Guest Post by Willis Eschenbach
OK, folks, for everyone who wanted to put forth your favorite theory about how downwelling radiation from the atmosphere is a fantasy, or how a cool atmosphere can’t leave the surface warmer than no atmosphere, or how pyrgeometers are fatally imprecise … this is the thread for you.
However, I’m going to ask that before you start, you understand my actual position on these questions. So I strongly request that before you comment, you read the following four posts. That way, you’ll be clear about my thoughts on the matter.
Can A Cold Object Warm A Hot Object? 2017-11-24
Short answer? Of course not, that would violate the Second Law of Thermodynamics —BUT it can leave the hot object warmer than it would be if the cold object weren’t there. Let me explain why this is so. Let me start by introducing the ideas of individual flows and ne…
Radiating the Ocean 2011-08-15
Once again, the crazy idea that downwelling longwave radiation (DLR, also called infra-red or IR, or “greenhouse radiation”) can’t heat the ocean has raised its ugly head on one of my threads. Figure 1. The question in question. There are lots of good arguments against the AGW consensus, but this…
The Steel Greenhouse 2009-11-17
There is a lot of misinformation floating around the web about the greenhouse effect works. It is variously described as a “blanket” that keeps the Earth warm, or a “mirror” that reflects part of the heat back to Earth, or “a pane of glass” that somehow keeps energy from escaping. It is none of these things.
People Living in Glass Planets 2010-11-27
Dr. Judith Curry notes in a posting at her excellent blog Climate Etc. that there are folks out there that claim the poorly named planetary “greenhouse effect” doesn’t exist. And she is right, some folks do think that. I took a shot at explaining that the “greenhouse effect” is a…
OK, now that y’all have read those four posts, and you are all clear about my position, let me offer some data to focus the discussion. Figure 1 shows the month-by-month surface shortwave (solar, “SW”) and longwave (thermal infrared, “LW”) radiant energy flows at the SURFRAD station in Goodwin Creek, Mississippi. The US maintains something called the SURFRAD (Surface Radiation Budget) Network of eight surface measuring stations. These have a variety of sensors that, as the name suggests, measure a variety of surface radiation flows. Each station has a Downwelling Pyranometer, Upwelling Pyranometer, Downwelling Pyrgeometer, Upwelling Pyrgeometer, UVB Sensor, Photosynthetically Active Radiometer, Normal Incidence Pyrheliometer, and a Shaded Pyranometer. These are calibrated annually to assure accurate measurements. They collect data on an almost continuous basis, 24/7/365. The stations have data from 1995 to the present.
So I picked a SURFRAD station at random, Goodwin Creek, Mississippi. And I picked a year at random, 2014, and downloaded the monthly average data from here. After I plotted it up I thought “I wonder how well this agrees with the CERES satellite-based dataset?” So I added the corresponding CERES data to the chart. Here is the result.

Figure 1. SURFRAD and CERES data, Goodwin Creek, Mississippi. The CERES data is for the 1° latitude by 1° longitude gridcell where the SURFRAD station is located. The background shows the Goodwin Creek SURFRAD station.
Now, folks have been questioning lately whether the CERES data is accurate enough for the type of analyses that I do, whether it is fit for the purpose … this should allay some of their concerns.
With all that as prologue, here’s the important part of this discussion.
The red|orange lines show the amount of solar energy that is absorbed by the surface. It’s the net of the downwelling solar minus the solar that is reflected back upwards from the ground. As you can see, the annual average solar energy absorbed by the surface is about 150 watts per square metre (W/m2).
The yellow|gold lines, on the other hand, show the upwelling longwave (thermal infrared) energy, energy that is radiated upwards from the surface. The annual average upwelling longwave energy is about 395 W/m2.
Now, for all of you that think that downwelling radiation from the atmosphere is a mirage, here’s the question.
If on an ongoing basis the surface is only absorbing 150 W/m2 of solar energy and is radiating 395 W/m2 of energy … why isn’t it frozen solid?
Seriously. If it is constantly radiating far more energy than it is absorbing … why isn’t it a block of ice?
To me, the obvious answer is, the surface is also absorbing downwelling radiation from the atmosphere. In Figure 1 above, the blue|cyan lines show the total of the net solar (SW, red|orange lines), plus the downwelling longwave thermal infrared (LW) from the atmosphere.
The annual average of the net downwelling radiation at the surface (SW +LW), the total energy absorbed by the surface, is about 490 W/m2. This is about a hundred W/m2 more than the energy that is lost to radiation, with the rest of the surface energy loss being in the form of the net of the sensible and latent heat lost gained and lost by the atmosphere via convection and conduction.
So there you have it. If you don’t think that downwelling LW radiation leaves the earth warmer than it would be if there was no atmosphere, you need to explain the mystery source of the additional energy necessary to keep the earth from freezing. And no, it’s not geothermal heat. We know from borehole measurements that geothermal heat, in general, is on the order of a tenth of a W/m2 or so … and we’re missing about 395 W/m2 emitted minus 150 W/m2 absorbed equals 245 W/m2 necessary to prevent freezing.
So what is the mystery source?
Let me add that the most excellent agreement between the SURFRAD and the CERES data means that it’s not instrumental error, or scientists who don’t know what they are measuring.
So where is the energy coming from?
My best to all, let the bunfight begin, and please, keep it civil … I may be wrong, but I’m not an idiot …
w.
As Usual I Politely But Loudly Request: QUOTE THE EXACT WORDS YOU ARE DISCUSSING. I can defend my own words. I can’t defend your interpretation of my words.
Nice posting Willis – I especially enjoyed how you packaged some older postings together as a coherent whole; I had never seen some of those other postings.
BUT…
I have some questions or nit-points. It seems to me that everyone assumes you can just simplify the climate and it all fits nicely together. I do not. My experience with natural systems is they are messy in the details, and sometimes the details are important after all.
Posting 1: Can A Cold Object Warm A Hot Object? I get the basic idea, it seems rather obvious to me in the simplified case. But atmosphere isn’t behaving the same way as a block of wood. It is a “fluid” that is moving about transporting heat both horizontally and vertically. The heat source (In this case the Earth) is not heated equally either – it is colder at the poles and warmer at the equator. I can agree that ignoring certain sources and sinks of energy are likely OK – nuclear half-life (emission of heat) and photosynthesis (sink for certain energies) for example, but the transport of heat is not trivial, especially since water is involved. So while I get the basic principle as you have explained it, I find myself wondering just how well it really behaves given reality of a complex climate system. I think real detailed measurements would be difficult to explain because the energy is moving about – one just has to infer a ground temperature if using a satellite.
Posting 2: Radiating the Ocean I understand this point as well, but I don’t think one can dismiss evaporation so easily. If the top layer (you use 1 mm) is warming, the evaporation should be increasing (assuming other contributing factors are stable). This provides a “ceiling” at which point warming will have an ever difficult time in increasing past – likely a logarithmic function. Also, the condition of the surface of the water likely plays a key part – I imagine strong winds blowing across the ocean’s surface will greatly increase evaporation (more surface area, dryer wind being introduced, sea spray droplets in the air). I wonder if our wonderful climate models take any of this into account?
Posting 3: The Steel Greenhouse My only problem with the term “Greenhouse Effect” is that a greenhouse works by physically preventing warm air from escaping once it is warmed by radiation. Our atmosphere is a highly active and changing system that behaves as it does BECAUSE it is moving about and because of water going through state changes as it moves. If you measure long-wave radiation up-welling from the Earth you do not necessarily know how it came to be where you measured it, only that the up-welling and down-welling totals should balance. Heat can move before it becomes radiation again.
I know I am nit-picking, but a long career in computers and programming has taught me the details are important (like round-off error).
Where does the actual Energy of the Radiation come in to the Calculations?
The Energy of UV, Short wave and white light is far higher than LWIR, this is obvious by the penetration of those frequencies in to the Oceans.
When those frequencies hit the surface and give up their energy how can that be compared to LWIR?
AC: You are confusing the total power flux density of radiation with the energy in an individual photon.
The energy in an individual photon is inversely proportional to wavelength (so directly proportional to frequency). For example, a single UV-B 300nm photon has 50 times the energy of a single LWIR 15um photon. This is why UV-B can break chemical bonds, leading to sunburn and skin cancer, and LWIR cannot.
But a 1 W/m2 power density flux of UV-B absorbed by an object transfers the same power as a 1 W/m2 power density flux of LWIR absorbed. It does take 50 times the number of 15um photons to produce this power density flux than 300nm photons.
No am not read it again.Quote “The Energy of UV, Short wave and white light is far higher than LWIR”
You absolutely are! Energy is measured in Joules, and the rate of energy transfer is measured in Watts. A Joule of LWIR has the same energy as a Joule of UV. As I have said, it takes many more photons of LWIR than UV to comprise a Joule, but two entities with the same Joules have the same energy.
This is such a basic point that it typically goes without saying.
Where in the calculations does it account for the Penetration of Water by higher energy Photons thus storing the energy making it unavailable for immediate Outgoing LWIR?
Glass absorbs your “higher energy” UV but allows “lower energy” visible light to pass through. Your paradigm is all wrong.
So you are saying it is not UV, SW and White Light that heats the oceans to depth?
That is new.
AC:
I simply pointed out through an obvious real-world counter-example that your assertion that some “higher energy” property of shorter wave radiation — seemingly that higher-energy photons can “push” further through material before being absorbed — was not correct.
I did not claim that visible light travels further through water than LWIR. That is directly observable.
Then, you agree about water, so where is that in the calculations
AC:
First, of course I meant to say: “I did not claim that visible light DOES NOT TRAVEL further through water than LWIR.” I’m glad you realized what I meant to say.
Your question “where is that in the calculations?” is a good one. The answer is that it depends on how detailed you want your calculations to be. As a first cut approximation, it doesn’t matter much whether it is absorbed in the first mm (LW) or the first few meters or tens of meters (SW). The immediate effect of a Joule absorbed at either level is to increase the thermal energy of the water, and losses must increase to balance this.
Going into more detail, consider the thin surface layer that absorbs all the incoming LWIR. This same surface layer also emits all the LWIR. Under “average” earth conditions, it is emitting about 400 W/m2, and absorbing about 300 W/m2, so there is a net radiative output of about 100 W/m2.
So the downwelling LWIR, reduces the net radiative losses from this surface layer by about 75%. This makes the surface layer far warmer than it otherwise would be. Compared to the case where there was no downwelling LWIR, this effectively acts as a layer of insulation, just like in the walls of your house, or the jacket you wear in cold weather. And this most certainly does have an effect on the temperature of the water further down.
I get your analysis but IMO, you leave something out. As a layman I only know that sometimes it is icy and sometimes it is warm on Earth. When it’s warm on Earth, then there must be retained heat. When it is icy, the heat is dissipating. To me the question is, why are we icy and why are we warm? No one seems to have a good explanation for that. So, if you want to say that it’s established science that the earth warms, you should say “under certain circumstances”. What am I missing?
Impossible to answer your question without knowing if you are talking about a single location on a daily basis, or over a year, or from one year to another, or between interglacial and glacial periods, or if you are talking about the difference between the poles and the tropics, or what?
The answer to each is different.
Sometimes it is warm because it is Summertime, and the part of the planet one is on is facing the Sun, and so the rays of the Sun hit the surface more directly, and the days are far longer, than when that part of the planet is facing away from the Sun.
But in some places and at other times, it is warm for other reasons.
Willis; the radiative impact of GHG’s in Earth’s atmosphere is to replace surface emission at the GHG wavelengths with emission from the top of the GHG column (ie: the tropopause). Since the tropopause is colder than the surface the total emission at these wavelengths is reduced therefore the GHG’s do reduce radiative loss which is in agreement with what you say in your article. HOWEVER
What I never hear discussed is that the atmosphere does mechanical work, for example winds, raising water to high altitudes. The energy for this work comes from absorbed solar energy. That means the atmosphere is a heat engine and heat engines are governed by Carnot’s laws, known since the 18th century. Most importantly this means there must be a hot junction where heat enters the working fluid (the surface in this case) and there must be a cold junction where waste heat leaves the working fluid, in this case the top of the convection loop ie; the tropopause (or lower stratosphere). This heat can only be lost by radiation and any gas that can radiate energy in the thermal IR is BY DEFINITION a GHG. Without green house gases there could not be a cold junction and that means the atmospheric heat engine could not operate. That in turn means no convection, no surface evaporation, no rain, no wind, no clouds, without convection no dust, no weather of any sort. The entire atmospheric column would be isothermal and saturated with water vapour. Because of the short time constant of dry soil (no rain) and other surface structures (think about how quickly beach sand or concrete heats up on a clear summers day) the temperature would reasonably closely track the instantaneous insolation. In the tropics this peaks at 1350 watts/sqM at noon giving a surface temperature of over 100C. Of course at night this would fall far below freezing. Far fetched? Not so, its the same as what happens inside a closed car left in the summer sun and even here in Melbourne (lat 37 south) the temperature can reach far over 60C killing children in minutes as alas too often happens. It also is similar to what happens on the moon (except the lunar “day” is 28 days not 1 as on Earth which changes the impacts of time constants)
The major net heat losses from the surface are due to evaporation and consequent convection and without GHG’s these would not occur. The total impact of GHG’s is to ameliorate surface temperatures reducing the maxima and increasing the minima to a point where life can exist on Earth. Unless someone can show there is a point of inflection, it follows that an increase in GHG’s is likely to lead to further amelioration ie: an even more benign climate not a more extreme one.
