Guest Post by Willis Eschenbach
Pushed by a commenter on another thread, I thought I’d discuss the R. W. Wood experiment, done in 1909. Many people hold that this experiment shows that CO2 absorption and/or back-radiation doesn’t exist, or at least that the poorly named “greenhouse effect” is trivially small. I say it doesn’t show anything at all. Let me show you the manifold problems with the experiment.
To start with, let me give a curious example of the greenhouse effect, that of the Steel Greenhouse. Imagine a planet in the vacuum of space. A residue of nuclear material reacting in the core warms it to where it is radiating at say 235 watts per square metre (W/m2). Figure 1 shows the situation.
 Figure 1. Planet in outer space, heated from the interior. Drawing show equilibrium situation
Figure 1. Planet in outer space, heated from the interior. Drawing show equilibrium situation
This planet is at equilibrium. The natural reactor in the core of the planet is generating energy that at the planet’s surface amounts to 235 W/m2. It is radiating the same amount, so it is neither warming nor cooling.
Now, imagine that without changing anything else, we put a steel shell around the planet. Figure 2 shows that situation, with one side of the shell temporarily removed so we can look inside.
 Figure 2. As in Figure 1, but with a solid steel shell surrounding the planet. Near side of the shell temporarily removed to view interior. Vertical distance of the shell from the surface is greatly exaggerated for clarity—in reality the shell and the shell have nearly the same surface area. (A shell 6 miles (10 km) above the Earth has an exterior area only 0.3% larger than the Earth’s surface area.)
Figure 2. As in Figure 1, but with a solid steel shell surrounding the planet. Near side of the shell temporarily removed to view interior. Vertical distance of the shell from the surface is greatly exaggerated for clarity—in reality the shell and the shell have nearly the same surface area. (A shell 6 miles (10 km) above the Earth has an exterior area only 0.3% larger than the Earth’s surface area.)
[UPDATE: Misunderstandings revealed in the comments demonstrated that I lacked clarity. To expand, let me note that because the difference in exterior surface area of the shell and the surface is only 0.3%, I am making the simplifying assumption that they are equal. This clarifies the situation greatly. Yes, it introduces a whopping error of 0.3% in the calculations, which people have jumped all over in the comments as if it meant something … really, folks, 0.3%? I am also making the simplifying assumption that both the planet and shell are “blackbodies”, meaning they absorb all of the infrared that hits them.]
Now, note what happens when we add a shell around the planet. The shell warms up and it begins to radiate as well … but it radiates the same amount inwards and outwards. The inwards radiation warms the surface of the planet, until it is radiating at 470 W/m2. At that point the system is back in equilibrium. The planet is receiving 235 W/m2 from the interior, plus 235 W/m2 from the shell, and it is radiating the total amount, 470 W/m2. The shell is receiving 470 W/m2 from the planet, and it is radiating the same amount, half inwards back to the planet and half outwards to outer space. Note also that despite the fact that the planetary surface ends up much warmer (radiating 470 W/m2), energy is conserved. The same 235 W/m2 of energy is emitted to space as in Figure 1.
And that is all that there is to the poorly named greenhouse effect. It does not require CO2 or an atmosphere, it can be built out of steel. It depends entirely on the fact that a shell has two sides and a solid body only has one side.
Now, this magical system works because there is a vacuum between the planet and the shell. As a result, the planet and the shell can take up very different temperatures. If they could not do so, if for example the shell were held up by huge thick pillars that efficiently conducted the heat from the surface to the shell, then the two would always be at the same temperature, and that temperature would be such that the system radiated at 235 W/m2. There would be no differential heating of the surface, and there would be no greenhouse effect.
Another way to lower the efficiency of the system is to introduce an atmosphere. Each watt of energy lost by atmospheric convection of heat from the surface to the shell reduces the radiation temperature of the surface by the same amount. If the atmosphere can conduct the surface temperature effectively enough to the shell, the surface ends up only slightly warmer than the shell.
Let me summarize. In order for the greenhouse effect to function, the shell has to be thermally isolated from the surface so that the temperatures of the two can differ substantially. If the atmosphere or other means efficiently transfers surface heat to the shell there will be very little difference in temperature between the two.
Now, remember that I started out to discuss the R. W. Wood experiment. Here is the report of that experiment, from the author. I have highlighted the experimental setup.
Note on the Theory of the Greenhouse
By Professor R. W. Wood (Communicated by the Author)
THERE appears to be a widespread belief that the comparatively high temperature produced within a closed space covered with glass, and exposed to solar radiation, results from a transformation of wave-length, that is, that the heat waves from the sun, which are able to penetrate the glass, fall upon the walls of the enclosure and raise its temperature: the heat energy is re-emitted by the walls in the form of much longer waves, which are unable to penetrate the glass, the greenhouse acting as a radiation trap.
I have always felt some doubt as to whether this action played any very large part in the elevation of temperature. It appeared much more probable that the part played by the glass was the prevention of the escape of the warm air heated by the ground within the enclosure. If we open the doors of a greenhouse on a cold and windy day, the trapping of radiation appears to lose much of its efficacy. As a matter of fact I am of the opinion that a greenhouse made of a glass transparent to waves of every possible length would show a temperature nearly, if not quite, as high as that observed in a glass house. The transparent screen allows the solar radiation to warm the ground, and the ground in turn warms the air, but only the limited amount within the enclosure. In the “open,” the ground is continually brought into contact with cold air by convection currents.
To test the matter I constructed two enclosures of dead black cardboard, one covered with a glass plate, the other with a plate of rock-salt of equal thickness. The bulb of a thermometer was inserted in each enclosure and the whole packed in cotton, with the exception of the transparent plates which were exposed. When exposed to sunlight the temperature rose gradually to 65 oC., the enclosure covered with the salt plate keeping a little ahead of the other, owing to the fact that it transmitted the longer waves from the sun, which were stopped by the glass. In order to eliminate this action the sunlight was first passed through a glass plate.
There was now scarcely a difference of one degree between the temperatures of the two enclosures. The maximum temperature reached was about 55 oC. From what we know about the distribution of energy in the spectrum of the radiation emitted by a body at 55 o, it is clear that the rock-salt plate is capable of transmitting practically all of it, while the glass plate stops it entirely. This shows us that the loss of temperature of the ground by radiation is very small in comparison to the loss by convection, in other words that we gain very little from the circumstance that the radiation is trapped.
Is it therefore necessary to pay attention to trapped radiation in deducing the temperature of a planet as affected by its atmosphere? The solar rays penetrate the atmosphere, warm the ground which in turn warms the atmosphere by contact and by convection currents. The heat received is thus stored up in the atmosphere, remaining there on account of the very low radiating power of a gas. It seems to me very doubtful if the atmosphere is warmed to any great extent by absorbing the radiation from the ground, even under the most favourable conditions.
I do not pretend to have gone very deeply into the matter, and publish this note merely to draw attention to the fact that trapped radiation appears to play but a very small part in the actual cases with which we are familiar.
Here would be my interpretation of his experimental setup:
 Figure 3. Cross section of the R. W. Wood experiment. The two cardboard boxes are painted black. One is covered with glass, which absorbs and re-emits infrared. The other is covered with rock salt, which is transparent to infrared. They are packed in cotton wool. Thermometers not shown.
Figure 3. Cross section of the R. W. Wood experiment. The two cardboard boxes are painted black. One is covered with glass, which absorbs and re-emits infrared. The other is covered with rock salt, which is transparent to infrared. They are packed in cotton wool. Thermometers not shown.
Bearing in mind the discussion of the steel greenhouse above, I leave it as an exercise for the interested reader to work out why this is not a valid test of infrared back-radiation on a planetary scale … please consider the presence of the air in the boxes, the efficiency of the convective heat transfer through that air from the box to the cover plates, the vertical temperature profile of that air, the transfer of energy from the “surface” to the “shell” through the walls of the box, and the relative temperatures of the air, the box, and the transparent cover.
Seems to me like with a few small changes it could indeed be a valid test, however.
Best regards,
w.
When exposed to sunlight the temperature rose gradually to 65 oC., the enclosure covered with the salt plate keeping a little ahead of the other, owing to the fact that it transmitted the longer waves from the sun, which were stopped by the glass. In order to eliminate this action the sunlight was first passed through a glass plate.
One of the (many) problems with this experiment is the ambiguity of the statement above. Passed through how? If glass directly atop the salt, it would render the two boxes effectively identical. Lots and lots of problems with the experiment itself, but how does one discuss it without knowing what the exact setup was?
I’m confused by the use of units of radiated flux. Conservation of energy would be measured as watts versus watts not watts/m^2 versus watts/m^2? Consider what happens as the size of the shell is increased to infinity?
Figure 2 text probably should be
“the planet and the shell have nearly the same surface area”
But in Diag 2, the steel shell has a much greater surface area than the core.
I hate to have to point this out, but your math on the 2nd diagram does not work.
Any possible shell around any possible sphere is going to have a surface area larger than the sphere it contains, so you cannot just split the “w/m^2” as seen on the (smaller) planet’s surface in half, and assume the same sum. The outer shell is larger, so the watts per METER SQUARED must be proportionately lower to get the total energy sums correct.
I may just be an engineer, not a scientist… but I can add.
i.e. if the core emits 235 W/m2 and the shell is say 10% greater area, its outward emission will be 211.5W/m2.
Ohh, Willis, you are inviting more posts from those who say that cooler bodies cannot transmit to warmer bodies (once again, yawn).
For those about to post such comments consider this:
There is a coolish radiator, emitting a small amount of infrared heat into a cold room. Then, another warmer radiator is placed in the room, a meter or so from the cooler radiator. Does the cooler radiator suddenly stop emitting infrared? And why would it do so?
(The cooler radiator does, of course, continue emitting just as before.)
.
Willis: This is a classic case of – “People’s ability to extrapolate FAR beyond any justifiable level” from one set of observations to another. I personally have NEVER considered Dr. Wood’s experiment to indicate ANYTHING OTHER than the conclusion that the “mechanism of action” of an actual GREENHOUSE is that of a “convective boundary”. It is interesting to note that YOUR theory of the importance of the virtually “unlimited” convection mechanisms in the REAL atmosphere leads to the “thunderstorm thermostat” conclusion.
Just as an aside on Dr. Wood’s GENERAL GENIUS is to point out his “rotating table liquid mercury lens” experiment, which allowed him to take photos of GALAXIES well before Eddington identified them in the 1930’s. (The disadvantage was that there was about a 5 degree (steradian) arc which could be covered by any apparatus set up anywhere in the world.) It cost MARKEDLY less than the Mt. Polomar telescope, however!
Max
This is stupid on stilts:
In order for the greenhouse effect to function, the shell has to be thermally isolated from the surface so that the temperatures of the two can differ substantially. If the atmosphere or other means efficiently transfers surface heat to the shell there will be very little difference in temperature between the two.
Yes. Just like a real greenhouse with atmosphere and everything.
That experiment doesn’t tell you much because you’re not capable of interpreting it correctly.
The experiment is done completely wrong anyway. To properly illustrate the “greenhouse” effect, Wood SHOULD have placed a brick inside each box with a temperature measurement device at the center of the brick. Shine the light until the center of both bricks stabilizes in temperature. Then turn off the lights expose both boxes to a clear cold sky, and record the rate at which the temperature drops in the center of each brick. Greenhouse effect is mainly a nighttime effect, not a daytime.
Problem #1
The troposphere ranges in thickness from 8km to 16km, Using 14km as an average, and a putative ghe of 33 degrees, that’s about 0.0023 degrees per meter. The apparatus used glass thermometers from 1906, suggesting an accuracy of perhaps 0.2 degrees at best. The boxes would have to have been about 1,000 meters on a side just to be big enough to produce enough ghe that it could even be measured by the apparatus used.
I am struggling with your thought-experiment because it seems to imply that you could raise the temperature of the planet to any arbitrarily high level by wrapping it in additional shells of steel. That is, if instead of wrapping your hypothetical planet in one steel shell, you used two nested shells, the outer shell would still stabilize at 235, the inner shell would stabilize at 470 and the planet would stabilize at 705. Keep adding shells until the inner-most steel melts. Yet the energy source, 235 W/m2, never changes. Am I understanding that correctly?
That would be a simple experiment to test in any good vacumn system. In fact, if true you’d expect the effect to be a significant source of difficulty in evacuated experiments. It’s been a long time since I did any experiments in a vacumn lab but I don’t remember having to make any such adjustments.
aaaaaaaaaaaaaaaaaaaagh!
100 meters on a side.
Silver Ralph,
You just flunked your first hourly in Thermo. The cooler radiator would be warmed by the warmer radiator, and begin radiating more. If you think this would warm the warmer radiator, then you will fail all your hourlies and never get through school.
Wilis, Joe Public has it exactly right. If you want to know what happens to the flux from a cooler source when it hits a warmer source, the answer is exactly nothing. It is not absorbed, but immediately re-emitted, transferring NO heat.
All these analogies are amusing but ignore Second Law.
To those who think the math in Figure 2 does not work: Please read the caption under Figure 2 as well as reading the picture.
It may be worth a revisit to Perpetuum Mobile WUWT Eschenbach Jan 19, 2012.
Rp = radius of Planet
Rs = radius of Shell.
Tsi, Tso = temp of Shell, inside and outside
Tp = temp of Planet.
By your setup, Rs > Re.
Tsi = Tso, since both are radiating the same energy flux.
By this, I conclude that the thermal conductivity of shell is very high and/or the shell is very thin.
Tp > Tsi (assuming black body).
As we reduce Rs to approach Rp in the limit, then Tp > Tsi and an infinite temperature gradiant which seems to be a logical impossibility.
You would agree, wouldn’t you that if the planet had no radioactive core, then Tp would have to equal Tsi. Yet the addition of a tiny radioactive energy source is now able to raise Tp to a level where it has twice the radiant flux as Tsi.
Doesn’t Tsi have to be a function of (Rs/Rp)^1/4?
To Mike M, Joe Public and Sarge – Energy is conserved and yes, you could reframe the entire example in watts instead of watts per square meter. It is irrelevant to the thought-experiment, however, because you can make the shell arbitrarily small as long as it is infinitesimally separated from the planet. The effect would be identical if the shell were one millimeter out rather than the severely exaggerated separation shown in Figure 2. And while that one-millimeter increase in radius would increase the surface area very slightly, it’s WAY below the rounding error of the system.
@Silver Ralph
Your example is not pertinent, the two radiators are two different sources of energy in the room. Very different than asserts that the exterior sphere warmed by the inner one can warm more that last.
@Mike M
“Consider what happens as the size of the shell is increased to infinity?”
And consider what happens as the size of the shell is the one of the inner sphere plus just an atomic layer?
Uhmmm… Still skeptic
A few typos, but correct to my understanding of heat transfer.
The difference in surface area of the actual atmosphere and the earth below it is not very big, and accounting for it would not change the argument. Integral over the surfaces of both would be equal number of Watts regardless of size.
So Co2 AGW is real. The point to argue is that it is benign, and probably swamped by larger natural variability over short and long time scales.
The Greenhouse Effect goes missing on certain nights at Penn State University.
This is an interesting paper especially as it comes from a source with no “spin” on the AGW debate.
The way I read the paper is it gives strong support for the conclusions of the famous Woods experiment.
Basically the project was to find if it made any sense to add Infra Red absorbers to polyethylene plastic for use in agricultural plastic greenhouses.
Polyethylene is IR transparent like the Rocksalt used in Woods Experiment.
The addition of IR absorbers to the plastic made it equivalent to “glass”
The results of the study show that( Page2 )
…”IR blocking films may occasionally raise night temperatures” (by less than 1.5C) “the trend does not seem to be consistent over time”
Conclusion is that it makes almost no difference whether the material radiates or not.
http://www.hort.cornell.edu/hightunnel/about/research/general/penn_state_plastic_study.pdf
There’s no sense trying to engage the sky dragon nutters on an intellectual basis.
The steel shell analogy does break down when you consider the atmosphere as a fluid instead of a solid.
Hot and cold air units circulate.Hot moves farther from the surface. The farther a unit of atmosphere gets from the surface, the less the earth obstructs its radiation into space. – just like your hand blocks more or less light as you move it closer or farther from your eyes.
Net result: Atmosphere is less reflective than a steel shell, and also because it circulates.
It’s been 30 or so years since I studied heat transfer in engineering school, but I seem to recall radiation heat transfer being proportional to delta-T to the fourth power, hence the shell would be radiating much (much!) more IR out to (nearly) absolute zero space than to the warmer planet surface. And as for the radiators-in-a-room example, I do believe that there indeed would be no IR radiated from the cooler unit to the warmer unit, but just in that particular direction — exactly at the points normal to the warmer radiator. I believe it would continue to emit IR in all other directions, however.
The biosphere is still in existing. I think the University of Arizona is operating it. Why not just carry out an experiment on the effect of various carbon dioxide concentration on the temperature inside the sphere. The experiment could be a super simplification but at least there will be some empirical data rather than all the computer models and assumptions.
Your diagram appears to be missing a glass plate that covered both enclosures to stop IR_in being a confounding factor. The relevent quote is
“In order to eliminate this action the sunlight was first passed through a glass plate.”
I’m probably wrong, but wouldn’t In order to eliminate this action the sunlight was first passed through a glass plate. imply a second glass plate above both the glass and rock salt plates in order to condition in incoming spectrum to be the same for both boxes?
Please pardon me if that’s silly or stupid. I’m too old and fuzzy minded to work through stuff like this quickly. I think I may have been smarter/quicker about 5 decades ago. Or at least I thought I was smarter/quicker.
Somehow I can’t see 235w radiating energy into 236w.
This: http://www.ems.psu.edu/~fraser/Bad/BadGreenhouse.html is in my opinion a much simpler, more elegant explanation.
Noud
Mike M says:
February 6, 2013 at 12:49 pm
“Conservation of energy would be measured as watts versus watts ”
Watts are a unit of POWER, that is joules/ second.
ENERGY is measured in joules. There is no law of conservation of power.
The earth get energy for only part of the day. At night half of the surface only loses energy.
Introducing the time dimension, that is averaging over 24 hours, as in this model and Trenberth’s, is not realistic.
Mike M says:
February 6, 2013 at 12:49 pm
I tried to head this incorrect argument off at the pass, but I was not emphatic enough. What I said was:
It is an acceptable simplification for a first-order analysis such as this one because the exterior surface are of the shell is approximately equal to the surface of the sphere ( less than half a percent difference).
If the shell size increases to infinity, you’re no longer in this thought experiment, but in a very different one.
w.
Silver Ralph says:
February 6, 2013 at 12:56 pm
There is a coolish radiator, emitting a small amount of infrared heat into a cold room. Then, another warmer radiator is placed in the room, a meter or so from the cooler radiator. Does the cooler radiator suddenly stop emitting infrared? And why would it do so?
Silver Ralph which radiator or combination of radiators determines the maximum temperature the room can attain? And why?
From a radiative heat transfer stand point picture two in wrong. As soon as Tsphere and Tshell are the same W/m^2 goes to zero. q/a= e SB (T1^4-T2^4) The surface area of the shell dictates that heat will only go in one direction from sphere to inner shell to outer shell to space.
Sarge, Joe Public,
If you read the whole piece, you will see that Willis did address that issue.
I’ve added the following update to the head post:
w.
TimTheToolMan says:
February 6, 2013 at 1:50 pm
True, thanks, Tim. I’ve updated the drawing.
w.
One atom of the planet transfers one unit of energy to one atom of the shell. The shell can radiate in any direction. How many units of energy does it have available to transfer to space and to the shell?
mikerossander says:
February 6, 2013 at 1:07 pm
Indeed, you can, you just need vacuum. Crazy but true, do the math. For each additional layer, you get an additional multiplication, i.e. 1 shell doubles the watts, 2 shells triples the watts, and so on. Of course, this does not double or triple the temperature. If you double the watts, the temperature goes up by only 20%, and tripling the watts only raises it by 30%.
w.
The number of errors in just the fundamentals associated with this thread, make it beyond redemption into anything associated with science.
[perhaps you would like to point these errors for the benefit of all. thanks . . mod]
The example and analysis of the steel shell given above is completely correct. If one was to add a second shell around the first the effect would be still greater. In fact, there is a form of commercial insulation made up of many layers of aluminium foil stacked one on top of the other which is highly effective and works on exactly this principle. It is also a commonly performed science experiment at secondary school level.
The claim that if there was sufficient conduction (or convection) so that the surface and steel shell were at the same temperature the effect would disappear is also correct. However in that case the radiation from surface to shell and from shell to surface would be the same so that radiation would no longer play a part – in effect the surface and the shell would become all the one body.
The thing is that in our planetary system the condition in the above paragraph is NOT met. The equivalent of the steel shell is the tropopause and it is NOT at the same temperature as the surface, it is quite a bit colder. In fact there is a non zero lapse rate between the surface and the tropopause whcih maintains that temperature difference. Why do I say the tropopause is the equivalent of the steel shell? Because this is the effective top of the CO2 and H2O columns and it is these gases that are capable of absorbing and radiating energy (oxygen and nitrogen do not absorb or radiate in the thermal infrared range of wavelengths).
The “steel shell” is not in fact opaque at all wavelengths, only at the green house gas wavelengths. Without green house gases there would be no shell and the surface would radiate freely to space. If green house gases absorbed all wavelengths the shell would be the equivalent of a steel shell and the surface temperature would be raised by in effect the integrated lapse rate from surface to shell. That explains why the temperature on Venus is so high. The extremely high concentration of GHG has caused so much line broadening that the GHG intercept virtually all long wave radiation and the atmosphere is so thick that the integrated lapse rate is huge.
With regard to the box experiment, this is complicated by the fact that a box as described can lose energy by radiation, convection or conduction whereas a planet surrounded by vacuum can only lose energy by radiation. The popular explanation is that the covered box works by blocking convection but consider the following. If convection occurs from the hot surface of the box why would it not also occur from the hot surface of the glass? Further if we argue that the important effect is interception of long wave radiation we again have a problem. If the glass is an effective absorber of long wave radiation it is also an effective emitter of long wave radiation so why would the glass if at the same temperature not emit as much long wave radiation as it absorbs? In that case it would make no difference whether or not the glass absorbed long wave radiation or not. Thus if the glass and box were at the same temperature, conduction, convection and radiation would be unchanged and there should be no difference in temperature.
The only answer to this apparent paradox (since clearly the glass covering does work) is that the glass is colder than the surface of the box – like the steel shell analogy. In fact what I think will be found is that the dominant energy transfer mechanism between box and glass is convection and that this is far from infinite so that there is a substantial temperature difference between box and glass, which is much closer to the steel shell example than one might think.
There are many inferences from this that should be testable but this post is already long and I think its appropriate to let others comment on the above first
cheers
Mike Hammer
I recently showed a film during middle school science class entitled “Heat”. The film makers conducted an experiment as follows.
4 identically sized double walled flasks: one no vacuum with silver coating, one no vacuum no silvering, one with vacuum with silvering, one with vacuum no silvering. Each flask equipped with a digital temperature sensor and insulated stopper.
Equal measures of water heated to the same temperature were introduced into each flask. Heat loss was measured over time and compared between the 4 flasks.
Result: Heat loss from the two vacuum flasks was markedly less than the two non-vacuum flasks. (I don’t recall any of the numbers). Heat loss from the two silvered flasks compared to the counterpart non-silvered flasks was minimal.
Their conclusion: In this experiment the predominant mechanism for heat loss was convection and conduction. Radiative heat loss accounted for a tiny fraction of total heat loss.
The term Green House Gas is a misnomer. Greenhouses work by stopping convective heat loss with a mechanical barrier. The equivalent barrier to convection in the atmosphere is called the tropopause. Greenhouse operators introduce enriched CO2 atmospheres into greenhouses to enhance growth, not to reduce radiative heat loss. Does this mean that CO2 does not change the radiative balance? No, radiative loss only becomes dominant at and above the tropopause which is above 80% of the worlds atmosphere. In other words above 80% of the atmosphere’s CO2. So I guess my question is: Do the radiative model calculation outputs need to be reduced by 80% to bring them into conformance with the real world?
Just asking.
Over at Climate, Etc., Pekka Pirilä says (February 5, 2013 at 5:27 am):
“It’s not totally clear what would happen for the atmosphere in total absence of all radiative gases, i.e. with exactly zero emissivity/absorptivity. I have been arguing for the mostly isothermal atmosphere but others have argued that the diurnal and latitudinal variability could still maintain circulation over an altitude range comparable to the present troposphere. As I haven’t heard of any credible analysis of this case i consider the case open.”
What about people who tried the standard scientific procedure of reproducible results? Apparently more “nutters”.
http://principia-scientific.org/supportnews/latest-news/34-the-famous-wood-s-experiment-fully-explained.html
Further to my previous post, from what I said there one would predict that two layers of glass over the box, separated from each other, would be more effective than one just as two concentric steel shells would be more effective than 1. In fact this is exactly the case and some glass houses are now made with two transparent layers (usually thin plastic not glass) for exactly that reason. Especially effective at retaining heat over night.
I am struggling with this. I am not a Sky dragon, and I accept the GH theory. But your diagram implies that there is no reason for heat energy to flow from a high energy state to a lower energy state. For instance, when a CO2 molecule is struck by radiation from the sun, it assumes a higher energy state than its surroundings. If, somehow, it were the last low energy CO2 molecule in the atmosphere, it would not emit the radiation. A blackbody the size of the universe, would emit no radiation, because there would be no lower energy state for the energy to flow to.
Now, let’s say that instead of a steel shell or atmosphere you envelop the radiating planet with water. The water has a much greater heat capacity than air or vacuum…
I’ve updated the drawing.
>>>>>>>>>>>>>
Which makes the problem worse. How far above the two boxes is the extra layer of glass? Glass absorbs LW, conducts well, and also radiates. So, upward LW from the rock salt box hits the glass, is absorbed, conducted, and re-radiated toward both the rock salt covered box and the glass covered box. Then you’ve got the glass in the glass covered box heating up by conduction, causing it to radiate, and the LW that it radiates being absorbed by the higher level of glass which then conducts and re-radiates to both boxes as well. The LW radiated from the upper layer of glass heats both boxes, one directly and one by heating the glass shield which then heats the box below by both radiance and conduction.
All of which happens at an order of magnitude in which the direct effects are easily measured with a glass thermometer but which isn’t even close when comes to the accuracy required to measure LW absorption and re-radiance.
Michael Moon says:
February 6, 2013 at 1:11 pm
So you agree that it is re-emitted. To be re-emitted, it had to be absorbed. You just say it happens really fast.
Actually, once energy is absorbed, there’s no way to distinguish it from any other energy, so there’s no way to tell when that particular energy was re-emitted.
Finally, you are correct that it transfers no heat, but not for the reason you think. Heat is NET energy transfer, which is different from radiative energy transfer from one body to another. It is the latter we are discussing, energy transfer.
Radiative energy doesn’t care where it is coming from or going go, or what the temperature is on either end. If I light a candle on the earth during the day, the sun ends up warmer than it would be if I didn’t light the candle. Of course the reverse is true as well, the candle ends up warmer than if there were no sun. Since NET heat flow is from the sun to the candle, no thermodynamic laws are broken … but that doesn’t mean that the light from the candle is not absorbed by the sun. It is definitely absorbed, and the sun ends up warmer because of that radiation.
Physics. Don’t leave home without it.
w.
W – “Seems to me like with a few small changes it could indeed be a valid test, however.”
Been there, done that –
http://i49.tinypic.com/34hcoqd.jpg
Two insulated boxes with double glazed LDPE film windows. Circulation fans and thermometers shielded from incoming light and out going IR. Matt black aluminium target plates. Halogen lights. One box filled with CO2 the other air. Which box heats up faster? Which box cools slower when the lights are switched off?
Answer – Over a 20 degree temperature change there is no measurable difference between the boxes using an electronic dual probe thermometer with 0.1 degree resolution. The reason is that while CO2 can intercept some outgoing IR from the target plate, it can also radiate energy it has acquired conductively from the target plate.
Willis you were almost there when you wrote on another thread –
W – “Some gases most assuredly absorb and emit infra-red, some gases don’t. In a mixture of the two, those that do absorb infrared immediately (nanoseconds) pass that energy on via collisions to the other gases that do not absorb or emit infrared and thus warm the mass of air. The reverse is true when they emit infrared, within nanoseconds they absorb energy from the other gases and cool the mass of air.”
However in modelling the role of radiative gases in the atmosphere the shell game is the wrong approach. This is the failed model used by the AGW pseudo scientists. It involves most of the “Do Nots” of atmospheric modelling. Let’s review –
1. Do not model the “earth” as a combined land/ocean/gas “thingy”
2. Do not model the atmosphere as a single body or layer
3. Do not model the sun as a ¼ power constant source without diurnal cycle
4. Do not model conductive flux to and from the surface and atmosphere based on surface Tav
5. Do not model a static atmosphere without moving gases
6. Do not model a moving atmosphere without Gravity
7. Do not model the surface as a combined land/ocean “thingy”
Avoid the “Do Nots” and you will fine the true role of radiative gases in the atmosphere. They cool at all concentrations above 0.0ppm. Without radiative gases full convective circulation below the tropopause will stall and the atmosphere will heat. Build this experiment to find out why convective circulation is critical to atmospheric temperatures and why radiative gases are critical to convective circulation –
http://i48.tinypic.com/124fry8.jpg http://tinypic.com/r/zmghtu/6 http://i49.tinypic.com/2a106x.jpg
Where are almost all the radiative gases in the atmosphere? Below the tropopause.
Where does almost all the vertical convective circulation occur? Below the tropopause.
Adding radiative gases to the atmosphere will not reduce the radiative cooling ability of the atmosphere. Play the shell game with the AGW pseudo scientists and you will always get the wrong answer.
And for the record – No, I am not one of the “slayers”. I do accept that the atmosphere radiates IR back to the surface. There is no easy out there.
mikerossander says:
February 6, 2013 at 1:07 pm
I am struggling with your thought-experiment because it seems to imply that you could raise the temperature of the planet to any arbitrarily high level by wrapping it in additional shells of steel.
**************************************
It implies it because it is true. Examine the construction of a high-end vacumm flask. Of course, the vacuum between the outside and the inside is to eliminate the gas conductive/convective heat transfer mechanism. But the good flasks also have multiple thin silverized plastic sheets that act as radiation barriers (combination of reflective and absorbed re-admitted) with non-thermally-conductive sheets in between. This is more difficult and expensive to manufacture than a single radiation barrier, but it is much more effective at reducing heat transfer.
If there were a heat source in the inner chamber (e.g. an electrical resistance heater) adding energy at a constant rate, each additional layer of radiative barrier would result in a higher temperature in the inner chamber.
bonsoir Mr Eschenbach
j’ai un problème avec votre figure 2
le “core” émet 235 W correspondant à une t* d’mission, disons TA et chauffe la sphère d’acier dont la t° s’établit (oublions les surfaces) à une t° correspondant à cette énergie reçue,soit TA
cette sphère émet à la fois vers l’extérieur et vers l’intérieur,(selon vous) mais de ce fait la surface d’émission à ainsi doublé!(surface extérieure plus surface intérieure)
et comme l’énergie reçue reste 235 w, et que pour rester en équilibre, la sphère d’acier doit émettre 235 w, ,on voit immédiatement que celle-ci n’a pu réémettre vers l’intérieur
en effet , dans votre configuration, il n’y a aucune raison que la t° de l’intérieur de la sphère soit différente de la t° de l’extérieur.
or pour rester en équilibre, il nous faut TA à la surface extérieure, et aussi, TA à l’intérieur, et dont la source doit émettre 2 fois plus d’énergie.
mais nous ne disposons que de 235 w
Maintenant, imaginons, que la surface de la sphère épouse parfaitement le core, et que la conduction soit parfaite,
croyez vous que sous la pellicule d’acier la t* du core va doubler?
Mr. Spencer nous avait déjà bien occupé avec ce petit paradoxe,(Yes Virginia…) dont mes amis et moi, n’avons jamais pu trouver une formulation valable(nous en discutons fréquemment sur le site suivant
http://www.skyfall.fr/
Quoi qu’il en soit, merci pour vos récits , je suis un voileux
W – “Seems to me like with a few small changes it could indeed be a valid test, however.”
Been there, done that –
http://i49.tinypic.com/34hcoqd.jpg
Two insulated boxes with double glazed LDPE film windows. Circulation fans and thermometers shielded from incoming light and out going IR. Matt black aluminium target plates. Halogen lights. One box filled with CO2 the other air. Which box heats up faster? Which box cools slower when the lights are switched off?
Answer – Over a 20 degree temperature change there is no measurable difference between the boxes using an electronic dual probe thermometer with 0.1 degree resolution. The reason is that while CO2 can intercept some outgoing IR from the target plate, it can also radiate energy it has acquired conductively from the target plate.
Willis you were almost there when you wrote on another thread –
W – “Some gases most assuredly absorb and emit infra-red, some gases don’t. In a mixture of the two, those that do absorb infrared immediately (nanoseconds) pass that energy on via collisions to the other gases that do not absorb or emit infrared and thus warm the mass of air. The reverse is true when they emit infrared, within nanoseconds they absorb energy from the other gases and cool the mass of air.”
However in modelling the role of radiative gases in the atmosphere the shell game is the wrong approach. This is the failed model used by the AGW pseudo scientists. It involves most of the “Do Nots” of atmospheric modelling. Let’s review –
1. Do not model the “earth” as a combined land/ocean/gas “thingy”
2. Do not model the atmosphere as a single body or layer
3. Do not model the sun as a ¼ power constant source without diurnal cycle
4. Do not model conductive flux to and from the surface and atmosphere based on surface Tav
5. Do not model a static atmosphere without moving gases
6. Do not model a moving atmosphere without Gravity
7. Do not model the surface as a combined land/ocean “thingy”
Avoid the “Do Nots” and you will fine the true role of radiative gases in the atmosphere. They cool at all concentrations above 0.0ppm. Without radiative gases full convective circulation below the tropopause will stall and the atmosphere will heat. Build this experiment to find out why convective circulation is critical to atmospheric temperatures and why radiative gases are critical to convective circulation –
http://i48.tinypic.com/124fry8.jpg http://tinypic.com/r/zmghtu/6 http://i49.tinypic.com/2a106x.jpg
Where are almost all the radiative gases in the atmosphere? Below the tropopause.
Where does almost all the vertical convective circulation occur? Below the tropopause.
Adding radiative gases to the atmosphere will not reduce the radiative cooling ability of the atmosphere. Play the shell game with the AGW pseudo scientists and you will always get the wrong answer.
And for the record – No, I am not one of the “slayers”. There is no easy out there.
Roger Clague says
“Watts are a unit of POWER, that is joules/ second.
ENERGY is measured in joules. There is no law of conservation of power.”
No, but thermal equilibrium is the condition described by zero net energy trasfer, or net zero joules per second, right? Its not a law, its a condition.
Please remove the all caps. Do you talk to people that way in person?
All these analogies are amusing but ignore Second Law.
>>>>>>>>>>>>>>>
You can’t pick and choose which laws of physics to use and when. They all exist at the same time. If the 2nd Law operates as you suggest, then it falsifies SB Law. You can have both, or neither, but you can’t have one and not the other.
Guest Post by Willis Eschenbach: “Pushed by a commenter on another thread, I thought I’d discuss the R. W. Wood experiment, done in 1909. Many people hold that this experiment shows that CO2 absorption and/or back-radiation doesn’t exist, or at least that the poorly named “greenhouse effect” is trivially small.”
==============================================================
I guess I am the “commenter on another thread”. I do not “hold that this experiment shows that CO2 absorption and/or back-radiation doesn’t exist”. Nor have I ever heard anyone claiming that. Nor did professor Wood hold that.
The Wood experiment demonstrates that “trapped/back radiation” has zero or negligible effect on the temperature of the source.
And this demonstrates that the underlying mechanism of the “greenhouse effect” as presented by the IPCC (http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-1-3.html) does not work at all or is negligible.
With the same internal heat source, the outgoing W/m^2 simply decrease with the increased surface area, but the total heat escaping will remain the same as that supplied by the internal source. Everything else is irrelevant garbage.
I’ve started using a IR non-contact thermometer to measure the temp of the sky on clear days, and so far on a 35F day, and a 28F day, it’s been lower than the temp the thermometer will read -40F. On a ~50F day, it read ~-35F.
My thermometer is measuring “back radiation”. Back radiation does add energy to anything it shines on, but the rate of transfer is very low, and while my 50F black driveway is getting radiated on, being at least 90F warmer, the rate of energy it’s radiating into space is much much higher than the other way around.
I plan to start logging the sky’s temp and logging it and air temp and humidity. I just have to wait for clear days, which I don’t get very often in NE Ohio.
From my work looking at the surface temperature record and night time cooling, I expect to see that humidity controls surface temps.
If the outer shell is radiating at 2x the flux of the inner core its temperature would have to be much higher than the inner core. How is that possible?
MiCro says:
February 6, 2013 at 2:42 pm
Is suppose to be “my 50F black driveway”
[Fixed. -w.]
Hi Willis!
In your own example with the metal sphere, or the shell, the back radiation to the planet will be as you describe. If you add a second shell the feedback process will start again and the outer shell will be the one radiating 235 W/m^2 (apart from differences in the areas, but don’t take that into consideration for practical means), and this will add another 235 W inwards which the planet will absorb and reradiate together with the original 235 and the first added 235, now resulting in 705 W/m^2 and a raised temperature at the surface as well. For each added shell there will be an additional 235 W/m^2 back to the planets surface. If the starting radiation is E, the full radiation from the surface with n shells will be E + n*E.
This is the same model as can be found in textbooks of climate physics, but now the metal shells are layers of air and the absorption materials are the greenhouse gases. The model looks convincing. But is it a reality? The energy budgets says that a little less than half of the incoming radiation reaches the surface of the earth as a mean value, and even less is pure reradiated energy that can start the process mentioned above. As an average value about 50-60 W can be an estimate for incoming/outcoming starting radiation. If I chose 65 W as an example I will at least not exaggerate in my example ahead. The mean surface temperature of the earth is said to be 15 C based upon measurements. And based upon this again, the mean radiation from the surface is said to be 390 w/m^2. So far so good. If the mean starting value is 65 W, the starting energy is multiplied by six by the greenhouse effect, 390/65 = 6. This would give an atmosphere of five completely absorbing/reradiating layers in the perspective of overall energy. But if this is good science for an average view, it also have to hold for a more special situation like a tropical desert with a zenith sun from a clear sky with very little water vapor. 1367 W/m^2 is coming in. Without an atmosphere it will give 120 C at the surface if it all is absorbed and reradiated, which is the common view. Well, some of it will be absorbed by the atmosphere. How much? What about 20%, that will leave us with about 1094 W/m^2 and a temperature of about 100 C according to Stephan-Boltzmans equation. That’s far beyond reality and still we haven’t taking the greenhouse effect into consideration which should give (1094 W/m^2)*6 = 6562 W/m^2 corresponding to a temperature of about 310 C, which is of course completely out of the question as the reality is about 55-60 C. And even if we take only half of the incoming radiation as start for the greenhouse effect, it will be far too much.
Will much more of the energy be transferred as convection? Maybe, but this can be tested precisely with the example of the Wood experiment, the one with the rock-salt plate. Because the rock-salt plate is transparent to both short and long wave radiation, both the start value radiation of 65 W in my example, and the added long wave back radiation up to 390 W will enter this greenhouse box and none of the energy will slip away due to convection because no convection is allowed. This little box should experience a full greenhouse effect and reach a temperature of more than 300 C. Well, as Wood showed, it didn’t. None greenhouse effect of that caliber. And even in the desert the atmosphere has a cooling effect, and for the most equals out the excessive conditions. Both ways. There is probably a certain greenhouse effect contributing to the convection, and greenhouse gases most probably give the atmosphere a certain kind of heat capacity that speeds up the energy flow and equals out the temperature, but a heavy back radiation? Hmm..
The basic physics is of course correct – but this hypothetical situation is not directly applicable to a planet warmed from an external source. For a start, the external radiation is both reflected and absorbed by the shell, and in a varying manner and in the case of a poorly conductive/convective and ‘reactive’ (or chaotic, if you prefer) atmosphere – the GHG properties are not constant. Once you have clouds, heat retaining liquid, surface solids, water vapour, aerosols, a ‘living’ Biosphere, etc, etc, etc – the situation is simply far too complicated to be realistically represented. Thus, when you realise just how complicated that it actually becomes in real life (as per our Earth) – it makes it even more highly suspicious to point to a SINGLE trace gas as a primary driver of GHG effect changes!
Further, when you then consider the actual carbon cycle and the natural CO2 present within the biosphere as a whole, and the potential natural variation of the ‘position/placement’ of CO2 within that biosphere (sinks and emitters!) – that adds even further complication.
Now, if someone wants to unravel that semi-chaotic non-linear mess and offer proof that CO2 based AGW is the proven real culprit, I’d be glad to hear it – as would millions of others!
Just sayin………..
What I meant was the outer shell is radiating over 2x the area of the inner shell at the same power so its temperature must be higher…
Vacuum between shell and planet = planet gets a lot hotter.
Perfect conduction between shell and planet = planet doesn’t get any hotter.
Imperfect conduction between shell and planet = AGW.
As KevinM has said above, AGW is real. The main argument is about how large or small the effect is.
Guest Post by Willis Eschenbach: ” let me give a curious example of the greenhouse effect, that of the Steel Greenhouse. Imagine a planet in the vacuum of space. A residue of nuclear material reacting in the core warms it to where it is radiating at say 235 watts per square metre (W/m2). … Now, note what happens when we add a shell around the planet. The shell warms up and it begins to radiate as well … but it radiates the same amount inwards and outwards. The inwards radiation warms the surface of the planet, …”
=============================================================
“Imagine”, I see.
I guess, it has never been proven experimentally that A warms B and then B warms A back, right? OK, this is a product of imagination, a fiction, and everyone has right to right a science-fictional story, no problem with that. But the readers need to be told clearly that this story is fictional, just to avoid confusion.
“The Wood experiment demonstrates that “trapped/back radiation” has zero or negligible effect on the temperature of the source.”
Unfortunately it doesnt test how the “greenhouse effect actually works and can never test that.”
The green house effect operates by raising the ERL. A raised ERL means a earth that radiates from a higher colder region. That means a slower rate of energy release to space and the surface cools less rapidly in response. back radiation is an EFFECT of the greenhouse effect not a cause. The theory is not that back radiation warms the source. It does not. The rate at which the source cools is slowed.
back radiation from the silver lining of a thermos does not warm the coffee. It slows the rate at whch the coffee cools and keeps it warmer than it would be otherwise. If that radiation shield is “leaky” the coffee cools more rapidily.
