From the “Montreal Protocol success is not weather, unless it is” department and NASA Goddard:
Using measurements from NASA’s Aura satellite, scientists studied chlorine within the Antarctic ozone hole over the last several years, watching as the amount slowly decreased. This is the first definitive evidence of the success of the Montreal Protocol on Substances that Deplete the Ozone Layer, which was ratified in 1987.
The international treaty banned the use of chlorofluorocarbons and related compounds, which break down in the stratosphere and release chlorine molecules. This chlorine depletes the ozone layer and is responsible for creating the hole in the ozone layer over Antarctica. The ozone hole fluctuates each year, reaching an annual maximum at the end of southern winter, usually in September. The hole has been trending smaller for the last few years, but as temperature has an effect on ozone-depletion, this was not definitive evidence of the Montreal Protocol’s efficacy.
Although scientists have been measuring levels of chlorine near the ground for decades, this study is the first time anyone accurately measured chlorine levels inside the ozone hole, confirming that the Montreal Protocol is doing its job.
Here is the Press release from January 4th, 2018:
Measurements show that the decline in chlorine, resulting from an international ban on chlorine-containing manmade chemicals called chlorofluorocarbons (CFCs), has resulted in about 20 percent less ozone depletion during the Antarctic winter than there was in 2005 — the first year that measurements of chlorine and ozone during the Antarctic winter were made by NASA’s Aura satellite.
“We see very clearly that chlorine from CFCs is going down in the ozone hole, and that less ozone depletion is occurring because of it,” said lead author Susan Strahan, an atmospheric scientist from NASA’s Goddard Space Flight Center in Greenbelt, Maryland.
CFCs are long-lived chemical compounds that eventually rise into the stratosphere, where they are broken apart by the Sun’s ultraviolet radiation, releasing chlorine atoms that go on to destroy ozone molecules. Stratospheric ozone protects life on the planet by absorbing potentially harmful ultraviolet radiation that can cause skin cancer and cataracts, suppress immune systems and damage plant life.
Two years after the discovery of the Antarctic ozone hole in 1985, nations of the world signed the Montreal Protocol on Substances that Deplete the Ozone Layer, which regulated ozone-depleting compounds. Later amendments to the Montreal Protocol completely phased out production of CFCs.
Past studies have used statistical analyses of changes in the ozone hole’s size to argue that ozone depletion is decreasing. This study is the first to use measurements of the chemical composition inside the ozone hole to confirm that not only is ozone depletion decreasing, but that the decrease is caused by the decline in CFCs.
The study was published Jan. 4 in the journal Geophysical Research Letters.
The Antarctic ozone hole forms during September in the Southern Hemisphere’s winter as the returning sun’s rays catalyze ozone destruction cycles involving chlorine and bromine that come primarily from CFCs. To determine how ozone and other chemicals have changed year to year, scientists used data from the Microwave Limb Sounder (MLS) aboard the Aura satellite, which has been making measurements continuously around the globe since mid-2004. While many satellite instruments require sunlight to measure atmospheric trace gases, MLS measures microwave emissions and, as a result, can measure trace gases over Antarctica during the key time of year: the dark southern winter, when the stratospheric weather is quiet and temperatures are low and stable.
The change in ozone levels above Antarctica from the beginning to the end of southern winter — early July to mid-September — was computed daily from MLS measurements every year from 2005 to 2016. “During this period, Antarctic temperatures are always very low, so the rate of ozone destruction depends mostly on how much chlorine there is,” Strahan said. “This is when we want to measure ozone loss.”
They found that ozone loss is decreasing, but they needed to know whether a decrease in CFCs was responsible. When ozone destruction is ongoing, chlorine is found in many molecular forms, most of which are not measured. But after chlorine has destroyed nearly all the available ozone, it reacts instead with methane to form hydrochloric acid, a gas measured by MLS. “By around mid-October, all the chlorine compounds are conveniently converted into one gas, so by measuring hydrochloric acid we have a good measurement of the total chlorine,” Strahan said.
Nitrous oxide is a long-lived gas that behaves just like CFCs in much of the stratosphere. The CFCs are declining at the surface but nitrous oxide is not. If CFCs in the stratosphere are decreasing, then over time, less chlorine should be measured for a given value of nitrous oxide. By comparing MLS measurements of hydrochloric acid and nitrous oxide each year, they determined that the total chlorine levels were declining on average by about 0.8 percent annually.