I know the above is too brief and sketchy. I put it all in far more detail in a post I sent to WUWT but unfortunately they chose not to post it. I would be interested to read your thoughts on the above.
Exactly.
”Without green house gases there could not be a cold junction and that means the atmospheric heat engine could not operate.”
Not exactly as there would still be a cold junction since O2,N2 are not IR active gases but they do both radiate and absorb feebly in the IR bands as they are matter. Absent the atm. IR active gas (water vapor,CO2, et. al.), Earth’s heat engine would still run but much more feebly i.e. not constant T(z) in the troposphere where there would still be weather.
Carnot has no “laws” named after him.
He has some principles, and a theorem.
Sometimes called a rule.
He is known for describing the limits of efficiency of heat engines.
I for one have no idea what anyone hopes to prove these endless hypotheticals regarding what the Earth would look like if something that will never happen was the case?
We cannot get ten people to line up and agree on the details what is happening in the actual world we are all sitting in the middle of for our entire lives.
If my Grandma had had balls, she would have been my Grandpa.
But then I woulda never been here, so she would not have been.
If sewer rat tasted like pumpkin pie, every day would be Thanksgiving for the homeless in New York City.
Or would it?
Nicholas, you have utterly missed the point I was trying to make. Subscribers to CAGW are claiming that the impact of GHG’s in Earth is to warm the planet. Less GHG and the planet gets too cold, more and it gets too warm so “lets stop increasing CO2 and frying the planet”.
The point is that GHG’s do NOT just make the Earth warmer. Yes they reduce radiative loss to space but they also create the conditions required for an atmospheric heat engine by creating a cold junction at the level of the tropopause ie: allowing energy loss to space from this region. That heat engine is what causes weather. Without it the daily temperature extremes (not to mention other minor issues such as no rain, no clouds etc) would be such that no life could exist at least on land. The total NET impact of GHG’s (among other things) is to ameliorate temperatures, reducing the temperature maxima and increasing temperature minima (via evaporation/condensation of water and convective processes). You can clearly see the impact by comparing conditions in a desert relative to a watery environment. With GHG’s conditions on our planet would be more extreme than the worst desert.
Now, in such a system there are really two alternatives. Firstly does the effect increase as GHG’s are increased (in which case more CO2 will result in an even more benign environment) or is there a point where the effect reverses and if that is the case where is that point of reversal. Which side of the reversal are we currently at and what causes the reversal in the first place? Surely questions that are VERY VERY far from trivial.
Is is a very different yet relevant point of view which is being completely overlooked by warmists simply claiming more GHG makes the planet warmer. It shows that their perspective is seriously deficient (at least in my view). So deficient that it makes their conclusions highly suspect. That is not irrelevant.
In answer to your point about Carnot, I think you are splitting hairs. Carnot pointed out that a heat engine cannot be 100% efficient, that heat must be lost from the working fluid via a cold junction that the maximium efficiency is defined as (Thot-Tcold)/Thot. He completely changed our understanding of how heat engines work. Whether you want to consider these simply insights or laws is entirely up to you but I don’t see that it changes his massive contribution in any way.
I can tell you know what you are talking about, but the point you were making above is drowned out by a long discussion of hypotheticals, which excuse me for saying so again, I have never observed to move any conversation forward in these matters.
In some comments below, I think you did clarify some things that needed to be clarified, but as was noted by others, you also said a few things that do not seem to be entirely true.
I might have skipped making that comment about Carnot and his work, but the thing is, when I read your comment, I had to stop and look to some reference material, looking for something that I did not recall.
So that is why I mentioned it. I was tired I suppose.
I do not think I missed what you were saying entirely, although it is difficult to parse long strings of hypothetical arguments to find the point.
And that was my point.
Heat engines are at their essence simple devices based on straightforward principles.
The atmosphere is anything but simple and encompasses nearly every aspect of several different branches of science.
Reducing this complexity to something simple is impossible, IMO.
Warmistas have gone wrong for the precise reason that they want to make it all very simple…more CO2 = hotter planet.
Which is very certainly wrong, unless everything we know about Earth history, as well as the more recent proxy and recorded temperature records, is wrong instead.
Greenhouse gases help provide a “cold junction” to drive the global heat engine, but they’re not the only one. Surface radiation from regions of the planet with lower insolation (e.g., the polar regions) also act as a heat sink that can help drive global convection.
No.
Moderating variations in surface temperatures is one of the effects of GHG’s and atmospheric (and oceanic) convective circulation.
But, this is not the only effect.
To see how GHGs can warm the surface, you might consider my essay Atmospheric Energy Recycling.
If the other gasses in the atmosphere don’t radiate much and the only way for energy off the planet is via radiation then I don’t see how adding more radiators makes the planet warmer. Less radiant gasses would seem to hang onto their energy longer and while more radiant ones might absorb energy they are also radiating faster and radiation is ultimately a cooling function. Perhaps the two balance to a certain energy level?
I do believe that when things warm they radiate faster thus cool faster. I believe that if the upper atmosphere is getting colder and the lower is getting warmer then convection accelerates, mixing the atmosphere between the two thus negating most of this effect. I believe in emergent phenomenon such as hurricanes, storms, clouds kicking into action, transporting or confining large amounts of heat to the upper more easily cooled atmosphere as needed. I believe energy in = energy out minus the emergent phenomenon called photosynthesis which both stores energy and accelerates when carbon dioxide is more readily available. This is probably a small effect but still they talk about energy imbalance and this must be at least part of this imbalance. I believe there are all sorts of physics and astronomics that have to be analyzed with empirical data in much more detail before we really get a handle on what tomorrow’s climate will bring.
The universe has a strange way of both expressing its self and gradually revealing its true nature in ways that we haven’t contemplated. The state of the climate models seems to show this very well.
I have been saying for some time that it is oxygen and nitrogen that are the real “retainers of heat”, it is GHGs that do the cooling, as they are only ones that can to any extent.
The thing is Greg that the additional radiators (GHG’s) do not operate in conjunction with the surface, but rather they replace part of the surface radiation. At the GHG wavelengths the surface emission is absorbed by the GHG’s low in the atmosphere. These GHG’s also radiate at the same wavelengths so, throughout the atmospheric column, energy at the GHG wavelengths is being continuously emitted and reabsorbed by the GHG. It cannot escape to space because it is reabsorbed by the GHG above or below before it can do so. Its only at the very top of the GHG column that the energy emitted is able to escape to space. To give some idea of the scale, spectroscopists talk about a parameter called absorbance. In essence it is a gas column that absorbs 90% of the light at the GHG wavelength incident on that column. Double that column length yields 2 absorbance absorbing 99% of the incident light (the first half absorbs 90% and the second half absorbs 90% of what remains). So what is the total absorbance of the atmospheric CO2 column? At the current concentration of CO2 its around 3000 abs. That means 90% of the surface emission is absorbed in the first 1/3000 of the atmosphere – around 10 meters, 99% in the first 20 meters and so on. It also means of course that only the bottom 10 meters or so of the CO2 column can radiate back to the surface. Any emission from the GHG is reabsorbed within a few meters of the point of emission. Its only the last 1/3000 of the CO2 column that can radiate to space and thats a really thin layer in the lower stratosphere (or tropopause).
So, if the total absorbance is 3000 that means its really really really saturated with respect to CO2 so why does increasing CO2 make any difference? The reason is that the absorption line is not a boxcar in shape but rather something close to a gaussian (its called a Lorenzian). Each time one doubles CO2 it is equivalent to convolving this gaussian with itself and the result of that convolution is a new gaussian which is wider ie: absorbs over a greater range of wavelengths. Each doubling of CO2 increases the wavelength range over which the gas absorbs by about the same amount which is what causes the logarithmic relationship. In fact, the unbroadened absorption lines are so narrow that GHG’s really only have any impact on radiative processes after the line center becomes saturated (ie: total absorbance of the gas column exceeds 1-2 abs at the line center).
hi Michael,
thank you for this. I rarely see this discussion get closer to the lower level physical transactions if you will. Can you explain what is causing this broadening of the absorption lines as more c02 is added? surely the ability of co2 to interact with. a photon of frequency X has not physically changed. is it just that the probability of the interaction decreases as you get away from the central absorption lines so that the lower probability transactions are adding to the absorption as co2 concentration increases ? also presumably the falloff in probability gets very steep at some point so that at some concentration full saturation is effectively reached. if that is so do you have any numbers around it?
thx d
Hi Davidh; good question. A molecule can absorb photons of some wavelengths because the molecule is made up of atoms (think of them as masses) connected by elastic bonds. The result is that these atoms can vibrate relative to each other in various ways. These resonances only occur in asymmetrical molecules which is why oxygen and nitrogen are not GHG’s. If a photon of the same frequency comes along it can excite this resonance and is absorbed in the process. So, you might think that only a photon of EXACTLY the right frequency can be so absorbed. Not quite; because all resonators have what is called a quality factor usually designated as Q. Because the Q is always finite (no resonator is perfect), the plot of resonance intensity versus frequency is not a line but instead a gaussian like curve (sometimes called a bell curve). That means that there is a non zero probability of a photon not quite at the resonance frequency being absorbed although the probability falls as the frequency difference between the photon and the resonance increases.
There are a number of factors that can broaden or shift the resonance. One is if the molecule is moving, its velocity will doppler shift the apparent frequency of the photon. Since in a gas, molecules are moving in all directions some will be travelling towards the photon some away from the photon so some absorb lower frequency photons and some higher frequency photons with the result the apparent resonance broadens. This is termed doppler broadening. Another way if if there are surrounding molecules which are hitting the absorbing molecule. These collision inject some energy so that the photon energy required to excite the resonance is changed. The higher the pressure the more such collisions there are and the more Q is lowered (ie the resonance broadened). It is termed pressure broadening. One could consider a solid to be the ultimate state of pressure broadening and in that case the absorption becomes a continuum rather then exhibiting a specific resonance. However it is not necessary to go quite that far. The high pressure discharge lamps used in digital projectors use pressure broadening with a pressure inside the bulb of up to 1000 psi so that the light emitted is more or less a continuum white light even though the emitting gas nominally shows resonances. While doppler and pressure broadening will occur they are not the significant factor here.
Consider we double the amount of CO2 in the atmosphere. That’s like 2 of our old atmospheres (pre doubling) placed one after the other. If the old atmosphere exhibited a gaussian absorption profile the new profile will show a profile which is the convolution of the old profile with itself (please look up “convolution” if you are not familiar with the term). If you convolve a gaussian with itself, the result is also a gaussian but one which is broader ie: a larger standard deviation. That essentially means the apparent line with is broadened. For example, if out in the wings of the absorption band a photon had say a 50% chance of being transmitted in our old atmosphere, in the new atmosphere it will have a 50% *50% = 25% chance of being transmitted. If the chance of transmission was 90% in the old atmosphere it will be 0.9*0.9 = 0.81 (81%) in the new atmosphere and so on.
I know its brief but I hope this answers your question.
The only problem with your description is that the KE exchange due to collissions happens much quicker than a photon release, therefore Convection will shift the heat up in to the atmosphere quicker.
That’s really not a problem.
When a GHG molecule absorbs a LW photon, yes, the absorbed energy is quickly thermalized, resulting in an incremental warming of the mixed gases in the air. The converse also happens, warm air in particular warms GHG molecules, and those molecules will sometimes radiate.
Convection doesn’t necessarily have much interaction with this process, because the layers of air above and below contain similar amounts of GHGs are are be warmed by very similar amounts. So, there is little temperature difference created, and little if any additional convection that results.
“The converse also happens, warm air in particular warms GHG molecules, and those molecules will sometimes radiate.”
I am so glad to hear someone say this.
Greg:
The key point you are missing is that radiators MUST also be absorbers (Kirchhoff’s Law). So an atmosphere with no radiating gases (O2 + N2 + Ar comes very close) has no absorption of surface radiation, permitting all surface radiation to radiate directly to space.
In our atmosphere, with H2O and CO2 and a few other absorbing gases, only about 10% of surface radiation escapes directly to space. Most is emitted from colder, higher elevations.
If downwelling LW radiation is important in any way, what best way to double or 1/2 it?
Hi Gbaikie; if you have a look at NASA’s earth’s energy budget diagram they show the surface loses NET 58 watts/sqM by radiation 86 watts/sqM by evaporation and 18 watts/sqM by convection. Only 40% is by radiation. Without GHG’s the evaporative and convective losses would not occur (see my earlier post this thread around 2:11 pm). Unless there is a point of inflection (a point where the action of GHG’s reverses) then, while increasing CO2 will slightly reduce surface radiative loss, it would increase convective and evaporate losses even more which would mean the net impact would be to cool the surface! How, by increasing convection leading to more evaporation and more rain!
Critical Radiative Theory (CRT)
From the top.
Any BB surface at 16 C, 289 K, radiates at 396 W/m^2. Check.
A surface at 16 C, 289 K, radiates at 63 W/m^2. Check.
