Guest Post by Bob Tisdale
UPDATE: Corrected the percentage of ocean heat loss though evaporation. Update 2: Added a link to a post by Willis Eschenbach at the end, and corrected a typo.
# # #
Ocean heat content and vertically averaged temperature data for the oceans have been the subjects of a couple of recent blog posts. As one might expect, the discussions on those threads tend to shift to the subject of whether or not the infrared (longwave) radiation from manmade greenhouse gases can cause any measureable ocean warming at the surface or at depth. According to the hypothesis of human-induced global warming, the warming of the global oceans to depth and the related ocean heat uptake are a function of the radiative imbalance caused by manmade greenhouse gases. There are a number of arguments for and against the hypothetical anthropogenic warming of the oceans.
So the topic of this post is ocean warming. I’ll present different opinions/arguments on anthropogenic ocean warming.
For a detailed overview of ocean heat content data, please see the post Is Ocean Heat Content Data All It’s Stacked Up to Be? And see the post AMAZING: The IPCC May Have Provided Realistic Presentations of Ocean Heat Content Source Data for another discussion by the IPCC.
INFRARED RADIATION CAN ONLY PENETRATE THE TOP FEW MILLIMETERS OF THE OCEAN SURFACE AND THAT’S WHERE EVAPORATION TAKES PLACE
It is often argued that infrared radiation from manmade greenhouse gases can only penetrate the top few millimeters of the ocean surface and that’s where evaporation occurs. That argument then continues that additional infrared radiation from anthropogenic greenhouse gases can only add to surface evaporation, and cannot heat the oceans. On the other hand, sunlight reaches into the oceans to depths of 100 meters or so, though most of it is absorbed in the top 10 meters. Even so, sunlight’s ability to warm the oceans is many orders of magnitude greater than infrared radiation. One of my earliest memories of this argument came from Robert E. Stevenson’s (Oceanographer Scripps) 2000 article Yes, the Ocean Has Warmed; No, It’s Not ‘Global Warming’. In April of this year, looking for solid answers on this topic, Roy Spencer presented the same arguments and a few counter arguments in his post, Can Infrared Radiation Warm a Water Body?
Field tests reported in the 2006 post Why greenhouse gases warm the oceans at RealClimate are often cited by those who believe infrared radiation is responsible for ocean warming. That guest post by Peter Minnett of the University of Miami includes:
However, some have insisted that there is a paradox here – how can a forcing driven by longwave absorption and emission impact the ocean below since the infrared radiation does not penetrate more than a few micrometers into the ocean?
So this argument was considered by climate scientists. The post then goes on to describe why it’s not an inconsistency and then to present the results of field tests. My Figure 1 is Figure 2 from that RealClimate post.
Figure 1 – The change in the skin temperature to bulk temperature difference as a function of the net longwave [infrared] radiation.
The summary text for the illustration at RealClimate reads:
There is an associated reduction in the difference between the 5 cm and the skin temperatures. The slope of the relationship is 0.002ºK (W/m2)-1. Of course the range of net infrared forcing caused by changing cloud conditions (~100W/m2) is much greater than that caused by increasing levels of greenhouse gases (e.g. doubling pre-industrial CO2 levels will increase the net forcing by ~4W/m2), but the objective of this exercise was to demonstrate a relationship.
That, however, creates a counter argument that has been discussed by others. See the HockeySchtick post RealClimate admits doubling CO2 could only heat the oceans 0.002ºC at most. Let me put this into more recent terms. According to the NOAA Annual Greenhouse Gas Index, infrared radiation has only increased about 1.2 watts/meter^2 from 1979 to 2013. Based on the findings at RealClimate, that rise in infrared radiation could only warm the sea surfaces by a little more than 0.002 deg C since 1979. Yet, looking at the global sea surface temperature data, Figure 2, the surfaces of the global oceans warmed more than 0.3 deg C from 1979 to 2013, leaving about 93% 99.3% of the ocean surface warming unexplained.
Figure 2
A continuation of the Minnett-field-test argument is that manmade greenhouse gases and ocean mixing will cause the warming of the mixed layer of the oceans. The HockeySchtick counter could be applicable here as well. The mixed layer ranges in depth from about 20 to 200 meters. Unfortunately, temperature data specifically for the mixed layer are not available in an easy-to-use format, so let’s assume that the NODC’s vertically averaged temperature data for the depths of 0-100 meters captures the vast majority of the mixed layer. As shown in Figure 2, the warming rate of the top 100 meters of the ocean is slightly less than the surface. In other words, the warming rate based on the field tests presented by RealClimate can’t explain the vast majority of the warming of the top 100 meters.
Further to the RealClimate post by Peter Minnett, see the very recent ClimateConversation post HotWhopper wrong on ocean heat. It includes links to a three part discussion titled “Anthropogenic Ocean Warming?” by Richard Cummings, which covers the Minnett findings and other proposed mechanisms of anthropogenic warming of the oceans:
- Part 1: Skeptical Science Offside
- Part 2: The Improbable IPCC Mechanism
- Part 3: Rahmstorf, Schmittner and Nuccitelli
“AIR-SEA FLUXES ARE THE PRIMARY MECHANISM BY WHICH THE OCEANS ARE EXPECTED TO RESPOND TO EXTERNALLY FORCED ANTHROPOGENIC AND NATURAL VOLCANIC INFLUENCES”
The quote in the heading is from Chapter 10 (WG1) of the IPCC’s 5th Assessment Report.
Richard Cummings comments from Part 2 of his series begins:
That’s it. 25 years and five assessment reports after its 1988 formation, the IPCC has not been able to firm up an anthropogenic ocean heating and thermal sea level rise mechanism. The one they have come up with is only “expected”, indicating that they are unable to cite studies of the real-world phenomenon of non-solar air => sea energy fluxes actually occurring on a scale that would explain 20th century ocean heat accumulation in the order of 18×10^22 J and subjugate a solar-only mechanism.
“…HEAT PENETRATES THE OCEANS FASTER IN A WARMER CLIMATE”
The heading is a quote from the concluding remarks by Stefan Rahmstorf in the RealClimate post Sea-level rise: Where we stand at the start of 2013 (my boldface).
My bottom line: The rate of sea-level rise was very low in the centuries preceding the 20th, very likely well below 1 mm/yr in the longer run. In the 20th Century the rate increased, but not linearly due to the non-linear time evolution of global temperature. The diagnosis is complicated by spurious variability due to undersampling, but in all 20th C time series that attempt to properly area-average, the most recent rates of rise are the highest on record. At the end of the 20th and beginning of the 21st Century the rate had reached 3 mm/year, a rather reliable rate measured by satellites. This increase in the rate of sea-level rise is a logical consequence of global warming, since ice melts faster and heat penetrates faster into the oceans in a warmer climate.
Is this a very simplified rewording of the argument that, although the atmosphere is cooler than the ocean surfaces, greenhouse gases will reduce the rate at which oceans can release heat to the atmosphere?
See Richard Cummings response in Part 3 of his series.
MECHANISMS FOR THE WARMING OF THE OCEANS
Donald Rapp presented a simple model to explain how manmade greenhouse gases could warm the oceans in his guest post at Judith Curry’s blog ClimateEtc, back in May 2014. See his post Mechanisms for the Warming of the Oceans. That post drew more than 400 comments. If you’re going to cut and paste one of your or someone else’s comments from that thread, please leave a hyperlink to it.
INFRARED RADIATION FROM MANMADE GREENHOUSE GASES HAS INCREASED SINCE 1979, WHILE TOTAL SOLAR IRRADIANCE HAS DECREASED. THEREFORE, INFRARED RADIATION CAUSED THE OCEAN WARMING.
This is one of the favorite arguments for anthropogenic warming of the oceans: Infrared radiation has increased since 1979 but total solar irradiance at the top of the atmosphere has decreased. Therefore, according to that ill-conceived argument, the sun can’t explain the warming.
Why is it ill-conceived? We’re interested in the amount of sunlight reaching the ocean surfaces and entering into them, not the amount of sunlight reaching the top of the atmosphere.
There is evidence the amount of sunlight reaching Earth’s surface increased from 1979 to 2013. It comes from a specialized climate model called a reanalysis, and the reanalysis being discussed is the NCEP-DOE R-2. Unlike the climate models used to hindcast and predict global warming, a reanalysis uses data (sea surface temperature data, cloud cover data, aerosol data, total solar irradiance data, and the like) as inputs and calculates variables that aren’t measured directly. It’s a climate model, so we still have to look at it with a skeptical eye, but even so, the sunlight reaching the surface of the Earth increased from 1979 to 2013, according to the NCEP-DOE R-2 reanalysis. See Figure 3.
Figure 3
I’ve added a note to the graph:
Above what value do the oceans accumulate heat?
That was to counter another ill-conceived argument. Someone might look at the graph and see that sunlight at the surface peaked around the year 2002 and has since dropped, expecting the oceans to lose heat during the decline. But that argument would fail to consider many things, including the one noted.
This also brings to mind something written by Carl-Gustaf Rossby in 1959. It is part of the opening chapter of the book The Atmosphere and Sea in Motion edited by Bert Bolin. That chapter is titled “Current problems in meteorology”. In it, Rossby made two suggestions while discussing ocean processes (my boldface):
a) The assumption that our planet as a whole stands in firm radiation balance with outer space cannot be accepted without reservations, even if periods of several decades are taken into account.
b) Anomalies in heat probably can be stored and temporarily isolated in the sea and after periods of the order of a few decades to a few centuries again influence the heat and water-vapour exchange with the atmosphere.
So, assuming the NCEP-DOE R2 reanalysis is correct, how long would the recent increase in the amount of sunlight entering the oceans impact climate? According to Rossby, it could be decades or centuries.
Something else to consider: according to the NODC’s vertically averaged temperature data to depths of 2000 meters, the North Atlantic and the Pacific Ocean show little to no warming since 2005. The other two ocean basins, the South Atlantic and Indian Oceans are showing warming, but they only cover about 1/3 of the ocean surface. See Figure 4.
Figure 4
That lack of warming to depths of 2000 meters for two ocean basins that cover 2/3 of the ocean surface (North Atlantic and Pacific) is hard to reconcile in a world where greenhouse gases are said to be well mixed, meaning they’re pretty well evenly distributed around the globe.
THE OCEANS HAVE THEIR OWN GREENHOUSE-LIKE EFFECT
In his post, The Deep Blue Sea, John L. Daly presented something that must be considered in every discussion of ocean warming: the oceans have their own greenhouse like effect (I’ve added a hyperlink to John Daly’s Figure 1):
A greenhouse effect, by definition, means that the medium through which radiation passes is more transparent at visible wavelengths, but more opaque at infra-red wavelengths, thus letting in visible energy but obstructing the escape of sufficient infra-red energy to maintain thermal equilibrium without a rise in temperature.
The oceans also behave this way.
Reference to fig. 1 shows that the oceans let in visible solar radiation right down to 100 metres depth. However, the oceans cannot radiate from such depths, as infra-red radiation can only take place from the top few millimetres of ocean. Thus, the oceans are also behaving in a greenhouse-like manner, taking in heat and then trapping some of it to cause a temperature rise.
Phrased differently, sunlight can warm the oceans to depths of 100 meters, but the oceans can only release heat at the surface. Now consider that the oceans release heat primarily through evaporation (if memory serves, somewhere in the neighborhood of 90% of the heat loss from the oceans is through evaporation). UPDATE: Sorry, in this instance my memory was off. Of the approximately 180+ watts/m^2 downward shortwave radiation reaching the ocean surface, about half (about 100 watts/m^2) is released through evaporation.
THERE ARE NATURALLY OCCURRING PROCESSES THAT CAN CAUSE THE LONG-TERM WARMING OF THE OCEANS TO DEPTH
The naturally occurring processes that can warm the oceans, of course, are not considered in the climate models used by the IPCC. Climate modelers’ force the warming of the oceans based on their assumptions of how the infrared radiation from manmade greenhouse gases warm the oceans.
We’re going to break the oceans down into ocean-basin subsets, because, for two of the subsets, climate scientists addressed those portions of the oceans in the studies linked to this post.
I’ve presented these discussions in previous posts using ocean heat content data. For a change of pace, I’m presenting the NODC depth-averaged temperature data for the depths of 0-700 meters.
THE WARMING OF THE NORTH ATLANTIC TO DEPTH
As a preface to our first discussion, Figure 5 presents the depth-averaged temperature anomalies (0-700 meters) for the North Atlantic and for the rest of the global oceans. To determine the depth-averaged temperature anomalies for the rest of the global oceans, I area-weighted the North Atlantic data (11.5%, see the NOAA webpage here) and subtracted it from the global data. The units are deg C.
Figure 5
It very obvious that the North Atlantic to depths of 700 meters warmed at a much faster rate than the rest of the oceans, about 3.3 times faster from 1955 to present. That ocean basin only covers 11.5% of the surface of the global oceans, yet it represents about 35% of the ocean warming to depths of 700 meters.
NOTE: It is unfortunate that the outputs of the climate model simulations of depth averaged temperature (or ocean heat content) are not available in an easy-to-use form so that the models can be compared to observations. We know climate models do not properly simulate the warming of ocean surfaces. They double the warming rate of the ocean surfaces over the past 33 years. See the model-data comparison graph here. Also see the posts here and here for additional discussions. It would be interesting to see how poorly the models simulate ocean warming to depth. [End note.]
Now consider what I wrote in that introductory portion from my upcoming book: It’s very obvious why the change in the ocean heat content is very important to the hypothesis of human-induced global warming. If the oceans could be shown to have warmed naturally, then the impacts of manmade greenhouse gases are much smaller than claimed by climate scientists.
And that’s exactly what a group of scientists did back in 2008. They determined the warming of the North Atlantic to 700 meters since 1955 was caused by naturally occurring processes, not by manmade greenhouse gases. We’ve discussed this paper a few times in recent years—in blog posts and in books. Here’s a portion of my ebook Who Turned on the Heat?
[START OF REPRINT FROM WHO TURNED ON THE HEAT?]
There is a study that provides an explanation for that additional warming. See Lozier et al (2008) The Spatial Pattern and Mechanisms of Heat-Content Change in the North Atlantic.
First, a quick introduction to one of the terms used in the following quotes: The North Atlantic Oscillation is an atmospheric climate phenomenon in the North Atlantic. Like the Southern Oscillation Index described in Chapter 4.3 ENSO Indices, the North Atlantic Oscillation is expressed as the sea level pressure difference between two points. The sea level pressures in Iceland, at the weather stations in Stykkisholmur or Reykjavik, can be used to calculate North Atlantic Oscillation Indices. Which Iceland location they elect to use as the high-latitude sea level pressure reference depends on the dataset supplier. The other point captures the sea level pressure at the mid-latitudes of the North Atlantic, and there are a number of locations that have been used for it: Lisbon, Portugal; Ponta Delgada, Azores; and Gibraltar. The North Atlantic Oscillation Index is primarily used for weather prediction. The direction and strength of the westerly winds in the North Atlantic are impacted by the sea level pressures in Iceland and the mid-latitudes of the North Atlantic, which, in turn, impact weather patterns in Europe and the East Coast of North America. If you live in those locations, you’ll often hear your weather person referring to the North Atlantic Oscillation. As will be discussed, winds in the North Atlantic can also impact Ocean Heat Content.
I’ll present two quotes from the Lozier et al (2008) paper. I’ll follow them with quotes from the press release that describes in layman terms how the North Atlantic Oscillation impacts the Ocean Heat Content of the North Atlantic. Back to Lozier et al (2008):
The abstract reads:
The total heat gained by the North Atlantic Ocean over the past 50 years is equivalent to a basinwide increase in the flux of heat across the ocean surface of 0.4 ± 0.05 watts per square meter. We show, however, that this basin has not warmed uniformly: Although the tropics and subtropics have warmed, the subpolar ocean has cooled. These regional differences require local surface heat flux changes (±4 watts per square meter) much larger than the basinwide average. Model investigations show that these regional differences can be explained by large-scale, decadal variability in wind and buoyancy forcing as measured by the North Atlantic Oscillation index. Whether the overall heat gain is due to anthropogenic warming is difficult to confirm because strong natural variability in this ocean basin is potentially masking such input at the present time.
In the paper, Lozier et al (2008) note, using NAO for North Atlantic Oscillation:
A comparison of the zonally integrated heat-content changes as a function of latitude (Fig. 4B) confirms that the NAO difference can largely account for the observed gyre specific heat-content changes over the past 50 years, although there are some notable differences in the latitudinal band from 35° to 45°N. Thus, we suggest that the large-scale, decadal changes in wind and buoyancy forcing associated with the NAO is primarily responsible for the ocean heat-content changes in the North Atlantic over the past 50 years.
Based on the wording of the two quotes, the paper appears to indicate that Lozier et al (2008) are describing the entire warming of ocean heat content in the North Atlantic. In other words, it seems that Lozier et al (2008) are not stating that the North Atlantic Oscillation is primarily responsible for the additional ocean heat-content changes in the North Atlantic, above and beyond the rest of the world, over the past 50 years; they’re saying it’s primarily responsible for all of the variability. The press release for the paper, on the other hand, leads you to believe the North Atlantic Oscillation is responsible for the North Atlantic warming above and beyond the global warming.
The Duke University press release for the paper is titled North Atlantic Warming Tied to Natural Variability. Though the other ocean basins weren’t studied by Lozier et al, the subtitle of the press release includes the obligatory reference to an assumed manmade warming in other basins: “But global warming may be at play elsewhere in the world’s oceans, scientists surmise”. To contradict that, we’ve found no evidence of an anthropogenic component in the warming of the other ocean basins.
The press release reads with respect to the North Atlantic Oscillation (NAO):
Winds that power the NAO are driven by atmospheric pressure differences between areas around Iceland and the Azores. “The winds have a tremendous impact on the underlying ocean,” said Susan Lozier, a professor of physical oceanography at Duke’s Nicholas School of the Environment and Earth Sciences who is the study’s first author.
