should be something like… speed up and slow down transfers only after 1st law accounting.
No matter, I know what you are trying to say and you are wrong.
The atmosphere subtracts from outgoing and adds to incoming so as to make the surface warmer than the S-B prediction whilst radiation to space matches S-B.
In effect the work done by the atmosphere mimics increased external insolation.
The evidence is the observation that for objects with atmospheres of more tha 0.1 bar the temperatures at the same pressure are similar after accounting for distance from the sun.
Radiative theory has no explanation for that.
I have the only detailed description as to why and how it works.
“Radiative theory has no explanation for that.”
Sure it does. Every atmosphere has GHG. Therefore every atmosphere should experience GHG warming.
What there *is* no explanation for is how work done by a falling atmosphere counts, but the energy input to raise the air to begin with is ignored.
Tim, I also disagree with Stephen as radiative-convective atm. theory is good enough to calculate T(z) for earth, venus to within instrumental accuracy.
I disagree with you though, in that the data to date indicate atm.s with not enough pressure to raise the atm. opacity very much looking up. So that the global surface T is about equal to planet brightness T such as for Mars by example. The global thermometer surface T for Mars is only sparsely measured but from what has been recorded this is close enough to planet brightness T for gov. work on the surface.
So to see if I can clarify things, let me ask a question of Tony and all the rest.
Do you think that a non-GHG atmosphere (say argon) can maintain the surface temperature of a planet at a level higher than the calculated Stefan-Boltzmann temperature of that planet given its incoming solar radiation?
Let’s set aside the question of the particular mechanism for the moment. Do you believe that such a continuing temperature elevation from the action of a non-GHG atmosphere alone is physically possible?
Serious yes or no question … I’m not looking for your explanation, just whether you think it’s possible.
w.
Willis, should I et. al. presume you are looking for serious answers for Earth orbit, similar rotating planet typical atm. parameters, like 1 atm. at surface, same L&O surface emissivity so forth?
Trick November 30, 2017 at 3:59 pm
Those don’t matter, although I’m leaving out planets made of radioactive materials and such. Just a ball floating in space, warmed by a sun, with an argon atmosphere. Some folks think that the pressure of the atmosphere, or the overturning of the atmosphere, or conduction, or some other atmospheric phenomenon, will permanently raise the surface temperature of the planet above the expected S-B temperature.
I’d like to know who those folks are so I can discuss it with them.
w.
“Some folks think that the pressure of the atmosphere…will permanently raise the surface temperature of the planet..
I’m one of those “folks” as the physics (measured in lab & theory) shows an increase in pressure adds to the first order opacity of an atmosphere. A rotating planet like Earth means you can reasonably spread insolation over the entire surface not just the illuminated half for a basic analogue to understand some basic atm. physics.
So I will go with those parameters as observed for Earth for basic calculation.
“Do you think that a non-GHG atmosphere (say argon) can maintain the surface temperature of a planet at a level higher than the calculated Stefan-Boltzmann temperature of that planet given its incoming solar radiation?”
If the water condenses out of current earth atm. as it changes to all argon in such a way that total albedo remains ~0.3, surface emissivity remains near enough 1.0 rounded, and the current atm. spectral emissivity looking up drops from about global median 0.8 to that of argon spectral emissivity (pure guess near 0.01 for calculations as I couldn’t quickly find a measured value), then a basic calculation shows 255.3K global median and with 0.0 argon emissivity 255.0K so the answer is: with pure argon atm. emissivity 0.01 find basic physics shows surface temperature a very little higher than a perfectly transparent atm. If find a measured emissivity applicable for argon Earth atm. then I’ll use it for better est.
“Do you believe that such a continuing temperature elevation from the action of a non-GHG atmosphere alone is physically possible?”
Yes, the 255.3K with argon would remain steady state global median, not be transient above the 255K completely transparent atm., at 0.3 albedo, current insolation (I used 1370), current rotation, current L&O surface at 1 atm. pressure.
I wonder if the various parties to this conversation could come to some sort of agreement of an online text of relevant information that can be referred to by everyone involved as well as those of us following along as best we can?
Maybe something like this, which I just found at random.
Not saying this one, but one.
Is that possible, or are some here of the opinion that the information they have is not written down in any texts on the subject?
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan.html
menicholas, the problem with the site you link is that it has no cites to source material and the testing that backs the source material. You are better off looking to gain an understanding of climate physics in a beginning college level meteorology text, several of which are on line if you have the chops (pre-req.s) to read them. The best ones willl cite past experimental work or publish their own weather & climate observations and instrumented lab tests.
Trick.
I studied meteorology on college.
And climatology.
And physics.
Interdisciplinary natural sciences.
My degree was physical chemistry.
The idea that there is a separate physics called climate physics seems as if it may be a problem, not a solution.
And I was the guy sitting in the front row getting an A in every one of those classes.
BTW, Trick, you are incorrect.
The site has an index and links to extensive references and such.
Menicholas, why not have this one instead.
http://ozonedepletiontheory.info/primary-problem-with-GG.html
Which contains many references.
I would prefer to have someone discuss not just the Physics, but also the Mechanics and Characteristics of how the CO2 part of the GHG Theory is so important.
“The site has an index and links to extensive references and such.”
Not on the page you linked. And no tags pointing to any of the references.
No
Whoops, Willis is asking about an argon atmosphere so you would not be a slayer just an agw proponent or a lukewarmer.
My answer would be yes on the basis of the conduction issue.
No — a purely non-GHG like argon could not raise the temperature above the S-B predicted temperature.
Paul says no, the atmosphere alone would not heat the surface.
wildeco2014 says yes, it would heat the surface.
Anyone else? Step up, here’s your chance, inter alia science is about taking a stand and defending it.
Regards to everyone, whether you have an opinion on the question you’re willing to share or not.
w.
Note that wildeco2014 and Stephen Wilde are the same. It just depends on which device I am using.
Sorry for any confusion but I thought people would realise.
I am still learning but my thoughts have been evolving from not being sure who to believe to believing that some people are just being hard headed and have confused the issue for people who are not physicists.
Gravitational collapse causes heating of a gas cloud, but there is no reason to think that heat is then stuck.
It seems implausible that an atmosphere with no (GHGs…are these the same as “radiative gasses”?) could just keep getting hotter and have no way to cool itself.
I say no.
I’m in the NO camp — the surface could radiate directly to space just as if there were no atmosphere. Since the atmosphere cannot absorb any energy from space or emit any energy to space, it can only exchange energy with the planet’s surface, so no net transfer to or from the surface.
Ed, how did you find an experiment that an argon gas atm. at STP cannot emit any energy to space? And thus emit no radiation to the surface either.
Ed, no net radiative transfer to or from the surface once the atmosphere is in place but there is a net conducted energy transfer from surface to atmosphere throughout the first convective overturning cycle.
That omission is the elephant in the room for the radiative theory.
Ed,
From conduction no?
So the initial heat from the collapse of the atmosphere would conduct to the surface which would then radiate to space?
The atmosphere would be warmed during the day by the ground, and convect and expand, and then be cooled at night and contract, no?
Seems the cooling part would be slower.
Trick:
A century’s worth of spectroscopic measurements in the laboratory has failed to find any IR emissions for argon gas. All of our theoretical knowledge as well says that there is no mechanism for argon gas to absorb or emit in the IR range.
Stephen and menicholas:
Since a transparent atmosphere has no mechanism to exchange energy with space, it can only exchange energy with the surface. This means that it must receive as much energy from the surface through conduction as it transmits to it — otherwise it would continually diverge in temperature from the surface.
The earth’s surface emits ~250 W/m2 more than the earth system absorbs from the sun (averaged over area and time). The zero-sum exchange between surface and atmosphere cannot remotely make up this difference.
What might have happened once billions of years ago cannot make any difference now. The residual geothermal flux density is less than 0.1 W/m2. There is no way that ancient event could still be providing 250 Joules every second over every square meter of the earth.
“A century’s worth of spectroscopic measurements in the laboratory..”
Cite just one for Ar Ed. Ought have a stacked high pile of ’em then.
NIST seems to indicate otherwise but I need to track down their 1973 ref. Also a chance these tables are in obscure handbooks, I’ll probably end up spending some time in the stacks. All I need is the spectral emissivity for Ar applicable to STP atm., can’t find it on the ‘net. Though I have developed a strange and sudden urge to get all my double glazed windows filled with Ar gas.
Tim Folkerts gives us another “No” vote … Tony, wickedwenchfan, Brett Keane, Steven Wilde, where do you guys stand on this issue?
w.
Dirty Argon with dust, water vapor, and the “usual” 0.65 to 0.75 atmosphere clarity, or laboratory pure clean Argon with nothing at all in it globally above a perfectly clean dust-free smooth surface with no oceans or seas?
(Heck, I’m still trying to find somebody who can re-create the 28 day lunar soil temperature cycle that we DO have measurements for in a “climate” model of a simple sphere in a vacuum. Not a flat-moon average temperature at equilibrium, but the pole-to-pole actual lunar soil surface temperature. )
RACookPE1978 November 30, 2017 at 6:47 pm Edit
Pure, undiluted, clean as clean argon. And water vapor is a GHG, no water vapor. No dust, it acts like a GHG although it’s an aerosol. Just argon.
Not sure why you’d want that, but the calculations are quite simple. The regolith (lunar dust) is a good insulator, so it heats and cools rapidly, and doesn’t transfer heat horizontally. Seems easy enough to do … I’m curious, though. What is it you hope to show with such a model?
And I’m curious about your answer to the argon question as well.
Thanks for the comment, always good to hear from you,
w.
Let me note that I bring this up because lots of folks say that either there is no downwelling radiation from GHGs, or that there is radiation but it doesn’t heat anything.
This leaves them trying to explain why the temperature of the earth stays above the S-B temperature. The usual explanation is that it’s the atmosphere itself that is keeping the earth warmer than S-B says it should be, and that it has nothing to do with GHGs.
So I ask again, can an atmosphere with no GHGs maintain a planetary surface temperature higher than Stefan-Boltzmann predicts from the solar energy radiating the planet.
w.
it’s the hydrosphere that captures the heat that a rock can’t.
the radiation goes deep and then it’s trapped cuz ir & water.
it’s not the atmosphere that heats the ocean. it’s the other way round.
Funny how Oleg Sorokhtin also agree with them about the The adiabatic theory of greenhouse effect.
But he is just some Russion Scientist without your grasp of things.
Oleg G. Sorokhtin – a graduate of the Leningrad Mining Institute in 1951, Dr.,., Academician (RANS), Honored Scientist of Russia, Honorary Polar Explorer. After graduating with honors from the LGI worked Hydroproject – hydrogeological exploration carried out by the “great construction projects of communism” (Kuibyshev HES, Turkmen channel). In 1953 he returned to Moscow and began work at the Institute of Physics of the Earth. Participated in three Antarctic expeditions, carried out a deep drilling and seismic studies, attended the Pole of Cold, the geomagnetic pole, opened the Pole of Inaccessibility. Since 1966 he has been working at the Institute of Oceanology. Shirshov USSR Academy of Sciences (RAS). He has participated in many oceanographic expeditions, fell to the bottom of the ocean, explore the underwater volcanoes, hot springs (black smokers). He has more than 300 scientific publications, including publications in “Reports of the USSR Academy of Sciences / RAS” and “Nature.” He was awarded the Order of Labor Red Banner, medals. He has two sons, two grandsons (the youngest – a year) and a granddaughter. The youngest son – Doctor of sciences, Professor of Murmansk State University (Apatity), corresponding member of Academy of Natural Sciences.
Or how about Hans Jelbring, no he won’t do as you have already made your mind up about him.
A C Osborn December 1, 2017 at 10:19 am Edit
Thanks, A C. Perhaps you decide whether a scientific claim is valid by looking at the resumes of people who agree with it. If you were to follow that to its reasonable conclusion, you’d have to believe Michael Mann, Peter Gleick, and a host of well-credentialed alarmists.
Me, I prefer running the numbers. I did that in my post The Mystery of Equation 8. No one has found flaws with it yet. I invite you to find one.
As to Jelbring, Dr. Robert Brown at Duke who teaches this stuff for a living wrote a very clear proof that Jelbring is wrong. Again, nobody has found any fault in his proof, but you’re welcome to try. That’s what science is about—not resumes, but logic and math.
Let me add that when Jelbring came out with his theory, I believed it and defended it.
However, Dr. Brown showed me I was wrong. So in contradiction to those who endlessly claim that my mind is made up, it’s not. When the facts change, I change my mind. Read Dr. Brown’s proof, it might change yours
w.
But it wasn’t just Oleg, it was quite a few others, with far more expertise than you who do not just believe it, they propose it.
Like I said why don’t you try argueing with all of them and see how far you get on.
And let me add that Nikolov and Zeller say that the answer is definitely “Yes” … and so do Volokin and ReLlez, so that’s four more in the “Yes” column.
w.
Trick, you basically said “Yes, if argon is a GHG”, because you specified that the emissivity of argon is 0.01.
However, the emissivity of argon for thermal IR is zero. It’s monatomic, so it is physically incapable of absorbing any thermal IR.
Given that case, a perfectly IR-transparent atmosphere, what would be your answer?
w.
“However, the emissivity of argon for thermal IR is zero.”
Zero or near zero? Tests show increasing pressure broadens the lines of any atm. gas in several unrelated ways. Can you cite an experiment showing your conclusion for argon emissivity 0.0 looking up at Earth atm. pressures? I get the feeling you are guessing 0.0 just like I guessed 0.01.
This is why I asked about pressure. Atm. so close to transparent as to be unmeasurably different from 0.0 atm. emissivity, then 255K results for no GHE, but there would still be an ATE over the moon as the surface emissivity and albedo sans atm. drive that physics.
Trick, the issue is that when a molecule absorbs IR, the IR is transformed into movement of the bonds between the atoms making up the molecule—they flex, they scissor, they twist, they stretch, depending on the number of atoms in the molecule they can have several flexing modes, and thus several different absorption bands.
However, monatomic gases can’t do that. There’s only one atom, no bands to flex. As a result, their absorption is not just low—monatomic gases can’t absorb at all because they have no bonds for the IR to grab on to and flex.
For example:
Finally, I’m sorry, but what is an “ATE”?
w.
Willis:
An “ATE” is an “atmospheric thermal effect” — it’s what Nikolov and Zeller (or is it Volokin and ReLllez?) assert is the alternative warming mechanism on planets.
Sometimes you’ll see it called the “atmospheric pressure effect” (APE). I like to think of it as the APE-sh*t effect.
Willis 8:55pm, I am unfamiliar with plasma jet spectroscopy, that paper is paywalled. If you think it holds the answer to finding an experiment for the emissivity of argon at Earth surface STP (looking up), I’ll go obtain a copy at the local college library.
”Finally, I’m sorry, but what is an “ATE”?”
The way you write about N&Z (V&R if you prefer), I was under the impression you had read their 2017 paper. Ed Bo is correct. See end of intro. paragraph and page 3, first paragraph under reference temperatures and reference pressure.
”monatomic gases can’t absorb at all”
Then you are the first in science to have discovered a pure white body object in argon gas. If argon can not absorb, it won’t emit and therefore is white (and black). However, I am willing to bet if you construct a very transparent container for some argon gas at say 1000C and place it in deep space, the gas will emit radiation and cool. If argon emits, argon can absorb.
Trick December 1, 2017 at 2:19 am
Trick, I just provided you with a citation saying, word for word,
Since they said it, how could I be the “first in science” to say that? And why would you think that all things MUST absorb and emit thermal radiation? Is there some law of nature I don’t know about, that says “All things must radiate thermal energy”? People mostly say that because that’s what they put in the high school textbooks, but what those textbooks mean is that all SOLID objects radiate thermal energy.
Finally, we are not talking about argon at 1,000°C. We are talking about thermal radiation at Earth-like temperatures. If that was not clear before, it is now. Nobody but you has said anything about gases at a thousand degrees, certainly not me. I suspect that if you torture argon enough it would confess, and that there could certainly be some combination of heat and pressure at which argon will do strange tricks … but argon in our normal environment neither absorbs nor emits thermal radiation.
Best regards,
w.
SpectraCalc.com provides some empirical support for Willis. They list the IR spectra for over 40 different atmospheric gases — but NOT argon (the 4th most common gas after N2, O2, & H2O). The only reason to leave it off is that it’s spectrum is completely negligible — less even than N2 and O2 that are listed.
Willis, it is a minor point debating the difference between steady state at 255.3K (argon .01) and 255.0 (argon 0.0). However, it’s sort of interesting as I don’t yet know the answer. Thanks to Tim F. doing some investigating but not yet finding Ar emissivity from experiment.
You have given a reference for argon plasmajet emissivity. I am not familiar and can’t read the paper; please explain emissivity in Ar plasmajet: is spin constrained? Ar being monatomic in earth atm. can spin and that spin is found quantized not continuous; also velocity differences will cause a doppler shift in the spin lines broadening them among other reasons.
All objects emit at all temperatures at all frequencies all the time, no exceptions. No means no so includes Ar gas. OK, make the very transparent container of 1atm. Ar in deep space start at 255K. I will bet a test will show it will emit and cool. I’ll look later for a ref. showing identified Ar spectrum in deep space and an emissivity test.
No steady state equilibrium earth surface & an Ar atm. is possible until the Ar emission spectrum shifts to regions for which the emissivity is not zero.
—-
Tim 4:12am, “completely negligible” is not zero. Ar emission cannot be identically zero as Willis is writing.
I was about to agree with Trick that it seems doubtful that there are substances that cannot emit any radiation, and so if they were hot and floating in space they would stay hot.
I used to read a lot about gas clouds in space, and how they are detected via various emission and absorption spectra.
So I did a quick search to see if any argon cloud have been discovered in space.
If they have, then they must be either emitting or absorbing.
I found this article, that a few years ago, they found argon in a gas cloud, the one around the crab nebula.
But…oooh…it was only detectable because it had formed a hydride, and unexpected result for a noble gas.
Reading between the lines, we know there is argon in these clouds, there has to be because supernovae produce argon…argon 36. The argon 40 we have a lot of here on earth comes from the decay of potassium.
And they can only see it when it forms an unlikely molecular pairing with a hydrogen ion.
I recall reading of some other instances a long time back that noble gasses can form molecules…it is unlikely but not impossible.
Here:
https://www.universetoday.com/107154/argon-the-first-noble-gas-discovered-in-space/
And then there are the argon windows…very efficient.
And we know why…no emissivity. Or really really low? *shrugs shoulders* Seems not…seems it may be goose eggs.
Golly, you can learn a lot by looking stuff up.
https://www.thebalance.com/cost-benefits-and-drawbacks-of-argon-gas-windows-844558
Trick, in thermodynamics and statistical mechanics, you quickly learn:
1) very few things are completely impossible.
2) it is easy to find things that are so improbably they would likely not occur in several lifetimes of the universe.
So, I am not going to say that argon @ 300 K categorically can’t emit thermal radiation. On the other hand, if 1 IR photon came out of a cloud of argon each year, I am not going to worry about that process when calculating the energy balance of earth’s atmosphere.
Trick December 1, 2017 at 6:30 am
All objects emit at all temperatures at all frequencies all the time, no exceptions. No means no so includes Ar gas. OK, make the very transparent container of 1atm. Ar in deep space start at 255K. I will bet a test will show it will emit and cool. I’ll look later for a ref. showing identified Ar spectrum in deep space and an emissivity test.
No steady state equilibrium earth surface & an Ar atm. is possible until the Ar emission spectrum shifts to regions for which the emissivity is not zero.
—-
Tim 4:12am, “completely negligible” is not zero. Ar emission cannot be identically zero as Willis is writing.
No, the energy levels for Ar are well known and will require excitation in the far UV at near ambient temperatures there is no way they will be populated so no emission also no way that Ar can absorb IR, there’re no available energy levels to accept those wavelengths.
Phil., if that is well known about Ar it ought to be easy to find, post up an experiment showing such.
—–
Tim F., “On the other hand, if 1 IR photon came out of a cloud of argon each year..”
Then the answer to Willis’ question:
“Do you think that a non-GHG atmosphere (say argon) can maintain the surface temperature of a planet at a level higher than the calculated Stefan-Boltzmann temperature of that planet given its incoming solar radiation?”
…is calculated YES by 1 IR photon each year. If 0 IR photons ever come out, then the answer is NO.
Trick December 1, 2017 at 12:30 pm
Phil., if that is well known about Ar it ought to be easy to find, post up an experiment showing such.
https://physics.nist.gov/PhysRefData/Handbook/Tables/argontable5.htm
Phil., thanks. I pounded around on many of the live links could not find any emissivity information for Ar or anything else. Maybe you have a link for measured emissivities?
The list of Ar “strong lines” in air shows many wavelengths in IR range though. As I wrote below, once the AR atm. is in place, no steady state equilibrium earth surface & an Ar atm. is possible until the Ar emission spectrum shifts to regions for which the emissivity is not zero.
Mr Eschenbach says “Is there some law of nature I don’t know about, that says “All things must radiate thermal energy”?”
Well you are contradicting Wiki
“Thermal radiation also known as heat is the emission of electromagnetic waves from all matter that has a temperature greater than absolute zero”
Note All Matter, no mention of solids, liquids or gases.
Credit to Willis for calmly narrowing the issue down to my single point.
The whole debate depends on one question and one alone.
Where does an atmosphere in the process of forming acquire its internal energy from?
If it comes from a temporary reduction in outgoing radiation then the pressure hypothesis is correct.
If it comes from a temporary reduction in surface temperature then the radiative theory is correct.
We have here the opportunity to destroy one hypothesis or the other.
Observations of multiple objects with atmospheres support the pressure based theory.
What else do we have either way?
I’ll explain why the only two available options are so brutally stark.
If the developing atmosphere takes the energy it needs whilst rising against gravity from outgoing radiation then there is no drop in surface temperature so that the surface must warm when convective descent begins.
If it takes its energy from the surface then the surface cools during the formation process but warms back up again when convective descent begins
In the first case you have a surface warming effect without DWIR
In the second case you need to add a warming effect from DWIR to get the surface temperature rise.
Stephen:
Here you say…..
“If the developing atmosphere takes the energy it needs whilst rising against gravity from outgoing radiation then there is no drop in surface temperature so that the surface must warm when convective descent begins.
If it takes its energy from the surface then the surface cools during the formation process but warms back up again when convective descent begins”
You are right about this being a crucial piece of logic to your premise that atmosphere alone can raise surface temperature but my position on this, based only on 1st law, is quite different. Here’s why……
If you have planet with no atmosphere, we all agree that EIN=EOUT and all transfer is by radiation. In previous comments, though you never outright said this, you implied that surface temperature is set by insolation. I have to deduce this when you say that even though your ‘developing atmosphere’ is siphoning off energy from the surface, the surface temperature is maintained constant by the ample supply of insolation.
I have two problems with this argument of yours.
One. You are making this argument while the system is in a state of disequilibrium. There is a net positive flow into the system during your `development` where outflow is inflow minus what is going into the new atmosphere. So the system is not in thermal equilibrium and should have no place in a discussion of 1st law energy accounting.
Two. If you insist that we go there, while developing atmosphere, the surface is shedding energy by two methods: radiation and conduction. It will radiate as a function of how much energy it needs to get rid of to maintain balance. By your own statements you acknowledge that outbound energy is being reduced by what is transferring to atmosphere. Radiation of a body is a property of the body as is its temperature. You simply can not claim that a constant temperature is being maintained by insolation. To do that implies that you are using insolation and T in S-B calculation. Surely you would agree that this is an invalid use of that equation.
Until we resolve this fundamental question i respectfully request that you don’t wander off to other planets or convection or lapse rates. There really is no point to going on about this topic (by any participant in the various atmosphere vs LWIR threads) and extending it to deeper considerations if we can’t all agree on something so basic.
Please address points one and two seperately.
Are we, after 4 billion years, in thermal equilibrium on a planet wide basis over a reasonable integration period?
Do you believe surface temp is f(EIN)?
i) It is perfectly reasonable to propose a temporary period of disequilibrium resulting in a stable surface temperature when the inflow remains constant and the outflow is split into two components adding up to the same energy value as the inflow. The point you seem to miss is that during that period the siphoned off energy goes to PE which does not register as thermal energy and so cannot manifest itself as a temperature rise until it gets back to the surface. That PE is ‘hidden’ from temperature sensors until it is returned to the surface as KE on closure of the first cycle. Thus the surface temperature stays stable during cycle one but rises as soon as the adiabatic loop closes.
ii) This is also dealt with by the point about the non thermal nature of PE. PE does not radiate so the temperature as viewed from space can drop whilst PE is building up within the atmosphere even though the surface remains at S-B. The thing is that as long as PE is in the process of being created it is not sufficient to rely on the S-B equation. The non thermal nature of PE breaks the radiative rules. The S-B equation is radiative only and does not deal with non radiative processes that create non thermal PE.
Here is the very important thing you’ve got wrong….
You keep insisting that the developing atmosphere is gaining energy by taking it from the outgoing radiation. If that were the case then you can make a nice looking diagram with a diversion of outgoing energy and a constant temperature surface so say, 240 w/m^2 In, 240 out in two vectors, one to space and one to atmosphere. Surface is in balance, T stays the same.
That is not what is happening. By your own definition you have an atmosphere that does not interact with IR. IT can only interact with surface in your definition.
That being the case, you have to account for the fact that the surface has Joules in it. Your developing atmosphere is not taking energy from radiant flow. It is taking it from the pool of joules in the surface. It is reducing surface energy content. That reduces surface radiation. The reduction of outgoing LW is not a diversion in a flow. It is an outcome of a reduction in surface energy content.
Surface energy content goes down when you fill your DA and it is exactly replaced once your new atmosphere equilibrates with the surface. In balance, after enough time, no change in surface temp and for T analysis atmosphere is irrelevant.
And BTW, I am perfectly ok with a system imbalance while your atmosphere comes to speed. Indeed, I would expect it. And while all that is going on, gravity and convection, and evaporation will all move around lots of energy but eventually the surface atmosphere
Boundary equilibrates.and when it does, it goes away from the math. Zeroes right out.
So you have a pool of joules at the surface which provided a pre atmosphere surface temperature for Earth of 255k as per S-B.
Those joules can either be radiated away by release of photons or conducted away into adjoining molecules. Both cannot occur simultaneously otherwise there is a breach of the first law.
During the formation of the atmosphere joules to the value of 222k release photons to space and joules to the value of 33k get conducted to the atmosphere where they do work in lifting mass up against the downward force of gravity.
How does that reduce surface temperature below 255k?
On completion of the formation process you still have the surface at 255k worth of joules as per S-B but you are also then getting an additional flow of joules arriving at the surface in adiabatic descent worth another 33k to make it 288k.
Thereafter the 288k surface radiates to space at 255k as per S-B and recycles 33k up and down to keep the atmosphere suspended off the surface against gravity indefinitely.
PLEASE take 10 minutes and draw a cartoon with your own ramblimgs. You can not reduce the energy content of something without a temperature drop.
During the formation process there is radiation worth 255 coming in constantly to replace the 222k going out to space and the 33k going into conduction.
How do you reduce the surface below 255k without being in breach of S-B?
The energy going into your atmosphere is not coming from radiation. It is coming from the energy that is contained in the surface.
Joules go down. T goes down. LW out goes down.
During your ‘devolopment’. EIN>EOUT for the system.
During the development of the atmosphere energy in is more than energy out but the excess of energy in is passed to PE which is not heat and which does not radiate. The resultant accumulation of energy in the system continues until the first convective cycle completes and then the additional energy becomes apparent at the surface as a 33k surface temperature enhancement.
Sigh
Suit yourself. I can see when I’m flogging a dead horse.
Imagine a really big bucket full of molten cheese in a mouse house. The mice that live there are very neat but not so bright.
For years, the mice kept putting cheese balls into the bucket until one day it overflowed. The neat mouse dullards scooped up the molten cheese and turned it into more cheese balls. Then they sent these extra cheese balls back to the cheese ball factory.
Since they weren’t too bright, it never occurred to them that stopping the cheese ball orders would have saved them from all the hard work making cheese balls to send back so it went on and on. Cheese balls in cheese balls out.
Then one day a mouse called Albert, said to all the tired cheese ball mouse workers, “I am going to town to buy a bucket. I will put my small bucket under your very large bucket and we will make a hole in yours to catch the extra melted cheese and you won’t have to make cheese balls anymore.”
The cheese ball worker mice were very happy. As soon as they opened the valve to the new bucket their lives improved. It wasn’t perfect because the hole wasn’t big enough to catch all the extra cheese. Each day 10 new cheese balls were delivered but only 9 cheese balls had to be cast from the overflowing messy big bucket. Better than before for sure.
For many months it was 10 cheese balls put into the big bucket and nine returned to the factory. Then one day, to their great dismay, the little bucket started to overflow. The cheese ball worker mice were moaning and cleaning up the mess, ready to turn it into cheese balls.
Happily, Albert came to the rescue with a cleanup plan. Look, if we just take the little bucket overflow and put it back in the big bucket every day, we won’t have to make more cheese balls we can keep on making 9.
So they did it. The next day, they went to the cheese room and found 10 cheese balls worth of melted cheese on the floor anyway. No matter how fast they moved the little bucket overflow into the big bucket, it was the same thing. 10 balls in 10 balls out. They were right back where they were months ago.
The moral of the story?
Don’t be a cheese ball! Just because Albert says it’s so, doesn’t mean you don’t have to do your own figgers’.
Ten cheese balls on a table. ( or 255k ready to go to space)
Send 5 to factory and put 5 in bucket. ( or 222k to space and 33k to atmosphere)
Return the 5 from the bucket to the table at the same time as 5 from the NEXT 10 made go into the bucket so you then have 10 on the table plus 5 in the bucket for a total of 15 ( or 222k + 33k = 255k back on table and 33k still in atmosphere being added to the surface in descent = 288k)
Send 10 to the factory.(255k to space) but since you waited for the next batch of 10 before despatch to the factory you still have 10 on the table PLUS 5 in the bucket
You have 5 in the bucket constantly and the most recent batch of 10 still on the table.
So, on the surface of the Earth you have 255k just arrived having just sent 255 to space and you still have 33k KE recovered from PE in descent at the surface which then warms above S-B.
You see, it is all in the TIMING.
The time taken for the first convective cycle causes the first 5 in the atmospheric bucket to be retained until the next 5 are ready so you always have 15 despite 10 in and 10 out.
That is why you MUST INTEGRATE BOTH radiative and non radiative processes to get the actual surface temperature for a planet with an atmosphere. S-B does not apply if non radiative processes interfere with the radiation budget.
It still applies from a point outside the atmosphere, however.
Can’t see any typos but you never know. It was tricky to formulate but readers should get the point anyway.
Nothing there but cheese, mice, and buckets. Don’t know what those 255,288,33 things are
Too painful to admit that a ten year old would know that energy loss delayed is temperature gained.
Glad to see you have come around.
Well at least you still have a sense of humour 🙂
The energy content of the system as a whole does not drop. It increases due to the creation of PE whilst the atmosphere is forming.
The energy content of the surface does not drop because it is still being fed new insolation at the same rate as pre atmosphere.
You can do a chart if you wish but it looks so simple to me that a ten year old could follow it and at present I don’t know how to get a chart into this thread and don’t intend to spend time learning how.
Ongoing convection is indeed net zero but due to the energy required to establish the atmosphere in the first place the system is always one cycle out of phase.
The result is a shedload of PE in the atmosphere that when it returns to the surface in descending air (half the atmosphere at any given moment) produces KE at the surface which raises the Earth’s surface temperature by 33k
did you read your last paragraph????
You have an atmosphere in thermal equilibrium (no net energy change) maintaining a surface T 33 degrees above what it HAS TO BE based on the inbound energy.
Please, please, please, draw some elementary diagrams and put your own numbers on them. What you are saying simply can not happen.
There is a net energy change during the formation of the atmosphere.
The S-B equation only works for a body without an atmosphere or from a viewpoint outside the atmosphere of a body with one.
“hereafter the 288k surface radiates to space at 255k as per S-B..”
No Stephen, the 288K surface radiates to space at 288K as per S-B.
The Earth has never radiated to space at 288k
If you can find a source that sets the Earth’s radiative loss to space at 288k I’d be interested to see it. I seem to recall that it is 255k.
“The Earth has never radiated to space at 288k”
Geez, Stephen, come on, the earth global surface on avg. radiates direct to space at 288K thermometer temperature in the so called window. Top of earth atm. (say defined by CERES orbit) radiates at brightness temperature 255K.
Well if top of atmosphere radiation to space is 255k then the surface at 288k is not radiating to space at 288k. Something gets in the way and that is conduction and convection. The additional internal energy acquired when the atmosphere formed is heating the surface by 33k.
“Well if top of atmosphere radiation to space is 255k then the surface at 288k is not radiating to space at 288k.”
Apparently, my ref. to “window” needs explanation, as the spectrum(s) posted above show, the atm. can be transparent to LWIR from the surface at certain wavelengths (frequencies) so some of surface radiation is not absorbed by the atm. (never gets to conduct and convect) and escapes to deep space. This radiation is shown in the energy budget arrows as an arrow traced straight up and out, that process is referred to as the window for radiation to escape directly to space.
This is possible at Earth surface pressures and atm. opacity; this window does not exist for Venus (is closed
to IR as surface atm. is totally IR opaque) and is much wider open on Mars due to its much lower total surface pressure.
I’ll explain why the only two available options are so brutally stark.
If the developing atmosphere takes the energy it needs whilst rising against gravity from outgoing radiation then there is no drop in surface temperature so that the surface must warm when convective descent begins.
If it takes its energy from the surface then the surface cools during the formation process but warms back up again when convective descent begins
In the first case you have a surface warming effect without DWIR
In the second case you need to add a warming effect from DWIR to get the surface temperature rise.
Your premise is built upon a ‘developing atmosphere’, right? Well that defines an atmosphere that is a net consumer of energy which means it is not in thermal equilibrium (defined as no net energy flow).
Do you have an energy flow accounting for thermal equilibrium? What do you think is a reasonable integration interval to use that would provide an equilibrated flow solution.
“If the developing atmosphere takes the energy it needs whilst rising against gravity from outgoing radiation …”
… and the only way to “take energy from outgoing radiation” is to absorb some of that radiation, which requires gases (or aerosols or clouds) that can absorb thermal IR. In other words — the greenhouse effect!
It takes it from the ground. Conduction reduces the ability of the surface to radiate to space thus it is taken from outgoing radiation and the surface temperature does not drop because fresh insolation maintains it.
And while this
And while this is going on what is the value of the outbound radiation?
“In the first case you have a surface warming effect without DWIR.”
This warming exists in Stephen’s imagination only until such time as Stephen can produce an observation of nature or lab test supporting his assertion.
The convection experiment above shows the low pressure system created by the rising red dye tracing the fluid is filled in with higher pressure surface” fluid at ambient temperature, no warming. Any red dye that makes it back to surface will be at surface ambient, no warming. The convective process tested has no surface warming effect.
Should be easy for Stephen to set up the test as shown, add some thermometers and prove it one way or the other. Simple logic means there is no surface warming demonstrated in the test and it shows no descending columns.
Yes. Sufficient insolation is needed to make that first rise.
The sun-warmed ground would conduct energy to the gas/ice.
A lapse rate could form, and phase changes at height could assist this. Depends on TSI.
Persistence of this atmosphere would be made harder if too much evaporative cooling was needed eg gas bleeding off into space, taking IR energy with it.
The existence of any atmosphere leads to an average emission height, and increasing energy density below that ie lapse rate. Isothermality seems very hard to achieve in an orbital situation.
Various planets and moons suggest the above, but it would be foolish to be dogmatic on my/our state of knowledge.
I take it that both Brett and Steven Wilde are in the “Yes” crowd. Anyone else have the courage to take a stand on this one? Tony? Anyone?
w.
yeah- but i’ll stand on water. this is a water planet.
it does not radiate like a rock. it can not. it traps radiant energy and then can not reradiate the energy as ir cuz it’s not transparent to ir.
that’s what heats the atmosphere.
Thanks, gnomish. As I said, I’m not interested in the proposed mechanism at the moment, just whether it’s possible. You say yes.
w.
https://www.britannica.com/technology/solar-pond
the question was ” can an atmosphere with no GHGs maintain a planetary surface temperature higher than Stefan-Boltzmann predicts from the solar energy radiating the planet.”
my answer is on a water planet, GHL does do it all day and night.
The Russian Scientist Oleg Sorokhtin also believes in The adiabatic theory of greenhouse effect.
But of course he obvioulsy doesn’t have Mr Eschenbach’s graps of the Science.
Oleg G. Sorokhtin – a graduate of the Leningrad Mining Institute in 1951, Dr.,., Academician (RANS), Honored Scientist of Russia, Honorary Polar Explorer. After graduating with honors from the LGI worked Hydroproject – hydrogeological exploration carried out by the “great construction projects of communism” (Kuibyshev HES, Turkmen channel). In 1953 he returned to Moscow and began work at the Institute of Physics of the Earth. Participated in three Antarctic expeditions, carried out a deep drilling and seismic studies, attended the Pole of Cold, the geomagnetic pole, opened the Pole of Inaccessibility. Since 1966 he has been working at the Institute of Oceanology. Shirshov USSR Academy of Sciences (RAS). He has participated in many oceanographic expeditions, fell to the bottom of the ocean, explore the underwater volcanoes, hot springs (black smokers). He has more than 300 scientific publications, including publications in “Reports of the USSR Academy of Sciences / RAS” and “Nature.” He was awarded the Order of Labor Red Banner, medals. He has two sons, two grandsons (the youngest – a year) and a granddaughter. The youngest son – Doctor of sciences, Professor of Murmansk State University (Apatity), corresponding member of Academy of Natural Sciences.
Plus of course there are massive holes in the current CO2 warming theory that involves the small matter of the number CO2 molecules and the Distance of them Surface that the Radiating molecules are and that the other 70% by Density of Air molecules between them and the Surface and add to that 70% of the Surface is not warmed by LWIR, but by SWIR.
I forgot so do G.V.ChilingarN.O.Sorokhtin, who work with him
Ho, and L. KhilyukM. V. Gorfunkel as well.
Oops I forgot this one S.A. Ushakov
Whoops there goes another one, how about John O Robertson.
My My, perhaps Mr Eschenbach should go and argue with with all those emminent Scientists instead of us LOL
Then od course there is Alberto Miatello, but he doesn’t count because he belongs to PSI.
This is getting embarrassing, look another one Charles R. Anderson, Ph.D., Physics.
Perhaps all you guys supporting Mr Eschenbach should have a rethink, don’t you?
To be fair to Willis, he seems more receptive to the idea now than he used to be unless I’ve misinterpreted his expressed wish to discuss it with us.
Do Scientists debunking AGW count?
https://arxiv.org/pdf/0707.1161v4.pdf
How about Theo Wolters, will he do?
Add M. Liu to the list.
Trick December 1, 2017 at 2:19 am
Why would I do that? I gave up on those jokers long ago, and when they tried the scam with the fake names, that was the last straw. However, reading over my posts from five years ago, I find that at one point I did know what ATE was, their so-called “Atmospheric Thermal Effect” … but obviously, I don’t try to retain such nonsense.
And Trick, if you are impressed by their equation and its ability to emulate the planetary temperatures, then you definitely should read my post entitled “The Miracle of Equation Eight“. It lays out all of the problems with their theory … I’ve written well over 500 scientific posts, and I’d totally forgotten about that one. It’s an interesting analysis, and it has stood the test of time. Not only did I show the problems with their bogus equation, I wrote and tested my own bogus equation which was both simpler and gave more accurate results than theirs. It’s just as meaningless as theirs, but it’s simpler and works better …
Finally, do you agree with N&Z that a non-GHG atmosphere, say argon, can permanently raise the temperature of a planet?
w.
I tend to agree with Willis that N & Z’s workings do not provide proof of anything for the technical reasons he stated but that does not mean they are wrong.
There is substantial evidence of a relationship between atmospheric masses and the surface temperature enhancement for various planets and moons in the solar system
The presentation by this chap provides several worked examples which are hard to ignore:
I prefer to get basic concepts and physical laws right before worrying about equations and it seems clear to me that the proposals I have put forward based on conduction and convection do provide an arguable alternative to the radiative hypothesis.
Is anyone else going to suggest a means of choosing which is more likely to be correct?
“provides several worked examples”
Here this “chap” curve fits an ideal equation with all measured data similar to N&Z (V&R) fit. There is no law against doing so but claiming curve fitting invalidates the GHE does not follow simple logic. All exoplanets can be curve fitted to the same eqn. once the data is measured but the point is to acquire enough knowledge to estimate exoplanet nature prior to having that measured data.
I don’t see that ‘chap’ as having engaged in curve fitting. He simply points out facts and gives an equation that actually works.
What no one has done other than me is to provide a conceptual description that is capable of explaining those particular facts better than the radiative theory can.
It comes down to this:
If the formation of an atmosphere causes a temporary drop in outgoing radiation then the pressure based theory is correct.
If that formation causes a temporary drop in surface temperature then the radiative theory is correct.
Which is it and why?
”I don’t see that ‘chap’ as having engaged in curve fitting.”
Then Stephen doesn’t understand curve fitting. What I mean here is the guy consulted papers (did you read them?) for information on measured planet median P(z), density(z) and he/they used the IGL to compute median T(z). So when he uses two of the parameters to back calculate the third, he is not adding anything new from the papers. And he just plots the various planets examined with the parameters chosen to show a good fit on the IGL curve like N&Z (V&R) did. No one typically measures avg. atm. density(z) in situ on a planet, it is typically found from probe (radio signal modulation) and T(z) calculated using IGL.
”Which is it and why?”
Consider the tank of water in the experiment above that should be teaching Stephen something about convection in a fluid warmed from the bottom in a gravity field.
Stephen now imagines the time of “formation of the atmosphere.” Let’s use that tank to add some climate formation realism. Imagine someone dropping in 4” diameter stones randomly different s[peeds,directions, 3” diameter iceballs even more randomly, someone sloshing the tank around, the tank glass walls and bottom still being poured from molten glass, then someone sloshes in very hot water & about 40% splashes out, all the time random temperature hot water cups are placed under the fluid on and off. THEN at some point while all this happening, the cup of hot water is placed as in the video and we are asked to answer 2 questions about the resulting convection.
Does this lab test exhibit a temporary drop in outgoing radiation or a temporary drop in surface temperature?
Alas, this experiment exhibits both answers to these questions at different times. To determine your answer, would require examination at a certain time & that is past history. No data is available. Thus, if you want to believe that there is drop in outgoing radiation or a drop in surface temperature, you may do so. No one can prove you wrong. But you cannot prove you are right. When faced with an undecidable proposition, you may believe whatever you wish. As you often do.
i) So you are suggesting that since the atmospheric parameters were calculated from probes by applying the gas laws the outcomes can safely be ignored? I don’t think so.
ii) With that tank there is no rotating sphere, no gas subject to the gas laws and no density gradient with height so that model is just silly. It is simple logic that if a flow of energy is split into two components then the first flow will drop by an amount equivalent to the second flow. Calling that ‘imagination’ is silly. One can easily resolve the answer to my rhetorical question by noting that conduction and convection work more slowly than radiation so introducing such processes can never reduce the surface to a temperature below S-B and the pressure hypothesis must be correct but if you can demonstrate otherwise then please do so.
”the outcomes can safely be ignored?”
No. What makes you write that? That the video invalidates the GHE does not follow simple logic.
The tank is on a rotating oblate spheroid. The tank contains a fluid. There is a density gradient with height. Find one for fluid called air. With particles used and find it demonstrates the same physics, no surface warming, particles return at ambient.
”..introducing such processes can never reduce the surface to a temperature below S-B..”
True all objects radiate at S-B not at a temperature below S-B. The pressure hypothesis must be correct only if you can demonstrate such in a test or observe such in nature. Until then all you have is imagination.
Until you have something new Stephen, I may not reply.
I didn’t say the outcomes from probes and the application of the gas laws could be safely ignored. I was pointing out that that was what you seemed to be saying.
As for the tank experiment it contains a liquid that does not observe the gas laws. Can you link to one that does use a gas ?
To study/test/learn about convective physics, all Stephen needs is a fluid warmed from below in a gravity field. The IGL is immaterial. Water and air are both fluids.
Willis, it is unfair to comment “nonsense” on anyone’s writing until you have read their exact words viz. “When you comment, please QUOTE THE EXACT WORDS THAT YOU ARE DISCUSSING, so that we can all understand the nature of your objections.” My memory also is not perfect and I prefer no abbreviations.
“do you agree with N&Z”…?
Those following are Willis’ words. Quote N&Z (V&R) exact words and I will keep it civil (top post also) and agree or disagree with them based on experiment and observation of nature. I’ve written my own words based on Willis’ proposition of Ar earth atm. above.
The N&Z name reversal was to demonstrate the nakedness of those who imagine their superiority. Sure worked. There have been other works showing up the same ritualistic beliefs in other ways, but also quite purposeful. The targets are expected to be the last to see the joke. Therein lies the effect.
ATE has been closely debated and tested for around a decade, maybe more, in its current debate. My own database concerning it is around 1GB. It took me on a journey into Quantum Theory and very much else. It will not go away, but nor do I mistake it for any ‘revealed truth’ that all must follow. Popper learnt his trade in my country, NZ, and I would be a fool to forget what he learnt and taught us.
heh- popper proved that nobody can prove anything – obviously a divinely revealed troof.
(i suppose i need to add an iron tag, but i was tempted to let it fly over you head again as it did the first time when popper zoomed ya.)
Trick, I linked to an entire post that I wrote about Nikolov and Zeller, which discussed their theory in detail. If that didn’t satisfy you, nothing will. I also invited you to find any flaws in my post. You have given me nothing.
Still waiting …
w.
Brett Keane December 1, 2017 at 10:26 am
If what you mean by “worked” was that it got their paper pulled from publication, got them mentioned in Retraction Watch, and exposed them to ridicule around the web, well yeah, it “sure worked” all right …
w.
Willis, your post was 2012. The 2017 paper didn’t exist back then. Quote N&Z (V&R) words from the 2017 paper being discussed per your note: “When you comment, please QUOTE THE EXACT WORDS THAT YOU ARE DISCUSSING, so that we can all understand the nature of your objections.” Also your be civil applies.
Still civilly waiting…
Trick December 1, 2017 at 7:24 pm Edit
Trick, obviously, somewhere I said the word “nonsense”. I’m sure I’ve said it lots of times. But since you have not quoted what I said, I fear you are making no sense. AFAIK I did NOT say that their 2017 paper was nonsense, because I have not read their 2017 paper. Sorry, not interested, they are puerile curve-fitters who’ve burnt their bridges with me. So … please quote whatever it was I said about “nonsense” that you are objecting to, and I’ll be happy to take a look at what I said.
In any case, it is totally unclear why you think I’m obliged to discuss a paper I haven’t read and won’t read …
w.
Willis, just yesterday you wrote specifically that a 2017 paper you haven’t read is nonsense. Then asked me a question about what they supposedly wrote using your words not theirs. Despite your top post request to always use the author’s words. The entire Willis quote is nearby:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2681969
” if you are impressed by their equation “
Which 2017 eqn.?
”do you agree with N&Z that a non-GHG atmosphere, say argon, can permanently raise the temperature of a planet?”
Where specifically do they write this in the 2017 paper? These are Willis’ words, I’ll respond to their words, point them out.
”In any case, it is totally unclear why you think I’m obliged to discuss a paper..”
You are not; you commented on the 2017 paper in your own block quote as “..such nonsense….just as meaningless as theirs..” and then asked me a question “do you agree” to something in it – so point out the something so I can answer. Simple re-re-request. Waiting.
Trick December 2, 2017 at 5:28 am
Trick, thanks for (finally) linking to what you are referring to. HOWEVER, what I actually said in the comment you linked to was as follows. YOU brought up the 2017 paper, viz:
I answered:
Please read that again, and point out to us where ANYTHING in what I said refers to the 2017 paper. I specifically said “five years ago”, which on my planet is NOT 2017. I also SPECIFICALLY SAID I DID NOT READ THEIR PAPER!!! So please, stop accusing me of claiming their 2017 paper was nonsense. I said no such thing.
Who said anything about a 2017 equation? Not me. I’m discussing their so-called “MIRACLE” equation.