The 20 percent decrease in ozone depletion during the winter months from 2005 to 2016 as determined from MLS ozone measurements was expected. “This is very close to what our model predicts we should see for this amount of chlorine decline,” Strahan said. “This gives us confidence that the decrease in ozone depletion through mid-September shown by MLS data is due to declining levels of chlorine coming from CFCs. But we’re not yet seeing a clear decrease in the size of the ozone hole because that’s controlled mainly by temperature after mid-September, which varies a lot from year to year.”
Looking forward, the Antarctic ozone hole should continue to recover gradually as CFCs leave the atmosphere, but complete recovery will take decades. “CFCs have lifetimes from 50 to 100 years, so they linger in the atmosphere for a very long time,” said Anne Douglass, a fellow atmospheric scientist at Goddard and the study’s co-author. “As far as the ozone hole being gone, we’re looking at 2060 or 2080. And even then there might still be a small hole.”
To read the study, visit: http://onlinelibrary.wiley.com/doi/10.1002/2017GL074830/abstract
However, just a couple of months ago, NASA claimed the most recent shrinkage of the Antarctic Ozone Hole was due to a warm winter, not the Montreal protocol. Next year, I wonder what they’ll say if the hole is bigger than 2017. They wrote then:
NASA and NOAA scientists work together to study the ozone layer, monitoring the hole over Antarctica as it fluctuates with the seasons.
This year, the ozone hole’s annual maximum set a record — the smallest it’s been since 1988.
The hole in the ozone layer is caused each year as ozone molecules react with chlorofluorocarbons (CFCs) in the atmosphere. The reactions occur at cold temperatures, so the hole reaches a maximum size each year at the end of southern winter, and then heals during the warmer summer months.
Although CFCs have been banned since 1987 under the Montreal Protocol on Substances that Deplete the Ozone Layer, the compounds decay very slowly, and still remain in the atmosphere. This year, the small ozone hole was mostly caused by warmer temperatures, which slowed down the reactions between ozone and CFCs.
NASA also claims the ozone hole will be gone by 2040, we’ll see then if this was just a natural feature all along, or if CFC reduction really did have an impact:
Big Ozone Holes Headed For Extinction By 2040
The next three decades will see an end of the era of big ozone holes. In a new study, scientists from NASA Goddard Space Flight Center say that the ozone hole will be consistently smaller than 8 million square miles by the year 2040.
Ozone-depleting chemicals in the atmosphere cause an ozone hole to form over Antarctica during the winter months in the Southern Hemisphere. Since the Montreal Protocol agreement in 1987, emissions have been regulated and chemical levels have been declining. However, the ozone hole has still remained bigger than 8 million square miles since the early 1990s, with exact sizes varying from year to year.
The size of the ozone hole varies due to both temperature and levels of ozone-depleting chemicals in the atmosphere. In order to get a more accurate picture of the future size of the ozone hole, scientists used NASA’s AURA satellite to determine how much the levels of these chemicals in the atmosphere varied each year. With this new knowledge, scientists can confidently say that the ozone hole will be consistently smaller than 8 million square miles by the year 2040. Scientists will continue to use satellites to monitor the recovery of the ozone hole and they hope to see its full recovery before the end of the century.
Research: Inorganic chlorine variability in the Antarctic vortex and implications for ozone recovery.
Journal: Geophysical Research: Atmospheres, December 18, 2014.
Link to paper: http://onlinelibrary.wiley.com/doi/10.1002/2014JD022295/abstract.
Ok, so they seem confident that the HCl is what the CFCs make when Ozone is depleted. So, as always, confounding studies have to be brought in by sceptics because the climate worriers never look to hard at unhelpful literature. My instant thought was that we are a chlorine salt water planet! It seems that ehat is “acidifying” the ocean is hydrochloric acid,wadyaknow!
https://www.google.com/amp/s/amp.livescience.com/2032-seawater-treatment-plants-combat-climate-change.html
And natural coastal aerosols (I’ve seen the faint haze from the sea spray in a wind with the waves pounding the shore) result in chlorine emissions into the atmosphere reactions of salt with the atmosphere and folks, i don’t have to look up the fact that chlorine dissolved in water makes HCl acid.
http://pubs.acs.org/doi/abs//es025793z
Now how many readers here, scientists and high school grads would conduct an experiment involving Cl and HCl in the atmosphere above a large basin of saltwater that isn’t listed as a part of the experiment?