Further to this, they write:
Her group’s analysis showed that water in the sub-polar ocean—roughly between 45 degrees North latitude and the Arctic Circle—became cooler as the water directly exchanged heat with the air above it.
By contrast, NAO-driven winds served to “pile up” sun-warmed waters in parts of the subtropical and tropical North Atlantic south of 45 degrees, Lozier said. That retained and distributed heat at the surface while pushing underlying cooler water further down.
The group’s computer model predicted warmer sea surfaces in the tropics and subtropics and colder readings within the sub-polar zone whenever the NAO is in an elevated state of activity. Such a high NAO has been the case during the years 1980 to 2000, the scientists reported.
“We suggest that the large-scale, decadal changes…associated with the NAO are primarily responsible for the ocean heat content changes in the North Atlantic over the past 50 years,” the authors concluded.
[END OF REPRINT FROM WHO TURNED ON THE HEAT?]
WHAT CAUSES THE WATER TO “PILE UP”, INCREASING OCEAN HEAT CONTENT?
Let’s discuss in more detail that “pile up” from the press release of Lozier et al. (2008). First, a few basics: The trade winds are a function of the temperature difference between the equator and higher latitudes. The warmer water near the equator causes warm air to rise there (convection). At the surface, winds blow from the mid latitudes toward the equator to make up for the deficit caused by the rising air, but the rotation of the Earth deflects that inrushing air to the west. Thus the trade winds blow from the northeast to the southwest in the Northern Hemisphere and from the southeast to the northwest in the Southern Hemisphere.
In the ocean basins, ocean circulation is driven primarily from the trade winds in the tropics blowing from east to west. That is, the trade winds push the surface waters from east to west in the tropics. Those westward-traveling waters warm under the tropical sun. They encounter a continental land mass and are directed toward the poles. In the North Atlantic, the poleward-flowing western boundary current is known as the Gulf Stream. It carries the warm tropical waters to the cooler high latitudes, where that water can release heat to the atmosphere more efficiently. At the mid-latitudes, those waters encounter the west to east winds known as westerlies and are blown eastward toward Europe and Africa. The eastern boundary current along Africa returns those cooler waters back toward the tropics, where they can be warmed again, completing the cycle. That ocean circulation loop is called a gyre.
Now for the “piling up”: Suppose the westerlies in the mid-latitudes slowed or reversed, while, at the same time, the trade winds were pushing the same amount of tropical water to the west and poleward. At mid-latitudes, the change in the strength or direction of the westerlies would resist the poleward transport of warm water from the tropics. That warm water would accumulate as a result. Here’s that quote from the press release again:
By contrast, NAO-driven winds served to “pile up” sun-warmed waters in parts of the subtropical and tropical North Atlantic south of 45 degrees, Lozier said. That retained and distributed heat at the surface while pushing underlying cooler water further down.
Presto. A naturally caused accumulation of heat in the North Atlantic.
Curiously, under the heading of “Beam Me Up, Scotty”, Stefan Rahmstorf of RealClimate presented a similar discussion in his post What ocean heating reveals about global warming. I, of course, commented on that in my post Comments on Stefan Rahmstorf’s Post at RealClimate “What ocean heating reveals about global warming”
Now suppose, at the same time, there were a series of strong El Niño events over a multidecadal period (1976 to the turn of the century for example), so that the tropical waters in the North Atlantic were naturally warmer than normal. Trenberth and Fasullo (2011) explain why some portions of the oceans remote to the tropical Pacific warm in response to an El Niño (my boldface):
But a major challenge is to be able to track the energy associated with such variations more thoroughly: Where did the heat for the 2009–2010 El Niño actually come from? Where did the heat suddenly disappear to during the La Niña? Past experience (Trenberth et al. 2002) suggests that global surface temperature rises at the end of and lagging El Niño, as heat comes out of the Pacific Ocean mainly in the form of moisture that is evaporated and which subsequently rains out, releasing the latent energy. Meanwhile, maximum warming of the Indian and Atlantic Oceans occurs about 5 months after the El Niño owing to sunny skies and lighter winds (less evaporative cooling), while the convective action is in the Pacific.
That additional sunlight during a period when El Niños dominated (1976 to the turn of the century) would add to the amount of accumulating warm water in the North Atlantic…and elsewhere.
And Trenberth now understands that the heat didn’t suddenly “disappear to during the La Niña”. It shows up as the “big jumps” in surface temperature in response to strong El Niño events. See the posts:
- Open Letter to the Royal Meteorological Society Regarding Dr. Trenberth’s Article “Has Global Warming Stalled?”
- The 2014/15 El Niño – Part 9 – Kevin Trenberth is Looking Forward to Another “Big Jump”
I also present those “big jumps” in the monthly sea surface temperature updates (November 2014 update is here). They stand out quite plainly in the sea surface temperature data for the South Atlantic, Indian and West Pacific Oceans. For a further discussion see the illustrated essay “The Manmade Global Warming Challenge” (42mb).
EXTRATROPICAL NORTH PACIFIC
The next paper to be discussed is Trenberth and Hurrell (1994): Decadal Atmosphere-Ocean Variations in the Pacific. In it, Trenberth and Hurrell were using an index derived from the sea level pressures of the extratropical North Pacific (30N-65N, 160E-140W), called the North Pacific Index, to explain shifts in the sea surface temperatures of the North Pacific. Again, a sea level pressure index reflects changes in the wind patterns. My Figure 6 is Figure 6 from Trenberth and Hurrell (1994).
Figure 6
That same shift appears in the depth-averaged temperature data for the extratropical North Pacific (24N-65N, 120E-80W) for the depths of 0-700 meters. But the shifts are delayed a year in the subsurface temperature data. See Figure 7.
Figure 7
I’ve color-coded 4 periods on the graph. The first period from 1955 to 1988 (dark blue) includes the downward shift in 1978. As a result of that shift in 1978 (that should be related to the shift in the sea level pressures and wind patterns), the depth-averaged temperature data shows a cooling trend from 1955 to 1988. That is, the extratropical North Pacific to depths of 700 meters cooled (not warmed) for more than 3 decades. The second period (red) captures the upward shift in 1988 and 1989 that, once again, should be related to the shift in the sea level pressures and wind patterns. From 1991 to 2002 (light blue), the extratropical North Pacific cooled once again to depths of 700 meters. And since the ARGO floats were deployed (black), the extratropical Pacific shows a slight warming to depth.
It’s blatantly obvious the extratropical North Pacific to depths of 700 meters would show no warming from 1955 to present if it wasn’t for that upward shift in 1988 and 1989. It’s also obvious that the downward shift in 1978 that extends to 1988 also impacts the long-term trend. That is, without the naturally caused downward shift in the late-1970s the long-term warming rate would be less. Obviously, natural variability, not manmade greenhouse gases, dominates the variability and long-term warming of the extratropical Pacific to the depths of 700 meters.
TROPICAL PACIFIC
We isolate the vertically averaged temperature data to depths of 700 meters for the tropical Pacific because the tropical Pacific is where El Niño and La Niña events take place, and El Niño and La Niña events, collectively, are the dominant forms of natural variability on Earth. A further clarification: while El Niño and La Niña events are focused on the equatorial Pacific, they directly impact the entire tropical Pacific. See the animation here for an extreme example of the effects of an El Niño on the sea level residuals of the tropical Pacific.
Let’s start with two quotes from (again) Kevin Trenberth. According to Trenberth, El Niño events are fueled by sunlight, not manmade greenhouse gases. In the much-cited Trenberth et al. (2002) The evolution of ENSO and global atmospheric surface temperatures, they stated (my boldface and brackets):
The negative feedback between SST and surface fluxes can be interpreted as showing the importance of the discharge of heat during El Niño events and of the recharge of heat during La Niña events. Relatively clear skies in the central and eastern tropical Pacific [during a La Niña] allow solar radiation to enter the ocean, apparently offsetting the below normal SSTs, but the heat is carried away by Ekman drift, ocean currents, and adjustments through ocean Rossby and Kelvin waves, and the heat is stored in the western Pacific tropics. This is not simply a rearrangement of the ocean heat, but also a restoration of heat in the ocean. Similarly, during El Niño the loss of heat into the atmosphere, especially through evaporation, is a discharge of the heat content, and both contribute to the life cycle of ENSO.
NOTE: That’s the source of my standard description of ENSO as a chaotic, naturally occurring, sunlight-fueled, recharge-discharge oscillator…with El Niños acting as the discharge phase and La Niñas acting as the recharge phase. But La Niñas also help to redistribute the leftover warm waters from the El Niños. [End note.]
Also see Trenberth and Fasullo (2011). They confirm that ENSO is sunlight-fueled during La Niña events:
Typically prior to an El Niño, in La Niña conditions, the cold sea waters in the central and eastern tropical Pacific create high atmospheric pressure and clear skies, with plentiful sunshine heating the ocean waters. The ocean currents redistribute the ocean heat which builds up in the tropical western Pacific Warm Pool until an El Niño provides relief (Trenberth et al. 2002).
Figure 8 presents the vertically averaged temperature anomalies (0-700 meters) for the tropical Pacific. El Niño and La Niña events directly impact the top 300 meters, so this depth captures their direct impacts. I’ve highlighted in maroon the three 3-year La Niña events of 1954 to 1957, 1973 to 1976, and 1998 to 2001. After those 3-year La Niña events, the tropical Pacific shows cooling, not warming. That indicates that the shorter La Niñas that follow El Niños only recharge part of the warm water released from the tropical Pacific by the El Niños. Also, I’ve highlighted in red the 7-month period associated with the 1995/96 La Niña. (See the old version of the NOAA ONI index.) The 1995/96 La Niña created the warm water that fueled the 1997/98 El Niño, which is responsible for the sharp drop in temperature following the heat uptake of the 1995/96 La Niña. The “overcharge” from the 1995/96 La Niña and the recharge during the 1998-01 La Niña obviously caused an upward shift in the subsurface temperatures of the tropical Pacific.
Figure 8
What is also blatantly obvious is the warming of the tropical Pacific to depth is dependent on 4 La Niña events. And according to Trenberth et al. (2002) and Trenberth and Fasullo (2011), sunlight warms the tropical Pacific during La Niñas, not infrared radiation from manmade greenhouse gases. (In the real world, downwelling longwave radiation decreases during La Niña events.)
BOTTOM LINE ON OCEAN TEMPERATURE DATA FOR THE DEPTHS OF 0-700 METERS
Subsurface temperature data (and ocean heat content data) for the North Atlantic, the Extratropical North Pacific and the Tropical Pacific all indicate that naturally occurring coupled ocean-atmosphere processes are the primary causes of ocean warming to depth, not manmade greenhouse gases. In fact, the data for the tropical Pacific and extratropical North Pacific show those oceans can cool for decadal and multidecadal periods between short-term naturally caused warming episodes. Those decadal and multidecadal cooling periods further suggest that manmade greenhouse gases have no measureable impact on ocean warming to depth.
NOTE: Someone is bound to note that I’ve only presented subsurface ocean temperature data for the top 700 meters and only for the oceans of the Northern Hemisphere and the tropical Pacific. If I receive a comment to that effect on the thread, I will refer that blogger to the 2 posts linked in the introduction. Here they are again:
- Is Ocean Heat Content Data All It’s Stacked Up to Be?
- AMAZING: The IPCC May Have Provided Realistic Presentations of Ocean Heat Content Source Data
CLOSING
I’m sure I’ve missed a few arguments for and against the anthropogenic ocean warming. If you introduce others, please provide links where possible.
UPDATE 2: While preparing this post, I overlooked an excellent post by Willis Eschenbach Radiating The Ocean.








At 1 bar atmospheric pressure evaporation takes up 5 times as much energy in latent form as is required to induce it.
That being the case I cannot see how there can be any left over DWIR to warm the sea surface or reduce the rate at which energy leaves from ocean to air.
The water vapour produced from more evaporation is lighter than air and so is whisked away upward taking that additional latent heat away with it.
In order for DWIR to warm a water surface the latent heat of evaporation would need to be the same as or less than the energy required to induce that evaporation.
A cooler atmosphere can never warm a warmer ocean except when the atmosphere itself warms and reduces the ocean’s ability to cool. Get over it. Whenever you violate the second law of thermodynamics, you are wrong.
Thanks, gymnosperm. The second law of thermodynamics doesn’t come into it, because you are asking the wrong question. The question is not whether “a cooler atmosphere can warm a warmer ocean”.
The question is whether the ocean is warmer when it is exposed to the ~360 W/m2 of downwelling radiation from the atmosphere, or if it would be warmer if it were exposed to the ~ 3W/m2 of radiation from outer space … the answer to that is quite obvious, and doesn’t involve any violation of the second law.
As I mentioned above, when you come in from the outside in the winter, your coat is much colder than you are … and yet you wear it to keep warm. Does this mean that a cold object (the coat) is warming a warm object (the person)?
Absolutely not, that would be a violation of the second law. We wear a coat because it leaves us warmer than the alternative of no coat.
And similarly (although for very different physical reasons) the GHGs in the atmosphere leave the world warmer than the alternative of no GHGs. Doesn’t mean a cold object warms a warm object, that’s not possible. It just means it leaves it warmer than having no GHGs.
w.
–The question is whether the ocean is warmer when it is exposed to the ~360 W/m2 of downwelling radiation from the atmosphere, or if it would be warmer if it were exposed to the ~ 3W/m2 of radiation from outer space … the answer to that is quite obvious, and doesn’t involve any violation of the second law.–
I don’t think it’s obvious.
But if you looking out a window of ISS into the blackness of the universe, I don’t your face would feel any cooler as compared looking out window in a house on Earth. Nor if touched the glass in space would it be any colder than a window on Earth.
And water is gas or solid in the vacuum of space. Or water would rapidly freezing and will cool to about -150 C rather quickly- but that is cooling from evaporation.
But for water near 0 C it does not need a lot of pressure to remain liquid. Or a strong plastic bag could withstand the pressure. So if had a clear strong mylar [other kinds of plastics might also evaporate] bag and filled it with water which was 5 C it will not instantly freeze.
And I am not sure how long it would take to freeze. It could take hours.
Or another thing water will form into a sphere if not touch something in microgravity. So one have say mylar bag 1 meter in diameter and put a 10 cm diameter sphere of water in the middle of it.
Put it in environment with say 2 psi- so bag of air will have 2 psig in a vacuum and have water sphere is in to middle of it.
And what happens?
I would guess the air in the bag would have to get to 0 C before the water could freeze. And I would guess it would take more than 3000 seconds before any ice formed on the outside of the sphere. And it would form on the outside of the sphere, first.
Anyone want provide another guess?
Or maybe someone done something like this already.
Willis, you (&warmists) keep on replacing isolation with a source of heat.
These are 2 different things and behave differently.
It is possible to replace one with the other – it may give almost same result for limited term, small variations, but will run into errors on long term or higher variations.
Repeating over and over again that isolation does help keep things warm will not fix the fact isolation is not a source of heat itself.
[“Isolation” or “Insulation” or (as I suspect) “Insolation” ? .mod]
Willis Eschenbach, December 13, 2014 at 10:21 pm: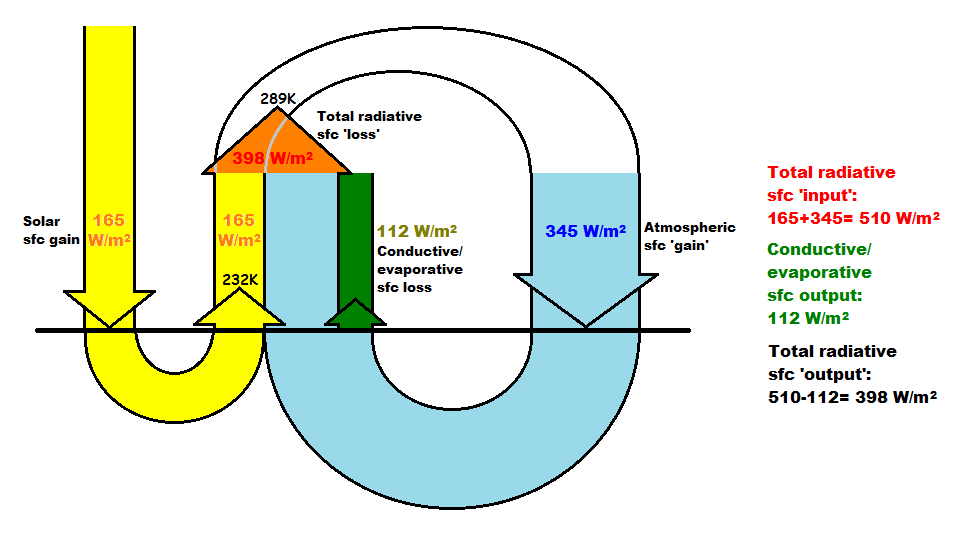
“The second law of thermodynamics doesn’t come into it, because you are asking the wrong question. The question is not whether “a cooler atmosphere can warm a warmer ocean”.
The question is whether the ocean is warmer when it is exposed to the ~360 W/m2 of downwelling radiation from the atmosphere, or if it would be warmer if it were exposed to the ~ 3W/m2 of radiation from outer space … the answer to that is quite obvious, and doesn’t involve any violation of the second law.”
This is saying the exact same thing, Willis. The warmer surface ends up having a higher temperature solely because of your 360 W/m^2 energy transfer from the cooler atmosphere. That means you transfer energy from a cool system to a warm system specifically to raise the temperature of the warm system. A 2nd Law violation could hardly be any more obvious than that …
“(…) when you come in from the outside in the winter, your coat is much colder than you are … and yet you wear it to keep warm. Does this mean that a cold object (the coat) is warming a warm object (the person)?