Again, what is it with the 2017 paper? I have been discussing their claim that they can predict the temperature of a planet by knowing only the pressure and the solar input. If that is so, then it must work whether there are GHGs in the atmosphere or not. It’s called “a logical deduction from their claims”.
When you quote a man, don’t strip out the context. Here is what I said:
Now … am I talking about their 2017 paper there? NO. I’m talking about the paper discussed in “The Mystery of Equation Eight“.
If you had actually read “The Mystery of Equation Eight”, perhaps you might have noticed that it was written in 2012 … so your claim that I was referring to 2017 is just more of your endless BS.
RE-READ WHAT YOU QUOTED OF MY WORDS, and point out to me where it says anything about their 2017 paper. Simple re-re-request. Waiting.
w.
Willis you are being temperamental, it must be a mirthy day for you. I had written a longer response point by point but it isn’t worth posting it. Made me feel better though.
”Finally, do you agree with N&Z that a non-GHG atmosphere, say argon, can permanently raise the temperature of a planet?”
You will have to point me to where 2012 N&Z write this if you want to discuss further. My first impression is they did not write this.
It’s okay, gnomish does it fine. Some heads are close to the ground.
A C Osborn December 1, 2017 at 12:45 pm
Back to the Original Post.
Who has heard of Marc-Auguste Pictet?
Who has seen his experiment?
Yes to both.
Do you still believe Cold Objects can make Warmer objects warmer without the warm object being heated some other way?
Of course the Pictet experiment proves it.
Do you believe that the Cold Radiation from a colder object can be focused using a Mirror, just like Heat can?
Of course, I liked Rumford’s explanation of Pictet’s experiment where he used the analogy with sound:
“According to this hypothesis, cold can with no more propriety by considered the absence of heat than a low or grave sound can be considered as the absence of a higher or more acute pitch; and the admission of rays which generate cold involves no absurdity and creates no confusion of ideas.”
Comports well with our current understanding.
So based on that logic if a cold object CAN warm a continually heated warm object, CO2 at -80C can only warm the Earth’s Surface while the Sun is also warming it.
-80ºC has nothing to do with anything!
Once the Sun goes down it will not warm it at all. So at the moment no CO2 induced Warming in the UK for 16 hours per day.
Actually it will, because in its absence the incoming radiation would be from a much colder source. Be thankful for the warming 24hrs/day.
It was established at the beginning of this post that Cold Object cannot warm a warmer object UNLESS THE WARMER OBJECT IS BEING HEATED, otherwise it can only slow the cooling.
At Night the Earth is COOLING, so the radiation from a -80C CO2 Molecule is a Cooler Object.
Whic part do you not understand?
How did the Pictet experiment prove that a cold object makes a warm object colder?
It made the Thermometer colder from 16ft away by focusing the Ice’s Radiant Energy on it.
Sorry this “How did the Pictet experiment prove that a cold object makes a warm object colder?” should have been
How did the Pictet experiment prove that a cold object makes a warm object warmer?
It made the Thermometer colder from 16ft away by focusing the Ice’s Radiant Energy on it.
AC says: “How did the Pictet experiment prove that a cold object makes a warm object colder?”
The analogy here would be if Pictet started with dry ice at the focus of the mirror, then switched to regular ice @ 0C. With the dry ice, the object at the focus of the other mirror would cool significantly (until some balance was achieved between heat in from the room and heat out to the dry ice). Perhaps that object would cool to 10C in a 20 C room. If the dry ice was replaced with regular ice, the object would warm (perhaps from 10 C to 15 C) — even though the object was already above the temperature of the ice! ‘
The radiation from the cold object (ice) makes the warm object (at the focus of the second mirror) warmer than it would have been with the radiation from the even colder object (dry ice).
The radiation from the cold object (the atmosphere) makes the warm object (the surface) warmer than it would have been with the radiation from the even colder object (the 3 K background radiation).
Tim Folkerts December 1, 2017 at 4:44 pm
AC says: “How did the Pictet experiment prove that a cold object makes a warm object
colderwarmer?”The analogy here would be if Pictet started with dry ice at the focus of the mirror, then switched to regular ice @ 0C. With the dry ice, the object at the focus of the other mirror would cool significantly (until some balance was achieved between heat in from the room and heat out to the dry ice). Perhaps that object would cool to 10C in a 20 C room. If the dry ice was replaced with regular ice, the object would warm (perhaps from 10 C to 15 C) — even though the object was already above the temperature of the ice!
Exactly, Pictet’s variation on the experiment did that. When he started with snow at the one focus to which Nitric acid had been added (colder than 0ºC) he achieved a temperature about 5ºC colder than if snow itself was used. Thus if the cold temperature is increased the thermometer recorded a higher temperature, this is exactly what happens when the cold background of space is replaced by the warmer atmosphere.
The radiation from the cold object (ice) makes the warm object (at the focus of the second mirror) warmer than it would have been with the radiation from the even colder object (dry ice).
The radiation from the cold object (the atmosphere) makes the warm object (the surface) warmer than it would have been with the radiation from the even colder object (the 3 K background radiation).
A C Osborn December 1, 2017 at 3:30 pm
It was established at the beginning of this post that Cold Object cannot warm a warmer object UNLESS THE WARMER OBJECT IS BEING HEATED, otherwise it can only slow the cooling.
At Night the Earth is COOLING, so the radiation from a -80C CO2 Molecule is a Cooler Object.
Whic part do you not understand?
Where you get the -80ºC CO2 from!
If you need to ask you do not even understand the Composition of the Atmosphere and where the CO2 window is that can absorb LWIR.
AC, if you are implying (as you indeed seem to be!) that the 15 um wavelength of radiation of CO2 implies that the radiation is “-80 C” based on Wein’s Law, then you have only a rudimentary understanding of thermal radiation! Wein’s law only gives the peak radiation for a blackbody.
Tim, I am merely quoting others, so please provide me with the actual temperature, if it is below Zero, or even below the Temperature of the Earth’s Surface then the same rule applies.
Back to the Original Post.
Who has heard of Marc-Auguste Pictet?
Who has seen his experiment?
Do you still believe Cold Objects can make Warmer objects warmer without the warm object being heated some other way?
Do you believe that the Cold Radiation from a colder object can be focused using a Mirror, just like Heat can?
So based on that logic if a cold object CAN warm a continually heated warm object, CO2 at -80C can only warm the Earth’s Surface while the Sun is also warming it.
Once the Sun goes down it will not warm it at all. So at the moment no CO2 induced Warming in the UK for 16 hours per day.
A C Osborn wrote, “Do you believe that the Cold Radiation from a colder object can be focused using a Mirror, just like Heat can?”
There’s no such thing as “cold radiation.” Radiation does not have temperature.
Perhaps you are confused because your light bulbs are specified with a “color temperature.” That doesn’t mean the light from the light bulb is any particular temperature, it is just a rough indication of the spectral distribution of the light which that bulb produces. It means that the light is supposed to LOOK similar to the human eye to light which WOULD be emitted by a black body of that temperature. Here’s a pretty good article:
http://www.soundandvision.com/content/led-vs-cfl-bulbs-color-temp-light-spectrum-and-more
Note that LED light bulbs have actual temperatures much lower than incandescent bulbs, but usually have a higher “color temperature.” That is possible because LED bulbs (unlike incandescent bulbs) are not at all like back-body emitters.
GHGs are nothing like black-bodies, either.
GHGs are colorants, because they change the absorption spectrum of the atmosphere. That is, they change its “color,” albeit in the far infrared part of the EM spectrum, rather than in the visible part. If you don’t think a color change can affect temperature then take your shoes off while standing on a light-colored sidewalk on a hot summer day, and then step, in bare feet, onto a black asphalt parking lot. That will certainly clear up your misconception!
A C Osborn wrote, “Do you still believe Cold Objects can make Warmer objects warmer without the warm object being heated some other way?”
Practically speaking, warmer objects are always being heated “in some other way.” Otherwise they would cool continually, and eventually become very, very cold, indeed.
But even it such a hypothetical case, if you were to wrap the object in a Space Blanket it would cool more slowly.
 
A C Osborn wrote, “…CO2 at -80C can only warm the Earth’s Surface while the Sun is also warming it.
Once the Sun goes down it will not warm it at all. So at the moment no CO2 induced Warming in the UK for 16 hours per day.”
Wrong. Most of the radiation which CO2 absorbs comes from the Earth. So it does, indeed, help make the surface a bit warmer even at night.
The first question I asked you is have you heard of “Marc-Auguste Pictet?”
And the second question was have you seen his experiment?
I suggest you take a look.
As to your answers, you obviously did NOT read what I said.
The whole point of this post is that if photons hit an object that is being constantly heated it will make it warmer (unless it is Argon Gas according to our Poster).
The Earth’s Surface is the object receiving sunlight, so photons from a colder object can warm it.
When the sunlight stops the heating stops, now the COOLING Earth’s surface can only have it’s Cooling Slowed, which is not Heating. This point was established at the begining of this post.
If the sun goes out will the Earth not ” cool continually, and eventually become very, very cold, indeed.”
Dave says: “Radiation does not have temperature.”
Thermal radiation DOES have a temperature — the temperature of the object that emitted it. This is most obvious with blackbody radiation but applies to non-black-bodies as well. Among other things, this mean that thermal radiation inside a 3000 K furnace cannot be focused to warm anything inside the furnace above 3000K, just like the thermal IR in a 300 K room cannot be focused to warm anything inside the room above 300 K. No fancy mirrors or lenses or filters can get around these limits — or the 2nd Law of Thermodynamics could be violated!
Tim, that’s wrong.
“Thermal radiation” is simply radiation emitted from a hot object or substance. It is emitted at a spectrum of wavelengths, which are determined by a combination of several factors, including temperature, and the physical characteristics of the object. Analysis of that emission spectrum can give clues to the temperature, which is how those no-touch IR thermometers work. It can also give clues about the object’s composition, which is how emission spectroscopy works.
However, the radiation, itself, has no temperature. There is no such thing as “20 C radiation.”
If you filter radiation from a hot source, to eliminate or attenuate some of the wavelengths, you’ll get a different spectrum, which might “fool” an IR thermometer, by mimicking the emissions of a blackbody at a different temperature.
That radiation will warm anything that absorbs it, regardless of the temperature of the emitter or the temperature of the absorber. There’s no little tag attached to a 15µ photon which says what the temperature was of the source that emitted it. That source could have been at 20°C or 20,000°C, there’s no way to tell, and it simply doesn’t matter.
Inference of an emitting object’s temperature from the spectrum of its emissions is basically a statistical exercise, like repeating an experiment many times to discovery the likelihood of various outcomes, but with a very large number of experiments (photons). But if you filter the radiation, e.g., though a tinted glass, or a prism, or a diffraction grating, it’s like throwing out undesired experimental outcomes, so you’ll draw incorrect inferences from it.
Here’s an example of that thought-experiment, with an object of unknown temperature emitting light, which is filtered by wavelength, and two sensors, sampling different slivers of the object’s emission spectrum
http://sealevel.info/temperature_through_prism.png
Do you think Sensor A can correctly determine the temperature of the light source?
Do you think Sensor B can correctly determine the temperature of the light source?
Do you think that if the two sensors tried to deduce a temperature they would deduce the same temperature?
Do you think that varying the temperature of the object would change the temperatures deduced by the two sensors?
Mr Burton, I am shocked do have no concept of Power & Energy?
Let me ask you
1 Would you be prepared to sit in a room being bathed in White Light Radiation from a Bulb?
2 Would you be prepared to sit in a room being bathed in Radiation from an IR Heater?
3 Would you be prepared to sit in a room being bathed in Ultra Violet Radiation?
4 Would you be prepared to sit in a room being bathed in X Ray Radiation?
5 Would you be prepared to sit in a room being bathed in Gamma Radiation?
6 Would you be prepared to sit in a room being bathed in Beta Radiation?
7 Would you be prepared to sit in a room being bathed in Neutron Radiation?
Dave, I think we are looking at two sides of the same coin.
Thermal radiation from a blackbody (or cavity) does indeed have a temperature. There is a thing called a “photon gas” with temperature and pressure and entropy, etc. Thermodynamics and statistical mechanics still applies to photons. https://en.wikipedia.org/wiki/Photon_gas.
“That radiation will warm anything that absorbs it, regardless of the temperature of the emitter or the temperature of the absorber. There’s no little tag attached to a 15µ photon which says what the temperature was of the source that emitted it. That source could have been at 20°C or 20,000°C, there’s no way to tell, and it simply doesn’t matter.”
Planck’s Law gives an upper limit on the intensity of radiation at any frequency (the spectral irradiance). No amount of filtering or focusing can make the thermal radiation from a cooler source as bright as the radiation from a warmer blackbody source. At 1 um, 5000K blackbody thermal radiation has a spectral radiance of ~ 7 kW / (sr*m^2*nm). 4000 K radiation is about 3.5 kW / (sr*m^2*nm). 3000 K radiation is about 1 kW / (sr*m^2*nm). No filter or focusing or different radiating surface can generate 7 kW / (sr*m^2*nm) from a 3000 K sources. If the intensity is 7 kW / (sr*m^2*nm), the surface must be (at least) 5000 K. Filtering and focusing cannot fool this sort of measurement into think bright light came from a cool source.
Thus the NET effect of ALL 15 um photons does have some limitations. No matter the sizes or shapes, no matter the materials or filters, no matter the mirrors or lenses — the net flow of 15um photons will always be from warmer to cooler. So there is no way (without other energy sources) to use the radiation from a 20 C object to raise the temperature of a 30 C object.
PS Astronomers routinely use a system like your Sensor A/Sensor B to find the temperature of stars. https://en.wikipedia.org/wiki/Color_index
Time’s up. The answers to the four questions are: No, No, No, No.
Tim Folkerts wrote, ” So there is no way (without other energy sources) to use the radiation from a 20 C object to raise the temperature of a 30 C object.”
There are always other energy sources. Otherwise it wouldn’t stay at 30°C.
Think about a 30°C “object” floating in the dark in deep, deep interstellar space, with no incoming radiation, but with enough of a radioisotopic heat source to maintain its temperature at a steady 30°C. (Of course the output of real radioisotopic heaters very slowly drops, as the radioisotope decays, but let’s pretend ours is steady.)
Now, if, instead of putting your object in the dark in interstellar space, you put the same object, with the same radioisotopic heat source, in a 20°C vacuum bottle container (with some sort of thermostatic control to keep the container at exactly 20°C), the “30°C” object will heat up very dramatically. In fact, it will become an 81.something°C object.
See if you can figure out how to calculate the new equilibrium temperature of the formerly-30°C object. I’ve given you the first two digits of the answer (81). See if you can figure out the next two digits.
Dave, I think we are talking past each other. You are focusing on certain details, while I am focusing on others.
“There are always other energy sources. Otherwise it wouldn’t stay at 30°C.”
First, I never claimed the object was staying at 30 C — only that it happened to be at 30 C at that particular moment. Second, it is quite possible for thermal radiation to be the only source of energy. Put the object inside an evacuated box where the walls are 20C.
My point is that any object inside the box will equilibrate to 20 C because it equilibrates with the *radiation* emitted by the walls. The radiation has a temperature of 20 C. There is no way around this. There is also no way for the object inside to equilibrate to any other temperature using passive devices to focus/reflect/filter the 20 C thermal IR inside the chamber. Fancy filters designed to ‘trick’ an IR thermometer cannot make the object warmer or cooler than 20 C once equilibrium is reached.
Also, measuring the intensity of the radiation inside the box (or the radiation coming from a blackbody surface) at any single wavelength is sufficient to determine the temperature. This radiation will follow the curve predicted by Planck’s Law. Here measuring the spectral irradiance at a single wavelength with “Sensor A” will indeed tell you the temperature.
“Now, if, instead of putting your object in the dark in interstellar space, you put the same object, with the same radioisotopic heat source, in a 20°C vacuum bottle container (with some sort of thermostatic control to keep the container at exactly 20°C), the “30°C” object will heat up very dramatically. In fact, it will become an 81.something°C object.”
Dave, it is pretty easy to do those calculations (assuming blackbody surfaces, as you seem to have done). It’s 81.56 C. Of course, any typical ‘vacuum bottle’ will have reflective walls with an emissivity of ~ 0 rather than ~ 1, so the heated object inside would get MUCH warmer than 81.56 C.
I wrote, “There are always other energy sources. Otherwise it wouldn’t stay at 30°C.”
Tim replied, “First, I never claimed the object was staying at 30 C — only that it happened to be at 30 C at that particular moment…”
Oh, come on. If there were no source of energy at all, it would equilibrate (well, asymtopically approach) 0 K. In the real world there are always other energy sources. How did your object get to 30°C?
I also wrote, “Now, if, instead of putting your object in the dark in interstellar space, you put the same object, with the same radioisotopic heat source, in a 20°C vacuum bottle container (with some sort of thermostatic control to keep the container at exactly 20°C), the “30°C” object will heat up very dramatically. In fact, it will become an 81.something°C object.”
Tim did the calculation correctly, and replied, “…(assuming blackbody surfaces, as you seem to have done). It’s 81.56 C. Of course, any typical ‘vacuum bottle’ will have reflective walls… so the heated object inside would get MUCH warmer than 81.56 C.”
Correct! Without reflective walls it would be 81.56°C, and if some of the object’s own radiation is reflected back to it then its temperature would rise even higher.
And so you have seen that the presence of a nearby (surrounding) 20°C object would raise the temperature of the formerly-stable-at-30°C object by more than 50 degrees!
In just the same way, warming the Earth’s chilly atmosphere can warm the already-warmer ground. Please explain this to A C Osborn et al!
Tim also wrote, “Put the object inside an evacuated box where the walls are 20C. … any object inside the box will equilibrate to 20 C because it equilibrates with the *radiation* emitted by the walls.”
Only if it has no other energy source. Such an object (with no other source of energy) would drop to near 0 K if it were in deep, deep interstellar space instead of in your 20°C box.
But restore the radioisotopic heater to the object that I described (the radioisotopic heater which puts out enough energy to keep the object at 30 °C), and put that object into your 20 °C box, and the object’s temperature will rise to 81.56 °C (or, as you correctly noted, even higher, if the box has reflective walls).
But then Tim wrote, The radiation has a temperature of 20 C. There is no way around this.”
Nooooo! Wrong! And you were doing so well. 😞
The radiation from the 20 °C box has no temperature. It is as readily absorbed by a 30 °C object or an 81°C object as it is by a 15°C object, and it has exactly the same effect: it warms them.
And Tim wrote, “Also, measuring the intensity of the radiation inside the box (or the radiation coming from a blackbody surface) at any single wavelength is sufficient to determine the temperature. This radiation will follow the curve predicted by Planck’s Law. Here measuring the spectral irradiance at a single wavelength with “Sensor A” will indeed tell you the temperature.”
Only you know the distance to the object, and only if the object is a true blackbody… and there are no true blackbodies, only approximations.
I wrote, “Only you know the distance to the object, and only if the object is a true blackbody…”
I should have added, “, and only if you don’t do something tricky, like put the object at the focal point of a parabolic reflector.”
Dave, we are getting pretty far afield. And I really do agree with much of what you are writing. My my difficulty is hwo you keep insisting on specific conditions that are different from what I am discussing – and then saying that disproves my point.
For example, I say ” it is quite possible for thermal radiation to be the only source of energy. ” You reply “If there were no source of energy at all, it would equilibrate (well, asymtopically approach) 0 K. ” You get rid of the thermal radiation and then claim a different temperature — which of course is possible without the thermal radiation that was posited.
Or my 30 C object was simply any old object that happened to be 30 C. Your 30 C object became a blackbody with a built-in heater that would maintain 30 C when in deep space far from any other heat sources.
———————–
As for the radiation within the evacuated chamber, that cavity radiation is basically perfect blackbody radiation. The intensity of the thermal radiation is independent of the size of the cavity or the distance from the walls to the detector. Furthermore, your fancy parabolic reflector will STILL not change this intensity (assuming the reflector has also been allowed to come to the temperature of the walls of hte chamber). That parabolic reflector will NOT change the temperature of the photon gas and will NOT change the temperture of any object placed at the focus.
———————–
But yes, I agree that “In just the same way, warming the Earth’s chilly atmosphere can warm the already-warmer ground. ” When there is an independently heated object with some relatively steady power supply (like the earth or your radioactive 30 block), then raising the temperature of hte surroundings will raise the temperature of the original, warmer object.
THIS IS THE IMPORTANT POINT as far as global warming goes. All the rest of what we are discussing is more esoteric phsyics. 🙂
Tom, what if the “surroundings” is a non-linear, chaotic system with huge forcings varying at all time scales? What if the minuscule increase in the temperature of the dynamic system caused directly by a minor gas is unmeasurable?
What if temperature measurements of the “surroundings” do not comport with assumed forced warming?
I think the above questions are your: “THIS IS THE IMPORTANT POINT as far as global warming goes.”
Tim Folkerts wrote, But yes, I agree that “In just the same way, warming the Earth’s chilly atmosphere can warm the already-warmer ground. ” When there is an independently heated object with some relatively steady power supply (like the earth or your radioactive 30 block), then raising the temperature of hte surroundings will raise the temperature of the original, warmer object. / THIS IS THE IMPORTANT POINT as far as global warming goes. All the rest of what we are discussing is more esoteric phsyics. :-)”
True, and of course it means the Slayers are all wrong.
Tim also wrote, “As for the radiation within the evacuated chamber, that cavity radiation is basically perfect blackbody radiation. The intensity of the thermal radiation is independent of the size of the cavity or the distance from the walls to the detector. Furthermore, your fancy parabolic reflector will STILL not change this intensity (assuming the reflector has also been allowed to come to the temperature of the walls of hte chamber). That parabolic reflector will NOT change the temperature of the photon gas and will NOT change the temperture of any object placed at the focus.”
I think there’s a misunderstanding about what I was talking about. The question is, can Sensor A tell what temperature the source object is?
http://sealevel.info/temperature_through_prism_with_parabolic_reflector.png
The answer is No, it cannot. Sensor A has only one clue about the temperature of the source: the intensity of the narrow band of orange light which the sensor receives. That intensity is affected by several factors, including the temperature of the source, the composition of the source, the size of the source, the distance of the source from the prism & sensor, and the existence of the parabolic reflector. All of those factors affect the intensity of the orange light which the sensor receives, and the sensor cannot tell them apart.
Adding the parabolic reflector increases the intensity of the orange light. Reducing the object’s temperature decreases the intensity of the orange light. Doing both could either increase or decrease the intensity, or leave the intensity unchanged. There’s no way for Sensor A to tell what has happened to the temperature merely from the intensity of the orange light, which is the only clue it has.
And there’s no such thing as “photon gas,” and photons have no temperature.
Dave a couple quick comments.
1) 88,000 hits for “photon gas” say you are wrong about the existence of photon gases. Some of the first links are to places like Nature magazine, Wikipedia, and several major research universities. If you dislike the idea of a “photon gas”, take it up with them.
2) Suppose I have a narrow band pass filter on my camera and take a picture of the sun. The pixels for the image of the sun will show some intensity — related to the brightness and temperature of the sun. A telephoto lens or a mirror will make MORE pixels bright, but will not make a specific pixel brighter. As such, the brightness of any pixel that is aimed at the sun can provide an estimate of the temperature of the sun. Certainly measure multiple wavelengths gives a better temperature estimate. Certainly anything between the sensor and the source can make the results inaccurate. Certainly tiny sources like stars that are too small to fully illuminate a pixel won’t measure accurately. But in principle, a single wavelength can tell you the temperature of a blackbody surface.
Tim wrote, “88,000 hits for “photon gas” say you are wrong about the existence of photon gases.”
I stand corrected, and thank you.
Tim also wrote, “2) Suppose I have a narrow band pass filter on my camera and take a picture of the sun. The pixels for the image of the sun will show some intensity — related to the brightness and temperature of the sun. A telephoto lens or a mirror will make MORE pixels bright, but will not make a specific pixel brighter.”
Assuming that your telephoto lens is the same aperture as the regular lens, it will make the originally lit pixels dimmer. But what’s your point?
Tim continued, “As such, the brightness of any pixel that is aimed at the sun can provide an estimate of the temperature of the sun.”
No, it can’t. It tells you only the intensity of the light which is falling on that camera pixel, which depends on many factors, including your distance from the Sun, the aperture of your lens, the focal length / size of the focused solar image, the clarity of you filter & lens, etc.
Tim continued, “But in principle, a single wavelength can tell you the temperature of a blackbody surface.”
No, it cannot.
Of course the object doesn’t necessarily resemble a blackbody, and certainly is not a perfect blackbody. But that actually doesn’t matter, in this case, because we’ve filtered out all but the orange light. Sensor A cannot possibly deduce the temperature of the emitting object merely from the intensity of the orange light which it emits.
“Do you believe that the Cold Radiation from a colder object can be focused using a Mirror, just like Heat can?”
Well any sort of EM radiation can be reflected and focused – lasers and radio waves and x-rays and thermal IR. So yes, “cold radiation” (thermal radiation from cold objects) can indeed be reflected and focused.
However, some care needs to be taken with your phrase “just like”. There are limits to how well thermal IR can be focused. For example, in a room at 20 C, a flat surface would receive about 400 W/m^2 of incoming thermal IR. With mirrors and lenses, you could focus this thermal IR to provide a maximum of …. still 400 W/m^2 of thermal IR! But 400 W/m^2 of sunlight could be focused to provide 1000’s of W/m^2 of incoming power.
Conversely, if your room was mostly at 20 C but a small portion was @ 0 C and radiating @ 315 W/m^2, then you could uses mirrors to block out the 20 C radiation and “focus” just on the 0C region so that only 315 W/m^2 radiation arrived at a surface (allowing the surface to cool well below 20 C). This is basically what Pictet’s experiment did.
Tim, Mr Eshenbach says it can’t be done for diffuse Radiation, would yor small portion be diffuse radiation or not?
I have just re-read the crap that comes from Mr Burton to make his point.
First of all he replaces a Heat sink at 3.5K with another heat sink at 293.15K and not only that but this new heat sink is not allowed to re-radiate at the same rate as the original Object it is receiving heat from and has to maintain a temperature 293.15K.
So he has introduced some kind of new Energy source in to the equation to keep the bottle cold.
And has the nerve to say “In just the same way, warming the Earth’s chilly atmosphere can warm the already-warmer ground. Please explain this to A C Osborn et al!”
Absolutely Typical.
We now have a few CO2 Molecules that do not re-radiate out to space, there is nothing between them and the Earth to absorb their energy before it gets to the Earth and they are no longer surrounded by any gases removing their Excess energy by collisions.
A C Osborn, I have no idea what you’re talking about. I said nothing about a “Heat sink at 3.5K.” In fact, I didn’t say anything about any heatsinks, nor about anything at 3.5K. None of what you wrote makes any sense.
If there’s something I wrote that doesn’t make sense to you, please quote it, or link to it, so I’ll know what you’re talking about.
And why don’t you see if you can do the calculation that Tim did, and figure out the next digit of the equilibrium temperature of the formerly-30°C object (the digit after “81.56”), assuming that the 20°C surrounding container reflects none of the formerly-30°C object’s radiation back to the formerly-30°C object.
I didn’t say the outcomes from probes and the application of the gas laws could be safely ignored. I was pointing out that that was what you seemed to be saying.
As for the tank experiment it contains a liquid that does not observe the gas laws. Can you link to one that does use a gas ?
Mr Eschenbach, would you like me to list the many many Scientific pieces of literature and courses that say exactly the same thing as that Wiki Quoute?
All matter above absolute Zero emit Thermal Radiation and it has nothing to to do with Molecules and all to do with Excited Atoms.
So do want the list or not, if so how many before you are convinced?
Here is just the Dictionary version
thermal radiation
See more synonyms on Thesaurus.com
noun, Thermodynamics.
1.
electromagnetic radiation emitted by all matter above a temperature of absolute zero because of the thermal motion of atomic particles.
A C Osborn December 2, 2017 at 4:34 am Edit
Someone here linked to the actual NIST spectrum for argon. Go study it.
Yes, there are lots of claims that “every object” radiates, which is 100% true for the common case in which people are referring to SOLID objects. And you can indeed find that statement all over the web.
However, it is not true for monatomic gases, AS THE LINK TO THE ACTUAL MEASURED SPECTRUM OF ARGON DEMONSTRATES CONCLUSIVELY.
And if you don’t see that in the NIST results, then you don’t know what you’re talking about, and I’ll bow out.
w.
A C Osborn December 1, 2017 at 3:10 pm Edit
No wonder you have trouble with scientific concepts, you’re getting your information from Wikipedia. You do realize that ANYONE IN THE WORLD could have written that sentence on Wikipedia, right? Including a 16-year old high school student? Because that is where I first heard that claim, in high school … and like you, I believed it. Until I found out how IR absorption happens and learned about monatomic gases.
Yes, it is widely believed, as you believe, that IR emission applies to everything. You can find that claim lots of places … but not in the spectroscopic lab.
Read the link to lab results that Phil gave above. It clearly shows that there are NO absorption bands for argon in the thermal IR range. NONE.
Sheesh … yes, A C, all SOLIDS emit thermal radiation, because all of them have molecular bonds between the atoms that can bend and flex. That’s how thermal IR is absorbed. The energy in the photon is converted into mechanical energy of the flexing of the bonds.
But monatomic gases (helium, neon, argon, etc.) have no molecular bonds. None. So they cannot either absorb or emit IR.
w.
Willis: “It clearly shows that there are NO absorption bands for argon in the thermal IR range. NONE.”
Click on the “strong lines” tab to find the absorption bands for argon in the thermal IR range.
Trick, that is not the same thing. These emission are for Argon that has been excited to higher levels (for example, in a gas discharge tube) and then falls back to some intermediate level. These lines are not available to cool Argon in the ground state.
It is much like the Pfund series or Humphreys Series for atomic hydrogen. Only very hot hydrogen (or hydrogen that has undergone an energetic collision) will emit such radiation. https://en.wikipedia.org/wiki/Hydrogen_spectral_series
Ar ref. please. The wiki page is only for hydrogen. The only ref. NIST has is G. Norlén, Phys. Scr. 8, 249 (1973). I’ll challenge my local college librarian to track it down. I have learned they like these, get a chance to practice their trade. Again, the only way that steady state equilibrium can be obtained in Willis’ challenge is for Ar to shift its emission spectrum into regions of nonzero emissivity. It doesn’t seem like nature should abhor a pure Ar atm., should be able to handle it.
Trick December 1, 2017 at 4:08 pm
Willis: “It clearly shows that there are NO absorption bands for argon in the thermal IR range. NONE.”
Click on the “strong lines” tab to find the absorption bands for argon in the thermal IR range.
Those are emission lines when you pass current through the Ar thereby electronically exciting it up that ladder of levels that I gave you, then it decays from one level to another emitting those wavelengths. Heat up the Ar so it’s not in the ground state then you could get some absorption in those bands but it would take ~2000ºC which is not what we’re talking about here.
Here’s the spectrum from an electronic discharge tube containing Argon.
Phil. 6:27pm, your NIST link does not have this detail. It merely references a 1973 paper. Perhaps you have a better link to an experiment that determines the spectral emissivity of Ar at earth atm. STP. Until then all I can do is look up the 1973 paper. Perhaps there are no tests for spectral emissivity of Ar at earth STP so that no one can ref. an experiment to form a reasoned response to Willis’ questions.
Trick December 1, 2017 at 7:31 pm
Phil. 6:27pm, your NIST link does not have this detail. It merely references a 1973 paper. Perhaps you have a better link to an experiment that determines the spectral emissivity of Ar at earth atm. STP. Until then all I can do is look up the 1973 paper. Perhaps there are no tests for spectral emissivity of Ar at earth STP so that no one can ref. an experiment to form a reasoned response to Willis’ questions.
There are no experiments for spectral emissivity of Ar at earth STP because there is no emission so there’s nothing that can be measured, it’s all in the ground state. When I used my FTIR spectrometer in my lab I had to take account of any background CO2 in the air, I didn’t have to do that for Argon because no lines exist.
“There are no experiments for spectral emissivity of Ar at earth STP..”
Every object radiates at all temperatures, all frequencies all the time, there are no exceptions. If Ar STP emissivity can’t be or hasn’t been measured by today’s instruments who knows about tomorrow’s. If there are no experiments, then any answer to Willis Ar atm. question is speculative at best, not borne out by test.
The cooling of a 1atm., 255K Ar transparent container in deep space would occur whether we can measure the Ar emissivity or not just like the atm. around Willis’ proposition.
Trick December 2, 2017 at 5:36 am
“There are no experiments for spectral emissivity of Ar at earth STP..”
Every object radiates at all temperatures, all frequencies all the time, there are no exceptions.
That’s your fundamental mistake, it’s not true, no gases do that for example.
If Ar STP emissivity can’t be or hasn’t been measured by today’s instruments who knows about tomorrow’s. If there are no experiments, then any answer to Willis Ar atm. question is speculative at best, not borne out by test.
There are experiments all the time, anytime anyone measures the emissions from air at STP, the emissions from Ar don’t exist. This is well known and the reason for it is well known, the measured energy levels for the Argon atoms have been measured and I gave you the data above. The lowest energy level is so far above the ground state that there is no chance of it being populated at STP. Take the Boltzmann distribution, the fraction of a gas that will occupy that excited state (or above) when at a temperature of 300K is ~2×10^-185, (there are only about 10^80 atoms in the universe)!
The cooling of a 1atm., 255K Ar transparent container in deep space would occur whether we can measure the Ar emissivity or not just like the atm. around Willis’ proposition.
Yes it will cool down because the container will emit to space, not the contents.
“There are experiments all the time, anytime anyone measures the emissions from air at STP, the emissions from Ar don’t exist.”
I’ve asked repeatedly for a cite to all these experiments Phil. So far nothing, no response except NIST which shows plenty of lines in Ar in air in IR band from 1973 testing. Just post up, cite your best published experiment confirming your comment, I’ll dig it up at college library if can’t find it online. I found the NIST ref. 73 today.
What is the energy needed to go from atomic Ar rotational base energy to the first excited quantized level for instance? Commonly for spinning constituents of air in meteorology applications this is about 1/3 kT with quantized spacing order of kT.
Ok, dispense with the container. Let the 255K Ar sample float free in space, large enough sample held together long enough by gravity say as large as Earth atm. Are you saying Ar won’t radiatively cool for the life of the universe? Universe heat death occurs and the Ar will still be 255K?
“That’s your fundamental mistake, it’s not true, no gases do that for example.”
Phil. there is a miscommunication going on here, the atm. radiation text I use Bohren 2006 does not agree with you (and his earlier one 1998). Possibly if we ask enough questions eventually get down to why you so vehemently disagree with the text.
All solids, all liquids, all gases, all plasmas emit at all frequencies at all temperatures all the time. The Planck radiance formula is never zero at any temperature and at any frequency. Whatever primoridial Ar existed at the temperatures of the big bang, it must still be at that temperature (or maybe a lower one above some cutoff) if Ar at STP cannot radiate or absorb according to you. My first impression was you really meant instruments aren’t sensitive enough, not sure now.
There is a big disconnect here and we ought to be able to find it. My interpretation is for the Ar atm. of Willis emissivity just shifts to regions for which the gas can emit.
Phil. this comment of yours might be a starting point to ask questions to iterate to reason for disconnect.
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2682654
Tim writes: “These emission are for Argon that has been excited to higher levels..”
The NIST ref. 73 informs that the testing pressure for the lines they report was at 0.2torr. This is LOT less than STP. It also says a current of 10mA was used, at 120v (which it doesn’t say) that is little over 1 watt. At STP, the global energy balances all show order of 100x this watt level. This seems to conflict with your 2000C electronic discharge tube example wattage, how does 1W get to a need for 2000C?
You write “Heat up the Ar so it’s not in the ground state…2000C “ yet Bohren writes to go up from base to 1st rotational quantum level for typical STP atomic constituent is 1/3kT (1998 p. 119), this is 1.1*10^-21 J. Hardly 2000C. Why?
Does this create a route to start to connect?
Trick, the Boltzmann constant is k = 8.6e-5 eV/K, or conversely that would be 11,600 K/eV.
* Since accelerating an electron through 120 V would give an electron up 120 eV of energy, that corresponds to about 1,400,000 K!
* the binding energy for most valence electrons is on the order of 10 eV, so 120 V is more than enough to ionize them!
* room temperature is about 0.025 eV, so thermal energies are typically insufficient to excite electrons to higher level.
However you look at it, 120 V is a LOT of energy and corresponds to very high temperatures! This can easily excite electrons to higher orbitals or ionize atoms completely — in ways that 300 K thermal vibrations never can.
Tim, what causes you to bring up electronic base level to 1st excited level energy? The 2000C electronic discharge tube? Yes, that is a diversion, way more energy ~100kT level jump not order of ~1/3kT typical for atomic rotationals (spinning), similar the collisional energy available in troposphere. Agree, no or very rare electronic transitions in Earth troposphere, don’t need to discuss those, atm. is not an electronic discharge tube. Trying to connect the dots on the atm. constituent rotational quantum jumps as in most common emission/absoption of photons in the troposphere.
Trick December 2, 2017 at 3:51 pm
“There are experiments all the time, anytime anyone measures the emissions from air at STP, the emissions from Ar don’t exist.”
I’ve asked repeatedly for a cite to all these experiments Phil. So far nothing, no response except NIST which shows plenty of lines in Ar in air in IR band from 1973 testing. Just post up, cite your best published experiment confirming your comment, I’ll dig it up at college library if can’t find it online. I found the NIST ref. 73 today.
The NIST report shows no emission lines from Ar in air at STP, it shows lines emitted from Ar in an electronic discharge tube. Such lines do not exist in the absence of such excitation.
What is the energy needed to go from atomic Ar rotational base energy to the first excited quantized level for instance? Commonly for spinning constituents of air in meteorology applications this is about 1/3 kT with quantized spacing order of kT.
You appear to have some severe misconceptions about the physical chemistry involved.
Molecules have three different modes of excitation:
Electronic, in which electrons occupy different energy levels, these are separated by the most energy and require UV light to excite them.
Vibrational, in which the molecular bonds vibrate and if the bond has a dipole can be excited by light. These exist as substructure to the electronic levels and are much more closely separated and so are excited by IR radiation.
Rotational, in which the molecule rotates and if the molecule has a dipole can be excited by light. These exist as substructure to the vibrational levels and are much more closely separated and so are excited by microwave radiation.
In all of these cases absorption will only occur if the incident light energy exactly matches the separation of two of the states. When emission occurs from an excited state to another lower state then the wavelength of the emitted light will exactly correspond to the separation between the states. Consequently there will be a discrete set of wavelengths emitted, the gas will not “radiate at all temperatures, all frequencies all the time”.
Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.
Consequently your question: “What is the energy needed to go from atomic Ar rotational base energy to the first excited quantized level for instance?”, makes no sense.
As the NIST site I showed you says the electronic ground state is separated from the first excited state by 93143.7653 cm-1 (in the far UV, separation over 1000kJ/mole).
Your statement: “Commonly for spinning constituents of air in meteorology applications this is about 1/3 kT with quantized spacing order of kT”, refers to rotational levels which Argon does not have.
Phil.: “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.”
Ok, this would be where you and Bohren differ, he writes ALL atoms have rotational states that are quantized without exception. So, thanks for that I have some reading to do. He is very good at citing source material.
The NIST ref. uses so much specialist wording I would have to spend time learning the lingo if it confirms this or not. You are then writing the spinning argon atoms do so as smoothly as they translate, that there is no quantum separation in rotational energy states as found in (all?) other atoms and molecules. Photons are not absorbed/emitted as rotational quanta in atomic Ar. Interesting. This strikes me as odd and not covered in Bohren’s work that I have found as Ar is not that important in meteorology. I may be interested enough to dig into this further.
“…it shows lines emitted from Ar in an electronic discharge tube.”
The NIST report uses an etalon to excite Ar in air at collisional energy levels MUCH lower than those found in the atmosphere. If it is only Ar electronic states being excited above base state by light quanta then possibly those electronic states for Ar can also be energized with the energy available in the lower atm. unlike the more prevalent constituents. Since you say these have not been found then they could be too feeble for modern instruments (which Bohren mentions) or the researchers weren’t looking for them.
Willis’ question excites more profound discussions than (possibly) expected.
Trick December 2, 2017 at 6:16 pm
Tim, what causes you to bring up electronic base level to 1st excited level energy? The 2000C electronic discharge tube? Yes, that is a diversion, way more energy ~100kT level jump not order of ~1/3kT typical for atomic rotationals (spinning), similar the collisional energy available in troposphere. Agree, no or very rare electronic transitions in Earth troposphere, don’t need to discuss those, atm. is not an electronic discharge tube. Trying to connect the dots on the atm. constituent rotational quantum jumps as in most common emission/absoption of photons in the troposphere.
It is not a diversion, Ar atoms don’t have rotational levels the 93,000 cm-1 ‘jump’ is the smallest one possible.
Trick December 2, 2017 at 4:07 pm
“That’s your fundamental mistake, it’s not true, no gases do that for example.”
Phil. there is a miscommunication going on here, the atm. radiation text I use Bohren 2006 does not agree with you (and his earlier one 1998). Possibly if we ask enough questions eventually get down to why you so vehemently disagree with the text.
All solids, all liquids, all gases, all plasmas emit at all frequencies at all temperatures all the time.
Not true, gases don’t do that.
Trick December 3, 2017 at 6:48 am
Phil.: “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.”
Ok, this would be where you and Bohren differ, he writes ALL atoms have rotational states that are quantized without exception. So, thanks for that I have some reading to do. He is very good at citing source material.
Then Bohren’s wrong, try reading any college level physical chemistry text.
The NIST ref. uses so much specialist wording I would have to spend time learning the lingo if it confirms this or not. You are then writing the spinning argon atoms do so as smoothly as they translate, that there is no quantum separation in rotational energy states as found in (all?) other atoms and molecules. Photons are not absorbed/emitted as rotational quanta in atomic Ar. Interesting. This strikes me as odd and not covered in Bohren’s work that I have found as Ar is not that important in meteorology. I may be interested enough to dig into this further.
A monatomic gas has three degrees of freedom which are the three translational modes, no monatomic gas has any rotational or vibrational modes. Neither do homonuclear diatomic such as N2 and O2 have IR active rotational or vibrational modes due to the lack of a dipole.
“…it shows lines emitted from Ar in an electronic discharge tube.”
The NIST report uses an etalon to excite Ar in air at collisional energy levels MUCH lower than those found in the atmosphere.
No, they’re much higher than this in the atmosphere see my post above.
If it is only Ar electronic states being excited above base state by light quanta then possibly those electronic states for Ar can also be energized with the energy available in the lower atm. unlike the more prevalent constituents. Since you say these have not been found then they could be too feeble for modern instruments (which Bohren mentions) or the researchers weren’t looking for them.
No they just do not exist.
“..try reading any college level physical chemistry text.”
I have one on order. On line texts (the 3 or 4 I found) tend to all agree with Bohren so far and not Phil. but nothing I can relate to test just words so not worth following up until I can find one with test data. I have at least a related source explaining misconceptions about this stuff that he cites but haven’t been to the library yet. Perhaps you could recommend a text that traces source material from actual testing. I’ve learned that atomic rotational quanta are so uninteresting they have been given comparatively little attention. Vibrationals having to do with chemical bonds have more significance.
“No, they’re much higher than this in the atmosphere see my post above.”
They? The etalon testing of NIST Ref. was done at much lower collisional energies than exist in the atm. so any rotational relaxation lines produced in those tests will also find enough energy in lower atm. collisions. The trouble is the ref. does not label them as rotational OR electronic unless the specialist lingo they use is not communicating to me (yet).
Trick December 3, 2017 at 6:30 pm
They? The etalon testing of NIST Ref. was done at much lower collisional energies than exist in the atm. so any rotational relaxation lines produced in those tests will also find enough energy in lower atm. collisions. The trouble is the ref. does not label them as rotational OR electronic unless the specialist lingo they use is not communicating to me (yet).
Look at the NIST report I cited, in the L column it shows you the electronic configuration, in the ground state it shows all 6 electrons in the 3P level, in the excited states one of the electrons has been raised to a higher level so 5 3P electrons plus one 4S electron and so on so they’re explicitly electronic.
[Italics dropped, second paragraph. .mod]
“Look at the NIST report I cited…”
I did, thanks for the decoding. If you look up the 1973 source material though there are way more pages of lines than the NIST link provides. It is at least possible those can be decoded into rotationals. It is also possible the rotational relaxation energies occur below the sensitivity of the etalon apparatus. The lingo is specialist and decoding will take some time.
Also, these listed electronic relaxations would statistically occur in a lower Earthian argon atm. as it would have more collisional energy available than in the collisional energy available in the etalon for the 1973 testing.
Trick December 4, 2017 at 1:17 am
“Look at the NIST report I cited…”
I did, thanks for the decoding. If you look up the 1973 source material though there are way more pages of lines than the NIST link provides. It is at least possible those can be decoded into rotationals. It is also possible the rotational relaxation energies occur below the sensitivity of the etalon apparatus. The lingo is specialist and decoding will take some time.
No it is not possible , Argon (or helium, neon etc.) does not have rotational states, you are confusing the emission lines from a discharge tube (which has many lines over a wide range of wavelengths) with the energy levels of the Argon atom.
Also, these listed electronic relaxations would statistically occur in a lower Earthian argon atm. as it would have more collisional energy available than in the collisional energy available in the etalon for the 1973 testing.
No they won’t, as I pointed out above the fraction of Ar atoms at 300K existing in the first excited state is about 10^-185. By the way an etalon is an interferometric devise used to measure the wavelengths with high accuracy, it is not a device for generating emissions.
The NIST data is from an etalon per the 1973 paper. So far that is the only test data you have cited supporting your position. The other authors I’ve found do not support your conclusions but I am still digging into their source material, too early to draw a conclusion.
Phil.: “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.”
https://www.bing.com/images/search?q=Rotational+states+of+Argon&qpvt=Rotational+states+of+Argon&FORM=IGRE
More non existent states
https://www.bing.com/images/search?q=Electronic+states+of+Argon&qpvt=Electronic+states+of+Argon&FORM=IGRE
A C Osborn December 4, 2017 at 11:09 am
More non existent states
https://www.bing.com/images/search?q=Electronic+states+of+Argon&qpvt=Electronic+states+of+Argon&FORM=IGRE
That shows the same electronic states given by the NIST report I linked to:
http://raptor.physics.wisc.edu/overview/over4.gif
Mr Eschenbach, would you like me to list the many many Scientific pieces of literature and courses that say exactly the same thing as that Wiki Quoute?
All matter above absolute Zero emit Thermal Radiation and it has nothing to to do with Molecules and all to do with Excited Atoms.
So do want the list or not, if so how many before you are convinced?
Here is just the Dictionary version
thermal radiation
See more synonyms on Thesaurus.com
noun, Thermodynamics.
1.
electromagnetic radiation emitted by all matter above a temperature of absolute zero because of the thermal motion of atomic particles.
A C Osborn December 2, 2017 at 4:38 am
Mr Eschenbach, would you like me to list the many many Scientific pieces of literature and courses that say exactly the same thing as that Wiki Quoute?
All matter above absolute Zero emit Thermal Radiation and it has nothing to to do with Molecules and all to do with Excited Atoms.
So do want the list or not, if so how many before you are convinced?
Since the statement is untrue I don’t care how many places you can find it, it’s still wrong.
Here is just the Dictionary version
thermal radiation
See more synonyms on Thesaurus.com
noun, Thermodynamics.
1.
electromagnetic radiation emitted by all matter above a temperature of absolute zero because of the thermal motion of atomic particles.
That is poorly written and misunderstood by you. For example Argon will emit thermal radiation if you heat it to about 2000K, it’s not in the case of Argon due to the motion of atomic particles but sub-atomic particles (electrons). In the case of CO2 it’s due to the motion of the atoms (vibration and rotation of the molecular bonds) and occurs at lower temperatures.
Phil, great, thank you.
Can you now explain how the the texts are all wrong.
They all say that any radiation from an object above Zero K is called Thermal Radiation, they do not call it anything else.
So can you provide me with the definitive Reference that says either that radiation is no emitted or it is called something else.
