Note: This is a contentious subject, and I have often shied away from it because it often erupts in food fights. However, Mr. Gill is making a good-faith effort here, and asks some relevant questions that I consider worth discussing. His original essay was sans graphics, and I’ve added two relevant graphics to aid in the discussion. – Anthony
Do Wien’s Law and Quantum Physics 101 prove CO2 can’t warm anything?
Guest essay by Rod Gill
WUWT has happily demonstrated many ways CO2 fails to produce measurable warming. I’ve thought of another way. It’s so simple I must have missed something, but I simply can’t work out what. It goes like this…
Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming. I suggest the quality of that 2W of radiation is crucial to determining whether or not it causes any atmospheric warming at all. First a few key points which I think are facts and not open to dispute.
My understanding of Thermodynamics and Radiation from CO2 is as follows:
In Thermodynamics, Temperature is the average kinetic energy of the particles in a body (solid or gas).
The temperature of a volume of air has nothing to do with the amount of radiation (sometimes mislabelled as heat by scientists) passing through it. Unless that radiation is at a frequency that can be absorbed by the air, its temperature is completely unaffected by the radiation (ignoring any convectional heating).For example at the top of Mount Everest, there is a lot of solar energy (long and short wave radiation) there when the sun is out but the temperature is still cold.
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law). This linear variation is not evident in this kind of plot since the intensity increases with the fourth power of the temperature (Stefan- Boltzmann law). The nature of the peak wavelength change is made more evident by plotting the fourth root of the intensity. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/wien.html
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Photons with not enough energy to raise the orbit of any of the electrons are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. If I gave you my references, which knowing the web may or may not be accurate, you might erroneously come to the same conclusions I have. However I have tried to limit my research to University papers and lecture notes hoping they are more reliable.
If I’ve got this right, CO2 caused global warming isn’t possible. If I haven’t got this right, then exactly how does LWIR radiated from CO2 warm anything?
Many thanks and please limit comments to specifics mentioned above. And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
I am not disagreeing with anything (yet). But I would like to make a few comments before all and sundry jump in:
Photon s are NOT real massless particles. The concept of a photon is a human construct from our mind in an attempt to observe the apparent “lumpiness” of certain phenomena with electro-magnetic radiation.
E-M Radiation CANNOT be directly observed, you can only observe the effect on real matter, and we know real matter is “lumpy” (particles). It MAY be the case that e-m radiation is not in any way “quantised” but is purely analogue and only the effects seem to suggest quantisation hence the concept of the photon.
Try, if possible, making your arguments work BOTH for photons (quantised e-m lumps) and also for e-m which is not quantised. You may need 2 completely different arguments. I suggest the results of doing this will (or may be) illuminating (pun intended).
My final comment is that the understanding of this, i.e. RADIATION, as it applies in the real world is THE most important thing we need to grasp in considering CAGW. and if we cannot grasp it correctly and securely by logical argument then let’s have some suggestions from you for REAL WORLD EXPERIMENTS to settle the issue(s).
The Reverend Badger – ” The concept of a photon is a human construct”
Exactly!
The concept of Photons represent half of a model. The duality model includes the ‘wave’. And when Earth’s release of heat is envisioned as a wave, the entire ‘trapping heat’ theory collapses.
Please don’t get into wave-particle duality. It’s a minefield. Your language is very engineer like rather than physical but seems to express the theory of the quantum model well. The problem with the AGW radiation theory from non physicists is that they are unable to separate quantum from classical physics. If you think in terms of energy rather than orbitals Neil Bohrs, it actually gets easier to explain what you have explained well here. The absorption energies are derived from the degrees of freedom within the molecule. CO² has two. Hence the 10 and 15µ absorption bands. However, I like your engineers approach. It’s good.
Gerontius – I thought a “degenerate pair of benders” were what we called Friday and Saturday nights at the frat house. This clearly has nothing to do with climate science!
O..Kay… Lets test your belief a little shall we. These elementary particles you call photons have a frequency (or wavelength) do they not? Can you tell me the frequency range please over which they exist. Do we have
60Hz/50Hz photons emanating from our power lines for example? Is there an upper frequency limit to them?
For further thinking please research the origin of the word/concept.
An object that instantly accelerates back to the speed of light when it leaves a variant local spacetime without additional energy *doesn’t exist*. There’s the rub. Its neither a wave NOR a particle. They have an infinite energy to mass ratio that is never reduced but have a quantifiable amount of energy. That means there’s nothing in them, not just “mass-less” but literally nothing. They no more exist than an electron exists outside it’s orbit.
Massless particles have zero rest mass. Their relativistic mass is simply their relativistic energy, divided by c^2, or mrel = E/c^2. (Can’t make the “rel” a subscript.)
The energy for photons is E = hf, where h is Planck’s constant and f is the photon frequency. This frequency and thus the relativistic energy are frame-dependent, a point I’ve been trying to make with LdB.
If an observer runs away from a photon in the direction the photon travels from a source, and it catches up with the observer, he or see will see it as having less energy than it had at the source. The faster the observer is traveling with regard to the source when the photon catches up, the less energy the photon has. As an observer approaches the speed of light with regard to the source, the photon looks redder and redder, by relativistic Doppler effect, and the energy of a very long-wavelength photon approaches zero. This is because photon is massless—the rest mass of a photon is zero.
In one of the Teaching Company’s Great Courses video series, the presenter talked about being asked the question “How big are photons?” during his thesis defense. He eventually replied that it depended on the wavelength. The proof of this is the pierced plate in the window of the microwave door. Photons of visible light fit through the holes and microwave photons (based on the holes in metal plates that they pass through) are disks within the uncertainty limits of a diameter of ten centimeters.
The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule
At this point I started to realise that the self-taught quantum physics was a little shaky. Despite numerous technical errors like “degrees Kelvin” ( the unit is kelvin ) ; writing Km for kilometres which shows a lack of basic understanding of the unit prefices; talking of “the” wavelength of thermally emitted IR when he should be referring to PEAK wavelength. I rather gave up hope on this article having any scientific merit at this point.
No one talks of the “altitude” of an electron orbit, they are not little Spuniks. Neither is the energy involved in photon absorption or emission the “kinetic” energy of the atomic electrons, it is potential energy which is transferred. Hence the idea of energy levels and dropping to a lower energy level producing the energy of the photon. The author also seems to be confusing electron states and the vibrational energy of the atoms within a molecule.
The IR interaction comes from vibrational energy of the three atoms in gages like CO2 and H2O ( a bit like a tuning fork ) . This is why diatomic molecules like O2 and N2 are not GHGs.
IR radiation only travels a few metres at ground level without being absorbed, so lets forget the notion that this only happens at a very narrow range of altitude around 90km.
I did not see much point in finishing the article.
Photons exist independently of your organs for detecting them, such as eyes and skin. But those organs wouldn’t have evolved were photons of visible and IR light not real.
Consider the process of pair production. A pair of “matter” particles (fermions) is produced by a pair of photons.
Because of momentum conservation laws, a pair of fermions can’t be created from a single photon. However, these laws permit matter creation when in the presence of another particle (another boson, or even a fermion) which can share the primary photon’s momentum. Thus, matter can be created out of two photons.
Greg,
As a general rule, most EM radiation cannot be observed directly. A limited region, between about 400 and 700 microns wavelength, can be perceived by human eyes and is generally referred to as “Light.”
Or consider the photoelectric effect, which is based upon the observation that EM radiation consists of a series of particles, ie photons. When a photon hits an electron on a metal surface, the electron can be emitted. The emitted electrons are called photoelectrons.
If photons don’t account for the PE, how do you imagine it works?
WUWT is an equal opportunity science “d@nial” site, except for “Sl@yers”. Those who d@ny gravity in favor of an imaginary “electric universe” are welcome here, along with those who d@ny EM radiation. Even creationists are permitted to comment, but not those skeptical of the GHE. As are Younger Dryas impact hypothesis proponents.
Full disclosure, IMO the GHE exists, but is negligible for CO2 beyond about 200 ppm. For the health of plants and the planet, 1200 ppm is about optimum. Evolution is a fact, as are gravity and EM radiation, while a YD impact is anti-scientific fantasy.
I concur…My BS meter went off immediately when it it looked like Rod Gill was trying to ‘manufacture’ a reason why there is no warming from CO2 (or presumably H2O for that matter) instead of perhaps hypothesizing how a negative feedback might make any warming a moot point. Why is it so hard for some here to rationalize that yes, there is a tiny weensy bit of warming from GHG’s? Wouldn’t it be better to make the argument that a bit of warming is good, and the additional CO2 is good for the Garden? I fear that arguments like this are very damaging to the skeptic cause, since then the alarmists just point at all of us and say we are very mistaken. We can’t win this battle about the notion that CO2 is a pollutant until we get real about basic atmospheric physics.
Bloke down the pub
November 19, 2017 6:41 am
‘Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.’
The behavior of electrons in atoms is still open to debate. But an electron “orbital” is a conflation of the prior molecular orbital theory and quantum dynamics to explain how electrons govern chemical reactions(and other things) in quantum mechanics. I believe the current theory is that orbitals are not a physical difference but an energy level. Atoms that absorb a photon and if it of the right frequency it increases the energy of some of the electrons. The physical size of the atom doesn’t change, but some of the electrons have more energy and the frequency associated with those electrons- E= h * f, the Planck constant times frequency.
Molecules, such as CO2, can also absorb energy as motion of the atoms in the molecule. This occurs at longer times and wavelengths. In CO2 the CO bonds can stretch in and out like a spring, both O atoms can move closer and further from each other(bend) and they wave, or twist the molecule around the plane of the O-C-O bonds along with the whole molecule rotating rather than just moving.
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit.” Not really. Temperature is a measure the the kinetic energy in matter. Radiation emittance and absorption is function of the energy within molecules or individual atoms and photons. A CO2 molecule can absorb a photon at any temperature and increase its energy without necessarily moving faster. But it can both absorb and transfer kinetic and internal energy from collisions and absorption to other molecules. The ozone reactions in the stratosphere are a good example. An ozone molecule can absorb a photon and split into an O atom and an O2 molecule. The O atom can end up with enough energy to react with another O2 molecule, rather than simply colliding and bouncing off at a slightly higher temperature.
All this stuff is very complicated and it really can’t be dealt with in words. Rod Gil’s essay is a good attempt to do physics with words and it doesn’t really work. CO2 can absorb and emit infrared photons but how the energy from those photons behaves needs math and physics models. CO2 is a trace gas in the atmosphere and doesn’t do much. The only major effect it has is through poor models of how the atmosphere reacts to energy from the sun that have a huge increased water vapor bias built into them. They model AN atmosphere, but not the atmosphere we have.
Exactly. Changing the total energy via having electrons in a higher orbital (a statistic) is not the same thing has having them move faster in 3 dimension translation, or in 3 dimension rotation, or in 3 dimension stretch along an axis, or in 3 dimension stretch perpendicular to an axis.
For light to heat something, the additional energy must be converted to kinetic energy increasing the total (and note, this is only possible to measure in a defined sample). For light to cool something, the loss of energy must result in a decrease in the kinetic energy. Energy that goes into phase changes that don’t change the kinetic energy can’t change the temperature.
Then we must remember that absorbers are emitters and that there is always incoming EM radiation. Even at night, there is still incoming long wave EM radiation. Otherwise, IR, microwave radio and long wave radio astronomy would not be possible. IR active gases (and aerosols) are two way screens. They screen some of the incoming and screen some of the outgoing. They can’t add extra energy. They can only time shift some of the energy.
This is actually pretty funny. We debate the supposedly critical problem of global warming and invoke the most fundamental basis of our understanding of particle physics in describing the issue. Nobody really understands the true nature of the underlying universe.
I kind of favour pilot wave theory but hey, that’s just me.
The funny thing is that they say the science is settled. A few years ago all the bright young physicists were in love with string theory. They stalled out and some other genius physicist called it, “not even wrong”. Surely one of the best putdowns ever.
And that’s physics! A whole lot brainier field than the climate swamp. They don’t even know what 90% of the universe is made of!
“They don’t even know what 90% of the universe is made of!”
Den!er! Dark matter is made of carbon dioxide. Human emitted carbon dioxide. Dark energy? That’s made out of human emitted carbon dioxide too. Pulsars, magnetars and quasars are also anthropogenic in origin.
String theory is just an idea Alan Guth thought of off of the top of his head one day.
The odds of it turning out to be an actual description of objective reality would be less than guessing the winning numbers in the Mega Millions lottery and the Powerball lottery with only two guesses. Not astronomical odds, but cosmological ones. IMO
And the 90% that is dark matter and dark energy?
These are ad hoc inventions because of some things that do not make sense according to current understanding of the four forces, such as the anomalous rotation rates of galaxies and inconsistencies in the red shift of distant Type A supernovae.
They create more questions than they answer.
>>
. . . inconsistencies in the red shift of distant Type A supernovae.
<<
It’s Type Ia supernovae. If Type ia supernovae are standard candles (like it is believed), then they point to a discrepancy in the expansion rate of the Universe–that is, the Universe expansion rate is speeding up.
Yes, I should know better than to comment off the cuff in the middle of the night.
I know the whole story, recall reading the research reports when they were first made public.
Maybe the expansion of the Universe is accelerating, and the invention of dark energy is justified.
But to me it hardly seems proven…hardly seems that there could be no other explanation.
In fact more recently, I have read that type 1a’s may not be the standard candle that has been thought.
But even if they are, something else could be going on.
as Greg stated earlier infra red comes from molecular vibrations, electronic transitions are for carbon dioxide are of far higher energy beyond that of visible light, Hence it is transparent. Indeed most gases are colourless and so thei electrons are not affected by visible (yes there are coloured gases NO2 is brown)
Greg, you are wrong with diatomic molecules not absorbing infrared light. Oxygen and Nitrogen do not interact because they are not polar. HCl, CO being polar have infrared absorptions.
DR
November 19, 2017 6:41 am
Ah, the upside down greenhouse effect. Things haven’t panned out quite the way it was explained ~30 years ago using basic high school physics as we’re constantly reminded. http://1clickurl.com/XYwCjxT
I have seen articles claiming glacier melting will cause sea level rise but what about the miles-thick layer of salt that underlie continental areas and more? Under most of Germany is a miles-thick salt layer. Under the
Great Lakes lie salt deposits mined in areas like Detroit. The Gulf of Mexico has a salt bed that dissolved could flood all land up to Yellowstone.
If the salt at the bottom of the gulf of Mexico was somehow brought to the surface and dissolved into the seawater, the ocean would go down, not up.
Obviously if you took miles of salt from a continent above sea level and dumped it into the ocean, the ocean would rise…but it would rise less than if you dumped an equal volume of insoluble rocks into the ocean.
x volume of NaCl + x volume of H2O is < 2x.
As for raising the oceans to the elevation above sea level of Yellowstone Park in Wyoming…impossible.
There is no where near enough land on all of the continents to raise the ocean that high.
The average height of all of the continents is about 2750', or about 840 meters.
The elevation of the Yellowstone Plateau is about 8000', or about 2,400 meters.
Since the continents are about 29% land and 71% ocean, putting all of the land above sea level into the oceans would raise the oceans less than one half of that 840 meters.
So…nope.
It’s really quite simple. CO2 at altitude is emitting at -80 deg C toward the surface which is 15 deg C. The energy levels of the surface equivalent to -80 dg C are full and the downward IR is reflected upwards. It is simply impossible for a cold body to heat a warmer body. We are done. No effect. Fantasy to think otherwise.
BTW, this is true for any IR absorbing gases in the atmosphere near the surface, as they are always warmer than -80 deg C. Same argument as above.
All this ignores the fact that we live in a pre-charged environment due to the magnetosphere and solar wind induction combined with cosmic rays. MOST reactions occur more readily in an heated beaker.
PR;
Huh?
Pre-charged?
What do you mean by that?
Reactions?
Absorption is not a reaction.
Not in the conventional meaning of these words.
Reactions refer to chemical processes.
Chemical reactions proceed more quickly in warmer conditions due to the required activation energy.
A “good-faith effort” indeed, but, IMHO, Rod Gill misapplies the equations he uses and misinterprets the results. Satellite views of long wave radiation from Earth to Space, looking down, and views of long radiation from Space to Earth, looking up from the surface, show the actual warming effects of water vapor, CO2, and other “greenhouse” gases.
Would you care to elaborate the equations and results you reference with what you consider the correct results ? Or will you just keep waving hands – I do like the steady flow of cool air that produces though.
” Wien’s Law ” is nothing more than a consequence of the ” Planck Radiation Formula. ”
The Planck formula is a theoretical derivation of the radiation properties of a theoretical (and non-existent) material object called a ” Black Body “, hence “Black Body Radiation. ”
Such a body does not, and CAN NOT exist. so BB radiation also does not exist.
BUT ! credible approximations to Black Bodies, and BB radiation do exist.
NO real material object can absorb 100.000….% of even a SINGLE frequency or wavelength of EM radiation, let alone ALL possible frequencies and wavelengths from ZERO frequency to ZERO Wavelength.
The remarkable thing about the Planck radiation formula is that it contains NO arbitrary constants, that need to be determined by experiment.
The Planck Formula : W(lambda) = C1 / (lambda)^5.(exp(C2/lambda.T – 1) where Lambda is the wavelength and T the kelvin temperature.
C1 and C2 are commonly called the first and second radiation constants. BUT !!
C1 has the value …. 2.pi.h.c^2 …. h being Planck’s constant, and c the velocity of light.
C2 has the value …. hc/k where k is Boltzmann’s constant.
Can you believe that !! ??? All that fictional stuff is completely described in terms of some of the most fundamental constants of Physics. It is one of the miracles of modern physics.
NOW ! Hidden in that Planck formula is the often not known fact that W(lambda) is a function of a …. SINGLE VARIABLE …. That single variable is (lambda x T) .
The best presentation of the Planck formula plots W(lambda) / W(lambdamax) versus lambda / lambdamax where lambdamax is the wavelength at which the peak spectral radiant emittance occurs, and W(lambdamax) is the value of the spectral radiant emittance at that peak wavelength.
On a logarithmic scale the useful range for lambda/lambdamax is 0.1 to 10.for a linear vertical scale for W(lambda)/W(lambdamax)
Only 1% of the total is emitted at wavelengths shorter than one half of the peak wavelength, and 25% is emitted at wavelengths below the peak wavelength.
Only 1% of the total remains at wavelengths longer than eight (8) times the peak wavelength.
Plotted on both logarithmic axes, a range of 0.1 to 50 for horizontal , and 1 down to 10^-5 for the vertical.
W(lambda)/W(lambdamax) drops to 10^-5 at values of 0.2 and 40 for Lambda/lambdamax.
So for most practical purposes, BB radiation has 98% of the energy between 0.2 and 8 times the peak wavelength.. Certainly for climate considerations the rest doesn’t matter although the physics of it is of great interest for other reasons.
I agree with the author EM radiation is NOT heat.
I don’t necessarily agree with the author’s thesis; and I don’t have time to critique it in detail.
I believe the sun warms the earth; and if you delay the cooling process, the sun keeps on radiating, so it must tend to get warmer. BUT ! that is if you ignore the fact that clouds will vary and compensate.
And NO, I am not concerned in the least with global warming; or climate change. I can drive ten miles down the road and get climate change.
G
PS for a really good Planck graph, see “Modern Optical Engineering”, by the late Warren J. Smith Page 194 in my 1966 edition.
@davidmhoffer, Ira glickstein – I do apologize for my snarky interjection – at first glance it appeared to look like “it’s wrong and finding the proof is left as an exercise to the reader” 🙁
The answer to this question, almost always, is plainly no. If you miraculously end up with blueprints of a perpetuum mobile, it is a mistake. And mistakes are easy to make, difficult to find and fix. Thus the tradition is that the professor has assistants to help students to find their errors. And errors they do, from hour to hour, week to week, year to year.
The question is not does an increase in greenhouse gas cause surface warming but rather how much surface warming. Increases in greenhouse gases in the atmosphere also increases convection cooling which reduces the lapse rate which reduces surface warming.
The infamous without ‘feedbacks’ cult of CAGW’s calculation (this is the so called 1 dimensional calculation that predicted 1.2C to 1.4C surface warming for a doubling of atmospheric CO2) incorrectly/illogical/irrationally/against the laws of physics held the lapse rate constant to determine (fudge) the estimated surface forcing for a doubling of atmospheric CO2. There is no scientific justification for fixing the lapse rate to calculate the no ‘feedback’ forcing of greenhouse gases.
Convection cooling is a physical fact not a theory and cannot be ignored in the without ‘feedbacks’ calculation. The change in forcing at the surface of the planet is less than the change in forcing higher in the atmosphere due to the increased convection cooling caused by greenhouse gases. We do not need to appeal to crank ‘science’ that there is no greenhouse gas forcing to destroy the cult of CAGW ‘scientific’ argument that there is a global warming crisis problem to solve.
P.S. The IPCC general circulation models (GCM) have more than a 100 free variables that are subjectively set to produce the ‘predicted’ warming.
Collapse of the Anthropogenic Warming Theory of the IPCC
4. Conclusions
In physical reality, the surface climate sensitivity is 0.1~0.2K from the energy budget of the earth and the surface radiative forcing of 1.1W.m2 for 2xCO2. Since there is no positive feedback from water vapor and ice albedo at the surface, the zero feedback climate sensitivity CS (FAH) is also 0.1~0.2K. A 1K warming occurs in responding to the radiative forcing of 3.7W/m2 for 2xCO2 at the effective radiation height of 5km. This gives the slightly reduced lapse rate of 6.3K/km from 6.5K/km as shown in Fig.2.
Transcript of a portion of Weart’s interview with Hansen.
Weart: This was a radiative convective model, so where’s the convective part come in. Again, are you using somebody else’s…
Hansen: That’s trivial. You just put in…
Weart: … a lapse rate…
Hansen: Yes. So it’s a fudge. That’s why you have to have a 3-D model to do it properly. In the 1-D model, it’s just a fudge, and you can choose different lapse rates and you get somewhat different answers (William: Different answers that invalidate CAGW, the 3-D models have more than 100 parameters to play with so any answer is possible. The 1-D model is simple so it possible to see the fudging/shenanigans). So you try to pick something that has some physical justification. But the best justification is probably trying to put in the fundamental equations into a 3-D model.
The modern anthropogenic global warming (AGW) theory began from the one dimensional radiative convective equilibrium model (1DRCM) studies with the fixed absolute and relative humidity utilizing the fixed lapse rate assumption of 6.5K/km (FLRA) for 1xCO2 and 2xCO2 [Manabe & Strickler, 1964; Manabe & Wetherald, 1967; Hansen et al., 1981]. Table 1 shows the obtained climate sensitivities for 2xCO2 in these studies, in which the climate sensitivity with the fixed absolute humidity CS (FAH) is 1.2~1.3K [Hansen et al., 1984].
In the 1DRCM studies, the most basic assumption is the fixed lapse rate of 6.5K/km for 1xCO2 and 2xCO2. The lapse rate of 6.5K/km is defined for 1xCO2 in the U.S. Standard Atmosphere (1962) [Ramanathan & Coakley, 1978]. There is no guarantee, however, for the same lapse rate maintained in the perturbed atmosphere with 2xCO2 [Chylek & Kiehl, 1981; Sinha, 1995]. Therefore, the lapse rate for 2xCO2 is a parameter requiring a sensitivity analysis as shown in Fig.1.
The followings are supporting data (William: In peer reviewed papers, published more than 20 years ago that support the assertion that convection cooling increases when there is an increase in greenhouse gases and support the assertion that a doubling of atmospheric CO2 will cause surface warming of less than 0.3C) for the Kimoto lapse rate theory above.
(A) Kiehl & Ramanathan (1982) shows the following radiative forcing for 2xCO2.
Radiative forcing at the tropopause: 3.7W/m2.
Radiative forcing at the surface: 0.55~1.56W/m2 (averaged 1.1W/m2).
This denies the FLRA giving the uniform warming throughout the troposphere in the 1DRCM and the 3DGCMs studies.
(B) Newell & Dopplick (1979) obtained a climate sensitivity of 0.24K considering the evaporation cooling from the surface of the ocean.
(C) Ramanathan (1981) shows the surface temperature increase of 0.17K with the direct heating of 1.2W/m2 for 2xCO2 at the surface.
William,
Thanks for the link to the work of Kyoji Kimoto. His figure 2 is most interesting in that it shows TOA warming without a change in height, precisely what we would expect if the tropopause is pressure dependent. Here is the abstract of a paper that shows this:-
A minimum atmospheric temperature, or tropopause, occurs at a pressure of around 0.1 bar in the atmospheres of Earth1, Titan2, Jupiter3, Saturn4, Uranus and Neptune4, despite great differences in atmospheric composition, gravity, internal heat and sunlight. In all of these bodies, the tropopause separates a stratosphere with a temperature profile that is controlled by the absorption of short-wave solar radiation, from a region below characterized by convection, weather and clouds5,6. However, it is not obvious why the tropopause occurs at the specific pressure near 0.1 bar. Here we use a simple, physically based model7 to demonstrate that, at atmospheric pressures lower than 0.1 bar, transparency to thermal radiation allows short-wave heating to dominate, creating a stratosphere. At higher pressures, atmospheres become opaque to thermal radiation, causing temperatures to increase with depth and convection to ensue. A common dependence of infrared opacity on pressure, arising from the shared physics of molecular absorption, sets the 0.1 bar tropopause. We reason that a tropopause at a pressure of approximately 0.1 bar is characteristic of many thick atmospheres, including exoplanets and exomoons in our galaxy and beyond. Judicious use of this rule could help constrain the atmospheric structure, and thus the surface environments and habitability, of exoplanets.
Robinson, T.D. & Catling, D.C. (2014) Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency.
Nature Geoscience 7 (1), 12-12. https://www.nature.com/articles/ngeo2020
A calculated value for CO2 of 0.02C using the ideal gas equation posted elsewhere by 1000Frolly works for me.
Ira: In what is your PhD? Art History? Gases warm nothing. They are not a source of energy. They can absorb, they can re-radiate, they can transmit, or they can reflect. But, they cannot warm.
Whether a gas can warm another mass is simply dependent on temperature, mass and thermal capacities of the masses. Steam is a gas and is used all the time in practice for heating.
Walter Sobchak November 19, 2017 at 7:45 am
Ira: In what is your PhD? Art History?
He has a PhD in Engineering and has authored some of the most detailed and accurate articles on GHE ever published in WUWT, including observational evidence that underscores the theory. As a hardcore skeptic with a lot of background in physics, I can suggest that rather than making snarky remarks, you could learn a lot from him, including why the author of this post is wrong:
Shearer, the question on what can ‘warm’ what is a semantic one. Only heat sources like radiators, fire or the Sun warm in meaning one that Sobchak employs above. Meaning two includes anything else that results in higher temperature somewhere.
Argon between my window glazing does not ‘heat’ my house, but it helps keeping it warmer. So does any other insulator by increasing the Km²/W value of the walls. Now my house is not heated with the insulator, but it surely is warmer because of them.
I’m tired of this ‘gas does not warm’, because it is always put forward and never any learning happens.
I’m tired of this ‘gas does not warm’, because it is always put forward and never any learning happens.
The difference in this case is water compensates for at least most of the changes of the other GHGS forcing. Because water’s is triggered by air temp and pressure.
IMO, a brief Internet search is generally preferable to casting aspersions. Not that art history is such a despicable discipline. It too requires pictures as well as words.
@Hugs November 19, 2017 at 10:21 am
“Now my house is not heated with the insulator, but it surely is warmer because of them.”
Only because the heat source (furnace) puts out more heat than can escape because of the insulation. The “warmth” of your home is dependent upon the heat source and the air temp outside (heat loss). It’s possible for it to get so cold outside that the insulation cannot keep the heat in with the furnace going full blast. Hence the insulation’s role is to slow the rate of heat loss. It doesnt make your home warmer, it makes it less cold.
I am with Hugs on this one.
Semantics…unhelpful and obfuscatory.
If CO2 retards heat loss through the atmosphere, it results in some amount of less-coldness.
If it is a radiative gas, perhaps it hastens heat loss at night when the sun is not shining…but we do not see greater nighttime coldness…we see less of that, and less winter coldness and less Artic coldness…all good things.
We also see less extreme daytime hotness, at least in the place where we have lots of good measurements over many decades.
A better question than all of this is…why does anyone think that a warmer Earth could possibly be bad?
When was this debate settled?
The answer is never…we never even had a discussion about it…it was merely asserted and for some incredibly strange reason people, even scientists, lapped it up.
Prior to 1988 every historian and everyone who studied Earth history knew that a warmer earth is a better place for life and for people.
All the rest of this is misdirection, stacked up against that undeniable fact.
This is what people say when they are shown how reality is not simple.
No, this is not semantics. In science it is imperative that the proper words are used to be precise and avoid ambiguity. AGW thrives on being vague and ambiguous.
Tell me, is a glass half full or half empty?
In science we would say a glass is half full if it was empty and filled with new water half way. Conversely, it is half empty if the glass was completely full, but half the water removed (what’s left is not new water).
The fact is, at night, for example, having temps under cloud doesnt get so cold is not warmer because getting warmed requires new energy to warm the object. Instead that warmth was already there just not as much of it was lost under cloud cover.
The fact is, at night, for example, having temps under cloud doesnt get so cold is not warmer because getting warmed requires new energy to warm the object. Instead that warmth was already there just not as much of it was lost under cloud cover.
I agree.
But to explain this
requires more energy.
Both of these nights were clear all night. And if you measure the sky with a IR thermometer you find it is still 80-100F colder than the ground, and air. And I have to wear a hat.
Another equal situation on the other side of the world, where they measured net radiation at 2m, you can see when the change in temp rate changes, the net flux changed, and it’s a regulatory response by water vapor.
I’m sure words matter but you just can’t define warming in some rare technical sense and expect people to talk about something else (what, less-colding?)
even when the dreaded warming is a small less-colding effect like GHE 2xCO2 without feedbacks.
Ira, can you give us a link to satellite measurements of “the actual warming effects of water vapor, CO2, and other greenhouse gases”? All I’ve seen are either radiometric measurements of temperature (not temperature of a particular gas) or measurements of the presence and quantity of greenhouse gases (but not the temperature of their emissions). For example:
Ira, can you give us a link to satellite measurements of “the actual warming effects of water vapor, CO2, and other greenhouse gases”?
Wrong question. The satellites see what is coming UP from the earth. If you want to see what’s coming DOWN from CO2, you have to measure it at earth surface, and that has in fact been done:
Note that they measured less than 0.2 w/m2 over a change of 22 ppm of CO2. In other words, very very small. You may also want to read through these which will explain in a lot more detail how things ACTUALLY work backed up by observational evidence. The argument is NOT about the effects of CO2, but the nature and magnitude of second and third order (feedback) effects, which are greatly exagerated:
Micro is exactly right…when the air reaches the dew point at night, cooling comes to a virtual halt.
And when it is hotter during the day, convection is enhanced.
Where it is hot enough and humid enough, convection causes thunderstorms which result in even greater cooling and transport of energy to the top portions of the troposphere.
If it is hotter, the thunderstorms either start sooner, or last longer, or grow to taller vertical extent.
IOW…more energy means stronger thunderstorms which means greater transport of energy to way up in the sky…where it is very cold, air is very dry, and heat/energy can far more readily escape to space.
“Micro is exactly right…when the air reaches the dew point at night, cooling comes to a virtual halt.”
AT the surface but not above it.
I’ve told Micro several times that he is looking at a small part of the system.
Just land FI.
Cooling to condensation point is absent over the majority of the Earth’s surface, due the fact that it is ocean.
Land cools quickly >> cooling of air above >>> condensation if moist/calm enough.
Ocean doesn’t cool (over 24 hours) >> no condenation (in absence of advection cooling).
If an inversion forms (most likely here) then in the drier air above cooling will still occur via radiation to space (given clear skies).
If fog forms then likewise the fog-top will cool (radiation to space).
The atmosphere has depth which micro totally ignores with his nonsense half-baked ideas.
Baloney Tone B
This is in regard to surface temps, no one ever said different.
For whole atmosphere we have the balloon data and satellites, and the satellite data includes over the oceans.
Funny how warmistas always want to play a game of switcheroo regarding which data is being discussed.
“No one lives in the troposphere”, they say.
OK, the surface stopped warming too, if they stop adjusting the damn data every year.
“If we include properly tortured ocean data, the pause disappears from the surface records”
No one lives in the ocean!
Lets get a straight story for one…which is it we care about Tone B…the surface, or the troposphere?
And what about that hot spot?
“Baloney Tone B
This is in regard to surface temps, no one ever said different.
For whole atmosphere we have the balloon data and satellites, and the satellite data includes over the oceans.
Funny how warmistas always want to play a game of switcheroo regarding which data is being discussed.
Don’t know what you’re on about my friend.
micro’s theory is that WV controls surface temperatures AT THE SURFACE.
And I’ve discussed it at length with him several times (on other Blogs).
Nothing to do with observational data.
It is simply basic meteorology.
And something I spent 32 years in the UKMO observing.
“And what about that hot spot?”
Oh, if you insist …
That’ll be the Tropical hot-spot – that would appear in ANY sort or warming.
It is merely a function of greater LH release aloft in the tropical high atmosphere via convection.
For one – it is difficult to find because of the nature of the instruments used. Radiosondes are imprecise for the job and have changed over the years, and sat obs are contaminated by Stratospheric cooling – which is a function of GHG theory.
“First, tropical warming is equally strong over both the 1959–2012 and 1979–2012 periods, increasing smoothly and almost moist-adiabatically from the surface (where it is roughly 0.14 K/decade) to 300 hPa (where it is about 0.25 K/decade over both periods), a pattern very close to that in climate model predictions. ”
That graph posted by micro6500 needs to also state that the rate of cooling remains fairly similar until RH approaches 100%, but at this point dew deposition occurs on ground and plant surfaces which releases latent heat. If further cooling occurs then fog forms with further latent heat release. So this is not just a straight forward water vapour greenhouse gas relationship but should also take into account latent heat release.
Rob
November 19, 2017 6:46 am
I’ve never heard a weather forecast yet that said, it’s going to be a high CO2 night, which is expected to keep temperatures warmer. They always say, lots of cloud cover tonight will help keep temperatures warmer, or the lack of cloud cover tonight will see temperatures plunge. If CO2 makes temperatures warmer, then we here in middle Alberta have been shortchanged for the last three weeks. So I don’t disagree with anything you say.
Cloud cover fluctuates considerably from day to day (or even hour to hour), but CO2 only varies (minutely) on a scale of months. The IPCC is forecasting (projecting?) temperature changes far less than Alberta sees in a typical day, and their forecast is for decades in the future. Including CO2 in a weather forecast would be ludicrous, as I’m sure you know.
But in the specific places where we would expect to see the most pronounced effect of CO2 caused warming…we do not see it.
A fact that is for some reason overlooked completely by 100% of warmistas.
Giving them all big giant credibility demerit.
To add to all of the other credibility demerits they have earned.
In fact, everything they assert is another demerit to their credibility.
Which by itself is incredibly unlikely…but there it is.
The big mystery is why anyone still believes anyone who has never been correct in any of their predictions?
Nylo
November 19, 2017 6:53 am
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
Why do you say that? Things do not emit radiation ONLY at their peak wavelength. They emit at that wavelength and also at others.
Pretending that only CO2 at -80C emits in that band is just so wrong. Any CO2 at any temperature above -80C will do so too, and also CO2 colder than that may emit a bit.
The problem is he is trying to treat everything as one effect there are two and he hasn’t worked that out.
It’s exactly the same as radio signals you have signals that are resonant to the reciever circuit and signals that are not hence the whole resonance thing. You can’t just use one law for both. Try working out how a radio reciever selectively amplifies one signal without using the proper calcs on the resonant frequencies.
It’s a standard EM wave (AKA a radio wave) stop treating it has heat and treat it like a radio wave at the resonant frequencies and you might get somewhere.
Does beg the question how much energy in the wavelength that CO2 absorbs is reflected back towards space. This will tell us the maximum possible amount of heat that CO2 can trap.
It will not stop warmists as CO2 will cause a positive feedback loop that increases H20 in the atmosphere.
But as H2O increases so the energy available to warm CO2 will therefore decrease.
How do I feel that this green house theory is not fully explained.
The real problem is not in the explanation but in the implications for the atmosphere.
Simply out, CO2 is not the temperature control knob of the atmosphere…never was, is not now, and never will it be.
Period.
Other factors are far more important…hence we have natural fluctuations at every time scale…it has been warmer, it has been colder.
Warmer has been a sweet deal for all living things, colder…not so much.
True. Electron configurations of atoms only change at a very high temperature. Configurations of atoms in a molecule (rotation, vibration) may change at atmospheric temperatures – that’s what makes water a “very bad” greenhouse gas, carbon dioxide not so bad.
As the illustration notes, Wien’s law applies to a a hypothetical black body (usually approximated as a hollow sphere with a small hole), an object with an infinite number of degrees of freedom of motion. It does not apply to the atmosphere, consisting of atoms and molecules with only few degrees of freedom.
ALL material that are above zero kelvin radiate ” …. Thermal Radiation …. ” as a consequence of the Temperature of that material. The ultimate source is the acceleration of electric charge; as a consequence of Maxwell’s equations of electro-magnetism.
When atoms of molecules collide (Temperature) the electric charge distributions are distorted, so during the collision, perfectly good radiating dipoles exist. There are also other more complex antenna radiators like quadrupoles and even hexadecapoles, that radiate in more complex radiation patterns than a dipole antenna.
But since we don’t have any gases that are totally opaque to any radiation, then gases would not be black bodies; but they DO emit “thermal” radiation. It’s called thermal because it is a consequence of Temperature of the material (even gases) but the radiation may be in the microwave or radio spectrum, and not in the LWIR spectrum.
George, I respectfully disagree. If a gas does not absorb radiation, then it can not emit it.”
A gas molecule can absorb radiation for very short period of time.
A volume of gas has temperature because billions and billions are traveling around the speed of a bullet and colliding [without friction] with each other, This kinetic energy remains indefinitely until
the gas molecules lose their kinetic energy by contacting other gas of lower average velocity [thereby increasing and averaging the combined average velocity. Or the kinetic is conserved.
Or gas can collide liquids or solids, and again transferring the kinetic energy [conserving the kinetic energy] but the solids of liquids can radiate the kinetic energy gained by the warmer gas molecules.
A gas molecule has no temperature, absorbing energy tends to indicate an increase in temperature, and one molecule has no temperature.
Well curious George, you are fee to disagree with anything I wrote; with no respect required.
You provide no support for your contention that ” If a gas does not absorb radiation, then it can not emit it..”
Also I don’t see anywhere that I wrote that gases do not or can not absorb EM radiation.
No point in citing the essence of Kirchoff’s law; that law only applies to objects in thermal equilibrium. Nothing in the climate system is ever in thermal equilibrium; the rotation of the earth simply will not allow thermal equilibrium to be achieved.
But if you are going to disagree with what somebody else has posted, please do the readers the courtesy of giving supporting data or evidence for your position.
Despite all the modern love affair with quantum mechanics; the ONLY physical constants which actually have accurate exact values, are fundamental elements of Maxwell’s theory of electro-magnetism which is classical Physics. And it is a direct consequence of that theory and the whole concept of “heat”, that ANY object with a thermo-dynamic Temperature greater than zero kelvin must radiate a thermal spectrum. It is NOT a quantum spectrum of specific lines or bands, but a continuum of frequencies, and is not related to electron energy levels in any quantum mechanical model of atoms or molecules.
We have a two mile long electron linear accelerator pinning the San Andreas fault together near Sand Hill road and Highway 280, that exists only because of the simple fact that accelerated electric charge MUST radiate EM radiation.
There IS a greenhouse gas effect. Here’s an example.
Let the flux from the sun to the ground be 4 joules/unit time*unit
area,
and stay constant.
–> SUN
–>
–>
–>
–>
With no greenhouse gases, the earth will either heat up or cool down
until
the outgoing flux from the earth is equal to the incoming flux from
the sun.
Sun –> O O O O <– <– <–O O–> <–
You've now got an unbalanced situation where 5 joules/unit time*unit
area
are hitting the earth, 4 from the sun and half of the 2 from the
atmosphere,
and only 3 joules per second are leaving the earth, the 2 not
absorbed by
the gas, and half of the 2 from the atmosphere. The atmosphere will
gradually
warm up until outgoing flux from the atmosphere, plus the fraction of
the flux from the
earth not intercepted by the atmosphere, equals the incoming amount
from the sun.
Since in my example, half of the outgong flux is intercepted by the
atmosphere,
the watts hitting the earth's surface will increase to
Remember the atmosphere is intercepting half of this, so 16/6 joules
(unit time*unit area)
is intercepted by the greenhouse gas atmosphere, and another half,
16/6 joules, escapes
directly to space.
The final equilibrium balance is
16/12 Earth
O–>16/12 16/12(from atmosphere)–>Earth
Sun –>4
16/6 to atmosphere <–
Earth
16/6 to space <–
Earth
So yes, a greenhouse gas will warm the earth, the amount of warming
depends on the fraction
of the outgoing (and incoming for that matter) radiation absorbed by
the atmosphere.
………………………………………………..
If the atmosphere was absorbing ALL radiation from the sun , you'd get a final balance of
4 watts/unit area from sun to atmosphere and 4 watts from atmosphere to space- in balance
4 watts from sun to atmosphere, 4 watts from earth's surface to atmosphere
4 watts from atmosphere to space, 4 watts from atmosphere to earth surface- in balance
4 watts from atmosphere to earth surface, 4 watts from earth surface to atmosphere – in balance.
So an atmosphere that absorbed ALL solar radiation would have a net zero greenhouse effect.
……………………………………………………….
When you look at the blackbody radiation curve, you'll notice that the shortwave frequencies, not affected by CO2, increase at a much greater rate with increasing temperature than longer wave radiation, which IS affected by the amount of CO2 in the air. The net result is that increasing absorption of long wave radiation by CO2 is MUCH smaller than increases in surface temperature. In addition, as the atmosphere warms up, a larger fraction of the sun's incoming radiation is absorbed directly by the atmosphere, reducing the overall greenhouse effect.
Alan, the only thing that alters that from an atm basis, is water’s actions are not linear over the standard range of pressure and temperatures.
That is what alters Earth from non condensing only atm.
Water does real work daily. And it moves that work around.
John
November 19, 2017 7:06 am
I agree with your findings, but not for the same reasons. Retired now, I used to be a laser engineer, and instrument design engineer, including weather stations, and all manner of energy measuring devices. I created infrared measurement systems and laser energy measurement systems. Thus, I know a little about gas behavior and thermal characteristics.
So, my argument against CO2 ‘warming’ anything goes like this? If it DID increase the surface temperature, the surface would radiate to space at the 4th power of the increase, instantly cooling itself back down. We know that everything radiates and we have laws for quantifying it.
Experiment: Try to heat a stove with a flashlight. There are lots of photons, you can see them. Yet the stove does not warm. Why? Because for each photon that strikes the stove, the stove emits millions, maybe billions of photons itself. Those few photons from the flashlight are simply overwhelmed, and the result is immeasurable. Applying that to CO2, how many surface molecules radiate for every CO2 molecule? There is NO WAY there is sufficient energy in CO2 radiation from the atmosphere to increase surface temperature, because the surface radiates FAR more than the CO2 does. And, again, if the CO2 DID somehow manage to ‘warm’ the surface, it’s millions of molecules per CO2 molecule would simply radiate at a higher rate instantaneously, and ‘cool’ back to equilibrium.
Lastly, consider that if there were CO2 ‘radiation’, it would cast a shadow when blocked – an invisible one, but still a shadow. If you create a filter that can filter out the CO2 spectrum, and place it on the window of an infrared thermometer, with another identical thermometer without the filter, you will find that they both measure the same temperature. That would not be possible, if CO2 was indeed ‘heating’ anything, those two thermometers would have to read differently.
Leave it to an engineer that understands the science better than the alleged experts. That’s because we operate in reality. A concept foreign to most pseudo theorists.
The author of this post has made so many errors that it would take a long time to go through them all. But above is observational evidence that fits with the theory. If you read carefully, and do the math, you’ll come to realize that the author of this post is wrong AND that the observational evidence, while confirming the theory, results in a sensitivity calculation so low as to be immaterial and so falsify the alarm.
The global warming alarm is NOT founded upon a GHE that does not exist. Continued efforts to discredit the first order effects of CO2 are not only futile, it gives the alarmists ammunition to show that we don’t know what we’re talking about. Global warming alarm is founded upon an exaggeration of second and third order effects (feedbacks) that are still not well understood because they cannot be directly observed, but for which there is increasing evidence are low, and decreasing evidence to show that they are high. At day’s end however, CO2 is logarithmic (that fact not only accepted by alarmist scientists, but repeatedly documented by them in their own IPCC reports) and so the more CO2 we have in the atmosphere, the less additional CO2 matters.
David H.
“CO2 we have in the atmosphere, the less additional CO2 matters.”
Exactly correct, except for plants, trees,… and crops, which are all parts of plants and trees.
IOW, for life. For the biosphere.
For the biosphere, it matters.
It is unambiguously very very good to have more, much more, CO2.
davidmhopper,
“The global warming alarm is NOT founded upon a GHE that does not exist. Continued efforts to discredit the first order effects of CO2 are not only futile, it gives the alarmists ammunition to show that we don’t know what we’re talking about.”
Exactly right. The post is utter nonsense. Trying to refute physics that has been well understood for a century or more is a waste of time, distracts from far more credible critiques of CAGW, and gives the crazy green left ammunition to attack perfectly reasonable arguments against CAGW. Please stop trying to help so very much. If Anthony never posted another article like this (and unfortunately, there have been many over the years), it would be a good decision.
It seems to me that Co2 molecules could only insulate – slow the amount IR radiation leaving the surface- and it’s a very small effect.
CO2 or any greenhouse gas can not increase the surface temperature. The surface is warmed by the sunlight.
Water can and does warm the surface at night…if it condenses into clouds.
I have had many a freeze and frost averted or reversed by a fortuitous streak of high cirrus streaming in while I was monitoring the temperature.
I have watched the mercury climb by several degrees in a matter of minutes when clouds raced in.
Many many times.
Not at all. It is more like building a dam across the river. After a small amount of time, the water behind the dam will be deeper (the temperature of the surface will be higher), but the amount of water flowing down stream will be the same as before.
The question is – Where is the CO2 part of the dam added? If it makes the dam higher, do we care?
If water is flowing over the top of the dam, then an extra inch of height makes a big difference. However, if water thru the dam is controlled by sluice gates, the the top of the dam will be dry and adding a couple of extra feet of height will have no effect.
My theory of the GHE is that water vapor (including clouds, rain, dew, etc.) controls the sluice gates.
Hmmm, very interesting to see the debate boiled down thusly…is CO2 more like a stream of piss into a fast moving river or an inch of manure on top of a leaking dam with open sluice gates?
“CO2 at 0.04% is no dam. There are billions more photons leaving the surface for every photon going to the surface from CO2.”
I suggest you study the Beer-Lambert equation and the importance of path-length.
Clue: the path-length to space is a tad long, enough to make the 0.04% (up 40% due anthro emissions) very much a dam.
I also suggest you consider CO2’s importance where WV is scarce.
There are billions more photons leaving the surface for every photon going to the surface from CO2.
Please provide a reference supporting that.
My computations indicate that at 15C, the Earth emits about 390 W/m2 and the first kilometer of CO2 would absorb about 54 W/m2 – except that water vapor absorbs about half of that. That is almost 14% of the radiated energy.
MODTRAN indicates that the downward radiation from 400 ppm CO2 is 81 W/m2 using the full atmosphere.
81/390 = 20.8%
When normal water vapor is added, the downward IR energy is 367 W/m2.
John,
Your real-world expertise is valuable in this struggle. You neatly in a few lines devastate the entire pseudo-science of GHG.
And you write clearly and concisely.
You should write a blog yourself.
Or hook up with another engineer who already has a blog destroying the GHG.
The discussions here fall into the trap of debating the scammers, on their own terms.
Please comment over here:
“…no way there is sufficient energy in CO2 radiation….” mostly correct John, but take my word as yet another old engineer who once did heat transfer calcs in combustion gases….if you go through these standard radiative calcs from our student days….. http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html
…….the CO2 does make a difference in the range of 1.2 degrees C per doubling. The real question is water vapour related. Is that 1.2 degrees C that is going to cause positive feedback, or is the extra water vapour going to cause more clouds to reflect incoming solar away ? It is probably the latter or Earth’s oceans would have boiled away long ago.
If you are a retired laser engineer (which itself is a weird term) then can I ask how you get population inversion on the CO2 gas inside the laser tube? Should be interesting in line with your opinion above.
I will not start arguiing about the physical phenomenon in a GH molecule, when a photon with the right wavelenght / frequency hits this molecule. This is a very basic stuff of molecular physics and there is enough evidence in which way it happnes. But I do not agree with the description of Rod Gill.
The GH effect in the atmosphere is a fact and it is caused by GH gases: H2O 81 %, CO2 13 %, O3 4 % CH4 & N2O 1 %, clouds 1 %. Link: http://www.sciencedomain.org/abstract/17484
Here is a figure from my web site, which shows the absorption wavelenghts of GH gases. A very bsic thing is that the absorption wavelenghts of other GH gases overlaps with water making them rather weak:
One thing more. The Earth’s surface emits about 395 W/m2 in 15 degrees and the LW radition emitted by the atmosphere to space is about 239 W/m2. The radiation loss is 396 – 239 = 157 W/2. Energy does not disappear but it can change its form. The radiation flux of 157 W/m2 maintains the temperature profile of the atmosphere together with the SW radiation absorpbed by the atmosphere, which is about 71 W/2.
It would be interesting if the Earth we not covered with water. Your model, does not include the Oceans which have 99.9% of the energy in the system. Go back and try again.
A nice essay, I don’t fully understand quantum physics, you have a much greater knowledge of this than me. Could I ask though there is a theory, I am not sure it is conclusive, that as CO2 concentration rises in the atmosphere, the increase in temperature is not linear, but logarithmic. If it is true how would this tie in with your theory? I have pasted my source below which is over 8 years old. https://wattsupwiththat.com/2010/03/08/the-logarithmic-effect-of-carbon-dioxide/
If you look at the figure above, you can see that CO2 can increase the area of its absorption peak in the wavelenght zone from 10 to 14 micrometers. In figure below are the increases of the absorptions for different CO2 concnetrations (hopefully cominf correctly). These curves are results of extensive spectral calculations. The logartimic relationship is the results of curve fitting of these calculation – not a result of a single calculation.
The theory is all hogwash. The claim of logarithmic relationship with CO2 abundance arises from the so called Beer’s Law (or Beer-Lambert law). Beer’s law applies strictly to linear transmission through non-scattering media. The photons are expected to travel in straight uni-axial direction through the medium, unless they get absorbed by some absorbing molecule. Then they are supposed to stay dead and not re-emit at some other wavelength and in an isotropic direction. The photons can’t stay dead. Eventually the absorbing medium must warm from the absorbed energy, so it must eventually emit LWIR photons.
Logarithmic means a change from one CO2 molecules to two CO2 molecules, has the same Temperature change effect as a change from 400 ppmm to 800 ppmm.
And there is no experimental evidence for logarithmic relationship. Sometimes CO2 and Temperature go in opposite directions.
There are no logarithms for negative numbers. and don’t bother me with Gamma functions.
You are wrong. Beer-Lambert law works only for very small concentrations like CH4 and N2O. CO2 and H2O do not follow the Beer-Lambert law anymore. Basic stuff of radiation physics.
Whatever the theory, CO2 obviously does not cause actual warming by experiment in the real atmosphere. The increase in CO2 in the ca. one hundred years from near 300PPM to near 400PPM does not correlate with global warming, or some would say, lack thereof.
Of course it “lacks”, because in the IPCC’s model, the warming effect is quite excatly 200 % too much. There are cosmic forces which explain the temperature history of the Earth since the Roman warm period. If there were no cosmic forces, the temperature graph would be like that of Mann’s graph: a straight line up to 1750.
joshv
November 19, 2017 7:17 am
You’ve got the physics wrong. Absorption spectra have little to do with electrons, and a lot to do with vibration modes of the CO2 molecule. CO2 is a complex molecule. It can move (translation), rotate, and vibrate. A molecule’s energy is equally partitioned between all of these degrees of freedom. The rotational and translational degrees of freedom cannot absorb IR, but the vibration degree of freedom can. The energy from IR, once absorbed by resonance with a vibrational mode, can then be distributed to the other modes. It does not stay ‘locked up’ in vibration, it can cause the molecule to rotate, and to move faster (heat up).
Think of a bunch of molecules bouncing around off of each other, in a collision, one molecule could easily lose much of it’s vibration or rotation and cause the second molecule to bounce off faster in another direction. Over many collisions, the vibrational energy from IR absorbance will be equally spread into all of the degrees of freedom, including translational, which increases the average kinetic energy of the gas molecules ‘heating’ them up.
Now, the opposite can also occur, translational energy (heat) can be converted into vibrational energy, and then that energy can be emitted as an IR photon.
Yes, you are right. The vibration of the atoms of carbon with respect to the oxygen in CO2 is how the energy at 15 microns is absorbed. It is not absorbed by the electrons in the atoms.
Photons only interact with charged particles.
A 15u photon might not be changing the energy level of a valance electron, but if it interacts, it is with the molecules electrons.
I’m not sure what the electrons in the bond is doing, while it’s vibrating, maybe it’s just oscillation along the length of that bond.
But there is an interaction of some kind with its electrons.
CO2 is a complex molecule but it isn’t magic. it is about 0.04% of the atmosphere. And the atmosphere is about 0.1% of the heat capacity of the ocean atmosphere system.
The CO2 molecule is linearly symmetrical, so it should have no electric dipole moment.
But it also has an elbow at the carbon atom and can bend in two different modes at right angles. The moment of inertia for such a vibration is quite high so the frequency is low hence the 15 micron wavelength. It’s a degenerate mode because of the two identical frequency oscillations, which I suppose are indistinguishable.
The asymmetrical stretch mode (all three atoms moving relative to the center of mass) radiates at around 4 microns, but the earth doesn’t emit much in the way of 4 micron radiation (the sun does; something less than 1% of solar spectrum)
The symmetrical stretch mode where the carbon is stationary as the center of mass, does not radiate in the infra-red. Well it shouldn’t have dipole radiation, but maybe it looks like two end to end dipoles in opposition so it might have a quadrupole or higher antenna configuration.
Anything that can radiate will radiate.
G
reallyskeptical
November 19, 2017 7:30 am
” LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.”
It’s not one way. Once LWIR is stopped, it (or it’s energy equivalent) has a 50% chance of escaping to space and 50% of escaping back to the surface.
To make sure you understand: I do know the net 66W/m² or whatever is a small portion of the total and I’m not confident on numbers given without error estimation. What I tried to say is the LWIR is not how energy gets up, it is just part of it, and you can’t put it shortly that half is emitted back down, it is awful.
R. Shearer,
You said, “That’s why the sky is blue. Scattering, especially via Raman effect.” You are confusing Raman inelastic scattering, which is volume scattering inside a solid, with elastic Rayleigh scattering, which is wavelength dependent. Thus, blue light is scattered more strongly than the longer wavelengths, and the appearance of a direct observation of the sun shifts from green to yellowish because the blue light has been removed, and reaches the surface through a different path than direct sunlight.
There are no flats on a sphere. The very smallest surface element has the exact same curvature as any other element.
Oh gee. I’m sure this is what are looking for.
Look, I’m not pushing flat-earth, just noting that not every calculation requires the second or third term, just simply because they are small compared to the other known errors.
” 15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.”
Question: Does CO2, or any molecule, radiate equally in all directions?
If so, at 95Km the earth is only in 25% of the radiation cone of CO2; and most of that at an oblique angle.
Actually, because the 2 oxygens are on one side of the carbon atom, and oxygen is heavier than carbon, this aligns the CO2 molecules like little arrows all pointing upward. So the radiation goes in that direction. Right?
I asked a similar question a few days ago, not nearly as well as you though, but then I’m not qualified in anything. However, it seemed to me that the radiative properties of CO2 are always assumed to be 100% directed back at the planets surface, which, to a layman like me, seems to defy logic.
Your radiation cone description is an easy way for me to visualise what’s happening. Thanks.
You are correct. Assume an earth radius of 6,378km and the straight line distance to horizon from 95km is 1104.9km (from internet horizon calculator). I calculated a cone of 160.4 degrees or 44.5% of potential 360 degree cone of radiation. Hope you find this number more to your liking.
I love the crazy stuff classical physics lead people to believe, let me guess the emission is in certain direction because in other directions it is going to bounce or crash into the nucleus 🙂
Question: Does CO2, or any molecule, radiate equally in all directions?
If so, at 95Km the earth is only in 25% of the radiation cone of CO2; and most of that at an oblique angle.
Far, far from true. The angle of depression of the horizon is arcCos(R/(R+h)), where R is the radius of the earth, 6380 km, and “h” is the height of observation. This gives us arcCos(6380/6475) = 9.8° … where you are claiming that the depression angle is 78.5°.
Yeah, but the part that goes sideways has an increased chance of hitting another molecule before it leaves the atmosphere, so half is a good approximation…is what the other argument is.
I am a retired EE, not trying to prove anything, just get science right. There are a lot of assumptions taken for granted that the “experts” don’t seem to have the time or inclination to answer or correct. So thanks for the courtesy of your reply to my comments.
My 44% calculation of Nov 19 at 3:46
“Assume an earth radius of 6,378km and the straight line distance to horizon from 95km is 1104.9km (from internet horizon calculator). I calculated a cone of 160.4 degrees or 44.5% of potential 360 degree cone of radiation”
Joshv, this is the logical issue I am trying to resolve: “Think of a ** bunch of molecules bouncing around off of each** other, in a collision, one molecule could easily **lose much of it’s vibration or rotation** and cause the second molecule to bounce off faster in another direction. Over many collisions, the vibrational energy from IR absorbance will be equally spread into all of the degrees of freedom, including translational, which increases the average kinetic energy of the gas molecules ‘heating’ them up.”
To me that screams that CONDUCTION being the premier cause of atmospheric warming. That then leads to a whole larger series of questions related to radiational warming and COOLING.
Conduction is quite insignificant. Gases conduct heat very poorly. However CONVECTION is very important, as a matter of fact more important than radiation for heat transport from the surface. Something that is almost never spoken about since it cannot be modelled realistically by GCM.
I agree. Convection trumps radiation every time. Just try holding your hand in front of a ‘radiator’ and then above it, and see where it gets warmer.
It always amazes me how all explanations of CO2 warming ignore convection as though it doesn’t happen or doesn’t matter. Yes, it’s very difficult to measure in the real world, but that’s not a good reason to ignore it!
It’s merely the mechanism of local equilibrium. Remember, these molecules also emit IR at their absorption spectra, and those photos travel at the speed of light.
Dr. Deanster
November 19, 2017 7:35 am
For the sake of discussion, the CO2 at 100 km in the atmosphere does not have to warm the liquid or solid surface below, it only need heat the gas directly below. Probably why these guys are always talking about a hot spot. …… but as we know, there is no hot spot.
My background is chemical movement in biological systems (pharmacokinetics) so you engineer guys feel free to correct me …. but the reason we don’t see a hot spot, IMO, is because radiation and energy move at the speed of light. From a biological perspective, this would equate to a 1/2 life in gases…. t-1/2, that for all practical purposes is near instantaneous. Thus, the only way for the atmosphere to stay warm is to have a continual supply of heat from solid and liquid surfaces, either directly or from flow (winds) from other places. That all goes back to heat capacity, where the oceans are king. We see this in observation, deserts cool very rapidly, and if not for an inflow of warmer air from surrounding areas, they would freeze every night. In contrast, oceans hold so much heat that they are capable of keeping the air above them warm within a very narrow max min range. …. for oceans it is an equation of energy input, which goes back to solar and clouds, and all that Jazz.
Thus, the atmospheric temp is 100% dependent on a constant inflow of heat. The GHG systems contribution is directly related to its heat capacity, and as you point out in your article, that would only be relevant at -80C, I’ll take your word for it regarding Wiens Law, but probabably also owing to the fact that relative to solids and liquid water, the GHG system has no heat capacity.
The oceans hold about 99.9% of the heat energy in the system. The mass of the oceans is about 24x the mass of the atmosphere. The heat holding capacity of water is 4.2x dry air. The clue that the oceans are the most important part is the seasonal fluctuation in the number and power of storms.
Oldie but goodie and a heck of a lot simpler and clearer.
References:
Trenberth et al 2011jcli24 Figure 10
This popular balance graphic and assorted variations are based on a power flux, W/m^2. A W is not energy, but energy over time, i.e. 3.4 Btu/eng h or 3.6 kJ/SI h. The 342 W/m^2 ISR is determined by spreading the average discular 1,368 W/m^2 solar irradiance/constant over the spherical ToA surface area. (1,368/4 =342) There is no consideration of the elliptical orbit (perihelion = 1,415 W/m^2 to aphelion = 1,323 W/m^2) or day or night or seasons or tropospheric thickness or energy diffusion due to oblique incidence, etc. This popular balance models the earth as a ball suspended in a hot fluid with heat/energy/power entering evenly over the entire ToA spherical surface. This is not even close to how the real earth energy balance works. Everybody uses it. Everybody should know better.
An example of a real heat balance based on Btu/h is as follows. Basically (Incoming Solar Radiation spread over the earth’s cross sectional area, Btu/h) = (U*A*dT et. al. leaving the lit side perpendicular to the spherical surface ToA, Btu/h) + (U*A*dT et. al. leaving the dark side perpendicular to spherical surface area ToA, Btu/h) The atmosphere is just a simple HVAC/heat flow/balance/insulation problem.
“Technically, there is no absolute dividing line between the Earth’s atmosphere and space, but for scientists studying the balance of incoming and outgoing energy on the Earth, it is conceptually useful to think of the altitude at about 100 kilometers above the Earth as the “top of the atmosphere.”
The top of the atmosphere is the bottom line of Earth’s energy budget, the Grand Central Station of radiation. It is the place where solar energy (mostly visible light) enters the Earth system and where both reflected light and invisible, thermal radiation from the Sun-warmed Earth exit. The balance between incoming and outgoing energy at the top of the atmosphere determines the Earth’s average temperature. The ability of greenhouses gases to change the balance by reducing how much thermal energy exits is what global warming is all about.”
ToA is 100 km or 62 miles. It is 68 miles between Denver and Colorado Springs. That’s not just thin, that’s ludicrous thin. 99% of the atmospheric mass is below 32 km. Above 32 km there are very few molecules. Without molecules, energy, heat, cold, hot concepts get a tad iffy.
The GHE/GHG loop as shown on Trenberth Figure 10 is made up of three main components: upwelling of 396 W/m^2 which has two sub parts: 63 W/m^2 LWIR and 333 W/m^2 and downwelling of 333 W/m^2.
The 396 W/m^2 is calculated by inserting 16 C or 279K in the S-B BB equation, a calculation that does not actually exist in the real world. The result is 55 W/m^2 of power flux more than ISR entering ToA, an obvious violation of conservation of energy, i.e. created out of nothing. That should have been a warning.
ISR of 341 W/m^2 enter ToA, 102 W/m^2 are reflected by the albedo, leaving a net 239 W/m^2 entering ToA. 78 W/m^2 are absorbed by the atmosphere leaving 161 W/m^2 for the surface. To maintain the overall energy balance and a steady temperature (not really a requirement) 160 W/m^2 rises from the surface (0.9 residual in ground) as 17 W/m^2 convection, 80 W/m^2 latent and 63 W/m^2 LWIR (S-B BB 183 K, -90 C or emissivity = .16) = 160 W/m^2. All of the graphic’s power fluxes are now present and accounted for. The remaining and perpetual looping 333 W/m^2 are the spontaneous creation of an inappropriate application of the S-B BB equation violating conservation of energy.
But let’s press on.
The 333 W/m^2 upwelling/downwelling constitutes a 100% efficient perpetual energy loop violating thermodynamics. There is no net energy left at the surface to warm the earth and there is no net energy left in the troposphere to impact radiative balance at ToA.
The 333 W/m^2, 97% of ISR, upwells into the troposphere where it is allegedly absorbed/trapped/blocked by a miniscule 0.04% of the atmosphere. That’s a significant heat load for such a tiny share of atmospheric molecules (aren’t any above 32 km) and they should all be hotter than two dollar pistols.
Except they aren’t.
The troposphere is cold, -40 C at 30,000 ft, 9 km, < -60 C at ToA. Depending on how one models the troposphere, an evenly distributed average or weighted by layers from surface to ToA, the S-B BB equation for the tropospheric temperatures ranges from 150 to 250 W/m^2, a considerable, 45% to 75% of, less than 333. Radiation is a surface phenomenon. There is no “surface.”
(99% of the atmosphere is below 32 km where molecular energy moves by convection/conduction/latent/radiation & where ideal S-B does not apply. Above 32 km the low molecular density does not allow for convection/conduction/latent and energy moves by S-B ideal radiation et. al.)
But wait!
The GHGs reradiate in all directions not just back to the surface. Say a statistical 33% makes it back to the surface that means 50 to 80 W/m^2. An even longer way away from the 333, 15% to 24% of.
But wait!
Because the troposphere is not ideal the S-B equation must consider emissivity. Nasif Nahle suggests CO2 emissivity could be around 0.1 or 5 to 8 W/m^2 re-radiated back to the surface. Light years from 333, 1.5% to 2.4% of.
But wait!
All of the above really doesn’t even matter since there is no net connection or influence between the 333 W/m^2 thermodynamically impossible loop and the radiative balance at 100 km ToA. Just erase this loop from the graphic and nothing else about the balance changes.
BTW 7 of the 8 reanalyzed (i.e. water board the data until it gives up the “right” answer) data sets/models show more power flux leaving OLR than entering ASR ToA or atmospheric cooling. Obviously those seven data sets/models have it completely wrong because there can’t possibly be any flaw in the GHE theory.
The GHE greenhouse analogy/theory not only does not apply to the atmosphere, it doesn’t even apply to warming a real greenhouse. (“The Discovery of Global Warming” Spencer Weart) In a real greenhouse the physical barrier of walls, glass, plastic trap the convective heat, not some kind of handwavium glassy, transparent, multi-layer, radiative thermal diode.
The surface of the earth is warm for the same reason a heated house is warm in the winter: Q = U * A * dT, the energy flow/heat resisting blanket of the insulated walls. Same for the atmospheric blanket. A blanket works by Q = U * A * dT, not S-B BB. The composite thermal conductivity of that paper-thin atmosphere, conduction, convection, latent, LWIR, resists the flow of energy, i.e. heat, from surface to ToA and to make that energy flow (heat) requires a temperature differential, 213 K ToA and 288 K surface = 75 C. The atmosphere is just a basic HVAC system boundary analysis.
Open for rebuttal. If you can explain how this upwelling/downwelling/”back” radiation actually really works be certain to copy Jennifer Marohasy as she has posted a challenge for such an explanation.
Yes, Trenberth’s Earth energy budget diagram is complete nonsense (as are all the other similar version). How it ever got published and is still used is a mystery. Well no, because it is all part of the AGW propaganda..
Don’t forget the oceans. Most of the solar radiation that is absorbed by the earth is absorbed by the oceans. Look at a photograph of the sunlit side of the earth from space. The dark spots are the oceans, where the radiation is absorbed and converted into heat. The oceans warm the atmosphere by evaporation and conduction.
“The oceans warm the atmosphere by evaporation and conduction.”
Not so much by conduction as by radiation. Water is impermeable to LWIR but this also means that it emits stronly in this band. Evaporation transfers (latent) heat to the atmosphere but does not, strictly speaking, heat it.
You identify the fundamental reason why the bulk of GCMs run far too hot when they try to close the radiation budget at the TOA. That is in a nutshell Trenberth’s missing heat problem and his lament that higher than justifiable aerosols were employed to cool historical calibrations of the GCMs.
NickS,
There are very few CO2 molecules above 32km altitude, yet these ad some from lower are supposed to do the heavy lifting to radiate away all of the Earth’s incoming radiation, at equilibrium.
This means these upper molecules must take in, then release,, quite a lot of energy.
Do you have any references to the energy a single CO2 molecule can handle? Or is there no upper limit? If no upper limit, where does the Planck curve come from? Geoff
Geoff,
I have a black ball way of thinking about radiative gases. Think of them as a set of small black balls, with numbers proportional to gas concentration, and disc area proportional to absorption cross section. They absorb and radiate as black bodies, but are not a black surface, globally. Now imagine looking down with IR vision. You’d see a general black body glow, but diffuse. A bit like looking into a fire. If they are sparse above 32km, there won’t be much glow there. You’ll see the layers beneath. There isn’t an issue of how much they can handle; if they are small balls, they absorb less. And of course, as well as radiating, in your view, they obstruct the balls beneath.
Latitude
November 19, 2017 7:43 am
If global warming theory went the other way……this would be just as feasible as what we are stuck with
The central error is here: “15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
CO2 absorbs and reradiates 15μm band radiation at all altitudes and all temperatures, just the same as the antenna on your FM radio works in your living room by absorbing 3m band radiation at room temperature, not close to 0°K.
Finally someone else realized the main problem with the article.
They seem to miss the point it is a radio wave and it doesn’t bang into every molecule in it’s path, they keep turning it into the solid photon tennis ball. The lies and tricks we use for classical physics comes back to haunt us at times.
PRECISELY the reason I suggested everyone try and explain things both with wave e-m as well as tennis ball photons ! It is useful. You try BOTH and see what happens.
Anthony Mills
November 19, 2017 7:46 am
A good understanding of this issue can be obtained from Staley,D.O., and Jurica,G.M., “Effective atmospheric emissivity under clear skies” J.Applied Meteorology,v.11,349-356,March 1972. The physics of radiation transport in gas mixtures was well established many years ago.
jim
November 19, 2017 7:49 am
Don’t disagree with that. However the logical outturn is the same as Rod’s. The translational energy created in turn creates vibrational energy, ie it ‘bounces around’ until it dissipates into space. Unless CO2 truly is ‘magical’ or ‘evil’, its degrees of freedom alternate as you describe, but don’t affect anything else.
GHG’s are a red herring. Radiation doesn’t heat , convection heats. And the Earth doesn’t have a glass roof.
Good, so sunlight does not heat, until it reaches the Earth where it heats. So radiation heats us after all.
Very close to correct, it passes straight thru the atmosphere unless it’s at a very specific frequency to a gas molecule that reacts at that frequency. It works exactly the same for your radio which is tuned to one station on the many that are transmitted thru the air.
At least now you don’t have solid ball photons banging into every molecule in it’s path like the article above 🙂
Martin Mason
November 19, 2017 7:49 am
Thanks for posting this Anthony, it is exactly how I as an engineer see it too. There is no other way that the first and second laws can be satisfied at real world and quantum level. It is the only interpretation of the theory that satisfies what we see happening and what has happened historically. Temperatures in the troposphere can be explained and calculated with no reference to back radiation so the conclusion surely has to be that back radiation doesn’t influence temperature.
Bill Illis
November 19, 2017 7:49 am
“For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.”
– They do NOT absorb photons in Long Wave but they absorb energy at the same “levels” through collisional energy exchange and pass on energy to other molecules at the same “levels” through the same collisions.
The most misunderstood aspect of this whole debate. Nitrogen and Oxygen just sit there all inert-like and are not part of the process is simply not correct. 98% of the atmosphere has the temperature/energy level of the “atmosphere”.
Bill Illis – a reprint of my question below – hope you can assist –
– since Oxygen and Nitrogen are not “greenhouse Gases”
Yet dont they serve some function of retaining heat and maintaining some balance of temp in the atmosphere.
Otherwise, it would seem that the total volume of Greenhouse gases is vastly too small (1%ish not counting water vapor) to retain any quanity of heat
Hard to fathom from a scientific point of view that CO2 or any of the other greenhouses gases are sufficiently powerful to act as the thermostat to the degree which they are credited.
As a matter of fact both oxygen and nitrogen are GHG though weak ones. Undisturbed N2 and O2 molecules do not absorb/emit LWIR, but when disturbed by collision or close proximity with other molecules they do so, though weakly.
As a matter of fact this has even been suggested as a solution for the “weak young sun” paradox, though this would require a very dense nitrogen atmosphere in the Archaean era.
“As a matter of fact this has even been suggested as a solution for the “weak young sun” paradox, though this would require a very dense nitrogen atmosphere in the Archaean era.” tty
AFAICS, you can hypothesize just about any remotely sane atmosphere you want in the Archean so long as it doesn’t contain free Oxygen. It’s not like there’s a lot of evidence to contradict whatever you set forth.
There is NO scattering, the N2 and O2 molecules are absorbing energy and entering an excited state.
It is just not through photon absorption.
Let’s say you have a N2 molecule 1 mm above a hot rock on the surface which got hot through absorbing solar photons throughout the day. The N2 molecule is moving around so fast, it is hitting other molecules 6 or 7 billion times per second.
So the N2 molecule is going to hit the rock at some point within a second or so since it started just 1 mm away. It is going to absorb 100 photons worth of energy in that collision with the rock molecule(s) and it is going to become extremely excited and become 1000 times “hotter” than the rest of the atmospheric molecules nearby. in a billionth of a second, it is going to collide with 6 or 7 other atmospheric molecules afterward and share that energy around and now a whole bunch of N2 and O2 molecules are hotter and more energetic and so on.
The atmosphere close to the rock is going to absorb up all that extra energy in the rock and become just as warm and energetic. The whole atmosphere, without a single GHG molecule in it, within 1 metre is all going to be the same temperature as the rock. AND, this all going to happen with 1 second. It is as close to,instantaneous as it gets.
Not a single GHG molecule is required to heat up the atmosphere. The atmosphere is going to warm up from collisions with the surface alone. They will then share that energy upwards in the atmosphere until something like a lapse rate develops.
An equilibrium temperature profile will be established since the N2 molecules can become hot enough to re-share that energy back to the rock. The rock can emit photons to space and it can warm up the atmosphere and the atmosphere can warm it, all without a single GHG molecule.
An equilibrium will be reached which is probably very much like the Greenhouse Effect without any greenhouse gases at all.
A person needs to think through this carefully to really get the point. Be an N2 molecule which is colliding with 6 billion other molecules every second and then translate that to 10 days in the atmosphere.
Molecules in the air also absorb solar radiation on its way toward the surface, most famously O2 high in the atmosphere which interacts with UVA and UVB photons to make and break O3.
And of course air molecules also scatter sunlight on its way down, which is why the sky looks blue, the color of shorter wavelengths of visible light.
Wait a second Bill.
I do not recall disagreeing with you before, but you seem to be saying that anytime a heated surface is in contact with air, within 1 second all of the air within 1 meter of the ground will be the same temp as that surface?
This is clearly not the case.
Whenever the sun is shining on a surface, it is very much hotter than the air more than a inch or a few inches from the ground.
Am I missing something, or did you perhaps misstate that?
This is IMO the most important rebuttal to the nontestable and nonverified Greenhouse Effect hypothesis (if one can actually call it a hypothesis). While N2 and O2 do not absorb or emit LWIR, they do heat and cool via All 3 energy transfer mediums. They also ,make up 99% of the 5000 Trillion tons of atmosphere with CO2 making up less than 1 trillion and H2O vapor making up around 11 Trillion. So roughly 4,987 Trillion tons of N2 and O2 contain almost all the energy that is absorbed by the atmosphere through whatever means is possible. The N2 and O2 are not without temp and most certainly emit half their radiation towards Earth and half out to space. Who cares if that energy is not LWIR, it is still radiation no matter its wavelength.
That’s 4,987 Trillion tons of matter versus some odd 13 Trillion absorbing and emitting energy.
Bill, N2 and O2 do radiate; they have to, else there is a contradiction to QM. They have QM predicted modes at 2338 and 1556 respectively, and these are detected only with Raman spectroscopy only.
Leonard Weinstein
November 19, 2017 7:50 am
The author is wrong on many points.The first is the point that CO2 only radiates to space from a very high altitude. In fact it radiates to space from a range of altitudes almost all the way through the atmosphere, although the peak amount of atmospheric radiation to space occurs relative high from about 5 to 15 km. Also radiation directly from the ground is a big factor, especially over deserts and very high latitudes. In addition, the radiation from water vapor and especially clouds is a major source of radiation to space. However with all of that said, increasing CO2 does increase the atmospheric trapping of radiation, which in effect acts like a radiation insulation, and by itself would increase the surface temperature. However, changes in clouds, transport of surface heating by ocean currents, and wind and storage and release by oceans may reduce the effect of the CO2, or may amplify it with the increased water vapor due to increased temperature. So far the data is inconclusive, but seems to indicate small to negligible net CO2 effect, but not due to the authors arguments. In the end, the lapse rate and effective average altitude of radiation to space determine the atmospheric greenhouse effect.
While it is true that CO2 acts as an insulator, it is as much of an insulator as a mesh made out of the lightest silk. I.E., not much. The warmistas write about CO2 as if it were an R30 fiberglass blanket. It isn’t
Comment to Leonard. I have carried out hundreds of spectral analyses. Majority of people have no idea what happens to the LW radiation as as function of altitude. For example, CO2 is so strong GH gas in its wave band zone (10-22 micrometer) that the absorption by CO2 does not increase after 1 km altitude. Why? Because there is no emission energy available. The GH gases cannot absorb more than the Earth’s surface emits. That is a simple physical fact. See a figure below. About 88 % of energy emitted by the surface is absorbed in the all-sky conditions.
To micro6500. The cloudeness-% of the average sky is 66 %. In the clody sky conditions, the emitted LW radiation by the surface is fully absorbed. All the climate scientists approve this fact. In clear sky conditions the absorption happens 78 %. Look at the first figure of mine. Optical window is not 30 %, it is much less.
” by itself would increase the surface temperature. ”
Take a large room with a pool. Heat it to 30C. Once at 30C fill the room with 100% CO2. Does the pool’s temp go up? No, impossible. I doubt it even cools slower.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C
If you look at a spectrum, the common form the Y axis is power in Watts, you are right that the energy at 15u per photon is slight, so to make an equal power as shorter wave lengths, there are a lot more photons.
So the flux is higher.
The interactions are photons bypass, they scatter, or they are absorbed.
This results in our experience, things are invisible to that wavelength, ie clear, it becomes reflective, or opaque.
But regardless of how 15u photons interact in the environment, water vapor regulates am temps to not drop much below dew point, at least until it all starts freezing.
When you monitor cooling at night, do you wonder why it’s clear, and yet the temp stops falling in the middle of the night. But most of the year it stops cooling around dew point. But what few realize is that it did not stop radiating to space, in the optical window temps are are nearly 100F cooler than air temps.
What does happen is the sensible heat, IR from condensing water, lights up the sky in all directions in the 14-16u water band, which lights up co2 at 15u. This is strongest at the surface, and it shows up as the rate temps falling while it’s still night out dropping towards zero. A side effect of using WV to regulate temps, it bleeds off atm water every night, this prevents the WV amplification of CS, the key to catastrophic warming. So not only is it not catastrophic, it doesnt even reallt cause any warming, WV will always prevent it.
micro6500
November 19, 2017 at 8:07 am: and possibly what Roy Spencer sees with his skyward pointing pyrgeometer. All real experiments including Hans Geiger’s monumental “The air above the ground”, those in the Australian outback, and Hartmann’s, show how CO2 is no different to any other gas except the one which cheats by phase-changing. Which is why radiators have caps, and we exist.
Micro6500
As stated above your assumption that water vapour is the key to cooling stopping is incorrect. It is heat release from latent heat as dew forms on surface of the ground and plants which slow cooling and then eventually fog forms and once it does then temps just flat line….Fog is liquid water…not the gaseous form water vapour and hence a different mechanism for cooling slowing down. Once fog forms…then the top of the fog layer becomes the radiating surface…not the ground, and as this fog layer cools further at the top, dewpoint is reached and the top of the fog layer rises. This is how fog layers deepen overnight.
As stated above your assumption that water vapour is the key to cooling stopping is incorrect. It is heat release from latent heat as dew forms on surface of the ground and plants which slow cooling and then eventually fog forms and once it does then temps just flat line….Fog is liquid water…not the gaseous form water vapour and hence a different mechanism for cooling slowing down. Once fog forms…then the top of the fog layer becomes the radiating surface…not the ground, and as this fog layer cools further at the top, dewpoint is reached and the top of the fog layer rises. This is how fog layers deepen overnight.
Dew is only a small part of it. But there’s a 30-40W/m^2 flux out the optical window at the same time the temperature stops falling.
Martin Mason
November 19, 2017 8:07 am
I can see the same circular arguments and the same immovable positions but nobody addressing the central point of the post. Can radiation from such a low temperature source warm surface matter which is at a much higher energy level, that is the only question to be answered surely. Also if back radiation causes warming how is it that surface temperature and tropospheric lapse rate can be calculated with no reference to radiation?
The sb-equation says no. There is no transfer but the heat, which is the difference in T⁴. Quantum physics is built on thermal physics, so it obeys the sb-law. Quantum is the microscopic details of heat.
The surface transfer 383W/m²-128W/m² to TOA, and TOA transfer nothing back. Neither does the rest of the atmosphere.
Sorry RS but that answers none of the questions I raised. It certainly doesn’t explain cold to hot heat transfer. It doesn’t even touch on why all tropospheric temperatures can be explained and calculated with no reference to back radiation. I really think that AGW is in desperate trouble?
The problem seems to exist in the word “warms”. Radiation from a cold object cannot “warm” a warmer object. However, it CAN leave the warm object warmer than it would have been without the cold object.
As an example. When we look up, the apparent temperature of the sky based on the amount of downwelling thermal radiation is on the order of -50°C … how can this radiation “warm” the much warmer earth?
The answer, of course, is that without the atmosphere the apparent temperature of the sky would be about -270°C … and as a result, we are warmer because of the existence of the atmosphere.
Does the radiation from the atmosphere “warm” the surface? No … but it does leave the surface much warmer than it would be without that radiation.
Willis Eschenbach
November 19, 2017 at 8:35 am: By its mass, and the sun. Just as explained by all real experimental Physicists from Maxwell (Theory of Heat) onwards. Not to mention the Mylar balloon expt, and all Nasa atmospheric readings through the solar system. Even Sagan admitted this eventually.
No, we are “warmer” because the oceans absorb a lot of the sun and release that energy slowly, which heats the atmosphere mostly by direct molecular contact with the air. The cooler air cannot warm the surface, only slow the rate of heat loss. It appears to have warmed because the temp number is higher, but that is not “warmer”. Example, day time heated by the sun gets to 30C, during the night it cools, losing that heat. If the lowest it gets on a clear night is 10C, then we have lost 20C worth of energy. But if on a cloudy night it instead goes to 20C, then we have NOT gained 10C in new energy (*warmer”). What has happened is less energy is lost. It’s not “warmer” on the cloudy nights, it’s less cooled. Because the temp is 10C higher numerically, we say it is “warmer”, but in reality it isnt warmer (no new energy added).
The cooler air cannot warm the surface, only slow the rate of heat loss.
This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.
Hugs:
“This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.”
To warm an object requires new energy to raise its temp. Losing less energy already there has a higher temp number, but it is not “warmer”. If you earn $1000 per month gross, and have a high tax rate of 25%, you lose $250, netting $750. But if the tax rate is lowered to 20%, you now lose only $200, does that mean you have earned $50 in new money? Nope. You earned the same, just lost less of it in taxes. Consider winter and night times as a tax on the energy the sun put to the earth during the day. Having a higher NET energy doesnt mean you are “warmer” because you dont have more gross energy.
Back radiation is competely irrelevant, as is sideways radiaton and slant radiation and every-which way radiation. The only important factor is the net upward radiation. Look at this formally correct but carefully doctored NASA diagram:
What it shows is that LWIR moves 66 Wm^-2 from the surface, 40 by direct radiation in the “window” and 26 by way of absorption/re-emission by GHG. It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat, 102 Wm^-2, of which 78 is latent heat of evaporated water, which condenses at high altitude and radiates the heat into space.
Also note that radiation is gross while convection is net (and that the radiation arrows are fat and red while the convection ones are as puny as possible). Taken at face value this diagram implies that the descending cooler/drier air and precipitation is at absolute zero since in this case there is no “back radiation” (=downward heat transport). The reason for this difference is that a honest diagram would show clearly that convection is the dominant process regulating surface temperature. And convection can’t be modelled by GCM, only “parameterized” (=guessed at) since it happens on a far to small spatial scale for GCM:s.
This is the flat earth model, and it is wrong. It overly simplifies and incorrectly depicts what is going on. Postma has written a lot about this flat earth model having no basis in reality.
The sun puts 1370W/m^2 to the surface, not 168 (which wouldnt power two 100watt light bulbs). The surface temp of the earth would be some 69C at that input, but it’s not. Why? Because the earth rotates. Only a small percent of the surface receives this input, the rest loses more than it gains. The earth’s rotation acts like a thermostat. If it rotated slower it would be too hot end of the day for life. It it rotated faster, it would not gain enough during the day to overcome the night time loss rate.
The sun puts 1370W/m^2 to the surface, not 168 (which wouldnt power two 100watt light bulbs).
Well, that 1370 watts is actually only 1362 watts/m^2.
But it is actually measured at 1362 watts/m^2 at the top of atmosphere, only about 1000 watts/m^2 gets through a clear atmosphere to the surface of the earth, at the equator. And that 1000 watts/m^2 only gets to the surface at noon on the equator, on a clear day, on average.
Over the whole year, the CAGW climacatastrophy community has accepted Trenberth’s [168] watts/m^2 absorbed + [30] watts/m*2 reflected as average values for the whole earth.
But that 198 watt/m^2 yearly average sunlight on the surface for every day and night is ONLY valid for the temperature regions ONLY between 55 north and 55 south latitudes. Any further north or south and the yearly total “average” sunlight goes much, much further down.
“It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat”
No,it’s not. The diagram is a budget. It records the fluxes that can be measured, and tests whether they are in balance. The flux that is affected by GHG concentration is the full 324 W/m2 downflux from air. The 390 W/m2 upflux at the surface responds to the temperature there. The difference, if you exclude AW, is small at 26 W/m2, but is pretty much locked. The 324 W/m2 is emitted from air near the surface, as you can tell from its magnitude. If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.
Dry convection is necessarily entered as a nett flux, because there are no measurements that can split it into separate streams. Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.
“If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.”
In that case You can kiss CAGW goodbye, because there is nothing else that GHG can affect.
” Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.”
True in so far as wet convection is of course by far dominant. Ever see cumulonimbus clouds? They are not close to the surface.
Martin Mason,
A cold object cannot warm a hot object by conduction, but a warm object (Earth) can warm a cold parcel of absorbing gas by radiation. The radiation that would have otherwise left the system, is retained and now available to contribute a slight warming of the dark side of the Earth where it is being subject to the IR radiation from the gas, which is above absolute zero. Consider the impact of an Earth radiating directly into space, versus an Earth radiating into space with an intervening blanket at a higher temperature than space.
I don’t understand the question in your last sentence.
You can test this experimentally. Two identical set ups one with no CO2 and one with lots. If back radiation exists and can transfer heat a measurable temperature difference can be seen. Relatively easy lab work and relatively inexpensive.
The answer of course can be answered by any five year old playing in the snow. No, radiation from a cooler object categorically doesn’t increase the temperature of a warmer object.
The ONLY^3 reason RGHE theory even exists is to explain how the average surface (1.5 m above ground) temperature of 288 K/15 C (K-T balance 289 K/16 C) minus 255 K/-18C , the average surface (now ground) temperature w/o an atmosphere (Which is just completely BOGUS!) equals 33 C warmer w/ than w/o atmosphere.
That Δ33 C notion is absolute rubbish and when it flies into the nearest dumpster it hauls RGHE “theory” in right behind it.
The sooner that is realized and accepted the sooner all of us will have to find something better to do with our time and the taxpayers’ money. Maybe that’s what keeps RGHE staggering down the road.
The genesis of RGHE theory is the incorrect notion that the atmosphere warms the surface (and that is NOT the ground). Explaining the mechanism behind this erroneous notion demands some truly contorted physics, thermo and heat transfer, i.e. energy out of nowhere, cold to hot w/o work, perpetual motion.
Is space cold or hot? There are no molecules in space so our common definitions of hot/cold/heat/energy don’t apply.
The temperatures of objects in space, e.g. the Earth, Moon, space station, Mars, Venus, etc. are determined by the radiation flowing past them. In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
But an object’s albedo reflects away some of that energy and reduces that temperature.
The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.
In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
Looked out. Can’t see much irradiance. I guess you should integrate to get the average surface value which is about quarter of the 1368W/m². Temperature was consequently around 278K. I guess it were colder without some warm gases and precipitation between my front yard and the 2.7K background.
Average is nonsense and does nothing to tell us what is going on. Example. Take a photograph of something, say a nice mountain range. Add up all the colour pixel numbers and divide by the number of pixels, then apply that number to each pixel. What do you get? A gray image with nothing to see.
This is exactly what you are doing by applying the average. You add up all the W/m^2 for the area, divide by the number of areas, and apply that to all the areas. It’s BS grayness that has no physical counterpart.
“It tells me much more than the peak value misleadingly used above.”
Yes, using JUST a peak value is just as meaningless. You want a real view of energy absorption and loss on this complicated and dynamic earth? Then do a proper 3D graphic of a tilted rotating spherical planet with large oceans and disproportional land masses that have different absorption. That flat earth graphic tells us nothing of what it really going on, and if anything has grossly mislead people into thinking a simple “budget” explains things. It doesnt. That graphic was invented to justify the AGW dogma. Again, AGW exists because of deliberate ambiguity.
Hiro Kawabata
November 19, 2017 8:17 am
The claims in this article are unfortunately not even wrong.
IR absorption by CO2 excites the vibrational modes of a CO2 molecule.
As the CO2 molecules collide, the excited vibrational modes transfer energy to the motion of other CO2 molecules. The average kinetic energy of molecules is what we measure as the temperature of a gas.
Nothing to do with the absorption and emission of visible light by electron level transitions
A correct summary of the process may be found here
As a simulation subject matter expert, you can’t use MODTRAN and run a single look through an average air sample and get anything useful.
If you do not run it over a days cycle as conditions change, all you’re doing is fooling yourself.
The excited modes of CO2 do NOT transfer energy to the motion of other CO2 molecules – or do it only in less than 0.04% of cases. What happens when an excited CO2 molecule collides with an N2 or O2 molecule or an Argon atom is very complex and I have not seen a satisfactory analysis.
Paywalled. But according to the abstract they handle CO2-CO2 collisions. As a concentration of CO2 is currently 0.04%, these collisions are very rare. And how about CO2-H2O collisions?
No CG, they do. That is why the absorption spectrum is in BANDS and not a single exact wavelength. The band of wavelengths show the range in energy needed to bump the molecule into a vibrational state, and the difference is caused by the very slight decrease or increase in kinetic energy from molecular collisions.
Eric
November 19, 2017 8:18 am
Wouldn’t a simple experiment (already conducted by John Tyndall) learn that 15 micrometer IR radiation is emitted and absorbed at other temperatures as well?
The mistake was made with Tyndall, he was using a thermoelectric transducer, a thermopile: he discovered the thermo electric gases – we call them the GHGs. Watch to see what I have discovered https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
Driller43
November 19, 2017 8:19 am
i think the author misapplies his second key point “The temperature of a (gas) directly affects the wavelength of the radiation it emits and absorbs.” Looks like he applies it incorrectly. Yes, for CO2, most of the outgoing radiation is mostly from the stratosphere; and in the atmospheric window region is mostly from the lower troposphere. From link article which concludes “The total temperature-dependent gas absorptivity effect (H2O, CO2, etc.) tends to result in a radiation energy convergence that decreases the total cooling of the atmosphere.” https://www.gfdl.noaa.gov/bibliography/related_files/yih0702.pdf
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
I never heard that about “too hot to exist”, and I don’t believe it.
I guess that he means that those bands are unimportant because they are at wavelengths where the Earth emits virtually no radiation at realistic ground temperatures. I agree that it is a very clumsy way of expressing this
Willis, you are a stickler for quoiting correctly when rebutting. I would like to point out the important qualifier that you are missing in your quote reference. He specifically said “to hot to exist in the atmosphere“. A distinction that is pertinent to his point..
Willis
The article is a mixture of facts and fallacies. The author is confused by a lot of ‘information’ gathered from various sources. Some of the bloggers are in the same boat. Too many things to correct so I just shut my mouth. Nevertheless, it’s entertaining to read some of the well-meaning comments.
Willis, re: “too hot to exist”. Rod Gill is confusing de-excitation radiation from the CO2 molecule with thermal radiation as described by Wien’s Law. That’s why he claims the CO2 emission bands at 2.7 and 4.3 microns only come from CO2 molecules at temperatures too hot to be found in the atmosphere (800° C and 400° C, respectively, if calculated from Wien’s Law). That’s also behind his ludicrous claim that CO2’s 15 micron emission band comes only from gas at -80° C.
Rod Gill fundamentally misunderstands the physics behind the so-called “greenhouse effect”, and he has no business publishing an article about it. I would have preferred that Anthony or one of his volunteers gently point that out to him and not publish such pseudoscience.
Of course the comments reveal that such confusion is fairly common, but we already knew that.
Willis,
Agreed, the first confusion is in assuming atomic excitation instead of molecular. Then electron states instead of rotational, vibrational, etc molecular states. Several other errors follow as a consequence.
However, I think the exercise is useful because it’s responses can correct those readers who were similarly starting from the same wrong point.
I tend to shut up because when my formal training in the 1960s was done, atomic was dominant and molecular seldom covered in lectures other than to say that molecular spectroscopy was a harder concept. Geoff
Willis, you are a stickler for quoiting correctly when rebutting. I would like to point out the important qualifier that you are missing in your quote reference. He specifically said “to hot to exist in the atmosphere“. A distinction that is pertinent to his point..
His claim is not true, whether he is talking about the atmosphere or the earth’s surface. So no, his qualifier is not important.
Which claim are you referring to? You are not being clear. “In the atmosphere” is definitely an important qualifier because he is talking about how the atmosphere actually operates and the range in which these interactions occur. You saying it isn’t does not make it so.
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
I’d love to invite you into a cave in a glacier sometime.
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
I’m sorry to hear that. Had you continued, and considered what I was saying, you might have learned something.
In the meantime, have you never been outside on a winter night when clouds came over? They leave the surface warmer than when there are no clouds. Clear winter nights are the coldest … why?
Because when there are no clouds, the back radiation is coming much more from outer space at a temperature of 3K or so, whereas the clouds are radiating at something like 225K …
Think about it. If a planet has no GHG atmosphere, it is exposed directly to outer space.
But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.
I’m not sure why this is so hard for some folks to grasp, but I assure you that it is settled science taught in the universities and backed up by the math. If you are interested in the math, there is an online calculator here. If you play with it for a while it may help you start to understand the nature of two-way radiative energy exchange … it spells out the formula for how it is calculated.
Willis, you are mixing apples and oranges. The clouds have a higher temperature than the rest of the atmosphere because of the latent heat of condensation being released. This is a phase change of water effect, not a GHG effect.
Secondly, your sentence
‘But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.’
is obvious and there is no need to shout. Everyone agrees that the atmosphere warms the earth compared to no atmosphere. The point at issue is does replacing a few O2 molecules with a few CO2 molecules make a difference?
Willis, you are mixing apples and oranges. The clouds have a higher temperature than the rest of the atmosphere because of the latent heat of condensation being released. This is a phase change of water effect, not a GHG effect.
The claim was that the cold atmosphere (containing cold clouds) could not leave the surface warmer than it would be without them, viz:
wickedwenchfan November 20, 2017 at 2:51 pm Edit
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
Since both the atmosphere and the clouds are colder than the surface, I am NOT mixing apples and oranges.
Secondly, your sentence
‘But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.’
is obvious and there is no need to shout. Everyone agrees that the atmosphere warms the earth compared to no atmosphere. The point at issue is does replacing a few O2 molecules with a few CO2 molecules make a difference?
No, “everyone” does NOT agree that the cold atmosphere warms the earth compared to no atmosphere. There are literally dozens of people who claim that it is impossible. They all say that because the atmosphere is colder than the earth, the atmosphere does not and cannot “warm the earth compared to no atmosphere”.
Which is why I was shouting … I’m getting tired of people saying the same thing over and over, when the answer is there from our own senses regarding winter clouds.
w.
PS—Can a block of ice leave a person warmer than no block of ice?
Sure … as long as the block of ice (at 0°C) is interposed between that person and a tank of liquid nitrogen (at -196°C). The ice provides more radiation to the person’s body than does the liquid nitrogen, so the person ends up warmer.
And the same is true about icy clouds which are interposed between a person and 3K background temperature of space … they leave the person warmer than no clouds.
Ryddegutt
November 19, 2017 8:26 am
CO2 molecules can absorb radiation and then transfer that energy to other gasses by conduction and versa visa.
At atmospheric pressure the conduction takes place with molecular collisions that theoretically occur faster than a CO2 molecule can radiate its heat — 10^-7 s.
It is pressure dependent as rgbatduke always said. But I don’t really know how this affects – probably surface CO2 absorbs only, and does not have time to emit, so the air eats the radiation band. And then complicated (parameterized) things happen, like evaporation, convection. I’m sure the problem is not high school physics as Gore said. He lied.
3) A Chinook demonstrates the effect of compression directly. Ne c’est pas?
4) This idea also satisfies Dr. Svalgaards assertion that TSI variance is too small to account for the variations of earth’s temperature.
5) One has to admit that the name “Greenhouse” in no way is descriptive of our atmosphere or how it works.
6) No matter how our atmosphere works, we are fortunate that the relative stability of temperature is within the range of water existing in all three states. As a carbon based life-form, I am grateful of this.
7) Ockham’s razor and plausibility Apply liberally, rinse, repeat.
( numbered for your rebuttal convenience and clarity, also inspected for politically free content )
Jer0me
November 19, 2017 at 1:10 pm: But in our air, they are struck kinetically thousands of times between each possible emission. Thusly, air eats radiation so convection and phase change of water dominates hugely to above the net emission height. Radiation is swamped by water anyway, which even Mann knows. It is all sleight of pen for the cause. Bring on the State Pen..
Rod, thanks for your post. However, you ask us “Am I right?” about the following:
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
Nope. You are wrong. However, given the depth of your misunderstanding, and your certainty that you understand it, I’m gonna leave the question of WHY you are wrong for you to work out for yourself.
Show us a reference for transfer of energy from co2 at lower temperature to a solid warmer body, in a controlled environment. If you can’t, there is no transfer.
I asked for a reference, not you making stuff up.
Fact: at equal temperature, no transfer. So why would there be transfer from cold to hot if one body is colder.
Show me a reference for any transfer outside of “net”, or shut up.
LdB, I’m not getting your point. Are you saying the CO2 laser beam CAN’T cut metal? Maybe you’re saying the laser cuts metal by COOLING it? Help me out here.
It would help if you actually understood “classical physics”. Then you wouldn’t be so confused and would understand the difference between cutting with a laser beam and laser cooling.
@Gary Young Hladik
The problem I was illustrating is even things we think of with one behaviour don’t always behave like that, you are saying the beam will always thermally act one way. That beam as it moves thru the air is a electromagnetic wave whether it heats or cools or even has any effect (think a mirror) is governed by a set of strange laws.
If that laser is a CO2 cutter and I replace the steel with aluminium, I assume you know what happens.
Try even cutting a very fine steel mesh 🙂
It’s a EM transfer you can’t equate it to thermal energy, you can heat,cool or reflect it.
Oh and for anyone who wants to know how it works.
It’s pretty straight forward you need 6 or more beams to do cooling, the frequency used matches one of the resonant frequencies of the atom you are trying to cool and it doesn’t actually see the beam as hot because it absorbs it and then re-emits it.
@ Gary Hladik
Yes I know, I was just firing a warning shot … yes but be careful.
If that metal is aluminium for example it will reflect and if the metal has a resonant frequency at that frequency it can be transparent or cool the metal.
@Gabro
No you are claiming there are cool lasers ™, you directly claimed it.
Yes, there are many differences between cutting and cooling lasers.
Now you obviously bothered to read and are now trying to dig yourself out of a hole, there is no such thing as a cool laser ™ they are all the same.
As I have already stated the laser can heat, cool, reflect, absorb or transmit thru any material which strangely enough is the same for every single EM frequency that exists including radio waves and thermal emissions. QM is very concise and very well tested on all this stuff/.
I like to use the analogy of an Eskimo igloo, where the snow, forming a temporary barrier, captures the heat inside the igloo long enough to maintain a comfortable temperature.
Which of course is an incorrect analogy as the igloo is a physical barrier to convection, which is how the surface loses most of the heat. It has zero to do with radiation effects.
CO2 is a physical barrier to some radiation. It is an analogy, not saying ice chunk is a greenhouse gas. Of course, ice IS a weak barrier to radiation and it does contribute to how igloo warms.
Jer0me,
You can heat a clear container of CO2 by radiation as from a laser beam of appropriate wavelength or a beam of sunlight passing through a small part of the wall. If the beam is turned off, the hot CO2 will heat the rest of the container wall as the CO2 cools by normal heat transfer equations. Is this at odds with what you mean? Geoff
joelobryan
November 19, 2017 at 10:09 am: You almost got there. The crooks know CO2 is too weak (at best, in fact it is a coolant). So they concocted an effect on water vapour to magnify things. This is on record. Other planets and large moons prove CO2 is not special except for life. We have that on record too.
A waterless planet with no tri or polyatomic gases, eg all Nitrogen, would still warm by conduction as we do. Conduction remains the dominant first mover, unless WV latent heat beats it on Earth, micro555? The emission from kinetic motion in the molecular force fields would still cool it. What is debateable is how far such an atmosphere has to expand outwards to radiate enough( by the Gas laws, which do indeed rule). Larger planets should somewhere reach sufficient gravity to keep hold of more gas than their net losses through what is really evaporation.
In infinity, I am fairly sure that such experiments are in progress.
The day you realize that you can warm yourself by standing in front of a block of (water) ice instead of a block of dry ice, or a tank of liquid nitrogen, is the day you get to be taken seriously, WWF.
So Andy: Suppose I, as an evil GHE believer, have you tied down to a big block of ice at 0C in a room at 25C. After a while, you get hypothermic and your body temperature drops to 35C.
I now offer to let you move to a big block of slate rock (which has the same thermal conductivity) at 25C in the same room instead, saying it could help you to increase your body temperature back to 37C.
By your logic, you would refuse, because the slate rock is still below your body temperature, so the change couldn’t possibly lead to an increase in your body temperature.
Now, if you were dead, that would be true. (You may be brain dead, but…)
Tom13 - the non climate scientist
November 19, 2017 8:29 am
Oxygen and Nitrogen are not “greenhouse Gases”
Yet dont they serve some function of retaining heat and maintaining some balance of temp in the atmosphere.
Otherwise, it would seem that the total volume of Greenhouse gases is vastly too small (1%ish not counting water vapor) to retain any quanity of heat
Hard to fathom from a scientific point of view that CO2 or any of the other greenhouses gases are sufficiently powerful to act as the thermostat to the degree which they are credited.
O2 and N2 certainly have molecular heat capacity. That is not the issue here. As diatomic molecules with limited vibrational modes, they only absorb in the UV band. Triatomic and higher molecules like CO2, H2O, O3, CH4, NO2 are the GHG’s because they have vibrational modes that allow absorption of IR wavelength photons.
But in terms of IR electromagnetic radiation at the temps under consideration for Earth’s atmosphere and surface temps, the three main gases, that is O2, N2, and Argon (that’s 99.5% of dry air), are transparent to IR wavelengths. Thus those 3 main components of dry air cannot absorb IR photons to warm. They are transparent to IR. Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar. But the GHE in the atmosphere also depends on the adiabatic lapse rate of the troposphere, which exists because pressure drops with altitude (gases are highly compressible of course). This temperature gradient creates an effective radiation level (ERL) where a point (vertically, a temperature profile) is reached where more photons escape to space than are re-radiated toward back toward the surface.
So now think of very dry desert air, how large a temperature swing there is from day to night. During the day, there is visible light and UV heating both the minimal water vapor and the surface. The air layer near the hot surface warms due to convective transfer. At low viewing angles, the shimmering desert convective heat flow is seen as a mirage because refracts the sunlights. But in a dry desert, with little water vapor, after the sun sets, the air cools rapidly, losing several degrees/hour because their is little vapor to help trap the escaping IR wavelength photons. That’s radiative cooling. The other non-condensing GHGs (CO2 in the troposphere, ozone in the stratosphere) help slow the cooling. This is why GHG theory predicts night-time lows will get warmer under increasing CO2 while having little measurable effect on day-time high temps.
The above descriptive physics is why the GCM outputs “predict” a tropospheric hotspot in the tropical latitudes.
And going from 3 CO2 molecules per 10,000 air molecules (0.03%) to 4 or 5 CO2 molecules per 10,000 air (0.04% to 0.05%) makes a negligible contribution to the trapping (back radiation) when the atmosphere (troposphere) contains a much higher molar quantity of (1% – 3%) H2O gas. If the atmosphere were completely dry then CO2 concentration changes would make a measurable impact on heat trapping.
This last fact is likely why the Tropospheric Hotspot in the tropics has not been observed. The GHE of CO2 is too weak to be detectable. This argues for the modification of the CO2 forcing theory from one of a strong GHE effect to a weak GHE effect for CO2. The evidence points to a weak GHE hypothesis for CO2. And that is something the current-day climateers, who are profiting from global warming alarmism, are loathe to admit.
Joel, the lapse rate is determined only by gravity and gas physical properties, it has no input or output from radiation. I don’t believe that the lapse rate creates the apparent radiation level, this depends only on the OLR magnitude and the surface area required to radiate it at the effective radiating temperature (-18C).
Surely there is no “trapping” of radiation by GHG’s?
Taking the relative humidity vs ghg analogy with deserts a step further –
the average relative humidity of a typical desert ranges from 10%-30% (various sources) while the ypical countryside ranges from 50-90%. the delta of the day/night temp swings between deserts vs countrysides is in the range of 20-30 degress. With the concentration of water vapor being 200-400x the concentration of co2, it would seem that an increase of 150ppm would be less than 1-2% of the effect that water vapor would have when you compare desert vs countryside.
Rh only matters when air temps are near dew point, that’s when it stops cooling.
Even though humidity is low in the desert, clear night min temp should be near dew point.
“Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar.”
No. The sink of heat energy is the oceans. They warm the atmosphere by evaporation. that is how the water vapor gets there. Confirmation sip a pina colada at a sea side bar at night. Try the same thing in the desert.
But in a dry desert, with little water vapor, after the sun sets, the air cools rapidly, losing several degrees/hour because their is little vapor to help trap the escaping IR wavelength photons. That’s radiative cooling. The other non-condensing GHGs (CO2 in the troposphere, ozone in the stratosphere) help slow the cooling. This is why GHG theory predicts night-time lows will get warmer under increasing CO2 while having little measurable effect on day-time high temps
It cools 3-4°F/hr in ohio at first too. It just stops at dew point, and the daily range is much higher in deserts.
These are both clear calm nights.
Good point top: if N2 and O2 do not emit or absorb IR, this is a contradiction to QM and thermodynamics.
They do radiate, they are GHGs and here how: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere.
The 15um radiation doesn’t warm anything, the sun at 500k does. All the 15um does is cancel out the 15um radiation coming up from the surface. The sun makes up the difference. Hence the atmosphere warms up a little.
That’s the theory, whether it happens or not or can actually be detected or not is the issue.
Peta of Newark
November 19, 2017 8:39 am
Quality not quantity.
My thinking for years now.
Sometimes called the Ultraviolet Catastrophe and the Perfect Example is solar panels.
(The UV Catastrophe was the title of a BBC Horizon program explaining a time when scientists couldn’t explain certain ‘wavelength’ or colour sensitive phenomena.)
Just exactly why do solar panels need direct (or reflected) sunlight?
Peta of Newark
November 19, 2017 at 8:39 am: We had a paper by warmist students a while back proposing panels to harvest the background radiation of Earthly IR return. Very excited they were at all that amperage (equalling solar input, after all). Only the sound of crickets since then, as they seek the voltage, I guess.
That pretty well explains the AGW myth, really. Magic and magic mushrooms.
The relationship stated in the quantum physics of electron orbitals and any relationship to temperature due to molecular kinetic energy is wrong. Dead wrong.
Sort of kills any value to discuss your other points when such a fundamental error is made.
a point nobody has tried to explain, the earth radiates the LW towards space because space is much cooler than the earth, what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? after co2 releases the tiny portion of the wave it grabbed for a picosecond again isnt a force REQUIRED to send in back towards earth?
It is perfectly possible for a cold molecule to emit a photon to be absorbed by a hotter one. But the flow of heat which is a statistical phenomenon will always go from warmer to colder (except for very small random fluctuations).
“what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? ”
Simply the ‘force’ of radiative exchange. Just because something is colder does not stop it radiating to something hotter. It just means that the net flow is from warm to cold. All things at temperature above 0K radiate. The atmosphere radiates, it just so happens that it is mostly transparent to LWIR (N2 +O2 = ~ 99% and there is an atmospheric window at around 12 micron for the GHGs – see graph) and at the Earth’s temp, 15 micron is the radiation it most strongly emits at. … which CO2 also happens to absorb/radiate most strongly at (just a bit more than H2O).
It comes back because the Earth’s surface is partially in the way of a measure of photons re-emitted. In the mean time (during daylight) shortwave has come in. It’s that extra SW that is causing the GHE.
The ‘delay’ in emission is due to the path-length of a photon through the trop and why it is far from saturated. At around 8km the molecular concentration falls off enough to allow most LWIR to get to space and that level is at the temp the Earth shows from space (255K) whilst here on the surface we live at it is 288K. So at that temp CO2 emits less strongly (being colder), yet it can still absorb at the same rate.
So molecules high up still radiate as normal but they are receiving less because of ‘blockage’ below – this causes stratospheric cooling while the trop warms.
“Net” flow of heat from warm to cold confuses the issue. the correct term is the only flow of heat is from warm to cold, surely there can be no actual flow of heat from cold to warm regardless of radiation flow if the radiation flow can’t be thermalized rather than reflected or transmitted.
In the sense than photons from the cold object impinge on the warm object there is ‘flow’, but the net flow is always from warm to cold unless work is done.
This is the fumdamental mistake made when people say “a cold object cannot heat a warm one”. Correct it cant – but it does add to its entropy and slow its cooling.
“The Second Law of Thermodynamics can be rephrased in several ways. Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction). It can also be expressed using the concept of entropy as saying that the system’s entropy will always naturally increase if no work is exerted to decrease it. These rephrasings mean fundamentally the same thing because heat deals with kinetic energy and increasing a system’s kinetic energy will increase the system’s entropy.”
A thought experiment. You have two flat metal plates somewhere out in space a foot apart. One is at 1000 degrees the other is at 500 degrees. Heat flows only in one direction, from the hotter to the colder plate. Now remove the colder plate. The hotter plate will cool down faster than before, despite that there was no heat flow to it from the colder plate.
>>
Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction).
<<
Not exactly. The Second Law is often stated as:
or the change in entropy for an isolated system not in equilibrium must increase until it reaches equilibrium. Not all systems are isolated. Entropy can decrease in a closed system or an open system. The Earth is usually modeled as a closed system, but it isn’t truly closed. Closed systems don’t allow mass transfer across the system boundary. Isolated systems allow neither mass nor energy transfer across the system boundary. Open systems allow both mass and energy transfer across the system boundary.
The Second Law for a closed system is:
where Q is a path variable and the equation is only valid for reversible processes (this is often stated as the Clausius definition of entropy). Notice that entropy has the same units as heat capacity or energy per unit temperature and in SI units: joules per Kelvin.
Since I mentioned Heat capacity, it is defined as:
So, in an isolated system, a colder object (say a black body) can transfer heat to a warmer object (say another black body) as long as the warmer object transfers more heat to the colder object–simple thermodynamics, and it doesn’t violate the Second Law.
Here is a picture of what natural gas does to IR.
.
.
Note how it blocks it out. Same thing happens to IR with CO2, but not to the same extent. If you were in orbit around the earth, and you “took a picture” in the IR band, CO2 would “darken” the image you get. This is how it “blocks” the IR escaping the planet.
This seems to demonstrate that we are wrong about the CO2 absorption range. A flame is putting out a frequency of well above 15u. But since no thermometer was inside the tube there is no demonstration that there was an increase in temperature.
The problem with his little demonstration is the candles flame is +500 degrees hotter than my shrubs, or the co2 in the atm.
This is propaganda. He knows better than this, that this isn’t really applicable to the problem that exists. He just had to sensationalize it to make an effect, and that’s what’s wrong with the consensus on AGW.
That’s still the candle video.
Besides the point, they filled it with 100% co2.
And so what, there’s an equal negative water feedback to the increases in co2 warming if there is any.
What you say about CO2 darkening the Earth viewed from space in the 15 um band is true. The informed, educated skeptic recognizes CO2 is GHG. But most of your darkening is due to water vapor that is unavoidably present on our 71% water world planet.
The real question for the informed, educated skeptic (that the alarmists avoid) though is is there appreciable (measurable) darkening when CO2 concentration goes from 3 CO2 molecules/10,000 air molecules to 6 CO2/10,000 air molecules. The 2 facts – 1) that the tropospheric hotspot has not been observed, and 2) that models are running too hot compared to 25+ years observation strongly suggests the CO2 strong GHE theory must be modified to a weak GHE theory.
But without a strong CO2 GHE theory, the alarmist argument collapses. That would mean the entire politicized Climate Change and the trillions of dollars hoped to be re-distrubuted vanishes as well.
Note two things in the picture. First the black asphalt roads are very “bright.” This picture is taken in the IR bands, not in the visible bands. The asphalt is “bright” because it is HOT. Also, natural gas does not block visible light, and the plume you are looking at is the natural gas escaping from an underground storage facility in California.
A good visualization on the effect The surface IR is absorbed into air which then does not re-emit it quickly at a wavelength visible in the picture. But, this is different from CO2 in multiple ways.
Satellites, often looking down from about 70km, see earth radiation at many different temperatures. You can’t just take a 15C/289K Planck curve and say, “hey, this only radiates at 10 microns”. Low energy IR radiation does not usually cause “electronic” transitions, that is bumping electrons to higher orbits. IR typically causes rotational and vibrational transitions. The significant CO2 transitions and their “bites” out of the spectrum are shown below. Their radiative temperatures are all different.
The 15um/667.4 fundamental bend (vibration) is seen radiating at a peak temperature of nearly 250K, and the surrounding rotations radiate at and conform to the 220K Planck curve.
The fundamental problem is we don’t live in a purely radiative world, as Bill and others have pointed out, collisional/conductive and convective interactions with the other (largely IR transparent) .996 of the atmosphere modulate the effective radiative temperatures. CO2 has a very low emissivity (tendency to emit photons). It prefers to dance.
Whatever you wish to call the disturbances in the electromagnetic/weak field, photons, or something we can detect as particle like, definitely exist; even though they have no mass. They do not control the earth’s energy balance alone.
“They do not control the earth’s energy balance alone.”
In a way they do, because the heat always finally leaves the Earth TOA as photons. But they do not alone control, or even dominate the heat balance of the surface of the Earth. Convection does.
In the summer, looking across an asphalt surface at low incidence angle over a long distance (long optical path length path length), one see the shimmer. That shimmering (mirage as it is called) is of course refraction due to rapidly warmed boundary-layer air rising from the hot black asphalt surface. The asphalt is dry. The air is mostly dry. Water vapor is present, it warms too. But the vast bulk of the heat transfer from a dry surface in this case is conduction at the boundary where air molecules collide with the hot asphalt molecules.
True, though part of the heating of the air is also due to LWIR emitted by the asphalt and absorbed by the air. And conduction is only important for the boundary and a very thin air layer because of the very low thermal conductivity of the air.
tty
November 19, 2017 at 9:49 am: Agreed tty. Next we have to get it that heat, being a process caused by Kinetic Energy, is what results in radiation. Radiation is not heat and only relatively weakly results in it if it interacts with matter in a kinetic manner. Another basis for warmist waffle.
Yes, my sloppy. tty has pointed this out. I should have said radiation does not alone control temperature between the surface and the effective radiative altitude.
Of course, it is actually far more complicated. The solar wind is incoming energy, but it is not EM radiation. Likewise, ions stripped from the earth’s atmosphere, reputedly as heavy as Oxygen, count as energy out.
Schrodinger's Cat
November 19, 2017 9:54 am
I have some doubts about some of your statements. Carbon dioxide does not undergo changes in electron levels as far as I know. Also, I’m not sure if the altitude of an electron from the nucleus is quite correct either. I think these are visualisations of differences in energy. However in the case of CO2, the extra energy is not sufficient to do any of that. It simply changes the vibrational energy. CO2 has four vibrational modes and the amplitude of the vibration is what changes on excitation.
I don’t think these change the the substance of your main claim, but since I am still grappling with that. I cannot comment either way. There is certainly something seriously wrong with the settled science.
As a footnote to Anthony, it may be a contentious topic but it is absolutely fundamental to the debate. The principles of spectroscopy are well known but when transferred to an atmospheric context there are masses of uncertainties to be resolved. This is where pseudo scientific assumptions and cherry picking of facts can support a credible argument on either side. Debates like this make gradual progress in revealing better insight. Thank you for posting this one.
ferdberple
November 19, 2017 10:00 am
under GHG theory the temperature of the earth would be unchanged if all the O2 and N2 was removed.
this makes no sense. the atmosphere has thermal inertia which would be dramatically reduced without O2 and N2. Nowhere is this accounted for.
So what would the earth be like without an atmosphere?
The average solar constant is 1,368 W/m^2 with an S-B BB temperature of 394 K or 17 C higher than the boiling point of water under sea level atmospheric pressure, which would no longer exist. The oceans would boil away removing the giga-tons of pressure that keeps the molten core in place. The molten core would push through the floor flooding the surface with dark magma changing both emissivity and albedo. With no atmosphere a steady rain of meteorites would pulverize the surface to dust same as the moon. The earth would be much like the moon with a similar albedo (0.12) and large swings in surface temperature from lit to dark sides. No clouds, no vegetation, no snow, no ice a completely different albedo, certainly not the current 30%. No molecules means no convection, conduction, latent energy and surface absorption/radiation would be anybody’s guess. Whatever the conditions of the earth would be without an atmosphere, it is most certainly NOT 240 W/m^2 and 255K.
The alleged 33C difference is between a) an average surface temperature composed of thousands of WAGs that must be +/- entire degrees and b) a theoretical temperature calculation 100 km (32 km) away that cannot even be measured (no molecules to compare) and c) all with an intact and fully functioning atmosphere.
The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.
“The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.”
Sorry, but that is not at all how it works. Hint: check the thermal conductivity of air (it is about the same as polyurethane foam) and calculate the temperature gradient needed to move 168 Wm^-2 through, say, 10 kilometers of polyurethane foam.
Also how do you explain that the temperature reaches a minimum at the tropopause and then starts increasing again?
The dominant heat-transfer mechanism in the atmosphere is convection and the lapse-rate is simply the amount of energy needed to drive the convection.
GHG theory predicts the tropospheric hotspot. This has failed to emerge.
in science a single failed prediction is absolute proof of a failed theory.
however, trying to prove why GHG theory failed is largely a fools errand, because we have no replacement theory.
for example. we know that Newtonian gravity fails to predict rotational speed of gravity. however, we cannot. explain why because we have no alternative theory. instead we are left to invent a new form of matter. dark matter that obeys some laws but not others. and then we postulate that the universe is 90% dark matter. this is GHG theory mistakes on a cosmic scale.
You are too lazy to follow the link I gave you,you also didn’t answer my question about the “evidence” in YOUR link.
“The researchers instead used observations and combined two well-known techniques—linear regression and Kriging”
Ha ha ha ha ha……..
You can’t be THAT stupid.
Meanwhile a rebuttal was posted on it:
Desperation — who needs thermometers? Sherwood finds missing hot spot with homogenized “wind” data
“The fingerprint test of the water-vapor feedback is the “hot-spot”, a warming of a band of the upper troposphere 10 km over the tropics. (See the reasons below at the end). The weather balloons were designed and calibrated to measure temperature and humidity as they rise through the sky and right through the hot-spot. Their results are unequivocal: red was not yellow; the spot was not hotter. Supporting this, the specific humidity was also supposed to rise, but fell instead. If the computer models worked on everything else, we might wonder if the millions of observations were biased, but the models didn’t predict the pause, were wrong about humidity, rainfall, drought, and clouds too. They didn’t work on regional, local, or continental scales and can’t explain long term historic climate either. At this point, a scientist would throw out the theory. The weather balloons independently agreed with each other, the humidity results fitted the temperature results, the whole lot was loosely supported by satellites. The data doesn’t need homogenising or kriging or obscure numerical witchcraft.
Instead Steven Sherwood and Nidhi Nishant of UNSW revisited their 2008 technique of homogenizing temperature data by using wind data as well. They homogenised it again. They have iterated the iteration? They’ve also extended it from 2005 to 2013 and changed the “wind shear” component to “vector wind”. Their new homogenized-temp-wind data is below (left). The model predictions of 2005 are centre, and the radiosonde temperature results (before homogenisation etc) are on the right.”
Layman’s interpretation of what you have just posted.
A scientific theory doesn’t work because something is missing. It’s not known what’s missing, but introducing an unknown makes the original theory work.
No wonder I abandoned science at secondary school. It’s like playing a game of chess, with a million pieces. Nothing is ever solved, just continually shuffled around with everyone having an opinion on which way to move a single pawn, that doesn’t exist.
RGHE/GHG theory assumes the earth is warmer w/ than w/o atmosphere. Attempts to explain this assumption are futile because it is fundamentally incorrect.
The atmospheric insulation blanket creates a thermal resistance (all 32km worth of molecules that matter) just like an electrical resistance or hydraulic resistance. In all three instances a potential difference is required to move energy: dT or dV or dH, from A to B. For heat flow it is: Q = U A dT where U, aka 1/resistance, is the combined effect of conduction, convection, latent and radiative properties together with wind, storms, lit side, dark side, etc. everything that impedes thermal movement from surface to ToA where 100% radiation takes over. (No molecules in space to take the energy handoff.)
The atmosphere is little more than a fundamental HVAC problem.
RGHE theory says the ground would get cold w/o “back” downwelling radiation. Reams of USCRN data show absolutely zero evidence of this.
The sun warms it all. Air temperature swings widely because of its low thermal capacity. The 2cm ground temperature swings very little because of its high thermal capacity. The 100 cm ground temperature hardly changes.
All night and in winter the air temperature spends many hours below ground temperature so the ground heats the air, NOT vice versa.
RGHE theory simply doesn’t stand up to physical observations. All the rest of the discussion is sound and fury signifying nothing.
However the resistance is gravitational, not thermal, since the dominating heat transfer mechanism is convection. The atmosphere has such low thermal conductivity that conduction is uttery insignificant. It is no coincidence that almost all insulating materials are mostly air.
Essentially correct. Warm air has lower density and therefore rises. As it rises it expands and cools down. Ultimately it will come down somewhere else, outside the convection cell.
“GHG theory predicts the tropospheric hotspot. This has failed to emerge.”
It is also the theory in any sort of warming. It is merely a function of greater LH release aloft in the tropical high atmosphere via convection.
One thing – it ois difficult to find because of the nature of the instruments used. Radiosondes are imprecise for the job and have changed over the years, and sat obs are contaminated by Stratospheric cooling – which is a function of GHG theory.
Another thing is that it has been found …
“First, tropical warming is equally strong over both the 1959–2012 and 1979–2012 periods, increasing smoothly and almost moist-adiabatically from the surface (where it is roughly 0.14 K/decade) to 300 hPa (where it is about 0.25 K/decade over both periods), a pattern very close to that in climate model predictions. ”
It is hard to know where to begin with such a complete pile of nonsense. One of the more obvious errors is confusing a black body radiation spectrum with discrete absorption lines due to molecular transitions. Then there is the weird description of electron orbitals which do not make any sense. If there was any attempt at quality control on this blog this post would have been thrown into the trash where it belongs.
I think the main problem is that the author seems to be completely unaware that the energy levels of a molecule are a lot more complex than for a single atom.
Germonio
November 19, 2017 at 10:42 am: Once again, explain not excoriate. Show how you are more knowledgeable, not just something else…..
ferdberple
November 19, 2017 10:55 am
radiation works by the exchange of photons. convection works by the exchange of virtual photons. thus if there is a GHG effect for co2 there must be the same effect for n2.
co2 absorbs a photon from the surface. this can be radiated up or down. n2 absorbs a virtual photon from the surface. this can be conducted up or down.
in all aspects the GHG effect operates like conduction at a distance, except that co2 can radiate to space while n2 cannot. thus co2 cools the surface on net as compared to n2.
Ferd,
I would suggest you need a dictionary. Convection refers to the movement of hot molecules from one place to another. It has nothing to do with virtual photons.
Uncle Gus
November 19, 2017 11:01 am
Fascinating!
I’m learning here just how little I know about gas absorbtion and emmission phenomena.
I’m also learning how much utter bilge can be written by someone who knows *nothing* about such things, but is willing to shoot their mouth off endlessly. And getting a fair idea of how difficult it must be for a relative layman to tell the difference.
For the record, Gill is wrong, but he’s at least honest. It would be nice if someone took him through his presentation point by point and explained (gently) where he went wrong. I’d do it, but, as I hinted, I’m not best qualified.
(Incidentally, I asked for something like this debate weeks ago – it’s good to see it happening. Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.)
Uncle Gus, why the rant after saying this: |… I’m not best qualified.”? But, you feel yourself qualified to claim: “Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.” I bet you don’t even understand your own hypocrisy.
“Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming.”
no.
the downwelling LWIR is a RESULT of GHGs ( like water) it is not the cause of warming.
When people “popularize” the science and dumb it down, they say LWIR causes the warming.
nope.
When you increase c02 two things happens.
1. the stratosphere cools. We have seen this. Its How we know the warming is GHG related and not the
sun.
2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.
a) radiating from a colder place entails a slow down in the cooling to space.
b) to maintain energy balance, the compensating increase in warmth at the surface has three
pathways:
1. it can go into the ocean
2. it can melt ice
3. it can warm the air.
Because in the stratosphere radiation dominates over convection, so more GHG increases the radiative energy loss. The temperature gradient is also the opposite – it gets warmer with altitude, since the main energy source is UV absorption in the ozone layer from which temperature decreases both upwards and downwards.
Even more explaining needed of the temperature changes as altitude increases:
In the Troposphere, temperature falls with altitude but, above the Troposphere comes the Stratosphere where temperatures increase with altitude but, above the Stratosphere comes the Mesosphere where temperatures fall again with altitude but, above the Mesosphere comes the Thermosphere where temperatures go up again with altitude!
Given the differences of opinion on this thread, I would love to hear everyone’s theories as to why all these temperature changes happen? 🙂
“2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.”
So you mean that when the temperature at the surface goes up the average temperature of the Earth seen from space will go down? That the temperature profile of the atmosphere is somehow unchangeable?
What does happen is that with more CO2 the average altitude from which the heat radiates out will rise slightly. All things equal this means that the surface temperature will also rise slightly, since more energy is needed to lift the convecting air.
However all things aren’t equal. Higher temperature means more evaporation and more H2O in the atmosphere More H2O in the atmosphere means a less steep lapse rate, i e a smaller temperature difference between the surface and a specific altitude. There will also be albedo effects (=clouds, snow, vegetation).
All things considered more CO2 in the atmosphere does seem likely to result in some warming, but the effect is probably quite slight.
“1. it can go into the ocean
2. it can melt ice
3. it can warm the air.”
NONE of which is happening.
1. Oceans are currently cooling, especially Southern Ocean
2. Only ice melt is down from the Highest level since the LIA (late 1970s)
Arctic sea ice is still in the top 10% of extent wrt the last 10,000 years
Purely in line with the approx 60 year AMO cycle
3. No warming in satellite data apart from ocean events.. ie release of energy from the oceans at El Nino events.
You are still selling moldy lemons , Mosh. Useless even as a car salesman.
No, if the magnitude of the effect cannot be calculated, then, Stephen Mosher has admitted that he, and BEST, are simple record-keepers, unable to predict Climate Sensitivity, and have also admitted, no one else can either.
No one can. From first principles, which means from the laws of Physics which have nothing to do with dodgy thermometer records, we cannot calculate Climate Sensitivity. Think about this.
Most people do not understand that the entire atmosphere radiates to space, including N2, O2, Argon, and the entire mass of the Atmosphere. All Matter above Absolute Zero radiates all the time.
Hotter Matter radiates to the Absolute Zero of Outer Space, which could maybe be a small fraction of a degree Kelvin above Absolute Zero, More than cooler matter.
There is this word Opaque. This word means No Radiation Penetrates. The Top of the Atmosphere radiates to space at a temperature far above Absolute Zero, much closer to the temperature at which CO2 has ceased to be opaque to the radiation it can absorb at its best absorption frequency, 15 Microns, corresponding to around -80 C.
More CO2 raises the altitude at which this happens. Due to the Lapse Rate, the higher the altitude at which the atmosphere no longer encounters an opaque layer, the cooler the temperature becomes at which the Entire Atmosphere can radiate to space. Heat Transfer is proportional to the Delta T, the difference in temperature between hotter and cooler.
More CO2 reduces the atmosphere’s flux to space, increasing the amount of heat retained in the atmosphere, warming the air at the surface, but no one can calculate how much.
More CO2 reduces the atmosphere’s flux to space, increasing the amount of heat retained in the atmosphere, warming the air at the surface, but no one can calculate how much.
Not much, because water compensates for the increase.
Steven – an honest question to answer please. If the average altitude at which th e Earth radiates to space is raised surely the radiating surface is also increased precisely because it is a now a larger spherical surface. Is that taken into account?
It’s easy to do the math. Let’s say, the “emission level” is increased by 100 meters (0.1km). The radius is increased from 6400km to 6400.1km, a 0.0015% increase. So the surface area is increased by 0.003%.
If you use a lapse rate of 6K per km, the raised layer would increase the surface temperature by 0.6K, other things being equal (yes, this is a highly simplified analysis). The increase in emission layer area of 3 parts in 100,000 is completely trivial in this analysis.
I think you missed something very important. Water vapor dominates the radiative heat transfer from the lower troposphere, blocking most of the spectrum that other GHGs would otherwise block. When the buoyant air reaches the tropopause, the water vapor condenses out. Water vapor ceases to be a factor at all in the process of radiating from the ground to space. So the effects of increasing the amounts of the other GHGs is no longer masked by water vapor.
b) to maintain energy balance, the compensating increase in warmth at the surface has three
pathways:
1. it can go into the ocean
2. it can melt ice
3. it can warm the air.
No wonder you can’t figure it out, you forgot the optical window to space.
No, he didn’t forget the sky window. He said the increase in warmth at the surface, he was not referring to the fact that some of the sun’s energy that reaches the earth’s surface goes back out into space through the sky window.
No, he didn’t forget the sky window. He said the increase in warmth at the surface, he was not referring to the fact that some of the sun’s energy that reaches the earth’s surface goes back out into space through the sky window.
Right, but he probably doesn’t expect the atm to self adjust how cold it gets, and there not being all that much additional warmth left to deal with. So none of his choices are correct, really.
a) radiating from a colder place entails a slow down in the cooling to space.
I’m throwing food now.
Can you explain “slow down cooling”? Does the emissivity of the planet change? Does CO2 change the emissivity of the planet? Where, then, is the back up that constitutes the “slow down”? — in a magical zone between the given emissivity and the given planetary radiation? How does that work? “Slow down” with respect to what time frame? Is Earth on a schedule to cool by a certain time? Who sets it? How long does it take for enough “slow down” to cause heating? WHAT exactly is being “slowed down”? What is the entity defining “cooling” that is being “slowed down”?
This “slow down the cooling to space” sounds a lot like a piece of glass inhibiting convection, … a roundabout way of resurrecting the idea of a barrier that keeps stuff from moving out. It’s a more indirect way of erecting that retaining barrier (via conceptual, rather than physical), but still it seems to beg holding onto a cherished, flawed idea.
I have yet to be convinced that a “colder place” can cause heating by any known physical means, either directly by adding heat or indirectly by “slowing down cooling”.
Energy comes in, energy goes out, all the time. If a little less energy goes out, then the atmosphere has a little more energy, and is a little warmer. Lapse rate means that the average temperature at the surface is determined by how much energy is leaving at the TOA.
It is unfortunate that you believe you have to restate the obvious in response to a rubbish post..
But fair enough. Yes, increasing GHG’s warm the surface (though the details are complicated and not homogeneous in time or space). Yes, continued use of fossil fuels will cause additional warming unless offsetting factors keep that from happening.
Now that we are past that, the real issues are (and have always been) how much warming, over how long, the negative consequences of that warming, and what offsetting benefits (eg greater crop production) balance in whole or in apart the negative consequences of that warming.
The Reverend Badger.
November 19, 2017 12:05 pm
LOOK HERE!
I got the first comment in this thread and I told you not to treat “photons” like they were real massless particles. I got my big red marker pen out and crossed out everybody who did.
Has anybody got another big red pen (or 2) please as I seem to have run out before I got to the end.
Furthermore ; Who the hell told more half of you that you could add radiative fluxes arithmetically?
Anyone who still thinks they can by tomorrow is invited round to my kitchen where I have a large number of radiative objects at all kinds of temperatures from 0 to 90 C which you will be required to arrange in an ensemble of your liking and invention in order to boil my egg.(Boil = 100 C)
Seriously guys and gals an actual experiment or even a thought experiment to show that adding radiative flux arithmetically is scientific nonsense is so flippin’ simple it is school physics level. Please – up your game!
If you cannot even grasp this the furthest you are going with atmospheric physics is horizontal dermatological absorption.
Double the W/m^2 at the sun (two suns) also doubles the W/m^2 at earth’s orbit – 1,368 * 2 = 2,736. S-B BB equivalent temperature rises to 468.7 K from 394 K.
Lets go a bit further. The Sun occupies a tiny percentage of the area of the sky, I think something like 0.002% IIRC . So never mind TWO suns, let the thought experiment go large and we can have 50,000 suns , should just be able to squeeze them into that sky. Now please redo the SB calculation like you did for 2 suns, nick. The answer should tell you something. Especially when you compare it with the temperature of EACH sun. A new definition of heat transfer from “cold” to “hot” !
This is of course the same as my numerous 90C radiating objects in my kitchen which you need to arrange to boil my egg. Yes, there are at least 50,000 of them and you can arrange them in a sphere around my saucepan.
You ask: “Who the hell told more half of you that you could add radiative fluxes arithmetically?”
The 1st Law of Thermodynamics (conservation of energy) told me that. It is any absolutely necessary consequence of a conserved quantity like energy.
In any actual thermodynamics class, virtually the first thing you learn is to add and subtract energy transfers to and from a “control mass” to determine changes in the energy of the control mass in “energy balance” calculations.
It’s no different from balancing your checkbook, which must observe “conservation of money”. In effect you are arguing that if you get a second job (let’s say lower paying than your first job), you can’t add the money you get from your second job to your bank account. It’s an absurd argument!
Badger, you are correct. If you could add w/m2 then it would be possible to cook steak with ice cubes.
Once an object has attained the max temperature it can adding a second source with the same temperature, as first, cannot cause the object to increase further. (T4 – T4) tells us that.
LdB
November 19, 2017 12:15 pm
Your Quantum facts are almost to a tee wrong
1.) Molecules have one or more electrons circling them. blah blah blah
NO
That is classical physics junk. In QM the electrons have complex distribution clouds only S orbitals 1s,2s,3s are circular. The reason is that electrons have half-integer QM spin and subject to Pauli exclusion principle that says two or more identical QM spin particles can not occupy the same space at the same time. They will instead by subject to pairing creating a waveform between them.
The reason for the half spin can be seen by seen by watching the field movement
You will also note the momentum is in the field it is not a real spin like in the physical sense.
So Quantum spin can be thought of as momentum but it can not be equated to a physical object spinning.
2.) For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
PARTLY
That is the process of excitation and it creates an excited state it equates to temperature in classical physics. Outside the two ways listed you can also excite an atom via the electric or magnetic fields.
You need to be careful the temperature however is not always positive, it can be negative 🙂
3.) For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit. blah blah
NO
You need to break that into Photoexcitation which says a Photon must have a frequency that matches the one of the excitation energy level to be absorbed. You can view it as a resonate frequency with one of the excitation states of the molecule. Any energy absorbed in this manner departs from the equilibrium Boltzmann distribution as viewed by classical physics.
If the energy does not match those special resonant frequencies absorption of the photon takes place in accordance with Planck’s quantum theory.
4.) Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum blah blah
NO
Photoexcitation is an independant process and will occur at any temperature
Laser cooling illustrates this in the most extreme way. https://en.wikipedia.org/wiki/Laser_cooling
The energies that are outside the resonant frequencies behave in the more normal way as you are trying to describe.
5.) Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them. blah blah blah
NO
see item 4, temperature does not play a part in photoexcitation only photon energy match
Now, you need to stop following the thermal collision effects and deal with only the photoexcitation effect and key to understanding that is bring Nitrogen into the mix. Nitrogen exists mainly as a simple diatomic atom in the atmosphere and it’s excited states are metastable and relatively long-lived and they happen to overlap a couple of CO2 frequencies. Classical collisions between CO2 and N2 will exchange energy in the photoexcitation frequencies. So the normal thermal energy and the photoexcitation energies can interchange. Without the interplay the two sets of energy would remain isolated.
Martin, do all your posts provide zero value add? Someone takes the time to write a detailed rebuttal of the author’s points, and all you have to say is blah blah blah. Truly the hallmark of a lightweight.
Much to read here in the comments, and I WILL get to it all later.
My overall observation, though, is that the talk still seems to center on warming.
The atmosphere keeps Earth’s surface from getting to hot too. The atmosphere, therefore, BOTH cools the surface AND warms the surface at the same time, depending on your perspective, … depending on what you are referencing as the main focus.
I think that talk should shift away from speaking of either extreme (warming or cooling) as the main focus and, instead, shift to the concept of REGULATION, which must consider both, or neither, depending on how you look at it.
Regulation involves an exchange between two processes, rather than either of those processes alone. And so it seems that CO2, H2O, and other gases REGULATE Earth’s temperature withing the habitable range familiar to us.
I agree Robert. As I noted earlier, the ocean, the soil, and the GHG merely serve as heat sinks for the atmosphere. The aspect of CO2 is limited by the amount of IR available to be absorbed. The equilibrium equation heat sink capacity can only reach a certain maximum simply because there is no more IR in that spectrum to work with.
So, as you say, it plays a small role in stabilizing the atmospheric temperature, but it plays little role to n the overall stabilization process, simply because it is insignificant compared to the ocean (the overwhelming #1 heat sink), and water vapor, the primary heat sink in atmosphere.
CheshireRed
November 19, 2017 12:28 pm
One thing that posts like this definitely demonstrate is there is no consensus. When multiple highly-qualified professionals and well-informed amateurs cannot agree on something as fundamental to ‘global warming’ as a GHE or high sensitivity…then it’s obvious there’s still oodles of uncertainty.
The back radiation is not the only energy flux warming the surface. There is also the direct solar irradiation. Below is an energy balance sheet of the Earth. It is the only presentation showing the three sky conditions: first number is all-sky, the second number is clear sky, and the third underlined number is cloudy sky. The total energy flux warming the surface is Sd + Ed = 168,8 W/m2 +344,7 = 513,5 W/m2. And
by the way, the both energy fluxes are based on direct measurements. The energy balance of the Earth’s surface shows that the incoming energy fluxes and the outgoing energy fluxes are in balance. If they were not, the surface would cool or warm.
Robert W Turner
November 19, 2017 12:37 pm
The major misconception presented here is treating all molecular energy the same. There are two distinct types, translational kinetic energy that all molecules have, and quantized vibrational energy that dipole molecules can have.
The GHG theory treats the later energy type as if it is not quantized, in other words they conceptualize that a dipole molecule will always absorb a certain wavelength of energy even if it is already in the energized vibrational state, that is NOT correct. If the gas molecule is already in its excited energy state, it can not continue to absorb that wavelength of light, that light will be transparent, reflected, or in some instances it will stimulate that molecule to radiate that energy and drop back to the non energized state.
However, very small levels of energy (factions of 1 eV) can be lost or gained from the quantized energy and given to translational kinetic energy upon collisions with other gas molecules — that’s why the absorption is a band instead of an exact wavelength. These collisions theoretically occur every 10^-7 s at atmospheric pressure which is faster than CO2 molecules theoretically radiate heat. Therefore, we should expect some minor heating caused by an increase in CO2 molecules in the atmosphere from conversion of quantized energy into translational kinetic energy from their collisions with non dipole molecules.
If all GHG molecules in the atmosphere are already absorbing and reemitting all available spectra able to bump the molecules into the energized state, then the only mechanism for additional retardation of heat escaping from the atmosphere can occur from the collisions described above. But that’s only if the available infrared energy is the limiting factor whereas more heating would occur if it is the available number of dipole molecules is the limiting factor.
In my opinion, we have empirical data showing that it is indeed the available IR that is the limiting factor and therefore any additional dipole molecules in the atmosphere can only heat by losing some of their quantized energy to kinetic energy. That is the CERES and TERRA data that has shown that certain regions of the atmosphere lose more heat into space than they receive from the sun, one region in particular being the Sahara desert.
This data not only shows that it is water vapor dominating the GHG feedback, but to me it also suggests that there is something to the theory that the temperature of planet’s atmosphere is largely dependent on adiabatic and gravity pressure induced compression heating. The most heat leaving the planet happens to be where cooled dry air descends within the atmosphere and inducing high pressure.
Notice the outgoing longwave radiation coincident with the descending air associated with Hadley cells. Please, I invite anyone to explain this with the GHG planetary warming theory and how it is not explained by adiabatic and gravity driven heating.
No such thing really. Pressure does not cause heating or cooling, only changes in pressure. Rising air cools down, sinking air warms up. This is the basic reason for the lapse rate.
That there is more outgoing radiation in the descending part of the Hadley cell is because the air there has lost most of the water vapor (the really important GHG) and is therefore much more transparent to LWIR, so it comes from a lower and warmer layer.
What do you mean no such thing really? Very basic physics, really.
“work done by gravity = W = mgh (h = height lost by the object)
An alternate way of looking at this is to call this the gravitational potential energy. An object with potential energy has the potential to do work. In the case of gravitational potential energy, the object has the potential to do work because of where it is, at a certain height above the ground, or at least above something.”
The process is started by solar heating of the ground, air masses warm up on the ground, they rise and then fall in the Hadley Cells. Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.
tty is correct. Static pressure does not cause any energy transfer.
Robert’s equation of “work done by gravity = W = mgh (h = height lost by the object)” actually proves this.
“h” in this equation is zero for the atmosphere as a whole. The atmosphere has no height to lose in toto. Any downdrafts are exactly balanced by updrafts elsewhere.
In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.
“Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.”
However the effect would be very weak in an atmosphere without GHG since very little heat would be transferred from the ground to the atmosphere (though it is rather unlikely that such an atmosphere exists anywhere).
“In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.”
Very true. As a matter of fact this is the main mechanism regulating the surface temperature of the Earth. And it can not be modeled realistically, now or in the foreseeable future (it would require increasing computational capacity by something like 10^12).
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
You are confusing the one-time effect of dynamic compression — which can and does result in “heating” (and can be immense on a planetary scale) — with the ongoing effect of static pressure — which cannot and does not.
If you compress the air in a bicycle tire pump, it gets hot. If you leave the compressed air in the pump, it does not stay hot, but your analysis says that it would
The gas giant planets go incredibly hot in the dynamic compression of their formation. Some think that Jupiter’s core got almost hot enough to initiate fusion. These planets still emit significantly more energy than they receive from the sun, which means they are still cooling.
The earth, however, does not emit any significant amount more than it absorbs from the sun. Its geothermal flux is only about 0.1 W/m2 averaged over the surface.
In what world is the atmosphere static? The down draft limits the warming, is that what’s observed?
However the effect would be very weak in an atmosphere without GHG since very little heat would be transferred from the ground to the atmosphere (though it is rather unlikely that such an atmosphere exists anywhere).
Complete rubbish. The composition of the gas has absolutely nothing to do with convection.
The sum is not zero in this process. Solar radiation does the work to lift the air and gravity does the work to compress it. Energy is actually input into the system this way, and lo and behold, it’s actually what is observed.
Doesn’t descending air become exposed to greater pressure as it descends? Isn’t gravity doing work on it? Doesn’t it’s density increase, from less dense to more dense? — and it’s kinetic energy increase from less to more? And doesn’t gravity cause this descending, compressing, increasing kinetic energy in conjunction with the already warmer level into which it descends?
I confuse nothing. It only requires “a one time heating”
The Kinetic energy at the bottom of the atmosphere doesn’t dissipate to the top again. To do that it must work against gravity. Take a look at Neptune, where you have much less solar input to confuse you. It gets warmer as you descend into it, until it is thousands of degrees C.
Outside the fraudulent area of climate change on earth, this sort of thing is universally accepted.
It’s about time people on here looked up from their narcissistic intellectual self aggrandisement and checked with the rest of the scientific world
yes it can continue to absorb at that or nearly at that wavelength. you missed out that vibration in a molecule has harmonics so two photon absorption is possible. or a photon of double that energy can be absorbed.
just like a harp string will have a fundamental frequency it will have higher energy harmonic frequencies. a harpist will pluck the string at a different height to change the harmonics of the string.
it can continue to absorb at that or nearly at that wavelength. you missed out that vibration in a molecule has harmonics so two photon absorption is possible. or a photon of double that energy can be absorbed
So you’re saying that a photon can be absorbed that is double the energy level at which the molecule actually absorbs? And given an infinite number of photons in the 15-20 nm range, a CO2 molecule will continue to absorb that thermal radiation regardless of all three normal modes energized? And then can theoretically emit at a higher energy level than the radiation it absorbs?
To R turner at 9:27 pm.
1 carbon dioxide has 4 modes of vibration. Symmetrical stretch, asymmetrical stretch, and two bending modes of equal energy. The word for equal energy in spectroscopy is DEGENERATE. so they are a degenerate pair of benders!
the symmetrical mode does not absorb infra red since there is no change in dipole moment. the other 3 modes are infra red active. there are an awful lot of vibrational energy level present in each mode so each mode can sequentially absorb a lot of almost equal energy photons one after an other. ( due to asymmetry in the vibration the second photon energy will be slightly less energetic than the first and so on). The molecule can absorb photons equal to 2 of these energy bands (or even 3) this is analogous to a musical string vibrating at twice its frequency ie one octave above the fundamental note.
there are not an infinite number of energy levels so it cannot absorb an infinite number of photons. if it absorbs too many photons then the molecule will vibrate so strongly that the atoms will fly apart and it no longer exists it will be CO and O. Double strength photons can come off but has much lower probability than sequential single photons. I some how remember that some lasers can produce double strength photons.
indeed at any one time there will be a fraction of the population of CO2 with excited vibrational modes, and these will be absorbing photons as well.
This is how molecules break down in heat. infra red lasers can be used to heat single bonds in a molecule and break it. however the method is in its infancy and may just as well break a nearby bond.
Robert W Turner ,
Note that your NASA illustration (second one) has a range of reflected light (0–700 W/m^2) that is more than twice the commonly accepted value of incoming TOA radiation (342 W/m^2). How can something reflect more than it receives?
If CO2 did affect climate, than the increase in CO2 over the past 30 years should have caused at least a measureable change in the dry lapse rate in the troposphere, but such has not happened.
There are many lapse rates in the atmosphere. g/Cp defines the Dry Adiabatic Lapse Rate (DALR) which is about 9.8 K/km. The more typical troposphere lapse rate is the Environmental Lapse Rate (ELR) which is 6.5 K/km. There are many more important lapse rates and one or more of them *might* be affected by CO2. However, all of them, except the DALR, are affected by radiation to/from CO2.
Whu should the dry lapse rate change? The small increase in CO2 is not enough to measurably change the specific heat of the dry atmosphere. If the increased CO2 resulted in an increased amount of H2O in the atmosphere (known. by friends and admirers as “water feedback”), then the average lapse rate would go down, but as far as I know this has not been observed.
willhaas. You forget that there is a possibility that the CO2 effect is much less than IPCC reports. In that case the other cosmic warming effects have decreased and therfore the temperature has paused, even though the warming by CO2 has increased slightly.
According to the IPCC, the warming effect of CO2: dT = 0.5 * 5.35 *ln(CO2/280) resulting to the climate sensitivity of 1.8 C degrees. I have reproduced the calculation of the warming formula of CO2 and it is: dT =0.27 * 3.12 *ln(CO2/280) resulting to the climate sensitivity of 0.6 C degrees.
The warming effects of different main forces are in 2015: the Sun 0.35 C, GH gases 0.28 C, and astronomic harmonic resonances 0.13 C, together 0.75 C. Link: https://www.climatexam.com/cosmic-theory-caws
The system is a lot more complex than many (including we token ‘warmists’) think.
angech
November 19, 2017 1:14 pm
“Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um”
–
This statement needs a lot of modification.
We are talking about our atmosphere.
Temperature is the sum total of all the energy in all the molecules in the atmosphere in any particular place.
Individually molecules will have different energy levels,
LWIR is produced by both the sun and the earth surface and the atmosphere and clouds.
So the atmosphere certainly has all LWIR frequencies trying to pass through it.
I guess the CO2 molecule can pick up these other two frequencies if needed if it runs across them?
–
If a CO2 molecule has picked up a higher “hotter” frequency energy packet and it subsequently emits it this does not mean that the whole atmosphere has to be at say 120C does it?
Yet that CO2 molecule will be buzzing around giving energy [heat[temperature]] to a lot of other O2, H20,N and other molecules while it is charged up.
–
Something is wrong in the state of Denmark but I am not good enough yet to explain it. There must be lots of people who do understand radiative physics to simply knock this contention out.
Where are they, please.
–
Quite happy to be a skeptic for lots of reasons but not on the CO2 is not a GHG as technically it will raise temperatures according to the laws of physics.
Andrew Hamilton
November 19, 2017 1:21 pm
Just a minor comment.
Should 274 K be 273 K and 15 C be 288 K, not 289 K?
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit” I stopped there, too many misconceptions about quantum mechanics. The IR absorption spectrum has nothing to do with the electronic excitations, but with rotational-vibrational spectrum of the molecule (cannot be purely vibrational, because the photon has helicity). I’m going to have a post on my blog about this sometime, specifically for CO2 and H2O, calculate some frequencies and explain a few things…
MODTRAN answers the question. MODTRAN, whose accuracy has been verified by satellite measurement calculates heat flux either outward or downward at any point in the atmosphere. The difference between heat flux outward at 0 altitude and at the top of the the atmosphere is the heat deposited in the atmosphere. If the GHG concentration is anything above 0 the heat deposition is positive. If it is 0, then all of the IR heat from the earth escapes to outer space and we are left with a frigid, uninhabitable earth.
Excuse me, but all the IR ultimately does escape to outer space (including geothermal heat).
“If the GHG concentration is anything above 0 the heat deposition is positive.”
Since it has been above zero for the last 4 billion years or so (and mostly much higher than now) according to your physics the planet should have melted and boiled off the atmosphere long ago. As a matter of fact even if the “heat deposition” was only 0.000001 degree per year the Earth should be almost as bright as the sun by now.
Yes, all the IR energy does escape to outer space, but it does so at a higher temperature than it would have done without the IR absorption of the atmosphere. It does it via the Stefan-Boltzmann equation. Since it loses some of the (discrete) IR levels leaving the earth’s atmosphere it has to increase its temperature to make up with a continuous spectrum what it has lost from the discrete emissions. This is the mechanism of GHG heating. You can read about the details by googling “Nature Abhors a Positive Feedback” which is a 3-year old post to this site (Watts Up With That).
Problem is no one uses it right.
It changes just by altering air temp and not composition. So, unless you run it for each temp, you get the wrong answer.
Ned Nikolov
November 19, 2017 1:44 pm
I agree – the notion that a gas such as CO2 or water vapor can “trap” radiant heat in a free atmosphere is simply unphysical. Heat trapping by gases is only possible by preventing/obstructing convective heat exchange (as in a glass greenhouse) or by using of IR-reflective materials such as polished aluminum that have VERY low IR emissivity/absorptivity and a high IR reflectivity (these materials are called “radiant barriers”).
Our latest paper addresses this issue and demonstrates using NASA planetary data that the thermal effect of planetary atmospheres has nothing to due with trapping of outgoing thermal radiation, but instead is due to the force of atmospheric pressure, which adiabatically enhances the energy received from the Sun. In other words, the so-called “Greenhouse effect” (more accurately named Atmospheric Thermal Enhancement or ATE) is a thermodynamic (pressure-induced) phenomenon that is completely independent of atmospheric composition:
Nikolov N, Zeller K (2017) New Insights on the Physical Nature of the Atmospheric Greenhouse Effect Deduced from an Empirical Planetary Temperature Model. Environ Pollut Climate Change 1: 112. doi:10.4172/2573-458X.1000112
URL: https://tinyurl.com/ydxlfwn7
Did you take high school physics? If you had, you would understand that for a force (such as atmospheric pressure) to transfer any energy, it must act over a distance (Work = Force * Distance, or more precisely, Work = Integral of Force over distance).
If an object is actually falling in a gravitational field, gravity is doing work on that object, transferring energy to it. But the atmosphere is not falling — it has already fallen. No distance, no energy transfer. (Any downdrafts must be exactly matched by updrafts, so these fully cancel out.)
Any reasonably bright high school physics student can recognize your argument for the nonsense it is!
Take your 5kg barbell and hang it from the ceiling on a hook. No movement, no work. The hook and the ceiling have no power supply.
If you held up the weight with a motorized winch, the motor would expend some energy holding up the weight (as the body does in your example). But engage the brake on the winch and turn off the motor, no energy is expended to hold up the weight.
You would fail high school physics, and not get started in any higher level physics.
To those who think that all the work of gravity has already been done and that this somehow falsifies the premise that there should be higher temperatures at the surface than at the top of the troposphere because of gravity:
When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located? Is it located at the top of the atmosphere? Or is it located at the bottom of the atmosphere?
If you want to distribute that energy back to the top of the atmosphere, what force will you have to work against to get it there? Would it be gravity by any chance? If you do work what do you expend? Oh yes! Kinetic energy? Which is converted to potential energy once more!
So the natural equilibrium of a large planetary atmosphere, is colder near the top, hotter at the bottom. See Neptune, Uranus, Saturn, Titan, Jupiter, Earth and Venus for and use the simple formula T=Pn/Rp to understand that the temperatures of all of these planets and moon can be precisely calculated using nothing else.
You ask: “When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?”
I see that you have never done this type of analysis. The question is not even properly posed. When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.
All of the planets you cite have a radiatively absorptive atmosphere between a relatively warm surface and the incredible cold (3K) of deep space. Like a steel bar with one end in boiling water and the other end in ice water, there will be a temperature gradient between the hot and cold ends. If the gradient of the atmosphere exceeds the adiabatic lapse rate, as it is on all of these bodies, the lapse rate is unstable, and convection will start to bring it back toward adiabatic.
Yes, I took physics in HS and in college and in graduate school. How about you? Did you forget that kinetic energy measured in Joules = Pressure*Volume, and that gas temperature is proportional to the internal kinetic energy of a gas? Perhaps these Wiki articles might help your memory:
Oh, and make sure to retake the class about adiabatic processes to understand how pressure relates to temperature in standard thermodynamics. You can also read carefully our paper, where these things are explained on pp. 6 – 15, while noting that our accurate model (Eq. 10a) is based on actual VETTED OBSERVED data, hence, it’s real!
But the atmosphere is not falling — it has already fallen.
I’m throwing food again.
How so? Is the atmosphere just sitting there static? I thought that the atmosphere was ALWAYS falling, … AND rising, … AND falling, … all the time. Doesn’t this mean that the atmosphere is WORKING all the time?, … under the influence of gravity? — gaining potential energy on the rise?, “unleashing” that potential energy on the way down? … in a continuous cycle?
There is complete confusion here by multiple commenters between the concepts of force, energy, and power.
The hook holding up the 5kg weight exerts a (continuous) force on it of F = m*g = (5 kg * 9.8 m/s2) = 49 Newtons.
To hold it in place, the hook does work on the weight of W = F * d = 49 N * 0 m = 0 Joules. So the power required is 0 Watts (= 0 Joules / time).
What are your equations for force, energy (work), and power for this case?
Andy cannot get it through his head that a one time increase in strain energy is NOT an ongoing continuous power transfer, no matter how many times this basic and trivial point is explained to him.
Ned: I am absolutely appalled that with all your credentials, you do not understand the most basic points you should have gotten in high school physics.
Pressure/volume work requires a CHANGE in volume to do the work. It’s really p*dV work. This is the same thing fundamentally as force needing to create movement to do work. The gas in a piston chamber must move the piston head to do work on it.
The weight force of the atmosphere on the earth’s surface does not move the earth’s surface. (Even if an initial application of this weight force caused some compression, there is no further change.) So the pressure/volume work done is zero.
These are basic, basic points of high school physics, and you get them completely wrong!
Pressure/volume work requires a CHANGE in volume to do the work. It’s really p*dV work. This is the same thing fundamentally as force needing to create movement to do work. The gas in a piston chamber must move the piston head to do work on it.
Height of the atm changes daily. Goes Up and Down like a thermometer.
Ned: I am absolutely appalled that with all your credentials, you do not understand the most basic points you should have gotten in high school physics.
Ed, it’s worse than that. He’s had the basic points explained to him here on WUWT, over and over, and he STILL doesn’t get it.
But heck, if you want a good laugh, you can read about Ned Nikolov’s work here …
You say: “Height of the atm changes daily. Goes Up and Down like a thermometer.”
The claim of “Atmospheric Pressure Effect” (APE) enthusiasts like Ned Nikolov is that the static weight force (pressure) of the atmosphere does continual work on the planet’s surface. To do this, it must move the surface downward.
If the height of the atmosphere varies up and down a little bit, that does not mean anything to the surface.
If you don’t count the downwelling longwave infrared flux (back radiation), the surface energy balance is out of whack by about 250 W/m2, averaged over the surface. The APE enthusiasts claim that the weight force of the atmosphere can make up this imbalance. That’s 250 Joules of work every second for every square meter of the planet’s surface. With an atmospheric pressure of about 100,000 N/m2, the earth surface would need to be continually compressing at 250/100,000 m/s, or 2.5 millimeters every second. This means that the earth’s radius would need to shrink by over 75 kilometers each year! Seriously???
Facepalm. It’s really hard to deal with ingrained blind ingnorance.
Willis, how about you accept that Ned has a much better understanding of physics than you and that it would be a good idea to take that as a starting point to try and understand where your misconceptions lie, rather than try to keep proving that cold heats hot and work done equals energy no longer in existence? Truly the level of your ignorant stubbornness could teach my 5 year old grandson a thing or two!
To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?
Why is it that Ned and Karl can accurately model the temperatures of every planetary atmosphere in the solar system with the same mathematical formula, but greenhouse effect proponents can’t even get their mathematics for earth to agree with itself, much less even one other planet?
Facepalm. It’s really hard to deal with ingrained blind ingnorance.
Willis, how about you accept that Ned has a much better understanding of physics than you and that it would be a good idea to take that as a starting point to try and understand where your misconceptions lie, rather than try to keep proving that cold heats hot and work done equals energy no longer in existence? Truly the level of your ignorant stubbornness could teach my 5 year old grandson a thing or two!
If you wish to follow the “science” of a man using an ad-hoc equation with more tunable parameters than data points to explain, you are welcome to.
To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?
He is right. The kinetic energy is converted to thermal energy. You sure you understand how this “science” thing works?
Why is it that Ned and Karl can accurately model the temperatures of every planetary atmosphere in the solar system with the same mathematical formula, but greenhouse effect proponents can’t even get their mathematics for earth to agree with itself, much less even one other planet?
BECAUSE NED HAS MORE TUNABLE PARAMETERS IN HIS EQUATION THAN HE HAS DATA POINTS TO EXPLAIN, PLUS FREE CHOICE OF EQUATION WITH NO NEED THAT IT BE PHYSICALLY BASED!
Sheesh! Given more parameters than data points to explain, it would be surprising if Ned could NOT explain the datapoints … but then I guess you don’t know how that works. Let me offer you the following, come back once you’ve read it. It explains very clearly why Ned is just fooling the rubes …
You say: ‘To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?’
BTW, it’s “Mr. Numpty” to you 😉
I already answered your question above, when I said: “When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.”
Since I was obviously too fast for you, I’ll break it down for you ask I would a high school physics problem to a struggling student.
Let’s take Newton’s apple of 0.5 kg, hanging 3 m above the ground in earth’s gravitational field of 9.8 m/s^2. It has a gravitational potential energy relative to the ground of PE = mgh = 0.5 kg * 9.8 m/s^2 * 3 m = 14.7 Joules.
Now the stem breaks and the apple falls toward the ground. Just before it hits the ground, the gravitational potential energy of 14.7 J has all been converted to kinetic energy of KE = (1/2) * m * v^2.
We can calculate v = sqrt (2 * 14.7 / 0.5) = 7.67 m/s.
Next it hits the ground in an inelastic collision and stops moving. Such an inelastic collision converts all of the kinetic energy of 14.7 J to internal (thermal) energy. For simplicity, we’ll say that all of this added thermal energy is in the apple as it splats on the ground.
The apple is mostly water, so has a thermal capacitance of about 4 kJ/kg/K. This leads to a temperature increase in the apple of:
Do you think the falling air of the Chinook wind leaves a vacuum at the higher elevations?
No. Do you?
The most significant point about an unstable air mass is that if a parcel of air at a given level within the air mass is forced upwards it will continue to rise; but equally important is this: – if an identical parcel of air from the same starting level in the same unstable air mass is forced downwards, it will continue to fall. We can all see the effects of the rising air in an unstable air mass when it cools sufficiently for the water vapour to condense and form cumulus clouds. What we fail to see, and this is what makes them so dangerous for aircraft, is the descending unstable air, the cold invisible down draft located alongside the rising cell. It is a mistake to assume that this cold downdraft is the same air that rose inside the convection cell. It is not. The rising air inside the storm becomes separated from the water vapour that formed the storm cloud as the rain falls out of the cell and the lifted separated and now dry air remains aloft in the anvil cloud at the top of the storm.
It is this process of drying by physical separation of moisture that makes convection that produces rain an irreversible process. Descending moist air in a cloud can evaporate the surrounding water droplets from the cloud, dissipating it and so slowing the rate of adiabatic warming as the moist cloud containing air parcel descends. Dry air cannot be cooled by evaporation of surrounding cloud moisture because there is none available. Consequently in the descending limb of the Hadley cell dry air is warmed as it is forced down by the Coriolis Effect and ends up at the surface, just like the Chinook wind, at a higher temperature than its initial starting point. And the reason for this change in temperature is because the latent heat of condensation of water vapour that powered the initial rise to the top of the troposphere in the convection storms of the equatorial zone made the rising air cool more slowly.
Nikolov claimed that the weight force of the atmosphere provides an ongoing energy transfer to the earth’s surface that keeps the surface at a substantially higher temperature than it would be otherwise.
I showed him the very basic high school physics point that static pressure provides no energy transfer, and that any deviations from the static case due to updrafts and downdrafts must cancel out.
Note carefully that I am talking about the mechanical force due to the weight of the atmosphere. You are talking about something completely different.
Yes, the convection circulation of air is an irreversible cycle, both due to the evaporation at the surface and the condensation at altitude, and the reduced IR opacity of the atmosphere at altitude. But this does not affect the weight force of the atmosphere on the surface.
Besides, what you describe has a net cooling effect on the surface (even if there is localized warming at downdrafts). Nikolov was arguing for an atmospheric warming effect.
Why do we need to continuously explain basic physics…err an elementary concept?
Solar energy lifts air. Literal energy from an outside source does work on the atmosphere, it has warmed and lifted a parcel of air into the atmosphere. At this point, the d-nyers of basic physics suggest that the energy simply goes away. That’s not what happens. The air has potential energy at this point, it falls and energy is converted to kinetic energy in the lower atmosphere.
Let’s work backwards now, we have kinetic energy added to the lower atmosphere from compression, the work was done by gravity. How did that air get there, it was lifted from being heated. How was it heated, from an outside energy source or an internal energy source? Uhh, an external source, still with me? So is the net result zero energy input into the system, no, energy is retained within the system that was obtained from an EXTERNAL ENERGY SOURCE.
If we must explain that a parcel of air in the upper troposphere is actually moving a distance, h, when it falls then I don’t know how much further back in explanation we need to go. Was that parcel of air magically moved into the upper atmosphere? Was that air simply pulled into the upper troposphere because falling air left a void for it to replace? Errr, no. The entire process is started from energy being input from the sun.
Does this really need further explanation to people. Well, I guess it might to people saying
Ed Bo,
I am all for a dynamic atmosphere that moves both mass and energy, causes local surface heating (Chinook winds) and elsewhere local surface cooling (virtually every rainstorm I have ever experienced).
Sadly, the very meaning of the term “adiabatic” is forgotten here in arguing that gravitationally induced compression of a parcel of air cannot increase its temperature in accordance with the Ideal Gas Law. The compression factor PV/nRT need not be unity, as for an ideal gas, for the temperature to rise adiabatically.
This conversation is absurd. You need to define the system boundaries. This old engineer still remembers his statics. If a mass is not moving, then no work is being done. For an object to be static, all the forces must add to zero and all the moments must add to zero.
It’s a different problem if you add in how the forces are created. Holding a weight motionless does no work. However, there is energy being expended in the muscles of your arm to create that force.
One person is ignoring how the forces are created and the others are trying to include the force creating process. Neither side is discussing the same problem.
I agree – the notion that a gas such as CO2 or water vapor can “trap” radiant heat in a free atmosphere is simply unphysical.
This from Ned Nikolov, the man so ashamed of his own work that he published it under a false name … well, actually, he would have published it, but I pointed out his craven deception to the journal, and they pulled his article.
His “scientific theory” involves an equation with more tunable parameters than there are data points … and while that kind of mathturbation causes anyone who thinks about things to laugh, I fear he has found a following among the credulous.
Ah well … just saying, folks, he’s a man who is willing to lie about his own name …
Still works better than your greenhouse effect mathematics. It also is based on basic principles that cold doesn’t raise the temperature of hot without work being performed. Concepts you still struggle to comprehend.
I guess you must have missed the rest of this post because you never addressed it in your “take down” of the original Nikolav paper. And you pretend you have actually refuted anything
John Day
@ Willis
> There is, of course, a technical term for what they have done,
> as there are no new mistakes under the sun. It is called “overfitting”.
I think you’re looking at this in the wrong way. You say ‘overfitting’, which suggests they are somehow dishonestly trying to ‘cook’ a formula to fit 8 examples.
I don’t think N&K (Ned&Karl) are dishonest. In fact, I think they are merely learning the relationships between pressure induced and radiative warming by trying to fit the set of parameters to a regression equation.
“Learning is compression” in the sense that they want to find the smallest set of parameters which fit the data. I.e Occam’s Razor: if two regressions, one with 5 parameters and another with 5000 parameters, both fit the data, which is better? Ans: keep it as simple as possible (but not too simple).
You’re also missing the main point:
“Pressure by itself is not a source of energy! Instead, it enhances (amplifies) the energy supplied by an external source such as the Sun through density-dependent rates of molecular collision. This relative enhancement only manifests as an actual energy in the presence of external heating. “
Note that the temperature T of a system in equilibrium can be computed from the just kinetic energy of the moving gas particles and their mutual collisions (density, implying pressure). We don’t need to know the radiative aspects of the system to compute the temperature! What part of the Ideal Gas Law do you not understand here?
So, having computed the temperature T we can then ask the question: where did the kinetic energy come from? Probably from solar heat energy absorbed by the surface.
But the point is we don’t need to know where the energy came from. Temperature T is soley dependent upon the internal kinetic energy of the gas and its density.
No change in pressure required. Yes, a pressure “gradient” necessarily exists on all planets with atmospheres, but that is accidental in the sense that even the gradient itself is not required to understand that at any point x,y,z the temperature is solely a function of kinetic energy and transfer of momentum by collisions.
N&K further make the claims that show no pressure change is needed
“In the case of an isobaric process, where pressure is constant and independent of temperature such as the one operating at the Earth surface, it is the physical force of atmospheric pressure that can only fully explain the observed near-surface thermal enhancement (NTE). “
Isobaric?
Yes, if you choose a long-enough time scale:
“the near-surface atmospheric dynamics can safely be assumed to be governed (over non-geological time scales) by nearly isobaric processes on average, i.e. operating under constant pressure. This isobaric nature of tropospheric thermodynamics implies that the average atmospheric volume varies in a fixed proportion to changes in the mean surface air temperature following the Charles/Gay-Lussac Law, i.e. Ts/V = const. “
Willis, please think about it some more before summarily rejecting it as nonsense.
AGAIN, we must ask, what is it you are missing about the physical explanation for which mathematical models exist to explain and match the temperature of rocky planets with atmospheres?
I guess you must have missed the rest of this post because you never addressed it in your “take down” of the original Nikolav paper. And you pretend you have actually refuted anything
I guess you must have missed the fact that Nikolov is using more tunable parameters than the data points that he is fitting. Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
When you do that you are GUARANTEED a good fit … but of course, that fit is meaningless. It would be hard to not get a good fit with that procedure.
Or perhaps you don’t realize what it means when you have more tunable parameters than data points to fit. Once again let me recommend Freeman Dyson’s clear takedown of the bogus Nikolov style of analysis …
Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
But there is a physically meaningful mechanism for how this works. It has been described numerous times on here and no one has refuted it with anything but sophistry.
The descending cool dry air within the Hadley cells retains energy within the climate system. This is a basic fact that keeps being rejected for no reason aside from conventional wisdom. Where is this retention through gravitational potential energy in the oft cited energy balance cartoons that you see above and below? Those flat earth cartoons are so laughably flawed that it’s amazing anyone is still using them.
Others have cited this process in gas giants. It is the same concept, but in the case of gas giants the energy source to start the convection is internal and therefore there is net energy loss, but not nearly as much energy loss if there wasn’t a gravity driven compression by the returning downdraft. On Earth, the energy source to kick off the convection is external, so therefore any returned energy is a net addition into the system, it limits the energy lost via the thermals and release of latent heat. This is true regardless of the composition of the atmosphere, it just matters that there is an atmosphere at all for convection to take place.
And I already discussed the empirical observations demonstrating this effect. The latitude belts between 23-30 degrees emit more LWIR back into space than any other region, even losing far more heat to space than received from the sun in places like the Atacama. It even holds true for the atmosphere over the oceans at these latitudes despite 30 mm of precipitable water per cubic meter.
Notice the subtropical south Atlantic. Greenhouse gases are surely present there, spreading that notorious back radiation around, yet this is among the highest outgoing radiative regions of the atmosphere. What is the source of that heat? I would say it from the additional heat added to that part of the atmosphere from gravity driven compressional heating at the descending leg of the Hadley Cells. Yes, that heat came from a different part of the atmosphere, but that heat in turn came from the sun. That means the process is retaining heat in the climate system, ironically much like how a greenhouse actually works, but instead of glass roof it is the force of gravity.
Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
But there is a physically meaningful mechanism for how this works. It has been described numerous times on here and no one has refuted it with anything but sophistry.
Thanks, RW. First, whatever the mechanism that you are describing might be, it’s not described by his equation. Look at the equation! It has little to do with anything.
Second, if your proposed mechanism were true, it would allow a planet with an IR-transparent atmosphere (e.g. argon) to be warmer than the S-B temperature … but as I showed in A Matter Of Some Gravity, that violates conservation of energy.
Third, regardless of all of that, he has more tunable parameters than he has data points to fit. This is a common enough mistake that it has its own name, “over-fitting”, and his is the most egregious example of overfitting that I’ve ever seen.
So let us assume that we have the airless perfectly evenly heated blackbody planet that I spoke of above, evenly surrounded by a sphere of mini-suns. The temperature of this theoretical planet is, of course, the theoretical S-B temperature.
Now suppose we add an atmosphere to the planet, a transparent GHG-free atmosphere. If the theories of N&K and Jelbring are correct, the temperature of the planet will rise.
But when the temperature of a perfect blackbody planet rises … the surface radiation of that planet must rise as well.
I don’t think that’s what’s implied by the theory at all. You can’t take a temperature of something (atmosphere at the surface) that is not there, but the surface of the planet itself would not heat up, no.
If you added an atmosphere of pure argon, there would be absolutely no condensed or solid particulate matter in the sky to absorb incoming radiation, and let’s assume this atmosphere refracts no incoming light. The surface of that planet will receive the full brunt of the solar radiation.
But, molecules in the atmosphere at the surface will heat up and convection starts. This then cools the planet’s lower atmosphere as heated air carried to less dense areas of the atmosphere, the heated air decompresses, hot molecules bump into cool molecules, and heat is radiated into space.
This process cools the surface and is the complete climastrology certified version of planetary energy budget models regarding pure thermal convection. But what happens as that air falls down to the surface again due to gravity? Unequivocally, that air is compressed and heats up, that is if you believe in the work of William Henry. Latitudinal dependent heating takes place in the area of the Hadley Cells where the gravity driven compression warms the air, exactly what we see on Earth. Far less of the heat is lost to space than is modeled, it’s ironically similar to the argument that the back radiation heats the surface by slowing the radiation that leaves the planet.
So, if you believe that the inherent gravity driven “heat retention” of the atmosphere actually exists at all, and I hope for William Henry that you do, then how do you think that compares to the importance of the previously discussed 0.001-1.7 eV molecular vibrations of the inaptly named GHG molecules and there emittance of this energy within the atmosphere?
And let’s imagine a planet with the same gravity as Earth, circling the same sun in the same orbit, but with an atmosphere of pure argon. Would that planet be hotter or cooler than Earth? With an atmosphere of the same molar mass, I bet that planet’s total average atmosphere would be cooler than Earth’s with no latent heat, but probably a much hotter surface due to higher SWR. Convection would probably actually be more active due to the extremely hot surface and argon doesn’t freeze until until -189 C so the structure of the lower atmosphere would be quite different. So theoretically if that atmosphere was able to stay gravitationally attached to the planet, where the pressure in this imaginary atmosphere is 1 atm, it would probably be about the same temperature as Earth at the same latitude.
I think this qualifies as a great example of how hard it is to defeat erroneous conventional wisdom.
Researchers have literally had to infect themselves with diseases to defeat it. Petroleum geologists were busy controlling oil blowouts in Oklahoma while others were still claiming commercial oil would never be found west of the Mississippi. But eventually, the science progresses.
Ned Nikolov
November 19, 2017 1:50 pm
A key point in this discussion is the fact that an enhanced IR absorption by some gases does NOT imply an ability of such gases to trap heat in an open convective environment. IR absorption and IR trapping are two distinctly different processes with different control mechanisms that have erroneously been conflated since the time of Fourier and Tyndall in the 1800s …
Ned Nikolov
November 19, 2017 at 1:50 pm: IIRC, Fourier and Tyndall have been misquoted by all warmista. They do this everywhere, thinking we won’t check. Sadly, they have been correct in that, too much.
One can clearly see that Arrhenius simply took Fourier’s conjecture/assumption about “heat trapping” by an open atmosphere as a “physical truth” without any empirical verification. He further developed the idea by proposing a “model” (his Equations 3 and 4) that relates Earth’s surface temperature (T) to atmospheric CO2 (K expressed in relative units) via equating T^4 to a ratio of dimensionless quantities! By failing to match measurement units between the left- and right-hand side of his equation, Arrhenius violated a basic principle of dimensional analysis in physics, which of course renders his model nonsensical from a mathematical and physical standpoint of view .. And this nonsense has been touted by the mainstream climate science for decades as one of the greatest scientific achievements of the 19th century!! Simply amazing …
Experiments by Arrhenius’ colleague, physicist Knut Ångström, the results of which were published in his 1900 paper “Über die Bedeutung des Wasserdampfes und der Kohlensäure bei der Absorption der Erdatmosphäre,” (Annalen der Physik 308(12): 720-732.), showed that that CO2 is transparent to 90% of infrared radiation applicable to temperature variation; and that those infrared bands which CO2 readily obstructs are already almost totally blocked by atmospheric H2O. His finding, that the relationship between the concentration of CO2 in the atmosphere and its effect on back radiation is logarithmic, has been replicated by many subsequent experimenters; all of whom show that doubling of the present carbon dioxide content in the atmosphere would only increase the back radiation by about 3.6 W/m ², which would, in the absence of other factors, give rise to an increase in temperature of between 0.6 and 0.8 C°.
Some think that doubling might yield a temperature increase of up to 1.2 C°. The only way that Warmunistas can get a scary range of 1.5 to 4.5 C° is by assuming unphysical positive water vapor feedbacks not in evidence.
A key point in this discussion is the fact that an enhanced IR absorption by some gases does NOT imply an ability of such gases to trap heat in an open convective environment. IR absorption and IR trapping are two distinctly different processes with different control mechanisms that have erroneously been conflated since the time of Fourier and Tyndall in the 1800s …
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field. Despite that, you have not defined the term, so we have no idea what it is that you mean.
How about you give us a clear definition of what you mean by “IR trapping”, so we can follow your ideas? Because for all we know it’s just a strawman that you’ve erected to knock down …
Willis said, on November 21, 2017 at 9:42 pm, in reply to Ned Nikolov :
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field.
Odd, because, from what I have observed, this term IS a common term in the field:
From a NASA website: https://climate.nasa.gov/causes/ Most climate scientists agree the main cause of the current global warming trend is human expansion of the “greenhouse effect” — warming that results when the atmosphere traps heat radiating from Earth toward space.
Someone needs to tell NASA that a common conception of the “greenhouse effect” is not to “trap heat”. Oh, and why they are at it, someone also needs to remind NASA that heat does NOT radiate, and that by continuing to speak of it as such, they continue to confuse the two concepts of “heat” and “radiation”.
From a Harvard University website: http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html Some of this terrestrial radiation is trapped by greenhouse gases and radiated back to the Earth, resulting in the warming of the surface known as the greenhouse effect.
Well now, Harvard some folks seem to think that greenhouse gases TRAP radiation. They relish the word, “trap”, and they use it in a way to confuse people into associating “heat” DIRECTLY with radiation, as to suggest that the two terms might be interchangeable (wrong).
A Columbia University website: http://www.columbia.edu/~vjd1/greenhouse.htm While the dominant gases of the atmosphere (nitrogen and oxygen) are transparent to infrared, the so-called greenhouse gasses, primarily water vapor (H2O), CO2, and methane (CH4), absorb some of the infrared radiation. They collect this heat energy and hold it in the atmosphere, delaying its passage back out of the atmosphere.
Let’s look at definition #18 for the verb, “trap” at Dictionary.com: 18. to stop and hold by a trap, as air in a pipe. So, it sure looks to me like Columbia University is talking about TRAP — they just avoid the term and describe what the term means in other words. Sorry, but “holding” and “delaying” is TRAPPING.
Consequently, I’m afraid that straw men are overseeing NASA, Harvard, and Columbia University, at the least. I wonder whether they are also spineless, … as in too timid to alter the momentum of confusion that they have helped nurture.
Willis said, on November 21, 2017 at 9:42 pm, in reply to Ned Nikolov :
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field.
Odd, because, from what I have observed, this term IS a common term in the field:
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
And THAT’s the problem — this simplistically WRONG explanation, using a word that WRONGLY compares a familiar plant-house structure with a gaseous atmosphere, perpetuates the very use of a term that IS ill-defined. If this term is so rampantly used with the “general public”, then how is the “general public” ever to come to terms with any reality?
The person you criticized initially for using this term was merely using it as a reference point, as many organizations state it. Nikolov was, in effect, kowtowing to this use, letting it slide in its amorphousness — as it is spread amorphously without any clear definition already by all who use it, as if this is okay. You made his using the term an issue, which it should not have been, in my opinion, which seems like a distraction, rather than a call for a definition that was significant to Nikolov’s statements. It is this nebulosity of definition that tends to give alternate views some credibility, I would say.
Oh, and not only does the term appear in press releases, but also it appears in the context of professional scientific organization’s, as a reflection of professional identities, like the following:
The warming effect of CO2 and other heat-trapping gases is well established and can be demonstrated with simple science experiments and satellite observations.
Earth’s atmosphere does the same thing as the greenhouse. Gases in the atmosphere such as carbon dioxide do what the roof of a greenhouse does. During the day, the Sun shines through the atmosphere. Earth’s surface warms up in the sunlight. At night, Earth’s surface cools, releasing the heat back into the air. But some of the heat is trapped by the greenhouse gases in the atmosphere. That’s what keeps our Earth a warm and cozy 59 degrees Fahrenheit, on average.
NASA’s spineless straw men apparently want to start indoctrinating America’s (and the world’s) youth as soon as possible with the TRAPPING idea, AND setting up future confusions in adulthood with the “earth’s-atmosphere-is-like-a-greenhouse” idea. Tragic!
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
Why would you think that I think the term has any scientific meaning. The term is crap. I think Nikolov knows that it’s crap too, but he used it as a device to hold people’s attention a bit, without saying that it’s crap, while he put forth more info from his point of view. The straw man that I am seeing is raising an issue about the term at all in Nikolov’s context, since it was used merely to set the stage of discourse, rather than as a label for stating a belief via the term’s clear definition.
The “clear” definition of “trapping heat” that you might seek is purposefully veiled in the totality of faulty discourse defending the “greenhouse effect”. All the math, all the elaborate descriptions, all the minutia devoted to the “greenhouse effect” is shadowed by this convenient umbrella idea of “trapping heat” or “trapping radiation”.
Supposed educators use the term haphazardly, aimed at people from childhood to adulthood, conditioning their minds to have a sense of heat or radiation as some “stuff” that gets caught in a trap, and then, after all the years of childhood and early adulthood, they then get blasted for using a term as adults that they were NEVER taught any better prior to adulthood. Very convenient, I’d say — nurturing child-like minds, so that sophisticated minds can dissect them as faulty-thinking minds, while sophisticated WRONG arguments are peddled, based primarily on appeal to adult-mind authority.
Carbon dioxide lasers have been used in laboratories for a long time. They rely on the property of CO2 to absorb IR, hold on to it for a time, and then release it in the form of a coherent beam of IR collimated by the laser apparatus. You can feel the warmth of a beam of IR produced by a small CO2 laser. Carbon dioxide in the atmosphere does the same thing, but an atmospheric CO2 molecule releases the IR in a random direction, half of the time upward toward space and the other half down to the earth. So a portion of the IR headed toward space is re-directed back to earth. This does not warm the earth, but it keeps it from cooling as fast, all other things being equal. The earth’s surface will therefore equilibrate at a somewhat higher temperature. Hence the greenhouse effect.
Gill’s original post talked about CO2 emitted IR “bouncing around for a while”. Yes, that’s what causes atmospheric temperatures to equilibrate at a higher level than they would without CO2.
You can also feel the warmth of a beam of IR produced by a large CO2 laser but I don’t recommend it. Actually these are fantastic inventions, now due to careful engineering, design and lots of associated hardware gizmos it is possible to get efficiencies of around 10% from a commercial CO2 laser. Yes , pump 10kW into it (effectively thermal energy) and you can get a cutting beam of 1kW out. Of course there is 9kW of heat you need to throw away so much of the apparatus is hot hot hot and there is coolant, pumps, radiators, etc to get rid of it.
I think we done well ! Compared to the equivalent gaia design where you shove 10kW off the surface of the earth, through all that wispy gas (including 0.04% CO2) and get a downward beam of [REDACTED #1] kW making an efficiency of [REDACTED #2] %.
It’s like that jar at the fair with loads and loads of sweets in it and you are supposed to guess the number and write it down with your name.
The persons who said 5kW and 50% should now identify themselves..
This does not warm the earth, but it keeps it from cooling as fast, all other things being equal. The earth’s surface will therefore equilibrate at a somewhat higher temperature. Hence the greenhouse effect.
CO2 lasers do not work with the ‘cold’ radiation of 15 um but a much warmer one that was stated in the above post: “Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.” https://en.wikipedia.org/wiki/Carbon_dioxide_laser The CO2 laser produces a beam of infrared light with the principal wavelength bands centering on 9.4 and 10.6 micrometers (μm).
Val Ryland
November 19, 2017 2:38 pm
As the author expects, there are some errors in this post that are responsible for the erroneous conclusion. The most important are these:
1. Wien’s law refers to the _peak_ wavelength of emitted radiation, not the only one. The distribution of the spectral radiance is given by Planck’s law. If the spectral radiance peaks at 10 micrometers, you can be certain sure there’s a lot of radiation around 15 micrometers as well.
2. For an object close to room temperature, no matter if solid, liquid, or gas, the atomic/molecular energies are largely unimportant (it happen to be important for CO2, which is the whole shebang). This is because at any ordinary temperature the occupancy of any state other than the ground state is pretty much zero, because typical atomic energies are separated by several eV. In order to get a fraction of 1/e molecules at an energy 1 eV above the ground state, the temperature would need to be >10,000 K. The important degrees of freedom are translational, and, depending on the substance, rotational and vibrational. The latter two are typically quantized, but the former is continuous. In other words, even if you don’t have some atomic energy accessible, you can ALWAYS knock particles around a little bit. Thus, a hydrogen gas at room temperature can be treated for all practical purposes as a gas of featureless, structureless point particles.
3. There is no physical law that states that molecules in liquids or solids “need more energy” in order to be excited, and I have no idea where this assumption could’ve come from.
All in all, the basic greenhouse mechanism remains unchallenged.
Thanks for posting this Anthony, it includes most of the misconceptions and errors about radiative transfer and CO2, so gives a good opportunity to rebut them in one place. References to the science can be found in any college textbook on Physical Chemistry and Molecular Spectroscopy. https://books.google.com/books/about/Physical_Chemistry_5th_Edition.html?id=nIggbG9i8qEC
My understanding of Thermodynamics and Radiation from CO2 is as follows:
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law).
It’s a reciprocal relationship not linear.
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
Wrong, those wavelengths exist in solar radiation and in surface emissions, so they are absorbed by CO2, just not in large amounts because those wavelengths are in the tails of the respective spectra.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is wrong and meaningless. Wien’s Law tells you the location of the peak wavelength, as the graphs added by Anthony shows wavelengths above and below that exist in the spectrum. More relevant to the IR emissions is the following:
As you can see the CO2 absorption band at 15micron is present at all the temperatures shown (dashed lines). Even though the maximum shifts with temperature so does the radiance increase as temperature increases. Consequently we see the following:
A blackbody at 300K emits 26.8522 W/m2/sr between 13 and 17 microns
at 270K it emits 18.4933 W/m2/sr
at 223K (-50ºC) it emits 8.53892 W/m2/sr
at 193K (-80ºC) it emits 4.32413 W/m2/sr
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
This is more like atomic structure and doesn’t apply to molecules (it’s more complex, bonding and non-bonding orbitals etc.)
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
No, that refers to part of the internal energy, the temperature is the kinetic energy of the molecule.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
No, it must receive a photon of exactly the right energy to excite the transition. However electronic transfers in molecules are excited by UV light. The transitions which we are talking about in the IR are excitations of the vibrational and rotational modes. In the case of CO2 absorption at 15micron it is the vibrational mode that is excited, specifically the bending of the CO2 molecule (with associated rotations).
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Again the photons must have exactly the right energy to reach the excited vibrational state. The molecule can emit the radiation but it’s far from immediate it will undergo millions of collisions with surrounding molecules during that time and in the lower atmosphere is more likely to transfer the energy to the atmosphere (thermalizes)
Photons with not enough energy to raise the orbit of any of the electrons excite the vibration are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Won’t be scattered or reflected.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
In the band from 13-17micron, as you can see from the diagram above it’s significant part of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
No, it depends on the molecule, and again it’s not electron excitation it’s bond vibration.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
As explained above this is not true for several reasons.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
No, the earth’s surface is very close to a black body and will readily absorb a photon emitted by CO2, or it could be absorbed by another CO2 molecule, or thermalize.
So am I right?
Mostly not I’m afraid, but thanks for the opportunity to correct these misconceptions which as you suggest are frequently encountered on the web.
And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
As pointed out the concept of CO2 molecules at -80ºC is a major misunderstanding of Wien’s law (it also makes more sense to use the form of that law which relates peak frequency to temperature since that is proportional to energy).
Go to MODTRAN and test this for yourself. You will see that CO2 doesn’t have any impact what so ever until you get up to about 3 to 5 km when H2O starts dropping out of the atmosphere. If you then take a look at the temperature of the atmosphere at those heights where CO2’s signature is present, you will see that it is about -80°C. What you are seeing is the thermalization of CO2/15µ, and it results in a temperature floor, not an increase in the ceiling. CO2 only warms the upper atmosphere, not the lower troposphere.
What a fascinating collection of comments. I never would have thought there would/could be so many different ways to visualize radiation, temperature, and heat flow.
Well worth reading in their entirety several times I think.
More simply, radiation from a cooler object cannot naturally raise the temperature of a warmer object. This is so basic and so obvious that WUWT should hang its head in shame over the amount of articles it has posted with convoluted explanations to the contrary.
The whole premise of the Greenhouse Effect has nothing to do with how much Radiation CO2 can absorb, but how much the radiation, emitted by the CO2 in the atmosphere, can warm the surface of the earth. The answer, of course, is zero! Radiation from a cooler source cannot increase the temperature of a warmer source.
End of story. You don’t need to invoke quantum physics to understand it. You just need to stop holding sophists with PhDs in awe and use some common sense and basic observations.
radio waves can be absorbed by a receiving antena that is warmer than the emitter and be warmed up such that it glows red hot. try putting an electrical conductor in a microwave oven
You are right – a cold object can not warm a warmer object.
However, the temperature of the atmosphere is between the maximum surface temperature and the minimum surface temperature. Therefore, the CO2 in the atmosphere can increase the *average* surface temperature by keeping the nigh time minimum warmer than it would be if there was no atmosphere.
Lars P.
November 19, 2017 3:30 pm
“This is a contentious subject, and I have often shied away from it because it often erupts in food fights. ”
Well yes this opens a can of worms.
Here my 2 worms, er, cents 😉
1) Basically what increasing CO2 in the atmosphere does is shortening the path of the 15 um radiation, from let’s say 10 meters to 9.
One could argue that, in the whole air column, the heat transfer through radiation made with 9 meter steps, instead of 10 meter steps, would increase the lapse rate and thus increase the temperature of the surface. However this is dependant on the percentage of energy that flows through the 15um from the total which I guess is in the order of less then 1/1000.
=> effect cannot be measured
2) Personally I think the whole greenhouse effect is being built on a wrong basis as the elephant in the room is excluded: the oceans.
It is not the atmosphere that has the greatest greenhouse effect, but the ocean, water is practically opaque to infrared radiation, not transparent as the atmosphere is. Light penetrates the oceans to depth up to 100 meters and warms it, however the ocean loses heat only at the surface. The puny effect of CO2 ‘backradiation’ (and all other atmosphere including water vapor) is dwarfed by a 0.5 meter water strata at the surface of the oceans.
The idea that the oceans would freeze without the atmosphere’s backradiation – as the warmists maintain is nonsense. The oceans receive a 1300 W/m2 at the Equator. That heat is absorbed, stored and keep the oceans warm in the night too. No danger of frozen waters.
Of course the atmosphere has its contribution too, but the oceans cannot be completely dismissed as it is done.
Geology proves that the ocean currents define the climate, not CO2 variations, but hey, maybe it is still too early to consider this…
Clyde Spencer
November 19, 2017 3:53 pm