Absolutely not, that would be a violation of the second law. We wear a coat because it leaves us warmer than the alternative of no coat.”
Yes, because it insulates us, making LESS ENERGY LEAVE our body per unit of time, almost exclusively by impeding convective/evaporative loss.
“And similarly (although for very different physical reasons) the GHGs in the atmosphere leave the world warmer than the alternative of no GHGs. Doesn’t mean a cold object warms a warm object, that’s not possible. It just means it leaves it warmer than having no GHGs.”
No, this is not what’s being claimed, Willis. You claim that the extra energy INPUT from these gases in our cooler atmosphere to the warmer surface is what makes the surface temperature 289K (from [165+345-112=] 398 W/m^2) rather than 255K (from 239 W/m^2) or 232K (from 165 W/m^2). The direct transfer of energy from a cooler object to a warmer object alone makes the temperature of the warmer object rise in absolute terms. This is a definite violation of the 2nd Law.
Insulation doesn’t work by the cooler layer feeding the heated object with more energy to warm it. It works by reducing the energy going OUT from the heated object per unit of time.
There’s a very important distinction to be made between these two scenarios. For the surface of the Earth, if you want to claim that it warms beyond pure solar radiative equilibrium by virtue of the “back radiation” from IR-active gases in the atmosphere, then it is this ‘cool’ atmospheric energy that piles up at/below the surface. Because there is no obstruction at any point of the outgoing IR from the surface. It is always free to leave.
If you instead were to claim that the atmosphere forces the surface to warm some more simply from having a higher temperature potential than space, making the transfer of energy from the warmer surface to the cooler atmosphere smaller per unit of time, then it is the ‘hot’ incoming solar energy that piles up at/below the surface. (Which is what actually happens in the real world.) Because now the absorbed solar energy isn’t able to escape back out again as fast as before. This was never the case in the first scenario.
Conclusion: You can’t transfer ‘cool’ energy to a warm object for energy gain, meaning an absolute increase in the warmer object’s ‘internal energy’ [U] and thus temperature [T]. Such accumulation with such a direct result must (by the laws of thermodynamics) come from a source hotter than the warm object. Because this would constitute a ‘heat transfer’ …
So you see, the atmospheric warming EFFECT is real. It does insulate the heated surface. But the “back radiation” (DWLWIR) EXPLANATION of how this effect comes about is wrong. Because it clearly violates the laws of thermodynamics. The EFFECT itself doesn’t. The “back radiation” EXPLANATION of it does.
[“Isolation” or “Insulation” or (as I suspect) “Insolation” ? .mod]
Thanks mod, sorry for that – “thermal insulation” should have been there, not “isolation”
http://en.wikipedia.org/wiki/Thermal_insulation
Kristian December 14, 2014 at 6:04 am
Willis Eschenbach, December 13, 2014 at 10:21 pm:
Conclusion: You can’t transfer ‘cool’ energy to a warm object for energy gain, meaning an absolute increase in the warmer object’s ‘internal energy’ [U] and thus temperature [T]. Such accumulation with such a direct result must (by the laws of thermodynamics) come from a source hotter than the warm object. Because this would constitute a ‘heat transfer’ …
You can via radiation because radiation transport does not depend on a temperature gradient.
A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.
Kristian 6:04am: “Conclusion: You can’t transfer ‘cool’ energy to a warm object for energy gain..”
Yes, you can. So inaccurate conclusion. Physics allow this, no 2LOT violation as you say since our warmer eyes can see cooler ice cubes absorbing their emitted radiant energy. Our warmer retinas absorb the” ‘cool’ “energy from the ice just fine. Focused rays thru a lens of ice can ignite flammable substance. Energy transfers both ways; 2LOT: net energy is one way to always increase entropy in real objects.
Phil., December 14, 2014 at 7:23 pm:
“You can via radiation because radiation transport does not depend on a temperature gradient.”
Yes, I know that in you people’s magical, pink little bubble world, radiation is free to violate the Laws of Thermodynamics, that it can do wonders and miracles. Fine. But I’m afraid your magical, pink little bubble world isn’t the real one. Out here in the real world, radiative transfers and their resulting effects must comply with the Laws of Thermodynamics before anything else, just like conductive transfers and their resulting effects have to. In the real world, in nature, it is not allowed for an energy transfer from a cool place to a warm place to make the temperature of that warm place rise. It simply doesn’t happen. If you observe something where it might LOOK like it does, it is your interpretation of what happens that’s wrong. Case in point:
“A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Your last sentence here describes a perfect violation of the 2nd Law of Thermodynamics, Phil. I’m surprised (or not) that you don’t realise yourself. It is pretty obvious. It’s in the very words you use. If you transfer energy (in this case, by radiation) from a cooler object to a warmer one … to HEAT it, then you’ve transferred HEAT to it. This cannot happen in nature.
The EFFECT you describe above is indeed real enough. Your “heating by back radiation” EXPLANATION of it, however, is blatantly wrong. Because it demands a direct violation of the 2nd Law.
Trick says:
A very precise and elegant example Trick
It is easy to be confused by the second law of thermodynamics. A net transfer of energy from a cold object to a warm would be a violation, but energy flows in both directions, only more from hot to cold than the other way.
/Jan
Kristian December 15, 2014 at 3:00 am
Phil., December 14, 2014 at 7:23 pm:
“A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Your last sentence here describes a perfect violation of the 2nd Law of Thermodynamics, Phil. I’m surprised (or not) that you don’t realise yourself. It is pretty obvious. It’s in the very words you use. If you transfer energy (in this case, by radiation) from a cooler object to a warmer one … to HEAT it, then you’ve transferred HEAT to it. This cannot happen in nature.
No, it just shows your ignorance of the correct application of the Laws of Thermodynamics to radiational heat transfer, I suggest you read up on it, ‘Hottel and Sarofim’ would be a good start.
Two bodies in radiational equilibrium with each other are both radiating proportional to the fourth power of their surface temperature. Raise the temperature of one of the bodies and the temperature of the other will rise accordingly, this does not violate any law of thermodynamics! Thus in the thermocouple example replacing a surface at 300K by a surface at 1000K (the radiation shield) causes the ThC to be hotter and register a higher temperature.
The EFFECT you describe above is indeed real enough. Your “heating by back radiation” EXPLANATION of it, however, is blatantly wrong. Because it demands a direct violation of the 2nd Law.
There is no violation of the 2nd Law, since you think the effect is not the result of radiational exchange, which has long been the accepted reason, perhaps you could explain the phenomenon.
See here for example:
http://eyrie.shef.ac.uk/eee/cpe630/comfun2.html
Phil., December 15, 2014 at 8:36 am:
“No, it just shows your ignorance of the correct application of the Laws of Thermodynamics to radiational heat transfer, I suggest you read up on it, ‘Hottel and Sarofim’ would be a good start.”
Sorry. I cannot but laugh! Phil, there are no concessitons made in the Laws of Thermodynamics for ‘radiational heat transfer’. You wrote: “Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Again, there is nothing wrong with the EFFECT you describe. It is real enough. But you EXPLAIN it like this: “… the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
No, Phil. No. No! NO!!! Read your own words again. And again. Until you understand.
How is it even possible for a grown person to not see what’s wrong with this picture!? You’re explicitly stating a violation of the 2nd Law here. And you just go on to argue that I am ignorant of the ‘correct application’ of this law when it comes to ‘radiational heat transfer’. HEAT is HEAT whether it is transferred by conductive, convective or radiative means. And the transfer of HEAT from one system to another has a very distinct effect indeed. Do you know what it is, Phil? And this effect, in nature, is only allowed to result in ONE particular distributional pattern. Can you guess which one, Phil?
It makes no difference what you call it. If you transfer energy to a system to make it warmer than what it was before, you have transferred HEAT to it (if not ‘work’). And HEAT cannot spontaneously transfer from a cold to a hot place. Radiation or no radiation.
Look, if you postulate two opposing ‘fluxes’ in a radiative heat transfer between two surfaces, the vector sum of which make up the ‘net radiation’, the net flow or transfer of energy between them, then it is your NET flow (which is equal to the radiative HEAT) which is doing the heating and cooling. Nothing else. No other ‘flux’. This is what makes it the HEAT. Get it, Phil?
And the ‘heating’ invariably occurs in the cooler object (from energy GAIN), the ‘cooling’ invariably in the warmer object (from an equal energy LOSS). Because HEAT always spontaneously moves from hot to cold, never the opposite way.
This is the REAL, the ACTUAL transfer of energy between the two objects at different temperatures in a heat transfer.
In the real world, not in the conceptual world, the HEAT FLUX is indivisible. All there is. It is ONE flow, one transfer of energy. There is no way you can physically split it into two separate, oppositely flowing streams of energy. The HEAT itself is all you’ll ever register. Meaning, the individual ‘hemifluxes’ that conceptually (according to the archaic (caloric theory-derived) bidirectional principle) make up the ‘net flux’ (the HEAT), are not themselves real, separate fluxes/transfers of energy. They are purely mathematical constructs (see below).
If you claim (as you definitely seem to be doing) that both of these are real and BY THEMSELVES AND SEPARATELY would achieve the same result as the ‘net’ of the two (the HEAT), namely heating/cooling of the two systems, then you are effectively saying that ‘HEAT GOES BOTH WAYS AND HEATS IN BOTH DIRECTIONS’, only more goes from hot to cold than from cold to hot. Which is clearly absurd and at odds with reality. The Laws of Thermodynamics do not allow it.
What’s the point in your world in emphasizing the ‘net flux’ (the heat) as being the only one that matters to the Laws of Thermodynamics, if there is no difference between the result it gives and the result the two conceptual hemifluxes supposedly comprising it gives? Then they are all ‘heats’.
“Two bodies in radiational equilibrium with each other are both radiating proportional to the fourth power of their surface temperature. Raise the temperature of one of the bodies and the temperature of the other will rise accordingly, this does not violate any law of thermodynamics! Thus in the thermocouple example replacing a surface at 300K by a surface at 1000K (the radiation shield) causes the ThC to be hotter and register a higher temperature.”
Yup, because you raise the ‘temperature potential’ of the cooler opposing surface, thus creating a gentler potential gradient away from the hotter (and externally heated) object. That’s what you see here:
P/A = e s (Th^4 – Tc^4)
On the righthand side you see two opposing ‘temperature potentials’, the potential being the HEAT FLUX each object, thermally isolated from the other, would emit to a perfect vacuum at 0 K. On the lefthand side you see the ACTUAL flux/transfer of energy between the two, the HEAT FLUX, its intensity determined by the difference in the two temperature potentials. The righthand side of this equation simply shows the mathematical operation needed to estimate the actual flux in the heat transfer.
“… since you think the effect is not the result of radiational exchange, which has long been the accepted reason, perhaps you could explain the phenomenon.”
Explained above. There is a change in the potential gradient through the radiation field between the heated object and its surrounding layer. Therefore there will be less energy moving per unit of time from this hot object to the nearby cooler one. The transfer of energy still (always) goes only one way, from hot to cold, but when cold becomes not so cold as before, the transfer is reduced. Simple as that.
Again, if your EXPLANATION of an observed physical effect ends up violating the Laws of Thermodynamics, then it’s wrong. Your explanation does end up violating the Laws of Thermodynamics. Mine doesn’t.
Hehe, that novel term ‘concessitons’ was of course supposed to be ‘concessions’.
Kristian December 15, 2014 at 1:58 pm
Phil., December 15, 2014 at 8:36 am:
“No, it just shows your ignorance of the correct application of the Laws of Thermodynamics to radiational heat transfer, I suggest you read up on it, ‘Hottel and Sarofim’ would be a good start.”
Sorry. I cannot but laugh! Phil, there are no concessions made in the Laws of Thermodynamics for ‘radiational heat transfer’. You wrote: “Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Again, there is nothing wrong with the EFFECT you describe. It is real enough. But you EXPLAIN it like this: “… the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
No, Phil. No. No! NO!!! Read your own words again. And again. Until you understand.
I do understand, unfortunately you continue to spout a load of pseudoscientific garbage which bears no resemblance to radiational heat transfer and the Second Law!
In the real world, not in the conceptual world, the HEAT FLUX is indivisible. All there is. It is ONE flow, one transfer of energy. There is no way you can physically split it into two separate, oppositely flowing streams of energy. The HEAT itself is all you’ll ever register. Meaning, the individual ‘hemifluxes’ that conceptually (according to the archaic (caloric theory-derived) bidirectional principle) make up the ‘net flux’ (the HEAT), are not themselves real, separate fluxes/transfers of energy. They are purely mathematical constructs (see below).
Far from being ‘mathematical constructs’ they are two separate flows, electromagnetic radiation flowing in both directions.
In the case of the thermocouple I cited you’ll see bright yellow light emitted in all directions which can be measured. Put the shield in place and it also emits yellow light in all directions, INCLUDING TOWARDS THE ThC, which can also be measured. Net energy flow as per the Second Law flows from hot to cold, the ThC will be slightly brighter than the shield. According to your weird theory the light emitted in the direction of the ThC somehow ceases to exist.
Phil., December 16, 2014 at 6:36 am:
“I do understand, unfortunately you continue to spout a load of pseudoscientific garbage which bears no resemblance to radiational heat transfer and the Second Law!”
This is a non-argument, Phil. It is just saying “DOES TOO”! You are not arguing. Why should the 2nd Law not apply to radiative heat transfer? Heat cannot move spontaneously from cold to hot. This is a thermodynamic absolute. If you know the thermodynamic definition of ‘heat’, then you know what it does. And you would know that a transfer of energy from cold to hot could never do in nature what you claim it does. Because it would constitute a transfer of HEAT.
You expect both of your ‘hemifluxes’ making up the NET, the ‘heat’, to give results as if they themselves were heat fluxes. Sorry. No go.
“Far from being ‘mathematical constructs’ they are two separate flows, electromagnetic radiation flowing in both directions.”
No, they are not. I too can play this game, Phil.
You just continuing to postulate it as truth doesn’t make it so.
<em"In the case of the thermocouple I cited you’ll see bright yellow light emitted in all directions which can be measured. Put the shield in place and it also emits yellow light in all directions, INCLUDING TOWARDS THE ThC, which can also be measured."
No, it can NOT be measured. It is CALCULATED. From the HEAT FLUX and TEMPERATURE. Are you being willfully obtuse?
“Net energy flow as per the Second Law flows from hot to cold, the ThC will be slightly brighter than the shield.”
Yes, you call it ‘net energy’. And your ‘net energy’ is all you’ll ever register, detect. It is the HEAT FLUX. Everything else you will have to CALCULATE based on your preconceived assumptions.
“According to your weird theory the light emitted in the direction of the ThC somehow ceases to exist.”
It is not a weird theory, Phil. It is reality. It is what we actually observe. The ‘weird theory’ is YOUR interpretation of this reality.
You are a person who truly believes that a transfer of energy from a cold to a hot place will raise the temperature of the hot place, not just in relative, but in absolute terms. This means you don’t understand the first thing about the basic Laws of Thermodynamics. And hence you are a person whose arguments cannot be taken seriously, Phil. I’m sorry.
Phil. says:
December 14, 2014 at 7:23 pm
You can via radiation because radiation transport does not depend on a temperature gradient.
A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.
Phil, you cannot transform insulation in a source of heat. This is why your ThC will never get warmer then the flame, no matter how much insulation you put around it.
If the insulation would be a source of heat as in your pink universe, then the ThC could get warmer then the flame.
It does not.
No matter what backradiation construct is done around it,, it will not get warmer then the source of heat = the flame. because insulation is not a source of heat, but a reduction in the heat flow.
Jeez.
Willis and Kristian.
I respect your efforts here and elsewhere and both of you are right in certain respects but nonetheless you both miss the essential issue.
Once convective ascent begins it requires an equal and opposite convective descent.
Convective ascent takes energy away from the surface that would otherwise have radiated to space and in the process converts kinetic energy (heat) to gravitational potential energy (not heat).
Convective descent the converts gravitational potential energy (not heat) to kinetic energy (heat) to the surface that can then be radiated to space.
Convective descent places adiabatically warmed air above the surface which reduces or eliminates (in an inversion) the lapse rate slope so that convection is in turn suppressed or eliminated.
Reducing or eliminating convection allows incoming solar radiation to raise surface temperature above that predicted by the S-B equation.
The mass of an atmosphere is the factor that allows convective ascent and descent NOT the radiative capability of that atmosphere.
Radiative fluxes between atmosphere and surface are a consequence of atmospheric mass subjected to external insolation and not a cause of the surface temperature.
The mass of an atmosphere acts exactly like a glass greenhouse roof in the descending convective phase.
The descent of adiabatically warmed air dissipates clouds so as to allow more solar radiation to reach the surface. That is equivalent to the transparency of a glass greenhouse roof.
The adiabatically warmed air above the surface reduces convection in exactly the same way as does a solid greenhouse roof.
The greenhouse effect was always an accurate description of how the mass of an atmosphere in adiabatic descent inhibited convection from the surface and thereby raised surface temperature above S-B.
The radiative theory is a complete dead end which offends basic thermodynamics.
That mass based description is consistent with Willis’s own thermostat hypothesis because it explains how the mass of an atmosphere uses the adiabatic warming of descending air in one location to trigger emergent convective phenomena in another location.
The observed global pattern of descending surface high pressure cells and ascending surface low pressure cells is the process in operation.
Change the proportion of GHGs in the atmosphere and all one does is change that circulation for a zero net change in surface temperature and since it is a matter of mass rather than the proportion of GHGs we could never measure the change from our emisssions..
–Stephen Wilde
December 14, 2014 at 10:00 am
Jeez.
Willis and Kristian.
I respect your efforts here and elsewhere and both of you are right in certain respects but nonetheless you both miss the essential issue.
Once convective ascent begins it requires an equal and opposite convective descent.