When you do that can you also provide a reference that says that that “something else” doesn’t involve photons being emitted?
More non existent states
https://www.bing.com/images/search?q=Electronic+states+of+Argon&qpvt=Electronic+states+of+Argon&FORM=IGRE
Phil, so I provide images of many examples of what you say does not exist and you refer to the NIST experiment once again. Which appears to prove – nothing.
All you have shown is how limited the NIST experiment is when it is contradicted by so many seperate sources.
Actually you haven’t in fact judging by what you’ve linked to it seems unlikely you’ve even looked at them, here’s one of them: http://pedalmag.com/wp-content/uploads/2016/05/Electron_Pro_Danish_FrameSet_cote_20160425.png
You haven’t showed a single example of a rotational transition but you have confirmed my reference to the electronic states of Argon: https://www.nature.com/articles/srep15254/figures/5
There are no images in the many you linked that had anything to do with rotational states of Argon, a few which show the same information about the electronic states as I linked to in the NIST report, and a lot of irrelevant images from various sources such as lasers, bicycles, helmets etc.
A C Osborn commented:
First, A C, you seem to think that I am the Editor of the journal. I am not. I pointed out to the Editor what was going on. He pulled the paper, as he should have, to protect the reputation of his journal—it was an attempt at underhanded deception. But notice … HE pulled the paper, not me. If you have a problem with that, I suggest you take it up with the Editor. I have no problems or regrets about my own actions, I’d notify the Editor again if I had it to do again.
It also appears that you are confused. The post you pointed to contains the “gory details”, but not the gory details of their “theory”. It has the gory details of N&Z getting their paper yanked for scientific malfeasance.
However, as you point out, that post doesn’t “do anything to destroy the theory” … for a simple reason. It wasn’t about the theory. It was about their attempt to scam the journal.
However, this post, which I’ve linked to three times now, shows that their theory is a scientific joke. If you find a real problem with my deconstruction of their curve-fitting, I’m all ears. But to date … nothing.
w.
“But to date…nothing”
Apart from:
https://tallbloke.wordpress.com/2012/02/09/nikolov-zeller-reply-eschenbach/
Tony December 2, 2017 at 12:04
Sorry for my lack of clarity. By “nothing”, I meant “nothing of substance”. If you think their defense of their own work holds water, more fool you. But as I proved above, there is no possible way that their MAGICAL! equation works without GHGs …
And N&Z still don’t understand that when you have 5 tunable parameters and only 8 data points, that it is very easy to fit them. In my linked post, The Mystery of Equation 8, I did a better job of fitting them with only 3 parameters.
So … how come you are not patting me on the back and congratulating me for improving on the N&Z equation?
Heck, Tony, believe what you want. Everyone here who does understand the issues knows that you don’t. You’ve completely trashed your own reputation … oh, wait, you don’t have a reputation, because you don’t even have the balls to sign your own name to your own words. So you can walk away, take up a new alias, and continue your nonsense.
Sadly,
w.
The post I linked to is a reply to “The Mystery of Equation 8”, by Nikolov and Zeller themselves. I recommend readers have a look at both and decide for themselves.
I note that you do not refer to my other reference which shows that many scientists and mathematicians ha very acceptably done exactly the same thing as N&Z.
Why is that?
A C Osborn December 2, 2017 at 3:41 am Edit
Because:
a) Science is not a democracy settled by votes, and
b) You haven’t even done yourself the favor of QUOTING YOUR OWN WORDS so that I could find the link you are mumbling about, and
c) If as you say they did “exactly the same thing as N&Z”, what N&Z did was a trivial and ludicrous curve fitting exercise involving 5 free parameters. Why would I want more of that?
I note you do not refer to my other post which shows that I have very acceptably done exactly the same thing as N&Z, and even better. I fitted their data with two less parameters. How come you are not congratulating me???
If that’s not enough reasons, let me know, I’m sure there are more ..
w.
when u burn ants with a magnifying glass, you are not concentrating temperature and the lens doesn’t get hot because it is transparent.
maybe some basic definitions are in order.
maybe start with temperature.
gnomish, thanks for the comment, but it’s not at all clear who or what you are referring to here. Please quote what you’re responding to, because as it stands, it’s too vague to be meaningful.
w.
well, there are some describing radiant energy as cold or hot – as if a thermometer could take its temperature, for one example.
that’s not going to get anybody to logic town.
then there’s a ‘thermal radiation’ meme. if IR is meant, then use that term, no? radiation is EM energy no matter if it’s IR or UV or X and any absorbed radiation of any frequency becomes kinetic energy, no?
if one wishes to refer to ‘color temperature’ of a black body- that’s a spectrum but color can not be measured with a thermometer.
imo, this language is but a baby step from phlogiston – but at least there’s a definition for that so i don’t have to parse idiosyncratic language of every person just to see if i can find any sense in a statement. i just can’t keep track of the babble. it is babel.
temperature is not an attribute of a 445nm blue laser’s monochromatic radiation but boy does it burn stuff.
gnomish December 1, 2017 at 10:34 pm
I repeat my request. Quote what you are talking about.
The infrared spectrum is roughly divided into two parts, generally called “near infrared” and “far infrared”. Near infrared is “near” to visible light in wavelength. We get a lot of that from the sun. Far infrared, on the other hand, comes from objects at earthlike temperatures. As a result it is often called “thermal IR”. And just to confuse matters, far infrared is also often called “LWIR”, or long-wave infrared. Sorry you don’t like it, but those are the terms in common use.
The temperature of a black-body for a given emitted radiation is often called “brightness temperature”, or the “S-B temperature”, for the Stefan-Boltzmann equation used to relate temperature and thermal radiation.
Again, however, your lack of quoting what you are referring to is biting you. The only reference in this thread to “color temperature” refers to light bulbs … and again, this is common usage, viz:
This relates to the fact that as you heat something, say a bar of steel, you can tell its temperature by the color of the light that it emits. See Westinghouse on the subject, they know about light bulbs.
I’m sorry, but it’s not babble in the slightest to anyone familiar with the field. These are all called “terms of art”, meaning words that have a special meaning in a particular field. Every specialty has them. You are obviously unfamiliar with them … but that’s no reason to get all huffy with people who are familiar with them.
Regards,
w.
dude, you want to be deliberately obtuse, fine.
off you feak, then.
Great word that Gnomish Obtuse.
Mr Eschenbach, what colour is an Argon Lamp?
So based on the colour what Temperature is it at?
You know, Argon, that stuff that cannot emit photons.
A C Osborn December 2, 2017 at 4:27 am
Great word that Gnomish Obtuse.
Mr Eschenbach, what colour is an Argon Lamp?
So based on the colour what Temperature is it at?
You know, Argon, that stuff that cannot emit photons.
Let’s get it straight as you’ve been told above Argon can’t absorb IR and therefore can’t emit at the temperatures experienced in the atmosphere.
Color temperature is applied to blackbody emitters which emit a continuum of light the peak of which shifts with temperature (Wien’s Law) so as temperature changes our perception of its color changes. In common parlance this is frequently used for incandescent light bulbs, but pyrometers used to measure furnace temperatures use the light spectrum to do so. Since incandescent bulbs have a hot filament which emits as a blackbody this was straight forward, however it’s not so with LEDs which only emit in the visible, but the manufacturers have an equivalent scale for them so that consumers can chose the bulb that best fits their application. Discharge lamps such as argon, helium etc are different again because they emit discrete line spectra (such as I showed you above for Argon), the color balance will change with temperature but it’s not as straightforward as in a continuous distribution. The temperature of an Argon lamp will depend on how much current you run through it and the perceived color will change as well.
No you get it straight.
Mr eschenbach incorrectly stated that there are “things” ( he did not state what things other than they are not solid objects) that CANNOT emit Thermal Radiation, no qualification that it is Infra Red Radiation.
All Radiation from any matter above Zero K is classed as Thermal Radiation and Argon is above Zero K.
The Atmosphere does not come in to this part of the conversation.
Up post you stated “No, the energy levels for Ar are well known and will require excitation in the far UV”.
Is there any far UV in the Radiation coming from the Sun?
.
Strictly speaking there is no such thing as thermal radiation. It’s a term that has (In common parlance) come to characterise electromagnetic radiation emitted by matter above absolute zero that we encounter in common environments.
It is all electromagnetic radiation and there is no diff between 10um Co2 photon from an atmospheric molecule and one that pops out of a Co2 laser.
It’s also misused a lot. Argon can radiate but not in the normal conditions encountered in this thread.
A C Osborn December 2, 2017 at 6:40 am
No you get it straight.
Mr eschenbach incorrectly stated that there are “things” ( he did not state what things other than they are not solid objects) that CANNOT emit Thermal Radiation, no qualification that it is Infra Red Radiation.
All Radiation from any matter above Zero K is classed as Thermal Radiation and Argon is above Zero K.
But it won’t emit until it gets to a couple of thousand degrees!
The Atmosphere does not come in to this part of the conversation.
Up post you stated “No, the energy levels for Ar are well known and will require excitation in the far UV”.
Is there any far UV in the Radiation coming from the Sun?
Not anywhere where you’ll find argon on this planet.
Again, you completely miss the point of the question.
If the whole Atmosphere is Argon as Mr Eshenbach suggests (probably an impossible situation considering where Argon comes from), would the Sun provide Far UV to warm it up?
A C Osborn December 2, 2017 at 4:27 am
Are you claiming that argon “cannot emit photons”? Because I never said that. I said that it does not emit in the range of “thermal IR”.
And no, “thermal IR” doesn’t mean “any temperature”. It is a term used interchangeably with “longwave IR” to indicate the part of the IR spectrum that occurs at earthlike temperatures.
w.
OK, Mr Eshenbach, I can see that you can never be pinned down as you change what you mean every time you are challenged on EXACTLY what you said.
What you originally said and I quote
Mr Eschenbach says “Is there some law of nature I don’t know about, that says “All things must radiate thermal energy”?”
Now you are saying that they DO radiate Thermal Energy, just not Thermal IR Energy.
There is a word for that.
A C Osborn December 2, 2017 at 1:25 pm Edit
Yeah, there is. It’s regarding you not noticing the context of the discussion, in which we’ve been talking about thermal IR. The word for that is unobservant.
And the word for your comment is “nitpicking”. If that’s all you can find wrong in my writings, I’m a happy man.
I do notice that nobody has found any problems with my proof that a non-GHG atmosphere cannot permanently warm the surface … including you. So you decide to nitpick instead.
Your choice … me I prefer science, but if you want to pick nits, be my guest.
w.
A C Osborn December 2, 2017 at 10:18 am
Again, you completely miss the point of the question.
If the whole Atmosphere is Argon as Mr Eshenbach suggests (probably an impossible situation considering where Argon comes from), would the Sun provide Far UV to warm it up?
Only far up in the thermosphere.
You may recall that in an attempt to simplify and clarify this matter, I asked the following question:
Willis Eschenbach November 30, 2017 at 3:48 pm
We’ve had responses of yes, it’s possible, and no, no way. Hans Jelbring has a whole theory about how a non-GHG atmospher can keep the surface warmer. Nikolov and Zeller say the same, with a different mechanism.
Now it’s time to settle the question. I say I can prove, not assert but prove, that not only is Jelbring proposed mechanism wrong—I can prove that there is no possible mechanism by which a non-GHG atmosphere can maintain a warmer surface.
(As an aside before we begin, before you claim that “nothing can be proven in science”, consider: you certainly can’t prove that there are no black swans … but it’s easy to prove that there are white swans …)
The type of proof that I will use is “proof by contradiction”. This involves assuming that what is under investigation is true, and then seeing if that assumption leads to any fatal contradictions.
SO: let us assume we have an airless black-body planet that has a non-GHG atmosphere. Say it is being evenly radiated from all sides at a rate of 340 W/m2, the strength of the sun. It is also emitting 340 W/m2, so it is in steady-state. And Stefan-Boltzmann gives that planet a temperature of about 5°C.
Now assume that we introduce an atmosphere of argon, which despite nay-sayers neither radiates nor emits in the thermal IR range. We let the transients settle down until it is once again at steady-state. (There may be transient heating or cooling from the expansion or compression of the atmosphere, but these are one-time events.)
Now, according to Hans Jelbring (and others), gravity acts on the atmosphere to make the surface permanently warmer. So let us ASSUME that he is right, and see what that gets us. Suppose his mechanism adds 10°C to the temperature. So the temperature of the planet settles down at a new steady-state, with a temperature of 15°C, maintained by the atmosphere. What’s not to like?
What’s not to like is that IF that is the case, then Stefan-Boltzmann says the planet must be radiating at the rate of about 390 W/2. But if the planet is at steady-state, this would violate the First Law of Thermodynamics. The planet at steady-state would constantly be radiating MORE than it is receiving, which is impossible.
Therefore, not only Jelbring’s hypothesis but any mechanism claiming to warm the planet by atmospheric action alone without GHGs is impossible. It leads to a violation of the First Law.
Q.E.D.
Comments invited.
w.
PS–As I said above, I believed Jelbring’s hypothesis when I first read it, and I said so publicly … but after I’d studied and learned more, I changed my mind. I’m curious if those of you who said yes, it is possible for a non-GHG atmosphere to permanently warm the surface, will have the courage to do the same …
For the longer version of this same proof, see A Matter Of Some Gravity
SB is a model that has validity in a defined context and not outside that context.
some soup is 95C but the thermos that contains it does not radiate the SB spectrum corresponding to its temperature cuz the heat can’t move by conduction or radiation (ok, the mirror returns only 99.9%, fine)
the thermos mirror is the radiant surface that is observable and it’s just not hot. but the soup is.
and this is a planet covered deep in soup.
so the surface of the thermos will be warmer than without the soup but it is heated by conduction, mostly.
the liquid storing the energy heats the surface and SB has no say in the matter.
http://www.westinghouselighting.com/images/pageassets/color-temperature-kelvin-light-appearance-ambience.jpg
and SB radiation calcs don’t confine radiation to long wave end of the spectrum.
http://hyperphysics.phy-astr.gsu.edu/hbase/wien.html big duh, right? when we have a stellar fusion generator so handy to look at any day…
lots of radiation has nothing to do with messrs s & b or wein or planck.
just cuz u got a hammafor…
Willis,
I like to read the longer version, as the gravity argument is used here too… But the reference points back to this discussion…
Thanks, Ferdinand, I fixed it.
w.
“..which despite nay-sayers neither radiates nor emits in the thermal IR range.”
A Willis’ speculation until proven by experiment. Hence any conclusion is likewise speculative. Willis cannot be proven wrong, nor can Willis be proven right.
Trick December 2, 2017 at 6:05 am
As mentioned repeatedly, you’ve been given the actual NIST spectrographic results that show exactly that. They are not theory. They are the results of an EXPERIMENT.
The fact that you don’t seem to understand them does not invalidate them.
w.
Willis: “..that show exactly that.”
What is it you mean by “that” Willis?
The NIST experimental data show plenty of lines emitted from atomic Ar in the IR in air. You must not have clicked on the right tab. Your Ar atm. will have an emissivity to multiply a nonzero Planck radiance at 255K and IR wavelength. Thus it will have a surface warming effect above an ideal transparent atm. The question is how much.
I guessed for an Ar emissivity of 0.01 (vs. normal atm. at 0.8 global median) then calculated around +0.3K (255.3K above 255K for transparency) assuming common Earth pressure, insolation, surface parameters.
As Phil. points out no one (so far as is yet discovered anyway) has run an emissivity test on Ar at typical atm. pressure (1atm.). Thus no one can prove your answer wrong or right for an argon atm. If you choose to change the proposition to an atm. consisting of a gas with a known emissivity (1atm) then experiment can support your proposition answer.
An immediate failure at the first point.
You cannot have a planet evenly radiated from all sides with 340 W/m2.
Is it totally surrounded by suns?
Therefore it cannot be in a steady state or thermal equilibrium, the Earth is not and has never been in equilibrium.
Seriously?
Nobody every claimed that 340 was an instantaneous value. Do you think you’ve made a new discovery with that rotation thingy?
Are you denying that the Earth’s Surface and Atmosphere is NOT in Equilibrium?
A C Osborn December 2, 2017 at 3:31 am
Dude, it’s a THOUGHT EXPERIMENT … I can absolutely have it surrounded by thousands of suns. Did you read “A Matter Of Some Gravity” as I suggested? Obviously not …
w.
Yes I know it is make believe and not any kind of Science.
A C Osborn December 2, 2017 at 1:02 pm
You’re really going to object to thought experiments all over again? Didn’t you learn from the first time? We went through that upthread, were you not paying attention? Maxwell’s Demon is a thought experiment. Schrodingers Cat is a thought experiment. Are you going to sit there and claim that neither of those are “any kind of science” just because they are thought experiments?
Sheesh … been there, done that …
w.
AC, if you don’t like the term “thought experiment” then try “homework problem”. That is essentially what these scenarios are. The scenarios focus in on a few concepts to see what textbook science would predict as the answer.
Another perspective on “A Matter of Some Gravity”:
http://theendofthemystery.blogspot.co.uk/2012/02/incompetent-skeptics-ii-willis.html
Tony, your “Other Perspective” is interesting and makes some good points. But it also makes some very fundamental mistakes about thermodynamics.
Tony December 2, 2017 at 4:52 pm
Tony, even you should be able to see the errors in this statement from “Another perspective” …
His claim is that when a warmer object absorbs radiated energy from a cooler object, the warmer object cannot then radiate the energy that it has received. Why? He doesn’t mention that part.
Now if you believe that, Tony, then you are as innocent of thermodynamic knowledge as is Harry Dale Huffman.
And if you don’t believe it, then why are you cluttering up this site with that kind of garbage?
Finally, there are lots of “competent scientists defending the greenhouse effect”, including a number on this very thread. Another point against Mr. Huffman’s screed.
w.
PS—I don’t trust men who use all three of their names. And Harry Dale Huffman is one more data point in favor of that rule of thumb.
Willis posts a link to a five year old article he’s written as if to say, “if you can’t find a fault with this, it’s right”. My point is that these discussions have already been had. If you don’t like (or can’t explain without misrepresenting) what Huffman is saying, there are blog posts and papers by others with a different take (or the same take written in a different way). There are also over one thousand comments in response to the original article, in the first place, many of which are not in agreement with Willis. I’m just making sure it’s realised that in each case (each time Willis links to one of his own articles) it’s known that this wasn’t the final word on the matter.
Willis:
i) Kinetic energy at the surface can either be conducted away or radiated away. Both cannot occur simultaneously. If one rises the other drops. So, if you have kinetic energy sufficient to produce a temperature of 288k as per the Earth’s measured surface temperature there is no physics that prevents 255k radiating to space and the other 33k being conducted to the atmosphere.
ii) If that 33k goes upwards from the surface in convective ascent (effectively disappearing from the radiative energy budget as PE) then it comes down in convective descent (reappearing within the radiative energy budget as KE at the surface) but at a later time. It is well established physics that adiabatic cooling in ascent and warming in descent is fully reversible.
iii) The original 33k taken from the surface by conduction in the formation of the atmosphere came from energy that would otherwise have radiated to space. That being the case it cannot have reduced surface temperature below 255k which was the surface temperature pre atmosphere as per S-B.
iv) If the surface temperature remained at 255k whilst the atmosphere was being formed then it MUST rise by 33k when the adiabatic loop closes.
v) Although the surface temperature is then 288k you still have ongoing conduction of 33k upward to maintain convective overturning PLUS 255k of radiation going out to space which keeps the combined radiative and adiabatic loops stable and the atmosphere in hydrostatic equilibrium indefinitely.
Your previous objection to such a scenario missed the issue of the process of creating the atmosphere in the first place when the first convective overturning cycle formed. That is where the non zero consequence of conduction and convection comes from so that there is no breach of the first law.
You can only counter that by suggesting that a surface at 288k can both radiate at 288k AND conduct at 33k simultaneously and THAT would be the true breach of the first law.
“Kinetic energy at the surface can either be conducted away or radiated away. Both cannot occur simultaneously.”
Yes! Please remember this! (also, it would be much more productive in general to talk about energy and power, rather than temperature.
“So, if you have kinetic energy sufficient to produce a temperature of 288k as per the Earth’s measured surface temperature there is no physics that prevents 255k radiating to space and the other 33k being conducted to the atmosphere.”
Actually, if you have kinetic energy sufficient that will be producing 390 W/m^2 of thermal IR, there IS physics that allows it to radiate away only 240 W/m^2 of those 390 W/m^2 to space . It’s called “Green house gases”. Without these gases, it would indeed radiate 390 W/m^2 if the surface were 288 K, causing very rapid cooling.
It could only be GHGs as a cause if the surface temperature drops whilst the atmosphere is forming.
If the surface temperature remains at 255 and radiation to space drops instead the cause is atmospheric mass within aa gravitational field.
Please explain how the surface temperature could drop during the formation of the atmosphere without a breach of S-B and the first law.
“You can only counter that by suggesting that a surface at 288k can both radiate at 288k AND conduct at 33k simultaneously and THAT would be the true breach of the first law.”
Objects routinely conduct and radiate as they independent processes, no breach Stephen. Planck’s radiation law was developed at room temperature and ~1atm. Conduction and convection were simply minimized.
The total of radiation and conduction cannot exceed the energy required to fulfil the S -B equation. If the Earth’s surface at 255k were to both radiate 255 to space and at the same time conduct 33k to the newly forming atmosphere the consequent surface temperature fall would be a breach of S-B.
Your proposal is that the same parcel of kinetic energy can both radiate photons and conduct the same energy that was released by the photons simultaneously. That is a breach of the first law.
Tim Folkerts, are you prepared to discuss the “Mechanics” of how those GHGs do there job?
AC, there are innumerable details, but the basic mechanism is pretty straightforward. The earth absorbs ~ 240 W/m^2 of power from the sun on average. To balance out (and in the long-term, earth does come quite close to balancing out), the earth must also radiate ~ 240 W/m^2 of thermal IR to space. This would correspond to an average temperature of ~ 255 K. GHGs in the cold upper atmosphere radiate at a temperature well below 255 K, and hence radiate weakly to space. To balance, some region MUST radiate at a higher temperature than 255 K, radiating more strongly to space to compensate. That region would be the surface.
Tim, that is what they keep saying and the diagrams show, it is “how it actually works” that I am interested in.
Let’s start with if CO2 is suplying the W/m2 where in the Atmosphere does this happen, ie how high and at what Temperature are they?
Next Moisture does the same, but presumably lower in the Atmosphere.
If that is correct why do dry Deserts get both Hotter & Colder than the tropics, aren’t all High temp records not registered in “dry” areas?
I completely understand that moisture (and the Seas) keep the Temperature more stable, but not Hotter.
“The total of radiation and conduction cannot exceed the energy required to fulfil the S -B equation.”
Sure it can Stephen. This is no breach of 1LOT. A molecule can emit a photon and bang into another slower molecule all at the same time, losing energy by a transfer of energy by conduction and radiation simultaneously. Possibly your wording is not conveying the math you are trying to verbalize, but I know you can’t read math so trying to get you to link or show what you really mean in math is probably futile.
You need a faster process of energy shedding than radiation to reduce the surface temperature when the atmosphere is forming. Since conduction and convection work slower than radiation the energy must come from a reduction in outgoing.
If a molecule bumps into another so as to reduce its energy content by conduction then the reduction in energy content is replaced by fresh incoming energy instead of that fresh incoming energy radiating out.
Conduction is an interruption in the free flow of radiation not an accelerant.
The atmosphere formed 4 billion years ago. Your insistence in continuing to bring it up is bright shining evidence that your theory can not support a simple energy flow accounting that demonstrates energy balance.
The planet is in dynamic equilibrium! Fit your theory to that unpleasant truth..
I thought you had given up when I used your own style of analogy to show you why you were wrong.
The events at the formation of the atmosphere when there was an interruption of the free flow of radiation through the system are critical to the surface temperature enhancement and will remain so for as long as the sun shines and thereby keeps the mass of the atmosphere suspended off the surface.
Stephen I didn’t go away. When I started our discussion I was trying to get you to demonstrate a simple flux accounting for our current, dynamically equilibrated atmosphere. Since you insisted on bringing up 4 billion year old developing atmosphere I engaged that argument.
We disagree on that accounting. I gave my best shot at trying to change your thinking on that formation and failed. Like you, i know when the horse is dead. I won’t go there anymore. I will even go one step further and grant that it all worked back then exactly as you say.
It is irrelevant to our initial discourse. Any ‘bump’ that is 4 billion years old is long gone from the system, right? By your own prior statements, you agree that today’s atmosphere has a net zero interchange of energy with the surface. The basis of your theory is that the atmosphere has no radiant energy interchange.
So we both agree that we are left with an atmosphere, by your reckoning that, from an energy consideration, can be completely eliminated from condideration. This leaves us with a surface at 288 k with an input of 240 watts/m^2. There is no reconciling that. It is not in balance. You need to explain the imbalance as some hitherto absent energy source
It is certainly not reconciled as an artifact of a 4 billion year old atmosphere formation under a vastly different sun.
For this question, I am still engaged and waiting for an answer devoid of the formation arguments.
You can’t ignore the imbalance created during the formation of the atmosphere.
As per the figures I gave you there were 10 cheese balls on the table at the start but by delaying the deliveries you are then actually holding 10 on the table and 5 in the bucket forever unless something else changes.
The effect of that initial temporary energy imbalance remains until the sun stops shining.
If one adds mass to the atmosphere the imbalance increases. If one removes mass the imbalance decreases.
Atmospheric mass used conduction and convection to delay radiative throughput and the surface temperature rose as a result.
That is why the temperature at 1 bar pressure on Venus is similar to that on Earth after adjusting for distance from the sun and why the surface beneath such a massive atmosphere is so hot.
More mass in the atmosphere created a bigger radiative imbalance during the formation of the Venusian atmosphere so the surface is much hotter after accounting for the distance from the sun.
So, in a nutshell, you claim that these 4 4 billion year old energies are still with us?
I respectfully repeat my request to show me the accounting.
I gave you the accounting in the cheese ball analogy. You start with 10 balls but by manipulating timing on one occasion you end up with 15 in stock forever.
I can see you never did stock control in retail.
No fair again. No cheese balls this time. That was a different problem for a different time. All those old balls got eaten in the cretacious. And, phase delays are not allowed in steady state accounting. All phase delays are moot after 1 cycle and the cyclical nature of our atmosphere has a frequency much much smaller than 4 billion years.
Once again I respectfully request the energy accounting for today’s dynamic equilibrium. BTW, that means no net energy exchange for the system, the atmosphere, and the surface. Please remember that this means you can’t include 4 billion year old bump in today’s accounting. That would be another no fair.
You are correct on the retail. Never did it.
Did however do microwave engineering, software engineering and physical oceanography, modeling boundary free ocean circulations, antenna field distributions, and network topology and capacity modeling.
Those bits have some accounting. Hope that is the last snark where we share the size of our d***s.
BTW. No fair bringing up Venus. No fair bringing up mass. No fair bringing up convection.
1. Net energy exchange at surface atmosphere Boundary is zero
2. No radiant interchange with atmosphere
3. 240 w/m^2 In
4. Surface is 288 K
Those are your claims and the only thing on the table.
Show the accounting!
You seem incapable of the mathematical agility required for retail stock control (multiple moving parts and variable timing) so no point engaging with you further since I’m sure other readers will get the idea.
Do you not realise that the radiatively inert atmosphere has a conductive energy exchange with the surface and the surface has a radiative energy exchange with space?
Adiabatically warmed air descending along the lapse rate slope places air at 255k adjacent to the surface and warms colder surfaces directly or causes warmer surfaces to cool less quickly.
By that means conduction and convection achieve exactly the same effect as that proposed for GHGs but it can’t be both or you get a surface temperature of 321k which we don’t.
So you have 33k up and down in the adiabatic loop with 255k in and out for the radiative loop.
But first you have to get the atmosphere up off the ground to create the adiabatic loop.
Paul Bahlin December 3, 2017 at 5:15 am
The planet is in dynamic equilibrium!
I assume that you mean “Thermodynamic equilibrium”
You do actually understand the concept of “Thermodynamic equilibrium”
What is Thermodynamic Equilibrium? Part-1
written by: Haresh Khemani • edited by: Swagatam • updated: 6/2/2011
Thermodynamic Equilibrium Defined
In an isolated system when there is no change in the macroscopic property of the system like entropy, internal energy etc, it is said to be in thermodynamic equilibrium. The state of the system which is in thermodynamic equilibrium is determined by intensive properties such as temperature, pressure, volume etc.
Whenever the system is in thermodynamic equilibrium, it tends to remain in this state infinitely and will not change spontaneously. Thus when the system is in thermodynamic equilibrium there won’t be any spontaneous change in its macroscopic properties.
Conditions for Thermodynamic Equilibrium
The system is said to be in thermodynamic equilibrium if the conditions for following three equilibrium is satisfied:
1) Mechanical equilibrium
2) Chemical equilibrium
3) Thermal equilibrium
1) Mechanical equilibrium: When there are no unbalanced forces within the system and between the system and the surrounding, the system is said to be under mechanical equilibrium. The system is also said to be in mechanical equilibrium when the pressure throughout the system and between the system and surrounding is same. Whenever some unbalance forces exist within the system, they will get neutralized to attain the condition of equilibrium. Two systems are said to be in mechanical equilibrium with each other when their pressures are same.
2) Chemical equilibrium: The system is said to be in chemical equilibrium when there are no chemical reactions going on within the system or there is no transfer of matter from one part of the system to other due to diffusion. Two systems are said to be in chemical equilibrium with each other when their chemical potentials are same.
2) Thermal equilibrium: When the system is in mechanical and chemical equilibrium and there is no spontaneous change in any of its properties, the system is said to be in thermal equilibrium. When the temperature of the system is uniform and not changing throughout the system and also in the surroundings, the system is said to be thermal equilibrium.
So let’s just take the last one.
When was the Earth’s Temperature Unchanging and when was the Temperature within the Earth System (Which includes the Core, the Crust, the Surface and the Atmopshere Unchanging?
Nice cut and paste diversion. I especially admire the part where you remind me that earth has parts. Never knew that.
Oh wait…..those other parts aren’t in what we agreed were the boundary parameters of the discussion. So go back , read the agreed upon parameters and answer the question.
BTW, your assumption is wrong. I specifically said dynamic equilibrium, no net change to energy flux. There could we’ll be millions of people jumping up and down trying to change our orbit at this very moment. They won’t change our radiation budget.
I am well aware that there are mouse farts that will cause momentary out of balance conditions. Fortunately for all of us, there aren’t enough mice on the planet now, or even 4 billion years ago to give that the slightest relevance to the discussion
Stephen Wilde December 3, 2017 at 7:27 am
I do get the idea. You think that by accusing your opponent in the debate of stupidity, that you get to walk away without answering his question.
You also think that some story about cheese balls is a proper accounting of the change in today’s energy flows that you claim exist because of a one-off event four billion years ago
So yes, Steven, “other readers will get the idea” … but you might not like the idea we get.
ANSWER THE MAN’S QUESTION! Other readers are getting the idea you don’t have an answer
And while you are thinking about how to answer it, consider. There are transient, one-off events all the time. Volcanoe erupt. Meteors crash. Hurricanes move immense amounts of energy around. These cause huge one-time changes to the energy content of the atmosphere and the earth.
Now if your theory is true, and the effect of one-time events persist forever as you claim … then where are the signatures of the other one-off events?
In fact, in a dynamic system like the climate, replete with homeostatic mechanisms, such events sink without a trace, and the status quo ante is quickly restored.
Best regards,
w.
Cheeseballs were introduced by the other guy.
Stock control is a perfect example of my point.
Energy used to raise an atmosphere off the ground is indubitably present for the life of the atmosphere.
Energy is not flux until it flows through a surface. You can have all the PE you wish up there but it will always be kg • m^2/s^2. Same for all the KE.
We are counting flux with units kg/m^3.
The fact that it sits on The surface and has been there for a long time has no bearing on the discussion.
It doesn’t ‘sit’ on the surface. It moves up and down in vast columns spread around the world and, being additional energy to that involved in the S-B equation it heats cooler surfaces and reduces the cooling rate of hotter surfaces.
Could not disagree with this more strongly. The atmospheric mass does just sit there. It has a PE that is function of mass and height. Parcels move up. Parcels move down. The mass of the atmosphere is not changing is it? Gravity? Height?
The PE is not a flux. You have already agreed that there is no net energy exchange with the surface from the day to day ups and downs of atmosphere.
Are you claiming that is the PE that creates your surface temperature enhancement?
Beneath downward columns PE is the only source of the ‘extra’ KE that arrives at the surface.
It is true that it is matched by the removal of KE beneath rising columns but there is a one cycle delay built in from the formation of the atmosphere so that they are out of phase and do not cancel at the surface provided fresh insolation to the surface continues.
You should have a closer look at my stock management example.
There is an ‘extra’ 33k at the surface which cannot be radiated to space because as fast as it appears beneath falling air it is taken up again in rising air.
Wow, just checked back on this thread and it is still going strong!
Willis,
Thanks for responding to my first post on Nov 26 2017 10:05 pm but you did not respond to my second post on Nov 28 2017 12:21 pm. I assume you lost it in all the other responses you are tracking so I thought I would ask the key question again in the hope that you are still tracking this thread.
It seems to me there is an elephant in the room. In my opinion, there is little doubt that, with bodies radiating back and forth to each other, you can work out the net energy flux using an ‘energy budget’ concept. However, this does not seem to work with temperatures.
For example, consider a white hot bar of steel weighing 1 Kg with a temperature of 1500 c. If an identical bar, also at 1500 c is placed next to it, the temperature of the first bar will not increase. If an infinite number of new bars, all at 1500 c, are added, the temperature of the first bar would still not increase.
Thus, despite an infinite increase in energy, there would be no temperature increase.
Yet, you argue that if an ice cube was put next to the white hot bar, the temperature of the bar would go up because the ice is blocking an even colder source (outer space). Somehow, this trivial change in energy would increase the temperature of the white hot bar when, in the other example, an infinite amount of new energy was not able to.
This does not seem to make sense.
It seems that , under certain circumstances, the energy budget approach works with joules but not with temperatures. Do you agree?
your argument is akin to galileo’s logic.
can something heavier fall faster? if you tie 2 lighter objects together do they suddenly fall faster than each other?
so i think you’ve shown that ‘warmer’ is not . but rate of cooling to some lower temperature than the original has changed for the bars that are surrounded.
What does get much warmer if we are talking about atmosphere is the air gap between the two or more steel plates. Which changes other parameters as well as radiation flow.
Yes you have.
But that does not meet the requirements of Cooler Warming Hotter for at least half the time on a day to day basis.
So as LWIR has little affect on water ie 70% of the Earth and it only warms the other 30% half the time from many kilometres away through a much thicker lower Atmosphere it is truly a mighty molecule.
so if you cut the hot steel bar in 2 pieces, each piece will make the other warmer than if they didn’t.
if you send one of them on a trip at near light speed,when it gets back, they will each be older than the other, as well.
the last ingredient to unlock the dream of infinite free energy is to put schrodinger’s cat in a transparent box and schrodinger’s dog in another transparent box where they can see each other.
by jove, i think i’ve got it! 😀
Every diversion to another hypothetical is evidence that you’ve abandoned the current one because you couldn’t make it stick.
Doesn’t make you wrong. Could be a tactical move, of course. But, if that’s ALL you ever do, It’s called dancing.
Paul Bahlin
December 3, 2017 at 9:25 am
Every diversion to another hypothetical is evidence that you’ve abandoned the current one because you couldn’t make it stick.
Doesn’t make you wrong. Could be a tactical move, of course. But, if that’s ALL you ever do, It’s called dancing.
Mr Bahlin, let me ask you thi as you are an avid believer in Cold making hot hotter.
Her is the ACTUAL situation
Two object in normal room temperature Air.
Object A at 25C and being heated by a constant energy source
Object B at 0C.
When I introduce Object B to within a few millimetres of Object A WHAT HAPPENS TO THE TEMPERATURE OF OBJECT A?
Before you answer bear in mind that I did this experiment 3 hours ago.
Don’t know or care. In my house object B would be a beer and I would drink it before A radiation was allowed to touch it.
Why do you keep dancing?
Bernard, thanks for the reminder.
With regards to the earth, we are talking about a steady-state situation. The earth’s temperature only changed by 0.2% over the twentieth century.
So to consider an equivalent situation, say we have a hot bar of steel weighing 1 Kg with a temperature of 50° c. Let’s assume for the moment that it is a blackbody floating in outer space. If so, it is radiating at about 620 W/m2. If it is in steady-state, that means it is also receiving 620 W/m2 from somewhere, say a radioactive element in the core of the bar.
So … it will sit there, just like the earth, in steady-state, for centuries. It gets 620 W/m2 from its radioactive core, basically 0 W/m2 from the surroundings, and it radiates the same total. Steady state, input = output.
Now, we plop down another bar of steel next to it. Let’s say that it is heated internally to 50°C as well, and radiates at the same rate as the other bar, 620 W/m2.
As a result, in the new situation, the first bar of steel is no longer receiving 0 W/m2 from its surroundings as before. Instead, it is receiving some of the energy that is being radiated by the second bar. Not all of it, obviously, most of the radiation of the second bar is going out to space. But some of the radiation from the second bar is hitting the first bar.
And when that happens, the first bar is no longer in steady-state. Now it is getting more energy than it is radiating … and when that happens, the first bar will warm up until the radiation going out matches the radiation coming in.
If this is not clear, I encourage you to play with the online calculator I linked to above …
Further questions or objections?
Bring’em on … that’s what this site is for, to try to find holes in or support for the claims of other scientists.
My best to you,
w.
(PS—Note that in this thought experiment, both bars will heat up until they reach a stable steady-state situation where they both are radiating what they absorb plus what comes from the radioactive core. Note also that this is the same thing that happens in my Steel Greenhouse. If you add a steel shell around an object heated by a constant energy flow, both the shell and the object will heat up.
For example: if we put a light bulb inside a thermos bottle, both the light bulb and the thermos bottle will get warmer …)
Mr Eschenbach, but the Earth’s surface is NOT in a heated steady state is it?
A heated steady state is NOT the same as an Average Radiation input if the input continuously varies from zero to 1300 W/m2 is it?
Every night the surface on the night side is no longer being heated, it is in a cooling state, do you agree that the surface cools at night?
A C Osborn December 2, 2017 at 3:24 am
Mr Eschenbach, but the Earth’s surface is NOT in a heated steady state is it?
A heated steady state is NOT the same as an Average Radiation input if the input continuously varies from zero to 1300 W/m2 is it?
Every night the surface on the night side is no longer being heated, it is in a cooling state, do you agree that the surface cools at night?
The Earth’s surface is continually emitting the same amount of radiation to space, if the surface is uniform, at any given time it will emit the same from the dark side as it did some hours previously. That’s a steady state.
At any given spot on the earth the budget will change in a periodic way, the amplitude of the fluctuation will depend on the rotation rate. Hence the amplitude of the fluctuation on the moon is higher than the earth is greater because of it’s very slow rotation rate. The amplitude will also depend on the presence of an atmosphere which will tend to smooth out the fluctuation, the extremely dense atmosphere of Venus leads to a very low amplitude.
Thanks Willis,
No fair! You replaced my thought experiment with your thought experiment and then defended your thought experiment. You did not address my elephant!
My point is that if you add infinity amounts of 1500 c white hot steel to a 1 Kg bar of 1500 c white hot steel, the 1 Kg bar will not increase its temperature. A split second later, everything will start to cool but that does not change the fact that the temperature of the bar did not go up despite the addition of an infinite amount of new energy! Thus, adding new energy does not always increase temperature!
You claim that if you place an ice cube next to my 1500 c white hot bar the temperature of the bar will actually increase because the ice cube is blocking an even colder source (outer space).
So, an ice cube increases the temperature of the white hot bar but a massive amount of white hot steel added does not?!
Doesn’t this drive a coach and horses through the ‘energy budget’ approach to predicting temperature?
Best regards
Sorry, Willis, I did not note which of your replies I was replying to.
My reply
Bernard Lodge December 2, 2017 at 8:55 am
was in response to your reply of:
Willis Eschenbach December 2, 2017 at 1:13 am
That is not the point Phil, only when an Object or Surface is being heated will the photons from a cooler object increase it’s temp.
So at night how can CO2 at well below Zero increase the Surface Temp as shown by the Trenberth diagram?
It can only very temporarily slow the cooling.
As I said below I conducted the test yesterday and again just now, 2 objects of equal tem cannot even warm each other let alone a colder one.
Seeing as you are answering my questions can I ask you why after around 30 years and Billions of $s the Climate Scientists cannot give a categorical answer of the affect of Water Vapour and Clouds?
Because I have a series of questions around the Mechanics of the Physics of GHG.
A C Osborn December 2, 2017 at 3:24 am
In the short term, no. In the long term, yes … and generally we have been talking here about the long-term steady-state average surface temperature.
w.
Yes you have.
But that does not meet the requirements of Cooler Warming Hotter for at least half the time on a day to day basis.
So as LWIR has little affect on water ie 70% of the Earth and it only warms the other 30% half the time from many kilometres away through a much thicker lower Atmosphere it is truly a mighty molecule.
Willis Eschenbach December 2, 2017 at 12:18 pm
Bernard Lodge December 2, 2017 at 9:05 am
Phil. December 2, 2017 at 6:00 am
Thanks Phil,
I think you would agree that the average temperature of both bars would be the same. My base point is that the the temperature of the first bar did not go up despite the addition of a new energy source.
Bernard, I did NOT say the temperature would go up.
I said, and very clearly I thought, that the temperature would be WARMER THAT IT WOULD BE WITHOUT THE SECOND BAR.
There is a very large difference between the two.
+++++++++++++++++++++++++++++++++++++++++++++++++
Willis,
Thanks for adding to my reply to Phil, but you did not actually reply to my original question to you which I will ask again:
Consider a white hot bar of steel weighing 1 Kg with a temperature of 1500 c. If an identical bar, also at 1500 c is placed next to it, the temperature of the first bar will not increase. In fact, if an infinite number of new bars, all at 1500 c, are added, the temperature of the first bar would still not increase.
Thus, despite an infinite increase in energy, there would be no temperature increase. This seems to contradict the energy budget approach to deriving temperatures in that no matter how much extra energy was added, the temperature would not increase.
I’m wondering how the Trenberth Energy Balance chart would cope with this scenario of extra energy with no temperature effect?
Best regards
Consider that you are using energy, as a term, a bit too sloppily. In all of these discussions we dwell way too much on temperature. Temperature is tertiary effect and we all talk about it too much because we live with it in our every day lives and we are very familiar with it.
Energy is what matters and even there that’s a bit over used. What is really really important is flux, the flow of energy, or in this case the exchange of energy. Flow causes energy variance which causes temp variance.
So we talk of our familiar, temperature, which is really tertiary effect of the most important physics which is flow, the most mysterious to our senses. If you asked 100 people about how their body senses heat they would all be confident that their skin is covered with little temperature sensors that somehow communicate temperature to our brains. Not true though.
Our skin senses flow. When you ‘feel’ cold It’s because you are losing energy and your skin sensors are telling you the direction of flow. You are NOT feeling cold. This is why you can walk into a 74 F room in winter and feel nice and cozy. Walk into the same room during a Florida summer and you’ll think you walked into a meat locker.
In the case of the iron bars this is how I approach it….
Each bar contains identical joules, therefore each bar is radiating identical flux. Any two bars that are exchanging flux with each other, must (with identical geometries) be having a net zero energy exchange and will therefore be incapable of changing each other’s energy content. The only thing left is that energy radiates away from both bars in the directions that are not facing each other.
The net loss of energy can only occur now over less geometry than would occur if they were not near each other. So you have two bars that will have identical temperature decay. It will take longer for them to stabilize (net zero exchange with environment)than either would alone.
You might even agree with me that temperature is not very relevant to the discussion and we figured it out without ever mentioning it.
Bernard Lodge December 1, 2017 at 9:52 pm
It seems to me there is an elephant in the room. In my opinion, there is little doubt that, with bodies radiating back and forth to each other, you can work out the net energy flux using an ‘energy budget’ concept. However, this does not seem to work with temperatures.
For example, consider a white hot bar of steel weighing 1 Kg with a temperature of 1500 c. If an identical bar, also at 1500 c is placed next to it, the temperature of the first bar will not increase.
Actually the adjacent sides of the two bars will increase. Assuming the bars are in a furnace then the first bar is at steady state with the furnace lining which will be cooler than the bar itself, when the second bar is placed next to the first it replaces the radiation from the cooler wall and therefore the bar will be hotter. This is the same principle as the heat shield used with thermocouples. In that case the temperature measured by a thermocouple is lower than the flame temperature because it is radiating heat to the surroundings, if a cylindrical shield is placed around the ThC that is much hotter than the surroundings and the measurement error is reduced. NACA produced reports on different designs of shields to be used in gas turbine engines back in the late 40s. Read any college text on radiation heat transfer and you’ll find many examples of these applications with worked calculations.
Phil. December 2, 2017 at 6:00 am
Thanks Phil,
I think you would agree that the average temperature of both bars would be the same. My base point is that the the temperature of the first bar did not go up despite the addition of a new energy source. A split second later they may start to cool but the second bar was added and the temperature of the first bar did not change. In fact, there could be an infinite amount of hot new bars added and the temperature of the first bar would still not go up.
Thus, not all new energy sources cause an object’s temperature to increase.
I conducted that experiment Bernard suggested yesterday with 2 objects at 60C and a few mms apart with cork between the two. Both were cooling, how much hotter did they both get when the cork was removed?
Zero, that is correct 0 not even 0.1C difference.
So you specify the steel plates to be in a furnace, where did Bernard Lodge mention a furnace?
Why would you change the parameters totally to make a point?
Bernard Lodge December 2, 2017 at 9:05 am
Bernard, I did NOT say the temperature would go up.
I said, and very clearly I thought, that the temperature would be WARMER THAT IT WOULD BE WITHOUT THE SECOND BAR.
There is a very large difference between the two.
Since you seemed to want a temperature that goes up, I gave the example of a bar heated by a constant energy source. Given your example, no, the temperature of the bar will NOT go up. But it will be warmer than it would have been without the second bar.
Regards,
w.
MMMMMMMMMM, “Given your example, no, the temperature of the bar will NOT go up. But it will be warmer than it would have been without the second bar.”
Temperature will NOT go Up = Warmer than it would have been.
The First Bar would be “Warmer”?
I think Mr Eschenbach must be getting tired.
I think he must mean that it would cool more slowly, so hold it’s heat longer.
A C Osborn December 2, 2017 at 9:06 am
I conducted that experiment Bernard suggested yesterday with 2 objects at 60C and a few mms apart with cork between the two. Both were cooling, how much hotter did they both get when the cork was removed?
Zero, that is correct 0 not even 0.1C difference.
So you specify the steel plates to be in a furnace, where did Bernard Lodge mention a furnace?
Why would you change the parameters totally to make a point?
The important parameter of the background temperature was not specified, without that the question was impossible, I chose a perfectly reasonable value.
Phil, as I was the one doing the experiment in my house the The Background Temperature was ambient in my kitchen, ie 20 C.
Now perhaps you will tell why this flood of photons being “Absorbed” by the objects did not raise the temperature of either at 60 C.
Because we keep hearing that Photons are photons and their Energy must make an [
obsorbingabsorbing] object hotter, even if they are coming from a colder object.Sorry Absorbing Object.
After all the second object at 60 C is obscuring an area of the kitchen that is at 20 C when the cork is removed.
Surely it must have [obscured] it from view just like a CO2 particle does with Space?
Damn, I wish there was an Edit facility on here.
I meant obscure.
Sounds like a really poorly thought out experiment, it appears that you think that the cork is at 20ºC? How are you measuring your objects’ temperature?
Willis Eschenbach
December 1, 2017 at 9:21 pm:
Just like on Earth, what is to stop conduction from the ground (heated by the sun) from warming the gas, and getting convection going? Also as here. If the gas is not warmable by radiation, only conduction could unfreeze it…..
So what, the conduction and convection don’t cause loss to space in the case of a non GHG atmosphere so the surface temperature will remain the same.
The surface temperature stays the same while the atmosphere is forming via convective ascent of atmospheric mass but as soon as that mass gets back to the surface in convective descent then the surface temperature rises above S-B.
