Note: This is a contentious subject, and I have often shied away from it because it often erupts in food fights. However, Mr. Gill is making a good-faith effort here, and asks some relevant questions that I consider worth discussing. His original essay was sans graphics, and I’ve added two relevant graphics to aid in the discussion. – Anthony
Do Wien’s Law and Quantum Physics 101 prove CO2 can’t warm anything?
Guest essay by Rod Gill
WUWT has happily demonstrated many ways CO2 fails to produce measurable warming. I’ve thought of another way. It’s so simple I must have missed something, but I simply can’t work out what. It goes like this…
Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming. I suggest the quality of that 2W of radiation is crucial to determining whether or not it causes any atmospheric warming at all. First a few key points which I think are facts and not open to dispute.
My understanding of Thermodynamics and Radiation from CO2 is as follows:
In Thermodynamics, Temperature is the average kinetic energy of the particles in a body (solid or gas).
The temperature of a volume of air has nothing to do with the amount of radiation (sometimes mislabelled as heat by scientists) passing through it. Unless that radiation is at a frequency that can be absorbed by the air, its temperature is completely unaffected by the radiation (ignoring any convectional heating).For example at the top of Mount Everest, there is a lot of solar energy (long and short wave radiation) there when the sun is out but the temperature is still cold.
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law). This linear variation is not evident in this kind of plot since the intensity increases with the fourth power of the temperature (Stefan- Boltzmann law). The nature of the peak wavelength change is made more evident by plotting the fourth root of the intensity. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/wien.html
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Photons with not enough energy to raise the orbit of any of the electrons are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. If I gave you my references, which knowing the web may or may not be accurate, you might erroneously come to the same conclusions I have. However I have tried to limit my research to University papers and lecture notes hoping they are more reliable.
If I’ve got this right, CO2 caused global warming isn’t possible. If I haven’t got this right, then exactly how does LWIR radiated from CO2 warm anything?
Many thanks and please limit comments to specifics mentioned above. And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C
If you look at a spectrum, the common form the Y axis is power in Watts, you are right that the energy at 15u per photon is slight, so to make an equal power as shorter wave lengths, there are a lot more photons.
So the flux is higher.
The interactions are photons bypass, they scatter, or they are absorbed.
This results in our experience, things are invisible to that wavelength, ie clear, it becomes reflective, or opaque.
But regardless of how 15u photons interact in the environment, water vapor regulates am temps to not drop much below dew point, at least until it all starts freezing.
When you monitor cooling at night, do you wonder why it’s clear, and yet the temp stops falling in the middle of the night. But most of the year it stops cooling around dew point. But what few realize is that it did not stop radiating to space, in the optical window temps are are nearly 100F cooler than air temps.
What does happen is the sensible heat, IR from condensing water, lights up the sky in all directions in the 14-16u water band, which lights up co2 at 15u. This is strongest at the surface, and it shows up as the rate temps falling while it’s still night out dropping towards zero. A side effect of using WV to regulate temps, it bleeds off atm water every night, this prevents the WV amplification of CS, the key to catastrophic warming. So not only is it not catastrophic, it doesnt even reallt cause any warming, WV will always prevent it.
micro6500
November 19, 2017 at 8:07 am: and possibly what Roy Spencer sees with his skyward pointing pyrgeometer. All real experiments including Hans Geiger’s monumental “The air above the ground”, those in the Australian outback, and Hartmann’s, show how CO2 is no different to any other gas except the one which cheats by phase-changing. Which is why radiators have caps, and we exist.
Micro6500
As stated above your assumption that water vapour is the key to cooling stopping is incorrect. It is heat release from latent heat as dew forms on surface of the ground and plants which slow cooling and then eventually fog forms and once it does then temps just flat line….Fog is liquid water…not the gaseous form water vapour and hence a different mechanism for cooling slowing down. Once fog forms…then the top of the fog layer becomes the radiating surface…not the ground, and as this fog layer cools further at the top, dewpoint is reached and the top of the fog layer rises. This is how fog layers deepen overnight.
As stated above your assumption that water vapour is the key to cooling stopping is incorrect. It is heat release from latent heat as dew forms on surface of the ground and plants which slow cooling and then eventually fog forms and once it does then temps just flat line….Fog is liquid water…not the gaseous form water vapour and hence a different mechanism for cooling slowing down. Once fog forms…then the top of the fog layer becomes the radiating surface…not the ground, and as this fog layer cools further at the top, dewpoint is reached and the top of the fog layer rises. This is how fog layers deepen overnight.
Dew is only a small part of it. But there’s a 30-40W/m^2 flux out the optical window at the same time the temperature stops falling.
Martin Mason
November 19, 2017 8:07 am
I can see the same circular arguments and the same immovable positions but nobody addressing the central point of the post. Can radiation from such a low temperature source warm surface matter which is at a much higher energy level, that is the only question to be answered surely. Also if back radiation causes warming how is it that surface temperature and tropospheric lapse rate can be calculated with no reference to radiation?
The sb-equation says no. There is no transfer but the heat, which is the difference in T⁴. Quantum physics is built on thermal physics, so it obeys the sb-law. Quantum is the microscopic details of heat.
The surface transfer 383W/m²-128W/m² to TOA, and TOA transfer nothing back. Neither does the rest of the atmosphere.
Sorry RS but that answers none of the questions I raised. It certainly doesn’t explain cold to hot heat transfer. It doesn’t even touch on why all tropospheric temperatures can be explained and calculated with no reference to back radiation. I really think that AGW is in desperate trouble?
The problem seems to exist in the word “warms”. Radiation from a cold object cannot “warm” a warmer object. However, it CAN leave the warm object warmer than it would have been without the cold object.
As an example. When we look up, the apparent temperature of the sky based on the amount of downwelling thermal radiation is on the order of -50°C … how can this radiation “warm” the much warmer earth?
The answer, of course, is that without the atmosphere the apparent temperature of the sky would be about -270°C … and as a result, we are warmer because of the existence of the atmosphere.
Does the radiation from the atmosphere “warm” the surface? No … but it does leave the surface much warmer than it would be without that radiation.
Willis Eschenbach
November 19, 2017 at 8:35 am: By its mass, and the sun. Just as explained by all real experimental Physicists from Maxwell (Theory of Heat) onwards. Not to mention the Mylar balloon expt, and all Nasa atmospheric readings through the solar system. Even Sagan admitted this eventually.
No, we are “warmer” because the oceans absorb a lot of the sun and release that energy slowly, which heats the atmosphere mostly by direct molecular contact with the air. The cooler air cannot warm the surface, only slow the rate of heat loss. It appears to have warmed because the temp number is higher, but that is not “warmer”. Example, day time heated by the sun gets to 30C, during the night it cools, losing that heat. If the lowest it gets on a clear night is 10C, then we have lost 20C worth of energy. But if on a cloudy night it instead goes to 20C, then we have NOT gained 10C in new energy (*warmer”). What has happened is less energy is lost. It’s not “warmer” on the cloudy nights, it’s less cooled. Because the temp is 10C higher numerically, we say it is “warmer”, but in reality it isnt warmer (no new energy added).
The cooler air cannot warm the surface, only slow the rate of heat loss.
This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.
Hugs:
“This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.”
To warm an object requires new energy to raise its temp. Losing less energy already there has a higher temp number, but it is not “warmer”. If you earn $1000 per month gross, and have a high tax rate of 25%, you lose $250, netting $750. But if the tax rate is lowered to 20%, you now lose only $200, does that mean you have earned $50 in new money? Nope. You earned the same, just lost less of it in taxes. Consider winter and night times as a tax on the energy the sun put to the earth during the day. Having a higher NET energy doesnt mean you are “warmer” because you dont have more gross energy.
Back radiation is competely irrelevant, as is sideways radiaton and slant radiation and every-which way radiation. The only important factor is the net upward radiation. Look at this formally correct but carefully doctored NASA diagram:
What it shows is that LWIR moves 66 Wm^-2 from the surface, 40 by direct radiation in the “window” and 26 by way of absorption/re-emission by GHG. It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat, 102 Wm^-2, of which 78 is latent heat of evaporated water, which condenses at high altitude and radiates the heat into space.
Also note that radiation is gross while convection is net (and that the radiation arrows are fat and red while the convection ones are as puny as possible). Taken at face value this diagram implies that the descending cooler/drier air and precipitation is at absolute zero since in this case there is no “back radiation” (=downward heat transport). The reason for this difference is that a honest diagram would show clearly that convection is the dominant process regulating surface temperature. And convection can’t be modelled by GCM, only “parameterized” (=guessed at) since it happens on a far to small spatial scale for GCM:s.
This is the flat earth model, and it is wrong. It overly simplifies and incorrectly depicts what is going on. Postma has written a lot about this flat earth model having no basis in reality.
The sun puts 1370W/m^2 to the surface, not 168 (which wouldnt power two 100watt light bulbs). The surface temp of the earth would be some 69C at that input, but it’s not. Why? Because the earth rotates. Only a small percent of the surface receives this input, the rest loses more than it gains. The earth’s rotation acts like a thermostat. If it rotated slower it would be too hot end of the day for life. It it rotated faster, it would not gain enough during the day to overcome the night time loss rate.
The sun puts 1370W/m^2 to the surface, not 168 (which wouldnt power two 100watt light bulbs).
Well, that 1370 watts is actually only 1362 watts/m^2.
But it is actually measured at 1362 watts/m^2 at the top of atmosphere, only about 1000 watts/m^2 gets through a clear atmosphere to the surface of the earth, at the equator. And that 1000 watts/m^2 only gets to the surface at noon on the equator, on a clear day, on average.
Over the whole year, the CAGW climacatastrophy community has accepted Trenberth’s [168] watts/m^2 absorbed + [30] watts/m*2 reflected as average values for the whole earth.
But that 198 watt/m^2 yearly average sunlight on the surface for every day and night is ONLY valid for the temperature regions ONLY between 55 north and 55 south latitudes. Any further north or south and the yearly total “average” sunlight goes much, much further down.
“It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat”
No,it’s not. The diagram is a budget. It records the fluxes that can be measured, and tests whether they are in balance. The flux that is affected by GHG concentration is the full 324 W/m2 downflux from air. The 390 W/m2 upflux at the surface responds to the temperature there. The difference, if you exclude AW, is small at 26 W/m2, but is pretty much locked. The 324 W/m2 is emitted from air near the surface, as you can tell from its magnitude. If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.
Dry convection is necessarily entered as a nett flux, because there are no measurements that can split it into separate streams. Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.
“If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.”
In that case You can kiss CAGW goodbye, because there is nothing else that GHG can affect.
” Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.”
True in so far as wet convection is of course by far dominant. Ever see cumulonimbus clouds? They are not close to the surface.
Martin Mason,
A cold object cannot warm a hot object by conduction, but a warm object (Earth) can warm a cold parcel of absorbing gas by radiation. The radiation that would have otherwise left the system, is retained and now available to contribute a slight warming of the dark side of the Earth where it is being subject to the IR radiation from the gas, which is above absolute zero. Consider the impact of an Earth radiating directly into space, versus an Earth radiating into space with an intervening blanket at a higher temperature than space.
I don’t understand the question in your last sentence.
You can test this experimentally. Two identical set ups one with no CO2 and one with lots. If back radiation exists and can transfer heat a measurable temperature difference can be seen. Relatively easy lab work and relatively inexpensive.
The answer of course can be answered by any five year old playing in the snow. No, radiation from a cooler object categorically doesn’t increase the temperature of a warmer object.
The ONLY^3 reason RGHE theory even exists is to explain how the average surface (1.5 m above ground) temperature of 288 K/15 C (K-T balance 289 K/16 C) minus 255 K/-18C , the average surface (now ground) temperature w/o an atmosphere (Which is just completely BOGUS!) equals 33 C warmer w/ than w/o atmosphere.
That Δ33 C notion is absolute rubbish and when it flies into the nearest dumpster it hauls RGHE “theory” in right behind it.
The sooner that is realized and accepted the sooner all of us will have to find something better to do with our time and the taxpayers’ money. Maybe that’s what keeps RGHE staggering down the road.
The genesis of RGHE theory is the incorrect notion that the atmosphere warms the surface (and that is NOT the ground). Explaining the mechanism behind this erroneous notion demands some truly contorted physics, thermo and heat transfer, i.e. energy out of nowhere, cold to hot w/o work, perpetual motion.
Is space cold or hot? There are no molecules in space so our common definitions of hot/cold/heat/energy don’t apply.
The temperatures of objects in space, e.g. the Earth, Moon, space station, Mars, Venus, etc. are determined by the radiation flowing past them. In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
But an object’s albedo reflects away some of that energy and reduces that temperature.
The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.
In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
Looked out. Can’t see much irradiance. I guess you should integrate to get the average surface value which is about quarter of the 1368W/m². Temperature was consequently around 278K. I guess it were colder without some warm gases and precipitation between my front yard and the 2.7K background.
Average is nonsense and does nothing to tell us what is going on. Example. Take a photograph of something, say a nice mountain range. Add up all the colour pixel numbers and divide by the number of pixels, then apply that number to each pixel. What do you get? A gray image with nothing to see.
This is exactly what you are doing by applying the average. You add up all the W/m^2 for the area, divide by the number of areas, and apply that to all the areas. It’s BS grayness that has no physical counterpart.
“It tells me much more than the peak value misleadingly used above.”
Yes, using JUST a peak value is just as meaningless. You want a real view of energy absorption and loss on this complicated and dynamic earth? Then do a proper 3D graphic of a tilted rotating spherical planet with large oceans and disproportional land masses that have different absorption. That flat earth graphic tells us nothing of what it really going on, and if anything has grossly mislead people into thinking a simple “budget” explains things. It doesnt. That graphic was invented to justify the AGW dogma. Again, AGW exists because of deliberate ambiguity.
Hiro Kawabata
November 19, 2017 8:17 am
The claims in this article are unfortunately not even wrong.
IR absorption by CO2 excites the vibrational modes of a CO2 molecule.
As the CO2 molecules collide, the excited vibrational modes transfer energy to the motion of other CO2 molecules. The average kinetic energy of molecules is what we measure as the temperature of a gas.
Nothing to do with the absorption and emission of visible light by electron level transitions
A correct summary of the process may be found here
As a simulation subject matter expert, you can’t use MODTRAN and run a single look through an average air sample and get anything useful.
If you do not run it over a days cycle as conditions change, all you’re doing is fooling yourself.
The excited modes of CO2 do NOT transfer energy to the motion of other CO2 molecules – or do it only in less than 0.04% of cases. What happens when an excited CO2 molecule collides with an N2 or O2 molecule or an Argon atom is very complex and I have not seen a satisfactory analysis.
Paywalled. But according to the abstract they handle CO2-CO2 collisions. As a concentration of CO2 is currently 0.04%, these collisions are very rare. And how about CO2-H2O collisions?
No CG, they do. That is why the absorption spectrum is in BANDS and not a single exact wavelength. The band of wavelengths show the range in energy needed to bump the molecule into a vibrational state, and the difference is caused by the very slight decrease or increase in kinetic energy from molecular collisions.
Eric
November 19, 2017 8:18 am
Wouldn’t a simple experiment (already conducted by John Tyndall) learn that 15 micrometer IR radiation is emitted and absorbed at other temperatures as well?
The mistake was made with Tyndall, he was using a thermoelectric transducer, a thermopile: he discovered the thermo electric gases – we call them the GHGs. Watch to see what I have discovered https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
Driller43
November 19, 2017 8:19 am
i think the author misapplies his second key point “The temperature of a (gas) directly affects the wavelength of the radiation it emits and absorbs.” Looks like he applies it incorrectly. Yes, for CO2, most of the outgoing radiation is mostly from the stratosphere; and in the atmospheric window region is mostly from the lower troposphere. From link article which concludes “The total temperature-dependent gas absorptivity effect (H2O, CO2, etc.) tends to result in a radiation energy convergence that decreases the total cooling of the atmosphere.” https://www.gfdl.noaa.gov/bibliography/related_files/yih0702.pdf
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
I never heard that about “too hot to exist”, and I don’t believe it.
