Note: I normally don’t publish anything related to the ideas of Nikolov and Zeller, for three reasons: 1) It’s just wrong, 2) It invariably descends into a shouting match. 3) These two guys published a paper under fake names to fool the peer-review process, which is a professional no-no.
But, here we are. I thought this was important to share. – Anthony
Giving Credit to Willis Eschenbach (originally published at drroyspencer.com)
by Roy W. Spencer, Ph. D.
The non-greenhouse theory of Nikolov (and now Zeller-Nikolov) continues to live on, most recently in this article I’ve been asked about on social media.
In short, it is the theory that there really isn’t a so-called “greenhouse effect”, and that the excess planetary surface temperatures on Earth, Venus, and other planets above the Stefan-Boltzmann (SB) temperature calculated from the rate of absorbed solar radiation is due to compressional heating by the atmosphere.
This is a popular alternative explanation that I am often asked about. Of course, if there is no “greenhouse effect”, we don’t have to worry about increasing CO2 in the atmosphere and all of the global warmmongers can go home.
I have posted on this blog many times over the years all of the evidences I can think of to show there really is a greenhouse effect, but it is never enough to change the minds of those who have already convinced themselves that planetary surface temperatures are only a function of (1) absorbed sunlight and (2) atmospheric pressure, as Zeller and Nikolov claim.
I’ve always had the nagging suspicion there was a simpler proof that the Zeller-Nikolov theory was wrong, but I could never put my finger on it. My co-worker, Danny Braswell (a PhD computational physicist) and I have joked over the years that we tend to make problems too difficult… we’ve spent days working a problem when the simple solution was staring us in the face all along.
Enter citizen scientist Willis Eschenbach, a frequent contributor at Wattsupwiththat.com, who back in 2012 posted there a “proof” that Nikolov was wrong. The simplicity of the proof makes it powerful, indeed. I don’t know why I did not notice it at the time. My apologies to Willis.
Basically, the proof starts with the simplified case of the average planetary temperature without an atmosphere, which can be calculated using a single equation (the Stefan-Boltzmann equation). Conceptually, in the absence of an atmosphere, sunlight will heat the surface and the temperature will rise until the rate of emitted infrared radiation from the surface to outer space equals the rate of absorbed solar energy. (To be accurate, one needs to take into account the fact the planet is rotating and spherical, the rate of heat conduction into the sub-surface, and you also need to know the planet’s albedo (solar reflectivity) and infrared emissivity).
The SB equation always results in a surface temperature that is too cold compared to surface temperatures when an atmosphere is present, and greenhouse theory is traditionally invoked to explain the difference.
Significantly, Willis pointed out that if atmospheric pressure is instead what raises the temperature above the S-B value, as the Zeller-Nikolov theory claims, the rate of energy loss by infrared radiation will then go up (for the same reason a hotter fire feels hotter on your skin at a distance). But now the energy loss by the surface is greater than the energy gained, and energy is no longer conserved. Thus, warming cannot occur from increasing pressure alone.
In other words, without the inclusion of the greenhouse effect (which has downward IR emission by the atmosphere reducing the net loss of IR by the surface), the atmospheric pressure hypothesis of Zeller-Nikolov cannot explain surface temperatures above the Stefan-Boltzmann value without violation of the fundamental 1st Law of Thermodynamics: Conservation of Energy.
This is a simple and elegant proof that radiation from the atmosphere does indeed warm the surface above the S-B value. This will be my first go-to argument from now on when asked about the no-greenhouse theory.
I like to give credit where credit is due, and Willis provided a valuable contribution here.
(For those who are not so scientifically inclined, I still like the use of a simple hand-held IR thermometer to demonstrate that the cold atmosphere can actually cause a warmer surface to become warmer still [and, no, the 2nd Law of Thermodynamics is not violated]).
When real science wins, we all win.
Real science should rely more on observation and less on conjecture. Observation: I see four inches of snow sitting on a steel cylinder of oxygen welding gas out next to my shed. I also see four inches of snow on everything else. The oxygen gas pressure in the cylinder is 2000 psi (138 Bar, 13.8 MegaPascal, 141 Kg/sq/centimeter, whatever). Leads me to the hypothesis that compressed gas does not retain its heat after the compression process is complete.
Dan
Exactly. My thoughts drifted to the compressed air on the back of divers – I couldn’t say that those air bottles were warm for long after filling…
Playing the devil’s advocate here. Is that what the Zeller-Nikolov hypothesis maintains, or rather that greater atmospheric compression results in higher molecular collision rates (increasing temperature) when heat is being added?
Icisil, as I understand, yes. The heating of the atmosphere in the N-Z theory has nothing to do with compression heating, as happens when a bicycle tire is blown up. The theory is completely misread.
Think atmospheric density and then this will be more along the right path.
Also, consider that if you were lying on the ground and the pressure weren’t equalized, you’d feel 20 grand pianos on top of you. This isn’t trivial, and that fact is significant but I don’t think really understood.
Don132
I think this theory makes perfect sense considering the lapse rate into the stratosphere. Different preasure creates different heat. We can measure it, and it stays the same.
Global warming is a bizarre phenomenon. A great event to be sure. Herd suggestion at its peak. We are a dumb race. No matter how many incisive and accurate observations can be made here, the sheeple will continue to believe. Oceanic outgassing or a volcanic event spits all over this stupidity.
…what this money could be used for…
Collisions do not cause heat. They just transfer one type of energy into another type of energy.
Global warming is measured via temperature, not heat. Energy imparted to a volume of gas increases average molecular kinetic energy, which increases temperature. Higher pressure creates denser arrangement of molecules, which translates to higher average kinetic energy and higher temperature. Is this not correct?
No that only works in a closed system … FYI the Earth isn’t a closed system 🙂
Under your theory if you compress something like a cylinder of compressed air it not only gets initially hot (If an 80 cf tank is filled too quickly it will get hot to the touch) but stays hot .. every scuba diver is carrying this charming little burning ember on there back and have 3rd degree burns on the middle of their back???
See the problem the idea doesn’t even work on a scuba tank.
Isicil, no higher pressure does not increase the average kinetic energy. Each molecule/atom has exactly the same amount of energy as before.
Right, higher pressure doesn’t increase kinetic energy, but it does increase atmospheric density near the surface, which means more kinetic energy imparted to the atmosphere via UV absorption, surface conduction, and increased gas molecule collisions.
I’m certainly not arguing that this theory makes sense, but it does seem that I had a misunderstanding of what they were saying. The actual claim seems to be that gas density “amplifies” the solar heating of the atmosphere due to an unspecified effect of collisions. This is not quite the same thing as “compressional heating by the atmosphere”. But I do not see any description of a physical mechanism for that “amplification”. It does not make sense to me that more collisions could somehow increase the total kinetic energy of the atmosphere since each collision merely reallocates the total kinetic energy of the colliding molecules.
From the actual paper
If the apologists for this hypothesis can’t come up with a physical mechanism to explain the “amplification” effect, then I would say it should be dismissed as a curve fitting exercise.
Physical work in needed to compress a gas. It is this INPUT of energy, ie the work done in compressing the gas which gives it more energy and thus ( temporarily ) makes it warmer.
This energy comes either from whatever is driving a pump, or from the change in gravitational potential energy when a mass of gas changes in altitude.
That is correct Greg you sort of have to invoke the first law, the problem is it is really a lie to try and make classical physics not break. There are countless examples a classic example I gave below … try working out how a fridge magnet stays on a door or have a super magnet stuck on a roof beam and hang a large mass indefinitely.
Under classical physics you have to assert the magnet is not doing work it is just holding the object at a given potential energy (AKA not doing work). Normally the layman sees the obvious problem why can’t you just put an object in the air and it not fall then?
Done properly under QM Field theory it is much easier to explain 🙂
If you need a hint, get two random shaped metal objects and put them on a catapult and toss them watch the motion. Now take a magnet and attach the two objects to each other and use the catapult again. Notice something different about the centre of gravity.
So the hint is how does the magnet change the centre of gravity so the two objects act as one?
There is a lot more going on than just classical physics energy and force 🙂
“Global warming is measured via temperature”
Global warming isn’t measured, it’s calculated, inferred, and wrong.
Rich Davis,
“If the apologists for this hypothesis can’t come up with a physical mechanism to explain the “amplification” effect, then I would say it should be dismissed as a curve fitting exercise.”
The “amplification” effect is simply the density of an atmosphere.
Why is Mars cold if it has an atmosphere of mostly CO2? Because it’s farther from the sun and because the atmosphere is so thin. If the atmosphere were denser would it matter? Yes. If it has less CO2, would it matter? No.
Why is it colder the higher we go? Because the atmosphere is thinner. It is not because of radiative effects.
Are radiative effects including in the lapse rate? No. That should be a clue. What governs the lapse rate? Mostly pressure, which directly affects density.
The “curve fitting” is this: looking at all the factors involved in planetary temperatures and performing dimensional analysis to determine which factors play a role in temperature, it was found that the presence of greenhouse gases had no influence. Maybe people are overlooking this. Where is the universal formula for which you can plug in insolation, greenhouse gas content/action, etc., and come up with temperature, and have that apply to our solar system? It doesn’t exist. This is what NV found. What matters is atmospheric density and solar insolation. Did it fit a curve? Yes. That’s what we might expect if they actually found a universal formula.
The physical mechanism is simple. If you add a fixed amount of heat to a gas until the gas molecules warm to the temperature of the heat source (i.e., the kinetic energy of the molecules of gas match the kinetic energy of the heat source) then the temperature of the gas will depend on its density: the temperature of a gas is the average kinetic energy of a volume of gas. Fewer molecules (thinner atmosphere) means lower temperature, many more molecules mean higher temperature, all else equal. This is by definition.
This is why the thermosphere is cold, even though the molecules have high energy.
On earth all the denser gas molecules are near the surface; therefore most of the atmospheric heat is near the surface, without any help from greenhouse gases. The atmosphere retains some of the heat from the surface; the oceans retain more. Between the oceans and the density of the atmosphere, there is no need to invoke the radiative greenhouse effect, which is not the same as saying that there is no radiative greenhouse effect. It’s just that according to NZ and others, the radiative greenhouse effect doesn’t drive the surface temperature. Pressure, along with the sun, does.
That is my take on it.
Don132
Don,
Density is indeed the critical issue as I’ve been saying since 2007.
The reason being that greater density leads to more effective conduction.
That is why I say that the lapse rate slope is an indicator for increasing conduction relative to radiation as one descends deeper into the mass of an atmosphere.
It is no coincidence that the lapse rate slope precisely follows the decline in density with height.
The denser the atmosphere the more conduction and the higher the temperature rises above S-B.
Conduction is a slower energy transfer process than radiation so the more of it you have the longer will be delayed the exit of solar energy back to space.
Downward radiation does not significantly delay the release of energy back to space because it is near instantaneous and therefore cannot be the cause of surface warming above S-B.
here is a model of a sterling engine showing how the atmosphere heats the surface.
The sun drives the shaft (solar panels not shown) and provides the average temperature between the cylinders. The hot cylinder is the surface, the cold the upper troposphere.
The shaft rotates once every 24 hours. Hopefully the animated gif works.
Moreover, the near-surface atmosphere, in close contact with a surface warmed intensely by the sun, is far denser than the much colder upper atmosphere, and thus the bulk of the “average heat” in concentrated at the surface.
It has nothing to do with compressive heating and thinking that it does is messing everyone up. It has everything to do with near-surface atmospheric density, caused by … the weight of the atmosphere, caused by … the bulk of the atmosphere, and …. gravity.
Don132
Thanks Don. Gravity is excluded in nearly all discussions of greenhouse theory.
It’s dismissed because it isn’t relevant.
The lower atmospheric is more dense because it is compressed by the upper atmosphere. Isn’t it semantics? Last airline flight I was on it was -60F at altitude and +60 when I landed. A pressure/altitude table predicts this.
Farmer Ch E retired – “Last airline flight I was on it was -60F at altitude and +60 when I landed. A pressure/altitude table predicts this.”
Kindly reference that table. The last time I flew at -60F at altitude, the local landing temperature was -30F (as in minus thirty).
Chad – here’s the table for Atmospheric Pressure at Different Altitudes.
https://www.avs.org/AVS/files/c7/c7edaedb-95b2-438f-adfb-36de54f87b9e.pdf
In an adiabatic system, compressed gas is hotter (think diesel engine compression). The atmospheric temperature at altitude is going to vary depending on cold fronts, warm fronts, jet stream from the poles, etc. so this table is not going to predict temperature at altitude perfectly. The audience to WUWT is pretty diverse so I never know if I’m commenting to a university professor, text book author, activist, or interested person w/o a science background.
This is exactly what I’m seeing in this discussion. I don’t know why NZ talk about amplification, but the mass of the lower atmosphere seems to provide a sufficient explanation of retained heat. Noting that moist air is more “massive” than dry air, it is easy to see why desert air cools quickly at sundown, while humid air does not. And of course the greater air pressure at low altitude also represents the relatively greater mass of the denser air. This seems to be the crux of NZ’s paper, and I don’t know why adiabatic heating is even mentioned here.
Much of this discussion seems to overlook the ever-changing solar input due to the earth’s rotation. Fortunately for life on the planet, the atmosphere provides not only essential gases but also molecules that retain some of the sun’s daily input.
With due respect to Dr. Spencer, I think much of this discussion tends to “make problems too difficult”.
Don, at very high altitudes and extremely thin atmosphere the temperature is very high (while the “heat” is very low!). See “thermosphere” https://en.wikipedia.org/wiki/Thermosphere
dwieland,
A couple comments on your post:
You refer to “retained heat”. Remember that “heat” is energy that is transferred and is not typically used in a noun form. Heat is something that flows.
Also, this comment is puzzling to me:
“Noting that moist air is more “massive” than dry air, it is easy to see why desert air cools quickly at sundown, while humid air does not.”
Water vapor has a very significant impact on how the atmosphere heats and cools, primarily due to the huge energy speed bumps that are encountered as water changes phases (solid to liquid, liquid to vapor, solid to vapor and all three of these in reverse). For your desert example, minimum nighttime temperature is controlled by the dew point (temperature at which the water vapor becomes saturated). To cool below this point requires crossing over one of the energy speed bumps – either it rains or it snows which incrementally lowers the dew point. A desert cools more at night because it is dry (low dew point) and there is minimal cloud cover to retain the nighttime heat.
You refer to “retained heat”. Remember that “heat” is energy that is transferred and is not typically used in a noun form.
Yet, you do just that:
A desert cools more at night because it is dry (low dew point) and there is minimal cloud cover to retain the nighttime heat.
To amend my “energy speed bump” analogy above, this is more than a speed bump, its almost like a wall. It’s like a jogger who runs the first part of a race on a dry track and then finishes the race running in 3 feet of water. The dry track represents cooling of the atmosphere and the water represents the extra energy required for the phase change (rain or snow).
thanks Leif – sometime we are creatures of habit to our own fault.
Plus 100.
With due respect to Dr. Spencer, I think much of this discussion tends to “make problems too difficult”.
The problem is that the table for Atmospheric Pressure at Different Altitudes is not for an atmosphere with lots and lots of water vapour in and a little bit of CO2.
NZ reference purely the pressure, not the atmospheric composition.
Their figures for an earth like planet will be out because a stock atmosphere without GHG will be at a lower temp than one with GHG. Not by a lot, But by enough for Roy and Anthony to distance themselves from the claim that GHG and by extension CO2 are not important.
The only way a planet of earth size pressure and albedo can have the same surface temp as planet earth is if they have used pressures including the effects of H2O and CO2 on said atmosphere.
In which case they should not diss the effects of CO2 and H20 which are important , real and scientifically alter the pressure and albedo significantly.
angech
Excellent comment.
angech,
NZ do not diss the effects of CO2 and H20. They say that pressure and insolation are much more important.
Don132
Good thinking Dan! The weight of a skyscraper resting on its foundation doesn’t heat the ground either.
Work, force times distance) is a form of energy and temperature is proportional to internal energy. Force alone is not energy, until it moves something. Imagine an Earth without an atmosphere far from any star. Then we allow air to fall from space to the surface to create an atmosphere. That air would certainly heat up. The force of gravity is doing work by moving air closer to the surface. Some PdV work will also be done. However, without the sun, that heat will soon be radiated away.
Frank, you will be interested to learn that we are dealing with different phases of matter, solid and gaseous, in your example. They act differently because of vastly different internal structure. Solids will heat and shift slightly under great force while gases do both vastly more easily. Just why is worth learning, I reckon.
It is however not doing N and Z justice to say something is wrong if the sun is required. Of course it is, for whtever theory, or the gases would be ice about 2m thick nevermind if CO2 was actually the miracle gas. No sun, no gas full stop in the real world.
Cold air sinks, warm air rises, that is work in the precise, physical definition, a heat engine in truth. In fact, forced compression is what ignites tender in a fire piston – an alternative to flint and steel or matches. The only debate seems to be where the bulk of the energy comes from and how much a biologically-critically important trace gas can influence things.
interesting analogy. I put 2 liters of water in a used soda bottle, set it outside and voila! It doesn’t rise and fall by several feet every day.
Whoa, you’re not addressing the obvious counter-example, that of dropping temperatures as you go to higher elevations, and hence lower pressures.
You got to look at the atmosphere as a system, the whole system has been heated by the Sun, and on average has a black-body-ish temperature, but gravity causes a temperature gradient, that at the surface is higher, but is compensated by the lower-than-blackbody temps much higher up.
Above 90,000 feet or so the atmosphere, while continuing to thin, becomes warmer: The thermosphere. https://en.wikipedia.org/wiki/Thermosphere
That statement whilst ‘theoretically’ correct, is rather misleading because there is all but no molecules of anything. This is not heat as we know it, eg., if you were to put your hand in it, it would not burn you.
Richard, “warmer” in my statement pertains to temperature, not how it feels to your hand. Temperature is not heat. This is why a discussion of global temperature is nearly meaningless as is exploration of the temperature of the air column. It has some use near the surface of the earth (within the troposphere in particular).
Dan, we do not say that. The energy input remains the sun of course. Not available to contained gas. Deeper understanding of Physics tells us, among other things, that energy fluxes always take the easiest route to increase entropy. Equipartition makes available the services of buoyancy for ground-heated gases, kinetically-thermalised molecules of all stripes and everywhere; and the massive power of water vapour. This can uplift five times more energy than needed here, being half the density of air and with specific heat capacity pretty near the top of any list for latent heat carriage.
Roy imagines his instrument is actually sensing and making sense of clear sky readings when they are outside the designed capacities of the receptor and the algorithm feeding the guage reader’s screen. Folk who helped develope these devices and with immense practical experience of the actual engineering involved, have shown this to those who seek the truth. Here in NZ at 35deg S latitude, summer, my device can read clearsky below zero C.
Reading Maxwell’s Theory of Heat from p330-350 would be a start. See Hockeyschtick for starters, and Tallblokes Blog. We have worked on this for many years now and understand that N and Z have many fellow scientists who have independently had to admit that the Ideal Gas Laws do not allow for unconfined (if constrained by gravity on their mass), atmospheres to be dominated by radiative transfer. Such a vector force is an effect of kinetic energy molecular vibration in the magnetic fields. It is relatively weak (-ve 4th power relationship), and its emissions happen so many orders of magnitude slower than KE collision transfer of energy that it hardly occurs. Indeed radiation is swamped by the instant expansion of energised gases – think gas-driven projectiles – followed by mass transfer uplift to where there is space for radiation to dominate. Say five to fifteen km for starters on Earth. Extrapolation of lapse rates tells the tale for those who wish to know….
Ditto for all measured solar system atmospheres thicker than 0.1bar. Gases are not surfaced, and steel greenhouses are irrelevant. I stand on the foundation built by Maxwell, and the null hypothesis remains intact. Understanding why the Gas Laws rule does take work on how gases are not the same in their nature, which is what’Physics’ means, as solids’ I repeat, ‘God’s Empiricism’ demonstrates what we say in the Solar System and all the various measurements of a wild assortment of atmospheric gases. They do act in concert, affected only by solar distance and atmospheric mass. All else follows. Brett
Right on , Brett!
Many people are having “black dog” moments.
Some might recall a comment made in another post where I stated that I thought I saw a black dog in my driveway, even a half day after I found out that I’d been burgled, when what I’d actually seen was the burglar jumping from the window.
Sometimes we see what we expect to see or are conditioned to see or want to see.
In order to believe that NZ are wrong you’d have to believe that a gas with “x” number of molecules would have the same temperature as a gas with “10x” molecules, when both are up against a heated surface that remains constantly heated, such as the surface of the earth during the daytime.
If NZ are wrong, then the implication is that it wouldn’t matter if surface pressure were 7 psi instead of 14.7 psi, and it wouldn’t matter if the surface pressure were 29 psi.
Once again, it’s not about compressive heating, which is the “black dog” story we’re telling ourselves.
Don132
The various proposals for terraforming Mars rely not upon increasing the radiative GHG effect, by increasing the number of molecules of GHGs in the Martian atmosphere, but rather upon adding mass to the Martian atmosphere thereby increasing the pressure of the atmosphere.
“Leads me to the hypothesis that compressed gas does not retain its heat after the compression process is complete.”
Certainly but the oxy welding gas cylinder is in an atmospheric environment where things come to an equilibrium temperature – typically ambient air temperature.
However an atmosphere is in an environment where the only means of exchange is radiation to space and this is entirely a different situation.
NASA’s Planetary Fact Sheets show that the outer solar system planets ALL have temperatures at 1 bar pressure that exceed their calculated blackbody temperature and exceed the temperature at 0.1 bar.
This characteristic is present in every planetor moon with an atmosphere. And using NASA’s data the temperatures can be calculated by the ideal gas laws.
Also all of the outer planets have extremely high core temperatures deep in their atmospheres with Uranus the lowest at ~4700°C.
None of that comes from the solar radiation and these have significantly lower traces- almost none – of GHG’s than Earth.
Some say this is the remnant “heat of formation” but that explanation is strange because, if it isn’t gravitational compression of gases responsible for the temperature, then where did the “heat of formation” originate ?
The outer planets also must have a gravitational core compressing the gases of their atmospheres because everything we know about gases indicates a “self compressing gas” is virtually impossible. We know the nature of a gas is to occupy any available space and therefore the accretion of free gases in a vacuum to form a self compressing mass is impossible.
Any discussion should consider these facts as listed by NASA.
I am for from convinced that that analogy is informative, since the gaseous system inside the gas bottle is stable and in equilibrium, which is not the position with Earth’s atmosphere.
Our atmosphere is constantly dynamic with air currents rising (via convection) and then falling. Further, the atmosphere is constantly being pulled/displaced by gravitational forces exerted by the sun and the moon, and the movement of tides etc, which gives rise to the atmospheric bulge. Hence work is constantly being performed in Earth’s atmosphere and the by product of this work may be sufficient to maintain temperature.
It is well known in motor racing that tyre temperature is a factor of work done by the tyre. The slight bulging/flexing of the tyre wall causes heat to be generated or maintained. Thus on racing cars, tyres are fitted out of a warm blanket, and if the car is idle or run slowly for any extended period the tyre temperature cools, but it does not cool if the car is driven at race speeds, just because of the small amount of work being inputted into the tyre.
The planet;s system is complex and a comparison with a simple analogy may not be appropriate.
Willis
If you took the sun out of the picture the planet would still be warmer with an atmosphere (whether it is composed of nitrogen or any other atmospheric gas ) than without an atmosphere. Adiabatic heating does not add energy nor enthalpy to a system, but it does increase temperature when the volume decreases; or correspondingly decreases temperature when the volume increases. Of course there is always some heat lost to surroundings.
Earth’s atmosphere acts like a pump which is moved by gravitational pressure which itself is influenced by the surrounding planets and sun. Even earth’s moon changes the gravitational pull of the earth. We may not understand gravity but we see its effects. It is this pumping action that gives the earth a basic temperature above what the moon has. There is no new energy source, thus no new energy to upset the energy balance. Adiabatic magnetization or demagnetization also has an effect on temperature. There is confusion between the definition of adiabatic in classical and quantum mechanics when referring to the speed of the process.
Heat Transfer, along with Thermodynamics, mass transfer, fluid dynamics and reaction kinetics are engineering subjects of which very few scientists understand even a little of anyone of these subjects.
If one looks at the original work of Stefan and Boltzmann it will be found that the S-B equation applies to surfaces (not gases) in a vacuum. Boltzmann wrote a proof for the second law of thermodynamics (or more correctly the 4th postulate of thermodynamics) in terms of entropy which is defined in the 3rd postulate of Thermodynamics. Baron Fourier found that a clear atmosphere (ie no clouds) acts somewhat like a vacuum with respect to infrared radiation.
N & Z’s propostion revolves around the 5th postulate which Willis and Spencer likely have ignored or do not understand.
Dimensional Analysis which N & Z say in their publication few scientists are aware of and do not understand has been an engineering subject since around 1900. Dimensional analysis is used in heat and transfer giving dimensional numbers such as the Nusselt No. (convection) Grashof number (evaporation) Schmidt No (mass transfer) and in Fluid dynamics ( Reynolds No.)
N&Z used dimensional analysis to find their relationship. They tried 12 dimensional relationships and found one which gave the least error based on measured values for different rocky planets (ie have a surface and an atmosphere-Venus, Earth, Mars, Titan and Triton). Their theory can be checked when more data is available for other moons and planets in the solar system .
I respect most of the work of Dr Spencer, he did reply to an email from me honestly saying he did not know the answer to my question (about methane) as he had no knowledge of organic chemistry.
I caught Willis out with a reference to a b*llSh*t paper you can see my response on my website (very little used) http://www.cementafriend.wordpress.com
Bad typing and editing meant to say dimensionless numbers -note Dr Gavin Schmidt did not know about the Schmidt number until he looked it up on Wiki and in a comment (on the website of an engineer in a post concerning a proposition by a Russian scientist about cyclones & tornados) admitted he did not know how the Schmidt number was used. Dr Schmidt certainly has no idea about dimensional analysis. It appears that most commentators here also do not understand.
cementafriend, you may understand dimensional analysis but you don’t seem to understand overfitting. Read The Mystery of Equation 8. I discuss overfitting there. In it, you’ll note that I developed an even simpler formula than that of N&Z that gives better answers than they got.
From that post:
Four free parameters and free choice of equation to fit eight points? That’s a scientific joke. It would only be surprising if they could NOT get a good result with those conditions, and as I said, I got an even better result.
Best of New Years to you,
w.
PS—I followed your link to what you claim was when you “caught me out” but I couldn’t find anything about what you claimed. A link to your entire blog is less than useful …
Willis it seems you did not try very hard about the comment here is a link https://cementafriend.wordpress.com/2011/10/14/methane-good-or-bad/#comments
to help you here is part of my comment
“Willis, nice to have a response from you. It is strange that with the paper, by Kasting et al 1983, you refer to that your BS indicator was not working.
Please note the first sentence of the abstract “A detailed model is presented of methane photochemistry in the primitive terrestrial atmosphere along with speculation about its interpretation” – model, primitive terrestrial atmosphere, speculation and interpretation.
The article refers to modelled anaerobic conditions ie no oxygen present. The present conditions in the atmosphere are completely different.”
I enjoy many of your articles but not this about the N&Z article which you may not have read in detail. I know plenty about curve fitting which is what all the so-called climate models do with temperature. Dimensional analysis is different especially when it looks at a range of measured parameters (with dimensions). The N&Z analysis may not be complete, there maybe better factors but at least it provides an equation which can be tested when data for other moons of Saturn and Jupiter is available.