Convective ascent takes energy away from the surface that would otherwise have radiated to space and in the process converts kinetic energy (heat) to gravitational potential energy (not heat).
Convective descent the converts gravitational potential energy (not heat) to kinetic energy (heat) to the surface that can then be radiated to space.
Convective descent places adiabatically warmed air above the surface which reduces or eliminates (in an inversion) the lapse rate slope so that convection is in turn suppressed or eliminated.–
[Agree to all above.
And then I wonder about the wording which follows:]
–Reducing or eliminating convection allows incoming solar radiation to raise surface temperature above that predicted by the S-B equation.–
I would instead say the “Reducing or eliminating convection” is the point when S-B equation applies to the surface. Or the convection process dominates before this point is reached.
Or the atmosphere is joined to the surface.
Or surface is a VW bug and atmosphere is massive trailer it’s pulling. And/or one could say as analogy the S-B equation is measuring the weight of VW bug but not the weight of the trailer.
Or less of an analogy, the kinetic energy of VW bug and not the trailer
Or another way to say it, is only once one has “Reducing or eliminating convection” then can the S-B equation starts to appear to work. Or the surface radiate less energy to space until the point where one is at the point of “Reducing or eliminating convection”.
Or S-B equation measures or concerned about a surface and when there is an atmosphere “the surface” includes the atmosphere which warmed and cooled each day. And this “thick surface” radiate mostly at it’s bottom and not much at the top of the “surface” and convection process is going to make the top and the bottom the same energy [per molecule of gas- it has same kinetic energy at bottom as at the top- and difference temperature due to difference of air density].
Though perhaps not sure what you referring to by S-B equation, because basically if stop convection losses then one can get the temperature predicted by the S-B equation. Such as
with sealed boxes or solar ponds- which work by stopping convection of water heated by sunlight, and so thereby get the max temperature predicted- a couple feet under the water of the solar pond’s surface.
An irony of all this is that Willis and I were debating from exactly opposite sides of the first law not so very long ago when he was claiming adiabatic warming from the mass of the atmosphere was a violation thereof. The difference there was that adiabatic warming takes place outside the realm of radiation. There are probably “outside the realm of radiation” effects hiding in these radiation budgets, but lets put all the cards on the same table.
Can’t see how that formats until I press post, but assuming it’s readable you can see that there is little difference between the ocean budget and the planetary budget and if one is a bit generous they are basically all the same. We are talking about a huge accounting problem on the scale of one TSI, which happens to be what atmospheric back radiation amounts to.
To my mind it all boils down to what I’ve annotated as the photon food fight. The circularity of this process is also shown in Kristian’s graphic. It’s a bit of a stretch but this process can be thought of as a cold plasma. Photons flung frenetically. The one TSI is absorbed by the ocean skin, but it does not warm it except when latent losses cool the surface to below atmospheric emission temperature. It just keeps it from cooling below the very same atmospheric emission temperature.
In this sense the average emission temperature of IR resonating atmospheric gasses, with the same power as the sun, controls ocean temperature. Yet we come full circle because the atmosphere derives 75% of its energy from the ocean. Like I said, a plasma…
Let me try this again. Here’s a planet in space, heated by an internal nuclear reaction that delivers 235 W/m2 at the surface. We’ll assume it is a blackbody.


This planet is at equilibrium. The natural reactor in the core of the planet is generating energy that at the planet’s surface amounts to 235 W/m2. It is radiating the same amount, so it is neither warming nor cooling.
Now, imagine that without changing anything else, we put a blackbody steel shell around the planet. The next graphic shows that situation, with one side of the shell temporarily removed so we can look inside.
Note that for the sake of the graphic, I’ve greatly exaggerated the distance from the planet to the shell. In fact it’s lets say a metre above the surface, so there’s only about 0.0001% difference in the areas, which for the sake of this first principles analysis we can ignore.
Now, note that in the second graphic, there is additional radiation striking the surface of the planet, radiation that was NOT present there in the first graphic. This inward-bound radiation exists because the shell is warmed by the radiation striking it from the inside.
So my question is …
Does the new radiation being absorbed by the planet leave the planet a) warmer, b) cooler, or c) the same temperature as before we added the shell?
Please start by explicitly saying whether you think the answer is a), b), or c) … and then explain why.
Best to all,
w.
How about this?
..
http://www.principia-scientific.org/the-relentless-pseudo-science-of-wuwt.html
David Socrates December 15, 2014 at 4:35 pm
David, thanks for the link to the discussion of my ideas over at Principia Anti-Scientifia. I can do no better in answer to your (and their) questions about the Steel Greenhouse than to quote what Dr. Robert Brown said in response to their ascientific claims over at your link:
Also look at what Joel Shore (jshore) has to say at their post. Look, I’ve disagreed with Dr. Brown a few times in the past … and almost always I was wrong. He’s a very bright guy. And Joel Shore someone who I’ve disagreed with any number of times … but he’s also a very bright guy. And unlike me, they are both physicists, Joel (I think) in the private sector and Robert teaches physics at Duke.
So when we all agree, it’s not that often, and because we do in this case … well, you might profitably consider the arguments that all of us are making in favor of the Steel Greenhouse not violating any laws in any fashion.
My restatement and clarification is here.
All the best,
w.
You can wrap the core in as many shells as you wish, the amount of energy radiated will remain the same. That’s a result of the first law of thermodynamics.
..
However, if each shell is 1 meter in height, eventually the 235 w/m2 will drop, as the area of the shell’s outer surface increases.
David, was there a part of this that you didn’t understand?
w.
If you are looking from the outside, the net outflow is 235 w/m (unless the surface area is increasing due to the shells)
…
Where are you placing your thermometer?
…
What happens inside the “iron shell” doesn’t really matter, the overriding issue is the 1st law of thermodynamics as viewed from the OUTSIDE.
..
Remember, the Earth is a closed thermodynamic system when viewed from the TOS
..
http://www.bluffton.edu/~bergerd/nsc_111/thermo2.html
David Socrates December 15, 2014 at 5:16 pm
Thanks, David. The temperature is taken at the planetary surface.
The overriding issue is surface temperature of the planet … because that’s where we live.
w.
Then tell us….what are the properties of the “iron sphere”….does it allow the transfer of energy in either direction without impeding it, or does it have an insinuative value.
You see, the core of the sun is at muti-milions of degrees C, bu the surface is just roughly 5800K The problem you have with your “iron core” model is that temperature is not a reliable measure of the flow of energy. I suggest you re-phrase the question as……”Is the flow of energy at the surface of the planet less, more or the same as the flow at the outer surface of the iron sphere”
The problem you have is you are focusing on temperature and forgetting to model the flow of energy.
In other words, the question you ask is irrelevant.
–So my question is …
Does the new radiation being absorbed by the planet leave the planet a) warmer, b) cooler, or c) the same temperature as before we added the shell?–
Any such planet could be considered to have shells before it’s radiated thru the atmosphere to this blackbody shell.
This added shell will not heat anything on the surface. It will not melt ice of a puddle and it will increase evaporation of the water.
Without the blackbody shell one could radiate heat so you make something which is cooler. Or one insulate against the heat from the surface and radiate something into a 2 K universe, and thereby passively cool something to say 100 K. So shell stops you from doing this- or you would have to get on the other side of the shell to do this.
–Please start by explicitly saying whether you think the answer is a), b), or c) … and then explain why.–
C.
Though it’s eliminated the possibility of cooling things by passive cooling. Though active refrigeration is about heat lost to convection of air, and so doesn’t stop this.
How about if half the world had geothermal heat.
The blackbody shell would help heat the side of planet without the geothermal heat- but not by much. And atmosphere would probably add as much heat to cool side as the blackbody shell
would.
Noooooooooooooooooooooooooooooooo! Not the steel shell again! Lol. How many comments did that post get?
Let me try to explain where people seem to be getting stuck. It is true that heat only ever flows from warm to cold. However, heat is the NET of the individual flows. Here’s an example from another arena. Suppose that I owe you a hundred bucks, and you owe me seventy five dollars. We decide to settle the debt. Here’s two views of the situation:

Now you can see that both of the situations are the same. One shows the individual flows, and the other shows the net flows. Now, the net flow of money goes from me to you. But it is identical to the result of considering the individual flows.
Now, if you didn’t owe me the $75 then I’d end up seventy five bucks richer …
Here’s the thing. The situation regarding radiation heat flows is IDENTICAL to the flows of money. If we have two planets radiating in both directions, one radiating 100 W/m2 and the other radiating 75 W/m2, there is a net flow of 25 W/m2 from the warmer to the cooler.
And if there were no 75 W/m2 flow from the cooler to the warmer planet, it would end up warmer by that amount … and there’s no violation of the Second Law either way.
w.
Well, Willis, you ignore the physics of the sea surface and the IR absorbency characteristics of the same. The sea surface absorbs IR from CO2 in the top 3 microns.
This is where your attention should be focused but for some reason you can’t do that. You go off on a tangent.
mpainter, perhaps that’s what YOU should be focused on. I focused on that earlier in the post. At this point I’m trying to get past the common misconception that a poor person can’t make a rich person richer than they would be without the poor person.
Which is the same as saying that the Earth’s surface is warmer because of the existence of the GHGs in the cool atmosphere than it would be without those GHGs.
So no, it’s not a tangent. It’s a different part of the same discussion.
And yes, the sea surface absorbs the DWIR in the skin … and that ends up leaving the entire mixed layer warmer than it would be without the DWIR..
w.
There is no mixing of the micro layer, Willis. That is the whole point. Has to do with rheology and the surface characteristics of water. And there is no conduction downward because of the temp. profile of the sub skin layer (under insolation, that is. The profile alters at night).
This is where the rubber meets the road but you are up in the wild blue yonder.
Are the sentences preceded by an ellipses bass ackwards ?
“If we have two planets radiating in both directions, one radiating 100 W/m2 and the other radiating 75 W/m2, there is a net flow of 25 W/m2 from the warmer to the cooler.”
We know of no planets which do anything like this.
We have two notable binary planet-like bodies. Earth and the Moon. And Pluto and it’s largest moon.
We also have some very large planets- the gas giants. And they have comparatively little moons.
So on these comparatively little moons, which can be a big as our moon, their gas giant may occupy a large part of their sky. And doubtful such large visibly apparent bodies are radiating much energy at this moon and far less likely the moon as radiating anything significant at the gas giant.
Take Saturn and it’s moon, Titan.
Saturn is 58,232 km radius. Or 116,464 km diameter
Earth is 6,371 km radius. Or 12,742 km diameter
http://nssdc.gsfc.nasa.gov/planetary/factsheet/saturnfact.html
And our Moon is 3475 km in diameter or 3 to 4 times smaller than Earth..
A thumb held at arm reach blocks the Moon, though from the lunar surface
your thumb doesn’t block Earth.
And replaced Saturn with Earth, you need to hold up something like a basketball to block it.
But Titan is about 1.2 million km from Saturn [Our Moon is .38 million km from Earth].
Saturn from it’s moon, Titan, looks about 3 to 4 times bigger than Earth from
our moon.
[Or about 10 times larger than our Moon looks like from Earth.]
And from Saturn looking at Titan it’s about 1/3 smaller and a lot dimmer than
our Moon looks like as observed from the Earth.
And blackbody temperature of Saturn: 81.1 K
And Earth is 254 K.
http://nssdc.gsfc.nasa.gov/planetary/factsheet/saturnfact.html
Or Earth will warm our Moon more than any other body, and our Moon warms Earth more
than any moon warms [radiantly] any other planet.
And it’s not in the range of more than 10 watts per square meter [in either direction]
Here is what the rGHE/AGW proponents claim: You can just ADD the DWLWIR ‘flux’ as an extra INPUT of energy to the surface, as an addition to the original solar heat flux, thus directly creating extra warming (as if they were two of a kind, as if they were both heat fluxes); 165 W/m^2, 232K >> [165+345-112=] 398 W/m^2, 289K.
If I get 100$ from my bank and then hand them all to my friend, upon which he hands 90 of them straight back, then I end up with 90 dollars.
The rGHE “back radiation” argument then goes as follows: I get 190$ IN, but give away 100$, so end up with 90$. The 190 dollars IN are counted like this: 100$ from the bank + 90$ back from my friend; the 100 dollars OUT are simply the 100$ I hand over to my friend.
In this world view it would thus seem that there is 190$ in circulation. But we all know that there is only the 100$ originally from the bank available.
The rGHE “back radiation” hypothesis creates extra energy out of nothing. Just to make their preconceived radiative (Stefan-Boltzmann) numbers add up. There simply is no extra radiative INPUT of energy from the atmosphere to the surface. No more than there being a conductive or evaporative (latent) input of energy from the atmosphere to the surface.
Willis
I am very pleased to see that you have returned to this article and provided your further views. Many of these are a restated of the views that you have expressed before in the past on other articles that have raised similar issues.
However, your response is not particularly helpful, since I suspect that we all understand the theory, and what is claimed of the theory. But that is not the issue. To just state the theory does not in any way prove what is claimed of the theory is in practice, correct.
The question is, does all this DWLWIR have the ability to do real work in the environ in which it finds itself? Just repeating the theory, and what is claimed of it, does not answer this; what you say is theoretical, and theoretical only, whereas what is needed is empirical observational data of real work done.
As far as I am aware, no one is doubting the measurements (subject to errors factors), but rather questioning what does this do. Whilst not an exact analogy, I can measure 12 volts on a flat car batter, but as soon as I seek to draw off some load, the voltage may drop, in the limit, to nil. There is a signal of 12 Volts on the flat battery, but in practice it does not have the ability to do any work, not even to run the indicator.
I do not doubt for one momemet that we can measure DWLWIR, but that does not mean that it does work in the environ that it finds itself. It may be a signal incapable of performing sensible work, and this is why no engineer has sought to tap into this power source which would solve the world’s energy demands in a clean/green manner.
As I have been saying to you for years, what is going on in the top microns of the oceans? What are the physical processes involved and at what rate are they handling energy? I want to know the physics and how energy is moving around.
As I say, may be there is some form of photonic exchange at the boundary surface layer. This would therefore be at a molecular level and if this is what is happening DWLWIR never enters the ocean. If that is the case, it would appear that it does not heat the ocean, and the present claims by teh warmists (which is the subject of Bob’s article0 is a non starter. .
Alternatively, DWLWIR is absorbed in accordance with its optical absorption characteristics which means that at about 200 W/m2 to 300 W/m2 is absorbed in just 3 microns (and this is on average figures for DWLWIR, it will be gigher in the tropics). That is a heck of a lot of energy concentrated in a very small volume of water, and unless this energy is quickly dissipated to volume, it would drive copious evaporation (which we do not see). Now what happens to that energy?
In the past you have suggested it is mixed by the action of wind, the action of waves and ocean over turning, but these are slow mechanical processes, and ocean over turning may be diurnal at that. I have severe reservations that such slow mechanical processes can sequester to depth all this energy at a rate fast enough to prevent the rapid and copious evaporation which would occur from the top microns if there is some 200 to 300 W/m2 of energy, capable of doing real work, absorbed in these microns. See my comment upon sea states at richard verney December 13, 2014 at 2:12 am. which considers the problems caused by sea conditions of say BF3 and less, or for that matter BF 8 and above.
To try and assess how effective the sl;ow mechanical processes that you invoke for mixing the energy that you claim is going into the oceans and preventing the oceans from freezing (ie., the slow mechanical processes of wind, waves and ocean over turning), I suggested that you should look at crater lakes (ie., lakes in caldera) which are usually protected from wind such that there is little mixing of the top layer through the action of wind and waves.
I also suggested that you should consider dew that lingers all day in the shady side of a hollow om a still winters day, yet which is burnt off in just an hour or so on the sunny side of the same hollow. The sunny side and shady side of the hollow are exposed to the same environmental conditions save other than sunshine, yet solar energy can burn off dew in hours and yet DWLWIR cannot do so all day even though the total DWLWIR over the course of a day is greater than the energy that the sunny side of the hollow received in just an hour or so of sumn up.
On a still winters day, there is no mixing of DWLWIR that is absorbed by the dew by the slow mechanical actions of wind and waves since those processes are not present. See my comments at: richard verney December 12, 2014 at 6:46 pm and richard verney December 13, 2014 at 1:30 am
Why not add to the equation ice. In ice there is no mixing (by wind, waves, ocean overturning) of DWLWIR that is absorbed in the first few microns of the surface that is exposed to DWLWIR. So why does ice not quickly melt during the night on a still night when the air temperature is just below freezing. Why os there not a pool of liquid water in the centre of the ice slowly spreading outwards?
I await your comments on the real world that we inhabit.
richard 5:56pm: “The question is, does all this DWLWIR have the ability to do real work in the environ in which it finds itself?….So why does ice not quickly melt during the night on a still night when the air temperature is just below freezing….await…comments on the real world that we inhabit.”
Yes LWIR can do real work in this real world as is shown by real world experiment in the picture below – night frost (or your ice) formation can be governed by the differing sky & surrounding LW radiation absorbed in still air. Explained in an “Essay on dew” by Dr. Craig Bohren in this google clip:
https://books.google.com/books?id=gHTDAgAAQBAJ&pg=PT111&lpg=PT111&dq=fig+9.2+bohren&source=bl&ots=jwicXAdT-z&sig=UiWp2bMjK7zxLfig1MO6lK6HjXo&hl=en&sa=X&ei=a6mPVNmGDdKdyATH9IHACQ&ved=0CB4Q6AEwAA#v=onepage&q=fig%209.2%20bohren&f=false
Ever seen frost on top of a car left outside at night, but not on the sides? I have. (No, frost does not fall from the sky.) Bring the top of the post down to ground level and understand why frost can linger in your hollow and the shade of trees as the sun rises.