Simple terms: Woods doesnt test the greenhouse hypothesis.
That hypothesis is.
1. Adding C02 RAISES the level of the ERL. woods experiment and any closed container experiment cannot test this.
2. Raising The ERL cause the source to cool less rapidly. The surface is not warmed by back radiation which is better understood as an effect of GHGs rather than the cause of warming.
back radiation in a thermos doesnt raise the temperature of the coffee, it slows the rate of energy loss.
Woods tested some other theory, some strawman version.
Correction: Because the rock-salt plate is transparent to both short and long wave radiation, both the start value radiation of 65 W in my example, and the added long wave back radiation up to 390 W will enter this greenhouse box and none of the energy will slip away due to convection because no convection is allowed.
Here it should be not 65 W but 1094 W, and instead of 390 W there should be 6562 W according to the example of the tropical desert. Sorry.
I think I am headed back to “Ilikebacon.com”, this thread makes my eyes bleed.
TR
The Wood experiment demonstrates that “trapped/back radiation” has zero or negligible effect on the temperature of the source.
>>>>>>>>>>>>>>>
Yes, the joules of energy exist, they just don’t do anything. Let’s just change the definition of joule to suit our belief system and prove it using an apparatus that can’t possibly measure with enough accuracy to support such a conclusion. And let’s further propose that conclusion based on an experiment in 1906 in opposition to the findings of Wien, Planck, Einstein, Bohr, and Milliken’s Nobel prizes in 1911, 1918, 1921, 1922 and 1923 respectively.
I agree with mkelly about figure 2 Willis. I can see what you were trying to say there but the arrows and amounts are confusing as presented.
I think it could be reworked to just key on what you were trying to say. Adding the shell makes the planet warmer without affecting the enery balance. At the steady state, planet produces 235, shell emits 235, but the produced 235 bounces around between the shell and the planet effectively warming it.
Still thinking about the Wood experiment.
Don K says:
February 6, 2013 at 1:50 pm
I’m probably wrong, but wouldn’t In order to eliminate this action the sunlight was first passed through a glass plate. imply a second glass plate above both the glass and rock salt plates in order to condition in incoming spectrum to be the same for both boxes?
Please pardon me if that’s silly or stupid. I’m too old and fuzzy minded to work through stuff like this quickly. I think I may have been smarter/quicker about 5 decades ago. Or at least I thought I was smarter/quicker.
<<<<<<<<<<<<<<<<<
Woods set out to debunk the claim that specific gases, were slowing down the escape of a class of radiation. That radiation class was infrared light.
Woods let infrared in one box,
and stopped infrared from getting in another:
proving it wasn't the amount of infrared-class light getting in, OR out,
that assigned temperature in any measureable way in atmospheric air.
Claims of not being able to "see the point" are of those who don't WANT to see the point.
They have what's called a 'belief' system.
They BELIEVE in the effect, no matter how many experiments show them, it's erroneous fantasy.
Woods proved the claim of infrared-resonant gases, being in a fundamental way responsible for temperature assignment in atmospheric gas mixture, is utter falsehood.
When two boxes were put out in sun, and identical gases inside: atmospheric mix wherever he was –
the box which didn't let infrared light in, warmed identically in time,
with the box that let it in.
If the atmospheric gas mix was somehow holding infrared heat, creating detectable temperature readings, the box that let the infrared light in,
*would have warmed up faster. *
It didn't.
The one that had the infrared in, blocked,
*would have had to have waited for portions of the visible light coming in, to convert to heat, before temperature climb appoximated the other's.*
Anyone who says "they can't see what that proved," is simply trying to hang on to their self-prescribed "dignity"
and popularity.
Because we've got enough instruments from optical telescopes to infrared telescopes to
check on the stories about the magic gas.
And it's a bullshoot story.
Woods knew it,
anyone who sees Woods' experiment, who's honest, knows it,
and the only people who even still cling to it are those who staked their reputations as public figures on it's being real.
Nobody believes in that crap except what are called 'true believers.'
Allen B. Eltor says:
February 6, 2013 at 2:14 pm
Thanks, Allen. Since you haven’t pointed out even one of your claimed errors, your opinion is worthless. This is a scientific site. If you see an error, quote my words and explain why I’m wrong. Otherwise, you’re just blowing smoke.
Regards,
w.
Tim Ball says:
February 6, 2013 at 2:24 pm
Tim, always good to hear from you. Unfortunately, your comment isn’t clear. Who are the “natters” you refer to?
w.
Konrad says:
February 6, 2013 at 2:34 pm
Thanks, Konrad. Unfortunately, that’s a totally different experiment that doesn’t help with this question. I fear it tells us nothing about whether the Woods experiment gives results valid for a planetary-sized greenhouse.
Remember, for the greenhouse to function, the shell has to be thermally insulated from the surface and thus able to adopt a different temperature. If not, then the shell will be at the same temperature as the surface and there will be no greenhouse effect.
w.
Greg House – “I guess, it has never been proven experimentally that A warms B and then B warms A back, right? OK, this is a product of imagination, a fiction, and everyone has right to right a science-fictional story, no problem with that. But the readers need to be told clearly that this story is fictional, just to avoid confusion.“.
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
The advantage of Roy Spencer’s example is that it can be set up and tested in a lab.
To my mind, it would be a very good idea for someone to actually do the experiment, properly, and (if Roy Spencer is correct) lay to rest the argument that AGW violates the 2nd law of thermodynamics.
Mike Jonas says:
February 6, 2013 at 2:57 pm
I don’t disagree in principle but as I observed earlier, in real life, we have a planet warmed from external radiation (with all the other associated effects) and also I take exception to using the term AGW instead of GHE (greenhouse effect) as that term is a false premise.
The top pain of glass was to make sure the IR that was reaching the boxes were equal in wavelength. Then the transfer was measured as longer wavelengths deduced by the temperature reading. Is this right?
joletaxi says:
February 6, 2013 at 2:36 pm
Si je vous comprends, je pense que le problème est dans les unités. Vous utilisez watts, et je me sers de watts par mètre carré. La coque reçoit 470 W m-2 de la surface, et parce qu’il a deux fois la superficie que la surface, il rayonne 235 W m-2 à la fois vers l’intérieur et vers l’extérieur.
Je suis heureux d’entendre que vous êtes un marin, pour moi, il ya toujours plus à apprendre sur la mer …
Bonne chance, prenez garde, parce que l’océan ne se soucie pas du tout …
w.
Greg House says:
February 6, 2013 at 2:41 pm
In this case, Greg, actually it’s not all about you, your guess is wrong … sorry, but there it is.
I quoted what Wood said. You can find examples of people claiming it means the greenhouse effect doesn’t exist all over the web. See the misunderstandings on this thread as one of far too many examples.
w.
I believe you touched on a point I’ve made that I’m surprised isn’t much more widely appreciated and distributed. The Catastrophic Anthropogenic Climate Change Alarmists state, as “proof” of their theories humans are destroying the planet through Dangerous Anthropogenic Global Warming, the apparent ESTIMATES that one amount of energy is coming to the Earth from the sun and a different, lower amount of energy is escaping from the Earth back to space. Where is the missing energy, they shout. Why Anthropogenic carbon dioxide in the atmosphere must be trapping it! Only we’re not seeing the necessary changes that would cause.
So where does the missing energy go? Anyone? Surely you know, it was mentioned (to some extent) in this article.
It goes to take part in various physical, chemical and life processes here on Earth, that’s where. It drives the winds, convection in the atmosphere. It moves huge quantities of ocean waters. It evaporates water from any and all places it is present on the surface and subject to being evaporated. It powers many endothermic chemical reactions. It allows plants to grow and creates “free” energy through the “magic” of the photoelectric effect. And, if after estimating and accounting for ALL THAT ENERGY, there is still a discrepancy, I have an answer for that, too.
Check your math, you didn’t carry or borrow correctly somewhere.
TomR,Worc,MA says:
I think I am headed back to “Ilikebacon.com”, this thread makes my eyes bleed.
________
As with most things, Science tastes better with bacon.
How is it possible for the shell to be emitting twice as much energy as the source? I think Figure 2 should be showing half being returned to earth and half going out to space. The temperature of the shell would then be lower than the earth based on Stephan-Boltzman.
Roger Clague says: “There is no law of conservation of power.”
Power is merely the flow of energy which is the topic here – heat.
Willis: “I tried to head this incorrect argument off at the pass, but I was not emphatic enough. What I said was: ….”
No need – Sorry, I missed the caption because I was mesmerized by the pretty graphic…
Steven Mosher says, February 6, 2013 at 2:59 pm: “The green house effect operates by raising the ERL. A raised ERL means a earth that radiates from a higher colder region. That means a slower rate of energy release to space and the surface cools less rapidly in response. back radiation is an EFFECT of the greenhouse effect not a cause. The theory is not that back radiation warms the source. It does not. The rate at which the source cools is slowed.
===========================================================
First the less important part about “warming vs slowing down cooling”. Normally, people would call a slowing down cooling effect a warming effect, too. I nevertheless prefer saying “affect the temperature” or “have an effect on temperature”. And, again, the Wood experiment demonstrates that trapped/back radiation has a zero or negligible effect on the temperature of the source. By the way, do you have any problem with “global warming” being called “global warming” and not “reduced global rate of cooling” (LOL)?
Second, your “ERL” etc is absurd (http://wattsupwiththat.com/2012/08/30/important-paper-strongly-suggests-man-made-co2-is-not-the-driver-of-global-warming/#comment-1068226) and, more important, not the politically relevant “greenhouse effect” as presented by the IPCC. You and anyone else is absolutely entitled to come up with whatever hypothesis on anything, but these private “greenhouse effect” hypotheses have zero political relevance. The official concept of the IPCC is different. And they mean exactly that very old concept of the effect of trapped/back radiation professor Wood so easily debunked back in 1909.
Here is the official version of the “greenhouse effect” as presented by the IPCC: “The Sun powers Earth’s climate, radiating energy at very short wavelengths, predominately in the visible or near-visible (e.g., ultraviolet) part of the spectrum. Roughly one-third of the solar energy that reaches the top of Earth’s atmosphere is reflected directly back to space. The remaining two-thirds is absorbed by the surface and, to a lesser extent, by the atmosphere. To balance the absorbed incoming energy, the Earth must, on average, radiate the same amount of energy back to space. Because the Earth is much colder than the Sun, it radiates at much longer wavelengths, primarily in the infrared part of the spectrum (see Figure 1). Much of this thermal radiation emitted by the land and ocean is absorbed by the atmosphere, including clouds, and reradiated back to Earth. This is called the greenhouse effect.”
http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-1-3.html
KevinM says:
February 6, 2013 at 2:40 pm
“No, but thermal equilibrium is the condition described by zero net energy trasfer, or net zero joules per second, right? Its not a law, its a condition.”
This Eshenbach model and Trenberth’s assume Watts/m2, that is Joules/sec/m2 in and out at the surface and in and out at the shell are equal at each place.
They apply the Law of Conservation of Energy and average horizontally over time and space. They add 2 extra dimensions . It is not a condition it is an incorrect application of a law.
The Law of Conservation of Energy can be correctly applied to a small vertical column of air. The column does not vary with time . Troposphere height is constant from day to night.
mgh = mcT
m = mass of a small volume of air
h = height above surface ( variable)
T = temperature ( variable )
c = heat capacity ( almost constant for small molecules )
g = acceleration due to gravity ( constant )
cancelling m and rearranging
h/T ( lapse rate ) = c/g
This shows that the temperature of the troposphere at any height ( including at the bottom, the earth’s surface ) does not depend on its composition, such as the % of CO2 in it.
Willis, nice, but not even wrong.
The total surface of your shell is not off by 0.3% from surface of your sphere, it is off by ~200%, the sphere has both an interior surface and and exterior surface. Assuming the shell is directly above the sphere and is infinitely thin then the error becomes exactly 200%.
Also, to do a proper energy budget you need to keep real energy sources separate from redirected energy flows. The energy from your sphere is an energy source (real energy input to the system supplied by breaking chemical bonds). The energy returning to the surface from the shell is just a redirected energy flux. Sure you can add them, but not if you want a useful answer. It’s like getting two tens as change for a five dollar bill.
As an empirical example; after space going satellites are assembled on the ground they are tested inside large vacuum chambers. So start with a satellite with no energy supplied (all electrical circuits turned OFF) at room temperature (a source of stored thermal energy), roll it into a steel vacuum chamber (also at room temperature). Close the door and evacuate the air (also at room temperature). Per your example the satellite (radiating it’s stored heat) would heat the vacuum chamber walls and the temperature of the satellite would rise. EXCEPT when you do this NOTHING MUCH happens to the temperature of the satellite. Assuming whatever structure is supporting the satellite (the floor of the chamber for example) remains at room temperature the satellite and vacuum chamber walls do not change temperature. I have witnessed this empirical experiment many times, what you describe DOES NOT HAPPEN, not even once, since satellites are fairly expensive and surprisingly delicate we would sure as heck notice if it started heating up. And it’s not because we don’t watch the temperature of the satellite, they are covered with tens of temperature sensors (telemetry sensors they are called in the trade).
Of course if we turn on electrical systems on the satellite the waste heat will cause the satellites temperature to rise, but it would also rise if you wrapped it with normal thermal insulation.
What really happens in your steel shell example (assuming an infinitely thin shell with infinitely high velocity of heat) is that at time = zero the shell has no thermal energy stored inside it. As the sphere radiates the shell “backradiates” zero. As the shell heats up it eventually “backradiates” exactly an amount equal to the radiation from the sphere and they reach the same temperature. The outside of the shell is now re-radiating energy from the real energy source (the sphere). Once one little tiny bit of energy is radiated by the shell it is immediately replaced by a little tiny bit of energy radiated by the sphere and the temperature remains at equilibrium. Of course since nothing useful is actually infinitely thin or has an infinitely high speed of heat delays are involved and no real system exhibits “thermal equilibrium”.
Thermal equilibrium is strictly a textbook creature, nobody has ever bagged an example in the wild.
Cheers, Kevin.
Whoops, my surface area error figure should have been 100% (2x shell surface area, 1x sphere surface area, thus (2-1)/1 = 100% error).
Cheers, Kevin.
Willis, for the outer shell to raise the temperature of the inner core its temperature must be higher than the inner core right? How can you raise the temperature of the outer shell higher than inner core so that there is net energy flow between the outer shell and inner core (a necessary condition to raise the temperature of the inner core) ?
The system will not reach an equilibrium if you posit that the outer shell will heat the inner core. The temperature will just rise higher and higher.
Great explication, WIllis. I particularly liked
“if for example the shell were held up by huge thick pillars that efficiently conducted the heat from the surface to the shell”
because, in fact, convection in the atmosphere – storms, clouds, winds, etc. are the huge thick pillars that reduce the temperature differential between Earth’s surface and the top of the atmsophere.
Haven’t read the comments but…if you put a steel shell around the earth aren’t you shielding the earth from the sun and wouldn’t that lead to immediate cooling?
Mike M says:
February 6, 2013 at 3:52 pm
Roger Clague says: “There is no law of conservation of power.”
Power is merely the flow of energy which is the topic here – heat.
The flow of energy to the earth is not constant. During daytime, energy flow in and out, but during
the night energy flow out but no energy flows in.
The Law of Conservation of Energy can only be applied vertically not horizontally and gives us the result
h/T = c/g.
As is confirmed by observation.
Steven Mosher says:
February 6, 2013 at 2:59 pm
“The green house effect operates by raising the ERL. A raised ERL means a earth that radiates from a higher colder region.”
———————————————————————————————————————-
No, running back to the ERL thing won’t work. The ERL game was only cooked up after it became impossible to ignore that most of the energy that radiative gases radiated to space was acquired through conduction and release of latent heat, not IR from the surface.
And of course we can see cloud tops radiating strongly in IR images from space. Far hotter than the surrounding air at their altitude. The altitude of radiative gases provably does not set the temperature of much of those gases at the time they are radiating the most IR. Try again.
Stephen Rasey
“As we reduce Rs to approach Rp in the limit, then Tp > Tsi and an infinite temperature gradiant which seems to be a logical impossibility.”
Firstly, without a conductive medium there is no gradient – was that not the reason why he stated no atmosphere.
Secondly, if Rs = Rp then the surface of the planet changes and will be first heated to a temperature at which it starts emitting at the equilibrium state; the surface energy state is doubled – but more importantly you’d also have conduction to deal with. The point of why the shell is off-surface is to avoid the point that Willis was trying to make: convection + conduction provide methods for thermal transfer from lower to upper atmosphere; however thermal conduction does not always translate to radiative transfer at some point and the conductive process obviously stops at the atmosphere-space interface, so it may just start doing different work such as expanding the volume of the total atmosphere rather than releasing IR to space (I don’t know). BTW a nominal distance will do for the purposes of the experiment.
Mike Jonas says, February 6, 2013 at 3:32 pm: “http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
The advantage of Roy Spencer’s example is that it can be set up and tested in a lab.
==========================================================
This is a wonderful idea real experimental testing, Mike, thank you. Has Roy Spencer done it yet?
Hello Willis;
If you have time please look at my post at 2:17. The glass covered box is far more interesting than it appears at first sight. Neither prevention of convection nor interception of thermal IR by the glass should make any difference at all if the glass is at the same temperature as the inside of the box. It will only work if the glass is colder than the inside of the box. The analogy to the steel shell is far closer than one might think at first sight.
cheers
Mike Hammer
“..Somehow I can’t see 235w radiating energy into 236w….”
There is a lot of this type of thinking by commenters. The radiation coming from a bright flashlight, trained on a light beam from a weaker flashlight doesn’t prevent the radiation from the weak flashlight (the batteries in the weak one rundown just as fast with or without its adversary). The light filaments in both glow and burn energy which is emitted as light. Ditto, the radiators. This is akin to the crystallization of salts in saturated solution: equilibrium is reached when dissolution from the salt crystals just equals the crystallization of solid salt – it doesn’t stop the action. Imagine turning on a weak flashlight just before turning on the strong one trained on it. What happens to the radiation that took off from the weak one just before it was “flooded” by the strong one? The fellow holding the strong flashlight in the dark can still see the light from the weak one. What about stars in the southern hemisphere shining “on” the stars from the northern hemisphere?
Willis:
I was referring to a snide comment about “nutters” in an earlier post. Here is the link to the experiments for those who didn’t look last time.
http://principia-scientific.org/supportnews/latest-news/34-the-famous-wood-s-experiment-fully-explained.html
Willis wrote (re the Woods experiment);
“Many people hold that this experiment shows that CO2 absorption and/or back-radiation doesn’t exist, or at least that the poorly named “greenhouse effect” is trivially small.”
I hold that “backradiation” does indeed exist; after all the walls of your house are “back radiating” towards you all the time. Did you ever notice (for folks in the Northern climes) that no matter how warm the air in your room is you still feel a little bit chillier in the winter than the spring or fall ? Why ? Because in the winter the inter walls of your house are just a few degrees cooler than in the spring. Thus they “back radiate” less and you feel just a bit chillier even though your furnace is holding (as well as it can) the air temperature constant.
The poorly named “greenhouse effect” is in fact equal to ZERO, not “trivally small”.
When you design a heating system for the interior of a house you consider the room size and select a heat source (say electric baseboard heaters) to warm that volume to a desired temperature. You add some overhead for really cold days and add a thermostat to regulate the average temperature. You DO NOT subtract the “back radiation” from the walls from the size of your heaters. The “back radiation” IS NOT an energy SOURCE, no way, no how. It is just re-directed energy and since it’s moving at the speed of heat it has no effect on the average temperature.
Now of course if you replaced the thermal insulation in your walls with steel you would have a very uncomfortable house. The speed of heat through the steel walls would mean that your furnace would never keep up and it would be damn chilly.
Sorry Willis, but R. W. Woods was correct, the temperature rise inside a greenhouse is ONLY caused by the restriction of convection. The opaque nature of some materials at some wavelengths only delays the flow of energy through the system. A real thermal insulator slows the velocity of heat flow, slowing and delaying (via multiple passes) are not interchangeable effects.
Cheers, Kevin.
Willis,
Please take some physics courses, or stop talking about it. You are embarassing yourself in front of thousands.
Your post is sense-free. 235 Watts per square meter would heat the steel, which would be cooler than the planet because it is 0.3% larger. The steel would radiate to space, but transfer ZERO heat back to the planet, as we all know that the Second Law is true. If you do not think the Second Law is true, well, I will read your fun stories about dolphins but nothing more concerning physics.
Absorbed and re-emitted, transferring NO heat, was I not clear? How about, absorbed and re-emitted in an infinitesimal? Does that help?
Do you know what heat is?
Problem #2
Since this is a closed system (unlike the earth) which is heated by SW that is absorbed into both systems in an identical manner, the question becomes, is this a model of what happens in the atmosphere? It is not.
In the atmosphere, 100% of all energy leaving the system does so via radiance. In this experiment, energy leaves the system by conductance/convection plus radiance. The ratio of conductance/convection to radiance is so large that in a system this small it could only be measured by instrumentation that did not exist in 1906.
Problem # 3
The ratio of conductance/convection to radiance is so large that in a system this small it could only be measured by instrumentation that did not exist in 1906.
Since both boxes heat up to about the same temperature, and both the salt rock and glass radiate the same amount at the same temperature, we’d need to be able to measure the energy radiated directly by each versus the amount of energy radiated by the salt rock plus the LW that passed through from the box itself. This would be also be a ratio to minute that instrumentation in 1906 could not possibly detect it.
Greg House says:
February 6, 2013 at 2:58 pm
Greg, of course it has been proven, many times over. It’s in every college thermo textbook. Go buy one. Read it. Come back. Participate intelligently, instead of just asserting your misconceptions.
Here’s an example to consider. Mount a light bulb socket on a table. Insert a 60-watt bulb. Measure the temperature, we’ll say it’s 150°C. Try a 100-watt bulb, it’s hotter, say 200°C.
Now, mount another socket right next to the first one. Insert a 100-watt bulb in one and a 60-watt bulb in the other. What will the final temperatures of the bulbs be?
I say both of them will run hotter in that situation, because A warms B, and B warms A. If you disagree, I urge you to buy a thermometer and bulbs and sockets and do the test. Me, I don’t need to do it, the physics of the situation is quite clear to me.
Regards,
w.
Steven Mosher says:
February 6, 2013 at 2:59 pm
What he said.
w.
What a silly example. Prior to the metal shell being put around the sphere, the temperature of the sphere would be just sufficient to radiate the wattage of the internal heat source to the vacuum of space. If the temperature was too low, the sphere would continue to heat until the temperature was just high enough to radiate away the energy. Adding the additional thermal resistance of a steel shell in series with the thermal resistance to the vacuum of space will actually reduce the temperature of the sphere as the emitting area is increased. Leaving a space, presumably containing a vacuum between the sphere and the shell would do nothing since this would have the same thermal resistance as from the sphere to space.
This would be the same as adding another heatsink on top of the existing heatsink on your cpu. The thermal resistance between the heatsink and the atmosphere is so high that adding additional metal actually helps cooling by increasing the surface area for emitting heat while adding only very slightly to the series thermal resistance between the chip and atmosphere.
For another example, adding a 1″ length of 20′ diameter pipe to a mile long length of 3/4″ hose will not appreciably increase the flow through the hose.
Also, note that your soup doesn’t get hotter when you put it into a Thermos in spite of the reflected IR radiation from the silvered sides of the Dewar flask.
Problem #4
The radiative ghe changes the observed temperature of the earth as seen from space by exactly zero. With no ghe the earth as seen from space would be 255K and the temperature at surface would be 255K. As seen from space with a ghe, the temperature of the earth IS 255K and the temperature of the surface IS 288K, However, the average height at which 255K occurs is 14 km instead of at surface.
As this experiment only measured equilibrium temperature, and only at one elevation in each box, it does not and cannot be used as a simulation of the radiative ghe.
Problem #5
The radiative ghe is distributed from earth surface to TOA. This experiment puts a sheet of glass in to represent the ghe of the atmosphere, but does so at what would be the TOA, but with conductance and convection still active, which doesn’t exist at the TOA.
Greg, of course it has been proven, many times over. It’s in every college thermo textbook.
>>>>>>>>>>>>>>>>
Not to mention that verifying the thermo laws via experimentation is part of the curriculum in many universities. A quick search for SB Law alone gets:
http://uregina.ca/~szymanss/uglabs/p242/Experiments/EXPT1.pdf
http://www.fiziks.net/lifesciences/exp54.htm
http://www3.wooster.edu/physics/jris/Files/Carter.pdf
http://personal.tcu.edu/zerda/manual/lab22.htm
http://wanda.fiu.edu/teaching/courses/Modern_lab_manual/stefan_boltzmann.html
http://sky.campus.mcgill.ca/Exp/Manuals/en0049.pdf
Is that enough or should I copy and paste the entire 177,000 results that searching “stefan-boltzmann law experiment” produced?
Not to mention that when a bunch of engineers design, oh, I don’t know, let’s go with a nuclear reactor…they figure out exactly how much power the fission process will produce, how much of it they can capture and use to run generators, how much will turn into waste heat, how much that waste heat will raise the temperature of everything from the boilers to the turbines, exactly how much water at a given temperature and flow rate will be required to keep the system cooled to a given operating temperature, and they nail it to fractions of a percent before the construction even starts, and they do so using the precise same laws of physics that we’re discussing right now.
Who out there thinks the laws of physics don’t work like the thermo texts say they do and the engineers just nailed their design by shear luck? A few hundred nuclear reactors in a row? C’mon, hands up. Greg and who else?
Michael Moon
I’m not saying Willis is right but I don’t think he’s wrong because of what you say.
“The steel would radiate to space, but transfer ZERO heat back to the planet, as we all know that the Second Law is true”
If by 2nd law you mean the movement of a system to maximum entropy this will not invalidate the above model. Remember this is a conceptual model? Why would it not radiate back to the planet, if the shell is cooler then the net transfer would be outward from the planet but there would be a some transfer of energy to the planet from the shell (radiative transfer is emitted from and toward bodies irrespective of current state (it doesn’t care about energy state); maximum entropy only requires that the net flow will be toward the cooler body). Eventually, if the planet were to run out of its internal heat then both would eventually become the same temperature (all other things being equal).
Your point about the steel being cooler. Yes to begin with, but with constant supply of energy it will warm, and yes it can’t get warmer than the emitter but it can get warmer and will return some energy back to the surface with the result that the surface of the planet will warm.
Willis wrote (re multiple light bulbs in close proximity);
”I say both of them will run hotter in that situation, because A warms B, and B warms A. If you disagree, I urge you to buy a thermometer and bulbs and sockets and do the test. Me, I don’t need to do it, the physics of the situation is quite clear to me.”
Please note that in this example you have TWO energy SOURCES.
Please replicate this thought experiment with two steel shells in close proximity (sans additional heat sources), do you speculate that steel shell A heats steel shell B (and vice versa) and the temperature of both rise ?
If that’s the case I could just replace my electric baseboard heaters with steel slabs, boy that would sure be a lot cheaper.
Cheers, Kevin.
@ Kev-in-Uk: Actually “Greenhouse Effect” is also an incorrect term/expression, as greenhouses rely on physical barriers, not just varying amounts of gasses, to do what they do. You are correct that while the planet does NATURALLY warm and cool, anyone who suggests there is any measurable ANTHROPOGENIC warming is only fooling themselves.
Willis Eschenbach says:
February 6, 2013 at 5:01 pm
“…60-watt bulb. Measure the temperature, we’ll say it’s 150°C. Try a 100-watt bulb, it’s hotter, say 200°C.”
“Now, mount another socket right next to the first one. Insert a 100-watt bulb in one and a 60-watt bulb in the other. What will the final temperatures of the bulbs be?”
The temperature will not exceed 160 watts.
cd says:
“Your point about the steel being cooler. Yes to begin with, but with constant supply of energy it will warm, and yes it can’t get warmer than the emitter but it can get warmer and will return some energy back to the surface with the result that the surface of the planet will warm.”
Not true. Thermodynamic entropy is defined in units of energy per degree of temperature. Once temperatures are matched (delta T = 0), then no energy is transferred between bodies.
Willis:
I don’t know how many times I’ve seen your steel shell-game, but I still ain’t buyin’ it. You are creating energy from nothing there, IMHO. I’m certainly not spending any time disputing this nonsense.
But my main interest is in the Wood experiments, and despite reading your post several times, I still haven’t figured out just how the shell-game is relevant. I just want to know why the IR backradiation from the glass does not make the inside of the glass greenhouse any warmer than the air in the “salt” greenhouse, which cannot have any such backradiation. And how the atmosphere can cause warming (or slow down cooling), if even the glass cannot do it.
Then you end the post with a puzzle for us:
“Bearing in mind the discussion of the steel greenhouse above, I leave it as an exercise for the interested reader to work out why this is not a valid test of infrared back-radiation on a planetary scale … please consider the presence of the air in the boxes, the efficiency of the convective heat transfer through that air from the box to the cover plates, the vertical temperature profile of that air, the transfer of energy from the “surface” to the “shell” through the walls of the box, and the relative temperatures of the air, the box, and the transparent cover.”
After all those words, you are leaving it as an exercise to the reader….?? WOW, this seems like a cop-out! Aren’t YOU the one who is supposed to be demonstrating why this experiment does not tell us anything about how backradiation helps heat (or prevents heat loss, or whatever)??
KevinK
Please replicate this thought experiment with two steel shells in close proximity (sans additional heat sources)
>>>>>>>>>>>
You’ve constructed a thought experiment which is in thermal equilibrium. By definition, net energy flux is zero. This tells you precisely nothing about a system which is not in thermal equilibrium (such as the baseboard heaters in your house).
Sparks says:
“The temperature will not exceed 160 watts.”
Temperature is not measured in watts. You have the right idea though. The temperature measured in any body will be the equivalent of having absorbed 160Watts of energy, which is defined by the heat capacity of the body defined in terms such as J / (kg K).
Who out there thinks the laws of physics don’t work like the thermo texts say they do and the engineers just nailed their design by shear luck? A few hundred nuclear reactors in a row? C’mon, hands up. Greg and who else?
>>>>>>>>>>>>>>>>
OK two hands. Greg and jae. Anyone else?
davidmhoffer says: “Greg, of course it [that A warms B and then B warms A back] has been proven, many times over. It’s in every college thermo textbook.“.
Unfortunately, as I read them, all the links you provided were only tests of Stefan Boltzmann Law, not of “A warms B and then B warms A back”.
I don’t doubt that “A warms B and then B warms A back” but it seems that a test of that specific hypothesis is needed to settle the matter. Roy Spencer’s set-up is definitely testable.
KevinK says:
February 6, 2013 at 5:31 pm
Willis wrote (re multiple light bulbs in close proximity);
Please note that in this example you have TWO energy SOURCES.
Your argument, as I understand it, is that a cooler object cannot radiate energy to a warmer object.
If that is correct, then the fact that they are transforming energy from electricity to heat is immaterial.
w.
jae says:
February 6, 2013 at 6:17 pm
I can’t tell you how I wish your final sentence were actually true … and no matter how many times you’ve seen it, you still haven’t found an error in it, or if you have you’re keeping it secret.
w.
Willis, pretty picture but,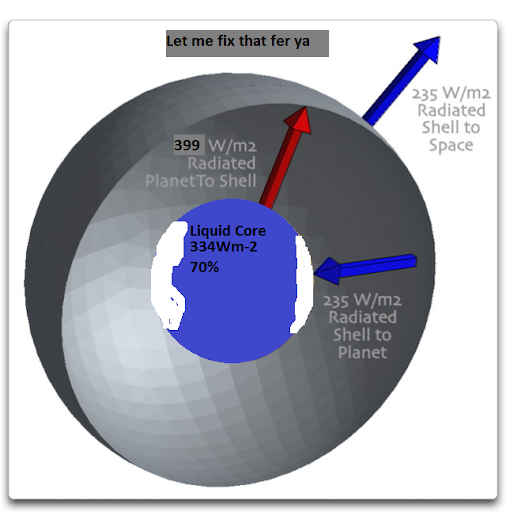
Let me fix that for ya.
KevinK @ 4:50 – During the winter there is generally less humidity, so that would cause the air to feel cooler than in the summer with the same temperature; but, in line with your point, the folks around here with brick houses require less wood to keep their homes heated once the bricks absorb the heat. I place a pot of water on my wood stove to increase the percentage of water vapor in the house to help with the sensation of heat.
Chad Jessup
the reason that houses with warm bricks require less ‘heat’ to maintain temperature is that the bricks are no longer absorbing heat, and their emissivity/conductivity is such that the rate of heat transfer from the exterior of the wall is very low. Hence the heating system only needs to keep up with the rate the bricks carry heat to the outside world .
All the units and quantities are irrelevant because the implication here is that a photon leaves the planet surface, interacts with an electron in the shell then somehow one photon is emitted back and another one into space.
Doesn’t seem right.
Mike Jonas;
Unfortunately, as I read them, all the links you provided were only tests of Stefan Boltzmann Law, not of “A warms B and then B warms A back”.
>>>>>>>>>>>>>>>>
My point was that the laws of thermodynamics are found in every university level text book and are validated through experimentation, of which SB Law is a single example. You really want me to go through the list of all the laws and post links to experiments validating each and every one of them?
On the other hand, your complaint that SB Law has nothing to do with a warms b suggests that you don’t have a clue what SB Law is or how to apply it. Otherwise you wouldn’t have said such a thing.
Gino says:
February 6, 2013 at 6:21 pm
Sparks says:
“The temperature will not exceed 160 watts.”
Temperature is not measured in watts. You have the right idea though. The temperature measured in any body will be the equivalent of having absorbed 160Watts of energy, which is defined by the heat capacity of the body defined in terms such as J / (kg K).
The temperature will not exceed 160 watts, convert it to joules to watts and watts to temperature. My point is valid.
Doing a simple calculation with the Stephan-Boltzman equation suggests that with black body radiation within a vacuum, the ratio of the planet surface temperature to the steel shell temperature is 2^.25 or (1.189). So if the surface of the planet was 100 degrees C, shell would have to be 40 degrees C.
joletaxi in English so all can understand the exchange:
Willis responds:
Sparks:
Watts do not convert to temperature without the heat capacity of the material. The point being that two items can have the same temperature but vastly different heat quantities.
Willis wrote;
“Your argument, as I understand it, is that a cooler object cannot radiate energy to a warmer object.”
NO, I have never said that. Energy flows back and forth between cooler objects and warmer objects all the time.
My argument is that you are mistaking these ENERGY FLOWS (fluxes) with ENERGY SOURCES.
An energy source ADDS energy to a system, an energy flow DIRECTS energy through a system. Think of cars leaving a parking lot, the number of people parking is like an energy source, the traffic light at the exit of the parking lot DIRECTS the flow of the energy (cars). Two totally different mechanisms.
If you go to a “Big City” Airport sometimes you miss the “exit here sign” and you have to loop back through the airport roads one more time to get on your way. You are not an “Extra” airport visitor; you just went through the ramp system twice before leaving. Same as the “greenhouse effect”, no more cars, they just looped through the airport roadways twice……….
Cheers, Kevin.
Greg House wrote (w.r.t. Roy Spencer’s”Yes Virginia there is a greenhouse effect”)
“This is a wonderful idea real experimental testing, Mike, thank you. Has Roy Spencer done it yet?”
As far as I know Dr. Spencer has never implemented this empirical experiment.
Please note that his description uses a “power supply” (a device that converts AC power from the electric company to DC current).
If instead you use an “energy supply” (i.e. a battery with a fixed supply of energy) you get a different result;
With a power supply you can indeed cause two metal slabs in a vacuum to achieve a higher temperature versus a single slab.
HOWEVER with an energy supply you simply cause a higher temperature for a short time and then the energy supply is exhausted and things cool back down.
With an energy supply you get a bit higher temperature for a time, but then things cool off and the average temperature is NEVER higher (although with proper techniques you might just “break even” although doubtful without “”perfect” (i.e. no efficiency loss) batteries).
Power supply, Energy Supply, two different things (apples vs oranges) as anybody that wished for a power supply to light up their flashlight when their energy supply (batteries) went dead should appreciate.
Cheers, Kevin.
Kev-in-Uk says:”I take exception to using the term AGW instead of GHE (greenhouse effect) as that term is a false premise.“.
Point taken. Much more is needed to establish AGW. I think it’s there but negligible, but again it needs proper testing (not easy, because I can’t see the testing being able to be done in a lab).
fhhaynie – If my understanding is correct, you need to use deg K not deg C. Also, the planet temperature you quote is the pre-shell temperature. Post-shell, the planet will be hotter.
Mike,
I used Kelvin in calculating the ratio and then converted to C with 100 degrees as an example. The point is at equilibrium, the radiation from the planet will always be twice as much as the outbound radiation from the shell.
davidmhoffer – It’s not me that has the problem. I’m suggesting a simple test to establish beyond doubt whether this interpretation of the law is correct – after all, science is supposed to be about testing. It is such a basic test, surely it is in a basic textbook somewhere, but no-one seems to have found it yet. If it’s not in the textbooks, then let’s encourage someone to do it.
I have a historical excerpt from the 1980’s, explaining the poorly named “greenhouse effect”.
5 March 1984,
UK: Scientist warn of the Green House Effect; Concern is growing that carbon dioxide, produced by burning fossil fuels, will affect the climate. Carbon dioxide acts like the glass of a green house, trapping the suns heat, and the amount in the atmosphere is growing. The latest pointer to its effects comes from scientists at the University of East Anglia who have found that 1981 and last year were among the warmest on record. A warmer climate could damage agriculture and cause flooding by melting the polar ice caps.
I may be a bit slow so correct me if I’m wrong but the take home message is that the experiment does *not* prove that the greenhouse effect (as described by radiation rather than convection or conduction) doesn’t exist but only shows that the greenhouse effect has virtually nothing to do with what keeps greenhouses warm. Have I got it or do I have to go sing about the rain in Spain while holding marbles in my mouth again?
Mike Jonas says:
February 6, 2013 at 7:22 pm
davidmhoffer – It’s not me that has the problem. I’m suggesting a simple test to establish beyond doubt whether this interpretation of the law is correct – after all, science is supposed to be about testing. It is such a basic test, surely it is in a basic textbook somewhere, but no-one seems to have found it yet. If it’s not in the textbooks, then let’s encourage someone to do it.
>>>>>>>>>>>>>>>>>>
Once again you demonstrate that you have no idea what is in the text books or how to apply it. Shall I add you to the list of people who think nuclear reactors get designed by accident?
Why isn’t the greatest experiment of all, nature, being discussed in all this?
There is no “hot spot” and the stratosphere is not cooling; the climate models are wrong. So why all the kerfuffle with pictures of spheres and such? Is the atmosphere behaving according to GHE theory or not?
Power is not conserved. Energy is.
Go back and learn from someone who knows engineering and physics and then get back to us. Your amateurish analysis is laughable. Like flux is conseved! Figure out how much energy the “blackbody” absorbed and what the energy loss is. Also, steel does not transmit light in, like say, the atmosphere does. Your oversimplification is a tell that you have no clue what you’re talking about.
This is from someone who agrees that man made “global climate change” is trivial at best.
I’ve taught 1st year Mech Eng. students who have a better grasp of heat transfer than you.
Thanks Willis. Agree totally with your article.
I quite like these discussions. They tend to be very amusing. The people who claim the people who get it right are idiots are a hoot. My absolute favorites are the guys who announce they are engineers. Fukushima anyone?
DR,
You are confusing two very different theories.
GHE theory is that the atmosphere makes the Earth warmer than it would be without the atmosphere. While there are arguments about whether this results from radiative effects or some other effect, GHE is easy to prove. The Earth and the Moon recieve about the same amount of incomming radiation per m2 from the sun, but the Earth which has an atmosphere has a higher average surface temp than the Moon.
AGW is a theory that human actions (burning fossil fuels) is increasing the strength of the GHE which will result in a catostrophic increase in the Earth’s Average surface temp.
The “hot spot”, and stratospheric cooling are products of climate models that allege to prove AGW.
AGW is dependent on GHE but GHE by itself does not imply any particular temp trend.
A thought experiment cannot refute a real experiment, ever.
Gino says:
February 6, 2013 at 7:00 pm
Sparks:
Watts do not convert to temperature without the heat capacity of the material. The point being that two items can have the same temperature but vastly different heat quantities.
a 60w bulb and a 100w bulb equals 160w, put the two bulbs together while live, their temperature will never exceed 160w. They do NOT exceed 160w, no mater how much you mess around with ‘energy budgets’ and the material capacity, 160w in 160w out.
this post may be alot of things but a good example of physics it most certainly is not …
at the start of your experiment the steel shell is radiating no energy … (we are assuming the hot planet is the only energy source) therefore when it does begin absorbing energy it will then radiate that energy in all directions when it drops back down to equilibrium … if the planet radiates X amount of energy the shell can only absorb X but it cannot radiate X back at the planet … it can only radiate at best .5 X … and since that radiation is essentially a spherical projection from each molecule in the steel shell we know that quite a bit less than .5 X is radiated back to strike and supposedly be absorbed by the planet …
so no, the planet would never double its radiated output … ever …
2 metal balls … 1 foot apart in a vacuum … one very hot, one not so hot …
according to the 2nd law the hotter ball cannot he heated by the cooler ball yet we know the cooler ball is radiating heat … what happens to that radiated energy when it strikes the hotter ball ? Is it absorbed or reflected ?
Snake Oil Baron wrote;
“I may be a bit slow so correct me if I’m wrong but the take home message is that the experiment does *not* prove that the greenhouse effect (as described by radiation rather than convection or conduction) doesn’t exist but only shows that the greenhouse effect has virtually nothing to do with what keeps greenhouses warm.”
Yes that is about right, the “effect” exists, but it has nothing to do with the average temperature of the Earth, that’s determined primarily by the massive thermal capacity of the oceans.
The ”effect” simply delays the flow of energy from the Sun to the Earth and onwards towards space by a few tens of milliseconds. Since there are about 86 million milliseconds in a day the “effect” is so small we probably could never afford to measure it.
Cheers, Kevin.
MattS says, February 6, 2013 at 7:50 pm: “GHE theory is that the atmosphere makes the Earth warmer than it would be without the atmosphere.”
==========================================================
No, this is not true, anyway the “greenhouse effect” as presented by the IPCC is about particular “greenhouse gases” making the Earth warmer specifically by by returning back some of the Earth’s outgoing IR radiation, it is not about the whole atmosphere. They do not tell people, however, that this 150 years old hypothesis was debunked 100 years ago by the Wood experiment.