I guess that he means that those bands are unimportant because they are at wavelengths where the Earth emits virtually no radiation at realistic ground temperatures. I agree that it is a very clumsy way of expressing this
Willis, you are a stickler for quoiting correctly when rebutting. I would like to point out the important qualifier that you are missing in your quote reference. He specifically said “to hot to exist in the atmosphere“. A distinction that is pertinent to his point..
Willis
The article is a mixture of facts and fallacies. The author is confused by a lot of ‘information’ gathered from various sources. Some of the bloggers are in the same boat. Too many things to correct so I just shut my mouth. Nevertheless, it’s entertaining to read some of the well-meaning comments.
Willis, re: “too hot to exist”. Rod Gill is confusing de-excitation radiation from the CO2 molecule with thermal radiation as described by Wien’s Law. That’s why he claims the CO2 emission bands at 2.7 and 4.3 microns only come from CO2 molecules at temperatures too hot to be found in the atmosphere (800° C and 400° C, respectively, if calculated from Wien’s Law). That’s also behind his ludicrous claim that CO2’s 15 micron emission band comes only from gas at -80° C.
Rod Gill fundamentally misunderstands the physics behind the so-called “greenhouse effect”, and he has no business publishing an article about it. I would have preferred that Anthony or one of his volunteers gently point that out to him and not publish such pseudoscience.
Of course the comments reveal that such confusion is fairly common, but we already knew that.
Willis,
Agreed, the first confusion is in assuming atomic excitation instead of molecular. Then electron states instead of rotational, vibrational, etc molecular states. Several other errors follow as a consequence.
However, I think the exercise is useful because it’s responses can correct those readers who were similarly starting from the same wrong point.
I tend to shut up because when my formal training in the 1960s was done, atomic was dominant and molecular seldom covered in lectures other than to say that molecular spectroscopy was a harder concept. Geoff
Willis, you are a stickler for quoiting correctly when rebutting. I would like to point out the important qualifier that you are missing in your quote reference. He specifically said “to hot to exist in the atmosphere“. A distinction that is pertinent to his point..
His claim is not true, whether he is talking about the atmosphere or the earth’s surface. So no, his qualifier is not important.
Which claim are you referring to? You are not being clear. “In the atmosphere” is definitely an important qualifier because he is talking about how the atmosphere actually operates and the range in which these interactions occur. You saying it isn’t does not make it so.
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
I’d love to invite you into a cave in a glacier sometime.
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
I’m sorry to hear that. Had you continued, and considered what I was saying, you might have learned something.
In the meantime, have you never been outside on a winter night when clouds came over? They leave the surface warmer than when there are no clouds. Clear winter nights are the coldest … why?
Because when there are no clouds, the back radiation is coming much more from outer space at a temperature of 3K or so, whereas the clouds are radiating at something like 225K …
Think about it. If a planet has no GHG atmosphere, it is exposed directly to outer space.
But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.
I’m not sure why this is so hard for some folks to grasp, but I assure you that it is settled science taught in the universities and backed up by the math. If you are interested in the math, there is an online calculator here. If you play with it for a while it may help you start to understand the nature of two-way radiative energy exchange … it spells out the formula for how it is calculated.
Willis, you are mixing apples and oranges. The clouds have a higher temperature than the rest of the atmosphere because of the latent heat of condensation being released. This is a phase change of water effect, not a GHG effect.
Secondly, your sentence
‘But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.’
is obvious and there is no need to shout. Everyone agrees that the atmosphere warms the earth compared to no atmosphere. The point at issue is does replacing a few O2 molecules with a few CO2 molecules make a difference?
Willis, you are mixing apples and oranges. The clouds have a higher temperature than the rest of the atmosphere because of the latent heat of condensation being released. This is a phase change of water effect, not a GHG effect.
The claim was that the cold atmosphere (containing cold clouds) could not leave the surface warmer than it would be without them, viz:
wickedwenchfan November 20, 2017 at 2:51 pm Edit
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
Since both the atmosphere and the clouds are colder than the surface, I am NOT mixing apples and oranges.
Secondly, your sentence
‘But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.’
is obvious and there is no need to shout. Everyone agrees that the atmosphere warms the earth compared to no atmosphere. The point at issue is does replacing a few O2 molecules with a few CO2 molecules make a difference?
No, “everyone” does NOT agree that the cold atmosphere warms the earth compared to no atmosphere. There are literally dozens of people who claim that it is impossible. They all say that because the atmosphere is colder than the earth, the atmosphere does not and cannot “warm the earth compared to no atmosphere”.
Which is why I was shouting … I’m getting tired of people saying the same thing over and over, when the answer is there from our own senses regarding winter clouds.
w.
PS—Can a block of ice leave a person warmer than no block of ice?
Sure … as long as the block of ice (at 0°C) is interposed between that person and a tank of liquid nitrogen (at -196°C). The ice provides more radiation to the person’s body than does the liquid nitrogen, so the person ends up warmer.
And the same is true about icy clouds which are interposed between a person and 3K background temperature of space … they leave the person warmer than no clouds.
Ryddegutt
November 19, 2017 8:26 am
CO2 molecules can absorb radiation and then transfer that energy to other gasses by conduction and versa visa.
At atmospheric pressure the conduction takes place with molecular collisions that theoretically occur faster than a CO2 molecule can radiate its heat — 10^-7 s.
It is pressure dependent as rgbatduke always said. But I don’t really know how this affects – probably surface CO2 absorbs only, and does not have time to emit, so the air eats the radiation band. And then complicated (parameterized) things happen, like evaporation, convection. I’m sure the problem is not high school physics as Gore said. He lied.
3) A Chinook demonstrates the effect of compression directly. Ne c’est pas?
4) This idea also satisfies Dr. Svalgaards assertion that TSI variance is too small to account for the variations of earth’s temperature.
5) One has to admit that the name “Greenhouse” in no way is descriptive of our atmosphere or how it works.
6) No matter how our atmosphere works, we are fortunate that the relative stability of temperature is within the range of water existing in all three states. As a carbon based life-form, I am grateful of this.
7) Ockham’s razor and plausibility Apply liberally, rinse, repeat.
( numbered for your rebuttal convenience and clarity, also inspected for politically free content )
Jer0me
November 19, 2017 at 1:10 pm: But in our air, they are struck kinetically thousands of times between each possible emission. Thusly, air eats radiation so convection and phase change of water dominates hugely to above the net emission height. Radiation is swamped by water anyway, which even Mann knows. It is all sleight of pen for the cause. Bring on the State Pen..
Rod, thanks for your post. However, you ask us “Am I right?” about the following:
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
Nope. You are wrong. However, given the depth of your misunderstanding, and your certainty that you understand it, I’m gonna leave the question of WHY you are wrong for you to work out for yourself.
Show us a reference for transfer of energy from co2 at lower temperature to a solid warmer body, in a controlled environment. If you can’t, there is no transfer.
I asked for a reference, not you making stuff up.
Fact: at equal temperature, no transfer. So why would there be transfer from cold to hot if one body is colder.
Show me a reference for any transfer outside of “net”, or shut up.
LdB, I’m not getting your point. Are you saying the CO2 laser beam CAN’T cut metal? Maybe you’re saying the laser cuts metal by COOLING it? Help me out here.
It would help if you actually understood “classical physics”. Then you wouldn’t be so confused and would understand the difference between cutting with a laser beam and laser cooling.
@Gary Young Hladik
The problem I was illustrating is even things we think of with one behaviour don’t always behave like that, you are saying the beam will always thermally act one way. That beam as it moves thru the air is a electromagnetic wave whether it heats or cools or even has any effect (think a mirror) is governed by a set of strange laws.
If that laser is a CO2 cutter and I replace the steel with aluminium, I assume you know what happens.
Try even cutting a very fine steel mesh 🙂
It’s a EM transfer you can’t equate it to thermal energy, you can heat,cool or reflect it.
Oh and for anyone who wants to know how it works.
It’s pretty straight forward you need 6 or more beams to do cooling, the frequency used matches one of the resonant frequencies of the atom you are trying to cool and it doesn’t actually see the beam as hot because it absorbs it and then re-emits it.
@ur momisugly Gary Hladik
Yes I know, I was just firing a warning shot … yes but be careful.
If that metal is aluminium for example it will reflect and if the metal has a resonant frequency at that frequency it can be transparent or cool the metal.
@Gabro
No you are claiming there are cool lasers ™, you directly claimed it.
Yes, there are many differences between cutting and cooling lasers.
Now you obviously bothered to read and are now trying to dig yourself out of a hole, there is no such thing as a cool laser ™ they are all the same.
As I have already stated the laser can heat, cool, reflect, absorb or transmit thru any material which strangely enough is the same for every single EM frequency that exists including radio waves and thermal emissions. QM is very concise and very well tested on all this stuff/.
I like to use the analogy of an Eskimo igloo, where the snow, forming a temporary barrier, captures the heat inside the igloo long enough to maintain a comfortable temperature.
Which of course is an incorrect analogy as the igloo is a physical barrier to convection, which is how the surface loses most of the heat. It has zero to do with radiation effects.
CO2 is a physical barrier to some radiation. It is an analogy, not saying ice chunk is a greenhouse gas. Of course, ice IS a weak barrier to radiation and it does contribute to how igloo warms.
Jer0me,
You can heat a clear container of CO2 by radiation as from a laser beam of appropriate wavelength or a beam of sunlight passing through a small part of the wall. If the beam is turned off, the hot CO2 will heat the rest of the container wall as the CO2 cools by normal heat transfer equations. Is this at odds with what you mean? Geoff
joelobryan
November 19, 2017 at 10:09 am: You almost got there. The crooks know CO2 is too weak (at best, in fact it is a coolant). So they concocted an effect on water vapour to magnify things. This is on record. Other planets and large moons prove CO2 is not special except for life. We have that on record too.
A waterless planet with no tri or polyatomic gases, eg all Nitrogen, would still warm by conduction as we do. Conduction remains the dominant first mover, unless WV latent heat beats it on Earth, micro555? The emission from kinetic motion in the molecular force fields would still cool it. What is debateable is how far such an atmosphere has to expand outwards to radiate enough( by the Gas laws, which do indeed rule). Larger planets should somewhere reach sufficient gravity to keep hold of more gas than their net losses through what is really evaporation.
In infinity, I am fairly sure that such experiments are in progress.
The day you realize that you can warm yourself by standing in front of a block of (water) ice instead of a block of dry ice, or a tank of liquid nitrogen, is the day you get to be taken seriously, WWF.
So Andy: Suppose I, as an evil GHE believer, have you tied down to a big block of ice at 0C in a room at 25C. After a while, you get hypothermic and your body temperature drops to 35C.
I now offer to let you move to a big block of slate rock (which has the same thermal conductivity) at 25C in the same room instead, saying it could help you to increase your body temperature back to 37C.
By your logic, you would refuse, because the slate rock is still below your body temperature, so the change couldn’t possibly lead to an increase in your body temperature.
Now, if you were dead, that would be true. (You may be brain dead, but…)
Tom13 - the non climate scientist
November 19, 2017 8:29 am
Oxygen and Nitrogen are not “greenhouse Gases”
Yet dont they serve some function of retaining heat and maintaining some balance of temp in the atmosphere.
Otherwise, it would seem that the total volume of Greenhouse gases is vastly too small (1%ish not counting water vapor) to retain any quanity of heat
Hard to fathom from a scientific point of view that CO2 or any of the other greenhouses gases are sufficiently powerful to act as the thermostat to the degree which they are credited.
O2 and N2 certainly have molecular heat capacity. That is not the issue here. As diatomic molecules with limited vibrational modes, they only absorb in the UV band. Triatomic and higher molecules like CO2, H2O, O3, CH4, NO2 are the GHG’s because they have vibrational modes that allow absorption of IR wavelength photons.
But in terms of IR electromagnetic radiation at the temps under consideration for Earth’s atmosphere and surface temps, the three main gases, that is O2, N2, and Argon (that’s 99.5% of dry air), are transparent to IR wavelengths. Thus those 3 main components of dry air cannot absorb IR photons to warm. They are transparent to IR. Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar. But the GHE in the atmosphere also depends on the adiabatic lapse rate of the troposphere, which exists because pressure drops with altitude (gases are highly compressible of course). This temperature gradient creates an effective radiation level (ERL) where a point (vertically, a temperature profile) is reached where more photons escape to space than are re-radiated toward back toward the surface.
So now think of very dry desert air, how large a temperature swing there is from day to night. During the day, there is visible light and UV heating both the minimal water vapor and the surface. The air layer near the hot surface warms due to convective transfer. At low viewing angles, the shimmering desert convective heat flow is seen as a mirage because refracts the sunlights. But in a dry desert, with little water vapor, after the sun sets, the air cools rapidly, losing several degrees/hour because their is little vapor to help trap the escaping IR wavelength photons. That’s radiative cooling. The other non-condensing GHGs (CO2 in the troposphere, ozone in the stratosphere) help slow the cooling. This is why GHG theory predicts night-time lows will get warmer under increasing CO2 while having little measurable effect on day-time high temps.
The above descriptive physics is why the GCM outputs “predict” a tropospheric hotspot in the tropical latitudes.
And going from 3 CO2 molecules per 10,000 air molecules (0.03%) to 4 or 5 CO2 molecules per 10,000 air (0.04% to 0.05%) makes a negligible contribution to the trapping (back radiation) when the atmosphere (troposphere) contains a much higher molar quantity of (1% – 3%) H2O gas. If the atmosphere were completely dry then CO2 concentration changes would make a measurable impact on heat trapping.
This last fact is likely why the Tropospheric Hotspot in the tropics has not been observed. The GHE of CO2 is too weak to be detectable. This argues for the modification of the CO2 forcing theory from one of a strong GHE effect to a weak GHE effect for CO2. The evidence points to a weak GHE hypothesis for CO2. And that is something the current-day climateers, who are profiting from global warming alarmism, are loathe to admit.
Joel, the lapse rate is determined only by gravity and gas physical properties, it has no input or output from radiation. I don’t believe that the lapse rate creates the apparent radiation level, this depends only on the OLR magnitude and the surface area required to radiate it at the effective radiating temperature (-18C).
Surely there is no “trapping” of radiation by GHG’s?
Taking the relative humidity vs ghg analogy with deserts a step further –
the average relative humidity of a typical desert ranges from 10%-30% (various sources) while the ypical countryside ranges from 50-90%. the delta of the day/night temp swings between deserts vs countrysides is in the range of 20-30 degress. With the concentration of water vapor being 200-400x the concentration of co2, it would seem that an increase of 150ppm would be less than 1-2% of the effect that water vapor would have when you compare desert vs countryside.
Rh only matters when air temps are near dew point, that’s when it stops cooling.
Even though humidity is low in the desert, clear night min temp should be near dew point.
“Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar.”
No. The sink of heat energy is the oceans. They warm the atmosphere by evaporation. that is how the water vapor gets there. Confirmation sip a pina colada at a sea side bar at night. Try the same thing in the desert.
But in a dry desert, with little water vapor, after the sun sets, the air cools rapidly, losing several degrees/hour because their is little vapor to help trap the escaping IR wavelength photons. That’s radiative cooling. The other non-condensing GHGs (CO2 in the troposphere, ozone in the stratosphere) help slow the cooling. This is why GHG theory predicts night-time lows will get warmer under increasing CO2 while having little measurable effect on day-time high temps
It cools 3-4°F/hr in ohio at first too. It just stops at dew point, and the daily range is much higher in deserts.
These are both clear calm nights.
Good point top: if N2 and O2 do not emit or absorb IR, this is a contradiction to QM and thermodynamics.
They do radiate, they are GHGs and here how: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere.
The 15um radiation doesn’t warm anything, the sun at 500k does. All the 15um does is cancel out the 15um radiation coming up from the surface. The sun makes up the difference. Hence the atmosphere warms up a little.