By the way if you have not guessed I am registered professional chemical engineer with considerable experience in heat transfer, combustion and process instrumentation and control. I also have a post graduate degree in business and understand the financial of the implications of the wasted efforts with so-called renewables.
Willis looked at your article. You make no mention of N & Z making dimensional analysis and looking at 12 sets of parameters and measured data from NASA and other country rockets (eg Russian for Venus). In their paper they did not include Europa because there was insufficient data particularly about its atmosphere and surface pressure. They did not include Mercury. I understand that only recently NASA has sent a rocket (space vehicle) to obtain data on surface temperature and atmosphere (if any) When accurate data for Mercury and Europa is available it should show if N&Z analysis applies to these rocky small sized planets.
cementafriend January 1, 2019 at 4:18 am
cementafriend, it’s not my job to root through your junk to find something. If you want me to read whatever you wrote, that’s your job.
Having just read it now, I haven’t the slightest idea why you brought it up. Near as I can tell it has nothing to do with the subject under discussion.
Pass.
You also say:
I couldn’t care less if they got there by way of dimensional analysis or ouija boards. All that matters is their final product. It contains 5 tunable parameters plus free choice of equations to fit eight data points. I said in the paper that it would only be surprising if they could NOT fit eight data points given those conditions.
Then I developed a better fit using less parameters.
Then I developed another equally good fit using one less variable.
And NONE of these equations, mine or theirs, mean a damn thing. They are all just the result of overfitting.
If at this point you don’t understand that we are dealing with meaningless curve fitting, then I encourage you to direct your comments elsewhere. If your understanding of overfitting is that poor there is no point in further discussion.
Have a wonderful New Year,
w.
Let’s not forget Ned Nikolov’s direct reply to Willis’ “The Mystery of Equation 8”:
https://tallbloke.wordpress.com/2012/01/17/nikolov-and-zeller-reply-to-comments-on-the-utc-part-1/comment-page-2/#comment-15281
I suppose he could have been more kind, but brutal honesty is something we all have to face at some time or another — I remember the time I was training in summer sessions of a professional arts school. Talk about brutal honesty !
Nikolov was brutal, but far from honest. If he had been he’d have noted that he used the less accurate of my two replacements for his curve fitting, rather than the one that has only three quarters of the RMS error of his curve fitting.
And if he were honest he wouldn’t have used a bogus temperature for Mars (180K) simply because it fit his curve to within 0.1°C. Instead, he would honestly have used the real Mars temperature of 201K, which gives a 30°C error …
w.
Willis,
I seem to be finding some wiggle room on mean temp of Mars. Does anybody really know what it is within the range being discussed?
Also, how do you answer Nikolov’s claim about your “confusion” over some of the values in his equations and how you characterize them as categories of measure that they are not? Specifically, the following are a couple of quotes in his critique of your critique of him:
He had a laundry list of five, I think. It would have been cool to see your exact responses to these over at tallbloke, but I’m gathering that maybe you and the tall one had some friction to hinder this.
Robert Kernodle January 4, 2019 at 12:42 pm
His claim of a Mars temperature of 180K is a long, long ways outside the “range being discussed”.
Side issue, unrelated to my two main points.
It is indeed a “tunable parameter”, it has no physical basis.
Tallbloke banned me from his site. I’ve asked him to lift the ban. He has refused. Which, no doubt, is why Nikolov posted his reply there.
To reiterate my two points:
1. Whether there are four tunable parameters or five plus a freely chosen non-physically based equation, he is only fitting eight data points, so it is wildly overfitted.
2. As my proof shows, the details of his claim are immaterial. if there are no GHGs in the atmosphere, it is physically impossible for any atmospheric-based (pressure, density, gravity, etc) processes to raise the surface temperature higher than the blackbody S-B temperature. Doing so means that the surface is radiating more than it receives, which is physically impossible.
Not only did he not falsify either of those points, he didn’t touch them at all. Instead he focused on a host of meaningless side issues like whether there are four or five tunable parameters …
w.
Willis:
“If there are no GHGs in the atmosphere, it is physically impossible for any atmospheric-based (pressure, density, gravity, etc) processes to raise the surface temperature higher than the blackbody S-B temperature. Doing so means that the surface is radiating more than it receives, which is physically impossible.”
Because … if the atmosphere is warmed, then that means that some of that warmth is necessarily transmitted to the surface, and if so, then that means that the surface is transmitting more energy than it receives! Got it! Thank you.
Now, please explain to us why an atmosphere that is heated by GHGs does not also transmit some of that energy to the surface, thereby causing the surface to radiate more than it receives. Divine heating?
The logic of the GHG warming position is impossible when set up against the atmospheric thermal effect. It’s an arbitrary logic that’s designed to support a position that gets weaker and weaker the more one examines it.
Don132
@Don
“explain to us why an atmosphere that is heated by GHGs does not also transmit some of that energy to the surface, thereby causing the surface to radiate more than it receives”
In a way this occurs. The atmosphere receives ~78 w/m^2 directly from solar radiation. It radiates ~333 w/m^2 to the surface and ~199 w/m^2 to space.
The surface receives ~161 w/m^2 of direct solar radiation. It radiates ~40 w/m^2 to space and ~356 w/m^2 to the atmosphere. It also moves ~97 w/m^2 to the atmosphere by evaporation (latent heat) and conduction/convection.
Confused? Most of this energy transfer occurs in a loop between surface and atmosphere and back again. It plays no direct role in net energy received or lost by the Earth. This energy loop is also what drives things like convection and thermals, which also play no direct role in Earth’s energy gain or loss.
Donb says of GHGs:
“Most of this energy transfer occurs in a loop between surface and atmosphere and back again. It plays no direct role in net energy received or lost by the Earth. This energy loop is also what drives things like convection and thermals, which also play no direct role in Earth’s energy gain or loss.”
Sounds a lot like the loop Stephen was describing.
In fact the theoretical mechanisms differ but the fact that the atmosphere is warmed by GHGs or by absorbed kinetic energy makes no difference to energy in/energy out. The only difference is that you arbitrarily say that one will cause more energy to be emitted than received.
Don132
Thanks for the reply, Willis.
I’m trying to wade through all this with my less-than-perfect [understatement] understanding of the math.
In one of your earlier comments, you wrote:
I’m thinking that there might be a better characterization of his (their) choice for Mars temperature than your implication of dishonesty. I took a quick look at this:
https://www.omicsonline.org/open-access/new-insights-on-the-physical-nature-of-the-atmospheric-greenhouse-effect-deduced-from-an-empirical-planetary-temperature-model.php?aid=88574
Nikolov N, Zeller K (2017) New Insights on the Physical Nature of the Atmospheric Greenhouse Effect Deduced from an Empirical Planetary Temperature Model. Environ Pollut Climate Change 1:112.s
… where the authors explain their choice of Mars temperature as follows:
“We found that quoted values for the mean global temperature and surface atmospheric pressure of Mars were either improbable or too uncertain to be useful to our analysis. Thus, studies published in the last 15 years report Mars’ GMAT being anywhere between 200 K and 240 K with the most frequently quoted values in the range 210–220 K [6,32,76-81]. However, in-situ measurements by Viking Lander 1 suggest that the average surface air temperature at a low-elevation site in the Martian subtropics does not exceed 207 K during the summerfall season (Appendix B). Therefore, the Red Planet’s GMAT must be lower than 207 K. The Viking records also indicate that average diurnal temperatures above 210 K can only occur on Mars during summertime. Hence, all such values must be significantly higher than the actual mean annual temperature at any Martian latitude. This is also supported by results from a 3-D global circulation model of the Red Planet obtained by Fenton et al. [82]. The surface atmospheric pressure on Mars varies appreciably with season and location. Its global average value has previously been reported between 600 Pa and 700 Pa [6,32,78,80,83,84], a range that was too broad for the target precision of our study. Hence our decision to calculate new annual global means of near-surface temperature and air pressure for Mars via a thorough analysis of available data from remote-sensing and in-situ observations. Appendix B details our computational procedure with the results presented in Table 2. It is noteworthy that our independent estimate of Mars’ GMAT (190.56 ± 0.7 K), while significantly lower than values quoted in recent years, is in perfect agreement with spherically integrated brightness temperatures of the Red Planet derived from remote microwave measurements in the late 1960s and early 1970s [85-87].”
Their reasons seem well thought out along a very structured line of argument, and I would not characterize this as dishonest.
I don’t have the command of the math to be a technical ref for you and Nikolov’s exchanges, but his ability to answer each of your objections indicates to me that he has a command for what he is doing.
What I find, in these sorts of higher level intellectual kung fu matches, is that inevitably one side claims that the other side does not understand some basic, while the other side claims the same of its respective other. It’s frustrating to be such a novice that I cannot declare a true winner of these debates. What I see, however, is that N&Z seem to be holding their own, despite your efforts to find something wrong with their ideas.
I, thus, cannot yet discount their approach to explaining Earth’s near-surface warmth via their alternative explanation, which seems consistent with banishing the insanity over the Satan molecule (CO2).
I have seen technical arguments against the “radiative greenhouse effect” that equal or surpass the rigor of your technical arguments against N&Z. Both approaches seem to have their proponents and detractors.
Robert Kernodle January 4, 2019 at 6:23 pm
Robert, every modern reference I can find puts the average temperature at ~210K—220K. I find nobody who puts it below 200K.
However, Nikolov used the value of 180K.
Now, you are free to believe that it is just a fortunate cosmic coincidence that 180K is within 0.1°C of the value predicted by Nikolov’s miracle equation.
Me … not so much …
w.
Here’s the reason why Mr. Eschenbach is right that almost all of those arguments are irrelevant: there would be no average conduction between the earth’s surface and the atmosphere if the atmosphere were perfectly non-radiative.
There are three components: the earth, its atmosphere, and space. If the atmosphere were completely non-radiative, then heat flow to and from the atmosphere could occur only by conduction, and that conduction would occur only between the atmosphere and the earth: there’s no conduction to space.
In such a situation there may be net local conduction over time between individual portions of the surface and the atmosphere. And for finite periods of time there may be net global conduction between the entire surface and the atmosphere. But on average over the entire surface and over time, the net heat flow by conduction has to be zero. Otherwise the atmosphere would eventually reach absolute zero or get so hot as to escape the earth’s potential well.
So it doesn’t matter whether the earth is spinning, whether convection is occurring, whether there’s one sun or many, or whether gravity imposes some temperature gradient on the atmosphere. Barring a long-term trend in the atmosphere’s total energy, all the energy received from the sun is either (1) retained by the earth to give its temperature a long-term trend or (2) radiated back out to space. Barring a long-term trend in the earth’s temperature, that is, the earth’s surface will on average radiate away all the power it receives from the sun, independently of the atmosphere’s size.
Yes, Holder’s inequality being what it is, the atmosphere and the spatial distribution of the incoming radiation will affect what the average temperature is that’s responsible for that outgoing radiation. But at steady state the average power the surface radiates has to equal the average power received from the sun(s).
In contrast, the real earth’s surface radiates more on average than the earth receives from the sun. Since that can’t occur with a non-radiative atmosphere, the earth’s surface temperature does depend on the atmosphere’s composition, not just on its the mass. And the overall conclusion of Nikolov and Zeller’s mental gyrations is that this isn’t true.
So, no matter what mental gyrations they go through to reach their conclusion, there’s something wrong with their reasoning, because their conclusion is wrong.
No, I’m under no illusion that this will convince anyone here. Having succumbed to morbid fascination at the appalling illogic of so many who have obviously studied some physical science, though, I had to vent.
“there would be no average conduction between the earth’s surface and the atmosphere if the atmosphere were perfectly non-radiative.”
That is perfectly correct but does not lead to the conclusion you think it does.
If you read my description you will see how a circulation of stored energy can lead to a warmed surface for the entire globe whilst not disrupting the energy in / energy out balance.
All you need is for the stored energy to cycle continually through a closed adiabatic loop.
Once the loop is established there is indeed then a net zero energy exchange with the surface but it still heats the surface because it represents a ‘rolling’ delay in the emission of solar energy to space.
I think it may be the ‘rolling over’ aspect that is causing some contributors a conceptual difficulty.
Joe Born:
“But on average over the entire surface and over time, the net heat flow by conduction has to be zero. Otherwise the atmosphere would eventually reach absolute zero or get so hot as to escape the earth’s potential well.”
This doesn’t make sense.
The atmosphere will conduct with the surface according to how dense the atmosphere is and how much insolation the surface receives. If the GHG-free atmosphere is extremely dense, then it’ll absorb lots of energy. It will in turn warm the surface; Willis admits this. The warmed surface will therefore radiate more energy than it would without an atmosphere, but this energy came from the atmosphere that had absorbed energy in the first place. No laws are violated.
The atmosphere (in a typical planet) will also conduct with polar regions and nighttime regions and with molecules that have been cooled by these. At some point an equilibrium will be reached that depends on atmospheric density and solar insolation, and there can be no runaway cooling or heating that violates conservation of energy.
Don132
Note the manner in which Mr. Wilde debates. Faced with an incontrovertible refutation of his position, he avoids addressing it directly but instead says there’s something the refutation failed to take into account. He then follows with word salad that no one understands.
The reason no one understands it is that it doesn’t make sense. And there’s no point in addressing the word salad, because he’ll always take the position that it means something other than what you think it does.
But someone who doesn’t yet have the background knowledge, or who just didn’t get the logic gene, blames his inability to understand on his shortcomings rather than on Mr. Wilde’s poor logic, and he assumes that because Mr. Wilde keeps on responding there must be something to what he says.
Unfortunately, this approach can be amazingly successful. Christopher Monckton has duped the Heartland Institute and Anthony Watts with it, for example. As a consequence, the world is dumber than it should be.
You will husband your time (and intelligence) best by skipping over Mr. Wilde’s comments.
I trust that others will see Joe’s comment for what it is, namely an attempt to divert from unpalatable truth.
Myself: “Now, please explain to us why an atmosphere that is heated by GHGs does not also transmit some of that energy to the surface, thereby causing the surface to radiate more than it receives. Divine heating?”
Myself: because some the energy is radiated out by the atmosphere and that’s how balance is achieved..
I make mistakes, and then I admit them. I’m trying to sort through all the logic here and I get tired, too.
But Willis is wrong when he says his hypothetical planet violates conservation of energy. Since there are no GHGs, the energy from the atmosphere is returned to the surface and not radiated out, and the surface radiates at a higher temperature than it would with GHGs, yes, but none of this violates conservation of energy.
It has to be. The atmosphere MUST absorb energy, and if it can’t radiate it away then that energy must be returned back to the surface from which it absorbed.
Don132
To Willis E, you wrote:
Yes, I am free to believe such, but to suggest that I actually do believe such would be a misrepresentation of my belief. What I believe is that N&Z have made a determined effort to establish a better estimate of Mars temp than all previous references that you rely on, and they explained this clearly in their latest paper to which I posted a link earlier.
I also have not relinquished belief that you might be muddying up their approach with higher-level errors than I can understand. The grander ones knowledge, the grander the mistakes that can be made. (^_^) Rest assured, though, that I continue to try to wade through your objections, hoping that glimmers of deeper understanding might shine through. I always read your stuff, and gain insights from it.
What I will say, in your defense, is that I believe Nikolov became a bit impatient and resorted to taking the lower ground when he brought your background into play in some of his comments. That is irrelevant to me. I try to look at the arguments and not the man, in these situations. This does nothing to reduce his credibility, however — it only shows that people who are passionate about what they do are human and get fed up with annoying situations, when they have to keep fighting the same battles over and over again to make any progress.
I’ve seen great genius in pissed off people. (^_^)
Don132:
I’ll try this once, but, no offense, nothing in the comments you’ve written so far gives me much optimism that you’ll get it.
That’s true. But make sure you keep a distinction in your mind between energy and power. The atmosphere can have prodigious amounts of energy without having any net heat flow occur between it and the surface at steady state. In fact, unless the atmosphere’s energy content—and presumable its temperature—is increasing or decreasing, there can be no net flow.
Not really. Yes, part of the surface could be warmed by the atmosphere—i.e., heat could flow on an ongoing basis from the atmosphere to that part of the surface—but only because heat is flowing from some other part of the surface to the atmosphere, for a net flow of zero. If the net flow into/out of the atmosphere weren’t zero, the atmosphere’s temperature would be changing.
Well, yes, that warmed part of the surface will indeed radiate more—because the other part, the part that’s warming the atmosphere, is cooler than it would be without the atmosphere, so it’s radiating less. The surface as a whole is not radiating more. (Because of the fourth-law relationship between temperature and radiation, the average temperature will be higher with such an atmosphere than without it, but that’s different from a higher level of radiation.)
I don’t know if it will help you understand this stuff, but here’s a highly simplified one-dimensional model that shows how the surface will emit more than system receives from the sun if the atmosphere radiates. If you then change the radiation/absorption percentages to zero to simulate non-greenhouse gases, you’ll see that it won’t.
We will (arbitrarily) divide the atmosphere into two lumped-parameter chunks. Because of convection and conduction, an altitude layer in the real atmosphere can emit more or less radiation than it absorbs. To keep things simple, though, let’s imagine that there’s no convection or conduction: at equilibrium each layer has to emit all it absorbs. Also, although the real atmosphere absorbs some solar radiation directly, the atmosphere in our hypothetical is completely transparent to solar radiation; it absorbs radiation only from the surface and other layers.
The following radiation quantities are consistent with those assumptions but show that the surface emits 2.2 W/m^2 for every 1 W/m^2 it absorbs from the sun. And only that 1 W/m^2 escapes back to space. Yet the emissions equal the absorptions: no energy is created or destroyed.
Each atmosphere layer in this (no-convection, no-conduction, lumped-parameter) hypothetical absorbs ¾ of the radiation it receives, and it emits all the radiation it absorbs. Also, 1 W/m^2 comes from space and the same amount is returned to space, but the surface emits 2.2 W/m^2. If you go through the arithmetic you can confirm this. If you so change it that each atmosphere layer absorbs all the radiation it receives, then the surface will emit 3.0 W/m^2. But, if you change the atmosphere to a non-greenhouse gas, then neither atmosphere layer will absorb any of the radiation that it receives, and it will therefore emit none. So the surface will receive no radiation from the atmosphere, and it therefore radiates only the 1.0 W/m^2 it receives from the sun.
And this is true independently of whether the total energy in an atmospheric layer is gargantuan or minuscule; the net conductive flow is zero.
Desperate, just desperate.
Converts a perfectly clear concept into utter gibberish.
Stephen in reply to Joe:
“Desperate, just desperate.
Converts a perfectly clear concept into utter gibberish.”
Disagree, Stephen. This is how we descend into name-calling and arguing.
I think Joe has valid points and they should be able to be logically answered. I’m still thinking of an answer myself. Where’s the flaw in Joe’s thinking? Point it out if you think it’s there.
We’re looking through different paradigms and in large part talking past each other.
Joe: “The following radiation quantities … show that the surface emits 2.2 W/m^2 for every 1 W/m^2 it absorbs from the sun. And only that 1 W/m^2 escapes back to space.” Say what, Joe? The earth is emitting more than it absorbs? I thought this entire discussion started because that can’t happen?
Joe: “.. . let’s imagine that there’s no convection or conduction: at equilibrium each layer has to emit all it absorbs.” OK, maybe, but cutting off conduction/convection is making another universe altogether, one that doesn’t work like ours.
So yes, I’m confused, and the people who understand this might be able to clarify.
Don132
Ok, then I await clarification and will then consider further but I don’t see what he is getting at currently.
Note that he was rude to me first, though.
Joe Born January 5, 2019 at 2:36 am
Thanks, Joe. The truth of it is so obvious that it staggers me that so many people absolutely refuse to face it. In a steady-state condition with no GHGs, there can be no net conduction of heat between the atmosphere and the surface. As you say, if there were the atmosphere would eventually either freeze or boil away.
I also had to laugh at your accurate description of Stephen Wilde’s method of debate …
All the best of the New Year to you,
w.
Adiabatic cooling denial ?
Thankyou cementafriend. You can show some folk the truth but they refuse to recognise it.
Just got to keep it out there…. Gas Laws Rule, Entropy and Gradients Win. Brett
The Stefan-Boltzmann equation (SB)_assumes a closed cavity with perfect reflecting surfaces inside, and a hole to measure the flux of the electromagnetic radiation leaving the hole.
The SB surface is an imaginary surface *INSIDE* the object.
SB works at estimating the surface temperature of the Sun because the Sun can be modeled as an ideal furnace, i.e., a closed cavity with perfect reflecting surfaces.
And even with these assumptions, it works pretty well.
But the Earth not a star.
When you apply the SB to the Earth, it produces the temperature measured by the satellite plus a piece of junk.
The resulting temperature is most likely in the stratosphere.
A greenhouse is a physical structure which reduces heat transfers to conduction.
A greenhouse gas is atmospheric gas enclosed by the greenhouse.
The atmosphere cools by convection.
Hence the atmosphere and the greenhouse have nothing common.
In order for the atmosphere to behave like greenhouse, one would have to introduce magical molecules and a hockey stick’.
Oh wait, Hansen provided the magical molecules and Mann provided the hockey stick.
Everyone in favor of consensus science say ‘Aye’.
Definitely. If surface temperatures are due to compressional heating, the added temperature would radiate away.
That is exactly what you can observe in cold places on earth, temperature inversion starting at the surface. Pressure does not keep the surface warm.
http://members.casema.nl/errenwijlens/co2/baroim190203.gif
No water vapor, no heat.
Perhaps, but the pressure must generate SOME heat, surely its not zero.
Klem, pressure doesn’t generate heat. CompressING a gas generates heat, but when it is at a steady pressure no heat is generated.
w.
Does increased pressure result in higher temperature when heat is added?
Icisil,
Yes. That is exactly the point. The highest pressure is nearest the surface.
I think we’re getting warmer!
Don132
For a given number of molecules, having a higher density means that a given amount of energy is distributed among a higher number of molecules, thus the amount by which each molecule is warmed is decreased by greater density: Less energy added per molecule.
The relationship between molecular velocity and temp is linear, so I believe that adding a given amount of energy to a less dense gas will heat it more, not less.
Sorry, I rewrote the description and did not change the first part of it.
It should start “For a given volume…”
Let’s keep volume constant and increase the number of molecules with pressure.
Volume x in the stratosphere containing y number of molecules each having z kinetic energy will not have the same temp as volume x at the earth’s surface with (let’s say) y^100 number of molecules each having z kinetic energy.
Is that correct?
It is not about weight heating the ground, it’s about the tremendous weight of the atmosphere at the surface being warmed by the surface, and the near-surface atmospheric density (a result of atmospheric weight due to atmospheric bulk and gravity) necessarily keeps the conducted heat near the surface: that’s where most of the atmospheric molecules are.
Without surface atmospheric density, there is no greenhouse effect at all. Try it! How about: Mars?
Don132
“The relationship between molecular velocity and temp is linear, so I believe that adding a given amount of energy to a less dense gas will heat it more, not less.”
That doesn’t make sense. A dense surface atmosphere will have more molecules heated by that surface through conduction, and therefore a given volume of gas will have more molecules that are sped up through conduction. As the temperature of a gas is the average of the speed of all the molecules in a volume of gas, the temperature of a denser gas warmed by surface conduction will be warmer than a less dense gas.
Why is it cooler three kilometers up? Primarily because the atmosphere is less dense three kilometers up.
Why is the thermosphere cold even though the molecules in it are moving exceedingly fast? Because the atmosphere is so thin there. The molecules are “hot” but a volume of gas in the thermosphere is cold.
Don132
” Klem, pressure doesn’t generate heat. CompressING a gas generates heat, but when it is at a steady pressure no heat is generated”
But our atmosphere is not at a steady pressure, every day it varies. meteorologists tell us daily that high and low pressure systems are sweeping through our region.
It sounds like pressure variability to me.
How does this not generate as least SOME heat?
*head spins around like little girl in The Exorcist*
The pressure is not steady. That would be the heat death of the planet. That would be the bicycle tire blown up once and done.
The atmosphere is free to expand (less the pressure of the currently diminished solar wind).
Sorry, yours and Roy’s argument fails to account for entropy as energy of position. Bang a cumulonimbus into the stratosphere (they dent it) and that suckah is coming down around the periphery of the cell. Energy is conserved, but part of the energy is potential energy, like a rock precariously perched on a canyon wall. Entropy statistically guarantees the rock will come down to reduce the energy of position. Mass over distance is work.
The tragedy here is that Ned Nikolov overstates the case just as you do. You’re both right. Your arguments are not exclusive. The greenhouse effect definitely exists (albeit in a form different than human CO2 reducing radiation to space). Ned’s gravitatinal warming also exists.
Menicholas December 31, 2018 at 2:24 pm said
No it isn’t. The relationship between energy and temperature is linear that means the (absolute)temperature is proportional to the √ of the molecular velocity. Pilots know this because they know that the delta T with increase in speed is (TAS /100)^2
KE = 1/2MV^2 applies to molecules just as surely as bigger lumps of stuff.
Willis (and Roy).
OK, now let’s substitute a totally non-GHG atmosphere (maybe pure nitrogen?) to 1 bar surface pressure.
Where is the surface from which the SB calculation is to be performed?
Is there no thermal conduction or convection and zero lapse rate?
Regards, just asking.
Bob Fernley-Jones has some very good questions that should not be ignored.
As for comparing a planet with no atmosphere with one with an atmosphere and using a law that does not apply (theoretically and empirically) to a body, for the said body, I think that Gerhard Gerlich and Ralf D. Tscheuschner already explained quite clearly why it’s not a good idea.
Bob Fernley-Jones December 31, 2018 at 8:18 pm
The S-B calculations are only valid for something which radiates. There are only two things in the system, the atmosphere and the surface. The atmosphere doesn’t radiate. The surface does. Where would you guess that the calculations take place?
There is indeed a lapse rate. However, since the surface is evenly heated and there is no loss of energy from the atmosphere there is neither convection nor conduction.
Regards,
w.
Willis Eschenbach: “There is indeed a lapse rate.”
There you lost me. I thought your hypothetical was a “perfectly evenly heated blackbody planet that I spoke of above, evenly surrounded by a sphere of mini-suns” with a perfectly non-radiative atmosphere.
That sounds as though the surface temperature would be uniform. What drives the adiabatic expansion normally thought to cause the lapse rate? What did I miss?
Joe Born January 1, 2019 at 11:23 am
Yeah, you’re right, I misspoke. The atmosphere would be isothermal.
There’s a good discussion of this question by Professor Brown here …
Always good to hear from you, have a wonderful New Year,
w.
Willis,
I forgot to mention that I agree that GHG effect is well demonstrated but that there is other stuff going on too, and yes, e.g. nitrogen apparently only absorbs incoming UV.
I disagree that there would be no convection. The modelled flat surface planet is spherical and rotating, with complications including tidal and Coriolis effects. Also, gas contact with the surface must result in conduction boosted by convection/advection even though gases have low conductivity. Consider an analogy of a vertical rod of low conductivity material. The taller the rod, (equivalent to higher atmospheric pressure) the slower will be the rate of heat escape from the surface.
Haven’t done the sums of course, just wondering.
Regards
Bob Fernley-Jones January 1, 2019 at 12:49 pm
My bad if I didn’t mention it, but in my mind the planet in my thought experiment is not rotating. As you can see, I wanted to simplify the situation as much as possible.
Next, there is no conduction. The air in the experiment will be isothermal, and will be at the same temperature as the surface. See Refutaion of Stable Thermal Equilibrium Lapse Rates for details.
Finally, the atmosphere can only lose heat to the surface. So your vertical rod doesn’t apply.