Note that the Wood experiment deals with the underlying mechanism of the “IPCC greenhouse effect”, namely the alleged effect of trapped/back radiation on the temperature of the source.
2nd Law of thermo
Net energy flux between two bodies will flow from hotter to colder.
SB Law
Calculates the energy flux from a body at a given temperature.
For two bodies in proximity to each other, suppose a net flux of C.
Via SB Law, calculate the energy flux from hotter = A
Via SB Law calculate the energy flux from colder = B
A – B = C
Any thermo text, any physics curriculum, and yes, this does explain that A heats B and B heats A. The net flux is C. Proven over and over again by experimentation. Used to design nuclear reactors, kitchen ovens, air conditioners, automobile cooling systems, and more on a daily basis
Or you can subscribe to the theory that these laws of physics are not in the thermo texts, haven’t been proven by experimentation, and that engineers design nuclear reactors, kitchen ovens, air conditioners and automobile cooling systems that functions as designed in advance of being built by a complete fluke.
Here’s another way to think about radiative heat exchange. Suppose we put up a detector, and we detect thermal infrared radiation coming from an object. Doesn’t matter where it’s coming from, but let’s say the temperature of the object is 15°C, so it’s putting out about 400 W/m2 of thermal radiation.
So let’s suppose we expose two objects to that 400 W/m2 of radiation, one of which is at 20°C, and the other of which is at 10°C.
Choices:
1. Both objects absorb absorb the radiation, why wouldn’t they?
2. Somehow the radiation can tell hot from cold, so it bounces off of the hot one.
3. ??
Here’s another example. You are standing next to a block of ice. It is suddenly replaced by a block of metal about 5° below body temperature. Do you feel warmer? Why?
w.
Snake Oil Baron says:
February 6, 2013 at 7:27 pm (Edit)
Got it in one …
w.
Graham Green says:
February 6, 2013 at 6:41 pm
All the units and quantities are irrelevant because the implication here is that a photon leaves the planet surface, interacts with an electron in the shell then somehow one photon is emitted back and another one into space.
Doesn’t seem right.
>>>>>>>>>>>>>>>>>>
Because it isn’t. The theory is that a single photon gets absorbed and a single photon gets emitted in a random direction. For a large number of photons emitted in a random direction, about 1/2 will be generally upward and about 1/2 will be generally downward, but the same number get emitted as absorbed.
davidmhoffer says:
February 6, 2013 at 7:29 pm
…” people who think nuclear reactors get designed by accident?”
__________
Perhaps you mean by trial and error?
Because safety is no accident. *rimshot*
davidmhoffer says, February 6, 2013 at 8:12 pm: “2nd Law of thermo
Net energy flux between two bodies will flow from hotter to colder.”
=========================================================
No, the known historical statements do not contain the word “net” nor do they imply it, nor is there apparently any real scientific experiment confirming this notion.
I guess, that “net” thing is a trick of “climate scientists” to justify their “greenhouse effect”.
greg house;
Note that the Wood experiment deals with the underlying mechanism of the “IPCC greenhouse effect”, namely the alleged effect of trapped/back radiation on the temperature of the source.
>>>>>>>>>>>>>>>
Is Lewandowsky paying you to blog?
Willis Eschenbach says, February 6, 2013 at 8:17 pm: “Here’s another example. You are standing next to a block of ice. It is suddenly replaced by a block of metal about 5° below body temperature. Do you feel warmer? Why?”
=======================================================
I guess, every warmist would indeed feel warmer and insist on that even after getting acquainted with the Wood experiment.
davidmhoffer says, February 6, 2013 at 8:12 pm: “For two bodies in proximity to each other, suppose a net flux of C.
Via SB Law, calculate the energy flux from hotter = A
Via SB Law calculate the energy flux from colder = B
A – B = C”
============================================================
There is no basis in real science for your “C”, so simple is that.
Willis said:
Here’s another example. You are standing next to a block of ice. It is suddenly replaced by a block of metal about 5° below body temperature. Do you feel warmer? Why?
w.
——————————————————————
Your body is transmitting less heat to the block. In other words, the energy flux (watts/m2) drops, and the draw on your bodies energy supply is lower. Your body requires less energy to maintain the same temperature.
LazyTeenager wrote;
“Fukushima anyone?”
Reliable (24/7/365 +/- a few minutes) AC electricity anyone ?
Fresh Water anyone ?
Safe transportation anyone ?
Bridges and building that stay put when you walk on/in them anyone ?
Cheap airplane travel across the globe anyone ?
Cheap (really really cheap) computing power anyone ?
Safe/warm houses in cold climates anyone ?
Apollo, SST, a safe end to the cold war anyone ?
Or how about endless doom and gloom predictions that NEVER EVERY MATCH REALITY anyone ?
I for one will take RESULTS instead of PREDICTIONS/PROJECTIONS/WILD ASS GUESSES.
Snake Oil Baron,
Congrats on your hole in one, Willis says you passed. I have a greenhouse, and my plants and I needed to hear this. This all needs to be cleared up.
For some, though, the rain in Spain will remain much too plain.
davidmhoffer says:
February 6, 2013 at 6:22 pm
“Who out there thinks the laws of physics don’t work like the thermo texts say they do and the engineers just nailed their design by shear luck? A few hundred nuclear reactors in a row? C’mon, hands up. Greg and who else?
>>>>>>>>>>>>>>>>
OK two hands. Greg and jae. Anyone else?”
Very funny, huff-man. NOW, please put up some detailed facts. Specific stuff, like a named text and page number. You’re good at broad insults, but I cannot think of any specific references to your snarks. Now let’s see something concrete, fella…
Richard G says:
February 6, 2013 at 8:33 pm
>>>>>>>>>>>>>>>
LOL
There is no basis in real science for your “C”, so simple is that.
>>>>>>>>>>>>>>>>>>>.
ROFLMAO
jae;
You’re good at broad insults, but I cannot think of any specific references to your snarks.
>>>>>>>>>>>>>>>
Is there some part of “any university level thermodynamics text” that you failed to understand? Do you want me to read it for you as well so that you don’t have to?
Wait… I already did.
No, the known historical statements do not contain the word “net”
>>>>>>>>>>>>>>>>>
There have been nearly as many formulations of the second law as there have been discussions of it.
—Philosopher / Physicist P.W. Bridgman, (1941)
Gino says:
February 6, 2013 at 8:54 pm
The “draw” on my body’s energy? The energy flux that I am radiating drops?
My friend, you desperately need to read about the great discovery of Stefan-Boltzmann. They showed that
RADIATION DEPEND ONLY ON TEMPERATURE AND EMISSIVITY
Period. No exceptions. No special clause that says you are radiating less energy because you are standing next to an ice block. No way to “draw” on something to make it radiate more or radiate less. No way for the energy flux to “drop”. Since for a given object emissivity is fixed, radiation depends only on temperature. Radiation does NOT depend on what is absorbing its radiation. It does NOT depend on the mass of the object, or the thermal mass. For a given emissivity, radiation is only and solely and completely a function of temperature.
In other words, your explanation is … well … wrong. The difference has nothing to do with you. It has to do with the fact that the ice block radiates less energy than the warm metal block … and with the fact that your body absorbs radiation from the ice block just as it does from the metal block. With the ice, your body gets less total radiation, so it feels colder.
Note that the flow of HEAT, which is different from ENERGY, is always and forever in one direction, from me to the ice block or to the metal block, because my body is warmer than they are … but the energy flows in both directions.
w.
Willis,
You must consider something here. Energy, in the form of heating your house, or keeping your lights on with the electrical bill, has been the subject of, shall we say, INTENSE scrutiny, since
Edison in the 1870’s. When a large-gigantic-immense-overwhelming amount of money is spent on the subject of energy, the community of suppliers tend to research this subject, to learn how to satisfy their customers at minimal cost.
Well, we did.
If there were a way to heat anything by putting a shell around it which would magically double the “heat input,” otherwise known as Flux, then it would have been seen and exploited many many years ago.
This is just a blog run by a weatherman, who is searching for something to post that he did not have to write, and you are a good writer who is supportive of our host. Why would you go here, with no fundamental understanding of the physics? You should stop.
You are not stupid, but you seem to be over-confident, posting science of which you know NOTHING. Please stop, otherwise people like GS and JH will shred you and destroy this blog. Anthony, I previously begged you to stop this.
If you would like me to edit posts concerning physics I could do it for a short time now….
Jeff Carlson said:
what happens to that radiated energy when it strikes the hotter ball ? Is it absorbed or reflected ?
=======================================
Higher temperatures emit more energy than lower temperatures so even though it “sees” the lower temperature object, the higher temperature object overpowers the lower on so to speak. That is the reason heat transfer calculations are based on temperature DIFFERENCE. Energy is transmitted at various wavelengths. Best explanation I’ve read involves wave theory. When two bodies at the same temp emit at the same wavelength, they form a standing wave. A standing wave does not transport energy. When they emit at different wavelengths, they ‘exchange’ energy as in absorb in one bandwidth and radiate in another with a net of zero to maintain a delta T of zero.
Cop; Do you know how fast you were going?
Heisenberg; No… but I know where I am.
Cop; You were going 80 in a 60 zone….
Heisenberg; Impossible. There was an experiment in 1906 proving that cars would never go faster than 40.
Cop; License and registration please…
[snip – take a 24 hour time out – Anthony]
Greg House says:
February 6, 2013 at 8:45 pm (Edit)
Greg, that is so precious, you actually think your unsupported opinion means something … you might try here for the relevant equations and a calculator.
There’s also an overview here … a text here …
And every one of them says the same thing … that C is the actual heat flow between the two bodies. If you have say 140 W/m2 of radiation going one way, and 290 W/m2 of radiation going the other way, the net heat flow is the difference “C”, of 150 W/m2.
Truly, my friend this is absolutely basic stuff, first year physics. You’re embarrassing yourself, or while you may not be, at least I’m embarrassed watching your ludicrous claims …
w.
Willis said:
Since for a given object emissivity is fixed, radiation depends only on temperature.
—————————–
Energy transmission however is dependent on temperature DIFFERENCE. Thank you for making my point. When the temperature difference drops (60F to say 5 f) then the energy flux drops as well. Your body maintains it’s surface temp with less energy flux. Your body is a generator that uses different amounts of energy to maintain a minimum core temp. It generates POWER from potential ENERGY. This manifests as TEMPERATURE, which is dependent on environment.
Willis Temperature is NOT energy, is NOT power. They are all different physical concepts and you seem to be having trouble separating them.
“[UPDATE: Note that because the difference in exterior surface area of the shell and the surface is only 0.03%, I am making the simplifying assumption that they are equal. This clarifies the situation greatly. Yes, it introduces a whopping error of 0.3% in the calculations, which people have jumped all over in the comments as if it meant something … really, folks, 0.3%?]”
Why does it have made of steel and and kilometers above earth?
Why not inch of dirt and inch of space separating it?
Why not do this 100 times. So it’s 200″ high.
Does prove anything other than air is pretty good insulator.
Now how much is earth being warmed per second?
Are suppose to think it’s somewhere around “235 watts per square meter”
heat coming from the surface?
Take a 10 meter by 10 meter floor space of a house- 100 square meter.
So 235 watts per square meter is 23500 watts of heat. Or using 23.5 kilowatts per
hour of electricity used to heat the house and the house is well insulated. And we suppose
or imagine it’s not going to get hot in the house?
Now, you can go into 100 square meter floor space room and measure the temperature of
the walls, floor and ceiling and convert this to watts per square, but if total area, this not how
much energy is being used to warm the room.
Say you planet and it’s geothermally heated so it’s uniform temperature of 15 C at it’s surface.
So no ice, and snow could not last very long, ocean will warm to 15 C at surface, and air will warmed to 15 C near the surface. So whenever surface of ocean cools below 15 C and air just above surface cools below 15 C, one added more heat. So we have a little Utopia, lack any exciting weather and somewhat cool- warmer for Russia and cooler for Tropics.
If mountains were considered surface, then you get no skiing. If you exclude mountains or certain mountains, then you can get surface and air temperature cold enough- and if high enough and made artificial snow- a mountain about 3000 meters gets cold enough for snow.
So for fun, let say in Utopia even though some might get injured, skiing is desired and allowed.
Now some genius decides putting a steel sphere around earth, to lower energy costs.
So we get the “Deathstar is too small project” and we going to put sphere of steel around the planet.
We have few option, we could try to make sphere strong enough, but that is
hopeless.
Or we orbit bands of steel at varying orbital height. This allows us use very thin plate steel. There going to be orbital mechanic issues, but we put say 1000 mile wide band in a polar orbit, and need 20 of them [circumference at equator 40,000 km]. if 50 km orbital height difference it’s 1000 km in total. So starting 100 km, and longitude 360, and second 50 km higher is 180 longitude, etc.
The question is would this reduce the heating costs. Simple answer is, no.
It would help [at least show that you trying] if you put shiny stuff on interior surface of it, but that would not keep the bands very warm [assuming earth was only thing keeping it warm].
Another plan is you want to do is keep the outside part of band as cold as possible. And this done by making layers of material. So say 10 layer of shining material which separated and insulated against heat being conducted. That should make the outside of bands fairly cold- very heat passing thru it.
And this whole band thing is quite cold with warmest part being the reflective surface facing Earth.
Now, how much is the this reflective sphere going warm the earth- near zero.
You would save a lot more energy if you decided to keep all mountains above 2000 meters non heated.
And if did this, these mountains would receive the most measurable heating from the sphere- so instead being at 0 C it might be 1/2 degree warmer.
One test this, have insulated box, put glass which is transparent to IR, put on balloon fly it to 60,000′ point window box at ground night side surface and see how warm it gets. If get a greenhouse effect which warms the air [or black plate] over 10 C- I am wrong.
Or easier use same box in room with a high ceiling and have ceiling 10 C warmer than floor. Put box on floor. See if it warms up by 5 C.
And I would be really impressed if the box which is warmed from the ceiling is warmer than the ceiling.
Willis Eschenbach says, February 6, 2013 at 9:49 pm: “you might try here for the relevant equations and a calculator. There’s also an overview here … a text here …”
==========================================================
Willis, your links show only that you are not the only one who is practising this “radiation arithmetic”. There are also others, I get it, thank you.
What I mean is that this “radiation arithmetic” has apparently no basis in real science, please, note this point. Apparently there is no real scientific physical experiment confirming that this “radiation arithmetic” is correct. This is the point.
Willis Eschenbach says:
February 6, 2013 at 9:31 pm
RADIATION DEPEND ONLY ON TEMPERATURE AND EMISSIVITY
Period. No exceptions.
———-
And the Gods laughed, and angels wept, at the temerity.
Of things never imagined.
Willis, HEAT is the transfer of energy. The transfer of energy is work (Joules), the rate at which energy is transferred is power (Watts). If no energy is transferred, no work is performed, If no work is performed, no power is used. If two bodies are at the same temperature the may “see each other” through radiation (see my standing wave post), but no work is performed transferring energy between the two bodies, therefore no energy moves between them, hence there is no power consumed.
Willis, a minor point/nitpick but I think you overstate to the percentage diffrence of your planetary globe and the steel shell areas by a factor of 10, assuming the globe is an earth sized and the shell height is top ~ top of our atmosphere . The area of the steel shell would be equal to 4*pi*(R+h)² and the area of the planet 4*pi*R² , where R is the radius of the globe and h is the heigth of the shell above the globe surface so the diffrence D comes out as D = 4*pi*[(R++h)² – R²] = 4*pi*(2*R+h²) , now using R= 6200 km and h= 10 km , giving D = 4*pi*12500 km² ~ 1.57*10⁵ km² , and the globe surface would be somthing like 4.85 * 10⁸ giving the ratio of D/Globearea ~ 0.0003 or 0.03%
not 0.3% , so the diffrence is even slighter than you state.
Michael Moon says (February 6, 2013 at 9:37 pm): “If you would like me to edit posts concerning physics I could do it for a short time now….”
When I could finally pick myself up off the floor, it occurred to me that I should nominate this comment for February Funny. 🙂
Perhaps I’m missing something.
If you chose a planet whose radius is r, and a shell with a radius of 2r, would not the radiative heat flux reach at the shell be 1/4 (235W/m^2)? Surface area being A=4pi*r^2
Polyethylene is transparent to IR but polytunnels work fine for friut and veg farming. (In fact an IR absorbent grade is sometimes used to prevent scorching.)
The Earth is never in equilibrium with space because there is virtually nothing in space to be in equilibrium with.Despite the Sun getting brighter and radioactivity in the Earths core the Earth has got colder over time no amount of insulation is going to slow that rate of cooling to space.Space has a density of almost nothing,there is no analogy that we can use to understand how the Earth and Space interact ,analogy is a poor argument.
Greg House says (February 6, 2013 at 4:37 pm): “This is a wonderful idea real experimental testing, Mike, thank you. Has Roy Spencer done it yet?”
As I’ve pointed out in other threads, Dr. Spencer has no incentive to perform the experiment, as it would only confirm what he and just about everybody else already knows.
The real question is why his critics haven’t performed it. If they really believe it would overturn established physics, it would be worth a Nobel Prize at least, and would earn the undying gratitude ot a world saved from the imaginary threat of Thermageddon. That they haven’t performed such a definitive experiment and claimed their laurels speaks volumes.
Gino:
“Willis, HEAT is the transfer of energy. The transfer of energy is work (Joules), the rate at which energy is transferred is power (Watts). If no energy is transferred, no work is performed, If no work is performed, no power is used. If two bodies are at the same temperature the may “see each other” through radiation (see my standing wave post), but no work is performed transferring energy between the two bodies, therefore no energy moves between them, hence there is no power consumed.”
Thanks Gino, Willis has always had this problem with power and flux.
Gino says:
February 6, 2013 at 9:41 pm
“Higher temperatures emit more energy than lower temperatures so even though it “sees” the lower temperature object, the higher temperature object overpowers the lower on so to speak. That is the reason heat transfer calculations are based on temperature DIFFERENCE. Energy is transmitted at various wavelengths. Best explanation I’ve read involves wave theory. When two bodies at the same temp emit at the same wavelength, they form a standing wave. A standing wave does not transport energy. When they emit at different wavelengths, they ‘exchange’ energy as in absorb in one bandwidth and radiate in another with a net of zero to maintain a delta T of zero.”
I agree with Dino. It is my opinion that Dino’s way of thinking is the way one applies physics.
And the word “Backradiation” was never mentioned in radiation physics classes back then. Maybe it is “Newspeak”?
Anthony Zeeman says (February 6, 2013 at 5:04 pm): “Leaving a space, presumably containing a vacuum between the sphere and the shell would do nothing since this would have the same thermal resistance as from the sphere to space.”
You’re forgetting that the shell radiates both out and in. Very different from the one-way radiation from the surface only. Think of the shell as giving the sphere a “rebate” on its radiated energy. 🙂
“This would be the same as adding another heatsink on top of the existing heatsink on your cpu.”
No. The heat sink is in contact with the microprocessor. Willis’s shell is about the same diameter as the “planet”, is separated from it by a vacuum, and the whole shebang is surrounded by vacuum. For obvious reasons, my laptop is designed to work in air. It even has a fan to convectively cool the heat sink, which cools the cpu by conduction. As with Wood’s model greenhouses, radiative cooling is a minor factor. If I were Greg House, I’d say my laptop disproves the so-called “greenhouse” effect., but I’m not. 🙂
“Also, note that your soup doesn’t get hotter when you put it into a Thermos in spite of the reflected IR radiation from the silvered sides of the Dewar flask.”
To compare with Willis’s example, you need to add a constant heat source to the inside of the thermos. Now what happens?
So what happens if the steel inner ball and the outer shell is replaced by nitrogen gas. Does the nitrogen gas temperature approach infinity as they say it can’t radiate heat away?
wayne says:
February 6, 2013 at 11:06 pm
But, you and Gino are begging the question, by implicitly asserting that equilibrium has been achieved and that the equilibrium with the steel shell is the same as that without it. Allow me to interject some math.
Basically, without the shell, the planet is receiving heat at a rate which I will call P_core, the power from the nuclear core. It is radiating it at
P_planet_outgoing = sigma*T_planet^4*(4*pi*R_planet^2)
where sigma is the SB constant, T_planet is the temperature of the planet, and the last term in parentheses is the the surface area with R_planet being the radius of the planet. Thus, the temperature of the planet at equilibrium is
T_planet = (P_core/(sigma*(4*pi*R_planet^2)))^0.25
Now, the shell has an inner radius, which I will call R_inner, and an outer radius, which I will call R_outer. It has incoming and outgoing power at
P_shell_incoming = sigma*T_planet^4*(4*pi*R_planet^2)
P_shell_outgoing = sigma*T_shell^4*(4*pi*(R_inner^2+R_outer^2)
This is the nub of the issue: The shell has at least twice the surface area of the planet over which it radiates. At equilibrium, we have
T_shell = T_planet * (R_planet^2/(R_inner^2+R_outer^2))^0.25
[Tshell is at most 2^0.25 := 20% lower in temperature than T_planet] but, these being absolute temperatures, 20% can be quite a lot.
What is T_planet now, though? Is it higher than it was before? Yes. The planet power balance is now
P_planet_incoming = P_core + sigma*T_shell^4*(4*pi*R_planet^2)
P_planet_outgoing = sigma*T_planet^4*(4*pi*R_planet^2)
At equilibrium, these are equal, so
sigma*T_planet^4*(4*pi*R_planet^2) = P_core + sigma*T_shell^4*(4*pi*R_planet^2)
= P_core + sigma*T_planet^4*(R_planet^2/(R_inner^2+R_outer^2))*(4*pi*R_planet^2)
Leading to
sigma*T_planet^4*(4*pi*R_planet^2)*(1 – R_planet^2/(R_inner^2+R_outer^2) ) = P_core
so, with the shell
T_planet_with_shell = T_Planet_without_shell / (1 – R_planet^2/(R_inner^2+R_outer^2) )^0.25
Again, the maximum is about 20% higher.
Things are slightly different for the atmosphere, in that gaseous particles can radiate in any direction, and not all those directions will intersect with the Earth’s surface. But, over 45% of them will at the top of the troposphere, instead of the ~50% from a thin solid shell, so this is not such a big deal.
A bigger deal is that, the atmosphere is in contact with the surface, and can exchange heat through conduction and convection. On that score, the steel shell thought experiment appears to become a bit of an academic exercise which may not be relevant for a planet with a gaseous atmosphere.
Bart says:
February 6, 2013 at 11:54 pm
Erratum: “Tshell is at most 2^0.25 := 20% lower in temperature than T_planet…”
“To compare with Willis’s example, you need to add a constant heat source to the inside of the thermos. Now what happens?”
I’ll have them on the market in a blink! ☺
Don’t you just hate cold coffee. Think 5‹W/m²› would do it?
Bob Roberts says:
February 6, 2013 at 5:34 pm
I am fully aware of the physics of the way a greenhouse works, thanks, Bob – and I am thus also fully aware of the ‘misnomer’ status of the GHE. Unfortunately, until someone comes up with an alternative well accepted description for the ‘warming effect’ of the atmosphere, I think the consensus use of GHE is the only one we can realistically apply at the moment! (If someone has created a better term that can be widely used, perhaps hey can let us know?)
regards
Kev
I still haven’t heard an explanation, why 6,000 ppm of CO2 in Martian atmosphere (which is exact equivalent of 400 ppm CO2 and thousands ppm of water vapor here) has no visible effect on its surface temperature, e.g. its black body and average temperature is the same 210K.
http://nssdc.gsfc.nasa.gov/planetary/factsheet/marsfact.html
Second, almost nobody considers one simple fact, that is, simple thermal insulation and heat retention of the bulk atmosphere – nitrogen and oxygen – does the job, which some are still trying to stretch on radiation diagram arrows. Moon night temperature is -150°C. Earth night is +10°C. Which kind of invisible, IR-based dragons breath warms our night by incredible 160°C? Photons bounced/radiated from tiny part of air molecules, really?
On my first day as a grammar school student (long, long ago but I recall it clearly) I became very upset when, after I gave my teacher the exact 50 cents required for the week’s “milk money”, she then gave “my” 50 cents to someone who had paid her a dollar. My mom eventually helped me to understand that the net result was correct and that it didn’t matter that my exact physical two quarters were no longer in the teacher’s possession; my milk was well and truly paid for.
It astonishes and dismays me that several rather didactic and even patronizing commentors are making similar conceptualization errors regarding basic radiative heat transfer as well as largely missing the point of Willis’ post.
A very wise man once wrote, “If any one thinks he is wise, let him become a fool, that he may become wise.” In other words, a humble, open mind is a prerequisite to learning.
Thank you for the clarifying and thought-provoking post, Willis. Well done.
Gino says:
February 6, 2013 at 5:59 pm
Not true. Thermodynamic entropy is defined in units of energy per degree of temperature. Once temperatures are matched (delta T = 0), then no energy is transferred between bodiesI
You didn’t read the post properly. Not the reference to NET transfer. If both bodies are at the same temperature there will be no net transfer of energy but both will continue to radiate to each other (balance therefore no change in energy state of either body). The vector defining the path of a IR photon will not stop because the surface it comes across is at the same temperature as its source. There will still be radiative transfer but NET transfer = 0. For your point to be true, that is no transfer, then the bodies would have to reach a state where they no longer emitted radiation.
Gino last point should have read:
Gino says:
February 6, 2013 at 5:59 pm
Not true. Thermodynamic entropy is defined in units of energy per degree of temperature. Once temperatures are matched (delta T = 0), then no energy is transferred between bodies.
You didn’t read the post properly. Note the reference to NET transfer. If both bodies are at the same temperature there will be no net transfer of energy, but both will continue to radiate to each other (balance: therefore no change in energy state of either body). The vector defining the path of a IR photon will not stop because the surface it comes across is at the same temperature as its source. There will still be radiative transfer but the net transfer = 0. For your point to be true, that is no transfer, then the bodies would have to reach a state where they no longer emitted radiation.
Mr. Eschenbach
thanks for attention
I remain convinced that whatever the conversion watt watt/m2, it does not change the fact that in Figure 2, you indicate that the core issue 235 w/m2 and, depending off the amount off energy in the core, and the surface (let suppose, it is an blackbody)and thus the surface of the sphere emits 235 w / m2 to stay équilibre.iff you forget a differences off the surface off the core, and the sphere.
Now you certainly indicate that, because You are aware that a diffrence in the surfaces matter.
and because you are in agreement that if the surface of the sphere is larger, the radiation for each squre m will decrease.
You then specify the sphere also emits 235 w/m2 inward.
But again, , you double the irradiation area!
By each square m off the sphere, You have 235 w émitted outwards, and also inwards, so the flux off energy is doubled?
Now, something else,
you indicate that the emission flux of 235 w/m2 of the core induce on the surface of the sphere equal flux irradiation outwards , but also inward.
I assume that if this time the core issues as indicated 470 w/m2, there is no reason that this time it would not be divided into two streams,
there should be 470 w/m2 inward as to the outside, if you follow your first pattern?
it is an circular patern?
–
Willis ….Lets reverse your set-up.
The steel hollow sphere is in deep space (-273C) with an initial internal vacuum.
Its heated by an external variable power supply carefully monitored to maintain the temperature at 50C.
The voltage and current are noted, say as V1 and I1, to get power P1
Now suddenly insert an object inside the sphere at -40C ( i can because its a thought experiment! ).
What happens next?
Does the real radiation from the colder object warm the steel sphere when absorbed?
I think not.
To restore the steel sphere to 50C the supplied power (P2) would have to increase for a time.
P2 > P1
Point being that in these apparent two object problems there is always a third (often ignored )temperature …..the surroundings.
In the example above the cold object at -40C is separated from the surrounding deep space by the steel sphere.
So no Virginia, colder objects don’t always ‘warm’ objects at a higher temperature.
Even If the steel shell was much larger than the planet surface, let’s say twice the surface area, it makes absolutely no difference to the argument. It this case the outer surface of the shell would have to radiate 117.5 W/sq.m to balance the 235 W/sq.m at the planet’s surface. This accords with the shell having a temperature of 213K. Because the shell is at 213K it also radiates from its interior surface 117.5 W/sq.m (Stefan’s Law). This energy falls on the planet which, because it has only half the surface area of the shell receives 235 W per sq.m of planetary surface.
So, the same calculation ensues. The planet is now receiving 235 W/sq.m from its interior and a further 235 W/sq.m ‘back-radiation’ from the shell, that is 470W/sq.m altogether and so the planet becomes much warmer because of the presence of the surrounding shell.
Did you notice the provocative ‘back-radiation’ I slipped in there?
Unfortunately, most commentators don’t seem to have the ability to grasp this, but it is so simple and fundamental it separates the sheep from the goats.
The shell game is a foolish game to play. The shell game is how the AGW pseudo scientist came up with their ludicrous 33 degrees warmer because of “GHGs” meme. Once again let’s review the critical “Do Nots” of atmospheric modelling-
1. Do not model the “earth” as a combined land/ocean/gas “thingy”
2. Do not model the atmosphere as a single body or layer
3. Do not model the sun as a ¼ power constant source without diurnal cycle
4. Do not model conductive flux to and from the surface and atmosphere based on surface Tav
5. Do not model a static atmosphere without moving gases
6. Do not model a moving atmosphere without Gravity
7. Do not model the surface as a combined land/ocean “thingy”*
As you can see the shell game commits the following scientific atrocities 2,3,5,6 & 7. It’s so bad at modelling the atmosphere it cannot even commit 4.
Does the surface emit IR? Yes. Does the atmosphere absorb some IR? Yes. Does the atmosphere radiate some IR back to the surface slowing it’s cooling rate? Yes. Is there a radiative GHE? Yes. Is the NET effect of radiative gases in our atmosphere warming? NO!
Radiative gases are critical for the continued vertical convective circulation observed below the tropopause. Without radiative energy loss at altitude, convective circulation would stall, and our atmosphere would heat and go isothermal. Increased radiative cooling of the surface under a non radiative atmosphere is no substitute for radiative cooling at altitude. Avoid the “Do Nots” and observe the science Willis avoids and you will find –
– Due to gravity, pressure gradient and resultant IR opacity gradient, an atmospheric column emits more IR to space than to the surface. 2 & 6
– Most energy emitted as IR to space from the atmosphere was obtained by the release of latent heat of evaporation above the level of max atmospheric IR opacity. 2 & 6
– Radiative gases emit more energy to space than the atmosphere ever intercepted from NET surface IR flux 2, 5 & 6
– Radiative gases return far, far less energy to the surface than emitted from the surface. 7*
– The conductive transfer of energy and the transfer of latent heat to the atmosphere is a function of surface Tmax not Tav. 3, 4 & 5
– Surface cooling by conduction + convection is more effective than gas conduction alone. 5
– Under a non-radiative atmosphere, increased radiative surface cooling will not result in significantly increased atmospheric cooling. 5 & 6
– Deep vertical convective circulation cannot continue under the tropopause without radiative gases. 5 & 6
And the summary? AGW is tripe. Radiative gases cool our atmosphere at all concentrations above 0.0ppm. Adding radiative gases to the atmosphere will not reduce the atmospheres radiative cooling ability.
* Willis has yet to concede that DWIR has no effect on the cooling rate of liquid water that is free to evaporatively cool. I base my claim on the several empirical experiments I have conducted myself. To this date I am unaware of any empirical work Willis has conducted in this area, or indeed any empirical experiment he has conducted.
Michael Moon says: February 6, 2013 at 9:37 pm
That shell is called ’insulation’ Mike; I think heating engineers know about it. But note, in Willis’s article he says nothing about doubling the heat input. That stays the same. Why not go back and read it very carefully and see if you can understand it this time.
.
There seems to be great confusion about fundamentals and the ‘imaginary’ 2nd Law of Thermodynamics.
Understanding electromagnetic radiation is a prerequisite to understanding the greenhouse effect. Radiation is a wave motion which transports energy through empty space at the speed of light by means of self-propagating electric and magnetic fields. It manifests itself in many forms, for example, as gamma rays, X-rays, light, infrared and radio waves. The only intrinsic difference between these various forms is that the waves have different wavelengths.
Radiation doesn’t ‘know’ if it is coming from a cold object or heading towards a hot object. How could it?
When radiation strikes the surface of a material one of three things may happen. It may be reflected, in which case it continues in a new direction with its energy conserved. It may pass through the material, like light through a window, radio waves through the walls of a house or like X-rays through your body. Or it may be absorbed by the material. Usually, a combination of two or three of these effects apply.
When radiation is absorbed the energy it conveys is also absorbed. Energy must be conserved and, most commonly, the absorption of radiation causes the absorbing material to heat up (the energy could, however, produce some alternative effects instead; photo-electric emission for example).
The Earth approximates to a black body in the long wave infrared region and so inevitably absorbs nearly all the down-welling radiation from the ‘cold’ atmosphere. And of course, this makes the surface warmer than it would otherwise be.
That shell is called ’insulation’ Mike
that something different no?
there is no matter off insulation here,
Willis
I have enjoyed your philosophy over the years, particularly your views on the safety valve action of thunderstorms, but your steel shell model here jars rather with my concept of the physics – bearing in mind that my degree is in archaeology, I might of course be missing a trick, but……
In my view your back radiation at 235 w.m-2 would only work if the steel shell is hotter than the core, which I don’t believe it is otherwise your model would be negated by the 2nd law of Thermodynamics. (Heat only flows from hot body to cold body, never in the other direction.) Otherwise the back radiation must just be deflected back to the steel shell, from whence it came and it wouldn’t heat that either, as it has just emanated from there and would eventually be re-radiated into space.
The same would apply, even if the core and shell were perfect black body radiators.
And as your analogy is, I assume, to ultimately represent in the end the earth and atmosphere, and particularly CO2 , the same principle applies – unless you assume that the CO2 molecules are hotter than the earth’s surface below the concept of ‘back radiation’ on which AGW seems to heavily revolve is not physically possible! Bit of a fatal flaw in the AGW concept.
Tx. Imarcus.
Your first model is OK for greenhouse gas theory, BUT IT IS COMPLETELY WRONG FOR PLANET EARTH!
Why is that? Well because the TOP os Earths’s atmosphere is actually HOTTER than the bottom. This is because UV radiation is absorbed by the top of the stratosphere to such an extent that the top of the atmosphere is the hottest part.
You might like to consider how this might change your simple metallic atmosphere model, Willis.
“And that is all that there is to the poorly named greenhouse effect. It does not require CO2 or an atmosphere, it can be built out of steel.”
This is untrue. What you see in the steel shell case is not “back-radiation” at all. It is merely reflection of the existing radiation from the planet – there is no change in wavelength. The steel shell will reflect the IR just as a mirrored surface will reflect visible light unchanged, and it will do so at the same angle of incidence too. CO2 actually re-radiates the IR but in all directions and at a different wavelength – quite different from your simplified model. Also, the CO2 only makes up 500ppm so it is nowhere near as dense as a steel shell at the photon level.
Furthermore, if the only heat source is actually outside the planet, how warm will the planet get then?
This steel shell model is typical of the over-simplified atmospheric models we get from Team-AGW to justify their claims. It has no business being considered here.
Konrad. says:
February 7, 2013 at 1:29 am
Konrad,
Your comment above contains many surprises, at least for me, but I find all of them plausible. You need to get published.
“Now, note what happens when we add a shell around the planet. The shell warms up and it begins to radiate as well … but it radiates the same amount inwards and outwards.”
Are you sure it warms up? If it is 100% reflective of IR it won’t absorb any energy – so it won’t warm up. What will be the temperature of the planets surface then? (Here’s a clue – it won’t just get hotter and hotter….)
My simple explanation of the Greenhouse effect.
Earth Shell Space
e1< e1< e1 e2> e2>
e3> e3/2>
<e3/2
e1 = energy recieved from the sun
e2 = energy directly radiated to space
e3 = energy indirectly radiated to space
Long term
e1 = e2 + e3/
Sorry. Should be
Long term
e1 = e2 + e3/2
Willis,
IR radiation has wave/partical duality properties as such it can be interferred with. Given your source in the thought experiment is the same the likelyhood is that the IR is coherent from both the steel plate and the core. This give an effective 0 W/M^2 between core and shell (much as there is no energy difference between core and slightly less core) as they are in equilibrium. No work is being done ergo no extra energy is needed for balance. If you place a thermometer between core and shell it will reach an equilibrium temperature faster than if no shell was there it will not be a higher temperature. As with all energy fields – it is not the amount that is important but the difference – in equilibrium there is no difference.
I really don’t get the quibbling by many here. Surely the point of R. Wood’s experiment is this . .
All things being the same, except that in one box infra red is retained by the glass and in the other it is released by the rock salt, it seems that the contribution to the temperature in the box made by reflected infra red is trivial at best.
The rest of the argy bargy is fine in that it would refine the experiment in various ways but it won’t change the observed fact by Wood that the greenhouse effect is not enhanced by the trapping of infra red radiation.
Ryan says: February 7, 2013 at 2:13 am
Ryan, Bad Clue! It will just get hotter and hotter. Or do you have some maths to show us why it won’t?
You have a constant heat source putting energy IN. You postulate a perfect reflecting surface that stops any energy getting out. The energy builds up ad infinitum. It will just get hotter and hotter.
Ryan says:
February 7, 2013 at 2:13 am
What part of “steel shell” leads you to think it is 100% reflective of IR? Steel has an IR reflectivity of about 5% or so …
Since nothing is 100% reflective, I fear I can’t answer you. It’s like the question about what happens when an irresistible force meets an immovable object. We can’t say because they are not possible even in theory, like a 100% reflective surface.
w.
Richard LH says:
February 7, 2013 at 2:17 am
That is so simple it is totally cryptic, I haven’t a clue what you’ve just said. What does e1 less than e1 less than e1 mean? (e1<e1<e1 in your explanation above)
w.
Keitho says:
February 7, 2013 at 2:37 am
Since the Wood experiment is totally unlike and has nothing to do with the greenhouse effect, it couldn’t reveal a dang thing about the greenhouse effect … read the post again. For the greenhouse effect to work, the shell has to/must/is required to be thermally isolated from the surface so it can take up a different temperature. In the Wood experiment the shell will always be at about the same temperature as the inside of the box, due to both convection and conduction.
So the two setups, the Wood experiment and the planetary greenhouse, have nothing to do with each other, and the experiment can’t show anything either way about the greenhouse.
w.
Thanks Willis, I was merely reflecting on the actual greenhouse that Wood was running and the fact that preventing the trapping of the IR made no measurable difference to the temperature in the boxes. The real cause of warming was the prevention of convection from cooling the box. The “reflected” IR is no big deal so surely the same thought could be applied to CO2, particularly in the presence of convection and conduction.
Your thought experiment at a global level is quite different and rather obvious assuming a vacuum between the planet and the steel shell where there is no convection or conduction, only radiation. Same difference really as the same point seems to be made.
Tim says:
February 7, 2013 at 2:32 am
My friend, if you can get coherent radiation from a chunk of steel, the laser scientists would like to talk to you … your whole claim is interesting, fanciful, and unfortunately, not possible. No coherent radiation comes from a planet or a steel shell, doesn’t happen.
w.
Willis Eschenbach says: February 6, 2013 at 2:33 pm
If I light a candle on the earth during the day, the sun ends up warmer than it would be if I didn’t light the candle.
____________________________________
Ahh, Willis, I did warn you that all the trolls would come out of the dark, claiming that a cool body cannot radiate to a warm body. (Cooler bodies have eyes, that can see a warmer body coming, and so they shut off their radiation immediately….)
But I must say that your analogy of a candle warming the Sun is much more elegant than my two radiator scenario. I like that idea that I can hold a candle up to the Sun and make it warmer. Anyone bold enough to say by how much?
.
“To compare with Willis’s example, you need to add a constant heat source to the inside of the thermos. Now what happens?”
Ah yes, but there is yet another flaw in Willis’ over simple thought experiment. In his experiment you have an infinite energy source of unknown temperature. This is not the case in the Earth/Sun system. In the Earth/Sun system we know how hot the Sun is, and we also know how hot it could theoretically make the surface of a body heated by the IR emitted by the Sun (which is rather smeared out by the time it reaches Earth).
Willis’ thought experiment would indeed by more honest if you put a body of known temperature in the center of the sphere and asked if it will get any hotter if you but a metal shell around the whole thing. Only it can’t be a metal shell in willis’ experiment because an ideal metal shell won’t absorb any heat energy and get warmer – it needs to be a “black body” shell to absorb the heat. But it can’t be a black body as such because a black body only radiates otuward and not out and in at the same time. And you can’t measure the temperature of the atmosphere is Willis’ experiment because there is no atmosphere so no sensible heat in the atmosphere. And if there was an atmosphere his experiment wouldn’t work at all.
Time to move on from this isn’t it?
I do wish Willis would wake up and realise that IR is not “heat” nor does it have a “temperature”. IR is a form of electro-magnetic energy that can be CONVERTED to heat by an ideal black body. If you talk about IR then you need to discuss the laws of thermodynamics at the quantum mechanical level. If you don’t want to discuss quantum mechanics then you must ignore IR and stick to discussing simple heat energy transfers using the laws of thermo-dynamics alone. Willis gets himself in knots by mixing IR into simple models of heat energy transfer that were developed before IR was known or understood and which don’t need a knowledge of IR to work.
Ryan says:
February 7, 2013 at 2:03 am (Edit)
The emissivity of various materials is given here. Reflectivity is equal to [ 1 – emissivity ]. The reflectivity of steel is about 10%-15%. So what you see in the steel shell case is indeed back radiation. I should have clarified that the shell and planet were blackbodies … I’ve added a note to the head post, thanks.
w.
This was your thought experiment – so if we take multiple IR wavelengths you still get to the problem that in equilibrium there is no energy difference between core and plate (or it is not equilibrium) . The “vaccuum” merely acts as a conduction/convection filter from core to plate – energy is a flow not a substance (no phlogiston in them thar hills) as such when the plate has expanded (well it is steel) and in equilibrium there is no more work being done on the plate – the plate becomes the core for IR transmission purposes. The flow is one way, net IR transmission at the inner surface is still zero.
davidmhoffer says:
February 6, 2013 at 8:12 pm
Or you can subscribe to the theory that these laws of physics are not in the thermo texts, haven’t been proven by experimentation, and that engineers design nuclear reactors, kitchen ovens, air conditioners and automobile cooling systems that functions as designed in advance of being built by a complete fluke.
I call that BULL SHIT.
Which of those systems rely on a Vacuum.
All of them rely on being IN CONTACT with the Heat Source or the Air being heated by the Heat Source or Air to radiate the heat away.
consider the implications of your thought experiment. with a finite amount of input energy you can heat an object to infinite temperature. you should patent it.