That’s the theory, whether it happens or not or can actually be detected or not is the issue.
Peta of Newark
November 19, 2017 8:39 am
Quality not quantity.
My thinking for years now.
Sometimes called the Ultraviolet Catastrophe and the Perfect Example is solar panels.
(The UV Catastrophe was the title of a BBC Horizon program explaining a time when scientists couldn’t explain certain ‘wavelength’ or colour sensitive phenomena.)
Just exactly why do solar panels need direct (or reflected) sunlight?
Peta of Newark
November 19, 2017 at 8:39 am: We had a paper by warmist students a while back proposing panels to harvest the background radiation of Earthly IR return. Very excited they were at all that amperage (equalling solar input, after all). Only the sound of crickets since then, as they seek the voltage, I guess.
That pretty well explains the AGW myth, really. Magic and magic mushrooms.
The relationship stated in the quantum physics of electron orbitals and any relationship to temperature due to molecular kinetic energy is wrong. Dead wrong.
Sort of kills any value to discuss your other points when such a fundamental error is made.
a point nobody has tried to explain, the earth radiates the LW towards space because space is much cooler than the earth, what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? after co2 releases the tiny portion of the wave it grabbed for a picosecond again isnt a force REQUIRED to send in back towards earth?
It is perfectly possible for a cold molecule to emit a photon to be absorbed by a hotter one. But the flow of heat which is a statistical phenomenon will always go from warmer to colder (except for very small random fluctuations).
“what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? ”
Simply the ‘force’ of radiative exchange. Just because something is colder does not stop it radiating to something hotter. It just means that the net flow is from warm to cold. All things at temperature above 0K radiate. The atmosphere radiates, it just so happens that it is mostly transparent to LWIR (N2 +O2 = ~ 99% and there is an atmospheric window at around 12 micron for the GHGs – see graph) and at the Earth’s temp, 15 micron is the radiation it most strongly emits at. … which CO2 also happens to absorb/radiate most strongly at (just a bit more than H2O).
It comes back because the Earth’s surface is partially in the way of a measure of photons re-emitted. In the mean time (during daylight) shortwave has come in. It’s that extra SW that is causing the GHE.
The ‘delay’ in emission is due to the path-length of a photon through the trop and why it is far from saturated. At around 8km the molecular concentration falls off enough to allow most LWIR to get to space and that level is at the temp the Earth shows from space (255K) whilst here on the surface we live at it is 288K. So at that temp CO2 emits less strongly (being colder), yet it can still absorb at the same rate.
So molecules high up still radiate as normal but they are receiving less because of ‘blockage’ below – this causes stratospheric cooling while the trop warms.
“Net” flow of heat from warm to cold confuses the issue. the correct term is the only flow of heat is from warm to cold, surely there can be no actual flow of heat from cold to warm regardless of radiation flow if the radiation flow can’t be thermalized rather than reflected or transmitted.
In the sense than photons from the cold object impinge on the warm object there is ‘flow’, but the net flow is always from warm to cold unless work is done.
This is the fumdamental mistake made when people say “a cold object cannot heat a warm one”. Correct it cant – but it does add to its entropy and slow its cooling.
“The Second Law of Thermodynamics can be rephrased in several ways. Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction). It can also be expressed using the concept of entropy as saying that the system’s entropy will always naturally increase if no work is exerted to decrease it. These rephrasings mean fundamentally the same thing because heat deals with kinetic energy and increasing a system’s kinetic energy will increase the system’s entropy.”
A thought experiment. You have two flat metal plates somewhere out in space a foot apart. One is at 1000 degrees the other is at 500 degrees. Heat flows only in one direction, from the hotter to the colder plate. Now remove the colder plate. The hotter plate will cool down faster than before, despite that there was no heat flow to it from the colder plate.
>>
Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction).
<<
Not exactly. The Second Law is often stated as:
or the change in entropy for an isolated system not in equilibrium must increase until it reaches equilibrium. Not all systems are isolated. Entropy can decrease in a closed system or an open system. The Earth is usually modeled as a closed system, but it isn’t truly closed. Closed systems don’t allow mass transfer across the system boundary. Isolated systems allow neither mass nor energy transfer across the system boundary. Open systems allow both mass and energy transfer across the system boundary.
The Second Law for a closed system is:
where Q is a path variable and the equation is only valid for reversible processes (this is often stated as the Clausius definition of entropy). Notice that entropy has the same units as heat capacity or energy per unit temperature and in SI units: joules per Kelvin.
Since I mentioned Heat capacity, it is defined as:
So, in an isolated system, a colder object (say a black body) can transfer heat to a warmer object (say another black body) as long as the warmer object transfers more heat to the colder object–simple thermodynamics, and it doesn’t violate the Second Law.
Here is a picture of what natural gas does to IR.
.
.
Note how it blocks it out. Same thing happens to IR with CO2, but not to the same extent. If you were in orbit around the earth, and you “took a picture” in the IR band, CO2 would “darken” the image you get. This is how it “blocks” the IR escaping the planet.
This seems to demonstrate that we are wrong about the CO2 absorption range. A flame is putting out a frequency of well above 15u. But since no thermometer was inside the tube there is no demonstration that there was an increase in temperature.
The problem with his little demonstration is the candles flame is +500 degrees hotter than my shrubs, or the co2 in the atm.
This is propaganda. He knows better than this, that this isn’t really applicable to the problem that exists. He just had to sensationalize it to make an effect, and that’s what’s wrong with the consensus on AGW.
That’s still the candle video.
Besides the point, they filled it with 100% co2.
And so what, there’s an equal negative water feedback to the increases in co2 warming if there is any.
What you say about CO2 darkening the Earth viewed from space in the 15 um band is true. The informed, educated skeptic recognizes CO2 is GHG. But most of your darkening is due to water vapor that is unavoidably present on our 71% water world planet.
The real question for the informed, educated skeptic (that the alarmists avoid) though is is there appreciable (measurable) darkening when CO2 concentration goes from 3 CO2 molecules/10,000 air molecules to 6 CO2/10,000 air molecules. The 2 facts – 1) that the tropospheric hotspot has not been observed, and 2) that models are running too hot compared to 25+ years observation strongly suggests the CO2 strong GHE theory must be modified to a weak GHE theory.
But without a strong CO2 GHE theory, the alarmist argument collapses. That would mean the entire politicized Climate Change and the trillions of dollars hoped to be re-distrubuted vanishes as well.
Note two things in the picture. First the black asphalt roads are very “bright.” This picture is taken in the IR bands, not in the visible bands. The asphalt is “bright” because it is HOT. Also, natural gas does not block visible light, and the plume you are looking at is the natural gas escaping from an underground storage facility in California.
A good visualization on the effect The surface IR is absorbed into air which then does not re-emit it quickly at a wavelength visible in the picture. But, this is different from CO2 in multiple ways.
Satellites, often looking down from about 70km, see earth radiation at many different temperatures. You can’t just take a 15C/289K Planck curve and say, “hey, this only radiates at 10 microns”. Low energy IR radiation does not usually cause “electronic” transitions, that is bumping electrons to higher orbits. IR typically causes rotational and vibrational transitions. The significant CO2 transitions and their “bites” out of the spectrum are shown below. Their radiative temperatures are all different.
The 15um/667.4 fundamental bend (vibration) is seen radiating at a peak temperature of nearly 250K, and the surrounding rotations radiate at and conform to the 220K Planck curve.
The fundamental problem is we don’t live in a purely radiative world, as Bill and others have pointed out, collisional/conductive and convective interactions with the other (largely IR transparent) .996 of the atmosphere modulate the effective radiative temperatures. CO2 has a very low emissivity (tendency to emit photons). It prefers to dance.
Whatever you wish to call the disturbances in the electromagnetic/weak field, photons, or something we can detect as particle like, definitely exist; even though they have no mass. They do not control the earth’s energy balance alone.
“They do not control the earth’s energy balance alone.”
In a way they do, because the heat always finally leaves the Earth TOA as photons. But they do not alone control, or even dominate the heat balance of the surface of the Earth. Convection does.
In the summer, looking across an asphalt surface at low incidence angle over a long distance (long optical path length path length), one see the shimmer. That shimmering (mirage as it is called) is of course refraction due to rapidly warmed boundary-layer air rising from the hot black asphalt surface. The asphalt is dry. The air is mostly dry. Water vapor is present, it warms too. But the vast bulk of the heat transfer from a dry surface in this case is conduction at the boundary where air molecules collide with the hot asphalt molecules.
True, though part of the heating of the air is also due to LWIR emitted by the asphalt and absorbed by the air. And conduction is only important for the boundary and a very thin air layer because of the very low thermal conductivity of the air.
tty
November 19, 2017 at 9:49 am: Agreed tty. Next we have to get it that heat, being a process caused by Kinetic Energy, is what results in radiation. Radiation is not heat and only relatively weakly results in it if it interacts with matter in a kinetic manner. Another basis for warmist waffle.
Yes, my sloppy. tty has pointed this out. I should have said radiation does not alone control temperature between the surface and the effective radiative altitude.
Of course, it is actually far more complicated. The solar wind is incoming energy, but it is not EM radiation. Likewise, ions stripped from the earth’s atmosphere, reputedly as heavy as Oxygen, count as energy out.
Schrodinger's Cat
November 19, 2017 9:54 am
I have some doubts about some of your statements. Carbon dioxide does not undergo changes in electron levels as far as I know. Also, I’m not sure if the altitude of an electron from the nucleus is quite correct either. I think these are visualisations of differences in energy. However in the case of CO2, the extra energy is not sufficient to do any of that. It simply changes the vibrational energy. CO2 has four vibrational modes and the amplitude of the vibration is what changes on excitation.
I don’t think these change the the substance of your main claim, but since I am still grappling with that. I cannot comment either way. There is certainly something seriously wrong with the settled science.
As a footnote to Anthony, it may be a contentious topic but it is absolutely fundamental to the debate. The principles of spectroscopy are well known but when transferred to an atmospheric context there are masses of uncertainties to be resolved. This is where pseudo scientific assumptions and cherry picking of facts can support a credible argument on either side. Debates like this make gradual progress in revealing better insight. Thank you for posting this one.
ferdberple
November 19, 2017 10:00 am
under GHG theory the temperature of the earth would be unchanged if all the O2 and N2 was removed.
this makes no sense. the atmosphere has thermal inertia which would be dramatically reduced without O2 and N2. Nowhere is this accounted for.
So what would the earth be like without an atmosphere?
The average solar constant is 1,368 W/m^2 with an S-B BB temperature of 394 K or 17 C higher than the boiling point of water under sea level atmospheric pressure, which would no longer exist. The oceans would boil away removing the giga-tons of pressure that keeps the molten core in place. The molten core would push through the floor flooding the surface with dark magma changing both emissivity and albedo. With no atmosphere a steady rain of meteorites would pulverize the surface to dust same as the moon. The earth would be much like the moon with a similar albedo (0.12) and large swings in surface temperature from lit to dark sides. No clouds, no vegetation, no snow, no ice a completely different albedo, certainly not the current 30%. No molecules means no convection, conduction, latent energy and surface absorption/radiation would be anybody’s guess. Whatever the conditions of the earth would be without an atmosphere, it is most certainly NOT 240 W/m^2 and 255K.
The alleged 33C difference is between a) an average surface temperature composed of thousands of WAGs that must be +/- entire degrees and b) a theoretical temperature calculation 100 km (32 km) away that cannot even be measured (no molecules to compare) and c) all with an intact and fully functioning atmosphere.
The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.
“The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.”
Sorry, but that is not at all how it works. Hint: check the thermal conductivity of air (it is about the same as polyurethane foam) and calculate the temperature gradient needed to move 168 Wm^-2 through, say, 10 kilometers of polyurethane foam.
Also how do you explain that the temperature reaches a minimum at the tropopause and then starts increasing again?
The dominant heat-transfer mechanism in the atmosphere is convection and the lapse-rate is simply the amount of energy needed to drive the convection.
GHG theory predicts the tropospheric hotspot. This has failed to emerge.
in science a single failed prediction is absolute proof of a failed theory.
however, trying to prove why GHG theory failed is largely a fools errand, because we have no replacement theory.
for example. we know that Newtonian gravity fails to predict rotational speed of gravity. however, we cannot. explain why because we have no alternative theory. instead we are left to invent a new form of matter. dark matter that obeys some laws but not others. and then we postulate that the universe is 90% dark matter. this is GHG theory mistakes on a cosmic scale.
You are too lazy to follow the link I gave you,you also didn’t answer my question about the “evidence” in YOUR link.
“The researchers instead used observations and combined two well-known techniques—linear regression and Kriging”
Ha ha ha ha ha……..
You can’t be THAT stupid.
Meanwhile a rebuttal was posted on it:
Desperation — who needs thermometers? Sherwood finds missing hot spot with homogenized “wind” data
“The fingerprint test of the water-vapor feedback is the “hot-spot”, a warming of a band of the upper troposphere 10 km over the tropics. (See the reasons below at the end). The weather balloons were designed and calibrated to measure temperature and humidity as they rise through the sky and right through the hot-spot. Their results are unequivocal: red was not yellow; the spot was not hotter. Supporting this, the specific humidity was also supposed to rise, but fell instead. If the computer models worked on everything else, we might wonder if the millions of observations were biased, but the models didn’t predict the pause, were wrong about humidity, rainfall, drought, and clouds too. They didn’t work on regional, local, or continental scales and can’t explain long term historic climate either. At this point, a scientist would throw out the theory. The weather balloons independently agreed with each other, the humidity results fitted the temperature results, the whole lot was loosely supported by satellites. The data doesn’t need homogenising or kriging or obscure numerical witchcraft.
Instead Steven Sherwood and Nidhi Nishant of UNSW revisited their 2008 technique of homogenizing temperature data by using wind data as well. They homogenised it again. They have iterated the iteration? They’ve also extended it from 2005 to 2013 and changed the “wind shear” component to “vector wind”. Their new homogenized-temp-wind data is below (left). The model predictions of 2005 are centre, and the radiosonde temperature results (before homogenisation etc) are on the right.”
Layman’s interpretation of what you have just posted.
A scientific theory doesn’t work because something is missing. It’s not known what’s missing, but introducing an unknown makes the original theory work.
No wonder I abandoned science at secondary school. It’s like playing a game of chess, with a million pieces. Nothing is ever solved, just continually shuffled around with everyone having an opinion on which way to move a single pawn, that doesn’t exist.
RGHE/GHG theory assumes the earth is warmer w/ than w/o atmosphere. Attempts to explain this assumption are futile because it is fundamentally incorrect.
The atmospheric insulation blanket creates a thermal resistance (all 32km worth of molecules that matter) just like an electrical resistance or hydraulic resistance. In all three instances a potential difference is required to move energy: dT or dV or dH, from A to B. For heat flow it is: Q = U A dT where U, aka 1/resistance, is the combined effect of conduction, convection, latent and radiative properties together with wind, storms, lit side, dark side, etc. everything that impedes thermal movement from surface to ToA where 100% radiation takes over. (No molecules in space to take the energy handoff.)
The atmosphere is little more than a fundamental HVAC problem.
RGHE theory says the ground would get cold w/o “back” downwelling radiation. Reams of USCRN data show absolutely zero evidence of this.
The sun warms it all. Air temperature swings widely because of its low thermal capacity. The 2cm ground temperature swings very little because of its high thermal capacity. The 100 cm ground temperature hardly changes.
All night and in winter the air temperature spends many hours below ground temperature so the ground heats the air, NOT vice versa.
RGHE theory simply doesn’t stand up to physical observations. All the rest of the discussion is sound and fury signifying nothing.
However the resistance is gravitational, not thermal, since the dominating heat transfer mechanism is convection. The atmosphere has such low thermal conductivity that conduction is uttery insignificant. It is no coincidence that almost all insulating materials are mostly air.
Essentially correct. Warm air has lower density and therefore rises. As it rises it expands and cools down. Ultimately it will come down somewhere else, outside the convection cell.