Best regards,
w.
“pressure doesn’t generate heat. Compressing a gas generates heat, but when it is at a steady pressure no heat is generated.”
–
There is potential energy, There is motion energy, heat is constantly being generated by the molecules in the gas until they run down.
If the gas is at constant steady pressure it is generating heat, the balloon is not deflating.
Your comment is apropos in a system with no energy input.
Ie no sun, the atmosphere would compress into ice sheets. Energy shut down.
So anywhere that pressure is preserved there is heat being generated to help it do so.
Even if the initial stimulus is external energy.
Now it may not be new energy [Your definition of generation I guess] but the collisions do occur and heat is generated.
Hence in any atmosphere with an energy input and a vaguely steady pressure there is heat being generated by the motion of the particles higher with higher pressures due to higher gravity.
That is why the gas stays as a gas under constant pressure.
NZ do have some science behind them.
–
Semantics is not science.
“pressure doesn’t generate heat” is not correct.
Pressure does not increase the energy in a system is much more precise.
The two do have a relationship
Gay-Lussac’s Law: The Pressure Temperature Law
This law states that the pressure of a given amount of gas held at constant volume is directly proportional to the Kelvin temperature.
Now how does it stay at constant volume again?
Willis,
Thanks for your interest,
Sorry, I was relating to Roy’s post and his model which involves a great deal of non-equilibrium stuff. For instance, the thermal inertia of the whatever regolith or rock surface (nothing to do with albedo) is a tricky one as the inclined sphere rotates from midday alignment to midnight. In demonstration, I can’t recall the details now but remember that NASA screwed-up on predicting the surface T’s on the moon at one stage (might have been from overestimation of the amount of dust, I vaguely recall).
At a quick look, I’m not satisfied with Robert Brown’s paper that it applies to Roy’s unstable model, and that therein a non-GHG column could be isothermal. In Roy’s rotating sphere I submit that the surface air T will mostly be different to that of the surface itself (in part because of convection/advection).
By definition, the non-GHG in contact with the surface is not heated by the sun (ignoring UV absorption) and so prima facie should be very cold at the surface. So how is it as you say that it is at the same T as the surface? What would the heat transfer process from the surface be? Might it be via conduction (curiously with zero interface resistance), and devoid of any of the thermal inertias? My long understanding has been that if you heat a fluid in a gravitational field from below, then convection and conduction will result and I can see no reason why that would not be true in Roy’s model.
The analogy was intended to show that an atmosphere slows the escape of heat from the surface even if it comprises only non-GHG’s.
Regards, Bob F-J
“The S-B calculations are only valid for something which radiates.”
That is not correct. They are not valid in general for ‘something which radiates’. A lot of problems are in the climastrological pseudo science because they consider as valid a law where it isn’t.
Willis states:
How can the surface of this planet be equally heated when it presents itself to the sun at different angles, and when the surface has different albedos?
If the surface was equally heated, there would not be a significant difference in temperatures between the Arctic, Antarctic, and the equator.
Further, a not insignificant area of this planet is covered by tropical rain forests. In a tropical rain forest, how much heating is there of the surface by the sun given the thickness of the canopy? I have seen estimates that the dense canopy blocks about 95% of the sun light such that there must be relatively little direct warming of the surface. Of course it is warm near the surface but this is due to high humidity, not due to direct solar irradiance.
The canopy is significantly higher than the surface (may be 30 to 40 metres above ground), and whilst the canopy receives solar energy some significant proportion of this is used to power photosynthesis, such that the absorption and re-radiation from the canopy can never be the same.
richard verney January 2, 2019 at 5:06 pm
READ THE DAMN LINK! I even provided a freakin’ PICTURE!
Sheesh … do your homework, you won’t look so foolish.
w.
I am talking about planet Earth, you are not talking about anything remotely like planet Earth (in your Link).
Your theoretical planet has even albedo (indeed it is a blackbody) and it is surrounded by 1000s of suns which evenly heat the theoretical planet. The surface is not presented to the sun at different angles because of the number of suns, and it is even stationary, and if it has an axial tilt that would be irrelevant because of the even distributions of the 1000s of suns. It does not have unequally distributed storage heaters (the oceans) etc. That conceptually is nothing like planet Earth.
In the real world, where there are differences one will normally see different responses. So for example if the population of this planet is about 7 billion people, the future population growth will depend upon the ratio of men to women and their distribution. So there will be a very different population growth if the split is about 50/50 with about 3.5 billion of each, but a very different population growth if there are say just 100 million women and about 6.9 billion men, or if 90% of the women are over 50 but 95% of the men are between 1 and 10 years old.
Thus, in the real world, on planet Earth, for example, the distribution of the land masses is pivotal. Even the slightest movement such as the opening up of the Panama Isthmuth can lead to a radical response to the same solar input and the same CO2 levels.
The devil is almost always in the detail, and this is a potential problem when one tries to extract a though experiment into the real world. Whilst thought experiments are interesting, one should not lose sight of their limitations.
Tom, for crying out loud we do NOT say that. SOL provides the energy. Brett
Tom, why assume there is no sunlight. We are not talking about something in steel, but atmosphere in space under that ol’ sun. Just because Willis cannot see the difference between the gas phase and solid steel, two totally different forms of matter (nope, no ‘ideal steel law’ applies), and he imagines he can make up equations that trump the work of fine Physicists, does not make the null hypothesis invalid. Which is what N and Z support. Brett
I was considering the equilibrium effect of gravitational compression, apart from the solar effects. Whatever warming one has from gravitational compression would radiate away, as would the effects from solar heating.
It was more a matter of dealing with one factor at a time.
Tom, except I deal with the real world, and input is punctuated variably but continuosly so. People should get out more….. Please stop making your assumptions about what I write. No models can approach reality in this situation. Brett
Once, as a college student, I figured this out. It was a matter of increasing refractive index approaching the black body, but that’s a detail, and one an atmosphere of increasing pressure supplies. The prof couldn’t figure out what was wrong with it (outside of breaking the laws of thermodynamics) and neither could I. I decided to trust the laws of thermodynamics.
Somewhere in grad school, I figured it out – black body radiation depends on the refractive index of the space it’s in. I’d love to claim a marvelous proof that was too large for the margins of the page, but I’d be lying. It was over fifty years ago, and I’ve simply forgotten the details.
Two comments on that.
SOURCE
Since the thermal radiation that we’re discussing is typically between 5,000 and 35,000 nm (5 – 35 microns), the error is meaninglessly small.
Next, the apparent conflict with the laws of thermodynamics is discussed here.
Best New Years wishes,
w.
I think an even simpler explanation is to observe what happens with a hand-operated air pump. Fill up the tire on your kid’s bicycle, and touch the metal outlet fitting with you finger. You will not forget the experience! You will pull your hand away quickly, and possibly teach the kids standing nearby some words they hadn’t heard before (at least from you!). Touch it 10 minutes later, and neither it or the air in the tire will be much different from the air temperature.
The message here is that, yes, compressing air will cause it to heat up as per Charles Law. But, through conduction and radiation, (as explained by the Second Law of Thermodynamics) the heated gas will come to be in equilibrium with its surroundings. That is, without the constant addition of energy equal to that which is lost, the compressed gas will decrease in temperature until equilibrium is reached.
That would apply to CONTINUOUS pressure increase only (work). A STABLE pressure is not the same..Bad analogy I would think..If you stop pumping, the pressure stays the same, but the temperature of the gas, when released, would be colder than the surrounding air..( I’ve used Acetylene/Oxygen tanks at work before and the released gas was always cold)..? What am I missing ? (probably lots..LOL) Or is it the release of pressure that makes the gas colder ?
Till you light it …D’OH !
Boom boom.
Nice one Marcus………..
In the case of acetylene (it’s actually dissolved in an acetone/support mixture) the liberation of acetylene involves a phase change that removes heat necessary for its vaporization and hence the tank cools.
Pressurize a gas, and it heats up (that’s why scuba tanks are usually in a tank of water while they are being filled to around 3300 PSI, so the water can adsorb the heat). Typically when the gas is later released, the gas absorbs roughly the same amount of heat as was created when it was pressurized. This is also roughly how A/C systems work. The net result being that heat is being moved around. Pressuring a gas does cause the gas to heat, but the heat is usually lost to something until, as Marcus says, it reaches ambient temperature. The once heated gas cannot stay at the pressure heated temperature unless it is in a perfectly insulated container. As I understand it, Earth’s atmosphere loses heat to space. Once the heat of pressurization is lost, it cannot continue to provide its heat – it is gone, else it would be a kind of perpetual motion (energy) machine.
Correct Clay all the junk being peddled assumes a closed system, the system isn’t closed exactly as the Scuba tank shows. As I said above if it worked like this every scuba tank diver would be swimming around with 3rd degree burns in the middle of there back.
Marcus I think that comment sounds like how the refrigeration cycle works in your ‘fridge ‘.
You have it. While air is being compressed, kinetic energy is changed to heat energy. Once the pumping stops there’s no more kinetic energy to produce heat. It’s just compressed air in a container. Atmospheric air is compressed by gravity but not in the active sense of a mechanical tire pump or air compressor. Gravity maintains the compression and the amount of compression is a function of altitude.
I’ve tried to rephrase what you wrote just like the famous science-popularizer Neil deGrasse Tyson habitually rephrases everything anyone says to him.
Me: “Hello.”
Neil: “You mean ‘Greetings’ or ‘How’s it going’ or some other phrase commonly understood to …blah blah…”
CKMoore and Marcus
The air IS heated during compression (ala Charles Law) and the pressure is maintained inside the tire. Yet, the tire does not stay hot.
Then why is it colder as you go higher in altitude, where the pressure is lower?
Clyde Spencer December 31, 2018 at 12:20 pm said
Charles’law does not have anything to do with the heating of a gas as it is compressed. Nothing At all.
Charles’ law: The volume of a given mass of a gas, held at constant pressure, is propotional to the absolute temerature. See, nothing to do with compression, in fact neccesarilly, by defintion, pressure is held constant.
acementhead
I stand corrected. I should have said Gay-Lussac’s law;
Gravity doesn’t cause a pressure gradient in a pile of books, but it does in the atmosphere, and that’s why the temperature drops as you go to higher altitudes and lower pressures.
There is a fundamental difference between solids and gases. But that said, if there is enough gravitational forces exerted on a solid object, solids will be heated, eg ., the Jovian moon Io. As Wikipedia notes:
Our planet’s atmosphere is also subject to constant gravitational tides which result in the atmospheric bulge. Our atmosphere is constantly being pulled by the moon and the sun, sometimes working in opposition and sometimes working in conjunction.
The atmosphere is constantly being displace from below by the action of the tides (as they come and go, and by the passing of mountain ranges as the planet spins on it’s axis. On top of that our planet’s atmosphere is constantly being heated from above by the sun and below by the surface and air currents are constantly circulating in 3 dimensions causing work to be done, the by product of which is heat.
Whether these processes are enough to explain why the atmosphere can maintain its heat is probably anyone’s guess. I have never seen anyone try and calculate the effect of all of this, and whether this point is sufficient to address and overcome the point made by Clay Sanborn December 31, 2018 at 11:15 am
a hand-operated air pump
=======
You are using the wring model. Consider a sterling engine. When you rotate the shaft you get a hot side and a cold side.
Now mount a solar panel and electric motor to the shaft. Put the hot side of the sterling engine on the surface and the cold side in the upper atmosphere.
The surface will now be warmer and the upper atmosphere cooler but the average will be unchanged.
When air compresses and heats, there is “work” done of some form to cause the compression (W= the integral of -PdV according to chapter 1 of my old P Chem book). This is a reversible process so when air in the atmosphere sinks and compresses, it heats, and when it rises, it cools (pretty basic stuff). There should be no “net” addition of energy to the system. On the other hand, the greenhouse effect takes energy emitted by the earth towards space and sends part of it back towards earth, so there is a net increase in energy at and near the surface (again pretty basic stuff). I think this is consistent with what Dr. Spencer is saying in a comment below.
Any experts out there want to add or correct my understanding here?
“On the other hand, the greenhouse effect takes energy emitted by the earth towards space and sends part of it back towards earth, so there is a net increase in energy at and near the surface (again pretty basic stuff).”
On the other hand, the atmospheric greenhouse effect (NZ) takes energy emitted by the earth towards space and keeps part of it near the surface due to the density of the near-surface atmosphere which has absorbed heat from the surface via conduction and convection, so there is a net increase in energy at and near the surface (pretty basic stuff.)
I’m not sure that any of the above means that the surface must necessarily have more energy than it’s supposed to have, i.e., that it’s radiating more than it receives.
It is not about compressive heating. If it is, then please state where NZ say explicitly that it is.
Don132
From the article above:
“In short, it is the theory that there really isn’t a so-called “greenhouse effect”, and that the excess planetary surface temperatures on Earth, Venus, and other planets above the Stefan-Boltzmann (SB) temperature calculated from the rate of absorbed solar radiation is due to compressional heating by the atmosphere.”
I consider compressional heating as a one-time event where once the atmosphere is compressed and in place, the temperature then equaliberates via various heat transfer mechanisms (convection, conduction, radiation) to a new equilibrium, unlike the GH effect where there is a continual boost in energy. In reading reviews of NZ, one of the reviewers stated NZ basically discovered the ideal gas law in an inefficient way with R2=0.9999. The ideal gas law connects temperature to pressure in a pretty ridged way (paraphrased from the reviewer).
I’m not well versed in the NZ topic and at this point will defer to others.
Farmer Ch E retired, you are quoting from Willis and Spencer who assume that NZ are talking about “compressional heating by the atmosphere.” NZ are not talking about compressional heating of the atmosphere, and they are not talking about the heating that takes place when a gas is compressed. Suggest that Willis and Spencer review the theory before making claims about it.
If the radiative greenhouse effect is what warms our atmosphere then we should be able to see a similar effect for all planets with GHGs, which is most of them, and we should be able to derive a universal formula for all of them. This has not happened. What NZ have done is search for a universal formula that captured the variables that mattered, using dimensional analysis. What they found is that the presence of GHGs did not matter; what mattered was atmospheric density and proximity to the sun. Those who are wedded to the radiative theory don’t like this and so assume that the theory says something else, so they can tear it down.
Over and over something about compressional heating is repeated. It is not about compressional heating. It is about atmospheric density and the concentration of warmed molecules where the atmosphere is most dense– namely, at the planetary surface.
Don132
I would consider that earth’s atmosphere acts like a continuous pump. The atmosphere expands and contracts. Whether gravity does this or not, I wouldn’t conjecture because no one understands or understood gravity (not even Einstein, but that is a topic for another day). However DWIR does exist as even Ned Nikolov is forced to admit or why would cloudy nights be warmer than non cloudy nights. WILLIS Could you please put your thinking cap on to try to destroy Thayer Watkins conclusions about cloudy nights ? I took Thayer’s conclusions and figured out the maximum effect of CO2 from that.
http://applet-magic.com/cloudblanket.htm
The following is my calculations given that Thayer is correct in his.
********************************************************************************
Clouds overwhelm the Downward Infrared Radiation (DWIR) produced by CO2. At night with and without clouds, the temperature difference can be as much as 11C. The amount of warming provided by DWIR from CO2 is negligible but is a real quantity. We give this as the average amount of DWIR due to CO2 and H2O or some other cause of the DWIR. Now we can convert it to a temperature increase and call this Tcdiox.The pyrgeometers assume emission coeff of 1 for CO2. CO2 is NOT a blackbody. Clouds contribute 85% of the DWIR. GHG’s contribute 15%. See the analysis in link. The IR that hits clouds does not get absorbed. Instead it gets reflected. When IR gets absorbed by GHG’s it gets reemitted either on its own or via collisions with N2 and O2. In both cases, the emitted IR is weaker than the absorbed IR. Don’t forget that the IR from reradiated CO2 is emitted in all directions. Therefore a little less than 50% of the absorbed IR by the CO2 gets reemitted downward to the earth surface. Since CO2 is not transitory like clouds or water vapour, it remains well mixed at all times. Therefore since the earth is always giving off IR (probably a maximum at 5 pm everyday), the so called greenhouse effect (not really but the term is always used) is always present and there will always be some backward downward IR from the atmosphere.
When there isn’t clouds, there is still DWIR which causes a slight warming. We have an indication of what this is because of the measured temperature increase of 0.65 from 1950 to 2018. This slight warming is for reasons other than just clouds, therefore it is happening all the time. Therefore in a particular night that has the maximum effect , you have 11 C + Tcdiox. We can put a number to Tcdiox. It may change over the years as CO2 increases in the atmosphere. At the present time with 409 ppm CO2, the global temperature is now 0.65 C higher than it was in 1950, the year when mankind started to put significant amounts of CO2 into the air. So at a maximum Tcdiox = 0.65C. We don’t know the exact cause of Tcdiox whether it is all H2O caused or both H2O and CO2 or the sun or something else but we do know the rate of warming. This analysis will assume that CO2 and H2O are the only possible causes. That assumption will pacify the alarmists because they say there is no other cause worth mentioning. They like to forget about water vapour but in any average local temperature calculation you can’t forget about water vapour unless it is a desert.
A proper calculation of the mean physical temperature of a spherical body requires an explicit integration of the Stefan-Boltzmann equation over the entire planet surface. This means first taking the 4th root of the absorbed solar flux at every point on the planet and then doing the same thing for the outgoing flux at Top of atmosphere from each of these points that you measured from the solar side and subtract each point flux and then turn each point result into a temperature field and then average the resulting temperature field across the entire globe. This gets around the Holder inequality problem when calculating temperatures from fluxes on a global spherical body. However in this analysis we are simply taking averages applied to one local situation because we are not after the exact effect of CO2 but only its maximum effect.
In any case Tcdiox represents the real temperature increase over last 68 years. You have to add Tcdiox to the overall temp difference of 11 to get the maximum temperature difference of clouds, H2O and CO2 . So the maximum effect of any temperature changes caused by clouds, water vapour, or CO2 on a cloudy night is 11.65C. We will ignore methane and any other GHG except water vapour.
So from the above URL link clouds represent 85% of the total temperature effect , so clouds have a maximum temperature effect of .85 * 11.65 C = 9.90 C. That leaves 1.75 C for the water vapour and CO2. CO2 will have relatively more of an effect in deserts than it will in wet areas but still can never go beyond this 1.75 C . Since the desert areas are 33% of 30% (land vs oceans) = 10% of earth’s surface , then the CO2 has a maximum effect of 10% of 1.75 + 90% of Twet. We define Twet as the CO2 temperature effect of over all the world’s oceans and the non desert areas of land. There is an argument for less IR being radiated from the world’s oceans than from land but we will ignore that for the purpose of maximizing the effect of CO2 to keep the alarmists happy for now. So CO2 has a maximum effect of 0.175 C + (.9 * Twet).
So all we have to do is calculate Twet.
Reflected IR from clouds is not weaker. Water vapour is in the air and in clouds. Even without clouds, water vapour is in the air. No one knows the ratio of the amount of water vapour that has now condensed to water/ice in the clouds compared to the total amount of water vapour/H2O in the atmosphere but the ratio can’t be very large. Even though clouds cover on average 60 % of the lower layers of the troposhere, since the troposphere is approximately 8.14 x 10^18 m^3 in volume, the total cloud volume in relation must be small. Certainly not more than 5%. H2O is a GHG. Water vapour outnumbers CO2 by a factor of 50 to 1 assuming 2% water vapour. So of the original 15% contribution by GHG’s of the DWIR, we have .15 x .02 =0.003 or 0.3% to account for CO2. Now we have to apply an adjustment factor to account for the fact that some water vapour at any one time is condensed into the clouds. So add 5% onto the 0.003 and we get 0.00315 or 0.315 % CO2 therefore contributes 0.315 % of the DWIR in non deserts. We will neglect the fact that the IR emitted downward from the CO2 is a little weaker than the IR that is reflected by the clouds. Since, as in the above, a cloudy night can make the temperature 11C warmer than a clear sky night, CO2 or Twet contributes a maximum of 0.00315 * 1.75 C = 0.0055 C.
Therfore Since Twet = 0.0055 C we have in the above equation CO2 max effect = 0.175 C + (.9 * 0.0055 C ) = ~ 0.18 C. As I said before; this will increase as the level of CO2 increases, but we have had 68 years of heavy fossil fuel burning and this is the absolute maximum of the effect of CO2 on global temperature.
So how would any average global temperature increase by 7C or even 2C, if the maximum temperature warming effect of CO2 today from DWIR is only 0.18 C? This means that the effect of clouds = 85%, the effect of water vapour = 13.5 % and the effect of CO2 = 1.5%.
Sure, if we quadruple the CO2 in the air which at the present rate of increase would take 278 years, we would increase the effect of CO2 (if it is a linear effect) to 4 X 0.18C = 0.72 C Whoopedy doo!!!!!!!!!!!!!!!!!!!!!!!!!!
While your calculated climate sensitivity to 4x CO2 is reassuring, I still call ‘bs’ on the whole premise. Switch the date to the late 30s or 1940-41, and then there has been no statistically significant warming in spite of a huge increase in CO2. Set the starting point back to the end of the Roman Republic or the Minoan Civilization, and there has been a huge cooling inspite of the doubling or more of CO2.
It would be awesome if someone could get funding for an experiment to finally probe things one way or the other. Say at least 2 large corrals or compounds, with 30m or more high walls (made with plastic sheeting probably), with different levels of CO2. CO2 is heavier than air and should stay in the corral, the high wall preventing winds from mixing things up with the outside air. One compound with 400ppm for control, the other at at least double.
Maybe telephone poles would be tall enough to support walls tall enough to keep the CO2 at the right concentration.
I’m sure someone here has a better, more creative idea for a definitive experiment, better than air in jar with heat lamps, bit I throw my idea out there to start the discussion.
“This slight warming is for reasons other than clouds” This should read
This slight warming is for reasons other than just clouds.
Fixed. I hate typos.
w.
Alan, I do not think that NZ say that DWIR does not exist. They say that it’s not as important as we believe. I think Stephen Wilde put it best: atmospheric pressure allows the radiative effects to work as they do.
Don132
My posting did not state that 0.18C was the actual climate temperature effect of CO2. What it did state was that 0.18C is the maximum possible effect of CO2 at 410ppm. The effect may well be ZERO C. My conclusions of a local temperature effect were derived and built upon from Thayer Watkin’s article. If Thayer is wrong then I am wrong. However my conclusions are from a local analysis. They do not conflict with either Ned Nikolov’s theory or the general GHG theory of the greenhouse effect of an atmosphere. I simply took the observation of a maximum observed effect of clouds of 11C and calculated the maximum possible effect of CO2 at today’s levels of 410ppm. The real effect of CO2 may be ZERO but at least I have shown that the maximum effect possible is 0.18C.
However there is another side to this whole scare. At the present rate of increase of net CO2 of 0.5 % , this represents that CO2 will exceed the UK workplace saftey limit of 5000 ppm in 500 years time. This does bother me because if the increase of net CO2 in the atmosphere does increase at that rate, then we can’t ignore that. If it was 5000 years, I wouldn’t worry about it but 500 years is a different matter. HOWEVER the rate of net increase of CO2 in the atmosphere goes up and down every year. The rate of change over the last 60 years has been 0.423 % calculated as a geometric average or 0.48% calculated as an arithmetic average. this may well level off to ZERO in the future or it may continue. If it does continue then we DO have a problem in 652 years at the geometrical rate of increase of 0.423 %. I am a total skeptic of temperature changes and of drowning in rising sea levels, but the possible choking to death on CO2 levels is a nagging problem in ~ 600 years.
Air raising takes energy away from the surface. Air descending brings energy back to the surface. Radiation takes energy away from the surface. Back radiation brings energy back to the surface. Neither process creates any energy, neither can result in any net energy increase.
You’re not missing anything, Marcus. That’s the point. Once the pressure stabilizes, there are no more temperature effects.
I don’t know why everyone else didn’t see this immediately. Yes, there was high temperature from the energy of the condensing of the planet and the atmosphere, but it was a temporary phenomenon. After things got stable, it would have cooled off to equilibrium.
Clyde Spencer December 31, 2018 at 9:58 am said
This is incorrect. Charles law does not predict the amount of adiabatic heating when a gas is compressed.
Classical Charles’ Law The volume of a given mass of gas, held at constant pressure, is proportional to the absolute temperature. It is incorporated in the formua pV=nRT which appears to be widely misunderstood on this site.
More at Wikipedia(which actually gets this sort of stuff right)
https://en.wikipedia.org/wiki/Gas_laws
Of course. Both points of view are right and legitimate. They are in fact complementary. In the standard atmosphere model, this observation results in the lower layers of atmosphere having a temperature higher than the upper ones. The gradient of temperature being derived from the variation of pressure with altitude dp/dh=-𝜌h and pressure being linked to temperature by the perfect gas law P=𝜌RT.
At the level of the ground, there is no radiation equilibrium, only 1/3 of the heat flux (approximately) is evacuated by IR radiation, 2/3 are evacuated by convection and as sensible heat (water vapour).
The effect of the presence of “greenhouse gases” is that the radiation equilibrium happens not at the surface of the earth, but at a higher altitude where temperatures (according to the standard atmosphere) are colder.
There is no easy way to determine the mean altitude of radiation equilibrium. The atmosphere itself not being a black body, the equilibrium altitude is not the same for every IR wavelength. Actually the temperature of the ground is determined by the position of the “transparent IR window” through which 1/3 of the heat evacuated by the ground leaks to space. The “edge” of the transparent window varies slightly when “greenhouse gases” (water vapour, carbon dioxide and others) concentrations increase, leading to a slight increase in the radiation temperature of the ground. The effect varies from place to place, instant to instant, and with the cloud coverage, so that, up to now, it has been impossible to hint its average value with mathematical models.
But we can calculate the maximum temperature effect of CO2 locally, which will also be its maximum effect everywhere. See my above post.
Hmm, I fill up wheelchair tires all the time, some up to 110psi. Never been burned by a hot valve stem, never even felt warmth, to be honest.
Does this mean that pressure has no effect or just that it is less than they predict ? I always thought gases got colder when compressed ?
“According to Charle’s – GayLussac’s Law, the volume of a fixed amount of gas maintained at constant pressure is directly proportional to its temperature.
or simply,
V
T
=
c
o
n
s
tan
t
When the gas is compressed it means that the volume decreases. As Charle’s – GayLussac’s Law states, we could predict that the temperature of the gas would also decrease.”
Marcus, gases actually get warmer when compressed, not colder. However, the part that Nikolov and Zeller (N&Z) don’t understand is that this is not a constant on-going process that can lead to a permanently higher temperature.
Best regards,
w.
W, i would like you to find a quote from NZ to support your assertion as to what they do not understand.
EdB, it doesn’t matter what their theory is. My proof shows that NO compression, pressure or gravity based theory can work in the absence of greenhouse gases.
As to quotes from N&Z, take a look at The Mystery of Equation 8.
w.
Willis, what you say is impossible in spite of the math.
In the absence of GHGs, a warmed planetary surface must warm (via conduction and convection) an atmosphere composed of N2 and O2 with the same pressure as ours, and that warmth must be concentrated near the surface (ie, the average kinetic energy of a volume of atmosphere [“temperature”] MUST be higher with greater pressure simply because there are most molecules per unit volume.) Furthermore, this heat wouldn’t be radiated away by the atmosphere. There is no violation of any physical law in any of this. Therefore pressure does not cause heating, but it allows heat to be retained– something like how GHGs work.
Don132
Having reviewed (but not read every reply), I’ll offer a couple of observations for the next flare-up of this debate.