— RADIATION DEPEND ONLY ON TEMPERATURE AND EMISSIVITY
Period. No exceptions. —
Actually there are some rather big exceptions. That’s because you forgot to mention when one can apply the SB-law.
“Sei ein absolut leerer Raum rings von für Wärmestrahlung undurchlässigen Wänden von
der absoluten Temperatur t umgeben;” – Bolzmann, 1879
You can only apply the law to a real blackbody in vacuum that doesn’t conduct heat to the other side. Steel is not a blackbody and you assume that it can conduct heat to the outside. So, you are not talking about the SB-law, but some madeup imaginary law.
By the way, your radiator also has to be fixed in space and it must have a defined surface. That’s why you cannot apply it to any gases. At least that’s what we learned in University here in Germany.
Whenever you use a ‘greybody’ you should first show that you are allowed to use some simplifications, and that this simplifications won’t change the essence of the law.
Sorry – bad ascii text formating.
My simple explanation of the ‘Greenhouse effect’.
Earth Atmosphere Space e1a> e1b> e1b> e2> e3> e3/m> e3/m> <e3/n <e3/ne1 = energy recieved from the sun
e1a = energy reflected by atmosphere
e1b = energy reflected by surface
e1c = energy absorbed by surface
e2 = energy directly radiated from surface to space
e3 = energy indirectly radiated from surface to space
e3 = e3/n + e3/m
n and m dermine the ratio of reflected energy to passthough.
Long term
e1c = e1 – e1a – e1b
e1c + e3/n = e2 + e3
e1 = e1a + e1b + e2 + e3/m
e3/n is the ‘Greenhouse effect’ as seen at the surface.
let’ s try another hypothesis
if I put a second shell around the first, and I make the same calculation like the mechanism described, this time, the core t° is 4 times higher?
if I repeat this process n times, can we say that the t ° of the core can become infinite?
weird is it not?
Might I suggest that the energy radiated by the planet is absorbed by the interior blackbody shell and an equal amount of radiation is radiated outwards by the shell. There is no temperature increase at the surface of the planet.
Wow, Willis, I have enjoyed so many of the things you have written here over the years, but this one, wow. Still clinging on to the “cold objects can make warmer objects warmer still” garbage? You “thought” experiment here is nothing more than a hodgepodge of card shuffling. I recently played this same game with my father over Dr. Spencer’s cold object/hot object claim. My father lost, and lost of one very simple reason, 2nd law. As indicated by others here, no matter how you slice it, twist it, dice it, a colder object cannot warm a warmer object, not in gas, not in steel, not in any way. The GHE either doesn’t exist, or is of such utterly small consequence that we will never be able to actually measure it. As for the IPCC form of the GHE theory, it is impossible.
Please, go back to writing your “good” articles. I really enjoy reading a lot of your stuff, but this article just blew away about a years worth of reading from you. argh….
A C Osborn says:
February 7, 2013 at 3:48 am
davidmhoffer says:
February 6, 2013 at 8:12 pm
Or you can subscribe to the theory that these laws of physics are not in the thermo texts, haven’t been proven by experimentation, and that engineers design nuclear reactors, kitchen ovens, air conditioners and automobile cooling systems that functions as designed in advance of being built by a complete fluke.
I call that BULL SHIT.
Which of those systems rely on a Vacuum.
All of them rely on being IN CONTACT with the Heat Source or the Air being heated by the Heat Source or Air to radiate the heat away.
>>>>>>>>>>>>>>>>>>>>>>.
Huh? Are you of the impression that systems being in contact suspend all other modes of energy transfer? It isn’t A or B. It is A and B. If the systems are in contact then they xfer energy via conduction and radiance. Makes the math even more complicated. But if you think the engineers can get it right by simply ignoring the laws of thermodynamics because the systems aren’t in a vacuum….I’ll add you to the list of people who think nuclear reactors work by shear fluke.
squid2112;
As indicated by others here, no matter how you slice it, twist it, dice it, a colder object cannot warm a warmer object, not in gas, not in steel, not in any way.
>>>>>>>>>>>>>>
When I was a kid we didn’t have enough money for heating fuel. We piled snow up against the side of the house to the point we could pretty much keep it warm with just the body heat of the people inside. Good to know that the snow must have been warmer than the house, because while shoveling it at -20C I could have sworn it was colder.
davidmhoffer says:
February 7, 2013 at 5:34 am
“When I was a kid we didn’t have enough money for heating fuel. We piled snow up against the side of the house to the point we could pretty much keep it warm with just the body heat of the people inside. Good to know that the snow must have been warmer than the house, because while shoveling it at -20C I could have sworn it was colder.”
What the snow did was increase the insulation factor for the house, it’s the same way igloo’s can be a lot warmer inside than out, while made of snow. Also note, snow has a lot of trapped air, which is a very good insulator.
“Mark Harvey aka imarcus says:
February 7, 2013 at 1:51 am ”
In my view your back radiation at 235 w.m-2 would only work if the steel shell is hotter than the core, which I don’t believe it is otherwise your model would be negated by the 2nd law of Thermodynamics. (Heat only flows from hot body to cold body, never in the other direction.) ”
When people read “heat flow can only flow from a hot body to a cold body” they nearly always make the same mistake. They take this statement from the point of view of an omnidirectional flow of heat. They mistakenly believe that it means that there can never be any transfer of the energy from the cooler body to the warmer body. This is an incorrect understanding.
The 2nd law always refers to coupled systems. It says that if you have 2 bodies of different temperatures there cannot be a spontaneous transfer of heat from the cooler to the warmer. If heat did in fact transfer in such a way, the warmer body would grower warmer still, at the expense of the cooler body. But it doesn’t say that energy cannot flow from cooler to warmer. Energy does in fact flow from the cooler to the warmer body, but at the SAME TIME, even MORE energy flows from the warm to the cooler body.
Before you say this can’t happen – it would lead to pertual motion, etc – just consider this. It is impossible to devise any hypothetical situation where you can allow energy to radiate from the cooler to the warmer body WHILST simultaneously preventing the energy flowing the other way. Energy will ALWAYS flow to the cooler body at a greater flux density than the other way round. The 2nd law is not violated.
Konrad. says:
February 7, 2013 at 1:29 am
“The shell game is a foolish game to play……………….”
Konrad’s explanation wins the debate IMO.
Beautifully summarized Konrad.
Perfect.
Where’s Al Gore? I want to tell him the science is settled. ;^)
Don’t think I’ve ever seen a thread where Mosher is right, and many other posters are wrong….
Greg House,
“No, this is not true, anyway the “greenhouse effect” as presented by the IPCC is about particular “greenhouse gases” making the Earth warmer specifically by by returning back some of the Earth’s outgoing IR radiation, it is not about the whole atmosphere. They do not tell people, however, that this 150 years old hypothesis was debunked 100 years ago by the Wood experiment.”
1 GHE theory in general predates the IPCC significantly.
2 Willis shows quite convincingly why the Wood experiment demonstrates/debunkes NOTHING.
3) IPCC claims the GHE is about particular gasses returning back some of the Earths’ outgoing IR radiation. Others here claim that the above applies to the whole atmosphere and others still claim it is a gravitational effect not radiative. Some here even claim simple convetive distribution of heat fully explains the difference.
I did note that the mechanism by which the atmosphere keeps the surface warmer than it would be without one was in dispute. However, a simple comparison with the Moon shows that whatever the mechanism is the atmosphere clearly affects the Earths average surface temp.
‘Thought experiments’ may fit comfortably into either Philosophy or Mathematics, but they are not a proper part of Physics. [Sorry, Einstein!]
When others try to repeat Willis’ ‘thought experiment’ and get different ‘results’, it is quite clear that there is confusion of mind somehere. I’m not the one to point my finger at anyone in particular, or waste my time trying to disentangle where the mistaken thoughts might be, because all (including me) are equally susceptible to mistakes in this regard.
One of the commonest mistakes in ‘thought experiments’ is introducing unspecified assumptions without realizing you are doing it. Merely stating that one “knows” what a certain hypothetical object will do in specified circumstances reeks of hubris.
So, Willis, please:
1. Build your radioactive, model ‘planet’;
2. Measure its equilibrium temperature;
3. Enclose it in a steel sphere of specific size (without contacting the ‘planet’);
4. Re-measure the temperatures of the two spheres (good luck with that!); and
5. Report the results.
All else is imaginary, with real numbers, i.e. Mathematical games. The gulf between ‘what we think will happen’ and ‘what actually happens in a real experiment’ is often surprising and should be educational.
“The emissivity of various materials is given here. Reflectivity is equal to [ 1 – emissivity ]. The reflectivity of steel is about 10%-15%.”
Ummm, I think you need to go back and re-read that table again!
Anyway, I’m glad you admit the shell cannot be made of steel at least. Rusty steel perhaps (iron oxide will get you closer to being a black body) but not steel. So now you know we are talking about black bodies – definitely not steel, not anything like steel.
Lets consider what ACTUALLY happens with a CO2 molecule when a IR photon hits it. Remember that IR is not HEAT. It is an electromagnetic radiation with energy that can be converted to heat in certain circumstances. So a photon with energy in the IR spectrum reaches a CO2 molecule. What happens to it? Well if it is within the scope of the covalent bonds of the CO2 molecule it could be absorbed by the electrons in the bonds causing the electrons to be lifted to a higher energy level. But here is the crux of the matter – if the electron is already at that higher energy level then an incoming IR photon WILL NOT BE ABSORBED (at least not at the same frequency) – the photon will just travel straight through. But also bear in mind that lifting an electron to a higher energy level does not in itself cause heat to occur – the electron must drop back to its lower energy level and the assumption is that the electron then loses energy and that energy (probably) ends up at some point exhibiting itself as heat (just following the laws of entropy) but maybe not right away because the CO2 may just emit IR of a different wavelength (i.e. not heat as such) which may then be absorbed (or then again may not) by some other object which then gets a bit warmer.
Does any of this actual description of what happens to CO2 with an incoming photon strike you as being in any way related to ideal black bodies suspended in space over planet earth with no atmosphere when the Earth is being heated from the inside from an essentially infinite energy source of no known specific reaction temperature? Because it seems the model you are creating is in no way related to reality and therefore not useful, before we even begin to get into the details of whether your description of the maths is plausible.
davidmhoffer says:
After reading all of these, I think you’re one of the ones who have it right.
Willis, Working from a flux rate (watt’s/sq) alone makes the analogy harder to follow. S-B is based on a difference in Temps, with that an an emissivity, you can calculate a flux rate. In the case of this example the 100/40 temps (fhhaynie’s calculation) for the inner/outer shells(if the steel was a blackbody) sounds about right. The net flux from the surface should then match your 235w flux (net) from the inner to the outer, the back radiation is why the inner sphere is warmer than the outer.
Ryan says:
February 7, 2013 at 3:10 am
“I do wish Willis would wake up and realise that IR is not “heat” nor does it have a “temperature”.”
It has an equivalent temp. CO2’s main absorption bands are ~4u @ ~850F, and then 10u-15u 60F- -112F But the 10u-15u band is very weak, and much of it is overlapped by water vapor.
Greg House, Please, please go to the hardware store, and buy yourself a handheld non contact IR thermometer. Once you can explain/understand how that works, you’ll at least get that S-B is well tested, and how everything radiates IR, even if it’s cold. Every things temp is the net of incoming radiation and out going radiation. Just as described by S-B.
My Ryobi IR thermometer on a clear sky 35F day, reads the skies temp at it’s minimum scale -40F, or it’s maximum scale of 608F. The less than -40F is the back radiation, instead of being the absolute zero of space, this lowers the temp difference between the earth and space slowing the cooling of the surface. It’s this -40F that all of the hubbub is about. But after digging through 120 million surface temp records, it doesn’t reduce reduce the amount of night time cooling any. The 608F is unexpected, but I think it’s just a reflection of solar IR off the atm. If there is a temp difference from increasing CO2, IMO it’s from this. Since a CO2 molecule will only absorb, and emit a single photon at a time, more Co2 will reflect more IR.
Lastly Dr Spencer did attempt to do this experiment.
Konrad. says:
February 7, 2013 at 1:29 am
“Does the surface emit IR? Yes. Does the atmosphere absorb some IR? Yes. Does the atmosphere radiate some IR back to the surface slowing it’s cooling rate? Yes. Is there a radiative GHE? Yes. Is the NET effect of radiative gases in our atmosphere warming? NO!”
This is a nice summary, but IMO the last answer is a possible slight warming, I put a max of maybe 25% of the measured trend, a slight beneficial warming to go along with all of the plant food. Plus if the Sun is getting ready to go on vacation, we’ll want all the extra warming we can get.
As a side note, we’ve had snow on the ground all but 4 or 5 days since a few days before Christmas in NE Ohio, it’s been quite a while since that’s happened!
Please explain how the low energy photons from the colder body gets absorbed by the atoms of the hot body already in higher excited states.
micro;
What the snow did was increase the insulation factor for the house, it’s the same way igloo’s can be a lot warmer inside than out, while made of snow. Also note, snow has a lot of trapped air, which is a very good insulator.
>>>>>>>>>>>>>>>>>>
Well gee, the air in the snow would be the same temperature as the snow, would it not? And how, exactly, do you think insulation works? It works by absorbing radiated energy and re-emitting some portion of it back.
So the summary of this thread reads pretty much like this:
Willis:
here’s how the Woods experiment was set up.
Whole Bunch of People Who Have Studied and Worked with Thermodynamics:
here’s about 20 different reasons why that experiment could not possibly demonstrate any meaningful data in regard to the radiative ghe
Whole Bunch of People Who Have NOT Studied or Worked with Thermodynamics:
you guys don’t know what you are talking about
I’ve scored the whole discussion and the winner is….
Lewandowsky
I’d like to make a quick comment about willis’ light bulb example and the assertion that a nice cool 60 watt bulb can increase the temperature of a hot 120 watt bulb.
Instead of light bulbs, imagine 2 batteries, one with 12 volts of electrostatic potential, one with 6. If you connect the two batteries in parallel (maybe through a resistance), the 6 volt battery doesn’t increase the potential of the 12 volt battery. That’s just obvious. Current only flows in one direction, from high to low. The potential of the 12 volt battery drops, and the potential of the 6 volt battery increases.
The exact same thing happens with a 120 and 60 watt bulb. The 60 watt bulb takes heat from the 120 watt bulb, cooling it.
Nevertheless, I completely agree with Willis, the 120 watt bulb is hotter because the 60 watt bulb is there. That’s because a 60 watt light bulb is a much poorer heat sink than the alternative, some nice cool air, which will absorb heat than waft away and be replaced by more cool air. The hot bulb replaces cool air and so the 120 watt bulb has a harder time getting rid of its heat and it runs hotter still.
So the 60 watt bulb is like a 6 volt battery and the air is like, say, a 1.5 volt battery. Less current flows from a 12 volt battery into a 6 volt battery than it would into a 1.5 volt battery, and as a result the voltage you’d measure at the junction of a 12 and 6 would be higher than at a 12 and 1.5. So in a sense, the 6 volt battery “increases” the potential of the 12 volt battery, but only in comparison to the alternative.
Now, before anyone jumps on me, let me state my awareness that electrical flow may not be a perfect analogy to heat flow. Electricity really really (pretty much really) only flows in one direction. What about heat? Does heat flow in one direction, or does it flow back and forth? Who Cares.
Quantum mechanics says that radiation scatters in all directions without preference. The presence of a hot light bulb doesn’t resist the flow of photons the way a positive electrical field would resist the flow of electrons, so the 60 watt bulb scatters in the direction of the 120 watt bulb exactly the same amount as it would in the direction of empty air or an ice cube or a vacuum. But thermodynamics says that heat only flows one direction, from hotter to cooler. That’s because in thermodynamics, which was come up with before corpuscles and turns out to be statistical law, you’re really talking about a net flow. In thermodynamics it’s ok to think of the 60 watt bulb as a heat sink, cooling the 120 watt bulb, even though the reason it’s a poorer heat sink, taking less heat away from the 120 than air would, is actually because it gives more back. Whether you think of heat as a flux flowing in only one direction, or as discrete bits of radiation bouncing between them back and forth, it really shouldn’t matter; as long as you just stay consistent about it you’ll get the same answer.
In other words, I think most, or at least a lot of, the arguing here is irrelevant and just people defending their initial semantic choices. But as for me and my own personal morality, I think it’s probably best when talking about “heat” to stick with thermodynamics convention and think of it as a flux and say it only flows from hot to cold, and if you’re talking about “radiation” it’s ok to say it goes everywhere.
Micro.
You can’t measure back radiation with an IR thermometer. They are specially designed to work within the IR atmospheric window, which means that they won’t see radiation from the atmosphere.
CO2’s absorption band relevant to the greenhouse effect is around 15 microns. It is not weak!
In fact the small amount of CO2 in the atmosphere will absorb 95% of radiation at 15 microns within a distance of 1 metre
lou says:
February 7, 2013 at 7:05 am
“Please explain how the low energy photons from the colder body gets absorbed by the atoms of the hot body already in higher excited states.”
Ok, I’ll have a go. Vibrating atoms of the “hot” body are not all moving at the same speed. Because they are colliding off each other, their movements are random. Some atoms will be moving at a speed higher than the temperature of the body would suggest, others moving slower. The slower moving atoms are able to absorb the photons from cooler body.
Though, having written that, I am not sure whether you are conflating two different things – the temperature of the body which consists of the average KE of the atoms, and whether the atoms exist in highly excited states. The latter means their electrons have moved to higher energy shells, and is not a function of their KE (temperature). Higher temperatures do not necessarily mean that their atoms exhibit highly excited (electronic) states – it merely refers to the average speed of motion of the atoms. This has nothing to do with whether or not the atom can absorb a photon, imo.
@ Willis,
Figure 2 is WRONG!
There is no 470W/m^2.
.
Even IF the steel shell was a perfect reflector and insulator – not allowing any losses. The internal volume will still be 235W/m^2.
.
What this theory does not cover is the rate of loss against the rate of heating. The effect of shutting the oven (or fridge) door.
.
To comprehend more clearly with fewer issues please see across temperatures as a “potential difference” the same mindset to Voltage differences. Then consider the rate of heating and cooling as Amps. The outcome is, of course, in Watts.
davidmhoffer says:
February 7, 2013 at 7:11 am
Fair enough.
@MattS
I quoted John Christy for a reason, as I think he knows a little bit about atmospheric physics hence it is “a real head scratcher” the atmosphere isn’t warming per the “theory”. Which theory was he speaking of? Atmospheric CO2 has been increasing. The upper atmosphere should be warming at a higher rate than the surface. The stratosphere should be cooling because we’re told the troposphere “traps” the heat. The opposite is true, so the idea of an “enhanced” GHE is wrong. If the basic physics according to Christy is the
then something is wrong with the “theory”.
We are constantly lectured that increasing CO2 levels WILL warm the atmosphere; it is all about basic high school physics we’re told. Well, I don’t think it is about basic physics. I think it is about how the climate system works apart from “basic physics”. The physics is not well understood in my view.
We’re also told OHC increases in the past 30 years is due to AGW. There is zero empirical evidence that even a doubling of atmospheric CO2 can warm the oceans to any measurable degree. Instead, we’re told there is mixing by ocean waves that magically transports the top few microns of “heated” water affected by “back radiation” from a few extra ppm’s of atmospheric CO2. Sure, everyone stirs their hot coffee to make it warmer. How can anyone try to pass off the idea that anything other than SW solar radiation can warm the oceans and expect thinking people to believe that is frankly, insulting.
We’re also told water vapor is a positive feedback. Ok, then explain why deserts with very little water vapor become much warmer than tropical regions with much higher water vapor content. There seems to be a limit to how much the earth can warm, and water in its various forms appears to be the mediator.
I understand radiation is the only method by which heat is transferred to space. However, it is convection that dominates during the day when the sun shines which cools the surface by transporting heat to the upper regions of the troposphere. The surface of the earth would be uninhabitable if not for convection. I question whether the “basic physics” properly account for these convective processes.
I am a bit disappointed Willis did not address Tim Ball’s reference to http://principia-scientific.org/supportnews/latest-news/34-the-famous-wood-s-experiment-fully-explained.html . I claim no scientific expertise, only that I have been following the “greenhouse effect” meme for the last 25+ years and it is not working as advertised. Obviously the physics are not all accounted for or that well understood.
If the Woods experiment did not demonstrate/disprove anything, then neither did Arrhenius.
Finally, we’re told the earth’s atmosphere is not like a real glass greenhouse, yet I have seen dozens of references by various government science agencies and universities describing the earth’s atmosphere being like a real glass greenhouse in which the troposphere “traps” the heat thereby cooling the stratosphere.
MikeB says:
February 7, 2013 at 7:24 am
They have to measure IR in the same range the meter can measure temps in, 608F to -40F is the full scale range of the meter. Now the meter’s emissivity setting is set to .95, and the sky might not be .95, so the temp displayed could be wrong, but it has to measure IR in the 4.5u to 12.5u range. I would love to have an IR spectrometer, but it is out of my price range. And it obviously reads a IR signal from the sky. Besides NASA thinks it can be done.
CO2’s absorption band relevant to the greenhouse effect is around 15 microns. It is not weak!
In fact the small amount of CO2 in the atmosphere will absorb 95% of radiation at 15 microns within a distance of 1 metre
Here’s the spectrum of Co2 from NIST. 15u IR has an equivalent temp of -112F, that might keep Co2 from freezing, but it isn’t going to keep all the water on the planet from freezing.
Sorry meant to quote this
Willis Eschenbach says, February 7, 2013 at 2:57 am: “Since the Wood experiment is totally unlike and has nothing to do with the greenhouse effect, it couldn’t reveal a dang thing about the greenhouse effect …”
==========================================================
I provided a quote from the IPCC explanation of the “greenhouse effect” and the link to it on this thread, you must have overlooked it.
The Wood experiment deals exactly with the underlying mechanism of the IPCC “greenhouse effect” (effect of trapped/back radiation) and demonstrates that it is bupkis.
cd says:
February 7, 2013 at 1:12 am
CD you are correct. I was sloppy in my expression at that point. I addressed that topic in later posts.
Bart says:
February 6, 2013 at 11:54 pm
P_shell_outgoing = sigma*T_shell^4*(4*pi*(R_inner^2+R_outer^2)
This is incorrect. There is no net energy flow from the shell to the core. The shell is not a generator. With no net energy there is no power flow from the inner surface. Net energy transfer back is zero. At best what you add is thermal resistance (what the rest of the world call insulation). But you need to be careful here because there is something called a critical thickness. This is the thickness of insulation that actually increases heat transfer due to greater surface area.
There’s waaaay too much here to respond to it all, but let me pick a few random bits to reply to.
“But here is the crux of the matter – if the electron is already at that higher energy level then an incoming IR photon WILL NOT BE ABSORBED (at least not at the same frequency) – the photon will just travel straight through.”
NO! That is more or less what happens for visible light, but not for IR. IR absorption is due to quantized VIBRATIONS of the molecules, not EXCITATIONS of electrons. The molecule can have multiple units of vibrational energy, so even if a molecule is already vibrating, it can pick up additional vibrational energy by absorbing another photon.
“… because all (including me) are equally susceptible to mistakes in this regard. ”
NO! People with more education and experience and intelligence are less susceptible to making mistakes. Not everyone is created equal when it comes to analyzing science.
“Energy does in fact flow from the cooler to the warmer body, but at the SAME TIME, even MORE energy flows from the warm to the cooler body.
YES! As an additional example, consider heat conduction between two pieces of metal at different temperatures — which is really just the sum total of the energy transferred at a molecular level by collisions at the interface between the two blocks of metal. Some of the collisions will involve faster-than-average atoms in the cool block hitting slower-than-average atoms in the warm block. ENERGY can and will occasionally be transferred from the cool block to the warm block but such collisions, but more often the transfer will be from warm to cool. The NET transfer of energy (ie the “heat”) will always be from warm to cool.
“Still clinging on to the “cold objects can make warmer objects warmer still” garbage?”
I am (unfortunately) not amazed that people still cling to this objection. It is very seductive to people to don’t really understand thermodynamics or the statistical nature of the 2nd Law.
> “heat” does not move from cool to warm.
> “energy” can and does move from cool to warm (just not as much as moves from warm to cool).
Others have given other examples, but try this one. Take a shed and put a 1,000 W heater inside. On a very cold day (say-20 C) the interior might reach 10 C. Now put the shed in contact with other cold air (say -10 C). Now the interior might reach 15 C.
You can slice and dice and discuss this anyway you want, but the simple fact is that the presence of “cold material around the heated object” (at -10C) was an integral part of “warming” the interior of the shed. We used merely “sort of cold surroundings” instead of “really cold surroundings” and the result was a warmer interior.
Just like using “sort of cold surroundings” (the atmosphere) can warm the surface compared to having “really cold surroundings” (2.7 K outerspace).
“if I repeat this process n times, can we say that the t ° of the core can become infinite?
weird is it not?”
Yes, it is weird, but it is not wrong! There is is a significant limitation to the thought experiment that would prohibit infinite temperatures (besides the mere fact that the whole thing would eventually melt). The heater itself must continue to provide the required power even when the surroundings are hot — ie the heater itself must be HOTTER than the surroundings so heat can still flow from the hotter heater to the cooler surroundings. Limitations on the actual heater used would eventually impose an upper limit. For example, if the sun (5780 K) is used as the heater, then the surface could never get above 5780 K no matter what sort of insulating layers (or mirrors or lenses) you tried to use.
davidmhoffer says:
February 7, 2013 at 7:11 am
And how, exactly, do you think insulation works? It works by absorbing radiated energy and re-emitting some portion of it back.
As a general statement I disagree. Most insulation tries to prevent convection and conduction.
Regardless of the Woods experiment; or irregardless, as the case may be, The Steel Shell experiment Willis presents simply doesn’t work.
Willis asserts his nuclear powered planet (sans shell) emitting 235 W/m^2, is in equilibrium.
Not true, it is cooling down, and eventually be quite cold when the finite amount of radioactive materials has all decaysed. OK so it may be a long time.
So now add the steel shell, and wait for it to reach equilibrium, which Willis asserts it does.
Well now we know for sure that it is not in equilibrium, since it is not all isothermal. The shell is presumably cooler than the planet. Furthermore, if it WAS in equilibrium, then Kirchoff’s Law would apply, and the shell would be absorbing and receiving the exact same spectrum of whatever radiation was in the cavity between the planet and the steel shell.
So a fundamental assumption of the experiment cannot be met.
Whatever the radiation flying around this shell game, it certainly is not black body radiation, so SB law and Planck’s radiation law do not apply here.
The Planck derivation was for the radiation escaping from a small hole in a THERMALLY ISOLATED ISOTHERMAL cavity, from which NO energy can escape through the insulating walls.
Steel of course is not a black body radiator, nor is any other known material.
The electromagnetic radiation spectrum, extends from down to but not including zero frequency (DC) and up to but not including infinite frequency, and a black body must perfectly absorb ALL of that radiation completely.
NO physical material has zero reflectance for EM radiation; that would reuire it to have unity refractive index like the vaccuum, and no material does.
Black body radiation itself is fiction, in fact it is fictionally fictional. It involves the physical properties of no physical material whatsoever. No electronic energy states, or any other trappings of quantum mechanics of real materials is involved in the derivation of the black body radiation formulae.
It is a quite hypothetical radiation spectrum that does not and cannot exist, and the knowledge of no material properties of real physical materials, was used in its derivation.
Yes it is an extremely useful and improtant result, but Willis’s shell planet, is not emitting BB radiation.
@davidmhoffer: February 7, 2013 at 5:34 am
Well davidmhoffer, you seem so ripe for being sold one of these new inventions today, run out and talk to one of the I.P.C.C. salesmen, get yourself one! I know, not quite as nifty as when you were heating your home with the backradiation from snow or you were staying so toasty warm in an igloo with all of that parabolic backradiation, but close.
http://www.ilovemycarbondioxide.com/IPCC_oven.HTML
☺
Seriously davidmhoffer, you and Willis have got some big problems here.
The shell as shown is outputting more energy, 470‹J/s/m2›, per time per area than is even available from the nuclear source in the first place, there is only one energy source in Fig. 2 and it’s maximum output through the sphere’s surface is 235‹J/s/m2›. Ever heard of the 1st law of thermodynamics? Don’t you see your problem? This is getting embarrassing for W.U.W.T.
Ever heard that an energy source can never heat itself?
I think now you too have mixed possible power with flux.
But this does shine a bright light on exactly where the Stefan-Boltzmann equation is being used and misapplied in climate science. Reflected energy from a source, in any manner, can never then be added back to the source’s original hypothesized flux by SB and then fed back recursively into the SB equation to hypothesize a new higher temperature of the source itself. This is where the I.P.C.C.’s Free Energy Oven fails.
This is also where Trenberth slips in that extra 23‹W/m²› in his “global” energy budget to boost the “backradiation”.
It is like taking a sheet of polished aluminum foil and holding it up to a wall radiating by SB per T at 400‹W/m²› and 400‹W/m²› is reflected back to the wall by the foil at the temperature of the foil so now you have 800‹W/m²› at the wall so of course the wall’s T must rise… if you can really see that this happening and being something physically real then, rather than trying to teach radiative energy science to all of the scientists and engineers coming here and to comment, maybe you should just stick to selling computer systems and Willis to construction.
However, David, if a cloud or the atmosphere gases absorb solar energy that came from an energy source external to the Earth’s climate system, the sun, then that energy can be deemed an original energy source in the system and that energy can be handled isotropically with down welling LW radiation that can raise the surface’s temperature if it ever increased.
I myself have been wrong in this respect too in the near past, to TFK09’s energy budget graphic, the only down welling source radiation is one-half of the 78, or 39, absorbed by the atmosphere and clouds plus the 161+1, or 162 absorbed by the surface. That gives the surface 200‹W/m²› of absorbed energy. From that the other one-half of the 78, or 39, goes to space plus the 98 sensible and evapotranspiration plus 102‹W/m²› net up radiated from the surface that gives 239‹W/m²› as expected, TFK’s one off. The net LW flux is the 102 from the surface, up, minus the one-half of the 78, or 39 down, giving the 63‹W/m²› net that you can see day in and day out of the net surface IR graphs at the ESRL.NOAA site for various locations. Finally even that now makes sense and balances and matches the data on the government climate and weather sites. I learn something new every day so I guess Willis’s so wrong post ends up with a good ending, for me anyway.
@tjfolkerts “Others have given other examples, but try this one. Take a shed and put a 1,000 W heater inside. On a very cold day (say-20 C) the interior might reach 10 C. Now put the shed in contact with other cold air (say -10 C). Now the interior might reach 15 C.”
This has absolutely nothing to do with the cold air making the warm air warmer!!! Once again this is due to conduction. Heat inside the shed will be conducted to the outside. The rate of conduction is dependent on the thermal gradient. The thermal gradient is dependent on the outside air temperature. Make the outside air warmer and the rate of conduction will decrease so the interior will be warmer. You can also slow the rate of conduction by covering the shed in rockwool. You cannot, however, significantly warm the shed by covering it with tin foil (although many a claim has been made that you can….)
I think it would be useful to back up one step and have -just- the argument about a hot plate and a not-so-hot plate. There are plenty of examples in textbooks to quote. Having the detailed discussion about how individual atoms in the “cold plate” can be hot and how individual atoms in the “hot plate” can be cold would be useful. Perhaps even a discussion over spectroscopy and how exceedingly hot things can -still- absorb IR photons – particularly if they’re molecules and happen to have vibrational modes as well.
And how many of the general thermodynamics rules are over NET energy transfers – while -statistical- thermo really lays out what temperature actually means atomically.
Until this particular discussion is firmly laid out, the entire “Steel Greenhouse” discussion inevitably latches onto misunderstandings.
“The Wood experiment deals exactly with the underlying mechanism of the IPCC “greenhouse effect” (effect of trapped/back radiation) and demonstrates that it is bupkis.”
The Wood experiment is similar in some ways, but very different in others, which is the point hat Willis was making and which you apparently missed.
> The earth is surrounded by extreme cold (outer space), but Wood’s experiment is surrounded by materials at very similar temperatures to the experiment itself.
> The atmosphere of the earth is many KILOmeters in size, but Wood’s experiment is many CENTImeters in size.
These (and other rather fundamental differences) make Wood’s experiment a rather poor analogy to earth’s situation.
Let me quote Wood himself on the fact that is experiments are applicable to real greenhouses, but may not be applicable to otehr situations like the earth:
wayne says (February 7, 2013 at 9:35 am): “The shell as shown is outputting more energy, 470‹J/s/m2›, per time per area than is even available from the nuclear source in the first place,”
Assume the surface area of the “planet” and the outer surface area of the “shell” are both 1 square meter (or if you must, make the outer surface of the shell 1.003 square meter). Now do the calculation in watts and get back to us.
Hint: The inner surface area of the shell equals the outer surface area.
Experiment to test “It is impossible to devise any hypothetical situation where you can allow energy to radiate from the cooler to the warmer body WHILST simultaneously preventing the energy flowing the other way. Energy will ALWAYS flow to the cooler body at a greater flux density than the other way round. The 2nd law is not violated.”
We have two bodies on either side of a one-way mirror which is mostly transparent to light and IR in one direction and mostly opaque or reflective in the other. On the side of the mirror which is reflective, we have a black body # 1, and on the on the opposite side of the one way mirror, we have similar black #2. The black bodies are equidistant from the one-way mirror, and we will, for this experiment, stipulate the black body on the reflective side of the mirror begins at 30 degrees C, and the similar black body on the transparent side of the mirror begins at 20 degrees C. Since we are interested in the radiative energy flows, we have created an experiment so that conductive and convective flows are minimized. What happens? To exaggerate the above, we will place similar parobolic dishes behind each of the two black bodies with the black bodies at the focal points. What happens? Does the cooler black body “heat” the warmer black body. Is energy flowing from the cooler black body at a greater flux density than the other way round?
Wayne says: “Ever heard of the 1st law of thermodynamics? Don’t you see your problem?”
No, I don’t see a problem:
* the inner sphere gains 235 W/m^2 from the internal heater.
* the inner sphere gains 235 W/m^2 from the shell
* the inner sphere radiates 470 W/m^2 to the outer shell
** Net transfer = 0 W/m^2 → No temperature change
*** Obeys 1st Law
* the outer shell gains 470 W/m^2 from the inner sphere.
* the outer shell radiates 235 W/m^2 to the inner sphere
* the inner sphere radiates 235 W/m^2 to space
** Net transfer = 0 W/m^2 → No temperature change
*** Obeys 1st Law
I am not creating or destroying any joules of energy.
“It is like taking a sheet of polished aluminum foil and holding it up to a wall radiating by SB per T at 400‹W/m²› and 400‹W/m²› is reflected back to the wall by the foil at the temperature of the foil so now you have 800‹W/m²› at the wall so of course the wall’s T must rise”
To the extent that “polished aluminum” is shorthand for “a material that reflects back all thermal radiation that reaches it”, then this is exactly what will happen! This is why “space blankets” are so effective.
Basically you are saying that you keep pumping energy into a system (400 J into each m^2 of surface area each second) but don’t let any energy out (the full 400 W/m^2 is reflected back). So of course it will keep warming up!
(Of course, if there is convection or conduction that will limit things . And some radiation will be absorbed by the Al and then emitted to the outside, so it is not reflecting 100% and so that also will limit the temperature inside.)
“This is getting embarrassing for W.U.W.T.”
hmmmmm ….
Doug Allen says “We have two bodies on either side of a one-way mirror which is mostly transparent to light and IR in one direction and mostly opaque or reflective in the other.”
Can’t happen. This would be an example of “Maxwell’s Demon”. As such, the rest of your argument is wrong before you even start.
(“One way mirrors” are not “one way” at all. They are simply mostly reflective from both sides. But one of the rooms is dark and one is bright, which makes it very difficult to see from the bright room to the dark room. If you make the formerly bright room dark and the formerly dark room bright, then the “one way mirror” will work in the opposite direction. Watch any cop show on TV and you will notice that the “interrogation room” is always brightly lit, while the “observation room” is always dimly lit. http://en.wikipedia.org/wiki/One-way_mirror)
Respectfully, it seems to me that the anti-GHers have a fundamental issue that they need to address before anyone else will take them seriously.
1. Per the Stefan-Boltzman law the *surface of the Earth* emits ~400W/m2
2. The Earth as a whole can only emit ~235W/m2 to *space*
As such, something must happen in the atmosphere (ie that which is between the surface of the Earth and space) to make up the difference. Given the complexity of the Earth-atmosphere system, it is easy to construct hypotheticals that *appear* to falsify the GH effect but much more difficult to do so and take into account 1&2.
Cheers, 🙂
Shawnet says
“1. Per the Stefan-Boltzman law the *surface of the Earth* emits ~400W/m2
2. The Earth as a whole can only emit ~235W/m2 to *space*
As such, something must happen in the atmosphere (ie that which is between the surface of the Earth and space) to make up the difference.”
You could also add that the Earth absorbs only 160W/m2 to make the apparent paradox more baffling!
However there are other examples in physics of a similar kind.
A child on a swing needs a very small input of energy to maintain much larger KE to PE interchange.
Energy can be stored in the system.
For our climate, the IPCC advocates say its all due to the GHE.
More rational people think that Latent Heat (both evaporation and fusion) and photochemistry are just two of the very many ways to store and release energy in the Earth system.
The bogus GHE theory (or theories because there are many variants) does not withstand rational scrutiny.
Ryan says: “You cannot, however, significantly warm the shed by covering it with tin foil (although many a claim has been made that you can….)”
ummm … yes you can.
http://littleshop.physics.colostate.edu/activities/atmos1/ColorAndCooling.pdf
Ryan says:
February 7, 2013 at 9:57 am
Ryan your comment about the shed made me think back to my SERE(survival evasion resistance escape) training in the Navy. One of the things they pounded into us was never use a downed aircraft body for shelter. Not in cold nor in hot areas. In cold the metal shell will suck the heat out of you and you freeze and in hot the metal shell will heat to oven like temps and bake you. small hyperbole.
I am sure if they had known that cold would warm us up we would not have been told that. sarc/
Shawnhet says:
February 7, 2013 at 10:35 am
Can you point to where this is defined and hopefully actually measured?
I’ll also note that if you look at nightly cooling since the 50’s (all 120 million NCDC records worth), there’s been no loss of cooling at night in the surface temperature record.
@davidmhoffer says:
February 7, 2013 at 5:34 am
So you are suggesting then that the IR from the snow kept you warm?
In Willis’ example, he mentions how you feel colder in the winter than in the summer within a house that is the same ambient temperature, incoherently attributing the extra chill you feel is from the colder walls. He may be correct about the walls, but in reverse. I would posit that you would feel cooler because the walls are absorbing more of the energy than you are, because they are cold, and you are NOT able to absorb energy from the cooler walls, because, well, errrrr, they are COOLER. Conversely, you may feel warmer in the summer because YOU are cooler than the walls and are absorbing more of the energy than the walls are, especially if the walls are warmer than the ambient temperature, in which case the walls are absorbing exactly ZERO energy from the ambient room (or YOU).
BTW, you stayed warmer in your house because you used the snow as INSULATION. An igloo comes to mind. When I was a scout, each year we would head up to the Blue Mountain in Washington for a week long camping trip. We did not stay in tents, we built igloos, because the snow provided such a good INSULATOR, not a back-radiation radiator…. sheeesh.. The only reason why it stayed warmer was because WE provided the heat. Without the heat from our bodies, the inside of the igloo was no warmer than the outside air, in fact, EXACTLY the same temperature!
Your house/snow/insulator example is a perfect test for Willis, and could be done this winter with the igloo example I just gave. This should be an easy, no brainer exercise for anyone to do. Make an igloo, does it get warmer inside when left to its own device? … I shall await your results.
Bryan:”More rational people think that Latent Heat (both evaporation and fusion) and photochemistry are just two of the very many ways to store and release energy in the Earth system.
The bogus GHE theory (or theories because there are many variants) does not withstand rational scrutiny.”
The problem with using latent heat as a proxy is that it doesn’t explain the difference. When latent heat is captured at the surface and released in the atmosphere, it acts to cool the surface (or bring the surface closer to the 235 w/m2 number). It doesn’t explain why the surface of the earth can remain warmer than the Earth as a whole). If there were no latent heat transfer at all (and nothing else changed) the surface of the Earth would emit 480W/m2 instead of 400W (IOW the GHE would be more intense *not* less).
Cheers, 🙂
MiCro:Can you point to where this is defined and hopefully actually measured?”
My number 1 is pulled directly from the Stefan-Boltzman law and the fact that the average temperature of the EArth is ~16C. Number 2 can be calculated from the TSI after accounting for the geometry of the Earth and its albedo.
HEre is a link to some good data for TSI IMO.
http://lasp.colorado.edu/sorce/data/data_product_summary.htm
Cheers, 🙂
tjfolkerts says:
February 7, 2013 at 10:27 am
Doug Allen says “We have two bodies on either side of a one-way mirror which is mostly transparent to light and IR in one direction and mostly opaque or reflective in the other.”
Can’t happen. This would be an example of “Maxwell’s Demon”. As such, the rest of your argument is wrong before you even start.
Certainly can, they’re called dichroic mirrors and are readily available, the type described is known as a ‘hot mirror’ whereby visible and IR below the cut-off wavelength is transmitted and IR above the cut-off wavelength is reflected. The Earth’s atmosphere acts as a dichroic although the mechanism is not reflection.
george e. smith says (February 7, 2013 at 9:32 am): “Black body radiation itself is fiction, in fact it is fictionally fictional. It involves the physical properties of no physical material whatsoever. No electronic energy states, or any other trappings of quantum mechanics of real materials is involved in the derivation of the black body radiation formulae.”
And don’t even get him started on the Ideal Gas Law! 🙂
Wayne says:
“The shell as shown is outputting more energy, 470‹J/s/m2›, per time per area than is even available from the nuclear source in the first place, there is only one energy source in Fig. 2 and it’s maximum output through the sphere’s surface is 235‹J/s/m2›. ”
NO! This is not what is happening. Go and look at Fig 2 again. The shell is radiating 235 w/m2 into space. In other words, the amount of energy leaving the system after the shell is the same as before the shell. All that happens is that the ball inside the shell will get a little warmer (the fourth root of 2 times warrmer to be exact).
It is like putting a coat on when you are cold. Your skin starts to warm up, but you have not created any energy from nothing.
Thanks for the interesting thought experiment, Willis. It kept me up mulling it over. For me, the “residue of nuclear material reacting in the core” is an unnecessary complication. If we get rid of it, I think I can see more clearly what back radiation from a blackbody shell does to the temperature of P in a dynamic rather than static case. The Shell doesn’t raise the temperature of an unpowered P, it slows its cooling. So if P is powered, then P will reach higher temperatures with a shell than without a shell.