“GHG theory predicts the tropospheric hotspot. This has failed to emerge.”
It is also the theory in any sort of warming. It is merely a function of greater LH release aloft in the tropical high atmosphere via convection.
One thing – it ois difficult to find because of the nature of the instruments used. Radiosondes are imprecise for the job and have changed over the years, and sat obs are contaminated by Stratospheric cooling – which is a function of GHG theory.
Another thing is that it has been found …
“First, tropical warming is equally strong over both the 1959–2012 and 1979–2012 periods, increasing smoothly and almost moist-adiabatically from the surface (where it is roughly 0.14 K/decade) to 300 hPa (where it is about 0.25 K/decade over both periods), a pattern very close to that in climate model predictions. ”
It is hard to know where to begin with such a complete pile of nonsense. One of the more obvious errors is confusing a black body radiation spectrum with discrete absorption lines due to molecular transitions. Then there is the weird description of electron orbitals which do not make any sense. If there was any attempt at quality control on this blog this post would have been thrown into the trash where it belongs.
I think the main problem is that the author seems to be completely unaware that the energy levels of a molecule are a lot more complex than for a single atom.
Germonio
November 19, 2017 at 10:42 am: Once again, explain not excoriate. Show how you are more knowledgeable, not just something else…..
ferdberple
November 19, 2017 10:55 am
radiation works by the exchange of photons. convection works by the exchange of virtual photons. thus if there is a GHG effect for co2 there must be the same effect for n2.
co2 absorbs a photon from the surface. this can be radiated up or down. n2 absorbs a virtual photon from the surface. this can be conducted up or down.
in all aspects the GHG effect operates like conduction at a distance, except that co2 can radiate to space while n2 cannot. thus co2 cools the surface on net as compared to n2.
Ferd,
I would suggest you need a dictionary. Convection refers to the movement of hot molecules from one place to another. It has nothing to do with virtual photons.
Uncle Gus
November 19, 2017 11:01 am
Fascinating!
I’m learning here just how little I know about gas absorbtion and emmission phenomena.
I’m also learning how much utter bilge can be written by someone who knows *nothing* about such things, but is willing to shoot their mouth off endlessly. And getting a fair idea of how difficult it must be for a relative layman to tell the difference.
For the record, Gill is wrong, but he’s at least honest. It would be nice if someone took him through his presentation point by point and explained (gently) where he went wrong. I’d do it, but, as I hinted, I’m not best qualified.
(Incidentally, I asked for something like this debate weeks ago – it’s good to see it happening. Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.)
Uncle Gus, why the rant after saying this: |… I’m not best qualified.”? But, you feel yourself qualified to claim: “Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.” I bet you don’t even understand your own hypocrisy.
“Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming.”
no.
the downwelling LWIR is a RESULT of GHGs ( like water) it is not the cause of warming.
When people “popularize” the science and dumb it down, they say LWIR causes the warming.
nope.
When you increase c02 two things happens.
1. the stratosphere cools. We have seen this. Its How we know the warming is GHG related and not the
sun.
2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.
a) radiating from a colder place entails a slow down in the cooling to space.
b) to maintain energy balance, the compensating increase in warmth at the surface has three
pathways:
1. it can go into the ocean
2. it can melt ice
3. it can warm the air.
Because in the stratosphere radiation dominates over convection, so more GHG increases the radiative energy loss. The temperature gradient is also the opposite – it gets warmer with altitude, since the main energy source is UV absorption in the ozone layer from which temperature decreases both upwards and downwards.
Even more explaining needed of the temperature changes as altitude increases:
In the Troposphere, temperature falls with altitude but, above the Troposphere comes the Stratosphere where temperatures increase with altitude but, above the Stratosphere comes the Mesosphere where temperatures fall again with altitude but, above the Mesosphere comes the Thermosphere where temperatures go up again with altitude!
Given the differences of opinion on this thread, I would love to hear everyone’s theories as to why all these temperature changes happen? 🙂
“2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.”
So you mean that when the temperature at the surface goes up the average temperature of the Earth seen from space will go down? That the temperature profile of the atmosphere is somehow unchangeable?
What does happen is that with more CO2 the average altitude from which the heat radiates out will rise slightly. All things equal this means that the surface temperature will also rise slightly, since more energy is needed to lift the convecting air.
However all things aren’t equal. Higher temperature means more evaporation and more H2O in the atmosphere More H2O in the atmosphere means a less steep lapse rate, i e a smaller temperature difference between the surface and a specific altitude. There will also be albedo effects (=clouds, snow, vegetation).
All things considered more CO2 in the atmosphere does seem likely to result in some warming, but the effect is probably quite slight.
“1. it can go into the ocean
2. it can melt ice
3. it can warm the air.”
NONE of which is happening.
1. Oceans are currently cooling, especially Southern Ocean
2. Only ice melt is down from the Highest level since the LIA (late 1970s)
Arctic sea ice is still in the top 10% of extent wrt the last 10,000 years
Purely in line with the approx 60 year AMO cycle
3. No warming in satellite data apart from ocean events.. ie release of energy from the oceans at El Nino events.
You are still selling moldy lemons , Mosh. Useless even as a car salesman.
No, if the magnitude of the effect cannot be calculated, then, Stephen Mosher has admitted that he, and BEST, are simple record-keepers, unable to predict Climate Sensitivity, and have also admitted, no one else can either.
No one can. From first principles, which means from the laws of Physics which have nothing to do with dodgy thermometer records, we cannot calculate Climate Sensitivity. Think about this.
Most people do not understand that the entire atmosphere radiates to space, including N2, O2, Argon, and the entire mass of the Atmosphere. All Matter above Absolute Zero radiates all the time.
Hotter Matter radiates to the Absolute Zero of Outer Space, which could maybe be a small fraction of a degree Kelvin above Absolute Zero, More than cooler matter.
There is this word Opaque. This word means No Radiation Penetrates. The Top of the Atmosphere radiates to space at a temperature far above Absolute Zero, much closer to the temperature at which CO2 has ceased to be opaque to the radiation it can absorb at its best absorption frequency, 15 Microns, corresponding to around -80 C.
More CO2 raises the altitude at which this happens. Due to the Lapse Rate, the higher the altitude at which the atmosphere no longer encounters an opaque layer, the cooler the temperature becomes at which the Entire Atmosphere can radiate to space. Heat Transfer is proportional to the Delta T, the difference in temperature between hotter and cooler.
More CO2 reduces the atmosphere’s flux to space, increasing the amount of heat retained in the atmosphere, warming the air at the surface, but no one can calculate how much.
More CO2 reduces the atmosphere’s flux to space, increasing the amount of heat retained in the atmosphere, warming the air at the surface, but no one can calculate how much.
Not much, because water compensates for the increase.
Steven – an honest question to answer please. If the average altitude at which th e Earth radiates to space is raised surely the radiating surface is also increased precisely because it is a now a larger spherical surface. Is that taken into account?
It’s easy to do the math. Let’s say, the “emission level” is increased by 100 meters (0.1km). The radius is increased from 6400km to 6400.1km, a 0.0015% increase. So the surface area is increased by 0.003%.
If you use a lapse rate of 6K per km, the raised layer would increase the surface temperature by 0.6K, other things being equal (yes, this is a highly simplified analysis). The increase in emission layer area of 3 parts in 100,000 is completely trivial in this analysis.
I think you missed something very important. Water vapor dominates the radiative heat transfer from the lower troposphere, blocking most of the spectrum that other GHGs would otherwise block. When the buoyant air reaches the tropopause, the water vapor condenses out. Water vapor ceases to be a factor at all in the process of radiating from the ground to space. So the effects of increasing the amounts of the other GHGs is no longer masked by water vapor.
b) to maintain energy balance, the compensating increase in warmth at the surface has three
pathways:
1. it can go into the ocean
2. it can melt ice
3. it can warm the air.
No wonder you can’t figure it out, you forgot the optical window to space.
No, he didn’t forget the sky window. He said the increase in warmth at the surface, he was not referring to the fact that some of the sun’s energy that reaches the earth’s surface goes back out into space through the sky window.
No, he didn’t forget the sky window. He said the increase in warmth at the surface, he was not referring to the fact that some of the sun’s energy that reaches the earth’s surface goes back out into space through the sky window.
Right, but he probably doesn’t expect the atm to self adjust how cold it gets, and there not being all that much additional warmth left to deal with. So none of his choices are correct, really.
a) radiating from a colder place entails a slow down in the cooling to space.
I’m throwing food now.
Can you explain “slow down cooling”? Does the emissivity of the planet change? Does CO2 change the emissivity of the planet? Where, then, is the back up that constitutes the “slow down”? — in a magical zone between the given emissivity and the given planetary radiation? How does that work? “Slow down” with respect to what time frame? Is Earth on a schedule to cool by a certain time? Who sets it? How long does it take for enough “slow down” to cause heating? WHAT exactly is being “slowed down”? What is the entity defining “cooling” that is being “slowed down”?
This “slow down the cooling to space” sounds a lot like a piece of glass inhibiting convection, … a roundabout way of resurrecting the idea of a barrier that keeps stuff from moving out. It’s a more indirect way of erecting that retaining barrier (via conceptual, rather than physical), but still it seems to beg holding onto a cherished, flawed idea.
I have yet to be convinced that a “colder place” can cause heating by any known physical means, either directly by adding heat or indirectly by “slowing down cooling”.
Energy comes in, energy goes out, all the time. If a little less energy goes out, then the atmosphere has a little more energy, and is a little warmer. Lapse rate means that the average temperature at the surface is determined by how much energy is leaving at the TOA.
It is unfortunate that you believe you have to restate the obvious in response to a rubbish post..
But fair enough. Yes, increasing GHG’s warm the surface (though the details are complicated and not homogeneous in time or space). Yes, continued use of fossil fuels will cause additional warming unless offsetting factors keep that from happening.
Now that we are past that, the real issues are (and have always been) how much warming, over how long, the negative consequences of that warming, and what offsetting benefits (eg greater crop production) balance in whole or in apart the negative consequences of that warming.
The Reverend Badger.
November 19, 2017 12:05 pm
LOOK HERE!
I got the first comment in this thread and I told you not to treat “photons” like they were real massless particles. I got my big red marker pen out and crossed out everybody who did.
Has anybody got another big red pen (or 2) please as I seem to have run out before I got to the end.
Furthermore ; Who the hell told more half of you that you could add radiative fluxes arithmetically?
Anyone who still thinks they can by tomorrow is invited round to my kitchen where I have a large number of radiative objects at all kinds of temperatures from 0 to 90 C which you will be required to arrange in an ensemble of your liking and invention in order to boil my egg.(Boil = 100 C)
Seriously guys and gals an actual experiment or even a thought experiment to show that adding radiative flux arithmetically is scientific nonsense is so flippin’ simple it is school physics level. Please – up your game!
If you cannot even grasp this the furthest you are going with atmospheric physics is horizontal dermatological absorption.
Double the W/m^2 at the sun (two suns) also doubles the W/m^2 at earth’s orbit – 1,368 * 2 = 2,736. S-B BB equivalent temperature rises to 468.7 K from 394 K.
Lets go a bit further. The Sun occupies a tiny percentage of the area of the sky, I think something like 0.002% IIRC . So never mind TWO suns, let the thought experiment go large and we can have 50,000 suns , should just be able to squeeze them into that sky. Now please redo the SB calculation like you did for 2 suns, nick. The answer should tell you something. Especially when you compare it with the temperature of EACH sun. A new definition of heat transfer from “cold” to “hot” !
This is of course the same as my numerous 90C radiating objects in my kitchen which you need to arrange to boil my egg. Yes, there are at least 50,000 of them and you can arrange them in a sphere around my saucepan.
You ask: “Who the hell told more half of you that you could add radiative fluxes arithmetically?”
The 1st Law of Thermodynamics (conservation of energy) told me that. It is any absolutely necessary consequence of a conserved quantity like energy.
In any actual thermodynamics class, virtually the first thing you learn is to add and subtract energy transfers to and from a “control mass” to determine changes in the energy of the control mass in “energy balance” calculations.
It’s no different from balancing your checkbook, which must observe “conservation of money”. In effect you are arguing that if you get a second job (let’s say lower paying than your first job), you can’t add the money you get from your second job to your bank account. It’s an absurd argument!
Badger, you are correct. If you could add w/m2 then it would be possible to cook steak with ice cubes.
Once an object has attained the max temperature it can adding a second source with the same temperature, as first, cannot cause the object to increase further. (T4 – T4) tells us that.
If you look at a spectrum, the common form the Y axis is power in Watts, you are right that the energy at 15u per photon is slight, so to make an equal power as shorter wave lengths, there are a lot more photons.
So the flux is higher.
The interactions are photons bypass, they scatter, or they are absorbed.
This results in our experience, things are invisible to that wavelength, ie clear, it becomes reflective, or opaque.
But regardless of how 15u photons interact in the environment, water vapor regulates am temps to not drop much below dew point, at least until it all starts freezing.
When you monitor cooling at night, do you wonder why it’s clear, and yet the temp stops falling in the middle of the night. But most of the year it stops cooling around dew point. But what few realize is that it did not stop radiating to space, in the optical window temps are are nearly 100F cooler than air temps.
What does happen is the sensible heat, IR from condensing water, lights up the sky in all directions in the 14-16u water band, which lights up co2 at 15u. This is strongest at the surface, and it shows up as the rate temps falling while it’s still night out dropping towards zero. A side effect of using WV to regulate temps, it bleeds off atm water every night, this prevents the WV amplification of CS, the key to catastrophic warming. So not only is it not catastrophic, it doesnt even reallt cause any warming, WV will always prevent it.
This is the real GHG effect.
micro6500
November 19, 2017 at 8:07 am: and possibly what Roy Spencer sees with his skyward pointing pyrgeometer. All real experiments including Hans Geiger’s monumental “The air above the ground”, those in the Australian outback, and Hartmann’s, show how CO2 is no different to any other gas except the one which cheats by phase-changing. Which is why radiators have caps, and we exist.
Well cheating is just a matter of which side you’re on.
Micro6500
As stated above your assumption that water vapour is the key to cooling stopping is incorrect. It is heat release from latent heat as dew forms on surface of the ground and plants which slow cooling and then eventually fog forms and once it does then temps just flat line….Fog is liquid water…not the gaseous form water vapour and hence a different mechanism for cooling slowing down. Once fog forms…then the top of the fog layer becomes the radiating surface…not the ground, and as this fog layer cools further at the top, dewpoint is reached and the top of the fog layer rises. This is how fog layers deepen overnight.
Dew is only a small part of it. But there’s a 30-40W/m^2 flux out the optical window at the same time the temperature stops falling.
I can see the same circular arguments and the same immovable positions but nobody addressing the central point of the post. Can radiation from such a low temperature source warm surface matter which is at a much higher energy level, that is the only question to be answered surely. Also if back radiation causes warming how is it that surface temperature and tropospheric lapse rate can be calculated with no reference to radiation?
The sb-equation says no. There is no transfer but the heat, which is the difference in T⁴. Quantum physics is built on thermal physics, so it obeys the sb-law. Quantum is the microscopic details of heat.
The surface transfer 383W/m²-128W/m² to TOA, and TOA transfer nothing back. Neither does the rest of the atmosphere.
I think your question is answered in this post:
https://izenmeme.wordpress.com/2017/10/29/back-radiation-and-the-2nd-law-of-thermodynamics/
Which is a variation of this post:
http://rabett.blogspot.co.uk/2017/10/an-evergreen-of-denial-is-that-colder.html
Sorry RS but that answers none of the questions I raised. It certainly doesn’t explain cold to hot heat transfer. It doesn’t even touch on why all tropospheric temperatures can be explained and calculated with no reference to back radiation. I really think that AGW is in desperate trouble?
The problem seems to exist in the word “warms”. Radiation from a cold object cannot “warm” a warmer object. However, it CAN leave the warm object warmer than it would have been without the cold object.