Emissivity = 63/396=0.16. Check.
IR thermometers are designed, fabricated and calibrated assuming BB.
They must be corrected with the 0.16.
Who says the surface radiates as a BB?
Trenberth does.
TFK_bams09 et al
You need to read up on Reflectance and integrating spheres.
Nick:
I repeat for the umpteenth time: You have absolutely zero understanding of the difference of gross and net flows of radiation. This is the MOST BASIC concept in radiative heat transfer, and it is COMPLETELY beyond you!
Nick S., I will point out for Earth global surface at 289K: emissivity + reflectivity + transmissivity = 1.0
Earth’s transmissivity is zero as incident light rays on the global surface do not come out the other side of the Earth. So for Earth global surface: emissivity + reflectivity + 0 = 1.0
Substitute in Nick’s derived emissivity of 0.16 for Earth global surface: 0.16 + reflectivity + 0 = 1.0
Simple arithmetic means Nick’s derivation has increased Earth’s surface global reflectivity to 0.84 albedo when satellite measurements of Earth’s global multiannual albedo are more like 0.3
As Ed Bo and Alexy point out, Nick S. needs to read up on the basics about which Nick S. is incorrectly writing.
The answer is right there on top , he said it himself.
Can A Cold Object Warm A Hot Object? Short answer? Of course not,that would violate the Second Law of Thermodynamics.
The second part of his claim – “BUT it can leave the hot object warmer than it would be if the cold object weren’t there” is just wording trick sleight of hand, because as far as physics are concerned, both of these scenarios are just one and exactly the same.
It should take even the slow ones among us no more than about two minutes to grasp once it is pointed out.
After that you don’t need to waste time reading the other links, they only contain mathematical psychobabble with no basis on the real physical world.
Hi Eben; sorry but your comment is incorrect. The difference is a hot object radiating into a cold environment or a hot object radiating to a warm environment. In the latter case the hot object loses less heat than in the former case. Practical example, go stand outside on a cold night and note how cold you feel, then, go stand inside a warm room and see if you feel just as cold or warmer. The room is still colder than you are yet you feel warmer. No its not due to conductive heat loss, its due to more back radiation from the warmer environment than you received from the cold environment. This is exactly what Willis was talking about.
The problem is the term “warmer than it would be”.
If this concept means “255 – a slowed cooling rate” then I can understand.
If it means (255 + heat) I can’t understand. The only way to increase the radiation temperature of 255 is to add heat. This means you have to have back radiation be more than what the earth’s surface is radiating. This of course assumes equilibrium between the earth/sun. It also assumes a transparent atmosphere. I know, I know, water absorbs a ton of “near IR” in the atmosphere so the atmosphere is not “transparent”. However, If you make this choice, then near IR absorption is not really “back radiation” either because the earth didn’t radiate it. Lots of radiation diagrams show the atmosphere absorbing about 76 W from the sun, but they then add that into back radiation also. That is wrong, it is really indirect sun radiation and should be added to the 161 W.
Also the term surface is defined very vaguely. Some people take it to mean earth’s surface, while other take it to be balanced radiation height.
Lastly, using the term GHG as an overarching term for radiating gases should be discouraged. From my investigations CO2 at best contributes 22% of the warming. I’m not convinced it is that high due to limited emissivity.
Water and water vapor are the big boys on the block. The latent heat capacity is tremendous and that is the real issue. Spending trillions upon trillions to reduce CO2 is ridiculous until a CO2 -> Water Vapor feedback can be proven and quantified.
Radiation is cooler in a room than the room temperature. This is because heated radiators heat the air molecules. A radiation panel can emit at room temperature making you feel at room temperature (instead of a radiator heating air molecules). Once the radiation panel is switched off you will feel the cooler air molecules. As radiation isn’t heat only the objects absorbing radiation produces heat. Desert vs Ocean. Over a 24hr period temperature barely changes over ocean, while desert increases by 10-15C in 8 hrs.
Should have mentioned, your confusion comes about because you are leaving out one word from your second law definition. The second law does NOT say a cold object cannot radiate energy to a warmer object it says NET heat flow is always from hotter to colder. Both objects radiate to each other all the time, its just that the warmer object radiates more so the NET flow is always from warmer to colder.
Read a little about the Pictet experiment. It will bend your brain. Some intuitive thoughts are not always correct.
Excellent!
Everyone here should read all about this sort of this, and this specific thing, as well as everything leading up to it:
Pictetâ•Žs experiment: The apparent radiation and reflection of cold (pugetsound.edu)
We have forgotten to account for frigorific rays!
I use them to cool the house in summer.
Eben:
A simple kitchen infrared thermometer computes the temperature of what it is pointed at by using the temperature-dependent electrical properties of the sensor.
Take your IR thermometer and point it at something in your freezer, anything that doesn’t have a polished metal surface. Read the reported temperature. Now point it at something in your refrigerator. It reports a higher temperature, but still below your kitchen ambient.
Think about this for a moment. The sensor temperature was higher when pointed at the frig than at the freezer, even though both objects were colder than the sensor. How is that possible? Hmm…
Now take an object from the frig and put it in the freezer in front of the object you had pointed to before. (I just did this with a carton of milk.) Quickly take the temperature of this object. It still reports the higher temperature of refrigerated objects, not the frozen objects.
So it did indeed “leave the temperature of the hot object [the sensor in the IR thermometer] warmer than it would be if the cold object [the carton of milk] weren’t there [and the sensor was just receiving radiation from the frozen object]”. Just as Willis claimed!
Similarly, the temperature of the hot object [earth’s surface] is warmer than it would be if the cold object [higher elevations of the atmosphere] weren’t there [and the surface was just receiving radiation from space]. In this case, the atmosphere typically has an effective radiating temperature of about -18C (255K) and space has an effective radiating temperature of about -270C (3K).
But this doesn’t explain how the surface W/O a GHG atmosphere radiates at 255, but with a GHG atmosphere, the earth’s surface radiates at 288.
“Warmer than it would be” is somewhat of a weasel wording. Does that mean GHG’s raise the surface temperature to 288? How does a cold atmosphere do that?
Or, does it mean the earth cools to 250 with GHG’s and 220 without GHG’s.
The radiation height explanation doesn’t suffice because all I can find is that GHG’s raise the 255 degree height from the surface to somewhere above it while just saying that means the surface has to warm. But then we loop around and how does 255 get higher from a cold atmosphere?
Jim, your comment “But this doesn’t explain how the surface W/O a GHG atmosphere radiates at 255, but with a GHG atmosphere, the earth’s surface radiates at 288.”
Firstly remember every surface above absolute zero radiates thermal energy. The surfaces are not sentient, their behaviour is not modified by any other surfaces in their proximity, the energy radiated depends only on the temperature and the emissivity. If we assume the surface is close to a black body (BB) emissivity=1 then the total energy radiated will be given by the Stefan Boltzmann law.
For a warm BB surface facing a cooler BB surface each radiates according to its temperature and each will absorb the energy emanating from the other surface which impinges on it. Since warm BB radiates more energy per sqM than the cooler BB the warm BB radiates more energy than it receives whereas the cooler BB will receive more energy per sqM than it radiates hence energy transfer is from warmer to cooler as required by second law.
Now image we start to adjust the temperature of the cooler surface. If we lower it, the cooler surface radiates less energy to the warmer surface. That means the difference between the energy radiated by the warm surface and the energy received by the warm surface increases. The NET energy loss from the warm surface is higher simply because it is receiving less energy back from the cooler surface.
Take the case in your sentence above which assumes Earth receives 240 watts/sqM of short wave radiation from the sun. The Earth’s temperature will adjust until it is losing 240 watts/sqM balancing the solar energy input. If the surface radiates to interstellar space it is radiating to an environment at 3 Kelvin so the energy it receives from that environment is extremely small and by Stefan Boltzmann the temperature will equilibrate at 255K. However imagine we interpose a surface at say 200K between Earth’s surface and interstellar space that 200K surface will radiate 91 watts/sqM which Earth’s surface will absorb. Now Earth’s surface needs to lose 240 + 91 watts/sqM for equilibrium and to do that the temperature has to rise to 276K.
No, the surface will continue to radiate (240 – 91). Your evaluation of (240+91) means heat is being added from a cold body to a hotter one. The formula is (T1 – T2) where T1 is the warm body and T2 is cold body.
What this means is that the gradient from the warm body will reduce and the temperature at “time +1” will be warmer than it would be with a colder body, but it will not increase from its original value. The body will continue to cool but just a slower rate.
That is why at equilibrium temperature the radiation energy in and out is equal for both bodies and heat is no longer being transferred. If you add the energies together you will never reach equilibrium and in essence will have created a perpetual motion machine. Run through the math. If the earth surface radiates (240 + 91 = 331), then the cold body will warm further and radiate even more back to the earth which will radiate more toward the cold body and on and on to infinity.
I am sorry Jim but you are not correct, the warm body does not radiate more and more to the cold body ad infinitum.
Imagine the hot body is a sphere – call it A. The cooler body is a spherical shell concentric with A -call it B. A absorbs 240 watts/sqM from the sun because B is transparent at visible (solar) wavelengths. Now imagine we consider the system from outside B. 240 watts/sqM goes in so 240 watts/sqM must go out if A and B are in equilibrium. But B is opaque at thermal IR wavelengths so this 240 watts MUST be radiating from the outer surface of B. That means according the SB equation B must be at a temperature of 255K. But if B radiates 240 watts/sqM from its outer face it must also radiate 240 watts/sqM from its inner face ie: towards A. That 240 watts/sqM will be absorbed by A so now A is receiving 240 watts/sqM from the sun plus 240 watts/sqM from B so it has to radiate 480 watts/sqM. To do that it’s temperature according to SB will be 303K. Note A radiates 480 wats/sqM to B whereas it only receives 240 wats/sqM from B therefore there is a net heat flow of 240 watts/sqM from A to B ie: from warmer to colder as required by second law.
So is this all just mathematical playing? NO! If you check you will find one can purchase a form of home insulation which is simply a stack of shiny metal foil sheets (usually aluminium) with air spaces between them. Metal is an excellent conductor so how does this form of insulation work if not by the above principle. Second example, rescue services make use of an emergency blanket which is simply a sheet of mylar plastic with an evaporated layer of aluminium on the surfaces so it looks like a mirror. It is very effective and robust being more or less water proof. The blanket is only microns thick so how does it keep the patient warm if not by the above analysed mechanism. If you argue its simply a wind shield then why go to the expense of coating it in an aluminium mirror surface, the mylar alone would be just as effective as a wind shield.
But where does the extra 240 watts from surface B come from?
Why isn’t surface B radiating 120 watts outward and 120 watts inward and at equilibrium with surface A.
Do you see how you just created energy out of nothing. You made a logical leap that isn’t justified and just stopped.
If surface A is now radiating 480 watts at 305, why doesn’t surface B radiate 480 watts outward and 480 watts inward? You just can’t stop this process when it gets you to the answer you want. That is what the warmists do!
Another quick question. When the first radiation hits surface B, is it radiated totally out or totally in. You have to answer this question before going on.
To do this properly, you need to start with an equation differentiate it so you can determine how temps and heat react at infinitesimal periods. Using averages and algebra will lead you to the wrong answers.
Jim, thanks for your comments. I completely understand where you are coming from because when I started learning physics I went though exactly the same agonising thought processes and had extreme difficulty understanding what my teachers were trying to explain. I realise that simply reiterating my previous analysis will not help.
I think the issue is that we as humans tend to think of things in total so we see A is hotter than B so how can B possibly radiate 240 watts to A. Surely that contravenes second law! The discipline of physics however analyses the world somewhat differently. It looks at each entity in isolation and then superimposes the analyses of all entities to come up with the net result. Looking just at the physics analysis from the point of view of B gives results which contradict our world view and we reject them. They only make sense when we add the analyses of all entities together but we don’t get that far before rejecting.
From Physics point of view entity B has a temperature above absolute zero so (assuming finite emissivity) it will radiate thermal IR. Entity B has 2 essentially identical surfaces so each will radiate equally. These surfaces are not sentient, they have no way of knowing to what they are radiating, they cant think “Oh there is a warmer surface in that direction so I cant radiate that way”. The radiation is simply defined by the temperature and emissivity. The warm surface also cannot think “oh these photons are coming from a colder object so I cant absorb them”, absorption has noting to do with where the photons came from. Similarly the photons are not sentient, they cannot think “Oh I am heading to a warmer surface which is not permitted so I will have to turn round”. The radiation occurs no matter what is in the direction of radiation. The feeling is that there is a paradox between physics and the real world. In fact, the paradox resolves when one adds in the activities of the other entities.
People always think second law states energy flow is always from warmer to colder thus a colder object cannot radiate to a warmer object. Great for people who already think in the framework of physics but misleading for those who do not. What is left out is the word NET. Second law does NOT state that a colder object cannot radiate to a warmer object, its just that if it does the warmer object will also radiate to the colder one and since it is warmer it will radiate more. Thus the colder will receive more energy than it radiates and the warmer will radiate more energy than it receives. Hence NET energy flow is always from warmer to colder.
It took me years to become completely comfortable with thinking in the framework of physics so I am not surprised by the continuous conflict over issues like this.