Lets take a planet P, of uniform blackbody steel with finite specific heat and high thermal conductivity, in a void. Via some method, nuclear or otherwise, P is raised to an arbitrarily high temperature above Tp= 255 deg K. Then the heat source is turned OFF.
The planet P in a vacuum will radiate according to SB into infinite background at 4 deg K. P will start to lose energy and cool off. The surface of P will be cooler than the interior, but we have specified that it has high thermal conductivity, so P will be nearly isothermal.
When the surface of P cools past Tp=253.75 deg K, the surface is radiating at Fp = 235 W/m^2 in Willis’ example. SUDDENLY (at T=0) two very thin blackbody steel foil hemispheres, who’s radii is 1.1x the radius of planet P fly in from the 4 deg K void, and Pac-Man-like snap shut around Planet P as shell S. At this moment, the shell is at Ts = 4 deg K, but is being warmed up by radiation from P at Tp = 253.75 deg K.
For simplicity, let’s assume that P and S are made of the same stuff and that the mass of S is 10^-6 of P and specific heat is constant from T = 4 to above 255 deg K. Because the S has much less mass than P, we could raise Ts 250 deg K by thermal conduction and Tp would drop less than 0.001 deg K. Finally assume the area of P, Ap=10^8 m^2, giving radius Rp = 2821 m. So radius of shell Rs= 1.1*Rp = 3103 m and Area of Shell As = 1.21 *10^8 m^2. When Tp = 253.75 deg K, Fp = 235 W/m^2 and Planet P is radiating 23.5 GW = Fp*Ap
There will be a Time A>0, where the temp of the Shell reaches 50 deg K. and radiates 0.35 W/m^2 from both the inside and the outside surface. Tp will drop by (50-4)/1000000 deg K. The shell hardly matters at this point.
At a later Time B>A, the temp of the shell reaches 100 deg K, radiating 5.67 W/m2 from both the inside and outside. The heat needed to raise the shell from 4 to 100 deg K only drops P by 96/1000000 deg K, so P no warmer than 253.749908 deg K, but the shell is radiating 0.6 GW into space while absorbing 23.5 GW from P. It is warming quickly.
At Time C>B, the temp of the shell reaches 150 deg K and radiates 28.7 W/m2 from both the inside and outside = 3.47 GW from each surface. The shell is receiving 23.5 GW from the planet and losing 6.94 GW from its own radiation, so it gaining energy and still warming up. To heat the shell 146 deg, planet P has had to give up at least heat equal to a drop in temperature of (150-4)/1000000 = 0.000146 deg. So P is still radiating 235 W/m2. But now we have a not insignificant 28.7 w/m2 back radiation from the shell.
Do we add that 28.7 W/m2 to the 235 W/m2 from the planet?
No. If we do, we have 263.7 W/m2 and if treated as from a black body, we get a temperature Tp = 261.15 deg K. We would think the planet warmed without a source of energy.
Do we subtract 28.7 W/m2 because the NET flow from the planet is 206.3 W/m2?
No. If we do, then 206.3 W/m2 from a blackbody is Tp = 249.6 deg K when it really still above Tp=253.749 deg K.
The back radiation flux cannot be used to change the temperature of P directly, but it does change the net energy flow, so it reduces the rate of P cooling. The planet is still at Tp = 253.749+ deg K and radiating 235 W/m2 at the same time it is absorbing 28.7 w/m2 from the shell.
To recap, we have a blackbody planet heated above 255 K, let to cool down to a point T=0 where it emits 235 W/m2 at 253.75 deg K. We wrap it in a blackbody foil shell (one millionth the mass of the planet) at 4 deg K. Via radiation, the planet transfers heat to warm the shell, by huge difference in mass, the planet cools 1/10000 deg for the shell to warm 100 deg. As the shell warms past 150 deg K at T=C, it is now back-radiating to the planet 28.7 W/m2. The Planet must still radiate at Tp = 253.74985 deg, Fp = 235 W/m2, losing energy into the shell at 23.5 GW. But the Back radiation from the shell of 28.7 W/m2 amounts to 2.8 GW of energy returned to the planet for a net planet energy loss of 20.7 GW instead of 23.5 GW when there was no shell.
Wrapping P in shell S, does not cause the temperature of P to rise, only allow P to cool more slowly.
In this setup, there is no equilibrium. Ts will rise until the net energy it receives from P (less back radiation) equals what it loses to the void. That would be 11.75 GW, over As, so Fs = 97.1 W/m2, which would be 203.4 deg K. At which point, both P and S begin a coupled cooling though they are at different temperatures.
Turn on P’s power source (a 23.5 GW, high temp) at the core, and P will indeed find an equilibrium Tp above its 253.75 deg K equilibrium when exposed to the 4 deg K void instead of a Shell at greater than 200 deg K.
Tosh! Do you realise that is a completely meaningless statement? Equivalent temperatures are based on power emitted, not on any particular chemical molecule.
There are two reactions you may get when you point someone in the right direction. Either they go in that direction or they stubbornly refuse because what they were seeking was confirmation of their belief in the wrong direction.
In which case, if you don’t want to learn, I can do no more.
As said on WUWT earlier this week,
(Mark Twain)
There are over 200 comments on this thread. At the time of my last comment only about 5 contributors seemed to understand what they were talking about (although a couple more have weighed in since). The rest are totally confused.
But the steel shell model is a simple example. If you don’t understand that then try to get up to speed. You make sceptics look bad.
joletaxi says:
February 7, 2013 at 4:40 am
It’s crazy but true. I discussed this above. Your numbers are a bit off, in that 1 shell gives double the watts, and two shells give triple the watts. N shells will multiply the watts by N+1.
However, the temperature of core does not go “infinite”. It goes up by the fourth root of N+1. So merely to double the temperature, you need 15 shells …
Long before that, of course, it would melt. Imagine putting a light bulb inside a vacuum flask (a Dewar or a Thermos). Then put that inside another vacuum flask, and then put the whole thing in another …
You will melt the glass or the light bulb.
As someone pointed out above, high end vacuum flasks use this exact principle by putting multiple layers of aluminium foil inside the flask … note that they have to be in vacuum for this to work efficiently. Even inefficiently it work well, that’s how they make the so-called “Space Blankets”.
w.
mkelly says:
“In cold the metal shell will suck the heat out of you ….
Yes, metal is a very good conductor, so a metal frame at 0C will cool you much faster than a rock or a tree at 0C.
“ and in hot the metal shell will heat to oven like temps and bake you”
Yes, metal is a very good conductor, so a metal frame at 45C will heat you much faster than a rock or a tree at 45C.
And then a second thought occurred to me. A quick search shows that metals tend to be more reflective for IR than for visible. http://www.tvu.com/PNextGenTFwebFig3.jpg
This means metal emits IR poorly but absorbs visible light (relatively) well. Or put the other way around, metal has a hard time absorbing sunlight, but an even harder time radiating IR. This make metal (in some sense) a “natural greenhouse material” (sunlight gets in, but IR can’t get out so well). Sun shining on metal makes it very warm (as everyone knows). Compare this to a light colored rock. The rock will absorb a little more sunlight than the metal, but will emit IR much better, so it will not warm up as much.
This could make an interesting little experiment …
q = ε σ (Th4 – Tc4) Ac
A typical radiative heat transfer equation from Engineering Tool Box. Where in this formula can the radiation from the shell cause an increase in the temperture of the globe? Again it seems to me that when Th = Tc then q is zero.
I may have to throw out my heat transfer book it may be all wrong.
Keitho says:
February 7, 2013 at 2:37 am
I really don’t get the quibbling by many here. Surely the point of R. Wood’s experiment is this . .
All things being the same, except that in one box infra red is retained by the glass and in the other it is released by the rock salt, it seems that the contribution to the temperature in the box made by reflected infra red is trivial at best.
The rest of the argy bargy is fine in that it would refine the experiment in various ways but it won’t change the observed fact by Wood that the greenhouse effect is not enhanced by the trapping of infra red radiation.
Wood’s experiment is fatally flawed as a description of the atmospheric GHE for several reasons. The fundamental one is that there is an atmosphere above the glass/salt plates, to be accurate it should be a vacuum and therefore only permit radiational heat transfer outwards. This could be achieved by putting the boxes inside a vacuum chamber with the top window being made from IR transparent material (preferably cooled to liq N2 temps). The other flaw which has been mentioned is that the size of the boxes is less than the convective length scale of the atmosphere contained in them, in order to get that right you’d either need a much bigger box or change the atmosphere inside to greatly change the length scale (Peclet No?)
I hear you Phil, and thank you for your reasoned response. I do wonder though how much more warming would be caused by the IR if the problems you outline were resolved. From my understanding it wouldn’t make a huge difference to the outcome, although it would greatly improve the experiment.
wayne:
In your post at February 7, 2013 at 9:35 am you say
Well, if by that you mean your post, then I agree.
For example, you ask about the hypothetical shell model
But nobody is suggesting that.
The shell inhibits the rate of heat loss from the planet by radiating some energy back towards the planet and, thus, reducing the net radiation from the planet. Thus, to obtain the same rate of heat loss in both cases the planet’s surface temperature is higher than it would be in the absence of the shell. But the shell does not ‘heat’ the planet.
Similarly, if you wrap yourself in a blanket on a very cold night then your surface temperature is higher than it would be if you were naked. The blanket inhibits the rate of heat loss from your skin by reducing some thermal conduction to the air. But the blanket does not ‘heat’ your skin.
The point you seem to be missing is that heat radiated to space from the hypothetical planet’s shell always equals the heat supplied to the surface from the hypothetical planet’s radioactive core. Similarly, when considering the Earth’s GHE, the heat radiated to space from the Earth’s surface and atmosphere always equals the heat supplied to the Earth from the Sun.
The effect of GHGs in the atmosphere is to inhibit the rate of heat loss from the Earth’s surface by radiating some energy back towards the surface. (This is like the shell of the hypothetical planet.) Calculating this GHE effect is not a simple application of the SB Law for the real Earth. Temperatures and concentrations of GHEs vary with time, geographical position and altitude over the Earth’s surface. It is difficult to conceive of an integration of SB which would enable direct calculation of the inhibition to radiative heat loss from the Earth’s surface.
Estimation of ‘back radiation’ is an attempt to quantify the magnitude of the inhibition to radiative heat loss from the Earth’s surface in the absence of the needed integration of the SB Law.
In my opinion, the Trenberth energy budget is very wrong. But it is not flawed in principle.
I hope that helps to overcome your embarrassment.
Richard
Greg House says:
February 6, 2013 at 2:58 pm
Guest Post by Willis Eschenbach: ” let me give a curious example of the greenhouse effect, that of the Steel Greenhouse. Imagine a planet in the vacuum of space. A residue of nuclear material reacting in the core warms it to where it is radiating at say 235 watts per square metre (W/m2). … Now, note what happens when we add a shell around the planet. The shell warms up and it begins to radiate as well … but it radiates the same amount inwards and outwards. The inwards radiation warms the surface of the planet, …”
=============================================================
“Imagine”, I see.
I guess, it has never been proven experimentally that A warms B and then B warms A back, right? OK, this is a product of imagination, a fiction, and everyone has right to right a science-fictional story, no problem with that. But the readers need to be told clearly that this story is fictional, just to avoid confusion.
Actually the readers need to be told repeatedly that you’re wrong and that you don’t understand undergraduate level radiation heat transfer! Try reading H C Hottel’s text for example. Read about the application of radiation shields for thermocouples as a practical example of the phenomenon, add a radiation shield around the ThC which is colder than the ThC and the measured temperature goes up!
Willis says: “The inwards radiation warms the surface of the planet, until it is radiating at 470 W/m2.”
tjflokerts says:
No, I don’t see a problem:
* the inner sphere gains 235 W/m^2 from the internal heater.
* the inner sphere gains 235 W/m^2 from the shell
* the inner sphere radiates 470 W/m^2 to the outer shell
** Net transfer = 0 W/m^2 → No temperature change
Willis used the word “warms” which means the temperature increased. So there was a temperature change. Does this change your thinking?
DR says:
February 7, 2013 at 8:00 am
We’re also told OHC increases in the past 30 years is due to AGW. There is zero empirical evidence that even a doubling of atmospheric CO2 can warm the oceans to any measurable degree. Instead, we’re told there is mixing by ocean waves that magically transports the top few microns of “heated” water affected by “back radiation” from a few extra ppm’s of atmospheric CO2. Sure, everyone stirs their hot coffee to make it warmer. How can anyone try to pass off the idea that anything other than SW solar radiation can warm the oceans and expect thinking people to believe that is frankly, insulting.
/////////////////////////////////////////////////////////////////////////////////////////////////////
I have for years been pointing out to Willis, the problems with DWLWIR and the oceans. Water is essentially opaque to DWLWIR. The absorption characterics of LWIR in water is that approximately 50% is fully absorbed within just 4 microns and virtually everything within 10 microns.
The energy flux is upwards not downwards in the top micron layers of the ocean so absorbed energy in those micron layers cannot be conducted downwards (ie., against the direction of flux) and ocean overturning (even if it could wrap and overturn the very top microns) is a mechanical process measured in many many hours whereas the absorption of DWLWIR is close to a light speed event.
I have often asked Willis to explain what physical process carries the absorbed DWLWIR down to the deep ocean in the light of the above facts, and, despite many requests for an explanation of the physical process, he has never provided one; instead he opts for the cop out, the oceans would freeze if they did not absorb DWLWIR.without actually proving that the tropical ocean would freeze (and of course, the tropical ocean acts as a heat pump distributing its heat to the other oceans).
But the problems with oceans is even more difficult. Oceans are covered with a fine mist of windswept spray and spume. This is significant since it would act as a LWIR block preventing DWLWIR even reaching the top micron layer of the ocean.
The average global wind speed over the oceans is about 7m per second, ie., about BF force 4. The human eyesight does not have good resolution and hence why TV works acceptably well (pre the digital era, TV in the UK had 405 lines per inch later increased to 625 lines per inch when colour TV was introduced, and for many years in the States, they had even lower resolution – nonetheless the human eye did not resolve these individual lines, just as it does not resolve the individual pixels in digital pictures). Between force 2 and force 3, the human eye can just begin to detect water being skimmed off the surface of the ocean. By force 3 we see scattered whitecrested wavelets. Force 4 white crested wavelets abound. What we are seeing here is the removal of more than just a few microns of water from the top layer of the ocean.. At this stage, immediately above the oceans is a fine layer of mist much more than 6 microns thick.. This mist is absorbing DWLWIR from above and it is preventing DWLWIR even reaching the top micron layer of the ocean below. It is only in still conditions that the main body of the ocean could actually receive any DWLWIR since in most conditions there is a layer of windswept spray and spume lying parellel and above the ocean below that acts as a complete LWIR block just like a sunumbrella/parasol would do for humans, or sunblock cream.
It would appear that DWLWIR cannot practically heat the oceans since in most conditions it cannot even reach the top of the ocean still less penetrate, or in someway be over-turned down into the deep ocean below.
IF DWLWIR cannot effectively heat the oceans since it is the oceans that ultimately drive the climate, CAGW is a non starter.
Greg House says:
February 6, 2013 at 8:34 pm
davidmhoffer says, February 6, 2013 at 8:12 pm: “2nd Law of thermo
Net energy flux between two bodies will flow from hotter to colder.”
=========================================================
No, the known historical statements do not contain the word “net” nor do they imply it, nor is there apparently any real scientific experiment confirming this notion.
I guess, that “net” thing is a trick of “climate scientists” to justify their “greenhouse effect”.
Nothing to do with ‘climate science’ it’s the basic physics of radiation heat transfer.
Arises from S-B law, light emitted by hot bb object = const.A.Th^4
light emitted by cool bb object = const.A.Tc^4
Net heat transferred between the two objects = const.A.(Th^4-Tc^4)
Since in our universe photons don’t carry with them a record of the temperature of the object which emitted them all photons of a given wavelength are absorbed equally regardless of the temperature of their source.
“mkelly says:
February 7, 2013 at 11:43 am
q = ε σ (Th4 – Tc4) Ac”
Emissivity is what changes when you add a shell to your greenhouse.
Cheers, 🙂
I live in the foothills of the mountains near the coast. Probably about 150 to 200m above sea level and about I km from the sea. From the back of my garden, the mountain slopes down to the sea and is covered in trees.
A couple of weeks ago, we had some windy weather. Probably force 5 gusting 6/7. The sun rises over the sea as seen from my back garden. Shortly after sunrise I was looking out and the low incidence of morning winter sun illuminated the spray being dragged off the top of the ocean. It was an impressive sight.
The skyline above the sea had a typical New York Skyscraper appearance. It is difficult to guage how high spray/spume was being sucked up but you could see a silhouette of what appeared to be mid to high level skyscrapers extending upwards perhaps 8 to 25 metres high. I am only guessing at the hieght in comparison to trees.
My point being that in these conditions force 5 gusting 6 to 7 which is not at all unusual for open ocean, and in these conditions there was clearly a layer of mist immediately above the ocean many metres high. That layer may often be transparent to the human eye, but in the backlit low incident sunlight conditions in which I was observing, it could clearly be seen. There is no reason to presume that a similar layer does not always exist in such windy conditions and to a lesser extent in lighter wind condiotions. Although this layer of fine mist may often be transparent to the human eye, it is not transparent to LWIR and would absorp DWLWIR as backradiated from upon high and would, because of the absorption characteristics of water, absorb that DWLWIR before it could reach the very top layer of the ocean itself.
Willis likes the real world but appears rarely to think about it when he comments upon DWLWIR (apart from his comments that clouds coming over head will quickly warm you up without actually dealing with convection)
Ryan says:
February 7, 2013 at 6:46 am
Lets consider what ACTUALLY happens with a CO2 molecule when a IR photon hits it. Remember that IR is not HEAT. It is an electromagnetic radiation with energy that can be converted to heat in certain circumstances. So a photon with energy in the IR spectrum reaches a CO2 molecule. What happens to it? Well if it is within the scope of the covalent bonds of the CO2 molecule it could be absorbed by the electrons in the bonds causing the electrons to be lifted to a higher energy level. But here is the crux of the matter – if the electron is already at that higher energy level then an incoming IR photon WILL NOT BE ABSORBED (at least not at the same frequency) – the photon will just travel straight through.
Again you are wrong, take a look at ro-vibrational spectra, Q-branch, just because a photon has been absorbed and a transition from v=0 to v=1 has occurred does not mean that a transition can not occur from v=1 to v=2.
But also bear in mind that lifting an electron to a higher energy level does not in itself cause heat to occur – the electron must drop back to its lower energy level and the assumption is that the electron then loses energy and that energy (probably) ends up at some point exhibiting itself as heat (just following the laws of entropy) but maybe not right away because the CO2 may just emit IR of a different wavelength (i.e. not heat as such) which may then be absorbed (or then again may not) by some other object which then gets a bit warmer.
At atmospheric pressure the most likely action is that collisional deactivation will occur due to collisions with surrounding molecules and those N2 and O2 molecules will heat up.
Michael Moon says:
February 6, 2013 at 9:37 pm
Willis,
You must consider something here. Energy, in the form of heating your house, or keeping your lights on with the electrical bill, has been the subject of, shall we say, INTENSE scrutiny, since
Edison in the 1870′s. When a large-gigantic-immense-overwhelming amount of money is spent on the subject of energy, the community of suppliers tend to research this subject, to learn how to satisfy their customers at minimal cost.
Check out double glazing!
If there were a way to heat anything by putting a shell around it which would magically double the “heat input,” otherwise known as Flux, then it would have been seen and exploited many many years ago.
Check out Dichroic Halogen Reflector (Lightbulb)!
mkelley asks: “Willis used the word “warms” which means the temperature increased. So there was a temperature change. Does this change your thinking?”
No. That doesn’t give me any reason to change my thinking 🙂
With no shell, the sphere will be 254 K (assuming it is a black body)
With a shell, the shell will be 254 K, and the sphere will be 302 K.
With two shells, the inner surface would be 334 K.
With three shells, the inner surface would be 359 K.
It all still works just fine. If you make it harder from IR radiation to leave, the system will warm up.
tjfolkerts:
“With no shell, the sphere will be 254 K (assuming it is a black body).
With two shells, the inner surface would be 334 K.”
This implies that the inner surface radiates at 3 times the flux density as with no shell. But surely it should be 4 times. This is my thinking –
The outer shell radiates at 1 times (say 1 unit) outwards and 1 unit inwards – 2 units in total. Therefore, the inner shell radiates 2 units outwards and hence 2 units downwards towards the sphere – 4 units in total. If it is radiating 4 units in total, then the sphere must be radiating 4 units upwards, not 3.
Where am I going wrong?
EForster says:
February 7, 2013 at 4:56 am
The missing link is that the shell radiates the same amount inwards as outwards. That radiation is absorbed by the planet, and adds to the energy of the planet. This leaves the surface warmer than it would be in the absence of the shell.
w.
Shawnhet says:
February 7, 2013 at 12:21 pm
“mkelly says:
February 7, 2013 at 11:43 am
q = ε σ (Th4 – Tc4) Ac”
Emissivity is what changes when you add a shell to your greenhouse.
Cheers, 🙂
The emissibity may or may not change, but you miss the point that when Th=Tc then the entire equation equals zero. No amount of change to the emissivity can fix that.
MikeB says:
February 7, 2013 at 11:37 am
As for being meaningless I’ll accepts Duke’s Physics Dept “Sloppy“, The reference to the molecule, was related to the 15u absorption band of Co2, as opposed to 4.5u to ~12u or 16.5 to 22u, where the photon is unlikely to be captured by Co2.
So, let me ask this, if a 15u wavelength photon is captured and thermalize by a molecule of Co2, what would it’s temperature be?
squid2112 says:
February 7, 2013 at 5:23 am
Wow, Willis, I have enjoyed so many of the things you have written here over the years, but this one, wow. Still clinging on to the “cold objects can make warmer objects warmer still” garbage?
I suppose I should write a post on the subject myself, but Yes, Virginia, Cooler Objects Can Make Warmer Objects Even Warmer Still.
Thanks, squid. I love it that when you agree with me everything’s fine, I’m a man worth listening to, but when you don’t I’m suddenly some kind of ignorant fool “clinging” to “garbage” … a bit less abuse might be in order, my friend.
In any case, I suspect this is a sematics problem so let me restate it. Note that my restatement changes nothing about what I have said in the head post.
This is because rather than the warmer object receiving radiation from the slightly cooler object of say 400 W/m2, it is only receiving a paltry 315 W/m2 from the block of ice. As a result, it cools faster when it is next to the ice.
Can we agree on that, that a block of ice cools an object faster than a block of warm material?
Let’s take the next step.
This is because rather than the warmer object receiving no radiation at all (it being in outer space), it is receiving an extravagant 315 W/m2 from the toasty block of ice next door. As a result, it cools slower when it is next to the ice than when the ice is not there.
Now, this is the important part. Since the object cools slower with the ice next to it , it ends up warmer than it would be without the ice.
Now in this situation, in common parlance, we say that the ice is warming the warmer object. You may object to that choice of words, which is why I have provided the alternate statement above, that in outer space, a block of ice will slow the cooling of a nearby warmer object compared to the same situation with no ice.
Can we agree on that? If so, we have no disagreement, because that is the exact situation in the head post. The shell slows the cooling rate of the planet. As a result, the planet warms up until it is able to remove a NET 235 watts/m2 from the surface to restore the equilibrium. To do so, it must warm to 470 W/m2.
All the best,
w.
PS—To ward off the pickers of nits, yes, cosmic background radiation is not really zero but 3 W/m2 … so sue me.
mkelly says:
February 7, 2013 at 1:05 pm:”The emissibity may or may not change, but you miss the point that when Th=Tc then the entire equation equals zero. No amount of change to the emissivity can fix that.”
You’re right I don’t follow you, but changing the emissivity can allow the differential btw Th and Tc to grow. That was what I was referring to to wit(from your previous post):”A typical radiative heat transfer equation from Engineering Tool Box. Where in this formula can the radiation from the shell cause an increase in the temperture of the globe?”
Cheers, 🙂
KevinM says:
February 6, 2013 at 1:22 pm
….So Co2 AGW is real. The point to argue is that it is benign, and probably swamped by larger natural variability over short and long time scales.
>>>>>>>>>>>>>>>>>>>>>>>>>
The other point everyone neglects is the warmists only focus on one side of the whole picture. They focus only on the interaction of GHGs with earthshine. A look at the actual wavelengths for energy interaction (see Graph ) shows both CO2 and H2O interact with wavelengths in the solar as well as the earthshine bands.
The most important vibrational and rotational transitions for CO2 is
Center……Band interval
667…………..540-800
961
plus…………..850-1250
1063.8
2349………….2100-2400
Visible and near-IR absorption bands
2526………….2000-2400
3703………….3400-3850
5000………….4700-5200
6250………….6100-6450
7143………….6850-7000
Chart from http://irina.eas.gatech.edu/EAS8803_Fall2009/Lec6.pdf
Since earthshine is much lower in energy per wavelength band than sunshine, (See graph ) increasing GHGs could actually have a cooling and not a heating effect on the earth.
As far as I can see the only change that GHGs actually make is to make the weather milder by moderating the temperature swings. Day times are not as hot and night times not as cool ( see comment 1 and comment 2 and comment 3 ~ The comparison of the humid Brazilian rain forest and the dry N. African Desert show the day-night variation of ~ 10C with a high humidity vs a day-night variation of 35C without and an overall average temperature 8C higher for the desert.)
I do not have the in-depth physics knowledge to put an accurate number on it, but I really do not think a photon emitted by the earth hangs around for several hours before finally escaping to space even with the pinball machine effect of GHGs. Therefore you are just seeing the short lived effect of modifying the day/night temperatures. As I said the Warmists focus on the warming effect of the night temperatures only and ignore the cooling effect on the day time temperatures.
…………………
As far as the Wood experiment goes, It proves the ‘greenhouse effect’ in greenhouses is a property of glass. The Wood’s apparatus was too crude to actually capture the warmist’s greenhouse effect.
It would be interesting to do the experiment with very precise equipment and four boxes.
1. Pure Nitrogen
2. Dry Air
3. Air at 100% humidity
4. Pure Carbon Dioxide.
The experiment should be repeated several times with the boxes used for a different gas each time. (I still think the effect is too slight to be captured even with modern equipment)
Gail Combs
Thanks for your interesting post.
I will follow up your links.
Your post is well supported by traceable evidence.
Well done!
richard verney – Downward IR barely penetrates the oceans, but that strongly affects the “skin layer”, the top _millimeter_ of water. Increased air temperature and DLWR reduce the temperature gradient across that skin layer, reduce the energy flow out of the (warmer) near-surface ocean, and change the energy balance. That _reduction in cooling_ means visible light energy (penetrating much further) accumulates and warms the oceans until the thermal gradient is reestablished, until the heat leaving the oceans matches that arriving.
All of which is but one aspect of increased greenhouse gases _reducing_ energy loss to space, causing the climate to warm until outgoing = incoming energy.
See http://www.realclimate.org/index.php/archives/2006/09/why-greenhouse-gases-heat-the-ocean/ for a discussion of this, including both references and data demonstrating this relationship.
wayne;
This is where the I.P.C.C.’s Free Energy Oven fails.
>>>>>>>>>>>>>>>
I’ve read AR4 WG1 front to back and nowhere is such a claim made. If you understood the first thing about the physics then you might be able to understand the claim they are actually making and attack that instead of demonstrating that you haven’t a clue what you are talking about.
squid2112;
So you are suggesting then that the IR from the snow kept you warm?
>>>>>>>>>>>>>>>>>>
No. I am stating it as a fact. The house is warmer with snow packed around it than without, the snow is colder than the house. The IR from the snow can be directly measured.
Willis, I agree with your description. It got me thinking how we might tie this closer to our real situation. What I found is interesting so please feel free to correct me where I go off the tracks.
Let’s modify this experiment a bit and see where it takes us. For simplicity lets assume 240 w/m2 as our heat source. In addition,
1) A perfect conducting wire connects the heat source to the outer shell and transfers 80 w/m2 to the shell. This is done to simulate our atmosphere absorbing about 1/3 of the energy from the Sun.
2) Another wire connects the surface to the shell and transfers half the energy the surface receives to the shell. This is done to simulate latent heat and conduction that is convected away from the surface.
OK, at time T1 we should see the shell receiving 240 m2 (80 directly via 1 + 80 directly from 2 + 80 radiated from the surface.) It would radiate 120 to space and 120 back to the surface. If we continue over time the shell will eventually warm up to 480 w/m2 with 240 going to space creating an equilibrium condition. However, the surface will be radiating 200 w/m2 and conducting 200 w/m2 for a total 400 w/m2.
Essentially, the surface warmed from the initial 160 w/m2 to a new equilibrium of 400 w/m2. Note that the surface does not reach the 480 w/m2 in the base case Willis described. What this shows is the energy that never makes it to the surface cannot warm the surface through the GHE. If we consider our own atmosphere this means only the 160 w/m2 that reaches the surface can be considered in computing the GHE. In other words, the GHE is much stronger than sometimes indicated.
Also, it did not matter how the energy is transferred to the shell. If I remove 2) the final result is still the same. This appears to mean the GHE effect is independent of how the energy reaches our atmosphere. This would appear to raise questions for the concept of an ERL. There is no dependence on the height of the shell.
Note that you can’t heat the surface any more unless we add another shell.
We know the real atmosphere does not absorb all the radiation from the surface. If it did we would be at the maximum GHE. So, how much radiation goes directly to space? According to the KT cartoon that is 40 w/m2. And, this number must be reduced anytime we want to increase the GHE.
So, let’s take our situation above and reduce the surface radiation by 40 w/m2. This reduces the surface to 360 w/m2. But we know from measurement that our surface temperature is 288K which corresponds to 390 w/m2. In other words, our temperature already exceeds the value that we would expect if 40 w/m2 was passing through the the atmosphere without being absorbed. Interesting.
This should tell us that my assumptions above are a little bit off. More energy must be reaching the surface than assumed using the 160 w/m2 KT cartoon value or more energy is produced internally.
One other thing to consider is the maximum temperature of the surface in this example is 290K (corresponds to 400 w/m2). There is no way the GHE can warm our planet above this value given my assumptions. And, even if you assume we could trap the 40 w/m2 that is not currently absorbed the maximum only rises to 295K. Warm, but no boiling oceans and no Venus.
I should add that this experiment only uses a single shell. If our real atmosphere more closely appears as multiple shells then it would mean the surface could warm more. And yeah, I’m taking a lot of license here in comparing the real situation with this thought experiment. However, there does seem to be something to the idea that 160 w/m2 reaching the surface has a maximum GHE and we are darn close.
Vince, I had to think a little to make sure I was right about the radiation of the shells. (I actually worked out the gory details for arbitrary emissivities once — I should write that up some time).
Anyway, at the bottom layer (let P = 235 W/m^2)
+P from heater + 2P from above – 3 P radiated up = balanced energy
At next layer
+ 3 P from below + 1 P from above – 2P down -2P up = balanced energy
At top
+2P from below -P down – P up = balanced energy.
tjfolkerts says, February 7, 2013 at 9:59 am: “The Wood experiment is similar in some ways, but very different in others, which is the point hat Willis was making and which you apparently missed.
> The earth is surrounded by extreme cold (outer space), but Wood’s experiment is surrounded by materials at very similar temperatures to the experiment itself.
> The atmosphere of the earth is many KILOmeters in size, but Wood’s experiment is many CENTImeters in size.
These (and other rather fundamental differences) make Wood’s experiment a rather poor analogy to earth’s situation.”
=============================================================
Yes, there are 1,000,000 differences between the boxes in the Wood experiment and the Earth, but those differences are irrelevant, and the Wood experiment was not meant to be an “analogy to earth’s situation”. It deals only with the underlying mechanism of the alleged “greenhouse effect” as presented by the IPCC: effect of the trapped/back radiation on the temperature of the source. It does not even deal directly with the so called “greenhouse gases”, because it is not necessary.
Willis — I think I will have to sue you 🙂
The temperature of space is about 3 K, but the power is about 0.000003 W/m^2. (Of course, that makes your point even more emphatically.)
And one other nit-pick. I was a little confused when you said “In the vacuum of outer space, a warm object with a block of ice nearby will cool faster than a warm object with a slightly cooler object nearby.” The “sightly cooler object” is slightly cooler than the ORIGINAL object, but the first time I read it, I was thinking slightly cooler than the BLOCK OF ICE. Obviously that makes a huge difference in the interpretation. I’m not sure how to say that so there is less room for misinterpretation.
Gail;
(I still think the effect is too slight to be captured even with modern equipment)
>>>>>>>>>>>>>>>>>>
Actually it isn’t.
http://www.john-daly.com/artifact.htm
There’s a zip file at the top with criticisms of this experiment that I consider valid. At least it is a real experiment though, done with precision equipment and not some joke with cardboard boxes in thermal equilibrium with the salt and glass windows, neither of which are thermally isolated by a vacuum, and measured glass thermometers that are two orders of magnitude less accurate than what would be required to measure the radiative ghe in the first place. Did I say joke? It doesn’t ascend to joke status and the manner in which it is being misused to promote misunderstanding isn’t at all funny.
Phil. says, February 7, 2013 at 11:45 am : “Wood’s experiment is fatally flawed as a description of the atmospheric GHE for several reasons.”
========================================================
The Wood experiment was not meant to be a description of the “atmospheric GHE”, it was only meant to check whether the underlying mechanism of that alleged “atmospheric GHE” (effect of the trapped/back radiation on the temperature of the source) worked or not. It did not. Bad for the “atmospheric GHE” concept (as presented by the IPCC).
Phil. says, February 7, 2013 at 12:08 pm: “Nothing to do with ‘climate science’ it’s the basic physics of radiation heat transfer.
Arises from S-B law, light emitted by hot bb object = const.A.Th^4
light emitted by cool bb object = const.A.Tc^4
Net heat transferred between the two objects = const.A.(Th^4-Tc^4)”
==========================================================
This “net” thing does not “arise” from S-B law, and it has apparently never been proven experimentally. As long as it has not been proven experimentally, it remains a fiction. A sort of “radiation arithmetic” without any basis in science.
Gale Combs says
“The other point everyone neglects is the warmists only focus on one side of the whole picture. They focus only on the interaction of GHGs with earthshine.”
I agree, and it is their downfall.
For these wavelengths > 3um the classical Rayleigh – Jeans formula holds.
http://hyperphysics.phy-astr.gsu.edu/hbase/mod6.html#c4
The classical model holds with no photons.
The Poynting Vector gives the direction of heat flow.
No reference whatsoever to backradiation.
Yet real heat transfer problems yield the correct answers!
Only with wavelengths < 3um(Solar) do we have to include quantum effects and the Rayleigh -Jeans model fails.
The Physics of Electromagnetic radiation in the vaccuum is a well founded robust theory. So much so, that the only exact fundamental constants of Physics are related to EM radiation theory.
The vaccuum of empty space has a Permittivity (Epsilon nought) that has an exact value. That constant gets involved in the capacitance relating to charge storage on conductors. Free space also has a magnetic Permeability (Mu nought) that has an exact value. That constant gets involved in the magnetic fields surrounding current carrying conductors.
Those two exact values also yield the velocity of electromagnetic radiation waves (c).
c = 1/sqrt (munought x epsilonnought) which thus also has an exact value.
Less well known or at least discussed is the remaining exact value fundamental physical constant which I will call Znouhgt, as I don’t recall right now what they really call it. It is the “characteristic impendance” of free space and is approximately 377 Ohms.
Well its exact value is 120 Pi Ohms. Paint your car with 377 Ohm paint and it will disappear. Well strictly speaking it will reflect no EM radiation of any knind, no matter what. It may not let you see through the car and out the other side .
I have no idea what if any effect either dark matter, or dark energy have on EM radiation if either exists.
So the point of this is that the vaccuum velocity of EM radiation, is not matched by the velocity of EM propagation in ANY real physical material medium (besides vaccuum). EM radiation always travels slower in any real physical material medium; well the phase veolicty is always lower.
The ratio of (c) to the velocity of EM radiation in a real medium is the refractive index of that medium.(N)
The characteristic impedance of the real medium is also different from 120 Pi Ohms (lower).
Whenever an EM wave travelling in a medium, encounters a different medium interface, the wave splits into two components; a transmitted wave which propagates into the denser medium, and a reflected wave which travels back into the original medium.
Going from the less dense medium, into the denser medium, also involves a phase change of pi radians, so the reflected wave is inverted. Going from the dense medium, into the less dense medium, there is no phase inversion. Most real media actually have a complex refractive index, due to surface phenomena at the interface, so there always can be a small phase shift, which manifests itself as an effective change in optical thickness of the medium.
The simplified reflection coefficient for EM radiation incident normally on the interface is given by:-
R = ((N1-N2) / (N1+N2))^2
N is 1.000…. for the vaccuum and something >1 for all normal real materials, so the reflection coefficient can never be zero except perhaps over some small frequency range (which might be of some use) But never over the whole DC -infinity sans ends range of EM radiation.
Maxwell’s Fisheye, and the Luneberg Lens are two hypotheticaloptical devices which have remarkable optical properties, but require that the material refractive index (graded index materials) be 1.00 at the surface of the device, and increase further from the surface. The Luneberg lens can be implemented approximately at microwave frequencies, with foam type materials. Otherwise they are hypothetical curiosities.
Ergo, no real physical body can absorb ALL em radiation that falls on it; so there can be no black bodies.
Luckily, we can emulate a BB over limited frequency ranges, so as to derive useful effects. And for such experimental devices, the bulk of the radiation closely approximates what the Planck and Stefan-Boltzmann, and Wien formulas for BB radiation predict. And that is why BB radiation theory is so important, even though it is quite fictional to the point that no physical observations we make of the real universe match it, except over small frequency or wavelength ranges.
Willis shell planet assumes that BB formulae are operating, yet his explanation of the model, with two completely different Spectra inside the shell, imply that the total radiant flux inside the shell is NOT a BB rtadiation spectrum at ANY isothermal Temperature.
All of which says nothing about the valididty of what we call the greenhouse effect, or whether real greenhouses or the Woods experiment, behave as is claimed.
Oddly there is a fifth Physical number that is exact.; but trivially so. That number is (g) the acceleration due to gravity. I’ll let you giggle the exact value for yourselves.
That just tells us when we say an acceleration is umpteen “g”s we meen umpteen times that number. OK so Ornithorynchus is a platypus. So we can just say umpteen platypi, rather than ornithorynchi; big deal. (g) is not exactly a fundamental physical quantity.
Willis and friends are wrongly attributing the atmospheric greenhouse effect with the greenhouse gas effect.
I have watched them go round and round for years: unable to establish any experiment that shows greenhouse gas effect to be true, they seize on the atmospheric greenhouse effect.
There is indeed an atmospheric greenhouse effect which buffers temperatures. Solar radiation in is buffered therefore temperatures aren’t able to get so high.
Earth & solar radiation out, are buffered therefore temperatures aren’t able to get so ow.
———–
I’ve watched these greenhouse gas effect people for ten years, trying to make believe they “understood” that “there really IS a greenhouse gas effect.”
Every single time someone shows them an actual experiment that disproves it, they’re off to another fantasy model. There’s no experiment that means anything to them, nothing proves anything.
*THE ABOVE are LINDZEN’S ALMOST EXACT WORDS.*
All greenhouse gas effect believers, MUST retreat to the nearest fantasy model because reality never bears out their claim.
So one thing they’re incapable of differentiating is the difference between the Atmospheric Greenhouse Effect: and the Greenhouse Gas Effect.
Mark it. Because once the scam’s gone viral, you’re going to be straightening them out on it as each new generation of suckers buys into it, till the day you die.
Another aspect none of them ever get: like-energy repulsion in thermodynamics.
In the Greenhouse Gas Effect voodoo believer’s head, classical like-energy repulsion simply doesn’t exist.
Here’s how that often works out: watch for it.
Remember the discussion “if you have a single light bulb in space, it won’t shine at normal, typical voltages and amperages, because lack of an infrared field around the bulb, demonstrates very low resistance to infrared emission, and the bulb dumps it’s radiation before it can glow.”
“If the bulb is taken back to temperatures it was designed to work in, it’ll glow and burn at design-assigned temps.
“If you put that lightbulb in a hot warehouse, it’s going to shine brighter:
“if you turn it on near another, each shines more, because field density of infrared energy between them is higher: and like energy fields, radiate into each other with more difficulty;
therefore on the side of the filament each bulb is on, like-force repulsion, makes each bulb able to dump a little less heat, in that direction. The filament has an area around it that is room temperature and emits normally except on one side a field where flux density is higher, therefore unable to dump as much energy to that side,
therefore temperatures rise slightly and each bulb shines correspondingly a little brighter.”
———————-
Well in “Greenhouse Gas Effect” pseudo-physics, this is now, “sharing energy” where it’s not the presence of field flux density stopping any radiation getting out, as long as the field has enough intensity,
it’s a new paradigm of each one radiating out : completely ignoring field flux-density as physical fact.
No they’re still radiating JUST LIKE THE OTHER FIELD ISN’T THERE
*and right there, they just DENIED a FUNDAMENTAL DRIVER of RADIATION MECHANICS*
They will do it and claim to you they never HEARD of such mechanics in radiative transfer.
Yet, every time they come into a discussion about the action of flow in energy, the hoop jumping begins:
* and ALL EXPERIMENTS now mean NOTHING *
and EVERY OPPORTUNITY to resort to an IMAGINARY FANTASY where they can DEFLECT YOU from the FACT THEIR PHYSICS DON’T WORK is taken.
they’re just “handing off, back and forth.
Go ask one how two light bulbs interact. *They will tell you to a man*
that those light bulbs are picking up therms from the space just outside the surface, internalizing them and immediately radiating another in a handoff effect.
Their physics must vanish the actual mechanism of radiation to be replaced with a social one where objects “share” energy by radiating into the field, then immediately receiving another from that field to replace it.
For Greenhouse Gas Effect Believers, every opportunity to avoid experimentation must be taken.
All experiments mean nothing except that there really is a Greenhouse Gas Effect, “You’re just confused.”
As they tell you the have a steel sphere at one temp on one side, the cold void of space on the other, yet that half of all therms touching the steel sphere must be re-radiated back into a field of higher flux density.
Willis tried to tell this forum he invented a steel sphere that must radiate back 1/2 of all thermal energy coming to it,
*no matter what the temperature hence field flux density, on the other side of that sphere*
and claims here proudly, that for years, not one human being has been able to find a hole in his story.