As an example. When we look up, the apparent temperature of the sky based on the amount of downwelling thermal radiation is on the order of -50°C … how can this radiation “warm” the much warmer earth?
The answer, of course, is that without the atmosphere the apparent temperature of the sky would be about -270°C … and as a result, we are warmer because of the existence of the atmosphere.
Does the radiation from the atmosphere “warm” the surface? No … but it does leave the surface much warmer than it would be without that radiation.
w.
Thanks I didn’t notice how you said this already so clearly.
Willis Eschenbach
November 19, 2017 at 8:35 am: By its mass, and the sun. Just as explained by all real experimental Physicists from Maxwell (Theory of Heat) onwards. Not to mention the Mylar balloon expt, and all Nasa atmospheric readings through the solar system. Even Sagan admitted this eventually.
No, we are “warmer” because the oceans absorb a lot of the sun and release that energy slowly, which heats the atmosphere mostly by direct molecular contact with the air. The cooler air cannot warm the surface, only slow the rate of heat loss. It appears to have warmed because the temp number is higher, but that is not “warmer”. Example, day time heated by the sun gets to 30C, during the night it cools, losing that heat. If the lowest it gets on a clear night is 10C, then we have lost 20C worth of energy. But if on a cloudy night it instead goes to 20C, then we have NOT gained 10C in new energy (*warmer”). What has happened is less energy is lost. It’s not “warmer” on the cloudy nights, it’s less cooled. Because the temp is 10C higher numerically, we say it is “warmer”, but in reality it isnt warmer (no new energy added).
Willis,
Expressed well!
This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.
“to warm” AND “to slow the rate of heat loss” are separated
geez my spelling…
Hugs:
“This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.”
To warm an object requires new energy to raise its temp. Losing less energy already there has a higher temp number, but it is not “warmer”. If you earn $1000 per month gross, and have a high tax rate of 25%, you lose $250, netting $750. But if the tax rate is lowered to 20%, you now lose only $200, does that mean you have earned $50 in new money? Nope. You earned the same, just lost less of it in taxes. Consider winter and night times as a tax on the energy the sun put to the earth during the day. Having a higher NET energy doesnt mean you are “warmer” because you dont have more gross energy.
Back radiation is competely irrelevant, as is sideways radiaton and slant radiation and every-which way radiation. The only important factor is the net upward radiation. Look at this formally correct but carefully doctored NASA diagram:

What it shows is that LWIR moves 66 Wm^-2 from the surface, 40 by direct radiation in the “window” and 26 by way of absorption/re-emission by GHG. It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat, 102 Wm^-2, of which 78 is latent heat of evaporated water, which condenses at high altitude and radiates the heat into space.
Also note that radiation is gross while convection is net (and that the radiation arrows are fat and red while the convection ones are as puny as possible). Taken at face value this diagram implies that the descending cooler/drier air and precipitation is at absolute zero since in this case there is no “back radiation” (=downward heat transport). The reason for this difference is that a honest diagram would show clearly that convection is the dominant process regulating surface temperature. And convection can’t be modelled by GCM, only “parameterized” (=guessed at) since it happens on a far to small spatial scale for GCM:s.
tty, always pleasure to read your comments. The red arrow is a red herring!
No oceans no model.
This is the flat earth model, and it is wrong. It overly simplifies and incorrectly depicts what is going on. Postma has written a lot about this flat earth model having no basis in reality.
The sun puts 1370W/m^2 to the surface, not 168 (which wouldnt power two 100watt light bulbs). The surface temp of the earth would be some 69C at that input, but it’s not. Why? Because the earth rotates. Only a small percent of the surface receives this input, the rest loses more than it gains. The earth’s rotation acts like a thermostat. If it rotated slower it would be too hot end of the day for life. It it rotated faster, it would not gain enough during the day to overcome the night time loss rate.
[??? .mod]
J. Richard Wakefield
Well, that 1370 watts is actually only 1362 watts/m^2.
But it is actually measured at 1362 watts/m^2 at the top of atmosphere, only about 1000 watts/m^2 gets through a clear atmosphere to the surface of the earth, at the equator. And that 1000 watts/m^2 only gets to the surface at noon on the equator, on a clear day, on average.
Over the whole year, the CAGW climacatastrophy community has accepted Trenberth’s [168] watts/m^2 absorbed + [30] watts/m*2 reflected as average values for the whole earth.
But that 198 watt/m^2 yearly average sunlight on the surface for every day and night is ONLY valid for the temperature regions ONLY between 55 north and 55 south latitudes. Any further north or south and the yearly total “average” sunlight goes much, much further down.
Spot on, tty! It’s not a scientific schematic, but a Soviet-style propaganda poster, with self-serving distortions of reality.
“It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat”
No,it’s not. The diagram is a budget. It records the fluxes that can be measured, and tests whether they are in balance. The flux that is affected by GHG concentration is the full 324 W/m2 downflux from air. The 390 W/m2 upflux at the surface responds to the temperature there. The difference, if you exclude AW, is small at 26 W/m2, but is pretty much locked. The 324 W/m2 is emitted from air near the surface, as you can tell from its magnitude. If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.
Dry convection is necessarily entered as a nett flux, because there are no measurements that can split it into separate streams. Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.
True, it doesn’t get too far. But it does get to the block called the atmosphere above.
“If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.”
In that case You can kiss CAGW goodbye, because there is nothing else that GHG can affect.
” Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.”
True in so far as wet convection is of course by far dominant. Ever see cumulonimbus clouds? They are not close to the surface.
Martin Mason,
A cold object cannot warm a hot object by conduction, but a warm object (Earth) can warm a cold parcel of absorbing gas by radiation. The radiation that would have otherwise left the system, is retained and now available to contribute a slight warming of the dark side of the Earth where it is being subject to the IR radiation from the gas, which is above absolute zero. Consider the impact of an Earth radiating directly into space, versus an Earth radiating into space with an intervening blanket at a higher temperature than space.
I don’t understand the question in your last sentence.
Martin Mason.
You can test this experimentally. Two identical set ups one with no CO2 and one with lots. If back radiation exists and can transfer heat a measurable temperature difference can be seen. Relatively easy lab work and relatively inexpensive.
The answer of course can be answered by any five year old playing in the snow. No, radiation from a cooler object categorically doesn’t increase the temperature of a warmer object.
The ONLY^3 reason RGHE theory even exists is to explain how the average surface (1.5 m above ground) temperature of 288 K/15 C (K-T balance 289 K/16 C) minus 255 K/-18C , the average surface (now ground) temperature w/o an atmosphere (Which is just completely BOGUS!) equals 33 C warmer w/ than w/o atmosphere.
That Δ33 C notion is absolute rubbish and when it flies into the nearest dumpster it hauls RGHE “theory” in right behind it.
The sooner that is realized and accepted the sooner all of us will have to find something better to do with our time and the taxpayers’ money. Maybe that’s what keeps RGHE staggering down the road.
The genesis of RGHE theory is the incorrect notion that the atmosphere warms the surface (and that is NOT the ground). Explaining the mechanism behind this erroneous notion demands some truly contorted physics, thermo and heat transfer, i.e. energy out of nowhere, cold to hot w/o work, perpetual motion.
Is space cold or hot? There are no molecules in space so our common definitions of hot/cold/heat/energy don’t apply.
The temperatures of objects in space, e.g. the Earth, Moon, space station, Mars, Venus, etc. are determined by the radiation flowing past them. In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
https://science.nasa.gov/science-news/science-at-nasa/2001/ast21mar_1/
But an object’s albedo reflects away some of that energy and reduces that temperature.
The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.
https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-723
The Earth’s albedo/atmosphere doesn’t keep the Earth warm, it keeps the Earth cool.
Bring science, I did. (6,000 views and zero rebuttals.)
http://writerbeat.com/articles/14306-Greenhouse—We-don-t-need-no-stinkin-greenhouse-Warning-science-ahead-
http://writerbeat.com/articles/15582-To-be-33C-or-not-to-be-33C
http://writerbeat.com/articles/16255-Atmospheric-Layers-and-Thermodynamic-Ping-Pong
Looked out. Can’t see much irradiance. I guess you should integrate to get the average surface value which is about quarter of the 1368W/m². Temperature was consequently around 278K. I guess it were colder without some warm gases and precipitation between my front yard and the 2.7K background.
Average is nonsense and does nothing to tell us what is going on. Example. Take a photograph of something, say a nice mountain range. Add up all the colour pixel numbers and divide by the number of pixels, then apply that number to each pixel. What do you get? A gray image with nothing to see.
This is exactly what you are doing by applying the average. You add up all the W/m^2 for the area, divide by the number of areas, and apply that to all the areas. It’s BS grayness that has no physical counterpart.
It tells me much more than the peak value misleadingly used above.
Hugs:
“It tells me much more than the peak value misleadingly used above.”
Yes, using JUST a peak value is just as meaningless. You want a real view of energy absorption and loss on this complicated and dynamic earth? Then do a proper 3D graphic of a tilted rotating spherical planet with large oceans and disproportional land masses that have different absorption. That flat earth graphic tells us nothing of what it really going on, and if anything has grossly mislead people into thinking a simple “budget” explains things. It doesnt. That graphic was invented to justify the AGW dogma. Again, AGW exists because of deliberate ambiguity.
The claims in this article are unfortunately not even wrong.
IR absorption by CO2 excites the vibrational modes of a CO2 molecule.
As the CO2 molecules collide, the excited vibrational modes transfer energy to the motion of other CO2 molecules. The average kinetic energy of molecules is what we measure as the temperature of a gas.
Nothing to do with the absorption and emission of visible light by electron level transitions
A correct summary of the process may be found here
“Has Global Warming Paused”
https://www.scribd.com/document/239526963/Argonne-National-Lab-Talk-Happer
A lecture by William Happer, a molecular spectroscopist and professor of physics at Princeton U.
One can run MODTRAN online here:
http://climatemodels.uchicago.edu/modtran/
As a simulation subject matter expert, you can’t use MODTRAN and run a single look through an average air sample and get anything useful.
If you do not run it over a days cycle as conditions change, all you’re doing is fooling yourself.
The excited modes of CO2 do NOT transfer energy to the motion of other CO2 molecules – or do it only in less than 0.04% of cases. What happens when an excited CO2 molecule collides with an N2 or O2 molecule or an Argon atom is very complex and I have not seen a satisfactory analysis.
Have a look here.
http://aip.scitation.org/doi/10.1063/1.4926880
Paywalled. But according to the abstract they handle CO2-CO2 collisions. As a concentration of CO2 is currently 0.04%, these collisions are very rare. And how about CO2-H2O collisions?
No CG, they do. That is why the absorption spectrum is in BANDS and not a single exact wavelength. The band of wavelengths show the range in energy needed to bump the molecule into a vibrational state, and the difference is caused by the very slight decrease or increase in kinetic energy from molecular collisions.
Wouldn’t a simple experiment (already conducted by John Tyndall) learn that 15 micrometer IR radiation is emitted and absorbed at other temperatures as well?
Eric
November 19, 2017 at 8:18 am: Tyndall made sure his absorber was cooled below his emitter, is why. The SB assumption, indeed, is to 0Kelvin.
The mistake was made with Tyndall, he was using a thermoelectric transducer, a thermopile: he discovered the thermo electric gases – we call them the GHGs. Watch to see what I have discovered https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
i think the author misapplies his second key point “The temperature of a (gas) directly affects the wavelength of the radiation it emits and absorbs.” Looks like he applies it incorrectly. Yes, for CO2, most of the outgoing radiation is mostly from the stratosphere; and in the atmospheric window region is mostly from the lower troposphere. From link article which concludes “The total temperature-dependent gas absorptivity effect (H2O, CO2, etc.) tends to result in a radiation energy convergence that decreases the total cooling of the atmosphere.” https://www.gfdl.noaa.gov/bibliography/related_files/yih0702.pdf
I got as far as this one …
I never heard that about “too hot to exist”, and I don’t believe it.
Citation?
w.
I guess that he means that those bands are unimportant because they are at wavelengths where the Earth emits virtually no radiation at realistic ground temperatures. I agree that it is a very clumsy way of expressing this
Thanks, tty, but I have no idea what he means, nor do I particularly care. I was asking for a citation about “the temperatures too hot to exist”.
w.
Willis, you are a stickler for quoiting correctly when rebutting. I would like to point out the important qualifier that you are missing in your quote reference. He specifically said “to hot to exist in the atmosphere“. A distinction that is pertinent to his point..
Clearly I try not to rely on spellcheck,…..perhaps I will start.
Willis
The article is a mixture of facts and fallacies. The author is confused by a lot of ‘information’ gathered from various sources. Some of the bloggers are in the same boat. Too many things to correct so I just shut my mouth. Nevertheless, it’s entertaining to read some of the well-meaning comments.
Willis, re: “too hot to exist”. Rod Gill is confusing de-excitation radiation from the CO2 molecule with thermal radiation as described by Wien’s Law. That’s why he claims the CO2 emission bands at 2.7 and 4.3 microns only come from CO2 molecules at temperatures too hot to be found in the atmosphere (800° C and 400° C, respectively, if calculated from Wien’s Law). That’s also behind his ludicrous claim that CO2’s 15 micron emission band comes only from gas at -80° C.
Rod Gill fundamentally misunderstands the physics behind the so-called “greenhouse effect”, and he has no business publishing an article about it. I would have preferred that Anthony or one of his volunteers gently point that out to him and not publish such pseudoscience.
Of course the comments reveal that such confusion is fairly common, but we already knew that.
Wien’s Law calculator:
https://www.ajdesigner.com/phpwien/wien_equation.php#ajscroll
Willis,
Agreed, the first confusion is in assuming atomic excitation instead of molecular. Then electron states instead of rotational, vibrational, etc molecular states. Several other errors follow as a consequence.
However, I think the exercise is useful because it’s responses can correct those readers who were similarly starting from the same wrong point.
I tend to shut up because when my formal training in the 1960s was done, atomic was dominant and molecular seldom covered in lectures other than to say that molecular spectroscopy was a harder concept. Geoff
David Ball November 20, 2017 at 12:07 am
His claim is not true, whether he is talking about the atmosphere or the earth’s surface. So no, his qualifier is not important.
w.
Willis Eschenbach November 20, 2017 at 1:13 am
Which claim are you referring to? You are not being clear. “In the atmosphere” is definitely an important qualifier because he is talking about how the atmosphere actually operates and the range in which these interactions occur. You saying it isn’t does not make it so.
David Ball November 20, 2017 at 11:07 am
His claim that:
w.
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
I’d love to invite you into a cave in a glacier sometime.
wickedwenchfan November 20, 2017 at 2:51 pm Edit
I’m sorry to hear that. Had you continued, and considered what I was saying, you might have learned something.
In the meantime, have you never been outside on a winter night when clouds came over? They leave the surface warmer than when there are no clouds. Clear winter nights are the coldest … why?
Because when there are no clouds, the back radiation is coming much more from outer space at a temperature of 3K or so, whereas the clouds are radiating at something like 225K …
Think about it. If a planet has no GHG atmosphere, it is exposed directly to outer space.
But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.
I’m not sure why this is so hard for some folks to grasp, but I assure you that it is settled science taught in the universities and backed up by the math. If you are interested in the math, there is an online calculator here. If you play with it for a while it may help you start to understand the nature of two-way radiative energy exchange … it spells out the formula for how it is calculated.
w.
Willis Eschenbach November 20, 2017 at 3:45 pm
Willis, you are mixing apples and oranges. The clouds have a higher temperature than the rest of the atmosphere because of the latent heat of condensation being released. This is a phase change of water effect, not a GHG effect.
Secondly, your sentence
‘But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.’
is obvious and there is no need to shout. Everyone agrees that the atmosphere warms the earth compared to no atmosphere. The point at issue is does replacing a few O2 molecules with a few CO2 molecules make a difference?
Bernard Lodge November 20, 2017 at 8:34 pm
The claim was that the cold atmosphere (containing cold clouds) could not leave the surface warmer than it would be without them, viz:
wickedwenchfan November 20, 2017 at 2:51 pm Edit
Since both the atmosphere and the clouds are colder than the surface, I am NOT mixing apples and oranges.