–Ever seen frost on top of a car left outside at night, but not on the sides? I have. (No, frost does not fall from the sky.) Bring the top of the post down to ground level and understand why frost can linger in your hollow and the shade of trees as the sun rises.–
Only frost I have seen fall from the sky is called snow.
But that frost/snow formed on top of the post. So question is why does water condense and form
ice or just sublimate from gas into ice.
When water forms into ice it has latent heat. So shiny or smooth surfaces do not radiate heat as well as rough surfaces. So for ice to form need something cold or something that radiates heat well.
So dew does settle from above it onto surfaces, but to form ice it need to shed heat- which reflective surface don’t do well.
So also every morning my car will be wet on the hood or roof- and not the sides of the car, but if it were cold enough the dew could form into ice.
richard verney December 15, 2014 at 5:56 pm
Dear heavens, man, do you not trust your own senses? Haven’t you been out on a bitter cold clear winter night, and then have a cloud come over and felt the warmth from the cloud? That is DWIR doing real work, warming your body in a perceptible manner. Of course the net heat flow is your body warming the cloud, but your body ends up warmer just the same.
And that is real, not theoretical, warming … which means that it is capable of doing real, not theoretical, work.
w.
Willis
Once again, you offer no answer to question put. Stop acting like a politician and start answering the question put, and not the question that you want to be put to you.
We have been discussing this for years. As I mentioned to you, what you have said in your posts of December 15, 2014 at 4:15 pm and December 15, 2014 at 4:27 pm are merely a restatement of comments that you have posted in the past. As you are aware, you are simply restating your views on the theory, and what you claim it does, without answering the question raised. I understand what is claimed of the theory, and how it is said to work. I do not need to see some drawings of personal finances to see what is claimed of the theory.
Your persistent failure, which has now gone on for several years, to deal with the question that I have raised ie., what is actually happening in the top few centimetres of the ocean on a micron by micron layer basis, and what are the physical processes involved and how and at what rate are they dealing with energy, confirms why modellers cannot model the ocean.
Until one properly understands what is going on in the atmosphere immediately above the ocean (say 50 metres above the ocean with resolution scaling down to microns as one approaches the boundary layer) and the top of the ocean itself (say the first 50 metres of ocean depth initially in sub micron resolution gradually extending out to micron resolution then centimetre resolution and then metre resolution) there is no prospect whatsoever of modelling oceans. Until we understand precisely what is going on, we cannot begin to model what is actually going on.
I know the reason why you do not answer my question. That is because you do not know the answer. I do not know the answer either, and that is why I am raising the question. I suspect no one knows the answer, and herein lies the problem. The devil of theories is in the detail. You claim that the oceans are being heated by the DWLWIR that they absorb, and I say let us assume that they are absorbing DWLWIR as claimed by you, then in which case how is that DWLWIR handled, and what problems arise in the handling of this DWLWIR?
Forgive my shouting: I DO NOT WANT TO HEAR ABOUT CLOUDS AND WHAT YOU CLAIM THAT CLOUDS DO. I WANT TO HEAR ABOUT ENERGY. IN PARTICULAR, I WANT TO HEAR ABOUT THE ENERGY IN THE TOP 3 MICRONS OF THE OCEANS, THE ENERGY IN THE TOP 3 MICRONS OF A CRATER LAKE, THE ENERGY IN THE TOP 3 MICRONS OF A DEW DROP.
The reason why I am interested in looking at crater lakes and dew droplets is that in the past you have claimed that the way the oceans are warmed is that the DWLWIR that is absorbed at the top of the ocean (without defining what is precisely meant) gets mixed with the bulk of the ocean, thereby warming the bulk, through various mechanical processes, namely by turbulence via wind, the action of waves and the process of ocean overturning.
I have my doubts that those mechanical processes are sufficient to mix the energy absorbed at the top of the ocean at a rate fast enough to dissipate and dilute that energy, because these are all slow mechanical processes. But it is possible to look at things where those processes are not seen. For the vast majority of the year, there is all but no wind on the surface of crater lakes, and no waves. On a still winter’s day there is no wind, no waves and no ocean over turning on dew drops. They do not encounter those processes, so we can eliminate them as a mechanism of distributing energy over a larger volume.
As you know due to the absorption characteristics of SWIR, there is all but no incoming solar energy absorbed in the top 3 microns of the oceans, or crater lakes. Fortunately for us (because life would be impossible in the form that we know it if this were not the case) Solar irradiance is absorbed at depth into a very large volume of water. It is not concentrated and thereby heats the bulk ocean/lake slowly and because it is absorbed over such a large volume and due to slow ocean currents which further distribute and the energy over an even greater volume, it heats the ocean only to a limited extent.
The absorption of LWIR is very different. 60% of it is absorbed in just 3 microns of perpendicular depth. Virtually no LWIR makes its way past 13 microns. Given that DWLWIR is omni-directional, with a significant proportion of DWLWIR having a grazing angle of say less than 40 degrees to the surface of the oceans/crater lake, more like 80% of all DWLWIR is absorbed within just 3 microns (on vertical basis) of the oceans/crater lake.
Accordingly, if DWLWIR is absorbed by the oceans, on average figures, we have about 200 W/m2 to 300 W/m2 of energy being absorbed and concentrated in just a 3 micron volume. In 1 hour that is upwards of 10,800,000 Watts of energy all concentrated in just 3 microns unless it has in some way been mixed into a larger volume of water. In a crater lake, or in a dew drop where there are no winds or waves to perform the mixing, this energy remains concentrated in just an extremely small volume.
The natural consequence of this is, IF the energy is not being mixed to volume at a speed greater than the speed that that energy would drive evaporation, evaporation will occur.
Now we know that copious evaporation is not occurring. This suggests one of two things. Either, the energy in some way is being mixed to volume at a speed which is fast enough to dissipate and dilute the energy before it drives evaporation. Alternatively, DWLWIR is incapable of performing sensible work in the environ in which it finds itself (alternatively, DWLWIR is never absorbed in the first place).
Let us consider the example of a dew drop on a cold winter day. Say sun up at 8am and on the sunny side of the hollow, the dew is burnt off (completely evaporated) by about 10am, yet on the shady side of the hollow, the dew lingers all day, it., it is not burnt off by 4 pm when the sun begins to set and the day begins to cool.
According to the K&T energy budget, the energy in is 168 W/m2 and DWLWIR of 324 W/m2 making a total energy in on the sunny side of the hollow of 492 W/m2, and the total energy in on the shady side of the hollow of just 324 W/m2.
Now if the dew drop is burnt off by 10 am, the dew drop has absorbed between 8 am and 10 am some 3, 542,400 W/m2 (492 x 3600 x 2). This amount of energy has fully burnt off the dew drop. By contrast on the shady side of the hollow, the de lingers all day and has not been burnt off by 4 pm. In this time, between 8am and 4 pm, the dew drop on the shady side of the hollow has absorbed DWLWIR of some 9, 331,200 W/m2 (324 x 3600 x 8).
Now of course, these are average figures and adjustment has to be made to reflect that we are dealing with winter in high latitudes, but the ratio of the calculation remains constant. Of course the K&T energy budget does not view solar as a day time phenomena but rather as a 24 hour constant. During the day there is more solar energy, but this fact will probably balanced by the fact that solar has relatively little energy at high latitudes between 8 am and 10 am in winter. Further, I have seen (many times) dew burnt off within an hour of sun up, but lingering all day in shady hollows.
Willis, PLEASE TELL ME
!. why DWLWIR cannot (and does not) burn off dew in the shady side of hollows.
2. how on a per second basis, the DWLWIR energy that is said to be absorbed in the top 3 microns of a crater lake (where there is no wind, no waves and if there is the equivalent of ocean over tunring this probably only occurs on a diurnal basis) get dissipated to depth at a rate quick enough to prevent copious evaoporation from the top few microns of the lake.
3. how on a per second basis, the DWLWIR energy that is said to be absorbed in the top 3 microns of the oceans in BF3 and less conditions gets dissipated to depth at a rate quick enough to prevent copious evaoporation from the top few microns of the lake.
I do not know what is going on. Please tell me what you think is going on.
Richard,
It’s very simple. Either the DWLWIR exists as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will HAVE TO be accounted for. Willis is absolutely correct. You can’t have it both ways. OR, the DWLWIR does NOT exist as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will NOT have to be accounted for.
Your argument that there IS indeed a DWLIWIR flux, but it cannot do ‘sensible work’ on the surface has no thermodynamic merit. If you transfer energy to a thermodynamic system and this system absorbs this energy, then this energy becomes part of (increases) the system’s internal energy [U], which corresponds to the system’s temperature [T]. There is no way around it.
You have to make up your mind.
–Let us consider the example of a dew drop on a cold winter day. Say sun up at 8am and on the sunny side of the hollow, the dew is burnt off (completely evaporated) by about 10am, yet on the shady side of the hollow, the dew lingers all day, it., it is not burnt off by 4 pm when the sun begins to set and the day begins to cool.
According to the K&T energy budget, the energy in is 168 W/m2 and DWLWIR of 324 W/m2 making a total energy in on the sunny side of the hollow of 492 W/m2, and the total energy in on the shady side of the hollow of just 324 W/m2.
Now if the dew drop is burnt off by 10 am, the dew drop has absorbed between 8 am and 10 am some 3, 542,400 W/m2 (492 x 3600 x 2). This amount of energy has fully burnt off the dew drop. By contrast on the shady side of the hollow, the de lingers all day and has not been burnt off by 4 pm. In this time, between 8am and 4 pm, the dew drop on the shady side of the hollow has absorbed DWLWIR of some 9, 331,200 W/m2 (324 x 3600 x 8).–
You got me thinking. A dew drop is massive compared other droplets in the air.
Or basically DWLWIR would control the size air droplets. And dew drop only has DWLWIR
coming at from 180 degrees, whereas droplet mid air would get from 360 degree.
Is there paper somewhere explaining the effect of DWLWIR effect upon water droplet size?
Apparently in a rain drop there is N = 4.716278×10^20 molecules of H20.
http://www.had2know.com/academics/how-many-molecules-drop-water.html
So instead of 10^20 one could much smaller on which has 10^10. Or drop with million or 1000.
Are some droplets too small to be affected by most of the LWIR?
Is a size of droplet which DWLWIR would evaporate instantaneous and therefore don’t exist?
Does DWLWIR eat up clouds and fog?
The absorption of LWIR is very different. 60% of it is absorbed in just 3 microns of perpendicular depth. Virtually no LWIR makes its way past 13 microns. Given that DWLWIR is omni-directional, with a significant proportion of DWLWIR having a grazing angle of say less than 40 degrees to the surface of the oceans/crater lake, more like 80% of all DWLWIR is absorbed within just 3 microns (on vertical basis) of the oceans/crater lake.
Accordingly, if DWLWIR is absorbed by the oceans, on average figures, we have about 200 W/m2 to 300 W/m2 of energy being absorbed and concentrated in just a 3 micron volume. In 1 hour that is upwards of 10,800,000 Watts of energy all concentrated in just 3 microns unless it has in some way been mixed into a larger volume of water. In a crater lake, or in a dew drop where there are no winds or waves to perform the mixing, this energy remains concentrated in just an extremely small volume.
The natural consequence of this is, IF the energy is not being mixed to volume at a speed greater than the speed that that energy would drive evaporation, evaporation will occur.
Now we know that copious evaporation is not occurring. This suggests one of two things. Either, the energy in some way is being mixed to volume at a speed which is fast enough to dissipate and dilute the energy before it drives evaporation. Alternatively, DWLWIR is incapable of performing sensible work in the environ in which it finds itself (alternatively, DWLWIR is never absorbed in the first place).
Or what actually happens, which is that the surface itself radiates back to the atmosphere/space. For ocean surface at 300K that’s about 450W/m^2 which is why the surface is cooler than the layer below.
http://ghrsst-pp.metoffice.com/pages/sst_definitions/sst_definitions.png
richard verney December 16, 2014 at 3:13 am
My unpleasant friend, here’s your exact “question put”, which I very clearly answered:
Now, you can claim I’m wrong in that answer.
But claiming that I’m not answering your questions is a damn lie, and I don’t take well to that. Come back when you can keep a civil tongue in your head and ask your question again, whatever it may be, and I may get around to answering in … but you just moved yourself to the end of the line with your slimy accusation. I answer more questions than any climate blogger I know of, and in more detail, and I’ll thank you to remember that.
You tell me to “stop acting like a politician and start answering the question”, when I JUST ANSWERED YOUR SPECIFIC QUESTION?
I answer, stop acting like a p***k.
w.
[I note that the mod has censored mpainter’s use of the above word, so I’ve censored my use of it here myself as well. Sauce for the goose, etc. -w.]
–Kristian
December 16, 2014 at 5:43 am
Richard,
It’s very simple. Either the DWLWIR exists as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will HAVE TO be accounted for. Willis is absolutely correct. You can’t have it both ways. OR, the DWLWIR does NOT exist as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will NOT have to be accounted for.–
Well let’s go back to Willis steel shell. If ground is 235 Watts per square meter then roughly the outside of shell will radiate 235 watts per square meter. And for the shell to radiate a 235 watts
it must be at the temperature that allows it to radiate. And since energy coming from the inside of shell the inside must be about as warm as the outside.
The shell will transfer heat to any side which can be heated, and since universe is 2 K, it radiate
it’s energy to the universe. And one to make something cold enough inside the shell it would radiate heat to that which is cold enough.
Or if you had equal conditions on either side of material which was the same it radiate equally to either side.
Now with such a large sphere a section 1 meter square is essentially flat- to think of it as curved in some way is simply wrong [it’s as flat as any table ever made].
On one side is the 2 K universe and radiant energy can go in 180 degree arc and it’s “field of view” is about 1/2 of the universe.
On the other side, is a universe which is confined to the inside of the shell. Which is mostly other parts of the shell plus the planet itself and “seeing” a portion of the surface of the planet.
[It should be noted that ideal blackbody would radiate equally in all directions- but we suppose to deal with a material which actually exists in your world, which we loosely identify as steel. But could assume that such steel is manufactured with exotic arts by the best engineering available on Earth to suit whatever our various purposes.]
If on surface and one has 1 meter square slab of something very cold, what will normal heat it the most would be conduction of heat or air convection. But the plan is we isolate the cold slab so these things or not heating it. Simple thing to do is not have slab on the surface, but have next to the steel shell. As there is no or little air up there and hang it from the shell itself. And you have to do is block or account for the radiate energy coming from the planet below.
We will make it simple and just account for radiate energy coming from the planet below. So have 4 wires hanging from shell, have the wires 2 meter long. Hang 1 square steel which the same stuff as shell. and have this 1 square meter steel be 2 K. What happens.
Roughly what happens is 2 K steel warms most from heat from the surface, and it’s blocking heat from the surface reaching the a section of shell, that section of shell continues radiating half whatever heat capacity it retains to 2 K universe and other half to 2 K 1 meter square slab of steel. Thus proving that inside of shell can radiate heat.
And within a few days [or minutes or hours] , the 1 meter square hanging from the shell will be the same temperature as the section of the shell above it.
And hanging it further from the shell merely makes it more complicated but gives same result in a few days.
One could imagine the shell is making uniform temperature, but what making the uniform temperature is mostly the ground radiating 235 watts per square meter. Or you can remove the shell and it makes little difference people living on the surface- though they can now launch rockets without hitting the shell. And see the stars.
gbaikie commented on Arguments For and Against Human-Induced Ocean Warming.
in response to Willis Eschenbach:
My heavens, dear fellow, it’s called a “thought experiment”. It’s how we conceptualize about things.
There are also no planets with a steel shell around them … but it’s still a very valuable thought experiment. It shows that the poorly named “greenhouse effect” depends only on the fact that the shell has twice the area of the surface.
w.
“Haven’t you been out on a bitter cold clear winter night, and then have a cloud come over and felt the warmth from the cloud?”
That is a consequence of the cloud reducing upward radiation to space from your skin. Your skin loses energy less fast and so the internal warmth of your body which is constantly being renewed is enabled to warm your skin once more.
Meanwhile the ground temperature will stop falling but will not rise as a result of DWIR from the cloud. The surface temperature will only actually rise if warmer air is advected in horizontally beneath the cloud.
In most cases the arriva of cloud is a sign of warmer air advecting in horizontally at a higher level.
A better knowledge of meteorology would help in this discussion.
A cloud moving over above a water surface reduces convection so that warmed humid air is unable to rise which is why it can become warmer at the surface. It is not a matter of DWIR from the cloud warming the surface.
50% of the atmosphere is descending at any one moment and being warmed adiabatically in the process. That descending air suppresses convection and allows incoming solar energy to heat the surface above the temperature that would be achievable from a purely radiative energy exchange as per the S-B equation.
On average that raises the surface temperature of the entire globe by 33C.
Stephen Wilde December 16, 2014 at 12:34 am
The clouds make my skin lose energy less fast? How do they do that? Block up the pores? Perhaps you could expand on the physics involved in clouds a kilometer above me being able to reduce the radiation from my body WITHOUT involving the true explanation of downwelling radiation.
I love the different bizarre physical theories that folks hold. Some say that there is no such thing as downwelling radiation, despite the fact that it has been measured all over the world thousands of times.
Others say there is downwelling radiation, but it can’t leave the surface warmer than it would be without the radiation because the surface is warmer than the clouds, and somehow the photons know that so they veer away from the surface or something.
Others say that there is downwelling radiation and that it can leave the land warmer but not the ocean. And once again, here there are a variety of explanations.
Fascinating.
w.
–The clouds make my skin lose energy less fast? How do they do that? Block up the pores? Perhaps you could expand on the physics involved in clouds a kilometer above me being able to reduce the radiation from my body WITHOUT involving the true explanation of downwelling radiation.–
So you are saying you have felt the warmth of downwelling radiation?
