By P Gosselin
A Nature study finds there’s very little risk that global warming would lead to more methane escaping from the oceans into the atmosphere.

Hat-tip: EIKE here.
Global warming alarmists have often used the scenario of increased methane in the atmosphere accelerating warming and climatic change.
But a recent study appearing in Nature, Negligible atmospheric release of methane from decomposing hydrates in mid-latitude oceans, dumps a lot cold water on this scenario. This is good news, which unfortunately the media refused to report.
At the bottom of the sea, there are large deposits of naturally occurring methane hydrate. There’s a fear that these ice-like deposits could melt and be released into the atmosphere if the oceans warmed. Methane is a far more powerful greenhouse gas than CO2. The researchers looked at the concentration and natural radiocarbon content of methane dissolved in the water column from the seafloor to the sea surface at seep fields along the US Atlantic and Pacific margins.
No methane reached the surface
Their measurements revealed no evidence of seep CH4 reaching surface waters when the water-column depth is greater than 430 ± 90 m. “Gas hydrates exist only at water depths greater than ~550 m in this region, suggesting that the source of methane escaping to the atmosphere is not from hydrate decomposition,” the authors add.
Dissolves in the ocean
In 2016, a paper published in the Reviews of Geophysics concluded that the annual emissions of methane to the ocean from degrading gas hydrates are far smaller than greenhouse gas emissions to the atmosphere from human activities and that most of the methane released by gas hydrates never even reaches the atmosphere. The methane often remains in the undersea sediments, dissolves in the ocean, or is converted to carbon dioxide by microbes.
Oceans retain virtually all the methane
The authors explain how methane sinks are increasingly incorporated into numerical models of climate-hydrate-interactions as knowledge becomes better established.
“Models are beginning to acknowledge that most CH4 bubbles emitted at the seafloor at water depths deeper than a few tens of meters will retain little or no methane by the time they reach the near-surface mixed layer, meaning that the primary repository of methane liberated by gas hydrate dissociation within any deepwater marine reservoir will be the ocean, not the atmosphere [e.g.,Biastoch et al., 2011],” the authors summarized.
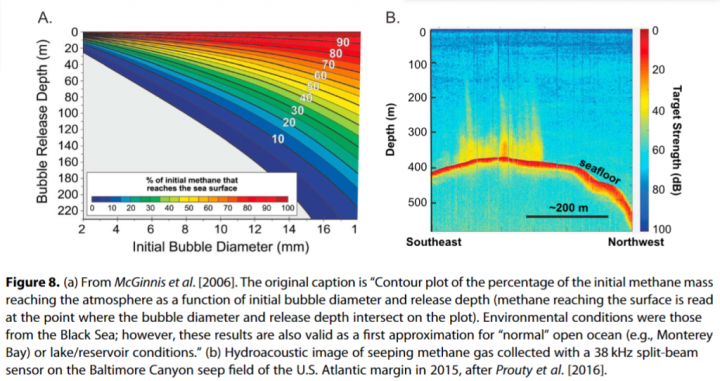
Image source: Reviews of Geophysics
Models are beginning to recognize? Are you joking?
Here is what NOAA says about methane hydrates:
Fuel of the FutureIn the past decade, ocean seismic surveys, deep-sea drilling and submersible studies provided the first hint at the amount of gas hydrates that exist on Earth. The concentration of gas molecules locked into hydrate crystals is up to 160 times greater than the same volume of pure gas. The result may be the largest single reservoir of carbon on the planet—at least twice as much as all other fossil fuels (e.g. oil, natural gas, coal) combined. The U.S. Exclusive Economic Zone (which extends to 200 m offshore) may contain as much as 200,000 trillion cubic feet of methane in hydrates (Kleinberg and Brewer, 2001). This is enough clean natural gas to power the United States for centuries. Read more about gas hydrates as a potential energy source.
Methane hydrates exist because the earth exudes methane. At a given temperature and pressure, it becomes like ‘ice’. It is not fossil in origin, the earth oozes methane all the time. It would be hard to harvest, but if the CO2 group’s expenditure on ‘stopping’ fossil fuel is any measure, it would be a relatively simply challenge to harvest it. Not solar. Not wind. But enough energy just sitting on the bottom of the ocean to power the world for centuries…
So far there is no economic method of harvesting gas from ocean hydrates but R&D efforts are making progress.