Go directly to his and his Greenhouse Gas Effect fan club and ask them why two light bulbs get hotter when burned near each other.
AGAIN: all acknowledgement of field flux density parameters vanishes instantly.
The bulbs aren’t trying to radiate into an elevated flux density field, and failing, because radiation can’t get out.
No this is the Amateurs-R-Us paradigm of utter disavowal of any acknowledgment of field flux density in radiative transfer mechanics, because it makes their voodoo line up PRECISELY with their experimental failure-to-appear.
“I’m blowing smoke.”
No Willis, YOU’RE THE ONE who lost sight of the entire concept, of impossibility of radiation,
into any field of identical flux density.
THESE WORDS DO NOT EXIST IN WILLIS’
and OTHER MAGIC GASSER’S VOCABULULARIES:
“Radiative transfer can not occur into a field with already identical levels of flux density.”
What they have, is “socialist physics” where the energy cooperates, and radiation into fields of identical intensity happen E.V.E.R.Y. STEP of the W.A.Y.
Go back and look.
They all do it,
because *THEIR BULLSHOOT DOESN’T FLY WITHOUT RADIATION,
INTO IDENTICAL FIELD DENSITIES;
which IS FORBIDDEN in RADIATIVE TRANSFER MECHANICS.*
Tell one of them, “Explain to me your story about why two lightbulbs near each other, will both be hotter,”
And watch what he tries to do.
davidmhoffer says:
February 7, 2013 at 3:00 pm
….
I was talking about “the Joke of an Experiment” was too crude to get meaningful data. Thanks for the link BTW. That method makes a lot more sense.
All Greenhouse Gas Effect believers,
(1) Can not differentiate between the Atmospheric Greenhouse Effect,
and the Greenhouse Gas Effect.
Ask one to sort that out for you. They can’t. For instance this article is about the Atmospheric Greenhouse Effect, which exists – attempted proven with the second great Magic Gasser Error,
(2)lack of acknowledgement of radiative transfer into identical energy-flux, fields being forbidden by thermodynamics.
They ALL: to a MAN: *DENY the IMPOSSIBILITY of RADIATIVE TRANSFER into a FIELD already EQUAL in energy density*
Go check with your local magic gasser, and ask him questions about the two things I just said.
Watch when he starts writhing like you’re pouring holy water on a vampire.
Ask him: if your radiative transfer model works, why can’t you show me with an experiment?
*Because they believe in radiative transfer into a field with identical flux density*
I lost a post in the ether so here’s the shortened re-write.
Ask a magic gasser to explain to you why two light bulbs are hotter when they are close together.
He will tell you that the two “share” energy; and that energy from one, enters the other then leaves again,
in direct contravention of the mandate in radiative transfer mechanics,
of the impossibility of radiative transfer into fields of equal or higher strength.
Go ask any magic gasser about the two light bulbs.
He’ll get all jacked up telling you how the energy from one, is entering the other: although the energy from the first will be much reduced by contact with the second.
He’ll act stunned when you explain to him field flux densities don’t allow a lower density field to radiate into a higher density field.
When you tell him the field density between the two rising, is keeps heat from leaving the bulbs in the first place, he’ll pretend he agrees but the very first time he explains it all his way, he will try to slip in radiation into a field of higher density energy.
Oh Yes
He Will.
Appended to the last post I made,
“Or, more likely, he will try to tell you he believes in radiative transfer into a field of identical energy, first: and if you buy that, he might then try to run with that and claim it’s possible for radiative transfer into a field of HIGHER energy density.
In any case when you examine all these Greenhouse Gas Effect wannabes, they all; TO A MAN
will try to tell you they believe in a radiative transfer mechanism in which lower density energy, migrates into a field of already higher density.
This is strictly – I repeat strictly and stringently and unyieldingly *forbidden*
in radiative transfer mechanics.
Richard M says (February 7, 2013 at 2:44 pm): “Willis, I agree with your description. It got me thinking how we might tie this closer to our real situation.”
Willis did a two-shell model here:
http://wattsupwiththat.com/2009/11/17/the-steel-greenhouse/
Figure 4.
Willis Eschenbach wrote:
“This is because rather than the warmer object receiving radiation from the cooler object of say 400 W/m2, it is only receiving a paltry 315 W/m2 from the block of ice. As a result, it cools faster when it is next to the ice.
Can we agree on that, that a block of ice cools an object faster than a block of warm material?”
Willis, sorry, because I believe you are correct in this thread, I cannot agree with you that a block of ice cools an object faster than a block of warm[er than ice] material.
stuff does not cool stuff
Because I agree with you on the wider points, I believe it is correct to say that a cooling (by radiation) object is warmed less by (the radiation from) a block of ice than it is by (the radiation from) a block of warm[er than ice] material.
If I’ve got that wrong, then I don’t understand your wider points -even though I agree with them! Nitpicking? Maybe, but this heat loss and transfer by radiation business needs careful wording in the absence of math (sadly, my default state).
stuff radiates regardless of its surroundings and loses temperature in the process
radiation from stuff will raise the temperature of other stuff regardless of that other stuff’s temperature
ugh?
tjfolkerts says:
February 7, 2013 at 2:59 pm
Dang, I’m forced to settle out of court on that one, of course you are correct. Well caught, I was moving too fast.
Clarity is never a niti-pick. Curiously, I had thought of that, was going to change it, and never got around to it, so rather than a nit-pick, given our agreement, it is an inspired truth …
Many thanks,
w.
Allen B. Eltor wrote:
“I’m blowing smoke.”
Well, that’s more an issue of convection, fluid dynamics and Brownian motion.
@KR says:
February 7, 2013 at 2:26 pm
/////////////////////////////////////////////////
To the extent that DWLWIR reaches the “skin layer” of the ocean nearly all of it is fully absorbed within the first 10 microns of that layer. Approx 50% of such DWLWIR is absorbed within 4 microns.
The ‘theoretical’ energy being absorbed within the first 4 or so microns of the “skin layer” is so great that it ought to lead to significant evaporation since the energy cannot easily make its way downward since the energy flux in this layer of the ocean is upwards, not downwards.. See
http://en.wikipedia.org/wiki/Sea_surface_temperature
from which you will note that temperature in the first 10nm is lower than the temperature in the first 1m. That cannot be conducted against the energy flow, so energy absorbed within the first 1, 2, 3, 4 microns etc cannot easily make its way down into the deep ocean. The realclimate article does not address this. It does not deal with hiow the energy theoretically absorbed in the first few microns is dissipated elsewhere before the first micron layer is boiled off leading to extensive evaporation. There is theoretically enough energy absorbed within the first 5 or so microns of the “skin layer” of the ocean to produce in excess of 12m worth of rainfall!
The other process which could dissipate the energy would be Ocean overturning, but this is a slow mechanical process which cannot operate quickly enough to drag the energy downwards into the deep ocean before the water in the first micron or two has absorbed so much energy that it is ‘boiled’ off.
But my point is that there is a LWIR block immediately above the oceans consisting of windswept spray and spume which is a fine mist of water droplets not vapour. This is seperated from the “skin layer” to which you refer and would absorb all (or nearly all) DWLWIR before it reaches the “skin layer” Since this windswept spray/spume consists of more than a few microns, it would act as a DLWIR block in the sameway as we use suncream to block harmful IR and UV rays. Once again the realclimate article does not address this point.
An assumption is being made that oceans are like a mill pond. Like a laboratory sinkfull of water. They are not. Given that the global average windspped over the oceans is BF force 4, oceans are overlaid with a mist of windswept spray and spume which DWLWIR must first encounter before it can reach the “surface skin” of the ocean and hence before any DWLWIR can go to heat the ocean.
Allen B. Eltor says:
February 7, 2013 at 4:32 pm
I fear that you have confused the impossibility of heat spontaneously flowing from a colder to a warmer surface with what happens to radiation.
You might think of radiation as a tiny BB whizzing through space. It doesn’t care about the temperature of what it hits, it just hits it, and transfers its energy to what it hits.
Similarly radiation passing through space just hits what it hits, and transfers its energy to that object.
This seems like it would be a violation of the Second Law of Thermodynamics, which seems to be what you are asserting.
What you are overlooking is this. Let’s assume there are two objects in outer space, I’ll call them C and H for cold and hot. Here’s the key piece to the puzzle:
If C can see H, H can see C.
This means that whenever a small amount of radiation is flowing from Cold to Hot, there is always MORE radiation simultaneously flowing from hot to cold.
The NET energy transferred by radiation, as you can see in any introductory textbook, is the incoming radiation minus the outgoing radiation, that is to say, energy gained minus energy lost. This NET energy transfer is called a flow of HEAT. It is energy gained minus energy lost.
The hot object is losing more that it is gaining from the cold object, so it cools as heat flows to the cold object in accordance with the second law.
Let me illustrate this with money. Suppose I give you a hundred dollars, and you give me seventy-five dollars. There’s two ways to look at that.
Both ways are equally valid. Now consider that I’m Hot and you’re Cold. Since all solid objects both radiate and absorb radiation, both objects are transferring radiative energy. I radiate 100 W/m2 and you radiate 75 W/m2 …
Net energy transfer, called a flow of heat, is 25 W/m2 from me to you. So the Second Law holds.
This is an exact analogue of the physical situation.
Hope this assists you,
w.
PJF says:
February 7, 2013 at 5:16 pm
Thanks, PJF. Again, this is a semantical problem. Let me say instead that stuff cools faster when it’s next to a block of ice than when it’s next to a block of wood.
The problem is the imprecision of language. When we say ice “warms” or it “cools”, the omitted part is “compared to X”. Compared to me, ice cools. Compared to dry ice, ice warms …
w.
Allen B. Eltor says (February 7, 2013 at 4:19 pm): “Go check with your local magic gasser, and ask him questions about the two things I just said.
Watch when he starts writhing like you’re pouring holy water on a vampire.
Ask him: if your radiative transfer model works, why can’t you show me with an experiment?”
Well, I’m not a “magic gasser”, whatever that is (at least I don’t think so), but my guess is your friendly MG will answer, “Why should I waste my time doing an experiment whose outcome I already know? Why don’t you do the definitive experiment* that proves me wrong? I don’t want to spoil it for you, Allen, but you rest assured: by the time the experiment is over, you get the girl, win the Nobel Prize, and SAVE THE ENTIRE PLANET!! Now you tell me, isn’t that worth a measly few weeks of your time?”
* If I’m reading you right, Dr. Spencer’s “Yes, Virginia” experiment should do the trick:
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
Allen B. Eltor says:
February 7, 2013 at 4:02 pm
Tell one of them, “Explain to me your story about why two lightbulbs near each other, will both be hotter,”
And watch what he tries to do.”
Answer: because they both shine on each other. Really, you need to calm down here. You can even test this out in your home. Get two lamps and put them next to each other as closely as you can. Then put your hand in between the two lamps. Then try again with only one lamp. Prediction: your hand will feel warmer with two lamps shining on it instead of one.
Cheers, 🙂
Gary Hladik says, February 7, 2013 at 5:51 pm: “* If I’m reading you right, Dr. Spencer’s “Yes, Virginia” experiment should do the trick: http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/“
===============================================================
This is not true, it was not an experiment. It was a fictional story, no reference to a real scientific physical experiment proving the main assertion was given in the article. The main assertion was given like that, in capital letters: “Well, I’m going to go ahead and say it: THE PRESENCE OF COOLER OBJECTS CAN, AND DO, CAUSE WARMER OBJECTS TO GET EVEN HOTTER.”
There is apparently no real scientific physical experiment proving that, exactly like in the case of Willis’ shell, ice block etc.
What would be really, really helpful here is if Willis and Huffer would get together (or go it alone!) and reference a textbook or some other reasonably credible source to back up their “theories/facts/whatever.” Blogging-talk does not constitute any proof of anything.
Yes, there are 1,000,000 differences between the boxes in the Wood experiment and the Earth, but those differences are irrelevant,
>>>>>>>>>>>>>>>>>>>>
It is my understanding that what you don’t know can’t hurt you. As a consequence, some people are invulnerable.
jae says:
February 7, 2013 at 6:38 pm
What would be really, really helpful here is if Willis and Huffer would get together (or go it alone!) and reference a textbook or some other reasonably credible source to back up their “theories/facts/whatever.”
>>>>>>>>>>>
Is there some part of “any university thermodynamics text book” that you fail to comprehend?
Thermal Engineering (Rajput)
Applied Thermodynamics (Rajput)
Thermodynamics and Chemistry (DeVoe)
Fundamentals of Thermodynamics (Sonntaq)
Modern Engineering Thermodynamics (Balmer)
Textbook of Thermodynamics (Epstein)
Engineering Thermodynamics (Nag)
Intro to Thermodynamics (Rao)
Thermodynamics (Cengel)
Fundamentals of Thermodynamics (Borgnakke)
Thermodynamics for Engineers (Somerton)
Thermodynamics Demystified (Potter)
Principles of Engineering Thermodynamics (Moran)
Commonly Asked Questions in Thermodynamics (Assael)
There are probably dozens of text books on thermodynamics.
There are also reasonably well written articles on the Second Law and Stefan-Boltzmann on the internet:
http://en.wikipedia.org/wiki/Second_law_of_thermodynamics
http://en.wikipedia.org/wiki/Stefan%E2%80%93Boltzmann_law
Greg House says (February 7, 2013 at 6:30 pm): “This is not true, it was not an experiment.”
Correct. That’s why our hypothetical MG was advising Allen to do the experiment himself. Personally, I think it was a pretty good sales pitch. So good, in fact, that if you don’t hurry up and do the experiment yourself, Greg, Allen may very well snatch that Nobel Prize right out of your hands. Why you’re still reading this thread when fame, fortune, and femmes beckon is beyond me. 🙂
Dr,
AGW is way out on a limb proof wise, an not in a good way.
“Finally, we’re told the earth’s atmosphere is not like a real glass greenhouse, yet I have seen dozens of references by various government science agencies and universities describing the earth’s atmosphere being like a real glass greenhouse in which the troposphere “traps” the heat thereby cooling the stratosphere.”
I will agree with you that there are plenty of people out there especially in the government pushing broken versions of GHE to support AGW.
However, I will repeat what I said earlier. The Earth and The Moon receive the same amount of energy per m2. The average surface temp of the Moon which has no atmosphere is lower than the average surface temp of the Earth which does have an atmosphere.
It is one thing to say we don’t really understand the mechanism behind GHE, however suggesting there is no GHE at all is counter to what is observable.
Second Law 1824 (Carnot)
2nd Law Revision 1851 (Kelvin)
2nd Law Revision 1854 (Clausius)
2nd Law Revision 2000 (Stoner)
Stefan 1879
Stefan-Boltzmann 1884
Wien’s Displacement Law 1893
Planck’s Constant 1900
For those lecturing me on physics, please note that the 2nd Law has been revised several times as our understanding of the physics has improved. In order for Stefan-Boltzmann Law and the Second Law to both be true, the second Law must refer to the net energy flux, and the energy flux must be two way (meaning from hot to cold and cold to hot at the same time). In fact, all the definitions over time of the 2nd Law refer to a statistical distribution, not a hard and fast one way street. The work of Wien and Planck improved our understanding of SB Law and 2nd Law and how they relate to each other, and have been proven experimentally too many times to count.
Those who yap on about what the 2nd Law says, or what SB Law says, but have no knowledge of the practical applications of these, ought to to some studying until they understand the formulas, how to apply them, and can prove that they have applied them properly through experimentation. I have. I’m betting Willis has. Richard Courtney most likely as well. Certainly Leif Svalgaard. Robert G Brown. Many of us have studied, learned, applied and proven. Yet we are lectured ad nauseum by those who have not and cannot.
jae says (February 7, 2013 at 6:38 pm): ‘What would be really, really helpful here is if Willis and Huffer would get together (or go it alone!) and reference a textbook or some other reasonably credible source to back up their “theories/facts/whatever.’
Science of Doom did just that:
http://scienceofdoom.com/2010/10/07/amazing-things-we-find-in-textbooks-the-real-second-law-of-thermodynamics/
“Blogging-talk does not constitute any proof of anything.”
I fear that even textbooks, in the eyes of some, do not constitute any proof of anything. See the comment thread following the SoD article.
Nice try, Huffer, but listing all the thermo books doesn’t cut it. Come on, if you have ANY integrity here, you will cite specific pages. What a piece of work!
Willis?? any specific references from the literature to your shell-game?
MattS says, February 7, 2013 at 7:23 pm: I will agree with you that there are plenty of people out there especially in the government pushing broken versions of GHE to support AGW.
===============================================================
The original official IPCC version has been broken since it came into existence around 1860, the Wood experiment (1909) proves that.
jae says:
February 7, 2013 at 7:33 pm
Nice try, Huffer, but listing all the thermo books doesn’t cut it. Come on, if you have ANY integrity here, you will cite specific pages. What a piece of work!
>>>>>>>>>>>>>.
Experimental proof was demanded and supplied.
Definitions were demanded and supplied.
Text books were demanded and supplied.
Now you want page numbers?
If you are such a genius, I’m certain that you can find the page numbers and quotes that prove what a complete idiot I am. Please do so.
davidmhoffer,
What I think jae is asking for, is for you to read it to him.
Greg House,
“The original official IPCC version has been broken since it came into existence around 1860”
This statement is simply absurd. The IPCC was created in 1988. Therefore the official IPCC version of GHE or anything else for that matter did not exist before 1988.
“the Wood experiment (1909) proves that”
Willis has demonstrated that the Wood experiment proves NOTHING. You have done noting to refute this except to keep repeating the above statement. Continually stating that the Wood experiment proves what you think it proves without even making an effort to refute anything Willis has presented in the main article brings to mind a certain definition of insanity.
MiCro says:
February 7, 2013 at 8:36 pm
davidmhoffer,
What I think jae is asking for, is for you to read it to him.
>>>>>>>>>>>>>>.
Yeah, and if I do, he’ll call it a fairy tale and ask for something else as proof. Funny thing is that Willis actually linked to the specific things being asked for upthread.
Gino says:
February 7, 2013 at 8:49 am
“P_shell_outgoing = sigma*T_shell^4*(4*pi*(R_inner^2+R_outer^2)
This is incorrect. There is no net energy flow from the shell to the core.”
It is correct. I did not say there was a net energy flow out of the shell. I said P_shell_outgoing = P_shell_incoming for equilibrium. The net is zero, because it is the outgoing minus the incoming, and they are equal. Note that the temperature of the shell is less than the temperature of the planet, so there is full consistency with the 2nd Law. Transfer of heat is only one way – outward. But, that does not mean that energy cannot pool up higher at the surface of the planet than it otherwise would without the shell, creating a higher temperature at the surface.
Failure to appreciate that last is a common misapprehension in this comment thread. A lot of people here are completely mixed up because they are not making the distinction between Joules and Watts. A Joule is a quantity of energy. A Watt is a Joule per second, the time rate of change of energy. Joules are conserved, Watts are not.
And then, other people are making a mistake thinking that temperature is proportional to radiated Watts, but it isn’t. Temperature to the fourth power is. So, you can have a big change in Watts without having a big change in temperature.
I solved the entire thing here. The question is not whether Willis is correct or not vis a vis his steel greenhouse model. He is correct. The question is whether this is a valid analogy to a situation in which the radiating bodies are not isolated from one another by a vacuum. A vacuum prevents energy swapping from any means but radiation, but that is not at all the situation with the Earth and its atmosphere.
Further to my previous comment, here is the bog-standard explanation of the radiative heat transfer. It says:
Note that the formula in that citation is the exact same formula I just scanned from my favorite text, the Engineer In Training Review Manual. The manual has more information per page than any other book I’ve seen. It differs from a college textbook in that if a college textbook is wrong, well, no big deal. But if the EIT Review Manual is wrong, buildings can collapse and boilers can explode and people can die … not only that, but budding engineers bitch like hell if an error in the manual makes them miss a question on the EIT exam … so it is very reliable. As you can see below, it says just what the cite above says:

Here’s another:
Now, there are three scholarly references. Every one of them makes the explicit claim that energy flows in both directions, from A to B and from B to A. That’s the physical meaning of the equation 5.10 above. We can factor that equation as follows.
q = A C s T1^4 – A C s T2^4 (5.10)
The first term reflects the flow in one direction, and the second term, the flow in the opposite direction. Note that since T1 and T2 are different temperatures, this explicitly means that energy is flowing in both directions.
SO … for all of you out there that hold on to the view that cold objects do not radiate energy to warm objects, I’ve just given three scholarly references which explicitly state that cold objects do indeed radiate energy to warm objects.
This being a scientific site, don’t bother telling me that I’m wrong. You are arguing, not with me, but with hundreds of years of people using those exact same formulas to build things from boilers and spaceships. So here’s the deal …
If you can’t come back with citations to established authorities which say that radiative energy DOESN’T flow from cold objects to warm objects … then don’t bother to come back. I’ve heard all the half-baked claims and pseudo-physics fantasies I can stand. Show up with science in your hand, citations saying cold objects CAN’T radiate to hot objects, or don’t show up at all.
w.
davidmhoffer says:
February 7, 2013 at 8:10 pm
j
While I agree with your science (the flows go both ways, hot to cold and cold to hot, but net flow only ever goes one way, hot to cold), regarding the page numbers I agree with jae. I hate it when somebody cites e.g. the IPCC Report for their claim, like somehow we’re supposed to guess what they are talking about. And that happens all the time in scientific papers, I find it shocking.
My old chemistry teacher, Mrs. Henniger, would whup our papers up and down with her red pencil if we did that, cited a reference and didn’t give page and if necessary paragraph. Science is not a guessing game, jae should not have to guess what you are referring to.
When I first started blogging, I’d do what you say above, go try to “find the page numbers and quotes” the person referred to … then after much effort I’d come back and get something along the lines of “You idiot, that’s not the information I’m talking about”.
About the third time someone tried that, I told them no, I’m not playing any more guessing games. If you have a reference, then man up and give it to me like an adult, or you lose all credibility. I’ve sworn off snipe hunts.
And you’re still right about the science … thanks for persevering.
w.
Just curious Willis,I passed my EIT exam way back in 1980 (or maybe 1981, the years get a bit cloudly with the passage of time). When exactly did you pass your EIT exam ?
YES heat fluxes flow both ways, but the NET heat flow is ALWAYS from warmer (Earth) to colder (Atmosphere) locations.
Cheers, Kevin.
Willis,
While I agree with your science (the flows go both ways, hot to cold and cold to hot, but net flow only ever goes one way, hot to cold), regarding the page numbers I agree with jae.
>>>>>>>>>>>>>>>>>
The specific definitions, experimental documentation, links to detailed articles and more were all supplied by me upthread, and on multiple occasions. At some point in the conversation “read it yourself” becomes a fair response.
Willis Eschenbach says:
February 6, 2013 at 5:01 pm
(to Greg House)
Greg, of course it has been proven, many times over. It’s in every college thermo textbook. Go buy one. Read it. Come back. Participate intelligently, instead of just asserting your misconceptions.
>>>>>>>>>>>>>>>>.
Those are your words Willis. But you bust my chops for (impolitely) telling jae the same thing?
Willis Eschenbach says, February 7, 2013 at 9:11 pm: ” Show up with science in your hand, citations saying cold objects CAN’T radiate to hot objects, or don’t show up at all.”
=============================================================
It is not about whether colder objects can or can not radiate to warmer objects.
It is about whether radiation of colder objects affects temperature of warmer objects. That is the point, please, note that.
I have never seen any valid link to a real scientific experiment proving it does. Nobody I talked to on various blogs was able to present such a link. No link, no description of a real scientific experiment proving that point directly, by actual temperature measurements was presented. People did present fictional stories or general references to the same unproven claims, but nothing real. The fictional stories about plates, shells etc have been around for years, but again, nothing real has come up until now.
On the other hand we have the Wood experiment where this trapped/back radiation apparently adds nothing or next to nothing to the temperature of the source. The “greenhouse effect” as presented by the IPCC is dead and no fictional shell can revive it. The question is for how long will it be possible to scare people by this dead body.
Willis Eschenbach says:
February 7, 2013 at 9:11 pm
“The first term reflects the flow in one direction, and the second term, the flow in the opposite direction.”
In large part, this is an argument of semantics, or of mental constructs which produce a particular perspective. There are not really individual flows of energy which bounce back and forth between the planet’s surface and the inside of the shell. Electromagnetic radiation is a wave phenomenon. A some points, the fields will interfere constructively, and at others destructively. So, there will be peaks of electric field and nulls at various places along the way, as there will of the magnetic fields. At equilibrium, there will be some dc level of electromagnetic energy filling the gap, as well as fluctuations from the non-stationary interference patterns which could appear to move in either direction on a finite time scale.
So, keep in mind that what you are describing is a model of what is going on, but there is no deep philosophical “truth” to it.
Let’s try something a little easier to wrap our minds around than flows of invisible energy fields.
Instead of the nuclear core, think of a spring gushing out of a cliffside into a stone channel. Beneath the source, there is some depth of water. Let’s assume that the channel is infinitely long and flat. In steady state, there is a uniform depth of water flowing outward.
Now, we put a low barrier, analogous to the steel shell, across the channel a few feet from the source. The water pools up behind it until it overcomes it. After an infinite amount of time to reach steady state again, the same volume of water, at uniform depth, is flowing downstream from the barrier. But, behind the barrier, the water is deeper.
You can think of it as that the barrier is sending water back to the source, and the water is simultaneously flowing to the barrier from the source. It’s fully equivalent to the observations. But, another way to look at it is that the water has simply pooled up behind the barrier.
Note the analogous action here: the flow rate is analogous to the Watts of power flowing out from the planet, but the water itself is analogous to the energy. We don’t have more Watts (flow of water) in the end. We have more retained energy (water).
Now, what is the analogy to filling the vacuum up with some medium which can transfer energy directly? It is akin to constructing not a barrier, but rather laying a slab in the channel below the spring at the height of the barrier. In the steady state, the water on top of the slab may only be slightly higher than the water beyond it, and this is due only to the fact that the water falling off the ledge of the slab will pick up a little energy from the change in potential, though it might lose it again due to viscous friction. But, there will not generally be much deeper water above the slab than below it, if at all.
And, that is how I see your analogy potentially failing, because it places a vacuum between the planet and the shell which is not present for the Earth and its atmosphere.
Micro asks,
This question makes no sense, Micro. The absorption or emission of a photon from CO2 does not define its temperature. CO2 will absorb radiation at any temperature and can emit at any temperature. You seem to be totally confused about the basics.
For those who want to learn, rather than just give the impression that sceptics are stupid, probably the most instructive site to teach the basics is ‘Science of Doom’
There, you can see an almost identical discussion of the Steel Shell scenario, this time with an insulated shell, at …
http://scienceofdoom.com/2010/07/26/do-trenberth-and-kiehl-understand-the-first-law-of-thermodynamics/
…..which demonstrates that there is no limit to the temperature that can be attained in the interior if the insulation is thick enough.
Can a cold body make a warmer one warmer? This is called the 3 body problem and is explained here…
http://scienceofdoom.com/2010/11/05/the-three-body-problem/
The woods experiment shows that there is no rise in temperature due to IR feedback on earth, the woods experiment does not show that feed backs do not exist, it does not show that the feedback climatologists call a “green house effect” does not exist, It shows that the “green house effect” is extremely weak, and shows the IR feedback being measured here on earth does not effect temperature or has a very weak unmeasurable effect on temperature.
The principle of the thought experiment Wills is explaining above shows an extreme example of a feedback by showing a hypothetical planet with a steel shell around it that in theory works on the same principle of how lasers work. Covering up a source of radiative heat like a light bulb will raise its temperature to a certain degree as the insulation will slow down the transportation heat from the source to beyond the insulation.
When you begin to add physical parameters like the mass of the planet and ‘shell’ and then add the distance between the planet and ‘shell’, the feed back will become weaker the greater the distance is between the shell the planet.
When you begin to add the parameters of our atmosphere in place of the ‘shell’ the Feedback will become weak to the point where it has no measurable effect on temperature, as ‘The woods experiment’ shows. improving the Woods experiment and the sensitivity of the instruments may in theory show an extremely weak feedback, although, finding the feed back with highly sensitive instruments will only show that this feedback does not effect our current instruments used for recording the planets temperature.
Figure 2 above Reminded me of a Dyson sphere which is a hypothetical megastructure originally described by Freeman Dyson. It should be a interesting starting point for further study exploring the theoretical principles around this subject.
“A Dyson sphere in the solar system, with a radius of one AU would have a surface area of at least 2.72e17 km^2, around 600 million times the surface area of the Earth. The sun has a energy output of around 4e26 W, of which most would be available to do useful work.”
http://www.aleph.se/Nada/dysonFAQ.html
There is also a limit in physics where it is imposable for a feedback to exist depending on mass and distance, one example is; if you scale the mass of the planet in figure 2. up to a point where IR can not escape the planet, therefor it will not reach the ‘shell’ and not cause any feedback. this can be worked out using the speed of light, basically E=Mc2.
[snip . . a list of your qualifications with a series of comments telling people they are wrong is basically content free. Please post your actual arguments rather than your CV, thanks . . . mod]
A reason it is hard to get into space is that chemical reaction have a limit to how hot they
can get. Chemical reaction produce a maximum velocity of their gases.
Related to this, is that gases at a particular temperature have difference velocity, helium or hydrogen have higher velocity than oxygen or nitrogen.
An important aspect with chemical rocket is how fast gases can leave the rocket nozzle. If you get a gas to leave at the speed of light, you very efficient rocket [uses less propellant-and 90% of the mass of chemical rocket are propellant [so this is important].
So there two things- how high of heat chemical reaction can reach and types of gasses with highest velocity.
And how hot a chemical reaction can get is one factor.
So certain fuels can reach certain temperature and it does not matter how much of this fuel is being burnt- it reaches a limit in terms of temperature.
This is similar to silly argument about 911, where it is said kerosene [jet fuel] doesn’t melt steel- regardless of how much kerosene is burning. But of course the issue is not about melting steel, steel is weaken with heat well below it’s melting point- and structural members need their strength- without this, 100 stories buildings don’t work- particularly when planes crash into them destroying some of their structural support.
Point is fire has limit to how hot it gets.
So you chemical reactions is adding heat, but without them increasing the temperature.
Obviously the combustion would adding energy but they not making things hotter.
And this article is about making things hotter.
So if burn certain amount fuel you can calculate that generates a certain amount watts per square. If doing this, one can calculate that adding so much fuel and therefore watts per square meter and then converting this to a certain temperature.
But it is NOT allowing for the limit of how hot a fuel can make something.
Now, nuclear reactions are quite different in terms of how hot they can heat something- it can heat things well over the boiling point of everything in existence.
Likewise electricity can used to reach any temperature- the limitation is the temperature the filament can withstand- a reason tungsten filaments are used [tungsten has very high melting point].
Sunlight which diffused and weaken at Earth distance- is weak in regards to the maximum temperature it can heat something. But if concentrated the sunlight one reach as a maximum
the temperature of the Sun [and vaporize anything].
So unmagnified sunlight is poor cousin in terms causing things to reach their maximum temperature- as compared to chemical, electrical or nuclear reactions which generate heat.
But if agree that Sunlight is weak in regards to heating stuff up in temperature, it’s spectrum
radiation is not all the same in this regard. The most amount energy and ability to heat anything is in the visible and near infrared part of spectrum.
The sun emits X-rays, UV, visible, infrared, microwave and radio. A photon of Xray is the most
powerful, followed by UV, visible, Near infrared, Mid- infrared, longwave IR, microwave and finally radio.
But even though the Sun emits X-rays it does not emit enough of them to up things, and the
sun emits more UV but not a lot:
“Sunlight’s composition at ground level, per square meter, with the sun at the zenith, is about 527 watts of infrared radiation, 445 watts of visible light, and 32 watts of ultraviolet radiation. ”
http://en.wikipedia.org/wiki/Sunlight
So because one has more infrared radiation [which mostly Near infrared] it does most of the warming.
But if you were to split sunlight and magnify only the infrared radiation, it would reach a lower
maximum temperature as compared to visible light. It would like magnifying the sunlight from a red star. It’s still hot enough melt almost anything, but it would not vaporize everything.
So infrared part gives the most heat, but is not the hottest light to use.
Which bring us to longwave IR [which there is so much fuss about]. We have lasers which use longwave IR and there are real niffy because the don’t destroy stuff you want to examine- if instead bounced say greenlight laser at something it heats it up and makes it more difficult to study.
It seems to be that if something is radiating a bodybody temperature then generally that is neighborhood of it’s max temperature. I think that was sort of the whole point of blackbody temperature stuff- find the max temperature of sunlight and such.
So for instance take a lightbulb with it’s filament at 3000 C, you should not be able to heat up the filament with something cooler than the filament temperature. Nor can magnify the light of filament to higher temperature than the filament.
Well I took the trouble to look at Willis’ shell model again last night and whilst I think it is a rather inaccurate model of the greenhouse effect it does rather demonstrate that you are ALL right and really arguing about the same point from different model perspectives. However, you are wrong if you think Willis’ model demonstrates GHE is real – it actually demontrates the opposite. Here are the two perspectives spelt out, starting with Willis model:
1] The Willis model is based on IR and therefore can be considered a qunatum mechanical model. Notice that in the first diagram the heat being lost to space is 235W/sqm. Willis tells us it is a black body so you could work out its temperature but there’s no real need. It will have a temperature related to its emissions which is fixed. In the second diagram the back radiation is 235W/sqm. However, notice similarly that in order to create an energy balance the rate of COOLING of the planet’s surface has also DOUBLED. Thus the NET emissions from the planets surface are exactly as they were before. Thus the temperature of the planet’s surface is exactly the same – all that is happening is that the back-radiation that is reflected towards the planet from the shell is simply reflected back towards the shell again (maybe the energy is absorbed and then re-emitted again but from an energy balance poitn of view we don’t need to worry about those details). In effect, Willis simple energy balance model demonstrates that back radiation of IR cannot heat the surface of the planet, even when it is 100% – it simply increases the local radiative losses from the surface of the planet.
2] Everyone else is using the classical model of thermodynamics based on heat flux. In this case heat flux cannot flow from a cold body to a hot body so there can be no net flow of heat from the shell to the planet. This means that the presence of the shell cannot impact the temperature of the surface of the planet and the temperature of the planet must remain the same regardless of the presence of the shell. You will notice that this is the same conclusion as in the Willis model. Similarly the conclusion is that the greenhouse effect cannot warm the surface of the planet.
The confusion that is causing so much argument here is the mixing of classical and quantum mechanical models. However, there is no disagreement in reality between the two approaches. The classical models talk about “heat flux” but heat flux doesn’t exist in a real manner in a system where radiation is the method of heat transfer except that there “appears” to be a net flow of heat between two objects – in reality this is an imaginary heat flux because in reality the radiative model involves a conversion from heat energy to photonic energy and then from photonic energy to heat energy in a 100% efficient process. The classical model doesn’t know anything about the details of how the heat flows from one body to another but it doesn’t need to – the laws still apply no matter the details.
A colder sky cannot possibly heat a warmer surface, any more than a trillion 1 ton blocks of frozen CO2 radiating at 194.56 K could melt a 1 inch water ice cube radiating at 273.15 K.
“CLASSICAL THERMODYNAMICS IS THE ONLY PHYSICAL THEORY OF UNIVERSAL CONTENT WHICH I AM CONVINCED WILL NEVER BE OVERTHROWN”….A Einstein.
Greg House:
At February 7, 2013 at 6:30 pm you write
That is not true. I conduct such an experiment most days and I use apparatus which I keep in my kitchen. The apparatus is called a microwave oven.
I put things in the apparatus, provide a power supply, and the apparatus heats the things I inserted to temperatures higher than the temperature of the apparatus.
In my experiment, as in “Willis shell”, the increase in temperature of the cooler object is induced by an effect of electromagnetic radiation and a supply of energy flow.
Richard
The things
*** all two-way entropic flow arguments fail at is dual***
(1) they won’t try to show you a diagrammatic explanation of how they conceive a low energy flux displacing another higher one in either free space or a matrix,
and
(2) when it comes time to check what they’re saying through experiment, the energy they always claim’s going to be there, never is.
You’ve got amateurs throwing around arguments all day long,
but the fact is,
the equations describing entropy are one-way. Bullshoot claims by wannabes aside, there’s no such phrase in mathematics, “and then, when entropy reverses…”
If there was math to describe the reversal of charge density progression, we’d all know what it is. Those of us who have to know if they exist, to keep our jobs, would know.
The proper diagrammatic representations of the events cried to the heavens to be occurring, never appear in the literature – although those purporting the mechanism to be very real, have had years to describe it, diagram it, and enter it into the literature.
Because I can diagram for a child how energy distribution in a solid winds up. It’s as easy as 1-2-3. Why can’t reverse-entropy people just give me a diagram showing where the charges distribute and I’ll teach it to my child. Ok my youngest child’s 22. I’ll draw it for my grandchild.
Why won’t they ever lay out exactly what they’re describing to you, properly notated and competently explained?
Answer: because they can’t, because there’s no such thing.
The predicted end result – that when THE INSTRUMENTS COME ON, the ENERGY is where they SAY it’s gonna be,
is always missing.
———-
Finally, look at the people purporting the two competing concepts:
the people who claimed to be satisfied with Woods’ experiment, went on to invent the electronic, and it’s associated, wireless, radiation-based space age.
The people who now claim entropy goes two directions are also, universally, associated with bad science. Fraud, intentional non-disclosure after fantastic claims, repeated evidence of gross incompetence.
Who are you going to believe: every equation ever devised showing energy flow in nature being from higher to lower,
every experiment ever done to check on the “Greenhouse Gas Effect Reverse Radiation,”
by the people who – literally – brought you the radiative transfer mechanism age?
Or the people who brought you Mannian Statistics, the entire Global Temperature Database at CRU trashed, and Manmade Catastrophic Global Warming? Which by the way ALSO: does not exist. Because it is based on – what?
Magical backward flowing energy.
It’s a scam, and the protests that the whole world hasn’t understood when we declared entropy to be a one way street, are as intelligent as the rest of the bad science surrounding the whole fraudulent debacle.
Greg House wrote “The original official IPCC version has been broken since it came into existence around 1860, the Wood experiment (1909) proves that.”
This is incorrect. The current understanding of the greenhouse effect stems from the work of Callendar, Kaplan and Plass in the 1950s and 60s, and involves energy balance at the top of the atmosphere, not the bottom. Therefore Woods experiment in no way refutes this, as the column of air inside the box is too small to exhibit a lapse rate, which is a key feature of the “official IPPC version” (not that there is such a thing).
Please read Spencer Wearts excellent book on the history of the discovery of global warming, also available on-line, see e.g. http://www.aip.org/history/climate/co2.htm .
Allen B. Eltor,
Your entire post above seems to be an Appeal to Authority — your own ‘authority’.
I can follow Willis Eschenbach’s and David Hoffer’s comments, but yours? Your comment addresses none of the substantive issues, except in extremely general terms.
If you want to be credible, comment on specifics. Your self-promoting wears thin. So you got a degree, so what? Scientific truth is the issue here. Your chest-thumping gets old fast.
MOD’s my comment has disappeared! I’ve tried to repost it but WP says it’s a duplicate.
[Reply: Sorry, nothing in the spam folder. Please re-submit, and always keep a copy of your comment until it is posted. — mod.]
For those who claim that a cooler object cannot radiate energy to a warmer object, the challenge for them is to explain how the cooler object can know in which directions it can safely radiate photons without them hitting a warmer object. This will be tricky as, for example, the warmer object may not be stationary, so the cooler object will need to be able to predict the trajectory of the warmer object to be sure that none of the photons that it radiates will hit it.
The conventional “net transfer” mechanism has no such difficulty; all bodies radiate energy in all directions, according to their temperature and emissivity, however more energy is radiated from the warm object to the cool object than vice versa, so the laws of thermodynamics are satisfied in terms of net averages over large numbers of photons.
It is not surprising that early work on thermodynamics did not stipulate “net” fluxes, because photons are a 20th century concept, so the idea that the measurable transfer of heat was actually the net result of an invisible exchange in both directions would have been an unnecessary complication.
The woods experiment shows that there is no rise in temperature due to IR feedback on earth, the woods experiment does not show that feed backs do not exist, it does not show that the feedback climatologists call a “green house effect” does not exist, It shows that the “green house effect” is extremely weak, and shows the IR feedback being measured here on earth does not effect temperature or has a very weak unmeasurable effect on temperature.
The principle of the thought experiment Wills is explaining above as I see it shows an extreme example of a feedback by showing a hypothetical planet with a steel shell around it that in theory works on the same principle of how lasers work. Covering up a source of radiative heat like a light bulb will raise its temperature to a certain degree as the insulation will slow down the transportation heat from the source to beyond the insulation.
When you begin to add physical parameters like the mass of the planet and ‘shell’ and then add the distance between the planet and ‘shell’, the feed back will become weaker the greater the distance is between the shell the planet.
When you begin to add the parameters of our atmosphere in place of the ‘shell’ the Feedback will become weak to the point where it has no measurable effect on temperature, as ‘The woods experiment’ shows. improving the Woods experiment and the sensitivity of the instruments may in theory show an extremely weak feedback, although, finding the feed back with highly sensitive instruments will only show that this feedback does not effect our current instruments used for recording the planets temperature.
Figure 2 above Reminded me of a Dyson sphere which is a hypothetical mega-structure originally described by Freeman Dyson. It should be a interesting starting point for further study.
A Dyson sphere in the solar system, with a radius of one AU would have a surface area of at least 2.72e17 km^2, around 600 million times the surface area of the Earth. The sun has a energy output of around 4e26 W, of which most would be available to do useful work.
http://www.aleph.se/Nada/dysonFAQ.html
There is also a limit in physics where it is imposable for a feedback to exist depending on mass and distance, one example is; if you scale the mass of the planet in figure 2. up to a point where IR can not escape the planet there for it will not reach the ‘shell’ and not cause any feedback. this can be worked out using the mass of an object and the speed of light, basically E=Mc2.
I have extensively checked out the greenhouse effect supposition (GHE). At the end of the day, however, I side with individuals such as astrophysicist, Joseph E. Postma, in relation to this issue. His work is sufficiently authoritative to dismiss the GHE supposition
Just one point… it is important to understand that the earth’s atmosphere does not act like a an actual greenhouse which, unlike the atmosphere, is a ‘closed system’. Consequently, at an elementary level, and using basic common sense, it becomes apparent that the GHE supposition is not valid.
The mother of all the heat-retention in the atmosphere is undoubtedly water vapour. CO2 is irrelevant in the overall scheme of things.