In answer to your point that I did not comment on your equation, the answer is; if Tc rises, the difference Th-Tc reduces so Q reduces. But that means energy in > energy out. The extra retained energy causes the hot surface to warm up so the temperature of Th rises until Q is restored to its original level.
cheers
Michael
You haven’t addressed my reference to the heat equation. I’ll list it out here.
Qnet/t = ε σ (Th4 – Tc4) Ah
where
Th = hot body absolute temperature (K)
Tc = cold surroundings absolute temperature (K)
Ah = area of the hot object (m2)
t = time
The only way for the net transfer of heat to raise the temp of the Thot body is for Qnet/t to become negative. You need to show how that is done when Ah∙ε∙σ∙Th^4 is always greater than or equal to Ah∙ε∙σ∙Tc^4.
Jim:
You ask, “Does that mean GHG’s raise the surface temperature to 288? How does a cold atmosphere do that?”
Think of an analogous situation, a sink with a faucet and (adjustable) drain. Fluid flow is analogous to energy flow, and the height of water in the sink is analogous to temperature.
With a given flow rate from the faucet (analogous to a constant solar input), the height of water in the sink will adjust until the outflow through the drain (analogous to the earth’s radiaion to space) matches the inflow from the faucet. The higher the height, the greater the pressure, so the greater the outflow through the drain to ambient pressure.
Now, constrict the drain somewhat. The level of water in the sink will increase until the added pressure restores an outflow through the drain equivalent to the inflow from the faucet (which has not changed).
But wait! How did the constriction of the drain below the sink push water uphill? The answer, of course, is that it didn’t. It simply reduced the “downhill” flow of water until the pressure difference between the sink and ambient was large enough again to balance the inflow from the faucet.
In this case, a surface without absorbing gases would be like the more open drain, reaching a level of 255K so the outflows matched the solar inflows. Adding absorbing gases restricts this outflow, causing the temperature to rise until its difference from ambient (3K) is large enough again to balance the inflow from the sun.
But it is vital to understand that the HEAT flow is always from the warmer surface to the cooler atmosphere, even as the surface increases in temperature. This HEAT flow is the difference between the larger upwelling radiative flow from the surface and the smaller downwelling flow from the GHGs in the atmosphere.
This concept of “radiative exchange”, as it is usually called, is the core of any textbook explanation of radiative heat transfer. The fact that the radiative flow from the warmer to the cooler body is always greater than the flow in the opposite direction means that HEAT flow is always from warmer to cooler, consistent with the 2nd Law
Again, your model assumes that the water in the drain is constricted. Radiation doesn’t work that way! If a body is at a given temp it will radiate a given amount regardless of what it is absorbing. The net flux will be reduced so that any rate of cooling will be reduced but that doesn’t mean that flux goes “negative”, i.e., heating. That process continues until equilibrium. That is, the hot body cools and the cool body warms until they are at equal temps.
Read this link.
https://www.engineeringtoolbox.com/radiation-heat-transfer-d_431.html
Notice the equation: q = ε σ (Th4 – Tc4) Ah
The energies are not additive. Nor does this equation include any time component.
Jim:
Constricting the water drain is conceptually equivalent to making the medium for radiative transfer less transparent, which is what GHGs do. Both increase the “resistance” to a flux. My old heat transfer textbooks analyze radiative heat transfer problems with resistive networks.
Let’s change the problem a little to overcome your objection. Now the drain pipe from the sink ends under water in a lower tank that spills over its brim to ambient. With that tank at a certain height, the level in the sink stabilizes at a height where the outflow from the sink to the tank matches the inflow from the faucet.
Now we raise the height of the tank. This reduces the pressure difference between ends of the drain pipe, reducing flow. This in turn causes the level in the sink to rise until the outflow through the drain pipe once again matches the inflow from the faucet.
Again, did the lower tank actually push water in the sink uphill? No!
As to the equation you cite, it is for the exchange between two bodies only, with the two gross flows in opposite directions. Things get much more complex when you have multiple bodies, with many flows both adding and subtracting, depending on directions.
But using the equation you cite, one way to look at it is this: With a fully transparent atmosphere, Tc in the equation is 3K. With a real atmosphere like ours, the effective Tc is usually about 255K (-18C). (This is equivalent to raising the lower tank height in my fluid flow example.) With Tc at 255K, the q in your equation is significantly reduced, so Th must be higher for the outgoing q to match the incoming solar radiation power.
You continue to make a jump in logic. “.. so Th must be higher …” How does this occur? Magic?
You keep adding the two fluxes together to get an answer. It may work for water in your model, yet radiation doesn’t work that way in the transfer of heat. Reexamine that equation.
Your model assumes that the water being “fed back” has a rate that is higher that that leaving the bucket so that it adds to the water level in the bucket. IOW, it turns the equation above negative.
To properly model with buckets of water, the “feed back” amount of water can not exceed the rate of drainage. The main bucket will continue draining, just at a slower rate. At some point the “feed back” can equal the rate of drainage and the buckets will be at equilibrium.
I don’t know how to describe it better. The water you add can never exceed the amount of water being drained. That 1st bucket is the source of water that can be “fed back” into the 1st bucket. If you add more that what is being drained, you are creating water out of thin air.
Jim:
No jump in logic, just VERY basic thermodynamic analysis, as you would learn in the first few weeks of an introductory thermodynamics course. I’m just employing conservation of energy.
You seem more comfortable treating radiative transfer as a net flow. So let’s use your equation. Starting from the case where the surface can radiate directly to space at a Tc of 3K, your equation gives us a certain net radiative flux out to space. With a given solar input Qsolar, the temperature Th of the earth’s surface stabilizes at a certain value where its outgoing power flow Qout matches the solar input — in many examples of this type, at 255K.
But if there is now a radiating substance between the earth’s surface and space, the earth’s surface sees a higher Tc value in your equation, and therefore there is a lower Qout (but it is still an OUTWARD NET FLOW). So far so good.
What are the implications of this lower Qout? Well, now there is higher power input to the surface than power output. By trivial energy balance calculations employing the 1st LoT, the energy of the earth’s surface MUST increase. This means the temperature increases. Again, I emphasize that this is true even though the net transfer toward space is still outward.
Note that this increase in temperature would not happen without the solar input, otherwise it would just reduce the rate of temperature reduction (as we note that evening cooling is slower on humid and/or cloudy nights compared to clear low humidity nights.
My fluid example is directly applicable. The drain flow is always net outward from the sink, but if it is reduced below the input from the faucet, the water level in the sink will rise.
Well said, Ed.
All of your comments here are well stated, at least so it seems to me.
What happens at night is an important part of this of course.
It took me a while to understand all of this, and I know I still have a lot to learn.
But so much of it is clear to me now.
For me, I had to empty my mind of opinions and preconceived notions, and go back to the beginning, and work through all of the history of physics.
“The net flux will be reduced so that any rate of cooling will be reduced but that doesn’t mean that flux goes “negative”, i.e., heating.”
In stars, the effect of radiation from a close binary is called the reflection effect, although note that the word “reflection” has a definition that is not the same as ordinary usage.
In fact, the amount of actual reflecting light is at most a few percent of the incident light on an irradiated star, as gleaned by modern studies that looked at polarization to determine this parameter:
Scientists prove that binary stars reflect light from one another (phys.org)
“Our modelling showed that stars are actually quite poor reflectors of light. The Sun, for example, reflects less that 0.1 percent of the light falling on it.
“However, for hotter stars, such as the components of Spica, with temperatures of 20,000 to 25,000 degrees Kelvin, the amount of reflection increases to a few per cent. The total amount of reflected light coming from the Spica system is, however, still very small.”
It has been well known for almost 100 years that each star heats the other. Eddington wrote about it in 1926 in a widely cited paper.
Heat transfer within stars is mainly radiative or convective.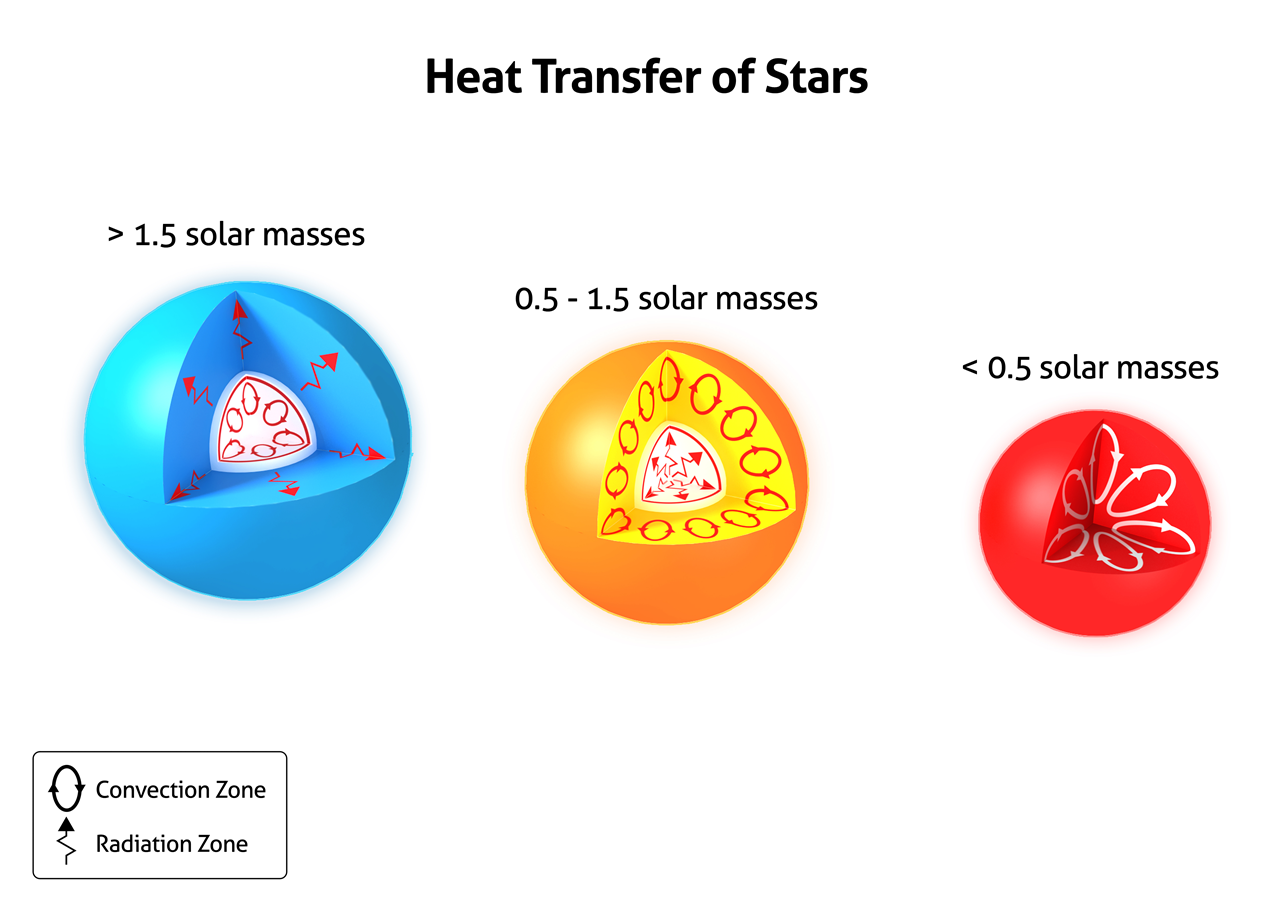
So there are many ways in which the situation is at least somewhat analogous to our atmosphere, but that is not what is important. What is important is, such questions of flux and absorption have been given close scrutiny in the specific case of photons from a cooler star impinging on the atmosphere of a warmer star, and it is well known that they heat each other.
See here:
“The Proximity Effects in Close Binary Systems. II. The Bolometric Reflection Effect for Stars with Deep Convective Envelopes”https://ui.adsabs.harvard.edu/link_gateway/1969AcA….19..245R/ADS_PDF
If you take the time to read through such discussions, it becomes obvious that physicists have always considered the effect of cooler bodies irradiating warmer ones, and the complexity of what happens to that radiation, including detailed discussions of flux, temperature, etc.
It would be better if people replying to my post were debating what I actually say instead debating the voices it their heads.
I thought of wording it a little differently, to make it as simple and clear as possible
———————————————————————————————————-
“Can A Cold Object Warm A Hot Object? Short answer? Of course not, that would violate the Second Law of Thermodynamics ”BUT it can leave the hot object warmer than it would be if the cold object weren’t there”
So he claims two opposite contradicting outcomes for the same scenario – in one sentence, as if twisting the words around a little made it something else.
He literally says in the second half of sentence that cold object makes the hot object even warmer , which in the first half of the sentence he says breaks the laws of thermodynamics.
Bizarro planet science at its finest.
The hot object cools as it radiates energy. The energy radiated by the cooler object replaces some of the energy lost by the hotter object such that it doesn’t lose heat as quickly.
That is exactly the the problem with GHG’s that are cool, somehow causing the surface temp to become warmer.
Think about heat capacities of soil and water. If instead of radiating everything away, a certain amount is “stored”, say through conduction, their temp would increase at least until the sun goes down.
How so?