…the surface temperature rises above S-B.”
No, the surface emits AT S-B. 288K T, emission is from an object at 288K. If at 255K, radiation is from an object at 255K.
Not if conduction and convection intervene it doesn’t.
Your require the same unit of kinetic energy to carry out two jobs simultaneously. Doesn’t operate like that.
“Your require the same unit of kinetic energy to carry out two jobs simultaneously.”
There is no such requirement. The energy transfer processes conductive, convective and radiative are all independent.
Stephen Wilde December 2, 2017 at 8:12 am
The surface temperature stays the same while the atmosphere is forming via convective ascent of atmospheric mass but as soon as that mass gets back to the surface in convective descent then the surface temperature rises above S-B.
And the emission increases which will drop the temperature back……
Air reaching the surface beneath falling columns produces KE which raises surface temperature but it is immediately taken up beneath the adjacent rising column and so cannot radiate to space.
Furthermore there is a phase delay of one cycle left over from the formation of the atmosphere which causes a net warming effect of 33k at the surface.
“Air reaching the surface beneath falling columns produces KE which raises surface temperature”
No. There are no falling columns. When surface air is warmed and rises, ambient higher pressure air at the surface moves in to replace the lower pressure surface air. Ambient surface air. As shown in the convection experiment above. Testing (and observation) is your imagination’s downfall.
Higher pressure IS a falling column.
if your falling air is forever supplying energy, What happens when It’s all down?
“Higher pressure IS a falling column.”
Again, simple convection testing shows there are no falling columns Stephen, the high pressure fluid moves in laterally at the surface to the low pressure fluid areas. The rising convective columns are mixed at upper levels. But i know Stephen will refuse to learn from testing and continue to imagine nature of meteorology.
Phil, such an atmosphere will be bleeding of energy in evaporative cooling of its own molecules raised to escape velocity. Happens now, here. At some stage, the idea that any gas can be non-radiative entirely will need proving too. Quantum Mechanics demands it…..
With hydrogen and helium maybe, not with argon.
Earth’s escape velocity is about 11km/sec, the probability of an argon atom exceeding 1km/sec at 300K is zero.
QM quite clearly demands that Ar does not emit at the conditions present in our atmosphere.
Except of course it would absorb all the High UV energy currently absorbed by Ozone and any that Ozone doesn’t currently stop, wouldnt it?
A C Osborn December 4, 2017 at 11:16 am
Except of course it would absorb all the High UV energy currently absorbed by Ozone and any that Ozone doesn’t currently stop, wouldnt it?
If it were the hypothetical pure Argon atmosphere then there would be no ozone, in any case it would only absorb the wavelengths given in the NIST table which are below even those absorbed by O2.
Repeated here in case it gets lost in the thread:
Willis:
i) Kinetic energy at the surface can either be conducted away or radiated away. Both cannot occur simultaneously. If one rises the other drops. So, if you have kinetic energy sufficient to produce a temperature of 288k as per the Earth’s measured surface temperature there is no physics that prevents 255k radiating to space and the other 33k being conducted to the atmosphere.
ii) If that 33k goes upwards from the surface in convective ascent (effectively disappearing from the radiative energy budget as PE) then it comes down in convective descent (reappearing within the radiative energy budget as KE at the surface) but at a later time. It is well established physics that adiabatic cooling in ascent and warming in descent is fully reversible.
iii) The original 33k taken from the surface by conduction in the formation of the atmosphere came from energy that would otherwise have radiated to space. That being the case it cannot have reduced surface temperature below 255k which was the surface temperature pre atmosphere as per S-B.
iv) If the surface temperature remained at 255k whilst the atmosphere was being formed then it MUST rise by 33k when the adiabatic loop closes.
v) Although the surface temperature is then 288k you still have ongoing conduction of 33k upward to maintain convective overturning PLUS 255k of radiation going out to space which keeps the combined radiative and adiabatic loops stable and the atmosphere in hydrostatic equilibrium indefinitely.
Your previous objection to such a scenario missed the issue of the process of creating the atmosphere in the first place when the first convective overturning cycle formed. That is where the non zero consequence of conduction and convection comes from so that there is no breach of the first law.
You can only counter that by suggesting that a surface at 288k can both radiate at 288k AND conduct at 33k simultaneously and THAT would be the true breach of the first law.
“You can only counter that by suggesting that a surface at 288k can both radiate at 288k AND conduct at 33k simultaneously and THAT would be the true breach of the first law.”
This wording makes no sense. An object radiates at 288K and conducts at 288K, if in equilibrium or transiently. Why would an object be at 288K and 33K at the same time (simultaneously)?
Heat (kinetic energy) is simply molecular motion. If a photon is emitted the motion reduces so that there is less conduction and if conduction occurs the motion reduces and there are less photons released.
If there is a constant energy supply immediately replacing what has been lost in both processes combined then the temperature doesn’t drop.
Once a blackbody reaches its S-B temperature thereby converting ALL incoming radiation to kinetic energy you can only reduce that kinetic energy if the outflow is faster than radiation.
Conduction and convection are slower than radiation. They inhibit the free flow of radiation but your proposal requires that they act as an accelerant.
Stephen, concur with that except for the last sentence: “They inhibit the free flow of radiation but your proposal requires that they act as an accelerant.”
I do not understand that I have a proposal for your “requires” that conduction and convection act as an accelerant to radiation. They are all independent processes producing entropy. If conduction and convection are minimized (as in deep space), radiation will dominate in energy transfer processes. Perhaps you can expand on your last sentence.
For example, many like to discuss farmer’s greenhouses. Consider one situated where it is very windy and without much insulation. Then consider one situated in calm conditions, same sun load but with much insulation. Different energy transfer eqn.s will need be considered for any design of supplemental HVAC to protect profits.
If an object receives 240W/m and radiates 240W/m and then you introduce some conduction of say 40 W/m then you say that the radiation out does not drop to 200 W/m ?
Stephen, you are not exactly clear enough. If I can guess your meaning correctly, an earth like planet in space is at equilibrium radiating 240 steady state in/out from TOA like earth as defined by CERES orbit (in thermo. this is termed a control volume).
Then an apparatus is added to independently conduct 40 to deep space sink. Ok, now an additional 40 out crosses orbit of CERES (and is not measured by CERES). Sure, there will be a transient condition existing while the newly conducted energy flowing away increases and as 240 radiated reduces. Eventually establishing new equilibrium 200 out radiated measured by CERES and 40 out conducted as measured by an inline instrument at orbit of CERES. Still in steady state with 240in.
If I get your meaning correctly, you have essentially added another window to space, now have one radiating window to space from L&O surface and one conducting window to space from L&O surface.
Willis, are you going to respond to my summary as to how conduction and convection cause a surface temperature enhancement?
I thought you were inviting something along those lines.
Stephen Wilde December 3, 2017 at 5:14 am
Thanks, Stephen. It appears that you still think that in a non-GHG atmosphere, conduction and convection will warm the surface.
However, IF that is the case, as I showed in my proof, the surface would continuously radiate more than it is absorbing … so your claim is physically impossible because it violates the First Law of Thermodynamics.
If you can explain why my proof is wrong, I’m all ears …
w.
Do you believe that the same unit of kinetic energy (molecular motion) can be radiated away as a photon simultaneously with it being conducted to another molecule?
Nice article.
However, they key to the Greenhouse effect is that the molecules are at a lower temperature than the surface. This means that more surface radiation is absorbed than emitted OUT TO SPACE by certain molecules like CO2 – BUT ONLY WHEN COOLER than the surface.
In contrast the article implies that the effect is because molecules are warmer than the background from space.
In fact, if a lapse rate mechanism could produce a atmospheric temperature lower than space – the planet would still warm. The reason this doesn’t happen is that you’d need an atmospheric heat pump.
Trick December 2, 2017 at 6:56 pm
And you can stuff your endless ad hominems where the solar constant is zero. I’m tired of your ceaseless whining.
Come back when you want to talk about the science instead of talking about me. If you want an answer, ask like a decent honest man does, instead of complaining about what you see as my faults. Your opinion about science might or might not be valuable.
Your opinion of me, on the other hand, is worthless, meaningless, useless, and usually unpleasant. Cut it out. It just makes you look like a jerk. I don’t think you actually are one, but you are sure doing a good imitation of one.
w.
“If you want an answer, ask like a decent honest man does, instead of complaining about what you see as my fault.”
I do not see this as a fault or even a complaint Willis, I’d say I see it as the human condition. We all prove we’re human at times. Ok, here’s my question (based on yours) asked like a decent honest man does, where does N&Z write that a non-GHG atmosphere, say argon, can permanently raise the temperature of a planet?
Trick December 3, 2017 at 12:01 am
If you don’t see calling a man “tempermental” as finding fault or a complaint, there’s little use talking to you.
And if you don’t understand that when N&Z say that pressure and solar input are solely responsible for temperature, that they are therefore saying that an argon atmosphere can permanently raise the planetary surface temperature, then you’re not paying attention.
I give up. Do what you want to do. Lots of people here, people who work in the field, people who teach in the field, have told you over and over that you are wrong on any number of your foolish claims, and exactly where you went off the rails.
Your response?
Nada. Nothing. Nil. Zip. Zero.
I give up. When a man is as determined to remain mired in ignorance and misunderstanding as you are, I gotta admit that you are far beyond my poor power to add or detract.
As I said, I used to believe what you believe and N&Z believe, that pressure alone can raise planetary temperatures. Heck, I even argued with Dr. Robert Brown about it …
But unlike you, I LISTENED TO HIM AND LEARNED FROM HIM … but noooo, not Trick, he knows everything already.
Spare me …
w.
“I used to believe what you believe and N&Z believe, that pressure alone can raise planetary temperatures.”
Total pressure is part of the equation for the opacity of an atm., not the partial pressures of each of its constituents so increasing total pressure alone is shown to increase atm. opacity by theory and confirmed by test. The mass mixing ratio and opacity of each absorber present are also part of the eqn. and tests/observations.
If you want me to spare you the details, I’ll stop there, per your request. However, you are the one raising the questions and seem to be interested in discussing the answers. Science appears to have at least partially answered them already. There are also some known unknowns and possibly unknown unknowns that are interesting to discuss in this field.
If it matters, I find little if anything novel in any of N&Z (V&R) work even in their latest (2017) paper.
Trick December 3, 2017 at 7:09 am
Thanks, Trick. I’m unclear about why you bring up atmospheric opacity. Are you saying that opacity can permanently increase the surface temperature?
Regards,
w.
”Are you saying that opacity can permanently increase the surface temperature?”
Yes.
For a completely transparent argon atm. of Earthian parameters the terrestrial LWIR 100% escapes to deep space and the atm. opacity eqn. is identically zero as there are 0.0 absorbers. If Ar has any nonzero absorptance of that LWIR at all, then global atm. opacity eqn. increases above 0.0 driving the surface energy balances and global median temperature increase above ~255K. Some could argue if the absorbance is not measurable then what’s the difference. I would agree no currently measurable difference is as good as none.
The answer to your original question hinges on this science.
Phil. comments 0.0 absorbency is the case for atomic Ar at normal temperatures in Earth lower atm. as the rotational 3-D spin of the atom is not quantized so absorbs no light quanta. Or at least it is not measurable. Thus, your answer is No. This differs from what is found in C. Bohren’s 2006 text on Atm. Radiation where the science answer is YES.
It is well recognized rotational energies associated with the spinning of atoms of finite extent about three mutually perpendicular axes do not enter into the determination of specific heats of atomic gases at normal temperatures. However, Ar not having quantized rotational energy levels is new for me. Argon does have a specific heat (about half that of air).
What is not well recognized so far as I know per Phil. is that atomic Ar spin energy levels are not quantized at normal temperatures. It could be well known in the specialist world though, since the NIST ref. is from 1973. This is interesting aspect for me to dig into, but it will take some time going through source material.
SkepticGoneWild December 3, 2017 at 1:47 am
I don’t think you’ve thought your example all the way through, SGW.
If you do that, the hairdryer is sure to get hotter … a perfect example of a cooler object (the mirror) making a warmer object hotter than it would be without the cooler object …
w.
You do realise the internet is like a permanent record of your comments?
Willis, I share your negative opinion of Trick but not your positive opinion of Robert Brown’s postings. When I dealt with him in another thread he appeared to have little knowledge of convection, lapse rates and adiabatic processes so I gave up on him.
Therefore, I would hope that you can look at those issues afresh in light of my post at 9.48 a m on the 2nd December.
Stephen Wilde December 3, 2017 at 5:21 am
If you “gave up” on Dr. Brown, then more fool you … regarding your posting.
Stephen Wilde December 2, 2017 at 9:48 am
Willis:
OK …
OK …
Well, we don’t know that. The energy might have come from vulcanism.
Me, I would say the extra 33K came from the sun, not the surface or from vulcanism. Here’s the accounting. Atmosphere forms, contains GHGs, sun warms the surface, GHGs intercept outgoing LW, send half of it back to the surface, surface warms up … what’s not to like?
However, you are missing something very important. If there is no atmosphere, there are no clouds … and with no clouds, the sun would put an additional 70 W/m2 into the system constantly. So after the atmosphere forms, the surface receives less solar energy …
This is why people are asking for a proper accounting … your method leaves things out.
No, because a) the 33k is a one-off change which will quickly equalize out, and 2) the system is now getting 70 W/m2 LESS because there are clouds.
I don’t understand why you think that if you add energy to the atmosphere or take energy out of the atmosphere, that will cause a permanent change in the energy balance. If the atmosphere contains GHGs, the excess energy will be radiated to space. If the atmosphere doesn’t contain GHGs it will be absorbed by the earth.
A one-time addition or subtraction of energy to or from the atmosphere cannot cause a persistent change to the energy flow in the system. If that were so, the energy lost due to every volcano would keep adding up, and we’d be freezing by now.
Say what? I don’t understand that last one at all. I counter your assertion that a one-time event four billion years ago affects today’s energy flows by pointing out that this is clearly not true for other such one-time events.
w.
i) The initial energy required to form an atmosphere does come from the sun.
ii) In order to ascertain the extent of that initial energy only the adiabatic component is relevant. Everything else is just variability around that initial addition of energy. For Earth we can see that the amount of that energy is enough to raise surface temperature by 33k and it is held in the atmosphere as potential energy that does not radiate and does not register as heat until it returns to the surface in adiabatic descent.
iii) If the atmosphere continues to be suspended against gravity by the upward pressure gradient force then that initial energy added during formation continues to be present. It cannot dissipate without collapsing the atmosphere.
iv) Energy added by vulcanism, ghgs or anything else is short term because it does dissipate via convective adjustments neutralising any internal radiative imbalances unlike the initial energy used to lift up the atmosphere.
v) I invite you to consider my workings showing how by using a stocktaking analogy you can start with a specific amount of material but by making a single change at the right time you then hold extra units in stock permanently. You can find that in my response to Paul after he first introduced such an analogy which was flawed. Conduction and convection cause a delay in the throughput of energy through a planetary body so that the amount of energy content is raised to a level that can support both radiative and adiabatic balance.
It really is as simple as that.
You have an up and down adiabatic loop running in parallel with the in and out radiative flow and you need enough molecular agitation at the surface (heat) to maintain both.
Everyone is hung up on unnecessary complexities.
Stephen, if I have a system with two 255 K rocks, with one moving with KE of 200 joules and the other stationery with 300 joules of PE, What is my system radiating?
Unfortunately, that is not a suitable analogy because it invites consideration only of radiative flows operating at the same speed.
One needs a gaseous body with internal movement within a gravitational field in contact with an irradiated solid body.
Your mind is still locked into radiation only scenarios.
What we have to consider is the interplay between radiative flows or fluxes in and out to one body which run at one speed and non radiative energy transformations between two types of energy one of which registers as heat whereas the other does not AND furthermore the two systems are in contact so that conduction can occur.
The most elegant and satisfactory solution is that which I supplied. Two independent energy ‘loops’ running in parallel after the gaseous part has stabilised at hydrostatic equilibrium.
Until you shed the radiative only scenario you won’t be able to grasp the concepts involved but I assure you that my description complies with basic physics.
I find your latest a bit vague and appreciate some clarificacation…..
Here you say,
“What we have to consider is the interplay between radiative flows or fluxes in and out to one body which run at one speed”
Specify the nature of these flows and put same example numbers or Labels on them.
Then you say,
” and non radiative energy transformations between two types of energy one of which registers as heat whereas the other does not ”
Again, nature of flow and some numbers or labels.
Finally…..
“The most elegant and satisfactory solution is that which I supplied. Two independent energy ‘loops’ running in parallel after the gaseous part has stabilised at hydrostatic equilibrium.”
Describe these loops, nature of flow and sample numbers or labels.
Thanks. It would really help my understanding to have some common labeling to discuss further
I’ve already told you several times.
33k up and down adiabatically which is a non radiative process.
255k in and out which is a radiative process.
The two processes combine at the surface to give 288k.
The ‘extra’ 33k was acquired from convection and conduction when the atmosphere formed and is not removed until the atmosphere falls to the ground.
You can’t ignore the 33k coming down simply because it matches 33k going up because there is a time delay of one convective cycle. Once the atmosphere is in place the 33k going up is not taken from the simultaneous downward leg but from the upward leg one cycle previously so the current downward leg of 33k is always a net positive and must be added to the radiative 255k.
That is implicit in the stock control example that I gave you previously.
Correction:
You can’t ignore the 33k coming down simply because it matches 33k going up because there is a time delay of one convective cycle. Once the atmosphere is in place the 33k going up is not taken from the simultaneous downward leg but from the DOWNWARD leg one cycle previously so the current downward leg of 33k is always a net positive and must be added to the radiative 255k.
“Me, I would say the extra 33K came from the sun, not the surface or from vulcanism. Here’s the accounting. Atmosphere forms, contains GHGs, sun warms the surface, GHGs intercept outgoing LW, send half of it back to the surface, surface warms up … what’s not to like? ”
So how does the atmosphere form other than by conduction from the irradiated surface followed by convective ascent followed by convective descent.
GHGs play no part in it because they don’t appear until after the gases have lifted off.
That being the case the surface temperature enhancement comes from conduction and convection. If you then add another 33k from DWIR the surface temperature would be 321k not 288k
I made this comment upthread but it seems to have gotten lost in the stream. For the people who are in the school that says cooler sourced IR can not be absorbed by warmer targets……..
Without changing the subject, and without any need for models or esoteric physics nobody is talking about, answer this simple question…….
Here’s the shot:
Your claim is that IR from a 90 k source incident on a 91 k target, does nothing to the target. If true it has to then either pass through it (albedo of 0) or be reflected from it (albedo of 1). You further claim if that very same source hits the very same target but now the target has a temperature of 89 k it is absorbed, at least partially.
Here’s the chaser:
If you would grant me the liberty of putting that in an equation, it would be albedo= f(T). Do you agree that my equation correctly supports your claim?
Ben Wouters December 3, 2017 at 6:31 am
Obvious? That’s not “obvious” at all. If it were, the moon would be up somewhere near the Earth’s temperature. In fact, we get less net radiation than the moon. We get 340 W/m2 from the sun, of which about a hundred are reflected back to space. The remainder, 240 W/m2, would heat a blackbody to a maximum temperature of about -18°C.
So no, Ben, Stefan-Bolzmann is quite clear about this. It isnot physically possible for 240 W/m2 from the sun to bring the earth up to its current temperature without the poorly-named “greenhouse effect”.
w.
Willis Eschenbach December 3, 2017 at 11:27 am
Since we have only one sun, incoming solar illuminates only half a planet. The other half is “illuminated” by the Cosmic Background Radiation. A blackbody in RADIATIVE balance with these radiation flows (using albedo 0,11 and emissivity 1.0) would have a temperature of ~162K.
Actual average temperature of the moon is ~35K higher at ~197K due to geothermal heat plus some heat carried over from the dayside.
Do you really believe that our atmosphere is the reason the average surface temperature on Earth is ~288K,
over 90K higher than the moons?
Follow up question:
How did our deep (below 1km) oceans get a temperature of around 275K, already 20K above the 255K (-18C) you mentioned as the maximum temperature the sun can create on Earth?
Ben, do you know how many Watts/m2 that 20K would represent?
A C Osborn December 4, 2017 at 11:29 am
Sorry. Don’t understand your question. The ~275K represent the energy content of the deep oceans.
W/m^2 is an energy FLUX.
Ben, thank you for the response, I do realise the difference between Flux and temp.
I was trying to go back to first principles in the Sphere/Shell Example.
I have calculated the mass of a Steel sphere with a 1M surface Area to establish how many Joules it would take to warm it 1K.
But can the Sphere also radiate with a heat flux of 240W/m2 at 0.1K, or 0.001K?
Adding a steel shell even 1mm thick, which obviously is not very realistic, would very slightly increase the amount of Steel to be heated and as the heat source is fixed would this only slow down when it got to radiate 240W/m2 and would the overall temperature be lower?
Ben Wouters December 4, 2017 at 10:33 am Edit
Thanks, Ben. I’m generally careful about what I say. Note that I said that 240 W/m2 (which is a 24/7 average over the surface of the sphere) would heat, not the Earth, but a blackbody, to a MAXIMUM of -18°C. I stand by that statement.
The actual temperature of the earth if there were no GHGs will be lower than that, but how much lower depends on your assumptions. There’s an excellent post on the question here by Dr. Robert Brown, who teaches physics at Duke.
w.
Ok. Do you agree that the sun only illuminates half a planet and that no physical mechanism can distribute that radiation around an entire planet and then create RADIATIVE balance with the incoming radiation?
If yes a simplistic blackbody calculation could be:
1364 W/m^2 /2 = 682 W/m^2 average radiation on the day side giving ~ 331K radiative balance temperature.
(albedo 0,0, emissivity 1,0)
The other half of the blackbody would be at ~3K so the average temperature of a blackbody in radiative balance would be ~167K (actually a bit lower still, since solar isn’t distributed evenly over the day side)
A theoretical blackbody has no heat storage capacity, so rotation rate is irrelevant.
Going from this simple model to the actual moon is pretty straightforward.
Earth is way more complicated.
Thanks for the Dr. Brown post. He seems to agree with this (simplistic) approach.
One essential element is missing in his post, geothermal energy.
To answer my own question on how the deep oceans got their ~275K temperature:
by cooling down from much higher temperatures (350K or even higher)
See this video between ~4:30 and 12:50 for a possible/likely scenario:
https://www.youtube.com/watch?v=gTsJubN68WE&t=2263s
I’m pretty confident I have a mechanism that explains how the temperature of the deep oceans has been maintained over time, how the sun only increases the temperature of the upper layers of the oceans creating the observed surface temperatures, and why the role of the atmosphere is ONLY reduction of energy loss to space, no increasing of the surface temperatures necessary or possible.
So yes, without atmosphere the surface would cool down, and no, this does not mean that the atmosphere increases the surface temperatures.
Ben, thankyou yet again.
I have been arguing with Mr Eshenbach and others about the fact that the the long term Average Temperature is not a Steady State or Equilibrium if everything in the Atmosphere including inputs, outputs and temperatures are in a state of continuous change.
Someone invoked “Dynamic Equilibrium”, but there again the Flux is varying from night to day, year to year and for other periods.
Otherwise how could the Earth have cooled from it’s beginning and from normal to Ice Age and Ice Age back to normal.
Surely Time is a factor in all Flux calculations, ie The SI derived unit of heat rate is joule per second.
A C Osborn December 6, 2017 at 9:31 am
Biggest problem imo in discussing Earth is the adherence to RADIATIVE balance. Works more or less for the moon since it has a surface with very little heat storage capacity. Totally different on Earth, ~70% of the surface is water and several kilometers of it 😉
Incoming solar is ~1364 W/m^2 times the area of a disc with the same radius as Earth.
~30% is send back to space and lost for planet Earth.
~20% is thermalized in the atmosphere (O2, O3, H2O and CO2 mostly) and increases its temperature.
~50% is thermalized at the surface (day side) and increases the temperature of the upper ~10m of soil and ~200m of the oceans.
This energy is in the end lost again as radiation into space from all around the world, mostly via the atmosphere which slows this energy loss (some radiation comes directly from the surface via the atmospheric window).
If the in- and outbound fluxes carry the same energy we have a balanced ENERGY budget, but nothing like a RADIATIVE balance.
Willis,
(I think my original attempt at this reply showed up in the wrong place so I will repeat it here – apologies for any confusion)
Thanks for adding to my reply to Phil, but you did not actually reply to my original question to you which I will ask again:
Consider a white hot bar of steel weighing 1 Kg with a temperature of 1500 c. If an identical bar, also at 1500 c is placed next to it, the temperature of the first bar will not increase. In fact, if an infinite number of new bars, all at 1500 c, are added, the temperature of the first bar would still not increase.
Thus, despite an infinite increase in energy, there would be no temperature increase. This seems to contradict the energy budget approach to deriving temperatures in that no matter how much extra energy was added, the temperature would not increase.
I’m wondering how the Trenberth Energy Balance chart would cope with this scenario of extra energy with no temperature effect?
Best regards
Thanks for persevering, Bernard. I fear that your question is not specific enough.
Let’s further assume that the bar is placed in outer space, nothing around. As a result, it will start to cool at a certain rate. It is radiating a lot (about a half million W/m2), and receiving basically nothing from the environment. Remember, the cooling rate is dependent on what it is gaining less what it is losing.
Now, let’s repeat the experiment, but this time place another identical bar next to the first bar. Again, both bars will cool … but the important point is thatthe first bar will cool more slowly than a single bar. Why? Because in part of the view field of the first bar is no longer receiving ~0 W/m2 from outer space, it is now intercepting and absorbing a part of the half million W/m2 of radiation from the second bar. This perforce must slow the rate of cooling of the first bar.
As a result, and as I’ve said many times, the first bar will end up warmer than it would be without the presence of the second bar.
Is there a “temperature increase” as you ask? No. But then I didn’t say there would be. I said it would end up warmer than without the presence of the other object.
Hope that helps …
w.
Willis Eschenbach December 3, 2017 at 1:06 pm
Thanks for the reply Willis,
I do understand that you are saying that the key effect is to slow the cooling rather than increase the starting temperature. That is not my question.
My question was: In my example, why does adding infinite amounts of new energy to a bar of metal not increase the temperature of the bar?. That seems to contradict the Trenberth Energy Balance logic?
Best regards
Bernard, if you added “… infinite amounts of new energy to a bar of metal …” not only would there be no bar, there would be no universe.
Willis Eschenbach
December 3, 2017 at 1:06 pm: Adding another bar is to completely alter the experiment. It is no longer the same set of conditions when you add MATTER to thereference frame. There is more MASS.
I could do the same with gas. Such is the case for Venus-Earth comparisons, allowing for AU distance and gravities. But it is also true that the reason there are gas laws is that their matter phase is different than solid metals’. So their means of balancing energy flows are not identical, another story. However, the addition of mass may be completely explainable by you, of course. That is what makes physics interesting…..
Bernard Lodge December 3, 2017 at 3:15 pm
Regards, Bernard. It only seems that way because you haven’t specified your situation clearly enough. If your bar is floating in space, then it is cooling and putting another identical bar next to it will slow the cooling.
On the other hand, if it is in a 1500C oven, then it is at steady-state with the environment and is not cooling. It is receiving and emitting the same amount of energy. However, it is already receiving the 1500°C worth of energy from the walls … so putting another 1500°C bar in does nothing. It blocks out the amount of energy it is supplying, so there is no net gain.
The key is the idea “in place of what”? If you put a 1500°C bar next to something in space, it is replacing zero watts of radiation from space with half a million watts of radiation … so things will change.
But if you put a 1500°C bar next to something in a 1500°C oven, it is replacing half a million watts of radiation with half a million watts of radiation … so there’s no temperature change.
Hope this helps, if not, ask questions …
w.
Willis Eschenbach December 3, 2017 at 8:05 pm
“Bernard. It only seems that way because you haven’t specified your situation clearly enough. If your bar is floating in space, then it is cooling and putting another identical bar next to it will slow the cooling.”
+++++++++++++++++++++++
If one bar of metal at 1500 c is then joined by a million bars of metal all at 1500 c, it does not matter where the first bar is – it could be in an oven or a fridge or in outer space. My question is why the extra trillion watts of energy from the new bars does not increase the temperature of the first bar? A really basic question.
If I had added a second bar at 3000 c, the temperature of the first bar would have gone up. However, the energy in one bar at 3000 c is much less than the total energy from a million bars at 1500 c so why does the little bit of energy from the 3000 c bar increase the temperature of the first bar when a trillion watts from 1500 c bars could not?
In the Trenberth Energy Balance, all the energy flows are converted into temperature increases. Yet, in my example above, I introduced a trillion new watts of energy flow and the temperature of the first bar did not increase. It seems in real life that not all energy flows increase the temperature of an absorbing body. Trenberth’s energy budget assumes they do. On the face of it, the Trenberth Energy Budget looks to be incomplete, or maybe even wrong. Perhaps it needs to include the temperature of the source of each radiation flow, not just its energy content?
I hope my question is now clear.
Best regards
It is always best to keep your first steps baby steps. It helps you understand the basic premise before you expand to a million bars.
Most importantly don’t divert the discussion as a debate tactic. Very very common in these topics.
So keep it simple, very simple. Start with two thin plates with geometry such that radiation off their edges is irrelevant. Put them in Vacuum facing each other and start experiment with exactly same temp for each.
What happens? Each plate radiates in two directions. One direction, away from other plate. The other towards its mate.
In the space between the plates there is no NET radiation. Neither plate can change the energy of the other. Each is blasting the other with the same flow, like filling your pool while the fire dept. Pumps it out just as fast.
In the direction away from plates, each plate is radiating away half of the energy it would have radiated if it was alone.
The plates will cool half as fast as they would have had they been alone.
Now if some clown enters the thread and says, “yeah but they’re not rotating and there’s no convection, and the micro wedgies don’t have enough freznoodles to heat anything.” Remember these are clowns, in the arena dirt because they couldn’t get a ride on the bull.
Focus on the example. It’s really simple. You can add 999,998 plates later. Forget temperature it is irrelevant to understanding. Energy is the nut
Bernard Lodge December 4, 2017 at 4:43 pm
Willis Eschenbach December 3, 2017 at 8:05 pm
Thanks, Bernard. Your question is clear. It is your assertion that “it could be in an oven or a fridge or in outer space” that is wrong. Those lead to very different outcomes.
The part you appear to be missing is the part about what the next bar replaces. If we put a 1500°C bar next to another one in a 1500°C oven, there will be no change. Think about it from the point of view of the first bar: where before there was an oven wall radiating at half a million W/m2, now there is a second bar radiating at half a million W/m2 … so absolutely no change in the radiation absorbed by the first bar.
HOWEVER, if the first bar is floating in space, then you’ve replaced basically zero radiation from space with half a million watts of radiated energy … so yes, that changes the radiation absorbed by the bar.
Finally, you seem to think that surrounding a bar of metal at 1500°C with a bunch of metal at 1500°C will somehow raise the temperature of the first bar … but consider the condition of a metal bar in a 1500°C oven. It is totally surrounded by the oven walls at 1500°C.
Does it warm to something more than 1500°C??
Hope this helps, if not, ask more questions. On my planet, the only stupid question is the one I didn’t ask …
w.
Paul Bahlin December 4, 2017 at 5:28 pm
Paul, while you certainly have my thanks for a very clear exposition of the issues, as an erstwhile cowboy I must object to this comparison.
In bull riding the people who keep the riders safe haven’t been called “clowns” for some time now. They are now called “bullfighters”, and for a damn good reason. Their job is to fight the bull and keep him from turning on the fallen cowboy. The bull riders entrust their lives to the bullfighters because without them a lot of good men would have been stomped to death. And despite your claim that “they couldn’t get a ride on the bull”, some of them are former bull riders who couldn’t get the smell of the bulls and the cloying arena dust and the roaring of the crowds out of their systems …
Just saying … the bullfighters are not a group you’d want to diss in a bar around where I grew up.
Other than that?
Your comment was spot on.
w.
Yeah. I figured I would get heat if a rodeo guy read that. I have great respect for those guys. They probably have more stones than the riders.
Anyway, hope you got my drift. It really frosts me when trolls step on an honest attempt to learn
“Me, I would say the extra 33K came from the sun, not the surface or from vulcanism. Here’s the accounting. Atmosphere forms, contains GHGs, sun warms the surface, GHGs intercept outgoing LW, send half of it back to the surface, surface warms up … what’s not to like? ”
So how does the atmosphere form other than by conduction from the irradiated surface followed by convective ascent followed by convective descent.
GHGs play no part in it because they don’t appear until after the gases have lifted off.
That being the case the surface temperature enhancement comes from conduction and convection. If you then add another 33k from DWIR the surface temperature would be 321k not 288k
Stephen Wilde December 3, 2017 at 1:10 pm Edit
From the Smithsonian:
If the gases were indeed “spewed from volcanoes”, your theory that we needed to put energy into the atmosphere as it formed goes out the window. The atmosphere would have been hotter than the surface to start with … and that’s the opposite of your theory.
But that’s not the main objection to your theory. The main objection is that even if your claim is correct and energy was pumped into the atmosphere four billion years ago, the effects of that one-time event on the flow system we call the climate are long gone.
For example, yes, dumping a truckload of water into a creek will raise the level of the creek … but not for long. And dumping a bunch of excess energy into the atmosphere, say by a solar flare or something, will raise the atmospheric temperature …
… but not for long, and certainly not for four billion years.
w.
In the absence of solar heating at the surface being conducted to all the materials from volcanoes those materials would cool and fall to the ground so your objection is invalid.
Anyway, you are distracting from the initial premise of a simple argon atmosphere.
As for your ‘main’ objection please explain the continuing presence of a vast amount of non radiative non thermal PE still in the atmosphere if you say it has all dissipated away.
How exactly do you say that energy could have been removed or otherwise cancelled by some sort of unspecified setoff.
Stephen, you often write the atm. is by and large in hydrostatic equilibrium. This means there is very little available PE to convert to KE. Only when the atm. is out of this equilibrium does it transfer PE to KE. By simple logic.
Whilst the atmosphere is in hydrostatic equilibrium overall the fact is that all rising air and all falling air is in disequilibrium.
If you study meteorology (as I have done for 60 years) and look at the global pressure chart you will see that the entire planet is divided into low pressure cells that are rising air and high pressure cells that are falling air.
Accordingly the entire mass of the atmosphere is constantly engaged in converting surface KE to PE or back again.
The total of KE and PE is vast and it is that energy which provides the upward pressure gradient force that opposes the downward force of gravity.
Actually, first order consideration is atmosphere has mass, all of it taken together, that is held up by the surface
More correctly, it is held up by the force exerted by the surface which is equal and opposite to the force exerted by gravity. It takes no work to do so
Work is required to launch it off the surface in the first place and more work is required to move it upwards in convection. That work converts KE to PE as the molecules move apart along the declining pressure gradient.
The gas is held off the surface by the upward pressure gradient force which requires kinetic energy at the surface to create it. A gas held off a surface is a very different to a solid held above a surface by contact.
Gee whiz, never knew that Stephen.
Atmospheric pressure is the weight of the atmosphere pressing on the surface. It has nothing at all to do with the structure of that atmosphere. The earth exerts an equal and opposite force to it.
If you consider a column, it doesn’t matter if the column has each molecule stacked on one other in a row extending to Pluto, or mashed into the ground like a pancake, or arranged in a magnificent arrangement of layers provided by a pressure gradient. The mass is the same.
You are conflating that which gives it structure, with that which keeps it where it is.
Stated more succinctly….A box of rocks has mass. Shaking it does not change it
You said:
“More correctly, it is held up by the force exerted by the surface which is equal and opposite to the force exerted by gravity. It takes no work to do so”
That gave me the impression that it was you who had conflated that which gives the atmosphere structure with that which keeps it where it is.
To resolve your apparent confusion I pointed out that the declining pressure gradient with height combined with kinetic energy at the surface does do work against gravity to create the upward pressure gradient force.
So, actually, that is what gives the atmosphere structure and keeps it where it is and NOT the mere presence of a surface beneath it.
If you are still using solids such as rocks as an analogy then you have still not got your head around the behaviour of gases as compared to solids.
An increase in molecular motion at the base of a column of gas will cause an increase in the upward pressure gradient force and allow it to expand further upward against gravity.
No molecular motion at the base will cause the gases to fall to the surface.
So 60 years in meteorology and you don’t think atmospheric pressure exerts a force on the Surface?
What’s the difference between
What is the pressure exerted on the surface by 1 kg of rocks? What’s the pressure exerted on the surface by a 1 kg column of air?
Not the point.
The point is that whatever the weight of an atmosphere the formation and maintenance of it depends on kinetic energy at the surface in excess of that predicted by the S-B equation and I’ve given you the same explanation multiple times in different formulations.
The atmospheric gases depart the surface without any input from GHGs so the conduction/convection explanation must be the correct one.
If one says that GHGs account for the surface temperature enhancement such that convection reduces the surface temperature from 255k to 222k and then returns it to 255k for a zero net effect and then GHGs radiate down to raise it to the observed temperature of 288k then you still have to account for yet another 33k worth of incoming radiation which was not being used by the system for the production of IR during the period of lowered surface temperature. What do you say happens to that further 33k?
It just won’t balance if GHGs are proposed as the cause because then you have to propose 321k instead of 288k.
Stephen the atmosphere is not ‘held up’by kinetic energy flowing in from the surface by conduction. It is ‘held up’, and I mean by that no net movement of mass, by the freaking surface.
The thermal energy fed to it by the surface can only heat air, causing differential density in the (fluid) atmosphere, which results in differential density, which results in buoyant forces, which results in air moving up. The mass in a convecting atmosphere is not changing. It is moving.
For every parcel moving up there is a parcel, somewhere, moving down, else we wouldn’t even be here. If you integrate these energies over a reasonable volume, say the planet, and time, say a million years, you must find that moving air and the lapse rate that develops from such movement does not, and can not effect the mass. It can not effect the net acceleration of that mass, which is 0. It effects the instantaneous structure, not the static properties of the entire system. Mass for instance.
I suspect this entire thread has been predicated on your assumption that the atmosphere needs energy to ‘stay up’ there. It doesn’t. It only needs a force to balance the force on it from gravity. Force is not energy. Potential energy is not energy. Energy requires mass to be displaced, net work to be done. The ‘atmosphere’ is not moving. Its parts are moving.
You seem to be describing a system kept in perpetual motion by events that occurred 4 billion years ago. All that PE that was put up there then is still there.
Finally, do a little thought experiment for me….
If there was suddenly no sun, What would keep the atmosphere ‘up there’ and what would the structure be? All that mass would still be above the surface and the temperature would be?????
Too many incorrect statements in there for me to have the will to respond.
Try them one at a time then…..
Does convection change the mass of the atmosphere?
Or this one. Does the atmosphere exert pressure on the surface of the planet?
Is potential-energy energy?
Does KE exchange energy by conduction?
Any one answer will do. You’ve made every one of these things into gobbly gook over the thread.
Stephen 4:43am, you just can’t have it both ways.
Either: “Accordingly the entire mass of the atmosphere is constantly engaged in converting surface KE to PE or back again.”
OR: “Whilst the atmosphere is in hydrostatic equilibrium overall”
If you study meteorology, then a paper written in the 1950s should have told you this. It is basic to the field. There is very little atm. PE available to transform into KE but you have to read the math to understand it and you will never do so. You will never understand this field of meteorology without mastering the math describing the changing processes. Simple logic.
Incorrect.
When energy up equals energy down both statements are compatible.
That doesn’t prevent lots of imbalances either side of the average.
When energy up equals energy down, energy net is zero.
Trick December 3, 2017 at 6:48 am Edit
Trick, once again I have to ask you to QUOTE THE EXACT WORDS THAT YOU ARE REFERRING TO!!!!
Maybe Bohren said something, maybe not. But until you QUOTE WHAT BOHREN SAID, it’s just your lips flapping …
w.
Bohren 2006 p. 4:
We often are told that when bodies are heated they radiate or that “hot” bodies radiate. True enough, but it is just as true that when bodies are cooled they radiate and that “cold” bodies radiate. All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times, although in varying amounts, possibly so small at some frequencies, for some materials, and at some temperatures as to be undetectable with today’s instruments (tomorrow’s, who knows?). Note that there is no hedging here: all means all. No exceptions. Never.
Bohren 1998 p. 119:
According to classical mechanics, a rotating object has rotational energy, which you can verify by trying tro stop a rotating bowling ball by your hand. According to quantum mechanics, the quantized levels of similarly spinning atoms are widely spaced relative to kT. This is why we obtained good agreement with measurement of (ratio of specific heats) for monatomic gases…
—–
Others:
Atomic and Molecular Processes edited by D.R. Bates
(Atomic) rotational quanta are much smaller than (molecular) vibrational quanta, and the probability of a translation-rotational energy transfer occurring in collision is correspondingly higher. Rotational relaxation times are therefore very small. Rotational relaxation phenomena thus play a comparatively unimportant role, and are difficult to measure. This, coupled with the fact that rotational energy is of less chemical significance than vibrational energy has led to rotational relaxation being given comparatively little attention.
Atoms By Jean Perrin
p. 73: Rotational energy does indeed vary by indivisible quanta like the atomic oscillation …dealing with rotations so rapid that each atom revolves more than a million times in one thousandth of a sec.
—–
There are more texts, papers I have yet to obtain & more leads from the 1973 NIST testing ref.s yet to be uncovered.
Thanks, Trick. The claim that
doesn’t even pass the laugh test. Take O3, for example. It only absorbs radiation in a few narrow bands. Kirchoff’s Law says that means it only emits radiation in a few narrow bands. I don’t know who Bohren is, but he doesn’t agree with lots of spectrographic results.
“All frequencies”, for example??? So every object we see is emitting radiation at every single frequency from one hertz to terahertz? Get real. That’s not happening.
I do love his CYA statement that although the most sensitive instruments known to man cannot detect such radiation, by gosh, he knows it is there … and you believe him? Really? HOW does he know it is there if nobody can either measure it or find it?
His are not scientific statements. They are statements of faith.
w.
“Take O3, for example. It only absorbs radiation in a few narrow bands.”
Now Willis is asserting (i.e. “it’s just your lips flapping”), please: “QUOTE THE EXACT WORDS THAT YOU ARE REFERRING TO!!!!”
“So every object we see is emitting radiation at every single frequency from one hertz to terahertz?”
Yes. And lower and higher freq.s. Plug any frequency and any temperature into the Planck Law and you will get an ideal nonzero radiance. This Law is what Bohren goes on to explain – found from actual testing! Maybe even Willis can learn from these discussions.
Trick December 4, 2017 at 4:20 am
“Take O3, for example. It only absorbs radiation in a few narrow bands.”
Now Willis is asserting (i.e. “it’s just your lips flapping”), please: “QUOTE THE EXACT WORDS THAT YOU ARE REFERRING TO!!!!”
“So every object we see is emitting radiation at every single frequency from one hertz to terahertz?”
Yes. And lower and higher freq.s. Plug any frequency and any temperature into the Planck Law and you will get an ideal nonzero radiance. This Law is what Bohren goes on to explain – found from actual testing! Maybe even Willis can learn from these discussions.
Planck’s Law applies to black bodies, gases are not black bodies and only emit at discrete wavelengths determined by quantum mechanics. Apparently Bohren doesn’t understand quantum mechanics. Planck’s Law gives an upper limit to emissions that’s all. Actual testing with gases bears this out, here’s one for Argon that I posted earlier:
Note it emits at discrete wavelengths not in a Planck continuum!
Phil, no fair; you are bringing data to a verbal gunfight.
Is there any recognised Scientist that MR Eshenbach is not prepared to rubbish other than Dr. Robert Brown and Leif, jesus what an EGO.
“They are statements of faith.” now who do we know that that description applies to?
A C Osborn December 4, 2017 at 11:39 am Edit
A C, if you assert as Bohren does that all object radiate at all frequencies at all times, and follow that with a statement that if we can’t find said radiation it’s because our instruments are not sensitive enough … sorry, but that is a statement of faith.
Remember Feynmann, he’s always worth listening to. He said:
w.
“Planck’s Law gives an upper limit to emissions that’s all.”
Yes, note the word ideal. Ideal radiance at all frequencies all temperatures all the time as Bohren notes.
To add to Willis’ comment: “Those of you who have heard the other two lectures will also find this lecture incomprehensible, but you know that that’s all right: as I explained in the first lecture, the way we have to describe Nature is generally incomprehensible to us.” Richard Feynman.
“Note it emits at discrete wavelengths not in a Planck continuum!”
What gas pressure? What power? Exposure time? To observe the continuum see results with much, much longer exposures.
The 1973 NIST data was produced at around 0.2-0.4torr and 1 to maybe 10 watts of power (assuming 120 volts), with such weak emissions some exposure times were for an hour. The apparatus used is not fully explained as it was in common use for a long time, they don’t even mention temperature just a 1.5L circulating water jacket with a thermostat. No setting given, just a ref. to earlier work to describe the apparatus maybe 20 years earlier (1953) that will be even harder to find.
Trick December 4, 2017 at 2:52 pm
“Planck’s Law gives an upper limit to emissions that’s all.”
Yes, note the word ideal. Ideal radiance at all frequencies all temperatures all the time as Bohren notes.
He doesn’t mention ‘ideal’.
Trick December 4, 2017 at 12:51 am
Bohren 2006 p. 4:
We often are told that when bodies are heated they radiate or that “hot” bodies radiate. True enough, but it is just as true that when bodies are cooled they radiate and that “cold” bodies radiate. All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times,
Not true this does not apply in the case of gases!
although in varying amounts, possibly so small at some frequencies, for some materials, and at some temperatures as to be undetectable with today’s instruments (tomorrow’s, who knows?). Note that there is no hedging here: all means all. No exceptions. Never.
The hedging is where he says that is might not be detectable!
Bohren 1998 p. 119:
According to classical mechanics, a rotating object has rotational energy, which you can verify by trying tro stop a rotating bowling ball by your hand. According to quantum mechanics, the quantized levels of similarly spinning atoms are widely spaced relative to kT. This is why we obtained good agreement with measurement of (ratio of specific heats) for monatomic gases…
There are no ‘quantized levels of similarly spinning atoms’ they are quantized levels of electronic excitation and as I’ve pointed out several times above their spacing far exceeds kT. It’s scary that someone can get a PhD in physics and publish books with such a fundamental misunderstanding of QM.
Trick December 4, 2017 at 12:51 am
Atomic and Molecular Processes edited by D.R. Bates
(Atomic) rotational quanta are much smaller than (molecular) vibrational quanta, and the probability of a translation-rotational energy transfer occurring in collision is correspondingly higher. Rotational relaxation times are therefore very small. Rotational relaxation phenomena thus play a comparatively unimportant role, and are difficult to measure. This, coupled with the fact that rotational energy is of less chemical significance than vibrational energy has led to rotational relaxation being given comparatively little attention.
Please note that the above ‘quotation’ from Bates is not accurate, the actual quotation is:
“Rotational quanta are much smaller than vibrational quanta, and the probability of a translation-rotational energy transfer occurring in collision is correspondingly higher. Rotational relaxation times are therefore very small. Rotational relaxation phenomena thus play a comparatively unimportant role, and are difficult to measure. This, coupled with the fact that rotational energy is of less chemical significance than vibrational energy has led to rotational relaxation being given comparatively little attention.”
The section it comes from is explicitly referring to Molecular processes, the addition of (Atomic) is deceptive!
The paragraph is comparing molecular rotational quanta with vibrational quanta, there are no atomic rotational or vibrational quanta. I don’t know where you got it from Trick but I would suggest treating that source with caution in future.
Atoms By Jean Perrin
p. 73: Rotational energy does indeed vary by indivisible quanta like the atomic oscillation …dealing with rotations so rapid that each atom revolves more than a million times in one thousandth of a sec.
This was published in 1913! He was explicitly talking about molecular rotations and citing the evidence for their quantization.
Just after Einstein and Bohr, before de Broglie, Heisenberg, Schrodinger, Born, Dirac, Pauli, Heitler and London to name just a few. The terms photon and quantum mechanics (quantenmechanik) didn’t exist yet!