No, “everyone” does NOT agree that the cold atmosphere warms the earth compared to no atmosphere. There are literally dozens of people who claim that it is impossible. They all say that because the atmosphere is colder than the earth, the atmosphere does not and cannot “warm the earth compared to no atmosphere”.
Which is why I was shouting … I’m getting tired of people saying the same thing over and over, when the answer is there from our own senses regarding winter clouds.
w.
PS—Can a block of ice leave a person warmer than no block of ice?
Sure … as long as the block of ice (at 0°C) is interposed between that person and a tank of liquid nitrogen (at -196°C). The ice provides more radiation to the person’s body than does the liquid nitrogen, so the person ends up warmer.
And the same is true about icy clouds which are interposed between a person and 3K background temperature of space … they leave the person warmer than no clouds.
CO2 molecules can absorb radiation and then transfer that energy to other gasses by conduction and versa visa.
Though much easier by re-radiation. Gasses do not conduct heat well. There is a reason why most good insulating materials are mostly air.
At atmospheric pressure the conduction takes place with molecular collisions that theoretically occur faster than a CO2 molecule can radiate its heat — 10^-7 s.
It is pressure dependent as rgbatduke always said. But I don’t really know how this affects – probably surface CO2 absorbs only, and does not have time to emit, so the air eats the radiation band. And then complicated (parameterized) things happen, like evaporation, convection. I’m sure the problem is not high school physics as Gore said. He lied.
yes they do, a dewer flask ( a vacuum flask) has a vacuum as an insulator, so your coffee keeps warmer for longer than if air was used to insulate.
1) The “compressor” is always on.
2) This, coupled with geothermal
( https://wattsupwiththat.com/2017/11/15/new-map-of-antarctic-geothermal-heat-suggests-steig-mann-2009-werent-measuring-global-warming/ ), account for our temperature range.
The earth is a magma filled balloon, at least the outer layers that we have been able to measure look that way.
3) A Chinook demonstrates the effect of compression directly. Ne c’est pas?
4) This idea also satisfies Dr. Svalgaards assertion that TSI variance is too small to account for the variations of earth’s temperature.
5) One has to admit that the name “Greenhouse” in no way is descriptive of our atmosphere or how it works.
6) No matter how our atmosphere works, we are fortunate that the relative stability of temperature is within the range of water existing in all three states. As a carbon based life-form, I am grateful of this.
7) Ockham’s razor and plausibility Apply liberally, rinse, repeat.
( numbered for your rebuttal convenience and clarity, also inspected for politically free content )
CO2 molecules can absorb radiation, and immediately emit said radiation, but in a random direction, ie scattered.
They cannot absorb radiation and convert it to kinetic energy (heat).
Jer0me
November 19, 2017 at 1:10 pm: But in our air, they are struck kinetically thousands of times between each possible emission. Thusly, air eats radiation so convection and phase change of water dominates hugely to above the net emission height. Radiation is swamped by water anyway, which even Mann knows. It is all sleight of pen for the cause. Bring on the State Pen..
Rod, thanks for your post. However, you ask us “Am I right?” about the following:
Nope. You are wrong. However, given the depth of your misunderstanding, and your certainty that you understand it, I’m gonna leave the question of WHY you are wrong for you to work out for yourself.
w.
Show us a reference for transfer of energy from co2 at lower temperature to a solid warmer body, in a controlled environment. If you can’t, there is no transfer.
There is always transfer in both directions but the net transfer is always from hot to cold.
I asked for a reference, not you making stuff up.
Fact: at equal temperature, no transfer. So why would there be transfer from cold to hot if one body is colder.
Show me a reference for any transfer outside of “net”, or shut up.
You ever use an IR thermometer and point it at a fire, and then in a freezer?
You got readings from both objects, what do you think they were measuring?
Oh, just to be clear, then imagine you point it at liquid nitrogen and compare that to the freezer. They all radiate.
Show me a reference that shows that an atom tells an incoming photon: “thank you, but no, since you com from a colder atom”.
What happens if the molecule/atom is already excited to the energy level of the incoming photon?
Then what the difference does it make?
lifeisthermal, carbon dioxide lasers operating at room temperature can cut through metal:
@Gary Young Hladik
So laser beams heat … errrrrr except when they cool
https://en.wikipedia.org/wiki/Laser_cooling
Yep you can use a laser beam to cool an object to near absolute zero explain away.
Love classical physics it is so simple except it’s totally wrong 🙂
LdB, I’m not getting your point. Are you saying the CO2 laser beam CAN’T cut metal? Maybe you’re saying the laser cuts metal by COOLING it? Help me out here.
LdB November 19, 2017 at 8:27 pm
It would help if you actually understood “classical physics”. Then you wouldn’t be so confused and would understand the difference between cutting with a laser beam and laser cooling.
So is there a difference in the beams between a cutting laser and laser cooling Gabro?
I like that concept I am going to get me some cool laser beams 🙂
@Gary Young Hladik
The problem I was illustrating is even things we think of with one behaviour don’t always behave like that, you are saying the beam will always thermally act one way. That beam as it moves thru the air is a electromagnetic wave whether it heats or cools or even has any effect (think a mirror) is governed by a set of strange laws.
If that laser is a CO2 cutter and I replace the steel with aluminium, I assume you know what happens.
Try even cutting a very fine steel mesh 🙂
It’s a EM transfer you can’t equate it to thermal energy, you can heat,cool or reflect it.
Oh and for anyone who wants to know how it works.
It’s pretty straight forward you need 6 or more beams to do cooling, the frequency used matches one of the resonant frequencies of the atom you are trying to cool and it doesn’t actually see the beam as hot because it absorbs it and then re-emits it.
LdB November 19, 2017 at 9:30 pm
Yes, there are many differences between cutting and cooling lasers.
LdB,
“…you are saying the beam will always thermally act one way.”
Actually, I’m saying “carbon dioxide lasers operating at room temperature can cut through metal”.
Oh really please tell me 🙂
@ur momisugly Gary Hladik
Yes I know, I was just firing a warning shot … yes but be careful.
If that metal is aluminium for example it will reflect and if the metal has a resonant frequency at that frequency it can be transparent or cool the metal.
We do that trick in QM making materials transparent
https://en.wikipedia.org/wiki/Electromagnetically_induced_transparency
I could make your laser beam pass right thru the metal plate .. but this isn’t something you would see in nature 🙂
Why does it surprise you that lasers can both heat and cool?
How lasers can be used both to cool to the coldest temperatures on earth and to heat to the highest:
http://physicscentral.com/explore/poster-spots.cfm
There are many techniques for laser cooling, although the Doppler technique is probably still the most common.
@Gabro
No you are claiming there are cool lasers ™, you directly claimed it.
Now you obviously bothered to read and are now trying to dig yourself out of a hole, there is no such thing as a cool laser ™ they are all the same.
As I have already stated the laser can heat, cool, reflect, absorb or transmit thru any material which strangely enough is the same for every single EM frequency that exists including radio waves and thermal emissions. QM is very concise and very well tested on all this stuff/.
Willis, I’m trying very hard to do this myself so please don’t cop out.
Martin, thanks, but I have no clue what you mean by that …
w.
I like to use the analogy of an Eskimo igloo, where the snow, forming a temporary barrier, captures the heat inside the igloo long enough to maintain a comfortable temperature.
Which of course is an incorrect analogy as the igloo is a physical barrier to convection, which is how the surface loses most of the heat. It has zero to do with radiation effects.
CO2 is a physical barrier to some radiation. It is an analogy, not saying ice chunk is a greenhouse gas. Of course, ice IS a weak barrier to radiation and it does contribute to how igloo warms.
Jer0me,
You can heat a clear container of CO2 by radiation as from a laser beam of appropriate wavelength or a beam of sunlight passing through a small part of the wall. If the beam is turned off, the hot CO2 will heat the rest of the container wall as the CO2 cools by normal heat transfer equations. Is this at odds with what you mean? Geoff
joelobryan
November 19, 2017 at 10:09 am: You almost got there. The crooks know CO2 is too weak (at best, in fact it is a coolant). So they concocted an effect on water vapour to magnify things. This is on record. Other planets and large moons prove CO2 is not special except for life. We have that on record too.
A waterless planet with no tri or polyatomic gases, eg all Nitrogen, would still warm by conduction as we do. Conduction remains the dominant first mover, unless WV latent heat beats it on Earth, micro555? The emission from kinetic motion in the molecular force fields would still cool it. What is debateable is how far such an atmosphere has to expand outwards to radiate enough( by the Gas laws, which do indeed rule). Larger planets should somewhere reach sufficient gravity to keep hold of more gas than their net losses through what is really evaporation.
In infinity, I am fairly sure that such experiments are in progress.
Willis, that’s a bit harsh on Rod. He clearly invited us to reveal the deficiencies in his argument. What more do you want? This is the debate.
The day you can warm yourself up by standing in front of a block of ice, is the day you get to be taken seriously, Willis
The day you realize that you can warm yourself by standing in front of a block of (water) ice instead of a block of dry ice, or a tank of liquid nitrogen, is the day you get to be taken seriously, WWF.
Off you go, Ed.. go and dip your head in a bucket of ice, again
Your brain has surely frozen.
Junior high physics really did stretch your limits didn’t it.
So Andy: Suppose I, as an evil GHE believer, have you tied down to a big block of ice at 0C in a room at 25C. After a while, you get hypothermic and your body temperature drops to 35C.
I now offer to let you move to a big block of slate rock (which has the same thermal conductivity) at 25C in the same room instead, saying it could help you to increase your body temperature back to 37C.
By your logic, you would refuse, because the slate rock is still below your body temperature, so the change couldn’t possibly lead to an increase in your body temperature.
Now, if you were dead, that would be true. (You may be brain dead, but…)
Oxygen and Nitrogen are not “greenhouse Gases”
Yet dont they serve some function of retaining heat and maintaining some balance of temp in the atmosphere.
Otherwise, it would seem that the total volume of Greenhouse gases is vastly too small (1%ish not counting water vapor) to retain any quanity of heat
Hard to fathom from a scientific point of view that CO2 or any of the other greenhouses gases are sufficiently powerful to act as the thermostat to the degree which they are credited.
O2 and N2 certainly have molecular heat capacity. That is not the issue here. As diatomic molecules with limited vibrational modes, they only absorb in the UV band. Triatomic and higher molecules like CO2, H2O, O3, CH4, NO2 are the GHG’s because they have vibrational modes that allow absorption of IR wavelength photons.
But in terms of IR electromagnetic radiation at the temps under consideration for Earth’s atmosphere and surface temps, the three main gases, that is O2, N2, and Argon (that’s 99.5% of dry air), are transparent to IR wavelengths. Thus those 3 main components of dry air cannot absorb IR photons to warm. They are transparent to IR. Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar. But the GHE in the atmosphere also depends on the adiabatic lapse rate of the troposphere, which exists because pressure drops with altitude (gases are highly compressible of course). This temperature gradient creates an effective radiation level (ERL) where a point (vertically, a temperature profile) is reached where more photons escape to space than are re-radiated toward back toward the surface.
So now think of very dry desert air, how large a temperature swing there is from day to night. During the day, there is visible light and UV heating both the minimal water vapor and the surface. The air layer near the hot surface warms due to convective transfer. At low viewing angles, the shimmering desert convective heat flow is seen as a mirage because refracts the sunlights. But in a dry desert, with little water vapor, after the sun sets, the air cools rapidly, losing several degrees/hour because their is little vapor to help trap the escaping IR wavelength photons. That’s radiative cooling. The other non-condensing GHGs (CO2 in the troposphere, ozone in the stratosphere) help slow the cooling. This is why GHG theory predicts night-time lows will get warmer under increasing CO2 while having little measurable effect on day-time high temps.
The above descriptive physics is why the GCM outputs “predict” a tropospheric hotspot in the tropical latitudes.
And going from 3 CO2 molecules per 10,000 air molecules (0.03%) to 4 or 5 CO2 molecules per 10,000 air (0.04% to 0.05%) makes a negligible contribution to the trapping (back radiation) when the atmosphere (troposphere) contains a much higher molar quantity of (1% – 3%) H2O gas. If the atmosphere were completely dry then CO2 concentration changes would make a measurable impact on heat trapping.
This last fact is likely why the Tropospheric Hotspot in the tropics has not been observed. The GHE of CO2 is too weak to be detectable. This argues for the modification of the CO2 forcing theory from one of a strong GHE effect to a weak GHE effect for CO2. The evidence points to a weak GHE hypothesis for CO2. And that is something the current-day climateers, who are profiting from global warming alarmism, are loathe to admit.
Joel, the lapse rate is determined only by gravity and gas physical properties, it has no input or output from radiation. I don’t believe that the lapse rate creates the apparent radiation level, this depends only on the OLR magnitude and the surface area required to radiate it at the effective radiating temperature (-18C).
Surely there is no “trapping” of radiation by GHG’s?
Taking the relative humidity vs ghg analogy with deserts a step further –
the average relative humidity of a typical desert ranges from 10%-30% (various sources) while the ypical countryside ranges from 50-90%. the delta of the day/night temp swings between deserts vs countrysides is in the range of 20-30 degress. With the concentration of water vapor being 200-400x the concentration of co2, it would seem that an increase of 150ppm would be less than 1-2% of the effect that water vapor would have when you compare desert vs countryside.
(apologies if my explanation isnt the best)
Rh only matters when air temps are near dew point, that’s when it stops cooling.
Even though humidity is low in the desert, clear night min temp should be near dew point.
“Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar.”
No. The sink of heat energy is the oceans. They warm the atmosphere by evaporation. that is how the water vapor gets there. Confirmation sip a pina colada at a sea side bar at night. Try the same thing in the desert.
It cools 3-4°F/hr in ohio at first too. It just stops at dew point, and the daily range is much higher in deserts.
These are both clear calm nights.
Good point top: if N2 and O2 do not emit or absorb IR, this is a contradiction to QM and thermodynamics.
They do radiate, they are GHGs and here how: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
The 15um radiation doesn’t warm anything, the sun at 500k does. All the 15um does is cancel out the 15um radiation coming up from the surface. The sun makes up the difference. Hence the atmosphere warms up a little.
That’s the theory, whether it happens or not or can actually be detected or not is the issue.
Quality not quantity.
My thinking for years now.
Sometimes called the Ultraviolet Catastrophe and the Perfect Example is solar panels.
(The UV Catastrophe was the title of a BBC Horizon program explaining a time when scientists couldn’t explain certain ‘wavelength’ or colour sensitive phenomena.)
Just exactly why do solar panels need direct (or reflected) sunlight?
Peta of Newark
November 19, 2017 at 8:39 am: We had a paper by warmist students a while back proposing panels to harvest the background radiation of Earthly IR return. Very excited they were at all that amperage (equalling solar input, after all). Only the sound of crickets since then, as they seek the voltage, I guess.
That pretty well explains the AGW myth, really. Magic and magic mushrooms.
The relationship stated in the quantum physics of electron orbitals and any relationship to temperature due to molecular kinetic energy is wrong. Dead wrong.
Sort of kills any value to discuss your other points when such a fundamental error is made.
joelobryan
November 19, 2017 at 8:53 am : Joel, please expound rather than excoriate. The poster requests this respectfully.
a point nobody has tried to explain, the earth radiates the LW towards space because space is much cooler than the earth, what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? after co2 releases the tiny portion of the wave it grabbed for a picosecond again isnt a force REQUIRED to send in back towards earth?
“…isnt a force REQUIRED to send in back towards earth?”
Cold to hot DEMANDS work per thermo, e.g. a refrigerator.
It is perfectly possible for a cold molecule to emit a photon to be absorbed by a hotter one. But the flow of heat which is a statistical phenomenon will always go from warmer to colder (except for very small random fluctuations).