Allen,
Suppose a warm metal block and a cool metal block are in contact. Do faster-than-average atoms in the cool block ever hit slower-than-average atoms in the warm block? Do such collisions ever transfer energy from the cool block to the warm block?
Is that ALSO “magical backward energy flow”?
Isn’t it very odd that all the Practical Experiments (including those at Universities) show that rather than warming the object DWIR actually cools them in relation to their surrounding. But Dr Spencer with his box sees this as proving that DWIR is heating the surface because the air inside could theoretically get to the same temperature as outer space?
Only a true believer can say this demonstrates heating.
richard verney – The “skin layer” measurements referred to in the RealClimate post (http://www.realclimate.org/index.php/archives/2006/09/why-greenhouse-gases-heat-the-ocean/) were taken in situ, on the oceans, not in a “mill pond”.
I’ll refer you to a description of heat loss through the thermal skin layer by Minnett (https://www.ghrsst.org/files/download.php?m=documents&f=120113121306-SSTDefinitionsDiscussion.pdf), primary research papers such as Minnett et al 2011 (http://www.sciencedirect.com/science/article/pii/S0967064510003024), Veron et al 2011 (http://journals.ametsoc.org/doi/abs/10.1175/2010JPO4491.1?journalCode=phoc), and for an overview Science of Doom (http://scienceofdoom.com/2011/01/18/the-cool-skin-of-the-ocean/) also discusses this in some detail.
Winds certainly do have an effect – the gradient between sub-skin temperatures and the skin layer is ~0.6K in low winds, dropping to perhaps ~0.13K in high winds. But the viscous surface water represents a thermal layer that energy must go through to leave the oceans, and energy transfer across that boundary is affected by the thermal gradient, by the difference between sub-skin water and the atmosphere.
A warmer atmosphere, with higher DLWR (even though _cooler_ than the sub-skin oceans), reduces the energy loss of the ocean through that boundary, meaning energy accumulation from incoming/penetrating sunlight, and hence ocean warming.
It may seem counter-intuitive, but those are the observations. If you disagree with the mechanism of outward energy transfer from the oceans, you will need to point to some supporting data.
Greg House says (February 7, 2013 at 10:01 pm): “It is not about whether colder objects can or can not radiate to warmer objects.
It is about whether radiation of colder objects affects temperature of warmer objects. That is the point, please, note that.”
Hi, Greg. I believe you’ve been referred to this experiment before, but just in case, check this out:
http://scienceofdoom.com/2010/07/31/the-amazing-case-of-back-radiation-part-three/
Greg House wrote:
“It is not about whether colder objects can or can not radiate to warmer objects.
It is about whether radiation of colder objects affects temperature of warmer objects. That is the point, please, note that.”
OK, I note that that is your point. So, if the radiation of colder objects doesn’t affect the temperature of warmer objects, what happens to the energy of that radiation?
Bart says (February 7, 2013 at 11:03 pm): “And, that is how I see your analogy potentially failing, because it places a vacuum between the planet and the shell which is not present for the Earth and its atmosphere.”
When teaching a new concept, it’s usually best to start with a simplified ideal model and work your way toward more complicated, more realistic cases. Willis’s model is step 1. The vacuum in the model is there to isolate the shell from conductive/convective contact with the surface. Of course the Earth’s upper atmosphere isn’t 100% isolated from the surface, but it’s still cooler than the surface and that’s the key factor illustrated in the model.
@richardscourtney:”I put things in the apparatus, provide a power supply, and the apparatus heats the things I inserted to temperatures higher than the temperature of the apparatus.”
That’s all very well Richard, but this is an energy conversion process, not a heat energy transfer in a closed system.The argument we are having is whether a cooler object can make a hotter source even hotter, or if this would break the First or Second Law of Thermodynamics.
Personally I would say that if it were possible to make a hot object even hotter by getting it intimate with a cooler object, then making my bath water cooler by adding cold water would be a futile exercise, since the energy inherent in “cold” water (i.e. water way above 0K) would still be enough to heat up the hot water. Or maybe not…..
If you were able to do that, then you could take buckets of water at the same temperature, mix them together and get a bucket of water at double the temperature. Oh if only! You could make a steam turbine just by “concentrating” the heat in water at room temperature! Sadly this would contradict the 2nd Law of Thermodynamics (i.e. the Laws of Entropy).
Mervyn says:
February 8, 2013 at 5:32 am
I have extensively checked out the greenhouse effect supposition (GHE). At the end of the day, however, I side with individuals such as astrophysicist, Joseph E. Postma, in relation to this issue. His work is sufficiently authoritative to dismiss the GHE supposition….
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
For those interested Dr. Postma posts a counter rebuttal to the “Skeptical Science” blog critique of the recent paper by astrophysicist Joseph E. Postma, ‘Copernicus meets the greenhouse effect,’
Ryan says (February 8, 2013 at 7:28 am): “The argument we are having is whether a cooler object can make a hotter source even hotter, or if this would break the First or Second Law of Thermodynamics.”
What’s your take on MikeB’s three-body reference above?
http://scienceofdoom.com/2010/11/05/the-three-body-problem/
Ryan (and many others),
You are making a false analogy when you say things like “Personally I would say that if it were possible to make a hot object even hotter by getting it intimate with a cooler object, then making my bath water cooler by adding cold water would be a futile exercise,”
The analogy is more like this. Fill a bath tub with water, put a small electric heater in the water, and set the whole thing outside on a very cold (for example -30 C) winter day (ie in contact with with the very cold surroundings). Measure the temperature of the water … maybe the heater can only heat the water to 5 C.
Then try it again on a sort of cold day (for example, 0C). Now maybe the heater (with the same power as before) will warm the water to 20 C. Putting the bathtub & water in contact with COLD air (0 C) instead of in contact with VERY COLD air is part of the cause allowing the water to warm from 5 C to 20 C. Even though the air is still COLD, it reduces the heat loss, thereby allowing the heater to be more effective.
THAT is what GHGs do. They let the earth be “in contact” (via thermal radiation) with COLD surroundings (the atmosphere) rather than in contact with VERY COLD surroundings (outer space). This, in conjunction with a continuous input of energy from the sun, makes the earth warmer.
To be more specific, that last paragraph should read “They let the earth‘s surface be “in contact” (via thermal radiation) … ” This allows the surface level to be warmer than it would be otherwise.
Ryan:
Greg house had claimed there was no “experiment” which shows a cooler object warming a hotter object. I pointed out that I own an apparatus – a microwave oven – which does exactly that by the action of a flow of energy providing electromagnetic radiation to the hotter object.
At February 8, 2013 at 7:28 am you have replied saying in total
I agree that I described an “energy conversion process”: the energy of electromagnetic radiation is converted to heat in the contents of the microwave oven and is powered by a source of energy flow (i.e. mains electricity in the microwave oven).
Similarly, the temperature rise of Willis’ hypothetical planet’s surface is an “energy conversion process”: the energy of electromagnetic radiation is converted to heat in the planet’s surface and is powered by a source of energy flow (i.e. the nuclear reactions in the planet)..
I fail to understand what you mean by it is “not a heat energy transfer in a closed system”: in each case electromagnetic energy is emitted from one object and is converted to heat in a hotter object such that the temperature of the hotter object is raised.
And neither case violates the First or Second Law of Thermodynamics.
Your statements about mixing water are irrelevant. They say nothing about how electromagnetic radiation interacts with matter.
Richard
“THAT is what GHGs do. They let the earth be “in contact” (via thermal radiation) with COLD surroundings (the atmosphere) rather than in contact with VERY COLD surroundings (outer space). This, in conjunction with a continuous input of energy from the sun, makes the earth warmer.”
No, the planet’s surface would be warm even if the planet were directly in contact with space. Furthermore the presence of an atmosphere means you have a temperature gradient developed across that atmosphere which will make the Earth’s surface even warmer as it will slow the rate of cooling – you don’t need GHGs for that. Thirdly, the top of the stratosphere is actually hotter than the Earth’s surface due to UV hitting the outer layer of the atmosphere, so the temperature gradient is actually inverted for cooling and inherently stable. Forthly, if we consider the cooling scenario separately then the Earth’s surface cannot itself get any hotter since it is effectively the source of the heating effect and the hottest part of the system – in your example you are only decreasing the temperature gradient across the system by raising the temperature of the outside of the system (i.e. you would be in effect increasing the temperature of outer space), which is NOT what GHGs do.
Go back to Willis’ shell system, but instead of a thermo-nuclear reaction consider a massive ball of water at a smidgen under 100Celsius. In the first diagram the water is cooling into space quite freely. But what about the second diagram. You are now reflecting a lot of IR back to the water, i.e. it seems to be receiving a lot more energy. Does that mean the water will now boil, or will it just stay a smidgen under 100Celsius?
@Ryan Spear: The argument we are having is whether a cooler object can make a hotter source even hotter,
Cooler than what? Cooler than the hotter source? Or cooler than the 4 deg K cosmic background?
Look at it from the point of view of the hotter object. Without the steel shell, it is staring into a 4 deg K “shell” of space. The hotter object gets wrapped in shell of 200 deg K. There will be a drop in Heat loss from the hotter object, and if the hotter object has an internal sourse of heat, its temperature will rise at equilibrium. “Come out of the cold and into my igloo.”
In Perpetuum Mobile, tjfolkerts (2/14/12 10:13am)
This exchange taught me the danger of taking an average energy flux in W/m2 and backing into a temperature. The tiny million sun example of 6000 deg K specks in a field of 4 deg K void can give the same flux as a uniform 279K field. (Thanks TJ).
[mods: please delete my 8:22 am with an unclosed Link ref. and use this one]
@Ryan Spear: The argument we are having is whether a cooler object can make a hotter source even hotter,
Cooler than what? Cooler than the hotter source? Or cooler than the 4 deg K cosmic background?
Look at it from the point of view of the hotter object. Without the steel shell, it is staring into a 4 deg K “shell” of space. The hotter object gets wrapped in shell of 200 deg K. There will be a drop in Heat loss from the hotter object, and if the hotter object has an internal sourse of heat, its temperature will rise at equilibrium. “Come out of the cold and into my igloo.”
In Perpetuum Mobile, tjfolkerts (2/14/12 10:13am)
This exchange taught me the danger of taking an average energy flux in W/m2 and backing into a temperature — there is more than one solution. The tiny million sun example of 6000 deg K specks in a field of 4 deg K void can give the same flux as a uniform 279K field. (Thanks TJ).
I suspect that no page numbers will be forthcoming to support the shell-game, because there is no support for the shell-game and its derivatives. I cannot weld steel with a propane torch. But according to the shell game, all I have to do is use 2 or 3 propane torches, adding their heat together, to do the trick. Sorry, no can do!!
tjfolkerts says:
February 8, 2013 at 7:51 am
>>>>>>>>>>>>>
An excellent explanation tj. Of course it will be refuted by some (snip) claiming an experiment in 1909 with cardboard boxes proves your explanation impossible followed by yet (snip) another shouting something about the second law being violated, and yet another (snip) complaining that he should be able to heat his house with two slabs of iron at the same temperature…
This thread reminds me of someone who sneers down their nose at the notion that the earth is flat and proceeds to prove that it isn’t by explaining how the sun orbits the earth.
If you want to improve Willis model to look like the real GHG system you should have a heat source outside the shell only, that turns on and off every 12hours and has a temperature of say 1000Celsius. Imagine the planet as a ball of water. This heat source will heat the ball of water so that when the heat source is on and there is no shell present the temp will reach 100Celsius, but when the heat source is off it will normally cool to space reaching 50Celsius.
Now re-introduce the shell during the periods when the heat source is off only. What will happen next is the ball of water inside the shell will not be able to cool either by conduction or radiation so it’s temp will remain at 100Celsius. Now remove the shell and re-apply the heat. Since the ball of water has now started at 50Celsius the temperature with the heat source on will reach 150Celsius (well, it will boil).
This is the greenhouse theory at its simplest. You notice it requires no detailed knowledge of DWIR or any other details to work – it only requires you to get the model right.
I did not say, however, that this would work on planet Earth.
jae says (February 8, 2013 at 8:40 am): “I suspect that no page numbers will be forthcoming to support the shell-game, because there is no support for the shell-game and its derivatives.”
Willis quoted chapter and verse (February 7, 2013 at 9:11 pm).
I pointed to a site that quotes several textbooks (February 7, 2013 at 7:32 pm).
Your claim is demonstrably wrong.
Bart says:
February 7, 2013 at 11:03 pm
Willis Eschenbach says:
February 7, 2013 at 9:11 pm
“The first term reflects the flow in one direction, and the second term, the flow in the opposite direction.”
In large part, this is an argument of semantics, or of mental constructs which produce a particular perspective. There are not really individual flows of energy which bounce back and forth between the planet’s surface and the inside of the shell. Electromagnetic radiation is a wave phenomenon. A some points, the fields will interfere constructively, and at others destructively. So, there will be peaks of electric field and nulls at various places along the way, as there will of the magnetic fields. At equilibrium, there will be some dc level of electromagnetic energy filling the gap, as well as fluctuations from the non-stationary interference patterns which could appear to move in either direction on a finite time scale.
Not correct there are indeed counter flowing flows of electromagnetic radiation, interference does not prevent the propagation of radiation in either direction, if it did a laser cavity wouldn’t work!
jae says:
February 8, 2013 at 8:40 am
“I suspect that no page numbers will be forthcoming to support the shell-game, because there is no support for the shell-game and its derivatives. I cannot weld steel with a propane torch. But according to the shell game, all I have to do is use 2 or 3 propane torches, adding their heat together, to do the trick. Sorry, no can do!!”
Oh, come on. You ask for specific pages of textbooks to give you an idea of where others are coming from and then once you are given them, you respond by arguing against a straw man position that has nothing to do with what you have been pointed to. I guess you can point someone to dozens of textbooks but you can’t make them understand any of them.
BTW, where does the extra heat come from that warms someone after they put on a parka when it is cold outside. Does that extra heat become any less real if you call it “backradiation”?
Cheers, 🙂
Greg House says:
February 7, 2013 at 3:08 pm
Phil. says, February 7, 2013 at 12:08 pm: “Nothing to do with ‘climate science’ it’s the basic physics of radiation heat transfer.
Arises from S-B law, light emitted by hot bb object = const.A.Th^4
light emitted by cool bb object = const.A.Tc^4
Net heat transferred between the two objects = const.A.(Th^4-Tc^4)”
==========================================================
This “net” thing does not “arise” from S-B law, and it has apparently never been proven experimentally. As long as it has not been proven experimentally, it remains a fiction. A sort of “radiation arithmetic” without any basis in science.
Just because you’re not prepared to read about something doesn’t mean it doesn’t exist. Feel free to continue in ignorance but don’t expect others to accept your nonsense. Either read some basic texts on radiation heat transfer (I suggest Hottell) and learn about the subject or shut up.
mkelly says:
February 7, 2013 at 11:43 am
q = ε σ (Th4 – Tc4) Ac
A typical radiative heat transfer equation from Engineering Tool Box. Where in this formula can the radiation from the shell cause an increase in the temperture of the globe? Again it seems to me that when Th = Tc then q is zero.
Increase Tc and the heat loss from the globe goes down, since heat input to the globe is constant the temperature will go up (Th) thus q will increase until q equals the input to the globe. If Th=Tc there will be no heat loss from the globe so there will be an increase in the globe temperature until q = input.
MiCro says:
February 7, 2013 at 1:27 pm
So, let me ask this, if a 15u wavelength photon is captured and thermalize by a molecule of Co2, what would it’s temperature be?
Its translational temperature will stay the same its rotational and vibrational temperatures will increase depending on which particular energy levels have just been populated. The excited state will now attempt to lose that excess energy by a combination of collisional deactivation and radiation. Which one is favored will depend on conditions, near the earth’s surface collisions are the major route, up near the tropopause it’s radiation.
Willis Eschenbach says:
February 7, 2013 at 9:11 pm
q = A C s T1^4 – A C s T2^4 (5.10)
Thanks for proving my point. When T1 and T2 are equal q goes to zero and heat transfer stops. So the shell cannot warm the globe.
Ryan says (February 8, 2013 at 8:16 am): “Go back to Willis’ shell system, but instead of a thermo-nuclear reaction consider a massive ball of water at a smidgen under 100Celsius. In the first diagram the water is cooling into space quite freely. But what about the second diagram. You are now reflecting a lot of IR back to the water, i.e. it seems to be receiving a lot more energy. Does that mean the water will now boil, or will it just stay a smidgen under 100Celsius?”
Neither. The water will gradually cool to the “temperature” of space, because the system as a whole (inner sphere plus shell) is still cooling to space via radiation from the shell.
Your question suggests that you don’t fully understand Willis’s example.
Ryan says:
“Thus the NET emissions from the planets surface are exactly as they were before. Thus the temperature of the planet’s surface is exactly the same – all that is happening is that the back-radiation that is reflected towards the planet from the shell is simply reflected back towards the shell again (maybe the energy is absorbed and then re-emitted again but from an energy balance point of view we don’t need to worry about those details). In effect, Willis simple energy balance model demonstrates that back radiation of IR cannot heat the surface of the planet, even when it is 100% – it simply increases the local radiative losses from the surface of the planet.”
Yes.
It seems with shell you could trap a lot low energy photons. I think one effect of doing this would
it would interfere with thermal goggles ability to see very far. And tend warm things which were fairly cool. Question is how bad could this IR pollution get and could get to point where there some mass to all this photons.
Greg House says:
February 7, 2013 at 10:01 pm
Willis Eschenbach says, February 7, 2013 at 9:11 pm: ” Show up with science in your hand, citations saying cold objects CAN’T radiate to hot objects, or don’t show up at all.”
=============================================================
It is not about whether colder objects can or can not radiate to warmer objects.
It is about whether radiation of colder objects affects temperature of warmer objects. That is the point, please, note that.
I have never seen any valid link to a real scientific experiment proving it does. Nobody I talked to on various blogs was able to present such a link. No link, no description of a real scientific experiment proving that point directly, by actual temperature measurements was presented. People did present fictional stories or general references to the same unproven claims, but nothing real. The fictional stories about plates, shells etc have been around for years, but again, nothing real has come up until now.
There’re plenty of such experiments and they have been documented ad nauseam, your refusal to read texts etc. doesn’t make them fiction it makes you a liar!
One I have described here involves the use of a thermocouple. If you immerse a thermocouple in flame gases it will measure a temperature which is lower than the adiabatic flame temperature because it is losing heat via radiation to its surroundings. If you mount a radiation shield around the ThC which still allows conduction and convection between the flame and ThC. The radiation shield gets hotter than the surroundings and so heats the ThC up and a higher temperature is recorded than in the absence of the shield, double shields increase it further. Well researched by NACA back in the 50’s for example. Below is a link with some examples of heat transfer problems from a text book, 12-89 shows the calculations for such a shield. Now shut up!
http://tinyurl.com/asffsbx
Genius; All horses are purple.
Horse Breeder; Uh…. no they aren’t.
Genius; Yes they are. I read it in a book from 1906.
Horse Breeder; Uhm… I’m a horse breeder, I’ve studied horses my whole life, and they aren’t purple.
Genius; The book from 1906 proves they are.
Horse Breeder; Look, here are 16 text books on horses, everything from the various breeds and strains to special cases like zebra and even half breed strains like mules, and not one of them mentions purple horses.
Genius; Those were all proven wrong by the book from 1906 that I read.
Horse Breeder; Uhm….tell you what. I have a horse in my trailer. Let’s go have a look.
Genius: No, I don’t want to look.
Horse Breeder; Why not? You said all horses are purple, I have a horse right here that you can look at and verify that it isn’t purple….
Genius; You’ve just proven my point.
Horse Breeder; Uhm…. and you figure that…how?
Genius; You said it isn’t purple. So it isn’t a horse. How stupid do you think I am?
Horse Breeder; Well, let’s just say I have to revise my initial opinion….
mkelly says:
February 8, 2013 at 11:04 am
Willis Eschenbach says:
February 7, 2013 at 9:11 pm
q = A C s T1^4 – A C s T2^4 (5.10)
Thanks for proving my point. When T1 and T2 are equal q goes to zero and heat transfer stops. So the shell cannot warm the globe.
You really don’t understand the physics at all! When q equals zero the globe will heat up like crazy and q will no longer be zero, q is the rate of heat transfer from globe to shell, when the globe is being heated as in Willis’s example Th will only equal Tc when Th is 0K.
mkelly says: ” Thanks for proving my point. When T1 and T2 are equal q goes to zero and heat transfer stops. So the shell cannot warm the globe.”
Let’s think about this for a moment. The inner sphere was at T1 = 254 K with a 235 W/m^2 heater when there was no shell around it. Now lets add that shell at T2. If T2 = T1 = 254 K, then there would indeed be no heat going from the shell to the surface, nor from the surface to the shell — heat transfer between the two stops just as you claim. BUT THERE IS STILL 235 W of heat (from the heater inside the sphere) going to sphere. Since the sphere is losing no energy to the shell, but gaining 235 W/m^2, it will necessarily warm up. It will warm up until it is radiating 2×235 W/m^2 @ 302 K.
Apropos to many internet discussions is the “Dunning–Kruger Effect”.
Shawnhet says:
February 8, 2013 at 10:21 am
“Oh, come on. You ask for specific pages of textbooks to give you an idea of where others are coming from and then once you are given them, you respond by arguing against a straw man position that has nothing to do with what you have been pointed to. I guess you can point someone to dozens of textbooks but you can’t make them understand any of them.
BTW, where does the extra heat come from that warms someone after they put on a parka when it is cold outside. Does that extra heat become any less real if you call it “backradiation”?
Shawnhet: I am NOT arguing that cooler objects can contribute energy to warmer ones. I’m arguing with the shell diagram. The “pages” w provided do nothing to support the shell diagram.
And your parka works primarily by INSULATING you. There is no “extra heat” there, and I doubt that the backradiation has much effect.
Phil. says:
February 8, 2013 at 10:56 am
Thanks, if the photon is thermalized, will the molecule’s kinetic temperature increase proportional to the energy of the photon?
Now, note what happens when we add a shell around the planet. The shell warms up and it begins to radiate as well … but it radiates the same amount inwards and outwards. The inwards radiation warms the surface of the planet, until it is radiating at 470 W/m2.
Two points:
1:
How does the shell acquire more W/m2 than is being generated by the planet?
At T=0, every thing is at 0K, then the reactor is turned on.
The planet warms up and eventually emits 235 W/m2, the shell follows this warming due to it eventually receiving 235 W/m2.
Then you say this shell radiates twice the power :- 235 W/m2 down and up!
How can the shell now deliver twice the power than that which it receives?
Valid equations can show you doubling the power but I would suggest that in this case it is the misapplication of valid equations is sending people down the wrong path.
2:
In the two bodies proposed experiment, it is suggested that a cold body can increase the temperature of a nearby hot body.
This is true if the colder body is treated as an insulator.
We can all agree that when fed with power, the hot body will continue to radiate and it will reach an equilibrium temperature with its heat sink.
If an insulator/reflector/path disruptor is placed between the hot plate and it heat sink, then the hot plate will increase in temperature until it can dissipate the same amount of energy as before via a more difficult path (via the insulator/cold body).
Its just insulation.
I would modify the two body experiment:
Hot plate as before (but made of aluminium), the cold plate was a sheet of super thin aluminium.
Cool the cool plate, insert into experiment,
I suggest that the radiation from/to the cold plate will have no effect except to get the cold plate up to the hot plate temperature, and have no measurable effect on the hot plate temperature.
Why?
Because the thin cold plate will have negligible insulation effect on the hot plate, so causing no temperature rise in the hot plate.
When I have run this experiment, I will let you know.
Steve Richards
“Willis Eschenbach says:
February 6, 2013 at 2:33 pm
Michael Moon says:
February 6, 2013 at 1:11 pm
Silver Ralph,
You just flunked your first hourly in Thermo. The cooler radiator would be warmed by the warmer radiator, and begin radiating more. If you think this would warm the warmer radiator, then you will fail all your hourlies and never get through school.
Wilis, Joe Public has it exactly right. If you want to know what happens to the flux from a cooler source when it hits a warmer source, the answer is exactly nothing. It is not absorbed, but immediately re-emitted, transferring NO heat.
All these analogies are amusing but ignore Second Law.
So you agree that it is re-emitted. To be re-emitted, it had to be absorbed. You just say it happens really fast.
Actually, once energy is absorbed, there’s no way to distinguish it from any other energy, so there’s no way to tell when that particular energy was re-emitted.”
I think point is are heating something?
Heating something with light requires transformation of energy.
So with solids and liquids they have a molecular structure- unlike gases.
Heat is vibrations of the molecular structures. Whereas heat for gas is the kinetic
motion of gas molecule. Gas molecules can freely move, the molecules of
liquids and solids are bound by their molecular structure.
So the conversion of electromagnetic energy into heat is the electromagnetic
energy increasing the vibrational energy of liquids and solids.
And this vibrational energy of liquids or solids is conducted to entire molecular
structure [thereby being heated]. This conduction of vibration not is not immediate
or at speed of light.
Putting cool frying pan on heating element does not convey the heat of element to
top surface of frying pan immediately- it takes a moment to heat up.
Better the conductor the faster this occurs.
Photon re-emitted can lack the time to heat something and also could not add to vibrational
energy of a solid or liquid- not convert the electromagnetic energy into heat.
Say you some surface which is 15 C. And heat it up by say 5 C, so it’s 20 C. The 20 C surface will emit more of wavelengths and more higher wavelength as compared to 15 C. The higher wavelength are a characteristic of the higher temperature, whereas more of lower wavelengths is not a characteristics of higher temperature- different material may emit more of certain wavelength as compared to other material with same temperature.
Or to heat up a gas, one needs higher vibration of the molecular structure- and less molecular vibration will cool the gas [a lot more of lower vibration state will not make the gas warmer].
jae says:
February 8, 2013 at 8:40 am
Since I gave citations to three different references above, including page numbers, I have no clue what you are complaining about.
In that comment, I also said, and I say again:
As a result, you showing up to tell me what you “suspect” is useless. Come back with references or I’m not interested in the slightest.
w.
Ryan says:
February 8, 2013 at 2:38 am
Thanks, Ryan. I’ve cut off your post above at the site where you made your mistake in logic.
The surface temperature is related, not to the NET radiation, but to the total radiation. How could it be otherwise, since the surface has no idea where the radiation is coming from or going to. So if a surface is radiating at e.g. 470 W/m2 the surface can’t say “well, half of these are from the shell, so they don’t count.”
470 W/m2 is 470 W/m2, and the temperature of the surface is related to only that, not the net radiation.
Hope this clarifies things,
w.
Will says:
February 8, 2013 at 2:49 am
A colder sky cannot possibly heat a warmer surface, any more than a trillion 1 ton blocks of frozen CO2 radiating at 194.56 K could melt a 1 inch water ice cube radiating at 273.15 K.
Thanks, Will. Consider this question. Which one will result in a warmer earth, either looking up from the surface to see:
1. An sky which is radiating something like 300 W/m2 back to the surface from the presence of GHGs, or
2. Outer space, which is radiating … well … nothing back to the surface.
When we replace the cold of outer space with the warmth of a GHG-containing atmosphere, the planetary temperature rises. In common parlance this is described as “the atmosphere warming the planet”. While this is not literally correct, it is the usual usage … but if you prefer, you can think of it as being equivalent to “the earth is warmer because of the presence of a cold atmosphere than it would be without it”. They mean the same thing.
w.
Tjfolkerts,
Thanks for taking the time to provide the shell energy budget. I can see where I went wrong. At the lowest level I had missed one of the back flows.
Regards, vc
Steve Richards says:
February 8, 2013 at 12:28 pm
Steve, it doesn’t. As the shell warms up it radiates back to the planet. As stated in the article,
So the shell receives 470 W/m2. I know this is difficult to grasp if you haven’t come across it before. But take a cold shower then check it out, this is what happens and everything is in balance.
I cannot express it clearer than explained by Willis:
No energy is being created and no energy is been destroyed.
Steve Richards says :
“At T=0, every thing is at 0K, then the reactor is turned on.
OK
“The planet warms up and eventually emits 235 W/m2, the shell follows this warming due to it eventually receiving 235 W/m2.”
Good so far. The sphere has warmed to 254 K.
“Then you say this shell radiates twice the power :- 235 W/m2 down and up!”
No, that is not what we are saying. The shell has TWICE the area of the sphere — it has an inside and an outside surface. So the shell radiates 235/2 W/m^2 out to space and 235/2 W/m^2 down to the surface = the power it receives. The shell is much cooler than the sphere: 213 K.
The presence of the “warm” shell (I call it “warm” because it is much warmer that outer space) adds 235/2 W/m^2 in addition to the 235 W/m^2 from the heater. This means the sphere is receiving 1.5 x 235 W/m^2 = 352 W/m^2.
So the sphere will warm up until it radiates 352 W/m^2 (from 254 K to 281 K). The shell will be radiating 352/2 W/m2 up and 352/2 down (warming from 213 K to 236 K).
But now the sphere is receiving 235 + 352/2 = 411 W/m^2, so it will warm up until it radiates 411 W/m^2 (warming from 281 K to 292 K) … but then the shell … but then the sphere …
We have an infinite series where the temperature of the sphere approaches 302 K ( 2X235 W/m^2) and the shell approaches 254 K (235 W/m^2). Energy is conserved at every location at every instant. Heat always flows from warmer to cooler.
Willis,
The problem of the steel shell is the steel and its 2 surfaces. Surface(or Side) A faces the planet, Surface (or Side) B faces cold outer space. You say at steady state both surfaces radiate 235 W/m^2.
Imagine the shell is perfectly transparent glass, then A radiates 0 and B radiates 235 that was passed through from A.
Imagine the shell is perfectly reflective silver, than A radiates 235 and B radiates 0.
In both cases, the shell is at 0 Kelvin because it can absorb no energy as a function of the sphere material.
But steel is a real material, so it reflects enery off sides A and B, transmits energy from side A to B, and over time absorbs energy and heats up. Also there is a non-zero distance from A to B and so a temperature gradient will develop between the surfaces.
The temperature of Side A will asymptotically approach the temperature of the planet. The temperature of Side B will asymptotically approach the temperature of outer space. Therefore, the sphere will radiate energy at a rate that will keep it about 1/2 the temperature of the surface of the planet. The exact temperature of the steel sphere is indeterminate from the information presented in the diagram since it partly depends on the reflective properties of the Side A and transmittal efficiency of Side B. At steady state, the radiation of Surface A + Surface B = 235 W/m^2 to satisfy the conservation of energy.
The other problem of the model is the surface of the planet at steady state has 2 temperatures corrsponding to 235 W/m^2 and 470 W/m^2. So, does the planet reflect all energy that it receives from the steel sphere or does it absorb it and then regulate its atomic reactions in such a way as to keep its surface temperature constant? Again, the model is indeterminate.
It does seem the planet surface will be warmer with the sphere but impossible to say by how much.
(A third material to consider is black hole stuff. Side A radiates 0 and Side B radiates 0 and the temperature of the sphere increases until the planet’s reactor is exhausted. So now we have a very hot black body radiating nothing.)
The problem with Willis’ steel BB is he’s never clear what it is he’s trying to relate. He has done this every time I’ve seen the BB posited.
It seems he takes a well known fact: that radiative conduction through solids is slower than free space
and tries to claim this, is the Greenhouse Gas Effect pertinent to infrared-resonant gas.
Yet he mistakenly – yet correctly – refers to the principles he discusses relevant to the BB as reflection on the “Greenhouse Effect” – which IS a real thing, it’s the “Atmospheric Greenhouse Effect.”
So by the time he’s discussed Woods’ work – the seminal expose on Arrhenius’ proven-wrong hypothesis that was tweaked by other failures until it’s claimed that solely infrared-resonant gases lend any additional temperature to the atmosphere at all – preposterous on it’s face yet touted far and wide among eco-wackos –
by the time he even invokes’ Woods’ work alone, he’s called one thing: Greenhouse Gas Effect –
another thing, the Atmospheric Greenhouse Effect.
This alone makes the article impossible to decipher and lends the idea Willis doesn’t know the difference. It wouldn’t be aggravating but I’ve seen him refer to these two utterly separate and distinct concepts by the same name, many times.
I’ve given him years as he’s dragged out the BB and that’s not ever been clear.
So that I’M clear, Willis
(1) refers to the Greenhouse Gas Effect, which Woods was checking, as the Greenhouse Effect, which Woods was NOT checking.
(2)refers to the model: which he then proceeds to describe under false tenets he ascribes very clearly as though he believes he’s talking about the Atmospheric Greenhouse Effect: come to think of it he later SAYS, “you don’t have to have CO2” – so I see now.
Willis thinks Woods was checking the Atmospheric Greenhouse Effect.
Willis also thinks the Atmospheric Greenhouse Effect is the Greenhouse Gas Effect.
Ok, so that’s now clearer.
—————
On to the next problem.
In Willis’ description of the BB he ascribes, arbitrarily, a 50-50 guaranteed absorption rate for radiation at the inner surface of his imaginary sphere. WHaT???
In Willis’ description of the BB he ascribes to the BB that it is steel: which has propagation delays due to interstitial resonance geometry irregularities, everybody knows why energy radiates through solids, slower.
In Willis’ description of the BB he then goes on to describe the steel BB as being a perfect black body object, with dramatically lower propagation speeds through it’s interstices.
So : there are at least three different descriptions of the functional radiative transfer qualities of the sphere:
(a) it’s steel
(b) it’s a blackbody
(c) it’s reflectance is 50% no matter what temperature it’s at, no matter what temperature the light coming in is at, and no matter what temperature the field on the outside is at, in spite of the fact it’s a blackbody which is a perfect absorber of infrared.
That’s PURE, SIMPLE, gobble-dee-guke
Willis then goes on to try to amateur-freestyle discuss energy migration,
to, eventually,
lay claim to the STUNNING reality he and apparently david hoffer believe fervently:
“The mathematics I do as an electronic engineer, regarding equilibria in everything I do in my job – which is calculating and checking the radiant transfer of electromagnetic energy through the atmosphere, through vacuum of space, and through industrial compounds needed to make that happen –
are LYING. There has been ALL ALONG, a TWO-WAY FLOW that neither mathematics, nor empirical demonstration ever showed before,”
and that only the people who believe in – whatever it is, they believe in – are smart enough to know.
That entropic migration during charge equalization
is actually not traveling at the speed of math.
It’s traveling at the speed of math one way, and too fast for anybody to have ever calculated,
the other way.
===============
That
is not science.
Not the part where Willis misunderstood what Woods checked: the question whether infrared-resonant gases are primary heat holders in atmospheric thermal cycling –
not the part where Willis described his Magic BB as a steel absorber,
then a 50/50 arbitrarily-always half & half absorber, whatever that is,
then a perfect blackbody absorber
which must by definition absorb all, conduct through and radiate perfectly out the other side, at the speed with which it would move through a vacuum adding only the additional time to make the change in direction which would allow the sphere to have that geometry –
that being said, what kind of amateur,
claims to have a perfect absorber & radiator, then talks about it heating up, because it can’t radiate?
Then claims others’ arguments don’t persuade ?
One who hasn’t ever had to pay the dues, to get a formalized education in energy transfer through radiative emissions law, guessing like a blind man in a crowd where his credibility went.
It wasn’t science, when Willis tried to tell me that when I became an electronic engineer and calculated radiative transfer as per entropy hundreds of times for a grade,
the math I learned was only part of the story.
No Willis the math you learned from those “introductory” textbooks you and david hoffer love so much,
was only part of the story.
The full story is that charge density dissipation travels one way because where there is no charge, there is nothing to counter an existing one. Therefore there really is a difference between five energy quanta, and three.
And when I have 5 in one object, and 3 in another, and calculate that 5 charges, giving up one charge, makes those two objects equal in energy contained,
I didn’t actually have a whole bunch of little unmeasurable-hence unwritable charges, secretly flying between the two, while I calculated the 1, migrating over to the three.
Now Willis you can pretend you and david hoffer didn’t spend an afternoon telling me that when I have
5 joules of energy in one globe, and 3 in another,
and that while 1 joule was migrating over to establish equality,
that 5 joules and 3 joules were furiously, but undetectably, swapping sides, between bars,
until just when that magically NOT furiously swapping sides 1, migrated OVER
but it’s not going to stop me pointing out that you tried to claim you think that happened.
None of that crap is science,
none of it is radiative transfer.
It’s gobble-dee-guke.
That starts out wrong and then goes to the point of telling me I don’t know how heat dissipates from an entity, and that while one value migrates, magically indiscernible and incalculable anti-quanta are hopping sides in my equations and in all objects in the universe.
jae:” I am NOT arguing that cooler objects can contribute energy to warmer ones. I’m arguing with the shell diagram. The “pages” w provided do nothing to support the shell diagram.
And your parka works primarily by INSULATING you. There is no “extra heat” there, and I doubt that the backradiation has much effect.”
Respectfully, jae, what Willis posted was exactly on point when it discussed how radiation always flows both ways between hot and cold bodies. His shell argument has nothing to do with having multiple low temperature torches heating something to higher than any one of them could get. Until you understand that, no amount of references will make sense to you.
The point about insulation and backradiation is that it is perfectly fine to think of GH gases as insulating (at least IMO) the Earth in pretty much the same way as your parka insulates your body in winter.
http://scienceofdoom.com/2012/07/23/how-the-greenhouse-effect-works-a-guest-post-and-discussion/
If, in fact, it is perfectly consistent to view GH gases as insulating the Earth, and you agree that insulation doesn’t produce extra heat, what is there left to disagree about at the end of the day?
Cheers, 🙂
I’m not here to be the most friendly nor most artistic skeptic. I’m here to be the sole person here, most likely, who’s calculated rate-of-dissipation of heat from one body to another THOUSANDS of times in my career.
I had to calculate radiant dissipation of energy through solids, and vacuum, and the atmosphere, by the end of 3-1/2 years’ combined school, on HUNDREDS of questions on which I was GRADED: give the right answer or FAIL.
These men on this forum, – Willis, david hoffer, all the usual
“entropy calculations don’t tell the truth, charge is actually flowing backwards while all ‘odd’ charges are migrating to equalization, forward”
crowd –
aren’t going to tell me there’s no mandate in charge based energy dynamics against unaided, reverse flow.
They’re just not.
No matter how many times they claim they have an “introductory text” that they think says undriven charge flow occurs.
My goodness… I forgot the /sarc at the end of that last comment. Thank you davidmhoffer for all of those kind words queueing me.
Allen B. Eltor:
I am writing this as a sincere attempt to be helpful.
I have read your long post at February 8, 2013 at 2:28 pm three times, and I still fail to understand what you are trying to say. I suspect that if I don’t ‘get it’ then others also won’t.
Perhaps you are trying to say several things at once and they are getting jumbled up?
I commend you to make a shorter post which attempts to make a single point in as clear and concise a manner as you can. You can make subsequent posts when that issue has been addressed.
I hope this helps.
Richard
A Willis Eschenbach starship.
Materials needed one asteroid- 1, 2, 5 or 20 Km diameter space rock.
Lots of steel sheeting.
Magnets. Lots of permanent magnets.
First make ceiling above asteroid.
Support ceiling with pillar which will needed to support the ceiling until
such time as it’s pressurized. After it’s pressured the pillars could be used
to hold the ceiling down. Make ceiling height: 30′.
The ceiling can made of steel and the thickness is related to how much you
want to pressurize the atmosphere. And it can be say less than 1/2″ thick
if you using enough pillars.
[One should keep in mind it’s very low gravity environment, less 1/100th of gee,
so making 1 ton car weigh less than 20 lb]
Above this ceiling we going use Willis Eschenbach’s insulation.
So 10 layers of very thin steel separated with a vacuum and supported
above the ceiling pillars with magnets {South South] which suspend
the weight of these spheres. Thus insuring there is no heat loss from
conduction of heat.
The question is once one has the ceiling pumped up with air and have 10 stacked
steel shells constructed, how much heating of 30′ room/atmosphere do we need?
And is there a certain temperature which dangerous- leading to dangerous
greenhouse effect?
Say we have floor heating which has a well insulated substrata, then 4″ concrete
with embedded thermal piping. With thermostat, which set to say 78 F [ 25.5 C 298.6 K]
which warmed by heated water.
So we know [and can adjust] temperature of the floor, the question what temperature of the ceiling and how much heating is needed.
We have low gravity so it seems ceiling fans which could provide well mixed air would
be good idea and it means air temperature at the ceiling will near temperature of the floor.
Now, a question arises by some [let’s call them deniers] of whether the ceiling
should have the conventional type insulation [say 6″ of fiberglass on the air
side of sphere ceiling] and others have faith it would inhibit Willis Eschenbach’s insulation,
thereby result in a increase of heating costs.
Which is the truth?
Willis: You must have missed a couple of my comments. I’m not challenging the citations you provided.
People are just not getting this are they? On one of the links I gave earlier ‘Science of Doom’ gives a possible explanation as to why.
MostCasualObserver says:
OK….but be aware that in this case the planet’s temperature will approach infinity because there is no heat loss.
Why? In fact it won’t; it will be at 253K and the surface of the planet will be much hotter (see later)
No it won’t; the outer surface of the shell will also be 253K (assuming it is a very thin conductive shell. For thick shells see http://scienceofdoom.com/2010/07/26/do-trenberth-and-kiehl-understand-the-first-law-of-thermodynamics/ )
Well, you are right about the 235 W/m2, but because you know this, the temperature of the sphere is not indeterminate. In fact you can work out precisely what it will be from the Stefan-Boltzmann Law. A blackbody which emits at 235 Watt/m2 must be at a temperature of 253K (we are told the shell is a blackbody). So the shell is at 253K.
We are told the planet is a blackbody so it doesn’t reflect anything. It absorbs everything.
Quote from article,
No, again from the Stefan-Boltzmann Law you can work out precisely how much. I leave that to you.
“Thanks, Will. Consider this question. Which one will result in a warmer earth, either looking up from the surface to see:
1. An sky which is radiating something like 300 W/m2 back to the surface from the presence of GHGs, or
2. Outer space, which is radiating … well … nothing back to the surface.”
Willis, consider this question. Why do you flip from one frame of reference to another half way through your question?
Earth as a whole v’s a localised phenomena. Surely you don’t think that the Earth as a whole is warmed by heat that is leaving the Earth via cloud formation do you Willis?
Surely even you can understand that this is merely localised warming resulting from global cooling?
Why do you have this tendency to double count?
Willis,
the flaw in your “shell game” is rather obvious. The shell never reaches the same temperature as the planet for the same reason that the surface of the sun is only 5,700º C while the core is 15,000,000º C.