Phil.: Thanks, these are just taken off the internet in quick scratch the surface searches to develop a reading list, Willis asked for what I had at the moment and I listed them (incomplete) for purpose – to attract a reply. I inserted the (words) as it seemed like that was the context reading the preceding page(s), so I differ with your comments. There is lot more work I need to do to complete dot connecting.
In 1913 I might point out the huge problems found between equipartition and specific heat testing were still a research subject from the late 19th century masters which wasn’t explained until QM. And this problem has been largely forgotten today. Would be interesting from a historical perspective to read Perrin.
“It’s scary that someone can get a PhD in physics and publish books with such a fundamental misunderstanding of QM.”
I would not recommend so hastily jumping to the classic internet futile tactic of attacking the person instead of the science. You need to read Bohren more thoroughly to appreciate his work in context, if interested enough. His source material includes a ref. to a delightful 1996 paper on this subject I would not have pulled had this discussion not occurred, for that I am grateful:
“Textbook treatments of specific heats of gases are discussed critically and thoroughly by Clayton A. Gearhart, 1996: Specific heats and the equipartition law in introductory textbooks. American Journal of Physics, Vol. 64, pp. 995-1000. Gearhart concludes that very few (of 27) physics textbooks treat specific heats correctly, yet another sad example of error propagation.”
Error propagation in textbooks is a recurring theme of Bohren’s as he repeatedly goes back to the source papers to compare current text book wording. Gearhart connects the dots from Perrin’s time to QM.
I’ve had 2or3 reads of the paper now and it will take more to absorb it all. For instance, the author explains why the 3 atomic rotational degrees of freedom are not needed for the heat capacity ratio that I mentioned earlier, only the translational DOF show up.
Plus this: “Atomic nuclei, of course, are no more rigid bodies than atoms or molecules. Nevertheless, collective “rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra.” A ref. to Cohen 1971 p. 147ff is given so I will now need to pull that work as this does not connect the dots to your writing. Then go back and try to connect the Gearhart dots to Bohren 1998 p. 119 treatment and your comments.
Dunno if my interest will persevere long enough (competing interests) but this task will likely outlive the life of this comment thread.
Ok Phil., I’m now convinced Gearhart 1996 adds enough to the answer that I’m satisfied dots are connected.
If still interested, what I find* re-reading Bohren, Gearhart is that the Ar atoms are indeed spinning and spectra from rotational base level to excited state has been measured for noble gases (see Cohen not NIST). Thus your “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.” is too simplistic an explanation for this very complex situation.
Classic physics calculates diatomic ratio of ideal gas specific heats 1.25 but all room temperature measurements showed closer to 1.4. This was a BIG problem at the time. Stumped all the late 1800s early 1900s physicists until QM was introduced. These guys at the time were offering odd ball solutions to protect classical physics similar to what you find in this comment thread et.al. .
Gearhart indicates when the QM was introduced it solved the problem of why certain DOF were excluded (from both monatomic and diatomic) in natural measurements but not by classical physics. Bohren writes this elimination of DOF guess had been eventually accurately made but no one knew why until QM explained it.
The quantum spacing of the rotational (monatomic spin and diatomic about the line joining the atoms) levels was too widely spaced relative to kT to matter at room temperatures where the specific heats/ratios were being measured, so some DOFs were ruled out by QM which then accounted for the measured 1.4 ratio. I am assuming for now this does go along with your base state comment iirc.
And it is consistent with the answer to Willis’ Ar atm. question being YES.
Note electronic excitation had nothing to do with the problem as they were much higher multiples of kT. NIST testing was a wild goose chase. It was the monatomic and diatomic QM rotational level DOF elimination considerations that explained the difference.
Gearhart explains this is now so uninteresting that it is being forgotten and written up wrongly in all but a few text books. He has a great story, must have spent a lot of time digging thru text books, something pissed him off to do so.
Says new authors are so hindered by the forgotten problem they seem afraid to rock the boat and just go along with other incorrect texts. Some word it incorrectly then put a small print footnote correction. Funny read.
*Recommend read the source material for the real stories first hand. I might pick up more on another re-re-read.
Trick December 4, 2017 at 6:57 pm
Ok Phil., I’m now convinced Gearhart 1996 adds enough to the answer that I’m satisfied dots are connected.
If still interested, what I find* re-reading Bohren, Gearhart is that the Ar atoms are indeed spinning and spectra from rotational base level to excited state has been measured for noble gases (see Cohen not NIST). Thus your “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.” is too simplistic an explanation for this very complex situation.
Is that the same Cohen referenced in Sandy Ashmore and Rog Donovan’s book? If so his work was on Argon hydrides not the same thing at all!
Gearhart mainly publishes on the history of quantum mechanics, however what I posted here is the current position of the subject, Argon does not have rotational or vibrational levels, your misreading and Gearhart’s misstatements notwithstanding.
Trick December 4, 2017 at 2:35 pm
Phil.: Thanks, these are just taken off the internet in quick scratch the surface searches to develop a reading list, Willis asked for what I had at the moment and I listed them (incomplete) for purpose – to attract a reply. I inserted the (words) as it seemed like that was the context reading the preceding page(s), so I differ with your comments. There is lot more work I need to do to complete dot connecting.
Yes I read the preceding page it refers to molecular spectra not atomic as you incorrectly stated. By the way when you claim to be quoting a reference it is important to clearly indicate any changes you made to it.
“It’s scary that someone can get a PhD in physics and publish books with such a fundamental misunderstanding of QM.”
I would not recommend so hastily jumping to the classic internet futile tactic of attacking the person instead of the science.
I’m not ‘attacking’ the author just expressing disappointment in his fundamental error!
”Is that the same Cohen referenced in Sandy Ashmore and Rog Donovan’s book?”
Dunno. I have the 1971 Cohen book on order, it is interesting that in 1996 Gearhart went all the way back to 1971 to find a ref. for noble gas spectra. But the NIST work goes all the way back to at least 1953 in their ref.s of the Ar apparatus used so there must have been some interest in Ar at those times.
”Argon does not have rotational…levels.”
Then your calculated ratio of Ar specific heats will be different than those you measure for Ar. You will have the same conundrum existing as before QM explained allowed atomic DOFs which Gearhart and Bohren explain.
”I’m not ‘attacking’ the author just expressing disappointment in his fundamental error!”
There is no error, experiments for the ratio of specific heats match calculations when the QM explanation for the atomic DOFs is understood to include quantum rotation.
Trick December 5, 2017 at 6:47 pm
”Is that the same Cohen referenced in Sandy Ashmore and Rog Donovan’s book?”
Dunno. I have the 1971 Cohen book on order, it is interesting that in 1996 Gearhart went all the way back to 1971 to find a ref. for noble gas spectra. But the NIST work goes all the way back to at least 1953 in their ref.s of the Ar apparatus used so there must have been some interest in Ar at those times.
If you can order the book surely you can give us the reference?
”Argon does not have rotational…levels.”
Then your calculated ratio of Ar specific heats will be different than those you measure for Ar. You will have the same conundrum existing as before QM explained allowed atomic DOFs which Gearhart and Bohren explain.
My calculated ratio is 1.66, that is also the measured ratio.
”I’m not ‘attacking’ the author just expressing disappointment in his fundamental error!”
There is no error, experiments for the ratio of specific heats match calculations when the QM explanation for the atomic DOFs is understood to include quantum rotation.
The error I referred to above was: “All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times,”.
However the ratio of specific heats for Argon does not allow for quantum rotation.
Trick December 4, 2017 at 2:35 pm
“Textbook treatments of specific heats of gases are discussed critically and thoroughly by Clayton A. Gearhart, 1996: Specific heats and the equipartition law in introductory textbooks. American Journal of Physics, Vol. 64, pp. 995-1000. Gearhart concludes that very few (of 27) physics textbooks treat specific heats correctly, yet another sad example of error propagation.”
Indeed, as he points out this is a failing of ‘Physics’ textbooks because they try to explain it without involving quantum mechanics, he also points out that ‘Physical Chemistry’ texts get it right because they involve quantum mechanics, specifically the one I used as an undergraduate, Fowler’s ‘Statistical Mechanics’.
He also says: “As it happens most if not all common monatomic gases have spherically symmetric nuclei , and hence cannot rotate.” (emphasis mine)
“:..spherically symmetric nuclei , and hence cannot rotate.”
The nuclei cannot rotate.
”If you can order the book surely you can give us the reference?”
See 2:35pm comment Gearhart ref. 21: ”ref. to Cohen 1971 p. 147ff..”
”My calculated ratio is 1.66, that is also the measured ratio.”
Because you made a lucky guess when calculating this nature of an atom, this same guess will not work for diatomic molecules. As Bohren writes: “It is not enough to be content with getting right answers by making a lucky guess. Without understanding why your guess was right, your next guess (about diatomic molecules using this guess) might not be so lucky.”
And this is what Gearhart 1996 explains, your lucky guess was known to be useful to calculate the measured 1.66 (independent of temperature) but not understood until QM explained it.
It will be a few more days until the Cohen book arrives.
“However the ratio of specific heats for Argon does not allow for quantum rotation.”
Gearhart notes from his ref. 21 Cohen 1971: “Nevertheless, the collective “rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra.”
He then goes on to explain why they do not show up which I take to mean do not show up as in his earlier discussed specific heat 1.66 calculation which is independent of temperature.
I won’t be able comment more than this until the Cohen book arrives.
Trick December 6, 2017 at 9:34 am
“:..spherically symmetric nuclei , and hence cannot rotate.”
The nuclei cannot rotate.
Exactly that’s the whole point, that’s where the mass of the Argon atom resides. As the paper you referenced points out the rotation of the electrons are not an issue.
Trick December 6, 2017 at 10:02 am
”If you can order the book surely you can give us the reference?”
See 2:35pm comment Gearhart ref. 21: ”ref. to Cohen 1971 p. 147ff..”
”My calculated ratio is 1.66, that is also the measured ratio.”
Because you made a lucky guess when calculating this nature of an atom, this same guess will not work for diatomic molecules. As Bohren writes: “It is not enough to be content with getting right answers by making a lucky guess. Without understanding why your guess was right, your next guess (about diatomic molecules using this guess) might not be so lucky.”
And this is what Gearhart 1996 explains, your lucky guess was known to be useful to calculate the measured 1.66 (independent of temperature) but not understood until QM explained it.
As a physical chemist I apply quantum mechanics, I did not make a ‘lucky guess’. Gearhart is referring to the erroneous attempt by physics text books to explain the DOF using classical mechanics, he’s quite clear that the correct application of quantum mechanics yields the correct result (and that physical chemistry textbooks do it the right way using QM). My assessment is based on the science of 2017, not a century ago!
Trick December 6, 2017 at 10:15 am
“However the ratio of specific heats for Argon does not allow for quantum rotation.”
Gearhart notes from his ref. 21 Cohen 1971: “Nevertheless, the collective “rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra.”
If you erroneously try to apply classical mechanics but as he points out if you correctly apply QM they do not.
He then goes on to explain why they do not show up which I take to mean do not show up as in his earlier discussed specific heat 1.66 calculation which is independent of temperature.
Here is what he says about the application of quantum mechanics to the equation:
“In particular, a symmetric rotator model can describe the rotational states of a spherically or axially symmetric “rigid body”; but all allowable wave functions for such systems must correspond to zero angular momentum about a symmetry axis [J=0 in Eq. (4)]. Thus a spherical nucleus cannot rotate.”
(My emphasis)
”Exactly that’s the whole point.”
Phi., not exactly, you are not reading Gearhart words closely enough to get his meaning. NUCLEI! He specifically means the nuclei cannot rotate wrt to the electron shell. Once the electron shell is included he calls that the collective “rigid body” modes which can rotate and cites Cohen.
”I did not make a ‘lucky guess’.”
You guessed the rotation DOFs could be ignored for your 1.66 calculation and only included the translational DOFs. You guessed right for an atom but this guess is wrong for a diatomic molecule gamma. QM explains why the guess is wrong.
”If you erroneously try to apply classical mechanics..”
No, Gearhart just explained the QM “symmetrical rotator” which assumes masses of finite size which he is applying here.
”Thus a spherical nucleus cannot rotate.”
NUCLEUS!! The QM “symmetrical rotator” which assumes masses of finite size includes the electron shell; collective “rigid body” is MORE than just the atomic nucleus & collectively exhibit rotational spectra. Have to await Cohen to go deeper.
Trick December 7, 2017 at 10:20 am
Trick, I’m not following this. It appears you are saying that the argon atom can rotate, but the nucleus isn’t rotating. This sounds highly improbable, but my knowledge of QM approaches 0K … what am I missing here?
w.
Willis, the 1996 paper by Gearhart is explaining the whole atom (nucleus and electrons) as a “rigid body” can rotate and the structure absorb photon quanta per QM as shown in spectra by his ref. to Cohen. Gearhart explains since most of the mass of the “rigid body” is in the nucleus, it is NOT just the nucleus rotating inside the electron shell absorbing the photon but the whole atomic structure. The nucleus does not rotate wrt to the electron shell. Gearhart points to Cohen for the testing, so I can’t make more progress until I find out what Cohen wrote & tested. No doubt he will ref. somebody else and the trail will continue.
The paper is easy to find at a college library and summarizes the errors found in 22 out of 27 physics texts on the subject. If not even the specialists can get this right all the time, or even bat .300 it is easy to understand how difficult is the subject.
Trick December 7, 2017 at 10:20 am
”Exactly that’s the whole point.”
Phi., not exactly, you are not reading Gearhart words closely enough to get his meaning. NUCLEI! He specifically means the nuclei cannot rotate wrt to the electron shell. Once the electron shell is included he calls that the collective “rigid body” modes which can rotate and cites Cohen.
No I’m reading it correctly, QM quite explicitly states there are no quantized atomic rotations, period!
,em>”I did not make a ‘lucky guess’.”
You guessed the rotation DOFs could be ignored for your 1.66 calculation and only included the translational DOFs. You guessed right for an atom but this guess is wrong for a diatomic molecule gamma. QM explains why the guess is wrong.
No I correctly applied QM which states exactly why there are only 3 DOF for an atom and also why there are only 5 DOF (two rotational) for a a homonuclear diatomic.
”If you erroneously try to apply classical mechanics..”
No, Gearhart just explained the QM “symmetrical rotator” which assumes masses of finite size which he is applying here.
”Thus a spherical nucleus cannot rotate.”
NUCLEUS!! The QM “symmetrical rotator” which assumes masses of finite size includes the electron shell; collective “rigid body” is MORE than just the atomic nucleus & collectively exhibit rotational spectra. Have to await Cohen to go deeper.
You’re already out of your depth!
Trick December 7, 2017 at 12:40 pm
Willis, the 1996 paper by Gearhart is explaining the whole atom (nucleus and electrons) as a “rigid body” can rotate and the structure absorb photon quanta per QM as shown in spectra by his ref. to Cohen. Gearhart explains since most of the mass of the “rigid body” is in the nucleus, it is NOT just the nucleus rotating inside the electron shell absorbing the photon but the whole atomic structure. The nucleus does not rotate wrt to the electron shell. Gearhart points to Cohen for the testing, so I can’t make more progress until I find out what Cohen wrote & tested. No doubt he will ref. somebody else and the trail will continue.
The paper is easy to find at a college library and summarizes the errors found in 22 out of 27 physics texts on the subject. If not even the specialists can get this right all the time, or even bat .300 it is easy to understand how difficult is the subject.
Yes the Physics texts because they try to do it without reference to QM and so get it wrong, on the other hand he points out that Physical Chemistry texts use QM and get it right.
Your first paragraph is incorrect, he shows that application of the Born-Oppenheimer approximation specifically excludes the electrons and categorically states that: “Molecular rotations are thus due to nuclear motion.” (referring to Herzberg, one of the undergraduate texts I used).
”You’re already out of your depth!”
Everyone has an opinion Phil. Let’s see: I pointed out Bohren’s text discussion differed from that of Phil., and dug out the Gearhart paper supporting Bohren’s views. Phil. put up a link to incomplete NIST spectral line data that Phil. does not or did not know:
1) The source i.e. who, what, when, where the spectra were run
2) The pressures the spectra were run at
3) The exposure times
4) The apparatus used
5) The temperature
6) The power applied, the source of the illumination, so forth.
What is more Phil. then confused Gearhart’s discussion of the nucleus with that of the atom.
”(Gearhart) points out that Physical Chemistry texts use QM and get it right.”
Gearhart in footnotes: “I have not explicitly stated which books give incorrect or misleading explanations.”
So, I also have an opinion of Phil. but I’ll keep it to myself. I will not pre-judge what the existing collective “rigid body” modes testing will reveal about nuclear rotational spectra in Cohen’s 1971 book until I’ve read and understood that material which Gearhart cites.
Trick December 8, 2017 at 5:16 am
”You’re already out of your depth!”
Everyone has an opinion Phil. Let’s see: I pointed out Bohren’s text discussion differed from that of Phil., and dug out the Gearhart paper supporting Bohren’s views. Phil. put up a link to incomplete NIST spectral line data that Phil. does not or did not know:
On the contrary I put up a link which showed all the energy levels between the ground state and the first ionization level, it is complete. It also included the reference to the original paper which answered all the questions about how the spectra were acquired, which I had read, I had to explain to you that the spectra included on the NIST site were the result of an electronic discharge and even had to explain what an etalon was to you which apparently you didn’t understand.
What is more Phil. then confused Gearhart’s discussion of the nucleus with that of the atom.
No I did not, you are the one who is confused, Gearhart explicitly states that “It is apparent that the electrons cannot participate in any collective rotational mode corresponding to the “rigid body” rotation of the molecule as a whole about an axis of symmetry.” Based on this it is clear that he regards the rotation of the nucleus as the important factor in determining the rotation of an atom and all of his subsequent discussion focuses on the nucleus.
As Gearhart says:
“Now in a multielectron atom or molecule, it is in principle possible that a suitable approximation would turn up a collective mode that would appear as a rotational energy spectrum. However, any approximate separation of a Hamiltonian into single electronic states on the one hand, and collective rotational states on the other, would have to rely on something like a Born-Oppenheimer approximation, which requires that rotational energy levels be very different in magnitude from single electronic states. But a rough estimate of the moment of inertia of an orbital electron about the molecular axis of symmetry shows that the rotational energies are on the order of eV -about the same as typical electronic energy states in atoms and diatomic molecules. It therefore appears that one cannot interpret atomic or molecular electronic excited states in terms of collective “rigid body” rotation. (Note, of course, that these electronic states, however regarded, typically have energies >> kT at room temperature and so cannot contribute to the specific heat.)”
“Atomic nuclei, of course, are no more rigid bodies than are atoms or molecules. Nevertheless, collective ”rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra. In principle, therefore, one might expect three rotational degrees of freedom for monatomic gas molecules such as mercury or the noble gases, corresponding to nuclear rotations about three mutually perpendicular axes through the center of the nucleus. These degrees of freedom do not show up for two reasons. First, as argued above, the lowest excited rotational energy state for such systems is on the order of 0.1 MeV, far greater than kT, and so the equipartition law could not apply.
A second, more fundamental argument, stemming from the symmetry of the wave function, provides yet another reason not to expect nuclear rotations about axes of symmetry. Consider a wave function rj, that is spherically symmetric. By a well-known theorem in quantum mechanics, such a state must have zero angular momentum. Similar considerations apply to systems that are axially symmetric. This theorem certainly applies to wave functions describing collective “rigid body” motion. Hence for spherically or axially symmetric nuclei, there can be no collective rotation about axes of symmetry. In particular, a symmetric rotator model can describe the rotational states of a spherically or axially symmetric “rigid body”; but all allowable wave functions for such systems must correspond to zero angular momentum about a symmetry axis [J=O in Eq. (4)]. Thus, a spherical nucleus cannot rotate.”
”(Gearhart) points out that Physical Chemistry texts use QM and get it right.”
Gearhart in footnotes: “I have not explicitly stated which books give incorrect or misleading explanations.”
That footnote is explicitly referring to the 27 Physics texts which he lists, not the physical chemistry texts he later references: “Since I am interested not in pointing fingers at any particular text, but in calling attention to a widespread problem, I have not explicitly stated which books give incorrect or misleading explanations. Readers will have little difficulty in making the identifications for themselves. ”
Gearhart is quite clear he takes issue with the physics text books’ attempt to explain atomic spectra using classical physics and not invoking QM and shows that their approach is wrong. He also points out that among the few that get it right: “It is noteworthy that two of the six texts that give correct explanations seem decidedly nervous about doing so. One states in the text that equipartition fails in diatomic molecules because the moment of inertia about the axis of symmetry is negligible, but then adds in small print in a footnote that ” A proper justification is provided by modern quantum mechanics.” A second book offers the same explanation with the observation that it was an “early attempt” which “leaves one feeling that the theory has been fudged,” and goes on to state that the “correct reason” is given by quantum mechanics. It is difficult to avoid the impression that both authors were a little intimidated by the weight of textbook tradition!”
He also comments that: “A full quantum mechanical treatment of atomic and mechanical rotations is neither trivial nor particularly well known to many physicists”
“By contrast, the quantum mechanical ”symmetric rotator,” which assumes masses of finite size, it is was not a standard textbook problem (although, interestingly, one of the earliest systems to receive a detailed quantum mechanical treatment). One typically sees it today in physical chemistry or molecular spectroscopy texts. One also sees it in older texts such as Pauling and Wilson’s Introduction to Quantum Mechanics and Fowler’s Statistical Mechanics.”
Texts which I used as an undergraduate, perhaps that is why I understand it better than ‘many physicists’?
So, I also have an opinion of Phil. but I’ll keep it to myself. I will not pre-judge what the existing collective “rigid body” modes testing will reveal about nuclear rotational spectra in Cohen’s 1971 book until I’ve read and understood that material which Gearhart cites.
Well Gearhart agrees with me that the only energy states that exist for Argon are the electronic levels indicated in the NIST report and that there are no rotational states, and therefore no absorption of IR under atmospheric conditions. Cohen’s book is about nuclear physics and may not give you the answers you seek.
”..even had to explain what an etalon was to you which apparently you didn’t understand.”
Ha, no Phil. that is just your opinion.
I’ll spend more time with your 9:07pm later if the thread doesn’t close, thanks for the major effort, but wanted to fill you in that Gearhart’s ref. Cohen does explain where the notion of “modern-physics courses” that write atoms do not spin comes from. I will let you find and enjoy that passage.
Cohen explains why atoms do spin and gives detailed measurements of photon absorption increasing the quanta of rotational energy for an atom. He lists (Fig. 6-17) the energies of excitation above the ground-state rotational band for some heavier atoms in a “low energy-level spectrum of this type.” Cites tests from a 1960 ref. So the trail for Ar goes cold in Cohen but possibly is discussed in the earlier text.
My interest will end there with 4 authors discussing atoms do have rotational energy level spectra as shown from experiment. These 4 authors (and possibly the 5 of 27) will vote with me that the answer to Willis’ challenge is YES.
SkepticGoneWild December 3, 2017 at 10:52 pm Edit
Oh, stop with the asinine snark. It makes you look ugly. The laws of thermo are just that, laws. Nobody can break them.
If the internal radioactivity of the sphere puts out 240 W/m2, then 240 W/m2 is emitted to space. Nothing is created.
NO. I’ll say it again using small words so you can grab it, because I’ve said it many times now and you’ve ignored it.
Heat can’t flow from cold to hot … but energy sure can.
The distinction between heat and energy still seems to be escaping you. Heat is is a special class of energy. It is the NET energy that that is moved by a temperature difference.
Radiation is a very different kind of energy. It just flows wherever it happens to flow. It can flow from cold to hot, as when my warm eyes can see cold bioluminescence in the ocean. According to you, that’s impossible. You’d claim that I’m saying that HEAT is flowing from the bioluminescence to my eyes. But it is not heat, it is energy. It flows from colder to hotter all the time. Put two objects near each other. Energy is radiated by the colder one and absorbed by the warmer one. At the same time, of course, MORE energy is also flowing from from the warmer one to the colder one. LOOK AT FIGURE 1. The flow goes both ways. We subtract one from the other, as I showed in the equation in the head post, to get the net flow. This net flow is called a “heat flux”, and as you would expect, it only flows from hot to cold.
As numerous people have noted, this is not complex. This is introductory thermodynamics. You take the larger energy flow, subtract the smaller energy flow, and the result is the heat flux. Standard stuff, well understood for a couple of centuries or so.
In the steel greenhouse, to pick a number, let’s say that in steady-state without a shell the sphere radiates 240 W/m2 to space. With a shell, the first law of thermo says it still must emit 240 W/m2 to space … but because a shell has two sides and a planet does not, the shell has twice the surface area of the planet.
So … we pop the shell around the planet. It is receiving 240 W/m2. But because it has twice the surface area, it only radiates 120 W/m2. Not only that, but half of it goes to space … and the other half is radiated inwards.
You seem to think that it cannot radiate inwards … but what is stopping it? If it radiates outwards it must radiate inwards. And what is absorbing that radiation?
The sphere. This makes the sphere heat up, and it continues to heat until 240 W/m2 is escaping to outer space from the shell. Then it is in steady-state, 240 W/m2 generated, 240 W/m2 emitted to space.
So. At steady-state, the shell MUST be radiating 240 W/m2 to space. This means it is also radiating 240 W/m2 inwards to the sphere. Now lets check first law.
The sphere is getting 480 W/m2, half from internal generation and half from the shell. It is radiating the exact same, 480 W/m2. No first law violation, no energy created.
The shell is receiving 480 W/m2. It is emitting 240 W/m2 through twice the area, which means it too is radiating exactly what it is receiving. No first law violation.
Now, let’s look at heat flow. The sphere is radiating 480 W/m2, and receiving 240 W/m2 from the shell. NET heat flow is outward to the shell, as we’d expect. It is a NET heat flow of 480 W/m2 emitted minus 240 W/m2 received from the shell, meaning the net heat flow is 240 W/m2 from the sphere to the shell … just as we would expect. Finally, the shell is emitting 240 W/m2 to space and receiving nothing in return. So it too has a net heat flow of 240 W/m2 to space.
Note that in all cases, heat is flowing from hot to cold—from the sphere to the shell, 240 W/m2, and from the shell to outer space, 240 W/m2. Everything balances, no thermodynamic laws broken.
What, am I your white knight? I’m supposed to defend your honor now? Sorry, pal, that’s on you. I’ll defend Ed Bo and many of the other commenters here, they don’t give me your nasty snark and personal attacks. I will not defend you, I view your actions as indefensible. That’s your job.
Truly, Skeptic, the first rule of holes applies here. Get a thermo text and stop making a public fool of yourself.
w.
Willis, you will receive no opposition from me.
Had you considered that, if there was a gap between the sphere and the shell, the shell would not be receiving 240 W/m^2?
Not sure what you mean, Dave. There is a gap between the sphere and the shell. It’s small, but it’s vacuum, so the exact dimensions don’t matter.
Well, if there is a gap, the interior area of the shell is greater than the area of the sphere. If the sphere is radiating at 240 W/m^2, the larger shell is receiving radiated energy over a larger area and must be receiving energy at a value less than 240 W/m^2. No?
This is a trivial matter, but illustrates the difference between per-unit values and absolute values.
Dave Fair December 4, 2017 at 2:32 pm
Thanks, Dave. Let’s put some numbers on it. The earth’s radius is about 6,378 km. The effective radiation altitude of the atmosphere is on the order of two km. The difference between the surface of the earth and the surface of a shell that is 2 km above the earth is a meaningless six hundredths of one measly percent (0.06%).
As such, it is customary to omit such trivial effects from the kind of first-order analysis I did in The Steel Greenhouse.
I suppose I should mention two other times I’ve explained this:
People Living in Glass Planets
and
The R. W. Wood Experiment
Perhaps those will clarify things for some people.
Regards,
w.
Dave Fair December 4, 2017 at 1:31 pm
Willis, you will receive no opposition from me.
Had you considered that, if there was a gap between the sphere and the shell, the shell would not be receiving 240 W/m^2?
That would be taken care of in the heat transfer equations by using the correct view factors, in the situation posited by Willis it will make a negligible change to the numbers.
Iterations to achieve 240 W/m2 out from Shell.
1. 240 out from sphere 60 out from shell 60 in to sphere at starting point
2. 300 out from sphere 75 out from shell 75 in to sphere
3. 375 out from sphere 93.7 out from shell 93.7 in to sphere
4. 468.75 out from sphere 117.1875 out from shell 117.1875 in to sphere
5. 585.9375 out from sphere 146.484375 out from shell 146.484375 in to sphere
6. 732.421875 out from sphere 183.1054688 out from shell 183.1054688 in to sphere
7. 915.5273438 out from sphere 228.8818359 out from shell 228.8818359 in to sphere
So we nearly have Your equilibrium, so to get to 240 out from shell we just need another 11.1 so that is doubled for the watts going in as well as out.
So the final temperature of the Sphere in now 937.5 W/m2 to obtain an output to space of 240 W/m2.
So unless you can point out where I went wrong does that look like a sphere at 480 = shell out at 240 to you Mr Eshenbach?
But we have a small problem here as the Sphere is now at 937.5 w/m2 and the shell is also exporting 240 W/m2 so although the 240 source heat to the Sphere is equal to the 240 out to space from the shell, the sphere is still getting 240 watt/m2 from the source and the shell is now putting 240 watt/m2 inwards.
Where can that be 240 W/m2 be going, OH I know someone said it must be being absorbed by the Sphere, after all what stops it from being absorbed.
So now the sphere is at 1177.5 and output from the shell is 394 W/m2.
Houston we have a problem.
Please explain what is wrong with my arithmetic as I have obviously gone badly wrong here.
Glad to, A C. For some reason, you seem to think that the output of the shell is 1/4 of the output of the sphere. It is not. The shell absorbs all of the radiation from the sphere. But because it has twice the surface area of the sphere, it emits at half the rate, not a quarter of the rate. So the iterations would be something like:
• 240 out from sphere 120 out from shell 120 in to sphere at starting point.
• 380 out from sphere 190 out from shell 190 in to sphere.
• 480 out from sphere 240 out from shell 240 in to sphere.
At that point the system is at steady state. The total amount generated in the sphere (240 W/m2) is being radiated to space.
w.
Also, A C, note that what the shell sends to the sphere is added to what the internal radioactivity sends to the sphere (240 W/m2). It is NOT added to whatever the sphere is radiating at the moment.
w.
I think you need to rethink that explanation.
I QUOTE YOUR EXACT WORDS.
“But because it has twice the surface area, it only radiates 120 W/m2. Not only that, but half of it goes to space … and the other half is radiated inwards.”
120/2 = 60 in the arithmatic they taught me at school.
OK, you say that it only adds to the 240 W/m2 and not the actual energy coming from the sphere.
The only problem left is how does the sphere increase it’s output without increasing it’s Temperature?
Willis,
A small remark, which doesn’t change the essence of the thought experiment:
When you insert the shell, its radiation in both directions only depends of its own temperature, it is not half the energy of the sphere in both directions as the surface is double that of the sphere. It may start at 3 K or room temperature. If it had the same initial temperature as the sphere, it would already send 240 W back to the sphere and 240 W to space.
But that is only for the initial temperature, once in equilibrium, the temperature of the shell would be such that the energy leaving it in both directions is always half that of the outgoing energy of the sphere. Or from the other viewpoint, the temperature of the sphere goes up until its outgoing energy doubles, thanks to the backradiation of the shell…
A C Osborn December 4, 2017 at 1:47 pm Edit
Thanks for the quote. I see the confusion. The shell is ALL radiating at half of the rate of the sphere, or 120 W/m2. The fact of where it radiates it doesn’t change how much it radiates. In other words, because half of the surface of the shell faces outer space, half of the 120 W/m2 radiation is directed to space … but that doesn’t cut the radiationper square metre in half.
If this is still not clear, please let me know.
A C Osborn December 4, 2017 at 1:43 pm Edit
Heck, I didn’t even realize that the Kardashians had a Scale 2 Civilization …
Best to you,
w.
The shell is ALL radiating at half of the rate of the sphere, or 120 W/m2. The fact of where it radiates it doesn’t change how much it radiates. In other words, because half of the surface of the shell faces outer space, half of the 120 W/m2 radiation is directed to space … but that doesn’t cut the radiationper square metre in half.
So let me get this right when you say half it doesn’t really mean half it actually means 120 W/m2 in BOTH directions.
Sorry my bad for reading the words wrong.
You made a little addition error on iteration 1. It doesn’t change your point at all. It still converges on the correct answer.
For anyone wanting a more formal approach here is why it works…..
If you assign b as the percentage of energy returned for any inbound value to the shell, E for the constant energy fed to the sphere and B as the total inbound from the shell you get inbound iterations from the sphere that sum to:
B=Eb+EB^2+Eb^3+…..Eb^n I want to add an E and take away an E for later trickiness so now I have…
B=E+Eb+EB^2+Eb^3+…..Eb^n -E then i factor to get B=E[( 1+b+B^2+b^3+…..b^n)-1]
Where n is the number of iterations and b is restricted to 0=<b=<1
The first term in the parenthetic expression is a power series equivalent to Taylor series expansion of 1/(1-b). So then B is simply B=E(1/(1-b)-1)
So for b=0, B= 0, transparent barrier. For b=1 B = infinity, no energy ever it gets out, exploding planet. And for everything in between the equation holds. Willis example is a b of 1/2. B=E, so planet receives 2E, shell receives 480, shell emits 240.
If you buy the argument that inbound energy from shell is absorbed and re-emitted, and you should, then this is the math that explains why it looks like something is being created from nothing. Remember that iterations can be thought of as little slices of time, each acting independent of the other. If you let the time slices approach zero, then n approaches infinity and this is the steady state math that represents it.
Paul Bahlin December 7, 2017 at 10:54 am
If you buy the argument that inbound energy from shell is absorbed and re-emitted, and you should, then this is the math that explains why it looks like something is being created from nothing.
No I shouldn’t without the proof that I keep asking for from Reputable Scientific Sites.
Let me ask you.
How many Joules per second per sq m is the heat source supplying to the Sphere, how many joules per second per sq m are leaving the Sphere and how many joules per second per sq m are leaving the Shell at iteration 0?
Upthread you will find a very detailed set of equations that you can plug all the numbers in. How do you learn anything if I do your arithmetic?
If you can’t follow what I wrote there is no shame in that. Maybe I wrote it crummy. If you have equation question fire away. What I won’t do is your arithmetic. Get straight on the math before you worry about the science. The math works for any system with feedback; money, yields on assembly lines, electronics….
Sorry, make that iteration1.
You are still not getting the actual situation.
You had the feedback from your Wife, it was something that you COULD NOT USE.
Paul, let me explain a bit further.
You are obviously a very talented Mathematician and have a passion for it.
In Thermodynamic Science there appears to be a written rule that Radiation from Cold can’t make hotter even hotter, I am trying to establish where reputable Scientific Physics establishments or Physics Scientists says it can.
So in Maths would you try and use a Trigonometry Formula for a Triangle to solve a problem for Circle?
Of course not as it would not be appropriate.
So why do you insist on using Positve Feedback where the Feedback is negative?
To go back to your Bank Analogy, assume that your Bank had a rule that it only accepted $s as depositing Currency and your Wife did actually manage to pay you some of your $100 dollars back but in Euros, You CAN’T put it in your Bank can you?
You could go elsewhere to spend it that does Accept Euros as you can with Radiation, if the Receiving object is cooler it will accept and Thermalise the Photon Energy.
A.C. Read carefully.
I come to this site to learn and I engage here when I think I have something to offer. I presume that everyone is here for similar reasons. But I am also not naive. Some come here with laser pointers to agonize the cats.
You are either a troll (laser) or you want to learn but are incapable of understanding how science works. My banking example was a freaking analogy. It doesn’t explain anything more than the math that would result for the hypothetical system I proposed.
Willis did the exact same thing for a hypothetical, closer in its definition to a, planet in space. The people who comment on that hypothetical with stupid shit like “come on, we don’t know how to build that” are assholes. Plain and simple!
I did the banking analogy in the hope that it would help you by deriving the math with a more tangible hypothetical than radiation. You have come back with a critique that changes the hypothetical and you never answered whether you agree with the math or not. You may be one that really wants to learn but when you play like that, It’s a laser and I won’t be your cat. It is very troll like.
So decide what it is that you are for. If you’re here to learn, answer the question. If not go away.
FYI, The question is do you agree with the math? Yes or no
So now let me pose my previous question as a Mathematical Formula.
Which one is correct bearing in mind X is a fixed Value and there is NO extra Energy in the system
X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = X (joules/second/M2 out of shell)
or
X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = 2 x X (joules/second/M2 out of shell)
Simplified
Output from Energy Source = Output from Shell
or
Output from Shell = 2 x Output from Energy Source
The Energy could just as easily be a dozen Apples being passed down a chain of people, there are no magic Apples allowed,
so 12 apples from person A to person B, 12 apples from person B to people C & D, 6 each, 12 apples eaten by people C & D (space)
Where do the extra dozen apples come from in this Steel Greenhouse Version.
12 apples from person A to person B, 12 apples from person B to people C & D, 6 each, 12 apples eaten by person C and 12 apples eaten by person D.
Or if you like you can use your $100 instead, as you seem to be cash orientated LOL.
AC, you are either breathtakingly ignorant or a particularly bad Troll.
I’ll say it only one more time: the sphere has an independent power source. The same as the earth’s solar input.
Do you deny that the earth’s surface is radiating at a rate greater than the sun’s input?
A.c. says:
“X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = X (joules/second/M2 out of shell)”
STOP RIGHT HERE….
Yes it is X out of she’ll but it is X/2 back to sphere and X/2 out to space.
“or
X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = 2 x X (joules/second/M2 out of shell)”
This part is wrong. It has the shell creating energy..
It is a recursive process the next things to hit shell are:
X/2 which splits X/4 in X/4 out, then
X/4 which splits X/8 in X/8 out, then on and on all the way to X/infinity in, X/infinity out.
Converges on a steady state solution. X in to system, X out of system. Try it with 100 apples a day.
Paul, read carefully.
I am not a troll and I am trying to understand how Mr Eshenbach is at odds with all the Physics that I can find, which is why I keep asking for examples of his physics.
And also how the mechanics of Global Warming works, not just the physics.
It is now obvious why both your Physics and Maths are wrong.
The power Source is supplying sufficient Watts the sphere to emitt 240 for every one of the 1000 sphere’s Square metres, there is none to spare.
The Sphere has a surface area of 1000M2 and has 240 Watts coming out of each one which would equate to 240,000 Watts.
The Shell has a Surface Area of 2000M2 so it Can only recieve 120 Watts for each of it’s square metres, so that would also equate to 240,000 Watts as well.
Mr Eshenbach says the Sphere is emitting 120W per M2 to space, so 2000 x 120 also equates to 240,000 Watts.
The shell’s input and output is halved because it’s Surface area is twice the size, not because half goes in and half goes out.
Now according to Mr Eshenbach he can add the inward 120 (against all the current rules) to the Sphere until the Shell is outputting 240 W/M2, so 240 x 2000 equates to 480,000 Watts, so where have the extra 240,000 watts come from if those from power source have gone out to space, the Shell cannot generate them?
Paul, to show you how earnest I am on this subject, I have been following AGW for about 10 years now) I purchased a twin probe high quality Digital Thermometer and I have been conducting experiments to prove or disprove some of the quotes being made on here about how photons don’t know where they come from or are going to and are absorbed and therefore must be thermalised even by hotter objects.
I think today’s is the 4th one, I have posted the others on here and no one comments about how they are proving or disproving what they think. The only comments I have had are from Mr Eshenbarch criticising my lack of sketches etc and my designattempts at trying to prove the claims.
I will now be posting today’s one in a moment.
They take quite a long time to do as you have to wait for Equilibrium to reached by each object at each stage, on top of that my wife of 48 years is giving me real grief about both “Communicating” with people on the various forums I frequent and doing these stupid experiments.
I will not stop experimenting until I have satisfied myself that I can or cannot find these special conditions where cold makes hot hotter.
Man, AC. Instead of trying to recreate hundreds of years of physics on your own, why not just buy a recent radiative physics text?
Correction
This sentence
Mr Eshenbach says the Sphere is emitting 120W per M2 to space, so 2000 x 120 also equates to 240,000 Watts.
Should be
Mr Eshenbach says the Shell is emitting 120W per M2 to space, so 2000 x 120 also equates to 240,000 Watts.
Dave Fair, if you can tell me which ones describe how Cold things make hot things even hotter by radiation alone I am quite happy to buy one.
As long as it is NOT written by a Climate Scientist like Trenberth or Mann.
The reason that I am doing it myself is because that is my nature, I have an enquiring mind, but like see things for myself.
AC, I have never claimed that a colder object will warm a hotter object by radiation from the colder object alone.
Is it possible for one to see things for oneself through reading a college physics textbook, AC? And take a class and attend labs?
Dave, I did so 50 years ago, Thermodynamics and Electrical & Eletronics, I am now 70, 71 in January and I do not feel like going back to School.
I also do not need to, there is this thing which we are comminicating on call the Internet, where if you want to know something you just ask, sometimes you have to ask multiple questions phrased differently and then follow up questions as new “terms” are given in reply.
Plus of course I also enjoy actually designing and doing the Experiments, even if my wife does give me grief for doing them.
A.C…..
I’ve read your last few comments, and all kidding aside, I think I get you now. Your real passion is the experimentation. So let me suggest a new experiment.
Sometimes it is very difficult to prove something affirmatively but relatively easy to prove an opposite reality to disprove the first. So let me suggest an alternative proof…..
The essence of adherents to the theory that green house warming is not real is that warm objects are unaffected by ‘cold’ radiation. So Let’s prove that.
So what you need is a constant source of ‘cold’ radiation and a way to trap it. If the theory is correct, the trapped energy will build to a readily measurable value. Indeed the experiment should blow up at some point😁
The source of cold radiation is the earth and we want to be relatively unaffected by moisture changes and the sun so i would pick an area of Sandy soil in a shady spot.
You need to cover this soil with a well insulated box with an open bottom. Let it sit for a week or so and graph the temperature in the box say every 2 hours.
Since the theory says warm bodies reject cold radiation, it will enter the box and get trapped by the relatively warmer exterior ( you need to either heat the outside or do it when ambient never goes below 60 or so). The radiation can’t reenter the surface because now it is back radiation, by the same reasoning.
You should see the temperature go up to a value and settle into some day-night oscillation with a RMS value you can calculate.
Next, take a polished aluminum plate and insert under the box, shutting off the earth’s radiation. Run your measurements again.
Compare your RMS values. Your first run should be considerably higher than second. I would love to see your results.
Of course it won’t blow up but if your insulation is good, the radiated box should get warmer than the non-radiated one. Conduction will be your nemesis so give that some thought.
It would be valuable data to concurrently record outside ambient (shaded).
While living in Alaska, I needed to pound dog tethering stakes in the frozen ground. I took a bucket and light bulb on an extension cord. The bucket reflected the light’s energy and interfered with conduction, which melted the soil to depths sufficient to pound in the needed stakes.
Depends on where in the Internet you look and the questions you ask.
Radiative physics is well-documented for anyone to follow.
I, too, took the courses you listed in the early 1970’s. I was astonished recently at the level of ignorance exhibited by nominally educated people.
Forgot to add….
Shutting off the radiation will be tricky. I would put it on sticks so it doesn’t touch the surface.
that way the surface still is the radiator but when radiation hits the plate it will be rejected per the premise of the experiment. You don’t want the plate picking up earth temp via conduction, else it becomes the
.
Dave Fair, I missed this insulting comment from you ie
“AC, you are either breathtakingly ignorant or a particularly bad Troll.
I’ll say it only one more time: the sphere has an independent power source. The same as the earth’s solar input.
Do you deny that the earth’s surface is radiating at a rate greater than the sun’s input?
”
If you bothered to read my experiments carefully you will see that in many cases the emitting objects also had independent fixed power sources and at no time has any of them risen in Temperature without Insulation or volume of air and surface being involved.
First of all the Sun is not a Constant Source of Energy, only It’s Average Energy is roughly constant
Day/Night it is the equivelent of turning off the Energy Source in the Sphere for 12 hours out of 24.
Which means it would lose all it’s heat (and the shell’s) to space and when the energy source is turned back on it has to heat the sphere back up from 3.5K to the temperature where it can emit 240W/M2, just as the sun does every morning.
The Earth’s Energy Input, Temperature and Thermal Output is at no time Constant.
And as to your last question, along with many Scientists I do not believe The Thermo Balance Chart is correct, due to the BB equations being incorrectly applied, as the Earth’s Surface is nothing like a Black Body, is not in a Vacuum and due to the Energy Types (which seem to be Totally ignored) and where they end up.
Consider this, 70% of the high Energy and White Light and the low level from the Sun end up in the Oceans, which they heat to a depth of many metres, the water has a much bigger energy store to give up at night compared to the rest of the Surface. The remainder of the radiation heats the top few feet at most of the Earthern Surface of all different Albedo and Conductivity levels which is quickly lost to space in comparison.
The Radiation leaving the Surface however is lower power only LWIR, therefore when it is thermalised it’s energy output and the number of Photons emitted is far below Sunlight, that is any photons that can be emitted after all the collisions with all the other molecules that have removed the energy from the CO2 Molecules before the photon can be emitted.
Add to that, the area in the Atmosphere where the LWIR is thermalised by CO2 is a long way above the surface, half goes to space and the other half has somehow to get past the much more densely packed Molecules and getting denser the closer to the surface between it and the Surface.
There is also the question of “Balance” does Atmospheric H2O and especially Cloud increase or decrease the Surface Temperature over a 24 hour, Month, Year, decade period etc.
Well there is Emperical evidence that Clouds Cool the Surface overall, even though it warms it at night.
Add to that the Psuedo Warming by “Adjustments” and mis-use of Equipment give me absolutely no confidence in CO2 having any affect whatsoever on Temperatures, even with them there is no correlation.
What I do believe is that the Insulation affect of the whole Atmosphere, could be the “Equivalent” of adding x amount of watts to the system, just as it does with a house, a furnace etc.
The important word is Equivalent. For me Downward Back Radiation does not come into it.
AC, I’m done here. You just can’t seem to believe theoretical physics, backed up by empirical physical evidence.
That doesn’t mean that anthropogenic CO2 has any meaningful impact on average terrestrial temperatures. That has yet to be proven by observations. There are too many other things going on in our atmosphere and oceans that have the ability to overwhelm anthropogenic CO2’s feeble forcings.
You also realise that a Kardashev Scale 2 Civilisation could not build a Dyson Sphere as it would fry every living thing inside sphere.
A C Osborn,
The increase in radiation is not cummulative, it is always the 240 W heat source input + what is back radiated the moment before. That makes:
1. Sphere: 240 W out; shell: 60 W back, 60 W out.
2. Sphere: 300 W (240 W + 60 W) out; shell: 75 W back, 75 W out.
3. Sphere: 315 W (240 W + 75 W) out; shell 93.7 W back, 93.7 W out.
4. Sphere: 313.7 W out; shell 117.2 W back, 117.2 W out.
5. Sphere: 357.2 W out; shell 146.5 W back, 146.5 W out.
6. Sphere: 386.5 W out; shell 183.1 W back, 183.1 W out.
7. Sphere: 423.1 W out; shell 228.9 W back, 228.9 W out.
—
N. Sphere: 480 W out; shell 240 W back, 240 W out.
Seems fine to me…
Well apart from your arithmetic that is 315/ 4 = is less than 80 not 93.7.
357/4 less than 90 not 146.5 etc.
However I had already accepted that the incoming is only added to th base 240.
As I said the only problem is how the Sphere absorbs the energy and adds it the base without increasing the actual temperature of the sphere.
Perhaps you can explain it to me?
A C Osborn December 4, 2017 at 2:17 pm
Huh? Who said the temperature of the sphere did not increase? It has to increase, because instead of getting 240 W/m2 from the interior plus zero from outer space, at final steady-state it is receiving an additional 240 W/m2 radiated back from the shell.
Regards,
w.
Ferdinand, you’re making the same mistake that A C made. The shell radiates half the energy back, not a quarter. Set the numbers aside for a moment. Of whatever the shell receives, half of that is then radiated by the inside of the shell and half by the outside. Same is true in our atmosphere. Whatever the GHGs absorb, half goes up, half goes down (with a minuscule correction for depression of the horizon).
w.