Energy is thermal property, heat is a thermal process. IR is a surface property not a bulk property. Where is the GHG surface?
“what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? ”
Simply the ‘force’ of radiative exchange. Just because something is colder does not stop it radiating to something hotter. It just means that the net flow is from warm to cold. All things at temperature above 0K radiate. The atmosphere radiates, it just so happens that it is mostly transparent to LWIR (N2 +O2 = ~ 99% and there is an atmospheric window at around 12 micron for the GHGs – see graph) and at the Earth’s temp, 15 micron is the radiation it most strongly emits at. … which CO2 also happens to absorb/radiate most strongly at (just a bit more than H2O).

It comes back because the Earth’s surface is partially in the way of a measure of photons re-emitted. In the mean time (during daylight) shortwave has come in. It’s that extra SW that is causing the GHE.
The ‘delay’ in emission is due to the path-length of a photon through the trop and why it is far from saturated. At around 8km the molecular concentration falls off enough to allow most LWIR to get to space and that level is at the temp the Earth shows from space (255K) whilst here on the surface we live at it is 288K. So at that temp CO2 emits less strongly (being colder), yet it can still absorb at the same rate.
So molecules high up still radiate as normal but they are receiving less because of ‘blockage’ below – this causes stratospheric cooling while the trop warms.
“Net” flow of heat from warm to cold confuses the issue. the correct term is the only flow of heat is from warm to cold, surely there can be no actual flow of heat from cold to warm regardless of radiation flow if the radiation flow can’t be thermalized rather than reflected or transmitted.
In the sense than photons from the cold object impinge on the warm object there is ‘flow’, but the net flow is always from warm to cold unless work is done.
This is the fumdamental mistake made when people say “a cold object cannot heat a warm one”. Correct it cant – but it does add to its entropy and slow its cooling.
“The Second Law of Thermodynamics can be rephrased in several ways. Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction). It can also be expressed using the concept of entropy as saying that the system’s entropy will always naturally increase if no work is exerted to decrease it. These rephrasings mean fundamentally the same thing because heat deals with kinetic energy and increasing a system’s kinetic energy will increase the system’s entropy.”
https://www.brightstorm.com/science/physics/heat-and-thermodynamics/second-law-of-thermodynamics/
A thought experiment. You have two flat metal plates somewhere out in space a foot apart. One is at 1000 degrees the other is at 500 degrees. Heat flows only in one direction, from the hotter to the colder plate. Now remove the colder plate. The hotter plate will cool down faster than before, despite that there was no heat flow to it from the colder plate.
>>
Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction).
<<
Not exactly. The Second Law is often stated as:

or the change in entropy for an isolated system not in equilibrium must increase until it reaches equilibrium. Not all systems are isolated. Entropy can decrease in a closed system or an open system. The Earth is usually modeled as a closed system, but it isn’t truly closed. Closed systems don’t allow mass transfer across the system boundary. Isolated systems allow neither mass nor energy transfer across the system boundary. Open systems allow both mass and energy transfer across the system boundary.
The Second Law for a closed system is:
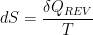
where Q is a path variable and the equation is only valid for reversible processes (this is often stated as the Clausius definition of entropy). Notice that entropy has the same units as heat capacity or energy per unit temperature and in SI units: joules per Kelvin.
Since I mentioned Heat capacity, it is defined as:

So, in an isolated system, a colder object (say a black body) can transfer heat to a warmer object (say another black body) as long as the warmer object transfers more heat to the colder object–simple thermodynamics, and it doesn’t violate the Second Law.
Jim
We live in a society exquisitely dependent on science and technology, in which hardly anyone knows anything about science and technology.
Carl Sagan
In questions of science, the authority of a thousand is not worth the humble reasoning of a single individual.
Galileo Galilei
“There are those who reason well, but they are greatly outnumbered by those who reason badly.”
― Galileo Galilei
Here is a picture of what natural gas does to IR.
.
.
Note how it blocks it out. Same thing happens to IR with CO2, but not to the same extent. If you were in orbit around the earth, and you “took a picture” in the IR band, CO2 would “darken” the image you get. This is how it “blocks” the IR escaping the planet.
Further….
https://m.youtube.com/watch?v=SeYfl45X1wo
This seems to demonstrate that we are wrong about the CO2 absorption range. A flame is putting out a frequency of well above 15u. But since no thermometer was inside the tube there is no demonstration that there was an increase in temperature.
CO2 expanding straight from a pressurized gas bottle……. DOH !!
Talk about ANTI-science…… and Toneb gets SUCKED IN by it.
And as mkelly says, frequency spectrum of a candle is nowhere near the absorption frequency of CO2
http://www.patarnott.com/phys625/images/candleAndLamp.JPG
This is the sort of NON-science we have come to expect from you, tone. !
The problem with his little demonstration is the candles flame is +500 degrees hotter than my shrubs, or the co2 in the atm.
This is propaganda. He knows better than this, that this isn’t really applicable to the problem that exists. He just had to sensationalize it to make an effect, and that’s what’s wrong with the consensus on AGW.
It all stinks.
“The problem with his little demonstration is the candles flame is +500 degrees hotter than my shrubs, or the co2 in the atm.”
My second vid isn’t.
Temp of the presenters hand.
That’s still the candle video.
Besides the point, they filled it with 100% co2.
And so what, there’s an equal negative water feedback to the increases in co2 warming if there is any.
“That’s still the candle video.”
No.
Here again ….
Starts with the same candle video.
https://urldefense.proofpoint.com/v2/url?u=https-3A__m.youtube.com_watch-3Fv-3DSeYfl45X1wo&d=DwMFaQ&c=RoP1YumCXCgaWHvlZYR8PZh8Bv7qIrMUB65eapI_JnE&r=KnDpXnbSBarnTnlVgl11aTGHo3AJb4b5nXkfcMmY5ZY&m=8hCX7splpO8_MCmKtspaCtnVqt0oVP0cy8wHXjDrmVQ&s=OcIjlaSaFTNnMZG18NxSgh2m5w9PzYk_ro1FEC351b8&e=
No it doesn’t.
And so what – look at the “hand” one.
Or are you gainsaying that as well.
Again there should be attenuation of the hand. 98.6 F is still well above the absorbing area of CO2. And again no thermometers.
What you say about CO2 darkening the Earth viewed from space in the 15 um band is true. The informed, educated skeptic recognizes CO2 is GHG. But most of your darkening is due to water vapor that is unavoidably present on our 71% water world planet.
The real question for the informed, educated skeptic (that the alarmists avoid) though is is there appreciable (measurable) darkening when CO2 concentration goes from 3 CO2 molecules/10,000 air molecules to 6 CO2/10,000 air molecules. The 2 facts – 1) that the tropospheric hotspot has not been observed, and 2) that models are running too hot compared to 25+ years observation strongly suggests the CO2 strong GHE theory must be modified to a weak GHE theory.
But without a strong CO2 GHE theory, the alarmist argument collapses. That would mean the entire politicized Climate Change and the trillions of dollars hoped to be re-distrubuted vanishes as well.
CPR is that not a blocking of reflected visible light not IR?
Note two things in the picture. First the black asphalt roads are very “bright.” This picture is taken in the IR bands, not in the visible bands. The asphalt is “bright” because it is HOT. Also, natural gas does not block visible light, and the plume you are looking at is the natural gas escaping from an underground storage facility in California.
A good visualization on the effect The surface IR is absorbed into air which then does not re-emit it quickly at a wavelength visible in the picture. But, this is different from CO2 in multiple ways.
Watch this and to see where the problem is: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
Satellites, often looking down from about 70km, see earth radiation at many different temperatures. You can’t just take a 15C/289K Planck curve and say, “hey, this only radiates at 10 microns”. Low energy IR radiation does not usually cause “electronic” transitions, that is bumping electrons to higher orbits. IR typically causes rotational and vibrational transitions. The significant CO2 transitions and their “bites” out of the spectrum are shown below. Their radiative temperatures are all different.
The 15um/667.4 fundamental bend (vibration) is seen radiating at a peak temperature of nearly 250K, and the surrounding rotations radiate at and conform to the 220K Planck curve.
The fundamental problem is we don’t live in a purely radiative world, as Bill and others have pointed out, collisional/conductive and convective interactions with the other (largely IR transparent) .996 of the atmosphere modulate the effective radiative temperatures. CO2 has a very low emissivity (tendency to emit photons). It prefers to dance.
Whatever you wish to call the disturbances in the electromagnetic/weak field, photons, or something we can detect as particle like, definitely exist; even though they have no mass. They do not control the earth’s energy balance alone.
“They do not control the earth’s energy balance alone.”
In a way they do, because the heat always finally leaves the Earth TOA as photons. But they do not alone control, or even dominate the heat balance of the surface of the Earth. Convection does.
Good clarification.
In the summer, looking across an asphalt surface at low incidence angle over a long distance (long optical path length path length), one see the shimmer. That shimmering (mirage as it is called) is of course refraction due to rapidly warmed boundary-layer air rising from the hot black asphalt surface. The asphalt is dry. The air is mostly dry. Water vapor is present, it warms too. But the vast bulk of the heat transfer from a dry surface in this case is conduction at the boundary where air molecules collide with the hot asphalt molecules.
True, though part of the heating of the air is also due to LWIR emitted by the asphalt and absorbed by the air. And conduction is only important for the boundary and a very thin air layer because of the very low thermal conductivity of the air.
tty
November 19, 2017 at 9:49 am: Agreed tty. Next we have to get it that heat, being a process caused by Kinetic Energy, is what results in radiation. Radiation is not heat and only relatively weakly results in it if it interacts with matter in a kinetic manner. Another basis for warmist waffle.
Gymnosperm, of course in the end, radiation is all that matters, as that is the only way energy actually leaves Earth.
Yes, my sloppy. tty has pointed this out. I should have said radiation does not alone control temperature between the surface and the effective radiative altitude.
Of course, it is actually far more complicated. The solar wind is incoming energy, but it is not EM radiation. Likewise, ions stripped from the earth’s atmosphere, reputedly as heavy as Oxygen, count as energy out.
I have some doubts about some of your statements. Carbon dioxide does not undergo changes in electron levels as far as I know. Also, I’m not sure if the altitude of an electron from the nucleus is quite correct either. I think these are visualisations of differences in energy. However in the case of CO2, the extra energy is not sufficient to do any of that. It simply changes the vibrational energy. CO2 has four vibrational modes and the amplitude of the vibration is what changes on excitation.
I don’t think these change the the substance of your main claim, but since I am still grappling with that. I cannot comment either way. There is certainly something seriously wrong with the settled science.
As a footnote to Anthony, it may be a contentious topic but it is absolutely fundamental to the debate. The principles of spectroscopy are well known but when transferred to an atmospheric context there are masses of uncertainties to be resolved. This is where pseudo scientific assumptions and cherry picking of facts can support a credible argument on either side. Debates like this make gradual progress in revealing better insight. Thank you for posting this one.
under GHG theory the temperature of the earth would be unchanged if all the O2 and N2 was removed.
this makes no sense. the atmosphere has thermal inertia which would be dramatically reduced without O2 and N2. Nowhere is this accounted for.
ferdberple,
So what would the earth be like without an atmosphere?
The average solar constant is 1,368 W/m^2 with an S-B BB temperature of 394 K or 17 C higher than the boiling point of water under sea level atmospheric pressure, which would no longer exist. The oceans would boil away removing the giga-tons of pressure that keeps the molten core in place. The molten core would push through the floor flooding the surface with dark magma changing both emissivity and albedo. With no atmosphere a steady rain of meteorites would pulverize the surface to dust same as the moon. The earth would be much like the moon with a similar albedo (0.12) and large swings in surface temperature from lit to dark sides. No clouds, no vegetation, no snow, no ice a completely different albedo, certainly not the current 30%. No molecules means no convection, conduction, latent energy and surface absorption/radiation would be anybody’s guess. Whatever the conditions of the earth would be without an atmosphere, it is most certainly NOT 240 W/m^2 and 255K.
The alleged 33C difference is between a) an average surface temperature composed of thousands of WAGs that must be +/- entire degrees and b) a theoretical temperature calculation 100 km (32 km) away that cannot even be measured (no molecules to compare) and c) all with an intact and fully functioning atmosphere.
The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.
“The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.”
Sorry, but that is not at all how it works. Hint: check the thermal conductivity of air (it is about the same as polyurethane foam) and calculate the temperature gradient needed to move 168 Wm^-2 through, say, 10 kilometers of polyurethane foam.
Also how do you explain that the temperature reaches a minimum at the tropopause and then starts increasing again?
The dominant heat-transfer mechanism in the atmosphere is convection and the lapse-rate is simply the amount of energy needed to drive the convection.
tty
“Also how do you explain that the temperature reaches a minimum at the tropopause and then starts increasing again?”
As molecular density decreases there is less molecular and more radiation. It’s a balance point.
Less molecular what?
GHG theory predicts the tropospheric hotspot. This has failed to emerge.
in science a single failed prediction is absolute proof of a failed theory.
however, trying to prove why GHG theory failed is largely a fools errand, because we have no replacement theory.
for example. we know that Newtonian gravity fails to predict rotational speed of gravity. however, we cannot. explain why because we have no alternative theory. instead we are left to invent a new form of matter. dark matter that obeys some laws but not others. and then we postulate that the universe is 90% dark matter. this is GHG theory mistakes on a cosmic scale.
correction. fails to predict rotational speed of galaxies.
Tropospheric hotspot : https://phys.org/news/2015-05-climate-scientists-elusive-tropospheric-hot.html
C. Paul Pierett,
Did you read what their “evidence” is for the hotspot, that Satellites still can’t find.
This one is much better article:
Study: Tropical Hotspot ‘Fingerprint’ Of Global Warming Doesn’t Exist In The Real World Data
https://wattsupwiththat.com/2016/09/22/study-tropical-hotspot-fingerprint-of-global-warming-doesnt-exist-in-the-real-world-data/
Satellite data shows no warming at all in one area and only 1/3 of the modeled warming in the other area.
You are easily fooled.
Got a citation other than a blog?
Something like this: http://iopscience.iop.org/article/10.1088/1748-9326/10/5/054007/meta
You are too lazy to follow the link I gave you,you also didn’t answer my question about the “evidence” in YOUR link.
“The researchers instead used observations and combined two well-known techniques—linear regression and Kriging”
Ha ha ha ha ha……..
You can’t be THAT stupid.
Meanwhile a rebuttal was posted on it:
Desperation — who needs thermometers? Sherwood finds missing hot spot with homogenized “wind” data
“The fingerprint test of the water-vapor feedback is the “hot-spot”, a warming of a band of the upper troposphere 10 km over the tropics. (See the reasons below at the end). The weather balloons were designed and calibrated to measure temperature and humidity as they rise through the sky and right through the hot-spot. Their results are unequivocal: red was not yellow; the spot was not hotter. Supporting this, the specific humidity was also supposed to rise, but fell instead. If the computer models worked on everything else, we might wonder if the millions of observations were biased, but the models didn’t predict the pause, were wrong about humidity, rainfall, drought, and clouds too. They didn’t work on regional, local, or continental scales and can’t explain long term historic climate either. At this point, a scientist would throw out the theory. The weather balloons independently agreed with each other, the humidity results fitted the temperature results, the whole lot was loosely supported by satellites. The data doesn’t need homogenising or kriging or obscure numerical witchcraft.
Instead Steven Sherwood and Nidhi Nishant of UNSW revisited their 2008 technique of homogenizing temperature data by using wind data as well. They homogenised it again. They have iterated the iteration? They’ve also extended it from 2005 to 2013 and changed the “wind shear” component to “vector wind”. Their new homogenized-temp-wind data is below (left). The model predictions of 2005 are centre, and the radiosonde temperature results (before homogenisation etc) are on the right.”
Charts in the below link.
http://joannenova.com.au/2015/05/desperation-who-needs-thermometers-sherwood-finds-missing-hot-spot-with-homogenized-wind-data/
Here is what the SATELLITE data was showing up to 2013:
http://www.climate4you.com/images/EquatorSurface300hPa200hPaDecadalTempChange%20BARCHART.gif
Professor Sherwood produced a dumb PDF.