–I love the different bizarre physical theories that folks hold. Some say that there is no such thing as downwelling radiation, despite the fact that it has been measured all over the world thousands of times.–
I have never measured downwelling radiation [nor can I say that I felt it].
From what I understand the measuring device for downwelling radiation doesn’t work indoors.
But Is there downwelling radiation indoors and one would need different equipment to measure it?
Can it be felt indoors?
I can tell feel a warmer or cooler room inside a house. But I have always assumed this is related to the air temperature.
And it is my understanding that a human body is not regulated by radiant energy- obviously a fire
or anything quite hot can warm someone with radiant energy, but generally the human body
controls heat by evaporation and convection and conduction of heat.
Or astronaut which would have about two square meter of surface area and does not lose much heat in space. And heater are not used in spacesuits, but rather instead a cooling system is used.
I am going to strongly disagree with you about your statement concerning “indoor” (downward, er, inbound) thermal radiation to a warm body. Spanish hotel and apartment bathrooms and bedrooms are an excellent test case for you: They are usually built of tile, smooth sheets of plastic and bare stone: ceiling, floor, walls, tub and bath enclosures, sinks, etc. Almost no surface is wooden, cloth, or cloth-coated. (Almost no surface has a low emissivity, almost no surface (lights excluded) has a surface temperature greater than the unclothed human body – winter or summer, spring or fall. Almost every surface will have that of the (unheated or poorly heated) room air. ) And, of course, I invite any of our Euro-speakin’ brethern to use their own test case and report their results who find themselves in similar locations.
Now, before a hot shower or cold bath water is run, yet while your body still dry, stand in free-space with no thermal insulation. (I will NOT request Willis provide evidence of this test condition!)
Assume your body is “normal” – you will be radiating thermal energy from every cm^2 skin surface because your skin is 37 deg C (98.6 F) = 310 K. The room walls and tiles and ceiling and floor will be receiving that thermal radiation, and will in turn, be radiating a much smaller amount of energy from their surface.
Stand still for a few minutes.
Cover the floor with a rug or bath towel. Cover the nearest wall with a towel or cotton sheet. Hang a sheet or large over the door. Again, stand still for a few minutes.
the difference in heat loss will be the “missing” LW energy from the cold tiles and floor being “shielded” from your body by the low emissivity cloth and wool and towels.
Now, wrap one of the large towels loosely about your legs, and, again, wait a few moments.
Move that large towel up higher (around your shoulders and neck) and, again, wait a few minutes.
The difference is the closeness of the wrap and the extra heat energy it has from your body to even more strongly shield you from the thermal radiant loss.
If no one is pounding on the bathroom door yet, wrap yourself in aluminum foil, and try the shielding again. (Do not re-use the foil for cooking later, or you will agian be subject to random poundings from the local cook.)
–Assume your body is “normal” – you will be radiating thermal energy from every cm^2 skin surface because your skin is 37 deg C (98.6 F) = 310 K. The room walls and tiles and ceiling and floor will be receiving that thermal radiation, and will in turn, be radiating a much smaller amount of energy from their surface. —
Since you not in spacesuit, your body has roughly about 1.6 square meter of area. How much is being radiated from your body and how much do think of being radiated from colder walls at your body.
Next is there anyway of increasing the surface area your body radiates and thereby be able cool down more?
Yes, but I will answer it by reversing the effect. Classically, a tall skinny person with very little body fat (such as myself) will lose much, much more heat by radiation and convection and better conduction (less insulation) per body mass than a shorter, rounder, fatter person who has much less surface area “per person” or “per pound”. Typically, you will see the example of the short Eskimo or Finn or northern Canadian native compared to a taller sub-Sahara African for example. lots of counter-examples of course, and diet and available food are more important. BUT – If “I” (the tall, handsome one with low body weight) have frozen to death early in life because “I” have lost more heat than my competitor for my future wife’s hand in marriage, then “I” do not reproduce. He (the short fat one with hairy chest, hairy face, and hairy legs and short arms) does reproduce.
Wow, a week has gone by and this topic is still being discussed. I’m glad I raised it once again. I’ll hold off on part 2 for another week.
No, Bob.
Willis has taken the discussion off on a tangent and invokes the ghe while ignoring
the physics of the sea surface.
He has yet to address sea surface effects except with a hand wave and some mumbles.
So Willis waxes forth on GHG and GHE while forgetting that it is the radiative properties of water that are the issue.
mpainter December 16, 2014 at 1:36 am
Gadzooks, sire, that’s nonsense. I’ve given the most detailed description I can of a variety of ways that the energy gets mixed downwards from the ocean skin. You can accuse me of being wrong … but saying I haven’t addressed the question is simply untrue.
w.
Willis
I consider that mpainter has a point. Not that you have taken the discussion off target, but rather that you have not addressed the important real world consequences of what is claimed of the theory.
You are correct that you have offered some explanation, namely you have suggested “…that the skin layer is not stable. Why? Because cold water is denser than warm water. As a result, the skin layer is constantly cooling, sinking, and being replaced by warmer water. It is overturning constantly.” and that “It is also mixed mechanically by the action of the wind. In anything but the weakest of winds, the skin layer is broken up both by the horizontal force of the wind, and by the mechanical action of the waves.”
But what you have not done is to address the speed at which these mechanisms take place, and to address whether they can realistically operate at a speed that is fast enough to disspiate the energy to depth/volume at a rate which the energy in the top 3 microns would otherwise drive evaporation.
Further, because of the explanation, you have put forward, I have raised examples where some of these processes are not present (crater lakes where there is no wind or wave action to mix the top to depth), dew drops and ice (where there is no wind or waves or ocean over turning to mix this energy to depth).
Further still, with dew drops even if there was the mixing that you suggest (cold layers sinking to depth) this would not prevent the total evaporation of the dew drop by DWLWIR.
What you need to consider is at what rate does ocean over turning mix DWLWIR, and what about dew and ice where this process is not present.
At what rate does wind and waves mix DWLWIR but what about crater lakes when this process does not takeplace, or for that matter the ocean when in BF3 or less conditions, and what about dew drops and ice where these processes are absent.
At what rate do you claim that cold water sinks? What about dew drops where if the top few microns sinks into the droplet it will gradually heat the droplet leading to the eventual evaporation of the droplet. Ditto ice.
You seem to overlook that somewhere as we exchange views there are large areas of the ocean encountering BF3 or less conditions. This is important since even if on average only 1% of DWLWIR is not mixed as you suggest (since on average some 1% or more of the ocean is experiencing BF3 conditions or less0 the gross energy budget claim fails because the energy being absorbed in the top of the ocean and mixed into the ocean is ever soo slightly less than that budget sets out such that it does not balnce and even a slight impbalance over some 4 billion years 9or so) causes problems.
I concur with Richard Verney.
I would add that the uppermost few microns of the skin is never involved in overturning because it is _always_ present, being due to evaporation which itself is incessant. Overturning does not mean the cessation of evaporation hence the cool layer is ever present. Does the wind have any effect? Yes, it increases the rate of evaporation and this maintains the cool layer, always present as the uppermost surface layer.
I should add that most of the IR is absorbed in this cool layer and that includes all of the IR emitted by CO2. This added energy is quickly transformed to latent heat as part of the dynamics of evaporative cooling.
I’ve addressed what actually happens at the surface but you studiously avoid discussing it!
Phil. December 16, 2014 at 7:54 am
Phil. December 14, 2014 at 7:42 pm
Phil. December 13, 2014 at 9:06 pm
I have to say the same thing to you as I did to richard verney, mpainter.
You cannot get anywhere with this “DWLWIR exists as an extra, separate radiative flux/transfer of energy to the surface’, but it cannot do anything except (in your case, it seems) cause evaporation” argument. If you agree with Willis that there is indeed a DWLWIR flux and it is absorbed by the surface, then this energy will HAVE TO be accounted for. Then a total of [165+345=] 510 W/m^2 is absorbed by the ocean surface and only 112 W/m^2 of this is lost again by way of conduction/evaporation. That leaves … 398 W/m^2. The potential emission flux of a black body surface at 289K.
You too have to make up your mind. I don’t get all these people that apparently agree completely with Willis that the DWLWIR flux is real, but who for some convoluted reason don’t agree with him that it helps keep the surface warmer than at pure solar radiative equilibrium, that it doesn’t do anything (other than provoke evaporation).
Kristian: you are picking bones. I do not buy the DWIR story, particularly where it is put at greater than insolation, as Willis does.
The issue is whether or not IR can heat water. Theory and experimental data both says no.
Kristian
I am sceptical as to whether DWLWIR is anything more than a signal, ie., it is something that can be measured, it is something that can tell us something about the temperature of an object, but whether it is something more than that, in particular capable of performing work, in the environ in which it finds itself, is moot. Accordingly, I am sceptical of its existance as an extra, separate radiative flux/transfer of energy from atmosphere to surface. My first post looks at the net flow position only. As i said, solar provides all the energy that the oceans need to prevent the oceans from freezing. It is solar, and only solar, that warms the oceans.
However, as far as my discussions with Willis are concerned, i am adopting the position; let us accept for the sake of argument that DWLWIR does exist as an energy flux, in which case how is it absorbed and how is it handled and what consequences flow from this? If it is a real energy flux (and goes towards warming the oceans) then I foresee problems with this due to the absorption characteristics of LWIR in water.
There is a heck of a lot of energy going into just 3 microns of water. This energy is highly concentrated and unless sequestered to depth at a rate faster than that energy would otherwise drive evaportation, there would be a heck of a lot of evaporation which we are not seeing. The annual rainfall would be far higher. We know the annual rainfall (within say a 100% error band) so we know the maximum evaporation from the oceans. This is far less than would occur given the amount of energy that DWLWIR would impart into the top 3 microns of the oceans unless that energy could quickly be diluted to volume, which means quickly sequestered to depth.
I want to explore what happens in the real world if the energy that Willis claims is absorbed by the oceans is truly so absorbed. I am testing that.
“The clouds make my skin lose energy less fast? How do they do that? Block up the pores? Perhaps you could expand on the physics involved in clouds a kilometer above me being able to reduce the radiation from my body WITHOUT involving the true explanation of downwelling radiation.”
Poor wording on my part, shouldn’t rush so much.
Clouds reduce convection and block upward radiation which reduces the rate of cooling for the ground surface and the air mass beneath the cloud. The air around your body therefore cools less quickly and draws heat from your bare skin less quickly.You will soon feel warmer than under a clear sky.
It isn’t DWIR from the cloud that makes you feel warmer.it is the reduced rate of cooling of the air around you relative to your own production of body heat.
Thanks, Stephen. Clouds don’t “reduce convection”, they have a variety of effects. In general, they simply move with the air.
And while they “block upward radiation”, this cannot directly “reduce the rate of cooling for the ground surface”. The ground radiates at the same amount whether or not there’s a cloud. The photons leave the ground at a rate determined by the TEMPERATURE, not by what may or may not be absorbing their radiation.
w.
Well, when one says that clouds reduce convection one should really say that warmer cloudy air carrying layer cloud reduces convection by reducing the rate of temperature decline with height.
As regards photons leaving the ground I still consider that the conductive exchange between surface and atmosphere reduces the number of photons leaving the ground as radiation.
The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.
Your previous point about the conductive convective exchange having no effect on surface temperature because it is a net zero energy exchange is not correct IMHO.
Even a net zero energy exchange requires a fund of energy (heat) at the base of the convective column to sustain its height.
That fund of heat at the base cannot be radiated away otherwise the atmosphere would subside to the ground.
But I don’t see you ever accepting that.
“Some say that there is no such thing as downwelling radiation, despite the fact that it has been measured all over the world thousands of times.”
There is downward radiation from the molecules around the sensor and their temperature depends on their position along the lapse rate slope set up by mass, gravity and insolation.
The sensor can be designed to measure temperature at a remote location determined by optical depth but the temperature of that location is in turn determined by its position along the lapse rate slope.
Of course there are regional and local variations over time as the atmospheric gases move around but in the end the average lapse rate slope prevails.
Stephen Wilde December 16, 2014 at 12:07 pm
As regards photons leaving the ground I still consider that the conductive exchange between surface and atmosphere reduces the number of photons leaving the ground as radiation.
The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.
Not true, radiation depends only on the temperature, any conduction/convection is an additional loss/gain.
I said:
“The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.”
and Phil replied:
“Not true, radiation depends only on the temperature, any conduction/convection is an additional loss/gain.”
But what if conduction/convection is a zero sum energy exchange as it is at the surface ?
If it is a zero sum exchange it cannot amount to a loss/gain but it still requires heat at the surface to sustain the height of the convective column.
And that heat cannot be allowed to radiate away otherwise the convective column would have to dissipate.
So, it seems that a given temperature of surface will radiate at a level determined by surface temperature BUT a portion of that radiation is constantly moving in and out of the conductive/convective exchange and so cannot escape to space from the surface.
Radiative theory does not consider the effect of conduction/convection on the ability of a surface to lose energy via radiation. Quite simply, conduction and convection do reduce radiative loss from a surface by tying up a portion of the surface radiation within the ongoing conductive/convective exchange between surface and the mass of the atmosphere.
–Radiative theory does not consider the effect of conduction/convection on the ability of a surface to lose energy via radiation. Quite simply, conduction and convection do reduce radiative loss from a surface by tying up a portion of the surface radiation within the ongoing conductive/convective exchange between surface and the mass of the atmosphere.–
Hmm. Take pot of water and shine laser thru water to bottom of the bottom of the pot.
The reflection of the laser off the bottom is probably not effected by conduction/convection
of water.
And with laser which powerful enough, one make a hole in bottom of pot with the water in it.
Or laser has to be powerful enough to overcome the molecular bond or molecule bond dissipate
the energy. A 100 kw laser might punch thru and 100 1 kw laser shining on different spots does not.
So with 100 kw laser burning hole in the pot, the water in pot would stop the edges of hole to conduct heat to rest of the pot.
With sunlight conduction/convection is going to stop heat from going lower into the surface.
And if have an hour of say 600 watts per square meter of sunlight any evaporation and conduction/convection is going to remove a good portion of the 600 watts per square meter
so it does “have” or “could” balance with a hour of 600 watts per square meter of net energy
returning to space.
gbaikie December 16, 2014 at 5:30 pm Edit
Sure have. Anyone who has spent a good deal of time outdoors at night in the winter has felt it.
I didn’t say you had measured it. I said it has been measured repeatedly. You might try googling “downwelling longwave observations” … I immediately find this. Also, both the TAO buoy system and the SURFRAD stations measure downwelling radiation 24/7.
Regards,
w.
“I didn’t say you had measured it. I said it has been measured repeatedly.”
Right, thousands of times you said. I would say perhaps millions of individual readings, but I have not measured it, nor has seemed to me that there has been any need to measure it.
It suppose to be important, right?
The device I believe is called a Pyrgeometer.
Wiki:
“Pyrgeometers are frequently used in meteorology, climatology studies. The atmospheric long-wave downward radiation is of interest for research into long term climate changes.”
It would seem to me to be a waste of money to buy one, but I am open to idea that they could
useful for some reason. And one thing I would like to know is what be good ones to buy.
I check amazon be none are sold there- though they sell books about it.
Now I read some bad product reviews:
“DLR and backradiation is thus fiction invented from an ad hoc formula without physical reality, which is not described in the physics literature. Nevertheless there are companies selling pyrgeometers at price of 4.000 Euro, but of course selling fiction can also serve as a business idea. But is it legal to sell fiction as science? As science fiction?”
http://claesjohnson.blogspot.com/2011/08/how-to-fool-yourself-with-pyrgeometer.html
And I would like to more balance in the product review.
Is something as useful as say thermometer. What do use it for? And etc.
oh, there is this less harsh review, here:
https://tallbloke.wordpress.com/2013/04/26/pyrgeometers-untangled/
But it still says:
“These devices do not measure “downwelling Infra Red” and the figure is a computed number based on the actual outgoing IR and the temperature of the instrument body.
Put in another way, such a figure is a backwards reading with human added offset.”
And:
“I repeat, heat flow is from the ground upwards. (under very rare meteorological conditions a minor reverse flow happens, the former is overwhelmingly dominant)”
But I am interested in more positive reviews and why it worth spending any money on.
Willis
the impression which you give is that you are unable to answer my specific questions, and have instead resulted to ad homs. That says it all.
But just in case I am mistaken since you assert “You tell me to “stop acting like a politician and start answering the question”, when I JUST ANSWERED YOUR SPECIFIC QUESTION?, and you have in fact answered my questions, I will set out my questions on a numerical basis, and you can then answer each question number by number, if necessqary cutting and pasting your earlier answer so that we can see whether your claim that you have “JUST ANSWERED [MY] SPECIFIC QUESTION is correct.
1 Given the absorption characteristics of DWLWIR in water, how much DWLWIR is absorbed within the top 3 microns of the ocean?
2. Do you accept that unless there is a process (and/or processes) that dissipate/sequester that energy to depth at a rate faster than the energy absorbed into and contained within the top 3 micron layer would otherwise drive evaporation, the energy absorbed and not so sequestered to depth would drive evaporation? If not, why do you not accept this, and what instead do you contend takes place?
3. What processes are claimed, by you, to mix that energy downwards and dissipate it to depth AND at what rate does each indiviidual process move/sequester that energy to depth?
4. In the case of crater lakes (ie., lakes in caldera) do you accept that since these are in a basin, they are, for main part, effectively shielded from wind, and consequently, it is relatively rare to see much in the way of waves on crater lakes? No doubt you have seen the usual Postcard Photographs with views of these lakes as still as a millpond with the mountain range well reflected in the lake.
5 Do you accept that in the case of crater lakes when compared to the ocean, there is for the main part (by which I mean on average for the better part of the year) far less wind and/or waves and/or swell to mix the top 3 micron layer of crater lakes when compared to the sea?. At what rate do you claim that waves on crater lakes mix the energy absorbed in the first 3 microns? What rate to you claim that wind mixes the energy absorbed in the top 3 microns?