I would love to wager that the ozone hole will be about the same size +or- the variance of the past 30 yrs. Indeed, a way to save the great grandchildren would be to set up a fund to bet against all the predicted disasters due to man’s activities. All Universities, doomer institutions should be required to contribute to the betting fund -at least in their minds it should be a no-brainer endowment for their institutions. I would even offer to the institutions that they can decide on the odds for each bet. Fair? We ate talking settled science here. The way they would do this is by giving a probability of their being correcct within shown error bars as part of their published works. E.g. a a 67% probability would be a payout of 2:1 to the winner. Contributions would be funded by each side stsrting now. I leave the detais to experts in rhe gambling field for paying in, setting up a trust and rules to make it work asit should.
More dubious information from the team who brought us the Apollo Moon Landing simulations and the Mars Rover on Devon Island. Let’s all have a very close examination of this new presentation first.
Yeah, I saw the video of the yearly changes of the ozone hole. OK, it looked like it decreased slightly last couple yrs.
Eco-nut taking an inch and making a mile: OH!! OH!! LOOK!! LOOK!! The Montreal Thingy is working!!! SEE!! TOLD YOU!!!
(note the accretion region surrounding the depletion region – try to account for it in your explanation)
======
Scientists from NASA and the National Oceanic and Atmospheric Administration (NOAA) have confirmed the ozone hole over the Antarctic this September is not only much smaller than it was in 2000 and 2001, but has split into two separate “holes.”
The researchers stressed the smaller hole is due to this year’s peculiar stratospheric weather patterns and that a single year’s unusual pattern does not make a long-term trend. Moreover, they said, the data are not conclusive that the ozone layer is recovering.

-NASA Earth Observatory, 2002
=====
(note the mountains surrounding the valley: try to account for the mountains in your explanation for the pattern)
Khwarizmi, I’ve been making this point for years but no one has shown much interest. I describe it like a turtleneck sweater rolled back and doubled over. There is no loss of ozone, it is simply pushed aside. Nasa’s simple coloured images always have this “roll neck” effect.
http://www.theozonehole.com
This is a great site but it has some weird blind spots. Oh well there are two of us who noticed this complete debunking of the human cause of the ozone hole sticking out of their own data. Eventually this could double sarc.
Gary, this winter anomaly was first described and explained in the early ’60s by Dobson himself. But it is not your fault that you missed that. The issue has become confused by the conflation of the ‘Spring hole’ (described by Farman, caused by CFCs) with the ‘Winter anomaly’ (cause by the vortex stopping Brewer-Dobson circulation). Now, with this video above explaining that the best time to measure the Chlorine (from CFCs) effect is in Winter, the issue is further confused. It’s hard to know what is going on both over Antarctica and in the interpretation of the evidence. No one who knows seems to have any interest in sorting out the confusions and distortions in the PR. I live in hope that one day some expert will provide a critique that will clarify the interpretation, the evidence.
Here is the original data from Halley Bay that lead Dobson to propose the anomaly and its cause:

berniel January 17, 2018 at 2:36 pm
Gary, this winter anomaly was first described and explained in the early ’60s by Dobson himself. But it is not your fault that you missed that. The issue has become confused by the conflation of the ‘Spring hole’ (described by Farman, caused by CFCs) with the ‘Winter anomaly’ (cause by the vortex stopping Brewer-Dobson circulation). Now, with this video above explaining that the best time to measure the Chlorine (from CFCs) effect is in Winter, the issue is further confused. It’s hard to know what is going on both over Antarctica and in the interpretation of the evidence. No one who knows seems to have any interest in sorting out the confusions and distortions in the PR. I live in hope that one day some expert will provide a critique that will clarify the interpretation, the evidence.
The top post refers to the paper which points out that during the spring O3 depletion event ultimately the Cl ends up as HCl, which is a stable Cl reservoir species. That is a good time to measure how much active Cl was involved in that spring
Basically during the winter the PSCs cause the reservoir species to react and form Cl2, once the UV hits the Cl2 the active O3 depleting molecules (Cl and ClO) are released and destroy the O3 and ultimately the reservoir species are reformed.
Here’s a graph showing the dramatic change in chemical composition as the vortex is entered.