For the geologists and mineral collectors here (and those of you old enough to have ever bellied up to the bar in a discotheque), you are probably familiar with the effect of shining a ‘Black Light’ on solid (e.g. scheelite) and liquid substances (e.g. hydrocarbons). Short-wavelength lights will invariably give different ‘glowing’ colors and intensities than long-wavelength lights. In general, the observed fluorescence and phosphorescence is of a shorter wavelength than the stimulating electromagnetic energy. As a rule of thumb, the fluorescence is of a longer wavelength than the stimulating wavelength, and is variable in duration with different materials. The fluorescence is usually confined to one or two narrow wavelengths, which distinguishes it from thermal emissions. That is to say, if a substance (including individual gas molecules) receives EM radiation of greater energy than an electronic orbital transition, some or all of the energy may be absorbed, and then re-emitted when, after a highly variable period of time, the electron(s) falls back to the ground state. So, strictly speaking, narrow wavelength emissions from CO2 should be considered fluorescence, and should be occurring at wavelengths longer than the absorption bands.
[ https://micro.magnet.fsu.edu/primer/techniques/fluorescence/fluorescenceintro.html ]
On the other hand, the molecules of solids, liquids, and gasses possess kinetic energy in the form of the velocity of individual molecules, straining in the chemical molecular bonds, and in the case of solids, vibration in the crystal lattice. All of these sources of kinetic energy have different energies; the integrated energy is related to the average velocity of molecules, and the average vibrational bonding energy. At high temperatures, Doppler shift will cause the apparent emission wavelengths to also be different. At certain energy levels, bonds can be broken, and a change in physical state will take place, Thus, the distribution of emitted thermal energy with respect to wavelength will approximate the classic Black Body emission.
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption. That is to say, the presence or absence of water vapor will strongly effect whether the atmosphere retains the CO2 emissions. The thermal emission should correspond to the average temperature of the gas molecules.
Once again, the situation is more complex than climatologists are either aware of, or want to admit.
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption.
You’re mistaken because you’re extrapolating from electronic transitions with vibrational fine structure to vibrational transitions with rotational fine structure where the ‘red shift’ is far less pronounced and you don’t have to worry about the Frank-Condon effect.
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
You are simply demonstrating your complete scientific illiteracy.
You see an equation, like the one for Wien’s Displacement Law. It expresses the peak radiative flux wavelength of a blackbody as a function of its temperature.
If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.
But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.
This is, simply put, complete nonsense!
Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target. Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
“If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.”
Uhhh, just what do you think I was saying? Just what is a black body to you? Yes, you warm up a body, and that body emits a spectrum. Just what do you think I was talking about?
“But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
Uhhh, no I don’t, and you can verify everything I say and write by simply going to MODTRAN or SpectralCalc. The calculators don’t lie.
“Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target.”
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Ed bo says : Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
If absolutely true why do the rings on my stove never show RED when the stove is off? Or why do we know that Blue stars have a higher temperature than brown dwarfs?
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
AndyHce November 20, 2017 at 3:04 am
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
The individual photons carry no information about the temperature of their source so an absorber can not distinguish the difference between a 15micron photon emitted at -80ºC and one emitted at 100ºC and will absorb them both.
Temperature measurements are made by looking at the distribution of photons (the spectrum) which is a function of temperature.
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. In fact plotting it versus wavelength distorts the distribution, it makes more sense to plot vs wavenumber, which is proportional to energy. If you do that you will see that the radiance peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution, by contrast water absorbs between 1500-2000 cm-1 (radiance ~0.02 W/m2/sr/cm-1).
The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such. http://www.azimuthproject.org/azimuth/files/earth_and_sun_emission.jpg
“The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.”
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm. There are plenty of creative ways for a scientist to isolate this effect. The fact that they don’t even attempt to demonstrate the basics through experimentation proves they aren’t looking for the truth, and why they rely on computer models.
co2islife November 20, 2017 at 9:19 am
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such.
No, I’m disagreeing with the statement you made, as shown in your graph the earth emits between 3 and 60µm with peak radiance at 10µm.
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm.
It certainly will!
All the 15µm light will be absorbed in a 10 cm path length if you put 1%CO2 in N2 in your container.
I said: “So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
You responded: “Uhhh, no I don’t”
But then you followed up with: “Absorbing a small fraction of that [earth’s IR spectrum] can’t warm the atmosphere above the 10µ temperature.”
So you just calculated a temperature AS A FUNCTION of wavelength. You don’t even understand that you don’t understand anything about this subject!
As to the (irrelevant) point you were clumsily trying to make, that the atmosphere’s absorption of some of earth’s IR emissions cannot increase the atmosphere’s temperature past that of the surface, no one is claiming that it does. The atmospheric greenhouse theory REQUIRES the atmosphere to be colder than the surface.
co2islife November 19, 2017 at 3:53 pm
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
co2islife November 19, 2017 at 3:53 pm
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is false.
co2islife November 20, 2017 at 9:11 am
That point isn’t debatable. It comes from the SpectralCalc Black Body Calculator. You are claiming the math is wrong.
No, I’m claiming that the guy looking at the graph doesn’t know what he’s talking about!
Run Spectralcalc Gas cell simulator with 0.0004 VMR of CO2 at 296K and plot absorption between 600cm-1 and 700cm-1. You’ll see that it absorbs just fine.
co2islife,
You said, “The quantum physics are well known and defined. I’ve pointed out almost all the points highlighted in this article. The physics are the physics, you can’t change that.” I wish it were that simple!
The impression I get from reading the comments is that most posting here are reasonably well-educated, but have varying backgrounds or disciplines. Yet, there is disagreement on what the “physics” says, even amongst those who share similar views about the validity of AGW. In part that may be the result of how an individual interprets the ‘facts.’ Often times the ease with which a problem can be solved depends on how it is expressed or framed. That area probably could use some work. Overall, I’d say that one of the problems is really a failure to communicate with one’s peers. Beyond that, there are some here with a vested interest in a particular viewpoint, and they have trouble looking at the problem from a different viewpoint. Few outside the area of geology are familiar with Chamberlain’s Method of Multiple Working Hypotheses.
If those of us, who are skeptical about the claims made by AGW alarmists, could get our act together and speak with a unified voice, I think that we would have a better chance of pointing out the errors of alarmists. But, with infighting, we are little more than an intellectual rabble.
It doesn’t help when the first comment is by a d@nier of EM radiation and photons.
John
November 19, 2017 3:59 pm
Well, I can fully understand why this topic is so debatable. I’m an Engineer myself, and I’m still having trouble with deciphering so many viewpoints. I only started becoming interested in the topic around 6 months ago. Probably the only real thing I’ve discovered is that it is much more complex than I had envisioned. Perhaps only time is the ultimate arbiter.
No it’s not complex, it’s second year thermo and heat transfer.
One popular RGHE theory power flux balance (“Atmospheric Moisture…. Trenberth et al 2011jcli24 Figure 10) has a spontaneous perpetual loop (333 W/m^2) flowing from cold to hot violating three fundamental thermodynamic laws. (1. Spontaneous energy out of nowhere, 2. perpetual loop w/o work, 3. cold to hot w/o work, 4. doesn’t matter because what’s in the system stays in the system)
“So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. ”
Almost everything discussed in this article can be found is article previously written on this topic. This Blog has all the resources to validate the points made in this article. The physics are the physics, they aren’t up for debate and Modtran and any Black Body calculator will demonstrate that. https://co2islife.wordpress.com/
This would have been a great thread if one were allowed to reference those unmentionable scientists that deny that CO2 can warm the surface. Especially the astrophysicist who just published a week ago or so on this topic.
I hope there comes a time when luke-warmers here can debate with the skeptics. Perhaps some day.
TTY, “Both of course.” is a perfect response! Most of the comments are citing warming of a molecule, parts of them, or atmosphere of them, but in reality are MOSTLY talking about reducing heat already gained.
Fact: Energy can neither be created nor destroyed, it can only be changed in form.
1) CO2’s only defined mechanism to affect climate change is through absorbing and thermalizing LWIR between 13 and 18µ
2) Those bands are only a small fraction of the emitted LWIR, earth’s peak is 10µ
3) There is no way through thermalization for CO2 to warm anything above the temperature of the emitting body, the energy simply doesn’t exist. Absorbing a fraction can’t account for more than the whole.
4) Radiation could trigger combustion, but that isn’t the phenomenon that we are talking about
5) Conduction, convection, and radiation all act to move heat away from the earth. Claims that the GHG effect is responsible for all the warmth of the atmosphere ignore conduction and convection.
6) This is easily proven in a lab by shining 15µ IR light into a flask of CO2. It won’t warm because the room temperature is way above -80°C
Technically energy can be “destroyed.” If you observe the spectra from distant stars, you’ll note that the hydrogen emission lines are red shifted. When the photon left the star of origin, it had the normal wavelength (freq) of the emission lines of hydrogen. As it traversed the expanding intergalactic space, it’s wavelength grew longer (red-shift.) There is a calculable loss of energy in this red-shifted photon according to E=hc/l where delta-E = hc/delta-l.
…
Where did the ‘lost’ energy go?
I already did. The photon that was emitted a million light years ago, “lost” some of it’s energy. The frequency has decreased (increased wavelength.) Where is the “lost” energy?
it went into potential energy. for the gravitational red shift it was demonstrated by t Cranshaw at Harwell in the 1960s. a photon fired from the ground level to the top of a hanger was found to be red shifted. a photon fired downwards was blue shifted. it is like a ball being throw upwards it looses speed but that does not mean that energy is destroyed. Similarly with a photon
Gerontius, that does not explain it. There is no gravitational field in the million light years between the star that emitted the photon, and the spectrometer here on earth that measured it. The red shift is due to the expansion of space, not to a gravitational field.
PS Gerontius, photons coming from the opposite side of the universe display the same red shift. You would think that if you looked in the opposite direction of the red shifted photon, you’d observe a blue shifted one if your “theory” were correct.
The energy doesn’t change so much as the wavelength stretches out, changing its color. If the source were heading toward you the waves would bunch up. It’s all due to the Doppler effect.
A passing car is a good example. You hear the sound change as the car passes. Sound at sea level and standard conditions travels at about 761 mph. So if a car is traveling toward you at 30 mph, then the sound is travelling at 761 + 30 mph = 791 mph. After it passes, the sound you hear is traveling at 761 – 30 Mph = 731 mph. So the energy does not particularly change. Turbulence has a small effect, but the pitch goes from higher to lower just as starlight red shifts.
It’s all down to the observer’s or listener’s point of view or frame of reference. The sound wave or photon just keeps doing its own thing, while the looker looks on.
As for the effect of gravity, red shift or blue shift is not due to any change in frequency of the photons, but rather to the time rates being different for observers in different potentials. No energy change occurs. If a photon of a given frequency is created by some atomic transition in the vicinity of a star, then it will appear red-shifted compared with the energy of the same transition at some distance from the star, but it hasn’t changed frequency.
Similar considerations apply for red or blue shift due to relative motion; the energy is unchanged relative to the location at which the photon was emitted, but appears different from a moving frame. The situation isn’t quite so clear-cut about red shift due to cosmological expansion, because it depends on how you describe the expansion, but even in that case the frequency of the photon is unchanged relative to its original rest frame.
“The energy doesn’t change so much as the wavelength stretches out, changing its color. If the source were heading toward you the waves would bunch up. It’s all due to the Doppler effect.”
That is a great point, the total energy is the same, but energy/m is less. You are stretching the energy over a larger distance.
The energy is not conserved no even conceptually both QM and GR demand it to be so. This is one of the problems with classical physics it leads you to stupid answers.
It is not a problem with “classical physics”, but with your lack of understanding thereof and misguided thinking about it.
As CO2 observes above in response to my prior comment, energy is conserved, but just delivered at a different rate per unit of EM radiation travel. That’s one way of explaining the paradox which you imagine.
Clearly you don’t understand QM or “classical physics”, by which you mean “physics”.
How many times do I need to explain it to you?
The apparent change in color, ie wavelength and frequency, or a photon of visible light traveling along at its accustomed C, is due to the observer’s position. In the Doppler effect, sound waves don’t change their energy content.
Again, if photons don’t exist, then neither do any of the other elementary particles.
Oh here he goes again trying to obviscate the problem, we have cool lasers(tm) again. You make a statement then just want to wave hands ignore it and try to deflect to something else.
the gravitational red shift is real and explicable and demonstrated . in that the energy lost is conserved in potential energy
the velocity red shift is real and is due to energy conservation, and not an example of non conservation of energy. You cannot look at the photon in isolation and must look at where it came. There is a fairly simple classical (although with a bit of QM thrown in explanation). It is readily available on the web, although if you look you will always get the Kernoodle reply that is non conservational. You need however to be rigorous in accounting your energy balance . There is no need to invoke expanding universes only relative motion between observer and emitter. You then get the Doppler effect. Now the Doppler effect and the red shift in electromagnetic waves is well known and its energy conservation is not in doubt. And as I said before the energy balance of the effect demonstrates that the red shift is an effect of energy conservation
Robert I leave you to search the web, ps Cranshaw used the velocity red shift to measure the gravitational shifts. Now that’s a big clue as to where the missing energy goes.
None of you here has mentioned it in this blog, so ignorance is bliss.
co2islife November 19, 2017 at 4:12 pm
Fact: Energy can neither be created nor destroyed, it can only be changed in form.
1) CO2’s only defined mechanism to affect climate change is through absorbing and thermalizing LWIR between 13 and 18µ
2) Those bands are only a small fraction of the emitted LWIR, earth’s peak is 10µ
Since you claim to use Spectracalc try the following:
Using the Blackbody calculator set it for a BB at 288K
Set the lower limit of the band to 13 micron and the upper limit to 17 micron.
Perform the calculation.
You should find that the band radiance is 23.3 W/m2/sr whereas the total radiance is 124.2 W/m2/sr.
By my reckoning that means the CO2 absorption band accounts for about 19% of the total, hardly a small fraction.
Remember “Everyone of my comments can be verified by MODTRAN or SPECTRALCALC.”
“By my reckoning that means the CO2 absorption band accounts for about 19% of the total, hardly a small fraction.”
In an atmosphere of 100% CO2, that would prove a whole lot, but add H2O to the mix and you will see CO2 is meaningless in the lower atmosphere. You only see a CO2 signature at 15µ after H2O precipitates out of the atmosphere. BTW, that 19% is 19% of the very low energy end of the LWIR spectrum. Now compare the energy of visible light, of which H2O in the oceans absorb 100% of. That is where the energy is, not LWIR.
co2islife November 20, 2017 at 5:57 am
“By my reckoning that means the CO2 absorption band accounts for about 19% of the total, hardly a small fraction.”
In an atmosphere of 100% CO2, that would prove a whole lot, but add H2O to the mix and you will see CO2 is meaningless in the lower atmosphere. You only see a CO2 signature at 15µ after H2O precipitates out of the atmosphere. BTW, that 19% is 19% of the very low energy end of the LWIR spectrum. Now compare the energy of visible light, of which H2O in the oceans absorb 100% of. That is where the energy is, not LWIR.
Not true, that 19% is of the total of the LWIR you can check it out on Spectralcalc. Also the CO2 absorption band is near the peak of the energy spectrum, it is water that is near the tail.
The Blackbody radiance peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution, by contrast water absorbs between 1500-2000 cm-1 (radiance ~0.02 W/m2/sr/cm-1).
You show the CO2 signature on the Modtran graphs on your own website from 1km, you also show the H2O signature off in the tail. If you do the calculations on Modtran comparing 0ppm CO2 with 400ppm CO2 you’ll find that ~10% of the total is removed by CO2.
“Also the CO2 absorption band is near the peak of the energy spectrum, it is water that is near the tail.”
Yep….
Dr. Deanster
November 19, 2017 4:13 pm
I see a lot of diagrams on radiation ,,,, incoming, outgoing, backwards. But all of this assumes that the majority of energy absorbed by the ocean is in some sort of magical equilibrium with the atmosphere. …. ie, a very large part of the incoming SW IR Is absorbed and NOT immediately released.
Exactly, but the ONLY outside source of energy to the earth, well, maybe some minuscule amount from plate tectonics, …. is SW, and it DOES penetrate the ocean surface. It doesn’t just warm the surface and immediately float back into the atmosphere.
Any equilibrium equation for the total earth can only be made n long term basis, as a good bit of energy is stored and released over time.
A close examination of USCRN soil data shows that solar IR hardly makes it 1 meter deep by conduction and then only during summer daylight which is the ONLY time the air is warmer than the ground.
Derek Colman
November 19, 2017 4:27 pm
My idea of an experiment is simple, maybe too simple. Take two identical sealed glass containers and fill each one with a mixture of gases which imitates the atmosphere. The only difference between them is that one has 0.028% of CO2 and the other has 0.04% of CO2. Both vessels should be fitted with highly accurate temperature probes. At the start ensure that both are at exactly the same temperature. Now radiate each with an IR lamp, tested to be identical in output. Now observe the temperature increase in the vessels. The data from this experiment can then be used to calculate and compare what happens to atmospheric temperature at pre-industrial and current CO2 levels. My guess is that the difference will be so small that even the accurate temperature probes can not distinguish it.
Sailboarder the earth is a closed system with the sun. Energy can enter but no mass. Also the video was done to correct numerous errors done for Al Gore by Bill Nye. Good video.
E=hv tells us how much energy is available to cause a CO2 molecule to increase its translation. And translation velocity is dependent on absolute temperature and molar mass. So heavier gas travels slower.
We know that Q = Cp m dT for how much energy is needed to raise a mass of something, air in this case.
I have yet to be able to show that hv * 400ppm CO2 = Cp m dT for a cubic meter air.
But a postulate of kinetic theory of gas says that all collisions are pure elastic and no energy is lost or gained, so if true CO2 cannot do this anyway.
That is all very interesting but it is a EM wave aka a radio wave. Just like your TV set the EM wave will only selectively react to certain frequencies. Do you really think the EM wave delivering your TV signal got absorbed and re-emited from every air molecule along the way or has anything to do with kinetic energy of molecules along the way?
I have no idea what GH theory works on I am not a climate scientist. All I am telling you is radiative transfer doesn’t occur in that manner described and energy can be lost and gained and attenuation can be measured. I am sure if you search Atmospheric Attenuation you can find the numbers for ll frequencies in the EM spectrum (from RF thru visible light up to X and Gamma rays).
LdB we have been shown graphs on this thread of CO2 absorbing at 15u. OK, then using E=hv we can calculate the amount of energy available to any CO2 molecule to increase its translation. Translation is directly related to temperature.
Due to the relationship of velocity to temperature we should be able to calculate the ability to raise a cubic meter of air via the energy in a 15u IR absorption.
Having taught basic radar theory and basic physics of underwater sound in the navy I have a idea of EM wave and attenuation etc. But that was many years ago. Thanks for your input.
Does anyone have a link to a research paper where 15µ IR was shined on a flask of CO2 and its temperature measured? Unless the CO2 spectrum is a lie, then the only contribution CO2 has to global warming is through the thermalization of IR at 15µ. That won’t warm anything. Simply focus on the basics. By what mechanism does CO2 affect climate change? Isolate that factor and test it. Has anyone tested the impact of CO2 thermalizing 15µ LWIR?
I too am working with this problem, I actually thought I had been ‘pipped to the post’, but after reading this, and the comments, nope – not even close. And Anthony, this may be ‘contentious’ but it is the key premise to greenhouse theory; without this, greenhouse theory collapses, so it is worth trashing out.
The way I see it the problem lies in the detectors of the greenhouse gases (see my youtube presentation below): they all use thermoelectric – Seebeck Effect transducers. What John Tyndall discovered In 1859 are really only the thermoelectric gases, N2 and O2 are not; nor is CO2s 1338cm mode. All thermograms and IR spectrograms are created using these detectors, but they discriminate the non thermoelectric modes and substances.
Greenhouse theory is 19th century science, pre Quantum mechanics . Quantum mechanics predicts and explains the vibrational modes, emission spectra, of all the molecules in the atmosphere including oxygen and nitrogen; It also says all matter radiates infrared. All matter! That oxygen and nitrogen are assumed not to radiate IR is a contradiction to QM. Either Quantum mechanics, or greenhouse theory is wrong: Quantum mechanics is not wrong. To observe the predicted Modes of oxygen and nitrogen – at 1556cm and 2338cm respectively – we must use 20th century technology, Raman spectroscopy (the complement instrument to thermoelectric IR spectroscopy) .
In the paper that I’m about to publish I will show the Raman laser technology is equivalent to Thermoelectric IR technology. Raman can do it all: it measures temperature, and the concentration of the gases. It is even used to measure the Keeling curve, and is the instrument of choice on solar system space probes for this reason. It can also show nitrogen and oxygen emit and absorb IR radiation, in total compliance with thermodynamic laws Stefan Boltzmann equation and Boltzmann constant. I can even show, by experiment and application, nitrogen radiates upon CO2 to heat the CO2 – figure that.
The following YouTube presentation is my rough beginnings – I have since developed my theory.
With oxygen and nitrogen being greenhouse gases, greenhouse theory collapses. https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s Reinterpreting John Tyndall’s 1859 Greenhouse Gas Experiment with Thermoelectric and Raman Theory
aGrimm
November 19, 2017 7:15 pm
Pretty much any wavelength of radiation can create the photons that we sense as heat. For example, the 662 kEv photons from Cs-137 when stopped by a lead shield will cause the lead to get hot if there are enough photons. Saw this with a cask of 55k Curies of Cs-137. The exterior of the cask was hot to the touch. Here is how I describe the conversion of the 662 kEv photons to heat EM. A loss of energy of the initial photon occurs as it impinges or interacts with an atom’s nucleus or electrons in a mass. There are number of processes by which the initial photon losses energy, but with each interaction of the photon with the orbiting electrons or the nucleus, some energy is “lost”. Of course the energy is not “lost” it is divided into lower or a different energy potential/form. One such loss might be when an orbital electron gains energy from the initial photon and ‘moves’ to a higher energy “excited” state. Generally, the electron does not want to stay in this state and will emit the excess energy as a low energy photon – sometimes immediately, sometimes after a passage of time. The process of the initial photon losing its energy can involve thousands of ever decreasing interactions. Another interaction might be where a photon strikes an electron and the electron is ejected from the atom. The initial photon loses energy equivalent to the binding energy of the electron. If the ejected photon received energy in excess of its binding energy, then that photon has energy to give to something else.
Because the eye is a measuring device for visible photons it is useful for describing the process of a photon being eventually degraded to heat. The eye has evolved to manage a reasonable level of visible photons without damage despite the fact that some of the dissipated energy becomes heat photons (whose energy is also dissipated). However, staring directly at the sun introduces too many photons and the heat dissipation mechanism cannot handle it and the retina will get burned. A burn is basically the rearrangement of a molecule, e.g. a protein molecule, due to the input of too much energy, whereby the new arrangement does not work biologically. Physics and biochemistry are fun fields to study in combination.
I have a few nit-picky points in Gill’s assessment, but overall I agree with his assessment.
Claude Harvey
November 19, 2017 7:42 pm
“This is a contentious subject, and I have often shied away from it because it often erupts in food fights.”
I think the stewed tomatoes now dripping from the ceiling have confirmed your reservations, Mr. Watts. I do hope Mr. Gill was properly attired for the occasion.
And for those who claim that gravity or atmospheric mass or some other phenomenon is responsible for the fact that the Earth is much warmer than we would expect given its distance from the sun, let me recommend my post entitled “A Matter Of Some Gravity“.
Or not, you could choose to ignore everything but the things that support your view …
Willis, I still see “The Steel Greenhouse” as one of the best articles I’ve ever read on the so-called “greenhouse effect.” In discussions on this site I’ve referred others to it several times. Thanks again.
The problem Willis is the way we still teach physics. Almost everyone who doesn’t get it has the image of a photon as something like a tennis ball flying thru the atmosphere crashing into every molecule in it’s path. They don’t understand or won’t accept it’s a radio signal that collapses to something we equate to a tennis ball.
“I think you might class the distance to that big shiny thingy in the sky …”
Exactly. FWIW, Venus receives about twice as much solar radiation per square meter as does the Earth. However the Venerian albedo is higher. More incoming radiation is reflected back to space. Also Venus rotates very slowly compared to Earth and Mars which probably alters the heat distribution considerably.
I considered adding solar radiation at ToA for each planet, but the differences are small compared to the enormous temperature differences. Naturally you’d expect Venus to be balmier and Mars chillier than Earth, all other things being equal, but of course other things aren’t. Not even close. The other things outweigh insolation.
Don,
Venus’ rotation is clearly another factor. But, even considering all other things, the fact that Venus and Mars both have high-CO2 atmospheres highlights the importance of density and surface pressure. The temperature at the point in the Venusian atmosphere with terrestrial surface pressure is about the same as on Earth.
Right on. The steel greenhouse ignores the pressure temperature effect.
The “pressure temperature effect”??? As I have shown, and as Dr. Brown has shown, that is a fantasy. READ THOSE LINKS, and then come back and tell us why Dr. Brown and I are wrong.
David, while a Chinook wind raises the temperature at the surface because the air is descending … what goes down must come up. So somewhere else the same amount of air is going up and cooling, so that there is no net gain or loss.
And from the tone of your comment, it is clear that you have NOT done as requested, which was:
As I have shown, and as Dr. Brown has shown, that is a fantasy. READ THOSE LINKS, and then come back and tell us why Dr. Brown and I are wrong.
If you want to argue with my ideas, at least do me the courtesy of reading them before starting in … both Dr. Brown and I have proven, not asserted but proven, that there is no “pressure temperature effect” capable of warming an entire planet on a continuous basis.
“Venus possesses a dense, hot atmosphere, composed primarily of carbon dioxide. A surface pressure of nearly 100 bars sustains the mean surface temperature of 740 K, which is essentially globally uniform except for topographic effects.”
Actually, Venus’ atmosphere is a little warmer than average Earth temperature at one bar, thanks to a bit more incident solar radiation. But close enough to make the point. For government work.
Put another way, IMO Boyle’s Law ought roughly to apply, since, while not a closed system, the atmosphere approximates one since incoming and outgoing radiation are close to in balance, while subject to minor perturbances.
Thus, greater pressure should yield higher temperature. The atmosphere expands and contracts under heating or cooling, thus lowering or increasing pressure and temperature at the margins to maintain a fairly constant temperature, until other factors such as Milankovitch cycles and albedo waxing and waning change the baseline. This process can be extrapolated out to even denser or yet lower pressures.
What would be the temperature of Venus at the distance of Mars from the Sun?
I’m sorry, but there are far too many unknowns in the thought experiment to even begin to say. FOR EXAMPLE, at the surface of Venus CO2 is a superfluid … would it still be a superfluid at the distance of Mars? And how would that change things?
Please explain why the temperature in Venus’ atmosphere at one bar is the same as on Earth. Thanks.
According to your graphic, the temperature of Venus’s atmosphere at one bar is on the order of 350K … which is 77°C or 170°F.
Look, my rule of thumb is that when someone starts talking about Venus to try to show something regarding the greenhouse effect, I stop listening. Venus is a hugely complex system about which we know little. Consider the trouble we have explaining the Earth, about which we know orders of magnitude more than we know about Venus …
Much as I respect Dr. Brown, “proof” is a strong word, appropriate for math, not technically applicable to science.
Say what? “Proof” is indeed a strong word, and I do not use it lightly. However, there are assuredly parts of science where things can indeed be proven, and this is one of them.
However, if you think that Dr. Browns proof is incorrect, how about you point out WHERE it is incorrect, and leave the grand overarching view of what “proof” is and isn’t for another time.
Note that I apply the same word to my proof regarding the same question, which takes a totally different path to the same conclusion. And again, I invite you to find something wrong with my proof.
Happens around 0.1 bar. on every planet with enough atmosphere.
angech
November 19, 2017 9:23 pm
“Once you have GHGs in the atmosphere, of course, some of the surface radiation can get absorbed in the atmosphere. In that case, the surface radiation is no longer constrained, and the surface is free to take up a higher temperature while the system as a whole emits the same amount of radiation to space that it absorbs.”
–
Thank you Willis.
I have never really understood you but this comment is great.
Since the same energy is going out that comes in there is never a real accumulation of energy in the system , just a change in the temperatures of the mediums of that system, if true.
More energy in the atmosphere less in the land and sea perhaps?
Without the CO2 the land under the sun would be a lot hotter during the middle of the day and would send out lots of IR at a higher temp than the atmosphere does.
Leo G
November 19, 2017 9:27 pm
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. ”
The kinetic energy of a gas is the sum of the energy of the translational motion of the centre of mass of each molecule. Electron energies are not involved.
Belief in AGW caused by CO2 = denying or ignoring the science of thermalization, Maxwell-Boltzmann distribution of molecule energy & quantum mechanics. The IR energy absorbed by CO2 is immediately (0.0002 microseconds) shared with surrounding molecules (thermalization) so, at low altitude, there is little chance for a CO2 molecule to emit a photon as a direct result of having absorbed one (relaxation time about 6 microseconds). Water vapor has many (>190) significant absorb/emit lines at substantially lower energy levels than the 15 micron absorb/emit band for CO2 and on average there are about 35 times as many WV molecules as CO2 molecules. At low altitude, energy absorbed by CO2 is effectively rerouted up via water vapor radiation and some convection. End result is CO2 has no significant effect on climate. http://globalclimatedrivers2.blogspot.com
Belief in AGW caused by CO2 = denying or ignoring the science of thermalization, Maxwell-Boltzmann distribution of molecule energy & quantum mechanics. The IR energy absorbed by CO2 is immediately (0.0002 microseconds) shared with surrounding molecules (thermalization) so, at low altitude, there is little chance for a CO2 molecule to emit a photon as a direct result of having absorbed one (relaxation time about 6 microseconds). Water vapor has many (>190) significant absorb/emit lines at substantially lower energy levels than the 15 micron absorb/emit band for CO2 and on average there are about 35 times as many WV molecules as CO2 molecules. At low altitude, energy absorbed by CO2 is effectively rerouted up via water vapor radiation and some convection. End result is CO2 has no significant effect on climate.
“Now my house is not heated with the insulator, but it surely is warmer because of them.”
Only because the heat source (furnace) puts out more heat than can escape because of the insulation. The “warmth” of your home is dependent upon the heat source and the air temp outside (heat loss). It’s possible for it to get so cold outside that the insulation cannot keep the heat in with the furnace going full blast. Hence the insulation’s role is to slow the rate of heat loss. It doesnt make your home warmer, it makes it less cold.
It doesn’t make it warmer, it makes it less cold??? That’s just a pointless semantic exercise trying to deny the fact that the earth is warmer with the atmosphere than it would be without the atmosphere.
J. Richard, if you asked someone who just put on their jacket “Are you warmer now?”, do you think they’d say “Yes”, or that they’d say “No, I’m not warmer now, but I’m less cold”?
If you think the latter, here’s a quick guide to things that exist on a spectrum:
J. Richard, if you asked someone who just put on their jacket “Are you warmer now?”, do you think they’d say “Yes”, or that they’d say “No, I’m not warmer now, but I’m less cold”?
Coming from a cold and damp (Scotland) part of the world a suitable response to that question would be “I’m not as cold as I was” 🙂
“It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat”
No,it’s not. The diagram is a budget. It records the fluxes that can be measured, and tests whether they are in balance. The flux that is affected by GHG concentration is the full 324 W/m2 downflux from air. The 390 W/m2 upflux at the surface responds to the temperature there. The difference, if you exclude AW, is small at 26 W/m2, but is pretty much locked. The 324 W/m2 is emitted from air near the surface, as you can tell from its magnitude. If the surface warms, that air warms too, and the flux difference remains near constant
Not true, according to CERES. As one example, for the Northern Hemisphere, as the average temperature swings from about 9°C to 22°C (a 4% change min to max), the net LW flux at the surface varies between about 52 and 60 W/m2 … since that is about a 15% change min to max, I’d hardly call it “near constant” W.R.T. temperature …
w.
(As a side note, CERES puts the upwelling LW at 398 W/m2, and the downwelling at 345 W/m2).
Willis,
The Trenberth budget deals with annual averages. That gives plenty of time for the air at DWLWIR emitting altitude to equilibrate with surface, if the (annual average) surface warms.
That makes no sense. You’ve said that “if the surface warms, that air warms too, and the flux difference remains constant”. You CANNOT determine that from a budget, whether Trenberth’s or someone else’s. A budget has no information at all about a “warming surface” …
Willis,
No, you can’t determine it from a budget. But you can reason about time scales. You are referring (I think) to a phase lag between surface and downward emitting layer temperature with the seasonal cycle. Averaging over a year is firstly a somewhat longer timescale, but also averages out the effect of such a seasonal lag. Basically the difference between upflux and down is determined by the temperature difference of emitting layer and ground temperatures, which is determined by lapse rate and GHG concentration. The latter fixes the altitude of the emitting layer.
Willis,
You might like to try this test with CERES (I am not so adept with it). Just look at different areas around the world. There will be big changes in upflux and down. But is it not always the case that upflux always slightly exceeds down, as they vary over such a range?
Willis,
You might like to try this test with CERES (I am not so adept with it). Just look at different areas around the world. There will be big changes in upflux and down. But is it not always the case that upflux always slightly exceeds down, as they vary over such a range?
Nick, interesting question. Here’s surface upwelling longwave minus surface downwelling longwave, in a couple of formats.:
As you point out, net flow is always from the surface upwards.
Willis,
Just adding some interpretation – I think the up-down difference is basically product of altitude of downward emitting layer and lapse rate. The dry lapse rate is higher than moist. And your map looks pretty much like a humidity map, except at poles, where flux in both directions is smaller.
Willis,
Further thought – altitude of downward emitting layer is dependent on GHG concentration – it is lower with more GHGs. So higher if water vapor is low. That adds to the difference being greater in dry places – it could be the main cause.
Martin A
November 20, 2017 12:20 am
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
I agree with your findings, but not for the same reasons. Retired now, I used to be a laser engineer, and instrument design engineer, including weather stations, and all manner of energy measuring devices. I created infrared measurement systems and laser energy measurement systems. Thus, I know a little about gas behavior and thermal characteristics.
So, my argument against CO2 ‘warming’ anything goes like this? If it DID increase the surface temperature, the surface would radiate to space at the 4th power of the increase, instantly cooling itself back down. We know that everything radiates and we have laws for quantifying it.
Experiment: Try to heat a stove with a flashlight. There are lots of photons, you can see them. Yet the stove does not warm.
Say what? If you shine a flashlight at a stove, it will end up warmer than without the flashlight. Not much warmer, but assuredly warmer. Look, John, a good chunk of the light from the flashlight will be ABSORBED by the stove, and the rest will be reflected.
Now, since energy cannot be either created or destroyed, what happens to the energy in the photons that are absorbed? Obviously, that energy must be changed into something … let’s see. It’s not converted to electricity. It’s not converted to chemical action. It’s not converted to mechanical energy … gosh, could it be converted to thermal energy?
And if (as you claim) the absorbed light is NOT converted to thermal energy … then just what are you claiming it is converted to?
You continue:
Why? Because for each photon that strikes the stove, the stove emits millions, maybe billions of photons itself. Those few photons from the flashlight are simply overwhelmed, and the result is immeasurable.
First, photons don’t get “overwhelmed” by photons going the other direction. If you are indeed an engineer you know that to be true. You can’t “overwhelm” a flashlight by shining fifty flashlights at it, that’s nuts.
Second, the resulting change in temperature in the stove is small. However, it is not “unmeasurable”, which you also know is true if you are an engineer.
Finally, your explanation that if CO2 “DID increase the surface temperature, the surface would radiate to space at the 4th power of the increase, instantly cooling itself back down” makes no sense at all. According to that theory, nothing can increase the surface temperature, because if it did the surface would “instantly cool” …
Willis, you’re close to right there, when you try to haul the lower atmosphere up by its own bootstraps, a humourous definition of futility. Any warming would indeed cause expansion by molecules moving at c. 1km/sec in the gas phase. The gas laws do rule, so the IR catastrophe will not happen. Ditto the never-observed ghe.
Cold objects must warm hotter objects, or even more strongly stated, heat the warmer object
Is this true?
In this case the fire and the light bulb
However the general truth comes out that is if the two objects are thermally isolated from their surroundings heat only flows from the higher temperature because that is the definition of HEAT
For example
Two metal blocks A and B sit separated inside a vacuum filled adiabatic enclosure.
Adiabatic enclosure consists of a perfect reflector face surrounded by a perfect insulator
Initially both at the same temperature. The zeroth law of thermodynamics applies.
Both emit and absorb equal amounts of radiation.
Neither one is said to heat the other.
Both objects remain at the same temperature
One block (A) has a power supply which is now switched on causing the temperature of the block to rise.
This in turn means that it will emit more radiation.
A will now heat B causing its temperature to rise.
B will in turn emit extra radiation which is absorbed by A but this ‘back’ radiation is caused by A.
Now comes the clincher
If B were not there at all the temperature A would be even higher.
So B cannot be said in any meaning of the word as a cause of heating A
in the case of the furnace(Tf) and light bulb(Tb)
If Tf >Tb heat will flow from furnace to bulb
If Tf <Tb heat will flow from bulb to furnace
You whole answer is so crazy you do realize you aren’t dealing directly with heat but with EM wave. We call it a thermal emission not because it is “hot” or “heat” but because the source of emission is from a thermal source. Don’t think of the emission as heat it’s just EM energy.
Lets give you this with lasers which are the most precise EM frequencies I can generate.
So I fire a CO2 infrared laser and a red laser at each other.
We know the beams will pass thru each other without interacting and in fact on a laser cutting machine we often use this so you can see where the cutting in what we call that a red dot pointer. The IR frequency is invisible to us but you inject the red laser thru one of the mirrors so it follows the path of the IR.
So got it I have an IR laser one way and a red laser beam the other way.
What happens when each beam hits the other laser device (and they will hit)?
No Willis I am absolutely correct
Before the power switch on A was turned on block A and block B were at the same temperature.
The adiabatic walls confines all energy within that enclosure
Lets say each has a mass of one kilogram
With both A and B the power source has to heat 2Kilograms
With B removed all the power source has to heat one kilogram which implies the temperature of A will rise twice as fast as before
Any physicist will confirm that I am correct
Bryan, your case is so specific that it has almost nothing to do with climate.
“Adiabatic enclosure consists of a perfect reflector face surrounded by a perfect insulator”
so you have created a situation where every last bit of radiation leaving the blocks gets reflected back perfectly. This is the exact opposite of the earth’s situation, where every last bit of radiation that leaves from the top of the atmosphere disappears and never comes back. If no energy can leave, then the two blocks will reach some equilibrium where both have the same temperature.
“If B were not there at all the temperature A would be even higher.”
While strictly true, this is not really very interesting. Basically all you have claimed is that an isolated, insulated system with mass “m” warms faster than an isolate, insulated system with mass “2m”.
I would challenge you to re-analyze with a system where the enclosure has perfectly ABSORBING walls and is held at, say, 3 K. You will find that in this more useful system, Block A does indeed warm faster than without block B, and Block B ends up at some temperature between that of Block A and the cold walls.
Hi tjfolkerts
My post was to correct the impression that the presence of any object irrespective of its temperature must ‘warm’ some neighbouring object.
This piece of fiction has become so prevalent in the last 20 years that normally rational folk like Willes have been blindsighted by it.
My example was to remind people that the surroundings need to be specified before any progress is made in analysing the flow of heat.
My scenario would be found in opening chapters of a thermodynamics book where definitions of adiabatic and diathermic walls, heat transfer and so on are introduced.
I am sure that ‘warming’ by cold objects meme has been carefully fostered by global warming advocates.
Look at some earlier comments upthread .
Block of ice in sitting room with person
Torch heating a furnace
Surely the Earths atmosphere in which radiative resistance rather than reverse heating gives a rational explanation of the situation and should be encouraged.
Its up to people who should know better to get back within the framework of physics
I agree with your point that it is valuable to examine all details of a system, and to be precise about language (the word “heat” being perhaps the biggest language offender). Your scenario certainly could be worked out with some basic undergraduate thermodynamics.
My main issue with your scenario is that it is SO far removed from scenarios related to the thermodynamics global warming that it could easily give a false assurance to people who simply want a post to confirm their biases. For planets, the default condition is blackbody surroundings at 3K. This is almost exactly the opposite of what you hypothesized.
I think it would be more productive to directly address issues like “warming with ice”. The radiation from cold things still feels warmer (and will help keep you warm) when compared to radiation from even colder things. If I am facing a wall of dry ice @ -79C and then someone interposes a wall of ice, then yes, I will feel warmer. A pot of water with an immersion heater will rise to a higher temperature when the radiation from the warmer ice replaces the radiation from the colder dry ice.
tjfolkerts
The block of ice in the sitting room example.
Everyday common sense tells people its not a good idea for keeping the house warm.
The walls floor and ceiling and air at around 20C (surroundings) will lose heat in melting the ice by conduction convection and radiation.
The fact that the ice block radiates to the wall is true but will not change the outcome .
If the surroundings were dry ice as you say it would be the ice block that would lose internal energy by heat transfer to the dry ice and so it would get colder.
Once the surroundings are specified it is much easier to keep track of the heat flow.
In reading posts on Global warming quite often only two items are mentioned
The hotter and colder object ( with no mention of surroundings).
Then comes an astonishing statement like….
Do you know that the cold object ‘warms’ the hotter object
It always seems that the main offenders are global warming advocates.
Also not all photons are created equal.
The ‘quality’ of the radiation was mentioned in the theme title but not discussed much any further.
Bryan says” In reading posts on Global warming quite often only two items are mentioned
The hotter and colder object ( with no mention of surroundings).”
Any decent discussion will include FOUR items
1) the warm object that is absorbing energy (eg the ground)
2) the source of energy (eg the sun)
3) a low temperature heat sink (eg 3 K outer space).
4) the object that is not absorbing energy from Item 2 but can absorb radiation from Item 1 (eg the atmosphere
Perhaps you were reading poor post where the authors did not really understand the GHE. Perhaps the authors figured the sun and outer space were simply givens that did not need specific mention. In any case, when all four items are included, it is very simple to see that adding Item 4 results in Item 1 getting warmer.
One place where intuition often goes astray is the “low temperature heat sink”. Normally our surroundings (around 20 C typically) are not all that cold. Then adding a wall of ice that is held at a steady 0 C (even colder than the surroundings) certainly will not warm you. But that would be like adding an atmosphere that is held steady at a temperature BELOW 3 K!
tjfolkerts: you state ” In any case, when all four items are included, it is very simple to see that adding Item 4 results in Item 1 getting warmer.” There is no atmosphere on the moon. Sunny side of it gets much higher temperature than the surface of the earth. How come?
esalil, this is a discussion of broad averages. The average surface temperature of the earth is MUCH higher than the average surface temperature of the moon. This despite the fact that the Moon absorbs a higher fraction of the incoming light.
Furthermore, the moon’s average temperature would rise if it had an atmosphere of GHGs (although the max temperature on the sunny side would probably drop a bit.
WUWT is an equal opportunity science “d@nial” site, except for “Sl@yers”. Those who d@ny gravity in favor of an imaginary “electric universe” are welcome here, along with those who d@ny EM radiation. Even creationists are permitted to comment, but not those skeptical of the GHE.
People skeptical of GHE are “not permitted to comment”? Say what???
READ THIS VERY THREAD. There are lots of people skeptical of the idea of GHE, and they are free to post, as are you. Your claim is a joke.
For me it looks like hopeless to explain the basic physics about the GHE for those who think that it is nonsense. I try another approach. If you think that the GHE does not exist, how do you explain the downward LW radiation at the surface which is about 345 W/2. Compare this to the direct solar radiation of 167 W/2 at the surface. (71 W/2 of solar radiation is absorbed by the atmosphere and therefore the outgoing LW radiation is totally 238 W/2). These figures are not nonsense, because they are both measurement based figures.We know that 99.97 % of the energy keeping the Earth warm originates from the Sun. Where this 345 W/2 comes from? How do you explain this, if there is no GHE?
It comes from the atmosphere (including clouds). The atmosphere radiates the energy absorbed from the sun and the surface. The energy from the surface is mostly nonradiative (evaporation and convection). This does not mean that the inceased atmospheric CO2 will result in a warmer surface.
There is a ghe, simply because there is an (insulating) atmosphere between the surface and the cold space. The atmosphere is in direct contact with the surface and is easily warmed by it, mostly by nonradiative processes. The bulk of the atmosphere (N2 and O2) plays a big role.
edimbukvarevic. Otherwise very good but you say that “The atmosphere is in direct contact with the surface and is easily warmed by it, mostly by nonradiative processes.” This is not correct: The upward LW radiation emitted by the surface is 396 W/2 (Excatly according to Max Planck’s formula). Latent heat flux 90 W/2, and thermal flux 25 W/2, together 115 W/2. According to basic mathematics 396 is more than three times greater than 115. Right?
Aveollila, the radiative heat flux surface-to-atmosphere is ~26 W/m2 (350 – 324). According to basic mathematics, that is less than 25% of the non-radiative fluxes (115 or 78 + 24 = 102 W/m2). It is only one fifth of the net surface-to-atmosphere heat flux (~128 W/m2).
edimbukvarevic You do not calculate the upward energy fluxes in the right way, because you subtract a donward flux from the upward fluxes (why you selected 324 and not the 168 ??). According to the energy balance of Kiehl an Trenberth (which is obsolete and based on the wrong atmosphere) the upward fluxes are 350 + 78 +24 = 492 and the downward fluxes are 168 + 324 = 492. That is called an energy balance. Your way of comparing fluxes is not based on the real world. Using your way, I can prove anything as I like.
Aveollila, I commmented on the atmosphere and its energy. In particular, the heat exchange between the atmosphere and the surface. The total heat transfer from the surface to the atmosphere is about 128 W/m2. Only a small part of this flux is radiative, about 20% (350 – 324 = 26 W/m2). This is not debatable, unless the Earth’s budgets are grossly inaccurate.
Furthermore, if we add solar radiation directly absorbed by the atmosphere (~67 W/m2), we get 195 W/m2. That means only ~14% of the total heat transfer to atmosphere is LWIR radiation.
Martin Mason
November 20, 2017 1:56 am
As always very confusing with so many different opinions, immovable positions and even contempt for anybody who doesn’t hold the same opinion. I’m an engineer but I’m constantly searching for an answer to how a cold body heats a warm body and how heat is apparently created from nothing.
Please allow me to ask a dumb question and hope that I can get an answer to at least help clear up some of my confusion. I can calculate solid surface temperature of the earth from incoming UV radiation (from a warmer body) and physical properties (absorptivity and emissivity of the surface). I can calculate a near surface air temperature based on conduction and convection. I can then calculate a lapse rate which gives me a vertical temperature profile which is pretty well matched by reality. I can do this with no reference to downwelling radiation. Why should the conclusion from this not be that this is because downwelling radiation doesn’t affect temperature and lapse rate?
Martin. Solar radiation is much more than UV radiation which has a wavelength shorter than the visible light.
I do not believe that you can show calculations that the solar radiation of 238 W/2 absorbed by the Earth produces the Earth’s surface temperature of 15 degrees Celcius. Why not? Because that absorbed radiation flux of 238 W/2 corresponds the surface temperature of -19 Celsius only as measured at TOA emitted by the Earth.
Nobody has shown that Max Planck’s equation is wrong. It shows excatly how the surface temperature produces an exact amount of radiation. The fact is that the surface has an average temperature of 15 C and it produces the radiation flux of 396 W/2. How it is possible that the Earth receives only 238 W/m2 but its surface can emit 396 W/? Perpetum mobile? Confirmed by measurements. Fighting against GHE like fighting against wind mills. You cannot win.
ave, the calculation for spot temperature is basic radiant heat transfer and is based on actual radiation not average hitting a flat plate and it doesn’t have an additive downwelling radiation component for it to work. The problem is that if you believe that downwelling IR is the only way that the surface can achieve it’s effective emitting radiation then you have to have 324 W/m2 of downwelling radiation that is higher than the sun gives us and a transfer of heat from cold regions to warmer ones.
If downwelling radiation is a key component in atmospheric temperature why is the lapse rate completely free of any influence from it. Why is Jericho warmer than Jerusalem or the temperature on a mountain lower than at the bottom.
Is it not so that any number of experiments of putting bodies of different temperature in proximity, the temperature of the highest temperature body is never increased by radiation from the lower temperature bodies (true?) no matter how many there are.
I believe that heat energy (as opposed to radiation) can never flow from a cold body to a warmer one at any level including quantum and once this is accepted, heating by downwelling radiation can’t be significant. No heat is added and none is trapped, no laws are broken
Maybe those who say that it is an atmospheric effect rather than a greenhouse effect could be correct. These wonderful gases though for sure moderate the temperature of the atmosphere but add heat to it?
To sailborder. Create a pressure of 100 atmosphere into a steel cylinder and leave it outside in the temperature of -10 degrees Celsius. What do think is the temperature after 2 hours. It is that -10 C degrees. A constant pressure cannot create heat into any system. Only if you do like in a diesel engine: apply a certain amount of mechanical work and turn it into heat.
“I believe that heat energy (as opposed to radiation) can never flow from a cold body to a warmer one at any level including quantum and once this is accepted, heating by downwelling radiation can’t be significant. No heat is added and none is trapped, no laws are broken”
What is so difficult to understand about the concept of slowed cooling?
Of course heat energy can impinge on a warm body from a colder one.
Photons from the colder object do not have any concept of the other object being hotter.
(and there will be photons unless it is at 0K)
And as a result the hotter body cools more slowly.
There is however no “Flow”, as in a net impingement of photons from the cold object such that it raises the temp of the warmer one.
It is like a tank loosing water at a rate of 10 gals/hr while at the same time you pour water from a bucket into the top at the rate of 1 gal/hr.
So the tank is actually leaking water at 9 gals/hr. It’s still leaking (cooling) but at a slower rate due to the small amount of water (energy) being added to it from the bucket (older object) leaking (cooling) at 1 gal/hr.
It is like a tank loosing water at a rate of 10 gals/hr while at the same time you pour water from a bucket into the top at the rate of 1 gal/hr.
So the tank is actually leaking water at 9 gals/hr. It’s still leaking (cooling) but at a slower rate due to the small amount of water (energy) being added to it from the bucket (older object) leaking (cooling) at 1 gal/hr.
I think this is probably the best visualization to explain what I’ve been talking about.
So, you have a big tank, with 2 holes in it. and over about 12 hr’s once a day, a big dump of water happens, pressure is a little higher, so it rushes out a bit faster at the peak level. One hole is the optical window, which is constant in size, and another that represents the energy through the other bands, part of this hole has grown slightly smaller from increases in GHG’s.
But together it’s a good size hole, and then the tank goes 12 hours between big dumps of water.
But, there’s a second smaller tank of water that is filled constantly, from far away.
And when the water level drops some, depending on the level of the second tank, some of the water in the second tank starts to flow into the bigger tank. This slows the drop in water level in the big tank, until the next big fill of water, where it shuts off till later.
If the second tank is really full, the big tank gets as much from it, as goes out the bottom once the valve opens, and the level doesn’t drop anymore, even though the main fill line is dry still.
This is why Co2 doesn’t have hardly any affect on the level. It’s the sheer size of the tanks compared to the tiny 3.7W.
“Unless the so called GHE is just the pressure density effect?”
Yes your bike tyre heats up as the air you pump into it increases in pressure.
But does it stay hot for you forever?
Obviously not.
Why?
You did the ‘work’ with the pump and then you stopped – then no work so the tyre cooled.
Now replace the ‘pump’ with gravity and the air by the Earth’s atmosphere.
(Hypothetically) if we could just switch on gravity from zero then yes the atmosphere would heat as it compressed.
But the work has then been done.
So it cools.
Gravity is NOT maintaining a surface temperature.
Full stop.
It does however set the LR by virtue of the -g/cp relation – the surface temp then being set by the GHE via raising the effective temp of Earth (255K) up to ~8km whilst the LR is maintained.
Rod
I believe that the simplest form of the question you ask is this:-
“Can something cold heat up something hot?”
To answer this question we need to establish the nature of collision:-
Can a slow moving object travelling behind ever catch up to and collide with a fast moving object travelling ahead of it and moving in the same direction?
Well of course not, only fast moving objects can ever catch up and collide with slow moving objects ahead. This is because collision is a vector process; fast objects collide into slow objects and not the other way round and so the transfer of momentum always proceeds from fast to slow and never from slow to fast (for objects moving in the same direction).
The issue of heating by “back radiation” is more subtle. The diagram that Anthony supplied demonstrates Wein’s displacement law and it shows the envelope curve for frequencies at different black body temperatures. In addition we have all seen the diagram that shows the effect of selective absorption of frequencies by greenhouse gases. What we have never seen is the diagram which shows an intensity mound in the low frequency tail above the envelope curve which is the absolute requirement for “cold” low frequency radiation to add energy to a high temperature body. This does not happen, it never has and it never will. Back radiation heating of the warm surface below by a cold surface above is a fiction and Wein’s displacement law proves this.
The radiative greenhouse effect is an attempt to explain how the low temperature isothermal stratosphere can heat a planet’s surface. It cannot. The troposphere (the weather layer) is a gigantic heat engine. It moves prodigious amounts of mass and energy all day and every day. Our atmosphere does work and it is powered by the heat of the sun and the speed of the Earth’s daily rotation. Like all heat engines it has an exhaust temperature, that exhaust temperature is observed in the isothermal shell of the stratosphere that surrounds our planet. Just as the exhaust temperature of an engine cannot be used to power that same engine, so it follows that the low temperature stratospheric shell aloft cannot provide back radiation “heat” to power the high temperature weather machine below.
So, Phillip, at night does a cloud overhead affect the temperature at the surface below and why as it is not cutting off any sunlight. Clouds are composed of GH effect water molecules at low temperature or do you have an alternate explanation?
angech,
I live in the British Isles. Almost every day somewhere here experiences the effect of warm moist cloudy air moving over night time cooled ground. My worst ever driving experience occurred a few years ago in Aberdeenshire when I lost control of my vehicle on black ice. One frosty winter’s evening I walked our dog alongside the River Don under crystal clear skies. The next morning, before dawn, the weather had changed to a mild 8C, with damp moist air, a thick haar (sea fog) and very poor visibility. As I drove south to work alongside the river I came to a small hill, the north facing slope of which was covered in black ice as moisture from the sea fog condensed onto the previously cooled road surface, so yes warm moist cloudy air can heat a cold ground. The question at issue however is can something cold heat up something hot?
I think that this is due to a change in wind direction rather than a change in the vertical temperature profile.
Certainly in Scotland we notice abrupt daily and hourly change.
If the wind is from south or west we have warmer weather.
From the north or northeast its much colder.
” so yes warm moist cloudy air can heat a cold ground. The question at issue however is can something cold heat up something hot?”
Yes and no.
I was meteorologist with the UKMO in a previous existence and one of our winter jobs was to monitor road temps over a wide part of the English Midlands for councils re gritting for ice/snow.
It is no mystery that cloud raises RST’s and hence air temps above.
It is added to the algorithms for RST prediction. On a otherwise frosty night it can make for a marked jump in road surface temp. In fact thin Ci cloud at 30,000ft at a temp of -40C will cause a road temp to either to halt or rise slightly when below 0C.
But it is NOT strictly raising it’s temp, as you will discover if you examine, say, a bridge in the same situation (air beneath).
What is happening is that the heat-flux from below the surface overcomes the surface emission to space (because of the cloud) and so the temp there rises.
In other words extra photons have impinged the road and added to it’s energy such that that entering from below is out of balance with the LWIR leaving.
It is though still a slowing of cooling and NOT cold heating up something hot.
Very interesting observation, thanks. I have often noticed that ground frost will form first on the metal skin of a car, before it sets into the pavement. Same principle I believe. I had never experienced black ice until that morning and had no idea how dangerous it can be, even with an overnight air temperature rise to well above freezing it is still possible to get into severe trouble on damp untreated roads.
I totally agree…… though I approach it from a heat capacity angle. The best the GHG can do is reach equilibrium with the ocean or ground …. simply because the GHG mass and heat capacity is minuscule compared to everything else.
Philip Mulholland November 20, 2017 at 6:52 am
It is standard knowledge that cloudy skies will be warmer than cloudless skies due to the blanketing affect of clouds. Surely it could also be that the air arrives already warm and clouds are part of the ‘package’? Here in the UK the majority of weather and associated temperatures come from somewhere else, either pre-heated or pre-cooled. However, we have cloudless days that can be very hot or very cold depending on the time of year. I understand the reason for this is that cloudless days tend to have low wind speeds as this is usually a high pressure weather system. In the summer when the sun is overhead the surface gets the maximum effect of the sun’s heat and the low wind speed does not remove this heat, much like a car can overheat if not moving. Conversely, with high pressure during the winter when the sun is week there is only slow out-flowing air and no in flowing warm air. Therefore, there are other considerations than just a cloudy sky keeps the temperature in.
Out of interest Adolf Galland gave four reasons why the Luftwaffe lost the Battle of Britain; number 1 was the unpredictable nature of Britain’s weather.
“The radiative greenhouse effect is an attempt to explain how the low temperature isothermal stratosphere can heat a planet’s surface.”
It doesn’t “heat it up”, you turkey, it reduces the rate of cooling!
Large amount of radiative energy going OUT. Smaller amount of radiative energy coming BACK. Result – less energy overall leaving.
So, with the Sun heating the ground, and the ground losing heat at a lesser rate than it would in vacuum, the ground gets warmer than it would in vacuum. It reaches equilibrium at a higher temperature.
Voila! Greenhouse Effect! Bleeding simple!
(No doubt someone is going to “debunk” this by nattering on about photons, or Wein, or by saying that the Earth is *never* in equilibrium. (Which it isn’t. And your point is?) Let someone else argue with them. Never debate with a fool, people might not be able to tell the difference.)
So, with the Sun heating the ground, and the ground losing heat at a lesser rate than it would in vacuum, the ground gets warmer than it would in vacuum. It reaches equilibrium at a higher temperature.
Here’s a thing; the maximum surface temperature measured on the Moon in a vacuum is 100C (373K) but on the Earth the maximum recorded surface temperate with an atmosphere of 100 kPa pressure is 58C (331K). The Moon orbits the Sun in tandem with the Earth at the same overall distance (Yes its absolute distance changes from New Moon to Full Moon and then back again) but, averaged over a year, the Moon experiences the same intensity of solar radiation as the Earth does.
So what do I conclude from this? That the sun heats more in a vacuum and that on Earth the atmosphere cools its surface.
Here’s a thing; the maximum surface temperature measured on the Moon in a vacuum is 100C (373K) but on the Earth the maximum recorded surface temperate with an atmosphere of 100 kPa pressure is 58C (331K).
The Moon orbits the Sun in tandem with the Earth at the same overall distance (Yes its absolute distance changes from New Moon to Full Moon and then back again) but, averaged over a year, the Moon experiences the same intensity of solar radiation as the Earth does.
So what do I conclude from this? That the sun heats more in a vacuum and that on Earth the atmosphere cools its surface.
Right facts, but I fear you’ve reached the wrong conclusion. It’s more complex than that. I discuss this question in a post called “The Moon Is A Cold Mistress” …
So, with the Sun heating the ground, and the ground losing heat at a lesser rate than it would in vacuum, the ground gets warmer than it would in vacuum. It reaches equilibrium at a higher temperature.
So, with the Sun heating the ground, and the ground losing heat at a lesser rate than it would in vacuum, the ground gets warmer than it would in vacuum. It reaches equilibrium at a higher temperature
Do you want to correct him or not?
I’m not sure what I have to do with this, as I haven’t commented on what Uncle Gus said. However, it is true that ceteris paribus, the surface with a GHG-containing atmosphere will indeed be warmer than it would be without said atmosphere … so it is not at all clear what you think I should “correct”.
so it is not at all clear what you think I should “correct”.
Try reading this:-
the ground gets warmer than it would in vacuum
Thanks for the reply, Philip. So your contention is that if we removed the earth’s atmosphere the surface would get warmer??? Seriously?
Stefan and Boltzmann disagree strongly with that … in a vacuum, the earth’s maximum possible temperature would be 5°C, assuming the earth were a superconducting sphere. Given that it is not a superconducting sphere, the temperature would be lower. For example, the moon, at the same distance from the sun as the earth, has an average temperature far below 0°C, because it rotates so slowly …
Willis,
One last time, I am talking about peak maximum temperature. In a vacuum, where there is no fluid (gas) to cool the surface the peak maximum temperature is higher under an equivalent radiation load.
Willis,
One last time, I am talking about peak maximum temperature. In a vacuum, where there is no fluid (gas) to cool the surface the peak maximum temperature is higher under an equivalent radiation load.
You may well have been talking about that, Philip.
But Uncle Gus, who we were discussing, definitely was NOT talking about that. You asked me, was there anything I would correct about the statement of UNCLE GUS, not about your statement … and he said nothing about max temperatures, he was talking about mean temperatures.
And what is with the “One last time …”, like you’re at the end of your tether and I’m too dumb to understand but you’ll give me one last chance. I don’t care which end of the tether you’re at, I was replying to your question about Uncle Gus.
w.
PS—the reason for the difference in max temperature of the earth and the moon is not the vacuum. It is a result of the fact that their rotation rates are different. Because the moon’s surface can cool or heat for a longer period, the cold side gets much colder and the warm side gets much warmer than the earth.
We know this because if the moon were spinning at say 1 rpm, where it would be evenly heated, given the moon’s albedo (0.12), the S-B equation says it would be at about -4°C. So clearly, the vacuum isn’t the reason for the high temperatures.
Willis,
Dinna fash. Here (I hope) is something we can agree on, slowly rotating planets heat more than rapidly rotating ones do.
pochas94
November 20, 2017 4:57 am
At thermal equilibrium, any time a molecule absorbs a photon, it will emit one (follows from Kirchoff’s radiation law.) There is an equal probability of emitting upward or downward. The greenhouse effect results because the sun / earth /space system is not in thermal equilibrium and an upward emitted photon may escape to space, while the ones emitted downward remain in the system. Missing from this discussion is convection, which controls surface temperature and indeed the whole temperature profile of the troposphere. Discussion of lapse rate and equivalent emission height is also missing. Also, the great source of stability of our climate, the Stephen-Boltzmann law which says that radiant emission goes as the fourth power of temperature, with the result that any increase in temperature is opposed by a massive increase in energy radiated to space.
Ian Macdonald
November 20, 2017 5:09 am
Infrared photons have far too little energy to change the orbit of an electron. The ‘greenhouse effect’ is entirely due to resonances in the elasticity of the chemical bonds between atoms in the gas molecule.
The 15um resonance is below the emission peak for typical ground temperature. However the photon distribution is wide enough that some will be in this range. Thus CO2 will have some effect on outgoing radiation.
When a CO2 molecule absorbs a photon, it may re-radiate it in a random direction, or it may transfer the energy to the bulk gas by way of colliding with a N2 or O2 molecule. Exactly which mechanism will predominate is hard to predict. The time interval of each is about the same, at 10ns.
N2 and O2 not being IR emitters, essentially have no way of liberating heat to space. However, if there is CO2 in the upper atmosphere, molecular collisions with this may provide a route for heat to escape to space, by way of exciting IR emission.
Hence the overall situation is far from simple, and it may even be that the presence of CO2 cools the upper atmosphere.
Perhaps more importantly there are numerous proofs of the fact that at sea level, CO2’s effect is already evident at very low concentrations, therefore further increases are unlikely to significantly increase its greenhouse effect. However at high altitudes this may not be the case. If so, adding CO2 to the atmosphere might actually cause planetary cooling rather than heating, since it will have little effect at sea level but will enhance the ability of the upper atmosphere to lose heat to space.
I’ve done a number of analyses of this on our website. For example:
Ian Macdonald November 20, 2017 at 5:09 am
Infrared photons have far too little energy to change the orbit of an electron. The ‘greenhouse effect’ is entirely due to resonances in the elasticity of the chemical bonds between atoms in the gas molecule.
The 15um resonance is below the emission peak for typical ground temperature. However the photon distribution is wide enough that some will be in this range. Thus CO2 will have some effect on outgoing radiation.
The 15 𝝻m resonance is very close to the emission peak at 288K.
When a CO2 molecule absorbs a photon, it may re-radiate it in a random direction, or it may transfer the energy to the bulk gas by way of colliding with a N2 or O2 molecule. Exactly which mechanism will predominate is hard to predict. The time interval of each is about the same, at 10ns.
The mean time between collisions in the lower troposphere is ~0.1ns the re-emission time is orders of magnitude longer.
When you say N2 and O2 cannot liberate ‘heat’ to space, are you saying they do not emit energy? I keep hearing that N2 and O2 do not absorb or emit IR, but I’m also aware that any matter above 0 Kelvin emits radiation at a rate determined by its avg temp. If an object or region of space emits radiation then it’s temperature decreases whether that energy is IR or not.
Given that N2 and O2 make up about 4950 Trillion tons of the total ~5,000 Trillion tons that comprise our atmosphere, it seems fairly obvious that the energy being emitted by N2 and O2 is where most of the energy is lost from the atmosphere radiating.
Steven Morris November 20, 2017 at 2:01 pm
When you say N2 and O2 cannot liberate ‘heat’ to space, are you saying they do not emit energy? I keep hearing that N2 and O2 do not absorb or emit IR, but I’m also aware that any matter above 0 Kelvin emits radiation at a rate determined by its avg temp. If an object or region of space emits radiation then it’s temperature decreases whether that energy is IR or not.
Does not apply to gases which can only emit at those wavelengths which correspond to allowed transitions between energy levels of the molecules. The blackbody relationship provides an upper limit to such emissions, there are no significant allowed transitions for either N2 or O2 within the range of frequencies emitted by a blackbody at the earth’s temperature.
Given that N2 and O2 make up about 4950 Trillion tons of the total ~5,000 Trillion tons that comprise our atmosphere, it seems fairly obvious that the energy being emitted by N2 and O2 is where most of the energy is lost from the atmosphere radiating.
Notice that the absorption/re-emission band of CO2 ranges from about 13 to 17 microns, corresponding to a temperature range of -51 to -103 degrees C, i.e., lower than is normally experienced at Earth’s surface (which corresponds to a range of about 9 to 13 microns), hence how could CO2 possibly behave like a greenhouse gas in the Earth surface environment? This is why infrared astronomer Michael Sanicola was able to report finding no back-radiation from CO2 that could interfere with his astronomical observations.
davidbennettlaing November 21, 2017 at 5:55 am
Notice that the absorption/re-emission band of CO2 ranges from about 13 to 17 microns, corresponding to a temperature range of -51 to -103 degrees C
There is no such correspondence. Blackbody radiation at the earth’s temperature peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution (~97% of max).
By the way you shouldn’t believe anything you read on Steve Goddard’s site, not even the names!
There is no such correspondence. Blackbody radiation at the earth’s temperature peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution (~97% of max).
When you look at the whole spectrum, being near the maximum, isn’t all that significant.
And remember water overlaps co2.
Here’s just co2
and Water
But, I think that as water condenses at night in the troposphere, that sensible heat, that IR will light up co2 @15u. And co2 might light up during the day, but IIRC that blocks about as much as it adds during the day. That why the focus on Min T. But they’re completely wrong about Min T, it just follows dew point, which follows the ocean cycles, and since air temps respond differently during the day over ocean than it does land, and the hemispheres are asymmetrical to land area, global Temp averages are going to cycle with the ocean cycles.
Then, what I found shows why co2 will never for the foreseeable oil reserves become to high for the atm water to not regulate Min T. If we doubled Co2, added another 3.7W/m^2, there’s just be ~3.7W/m^2 decrease in the complementary water vapor forcing. It condenses as much water vapor as it can, to try and stay above dew point. And it looks like it’s supplying 40 or 50W/m^2, That’s a lot of doubling.
Not to say 2,000ppm is acceptable, but even an absurdly high 800ppm, isn’t a temp problem for over heating the planet.
micro6500 November 21, 2017 at 8:50 am
“There is no such correspondence. Blackbody radiation at the earth’s temperature peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution (~97% of max).”
When you look at the whole spectrum, being near the maximum, isn’t all that significant.
If you run it through Modtran you’ll find that about 10% of the total is absorbed by CO2.
And remember water overlaps co2.
If you’d run the spectra at a realistic resolution you’d find that both spectra are composed of discrete lines which in general are a different wavelengths and therefore don’t interfere with each other.
Run Spectralcalc between 600 and 700 cm-1 for both CO2 and H2O and you’ll see how sparse the H2O lines are compared with the CO2.
Phil; Who is “Steve Goddard?” I know that Wien temperatures can’t actually be inferred from the line spectra of gases, but my point is that even if you were to construct a continuous, bell-shaped, Planck-like curve from the radiation within the band of CO2’s output (13to 17 microns), the closest thing to a Wien temperature you’d get would be the most intense line, which is 14.95 microns. That would correspond to a Wien temperature of -79 degrees C, which is well below any temperature experienced at Earth’s surface. In fact, of course, gases acquire their temperatures not from radiation, but from the kinetic energy of their constituent molecules, which is a function of pressure, but sticking with the radiative argument on which greenhouse theory is based, the band within which CO2 can emit just doesn’t qualify the gas for greenhouse function within Earth’s atmosphere.
davidbennettlaing November 22, 2017 at 5:54 am
Phil; Who is “Steve Goddard?”
It’s the pseudonym of the host of the website where the bogus post purported to be by a non-existent IR astronomer Michael Sanicola, which you referred to, was posted.
I know that Wien temperatures can’t actually be inferred from the line spectra of gases, but my point is that even if you were to construct a continuous, bell-shaped, Planck-like curve from the radiation within the band of CO2’s output (13to 17 microns), the closest thing to a Wien temperature you’d get would be the most intense line, which is 14.95 microns. That would correspond to a Wien temperature of -79 degrees C, which is well below any temperature experienced at Earth’s surface.
So what? A blackbody at -80ºC emits its peak radiation at 15 microns, that has no relevance to the BB emission from the earth’s surface. The earth’s surface at 288K emits much more 15 micron radiation than a BB at -80ºC (193K), 5.8 W/m2/sr/µm vs 1.1 W/m2/sr/µm. What’s of interest is the IR emitted by the surface.
Phil; Michael Sanicola is apparently a pseudonym for Morgan Wright, who states “I’m an opticist, who specializes in optics and IR. I worked for GE’s infrared department and designed infrared telescopes for GE that were used by NASA in outer space. I invented the ambient temperature microbolometer.” By whatever name, the individual appears to know what he’s talking about. As to your contention that its all about Earth’s IR output, that’s just not the case. The important consideration is the radiation that CO2 gas is capable of re-emitting, and that lies between the limits of 13 and 19 microns, as I (and MODTRAN6) said.This band is represented in Earth’s BB spectrum, but it’s in the right hand, “cold” wing of the spectrum, corresponding to temperatures below those of Earth’s surface, where none of it can therefore influence Earth’s actual surface temperature range.
Jeffrey Westcott
November 20, 2017 8:01 am
I dare anyone who believes that the science is settled on the GHE to read this entire discussion! The inability of anyone to clearly and convincingly explain this effect is confirmation of its complexity exceeding our current knowledge of the physics, chemistry, and meteorology involved. I’m not sure we even know the sign (positive or negative) of the climate’s sensitivity to increased CO2 ! I know that it’s a “wicked” problem to solve, but the wide range of currently proposed hypotheses is bigger than it ought to be, and that’s an embarrassment for science.
Very strange, somehow there are comments above under my name, “Robert Kernodle”, that I DID NOT WRITE. Somebody else wrote them and somehow was able to post under my name. How is this possible?
Martin Mason
November 20, 2017 8:48 am
Aveollila, that is true for a static mass of air at constant pressure. That isn’t the case in the troposphere where packages of air rise and cool and then fall and heat by compression. Surely this has to taken into the big picture.
Also recognize that the atmospheres volume is not static, thus allowing for cooling via expansion. This is the biggest problem with arhennious and Tyndalls experiments, they are in test tubes where the mass of air being heated with IR is not allowed to expand or contract with addition or subtraction of energy.
The Reverend Badger
November 20, 2017 9:19 am
As expected this thread has very rapidly accumulated hundreds of comments. Very nice! As there have been a number of criticism of my initial comment (pure luck got in first) concerning “photons” I would now like to add some additional comments.
1. The concept of a “photon” was invented to help explain certain experimental results involving electro-magnetic radiation. Specifically that in certain experiments it looked like there was quantisation as opposed to discrete analogue variation.
2. As a concept it has worked and aided our understanding but THERE IS NO EXPERIMENTAL EVIDENCE that an elementary particle , The “photon”, exists. All results can equally be explained by assuming electromagnetic radiation is a wave, with analogue variability but the interaction of this wave with matter causes phenomena which are discrete/quantised because matter itself is particulate. In other words the e-m is a wave, the matter is particles, the results can be “lumpy” BECAUSE of the matter, NOT because of the wave i.e. not because the wave is really “lumpy” itself or comprised of “photons”.
3. This is why I asked readers to use both explanations when trying to explain things in radiative physics. Try explaining it both from e-m being only a wave as well as your favourite photon/particle explanation.
4. The belief that photons are real elementary particles is very very widespread but you will find the better PhD’s referring to it as quantised e-m radiation (that’s sort of a fudge/middle ground idea).
5. Anyone who thinks “photons” are real elementary particles is advised to read up on the history of how the word came to be invented and what various eminent scientists at the time thought of it.
6. Finally, if anyone really thinks they do have a link to experimental evidence which conclusively shows that photons are real elementary particles I would be happy to look at it. Before giving me the link please be sure to consider the alternative explanation (e-m wave interacting with lumpy matter) and tell me why that is NOT an equally valid explanation.
Please post the work of the PhDs whom you consider better regarding the physical reality of photons. A photon is a quantum of EM radiation, so the formulation which “they” allegedly use is a distinction without a difference.
I’m familiar with how photons were discovered and named. In reply to your original comment, I’ve already given you examples demonstrating the reality of photons and their relationship to “matter”, to which you haven’t bothered to respond.
I, Robert Kernodle, did NOT post the following FOUR comments, even though each appears separately under my name (linked to my website) as indicated here:
Robert Kernodle November 19, 2017 at 4:35 pm Technically energy can be “destroyed.” If you observe the spectra from distant stars, you’ll note that the hydrogen emission lines are red shifted. When the photon left the star of origin, it had the normal wavelength (freq) of the emission lines of hydrogen. As it traversed the expanding intergalactic space, it’s wavelength grew longer (red-shift.) There is a calculable loss of energy in this red-shifted photon according to E=hc/l where delta-E = hc/delta-l.
…
Where did the ‘lost’ energy go?
Robert Kernodle November 19, 2017 at 5:34 pm I already did. The photon that was emitted a million light years ago, “lost” some of it’s energy. The frequency has decreased (increased wavelength.) Where is the “lost” energy?
Robert Kernodle November 19, 2017 at 6:02 pm Gerontius, that does not explain it. There is no gravitational field in the million light years between the star that emitted the photon, and the spectrometer here on earth that measured it. The red shift is due to the expansion of space, not to a gravitational field.
Robert Kernodle November 19, 2017 at 6:05 pm PS Gerontius, photons coming from the opposite side of the universe display the same red shift. You would think that if you looked in the opposite direction of the red shifted photon, you’d observe a blue shifted one if your “theory” were correct.
Either there is a bug, or my ID has somehow been hacked, or an alter-universe spawn of myself is phasing in and out of this universe. If one of his posts follows one of mine (the real me), then will the universe annihilate itself?
Alexander Vissers
November 20, 2017 12:47 pm
You should from the comments above by now understand that the -80 degree is the lowest temperature at which CO2 can emit radiation and from your own emission graph that it will emit more in any wavelength the warmer it gets. It absorbs radiation (your graph is a solar radiation absorbtion graph) from the sun which is approx 5770 K so a great deal warmer. The interesting thing of the “finger graph (spectrum figure)” is that heat can “escape” the CO2 trap if other substances emit the heat in a wavelength for which CO2 is “transparent” and that some solar radiation in the infra is absorbed by CO2 in the stratosphere and high altitude troposphere and never makes it to the surface of the planet. As to CO2 the absorbtion of light and heat by different types of gasses please read the treaty by Arrhenius. This may be a first introduction. https://earthobservatory.nasa.gov/Features/Arrhenius/
davidbennettlaing
November 20, 2017 2:32 pm
Key points:
• Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
Correct, but strictly applicable only to molecular bonds. The fixed levels at which electrons exist in such bonds are “quantized” due to resonance and harmonics. When radiation of the exact same frequency as the natural resonant vibration of a particular molecular bond, or a harmonic thereof, is received, the electron in the bond in question makes an integral leap (1,2,3…,) to the next higher level. When a natural resonance or harmonic is equaled by incoming radiation, the ensuing leap occurs without requiring an input of energy.
• For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
Correct, again, for bonds. Where radiation is involved, this happens by resonance without requiring energy input.
• For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
No. As mentioned above, no energy input is required for the resonant leap of a bonding electron to a higher quantum level. Einstein couldn’t have known this, so he invented the “photon” to explain the phenomenon. In reality, EMR is simply a frequency field containing no energy whatsoever.
• Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
EMR of frequencies between resonant levels don’t interact (aren’t absorbed) but are simply reflected or transmitted. Only resonant frequencies are absorbed.
• Photons with not enough energy to raise the orbit of any of the electrons are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
Correct.
• The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
For “photon,” read “radiation.” The absorption/emission bandwidth of CO2 is from 13 to 17 microns, according to MODTRAN6.
• Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
No. Again, quantum leaps only occur at resonant frequencies. In condensed phases (solids and liquids), in which atoms are in constant mutual contact, heat conduction occurs, leading to the familiar Planck distribution consisting of a continuous, bell-shaped, broad range of frequencies of which the peak frequency corresponds to the (Wien) temperature of the emitting object. In gases, atoms are seldom in contact (except for pressure-broadening in dense gases), so such thermal conduction can’t occur, so gases exhibit line spectra in which spectral lines are separated by resonant and harmonic intervals (Rydberg-Ritz combination principle). CO2 can only absorb and emit radiation in the bandwidth 13 to 17 microns, and if a Planck spectrum were to be assembled from radiation in this range, its Wien temperature would be well below Earth surface temperatures, hence CO2 is incapable of transferring heat to the warmer Earth.
• LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
I was going to respond to some of these ‘corrections’ but when I got to: “In reality, EMR is simply a frequency field containing no energy whatsoever.”
I realized it was pointless. With such a flawed view of radiation, it would take way too much effort to address all the errors in the original post PLUS all the errors in the ‘correction’.
Can you actually refute my allegations, Mr. Folkerts, or would it be “too much effort?” It’s easy to dismiss things you don’t agree with, but that doesn’t mean that you’ve decided the issue. Science is too heavily burdened by people who simply accept long-established theory. As a polymath, I see definite value in questioning old thinking and in seeking truth in hard datasets rather than in the weight of well-established ideas. The Universe is what it is, not what we think it is, and the proper access to truth is a willingness to question old thoughts.
“When radiation of the exact same frequency as the natural resonant vibration of a particular molecular bond, or a harmonic thereof, is received, the electron in the bond in question makes an integral leap (1,2,3…,) to the next higher level. “
The absorption of this long wavelength IR involves vibrations and rotations of molecules. Not electrons jumping to higher levels. The “Bohr model” thinking with electrons jumping to higher energy orbitals is not correct here.
“Where radiation is involved, this happens by resonance without requiring energy input.”
This violates conservation of energy. If a molecule gains energy (warms up), it requires energy input.
“CO2 can only absorb and emit radiation in the bandwidth 13 to 17 microns, and if a Planck spectrum were to be assembled from radiation in this range, its Wien temperature would be well below Earth surface temperatures”
The “Wein temperature” strictly only applies to a full blackbody curve. The wavelength from Wein’s Law tells the wavelength that is most strongly emitted by a blackbody radiator. When the emitting material is not a blackbody, the strongest wavelength is not necessarily related to the wavelength predicted by Wein’s Law.
Can you actually refute my allegations, Mr. Folkerts, or would it be “too much effort?”
David, you have claimed that “In reality, EMR [electromagnetic radiation] is simply a frequency field containing no energy whatsoever.”.
Say what? Light is EMR and it definitely contains energy. We know this because we can feel the energy in sunlight warm our skins.
Microwaves are EMR. We use the energy in microwaves, the energy you claim doesn’t exist, to cook our food.
Given such a bizarre statement, one that is so divorced from reality, I can understand tjf not wanting to get into a discussion with you. The problem is that you are starting from a point that is so far away from well-established basic physics that there is little hope of bridging the gap.
You say “As a polymath, I see definite value in questioning old thinking and in seeking truth in hard datasets”, and I agree completely.
However, I also see no value in ignoring old thinking as you are doing. The fact that electromagnetic fields contain energy, energy that is used e.g. in electric motors to do practical work, has been known since someone, maybe Faraday, noticed that a wire containing an electrical current could move the needle of a compass. Moving a compass needle requires energy …
And regarding questioning old thinking, here’s some old thinking you might consider in your quest for new understanding …
1. Bond responses include rotations, vibrations, and ionizations, in order of resonant energy induction. The first doesn’t involve jumps to higher energy levels. The latter two do, creating increasingly greater distance between bonded atoms until the final distancing, ionization.
2. The first law of thermodynamics holds only in thermally isolated systems in equilibrium, which Earth/radiation interactions clearly are not. The principle of resonant response (unknown to Einstein) is well understood, now, and all that is required for an energyless response in a frictionless system (such as molecular bonds) is that incoming radiation has exactly the same frequency as a resonant frequency or overtone of the receiving bond. The resulting increased motion and acceleration of charged particles (electrons) in the bond results in the LOCAL generation of energy. This occurs in quantized steps, corresponding to resonant frequencies or overtones thereof. This is what Einstein interpreted as “Lichtquanten,” or “photons,” which are locally produced by the resonant interaction of EMR with matter and not carried by incoming EMR.
3. Quite true that the Wien temperature strictly only applies to the most intense frequency of a continuous Planck spectrum. However, I challenge you to assemble anything resembling a Planck curve from the discrete line spectra composing CO2’s input and output and have the Wien temperature of its most intense spectral line (about 14.95 microns) fall within a range that corresponds to normal Earth temperatures. In other words, CO2 can only return infrared radiation to Earth’s surface that is colder than Earth’s normal surface temperature range, and as is well-known, cooler objects (here, CO2) can’t transfer heat to warmer ones (here, Earth’s surface).
“Light is EMR and it definitely contains energy. We know this because we can feel the energy in sunlight warm our skins.”
What we actually feel is our skins warming under the impact of energyless EMR. In all cases of the detection of “photons,” interaction of EMR with matter is involved. Even our detection devices are material, and so, logically, they respond to incoming EMR. “Photons,” to my knowledge, have never been “detected” in EMR in a context apart from matter. This removal of energy from EMR solves a lot of problems. First, EMR is known to have no mass and it travels at light speed, neither of which is possible if it contains energy. Second, complex mathematics with imaginary numbers are required to get around this, and these spin off all kinds of imaginary, Alice-in-Wonderland worlds for us to contemplate. Third, it relegates energy to its proper state as a property of matter, not as some two-faced protean phenomenon that is mass-bound in most forms and mass-independent in another. With appropriate dimensional transforms, all forms of energy (except, of course, that supposedly in EMR) can be seen to have a mass term. Mass is even present in Planck’s constant h, in that purest of equations dealing with EMR, E=hv.
The statement here is not bizarre. It is simply a rethink of old, entrenched theor based on new information. Once resonance and the consequent local generation of energy (“photons”) by EMR are taken into account, things become a whole lot simpler.
1) I don’t see how rotation and vibration could be considered “bond responses”. Rotation is a physical motion of the atoms (which you seem to acknowledge with “The first doesn’t involve jumps to higher energy levels”). Vibration could, I suppose, be considered 1/2 bond energy and 1/2 KE. In any case, this difference in terminology is only a minor point.
2) Conservation of energy always holds (with no known counterexamples). Your claim that “The first law of thermodynamics holds only in thermally isolated systems in equilibrium” is patently false. ΔU = Q – W applies when you use a calorimeter. It applies to heat engines. This law is ROUTINELY applied to non-isolated, non-equilibrium scenarios. For isolated systems in thermodynamic equilibrium, the first law becomes simply ΔU = 0!
Your claim of “results in the LOCAL generation of energy” simply is not observed in nature. Any local gain in energy of a molecule is the result of the simultaneous equal local loss of energy of a photon. Einstein said nothing to contradict this conclusion.
3) This whole paragraph seems to be a misapplication of some correct laws of physics. You say “as is well-known, cooler objects (here, CO2) can’t transfer heat to warmer ones”. This is true as far as it goes, but cooler objects (like CO2 in the air) CAN slow the loss of heat (radiation from the warm surface) when interposed in front of an even colder background (3K outer space). With a steady input of heat from the sun, this can and does result in a warmer temperature for the surface.
1) Right you are regarding rotations. Vibrations involve accelerations, as the bond stretches and contracts with each cycle. This does two things: first, accelerating charged particles emit EMR, and second, increased vibrational motion of the bonded atoms represents increased kinetic energy output.
2) Patently false? Wikpedia (e.g.,) says, “The law of conservation of energy states that the total energy OF AN ISOLATED SYSTEM is constant” (my emphasis). Perhaps I should have stressed “isolated,” which Sun/Earth certainly isn’t. If my view is correct, the local generation of energy is seen all the time in EMR resonantly inducing increased vibrations and ionizations with the release of photons in receiving matter, given a high enough frequency of radiation. Also, every time you drive your car, local generation of energy occurs in its internal combustion engine, given a sufficient fuel input. Again, not a closed system.
3) Absolutely! This is evident in the difference between enjoying a cool evening in humid Kent, Connecticut as opposed to a chilly one in the dry lower Sonoran desert of southern Arizona, even though the daytime temperature only hit 78 in Kent, whereas it hit 109 in Catalina. We’re talking H20, here, but CO2 works the same way, as Wm. Ferrell said back in 1884, or thereabouts. The thing is, CO2 absorption and back-radiation can retard heat loss, but it can’t add heat. It’s sort of like a blanket over a sleeping person. It can retard heat loss from the body, but it can’t raise the body temperature past its metabolic norm of 37 C unless, of course, it’s electric. Similarly, increased solar input can raise Earth’s surface temperature, but back-radiation from CO2 can’t. Interestingly, I haven’t seen the exothermic reactions involving O2 and O3 in the stratosphere as significant sources of added heat for the Earth system.
“[A blanket] can’t raise the body temperature past its metabolic norm of 37 C “
Ah! But here the body has an active thermostat the can adjust the power input (ie regulate metabolism rates) to deliberately seek a specific temperature of 37 C. The earth doesn’t in a similar way actively adjust the power input from the sun to deliberately seek a temperature of, say, 15 C.
The earth doesn’t in a similar way actively adjust the power input from the sun to deliberately seek a temperature of, say, 15 C.
Ah, but it does. First there’s trillions of tons of solar heated liquid all over, and as Willis has shown, water vapor clouds and then storms when it gets too how, there by regulating max T, and I’ve shown that water vapor regulates cooling using this stored energy to augment surface temps to limit cooling at night to dew point.
Steve Zell
November 20, 2017 3:12 pm
Rod Gill is not completely correct in saying that just because the temperature at which the maximum radiation is emitted at 15 um is -80 C, that IR radiation at 15 um cannot be absorbed at higher temperatures.
Rod Gill’s top graph shows the Planck distribution of intensity of emission as a function of wavelength at given values of temperature. If the wavelength of maximum intensity is about 500 nm for 6000 K (about the temperature of the surface of the sun), there is still substantial energy emitted at wavelengths above and below 500 nm.
The Planck distribution for the Earth’s surface at 289 K would reach a maximum intensity for a wavelength of 10 um, but there would still be a substantial intensity at the slightly higher wavelength (lower frequency) of 15 um.
The Planck distribution and Wien’s law (which is merely differentiating the Planck distribution and setting the derivative to zero) apply to EMISSION spectra. The ability of a gas to ABSORB infrared radiation is based on the photon energy levels that cause an electron to change from one orbital to a higher-energy orbital, which vary from one gas to another.
One of the keys to debunking global-warming theory is in the second figure in Rod Gill’s article. For the 13-17 um peak absorption range for CO2, there is also substantial absorption by water vapor. It is not clear from the graphs at what concentration these absorption spectra were measured, but it can be assumed that all were measured at the same concentration for an apples-to-apples comparison.
Over an ocean, it is common for the water-vapor concentration to be 1 to 2 percent, or 10,000 to 20,000 ppm, as compared to about 400 ppm CO2 (currently at Mauna Loa). If water vapor at 400 ppm would absorb 40 to 60% of the available energy at a wavelength between 13 and 17 um, at 10,000 ppm it would absorb over 99.9% of the available energy (the energy transmitted, or not absorbed, is a negative exponential of the concentration * distance), and the CO2 absorbs most of what’s left, which is less than 0.1% of the total energy absorbed.
Since 70% of the earth’s surface is covered by water, there is always water vapor more or less in equilibrium with it in the air over the oceans, and CO2 has a very minor effect on climate over the oceans, because water vapor has already absorbed the vast majority of the emitted IR energy.
CO2 can only affect the climate in areas with extremely low water vapor concentrations, either over deserts or in very cold climates where the air is too cold to hold much water vapor. This may occur over high-latitude areas in winter, but this does not prevent temperatures from dropping well below freezing.
During the Arctic spring and summer, some of the sea ice along the northern shores of Russia, Scandinavia, Alaska, and Canada does melt (primarily because of warmer sun-heated air blowing northward off the continents). But the open water allows for evaporation, which raises the humidity of the atmosphere and allows clouds and storms to form, which limits the amount of incident sunlight and keeps temperatures only slightly above freezing during the summer months.
The major fear of global-warming alarmists is that runaway warming will melt the Greenland and Antarctic ice caps and cause flooding of coastal cities.
The facts show that, over temperate and tropical oceans, the vast majority of the available IR energy is already absorbed by water vapor, and the CO2 effect is like the weight of a fly on top of an elephant (of water vapor absorption). CO2 can only absorb appreciable energy in very cold and/or dry environments, but in the polar regions, most of the “warming” is in the winter, when it is too cold to melt ice, and humidities increase in summer, damping out the effect of CO2. Also, most of the Greenland and Antarctic ice caps are at high altitudes (over 2000 meters) where midsummer temperatures never get above freezing, so that no melting occurs.
“CO2 can only affect the climate in areas with extremely low water vapor concentrations, either over deserts or in very cold climates where the air is too cold to hold much water vapor. This may occur over high-latitude areas in winter, but this does not prevent temperatures from dropping well below freezing. “
This also occurs at high ALTITUDES. The emission of 15 um IR to space occurs mostly up near the tropopause. This is confirmed by various data like, which shows the IR emission to space in the 15 um range radiates as well as a ~ 215 K blackbody. At these low temperatures, there is basically no water vapor in the atmosphere.
So from your own logic, CO2 near the tropopause affects climate. And since the tropopause circles the earth, CO2 impacts the climate globally.
The main point that seems to be missing here is that the ‘greenhouse effect’ is about ‘trapping’ heat by radiative/greenhouse gasses. RGs don’t trap heat they just delay its transfer to space. The atmosphere is awash with collision induced IR that is constantly moving energy about. How fast? The key question is how long this delay is. Eventually the energy is radiated to space or the atmosphere would just keep heating up.
If the delay is long the energy involved will heat the atmosphere significantly. If it is short, the energy is transferred to space rapidly and there is no significant heating of the atmosphere. I calculate the heating to be negligible.
See: brindabella.id.au/climarc in the RadiativeDelayInContext article.
dai davies, I agree with your analysis with the exception that it would be clearer if you added the time frame to: ” calculate the heating to be negligible…( for a millisecond, second, hour, day, month years etc.). As I understand the average residence time of a photon in a GHG molecule is in the nano to microsecond time frame. So although “trapping” may occur, on a molecular basis, it is nearly instantaneous while leaving little if any energy transfer.
I agree with this statement of your: “The atmosphere is awash with collision induced IR that is constantly moving energy about.”, but would add molecular collisions to the concept. When these molecules collide they can and often do transfer energy. That function is called conduction. For me that is the best explanation for energy/heat “trapping” in the atmosphere.
It is the same for the surface, EXCEPT FOR WATER, where that IR energy can and most often is trapped for a significant time. It doesn’t mater whether that IR energy originated from a molecule in the atmosphere or from the Sun.
CoRev November 21, 2017 at 4:05 am
dai davies, I agree with your analysis with the exception that it would be clearer if you added the time frame to: ” calculate the heating to be negligible…( for a millisecond, second, hour, day, month years etc.). As I understand the average residence time of a photon in a GHG molecule is in the nano to microsecond time frame. So although “trapping” may occur, on a molecular basis, it is nearly instantaneous while leaving little if any energy transfer.
No, for CO2 the time to emit is of the order of msec whereas a typical molecule will undergo about 10 collisions per nanosec in the lower atmosphere so there is plenty of time for energy transfer.
Phil, as I stated the residence time of a photon is” “is in the nano to microsecond time frame.” I need a scientific reference to assure such a long time as milli-second. Also do you have a reference for your claim of multiple molecular collisions for lower atmosphere resident molecules.
Regardless, i think you are confirming my contention that conduction/collisions are a/the major energy transfer mechanism.
I asked for a reference, not you making stuff up.
Fact: at equal temperature, no transfer. So why would there be transfer from cold to hot if one body is colder.
Show me a reference for any transfer outside of “net”, or shut up.
Glad to. I have more references if you wish, it is bog-standard thermodynamics. Or you could just google “two-way radiative transfer” …
Now, until you understand the equation at the bottom of that webpage I linked to, and you can explain it to us, how about YOU shut up …
lifeisthermal, in reference to you saying “Show me a reference for any transfer outside of “net”, or shut up.”, in fact, the very term “net transfer” should give you a clue that there are energy transfers other than the net transfer …
You see,”net energy transfer” means energy gains minus energy losses, and each of those IS AN ENERGY TRANSFER.
Willis, surely though there is radiative transfer from cold to hot but never a transfer of heat energy. The colder body can never warm the warmer body?
Willis, surely though there is radiative transfer from cold to hot but never a transfer of heat energy. The colder body can never warm the warmer body?
Thanks, Martin, and you are right. However, this gets us into semantical trouble because of our common meaning of “warm”, which differs from a strict scientific meaning.
For example, we think nothing of putting on a cold coat and noting that it has warmed us up … which of course can’t happen, as you point out. As they say:
The Second Law of Thermodynamics—It’s More Than Just A Good Idea
So I’m very careful in my use of words around this issue. If you look at my comments, I say things like “the cold atmosphere leaves the surface warmer than it would be without the atmosphere”, which is absolutely true and does not violate the Second Law.
When you say N2 and O2 cannot liberate ‘heat’ to space, are you saying they do not emit energy?
N2 and O2 do both absorb and radiate, but they do so in only a very limited part of the IR spectrum.
I keep hearing that N2 and O2 do not absorb or emit IR, but I’m also aware that any matter above 0 Kelvin emits radiation at a rate determined by its avg temp.
Not quite true. One exception is xenon gas, which is monatomic and has no free electrons. As a result, it neither radiates nor absorbs energy.
If an object or region of space emits radiation then it’s temperature decreases whether that energy is IR or not.
Yes, assuming it is not receiving any radiation …
Given that N2 and O2 make up about 4950 Trillion tons of the total ~5,000 Trillion tons that comprise our atmosphere, it seems fairly obvious that the energy being emitted by N2 and O2 is where most of the energy is lost from the atmosphere radiating.
Often in science, things that are “fairly obvious” are not true. In this case, because of the narrowness of the O2 and N2 absorption bands, they physically cannot absorb (or radiate) much of the upwelling radiant energy, because it is the wrong wavelength.
🙂
Who cares whether emissions are in the Infrared part of the spectrum or not? If an object radiates outside of the IR, are we to believe that the amount of energy within that region of space has not decreased and therefore cooled? Given no or less energy has been absorbed of course.
So our science books should say, all matter above 0 Kelvin, except Xenon, radiate energy. Oddly enough one can buy Xenon lights.
N2 and O2 do not need to absorb or emit IR in order to heat or cool. Energy is conducted via contact with the surface and they are constantly colliding with H2O vapor, CO2, CH4, etc reducing the energy within those molecules if the they have higher energy and increasing it if they have less. Once they have attained the Avg temp of say 15C, they are free to radiate that energy at a wavelength determined by Wein’s Law, correct?
Entropy be damned if objects cannot radiate unless they are some exact temp.
Steven Morris November 21, 2017 at 3:00 am
N2 and O2 do not need to absorb or emit IR in order to heat or cool. Energy is conducted via contact with the surface and they are constantly colliding with H2O vapor, CO2, CH4, etc reducing the energy within those molecules if the they have higher energy and increasing it if they have less. Once they have attained the Avg temp of say 15C, they are free to radiate that energy at a wavelength determined by Wein’s Law, correct?
No, they are unable to emit in that wavelength range because there is no excited dipole. The energy you refer to is just translational energy.
Not quite true. One exception is xenon gas, which is monatomic and has no free electrons. As a result, it neither radiates nor absorbs energy.
Actually Willis even Xenon emits, in fact it has a very large number of lines in the visible which is why it’s often used in lamps. http://astro.u-strasbg.fr/~koppen/discharge/
Not quite true. One exception is xenon gas, which is monatomic and has no free electrons. As a result, it neither radiates nor absorbs energy.
Actually Willis even Xenon emits, in fact it has a very large number of lines in the visible which is why it’s often used in lamps.
Sorry for my lack of clarity, Phil. You are correct. However, I was referring to the current context, which is thermal longwave (IR) radiation. Xenon neither absorbs nor emits thermal longwave radiation.
However, when you get xenon up to very high temperatures, it can indeed emit light … but that is happening by an entirely different mechanism which has nothing to do with the subject under discussion.
Thanks,
w.
Michael S. Kelly
November 20, 2017 8:50 pm
I don’t have a definitive answer, here, but do submit the following criticisms. The idea that IR photons just bounce around the atmosphere, being absorbed and re-emitted by CO2 molecules, producing no warming, is probably wrong. If absorption were followed instantaneously by emission, it would have a better chance of being true, but that isn’t the case.
For example, the radiative lifetime of a CO2 molecule for the 106 micron transition is on the order of 2 msec for CO2 in gases at a pressure of about 8 torr. That’s the only reason a CO2 laser can work, especially a Q-switched, high-power laser. It also means that at higher pressures, the probability of collisional, rather than radiative, de-excitation grows substantially. As a matter of fact, the transition I just cited – the 10.6 micron one – is produced by a collision with a nitrogen molecule with a rotational energy of about the same magnitude. De-excitation occurs exactly the same way.
I rather think that the absorbed energy by a photon exciting a CO2 molecule is more likely to be thermalized by collision than re-radiated, especially at lower altitudes.
I don’t have a definitive answer, here, but do submit the following criticisms. The idea that IR photons just bounce around the atmosphere, being absorbed and re-emitted by CO2 molecules, producing no warming, is probably wrong. If absorption were followed instantaneously by emission, it would have a better chance of being true, but that isn’t the case.
For example, the radiative lifetime of a CO2 molecule for the 106 micron transition is on the order of 2 msec for CO2 in gases at a pressure of about 8 torr. That’s the only reason a CO2 laser can work, especially a Q-switched, high-power laser.
Michael, I find the CO2 laser talk contains an error, as CO2 lasers work with 10 um frequencies and not with 15um as specified in the text above:
Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um
Michael, I find the CO2 laser talk contains an error, as CO2 lasers work with 10 um frequencies and not with 15um as specified in the text above:
The radiative lifetime of the 15μm excited state of the CO2 is the same order of magnitude as the 10μm state (msec) far longer than the mean time between collisions (less than nsec).
Bernard Lodge
November 21, 2017 12:37 am
‘This is a contentious subject, and I have often shied away from it because it often erupts in food fights’
Thanks Anthony for having this discussion. It may be a bit messy, but only by repeatedly discussing this key issue will we be able to understand and either prove or disprove the GHG effect to the general satisfaction of the WUWT audience.
Thanks also to Willis in particular for engaging so deeply in this discussion despite his obvious frustration with so many people. I fear I am about to add myself to that group! I am a firm believer that if you can’t explain something to a willing listener, you don’t really understand it yourself. I am still doubtful about the GHG effect and will try to explain my doubts simply.
Some initial comments:
The Steel Greenhouse essay written by Willis explains how his definition of the greenhouse effect works, using black body objects radiating back and forth to each other. He assumes that there is perfect energy radiation and absorption and hence, any back radiation to the Earth’s surface will result in an increase in temperature. This model even works in a vacuum. He then says that this is how CO2 warms the Earth because back radiation from the CO2 will warm the Earth’s surface.
The implicit assumption seems to be that any back radiation must raise the temperature of the Earth above what it would otherwise be. This assumes that all emissions are additive with respect to temperature.
My first problem with this is that although all emissions are additive with regard to energy, I don’t believe that all energy emissions are additive with regard to temperature. For instance, sometimes electromagnetic (EM) emissions pass through material, sometimes they are reflected, sometimes they absorbed, sometimes they are immediately re-radiated, sometimes they are scattered etc. Many of these events do not change the temperature. An obvious example of this is that two bars of white hot metal placed together have double the energy but no change in temperature. From this, it is not certain to me that CO2 back radiation would for sure raise temperature in the real world. I need more proof than Willis’ black body model which by definition would increase temperature with any emissions.
My second problem relates to whether a cold body can raise the temperature of a warmer body. This is vital to understand because Willis himself stated in the thread that the approximate average temperature of the atmosphere is -50 C so how can that raise the temperature of the surface which is already at a higher temperature? Willis says that -50 C is warmer than the -270 C vacuum of space which means that the Earth will cool slower which is the same as being warmer.
I can think of two challenges to this logic:
a) Whether an atmosphere slows the cooling of the earth is not the point. The real question is does replacing a few -50 C molecules of oxygen with a few -50 C molecules of CO2 make any difference to the Earth’s temperature? The temperature differential between the surface and the atmosphere is the same either way so why would the rate of cooling of the Earth’s surface change at all? That is the real GHG effect that needs to be proven. In the 600+ comments in this thread, none of them addresses this.
b) Logically, I cannot see how CO2 at -50 C can warm the Earth’s surface which is already at +15 C. I do accept that cold objects emit EM radiation but do not accept that they can raise the temperature of an already warmer object. For instance, how many cubes of ice do I have to surround a one liter pan of water with to raise the water to boiling point? If someone can do the math on that and tell me the answer then I will be persuaded.
Those are my reasons for doubting the existence of the GHG effect.
“My second problem relates to whether a cold body can raise the temperature of a warmer body.”
Again what is so difficult about the concept of “slowed cooling” that cannot get through to some people and they continue to talk of “raise the temperature”.
Please look at it as energy in (from the Sun) vs energy out at TOA.
More is coming in (SW absorbed) than going out (LWIR emitted).
If there were no enhanced GHE then the two would balance.
The surface temp has to rise in order to equilibriate via the SB equation
Added anthro CO2 is “slowing the cooling” of Earth’s surface by back-radiating some of the LWIR to the surface where it “acts twice” to cause warming.
It isn’t a cold object warming a hot one at all.
The energy that is “causing” the warming is that that left from the warm body in the first place.
It is a reflection/insulation effect.
No point as you will always have the last hand-waving point, with a bit of SHOUTING, that you seem to think is intelligent discourse.
As I said – if you say so.
How about you post some rather than ranting at me for not.
You’re the one hand-waving as usual.
I say again – If you say so – I believe you.
And the rest of the scientific community also.
Martin Mason
November 21, 2017 2:55 am
Tone, slowing down cooling isn’t heating. Trapping heat is heating and that doesn’t happen. I also can’t see anywhere that a consistent radiation imbalance exists at the TOA
“Tone, slowing down cooling isn’t heating. Trapping heat is heating and that doesn’t happen. I also can’t see anywhere that a consistent radiation imbalance exists at the TOA”
Of course slowing down cooling is heating.
While it is slowed down the Sun is still shining on half the globe is it not?
So there is incoming SW being absorbed during the “slowing”.
That is the imbalance shown by TOA observations and also by calculation of OHC being accrued – which is where most of the imbalance is being stored.
For a blackbody receiving P input which is in a steady state with space at 3K
P=kA(T1^4-3^4)
For the same body in steady state with surroundings at 220K
P=kA(T2^4-220^4)
therefore T2^4=T1^4 + 220^4 – 3^4
Clearly as a result of this change the blackbody will be warmer.
AndyG55 November 21, 2017 at 11:50 am
Earth is NOT a black body.
Stop using non-applicable theoretical maths.
Deal with reality.
Realty is that the earth emits within a few % of a true Blackbody in the IR, those equations are applicable to the earth. Perhaps you should stop your content-free ignorant posts?
Personally I blame the astronomers for all this and yet it all started with such good intentions (The road to hell and all that). The simple question they wished to answer was how to devise a technique to calculate the average temperature of a planet given the following series of knowns:-
1. The energy intensity of the sun’s beam of light at a given distance from the star determined using the inverse square law of radiation.
2. The average orbital distance of a planet from the sun – this determines the average intensity of the sunlight that the planet intercepts.
3. The size of a planet’s disk – this determines the amount of sunlight that the planet collects.
4. Then there are the twin problems of daily planetary rotation and axial tilt.
So they made a perfectly valid simplification. They decided to ignore all the complicated spherical geometry of trying to determine how the surface of a spinning globe is lit and by what intensity and instead made the following correct assertions:-
1. Total energy in is equal to the total energy out.
2. That the cross-sectional area of the incoming radiant beam intercepted by a globe equates to the intercept area of a notional disk of equivalent radius BUT that the surface area of the outgoing emissions from that planet is spread over an area that is four times the area of the notional intercept disk. This is because the surface area of a sphere is 4πr^2; that is four times the area of a disk that has the same radius. (Clever but lazy because it has caused all sorts of problems).
So having divided the total intensity of the incoming beam by 4 they then applied the Stefan Boltzmann equation to this value to compute the average temperature of the planet and (horror of horrors) the answer they got when the calculation was applied to the Earth was obviously wrong. The SB equation gave a value that is 33C too low. Oh dear.
And then they did something really stupid, they invented the radiant greenhouse effect to heat the planet’s surface in a curve fitting extrapolation of an equation of state that is applicable to the stratosphere, but not to the troposphere where its application is invalid. After all they were astronomers used to dealing with radiation so what could be wrong with that? It seems that they forgot all about Maxwell’s ideal gas law and its application to pressures above 10kPa. They forgot about the gravitational binding to the surface of a planetary atmosphere and the consequent storage of energy that this gravitational binding represents. They did not consider the effect of insolation intensity and gravitationally determined escape velocity on selective loss to space of atmospheric volatiles with a low molecular weight. And don’t get me started on the effect on the latitudinal reach of the Hadley cell of the daily rotation rate of a planet (an angular momentum effect and not a temperature determined property of an atmosphere). All of these are easy things to forget after all.
And then we come to the piece de resistance, the crowning glory of this sick sad farce, the dangerous greenhouse gas carbon dioxide. Using their logic they claim that adding CO2 to the troposphere will raise the radiant emission level at the TOA and so dangerously warm the surface. The fact that the Tropopause is a pressure and not a temperature induced phenomena of change of atmospheric state is ignored. IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall below their radiative balance equation calculated from first principles (the distance of the Earth from the Sun) and as this cannot happen it necessarily follows that CO2 cannot warm the planet because the outgoing emission temperature can never be set at a lower value than the notional incoming collection temperature.
I was with you until “And then they did something really stupid, they invented the radiant greenhouse effect to heat the planet’s surface in a curve fitting extrapolation “
The greenhouse effect is not an “invention — it is a very basic application of the laws of thermodynamics. If radiation is blocked as it leaves a planet’s surface, the result is an increase in the surface temperature. Nothing in this theory contradicts the gas laws. Nothing denies gravitational potential energy. Nothing is invalidated by lighter gases escaping off to space.
I will grant that the super-simple models are not sufficient to calculate actual temperatures, but they are not designed to be. More intricate models would be needed for that.
“IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall below their radiative balance equation calculated from first principles (the distance of the Earth from the Sun) and as this cannot happen it necessarily follows that CO2 cannot warm the planet”
You seem to have this rather reversed! There is no “first principle” that says that radiation must be balanced. *IF* radiation is balanced, then the temperature will remain steady. However, if the radiation becomes imbalanced, then the temperature will change. . The correct conclusion is IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall … causing net gain in thermal energy and an increase in temperature at the surface until balance was restored.
Quantum mechanics and absorption spectra aside – there is still no correlation between increasing CO2 conc and atmospheric warming. Temperature measurements over the past upteen years have shown that the latter is flatlining
Quantum mechanics and absorption spectra aside – there is still no correlation between increasing CO2 conc and atmospheric warming. Temperature measurements over the past upteen years have shown that the latter is flatlining
Good observation.
Either:
With all the increasing GHG’s, theory demands warming. Yet you are correct, other than el ninos, flatline.
To be flat, there has to be a counter regulating force.
Or
There is no GHG effect.
And I found the counter regulating force.
In either case, there is no chance of catastrophic warming.
Roger Clague
November 21, 2017 4:16 am
Philip Mulholland
November 21, 2017 at 3:51 am
says: Tropopause is a pressure and not a temperature induced phenomena
The Tropopause is a temperature phenomenon. Almost constant over the whole surface. Much more interesting than the surface temperature.
I don’t know what causes it, so am interested in suggestions like yours.
Pressure continues to fall after the tropopause height. That is while the temperature is not falling
I am puzzled that falling pressure can leave temperature to stay the same.
Roger,
Thank you for your comment.
Let me try this idea on you:-
The process by which the surface of a spherical, rotating, orbiting planet unevenly intercepts high grade solar energy from its parent sun and subsequently emits to space a uniform evenly distributed output of low grade thermal radiation forms the defining character of planetary climate.
The Stratosphere is an isothermal shell of low pressure gas that surrounds the Troposphere and provides a thermal datum that governs the operational parameters of the high pressure gases in the weather machine below. In a very real sense the Stratosphere is the exhaust system of the planet’s atmosphere. Just as the temperature of the exhaust gases from a heat engine cannot be used to power the engine itself, so it is also impossible for the exhaust heat content of the Stratosphere to power the weather machine by warming the planet’s surface below.
Uh, you do know that the exhaust gas from an engine is exactly what powers a turbo charger, right? That is, with the appropriate mechanism (in this case a turbo charger), you can harness a bit more of that thermal energy escaping the engine. To continue your analogy, it’s not even necessary to introduce the turbo mechanism to affect the engine. Merely, changing the parameters of the exhaust system, like downpipe diameter for example, will change flow from the system which can affect the power generated. I think one could fairly make the argument that this is analogous to changing the ratios of the radiatively-relevant molecules in our atmosphere.
Thus, I find your explanation lacking due to the simple fact that coupled systems are…well…coupled. Changes to one part will necessarily affect others. The amplitude of the effect can be debated, as well as whether or not the effect is physically significant at standard operating parameters, but again, the effect is real.
Philip Mulholland November 21, 2017 at 4:54 am
Just as the temperature of the exhaust gases from a heat engine cannot be used to power the engine itself,
Never heard of a turbocharger or a Turbojet I guess?
Just as the temperature of the exhaust gases from a heat engine cannot be used to power the engine itself,
Never heard of a turbocharger or a Turbojet I guess?
Yes, but they lose efficiency the closer the exhaust temp is to air temp. So usually turbo’s are at the exhaust where the gas is as hot as possible. When you account to pressure differences, that’s the temp differential?
Guys,
Lots of kick back here, but I will stand my ground. Every heat engine has an exhaust, even turbo charged ones. The genius of Robert Stevenson was to take the exhaust steam from the driving piston and use it to force ventilate the fire box in a feedback loop of increased power. But at some point the law of diminishing returns kicks in and the friction within your machine is greater than the added power you can glean from the exhaust.
The real idea I want criticism on is the observation that the isothermal shell of the stratosphere is in fact an exhaust thermal datum to the weather machine of the troposphere. It is the uniform planet wide distribution of this isothermal shell that is most interesting. My biggest problem has always been that this uniformity is in effect a friction-less system, so how can this form the starting point to a weather machine that clear moves mass? It makes much more sense to me to view the stratosphere as an exit system with waste heat and not an input system.
Phillip says: “Stratosphere is the exhaust system of the planet’s atmosphere.”
Why would you say this? The ‘exhaust’ from the earth is the thermal IR that escapes to space. Some of the ‘exhaust’ comes directly from the surface through the ‘atmospheric window’. Some of the ‘exhaust’ comes from clouds; some comes from GHGs in the atmosphere. Very little comes from the stratosphere.
“I don’t know what causes it, so am interested in suggestions like yours.”
Simply put, it is the inversion level where heating of O3 in the stratosphere meets and overcomes the -ve LR of the Trop – the level at which (mostly) convection can reach in the Troposphere. The level is a trade off between the O3 induced warming and the warmth of the Trop – it is highest in the tropics and lowest at the Poles.
Roger,
I refer you to the figure in the following paper:
Robinson, T.D. & Catling, D.C. (2014) Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent intrared transparency. Nature Geoscience 7 (1), 12-15. https://www.nature.com/articles/ngeo2020
Venus is an exception. At its tropopause, temperature and pressure reach Earth-like levels, ie ~1.0 bar. Also, at its searing surface the winds are slow, but at the top of the troposphere, clouds pick up speed to 100 m/s, thus showing yet again colder is stormier, contrary to false CACA dogma regarding “extreme weather” from global warming.
The thin atmosphere of Mars is essentially stratospheric at the surface.
Richard Greene November 21, 2017 at 6:50 am
The science of climate change is obviously not settled.
The science points raised in the head post are settled, unfortunately many of them are wrongly stated or misunderstood as I, Willis and others have pointed out. Just because people unfamiliar with the science such as the author (as he admitted) get it wrong doesn’t mean the science isn’t settled.
E.g.
Phil. November 19, 2017 at 2:58 pm
The worst of these errors is the misrepresentation of Wien’s Law which has also appeared in numerous other places, including by a fictional astronomer on Steve site.
The mis-statement is that because Wien’s Law says the temperature at which the maximum emission occurs at a wavelength of 15microns is -80ºC then “Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
CO2 does indeed have a maximum in the radiance-wavelength curve at 15 microns for a temperature of -80ºC, however for a higher temperature the emissions at 15microns are always higher.
At 15 microns and 193K radiance is 1.09 W/m2/sr/µm, at 203K it’s 1.41 W/m2/sr/µm, at 213K it’s 1.80 W/m2/sr/µm, and at 223K it’s 2.26 W/m2/sr/µm.
Dr. Deanster
November 21, 2017 8:07 am
Just my uninformed opinion on back radiation, but it only becomes relevant in the absence of an external heat source. My observational evidence:
1) increased GHG does not increase day time highs. This says that the normal heating process of the atmosphere completely overwhelms any contribution from back radiation. During the day, the GHG compartment absorbs all the energy it can, which is a finite quantity. To some extent, the GHG compartment seems to prevent overheating, as it seems places of high WV can’t seem to reach the high temperatures achieved in desert environments.
2) most of the science-ee stuff I’ve seen show graphs where it is the night time lows that are increasing. IMO, this is because as the temperature drops, the GHG heat sink begins to release its energy to its surrounding, slowing the rate of cooling. This is supported by the comments of others, deserts with no WV cool rapidly, coastal areas like New Orleans don’t.
3) local temps are more influenced by air movement than than local radiation. When a cool front comes in, it gets cold, when a warm front comes in, it gets warmer ….. doesn’t seem to matter much what the back radiation is doing.
“During the day, the GHG compartment absorbs all the energy it can,”
And releases it as well, just as it does at night. It both absorbs and emits all the time on short time frames.
Most of the heat energy of the atmosphere is in molecular motion not stored protons, that energy has been transferred to molecular motion.
I just noticed this claim in the head post. There are two huge problems with it. Here’s what it says:
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit.
I’m sorry, but this is not true. A gas at 50°C has more kinetic energy than a gas at 20°C, without any electrons moving to more energetic orbits.
The word “kinetic” in “kinetic energy” is from the Greek, meaning movement or motion. Similarly, “kinetic” energy is specifically energy due to MOTION. It is not energy due to different energy levels of electrons, the name itself says that.
Second, the absorption of thermal longwave radiation does NOT result in an electron being kicked to a higher orbit. That is the photoelectric effect, but there’s not enough energy in thermal LR to move an electron.
Instead, when a molecule of a gas absorbs longwave radiation, it starts the molecule vibrating. Depending on the complexity of the molecule, this vibration can include a variety of vibrational modes including stretching, asymmetric stretching, scissoring, rocking, wagging, and twisting.
The more modes of vibration a molecule has, the more different frequencies of longwave radiation it can absorb (or radiate). Obviously, a simple two-atom molecule like N2 or O2 can only stretch, it can’t twist or flex … which is why they can only absorb or radiate in a narrow frequency band. Molecules with three atoms like CO2 and H2O have many more vibrational modes, so they absorb over a wide range. Solids, of course, made of millions of atoms or molecules, have a host of frequencies at which they absorb and radiate. And monatomic gases like argon have no vibrational modes at all, so they can neither absorb nor radiate longwave thermal radiation.
However, within microseconds, this vibrational energy is transferred to thousands and thousands of neighboring gas molecules. This converts the vibration into motion of the surrounding molecules. The net effect is to heat the body of the gas, even when the amount of GHG is quite small.
Man … the incorrect statements in the head post just keep on coming …
However, within microseconds, this vibrational energy is transferred to thousands and thousands of neighboring gas molecules. This converts the vibration into motion of the surrounding molecules. The net effect is to heat the body of the gas, even when the amount of GHG is quite small.
Quite true, it’s the main reasoning for the broadening of the absorption bands, and I believe this is the main mechanism for “GHG” warming after the concentration of such molecules reaches the point of absorbing the available LWIR. However, the energy of the vibration of these normal modes is between 0.0001-1.7 eV and the energy lost or gained upon molecular collisions is a fraction of this energy. And then we must ask, does the donation of molecular vibrational energy to kinetic energy increase convection?
@corev
The time scale I’m referring to is the mean transition time for energy to be transferred from the lower atmosphere to space. This is the upward component of the omnidirectional collision induced radiation. The timescale is a few hours. Compared with typical molecular timescales this is an eternity, but the intuitive comparison I make is that taking a square meter column of atmosphere (10T) and an upward flux of 200W, it’s like a 200W lightbulb heating the air in a gym for a few hours. A small effect of around 0.14C, cf the 33C GHE assumed by the IPCC science.
Yes, the average lifetime of an excited RG molecule is small – usually de-excited in the next molecular collision it experiences. A very small portion get to emit a photon, but since there are a lot of molecules the radiation levels are significant.
True, most of the energy in the atmosphere (ignoring mass energy) is KE of molecules, but conduction is slow. Convection and evaporation/condensation cycle are major players.
The essence of the GHE is not back radiation, but how much of the surface emitted energy remains in the lower atmosphere to contribute to it, and how rapidly it is transported upward radiatively rather than being ‘trapped’.
@tjfolkerts
“The correct conclusion is IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall … causing net gain in thermal energy and an increase in temperature at the surface until balance was restored.”
No. Again consensus CS is ignoring the time factor. Even though rates of emission in the upper troposphere will drop if the tropopause rises, global atmospheric circulation continues poleward, radiating as it goes, until the upper temps drop and density increases until upper air drops giving Hadley cells.
dai
Brett Keane
November 21, 2017 6:49 pm
Yes indeed, dai. This is all almost as good as Saturday’s Wales-All Blacks match should be.
About 1902, Prof Robert Woods refuted the existence of an atmospheric greenhouse, and soon all those other great experimemtalists agreed. Arrhenius realised this, and went on to do other good works. It is a mere latter-day contrivance of hippy science, magic mushroom stuff. Nasa’s solar system data shows the truth. It is all the gravito-thermal effect. Convectively enabled and phase change accelerated to easily cope with imbalances of input and distribution. Water is the real magic stuff…..
Radiation is an effect of KE, not a prime cause. Except of seemingly endless misconceptions. In furnace engineering, CO2 speeds heat movement to furnace walls etc.. Above 200ppm, no significant increase. That is all, no blanket to suck on, sorry Linus.
About 1902, Prof Robert Woods refuted the existence of an atmospheric greenhouse, and soon all those other great experimemtalists agreed.
The Wood experiment was fatally flawed for reasons I explained in detail in “The R. W. Wood Experiment“.
Now, let’s do this scientifically. I am not interested in your opinion of my analysis of the poorly-designed Wood experiment. I am interested in your scientific objections to my analysis. However, please QUOTE THE EXACT WORDS THAT YOU OBJECT TO in my analysis, so we can all understand just what you think is wrong.
Or you could either not read my analysis, or read it and know it is right and blow it off … your choice. However, note if you do not raise any scientific objections to my analysis, I will take that as evidence.
w.
Brett Keane
November 22, 2017 12:41 am
Willis Eschenbach
November 21, 2017 at 8:33 pm: Willis, this is not your post. Of course I read yours, referenced. It did not apply to the Optical Physicist’s propositition. Ask Konrad Hartmann. So I see no reason to debate it here,except to note that lids are out of place in a real atmosphere, as are surfaces also..
Actually studying Fourier, Tyndall and Maxwell. for instance, shows where Wood was coming from. Not what flower children claim they said, which is something else.
I gave plenty of data sources in my entries above. Argue them if you wish. The header Post requests such for its own propositions. This is the basis of all real scientific method. A thing of beauty….
Willis Eschenbach November 21, 2017 at 8:33 pm: Willis, this is not your post. Of course I read yours, referenced. It did not apply to the Optical Physicist’s propositition. Ask Konrad Hartmann. So I see no reason to debate it here,except to note that lids are out of place in a real atmosphere, as are surfaces also..
Hey, Brett, if you can’t find anything wrong with my post on the R. W. Wood experiment, that’s fine. Trying to blow it off by claiming it “did not apply to the Optical Physicist’s proposition”, whatever that might be, is just handwaving to distract us from the fact that apparently you can’t find anything wrong with my post.
You made a claim about the R. W. Wood experiment. I gave you a detailed analysis and asked what is wrong with the analysis. In response, you’ve just headed for the door …
Willis Eschenbach November 22, 2017 at 11:24 am:
Willis, Wood was a brilliant experimentalist. So is Hartmann, so is Berthold Klein of the Mylar balloon expt. Such are not refuted by a thought expt. Nor are the Nasa solar system data and the many papers based on what it teaches us. Which is that atmospheric gases handle energy inpiut according to the combined gas laws under the Poissin relation, as Maxwell noted in his “Theory of Heat”. By vigorous convection until radiation can finally leave ie less than one optical depth. Window EMF alone takes that door, not I.
Willis Eschenbach November 22, 2017 at 11:24 am:
Willis, Wood was a brilliant experimentalist. So is Hartmann, so is Berthold Klein of the Mylar balloon expt. Such are not refuted by a thought expt. Nor are the Nasa solar system data and the many papers based on what it teaches us. Which is that atmospheric gases handle energy inpiut according to the combined gas laws under the Poissin relation, as Maxwell noted in his “Theory of Heat”. By vigorous convection until radiation can finally leave ie less than one optical depth. Window EMF alone takes that door, not I.
Thanks, Brett. Yes, I know that Wood was an experimentalist, although given his experiment I would not class him as “brilliant” … and more to the point, l+
et me remind you of a quote by the amazing RIchard Feynman, a true scientific genius. He said:
“Science is the belief in the ignorance of experts,”
If we took previous claims and experiments as gospel, science would never advance again …
Second, I note that once again you have not brought up a single argument against a single one of my claims. Instead, you are indulging yourself in argumentum ad verecundiam, which was recognized as a logical error a couple of millennia ago.
Look, I made a bunch of very clear claims in that post. If you think one of them is wrong, then quote it and tell us why. Because simply mumbling a bunch of scientists’ names as though they were a magical incantation doesn’t work here at WUWT.
Regards,
w.
Martin Mason
November 22, 2017 1:12 am
Willis, with all due respect it’s absolutely up to you to prove that the steel greenhouse theory is correct. If the work done by Wood was in isolation then I’d agree but it seems that the same conclusion is arrived by many others since. There are some things that are worth taking the effort doing but arguing about the steel greenhouse on here definitely isn’t one of them because of the fixed positions that all parties exhibit and the fact that when it comes down to it we are like minded on the issues. With all due respect Willis (and that is sincere), you have a very good brain but your positions on all issues are set in concrete.
Willis, with all due respect it’s absolutely up to you to prove that the steel greenhouse theory is correct.
Martin, with all due respect, you absolutely don’t understand how science works. There is no way in science to “prove” that something is correct, nor do scientist try to do so. Science works like this:
Someone puts up a scientific claim, complete with all of the data, math, observations, and the like that support the claim.
Then, other scientists try to poke holes in the claim, showing that the math is wrong or the logic doesn’t work or in any other manner.
If other scientists cannot poke holes in the claim, then it is accepted as scientifically valid … until such time as it is overthrown.
Note that this process is not about proof. It is about falsification.
Next, you claim that my positions are set in concrete. That’s madness. When I’m shown to be wrong I’m the first to admit it, although like anyone I don’t like it. I’m one of the few bloggers with posts titled things like “Wrong Again” and “Wrong Again, Again“. People had shown that I was wrong, and I not only admitted it, I wrote a whole post about it. And then a few years later it happened again, and I did the same.
So I totally reject your claim. As the man said, if the facts change, I change my opinion …
Anyhow, here is your opportunity to show everyone just how wrong I am and how right you are. Point out to us just which of my claims about the steel greenhouse is incorrect in any way. If you are right, I’ll be the first to say so.
Or you can just give us some flimsy excuse about why you don’t want to do that. Be forewarned, however, that at this point, and after the claims and untrue accusations you’ve made, and after your implication that you already know of some faults in my steel greenhouse analysis, at this point if you do not tell us just exactly what is wrong with my steel greenhouse claims, I’m going to assume that it’s because you can’t find one single thing wrong with it …
On the other hand, if you do want to dispute something I wrote in that post, please quote the exact words of my claim that you are disputing so we can all understand exactly where you think the error is.
Willis, I don’t mean prove in a legal basis only by repeatable and observational basis such as gravity can be. Should I say validated beyond reasonable doubt? Heating by cold downwelling radiation hasn’t passed that test yet, does it have a falsification test?
The tone of your response is the reason why I don’t want to get into a discussion. I used the wrong description perhaps in saying that your positions are set in concrete but you don’t communicate well with those who may have a different position. 🙂
Heating by cold downwelling radiation hasn’t passed that test yet, does it have a falsification test?
SB is well tested, it has not been falsified.
Heat never flows from cold to warm, but energy sure does. And SB describes energy flow between objects both hot and cold.
You can easily prove all solid objects radiate, whether hot or cold with an ir thermometer.
Martin, your position is not supported by over 100 years of evidence.
Frankly, “destruction” at Climate of Sophistry is a vote in favor of Willis’ theory.
PS, the error in the page you linked to starts with “The shell’s surface would emit on its interior as well, however, internal emission by the shell will always meet another interior side of the shell (or the sphere), and hence will not leave the shell.”
Up until this point, the the shell and the sphere were treated as two separate “systems” that can exchange energy. Suddenly the shell is not allowed to transfer energy to the sphere.
This error gets manifest in equation 3b. When the sphere is inside the shell, then the “universal ambient-temperature environment ” for the sphere is no longer T_0, but now is T_sh. With these corrections in understanding and math, the correct answer (Willis’ answer) pops up. Any physics or engineering professor will agree with Willis.
No, it’s demolished. And all your concerns, or even anything else you might be paid to invent, are all well and truly addressed. Done and dusted, dusted and done. That’s that!
Willis, I don’t mean prove in a legal basis only by repeatable and observational basis such as gravity can be. Should I say validated beyond reasonable doubt? Heating by cold downwelling radiation hasn’t passed that test yet, does it have a falsification test?
You still don’t understand. Science is not a process of “validation”, it is a process of falsification.
As to whether “heating by cold downwelling radiation” exists, the two-way model of radiation exchange is so well accepted that it is in all the thermo texts I’ve seen. There’s a good online calculator here that contains the actual equation used to calculate the effect. You might profitably spend some time studying that equation.
Sure, you could falsify it, all it would take is some thermometers and some radiation measuring instruments and you could falsify it in one day. Or you could just walk outside, measure the downwelling radiation, and consider the following question:
Since energy is neither created nor destroyed, only converted to a different form, if that downwelling radiation is not leaving the object it strikes warmer than it would be without the radiation … then what is happening to the absorbed radiative energy? What is it converted to if not thermal energy? It’s not converted to light, or chemical action, or motion …
The tone of your response is the reason why I don’t want to get into a discussion. I used the wrong description perhaps in saying that your positions are set in concrete but you don’t communicate well with those who may have a different position. 🙂
Oh, I see. Your excuse for not showing us the errors that you claim to have found in my work is that you don’t like my “tone” … let me quote what I said before:
Or you can just give us some flimsy excuse about why you don’t want to do that.
My “tone”? That’s hilarious. Come back when you care enough about science to get your hands dirty. Science is a blood sport, where people do their level best to destroy some other person’s treasured and precious ideas and claims. It’s not some appeal to your feelz. Nobody likes it when their scientific sacred ox is gored, whether it’s done gracefully as a ballet or not …
I deal with folks whose tone I don’t like all day long regarding scientific questions and answers, including your tone in this very discussion. Get over it. Either you can find errors in my work or not, and so far all I’ve seen is handwaving.
Like I said, it’s now time to either bet or fold …
No, it’s demolished. And all your concerns, or even anything else you might be paid to invent, are all well and truly addressed. Done and dusted, dusted and done. That’s that!
Oh, Tony, you are practicing my favorite kind of science … science by vehement assertion including exclamation marks and meaningless repetition of blanket statements. That’ll convince ’em!
Read tj’s objections again. They are clear and to the point. If you disagree with them, quote the one(s) you disagree with and tell us why they are wrong.
Tony, have you ever critically read that post? Let’s start with: “If the sphere does produce any power then its temperature will rise above T0 [the ambient temperature of the environment surrounding the sphere], and its energy production would then be given by
1b) Psp = 4π Rsp^2 σ(Tsp^4 – To^4)”
So for example, if the radius of the sphere were 0.282 m (ie an area of 1 m^2), then the power from the sphere to the surroundings would be
* 240 W/m^2 if Tsp = 255.1 K and To = 0
* 480 W/m^2 if Tsp = 303.3 K and To = 0
* 240 W/m^2 if Tsp = 255.1 K and To = 0
This is the standard physics of radiation transfer (assuming blackbody surfaces, of course). Do you accept this?
Silly little Willy. Read Postma’s articles, which refute the Steel Greenhouse conjecture, and stop practicing science by vehement assertion. It’s OK that you’ve lost.
Oops, that should have been:
* 240 W/m^2 if Tsp = 255.1 K and To = 0
* 480 W/m^2 if Tsp = 303.3 K and To = 0 * 240 W/m^2 if Tsp = 303.3 K and To = 255.1
Tim starts his process…all too familiar. Sorry Tim, it’s done. You’ll have to find another means of employment. You’ll be OK. Politics awaits…off you pop.
“Tim starts his process … ” — of using basic, established equations from physics to figure out how the universe shold work.
Meanwhile, Tony starts his process of avoiding equations and tough questions – instead using the very tactic he accuses others of: “practicing science by vehement assertion.”
Who’s tactic? Lol. Have a catch up and get back to me. You seem stressed…not like you to make so many mistakes. Have a couple of days holiday, you’ve earned them. They must have been paying you overtime these last few weeks. So many articles recently you have to comment on! Poor you.
We all get it Tony. You fear to respond meaningfully to basic questions about science so you deflect.
It is really very simple — assuming you are willing to make a good faith effort to defend your positions. Once again, if a sphere of radius 0.282 m (1m^2 surface area) is surrounded by an environment at an ambient temperature To, are the following accurate for the power from the sphere. (Again, remember — the equation came from your own link). Let me add one more for fun:
* 240 W if Tsp = 255.1 K and To = 0 K
* 480 W if Tsp = 303.3 K and To = 0 K
* 240 W if Tsp = 303.3 K and To = 255.1 K
* 240 W if Tsp = 282.3 K and To = 214.5 K
This is merely to establish that we can both do the calculations and can both agree that the SB law is valid here.
Ha ha, don’t be silly Tim. You don’t control the conversation, though I know you wished you did. But wishing something doesn’t make it so. Unfortunately, Postma refuted the Steel Greenhouse conjecture. You’ll just have to come up with something else to try to dupe your betters.
I am not “controlling the conversation — i am exactly following the conversion in your own link! And yet even agreeing with you is somehow too dangerous for you to engage with!
“I was just linking to the refutation” … and I was just pointing out the glaring errors in that rather feeble refutation. Sorry if you thought that some second-rate blog is actually the last word in science.
Tony, you talk about “my religion” and yet all I have done is try to discuss equations THAT COME FROM YOUR OWN LINK! That fact that you are not willing to engage in scientific discussions indicated clearly who is basing their position on faith.
I have absolutely no idea how you are going to pretend this up for discussion, but I understand that due to your profession, you are unable to refrain from responding. Amusing for me, though. Don’t let it get you down. You’ll find something else.
“SB is well tested, it has not been falsified.
Heat never flows from cold to warm, but energy sure does. And SB describes energy flow between objects both hot and cold.
You can easily prove all solid objects radiate, whether hot or cold with an ir thermometer.”
A very useful difference you brought up,that too many trip over.
“Heat never flows from cold to warm, but energy sure does”
When I first studied thermodynamics (at MIT), the professor insisted we spend the first several weeks rigorously defining systems and subsystems, and carefully tallying the energy flows in and out of each subsystem, plus the system as a whole, in doing energy balance calculations.
I grew impatient with this, as I was eager to get on to fun subjects like turbochargers and cogeneration, but I am very glad he did this, because it has kept me from making a fool of myself like the author of your linked post does.
Tim spotted a few of his key mistakes, such as when considering the shell as a subsystem, not counting emissions from one side of the shell as energy output from the subsystem. This is the type of mistake a weak student makes in the first weeks of an introductory undergraduate course. But anyone who wants to pass the course needs to stop making that kind of mistake.
It is one thing for an 18-year-old student to make that mistake on an early problem set. It is another thing completely for someone claiming professional expertise to make so basic an error, and still not be able to see it after it has been pointed out to him multiple times.
I note with amusement that you have not even attempted any kind of substantive argument.
I don’t think you’re capable of it.
Ed, where is your substantive argument? I’ve invited people to quote the exact words I wrote that they think is wrong. That’s the start of a substantive argument, when you clearly define what it is that you object to. Then you say just exactly what you think is wrong with those specific words, why they are not true.
So … you are welcome to quote whatever it was that I said in the Steel Greenhouse that you think is wrong, and tell us why you think it is wrong. Please don’t bother pointing to the arguments of others, I am interested in YOUR OPINION, not that of some random blogger who is not here to defend his claims.
Or not, you could do what the rest have done and come up with some feeble excuse why you don’t want to put your money where your mouth is … up to you.
That’s right. I haven’t made an argument and have no intention to. As you should have read and understood since it was made pretty clear in earlier comments. There is nothing Tim said which isn’t answered in the articles at CoS or the comments in the articles, and you’ve added nothing to what Tim said.
You guys are losing your cool, I get it, your religion is collapsing in on itself all around you. It must be scary. I try to sympathise, amidst the chuckles. Don’t worry though, your skills are transferable.
Now you say you can’t “prove” anything, but previously you claimed that you and Dr. Brown had shown “proof” of the correctness of your position on this question.
I am not disagreeing with anything YOU have said.Tony pointed to the ridiculous post at CoS that tried to refute your “steel greenhouse” analysis.
I specifically pointed out a key error made at CoS (which Tim also pointed out even before me) in analyzing the “shell” subsystem — namely the mistake that radiation from the inside of the shell does not “count” as an energy output from that shell.
Tony, on the other hand, has not made a single substantive point in all of this posts.
WIllis,
It is YOU who does not understand how science operates. The steps of the scientific method are generally as follows:
1. Define a question
2. Gather information and resources (observe)
3. Form an explanatory hypothesis
4. Test the hypothesis by performing an experiment and collecting data in a reproducible manner
5. Analyze the data
6. Interpret the data and draw conclusions that serve as a starting point for new hypothesis
7. Publish results
8. Retest (frequently done by other scientists)
You are at step 3. You have not performed any real science so far, so there is nothing to rebut, since all you have is a silly steel greenhouse thought experiment.
Anthony, thanks for hosting Rod Gill’s essay, the nearly 800 posts (with no screaming, SHOUTING or name calling) discussing this fundamental but complex subject, are testament to the interest & wealth of knowledge on this site.
May I suggest that you add to the Atmospheric page another sub-heading of ‘GHG/Radiative Gases’ where this thread & related info can be easily found (particularly by someone new to the site or unaware of the obtuse subject names & refs they need to look for).
Jorge Oliveira
November 22, 2017 3:39 am
Is it legitimate to apply the Stefan-Boltzman law to the Earth’s surface – what happens, for instance, in the Trenberth diagram – when this surface is covered by an (active) atmosphere some kilometres thick ?
Shouldn’t it be more accurate to displace the relevant surface a few kilometres out of the Earth’s surface ? If so, what distance should be appropriate ?
Blair Macdonald
November 22, 2017 4:13 am
Blair Macdonald
November 22, 2017 4:46 am
From first principles I offer my hypothesis of the atmosphere: I am currently writing up my discoveries.
1) The below diagram shows the complete (augmented) IR atmosphere; thermoelectric and Raman, with all the quantum mechanics (QM) predicted vibration modes.
2) The said ‘special’ GHGs (CO2 etc) are really only the thermo-electric gases (TEGs): first discovered by John Tyndall in 1859 using thermo-electric thermopile transducers. Here’s where the misconception begun – he interpreted them to be absorbs when they were really transducing an electromotive force (volts). N2 and O2 will not generate electricity at any temperature.
3) QM predicts N2 and O2 at 1556 and 2338 respectively; these Raman (non ‘electric’ dipole) modes can only be observed with modern (late 20th Century) laser dependent Raman spectrometers. Raman also detects CO2’s and CH4’s non TE (IR) modes.
Raman and Thermo-electric (IR) can both determine temperature of molecules and thus are not only complementary, but also equivalent: the temperature of O2 N2 H2O CO2 .. can all be determined from Raman alone as can the concentrations of the gases. The Keeling curve can be determined by Raman Spectroscopy
4) From the above: Raman spectroscopy is an instrument of choice on solar system space probes. It is also used in monitoring the Earths atmosphere – by means of Raman Lidar.
5) From the above it is implied O2 and N2 radiate IR: they emit and absorb as by the Boltzmann constant. A molecule of N2 in the thermosphere is 2500K – excited only from radiation. This temperature is measured from Raman Lidar.
6) In a CO2 laser, N2’s 2338cm mode is radiated by electron collisions (equivalent to photons) to ‘pump’ the CO2s 2349cm mode. If this did not happen, the CO2 laser would not operate and no cosmetic surgery.
Conclusion
N2 and O2 are GHGs – the whole atmosphere consists of GHGs, no special ones. It is the instruments we measure them with that makes them special.
Note: Raman spectroscopy is an instrument to detect vibrational spectra and should not be confused with the Raman effect – this is not my claim.
Yes N2 and O2 have Raman active vibrations (which are not IR active) and so can be detected if excited by a focussed laser beam, what possible relevance does this have to the atmosphere?
Everything: it ends the climate debate.
There are two sides to my theory: Tyndall discovered thermo-electric gases (what we know as the GHGs); and Raman shows what and why he didn’t measure, N2 and O2. Raman is a very good thermometer.
In the 21st Century, to understand IR behaviour of matter we use two instruments: Raman and thermoelectric-IR (thermoelectric, my words cause that is what they are). We do the same in the atmosphere, Raman is used already there, even NASA uses it; I am bringing them together, to solve the problem.
The standard model of GH theory can only hold with O2 and N2 are non-GHGs; that they are benign, that they are ‘forced’ by collision from the GHGs. With Raman spectroscopy we see they are IR radiation active – if a the molecule is radiated, the temperature will rise in accordance with the Stefan Boltzmann equation, Raman can measure that temperature of N2 and O2. In fact, it may even that N2 excites/ heats CO2 directly through its 2338/2349cm-1 close modes (my claim). Figure that?
How could we think anything else; the air is a near perfect insulator, it has a thermal conduction value of near 0 (0.024 – no units); air has to radiate on those grounds alone; not to mention that if it didn’t, it would contradict QM and thermal dynamics where all matter above absolute 0K radiates.
I am not creating anything new; only putting what we know together.
Lets do experiments and test. Actually, I have found the experiments; it holds.
Blair Macdonald November 22, 2017 at 12:39 pm
Everything: it ends the climate debate.
In your dreams, Raman spectroscopy is a technique for detecting certain species, only a very small proportion of the population undergo the transitions and usually a strongly focussed light source is needed and sophisticated filtering techniques to separate the dominant elastically scattered light. It’s certainly not an effect which is capable of transferring significant amounts of energy in the atmosphere.
The standard model of GH theory can only hold with O2 and N2 are non-GHGs; that they are benign, that they are ‘forced’ by collision from the GHGs. With Raman spectroscopy we see they are IR radiation active
No we do not we see that the vibrational modes of N2 and O2 are not IR active but are Raman active (something I was taught as an undergrad many years ago, so it’s nothing new). Which is exactly what the ‘standard theory’ accounts for, N2 and O2 can’t participate in absorbing the Ir emitted by the earth.
Argon is interesting; it does not transduce electricity from its IR radiation and it does not seem to have a clear Raman active mode or spectra – it must have. I’ll keep hunting that one.
Blair Macdonald November 23, 2017 at 4:48 am
Argon is interesting; it does not transduce electricity from its IR radiation and it does not seem to have a clear Raman active mode or spectra – it must have. I’ll keep hunting that one.
Yet more misunderstanding by you of Raman spectroscopy, Argon doesn’t have vibrational energy levels, neither IR active nor Raman active so don’t waste your time hunting.
Martin Mason
November 22, 2017 6:08 am
Very interesting Blair but what excites the non GHG molecules. What does it mean in real life?
Blair Macdonald
November 22, 2017 12:08 pm
“..what excites the non GHG molecules” They, N2 and O2( and H2 I believe?) are excited by the Sun, by photons, just as we understand QM radiation, nothing new there. It is just to say N2 and O2 have spectra lines predicted, and they are observed (with Raman) and they behave just as they should when excited; all in accordance, I would add to say, as the Stefan Boltzmann law.
What does it mean for real life? It updates the 150 year old, pre quantum understanding of the atmosphere. GH theory is updated, there are no special GHGs, but only GHGs – after that the 0th and 1st laws follow.
We cannot have the out standing pillar of science, QM, in contradiction over the non GHGs: they must radiate – and they do. Everything with temperature, and spectra lines, radiates – all and in compliance with Boltzmann’s constant. Else we have a catastrophe – what I term ‘the IR catastrophe’.
Blair, dividing gases into “GHGs” and “non-GHGs” is not intended to imply that non-GHGs don’t absorb at all. It is simply intended to sort into two broad categories — those that absorb well and those that absorb poorly. (much like materials are often divided into “conductors” and “insulators”, even though good conductors have some resistance, and good insulators have some conductance.)
The point is that a GHG like CO2 absorbs orders of magnitude more IR than a non-GHG like N2. Unless you are trying to predict IR intensities to within a few ppm, you can ignore N2 completely when dealing with atmospheric IR.
tjfolkerts No, N2 and O2 do not by GH theory emit or radiate any IR and not ‘poorly’, at any temperature – check the definitions. This is a catastrophe: all matter radiates.
You are relying on thermoelectrics as you paradigm of thinking: if we used Raman spectroscopy alone (as shown in the diagram) we would get a different set of gases; but they would be equivalent to the ‘GHG’s but for N2 and O2 and some others. The Raman gases all have (by early 20th Century quantum experiments – namely the Franck Hertz experiment) spectra lines, they all vibrate, and they have temperature.
As a thought experiment: take all the said GHGs out of the atmosphere leaving only N2 O2; would the air temperature change during the day change? Yes – just as it does today, and it would by radiation, and not conduction as N2 and O2 (the air) are near perfect thermal insulators.
Another thing, by current greenhouse reasoning; glass is a greenhouse solid: it is transparent to the eye, and ‘opaque’ to the IR – just like CO2. Why is there no issue with glass?
Water is – for the same reasons – a greenhouse liquid.
tjfolkerts No, N2 and O2 do not by GH theory emit or radiate any IR and not ‘poorly’, at any temperature – check the definitions.
We don’t have to “check the definitions” to know this is 100% wrong. The absorption bands for 02 are shown in the head post graphic … read’m and weep …
This is a catastrophe: all matter radiates.
While this is widely believed, it is not completely true. Monatomic gases (helium, neon, argon, etc.) neither absorb nor emit thermal radiation.
The only “catastrophe” is that you don’t seem to understand what you are talking about … or more accurately, the “catastrophe” is that you are firmly convinced you understand what you are talking about …
While I appreciate the passion with which you hold your views, that doesn’t make them correct …
“No, N2 and O2 do not by GH theory emit or radiate any IR”
This is not any key tenet of GH theory. All that is required is that
1) sunlight can get in easily and
2) “earthlight” cannot get out easily.
It turns out that for (2) (the blocking of IR)
* H20 is the most important gas
* CO2 is the next most important gas
* polyatomic molecules like CH4, O3, and NO2 are also fairly important.
* monatomic and diatomic molecules like Ar, N2 and O2 contribute almost nothing.
GH theory would still work if N2 or Ar absorbed significant amounts of IR — but experimentally they don’t. GH theory still works when N2 absorbs some IR. The key factor here is that even small changes in CO2 (from 300 ppm to 400 ppm) STILL has a MUCH greater effect than the entire 780,000 ppm of N2. So N2 can be pretty much ignored when doing calculations. (Or — you could include N2 and get an answer that differs by an imperceptible amount).
“You are relying on thermoelectrics as you paradigm of thinking “
By this, it seems you are referring to gases that cause a noticeable change in temperature when IR passes through (ie thermoelectric detectors that create voltages in response to changes in temperature show a signal). In this case, this is EXACTLY the sort of change we are interested in. If N2 happens to show some weak absorption using something like Raman spectroscopy but DOESN’T register when you are measuring actual temperature change due to actual IR, then — almost by definition — N2 is not contributing (in a measurable way) to the GH effect.
Blair Macdonald
November 22, 2017 11:55 pm
tjfolkerts ‘..dividing gases into “GHGs” and “non-GHGs” is not intended to imply that non-GHGs don’t absorb at all.’
That is not how it is read or interpreted, check any definition of a GHG or n – GHG and it reads clear, ‘N2 and O2 do not absorb or emit IR’.
The rest of your comment is all based on readings from thermoelectric transducers: then you have go back and again break it down into ‘first principles’; how does the device work that you have come to your conclusion with – it is all by thermoelectrics. You are just repeating the standard model, and it just does not stack up.
Watch my basic/nervous presentation I did a few years ago here: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
We must lose the IR from IR spectroscopy: and term it TE spectroscopy and the TE gases – ThermoElectric spectroscopy and gases.
Here’s a question: what if it was only Raman devices we had to measure the IR atmosphere, and not thermo electric (just as we do for Mars): what would the known GHGs be? They would be every gas (if I haven’t missed any non Raman gases), as all gases are Raman active (I’m pretty sure). They would be Raman active and equivalent to thermoelectric ‘IR’ through the equipartition principle.
What I have here is just the ‘tip of the iceberg’, and the truth shocking: what I have found is all radiation theory (black body, emissivity and the like) done by Kirchhoff, Stefan, Boltzmann and others is all based on 19th Century thermoelectric detectors/transducers – totally neglecting QM and laser Raman observations and measurements. It will be my life’s work – with help hopefully – to clear this up.
Blair, statements in science like “N2 and O2 do not absorb or emit IR” often mean “do not absorb or emit in sufficient amount to be significant”. So I might “Pyrex is an insulator and does not conduct electricity” — and most people would go along with that. But I can look up the resistivity (http://glassfab.com/wp-content/uploads/2015/08/Corning-Pyrex.pdf) and find that it is about 10^8 Ohm*cm. This makes it an extremely good insulator, but it does (at some infinitesimal level) conduct.
” check any definition of a GHG “
Well, wikipedia (the first hit when I searched) disagrees with you. “Hence they [non GHGs] are almost totally unaffected by infrared radiation. — Wikipedia
If this is your entire thesis (‘non-GHGs do actually absorb some small amount of IR’) then you are barking up the wrong tree.
Blair Macdonald
November 23, 2017 5:40 am
I need help: anyone reading my claims and and are interested and would like to support me, financially, or otherwise, I need it. I am doing this on my own, I have a paper nearing completion, and it now needs the best minds – I’ve done my best. The pay-off: the greatest upset in scientific history. Message me through my blog and otherwise.
Also, Anthony: this is the area of movement, of thinking, 870 in three days! This is first principles, and this is where the debate is, and where it will be settled, and where GH theory as we know it will collapse. Let it be aired: better out than in.
I need help: anyone reading my claims and and are interested and would like to support me, financially, or otherwise, I need it. I am doing this on my own, I have a paper nearing completion, and it now needs the best minds – I’ve done my best. The pay-off: the greatest upset in scientific history.
Whew, that’s a relief. I was afraid that your astoundingly brilliant breakthrough would only qualify as the second greatest upset in scientific history …
I was also curious as to who is the current record holder for the “greatest upset in scientific history” … curiously, the Guinness Book of World Records doesn’t list that one.
I’m glad, however, that all your fantastic scientific successes haven’t given you a swelled head.
Thanks, Gary. It appears that like many others, you don’t have the albondigas to point to one single statement in my post about the Steel Greenhouse and tell us what’s wrong with it. Not one.
No surprise there, lots of folks on this thread have more mouth than they have facts or brains … come back when you actually want to discuss the science, I’ll be happy to do it.
I do not have adequate qualifications to argue your steel greenhouse but I would like to ask a few layman’s questions if you don’t mind.
Do you believe in the Trenbeth diagram, ie do you believe that there’s around 333w/m2 of downward LWIR?
What value do you put on the equivalent Solar energy hitting the surface?
Why can the Solar Energy be made to perform work and the DWLWR can’t?
Yeah right Willis, remember this, ‘“That’s just a rounding error in the model”. when your model violated conservation of energy.
You had it it explained to you over and over, Joe may as well have been talking to a plank.
Further up the comments you scolded a commentator for taking offence to your forth-right attitude, that made made me chuckle remembering how you whined and whined about Joseph’s dismissive attitude to your ”citizen science”.
Why don’t you grow a set and finish the debate ?.
As for the rest here, the GHE fraud is on its knees you need to start re-aliening to the no GHE line because Anthony Spencer et al subtly are, and have been for months.
The 2nd law violation of energy from cold environs being unable to increase temperature in warm environs is the end of climatism and the climatists, once the blue/red team exercises into the science of CO2 get underway., they will schooled.
Quite a lot of enraged ranting and insults at first, but Postma does eventually get to a straightforward walk through of the main errors. Then there are 937 comments of further discussion on the matter. Yes, all objections you could pretty much imagine have been answered. Then there’s the most recent three articles at CoS as well to wrap it up, and the comments there too.
Hope that helps. Don’t expect anything from Willy, there will never be an admission of error on this matter, I doubt. He’s best just ignored at this stage.
Don’t expect anything from Willy, there will never be an admission of error on this matter, I doubt.
When I’m in a discussion and someone starts playing grade-school snarky games with my name, I know I’ve won. The only reason a man starts throwing mud like that is because he’s out of real ammunition …
I’ve invited Tony to point out here on this thread one single statement I made in the Steel Greenhouse that he thinks is wrong.
Instead of taking his chance and showing us how he can prove me wrong, instead of putting his brilliant claims out where they can be examined by all and judged fairly, he plays name games and wants me to talk about some mysterious discussion from years ago …
… and apparently, he actually mistakes that for winning the discussion.
Amazing. Ah, well, I suppose I should remember, DFTT.
Yeah right Willis, remember this, ‘“That’s just a rounding error in the model”. when your model violated conservation of energy.
You had it it explained to you over and over, Joe may as well have been talking to a plank.
Further up the comments you scolded a commentator for taking offence to your forth-right attitude, that made made me chuckle remembering how you whined and whined about Joseph’s dismissive attitude to your ”citizen science”.
Why don’t you grow a set and finish the debate ?.
Who is “Joe”, and what on earth are you babbling about?
As for the rest here, the GHE fraud is on its knees you need to start re-aliening to the no GHE line because Anthony Spencer et al subtly are, and have been for months.
And what is “re-aliening”, the only alien I see here is you.
The 2nd law violation of energy from cold environs being unable to increase temperature in warm environs is the end of climatism and the climatists, once the blue/red team exercises into the science of CO2 get underway., they will schooled.
“They will schooled”??? Don’t you want to buy a verb for that sentence?
All I can get from your cryptic message is:
1. You definitely need to cool down. None of this is worth getting your blood all angrified.
2. You don’t understand the difference between heat flows and energy flows. I’m writing a post on the subject right now, because you are far from the only one who doesn’t get it. Short version? Heat can’t flow from cold to hot … but radiated energy sure can.
3. You have a lot of trouble communicating clearly.
Gary, I’m more than happy to discuss any objections you might have to my post on the Steel Greenhouse. I will say that physicists that I admire, people who teach physics for a living, say there is no violation of the Second Law involved.
You seem to believe that thousands of scientists in dozens of countries are either ignoring an egregious Second Law violation in the greenhouse effect, or they are simply too stupid to see it … but you, you can see it …
I could give you the odds on that being true … but you’d probably just yell at me some more …
So to clarify, Tony supports this statement, taken directly from his site.
“Heat flow, which is typically denoted as “Q”, is produced by the difference in energy emission between two objects. If we wish that positive Q(sp-sh) means heat flow from the sphere to the shell, then the heat flow equations are
So if we use this equation for a sphere a radius Rsp = 1m and temperature Tsp = 300K inside a larger, cooler shell of radius Rsh = 2m and temperature Tsp = 290 K, we get
Q(sp-sh) = 5770 W – 20160 W = -14390 W
As stated above, a positive Q(sp-sh) means heat flow from the sphere to the shell, so this negative result predicts a heat flow the other way — from the shell to the sphere. Yes, heat flows from the cooler shell to the warmer sphere according to these esteemed scientists.
So, like I say, any and all objections are covered at CoS, in the articles and comments. So that’s that. Poor old Willy, and poor old Tim. But what’s done is done,
“Why can the Solar Energy be made to perform work and the DWLWR can’t?”
The main reason is that solar energy arrives as a near-parallel beam and can be focussed. So you can get a very high temperature relative to terrestrial sinks.
DWLWIR is diffuse, and can’t be focussed. The only way it could be made to do work is if a sink colder than where it comes from could be found.
That doesn’t mean that DWLWIR doesn’t add heat to the surface it falls on. It just means that that surface must be fully exposed to the source region, and so loses more heat than it gains. So no work. But it doesn’t lose as much heat as it would have if exposed to space.
So do tell, Tony! How was this objection handled? Which comments justify an equation that clearly predict heat flowing from large, cool objects to small warm object?
Tim, you are referring to equation 4a from the article at CoS that Gary linked to (as did I, further up in a different discussion). All anyone needs to do is read through that article in its entirety to see where you’ve “gone wrong”. Your “misunderstanding” would be amusing if I thought it wasn’t deliberate. However, I’m aware that you have indeed read the entire article, so it’s unlikely you would make that mistake genuinely. As it is, I suspect it’s simply an attempt to bait me into a discussion whereupon your usual deception can take place.
It’s funny, you and Willy are so alike. You just approach it from two different angles. Willy plays the game of insisting everyone has to discuss things under the terms of his original article. “Quote my exact words! Tell me where I’m wrong!”
Then when somebody does just that, and indeed writes a whole series of articles about it, responding to a huge variety of different objections and “misunderstandings” in the comments below, Willy can deflect from any of that by simply insisting that anyone talking to him now has to go through the whole thing again. The whole discussion must be had again! Lol. Meanwhile, people like Tim are on hand to take a snippet from that discussion completely out of context, in an attempt to bait people into once again repeating the whole discussion…
“Tim, you are referring to equation 4a “
Yes.
Exactly.
The equation that says heat flows from large cool objects to small hotter objects. It’s right there for all the world to see. No ‘context’ can fix that sort of blatant error.
So you are going to continue to be deliberately obtuse?
Well, let’s take the bait. If nothing else, your responses will serve to demonstrate the folly of entering into further discussion, with folk such as yourself…
When Q=0 (i.e at equilibrium), equation 5a shows the temperature of the shell (Tsh) in relation to the temperature of the sphere (Tsp), and the radius of both sphere and shell. From this equation it’s clear that the temperature of the sphere is always going to be higher than the shell, at equilibrium. This is because the shell is necessarily larger in size than the sphere, and will therefore (possessing a larger surface area) always emit more energy in total than does the sphere, if they were at the same temperature, all else being equal. Energy is conserved, not temperature.
You have “arbitrarily” chosen a value for the temperature of the shell which would not be reached spontaneously were the shell introduced at 0 K, when the sphere is at 300 K (as you also specify). Equation 5a shows that with the sphere at 300 K and with the radius of the sphere and the shell as you specify, the shell would equilibrate at a lower temperature than 290 K. So, for the shell to be at 290 K, it would require a source of additional (external) energy besides what it receives from the sphere. Which is why you get the result you do when you insert those temperature and radius numbers into equation 4a.
You know, for practical purposes you can consider a light bulb as a physical implementation of the steel sphere.
The filament isn’t a sphere, but being mostly 2d I think it serves the purpose of a sphere for an example that can be tested.
“When Q=0 (i.e at equilibrium) …
… and this is the next error. (Not that it fixes anything in the previous error– it was derived as a general result and still predicts heat from large cool shells to small warm spheres.)
When Q=0, then there is no heat flowing from the sphere to the shell. But the discussion starts by positing “a power-generating sphere”. We are told that power goes IN to the sphere, but now we are asked to accept that no power goes OUT from the sphere. If the sphere has power in but no power out, it must be warming. But a warming sphere by definition is not at equilibrium.
The errors just keep cascading into more and more contradictions — contradictions not only with every thermodynamics textbook, but internal contradictions within the so-called ‘proof’ itself.
“we are told that power goes IN to the sphere, but now we are asked to accept that no power goes OUT from the sphere”
Yes, great demonstration, Tim. As soon as your (no doubt deliberate) error is exposed you immediately change tack onto something completely different…something you just absolutely fabricate out of thin air. I’m glad I took the bait, I knew you would immediately show your true colours. It’s like I said before, though, you’ll be OK. Politics beckons.
Rob G
November 23, 2017 12:18 pm
This is not for any fights, but I am surprised that after four years when I came back to this site, I still see the same old skeptical discussions essentially on whether or not humans are playing a role in climate change, or even more basic, whether CO2 is a greenhouse gas that even most skeptics of AGW don’t question. On the other side, countries like China and India are well ahead of their targets set by Paris agreement even without the US. Although they had some setbacks from nuclear issues, Germany is well on its way to have almost all of their energy from renewable sources. US on the other hand is going back, making knowledgeable people around the world laugh at the US’s ideologically based skepticism coming mainly from those with little training and no real credentials in the area – which seems to start with the new EPA head.
Yes, water vapor has a major role, but excess water vapor (determine by pressure, temperature) will condense. CO2 doesn’t do that at earth’s temperatures, hence serve as the cause for temperature change. Most scientsts from Arrhenius onwards (from 1895) accepted CO2’s greenhouse capability.
When was the last time Earth’s atm didn’t have any water vapor? But besides that, you didn’t understand the significance of net radiation dropping by over 60% in the middle of the night. Same thing scientists since Arrhenius have all missed.
micro6500
November 23, 2017 at 12:51 pm: You are darn right there. Latent heat, half the density of air, massive lifting power. Well-measured, non GH mechanism. The arch warmista rely on water to get them over the line because they cannot claim CO2 has the oomph. Little realising water was doing it all anyway, without any GH. And plenty of capacity to spare…..
Small wonder deserts are hotter than jungles, and the Models have failed. They do after all use GH factors
micro6500, “When was the last time Earth’s atm didn’t have any water vapor? But besides that, you didn’t understand the significance of net radiation dropping by over 60% in the middle of the night. Same thing scientists since Arrhenius have all missed.” I don’t know what any of that has to do with the effect of CO2? With or without CO2 change, there will be greenhouse effect from water. Increasing temperature from increased CO2 will increase water vapor in the atm that in turn will further heat the globe – as a simple explanation. The reality is more complcated but the effect is the same. I am not sure what you mean by the radiation drop, such variations are there with or without water vapor in atmosphere like in deserts. In any case, we don’t have much control over water vapor and even if we all change to hydrogen fuel giving water vapor as the reaction product, unlike CO2 this is not going to change anything since there are limits how much water atmosphere can carry. Excess water will precipitate. So I am not sure what you are suggesting. All scientists account for the effect of water vapor since it is significant.
No, Micro6500, I said it is more complcated, there are both positive and negative effects and that is why I did not want to go into the details of it. But when you publish your work showing “….not co2, and in fact any additional warming from Co2 has to be lost to space, before the change to the slower cooling rate….” in a real journal with statistical data, I will certainly pay more attention.
Did you see that Min T follows dew point temp with a 97% correlation, over 75 million station records.
And my code is published for you can validate my work
You see, my work is published there, everything anyone needs to confirm my work. It’s just not to many ppl understand the signals in a regulator. If they did, they would see that’s what happens at night under clear skies. Can you explain why the temp stops dropping in the middle of the night, while T zenith is still 90F colder than aur temps? But you can’t, or won’t because you know it ends AGW concerns. As a regulating mechanism, water vapor has well over 10x the power co2 has, we will never burn enough fossil fuels to alter temps with co2, the condensing water vapor never runs out!
Well… Micro6500, I would strongly encourage you to send your work to a journal. If it is important, people who don’t read blogs (this is the first time I came here in five or six years, that goes for all other climate related blogs) needs to read it, and if there are problems with your work, the reviewers will hopefully address that. Everyone knows that water vapor has a substantial effect, especially because there is more of that than CO2. But most researchers will object to your other characterization, that CO2 effect is somehow negated by water vapor, or calling them fools.
Then you do not note that net radiation drops significantly while it’s still dark out? If you’ve looked at night time temp data you’ve seen it before. That how I found it, 4am clear skies, and the temp isn’t dropping, and yet my IR thermometer says the sky is -40° or -50°F.
Radiatively, that’s about what the optical window sees from the surface. It has to be dumping 40 or 50W/m^2.
Why doesn’t the temp keep dropping?
And what air temp does it stop near?
I don’t expect there are any amount if changes I could make in writing a paper, that would get it accepted.
It’s available on the internet, and a lot of ppl read WUWT, and Climate Etc, so I know a lot of climate scientists have seen it.
Did you see that Min T follows dew point temp with a 97% correlation, over 75 million station records.
And my code is published for you can validate my work
You see, my work is published there, everything anyone needs to confirm my work.
Thanks, Micro. The problem with that 97% correlation is that it is expected, since dewpoint is a function inter alia of temperature. There are various ways to calculate dewpoint from temperature and relative humidity. Here’s one from the Bulletin of the American Meteorological Society:
Td = T – ((100 – RH)/5.)
where Td is dewpoint, T is temperature, and RH is relative humidity.
Now, relative humidity is not too useful to us here, because it changes with temperature. But RH is a function of the absolute humidity, the actual amount of water in the air.
Now, what this says is that Td, the dewpoint temperature, is a function of T, the temperature, and AH, the amount of water in the air. However, the actual amount of water in the air doesn’t change all that fast, particularly when temperatures are dropping. And this means that if the amount of water in the air doesn’t change, that dewpoint is a function solely of temperature, and that the correlation will be best at minimum temperature.
Finally, whenever a falling temperature causes the dew to form, the dewpoint is equal to the temperature. So of course they vary in tandem, as the temperature drops below dewpoint they both move downwards in lockstep.
Given all of that, we would absolutely expect a very good correlation between Td and T, simply because Td is a function of T and AH, and AH changes slowly.
So while your finding is true … it is also exactly what we’d expect to find. And as a result, it’s not at all clear what use that might be. How does finding something that we’d expect to find advance our knowledge?
Willis, there is an energy barrier as air temps drop to dew point. That forces the release of stored energy, the water has to release it to condense. What RH really means, is the percentage of water vapor condensing is a vol of air increases, it has to be giving up energy, and QM requires that distribution. So some is droplets, most is still gas, but as it cools to dew point that ratio changes.
It’s all that latent energy radiating from the condensing atm water vapor that’s supplying the surface energy that compensates for energy going out the optical window.
Just like you found water regulates ssts by breaking out into cooling thunderstorms, thus process kicks in only after the air has cooled, then there’s a large increase in IR, latent heat.
That’s how it stops cooling in the middle of the night
Or
And everyone just thought it has reached equilibrium, but in reality it’s quite active, and it’s being regulated.
Willis,
Since you didn’t respond, I’m not sure if we are done yet. Did you check out the paper I linked yet?
And why doesn’t air temp drop below dew point(rhetorical)? There’s at least 35W/m^2 to space even when temps are not falling at all in the data collected there.
Can we agree there’s an energy barrier, ie that more radiation has to be lost to maintain an equal temp drop once air temps are at dew point temp?
That by definition is a non-linear function.
Given all of that, we would absolutely expect a very good correlation between Td and T, simply because Td is a function of T and AH, and AH changes slowly.
And conversely this is proof Tmin is a function of dew point, not co2.
It also is a negative feedback to water vapor amplification that is a requirement for catastrophe.
In fact it is non-linear enough to remove most of the energy accumulated by co2 before morning mist nights.
Where there’s enough water vapor to work with. Why deserts have such a large range, dew point is low, max T is high.
Willis,
Since you didn’t respond, I’m not sure if we are done yet. Did you check out the paper I linked yet?
And why doesn’t air temp drop below dew point(rhetorical)? There’s at least 35W/m^2 to space even when temps are not falling at all in the data collected there.
Sorry, Mike, I’m on the road right now. I haven’t answered because I’m not clear exactly what your point is about dew point and min temp. You seem to think it is significant that min temp and dew point are often either close or the same … which is true but I don’t see the relevance.
There’s an energy barrier to air temps dropping below dew point, because most of the cooling is radiative, and when it radiates, half or more runs into water or co2 and is captured. The higher % of the water
“The higher % of the water” that has cooled to dew point, but for every photon it can emit, it captures another photon.
You’re exchanging money analogy, thus us putting some in the bank during the day, and withdrawals it only to keep air temps from falling too much below dew point.
What I can’t explain to people is it acts like a switching power supply regulating min T, which is normal climate that you explained. It’s just they are dependant functions, consuming about 10x the energy of co2, and it’s regulated by temp, it doesn’t slow cooling until after it gets near dew point, the same fixed point whether Tmax was 75, or 76. If you look at the rate temp changes at dusk, it’s 3-4°F/hr, some clear nights it stops cooling, others it slows. On the slow nights, if temps are warmer, say 1° as my example, the transition switches at the same absolute temp in either case. In effect, all you do is exchange cooling that 1° at the high cooling rate, for the time required to drop 1°, for that time at the slowest cooling before sunrise. And on nights that’s 0, well all the excess is lost, ie min T is entirely dependent on water vapor, co2 had zero affect.
“Germany breaks renewables record with coal and nuclear power responsible for only 15% of country’s total energy” Link posted above … there are many other links one can look into.
No, Gabro: It says “At one point on the sunny and breezy Sunday, sustainable energy from wind, solar, biomass and hydro power provided a record 85 per cent of the country’s total energy.” There are not there yet, but with proper conditions they are already close.
No, Gabro: It says “At one point on the sunny and breezy Sunday, sustainable energy from wind, solar, biomass and hydro power provided a record 85 per cent of the country’s total energy.” There are not there yet, but with proper conditions they are already close.
You guys are talking at cross purposes, and both of you are right.
Rob, you have the curious idea that because on a Sunday when no industry is running and it is sunny and breezy so there is no need for either heating or air conditioning, for ONE INSTANT renewables were providing 85% of the power, that that has some meaning. But all it means that the demand was really, really low, and the sun plus wind was at its highest.
However, Andy is showing the long-term reality, which is that sun plus wind in Germany generates 3.3% of the energy used.
After billions of dollars have been spent on solar and wind in Germany … THREE POINT THREE PERCENT.
Now it’s clear that you are both right. Average of wind and solar is 3.3% over the whole year. On the other hand, on one day when conditions were perfect, demand at its lowest, wind and solar at their highest, for one instant they got 85% … but so what?
Rob, do you make business decisions based on a best case ever scenario, or an average scenario?
Because me, when I want to judge how something is doing, I would NEVER just consider how she sails on a good day with a following wind … I want to see what the long-term situation is.
So while both of you are right, I know which numbers I pay attention to, and it is not how it did on the best day of the year. Hyping that kind of data is a cheap salesman’s trick …
W., Germany is still not there, but they are still investing and they have a plan to get the average energy supply from renewable sources high. On your question “Rob, do you make business decisions based on a best case ever scenario, or an average scenario?” Germany had already made that decision, but they made that based on the average anticipated power generation, not current average power supply, from such sources. The advantage here is, apart from the capital investments (which is required for any power plants) there are no expenses for buying the sources of energy (like coal, natural gas, uranium, etc.) except for biogas. Maintainance expenses are there for all plants. So overall it works out much cheaper to have renewable sources, and it is a wise business decision for consumers and corporations.
W., Germany is still not there, but they are still investing and they have a plan to get the average energy supply from renewable sources high.
Indeed they do have a plan, and after spending billions and billions of dollars on the plan, solar plus wind supply 3.3% of their energy. Look, even the Germans admit that their “energiewende” has been a colossal and expensive failure that has made their electricity prices skyrocket …
So yes, they have a plan, and they are now in the process of abandoning that plan as an expensive failure.
w.
PS—You describe 3.3% of energy coming from solar and wind as “still not there” … a more accurate description would be “still barely begun”.
W. That link is one person’s opinion. I know there are some issues with energy conversion in Germany, even NY Times reported that, but it is not a failure and there are no plans of abandoning it. We will see what happens in the next 10 years. I predict you will see substantially more solar and wind based energy production in Germany in that time. CO2 is harmful or not is one side of the story, when solar panels are becoming more efficient with falling cost, it simply becomes a better/cheaper route. Fusion energy is still an iffy solution for the growing energy needs. Availabilty of fossil fuel will only last so long, whether it is 36 years or 100 years. So alternate energy sources are going to come up, and as calculators kicked slide rules out, these new sources are eventually going to make fossil fuel obsolate. Even with friendly policies for coal, coal power plants in the US are still shutting down because of other reasons, and that trend will continue. https://www.nytimes.com/2017/10/07/business/energy-environment/german-renewable-energy.html
No, W., the basis for everything they wrote there comes from various reports, which one can verify, and I have not seen anyone offering data to counter it.
No, W., the basis for everything they wrote there comes from various reports, which one can verify, and I have not seen anyone offering data to counter it.
Rob, you are welcome to believe the NYT. I don’t trust a word they say. And yes, they always base what they claim on “various reports” … me, I prefer news organizations that base their work on “various facts“. Their reporting was so bad during the last election that the Editor famously APOLOGIZED for it.
Of course, the apology was as fake as their “news”, they just kept doing the same thing … no surprise there.
In addition, the article is not available, I’ve used up my two free views for the month.
So you’ll have to argue with yourself on this one. Inter alia, however, I would note that China didn’t agree to much at Paris except to have their CO2 emissions peak by 2030 … and if you believe that will happen, you’re beyond my poor power to add or detract …
I do not have adequate qualifications to argue your steel greenhouse but I would like to ask a few layman’s questions if you don’t mind.
Do you believe in the Trenbeth diagram, ie do you believe that there’s around 333w/m2 of downward LWIR?
What value do you put on the equivalent Solar energy hitting the surface?
Why can the Solar Energy be made to perform work and the DWLWR can’t?
Excellent questions all.
Regarding downward longwave infrared (LWIR), this is routinely measured by scientists all around the world. You can measure it yourself (with admittedly poor accuracy) with an infrared standoff thermometer. The CERES satellite data puts it at about 345 W/m2 on a global 24/7 average.
Again per ceres, solar hitting the surface is on the order of a 186 W/m2. However, about 24 W/m2 is reflected by the surface.
Finally, both solar and LW can be made to do work. Any heat can be used in a heat engine. However, for any heat engine to work, you need to discharge the rejected heat into a cooler environment … and the atmosphere, in general, is cooler than the surface—so there is nowhere on the surface to discharge the waste heat.
What about photovoltaics? The problem is that there is not enough energy in thermal radiation to bust loose an electron and kick it up to a higher orbital, as required for the photoelectric effect … so no photovoltaics as far as anyone has found so far.
Best regards, and if you have further questions I’m happy to discuss them.
Well the question is now that Solar can be used for work on the surface as demonstrated by any Solar Still or Solar Array like Ivanpah.
Ivanpah Generates almost 1000Gw per year according to Wiki by collecting, focusing and using the miserely 186W/m2 and yet it can’t Generate anything from the 345W/m2 of LWIR.
So why is that?
Aren’t all Watts created equal?
A. C. Osborn, another good question. The answer is, solar stills and solar arrays are not driven by average solar energy. They are driven by instantaneous solar energy,
At the tropics at noon, that’s over a thousand watts per square metre … and at 38°N where I live, peak solar is on the order of 750 W/m2.
Bear in mind that 30°C (86°F) equates to a S-B blackbody radiation of 480 W/m2 … so the sun is much stronger than ambient. That’s why the sun can do useful work at the surface, while the radiation from the cold atmosphere cannot.
Not exactly, it’s delivered at near 1,000W,m^2, Not 186, and whatever the lwir amount is, it’s not different from the exhaust, other that radiative cooling to space, and it too would need concentrated. But Ivanpah doesn’t run at night because when you look up it’s cold, not hot.
Sorry, that won,t quite do as an answer, because even if it was 900W you should still be able to get 1/3 of the energy by concentrating 300W of DWLIR.
With a simple 3″ hyperbolic reflector I can raise temperature of a piece of steel to 450C in UK and yet a Solar still extracts the heat from an object at night, cooling it to around -5C.
So why can’t we heat anything by concentrating 300+ W/m2, but instead it cools it?
These experiments have been done countless times with the same results.
My response was to Willis, not you micro.
But perhaps you can answer the question of why a Solar Still or Oven becomes a Refrigerator at night?
I have come to understand that we do not understand energy as much as some like to think, for instance why hot objects cool quicker than cooler objects.
Or why a surface with an LED light shining on it Frost’s over quicker than an area of it shaded from that same light.
1000s of degrees Sun!
You are having a laugh.
Watts are Watts, that is how ghgs work isn’t it?
So you are confirming that all Watts are NOT EQUAL, thank you.
“Ivanpah Generates almost 1000Gw per year according to Wiki by collecting, focusing and using the miserely 186W/m2 and yet it can’t Generate anything from the 345W/m2 of LWIR.
So why is that?
Aren’t all Watts created equal?”
AC, Let me expand on what Willis said (here and before). The word “focusing” is critical.
The 100’s of W/m^2 of sunlight comes in the form of a very intense beam from a small part of the sky. With a lens or mirror, you can focus in sunlight from other directions, multiplying the total to many 1000’s of W/m^2. Basically, you can make it look like there are thousands of suns all around.
The 100’s of W/m^2 of thermal IR comes in the form of a very diffuse glow from every part of the sky. As such it is impossible to focus that IR. If you put up a mirror to reflect IR, you are blocking an equal amount of IR that was already coming from that direction.
Wrong, you can focus it, that is precisely what a Solar still does and it makes objects at the focal point colder.
Let me provide you with a thought, suppose it makes them colder because it is coming from a colder place?
Can you prove that to be wrong?
“Aren’t all Watts created equal?”
Of course they aren’t. That’s what the second law of thermo is about. There is energy (Watts), which is conserved, and associated free energy (Watts), which can be dissipated (or used). Free energy is the capability of doing work. The Watts of electricity that you might use to warm your house are valuable. The warmth they are converted to has much reduced utility.
Thank you, you have just blown GHG theory out of the water, because obviously DWLIR Watts are not equal to REAL watts either.
Watts that can’t do any work are worthless.
AC I am confused by what you mean by a “solar still”. Google searches reveal devices designed to distill water by warming it. Perhaps you mean something akin to this idea: https://en.wikipedia.org/wiki/Radiative_cooling#Nocturnal_ice_making This is the same reason front can form on the tops of cars parked outside, even when the air temperature stays above freezing. I have seen this principle extended to roof-top panels.
If this is what you mean, then yes, some “focusing” is possible. in this case, “warm” IR from the warm ground (or roof) below is blocked and “cool” IR from the sky is substituted.
Sorry, that won,t quite do as an answer, because even if it was 900W you should still be able to get 1/3 of the energy by concentrating 300W of DWLIR.
With a simple 3″ hyperbolic reflector I can raise temperature of a piece of steel to 450C in UK and yet a Solar still extracts the heat from an object at night, cooling it to around -5C.
So why can’t we heat anything by concentrating 300+ W/m2, but instead it cools it?
I’ve never heard of a way to do that. The problem is that unlike sunlight, longwave IR doesn’t arrive at the surface as a beam of radiation. You can focus and concentrate and reflect a beam of radiation.
But the longwave infrared is coming from the entire hemispherical dome of the sky, in all directions. I know of no way to focus or concentrate that kind of radiation
These experiments have been done countless times with the same results.
In the headline to this post is the following question:- “what’s the quality of your radiation?”
Now here’s a thing. When I was young in the 1960s and before the political parasites had debased the currency, it used to be possible to collect bronze pennies from the reign of Queen Victoria. The oldest coin in my collection was one dated 1860, faint, worn and of no numismatic value, but it was fascinating to me that something minted one hundred years previously was still in circulation. My coin set was a lesson in the past of my country and gave a perspective of history that no modern child can experience.
My historical coin collection was also an economics lesson, silver sixpences minted before 1920 really were just that, sterling coins containing 92.5% silver, while none of the sixpence coins minted after 1946 contained any silver at all (how the mighty were fallen). But the key point I learned from my collection was this, no matter how many bronze pennies I collected, and no matter how large the sum became, none of these pennies ever turned into a silver sixpence.
Now will someone please explain to me how “cold” low quality, low frequency electromagnetic radiation can spontaneously turn into “hot” high quality, high frequency rays? The Wein’s displacement law proves that this is impossible. In order to raise temperature of a surface you need to add high frequencies, adding more low frequency energy, no matter how much, never works. You can never turn bronze pennies into silver sixpences and certainly never bright silver into glowing gold.
Good to introduce discussion on the ‘quality’ of the energy
I think that in all quoted instances of this kind the source of energy is still pumping away.
An example would be an electric light bulb.
Without shading it would glow at a certain temperature
With a reflecting partial covering it would glow brighter or more ‘blue’
Now one interpretation of this favoured by warmists is that reflected low quality energy is converted to a higher quality energy.
The way I would interpret this is that additional radiative resistance is introduced by the reflective covering reducing the energy loss of the bulb.
To continue with your ‘coin’ metaphor electrical energy is the ‘gold ‘ standard.
“You can never turn bronze pennies into silver sixpences and certainly never bright silver into glowing gold.”
Electrical energy is of the highest quality and can be used to top up the higher frequencies.
With no electric power however.
“You can never turn bronze pennies into silver sixpences and certainly never bright silver into glowing gold.”
Holds true
Well expressed! CO2 can only emit (and absorb)in the waveband 13 to 17 microns, according to MODTRAN6. These are line spectra, and if you spread them out somehow into a continuous black body Planck spectrum, such as Earth produces, the warmest “Wien” temperature you could get out of the mix would be -79 degrees C, corresponding to the most intense line at 14.95 microns, and this is way below normal Earth surface temperatures. Cooler objects (here, CO2) can’t transfer heat to warmer ones (here, Earth’s surface), so the radiative argument for a greenhouse effect for CO2 on Earth is nonsense. In any case, gases actually derive their temperature much more from from pressure than from radiation, and that applies to ALL atmospheric gases, not just CO2, and even here, due to the lapse rate, atmospheric CO2 is never warmer than Earth’s surface. CO2 radiation can retard Earth’s infrared output (that of 13 microns wavelength and above), slowing heat loss, but heat addition can only come from Sun or from ozone creation/destruction reactions in the stratosphere.
These are line spectra, and if you spread them out somehow into a continuous black body Planck spectrum, such as Earth produces, the warmest “Wien” temperature you could get out of the mix would be -79 degrees C, corresponding to the most intense line at 14.95 microns, and this is way below normal Earth surface temperatures. Cooler objects (here, CO2) can’t transfer heat to warmer ones (here, Earth’s surface), so the radiative argument for a greenhouse effect for CO2 on Earth is nonsense.
What you leave out is if absorbed, energy is energy, and if there are more photons, there’s more energy. So your assumption is incorrect, co2 can warm the surface, it just takes more 15u photons to do so.
And if you look at the y axis of a atm spectum, it’s in energy, not photon count.
micro6500, that’s an older and outmoded way of looking at things. In reality, EMR doesn’t contain photons at all, but is simply a frequency field devoid of energy. Photons arise when the energy field impinges on matter, resonantly inducing increased bond vibrations at fundamental or harmonic frequencies.The increased motion of bonded atoms generates kinetic energy, and this is what Einstein correctly interpreted as “Lichtquanten” (photons), except that he made the mistake of thinking that the Lichtquanten came in with the EMR instead of being resonantly generated on the spot (resonant vibrations in frictionless bonds require no energy input, but Einstein couldn’t have known this in 1905).
Energy is really a property of matter, and in every case except that of the supposed radiation-borne photon, its dimensions contain, or can be converted to contain, a mass term. We know that EMR is massless and that it travels at light speed, neither of which would be possible if it contained energy. All our detection devices are material, so we can only detect photons after they’ve interacted with matter. We can’t detect them in massless contexts such as outer space. All energy is mass-bound, and therefore contains a mass term, which is far more reasonable than a situation in which all energy is mass-bound except for the kind that goes whizzing through space like some protean chimaera and is somehow mass-independent.
Be that as it may, any back-radiation from CO2 must be in the range 13 to 17 microns. These wavelengths would correspond to Wien temperatures of -51 and -103 degrees C if they were in a continuous Planck distribution, and their most intense line is 14.95 microns, corresponding to a Wien temperature of -79 degrees C, all temperatures well below those characteristic of Earth’s surface, in consequence of which they can’t transfer heat to the warmer surface of Earth but are reflected away instead.
I think it can, reduce cooling by about 3.7W/m^2, but that’s not what really slows cooling, that’s from water vapor.
And you can see it in net radiation.
And if you look at the cooling curve, it’s also not the expected exp decay.
Why should it keep cooling? Because the sky is 40 or 50 below F.
Sure. Top chart just has Temp, Rel humidity, and net radiation, covers about 3 days, and in general they were mostly clear, the temps change smoothly except in the afternoon. You can see how temps fall at night, and once rh gets over about 50%, you can see a bend in the temp curve, and at the same time net radiation drops. This was recorded by the group that wrote the paper I mentioned to Willis, in Australia.
As air cools, and nears dew point more water has to condense, but there a lot of energy that water vapor has to release before it can do that. And is a gas like this, as it does, some of that energy is given to water that just condensed, causing it to reevaporate.
The second was taken by me in Ohio, you can see the distinctive cooling rate slow down under clear dark skies, and in the upper section you see IR thermometer readings from the same night showing the BB temp for the optical window to be about -50°F.
SB equations show about 80W/m^2 for that difference in temp. I think the optical window is about 40% of that, so 32W/m^2 Is radiating to space, and temps are not changing.
Net rad can drop because the window closes, or more energy comes from the surface.
And the evidence is in more energy, and that aligns with water vapor having to dump energy to cool.
Sure. Top chart just has Temp, Rel humidity, and net radiation, covers about 3 days, and in general they were mostly clear, the temps change smoothly except in the afternoon. You can see how temps fall at night, and once rh gets over about 50%, you can see a bend in the temp curve, and at the same time net radiation drops. This was recorded by the group that wrote the paper I mentioned to Willis, in Australia.
As air cools, and nears dew point more water has to condense, but there a lot of energy that water vapor has to release before it can do that. And is a gas like this, as it does, some of that energy is given to water that just condensed, causing it to reevaporate.
The second was taken by me in Ohio, you can see the distinctive cooling rate slow down under clear dark skies, and in the upper section you see IR thermometer readings from the same night showing the BB temp for the optical window to be about -50°F.
SB equations show about 80W/m^2 for that difference in temp. I think the optical window is about 40% of that, so 32W/m^2 Is radiating to space, and temps are not changing.
Net rad can drop because the window closes, or more energy comes from the surface.
And the evidence is in more energy, and that aligns with water vapor having to dump energy to cool.
micro6500, I definitely think you’re on to something here. I’ll have to spend some time with your blog to appreciate it fully, but the importance of H2O in radiative exchange has been sorely neglected, IMHO.
Be that as it may, any back-radiation from CO2 must be in the range 13 to 17 microns. These wavelengths would correspond to Wien temperatures of -51 and -103 degrees C if they were in a continuous Planck distribution, and their most intense line is 14.95 microns, corresponding to a Wien temperature of -79 degrees C, all temperatures well below those characteristic of Earth’s surface, in consequence of which they can’t transfer heat to the warmer surface of Earth but are reflected away instead.
So what, application of a nonexistent ‘inverse Wien’s Law’ means nothing. The surface of the earth quite happily absorbs 15 micron light whether it originates from T=400K or T=230K, in fact the surface is not aware of the source temperature.
“Now will someone please explain to me how “cold” low quality, low frequency electromagnetic radiation can spontaneously turn into “hot” high quality, high frequency rays?”
They don’t. No one claims it does.
“In order to raise temperature of a surface you need to add high frequencies, adding more low frequency energy, no matter how much, never works.”
Tell that to people who use CO2 lasers to melt steel. You need to be much more precise in your thinking, since a simple couter-example shows your throught process is faulty here.
So tjf, if I said that water cannot run uphill and you said yes it can, here is a pump storage scheme that proves that it can, would I not be correct in questioning your faulty thinking? After all is a laser not a precisely designed machine that need to consume power in order to work?
Phillip, you specifically claimed “adding more low frequency energy, no matter how much, never works [to raise temperatures]”. Now you seem to have reversed your position, accepting that lots of low frequency energy (ie 15 um photons) can indeed raise temperatures to quite high level. This simply shows how challenging all this can be so state ideas precisely and accurately.
Certainly it is impossible to use thermal photons from a source at T1 (and only those photons) to raise an object to a temperature T2 where T2 > T1. But those photons from the source at T1 PLUS other power inputs can raise the temperature above T1.
Philip Mulholland November 24, 2017 at 10:30 am
So tjf, if I said that water cannot run uphill and you said yes it can, here is a pump storage scheme that proves that it can, would I not be correct in questioning your faulty thinking? After all is a laser not a precisely designed machine that need to consume power in order to work?
It’s your faulty thinking which tries to apply a nonexistent ‘inverse Wien’s Law’, your logic is akin to saying red hot steel is at 650ºC, therefore the rose of the same color in your garden must also be at 650ºC.
Now will someone please explain to me how “cold” low quality, low frequency electromagnetic radiation can spontaneously turn into “hot” high quality, high frequency rays? The Wein’s displacement law proves that this is impossible. In order to raise temperature of a surface you need to add high frequencies, adding more low frequency energy, no matter how much, never works.
This is based on the false use of Wien’s Law, which proves no such thing. Wien’s law just tells you at what wavelength the peak of the distribution will be, however both higher and lower wavelengths will be present.
Using an ‘inverse Wien’s Law’ does not work, you can not take the wavelength and infer what temperature it must have been emitted at. This is a common mistake which many who have posted in this thread have made!
An example of their error is that they assume that via Wien’s Law a 10.6 micron photon originated at 273K and therefore cannot raise the temperature of a 300K object, and yet a laser beam of 10.6 micron photons can melt steel. The ability to heat an object is not related to the frequency of the light (other than via the reflectivity of the object).
micro6500 November 27, 2017 at 8:50 am
“Using an ‘inverse Wien’s Law’ does not work, you can not take the wavelength and infer what temperature it must have been emitted at.”
No, but IMO it is a useful measure of energy and potential work that can be extracted.
You’re welcome to your opinion but I see no basis for it.
Martin Mason
November 24, 2017 6:58 am
tj, that is what flummoxed me but surely lasers have a heat input and work by amplifying and focusing low energy light into high energy. I don’t believe that it’s an example of cold transferring heat to warm.
Martin Mason
November 24, 2017 7:44 am
Another question that I’ve never had a sensible answer to is that if there is a downwelling radiation which has the “energy” to significantly raise the temperature of the planet, why can’t we recover what is apparently a real and significant heat? One answer I had was that it is low grade energy and that is surely the point
I have just asked Willis that very question up post, Inanpah and other Solar collectors convert it to heat and electricity and yet they do not convert the higher wattage lwr at all.
Watts Up With That then?
Brett Keane
November 24, 2017 11:15 am
tj is an inveterate troll of the sort who can produce formulae and seeming facts at will which, on inspection, always turn out to be deliberately misleading. We know him of old…..
Brett Keane
November 24, 2017 11:32 am
tj deceptively leaves out that CO2 does not laser anything. His tone displays his intent. I t can respond to large voltage differences, and transfer that to another gas (Argon IIRC). This gas can produce stimulated emissions at steel-cutting power..
I am not disagreeing with anything (yet). But I would like to make a few comments before all and sundry jump in:
Photon s are NOT real massless particles. The concept of a photon is a human construct from our mind in an attempt to observe the apparent “lumpiness” of certain phenomena with electro-magnetic radiation.
E-M Radiation CANNOT be directly observed, you can only observe the effect on real matter, and we know real matter is “lumpy” (particles). It MAY be the case that e-m radiation is not in any way “quantised” but is purely analogue and only the effects seem to suggest quantisation hence the concept of the photon.
Try, if possible, making your arguments work BOTH for photons (quantised e-m lumps) and also for e-m which is not quantised. You may need 2 completely different arguments. I suggest the results of doing this will (or may be) illuminating (pun intended).
My final comment is that the understanding of this, i.e. RADIATION, as it applies in the real world is THE most important thing we need to grasp in considering CAGW. and if we cannot grasp it correctly and securely by logical argument then let’s have some suggestions from you for REAL WORLD EXPERIMENTS to settle the issue(s).
The Reverend Badger – ” The concept of a photon is a human construct”
Exactly!
The concept of Photons represent half of a model. The duality model includes the ‘wave’. And when Earth’s release of heat is envisioned as a wave, the entire ‘trapping heat’ theory collapses.
Please don’t get into wave-particle duality. It’s a minefield. Your language is very engineer like rather than physical but seems to express the theory of the quantum model well. The problem with the AGW radiation theory from non physicists is that they are unable to separate quantum from classical physics. If you think in terms of energy rather than orbitals Neil Bohrs, it actually gets easier to explain what you have explained well here. The absorption energies are derived from the degrees of freedom within the molecule. CO² has two. Hence the 10 and 15µ absorption bands. However, I like your engineers approach. It’s good.
last time I counted it had 4 sym, asym and a degenerate pair of benders
Gerontius – I thought a “degenerate pair of benders” were what we called Friday and Saturday nights at the frat house. This clearly has nothing to do with climate science!
rip
IMO photons, like other elementary particles, are objectively real physical phenomena.
We have models of them, but that doesn’t mean that they don’t actually exist.
O..Kay… Lets test your belief a little shall we. These elementary particles you call photons have a frequency (or wavelength) do they not? Can you tell me the frequency range please over which they exist. Do we have
60Hz/50Hz photons emanating from our power lines for example? Is there an upper frequency limit to them?
For further thinking please research the origin of the word/concept.
Of course I can tell you the frequency and wavelengths of photons.
The energy E, frequency f, and wavelength λ of a photon are related thus:
E=hf=hc/λ,
where c is the speed of light and h is Planck’s constant. So, given any one term, the other two can readily be calculated.
This will give you an idea of the range of possible energies, wavelengths and frequencies of photons:

PS: Physics is fascinating and fun. You really ought to study it sometime.
An object that instantly accelerates back to the speed of light when it leaves a variant local spacetime without additional energy *doesn’t exist*. There’s the rub. Its neither a wave NOR a particle. They have an infinite energy to mass ratio that is never reduced but have a quantifiable amount of energy. That means there’s nothing in them, not just “mass-less” but literally nothing. They no more exist than an electron exists outside it’s orbit.
Prj,
Massless particles have zero rest mass. Their relativistic mass is simply their relativistic energy, divided by c^2, or mrel = E/c^2. (Can’t make the “rel” a subscript.)
The energy for photons is E = hf, where h is Planck’s constant and f is the photon frequency. This frequency and thus the relativistic energy are frame-dependent, a point I’ve been trying to make with LdB.
If an observer runs away from a photon in the direction the photon travels from a source, and it catches up with the observer, he or see will see it as having less energy than it had at the source. The faster the observer is traveling with regard to the source when the photon catches up, the less energy the photon has. As an observer approaches the speed of light with regard to the source, the photon looks redder and redder, by relativistic Doppler effect, and the energy of a very long-wavelength photon approaches zero. This is because photon is massless—the rest mass of a photon is zero.
But, again, in motion it has relativistic mass.
In one of the Teaching Company’s Great Courses video series, the presenter talked about being asked the question “How big are photons?” during his thesis defense. He eventually replied that it depended on the wavelength. The proof of this is the pierced plate in the window of the microwave door. Photons of visible light fit through the holes and microwave photons (based on the holes in metal plates that they pass through) are disks within the uncertainty limits of a diameter of ten centimeters.
Richard Bell November 20, 2017 at 1:00 pm
That’s a neat demonstration. Thanks.
Are you aware what your eyes are for ??
At this point I started to realise that the self-taught quantum physics was a little shaky. Despite numerous technical errors like “degrees Kelvin” ( the unit is kelvin ) ; writing Km for kilometres which shows a lack of basic understanding of the unit prefices; talking of “the” wavelength of thermally emitted IR when he should be referring to PEAK wavelength. I rather gave up hope on this article having any scientific merit at this point.
No one talks of the “altitude” of an electron orbit, they are not little Spuniks. Neither is the energy involved in photon absorption or emission the “kinetic” energy of the atomic electrons, it is potential energy which is transferred. Hence the idea of energy levels and dropping to a lower energy level producing the energy of the photon. The author also seems to be confusing electron states and the vibrational energy of the atoms within a molecule.
The IR interaction comes from vibrational energy of the three atoms in gages like CO2 and H2O ( a bit like a tuning fork ) . This is why diatomic molecules like O2 and N2 are not GHGs.
IR radiation only travels a few metres at ground level without being absorbed, so lets forget the notion that this only happens at a very narrow range of altitude around 90km.
I did not see much point in finishing the article.
I see where you are going with that… but my eyeballs are real matter…
The Reverend Badger November 19, 2017 at 2:51 pm
Photons exist independently of your organs for detecting them, such as eyes and skin. But those organs wouldn’t have evolved were photons of visible and IR light not real.
Consider the process of pair production. A pair of “matter” particles (fermions) is produced by a pair of photons.
Because of momentum conservation laws, a pair of fermions can’t be created from a single photon. However, these laws permit matter creation when in the presence of another particle (another boson, or even a fermion) which can share the primary photon’s momentum. Thus, matter can be created out of two photons.
Greg,
I agree. It was almost like a test to see who could recognize garbage fiziks.
Greg,
As a general rule, most EM radiation cannot be observed directly. A limited region, between about 400 and 700 microns wavelength, can be perceived by human eyes and is generally referred to as “Light.”
Clyde Spencer November 19, 2017 at 4:18 pm
Our skin feels IR light and burns under UV rays. For higher and lower energy photons, we have instruments to detect them.
Sorry, Rev, but photons and EM radiation are the real deal meal.
Rev Bad,
Or consider the photoelectric effect, which is based upon the observation that EM radiation consists of a series of particles, ie photons. When a photon hits an electron on a metal surface, the electron can be emitted. The emitted electrons are called photoelectrons.
If photons don’t account for the PE, how do you imagine it works?
WUWT is an equal opportunity science “d@nial” site, except for “Sl@yers”. Those who d@ny gravity in favor of an imaginary “electric universe” are welcome here, along with those who d@ny EM radiation. Even creationists are permitted to comment, but not those skeptical of the GHE. As are Younger Dryas impact hypothesis proponents.
Full disclosure, IMO the GHE exists, but is negligible for CO2 beyond about 200 ppm. For the health of plants and the planet, 1200 ppm is about optimum. Evolution is a fact, as are gravity and EM radiation, while a YD impact is anti-scientific fantasy.
The “expanding earth” is yet more fantastic and more anti-scientific. I’m sorry. Continents move. Get over it.
Rev Bad,
What on your planet makes X ray photographs?
How does radar work?
How do you receive cell phone, TV and radio signals?
Thanks.
Yes, this article made me cringe and face-palm. Talk about totally getting the basics wrong.
I have to agree.
With all due respect, this article is cringeworthy regarding physics, and for some reason ignores the terminology thereof.
I concur…My BS meter went off immediately when it it looked like Rod Gill was trying to ‘manufacture’ a reason why there is no warming from CO2 (or presumably H2O for that matter) instead of perhaps hypothesizing how a negative feedback might make any warming a moot point. Why is it so hard for some here to rationalize that yes, there is a tiny weensy bit of warming from GHG’s? Wouldn’t it be better to make the argument that a bit of warming is good, and the additional CO2 is good for the Garden? I fear that arguments like this are very damaging to the skeptic cause, since then the alarmists just point at all of us and say we are very mistaken. We can’t win this battle about the notion that CO2 is a pollutant until we get real about basic atmospheric physics.
‘Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.’
The closer to the nucleus?
The behavior of electrons in atoms is still open to debate. But an electron “orbital” is a conflation of the prior molecular orbital theory and quantum dynamics to explain how electrons govern chemical reactions(and other things) in quantum mechanics. I believe the current theory is that orbitals are not a physical difference but an energy level. Atoms that absorb a photon and if it of the right frequency it increases the energy of some of the electrons. The physical size of the atom doesn’t change, but some of the electrons have more energy and the frequency associated with those electrons- E= h * f, the Planck constant times frequency.
Molecules, such as CO2, can also absorb energy as motion of the atoms in the molecule. This occurs at longer times and wavelengths. In CO2 the CO bonds can stretch in and out like a spring, both O atoms can move closer and further from each other(bend) and they wave, or twist the molecule around the plane of the O-C-O bonds along with the whole molecule rotating rather than just moving.
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit.” Not really. Temperature is a measure the the kinetic energy in matter. Radiation emittance and absorption is function of the energy within molecules or individual atoms and photons. A CO2 molecule can absorb a photon at any temperature and increase its energy without necessarily moving faster. But it can both absorb and transfer kinetic and internal energy from collisions and absorption to other molecules. The ozone reactions in the stratosphere are a good example. An ozone molecule can absorb a photon and split into an O atom and an O2 molecule. The O atom can end up with enough energy to react with another O2 molecule, rather than simply colliding and bouncing off at a slightly higher temperature.
All this stuff is very complicated and it really can’t be dealt with in words. Rod Gil’s essay is a good attempt to do physics with words and it doesn’t really work. CO2 can absorb and emit infrared photons but how the energy from those photons behaves needs math and physics models. CO2 is a trace gas in the atmosphere and doesn’t do much. The only major effect it has is through poor models of how the atmosphere reacts to energy from the sun that have a huge increased water vapor bias built into them. They model AN atmosphere, but not the atmosphere we have.
If we don’t do physics with words, how do we do physics? Math but no verbal component?
Exactly. Changing the total energy via having electrons in a higher orbital (a statistic) is not the same thing has having them move faster in 3 dimension translation, or in 3 dimension rotation, or in 3 dimension stretch along an axis, or in 3 dimension stretch perpendicular to an axis.
For light to heat something, the additional energy must be converted to kinetic energy increasing the total (and note, this is only possible to measure in a defined sample). For light to cool something, the loss of energy must result in a decrease in the kinetic energy. Energy that goes into phase changes that don’t change the kinetic energy can’t change the temperature.
Then we must remember that absorbers are emitters and that there is always incoming EM radiation. Even at night, there is still incoming long wave EM radiation. Otherwise, IR, microwave radio and long wave radio astronomy would not be possible. IR active gases (and aerosols) are two way screens. They screen some of the incoming and screen some of the outgoing. They can’t add extra energy. They can only time shift some of the energy.
This is actually pretty funny. We debate the supposedly critical problem of global warming and invoke the most fundamental basis of our understanding of particle physics in describing the issue. Nobody really understands the true nature of the underlying universe.
I kind of favour pilot wave theory but hey, that’s just me.
The funny thing is that they say the science is settled. A few years ago all the bright young physicists were in love with string theory. They stalled out and some other genius physicist called it, “not even wrong”. Surely one of the best putdowns ever.
And that’s physics! A whole lot brainier field than the climate swamp. They don’t even know what 90% of the universe is made of!
“They don’t even know what 90% of the universe is made of!”
Den!er! Dark matter is made of carbon dioxide. Human emitted carbon dioxide. Dark energy? That’s made out of human emitted carbon dioxide too. Pulsars, magnetars and quasars are also anthropogenic in origin.
String theory is just an idea Alan Guth thought of off of the top of his head one day.
The odds of it turning out to be an actual description of objective reality would be less than guessing the winning numbers in the Mega Millions lottery and the Powerball lottery with only two guesses. Not astronomical odds, but cosmological ones. IMO
And the 90% that is dark matter and dark energy?
These are ad hoc inventions because of some things that do not make sense according to current understanding of the four forces, such as the anomalous rotation rates of galaxies and inconsistencies in the red shift of distant Type A supernovae.
They create more questions than they answer.
Correction…Alan Guth…cosmic inflation, not string theory.
>>
. . . inconsistencies in the red shift of distant Type A supernovae.
<<
It’s Type Ia supernovae. If Type ia supernovae are standard candles (like it is believed), then they point to a discrepancy in the expansion rate of the Universe–that is, the Universe expansion rate is speeding up.
Jim
Yes, I should know better than to comment off the cuff in the middle of the night.
I know the whole story, recall reading the research reports when they were first made public.
Maybe the expansion of the Universe is accelerating, and the invention of dark energy is justified.
But to me it hardly seems proven…hardly seems that there could be no other explanation.
In fact more recently, I have read that type 1a’s may not be the standard candle that has been thought.
But even if they are, something else could be going on.
>>
In fact more recently, I have read that type 1a’s may not be the standard candle that has been thought.
<<
Yes, I’ve read that too. It just might throw a monkey wrench into the whole dark energy works.
Jim
as Greg stated earlier infra red comes from molecular vibrations, electronic transitions are for carbon dioxide are of far higher energy beyond that of visible light, Hence it is transparent. Indeed most gases are colourless and so thei electrons are not affected by visible (yes there are coloured gases NO2 is brown)
Greg, you are wrong with diatomic molecules not absorbing infrared light. Oxygen and Nitrogen do not interact because they are not polar. HCl, CO being polar have infrared absorptions.
Ah, the upside down greenhouse effect. Things haven’t panned out quite the way it was explained ~30 years ago using basic high school physics as we’re constantly reminded.
http://1clickurl.com/XYwCjxT
I have seen articles claiming glacier melting will cause sea level rise but what about the miles-thick layer of salt that underlie continental areas and more? Under most of Germany is a miles-thick salt layer. Under the
Great Lakes lie salt deposits mined in areas like Detroit. The Gulf of Mexico has a salt bed that dissolved could flood all land up to Yellowstone.
If the salt at the bottom of the gulf of Mexico was somehow brought to the surface and dissolved into the seawater, the ocean would go down, not up.
Obviously if you took miles of salt from a continent above sea level and dumped it into the ocean, the ocean would rise…but it would rise less than if you dumped an equal volume of insoluble rocks into the ocean.
x volume of NaCl + x volume of H2O is < 2x.
As for raising the oceans to the elevation above sea level of Yellowstone Park in Wyoming…impossible.
There is no where near enough land on all of the continents to raise the ocean that high.
The average height of all of the continents is about 2750', or about 840 meters.
The elevation of the Yellowstone Plateau is about 8000', or about 2,400 meters.
Since the continents are about 29% land and 71% ocean, putting all of the land above sea level into the oceans would raise the oceans less than one half of that 840 meters.
So…nope.
‘Scuse me…earth is 29% land and 71% ocean.
It’s really quite simple. CO2 at altitude is emitting at -80 deg C toward the surface which is 15 deg C. The energy levels of the surface equivalent to -80 dg C are full and the downward IR is reflected upwards. It is simply impossible for a cold body to heat a warmer body. We are done. No effect. Fantasy to think otherwise.
BTW, this is true for any IR absorbing gases in the atmosphere near the surface, as they are always warmer than -80 deg C. Same argument as above.
All this ignores the fact that we live in a pre-charged environment due to the magnetosphere and solar wind induction combined with cosmic rays. MOST reactions occur more readily in an heated beaker.
PR;
Huh?
Pre-charged?
What do you mean by that?
Reactions?
Absorption is not a reaction.
Not in the conventional meaning of these words.
Reactions refer to chemical processes.
Chemical reactions proceed more quickly in warmer conditions due to the required activation energy.
A “good-faith effort” indeed, but, IMHO, Rod Gill misapplies the equations he uses and misinterprets the results. Satellite views of long wave radiation from Earth to Space, looking down, and views of long radiation from Space to Earth, looking up from the surface, show the actual warming effects of water vapor, CO2, and other “greenhouse” gases.
Would you care to elaborate the equations and results you reference with what you consider the correct results ? Or will you just keep waving hands – I do like the steady flow of cool air that produces though.
” Wien’s Law ” is nothing more than a consequence of the ” Planck Radiation Formula. ”
The Planck formula is a theoretical derivation of the radiation properties of a theoretical (and non-existent) material object called a ” Black Body “, hence “Black Body Radiation. ”
Such a body does not, and CAN NOT exist. so BB radiation also does not exist.
BUT ! credible approximations to Black Bodies, and BB radiation do exist.
NO real material object can absorb 100.000….% of even a SINGLE frequency or wavelength of EM radiation, let alone ALL possible frequencies and wavelengths from ZERO frequency to ZERO Wavelength.
The remarkable thing about the Planck radiation formula is that it contains NO arbitrary constants, that need to be determined by experiment.
The Planck Formula : W(lambda) = C1 / (lambda)^5.(exp(C2/lambda.T – 1) where Lambda is the wavelength and T the kelvin temperature.
C1 and C2 are commonly called the first and second radiation constants. BUT !!
C1 has the value …. 2.pi.h.c^2 …. h being Planck’s constant, and c the velocity of light.
C2 has the value …. hc/k where k is Boltzmann’s constant.
Can you believe that !! ??? All that fictional stuff is completely described in terms of some of the most fundamental constants of Physics. It is one of the miracles of modern physics.
NOW ! Hidden in that Planck formula is the often not known fact that W(lambda) is a function of a …. SINGLE VARIABLE …. That single variable is (lambda x T) .
The best presentation of the Planck formula plots W(lambda) / W(lambdamax) versus lambda / lambdamax where lambdamax is the wavelength at which the peak spectral radiant emittance occurs, and W(lambdamax) is the value of the spectral radiant emittance at that peak wavelength.
On a logarithmic scale the useful range for lambda/lambdamax is 0.1 to 10.for a linear vertical scale for W(lambda)/W(lambdamax)
Only 1% of the total is emitted at wavelengths shorter than one half of the peak wavelength, and 25% is emitted at wavelengths below the peak wavelength.
Only 1% of the total remains at wavelengths longer than eight (8) times the peak wavelength.
Plotted on both logarithmic axes, a range of 0.1 to 50 for horizontal , and 1 down to 10^-5 for the vertical.
W(lambda)/W(lambdamax) drops to 10^-5 at values of 0.2 and 40 for Lambda/lambdamax.
So for most practical purposes, BB radiation has 98% of the energy between 0.2 and 8 times the peak wavelength.. Certainly for climate considerations the rest doesn’t matter although the physics of it is of great interest for other reasons.
I agree with the author EM radiation is NOT heat.
I don’t necessarily agree with the author’s thesis; and I don’t have time to critique it in detail.
I believe the sun warms the earth; and if you delay the cooling process, the sun keeps on radiating, so it must tend to get warmer. BUT ! that is if you ignore the fact that clouds will vary and compensate.
And NO, I am not concerned in the least with global warming; or climate change. I can drive ten miles down the road and get climate change.
G
PS for a really good Planck graph, see “Modern Optical Engineering”, by the late Warren J. Smith Page 194 in my 1966 edition.
Or will you just keep waving hands
I suggest you read these, all authored by Ira, and with the equations and results you seek included:
http://wattsupwiththat.com/2011/05/07/visualizing-the-greenhouse-effect-light-and-heat/
http://wattsupwiththat.com/2011/03/29/visualizing-the-greenhouse-effect-molecules-and-photons/
http://wattsupwiththat.com/2011/02/28/visualizing-the-greenhouse-effect-atmospheric-windows/
@davidmhoffer, Ira glickstein – I do apologize for my snarky interjection – at first glance it appeared to look like “it’s wrong and finding the proof is left as an exercise to the reader” 🙁
davidmhoffer: THANKS for the links and great to hear from you again. For still more context on the scientific and moderate skeptic view, see my https://wattsupwiththat.com/2014/01/12/global-warming-is-real-but-not-a-big-deal-2/ Love, Ira
Excellent comment George!
Ira is pretty solid, so listen up when he waves his hands. He knows whereof he waves.
Can you tell us what percent of that warming is from water vapor(?), carbon dioxide(?), other greenhouse gases?
So am I right?
The answer to this question, almost always, is plainly no. If you miraculously end up with blueprints of a perpetuum mobile, it is a mistake. And mistakes are easy to make, difficult to find and fix. Thus the tradition is that the professor has assistants to help students to find their errors. And errors they do, from hour to hour, week to week, year to year.
So I agree with Ira G…
The question is not does an increase in greenhouse gas cause surface warming but rather how much surface warming. Increases in greenhouse gases in the atmosphere also increases convection cooling which reduces the lapse rate which reduces surface warming.
The infamous without ‘feedbacks’ cult of CAGW’s calculation (this is the so called 1 dimensional calculation that predicted 1.2C to 1.4C surface warming for a doubling of atmospheric CO2) incorrectly/illogical/irrationally/against the laws of physics held the lapse rate constant to determine (fudge) the estimated surface forcing for a doubling of atmospheric CO2. There is no scientific justification for fixing the lapse rate to calculate the no ‘feedback’ forcing of greenhouse gases.
Convection cooling is a physical fact not a theory and cannot be ignored in the without ‘feedbacks’ calculation. The change in forcing at the surface of the planet is less than the change in forcing higher in the atmosphere due to the increased convection cooling caused by greenhouse gases. We do not need to appeal to crank ‘science’ that there is no greenhouse gas forcing to destroy the cult of CAGW ‘scientific’ argument that there is a global warming crisis problem to solve.
P.S. The IPCC general circulation models (GCM) have more than a 100 free variables that are subjectively set to produce the ‘predicted’ warming.
http://hockeyschtick.blogspot.ca/2015/07/collapse-of-agw-theory-of-ipcc-most.html
https://drive.google.com/file/d/0B74u5vgGLaWoOEJhcUZBNzFBd3M/view?pli=1
Transcript of a portion of Weart’s interview with Hansen.
William,
Thanks for the link to the work of Kyoji Kimoto. His figure 2 is most interesting in that it shows TOA warming without a change in height, precisely what we would expect if the tropopause is pressure dependent. Here is the abstract of a paper that shows this:-
Robinson, T.D. & Catling, D.C. (2014) Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency.
Nature Geoscience 7 (1), 12-12.
https://www.nature.com/articles/ngeo2020
A calculated value for CO2 of 0.02C using the ideal gas equation posted elsewhere by 1000Frolly works for me.
Actually what the surface radiation shows is water vapor regulating cooling as air temps nears dew point.
https://micro6500blog.wordpress.com/2016/12/01/observational-evidence-for-a-nonlinear-night-time-cooling-mechanism/
Indeed I tend to find this out every year when dew freezes over at the driveway, keeping temperature stable but not keeping me stable.
You’re still radiating. Month back at 4:00am, it was like -50F, but cooling was almost stopped.
Ira: In what is your PhD? Art History? Gases warm nothing. They are not a source of energy. They can absorb, they can re-radiate, they can transmit, or they can reflect. But, they cannot warm.
Foodfight!
Walter, the enthalpy of air at sea level in the tropics is ~73kJ/m^3, hardly nothing.
Whether a gas can warm another mass is simply dependent on temperature, mass and thermal capacities of the masses. Steam is a gas and is used all the time in practice for heating.
Walter Sobchak November 19, 2017 at 7:45 am
Ira: In what is your PhD? Art History?
He has a PhD in Engineering and has authored some of the most detailed and accurate articles on GHE ever published in WUWT, including observational evidence that underscores the theory. As a hardcore skeptic with a lot of background in physics, I can suggest that rather than making snarky remarks, you could learn a lot from him, including why the author of this post is wrong:
http://wattsupwiththat.com/2011/05/07/visualizing-the-greenhouse-effect-light-and-heat/
http://wattsupwiththat.com/2011/03/29/visualizing-the-greenhouse-effect-molecules-and-photons/
http://wattsupwiththat.com/2011/02/28/visualizing-the-greenhouse-effect-atmospheric-windows/
Shearer, the question on what can ‘warm’ what is a semantic one. Only heat sources like radiators, fire or the Sun warm in meaning one that Sobchak employs above. Meaning two includes anything else that results in higher temperature somewhere.
Argon between my window glazing does not ‘heat’ my house, but it helps keeping it warmer. So does any other insulator by increasing the Km²/W value of the walls. Now my house is not heated with the insulator, but it surely is warmer because of them.
I’m tired of this ‘gas does not warm’, because it is always put forward and never any learning happens.
The difference in this case is water compensates for at least most of the changes of the other GHGS forcing. Because water’s is triggered by air temp and pressure.
If this be the same Dr. Ira Glickstein, he might want to check out the difference between a howitzer and a recoilless rifle:
https://plus.google.com/111810954238686745033
The good doctor’s blog:
http://visualira.blogspot.com/
Ratings by students:
http://www.ratemyprofessors.com/ShowRatings.jsp?tid=530927
IMO, a brief Internet search is generally preferable to casting aspersions. Not that art history is such a despicable discipline. It too requires pictures as well as words.
@Hugs November 19, 2017 at 10:21 am
“Now my house is not heated with the insulator, but it surely is warmer because of them.”
Only because the heat source (furnace) puts out more heat than can escape because of the insulation. The “warmth” of your home is dependent upon the heat source and the air temp outside (heat loss). It’s possible for it to get so cold outside that the insulation cannot keep the heat in with the furnace going full blast. Hence the insulation’s role is to slow the rate of heat loss. It doesnt make your home warmer, it makes it less cold.
OK, let’s call it then global less-colding if it sounds better.
As I said, this is just semantics, and not really a very constructive foodfight.
I am with Hugs on this one.
Semantics…unhelpful and obfuscatory.
If CO2 retards heat loss through the atmosphere, it results in some amount of less-coldness.
If it is a radiative gas, perhaps it hastens heat loss at night when the sun is not shining…but we do not see greater nighttime coldness…we see less of that, and less winter coldness and less Artic coldness…all good things.
We also see less extreme daytime hotness, at least in the place where we have lots of good measurements over many decades.
A better question than all of this is…why does anyone think that a warmer Earth could possibly be bad?
When was this debate settled?
The answer is never…we never even had a discussion about it…it was merely asserted and for some incredibly strange reason people, even scientists, lapped it up.
Prior to 1988 every historian and everyone who studied Earth history knew that a warmer earth is a better place for life and for people.
All the rest of this is misdirection, stacked up against that undeniable fact.
Hugs
“As I said, this is just semantics,”
This is what people say when they are shown how reality is not simple.
No, this is not semantics. In science it is imperative that the proper words are used to be precise and avoid ambiguity. AGW thrives on being vague and ambiguous.
Tell me, is a glass half full or half empty?
In science we would say a glass is half full if it was empty and filled with new water half way. Conversely, it is half empty if the glass was completely full, but half the water removed (what’s left is not new water).
The fact is, at night, for example, having temps under cloud doesnt get so cold is not warmer because getting warmed requires new energy to warm the object. Instead that warmth was already there just not as much of it was lost under cloud cover.
I agree.
But to explain this
requires more energy.
Both of these nights were clear all night. And if you measure the sky with a IR thermometer you find it is still 80-100F colder than the ground, and air. And I have to wear a hat.
Another equal situation on the other side of the world, where they measured net radiation at 2m, you can see when the change in temp rate changes, the net flux changed, and it’s a regulatory response by water vapor.
Sorry, forgot the second graph
Wakefield,
I’m sure words matter but you just can’t define warming in some rare technical sense and expect people to talk about something else (what, less-colding?)
even when the dreaded warming is a small less-colding effect like GHE 2xCO2 without feedbacks.
Ira. Right so. This is very simple,because there direct measurements showing the basic fluxes.
Ira, can you give us a link to satellite measurements of “the actual warming effects of water vapor, CO2, and other greenhouse gases”? All I’ve seen are either radiometric measurements of temperature (not temperature of a particular gas) or measurements of the presence and quantity of greenhouse gases (but not the temperature of their emissions). For example:
Temperature:
https://www.nsstc.uah.edu/climate/
http://www.remss.com/measurements/upper-air-temperature/
Greenhouse gases:
https://oco.jpl.nasa.gov/
https://www.nasa.gov/jpl/oco2/pia18934
Do you have a reference to satellite measurements of temperatures of specific gases?
Ira, can you give us a link to satellite measurements of “the actual warming effects of water vapor, CO2, and other greenhouse gases”?
Wrong question. The satellites see what is coming UP from the earth. If you want to see what’s coming DOWN from CO2, you have to measure it at earth surface, and that has in fact been done:
https://phys.org/news/2015-02-carbon-dioxide-greenhouse-effect.html
Note that they measured less than 0.2 w/m2 over a change of 22 ppm of CO2. In other words, very very small. You may also want to read through these which will explain in a lot more detail how things ACTUALLY work backed up by observational evidence. The argument is NOT about the effects of CO2, but the nature and magnitude of second and third order (feedback) effects, which are greatly exagerated:
http://wattsupwiththat.com/2011/05/07/visualizing-the-greenhouse-effect-light-and-heat/
http://wattsupwiththat.com/2011/03/29/visualizing-the-greenhouse-effect-molecules-and-photons/
http://wattsupwiththat.com/2011/02/28/visualizing-the-greenhouse-effect-atmospheric-windows/
Here’s 18°F of warming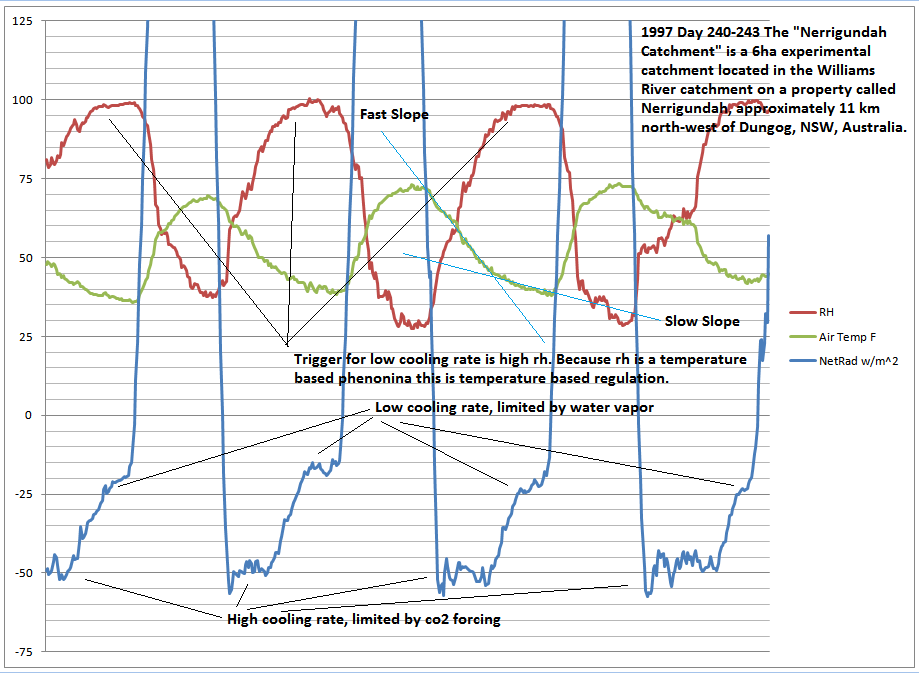
Micro is exactly right…when the air reaches the dew point at night, cooling comes to a virtual halt.
And when it is hotter during the day, convection is enhanced.
Where it is hot enough and humid enough, convection causes thunderstorms which result in even greater cooling and transport of energy to the top portions of the troposphere.
If it is hotter, the thunderstorms either start sooner, or last longer, or grow to taller vertical extent.
IOW…more energy means stronger thunderstorms which means greater transport of energy to way up in the sky…where it is very cold, air is very dry, and heat/energy can far more readily escape to space.
“Micro is exactly right…when the air reaches the dew point at night, cooling comes to a virtual halt.”
AT the surface but not above it.
I’ve told Micro several times that he is looking at a small part of the system.
Just land FI.
Cooling to condensation point is absent over the majority of the Earth’s surface, due the fact that it is ocean.
Land cools quickly >> cooling of air above >>> condensation if moist/calm enough.
Ocean doesn’t cool (over 24 hours) >> no condenation (in absence of advection cooling).
If an inversion forms (most likely here) then in the drier air above cooling will still occur via radiation to space (given clear skies).
If fog forms then likewise the fog-top will cool (radiation to space).
The atmosphere has depth which micro totally ignores with his nonsense half-baked ideas.
Baloney Tone B
This is in regard to surface temps, no one ever said different.
For whole atmosphere we have the balloon data and satellites, and the satellite data includes over the oceans.
Funny how warmistas always want to play a game of switcheroo regarding which data is being discussed.
“No one lives in the troposphere”, they say.
OK, the surface stopped warming too, if they stop adjusting the damn data every year.
“If we include properly tortured ocean data, the pause disappears from the surface records”
No one lives in the ocean!
Lets get a straight story for one…which is it we care about Tone B…the surface, or the troposphere?
And what about that hot spot?
“Baloney Tone B
This is in regard to surface temps, no one ever said different.
For whole atmosphere we have the balloon data and satellites, and the satellite data includes over the oceans.
Funny how warmistas always want to play a game of switcheroo regarding which data is being discussed.
Don’t know what you’re on about my friend.
micro’s theory is that WV controls surface temperatures AT THE SURFACE.
And I’ve discussed it at length with him several times (on other Blogs).
Nothing to do with observational data.
It is simply basic meteorology.
And something I spent 32 years in the UKMO observing.
“And what about that hot spot?”
Oh, if you insist …
That’ll be the Tropical hot-spot – that would appear in ANY sort or warming.
It is merely a function of greater LH release aloft in the tropical high atmosphere via convection.
For one – it is difficult to find because of the nature of the instruments used. Radiosondes are imprecise for the job and have changed over the years, and sat obs are contaminated by Stratospheric cooling – which is a function of GHG theory.
“First, tropical warming is equally strong over both the 1959–2012 and 1979–2012 periods, increasing smoothly and almost moist-adiabatically from the surface (where it is roughly 0.14 K/decade) to 300 hPa (where it is about 0.25 K/decade over both periods), a pattern very close to that in climate model predictions. ”
http://iopscience.iop.org/article/10.1088/1748-9326/10/5/054007/meta
And the instruments used introduce some errors but IRA is not an arm waver.
That graph posted by micro6500 needs to also state that the rate of cooling remains fairly similar until RH approaches 100%, but at this point dew deposition occurs on ground and plant surfaces which releases latent heat. If further cooling occurs then fog forms with further latent heat release. So this is not just a straight forward water vapour greenhouse gas relationship but should also take into account latent heat release.
I’ve never heard a weather forecast yet that said, it’s going to be a high CO2 night, which is expected to keep temperatures warmer. They always say, lots of cloud cover tonight will help keep temperatures warmer, or the lack of cloud cover tonight will see temperatures plunge. If CO2 makes temperatures warmer, then we here in middle Alberta have been shortchanged for the last three weeks. So I don’t disagree with anything you say.
Cloud cover fluctuates considerably from day to day (or even hour to hour), but CO2 only varies (minutely) on a scale of months. The IPCC is forecasting (projecting?) temperature changes far less than Alberta sees in a typical day, and their forecast is for decades in the future. Including CO2 in a weather forecast would be ludicrous, as I’m sure you know.
But in the specific places where we would expect to see the most pronounced effect of CO2 caused warming…we do not see it.
A fact that is for some reason overlooked completely by 100% of warmistas.
Giving them all big giant credibility demerit.
To add to all of the other credibility demerits they have earned.
In fact, everything they assert is another demerit to their credibility.
Which by itself is incredibly unlikely…but there it is.
The big mystery is why anyone still believes anyone who has never been correct in any of their predictions?
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
Why do you say that? Things do not emit radiation ONLY at their peak wavelength. They emit at that wavelength and also at others.
IF what you say is true spectrometers would not work. It is not a peak it is a band… like -78 to -82C.
Oh this is lovely. Think about the Sun. It emits not only at the peak wavelength. And yes you can measure its surface temperature from the spectrum.
Pretending that only CO2 at -80C emits in that band is just so wrong. Any CO2 at any temperature above -80C will do so too, and also CO2 colder than that may emit a bit.
Yes, this is wrongly said. It is far too much to try to fix it up.
The problem is he is trying to treat everything as one effect there are two and he hasn’t worked that out.
It’s exactly the same as radio signals you have signals that are resonant to the reciever circuit and signals that are not hence the whole resonance thing. You can’t just use one law for both. Try working out how a radio reciever selectively amplifies one signal without using the proper calcs on the resonant frequencies.
It’s a standard EM wave (AKA a radio wave) stop treating it has heat and treat it like a radio wave at the resonant frequencies and you might get somewhere.
Yes, and also consider this:
https://en.wikipedia.org/wiki/Two-photon_absorption
Does beg the question how much energy in the wavelength that CO2 absorbs is reflected back towards space. This will tell us the maximum possible amount of heat that CO2 can trap.
It will not stop warmists as CO2 will cause a positive feedback loop that increases H20 in the atmosphere.
But as H2O increases so the energy available to warm CO2 will therefore decrease.
How do I feel that this green house theory is not fully explained.
You do understand it’s a EM wave?
Can I asked you how much of a microwave TV signal from a satelitte in space beaming to earth do you think a CO2 molecule reflects back to space?
The real problem is not in the explanation but in the implications for the atmosphere.
Simply out, CO2 is not the temperature control knob of the atmosphere…never was, is not now, and never will it be.
Period.
Other factors are far more important…hence we have natural fluctuations at every time scale…it has been warmer, it has been colder.
Warmer has been a sweet deal for all living things, colder…not so much.
The very first key point is dead wrong. Electron ‘orbit height’ (energy level) does NOT change with temperature. ‘Temperature’ is the molecular level vibration/velocity/Kinetic energy, not subatomic (within the atoms). That’s what I was taught. Google can get this right, https://www.quora.com/By-lowering-temperature-the-atoms-slow-down-So-do-the-electrons-in-the-atom-also-slow-down
True. Electron configurations of atoms only change at a very high temperature. Configurations of atoms in a molecule (rotation, vibration) may change at atmospheric temperatures – that’s what makes water a “very bad” greenhouse gas, carbon dioxide not so bad.
As the illustration notes, Wien’s law applies to a a hypothetical black body (usually approximated as a hollow sphere with a small hole), an object with an infinite number of degrees of freedom of motion. It does not apply to the atmosphere, consisting of atoms and molecules with only few degrees of freedom.
ALL material that are above zero kelvin radiate ” …. Thermal Radiation …. ” as a consequence of the Temperature of that material. The ultimate source is the acceleration of electric charge; as a consequence of Maxwell’s equations of electro-magnetism.
When atoms of molecules collide (Temperature) the electric charge distributions are distorted, so during the collision, perfectly good radiating dipoles exist. There are also other more complex antenna radiators like quadrupoles and even hexadecapoles, that radiate in more complex radiation patterns than a dipole antenna.
But since we don’t have any gases that are totally opaque to any radiation, then gases would not be black bodies; but they DO emit “thermal” radiation. It’s called thermal because it is a consequence of Temperature of the material (even gases) but the radiation may be in the microwave or radio spectrum, and not in the LWIR spectrum.
G
George, I respectfully disagree. If a gas does not absorb radiation, then it can not emit it.
” Curious George
November 19, 2017 at 10:04 am
George, I respectfully disagree. If a gas does not absorb radiation, then it can not emit it.”
A gas molecule can absorb radiation for very short period of time.
A volume of gas has temperature because billions and billions are traveling around the speed of a bullet and colliding [without friction] with each other, This kinetic energy remains indefinitely until
the gas molecules lose their kinetic energy by contacting other gas of lower average velocity [thereby increasing and averaging the combined average velocity. Or the kinetic is conserved.
Or gas can collide liquids or solids, and again transferring the kinetic energy [conserving the kinetic energy] but the solids of liquids can radiate the kinetic energy gained by the warmer gas molecules.
A gas molecule has no temperature, absorbing energy tends to indicate an increase in temperature, and one molecule has no temperature.
Well curious George, you are fee to disagree with anything I wrote; with no respect required.
You provide no support for your contention that ” If a gas does not absorb radiation, then it can not emit it..”
Also I don’t see anywhere that I wrote that gases do not or can not absorb EM radiation.
No point in citing the essence of Kirchoff’s law; that law only applies to objects in thermal equilibrium. Nothing in the climate system is ever in thermal equilibrium; the rotation of the earth simply will not allow thermal equilibrium to be achieved.
But if you are going to disagree with what somebody else has posted, please do the readers the courtesy of giving supporting data or evidence for your position.
Despite all the modern love affair with quantum mechanics; the ONLY physical constants which actually have accurate exact values, are fundamental elements of Maxwell’s theory of electro-magnetism which is classical Physics. And it is a direct consequence of that theory and the whole concept of “heat”, that ANY object with a thermo-dynamic Temperature greater than zero kelvin must radiate a thermal spectrum. It is NOT a quantum spectrum of specific lines or bands, but a continuum of frequencies, and is not related to electron energy levels in any quantum mechanical model of atoms or molecules.
We have a two mile long electron linear accelerator pinning the San Andreas fault together near Sand Hill road and Highway 280, that exists only because of the simple fact that accelerated electric charge MUST radiate EM radiation.
G
Driller43,
Yes, you are quite right.
Here’s a references to an n layer atmosphere:
http://en.wikipedia.org/wiki/Idealized_greenhouse_model
GREENHOUSE WARMING Part I
There IS a greenhouse gas effect. Here’s an example.
Let the flux from the sun to the ground be 4 joules/unit time*unit
area,
and stay constant.
–> SUN
–>
–>
–>
–>
With no greenhouse gases, the earth will either heat up or cool down
until
the outgoing flux from the earth is equal to the incoming flux from
the sun.
Sun –> O O O O <– <– <–O O–> <–
You've now got an unbalanced situation where 5 joules/unit time*unit
area
are hitting the earth, 4 from the sun and half of the 2 from the
atmosphere,
and only 3 joules per second are leaving the earth, the 2 not
absorbed by
the gas, and half of the 2 from the atmosphere. The atmosphere will
gradually
warm up until outgoing flux from the atmosphere, plus the fraction of
the flux from the
earth not intercepted by the atmosphere, equals the incoming amount
from the sun.
Since in my example, half of the outgong flux is intercepted by the
atmosphere,
the watts hitting the earth's surface will increase to
1/(1-1/4) = 4/3 of 4 joules/(unit time*unit area) = 5 1/3 joules/(unit
time*unit area).
Remember the atmosphere is intercepting half of this, so 16/6 joules
(unit time*unit area)
is intercepted by the greenhouse gas atmosphere, and another half,
16/6 joules, escapes
directly to space.
The final equilibrium balance is
16/12 Earth
O–>16/12 16/12(from atmosphere)–>Earth
Sun –>4
16/6 to atmosphere <–
Earth
16/6 to space <–
Earth
So yes, a greenhouse gas will warm the earth, the amount of warming
depends on the fraction
of the outgoing (and incoming for that matter) radiation absorbed by
the atmosphere.
………………………………………………..
If the atmosphere was absorbing ALL radiation from the sun , you'd get a final balance of
4 watts/unit area from sun to atmosphere and 4 watts from atmosphere to space- in balance
4 watts from sun to atmosphere, 4 watts from earth's surface to atmosphere
4 watts from atmosphere to space, 4 watts from atmosphere to earth surface- in balance
4 watts from atmosphere to earth surface, 4 watts from earth surface to atmosphere – in balance.
So an atmosphere that absorbed ALL solar radiation would have a net zero greenhouse effect.
……………………………………………………….
When you look at the blackbody radiation curve, you'll notice that the shortwave frequencies, not affected by CO2, increase at a much greater rate with increasing temperature than longer wave radiation, which IS affected by the amount of CO2 in the air. The net result is that increasing absorption of long wave radiation by CO2 is MUCH smaller than increases in surface temperature. In addition, as the atmosphere warms up, a larger fraction of the sun's incoming radiation is absorbed directly by the atmosphere, reducing the overall greenhouse effect.
Alan, the only thing that alters that from an atm basis, is water’s actions are not linear over the standard range of pressure and temperatures.
That is what alters Earth from non condensing only atm.
Water does real work daily. And it moves that work around.
I agree with your findings, but not for the same reasons. Retired now, I used to be a laser engineer, and instrument design engineer, including weather stations, and all manner of energy measuring devices. I created infrared measurement systems and laser energy measurement systems. Thus, I know a little about gas behavior and thermal characteristics.
So, my argument against CO2 ‘warming’ anything goes like this? If it DID increase the surface temperature, the surface would radiate to space at the 4th power of the increase, instantly cooling itself back down. We know that everything radiates and we have laws for quantifying it.
Experiment: Try to heat a stove with a flashlight. There are lots of photons, you can see them. Yet the stove does not warm. Why? Because for each photon that strikes the stove, the stove emits millions, maybe billions of photons itself. Those few photons from the flashlight are simply overwhelmed, and the result is immeasurable. Applying that to CO2, how many surface molecules radiate for every CO2 molecule? There is NO WAY there is sufficient energy in CO2 radiation from the atmosphere to increase surface temperature, because the surface radiates FAR more than the CO2 does. And, again, if the CO2 DID somehow manage to ‘warm’ the surface, it’s millions of molecules per CO2 molecule would simply radiate at a higher rate instantaneously, and ‘cool’ back to equilibrium.
Lastly, consider that if there were CO2 ‘radiation’, it would cast a shadow when blocked – an invisible one, but still a shadow. If you create a filter that can filter out the CO2 spectrum, and place it on the window of an infrared thermometer, with another identical thermometer without the filter, you will find that they both measure the same temperature. That would not be possible, if CO2 was indeed ‘heating’ anything, those two thermometers would have to read differently.
Leave it to an engineer that understands the science better than the alleged experts. That’s because we operate in reality. A concept foreign to most pseudo theorists.
Here’s reality for you:
https://phys.org/news/2015-02-carbon-dioxide-greenhouse-effect.html
The author of this post has made so many errors that it would take a long time to go through them all. But above is observational evidence that fits with the theory. If you read carefully, and do the math, you’ll come to realize that the author of this post is wrong AND that the observational evidence, while confirming the theory, results in a sensitivity calculation so low as to be immaterial and so falsify the alarm.
The global warming alarm is NOT founded upon a GHE that does not exist. Continued efforts to discredit the first order effects of CO2 are not only futile, it gives the alarmists ammunition to show that we don’t know what we’re talking about. Global warming alarm is founded upon an exaggeration of second and third order effects (feedbacks) that are still not well understood because they cannot be directly observed, but for which there is increasing evidence are low, and decreasing evidence to show that they are high. At day’s end however, CO2 is logarithmic (that fact not only accepted by alarmist scientists, but repeatedly documented by them in their own IPCC reports) and so the more CO2 we have in the atmosphere, the less additional CO2 matters.
“The global warming alarm is NOT founded upon a GHE that does not exist.”
Couldn’t Anthony Watts acknowledged this misunderstanding instead of promoting it?
Tony McLeod
Welching on your bet? You lost our bet and said you would never post here again, I took you as a man of honor sir, was that a mistake?
Mods the bet was made as many here know an should be honored.
David H.
“CO2 we have in the atmosphere, the less additional CO2 matters.”
Exactly correct, except for plants, trees,… and crops, which are all parts of plants and trees.
IOW, for life. For the biosphere.
For the biosphere, it matters.
It is unambiguously very very good to have more, much more, CO2.
davidmhopper,
“The global warming alarm is NOT founded upon a GHE that does not exist. Continued efforts to discredit the first order effects of CO2 are not only futile, it gives the alarmists ammunition to show that we don’t know what we’re talking about.”
Exactly right. The post is utter nonsense. Trying to refute physics that has been well understood for a century or more is a waste of time, distracts from far more credible critiques of CAGW, and gives the crazy green left ammunition to attack perfectly reasonable arguments against CAGW. Please stop trying to help so very much. If Anthony never posted another article like this (and unfortunately, there have been many over the years), it would be a good decision.
The first order effect is ENHANCED PLANT GROWTH, who is trying to discredit it ?
The nth-order effect, warming, has never been measured, and only exists in erroneous theory and models..
It seems to me that Co2 molecules could only insulate – slow the amount IR radiation leaving the surface- and it’s a very small effect.
CO2 or any greenhouse gas can not increase the surface temperature. The surface is warmed by the sunlight.
Water can and does warm the surface at night…if it condenses into clouds.
I have had many a freeze and frost averted or reversed by a fortuitous streak of high cirrus streaming in while I was monitoring the temperature.
I have watched the mercury climb by several degrees in a matter of minutes when clouds raced in.
Many many times.
CO2 warming the surface with IR is like pissing against a fast flowing river.
Not at all. It is more like building a dam across the river. After a small amount of time, the water behind the dam will be deeper (the temperature of the surface will be higher), but the amount of water flowing down stream will be the same as before.
The question is – Where is the CO2 part of the dam added? If it makes the dam higher, do we care?
If water is flowing over the top of the dam, then an extra inch of height makes a big difference. However, if water thru the dam is controlled by sluice gates, the the top of the dam will be dry and adding a couple of extra feet of height will have no effect.
My theory of the GHE is that water vapor (including clouds, rain, dew, etc.) controls the sluice gates.
Robert Clemenzi :
“Not at all. It is more like building a dam across the river.”
CO2 at 0.04% is no dam. There are billions more photons leaving the surface for every photon going to the surface from CO2.
Hmmm, very interesting to see the debate boiled down thusly…is CO2 more like a stream of piss into a fast moving river or an inch of manure on top of a leaking dam with open sluice gates?
“CO2 at 0.04% is no dam. There are billions more photons leaving the surface for every photon going to the surface from CO2.”
I suggest you study the Beer-Lambert equation and the importance of path-length.
Clue: the path-length to space is a tad long, enough to make the 0.04% (up 40% due anthro emissions) very much a dam.
I also suggest you consider CO2’s importance where WV is scarce.
Please provide a reference supporting that.
My computations indicate that at 15C, the Earth emits about 390 W/m2 and the first kilometer of CO2 would absorb about 54 W/m2 – except that water vapor absorbs about half of that. That is almost 14% of the radiated energy.
MODTRAN indicates that the downward radiation from 400 ppm CO2 is 81 W/m2 using the full atmosphere.
81/390 = 20.8%
When normal water vapor is added, the downward IR energy is 367 W/m2.
John,
Your real-world expertise is valuable in this struggle. You neatly in a few lines devastate the entire pseudo-science of GHG.
And you write clearly and concisely.
You should write a blog yourself.
Or hook up with another engineer who already has a blog destroying the GHG.
The discussions here fall into the trap of debating the scammers, on their own terms.
Please comment over here:
https://realclimatescience.com/
This electronic engineer agrees. The last paragraph is a gem. If this experiment were conducted it would blow the GHE to smithereens
“…no way there is sufficient energy in CO2 radiation….” mostly correct John, but take my word as yet another old engineer who once did heat transfer calcs in combustion gases….if you go through these standard radiative calcs from our student days…..
http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html
…….the CO2 does make a difference in the range of 1.2 degrees C per doubling. The real question is water vapour related. Is that 1.2 degrees C that is going to cause positive feedback, or is the extra water vapour going to cause more clouds to reflect incoming solar away ? It is probably the latter or Earth’s oceans would have boiled away long ago.
If you are a retired laser engineer (which itself is a weird term) then can I ask how you get population inversion on the CO2 gas inside the laser tube? Should be interesting in line with your opinion above.
I will not start arguiing about the physical phenomenon in a GH molecule, when a photon with the right wavelenght / frequency hits this molecule. This is a very basic stuff of molecular physics and there is enough evidence in which way it happnes. But I do not agree with the description of Rod Gill.
The GH effect in the atmosphere is a fact and it is caused by GH gases: H2O 81 %, CO2 13 %, O3 4 % CH4 & N2O 1 %, clouds 1 %. Link: http://www.sciencedomain.org/abstract/17484
Here is a figure from my web site, which shows the absorption wavelenghts of GH gases. A very bsic thing is that the absorption wavelenghts of other GH gases overlaps with water making them rather weak:

One thing more. The Earth’s surface emits about 395 W/m2 in 15 degrees and the LW radition emitted by the atmosphere to space is about 239 W/m2. The radiation loss is 396 – 239 = 157 W/2. Energy does not disappear but it can change its form. The radiation flux of 157 W/m2 maintains the temperature profile of the atmosphere together with the SW radiation absorpbed by the atmosphere, which is about 71 W/2.
It would be interesting if the Earth we not covered with water. Your model, does not include the Oceans which have 99.9% of the energy in the system. Go back and try again.
Agree X 1 Million.
A nice essay, I don’t fully understand quantum physics, you have a much greater knowledge of this than me. Could I ask though there is a theory, I am not sure it is conclusive, that as CO2 concentration rises in the atmosphere, the increase in temperature is not linear, but logarithmic. If it is true how would this tie in with your theory? I have pasted my source below which is over 8 years old.
https://wattsupwiththat.com/2010/03/08/the-logarithmic-effect-of-carbon-dioxide/
If you look at the figure above, you can see that CO2 can increase the area of its absorption peak in the wavelenght zone from 10 to 14 micrometers. In figure below are the increases of the absorptions for different CO2 concnetrations (hopefully cominf correctly). These curves are results of extensive spectral calculations. The logartimic relationship is the results of curve fitting of these calculation – not a result of a single calculation.

