From NOT A LOT OF PEOPLE KNOW THAT
By Paul Homewood
h/t Philip Bratby/Paul Kolk
In BBC world, everything bad is due to climate change!

For Antarctic wildlife, exposure to the Sun’s damaging rays has increased in recent years, scientists say.
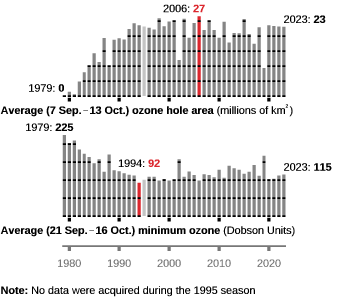
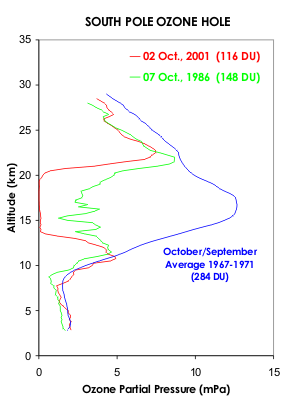
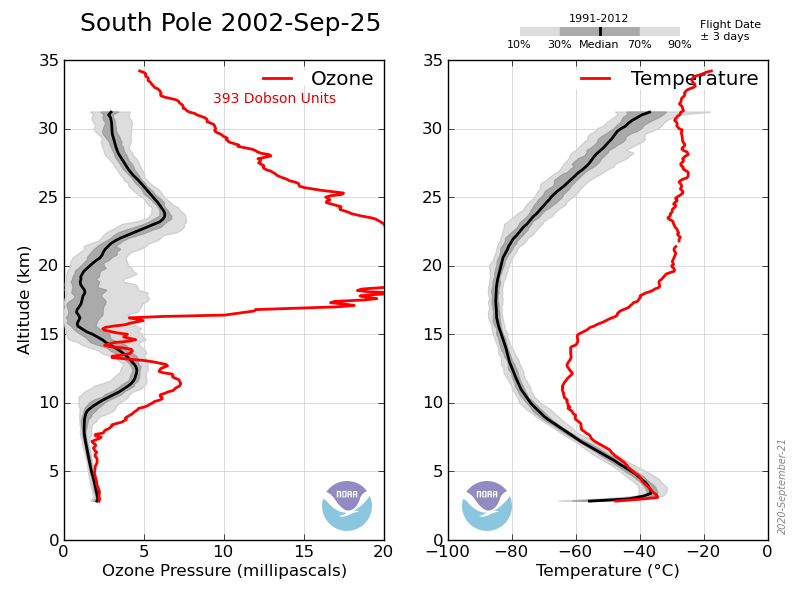
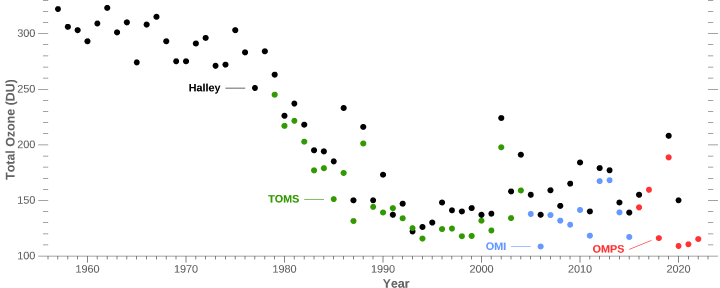
A hole in the ozone layer – the protective barrier of gas in the upper atmosphere – now lingers over the frozen continent for more of the year.
A major cause of ozone loss is believed to be the amount of smoke from unprecedented Australian wildfires, which were fuelled by climate change.
https://www.bbc.co.uk/news/science-environment-68906013
As we know, the 2019 drought was far from being unprecedented in SE Australia, the region worse affected, so climate change had no effect. Instead there is plenty of evidence that poor forestry management was the biggest factor.

In fact the paper itself makes no claims about “unprecedented Australian wildfires”, and the factors behind ozone loss are much more complex and nuanced than the BBC reports. In particular, La Nina, the polar vortex and the Hunga-Tongo volcano all play a role alongside Australian bushfires. The BBC report makes no mention of any of these other factors.


https://onlinelibrary.wiley.com/doi/epdf/10.1111/gcb.17283
As with all these sort of studies, the scientists are only looking at a few years worth of data, so they have absolutely no idea whether similar cycles of ozone loss occurred naturally and regularly in the past.
But that is of no concern to the BBC, who would rather persuade you that Antarctic animals are being sunburnt because of your consumption of fossil fuels.