Rh measurements falling.
ferdberple
Layman’s interpretation of what you have just posted.
A scientific theory doesn’t work because something is missing. It’s not known what’s missing, but introducing an unknown makes the original theory work.
No wonder I abandoned science at secondary school. It’s like playing a game of chess, with a million pieces. Nothing is ever solved, just continually shuffled around with everyone having an opinion on which way to move a single pawn, that doesn’t exist.
“Nothing is ever solved, just continually shuffled around with everyone having an opinion on which way to move a single pawn, that doesn’t exist.”
You just described all of life.
ferdberple
RGHE/GHG theory assumes the earth is warmer w/ than w/o atmosphere. Attempts to explain this assumption are futile because it is fundamentally incorrect.
The atmospheric insulation blanket creates a thermal resistance (all 32km worth of molecules that matter) just like an electrical resistance or hydraulic resistance. In all three instances a potential difference is required to move energy: dT or dV or dH, from A to B. For heat flow it is: Q = U A dT where U, aka 1/resistance, is the combined effect of conduction, convection, latent and radiative properties together with wind, storms, lit side, dark side, etc. everything that impedes thermal movement from surface to ToA where 100% radiation takes over. (No molecules in space to take the energy handoff.)
The atmosphere is little more than a fundamental HVAC problem.
RGHE theory says the ground would get cold w/o “back” downwelling radiation. Reams of USCRN data show absolutely zero evidence of this.
The sun warms it all. Air temperature swings widely because of its low thermal capacity. The 2cm ground temperature swings very little because of its high thermal capacity. The 100 cm ground temperature hardly changes.
All night and in winter the air temperature spends many hours below ground temperature so the ground heats the air, NOT vice versa.
RGHE theory simply doesn’t stand up to physical observations. All the rest of the discussion is sound and fury signifying nothing.
What shall occupy our empty hours then?
However the resistance is gravitational, not thermal, since the dominating heat transfer mechanism is convection. The atmosphere has such low thermal conductivity that conduction is uttery insignificant. It is no coincidence that almost all insulating materials are mostly air.
Cold air settles, warm air rises by gravity.
And heat rises through my due to anti-gravity waves.
attic
“Cold air settles, warm air rises by gravity.”
Essentially correct. Warm air has lower density and therefore rises. As it rises it expands and cools down. Ultimately it will come down somewhere else, outside the convection cell.
“GHG theory predicts the tropospheric hotspot. This has failed to emerge.”
It is also the theory in any sort of warming. It is merely a function of greater LH release aloft in the tropical high atmosphere via convection.
One thing – it ois difficult to find because of the nature of the instruments used. Radiosondes are imprecise for the job and have changed over the years, and sat obs are contaminated by Stratospheric cooling – which is a function of GHG theory.
Another thing is that it has been found …
“First, tropical warming is equally strong over both the 1959–2012 and 1979–2012 periods, increasing smoothly and almost moist-adiabatically from the surface (where it is roughly 0.14 K/decade) to 300 hPa (where it is about 0.25 K/decade over both periods), a pattern very close to that in climate model predictions. ”
http://iopscience.iop.org/article/10.1088/1748-9326/10/5/054007/meta
It is hard to know where to begin with such a complete pile of nonsense. One of the more obvious errors is confusing a black body radiation spectrum with discrete absorption lines due to molecular transitions. Then there is the weird description of electron orbitals which do not make any sense. If there was any attempt at quality control on this blog this post would have been thrown into the trash where it belongs.
As would yours.
I think the main problem is that the author seems to be completely unaware that the energy levels of a molecule are a lot more complex than for a single atom.
Germonio
November 19, 2017 at 10:42 am: Once again, explain not excoriate. Show how you are more knowledgeable, not just something else…..
radiation works by the exchange of photons. convection works by the exchange of virtual photons. thus if there is a GHG effect for co2 there must be the same effect for n2.
co2 absorbs a photon from the surface. this can be radiated up or down. n2 absorbs a virtual photon from the surface. this can be conducted up or down.
in all aspects the GHG effect operates like conduction at a distance, except that co2 can radiate to space while n2 cannot. thus co2 cools the surface on net as compared to n2.
Ferd,
I would suggest you need a dictionary. Convection refers to the movement of hot molecules from one place to another. It has nothing to do with virtual photons.
Fascinating!
I’m learning here just how little I know about gas absorbtion and emmission phenomena.
I’m also learning how much utter bilge can be written by someone who knows *nothing* about such things, but is willing to shoot their mouth off endlessly. And getting a fair idea of how difficult it must be for a relative layman to tell the difference.
For the record, Gill is wrong, but he’s at least honest. It would be nice if someone took him through his presentation point by point and explained (gently) where he went wrong. I’d do it, but, as I hinted, I’m not best qualified.
(Incidentally, I asked for something like this debate weeks ago – it’s good to see it happening. Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.)
Uncle Gus, why the rant after saying this: |… I’m not best qualified.”? But, you feel yourself qualified to claim: “Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.” I bet you don’t even understand your own hypocrisy.
Please try, we, the great unwashed, are waiting to be educated.
“I’m learning here just how little I know ”
That has been obvious to us in basically every one of your posts. !
“Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming.”
no.
the downwelling LWIR is a RESULT of GHGs ( like water) it is not the cause of warming.
When people “popularize” the science and dumb it down, they say LWIR causes the warming.
nope.
When you increase c02 two things happens.
1. the stratosphere cools. We have seen this. Its How we know the warming is GHG related and not the
sun.
2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.
a) radiating from a colder place entails a slow down in the cooling to space.
b) to maintain energy balance, the compensating increase in warmth at the surface has three
pathways:
1. it can go into the ocean
2. it can melt ice
3. it can warm the air.
Or 4. or it might mean the earth is cooler.
Why does the stratosphere cool? I’m not disagreeing, I just would like to hear your explanation.
Because in the stratosphere radiation dominates over convection, so more GHG increases the radiative energy loss. The temperature gradient is also the opposite – it gets warmer with altitude, since the main energy source is UV absorption in the ozone layer from which temperature decreases both upwards and downwards.
Tom November 19, 2017 at 12:10 pm
Even more explaining needed of the temperature changes as altitude increases:
In the Troposphere, temperature falls with altitude but, above the Troposphere comes the Stratosphere where temperatures increase with altitude but, above the Stratosphere comes the Mesosphere where temperatures fall again with altitude but, above the Mesosphere comes the Thermosphere where temperatures go up again with altitude!
Given the differences of opinion on this thread, I would love to hear everyone’s theories as to why all these temperature changes happen? 🙂
“2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.”
So you mean that when the temperature at the surface goes up the average temperature of the Earth seen from space will go down? That the temperature profile of the atmosphere is somehow unchangeable?
What does happen is that with more CO2 the average altitude from which the heat radiates out will rise slightly. All things equal this means that the surface temperature will also rise slightly, since more energy is needed to lift the convecting air.
However all things aren’t equal. Higher temperature means more evaporation and more H2O in the atmosphere More H2O in the atmosphere means a less steep lapse rate, i e a smaller temperature difference between the surface and a specific altitude. There will also be albedo effects (=clouds, snow, vegetation).
All things considered more CO2 in the atmosphere does seem likely to result in some warming, but the effect is probably quite slight.
Stephen, what do you, as an English graduate, know about the science?
Mosher finally gets something right. Thing is, this effect cannot be calculated, so all attempts at defining Climate Sensitivity are un-scientific.
Stephen knows a hell of a lot more about the science than you do, Martin.
I don’t always agree with Stephen, but I take his analysis (not his snark) seriously. You, on the other hand…
You, as an engineer, should be ashamed that you have been “schooled” (as the kids say) by an English major.
“1. it can go into the ocean
2. it can melt ice
3. it can warm the air.”
NONE of which is happening.
1. Oceans are currently cooling, especially Southern Ocean
2. Only ice melt is down from the Highest level since the LIA (late 1970s)
Arctic sea ice is still in the top 10% of extent wrt the last 10,000 years
Purely in line with the approx 60 year AMO cycle
3. No warming in satellite data apart from ocean events.. ie release of energy from the oceans at El Nino events.
You are still selling moldy lemons , Mosh. Useless even as a car salesman.
No, if the magnitude of the effect cannot be calculated, then, Stephen Mosher has admitted that he, and BEST, are simple record-keepers, unable to predict Climate Sensitivity, and have also admitted, no one else can either.
No one can. From first principles, which means from the laws of Physics which have nothing to do with dodgy thermometer records, we cannot calculate Climate Sensitivity. Think about this.
Most people do not understand that the entire atmosphere radiates to space, including N2, O2, Argon, and the entire mass of the Atmosphere. All Matter above Absolute Zero radiates all the time.
Hotter Matter radiates to the Absolute Zero of Outer Space, which could maybe be a small fraction of a degree Kelvin above Absolute Zero, More than cooler matter.
There is this word Opaque. This word means No Radiation Penetrates. The Top of the Atmosphere radiates to space at a temperature far above Absolute Zero, much closer to the temperature at which CO2 has ceased to be opaque to the radiation it can absorb at its best absorption frequency, 15 Microns, corresponding to around -80 C.
More CO2 raises the altitude at which this happens. Due to the Lapse Rate, the higher the altitude at which the atmosphere no longer encounters an opaque layer, the cooler the temperature becomes at which the Entire Atmosphere can radiate to space. Heat Transfer is proportional to the Delta T, the difference in temperature between hotter and cooler.
More CO2 reduces the atmosphere’s flux to space, increasing the amount of heat retained in the atmosphere, warming the air at the surface, but no one can calculate how much.
Mosher is right, this time…
Not much, because water compensates for the increase.
“Stephen Mosher has admitted that he, and BEST, are simple record-keepers”
Don’t forget .. Data Molesters. !!
“Don’t forget .. Data Molesters. !!”
Stupid comment of the day. Evidence of a 3rd grade intellect.
No need to put a title on your posts Chris. They are always stupid. !
Do you DENY that BEST massively manipulate/torture the data to meet their “regional expectations” ?
Steven – an honest question to answer please. If the average altitude at which th e Earth radiates to space is raised surely the radiating surface is also increased precisely because it is a now a larger spherical surface. Is that taken into account?
This effect is negligible.
MCoEA:
It’s easy to do the math. Let’s say, the “emission level” is increased by 100 meters (0.1km). The radius is increased from 6400km to 6400.1km, a 0.0015% increase. So the surface area is increased by 0.003%.
If you use a lapse rate of 6K per km, the raised layer would increase the surface temperature by 0.6K, other things being equal (yes, this is a highly simplified analysis). The increase in emission layer area of 3 parts in 100,000 is completely trivial in this analysis.
Ed Bo. – thanks.
I think you missed something very important. Water vapor dominates the radiative heat transfer from the lower troposphere, blocking most of the spectrum that other GHGs would otherwise block. When the buoyant air reaches the tropopause, the water vapor condenses out. Water vapor ceases to be a factor at all in the process of radiating from the ground to space. So the effects of increasing the amounts of the other GHGs is no longer masked by water vapor.
No wonder you can’t figure it out, you forgot the optical window to space.
No, he didn’t forget the sky window. He said the increase in warmth at the surface, he was not referring to the fact that some of the sun’s energy that reaches the earth’s surface goes back out into space through the sky window.
Right, but he probably doesn’t expect the atm to self adjust how cold it gets, and there not being all that much additional warmth left to deal with. So none of his choices are correct, really.
I’m throwing food now.
Can you explain “slow down cooling”? Does the emissivity of the planet change? Does CO2 change the emissivity of the planet? Where, then, is the back up that constitutes the “slow down”? — in a magical zone between the given emissivity and the given planetary radiation? How does that work? “Slow down” with respect to what time frame? Is Earth on a schedule to cool by a certain time? Who sets it? How long does it take for enough “slow down” to cause heating? WHAT exactly is being “slowed down”? What is the entity defining “cooling” that is being “slowed down”?
This “slow down the cooling to space” sounds a lot like a piece of glass inhibiting convection, … a roundabout way of resurrecting the idea of a barrier that keeps stuff from moving out. It’s a more indirect way of erecting that retaining barrier (via conceptual, rather than physical), but still it seems to beg holding onto a cherished, flawed idea.
I have yet to be convinced that a “colder place” can cause heating by any known physical means, either directly by adding heat or indirectly by “slowing down cooling”.
Energy comes in, energy goes out, all the time. If a little less energy goes out, then the atmosphere has a little more energy, and is a little warmer. Lapse rate means that the average temperature at the surface is determined by how much energy is leaving at the TOA.
Steve Mosher,
It is unfortunate that you believe you have to restate the obvious in response to a rubbish post..
But fair enough. Yes, increasing GHG’s warm the surface (though the details are complicated and not homogeneous in time or space). Yes, continued use of fossil fuels will cause additional warming unless offsetting factors keep that from happening.
Now that we are past that, the real issues are (and have always been) how much warming, over how long, the negative consequences of that warming, and what offsetting benefits (eg greater crop production) balance in whole or in apart the negative consequences of that warming.
LOOK HERE!
I got the first comment in this thread and I told you not to treat “photons” like they were real massless particles. I got my big red marker pen out and crossed out everybody who did.
Has anybody got another big red pen (or 2) please as I seem to have run out before I got to the end.
Furthermore ; Who the hell told more half of you that you could add radiative fluxes arithmetically?
Anyone who still thinks they can by tomorrow is invited round to my kitchen where I have a large number of radiative objects at all kinds of temperatures from 0 to 90 C which you will be required to arrange in an ensemble of your liking and invention in order to boil my egg.(Boil = 100 C)
Seriously guys and gals an actual experiment or even a thought experiment to show that adding radiative flux arithmetically is scientific nonsense is so flippin’ simple it is school physics level. Please – up your game!
If you cannot even grasp this the furthest you are going with atmospheric physics is horizontal dermatological absorption.
It’s not clear what you mean. If we had two suns, wouldn’t it be a lot hotter?
Double the W/m^2 at the sun (two suns) also doubles the W/m^2 at earth’s orbit – 1,368 * 2 = 2,736. S-B BB equivalent temperature rises to 468.7 K from 394 K.
Tom and nickreality65.
Lets go a bit further. The Sun occupies a tiny percentage of the area of the sky, I think something like 0.002% IIRC . So never mind TWO suns, let the thought experiment go large and we can have 50,000 suns , should just be able to squeeze them into that sky. Now please redo the SB calculation like you did for 2 suns, nick. The answer should tell you something. Especially when you compare it with the temperature of EACH sun. A new definition of heat transfer from “cold” to “hot” !
This is of course the same as my numerous 90C radiating objects in my kitchen which you need to arrange to boil my egg. Yes, there are at least 50,000 of them and you can arrange them in a sphere around my saucepan.
Looks like I am having just the toast then.
Badger:
You ask: “Who the hell told more half of you that you could add radiative fluxes arithmetically?”
The 1st Law of Thermodynamics (conservation of energy) told me that. It is any absolutely necessary consequence of a conserved quantity like energy.
In any actual thermodynamics class, virtually the first thing you learn is to add and subtract energy transfers to and from a “control mass” to determine changes in the energy of the control mass in “energy balance” calculations.
It’s no different from balancing your checkbook, which must observe “conservation of money”. In effect you are arguing that if you get a second job (let’s say lower paying than your first job), you can’t add the money you get from your second job to your bank account. It’s an absurd argument!
Actually you cannot add temperature fields, that is nonsense, but if you concert it to flux, W/m^2, you can, then concert it back to a temp.
You got flux and field confused.
Oooh, yes, Reverend! I see, now! Please don’t beat me again! Pleeeasse!
Badger, you are correct. If you could add w/m2 then it would be possible to cook steak with ice cubes.
Once an object has attained the max temperature it can adding a second source with the same temperature, as first, cannot cause the object to increase further. (T4 – T4) tells us that.