What I have not seen in this discussion are the terms Path Function and State Function. The error of much of this discussion is confusing the two. A gas equation of state of the form PV = f(nT) is a State Function (the ideal gas law being the most popular form) and can be arranged to predict the tempeature of a closed system of gas at a given P,V,n. What is overlooked is that for a given slice of the atmosphere near the surface, the current combination of PVT is a Path Function becasue the system is not closed (hot air can move in/it can move out, heat is radiated in/out, heat is conducted in/out, etc., etc., etc.).
Thus, your perferred equation of state can only be used to predict the change in temperature from State 1 to State 2. One might be tempted to use the (relatively) constant (averaged?) condtions of outside the atmosphere (State 1) to predict a ground-level State 2; however, because your selected PVn slice of atmosphere is not a closed system, the prediction is only transiently correct. The discussion around filling scub tanks (with and without the water bath) illustrate the problem exactly. Consequently, it is an improper use of thermodynamics to attempt to predict surface temperature from PV work alone.
As many have pointed-out, the N&Z work is not a thermodynamic prediction. It is rather an attempt at heat transfer prediction. We don’t all freeze to death when the sun goes down becasue the earth’s atmosphere is an insulating system.
In engineering, heat transfer rate (Q) is generally writen in the form:
Q = U x f(T1,T2)
There are many forms of the equation: U can be modeled by a thermal conductivity; the temperature difference can be a linear difference, a difference of squares, a difference of 4th power (common in radiation transfer), log-mean difference, and others. Generally, engineers only model the coeficient U from first principals (like thermal conductivity) in very narrowly defined and simplistic systems. The N&Z work spiffys-up the general heat transfer equation:
Q = f(P, other stuff) x f(T).
Perform a heat balance (Qin = Qout) and pick a reference temperature and one has a predictive equation for air temperature based on heat transfer. Here’s the rub, if you look at Equation 8, it has squishy parameters and a numeric coefficient. Consequently, it’s curve fitting as Willis has explained.
Now, in N&Z’s defense, curve fitting has a long and distinquished career in heat transfer. In the general equation above, U is rarely derived from first principals: real engineering systems are seldom sufficiently neat/simple. Consequently, we measure/estimate/guess a simple numeric coefficient and pair it with the form of tempature difference (linear, square, etc.) that provided the best fit over the narrow range of temperature for which we’ll accept this approximation. Useful tool.
However, as has been discussed on this site numerous times, curve-fitting parameters is at best sketchy proof that the parameters so-fitted rise to the exalted state of first principals. The greater the number of fitting parameters in the equation, the more dubious the conclusion. The dimensional analysis is a good way to avoid terms for your fit that are a priori incorrect (eg: P/V for energy); but, it is in no way proof that the term is a proper element of the model. In the jargon, a necessary but not sufficient condition.
So, of your kindness, please lay down your thermodyanmic clubs and take up your heat transfer clubs. The discussion may now resume.
Don said: “Willis, what you say is impossible in spite of the math.
In the absence of GHGs, a warmed planetary surface must warm (via conduction and convection) an atmosphere composed of N2 and O2 with the same pressure as ours, and that warmth must be concentrated near the surface (ie, the average kinetic energy of a volume of atmosphere [“temperature”] MUST be higher with greater pressure simply because there are most molecules per unit volume.) ”
There is no contradiction there. The temperature gradient results from the hydrodynamic pressure of the gas column. What changes in a transparent atmosphere (without GHG) is that the radiative equilibrium must take place at the level of the ground when it takes place at different altitudes depending on IR wavelengths with GHGs.
As a result, the radiative temperature equilibrium takes place higher in the atmosphere and, thanks to the temperature gradient of a normal atmosphere, the surface is warmer.
What actually determines the temperature “at the level of the ground” is the wavelength of the edge of the atmospheric windows which depends mostly on water vapor and a little on carbon dioxide concentrations.
What actually determines the temperature at the level of the ground is the amount of KE released within descending air masses.
Any radiative imbalances are neutralised by convective adjustments.
It is established science that convective adjustments can stabilise or neutralise radiative imbalances
http://www.public.asu.edu/~hhuang38/mae578_lecture_06.pdf
“Radiative equilibrium profile could be unstable; convection restores it to stability (or neutrality)”
and:
Note that the hydrostatic equation depicts the vertical balance of force for a piece of fluid at rest. The balance is between the upward pressure gradient force and downward gravitational force.
The hydrostatic equation is the vertical component of the momentum equation (Newton’s equation of motion) for the fluid parcel when the forces are in perfect balance and the net acceleration = 0.”
Readers should study that lecture since it explains the concept of hydrostatic balance within atmospheres.
It appears that those climate scientists who apply the radiative gases theory of climate change have overlooked the means by which convection neutralises radiative imbalances.
Paul says, in reference to GHGs:
“As a result, the radiative temperature equilibrium takes place higher in the atmosphere and, thanks to the temperature gradient of a normal atmosphere, the surface is warmer.”
I’m modifying my position to say not only has atmospheric heat been concentrated toward the surface by pressure, but also that density caused by pressure allows the atmosphere to absorb heat from the surface, and hold it there. I change my position because of the thinking I’ve gone through that shows me that my understanding of NZ, Wilde, and Holmes was incomplete in that I didn’t realize (explicitly) that an atmosphere without GHGs must be able to absorb energy, which is the only way to resolve Willis’ hypothetical planet riddle. Stephen has been saying this all along.
So if pressure is doing something, is radiation doing something on top of that? Or are the radiation calculations arbitrarily designed to make up the difference for surface T versus incoming, because we assume that pressure plays no role? Radiative theory is self-consistent within its own paradigm; we’d expect that. But are the underlying assumptions of the paradigm correct?
In the view that we count down from emissions height, pressure is just a bystander, waiting on the sidelines to make equations come out correctly but not doing anything.
Don132
Willis, “gases actually get warmer when compressed” = logic, but when a gas is released into space, does it take the heat with it ? I’m so confused….(and it has nothing to do with my overindulgence on N.Y. Eve.) lol ..D’OH !
Willis,
I think I’m on your side on this, but cannot gravity be considered a “constant ongoing process”?
It doesn’t stop when the compression is ‘complete’ even if convection is acting in the opposite direction
This is hard for my brain, so please be generous in your response.
Mothcatcher, gravity cannot do constant work. If it could we’d just harness it for a perpetual motion machine. It is a force, not energy.
w.
That’s why my brain hurts. It’s a force, it’s not energy. Agreed. But it’s still a force. Doesn’t stop being a force when it has captured an atmosphere.
Willis, I still don’t understand your proof. The atmosphere doesn’t have to do work. (You have proved above that it does not.)
However, your thought experiment does deviate from an ideal blackbody. I believe the addition of an atmosphere (with no GHGs) does change the radiative physics in your thought experiment. [Even using an atmosphere that cannot radiate energy.]
As another person stated the problem:
“If the planet without an atmosphere has temperature T1 and you were to magically add an atmosphere, let’s say with no GHGs that cannot radiate at all in IR wavelengths, according to the N-Z theory the temperature at the surface should rise to T2.
But if it does rise, surface radiation going out to space should increase, which would not be captured by the atmosphere; this would create a state of disequilibrium.”
I do not believe this is correct, because the thought experiment planet significantly deviates from an ideal blackbody in the distribution of energy it is radiating.
In the initial thought experiment planet (no atmosphere), the bands between 30N and 30S of the planet will be absorbing the bulk of the incoming solar radiation. This area would be far hotter than the poles, and would be re-radiating a massive amount of infrared energy back out to space. After the planet reached thermal equilibrium, the outgoing radiation necessarily must equal the incoming radiation. However, the equatorial band is going to be blazing hot and will be radiating out the bulk of the energy budget for the planet. T1 is the integral of the temperature of the planet.
Now consider the second planet with a magically introduced atmosphere (with no greenhouse gases including no water vapor). Once again, the bands between 30N and 30S will be receiving the exact same amount of incoming solar radiation (since our atmosphere is effectively transparent). However, in this scenario, the atmosphere is warmed by conduction from the heated surface of the planet. A heat engine now begins to operate as some of the atmospheric warmth is transported to the poles. The net effect is that the near equator area is partially cooled by some process other than radiation. The poles are also warmed to some degree by their contact to a warmer atmosphere .
Consider that the S-B equation states that the amount of heat a surface radiates is proportional to the fourth power of its temperature. In our thought experiment, the radiation at the equator would decline by a 4th power function of the equilibrium temperature decrease due to the atmospheric transfer of heat. The amount of outgoing radiation from this particular part of the planet will drop substantially, based on how much the temperature can be reduced by conduction to the planetary atmosphere.
The bands from 30N-90N and 30S-90S could warm substantially while only moderately increasing their outgoing infrared radiation. It would therefore be possible for T2 (as the integral of the temperature) to be higher on this planet since these bands cover double the surface area. Average outgoing infrared radiation will exactly balance the incoming solar radiation. However, the distribution of the outgoing radiation will obviously be much different relative to the planet with no atmosphere.
The S-B equation assumes outgoing energy is uniformly radiated from the spherical body. This is certainly not the case for our thought experiment planets. The equatorial band will be the hottest part of the planet by far, and will radiate a massive portion of the energy budget. Any drop in temperature of the hottest portion of the planet due to atmospheric heat transport will make the equatorial band a much less efficient radiator of thermal energy due to the 4th power rule.
The “average” temperature of the planet with an atmosphere can now be higher, even with the exact same amount of outgoing infrared radiation.
Willis
Gravity + Solar energy can do constant work, the water cycle is a prime example. Atmospheric density plays an important part in the cycle. You can’t say that density is not important to temperature.
mothcatcher. Like any other problem in statics, movement stops when forces equal. Compression is “complete” when the pressure from the internal kinetic energy (the “temperature”) balances out the compression from gravity. Of course you have to look at each altitude separately, which is a basic calculus problem: the higher the altitude the less stuff is on top of each molecule and the lower the pressure. (Of course all bets are off it the air isn’t at thermal equilibrium and starts convecting!)
Willis i’m not understanding where the free energy comes from, if the gas when released is same temperature as before being compressed
A , You compress a gas and it warms, then cools via radiation or conduction.
That warming and cooling was a transfer of some of the gases energy to the outside environ from the gas inside the pressurised environment.
The temp of both environs is equal after cooling as per the gas bottles example is that right.
So the gas when released must be colder when released it doesnt contain the same amount of energy per molecule as it did before pressurisation.
Well thats how it comes over to me, but i’m asking, not telling as these things aren’t intuitive.
Or was the force required to compress the gas, as in mechanical energy transformed into the heat.
Gravity IS constantly working. And gravity as an attribute of every matter works as force only on cosmic/planetary scales so any comparison to an experiment with air in a bottle is by definition an unfitting allegory or even completely meaningless.
The dis-/approval of the NZ theorem can therefore only come from more data about rocky planets. So long I will enjoy my popcorn watching this debate.
Willis, you are usually pretty sharp, but you have completely missed the boat on this one. A column of gas in a gravitational field isolated from its environment is NOT isothermal. RGB’s counterexample is fundamentally flawed, so don’t try to rely on that. The basics are simple. Here they are:
Gas molecules in a column are moving. Any object that is moving up or down in a gravitational field is constantly trading off kinetic energy against potential energy. That includes molecules. Molecules high up in the column have a lot of potential energy, relative to the gravitational field, but they gain that at the expense of kinetic energy, like anything else. So molecules lower down have less potential energy, and more kinetic. The problem that I suspect you are running into is that you are confusing temperature with energy, but temperature doesn’t measure ALL energy – only the kinetic part. So the column is isoENERGETIC, yes, but not isoTHERMAL.
No laws of thermodynamics are being violated here, and no, you can’t turn such a column into a perpetual motion machine no matter how hard you try. And no, the result does not depend on work being done on the system such as in a bicycle pump. Also you will be badly confused if you try to rely on the Ideal Gas Law, because it doesn’t apply to real gases.
Note that gravity is not magically “heating up the system” or any such thing. It is merely applying a gradient of force, which results in moving molecules rearranging themselves according to their distribution of kinetic and potential energies, which changes as they move around and bounce off each other.
Please get this right, because you are confusing poor Anthony… and now he’s giving you credit for a wrong result. I am embarrassed for both of you.
You don’t have to believe me, just try the experiment. Apparently some Germans have done it, and verified the obvious result. Richard Feynman has also apparently explained this, probably better than I can, and doubtless more than once, but the physics are really not that hard.
Steve Keppel-Jones,
What you’ve shown is that an atmosphere with a pressure gradient can’t be isothermal, but you haven’t shown how it gets extra KE to raise surface temp above BB.
If only so much KE comes from surface conduction, then how do we get extra?
Don132
Don, as best I can tell, there is no “extra”. PE and KE are distributed throughout the atmospheric column, in what would be a smooth gradient if there were no other factors, and therefore somewhere in the middle is the average – which has to be the BB temperature in order to maintain radiative equilibrium (more or less). It would be very surprising if the average were to be found either at the top or the bottom.
Of course I am not trying to say that there is no surface warming effect from radiant greenhouse gases, or surface conduction, tidal compression, etc., so that changes things up a bit in the real atmosphere. But I suspect not by more than a few degrees. And radiant gases have cooling effects higher in the atmosphere too, so the whole thing is quite complex.
(I haven’t tried to calculate the relative contribution ratio of atmospheric pressure vs. radiant heating on the surface temperature, I’ll leave that to more dedicated and capable individuals such as N&Z. But it definitely isn’t either 0 or 1. I would suspect upwards of 0.8 in favour of atmospheric pressure.)
Steve Keppel-Jones:
“PE and KE are distributed throughout the atmospheric column, in what would be a smooth gradient if there were no other factors, and therefore somewhere in the middle is the average – which has to be the BB temperature in order to maintain radiative equilibrium (more or less).”
In a GHG-free atmosphere only the surface is radiating.
In a GHG-free atmosphere, the molecules can’t absorb any radiation from the surface; they can only conduct with the surface, and there’s a net KE that can be conducted. Molecules go up, become PE, come down, become KE. Surface radiates at BB temp, and molecules at the surface have the KE that reflect that BB temp. The surface can’t conduct more KE than is present at the surface. The lapse rate then goes from surface to tropopause. I fail to see how the temperature at the surface can be anything more than the BB KE allows.
Don132
If the surface is at BB then there is no KE available to maintain convection. It has to be higher than BB and I’ve told you how it works.
“In a GHG-free atmosphere only the surface is radiating.”
Don, that’s not quite right, that atm. would radiate less of course but since the remaining atm. has mass, the GHG-free atm. radiates in addition to the surface.
@Trick
A GHG free atmosphere would radiate VERY little. Radiation by N2 & O2 in the IR is exceedingly low (as is IR absorption). N2 and O2 can radiate in the UV, but where on Earth is it sufficiently hot for that to occur?
N2 and O2 have mass Don, so they radiate at each temperature and at all frequencies since you can plug any temperature and any frequency into the ideal Planck formula and obtain a non-zero irradiance on the surface from such an atmosphere.
Mass does not permit IR radiation. Gases like N2 and O2 are permitted to radiate ONLY in permitted quantum energy jumps of the involved bond. Because N2 and O2 have quite strong bonds, these quantum jumps, which release or absorb energy, occur in the UV, not IR
Stephen:
“If the surface is at BB then there is no KE available to maintain convection. It has to be higher than BB and I’ve told you how it works.” But here you don’t re-state what you say and force me to look for it. So, here it is:
“The energy initially required to provide the PE in the atmosphere is drawn from energy that would otherwise have radiated to space.” Again, I think you mean KE, even though the PE comes from a molecule lower down losing KE.
So the energy (the extra KE you need) is from radiation? Even in a GHG-free atmosphere? How does that work?
Is everyone agreed on the issue that a radiating molecule loses kinetic energy? Because I’m confused on that point. Can radiation and conduction happen at the same time? I’ve always thought they could.
Don132
Don,
The KE one needs is from conduction.
Radiation and conduction can occur at the same time but the same unit of KE cannot be simultaneously radiated and conducted.
So, a surface at 288k can radiate 255k to space with the other 33k being conducted.
“Gases like N2 and O2 are permitted to radiate ONLY in permitted quantum energy jumps of the involved bond.”
No, when that photon is emitted, the molecule is moving thus a doppler shift in the frequency away from the line occurs, this is what is known as doppler line broadening in a gas. There are also other independant broadening processes for which the observed irradiance of any gas specie spectra is smoothed like that from a solid, these were discovered by experiment and subsequently explained. Better more sensitive photon detectors like CCDs have been developed since the originals were done on less sensitive photographic plates with long exposure times.
Stephen:
“The energy initially required to provide the PE in the atmosphere is drawn from energy that would otherwise have radiated to space.”
So how does that work, such that we end up with the extra KE needed for thermal enhancement? Initially, that is: how do we initially get the extra KE? I don’t care about atmospheric circulation at this point.
Just the bare-bones statement of where the extra KE comes from, in the most concise and precise way you can state it.
Don132
Atmospheric circulation is the cause so I can’t exclude it. It is perfectly clear in my original narrative.
A discrete zero sum circulation requires a kinetic energy store at the base to maintain it. Zero sum does not mean zero energy.
“Atmospheric circulation is the cause so I can’t exclude it. It is perfectly clear in my original narrative.
A discrete zero sum circulation requires a kinetic energy store at the base to maintain it. Zero sum does not mean zero energy.”
I don’t mean to be stubborn but OK, atmospheric circulation is important. What I want to know is where the extra KE comes from for surface thermal enhancement.
Sorry to be dense. It’s got to be crystal-clear for me. So please just state the mechanism for the extra boost of KE that raises temp above BB. I’m just not understanding the mechanism. If you’ve already stated it, can you please show me exactly where? In your original narrative, for example? I don’t have much time to spend on this so right now I can’t afford to go searching for it, even in the narrative; please post excerpt or exact link to location, or tell me which paragraph, etc.
Don132
Last try, because I have better things to do and we are in the company of trolls.
It comes from the recirculating atmosphere.
Once the circulation completes the first ‘tour’ it feeds on itself because what goes around comes around in a zero sum loop.
Meanwhile the sunlight continues at full strength so that gives you minimum surface temperature of 255k
Additionally , you have 33k going up at the same rate as 33k is coming down so there is a constant extra 33k at the surface all the time.
If you don’t get it now, I give up.
Stephen: “What matters is that there is a temperature difference between the S-B prediction and the reality.
It is downward radiation or it is KE released from descending mass.”
Net KE released from descending mass is the same as net KE at the surface. How do you get KE enhancement?
“If radiative energy leaves a molecule it cools down and radiates less UNLESS the lost energy is replaced, as it is, by fresh insolation.” How does that affect anything in a non-GHG atmosphere?
I understand pressure and the lapse rate. I don’t understand how you get KE beyond BB kinetic energy in a non-GHG atmosphere.
“The energy initially required to provide the PE in the atmosphere is drawn from energy that would otherwise have radiated to space.” What energy initially required? To lift the atmosphere? How is that energy acquired in a non-GHG atmosphere? Does the surface stop radiating temporarily and devote energy to KE? Why? How? What mechanism forces the surface to stop radiating so much to increase KE, if that’s what you’re claiming?
If you can’t explain it clearly and distinctly then there’s a problem somewhere. Your central problem is the acquisition of extra KE, and you say that no one understands except those who “get it.” You should be able to state how simply and clearly without referring us to things we might not have to look up and read; you should be able to state it in a sentence or two.
“Thus you get the full effect from continuing insolation PLUS the extra energy at the surface needed to keep convection running.” What extra energy, once circulation is in place?
“I can now more precisely describe the role of pressure.
A non radiative cloud of gas outside a gravity field will be almost all potential energy beimg at the temperature of space.
Applying pressure forces molecules closer together thereby converting PE to KE and the temperature rises.
Wrap it around a rocky planet using the force of gravity and the density gradient sorts the molecules so that they are closest together at the base.
Pressure at the base squeezes KE out of PE to generate heat.
The more pressure, the more KE can be derived from the gas at the surface.
The density gradient then determines the angle of the lapse rate slope and the lapse rate slope inevitably induces convection which prevents the KE at the bottom from dissipating by constantly renewing in a recycling process.”
None of that explains how you get the extra KE.
Don132
Don, it does get more complicated when you add incoming radiation to heat the surface, and therefore convection to produce a convective (not just molecular-kinetic) lapse rate, but still leave out the greenhouse gases. That’s not Earth, of course, nor my isolated column of gas, so I’ll defer to Stephen Wilde on that scenario. On that planet, maybe the BB temperature would be at the surface? But still warmer than the BB temperature of a planet with no atmosphere, because the kinetic energy required to keep the atmosphere from condensing has to be balanced by a higher surface temperature? I think that’s what Stephen W is saying. In any case, my explanation was only to point out the error of Willis’s statement, namely that an isolated column of gas in a gravitational field must be isothermic. I did not include incoming or outgoing radiation (or convection) to make my point, because those are more complex, and not necessary for the specific explanation I was making. But until he gets over that, he will never understand Earth’s atmosphere properly…
Wouldn’t be Mr Roderich Graeff by any chance?
A C Osborn, if you are referring to my reference to “RGB”, that is Robert G Brown, physics professor at Duke. He tried to make a counterexample to the statement that an isolated column of gas in a gravitational field cannot be isothermal, but his counterexample is full of holes. Willis does not do himself any favours by trying to rely on it.
No, it was the Gas Column work in Germany.
Oh right, thanks for that tip, A C. It’s hard to argue with experimental results, but that doesn’t stop people from trying, apparently!
Steve Keppel-Jones:
Mr. Eschenbach and Dr. Brown did not forget that kinetic energy converts to potential energy with altitude. Instead, they remembered that lower-velocity molecules are culled from the gas as it ascends, so the temperature remains the same. The Coombes & Laue paper I cited nearby explains that; here’s a summary.
Suppose that at some altitude the number
the number  of upward-traveling molecules per joule per meter whose vertical component of translational kinetic energy is
of upward-traveling molecules per joule per meter whose vertical component of translational kinetic energy is 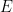 is distributed in accordance with
is distributed in accordance with
where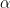 is a constant inversely proportional to temperature. The molecules for which
is a constant inversely proportional to temperature. The molecules for which  at altitude
at altitude  will have
will have  at
at  , so the distribution
, so the distribution  at
at  is shifted from
is shifted from  :
:
which means:
or
where
That is, the molecular density is lower at the higher altitude. But, although the molecule densities are different, the energy probability distributions are the same at both altitudes. Specifically, there are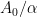 molecules per meter at
molecules per meter at  and
and 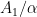 molecules per meter at
molecules per meter at  , and if we divide those values into the respective distributions above we get the same energy probability distribution
, and if we divide those values into the respective distributions above we get the same energy probability distribution  for both: the temperature is the same.
for both: the temperature is the same.
So Mr. Eschenbach and Dr. Brown are basically correct about isothermality in an equilibrium gas column subject to a uniform gravitational field. I disagree with them only in the theoretical detail that, strictly speaking, the kinetic-energy distribution is only approximately exponential. But the approximation is so good that there’s no practical situation in which that theoretical detail makes a difference.
It is NOT basically correct for isothermality in a perfectly isolated gas column subject to a uniform gravitational field at thermodynamic equilibrium. That max. entropy solution is the Poisson T(p) non-isothermal as the isothermal solution T(z) = constant has been shown to have less entropy therefore not yet at thermodynamic equilibrium (heat death).
Dr. Brown, as I recall, drew a wall of insulation around his column but treated it as an isolated column mathematically for a confusing story.
Trick:
I don’t think we can meaningfully discuss this.
I don’t see how your conclusion that “It is NOT basically correct for isothermality in a perfectly isolated gas column subject to a uniform gravitational field at thermodynamic equilibrium” follows from the other things you say. (Yes, if it would be compelling that entropy is maximized for some other configuration, but your just saying it doesn’t make it so.)
And you apparently were unable to follow my math.
So little purpose will be served by pursuing this further.
Joe 7:26am, f it is of interest to you to pursue, my prose follows the entropy maximization math in a beginner’s text Bohren 1998 sec. 4.4
“Molecules high up in the column have a lot of potential energy, relative to the gravitational field, but they gain that at the expense of kinetic energy, like anything else.”
You seem to assume that the molecule expended its own kinetic energy to get up there. More likely it was pushed up there and still has its kinetic energy (heat). Whatever pushed it gave up some energy and didn’t move up the column.
The result is fewer molecules up high, but some of them will be energetic. This can produce an interesting effect such as the “thermosphere”, very high temperature at high altitude where molecules cannot give up their energy by radiation nor by collision; so they stay “hot”.
An atmosphere of, say, nitrogen might be very hot at all altitudes, heated by ground contact but cooled by nothing. Methane, ozone and carbon dioxide cool the top of atmosphere while helping accumulate heat near the surface.
Michael 2, I’m not assuming anything. A single molecule could expend its own energy to gain altitude, yes, but in a gas consisting of many molecules, each one is bumping into others (and the wall of a container, if they are contained) all the time. In each collision, the total energy is conserved. So whenever any molecule is moving upward, it is exchanging kinetic energy for potential energy, and vice versa. Whether it is the same molecule that goes all the way from the bottom of a column to the top or not, is irrelevant – as long as energy is conserved at each collision.
The fallacy is to consider pressure to be the independent variable. The physical reality is that temperature drives volitilization and thus the amount of atmosphere. The anount of atmosphere (moles n in PV=nRT) determines pressure.
Most gases heat when compressed. This is due to conservation of energy. The work put into the system cannot disappear. The static atmosphere is not doing work. Its primary source of energy is heat from the sun. Without that, the atmosphere would condense out and pressure would drop.
If you take a specific volume of gas and compress it, what you say is true… then temperature will rise. But for the global atmosphere, any air sinking and compressing (and warming) is exactly matched by an equal amount of rising air at the same altitude that is doing the opposite. There is no net temperature change.
There is no net temperature change.
==========
Exactly. The average temperature remains unchanged. However, so long as there is adiabatic vertical circulation, the gas will be cooler at the top and warmer at the bottom.
The vertical circulation is a result of uneven heating by the sun as the earth rotates and orbits.
Sort of like an enormous sterling engine that cycles every 24 hours. The sun turns the shaft on the engine. One side of the engine will get hot and the other side will get cold, while the average remains unchanged.
The hot side is the surface and the cold side is the upper troposphere, and the work to turn the engine is provided by the sun.
OK, maybe I’m being picky …
If we have convection, we’re moving heat upward in the atmosphere. If we didn’t have convection, more heat would be retained at the surface. I would say that convection results in cooling.
commieBob December 31, 2018 at 1:52 pm
Bob, you’re forgetting that what goes up must come down. For every parcel of air moving upwards and cooling, another equal parcel is moving downwards and warming … net result?
No change.
w.
Unless the environment that the air convects into is colder than the rising air, in which case the rising air parcels warm the upper atmosphere by conduction.
This happens all day long in summer, as rising parcels of air warmed by contact with the surface rise and condense into clouds, in this case fair weather cumulus clouds, which then evaporate.
As the rising parcels warm the layers above and thus cool down, they descend back to the surface.
So over the course of the day the heat from the surface is distributed to altitude.
If these parcels did not transfer heat while they were aloft, they would be unable to descend back to where they started.
What you’re saying is true if, and only if, the only heating and cooling are due to the ideal gas law.
At the bottom, the atmosphere gains heat from the surface and loses heat to outer space due to radiation at the top.
That’s the simple version. Here’s a more complete version. The moisture content of the air adds a tiny bit to the complexity of the problem … LOL.