What matters is the relative temperature of the surface and subsurface, not if the surface is still getting some incoming radiation.
The surface can be getting incoming radiation, and yet be cooling, if it is radiating more than it is absorbing at that instant.
Equilibrium vs disequilibrium.
I probably didn’t make myself clear. This is where a lot of climate science falls down when trying to use simple algebra and averages.
In the morning when the first bit of radiation from the sun strikes the surface, two things can occur. One, the molecule absorbing the radiation can re-radiate it, or two, it can conduct that energy further into the surface in which case no re-radiation will occur. That process continues until an equilibrium temperature is established throughout the surface and radiation in equals radiation out. One needs to analyze this with something more complicated than algebra. It happens with a time varying gradient that is dependent upon a number of factors.
Now, when the incoming radiation disappears, the reverse occurs and depending on the temperature of the surface, its emissivity, and the mass involved, radiation continues, again with a decreasing gradient until a new equilibrium is established.
What I was trying to summarize, albeit poorly, is that there is more going on than just “back radiation” that causes temperature and radiation from the surface to change throughout the day. Simple arithmetic averages on time varying functions will hide a lot of information. When was the last time you read anything on WUWT or even in peer reviewed papers where calculus was used to evaluate the climate system. Climate science has become inured to using simple arithmetic and statistics to try and describe time dependent phenomena and overlooks much.
Oh, and on that we agree completely.
I made this comment several days before this thread opened up, on the previous thread by W.E.
I am copying part of my comment, which is here:
https://wattsupwiththat.com/2021/05/26/modeling-unreality/#comment-3255582:
“What really happens is, the Sun rises in the morning (after a dark and stormy night…), and for a while there is more incoming than outgoing energy to and from the ground, and so the surface heats up.
The ground started the day with some heat left over from all the previous days, so it is not starting or ending with zero energy.
It keeps radiating more strongly as long as it (the ground) is getting warmer, and at some point in mid afternoon, the outgoing flux is first exactly equal to the incoming, and the ground stops warming, and then because the Sun is getting lower in the sky, and the ground is initially still about the same temp, for the rest of the day the ground is cooling as outgoing flux exceeds incoming.
At Sunset, incoming stops, and the ground continues to cool, until a temperature is reached where the ground is in equilibrium with the air temp and the sky temp. (and conduction of heat from below…which is slow)
Sidenotes:
We have neglected throughout so far, and it is neglected in many of the diagrams, that there is also energy transfer from the ground to the air via conduction the whole time.
-If the air is warmer than the ground, the air is warming the ground by conduction by some amount or rate.
-When the ground is warmer than the air, the ground is warming the air by some amount or rate.
Since air is far less dense and has far less thermal mass than solid ground, heat transfer from air to ground proceeds far more slowly when the air is warmer. This helps explain, for example, how and why frost can sit on the ground when the air temp is 38°: The ground is radiating more quickly than air can provide energy to melt the ice crystals. Note this only happens in clear skies and light to absent wind. Any significant wind lets the air deliver more energy to the ground, and any clouds or even haze, slows outgoing radiation below the amount needed to get frost at 38°. So this explanation satisfies observations of this common occurrence, as well as textbook descriptions of when frost can form. I myself, having been born and raised in the downtown of a big city, never saw this occurring until I was in college and bought some land way out in what city folks call “the country”. I had previously been told this is true in classes in such subjects as physical geography and meteorology, and can happily report that I live far enough away from cities that I see it all the time, starting from my plant nursery days. Imagine my surprise…ice on the ground and cars and grass in tiny crystals when it is way above freezing. But only if there is no wind and the dew point is low and there are no clouds. Hot damn, I have seen it with me own eyes, and so can you. But you have to go outside on cold nights with thermometers and look for it. Not to see the frost, but to know it is actually well above freezing even an inch above the frost. And you have to do it many times to confirm that even a mild breeze of 4mph or so, or any clouds, will disallow frost at these temps (32°-38°F)
OK, end of side note and back to discussion of my plain language description of what the hell exactly is going on with all of this incredibly controversial and utterly unproductive morass...
If there was no incoming back radiation at night, then the Earth would be almost like the dark side of the moon, with the night time temp FALLING Falling falling …down to extremely low temps limited only by how fast heat could conduct up through the ground or conduct back to the dense surface from the thin air…but the air would be cooling by contact with the cold cold cold of space ground.
As we can see from our side notes, we know that conduction up from the ground or to the surface from the thin air, is not even sufficient to melt tiny crystals of ice, or keep them from forming, even when the ground is over 70° just an inch or two down, and the air is well above freezing.
If there were no such thing as back radiation, why would not the ground keep cooling all night long?
We know air radiates, because everything does. We know moist air cools more slowly than dry air. Why does it? Because all that moisture is radiating, all the time, and at night it becomes a significant influence on how fast and how much the ground cools.
We know the ground cools faster than the air after sunset, because it can be observed that first dew and then frost, if it is cold enough, will form on ground surface long before the air is at the dew point (in the case of dew) or at the freezing point, (in the case of frost).
On Summer evenings, anyone can go to anyplace with grass and see the dew form, and do so before the Sun is even all the way set in some cases.
And we know the air near the ground cools by it’s own radiation and also from contact with the more rapidly cooling ground surfaces. How do we know this?
Because we can put thermometers outside the windows of tall buildings for one thing, or we can look at soundings from radiosonde balloons for another thing. And when we do that we see that the air is cooling off at all levels, but fastest at the ground, and we can infer how fast heat can conduct through air and know the entire air column could not be cooling by conduction from the ground. Air conducts poorly. Anyone can test this in any kitchen. Or look it up.”
Do you have problems balancing your checkbook? Radiation (money) is still radiation, in or out.
You might try adding something relevant to the discussion. Being a troll just has to be boring for you.
Eben,
Are we to take it from you what Willis has said, rather than read it ourselves?
According to you, what matters is how someone can interpret words, rather than the facts that are being described with those words, is that it?
But it is really impossible to believe that anyone capable of rational discussion of such matters is unable to discern any difference in what is being said between the two statements.
You are leaving out part of the explanation, and arguing from there that the explanation makes no sense.
It is not merely a different wording to say that a cool object can slow heat loss from a warmer object, vs a cool object warming a warmer object.
If one leaves out the part of the explanation wherein the cool object in question, has replaced an even cooler one, one has changed the conditions of the whole story.
You cannot leave out parts of what is going on and claim they do not matter, unless you first provide some explanation for why it is valid to leave out those parts!
Likewise, when you claim that all that is different between the two statements is “twisting around the words a little”, you are revealing that you are either suffering from a comprehension problem, or deliberately misrepresenting what was said.
We can all read what was said:
“Can A Cold Object Warm A Hot Object? 2017-11-24
Short answer? Of course not, that would violate the Second Law of Thermodynamics —BUT it can leave the hot object warmer than it would be if the cold object weren’t there.”
These are two different propositions, not a twisting of words around a little.
But let us consider some cases of changing words around a little, and see if the result can be saying something entirely different.
Or better yet, how about we leave the words the same but add a simple punctuation mark.
Let’s eat Grandma!
Let’s eat, Grandma!
Are those the same statement?
No one has any trouble understanding what happens when someone walks in between themselves and a roaring campfire.
Sometimes I do not know what is wrong with people, but then I remember that even though some people had invented complex mathematics thousands of years ago, in 2021 after going to school five days a week for a dozen years, there are people who cannot even do simple arithmetic.
The only thing bizarre going on that I can see is that some people, who are busily being little more than willfully ignorant, are trying to lecture educated people on a science website, when they themselves seem to have failed to grasp the basics of verbal communication.
What proportion of the Greenhouse effect is attributable to Water vapor and to Carbon Dioxide?
Tom, answered a few times in recent WE post comments, Depends on your frame of reference as to specifics. AR4 implied no feedbacks CO2 ECS was about 1.1, same value as about 2011 Climate Etc calculations. Lindzen 2012 used 1.2. Moncktons equation and values calculates 1.16–so maybe Lindzen just rounded up. That is the CO2 alone ballpark.
AR4 also said water vapor by itself about doubles this value. So 2.2-2.4. Using AR4 ECS~3, and Lindzen’s f/(1-f) 2012 Bode curve, ECS is about Bode 0.65, water vapor is about 0.5, so clouds (all else per AR4 cancelling) must be about 0.15 since Bode is linear additive.
Answering your question two ways, wvf is twice no feedback CO2 by itself, and via Bode comprises ~0.5/0.65 or about two thirds of all feedback above CO2 alone, the rest being mostly clouds.
So at least half and half.
An insignificant amount that is inflated in computer models(as is the temperature of the earth for a greenhouse effect to exist). What’s fooling you in this article is the average suns energy over a month(including nights) will make it lower than what is being emitted by the ground including nights. Only 25% of the surface is heated by the sun 75% of the earth’s cloud cover. 1360/4=340.Land absorbs 100 watts more than ocean. Averaged over earth around 36 watts 329 January ( cooled by 11 watts) and 360 watts in the NH summer, gain of 20 watts. The earth is an ideal thermal blackbody so energy from the sun only adds heat (max to min) to the land, oceans and atmosphere. Atmospheric pressure provides 98 watts on top of the 240 watts the surface emits.
Willis, this has been a lot of fun. It’s interesting to see the mental knots that people tie themselves into to try and prove to themselves that we’re all wrong.
Here’s an interesting observation that anyone can make who has spent time on a tropical island and in a desert. After dark, on the tropical island, it gets cooler than the day, but still uncomfortably warm (for those of us who grew up in no-central-heating UK). In the desert, it cools down very quickly when the sun goes down, and it can get right chilly.
Why is the tropical island showing a much stronger greenhouse effect (i.e. slower night time cooling) than the desert? The CO2 in the atmosphere is much the same all over (“a well mixed gas”), so if it controls heat retention at night, they should both cool down at the same rate. Of course, it’s the H2O, which is not a well-mixed gas due to its habit of condensing and falling back to earth. This simple pair of observations demonstrates to an open mind that (a) the greenhouse effect is real and (b) water vapour is the dominant greenhouse gas. The evil genius carbon dioxide has a minor walk-on role in the great greenhouse drama/farce/tragedy.
SR wrote:
What you observed is the result of a condensible gas.
You need to define “Greenhouse Effect” before you make the claim that it is real.
Smart Rock,
With regard to your point that tropical night time is warmer than desert night time. I agree with you that it seems obvious that it is much more likely the effect of water vapor rather than CO2. However, why do you attribute it to the greenhouse effect and not simply latent heat released as the water vapor cools and condenses? That would seem to me to be the simplest explanation … which would not be a demonstration that the greenhouse effect is real. It just demonstrates that a lot of latent heat of condensation is being released. Deserts are dryer so there is less condensation and so they are cooler at night.
Best regards
“… not simply latent heat released as the water vapor cools and condenses?”
Because the effect is apparent as soon as the Sun sets, and even before, and not only after the air has cooled to the point that some of it is beginning to condense.
Right?
In fact, the effect of moisture in the air on slowing the rate of nighttime cooling is not even specific to only that moisture that is in the layer of air near the surface.
One has the same effect from water vapor no matter where it resides in the air column.
In reality, many things are always going on simultaneously, and indeed water vapor has multiple effects on the temperature of the air, besides for the other one you mention, that of releasing latent heat when it condenses.
One of these other things is that water vapor has a very high specific heat, and so more energy must be added in order to warm humid air than dry air, by a given number of degrees, and likewise more energy must be subtracted to cool humid air a given number of degrees than air which is less humid.
But none of this means that one can pick and choose which details are relevant.
What it does illustrate is that in order to understand complex phenomena, one must have knowledge of all of the relevant parameters, and also one must have some method of discerning the relative contribution of each.
One must keep it all in mind at once, and be aware of all of the relevant observations.
Water vapor in the layers of air removed from the surface will retard the rate of surface heat loss, but will not change the specific heat of air at ground level where the temperature is being measured.
If I am sure of anything, it is that the wrong ideas of the warmistas cannot be neutralized or counteracted by other wrong ideas.
Also the day time temperature is typically less on the tropical island.
Moisture stops your body cooling (human experience of a greenhouse, not an atmospheric one) on a tropical island than the desert(very low moisture content).Cooling happens upwards as weight of atmosphere decreases and molecules are further apart there are lower collisions. Atmosphere above the equator cools 30°C more than atmosphere over the polar regions at times. Not all the time.
Let’s face it – the greenhouse back-radiation theory is correct. Radiation does indeed dominate temperature. So let’s all just accept the consensus and move on…
Except that there’s a tiny condition in the small print. The theory is correct if you happen to live in the earliest 300,000 years of the universe’s history, after the Big Bang.
This was the early light dominated epoch, during which light dominated everything (hence the name). Quarks may have been flying around in the photon soup but photon intensity was too high to let atoms form.
But we no longer live in the light dominated epoch. We haven’t for nearly 14 billion years. Now we’re in the matter dominated epoch.
That means that in the atmosphere, although it’s heated radiatively by the sun, that heat quickly turns into heat of atoms in the atmosphere, ocean and land and thereafter photons take a back seat. Hard to hear, but that’s the reality. In terms of heat movement in the atmosphere convection is overwhelmingly dominant and radiative effects, while measurable, are not very significant.