6 Do you claim that ocean overturning (or its equivalent) is a process that takes place in crater lakes? If yes, is this a diurnal process, or is it a process that on goes 24/7? At what speed/rate does this process (if it is claimed to take place) dissipate/sequester the energy in the top 3 micron layer to depth?
7. Please review my earlier comments on dew drops (where I mention how dew on the sunny side of a hollow can be burnt off within an hour or so of sun up on a winter’s day, but on the shady side of the same hollow the dew can linger all day) . What processes do you claim mix the DWLWIR that is absorbed within the top 3 microns of a dew drop on a still winters day (ie., one which has no significant wind), and at what rate does each process mix the energy absorbed in the top 3 microns?.
8. Further to the above dew drop point, if the energy absorbed in the top 3 microns is mixed in accordance with the processes that you claim to mix that energy, why does the dew drop not eventually warm and evaporate. After all there is little volume of liquid in a dew drop ( perhaps in the region of just 0.0648524 millilitres or a few times that amount) and 8 hours of DWLWIR (at average figures in the K&T energy budget) is 9, 331,200 W/m2 (324 x 3600 x 8).. Of course, i accept that the average figure for DWLWIR needs to be reduced, but the ratio between solar and DWLWIR would still remain the same such that the forcing from weak winter solar burns off a dew drop within an hour or so, but DWLWIR does not even after exposure for the entire day.
9. In conditions where the ambient air temperature is just a little below freezing, why can solar begin to melt ice after just an hour or so of exposure (eg., cloudless sunny day) but DWLWIR cannot melt ice on a cloudy day even though the ice is exposed to DWLWIR for many many hours? After all it is receiving the full benefit of those warming clouds you like
10, How much energy is absored in the first 3 microns of ice on a cloudy day, and what process do you claim distributes and dissipates that energy to depth at a rate faster than the surface would melt if that energy remained concentrated in the top 3 microns. What rate do each of these processes move/sequester/distribute the energy absorbed in the first 3 microns to depth?
11.When considering 1 to 3 above, what happens in the ocean when the prevailing conditions are BF3 or less, BF2 or less, BF1 or less? ie., what processes do you claim mix the energy absorbed in the top 3 microns of the oceans and at what rate does each process mix that energy to depth in these condfitions?
I look forward to receiving your answer to the specific questions detailed above.
Many thanks .
richard verney December 16, 2014 at 7:24 pm Edit
Richard, as I implied above, if you kept resorting to attacking me there was no sense in answering you. You have not answered my four questions in the slightest. I’ve answered yours, and you are still attacking me.
I give up. You win. I’m tired of your attacks. I’m done with your unpleasantness. Go bother someone else.
I can see that nothing I’ve said has made the slightest impression on you. Since you have claimed that your statement above “says it all”, I’ll take you at your word and let you go. You’ve said it all. I’ve said it all. Please address any further remarks to anyone but me.
w.
Willis
As you are aware, I have not attacked you once. At most I have suggested that your answering is like that of a politician. That is not an attack, that is an observation on an approach/style to dealing with issues/questions. It is not even a derogatory comment, since many politicians are quite intelligent.
When mpainter suggested/implied that you had given no answer as to mechanisms that you claim mix the DWLWIR into the oceans, I suggested that was not so and that you had given an indication of mechanisms, but what was missing was the speed at which these processes mix DWLWIR absorbed in the top 3 microns to depth, and hence dilute the energy to volume. So when you read the posts, you will see that I have even sprung to yoru defence and supported you.
On the other hand you have suggested that I am acticg like a prick. That is an attack on character, and a derogatory one at that. It is an ad hom.
The issue here is quite simple. The claim that DWLWIR is absorbed in the ocean leads to a number of issues. In particular, the energy absorbed is extremely concentrated somewhere betweem 60 to 80% of DWLWIR is concentrated in just 3 microns, and that is a heck of a lot of energy. That energy (if capable of performing sensible work in the environ in which it finds itself) would drive copious amounts of evaporation and/or carry the water vapour evaporated to great height, unless it is sequestered to depth (thereby dissipating and diluting the energy into a larger volume) at a rate faster than the rate that that energy would if not sequestered to depth, drive evaporation from the top microns. So the speed of the mixing proceeses that you cite is the central issue. You may be right that those proceeses exist and do mix energy at the very top microns of the ocean, but the issue is at what speed do they perform this mixing.
So that we can consider the effectiveness of those processes in more detail, I have sought to identify scenarios where those processes must be less effective, perhaps even no existent. Eg. in BF2 and blow there is no significant wind or waves, in crater lakes there is no significant wind or waves. On a still winter’s day dew drops are not subjected to significant wind or waves, nor ocean overturning. etc etc.
When comnsidering the oceans, it is claimed that the average wind conditions/sea state is globally on average just over BF4. If that is so, since this is an average figure, it must mean that at least 20% of the oceans on average experience average conditions of BF2 or less. This is material since if for 205 of the year there is no effective mixing of the top 3 micron layer by the action of wind and waves, how is ALL the DWLWIR effectively being mixed into the oceans. In these conditions only 2 mechanisms claimed by you can be operative, namely ocean overturning, and cold water sinking. Is ocean overturning operative 24/7 or is it only operative on a diurnal basis, and if a diurnal process what happens when it is not operative? What is the speed/rate at which ocean overturning mixes the top 3 micron layer to depth? What is the speed at which cold water sinking mixes the 3 micron layer to depth?
As I have said to you on a number of previous occassions, let us discuss the science. If you have an answer to the points I raise, then please detail it. If you do not know the answer,so be it, there is no discrace in that. I do not know the answers since, as far as I am aware, no one is carrying out the empirical testing and collecting impirical observational data on this.
That being the case, we are left to grasp with what may be objectively viewed as likely. I suggest that the action of wind and waves can not effectively mix DWLWIR absorbed in the top 3 microns of the oceans when the prevailing conditions are BF2 or less, ditto in crater lakes, ditto in dew drops on a still winter’s day.
I would suggest that ocean overturning is a slow mechanical process and appears to be a diurnal phenomena such that either way, it is not capable of mixing DWLWIR absorbed in the top 3 microns to depth at a rate fast enough to prevent DWLWIR absorbed in the 3 micron layer simply driving evaporation (including carrying it to height) and latent heat loss. In any event, ocean over turning cannot explain why a dew drop on a still winter’s day is not burnt off/evaportaed by DWLWIR.
The cold water sinking claim has a number of issues niot least that the top microns of the oceans are cooler only because of the effects of evaporation taking place at the very top of the ocean and the difference in buoyancy is such that cold water would be sinking at a ver slow rate and so any energy contained in that cold water is being seqestered to depth at a very slow rate not sufficient to prevent the DWLWIR absorbed in the top 3 microns from simply driving evaportion.
In would like to hear your views on the science since you coukd usefully contribute towards this debate if you were to address the specifics,
Richard Verney,
You see what happens when you try to force Willis to the issue: you get a sailor, not a scientist.
richard verney December 17, 2014 at 5:34 am
Richard, this is why I’ve given up dealing with you. As you know, you’ve lied about whether I answered you questions. That is assuredly an attack on my honesty.
w.
mpainter December 17, 2014 at 7:49 am
I see … lying about whether I’ve answered richard’s questions is now called “forcing” me to the issue.
I’ve got news for you, mpainter. Trying to “force” an honest man to do anything by lying about him gets you nowhere, whether the man is a sailor, a scientist, or both … but then if you hung out with honest men I wouldn’t have to explain this to you.
I answer more questions and in more detail than any other climate researcher you can name. I’ve answered your questions. I’ve made a good faith attempt to answer all honest questions.
And in particular, I’ve answered richard’s questions, over and over as nauseum, on this and previous threads. He just keeps asking the same questions in a new and different guise … and all the while he hasn’t answered my questions.
As a result, when he topped that off by claiming I’m not answering his questions, I pulled the plug on his endless whinging…. so sue me. Clearly you think that kind of passive-aggressive attack is fine. Your call.
w.
It seems if there isn’t back radiation then greenhouse gases do not warm Earth.
It seems most agree that clouds add warmth at night.
I think most agree that lower elevation [a big hole in the ground] would increase
air temperature. The hottest air temperature ever recorded was slightly below sea level and
it seems good rule that if you looking the highest air temperature one look at regions below
sea level.
It also well know that UHI increases mostly night time temperatures, but also seems to a some increase in daytime temperatures. And such thing as inversion layers also tend to increase daytime temperatures.
So in addition to below sea level, some kind UHI effect and inversion layers could cause the highest day time air temperature.
Ans I think a common aspect of all of the above can be related to having some kind of elevated surface. So clouds are elevated surface, as are inversion layers [sort of] and below sea level is the rest of world is an elevated surface, and cities have a bunch elevated surfaces in the form of buildings.
Though clouds are related to warming at night and I suspect that cloud are related to a general idea of back radiation. Or radiation from ground is intersecting a cloud which is a elevated
surface. And clouds at a higher elevation could be must cooler than the surface, so one violating colder things warming warmer things, instead something warmer is warming a colder thing.
So doesn’t seem too problematic that the ground could heat the clouds- the problem is related to
how could the cloud warm the ground. There seems to be many possible mechanisms, and perhaps there are more than one or two mechanism involved. For example clouds are water droplets which unlike gases reflect light. Another factor is clouds are where lots H2O gas still condensing into liquid, so creating heat.
Now regarding clouds, one can clouds at varying elevation. I don’t think fog [or clouds on the ground] make nights warmer, though perhaps it’s the dampness just make feel cooler and does
tend to keep it warmer. But do clouds cause more warming depending upon what elevation they
are at?
Stephen Wilde December 17, 2014 at 12:41 am
I said:
“The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.”
and Phil replied:
“Not true, radiation depends only on the temperature, any conduction/convection is an additional loss/gain.”
But what if conduction/convection is a zero sum energy exchange as it is at the surface ?
If it is a zero sum exchange it cannot amount to a loss/gain but it still requires heat at the surface to sustain the height of the convective column.
And that heat cannot be allowed to radiate away otherwise the convective column would have to dissipate.
Where do you get this fictitious ‘law’ from?
It’s about time you read up on heat transfer instead of making stuff up.
If you consider a control element at the surface, say a 1 micron cube, the top surface will be radiating at ɛ𝜎ATtop^4, the element will be absorbing some of the radiation incident on it, mostly IR. There will also be conductive and convective heat transfer at both the top and bottom surfaces, given by equations of the form Q=hA∆T. If the element is net losing heat it will cool down until a balance is achieved, in the case of the night-time ocean shown, by about 0.3K. The only way that conduction/convection will effect the radiation loss is due to their influence on the temperature, however the radiation is the dominant loss mechanism so their influence is small.
So, it seems that a given temperature of surface will radiate at a level determined by surface temperature BUT a portion of that radiation is constantly moving in and out of the conductive/convective exchange and so cannot escape to space from the surface.
This is gobbledygook do a proper analysis.
Radiative theory does not consider the effect of conduction/convection on the ability of a surface to lose energy via radiation. Quite simply, conduction and convection do reduce radiative loss from a surface by tying up a portion of the surface radiation within the ongoing conductive/convective exchange between surface and the mass of the atmosphere.
As stated above their only role is to reduce the surface temperature which has a negligibly small effect on radiation loss.
Conduction and convection reduce surface temperature on uplift and increase it on descent. The two balance in order to sustain atmospheric height.
The heating of the surface on descent is effected not by direct transfer of energy from air to ground but via adiabatically warmed descending air reducing the lapse rate slope and thereby inhibiting convection.
It is the inhibiting of convection globally beneath columns of adiabatically warmed descending air within surface high pressure cells that acts as a greenhouse effect.
Just like in a true greenhouse the warmed descending air becomes transparent (just like a greenhouse roof) through the dissipation of clouds and the layer of warmed air inhibits convection (just like a greenhouse roof).
That is why the mass induced greenhouse effect was so decribed by meteorologists from the very beginning.
The astrophysicists who took over climate science didn’t know much about meteorology.
This is just an observation I have found. Perhaps someone can explain it.
DWLWR shines both day and night, correct? And the figures I see batted about are roughly 320 w/m-2. So how come on clear night I don’t feel this 320 w/m-2 when I am out in the open? 320 w/m-2 is a lot of energy. That’s the equivalent of standing out in direct sunlight at about 9 to 10 in the morning. You can feel that heat on your face. But at night, I don’t feel a thing when walking out of a covered area into this 320 w/m-2 of intense radiation. I mean this is the equivalent of over three 100 watt light bulbs shining over a square meter of surface. I should feel the heat on my face. This should warm up exposed pavement or concrete, no? But the concrete under my car feels the same temperature as the concrete exposed to this DWLWR.
Forget the ocean warming business. Does DWLWR heat up the earth’s ground surface? Seems like an easier experiment to perform.
Thanks for the question, SkepticGW.
At about nine or ten AM, depending on location, you get about 320 W/m2 from the sun PLUS about the same amount from longwave radiation. This gives a total of about 640 W/m2 which is clearly perceptible.
Next, you say:
Nor should you … because you also get radiation from the covered area. Folks think that a roof shades you from the IR just like it shades you from the sun. And in fact it does … but while the roof is not radiating light, it’s radiating IR.
As a result there’s a big difference between what a roof does to visible light (cuts it off) and what it does to the IR (replaces DWIR with IR from the roof.)
Finally, you need to remember that 320 W/m2 is not “intense radiation”. It has the black-body temperature of about 0°C … so it has the same effect on your body as the radiation from a block of ice.
w.
–SkepticGoneWild
December 17, 2014 at 8:26 am
This is just an observation I have found. Perhaps someone can explain it.
DWLWR shines both day and night, correct? And the figures I see batted about are roughly 320 w/m-2. So how come on clear night I don’t feel this 320 w/m-2 when I am out in the open? 320 w/m-2 is a lot of energy. That’s the equivalent of standing out in direct sunlight at about 9 to 10 in the morning. You can feel that heat on your face. But at night, I don’t feel a thing when walking out of a covered area into this 320 w/m-2 of intense radiation. I mean this is the equivalent of over three 100 watt light bulbs shining over a square meter of surface. —
The 3 lightbulbs is more like the direct light of sunlight.
Or 3 100 watt halogen spotlights would be even more like sunlight.
DWLWR is not directed light.
Some people claim that DWLWR is like the infrared light of the walls of a dark cave or the IR of a ice cube.
Generally speaking DWLWR seems to me to be ill defined, but everyone would agree it’s not
direct light. Or wiki says about sunlight
“When the direct solar radiation is not blocked by clouds, it is experienced as sunshine, a combination of bright light and radiant heat. When it is blocked by the clouds or reflects off other objects, it is experienced as diffused light.”
And I would add that such diffused sunlight is more directed light than DWLWR.
Of course also everyone would agree that DWLWR is not the high energy of visible light.
So one point to the blue sky as something like DWLWR, except the blue sky is scattered blue visible light which very different species as compared to longwave IR.
Though if want to use visible light as analogy, photography deals with direct and indirect light:
“If you really think about it, there is no fundamental difference between direct light and indirect light. One is simply the overabundance of another. When you say the light is direct, you are saying a lot more about what isn’t happening than what is. Direct light is the absence of light on any other side except the side with a single light source.
Indirect light, by contrast, is light coming in from all sides. Technically speaking, there’s always some small amount of light bouncing around and hitting your subject on all sides too. The difference with indirect light is that it is more evenly balanced. In other words, the light reflecting onto the other side of your subject is nearly the same intensity as the source itself.
Most indirect light isn’t completely indirect either. The original source is usually a little brighter than the sources of reflected light. To see what I mean, just think of an overcast day. We would say this is an indirect lighting situation, but if you look at the sun, it’s still brighter than anything else around. The real difference, then, is the degree to which it is bright. On an overcast day, it’s relatively less bright than on a fully sunny day.”
– See more at: http://www.digital-photo-secrets.com/tip/2099/direct-and-indirect-light/#sthash.6APovvXd.dpuf
richard verney December 16, 2014 at 7:42 pm
Kristian
I am sceptical as to whether DWLWIR is anything more than a signal, ie., it is something that can be measured, it is something that can tell us something about the temperature of an object, but whether it is something more than that, in particular capable of performing work, in the environ in which it finds itself, is moot. Accordingly, I am sceptical of its existance as an extra, separate radiative flux/transfer of energy from atmosphere to surface. My first post looks at the net flow position only. As i said, solar provides all the energy that the oceans need to prevent the oceans from freezing. It is solar, and only solar, that warms the oceans.
However, as far as my discussions with Willis are concerned, i am adopting the position; let us accept for the sake of argument that DWLWIR does exist as an energy flux, in which case how is it absorbed and how is it handled and what consequences flow from this? If it is a real energy flux (and goes towards warming the oceans) then I foresee problems with this due to the absorption characteristics of LWIR in water.
There is a heck of a lot of energy going into just 3 microns of water. This energy is highly concentrated and unless sequestered to depth at a rate faster than that energy would otherwise drive evaportation, there would be a heck of a lot of evaporation which we are not seeing.
That’s because it’s radiating from the surface as I’ve pointed out to you several times but you choose to ignore! How do you think the SST is measured by satellites?
The annual rainfall would be far higher. We know the annual rainfall (within say a 100% error band) so we know the maximum evaporation from the oceans. This is far less than would occur given the amount of energy that DWLWIR would impart into the top 3 microns of the oceans unless that energy could quickly be diluted to volume, which means quickly sequestered to depth.
I want to explore what happens in the real world if the energy that Willis claims is absorbed by the oceans is truly so absorbed. I am testing that.
Except when it conflicts with your preconceived ideas, then you ignore it!
Either do a proper energy balance for the skin layer or stop wasting space here.