And in fact that the environment the rising parcel is ascending into is cooler than the rising parcel is a precondition for it to rise at all.
If it is not cooler, the parcel will not, and cannot rise (except to the extent that a rising parcel may acquire a certain amount of momentum and overshoot the level at which it is the same temp in the environment) to begin with. How high it rises is thus a function of how rapidly the atmosphere is cooling with height…unstable air is air that has an ELR greater that the dry adiabatic rate and the moist adiabatic rate…this the rising parcel will keep rising even after it condenses into a cloud. It gets complicated though, because if the air aloft is very dry, the cloud will evaporate…
You will not get vertical convection currents if the rising air is not shedding heat in it’s journey.
The lapse rate of the atmosphere is due to adiabatic expansion of rising warm air.
Hint: adiabatic-In thermodynamics, an adiabatic process is one that occurs without transfer of heat or mass of substances between a thermodynamic system and its surroundings. In an adiabatic process, energy is transferred to the surroundings only as work.
Commie Bob, yes, it is a simplified version for sure.
For the whole story, take a bunch of college level physical geography and meteorology classes.
Or do lots and lots of reading.
“The lapse rate of the atmosphere is due to adiabatic expansion of rising warm air.”
Incorrect.
Numerous factor influence the environmental lapse rate.
It exists even where no air is rising or has risen.
For a simple example, in Antarctica in Winter, under a dome of high pressure, the air is descending and warming, creating a certain lapse rate. The air also contains radiative gasses, causing it to cool, which obviously is a further influence.
Air descending on the lee side of a mountain range is heating from compression, and may also be warmed by contact with the ground surface, or cooled. Rain falling as verge may cool a part of the atmosphere, but not some other part below the point it has all evaporated.
Etc.
like rhe atmosphere, a sterling engine has no net movement of gas, yet one side gets warm and the other gets cold.
What is important is the relative phase angle between the parcels of air moving up and down.
The phase angle allows you to determine which side of the engine gets hot and which gets hot.
Only if the phase angle is 180 degrees is the engine isothermal.
Not during the formation of the atmosphere. See my reply to Roy above
“For every parcel of air moving upwards and cooling, another equal parcel is moving downwards and warming … net result? No chance.”
This is at equilibrium in the heat engine that Ferdberple describes. So Willis is correct: there is no overall change. But that fact is not significant, neither for NZ theory nor for the radiative greenhouse theory.
Don132
Some folks think the greenhouse effect is due to adiabatic heating. As Roy points out, that’s bogus. My quibble is not that Roy was wrong, per se. It’s just that convection is almost never an adiabatic process. Energy is almost always added and lost.
Willis, on the other hand was wrong because, I think, he missed my point.
” For every parcel of air moving upwards and cooling, another equal parcel is moving downwards and warming … net result?”
Oh dear, someone’s never heard of/understood entropy!
Roy,
There is a net temperature change during the formation of an atmosphere suspended off the surface.
What do you think happens to the energy required to enable ongoing convective overturning?
If it were ever radiated to space then the atmosphere would fall back to the surface.
Roy,
OK, now let’s substitute a totally non-GHG atmosphere (maybe pure nitrogen?) to 1 bar surface pressure.
Where is the surface from which the SB calculation is to be performed?
Is there no thermal conduction or convection and zero lapse rate?
Regards, just asking.
Roy,
I should add that I totally agree that there is a GHG effect but that there is a lot of other stuff going on that makes its net contribution very uncertain. I suspect that apparently big negative feedbacks such as evapotranspiration are not adequately studied while everyone hyperventilates over radiative effects.
I admire your work
“But for the global atmosphere, any air sinking and compressing (and warming) is exactly matched by an equal amount of rising air at the same altitude that is doing the opposite.”
It may not rise and fall at the same latitude. Think Hadley Cell, it’s drier when it falls too.
“If you take a specific volume of gas and compress it, what you say is true… then temperature will rise. But for the global atmosphere, any air sinking and compressing (and warming) is exactly matched by an equal amount of rising air at the same altitude that is doing the opposite. There is no net temperature change.”
.
No, wrong.
Temperature in a gas is just a measure of the average kinetic energy of the particles in the gas.
A temperature gradient/enhancement is set up in all convecting atmospheres (those >10kPa), including Earth’s.
This is because when a gas parcel expands adiabatically, as it does when rising in a gravitational field, it does positive work – and the kinetic energy drops and so the temperature drops. However, when a gas parcel is compressed, as it is when it descends adiabatically in a gravitational field, then it does negative work, and its kinetic energy rises and so its temperature goes up.
Why does the kinetic energy of the gas rise when descending? It’s because some of its potential energy is converted to enthalpy, so producing an increase in pressure, specific internal energy and hence, temperature in accordance with the following equation;
H = PV + U
Where;
H = enthalpy (J/kg)
P = pressure (Pa)
V = specific volume (m³)
U = specific internal energy (kinetic energy)
There is no ‘greenhouse effect’ because there are no ‘special’ gases which can cause it.
Read my paper and learn something;
Holmes, R. I. (2018). Thermal Enhancement on Planetary Bodies and the Relevance of the Molar Mass Version of the Ideal Gas Law to the Null Hypothesis of Climate Change. Earth, 7(3), 107-123.
That paper at
http://www.sciencepublishinggroup.com/journal/paperinfo?journalid=161&doi=10.11648/j.earth.20180703.13
is packed with references indeed.
Maybe totally off topic question : What would happen to a sufficiently dense gas giant or “brown dwarf” with an intense radiative pulse ( such as a nearby supernova , GRB or large flare)? Enhanced heating would then not depend on molecular particulars. I wonder if this pressure enhanced heating is actually used today in thermonuclear fusion design?
It seems N-Z say gas giants are to be handled differently.
Marcus
You have it exactly backwards! As a parcel of air is lifted orographically, it expands and cools, often producing precipitation. As the parcel of air descends on the other side of the mountain range it is compressed and heats. That’s what gives places like Death Valley their extraordinary Summer temperatures. But, radiative cooling at night allows the heated air to cool down.
If there are no clouds.
Backwards. Compress a gas and it concentrates its energy in a smaller volume, heating up proportionately. However, it then cools off to the ambient temperature outside the compressed chamber. Release a gas from a pressurized tank and it gets frost around the exit. It, too, then warms up to match the ambient temperature.
Some years ago the relief valve on a large Nitrogen tank (3500psi) outside my lab burst. For a short while there was a supersonic jet of nitrogen emerging, so cold that it was condensing as liquid N2, quite impressive! Freaked out the emergency staff who turned up, had to restrain them from evacuating the whole area. Had to author a report though and come up with a design modification to the relief valve.
Marcus, same amount of molecules and same amount of internal energy, Q, in a smaller volume means that the temp rises.
This can be observed during any compression process in air, or any other gas.
Are Santa Ana winds cold? They are compressional winds.
Does air get colder when it rises? Yes, because it is under less pressure, i.e. same amount of Q in a larger volume = lower temp.
This can also be observed when you spray something out of an aerosol can.
Do you have a can of compressed air for cleaning your computer?
You have it backwards.
Hilarious comment here :
http://notrickszone.com/2018/09/24/climate-scientist-karl-zeller-sums-up-the-discovery-that-pressure-not-co2-determines-planets-temps/
https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-723
On the average temperature of airless spherical bodies and the magnitude of Earth’s atmospheric thermal effect Den Volokin and Lark ReLlez
For the humor challenged try spelling their names backwards!
LOL!
Here we go again!
I love this stuff because it makes me think things through. Willis may be right, and I’ll honestly give him and Spencer and everyone else credit. But … I think N-Z are correct. I don’t have time now right but look forward to responses! I’ll participate as time allows.
But let’s just keep this a fun back-and-forth. It’s different paradigms, that’s all, and maybe some people are confused. Maybe I’m the confused one; it wouldn’t be the first time.
As to Anthony’s objections to N-Z, number two can be remedied if people tread a little lightly and remember than it’s not personal. Avoid ad hominem attacks and stick to the facts. Regarding number three, I think that the “fake names” issue is a non-issue, as the names were so obviously fake, and so obviously really Nikolov and Zeller (spelled backwards! hello?) that it’s also obvious that they did it to prove a point: they couldn’t get published as Nikolov and Zeller.
But to start, I’ll venture to say that the N-Z theory does NOT depend on compressional heating by the atmosphere. That’s a major misinterpretation. There is no compressional heating by the atmosphere, and N-Z never claim there is. Yes, gravity matters, but…. So right off the bat I’m sticking my neck out on the chopping block– all in good fun!
It’s really a beautiful and elegant theory, but I think people are so used to looking through a certain paradigm that they can’t see it.
Don132
Don, that’s par for the course of those who are left brain dominant; they get fixated on certain beliefs they think are unchallengeable because it’s all they know. They aren’t known for creativity or not conventional thinking because they have no balance between logic and emotion, which is necessary when attempting to progress in life.
It’s not an attack either, it’s just how different people operate. The modern left is stuck in right brain la la Land with no attempt to employ logic. It should be obvious to any outside observer who is balanced how these polar types aren’t much different, just in approach
“The modern left is stuck in right brain la la Land with no attempt to employ logic.”
Matthew,
Are you suggesting that political tendency is a product of which side of the brain is dominant? That doesn’t seem to be a logical idea. Left- vs. right-hemispheric dominance and the association with specific traits is largely a myth, though there are some tendencies, particularly in language.
Attempting to employ logic is not a product or determinant of political ideology. It’s the premises that differ.
(Interestingly, there’s evidence of a substantial genetic component of political leaning – but that doesn’t mean it’s immutable, of course.)
I’ve never understood why people can’t accept that the answer could be: “All of the Above”, not a few personally preferred combinations..The perplexity of the atmosphere is most likely beyond our ability to understand at the moment, so i accept most concepts as “possible”, Vuk’s hypothesis being the most valid.
Only time will tell. As a Canadian/American stuck in Canada, I hope it doesn’t colder…
ok, rant done. lol
Thanks Marcus, such a fate deserves a most sincere ‘Happy New Year Greetings’ from me to you. I’ve got some close Canadian relatives, every winter they migrate to Florida for 4-6 weeks, often longer. My younger daughter exactly a year ago left balmy London, UK, to spend two weeks just outside Canadian Arctic circle. Even London so called winter I often find on depressing side, so I migrate to sunny Mediterranean for 2-3 weeks at the time.
There was that movie series where the immortals with swords were running about throughout history trying to cut off each others’ heads…. “There can be only one!”
I kinda liked the movie series, but I never understood why “there could be only one”. At times there were immortals hanging out together, having a good time, not cutting each others’ heads off. Didn’t make sense. In the beginning there were scores of them (immortals), sometimes interacting, and sometimes not.
Seemed like in the end there could have been two, or three, or even more very important immortals. They could have gotten along; maybe one of them being more important or successful than the others, but still accepting of the comradery & interaction of the others.
Seems that the only real trouble was the one stubborn outspoken jackass, waving his sword around shouting “there can be only one!!!”. Sometimes the jackass didn’t even seem to care if he was the only one … he was just want to cause trouble by demanding that “there could be only one”. And this jackass demanded so much attention with his arrogance and attitude that the good part of the show was overwhelmed by his nuisance.
Anyway, allowing for more than one seems like it would have been a much better story line.
“Mom!”
https://youtu.be/8FurnHg4by8
But you gotta admit, when he used safety pins to glue his neck back together…that was cinema gold, baby!
There could be only one in order to achieve the goal of enlightenment, or whatever it was. The one left could see everything, hear everything, hear thoughts of everyone, that sort of nonsense.
Is the Eschenbach “proof” formulated in an equation(s)? I’m having difficulty understanding the exact meaning of the words used for this simple proof.
R, someone up above stated it quite clearly.
I hope that clarifies it. The only equation involved is the Stefan Boltzmann equation that relates thermal radiation and temperature. It says that when temperature increases, thermal radiation must also increase.
w.
Wrong. The added atmosphere will cool the surface by conduction and resulting convection will lead to winds carrying the heat to the poles where the atmosphere will heat the surface in return and the heat will radiate away.
Sounds good. I think people are looking for proof of how N-Z must be wrong instead of looking carefully at what they’re saying.
Don132
Temperature in a gas is the average kinetic energy of the molecules in a volume of the gas If the pressure is increased the number of molecules increase (Avogadro’s hypothesis/law). If the kinetic energy of the molecules remains the same then the temperature of that volume of gas has increased as the number of molecules has increased. This is the basis of The Gas Laws.
The Willis approach appears to be a denial of the adiabatic lapse rates. These are the rates at which heat content is lost/gained due to the atmospheric level/pressure.
As these lapse rates are well established this seems a rather strange position to take. Yes convection occurs when gases are warmer (less dense due to kinetic energy and speed of molecules) or colder (more dense due to reduced kinetic energy/speed of molecules).
Given a heat source – whatever that is conduction from the surface or infrared absorption, the kinetic energy increases and the gas expands and convection raises the volume of air to a level where the adiabatic heat/volume is balanced.
I have yet to see an experimental proof of infrared heating a water surface – it always results in increased evaporation and evaporative -latent- heat loss same with warm air over a water surface (it is why you blow on hot drinks/food). 70% of the Earth’s Surface is water another 20% is covered with transpiring vegetation. These surfaces will cool when exposed to infrared or breezes and the heat is removed as latent heat not sensible heat.
So by all means assume that a bare rocky earth – about 15% of the surface, will warm but you cannot take S/B as a case for a water or vegetation covered surface.
Yet the same Willis Eisenbach has many posts on here showing how the hydrologic cycle cools the surface – while at the same time claiming that it warms ???
This is a very simple subject for experimentation – but nobody is doing it. This lack of simple experimentation implies that the answer is known but unwelcome.
“If the planet without an atmosphere has temperature T1 and you were to magically add an atmosphere, let’s say with no GHGs that cannot radiate at all in IR wavelengths, according to the N-Z theory the temperature at the surface should rise to T2.
But if it does rise, surface radiation going out to space should increase, which would not be captured by the atmosphere; this would create a state of disequilibrium.”
But the idea is that the surface temperature rises because of a warmer lower atmosphere.
Willis If Thayer Watkins is correct with his finding that clouds are responsible for 85% of the GHG effect then I calculated that the maximum effect of CO2 would be 1.5%. That would leave water vapour for the other 13.5%. Nikolov’s theory could work on the grand scale and Watkins theory on the local scale. Those 2 combined would take care of your disequilbrium.
Ned Nikolov tends to forget about the local effects of clouds,water vapour and CO2. Once you calculate the maximum effect of CO2 on a local scale, that maximum is still the maximum everywhere else. If Thayer is correct about the 85% effect of clouds that would mean clouds and water vapour together account for 98.5% of the GHG effect on a local scale. With Ned’s grand scale theory there wouldnt be a disequilibrium when you combine it with the local effects . http://applet-magic.com/cloudblanket.htm
Alan Tomalty January 1, 2019 at 8:53 pm
The greenhouse effect can be measured as the percentage of upwelling surface longwave radiation absorbed by the atmosphere.
CERES data shows that where there are no clouds, ~ 31% of upwelling LW radiation is absorbed. When we add in the clouds, ~ 38*% of upwelling LW is absorbed.
This means that clouds are responsible for 7 / 38 = 18% of the total absorption. So I would argue that whoever Thayer Watkins is, his finding that clouds are responsible for 85% of the GHG effect is not true.
Best regards,
w.
Comparing 31% of ‘X’ to 38% of ‘Y’ needs to include the values of ‘X’ & ‘Y’.
With clouds, the incoming radiation (‘Y’) is less; your simplification, in this case, does not appear to be invalid.
… incoming radiation (associated with ‘Y’) is less …
Willis I assume that you have read both of Ned’s papers. Do you agree with his finding that all calculations of an earth’s temperature without an atmosphere, are wrong because of Holder’s inequality?
Alan, Ned said:
===
“Due to Holder’s inequality, one CANNOT estimate the true MEAN temperature of a spherical body from the AVERAGE absorbed radiation by that body as currently attempted.”
===
I agree with that. However, I do not agree that “all calculations of an earth’s temperature without an atmosphere, are wrong because of Holder’s inequality”. For example, in my thought experiment with an even heating it can indeed be estimated.
All that Holder’s inequality tells us is that with a given energy input to a planet, if there is any variation in temperature, the average temperature will be lower than if the heating and cooling is evenly distributed.
Finally, there are many more roadblocks to estimating the blackbody or other temperature of some earth with changed conditions. See Dr. Brown’s post on the subject,
w.
If not compressional heating, how else would high pressure in a gas lead to higher temperature (in their view)?
Sun plus lapse rate.
lapse rate is not a physical process, it is just the empirically observed temperature gradient.
The lapse rate is a physical process observed in the troposhere and is described mathematically by gravity and height.
Atmospheric lapse rate is a well known physical process. It is analytically determined by the specific heat of the gas, and gravity. It is modified by phase change of water vapor from gas to ice or rain, which lowers it’s value from near 8 to about 6.5 degrees C per km. The process only requires a reasonably well mixing of the atmosphere (convection). It is due to the adiabatic compression of gas moving down, and expansion of gas rising. The process produces a temperature gradient, not a specific temperature level. The greenhouse effect require both a lapse rate and an optically absorbing atmosphere from either molecular absorption, or particles (dust, ice particles, or droplets).
There is something called the environmental lapse rate (ELR), which exists in the atmosphere and describes how much the air cools with height.

It varies, and this variance is the cause for much of the weather we get, but also a consequence of it.
Then there is the amount a parcel will cool if it is lifted into the atmosphere, whether by being forced to rise over a mountain, or by being heated above the temp of the air above it.
If the air above the heated parcel is warmer than the parcel, it cannot rise, and will just keep getting hotter, until it finally is able to rise due to being warmer.
When a parcel of unsaturated air rises, it cools at a certain rate called the dry adiabatic lapse rate, and if it is saturated, it cools at the moist adiabatic rate. The opposite occurs of the parcel descends.
Also, the rate is not constant, but depends on barometric pressure and temperature, and so the graph of the rate is actually a crosshatched plot of curved lines.
So, this misunderstanding you guys are having is because you are talking about or conflating two different concepts…the ELR, and the adiabatic lapse rates.
…talking about OR conflating…
Fixed … I hate typos.
w.
Temperature in a gas is the average kinetic energy of the molecules in a volume of the gas If the pressure is increased the number of molecules increase (Avogadro’s hypothesis/law). If the kinetic energy of the molecules remains the same then the temperature of that volume of gas has increased as the number of molecules has increased. This is the basis of The Gas Laws.
The Willis approach appears to be a denial of the adiabatic lapse rates. These are the rates at which heat content is lost/gained due to the atmospheric level/pressure.
As these lapse rates are well established this seems a rather strange position to take. Yes convection occurs when gases are warmer (less dense due to kinetic energy and speed of molecules) or colder (more dense due to reduced kinetic energy/speed of molecules).
Given a heat source – whatever that is conduction from the surface or infrared absorption, the kinetic energy increases and the gas expands and convection raises the volume of air to a level where the adiabatic heat/volume is balanced.
I have yet to see an experimental proof of infrared heating a water surface – it always results in increased evaporation and evaporative -latent- heat loss same with warm air over a water surface (it is why you blow on hot drinks/food). 70% of the Earth’s Surface is water another 20% is covered with transpiring vegetation. These surfaces will cool when exposed to infrared or breezes and the heat is removed as latent heat not sensible heat.
So by all means assume that a bare rocky earth – about 15% of the surface, will warm but you cannot take S/B as a case for a water or vegetation covered surface.
Yet the same Willis Eisenbach has many posts on here showing how the hydrologic cycle cools the surface – while at the same time claiming that it warms ???
This is a very simple subject for experimentation – but nobody is doing it. This lack of simple experimentation implies that the answer is known but unwelcome.
Ian, Solar Radiation (short wave) heats up the oceans nicely, but the heat is released gradually primarily through evaporation.
That is the primary Green house affect in a nut shell.
Ian,
that’s fundamentally incorrect. The number of moles of gas does not change because you increase pressure. That would require a violation of the conservation of mass!
The ideal gas law PV=nRT requires that the volume must decrease if the pressure increases at the same temperature, or the temperature must rise for the pressure to increase at the same volume. For the most part, the number of moles of gas in the atmosphere is a constant. Water evaporated is about equal to water condensed. Net losses to space, outgassing from oceans and volcanoes notwithstanding, average atmospheric pressure is pretty constant.
I agree.
Ian, your explanation is a jumble of mixed up terminology and incorrect statements, such as this one:
“The Willis approach appears to be a denial of the adiabatic lapse rates. These are the rates at which heat content is lost/gained due to the atmospheric level/pressure.””
The definition of an adiabatic process is that no energy is lost of gained during the process.
It is impossible to tell if you simply explained what you meant incorrectly or if you misunderstand what you are talking about.
In scientific discussions, particularly those involving disagreements, terminology and using concise and correct language is vital.
Telling someone they are wrong and using an incorrect statement to do so does not get anyone anywhere.
That passage I quoted is as far as I got when I wrote this.
But even before that, it was hard to discern exactly what you were saying. I am trying to be descriptive and not judgmental, but when you say increasing the pressure increases the number of molecules, it is confusing. Typically such discussions are done using a fixed parcel of a gas. Greater density means that there are more molecules in a given volume…when all else is equal. But unless you say so, it is hard to determine exactly what you mean to say.
Just sayin’.
I agree.
While having a dense atmosphere does not make a planet warmer, it makes the surface warmer through lapse rate, all the heat that atmosphere absorbs is not evenly distributed, it is colder at the top and warmer at the bottom, the more atmosphere you have the more extreme it becomes.
I’ll expand EdB’s comment, a bit.
Venus takes 243 days to rotate. Making for a very long day.
Only Venus’s light/dark periods are 117 days long, since Venus rotates backwards causing the sun to appear to rise in the West and set in the East. Venus’s slow spin is complicated by Venus’s full rotation around the sun.
That is a long time to absorb a much stronger level of insolation.
ATK: Also a long night to lose it except that strong venu-strophic winds redistribute the solar energy evenly. All that CO2 should cause night freezes if it was so good, but no….. Brett
Roy, it is not that high pressure leads to high temperature, it’s that high pressure leads to increased molecular density. These dense molecules conduct/convect with the surface warmed by the sun. They necessarily hold the heat conducted from the surface close to the surface, as that’s where the vast majority of the atmospheric molecules are, which in the absence of GHGs could not radiate that heat away, and this fact would NOT violate any law of physics, and would not necessarily make the earth radiate more energy than it receives, as Willis seems to claim.
The dense atmosphere is warmed by the surface that’s warmed by the sun, and we could argue that without GHGs the atmosphere could not cool as effectively, which is maybe what Willis wanted to say. But, the atmosphere could cool through conduction with cooler molecules higher up or more toward the poles, or at nighttime with the cooling surface.
14.7 psi at the surface isn’t a trivial amount of pressure, and makes for a very dense surface atmosphere.
Why is the Grand Canyon warmer at the bottom? When cold air sinks?
Well, it is New Year’s Eve and that’s all I have time for!
Don132
The volume of the atmosphere is always changing. Right now the thermosphere is cooling, thus it is shrinking, assuming mass stays the same and pressure stays the same. PV =nRT where R is the ideal gas constant which itself = Boltzmann constant * Avogadro constant.
https://spaceweatherarchive.com/2018/09/27/the-chill-of-solar-minimum/
The Langley Space Research Centre did not receive Gavin Schmidt’s email to GET WITH THE PROGRAM. Folks This is going to destroy the CAGW meme quickly. In the link they go on to say
“The thermosphere always cools off during Solar Minimum. It’s one of the most important ways the solar cycle affects our planet,” explains Mlynczak, who is the associate principal investigator for SABER.”
THIS CANT GO WELL FOR AL GORE’S CHURCH OF CLIMATOLOGY. They measured the infrared glow of the molecules of CO2 and NO at the very top of the atmosphere.
“We see a cooling trend,” says Martin Mlynczak of NASA’s Langley Research Center. “High above Earth’s surface, near the edge of space, our atmosphere is losing heat energy. If current trends continue, it could soon set a Space Age record for cold.”
They developed a Thermosphere Climate Index
As 2018 comes to an end, the Thermosphere Climate Index is on the verge of setting a Space Age record for Cold. “We’re not there quite yet,” says Mlynczak, “but it could happen in a matter of months.”
I tried to put the graph in this post but the png format didnt seem to work.
However if you look at the graph, they extended SABER’S 17 year data back to 1950 by using geomagnetic activity and the sun’s UV output which have been measured since 1950. This will not go well for Al Gore’s Church of Climatology and his main disciple Gavin Schmidt at GISS.
The graph shows an ice age type of pattern with the y axis being POWER 10^11 W. There is a total variation of 5x. The graph corresponds very well with the sunspot solar cycles.
FOLKS IT GETS EVEN BETTER
https://spaceweatherarchive.com/category/thermosphere/
We now have a US government agency that is tracking the solar minimums and maximums and showing actual data of Power being emitted to space. It seems that variation is so huge that Willis; it is the SUN after all. Prepare for much colder weather in next 5 years that will be colder than in the 70’s. I will accept cold weather if it destroys Al Gore’s Church of Climatology .
http://www.spaceweather.com/
I led you astray slightly. NASA seems to have decreed that they will not show the Thermosphere Climate Index. You can get it at the above link (go figure, a private site). It looks like the Langley Research Centre (LRC) in Virginia is also a Global Warming shop.
https://www.nasa.gov/langley/overview I predict that the internal program funding will cut for SABRE.. Trump needs to fire both Gavin Schmidt (GISS) and David Bowles (LRC)
“We are especially pleased that SABER is gathering information so important for tracking the effect of the Sun on our atmosphere,” says James Russell, SABER’s Principal Investigator at Hampton University. “A more than 16-year record of long-term changes in the thermal condition of the atmosphere more than 70 miles above the surface is something we did not expect for an instrument designed to last only 3-years in-orbit.”
So that explains what happened. When they originally planned this program whoever was in charge realized that this would destroy Al Gore’s Church of Climatology. So they only gave it a 3 year run which is ridiculous. Don’t forget that this machinery is piggybacking onboard NASA’s TIMED satellite. However the machinery outperformed its wear date and lasted a full 16.5 years. Somehow whoever was in charge did not manage to sabotage its mission with a real best before date coding strike. So now we have another piece of the radiation puzzle which will this time rear its beautiful head and show us the reality of COLD climate like we had in the 70’s which of course will cause Al Gore’s Church of Climatology to crumble to dust like make up clouds. But this time it will be the SUN stupid.
Alan Tomalty January 1, 2019 at 10:46 pm
Alan, I’ve never denied that the sunspot-related solar variations affect the temperature of the thermosphere. However, it’s a temperature difference that makes no difference. Why? Because the thermosphere is so thin. How thin? So thin that there are so few electrons that it won’t carry sound. It’s so thin that it’s almost empty space. The Space Station travels through the thermosphere.
How much effect does it have on the surface? Well, the temperature of the thermosphere is on the order of 1000K, which is about 730°C, or 1,340°F … but there are so few atoms that if you could feel it, it would feel cold. For example, despite the 1,340°F temperature, the Space Station isn’t burning hot on the outside, it’s freezing cold …
So yes, the sun does change the temperature of the thermosphere … but as near as I can tell, that makes zero difference down here on the surface.
w.
However, it is one indicator that the sun must have some effect on the lower atmosphere if the total visible and IR radiation escaping to space from the thermopshere is 5 to 10 times smaller in a low sun spot cycle than a high one, Of course those calculations do not include the amount of xrays, Extreme UV, Far UV and near UV that is escaping. Those quantities may be higher which may balance out the visible and IR radiation. Nonetheless in general low sunspot activity is somewhat associated with cooler global temperatures perhaps with some type of lag mechanism. I believe that Willie Soon is working on this.