The theory is all hogwash. The claim of logarithmic relationship with CO2 abundance arises from the so called Beer’s Law (or Beer-Lambert law). Beer’s law applies strictly to linear transmission through non-scattering media. The photons are expected to travel in straight uni-axial direction through the medium, unless they get absorbed by some absorbing molecule. Then they are supposed to stay dead and not re-emit at some other wavelength and in an isotropic direction. The photons can’t stay dead. Eventually the absorbing medium must warm from the absorbed energy, so it must eventually emit LWIR photons.
Logarithmic means a change from one CO2 molecules to two CO2 molecules, has the same Temperature change effect as a change from 400 ppmm to 800 ppmm.
And there is no experimental evidence for logarithmic relationship. Sometimes CO2 and Temperature go in opposite directions.
There are no logarithms for negative numbers. and don’t bother me with Gamma functions.
G
You are wrong. Beer-Lambert law works only for very small concentrations like CH4 and N2O. CO2 and H2O do not follow the Beer-Lambert law anymore. Basic stuff of radiation physics.

try the Beer Lambert Law
Whatever the theory, CO2 obviously does not cause actual warming by experiment in the real atmosphere. The increase in CO2 in the ca. one hundred years from near 300PPM to near 400PPM does not correlate with global warming, or some would say, lack thereof.
Of course it “lacks”, because in the IPCC’s model, the warming effect is quite excatly 200 % too much. There are cosmic forces which explain the temperature history of the Earth since the Roman warm period. If there were no cosmic forces, the temperature graph would be like that of Mann’s graph: a straight line up to 1750.
You’ve got the physics wrong. Absorption spectra have little to do with electrons, and a lot to do with vibration modes of the CO2 molecule. CO2 is a complex molecule. It can move (translation), rotate, and vibrate. A molecule’s energy is equally partitioned between all of these degrees of freedom. The rotational and translational degrees of freedom cannot absorb IR, but the vibration degree of freedom can. The energy from IR, once absorbed by resonance with a vibrational mode, can then be distributed to the other modes. It does not stay ‘locked up’ in vibration, it can cause the molecule to rotate, and to move faster (heat up).
Think of a bunch of molecules bouncing around off of each other, in a collision, one molecule could easily lose much of it’s vibration or rotation and cause the second molecule to bounce off faster in another direction. Over many collisions, the vibrational energy from IR absorbance will be equally spread into all of the degrees of freedom, including translational, which increases the average kinetic energy of the gas molecules ‘heating’ them up.
Now, the opposite can also occur, translational energy (heat) can be converted into vibrational energy, and then that energy can be emitted as an IR photon.
Yes, you are right. The vibration of the atoms of carbon with respect to the oxygen in CO2 is how the energy at 15 microns is absorbed. It is not absorbed by the electrons in the atoms.
Photons only interact with charged particles.
A 15u photon might not be changing the energy level of a valance electron, but if it interacts, it is with the molecules electrons.
I’m not sure what the electrons in the bond is doing, while it’s vibrating, maybe it’s just oscillation along the length of that bond.
But there is an interaction of some kind with its electrons.
CO2 is a complex molecule but it isn’t magic. it is about 0.04% of the atmosphere. And the atmosphere is about 0.1% of the heat capacity of the ocean atmosphere system.
Yes, his second bullet is just plain incorrect, as is the second to last.
The CO2 molecule is linearly symmetrical, so it should have no electric dipole moment.
But it also has an elbow at the carbon atom and can bend in two different modes at right angles. The moment of inertia for such a vibration is quite high so the frequency is low hence the 15 micron wavelength. It’s a degenerate mode because of the two identical frequency oscillations, which I suppose are indistinguishable.
The asymmetrical stretch mode (all three atoms moving relative to the center of mass) radiates at around 4 microns, but the earth doesn’t emit much in the way of 4 micron radiation (the sun does; something less than 1% of solar spectrum)
The symmetrical stretch mode where the carbon is stationary as the center of mass, does not radiate in the infra-red. Well it shouldn’t have dipole radiation, but maybe it looks like two end to end dipoles in opposition so it might have a quadrupole or higher antenna configuration.
Anything that can radiate will radiate.
G
” LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.”
It’s not one way. Once LWIR is stopped, it (or it’s energy equivalent) has a 50% chance of escaping to space and 50% of escaping back to the surface.
Err, I’m always dumbed by this argument. No, the atmosphere is not transparent. No, the wavelength changes. It is far more complicated.
The atmosphere may not be transparent, but it is pretty darn close. Else, we would starve to death.
no, itis not transparent enough for lwir to just go away…
And LWIR is what % of the total energy flux?
Look at the scary red arrows in the pic below. The net flow up is 64W/m², gross is a bit larger.
To make sure you understand: I do know the net 66W/m² or whatever is a small portion of the total and I’m not confident on numbers given without error estimation. What I tried to say is the LWIR is not how energy gets up, it is just part of it, and you can’t put it shortly that half is emitted back down, it is awful.
I think I’m too tired to distinguish IR from LWIR. Time to go to bed.
That’s why the sky is blue. Scattering, especially via Raman effect.
R. Shearer,
You said, “That’s why the sky is blue. Scattering, especially via Raman effect.” You are confusing Raman inelastic scattering, which is volume scattering inside a solid, with elastic Rayleigh scattering, which is wavelength dependent. Thus, blue light is scattered more strongly than the longer wavelengths, and the appearance of a direct observation of the sun shifts from green to yellowish because the blue light has been removed, and reaches the surface through a different path than direct sunlight.
planet is not flat….
Flat to first approximation. The atmosphere is dozens of km thick, the planet is thousands of km deep. It is thinner than the peel of an apple.
There are no flats on a sphere. The very smallest surface element has the exact same curvature as any other element.
There also are no spheres anywhere.
G
Oh gee. I’m sure this is what are looking for.
Look, I’m not pushing flat-earth, just noting that not every calculation requires the second or third term, just simply because they are small compared to the other known errors.
” 15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.”
Question: Does CO2, or any molecule, radiate equally in all directions?
If so, at 95Km the earth is only in 25% of the radiation cone of CO2; and most of that at an oblique angle.
Actually, because the 2 oxygens are on one side of the carbon atom, and oxygen is heavier than carbon, this aligns the CO2 molecules like little arrows all pointing upward. So the radiation goes in that direction. Right?
pmhinsc
I asked a similar question a few days ago, not nearly as well as you though, but then I’m not qualified in anything. However, it seemed to me that the radiative properties of CO2 are always assumed to be 100% directed back at the planets surface, which, to a layman like me, seems to defy logic.
Your radiation cone description is an easy way for me to visualise what’s happening. Thanks.
reallskeptical are you trying to be funny? The carbon atom in CO2 is in the center of the molecule. Recall that carbon atom forms 4 covalent bonds.
‘If so, at 95Km the earth is only in 25% of the radiation cone of CO2; and most of that at an oblique angle’
No, it is not 25%.
That would be nearer to 950 km. I’m too tired at this time to calculate the 95 km value.
Hugs: “No, it is not 25%”
You are correct. Assume an earth radius of 6,378km and the straight line distance to horizon from 95km is 1104.9km (from internet horizon calculator). I calculated a cone of 160.4 degrees or 44.5% of potential 360 degree cone of radiation. Hope you find this number more to your liking.
I love the crazy stuff classical physics lead people to believe, let me guess the emission is in certain direction because in other directions it is going to bounce or crash into the nucleus 🙂
pmhinsc November 19, 2017 at 8:40 am
Far, far from true. The angle of depression of the horizon is arcCos(R/(R+h)), where R is the radius of the earth, 6380 km, and “h” is the height of observation. This gives us arcCos(6380/6475) = 9.8° … where you are claiming that the depression angle is 78.5°.
w.
Yeah, but the part that goes sideways has an increased chance of hitting another molecule before it leaves the atmosphere, so half is a good approximation…is what the other argument is.
Hugs and Willis Eschenbach,
I am a retired EE, not trying to prove anything, just get science right. There are a lot of assumptions taken for granted that the “experts” don’t seem to have the time or inclination to answer or correct. So thanks for the courtesy of your reply to my comments.
My 44% calculation of Nov 19 at 3:46
“Assume an earth radius of 6,378km and the straight line distance to horizon from 95km is 1104.9km (from internet horizon calculator). I calculated a cone of 160.4 degrees or 44.5% of potential 360 degree cone of radiation”
In excel =2*degrees(aCos(1101/(6378+95))) where straight line distance to horizon is 1101.4km, earth radius is 6,378km, and observation height is 95km.
here is where I got straight ine distance to horizon.
don’t see how radiation to earth from 95km can extend beyond horizon.
If distance to horizon link doesn’t work try:
http://www.ringbell.co.uk/info/hdist.htm
Joshv, this is the logical issue I am trying to resolve: “Think of a ** bunch of molecules bouncing around off of each** other, in a collision, one molecule could easily **lose much of it’s vibration or rotation** and cause the second molecule to bounce off faster in another direction. Over many collisions, the vibrational energy from IR absorbance will be equally spread into all of the degrees of freedom, including translational, which increases the average kinetic energy of the gas molecules ‘heating’ them up.”
To me that screams that CONDUCTION being the premier cause of atmospheric warming. That then leads to a whole larger series of questions related to radiational warming and COOLING.
Conduction is quite insignificant. Gases conduct heat very poorly. However CONVECTION is very important, as a matter of fact more important than radiation for heat transport from the surface. Something that is almost never spoken about since it cannot be modelled realistically by GCM.
Evaporative cooling is probably more important than radiative warming, which is why IMO, net feedbacks from water vapor are likely negative.
I agree. Convection trumps radiation every time. Just try holding your hand in front of a ‘radiator’ and then above it, and see where it gets warmer.
It always amazes me how all explanations of CO2 warming ignore convection as though it doesn’t happen or doesn’t matter. Yes, it’s very difficult to measure in the real world, but that’s not a good reason to ignore it!
It’s merely the mechanism of local equilibrium. Remember, these molecules also emit IR at their absorption spectra, and those photos travel at the speed of light.
For the sake of discussion, the CO2 at 100 km in the atmosphere does not have to warm the liquid or solid surface below, it only need heat the gas directly below. Probably why these guys are always talking about a hot spot. …… but as we know, there is no hot spot.
My background is chemical movement in biological systems (pharmacokinetics) so you engineer guys feel free to correct me …. but the reason we don’t see a hot spot, IMO, is because radiation and energy move at the speed of light. From a biological perspective, this would equate to a 1/2 life in gases…. t-1/2, that for all practical purposes is near instantaneous. Thus, the only way for the atmosphere to stay warm is to have a continual supply of heat from solid and liquid surfaces, either directly or from flow (winds) from other places. That all goes back to heat capacity, where the oceans are king. We see this in observation, deserts cool very rapidly, and if not for an inflow of warmer air from surrounding areas, they would freeze every night. In contrast, oceans hold so much heat that they are capable of keeping the air above them warm within a very narrow max min range. …. for oceans it is an equation of energy input, which goes back to solar and clouds, and all that Jazz.
Thus, the atmospheric temp is 100% dependent on a constant inflow of heat. The GHG systems contribution is directly related to its heat capacity, and as you point out in your article, that would only be relevant at -80C, I’ll take your word for it regarding Wiens Law, but probabably also owing to the fact that relative to solids and liquid water, the GHG system has no heat capacity.
The oceans hold about 99.9% of the heat energy in the system. The mass of the oceans is about 24x the mass of the atmosphere. The heat holding capacity of water is 4.2x dry air. The clue that the oceans are the most important part is the seasonal fluctuation in the number and power of storms.
Oldie but goodie and a heck of a lot simpler and clearer.
References:
Trenberth et al 2011jcli24 Figure 10
This popular balance graphic and assorted variations are based on a power flux, W/m^2. A W is not energy, but energy over time, i.e. 3.4 Btu/eng h or 3.6 kJ/SI h. The 342 W/m^2 ISR is determined by spreading the average discular 1,368 W/m^2 solar irradiance/constant over the spherical ToA surface area. (1,368/4 =342) There is no consideration of the elliptical orbit (perihelion = 1,415 W/m^2 to aphelion = 1,323 W/m^2) or day or night or seasons or tropospheric thickness or energy diffusion due to oblique incidence, etc. This popular balance models the earth as a ball suspended in a hot fluid with heat/energy/power entering evenly over the entire ToA spherical surface. This is not even close to how the real earth energy balance works. Everybody uses it. Everybody should know better.
An example of a real heat balance based on Btu/h is as follows. Basically (Incoming Solar Radiation spread over the earth’s cross sectional area, Btu/h) = (U*A*dT et. al. leaving the lit side perpendicular to the spherical surface ToA, Btu/h) + (U*A*dT et. al. leaving the dark side perpendicular to spherical surface area ToA, Btu/h) The atmosphere is just a simple HVAC/heat flow/balance/insulation problem.
http://earthobservatory.nasa.gov/IOTD/view.php?id=7373
“Technically, there is no absolute dividing line between the Earth’s atmosphere and space, but for scientists studying the balance of incoming and outgoing energy on the Earth, it is conceptually useful to think of the altitude at about 100 kilometers above the Earth as the “top of the atmosphere.”
The top of the atmosphere is the bottom line of Earth’s energy budget, the Grand Central Station of radiation. It is the place where solar energy (mostly visible light) enters the Earth system and where both reflected light and invisible, thermal radiation from the Sun-warmed Earth exit. The balance between incoming and outgoing energy at the top of the atmosphere determines the Earth’s average temperature. The ability of greenhouses gases to change the balance by reducing how much thermal energy exits is what global warming is all about.”
ToA is 100 km or 62 miles. It is 68 miles between Denver and Colorado Springs. That’s not just thin, that’s ludicrous thin. 99% of the atmospheric mass is below 32 km. Above 32 km there are very few molecules. Without molecules, energy, heat, cold, hot concepts get a tad iffy.
The GHE/GHG loop as shown on Trenberth Figure 10 is made up of three main components: upwelling of 396 W/m^2 which has two sub parts: 63 W/m^2 LWIR and 333 W/m^2 and downwelling of 333 W/m^2.
The 396 W/m^2 is calculated by inserting 16 C or 279K in the S-B BB equation, a calculation that does not actually exist in the real world. The result is 55 W/m^2 of power flux more than ISR entering ToA, an obvious violation of conservation of energy, i.e. created out of nothing. That should have been a warning.
ISR of 341 W/m^2 enter ToA, 102 W/m^2 are reflected by the albedo, leaving a net 239 W/m^2 entering ToA. 78 W/m^2 are absorbed by the atmosphere leaving 161 W/m^2 for the surface. To maintain the overall energy balance and a steady temperature (not really a requirement) 160 W/m^2 rises from the surface (0.9 residual in ground) as 17 W/m^2 convection, 80 W/m^2 latent and 63 W/m^2 LWIR (S-B BB 183 K, -90 C or emissivity = .16) = 160 W/m^2. All of the graphic’s power fluxes are now present and accounted for. The remaining and perpetual looping 333 W/m^2 are the spontaneous creation of an inappropriate application of the S-B BB equation violating conservation of energy.
But let’s press on.
The 333 W/m^2 upwelling/downwelling constitutes a 100% efficient perpetual energy loop violating thermodynamics. There is no net energy left at the surface to warm the earth and there is no net energy left in the troposphere to impact radiative balance at ToA.
The 333 W/m^2, 97% of ISR, upwells into the troposphere where it is allegedly absorbed/trapped/blocked by a miniscule 0.04% of the atmosphere. That’s a significant heat load for such a tiny share of atmospheric molecules (aren’t any above 32 km) and they should all be hotter than two dollar pistols.
Except they aren’t.
The troposphere is cold, -40 C at 30,000 ft, 9 km, < -60 C at ToA. Depending on how one models the troposphere, an evenly distributed average or weighted by layers from surface to ToA, the S-B BB equation for the tropospheric temperatures ranges from 150 to 250 W/m^2, a considerable, 45% to 75% of, less than 333. Radiation is a surface phenomenon. There is no “surface.”
(99% of the atmosphere is below 32 km where molecular energy moves by convection/conduction/latent/radiation & where ideal S-B does not apply. Above 32 km the low molecular density does not allow for convection/conduction/latent and energy moves by S-B ideal radiation et. al.)
But wait!
The GHGs reradiate in all directions not just back to the surface. Say a statistical 33% makes it back to the surface that means 50 to 80 W/m^2. An even longer way away from the 333, 15% to 24% of.
But wait!
Because the troposphere is not ideal the S-B equation must consider emissivity. Nasif Nahle suggests CO2 emissivity could be around 0.1 or 5 to 8 W/m^2 re-radiated back to the surface. Light years from 333, 1.5% to 2.4% of.
But wait!
All of the above really doesn’t even matter since there is no net connection or influence between the 333 W/m^2 thermodynamically impossible loop and the radiative balance at 100 km ToA. Just erase this loop from the graphic and nothing else about the balance changes.
BTW 7 of the 8 reanalyzed (i.e. water board the data until it gives up the “right” answer) data sets/models show more power flux leaving OLR than entering ASR ToA or atmospheric cooling. Obviously those seven data sets/models have it completely wrong because there can’t possibly be any flaw in the GHE theory.
The GHE greenhouse analogy/theory not only does not apply to the atmosphere, it doesn’t even apply to warming a real greenhouse. (“The Discovery of Global Warming” Spencer Weart) In a real greenhouse the physical barrier of walls, glass, plastic trap the convective heat, not some kind of handwavium glassy, transparent, multi-layer, radiative thermal diode.
The surface of the earth is warm for the same reason a heated house is warm in the winter: Q = U * A * dT, the energy flow/heat resisting blanket of the insulated walls. Same for the atmospheric blanket. A blanket works by Q = U * A * dT, not S-B BB. The composite thermal conductivity of that paper-thin atmosphere, conduction, convection, latent, LWIR, resists the flow of energy, i.e. heat, from surface to ToA and to make that energy flow (heat) requires a temperature differential, 213 K ToA and 288 K surface = 75 C. The atmosphere is just a basic HVAC system boundary analysis.
Open for rebuttal. If you can explain how this upwelling/downwelling/”back” radiation actually really works be certain to copy Jennifer Marohasy as she has posted a challenge for such an explanation.
Nick Schroeder, BSME, PE
Yes, Trenberth’s Earth energy budget diagram is complete nonsense (as are all the other similar version). How it ever got published and is still used is a mystery. Well no, because it is all part of the AGW propaganda..
Don’t forget the oceans. Most of the solar radiation that is absorbed by the earth is absorbed by the oceans. Look at a photograph of the sunlit side of the earth from space. The dark spots are the oceans, where the radiation is absorbed and converted into heat. The oceans warm the atmosphere by evaporation and conduction.
“The oceans warm the atmosphere by evaporation and conduction.”
Not so much by conduction as by radiation. Water is impermeable to LWIR but this also means that it emits stronly in this band. Evaporation transfers (latent) heat to the atmosphere but does not, strictly speaking, heat it.
“Evaporation transfers (latent) heat to the atmosphere but does not, strictly speaking, heat it.”
Only if the water vapor is not part of the atmosphere.
You identify the fundamental reason why the bulk of GCMs run far too hot when they try to close the radiation budget at the TOA. That is in a nutshell Trenberth’s missing heat problem and his lament that higher than justifiable aerosols were employed to cool historical calibrations of the GCMs.
One of them, but I don’t think the main one, they parameterize what % of water molecules that leave the surface, not fall back into the water.
That’s how they got water vapor amplification, they stepped on the physics to make it work to how they thought it worked.
Perfect example of bias.
NickS,
There are very few CO2 molecules above 32km altitude, yet these ad some from lower are supposed to do the heavy lifting to radiate away all of the Earth’s incoming radiation, at equilibrium.
This means these upper molecules must take in, then release,, quite a lot of energy.
Do you have any references to the energy a single CO2 molecule can handle? Or is there no upper limit? If no upper limit, where does the Planck curve come from? Geoff
Geoff,
I have a black ball way of thinking about radiative gases. Think of them as a set of small black balls, with numbers proportional to gas concentration, and disc area proportional to absorption cross section. They absorb and radiate as black bodies, but are not a black surface, globally. Now imagine looking down with IR vision. You’d see a general black body glow, but diffuse. A bit like looking into a fire. If they are sparse above 32km, there won’t be much glow there. You’ll see the layers beneath. There isn’t an issue of how much they can handle; if they are small balls, they absorb less. And of course, as well as radiating, in your view, they obstruct the balls beneath.
If global warming theory went the other way……this would be just as feasible as what we are stuck with
The central error is here: “15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
CO2 absorbs and reradiates 15μm band radiation at all altitudes and all temperatures, just the same as the antenna on your FM radio works in your living room by absorbing 3m band radiation at room temperature, not close to 0°K.
Finally someone else realized the main problem with the article.
They seem to miss the point it is a radio wave and it doesn’t bang into every molecule in it’s path, they keep turning it into the solid photon tennis ball. The lies and tricks we use for classical physics comes back to haunt us at times.
PRECISELY the reason I suggested everyone try and explain things both with wave e-m as well as tennis ball photons ! It is useful. You try BOTH and see what happens.
A good understanding of this issue can be obtained from Staley,D.O., and Jurica,G.M., “Effective atmospheric emissivity under clear skies” J.Applied Meteorology,v.11,349-356,March 1972. The physics of radiation transport in gas mixtures was well established many years ago.
Don’t disagree with that. However the logical outturn is the same as Rod’s. The translational energy created in turn creates vibrational energy, ie it ‘bounces around’ until it dissipates into space. Unless CO2 truly is ‘magical’ or ‘evil’, its degrees of freedom alternate as you describe, but don’t affect anything else.
GHG’s are a red herring. Radiation doesn’t heat , convection heats. And the Earth doesn’t have a glass roof.
sure it does, ever see a laser blast a hole in 8″ steel armor?
That is radiation warming.
But the laser didn’t heat anything until it reached the target as it was just energy.
True, though it does sometimes interacts with stuff, and that causes the air to heat some.
Good, so sunlight does not heat, until it reaches the Earth where it heats. So radiation heats us after all.
When sunlight hits you, you heat. Same for most of the ground.
Naughty, naughty, I see what you are doing here…..
Stimulated emission reminds us of a certain leisure actuvity, and has no other relevance!
Brett, what do you think lasars laze?
The first initial is Light
@Hugs
Very close to correct, it passes straight thru the atmosphere unless it’s at a very specific frequency to a gas molecule that reacts at that frequency. It works exactly the same for your radio which is tuned to one station on the many that are transmitted thru the air.
At least now you don’t have solid ball photons banging into every molecule in it’s path like the article above 🙂
Thanks for posting this Anthony, it is exactly how I as an engineer see it too. There is no other way that the first and second laws can be satisfied at real world and quantum level. It is the only interpretation of the theory that satisfies what we see happening and what has happened historically. Temperatures in the troposphere can be explained and calculated with no reference to back radiation so the conclusion surely has to be that back radiation doesn’t influence temperature.
“For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.”
– They do NOT absorb photons in Long Wave but they absorb energy at the same “levels” through collisional energy exchange and pass on energy to other molecules at the same “levels” through the same collisions.
The most misunderstood aspect of this whole debate. Nitrogen and Oxygen just sit there all inert-like and are not part of the process is simply not correct. 98% of the atmosphere has the temperature/energy level of the “atmosphere”.
(Edited) MOD
– They do NOT absorb photons …
Bill Illis – a reprint of my question below – hope you can assist –
– since Oxygen and Nitrogen are not “greenhouse Gases”
Yet dont they serve some function of retaining heat and maintaining some balance of temp in the atmosphere.
Otherwise, it would seem that the total volume of Greenhouse gases is vastly too small (1%ish not counting water vapor) to retain any quanity of heat
Hard to fathom from a scientific point of view that CO2 or any of the other greenhouses gases are sufficiently powerful to act as the thermostat to the degree which they are credited.
As a matter of fact both oxygen and nitrogen are GHG though weak ones. Undisturbed N2 and O2 molecules do not absorb/emit LWIR, but when disturbed by collision or close proximity with other molecules they do so, though weakly.
As a matter of fact this has even been suggested as a solution for the “weak young sun” paradox, though this would require a very dense nitrogen atmosphere in the Archaean era.
Tom, GHG don’t retain any IR. They scatter it. Until, and only if, this IR returns to the surface, it causes no heating.
The CAGW hypothesis relies on that being significant. As I understand it, actual real-world tests show that it’s not very significant.
“As a matter of fact this has even been suggested as a solution for the “weak young sun” paradox, though this would require a very dense nitrogen atmosphere in the Archaean era.” tty
AFAICS, you can hypothesize just about any remotely sane atmosphere you want in the Archean so long as it doesn’t contain free Oxygen. It’s not like there’s a lot of evidence to contradict whatever you set forth.
There is NO scattering, the N2 and O2 molecules are absorbing energy and entering an excited state.
It is just not through photon absorption.
Let’s say you have a N2 molecule 1 mm above a hot rock on the surface which got hot through absorbing solar photons throughout the day. The N2 molecule is moving around so fast, it is hitting other molecules 6 or 7 billion times per second.
So the N2 molecule is going to hit the rock at some point within a second or so since it started just 1 mm away. It is going to absorb 100 photons worth of energy in that collision with the rock molecule(s) and it is going to become extremely excited and become 1000 times “hotter” than the rest of the atmospheric molecules nearby. in a billionth of a second, it is going to collide with 6 or 7 other atmospheric molecules afterward and share that energy around and now a whole bunch of N2 and O2 molecules are hotter and more energetic and so on.
The atmosphere close to the rock is going to absorb up all that extra energy in the rock and become just as warm and energetic. The whole atmosphere, without a single GHG molecule in it, within 1 metre is all going to be the same temperature as the rock. AND, this all going to happen with 1 second. It is as close to,instantaneous as it gets.
Not a single GHG molecule is required to heat up the atmosphere. The atmosphere is going to warm up from collisions with the surface alone. They will then share that energy upwards in the atmosphere until something like a lapse rate develops.
An equilibrium temperature profile will be established since the N2 molecules can become hot enough to re-share that energy back to the rock. The rock can emit photons to space and it can warm up the atmosphere and the atmosphere can warm it, all without a single GHG molecule.
An equilibrium will be reached which is probably very much like the Greenhouse Effect without any greenhouse gases at all.
A person needs to think through this carefully to really get the point. Be an N2 molecule which is colliding with 6 billion other molecules every second and then translate that to 10 days in the atmosphere.
Molecules in the air also absorb solar radiation on its way toward the surface, most famously O2 high in the atmosphere which interacts with UVA and UVB photons to make and break O3.
And of course air molecules also scatter sunlight on its way down, which is why the sky looks blue, the color of shorter wavelengths of visible light.
Wait a second Bill.
I do not recall disagreeing with you before, but you seem to be saying that anytime a heated surface is in contact with air, within 1 second all of the air within 1 meter of the ground will be the same temp as that surface?
This is clearly not the case.
Whenever the sun is shining on a surface, it is very much hotter than the air more than a inch or a few inches from the ground.
Am I missing something, or did you perhaps misstate that?
Yes, in addition N2 and O2 scatter light via the Raman effect, which is due to rotational energy levels.
This is IMO the most important rebuttal to the nontestable and nonverified Greenhouse Effect hypothesis (if one can actually call it a hypothesis). While N2 and O2 do not absorb or emit LWIR, they do heat and cool via All 3 energy transfer mediums. They also ,make up 99% of the 5000 Trillion tons of atmosphere with CO2 making up less than 1 trillion and H2O vapor making up around 11 Trillion. So roughly 4,987 Trillion tons of N2 and O2 contain almost all the energy that is absorbed by the atmosphere through whatever means is possible. The N2 and O2 are not without temp and most certainly emit half their radiation towards Earth and half out to space. Who cares if that energy is not LWIR, it is still radiation no matter its wavelength.
That’s 4,987 Trillion tons of matter versus some odd 13 Trillion absorbing and emitting energy.
The end is is near for the AGW scam.
Bill, N2 and O2 do radiate; they have to, else there is a contradiction to QM. They have QM predicted modes at 2338 and 1556 respectively, and these are detected only with Raman spectroscopy only.
The author is wrong on many points.The first is the point that CO2 only radiates to space from a very high altitude. In fact it radiates to space from a range of altitudes almost all the way through the atmosphere, although the peak amount of atmospheric radiation to space occurs relative high from about 5 to 15 km. Also radiation directly from the ground is a big factor, especially over deserts and very high latitudes. In addition, the radiation from water vapor and especially clouds is a major source of radiation to space. However with all of that said, increasing CO2 does increase the atmospheric trapping of radiation, which in effect acts like a radiation insulation, and by itself would increase the surface temperature. However, changes in clouds, transport of surface heating by ocean currents, and wind and storage and release by oceans may reduce the effect of the CO2, or may amplify it with the increased water vapor due to increased temperature. So far the data is inconclusive, but seems to indicate small to negligible net CO2 effect, but not due to the authors arguments. In the end, the lapse rate and effective average altitude of radiation to space determine the atmospheric greenhouse effect.
While it is true that CO2 acts as an insulator, it is as much of an insulator as a mesh made out of the lightest silk. I.E., not much. The warmistas write about CO2 as if it were an R30 fiberglass blanket. It isn’t
Leo, from the surface, there a regulation effect of WV.
https://micro6500blog.wordpress.com/2016/12/01/observational-evidence-for-a-nonlinear-night-time-cooling-mechanism/
Comment to Leonard. I have carried out hundreds of spectral analyses. Majority of people have no idea what happens to the LW radiation as as function of altitude. For example, CO2 is so strong GH gas in its wave band zone (10-22 micrometer) that the absorption by CO2 does not increase after 1 km altitude. Why? Because there is no emission energy available. The GH gases cannot absorb more than the Earth’s surface emits. That is a simple physical fact. See a figure below. About 88 % of energy emitted by the surface is absorbed in the all-sky conditions.

the optical window covers at least 30% of the spectrum, if not closer to 40%.
I don’t see how it can be 88% absorbed.
To micro6500. The cloudeness-% of the average sky is 66 %. In the clody sky conditions, the emitted LW radiation by the surface is fully absorbed. All the climate scientists approve this fact. In clear sky conditions the absorption happens 78 %. Look at the first figure of mine. Optical window is not 30 %, it is much less.
It’s clear above the clouds too.
What then is the GHE-difference between NYC and Mexico City (alt. 2 250 m / 7 375 ft)?
” by itself would increase the surface temperature. ”
Take a large room with a pool. Heat it to 30C. Once at 30C fill the room with 100% CO2. Does the pool’s temp go up? No, impossible. I doubt it even cools slower.
An excellent thought experiment and also the basis for a real world experiment at relatively low cost.
The Reverend Badger November 20, 2017 at 9:40 am Edit
Actually, it’s a lousy thought experiment. It’s quite similar to the R. W. Wood experiment. I discuss the problems with your thought experiment here.
w.
If you look at a spectrum, the common form the Y axis is power in Watts, you are right that the energy at 15u per photon is slight, so to make an equal power as shorter wave lengths, there are a lot more photons.
So the flux is higher.
The interactions are photons bypass, they scatter, or they are absorbed.
This results in our experience, things are invisible to that wavelength, ie clear, it becomes reflective, or opaque.
But regardless of how 15u photons interact in the environment, water vapor regulates am temps to not drop much below dew point, at least until it all starts freezing.
When you monitor cooling at night, do you wonder why it’s clear, and yet the temp stops falling in the middle of the night. But most of the year it stops cooling around dew point. But what few realize is that it did not stop radiating to space, in the optical window temps are are nearly 100F cooler than air temps.
What does happen is the sensible heat, IR from condensing water, lights up the sky in all directions in the 14-16u water band, which lights up co2 at 15u. This is strongest at the surface, and it shows up as the rate temps falling while it’s still night out dropping towards zero. A side effect of using WV to regulate temps, it bleeds off atm water every night, this prevents the WV amplification of CS, the key to catastrophic warming. So not only is it not catastrophic, it doesnt even reallt cause any warming, WV will always prevent it.
This is the real GHG effect.
micro6500
November 19, 2017 at 8:07 am: and possibly what Roy Spencer sees with his skyward pointing pyrgeometer. All real experiments including Hans Geiger’s monumental “The air above the ground”, those in the Australian outback, and Hartmann’s, show how CO2 is no different to any other gas except the one which cheats by phase-changing. Which is why radiators have caps, and we exist.
Well cheating is just a matter of which side you’re on.
Micro6500
As stated above your assumption that water vapour is the key to cooling stopping is incorrect. It is heat release from latent heat as dew forms on surface of the ground and plants which slow cooling and then eventually fog forms and once it does then temps just flat line….Fog is liquid water…not the gaseous form water vapour and hence a different mechanism for cooling slowing down. Once fog forms…then the top of the fog layer becomes the radiating surface…not the ground, and as this fog layer cools further at the top, dewpoint is reached and the top of the fog layer rises. This is how fog layers deepen overnight.
Dew is only a small part of it. But there’s a 30-40W/m^2 flux out the optical window at the same time the temperature stops falling.
I can see the same circular arguments and the same immovable positions but nobody addressing the central point of the post. Can radiation from such a low temperature source warm surface matter which is at a much higher energy level, that is the only question to be answered surely. Also if back radiation causes warming how is it that surface temperature and tropospheric lapse rate can be calculated with no reference to radiation?
The sb-equation says no. There is no transfer but the heat, which is the difference in T⁴. Quantum physics is built on thermal physics, so it obeys the sb-law. Quantum is the microscopic details of heat.
The surface transfer 383W/m²-128W/m² to TOA, and TOA transfer nothing back. Neither does the rest of the atmosphere.
I think your question is answered in this post:
https://izenmeme.wordpress.com/2017/10/29/back-radiation-and-the-2nd-law-of-thermodynamics/
Which is a variation of this post:
http://rabett.blogspot.co.uk/2017/10/an-evergreen-of-denial-is-that-colder.html
Sorry RS but that answers none of the questions I raised. It certainly doesn’t explain cold to hot heat transfer. It doesn’t even touch on why all tropospheric temperatures can be explained and calculated with no reference to back radiation. I really think that AGW is in desperate trouble?
The problem seems to exist in the word “warms”. Radiation from a cold object cannot “warm” a warmer object. However, it CAN leave the warm object warmer than it would have been without the cold object.
As an example. When we look up, the apparent temperature of the sky based on the amount of downwelling thermal radiation is on the order of -50°C … how can this radiation “warm” the much warmer earth?
The answer, of course, is that without the atmosphere the apparent temperature of the sky would be about -270°C … and as a result, we are warmer because of the existence of the atmosphere.
Does the radiation from the atmosphere “warm” the surface? No … but it does leave the surface much warmer than it would be without that radiation.
w.
Thanks I didn’t notice how you said this already so clearly.
Willis Eschenbach
November 19, 2017 at 8:35 am: By its mass, and the sun. Just as explained by all real experimental Physicists from Maxwell (Theory of Heat) onwards. Not to mention the Mylar balloon expt, and all Nasa atmospheric readings through the solar system. Even Sagan admitted this eventually.
No, we are “warmer” because the oceans absorb a lot of the sun and release that energy slowly, which heats the atmosphere mostly by direct molecular contact with the air. The cooler air cannot warm the surface, only slow the rate of heat loss. It appears to have warmed because the temp number is higher, but that is not “warmer”. Example, day time heated by the sun gets to 30C, during the night it cools, losing that heat. If the lowest it gets on a clear night is 10C, then we have lost 20C worth of energy. But if on a cloudy night it instead goes to 20C, then we have NOT gained 10C in new energy (*warmer”). What has happened is less energy is lost. It’s not “warmer” on the cloudy nights, it’s less cooled. Because the temp is 10C higher numerically, we say it is “warmer”, but in reality it isnt warmer (no new energy added).
Willis,
Expressed well!
This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.
“to warm” AND “to slow the rate of heat loss” are separated
geez my spelling…
Hugs:
“This is the umpteenth time when “to warm” to “to slow the rate of heat loss” are separated, yet, “slowing the rate of heat loss” results in a warmer average, i.e. warming.”
To warm an object requires new energy to raise its temp. Losing less energy already there has a higher temp number, but it is not “warmer”. If you earn $1000 per month gross, and have a high tax rate of 25%, you lose $250, netting $750. But if the tax rate is lowered to 20%, you now lose only $200, does that mean you have earned $50 in new money? Nope. You earned the same, just lost less of it in taxes. Consider winter and night times as a tax on the energy the sun put to the earth during the day. Having a higher NET energy doesnt mean you are “warmer” because you dont have more gross energy.
Back radiation is competely irrelevant, as is sideways radiaton and slant radiation and every-which way radiation. The only important factor is the net upward radiation. Look at this formally correct but carefully doctored NASA diagram:

What it shows is that LWIR moves 66 Wm^-2 from the surface, 40 by direct radiation in the “window” and 26 by way of absorption/re-emission by GHG. It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat, 102 Wm^-2, of which 78 is latent heat of evaporated water, which condenses at high altitude and radiates the heat into space.
Also note that radiation is gross while convection is net (and that the radiation arrows are fat and red while the convection ones are as puny as possible). Taken at face value this diagram implies that the descending cooler/drier air and precipitation is at absolute zero since in this case there is no “back radiation” (=downward heat transport). The reason for this difference is that a honest diagram would show clearly that convection is the dominant process regulating surface temperature. And convection can’t be modelled by GCM, only “parameterized” (=guessed at) since it happens on a far to small spatial scale for GCM:s.
tty, always pleasure to read your comments. The red arrow is a red herring!
No oceans no model.
This is the flat earth model, and it is wrong. It overly simplifies and incorrectly depicts what is going on. Postma has written a lot about this flat earth model having no basis in reality.
The sun puts 1370W/m^2 to the surface, not 168 (which wouldnt power two 100watt light bulbs). The surface temp of the earth would be some 69C at that input, but it’s not. Why? Because the earth rotates. Only a small percent of the surface receives this input, the rest loses more than it gains. The earth’s rotation acts like a thermostat. If it rotated slower it would be too hot end of the day for life. It it rotated faster, it would not gain enough during the day to overcome the night time loss rate.
[??? .mod]
J. Richard Wakefield
Well, that 1370 watts is actually only 1362 watts/m^2.
But it is actually measured at 1362 watts/m^2 at the top of atmosphere, only about 1000 watts/m^2 gets through a clear atmosphere to the surface of the earth, at the equator. And that 1000 watts/m^2 only gets to the surface at noon on the equator, on a clear day, on average.
Over the whole year, the CAGW climacatastrophy community has accepted Trenberth’s [168] watts/m^2 absorbed + [30] watts/m*2 reflected as average values for the whole earth.
But that 198 watt/m^2 yearly average sunlight on the surface for every day and night is ONLY valid for the temperature regions ONLY between 55 north and 55 south latitudes. Any further north or south and the yearly total “average” sunlight goes much, much further down.
Spot on, tty! It’s not a scientific schematic, but a Soviet-style propaganda poster, with self-serving distortions of reality.
“It is only these 26 Wm^-2 that are effected by the amount of GHG in the atmosphere. Convection moves much more heat”
No,it’s not. The diagram is a budget. It records the fluxes that can be measured, and tests whether they are in balance. The flux that is affected by GHG concentration is the full 324 W/m2 downflux from air. The 390 W/m2 upflux at the surface responds to the temperature there. The difference, if you exclude AW, is small at 26 W/m2, but is pretty much locked. The 324 W/m2 is emitted from air near the surface, as you can tell from its magnitude. If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.
Dry convection is necessarily entered as a nett flux, because there are no measurements that can split it into separate streams. Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.
True, it doesn’t get too far. But it does get to the block called the atmosphere above.
“If the surface warms, that air warms too, and the flux difference remains near constant. It is not sensitive to the amount of GHG.”
In that case You can kiss CAGW goodbye, because there is nothing else that GHG can affect.
” Its magnitude is often exaggerated by commentators. It is small because, while the lapse rate is less than the theoretical dry adiabatic, the air is convectively stable. Air that acquires heat cools at DALR as it rises, and doesn’t get far before it reaches ambient.”
True in so far as wet convection is of course by far dominant. Ever see cumulonimbus clouds? They are not close to the surface.
Martin Mason,
A cold object cannot warm a hot object by conduction, but a warm object (Earth) can warm a cold parcel of absorbing gas by radiation. The radiation that would have otherwise left the system, is retained and now available to contribute a slight warming of the dark side of the Earth where it is being subject to the IR radiation from the gas, which is above absolute zero. Consider the impact of an Earth radiating directly into space, versus an Earth radiating into space with an intervening blanket at a higher temperature than space.
I don’t understand the question in your last sentence.
Martin Mason.
You can test this experimentally. Two identical set ups one with no CO2 and one with lots. If back radiation exists and can transfer heat a measurable temperature difference can be seen. Relatively easy lab work and relatively inexpensive.
The answer of course can be answered by any five year old playing in the snow. No, radiation from a cooler object categorically doesn’t increase the temperature of a warmer object.
The ONLY^3 reason RGHE theory even exists is to explain how the average surface (1.5 m above ground) temperature of 288 K/15 C (K-T balance 289 K/16 C) minus 255 K/-18C , the average surface (now ground) temperature w/o an atmosphere (Which is just completely BOGUS!) equals 33 C warmer w/ than w/o atmosphere.
That Δ33 C notion is absolute rubbish and when it flies into the nearest dumpster it hauls RGHE “theory” in right behind it.
The sooner that is realized and accepted the sooner all of us will have to find something better to do with our time and the taxpayers’ money. Maybe that’s what keeps RGHE staggering down the road.
The genesis of RGHE theory is the incorrect notion that the atmosphere warms the surface (and that is NOT the ground). Explaining the mechanism behind this erroneous notion demands some truly contorted physics, thermo and heat transfer, i.e. energy out of nowhere, cold to hot w/o work, perpetual motion.
Is space cold or hot? There are no molecules in space so our common definitions of hot/cold/heat/energy don’t apply.
The temperatures of objects in space, e.g. the Earth, Moon, space station, Mars, Venus, etc. are determined by the radiation flowing past them. In the case of the Earth, the solar irradiance of 1,368 W/m^2 has a Stefan Boltzmann black body equilibrium temperature of 394 K, 121 C, 250 F. That’s hot. Sort of.
https://science.nasa.gov/science-news/science-at-nasa/2001/ast21mar_1/
But an object’s albedo reflects away some of that energy and reduces that temperature.
The Earth’s albedo reflects away about 30% of the Sun’s 1,368 W/m^2 energy leaving 70% or 958 W/m^2 to “warm” the surface (1.5 m above ground) and at an S-B BB equilibrium temperature of 361 K, 33 C cooler (394-361) than the earth with no atmosphere or albedo.
https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-723
The Earth’s albedo/atmosphere doesn’t keep the Earth warm, it keeps the Earth cool.
Bring science, I did. (6,000 views and zero rebuttals.)
http://writerbeat.com/articles/14306-Greenhouse—We-don-t-need-no-stinkin-greenhouse-Warning-science-ahead-
http://writerbeat.com/articles/15582-To-be-33C-or-not-to-be-33C
http://writerbeat.com/articles/16255-Atmospheric-Layers-and-Thermodynamic-Ping-Pong
Looked out. Can’t see much irradiance. I guess you should integrate to get the average surface value which is about quarter of the 1368W/m². Temperature was consequently around 278K. I guess it were colder without some warm gases and precipitation between my front yard and the 2.7K background.
Average is nonsense and does nothing to tell us what is going on. Example. Take a photograph of something, say a nice mountain range. Add up all the colour pixel numbers and divide by the number of pixels, then apply that number to each pixel. What do you get? A gray image with nothing to see.
This is exactly what you are doing by applying the average. You add up all the W/m^2 for the area, divide by the number of areas, and apply that to all the areas. It’s BS grayness that has no physical counterpart.
It tells me much more than the peak value misleadingly used above.
Hugs:
“It tells me much more than the peak value misleadingly used above.”
Yes, using JUST a peak value is just as meaningless. You want a real view of energy absorption and loss on this complicated and dynamic earth? Then do a proper 3D graphic of a tilted rotating spherical planet with large oceans and disproportional land masses that have different absorption. That flat earth graphic tells us nothing of what it really going on, and if anything has grossly mislead people into thinking a simple “budget” explains things. It doesnt. That graphic was invented to justify the AGW dogma. Again, AGW exists because of deliberate ambiguity.
The claims in this article are unfortunately not even wrong.
IR absorption by CO2 excites the vibrational modes of a CO2 molecule.
As the CO2 molecules collide, the excited vibrational modes transfer energy to the motion of other CO2 molecules. The average kinetic energy of molecules is what we measure as the temperature of a gas.
Nothing to do with the absorption and emission of visible light by electron level transitions
A correct summary of the process may be found here
“Has Global Warming Paused”
https://www.scribd.com/document/239526963/Argonne-National-Lab-Talk-Happer
A lecture by William Happer, a molecular spectroscopist and professor of physics at Princeton U.
One can run MODTRAN online here:
http://climatemodels.uchicago.edu/modtran/
As a simulation subject matter expert, you can’t use MODTRAN and run a single look through an average air sample and get anything useful.
If you do not run it over a days cycle as conditions change, all you’re doing is fooling yourself.
The excited modes of CO2 do NOT transfer energy to the motion of other CO2 molecules – or do it only in less than 0.04% of cases. What happens when an excited CO2 molecule collides with an N2 or O2 molecule or an Argon atom is very complex and I have not seen a satisfactory analysis.
Have a look here.
http://aip.scitation.org/doi/10.1063/1.4926880
Paywalled. But according to the abstract they handle CO2-CO2 collisions. As a concentration of CO2 is currently 0.04%, these collisions are very rare. And how about CO2-H2O collisions?
No CG, they do. That is why the absorption spectrum is in BANDS and not a single exact wavelength. The band of wavelengths show the range in energy needed to bump the molecule into a vibrational state, and the difference is caused by the very slight decrease or increase in kinetic energy from molecular collisions.
Wouldn’t a simple experiment (already conducted by John Tyndall) learn that 15 micrometer IR radiation is emitted and absorbed at other temperatures as well?
Eric
November 19, 2017 at 8:18 am: Tyndall made sure his absorber was cooled below his emitter, is why. The SB assumption, indeed, is to 0Kelvin.
The mistake was made with Tyndall, he was using a thermoelectric transducer, a thermopile: he discovered the thermo electric gases – we call them the GHGs. Watch to see what I have discovered https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
i think the author misapplies his second key point “The temperature of a (gas) directly affects the wavelength of the radiation it emits and absorbs.” Looks like he applies it incorrectly. Yes, for CO2, most of the outgoing radiation is mostly from the stratosphere; and in the atmospheric window region is mostly from the lower troposphere. From link article which concludes “The total temperature-dependent gas absorptivity effect (H2O, CO2, etc.) tends to result in a radiation energy convergence that decreases the total cooling of the atmosphere.” https://www.gfdl.noaa.gov/bibliography/related_files/yih0702.pdf
I got as far as this one …
I never heard that about “too hot to exist”, and I don’t believe it.
Citation?
w.
I guess that he means that those bands are unimportant because they are at wavelengths where the Earth emits virtually no radiation at realistic ground temperatures. I agree that it is a very clumsy way of expressing this
Thanks, tty, but I have no idea what he means, nor do I particularly care. I was asking for a citation about “the temperatures too hot to exist”.
w.
Willis, you are a stickler for quoiting correctly when rebutting. I would like to point out the important qualifier that you are missing in your quote reference. He specifically said “to hot to exist in the atmosphere“. A distinction that is pertinent to his point..
Clearly I try not to rely on spellcheck,…..perhaps I will start.
Willis
The article is a mixture of facts and fallacies. The author is confused by a lot of ‘information’ gathered from various sources. Some of the bloggers are in the same boat. Too many things to correct so I just shut my mouth. Nevertheless, it’s entertaining to read some of the well-meaning comments.
Willis, re: “too hot to exist”. Rod Gill is confusing de-excitation radiation from the CO2 molecule with thermal radiation as described by Wien’s Law. That’s why he claims the CO2 emission bands at 2.7 and 4.3 microns only come from CO2 molecules at temperatures too hot to be found in the atmosphere (800° C and 400° C, respectively, if calculated from Wien’s Law). That’s also behind his ludicrous claim that CO2’s 15 micron emission band comes only from gas at -80° C.
Rod Gill fundamentally misunderstands the physics behind the so-called “greenhouse effect”, and he has no business publishing an article about it. I would have preferred that Anthony or one of his volunteers gently point that out to him and not publish such pseudoscience.
Of course the comments reveal that such confusion is fairly common, but we already knew that.
Wien’s Law calculator:
https://www.ajdesigner.com/phpwien/wien_equation.php#ajscroll
Willis,
Agreed, the first confusion is in assuming atomic excitation instead of molecular. Then electron states instead of rotational, vibrational, etc molecular states. Several other errors follow as a consequence.
However, I think the exercise is useful because it’s responses can correct those readers who were similarly starting from the same wrong point.
I tend to shut up because when my formal training in the 1960s was done, atomic was dominant and molecular seldom covered in lectures other than to say that molecular spectroscopy was a harder concept. Geoff
David Ball November 20, 2017 at 12:07 am
His claim is not true, whether he is talking about the atmosphere or the earth’s surface. So no, his qualifier is not important.
w.
Willis Eschenbach November 20, 2017 at 1:13 am
Which claim are you referring to? You are not being clear. “In the atmosphere” is definitely an important qualifier because he is talking about how the atmosphere actually operates and the range in which these interactions occur. You saying it isn’t does not make it so.
David Ball November 20, 2017 at 11:07 am
His claim that:
w.
I got as far on your posts as you claiming that radiation from a cooler shell could increase the temperature of a warmer sphere.
I’d love to invite you into a cave in a glacier sometime.
wickedwenchfan November 20, 2017 at 2:51 pm Edit
I’m sorry to hear that. Had you continued, and considered what I was saying, you might have learned something.
In the meantime, have you never been outside on a winter night when clouds came over? They leave the surface warmer than when there are no clouds. Clear winter nights are the coldest … why?
Because when there are no clouds, the back radiation is coming much more from outer space at a temperature of 3K or so, whereas the clouds are radiating at something like 225K …
Think about it. If a planet has no GHG atmosphere, it is exposed directly to outer space.
But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.
I’m not sure why this is so hard for some folks to grasp, but I assure you that it is settled science taught in the universities and backed up by the math. If you are interested in the math, there is an online calculator here. If you play with it for a while it may help you start to understand the nature of two-way radiative energy exchange … it spells out the formula for how it is calculated.
w.
Willis Eschenbach November 20, 2017 at 3:45 pm
Willis, you are mixing apples and oranges. The clouds have a higher temperature than the rest of the atmosphere because of the latent heat of condensation being released. This is a phase change of water effect, not a GHG effect.
Secondly, your sentence
‘But if it has a GHG atmosphere, it is receiving radiation from that atmosphere, and this leaves the planetary surface WARMER THAN IT WOULD BE WITHOUT THE ATMOSPHERE.’
is obvious and there is no need to shout. Everyone agrees that the atmosphere warms the earth compared to no atmosphere. The point at issue is does replacing a few O2 molecules with a few CO2 molecules make a difference?
Bernard Lodge November 20, 2017 at 8:34 pm
The claim was that the cold atmosphere (containing cold clouds) could not leave the surface warmer than it would be without them, viz:
wickedwenchfan November 20, 2017 at 2:51 pm Edit
Since both the atmosphere and the clouds are colder than the surface, I am NOT mixing apples and oranges.
No, “everyone” does NOT agree that the cold atmosphere warms the earth compared to no atmosphere. There are literally dozens of people who claim that it is impossible. They all say that because the atmosphere is colder than the earth, the atmosphere does not and cannot “warm the earth compared to no atmosphere”.
Which is why I was shouting … I’m getting tired of people saying the same thing over and over, when the answer is there from our own senses regarding winter clouds.
w.
PS—Can a block of ice leave a person warmer than no block of ice?
Sure … as long as the block of ice (at 0°C) is interposed between that person and a tank of liquid nitrogen (at -196°C). The ice provides more radiation to the person’s body than does the liquid nitrogen, so the person ends up warmer.
And the same is true about icy clouds which are interposed between a person and 3K background temperature of space … they leave the person warmer than no clouds.
CO2 molecules can absorb radiation and then transfer that energy to other gasses by conduction and versa visa.
Though much easier by re-radiation. Gasses do not conduct heat well. There is a reason why most good insulating materials are mostly air.
At atmospheric pressure the conduction takes place with molecular collisions that theoretically occur faster than a CO2 molecule can radiate its heat — 10^-7 s.
It is pressure dependent as rgbatduke always said. But I don’t really know how this affects – probably surface CO2 absorbs only, and does not have time to emit, so the air eats the radiation band. And then complicated (parameterized) things happen, like evaporation, convection. I’m sure the problem is not high school physics as Gore said. He lied.
yes they do, a dewer flask ( a vacuum flask) has a vacuum as an insulator, so your coffee keeps warmer for longer than if air was used to insulate.
1) The “compressor” is always on.
2) This, coupled with geothermal
( https://wattsupwiththat.com/2017/11/15/new-map-of-antarctic-geothermal-heat-suggests-steig-mann-2009-werent-measuring-global-warming/ ), account for our temperature range.
The earth is a magma filled balloon, at least the outer layers that we have been able to measure look that way.
3) A Chinook demonstrates the effect of compression directly. Ne c’est pas?
4) This idea also satisfies Dr. Svalgaards assertion that TSI variance is too small to account for the variations of earth’s temperature.
5) One has to admit that the name “Greenhouse” in no way is descriptive of our atmosphere or how it works.
6) No matter how our atmosphere works, we are fortunate that the relative stability of temperature is within the range of water existing in all three states. As a carbon based life-form, I am grateful of this.
7) Ockham’s razor and plausibility Apply liberally, rinse, repeat.
( numbered for your rebuttal convenience and clarity, also inspected for politically free content )
CO2 molecules can absorb radiation, and immediately emit said radiation, but in a random direction, ie scattered.
They cannot absorb radiation and convert it to kinetic energy (heat).
Jer0me
November 19, 2017 at 1:10 pm: But in our air, they are struck kinetically thousands of times between each possible emission. Thusly, air eats radiation so convection and phase change of water dominates hugely to above the net emission height. Radiation is swamped by water anyway, which even Mann knows. It is all sleight of pen for the cause. Bring on the State Pen..
Rod, thanks for your post. However, you ask us “Am I right?” about the following:
Nope. You are wrong. However, given the depth of your misunderstanding, and your certainty that you understand it, I’m gonna leave the question of WHY you are wrong for you to work out for yourself.
w.
Show us a reference for transfer of energy from co2 at lower temperature to a solid warmer body, in a controlled environment. If you can’t, there is no transfer.
There is always transfer in both directions but the net transfer is always from hot to cold.
I asked for a reference, not you making stuff up.
Fact: at equal temperature, no transfer. So why would there be transfer from cold to hot if one body is colder.
Show me a reference for any transfer outside of “net”, or shut up.
You ever use an IR thermometer and point it at a fire, and then in a freezer?
You got readings from both objects, what do you think they were measuring?
Oh, just to be clear, then imagine you point it at liquid nitrogen and compare that to the freezer. They all radiate.
Show me a reference that shows that an atom tells an incoming photon: “thank you, but no, since you com from a colder atom”.
What happens if the molecule/atom is already excited to the energy level of the incoming photon?
Then what the difference does it make?
lifeisthermal, carbon dioxide lasers operating at room temperature can cut through metal:
@Gary Young Hladik
So laser beams heat … errrrrr except when they cool
https://en.wikipedia.org/wiki/Laser_cooling
Yep you can use a laser beam to cool an object to near absolute zero explain away.
Love classical physics it is so simple except it’s totally wrong 🙂
LdB, I’m not getting your point. Are you saying the CO2 laser beam CAN’T cut metal? Maybe you’re saying the laser cuts metal by COOLING it? Help me out here.
LdB November 19, 2017 at 8:27 pm
It would help if you actually understood “classical physics”. Then you wouldn’t be so confused and would understand the difference between cutting with a laser beam and laser cooling.
So is there a difference in the beams between a cutting laser and laser cooling Gabro?
I like that concept I am going to get me some cool laser beams 🙂
@Gary Young Hladik
The problem I was illustrating is even things we think of with one behaviour don’t always behave like that, you are saying the beam will always thermally act one way. That beam as it moves thru the air is a electromagnetic wave whether it heats or cools or even has any effect (think a mirror) is governed by a set of strange laws.
If that laser is a CO2 cutter and I replace the steel with aluminium, I assume you know what happens.
Try even cutting a very fine steel mesh 🙂
It’s a EM transfer you can’t equate it to thermal energy, you can heat,cool or reflect it.
Oh and for anyone who wants to know how it works.
It’s pretty straight forward you need 6 or more beams to do cooling, the frequency used matches one of the resonant frequencies of the atom you are trying to cool and it doesn’t actually see the beam as hot because it absorbs it and then re-emits it.
LdB November 19, 2017 at 9:30 pm
Yes, there are many differences between cutting and cooling lasers.
LdB,
“…you are saying the beam will always thermally act one way.”
Actually, I’m saying “carbon dioxide lasers operating at room temperature can cut through metal”.
Oh really please tell me 🙂
@ Gary Hladik
Yes I know, I was just firing a warning shot … yes but be careful.
If that metal is aluminium for example it will reflect and if the metal has a resonant frequency at that frequency it can be transparent or cool the metal.
We do that trick in QM making materials transparent
https://en.wikipedia.org/wiki/Electromagnetically_induced_transparency
I could make your laser beam pass right thru the metal plate .. but this isn’t something you would see in nature 🙂
Why does it surprise you that lasers can both heat and cool?
How lasers can be used both to cool to the coldest temperatures on earth and to heat to the highest:
http://physicscentral.com/explore/poster-spots.cfm
There are many techniques for laser cooling, although the Doppler technique is probably still the most common.
@Gabro
No you are claiming there are cool lasers ™, you directly claimed it.
Now you obviously bothered to read and are now trying to dig yourself out of a hole, there is no such thing as a cool laser ™ they are all the same.
As I have already stated the laser can heat, cool, reflect, absorb or transmit thru any material which strangely enough is the same for every single EM frequency that exists including radio waves and thermal emissions. QM is very concise and very well tested on all this stuff/.
Willis, I’m trying very hard to do this myself so please don’t cop out.
Martin, thanks, but I have no clue what you mean by that …
w.
I like to use the analogy of an Eskimo igloo, where the snow, forming a temporary barrier, captures the heat inside the igloo long enough to maintain a comfortable temperature.
Which of course is an incorrect analogy as the igloo is a physical barrier to convection, which is how the surface loses most of the heat. It has zero to do with radiation effects.
CO2 is a physical barrier to some radiation. It is an analogy, not saying ice chunk is a greenhouse gas. Of course, ice IS a weak barrier to radiation and it does contribute to how igloo warms.
Jer0me,
You can heat a clear container of CO2 by radiation as from a laser beam of appropriate wavelength or a beam of sunlight passing through a small part of the wall. If the beam is turned off, the hot CO2 will heat the rest of the container wall as the CO2 cools by normal heat transfer equations. Is this at odds with what you mean? Geoff
joelobryan
November 19, 2017 at 10:09 am: You almost got there. The crooks know CO2 is too weak (at best, in fact it is a coolant). So they concocted an effect on water vapour to magnify things. This is on record. Other planets and large moons prove CO2 is not special except for life. We have that on record too.
A waterless planet with no tri or polyatomic gases, eg all Nitrogen, would still warm by conduction as we do. Conduction remains the dominant first mover, unless WV latent heat beats it on Earth, micro555? The emission from kinetic motion in the molecular force fields would still cool it. What is debateable is how far such an atmosphere has to expand outwards to radiate enough( by the Gas laws, which do indeed rule). Larger planets should somewhere reach sufficient gravity to keep hold of more gas than their net losses through what is really evaporation.
In infinity, I am fairly sure that such experiments are in progress.
Willis, that’s a bit harsh on Rod. He clearly invited us to reveal the deficiencies in his argument. What more do you want? This is the debate.
The day you can warm yourself up by standing in front of a block of ice, is the day you get to be taken seriously, Willis
The day you realize that you can warm yourself by standing in front of a block of (water) ice instead of a block of dry ice, or a tank of liquid nitrogen, is the day you get to be taken seriously, WWF.
Off you go, Ed.. go and dip your head in a bucket of ice, again
Your brain has surely frozen.
Junior high physics really did stretch your limits didn’t it.
So Andy: Suppose I, as an evil GHE believer, have you tied down to a big block of ice at 0C in a room at 25C. After a while, you get hypothermic and your body temperature drops to 35C.
I now offer to let you move to a big block of slate rock (which has the same thermal conductivity) at 25C in the same room instead, saying it could help you to increase your body temperature back to 37C.
By your logic, you would refuse, because the slate rock is still below your body temperature, so the change couldn’t possibly lead to an increase in your body temperature.
Now, if you were dead, that would be true. (You may be brain dead, but…)
Oxygen and Nitrogen are not “greenhouse Gases”
Yet dont they serve some function of retaining heat and maintaining some balance of temp in the atmosphere.
Otherwise, it would seem that the total volume of Greenhouse gases is vastly too small (1%ish not counting water vapor) to retain any quanity of heat
Hard to fathom from a scientific point of view that CO2 or any of the other greenhouses gases are sufficiently powerful to act as the thermostat to the degree which they are credited.
O2 and N2 certainly have molecular heat capacity. That is not the issue here. As diatomic molecules with limited vibrational modes, they only absorb in the UV band. Triatomic and higher molecules like CO2, H2O, O3, CH4, NO2 are the GHG’s because they have vibrational modes that allow absorption of IR wavelength photons.
But in terms of IR electromagnetic radiation at the temps under consideration for Earth’s atmosphere and surface temps, the three main gases, that is O2, N2, and Argon (that’s 99.5% of dry air), are transparent to IR wavelengths. Thus those 3 main components of dry air cannot absorb IR photons to warm. They are transparent to IR. Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar. But the GHE in the atmosphere also depends on the adiabatic lapse rate of the troposphere, which exists because pressure drops with altitude (gases are highly compressible of course). This temperature gradient creates an effective radiation level (ERL) where a point (vertically, a temperature profile) is reached where more photons escape to space than are re-radiated toward back toward the surface.
So now think of very dry desert air, how large a temperature swing there is from day to night. During the day, there is visible light and UV heating both the minimal water vapor and the surface. The air layer near the hot surface warms due to convective transfer. At low viewing angles, the shimmering desert convective heat flow is seen as a mirage because refracts the sunlights. But in a dry desert, with little water vapor, after the sun sets, the air cools rapidly, losing several degrees/hour because their is little vapor to help trap the escaping IR wavelength photons. That’s radiative cooling. The other non-condensing GHGs (CO2 in the troposphere, ozone in the stratosphere) help slow the cooling. This is why GHG theory predicts night-time lows will get warmer under increasing CO2 while having little measurable effect on day-time high temps.
The above descriptive physics is why the GCM outputs “predict” a tropospheric hotspot in the tropical latitudes.
And going from 3 CO2 molecules per 10,000 air molecules (0.03%) to 4 or 5 CO2 molecules per 10,000 air (0.04% to 0.05%) makes a negligible contribution to the trapping (back radiation) when the atmosphere (troposphere) contains a much higher molar quantity of (1% – 3%) H2O gas. If the atmosphere were completely dry then CO2 concentration changes would make a measurable impact on heat trapping.
This last fact is likely why the Tropospheric Hotspot in the tropics has not been observed. The GHE of CO2 is too weak to be detectable. This argues for the modification of the CO2 forcing theory from one of a strong GHE effect to a weak GHE effect for CO2. The evidence points to a weak GHE hypothesis for CO2. And that is something the current-day climateers, who are profiting from global warming alarmism, are loathe to admit.
Joel, the lapse rate is determined only by gravity and gas physical properties, it has no input or output from radiation. I don’t believe that the lapse rate creates the apparent radiation level, this depends only on the OLR magnitude and the surface area required to radiate it at the effective radiating temperature (-18C).
Surely there is no “trapping” of radiation by GHG’s?
Taking the relative humidity vs ghg analogy with deserts a step further –
the average relative humidity of a typical desert ranges from 10%-30% (various sources) while the ypical countryside ranges from 50-90%. the delta of the day/night temp swings between deserts vs countrysides is in the range of 20-30 degress. With the concentration of water vapor being 200-400x the concentration of co2, it would seem that an increase of 150ppm would be less than 1-2% of the effect that water vapor would have when you compare desert vs countryside.
(apologies if my explanation isnt the best)
Rh only matters when air temps are near dew point, that’s when it stops cooling.
Even though humidity is low in the desert, clear night min temp should be near dew point.
“Our atmosphere’s temperature depends on the presence of trace gases that do absorb IR (mainly H2O in its gas phase.) and then transfer their kinetic energy through collisions to N2, O2, and Ar.”
No. The sink of heat energy is the oceans. They warm the atmosphere by evaporation. that is how the water vapor gets there. Confirmation sip a pina colada at a sea side bar at night. Try the same thing in the desert.
It cools 3-4°F/hr in ohio at first too. It just stops at dew point, and the daily range is much higher in deserts.
These are both clear calm nights.
Good point top: if N2 and O2 do not emit or absorb IR, this is a contradiction to QM and thermodynamics.
They do radiate, they are GHGs and here how: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
The 15um radiation doesn’t warm anything, the sun at 500k does. All the 15um does is cancel out the 15um radiation coming up from the surface. The sun makes up the difference. Hence the atmosphere warms up a little.
That’s the theory, whether it happens or not or can actually be detected or not is the issue.
Quality not quantity.
My thinking for years now.
Sometimes called the Ultraviolet Catastrophe and the Perfect Example is solar panels.
(The UV Catastrophe was the title of a BBC Horizon program explaining a time when scientists couldn’t explain certain ‘wavelength’ or colour sensitive phenomena.)
Just exactly why do solar panels need direct (or reflected) sunlight?
Peta of Newark
November 19, 2017 at 8:39 am: We had a paper by warmist students a while back proposing panels to harvest the background radiation of Earthly IR return. Very excited they were at all that amperage (equalling solar input, after all). Only the sound of crickets since then, as they seek the voltage, I guess.
That pretty well explains the AGW myth, really. Magic and magic mushrooms.
The relationship stated in the quantum physics of electron orbitals and any relationship to temperature due to molecular kinetic energy is wrong. Dead wrong.
Sort of kills any value to discuss your other points when such a fundamental error is made.
joelobryan
November 19, 2017 at 8:53 am : Joel, please expound rather than excoriate. The poster requests this respectfully.
a point nobody has tried to explain, the earth radiates the LW towards space because space is much cooler than the earth, what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? after co2 releases the tiny portion of the wave it grabbed for a picosecond again isnt a force REQUIRED to send in back towards earth?
“…isnt a force REQUIRED to send in back towards earth?”
Cold to hot DEMANDS work per thermo, e.g. a refrigerator.
It is perfectly possible for a cold molecule to emit a photon to be absorbed by a hotter one. But the flow of heat which is a statistical phenomenon will always go from warmer to colder (except for very small random fluctuations).
Energy is thermal property, heat is a thermal process. IR is a surface property not a bulk property. Where is the GHG surface?
“what force gives co2 the POWER needed to reverse that natural direction and send anything back towards the earth????? ”
Simply the ‘force’ of radiative exchange. Just because something is colder does not stop it radiating to something hotter. It just means that the net flow is from warm to cold. All things at temperature above 0K radiate. The atmosphere radiates, it just so happens that it is mostly transparent to LWIR (N2 +O2 = ~ 99% and there is an atmospheric window at around 12 micron for the GHGs – see graph) and at the Earth’s temp, 15 micron is the radiation it most strongly emits at. … which CO2 also happens to absorb/radiate most strongly at (just a bit more than H2O).