Japan is so far leading the world in gas extraction from ocean hydrates (China might be now close). Japan have now years of research that is gradually identifying the technical issues:
https://www.mh21japan.gr.jp/english/results.html#ach-01
Australia has enough easily accessible lignite to power the country for centuries. This fuel is not economic to export so there is only a domestic market with power stations sited on the coal fields.
Photo of methane hydrate in a grapefruit-size chunk of seafloor mud. If you want to ‘harvest’ it, you either heat it (and the mud) in place, or mine it and bring it up to higher depth or the surface to reduce the pressure. Both ways are costly, more than the gas will likely bring.
The stuff just isn’t concentrated enough, and merely poking a drill pipe into an area of hydrates won’t produce anything because it’s a solid and doesn’t flow as oil and gas do. If our descendants get desperate enough, maybe they can afford to mine it. The limitation there is whether they’re using more energy to produce it than they’re getting out of it.
It floats!
“gas extraction from ocean hydrates “
There is the problem, “the reaction that releases methane is “endothermic.” The significance of that, she says, is that the methane absorbs heat from the surroundings, and the methane “keeps shutting itself down.” (Carolyn Ruppel, PhD, who heads the gas hydrates research project for the United States Geological Survey.)
Methane is found on some planets and moons in the solar system. It may be that methane inder pressure and heat and catalysts over time becomes oil – no dinosaurs involved.
You are missing a step. How do you get the organic material to the bottom of the ocean so that it can rot and create the methane.
Any methane in the atmosphere would have been destroyed as soon as plants started creating oxygen.
Any methane brought to the planet during its formation would have floated to the top (atmosphere) while the earth was still molten.
What are these catalysts that turn methane into oil and tars?
Temperature causes things to go the other way.
But at what pressure? Anyone who wants to calculate the amount of gas in a volume, but leaves out pressure is indicating a complete lack of seriousness.
Pressure at 500m depth in the ocean is 49 atm. Pressure in a portable container is 200 atm, four times as much. If it could be fragmented easily (say, with a remote robot ’tiller’), it floats in seawater and would rise up a large diameter pipe, shedding first mineral material and then water at near-surface temperatures.
One m³ of methane clathrate contains 169m³ of methane at STP. It seems with the right apparatus configuration, it could be produced already significantly pressurized.
We must discover ways to prevent microbes from converting CH4 into CO2 to save the planet. Your children’s children future depends on it.
Let’s start a movement to save the planet (from nature ) …
‘Just Stop Microbes’
It’s usually O2 that converts CH4 into CO2 and H2O.
Sometimes slow, sometimes fast
You say that methane is a far more powerful greenhouse gas than CO2. Happer found from a line by line spectral analysis that methane is a week warming agent. Where did you get your information?
Where did you get your information?
__________________________________
Oh it’s easy to figure that out, just do a Google [News] search on “Methane times” and you will come up with oodles of stories claiming it’s 80 times more powerful than CO2 and zero stories telling us how much warming it will cause. Sort of like the dearth of stories these days about polar bear extinction.
The warming potential of methane is essentially zero.
https://wattsupwiththat.com/2014/04/11/methane-the-irrelevant-greenhouse-gas/
“ is a week warming agent.”
Cyclical?
Yup. Twice fortnightly!
Everyone is a little wrong, OK orders of magnitude wrong at some wavelengths. The attached is an Illustration of absorption cross-sections for CH4 (black), CO2 (grey), and H2O vapor (blue) as a function of (a) solar and (b) thermal infrared wavelengths (right) in a 1-bar atmosphere. Wavelength in microns is 10,000/wavenumber. So the atmospheric window 8 to 14 microns with Wein’s law Tmax of roughly 90 C to -65 C (most all Earthly temperatures) is wave numbers 1250 to 715….Well, its clear that the one number published by the IPCC is invalid over any reasonable range, not to mention the chart is per molecule and there is roughly 10^-2 as much methane as CO2 and 10^2 as much H2O as CO2 in the real atmosphere.
So at say 15 C, 288 K, Weins law Tmax, so 10 microns or 1000 waves /cm…from the following graph CH4 has an absorption cross section of about 1 thousandth of a CO2 molecule, and 1 hundredth the concentration….so a nothing burger as far as ability to warm surrounding air molecules compared to CO2.
Criticism accepted with appreciation, I’ve never used this particular chart before…
Weak
P Gosselin says, “Methane is a far more powerful greenhouse gas than CO2”
______________________________________________________________
Please stop buying into the bullshit.