Please consider Roy, how energy flows down the gradient, up the Entropic, with deference to Equipartition.
If it is less expensive to use gaseous buoyancy differences and in our case WV LH transport, and that is so, then how can all this about radiant Flux (a VECTOR force), mean as much as claimed.
We know empirically that convection rules. We know that the gas laws Poisson Relationship means gas specie is inconsequential except for tiny mass effects. N and Z and others have come to this from several angles and the empirical solar system data make it as close as possible to incontrovertible. Radiative flux only rules above the c.0.1bar level, any world, any gas. Maxwell deduced this in the 1860s and he was a great experimentalist as well as theorist. Brett from the land of Rutherford and Popper…..
Brett It would help if you explained yourself in plain English scientific terms. Most of us havent got a clue of what you are saying.
Don – As I read it, Willis (as represented by Roy Spencer) doesn’t assume anything about N-Z, he simply shows that if the atmosphere does not interfere with radiation then the temperature can’t increase. Willis’ explanation is indeed elegant.
No, you ignored conduction, convection and thus cooling.
Infra red rad takes care of the cooling. The atmosphere without adsorptive- re-emittive gases are transparent to infra red going out (and shortwave coming in) If you assume an ocean, yeah huge convection because the much lighter water molecules evaporating from the water surface plus being heated transport huge volumes of heat upwards and “make” weather. With just a cold rock and, say, nitrogen “weather” isn’t much in evidence.
Conduction and convection transport energy. and then it stops if the transported energy is not removed (you can’t accumulate the energy, it would make the higher level hotter than below, and conduction and convection would reverse). Without radiation to space you can’t remove the solar energy.
Don
There is decompression in rising air and compression in descending air each of which comprise half the atmosphere at any given moment.
The extra surface heating develops during the initial formation of an atmosphere and convective overturning can never be prevented due to uneven surface heating.
As long as convective overturning continues the extra surface heating will be retained.
My description of the mechanics of the process is supplemental to the N-Z findings
You’re saying that there is a primordial reservoir of excess heat maintained in the atmosphere so long as the sun continues to supply energy to drive convection?
Berthold Klein and Konrad Hartmann are two who have shown that N and Z are right, by physical experiment. Nasa’s solar system atmospheric probe readings do too. Allmendinger and other gas engineers show that all gases do radiate in both Light and Raaman spectroscopics. Others just have not looked hard enough, taking’expert’s’ words for it. Nand Z are two of many, but blindness seems to prevail, and doubling down on failed hypotheses. Very Feinmannesque/Popperesque.
Weathermen shoud consider how much radiative theory figures in building the Standard Atmosphere. Or how little Hoyt and Hottel and all Process Engineers care for the magic powers of CO2.
Don, there were perfectly good reasons for their jape, mainly to do with the dishonesty of warmista including reviewers etc.. Ask Peter Ridd and so many other sceptics.The interesting thing is how Willis and Anthony jumped on it and took the warmista line, and harshly. Including being blind to real Physicists’ findings. Starting with Poisson and Maxwell also Wood. Not in a pleasant manner either. However I don’t give up on them so long as mutual human respect is shown all around, we will get there.
Which in Science is often someplace we never imagined – that’s the beauty of it. Brett
Actually, I did manage to rebut Willis’s alleged ‘proof’ at the time but just as I got to the point where he would have had to back down he went off at an irrelevant tangent and became somewhat shouty.
Since this has come up again I must address it again and as a starting point I refer to my description of the conductive / convective process involved here:
https://tallbloke.wordpress.com/2017/06/15/stephen-wilde-how-conduction-and-convection-cause-a-greenhouse-effect-arising-from-atmospheric-mass/
One can readily see that the excess surface heat cannot be radiated away to space so long as a conducting and convecting atmosphere is held off the surface in hydrostatic equilibrium since that excess is being constantly recycled in and out of potential energy (which is not heat and does not radiate) within ascending and descending columns of atmospheric gases.
The same parcel of energy in the form of surface heat cannot be in two places at once so energy that is being constantly recycled between KE and PE within convective overturning is simply not available for radiation to space.
It follows that due to the conversion of surface heat to potential energy aloft as air rises there can never be an isothermal atmosphere as Roy Spencer proposes and the inevitable existence of a lapse rate slope even in a completely non radiative atmosphere would be the necessary proof.
There is no breach of any law of thermodynamics in my description and it is fully compliant with the gas laws.
The problem with an upward facing IR thermometer is simply that it will measure a temperature at the height where atmospheric density triggers the sensor. Thus if pointed at a clear sky it will register a low temperature high up in the atmosphere but if clouds or particulates increase density so as to trigger the sensor at a lower height then it will register a warmer temperature. The reading provided by such a thermometer just shows the temperature of the point along the lapse rate slope where density triggers the sensor. That is therefore not a proof of downward radiation in place of compressional heating since it is consistent with both.
The reality is that downward radiation is neutralised by conduction and convection as it moves down along the lapse rate slope so that the surface effect at the surface becomes zero.
Stands back and waits for chaos to ensue 🙂
As a layman, I thoroughly enjoy reading the comments for exactly these comments mr. Wilde, as well as the debate that ensue
Thanks all for your input and effort. It helps me learn slowly the science I don’t understand.
You and me too. I love these threads. I read them and change my mind back and forth until I’m all messed up. Then I go to bed 🙂
I learn tons on sites like this. Sadly they are few and far between where real discussions can be carried on politely (for the most part).
I’m not seeing the logic in this.
If the planet without an atmosphere has T1 and you were to magically add an atmosphere, lets say with no GHGs that cannot radiate at all in IR wavelengths, according to the N-Z theory the temperature at the surface should rise to T2. But if it does rise, surface radiation going out to space should increase, which would not be captured by the atmosphere; this would create a state of disequilibrium.
You say “excess surface heat cannot be radiated away to space so long as a conducting and convecting atmosphere is held off the surface in hydrostatic equilibrium since that excess is being constantly recycled in and out of potential energy.” My recollection of heat transfer says that this is wrong. A body radiates only according to it’s present temperature. If its temperature goes up, it must radiate more; conduction and convection cannot stop a surface from emitting more radiation if its surface temperature rises.
Similarly, you cannot cause temperatures on a surface to rise at all if there is always a theoretical “hydrostatic equilibrium” between the surface and the atmosphere since the exchange of equal amounts of heat changes the temperature of neither body. Your “hydrostatic equilibrium” theory itself contradicts the N-Z theory.
Kurt,
Are you not suggesting that heat can be radiated away and conducted/convected away simultaneously?
You need the same parcel of energy to be in two places at once.
At hydrostatic equilibrium the net energy exchange is indeed zero but you need an injection of energy to get the atmosphere to hydrostatic equilibrium in the first place and that slug of energy is then recycled up and down indefinitely as long as the atmosphere remains in place. That slug of energy heats the surface but cannot radiate to space.
A body only radiates according to its temperature if in a vacuum i.e. no ongoing non radiative processes. The S-B equation refers to a vacuum.
Of course heat can be simultaneously conducted, convected, and radiated away. But radiation from a surface always increases when its temperature increases according to the fourth power of its temperature. What you are saying is that a surface can two different temperatures, radiating the same at each of those temperatures. That’s not possible.
You’re missing the entire thrust of Willis’ proof. You start with a planet having no atmosphere, and that planet is going to have an equilibrium temperature. Then ask yourself whether the addition of an atmosphere having no GHGs would increase that equilibrium temperature. Willis’ proof shows that it would not.
Your “slug of energy” explanation has no relation to how heat transfer and equilibrium actually work. Ask yourself where that slug of energy into the atmosphere comes from, and what happens to the body that sends that slug of energy into the atmosphere so it can heat itself to achieve equilibrium with the surface.
What would really happen if you add an atmosphere to an otherwise barren planet is that the surface, already in equilibrium with the incoming radiation from space would rapidly cool since it now can conduct and convect quite a bit of its incoming heat into the atmosphere. But once the surface reaches thermal equilibrium with the atmosphere as you envision it, the surface temperature has to have risen right back to the same temperature it was before the addition of the atmosphere, since its net exchange with the atmosphere is zero, and it is still getting the exact same amount of incoming radiation from the sun.
Willis does not show that the surface will be warmer. He ignores the surface heating the atmosphere through conduction thus cooling the surface below his SB temperature.
Kurt
Conduction and convection can of course occur at the same time as radiation but cannot both involve the same unit of surface energy. Once a unit is radiated away it is gone and cannot also be conducted.
You have to view the planet with an atmosphere from outside the atmosphere for S-B to apply. Viewed from space the planet drops below S-B whilst the atmosphere forms and returns to S-B when the atmosphere is in place but then has a surface above S-B. The excess above S-B is the greenhouse effect (non radiative, mass induced) and is constantly recycled up and down in ongoing convection.
Where else would you place the energy required for ongoing convective overturning?
“Viewed from space the planet drops below S-B whilst the atmosphere forms and returns to S-B when the atmosphere is in place but then has a surface above S-B. The excess above S-B is the greenhouse effect (non radiative, mass induced) and is constantly recycled up and down in ongoing convection.”
I’m trying to make some sense out of this, but I can’t. A temperature is not something that can be recycled, or moved around. Heat can; but if your theory is that there is some amount of stored atmospheric heat in constant transport up and down in the climate system that never gets a chance to be radiated by the surface, then I can just ignore it since it has no effect on the temporal equilibrium temperature of the surface, which receives a certain heat flux (Joules per second per square meter) and has to radiate a flux over that same time interval that sheds the energy it got. Whatever heat exchange you are contemplating between the surface and the atmosphere, in the absence of radiating greenhouse gasses, can only be a zero-sum game in the accounting that matters.
I think of the atmosphere, in the absence of GHGs, as largely just a buffer. It can temporarily let the surface of the earth maintain a lower temperature when the sun is blasting it during daytime by dumping heat into the buffer, and it can let the surface of the earth maintain a higher temperature during nighttime when the buffer dumps heat back to the surface while the surface radiates more to space than it gets from the stars. But the heat exchange in and out of the buffer does not affect the long-term throughput of the system.
Kurt:
You mistakenly assert,
“What would really happen if you add an atmosphere to an otherwise barren planet is that the surface, already in equilibrium with the incoming radiation from space would rapidly cool since it now can conduct and convect quite a bit of its incoming heat into the atmosphere. But once the surface reaches thermal equilibrium with the atmosphere as you envision it, the surface temperature has to have risen right back to the same temperature it was before the addition of the atmosphere, since its net exchange with the atmosphere is zero, and it is still getting the exact same amount of incoming radiation from the sun.”
Sorry, but that displays inadequate understanding. And such misunderstanding is common on all sides of this discussion. Decades ago Hans Jelbring tried to ‘cut through’ the misunderstandings but his attempt seems to have only encouraged people to entrench their views.
I explain as follows.
The “otherwise barren planet” does NOT have a single surface temperature for the same reason that the Moon does not have a single surface temperature; i.e. it obtains solar heating on its day side but not its night side while all its surface radiates energy to space.
If the length of each day on the “otherwise barren planet” equals one of its years then half its surface is solar heated and its other hemisphere is not. Both hemispheres radiate to space but the not-solar- heated region only obtains heat by conduction through the solid material of the planet. Also, the planet obtains most heat in its equatorial region and little heat at its poles. And provision of any difference between the length of its year and the length of its day provides varying solar heating to each region of the planet’s surface.
The S-B temperature of this planet is not a simple average of its surface temperatures. The S-B temperature is the effective temperature the planet would have if it were a grey body radiating an amount of energy equivalent to the solar energy the planet absorbs.
Any alteration to the system alters the greyness of the planet and, thus, the planet’s S-B temperature.
For example, change length of day of the “otherwise barren planet” and its actual surface temperatures all change. But radiative output is proportional to the fourth power of the absolute temperature. A reduction to e.g. its day surface temperature of say x produces a reduction of radiated output of say -y, but an increase to its night surface temperature of x provides an increase to its radiative output of LESS THAN +y (because the day surface has much higher temperature than the night surface and radiation is proportional to the fourth power of the absolute temperature of the radiating surface). This alters radiative equilibrium (i.e. the planet absorbs more heat than it radiates) so the planet’s S-B temperature rises until radiative equilibrium is re-established.
Similarly, add a GHG-free atmosphere and solar heat absorbed by the planet’s hot regions is conducted to the atmosphere then transported to cooler regions by convection where it heats the cooler regions. This, too, alters the planet’s S-B temperature.
Then add GHG’s to the atmosphere and the magnitude of both surface heating and heat distribution is altered some more. This also alters the planet’s S-B temperature.
PLEASE NOTE THAT NONE OF THIS REQUIRES ANY COMPRESSIVE HEATING.
However, Jelbring noticed something interesting.
He observed that the S-B temperature of the Earth and the S-B of each other observable planet with an atmosphere (except Mars that has small and variable temperature) fits an equation which relates the temperature to only the plant’s distance from the Sun and the surface density of its atmosphere.
Jelbring considered this observation to be a remarkable coincidence. So, he published a paper in which proposed that all radiative, conductive and convective effects may adjust in a planetary atmosphere as though they were determined by gravity.
This suggestion could have been expected to be seminal but it was not. This is sad because investigation of possible ways such a coincidence could occur may have revealed much whether or not the Jelbring conjecture is true. But, instead of such investigations, some people claimed that compressive heating could not determine temperature while others claimed that it does, and they argued to defend those positions.
Richard
PS Happy New Year to all.
CORRIGENDUM
Mars has small and variable ATMOSPHERE (not temperature).
Sorry, but my medication does not assist me avoiding mistakes.
Richard
A proper calculation of the mean physical temperature of a spherical body requires an explicit integration of the Stefan-Boltzmann equation over the entire planet surface. This means first taking the 4th root of the absorbed solar flux at every point on the planet and then doing the same thing for the outgoing flux at Top of atmosphere from each of these points that you measured from the solar side and subtract each point flux and then turn each point result into a temperature field and then average the resulting temperature field across the entire globe. This gets around the Holder inequality problem when calculating temperatures from fluxes on a global spherical body.
Alan Tomalty:
Yes. You have restated my explanation but (I think) in a less clear manner and without explanation of why the procedure you suggest is required to determine S-B temperature from radiative fluxes.
Richard
But doesn’t the temperature have various points where it gets warmer as it goes up? If so how does kinetic energy turn to potential energy and then back into kinetic energy etc as it goes up?
It only turns back into kinetic energy when it descends.
Rising air will never heat back up simply due to rising further, unless something else is going on to add energy to it.
You have actual struck another hole in the proposal there is no green house effect, try and explain it 🙂
It is actually funny watching people use classical physics to try and solve this, try looking what happens when you fire a laser into space (search laser transmission thru atmosphere) it will help you a lot 🙂
“A body only radiates according to its temperature if in a vacuum”
That seems unlikely. Bodies do not know or care if they exist in a vacuum. Nothing about S-B requires a vacuum (but DOES require a blackbody; emissivity=1).
Michael 2 January 2, 2019 at 7:56 am
No, it does NOT require a blackbody. Emissivity (epsilon) can be any value because it is included in the S-B equation.
Regards,
w.
Any value between 0 and 1 including 1.
I sit corrected. As commonly used in global warming arguments seems to assume the existence of a blackbody (emissivity=1) and from that starting point emanates many arguments, some of which appear in the comments on this page.
At any rate, I observe no disagreement about the non-requirement of a vacuum. It was the first I’ve encountered that particular argument.
That’s odd because it appears that radiation is described as being at maximum efficiency in a vacuum.
That implies that it somehow becomes less efficient in a non vacuum.
Consider an extreme example of a group of molecules surrounded by solid material. In that case no radiation gets out and all energy transfer is via conduction.
That is germane to the density issue because one would assume that if the density of the surrounding material were to decline then radiation could escape and would start to increase relative to conduction.
It seems clear that matter placed in the path of radiative emissions leads to conduction which attenuates the radiative output. Of course conduction will only occur if the surrounding material is in contact with the source but that is the case for an atmosphere in contact with a surface.
So, the issue is whether non radiative gases in an atmosphere will attenuate the radiation from the surface below via conductive absorption rather than radiative absorption.
Read Ned Nikolov’s 1st paper where sprlled his name backwards.
https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-723
You have to get around Holder’s inequality when evaluating temperatures in a spherical globe.
conduction and convection cannot stop a surface from emitting more radiation if its surface temperature rises.
≠=========
Kinetic energy can be radiated but potential energy cannot. Convection converts kinetic energy to potential energy and back again in sync with the rotation of the earth, which delays the radiation to space.
Fred – show that on a water surface where the increase in energy results in evaporative cooling of the surface as it loses latent heat of evaporation. Do remember that at least 70% of the surface of the Earth is water/transpiring plants.
Kurt December 31, 2018 at 11:05 am
Absolutely. You got it in one.
Stephen Wilde says that the heat is “conducted/convected away” … but “away” in this case means “to the atmosphere”, which makes no sense. You cannot continuously pump heat into the atmosphere. As soon as the atmosphere reaches the temperature of the surface, no more heat will flow into the atmosphere … so perforce it must radiate to space.
But as you say … “this would create a state of disequilibrium”, so it’s not possible.
Thanks,
w.
Willis,
So all your posts on hydrologic cooling of the Earth’s surface by thundershowers are incorrect?
Ian W December 31, 2018 at 1:40 pm
Say what? My thought experiment involves a blackbody planet with no GHGs, which includes water vapor.
Why would this have something to do with thunderstorm cooling the surface?
w.
You invent a virtual world unlike Earth then claim on the basis of that the atmosphere of Earth that is full of latent heat, must behave like your virtual world?
Your dry rock virtual world can be claimed to follow S/B – but a water world cannot. You have oversimplified your argument.
Ian, it’s called a “thought experiment”. Einstein was famous for using them. They are invaluable for understanding things when we cannot do real experiments.
Now, N&Z claim that their theory does NOT require GHGs to raise the temperature of the surface of a planet above the S-B temperature. My thought experiment proves that their claim is not possible. Without GHGs the only way for the planet to lose energy is radiation from the surface … and it cannot radiate more than it is receiving.
Does this apply to earth? Nope, nor was it intended to do so. It applies to the N&Z claim, and it shows that their claim violates the laws of thermodynamics. Q.E.D.
w.
Ian, I support your view. Fynman commented that if your guess does not conform to reality then it is wrong. W uses a non physical model. It is not real. Einstein started with a physically real thing, that the speed of light as measured in many experiments was constant. W is misrepresenting E’s model.
Would not a much more dense atmosphere have greater laten energy than a less dense atmosphere simply because of convection of heat from the surface?
It doesnt really matter whichever theory is correct because temperatures are determined locally via clouds,water vapour and non condensing GHGs. Sure there is some basic reason that raises the non atmosphere moon temperature from 197.3K to the earth temperature of 287.6K , but since in the end we are trying to rid this world of the CO2 scam, it is the effect of CO2 that we need to know.
http://applet-magic.com/cloudblanket.htm
Clouds overwhelm the Downward Infrared Radiation (DWIR) produced by CO2. At night with and without clouds, the temperature difference can be as much as 11C. The amount of warming provided by DWIR from CO2 is negligible but is a real quantity. We give this as the average amount of DWIR due to CO2 and H2O or some other cause of the DWIR. Now we can convert it to a temperature increase and call this Tcdiox.The pyrgeometers assume emission coeff of 1 for CO2. CO2 is NOT a blackbody. Clouds contribute 85% of the DWIR. GHG’s contribute 15%. See the analysis in link. The IR that hits clouds does not get absorbed. Instead it gets reflected. When IR gets absorbed by GHG’s it gets reemitted either on its own or via collisions with N2 and O2. In both cases, the emitted IR is weaker than the absorbed IR. Don’t forget that the IR from reradiated CO2 is emitted in all directions. Therefore a little less than 50% of the absorbed IR by the CO2 gets reemitted downward to the earth surface. Since CO2 is not transitory like clouds or water vapour, it remains well mixed at all times. Therefore since the earth is always giving off IR (probably a maximum at 5 pm everyday), the so called greenhouse effect (not really but the term is always used) is always present and there will always be some backward downward IR from the atmosphere.
When there isn’t clouds, there is still DWIR which causes a slight warming. We have an indication of what this is because of the measured temperature increase of 0.65 from 1950 to 2018. This slight warming is for reasons other than just clouds, therefore it is happening all the time. Therefore in a particular night that has the maximum effect , you have 11 C + Tcdiox. We can put a number to Tcdiox. It may change over the years as CO2 increases in the atmosphere. At the present time with 409 ppm CO2, the global temperature is now 0.65 C higher than it was in 1950, the year when mankind started to put significant amounts of CO2 into the air. So at a maximum Tcdiox = 0.65C. We don’t know the exact cause of Tcdiox whether it is all H2O caused or both H2O and CO2 or the sun or something else but we do know the rate of warming. This analysis will assume that CO2 and H2O are the only possible causes. That assumption will pacify the alarmists because they say there is no other cause worth mentioning. They like to forget about water vapour but in any average local temperature calculation you can’t forget about water vapour unless it is a desert.
A proper calculation of the mean physical temperature of a spherical body requires an explicit integration of the Stefan-Boltzmann equation over the entire planet surface. This means first taking the 4th root of the absorbed solar flux at every point on the planet and then doing the same thing for the outgoing flux at Top of atmosphere from each of these points that you measured from the solar side and subtract each point flux and then turn each point result into a temperature field and then average the resulting temperature field across the entire globe. This gets around the Holder inequality problem when calculating temperatures from fluxes on a global spherical body. However in this analysis we are simply taking averages applied to one local situation because we are not after the exact effect of CO2 but only its maximum effect.
In any case Tcdiox represents the real temperature increase over last 68 years. You have to add Tcdiox to the overall temp difference of 11 to get the maximum temperature difference of clouds, H2O and CO2 . So the maximum effect of any temperature changes caused by clouds, water vapour, or CO2 on a cloudy night is 11.65C. We will ignore methane and any other GHG except water vapour.
So from the above URL link clouds represent 85% of the total temperature effect , so clouds have a maximum temperature effect of .85 * 11.65 C = 9.90 C. That leaves 1.75 C for the water vapour and CO2. CO2 will have relatively more of an effect in deserts than it will in wet areas but still can never go beyond this 1.75 C . Since the desert areas are 33% of 30% (land vs oceans) = 10% of earth’s surface , then the CO2 has a maximum effect of 10% of 1.75 + 90% of Twet. We define Twet as the CO2 temperature effect of over all the world’s oceans and the non desert areas of land. There is an argument for less IR being radiated from the world’s oceans than from land but we will ignore that for the purpose of maximizing the effect of CO2 to keep the alarmists happy for now. So CO2 has a maximum effect of 0.175 C + (.9 * Twet).
So all we have to do is calculate Twet.
Reflected IR from clouds is not weaker. Water vapour is in the air and in clouds. Even without clouds, water vapour is in the air. No one knows the ratio of the amount of water vapour that has now condensed to water/ice in the clouds compared to the total amount of water vapour/H2O in the atmosphere but the ratio can’t be very large. Even though clouds cover on average 60 % of the lower layers of the troposhere, since the troposphere is approximately 8.14 x 10^18 m^3 in volume, the total cloud volume in relation must be small. Certainly not more than 5%. H2O is a GHG. Water vapour outnumbers CO2 by a factor of 50 to 1 assuming 2% water vapour. So of the original 15% contribution by GHG’s of the DWIR, we have .15 x .02 =0.003 or 0.3% to account for CO2. Now we have to apply an adjustment factor to account for the fact that some water vapour at any one time is condensed into the clouds. So add 5% onto the 0.003 and we get 0.00315 or 0.315 % CO2 therefore contributes 0.315 % of the DWIR in non deserts. We will neglect the fact that the IR emitted downward from the CO2 is a little weaker than the IR that is reflected by the clouds. Since, as in the above, a cloudy night can make the temperature 11C warmer than a clear sky night, CO2 or Twet contributes a maximum of 0.00315 * 1.75 C = 0.0055 C.
Therfore Since Twet = 0.0055 C we have in the above equation CO2 max effect = 0.175 C + (.9 * 0.0055 C ) = ~ 0.18 C. As I said before; this will increase as the level of CO2 increases, but we have had 68 years of heavy fossil fuel burning and this is the absolute maximum of the effect of CO2 on global temperature.
So how would any average global temperature increase by 7C or even 2C, if the maximum temperature warming effect of CO2 today from DWIR is only 0.18 C? This means that the effect of clouds = 85%, the effect of water vapour = 13.5 % and the effect of CO2 = 1.5%.
Sure, if we quadruple the CO2 in the air which at the present rate of increase would take 278 years, we would increase the effect of CO2 (if it is a linear effect) to 4 X 0.18C = 0.72 C Whoopedy doo!!!!!!!!!!!!!!!!!!!!!!!!!!
G’ Day Kurt, there is no such thing as a gas that cannot radiate in the IR spectrum. All matter radiates Black Body radiation in accordance with SB’s law. I think you are proposing an atmosphere that does not absorb at any of the BB frequencies that the surface radiates. Cheers Bruiser
The SB equation does not describe gas emissions. They depend upon quantum mechanics and CO2 has multiple ways to absorb photons and emit them. Nitrogen by contrast is almost unable to absorb and radiate heat.
This appears a rather novel assertion. Are you suggesting that if we were able to measure the temperature of individual components of the atmosphere they would have different temperatures, such that, at any given altitude, nitrogen which has a different temperature to that of CO2?
If that were the case, we ought to be able to see great fluctuations in temperature as CO2 levels vary during the course of the day, or where CO2 is being out gased.
If nitrogen cannot be heated (according to you, it cannot absorb heat), how do you explain the following table which shows its conductivity at temperatures between -200 deg C to 1300 degC?
Richard, you’ve got to learn to read between the lines. He means NO2 is unable to absorb and radiate longwave thermal radiation. Nothing to do with conduction or convection.
w.
Well he should have said that then.
Bruiser December 31, 2018 at 1:29 pm
Bruiser, this is widely believed but is not true. All SOLIDS both absorb and emit blackbody (thermal) radiation, but not all gases. That’s why we divide them into greenhouse gases (CH4, CO2, H2O, etc) and non-greenhouse gases.
To absorb the thermal radiation energy, it must be converted into mechanical motion within the molecule. It does this by stretching or flexing or scissoring the atomic bonds that hold the atoms of the molecule together.
But monatomic gases like argon, neon, zenon, and the like do not have atomic bonds, so there is no way for them to either absorb or radiate thermal energy.
Regards,
w.
Willis you add an atmosphere to a planet, that atmosphere is going to absorb heat, the same amount as the planet, the bigger the mass of atmosphere the bigger the heat, so the temperature should stay the same and it does, But not evenly, this is where lapse rate screws everything up.
The t1 t2 is not changed overall but it is changed at the bottom of the atmosphere,
Even Loschmidt or Boltzmann/Maxwell could not agree on lapse rate, I favor Loschmidt.
Why “thermal radiation energy”. It is all just electromagnetic radiation, from near infinite wave length to near infinite frequency, is it not? Other than the particular absorption./emission spectrum of any particular substance there isn’t any difference in the radiation beyond frequency and amplitude, is there?
There is a device whose name I unfortunately can’t recall at the moment. I think it is something different than a plasma torch. Some particular radio frequency EM is transmitted via an appropriately designed antenna into a stream of gas. Electrons in the gas atoms are raised to a higher quantum level. Passing out of the transmission field, the electrons fall back down to their ground state, releasing the energy they absorbed. Extremely high temperatures can be achieved. Does this only work with non-monatomic gases?