It comes back because the Earth’s surface is partially in the way of a measure of photons re-emitted. In the mean time (during daylight) shortwave has come in. It’s that extra SW that is causing the GHE.
The ‘delay’ in emission is due to the path-length of a photon through the trop and why it is far from saturated. At around 8km the molecular concentration falls off enough to allow most LWIR to get to space and that level is at the temp the Earth shows from space (255K) whilst here on the surface we live at it is 288K. So at that temp CO2 emits less strongly (being colder), yet it can still absorb at the same rate.
So molecules high up still radiate as normal but they are receiving less because of ‘blockage’ below – this causes stratospheric cooling while the trop warms.
“Net” flow of heat from warm to cold confuses the issue. the correct term is the only flow of heat is from warm to cold, surely there can be no actual flow of heat from cold to warm regardless of radiation flow if the radiation flow can’t be thermalized rather than reflected or transmitted.
In the sense than photons from the cold object impinge on the warm object there is ‘flow’, but the net flow is always from warm to cold unless work is done.
This is the fumdamental mistake made when people say “a cold object cannot heat a warm one”. Correct it cant – but it does add to its entropy and slow its cooling.
“The Second Law of Thermodynamics can be rephrased in several ways. Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction). It can also be expressed using the concept of entropy as saying that the system’s entropy will always naturally increase if no work is exerted to decrease it. These rephrasings mean fundamentally the same thing because heat deals with kinetic energy and increasing a system’s kinetic energy will increase the system’s entropy.”
https://www.brightstorm.com/science/physics/heat-and-thermodynamics/second-law-of-thermodynamics/
A thought experiment. You have two flat metal plates somewhere out in space a foot apart. One is at 1000 degrees the other is at 500 degrees. Heat flows only in one direction, from the hotter to the colder plate. Now remove the colder plate. The hotter plate will cool down faster than before, despite that there was no heat flow to it from the colder plate.
>>
Fundamentally, it says that heat always flows from hot objects to cold objects (unless work is exerted to make it flow the other direction).
<<
Not exactly. The Second Law is often stated as:

or the change in entropy for an isolated system not in equilibrium must increase until it reaches equilibrium. Not all systems are isolated. Entropy can decrease in a closed system or an open system. The Earth is usually modeled as a closed system, but it isn’t truly closed. Closed systems don’t allow mass transfer across the system boundary. Isolated systems allow neither mass nor energy transfer across the system boundary. Open systems allow both mass and energy transfer across the system boundary.
The Second Law for a closed system is:
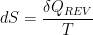
where Q is a path variable and the equation is only valid for reversible processes (this is often stated as the Clausius definition of entropy). Notice that entropy has the same units as heat capacity or energy per unit temperature and in SI units: joules per Kelvin.
Since I mentioned Heat capacity, it is defined as:

So, in an isolated system, a colder object (say a black body) can transfer heat to a warmer object (say another black body) as long as the warmer object transfers more heat to the colder object–simple thermodynamics, and it doesn’t violate the Second Law.
Jim
We live in a society exquisitely dependent on science and technology, in which hardly anyone knows anything about science and technology.
Carl Sagan
In questions of science, the authority of a thousand is not worth the humble reasoning of a single individual.
Galileo Galilei
“There are those who reason well, but they are greatly outnumbered by those who reason badly.”
― Galileo Galilei
Here is a picture of what natural gas does to IR.
.
.
Note how it blocks it out. Same thing happens to IR with CO2, but not to the same extent. If you were in orbit around the earth, and you “took a picture” in the IR band, CO2 would “darken” the image you get. This is how it “blocks” the IR escaping the planet.
Further….
https://m.youtube.com/watch?v=SeYfl45X1wo
This seems to demonstrate that we are wrong about the CO2 absorption range. A flame is putting out a frequency of well above 15u. But since no thermometer was inside the tube there is no demonstration that there was an increase in temperature.
CO2 expanding straight from a pressurized gas bottle……. DOH !!
Talk about ANTI-science…… and Toneb gets SUCKED IN by it.
And as mkelly says, frequency spectrum of a candle is nowhere near the absorption frequency of CO2
http://www.patarnott.com/phys625/images/candleAndLamp.JPG
This is the sort of NON-science we have come to expect from you, tone. !
The problem with his little demonstration is the candles flame is +500 degrees hotter than my shrubs, or the co2 in the atm.
This is propaganda. He knows better than this, that this isn’t really applicable to the problem that exists. He just had to sensationalize it to make an effect, and that’s what’s wrong with the consensus on AGW.
It all stinks.
“The problem with his little demonstration is the candles flame is +500 degrees hotter than my shrubs, or the co2 in the atm.”
My second vid isn’t.
Temp of the presenters hand.
That’s still the candle video.
Besides the point, they filled it with 100% co2.
And so what, there’s an equal negative water feedback to the increases in co2 warming if there is any.
“That’s still the candle video.”
No.
Here again ….
Starts with the same candle video.
https://urldefense.proofpoint.com/v2/url?u=https-3A__m.youtube.com_watch-3Fv-3DSeYfl45X1wo&d=DwMFaQ&c=RoP1YumCXCgaWHvlZYR8PZh8Bv7qIrMUB65eapI_JnE&r=KnDpXnbSBarnTnlVgl11aTGHo3AJb4b5nXkfcMmY5ZY&m=8hCX7splpO8_MCmKtspaCtnVqt0oVP0cy8wHXjDrmVQ&s=OcIjlaSaFTNnMZG18NxSgh2m5w9PzYk_ro1FEC351b8&e=
No it doesn’t.
And so what – look at the “hand” one.
Or are you gainsaying that as well.
Again there should be attenuation of the hand. 98.6 F is still well above the absorbing area of CO2. And again no thermometers.
What you say about CO2 darkening the Earth viewed from space in the 15 um band is true. The informed, educated skeptic recognizes CO2 is GHG. But most of your darkening is due to water vapor that is unavoidably present on our 71% water world planet.
The real question for the informed, educated skeptic (that the alarmists avoid) though is is there appreciable (measurable) darkening when CO2 concentration goes from 3 CO2 molecules/10,000 air molecules to 6 CO2/10,000 air molecules. The 2 facts – 1) that the tropospheric hotspot has not been observed, and 2) that models are running too hot compared to 25+ years observation strongly suggests the CO2 strong GHE theory must be modified to a weak GHE theory.
But without a strong CO2 GHE theory, the alarmist argument collapses. That would mean the entire politicized Climate Change and the trillions of dollars hoped to be re-distrubuted vanishes as well.
CPR is that not a blocking of reflected visible light not IR?
Note two things in the picture. First the black asphalt roads are very “bright.” This picture is taken in the IR bands, not in the visible bands. The asphalt is “bright” because it is HOT. Also, natural gas does not block visible light, and the plume you are looking at is the natural gas escaping from an underground storage facility in California.
A good visualization on the effect The surface IR is absorbed into air which then does not re-emit it quickly at a wavelength visible in the picture. But, this is different from CO2 in multiple ways.
Watch this and to see where the problem is: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
Satellites, often looking down from about 70km, see earth radiation at many different temperatures. You can’t just take a 15C/289K Planck curve and say, “hey, this only radiates at 10 microns”. Low energy IR radiation does not usually cause “electronic” transitions, that is bumping electrons to higher orbits. IR typically causes rotational and vibrational transitions. The significant CO2 transitions and their “bites” out of the spectrum are shown below. Their radiative temperatures are all different.
The 15um/667.4 fundamental bend (vibration) is seen radiating at a peak temperature of nearly 250K, and the surrounding rotations radiate at and conform to the 220K Planck curve.
The fundamental problem is we don’t live in a purely radiative world, as Bill and others have pointed out, collisional/conductive and convective interactions with the other (largely IR transparent) .996 of the atmosphere modulate the effective radiative temperatures. CO2 has a very low emissivity (tendency to emit photons). It prefers to dance.
Whatever you wish to call the disturbances in the electromagnetic/weak field, photons, or something we can detect as particle like, definitely exist; even though they have no mass. They do not control the earth’s energy balance alone.
“They do not control the earth’s energy balance alone.”
In a way they do, because the heat always finally leaves the Earth TOA as photons. But they do not alone control, or even dominate the heat balance of the surface of the Earth. Convection does.
Good clarification.
In the summer, looking across an asphalt surface at low incidence angle over a long distance (long optical path length path length), one see the shimmer. That shimmering (mirage as it is called) is of course refraction due to rapidly warmed boundary-layer air rising from the hot black asphalt surface. The asphalt is dry. The air is mostly dry. Water vapor is present, it warms too. But the vast bulk of the heat transfer from a dry surface in this case is conduction at the boundary where air molecules collide with the hot asphalt molecules.
True, though part of the heating of the air is also due to LWIR emitted by the asphalt and absorbed by the air. And conduction is only important for the boundary and a very thin air layer because of the very low thermal conductivity of the air.
tty
November 19, 2017 at 9:49 am: Agreed tty. Next we have to get it that heat, being a process caused by Kinetic Energy, is what results in radiation. Radiation is not heat and only relatively weakly results in it if it interacts with matter in a kinetic manner. Another basis for warmist waffle.
Gymnosperm, of course in the end, radiation is all that matters, as that is the only way energy actually leaves Earth.
Yes, my sloppy. tty has pointed this out. I should have said radiation does not alone control temperature between the surface and the effective radiative altitude.
Of course, it is actually far more complicated. The solar wind is incoming energy, but it is not EM radiation. Likewise, ions stripped from the earth’s atmosphere, reputedly as heavy as Oxygen, count as energy out.
I have some doubts about some of your statements. Carbon dioxide does not undergo changes in electron levels as far as I know. Also, I’m not sure if the altitude of an electron from the nucleus is quite correct either. I think these are visualisations of differences in energy. However in the case of CO2, the extra energy is not sufficient to do any of that. It simply changes the vibrational energy. CO2 has four vibrational modes and the amplitude of the vibration is what changes on excitation.
I don’t think these change the the substance of your main claim, but since I am still grappling with that. I cannot comment either way. There is certainly something seriously wrong with the settled science.
As a footnote to Anthony, it may be a contentious topic but it is absolutely fundamental to the debate. The principles of spectroscopy are well known but when transferred to an atmospheric context there are masses of uncertainties to be resolved. This is where pseudo scientific assumptions and cherry picking of facts can support a credible argument on either side. Debates like this make gradual progress in revealing better insight. Thank you for posting this one.
under GHG theory the temperature of the earth would be unchanged if all the O2 and N2 was removed.
this makes no sense. the atmosphere has thermal inertia which would be dramatically reduced without O2 and N2. Nowhere is this accounted for.
ferdberple,
So what would the earth be like without an atmosphere?
The average solar constant is 1,368 W/m^2 with an S-B BB temperature of 394 K or 17 C higher than the boiling point of water under sea level atmospheric pressure, which would no longer exist. The oceans would boil away removing the giga-tons of pressure that keeps the molten core in place. The molten core would push through the floor flooding the surface with dark magma changing both emissivity and albedo. With no atmosphere a steady rain of meteorites would pulverize the surface to dust same as the moon. The earth would be much like the moon with a similar albedo (0.12) and large swings in surface temperature from lit to dark sides. No clouds, no vegetation, no snow, no ice a completely different albedo, certainly not the current 30%. No molecules means no convection, conduction, latent energy and surface absorption/radiation would be anybody’s guess. Whatever the conditions of the earth would be without an atmosphere, it is most certainly NOT 240 W/m^2 and 255K.
The alleged 33C difference is between a) an average surface temperature composed of thousands of WAGs that must be +/- entire degrees and b) a theoretical temperature calculation 100 km (32 km) away that cannot even be measured (no molecules to compare) and c) all with an intact and fully functioning atmosphere.
The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.
“The surface of the earth is warm because the atmosphere provides an insulating blanket, a thermal resistance, no different from the insulation in the ceiling and walls of a house with the temperature differential determined per the equation Q = U * A * dT, simple to verify and demonstrate.”
Sorry, but that is not at all how it works. Hint: check the thermal conductivity of air (it is about the same as polyurethane foam) and calculate the temperature gradient needed to move 168 Wm^-2 through, say, 10 kilometers of polyurethane foam.
Also how do you explain that the temperature reaches a minimum at the tropopause and then starts increasing again?
The dominant heat-transfer mechanism in the atmosphere is convection and the lapse-rate is simply the amount of energy needed to drive the convection.
tty
“Also how do you explain that the temperature reaches a minimum at the tropopause and then starts increasing again?”
As molecular density decreases there is less molecular and more radiation. It’s a balance point.
Less molecular what?
GHG theory predicts the tropospheric hotspot. This has failed to emerge.
in science a single failed prediction is absolute proof of a failed theory.
however, trying to prove why GHG theory failed is largely a fools errand, because we have no replacement theory.
for example. we know that Newtonian gravity fails to predict rotational speed of gravity. however, we cannot. explain why because we have no alternative theory. instead we are left to invent a new form of matter. dark matter that obeys some laws but not others. and then we postulate that the universe is 90% dark matter. this is GHG theory mistakes on a cosmic scale.
correction. fails to predict rotational speed of galaxies.
Tropospheric hotspot : https://phys.org/news/2015-05-climate-scientists-elusive-tropospheric-hot.html
C. Paul Pierett,
Did you read what their “evidence” is for the hotspot, that Satellites still can’t find.
This one is much better article:
Study: Tropical Hotspot ‘Fingerprint’ Of Global Warming Doesn’t Exist In The Real World Data
https://wattsupwiththat.com/2016/09/22/study-tropical-hotspot-fingerprint-of-global-warming-doesnt-exist-in-the-real-world-data/
Satellite data shows no warming at all in one area and only 1/3 of the modeled warming in the other area.
You are easily fooled.
Got a citation other than a blog?
Something like this: http://iopscience.iop.org/article/10.1088/1748-9326/10/5/054007/meta
You are too lazy to follow the link I gave you,you also didn’t answer my question about the “evidence” in YOUR link.
“The researchers instead used observations and combined two well-known techniques—linear regression and Kriging”
Ha ha ha ha ha……..
You can’t be THAT stupid.
Meanwhile a rebuttal was posted on it:
Desperation — who needs thermometers? Sherwood finds missing hot spot with homogenized “wind” data
“The fingerprint test of the water-vapor feedback is the “hot-spot”, a warming of a band of the upper troposphere 10 km over the tropics. (See the reasons below at the end). The weather balloons were designed and calibrated to measure temperature and humidity as they rise through the sky and right through the hot-spot. Their results are unequivocal: red was not yellow; the spot was not hotter. Supporting this, the specific humidity was also supposed to rise, but fell instead. If the computer models worked on everything else, we might wonder if the millions of observations were biased, but the models didn’t predict the pause, were wrong about humidity, rainfall, drought, and clouds too. They didn’t work on regional, local, or continental scales and can’t explain long term historic climate either. At this point, a scientist would throw out the theory. The weather balloons independently agreed with each other, the humidity results fitted the temperature results, the whole lot was loosely supported by satellites. The data doesn’t need homogenising or kriging or obscure numerical witchcraft.
Instead Steven Sherwood and Nidhi Nishant of UNSW revisited their 2008 technique of homogenizing temperature data by using wind data as well. They homogenised it again. They have iterated the iteration? They’ve also extended it from 2005 to 2013 and changed the “wind shear” component to “vector wind”. Their new homogenized-temp-wind data is below (left). The model predictions of 2005 are centre, and the radiosonde temperature results (before homogenisation etc) are on the right.”
Charts in the below link.
http://joannenova.com.au/2015/05/desperation-who-needs-thermometers-sherwood-finds-missing-hot-spot-with-homogenized-wind-data/
Here is what the SATELLITE data was showing up to 2013:
http://www.climate4you.com/images/EquatorSurface300hPa200hPaDecadalTempChange%20BARCHART.gif
Professor Sherwood produced a dumb PDF.
Rh measurements falling.
ferdberple
Layman’s interpretation of what you have just posted.
A scientific theory doesn’t work because something is missing. It’s not known what’s missing, but introducing an unknown makes the original theory work.
No wonder I abandoned science at secondary school. It’s like playing a game of chess, with a million pieces. Nothing is ever solved, just continually shuffled around with everyone having an opinion on which way to move a single pawn, that doesn’t exist.
“Nothing is ever solved, just continually shuffled around with everyone having an opinion on which way to move a single pawn, that doesn’t exist.”
You just described all of life.
ferdberple
RGHE/GHG theory assumes the earth is warmer w/ than w/o atmosphere. Attempts to explain this assumption are futile because it is fundamentally incorrect.
The atmospheric insulation blanket creates a thermal resistance (all 32km worth of molecules that matter) just like an electrical resistance or hydraulic resistance. In all three instances a potential difference is required to move energy: dT or dV or dH, from A to B. For heat flow it is: Q = U A dT where U, aka 1/resistance, is the combined effect of conduction, convection, latent and radiative properties together with wind, storms, lit side, dark side, etc. everything that impedes thermal movement from surface to ToA where 100% radiation takes over. (No molecules in space to take the energy handoff.)
The atmosphere is little more than a fundamental HVAC problem.
RGHE theory says the ground would get cold w/o “back” downwelling radiation. Reams of USCRN data show absolutely zero evidence of this.
The sun warms it all. Air temperature swings widely because of its low thermal capacity. The 2cm ground temperature swings very little because of its high thermal capacity. The 100 cm ground temperature hardly changes.
All night and in winter the air temperature spends many hours below ground temperature so the ground heats the air, NOT vice versa.
RGHE theory simply doesn’t stand up to physical observations. All the rest of the discussion is sound and fury signifying nothing.
What shall occupy our empty hours then?
However the resistance is gravitational, not thermal, since the dominating heat transfer mechanism is convection. The atmosphere has such low thermal conductivity that conduction is uttery insignificant. It is no coincidence that almost all insulating materials are mostly air.
Cold air settles, warm air rises by gravity.
And heat rises through my due to anti-gravity waves.
attic
“Cold air settles, warm air rises by gravity.”
Essentially correct. Warm air has lower density and therefore rises. As it rises it expands and cools down. Ultimately it will come down somewhere else, outside the convection cell.
“GHG theory predicts the tropospheric hotspot. This has failed to emerge.”
It is also the theory in any sort of warming. It is merely a function of greater LH release aloft in the tropical high atmosphere via convection.
One thing – it ois difficult to find because of the nature of the instruments used. Radiosondes are imprecise for the job and have changed over the years, and sat obs are contaminated by Stratospheric cooling – which is a function of GHG theory.
Another thing is that it has been found …
“First, tropical warming is equally strong over both the 1959–2012 and 1979–2012 periods, increasing smoothly and almost moist-adiabatically from the surface (where it is roughly 0.14 K/decade) to 300 hPa (where it is about 0.25 K/decade over both periods), a pattern very close to that in climate model predictions. ”
http://iopscience.iop.org/article/10.1088/1748-9326/10/5/054007/meta
It is hard to know where to begin with such a complete pile of nonsense. One of the more obvious errors is confusing a black body radiation spectrum with discrete absorption lines due to molecular transitions. Then there is the weird description of electron orbitals which do not make any sense. If there was any attempt at quality control on this blog this post would have been thrown into the trash where it belongs.
As would yours.
I think the main problem is that the author seems to be completely unaware that the energy levels of a molecule are a lot more complex than for a single atom.
Germonio
November 19, 2017 at 10:42 am: Once again, explain not excoriate. Show how you are more knowledgeable, not just something else…..
radiation works by the exchange of photons. convection works by the exchange of virtual photons. thus if there is a GHG effect for co2 there must be the same effect for n2.
co2 absorbs a photon from the surface. this can be radiated up or down. n2 absorbs a virtual photon from the surface. this can be conducted up or down.
in all aspects the GHG effect operates like conduction at a distance, except that co2 can radiate to space while n2 cannot. thus co2 cools the surface on net as compared to n2.
Ferd,
I would suggest you need a dictionary. Convection refers to the movement of hot molecules from one place to another. It has nothing to do with virtual photons.
Fascinating!
I’m learning here just how little I know about gas absorbtion and emmission phenomena.
I’m also learning how much utter bilge can be written by someone who knows *nothing* about such things, but is willing to shoot their mouth off endlessly. And getting a fair idea of how difficult it must be for a relative layman to tell the difference.
For the record, Gill is wrong, but he’s at least honest. It would be nice if someone took him through his presentation point by point and explained (gently) where he went wrong. I’d do it, but, as I hinted, I’m not best qualified.
(Incidentally, I asked for something like this debate weeks ago – it’s good to see it happening. Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.)
Uncle Gus, why the rant after saying this: |… I’m not best qualified.”? But, you feel yourself qualified to claim: “Not so good to see that the Greenhouse Deniers are not giving up, Against stupidity the Gods themselves strive in vain.” I bet you don’t even understand your own hypocrisy.
Please try, we, the great unwashed, are waiting to be educated.
“I’m learning here just how little I know ”
That has been obvious to us in basically every one of your posts. !
“Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming.”
no.
the downwelling LWIR is a RESULT of GHGs ( like water) it is not the cause of warming.
When people “popularize” the science and dumb it down, they say LWIR causes the warming.
nope.
When you increase c02 two things happens.
1. the stratosphere cools. We have seen this. Its How we know the warming is GHG related and not the
sun.
2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.
a) radiating from a colder place entails a slow down in the cooling to space.
b) to maintain energy balance, the compensating increase in warmth at the surface has three
pathways:
1. it can go into the ocean
2. it can melt ice
3. it can warm the air.
Or 4. or it might mean the earth is cooler.
Why does the stratosphere cool? I’m not disagreeing, I just would like to hear your explanation.
Because in the stratosphere radiation dominates over convection, so more GHG increases the radiative energy loss. The temperature gradient is also the opposite – it gets warmer with altitude, since the main energy source is UV absorption in the ozone layer from which temperature decreases both upwards and downwards.
Tom November 19, 2017 at 12:10 pm
Even more explaining needed of the temperature changes as altitude increases:
In the Troposphere, temperature falls with altitude but, above the Troposphere comes the Stratosphere where temperatures increase with altitude but, above the Stratosphere comes the Mesosphere where temperatures fall again with altitude but, above the Mesosphere comes the Thermosphere where temperatures go up again with altitude!
Given the differences of opinion on this thread, I would love to hear everyone’s theories as to why all these temperature changes happen? 🙂
“2. The average altitude at which the earth radiates to space is raised. Since we have a negative lapse rate
that means the earth radiates from a higher and thus colder place.”
So you mean that when the temperature at the surface goes up the average temperature of the Earth seen from space will go down? That the temperature profile of the atmosphere is somehow unchangeable?
What does happen is that with more CO2 the average altitude from which the heat radiates out will rise slightly. All things equal this means that the surface temperature will also rise slightly, since more energy is needed to lift the convecting air.
However all things aren’t equal. Higher temperature means more evaporation and more H2O in the atmosphere More H2O in the atmosphere means a less steep lapse rate, i e a smaller temperature difference between the surface and a specific altitude. There will also be albedo effects (=clouds, snow, vegetation).
All things considered more CO2 in the atmosphere does seem likely to result in some warming, but the effect is probably quite slight.
Stephen, what do you, as an English graduate, know about the science?
Mosher finally gets something right. Thing is, this effect cannot be calculated, so all attempts at defining Climate Sensitivity are un-scientific.
Stephen knows a hell of a lot more about the science than you do, Martin.
I don’t always agree with Stephen, but I take his analysis (not his snark) seriously. You, on the other hand…
You, as an engineer, should be ashamed that you have been “schooled” (as the kids say) by an English major.
“1. it can go into the ocean
2. it can melt ice
3. it can warm the air.”
NONE of which is happening.
1. Oceans are currently cooling, especially Southern Ocean
2. Only ice melt is down from the Highest level since the LIA (late 1970s)
Arctic sea ice is still in the top 10% of extent wrt the last 10,000 years
Purely in line with the approx 60 year AMO cycle
3. No warming in satellite data apart from ocean events.. ie release of energy from the oceans at El Nino events.
You are still selling moldy lemons , Mosh. Useless even as a car salesman.
No, if the magnitude of the effect cannot be calculated, then, Stephen Mosher has admitted that he, and BEST, are simple record-keepers, unable to predict Climate Sensitivity, and have also admitted, no one else can either.
No one can. From first principles, which means from the laws of Physics which have nothing to do with dodgy thermometer records, we cannot calculate Climate Sensitivity. Think about this.
Most people do not understand that the entire atmosphere radiates to space, including N2, O2, Argon, and the entire mass of the Atmosphere. All Matter above Absolute Zero radiates all the time.
Hotter Matter radiates to the Absolute Zero of Outer Space, which could maybe be a small fraction of a degree Kelvin above Absolute Zero, More than cooler matter.
There is this word Opaque. This word means No Radiation Penetrates. The Top of the Atmosphere radiates to space at a temperature far above Absolute Zero, much closer to the temperature at which CO2 has ceased to be opaque to the radiation it can absorb at its best absorption frequency, 15 Microns, corresponding to around -80 C.
More CO2 raises the altitude at which this happens. Due to the Lapse Rate, the higher the altitude at which the atmosphere no longer encounters an opaque layer, the cooler the temperature becomes at which the Entire Atmosphere can radiate to space. Heat Transfer is proportional to the Delta T, the difference in temperature between hotter and cooler.
More CO2 reduces the atmosphere’s flux to space, increasing the amount of heat retained in the atmosphere, warming the air at the surface, but no one can calculate how much.
Mosher is right, this time…
Not much, because water compensates for the increase.
“Stephen Mosher has admitted that he, and BEST, are simple record-keepers”
Don’t forget .. Data Molesters. !!
“Don’t forget .. Data Molesters. !!”
Stupid comment of the day. Evidence of a 3rd grade intellect.
No need to put a title on your posts Chris. They are always stupid. !
Do you DENY that BEST massively manipulate/torture the data to meet their “regional expectations” ?
Steven – an honest question to answer please. If the average altitude at which th e Earth radiates to space is raised surely the radiating surface is also increased precisely because it is a now a larger spherical surface. Is that taken into account?
This effect is negligible.
MCoEA:
It’s easy to do the math. Let’s say, the “emission level” is increased by 100 meters (0.1km). The radius is increased from 6400km to 6400.1km, a 0.0015% increase. So the surface area is increased by 0.003%.
If you use a lapse rate of 6K per km, the raised layer would increase the surface temperature by 0.6K, other things being equal (yes, this is a highly simplified analysis). The increase in emission layer area of 3 parts in 100,000 is completely trivial in this analysis.
Ed Bo. – thanks.
I think you missed something very important. Water vapor dominates the radiative heat transfer from the lower troposphere, blocking most of the spectrum that other GHGs would otherwise block. When the buoyant air reaches the tropopause, the water vapor condenses out. Water vapor ceases to be a factor at all in the process of radiating from the ground to space. So the effects of increasing the amounts of the other GHGs is no longer masked by water vapor.
No wonder you can’t figure it out, you forgot the optical window to space.
No, he didn’t forget the sky window. He said the increase in warmth at the surface, he was not referring to the fact that some of the sun’s energy that reaches the earth’s surface goes back out into space through the sky window.
Right, but he probably doesn’t expect the atm to self adjust how cold it gets, and there not being all that much additional warmth left to deal with. So none of his choices are correct, really.
I’m throwing food now.
Can you explain “slow down cooling”? Does the emissivity of the planet change? Does CO2 change the emissivity of the planet? Where, then, is the back up that constitutes the “slow down”? — in a magical zone between the given emissivity and the given planetary radiation? How does that work? “Slow down” with respect to what time frame? Is Earth on a schedule to cool by a certain time? Who sets it? How long does it take for enough “slow down” to cause heating? WHAT exactly is being “slowed down”? What is the entity defining “cooling” that is being “slowed down”?
This “slow down the cooling to space” sounds a lot like a piece of glass inhibiting convection, … a roundabout way of resurrecting the idea of a barrier that keeps stuff from moving out. It’s a more indirect way of erecting that retaining barrier (via conceptual, rather than physical), but still it seems to beg holding onto a cherished, flawed idea.
I have yet to be convinced that a “colder place” can cause heating by any known physical means, either directly by adding heat or indirectly by “slowing down cooling”.
Energy comes in, energy goes out, all the time. If a little less energy goes out, then the atmosphere has a little more energy, and is a little warmer. Lapse rate means that the average temperature at the surface is determined by how much energy is leaving at the TOA.
Steve Mosher,
It is unfortunate that you believe you have to restate the obvious in response to a rubbish post..
But fair enough. Yes, increasing GHG’s warm the surface (though the details are complicated and not homogeneous in time or space). Yes, continued use of fossil fuels will cause additional warming unless offsetting factors keep that from happening.
Now that we are past that, the real issues are (and have always been) how much warming, over how long, the negative consequences of that warming, and what offsetting benefits (eg greater crop production) balance in whole or in apart the negative consequences of that warming.
LOOK HERE!
I got the first comment in this thread and I told you not to treat “photons” like they were real massless particles. I got my big red marker pen out and crossed out everybody who did.
Has anybody got another big red pen (or 2) please as I seem to have run out before I got to the end.
Furthermore ; Who the hell told more half of you that you could add radiative fluxes arithmetically?
Anyone who still thinks they can by tomorrow is invited round to my kitchen where I have a large number of radiative objects at all kinds of temperatures from 0 to 90 C which you will be required to arrange in an ensemble of your liking and invention in order to boil my egg.(Boil = 100 C)
Seriously guys and gals an actual experiment or even a thought experiment to show that adding radiative flux arithmetically is scientific nonsense is so flippin’ simple it is school physics level. Please – up your game!
If you cannot even grasp this the furthest you are going with atmospheric physics is horizontal dermatological absorption.
It’s not clear what you mean. If we had two suns, wouldn’t it be a lot hotter?
Double the W/m^2 at the sun (two suns) also doubles the W/m^2 at earth’s orbit – 1,368 * 2 = 2,736. S-B BB equivalent temperature rises to 468.7 K from 394 K.
Tom and nickreality65.
Lets go a bit further. The Sun occupies a tiny percentage of the area of the sky, I think something like 0.002% IIRC . So never mind TWO suns, let the thought experiment go large and we can have 50,000 suns , should just be able to squeeze them into that sky. Now please redo the SB calculation like you did for 2 suns, nick. The answer should tell you something. Especially when you compare it with the temperature of EACH sun. A new definition of heat transfer from “cold” to “hot” !
This is of course the same as my numerous 90C radiating objects in my kitchen which you need to arrange to boil my egg. Yes, there are at least 50,000 of them and you can arrange them in a sphere around my saucepan.
Looks like I am having just the toast then.
Badger:
You ask: “Who the hell told more half of you that you could add radiative fluxes arithmetically?”
The 1st Law of Thermodynamics (conservation of energy) told me that. It is any absolutely necessary consequence of a conserved quantity like energy.
In any actual thermodynamics class, virtually the first thing you learn is to add and subtract energy transfers to and from a “control mass” to determine changes in the energy of the control mass in “energy balance” calculations.
It’s no different from balancing your checkbook, which must observe “conservation of money”. In effect you are arguing that if you get a second job (let’s say lower paying than your first job), you can’t add the money you get from your second job to your bank account. It’s an absurd argument!
Actually you cannot add temperature fields, that is nonsense, but if you concert it to flux, W/m^2, you can, then concert it back to a temp.
You got flux and field confused.
Oooh, yes, Reverend! I see, now! Please don’t beat me again! Pleeeasse!
Badger, you are correct. If you could add w/m2 then it would be possible to cook steak with ice cubes.
Once an object has attained the max temperature it can adding a second source with the same temperature, as first, cannot cause the object to increase further. (T4 – T4) tells us that.
Your Quantum facts are almost to a tee wrong
1.) Molecules have one or more electrons circling them. blah blah blah
NO
That is classical physics junk. In QM the electrons have complex distribution clouds only S orbitals 1s,2s,3s are circular. The reason is that electrons have half-integer QM spin and subject to Pauli exclusion principle that says two or more identical QM spin particles can not occupy the same space at the same time. They will instead by subject to pairing creating a waveform between them.
The reason for the half spin can be seen by seen by watching the field movement#/media/File:Spin_One-Half_(Slow).gif)
You will also note the momentum is in the field it is not a real spin like in the physical sense.
So Quantum spin can be thought of as momentum but it can not be equated to a physical object spinning.
2.) For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
PARTLY
That is the process of excitation and it creates an excited state it equates to temperature in classical physics. Outside the two ways listed you can also excite an atom via the electric or magnetic fields.
You need to be careful the temperature however is not always positive, it can be negative 🙂
3.) For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit. blah blah
NO
You need to break that into Photoexcitation which says a Photon must have a frequency that matches the one of the excitation energy level to be absorbed. You can view it as a resonate frequency with one of the excitation states of the molecule. Any energy absorbed in this manner departs from the equilibrium Boltzmann distribution as viewed by classical physics.
If the energy does not match those special resonant frequencies absorption of the photon takes place in accordance with Planck’s quantum theory.
4.) Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum blah blah
NO
Photoexcitation is an independant process and will occur at any temperature
Laser cooling illustrates this in the most extreme way.
https://en.wikipedia.org/wiki/Laser_cooling
The energies that are outside the resonant frequencies behave in the more normal way as you are trying to describe.
5.) Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them. blah blah blah
NO
see item 4, temperature does not play a part in photoexcitation only photon energy match
Now, you need to stop following the thermal collision effects and deal with only the photoexcitation effect and key to understanding that is bring Nitrogen into the mix. Nitrogen exists mainly as a simple diatomic atom in the atmosphere and it’s excited states are metastable and relatively long-lived and they happen to overlap a couple of CO2 frequencies. Classical collisions between CO2 and N2 will exchange energy in the photoexcitation frequencies. So the normal thermal energy and the photoexcitation energies can interchange. Without the interplay the two sets of energy would remain isolated.
blah blah blah.
Martin, do all your posts provide zero value add? Someone takes the time to write a detailed rebuttal of the author’s points, and all you have to say is blah blah blah. Truly the hallmark of a lightweight.
Much to read here in the comments, and I WILL get to it all later.
My overall observation, though, is that the talk still seems to center on warming.
The atmosphere keeps Earth’s surface from getting to hot too. The atmosphere, therefore, BOTH cools the surface AND warms the surface at the same time, depending on your perspective, … depending on what you are referencing as the main focus.
I think that talk should shift away from speaking of either extreme (warming or cooling) as the main focus and, instead, shift to the concept of REGULATION, which must consider both, or neither, depending on how you look at it.
Regulation involves an exchange between two processes, rather than either of those processes alone. And so it seems that CO2, H2O, and other gases REGULATE Earth’s temperature withing the habitable range familiar to us.
Damn typos above that I cannot fix. Live with them and groan.
I agree Robert. As I noted earlier, the ocean, the soil, and the GHG merely serve as heat sinks for the atmosphere. The aspect of CO2 is limited by the amount of IR available to be absorbed. The equilibrium equation heat sink capacity can only reach a certain maximum simply because there is no more IR in that spectrum to work with.
So, as you say, it plays a small role in stabilizing the atmospheric temperature, but it plays little role to n the overall stabilization process, simply because it is insignificant compared to the ocean (the overwhelming #1 heat sink), and water vapor, the primary heat sink in atmosphere.
One thing that posts like this definitely demonstrate is there is no consensus. When multiple highly-qualified professionals and well-informed amateurs cannot agree on something as fundamental to ‘global warming’ as a GHE or high sensitivity…then it’s obvious there’s still oodles of uncertainty.
The back radiation is not the only energy flux warming the surface. There is also the direct solar irradiation. Below is an energy balance sheet of the Earth. It is the only presentation showing the three sky conditions: first number is all-sky, the second number is clear sky, and the third underlined number is cloudy sky. The total energy flux warming the surface is Sd + Ed = 168,8 W/m2 +344,7 = 513,5 W/m2. And

by the way, the both energy fluxes are based on direct measurements. The energy balance of the Earth’s surface shows that the incoming energy fluxes and the outgoing energy fluxes are in balance. If they were not, the surface would cool or warm.
The major misconception presented here is treating all molecular energy the same. There are two distinct types, translational kinetic energy that all molecules have, and quantized vibrational energy that dipole molecules can have.
The GHG theory treats the later energy type as if it is not quantized, in other words they conceptualize that a dipole molecule will always absorb a certain wavelength of energy even if it is already in the energized vibrational state, that is NOT correct. If the gas molecule is already in its excited energy state, it can not continue to absorb that wavelength of light, that light will be transparent, reflected, or in some instances it will stimulate that molecule to radiate that energy and drop back to the non energized state.
However, very small levels of energy (factions of 1 eV) can be lost or gained from the quantized energy and given to translational kinetic energy upon collisions with other gas molecules — that’s why the absorption is a band instead of an exact wavelength. These collisions theoretically occur every 10^-7 s at atmospheric pressure which is faster than CO2 molecules theoretically radiate heat. Therefore, we should expect some minor heating caused by an increase in CO2 molecules in the atmosphere from conversion of quantized energy into translational kinetic energy from their collisions with non dipole molecules.
If all GHG molecules in the atmosphere are already absorbing and reemitting all available spectra able to bump the molecules into the energized state, then the only mechanism for additional retardation of heat escaping from the atmosphere can occur from the collisions described above. But that’s only if the available infrared energy is the limiting factor whereas more heating would occur if it is the available number of dipole molecules is the limiting factor.
In my opinion, we have empirical data showing that it is indeed the available IR that is the limiting factor and therefore any additional dipole molecules in the atmosphere can only heat by losing some of their quantized energy to kinetic energy. That is the CERES and TERRA data that has shown that certain regions of the atmosphere lose more heat into space than they receive from the sun, one region in particular being the Sahara desert.
This data not only shows that it is water vapor dominating the GHG feedback, but to me it also suggests that there is something to the theory that the temperature of planet’s atmosphere is largely dependent on adiabatic and gravity pressure induced compression heating. The most heat leaving the planet happens to be where cooled dry air descends within the atmosphere and inducing high pressure.
http://pages.mtu.edu/~scarn/teaching/GE4250/absorption_lecture_slides.pdf

https://www.researchgate.net/publication/317570648_New_Insights_on_the_Physical_Nature_of_the_Atmospheric_Greenhouse_Effect_Deduced_from_an_Empirical_Planetary_Temperature_Model
Notice the outgoing longwave radiation coincident with the descending air associated with Hadley cells. Please, I invite anyone to explain this with the GHG planetary warming theory and how it is not explained by adiabatic and gravity driven heating.
“gravity pressure induced compression heating.”
No such thing really. Pressure does not cause heating or cooling, only changes in pressure. Rising air cools down, sinking air warms up. This is the basic reason for the lapse rate.
That there is more outgoing radiation in the descending part of the Hadley cell is because the air there has lost most of the water vapor (the really important GHG) and is therefore much more transparent to LWIR, so it comes from a lower and warmer layer.
What do you mean no such thing really? Very basic physics, really.
“work done by gravity = W = mgh (h = height lost by the object)
An alternate way of looking at this is to call this the gravitational potential energy. An object with potential energy has the potential to do work. In the case of gravitational potential energy, the object has the potential to do work because of where it is, at a certain height above the ground, or at least above something.”
http://physics.bu.edu/~duffy/py105/Energy.html
The process is started by solar heating of the ground, air masses warm up on the ground, they rise and then fall in the Hadley Cells. Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.
tty is correct. Static pressure does not cause any energy transfer.
Robert’s equation of “work done by gravity = W = mgh (h = height lost by the object)” actually proves this.
“h” in this equation is zero for the atmosphere as a whole. The atmosphere has no height to lose in toto. Any downdrafts are exactly balanced by updrafts elsewhere.
In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.
“Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.”
However the effect would be very weak in an atmosphere without GHG since very little heat would be transferred from the ground to the atmosphere (though it is rather unlikely that such an atmosphere exists anywhere).
“In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.”
Very true. As a matter of fact this is the main mechanism regulating the surface temperature of the Earth. And it can not be modeled realistically, now or in the foreseeable future (it would require increasing computational capacity by something like 10^12).
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
WWF:
You are confusing the one-time effect of dynamic compression — which can and does result in “heating” (and can be immense on a planetary scale) — with the ongoing effect of static pressure — which cannot and does not.
If you compress the air in a bicycle tire pump, it gets hot. If you leave the compressed air in the pump, it does not stay hot, but your analysis says that it would
The gas giant planets go incredibly hot in the dynamic compression of their formation. Some think that Jupiter’s core got almost hot enough to initiate fusion. These planets still emit significantly more energy than they receive from the sun, which means they are still cooling.
The earth, however, does not emit any significant amount more than it absorbs from the sun. Its geothermal flux is only about 0.1 W/m2 averaged over the surface.
For a clearer understanding of adiabatic heating/cooling in the atmosphere, see: https://www.cengage.com/resource_uploads/downloads/0495555061_137425.pdf
Earth pressure gradient allows it maintain a temperature gradient.
This is immediately and obviously MEASURABLE.
Unlike any mythical warming by CO2.
never been measured…. a fantasy !!
In what world is the atmosphere static? The down draft limits the warming, is that what’s observed?
Complete rubbish. The composition of the gas has absolutely nothing to do with convection.
The sum is not zero in this process. Solar radiation does the work to lift the air and gravity does the work to compress it. Energy is actually input into the system this way, and lo and behold, it’s actually what is observed.
Doesn’t descending air become exposed to greater pressure as it descends? Isn’t gravity doing work on it? Doesn’t it’s density increase, from less dense to more dense? — and it’s kinetic energy increase from less to more? And doesn’t gravity cause this descending, compressing, increasing kinetic energy in conjunction with the already warmer level into which it descends?
I confuse nothing. It only requires “a one time heating”
The Kinetic energy at the bottom of the atmosphere doesn’t dissipate to the top again. To do that it must work against gravity. Take a look at Neptune, where you have much less solar input to confuse you. It gets warmer as you descend into it, until it is thousands of degrees C.
Outside the fraudulent area of climate change on earth, this sort of thing is universally accepted.
It’s about time people on here looked up from their narcissistic intellectual self aggrandisement and checked with the rest of the scientific world
yes it can continue to absorb at that or nearly at that wavelength. you missed out that vibration in a molecule has harmonics so two photon absorption is possible. or a photon of double that energy can be absorbed.
just like a harp string will have a fundamental frequency it will have higher energy harmonic frequencies. a harpist will pluck the string at a different height to change the harmonics of the string.
So you’re saying that a photon can be absorbed that is double the energy level at which the molecule actually absorbs? And given an infinite number of photons in the 15-20 nm range, a CO2 molecule will continue to absorb that thermal radiation regardless of all three normal modes energized? And then can theoretically emit at a higher energy level than the radiation it absorbs?
To R turner at 9:27 pm.
1 carbon dioxide has 4 modes of vibration. Symmetrical stretch, asymmetrical stretch, and two bending modes of equal energy. The word for equal energy in spectroscopy is DEGENERATE. so they are a degenerate pair of benders!
the symmetrical mode does not absorb infra red since there is no change in dipole moment. the other 3 modes are infra red active. there are an awful lot of vibrational energy level present in each mode so each mode can sequentially absorb a lot of almost equal energy photons one after an other. ( due to asymmetry in the vibration the second photon energy will be slightly less energetic than the first and so on). The molecule can absorb photons equal to 2 of these energy bands (or even 3) this is analogous to a musical string vibrating at twice its frequency ie one octave above the fundamental note.
there are not an infinite number of energy levels so it cannot absorb an infinite number of photons. if it absorbs too many photons then the molecule will vibrate so strongly that the atoms will fly apart and it no longer exists it will be CO and O. Double strength photons can come off but has much lower probability than sequential single photons. I some how remember that some lasers can produce double strength photons.
indeed at any one time there will be a fraction of the population of CO2 with excited vibrational modes, and these will be absorbing photons as well.
This is how molecules break down in heat. infra red lasers can be used to heat single bonds in a molecule and break it. however the method is in its infancy and may just as well break a nearby bond.
Robert W Turner ,
Note that your NASA illustration (second one) has a range of reflected light (0–700 W/m^2) that is more than twice the commonly accepted value of incoming TOA radiation (342 W/m^2). How can something reflect more than it receives?
That particular image is for the reflected SWR.
If CO2 did affect climate, than the increase in CO2 over the past 30 years should have caused at least a measureable change in the dry lapse rate in the troposphere, but such has not happened.
Will, CO2 radiation can’t change the lapse rate, it’s dependent only on g/Cp
… and the slight change in aCO2 decreases Cp by a tiny amount , hence increases lapse rate.. immeasurable with so much H2O about.
There are many lapse rates in the atmosphere. g/Cp defines the Dry Adiabatic Lapse Rate (DALR) which is about 9.8 K/km. The more typical troposphere lapse rate is the Environmental Lapse Rate (ELR) which is 6.5 K/km. There are many more important lapse rates and one or more of them *might* be affected by CO2. However, all of them, except the DALR, are affected by radiation to/from CO2.
Only atmospheric warming has come from releases of energy from the oceans via El Ninos.
Nothing to do with CO2 or human anything.
Fixed it 🙂
Whu should the dry lapse rate change? The small increase in CO2 is not enough to measurably change the specific heat of the dry atmosphere. If the increased CO2 resulted in an increased amount of H2O in the atmosphere (known. by friends and admirers as “water feedback”), then the average lapse rate would go down, but as far as I know this has not been observed.
willhaas. You forget that there is a possibility that the CO2 effect is much less than IPCC reports. In that case the other cosmic warming effects have decreased and therfore the temperature has paused, even though the warming by CO2 has increased slightly.
According to the IPCC, the warming effect of CO2: dT = 0.5 * 5.35 *ln(CO2/280) resulting to the climate sensitivity of 1.8 C degrees. I have reproduced the calculation of the warming formula of CO2 and it is: dT =0.27 * 3.12 *ln(CO2/280) resulting to the climate sensitivity of 0.6 C degrees.
Link: https://wattsupwiththat.com/2017/03/17/on-the-reproducibility-of-the-ipccs-climate-sensitivity/
The warming effects of different main forces are in 2015: the Sun 0.35 C, GH gases 0.28 C, and astronomic harmonic resonances 0.13 C, together 0.75 C. Link: https://www.climatexam.com/cosmic-theory-caws
Very interesting comments on the greenhouse effect from Ray Pierrehumbert here: https://www.youtube.com/watch?v=slPMD5i5Phg
The system is a lot more complex than many (including we token ‘warmists’) think.
“Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um”
–
This statement needs a lot of modification.
We are talking about our atmosphere.
Temperature is the sum total of all the energy in all the molecules in the atmosphere in any particular place.
Individually molecules will have different energy levels,
LWIR is produced by both the sun and the earth surface and the atmosphere and clouds.
So the atmosphere certainly has all LWIR frequencies trying to pass through it.
I guess the CO2 molecule can pick up these other two frequencies if needed if it runs across them?
–
If a CO2 molecule has picked up a higher “hotter” frequency energy packet and it subsequently emits it this does not mean that the whole atmosphere has to be at say 120C does it?
Yet that CO2 molecule will be buzzing around giving energy [heat[temperature]] to a lot of other O2, H20,N and other molecules while it is charged up.
–
Something is wrong in the state of Denmark but I am not good enough yet to explain it. There must be lots of people who do understand radiative physics to simply knock this contention out.
Where are they, please.
–
Quite happy to be a skeptic for lots of reasons but not on the CO2 is not a GHG as technically it will raise temperatures according to the laws of physics.
Just a minor comment.
Should 274 K be 273 K and 15 C be 288 K, not 289 K?
Andrew Hamilton,
Yes
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit” I stopped there, too many misconceptions about quantum mechanics. The IR absorption spectrum has nothing to do with the electronic excitations, but with rotational-vibrational spectrum of the molecule (cannot be purely vibrational, because the photon has helicity). I’m going to have a post on my blog about this sometime, specifically for CO2 and H2O, calculate some frequencies and explain a few things…
Yes he hasn’t got there are two totally different things and they behave differently.
MODTRAN answers the question. MODTRAN, whose accuracy has been verified by satellite measurement calculates heat flux either outward or downward at any point in the atmosphere. The difference between heat flux outward at 0 altitude and at the top of the the atmosphere is the heat deposited in the atmosphere. If the GHG concentration is anything above 0 the heat deposition is positive. If it is 0, then all of the IR heat from the earth escapes to outer space and we are left with a frigid, uninhabitable earth.
Excuse me, but all the IR ultimately does escape to outer space (including geothermal heat).
“If the GHG concentration is anything above 0 the heat deposition is positive.”
Since it has been above zero for the last 4 billion years or so (and mostly much higher than now) according to your physics the planet should have melted and boiled off the atmosphere long ago. As a matter of fact even if the “heat deposition” was only 0.000001 degree per year the Earth should be almost as bright as the sun by now.
Yes, all the IR energy does escape to outer space, but it does so at a higher temperature than it would have done without the IR absorption of the atmosphere. It does it via the Stefan-Boltzmann equation. Since it loses some of the (discrete) IR levels leaving the earth’s atmosphere it has to increase its temperature to make up with a continuous spectrum what it has lost from the discrete emissions. This is the mechanism of GHG heating. You can read about the details by googling “Nature Abhors a Positive Feedback” which is a 3-year old post to this site (Watts Up With That).
” It does it via the Stefan-Boltzmann equation” The atmosphere is not a black body. Ex falso, quodlibet.
Problem is no one uses it right.
It changes just by altering air temp and not composition. So, unless you run it for each temp, you get the wrong answer.
I agree – the notion that a gas such as CO2 or water vapor can “trap” radiant heat in a free atmosphere is simply unphysical. Heat trapping by gases is only possible by preventing/obstructing convective heat exchange (as in a glass greenhouse) or by using of IR-reflective materials such as polished aluminum that have VERY low IR emissivity/absorptivity and a high IR reflectivity (these materials are called “radiant barriers”).
Our latest paper addresses this issue and demonstrates using NASA planetary data that the thermal effect of planetary atmospheres has nothing to due with trapping of outgoing thermal radiation, but instead is due to the force of atmospheric pressure, which adiabatically enhances the energy received from the Sun. In other words, the so-called “Greenhouse effect” (more accurately named Atmospheric Thermal Enhancement or ATE) is a thermodynamic (pressure-induced) phenomenon that is completely independent of atmospheric composition:
Nikolov N, Zeller K (2017) New Insights on the Physical Nature of the Atmospheric Greenhouse Effect Deduced from an Empirical Planetary Temperature Model. Environ Pollut Climate Change 1: 112. doi:10.4172/2573-458X.1000112
URL: https://tinyurl.com/ydxlfwn7
Ned:
Did you take high school physics? If you had, you would understand that for a force (such as atmospheric pressure) to transfer any energy, it must act over a distance (Work = Force * Distance, or more precisely, Work = Integral of Force over distance).
If an object is actually falling in a gravitational field, gravity is doing work on that object, transferring energy to it. But the atmosphere is not falling — it has already fallen. No distance, no energy transfer. (Any downdrafts must be exactly matched by updrafts, so these fully cancel out.)
Any reasonably bright high school physics student can recognize your argument for the nonsense it is!
Take a 5kg barbell, hold it with your arm horizontal out in front of you.
No movement.
so, no work involved….. Right Ed
That’s the trouble with your high school physics, Eb.
ITS “LIMITED”. !!
Take your 5kg barbell and hang it from the ceiling on a hook. No movement, no work. The hook and the ceiling have no power supply.
If you held up the weight with a motorized winch, the motor would expend some energy holding up the weight (as the body does in your example). But engage the brake on the winch and turn off the motor, no energy is expended to hold up the weight.
You would fail high school physics, and not get started in any higher level physics.
To those who think that all the work of gravity has already been done and that this somehow falsifies the premise that there should be higher temperatures at the surface than at the top of the troposphere because of gravity:
When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located? Is it located at the top of the atmosphere? Or is it located at the bottom of the atmosphere?
If you want to distribute that energy back to the top of the atmosphere, what force will you have to work against to get it there? Would it be gravity by any chance? If you do work what do you expend? Oh yes! Kinetic energy? Which is converted to potential energy once more!
So the natural equilibrium of a large planetary atmosphere, is colder near the top, hotter at the bottom. See Neptune, Uranus, Saturn, Titan, Jupiter, Earth and Venus for and use the simple formula T=Pn/Rp to understand that the temperatures of all of these planets and moon can be precisely calculated using nothing else.
The greenhouse effect is falsified.
WWF:
You ask: “When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?”
I see that you have never done this type of analysis. The question is not even properly posed. When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.
All of the planets you cite have a radiatively absorptive atmosphere between a relatively warm surface and the incredible cold (3K) of deep space. Like a steel bar with one end in boiling water and the other end in ice water, there will be a temperature gradient between the hot and cold ends. If the gradient of the atmosphere exceeds the adiabatic lapse rate, as it is on all of these bodies, the lapse rate is unstable, and convection will start to bring it back toward adiabatic.
Poor Ed, you really don’t have the faintest clue past junior high level, do you?
Keep expounding your ignorance of how materials deflect and absorb strain..
Its funny to watch 🙂
“No movement, no work.”
Your base-level ignorance is exposed in that one statement.
“no energy is expended to hold up the weight.”
Again.. a junior high understanding of materials.
As expected.
“Take a 5kg barbell, hold it with your arm horizontal out in front of you.
No movement.
so, no work involved….. Right Ed”
It is noted that you immediately run away from the answer, try and distract, and in doing so highlight your ignorance of basic materials.
Ed says there is no work involved in holding a 5kg barbell horizontally in front him.
He must be superman or something. !
Yes, I took physics in HS and in college and in graduate school. How about you? Did you forget that kinetic energy measured in Joules = Pressure*Volume, and that gas temperature is proportional to the internal kinetic energy of a gas? Perhaps these Wiki articles might help your memory:
https://en.wikipedia.org/wiki/Joule
https://en.wikipedia.org/wiki/Ideal_gas_law
Oh, and make sure to retake the class about adiabatic processes to understand how pressure relates to temperature in standard thermodynamics. You can also read carefully our paper, where these things are explained on pp. 6 – 15, while noting that our accurate model (Eq. 10a) is based on actual VETTED OBSERVED data, hence, it’s real!
And just for Ed’s edumacation
Strain Energy within materials is also measured in Joules.
Ed writes,
“Take your 5kg barbell and hang it from the ceiling on a hook. No movement, no work. The hook and the ceiling have no power supply.”
There is still work in it,Ed or it would have IMMEDIATELY fallen to the floor. What is holding it to the surface of the ceiling,Ed?
I can stand still therefore I don’t float away from the surface?
As I said, Sunset…..
….poor ed is stuck with a junior high school understanding of structures and how they work.
Ed Bo
Have you never heard of the Chinook wind and seen what it does to snow on the ground?
I’m throwing food again.
How so? Is the atmosphere just sitting there static? I thought that the atmosphere was ALWAYS falling, … AND rising, … AND falling, … all the time. Doesn’t this mean that the atmosphere is WORKING all the time?, … under the influence of gravity? — gaining potential energy on the rise?, “unleashing” that potential energy on the way down? … in a continuous cycle?
How is this merely “already fallen”?
There is complete confusion here by multiple commenters between the concepts of force, energy, and power.
The hook holding up the 5kg weight exerts a (continuous) force on it of F = m*g = (5 kg * 9.8 m/s2) = 49 Newtons.
To hold it in place, the hook does work on the weight of W = F * d = 49 N * 0 m = 0 Joules. So the power required is 0 Watts (= 0 Joules / time).
What are your equations for force, energy (work), and power for this case?
Andy cannot get it through his head that a one time increase in strain energy is NOT an ongoing continuous power transfer, no matter how many times this basic and trivial point is explained to him.
Ned: I am absolutely appalled that with all your credentials, you do not understand the most basic points you should have gotten in high school physics.
Pressure/volume work requires a CHANGE in volume to do the work. It’s really p*dV work. This is the same thing fundamentally as force needing to create movement to do work. The gas in a piston chamber must move the piston head to do work on it.
The weight force of the atmosphere on the earth’s surface does not move the earth’s surface. (Even if an initial application of this weight force caused some compression, there is no further change.) So the pressure/volume work done is zero.
These are basic, basic points of high school physics, and you get them completely wrong!
Height of the atm changes daily. Goes Up and Down like a thermometer.
Ed Bo November 20, 2017 at 9:33 am
Ed, it’s worse than that. He’s had the basic points explained to him here on WUWT, over and over, and he STILL doesn’t get it.
But heck, if you want a good laugh, you can read about Ned Nikolov’s work here …
w.
micro6500:
You say: “Height of the atm changes daily. Goes Up and Down like a thermometer.”
The claim of “Atmospheric Pressure Effect” (APE) enthusiasts like Ned Nikolov is that the static weight force (pressure) of the atmosphere does continual work on the planet’s surface. To do this, it must move the surface downward.
If the height of the atmosphere varies up and down a little bit, that does not mean anything to the surface.
If you don’t count the downwelling longwave infrared flux (back radiation), the surface energy balance is out of whack by about 250 W/m2, averaged over the surface. The APE enthusiasts claim that the weight force of the atmosphere can make up this imbalance. That’s 250 Joules of work every second for every square meter of the planet’s surface. With an atmospheric pressure of about 100,000 N/m2, the earth surface would need to be continually compressing at 250/100,000 m/s, or 2.5 millimeters every second. This means that the earth’s radius would need to shrink by over 75 kilometers each year! Seriously???
Philip:
You say: “Have you never heard of the Chinook wind and seen what it does to snow on the ground?”
Do you think the falling air of the Chinook wind leaves a vacuum at the higher elevations?
Facepalm. It’s really hard to deal with ingrained blind ingnorance.
Willis, how about you accept that Ned has a much better understanding of physics than you and that it would be a good idea to take that as a starting point to try and understand where your misconceptions lie, rather than try to keep proving that cold heats hot and work done equals energy no longer in existence? Truly the level of your ignorant stubbornness could teach my 5 year old grandson a thing or two!
To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?
Why is it that Ned and Karl can accurately model the temperatures of every planetary atmosphere in the solar system with the same mathematical formula, but greenhouse effect proponents can’t even get their mathematics for earth to agree with itself, much less even one other planet?
wickedwenchfan November 20, 2017 at 3:36 pm
If you wish to follow the “science” of a man using an ad-hoc equation with more tunable parameters than data points to explain, you are welcome to.
He is right. The kinetic energy is converted to thermal energy. You sure you understand how this “science” thing works?
BECAUSE NED HAS MORE TUNABLE PARAMETERS IN HIS EQUATION THAN HE HAS DATA POINTS TO EXPLAIN, PLUS FREE CHOICE OF EQUATION WITH NO NEED THAT IT BE PHYSICALLY BASED!
Sheesh! Given more parameters than data points to explain, it would be surprising if Ned could NOT explain the datapoints … but then I guess you don’t know how that works. Let me offer you the following, come back once you’ve read it. It explains very clearly why Ned is just fooling the rubes …
w.
WWF:
You say: ‘To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?’
BTW, it’s “Mr. Numpty” to you 😉
I already answered your question above, when I said: “When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.”
Since I was obviously too fast for you, I’ll break it down for you ask I would a high school physics problem to a struggling student.
Let’s take Newton’s apple of 0.5 kg, hanging 3 m above the ground in earth’s gravitational field of 9.8 m/s^2. It has a gravitational potential energy relative to the ground of PE = mgh = 0.5 kg * 9.8 m/s^2 * 3 m = 14.7 Joules.
Now the stem breaks and the apple falls toward the ground. Just before it hits the ground, the gravitational potential energy of 14.7 J has all been converted to kinetic energy of KE = (1/2) * m * v^2.
We can calculate v = sqrt (2 * 14.7 / 0.5) = 7.67 m/s.
Next it hits the ground in an inelastic collision and stops moving. Such an inelastic collision converts all of the kinetic energy of 14.7 J to internal (thermal) energy. For simplicity, we’ll say that all of this added thermal energy is in the apple as it splats on the ground.
The apple is mostly water, so has a thermal capacitance of about 4 kJ/kg/K. This leads to a temperature increase in the apple of:
DeltaT = 0.0147 kJ / 0.5 kg / 4 [kJ/kg/K] = 0.00735K
This is a trivial high school physics problem. But neither you nor Ned has enough of a grasp of the basic concepts to get it.
Ed Bo is simply explaining standard, correct physics. He (and Willlis) are absolutely right.
Ed Bo (Again)
So you think that convectional overturn is a zero sum process?
You have forgotten about the water vapour that got left behind and fell out from the rising limb.
Here is a picture of a condensed greenhouse gas falling under gravity. This is clearly not the result of a zero sum process.
http://1.bp.blogspot.com/-NeGDYGjuBI8/UdFRSl28cbI/AAAAAAAAWrw/tJNkpRp4QxI/s1024/Waterfalls+Scenery+Wallpapers+%25281%2529.jpg
Ed Bo,
No. Do you?
The most significant point about an unstable air mass is that if a parcel of air at a given level within the air mass is forced upwards it will continue to rise; but equally important is this: – if an identical parcel of air from the same starting level in the same unstable air mass is forced downwards, it will continue to fall. We can all see the effects of the rising air in an unstable air mass when it cools sufficiently for the water vapour to condense and form cumulus clouds. What we fail to see, and this is what makes them so dangerous for aircraft, is the descending unstable air, the cold invisible down draft located alongside the rising cell. It is a mistake to assume that this cold downdraft is the same air that rose inside the convection cell. It is not. The rising air inside the storm becomes separated from the water vapour that formed the storm cloud as the rain falls out of the cell and the lifted separated and now dry air remains aloft in the anvil cloud at the top of the storm.
It is this process of drying by physical separation of moisture that makes convection that produces rain an irreversible process. Descending moist air in a cloud can evaporate the surrounding water droplets from the cloud, dissipating it and so slowing the rate of adiabatic warming as the moist cloud containing air parcel descends. Dry air cannot be cooled by evaporation of surrounding cloud moisture because there is none available. Consequently in the descending limb of the Hadley cell dry air is warmed as it is forced down by the Coriolis Effect and ends up at the surface, just like the Chinook wind, at a higher temperature than its initial starting point. And the reason for this change in temperature is because the latent heat of condensation of water vapour that powered the initial rise to the top of the troposphere in the convection storms of the equatorial zone made the rising air cool more slowly.
Philip:
You are confusing very separate issues here.
Nikolov claimed that the weight force of the atmosphere provides an ongoing energy transfer to the earth’s surface that keeps the surface at a substantially higher temperature than it would be otherwise.
I showed him the very basic high school physics point that static pressure provides no energy transfer, and that any deviations from the static case due to updrafts and downdrafts must cancel out.
Note carefully that I am talking about the mechanical force due to the weight of the atmosphere. You are talking about something completely different.
Yes, the convection circulation of air is an irreversible cycle, both due to the evaporation at the surface and the condensation at altitude, and the reduced IR opacity of the atmosphere at altitude. But this does not affect the weight force of the atmosphere on the surface.
Besides, what you describe has a net cooling effect on the surface (even if there is localized warming at downdrafts). Nikolov was arguing for an atmospheric warming effect.
Why do we need to continuously explain basic physics…err an elementary concept?
Solar energy lifts air. Literal energy from an outside source does work on the atmosphere, it has warmed and lifted a parcel of air into the atmosphere. At this point, the d-nyers of basic physics suggest that the energy simply goes away. That’s not what happens. The air has potential energy at this point, it falls and energy is converted to kinetic energy in the lower atmosphere.
Let’s work backwards now, we have kinetic energy added to the lower atmosphere from compression, the work was done by gravity. How did that air get there, it was lifted from being heated. How was it heated, from an outside energy source or an internal energy source? Uhh, an external source, still with me? So is the net result zero energy input into the system, no, energy is retained within the system that was obtained from an EXTERNAL ENERGY SOURCE.
If we must explain that a parcel of air in the upper troposphere is actually moving a distance, h, when it falls then I don’t know how much further back in explanation we need to go. Was that parcel of air magically moved into the upper atmosphere? Was that air simply pulled into the upper troposphere because falling air left a void for it to replace? Errr, no. The entire process is started from energy being input from the sun.
Does this really need further explanation to people. Well, I guess it might to people saying
Ed Bo,
I am all for a dynamic atmosphere that moves both mass and energy, causes local surface heating (Chinook winds) and elsewhere local surface cooling (virtually every rainstorm I have ever experienced).
Sadly, the very meaning of the term “adiabatic” is forgotten here in arguing that gravitationally induced compression of a parcel of air cannot increase its temperature in accordance with the Ideal Gas Law. The compression factor PV/nRT need not be unity, as for an ideal gas, for the temperature to rise adiabatically.
This conversation is absurd. You need to define the system boundaries. This old engineer still remembers his statics. If a mass is not moving, then no work is being done. For an object to be static, all the forces must add to zero and all the moments must add to zero.
It’s a different problem if you add in how the forces are created. Holding a weight motionless does no work. However, there is energy being expended in the muscles of your arm to create that force.
One person is ignoring how the forces are created and the others are trying to include the force creating process. Neither side is discussing the same problem.
Jim
Absurd!!! This site needs an editing feature.
Jim
Jim Masterson November 21, 2017 at 9:24 pm
That “editing feature” would be me … your error is fixed, I hate typos. Unfortunately, WordPress doesn’t offer editing.
w.
Thanks Willis–you are a great editor.
Jim
Ned Nikolov November 19, 2017 at 1:44 pm
This from Ned Nikolov, the man so ashamed of his own work that he published it under a false name … well, actually, he would have published it, but I pointed out his craven deception to the journal, and they pulled his article.
His “scientific theory” involves an equation with more tunable parameters than there are data points … and while that kind of mathturbation causes anyone who thinks about things to laugh, I fear he has found a following among the credulous.
Ah well … just saying, folks, he’s a man who is willing to lie about his own name …
w.
Still works better than your greenhouse effect mathematics. It also is based on basic principles that cold doesn’t raise the temperature of hot without work being performed. Concepts you still struggle to comprehend.
I guess you must have missed the rest of this post because you never addressed it in your “take down” of the original Nikolav paper. And you pretend you have actually refuted anything
AGAIN, we must ask, what is it you are missing about the physical explanation for which mathematical models exist to explain and match the temperature of rocky planets with atmospheres?
RWturner November 21, 2017 at 10:18 am
I guess you must have missed the fact that Nikolov is using more tunable parameters than the data points that he is fitting. Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
When you do that you are GUARANTEED a good fit … but of course, that fit is meaningless. It would be hard to not get a good fit with that procedure.
Or perhaps you don’t realize what it means when you have more tunable parameters than data points to fit. Once again let me recommend Freeman Dyson’s clear takedown of the bogus Nikolov style of analysis …
Regards,
w.
But there is a physically meaningful mechanism for how this works. It has been described numerous times on here and no one has refuted it with anything but sophistry.
The descending cool dry air within the Hadley cells retains energy within the climate system. This is a basic fact that keeps being rejected for no reason aside from conventional wisdom. Where is this retention through gravitational potential energy in the oft cited energy balance cartoons that you see above and below? Those flat earth cartoons are so laughably flawed that it’s amazing anyone is still using them.
Others have cited this process in gas giants. It is the same concept, but in the case of gas giants the energy source to start the convection is internal and therefore there is net energy loss, but not nearly as much energy loss if there wasn’t a gravity driven compression by the returning downdraft. On Earth, the energy source to kick off the convection is external, so therefore any returned energy is a net addition into the system, it limits the energy lost via the thermals and release of latent heat. This is true regardless of the composition of the atmosphere, it just matters that there is an atmosphere at all for convection to take place.
And I already discussed the empirical observations demonstrating this effect. The latitude belts between 23-30 degrees emit more LWIR back into space than any other region, even losing far more heat to space than received from the sun in places like the Atacama. It even holds true for the atmosphere over the oceans at these latitudes despite 30 mm of precipitable water per cubic meter.

Notice the subtropical south Atlantic. Greenhouse gases are surely present there, spreading that notorious back radiation around, yet this is among the highest outgoing radiative regions of the atmosphere. What is the source of that heat? I would say it from the additional heat added to that part of the atmosphere from gravity driven compressional heating at the descending leg of the Hadley Cells. Yes, that heat came from a different part of the atmosphere, but that heat in turn came from the sun. That means the process is retaining heat in the climate system, ironically much like how a greenhouse actually works, but instead of glass roof it is the force of gravity.
P.S. You forgot to add the link to Dyson’s takedown of Ned’s models.
RWturner November 21, 2017 at 2:11 pm
Thanks, RW. It’s fixed now, I’ll put the link here as well.
w.
RWturner November 21, 2017 at 2:10 pm
Thanks, RW. First, whatever the mechanism that you are describing might be, it’s not described by his equation. Look at the equation! It has little to do with anything.
Second, if your proposed mechanism were true, it would allow a planet with an IR-transparent atmosphere (e.g. argon) to be warmer than the S-B temperature … but as I showed in A Matter Of Some Gravity, that violates conservation of energy.
Third, regardless of all of that, he has more tunable parameters than he has data points to fit. This is a common enough mistake that it has its own name, “over-fitting”, and his is the most egregious example of overfitting that I’ve ever seen.
w.
I don’t think that’s what’s implied by the theory at all. You can’t take a temperature of something (atmosphere at the surface) that is not there, but the surface of the planet itself would not heat up, no.
If you added an atmosphere of pure argon, there would be absolutely no condensed or solid particulate matter in the sky to absorb incoming radiation, and let’s assume this atmosphere refracts no incoming light. The surface of that planet will receive the full brunt of the solar radiation.
But, molecules in the atmosphere at the surface will heat up and convection starts. This then cools the planet’s lower atmosphere as heated air carried to less dense areas of the atmosphere, the heated air decompresses, hot molecules bump into cool molecules, and heat is radiated into space.
This process cools the surface and is the complete climastrology certified version of planetary energy budget models regarding pure thermal convection. But what happens as that air falls down to the surface again due to gravity? Unequivocally, that air is compressed and heats up, that is if you believe in the work of William Henry. Latitudinal dependent heating takes place in the area of the Hadley Cells where the gravity driven compression warms the air, exactly what we see on Earth. Far less of the heat is lost to space than is modeled, it’s ironically similar to the argument that the back radiation heats the surface by slowing the radiation that leaves the planet.
So, if you believe that the inherent gravity driven “heat retention” of the atmosphere actually exists at all, and I hope for William Henry that you do, then how do you think that compares to the importance of the previously discussed 0.001-1.7 eV molecular vibrations of the inaptly named GHG molecules and there emittance of this energy within the atmosphere?
And let’s imagine a planet with the same gravity as Earth, circling the same sun in the same orbit, but with an atmosphere of pure argon. Would that planet be hotter or cooler than Earth? With an atmosphere of the same molar mass, I bet that planet’s total average atmosphere would be cooler than Earth’s with no latent heat, but probably a much hotter surface due to higher SWR. Convection would probably actually be more active due to the extremely hot surface and argon doesn’t freeze until until -189 C so the structure of the lower atmosphere would be quite different. So theoretically if that atmosphere was able to stay gravitationally attached to the planet, where the pressure in this imaginary atmosphere is 1 atm, it would probably be about the same temperature as Earth at the same latitude.
I think this qualifies as a great example of how hard it is to defeat erroneous conventional wisdom.
Researchers have literally had to infect themselves with diseases to defeat it. Petroleum geologists were busy controlling oil blowouts in Oklahoma while others were still claiming commercial oil would never be found west of the Mississippi. But eventually, the science progresses.
A key point in this discussion is the fact that an enhanced IR absorption by some gases does NOT imply an ability of such gases to trap heat in an open convective environment. IR absorption and IR trapping are two distinctly different processes with different control mechanisms that have erroneously been conflated since the time of Fourier and Tyndall in the 1800s …
Ned Nikolov
November 19, 2017 at 1:50 pm: IIRC, Fourier and Tyndall have been misquoted by all warmista. They do this everywhere, thinking we won’t check. Sadly, they have been correct in that, too much.
The classic paper by Arrhenius (1896) illustrates quite well the roots of confusion in the modern radiative “Greenhouse” theory:
“On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground”
http://blogs.dickinson.edu/cop20/files/2014/08/1896-publication.pdf
One can clearly see that Arrhenius simply took Fourier’s conjecture/assumption about “heat trapping” by an open atmosphere as a “physical truth” without any empirical verification. He further developed the idea by proposing a “model” (his Equations 3 and 4) that relates Earth’s surface temperature (T) to atmospheric CO2 (K expressed in relative units) via equating T^4 to a ratio of dimensionless quantities! By failing to match measurement units between the left- and right-hand side of his equation, Arrhenius violated a basic principle of dimensional analysis in physics, which of course renders his model nonsensical from a mathematical and physical standpoint of view .. And this nonsense has been touted by the mainstream climate science for decades as one of the greatest scientific achievements of the 19th century!! Simply amazing …
Experiments by Arrhenius’ colleague, physicist Knut Ångström, the results of which were published in his 1900 paper “Über die Bedeutung des Wasserdampfes und der Kohlensäure bei der Absorption der Erdatmosphäre,” (Annalen der Physik 308(12): 720-732.), showed that that CO2 is transparent to 90% of infrared radiation applicable to temperature variation; and that those infrared bands which CO2 readily obstructs are already almost totally blocked by atmospheric H2O. His finding, that the relationship between the concentration of CO2 in the atmosphere and its effect on back radiation is logarithmic, has been replicated by many subsequent experimenters; all of whom show that doubling of the present carbon dioxide content in the atmosphere would only increase the back radiation by about 3.6 W/m ², which would, in the absence of other factors, give rise to an increase in temperature of between 0.6 and 0.8 C°.
Some think that doubling might yield a temperature increase of up to 1.2 C°. The only way that Warmunistas can get a scary range of 1.5 to 4.5 C° is by assuming unphysical positive water vapor feedbacks not in evidence.
English translation of Ångström’s paper:
https://ozonedepletiontheory.info/Papers/Angstrom1900-English.pdf
Ned Nikolov November 19, 2017 at 1:50 pm
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field. Despite that, you have not defined the term, so we have no idea what it is that you mean.
How about you give us a clear definition of what you mean by “IR trapping”, so we can follow your ideas? Because for all we know it’s just a strawman that you’ve erected to knock down …
w.
Willis said, on November 21, 2017 at 9:42 pm, in reply to Ned Nikolov :
Odd, because, from what I have observed, this term IS a common term in the field:
From a NASA website:
https://climate.nasa.gov/causes/
Most climate scientists agree the main cause of the current global warming trend is human expansion of the “greenhouse effect” — warming that results when the atmosphere traps heat radiating from Earth toward space.
Someone needs to tell NASA that a common conception of the “greenhouse effect” is not to “trap heat”. Oh, and why they are at it, someone also needs to remind NASA that heat does NOT radiate, and that by continuing to speak of it as such, they continue to confuse the two concepts of “heat” and “radiation”.
From a Harvard University website:
http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html
Some of this terrestrial radiation is trapped by greenhouse gases and radiated back to the Earth, resulting in the warming of the surface known as the greenhouse effect.
Well now, Harvard some folks seem to think that greenhouse gases TRAP radiation. They relish the word, “trap”, and they use it in a way to confuse people into associating “heat” DIRECTLY with radiation, as to suggest that the two terms might be interchangeable (wrong).
A Columbia University website:
http://www.columbia.edu/~vjd1/greenhouse.htm
While the dominant gases of the atmosphere (nitrogen and oxygen) are transparent to infrared, the so-called greenhouse gasses, primarily water vapor (H2O), CO2, and methane (CH4), absorb some of the infrared radiation. They collect this heat energy and hold it in the atmosphere, delaying its passage back out of the atmosphere.
Let’s look at definition #18 for the verb, “trap” at Dictionary.com: 18. to stop and hold by a trap, as air in a pipe. So, it sure looks to me like Columbia University is talking about TRAP — they just avoid the term and describe what the term means in other words. Sorry, but “holding” and “delaying” is TRAPPING.
Consequently, I’m afraid that straw men are overseeing NASA, Harvard, and Columbia University, at the least. I wonder whether they are also spineless, … as in too timid to alter the momentum of confusion that they have helped nurture.
Robert Kernodle November 22, 2017 at 8:13 am Edit
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
Thanks in advance,
w.
Willis on November 22, 2017 at 11:12 am wrote:
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
And THAT’s the problem — this simplistically WRONG explanation, using a word that WRONGLY compares a familiar plant-house structure with a gaseous atmosphere, perpetuates the very use of a term that IS ill-defined. If this term is so rampantly used with the “general public”, then how is the “general public” ever to come to terms with any reality?
The person you criticized initially for using this term was merely using it as a reference point, as many organizations state it. Nikolov was, in effect, kowtowing to this use, letting it slide in its amorphousness — as it is spread amorphously without any clear definition already by all who use it, as if this is okay. You made his using the term an issue, which it should not have been, in my opinion, which seems like a distraction, rather than a call for a definition that was significant to Nikolov’s statements. It is this nebulosity of definition that tends to give alternate views some credibility, I would say.
Oh, and not only does the term appear in press releases, but also it appears in the context of professional scientific organization’s, as a reflection of professional identities, like the following:
American Association for the Advancement of Science:
http://whatweknow.aaas.org/get-the-facts/
The warming effect of CO2 and other heat-trapping gases is well established and can be demonstrated with simple science experiments and satellite observations.
TRAPPING, right? — I didn’t misread that?
Australian Academy of Science:
https://www.science.org.au/curious/earth-environment/enhanced-greenhouse-effect
The result is that some of the sun’s energy becomes ‘trapped’—making the lower part of the atmosphere, and Earth, warmer than it would be otherwise.
TRAP, yes?
NASA website for childhood education:
https://climatekids.nasa.gov/greenhouse-effect/
Earth’s atmosphere does the same thing as the greenhouse. Gases in the atmosphere such as carbon dioxide do what the roof of a greenhouse does. During the day, the Sun shines through the atmosphere. Earth’s surface warms up in the sunlight. At night, Earth’s surface cools, releasing the heat back into the air. But some of the heat is trapped by the greenhouse gases in the atmosphere. That’s what keeps our Earth a warm and cozy 59 degrees Fahrenheit, on average.
NASA’s spineless straw men apparently want to start indoctrinating America’s (and the world’s) youth as soon as possible with the TRAPPING idea, AND setting up future confusions in adulthood with the “earth’s-atmosphere-is-like-a-greenhouse” idea. Tragic!
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
Why would you think that I think the term has any scientific meaning. The term is crap. I think Nikolov knows that it’s crap too, but he used it as a device to hold people’s attention a bit, without saying that it’s crap, while he put forth more info from his point of view. The straw man that I am seeing is raising an issue about the term at all in Nikolov’s context, since it was used merely to set the stage of discourse, rather than as a label for stating a belief via the term’s clear definition.
The “clear” definition of “trapping heat” that you might seek is purposefully veiled in the totality of faulty discourse defending the “greenhouse effect”. All the math, all the elaborate descriptions, all the minutia devoted to the “greenhouse effect” is shadowed by this convenient umbrella idea of “trapping heat” or “trapping radiation”.
Supposed educators use the term haphazardly, aimed at people from childhood to adulthood, conditioning their minds to have a sense of heat or radiation as some “stuff” that gets caught in a trap, and then, after all the years of childhood and early adulthood, they then get blasted for using a term as adults that they were NEVER taught any better prior to adulthood. Very convenient, I’d say — nurturing child-like minds, so that sophisticated minds can dissect them as faulty-thinking minds, while sophisticated WRONG arguments are peddled, based primarily on appeal to adult-mind authority.
Very scientific! [sarcasm intended]
Carbon dioxide lasers have been used in laboratories for a long time. They rely on the property of CO2 to absorb IR, hold on to it for a time, and then release it in the form of a coherent beam of IR collimated by the laser apparatus. You can feel the warmth of a beam of IR produced by a small CO2 laser. Carbon dioxide in the atmosphere does the same thing, but an atmospheric CO2 molecule releases the IR in a random direction, half of the time upward toward space and the other half down to the earth. So a portion of the IR headed toward space is re-directed back to earth. This does not warm the earth, but it keeps it from cooling as fast, all other things being equal. The earth’s surface will therefore equilibrate at a somewhat higher temperature. Hence the greenhouse effect.
Gill’s original post talked about CO2 emitted IR “bouncing around for a while”. Yes, that’s what causes atmospheric temperatures to equilibrate at a higher level than they would without CO2.
You can also feel the warmth of a beam of IR produced by a large CO2 laser but I don’t recommend it. Actually these are fantastic inventions, now due to careful engineering, design and lots of associated hardware gizmos it is possible to get efficiencies of around 10% from a commercial CO2 laser. Yes , pump 10kW into it (effectively thermal energy) and you can get a cutting beam of 1kW out. Of course there is 9kW of heat you need to throw away so much of the apparatus is hot hot hot and there is coolant, pumps, radiators, etc to get rid of it.
I think we done well ! Compared to the equivalent gaia design where you shove 10kW off the surface of the earth, through all that wispy gas (including 0.04% CO2) and get a downward beam of [REDACTED #1] kW making an efficiency of [REDACTED #2] %.
It’s like that jar at the fair with loads and loads of sweets in it and you are supposed to guess the number and write it down with your name.
The persons who said 5kW and 50% should now identify themselves..
AN ORDERLY QUEUE PLEASE, and stop pushing…
That’s just it, it doesn’t stay the same.
CO2 lasers do not work with the ‘cold’ radiation of 15 um but a much warmer one that was stated in the above post:
“Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.”
https://en.wikipedia.org/wiki/Carbon_dioxide_laser
The CO2 laser produces a beam of infrared light with the principal wavelength bands centering on 9.4 and 10.6 micrometers (μm).
As the author expects, there are some errors in this post that are responsible for the erroneous conclusion. The most important are these:
1. Wien’s law refers to the _peak_ wavelength of emitted radiation, not the only one. The distribution of the spectral radiance is given by Planck’s law. If the spectral radiance peaks at 10 micrometers, you can be certain sure there’s a lot of radiation around 15 micrometers as well.
2. For an object close to room temperature, no matter if solid, liquid, or gas, the atomic/molecular energies are largely unimportant (it happen to be important for CO2, which is the whole shebang). This is because at any ordinary temperature the occupancy of any state other than the ground state is pretty much zero, because typical atomic energies are separated by several eV. In order to get a fraction of 1/e molecules at an energy 1 eV above the ground state, the temperature would need to be >10,000 K. The important degrees of freedom are translational, and, depending on the substance, rotational and vibrational. The latter two are typically quantized, but the former is continuous. In other words, even if you don’t have some atomic energy accessible, you can ALWAYS knock particles around a little bit. Thus, a hydrogen gas at room temperature can be treated for all practical purposes as a gas of featureless, structureless point particles.
3. There is no physical law that states that molecules in liquids or solids “need more energy” in order to be excited, and I have no idea where this assumption could’ve come from.
All in all, the basic greenhouse mechanism remains unchallenged.
Thanks for posting this Anthony, it includes most of the misconceptions and errors about radiative transfer and CO2, so gives a good opportunity to rebut them in one place. References to the science can be found in any college textbook on Physical Chemistry and Molecular Spectroscopy.
https://books.google.com/books/about/Physical_Chemistry_5th_Edition.html?id=nIggbG9i8qEC
My understanding of Thermodynamics and Radiation from CO2 is as follows:
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law).
It’s a reciprocal relationship not linear.
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
Wrong, those wavelengths exist in solar radiation and in surface emissions, so they are absorbed by CO2, just not in large amounts because those wavelengths are in the tails of the respective spectra.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is wrong and meaningless. Wien’s Law tells you the location of the peak wavelength, as the graphs added by Anthony shows wavelengths above and below that exist in the spectrum. More relevant to the IR emissions is the following:
http://lasp.colorado.edu/~bagenal/3720/CLASS5/EarthBB.jpg
As you can see the CO2 absorption band at 15micron is present at all the temperatures shown (dashed lines). Even though the maximum shifts with temperature so does the radiance increase as temperature increases. Consequently we see the following:
A blackbody at 300K emits 26.8522 W/m2/sr between 13 and 17 microns
at 270K it emits 18.4933 W/m2/sr
at 223K (-50ºC) it emits 8.53892 W/m2/sr
at 193K (-80ºC) it emits 4.32413 W/m2/sr
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
This is more like atomic structure and doesn’t apply to molecules (it’s more complex, bonding and non-bonding orbitals etc.)
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
No, that refers to part of the internal energy, the temperature is the kinetic energy of the molecule.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
No, it must receive a photon of exactly the right energy to excite the transition. However electronic transfers in molecules are excited by UV light. The transitions which we are talking about in the IR are excitations of the vibrational and rotational modes. In the case of CO2 absorption at 15micron it is the vibrational mode that is excited, specifically the bending of the CO2 molecule (with associated rotations).
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Again the photons must have exactly the right energy to reach the excited vibrational state. The molecule can emit the radiation but it’s far from immediate it will undergo millions of collisions with surrounding molecules during that time and in the lower atmosphere is more likely to transfer the energy to the atmosphere (thermalizes)
Photons with not enough energy to
raise the orbit of any of the electronsexcite the vibration are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Won’t be scattered or reflected.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
In the band from 13-17micron, as you can see from the diagram above it’s significant part of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
No, it depends on the molecule, and again it’s not electron excitation it’s bond vibration.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
As explained above this is not true for several reasons.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
No, the earth’s surface is very close to a black body and will readily absorb a photon emitted by CO2, or it could be absorbed by another CO2 molecule, or thermalize.
So am I right?
Mostly not I’m afraid, but thanks for the opportunity to correct these misconceptions which as you suggest are frequently encountered on the web.
And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
As pointed out the concept of CO2 molecules at -80ºC is a major misunderstanding of Wien’s law (it also makes more sense to use the form of that law which relates peak frequency to temperature since that is proportional to energy).
Go to MODTRAN and test this for yourself. You will see that CO2 doesn’t have any impact what so ever until you get up to about 3 to 5 km when H2O starts dropping out of the atmosphere. If you then take a look at the temperature of the atmosphere at those heights where CO2’s signature is present, you will see that it is about -80°C. What you are seeing is the thermalization of CO2/15µ, and it results in a temperature floor, not an increase in the ceiling. CO2 only warms the upper atmosphere, not the lower troposphere.
http://lasp.colorado.edu/~bagenal/3720/CLASS5/EarthBB.jpg
What a fascinating collection of comments. I never would have thought there would/could be so many different ways to visualize radiation, temperature, and heat flow.
Well worth reading in their entirety several times I think.
More simply, radiation from a cooler object cannot naturally raise the temperature of a warmer object. This is so basic and so obvious that WUWT should hang its head in shame over the amount of articles it has posted with convoluted explanations to the contrary.
The whole premise of the Greenhouse Effect has nothing to do with how much Radiation CO2 can absorb, but how much the radiation, emitted by the CO2 in the atmosphere, can warm the surface of the earth. The answer, of course, is zero! Radiation from a cooler source cannot increase the temperature of a warmer source.
End of story. You don’t need to invoke quantum physics to understand it. You just need to stop holding sophists with PhDs in awe and use some common sense and basic observations.
radio waves can be absorbed by a receiving antena that is warmer than the emitter and be warmed up such that it glows red hot. try putting an electrical conductor in a microwave oven
It would work if it wasn’t for the fact that there are too many closed circuit loops that would generate heat and eddy currents and destroy it.
In fact many RF device send and receive on the same antenna all the time 🙂
Try a search on
“can two RF frequencies share the same antenna”
Or even lets try putting two full transmitters on the same antenna so try
“single antenna multiple radios”
It definitively answers your question 🙂
You are right – a cold object can not warm a warmer object.
However, the temperature of the atmosphere is between the maximum surface temperature and the minimum surface temperature. Therefore, the CO2 in the atmosphere can increase the *average* surface temperature by keeping the nigh time minimum warmer than it would be if there was no atmosphere.
“This is a contentious subject, and I have often shied away from it because it often erupts in food fights. ”
Well yes this opens a can of worms.
Here my 2 worms, er, cents 😉
1) Basically what increasing CO2 in the atmosphere does is shortening the path of the 15 um radiation, from let’s say 10 meters to 9.
One could argue that, in the whole air column, the heat transfer through radiation made with 9 meter steps, instead of 10 meter steps, would increase the lapse rate and thus increase the temperature of the surface. However this is dependant on the percentage of energy that flows through the 15um from the total which I guess is in the order of less then 1/1000.
=> effect cannot be measured
2) Personally I think the whole greenhouse effect is being built on a wrong basis as the elephant in the room is excluded: the oceans.
It is not the atmosphere that has the greatest greenhouse effect, but the ocean, water is practically opaque to infrared radiation, not transparent as the atmosphere is. Light penetrates the oceans to depth up to 100 meters and warms it, however the ocean loses heat only at the surface. The puny effect of CO2 ‘backradiation’ (and all other atmosphere including water vapor) is dwarfed by a 0.5 meter water strata at the surface of the oceans.
The idea that the oceans would freeze without the atmosphere’s backradiation – as the warmists maintain is nonsense. The oceans receive a 1300 W/m2 at the Equator. That heat is absorbed, stored and keep the oceans warm in the night too. No danger of frozen waters.
Of course the atmosphere has its contribution too, but the oceans cannot be completely dismissed as it is done.
Geology proves that the ocean currents define the climate, not CO2 variations, but hey, maybe it is still too early to consider this…
For the geologists and mineral collectors here (and those of you old enough to have ever bellied up to the bar in a discotheque), you are probably familiar with the effect of shining a ‘Black Light’ on solid (e.g. scheelite) and liquid substances (e.g. hydrocarbons). Short-wavelength lights will invariably give different ‘glowing’ colors and intensities than long-wavelength lights. In general, the observed fluorescence and phosphorescence is of a shorter wavelength than the stimulating electromagnetic energy. As a rule of thumb, the fluorescence is of a longer wavelength than the stimulating wavelength, and is variable in duration with different materials. The fluorescence is usually confined to one or two narrow wavelengths, which distinguishes it from thermal emissions. That is to say, if a substance (including individual gas molecules) receives EM radiation of greater energy than an electronic orbital transition, some or all of the energy may be absorbed, and then re-emitted when, after a highly variable period of time, the electron(s) falls back to the ground state. So, strictly speaking, narrow wavelength emissions from CO2 should be considered fluorescence, and should be occurring at wavelengths longer than the absorption bands.
[ https://micro.magnet.fsu.edu/primer/techniques/fluorescence/fluorescenceintro.html ]
On the other hand, the molecules of solids, liquids, and gasses possess kinetic energy in the form of the velocity of individual molecules, straining in the chemical molecular bonds, and in the case of solids, vibration in the crystal lattice. All of these sources of kinetic energy have different energies; the integrated energy is related to the average velocity of molecules, and the average vibrational bonding energy. At high temperatures, Doppler shift will cause the apparent emission wavelengths to also be different. At certain energy levels, bonds can be broken, and a change in physical state will take place, Thus, the distribution of emitted thermal energy with respect to wavelength will approximate the classic Black Body emission.
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption. That is to say, the presence or absence of water vapor will strongly effect whether the atmosphere retains the CO2 emissions. The thermal emission should correspond to the average temperature of the gas molecules.
Once again, the situation is more complex than climatologists are either aware of, or want to admit.
Clyde Spencer November 19, 2017 at 3:53 pm
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption.
You’re mistaken because you’re extrapolating from electronic transitions with vibrational fine structure to vibrational transitions with rotational fine structure where the ‘red shift’ is far less pronounced and you don’t have to worry about the Frank-Condon effect.
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
Climate “Science” on Trial; CO2 is a Weak GHG, it has no Permanent Dipole
If anything CO2 helps to act as a temperature floor for the globe, as it’s main contribution is to thermalize energy consistent with a blackbody temperature of -80º C (-50º C to -110º C).
https://co2islife.wordpress.com/2017/01/30/climate-science-on-trial-co2-is-a-weak-ghg-it-has-no-dipole/
co2islife:
You are simply demonstrating your complete scientific illiteracy.
You see an equation, like the one for Wien’s Displacement Law. It expresses the peak radiative flux wavelength of a blackbody as a function of its temperature.
If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.
But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.
This is, simply put, complete nonsense!
Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target. Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
“If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.”
Uhhh, just what do you think I was saying? Just what is a black body to you? Yes, you warm up a body, and that body emits a spectrum. Just what do you think I was talking about?
“But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
Uhhh, no I don’t, and you can verify everything I say and write by simply going to MODTRAN or SpectralCalc. The calculators don’t lie.
“Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target.”
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Ed bo says : Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
If absolutely true why do the rings on my stove never show RED when the stove is off? Or why do we know that Blue stars have a higher temperature than brown dwarfs?
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
AndyHce November 20, 2017 at 3:04 am
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
The individual photons carry no information about the temperature of their source so an absorber can not distinguish the difference between a 15micron photon emitted at -80ºC and one emitted at 100ºC and will absorb them both.
Temperature measurements are made by looking at the distribution of photons (the spectrum) which is a function of temperature.
co2islife November 19, 2017 at 4:41 pm
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. In fact plotting it versus wavelength distorts the distribution, it makes more sense to plot vs wavenumber, which is proportional to energy. If you do that you will see that the radiance peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution, by contrast water absorbs between 1500-2000 cm-1 (radiance ~0.02 W/m2/sr/cm-1).
The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such.
http://www.azimuthproject.org/azimuth/files/earth_and_sun_emission.jpg
“The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.”
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm. There are plenty of creative ways for a scientist to isolate this effect. The fact that they don’t even attempt to demonstrate the basics through experimentation proves they aren’t looking for the truth, and why they rely on computer models.
co2islife November 20, 2017 at 9:19 am
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such.
No, I’m disagreeing with the statement you made, as shown in your graph the earth emits between 3 and 60µm with peak radiance at 10µm.
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm.
It certainly will!
All the 15µm light will be absorbed in a 10 cm path length if you put 1%CO2 in N2 in your container.
co2islife:
I said: “So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
You responded: “Uhhh, no I don’t”
But then you followed up with: “Absorbing a small fraction of that [earth’s IR spectrum] can’t warm the atmosphere above the 10µ temperature.”
So you just calculated a temperature AS A FUNCTION of wavelength. You don’t even understand that you don’t understand anything about this subject!
As to the (irrelevant) point you were clumsily trying to make, that the atmosphere’s absorption of some of earth’s IR emissions cannot increase the atmosphere’s temperature past that of the surface, no one is claiming that it does. The atmospheric greenhouse theory REQUIRES the atmosphere to be colder than the surface.
co2islife November 19, 2017 at 3:53 pm
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
And you’ve been wrong every time you’ve done so!
That point isn’t debatable. It comes from the SpectralCalc Black Body Calculator. You are claiming the math is wrong.
co2islife November 19, 2017 at 3:53 pm
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is false.
co2islife November 20, 2017 at 9:11 am
That point isn’t debatable. It comes from the SpectralCalc Black Body Calculator. You are claiming the math is wrong.
No, I’m claiming that the guy looking at the graph doesn’t know what he’s talking about!
Run Spectralcalc Gas cell simulator with 0.0004 VMR of CO2 at 296K and plot absorption between 600cm-1 and 700cm-1. You’ll see that it absorbs just fine.
“Note: This is a contentious subject, and I have often shied away from it because it often erupts in food fights.”
Mr Watts, why is this a contentious issue? The quantum physics are well known and defined. I’ve pointed out almost all the points highlighted in this article. The physics are the physics, you can’t change that.
How to Discuss Global Warming with a “Climate Alarmist.” Scientific Talking Points to Win the Debate.
https://co2islife.wordpress.com/2017/01/16/how-to-discuss-global-warming-with-a-climate-alarmist-scientific-talking-points-to-win-the-debate/
co2islife,
You said, “The quantum physics are well known and defined. I’ve pointed out almost all the points highlighted in this article. The physics are the physics, you can’t change that.” I wish it were that simple!
The impression I get from reading the comments is that most posting here are reasonably well-educated, but have varying backgrounds or disciplines. Yet, there is disagreement on what the “physics” says, even amongst those who share similar views about the validity of AGW. In part that may be the result of how an individual interprets the ‘facts.’ Often times the ease with which a problem can be solved depends on how it is expressed or framed. That area probably could use some work. Overall, I’d say that one of the problems is really a failure to communicate with one’s peers. Beyond that, there are some here with a vested interest in a particular viewpoint, and they have trouble looking at the problem from a different viewpoint. Few outside the area of geology are familiar with Chamberlain’s Method of Multiple Working Hypotheses.
If those of us, who are skeptical about the claims made by AGW alarmists, could get our act together and speak with a unified voice, I think that we would have a better chance of pointing out the errors of alarmists. But, with infighting, we are little more than an intellectual rabble.
Everyone of my comments can be verified by MODTRAN or SPECTRALCALC. I simply reading a calculator’s output. If the computer is wrong, then I am wrong.
It doesn’t help when the first comment is by a d@nier of EM radiation and photons.
Well, I can fully understand why this topic is so debatable. I’m an Engineer myself, and I’m still having trouble with deciphering so many viewpoints. I only started becoming interested in the topic around 6 months ago. Probably the only real thing I’ve discovered is that it is much more complex than I had envisioned. Perhaps only time is the ultimate arbiter.
John,
No it’s not complex, it’s second year thermo and heat transfer.
One popular RGHE theory power flux balance (“Atmospheric Moisture…. Trenberth et al 2011jcli24 Figure 10) has a spontaneous perpetual loop (333 W/m^2) flowing from cold to hot violating three fundamental thermodynamic laws. (1. Spontaneous energy out of nowhere, 2. perpetual loop w/o work, 3. cold to hot w/o work, 4. doesn’t matter because what’s in the system stays in the system)
“So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. ”
Almost everything discussed in this article can be found is article previously written on this topic. This Blog has all the resources to validate the points made in this article. The physics are the physics, they aren’t up for debate and Modtran and any Black Body calculator will demonstrate that.
https://co2islife.wordpress.com/
This would have been a great thread if one were allowed to reference those unmentionable scientists that deny that CO2 can warm the surface. Especially the astrophysicist who just published a week ago or so on this topic.
I hope there comes a time when luke-warmers here can debate with the skeptics. Perhaps some day.
First have to agree on whether atmosphere warms or cool the surface. Until then the debate will be infinite.
Both of course.
TTY, “Both of course.” is a perfect response! Most of the comments are citing warming of a molecule, parts of them, or atmosphere of them, but in reality are MOSTLY talking about reducing heat already gained.
Fact: Energy can neither be created nor destroyed, it can only be changed in form.
1) CO2’s only defined mechanism to affect climate change is through absorbing and thermalizing LWIR between 13 and 18µ
2) Those bands are only a small fraction of the emitted LWIR, earth’s peak is 10µ
3) There is no way through thermalization for CO2 to warm anything above the temperature of the emitting body, the energy simply doesn’t exist. Absorbing a fraction can’t account for more than the whole.
4) Radiation could trigger combustion, but that isn’t the phenomenon that we are talking about
5) Conduction, convection, and radiation all act to move heat away from the earth. Claims that the GHG effect is responsible for all the warmth of the atmosphere ignore conduction and convection.
6) This is easily proven in a lab by shining 15µ IR light into a flask of CO2. It won’t warm because the room temperature is way above -80°C
Technically energy can be “destroyed.” If you observe the spectra from distant stars, you’ll note that the hydrogen emission lines are red shifted. When the photon left the star of origin, it had the normal wavelength (freq) of the emission lines of hydrogen. As it traversed the expanding intergalactic space, it’s wavelength grew longer (red-shift.) There is a calculable loss of energy in this red-shifted photon according to E=hc/l where delta-E = hc/delta-l.
…
Where did the ‘lost’ energy go?
“Technically energy can be “destroyed.””
Prove that and you repeal Newton’s Law, and you will get a Nobel Prize.
I already did. The photon that was emitted a million light years ago, “lost” some of it’s energy. The frequency has decreased (increased wavelength.) Where is the “lost” energy?
it went into potential energy. for the gravitational red shift it was demonstrated by t Cranshaw at Harwell in the 1960s. a photon fired from the ground level to the top of a hanger was found to be red shifted. a photon fired downwards was blue shifted. it is like a ball being throw upwards it looses speed but that does not mean that energy is destroyed. Similarly with a photon
Gerontius, that does not explain it. There is no gravitational field in the million light years between the star that emitted the photon, and the spectrometer here on earth that measured it. The red shift is due to the expansion of space, not to a gravitational field.
PS Gerontius, photons coming from the opposite side of the universe display the same red shift. You would think that if you looked in the opposite direction of the red shifted photon, you’d observe a blue shifted one if your “theory” were correct.
They never get that, but you are being mean 🙂
The energy doesn’t change so much as the wavelength stretches out, changing its color. If the source were heading toward you the waves would bunch up. It’s all due to the Doppler effect.
A passing car is a good example. You hear the sound change as the car passes. Sound at sea level and standard conditions travels at about 761 mph. So if a car is traveling toward you at 30 mph, then the sound is travelling at 761 + 30 mph = 791 mph. After it passes, the sound you hear is traveling at 761 – 30 Mph = 731 mph. So the energy does not particularly change. Turbulence has a small effect, but the pitch goes from higher to lower just as starlight red shifts.
It’s all down to the observer’s or listener’s point of view or frame of reference. The sound wave or photon just keeps doing its own thing, while the looker looks on.
As for the effect of gravity, red shift or blue shift is not due to any change in frequency of the photons, but rather to the time rates being different for observers in different potentials. No energy change occurs. If a photon of a given frequency is created by some atomic transition in the vicinity of a star, then it will appear red-shifted compared with the energy of the same transition at some distance from the star, but it hasn’t changed frequency.
Similar considerations apply for red or blue shift due to relative motion; the energy is unchanged relative to the location at which the photon was emitted, but appears different from a moving frame. The situation isn’t quite so clear-cut about red shift due to cosmological expansion, because it depends on how you describe the expansion, but even in that case the frequency of the photon is unchanged relative to its original rest frame.
E= hv
E= Energy
h = Plancks constant
v = frequency
The formula begs to differ 🙂
“The energy doesn’t change so much as the wavelength stretches out, changing its color. If the source were heading toward you the waves would bunch up. It’s all due to the Doppler effect.”
That is a great point, the total energy is the same, but energy/m is less. You are stretching the energy over a larger distance.
You haven’t dealt with the issue that all your own formulas tell you that you lost energy.
Every real physicist will give you the same answer
https://physics.stackexchange.com/questions/21603/have-red-shifted-photons-lost-energy-and-where-did-it-go
https://physics.stackexchange.com/questions/7060/redshifting-of-light-and-the-expansion-of-the-universe
The energy is not conserved no even conceptually both QM and GR demand it to be so. This is one of the problems with classical physics it leads you to stupid answers.
LdB November 20, 2017 at 8:43 am
It is not a problem with “classical physics”, but with your lack of understanding thereof and misguided thinking about it.
As CO2 observes above in response to my prior comment, energy is conserved, but just delivered at a different rate per unit of EM radiation travel. That’s one way of explaining the paradox which you imagine.
What you are again showing is you don’t have a clue about QM.
So if you energy is coming in over time perhaps you would like to tell me how long your photon is and what time period 🙂
LdB November 20, 2017 at 11:45 am
Clearly you don’t understand QM or “classical physics”, by which you mean “physics”.
How many times do I need to explain it to you?
The apparent change in color, ie wavelength and frequency, or a photon of visible light traveling along at its accustomed C, is due to the observer’s position. In the Doppler effect, sound waves don’t change their energy content.
Again, if photons don’t exist, then neither do any of the other elementary particles.
Oh here he goes again trying to obviscate the problem, we have cool lasers(tm) again. You make a statement then just want to wave hands ignore it and try to deflect to something else.
Robert Kernoodle, Gabro,
Sorry for the tardy reply
the gravitational red shift is real and explicable and demonstrated . in that the energy lost is conserved in potential energy
the velocity red shift is real and is due to energy conservation, and not an example of non conservation of energy. You cannot look at the photon in isolation and must look at where it came. There is a fairly simple classical (although with a bit of QM thrown in explanation). It is readily available on the web, although if you look you will always get the Kernoodle reply that is non conservational. You need however to be rigorous in accounting your energy balance . There is no need to invoke expanding universes only relative motion between observer and emitter. You then get the Doppler effect. Now the Doppler effect and the red shift in electromagnetic waves is well known and its energy conservation is not in doubt. And as I said before the energy balance of the effect demonstrates that the red shift is an effect of energy conservation
Robert I leave you to search the web, ps Cranshaw used the velocity red shift to measure the gravitational shifts. Now that’s a big clue as to where the missing energy goes.
None of you here has mentioned it in this blog, so ignorance is bliss.
Robert Kernodle
in answer to your question
Please read Rev Mod Phys 4.87 1932 Enrico Fermi “Quantum Theory of Radiation”
It explains that red shift is an example of energy conservation!
co2islife November 19, 2017 at 4:12 pm
Fact: Energy can neither be created nor destroyed, it can only be changed in form.
1) CO2’s only defined mechanism to affect climate change is through absorbing and thermalizing LWIR between 13 and 18µ
2) Those bands are only a small fraction of the emitted LWIR, earth’s peak is 10µ
Since you claim to use Spectracalc try the following:
Using the Blackbody calculator set it for a BB at 288K
Set the lower limit of the band to 13 micron and the upper limit to 17 micron.
Perform the calculation.
You should find that the band radiance is 23.3 W/m2/sr whereas the total radiance is 124.2 W/m2/sr.
By my reckoning that means the CO2 absorption band accounts for about 19% of the total, hardly a small fraction.
Remember “Everyone of my comments can be verified by MODTRAN or SPECTRALCALC.”
“By my reckoning that means the CO2 absorption band accounts for about 19% of the total, hardly a small fraction.”
In an atmosphere of 100% CO2, that would prove a whole lot, but add H2O to the mix and you will see CO2 is meaningless in the lower atmosphere. You only see a CO2 signature at 15µ after H2O precipitates out of the atmosphere. BTW, that 19% is 19% of the very low energy end of the LWIR spectrum. Now compare the energy of visible light, of which H2O in the oceans absorb 100% of. That is where the energy is, not LWIR.
co2islife November 20, 2017 at 5:57 am
“By my reckoning that means the CO2 absorption band accounts for about 19% of the total, hardly a small fraction.”
In an atmosphere of 100% CO2, that would prove a whole lot, but add H2O to the mix and you will see CO2 is meaningless in the lower atmosphere. You only see a CO2 signature at 15µ after H2O precipitates out of the atmosphere. BTW, that 19% is 19% of the very low energy end of the LWIR spectrum. Now compare the energy of visible light, of which H2O in the oceans absorb 100% of. That is where the energy is, not LWIR.
Not true, that 19% is of the total of the LWIR you can check it out on Spectralcalc. Also the CO2 absorption band is near the peak of the energy spectrum, it is water that is near the tail.
The Blackbody radiance peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution, by contrast water absorbs between 1500-2000 cm-1 (radiance ~0.02 W/m2/sr/cm-1).
You show the CO2 signature on the Modtran graphs on your own website from 1km, you also show the H2O signature off in the tail. If you do the calculations on Modtran comparing 0ppm CO2 with 400ppm CO2 you’ll find that ~10% of the total is removed by CO2.
“Also the CO2 absorption band is near the peak of the energy spectrum, it is water that is near the tail.”
Yep….