Phil., December 17, 2014 at 9:06 am:
“That’s because it’s radiating from the surface as I’ve pointed out to you several times but you choose to ignore! How do you think the SST is measured by satellites?“
Thanks for the good laugh, Phil 🙂 So, the satellite-borne instruments in space detect radiative ocean surface fluxes of 369 W/m^2 here, 424 W/m^2 there and 473 W/m^2 over there and from this they can easily calculate the SST in each place, courtesy of the Stefan-Boltzmann equation: 11, 21 and 29 degrees Celsius respectively. Is that it, Phil? Is that how they do it? Tell me that’s what you think …
richard verney December 17, 2014 at 5:34 am
Willis
As you are aware, I have not attacked you once. At most I have suggested that your answering is like that of a politician. That is not an attack, that is an observation on an approach/style to dealing with issues/questions. It is not even a derogatory comment, since many politicians are quite intelligent.
When mpainter suggested/implied that you had given no answer as to mechanisms that you claim mix the DWLWIR into the oceans, I suggested that was not so and that you had given an indication of mechanisms, but what was missing was the speed at which these processes mix DWLWIR absorbed in the top 3 microns to depth, and hence dilute the energy to volume.
But the predominant mechanism by which energy is lost from the surface is radiation upwards, not mixing to the depths, a fact you continue to ignore.
The issue here is quite simple. The claim that DWLWIR is absorbed in the ocean leads to a number of issues. In particular, the energy absorbed is extremely concentrated somewhere betweem 60 to 80% of DWLWIR is concentrated in just 3 microns, and that is a heck of a lot of energy. That energy (if capable of performing sensible work in the environ in which it finds itself) would drive copious amounts of evaporation and/or carry the water vapour evaporated to great height, unless it is sequestered to depth (thereby dissipating and diluting the energy into a larger volume) at a rate faster than the rate that that energy would if not sequestered to depth, drive evaporation from the top microns.
Or what actually happens, radiation from the surface, if you want to do heat transfer analysis you should include all the processes, not pick and choose.
–But the predominant mechanism by which energy is lost from the surface is radiation upwards, not mixing to the depths, a fact you continue to ignore.–
Energy is not lost “mixing to the depths”.
Mixing causes heat to be more uniform. Mixing is heating something which is colder- causing a increase of the overall amount of energy content- it’s heating something..
Though nearly all energy leaving Earth comes from some kind surface and things which can have a surface are liquids or solids- as compared to gases – a surface being comprised molecules in structural molecular bond.
Only a surface can reflect or direct radiant energy and only directed radiation can warm anything
by any significant amount. And only a surface can have a blackbody spectrum of light.
The surface [skin surface] of ocean and land is different, with the land most of the sunlight reaching the surface is radiated back into the universe and large amount is re-radiated in less than a second.
With ocean most of the energy of sunlight penetrates below couple feet under surface of the water.
Since most of Earth is covered with ocean, most of the energy of sunlight reaching Earth enter the ocean below it’s surface. And any heat under the water can not radiate up to the universe.
What can travel thru many meter of water is visible light, blue visible light can travel to furthest
thru to water and this is related to why the ocean is blue.
So for instance at 100 meter under water only blue light [and some UV ] can go thru so much
water as you go deeper than 100 meters under the surface eventually all visible light is stopped.
“Light may be detected as far as 1,000 meters down in the ocean,
but there is rarely any significant light beyond 200 meters.”
http://oceanservice.noaa.gov/facts/light_travel.html
Phil. December 17, 2014 at 9:22 am
Phil … now you’re starting to see why discussing things with richard goes nowhere. He’s not looking for an explanation. He’s looking to nit-pick, and he will happily ignore the facts and your answers, over and and over, until you get tired of endlessly answering.
And of course, at that point he’ll claim victory on the basis that, despite the fact that you’ve answered him over and over, you’re not answering …
w.
Hi Kristian, I see you’re back reprising your role of class clown. Interesting that accurate science amuses you so much.
If you’d have taken the trouble to look it up you would have found that the SST is indeed measured using IR radiometry, a slightly more sophisticated approach than you describe though. The Advanced Very High Resolution Radiometer (AVHRR) measures at 3.7, 11 and 12 microns as does MODIS, AMSR-E uses microwave in a similar manner in order to see through the clouds. So yes SST is measured using the very process that Verney and Painter exclude from their analysis of the ocean surface.
Avoiding the question, are we, Phil? Are the satellites estimating SST based on ‘flux intensity’ (like the rGHE is) or based on ‘frequency distribution’?
Avoiding the question, are we, Phil? Are the satellites estimating SST based on ‘flux intensity’ (like the rGHE is) or based on ‘frequency distribution’?
No I answered the question you asked. The SST is measured by satellites based on IR radiometry, tough to do if it doesn’t exist as Verney seems to think.
Phil. December 17, 2014 at 6:52 pm
“No I answered the question you asked. The SST is measured by satellites based on IR radiometry, tough to do if it doesn’t exist as Verney seems to think.”
No, you’re specifically avoiding what I’m getting at. You’re building a straw man to tear down. No one is claiming (not Verney either) that there is no IR being emitted from the surface. The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating. And even when confronted with it, you don’t fully want to scrap the idea. You state in your last answer: “(…) the SST is indeed measured using IR radiometry, a slightly more sophisticated approach than you describe though.”
Of course it’s using IR radiometry. That’s not the point. The point is: Do the satellite instruments estimate the SST based on detecting a BB ‘flux intensity’ from the surface of 395 W/m^2 (like what the rGHE hypothesis and its proponents would suggest, THAT’S the point) or do they do it based on a specific ‘frequency/wavelength distribution’ of the IR detected? If the latter rather than the former method is what’s used, then your original straw man argument has no bearing on the issue we’re discussing here at all.
It’s been shown
http://wattsupwiththat.com/2014/12/09/arguments-for-and-against-human-induced-ocean-warming/#comment-1812746
that the postulated BB emission flux from the surface is NOT measured in the sense of being ‘detected’. It is ONLY calculated by directly applying the Stefan-Boltzmann equation to the assumption that the surface is a thermally isolated, pure emitter (that is, in a purely radiative setting), which it is NOT. The 395 W/m^2 is not an actual flux/transfer of energy, no more so than the DWLWIR 340 W/m^2 is an actual flux/transfer of energy. Only the HEAT is. The two ‘hemifluxes’ are both merely mathematical constructs, conceptual, potential heat fluxes. The evidence is right there in the method descriptions of how they’re ‘found’, Phil.
Kristian December 17, 2014 at 11:04 pm
Phil. December 17, 2014 at 6:52 pm
“No I answered the question you asked. The SST is measured by satellites based on IR radiometry, tough to do if it doesn’t exist as Verney seems to think.”
No, you’re specifically avoiding what I’m getting at. </em.
No you keep changing what you're 'getting at' and it bears no relevance to what I said in any case.
You’re building a straw man to tear down. No one is claiming (not Verney either) that there is no IR being emitted from the surface.</em.
As pointed out above, by explicitly omitting the role of IR emission at the surface of the ocean in his analysis and refusing to even discuss it, that's exactly what he's doing.
The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating.
No I explicitly state that, it’s been measured many times, here’s an example:
http://www.azimuthproject.org/azimuth/files/emission.png
The measurement shows the radiance from the surface with absorption from atmospheric trace gases such CO2, H2O, O3 and CH4. Satellite measurements of the radiance are made in the region indicated as ‘Window’ to avoid interference by these species.
And even when confronted with it, you don’t fully want to scrap the idea. You state in your last answer: “(…) the SST is indeed measured using IR radiometry, a slightly more sophisticated approach than you describe though.”
Of course it’s using IR radiometry. That’s not the point. The point is: Do the satellite instruments estimate the SST based on detecting a BB ‘flux intensity’ from the surface of 395 W/m^2 (like what the rGHE hypothesis and its proponents would suggest, THAT’S the point) or do they do it based on a specific ‘frequency/wavelength distribution’ of the IR detected? If the latter rather than the former method is what’s used, then your original straw man argument has no bearing on the issue we’re discussing here at all.
As stated they do it by measuring the radiance in the ‘Window’ region so by your own admission I’m correct.
It’s been shown
No it hasn’t that’s just your repeating the same mantra.
that the postulated BB emission flux from the surface is NOT measured in the sense of being ‘detected’. It is ONLY calculated by directly applying the Stefan-Boltzmann equation to the assumption that the surface is a thermally isolated, pure emitter (that is, in a purely radiative setting), which it is NOT. The 395 W/m^2 is not an actual flux/transfer of energy, no more so than the DWLWIR 340 W/m^2 is an actual flux/transfer of energy. Only the HEAT is. The two ‘hemifluxes’ are both merely mathematical constructs, conceptual, potential heat fluxes.
No that’s your pet theory which no text on radiation heat transfer endorses, try ‘Hottell and Sarofim’ or ‘Holman’ for example.
Phil., December 18, 2014 at 6:25 am:
Me: “The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating.”
Phil: “No I explicitly state that, it’s been measured many times, here’s an example:
The measurement shows the radiance from the surface with absorption from atmospheric trace gases such CO2, H2O, O3 and CH4. Satellite measurements of the radiance are made in the region indicated as ‘Window’ to avoid interference by these species.”
For crying out loud, are you pulling my leg!? Why are you posting a ToA spectrum when arguing about surface emissions!? And how are these spectra actually generated? Any idea? Read up on it?
“As stated they do it by measuring the radiance in the ‘Window’ region so by your own admission I’m correct.”
*Sigh* Avoiding the issue ………..
“No it hasn’t [been shown] that’s just your repeating the same mantra.”
In the link provided it is shown, yes. It says so right in the text of the article discussed. Surface emission is NOT ‘measured’. It is CALCULATED using a specific S-B formula. It’s right there, Phil. You can deny it all you want. Read the bloody paper!!!
You’re looking more and more silly, Phil.
“No that’s your pet theory which no text on radiation heat transfer endorses (…)”
Heh, I’m sorry, Phil, but the physical discipline concerning itself with system/object temperature change from energy transfer is called … THERMODYNAMICS.
It is quite obvious that you have never opened even an introductory textbook on this particular subject.
I’ll repeat what I told you upthread: “Out here in the real world, radiative transfers and their resulting effects must comply with the Laws of Thermodynamics before anything else, just like conductive transfers and their resulting effects have to. In the real world, in nature, it is not allowed for an energy transfer from a cool place to a warm place to make the temperature of that warm place rise. It simply doesn’t happen. If you observe something where it might LOOK like it does, it is your interpretation of what happens that’s wrong.”
Yes Willis, I’m very patient though, I more interested in rebutting his nonsense so that other readers will realize he doesn’t have a clue about the subject and that he’s the one who doesn’t address the point.
Well then, proceed, Phil., rebut . I am interested.
I’ve already rebutted it, verney doesn’t answer.
Willis Eschenbach
December 17, 2014 at 9:51 am
/////
Willis
First you suggest that I am acting like a prick.
Now you suggest that I am lying.
Please quote my questions (together with time/date of posting) and please quote your answers thereo (together with time/date of posting)..
Lets see to what extent you have answered my questions.
As I mentioned above, richard, there is no end to your questions. I already answered this very question of yours above, and now you want me to answer it again.
I’m done with you.
w.
Are you done with me Willis? Because I’ve got some more questions for you but I’m afraid to ask lest I get called a p***k.
[language. .mod]
Thanks for the offer, mpainter, but I’ll pass. You wanted to jump in on richard’s behalf. There was no reason for you to be involved at all. As a result, I fear you have cancelled your vote on my planet.
Best regards,
w.
Goodness, gracious, moderator, you have some more work up thread.
“You better watch out,
You better not cry,
You better not pout,
I’m telling you why:
Santa Claus is coming to town!
He’s making a list,
And checking it twice,
Gonna find out who’s naughty or nice.
Santa Claus is coming to town!”
Read more: Christmas Song – Santa Claus Is Coming To Town Lyrics | MetroLyrics
mpainter December 17, 2014 at 6:21 pm
Not on my behalf he doesn’t … been there, done that several hours ago after reading your previous comment. You’re late to the party.
However, there may be someone other than me upthread, I haven’t read all the replies. I gave up on that a much earlier.
w.
Willis
It is a very serious allegation to call someone a liar, and I note that you have not substantiated that your derogatory allegation made against me is true.
As you are aware, I have not been asking you what is the energy budget of the oceans. Nor have I been asking you whether clouds warm, or slow the rate of cooling. I have not assked you rto restate your steel greenhouse etc. All of those issues may or may not be interesting, but they were not relevant to the specific questions that I was asking. To talk about them, is not to answer my questions..
My questions centre upon exactly what is going on in the top 3 microns of the oceans, and if DWLWIR is absorbed and capable of performing sensible work, whether there are processes that can mix the energy so absorbed (which is in the region of 200 to 300 W/sq.m) and sequester this energy to depth at a rate faster than the rate that the energy absorbed in the top 3 microns would drve evaporation if that energy remained concentrated in the top 3 microns and was not sequestered to depth and thereby diluted by volume. I have asked you about the physics of the top 3 microns and in particular the rate at which any physical process can effectively mix energy and sequester it to depth at a rate which is fast enough to evaporation from the very top of the ocean. .
I have not checked the above, but if I recall correctly, you have not mentioned to me (but rather to someone else) that the energy in the top microns is mixed by wind and waves and cold water sinking. You may or may not also have mentioned ocean over turning. I recall in the past you have mentioned that.
Accordingly, although I believe that those processes were never directed to me in a direct answer to my question, since those processes have been mentioned in the comment section of this Article, I desired to explore the rate at which these proceesses could effectively mix the energy in the top few microns of water.
I then suggested that one should look at crater lakes where there is little in the way of wind and waves, so that those processes could be ruled out. This would leave just ocean overturning, and I asked you whether that was a 24/7 process, or a diurnal process, and cold water sinking.
I asked you about the oceans in BF3 or less (particularly BF2 or less which given that the global average wind speed over the oceans is said to be a little over BF4, it follows that BF2 or less must be being experienced some 20% or so of the time), since again the action of wind and waves could largely be ignored. That again would leave just ocean overturning (to which whether it is a diurnal process would be relevant) and cold water sinking.
I asked you about a dew drop on a still winter’s day where there is no action of wind, waves or ocean overturning, so those processes could be completely ruled out. This would leave just your cold water sinking theory, and I pointed out that even if cold water sinks as you suggest this would go to mix energy into the very small volume of the dew drop and would still result in the dew drop being burnt off/evaporated if DWLWIR is being absorrbed by the dew drop is capable of sensible work and actually heats the dew drop.
You have not answered those questions.
Let us not waste time arguing whether you have or have not answered those questions. Instead, now set out your answers..Of course, you may cut and paste any previous answer that deals with the rate of mixing wwith respect to each process that you claim mixes the energy, and/or which specifically deals with crater lakes, with oceanss in BF3 or less, with dew drops.
I hope that you will now constructively respond taking the debate forward.
richard verney December 18, 2014 at 1:34 am
I then suggested that one should look at crater lakes where there is little in the way of wind and waves, so that those processes could be ruled out. This would leave just ocean overturning, and I asked you whether that was a 24/7 process, or a diurnal process, and cold water sinking.
I asked you about the oceans in BF3 or less (particularly BF2 or less which given that the global average wind speed over the oceans is said to be a little over BF4, it follows that BF2 or less must be being experienced some 20% or so of the time), since again the action of wind and waves could largely be ignored. That again would leave just ocean overturning (to which whether it is a diurnal process would be relevant) and cold water sinking.
I asked you about a dew drop on a still winter’s day where there is no action of wind, waves or ocean overturning, so those processes could be completely ruled out. This would leave just your cold water sinking theory, and I pointed out that even if cold water sinks as you suggest this would go to mix energy into the very small volume of the dew drop and would still result in the dew drop being burnt off/evaporated if DWLWIR is being absorrbed by the dew drop is capable of sensible work and actually heats the dew drop.
In all of those cases it leaves what you don’t wish to face up to, radiational loss from the surface, in the case of a dew drop at ~5ºC that would be ~330W/m^2.
Phil. ,
Thanks for the temp. profile of the sea surface that you provided in your comment above at 12-16, 7:45am. I have studied this before, but you have handily provided it for this dicussion.
This profile pretty well refutes the notion that energy from incident IR is mixed or conducted downward before it is converted to latent heat., as put by Willis, Judith Curry, and others. Specifically, the day temp. profile shows that there is no overturning of the water down to 10 meters. Nor can heat be conducted downward from this cooler interval to the water below. The night temp. profile, in comparison, shows that overturning, or convection, is the dominant process at the sea surface. Note that there is no temp .differential in night from aprox. 1 mm to 10 meters depth, and only about 0.2 C difference from 1 mm to the surface. This contrasts with the temperature “bump” seen in the day temp. profile which is the proof that no convection is occurring during the day, hence no mixing downward.
So far, no one has shown that IR has any effect on SST (or OHC), and indeed, the basic physics refutes the notion.
Clarification: “Nor can heat be conducted downward from this cooler interval..”, meaning the uppermost micro-layer where the IR photons are absorbed. Note the warming from this to the 1 mm point, hence no conduction of heat from incident IR is possible.
It shows quite clearly that heat is lost from the surface via radiation before any other mechanism, setting up a gradient of ~0.3ºC/mm over the first mm at both day and night, allowing conduction from below. During the day the layer from 1mm to 10m is warmed by solar insolation. The air at the air/water interface is saturated and at about 2m above the surface it’s typically 0.8RH so the amount of latent heat is controlled by mixing in this layer.
So far, no one has shown that IR has any effect on SST (or OHC), and indeed, the basic physics refutes the notion.
On the contrary the basic physics shows that it has a major effect.
Phil.,
Is this some kind of game?
You are not to be taken seriously. Once more, the issue is IR incident on the sea surface and its contribution to SST and OHC.