AndyHce January 1, 2019 at 1:24 am
Andy, the term “thermal radiation” is a generally used synonym for “longwave radiation”. It specifically refers to the radiation from things at earthlike temperatures.
w.
And my understanding is that even even diatomic molecules like N2 and O2 radiate at much higher temperatures than IR. So in order for any other gasses than GHGs like CO2, water vapor, methane, etc. in the atmosphere to radiate to space, the atmophere’s temperature would first have to rise to temperatures so high that the atmosphere wouldn’t be able to absorb enough energy from the surface to sustain such temperatures.
The satellite temperatures measures are of microwave emitted by O2 and that is mainly at a much lower temperature than the earth’s surface
Stephen, winter temperature inversion falsifies the theory. The atmosphere cools from the bottom up, akso neasured in daily observations during the Koorin expedition
Clarke, R. H.; Brook, R. R. The Koorin Expedition: atmospheric boundary layer data over tropical savanna land. In: Canberra: Department of Science and the Environment; 1979.
Hans,
That is a local short term exception, not a falsification. It is the ground that cools faster than warmth from compression can be supplied from above, indeed the inversion blocks the descent from above. In due course an inversion always dissipates.
Stephen Wilde December 31, 2018 at 10:22 am
Without a link to back up your nonsense that’s just an ad hominem attack. So let’s set that aside and take a look at your current argument. You say:
I don’t understand this. Let me restate the experimental situation. We have a blackbody planet with an atmosphere which does NOT absorb or emit thermal radiation. Argon will do nicely. The surface is evenly heated from all sides by thousands of suns. It gets up the to Stefan-Boltzmann corresponding temperature, where it radiates exactly as much energy as it receives.
In this system, the surface radiation is the only way that the planet can lose heat to space—the atmosphere cannot radiate.
Now IF by some unknown method the surface is heated above the Stefan-Boltzmann temperature, perhaps involving “a conducting and convecting atmosphere which is held off the surface in hydrostatic equilibrium since that excess is being constantly recycled in and out of potential energy”, or perhaps by some other method, then again by Stefan-Boltzmann, the surface perforce will be radiating more energy. It has to radiate more because it is warmer and there is no place else for the excess heat to go.
But if that is the case, then the surface is radiating more energy than it is receiving … which is a violation of the Laws of Thermodynamic.
As a result, Stephen, it doesn’t matter what your explanation is of how the surface might be warmed. If the atmosphere doesn’t contain greenhouse gases, then if the surface is warmed by whatever atmospheric or pressure-based means, it will have to radiate more energy than it is receiving and that can’t happen.
Best of the New Year to you,
w.
PS—a comment to you from my original post containing my proof, from Dr. Robert Brown, a brilliant guy who actually teaches this stuff at the college level:
Robert Brown January 15, 2012 at 8:51 am
What he said …
A body heated on all sides by thousands of suns will heat up until it reaches the temperature of those thousands is suns.
EdB December 31, 2018 at 11:57 am
Nope. Depends on how far away the suns are … for example, every star in the sky is a sun, so the earth is heated by thousands of suns …
w.
Nope. The universe is dominated by mass that is not a burning sun. Your model is not correct. Try considering molecule surrounded by hot molecules.
Sigh
Willis, now repeat your experiment with a water word like Earth with evaporative cooling of the type you also write about with a powerful hydrologic cycle.
Ian W December 31, 2018 at 1:17 pm
Sorry, but my experiment specified no greenhouse gases, so I can’t “repeat” it with GHGs.
w.
I disagree with add Spencer’s assertion that W has refuted NZ. Using a non real model of a body surrounded by suns is not a refutation.
So Willis your ‘experiment’ is not valid for Earth and is therefore not applicable to Earth’s climate. As such claiming that another experiment that was intended for Earth is invalid due to your experiment on a virtual world unlike Earth seems to be stretching a point – would you agree?
Ian and EdB, it’s called a “thought experiment”. Einstein was famous for using them. They are invaluable for understanding things when we cannot do real experiments.
Now, N&Z claim that their theory does NOT require GHGs to raise the temperature of the surface of a planet above the S-B temperature. My thought experiment proves that their claim is not possible. Without GHGs the only way for the planet to lose energy is radiation from the surface … and it cannot radiate more than it is receiving.
Does this apply to earth? Nope. It applies to the N&Z claim, and it shows that their claim violates the laws of thermodynamics.
w.
I can’t figure out if Mr. Eschenbach’s proof is too simple or too complicated for me to understand.
Start with the simplest experiment to measure radiation. A heated iron sphere is suspended (by a perfectly insulated material) within a glass vacuum cylinder located in a room (of infinite size) with an air temperature of 70 degrees. We all agree that the sphere will radiate energy until the temperature of the sphere comes into equilibrium with the temperature of the room. 100% of the energy transfer from the sphere would be due to radiation.
Now consider a second experiment. Same situation, except the cylinder is now filled with nitrogen. IMO, the sphere will now reach equilibrium with the surrounding room temperature more rapidly – the sphere can now transfer energy via radiation, conduction, and convection. The “atmosphere” around the sphere does not act as an insulator. In this situation, I believe that Mr. Eschenbach’s proof would hold true.
However, the second experiment does not match the situation of a hypothetical earth with no greenhouse gases. Everyone keeps talking about the lapse rate in the troposphere. However, no one is discussing the incredibly hot temperatures in the thermosphere (up to 3,600 F). This area is directly heated by the sun due to the absorption of UV, visible light, and high energy gamma rays.
I believe it is possible to create a set of conditions in my “room” experiment wherein the temperature of the iron sphere matches the actual observed temperature of the earth – with the surrounding room being set at the temperature of interstellar space. One experiment would use CO2 in the cylinder surrounding the sphere and have a hot thermosphere separating the glass cylinder from “space”. The thermosphere/atmosphere system would certainly retard the heat loss from the sphere into space.
However, we would also set up a second experiment using N2 in the cylinder. I believe this system would also retard the heat loss from the sphere into space – without the use of any greenhouse gas in the cylinder.
I believe the heat and density of the thermosphere in the second experiment (for the same equilibrium sphere temperature) would have to be different than the conditions of the first experiment. However, I do not believe greenhouse gasses are required for the actual earth temperature to exceed the calculated blackbody temperature. IF TRUE, THIS DOES NOT DISPROVE THE GREENHOUSE GASSES THEORY. HOWEVER, IF TRUE, THE FACT THE EARTH’S TEMPERATURE EXCEEDS THE BLACKBODY IS LIKEWISE NOT PROOF OF A GREENHOUSE EFFECT.
[I am certainly not a physicist, and the hypothetical experiment is simplified. However, I don’t understand the requirement for greenhouse gasses in the much more complicated real-world situation.]
Pillage
You said, “However, no one is discussing the incredibly hot temperatures in the thermosphere (up to 3,600 F).” The temperature of the gas molecules (will determine the distribution, with respect to wavelength, of energy radiated. However, what I think that you are missing is that the total energy radiated is directly related to the number of molecules radiating per unit volume, which is small for that rarefied region of the atmosphere. So, yes, there is radiation coming from the thermosphere, but the quantity is small.
I agree that the amount of energy is quite small.
I do not understand how this layer, plus a pure nitrogen atmosphere, would not raise the temperature of the earth above the theoretical blackbody earth temperature.
Is the answer that the measured temperature increase would be infinitesimal?
“However, I do not believe greenhouse gasses are required for the actual earth temperature to exceed the calculated blackbody temperature.”
So what else? Here is a simple version of Willis’ energy balance:
1. The surface receives about 240 W/m2 solar, on average. Solar is the only overall energy source here (geothermal is tiny). The mass of the atmosphere is not a sustainable energy source.
2. The surface, from S-B and its temperature, emits about 396 W/m2. With no GHG’s to obstruct it, that heat would be lost to the Earth. And then, how can the surface temperature be maintained, with a nett loss of 156 W/m2 and no energy source of its own?
The answer of course is that some of the outgoing radiation is intercepted by GHG and returned, mostly as down IR, restoring the balance. What else?
Why are you ignoring conduction and convection which leads to winds, thus completely changing the temperature distribution, starting with a cooler equator?
“Why are you ignoring conduction and convection . . . ?”
Because conduction and convection cannot transport heat into space.
Conduction and convection can only store heat from the surface temporarily when the surface is warmer than the atmosphere, and return it when the surface is cooler than the atmosphere. It’s like a buffer in a computer system that stores data, but is not the source of it. Since the atmosphere cannot create heat and provide it to the surface on a sustained basis, the only thing that can heat the surface from its black body equilibrium temperature is having an atmosphere that captures heat that the surface would otherwise have emitted directly to space and radiate a portion of that back to the surface.
The new year celebrations at work, Nick.
On reflection you might like to correct your post.
The surface receives 240 solar and 156 IR back radiation which is just recycling the 240 W/m2. It is not new energy.
The 240 coming in is the 240 going out, there is no extra 156 of extra energy.
The GHG and their accompanying atmospheric gases do heat up purely to get the energy to the effective emission level to our 240 back into space from the higher effective level.
There is no balance to restore, the earth never could lose 156 extra energy, it never had it to lose in the first place.
The new year celebrations at work, Nick.
On reflection you might like to correct your post.
The surface receives 240 solar and 156 IR back radiation which is just recycling the 240 W/m2. It is not new energy.
The 240 coming in is the 240 going out, there is no extra 156 of extra energy.
The GHG and their accompanying atmospheric gases do heat up purely to get the energy to the effective emission level to our 240 back into space from the higher effective level.
There is no balance to restore, the earth never could lose 156 extra energy, it never had it to lose in the first place.
1)The surface doesnt get 240 W/m^2.
77W/m^2 is absorbed by the atmosphere before it has a chance to get to the surface.
2) Nick since you are combining the energy balance numbers from a real earth and transposing them to a fictititious earth, your attempt at balancing doesnt make sense.
However let us stick to the real earth AND Before figuring out the DWIR, let us calculate the absorptions and emissions 1st. Since only 163 gets to the surface , 114 of that hits the oceans (70%)and 49 hits the land(30%).
Of the 49 that hits the land, 10% of 86.4 (total evapotranspiration) =(transpiration) = 8.64
is emitted back to atmosphere. that leaves ~40.4 left. Another emission is 18.4 conduction that leaves 22 left to be emitted to atmosphere from land.
From the oceans we have the rest of the evaporation which is 86.4-8.64 = ~ 77.7 So subtract that from the oceans that leaves you 36.3. Add this to the amount left to be emitted from land 36.3 + 22 = 58.3 BUT satellite measurements show a window of 66W/m^2 from surface direct to space NOT 40 as Trenberth originally wrote. To quote Cementafriend “Prof Trenberth wrote us that he knows this. But but he kept his 40 disregarding measurements”
If it really is 66 then there would already be a minus 8 W/m^2 to be emitted from surface. SOMETHING DOES NOT COMPUTE. We don’t have any IR left for the DWIR.
Alan Tomalty January 2, 2019 at 11:17 pm
Sorry, but that makes no sense, and it indicates that you truly don’t understand what you are talking about. Both the land and the ocean receive the same amount of 163 W/m2. It is a PER SQUARE METRE amount, whether the square metre is land or sea.
w.
“Same situation, except the cylinder is now filled with nitrogen. IMO, the sphere will now reach equilibrium with the surrounding room temperature more rapidly – the sphere can now transfer energy via radiation, conduction, and convection.”
If I understand your experiment correctly, it doesn’t quite fit what happens with the Earth. Your sphere starts with a fixed amount of heat in it that slowly dissipates into surrounding air, first by radiating to a glass cylinder that then conducts and/or convects to surrounding air. You modify that to fill the vacuum with a radiatively non-absorbing gas and say that the fixed amount of heat in the sphere can now be transmitted to the glass more effectively via the gas, which then conducts/convects to the surrounding air at the surface of the glass.
But the Earth is a system where the surface is surrounded by air, which is in turn surrounded by a vacuum. The vaccum of space isn’t surrounded by anything and can’t be filled by gas. Gas can surround the earth, but can’t act as a conductive conduit to something outside the Earth. And rather than just having an initial charge of heat to dissipate, the Earth (your cylinder) is constantly heated by an external source – the Sun. So in the real world, the atmosphere won’t help the surface of the Earth conduct or convect to something outside the boundary of the atmosphere, as is the case in your example. And since the surface of the Earth is constantly receiving an energy flux from the sun, convection and conduction can’t do anything to affect the equilibrium temperature that the Earth has to maintain against its surrounding vacuum.
“convection and conduction can’t do anything to affect the equilibrium temperature that the Earth has to maintain against its surrounding vacuum.”
They do create a potential energy store that constantly returns to the surface as heat over half the surface and which then travels to the base of the next region of uplift where the extra heat has to be added to continuing solar input which raises surface temperature above S-B
Pillage,
Three points. First, don’t forget that the Earth – and its atmosphere – is in a vacuum. In the overall scheme, only radiative energy can leave the system, there’s no air out there to convect into. Second, don’t confuse temperature with heat energy, which is temperature multiplied by thermal mass. The thermosphere is hot, but not dense; high temperature with little total energy.
Finally, note that with the addition of an atmosphere, the radiative surface of the iron sphere isn’t the surface of the iron any more, it’s the surface of the gas, subject to the transmission characteristics of that gas. The entire system will be at an equilibrium temperature where the total radiated power is equal to that received; anything below the radiative surface can be at a higher temperature (or perhaps lower, if the gas has a low enough emissivity).
Finally I see someone talk about the emissivity of the atmosphere. As an engineer I know the emissivity is proportional to the partial pressures of the SO2, CO2 and H2O. So the higher the atmospheric pressure the more the “green house” gases will absorb and reradiate. This is how pressure comes in to the equation.
And the more conduction will compete with radiation.
Willis’ proof makes no sense.
If you have an argon atmosphere surrounded by thousands of suns, then the planet will radiate what it radiates, AND the near-surface temperature of the atmosphere will be significantly warmer than the atmosphere 10 km up. It would have to be, as there are more molecules of argon nearer the surface which have conducted with the very hot surface, and far fewer argon molecules 10 km up which are not conducting with the very hot surface. The molecules MAY contain the same kinetic energy above as below, but we measure atmospheric temperature not by individual molecules but by the average kinetic energy of a volume of gas; hence, all else equal, the temperature of a thinner atmosphere MUST be less than that of a thicker atmosphere. Such a situation violates no physical laws. We can’t make any assumptions about what “target” temperature that planet’s surface will be, but we do know that whatever it is, no physical laws are violated.
If you add GHGs the planet will still radiate what it radiates and violate no laws of physics, and the near-surface atmosphere will still be warmer than the atmosphere 10 km up.
Don132
“we measure atmospheric temperature not by individual molecules but by the average kinetic energy of a volume of gas”
That statement may have a latent ambiguity, but it seems at odds with the ideal gas law:
PV=NkT -> T=(P/N)(V/k): The temperature of a given volume of an ideal gas is proportional in accordance with the reciprocal of Boltzmann’s constant to the ratio that the volume’s pressure bears to the number of molecules that occupy that volume.
So temperature is a measure of the kinetic energy per molecule per degree of freedom (where kinetic energy is computed in accordance with the molecular velocities with respect to gas’s center of mass).
“hence, all else equal, the temperature of a thinner atmosphere MUST be less than that of a thicker atmosphere.”
But what’s usually not equal in a thinner atmosphere is the pressure, and if the pressure were lower in proportion to the molecular concentration–i.e., if P/N were the same at 10 km as at the surface–then the temperature would be the same even though the number of molecules is much less.
Joe Born,
Does not less pressure lead to a thinner atmosphere?
” Clearly, temperature has to do with the kinetic energy of the molecules, and if the molecules act like independent point masses, then we could define temperature in terms of the average translational kinetic energy of the molecules, the so-called ‘kinetic temperature’.
“It is important to note that the average kinetic energy used here is limited to the translational kinetic energy of the molecules. That is, they are treated as point masses and no account is made of internal degrees of freedom such as molecular rotation and vibration. This distinction becomes quite important when you deal with subjects like the specific heats of gases.”
When we speak of gas temperature we speak of the average translational energy of the molecules within a volume of gas.
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
and
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
Don132
Stephen, I totally agree with you, and that has been my belief for over 2 decades. Of course, before I retired, I was an engineer in lasers, instrumentation, and weather instruments. I learned the thermal laws and the gas laws, and understand them fully.
But I do have one comment to add. We all know that the sun heats the surface, and the air at the surface every day. We also know that heated air rises through convection. What seems to be ignored is what happens to cause the atmosphere to cool back down at night. We KNOW it gets hot from the sun, but we don’t realize how the cooling takes effect. Hint: It is from the surface UP. The surface radiates, which cools it (ever stick your feet into sand at night?) and the air in contact with that cooled surface is cooled to the temperature of the surface. But that air does not rise – it pools as a cool layer and that layer increases in thickness during the night. Balloon measurements confirm this. Thermometers are at 2 meters to prevent measuring this cooled air at the surface. Simply stated, the atmosphere is heated mostly by the heated surface when sun is present, and it cools mostly by conduction at night. It takes LONGER to cool than to heat, which is why it is coolest JUST before the sun begins warming again. (All comments are without wind.) The surface radiates 24×7, the atmosphere near the surface is warmed only when the sun is present, and cools when it is not. The whole atmosphere is a reservoir of heat that is constantly applied to the earth as it rotates. It does not take downwelling radiation to explain any of this. Oh, and since gh gases can radiate, they also help cool the atmosphere at night, but not by much.
Well described imo. Wind, water, oceans, ice, clouds, etc complicate it all of course, and makes it impossible to compute.
And this is shown by the layers of fog in valleys.
Excellent common-sense explanation, John.
Don132
Infra red rad takes care of the cooling. The atmosphere without adsorptive- re-emittive gases are transparent to infra red going out (and shortwave coming in) If you assume an ocean, yeah huge convection because the much lighter water molecules evaporating from the water surface plus being heated transport huge volumes of heat upwards and “make” weather. With just a cold rock and, say, nitrogen “weather” isn’t much in evidence.
Posted at Dr. Spencer’s blog:
“… radiation from the atmosphere does indeed warm the surface…”
Can I rephrase that? How about:
“…radiation from the atmosphere does indeed result in warming of the surface…”
Words mean things. The sun does the warming, the greenhouse gas interferes with the cooling. The result is warming.
Dr. Spencer agreed that was correct.
What I didn’t post over there was the notion expressed in the first quote above that back radiation warms the earth. It’s semantics. It’s easy to say CO2 warms the earth – rolls off the tongue better than slows the cooling or any other more accurate phrase does. But when you say, “Radiation from the atmosphere warms the surface.” you’re going to get jumped on by a lot of people, and for darn good reason. Because it doesn’t, it can’t – a brick of dry ice, which is pretty close to a 15µ radiating black body, isn’t going to warm anything that’s in the temperate range. It’s just that the background of space at essentially absolute zero will facilitate more rapid cooling.
will facilitate more rapid cooling.
====≠==
One could argue the same for conduction. Instead if radiating energy to space, the surface energy can be conducted to the atmosphere such as when the sun comes up, and then this energy could be conducted from the atmosphere back to the surface 12 hours later when the sum goes down.
However in this case the cooling would be delayed for hours rather than fractions of a millisecond as for radiation, which would appear to make conduction even more powerful than radiation at creating a greenhouse effect.
I agree with your model. I might add that until someone can write code to describe the interaction of clouds, convection, winds, conduction, evaporation, etc., no one will be able to tell which cooling mechanism dominates each minute of each day, to average out to what we know as the climate. It’s a fools errand. We need to take a pill and just wait and see how much if any our added CO2 causes. I still vote for very little, as I doubt we will be able to measure it for certain.
This is certainly a point of common ground between us, though certainly not with the alarmists. If ECS is <1.5K as the evidence implies, it doesn’t practically speaking matter if it is your zero or my 1.3. It is either of no consequence to global surface temperature or slightly beneficial. (Given the practical limits on how much more CO2 we could potentially emit, and how that limits the upper bound on CO2 concentration and thus warming if ECS is ~1.3-1.5).
Either way, the higher CO2 concentration is beneficial to agriculture and plant life in general. The continued use of fossil fuels is the only realistic way that the poor countries will raise themselves from poverty. Even if we are selfishly only interested in the impact that using or eschewing fossil fuels would have on our own standard of living, we must be concerned about that.
Apart from a pure love of knowledge which I don’t denigrate at all, there isn’t a practical aspect to this argument we’re having. Or maybe there is after all. Because in a democracy, it matters less what is true and more what is believed to be true. If the voting public decide that the alarmist view must be right because they mistakenly associate the skeptical view with an unscientific hypothesis that is easily discredited, then the alarmists carry the day and our standard of living crashes.
FACT: Air is an insulator.
Now please explain to me [or provide a link]: How much of the “Green House Effect” is caused simply by the atmosphere surrounding the planet?
Usurbrain – 10:42 am
“Sometimes the first duty of intelligent men is the restatement of the obvious” – George Orwell.
That is wrong. It’s just not a good conductor. It still is a conductor.
all insulation is a conductor (in fact I believe ALL matter is a conductor of heat, some better than others) … “not a good conductor” is what makes it good as insulation …
Heat is transferred by Brownian random walk
 er.com/1F2r.mp4
er.com/1F2r.mp4
Usurbrain
Non-convecting air is a poor conductor. However, it does not impede radiation unless there are gas molecules that absorb at the wavelength(s) of radiation.
“However, it does not impede radiation ” That is not logical. The atmosphere on earth is NOT invisible to radiation. Therefore it does “impede” radiation.
For heat transfer from a pipe in a heat exchanger or a fuel rod in a nuclear reactor to work properly the pipe must be in contact to the water. When a film of gas or even a significant number of microscopic bubbles of gas form on the pipe the temperature of the pipe can get hot enough to damage the pipe in the area where these bubbles or film develops. This can happen in a heat exchanger in a low flow area or from an eddy in the pipe from an elbow, flaw, etc. Usually, when bubbles of steam develop the microscopic bubbles can help transfer heat, however after there is a departure from nucleate boiling, the bubbles form a film of steam vapor and can cause the pipes to melt. This film is measured in fractions of inches/millimeters. When there is a departure from nucleate boiling with nuclear fuel rods they can melt.
If a microscopic film of water vapor can act as an insulator, then several miles of air will act as ian insulator.
Usurbrain,
You took half a sentence and said it is not logical, when what makes it true is the part of the sentence you cut off!
Come on!
WTF is going on here with this discussion?
If the GHG absorb/reflect the radiation they impede it. PERIOD. What logic negates that? How else would there be a lapse rate?
“However, it does not impede radiation unless there are gas molecules that absorb at the wavelength(s) of radiation.”
This is what Clyde wrote.
You then took this part:
“However, it does not impede radiation”
And said it is not logical.
Taking half a sentence and arguing against it…?
The “unless” part is what makes the first part true.
“unless there are gas molecules that absorb at the wavelength(s) of radiation.” You have STILL not convinced me that the gas that does NOT absorb does not impede.
When you travel through a crowd you do not get absorbed and you do get impeded. When a neutron passes through material it can take longer to get through the material without being absorbed. Same with photons – as shown by a prism, other electromagnetic particles also “bounce around”.
Yes, the speed of light through a gas is slower than the speed of light through a vacuum.
But it just gets where it is going a little slower.
Radiation outbound from the surface will be in space in a matter of a tiny fraction of a second.
Going slower will not make enough difference to matter for the purposes of this discussion.
Your disagreement did not seem to be one of semantics, but maybe I was wrong.
Usurbrain
You are confusing conduction in a heat exchanger with radiative transfer in the atmosphere or a vacuum.
Only used that discussion to show that gas is an insulator. Which you agreed with.
Repeating – If a microscopic film of water vapor can act as an insulator, then several miles of air will act as an insulator.
Userbrain
Both “insulator” and “conductor” are imprecise terms with approximately opposite meanings. To be more precise, one should quote the quantitative properties, because the ‘insulating’ properties of a given thickness of material are the same as that for a substance of twice the thickness with twice the conductivity. Everything except an absolute vacuum will have some conductivity. The decision to call something an insulator or conductor depends on whether the conductivity is high or low, and to some extent on the application.
Perhaps this report will fill some gaps in your knowledge.
http://www.sfu.ca/phys/346/121/lecture_notes/lecture33_heat_loss.pdf
Pay special attention to the section on “Windows: Convective/conductive layer.” This also happens to the Earth, the reason that the weather instruments are at four to five feet above ground. And the reason the ground stays warmer longer than the air above four feet.
A Stagnant layer prevents and impeded conduction, the air itself still has some insolation. That are an awful lot of books are wrong.
I don’t believe compression or GHG explain why a planet with an atmosphere is warmer than one without.
What explains the SURFACE warming is the lapse rate. This warms the surface while cooling the upper atmosphere, while the average temperature remains unchanged, matching the S-B prediction.
Thus, if you can explain the cause of the lapse rate you can explain why the surface is warmer than predicted by S-B.
I do think it is important to note that the equation for the lapse rate is not dependent on the greenhouse effect.
Rather the lapse rate would appear to be a result of the work performed by the vertical circulation of the atmosphere driven by solar radiation, in a gravity well.
It is thus likely the work performed by the vertical circulation that transfers energy from the upper atmosphere to the lower atmosphere, creating a lapse rate, heating the surface, and cooling the upper atmosphere.
Otherwise the atmosphere would be isothermal. The same temperature at the surface as at altitude.
Ferd,
The lapse rate slope follows the decline in density with height and so is a product of increasing compression as one descends through an atmosphere.
Moving air up or down moves it along that lapse rate slope so the degree of compression changes and so does the temperature as per the gas laws.
The greenhouse effect is a product of compression and not of radiation.
Stephen, likely the GHG effect and the lapse rate are two different names for the same thing, because they both predict the surface to be 32C warmer than the average temperature of the atmosphere.
And equally the GHG effect would occur even without any CO2 because conduction and convection in an atmosphere are no less efficient than radiation at transferring energy.
Conduction and convection are simply slower than radiation, but since the GHG effect is due to slowing the cooling, conduction and convection are if anything better suited than radiation at creating a GHG effect.
The greenhouse effect is a combined cause of radiation absorption in the atmosphere and the lapse rat. Nothing else. The lapse rate exists even with no absorbing gas, but is a gradient, not a level, and the surface cannot warm (on average) unless there is radiation absorption. Willis hit the nail on the head about conservation of energy prohibiting heating above the direct balance with the surface unless there is radiation absorbing gas (or aerosols)..
No, Willis used a non physical body surrounded by suns.
Until that model is binned…. nothing proven.
Leonard has it right. The lapse rate will exist even without any GHGs. However, without them you cannot raise the temperature of the planet’s surface. Hence, the surface temperature would be the SB value based on the energy received.
Adding GHGs allows more energy to be moved into the atmosphere but more importantly provides the ability to raise the height of the radiating temperature above the surface. This allows the lapse rate to provide additional warming of the surface.
You’ve got to have GHGs to do the radiating.
Where I disagree with the current theory is that I think a given lapse rate will always have a maximum radiating temperature. Since the lapse rate is a function of the mass of the atmosphere and the gravitational field, it will be a related to the density/pressure of that atmosphere. I think this is why Z-N found the relationship they found.
Every planet they examined has GHGs. Hence, they all will allow energy to be moved from the surface. This will allow the elevation of the radiating temperature due to the lapse rate.