I see a lot of diagrams on radiation ,,,, incoming, outgoing, backwards. But all of this assumes that the majority of energy absorbed by the ocean is in some sort of magical equilibrium with the atmosphere. …. ie, a very large part of the incoming SW IR Is absorbed and NOT immediately released.
LWIR between 13 and 18µ doesn’t penetrate or warm the oceans.
Exactly, but the ONLY outside source of energy to the earth, well, maybe some minuscule amount from plate tectonics, …. is SW, and it DOES penetrate the ocean surface. It doesn’t just warm the surface and immediately float back into the atmosphere.
Any equilibrium equation for the total earth can only be made n long term basis, as a good bit of energy is stored and released over time.
A close examination of USCRN soil data shows that solar IR hardly makes it 1 meter deep by conduction and then only during summer daylight which is the ONLY time the air is warmer than the ground.
My idea of an experiment is simple, maybe too simple. Take two identical sealed glass containers and fill each one with a mixture of gases which imitates the atmosphere. The only difference between them is that one has 0.028% of CO2 and the other has 0.04% of CO2. Both vessels should be fitted with highly accurate temperature probes. At the start ensure that both are at exactly the same temperature. Now radiate each with an IR lamp, tested to be identical in output. Now observe the temperature increase in the vessels. The data from this experiment can then be used to calculate and compare what happens to atmospheric temperature at pre-industrial and current CO2 levels. My guess is that the difference will be so small that even the accurate temperature probes can not distinguish it.
Derek Anthony already did this with his two glass jar experiment. One filled with air the other with CO2. Search for it and watch the video.
A glass jar is a closed system. The earth is an open system. Thus a false experiment.
Sailboarder the earth is a closed system with the sun. Energy can enter but no mass. Also the video was done to correct numerous errors done for Al Gore by Bill Nye. Good video.
E=hv tells us how much energy is available to cause a CO2 molecule to increase its translation. And translation velocity is dependent on absolute temperature and molar mass. So heavier gas travels slower.
We know that Q = Cp m dT for how much energy is needed to raise a mass of something, air in this case.
I have yet to be able to show that hv * 400ppm CO2 = Cp m dT for a cubic meter air.
But a postulate of kinetic theory of gas says that all collisions are pure elastic and no energy is lost or gained, so if true CO2 cannot do this anyway.
That is all very interesting but it is a EM wave aka a radio wave. Just like your TV set the EM wave will only selectively react to certain frequencies. Do you really think the EM wave delivering your TV signal got absorbed and re-emited from every air molecule along the way or has anything to do with kinetic energy of molecules along the way?
To your question my answer is NO. But since the present GH theory is based on CO2 absorbing IR we should be able to reconcile these two items.
I have no idea what GH theory works on I am not a climate scientist. All I am telling you is radiative transfer doesn’t occur in that manner described and energy can be lost and gained and attenuation can be measured. I am sure if you search Atmospheric Attenuation you can find the numbers for ll frequencies in the EM spectrum (from RF thru visible light up to X and Gamma rays).
LdB we have been shown graphs on this thread of CO2 absorbing at 15u. OK, then using E=hv we can calculate the amount of energy available to any CO2 molecule to increase its translation. Translation is directly related to temperature.
Due to the relationship of velocity to temperature we should be able to calculate the ability to raise a cubic meter of air via the energy in a 15u IR absorption.
Having taught basic radar theory and basic physics of underwater sound in the navy I have a idea of EM wave and attenuation etc. But that was many years ago. Thanks for your input.
Does anyone have a link to a research paper where 15µ IR was shined on a flask of CO2 and its temperature measured? Unless the CO2 spectrum is a lie, then the only contribution CO2 has to global warming is through the thermalization of IR at 15µ. That won’t warm anything. Simply focus on the basics. By what mechanism does CO2 affect climate change? Isolate that factor and test it. Has anyone tested the impact of CO2 thermalizing 15µ LWIR?
Testing
I too am working with this problem, I actually thought I had been ‘pipped to the post’, but after reading this, and the comments, nope – not even close. And Anthony, this may be ‘contentious’ but it is the key premise to greenhouse theory; without this, greenhouse theory collapses, so it is worth trashing out.
The way I see it the problem lies in the detectors of the greenhouse gases (see my youtube presentation below): they all use thermoelectric – Seebeck Effect transducers. What John Tyndall discovered In 1859 are really only the thermoelectric gases, N2 and O2 are not; nor is CO2s 1338cm mode. All thermograms and IR spectrograms are created using these detectors, but they discriminate the non thermoelectric modes and substances.
Greenhouse theory is 19th century science, pre Quantum mechanics . Quantum mechanics predicts and explains the vibrational modes, emission spectra, of all the molecules in the atmosphere including oxygen and nitrogen; It also says all matter radiates infrared. All matter! That oxygen and nitrogen are assumed not to radiate IR is a contradiction to QM. Either Quantum mechanics, or greenhouse theory is wrong: Quantum mechanics is not wrong. To observe the predicted Modes of oxygen and nitrogen – at 1556cm and 2338cm respectively – we must use 20th century technology, Raman spectroscopy (the complement instrument to thermoelectric IR spectroscopy) .
In the paper that I’m about to publish I will show the Raman laser technology is equivalent to Thermoelectric IR technology. Raman can do it all: it measures temperature, and the concentration of the gases. It is even used to measure the Keeling curve, and is the instrument of choice on solar system space probes for this reason. It can also show nitrogen and oxygen emit and absorb IR radiation, in total compliance with thermodynamic laws Stefan Boltzmann equation and Boltzmann constant. I can even show, by experiment and application, nitrogen radiates upon CO2 to heat the CO2 – figure that.
The following YouTube presentation is my rough beginnings – I have since developed my theory.
With oxygen and nitrogen being greenhouse gases, greenhouse theory collapses.
https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s Reinterpreting John Tyndall’s 1859 Greenhouse Gas Experiment with Thermoelectric and Raman Theory
Pretty much any wavelength of radiation can create the photons that we sense as heat. For example, the 662 kEv photons from Cs-137 when stopped by a lead shield will cause the lead to get hot if there are enough photons. Saw this with a cask of 55k Curies of Cs-137. The exterior of the cask was hot to the touch. Here is how I describe the conversion of the 662 kEv photons to heat EM. A loss of energy of the initial photon occurs as it impinges or interacts with an atom’s nucleus or electrons in a mass. There are number of processes by which the initial photon losses energy, but with each interaction of the photon with the orbiting electrons or the nucleus, some energy is “lost”. Of course the energy is not “lost” it is divided into lower or a different energy potential/form. One such loss might be when an orbital electron gains energy from the initial photon and ‘moves’ to a higher energy “excited” state. Generally, the electron does not want to stay in this state and will emit the excess energy as a low energy photon – sometimes immediately, sometimes after a passage of time. The process of the initial photon losing its energy can involve thousands of ever decreasing interactions. Another interaction might be where a photon strikes an electron and the electron is ejected from the atom. The initial photon loses energy equivalent to the binding energy of the electron. If the ejected photon received energy in excess of its binding energy, then that photon has energy to give to something else.
Because the eye is a measuring device for visible photons it is useful for describing the process of a photon being eventually degraded to heat. The eye has evolved to manage a reasonable level of visible photons without damage despite the fact that some of the dissipated energy becomes heat photons (whose energy is also dissipated). However, staring directly at the sun introduces too many photons and the heat dissipation mechanism cannot handle it and the retina will get burned. A burn is basically the rearrangement of a molecule, e.g. a protein molecule, due to the input of too much energy, whereby the new arrangement does not work biologically. Physics and biochemistry are fun fields to study in combination.
I have a few nit-picky points in Gill’s assessment, but overall I agree with his assessment.
“This is a contentious subject, and I have often shied away from it because it often erupts in food fights.”
I think the stewed tomatoes now dripping from the ceiling have confirmed your reservations, Mr. Watts. I do hope Mr. Gill was properly attired for the occasion.
For those still confused about how the poorly named “greenhouse effect” works, please see two posts on the subject:
The Steel Greenhouse
People Living in Glass Planets
And for those who claim that gravity or atmospheric mass or some other phenomenon is responsible for the fact that the Earth is much warmer than we would expect given its distance from the sun, let me recommend my post entitled “A Matter Of Some Gravity“.
Or not, you could choose to ignore everything but the things that support your view …
w.
Willis, I still see “The Steel Greenhouse” as one of the best articles I’ve ever read on the so-called “greenhouse effect.” In discussions on this site I’ve referred others to it several times. Thanks again.
Seriously?
The problem Willis is the way we still teach physics. Almost everyone who doesn’t get it has the image of a photon as something like a tennis ball flying thru the atmosphere crashing into every molecule in it’s path. They don’t understand or won’t accept it’s a radio signal that collapses to something we equate to a tennis ball.
Neither of the first two bare the slightest resemblance to Earths’s atmosphere.
And everyone knows that an atmospheric pressure gradient, and related temperature gradient, exists on every known planet with an atmosphere.
Venus:
Atmospheric composition: 96.5% carbon dioxide, 3.5% nitrogen, various trace gases.
Surface atmospheric pressure: 93 bar
Temperature: Over 450 °C
Earth:
Atmospheric composition: 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% water vapor (much higher at sea level), 0.04% carbon dioxide, other trace gases
Surface atmospheric pressure: 1.013 bar
Temperature: 15 °C
Mars:
Atmospheric composition: 95.32% carbon dioxide, 2.7% nitrogen, 1.6% oxygen, 0.08% carbon monoxide, 0.3% various trace gases.
Surface atmospheric pressure: 0.006 bar
Temperature: -60 °C
Thus, at first glance, mass of the atmosphere matters, but GHG concentration, not at all. No doubt there are extenuating circumstances.
I think you might class the distance to that big shiny thingy in the sky as an “extenuating circumstance”.
“I think you might class the distance to that big shiny thingy in the sky …”
Exactly. FWIW, Venus receives about twice as much solar radiation per square meter as does the Earth. However the Venerian albedo is higher. More incoming radiation is reflected back to space. Also Venus rotates very slowly compared to Earth and Mars which probably alters the heat distribution considerably.
Tty,
I considered adding solar radiation at ToA for each planet, but the differences are small compared to the enormous temperature differences. Naturally you’d expect Venus to be balmier and Mars chillier than Earth, all other things being equal, but of course other things aren’t. Not even close. The other things outweigh insolation.
Don,
Venus’ rotation is clearly another factor. But, even considering all other things, the fact that Venus and Mars both have high-CO2 atmospheres highlights the importance of density and surface pressure. The temperature at the point in the Venusian atmosphere with terrestrial surface pressure is about the same as on Earth.
Right on. The steel greenhouse ignores the pressure temperature effect.
sailboarder November 20, 2017 at 4:09 am
The “pressure temperature effect”??? As I have shown, and as Dr. Brown has shown, that is a fantasy. READ THOSE LINKS, and then come back and tell us why Dr. Brown and I are wrong.
w.
A Chinook demonstrates that you are wrong.
David,
How about Walla Walla winds, the reverse of a Chinook?
David Ball November 20, 2017 at 12:23 pm
David, while a Chinook wind raises the temperature at the surface because the air is descending … what goes down must come up. So somewhere else the same amount of air is going up and cooling, so that there is no net gain or loss.
And from the tone of your comment, it is clear that you have NOT done as requested, which was:
If you want to argue with my ideas, at least do me the courtesy of reading them before starting in … both Dr. Brown and I have proven, not asserted but proven, that there is no “pressure temperature effect” capable of warming an entire planet on a continuous basis.
w.
Willis,
What would be the temperature of Venus at the distance of Mars from the Sun?
Please explain why the temperature in Venus’ atmosphere at one bar is the same as on Earth. Thanks.
https://www.sciencedirect.com/science/article/pii/B9780124158450000141
“Venus possesses a dense, hot atmosphere, composed primarily of carbon dioxide. A surface pressure of nearly 100 bars sustains the mean surface temperature of 740 K, which is essentially globally uniform except for topographic effects.”
http://physics.uoregon.edu/~jimbrau/BrauImNew/Chap09/7th/AT_7e_Figure_09_17.jpg
Much as I respect Dr. Brown, “proof” is a strong word, appropriate for math, not technically applicable to science.
Actually, Venus’ atmosphere is a little warmer than average Earth temperature at one bar, thanks to a bit more incident solar radiation. But close enough to make the point. For government work.
Put another way, IMO Boyle’s Law ought roughly to apply, since, while not a closed system, the atmosphere approximates one since incoming and outgoing radiation are close to in balance, while subject to minor perturbances.
Thus, greater pressure should yield higher temperature. The atmosphere expands and contracts under heating or cooling, thus lowering or increasing pressure and temperature at the margins to maintain a fairly constant temperature, until other factors such as Milankovitch cycles and albedo waxing and waning change the baseline. This process can be extrapolated out to even denser or yet lower pressures.
“that there is no “pressure temperature effect”
Yet it exist on all known planets with an atmosphere.
hmmm !
Gabro November 20, 2017 at 12:57 pm
I’m sorry, but there are far too many unknowns in the thought experiment to even begin to say. FOR EXAMPLE, at the surface of Venus CO2 is a superfluid … would it still be a superfluid at the distance of Mars? And how would that change things?
According to your graphic, the temperature of Venus’s atmosphere at one bar is on the order of 350K … which is 77°C or 170°F.
Look, my rule of thumb is that when someone starts talking about Venus to try to show something regarding the greenhouse effect, I stop listening. Venus is a hugely complex system about which we know little. Consider the trouble we have explaining the Earth, about which we know orders of magnitude more than we know about Venus …
Say what? “Proof” is indeed a strong word, and I do not use it lightly. However, there are assuredly parts of science where things can indeed be proven, and this is one of them.
However, if you think that Dr. Browns proof is incorrect, how about you point out WHERE it is incorrect, and leave the grand overarching view of what “proof” is and isn’t for another time.
Note that I apply the same word to my proof regarding the same question, which takes a totally different path to the same conclusion. And again, I invite you to find something wrong with my proof.
Thanks,
w.
AndyG55 November 20, 2017 at 2:28 pm
“that there is no “pressure temperature effect”
Yet it exist on all known planets with an atmosphere.
Like the one where the pressure drops from 20,000 Pa to 110 Pa and the temperature increases from 210K to 270K?
You mean virtually a vacuum, where radiation starts to take over.
DOH !,
Phil, there has to be enough atmosphere to sustain the gradient
Do try to keep up.
Happens around 0.1 bar. on every planet with enough atmosphere.
“Once you have GHGs in the atmosphere, of course, some of the surface radiation can get absorbed in the atmosphere. In that case, the surface radiation is no longer constrained, and the surface is free to take up a higher temperature while the system as a whole emits the same amount of radiation to space that it absorbs.”
–
Thank you Willis.
I have never really understood you but this comment is great.
Since the same energy is going out that comes in there is never a real accumulation of energy in the system , just a change in the temperatures of the mediums of that system, if true.
More energy in the atmosphere less in the land and sea perhaps?
Without the CO2 the land under the sun would be a lot hotter during the middle of the day and would send out lots of IR at a higher temp than the atmosphere does.
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. ”
The kinetic energy of a gas is the sum of the energy of the translational motion of the centre of mass of each molecule. Electron energies are not involved.
Belief in AGW caused by CO2 = denying or ignoring the science of thermalization, Maxwell-Boltzmann distribution of molecule energy & quantum mechanics. The IR energy absorbed by CO2 is immediately (0.0002 microseconds) shared with surrounding molecules (thermalization) so, at low altitude, there is little chance for a CO2 molecule to emit a photon as a direct result of having absorbed one (relaxation time about 6 microseconds). Water vapor has many (>190) significant absorb/emit lines at substantially lower energy levels than the 15 micron absorb/emit band for CO2 and on average there are about 35 times as many WV molecules as CO2 molecules. At low altitude, energy absorbed by CO2 is effectively rerouted up via water vapor radiation and some convection. End result is CO2 has no significant effect on climate. http://globalclimatedrivers2.blogspot.com
Belief in AGW caused by CO2 = denying or ignoring the science of thermalization, Maxwell-Boltzmann distribution of molecule energy & quantum mechanics. The IR energy absorbed by CO2 is immediately (0.0002 microseconds) shared with surrounding molecules (thermalization) so, at low altitude, there is little chance for a CO2 molecule to emit a photon as a direct result of having absorbed one (relaxation time about 6 microseconds). Water vapor has many (>190) significant absorb/emit lines at substantially lower energy levels than the 15 micron absorb/emit band for CO2 and on average there are about 35 times as many WV molecules as CO2 molecules. At low altitude, energy absorbed by CO2 is effectively rerouted up via water vapor radiation and some convection. End result is CO2 has no significant effect on climate.
J. Richard Wakefield November 19, 2017 at 1:28 pm
It doesn’t make it warmer, it makes it less cold??? That’s just a pointless semantic exercise trying to deny the fact that the earth is warmer with the atmosphere than it would be without the atmosphere.
J. Richard, if you asked someone who just put on their jacket “Are you warmer now?”, do you think they’d say “Yes”, or that they’d say “No, I’m not warmer now, but I’m less cold”?
If you think the latter, here’s a quick guide to things that exist on a spectrum:
Less tall = shorter.
Less heavy = lighter.
Less dumb = smarter.
Less cold = warmer.
w.
Willis
Coming from a cold and damp (Scotland) part of the world a suitable response to that question would be “I’m not as cold as I was” 🙂
As I come from the “superhot” Arctic, I find this very amusing.
Nick Stokes November 19, 2017 at 5:32 pm
Not true, according to CERES. As one example, for the Northern Hemisphere, as the average temperature swings from about 9°C to 22°C (a 4% change min to max), the net LW flux at the surface varies between about 52 and 60 W/m2 … since that is about a 15% change min to max, I’d hardly call it “near constant” W.R.T. temperature …
w.
(As a side note, CERES puts the upwelling LW at 398 W/m2, and the downwelling at 345 W/m2).
Willis,
The Trenberth budget deals with annual averages. That gives plenty of time for the air at DWLWIR emitting altitude to equilibrate with surface, if the (annual average) surface warms.
That makes no sense. You’ve said that “if the surface warms, that air warms too, and the flux difference remains constant”. You CANNOT determine that from a budget, whether Trenberth’s or someone else’s. A budget has no information at all about a “warming surface” …
w.
Willis,
No, you can’t determine it from a budget. But you can reason about time scales. You are referring (I think) to a phase lag between surface and downward emitting layer temperature with the seasonal cycle. Averaging over a year is firstly a somewhat longer timescale, but also averages out the effect of such a seasonal lag. Basically the difference between upflux and down is determined by the temperature difference of emitting layer and ground temperatures, which is determined by lapse rate and GHG concentration. The latter fixes the altitude of the emitting layer.
Willis,
You might like to try this test with CERES (I am not so adept with it). Just look at different areas around the world. There will be big changes in upflux and down. But is it not always the case that upflux always slightly exceeds down, as they vary over such a range?
Nick Stokes November 20, 2017 at 2:25 am
Nick, interesting question. Here’s surface upwelling longwave minus surface downwelling longwave, in a couple of formats.:
As you point out, net flow is always from the surface upwards.
All the best,
w.
Willis,
Thanks
Willis,
Just adding some interpretation – I think the up-down difference is basically product of altitude of downward emitting layer and lapse rate. The dry lapse rate is higher than moist. And your map looks pretty much like a humidity map, except at poles, where flux in both directions is smaller.
Willis,
Further thought – altitude of downward emitting layer is dependent on GHG concentration – it is lower with more GHGs. So higher if water vapor is low. That adds to the difference being greater in dry places – it could be the main cause.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and
absorbs.John November 19, 2017 at 7:06 am
Say what? If you shine a flashlight at a stove, it will end up warmer than without the flashlight. Not much warmer, but assuredly warmer. Look, John, a good chunk of the light from the flashlight will be ABSORBED by the stove, and the rest will be reflected.
Now, since energy cannot be either created or destroyed, what happens to the energy in the photons that are absorbed? Obviously, that energy must be changed into something … let’s see. It’s not converted to electricity. It’s not converted to chemical action. It’s not converted to mechanical energy … gosh, could it be converted to thermal energy?
And if (as you claim) the absorbed light is NOT converted to thermal energy … then just what are you claiming it is converted to?
You continue:
First, photons don’t get “overwhelmed” by photons going the other direction. If you are indeed an engineer you know that to be true. You can’t “overwhelm” a flashlight by shining fifty flashlights at it, that’s nuts.
Second, the resulting change in temperature in the stove is small. However, it is not “unmeasurable”, which you also know is true if you are an engineer.
Finally, your explanation that if CO2 “DID increase the surface temperature, the surface would radiate to space at the 4th power of the increase, instantly cooling itself back down” makes no sense at all. According to that theory, nothing can increase the surface temperature, because if it did the surface would “instantly cool” …
w.
Willis, you’re close to right there, when you try to haul the lower atmosphere up by its own bootstraps, a humourous definition of futility. Any warming would indeed cause expansion by molecules moving at c. 1km/sec in the gas phase. The gas laws do rule, so the IR catastrophe will not happen. Ditto the never-observed ghe.
“Ditto the never-observed ghe.”
Except it has been, and the link has been posted on this thread at least twice.
How about you read it?
https://phys.org/news/2015-02-carbon-dioxide-greenhouse-effect.html
The problem is that this only matters if all else is the same, and it’s not.
Proposition!
Cold objects must warm hotter objects, or even more strongly stated, heat the warmer object
Is this true?
In this case the fire and the light bulb
However the general truth comes out that is if the two objects are thermally isolated from their surroundings heat only flows from the higher temperature because that is the definition of HEAT
For example
Two metal blocks A and B sit separated inside a vacuum filled adiabatic enclosure.
Adiabatic enclosure consists of a perfect reflector face surrounded by a perfect insulator
Initially both at the same temperature. The zeroth law of thermodynamics applies.
Both emit and absorb equal amounts of radiation.
Neither one is said to heat the other.
Both objects remain at the same temperature
One block (A) has a power supply which is now switched on causing the temperature of the block to rise.
This in turn means that it will emit more radiation.
A will now heat B causing its temperature to rise.
B will in turn emit extra radiation which is absorbed by A but this ‘back’ radiation is caused by A.
Now comes the clincher
If B were not there at all the temperature A would be even higher.
So B cannot be said in any meaning of the word as a cause of heating A
in the case of the furnace(Tf) and light bulb(Tb)
If Tf >Tb heat will flow from furnace to bulb
If Tf <Tb heat will flow from bulb to furnace
You whole answer is so crazy you do realize you aren’t dealing directly with heat but with EM wave. We call it a thermal emission not because it is “hot” or “heat” but because the source of emission is from a thermal source. Don’t think of the emission as heat it’s just EM energy.
Lets give you this with lasers which are the most precise EM frequencies I can generate.
So I fire a CO2 infrared laser and a red laser at each other.
We know the beams will pass thru each other without interacting and in fact on a laser cutting machine we often use this so you can see where the cutting in what we call that a red dot pointer. The IR frequency is invisible to us but you inject the red laser thru one of the mirrors so it follows the path of the IR.
So got it I have an IR laser one way and a red laser beam the other way.
What happens when each beam hits the other laser device (and they will hit)?
Bryan, you were correct until you got to this statement:
Why on earth would that be? You’ve already said that B radiates energy to A. How can A be warmer when you remove that source of energy?
As LdB said, you are conflating heat with thermal emission, when they are two very different things.
Regards,
w.
Here is an RGB laser in operation and according to you each colour is at different temperatures 🙂
Hopefully you may care to rethink all that.
No Willis I am absolutely correct
Before the power switch on A was turned on block A and block B were at the same temperature.
The adiabatic walls confines all energy within that enclosure
Lets say each has a mass of one kilogram
With both A and B the power source has to heat 2Kilograms
With B removed all the power source has to heat one kilogram which implies the temperature of A will rise twice as fast as before
Any physicist will confirm that I am correct
Bryan, your case is so specific that it has almost nothing to do with climate.
“Adiabatic enclosure consists of a perfect reflector face surrounded by a perfect insulator”
so you have created a situation where every last bit of radiation leaving the blocks gets reflected back perfectly. This is the exact opposite of the earth’s situation, where every last bit of radiation that leaves from the top of the atmosphere disappears and never comes back. If no energy can leave, then the two blocks will reach some equilibrium where both have the same temperature.
“If B were not there at all the temperature A would be even higher.”
While strictly true, this is not really very interesting. Basically all you have claimed is that an isolated, insulated system with mass “m” warms faster than an isolate, insulated system with mass “2m”.
I would challenge you to re-analyze with a system where the enclosure has perfectly ABSORBING walls and is held at, say, 3 K. You will find that in this more useful system, Block A does indeed warm faster than without block B, and Block B ends up at some temperature between that of Block A and the cold walls.
Hi tjfolkerts
My post was to correct the impression that the presence of any object irrespective of its temperature must ‘warm’ some neighbouring object.
This piece of fiction has become so prevalent in the last 20 years that normally rational folk like Willes have been blindsighted by it.
My example was to remind people that the surroundings need to be specified before any progress is made in analysing the flow of heat.
My scenario would be found in opening chapters of a thermodynamics book where definitions of adiabatic and diathermic walls, heat transfer and so on are introduced.
I am sure that ‘warming’ by cold objects meme has been carefully fostered by global warming advocates.
Look at some earlier comments upthread .
Block of ice in sitting room with person
Torch heating a furnace
Surely the Earths atmosphere in which radiative resistance rather than reverse heating gives a rational explanation of the situation and should be encouraged.
Its up to people who should know better to get back within the framework of physics
Sorry (profuse apologies) should have written Willis rather than Willes
Bryan,
I agree with your point that it is valuable to examine all details of a system, and to be precise about language (the word “heat” being perhaps the biggest language offender). Your scenario certainly could be worked out with some basic undergraduate thermodynamics.
My main issue with your scenario is that it is SO far removed from scenarios related to the thermodynamics global warming that it could easily give a false assurance to people who simply want a post to confirm their biases. For planets, the default condition is blackbody surroundings at 3K. This is almost exactly the opposite of what you hypothesized.
I think it would be more productive to directly address issues like “warming with ice”. The radiation from cold things still feels warmer (and will help keep you warm) when compared to radiation from even colder things. If I am facing a wall of dry ice @ -79C and then someone interposes a wall of ice, then yes, I will feel warmer. A pot of water with an immersion heater will rise to a higher temperature when the radiation from the warmer ice replaces the radiation from the colder dry ice.
tjfolkerts
The block of ice in the sitting room example.
Everyday common sense tells people its not a good idea for keeping the house warm.
The walls floor and ceiling and air at around 20C (surroundings) will lose heat in melting the ice by conduction convection and radiation.
The fact that the ice block radiates to the wall is true but will not change the outcome .
If the surroundings were dry ice as you say it would be the ice block that would lose internal energy by heat transfer to the dry ice and so it would get colder.
Once the surroundings are specified it is much easier to keep track of the heat flow.
In reading posts on Global warming quite often only two items are mentioned
The hotter and colder object ( with no mention of surroundings).
Then comes an astonishing statement like….
Do you know that the cold object ‘warms’ the hotter object
It always seems that the main offenders are global warming advocates.
Also not all photons are created equal.
The ‘quality’ of the radiation was mentioned in the theme title but not discussed much any further.
Bryan says” In reading posts on Global warming quite often only two items are mentioned
The hotter and colder object ( with no mention of surroundings).”
Any decent discussion will include FOUR items
1) the warm object that is absorbing energy (eg the ground)
2) the source of energy (eg the sun)
3) a low temperature heat sink (eg 3 K outer space).
4) the object that is not absorbing energy from Item 2 but can absorb radiation from Item 1 (eg the atmosphere
Perhaps you were reading poor post where the authors did not really understand the GHE. Perhaps the authors figured the sun and outer space were simply givens that did not need specific mention. In any case, when all four items are included, it is very simple to see that adding Item 4 results in Item 1 getting warmer.
One place where intuition often goes astray is the “low temperature heat sink”. Normally our surroundings (around 20 C typically) are not all that cold. Then adding a wall of ice that is held at a steady 0 C (even colder than the surroundings) certainly will not warm you. But that would be like adding an atmosphere that is held steady at a temperature BELOW 3 K!
tjfolkerts: you state ” In any case, when all four items are included, it is very simple to see that adding Item 4 results in Item 1 getting warmer.” There is no atmosphere on the moon. Sunny side of it gets much higher temperature than the surface of the earth. How come?
esalil, this is a discussion of broad averages. The average surface temperature of the earth is MUCH higher than the average surface temperature of the moon. This despite the fact that the Moon absorbs a higher fraction of the incoming light.
Furthermore, the moon’s average temperature would rise if it had an atmosphere of GHGs (although the max temperature on the sunny side would probably drop a bit.
Gabro November 19, 2017 at 5:45 pm
People skeptical of GHE are “not permitted to comment”? Say what???
READ THIS VERY THREAD. There are lots of people skeptical of the idea of GHE, and they are free to post, as are you. Your claim is a joke.
w.
For me it looks like hopeless to explain the basic physics about the GHE for those who think that it is nonsense. I try another approach. If you think that the GHE does not exist, how do you explain the downward LW radiation at the surface which is about 345 W/2. Compare this to the direct solar radiation of 167 W/2 at the surface. (71 W/2 of solar radiation is absorbed by the atmosphere and therefore the outgoing LW radiation is totally 238 W/2). These figures are not nonsense, because they are both measurement based figures.We know that 99.97 % of the energy keeping the Earth warm originates from the Sun. Where this 345 W/2 comes from? How do you explain this, if there is no GHE?
It comes from the atmosphere (including clouds). The atmosphere radiates the energy absorbed from the sun and the surface. The energy from the surface is mostly nonradiative (evaporation and convection). This does not mean that the inceased atmospheric CO2 will result in a warmer surface.
There is a ghe, simply because there is an (insulating) atmosphere between the surface and the cold space. The atmosphere is in direct contact with the surface and is easily warmed by it, mostly by nonradiative processes. The bulk of the atmosphere (N2 and O2) plays a big role.
edimbukvarevic. Otherwise very good but you say that “The atmosphere is in direct contact with the surface and is easily warmed by it, mostly by nonradiative processes.” This is not correct: The upward LW radiation emitted by the surface is 396 W/2 (Excatly according to Max Planck’s formula). Latent heat flux 90 W/2, and thermal flux 25 W/2, together 115 W/2. According to basic mathematics 396 is more than three times greater than 115. Right?
Aveollila, the radiative heat flux surface-to-atmosphere is ~26 W/m2 (350 – 324). According to basic mathematics, that is less than 25% of the non-radiative fluxes (115 or 78 + 24 = 102 W/m2). It is only one fifth of the net surface-to-atmosphere heat flux (~128 W/m2).
http://www.ipcc.ch/publications_and_data/ar4/wg1/en/fig/faq-1-1-figure-1-l.png
Pressure/temperature effect?
edimbukvarevic You do not calculate the upward energy fluxes in the right way, because you subtract a donward flux from the upward fluxes (why you selected 324 and not the 168 ??). According to the energy balance of Kiehl an Trenberth (which is obsolete and based on the wrong atmosphere) the upward fluxes are 350 + 78 +24 = 492 and the downward fluxes are 168 + 324 = 492. That is called an energy balance. Your way of comparing fluxes is not based on the real world. Using your way, I can prove anything as I like.
Aveollila, I commmented on the atmosphere and its energy. In particular, the heat exchange between the atmosphere and the surface. The total heat transfer from the surface to the atmosphere is about 128 W/m2. Only a small part of this flux is radiative, about 20% (350 – 324 = 26 W/m2). This is not debatable, unless the Earth’s budgets are grossly inaccurate.
Furthermore, if we add solar radiation directly absorbed by the atmosphere (~67 W/m2), we get 195 W/m2. That means only ~14% of the total heat transfer to atmosphere is LWIR radiation.
As always very confusing with so many different opinions, immovable positions and even contempt for anybody who doesn’t hold the same opinion. I’m an engineer but I’m constantly searching for an answer to how a cold body heats a warm body and how heat is apparently created from nothing.
Please allow me to ask a dumb question and hope that I can get an answer to at least help clear up some of my confusion. I can calculate solid surface temperature of the earth from incoming UV radiation (from a warmer body) and physical properties (absorptivity and emissivity of the surface). I can calculate a near surface air temperature based on conduction and convection. I can then calculate a lapse rate which gives me a vertical temperature profile which is pretty well matched by reality. I can do this with no reference to downwelling radiation. Why should the conclusion from this not be that this is because downwelling radiation doesn’t affect temperature and lapse rate?
Martin. Solar radiation is much more than UV radiation which has a wavelength shorter than the visible light.
I do not believe that you can show calculations that the solar radiation of 238 W/2 absorbed by the Earth produces the Earth’s surface temperature of 15 degrees Celcius. Why not? Because that absorbed radiation flux of 238 W/2 corresponds the surface temperature of -19 Celsius only as measured at TOA emitted by the Earth.
Nobody has shown that Max Planck’s equation is wrong. It shows excatly how the surface temperature produces an exact amount of radiation. The fact is that the surface has an average temperature of 15 C and it produces the radiation flux of 396 W/2. How it is possible that the Earth receives only 238 W/m2 but its surface can emit 396 W/? Perpetum mobile? Confirmed by measurements. Fighting against GHE like fighting against wind mills. You cannot win.
Unless the so called GHE is just the pressure density effect?
ave, the calculation for spot temperature is basic radiant heat transfer and is based on actual radiation not average hitting a flat plate and it doesn’t have an additive downwelling radiation component for it to work. The problem is that if you believe that downwelling IR is the only way that the surface can achieve it’s effective emitting radiation then you have to have 324 W/m2 of downwelling radiation that is higher than the sun gives us and a transfer of heat from cold regions to warmer ones.
If downwelling radiation is a key component in atmospheric temperature why is the lapse rate completely free of any influence from it. Why is Jericho warmer than Jerusalem or the temperature on a mountain lower than at the bottom.
Is it not so that any number of experiments of putting bodies of different temperature in proximity, the temperature of the highest temperature body is never increased by radiation from the lower temperature bodies (true?) no matter how many there are.
I believe that heat energy (as opposed to radiation) can never flow from a cold body to a warmer one at any level including quantum and once this is accepted, heating by downwelling radiation can’t be significant. No heat is added and none is trapped, no laws are broken
Maybe those who say that it is an atmospheric effect rather than a greenhouse effect could be correct. These wonderful gases though for sure moderate the temperature of the atmosphere but add heat to it?
To sailborder. Create a pressure of 100 atmosphere into a steel cylinder and leave it outside in the temperature of -10 degrees Celsius. What do think is the temperature after 2 hours. It is that -10 C degrees. A constant pressure cannot create heat into any system. Only if you do like in a diesel engine: apply a certain amount of mechanical work and turn it into heat.
“I believe that heat energy (as opposed to radiation) can never flow from a cold body to a warmer one at any level including quantum and once this is accepted, heating by downwelling radiation can’t be significant. No heat is added and none is trapped, no laws are broken”
What is so difficult to understand about the concept of slowed cooling?
Of course heat energy can impinge on a warm body from a colder one.
Photons from the colder object do not have any concept of the other object being hotter.
(and there will be photons unless it is at 0K)
And as a result the hotter body cools more slowly.
There is however no “Flow”, as in a net impingement of photons from the cold object such that it raises the temp of the warmer one.
It is like a tank loosing water at a rate of 10 gals/hr while at the same time you pour water from a bucket into the top at the rate of 1 gal/hr.
So the tank is actually leaking water at 9 gals/hr. It’s still leaking (cooling) but at a slower rate due to the small amount of water (energy) being added to it from the bucket (older object) leaking (cooling) at 1 gal/hr.
I think this is probably the best visualization to explain what I’ve been talking about.
So, you have a big tank, with 2 holes in it. and over about 12 hr’s once a day, a big dump of water happens, pressure is a little higher, so it rushes out a bit faster at the peak level. One hole is the optical window, which is constant in size, and another that represents the energy through the other bands, part of this hole has grown slightly smaller from increases in GHG’s.
But together it’s a good size hole, and then the tank goes 12 hours between big dumps of water.
But, there’s a second smaller tank of water that is filled constantly, from far away.
And when the water level drops some, depending on the level of the second tank, some of the water in the second tank starts to flow into the bigger tank. This slows the drop in water level in the big tank, until the next big fill of water, where it shuts off till later.
If the second tank is really full, the big tank gets as much from it, as goes out the bottom once the valve opens, and the level doesn’t drop anymore, even though the main fill line is dry still.
This is why Co2 doesn’t have hardly any affect on the level. It’s the sheer size of the tanks compared to the tiny 3.7W.
“Unless the so called GHE is just the pressure density effect?”
Yes your bike tyre heats up as the air you pump into it increases in pressure.
But does it stay hot for you forever?
Obviously not.
Why?
You did the ‘work’ with the pump and then you stopped – then no work so the tyre cooled.
Now replace the ‘pump’ with gravity and the air by the Earth’s atmosphere.
(Hypothetically) if we could just switch on gravity from zero then yes the atmosphere would heat as it compressed.
But the work has then been done.
So it cools.
Gravity is NOT maintaining a surface temperature.
Full stop.
It does however set the LR by virtue of the -g/cp relation – the surface temp then being set by the GHE via raising the effective temp of Earth (255K) up to ~8km whilst the LR is maintained.
Toneb, the pressure is constantly ON. Are you intentionally being obtuse?
Rod
I believe that the simplest form of the question you ask is this:-
“Can something cold heat up something hot?”
To answer this question we need to establish the nature of collision:-
Can a slow moving object travelling behind ever catch up to and collide with a fast moving object travelling ahead of it and moving in the same direction?
Well of course not, only fast moving objects can ever catch up and collide with slow moving objects ahead. This is because collision is a vector process; fast objects collide into slow objects and not the other way round and so the transfer of momentum always proceeds from fast to slow and never from slow to fast (for objects moving in the same direction).
The issue of heating by “back radiation” is more subtle. The diagram that Anthony supplied demonstrates Wein’s displacement law and it shows the envelope curve for frequencies at different black body temperatures. In addition we have all seen the diagram that shows the effect of selective absorption of frequencies by greenhouse gases. What we have never seen is the diagram which shows an intensity mound in the low frequency tail above the envelope curve which is the absolute requirement for “cold” low frequency radiation to add energy to a high temperature body. This does not happen, it never has and it never will. Back radiation heating of the warm surface below by a cold surface above is a fiction and Wein’s displacement law proves this.
The radiative greenhouse effect is an attempt to explain how the low temperature isothermal stratosphere can heat a planet’s surface. It cannot. The troposphere (the weather layer) is a gigantic heat engine. It moves prodigious amounts of mass and energy all day and every day. Our atmosphere does work and it is powered by the heat of the sun and the speed of the Earth’s daily rotation. Like all heat engines it has an exhaust temperature, that exhaust temperature is observed in the isothermal shell of the stratosphere that surrounds our planet. Just as the exhaust temperature of an engine cannot be used to power that same engine, so it follows that the low temperature stratospheric shell aloft cannot provide back radiation “heat” to power the high temperature weather machine below.
So, Phillip, at night does a cloud overhead affect the temperature at the surface below and why as it is not cutting off any sunlight. Clouds are composed of GH effect water molecules at low temperature or do you have an alternate explanation?
angech,
I live in the British Isles. Almost every day somewhere here experiences the effect of warm moist cloudy air moving over night time cooled ground. My worst ever driving experience occurred a few years ago in Aberdeenshire when I lost control of my vehicle on black ice. One frosty winter’s evening I walked our dog alongside the River Don under crystal clear skies. The next morning, before dawn, the weather had changed to a mild 8C, with damp moist air, a thick haar (sea fog) and very poor visibility. As I drove south to work alongside the river I came to a small hill, the north facing slope of which was covered in black ice as moisture from the sea fog condensed onto the previously cooled road surface, so yes warm moist cloudy air can heat a cold ground. The question at issue however is can something cold heat up something hot?
I think that this is due to a change in wind direction rather than a change in the vertical temperature profile.
Certainly in Scotland we notice abrupt daily and hourly change.
If the wind is from south or west we have warmer weather.
From the north or northeast its much colder.
Angech, are clouds not condensed water with no greenhouse effect?
” so yes warm moist cloudy air can heat a cold ground. The question at issue however is can something cold heat up something hot?”
Yes and no.
I was meteorologist with the UKMO in a previous existence and one of our winter jobs was to monitor road temps over a wide part of the English Midlands for councils re gritting for ice/snow.
It is no mystery that cloud raises RST’s and hence air temps above.
It is added to the algorithms for RST prediction. On a otherwise frosty night it can make for a marked jump in road surface temp. In fact thin Ci cloud at 30,000ft at a temp of -40C will cause a road temp to either to halt or rise slightly when below 0C.
But it is NOT strictly raising it’s temp, as you will discover if you examine, say, a bridge in the same situation (air beneath).
What is happening is that the heat-flux from below the surface overcomes the surface emission to space (because of the cloud) and so the temp there rises.
In other words extra photons have impinged the road and added to it’s energy such that that entering from below is out of balance with the LWIR leaving.
It is though still a slowing of cooling and NOT cold heating up something hot.
“I was meteorologist with the UKMO”
roflmao.
And you actually “advertise” that…. that’s funny !
Toneb,
Very interesting observation, thanks. I have often noticed that ground frost will form first on the metal skin of a car, before it sets into the pavement. Same principle I believe. I had never experienced black ice until that morning and had no idea how dangerous it can be, even with an overnight air temperature rise to well above freezing it is still possible to get into severe trouble on damp untreated roads.
I totally agree…… though I approach it from a heat capacity angle. The best the GHG can do is reach equilibrium with the ocean or ground …. simply because the GHG mass and heat capacity is minuscule compared to everything else.
Philip Mulholland November 20, 2017 at 6:52 am
It is standard knowledge that cloudy skies will be warmer than cloudless skies due to the blanketing affect of clouds. Surely it could also be that the air arrives already warm and clouds are part of the ‘package’? Here in the UK the majority of weather and associated temperatures come from somewhere else, either pre-heated or pre-cooled. However, we have cloudless days that can be very hot or very cold depending on the time of year. I understand the reason for this is that cloudless days tend to have low wind speeds as this is usually a high pressure weather system. In the summer when the sun is overhead the surface gets the maximum effect of the sun’s heat and the low wind speed does not remove this heat, much like a car can overheat if not moving. Conversely, with high pressure during the winter when the sun is week there is only slow out-flowing air and no in flowing warm air. Therefore, there are other considerations than just a cloudy sky keeps the temperature in.
Out of interest Adolf Galland gave four reasons why the Luftwaffe lost the Battle of Britain; number 1 was the unpredictable nature of Britain’s weather.
“The radiative greenhouse effect is an attempt to explain how the low temperature isothermal stratosphere can heat a planet’s surface.”
It doesn’t “heat it up”, you turkey, it reduces the rate of cooling!
Large amount of radiative energy going OUT. Smaller amount of radiative energy coming BACK. Result – less energy overall leaving.
So, with the Sun heating the ground, and the ground losing heat at a lesser rate than it would in vacuum, the ground gets warmer than it would in vacuum. It reaches equilibrium at a higher temperature.
Voila! Greenhouse Effect! Bleeding simple!
(No doubt someone is going to “debunk” this by nattering on about photons, or Wein, or by saying that the Earth is *never* in equilibrium. (Which it isn’t. And your point is?) Let someone else argue with them. Never debate with a fool, people might not be able to tell the difference.)
Gus,
Here’s a thing; the maximum surface temperature measured on the Moon in a vacuum is 100C (373K) but on the Earth the maximum recorded surface temperate with an atmosphere of 100 kPa pressure is 58C (331K). The Moon orbits the Sun in tandem with the Earth at the same overall distance (Yes its absolute distance changes from New Moon to Full Moon and then back again) but, averaged over a year, the Moon experiences the same intensity of solar radiation as the Earth does.
So what do I conclude from this? That the sun heats more in a vacuum and that on Earth the atmosphere cools its surface.
Earth’s atmosphere and oceans move the heat around. On the moon, it stays put, except for moving through the ground.
The moon is thus also colder than earth ever gets. Our air and water both cool and warm the surface.
Philip Mulholland November 20, 2017 at 1:57 pm
Right facts, but I fear you’ve reached the wrong conclusion. It’s more complex than that. I discuss this question in a post called “The Moon Is A Cold Mistress” …
w.
Willis,
This is what Uncle Gus wrote:-
Do you want to correct him or not?
Philip Mulholland November 20, 2017 at 10:17 pm
I’m not sure what I have to do with this, as I haven’t commented on what Uncle Gus said. However, it is true that ceteris paribus, the surface with a GHG-containing atmosphere will indeed be warmer than it would be without said atmosphere … so it is not at all clear what you think I should “correct”.
What am I missing here?
w.
Willis
Try reading this:-
Gus thinks that a surface in the presence of an atmosphere can be warmed to a higher temperature than a surface in a vacuum can be.
The invention of the vacuum flask
Philip Mulholland November 21, 2017 at 1:34 am
Thanks for the reply, Philip. So your contention is that if we removed the earth’s atmosphere the surface would get warmer??? Seriously?
Stefan and Boltzmann disagree strongly with that … in a vacuum, the earth’s maximum possible temperature would be 5°C, assuming the earth were a superconducting sphere. Given that it is not a superconducting sphere, the temperature would be lower. For example, the moon, at the same distance from the sun as the earth, has an average temperature far below 0°C, because it rotates so slowly …
w.
Willis,
One last time, I am talking about peak maximum temperature. In a vacuum, where there is no fluid (gas) to cool the surface the peak maximum temperature is higher under an equivalent radiation load.
Philip Mulholland November 21, 2017 at 11:14 am
You may well have been talking about that, Philip.
But Uncle Gus, who we were discussing, definitely was NOT talking about that. You asked me, was there anything I would correct about the statement of UNCLE GUS, not about your statement … and he said nothing about max temperatures, he was talking about mean temperatures.
And what is with the “One last time …”, like you’re at the end of your tether and I’m too dumb to understand but you’ll give me one last chance. I don’t care which end of the tether you’re at, I was replying to your question about Uncle Gus.
w.
PS—the reason for the difference in max temperature of the earth and the moon is not the vacuum. It is a result of the fact that their rotation rates are different. Because the moon’s surface can cool or heat for a longer period, the cold side gets much colder and the warm side gets much warmer than the earth.
We know this because if the moon were spinning at say 1 rpm, where it would be evenly heated, given the moon’s albedo (0.12), the S-B equation says it would be at about -4°C. So clearly, the vacuum isn’t the reason for the high temperatures.
I go into this in some detail in “The Moon Is A Cold Mistress“, which you might enjoy.
Willis,
Dinna fash. Here (I hope) is something we can agree on, slowly rotating planets heat more than rapidly rotating ones do.
At thermal equilibrium, any time a molecule absorbs a photon, it will emit one (follows from Kirchoff’s radiation law.) There is an equal probability of emitting upward or downward. The greenhouse effect results because the sun / earth /space system is not in thermal equilibrium and an upward emitted photon may escape to space, while the ones emitted downward remain in the system. Missing from this discussion is convection, which controls surface temperature and indeed the whole temperature profile of the troposphere. Discussion of lapse rate and equivalent emission height is also missing. Also, the great source of stability of our climate, the Stephen-Boltzmann law which says that radiant emission goes as the fourth power of temperature, with the result that any increase in temperature is opposed by a massive increase in energy radiated to space.
Infrared photons have far too little energy to change the orbit of an electron. The ‘greenhouse effect’ is entirely due to resonances in the elasticity of the chemical bonds between atoms in the gas molecule.
The 15um resonance is below the emission peak for typical ground temperature. However the photon distribution is wide enough that some will be in this range. Thus CO2 will have some effect on outgoing radiation.
When a CO2 molecule absorbs a photon, it may re-radiate it in a random direction, or it may transfer the energy to the bulk gas by way of colliding with a N2 or O2 molecule. Exactly which mechanism will predominate is hard to predict. The time interval of each is about the same, at 10ns.
N2 and O2 not being IR emitters, essentially have no way of liberating heat to space. However, if there is CO2 in the upper atmosphere, molecular collisions with this may provide a route for heat to escape to space, by way of exciting IR emission.
Hence the overall situation is far from simple, and it may even be that the presence of CO2 cools the upper atmosphere.
Perhaps more importantly there are numerous proofs of the fact that at sea level, CO2’s effect is already evident at very low concentrations, therefore further increases are unlikely to significantly increase its greenhouse effect. However at high altitudes this may not be the case. If so, adding CO2 to the atmosphere might actually cause planetary cooling rather than heating, since it will have little effect at sea level but will enhance the ability of the upper atmosphere to lose heat to space.
I’ve done a number of analyses of this on our website. For example:
https://iwrconsultancy.co.uk/science/modtran