Even when radiation does heat gas, it does so in the manner of gas molecules heating each other, and photonic phenomena such as absorption and emission frequencies are of negligible relevance.
That’s why Einstein said that even when electromagnetic radiation heats a gas, it does so by momentum transfer interactions very similar to the transfer of thermal energy between atoms. Following the same Maxwell / Planck distributions and statistics. Photon heat is immediately turned into heat of matter and from then on it’s matter interactions that dominate.
To quote the great man himself:
“During absorption and emission of radiation there is also present a transfer of momentum to the molecules. This means that just the interaction of radiation and molecules leads to a velocity distribution of the latter. This must surely be the same as the velocity distribution which molecules acquire as the result of their mutual interaction by collisions, that is, it must coincide with the Maxwell distribution. We must require that the mean kinetic energy which a molecule per degree of freedom acquires in a Plank radiation field of temperature T be
kT / 2
this must be valid regardless of the nature of the molecules and independent of frequencies which the molecules absorb and emit.
https://ptolemy2.wordpress.com/2020/02/16/albert-einstein-said-no-to-co2-radiative-warming-of-the-atmosphere/
Near the end of Homer’s Iliad, Odysseus and his son plot the recapture from squatters of their family home. They have received the support of the goddess Athena in this endeavour. Odysseus says to his still nervous son, “we have Athena with us – we hardly need to cudgel our brains for further allies!”
We have Einstein on our side. We likewise do not need to “cudgel our brains for further allies”.
Yes – Doing EMR balances at the surface of the Earth amongst all that matter is meaningless.
Willis would get more meaningful results by working at the top of the atmosphere where there is much less energy transfer involving matter.
The fact that he is suggesting the surface is radiating OLR at 395W/sq.m shows he has moved beyond the bounds of reality.
Rick, why do you have to turn everything into a personal attack? It makes you look vindictive, arrogant, and unpleasant. I doubt that you are that in real life, but for some reason you want to insult me as though that would make your … curious … claims more believable.
It doesn’t make them believable. If you want to claim that the ground is NOT radiating at ~ 390 W/m2, you’ll have to argue with Stefan and Boltzmann. You know, the guys who showed that
W/m2 = 5.67E-8 * epsilon * temperature (K)^4.
where epsilon is emissivity, typically above 0.95 for the earth. If we take the surface temperature as say 65°F (18.3°C, 291.5K) of an afternoon in Goodwin Creek, that gives us something like:
5.67E-8 * 0.97 * 291.5^4 = 397 W/m2
And if you disagree, as I said, you’ll have to take it up with Mssrs Stefan and Boltzmann, and insulting me in the process just makes you look afraid.
w.
Willis wrote:
No it doesn’t. The only way that relationship is correct is if the target is at 0K. The relationship is a radiating potential. It only becomes energy when it has a target at different potential. There is no energy transfer between objects at the same potential. This is where your confusion stems.
How could EMR possibly get any distance through moisture at 100% humidity near an ocean surface. Most of the heat is transported by the air circulation and phase change of water to vapour.
As I point out below you would be much wiser to stick with ToA EMR fluxes than down at the surface where the matter makes it very complicated knowing what is transferred by sensible heat, latent heat and EMR.
“The only way that relationship is correct is if the target is at 0K.”
No, Rick. The relationship is practically used well at STP with my Ryobi IR002 IR thermometer with fixed emissivity ~0.95.
Pointed at a lab glass of ice water in my kitchen the IR002 unit reads: 32F
Pointed at my boiling tea kettle my IR002 unit reads: 212F
For about $30, you can prove to yourself instrumentally that the target being at 0K is not the only way the relationship is correct.
RickWill – “There is no energy transfer between objects at the same potential. This is where your confusion stems.”
If you accept that, it violates causality. This assumes that a body will only emit radiation towards a body that is cooler, and knows the temperature of that body so that it emits just the right amount of energy, and can also take into account any refractive index change or mirors that will change the direction of the emitted photons before they get absorbed by the receiving body. If we take stars as our example emitting body, then billions of years ago those stars sent out photons specifically for our eyes when we happened to look at the sky. You can get around this by saying they are virtual photons until they are detected by something, but that ends up somewhat paradoxical.
It’s more logical to state that the body emits in all directions according to its absolute temperature according to the S-B rule. Those are thus real photons (packets of energy) and not virtual ones. Thus the radiation between two bodies at the same temperature is not zero, but instead equal in either direction. It may be net zero, but if we place a radiometer between them we can see that each body is radiating.
I know it is standardly stated that in equilibrium there is no energy transfer, but we need to state that that is a net measurement, and that energy-transfer itself is as great as if each body was radiating or conducting energy to something at 0K (zero degrees absolute). Every time we state something as “net” or as “average” we’re throwing data away, which is OK if you actually don’t need it but not good if you understand the net or average number as being real data. It’s a derivative.
It seems logical that the Earth will only receive and transmit energy via radiation, and thus that the long-wave radiation from environmental heat, which is absorbed by the gases radiative in that part of the spectrum within a few tens of metres at ground level and re-radiated each time in all directions, will mainly be finally radiated from the Earth within the top absorbance-length of the upper atmosphere. I’ve seen statements of the absorbance length at ground level as being around 10-20m, but couldn’t find out whether they were defining absorbance length as 1/2, 1/e, or 90%. Whatever, the actual atmosphere is a lot of those lengths high, thus changes in CO2 concentration won’t make a lot of difference to overall energy radiation. There will be some changes due to the actual height (and thus overall area) of that final emission shell. Increased concentration of radiative gases will also lengthen the random walk needed for the LW radiated energy to exit the Earth, and thus delay the time between the emission of energy by radiation from the Earth, to when it finally leaves the Earth altogether. It’s that extra delay that’s going to cause an increase in measured temperatures, but it’s not going to be a lot.
Though there seems to be a correlation between global temperatures and CO2 sufficient to cause people to state that CO2 controls the temperature, especially if you only look back a short time, Willis has previously shown that correlation breaks down dramatically on the 100My and greater timescale. CO2 has a minor effect on temperature, based on history, even when we only look at the history from the end of the Little Ice Age until around 1950, and compare that to 1950 until now. It can’t be invoked to explain the Mediaeval Warm period, or the Roman one, or the Ionian one before that. 1000 years ago the Vikings were growing barley in Greenland, and the evidence for that is good. We’re nowhere near that temperature now.
The big problem in this discussion is that the Global Average Temperature really isn’t well-defined. Even with satellite measurements, which can give a figure for places that haven’t got a ground-based thermometer and never have done, the satellite needs to measure the radiative temperature and assume the emissivity of the surface. Thus we only really have an apples-to-apples database from around 1979 onwards, and accuracy leaves a bit to be desired even then. Before that, temperatures and other weather measurements were largely taken where people were, and so as cities grew the measured temperatures no longer corresponded to the same conditions, and of course airports really need to know the temperature and humidity to ensure that take-offs and landings are safe. So: we take the whole lot of available data and take an average (by various mathematical methods) of the whole lot, and it’s not actually measuring the same thing now as it was 10 years ago. If you find a long-term weather-station that’s not had urban or airport encroachment, in general they show a slight drop over the last 70 years or so, and not a rise.
Can we explain why today’s fishing area Dogger Bank used to be Doggerland around 7000 years ago, and was farmed? Can we explain why the Sahara was a lush savannah 5000 years ago before the monsoons failed and it turned into a desert instead? I don’t think human CO2 emissions had anything to do with those events, or even that global CO2 concentrations could be blamed. We’re pretty certain the various Warm Periods and the Little Ice Age were widespread over the Earth, but we can’t explain those either using CO2.
A problem I see with the standard diagrams showing downwelling LWIR is that it is not specified as to the height of the emission of that radiation. I’d figure that height to be a few tens of metres for some bands (absorbance from CO2 and H2O), with the clouds reflecting some more from a greater height. The wavelength is going to be important here. Maybe especially the 10-micron band, where there is almost no absorption through the whole atmosphere and so it’s a “window to space” we can use for passive cooling using metamaterials. Lumping everything together into SW radiation and LW radiation is a bit cavalier.
More importantly, global average temperature is a meaningless number that certainly does not represent “climate”.
Simon
Just to be pedantic, light does not take time to travel. A light photon “travels” at c meaning that it is a massless field and experiences no passing off time. This often overlooked fact is basic relativity.
Photons from a distant star take no time to travel to earth. However time itself is shifted relative to earth at the photon’s home star by many years.
This urban myth of light arriving at earth having departed the star of origin thousands of years ago is patently false and has no place in a serious scientific discussion. Light does not do time.
The interesting question is – is it possible for both ends of a photon to be entangled? Photons are bridges of causality and the “speed” of light c is not about light. It is the speed of causality.
Hatter – if you dig deeper here you start bringing up paradoxes. In our reference frame, light has a velocity, and that velocity varies with gravitational potential so that the path of the photon is diverted from “straight”. Einstein showed that it is bent by twice as much as a massy particle would be in the same gravitational field (sorry, clumsy wording, but saves a few paragraphs of precise wording), which shows that the equivalence principle is in fact wrong – you can tell the difference between a gravitational field and acceleration if you are in a closed elevator cubicle by measuring the photon path.
If you travel alongside a photon, and as you say it experiences no time from emission to absorption, then it could also not oscillate since there is no time to oscillate in – and yet we can measure those oscillations in space. Paradox. Also, any mass at all in the vicinity would appear to be infinite (relative velocity is the speed of light) and have an infinite gravitational attraction – another paradox. The photon does carry momentum, which is defined as force times time, and again if the photon has zero time then what is the force exerted over that zero time to give us a specific amount of momentum?
Thus I’d say we haven’t yet produced an explanation that is internally consistent and without paradox.
I think we can say that the source of the photon is just beyond its horizon while it travels, so that the photon carries no information about its source, and that the destination is similarly outside its horizon of knowledge. It’s simply a packet of energy with momentum. One photon thus tells you nothing about its source, though if you have enough of them you can statistically infer the temperature of the source.
Entanglement is difficult, since although we know it happens (and can engineer that to happen between photons or between particles) I haven’t seen any explanation of the mechanism by which the information is passed, maybe especially since that information seems to be passed at infinite velocity and not at c.
When I was a student I knew the accepted answers for all this and accepted them as valid. Now, almost half a century on, it isn’t as simple and I see the paradoxes in the answers, but haven’t yet managed to resolve them. Though I figure that Mike McCulloch’s QI theory fits the observations of gravitational acceleration at parsec-scale distances without needing any fitting parameters, and thus has a high probability of being true, it also needs the transfer of information (entanglement?) to work, so your last paragraph is definitely relevant. Strange thing is that it is possible to send data down an unterminated coax which is electrically short (less than 1/4 wavelength) faster than c – measured up to 8c. Thus experimentally the speed of light is not the absolute limit to data transmission that we were taught. Near-field is definitely odd. I’ll add in links to the relevant articles if anyone wants them. Still, note that causality is not violated by being able to transmit information at infinite velocity, even though an observer could then see an event happen before its cause when limited to the information about both traveling at c to the observer. I think causality is actually inviolable, though a lot of the other laws have exceptions if we engineer the right circumstances.
Maybe not quite the answer you expected….
If what you say is true then a photon has infinite energy and we have no universe
Bob – that’s why I’m saying that the standard explanation isn’t correct. Too many paradoxes. Here, though, it wouldn’t be infinite energy, just infinite force for zero time to transfer the momentum.
I’m open to suggestions as to how to avoid the paradox, though.
True
“…light does not take time to travel.”
We do not take account of events from the reference frame of a photon.
“Just to be pedantic, light does not take time to travel. A light photon “travels” at c meaning that it is a massless field and experiences no passing off time. This often overlooked fact is basic relativity.
Photons from a distant star take no time to travel to earth. However time itself is shifted relative to earth at the photon’s home star by many years.
This urban myth of light arriving at earth having departed the star of origin thousands of years ago is patently false and has no place in a serious scientific discussion. Light does not do time.”
Light certainly does ‘do time’.
For example, a laser diagnostic i worked with required light of two different colors to hit the target molecules simultaneously. I was using a pulsed laser with a pulse of 8ns, I split the light into two pulses, one blue and one green, because of the process the blue beam travelled 8 feet further than the green. As a result the diagnostic didn’t work because the two pulses weren’t in the test cell simultaneously!. In order to make it work the path length of the green beam had to be increased by 8 feet to ensure that the two beams overlapped in the test cell.
Excellent post, Simon.
Simon,
There are a huge number of specific examples showing that CO2 and temperature are not correlated, that they are only positively correlated in a few specific instances, and in these instances, it can almost always be shown to be the case that temperature led CO2, and not the other way around.
In fact, interglacials always start and temps warm when CO2 is at or near a minimum value, and reglaciation always commences when CO2 is at a local maximum value.
Looking at the past, it is literally impossible to make the case for CO2 controlling the temperature of the Earth.
Personally, I think this type of analysis, that is, an historical one, is overwhelmingly persuasive.
Rick
The mean free path of IR in regard to present CO2 level in air is 25 meters. In regard to water vapour it is even shorter.
Emitted radiation has no ‘target,’ an object emits in all possible direction based on the temperature of of the object. Everything else in the S/B equation is a constant.