The clue for Mr. Gosselin is that we are never told how much methane is going to warm the planet. Telling us that methane is a powerful greenhouse gas, needs a second clause telling how much warming it will cause. We are NEVER told how much that is. Various posts here at WattsUpWithThat indicate that by 2100 methane might cause something less than a tenth of a degree of warming. Perhaps ~0.05°C. If anyone thinks would be more than that, they should pipe up and show their source or work.
Methane is irrelevant.
First, there isn’t much of it – 2 ppm – as opposed to CO2’s ~420 ppm, and water vapor’s 40.000 – 50,000 ppm.
Second, like CO2, its IR response is almost entirely masked by water vapor.
The idea that Methane is a ‘greenhouse gas much more powerful than CO2’ is based on early estimates of the ‘forcing’, estimates made by amateurs and dilettantes who thought that such calculations were simple schoolboy physics. Witness: their overestimate of the forcing by a factor of at least 3 for CO2. If you do the correct analysis following the methods set out by Happer and Wijngaarden then you get that even if you increase the atmospheric Methane content by a factor of 10 the surface temperature response is less than 0.4C. It is a complete red herring.
“estimates”
Guesstimates.
Decades ago Forbes magazine had an article about methane hydrates and concluded that there was over 3000 years available based on then present usage.
There was also years ago an idea put forth that the large depression in the Voring Plateau was a release of massive amounts of methane that led to the end of last glaciation.
https://geographic.org/geographic_names/name.php?uni=-242672&fid=6438&c=undersea_features
Unless God created ocean methane hydrates in place, they must have been deposited. Does that process continue today?
Methane is part of the earth to start with. It is now and always has been seeping into the atmosphere. Methane is common in coal mines. The coal doesn’t make the methane, the methane makes the coal. Read ‘The Deep Hot Biosphere’ by the late Thomas Gold for enlightenment. He showed pictures of fossilized trees where only a portion of the tree became ‘coalified’ due to a methane path through that part of the tree.
This whole idea of fossilized plants and animals becoming oil is simply ridiculous. Everyone knows what happens to plants when they die – they compost. And that becomes food for more plants. Gold hypothesized that there have never been enough plants grown on earth to equal the amount of gas, coal and oil that exist, if it ALL turned into ‘fuel’. It doesn’t.
jshot,
I think I’ll go with the geological consensus on this. Thanks anyway.
According to the IPCC reports, the oceans have warmed about 0.01C. I’m going to guess that most of that warming is occurring in the surface waters, not the deeps.
So how much of the hydrates are going to melt based on this less than 0.01C warming?
Methane is a high energy food source for bacteria in the sea. This is also true for hydrocarbon seeps on the ocean floor. The reason the huge release of petroleum from BP’s ruptured drill platform pipe in the Gulf of Mexico in 2007 did not cause massive fouling of beaches in Florida as was expected, is because of the large population of oil-eating bacteria in Gulf waters. Countless natural oil seeps in the gulf maintain the population.
Similarly, oil seeps abound offshore of California. Ironically, allowing oil production in these prolific resource areas reduce formation pressures, thereby reducing and eliminating the seeps. Over time, the seeps actually end up producing the oil into the environment anyway.
There is a coal mine in Colombia where they find fossils of extinct 20 foot crocs…40 foot snakes…the coal apparently preserved the fossils and the coal was not made from them…..a petroleum co. once had a dinosaur image as part of its logo….but sorry….no oil from dino…..Sinclair oil co.
Coal also preserved giant fern trees 300ft or more that are part of the coal itself. The trees grew in giant swamps, so the crocs and snakes were at home in this foetid wet environment.
The coal seams built up in slowly sinking areas with new generations of swamp forest growing on top of old fallen ones. Eventually these deposits would sink below sealevel and become layered over with and silt and clay. With deeper burial, lithostatic pressure would squeeze the layer, carbonizing the wood and other plant matter. Probably, the crocs and snakes fossilized and preserved were in the final cycle of coal creation.
And the carboniferous era ended when fungii evolved that rotted the dead wood…which had previously been piling up in many places dozens of feet deep, eventually compressed about 10:1 into a 10 foot or so thick coal seam. This was fortunate as the fungii released CO2 back into the air instead of it all being sequestered in coal. There must have also been massive forest fires at the time, but without much evidence in the coal beds, where one would expect to see ash layers.
a study on Eemian period methane releases came out a couple months before the one cited by Nature in the above article – it was reported on by WaPo in Aug 2022 (https://www.washingtonpost.com/climate-environment/2022/08/24/methane-hydrates-ocean-global-warming/) – which took a surprisingly moderate position – “…some experts say it’s not cause for alarm”