A C Osborn,
Should have refreshed the discussion some time earlier, as I see that Willis had already responded…
Of course the actual sphere increases in temperature: as long as more energy is coming in than is going out, that energy increases the temperature of the sphere, or its outgoing energy can’t go up: the incoming radiation is absorbed, not reflected.
See what happens with the temperature of the original plate after I have inserted a second one in my own calculations above. That is as good the case for a sphere + shell as for two plates. As long as you have a continuous source of energy, the temperature of the heated part goes up with adding any hindrance to the outside world until the outside loss of energy is the same as the energy supply.
The calculations of how fast the sphere and shell get in equilibrium is a matter of mass of sphere and shell.
I only used your own figures for convenience, but it is in fact a factor 2, not a factor 4, at light speed if the sphere and shell had no mass and/or zero specific heat…
Willis,
Indeed it is a factor 2, not 4, but only used his own figures to show that it goes (slower) to the right equilibrium… In fact a matter of initial temperature of the shell and also a matter of mass and specific heat of sphere and shell…
Stephen, Bernard, Phil etc.:We now seem to be deep in the parallel world of the Feynmannian “eternal sidestep of hypothesis” again. Seems endemic in certain circles. Circles being apt as of now…..
From my ‘First Metric’ edition of ‘Mechanical Engineering Science’ by Hannah and Hillier, p.275; 14.15 Radiation: “The transfer of heat by radiation is usually important at high temperatures or when conduction and convection are negligible.” I also note that air has greater heat or thermal capacity (Cp 1:0.85) than CO2. Once gasified by increased Kinetic Energy (of which radiation is just a byproduct, feeble at STP), convection dominates to the emission height. Pure argon may not remain for aeons, as Mr Wilde pointed out years ago in relation to non-ghgs. On Earth, water’s LH uplift supercharges cooling, so CO2 is superfluous and can get on with making life possible. Of course, no life on a waterless planet either. But Willis posits an argon body, and I have said it can be both gaseously covered and also cooled in that state.
Some seem to be unable to see that solids must be phase-changed by application of energy ie work done or heat, if they are to become gases eg an atmosphere. Conduction does that here, and would do it for argon. Not for the first time, the cart needs to be behind the horse or Neddy gets very unhappy. I note that Bull(s) are better at pushing, also Billygoats. Lest we get too serious about this debate among people hopefully looking in the same direction……
Brett Keane December 4, 2017 at 2:57 pm
I posted above a quote from the Smithsonian. It said that the initial atmosphere was vented from volcanoes, so no need to involve “conduction” …
w.
And I told you why that is insufficient on its own.
Brett Keane December 4, 2017 at 2:57 pm
I also note that air has greater heat or thermal capacity (Cp 1:0.85) than CO2.
Actually CO2 has a higher heat capacity than air.
Does that “Air” actually mean the rewst of the Atmosphere on Earth where the “Air” has so much Water Vapour in it?
Well, after having read most of the 1,582 comments so far, I still have not seen anyone come up with a viable, rational objection to Willis’s basic contention that a cool object really can warm a warmer object. I have seen numerous digressions into interesting but irrelevant side issues, some unintelligible expositions of alternative theories of planetary warming involving convection, KE and PE, and the usual quota of unnecessary personal criticisms and cynical ad homs, but no solid, coherent scientific arguments that decisively refute his proposition. I’m left with the impression that his detractors don’t really have one.
I don’t understand why they seem to be making such a complicated issue out of something that is basically very simple. We have an earth’s surface that is radiating somewhere in the region of 390 W/m² of power on average (corresponding with a global mean temperature of about 14°C) but which is receiving only about 240 W/m² of power continuously from the sun (corresponding with a global mean temperature of about –19°C). So where is the roughly 150 W/m² of power coming from to make up the shortfall and keep the surface radiating at 390 W/m²?
Since the surface can only receive power-inputs from two directions (i.e. above and below) and the amount of power emanating constantly and continuously from below the surface is negligible (discounting temporary power-surges from storage in oceans, etc.), the shortfall has to be made up by power coming from above, i.e. from the direction of the atmosphere and outer space beyond it. But the only significant power-source in outer space is the sun and we have already taken that into account (i.e. as ~240 W/m² of insolation at the surface), so we are left with the atmosphere itself as the only possible source of the ~150 W/m² that is required to make up the shortfall at the surface.
Now the question arises as to where the atmosphere is getting its ~150 W/m² from to give continuously to the surface. The obvious answer to that question is that it is getting it from the surface radiance, so that the atmosphere is effectively recycling back to the surface ~150 W/m² out of the ~390 W/m² which the surface is continuously radiating into it.
This answer is confirmed when we consider that the amount of power radiating out of the topo of the atmosphere is, again, ~240 W/m². This situation implies that although the atmosphere is receiving ~390 W/m² of power from the surface in the form of IR-radiation, it is passing only ~240 W/m² of it to outer space, whereby it has acquired a power-surplus of ~150 W/m² which it must output somewhere to maintain its internal thermodynamic equilibrium of the system. But, again, there are only two possible directions in which it can output that power-surplus, namely above into outer space and below into the planet’s surface. And since we already know that it is only outputting ~240 W/m² to outer space, the only direction left for it to be outputting the surplus ~150 W/m² is back into the surface.
This, then, is the unavoidable conclusion to which this analysis of power inputs and outputs in the climate system brings us: that the atmosphere must be continuously recycling some of the power of surface radiance (~150 W/m²) back to the surface in order to maintain the surface radiance at the level at which we find it (~390 W/m²). Applying the Stefan-Boltzmann law to these power-ratings we find that this recycling of surface radiant power maintains the average surface temperature at ~33°C above the ~-19°C that insolation of ~240 W/m² could support by itself. Thus, we can say that the atmosphere really does warm the planetary surface and keep it warmed well above the temperature that it would otherwise have if it was warmed by incoming solar radiation alone.
But the average temperature of the atmosphere is well below that of the surface. Thus, the atmosphere demonstrates existentially and continuously how a cold object really can warm a warmer one, just as Willis has proposed.
Dang, Cassio, yer bringing that “logic” stuff into the discussion …
Thanks very much for your clear and compelling comment. As you point out, if it is not the downwelling radiation that is keeping the oceans from freezing solid, then what is keeping the surface warm? I’ve never seen anyone even try to answer that except N&Z and Jelbring and the like, who insist that the energy comes from gravity acting on the atmosphere … not.
w.
I really don’t understand why it is so confusing to propose adiabatic descent plus a time delay as the source of the energy being returned to the surface.
Because putting money in the bank on monday and taking it out on thursday doesn’t leave you richer.
Stephen Wilde December 5, 2017 at 12:48 am
I really don’t understand why it is so confusing to propose adiabatic descent plus a time delay as the source of the energy being returned to the surface.
It’s some positive feedback not a source, your explanation of it makes it seem as if there’s one concerted cycle as opposed to the many out of phase cycles which actually take place.
It’s worse tha what you think.
From what I can piece together from his word generator, his theory is thus:
The early atmosphere accumulated potential energy. This potential energy is what is supplying the eternal net positive flow from atmosphere to surface today. Convection is a component in a ke-pe dance fueled by a net zero flow via conduction with the surface. The atmosphere is not a fluid kept in place in the gravity field by the surface, rather it is ‘held up’ by convection. And finally, his newest wordsmithing is that LW from the earth system interacts with SW from the sun to form standing wave that keeps the PE there.
Haven’t seen any equations for his theory. I am working on it though. The model is for the PE of a 4 billion year old rock on a mountain top, raining down energy to keep the mountain warmer than it would be if the rock fell down.
Making progress but so far, can’t seem to get the units right.
All out of phase cycles within the atmosphere net out to leave the atmosphere in hydrostatic equilibrium.
Willis Eschenbach December 4, 2017 at 5:13 pm
Thanks for those kind remarks, Willis. I’m glad you found it so.
As you say – “not”. I don’t buy that theory either for two reasons, viz.:
1: It seems to be in conflict with the basic laws of mechanics to me. Gravity can certainly produce a force, but a force is not the same thing as energy, nor is it the same thing as energy-flux, a.k.a. “power”. I think gravity can produce energy only when it causes a net displacement of matter (as per the fundamental definition of work as Force X Distance moved in the direction of the force). But in an atmosphere in hydrostatic equilibrium there is no net displacement of matter taking place.
2: It proposes a new, superfluous, unconventional theoretical principle to explain a phenomenon (i.e. the surface temperature elevations of planets) that is already explicable with the existing conventional principles of radiative physics, as shown in my previous comment above. Thus, it apparently falls foul of Occam’s Razor.
I wish N&K et al would wise up to these fundamental objections to their theory from conventional physics and address them honestly in public so that we can all move on, but I confess that I have little hope of them doing so in the foreseeable future as they seem to me too preoccupied with proving it to be capable of hearing how it might actually be wrong at present.
“Applying the Stefan-Boltzmann law to these power-ratings we find that this recycling of surface radiant power maintains the average surface temperature at ~33°C above the ~-19°C that insolation of ~240 W/m² could support by itself.”
We don’t need to apply the Stefan-Boltzmann law to the fluxes either, that’s the great thing. The surface temperature at approx. 288 K, and the temperature at the mass-weighted mean temperature of the atmosphere of 255 K (see page 3, here: http://paoc.mit.edu/labweb/notes/chap3.pdf) are both directly measured. So we know that this surplus ~150 W/m2 we’re all familiar with must equate to a 33 K difference. Without the need for any conversion of fluxes to temperatures.
Tony, December 4, 2017 at 6:06 pm
Thanks for that useful reference, Tony. OK, so the average temperatures of the surface and the atmosphere have been measured and confirm that the surface is 33°K/C warmer than the atmosphere. Now, what about their radiances, which we also need to know in order to track the power-flows through the system?
But if we’ve only measured their temperatures, how do we know about the ~150 W/m² difference between the radiances of the atmosphere and the surface without using Stephan-Boltzmann? OK, satellites can measure the radiance coming out of the top of the atmosphere, but last I heard they still cannot measure the surface radiance with current state-of-art technology and there aren’t enough ground stations to do it either. So, humbling as it might be to admit the truly primitive state of modern day “climate science”, I think the Stephan-Boltzmann law is indispensable for the time being.
True, Cassio, we don’t know whether that flux difference of ~150 W/m2 will be correct. Whatever that flux difference is, though, in reality; we know it cannot account for a temperature difference greater than 33 K. Since, as you agree, the temperature difference of 33 K has been measured and confirmed. It may well be that the flux difference is something other than ~150 W/m2, we’ll just have to wait and see, at such time as the surface radiance can be measured.
I believe what you say but am lost. The earth receives 240 w/m2 and 240 w/m2 leaves into space. where does the 150 w/m2 at the surface come from? there are zero watts available to reradiate is 240 come in and 240 leave. what warms the surface?
@cjw,
Yes, cjw, I can see why you’re lost…you’ve got all these watts/sq.m. rattling around in your head in great confusion. Cassio and Willis have put you wrong because they both subscribe to the Earth Energy Budget diagrams of Trenberth, which shows that there is insufficient solar radiation arriving at the Earth’s surface…and using the Stephan Boltzmann equation with an emissivity just rounded at 1….they get the surface average temperature showing an unreal frozen minus 18deg C.
The reason is because these EEB diagrams only show about 161 , 163, 168.. and this latest one of Willis’s…169 w/sq.m solar radiation at the Earth’s surface ( they are both rabbiting on about 240w/sq.m. at the moment…increasing all the time.. 🙂 )
In reality , the TSI of about 1360w/sq.m is what arrives at the Top of the Atmosphere (TOA). It’s real and measured by the satellites. It’s a yearly global average…a bulk load at the TOA which is not to be divided down by 4 . …at the TOA. The sun never sets in space, and space is right there at the TOA. The sun just sits there 24/7. It’s 1360 w/sq.m at the TOA…and this is sufficient solar radiation to keep Earth’s surface temperatures at what they really are.
If you take this 1360w/sq.m. ,you can then geometrically divide it down by 4 to account for the night and day geometric attenuation, to get your 340w/sq.m, but now that is at the EARTH’S SURFACE…not at the TOA.
Taking this 340 w/sq.m solar flux at the Earth’s surface, and an emissivity of 0.82 (courtesy of Nasif Nahle) we can slot those numbers into the Stephan Boltzmann equation and you come up with a surface temperature of about 19deg C….this is a bit high.
They say the real surface temperature is measured at about 15 deg.C …so there would be less than that average of 340w/sq.m at the Earth’s surface…caused by the atmospheric attenuation…clouds,etc.
Geometric attenuation plus atmospheric attenuation on the real ,measured 1360w/sq.m using the SB equation will give you the real global average temperature, with no such thing as a “greenhouse effect” from the atmosphere. End of story.
Mack December 5, 2017 at 1:37 am Edit
Man, I don’t know if I can explain all the ways that is wrong. Let me just say what is true, it might be shorter.
• TOA solar radiation is about 1360 W/m2
• The earth intercepts an amount of sun equal to the cross-sectional area of the planet.
• To average it over the surface of the planet, you divide by four. However, this is NOT what makes it to the surface. It is the 24/7 average of the 1360 W/m2 instantaneous radiation at the TOA. Not the surface. The TOA
• Of this 340 W/m2 at the TOA, about 100 W/m2 is reflected by the surface and the clouds. How do we know this? It is measured from satellites. This leaves 240 W/m2 making it past the reflections.
• Of this 240 W/m2 making it past the clouds and reflections, about 70 W/m2 is absorbed in the atmosphere before hitting the surface. The atmosphere itself absorbs some, and some is absorbed by various small particles, airborne chemicals, clouds, and aerosols. This leaves about 170 W/m2 hitting the surface.
Note that this all is either measured or estimated from measurements and cross-checked in a number of fashions. Your claim that you just divide the TOA sunlight by four and that 340 W/m2 is what hits the surface ignores cloud reflections, ground reflections, and atmospheric absorption.
However, the errors don’t stop there. You say:
Don’t know who Nasif Nahle is, but the satellites say that he is wrong about an emissivity of 0.82. That’s far too low. Only a small part of earth’s surface (deserts) have an emissivity of less than 0.85, and most of it is up around 0.95 or higher. From the Terra Satellite folks who measure the emissivity from space:
Bearing in mind that 72% of earth is covered with water, and that the rest (except deserts and arid areas) is covered with either vegetation or ice, it is clear why the average emissivity of the planet is around 0.95.
Sigh …
w.
@CJW
I mean no disrespect by this response. This is not simple and if it was then there would be no controversy and no endless discussion. Since it is not clear where you are baffled and I do not want to be the millionth explanation, permit me to speculate and offer a simple attempt to unconfuse you, and hopefully others.
Since you say you ‘believe’ Willis’ explanations, I’ll make a giant leap here and speculate that you either can’t or won’t do the math yourself and consequently are relying on the argumentation and kind of sense of the players. There is no shame in that and again please don’t think I am taking a poke at you.
There are tons of topics on this blog that are so far beyond my own abilities that sometimes I feel very small from reading. I read those and keep my mouth shut so I don’t embarrass myself. This topic is not one of those so here is a possible way out of your dilemma.
This topic is confusing because it is both in and out of our ‘scale’. Humans have innate skill at understanding the things in our ‘scale’: temperature, the distance to the next city, the time to dinner, etc. Conversely we are not good at things out of our ‘scale’: the distances to galaxies, the age of the earth, massive numbers, tiny numbers, energy, radiation, etc.
This radiation problem conflates temperature (very familiar to us and our scale), and radiant energy flow ( one of the most mysterious and furthest from our scale).
You are very reasonably conflicted by a system that claims to raise temperate by depositing nothing. It’s like getting rich by depositing money in the bank while your spouse takes it out the back door.
Let me suggest this as a solution. Stop thinking about temperature. It is just a proxy for what counts, energy. Think of radiation energy as water flowing into a swimming pool. When the pool is full it overflows and the water out = water in. That is the planet with no atmosphere.
Now add an atmosphere. It is like raising the sides of the pool. After a while the new pool will overflow too but now it has more water in it.
And oh, by the way, more water means more energy which will have a higher temperature.
Now I will be attacked for this post because the pool isn’t rotating , and you can’t put sides on a kardashian atmosphere, and the microdoodles don’t allow it, and you haven’t considered the spiral arms of the galaxies. Just remember that half of what you read in comments is bullshit spewed by people who should be keeping their mouths shut.
Energy is the nut. Temperature is distraction
cjw, December 4, 2017 at 10:40 pm:
Please don’t believe anything I say that you don’t understand. If you do that you will surely get hopelessly lost.
I can only repeat what I already said in my comment above to which you are replying: it has to come from the atmosphere.
There are substances residing in the atmosphere (i.e. gases, clouds, aerosols and particulates) which capture some of the outgoing surface radiance on its way through to outer space and which redirect some of that captured energy (~150 W/m² altogether) back to the surface, thereby supplementing the energy which the surface receives from the sun (~240 W/m²). This is what I think warms the surface above the temperature which the solar input of 240 W/m² could sustain by itself.
“There are substances residing in the atmosphere (i.e. gases, clouds, aerosols and particulates) which capture some of the outgoing surface radiance on its way through to outer space and which redirect some of that captured energy (~150 W/m² altogether) back to the surface, thereby supplementing the energy which the surface receives from the sun (~240 W/m²). This is what I think warms the surface above the temperature which the solar input of 240 W/m² could sustain by itself.“
Cassio, given what we discussed earlier, I would assume you agree that this captured energy of ~150 W/m2, could not possibly be warming the surface by an amount greater than 33 K?
Tony, December 6, 2017 at 3:49 pm:
I do agree, Tony, so long as you’re treating the 33 K figure as approximate and you’re not wanting to change any of the other parameters of the situation like albedo and insolation. The ~150 W/m² recycled power only produces a surface temperature elevation of ~33°K under very particular circumstances, you see.
The main factor here, though, is usually the 4th power relationship between radiant intensity and absolute temperature in the Stefan-Boltzmann formula, which we’re using to convert temperatures into radiances and vice versa. Because of this relationship, a ~150 W/m² increase at the lower end of the radiance-scale equates to a larger temperature-elevation than it does higher up the scale. So the ~150 W/m² of extra surface radiance that is boosting the surface temperature by ~33°K is specific to the effective insolation temperature of ~–19°C and the (estimated) actual surface temperature of ~14°C (give or take a degree here or there to allow for the large uncertainties that we’re having to deal with here).
Sounds about right. All seems pretty straight-forward to me. It’s the funniest thing, but I had a heck of a job trying to explain that to someone else, in an earlier conversation. They were doing absolutely anything they could to avoid agreeing to that point.
Here’s a link to an important part of that conversation:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2680092
Tony, you obviously believe that Back Radiation is warming the Surface, so can I ask.
Do you believe that this Results from the Radiation actually striking, being absorbed and also thermalised by the Surface?
No, AC, I don’t think that.
Tony, if your answer to my question is yes, what part of the atmosphere do believe that the Radiation is coming from.
Also which Molecules are responsible CO2,Water Vapour or Clouds?
And to be clear, my “no” is in response to:
“Tony, you obviously believe that Back Radiation is warming the Surface”
Tont, thanks for the response, I obviously misread your chat with Cassio, sorry about that.
Tony it would help if I could accurately hit the Y and not the T.
Ha ha, no worries A C!
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2688993
Cassio
December 4, 2017 at 3:46 pm: The Moon’s surface is indeed that of its mass. That of Earth is higher, and gas properties render it higher in temperature than the gas above ie the lapse rate. The lapse rate is an effect of gravity.
Willis, thanks for your reply. The atmosphere we have now is very much not the one we started with from initial outgassing. Whether or not there was enough insolation at a critical point after collisions lessened enough for geothermal heat not to float an atmosphere, the fact remains that every night on average the daily increase is lost with time to spare. And total freezing would soon ensue if the sun didn’t rise. As the PE went unreplaced.
So, instead of a steel shell, try this thought expt with no gravity…..:)
You’r one of those automated story generators aren’t you?
For those of you interested in thermodynamics, let me recommend that if you have not heard of it you read about the Constructal Law. It is a fundamental thermodynamic law first enunciated by Adrian Bejan in 1996, so if you studied thermo before that, you might have missed it. Here are a couple of posts of mine on the subject:
The Constructal Law of Flow Systems 2010-11-15
One of the most fundamental and far-reaching discoveries in modern thermodynamics is the Constructal Law (see the wiki entry as well). It was first formulated by Adrian Bejan in 1996. In one of his descriptions, the Constructal Law is: For a finite-size (flow) system to persist in time (to live),…
Constructal GDP 2010-11-16
Encouraged by the response to my post on Adrian Bejan and the Constructal Law, which achieved what might be termed unprecedented levels of tepidity, I persevere. Here’s a lovely look at the energy use of the United States: Figure 1. US 2002 Energy production and consumption by sector. There are…
My regards to everyone,
w.
Willis,
You accepted the first three points of my elevator speech in favour of the adiabatic hypothesis but balked on the other two which were:
“iv) If the surface temperature remained at 255k whilst the atmosphere was being formed then it MUST rise by 33k when the adiabatic loop closes.
and
v) Although the surface temperature is then 288k you still have ongoing conduction of 33k upward to maintain convective overturning PLUS 255k of radiation going out to space which keeps the combined radiative and adiabatic loops stable and the atmosphere in hydrostatic equilibrium indefinitely.
Your previous objection to such a scenario missed the issue of the process of creating the atmosphere in the first place when the first convective overturning cycle formed. That is where the non zero consequence of conduction and convection comes from so that there is no breach of the first law.”
Your response was:
“If the gases were indeed “spewed from volcanoes”, your theory that we needed to put energy into the atmosphere as it formed goes out the window. The atmosphere would have been hotter than the surface to start with … and that’s the opposite of your theory.
But that’s not the main objection to your theory. The main objection is that even if your claim is correct and energy was pumped into the atmosphere four billion years ago, the effects of that one-time event on the flow system we call the climate are long gone.
For example, yes, dumping a truckload of water into a creek will raise the level of the creek … but not for long. And dumping a bunch of excess energy into the atmosphere, say by a solar flare or something, will raise the atmospheric temperature …
… but not for long, and certainly not for four billion years.”
and I replied:
“In the absence of solar heating at the surface being conducted to all the materials from volcanoes those materials would cool and fall to the ground so your objection is invalid.
Anyway, you are distracting from the initial premise of a simple argon atmosphere.
As for your ‘main’ objection please explain the continuing presence of a vast amount of non radiative non thermal PE still in the atmosphere if you say it has all dissipated away.
How exactly do you say that energy could have been removed or otherwise cancelled by some sort of unspecified setoff?”
In addition the creek example doesn’t work because the surplus water can be washed away downstream and lost in the much larger body of the ocean. That can’t happen for an atmosphere because the extra energy plonked into it has no means of escape to space whist fresh insolation and further conduction continue. A more apt analogy would be a standing wave above an obstacle such as a stone in the creek.
I think we should pursue the discussion further since I am genuinely curious as to what your further response might be.
Radiated away into space … it’s the only way to remove energy from the planetary system.
w.
When and how did that happen if radiation to space is limited to radiation received from space?
and
If it were radiated to space why did the atmosphere not fall to the ground given that there is a one cycle energy store in the atmosphere that would then have disappeared.
During the course of such radiation to space the atmosphere would have to shrunk progressively until it were all gone.
You seem to think that inflation does not require any energy and inflation of the volume of denser surface materials into a less dense gaseous form is all that an atmosphere represents.
I think the standing wave / feedback loop proposition is as good as it could be.
I think I finally get it. It’s the PE doing the work, correct?
Almost, PE is a consequence of work.
Work is measured in Joules thus:
“One joule is equal to the energy used to accelerate a body with a mass of one kilogram using one newton of force over a distance of one meter”
so you need one joule to raise 1kg of atmospheric mass one metre in height and that means work is being done.
In the process of rising, the molecules move further apart due to declining pressure and become cooler as per the gas laws (greater volume).
The energy from that KE cannot be eliminated (conservation of energy) so we say it has become potential energy. The term ‘potential’ means it does not show up on sensors as heat.
Not being heat PE cannot be radiated to space so it is wholly recoverable on the subsequent descent. If the atmosphere cannot radiate then it is only going to appear as heat again when it gets back to the surface one convective cycle later than when it went up.
Due to that one cycle delay the surface is then being heated both by that KE returning to the surface PLUS continuing insolation so you get 288k instead of 255k at the surface.
You can’t offset it with the KE going to PE in the simultaneous ascent, as Willis suggests, because that energy is derived from the previous descent. Refer to the stock control analogy that I gave you.
Work done in uplift (provoked by density differentials at the surface) creates PE from KE as the rising molecules move up along the declining density gradient caused by gravity.
PE is a consequence of work done in uplift and being an adiabatic process (PE cannot radiate to space) is fully reversible in descent.
It works as long as there are density variations at the surface and applies whether there are GHGs or not since you cannot eliminate such variations on a sphere lit from a point source of light.
Stephen you are creating system where uplift is derived from returning air which fuels the next uplift while simultaneouly delivering that same energy to a surface feeding in even more energy that does nothing. The phase delay is meaningless over any integration interval.
For crying out load, stop writing and draw your first picture of it.
Think of the phase delay like this….
Every morning i put a million bucks in the bank. Every evening I take a million bucks out.
Every Monday morning my wife puts in a million bucks. Every sunday evening she takes a million bucks out. I’m a millionaire all week but if I add up all my monthly statements I got nothing.
And that is exactly the amount of net energy your theory will deliver to the surface.
Sad.
You are not contributing here in good faith.
You are quite wrong. I have posed many many questions to you that remain unanswered. I am trying very hard to get you to answer them.
Every time you can’t answer you change the subject. That is demonstrable bad faith
Well between you there is 2 million somewhere.
In my dreams😃. More like rubber checks going in and out too fast for the bankers to catch up with me.
A good analogy for other features of your personality then?
Anyway, you’ve given me good exercise in boiling my proposals down to the simplest possible form but I don’t need to spend any more time on that.
I just now need to await Willis’s further comments since they are what I am after here.
Remember that this is a place of public access and likely to be a permanent record unless closed down or interfered with and even then there is still the so called ‘wayback machine’.
Pursuing the ‘creek’ analogy, better to regard radiation through space as a vast river.
If you put a planet in the way then the flow is slowed down and a standing wave forms, due to the presence of matter, which manifests as the generation of IR from the background flow to give a surface temperature for the obstruction.
If you then add an atmosphere the extra mass increases the size of the standing wave so that there is more IR but, due to the atmosphere being gaseous and therefore able to conduct and convect, a feedback loop forms between the mass of the planet and the mass of the atmosphere with the additional energy unable to escape to space so that the planet’s surface warms up instead of the atmosphere’s energy radiating to space.
Hope that is easier for those confused by the various more involved forms of words that I used previously in response to various objections.
So then your river analogy is proposing a river of SW developing a standing wave via the interaction with LW in the stream which is increased by the new gasses that don’t radiate LW.
GOT IT!
YES, hallelujah !
Interaction with matter, though.
You’ve worked hard to get away from the radiative only scenario.
Now we need a few others to see it 🙂
You forgot the sarc tag, right?
I don’t do sarc.
Was your comment sarc ?
Actually it was loaded with it. Your radiation river has SW interacting with LW out in vacuum of space.
That would be a fabulous discovery. For now it is laughably absurd. Could be wrong I suppose but you’d have to cite it for me.
A solid object in space absorbs shortwave, converts it to heat and emits longwave.
That is clear from my post.
You summary is inaccurate and misleading.
I thought we were talking about radiation in radiation rivers. Where did the matter come from?
We might as well add some ancient Greek philosophy here–
“No man ever steps in the same river twice”–Heraclitus of Ephesus
and then–
According to Aristotle, Cratylus went a step beyond his master’s doctrine and proclaimed that it cannot even be done once (to step in the same river).
It’s about the only thing I remember from my engineering HSS classes.
Jim
Willis,
Firstly, thanks for a stimulating article. The accounting analogy is excellent… (and never mind the ever-present trolls!)
Way back on November 25th at 9:56am, I responded to a comment by Philip Mullholland as follows: Philip, You say: What I had never noticed in the Trenberth diagram, until you pointed it out just now, is that there is no downward return vector to balance the upward mass transport of air that occurred during precipitation. That is because you were [not] aware that the two non-radiative energy transfers from the surface, for sensible heat (22W/m^2) and for latent heat (76W/m^2), are the net flows of energy due to these two phenomena. This is explained in the original paper that accompanied the original Trenberth diagram (I don’t know where Willis got his diagram from but the figures are in more or less the same ballpark as Trenberth’s and are definitely net figures.)
Philip did not respond to me but, browsing just now I noticed that I had missed Stephen Wilde’s response to me which was: Please substantiate that. Even Willis accepts that the net effect of convective overturning is zero so you cannot assert that the Trenberth diagram represents a net outturn. Trenberth just erroneously includes the upward leg alone (leaving out the downward leg) and compensates for that omission by incorrectly asserting a surface warming effect from DWIR.
Two questions for you:
1. My assumption is that some non-zero proportion of the sensible and latent heat moving up the atmospheric column by atmospheric processes gets radiated by GHGs to space in the upper atmosphere. If this were not the case then those mechanisms would provide no contribution to the balancing long-term steady-state LW energy flow from surface to space!
Can you please confirm whether you agree with this, contrary to Stephen’s claim that you think the ‘net effect of convective overturning is zero’.
2. He then suggests that the figure given for sensible heat convection from the surface is the ‘upward component only’ and that the downward component is in some way hidden in the general atmosphere-surface downward radiation figure.
Do you agree that all the energy flux values in your energy balance diagram (and in the original Trenberth diagram) are net figures, except for the two opposing up-and-down LW radiation values at the surface-atmosphere interface, which automatically offset one another, leaving a small net upward radiative flow?
All the best,
David
David,
It is only the adiabatic portion that nets out to zero. We were discussing an Argon atmosphere not radiating to space at all.
In the real world there is radiative leakage to space from within the atmosphere.
The Trenberth figures for thermals and latent heat appear to be IN ADDITION TO the radiative leakage from within the atmosphere since that portion is accommodated within other radiative outflows. They are also shown as a different colour which places them outside the yellow incoming and the blue outgoing radiation figures.
I did discuss an earlier version of the diagram in 2014 here:
http://www.newclimatemodel.com/correcting-the-kiehl-trenberth-energy-budget/
The same principles apply to the new diagram but the figures would need reworking.
What is the ‘part’ that does not net out to 0?
The first convective cycle during which KE is going into PE through conduction and convection. During that time the planet as viewed from space would appear to get cooler because some of the outgoing radiation is going to convection instead and the surface stays at 255k.
Once the atmosphere is in place the view from space goes to 255k and the surface goes to 288k.
Stephen, how do you know in your 1st convective cycle that the atm. was cooler than the surface? Your imagined 1st convective cycle must have had the the atm. warmer than the surface (volcano source, super heated steam source, so forth). Thus in your imagined 1st cycle the lower atm. fluid was warmed from above in a gravity field and no convection would be possible except in your imagination.
Trick 8.06 am
Output from volcanos cools very fast both by radiation to space and by expansion into the, much lower pressure, surrounding atmosphere as per the gas laws. The higher they go the faster they cool.
Shortly after each injection of new material conduction from the constantly irradiated surface takes over again.
Nice imagination Stephen. I get it. If in the first cycle up the atm. was cooler than the surface, then the 33K we enjoy today comes from some sort of imaginary PE/KE transformation that happened way back then. If in the first cycle up the atm. was warmer than the surface, then the 33K we enjoy today comes from some sort of imaginary volcano puff and/or super-heated steam outgassing being retained from way back then.
The atmosphere as a whole can never become warmer than the average surface temperature even with vast amounts of local or regional volcanic activity because if it did then the combination of KE plus PE within molecules high up would drive mass from the atmosphere out to space due to their high total energy content.
In that scenario the upward pressure gradient force would substantially exceed the downward force of gravity.
This is all pretty basic science and I’m increasingly surprised at how little you know.
“..because if it did then the combination of KE plus PE within molecules high up would drive mass from the atmosphere out to space due to their high total energy content.”
PE does not contribute to escape velocity Stephen. Sure the H2 could get out as it has done, and some helium but escape velocity could not be attained by (much) N2, O2 and higher molecular mass constituents.
So, would Stephen care to fill me in on the escape velocity and temperature that is needed for your original atm. N2 (or whatever molecules you imagine it consisted of) that you’ve computed for each constituent escape to space in order to come to this conclusion, just because of how little science you claim I know.
Total energy content does contribute to escape velocity and PE is part of total energy content.
If a molecule of N2 has its full load of PE for its actual height then to keep it below escape velocity it needs a commensurately reduced load of KE.
If it has too much KE for its position along the lapse rate slope then it will keep rising and unless something else puts a stop to that rise such as the ozone induced temperature inversion at the tropopause or unless it dissipates surplus KE by conduction to adjoining molecules (which it usually does) then it will keep rising until it is lost to space.
So you don’t need the details. You just need to know how basic physics plays out (often counterintuitively) in a real atmosphere which is a matter for the specialised discipline of meteorology. Not many atmospheric physicists seem to be aware of basic meteorological principles since they rely on radiative energy exchanges and fail to consider non radiative energy exchanges.
“If a molecule of N2 has its full load of PE for its actual height then to keep it below escape velocity it needs a commensurately reduced load of KE.”
LOL, really. Stephen you hardly ever cease to amuse. And you do not seem to be aware of basic radiative details so have to imagine what actually happens in radiative energy transfer. Adv. math in thermo. is far beyond your capability. You don’t understand even basic atm. convection let alone the small amount of available atm. PE as understood from a foundational 1950s paper.
Evidence, please.
Evidence? Margules 1903, Haurwitz 1941 and Lorenz 1955: “Evidently the total potential energy is not a good measure of the amount of energy available for conversion into kinetic energy under adiabatic flow….Consider first an atmosphere whose density stratification is everywhere horizontal. In this case, although total potential energy is plentiful, none at all is available for conversion into kinetic energy. .” so forth.
More than likely Stephen will not look these up as he’d rather imagine the atm. physics, since it is easier, no work involved.
I’ve not read every comment here, but most have nothing to do with thermodynamics.
The first thing one must do is define the system and its boundaries. Without that step, you can’t even apply the laws. Heat is defined as the energy crossing a system boundary due to a temperature difference–hotter to colder. They usually add in the phrase, not due to work. Systems do not contain heat or work–they only appear at their boundaries. Heat can be transferred by conduction, convection, or radiation. I don’t understand the phrase: “heat is not radiation.” Yes it is.
The first law in differential form using the Clausius standard is:

where U is internal energy, Q is heat, and W is work. The squigglely D’s are to remind you that heat and work are path variables. Internal energy is a state variable. Positive heat is heat added to the system, negative heat is heat removed from the system, positive work is work done by the system, and negative work is work done on the system. This isn’t the only standard or form for the first law.
The statement “ENERGY is not HEAT” is a problem. Maybe all energy is not heat, but all heat is energy. The SI unit for heat is the joule. In the past, they used BTUs and calories. Both are easily converted to joules by simple conversion factors.
The second law only applies to isolated systems. Closed systems can lose heat and entropy.
For example, here’s an isolated system:
Object B is hotter than object A and is transferring heat Q from B to A. Objects A and B are subsystems (otherwise we couldn’t talk about heat transfers) and they are closed systems–we’re allowing energy to transfer across their system boundaries. The entropy for A is where
where  is the absolute temperature of object A. The entropy for B is
is the absolute temperature of object A. The entropy for B is 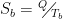 where
where 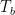 is the absolute temperature of object B.
is the absolute temperature of object B.
Now if , then
, then 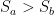 .
.
Using the second law we get:
 .
.
So this isolated system does not violate the second law. But notice that object B is losing entropy. The entropy lost by B is less than the entropy gained by A–for the same heat transfer. If a closed system couldn’t lose entropy, then nothing would ever cool down.
The internal energy of B is decreasing, while the internal energy of A is increasing. This will cause the temperature of A to increase, and the temperature of B to decrease. This will continue until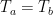 . Heat will stop being transferred, entropy will reach a maximum for this system, and
. Heat will stop being transferred, entropy will reach a maximum for this system, and  at equilibrium.
at equilibrium.
Jim (I hope this post works correctly.)
Jim, that is an excellent introduction to systems, entropy, heat, etc.
To use this approach to Willis’ scenario will take a bit more effort. We would need to consider a heat input (eg from the sun, an electric heater, or radioactive decay). We would need to consider a fixed temperature heat sink (eg outer space at 2.7 K or a room at 20 C). With these additions, the system of A+B (for example the earth + the atmosphere) is no longer isolated and A & B do not need to approach a common final temperature with Q=0.
Mr Folkerts, in your response to Jim Masterson you stated “To use this approach to Willis’ scenario will take a bit more effort. We would need to consider a heat input (eg from the sun, an electric heater, or radioactive decay). We would need to consider a fixed temperature heat sink (eg outer space at 2.7 K or a room at 20 C).”
Can you explain your logic here please?
It has already been admitted on here that 2 objects at the same temperature in a room cannot make each other warm, despite flooding each other with Photons, so how does one colder object increase the temperature of the hotter one as in “object A being Earth’s Surface and Object B being CO2 Molecules.”
I asked Paul Bahlin at
A C Osborn
December 3, 2017 at 10:10 am
what would be the result of one heated object A at equilibrium and 25 degrees C and an object B at 0 degrees C.
When I introduce Object B to within a few millimetres of Object A WHAT HAPPENS TO THE TEMPERATURE OF OBJECT A?
His answer
Paul Bahlin
December 3, 2017 at 10:33 am
Don’t know or care. In my house object B would be a beer and I would drink it before A radiation was allowed to touch it.
So perhaps you are prepared to offer a more “Scientific” answer to the question?
For clarification the “heat Sink” is my kitchen walls & ceiling, at 19 C.
Steven Wilde, perhaps an example will help. You seem to think that the atmosphere is kept warm by the 33K of warming from its original formation. If I understand you, the rest is just a constant exchange of PE and KE. Here’s a parallel.
Staying alive doesn’t require a static amount of energy. It requires a flow of energy in the form of money.
So suppose I give you $33,000 dollars. Then for fifty years, every year I give you another $33,000, and you give me the same amount, $33,000. And that’s all the money in the equation.
The question is, what are you going to live on?
Similarly, staying warm doesn’t require a static amount of energy. It requires a flow of energy in the form of heat.
So suppose I give the atmosphere some large amount of energy. Then for fifty years, I give the atmosphere another large amount of energy, and it gives me the same amount back. And that’s all the energy in the equation.
The question is, what will keep the atmosphere warm?
That’s why folks keep trying to point out that a one-time injection of either money or energy is not enough to keep you alive or to keep the atmosphere warm over an extended period of time. That requires a flow, a flow where more energy or more heat is required on a constant, ongoing basis.
Regards,
w.
Fair question, Willis, and the same as the one that Paul raised and which I dealt with in the stock control analogy.
I’ll deal with it afresh here for your convenience using your dollars instead of his cheeseballs.
i ) You give me 33000 dollars on 1st January 2000
ii) I give you 33000 dollars on 1st January 2001 and receive another 33000 dollars at the same time.
iii) I still have 33000 dollars do I not?
iv) If we then continue forever then I have 33000 dollars forever to do with as I will or as per your analogy ‘to live on’.
v) When I die you don’t pay me 33000 dollars on 1st January following my demise and my estate owes you 33000 dollars from which you are repaid (unless I’ve spent it on having fun – which these online exchanges are for me -it’s a neat pastime)
So, when the atmosphere falls to the ground it repays its PE but that won’t happen until the sun stops shining which is when the atmosphere dies.
What is happening is that there is a one cycle delay in refunding your 33000 dollars just as there is a one cycle delay for energy held in the atmosphere escaping to space.
Is there a flaw I haven’t thought of ?
How did Stephen live over the years without spending any of the 33000?
The atmosphere doesn’t have a constant energy demand as we do.
It simply needs the initial slug of energy to get it hoisted aloft. Once aloft it needs no further input so simple recycling of the available energy is sufficient.
Willis concedes that ongoing convection is a zero sum game so if it were to deplete that initial 33000 dollars by some means it would not be a zero sum game and the atmosphere would gradually deflate.
But we know that it does not.
“The atmosphere doesn’t have a constant energy demand as we do.”
It does Stephen. The atm. loses calories continously just as you do. You need to eat & the atm. needs the sun. A fuel needs to be burned to maintain your temperature & the atm. temperature near steady state.
The sun supplies fresh calories at a constant rate. That is sufficient to hold the mass of the atmosphere off the ground since the atmosphere has no metabolism to burn calories as we do.
Doe you think the atmosphere has something equivalent to a metabolism?
So, after a little diversion, how did Stephen live over the years without spending any of the 33000?
Huh? As others have noted, WHAT ARE YOU LIVING ON?
Your claim seems to be that you just heat something up by 33K and it stays at that temperature forever. But the atmosphere is constantly losing heat.
You’re still not accounting for the fact that it takes a continuous supply of energy to do continuous work, like keeping an object warm.
w.
This had me baffled for a long time but I think I’ve discovered the disconnect. Stephen, I know you’re pissed off at me and I have dished a lot of snark your way but I really am trying to understand your position.
Could it be that you think PE is Energy? You seem to be ‘living on It’ in Willis’ example because your daily money exchange is with money that’s never been touched. There’s no other money available.
Do you count the atmosphere’s PE in the PE-KE exchange of convecting parcels?
Willis, Stephen knows as should you, that after the initial input, the continuation of an atmosphere not an icefield depends on daily input until the sun returns to its original low or non-radiative state.. No need for confusion on something as basic and simple as this. But then again, I’m just a Kiwi cowboy and seaman…..who only got serious about physics late in life. Cheers, Brett
Thanks Brett.
If Willis can’t get past that then I’ll just have to give up here and revisit the issue in the next thread where it becomes relevant.
The thing is that Willis agrees my first three bullet points in support of the elevator description of the adiabatic effect on surface temperature.
He has only balked on points 4 and 5 but they follow naturally and logically from the previous points so he really has nowhere to go but doesn’t seem to realise that yet.
For example, he insists that an atmosphere has some sort of ‘burn’ rate which sheds adiabatic energy to space despite PE being non radiative yet at the same time he agrees that convection up equals convection down.
I’m only focusing on Willis now because the other critics seem to be beyond redemption.
Brett Keane December 6, 2017 at 12:08 am
Brett, I fear I have no idea what you are talking about. It appears you think I “should” know something, but what it is and why you think I don’t know it is a mystery. Also, you have no idea what Steven knows, and unless he’s appointed you his spokesdude, you have no right to speak for him as to what he knows.
As to “no need for confusion”, to avoid the confusion that you are adding to with this comment, please follow my polite request to QUOTE WHATEVER THE HELL IT IS YOU ARE BABBLING ABOUT.
I’m sorry to shout, but you and Stephen just toss out things and assume that I know what you are referring to. I don’t really understand Stephen’s claim, yet he keeps saying that I “agree with his first three bullet points” … and since there are 1800 comments in this thread and I have no idea how to find whatever bullet points he’s talking about, just how on earth am I supposed to answer?
People keep assuming that I’m having a one-on-one conversation with them, and that I can remember every detail of every claim they made and every response of mine … bad news. I’m holding like twenty conversations simultaneously, often with people using totally forgettable aliases, many of them making similar points.
Now, I know that you’re a cowboy and a seaman. But that’s no excuse for ignoring a polite request. I have no idea what Stephen knows or should know. I have no idea what you know or should know. So please, stop waving your hands and acting like it’s going somewhere. I’m tired of filtering through comment after comment making claims about what I said or they said or you said, WITHOUT ANY QUOTATION TO TELL ME WHAT IT IS THAT HAS THEIR KNICKERS IN A TWIST!
Why is following a simple polite request so damn hard? If you cowboy and go to sea in this slapdash fashion, god help your crew, your bunkmates, and your horse …
Sadly,
w.
Stephen Wilde December 6, 2017 at 1:21 am
“Get past” WHAT? WHICH “issue”? You and Brett think you are having a discussion, and your lips are moving, but the words make no sense.
Stephen, you have been asked many times, including just below (again) by TJF, to provide a coherent description of a) what it is that you are claiming, and b) EXACTLY how it works. In response we get bafflegab. My patience is run out.
So I will say, along with all the rest, again, show us the numbers!
Truly, at this point I don’t know what your basic claim is. Boil it down into an elevator speech about how it all works, and then PUT NUMBERS TO IT.
I’ve had it with the handwaving. What is your basic claim? What numbers support it? What is your ensuing claim assuming the first one is true? What numbers support it?
Exasperatedly,
w.
Mr Eschenbach is making ridiculous statements again.
“Can’t live on a single injection of cash”
Without any qualification of a single injection amount.
Give any reasonable person $1billion and they will live very nicely for the rest of their lives and so will their families.
Probably $10million would do it for most people.
A C Osborn December 6, 2017 at 12:33 pm
You do know that such snark just makes you look like you are worried about losing the debate, I hope …
You miss the point entirely, likely my fault for lack of clarity. Let me take another shot at it:
“Can’t live on a single injection of cash, you have to spend some to stay alive.”
In Steven’s explanation, he keeps the atmosphere warm indefinitely, but he’s not spending any energy to do so. That was what I was trying to point out with my example.
w.
But my comment is no where as “snarky” as many of yours.
And not only that, it was perfectly accurate, as was my previous correcting of your statement about “thermal energy” when you really meant IR energy.
It is not my fault you get carried away and make incorrect statements to make your points, it was yours.
So maybe if you cut out the rhetoric and just stuck to the facts I wouldn’t feel the need to set the record straight.
A C. .no matter how much time, effort and energy you expend setting the record straight, it will do nothing to convince Willis that he does not know everything, nor will it cure him of the problem of his over inflated ego.
I know, it is not for him it is for the lurkers, who may take what he says at face value.
Does that sound familiar?
Jesus, AC! Can’t you just address Willis’ science and avoid verbal coup-counting.
Dave, Mr Eshenbach does not need your.
Let me correct your statement for you.
Stop mimicking Willis just address Willis’ Fantasy science and avoid verbal coup-counting.
But as you are still asking questions.
Please show me any reputable (ie non Climate Science) sites that say that the radiation from a cold object makes a warmer one warmer and please do not quote a “Thought Experiment”.
That is all I am trying to Establish, what conditions that are currently not described by the Science allows photonic energy from whatever colder source to warm up a warmer object.
The definition of heat flow is the amount of Joules per second (Watts) per Metre2. Therefore at Equilibrum the amount of joules is a finite amount from the energy source without changing the energy source.
So that just leaves Emitting Surface Area as the variable.
Let’s assume that his sphere is spitting 2000 Joules per second per metre2 at Equilibrium, if we just take his sphere and expand it to the size of the Shell it’s Output will also drop by half as in the Steel Greenhouse Shell to 1000 Watts/M2 ie half the number of joules available per second per M2.
Please note that the System is still at Equilibrium putting out the same total of 2000 Joules per second when the total surface is considered, ie over twice as many square metres.
Also note that it is NOT at 4000 Joules per second, which are NOT available, totally impossible and are totally unnecessary for the sphere to obtain its original state of Equilibrium.
In fact I am not sure if it willactually be below 1000 joules per second as it takes twice as many joules to heat twice as much steel (Specific Heat) to the same Temperature, so am not sure if they can still be radiating the same.
As it would also take a little more to heat the extra Steel in the Shell.
I have been conducting REAL Experiments to try and find those magical conditions where the Colder surface Radiation heats the warmer surface and up till now I have failed.
As to the Light Bulb in a jar and foil which you so heartily endorsed, I am absolutely staggered that anyone believes that Back Radiation is directly involved in the warming of the Bulb.
I did not need to be shown that experiment as I had already done it for myself. Of course I used a Thermometer with 2 probes where it can provide not only the temperature from the probes, but also their difference in temp.
The extra heat comes from standard “Insulation” plus one other factor which comes after this one.
So what has changed, the addition of the Jar and the Foil have drastically reduced the heat loss via both Conduction and Convection. So what about the extra heat from adding the Foil, well that is where any back Radiation comes in.
Not only is the Foil doing the same to Conduction & Convection now the foil heats much quicker than the air between it and the bulb as it is a much better Conducter. The much hotter foil’s radiation excites the Air between the Foil and the Bulb increasing Temperature and thus slowing the cooling even more.