Not everything in the SB equation is a constant. ε and surface area are major parts,. Not all substances have the same value.
I’m sorry for expressing myself poorly. I should have simply said there is no “target” term in the S-B equation.
I understand. Using vernacular descriptions of physical phenomena is a danger and far to easy to do.
Not that stupidity again. How come there is high ‘radiating potential’, whatever magic that is, in the atmospheric window seen from space, but not in the bands where the GHGs are active?
How come it’s the opposite seen from the surface? High radiating potential in the GHG-bands, an almost zero in the atmospheric window in the arctic. Emission spectra.
How about this:
What does it mean when relative humidity is at 100%?
It means that there is no more net evaporation from the surface.
Now, evaporation is when water molecules in the liquid phase leave the surface due to having acquired a higher than average velocity than the rest of the water, and that molecule can thus fly right out of the surface into the air.
This does not stop happening when the air is very humid, or even slow down.
What happens instead is that as many water molecules are entering the water from the air, as are entering the air from the water.
In the same way, if the water is at the same temperature as the air, and no heat is being conducted between the air and the water, it is because the same amount of heat is flowing each way.
IOW, the same amount of energy is flowing in each direction (there are always some slow and some fast molecules compared to the average velocity, and so these can exchange energy amongst each other), so the net is zero.
What about radiation?
Photons from the water in the air are being emitted at the water, and photons from the water are being emitted upwards into the air.
But water vapor will only emit, and therefore only absorb, certain discrete bands of wavelengths of photons. Is liquid water so constrained?
I had to look it up, to be sure, but my understanding is that condensed matter is not constrained in the same way that gas molecules are.
So it would seem that liquid water can emit at wavelengths that shine right through water vapor, but all the the photons emitted by the vapor towards the water can be absorbed by water molecules.
So, I am not sure, although others here probably know for sure, but I think the ocean can still emit to the sky even when the air above it is 100% humidity, and the same temperature.
Yes, no?
I did find this when I looked:

“Infrared emission spectra of liquid, bulk water at 37 and 47 ◦ C compared with black-body emission curves of the same temperatures. The letters signify the different peaks: (a) broad emission peak centred around 2150 cm − 1 , (b) CO 2 absorption peak, (c) water vapour absorption lines arising from H 2 O molecules in the air above the water surface.”
And this:
“Absorption spectrum (attenuation coefficient vs. wavelength) of liquid water (red),[1][2][3] atmospheric water vapor (green)[4][5][6][4][7] and ice (blue line)[8][9][10] between 667 nm and 200 μm.[11] The plot for vapor is a transformation of data Synthetic spectrum for gas mixture ‘Pure H2O’ (296K, 1 atm) retrieved from Hitran on the Web Information System.[6]”
Does this mean the ocean can radiate through humid air?
I think just like on land, it is slower, but does happen.
I’ve been looking for that Einstein quote. Now I have a copy. Thanks. 🙂
If you think only 150 watts of solar energy is absorbed you can think again. Tops of clouds average 3km above the ground. Direct solar 185 + diffused solar 75 = 260 watts is above the clouds. Then the 150 watts of solar energy absorbed with 20 convection makes surface 390 watts for Goodwin Creek. 240 + 260 = 500 watts solar input.
So SURFRAD stations
Is there a place/database of CO2 readings taken at or very near those same SURFRAD stations? So we can take the SURFRAD data and make a scatterplot against the corresponding CO2 data, and see if we get anything other than a random blob? I would think this would prove/disprove the CO2-controls-temperature once and for all, wouldn’t it?
Probably can’t do that. The instrument(s) is not sensitive enough. It’s not designed to be.
Spectroradiometric measurements out to the far IR would give much better wavelength resolution over the simple two-band SW-LW SURFRAD setups. But such an instrument does not exist.
And also, could we get simultaneous CO2 measurements for each radiation measurement we record? At the same, or nearly the same, location? I doubt it. Furthermore, is a CO2 measurement at the surface sufficient, or do I need to go all the way up to the top of the troposphere, recording measurements every 10,000 (1,000? 100?) m or so?
You request we read these 4 other posts as well. That’s 5 posts of conjecture. Does Wallis think we’ve never heard these arguments? It’s still just conjecture; like it was 54 years ago when this GHGE argument appeared in its current form. It is not science. Evidence will convince me. Another thought experiment, or appeal to authority will not. 54 years waiting for modelers to attempt to falsify their hypothesis. 54 years waiting for validations = careful, controlled studies showing the effect. Still waiting for it; 54 years later.
B: Why can’t I be convinced by speculation and conjecture?
A: Because I have empirical evidence against a greenhouse gas effect. Here it is:
1st. Here’s what I think GHGE fans claim:
1. Radiatively active gases, so-called greenhouse gases, GHG, mainly CO2 and H2O warm the surface of the earth by 32 C, on average, due to back radiation.
2. CO2 does ¼, 8C, and water vapor, WV, does ¾, ~24C. CO2 and WV are the 2 main greenhouse gases.
3. None of the main atmospheric gases (N2, O2, Ar), which make up over 99% of the atmosphere, warm the surface.
4. CO2 is the “forcing” gas due to its long atmospheric lifespan of at least 5 years. WV is not “forcing”, due to its short atmospheric lifespan of about 8 to 9 days. By “forcing”, they mean a change in CO2 is determinative; so much so that greater CO2 atmospheric concentrations lead to more WV, which leads to a warmer surface.
That, above, is conjecture. It is not The Science. The science is what we empirically observe.
Here is a GHGE falsification.
After arid regions of China were irrigated, WV in the atmosphere above must certainly have increased. Q: Did the resultant GHG back radiation (due to more WV) warm the surface below? A: No, or at least not nearly as much as the production of WV by evaporative cooling cooled the surface. In daytime, during the growing season, irrigated regions were over 6C cooler than adjacent, non-irrigated regions. More WV is associated with surface cooling, not warming. This study falsifies the GHGE effect.
ICE = irrigation cooling effect; LST = land surface temperature
See: Yang / Huang / Tang; 2019; ‘Irrigation cooling effect on land surface temperature across China based on satellite observations‘
Link: https://doi.org/10.1016/j.scitotenv.2019.135984
Pdf: https://www.researchgate.net/publication/337836655
Mark, here’s John Christy of MSU satellite fame discussing the question. It’s nowhere near as clear-cut as you seem to think.
w.
Willis, did you mean to include a link?
Indeed I did. Thanks, fixed.
w.
Willis asked:
You need to justify these two values. Where do they come from. They are not real.
OLR exiting the surface most often leaves via the clouds – certainly via water vapour if it is present. So the surface radiation is not radiating directly to space. The energy that is eventually lost as OLR has worked its way up the atmospheric column in a myriad of ways. Very little OLR departs the surface directly to space over water, where the energy balance actually occurs.
Over tropical warm pools the “surface” radiated fluxes balance around 210W/sq.m but the OLR radiating surface is top of the clouds between 7 and 14km. Most of that heat worked its way up the column as sensible heat before exiting.
The attached table shows the measured surface insolation at moored buoys in the three tropical oceans when the buoys were each in a warm pool. Surface insolation ranged from 184W/sq.m to 223W/sq.m. The ToA OLR ranged from 215 to 235W/sq.m. These were for a month period.
You are far better off taking any EMR balance to the ToA than playing around with silly stuff like downwelling SW. It is just a fiddle. It is not real.
Willis! Please don’t fall for Gavin’s slight-of-hand, which he repeats <i>ad nauseum</i>.
“So there you have it. If you don’t think that downwelling LW radiation leaves the earth warmer than it would be <b>if there was no atmosphere</b>, you need to explain the mystery source of the additional energy necessary to keep the earth from freezing.”
We are trying to find the effect of the GHG’s and the change in concentration of GHG’s. We are not trying to compare an atmosphere with GHG’s with “no atmosphere at all”.
We trying to compare the status quo with an atmosphere with no GHG’s at all – that is at least a fair comparison.
Your calculation about absorbed radiation reaching the surface is relevant. The radiation reaching TOA is well above what reaches the surface. When GHG’s are removed far more radiation reaches the surface – essentially all of it would. In that condition, far more radiation is absorbed by the surface.
The amount of absorbed energy will never equal the radiation <i>from the surface </i>because a large amount of hot-surface-energy is convected to the air – certainly more than half. It is true that all radiation in and out of the TOA is measurable using radiation measuring device(s). That doesn’t help us measure what is going on immediately above the surface. The surface air is heated by the hot surface. The heated air rises and eventually (if there are some GHG’s) it is radiated into space. If there were no GHG’s, the air would be warmed and never cool – because it can’t (unless it is pulled against the surface).
Considering the convective heat transfer to the air at the surface might balance the surface equation. Ignoring it creates confusion.
My short response to this is:
1. Back radiation is evidence for a greenhouse effect, but is not the effect itself. It just represents the surface and lower atmosphere trying to come to thermal equilibrium, as thermodynamics shows they must tend.
2. There is an alternative explanation for Earth’s temperature being about 33C above that calculated for a black body in our orbit. It is the Diurnal Smoothing Effect (DSE) demonstrated in NASA’s DIVINER lunar data – and yes, the moon has no significant atmosphere but thermal buffering but the rock surface provides it.
I discuss these points and more in my Climate Confusion and Fear article on the front page of my legacy site http://brindabella.id.au.
The confusion comes from the lack of a meaningful definition of the GhE. Ie one that can lead to a calculation of its magnitude. If the energy “trapped” by GHGs was transmitted rapidly up to the upper atmosphere and radiated there would be minimal stored heat and consequent temperature rise. The critical issue is how long does this take.
If the GhE=33C assumption was correct this would imply, by somple undergraduate physics, a delay of 19 days. I list four distinct lines of evidence that it is just a few hours. This is a factor of about 200, which tallys with my detailed calculations and those of Nahle (2011).
Why is delay the issue? Imagine an exhibition building with people entering at the rate of one per minute and average stay of one hour. First person enters and by the time they leave 60 others have entered. An average exit rate of one per minute is established. The mean occupancy is remains at around 60. Translate body temperature to extra atmospheric temperature and you have the GhE.
No. Effects that make surface temperature more uniform in time or space can produce a certain amount of warming, but there is a well-defined limit to how much warming this can explain. Such smoothing can never raise the temperature above the effective radiative temperature Tₑ, defined by 𝜀𝜎⋅Tₑ⁴ = Savg, where Savg is the average insolation. For Earth, Tₑ is 30 to 33℃ colder than Earth’s observed temperature. DSE can explain warming up to Tₑ, but not warming beyond that.
I glanced at your article. The “the transit or delay time” is NOT the key to the GHE.
It’s extremely easy to get arguments about the role of time in thermodynamic systems wrong, and your essay seems to offer a creative example of that. Energy retention time does, in some sense, relate to temperature increase, but your reasoning about the issue is erroneous.
You might want to consider a long comment I wrote about the role of energy “residence time.” (See also this comment or this one offering an analogy of a river and a lake. These might help, though they don’t directly address the particular error you are making.)
Willis,
You wrote –
“If on an ongoing basis the surface is only absorbing 150 W/m2 of solar energy and is radiating 395 W/m2 of energy … why isn’t it frozen solid?”
A remarkably silly thing to say.
Much of the Earth’s surface is, indeed, frozen solid. Your silliness of conflating (apparently) w/m2 with measurements of temperature is just, well, silly.
The nonsensical approach of alarmists, blathering endlessly about averages and energy balances, is also just silly.
The Earth is not frozen where it absorbs sufficient energy to remain – unfrozen! Like, most of it.
If alarmist calculations show the temperature “should be” other than what is measured, then the calculations are wrong, wrong, wrong.
Air has a temperature. If you are surprised that the the temperature of about a ton of air sitting over every square foot of the surface beneath it can be measured, you need to learn some basic physics.
I find the entire treatment of atmospheric thermodynamics unsatisfactory. If I may try to simplify the process as described (and I may be wrong), incoming visible and some IR radiation heat the ground, which causes convection currents to carry the air aloft. IR emissions from the ground are intercepted by the greenhouse gases in a column of air whose density is steadily declining. The upward convecting air reaches an altitude where it can finally radiate to space, but also radiates back to the ground.
But the radiation going back to the ground (infrared) has to penetrate an atmosphere whose density is steadily increasing. How exactly does that work. The outgoing infrared was blocked by a steadily decreasing density atmosphere, but comes right back through one that is steadily increasing density?
Another aspect of the whole problem is the idea of “adiabatic lapse rate.” The term “adiabatic” refers to processes in which no heat (energy) enters or leaves a system. But the parcels of air convecting upward still contain greenhouse gases, and thus must be constantly radiating and absorbing energy. This is the opposite of adiabatic. And, oh, yes, since the air temperature for the convecting parcel is dropping, the amount of energy leaving the system will be constantly changing with respect to that being absorbed.
Though my career has been in rocket propulsion and space system engineering, my hobbies in the past decade have included the study of nuclear engineering, ranging from reactors to bombs. The degree of sophistication involved in the neutronics of either a reactor or a bomb (the two are way different) is very high, and was achieved in the pre-computer era. Atmospheric radiation transport phenomena involve all of the same issues, but I’ve seen very, very little of the same kind of sophistication in any global warming paper, or online discussion. If you have access to The Los Alamos Primer, read it. The atomic bomb was simple compared to “global warming,” but you’ll find the sophistication in that little book (used to brief incoming Manhattan Project scientists) rather breathtaking.