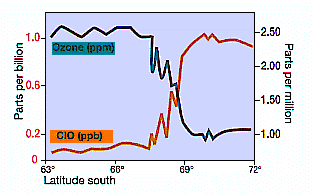
Measurements of ClO concentration and ozone from aircraft (NASA, 1987). Note the rapid incease of ClO as the aircraft enters the polar vortex and the Antactic ozone ‘hole’ (about 67ºS) and the inverse correlation of ClO with ozone decline in mid-September when the chemically unbalanced area in the vortex was sunlit.
Thanks Phil. I get the smoking gun reverse correlation of O3 and ClO in the spring sunlight. What remains confusing for me is when they say that the best time to measure Cl destruction of ozone is to measure ozone depletion in the winter dark. The top post says: During winter “Antarctic temperatures are always very low, so the rate of ozone destruction depends mostly on how much chlorine there is,” Strahan said. “This is when we want to measure ozone loss.” ” They want to measure then because they want to detect the Cl-from-CFCs effect. But the post also says “The Antarctic ozone hole forms during September in the Southern Hemisphere’s winter as the returning sun’s rays catalyze ozone destruction cycles involving chlorine and bromine that come primarily from CFCs.” Does the CFC effect require spring sunlight or is it an exacerbation of the winter anomaly? Or both? Are they playing hide-the-pea or have I just got distracted?
in a hundred years, if the human race hasn’t disappeared up its own backside due to the hubris of the well educated but terminally stupid ,this will be one of those paradigms that will be laughed at and used as an example of correlation not automatically mean causation.
oops, forgot the “ing” on the end of mean. oh well, how sad, never mind. obviously tired shovelling viner off the drive this evening. at least the children know what snow is this winter.
Perhaps the hurricane drought can be credited with the decrease in stratospheric chlorine more than the Montreal Accords. It would seem that oceanic chlorine and bromine would be a much more prolific source of stratospheric halides than would an industrial refrigerant. Just looking at an Atlantic hurricane, one has to conclude that quite a bit of salt has to be lofted into the upper atmosphere. I find a number of non-global warming and non-ozone depletion references on line regarding atmospheric halides. A real study of this would use isotopic tracers to determine where the chlorine comes from. But that is difficult, and doesn’t serve the narrative.
However the halides swept up from the ocean are water soluble and are precipitated out of the atmosphere in the form of rain (and snow). Measurements show that the HCl mixing ratio is less than 0.1 ppbv at
elevations above 7 km, and less than 0.04 ppbv at 13.7 km.
Stratospheric chlorine is ~80% from CFC’s and related manmade organic chlorine compounds,
such as carbon tetrachloride and methyl chloroform and ~15-20% from methyl chloride (CH3Cl), most of which is natural.
F. S. Rowland, “Stratospheric Ozone Depletion”, Ann. Rev. Phys. Chem. 42, 731, 1991.
Read this from the wikipedia site on ozone
“Computer modeling
Scientists have attributed ozone depletion to the increase of man-made (anthropogenic) halogen compounds from CFCs by combining observational data with computer models. These complex chemistry transport models (e.g. SLIMCAT, CLaMS—Chemical Lagrangian Model of the Stratosphere) work by combining measurements of chemicals and meteorological fields with chemical reaction rate constants. They identify key chemical reactions and transport processes that bring CFC photolysis products into contact with ozone.
“Ozone depletion and global warming[edit]
Main article: Ozone depletion and global warming
Among others, Robert Watson had a role in the science assessment and in the regulation efforts of ozone depletion and global warming.[31] Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a crucial role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment[31] to reach a consensus to provide the IPCC Summary for Policymakers.