https://iwrconsultancy.co.uk/science/computed
https://iwrconsultancy.co.uk/science/co2maths
That is a very interesting graphic.
Ian Macdonald November 20, 2017 at 5:09 am
Infrared photons have far too little energy to change the orbit of an electron. The ‘greenhouse effect’ is entirely due to resonances in the elasticity of the chemical bonds between atoms in the gas molecule.
The 15um resonance is below the emission peak for typical ground temperature. However the photon distribution is wide enough that some will be in this range. Thus CO2 will have some effect on outgoing radiation.
The 15 𝝻m resonance is very close to the emission peak at 288K.
When a CO2 molecule absorbs a photon, it may re-radiate it in a random direction, or it may transfer the energy to the bulk gas by way of colliding with a N2 or O2 molecule. Exactly which mechanism will predominate is hard to predict. The time interval of each is about the same, at 10ns.
The mean time between collisions in the lower troposphere is ~0.1ns the re-emission time is orders of magnitude longer.
Ian – Where did the 10ns time come from? I calculated approx 0.2 ns average time between contacts using the calculator at http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/frecol.html and found relaxation time of 6000 ns at 210 K at http://onlinelibrary.wiley.com/doi/10.1002/qj.49709540302/abstract. Other refs allowed deternination of effect of temperature resulting in approx 5300 ns at 280 K.
When you say N2 and O2 cannot liberate ‘heat’ to space, are you saying they do not emit energy? I keep hearing that N2 and O2 do not absorb or emit IR, but I’m also aware that any matter above 0 Kelvin emits radiation at a rate determined by its avg temp. If an object or region of space emits radiation then it’s temperature decreases whether that energy is IR or not.
Given that N2 and O2 make up about 4950 Trillion tons of the total ~5,000 Trillion tons that comprise our atmosphere, it seems fairly obvious that the energy being emitted by N2 and O2 is where most of the energy is lost from the atmosphere radiating.
Steven Morris November 20, 2017 at 2:01 pm
When you say N2 and O2 cannot liberate ‘heat’ to space, are you saying they do not emit energy? I keep hearing that N2 and O2 do not absorb or emit IR, but I’m also aware that any matter above 0 Kelvin emits radiation at a rate determined by its avg temp. If an object or region of space emits radiation then it’s temperature decreases whether that energy is IR or not.
Does not apply to gases which can only emit at those wavelengths which correspond to allowed transitions between energy levels of the molecules. The blackbody relationship provides an upper limit to such emissions, there are no significant allowed transitions for either N2 or O2 within the range of frequencies emitted by a blackbody at the earth’s temperature.
Given that N2 and O2 make up about 4950 Trillion tons of the total ~5,000 Trillion tons that comprise our atmosphere, it seems fairly obvious that the energy being emitted by N2 and O2 is where most of the energy is lost from the atmosphere radiating.
Not true as it’s based on your false premise.
Notice that the absorption/re-emission band of CO2 ranges from about 13 to 17 microns, corresponding to a temperature range of -51 to -103 degrees C, i.e., lower than is normally experienced at Earth’s surface (which corresponds to a range of about 9 to 13 microns), hence how could CO2 possibly behave like a greenhouse gas in the Earth surface environment? This is why infrared astronomer Michael Sanicola was able to report finding no back-radiation from CO2 that could interfere with his astronomical observations.
davidbennettlaing November 21, 2017 at 5:55 am
Notice that the absorption/re-emission band of CO2 ranges from about 13 to 17 microns, corresponding to a temperature range of -51 to -103 degrees C
There is no such correspondence. Blackbody radiation at the earth’s temperature peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution (~97% of max).
By the way you shouldn’t believe anything you read on Steve Goddard’s site, not even the names!
When you look at the whole spectrum, being near the maximum, isn’t all that significant.
And remember water overlaps co2.
Here’s just co2
and Water
But, I think that as water condenses at night in the troposphere, that sensible heat, that IR will light up co2 @15u. And co2 might light up during the day, but IIRC that blocks about as much as it adds during the day. That why the focus on Min T. But they’re completely wrong about Min T, it just follows dew point, which follows the ocean cycles, and since air temps respond differently during the day over ocean than it does land, and the hemispheres are asymmetrical to land area, global Temp averages are going to cycle with the ocean cycles.
Then, what I found shows why co2 will never for the foreseeable oil reserves become to high for the atm water to not regulate Min T. If we doubled Co2, added another 3.7W/m^2, there’s just be ~3.7W/m^2 decrease in the complementary water vapor forcing. It condenses as much water vapor as it can, to try and stay above dew point. And it looks like it’s supplying 40 or 50W/m^2, That’s a lot of doubling.
Not to say 2,000ppm is acceptable, but even an absurdly high 800ppm, isn’t a temp problem for over heating the planet.
micro6500 November 21, 2017 at 8:50 am
“There is no such correspondence. Blackbody radiation at the earth’s temperature peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution (~97% of max).”
When you look at the whole spectrum, being near the maximum, isn’t all that significant.
If you run it through Modtran you’ll find that about 10% of the total is absorbed by CO2.
And remember water overlaps co2.
If you’d run the spectra at a realistic resolution you’d find that both spectra are composed of discrete lines which in general are a different wavelengths and therefore don’t interfere with each other.
Run Spectralcalc between 600 and 700 cm-1 for both CO2 and H2O and you’ll see how sparse the H2O lines are compared with the CO2.
Phil; Who is “Steve Goddard?” I know that Wien temperatures can’t actually be inferred from the line spectra of gases, but my point is that even if you were to construct a continuous, bell-shaped, Planck-like curve from the radiation within the band of CO2’s output (13to 17 microns), the closest thing to a Wien temperature you’d get would be the most intense line, which is 14.95 microns. That would correspond to a Wien temperature of -79 degrees C, which is well below any temperature experienced at Earth’s surface. In fact, of course, gases acquire their temperatures not from radiation, but from the kinetic energy of their constituent molecules, which is a function of pressure, but sticking with the radiative argument on which greenhouse theory is based, the band within which CO2 can emit just doesn’t qualify the gas for greenhouse function within Earth’s atmosphere.
davidbennettlaing November 22, 2017 at 5:54 am
Phil; Who is “Steve Goddard?”
It’s the pseudonym of the host of the website where the bogus post purported to be by a non-existent IR astronomer Michael Sanicola, which you referred to, was posted.
I know that Wien temperatures can’t actually be inferred from the line spectra of gases, but my point is that even if you were to construct a continuous, bell-shaped, Planck-like curve from the radiation within the band of CO2’s output (13to 17 microns), the closest thing to a Wien temperature you’d get would be the most intense line, which is 14.95 microns. That would correspond to a Wien temperature of -79 degrees C, which is well below any temperature experienced at Earth’s surface.
So what? A blackbody at -80ºC emits its peak radiation at 15 microns, that has no relevance to the BB emission from the earth’s surface. The earth’s surface at 288K emits much more 15 micron radiation than a BB at -80ºC (193K), 5.8 W/m2/sr/µm vs 1.1 W/m2/sr/µm. What’s of interest is the IR emitted by the surface.
Phil; Michael Sanicola is apparently a pseudonym for Morgan Wright, who states “I’m an opticist, who specializes in optics and IR. I worked for GE’s infrared department and designed infrared telescopes for GE that were used by NASA in outer space. I invented the ambient temperature microbolometer.” By whatever name, the individual appears to know what he’s talking about. As to your contention that its all about Earth’s IR output, that’s just not the case. The important consideration is the radiation that CO2 gas is capable of re-emitting, and that lies between the limits of 13 and 19 microns, as I (and MODTRAN6) said.This band is represented in Earth’s BB spectrum, but it’s in the right hand, “cold” wing of the spectrum, corresponding to temperatures below those of Earth’s surface, where none of it can therefore influence Earth’s actual surface temperature range.
I dare anyone who believes that the science is settled on the GHE to read this entire discussion! The inability of anyone to clearly and convincingly explain this effect is confirmation of its complexity exceeding our current knowledge of the physics, chemistry, and meteorology involved. I’m not sure we even know the sign (positive or negative) of the climate’s sensitivity to increased CO2 ! I know that it’s a “wicked” problem to solve, but the wide range of currently proposed hypotheses is bigger than it ought to be, and that’s an embarrassment for science.
Very strange, somehow there are comments above under my name, “Robert Kernodle”, that I DID NOT WRITE. Somebody else wrote them and somehow was able to post under my name. How is this possible?
Aveollila, that is true for a static mass of air at constant pressure. That isn’t the case in the troposphere where packages of air rise and cool and then fall and heat by compression. Surely this has to taken into the big picture.
Also recognize that the atmospheres volume is not static, thus allowing for cooling via expansion. This is the biggest problem with arhennious and Tyndalls experiments, they are in test tubes where the mass of air being heated with IR is not allowed to expand or contract with addition or subtraction of energy.
As expected this thread has very rapidly accumulated hundreds of comments. Very nice! As there have been a number of criticism of my initial comment (pure luck got in first) concerning “photons” I would now like to add some additional comments.
1. The concept of a “photon” was invented to help explain certain experimental results involving electro-magnetic radiation. Specifically that in certain experiments it looked like there was quantisation as opposed to discrete analogue variation.
2. As a concept it has worked and aided our understanding but THERE IS NO EXPERIMENTAL EVIDENCE that an elementary particle , The “photon”, exists. All results can equally be explained by assuming electromagnetic radiation is a wave, with analogue variability but the interaction of this wave with matter causes phenomena which are discrete/quantised because matter itself is particulate. In other words the e-m is a wave, the matter is particles, the results can be “lumpy” BECAUSE of the matter, NOT because of the wave i.e. not because the wave is really “lumpy” itself or comprised of “photons”.
3. This is why I asked readers to use both explanations when trying to explain things in radiative physics. Try explaining it both from e-m being only a wave as well as your favourite photon/particle explanation.
4. The belief that photons are real elementary particles is very very widespread but you will find the better PhD’s referring to it as quantised e-m radiation (that’s sort of a fudge/middle ground idea).
5. Anyone who thinks “photons” are real elementary particles is advised to read up on the history of how the word came to be invented and what various eminent scientists at the time thought of it.
6. Finally, if anyone really thinks they do have a link to experimental evidence which conclusively shows that photons are real elementary particles I would be happy to look at it. Before giving me the link please be sure to consider the alternative explanation (e-m wave interacting with lumpy matter) and tell me why that is NOT an equally valid explanation.
Please post the work of the PhDs whom you consider better regarding the physical reality of photons. A photon is a quantum of EM radiation, so the formulation which “they” allegedly use is a distinction without a difference.
I’m familiar with how photons were discovered and named. In reply to your original comment, I’ve already given you examples demonstrating the reality of photons and their relationship to “matter”, to which you haven’t bothered to respond.
PS: In terms of QM theory, photons are no more nor less real than electrons.
Do you also consider electrons not to exist?
As mass and energy are equivalent, so are particles and waves at relativistic and quantum scales.
I, Robert Kernodle, did NOT post the following FOUR comments, even though each appears separately under my name (linked to my website) as indicated here:
Robert Kernodle November 19, 2017 at 4:35 pm
Technically energy can be “destroyed.” If you observe the spectra from distant stars, you’ll note that the hydrogen emission lines are red shifted. When the photon left the star of origin, it had the normal wavelength (freq) of the emission lines of hydrogen. As it traversed the expanding intergalactic space, it’s wavelength grew longer (red-shift.) There is a calculable loss of energy in this red-shifted photon according to E=hc/l where delta-E = hc/delta-l.
…
Where did the ‘lost’ energy go?
Robert Kernodle November 19, 2017 at 5:34 pm
I already did. The photon that was emitted a million light years ago, “lost” some of it’s energy. The frequency has decreased (increased wavelength.) Where is the “lost” energy?
Robert Kernodle November 19, 2017 at 6:02 pm
Gerontius, that does not explain it. There is no gravitational field in the million light years between the star that emitted the photon, and the spectrometer here on earth that measured it. The red shift is due to the expansion of space, not to a gravitational field.
Robert Kernodle November 19, 2017 at 6:05 pm
PS Gerontius, photons coming from the opposite side of the universe display the same red shift. You would think that if you looked in the opposite direction of the red shifted photon, you’d observe a blue shifted one if your “theory” were correct.
________________________________________________________
I, Robert Kernodle DID, however, post SIX comments, as indicated on the dates below:
Robert Kernodle November 20, 2017 at 7:49 am
Robert Kernodle November 19, 2017 at 12:22 pm
Robert Kernodle November 19, 2017 at 12:24 pm
Robert Kernodle November 20, 2017 at 8:03 am
Robert Kernodle November 20, 2017 at 8:14 am
Robert Kernodle November 20, 2017 at 8:31 am
Either there is a bug, or my ID has somehow been hacked, or an alter-universe spawn of myself is phasing in and out of this universe. If one of his posts follows one of mine (the real me), then will the universe annihilate itself?
You should from the comments above by now understand that the -80 degree is the lowest temperature at which CO2 can emit radiation and from your own emission graph that it will emit more in any wavelength the warmer it gets. It absorbs radiation (your graph is a solar radiation absorbtion graph) from the sun which is approx 5770 K so a great deal warmer. The interesting thing of the “finger graph (spectrum figure)” is that heat can “escape” the CO2 trap if other substances emit the heat in a wavelength for which CO2 is “transparent” and that some solar radiation in the infra is absorbed by CO2 in the stratosphere and high altitude troposphere and never makes it to the surface of the planet. As to CO2 the absorbtion of light and heat by different types of gasses please read the treaty by Arrhenius. This may be a first introduction.
https://earthobservatory.nasa.gov/Features/Arrhenius/
Key points:
• Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
Correct, but strictly applicable only to molecular bonds. The fixed levels at which electrons exist in such bonds are “quantized” due to resonance and harmonics. When radiation of the exact same frequency as the natural resonant vibration of a particular molecular bond, or a harmonic thereof, is received, the electron in the bond in question makes an integral leap (1,2,3…,) to the next higher level. When a natural resonance or harmonic is equaled by incoming radiation, the ensuing leap occurs without requiring an input of energy.
• For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
Correct, again, for bonds. Where radiation is involved, this happens by resonance without requiring energy input.
• For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
No. As mentioned above, no energy input is required for the resonant leap of a bonding electron to a higher quantum level. Einstein couldn’t have known this, so he invented the “photon” to explain the phenomenon. In reality, EMR is simply a frequency field containing no energy whatsoever.
• Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
EMR of frequencies between resonant levels don’t interact (aren’t absorbed) but are simply reflected or transmitted. Only resonant frequencies are absorbed.
• Photons with not enough energy to raise the orbit of any of the electrons are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
Correct.
• The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
For “photon,” read “radiation.” The absorption/emission bandwidth of CO2 is from 13 to 17 microns, according to MODTRAN6.
• Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
No. Again, quantum leaps only occur at resonant frequencies. In condensed phases (solids and liquids), in which atoms are in constant mutual contact, heat conduction occurs, leading to the familiar Planck distribution consisting of a continuous, bell-shaped, broad range of frequencies of which the peak frequency corresponds to the (Wien) temperature of the emitting object. In gases, atoms are seldom in contact (except for pressure-broadening in dense gases), so such thermal conduction can’t occur, so gases exhibit line spectra in which spectral lines are separated by resonant and harmonic intervals (Rydberg-Ritz combination principle). CO2 can only absorb and emit radiation in the bandwidth 13 to 17 microns, and if a Planck spectrum were to be assembled from radiation in this range, its Wien temperature would be well below Earth surface temperatures, hence CO2 is incapable of transferring heat to the warmer Earth.
• LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
Correct.
I was going to respond to some of these ‘corrections’ but when I got to:
“In reality, EMR is simply a frequency field containing no energy whatsoever.”
I realized it was pointless. With such a flawed view of radiation, it would take way too much effort to address all the errors in the original post PLUS all the errors in the ‘correction’.
Can you actually refute my allegations, Mr. Folkerts, or would it be “too much effort?” It’s easy to dismiss things you don’t agree with, but that doesn’t mean that you’ve decided the issue. Science is too heavily burdened by people who simply accept long-established theory. As a polymath, I see definite value in questioning old thinking and in seeking truth in hard datasets rather than in the weight of well-established ideas. The Universe is what it is, not what we think it is, and the proper access to truth is a willingness to question old thoughts.
David … here are a few.
“When radiation of the exact same frequency as the natural resonant vibration of a particular molecular bond, or a harmonic thereof, is received, the electron in the bond in question makes an integral leap (1,2,3…,) to the next higher level. “
The absorption of this long wavelength IR involves vibrations and rotations of molecules. Not electrons jumping to higher levels. The “Bohr model” thinking with electrons jumping to higher energy orbitals is not correct here.
“Where radiation is involved, this happens by resonance without requiring energy input.”
This violates conservation of energy. If a molecule gains energy (warms up), it requires energy input.
“CO2 can only absorb and emit radiation in the bandwidth 13 to 17 microns, and if a Planck spectrum were to be assembled from radiation in this range, its Wien temperature would be well below Earth surface temperatures”
The “Wein temperature” strictly only applies to a full blackbody curve. The wavelength from Wein’s Law tells the wavelength that is most strongly emitted by a blackbody radiator. When the emitting material is not a blackbody, the strongest wavelength is not necessarily related to the wavelength predicted by Wein’s Law.
davidbennettlaing November 20, 2017 at 6:56 pm
David, you have claimed that “In reality, EMR [electromagnetic radiation] is simply a frequency field containing no energy whatsoever.”.
Say what? Light is EMR and it definitely contains energy. We know this because we can feel the energy in sunlight warm our skins.
Microwaves are EMR. We use the energy in microwaves, the energy you claim doesn’t exist, to cook our food.
Given such a bizarre statement, one that is so divorced from reality, I can understand tjf not wanting to get into a discussion with you. The problem is that you are starting from a point that is so far away from well-established basic physics that there is little hope of bridging the gap.
You say “As a polymath, I see definite value in questioning old thinking and in seeking truth in hard datasets”, and I agree completely.
However, I also see no value in ignoring old thinking as you are doing. The fact that electromagnetic fields contain energy, energy that is used e.g. in electric motors to do practical work, has been known since someone, maybe Faraday, noticed that a wire containing an electrical current could move the needle of a compass. Moving a compass needle requires energy …
And regarding questioning old thinking, here’s some old thinking you might consider in your quest for new understanding …
Best to you,
w.
T.J. Folkerts, to your attempted refutations:
1. Bond responses include rotations, vibrations, and ionizations, in order of resonant energy induction. The first doesn’t involve jumps to higher energy levels. The latter two do, creating increasingly greater distance between bonded atoms until the final distancing, ionization.
2. The first law of thermodynamics holds only in thermally isolated systems in equilibrium, which Earth/radiation interactions clearly are not. The principle of resonant response (unknown to Einstein) is well understood, now, and all that is required for an energyless response in a frictionless system (such as molecular bonds) is that incoming radiation has exactly the same frequency as a resonant frequency or overtone of the receiving bond. The resulting increased motion and acceleration of charged particles (electrons) in the bond results in the LOCAL generation of energy. This occurs in quantized steps, corresponding to resonant frequencies or overtones thereof. This is what Einstein interpreted as “Lichtquanten,” or “photons,” which are locally produced by the resonant interaction of EMR with matter and not carried by incoming EMR.
3. Quite true that the Wien temperature strictly only applies to the most intense frequency of a continuous Planck spectrum. However, I challenge you to assemble anything resembling a Planck curve from the discrete line spectra composing CO2’s input and output and have the Wien temperature of its most intense spectral line (about 14.95 microns) fall within a range that corresponds to normal Earth temperatures. In other words, CO2 can only return infrared radiation to Earth’s surface that is colder than Earth’s normal surface temperature range, and as is well-known, cooler objects (here, CO2) can’t transfer heat to warmer ones (here, Earth’s surface).
Willis,you said:
“Light is EMR and it definitely contains energy. We know this because we can feel the energy in sunlight warm our skins.”
What we actually feel is our skins warming under the impact of energyless EMR. In all cases of the detection of “photons,” interaction of EMR with matter is involved. Even our detection devices are material, and so, logically, they respond to incoming EMR. “Photons,” to my knowledge, have never been “detected” in EMR in a context apart from matter. This removal of energy from EMR solves a lot of problems. First, EMR is known to have no mass and it travels at light speed, neither of which is possible if it contains energy. Second, complex mathematics with imaginary numbers are required to get around this, and these spin off all kinds of imaginary, Alice-in-Wonderland worlds for us to contemplate. Third, it relegates energy to its proper state as a property of matter, not as some two-faced protean phenomenon that is mass-bound in most forms and mass-independent in another. With appropriate dimensional transforms, all forms of energy (except, of course, that supposedly in EMR) can be seen to have a mass term. Mass is even present in Planck’s constant h, in that purest of equations dealing with EMR, E=hv.
The statement here is not bizarre. It is simply a rethink of old, entrenched theor based on new information. Once resonance and the consequent local generation of energy (“photons”) by EMR are taken into account, things become a whole lot simpler.
Cheers,
David
David, an interesting discussion.
1) I don’t see how rotation and vibration could be considered “bond responses”. Rotation is a physical motion of the atoms (which you seem to acknowledge with “The first doesn’t involve jumps to higher energy levels”). Vibration could, I suppose, be considered 1/2 bond energy and 1/2 KE. In any case, this difference in terminology is only a minor point.
2) Conservation of energy always holds (with no known counterexamples). Your claim that “The first law of thermodynamics holds only in thermally isolated systems in equilibrium” is patently false. ΔU = Q – W applies when you use a calorimeter. It applies to heat engines. This law is ROUTINELY applied to non-isolated, non-equilibrium scenarios. For isolated systems in thermodynamic equilibrium, the first law becomes simply ΔU = 0!
Your claim of “results in the LOCAL generation of energy” simply is not observed in nature. Any local gain in energy of a molecule is the result of the simultaneous equal local loss of energy of a photon. Einstein said nothing to contradict this conclusion.
3) This whole paragraph seems to be a misapplication of some correct laws of physics. You say “as is well-known, cooler objects (here, CO2) can’t transfer heat to warmer ones”. This is true as far as it goes, but cooler objects (like CO2 in the air) CAN slow the loss of heat (radiation from the warm surface) when interposed in front of an even colder background (3K outer space). With a steady input of heat from the sun, this can and does result in a warmer temperature for the surface.
tjfolkerts, Thank you!
1) Right you are regarding rotations. Vibrations involve accelerations, as the bond stretches and contracts with each cycle. This does two things: first, accelerating charged particles emit EMR, and second, increased vibrational motion of the bonded atoms represents increased kinetic energy output.
2) Patently false? Wikpedia (e.g.,) says, “The law of conservation of energy states that the total energy OF AN ISOLATED SYSTEM is constant” (my emphasis). Perhaps I should have stressed “isolated,” which Sun/Earth certainly isn’t. If my view is correct, the local generation of energy is seen all the time in EMR resonantly inducing increased vibrations and ionizations with the release of photons in receiving matter, given a high enough frequency of radiation. Also, every time you drive your car, local generation of energy occurs in its internal combustion engine, given a sufficient fuel input. Again, not a closed system.
3) Absolutely! This is evident in the difference between enjoying a cool evening in humid Kent, Connecticut as opposed to a chilly one in the dry lower Sonoran desert of southern Arizona, even though the daytime temperature only hit 78 in Kent, whereas it hit 109 in Catalina. We’re talking H20, here, but CO2 works the same way, as Wm. Ferrell said back in 1884, or thereabouts. The thing is, CO2 absorption and back-radiation can retard heat loss, but it can’t add heat. It’s sort of like a blanket over a sleeping person. It can retard heat loss from the body, but it can’t raise the body temperature past its metabolic norm of 37 C unless, of course, it’s electric. Similarly, increased solar input can raise Earth’s surface temperature, but back-radiation from CO2 can’t. Interestingly, I haven’t seen the exothermic reactions involving O2 and O3 in the stratosphere as significant sources of added heat for the Earth system.
“[A blanket] can’t raise the body temperature past its metabolic norm of 37 C “
Ah! But here the body has an active thermostat the can adjust the power input (ie regulate metabolism rates) to deliberately seek a specific temperature of 37 C. The earth doesn’t in a similar way actively adjust the power input from the sun to deliberately seek a temperature of, say, 15 C.
Ah, but it does. First there’s trillions of tons of solar heated liquid all over, and as Willis has shown, water vapor clouds and then storms when it gets too how, there by regulating max T, and I’ve shown that water vapor regulates cooling using this stored energy to augment surface temps to limit cooling at night to dew point.
Rod Gill is not completely correct in saying that just because the temperature at which the maximum radiation is emitted at 15 um is -80 C, that IR radiation at 15 um cannot be absorbed at higher temperatures.
Rod Gill’s top graph shows the Planck distribution of intensity of emission as a function of wavelength at given values of temperature. If the wavelength of maximum intensity is about 500 nm for 6000 K (about the temperature of the surface of the sun), there is still substantial energy emitted at wavelengths above and below 500 nm.
The Planck distribution for the Earth’s surface at 289 K would reach a maximum intensity for a wavelength of 10 um, but there would still be a substantial intensity at the slightly higher wavelength (lower frequency) of 15 um.
The Planck distribution and Wien’s law (which is merely differentiating the Planck distribution and setting the derivative to zero) apply to EMISSION spectra. The ability of a gas to ABSORB infrared radiation is based on the photon energy levels that cause an electron to change from one orbital to a higher-energy orbital, which vary from one gas to another.
One of the keys to debunking global-warming theory is in the second figure in Rod Gill’s article. For the 13-17 um peak absorption range for CO2, there is also substantial absorption by water vapor. It is not clear from the graphs at what concentration these absorption spectra were measured, but it can be assumed that all were measured at the same concentration for an apples-to-apples comparison.
Over an ocean, it is common for the water-vapor concentration to be 1 to 2 percent, or 10,000 to 20,000 ppm, as compared to about 400 ppm CO2 (currently at Mauna Loa). If water vapor at 400 ppm would absorb 40 to 60% of the available energy at a wavelength between 13 and 17 um, at 10,000 ppm it would absorb over 99.9% of the available energy (the energy transmitted, or not absorbed, is a negative exponential of the concentration * distance), and the CO2 absorbs most of what’s left, which is less than 0.1% of the total energy absorbed.
Since 70% of the earth’s surface is covered by water, there is always water vapor more or less in equilibrium with it in the air over the oceans, and CO2 has a very minor effect on climate over the oceans, because water vapor has already absorbed the vast majority of the emitted IR energy.
CO2 can only affect the climate in areas with extremely low water vapor concentrations, either over deserts or in very cold climates where the air is too cold to hold much water vapor. This may occur over high-latitude areas in winter, but this does not prevent temperatures from dropping well below freezing.
During the Arctic spring and summer, some of the sea ice along the northern shores of Russia, Scandinavia, Alaska, and Canada does melt (primarily because of warmer sun-heated air blowing northward off the continents). But the open water allows for evaporation, which raises the humidity of the atmosphere and allows clouds and storms to form, which limits the amount of incident sunlight and keeps temperatures only slightly above freezing during the summer months.
The major fear of global-warming alarmists is that runaway warming will melt the Greenland and Antarctic ice caps and cause flooding of coastal cities.
The facts show that, over temperate and tropical oceans, the vast majority of the available IR energy is already absorbed by water vapor, and the CO2 effect is like the weight of a fly on top of an elephant (of water vapor absorption). CO2 can only absorb appreciable energy in very cold and/or dry environments, but in the polar regions, most of the “warming” is in the winter, when it is too cold to melt ice, and humidities increase in summer, damping out the effect of CO2. Also, most of the Greenland and Antarctic ice caps are at high altitudes (over 2000 meters) where midsummer temperatures never get above freezing, so that no melting occurs.
“CO2 can only affect the climate in areas with extremely low water vapor concentrations, either over deserts or in very cold climates where the air is too cold to hold much water vapor. This may occur over high-latitude areas in winter, but this does not prevent temperatures from dropping well below freezing. “
This also occurs at high ALTITUDES. The emission of 15 um IR to space occurs mostly up near the tropopause. This is confirmed by various data like , which shows the IR emission to space in the 15 um range radiates as well as a ~ 215 K blackbody. At these low temperatures, there is basically no water vapor in the atmosphere.
, which shows the IR emission to space in the 15 um range radiates as well as a ~ 215 K blackbody. At these low temperatures, there is basically no water vapor in the atmosphere.
So from your own logic, CO2 near the tropopause affects climate. And since the tropopause circles the earth, CO2 impacts the climate globally.
The main point that seems to be missing here is that the ‘greenhouse effect’ is about ‘trapping’ heat by radiative/greenhouse gasses. RGs don’t trap heat they just delay its transfer to space. The atmosphere is awash with collision induced IR that is constantly moving energy about. How fast? The key question is how long this delay is. Eventually the energy is radiated to space or the atmosphere would just keep heating up.
If the delay is long the energy involved will heat the atmosphere significantly. If it is short, the energy is transferred to space rapidly and there is no significant heating of the atmosphere. I calculate the heating to be negligible.
See: brindabella.id.au/climarc in the RadiativeDelayInContext article.
dai
dai davies, I agree with your analysis with the exception that it would be clearer if you added the time frame to: ” calculate the heating to be negligible…( for a millisecond, second, hour, day, month years etc.). As I understand the average residence time of a photon in a GHG molecule is in the nano to microsecond time frame. So although “trapping” may occur, on a molecular basis, it is nearly instantaneous while leaving little if any energy transfer.
I agree with this statement of your: “The atmosphere is awash with collision induced IR that is constantly moving energy about.”, but would add molecular collisions to the concept. When these molecules collide they can and often do transfer energy. That function is called conduction. For me that is the best explanation for energy/heat “trapping” in the atmosphere.
It is the same for the surface, EXCEPT FOR WATER, where that IR energy can and most often is trapped for a significant time. It doesn’t mater whether that IR energy originated from a molecule in the atmosphere or from the Sun.
CoRev November 21, 2017 at 4:05 am
dai davies, I agree with your analysis with the exception that it would be clearer if you added the time frame to: ” calculate the heating to be negligible…( for a millisecond, second, hour, day, month years etc.). As I understand the average residence time of a photon in a GHG molecule is in the nano to microsecond time frame. So although “trapping” may occur, on a molecular basis, it is nearly instantaneous while leaving little if any energy transfer.
No, for CO2 the time to emit is of the order of msec whereas a typical molecule will undergo about 10 collisions per nanosec in the lower atmosphere so there is plenty of time for energy transfer.
Phil, as I stated the residence time of a photon is” “is in the nano to microsecond time frame.” I need a scientific reference to assure such a long time as milli-second. Also do you have a reference for your claim of multiple molecular collisions for lower atmosphere resident molecules.
Regardless, i think you are confirming my contention that conduction/collisions are a/the major energy transfer mechanism.
lifeisthermal November 19, 2017 at 10:48 am
Glad to. I have more references if you wish, it is bog-standard thermodynamics. Or you could just google “two-way radiative transfer” …
Now, until you understand the equation at the bottom of that webpage I linked to, and you can explain it to us, how about YOU shut up …
w.
lifeisthermal, in reference to you saying “Show me a reference for any transfer outside of “net”, or shut up.”, in fact, the very term “net transfer” should give you a clue that there are energy transfers other than the net transfer …
You see,”net energy transfer” means energy gains minus energy losses, and each of those IS AN ENERGY TRANSFER.
w.
Willis, surely though there is radiative transfer from cold to hot but never a transfer of heat energy. The colder body can never warm the warmer body?
Martin Mason November 21, 2017 at 2:05 am
Thanks, Martin, and you are right. However, this gets us into semantical trouble because of our common meaning of “warm”, which differs from a strict scientific meaning.
For example, we think nothing of putting on a cold coat and noting that it has warmed us up … which of course can’t happen, as you point out. As they say:
So I’m very careful in my use of words around this issue. If you look at my comments, I say things like “the cold atmosphere leaves the surface warmer than it would be without the atmosphere”, which is absolutely true and does not violate the Second Law.
Regards,
w.
Steven Morris November 20, 2017 at 2:01 pm
N2 and O2 do both absorb and radiate, but they do so in only a very limited part of the IR spectrum.
Not quite true. One exception is xenon gas, which is monatomic and has no free electrons. As a result, it neither radiates nor absorbs energy.
Yes, assuming it is not receiving any radiation …
Often in science, things that are “fairly obvious” are not true. In this case, because of the narrowness of the O2 and N2 absorption bands, they physically cannot absorb (or radiate) much of the upwelling radiant energy, because it is the wrong wavelength.
w.
🙂
Who cares whether emissions are in the Infrared part of the spectrum or not? If an object radiates outside of the IR, are we to believe that the amount of energy within that region of space has not decreased and therefore cooled? Given no or less energy has been absorbed of course.
So our science books should say, all matter above 0 Kelvin, except Xenon, radiate energy. Oddly enough one can buy Xenon lights.
N2 and O2 do not need to absorb or emit IR in order to heat or cool. Energy is conducted via contact with the surface and they are constantly colliding with H2O vapor, CO2, CH4, etc reducing the energy within those molecules if the they have higher energy and increasing it if they have less. Once they have attained the Avg temp of say 15C, they are free to radiate that energy at a wavelength determined by Wein’s Law, correct?
Entropy be damned if objects cannot radiate unless they are some exact temp.
Steven Morris November 21, 2017 at 3:00 am
N2 and O2 do not need to absorb or emit IR in order to heat or cool. Energy is conducted via contact with the surface and they are constantly colliding with H2O vapor, CO2, CH4, etc reducing the energy within those molecules if the they have higher energy and increasing it if they have less. Once they have attained the Avg temp of say 15C, they are free to radiate that energy at a wavelength determined by Wein’s Law, correct?
No, they are unable to emit in that wavelength range because there is no excited dipole. The energy you refer to is just translational energy.
Willis Eschenbach November 20, 2017 at 4:37 pm
Not quite true. One exception is xenon gas, which is monatomic and has no free electrons. As a result, it neither radiates nor absorbs energy.
Actually Willis even Xenon emits, in fact it has a very large number of lines in the visible which is why it’s often used in lamps.
http://astro.u-strasbg.fr/~koppen/discharge/
Phil. November 22, 2017 at 4:53 am
Sorry for my lack of clarity, Phil. You are correct. However, I was referring to the current context, which is thermal longwave (IR) radiation. Xenon neither absorbs nor emits thermal longwave radiation.
However, when you get xenon up to very high temperatures, it can indeed emit light … but that is happening by an entirely different mechanism which has nothing to do with the subject under discussion.
Thanks,
w.
I don’t have a definitive answer, here, but do submit the following criticisms. The idea that IR photons just bounce around the atmosphere, being absorbed and re-emitted by CO2 molecules, producing no warming, is probably wrong. If absorption were followed instantaneously by emission, it would have a better chance of being true, but that isn’t the case.
For example, the radiative lifetime of a CO2 molecule for the 106 micron transition is on the order of 2 msec for CO2 in gases at a pressure of about 8 torr. That’s the only reason a CO2 laser can work, especially a Q-switched, high-power laser. It also means that at higher pressures, the probability of collisional, rather than radiative, de-excitation grows substantially. As a matter of fact, the transition I just cited – the 10.6 micron one – is produced by a collision with a nitrogen molecule with a rotational energy of about the same magnitude. De-excitation occurs exactly the same way.
I rather think that the absorbed energy by a photon exciting a CO2 molecule is more likely to be thermalized by collision than re-radiated, especially at lower altitudes.
Michael S. Kelly
November 20, 2017 at 8:50 pm
I don’t have a definitive answer, here, but do submit the following criticisms. The idea that IR photons just bounce around the atmosphere, being absorbed and re-emitted by CO2 molecules, producing no warming, is probably wrong. If absorption were followed instantaneously by emission, it would have a better chance of being true, but that isn’t the case.
For example, the radiative lifetime of a CO2 molecule for the 106 micron transition is on the order of 2 msec for CO2 in gases at a pressure of about 8 torr. That’s the only reason a CO2 laser can work, especially a Q-switched, high-power laser.
Michael, I find the CO2 laser talk contains an error, as CO2 lasers work with 10 um frequencies and not with 15um as specified in the text above:
Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um
Lars P. November 21, 2017 at 5:31 am
Michael, I find the CO2 laser talk contains an error, as CO2 lasers work with 10 um frequencies and not with 15um as specified in the text above:
The radiative lifetime of the 15μm excited state of the CO2 is the same order of magnitude as the 10μm state (msec) far longer than the mean time between collisions (less than nsec).
‘This is a contentious subject, and I have often shied away from it because it often erupts in food fights’
Thanks Anthony for having this discussion. It may be a bit messy, but only by repeatedly discussing this key issue will we be able to understand and either prove or disprove the GHG effect to the general satisfaction of the WUWT audience.
Thanks also to Willis in particular for engaging so deeply in this discussion despite his obvious frustration with so many people. I fear I am about to add myself to that group! I am a firm believer that if you can’t explain something to a willing listener, you don’t really understand it yourself. I am still doubtful about the GHG effect and will try to explain my doubts simply.
Some initial comments:
The Steel Greenhouse essay written by Willis explains how his definition of the greenhouse effect works, using black body objects radiating back and forth to each other. He assumes that there is perfect energy radiation and absorption and hence, any back radiation to the Earth’s surface will result in an increase in temperature. This model even works in a vacuum. He then says that this is how CO2 warms the Earth because back radiation from the CO2 will warm the Earth’s surface.
The implicit assumption seems to be that any back radiation must raise the temperature of the Earth above what it would otherwise be. This assumes that all emissions are additive with respect to temperature.
My first problem with this is that although all emissions are additive with regard to energy, I don’t believe that all energy emissions are additive with regard to temperature. For instance, sometimes electromagnetic (EM) emissions pass through material, sometimes they are reflected, sometimes they absorbed, sometimes they are immediately re-radiated, sometimes they are scattered etc. Many of these events do not change the temperature. An obvious example of this is that two bars of white hot metal placed together have double the energy but no change in temperature. From this, it is not certain to me that CO2 back radiation would for sure raise temperature in the real world. I need more proof than Willis’ black body model which by definition would increase temperature with any emissions.
My second problem relates to whether a cold body can raise the temperature of a warmer body. This is vital to understand because Willis himself stated in the thread that the approximate average temperature of the atmosphere is -50 C so how can that raise the temperature of the surface which is already at a higher temperature? Willis says that -50 C is warmer than the -270 C vacuum of space which means that the Earth will cool slower which is the same as being warmer.
I can think of two challenges to this logic:
a) Whether an atmosphere slows the cooling of the earth is not the point. The real question is does replacing a few -50 C molecules of oxygen with a few -50 C molecules of CO2 make any difference to the Earth’s temperature? The temperature differential between the surface and the atmosphere is the same either way so why would the rate of cooling of the Earth’s surface change at all? That is the real GHG effect that needs to be proven. In the 600+ comments in this thread, none of them addresses this.
b) Logically, I cannot see how CO2 at -50 C can warm the Earth’s surface which is already at +15 C. I do accept that cold objects emit EM radiation but do not accept that they can raise the temperature of an already warmer object. For instance, how many cubes of ice do I have to surround a one liter pan of water with to raise the water to boiling point? If someone can do the math on that and tell me the answer then I will be persuaded.
Those are my reasons for doubting the existence of the GHG effect.
“My second problem relates to whether a cold body can raise the temperature of a warmer body.”
Again what is so difficult about the concept of “slowed cooling” that cannot get through to some people and they continue to talk of “raise the temperature”.
Please look at it as energy in (from the Sun) vs energy out at TOA.
More is coming in (SW absorbed) than going out (LWIR emitted).
If there were no enhanced GHE then the two would balance.
The surface temp has to rise in order to equilibriate via the SB equation
Added anthro CO2 is “slowing the cooling” of Earth’s surface by back-radiating some of the LWIR to the surface where it “acts twice” to cause warming.
It isn’t a cold object warming a hot one at all.
The energy that is “causing” the warming is that that left from the warm body in the first place.
It is a reflection/insulation effect.
No warming in 39 years apart from El Nino and ocean events
CO2 has NO effect on oceans.
Warming by CO2 in a convectively control atmosphere has NEVER been measured.
If you say so.
Notice you have no other come-back.
EMPTY.
End of story, hey !!
No point as you will always have the last hand-waving point, with a bit of SHOUTING, that you seem to think is intelligent discourse.
As I said – if you say so.
Good to see you have ZERO !
Not one counter to the FACTS.
Poor little petal
How about you post some rather than ranting at me for not.
You’re the one hand-waving as usual.
I say again – If you say so – I believe you.
And the rest of the scientific community also.
Tone, slowing down cooling isn’t heating. Trapping heat is heating and that doesn’t happen. I also can’t see anywhere that a consistent radiation imbalance exists at the TOA
Martin:
“Tone, slowing down cooling isn’t heating. Trapping heat is heating and that doesn’t happen. I also can’t see anywhere that a consistent radiation imbalance exists at the TOA”
Of course slowing down cooling is heating.
While it is slowed down the Sun is still shining on half the globe is it not?
So there is incoming SW being absorbed during the “slowing”.
That is the imbalance shown by TOA observations and also by calculation of OHC being accrued – which is where most of the imbalance is being stored.
Tone, define “slowing down heating?”
For a blackbody receiving P input which is in a steady state with space at 3K
P=kA(T1^4-3^4)
For the same body in steady state with surroundings at 220K
P=kA(T2^4-220^4)
therefore T2^4=T1^4 + 220^4 – 3^4
Clearly as a result of this change the blackbody will be warmer.
Earth is NOT a black body.
Stop using non-applicable theoretical maths.
Deal with reality.
AndyG55 November 21, 2017 at 11:50 am
Earth is NOT a black body.
Stop using non-applicable theoretical maths.
Deal with reality.
Realty is that the earth emits within a few % of a true Blackbody in the IR, those equations are applicable to the earth. Perhaps you should stop your content-free ignorant posts?
“Tone, define “slowing down heating?””
Why?
That’s not what I said.
Personally I blame the astronomers for all this and yet it all started with such good intentions (The road to hell and all that). The simple question they wished to answer was how to devise a technique to calculate the average temperature of a planet given the following series of knowns:-
1. The energy intensity of the sun’s beam of light at a given distance from the star determined using the inverse square law of radiation.
2. The average orbital distance of a planet from the sun – this determines the average intensity of the sunlight that the planet intercepts.
3. The size of a planet’s disk – this determines the amount of sunlight that the planet collects.
4. Then there are the twin problems of daily planetary rotation and axial tilt.
So they made a perfectly valid simplification. They decided to ignore all the complicated spherical geometry of trying to determine how the surface of a spinning globe is lit and by what intensity and instead made the following correct assertions:-
1. Total energy in is equal to the total energy out.
2. That the cross-sectional area of the incoming radiant beam intercepted by a globe equates to the intercept area of a notional disk of equivalent radius BUT that the surface area of the outgoing emissions from that planet is spread over an area that is four times the area of the notional intercept disk. This is because the surface area of a sphere is 4πr^2; that is four times the area of a disk that has the same radius. (Clever but lazy because it has caused all sorts of problems).
So having divided the total intensity of the incoming beam by 4 they then applied the Stefan Boltzmann equation to this value to compute the average temperature of the planet and (horror of horrors) the answer they got when the calculation was applied to the Earth was obviously wrong. The SB equation gave a value that is 33C too low. Oh dear.
And then they did something really stupid, they invented the radiant greenhouse effect to heat the planet’s surface in a curve fitting extrapolation of an equation of state that is applicable to the stratosphere, but not to the troposphere where its application is invalid. After all they were astronomers used to dealing with radiation so what could be wrong with that? It seems that they forgot all about Maxwell’s ideal gas law and its application to pressures above 10kPa. They forgot about the gravitational binding to the surface of a planetary atmosphere and the consequent storage of energy that this gravitational binding represents. They did not consider the effect of insolation intensity and gravitationally determined escape velocity on selective loss to space of atmospheric volatiles with a low molecular weight. And don’t get me started on the effect on the latitudinal reach of the Hadley cell of the daily rotation rate of a planet (an angular momentum effect and not a temperature determined property of an atmosphere). All of these are easy things to forget after all.
And then we come to the piece de resistance, the crowning glory of this sick sad farce, the dangerous greenhouse gas carbon dioxide. Using their logic they claim that adding CO2 to the troposphere will raise the radiant emission level at the TOA and so dangerously warm the surface. The fact that the Tropopause is a pressure and not a temperature induced phenomena of change of atmospheric state is ignored. IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall below their radiative balance equation calculated from first principles (the distance of the Earth from the Sun) and as this cannot happen it necessarily follows that CO2 cannot warm the planet because the outgoing emission temperature can never be set at a lower value than the notional incoming collection temperature.
I was with you until “And then they did something really stupid, they invented the radiant greenhouse effect to heat the planet’s surface in a curve fitting extrapolation “
The greenhouse effect is not an “invention — it is a very basic application of the laws of thermodynamics. If radiation is blocked as it leaves a planet’s surface, the result is an increase in the surface temperature. Nothing in this theory contradicts the gas laws. Nothing denies gravitational potential energy. Nothing is invalidated by lighter gases escaping off to space.
I will grant that the super-simple models are not sufficient to calculate actual temperatures, but they are not designed to be. More intricate models would be needed for that.
“IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall below their radiative balance equation calculated from first principles (the distance of the Earth from the Sun) and as this cannot happen it necessarily follows that CO2 cannot warm the planet”
You seem to have this rather reversed! There is no “first principle” that says that radiation must be balanced. *IF* radiation is balanced, then the temperature will remain steady. However, if the radiation becomes imbalanced, then the temperature will change. . The correct conclusion is
IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall … causing net gain in thermal energy and an increase in temperature at the surface until balance was restored.
Quantum mechanics and absorption spectra aside – there is still no correlation between increasing CO2 conc and atmospheric warming. Temperature measurements over the past upteen years have shown that the latter is flatlining
Good observation.
Either:
With all the increasing GHG’s, theory demands warming. Yet you are correct, other than el ninos, flatline.
To be flat, there has to be a counter regulating force.
Or
There is no GHG effect.
And I found the counter regulating force.
In either case, there is no chance of catastrophic warming.
Philip Mulholland
November 21, 2017 at 3:51 am
says: Tropopause is a pressure and not a temperature induced phenomena
The Tropopause is a temperature phenomenon. Almost constant over the whole surface. Much more interesting than the surface temperature.
I don’t know what causes it, so am interested in suggestions like yours.
Pressure continues to fall after the tropopause height. That is while the temperature is not falling
I am puzzled that falling pressure can leave temperature to stay the same.
Roger,
Thank you for your comment.
Let me try this idea on you:-
The process by which the surface of a spherical, rotating, orbiting planet unevenly intercepts high grade solar energy from its parent sun and subsequently emits to space a uniform evenly distributed output of low grade thermal radiation forms the defining character of planetary climate.
The Stratosphere is an isothermal shell of low pressure gas that surrounds the Troposphere and provides a thermal datum that governs the operational parameters of the high pressure gases in the weather machine below. In a very real sense the Stratosphere is the exhaust system of the planet’s atmosphere. Just as the temperature of the exhaust gases from a heat engine cannot be used to power the engine itself, so it is also impossible for the exhaust heat content of the Stratosphere to power the weather machine by warming the planet’s surface below.
Philip,
Uh, you do know that the exhaust gas from an engine is exactly what powers a turbo charger, right? That is, with the appropriate mechanism (in this case a turbo charger), you can harness a bit more of that thermal energy escaping the engine. To continue your analogy, it’s not even necessary to introduce the turbo mechanism to affect the engine. Merely, changing the parameters of the exhaust system, like downpipe diameter for example, will change flow from the system which can affect the power generated. I think one could fairly make the argument that this is analogous to changing the ratios of the radiatively-relevant molecules in our atmosphere.
Thus, I find your explanation lacking due to the simple fact that coupled systems are…well…coupled. Changes to one part will necessarily affect others. The amplitude of the effect can be debated, as well as whether or not the effect is physically significant at standard operating parameters, but again, the effect is real.
rip
Philip Mulholland November 21, 2017 at 4:54 am
Just as the temperature of the exhaust gases from a heat engine cannot be used to power the engine itself,
Never heard of a turbocharger or a Turbojet I guess?
Yes, but they lose efficiency the closer the exhaust temp is to air temp. So usually turbo’s are at the exhaust where the gas is as hot as possible. When you account to pressure differences, that’s the temp differential?
Guys,
Lots of kick back here, but I will stand my ground. Every heat engine has an exhaust, even turbo charged ones. The genius of Robert Stevenson was to take the exhaust steam from the driving piston and use it to force ventilate the fire box in a feedback loop of increased power. But at some point the law of diminishing returns kicks in and the friction within your machine is greater than the added power you can glean from the exhaust.
The real idea I want criticism on is the observation that the isothermal shell of the stratosphere is in fact an exhaust thermal datum to the weather machine of the troposphere. It is the uniform planet wide distribution of this isothermal shell that is most interesting. My biggest problem has always been that this uniformity is in effect a friction-less system, so how can this form the starting point to a weather machine that clear moves mass? It makes much more sense to me to view the stratosphere as an exit system with waste heat and not an input system.
Philip Mulholland November 21, 2017 at 10:58 am
Philip, modeling the climate system as a heat engine is a very productive way to look at it. Unfortunately, this path is not followed a lot.
You might be interested in this paper, describing the application of the Constructal Law to the grand heat engine we call the climate …
w.
Thanks Willis,
I will study the Bejan & Reis paper.
Always more to learn.
Phillip says: “Stratosphere is the exhaust system of the planet’s atmosphere.”
Why would you say this? The ‘exhaust’ from the earth is the thermal IR that escapes to space. Some of the ‘exhaust’ comes directly from the surface through the ‘atmospheric window’. Some of the ‘exhaust’ comes from clouds; some comes from GHGs in the atmosphere. Very little comes from the stratosphere.
IMO, the poles and extratropics are, that where tropical air cools, and dehumidifier as it goes poleward.
“I don’t know what causes it, so am interested in suggestions like yours.”
Simply put, it is the inversion level where heating of O3 in the stratosphere meets and overcomes the -ve LR of the Trop – the level at which (mostly) convection can reach in the Troposphere. The level is a trade off between the O3 induced warming and the warmth of the Trop – it is highest in the tropics and lowest at the Poles.
https://www.atoptics.co.uk/highsky/htrop.htm
Yep, Convection and conduction RULE when the atmosphere is thick enough.
Thanks, Tone.. you are getting there.. finally !!
Roger,
I refer you to the figure in the following paper:
Robinson, T.D. & Catling, D.C. (2014) Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent intrared transparency. Nature Geoscience 7 (1), 12-15.
https://www.nature.com/articles/ngeo2020
Thanks for the link, Philip, most fascinating.
w.
Interesting. Thanks.
Venus is an exception. At its tropopause, temperature and pressure reach Earth-like levels, ie ~1.0 bar. Also, at its searing surface the winds are slow, but at the top of the troposphere, clouds pick up speed to 100 m/s, thus showing yet again colder is stormier, contrary to false CACA dogma regarding “extreme weather” from global warming.
The thin atmosphere of Mars is essentially stratospheric at the surface.
The science of climate change is obviously not settled.
But does it really matter when the average temperature
of our planet has remained in a narrow 1 degree C. range since 1880?
And that range could easily have been doubled by measurement errors.
The current climate is the best it has ever been for humans,
and has improved for green plants since the 1800s.
What climate change problem?
The understanding of climate change (“settled science”)
does not matter, because there is no problem that needs to be solved.
My climate blog for non-scientists:
http://www.elOnionBloggle.Blogspot.com
Richard Greene November 21, 2017 at 6:50 am
The science of climate change is obviously not settled.
The science points raised in the head post are settled, unfortunately many of them are wrongly stated or misunderstood as I, Willis and others have pointed out. Just because people unfamiliar with the science such as the author (as he admitted) get it wrong doesn’t mean the science isn’t settled.
E.g.
Phil. November 19, 2017 at 2:58 pm
The worst of these errors is the misrepresentation of Wien’s Law which has also appeared in numerous other places, including by a fictional astronomer on Steve site.
The mis-statement is that because Wien’s Law says the temperature at which the maximum emission occurs at a wavelength of 15microns is -80ºC then “Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
CO2 does indeed have a maximum in the radiance-wavelength curve at 15 microns for a temperature of -80ºC, however for a higher temperature the emissions at 15microns are always higher.
At 15 microns and 193K radiance is 1.09 W/m2/sr/µm, at 203K it’s 1.41 W/m2/sr/µm, at 213K it’s 1.80 W/m2/sr/µm, and at 223K it’s 2.26 W/m2/sr/µm.
Just my uninformed opinion on back radiation, but it only becomes relevant in the absence of an external heat source. My observational evidence:
1) increased GHG does not increase day time highs. This says that the normal heating process of the atmosphere completely overwhelms any contribution from back radiation. During the day, the GHG compartment absorbs all the energy it can, which is a finite quantity. To some extent, the GHG compartment seems to prevent overheating, as it seems places of high WV can’t seem to reach the high temperatures achieved in desert environments.
2) most of the science-ee stuff I’ve seen show graphs where it is the night time lows that are increasing. IMO, this is because as the temperature drops, the GHG heat sink begins to release its energy to its surrounding, slowing the rate of cooling. This is supported by the comments of others, deserts with no WV cool rapidly, coastal areas like New Orleans don’t.
3) local temps are more influenced by air movement than than local radiation. When a cool front comes in, it gets cold, when a warm front comes in, it gets warmer ….. doesn’t seem to matter much what the back radiation is doing.
🙂
“During the day, the GHG compartment absorbs all the energy it can,”
And releases it as well, just as it does at night. It both absorbs and emits all the time on short time frames.
Most of the heat energy of the atmosphere is in molecular motion not stored protons, that energy has been transferred to molecular motion.
I just noticed this claim in the head post. There are two huge problems with it. Here’s what it says:
I’m sorry, but this is not true. A gas at 50°C has more kinetic energy than a gas at 20°C, without any electrons moving to more energetic orbits.
The word “kinetic” in “kinetic energy” is from the Greek, meaning movement or motion. Similarly, “kinetic” energy is specifically energy due to MOTION. It is not energy due to different energy levels of electrons, the name itself says that.
Second, the absorption of thermal longwave radiation does NOT result in an electron being kicked to a higher orbit. That is the photoelectric effect, but there’s not enough energy in thermal LR to move an electron.
Instead, when a molecule of a gas absorbs longwave radiation, it starts the molecule vibrating. Depending on the complexity of the molecule, this vibration can include a variety of vibrational modes including stretching, asymmetric stretching, scissoring, rocking, wagging, and twisting.
The more modes of vibration a molecule has, the more different frequencies of longwave radiation it can absorb (or radiate). Obviously, a simple two-atom molecule like N2 or O2 can only stretch, it can’t twist or flex … which is why they can only absorb or radiate in a narrow frequency band. Molecules with three atoms like CO2 and H2O have many more vibrational modes, so they absorb over a wide range. Solids, of course, made of millions of atoms or molecules, have a host of frequencies at which they absorb and radiate. And monatomic gases like argon have no vibrational modes at all, so they can neither absorb nor radiate longwave thermal radiation.
However, within microseconds, this vibrational energy is transferred to thousands and thousands of neighboring gas molecules. This converts the vibration into motion of the surrounding molecules. The net effect is to heat the body of the gas, even when the amount of GHG is quite small.
Man … the incorrect statements in the head post just keep on coming …
w.
Quite true, it’s the main reasoning for the broadening of the absorption bands, and I believe this is the main mechanism for “GHG” warming after the concentration of such molecules reaches the point of absorbing the available LWIR. However, the energy of the vibration of these normal modes is between 0.0001-1.7 eV and the energy lost or gained upon molecular collisions is a fraction of this energy. And then we must ask, does the donation of molecular vibrational energy to kinetic energy increase convection?
@corev
The time scale I’m referring to is the mean transition time for energy to be transferred from the lower atmosphere to space. This is the upward component of the omnidirectional collision induced radiation. The timescale is a few hours. Compared with typical molecular timescales this is an eternity, but the intuitive comparison I make is that taking a square meter column of atmosphere (10T) and an upward flux of 200W, it’s like a 200W lightbulb heating the air in a gym for a few hours. A small effect of around 0.14C, cf the 33C GHE assumed by the IPCC science.
Yes, the average lifetime of an excited RG molecule is small – usually de-excited in the next molecular collision it experiences. A very small portion get to emit a photon, but since there are a lot of molecules the radiation levels are significant.
True, most of the energy in the atmosphere (ignoring mass energy) is KE of molecules, but conduction is slow. Convection and evaporation/condensation cycle are major players.
The essence of the GHE is not back radiation, but how much of the surface emitted energy remains in the lower atmosphere to contribute to it, and how rapidly it is transported upward radiatively rather than being ‘trapped’.
This is a perspective and paradigm shift.
dai
@tjfolkerts
“The correct conclusion is IF the thermal emission layer is raised in a gravitational field then the thermal emission temperature would fall … causing net gain in thermal energy and an increase in temperature at the surface until balance was restored.”
No. Again consensus CS is ignoring the time factor. Even though rates of emission in the upper troposphere will drop if the tropopause rises, global atmospheric circulation continues poleward, radiating as it goes, until the upper temps drop and density increases until upper air drops giving Hadley cells.
dai
Yes indeed, dai. This is all almost as good as Saturday’s Wales-All Blacks match should be.
About 1902, Prof Robert Woods refuted the existence of an atmospheric greenhouse, and soon all those other great experimemtalists agreed. Arrhenius realised this, and went on to do other good works. It is a mere latter-day contrivance of hippy science, magic mushroom stuff. Nasa’s solar system data shows the truth. It is all the gravito-thermal effect. Convectively enabled and phase change accelerated to easily cope with imbalances of input and distribution. Water is the real magic stuff…..
Radiation is an effect of KE, not a prime cause. Except of seemingly endless misconceptions. In furnace engineering, CO2 speeds heat movement to furnace walls etc.. Above 200ppm, no significant increase. That is all, no blanket to suck on, sorry Linus.
Brett Keane November 21, 2017 at 6:49 pm
The Wood experiment was fatally flawed for reasons I explained in detail in “The R. W. Wood Experiment“.
Now, let’s do this scientifically. I am not interested in your opinion of my analysis of the poorly-designed Wood experiment. I am interested in your scientific objections to my analysis. However, please QUOTE THE EXACT WORDS THAT YOU OBJECT TO in my analysis, so we can all understand just what you think is wrong.
Or you could either not read my analysis, or read it and know it is right and blow it off … your choice. However, note if you do not raise any scientific objections to my analysis, I will take that as evidence.
w.
Willis Eschenbach
November 21, 2017 at 8:33 pm: Willis, this is not your post. Of course I read yours, referenced. It did not apply to the Optical Physicist’s propositition. Ask Konrad Hartmann. So I see no reason to debate it here,except to note that lids are out of place in a real atmosphere, as are surfaces also..
Actually studying Fourier, Tyndall and Maxwell. for instance, shows where Wood was coming from. Not what flower children claim they said, which is something else.
I gave plenty of data sources in my entries above. Argue them if you wish. The header Post requests such for its own propositions. This is the basis of all real scientific method. A thing of beauty….
Brett Keane November 22, 2017 at 12:41 am Edit
Hey, Brett, if you can’t find anything wrong with my post on the R. W. Wood experiment, that’s fine. Trying to blow it off by claiming it “did not apply to the Optical Physicist’s proposition”, whatever that might be, is just handwaving to distract us from the fact that apparently you can’t find anything wrong with my post.
You made a claim about the R. W. Wood experiment. I gave you a detailed analysis and asked what is wrong with the analysis. In response, you’ve just headed for the door …
w.
Willis Eschenbach November 22, 2017 at 11:24 am:
Willis, Wood was a brilliant experimentalist. So is Hartmann, so is Berthold Klein of the Mylar balloon expt. Such are not refuted by a thought expt. Nor are the Nasa solar system data and the many papers based on what it teaches us. Which is that atmospheric gases handle energy inpiut according to the combined gas laws under the Poissin relation, as Maxwell noted in his “Theory of Heat”. By vigorous convection until radiation can finally leave ie less than one optical depth. Window EMF alone takes that door, not I.
Brett Keane November 22, 2017 at 12:40 pm
Thanks, Brett. Yes, I know that Wood was an experimentalist, although given his experiment I would not class him as “brilliant” … and more to the point, l+
et me remind you of a quote by the amazing RIchard Feynman, a true scientific genius. He said:
If we took previous claims and experiments as gospel, science would never advance again …
Second, I note that once again you have not brought up a single argument against a single one of my claims. Instead, you are indulging yourself in argumentum ad verecundiam, which was recognized as a logical error a couple of millennia ago.
Look, I made a bunch of very clear claims in that post. If you think one of them is wrong, then quote it and tell us why. Because simply mumbling a bunch of scientists’ names as though they were a magical incantation doesn’t work here at WUWT.
Regards,
w.
Willis, with all due respect it’s absolutely up to you to prove that the steel greenhouse theory is correct. If the work done by Wood was in isolation then I’d agree but it seems that the same conclusion is arrived by many others since. There are some things that are worth taking the effort doing but arguing about the steel greenhouse on here definitely isn’t one of them because of the fixed positions that all parties exhibit and the fact that when it comes down to it we are like minded on the issues. With all due respect Willis (and that is sincere), you have a very good brain but your positions on all issues are set in concrete.
Martin Mason November 22, 2017 at 1:12 am
Martin, with all due respect, you absolutely don’t understand how science works. There is no way in science to “prove” that something is correct, nor do scientist try to do so. Science works like this:
Someone puts up a scientific claim, complete with all of the data, math, observations, and the like that support the claim.
Then, other scientists try to poke holes in the claim, showing that the math is wrong or the logic doesn’t work or in any other manner.
If other scientists cannot poke holes in the claim, then it is accepted as scientifically valid … until such time as it is overthrown.
Note that this process is not about proof. It is about falsification.
Next, you claim that my positions are set in concrete. That’s madness. When I’m shown to be wrong I’m the first to admit it, although like anyone I don’t like it. I’m one of the few bloggers with posts titled things like “Wrong Again” and “Wrong Again, Again“. People had shown that I was wrong, and I not only admitted it, I wrote a whole post about it. And then a few years later it happened again, and I did the same.
So I totally reject your claim. As the man said, if the facts change, I change my opinion …
Anyhow, here is your opportunity to show everyone just how wrong I am and how right you are. Point out to us just which of my claims about the steel greenhouse is incorrect in any way. If you are right, I’ll be the first to say so.
Or you can just give us some flimsy excuse about why you don’t want to do that. Be forewarned, however, that at this point, and after the claims and untrue accusations you’ve made, and after your implication that you already know of some faults in my steel greenhouse analysis, at this point if you do not tell us just exactly what is wrong with my steel greenhouse claims, I’m going to assume that it’s because you can’t find one single thing wrong with it …
On the other hand, if you do want to dispute something I wrote in that post, please quote the exact words of my claim that you are disputing so we can all understand exactly where you think the error is.
Time to bet or fold, Martin …
Regards to you and yours,
w.
OK, well the Steel Greenhouse has been comprehensively demolished here, and in numerous other posts, so that’s that.
https://climateofsophistry.com/2017/10/19/the-steel-greenhouse-in-an-ambient-temperature-environment/
Willis, I don’t mean prove in a legal basis only by repeatable and observational basis such as gravity can be. Should I say validated beyond reasonable doubt? Heating by cold downwelling radiation hasn’t passed that test yet, does it have a falsification test?
The tone of your response is the reason why I don’t want to get into a discussion. I used the wrong description perhaps in saying that your positions are set in concrete but you don’t communicate well with those who may have a different position. 🙂
I may
SB is well tested, it has not been falsified.
Heat never flows from cold to warm, but energy sure does. And SB describes energy flow between objects both hot and cold.
You can easily prove all solid objects radiate, whether hot or cold with an ir thermometer.
Martin, your position is not supported by over 100 years of evidence.
Frankly, “destruction” at Climate of Sophistry is a vote in favor of Willis’ theory.
PS, the error in the page you linked to starts with “The shell’s surface would emit on its interior as well, however, internal emission by the shell will always meet another interior side of the shell (or the sphere), and hence will not leave the shell.”
Up until this point, the the shell and the sphere were treated as two separate “systems” that can exchange energy. Suddenly the shell is not allowed to transfer energy to the sphere.
This error gets manifest in equation 3b. When the sphere is inside the shell, then the “universal ambient-temperature environment ” for the sphere is no longer T_0, but now is T_sh. With these corrections in understanding and math, the correct answer (Willis’ answer) pops up. Any physics or engineering professor will agree with Willis.
No, it’s demolished. And all your concerns, or even anything else you might be paid to invent, are all well and truly addressed. Done and dusted, dusted and done. That’s that!
Tony November 22, 2017 at 3:39 am
If you believe that analysis, I have a bridge I’d be happy to sell you.
w.
Nice to see you conceding the point, finally. You couldn’t carry on lying forever.
Martin Mason November 22, 2017 at 4:22 am
You still don’t understand. Science is not a process of “validation”, it is a process of falsification.
As to whether “heating by cold downwelling radiation” exists, the two-way model of radiation exchange is so well accepted that it is in all the thermo texts I’ve seen. There’s a good online calculator here that contains the actual equation used to calculate the effect. You might profitably spend some time studying that equation.
Sure, you could falsify it, all it would take is some thermometers and some radiation measuring instruments and you could falsify it in one day. Or you could just walk outside, measure the downwelling radiation, and consider the following question:
Since energy is neither created nor destroyed, only converted to a different form, if that downwelling radiation is not leaving the object it strikes warmer than it would be without the radiation … then what is happening to the absorbed radiative energy? What is it converted to if not thermal energy? It’s not converted to light, or chemical action, or motion …
Oh, I see. Your excuse for not showing us the errors that you claim to have found in my work is that you don’t like my “tone” … let me quote what I said before:
My “tone”? That’s hilarious. Come back when you care enough about science to get your hands dirty. Science is a blood sport, where people do their level best to destroy some other person’s treasured and precious ideas and claims. It’s not some appeal to your feelz. Nobody likes it when their scientific sacred ox is gored, whether it’s done gracefully as a ballet or not …
I deal with folks whose tone I don’t like all day long regarding scientific questions and answers, including your tone in this very discussion. Get over it. Either you can find errors in my work or not, and so far all I’ve seen is handwaving.
Like I said, it’s now time to either bet or fold …
w.
Tony November 22, 2017 at 7:15 am
In regards to tjfolkerts comment …
Oh, Tony, you are practicing my favorite kind of science … science by vehement assertion including exclamation marks and meaningless repetition of blanket statements. That’ll convince ’em!
Read tj’s objections again. They are clear and to the point. If you disagree with them, quote the one(s) you disagree with and tell us why they are wrong.
w.
Tony, have you ever critically read that post? Let’s start with:
“If the sphere does produce any power then its temperature will rise above T0 [the ambient temperature of the environment surrounding the sphere], and its energy production would then be given by
1b) Psp = 4π Rsp^2 σ(Tsp^4 – To^4)”
So for example, if the radius of the sphere were 0.282 m (ie an area of 1 m^2), then the power from the sphere to the surroundings would be
* 240 W/m^2 if Tsp = 255.1 K and To = 0
* 480 W/m^2 if Tsp = 303.3 K and To = 0
* 240 W/m^2 if Tsp = 255.1 K and To = 0
This is the standard physics of radiation transfer (assuming blackbody surfaces, of course). Do you accept this?
Silly little Willy. Read Postma’s articles, which refute the Steel Greenhouse conjecture, and stop practicing science by vehement assertion. It’s OK that you’ve lost.
Oops, that should have been:
* 240 W/m^2 if Tsp = 255.1 K and To = 0
* 480 W/m^2 if Tsp = 303.3 K and To = 0
* 240 W/m^2 if Tsp = 303.3 K and To = 255.1
Tim starts his process…all too familiar. Sorry Tim, it’s done. You’ll have to find another means of employment. You’ll be OK. Politics awaits…off you pop.
“Tim starts his process … ” — of using basic, established equations from physics to figure out how the universe shold work.
Meanwhile, Tony starts his process of avoiding equations and tough questions – instead using the very tactic he accuses others of: “practicing science by vehement assertion.”
Who’s tactic? Lol. Have a catch up and get back to me. You seem stressed…not like you to make so many mistakes. Have a couple of days holiday, you’ve earned them. They must have been paying you overtime these last few weeks. So many articles recently you have to comment on! Poor you.
We all get it Tony. You fear to respond meaningfully to basic questions about science so you deflect.
It is really very simple — assuming you are willing to make a good faith effort to defend your positions. Once again, if a sphere of radius 0.282 m (1m^2 surface area) is surrounded by an environment at an ambient temperature To, are the following accurate for the power from the sphere. (Again, remember — the equation came from your own link). Let me add one more for fun:
* 240 W if Tsp = 255.1 K and To = 0 K
* 480 W if Tsp = 303.3 K and To = 0 K
* 240 W if Tsp = 303.3 K and To = 255.1 K
* 240 W if Tsp = 282.3 K and To = 214.5 K
This is merely to establish that we can both do the calculations and can both agree that the SB law is valid here.
Ha ha, don’t be silly Tim. You don’t control the conversation, though I know you wished you did. But wishing something doesn’t make it so. Unfortunately, Postma refuted the Steel Greenhouse conjecture. You’ll just have to come up with something else to try to dupe your betters.
I am not “controlling the conversation — i am exactly following the conversion in your own link! And yet even agreeing with you is somehow too dangerous for you to engage with!
I was just linking to the refutation to let people know that was all finished with. Sorry if you thought there was anything left to discuss.
“I was just linking to the refutation” … and I was just pointing out the glaring errors in that rather feeble refutation. Sorry if you thought that some second-rate blog is actually the last word in science.
I’m sorry that your religion is falsified. Still, humanity has taken a step forward, despite you, so it’s not all bad.
Tony, you talk about “my religion” and yet all I have done is try to discuss equations THAT COME FROM YOUR OWN LINK! That fact that you are not willing to engage in scientific discussions indicated clearly who is basing their position on faith.
I have absolutely no idea how you are going to pretend this up for discussion, but I understand that due to your profession, you are unable to refrain from responding. Amusing for me, though. Don’t let it get you down. You’ll find something else.
Micro, writes,
“SB is well tested, it has not been falsified.
Heat never flows from cold to warm, but energy sure does. And SB describes energy flow between objects both hot and cold.
You can easily prove all solid objects radiate, whether hot or cold with an ir thermometer.”
A very useful difference you brought up,that too many trip over.
“Heat never flows from cold to warm, but energy sure does”
Tony:
When I first studied thermodynamics (at MIT), the professor insisted we spend the first several weeks rigorously defining systems and subsystems, and carefully tallying the energy flows in and out of each subsystem, plus the system as a whole, in doing energy balance calculations.
I grew impatient with this, as I was eager to get on to fun subjects like turbochargers and cogeneration, but I am very glad he did this, because it has kept me from making a fool of myself like the author of your linked post does.
Tim spotted a few of his key mistakes, such as when considering the shell as a subsystem, not counting emissions from one side of the shell as energy output from the subsystem. This is the type of mistake a weak student makes in the first weeks of an introductory undergraduate course. But anyone who wants to pass the course needs to stop making that kind of mistake.
It is one thing for an 18-year-old student to make that mistake on an early problem set. It is another thing completely for someone claiming professional expertise to make so basic an error, and still not be able to see it after it has been pointed out to him multiple times.
It’ll be OK, Ed.
Tony:
I note with amusement that you have not even attempted any kind of substantive argument.
I don’t think you’re capable of it.
You have trouble reading? Or understanding?
Still not a single actual substantive argument.
Yep, you have no capabilities whatsoever. You don’t even do snarky trolling well.
Ed Bo November 22, 2017 at 2:51 pm
Ed, where is your substantive argument? I’ve invited people to quote the exact words I wrote that they think is wrong. That’s the start of a substantive argument, when you clearly define what it is that you object to. Then you say just exactly what you think is wrong with those specific words, why they are not true.
So … you are welcome to quote whatever it was that I said in the Steel Greenhouse that you think is wrong, and tell us why you think it is wrong. Please don’t bother pointing to the arguments of others, I am interested in YOUR OPINION, not that of some random blogger who is not here to defend his claims.
Or not, you could do what the rest have done and come up with some feeble excuse why you don’t want to put your money where your mouth is … up to you.
w.
That’s right. I haven’t made an argument and have no intention to. As you should have read and understood since it was made pretty clear in earlier comments. There is nothing Tim said which isn’t answered in the articles at CoS or the comments in the articles, and you’ve added nothing to what Tim said.
You guys are losing your cool, I get it, your religion is collapsing in on itself all around you. It must be scary. I try to sympathise, amidst the chuckles. Don’t worry though, your skills are transferable.
Yeah, Ed! Put up or shut up! Lol. Poor old Willy.
Willis,
Now you say you can’t “prove” anything, but previously you claimed that you and Dr. Brown had shown “proof” of the correctness of your position on this question.
Which is it? Proof or no proof?
Willis:
I am not disagreeing with anything YOU have said.Tony pointed to the ridiculous post at CoS that tried to refute your “steel greenhouse” analysis.
I specifically pointed out a key error made at CoS (which Tim also pointed out even before me) in analyzing the “shell” subsystem — namely the mistake that radiation from the inside of the shell does not “count” as an energy output from that shell.
Tony, on the other hand, has not made a single substantive point in all of this posts.
Poor Ed. Still unable to read, or understand. Or both. He’s almost as confused as Willy.
WIllis,
It is YOU who does not understand how science operates. The steps of the scientific method are generally as follows:
1. Define a question
2. Gather information and resources (observe)
3. Form an explanatory hypothesis
4. Test the hypothesis by performing an experiment and collecting data in a reproducible manner
5. Analyze the data
6. Interpret the data and draw conclusions that serve as a starting point for new hypothesis
7. Publish results
8. Retest (frequently done by other scientists)
You are at step 3. You have not performed any real science so far, so there is nothing to rebut, since all you have is a silly steel greenhouse thought experiment.
Anthony, thanks for hosting Rod Gill’s essay, the nearly 800 posts (with no screaming, SHOUTING or name calling) discussing this fundamental but complex subject, are testament to the interest & wealth of knowledge on this site.
May I suggest that you add to the Atmospheric page another sub-heading of ‘GHG/Radiative Gases’ where this thread & related info can be easily found (particularly by someone new to the site or unaware of the obtuse subject names & refs they need to look for).
Is it legitimate to apply the Stefan-Boltzman law to the Earth’s surface – what happens, for instance, in the Trenberth diagram – when this surface is covered by an (active) atmosphere some kilometres thick ?
Shouldn’t it be more accurate to displace the relevant surface a few kilometres out of the Earth’s surface ? If so, what distance should be appropriate ?
From first principles I offer my hypothesis of the atmosphere: I am currently writing up my discoveries.
1) The below diagram shows the complete (augmented) IR atmosphere; thermoelectric and Raman, with all the quantum mechanics (QM) predicted vibration modes.
2) The said ‘special’ GHGs (CO2 etc) are really only the thermo-electric gases (TEGs): first discovered by John Tyndall in 1859 using thermo-electric thermopile transducers. Here’s where the misconception begun – he interpreted them to be absorbs when they were really transducing an electromotive force (volts). N2 and O2 will not generate electricity at any temperature.
3) QM predicts N2 and O2 at 1556 and 2338 respectively; these Raman (non ‘electric’ dipole) modes can only be observed with modern (late 20th Century) laser dependent Raman spectrometers. Raman also detects CO2’s and CH4’s non TE (IR) modes.
Raman and Thermo-electric (IR) can both determine temperature of molecules and thus are not only complementary, but also equivalent: the temperature of O2 N2 H2O CO2 .. can all be determined from Raman alone as can the concentrations of the gases. The Keeling curve can be determined by Raman Spectroscopy
4) From the above: Raman spectroscopy is an instrument of choice on solar system space probes. It is also used in monitoring the Earths atmosphere – by means of Raman Lidar.
5) From the above it is implied O2 and N2 radiate IR: they emit and absorb as by the Boltzmann constant. A molecule of N2 in the thermosphere is 2500K – excited only from radiation. This temperature is measured from Raman Lidar.
6) In a CO2 laser, N2’s 2338cm mode is radiated by electron collisions (equivalent to photons) to ‘pump’ the CO2s 2349cm mode. If this did not happen, the CO2 laser would not operate and no cosmetic surgery.
Conclusion
N2 and O2 are GHGs – the whole atmosphere consists of GHGs, no special ones. It is the instruments we measure them with that makes them special.
Note: Raman spectroscopy is an instrument to detect vibrational spectra and should not be confused with the Raman effect – this is not my claim.
Yes N2 and O2 have Raman active vibrations (which are not IR active) and so can be detected if excited by a focussed laser beam, what possible relevance does this have to the atmosphere?
Everything: it ends the climate debate.
There are two sides to my theory: Tyndall discovered thermo-electric gases (what we know as the GHGs); and Raman shows what and why he didn’t measure, N2 and O2. Raman is a very good thermometer.
In the 21st Century, to understand IR behaviour of matter we use two instruments: Raman and thermoelectric-IR (thermoelectric, my words cause that is what they are). We do the same in the atmosphere, Raman is used already there, even NASA uses it; I am bringing them together, to solve the problem.
The standard model of GH theory can only hold with O2 and N2 are non-GHGs; that they are benign, that they are ‘forced’ by collision from the GHGs. With Raman spectroscopy we see they are IR radiation active – if a the molecule is radiated, the temperature will rise in accordance with the Stefan Boltzmann equation, Raman can measure that temperature of N2 and O2. In fact, it may even that N2 excites/ heats CO2 directly through its 2338/2349cm-1 close modes (my claim). Figure that?
How could we think anything else; the air is a near perfect insulator, it has a thermal conduction value of near 0 (0.024 – no units); air has to radiate on those grounds alone; not to mention that if it didn’t, it would contradict QM and thermal dynamics where all matter above absolute 0K radiates.
I am not creating anything new; only putting what we know together.
Lets do experiments and test. Actually, I have found the experiments; it holds.
Blair Macdonald November 22, 2017 at 12:39 pm
Everything: it ends the climate debate.
In your dreams, Raman spectroscopy is a technique for detecting certain species, only a very small proportion of the population undergo the transitions and usually a strongly focussed light source is needed and sophisticated filtering techniques to separate the dominant elastically scattered light. It’s certainly not an effect which is capable of transferring significant amounts of energy in the atmosphere.
The standard model of GH theory can only hold with O2 and N2 are non-GHGs; that they are benign, that they are ‘forced’ by collision from the GHGs. With Raman spectroscopy we see they are IR radiation active
No we do not we see that the vibrational modes of N2 and O2 are not IR active but are Raman active (something I was taught as an undergrad many years ago, so it’s nothing new). Which is exactly what the ‘standard theory’ accounts for, N2 and O2 can’t participate in absorbing the Ir emitted by the earth.
How about Argon?
Argon is monatomic, so it doesn’t absorb or emit longwave IR.
Argon is interesting; it does not transduce electricity from its IR radiation and it does not seem to have a clear Raman active mode or spectra – it must have. I’ll keep hunting that one.
Blair Macdonald November 23, 2017 at 4:48 am
Argon is interesting; it does not transduce electricity from its IR radiation and it does not seem to have a clear Raman active mode or spectra – it must have. I’ll keep hunting that one.
Yet more misunderstanding by you of Raman spectroscopy, Argon doesn’t have vibrational energy levels, neither IR active nor Raman active so don’t waste your time hunting.
Very interesting Blair but what excites the non GHG molecules. What does it mean in real life?
“..what excites the non GHG molecules” They, N2 and O2( and H2 I believe?) are excited by the Sun, by photons, just as we understand QM radiation, nothing new there. It is just to say N2 and O2 have spectra lines predicted, and they are observed (with Raman) and they behave just as they should when excited; all in accordance, I would add to say, as the Stefan Boltzmann law.
What does it mean for real life? It updates the 150 year old, pre quantum understanding of the atmosphere. GH theory is updated, there are no special GHGs, but only GHGs – after that the 0th and 1st laws follow.
We cannot have the out standing pillar of science, QM, in contradiction over the non GHGs: they must radiate – and they do. Everything with temperature, and spectra lines, radiates – all and in compliance with Boltzmann’s constant. Else we have a catastrophe – what I term ‘the IR catastrophe’.
Yes Blair, the IR Catastrophe is the fox in the henhouse for all warmista.
Blair, dividing gases into “GHGs” and “non-GHGs” is not intended to imply that non-GHGs don’t absorb at all. It is simply intended to sort into two broad categories — those that absorb well and those that absorb poorly. (much like materials are often divided into “conductors” and “insulators”, even though good conductors have some resistance, and good insulators have some conductance.)
The point is that a GHG like CO2 absorbs orders of magnitude more IR than a non-GHG like N2. Unless you are trying to predict IR intensities to within a few ppm, you can ignore N2 completely when dealing with atmospheric IR.
tjfolkerts No, N2 and O2 do not by GH theory emit or radiate any IR and not ‘poorly’, at any temperature – check the definitions. This is a catastrophe: all matter radiates.
You are relying on thermoelectrics as you paradigm of thinking: if we used Raman spectroscopy alone (as shown in the diagram) we would get a different set of gases; but they would be equivalent to the ‘GHG’s but for N2 and O2 and some others. The Raman gases all have (by early 20th Century quantum experiments – namely the Franck Hertz experiment) spectra lines, they all vibrate, and they have temperature.
As a thought experiment: take all the said GHGs out of the atmosphere leaving only N2 O2; would the air temperature change during the day change? Yes – just as it does today, and it would by radiation, and not conduction as N2 and O2 (the air) are near perfect thermal insulators.
Another thing, by current greenhouse reasoning; glass is a greenhouse solid: it is transparent to the eye, and ‘opaque’ to the IR – just like CO2. Why is there no issue with glass?
Water is – for the same reasons – a greenhouse liquid.
Blair Macdonald November 23, 2017 at 5:16 am
We don’t have to “check the definitions” to know this is 100% wrong. The absorption bands for 02 are shown in the head post graphic … read’m and weep …
While this is widely believed, it is not completely true. Monatomic gases (helium, neon, argon, etc.) neither absorb nor emit thermal radiation.
The only “catastrophe” is that you don’t seem to understand what you are talking about … or more accurately, the “catastrophe” is that you are firmly convinced you understand what you are talking about …
While I appreciate the passion with which you hold your views, that doesn’t make them correct …
w.
“No, N2 and O2 do not by GH theory emit or radiate any IR”
This is not any key tenet of GH theory. All that is required is that
1) sunlight can get in easily and
2) “earthlight” cannot get out easily.
It turns out that for (2) (the blocking of IR)
* H20 is the most important gas
* CO2 is the next most important gas
* polyatomic molecules like CH4, O3, and NO2 are also fairly important.
* monatomic and diatomic molecules like Ar, N2 and O2 contribute almost nothing.
GH theory would still work if N2 or Ar absorbed significant amounts of IR — but experimentally they don’t. GH theory still works when N2 absorbs some IR. The key factor here is that even small changes in CO2 (from 300 ppm to 400 ppm) STILL has a MUCH greater effect than the entire 780,000 ppm of N2. So N2 can be pretty much ignored when doing calculations. (Or — you could include N2 and get an answer that differs by an imperceptible amount).
“You are relying on thermoelectrics as you paradigm of thinking “
By this, it seems you are referring to gases that cause a noticeable change in temperature when IR passes through (ie thermoelectric detectors that create voltages in response to changes in temperature show a signal). In this case, this is EXACTLY the sort of change we are interested in. If N2 happens to show some weak absorption using something like Raman spectroscopy but DOESN’T register when you are measuring actual temperature change due to actual IR, then — almost by definition — N2 is not contributing (in a measurable way) to the GH effect.
tjfolkerts ‘..dividing gases into “GHGs” and “non-GHGs” is not intended to imply that non-GHGs don’t absorb at all.’
That is not how it is read or interpreted, check any definition of a GHG or n – GHG and it reads clear, ‘N2 and O2 do not absorb or emit IR’.
The rest of your comment is all based on readings from thermoelectric transducers: then you have go back and again break it down into ‘first principles’; how does the device work that you have come to your conclusion with – it is all by thermoelectrics. You are just repeating the standard model, and it just does not stack up.
Watch my basic/nervous presentation I did a few years ago here: https://www.youtube.com/watch?v=T0IHKKkOwdU&t=860s
We must lose the IR from IR spectroscopy: and term it TE spectroscopy and the TE gases – ThermoElectric spectroscopy and gases.
Here’s a question: what if it was only Raman devices we had to measure the IR atmosphere, and not thermo electric (just as we do for Mars): what would the known GHGs be? They would be every gas (if I haven’t missed any non Raman gases), as all gases are Raman active (I’m pretty sure). They would be Raman active and equivalent to thermoelectric ‘IR’ through the equipartition principle.
What I have here is just the ‘tip of the iceberg’, and the truth shocking: what I have found is all radiation theory (black body, emissivity and the like) done by Kirchhoff, Stefan, Boltzmann and others is all based on 19th Century thermoelectric detectors/transducers – totally neglecting QM and laser Raman observations and measurements. It will be my life’s work – with help hopefully – to clear this up.
Blair, statements in science like “N2 and O2 do not absorb or emit IR” often mean “do not absorb or emit in sufficient amount to be significant”. So I might “Pyrex is an insulator and does not conduct electricity” — and most people would go along with that. But I can look up the resistivity (http://glassfab.com/wp-content/uploads/2015/08/Corning-Pyrex.pdf) and find that it is about 10^8 Ohm*cm. This makes it an extremely good insulator, but it does (at some infinitesimal level) conduct.
” check any definition of a GHG “
Well, wikipedia (the first hit when I searched) disagrees with you.
“Hence they [non GHGs] are almost totally unaffected by infrared radiation. — Wikipedia
If this is your entire thesis (‘non-GHGs do actually absorb some small amount of IR’) then you are barking up the wrong tree.
I need help: anyone reading my claims and and are interested and would like to support me, financially, or otherwise, I need it. I am doing this on my own, I have a paper nearing completion, and it now needs the best minds – I’ve done my best. The pay-off: the greatest upset in scientific history. Message me through my blog and otherwise.
Also, Anthony: this is the area of movement, of thinking, 870 in three days! This is first principles, and this is where the debate is, and where it will be settled, and where GH theory as we know it will collapse. Let it be aired: better out than in.
You may find this paper to be of interest:
http://www.academicjournals.org/journal/IJPS/article-full-text-pdf/E00ABBF60017
All the best.
Thank you.
B
Link doesn’t work for me Tony
OK, try this link: https://orcid.org/0000-0002-3340-3063
Then scroll down to the “Works” section and click on the URL under “The thermal behaviour of gases under the influence of infrared-radiation”.
Blair Macdonald November 23, 2017 at 5:40 am
Whew, that’s a relief. I was afraid that your astoundingly brilliant breakthrough would only qualify as the second greatest upset in scientific history …
I was also curious as to who is the current record holder for the “greatest upset in scientific history” … curiously, the Guinness Book of World Records doesn’t list that one.
I’m glad, however, that all your fantastic scientific successes haven’t given you a swelled head.
w.
Why do you not debate your fantasy phizzacks here Willis, https://climateofsophistry.com/2017/10/19/the-steel-greenhouse-in-an-ambient-temperature-environment/#comment-32084 oh that’s right you did, and you slowed/delayed cooling thought car wreck experiment got flushed and you along with it.
PS Jesus was a carpenter aswell, stick to hanging doors Willis.
Thanks, Gary. It appears that like many others, you don’t have the albondigas to point to one single statement in my post about the Steel Greenhouse and tell us what’s wrong with it. Not one.
No surprise there, lots of folks on this thread have more mouth than they have facts or brains … come back when you actually want to discuss the science, I’ll be happy to do it.
w.
I do not have adequate qualifications to argue your steel greenhouse but I would like to ask a few layman’s questions if you don’t mind.
Do you believe in the Trenbeth diagram, ie do you believe that there’s around 333w/m2 of downward LWIR?
What value do you put on the equivalent Solar energy hitting the surface?
Why can the Solar Energy be made to perform work and the DWLWR can’t?
Yeah right Willis, remember this, ‘“That’s just a rounding error in the model”. when your model violated conservation of energy.
You had it it explained to you over and over, Joe may as well have been talking to a plank.
Further up the comments you scolded a commentator for taking offence to your forth-right attitude, that made made me chuckle remembering how you whined and whined about Joseph’s dismissive attitude to your ”citizen science”.
Why don’t you grow a set and finish the debate ?.
As for the rest here, the GHE fraud is on its knees you need to start re-aliening to the no GHE line because Anthony Spencer et al subtly are, and have been for months.
The 2nd law violation of energy from cold environs being unable to increase temperature in warm environs is the end of climatism and the climatists, once the blue/red team exercises into the science of CO2 get underway., they will schooled.
[tone it down -mod]
A C Osborn, here is a link to the first article at CoS on the steel greenhouse.
https://climateofsophistry.com/2013/03/08/the-fraud-of-the-aghe-part-11-quantum-mechanics-the-sheer-stupidity-of-ghe-science-on-wuwt/
Quite a lot of enraged ranting and insults at first, but Postma does eventually get to a straightforward walk through of the main errors. Then there are 937 comments of further discussion on the matter. Yes, all objections you could pretty much imagine have been answered. Then there’s the most recent three articles at CoS as well to wrap it up, and the comments there too.
Hope that helps. Don’t expect anything from Willy, there will never be an admission of error on this matter, I doubt. He’s best just ignored at this stage.
(Keep it civil) MOD
Tony November 23, 2017 at 3:59 pm
When I’m in a discussion and someone starts playing grade-school snarky games with my name, I know I’ve won. The only reason a man starts throwing mud like that is because he’s out of real ammunition …
I’ve invited Tony to point out here on this thread one single statement I made in the Steel Greenhouse that he thinks is wrong.
Instead of taking his chance and showing us how he can prove me wrong, instead of putting his brilliant claims out where they can be examined by all and judged fairly, he plays name games and wants me to talk about some mysterious discussion from years ago …
… and apparently, he actually mistakes that for winning the discussion.
Amazing. Ah, well, I suppose I should remember, DFTT.
w.
I am keeping it perfectly civil. Thank you.
Gary Ashe November 23, 2017 at 2:59 pm Edit
Who is “Joe”, and what on earth are you babbling about?
And what is “re-aliening”, the only alien I see here is you.
“They will schooled”??? Don’t you want to buy a verb for that sentence?
All I can get from your cryptic message is:
1. You definitely need to cool down. None of this is worth getting your blood all angrified.
2. You don’t understand the difference between heat flows and energy flows. I’m writing a post on the subject right now, because you are far from the only one who doesn’t get it. Short version? Heat can’t flow from cold to hot … but radiated energy sure can.
3. You have a lot of trouble communicating clearly.
Gary, I’m more than happy to discuss any objections you might have to my post on the Steel Greenhouse. I will say that physicists that I admire, people who teach physics for a living, say there is no violation of the Second Law involved.
You seem to believe that thousands of scientists in dozens of countries are either ignoring an egregious Second Law violation in the greenhouse effect, or they are simply too stupid to see it … but you, you can see it …
I could give you the odds on that being true … but you’d probably just yell at me some more …
w.
So to clarify, Tony supports this statement, taken directly from his site.
”
So if we use this equation for a sphere a radius Rsp = 1m and temperature Tsp = 300K inside a larger, cooler shell of radius Rsh = 2m and temperature Tsp = 290 K, we get
Q(sp-sh) = 5770 W – 20160 W = -14390 W
As stated above, a positive Q(sp-sh) means heat flow from the sphere to the shell, so this negative result predicts a heat flow the other way — from the shell to the sphere. Yes, heat flows from the cooler shell to the warmer sphere according to these esteemed scientists.
So, like I say, any and all objections are covered at CoS, in the articles and comments. So that’s that. Poor old Willy, and poor old Tim. But what’s done is done,
Tony, I do not need any help from you thanks, I have read both sides of this debate at both sites.
Willis, I realise that you have been sidetracked by Gary & Tony, but could you find the time to answer my questions?
Or anybody else?
Yes, A C Osborn, Willy did answer your questions, further below. Just keep scrolling down. Happy to help.
“Why can the Solar Energy be made to perform work and the DWLWR can’t?”
The main reason is that solar energy arrives as a near-parallel beam and can be focussed. So you can get a very high temperature relative to terrestrial sinks.
DWLWIR is diffuse, and can’t be focussed. The only way it could be made to do work is if a sink colder than where it comes from could be found.
That doesn’t mean that DWLWIR doesn’t add heat to the surface it falls on. It just means that that surface must be fully exposed to the source region, and so loses more heat than it gains. So no work. But it doesn’t lose as much heat as it would have if exposed to space.
So do tell, Tony! How was this objection handled? Which comments justify an equation that clearly predict heat flowing from large, cool objects to small warm object?
Tim, you are referring to equation 4a from the article at CoS that Gary linked to (as did I, further up in a different discussion). All anyone needs to do is read through that article in its entirety to see where you’ve “gone wrong”. Your “misunderstanding” would be amusing if I thought it wasn’t deliberate. However, I’m aware that you have indeed read the entire article, so it’s unlikely you would make that mistake genuinely. As it is, I suspect it’s simply an attempt to bait me into a discussion whereupon your usual deception can take place.
It’s funny, you and Willy are so alike. You just approach it from two different angles. Willy plays the game of insisting everyone has to discuss things under the terms of his original article. “Quote my exact words! Tell me where I’m wrong!”
Then when somebody does just that, and indeed writes a whole series of articles about it, responding to a huge variety of different objections and “misunderstandings” in the comments below, Willy can deflect from any of that by simply insisting that anyone talking to him now has to go through the whole thing again. The whole discussion must be had again! Lol. Meanwhile, people like Tim are on hand to take a snippet from that discussion completely out of context, in an attempt to bait people into once again repeating the whole discussion…
“Tim, you are referring to equation 4a “
Yes.
Exactly.
The equation that says heat flows from large cool objects to small hotter objects. It’s right there for all the world to see. No ‘context’ can fix that sort of blatant error.
So you are going to continue to be deliberately obtuse?
Well, let’s take the bait. If nothing else, your responses will serve to demonstrate the folly of entering into further discussion, with folk such as yourself…
When Q=0 (i.e at equilibrium), equation 5a shows the temperature of the shell (Tsh) in relation to the temperature of the sphere (Tsp), and the radius of both sphere and shell. From this equation it’s clear that the temperature of the sphere is always going to be higher than the shell, at equilibrium. This is because the shell is necessarily larger in size than the sphere, and will therefore (possessing a larger surface area) always emit more energy in total than does the sphere, if they were at the same temperature, all else being equal. Energy is conserved, not temperature.
You have “arbitrarily” chosen a value for the temperature of the shell which would not be reached spontaneously were the shell introduced at 0 K, when the sphere is at 300 K (as you also specify). Equation 5a shows that with the sphere at 300 K and with the radius of the sphere and the shell as you specify, the shell would equilibrate at a lower temperature than 290 K. So, for the shell to be at 290 K, it would require a source of additional (external) energy besides what it receives from the sphere. Which is why you get the result you do when you insert those temperature and radius numbers into equation 4a.
You know, for practical purposes you can consider a light bulb as a physical implementation of the steel sphere.
The filament isn’t a sphere, but being mostly 2d I think it serves the purpose of a sphere for an example that can be tested.
“When Q=0 (i.e at equilibrium) …
… and this is the next error. (Not that it fixes anything in the previous error– it was derived as a general result and still predicts heat from large cool shells to small warm spheres.)
When Q=0, then there is no heat flowing from the sphere to the shell. But the discussion starts by positing “a power-generating sphere”. We are told that power goes IN to the sphere, but now we are asked to accept that no power goes OUT from the sphere. If the sphere has power in but no power out, it must be warming. But a warming sphere by definition is not at equilibrium.
The errors just keep cascading into more and more contradictions — contradictions not only with every thermodynamics textbook, but internal contradictions within the so-called ‘proof’ itself.
“we are told that power goes IN to the sphere, but now we are asked to accept that no power goes OUT from the sphere”
Yes, great demonstration, Tim. As soon as your (no doubt deliberate) error is exposed you immediately change tack onto something completely different…something you just absolutely fabricate out of thin air. I’m glad I took the bait, I knew you would immediately show your true colours. It’s like I said before, though, you’ll be OK. Politics beckons.
This is not for any fights, but I am surprised that after four years when I came back to this site, I still see the same old skeptical discussions essentially on whether or not humans are playing a role in climate change, or even more basic, whether CO2 is a greenhouse gas that even most skeptics of AGW don’t question. On the other side, countries like China and India are well ahead of their targets set by Paris agreement even without the US. Although they had some setbacks from nuclear issues, Germany is well on its way to have almost all of their energy from renewable sources. US on the other hand is going back, making knowledgeable people around the world laugh at the US’s ideologically based skepticism coming mainly from those with little training and no real credentials in the area – which seems to start with the new EPA head.
No, they’re fools.
Water vapor regulates surface temps, not co2.
Yes, water vapor has a major role, but excess water vapor (determine by pressure, temperature) will condense. CO2 doesn’t do that at earth’s temperatures, hence serve as the cause for temperature change. Most scientsts from Arrhenius onwards (from 1895) accepted CO2’s greenhouse capability.
When was the last time Earth’s atm didn’t have any water vapor? But besides that, you didn’t understand the significance of net radiation dropping by over 60% in the middle of the night. Same thing scientists since Arrhenius have all missed.
micro6500
November 23, 2017 at 12:51 pm: You are darn right there. Latent heat, half the density of air, massive lifting power. Well-measured, non GH mechanism. The arch warmista rely on water to get them over the line because they cannot claim CO2 has the oomph. Little realising water was doing it all anyway, without any GH. And plenty of capacity to spare…..
Small wonder deserts are hotter than jungles, and the Models have failed. They do after all use GH factors
micro6500, “When was the last time Earth’s atm didn’t have any water vapor? But besides that, you didn’t understand the significance of net radiation dropping by over 60% in the middle of the night. Same thing scientists since Arrhenius have all missed.” I don’t know what any of that has to do with the effect of CO2? With or without CO2 change, there will be greenhouse effect from water. Increasing temperature from increased CO2 will increase water vapor in the atm that in turn will further heat the globe – as a simple explanation. The reality is more complcated but the effect is the same. I am not sure what you mean by the radiation drop, such variations are there with or without water vapor in atmosphere like in deserts. In any case, we don’t have much control over water vapor and even if we all change to hydrogen fuel giving water vapor as the reaction product, unlike CO2 this is not going to change anything since there are limits how much water atmosphere can carry. Excess water will precipitate. So I am not sure what you are suggesting. All scientists account for the effect of water vapor since it is significant.
That’s because you do not recognize a regulator when you see one.
https://micro6500blog.wordpress.com/2016/12/01/observational-evidence-for-a-nonlinear-night-time-cooling-mechanism/
And while you are quick to claim positive water vapor feedback, you forget about the negative feedback response at night. Thing is, it cancels out the excess days warming, if there is any. Read the link, understand it. Then we’ll talk.
No, Micro6500, I said it is more complcated, there are both positive and negative effects and that is why I did not want to go into the details of it. But when you publish your work showing “….not co2, and in fact any additional warming from Co2 has to be lost to space, before the change to the slower cooling rate….” in a real journal with statistical data, I will certainly pay more attention.
Did you see that Min T follows dew point temp with a 97% correlation, over 75 million station records.
And my code is published for you can validate my work
You see, my work is published there, everything anyone needs to confirm my work. It’s just not to many ppl understand the signals in a regulator. If they did, they would see that’s what happens at night under clear skies. Can you explain why the temp stops dropping in the middle of the night, while T zenith is still 90F colder than aur temps? But you can’t, or won’t because you know it ends AGW concerns. As a regulating mechanism, water vapor has well over 10x the power co2 has, we will never burn enough fossil fuels to alter temps with co2, the condensing water vapor never runs out!
Well… Micro6500, I would strongly encourage you to send your work to a journal. If it is important, people who don’t read blogs (this is the first time I came here in five or six years, that goes for all other climate related blogs) needs to read it, and if there are problems with your work, the reviewers will hopefully address that. Everyone knows that water vapor has a substantial effect, especially because there is more of that than CO2. But most researchers will object to your other characterization, that CO2 effect is somehow negated by water vapor, or calling them fools.
Then you do not note that net radiation drops significantly while it’s still dark out? If you’ve looked at night time temp data you’ve seen it before. That how I found it, 4am clear skies, and the temp isn’t dropping, and yet my IR thermometer says the sky is -40° or -50°F.
Radiatively, that’s about what the optical window sees from the surface. It has to be dumping 40 or 50W/m^2.
Why doesn’t the temp keep dropping?
And what air temp does it stop near?
I don’t expect there are any amount if changes I could make in writing a paper, that would get it accepted.
It’s available on the internet, and a lot of ppl read WUWT, and Climate Etc, so I know a lot of climate scientists have seen it.
micro6500 November 24, 2017 at 7:06 am
Thanks, Micro. The problem with that 97% correlation is that it is expected, since dewpoint is a function inter alia of temperature. There are various ways to calculate dewpoint from temperature and relative humidity. Here’s one from the Bulletin of the American Meteorological Society:
Td = T – ((100 – RH)/5.)
where Td is dewpoint, T is temperature, and RH is relative humidity.
Now, relative humidity is not too useful to us here, because it changes with temperature. But RH is a function of the absolute humidity, the actual amount of water in the air.
RH = 75.2 AH e^(-((17.6 T)/(243 + T))) (273 + T)
Substituting above, we get:
Td = T + AH e^(-((17.6 T)/(243 + T))) (4110 + 15 T) – 20
Now, what this says is that Td, the dewpoint temperature, is a function of T, the temperature, and AH, the amount of water in the air. However, the actual amount of water in the air doesn’t change all that fast, particularly when temperatures are dropping. And this means that if the amount of water in the air doesn’t change, that dewpoint is a function solely of temperature, and that the correlation will be best at minimum temperature.
Finally, whenever a falling temperature causes the dew to form, the dewpoint is equal to the temperature. So of course they vary in tandem, as the temperature drops below dewpoint they both move downwards in lockstep.
Given all of that, we would absolutely expect a very good correlation between Td and T, simply because Td is a function of T and AH, and AH changes slowly.
So while your finding is true … it is also exactly what we’d expect to find. And as a result, it’s not at all clear what use that might be. How does finding something that we’d expect to find advance our knowledge?
Best to you,
w.
Willis, there is an energy barrier as air temps drop to dew point. That forces the release of stored energy, the water has to release it to condense. What RH really means, is the percentage of water vapor condensing is a vol of air increases, it has to be giving up energy, and QM requires that distribution. So some is droplets, most is still gas, but as it cools to dew point that ratio changes.
It’s all that latent energy radiating from the condensing atm water vapor that’s supplying the surface energy that compensates for energy going out the optical window.
Just like you found water regulates ssts by breaking out into cooling thunderstorms, thus process kicks in only after the air has cooled, then there’s a large increase in IR, latent heat.
That’s how it stops cooling in the middle of the night
Or
And everyone just thought it has reached equilibrium, but in reality it’s quite active, and it’s being regulated.
Have you read this https://www.researchgate.net/publication/253127549_What_determines_the_nocturnal_cooling_timescale_at_2_m
?
Willis,
Since you didn’t respond, I’m not sure if we are done yet. Did you check out the paper I linked yet?
And why doesn’t air temp drop below dew point(rhetorical)? There’s at least 35W/m^2 to space even when temps are not falling at all in the data collected there.
Can we agree there’s an energy barrier, ie that more radiation has to be lost to maintain an equal temp drop once air temps are at dew point temp?
That by definition is a non-linear function.
And conversely this is proof Tmin is a function of dew point, not co2.
It also is a negative feedback to water vapor amplification that is a requirement for catastrophe.
In fact it is non-linear enough to remove most of the energy accumulated by co2 before morning mist nights.
Where there’s enough water vapor to work with. Why deserts have such a large range, dew point is low, max T is high.
Mike
RobG,
this GERMAN website (In English) has a lot of information about the failing Solar and Wind power.
http://notrickszone.com/?s=Energiewende#sthash.DrHJHget.dpbs
micro6500 November 25, 2017 at 6:59 pm
Sorry, Mike, I’m on the road right now. I haven’t answered because I’m not clear exactly what your point is about dew point and min temp. You seem to think it is significant that min temp and dew point are often either close or the same … which is true but I don’t see the relevance.
What am I missing here?
w.
There’s an energy barrier to air temps dropping below dew point, because most of the cooling is radiative, and when it radiates, half or more runs into water or co2 and is captured. The higher % of the water
“The higher % of the water” that has cooled to dew point, but for every photon it can emit, it captures another photon.
You’re exchanging money analogy, thus us putting some in the bank during the day, and withdrawals it only to keep air temps from falling too much below dew point.
What I can’t explain to people is it acts like a switching power supply regulating min T, which is normal climate that you explained. It’s just they are dependant functions, consuming about 10x the energy of co2, and it’s regulated by temp, it doesn’t slow cooling until after it gets near dew point, the same fixed point whether Tmax was 75, or 76. If you look at the rate temp changes at dusk, it’s 3-4°F/hr, some clear nights it stops cooling, others it slows. On the slow nights, if temps are warmer, say 1° as my example, the transition switches at the same absolute temp in either case. In effect, all you do is exchange cooling that 1° at the high cooling rate, for the time required to drop 1°, for that time at the slowest cooling before sunrise. And on nights that’s 0, well all the excess is lost, ie min T is entirely dependent on water vapor, co2 had zero affect.
Sorry, I hit send in the middle of writing this on my phone, and had to figure where I was before I coukd continue.
“Germany is well on its way to have almost all of their energy from renewable sources.
ROFLMAO
Of course they are, Rob. 😉
Get some actual FACTS before you comment next time.
wind, 2.1%

Solar 1.2%
Biomess 7.3%
http://www.independent.co.uk/news/world/europe/germany-renewable-energy-record-coal-nuclear-power-energiewende-low-carbon-goals-a7719006.html
“Germany breaks renewables record with coal and nuclear power responsible for only 15% of country’s total energy” Link posted above … there are many other links one can look into.
Rob,
You left out natural gas and oil.
No, Gabro: It says “At one point on the sunny and breezy Sunday, sustainable energy from wind, solar, biomass and hydro power provided a record 85 per cent of the country’s total energy.” There are not there yet, but with proper conditions they are already close.
Rob G November 23, 2017 at 5:05 pm
You guys are talking at cross purposes, and both of you are right.
Rob, you have the curious idea that because on a Sunday when no industry is running and it is sunny and breezy so there is no need for either heating or air conditioning, for ONE INSTANT renewables were providing 85% of the power, that that has some meaning. But all it means that the demand was really, really low, and the sun plus wind was at its highest.
However, Andy is showing the long-term reality, which is that sun plus wind in Germany generates 3.3% of the energy used.
After billions of dollars have been spent on solar and wind in Germany … THREE POINT THREE PERCENT.
Now it’s clear that you are both right. Average of wind and solar is 3.3% over the whole year. On the other hand, on one day when conditions were perfect, demand at its lowest, wind and solar at their highest, for one instant they got 85% … but so what?
Rob, do you make business decisions based on a best case ever scenario, or an average scenario?
Because me, when I want to judge how something is doing, I would NEVER just consider how she sails on a good day with a following wind … I want to see what the long-term situation is.
So while both of you are right, I know which numbers I pay attention to, and it is not how it did on the best day of the year. Hyping that kind of data is a cheap salesman’s trick …
w.
W., Germany is still not there, but they are still investing and they have a plan to get the average energy supply from renewable sources high. On your question “Rob, do you make business decisions based on a best case ever scenario, or an average scenario?” Germany had already made that decision, but they made that based on the average anticipated power generation, not current average power supply, from such sources. The advantage here is, apart from the capital investments (which is required for any power plants) there are no expenses for buying the sources of energy (like coal, natural gas, uranium, etc.) except for biogas. Maintainance expenses are there for all plants. So overall it works out much cheaper to have renewable sources, and it is a wise business decision for consumers and corporations.
Rob G November 24, 2017 at 6:53 am
Indeed they do have a plan, and after spending billions and billions of dollars on the plan, solar plus wind supply 3.3% of their energy. Look, even the Germans admit that their “energiewende” has been a colossal and expensive failure that has made their electricity prices skyrocket …
So yes, they have a plan, and they are now in the process of abandoning that plan as an expensive failure.
w.
PS—You describe 3.3% of energy coming from solar and wind as “still not there” … a more accurate description would be “still barely begun”.
W. That link is one person’s opinion. I know there are some issues with energy conversion in Germany, even NY Times reported that, but it is not a failure and there are no plans of abandoning it. We will see what happens in the next 10 years. I predict you will see substantially more solar and wind based energy production in Germany in that time. CO2 is harmful or not is one side of the story, when solar panels are becoming more efficient with falling cost, it simply becomes a better/cheaper route. Fusion energy is still an iffy solution for the growing energy needs. Availabilty of fossil fuel will only last so long, whether it is 36 years or 100 years. So alternate energy sources are going to come up, and as calculators kicked slide rules out, these new sources are eventually going to make fossil fuel obsolate. Even with friendly policies for coal, coal power plants in the US are still shutting down because of other reasons, and that trend will continue. https://www.nytimes.com/2017/10/07/business/energy-environment/german-renewable-energy.html
https://www.nytimes.com/2017/05/22/opinion/paris-agreement-climate-china-india.html For example.
Gosh … you meant the New York Times said it?
Sorry, Rob, but these days, if the NYT said it, that actually reduces the odds that it’s true …
w.
No, W., the basis for everything they wrote there comes from various reports, which one can verify, and I have not seen anyone offering data to counter it.
Rob G November 24, 2017 at 6:22 am
Rob, you are welcome to believe the NYT. I don’t trust a word they say. And yes, they always base what they claim on “various reports” … me, I prefer news organizations that base their work on “various facts“. Their reporting was so bad during the last election that the Editor famously APOLOGIZED for it.
Of course, the apology was as fake as their “news”, they just kept doing the same thing … no surprise there.
In addition, the article is not available, I’ve used up my two free views for the month.
So you’ll have to argue with yourself on this one. Inter alia, however, I would note that China didn’t agree to much at Paris except to have their CO2 emissions peak by 2030 … and if you believe that will happen, you’re beyond my poor power to add or detract …
w.
I have been to India and China looking at their solar initiatives, in India you will see solar panels in many homes, it is becoming a trend. China is slightly behind but they will be investing a lot of money and within five years you will see China ahead of India. Those people are not really being silly, I think in a decade you will see US far behind those contries for cheaper energy sources (as well as in technical capabilities related to solar panels, China already has the dominant market share for solar panel manufacturing) https://economictimes.indiatimes.com/industry/energy/power/india-ranked-second-in-renewable-energy-attractiveness-index/articleshow/58698180.cms
https://www.reuters.com/article/us-china-energy-renewables/china-to-plow-361-billion-into-renewable-fuel-by-2020-idUSKBN14P06P
A C Osborn November 23, 2017 at 2:58 pm
Excellent questions all.
Regarding downward longwave infrared (LWIR), this is routinely measured by scientists all around the world. You can measure it yourself (with admittedly poor accuracy) with an infrared standoff thermometer. The CERES satellite data puts it at about 345 W/m2 on a global 24/7 average.
Again per ceres, solar hitting the surface is on the order of a 186 W/m2. However, about 24 W/m2 is reflected by the surface.
Finally, both solar and LW can be made to do work. Any heat can be used in a heat engine. However, for any heat engine to work, you need to discharge the rejected heat into a cooler environment … and the atmosphere, in general, is cooler than the surface—so there is nowhere on the surface to discharge the waste heat.
What about photovoltaics? The problem is that there is not enough energy in thermal radiation to bust loose an electron and kick it up to a higher orbital, as required for the photoelectric effect … so no photovoltaics as far as anyone has found so far.
Best regards, and if you have further questions I’m happy to discuss them.
w.
Well the question is now that Solar can be used for work on the surface as demonstrated by any Solar Still or Solar Array like Ivanpah.
Ivanpah Generates almost 1000Gw per year according to Wiki by collecting, focusing and using the miserely 186W/m2 and yet it can’t Generate anything from the 345W/m2 of LWIR.
So why is that?
Aren’t all Watts created equal?
Oh, I forgot, of course DWLIR can do a sort of work because at night a Solar still extracts the heat out of objects and makes them colder, don’t they?
A. C. Osborn, another good question. The answer is, solar stills and solar arrays are not driven by average solar energy. They are driven by instantaneous solar energy,
At the tropics at noon, that’s over a thousand watts per square metre … and at 38°N where I live, peak solar is on the order of 750 W/m2.
Bear in mind that 30°C (86°F) equates to a S-B blackbody radiation of 480 W/m2 … so the sun is much stronger than ambient. That’s why the sun can do useful work at the surface, while the radiation from the cold atmosphere cannot.
Best regards,
w.
Not exactly, it’s delivered at near 1,000W,m^2, Not 186, and whatever the lwir amount is, it’s not different from the exhaust, other that radiative cooling to space, and it too would need concentrated. But Ivanpah doesn’t run at night because when you look up it’s cold, not hot.
Sorry, that won,t quite do as an answer, because even if it was 900W you should still be able to get 1/3 of the energy by concentrating 300W of DWLIR.
With a simple 3″ hyperbolic reflector I can raise temperature of a piece of steel to 450C in UK and yet a Solar still extracts the heat from an object at night, cooling it to around -5C.
So why can’t we heat anything by concentrating 300+ W/m2, but instead it cools it?
These experiments have been done countless times with the same results.
Because you are concentrating the many thousands of degree Sun, vs air temp.
My response was to Willis, not you micro.
But perhaps you can answer the question of why a Solar Still or Oven becomes a Refrigerator at night?
I have come to understand that we do not understand energy as much as some like to think, for instance why hot objects cool quicker than cooler objects.
Or why a surface with an LED light shining on it Frost’s over quicker than an area of it shaded from that same light.
1000s of degrees Sun!
You are having a laugh.
Watts are Watts, that is how ghgs work isn’t it?
So you are confirming that all Watts are NOT EQUAL, thank you.
Goes with the nearly 1,000 W/m^2 near the equator.
“Ivanpah Generates almost 1000Gw per year according to Wiki by collecting, focusing and using the miserely 186W/m2 and yet it can’t Generate anything from the 345W/m2 of LWIR.
So why is that?
Aren’t all Watts created equal?”
AC, Let me expand on what Willis said (here and before). The word “focusing” is critical.
The 100’s of W/m^2 of sunlight comes in the form of a very intense beam from a small part of the sky. With a lens or mirror, you can focus in sunlight from other directions, multiplying the total to many 1000’s of W/m^2. Basically, you can make it look like there are thousands of suns all around.
The 100’s of W/m^2 of thermal IR comes in the form of a very diffuse glow from every part of the sky. As such it is impossible to focus that IR. If you put up a mirror to reflect IR, you are blocking an equal amount of IR that was already coming from that direction.
Wrong, you can focus it, that is precisely what a Solar still does and it makes objects at the focal point colder.
Let me provide you with a thought, suppose it makes them colder because it is coming from a colder place?
Can you prove that to be wrong?
“Aren’t all Watts created equal?”
Of course they aren’t. That’s what the second law of thermo is about. There is energy (Watts), which is conserved, and associated free energy (Watts), which can be dissipated (or used). Free energy is the capability of doing work. The Watts of electricity that you might use to warm your house are valuable. The warmth they are converted to has much reduced utility.
Thank you, you have just blown GHG theory out of the water, because obviously DWLIR Watts are not equal to REAL watts either.
Watts that can’t do any work are worthless.
AC I am confused by what you mean by a “solar still”. Google searches reveal devices designed to distill water by warming it. Perhaps you mean something akin to this idea: https://en.wikipedia.org/wiki/Radiative_cooling#Nocturnal_ice_making This is the same reason front can form on the tops of cars parked outside, even when the air temperature stays above freezing. I have seen this principle extended to roof-top panels.
If this is what you mean, then yes, some “focusing” is possible. in this case, “warm” IR from the warm ground (or roof) below is blocked and “cool” IR from the sky is substituted.
A C Osborn November 24, 2017 at 2:47 pm Edit
I’ve never heard of a way to do that. The problem is that unlike sunlight, longwave IR doesn’t arrive at the surface as a beam of radiation. You can focus and concentrate and reflect a beam of radiation.
But the longwave infrared is coming from the entire hemispherical dome of the sky, in all directions. I know of no way to focus or concentrate that kind of radiation
I don’t know what this means. What experiments?
Let me know if this is not clear.
w.
In the headline to this post is the following question:- “what’s the quality of your radiation?”
Now here’s a thing. When I was young in the 1960s and before the political parasites had debased the currency, it used to be possible to collect bronze pennies from the reign of Queen Victoria. The oldest coin in my collection was one dated 1860, faint, worn and of no numismatic value, but it was fascinating to me that something minted one hundred years previously was still in circulation. My coin set was a lesson in the past of my country and gave a perspective of history that no modern child can experience.
My historical coin collection was also an economics lesson, silver sixpences minted before 1920 really were just that, sterling coins containing 92.5% silver, while none of the sixpence coins minted after 1946 contained any silver at all (how the mighty were fallen). But the key point I learned from my collection was this, no matter how many bronze pennies I collected, and no matter how large the sum became, none of these pennies ever turned into a silver sixpence.
Now will someone please explain to me how “cold” low quality, low frequency electromagnetic radiation can spontaneously turn into “hot” high quality, high frequency rays? The Wein’s displacement law proves that this is impossible. In order to raise temperature of a surface you need to add high frequencies, adding more low frequency energy, no matter how much, never works. You can never turn bronze pennies into silver sixpences and certainly never bright silver into glowing gold.
Good to introduce discussion on the ‘quality’ of the energy
I think that in all quoted instances of this kind the source of energy is still pumping away.
An example would be an electric light bulb.
Without shading it would glow at a certain temperature
With a reflecting partial covering it would glow brighter or more ‘blue’
Now one interpretation of this favoured by warmists is that reflected low quality energy is converted to a higher quality energy.
The way I would interpret this is that additional radiative resistance is introduced by the reflective covering reducing the energy loss of the bulb.
To continue with your ‘coin’ metaphor electrical energy is the ‘gold ‘ standard.
“You can never turn bronze pennies into silver sixpences and certainly never bright silver into glowing gold.”
Electrical energy is of the highest quality and can be used to top up the higher frequencies.
With no electric power however.
“You can never turn bronze pennies into silver sixpences and certainly never bright silver into glowing gold.”
Holds true
Well expressed! CO2 can only emit (and absorb)in the waveband 13 to 17 microns, according to MODTRAN6. These are line spectra, and if you spread them out somehow into a continuous black body Planck spectrum, such as Earth produces, the warmest “Wien” temperature you could get out of the mix would be -79 degrees C, corresponding to the most intense line at 14.95 microns, and this is way below normal Earth surface temperatures. Cooler objects (here, CO2) can’t transfer heat to warmer ones (here, Earth’s surface), so the radiative argument for a greenhouse effect for CO2 on Earth is nonsense. In any case, gases actually derive their temperature much more from from pressure than from radiation, and that applies to ALL atmospheric gases, not just CO2, and even here, due to the lapse rate, atmospheric CO2 is never warmer than Earth’s surface. CO2 radiation can retard Earth’s infrared output (that of 13 microns wavelength and above), slowing heat loss, but heat addition can only come from Sun or from ozone creation/destruction reactions in the stratosphere.
What you leave out is if absorbed, energy is energy, and if there are more photons, there’s more energy. So your assumption is incorrect, co2 can warm the surface, it just takes more 15u photons to do so.
And if you look at the y axis of a atm spectum, it’s in energy, not photon count.
micro6500, that’s an older and outmoded way of looking at things. In reality, EMR doesn’t contain photons at all, but is simply a frequency field devoid of energy. Photons arise when the energy field impinges on matter, resonantly inducing increased bond vibrations at fundamental or harmonic frequencies.The increased motion of bonded atoms generates kinetic energy, and this is what Einstein correctly interpreted as “Lichtquanten” (photons), except that he made the mistake of thinking that the Lichtquanten came in with the EMR instead of being resonantly generated on the spot (resonant vibrations in frictionless bonds require no energy input, but Einstein couldn’t have known this in 1905).
Energy is really a property of matter, and in every case except that of the supposed radiation-borne photon, its dimensions contain, or can be converted to contain, a mass term. We know that EMR is massless and that it travels at light speed, neither of which would be possible if it contained energy. All our detection devices are material, so we can only detect photons after they’ve interacted with matter. We can’t detect them in massless contexts such as outer space. All energy is mass-bound, and therefore contains a mass term, which is far more reasonable than a situation in which all energy is mass-bound except for the kind that goes whizzing through space like some protean chimaera and is somehow mass-independent.
Be that as it may, any back-radiation from CO2 must be in the range 13 to 17 microns. These wavelengths would correspond to Wien temperatures of -51 and -103 degrees C if they were in a continuous Planck distribution, and their most intense line is 14.95 microns, corresponding to a Wien temperature of -79 degrees C, all temperatures well below those characteristic of Earth’s surface, in consequence of which they can’t transfer heat to the warmer surface of Earth but are reflected away instead.
I think it can, reduce cooling by about 3.7W/m^2, but that’s not what really slows cooling, that’s from water vapor.

And you can see it in net radiation.
And if you look at the cooling curve, it’s also not the expected exp decay.
Why should it keep cooling? Because the sky is 40 or 50 below F.
micro6500, I think I get your drift, but a little more explanation of your graphics would be helpful. Thanks.
Sure. Top chart just has Temp, Rel humidity, and net radiation, covers about 3 days, and in general they were mostly clear, the temps change smoothly except in the afternoon. You can see how temps fall at night, and once rh gets over about 50%, you can see a bend in the temp curve, and at the same time net radiation drops. This was recorded by the group that wrote the paper I mentioned to Willis, in Australia.
As air cools, and nears dew point more water has to condense, but there a lot of energy that water vapor has to release before it can do that. And is a gas like this, as it does, some of that energy is given to water that just condensed, causing it to reevaporate.
The second was taken by me in Ohio, you can see the distinctive cooling rate slow down under clear dark skies, and in the upper section you see IR thermometer readings from the same night showing the BB temp for the optical window to be about -50°F.
SB equations show about 80W/m^2 for that difference in temp. I think the optical window is about 40% of that, so 32W/m^2 Is radiating to space, and temps are not changing.
Net rad can drop because the window closes, or more energy comes from the surface.
And the evidence is in more energy, and that aligns with water vapor having to dump energy to cool.
Sure. Top chart just has Temp, Rel humidity, and net radiation, covers about 3 days, and in general they were mostly clear, the temps change smoothly except in the afternoon. You can see how temps fall at night, and once rh gets over about 50%, you can see a bend in the temp curve, and at the same time net radiation drops. This was recorded by the group that wrote the paper I mentioned to Willis, in Australia.
As air cools, and nears dew point more water has to condense, but there a lot of energy that water vapor has to release before it can do that. And is a gas like this, as it does, some of that energy is given to water that just condensed, causing it to reevaporate.
The second was taken by me in Ohio, you can see the distinctive cooling rate slow down under clear dark skies, and in the upper section you see IR thermometer readings from the same night showing the BB temp for the optical window to be about -50°F.
SB equations show about 80W/m^2 for that difference in temp. I think the optical window is about 40% of that, so 32W/m^2 Is radiating to space, and temps are not changing.
Net rad can drop because the window closes, or more energy comes from the surface.
And the evidence is in more energy, and that aligns with water vapor having to dump energy to cool.
Oh, if you haven’t, follow the url in my name.
micro6500, I definitely think you’re on to something here. I’ll have to spend some time with your blog to appreciate it fully, but the importance of H2O in radiative exchange has been sorely neglected, IMHO.
davidbennettlaing November 25, 2017 at 11:57 am
Be that as it may, any back-radiation from CO2 must be in the range 13 to 17 microns. These wavelengths would correspond to Wien temperatures of -51 and -103 degrees C if they were in a continuous Planck distribution, and their most intense line is 14.95 microns, corresponding to a Wien temperature of -79 degrees C, all temperatures well below those characteristic of Earth’s surface, in consequence of which they can’t transfer heat to the warmer surface of Earth but are reflected away instead.
So what, application of a nonexistent ‘inverse Wien’s Law’ means nothing. The surface of the earth quite happily absorbs 15 micron light whether it originates from T=400K or T=230K, in fact the surface is not aware of the source temperature.
“Now will someone please explain to me how “cold” low quality, low frequency electromagnetic radiation can spontaneously turn into “hot” high quality, high frequency rays?”
They don’t. No one claims it does.
“In order to raise temperature of a surface you need to add high frequencies, adding more low frequency energy, no matter how much, never works.”
Tell that to people who use CO2 lasers to melt steel. You need to be much more precise in your thinking, since a simple couter-example shows your throught process is faulty here.
So tjf, if I said that water cannot run uphill and you said yes it can, here is a pump storage scheme that proves that it can, would I not be correct in questioning your faulty thinking? After all is a laser not a precisely designed machine that need to consume power in order to work?
Phillip, you specifically claimed “adding more low frequency energy, no matter how much, never works [to raise temperatures]”. Now you seem to have reversed your position, accepting that lots of low frequency energy (ie 15 um photons) can indeed raise temperatures to quite high level. This simply shows how challenging all this can be so state ideas precisely and accurately.
Certainly it is impossible to use thermal photons from a source at T1 (and only those photons) to raise an object to a temperature T2 where T2 > T1. But those photons from the source at T1 PLUS other power inputs can raise the temperature above T1.
Philip Mulholland November 24, 2017 at 10:30 am
So tjf, if I said that water cannot run uphill and you said yes it can, here is a pump storage scheme that proves that it can, would I not be correct in questioning your faulty thinking? After all is a laser not a precisely designed machine that need to consume power in order to work?
It’s your faulty thinking which tries to apply a nonexistent ‘inverse Wien’s Law’, your logic is akin to saying red hot steel is at 650ºC, therefore the rose of the same color in your garden must also be at 650ºC.
That would be it’s color temp.
micro6500 November 27, 2017 at 8:53 am
That would be it’s color temp.
For the steel yes, for the rose no.
Philip Mulholland November 24, 2017 at 2:49 am
Now will someone please explain to me how “cold” low quality, low frequency electromagnetic radiation can spontaneously turn into “hot” high quality, high frequency rays? The Wein’s displacement law proves that this is impossible. In order to raise temperature of a surface you need to add high frequencies, adding more low frequency energy, no matter how much, never works.
This is based on the false use of Wien’s Law, which proves no such thing. Wien’s law just tells you at what wavelength the peak of the distribution will be, however both higher and lower wavelengths will be present.
Using an ‘inverse Wien’s Law’ does not work, you can not take the wavelength and infer what temperature it must have been emitted at. This is a common mistake which many who have posted in this thread have made!
An example of their error is that they assume that via Wien’s Law a 10.6 micron photon originated at 273K and therefore cannot raise the temperature of a 300K object, and yet a laser beam of 10.6 micron photons can melt steel. The ability to heat an object is not related to the frequency of the light (other than via the reflectivity of the object).
No, but IMO it is a useful measure of energy and potential work that can be extracted.
micro6500 November 27, 2017 at 8:50 am
“Using an ‘inverse Wien’s Law’ does not work, you can not take the wavelength and infer what temperature it must have been emitted at.”
No, but IMO it is a useful measure of energy and potential work that can be extracted.
You’re welcome to your opinion but I see no basis for it.
tj, that is what flummoxed me but surely lasers have a heat input and work by amplifying and focusing low energy light into high energy. I don’t believe that it’s an example of cold transferring heat to warm.
Another question that I’ve never had a sensible answer to is that if there is a downwelling radiation which has the “energy” to significantly raise the temperature of the planet, why can’t we recover what is apparently a real and significant heat? One answer I had was that it is low grade energy and that is surely the point
I have just asked Willis that very question up post, Inanpah and other Solar collectors convert it to heat and electricity and yet they do not convert the higher wattage lwr at all.
Watts Up With That then?
tj is an inveterate troll of the sort who can produce formulae and seeming facts at will which, on inspection, always turn out to be deliberately misleading. We know him of old…..
tj deceptively leaves out that CO2 does not laser anything. His tone displays his intent. I t can respond to large voltage differences, and transfer that to another gas (Argon IIRC). This gas can produce stimulated emissions at steel-cutting power..
I they lase at 10.6u.