Experiment 1 with and without Foil box with a hot object
Without Box
Heated Object Temp (Facing Upwards) = 24.5C
Air above temp = 20.2C
Difference = 4.1C
With Box (with Foil) after 15 minutes
Heated Object Temp (Facing Upwards) = 26.0C
Air above temp = 24.0C
Difference = 2.0C
With Box (with Foil) after 20 minutes
Heated Object Temp (Facing Upwards) = 27.2C
Air above temp = 24.2C
Difference = 3.2C
With Box (with Foil) after 30 minutes
Heated Object Temp (Facing Upwards) = 27.5C
Air above temp = 24.4C
Difference = 3.2C
Foil at this point 22.5C
So do you see what happened, the Air heated most at 4.0C first due to the foil which in turn meant the heat sink was no longer as effective and the heated object warmed 2.0C.
But the air had started to stabilise at 24.2C and only rose another 0.2C while the object was still trying to reach stability.at 27.2C and then 27.5C.
However the Actual Heat Source which was a bulb around 25mm below the object could not be affected because all the heating was taking place above the Object, whereas the temperature to the side and below the object was only raised by 0.4C despite all that heat above neing reflected downwards by the Foil and By Conduction within the Air.
So now to the other factor involved, lets assume a warehouse with 20000 cubic metres of space. It has no heating so it remains at roughly outside ambient. We add X number of watts/m2 of Radiators with a large enough boiler to maintain a temp of say 30 degrees. Now if I put you in that Wharehouse with the Heating running at max you would be quite a bit hot, but not too uncomfortable. If I now take all thus radiators and stuff them into a room of 200 cubic metres with much better thermal Insulation how do you think you would feel.
Well you probably wouldn’t feel anything for very long as you would be Dead from heat exhaustion.
That is precisely what they did with the bulb in a box, took it out of a very spacous room and placed it in a very tiny one by comparison and added additional Thermal cladding..
Now tell me if you are surpised by the Air getting much hotter and thereby preventing the Bulb from cooling.
Perhaps they should fill the Box with CO2 at -180C to see how much it heats the bulb, I might try that with Cold air as I don’t have any CO2.
Experiment 2 with and without Foil box with 2 cold objects 13grms in weight at 12.0C and 12.7C in the box.
It is an established fact that a hotter object can warm quicker than a colder object so the one in the box may cool quicker. The Purpose of experiment is to demonstrate further that it insulation
After 0 minutes
Object out of box = 12.0C
Object in box = 12.7C
Difference = -.0.7C colder than outside
After 10 minutes
Object out of box 14.5C
Object in box = 14.9C
Difference = -0.5C
After 20 minutes
Object out of box = 17.3C
Object in box = 17,2C
Difference = +0.1C
After 30 minutes
Object out of box = 18.4C
Object in box = 18.1C
Difference = +0.3C
So there you have it A Foil Box also slows how much a cold object warms up, so it is insulating it from it’s surrounding, it did not add a single bit of back radiation to speed up the warming compared the one outside the box.
More Real Experiments to follow.
Well, AC, I was responding to a question about a steel sphere with an independent power source surrounded by a steel shell. It is a complete system suspended in outer space.
I assert that energy from the sphere is totally incident on the interior of the shell.
I assert that the shell radiates energy at both surfaces equally at all times; half of the shell’s energy is radiated outward to space, half radiated inward to the sphere.
I assert that all of the shell’s energy is supplied by the sphere.
I assert that the radiated energy of the interior of the shell is absorbed by the sphere, heating the sphere.
I assert that the heated sphere is emitting additional energy to the interior of the shell.
I assert that the process continues until the energy input to the sphere/shell system is at energy equilibrium with its surroundings; 1/2 of the energy supplied by the sphere is radiated by the outer shell, 1/2 back-radiated at the sphere.
Those are the only things I assert on this Thread.
You know, I think future generations (if they can be bothered) are going to look at some of my opponent’s comments with amusement as I do,so Trick and Paul, be more careful what you say.
As for Willis I currently believe him to be an honest broker.
Stephen, your theory all seems to hinge on the “initial uplift” of your “adiabatic loop” as the atmosphere is added. So let me ask you about that.
Suppose there is a planet with no atmosphere and a blackbody surface. I has a 240 W/m^2 power supply, which makes the surface 255 K. Now add an atmosphere (pure argon that doesn’t radiate noticeable thermal IR). I can imagine a few different options.
1) The atmosphere starts at 255 K. In this case, it is the same temperature as the surface and no convection is started. There is never energy lost from the surface to the atmosphere, never any ‘adiabatic loop’, and never any energy returned to the surface to warm it about 255 K.
2) the atmosphere starts at some colder temperature — say 200 K. The atmosphere will warm by contact with the ground and start to convect. However, that same contact must necessarily cool the surface below 255 K. That is how conduction work — the cool object warms and the warm object cools. The surface will continue to be held below 255 K and the uplifting air will also be below 255 K — until the “initial adiabatic loop” has been set up and the air returns. However, since the the loop is adiabatic, the air will return at the same temperature it left — somewhere below 255 K (having cooled on the way up and warmed again on the way down). Air below 255K returns to a surface below 255K, so that col air can’t warm surface above 255 K.
3) the atmosphere starts at a temperature about 255 K — say 288 K. Now the bottom of the atmosphere will cool by conduction, and the surface will warm above 255K. But no convection is started so there is no “adiabatic loop”. The surface — now warmer than 255 K will radiate more that 240W/m^2, so there is continuously energy lost and continuous over all cooling. The atmosphere above 255 K cools by conduction to the surface below 255 K. This continues (with no ‘adiabatic loop’) until everything equilibrates to 255 K.
None of these works at all to get the surface to 288K. Perhaps you can describe specifically your scenario for adding an atmosphere.
TJF: Yet again, still, after all this time, you act as if you do not know what makes a gas, any gas, different from other phases of matter. From above, you do not admit that a gas is matter. Or surely you would write different words. Hint: the effective surface is changed, and gravity is able to squeeze more matter with its energy into smaller spaces, lower down.
Enjoy.
Well, they can’t accept the effect of the gas laws can they?
If they do, it is game over.
Brett Keane December 6, 2017 at 12:36 am Edit
Could you be more unclear, please? There were times when I thought I could actually understand your point …
Setting the sarcasm aside, if you disagree with a man, QUOTE HIS EXACT WORDS, and then tell us exactly where they are wrong. Saying “From above, you do not admit that gas is matter” is a handwaving joke … where the hell did TJF say that?
Etcetera for your other points …
w.
Hmmmm … i said:
* gases warm when put in contact with warmer objects and cool when put in contact with cooler objects..
* gases expand when they get warm.
* warm, less dense gases will rise.
* rising gases in an atmosphere will expand and cool.
* sinking gases in an atmosphere will compress and warm.
* If a gas expends and then compresses back to the original volume adiabatically, it will return to its original temperature.
That all sounds exactly like the behavior of gases to me!
Paul Bahlin asked:
“Could it be that you think PE is Energy? You seem to be ‘living on It’ in Willis’ example because your daily money exchange is with money that’s never been touched. There’s no other money available.
Do you count the atmosphere’s PE in the PE-KE exchange of convecting parcels?”
Yes, of course it counts as energy. It just isn’t heat and doesn’t radiate.
Molecules of gases have electromagnetic forces between them.
If you move them closer together then those interactions become more intense because the same energy is focused within a smaller area and they become hotter.
If you move them further apart the interactions become less intense and the available energy becomes spread over a larger volume so they cool down.
That is the essence of the gas laws and the reason why the pressure gradient set up by gravity is so effective in creating a lapse rate with height.
The changing of the distance between molecules does not imply any loss of total energy (conservation of energy) but it does involve a change in the amount of sensible heat within the volume occupied by the gas.
And because an atmosphere is inanimate, with no metabolism, it has no energy burn rate once established and the PE content is unable to radiate to space so it just recycles constantly between surface and atmosphere.as long as the sun keeps shining.
That is a trivial and well known physical principle that you should all be well aware of, as Brett kindly pointed out.
The entire universe runs in a background of potential energy derived from the expansion of gases after the big bang but that is another story.
“And because an atmosphere is inanimate, with no metabolism…”
The atm. of course has no metabolism, YOU do though Stephen and you needed to spend some of the $33000 each year to support that metaboliosm which is the fatal flaw you hadn’t thought of in your 1:16pm comment. Likewise the sun/earth system also has to burn a fuel to support its energy loss.
Stephen Wilde December 6, 2017 at 1:47 am
Molecules of gases have electromagnetic forces between them.
If you move them closer together then those interactions become more intense because the same energy is focused within a smaller area and they become hotter.
If you move them further apart the interactions become less intense and the available energy becomes spread over a larger volume so they cool down.
That is the essence of the gas laws and the reason why the pressure gradient set up by gravity is so effective in creating a lapse rate with height.
The changing of the distance between molecules does not imply any loss of total energy (conservation of energy) but it does involve a change in the amount of sensible heat within the volume occupied by the gas.
You have a misunderstanding of the gas laws. The ideal gas law which applies under the conditions of our atmosphere is based on there being no attractions between the molecules. Under conditions of very low temperature and high pressure there are deviations from the ideal gas law and a different equation of state is used and attraction between molecules become significant (the a/V^2 term in the van der Waals equation for example)
No gas is ideal.
The concept of an ideal gas is just a construct upon which one then superimposes the characteristics of real gases in real atmospheres.
In real life the interactions between molecules are indeed significant hence the need for the gas laws. I think you are just thrashing about.
Do you really deny the relationship PV=nRT?
Stephen Wilde December 7, 2017 at 8:17 am
No gas is ideal.
The concept of an ideal gas is just a construct upon which one then superimposes the characteristics of real gases in real atmospheres.
In real life the interactions between molecules are indeed significant hence the need for the gas laws. I think you are just thrashing about.
Do you really deny the relationship PV=nRT?
No, that is the ideal gas law!
A good description is the one from Hyperphysics:
“An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in which there are no intermolecular attractive forces. One can visualize it as a collection of perfectly hard spheres which collide but which otherwise do not interact with each other. In such a gas, all the internal energy is in the form of kinetic energy and any change in internal energy is accompanied by a change in temperature.”
Under conditions when it’s necessary to take account of attraction between molecules and finite molecular size you need a different equation of state, a good one being the van de Waals equation:
(P+a(n/V)^2)(V/n-b)=RT
Hi Phil.
Thanks for that clarification of the appropriate formula in relation to real gases.
If the atmosphere can be treated as an ideal gas with no intermolecular forces and therefore no potential energy (all being kinetic energy) then why do we hear so much about potential energy in the atmosphere when one studies meteorology?
If one takes an ideal gas at a specific temperature and pressure and then expand it does the gas cool due to creation of PE at the expense of KE or not?
Stephen Wilde December 8, 2017 at 7:33 am
Hi Phil.
Thanks for that clarification of the appropriate formula in relation to real gases.
If the atmosphere can be treated as an ideal gas with no intermolecular forces and therefore no potential energy (all being kinetic energy) then why do we hear so much about potential energy in the atmosphere when one studies meteorology?
I don’t know I’ve not taught meteorology so I don’t know what the context is. I suspect it’s related to instability and buoyancy of an air parcel?
If one takes an ideal gas at a specific temperature and pressure and then expand it does the gas cool due to creation of PE at the expense of KE or not?
Assuming it’s done adiabatically then the gas cools because of the work done by expanding it.
Yes, the gas cools as a result of work done but work done in the reversible process of convective overturning doesn’t destroy energy. Instead it transforms KE to PE and if we are considering an adiabatic process then it is fully reversible.
An atmosphere is loaded with PE and KE in equal proportions and it is the interplay between them that creates weather and climate. Molecules closer together increases the interaction between molecules which then oscillate faster and the temperature rises.
So, reverting to my initial description, the intermolecular forces are important within a real atmosphere loaded with PE but as long as one adjusts for position along the lapse rate slope, which the standard atmosphere does, then you can use the ideal gas laws well enough for all practical purposes.
Themore refined equation you mentioned makes it even more accurate but that isn’t necessary in practice.
Stephen Wilde December 9, 2017 at 10:15 am
Yes, the gas cools as a result of work done but work done in the reversible process of convective overturning doesn’t destroy energy. Instead it transforms KE to PE and if we are considering an adiabatic process then it is fully reversible.
An atmosphere is loaded with PE and KE in equal proportions and it is the interplay between them that creates weather and climate. Molecules closer together increases the interaction between molecules which then oscillate faster and the temperature rises.
So, reverting to my initial description, the intermolecular forces are important within a real atmosphere loaded with PE but as long as one adjusts for position along the lapse rate slope, which the standard atmosphere does, then you can use the ideal gas laws well enough for all practical purposes.
Themore refined equation you mentioned makes it even more accurate but that isn’t necessary in practice.
The intermolecular forces are not important within our atmosphere, if they were the ideal gas laws would not accurately represent the atmosphere. Van de Waals equation isn’t used in gases at pressures of one atmosphere or less because the corrections are too small to be meaningful. Molecules in our atmosphere are about 10 diameters apart on average, if you look at the Lennard-Jones equation you’ll see that the attractive force falls off as r^-6, hence no meaningful attractive force.
Willis,
May I suggest that you find and print out my elevator post and our subsequent communications and then address my latest responses to you. Create a new head post on that very issue if it helps.
I find it very difficult to negotiate such a long thread too but it is your thread and you must be able to use the system to make things easier.
As I see it you have now been given exactly what you asked for by way of a coherent explanation as how the adiabatic theory can raise a surface temperature above S-B and I’ve done it by using the most basic and incontrovertible scientific laws.
Stephen, let me say that I don’t go on a snipe hunt for any man. I used to do that, go hunting … and then the other person would say “No, not that elevator post, you idiot, I meant THE elevator post” … so I gave it up.
If you have something that you think is important, let me suggest that YOU find and print whatever it is that you think is important.
Next, you claim that “adiabatic theory can raise a surface temperature above S-B”. However, I showed above that this is physically impossible for a non-GHG atmosphere. Why? Because if it did so, then the surface would be radiating more energy on a continuous basis than it receives. Re-read my post, “A Matter Of Some Gravity“, or Robert Brown’s post, “A Refutation Of Stable Thermal Equilibrium Lapse Rates“. I note that you have not said one damn word to dispute either my post or Robert Brown’s post, and they both PROVE, not assert but prove, that you are wrong.
Both of the proofs are very broad in scope, and as near as I can understand your claims, they are specifically ruled out by either proof.
Come back when you are willing to face the ugly facts. And no, I’m not doing your homework for you.
w.
Here is my elevator speech again:
“Willis:
i) Kinetic energy at the surface can either be conducted away or radiated away. Both cannot occur simultaneously. If one rises the other drops. So, if you have kinetic energy sufficient to produce a temperature of 288k as per the Earth’s measured surface temperature there is no physics that prevents 255k radiating to space and the other 33k being conducted to the atmosphere.
ii) If that 33k goes upwards from the surface in convective ascent (effectively disappearing from the radiative energy budget as PE) then it comes down in convective descent (reappearing within the radiative energy budget as KE at the surface) but at a later time. It is well established physics that adiabatic cooling in ascent and warming in descent is fully reversible.
iii) The original 33k taken from the surface by conduction in the formation of the atmosphere came from energy that would otherwise have radiated to space. That being the case it cannot have reduced surface temperature below 255k which was the surface temperature pre atmosphere as per S-B.
iv) If the surface temperature remained at 255k whilst the atmosphere was being formed then it MUST rise by 33k when the adiabatic loop closes.
v) Although the surface temperature is then 288k you still have ongoing conduction of 33k upward to maintain convective overturning PLUS 255k of radiation going out to space which keeps the combined radiative and adiabatic loops stable and the atmosphere in hydrostatic equilibrium indefinitely.
Your previous objection to such a scenario missed the issue of the process of creating the atmosphere in the first place when the first convective overturning cycle formed. That is where the non zero consequence of conduction and convection comes from so that there is no breach of the first law.”
You previously replied ‘0k’ to points i) ii) and iii) but balked at points iv) and v)
Having so replied to point i) it follows that a surface at 288k need not radiate to space at 255k since 33k can go into conduction instead and as per point ii) be recycled up and down indefinitely.
Your objection to points iv and v amounts to a disbelief that an atmosphere cannot be held off the ground indefinitely once the first convective cycle completes because its initial slug of energy will somehow be radiated off to space without the atmosphere falling to the ground.
I asked you to explain how that might work given that the energy involved is PE that does not radiate and you gave me a stocktaking analogy which was flawed so I gave you a corrected one which showed that the extra energy acquired from the surface during formation of the atmosphere need not be lost if it is being constantly recycled up and down within an adiabatic loop. You accepted in your reply to point ii) that such a loop is fully reversible.
There was then a suggestion from you or someone else that the atmosphere needed something to ‘live on’ but I pointed out that as an inanimate object an atmosphere has no metabolism and requires no such additional energy once it is in place and supported by the continuing arrival of fresh insolation. That is the issue that Brett picked up on quite correctly.
As for your demand for ‘numbers’ it is very simple.
255k in and 255k out in the radiative loop.
33k up and 33k down in the adiabatic loop
That is the current position.
Over to you for the next step.
Stephen Wilde December 6, 2017 at 3:29 am
TL;DR. An “elevator speech” boils your central ideas down to a few crucial ideas. It’s called an elevator speech because the scenario is as follows. You get on an elevator, and there is the very man you hope to convince. You’ve only got the very small length of the elevator ride to make your pitch … so you have to make every single word count.
Please boil it down to a quarter of that size, throw away all the extraneous stuff, restrict it to the very important. I ask you to do this for me, but more importantly, for yourself. If you can’t get your idea across in a very short time, you don’t really understand what’s important in it and what’s secondary.
Finally, if the numbers are what you claim, viz:
then I have no idea what your central claim is. You’ve claimed (I think) that from somewhere you get energy to keep the atmosphere warm on a constant basis … how much energy, and where does it come from? Whatever it is, it has nothing to do with those numbers.
Finally, you cannot do accounting in Kelvin, because temperature is not conserved. An accounting must, must be done in W/m2, because they are conserved … and the fact that you seem innocent of that concept is certainly worrisome,
In any case, I still look forward to your elevator speech, because I still have no clue what you think warms the atmosphere in your scenario.
w.
From: A Matter Of Some Gravity
“NOTE 1: Here’s the thing about a planet with a transparent atmosphere. There is only one object that can radiate to space, the surface. As a result, it is constrained to emit the exact amount of radiation it absorbs. So there are no gravity/atmospheric phenomena that can change that. It cannot emit more or less than what it absorbs while staying at the same temperature, conservation of energy ensures that. This means that while the temperature can be lower than the theoretical S-B temperature, as is the case with the moon, it cannot be more than the theoretical S-B temperature. To do that it would have to radiate more than it is receiving, and that breaks the conservation of energy.”
Willis has now accepted that a surface at 288k can radiate 255k and conduct / convect at 33k since you cannot use the same unit of kinetic energy for two independent processes.
Thus his above conclusion is incorrect.
So, the next step is to ask how that 288k can be maintained without increasing radiation TO SPACE to 288k.
Quite simply, the same 33k is recycled up and down constantly with a single cycle delay. There is a total of energy (PE plus KE) ‘worth’ 66k in the atmosphere of which 33k is constantly rising and 33k is constantly falling and there is no need to ‘consume’ any of that energy in order to keep it going because the movement up and down arises solely from temperature induced density differentials in the horizontal plane which cancel out over a single cycle.
I have also looked at Robert Brown’s work again and he talks only about a vertical column constrained by vertical sides. That setup contains no provision for the creation of PE from KE as in the real atmosphere which has no constraints on expansion with height.
Both articles are fatally flawed IMHO.
In previous post I asked:
“Do you count the atmosphere’s PE in the PE-KE exchange of convecting parcels?”
You answered:
“Yes, of course it counts as energy. It just isn’t heat and doesn’t radiate.”
Could this be the root of our disconnect?
First off, the atmosphere has a mass and some effective center of gravity that you can use to calculate its PE value. If you would, please state whether you agree with this point with yes or no.
Secondly, I hold that this PE value is a practical constant unless the effective center of gravity moves over some vertical distance. Please state whether you agree with this, yes or no.
Thirdly, I hold that this PE is not energy, precisely because it is not, and can not move vertically. In similar fashion oil is not energy, and fire-wood is not energy, despite having fire in its description. Do you agree, yes or no.
Lastly, I hold that INDIVIDUAL parcels of air, can and do exhibit changes to their KE-PE exchange and these changes do not, and can not change or interact with the total PE of the atmosphere. Agree, yes or no.
PS:
You seem to be using the POTENTIAL energy of the entire mass of the atmosphere as an eternal flux generator and this a perpetual motion machine.
The PE of the ENTIRE atmosphere is a net positive flow, into the atmosphere at time 0 and out when the sun winks out. For the eons between it does squat.
Stephen, your very first point is rather befuddled and poorly expressed. That needs to be cleared up before ANY of the rest can be discussed.
“i) Kinetic energy at the surface …”
Clearly you actually mean “internal energy,” U — the energy associated with random microscopic thermal energy of atoms.
” … [internal energy] can either be conducted away or radiated away. Both cannot occur simultaneously.
Clearly you actually mean that a given joule of energy can either be conducted away or radiated away. Later on you specifically say that both process do indeed happen simultaneously — ” there is no physics that prevents [some energy[ radiating to space and the other [energy] being conducted to the atmosphere”
” … a temperature of 288k as per the Earth’s measured surface temperature
If this tempeature is true (and the surface is a blackbody emitter as we have been assuming), then SB says that it will be radiating
P/A = sigma { (288K)^4 – T(cold)^4) }
” … no physics that prevents [240W/m^2] radiating to space”
Yes,physics DOES prevent this — the SB equation quoted above! A 288 K blackbody surface “radiating to [T(cold)=3K] space” will radiate 390 W/m^2. The only way to radiate 240 W/m^2 “to space” from the 288 K surface would be if “space” were T(cold)=227 K!
So, Stephen, which is it?
* Space is 227K.
* The SB equation is wrong.
* The surface radiates 390 W/m^2 to space?
S-B should not be applied to a surface beneath a convecting atmosphere.
“Willis has now accepted that a surface at 288k can radiate 255k..”
No, only in Stephen’s imagination.
“S-B should not be applied to a surface beneath a convecting atmosphere.”
Why not Stephen? You will have a hard time explaining yourself when S-B was developed by tests within a convecting atm.
Evidence please.
As if Stephen will actually look it up. Or be able to absorb it. But there is the nonzero possibility Stephen will do so and at least look at the sketches of the lab equipment, as cited by Planck:
H. Rubens, F. Kurlbaum, Ann. d. Phys. 4, p. 649, 1901. Available for free on the internet. Also Lummer, Pringsheim, Ann. d. Phys. 6, p. 210, 1901.
“S-B should not be applied to a surface beneath a convecting atmosphere.”
*You* are the one who needs to supply evidence! I have never once heard any such claim. I have never read such an exception in a textbook. S-B depends only on emissivity, surface temperature, and surrounding temperature. There is not and have never has been any other factors in the equation for air pressure, rate of convection, etc.
So … what textbook or source tells us how to calculate the radiation “beneath a convecting atmosphere” and what factors do we need to include?
The S-B definition and equation contains no provision for non radiative processes, just as you say.
How then, can you apply it regardless when non radiative processes are active?
It was never designed for that scenario.
Stephen Wilde December 6, 2017 at 10:13 am
Say what?
Stephen Wilde December 6, 2017 at 1:39 pm
Wow … just … wow.
I’ve read huge amounts on this subject, as you might imagine. You are the first and the only person that I have ever heard make this claim. Ever.
As such, I fear that you will need to substantiate it solidly with scientific references. It is an extraordinary claim, and as the saying goes, extraordinary claims require extraordinary evidence.
One reason I don’t think your claim is true is that virtually every object around us has active non-radiative processes going on—convection and conduction go on almost everywhere except the vacuum of outer space.
If you were right, that would mean that the S-B equation wouldn’t be valid for anything except space exploration … whereas in fact it is used in night-vision glasses and stand-off thermometers and it is employed in calculations daily in situations where you say it is invalid.
So let’s make this the test case for your understanding of thermodynamics. Come up with the references, or admit you were wrong.
I truly hope you follow up on this, because admitting you are wrong will give you some hope of being right … whereas the way it stands, you remain mired in misconceptions while loudly claiming incorrect ideas are gospel truth.
w.
PS—you say:
Huh? Kelvin IS temperature … so it is NOT conserved.
There is a reason that we do energy budgets in W/m2 and not in Kelvin. It appears I am not able to get this across to you, but you might profitably ponder why it is we use W/m2 … you might start by looking up the difference between “extensive” and “intensive” properties of matter. Mass is an extensive property. It can be added and subtracted when the objects are combined or split. Three kilos of water plus two kilos of water is five kilos of water.
Temperature and density, on the other hand, are intensive properties. They cannot be added or subtracted when objects are combined or split.
For example. If you split a 2kg block of steel in half, you get two 1kg blocks of steel, because mass is conserved.
But if you split a 2kg block of steel at 288K in half, you do NOT get two 1kg blocks of steel at 144K … because Kelvin are NOT conserved.
Which is why attempting an accounting in Kelvin is … well … let me call it “not done in scientific circles” and leave it at that.
S-B can be used within an atmosphere for such utilitarian purposes because the net effect of the non radiative processes is pretty much the same anywhere in the atmosphere after accounting for the lapse rate slope once hydrostatic equilibrium has been achieved. Hence the utility of the standard atmosphere.
What I mean by not applying it to a planetary surface beneath an atmosphere is a different issue. To apply it correctly one must observe the planet from outside the atmosphere and as we see the Earth from space it is indeed radiating at 255k and not at 288k.
I have given you a fuller explanation below as to why the surface is warmer than S-B.
As regards the units I’ve been using I have repeatedly referred to the amount of kinetic energy that gives rise to a temperature in kelvin. Since the big debate is always about where the extra surface temperature of 33k comes from that is by far the simplest way of looking at it. W/m2 is not as straightforward when readers have been conditioned by the 255k 288k and 33k numbers and I have been on threads where there has been much consequent confusion.
To say it is ‘not done’ is simple snobbery.
Stephen:
In my professional career, I have to (among other things) design thermal systems to keep power electronic devices from overheating.
We mount the power electronic devices on metal heat sinks to conduct the thermal energy from the small transistor to a large surface. We often black-anodize the outer heat sink surface to increase its emissivity. Sometimes we will also add fans for forced convective cooling.
The idea that adding the fans for more convective transfer would reduce the ability of the surface to radiate (for a given temperature) is simply absurd — we have never detected this, and no one in our group would give the idea a moment’s consideration.
Throughout all your comments, you display a very basic and repeated confusion between energy and power, which causes you to conclude all sorts of ridiculous things.
The 1st Law of Thermodynamics is about conservation of energy, not conservation of power. You are arguing that if a square meter of surface can radiate 400 watts (power) into a vacuum at a given temperature, then if it starts conducting/convecting 100 watts into the atmosphere, its radiative output will reduce to 300 watts. That is a “conservation of power” argument, which has no validity.
Using the 1st Law properly, you can determine that if the surface starts outputting 100 watts conductively/convectively as well as the 400 watts radiatively, the total output is 500 Joules every second, thus reducing the internal energy of the body by 500 Joules per second. If all of the inputs to the object sum up to 400 watts, the internal energy of the body decreases by 100 Joules per second, and the body cools.
Even your financial analogies suffer from this same confusion between the conserved substance (money) and its rate of transfer (e.g. $/week).
Ed,
You are perfectly correct for the situation within an atmosphere where there is a haze of radiative and conductive energy governed by the lapse rate slope.
I am only considering the relationship between and irradiated planet, it’s atmosphere and radiation actually reaching space which is an entirely different scenario.
33k of the Earth’s potential ability to radiate to space just doesn’happen.
AGW theory says it is because of DWIR and I say it is because of convective overturning.
Stephen:
You say what you are considering “is an entirely different scenario”.
No, it’s not!!!
Both are the same scenario — a warm surface both radiating away and conducting/convecting away thermal energy to a colder ambient. Both follow the same laws of physics.
The only mechanism through which the atmosphere can attenuate the radiative power to space is through absorptive constituents (aka “greenhouse gases”). Transparent gases, even if moving up or down, do not affect the radiative transmission from surface to space at all.
TJF said:
“1) The atmosphere starts at 255 K. In this case, it is the same temperature as the surface and no convection is started. There is never energy lost from the surface to the atmosphere, never any ‘adiabatic loop’, and never any energy returned to the surface to warm it about 255 K.”
Which is utter nonsense. Convective overturning cannot be prevented over an unevenly irradiated surface so the entire atmosphere can never be at 255k due to the lapse rate slope that then ensues.
TJF comment is NOT utter nonsense, only in Stephen’s imagination is it such, as there is no unevenly radiated surface if the surface is all at 255K. The lapse rate slope simply STARTS everywhere at 255K. Simple logic.
You keep putting the cart before the horse, Stephen. You talk about some “initial loop” seting everything up, but never describe the initial conditions! The atmosphere could start at a uniform 255 K if “god” created it that way. But you are welcome to describe YOUR initial conditions.
For example, you could average 240 W/m^2 to the surface by having half receive 390 W/m^2 (288K) and half receive 90 W/m^2 (200 K). This would certainly set off convective overturning if you added an atmosphere. Warm 288 K air rising in the warm areas and returning at 288 K in the cold areas would warm the ground above 200 K — but also cools the air below 288. This intermediate-temperature air would return to the warm areas, but the ground would below 288 K due to contact with the air. So the NEXT “loop” would start BELOW 288 K on the way up and return at the same temperature to the cold areas.
So now even the warmest areas are cooler than 288 and the cool areas are WAY cooler than 288.
I can guarantee that no matter what combinations of radiation (averaging to 240 W/m^2 incoming), initial temperature of the surface and atmosphere, and heat capacities you postulate, you will NEVER be able to get above the 255 K average temperature limit imposed by SB.
Three recent articles about the steel greenhouse. They’re all part of one overall refutation, so it’s best to read them all. There are prior articles there too on the same subject.
Just on the off-chance that there is anyone out there still reading through at this point, other than the handful of people still commenting, who haven’t seen these posts already:
https://climateofsophistry.com/2017/10/19/the-steel-greenhouse-in-an-ambient-temperature-environment/#comment-32262
https://climateofsophistry.com/2017/10/22/incomplete-thermodynamics/
https://climateofsophistry.com/2017/11/03/the-alarmist-radiative-greenhouse-effects-final-end/
Tony December 6, 2017 at 2:48 am
Three recent articles about the steel greenhouse. They’re all part of one overall refutation, so it’s best to read them all. There are prior articles there too on the same subject.
Just on the off-chance that there is anyone out there still reading through at this point, other than the handful of people still commenting, who haven’t seen these posts already:
The analysis presented there is based on the following error;
“The shell’s surface would emit on its interior as well, however, internal emission by the shell will always meet another interior side of the shell (or the sphere), and hence will not leave the shell. Internal emission by the shell’s surface hence does not lead to a loss of energy for the shell, and hence the energy produced by the sphere will be conserved with the outward emission of the shell to the environment.”
You’re welcome to go over there but be prepared for the foulmouthed, abusive host, it’s easy to see why he isn’t allowed to post here!
Here’s the disingenuous trick they are playing. They are using the very same mechanism that they deny exists to disprove the effect.
Note that they aren’t telling you (at least by your summary) what happens next…. so that radiation doesn’t leave. Fine. Where does it go and what does it do when it gets there.
Like I said, it’s best to read them all.
As Phil says, it is EASY to find very fundamental flaws in those critiques. THe ironic thing is that the correct answer is presented — but then excuses are invented as to why the actually correct answer must be wrong!
OK, Tim.
Tony will also find that testing is completely absent from any of the 3 links & more that he posted up. Not having to comply with 1LOT, 2LOT as all testing does allows for much assertion and imagination on display.
OK, Trick.
Typo alert:
Should be:
Your objection to points iv and v amounts to a belief that an atmosphere can be held off the ground indefinitely once the first convective cycle completes despite its initial slug of energy somehow being radiated off to space.
No fair! We are talking about a transparent atmosphere that does not radiate to space. Plus where does the atmosphere go when the sun winks out?
Ask Willis, not me.
Do not have to ask Willis, he was indeed writing about a transparent atm. “Here’s the thing about a planet with a transparent atmosphere.” which cannot exist in nature as a transparent atm. will not produce entropy as light passes through, a process ruled out by 2LOT.
Willis, I think that is a pretty poor response to a good faith attempt to fulfil your specific request. The points that need addressing by you are as clear as they can be.
To suggest that my elevator speech is too long when it contains only 5 points and a little exposition is simply perverse.
“To suggest that my elevator speech is too long..”
Not if the listener already got off the elevator and you continue to rattle on about something or other.
Stephen, as I’m sure you’re aware, it is futile to attempt to argue with those who do not do so in good faith. Just acknowledge receipt of their response with a simple, non-commital, “OK”, and hope that someone else comes along who isn’t simply out to sow as much confusion as possible.
I’ll just wait and see if Willis comments further.
Whatever he does will speak volumes either way.
I’m confident that many non commenting readers will get the point and I’ll be raising it on future occasions where it is relevant to the thread both here (if Anthony doesn’t censor me) and elsewhere.
Tony December 6, 2017 at 11:06 am
Oh, piss off with your underhanded accusations that I am not arguing in good faith. You don’t even have the pelotas to accuse me by name, and that’s lower than a snake’s belly. You don’t even have the courage of your own convictions.
I have made every effort to engage with Stephen, and I continue to do so.
To date, Tony, you’ve added nothing to this conversation but nastiness. Seriously, when are you going to actually make a scientific comment?
w.
That’s right, I didn’t say your name. Seems strange you responded, given that. Do at least TRY to be civil.
Stephen Wilde December 6, 2017 at 3:58 am
Stephen, thanks for the response. My reply was also in good faith. But if you cannot explain your ideas simply, I’m not interested. As I said, that is as much for your benefit as for mine. I am trying to force you to think more deeply, because to date, your “explanations” simply have not made sense.
I note also that you have not commented on your attempt to do an accounting in Kelvin …
w.
Clear and simple enough to me and I suspect for other readers who do not get involved but OK Willis.
I’ll catch up with you again next time the issue becomes relevant and maybe by then you might have given it more thought than is apparent from your replies here.
Man, I hate being right about this stuff, but I figured that as soon as you were actually pressed about things like doing accounting in Kelvin and keeping an atmosphere warm without expending any energy to do so, you’d be out the door …
I am sorry to see you go. If you stayed and tried to say defend accounting for energy in Kelvin, you might have learned something.
Morning here, I’m off to work. I wish you the best.
w.
“Man, I hate being right about this stuff, but I figured that as soon as you were actually pressed about things like doing accounting in Kelvin and keeping an atmosphere warm without expending any energy to do so, you’d be out the door“
Or perhaps you’re wrong, and Stephen just got tired of talking to a brick wall.
And all my numbers are specifically described as kinetic energy sufficient to produce the quoted temperatures so criticism of that aspect is inappropriate too.
I await hearing on the basic scientific issues that I have put in play.
“I await hearing on the basic scientific issues that I have put in play.”
Many comments by Stephen are unscientific by simple logic having been shown wrong by test and more informed study of authors 60or70 years ago or more. So actually Stephen has already heard the science but prefers to continue with imagined meteorology.
Replace Stephen with Mr Eshenbach and your statement I just as true.
Only in his fantasy thought experiments do cold things make hot things hotter.
Whereas back here in the real world practically every physics statements and test says it doesn’t and can’t happen.
Only climate science with a very small s thinks otherwise.
So Trick point us to all the scientific papers and quotes by real Scientists that back him up please.
“So Trick point us to all the scientific papers and quotes by real Scientists that back him up please.”
Quote the exact words you are referring to in order to make this possible.
In the steel greenhouse the hotter body (Sphere) absorbs and thermalises the lower power radiation from the cooler body (Shell) and gets hotter and emits even higher energy radiation.
To summarise a Cold body makes a warm body warmer by back radiation or any of it’s radiation.
No, AC: The sphere has an independent power source. Without that, the entire sphere/shell system radiates until it is at ambient temperature, presumably about 3K.
Stephen Wilde December 6, 2017 at 4:12 am Edit
No. You call them “kinetic energy”, but your numbers were in KELVIN, which is not kinetic energy in any form. One example among many:
You can’t add Kelvin like that, temperature is not conserved. And as I said before, the fact that you are innocent of any idea that temperature is not conserved is worrisome.
w.
Oh dear.
Kinetic energy is simply molecular motion that leads to a temperature that can be measured in kelvin.
I can ‘add kelvin’ like that because energy is conserved whereas temperature is not. I tried to show that total energy in the atmosphere is a lot more than is apparent from the temperature along the lapse slope and it only becomes fully apparent as temperature when descending columns return heat to the surface.
The total PE plus KE in the atmosphere originally came from the surface via the surface temperature caused by molecular motion induced by the sun.
I think you are too far down a rabbit hole due to the convolutions caused by your adherence to the radiation theory which is what leads to the ever raging confusion over the steel greenhouse.. You need to start over from first principles and keep it simple.
Maybe another time.
Stephen, I fear you are beyond my poor power to add or detract. I gave you a clear description of intensive and extensive properties. I pointed out that intensive properties are NOT conserved. If you divide a 2 kg block in two, you get two 1-kg blocks. Mass is conserved.
However, if you divide a 2 kg block at 200°C in two, you do NOT get two 1-kg blocks at 100°C … why not?
Rather than commenting on that, rather than thinking about that, rather than deal with the important concept of intensive and extensive properties or even mention them, you just blow off what I wrote and say that temperature is just kinetic energy, and energy is conserved, so you can add temperatures.
If that is the case, then why don’t we get two 1-kg blocks at 100°C? I mean, temperature is just kinetic energy and kinetic energy is conserved … so why don’t we get two blocks at 100°C? You’ve assured me that because energy is conserved I could do just that, so why doesn’t it work?
Or how about if we combine two buckets of water at 300K? Do we get one bucket of water at 600K? Temperature is just energy so we can add them, you said … so when I add 300K and 300K I get 600K. You said it could be done, so why can’t I do it?
The answer is yes, temperature is a measure of the average kinetic energy of the molecules, but it is an INTENSIVE PROPERTY, which means that you can’t add it or subtract it.
And given that you truly a) don’t understand that, and b) think you understand it perfectly, and c) are totally unwilling to listen to any number of folks trying to set you straight, including folks who teach this for a living, and d) you show no interest in learning anything anywhere anytime, and e) you scatter insults along your trail like confetti … like I said, you’re clearly beyond my poor power to add or detract.
Best wishes,
w.
Paging Mr Eshenbach and all the adherents to the Steel Greenhouse thought experiment.
I have a thought experiment based on that experiment that can actually be easily tested in the real world.
The original experiment required one major premise to make it work ie a continuously heated object receiving Radiation of any energy value from another object which will increase it’s Temperature.
Is that correct?
So we have fixed energy and another energy source of what ever temperature.
The thought experiment
An energy source (Hot Water, electric blanket etc)
A Shell which has a thermocouple to measure its temperature.
Surround the Shell with the energy source to heat it up above Ambient.
Now according to the theory any energy impacting the Shell Surface from whatever source will increase the Temperature.
So the Flux from the inner side of the shell is for instance 1 Watt/ metre2 based on the heater.
The Photons from any point on the inner Shell Surface will now strike the Inner Surface of the Sphere somewhere else, be asborbed and warm it up while it is still being heated by the exterior heat source?
If this IS the case will the Sphere attain a temperature higher than the surrounding heat source and hence increase it’s temperature as well.
So does this experiment meet the Steel Greenhouse Experiment criteria of constantly heated Surface receiving photon energy from another source?
If not in what special way does it differ
I will conduct this experiment tomorrow so would anyone like to predict what temperature the Shell will get to above the heat source?
I have already conducted a Hot/Cold Objects with no increase, two objects at the same temp with no increase and finally a continuously heated object/cooler object with no increase.
I’m sorry, but I don’t understand either your experimental setup or what it has to do with the Steel Greenhouse. Perhaps if you were to point us to an illustration of the setup it would help. I don’t like to comment on something I don’t understand.
Thanks,
w.
Forget the setup, if you can’t work it out for yourself there is no point in talking to you.
Perhaps one of your adherents can figure it out and give me an answer.
“I don’t like to comment on something I don’t understand.“
Odd. It’s never stopped you before.
Willis is entitled to adult-like responses. Above – well it is the normal food fight behaviour.
A C Osborn December 6, 2017 at 1:00 pm
I civilly and politely ask for a drawing and a clarification and I get abuse … charming.
w.
My God you must be a snowflake if you call that abuse.
I tell you what, go back and read your own responses to me and many others if you want to see verbal abuse, sarcasm, character assassination and much more.
A C Osborn December 6, 2017 at 2:23 pm
Call it what you like, that wasn’t the point. The point was that you refused a polite request for further explanation and coupled it with an attack on my ability to “work it out”.
I don’t want to “work it out for myself”, A C. I don’t go on snipe hunts like that, and I am unwilling to guess what it is that you mean.
Why? Because when I guess, people have a habit of saying “You idiot, that’s not what I meant!!”
So, being unwilling to expose myself to attack for guessing wrong, I asked politely for a drawing and further explanation … and I got an attack in return.
Is that a better description of your unwillingness to simply explain your ideas?
Look, you actually had someone interested enough in your thought experiment to want to see where it went, someone curious enough to ask you to explain it further … and instead of just explaining it and providing a sketch of your experimental setup, you pissed all over your chance to discuss it.
Now, you have no one who wants to discuss it with you, because I sure don’t.
Congratulations. Well done.
w.
For a start I have no idea how to provide a sketch even if I had one.
I suggested that your steel greenhouse would proved that a Dyson sphere would probably kill everyone in it.
Your response was I didn’t know the Kardashians had class 2 civilisation.
Now THAT is what you call snarky & rude.
The Dyson Sphere would have to heat up sufficiently to radiate to space the entire output of the sun. That would certainly fry anyone between the two.
A C:
This experiment that was written up several years ago on WUWT tests what I think you are trying to express:
https://wattsupwiththat.com/2013/05/28/slaying-the-slayers-with-watts-part-2/
It looks to me like it backs up Willis’ analysis.
“It looks to me like it backs up Willis’ analysis.”
Hugely, Ed.
A C Osborn December 6, 2017 at 2:58 pm
There’s a well-established procedure for dealing with that kind of dilemma. It’s called “asking”. See, you say “How do I post a sketch?” Then I say “Draw it, scan it, put it up on Photobucket or Pinterest or some other hosting site, and provide a link to it”.
…
This is why I ask people to QUOTE MY EXACT WORDS, a polite request which you frequently ignore. You have totally misrepresented what went on. Actually, the conversation went like this:
“Snarky and rude”??? It’s called HUMOR, A C, you ought to look into it.
I have no idea what a “Kardashev Scale 2 Civilization” or a Dyson sphere is, and I assumed that whatever it was it was science fiction and thus had nothing to do with a steel greenhouse, and I presumed you were making a humorous aside … so I responded with humor.
But OK, if you were serious, no need to angrify your blood, let me look this up
…
I find this:
(As a trivial aside, I note that it is not called a “Kardashev Scale 2” civilization, as you had stated, it’s a “Type II” … moving on …)
Next, a “Dyson Sphere”, presumably Freeman has to be in there somewhere, hang on again …
… OK, got it.
So it was science fiction, as I thought …
In any case, no, a “Dyson Sphere” wouldn’t fry everyone. With a single shell, the maximum achievable surface gain at the sphere is a doubling of the radiation of the planet or sun at the center of the sphere. It will not heat up indefinitely as you seem to think. That wouldn’t be enough energy to boil water at the distance of the earth … S-B blackbody temperature at earth’s distance from the sun with doubled solar radiation would be 57°C.
=======================
So now, cue the chorus of people saying things like “Willis can’t be convinced of anything, it’s no use trying, he doesn’t think a Dyson sphere would fry everyone” … folks, when I am convinced, I change my mind publicly. I have two posts from a while ago where that exact thing happened. One post was called “Wrong Again”. The second post, a few years later on another subject, was called “Wrong Again, Again”.
In addition, I disagreed strongly with Robert Brown about Jelbring’s hypothesis that atmospheric pressure can cause ongoing atmospheric warming … but Robert convinced me, and I admitted it in public.
Here’s another example, from an addendum to a head post in which I’d made an error:
When I am wrong, as happens to everyone, I have consistently and publicly admitted it. And not in any half-arsed fashion either. I don’t hedge it about, I say flat out “I was 100% wrong.”
Now, can any of my attackers say the same thing? Anyone? Tony, where have you publicly admitted you were wrong? … oh, wait, I forgot. He’s cowering behind an alias, and having an alias means never having to say you’re wrong. You just change names and keep going. Robert Kermodle, how about you? Can you point to where you have very publicly and humiliatingly admitted you were in error, as I have done a number of times?
Look, I have demonstrated over and over that when the facts change, I admit publicly, clearly, and unequivocally that I was wrong, and I change my mind. Since you guys have not done the same, how about y’all cut back on the false claim that I cannot be convinced of anything? That’s a demonstrable lie.
w.
PS—Prepare for further brain explosions when I say … if you want to fry everyone, put more concentric shells (Dyson spheres) around the sun. The maximum radiation increase for a steel greenhouse is input radiation times (N+1), where N is the number of shells. With one shell, instead of 340 W/m2 at earths distance you get 2 * 340 = 640 W/m2. With two shells you get 3 * 340 = 1020 W/m2. This corresponds to a blackbody temperature of 97°C, hot, but not hot enough to sterilize the planet.
How about three shells? That would give a maximum of 4 * 340 = 1360 W/m2. This corresponds to a blackbody temperature of 120°C, should be enough to kill everything except the extremophiles.
Now, if this seems counterintuitive, consider a light bulb. It radiates a lot of energy. Suppose you put it in a thermos bottle. It will heat up further, of course, until it is hot enough that the thermos bottle is losing the amount of energy coming from the light bulb. At that point, it will be in steady-state, no more heating.
(I note in passing that the thermos bottle, which is cooler than the light bulb, makes the bulb warmer than it would be without the thermos, as I said to open this discussion … but I digress.)
Now, put the system, composed of the light bulb and the thermos bottle, inside another thermos bottle. What happens? The light bulb gets hotter still, and the inner thermos gets hotter still.
Q. E. D.
My hasty, smarmy quip that anything between the sun and a Dyson Sphere would fry was greatly in error, Willis. Thanks for the heads-up.
TL:DR
Here is a refutation of the “Slaying the Slayers With Watts” experiments parts I and II:
https://climateofsophistry.com/2013/06/05/slaying-watts-with-watts/
Ok, Tony. I see the amount of testing performed in the linked site is same amount of science supporting their refutation.
OK, Trick.
Just a question: What if we reverse the steel shell problem?
A sphere existing in space and at equilibrium with space is subsequently surrounded by a shell with a separate energy source such that it is radiating energy at a constant rate to the sphere. The sphere heats to equilibrium WRT the shell and the system is in balance.
A separate energy source is introduced to the sphere. Does the sphere heat up in relation to the prior equilibrium? If so, does that cause the sphere to radiate more energy?
If the sphere is radiating more, does that radiation impact the shell? If the shell is receiving a net increase of energy from the sphere, does the shell increase in temperature?
If the shell begins radiating more energy because of an increase in temperature, does the increased radiation impact the sphere?
Over time, given two separate, unvarying energy sources, does the sphere/shell system settle to a particular temperature dependent on the physical nature of the system?
Replace the system with the earth as the sphere and its atmosphere as the shell. Does an increase in radiative molecules (GHG’s) in the atmosphere increase the amount of radiation being emitted by the atmosphere?
Rinse and repeat.
And, yes, the atmosphere is dynamic with many energy exchanges occurring on all time scales. That doesn’t mean that additional radiative gases (GHG’s) won’t add energy to the system. But it also means that the contributions of an increase in CO2 is basically unmeasurable in the system, given the large, overwhelming changes in water vapor and clouds in the system.
Dave Fair December 6, 2017 at 12:45 pm
Yep.
The sphere has to heat up if it is receiving more energy. And in turn, it will radiate more.
Yes.
Yes.
Yes. It will settle down to steady-state when the total energy radiated to space is equal to the total energy input.