There are various areas of linkage between ozone depletion and global warming science:
Radiative forcing from various greenhouse gases and other sources
The same CO
2 radiative forcing that produces global warming is expected to cool the stratosphere.[111] This cooling, in turn, is expected to produce a relative increase in ozone (O
3) depletion in polar area and the frequency of ozone holes.[112]
Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the troposphere; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes “observed stratospheric O
3 losses over the past two decades have caused a negative forcing of the surface-troposphere system”[14] of about −0.15 ± 0.10 watts per square meter (W/m2).[86]
One of the strongest predictions of the greenhouse effect is that the stratosphere will cool.[111] Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of greenhouse gases and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the National Oceanic and Atmospheric Administration’s Geophysical Fluid Dynamics Laboratory show that above 20 km (12 mi), the greenhouse gases dominate the cooling.[113]
As noted under ‘Public Policy’, ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m2 of radiative forcing, corresponding to about 14 percent of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.[86]
The long term modeling of the process, its measurement, study, design of theories and testing take decades to document, gain wide acceptance, and ultimately become the dominant paradigm. Several theories about the destruction of ozone were hypothesized in the 1980s, published in the late 1990s, and are currently being investigated. Dr Drew Schindell, and Dr Paul Newman, NASA Goddard, proposed a theory in the late 1990s, using computational modeling methods to model ozone destruction, that accounted for 78 percent of the ozone destroyed. Further refinement of that model accounted for 89 percent of the ozone destroyed, but pushed back the estimated recovery of the ozone hole from 75 years to 150 years. (An important part of that model is the lack of stratospheric flight due to depletion of fossil fuels.)”
THE FOLLOWING IS IN CAPS SO THAT YOU KNOW IT IS MY COMMENT
NOW I KNOW WHY THIS WHOLE SITUATION ABOUT THE OZONE DEPLETING REGS AND LAWS AND AGW REGS AND LAWS AROUND THE WORLD ARE CONNECTED. THE CONNECTION IS THROUGH THE IPCC
AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAGH
i AM ASHAMED OF THE HUMAN RACE THAT ONE PANEL CAN CAUSE SO MUCH HARDSHIP. I AM NOT AS MUCH CONVINCED THAT THE OZONE HOLE IS A SCAM AS MUCH AS I AM CONVINCED THAT AGW IS A SCAM BUT AFTER READING THE WIKI ARTICLE MY SCAM DETECTOR IS GROWING ABOUT THE OZONE HOLES AS E WELL
I haven’t looked at it for a while but the BAS web site had data for 2009-10 and reported that:
“Ozone values dropped, to reach a minimum of around 125 DU (60% depletion) in late September, (Antarctic spring). The lowest daily value measured was 107 DU on October 1. This minimum value is similar to those recorded each October since the early 1990s.”
It was also similar to those in the spring of 1958 at the French Antarctic Observatory at Dumont d’Urville [opposite side of the South Pole from Halley Bay], when Rigaud and Leroy [quoted in Annales Geophysicae (November, 1990)] reported atmospheric ozone levels as low as 110 DU.
Don’t forget Mount Erebus, which started erupting in 1971 and has been active ever since. It is one of the largest, if not the largest, natural source of stratospheric halogens and situated right in the middle of the Antarctic ozone hole.
https://www.sciencedirect.com/science/article/pii/S1352231015304246
Every time I see an article on the Ozone Hole and the impact of CFC’s, I’m reminded of a sidebar in the 1969 Time Life Life Science Series book on Mathematics.
The side bar, A Topological Redhead, was accompanied by a photo of a boy showing his hair swirl and read,
“…most human heads have a fixed point, in the form of a whorl, from which all hair radiates. Topologically, it would be impossible to cover a sphere with hair — or with radiating lines — without at least ONE such fixed point. For the same reason, the wind cannot blow everywhere over the earth’s surface at once; there must be a point of calm.”
Just because the ozone hole was discovered in 1985 and CFC measurements were first obtained in 2005 as noted in this post, does not mean the hole, whatever we choose to call it, recently emerged. It, and potentially many more, have existed for ever.
Joe Armstrong January 17, 2018 at 8:30 am
Just because the ozone hole was discovered in 1985 and CFC measurements were first obtained in 2005 as noted in this post, does not mean the hole, whatever we choose to call it, recently emerged. It, and potentially many more, have existed for ever.
The stratospheric CFCs were measured starting at least in 1985.
I’ll try one more time! There is no depletion of ozone! it’s pushed out of the “hole” and banked in a ring around the hole,. Like shovelling snow there is no disappearance its just pushed aside. See the images from Nasa:
http://www.abc.net.au/reslib/200906/r380242_1771273.jpg
A chemical reaction can’t do this! The ozone has simply been redistributed. I ‘ve been trying to attract notice to this here in vain for several years. Im now joined by one other comenter who has an excellent 3D graphic of it.
https://wattsupwiththat.com/2018/01/16/nasa-claim-definitive-evidence-of-the-montreal-protocols-success-on-ozone-hole-but-may-be-premature/comment-page-1/#comment-2718899
Com’on people, this is science.Are there only two of us that get It?