Basically, the mass of the atmosphere plus the mass of the planet define a unique lapse rate. Given almost any amount of GHGs the height of the radiating temperature will then depend uniquely on the amount of energy received. Adding more GHGs will have no effect.
If the greenhouse effect were a result of radiative effects (not denying that these exist, just denying that they are as important as everyone believes) then why are there no terms for radiative effects in lapse rate equations? But there are most certainly terms for pressure in lapse rate equations.
Don132
Fredberple. Rather “equation for the lapse rate IS dependent on the greenhouse effect – by a small factor“. See the quantitative thermodynamic development of standard atmospheric lapse rate by RH Essenhigh using S-S integral equation method:
Essenhigh RH. Prediction of the standard atmosphere profiles of temperature, pressure, and density with height for the lower atmosphere by solution of the (S− S) integral equations of transfer and evaluation of the potential for profile perturbation by combustion emissions. Energy & fuels. 2006 May 17;20(3):1057-67.
Abstract
The greenhouse effect enters as the small “value of the group-pair (kp)o representing the ground-level value of (kp), the product of the effective absorption coefficient and concentration of the mixed gases, written as a single parameter but decomposable into constituent gases and/or gas bands.”
Essenhigh’s development was extended above the troposphere by:
Kolan, S., 2009. Study of energy balance between lower and upper atmosphere (Doctoral dissertation, The Ohio State University).
Marcus says that he thought that gases got colder when compressed. I thought that it was basic school physics that gases heated up when compressed. There is a type of engine that was invented by a guy named Diesel that relies on air becoming hot when compressed to work. My car is equipped with this type of engine and seeing as it always gets me where I want to go, I suspect that basic school physics got it right.
“The SB equation always results in a surface temperature that is too cold compared to surface temperatures when an atmosphere is present, and greenhouse theory is traditionally invoked to explain the difference.”
Traditionally invoked? If we invoke it then it must exist?
How about if we prove the existence of the ‘greenhouse theory’ rather than just invoke it to explain away a problem. If this ‘greenhouse’ property exists, then it must manifest itself on Mars (95% CO2 atmosphere), even if close to negligible. Why haven’t we measured it?
Isn’t it interesting that the Stefan-Boltzmann equation is used to determine that the ‘greenhouse theory’ exists which has no equations itself. We’re using a deterministic theory to prove the existence of a non-deterministic theory.
Measuring the ‘greenhouse theory’ would go a long way to proving its existence.
Do Celestial Spheres exist because they were introduced to support a scientific theory? The Geo-centric theory could not explain planetary orbits so Celestial Spheres were ‘invoked’ to explain away the issue. Therefore they exist, right?
The claim is that the Earth is 33C above the temperature derived from the Stefan-Boltzmann equation.
What value does the Stefan-Boltzmann equation give for Mars? Is there ‘excess’ heat on Mars as well?
The “excess heat” due to the greenhouse effect on Mars is manifested by a surface temperature excess of about 5K. The greenhouse effect on Mars , despite its 95% CO2 atmosphere composition is affected by the much lower pressure, the lower solar input and the effect of dust storms which have a pronounced anti – greenhouse effect .
The following discussion aid for science teachers contains Trenberth type heat budget diagrams for both Earth and Mars and you can compare the downward longwave radiation for both planets :
https://www.scienceinschool.org/content/planetary-energy-budgets
mikewaite: [ The “excess heat” due to the greenhouse effect on Mars is manifested by a surface temperature excess of about 5K. ]
Would you show your work on how you arrived at “about 5K”? I’d like to see the equation, what science did you apply? What assumptions are used to derive your answer? “About 5K” is not exact but rather implies this value falls within some range, is that range as large as +-5K? How are you setting your boundaries?
>>
What value does the Stefan-Boltzmann equation give for Mars? Is there ‘excess’ heat on Mars as well?
<<
The equations and procedure are not very complicated. You can solve it for your self.
Jim
Jim Masterson – “The equations and procedure are not very complicated. You can solve it for your self.”
Thanks. I’m not asking if the equations and procedures are complicated or simplistic, I’m asking you to reproduce them here and now. What assumptions are required before applying those simplistic equations and procedures?
First we need the TSI. The formula for TSI is a modified version of the Stephen-Boltzmann law:
where σ is the Stephen-Boltzmann constant, Ts is the Sun’s surface temperature, Rs is the radius of the Sun, and Rp is the planetary distance. If sigma = 5.670373e-8 W/(m^2 K^4); Ts = 5,778K; Rs = 6.96392e5 Km; and Rp = 1 Astronomical Unit (AU) or 1.496e8 Km; we get: 1,369 W/m^2–which is in the ballpark.
To obtain the surface temperature of the Earth, we use the Stephen-Boltzmann law solved for temperature, or:
But our computed TSI is too high. The logic goes like this: the Earh is a sphere, it captures the solar radiation in a circular area, and because it rotates, it spreads this radiation “evenly” over its surface. We can compute this as follows:
And we also have to take into consideration the planetary albedo. The Earth’s albedo is about 0.30, or it reflects about 30% of the incoming radiation back to space. So now we can solve for temperature:
If we do the same thing for Mars, we get about 590 W/m^2 for the TSI at Mars (since we were using AU for Rp, the Martian AU is 1.523679–we increase Rp by that factor). The Martian albedo is about 0.25. Solving for the Martian surface temperature, we get 210K. Considering that the average surface temperature for Mars is from 208k to 210K, there’s not much “excess” to play with. Of course, you can “play” with the numbers as you like.
Jim
Thanks, Jim, clearly stated.
I note that you correctly say:
In that regard, note that Ned Nikolov simply made up a Martian temperature of 180K so it would fit with his bogus “Miracle Equation” … oooh, very, very bad boy …
I note that Stephen Wilde and his other defenders here won’t touch that fact with a 10 metre pole …
w.
Thanks Willis for your kind comments.
Jim
Jim Masterson – Thanks for the response, that’s excellent.
[But our computed TSI is too high. The logic goes like this: the Earth is a sphere, it captures the solar radiation in a circular area, and because it rotates, it spreads this radiation “evenly” over its surface.]
I don’t believe the sun’s radiation is ‘spread evenly over the Earth’s surface’, do you? That’s a bad assumption that directly impacts the result.
[The Earth’s albedo is about 0.30]
“About 0.30” is still the value used? Climate science has had a tremendous amount of time and computer processing power and there has been no improvement on this value which directly impacts the result?
Are your derived values, based entirely upon these bad assumptions, the only evidence that the ‘greenhouse gas’ property exists? That is truly pathetic!
>>
Thanks for the response, that’s excellent.
<<
Thanks, I appreciate compliments.
>>
I don’t believe the sun’s radiation is ‘spread evenly over the Earth’s surface’, do you? That’s a bad assumption that directly impacts the result.
<<
You didn’t ask me initially what I thought–you wanted to know how climatologists possibly make the calculations. This calculation ranks as a back-of-the-envelope type; because the equations fit on the back of an envelope, and the answer is available in a few minutes. If you really want an more “correct” answer it will take a lot of work. From first principles you’ll need trigonometry, differential and integral calculus, and lots of analytical geometry. The result will probably be an unwieldy, non-linear equation that needs to be integrated (to compute the average) and has no analytical integral. That means it will have to be integrated using limit theory and (horrors) a computer. But by all means, you go ahead–I’m content with the equations and assumptions I used.
However, let’s say the 255K answer has an error of 10%. (I don’t think it’s anywhere that big.) Ten percent is 26K or ±26K. It then ranges from 229K to 281K. You still can’t get rid of that last 7K; and it could be as bad as 59K.
>>
“About 0.30” is still the value used?
<<
Roughly. I think it’s decreasing slightly.
>>
Are your derived values, based entirely upon these bad assumptions, the only evidence that the ‘greenhouse gas’ property exists? That is truly pathetic!
<<
It’s only pathetic, if you’re trying to get rid of the GHE. I think one exists. Ever spent time in a desert? A tropical forest? I have. That water vapor makes a big difference in temperature ranges.
Jim
..I’ve been waiting for this discussion all year. Good timing for it. I really want to know, is it 10% possible, 40%, 60% ? Way beyond my “pay grade” to understand BUT, I do understand that the possibility cannot be 0%…IMHO
“The same parcel of energy in the form of surface heat cannot be in two places at once so energy that is being constantly recycled between KE and PE within convective overturning is simply not available for radiation to space.”
Eh?
Steven a parcel of air has a temperature …. and therefore radiates. To space.
“The problem with an upward facing IR thermometer is simply that it will measure a temperature at the height where atmospheric density triggers the sensor. ”
Steve, an IR thermo is measuring LWIR photons that have entered thermo HAVING downwelled from the atmosphere. It isn’t measuring a height. Those photons have come from many heights tho chiefly from below the level of effective ratitive cooling (-18C).
As Clyde said (beat me to it) – the “bike tyre” is sufficient debunking of the “theory”.
In order for atmospheric density to be the cause of the excess 33C above S-B then the compressional heating contributed by the atmosphere due to it being at an average 1013mb at the surface MUST be kept up continually. By that I mean constantly be compressed and decompressed (which is zero-sum anyway)
Otherwise it is a violation of the 1st LoT.
Perpetual free energy!
Hypothetically, were we able to take a model airless Earth and suddenly introduce our atmosphere under gravity, then yes there would be compressional heating BUT it will then cool to space.
This is where the fallacy falls down.
It is not the fact that the atmosphere is in a permanently compressed state that gives the compressional heating.
It is the ACT of compressing it.
An action – the reaction – conservation of energy.
Then THAT’S it.
Your bike tyre doesn’t stay perpetually hot because it’s at 80 Bar!
It heats on the act of getting compressed to 80 bar.
Then it cools, because the heating action has stopped.
The answer to our energy problems if it did.
Incidentally gravity works on the atmosphere to create the LR via the relation -g/Cp.
Which is then further modified by the GHE and LH release aloft.
AB
We are actually in agreement for a change! 🙂
Same for Compressed Air bottles used for divers and firemen. I helped refill the bottles when I was a volunteer fireman. We placed them in a farm water trough/tub of water to help cool the tanks as we filled them. They still got rather warm to the touch. Next day they were all at room temperature.
Although I’ve long since lost patience with Nikolov and Zeller, I remain receptive to a theory, which Luboš Motl discussed at https://motls.blogspot.com/2010/05/hyperventilating-on-venus.html, that can easily be confused with theirs.
The theory is that beyond a certain point a planet’s surface temperature depends more on its atmosphere’s thickness than on its greenhouse-gas content.
Perhaps that theory is wrong, but I haven’t seen a compelling refutation.
Patience is a card game, nothing scientific.
Yup, exactly what I have been saying for many years too. I suspect the profile of an atmosphere in a gravitational field will define a lapse rate AND an average radiating altitude independent of the amount of GHGs (you just need enough to radiate away the incoming energy).
This is what Z-N found. They didn’t understand it and hence tried to invent something else to explain their finding.
“independent of the amount of GHGs”
I think I agree with you and that you agree with Dr. Motl’s (explanation of Mr. Heller’s) theory, but that quoted phrase is not quite correct, at least as I understand Dr. Motl’s theory.
As I understand that theory, the surface won’t radiate more power than that of the (virtually all solar) incoming radiation at the top of the atmosphere, no matter what the atmosphere’s mass is, unless the atmosphere includes at least some greenhouse gases. So according to Dr. Motl it’s not true that the surface radiation is completely independent of greenhouse gases.
But, as the atmosphere’s greenhouse-gas-imparted infrared opacity increases, the surface radiation’s sensitivity to greenhouse-gas concentration falls, and its sensitivity to lapse rate increases. When the resultant opacity becomes high, the surface radiation’s sensitivity to greenhouse-gas concentration therefore becomes negligible, and the integral of the lapse rate with respect to altitude becomes the variable that principally determines the surface radiation.
As (I believe) you say, this may be the effect that caused Nokolov & Zeller to arrive at their theory. (Or maybe not; I found their exposition confusing.)
Zeller and Nikolov, aren’t they two russkies who were fascinated by the Salisbury cathedral height ?
That was yer man Pablo Miller, Skripal’s MI6 handler, since served with a Gov’t D-notice. Maybe ye need to check some more?
My thanks to both Dr. Roy Spencer, one of my scientific heroes, and Anthony Watts, another of the same, for pointing out my proof that gravity or pressure cannot cause a permanent elevation in planetary surface temperature. Ned Nikolov’s nonsense has more lives than a cat.
For another look at the kind of goofy claims that Nikolov believes are valid, see my post entitled “The Mystery of Equation Eight“.
w.
That I can follow. Their Nte looks a lot like the lifetime of CO2 according to the Bern model.
Your proof is not valid. Your model is non physical. A body surrounded by suns as you describe would heat up to match the suns mean radiative temperature. Your explaination using the cold universe is false, as the non radiative mass in the universe keeps the mean universe temperature near absolute zero.
I derived the parameterized equations NZ used and I think they are on to something. I await some better probe data from new source moons to verify their work.
So much wrong with that statement .. where to start.
The lack of any molecules is what makes space cold because in the whacky world of classical physics you define temperature as roughly the average kinetic energy of being struck by a molecule. Under classical physics you then end up with a strange plausable fate of the universe called a heat death
https://en.wikipedia.org/wiki/Heat_death_of_the_universe
In that lovely end to the universe space is still cold even though the universe has died from heat (because of the lack of molecules) … strange hey.
The lesson here is be very careful when evoking classical physics on the universe as all teh theories usually drop into a very very big hole.
Oh I really should link the paradox as worked out a century ago, you might not get the background from wiki it’s a bit lacking on background
https://en.wikipedia.org/wiki/Heat_death_paradox
Does this same proof disprove the idea that adding GHGs to the atmosphere beyond what is needed to completely capture surface radiation, nonetheless further increases temperatures at the surface by raising the theoretical escape level of emission in the atmosphere, thereby raising surface surface temperatures due the lapse rate (what I have often heard referred to as the “enhanced greenhouse effect”) ?
I ask this because of the post by Stephen Wilde above. It seems to me that both he and the proponents of the enhanced greenhouse effect are making the same logical error – they both simply create this theoretically-unbounded equal exchange of heat between the atmosphere and the surface to get as much of a surface temperature increase as you want.
Don’t mistake me here. I completely agree that an atmosphere that traps any portion of radiation emitted from the surface that otherwise would have escaped directly to space would cause surface temperatures to rise. It’s a textbook feedback effect. What I’m questioning is whether you can get a further increase in temperature at the surface by having a top layer of the atmosphere intercept what was emitted outward by a bottom layer of the atmosphere, and cause the bottom layer’s temperature (and the air temperature at the surface) to rise due merely to the pressure gradient that already exists in the atmosphere. That theory has always been where I jump off the greenhouse theory bandwagon.
Kurt,
Read my linked description.
You can only raise the surface temperature by enough to produce such an upward pressure gradient force as will offset the downward force of gravity.
There is no logical error.
“You can only raise the surface temperature by enough to produce such an upward pressure gradient force as will offset the downward force of gravity.”
That doesn’t even begin to make sense. The downward force of gravity is the same in Phoenix on a bright summer afternoon as it is in Anchorage on a clear winter night. The surface temperatures at these locations differ vastly, and that difference has nothing to do with gravity. Gravity does not change over the course of a day or a year, yet temperatures do vary significantly over the these intervals.
Kurt
You must consider the globe as a whole. There are bound to be differences from place to place on the surface.
“What I’m questioning is whether you can get a further increase in temperature at the surface by having a top layer of the atmosphere intercept what was emitted outward by a bottom layer of the atmosphere, and cause the bottom layer’s temperature (and the air temperature at the surface) to rise due merely to the pressure gradient that already exists in the atmosphere. That theory has always been where I jump off the greenhouse theory bandwagon.”
Kurt:
Don’t see why you would question that.
And the “pressure gradient” has nothing to do with it (if I understand you correctly)
It is simply a furtherance of the affect you accept.
The GHE is a function of path-length of terrestrial IR through the atmosphere to space. (Beer-Lambert Law).
https://www.britannica.com/science/Beers-law
The longer the path the more GHG molecules photons encounter exiting to space.
It’s like a mist/fog into which you shine a torch beam – you will see the fog particles intercept the light and reflect (insert back-radiate for LWIR in the GHE) back to you. The more powerful the beam the further back through the fog they will travel and have more particles in their path.
At the level of effective emission to space (where more photons escape up and out to space than get back-radiated (this is at a height of -18C – Earth’s S-B equilibrium temp) though, emitting molecules are colder and so less efficient at the job of emission to space than are the molecules lower down.
It is this level that rises as concentrations of GHGs increase – BOTH increasing the path-length (more intercepting molecules AND a colder effective emitting level).
So you predict a hot spot but yet it does not happen. Thus your model must be deficient.
Edb:
Seems you didn’t get the message.
Mind you wouldn’t on here.
It has been….
http://iopscience.iop.org/article/10.1088/1748-9326/10/5/054007/meta
Also a “Tropical hotspot” would happen under any form of heating, as it is caused simply by the release of excess LH aloft under enhance Oceanic tropical heating.
Anthony – Sherwood’s analysis doesn’t count. It is unreliable because it uses wind data as a proxy for temperature. Christy et al challenged this in 2010, and the IPCC (referring inter alia to Christy) gave this method a “low confidence” in AR5 as follows:
“Atmospheric winds are driven by thermal gradients. Radiosonde winds
are far less affected by time-varying biases than their temperatures (Gruber and Haimberger, 2008; Sherwood et al., 2008; Section 2.7.3). Allen and Sherwood (2007) initially used radiosonde wind to infer temperatures within the Tropical West Pacific warm pool region, then extended this to a global analysis (Allen and Sherwood, 2008) yielding a distinct tropical upper tropospheric warming trend maximum within
the vertical profile, but with large uncertainty. Winds can only quantify
relative changes and require an initialization (location and trend at that location) (Allen and Sherwood, 2008). The large uncertainty range was predominantly driven by this initialization choice, a finding later confirmed by Christy et al. (2010), who in addition questioned the stability given the sparse geographical sampling, particularly in the
tropics, and possible systematic sampling effects amongst other potential issues. Initializing closer to the tropics tended to reduce or remove the appearance of a tropical upper tropospheric warming trend maximum (Allen and Sherwood, 2008; Christy et al., 2010). There is only low confidence in trends inferred from ‘thermal winds’ given the relative immaturity of the analyses and their large uncertainties.”
Please Anthony, no more references to Sherwood. It will not resolve anything.
Jordan:
This study is not by Sherwood ….
https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/grl.50465
“Using these approaches, it is shown that within observational uncertainty, the 5–95 percentile range of temperature trends from both coupled‐ocean and atmosphere‐only models are consistent with the analyzed observations at all but the upper most tropospheric level (150 hPa), and models with ultra‐high horizontal resolution (≤ 0.5° × 0.5°) perform particularly well.”
Anthony, your alternative paper resolves nothing. It says
“Nevertheless, the models still exhibit more warming than the observations in the upper troposphere (above ∼250 hPa), and the upper most level (150 hPa) of model simulated temperature trends is not consistent with observations over the 5–95 percentile range.”
OK, we’re no further forward than Christy’s 2010 conclusion that predicted scaling ratio (indicating a hot spot) is not evident in the observational data.
If you cannot provide conclusive evidence, why toss-in references to articles that contribute nothing to the discussion?
“If you cannot provide conclusive evidence, why toss-in references to articles that contribute nothing to the discussion?”
Because it has.
That is your interpretation.
I beg to differ.
OK?
Come back to EdB’s question “So you predict a hot spot but yet it does not happen. Thus your model must be deficient”
You have tried Sherwood (nope) and then another reference. With two shots, you added nothing to make a dent in Christy’s 2010 conclusion that there is a significant discrepancy between the models and observations on the measure of scaling ratio (predicted structure of warming due to GHGs) . EdB raised valid point and your responses fail to address it. OK.
“So you predict a hot spot but yet it does not happen. Thus your model must be deficient”
I have addressed it.
With that study.
It has been predicted and it has been found.
That denizens here naysay that is a self-fulfilling prophecy.
Yes, the model is deficient (at that close-up detailed part of the atmosphere).
Are you saying that there is no global warming at all?
Because it would be there with any kind of warming it is not dependent on the GHE
Again from the abstract…
“Using these approaches, it is shown that within observational uncertainty, the 5–95 percentile range of temperature trends from both coupled‐ocean and atmosphere‐only models ARE CONSISTENT with the analyzed observations at all but the upper most tropospheric level (150 hPa), and models with ultra‐high horizontal resolution (≤ 0.5° × 0.5°) perform particularly well….”
So correct (within uncertainties) other that the topmost level.
Of which they say “Other than model resolution, it is hypothesized that this remaining discrepancy could be due to a poor representation of stratospheric ozone or remaining observational uncertainty.
No one is saying that models are perfect, especially when zooming into that amount of detail.
That level is obviously hard for the models to resolve being just under the Tropopause with the stratospheric O3 just above
That without the observational difficulties inherent at that level.
It seems to be a case of it’s not perfect so it’s completely wrong.
We don’t know everything – so we know nothing.
Now where have I come across that before.
Oh yeah.
The idea that the enhanced GHG effect is dependent on lapse rate (temperature and pressure of a gas are related by P is equal to kVT) is commonly proffered as an explanation as to why surface temperatures increase as the optical depth of the atmosphere increases. See these links:
http://www.realclimate.org/index.php/archives/2010/07/a-simple-recipe-for-ghe/
https://atmos.washington.edu/2007Q1/211/Notes/old_notes/Section4.pdf
The Beer-Lambert law only deals with the depth at which radiation from the Earth’s surface can be absorbed, and only tells you that as the concentration of GHGs increase, the atmosphere gets more efficient at absorbing the radiation from the surface, intercepting closer to the Earth’s surface or intercepting more of it of some of the surface radiation was escaping to space. But the atmosphere can’t capture more than what the Earth throws up at it.
GHG molecules don’t reflect energy, like your fog example. They absorb it and then kinetically transfer the heat absorbed to the remainder of the atmosphere, which can’t directly absorb radiation. Thus, the GHG molecules’ temperature is locked to the temperature of the bulk atmosphere surrounding them. GHGs also radiate in proportion to their temperature, but because their temperature is locked to the non-GHGs surrounding them, the heat GHGs radiate comes from the bulk atmosphere. The common perception that GHGs capture radiation and re-radiate it is wrong; you have to analytically separate the absorptive properties of GHGs from their radiative properties since absorption and radiation by GHGs in an atmosphere have completely separate mathematical behaviors.
My question remains unanswered: once all the radiation emitted to space comes from the atmosphere at thermal equilibrium because the atmosphere absorbs everything from the surface, how can the temperature of the atmosphere increase from top to bottom while maintaining thermal equilibrium? If it radiates more, where does the additional heat that it radiates first come from, and what cools to provide that heat?
I agree that GHGs virtually do not reflect imparted IR photons, like a tennis ball. Thousands of thermalizing interactions with N2, O2, etc, happen first. Thus the net effect of more CO2 is merely to reduce the average distance a photon travels before being absorbed. So what if that is reduced from say ten meters to five? The action of convection and conduction shifts a bit but is all averaged out within minutes. The only temperature change I see is a result of ‘friction’ in the system,ie, heat transfer is less than instantaneous and ideal.
Kurt, I believe the claim is that the atmosphere absorbs more radiation at the wings of the CO2 absorption band. So, it hasn’t absorbed it all yet.
That’s certainly part of it. Not only is there more radiation at the sidebands of CO2 that water vapor does not capture, but in dry, cold regions there is not enough water vapor to capture all of the radiation from the surface to space. I’m not arguing that there is no more warming to be done by adding GHGs to the atmosphere. What I am arguing is that the amount of warming is overstated because the IPCC and other warmists think that even over areas of the Earth where water vapor and other GHGs already capture all of the emitted radiation, adding GHGs in such regions still increases surface temperature by lifting the effective altitude of emission, raising the temperature aloft which propagates downward due to the lapse rate. It’s only this part of the theory that I jump ship on.
If gravitational energy is converted into heat, then the planet will slowly lose mass.
Energy will be conserved, and there is no violation of Thermodynamics.
I’m no supporter of Zeller-Nikolov theory, but suspect that it does not violate the 1st Law as supposed.
Pat
Gravity in a star causes heat – that’s how stars ignite by fusion above a certain size. If this heating per se is associated with mass loss, that’s a very strange and unfamiliar nuclear reaction. I think you’ll find that if pressure-temperature from gravity cause mass loss, the universe would have lost all its baryonic mass long ago.
Tasfay Martinov
BUT, stars radiate energy — rather spectacularly! One might say that they are stellar performers.
Tasfay, gravity causes compressional heat in a star by the conventional PV=nrT.
My understanding of the surmise here, is that gravitational energy is converted into heat energy. Loss of gravitational energy requires loss of mass, however that might happen. There’d be no violation of the first law.
I’m confused by the whole thing. The average temperature of the planet is something like 1500°C. Black body nor anything else I’ve seen here applies.
Is that you Mr. Gore?
If you mean the average temperature including the core, it’s irrelevant. You’re forgetting the insulating characteristics of rock, which is what keeps us from getting roasted by magma. The heat leakage through the crust is relatively minor in the climactic scheme of things (barring another Deccan or Siberian Trap episode!).
What matters is the balance between energy received from the Sun and energy radiated by the earth’s surface and upper atmosphere. The temperature that’s important to us, at the surface and in the lower kilometer or so of the atmosphere, depends on how much the atmosphere above it absorbs or passes IR radiation. See my rather lengthy post elsewhere in these comments.
My total knowlwdge of thermodynamics
P1*V1/T1 = P2*V2/T2 , or P*V = n* R*T
is a one off short term event, so these guys (in my book) must be wrong.
The atmospheric lapse rate does in fact result in a temperature gradient (increasing temperature as you approach the surface) even with no greenhouse gases. However this is a gradient, not a specific level of temperature. One more piece of information is needed to set the surface level. With no greenhouse gases, the radiation from the surface has to match (on average) the absorbed solar radiation, so Willis is correct. It is the fact that with greenhouse gases, the average location of the source of radiation balance between incoming and outgoing radiation is well above the surface (near 5 km for Earth and about 50 km for Venus) that results in those locations having the required temperatures to cause the balance. With the temperature set at this average altitude to obtain balance, it is in fact the lapse rate time the average altitude that determines the surface temperature increase over the no greenhouse gas case. N & Z confuse a gradient with a temperature level.
That’s a better explanation, at least to me.
Their temperature level is determined by the distance to the sun.
“With no greenhouse gases, the radiation from the surface has to match (on average) the absorbed solar radiation…”
At the same time, the GHG-free atmosphere MUST retain some heat conducted from the surface and it cannot radiate that heat away, with no violation of any physical law! Maybe we’re missing something?
I think we’re throwing globs at the problem in order to dismiss NZ.
Don132
One thought I have here: Given the surface temperature predicted by S-B, corrected for albedo, IR emissivity, etc., given the amount of warming expected from greenhouse gases, given an expected lapse rate, the thickness of the troposphere can be roughly predicted.
Anyone who resorts to a “proof” based on Leibniz’s conservation of force (yes, not energy) should check Helmholz who made a complete fool of himself refuting Weber’s electrodynamics.
He is the template for the sheer unimaginative sterile consensus. As Dr. Bohm said, “impossibility proofs” are only proof of the lack of imagination, a Kan’tian disease (See J.S. Bell Unspeakable in QM). It takes a poet like Edgar Allan Poe, or Heinrich Heine to sent obscurantist rubbish out the window. 3 References available for the brave.
According to the adored 2nd Law, all life would not exist, nor WUWT!