That one is easy because we can actually measure it from space. However, there is one further question:
Does an increase in downwelling GHG radiation perforce mean the surface of the sphere heats up?
I say no, because it is offset by small, almost undetectable changes in the natural thermoregulatory mechanisms. We get earlier daily formation of tropical clouds and thunderstorms to reduce solar input; a slight increase in the El Nino/La Nina pump moving warm water to the poles; a few more dust devils moving energy from the surface to the atmosphere, and the status quo ante is restored.
w.
Thanks, Willis. I was going to go there with my next series of questions.
Delta-CO2 forcings are lost in the vastly larger climate energy systems. The is no practical mechanism by which temperatures can go up to drive 3X H2O forcings.
I think you will find that the blanket is a constant temp device.
If that response is to me, who said so?
You are suggesting is has a thermostat only position.
There is a maximum output position for fast heating on mine, but it runs for a maximum of 90 minutes which is easily long enough to do this.
Then again so would a bath full of hot water, as it would take quite a while to cool significantly.
So would a large pot of water that is boiling or maintained at a simmer.
But perhaps you could answer the question instead, in a heated sphere the inner surface is emmitting photons which are impacting another heated surface directly opposite it. Do those absorbed photons warm the surface or not?
If not why not?
Here’s the steel greenhouse with all the science removed. Just the math. I know It’s more fun to lip flap opinions but sooner or later the math will out and when it does it is often counter intuitive but always correct.
I have a bank account that I put the same amount of money in every night. Every morning my wife takes it all out as cash then feels bad and puts 20% back.
Let’s say for demonstration purposes it is $100. The first day she walks away with $80 and the bank has $20.
The next day she takes the $120, walks away with $96 and the bank has $24. And so it goes. Next day she keeps $99.20 and the bank has 24.80. Then It’s $99.84 and $24.96. Then $99.97 and $24.99.
You can keep it going if you like. But, i think you can see that by the end of the second week $125 is coming out of that bank every day and I only put in $100 Every day. My wife is taking all my money and the bank is a perpetual money machine. ESPECIALLY WHEN I LEAVE OUT THE wife’s guilt.
It’s the magic of feedback and the bank math is exactly the same as the greenhouse math. Before you get anywhere near discussing the galactic implications of the Microstates of argon in the galactic spiral on a rotating water planet you should get crystal clear on the simple math.
The greenhouse is not making energy. It’s distributing it in counterintuitive ways and you can’t figure it out with a freakin electric blanket
Maths, simple or otherwise can be incorrectly applied.
Show where the Science says energy from an object that is cold is absorbed by and makes a hotter object warmer.
Apart from Climate Science that is.
Please provide the evidence that the Maths is acceptable for that condition.
Oh no no no. First you must agree that feedback to a source can make that source supply more than what is being supplied by its primary, constant, source.
Do that and we will proceed. I want a concise statement of agreement or a demonstration as to where the math in my bank example is wrong.
AC, I think the operative term is “warmer than it would otherwise be.” With no outside energy source, the two-body system loses energy and cools, as a system. The warmer body cools at a slightly lower rate because of the surrounding body’s energy inputs. Likewise, the surrounding material cools at a lower rate than it would without the interior body’s energy contribution.
Throw in an independent power source for the warmer interior object, it warms to bring the entire system into thermal equilibrium, including the surrounding medium. The surrounding cooler object warms to bring net energy output into balance with the inner body’s energy input.
The interior body must increase in temperature for its energy output to increase.
As to your last statement I may or may not be able to “figure it out”, but I do not need to if I prove it wrong by experiment.
Remember Einstein’s quote about 1 experiment?
Well I am on number 3 which all prove it wrong, number 4 will be enlightening.
How no no no, you made the statement it is encumbent on you to back it up.
Paul Bahlin wrote, “i think you can see that by the end of the second week $125 is coming out of that bank every day and I only put in $100 Every day.”
You mean 80% of $125 = $100 is coming out every day = the $100 that you put in every day.
No! I meant what i said. $125 is being taken out, period full stop. Go get the withdrawal slips. 80% of that is being taken away by wifey. She gets the net. What she walks with is not what is being taken out.
Language is a beech, right?
A C Osborn on December 6, 2017 at 3:37 pm
Says,
“How no no no, you made the statement it is encumbent on you to back it up.”
If you reread my comment, I never
Sorry about the broken comment….
I never mentioned more than banking. It’s a math problem. Answer the math question.
Is it right or wrong?
Paul, I don’t think there is anything wrong with your basic arithmetic, but there is an awful lot wrong with your logic.
Let me try and straighten it out for you.
Your bank account has a trillion dollars debt (Space), so you put in your money and when your wife goes to the bank there is nothing in there because the bank has taken the lot to service your debt to space.
So your wife who is really pissed off with you because she knows your snarkiness thinks you didn’t put any in.
So she either never speaks to you again, divorces and marries someone who doesn’t have your debt, or sends you an IOU for the $100.
Now do you see about whether your arithmetic applies or not?
Yeah, well that already happened to me once. Now I have a much much more tolerant one.😄
Paul,
RUN YOUR OWN MATH using your specified formula.
By day #7, the bank keeps $25 and your wife withdraws $100. Every single day after that, the bank keeps $25, and your wife takes $100. Period. Full stop.
Law of diminishing returns. The amount of “feed back” on the initial $100 was $20, then on the $124 it was only $4, then it was only $0.80 then $0.16 then $0.03 then $0.01. Once it reaches $25.00 remaining in the bank, the daily input and withdrawals by your wife MATCH. 20% of 125.00 is always going to be $25.00.
Math is math. Right?
What’s your point?
Paul,
My point:
You stated:
“You can keep it going if you like. But, i think you can see that by the end of the second week $125 is coming out of that bank every day and I only put in $100 Every day. ”
You are wrong.
By day 7, the amount going into the bank every day from you, and being withdrawn by your wife every day, are BOTH $100.
According to your scenario in which your wife’s “guilt” makes her put back 20% of the total in the bank, once the amount being left in the bank reaches $25, the account reaches equilibrium…same amount in on one side, same amount out on the other. At NO POINT in time (unless you change your formula somehow) is “$125.00 coming out of that account every day”.
To fulfill YOUR formula, your wife must leave 20% of each morning’s total in the bank. At $25 in the bank, when you deposit $100, she can only take out 80% of $125.00. 80% of $125 is……$100. So UNLESS you change your “simple” thought experiment somehow, on day 7, the account reaches equilibrium and at no point does your wife withdraw $125.00. Not even once, much less “every day”.
Read again. $125 withdrawal, $25 deposit, $100 to walk away with. 2 transactions.
AC, you start with:
Come back when you can tell us why those two are totally different and will lead to different outcomes for your thought experiment.
As I said, I don’t understand your experiment. More to the point, however, it seems that you don’t understand your experiment.
w.
Well here is the question, you say that the lower level energy MUST be absorbed and thermalised by the warmer object.
So please explain why it MUST NOT be absorbed and thermalised by an empty Shell’s opposite wall?
Or in the case of the steel greenhouse with a much larger shell, all of the wall still visible beyond the sphere, ie a sphere at 1 metre diameter with a shell of 1000 metres.
A C Osborn December 7, 2017 at 12:25 pm
Well here is the question, you say that the lower level energy MUST be absorbed and thermalised by the warmer object.
So please explain why it MUST NOT be absorbed and thermalised by an empty Shell’s opposite wall?
Or in the case of the steel greenhouse with a much larger shell, all of the wall still visible beyond the sphere, ie a sphere at 1 metre diameter with a shell of 1000 metres.
Why do you think that should be the case?
Tony
“refutation of the “Slaying the Slayers With Watts” experiments’
At the outset they have a light bulb next to a mirror saying something like ‘obviously this bulb will not heat up or shine brighter”
But then when they discuss the experiments, that is exactly what is found! The light bulb did got hotter! There is yada yada about why that happened, but with no real refutation of it.
I don’t why you find this impressive?
I also don’t understand why you think Postma understands thermodynamics, but somehow Spencer, Christy, Lindzen, Freeman Dyson (!) and countless others do not?
It tends to be best to read it all. Plus try to understand it.
Read it. Not impressed. What part do you find persuasive?
OK, Nate.
Willis said:
“keeping an atmosphere warm without expending any energy to do so,”
Energy can either be expended as a result of work done OR it can be transformed as a result of work done.
The former generates heat as a by product but the latter just moves heat around.
The ability of convection to move energy around by transforming it rather than by expending it appears to be a new concept for people here.
I’ll only comment further here if I get some positive indication of progress (unlikely, I know)
Stephen Wilde December 6, 2017 at 1:16 pm
Thanks, Steven. Convection in our atmosphere indeed moves matter around, transforming potential energy into kinetic energy and vice versa as it does so.
However, this is not sufficient to warm anything, because it is a zero-sum process. For every parcel of air that is raised, an equivalent parcel is lowered. As a result, there is no gain.
And because there is no gain, such a transformation cannot keep anything permanently warm.
To keep something warm, you need to constantly add energy to it to offset the constant heat loss. The unanswered question with your theory is, where does that constant energy flux come from? It cannot come from the original energy you say was necessary to get the atmosphere started. That would have been used up long ago keeping the atmosphere warm.
So … where does it come from?
w.
That is reasonably put so I’ll continue.
Since convection is a zero sum process no energy is being lost or gained within the adiabatic loop but new solar energy is constantly flowing through the loop such that every convective cycle 33k enters the loop from solar heating of the surface and 33k leaves the loop via solar radiation from the surface. Thus the total of solar radiation flowing through the entire system is also net zero with the same out as in.
Then the stock control analogy comes in.
Where there is a one cycle delay in releasing solar radiation to space you constantly have 33k of solar radiation backed up in the adiabatic loop.
Radiation to space from the surface beneath the adiabatic loop is not from the 33k taken up simultaneously but rather from the previous ascent of 33k.
It obviously isn’t the exact same block of energy that lifted up the atmosphere but it might as well be because the pool of energy in the adiabatic loop is being constantly topped up again as fast as it departs.
Now the lapse rate slope clearly arranges 33k of KE at the surface and 33k of PE at the top (roughly) once hydrostatic equilibrium is achieved so you naturally have an extra 33k of heat at the surface in addition to continuing insolation from the sun.
It is like a standing wave in a river where an obstacle beneath the water slows down one portion of the flow and speeds up another portion in a net zero effect on total flow but it produces a higher water surface in the form of the wave.
Some may balk at my use of the term 33k of PE since PE is not heat but that is just shorthand for ‘ the amount of kinetic energy that would provide a temperature of 33k if all the potential energy present were to be transformed to KE’
Given that I have been accused of using too many words I think that stylistic convention is reasonable.
I awoke this morning expecting to see a reasoned response but nothing thus far.
Do you really think convection just moves energy around? Pretty astounding. No work is done by the air that’s pushing it up? No buoyant force lifting it against gravity?
Really?
Jeez.
Convection IS the work and Is a consequence of buoyancy differentials in the horizontal plane.
Spare me.
Paul, I’ve noted that you have a sense of humour but there is a limit.
Stephen Wilde
December 6, 2017 at 1:16 pm: Yup, Stephen, just eternal deflection. Of course we know tj and Trick of old, sadly. While one of the previous times when I think I
met EdBo, he was pretenting to be an ex- Navy/CG Officer. But he did not know the differences between steam, smoke or photoshopping of chimneys.
Fancy saying the thoughty’s surface must cool and remain so on contact with argon, when it has a thoughty constant irradiation? Just makin’ it up, I see.
And by the way Willis, re aspersions on my horse care and seakeeping, quite amusing: “There y’go again” said the Gypper. My horses could run, but not the steel greenhouse and any variants. You have to get serious about understanding gases………
After learning about the way you can’t violate 2nd law, I’ve come up with a fantastic idea. It’s called Earth Oven. We know that the earth, about 10 feet down, is a virtually constant 55 degrees F. This means it is a listless supply of cold radiation.
You could say it is a constant supply of 303 k energy as I’ve heard it described here. We also know that cold things can’t get absorbed by hot things. So here goes….
We make a double walled glass container with an open bottom and put it in the yard. Of course we would have to make a vacuum between the walls.
Then we just put in the chicken and wait. The cold 303 k energy flow comes out of the ground and hits the glass. The glass is warmer than the 303 k energy so none of it can get out.
It bounces around and some of it even goes back and hits the ground but it’s warmer than the 303 k energy flow too and anyway it is back radiation and we know that can’t do anything either.
The Earth Oven has a limitless supply of energy. The outside never gets hot and It’s free cooking.
What do you think? Haven’t worked all the details out. It will only work when It’s more than 55 F outside but that wouldn’t matter much in poor countries that are ALWAYS hot
Yeah, listless should be limitless. Spend more time fixing stupid auto correction then typos.
After some thinking, I think I’ve solved the problem where Earth Oven doesn’t work when It’s colder than 55F in my yard.
Since albedo is a function of temperature, all I have to do is put a little heater on the outside surface. As soon as it gets to 55F the glass switches to albedo = 1 and nothing gets out.
No movement of energy there from KE to PE and back again and no time delay in the background flow of energy so a worthless analogy.
Steven comments:
“No movement of energy there from KE to PE and back again and no time delay in the background flow of energy so a worthless analogy.”
Well, never said it was a convection oven did I? Never said there was air in it either. But if you insist, make it a 1 meter cube. Then go figure out the velocity of 1.3 kg of air cranking around as convection then compare It’s KE to the 482 w/m^2 pouring out of the ground.
Come back to me with some numbers.
Until then; worthless comment!
And oh btw. It’s not an analogy.
It’s a sure fired marketplace winner of a fuel free oven built on the science you are putting forth. It has nothing to do with atmosphere. Never mentioned it.
I would appreciate it if your commentary sticks to what I am going to patent.
Willis, in a long post above you criticised me for using temperature as if it were an extensive property that could be conserved rather than more correctly treating it as an intensive property that cannot be preserved.
I have said that I was using temperature in rising and falling columns as :
“shorthand for ‘ the amount of kinetic energy that would provide a temperature of 33k if all the potential energy present were to be transformed to KE’”
Kinetic energy, being molecular movement is energy that must be preserved and so has an extensive property that can be added and subtracted.
So, I am correctly talking about kinetic energy with its extensive property but in order to link that to PE which does not have a temperature it is necessary to consider the thermal potential of the energy held as PE when it converts to KE.
That is all I am doing.
Your buckets of water and 1kg blocks being liquids and solids rather than gases are not appropriate illustrations since there is no significant interchange of KE and PE when one moves them up or down.
I know that I cannot add and subtract the temperatures of PE and KE since PE has no temperature and temperature is an intensive property but PE does have the potential of affecting temperature when work is done on it and one must take that potential into account somehow.
I am therefore adding and subtracting kinetic energy and NOT adding and subtracting temperature.
Anyway, can we just ignore that straw man and have your comment on my above post at 2.39 pm on the 6th?
Stephen Wilde December 7, 2017 at 4:21 am
Perhaps you missed the part of my last comment where I said:
I’m sorry, but I cannot deal any more with your willful, stubborn, and unpleasant ignorance. The ideas of intensive and extensive energy are not “straw men” that can be “ignored”, any more than we can “ignore” the laws of thermodynamics. You’ll have to find some other way to learn something about the subject you are convinced you know better than anyone else. I’m clearly not able to penetrate the walls you’ve built up.
Pass …
w.
PS—and no … you cannot add temperatures … despite being intimately related to kinetic and potential energy, TEMPERATURE IS NOT CONSERVED! And since you are either unable or unwilling to understand that, I give up. Go try to convince someone else that temperatures can be added and subtracted, it’s not gonna happen with me.
It is perfectly clear to any neutral observer that I am adding and subtracting kinetic energy and NOT temperature.
That is entirely legitimate.
That kinetic energy influences temperature in a way that does not suit you is not my problem.
What insults have I issued ?
Are you confusing me with others?
Willis said:
“The ideas of intensive and extensive energy are not “straw men” that can be “ignored”, any more than we can “ignore” the laws of thermodynamics”
I didn’t suggest that those ideas were straw men that can be ignored. I said that your allegation that I was ignoring them was a straw man.
As explained above, I am adding and subtracting kinetic energy and NOT temperature.
I know you are a busy man but I have spent a lot of time carefully narrowing down the issues to a couple of points that need clarification from you.
All I ask is that you attend to them.
Stephen Wilde December 7, 2017 at 1:55 pm
Stephen, you are the only person that I have ever heard make the claim that because temperature is a reflection of the average kinetic energy of the molecules involved, that means that you can add and subtract temperatures in Kelvin. The only one. You have not provided any logical reason why that should be so. I’ve pointed out that when you add two 1-kg masses at 200K you don’t get one 2-kg mass at 400K, but you persist in your claim that you can add temperatures.
You have also claimed that a one-time injection of energy in the atmosphere is what is keeping your imaginary system going. I pointed out that there are two proofs that that doesn’t work, mine and Dr. Robert Brown’s. Your response?
Say what? You don’t get to claim that two separate proofs, proofs that many people have examined and found no flaws with, are “fatally flawed” without pointing out the fatal flaws. That’s just more of your endless and baseless denial of anyone who doesn’t agree with you.
I have done my level best to “attend” to the issues you raised. But neither citing scientific works, nor others engaged in trying to get you to see just where your ideas are wrong, nor two separate proofs, nor pointing out things like the difference between intensive and extensive properties, have done any good.
So, as I said above, I can’t take any more your endless rejection of my good-will efforts and those of others. Nothing that I or anyone has said, nothing the citations have said, nothing the other people on the thread have said, has made the slightest dent in your unfounded certitude that you alone know how it all works. Nobody agrees with you, the citations don’t agree with you, but that makes absolutely no difference to you.
Let me suggest that if you want to go further, you point out the flaw in my proof that a non-GHG atmosphere alone cannot keep the surface temperature warmer than the S-B temperature corresponding to the amount of incoming radiation. That proof is here, and I don’t care in the slightest that you CLAIM it is fatally flawed. You need to SHOW that it is fatally flawed, or your whole premise that the atmosphere can warm the surface on a continuous basis goes up in smoke.
If you can show me the flaw in my proof, we’ll have something to discuss. Until then I’m getting off your merry-go-round, it’s making me dizzy …
w.
Stephen, I think I have your “elevator speech”.
The normal version of the Stefan-Boltzmann Law governing thermal radiation is incorrect because it fails to account for convection. With the correct version of the S-B Law, a 288 K surface below a convecting atmosphere will only radiate as if it were 255 K, allowing a mere 240 W/m^2 of incoming sunlight to maintain the surface and lower atmosphere at 288 K as long as convection occurs.
You missed out the conversion to and fro between KE and PE and the consequent delay in energy flowing through the system so you are talking rot.
S-B is based on radiation in and radiation out. It does not deal with a non radiative feedback loop between surface and atmosphere.
S-B is perfectly correct but it must be also be applied correctly.
The surface radiates at 288k but 33k doesn’t get out because it is absorbed by conduction and convection along the lapse rate slope.
Viewed from space the combined surface and atmosphere do observe S-B. It is only below top of atmosphere that S-B becomes inadequate.
“The surface radiates at 288k but 33k doesn’t get out because it is absorbed by conduction and convection along the lapse rate slope.”
In other words, the surface radiates at 390 W/m^2 (@288 K), but (390 W/m^2 – 240 W/m^2) = 150 W/m^2 (@ 288K – 255K = 33 K) of that upward radiation doesn’t get out because it is ” absorbed by conduction and convection along the lapse rate slope”. What mechanism allows 150 W/m^2 of outward travelling thermal radiation to get absorbed ‘along the lapse rate slope’? Where along that slope is it absorbed?
So you don’t believe that matter absorbs radiation and can then pass it to other particles of matter by conduction and convection?
If you don’t believe that conduction can draw energy away from radiation you are in conflict with Willis who ok’d that point. For a non radiative atmosphere it would happen at the surface.
You can’t have the same unit of kinetic energy in two places at once or performing two discrete tasks simultaneously.
Are you really serious?
Stephen, the very way you express your ideas and ask your questions belies fundamental misunderstandings — both about what I am saying and about how the energy transfers work. We could maybe try to reach a common understanding somewhere else — like your blog. But I suspect we have gone about as far as we can go here.
“We could maybe try to reach a common understanding..”
This will not happen Tim since Stephen ignores the testing, modern texts, and prominent papers that you et. al. rely on to understand meteorology. And Tim will not accept Stephen’s imagined meteorology.
Stephen — You ask: “So you don’t believe that matter absorbs radiation and can then pass it to other particles of matter by conduction and convection?”
I believe that matter with absorptivity greater than zero absorbs radiation and can then pass it to other particles of matter by conduction and convection.
I also believe — because of over a century of repeatable spectroscopic measurements — that N2, O2, and Ar in the atmosphere DO NOT have absorptivity noticeably greater than zero in the thermal IR band emitted by the earth’s surface.
I further believe — again because of over a century of repeatable spectroscopic measurements — that H2O, CO2, and other polyatomic gas molecules in the atmosphere with polar covalent bonds DO have absorptivity significantly greater than zero in the thermal IR band emitted by the earth’s surface.
We call this second class of molecules “greenhouse gases”, and it appears from your argument that your theories are dependent on their presence — that is, the metaphoric “greenhouse effect”. By your own logic, it would not happen with just the “transparent gases”.
Ed Bo 8:55pm: “.. N2, O2, and Ar in the atmosphere DO NOT have absorptivity noticeably greater than zero in the thermal IR band emitted by the earth’s surface.”
A little google-fu and your searching will find paper(s) on the noticeably nonzero collision-induced absorption by molecular N2, O2 & their individual effects quantified on the OLR of Earth’s atmosphere. Ar not so much.
Trick:
I’m well aware of that, which is why I added a qualifier to my statement. But the absorptivity is so low — many, many orders of magnitude less that that of CO2 and H2O — that there is no way is has any practically significant effect.
Mr Folkerts, I know that. You are busy at the moment with Mr Wilde.
But I asked you a question much further up post when you commented on post by Jim Masterson which I hoped you would answer.
It involves the very comment you made about adding a heat source and heat sink.
Could you find the time to answer it now please?
Dave Fair up post, which is getting lost now due to the still heavy traffic with Mr Wilde, I responded to you defense of Mr Eschenbach, as it will probably not get read by you if you are just staying with the current debate I am going to repeat it here
Dave, Mr Eshenbach does not need your.
Let me correct your statement for you.
Stop mimicking Willis just address Willis’ Fantasy science and avoid verbal coup-counting.
But as you are still asking questions.
Please show me any reputable (ie non Climate Science) sites that say that the radiation from a cold object makes a warmer one warmer and please do not quote a “Thought Experiment”.
That is all I am trying to Establish, what conditions that are currently not described by the Science allows photonic energy from whatever colder source to warm up a warmer object.
The definition of heat flow is the amount of Joules per second (Watts) per Metre2. Therefore at Equilibrum the amount of joules is a finite amount from the energy source without changing the energy source.
So that just leaves Emitting Surface Area as the variable.
Let’s assume that his sphere is spitting 2000 Joules per second per metre2 at Equilibrium, if we just take his sphere and expand it to the size of the Shell it’s Output will also drop by half as in the Steel Greenhouse Shell to 1000 Watts/M2 ie half the number of joules available per second per M2.
Please note that the System is still at Equilibrium putting out the same total of 2000 Joules per second when the total surface is considered, ie over twice as many square metres.
Also note that it is NOT at 4000 Joules per second, which are NOT available, totally impossible and are totally unnecessary for the sphere to obtain its original state of Equilibrium.
In fact I am not sure if it willactually be below 1000 joules per second as it takes twice as many joules to heat twice as much steel (Specific Heat) to the same Temperature, so am not sure if they can still be radiating the same.
As it would also take a little more to heat the extra Steel in the Shell.
I have been conducting REAL Experiments to try and find those magical conditions where the Colder surface Radiation heats the warmer surface and up till now I have failed.
As to the Light Bulb in a jar and foil which you so heartily endorsed, I am absolutely staggered that anyone believes that Back Radiation is directly involved in the warming of the Bulb.
I did not need to be shown that experiment as I had already done it for myself. Of course I used a Thermometer with 2 probes where it can provide not only the temperature from the probes, but also their difference in temp.
The extra heat comes from standard “Insulation” plus one other factor which comes after this one.
So what has changed, the addition of the Jar and the Foil have drastically reduced the heat loss via both Conduction and Convection. So what about the extra heat from adding the Foil, well that is where any back Radiation comes in.
Not only is the Foil doing the same to Conduction & Convection now the foil heats much quicker than the air between it and the bulb as it is a much better Conducter. The much hotter foil’s radiation excites the Air between the Foil and the Bulb increasing Temperature and thus slowing the cooling even more.
Experiment 1 with and without Foil box with a hot object
Without Box
Heated Object Temp (Facing Upwards) = 24.5C
Air above temp = 20.2C
Difference = 4.1C
With Box (with Foil) after 15 minutes
Heated Object Temp (Facing Upwards) = 26.0C
Air above temp = 24.0C
Difference = 2.0C
With Box (with Foil) after 20 minutes
Heated Object Temp (Facing Upwards) = 27.2C
Air above temp = 24.2C
Difference = 3.2C
With Box (with Foil) after 30 minutes
Heated Object Temp (Facing Upwards) = 27.5C
Air above temp = 24.4C
Difference = 3.2C
Foil at this point 22.5C
So do you see what happened, the Air heated most at 4.0C first due to the foil which in turn meant the heat sink was no longer as effective and the heated object warmed 2.0C.
But the air had started to stabilise at 24.2C and only rose another 0.2C while the object was still trying to reach stability.at 27.2C and then 27.5C.
However the Actual Heat Source which was a bulb around 25mm below the object could not be affected because all the heating was taking place above the Object, whereas the temperature to the side and below the object was only raised by 0.4C despite all that heat above neing reflected downwards by the Foil and By Conduction within the Air.
So now to the other factor involved, lets assume a warehouse with 20000 cubic metres of space. It has no heating so it remains at roughly outside ambient. We add X number of watts/m2 of Radiators with a large enough boiler to maintain a temp of say 30 degrees. Now if I put you in that Wharehouse with the Heating running at max you would be quite a bit hot, but not too uncomfortable. If I now take all thus radiators and stuff them into a room of 200 cubic metres with much better thermal Insulation how do you think you would feel.
Well you probably wouldn’t feel anything for very long as you would be Dead from heat exhaustion.
That is precisely what they did with the bulb in a box, took it out of a very spacous room and placed it in a very tiny one by comparison and added additional Thermal cladding..
Now tell me if you are surpised by the Air getting much hotter and thereby preventing the Bulb from cooling.
Perhaps they should fill the Box with CO2 at -180C to see how much it heats the bulb, I might try that with Cold air as I don’t have any CO2.
Experiment 2 with and without Foil box with 2 cold objects 13grms in weight at 12.0C and 12.7C in the box.
It is an established fact that a hotter object can warm quicker than a colder object so the one in the box may cool quicker. The Purpose of experiment is to demonstrate further that it insulation
After 0 minutes
Object out of box = 12.0C
Object in box = 12.7C
Difference = -.0.7C colder than outside
After 10 minutes
Object out of box 14.5C
Object in box = 14.9C
Difference = -0.5C
After 20 minutes
Object out of box = 17.3C
Object in box = 17,2C
Difference = +0.1C
After 30 minutes
Object out of box = 18.4C
Object in box = 18.1C
Difference = +0.3C
So there you have it A Foil Box also slows how much a cold object warms up, so it is insulating it from it’s surrounding, it did not add a single bit of back radiation to speed up the warming compared the one outside the box.
I agree AC; it is frustrating to carry on a dialogue with all the Thread hopping.
I have never said a cooler object can warm a warmer object. Without an independent power source, at best the cooler object would slow cooling of the warmer.
I have already done that experiment.
But no one on here wants to predict the result.
So I will tell you anyway the heated object at equilibrium exposed to a cold object, absolutely no warming at all, it instantly cooled at 0.4C/second.
A C — You request: “Please show me any reputable (ie non Climate Science) sites that say that the radiation from a cold object makes a warmer one warmer and please do not quote a “Thought Experiment”.”
You can check out any college textbook that explains radiative heat transfer, whether in physics or engineering. EVERY one shows this. For example, here is a link to MIT engineering professor Lienhard’s heat transfer textbook:
http://web.mit.edu/lienhard/www/ahttv211.pdf
Check out the very first explanation of radiation heat transfer in the introductory chapter (p 32). It states:
“Suppose that a heated object (1 in Fig. 1.16a) radiates only to some other object (2) and that both objects are thermally black. All heat leaving object 1 arrives at object 2, and all heat arriving at object 1 comes from object 2. Thus, the net heat transferred from object 1 to object 2, Qnet, is the difference between Q(1 to 2) = A1 eb(T1) and Q(2 to 1) = A1 eb(T2)…”
Note carefully that it describes the energy transfer as going both ways. Note in particular that it does not even say whether T1 is greater than T2 or less than T2.
There is a lot more detail in the chapter dedicated to radiative heat transfer, starting on page 529.
As I said, EVERY heat transfer textbook I have ever seen explains it in the same way, with bi-directional exchange of power. I have NEVER seen such a textbook claim that radiated energy from a colder object cannot be absorbed by a warmer object.
So I will turn the challenge around on you: Please show me any reputable sites that say that the radiation from a cold object cannot be absorbed by a warmer object, thereby increasing its energy level above what it would be without that radiative transfer.
A C Osborn asked, “Please show me any reputable (ie non Climate Science) sites that say that the radiation from a cold object makes a warmer one warmer…”
Ed Bo replied, “check out any college textbook that explains radiative heat transfer, whether in physics or engineering. EVERY one shows this. For example…”
It would be a lot more fun to perform the experiment which I suggested to Michael Moon:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/#comment-2677988
http://sealevel.info/man-and-woman-snuggling-under-blanket-BYAG9P_30pct.png
One of the other of you surely starts out a bit chillier than the other, yet when you snuggle up close to her you will both get warmer.
More experiments to come.
Stephen Wilde December 7, 2017 at 1:33 pm
Nope. And I called you on it before:
============
Stephen Wilde December 2, 2017 at 1:21 pm
Too painful to admit that a ten year old would know that energy loss delayed is temperature gained.
Stephen Wilde December 3, 2017 at 7:18 am
I gave you the accounting in the cheese ball analogy. You start with 10 balls but by manipulating timing on one occasion you end up with 15 in stock forever. I can see you never did stock control in retail.
Stephen Wilde December 3, 2017 at 7:27 am
You seem incapable of the mathematical agility required for retail stock control (multiple moving parts and variable timing) so no point engaging with you further since I’m sure other readers will get the idea.
Willis Eschenbach December 3, 2017 at 11:44 am
I do get the idea. You think that by accusing your opponent in the debate of stupidity, that you get to walk away without answering his question.
Paul Bahlin December 5, 2017 at 9:41 am
Stephen Wilde December 5, 2017 at 9:54 am
A good analogy for other features of your personality then?
Stephen Wilde December 5, 2017 at 1:42 am
Hope that is easier for those confused by the various more involved forms of words that I used previously in response to various objections.
Stephen Wilde December 5, 2017 at 12:27 pm
This is all pretty basic science and I’m increasingly surprised at how little you know.
==================
Clearly, we are all just too stupid to appreciate the true brilliance and insight of your claims …
w.
Willis, yes and after 8-10 years of Stephen attempting to get his comments generally accepted. If Stephen’s meteorological points were valid and backed by experiment & observation, consistent with the bulk of the foundational science work in the field, his efforts would have been fruitful LONG ago.
Then there is a disconnect between our opinions as to what constitutes insults. I saw my asides as pitched at a level similar to what was directed at me.
I’ll avoid any such asides in future but maybe others should do the same.
I agree that there is no point going further into the science points given that you won’t say how you think the energy used to form an atmosphere could have been dissipated away when the atmosphere is still present.
Nor will you accept that heat removed in ascent can cause a temperature rise when it is returned at a later date in descent whilst insolation continues throughout.
Neither your ‘proof’ nor that of Robert Brown considers adiabatic heating and cooling with its consequent delay in energy throughput at all.
Will contribute further in future threads (without any asides) if permitted to do so because many others see what I see and there should be some representation of that fact on this site.
Best wishes.
Stephen Wilde December 8, 2017 at 5:51 am:
Stephen, I distinctly remember Willis answering that question in a previous comment, but I don’t have time to search through 1800-odd comments right now to locate it for you. So I’ll just repeat it again here and now as it’s a really short and succinct answer. It is: Radiation.
This is the standard answer that you will also get from any conventional physical scientist, any competent geophysics textbook and any decent encyclopedia under “History of the earth” or the like. Disagree with it by all means if you want to, but you cannot expect others to join you in disagreeing with it unless you give them good reason to do that. And as far as I can see, you still haven’t given one.
I don’t accept that either, since heat removed from the surface by upward convection does not normally come back down again in the descending phase of the cycle. Normal convection cycles are net-transporters of heat from lower altitudes, where the air is warmer, to higher ones, where the air is cooler. I’ll grant that there might be occasional exceptions to this rule, mysterious nature being what she is, but in the main this is what standard meteorology says happens. Thus, their overall effect on the global surface is apparently to cool it, not to warm it or to re-deposit heat in it that they have previously extracted from it. I am surprised that someone who says he has been studying meteorology for sixty years should appear to be unaware of this.
I suspect that is because they would not need to consider it since convective overturning is not adiabatic. On the contrary, it is precisely the heat-input at the bottom of the cycle and the heat-output at the top that drives the cycle.
“In thermodynamics, an adiabatic process is one that occurs without transfer of heat or matter between a thermodynamic system and its surroundings. In an adiabatic process, energy is transferred to its surroundings only as work.” as Wikipedia aptly puts it.
Sorry, but I don’t see how you can get any surface temperature enhancement out of this process, with or without a time delay.
Cassio,
I had hoped to move on but since your post contains an insult I feel obliged to politely respond:
i) Willis did answer ‘radiation’ but the outstanding question is as to why the atmosphere has not collapsed to the ground. Can you help on that?
ii) Willis accepts that an adiabatic process is fully reversible. Heat at the surface that becomes PE higher up cannot radiate to space. Can you suggest how it would fail to return as heat in the descent?
iii) It is true that where an atmosphere contains radiative material there is some leakage of radiative energy out of the adiabatic cycle but most is recovered in the descent otherwise there would be no adiabatic warming in descent but there clearly is, at a rate that exactly matches the cooling in ascent if one disregards the effect of water vapour which is a separate issue. If you have some meteorological knowledge to the contrary please produce it.
iv)The radiative theory relies on a time delay to cause surface warming. Why would it be any different for a non adiabatic process especially when the latter is far slower than the former? Willis and Robert should reconsider their works accordingly.
Stephen: to your question.
“Willis did answer ‘radiation’ but the outstanding question is as to why the atmosphere has not collapsed to the ground. Can you help on that?”
We are still talking about an adiabaticc system of transparent gas, so no energy exchange except surface boundary via conduction, right.
So imagine a cold column with no convection. The warm surface passes energy to the gas. It expands and accepts energy until the surface equilibrates. At that point, the energy exchange stops forever. The potential energy is established forever. The ke is zero forever. That pe is no longer energy.
It takes no energy to .maintain this state forever.
So how did you get that cold column off the ground in the first place?
Go read it.
Energy goes in. One time. Never comes out.
You need 4 blocks of energy.
First, conduction from incoming solar hitting the surface to the rising column to get it into place.
Second, refresh the energy in the rising column as the falling leg completes.
Third, conduction back to the ground so that radiative balance with space is maintained.
Fourth, refresh the energy in the rising column for the second cycle from more incoming solar simultaneously with number 3.
Then you have the two separate energy loops in balance and surplus KE at the surface keeping the ‘motor’ running. KE is most definitely not zero because the air at the surface is all KE and no PE with a lapse rate slope marking the steady transition of KE to PE with height.
Refer back to my stock control analogy.
The energy for the first column cannot leave until you return the entire mass of the atmosphere to the surface. One needs to cut off the conduction in step 1 (sun stops shining) for the same time interval as one cycle and that causes the columns to collapse. That seems to be the issue that Willis and you have a problem with.
Your mistake is that PE is energy but not heat and the exchange between KE and PE is constant but in balance. I’m not sure what Willis’s problem is because he hasn’t said.
Yes PE is energy. But It’s not flux. It’s not flowing. You can’t use it in an accounting of flux values moving in and out of a system or subsystem
First, we are
First, we are not talking about blocks. We are talking about flows.
Your four flows are:
An initial flow of energy into the atmosphere during formation by conduction. This will continue, unbalanced, until the atmosphere is formed. At this point it has a mass, a center of gravity, a PE, and a lower boundary temperature equal to the surface temperature (integrated over reasonable time).
At this point conduction stops. Absent something gross happening, that’s the fourth flow, (when the sun winks out). You won’t see that energy ever again as a flow(), right. That energy can no longer cross that equilibrated boundary by conduction so it takes no energy, forever, to keep it there. Remember, by your own definition it has no other way to gain or lose.
Now while all that gas is in place, it has ke-pe exchanges, driven by surface conduction where there are localized imbalances in temperature, that exactly balance. That is flows 2 and three.
These are occurring with individual portions or parcels of the gas, not the entire mass. You have to think of system states over temporal range. If i take that range as time 0 to time infinity, then that initial flow is relevant and included. If i take the temporal range as, say +- thousand years it is irrelevant and not included.
There is nothing in Newtonian physics that says mass is a function of where its parts are.
I think you are trying to make a flow from time 0 PE and that is the energy you are using to maintain the surface temp at 288k.
You can’t. The atmosphere (entire mass) is not participating in ke-pe exchange, parts are.
Well a flow of energy between two points over a specified period of time can be legitimately regarded as a discrete ‘block’ of energy.
Anyway, you’ve confirmed what I was suspecting about your view of the formation of an atmosphere.
You have this mental picture of the entire atmosphere rising off the ground evenly around the globe until it reaches maximum height at which point it settles into a static condition with no top to bottom convection.
Even then maximum KE would be at the base and maximum PE at the top and the KE at the base would still have to be added to the KE from continuing insolation so even then you can’t get a surface at S-B.
It is that ‘extra’ KE at the surface which supplies the upward pressure gradient force which offsets the downward force of gravity whilst radiation in from space equals radiation out to space.
In reality, a planet is unevenly heated with a dark side and an illuminated side so that there is always a preferred hotter location for the beginning of the process. You will always start with discrete rising columns and they will fall again in discrete descending columns. No way to avoid it even with no GHGs so convection can never stop and you permanently have convective overturning converting KE to PE and PE to KE.
PE is simply former kinetic energy with the potential to become KE again when it is compressed once more.
All the jumbled up events within the atmosphere are just the interplay of irregularities in the PE / KE balance along the lapse rate slope caused by gravity having created a density gradient.
If you could adjust your mental picture then maybe you can start to see it?
Before the atmosphere there is indeed 0 PE.
After the atmosphere has formed there is a permanent store of PE which is proportionate to the weight of atmospheric mass and that weight is dependent on both the amount of mass and the power of the gravitational field.
Look, Cassio, why be so quick to reject Stephen’s idea? You guys are going to need SOMETHING to explain the remainder of the total atmospheric thermal enhancement, beyond the ~33 K Cassio has agreed is the maximum the radiative GHE can be producing. You can still hang on to your “back radiation heating” nonsense for the time being, if you like, but it only gets you so far. As Ed Bo agrees, whatever the true ATE value is for Earth, it MUST be greater than ~33 K.
Cassio’s agreement:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2687269
Ed Bo’s agreement:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2680092
No Tony, you have a complete inability to comprehend the simplest of arguments.
I “agreed” that the GHE “is more” than 33K (that is, the average surface temperature of the earth is more than 33K higher than an alternate imaginary earth without it.
That is completely different from — and in fact, pretty much the opposite of — agreeeing that it “cannot” be greater than 33K.
I pointed out that the argument that it cannot be greater than 33K was based on the same erroneous analysis that your heroes N&Z based their paper on.
When will you have anything constructive and honest to contribute to the discussion?
Pretending not to understand is not going to work at this stage, Ed. Sorry.
“I pointed out that the argument that it cannot be greater than 33K was based on the same erroneous analysis that your heroes N&Z based their paper on.“
Plus, you might want to click on the link under “Cassio’s agreement”, read that comment and the preceding discussion, then if you have any questions, take it up with Cassio.
“ii) Willis accepts that an adiabatic process is fully reversible. Heat at the surface that becomes PE higher up cannot radiate to space. Can you suggest how it would fail to return as heat in the descent?”
It would fail to return as heat in the descent because no real process is fully reversible. There are always losses in any real process thus the 33K cannot be held in place over time by the process Stephen describes.
“There are no “losses” in either of these processes.”
And yet universe entropy increased during the process (as this is a real process as noted) thus somewhere there was a loss which is easy to explain. Perhaps Rob can cite a source for his assertion explaining evidence for no loss but I doubt it.
Another Experiment, I only had time for one today.
This time with a heated object at equilibrium, an object at ambient and an object slightly warmer than ambient, this target object partially obscures an inside wall 0.2 above ambient Air when in place.
The air space between the heated object and the target objects, is swept by a low power fan to help prevent heated air affecting the results as I cannot make a vacuum of my room. But the airflow is masked from the target.
Starting position with fan on and heated object at equilibrium
Ambient Air Temperature = 17.6C Ambient Wall Temperature 17.8C
Temperature of heated object = 36.7C on Probe T1 now known as T1
Temperature of target object = 17.6C on Probe T2 now known as T2
After 10 minutes
T2 = 18.3C
T1 = 33.4C = 3.3C lower than start point
After 15 minutes
T2 = 18.3C
T1 = 33.2C = 3.5C lower than start point
After 20 minutes
T2 = 18.3C
T1 = 33.2C = 3.5C lower than start point
So that looks like Equilibrium, unfortunately the heat transfer to the target (T2) is rather low, but it still caused a 3.5C drop in the heated object (T1) by it’s presence, note it is Cooler, NOT hotter.
The original object (T2) is now warmed from the opposite side to T1 to try and generate that elusive Extra warming.
After 10 minutes
T2 = 22.2C A rise of 3.9C
T1 = 33.4C so a Massive rise of 0.1C even though the T2 is much warmer
After 20 minutes
T2 = 22.3C A rise of 3.9C
T1 = 33.1C the original gain has now been lost but Ambient is now at 17.4C, which is probably the cause.
Still no magic photons from a colder place making a hotter object even hotter.
I will keep trying to find them though, I haven’t given up yet.
Has anyone got any ideas for a simple experiment that I can do at home to find them?
Sorry T2 = 22.3C A rise of 3.9C should be
T2 = 22.3C A rise of 4.0C
OK, 2 Experiments to go.
One to test the theory that a mirror will increase the temperature of heated or non heated object.
The second is to get as close to the steel greenhouse as possible with what I can make, it will consist of
1. A ball, with most of the air extracted and resealed
2. A light bulb suspended in the ball with temperature transducer attached.
3. A second temperature transducer attached to the outside.
4. Either a fridge it ice to surround the ball.
Anyone prepared to offer what results I will get?
Great idea, AC.
First suspend the light bulb (the surface of the bulb is the iron sphere) by itself surrounded by your ice container. No ice can face the bulb directly (phase changes, you know). Let the system reach equilibrium. I guess you can replace melted ice in the surround-container to maintain a constant-temperature heat sink.
The interior of the surround-container must be maintained at a constant temperature at all times. No cheating.
Measure the temperature of the surface of the light bulb.
Then introduce the ball (iron shell), completely surrounding the light bulb and re-surround the whole shebang with the ice-filled surround-container. The interior of the surround-container must maintain the same exact constant temperature as described above.
The surface of the light bulb will heat up. If not, you have performed an Al Gore/Bill Nye level experiment.
You’re right, of course. But CO2 is such a strong absorber/emitter at 667 cm^-1 that it absorbs essentially 100% within tens of metres from the Earth’s surface. By Kirchhoff’s Law that a good absorber is a good emitter, it then radiates in both the forward and backward directions. Therefore your model of using a reflector which sends radiation only backward is not relevant. For Conservation of Total Energy, the back-radiation from CO2 must be powered by an equal amount of emission from the Earth’s surface, resulting in zero net warming of the surface (there is negligible 667 cm^-1 radiation from the Sun itself to be absorbed by CO2). The forward radiation from the CO2 in the atmosphere is that portion of other photons emitted from the surface that is not net absorbed by CO2 and transferred via inelastic collisions to the non-radiating N2, O2 and Ar of the troposphere. What then powers warming of the Earth’s surface? The incoming Solar visible and UV radiation that reaches the surface, which is strong enough to warm the Moon’s surface well above 40 Celsius in the daytime.
At no stage have the affirmatuve team in this post come to grips with the solar system empirical data. If this is a test, there has been, also, failure at the poster’s (WE) end re respect of contrary ideas, data, and persons. As if this was a warmunista blog.
Unvalidated models have failed. Allowing for real honest margins of error, no warming has happened during two decades of CO2 increase. What are we left with? Thought experiments here, also unverifiable. Jumping Jehasophat, Doc!
And if the steel shell was instead steel gas, it would behave as a gas, by gaseous equations of state. By physical Laws, cause and effect, which govern such to act differently from all solids . As a whole in spite of any trace gases including CO2. But remove gravity and the game changes……
A C Osborn December 8, 2017 at 3:25 pm
Thanks, A C, but as far as I know neither I nor anyone else has said that cold things can make hot things even hotter by radiation alone.
I have said that if you have an object at steady-state in a given environment, it will get warmer if you replace a cold object in its view field with a warm object. HOWEVER, the assumption of a steady-state pre-supposes a constant energy supply to the object. It is the constant energy supply which is making the object hotter, not the decreased net heat loss due to the change in the environment.
When you decrease the energy loss of an object receiving a steady flow of energy, it will warm up. Put mirrors on all sides of an electric light bulb, for example. Or put a blanket around an electric light bulb. The blanket or the mirrors are COOLER than the light bulb. But if you put them around the light bulb it gets WARMER.
Now, it appears that because the mirrors or the blanket are cooler than the light bulb, your claim is that the light bulb WILL NOT GET WARMER.
Me, I can do that experiment in my head and have 100% certainty about what will happen … the bulb will get warmer.
Heck, put a light bulb inside a thermos bottle and see what happens …
Think about it. You and others are seriously claiming that if you put a light bulb into a thermos and evacuate the air so all heat transmission is by radiation, the light bulb will NOT get warmer simply because the thermos is cooler than the light bulb …
Now, if you have to do experiments to verify that, I’d suggest you take a small incandescent light bulb, tape a thermistor to it, stick it in a thermos bottle, pump out the air, and see what happens … for me, the thought experiment plus the equations I gave in the head post are sufficient, but YMMV.
w.
PS—you say:
A C Osborn December 9, 2017 at 3:39 am
In that case, you should have no problem interpreting the equations that I gave at the end of the head post … and yet you and many others keep either denying them or ignoring them. And if you’ve forgotten how to do the equations, I included an online calculator to allow you to skip the calculations and go straight to the answer … let me suggest that you look at both, as they agree with me, Ed Bo, and many others here on this thread.
A C:
You may want to ponder why the agencies that build spacecraft and use large vacuum chambers to test them before launch provide the capability for supercooling the walls of the vacuum chambers.
They commonly use liquid nitrogen to cool “shrouds” inside the structural walls of the chamber. Nitrogen boils at about 70K, so this keeps the walls below 100K. 100K is 1/3 the temperature of typical ambient of about 300K, so the radiative flux density is 1/81.
When they really want to test deep space environment, they add supercooled helium shrouds inside the nitrogen shrouds to get down to temperatures of about 20 – 30K. With 30K at 1/10 of 300K, the radiative flux density is 1/10000. The sensor electronics of the Webb space telescope just went through several months of testing in this environment. They did this because they want these electronics to operate at about 30K in actual operation.
This supercooling of vacuum chamber walls costs untold millions of dollars. It is done to minimize the “back radiation” from the walls of the chamber (which would be about 400 W/m2 at typical surface ambient temperatures).
By your logic, these agencies are wasting their money, because there is no way the walls, even at 300K, could serve to increase the temperature of the electronics (which have their own power supply).
Do you really think they are wasting their (our) money??? Have you written them to complain about this incredible waste of taxpayer money?