Gary Pearse January 17, 2018 at 11:43 am
I’ll try one more time! There is no depletion of ozone! it’s pushed out of the “hole” and banked in a ring around the hole,. Like shovelling snow there is no disappearance its just pushed aside. See the images from Nasa:
Not true, the ozone is mainly generated in the tropics and flows towards the poles, when the S polar vortex forms it prevents further ozone entering the polar region and O3 accumulates outside the vortex. Meanwhile the previous ozone trapped inside the vortex remains at about 300DU until the sunlight returns and causes destruction of this ozone, thus forming the hole. As the atmosphere inside the vortex warms up the vortex breaks down and the ozone which has accumulated outside is able to mix with the depleted atmosphere inside.
A chemical reaction can’t do this! The ozone has simply been redistributed. I ‘ve been trying to attract notice to this here in vain for several years. Im now joined by one other comenter who has an excellent 3D graphic of it.
Com’on people, this is science.Are there only two of us that get It?
No the science is as I describe it above.
Gary Pearse January 17, 2018 at 11:43 am
I’ll try one more time! There is no depletion of ozone! it’s pushed out of the “hole” and banked in a ring around the hole,. Like shovelling snow there is no disappearance its just pushed aside. See the images from Nasa:
Not true, the ozone is mainly generated in the tropics and flows towards the poles, when the S polar vortex forms it prevents further ozone entering the polar region and O3 accumulates outside the vortex. Meanwhile the previous ozone trapped inside the vortex remains at about 300 DU until the sunlight returns and causes destruction of this ozone, thus forming the hole. As the atmosphere inside the vortex warms up the vortex breaks down and the ozone which has accumulated outside is able to mix with the depleted atmosphere inside.
A chemical reaction can’t do this! The ozone has simply been redistributed. I ‘ve been trying to attract notice to this here in vain for several years. Im now joined by one other comenter who has an excellent 3D graphic of it.
https://wattsupwiththat.com/2018/01/16/nasa-claim-definitive-evidence-of-the-montreal-protocols-success-on-ozone-hole-but-may-be-premature/comment-page-1/#comment-2718899
Com’on people, this is science.Are there only two of us that get It?
No the science is as I describe it above.
Mods, why is my reply to Gary Pearse being blocked?
[? Nothing in queue. .mod]
Just tried it for the fourth time, still no show!
No, you have this backwards, the ozone is mainly formed in the tropics and is transported towards the poles.
When the S Polar vortex forms the ozone is prevented from entering the polar region and accumulates outside the vortex. Meanwhile as a result of the heterogeneous reactions on the PSCs chlorine is released in the polar stratosphere and when the sunshine returns the ozone trapped inside the vortex is rapidly destroyed. As the atmosphere warms up the vortex breaks down and the ozone outside mixes with the ozone depleted atmosphere inside.
Clearly a chemical reaction can do it, the science is as I described it above.
Phil. January 18, 2018 at 9:09 am
Mods, why is my reply to Gary Pearse being blocked?
[? Nothing in queue. .mod]
Two versions predating this post by the Mod have just appeared so I guess they were in the queue!
Apparently Gary’s post contains a poison pill because whenever I tried to quote him it didn’t post!
I find it quite incomprehensible that a CFC thinned ozone layer is not considered,even by those critical of the dominant paradigm of CO2/warming, as the most probable cause of global warming instead of CO2. A thinned ozone layer allows greater irradiation of the atmosphere and Earth’s surface with ionizing solar UV-B radiation, which is near the top of Sun’s radiative spectrum, so a little goes a long way. Further, the record of temperature anomalies over the past 50 years correlates much better with total equivalent atmospheric chlorine concentration than it does with CO2 concentration. The latter has been steadily rising since records have been kept, but global temperature has leveled off following a strong spurt of global warming from 1975 to 1998, in the now two-decade-long, level trend of the so-called “global warming hiatus.” Since the Montreal Protocol took effect through the ’90s, the temperature slowdown is entirely consistent with a chlorine-thinned ozone layer being the true cause of global warming. Certainly, if ionizing UV-B radiation is capable of causing severe sunburn and genetic alteration, it is also capable of causing global warming.
More on this can be found by Googling “David Bennett Laing WUWT” and selecting any of the top four urls.