You are correct—climate modelers over-simplify this very complex subject.
The quality of this comment makes me wish there was a scale for upvoting/downvoting – 5 Stars!
The gap described in your last two paragraphs – between nuclear physicists and climate scientists includes respect: The nuclear scientists had a deep respect (and healthy fear) of the material they were studying and working with, as well as the implications of their work. Mainstream climate science, on the other hand, is brazenly disrespectful and cavalier. They use and abuse every aspect of their field, as well as every lever available to them.
Worse, these unqualified/disqualified scientists have coagulated with politicians and journalists of similar quality into worker bees performing various roles in the same noxious hive. Since their lack of integrity does not diminish their work ethic, their coordinated efforts have made their hive a real threat.
Have you actually read any real climate science papers?
I think you’re speaking out of some image of climate scientists as cartoon villains.
As a scientist who has worked in particle physics, quantum optics, and physical chemistry, but not in climate science, I don’t think you’re being fair. The climate science papers I’ve read seem to me to involve a far higher degree of sophistication than is consistent with this narrative.
I have made atmosphere density issue many times, along with the mean free path and the long time to emit a co2 photon compared to th short time to collision.
“The Los Alamos Primer”
Just ordered it. Thanks for the tip.
Online discussion and climate papers are two different worlds. I’ve seen a fair amount of sophistication in scientific climate papers. But, there is often a breath-taking lack of sophistication in online discussion.
Sometimes. Convection happens under some conditions, and not under others. And sometimes convection moves air through only a modest vertical range, while under other circumstances it shoots air (and water vapor) up into the stratosphere.
Sure.
While this statement has elements of truth, it’s really muddled.
While may be “upward convecting air”, the process of radiation reaching space is not necessarily dependent on this.
One way to think about this is that there are many “layers” in the atmosphere, and there are heat transfer processes between layers, and between the planetary surface and the various layers.
Convection (of sensible and latent heat) conveys heat away from the surface to the lowest layer of the atmosphere. Convection may further transfer heat between adjacent layers, within parts of the atmosphere where convection is active.
Longwave radiation also conveys heat between the surface and any layer of the atmosphere that absorbs that radiation. LW radiation also conveys heat between atmospheric layers. And, eventually some LW radiation is radiated to space.
Both convective and radiative processes affect temperatures throughout the troposphere.
At every point in the atmosphere, the amount of LW radiation emitted is proportional to the concentration of greenhouse gases and (more or less) the fourth power of the temperature of the air.
For lower layers of the air, much of the upward emitted LW radiation will be absorbed by a higher layer. But, at a certain altitude (that altitude depends strongly on the particular radiation wavelength) the air above is transparent enough that the radiation is likely to reach space.
You seem to have a mistaken mental model in which LW radiation is only emitted at certain locations in the atmosphere.
Yet, what actually happens is that every layer of the atmosphere is absorbing LW radiation from both directions and emitting LW radiation from both directions.
Ultimately, this is modeled by writing differential equations for the upward and downward fluxes of LW radiation at each point in the atmosphere.
The fluxes high in the atmosphere (where the atmosphere is relatively cool and LW fluxes are lower) are what determines how much LW radiation escapes to space.
The fluxes low in the atmosphere (where the atmosphere is relatively warm and LW fluxes are higher) is what determines how much LW radiation reaches the surface.
Make sense?
To model a process as “adiabatic” it is often sufficient that only relatively “small” amounts of heat energy are being gained or lost.
Atmospheric convection is sometimes termed “pseudo-adiabatic”, because the condensation and evaporation of moisture (i.e., interchange between latent and sensible heat) is an important part of the process, but it’s not technically adiabatic.
The magnitude of radiative heat flows in convecting air tend to be modest enough that an “adiabatic” or “pseudo-adiabatic” model still offers a pretty good description of much of the physics. I suspect it works to model the radiative processes as a “perturbation” on the more-or-less adiabatic process.
Bottom line: both convection and radiation matter, but the energies involved in convection can be enormous, and radiation doesn’t greatly distort the dynamics of convection.
Yes. And, there are equations that capture all this.
The details are usually omitted in popular accounts of how meteorology and the Greenhouse Effect work. But, that doesn’t mean that those working on the science don’t know how to deal with it.
The radiative physics aspect is, by physics standards, quite straightforward, even if it would appear complicated to someone who isn’t comfortable with a lot of math.
The complexity is of climate modeling is other aspects of the physics.
Does any of this help?
Willis, this dumb ol’ retired engineer thinks this is one fine post.
I see data from two sources and they agree quite well enough.
The squiggly lines on the graphs support your points.
The explanations are clear and easy to follow, even by me.
What’s not to like?
.
.
I’ve been paying attention to GW => AGW => CAGW => CC => ACC => CACC => …. Climate Catastrophe => ………….. Climate Collapse! since Big Al put out “An Inconvenient Truth” and proposed a Carbon Credit Exchange as the solution, which by happenstance, ALGore was heavily invested in.
Over the years, I’ve come to value WUWT that because it covered the political aspects of……… it’s Friday, 5/28/21 here……. who knows what it’s called today?
And I’ve also appreciated the fact that the efforts to really examine and figure out just WTH?!? is going on with the Earth’s climate are largely focused here and a few other places. And so I learn actual climate science.
I’ve been well aware of the food fight you warned of at the beginning of this post. But this particular post put all of the fuss (and fuzz) I’ve read about on this topic over the years into one nice, tidy, easily understandable package. This dumb ol’ engineer could easily read and comprehend it all.
Thank you. 👍
(Screw the typos and semantics arguments. The overall point and evidence is clear, at least to me.)
As I read all this about averages of averages of averages and simple linear algebra my head spins. We are dealing with energy and heat and a multitude of factors that all occur as a continuous function like sin waves. Daily (sun comes up, sun goes down), monthly, annually, solar cycles, ENSO, AMO and others.
We are never going to reach a definitive conclusion of anything by trying to find averages of time varying phenomena. It like trying to average sine and cosine waves with simple averages. Using statistics to analyze data points on a continuous function is useless also. We are not analyzing characteristics of unique independent data points from a population. Climate science is trying to statistically analyze a series of min and max values from various “frequencies” to try and arrive at a unique solution. That’s like trying to statistically analyze the min and max volumes over time of all the instruments in a Bach concert. The answer you get will tell you nothing.
I see some down votes. Perhaps those folks would like to tell us when they used plain old algebra and averages in their engineering thermodynamics or physics classes to do calculations. Mine always dealt with differentials and integrals based on time and exponential functions. Why is climate science any less exacting?
There are some people on all of the WUWT threads lately just going along and downvoting every comment by every skeptic, especially the ones that make some good points.
Denying accepted physics is not skepticism. One must replace it with something that makes physical sense, not just poorly supported statements about how things might work.
Well, Jim, why don’t you explain the difference between the average energy emitted at the surface of the earth from that emitted at TOA? You can only use generally accepted scientifically validated concepts and measurements, provisional or not.
There are numerous factors in the earth’s atmosphere other than radiation. This article only deals with radiation in a somewhat ,”ideal” situation. There has only been brief mention of other things like molecular collisions, convection/advection, lapse rate, and heat capacities, and specific heat.
Here is something to think about. What happens to the energy of a CO2 molecule ejected at 200 degrees from a burner as it transits to the stratosphere and it’s temperature drops? I know it is a hypothetical but it is an important part of convection.
That is why it is hard to deal with radiation at TOA. Radiation can disappear in the system and the energy used to perform “work” against gravity in convection.
Again, its a thought experiment on back radiation, not a treatise on the dynamics of the Earth’s oceans, land, ice and atmosphere which includes clouds. [The outer sphere radiates as a plate; both sides must have equal radiation emissions. The core absorbs the inward radiation.]
Both sides must radiate what radiation is absorbed. Incoming radiation is not divided by two and half goes out each side if the ppate. If a plate is at a given temp, it radiates the calculated amount out both sides.
Thanks, Jim. This might be clearer with an example.
Assume a one square metre plate in outer space that is receiving 470 W/m2 on one side. And assume that it is at steady-state, neither warming nor cooling. Thus, the amount it is radiating must equal the amount it is receiving.
Q1. At what rate (in W/m2) is it radiating?
Q2. Assuming it is a blackbody, what is its temperature?
Best regards,
w
At equilibrium the temperature of the body will be determined by the SB equation -> q = εσT⁴ which gives T = [{470/(1)(5.6697×10^-8)}]^¼ = 302 K.
Let me quote from Planck’s thesis on heat and radiation. (1)
(Bold by me.)
What does this all mean? It means a body at equilibrium radiates in all directions uniformly. So a body at 302 K radiates 470 w/m^2 in all directions. This applies to any homogenous isotropic substance with a volume. Total heat radiation is not divided by the number of surfaces a volume has.
(1) Title: The Theory of Heat Radiation
Author: Max Planck
Translator: Morton Masius
Try again, the body will be emitting at 235W/m^2 in all directions uniformly, therefore its temperature is ~254 K.
And the interior core heats up in response to the inner radiation of the outer core in the purely thought experiment.
Are you sure about that? The material of the inner core is already at a given temp. Molecules are both traveling faster and vibrating. Can a molecule of say CO2 absorb another unit of energy by moving even faster and vibrating at another level?
Find a reference that says this is true.
So how much slower surface cooling is back radiation from CO2 went it’s equivalent to -80C. No hotspot.?!
Weins displacement law shows the peak on a Planck curve. Things radiate without having to be on the peak. I radiate 15 micron without being in a cryogenic container.
Be careful. Planck curves are based on black body radiation, that is, an ideal body. If you believe that atoms and molecules have unique spectrums then you also must admit they are not black bodiescand don’t meet the curves exactly.
Astronomy would be hamstrung if everything radiates everywhere! How could you identify individual elements.
I separate the two. Blackbody curve and Planck curve. My Planck curve is derived from the Planck formula but applying emissivity of separate wavelengths. The curve varies according to the material studied and the temperature. I don’t apply an emissivity to the whole. That would make it a grey body. Blackbodies and grey bodies don’t exist. There are only non-grey bodies. SB calculations from others that put emissivity into the formula make me want to vomit.
It’s not “equivalent to -80℃.” That’s just a meme going around that is based on a stunning lack of understanding of thermal physics. I wrote an essay that unpacks this issue.
“There are lots of good arguments against the AGW consensus, but this…”
I did not click on these links and read them yet, although I am pretty sure I read two of them yesterday and all of them at various times…
But I can finish this sentence: “…is not one of them.”
For me, this topic and the arguments that ensue from it, represents a classic example of how difficult is is for people to be persuaded to change their mind, or to decide for themselves that perhaps they ought to learn more and then reevaluate their position or belief.
Personally, I came across this argument without knowing about it ahead of time, and knew right away that I had no way to decide which side of the argument was correct, or more correct.
And I also knew I was not gonna take a position on any of it until I not only knew exactly what the people having the argument were each saying and apparently believing, and then, at that point, finding out independently what the actual physics was, preferably from sources that had nothing to do with anything involving the atmosphere.
Laws of physics do not apply in one certain place only, or a single circumstance.
They are general rules for how the universe works.
They operate in the realm of objective reality.
The way I see it, the big problem we have today with the whole warmista problem, is that a bunch of people have decided that one certain thing must be true, therefore it is true, and have let that belief obscure every other tiny bit of contrary information. They are literally blind to anything that contradicts their one core belief.
It is the central paradigm of their entire worldview of the Earth, and of science, and hence of reality.
One cannot be a scientist if one makes up one’s mind about things and insisting on the correctness of that thing.
Cargo Cult Science: Richard Feynman On Believing What Isn’t True (fs.blog)
My other thought in this is, I think there are a lot of people who have never spent large amounts of time outside carefully observing the Earth and the sky and everything on it and in it.
Exactly!!! When I say, “My tinnitus sounds like a summer night, without the sudden silent pauses.” WE and probably you will know exactly what I mean, but how many others will get it? It helps if you grew up in a house with no air conditioning in Brazoria County, Texas (that’s just south of Houston).
“The way I see it, the big problem we have today with the whole warmista problem, is that a bunch of people have decided that one certain thing must be true, therefore it is true, and have let that belief obscure every other tiny bit of contrary information. They are literally blind to anything that contradicts their one core belief.”
That’s a pretty good description of the situation today.
Their beliefs about the “climate truth” (CO2 is a problem) certainly are not based on any evidence.
I know CO2 cools the planet. This is how.
Lovelock’s Gaia theory has it that life moves the conditions for life to some sort of optimum. This is wildly incorrect. Deserts and the arctic ice sheets are hardly optimum.
Instead, life moves everything to the edge of existence. If there is some parameter or resource in excess, and some form of life can take advantage of it, and in doing so decrease the availability of that parameter or resource, then it will, moving the conditions of existence even more towards the edge.
Right now, the edge of existence is coldness and lack of CO2. Artificially adding CO2 will mean those forms of life able to take advantage will do so, and by doing so, make things even colder for the rest of the biosphere.
Just my inexpert thought bubble.