The temperature sink of Space is the only true non-variable.
mkelly says:
February 8, 2013 at 11:04 am (Edit)
mkelly, I suggest you plot the temperatures starting with the surface at 235 W/m2 and the shell at 0 W/m2
At t1, the shell starts to warm. It gets to where it is radiating say 10 W/m2. That radiation goes both outwards and inward, so the surface temperature of the planet immediately jumps to the equivalent of 245 W/m2.
Ok, so by t2 the additional heat from the planet brings up the shell to radiate 20 W/m2 … and the surface goes to 255 W/m2.
I’m sure you can see the problem … the shell and the surface are never at the same temperature. They can’t be. Even if they were the same temperature when they were created, the surface would immediately start to warm because of the additional radiation coming back from the shell.
All the best,
w.
Alan B Eltor;
These men on this forum, – Willis, david hoffer, all the usual
“entropy calculations don’t tell the truth, charge is actually flowing backwards while all ‘odd’ charges are migrating to equalization, forward”
crowd –
>>>>>>>>>>>>>>
Like richardscourtney I really can’t figure out what the heck you are talking about. As for suggesting that entropy calculations don’t tell the truth, I challenge you to produce a quote by me that makes any such suggestion. Similarly, I said nothing about “charge”, nothing about which way charge flows let alone some being odd or not. You want to stick my name in a rant, by all means, but quote the specific words you are referring to, and explain what the terminology is that you are using.
jae says:
February 8, 2013 at 3:14 pm
Willis: You must have missed a couple of my comments. I’m not challenging the citations you provided.
>>>>>>>>>>>>>>>
You griped that neither Willis nor I had provided you the citations you demanded, Willis provided exactly what you asked for, and yet you still gripe about not getting the citations you demanded… but you aren’t challenging the ones Willis gave you. Are horses purple in your world?
richardscourtney says:
February 8, 2013 at 2:58 pm
Allen B. Eltor:
I am writing this as a sincere attempt to be helpful.
I have read your long post at February 8, 2013 at 2:28 pm three times, and I still fail to understand what you are trying to say. I suspect that if I don’t ‘get it’ then others also won’t.
Perhaps you are trying to say several things at once and they are getting jumbled up?
I commend you to make a shorter post which attempts to make a single point in as clear and concise a manner as you can. You can make subsequent posts when that issue has been addressed.
I hope this helps.
Richard
<<<<<Low energy distribution, aren’t telling the full story;
and that during the drift from unequal to equal energy density, there’s an entire undiscussed exchange of energy therefore energy charge, from the lower field, into the higher density one:
and that because mathematics can’t be found, describing this simultaneous and incalculable,
temporary reversal
of charge density distribution laws and mechanics means nothing to him. It can’t be proven it’s not true, so it’s not true, that energy MUST, ALWAYS, go from High->Low distribution when left to the devices of charge mechanics.”
That’s not science. That’s a mind adrift amid an ocean of radiative mechanical potentials, without the anchor of formal instruction on why energy can’t infuse light of a lower flux density, into interstice resonances that are already charged to a higher density.
It’s amateur hour on steroids and I can prove it.
Have him explain to you step by step about the magical sharing light bulbs,
that take each other’s light in, apply it to their own temperature, then give off a little of their own to the other bulb, each applying the others’ energy to raise their own temperature.
Try breaking the news to Willis and david hoffer and all these reverse-entropic, against-charge flow zombies that all the atomic interstices which could accept any energy, are already filled to higher energy density – charge – numbers.
===============================
You can’t charge a container with an average pressure of X pounds per square inch, then put on a hose, and insert air at LESS pressure.
================================
All entropic flow must obey classical pressure-differential calculations
in absolute algebraic terms. Period.
Without exception.
Or my electronic mathematics can’t predict and track it.
===============================
And mathematics can predict and track it,
as proven by the electronic radiation-age
Willis and david hoffer and all the other reverse-flow, against-charge, physics amateurs here
watch me, and the other radiative-energy management professionals around the world
make work.
Huffer:
Willis is responding as he should. With facts, reason, and integrity. YOU, on the other hand, have not added ANYTHING to this conversation, except insults. Fella, I’m beginning to think that that is because you CAN’T offer something of substance! Facts, not polemics, run science. Buck up!
REPLY: ya know, you aren’t blameless in this either with your own rhetoric. And if you want to get anywhere I suggest you stop insulting the man as “huffer” where his name is
HoffmanHoffer. I’m growing quite tired of it all.Take a 48 hour time out this time. There won’t be a third. – Anthony
I repeat my post from above, since Willis (and some others) seem to have missed it (or are ignoring it). I expect a response that is direct and not some type of strawman/sidestep/subject change. For once.
jae says:
February 8, 2013 at 8:40 am
“I suspect that no page numbers will be forthcoming to support the shell-game, because there is no support for the shell-game and its derivatives. I cannot weld steel with a propane torch. But according to the shell game, all I have to do is use 2 or 3 propane torches, adding their heat together, to do the trick. Sorry, no can do!!”:
I think it might help if people realized that it doesn’t matter how the shell is heated. That is, assume the sphere does not radiate energy outward. Assume it radiates at specific frequencies and is covered in a special reflective material that prevents outward radiation while allowing down-welling radiation to pass through. Now, also assume the sphere is connected to the shell by a conductor so all the energy has new route to get to the shell. This prevents the shell from overheating.
The bottom line is the equilibrium situation is exactly the same. The shell will still end up radiating 470 w/m2, half in each direction. Notice that the surface of the sphere now appears to the shell to be 0K as no radiation is being emitted. Hence, no seeming violation of the 2nd law while the surface warms just as it did in the case with outward radiation.
Now, what happens if we also coat the inside of the shell with our special reflective material? Since no radiation can be directed to the surface it must all go to space. The equilibrium will now be 235 w/m2 with no additional heating of the surface. Imagine that, it is the downward radiation that is heating the surface.
My cut and paste with WordPress always sucks as I’m putting posts together.
Hey, Anthony:
It is your blog, and what you do is OK by me. BUT, I want to say that you are not being fair at all here; you seem to be ignoring all the insults from Hoffer and concentrating on my replies only. For the record, you are being unfair. Twice, now!
Snip, if you wish, but you should still get the message, sir
AND, your “punishment” of 48 hours certainly does not achieve anything that I can see… I don’t give a “—-” about your punishment. Truth will prevail, censorship be damned. MORON.
Allan B Eltor;
You can’t charge a container with an average pressure of X pounds per square inch, then put on a hose, and insert air at LESS pressure.
>>>>>>>>>>>>>
I understand what you are trying to get at, but the analogy is not apt. Radiant energy is transmitted as photons, which have no mass, and are neither a wave nor a particle, but share the characteristics of both. If you’ve ever done a ripple tank experiment, one of the things you might have observed is that waves travelling in opposite directions can and do pass right through each other. At the point of intersection, you do indeed get some interesting effects such as areas of complete calm, and standing waves. But then the ripples appear again on the other side, seemingly unaffected by each other (of course there are some effects, but minor by comparison) seemingly undisturbed. You can simulate this quite easily by throwing two pebbles into a pond at the same time and watching what happens to the ripples as they intersect than then continue on past each other. My point is that radiated energy shares some of these wave type behaviours, which is part of why radiated energy from two surfaces don’t interact as they pass through each other either.
“REPLY: ya know, you aren’t blameless in this either with your own rhetoric. And if you want to get anywhere I suggest you stop insulting the man as “huffer” where his name is Hoffman. I’m growing quite tired of it all.
Take a 48 hour time out this time. There won’t be a third. – Anthony”
LOL. Heil Hitler!
[Reply: Labeling Anthony a “MORON” and equating him to Hitler is immature. Anthony has put up with mmore from you than I would have. I doubt there is anyone here who has any sympathy for your juvenile behavior. And you are wasting your time trying to comment for the next two days. I will simply delete anything you try to post. — mod.]
REPLY to “JAE”
Since you’ve just called me a moron and equated me to Hitler, let’s just dispense with the 48 hours and move straight to a permanent ban.
I have no use for people that hide behind acronyms and hurl insults. Be as upset as you wish, but all further comments from you will be deleted.
Anthony
Allen B. Eltor
Allen, that statement you made, right there on pressure, lights up a concept that dates way back to college taking astronomy but some clarity on that topic of radiative pressure and radiation density has faded. I’ve looked for it on the web but to no avail.
Are you saying in so many words that radiation density has a pressure and through quantum wave interaction there is even a reflection component of radiation on radiation depending on that radiation density at any point in space? That is the gist of that faint thought that I seem to have learned long ago and let it fade. I believe it was in astronomy.
Could you expound on that a bit. Some here are following your statements closely.
Could this touch a bit on diametrically opposed anti-polarity quantum waves of like frequency and cancellation of effect?
Anthony;
REPLY: ya know, you aren’t blameless in this either with your own rhetoric. And if you want to get anywhere I suggest you stop insulting the man as “huffer” where his name is Hoffman.
>>>>>>>>>>>>>>
Ouch ouch ouch. First Willis calls me out for failing to provide cites and now our host gets my name wrong. My feelings are hurt. Wait… I forgot… I’m a salesman. We don’t have feelings.
I’ll try and tone it down Anthony. My frustration with the repetitive claims over the course of several threads is showing. Apologies.
REPLY: I’m sorry Mr. Hoffer. I work with somebody named Hoffman, so it is an innocent slip. I’m getting really tired of all the sniping, as it is a waste of everybody’s time, especially me and the moderators, who have to decide whether to pass it or not. – Anthony
jae;
I cannot weld steel with a propane torch. But according to the shell game, all I have to do is use 2 or 3 propane torches, adding their heat together, to do the trick. Sorry, no can do!!
>>>>>>>>>>>.
Correct, you can’t do that. But there’s no shell game to be had in this example. Flames from propane torches are burning gasses. Stefan-Boltzmann Law doesn’t apply easily to gasses. Photons emitted from one flame may well pass right through the other flame because the molecules are already in an excited state and unable to absorb another photon. Physical surfaces don’t operate like that. This is part of the confusion regarding the ghe of the atmosphere. As seen from space, the “temperature” of the earth is about -18 degrees based on the radiated energy and SB Law. But when measuring that radiated energy, all we can do is measure the total emitted. Some may have come from the top of the atmosphere, and some from the bottom, and some from anywhere in between. We don’t actually know where any given photon came from. So what are we measuring the temperature of? The system as a whole, not any given point in the system. There’s just no surface to measure!
So, for solid matter, such as the model Willis has used, SB Law is a relatively easy calculation. For two surfaces radiating toward each other, it is still an easy calculation. For two gasses from propane torches radiating toward each other, it is a devil of a calculation and I will freely admit I don’t know how. My point however is that not having surfaces, the “shell game” as you call it doesn’t apply in the first place because there is no “shell”.
However, if we change the analogy a bit, try and melt iron with coal. Pretty tough to do. Put the coal in a blast furnace though, and no problem. Yes, part of the heat from the blast furnace is due to the air being driven through the burning coal, but if you take away the walls of the blast furnace, the blast alone isn’t enough to melt the iron. Put the “shell” on, and presto, melted iron.
Willis Eschenbach says:
February 6, 2013 at 2:33 pm
If I light a candle on the earth during the day, the sun ends up warmer than it would be if I didn’t light the candle. Of course the reverse is true as well, the candle ends up warmer than if there were no sun. Since NET heat flow is from the sun to the candle, no thermodynamic laws are broken … but that doesn’t mean that the light from the candle is not absorbed by the sun. It is definitely absorbed, and the sun ends up warmer because of that radiation.
Please tell me you are not serious. Wrong on so many levels. But first let’s perform a simple experiment. Take an ice cube out of the freezer with a pair of tongs to hold it. Place your hand above the ice cube. Feel any cold radiating from it? No. Does the ice cube “warm” up your hand? Why then would a candle warm up the sun?
Your candle/sun example violates the Second Law; the general Clausius statement of it being: “No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature. EM radiation can travel from a colder body to a warmer one, but it will not be converted to thermal energy, as it must obey the Second Law. This idea of “net” heat flow is not supported. There are two distinct radiation beams; the one from the candle, and the one from the sun, and the Second Law must apply to each.
Here is a u-tube video of the kind ripple tank experiment I was talking about previously. The physicist even explains how the ripples in the water simulate certain aspects of light. As one can easily see, the ripples pass through each other while travelling in opposite directions.
EM radiation can travel from a colder body to a warmer one, but it will not be converted to thermal energy, as it must obey the Second Law.
>>>>>>>>>>>>>>>
By violating the First Law?
http://en.wikipedia.org/wiki/First_law_of_thermodynamics
http://en.wikipedia.org/wiki/Conservation_of_energy
David,
I am not going to read those references and attempt to guess your point. Please give me an explanation in your own words.
I am assuming you think the ice cube will warm my hand, and the candle will warm the sun? Yes, no?
ThePhysicsGuy says, February 8, 2013 at 8:33 pm: “David,
I am assuming you think the ice cube will warm my hand, and the candle will warm the sun? Yes, no?”
===========================================================
I am really not an expert on “mutual warming theory”, but there is an interesting implication. If the temperature of the Sun was constant, let us say due to the internal solar processes, then the Willis candle has warmed the Sun. Well, the warmer Sun in turn warms additionally not just the candle, but also the surface of the Earth. Now, the warmer surface of the Earth warms additionally the Sun. The Sun is pleased with the Earth warming it and warms the Earth further. The Earth is getting warmer and warmer this way, so is the Sun and we have global warming on both the Earth and the Sun.
ThePhysicsGuy says:
February 8, 2013 at 8:33 pm
David,
I am not going to read those references and attempt to guess your point. Please give me an explanation in your own words.
>>>>>>>>>>>>>>>>
Your contention that EM radiation can travel from a colder body to a warmer one, but it will not be converted to thermal energy would violate the First Law of Thermodynamics and the Law of Conservation of Energy. I’ve provided wikipedia links to both for your reference.
ThePhysicsGuy says:
I am assuming you think the ice cube will warm my hand, and the candle will warm the sun? Yes, no?
>>>>>>>>>>>>>>>>>.
Yes to both. The ice cube however will warm your hand a lot less than, for example, a puppy, and a lot more, for example, than dry ice, which would be still more than outer space. Your hand will warm the ice cube a lot more than the ice cube warms your hand. The candle will warm the sun. For the candle to NOT warm the sun would be a violation of the First Law, Conservation of Energy, and Stefan-Boltzmann Law. All of these laws can be reconciled one way, and one way only: By the Second Law referring to the net energy flux which can be determined by subtracting the SB Law flux of the cooler body from the SB Law flux of the hotter body to arrive at the net flux.
On the other hand, I think Willis picked a bad example in terms of the candle and the sun because verifying by experimentation would be…. problematic. But as far as the laws of thermodynamics go, yes.
With a name like “ThePhysicsGuy” I was hoping you would get the physics right, but alas you messed up here.
“Please tell me you are not serious.”
In Willis’ example of the candle and the sun, the warming effect of candle around the sun would be so infinitesimally small that it could never be measured even in principle. But is does exist.
Imagine lighting a gazillion candles around the sun, so that the entire sphere surrounding the sun was covered with candle flames. This would throw a lot of light back to the sun, and would warm the surface of the sun (or “slow the cooling” if you prefer — the net result would be a surface temperature higher than 5780 K). If you remove the candles one at a time, the surface of the sun would cool until you removed the last candle and the sun returns to the original 5780 K.
“Take an ice cube out of the freezer with a pair of tongs to hold it. Place your hand above the ice cube. Feel any cold radiating from it? No. Does the ice cube “warm” up your hand?”
To make this analogy work, the rest of the room would have to be EVEN COLDER than the ice — dry ice perhaps. Then the “warm” ice would indeed help keep your hand warmer than it would have been when surrounded by dry ice. Dry Ice at -80 C radiates only about 80 W/m^2 toward your hand, but the regular ice (0 C) radiates about 315 W/m^2. Reducing the heat loss would definitely make your hand get frost bite more slowly.
” “No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature.
Process = IR radiation.
IR radiation does not transfer heat (net energy) from the colder object to the warmer.
There is no violation.
PS. See my note about conduction above before objecting to the word “net”.
http://wattsupwiththat.com/2013/02/06/the-r-w-wood-experiment/#comment-1219292
PPS. If you really want to go deeper and decide if the word “net” is implied, then try the more fundamental statistical mechanics definition of entropy, and see if entropy of the universe increases when SOME energy moves from cold to warm as long as there is MORE energy moving from warm to cold.
davidmhoffer says:
February 8, 2013 at 5:24 pm
“Like richardscourtney I really can’t figure out what the heck you are talking about.”
…. Similarly, I said nothing about “charge”, nothing about which way charge flows, let alone some being odd or not. ”
————————
You talk so much you don’t know WHAT you said.
” davidmhoffer says:
February 7, 2013 at 7:28 pm
“…In order for Stefan-Boltzmann Law and the Second Law to both be true, the second Law must refer to the net energy flux, and the energy flux must be two way (meaning from hot to cold and cold to hot at the same time).”
———-
So much for your word being worth the time it takes to read it.
———
You’re such a grasping amateur you don’t even know all energy computation is done in pure algebraic terms; that if it says one-way flow, then your false claim,
*of reverse undocumented, incalculable flow *
is you, the turbo-posting amateur, on the internet;
pretending you know things are happening that mathematics can not express.
To me.
Electronic energy in an entity is potential heat; heat in electronic engineering, is disruption, spurious emissions, and death to quality signal processing and radiative emissions;
so don’t try to claim you’re talking about “other radiative transfer”.
I calibrate analyze & maintain some of the most sophisticated RADIATIVE TRANSFER APPARATUS in the HISTORY of HUMANITY.
I earned that lofty position doing something called “Knowing the right answers when amateurs get confused and think energy spontaneously flows backward.”
I have my bona fides, that precious lambskin: after completion of four years’ BLISTERING work in 3-1/2, in the radiative transfer principles of everything mankind ever witnessed.
Not that he ever built. That he ever witnessed.
And no one ever witnessed “reverse flow undocumented by the calculations.”
There’s only you, and a very few other people –
who, incidentally are connected with the most mindlessly stupid, & egregious scientific postulates, maybe in the history of humanity –
making claim that “energy flows both ways”, when mathematics says CLEARLY: it’s flowing one.
———-
We have incredibly sensitive instruments; and incredibly innovative ways of deploying them, to trigger when previously unnoticed, or undesigned-for phenomena, show themselves.
Not one of the men I have ever worked with, has EVER said,
“Did you know that in spite of charge density demands of pressure-gradient energy transfer principles,
energy actually flows AGAINST the algebraic mandate?”
Not once.
Just you, the amateur on the internet,
and some crooked government employees whose science is notoriously bad.
I seeas I’ve returned you’re STILL turbo-posting, affecting pretense we’ve had some conversation about dropping rocks in swimming pools.
You just never know when to stop because you’re behind.
I’ve seen other working radiation-transfer professionals be horned out because turbo-posting amateurs can’t be confronted with the fact they laid claim to having special elevated knowledge
of incalculable and undocumentable –
and unmeasureable for that matter, “reverse” or “two-way flow” against CLASSICAL pressure-gradient energy migration demands.
You might think because you talk more than nearly anyone, the working professionals in radiative emissions are intimidated. I’m certainly not.
I could take you into the lunch room and let you tell that
“it’s incalculable and unmeasurable, but if you really understood, YOU’D SEE like ME: there’s magic BACKWARD flux-density assignments! REALLy! That’s the only way, all the EQUATIONS work out!”
You’re an embarrassment to me, and to my kind: the radiative transfer & emissions
expert professional kind.
Don’t even try to make the claim you’ve got some secret telephone line to Entropy and that it calls you up and whispers “when it’s just not right” and you have to come here and proclaim migration of energy against principles that govern a heater.
You’ve already been shown by me to be capable of saying you never made utterances to particular effect, and shamelessly then, affecting pretense you, and I, had some other discussion than the one we HAVE had:
that I told you, there is not a single equation or mathematical inference ANYWHERE
that any energy in a system EVER flowed against the classical pressure-gradient mathematics that make it possible for a redneck with a ball cap to understand how his CB works.
I told you, not one single time in my entire 18 years PLUS three, has anyone EVER mentioned to me they believe in incalculable and unmeasurable “reverse entropic flow.”
Not once. Not ever.
Anything you’ve said here that implies that, is utter fabrication by a wannabe who never made past those “introductory texts” you claim you’re familiar with.
Radiative transfer mechanics is organized according to pressure-gradient concepts very much like pressurization of anything else, including an air tank. Not everything crosses exactly but
that’s why light energy is often seen referred to as “pressure”
and that’s why thermal energy is often referred to as “pressure”
and that’s why electronic flow is often referred to as “pressure.”
And you have been in here for MONTHS if not YEARS telling people *you saw a hose get connected to a PRESSURE and INJECT, using LOWER PRESSURE than the vessel that energy was injected into.
Bullshoot. Period. By a rank amateur who has no idea how few words it takes to chop that up and leave it on the deck, bait for GULLS.
Allen B. Eltor says:
February 8, 2013 at 9:00 pm
>>>>>>>>>>>>>>>>>>.
I refer you to Willis’ comment upthread:
Willis Eschenbach says:
February 7, 2013 at 9:11 pm
If you feel strongly about the matter, then I suggest you take it up with the authors of those text books and reference manuals in use at universities and engineering companies all over the world.
davidmhoffer says:
February 8, 2013 at 9:32 pm
If you feel strongly about the matter, then I suggest you take it up with the authors of those text books and reference manuals in use at universities and engineering companies all over the world.
<<<<<<<
I already did. They gave me a degree in electronic engineering, after thousands of hours face to face instruction,
and told me fakes on the internet would be making claims of magical "non pressure-gradient prescribed flow," that "no mathematics can show: it shows one way flow but actually, it's going both directions!"
and not to let them get my identification card and use it to get into the building.
Yes I agree with Willis that in his model of black bodies, they will work as described.
However, as BB are a theoretical construct, not able to be realised, give me and many others a problem when moving into the real world.
Perhaps this is the problem we should address, using a theoretical construct as though it is real?
We all know that a Parker warms you in the winter by increasing the thermal gradient between your body and the ambient air, no back radiation what so ever.
We all know that igloos are cold inside unless you are inside as well to warm them up, no back radiation what so ever.
Perhaps all of this confusion has been brought about by too enthusiastic application of an interesting theory into areas where is might be best to use others?
PS: we all know the earth is not a BB but some persist in using BB calculations as though they are a perfect fit.
Steve Richards
So you have planet and it’s radiating 235 watts per square meter. Or surface is about
-18 C [255 K].
You put steel shell around it and then planet surface radiates 470 watts per square meter.
What is surface temperature? Is about 26 C [300 K]?
Or what is the temperature on this surface which radiating 470 watts?
And could someone also describe this as heat from core [235 watts per square meter] is inhibited
from radiating enough energy as would be able to do if was radiating into the vacuum of space, therefore the ground temperature increases to 26 C, at which point the temperature is sufficient
to radiate the 235 watts per square generated from the core generated heat plus the increase heat of the surface which adding another 235 watts per square meter?
Steve Richards says (February 8, 2013 at 10:03 pm): “We all know that igloos are cold inside unless you are inside as well to warm them up, no back radiation what so ever.”
Funny you should mention igloos. According to Figure 18 on page 85 of this paper,
http://www.atmos.washington.edu/sootinsnow/PDF_Documents/Warren_Optical%20Properties%20of%20Snow.pdf
the infrared emissivity of snow varies from about 0.95 to very nearly 1.00, depending on temp, wavelength, and angle. In other words, snow is a very good emitter/absorber of infrared radiation, and the snow igloo is emitting it in all directions, inside and out. If you enter the igloo (or put a Willis-type nuclear heater in it), the inner surface of the igloo will absorb your IR radiation and send so-called “back radiation” in your direction.
Of course down here deep in our atmosphere, conduction and convection are the predominant methods of heat transfer, and the igloo “works” mainly by curtailing these processes. When you’re considering the heating and cooling of the Earth from and to outer space, however, radiation is the only game in town.
Phil. says:
February 8, 2013 at 10:18 am
“Not correct there are indeed counter flowing flows of electromagnetic radiation, interference does not prevent the propagation of radiation in either direction, if it did a laser cavity wouldn’t work!”
Optical resonators form a standing wave oscillation. This is a singular condition, and there is no net flow of energy in either direction. So, here also, your interpretation of what is happening is a mental construct, a “useful fiction” to help understand the system, and what it will do when you perturb it from that condition.
Don’t you think it is ironic on these blogs that sceptics like myself have to spend all our time defending the greenhouse effect because so many commentators are unable to grasp even basic physics?
I would rather be debating how limited its future effect will be.
And now, in addition to the inappropriately named Greenhouse Effect we have inappropriately named ‘ThePhysicsGuy’
Sad, n’est pas.
There is an error in my equations above. If others had been interested in doing the math, instead of insisting on their individual infallible arrangement of words, it might have been detected earlier. I want to lay this out very carefully, so please check my math, any who are interested.
The total incoming power to the planet is
This is the power from the nuclear core, plus the reflected power from the inner shell. I had it incorrect previously because the reflected power must be integrated over the area of its source, not its sink.
The total outgoing is
Thus, at equilibrium when these two quantities balance, we have
(1)
For the shell, we have as before (I got it right in this one, for some reason)
When these balance, we have, as before
(2)
Plugging (2) into (1), we get
(3)
We know that without the shell
(4)
Hence,
(5)
Please, by all means, check my math and make constructive comments using math because this is a rather astounding result, the implications of which we can explore when others have verified the formula.
If others verify the formula. It is late, and I could be befogged. If this is right, it stands the whole discussion up on its head, so I am filled with self-doubt at the moment. It will not surprise me if I have made an error somewhere. But, if I haven’t…
Allen B. Eltor:
My post at February 8, 2013 at 2:58 pm genuinely tried to help you by suggesting your posts would be more understandable if they each provided one point clearly expressed.
Your post at February 8, 2013 at 6:01 pm has replied to that but, sadly, I am still failing to understand what you are trying to say. I read your words but I fail to see any clear point. There is no summarising statement and I fail to read any explanation, justification or exposition of it.
When reading your words I am reminded of the response by Eric Morecombe when answering complaint at his piano playing; i.e.
I regret this because you may be trying to tell me something which I would like to know.
Richard
Mr Hoffer has a serious problem distinguishing between thermal insulation and radiation.
This thread is strewn with examples where he continually confuses the two. Using his igloo analogy. The snow acts as a insulator. There will be “back-radiation” but no thermalisation can occur from it to anything above 273 K.
If I am wrong then ice would melt ice. Or rather no ice could ever form because everything would be continuously making everything else ever hotter and hotter.
As I said above, a colder sky cannot possibly heat a warmer surface, any more than a trillion 1 ton blocks of frozen CO2 radiating at 194.56 K could melt a 1 inch water ice cube radiating at 273.15 K.
In both cases it is forbidden by the laws of thermodynamics.
The colder substance/object radiates IR with a lower flux density than the warmer object. The IR from the colder substance/object cannot be thermalised by the warmer substance/object and therefore is just scattered/re-emitted at light speed.
No thermalisation occurs when IR travels from cold to hot.
Willis’ “shell game” generates a paradox. There is no mechanism preventing the system from getting ever hotter and hotter. You could not maintain the inner vacuum in reality, for a start. Then the system would simply return to radiative equilibrium with the core.
It’s a “thought experiment” for a very good reason.
Bart,
The radius of the shell doesn’t matter. For a thin shell it cancels out. You get the same result whatever the radius of the shell (see my first post).
By the way, the shell is not ‘reflecting’, it is absorbing and then emitting.
Sorry Willis this is complete sophistry.
Your model is nothing like reality and reality shows a different scenario. The planet is a revolving sphere with insolation at 1370W/m2. This is seen on only half the planet, since reality has night time, and is reduced by albedo and adsorption to 960W/m2, over half the planet. since this power, yes power since a Watt is a unit of power not energy, arrives as a disc and has to spread over a hemisphere, we divide by 2 since a hemisphere has twice the area if the subtended disc, giving 480W/m2 average over the hemisphere. True radiated energy is at 240W/m2 but this is for all the planet’s area. 480W/m2 gives a temperature of +33C.
Wood’s experiment had nothing to do with re-radiated heat but to confirm that despite the IR opaqueness of glass visible light gives heat as well. This was confirmed using a rock salt window and glass window in side by side vessels where temperatures were similar despite the glass being IR opaque.
See:- http://www.climateofsophistry.com
Will wrote “Willis’ “shell game” generates a paradox. There is no mechanism preventing the system from getting ever hotter and hotter.”
This is incorrect, the only source of heat involved is the nuclear core, and as the shell radiates heat from its outer surface. The rate of heat loss from the planet/shell system is proportional to the fourth power of the temperature of the outer shell, so as soon as the outer shell warms enough to radiate energy at the same rate it is generated by the nuclear core, the system will reach thermal equilibrium and will cease to get any hotter. There is no paradox.
Allen B. Eltor:
Perhaps my difficulty in grasping your words would be understandable by comparing these quotations of your words.
At February 8, 2013 at 9:18 pm you wrote
Well, your maths are wrong when your maths dispute reality.
There are several systems where some energy in a system flows against the pressure-gradient; e.g.
ater pumps which use some the energy of the flowing water to raise some of the water to above its pressure head,
refrigeration systems,
etc.
At February 8, 2013 at 9:00 pm you wrote
So what? I rely on an auto mechanic to “calibrate analyze & maintain” my car but I would not expect him to understand the combustion kinetics in the car’s cylinders.
The only way I can make sense of your rants in this thread is to assume you are one of the ‘new bread’ of scientists and engineers who graduate with a good understanding of how to ask a computer to provide an “answer”, and who think they understand what the computer does, but they don’t really understand because they only have a superficial theoretical knowledge.
At the moment, all I can see in your several posts on this thread is assertions that everybody except you is wrong because you are qualified to say they are but you have not shown their errors.
Perhaps you can try to provide a coherent and cogent post based on evidence and/or logic instead of claims to your own authority. That would alter my opinion of your posts which I have here explained.
Richard
Willis,
Over at Tallbloke’s Talkshop we are debating the same issue:
http://tallbloke.wordpress.com/2013/02/04/david-cosserat-atmospheric-thermal-enhancement-part-i-the-great-debate-begins
Coincidence? 🙂
We are currently trying to establish sensible ground rules there on a number of technical issues one of which is this vexed issue of so-called back radiation. I describe there a thought experiment which may help people understand that radiative energy flowing between bodies separated by a vacuum does of necessity flow in both directions. Like you, I have found it a tough task to persuade some commentators that two-way flow really does (and must) happen.
The difficulty, I think is psychological. Skeptics such as your ludicrous, bombastic Allen B. Eltor, who have a perfect right not to believe in CAGW or AGW, appear to think that they are at Custer’s Last Stand. So they try to defend the indefensible without thinking whether they need to do so in order to sustain their position.
In fact, as I have demonstrated at the above link, skeptics should welcome the concept of back radiation because it shows that the fully-thermalised lower atmosphere is behaving as it should, given its elevated temperature. It is hardly surprising that it radiates back to the surface nearly as much power (333Wm-2) as the surface radiates upwards (356Wm-2).
The net difference beween the up and down radiative flows is a piddling 23Wm-2. This net flow is upwards of course from warmer surface to slightly cooler lower atmosphere in full conformance with the 2nd Law. That 23Wm-2 is in any case almost immediately thermalised in the atmosphere and joins the 17Wm-2 of conduction/convection thermalisation and the 80Wm-2 of latent heat (later thermalised at cloud level) to provide an overall thermalised through-flow of ~200Wm-2 of energy from Sun-to-Earth-to-space.
People need to understand that the enhanced radiation of 333Wm-2 apparently ‘going round in circles’ between surface and atmosphere is is not the cause of their respective elevated temperatures. It is simply a consequence of them! It does no work. It is not dissipated. So it does not count in the overall (relatively modest) 199Wm-2 through-flow of energy from Sun-to-Earth-to-space that is needed to make up for energy losses, and which keeps the surface and atmosphere at the stable enhanced mean temperature levels that we humans enjoy.
Rather than call it the ‘Greenhouse Effect’ I prefer to use the term ‘Atmospheric Thermal Enhancement effect’ or ATE for short. This is a perfectly neutral term which allows us to go on to debate in a mature, and non-confrontational, way whether or not the thermal enhancement is due to GHGs. This we intend to discuss, rationally, in our upcoming Part II.
johnmarshall, you are being grossly unfair to Willis, who is doing a good job of explaining some very basic concepts. The shell model is obviously intended to be only the most basic representation of one aspect of the greenhouse effect (backradiation), only complex enough to reveal the problem with the Woods experiment. nothing more. Using such simplified models is common place in science, for good reason, and is hardly sophistry.
If you want a more realistic model, including features such as rotation, then I suggest you try Raymond Pierrhumberts book, “Principles of Planetary Climate”, chapter 3 deals with radiation balance of planets.
For those who argue there’s no back radiation, no “Net”, notice SB equations have a T1-T2 term, if you look up the definition of Net, it say one definition is a difference. This difference does always flow from hot to cold, but to get the right answer you have to account for radiation being exchanged between both objects.
What I think we should discuss next is that the temp of the sky is lower that the earth( 75 F colder on a clear 35F day in NE Ohio). Much lower when there’s little water vapor in the atm. These facts explain why when the Sun goes down the temp drops, a lot in dry locations. And the difference in day night cycle controls whether It’s warm or cold, ie the seasons.
The average temp swing is about 18F when averaged over a year (follow the link in my name to see the graphs).
Yet, it can be as much higher in dry locations, and much lower in humid locations. This average has not really changed over the last 60+ years.
dikranmarsupial says:
February 9, 2013 at 3:30 am
“Will wrote “Willis’ “shell game” generates a paradox. There is no mechanism preventing the system from getting ever hotter and hotter.”
This is incorrect, the only source of heat involved is the nuclear core, and as the shell radiates heat from its outer surface. The rate of heat loss from the planet/shell system is proportional to the fourth power of the temperature of the outer shell, so as soon as the outer shell warms enough to radiate energy at the same rate it is generated by the nuclear core, the system will reach thermal equilibrium and will cease to get any hotter. There is no paradox.
You have only half quoted me and then simply repeated the rest of my comment in your own words and then claim that I am incorrect. This is an over used trick of semantics.
My quote in full:
“Willis’ “shell game” generates a paradox. There is no mechanism preventing the system from getting ever hotter and hotter. You could not maintain the inner vacuum in reality, for a start. Then the system would simply return to radiative equilibrium with the core.
Which is the same as yours, sans waffle. Neither of which are anywhere near Willis’ impossible thought experiment because he double counts energy in order to imply additional heating.
So in effect you are agreeing with me while implying that you support Willis.
Very tricksy.
David Socrates,
I took a look at Tallbloke’s “The Great Debate”, and the ground rules establish essentially the “alarmist” GHG model as developed by Trenberth as the starting point. So if you don’t agree with the basic concepts of the model as set forth, too bad, that is not up for debate. No thanks. Sounds more like an indoctrination session.
For you electronics guys who still struggle with this.
Picture 3 resistors in series, with each end connected to separate 12v power supplies, now calculate current, voltage and power for each node. Now change one supply to 6v, recalculate, now set it to 0v, recalculate again.
It’s not exactly the same as SB between two objects, but its similar.
Will says:
February 9, 2013 at 2:41 am
Mr Hoffer has a serious problem distinguishing between thermal insulation and radiation.
This thread is strewn with examples where he continually confuses the two. Using his igloo analogy. The snow acts as a insulator. There will be “back-radiation” but no thermalisation can occur from it to anything above 273 K.
>>>>>>>>>>>>>>>>>>>>>>..
Mr Hoffer did not propose an igloo analogy in this thread. Your statement that back radiation exists but no thermalisation occurs is a violation of the Law of Conservation of Energy, First Law of Thermodynamics and Stefan-Boltzmann Law and to be true would require that both the joule and watt as fundamental units of physics be re-defined. The rest of your rant suffers from the same errors as your first paragraph.
Bart says:
1) As mentioned before, the shell “emits” its own power, it doesn’t “reflect” power.
2) More importantly, you missed power transfer from the inner surface of the shell back to the inner surface of the shell. In the limit that the shell is the same radius as the planet, this term doesn’t matter. In the limit that the planet is small compared to the shell, then the thermal radiation emitted by the inner surface of the shell will simply hit some other part of the inner surface of the shell.
I started to write out all the equations, but it got too messy. Suffice it to say that not all the power emitted by the inner surface of the shell hits the planet. If you want to look at the “realistic” situation where the planet is some finite distance above the planet’s surface, then you will have to account for the photons the get emitted by the inner surface of the shell but “miss” the planet and instead land back on the shell. (For earth, this amounts to a fraction of a percent.)
P(in to the planet from the shell) = σ (T_shell)^4 (area of PLANET)
P(out from the shell) = σ (T_shell)^4 (area of SHELL)
That would mean
P(from shell back to shell) would be σ (T_shell)^4 (area of SHELL – area of PLANET)
richardscourtney;
Re Eltor
>>>>>>>>>>>>>>..
Richard, we may fail to grasp the stature of Mr Eltor. According to him:
I have my bona fides, that precious lambskin: after completion of four years’ BLISTERING work in 3-1/2, in the radiative transfer principles of everything mankind ever witnessed. Not that he ever built. That he ever witnessed.
When one is in the presence of a person who in 3.5 years studied all the radiative transfer principles that mankind has ever witnessed, refuted all the thermodynamics texts (which nonetheless remain in use to this day) while earning a degree in electronics engineering one is the presence of someone very special.
Micro,
The SB equation has no T1-T2 term. It is simply
P = σ T4
Where P is the power in watts emitted per square metre of blackbody surface, sigma (σ) is the Stefan-Boltzmann constant and T is the absolute temperature in kelvin. The value of the constant σ is 5.67 * 10-8 (this is an easy constant to remember because the significant digits to remember go in sequence, 5,6,7,8 ). The power P is variously called the ‘emissive power’, ‘radiant power’, ‘radiant exitance’ or ‘radiant emittance’
For non-blackbodies the power is simply factored down by the emissivity.
You don’t
.
The amount of energy radiated by an object depends only on its own temperature and emissivity. It is independent of where the object is or what is or the temperatures of things surrounding it.
Will, no, they are not equivalent. The system will reach thermal equilibrium whether or not the vacuum is maintained.
The nuclear core generates energy at a constant rate, the outer shell radiates heat at a rate proportional to the fourth power of its temperature. Thus as the temperature of the outer shell increases, there will come a point where it radiates as much energy as the core generates and thermal equilibrium is reached. This is true whether the energy is transmitted from the core to the shell by radiation, conduction or convection, so whether the internal vacuum is maintained is irrelevant.
Willis is correct, there is no paradox, there is no double counting of energy.
Willis Eschenbach says:
February 6, 2013 at 5:01 pm
Here’s an example to consider. Mount a light bulb socket on a table. Insert a 60-watt bulb. Measure the temperature, we’ll say it’s 150°C. Try a 100-watt bulb, it’s hotter, say 200°C.
Now, mount another socket right next to the first one. Insert a 100-watt bulb in one and a 60-watt bulb in the other. What will the final temperatures of the bulbs be?
I say both of them will run hotter in that situation, because A warms B, and B warms A
You also stated that, “..of course it has been proven, many times over. It’s in every college thermo textbook”.. In 1850 Clausius stated, “Heat cannot of itself pass from a colder to a hotter body.” That statement (or similar version) of the Second Law of Thermodynamics has been in every college textbook since that time. Yet you indicate that the colder light bulb will warm the warmer light bulb. So, what will happen with your version of thermodynamics? The 150°C bulb will cause the 200°C. bulb to become warmer. But now the warmer 200°C+ bulb (B) will then warm bulb (A) even more, with the cycle going back and forth until both bulbs thankfully explode, before melting a hole to China.
Care to explain yourself?
REPLY: Seems like an easy experiment to perform to find out. I think I’ll actually do this – Anthony
Anthony – light bulbs are not designed to radiate heat but light, and also have a rather low mass and are hollow, so it may be difficult to get reliable temperature measurements. I think this would be a useful experiment, but I would suggest electrically heated metal spheres instead, so the thermal inertia makes the equilibrium temperature easier to measure. I’d certainly be interested to see the results.
MikeB,
Here’s the equation
http://upload.wikimedia.org/math/9/c/a/9ca1177f3d75ec3bb4a98ab7ce668297.png
And it indeed has a T1 – T2 term.
Anthony, if the bulbs are say 6″ apart, measure between them, then 3″ on the outside of both, and with a say 60w and 100w bulbs you will get 3 different readings. Also note the filaments will both be similar temps, since they both output similar color spectrums (color temps).
ThePhysicsGuy asks ” … with the cycle going back and forth until both bulbs thankfully explode, before melting a hole to China.
Care to explain yourself?”
Every true physics guy is quite familiar with convergent series.
http://en.wikipedia.org/wiki/Convergent_series
MiCro says, February 9, 2013 at 5:28 am: “For those who argue there’s no back radiation, no “Net”, notice SB equations have a T1-T2 term, if you look up the definition of Net, it say one definition is a difference.”
============================================================
Stefan–Boltzmann law has nothing to do with your “T1-T2 term”. Your “T1-T2 term” has apparently no basis in real science, because it has apparently never been confirmed experimentally. The same goes for this “Net”, it is equivalent to that fictional “T1-T2 term”.
Anthony,
You might want to modify the experiment some since the two filaments will have roughly the same temp (but different visible light flux). Test with two 100w clear bulbs, with one on a dimmer circuit. Also align both filaments in parallel, and it might be interesting to do one test with one perpendicular to the other.
davidmhoffer:
re your post addressed to me at February 9, 2013 at 6:50 am.
I admit to being bemused at this thread, and it is not only Eltor whose posts I fail to understand. The following listed history explains my puzzlement.
1.
At February 7, 2013 at 11:48 am I wrote a post to ‘wayne’ which I mistakenly thought would overcome any confusion about the difference between ‘heating’ and ‘reducing heat loss’.
2.
But that failed, and Greg House explained why at February 7, 2013 at 6:30 pm. He asserted that there is no physical demonstration of a cooler object making a warmer object hotter.
3.
So, at February 8, 2013 at 3:37 am, I refuted that by saying my microwave oven IS a cooler object that makes a warmer object hotter.
4.
Ryan responded (at February 8, 2013 at 7:28 am ) saying
5.
I replied to that at February 8, 2013 at 8:14 am, saying
6.
At that point I had thought there was nothing left to say except to explain the issues of mechanism and mathematical description of the kind you have been providing.
7.
Clearly, I was wrong because subsequently a series of people have ignored the contents of my posts and have iterated the same points which I had explained are erroneous.
Those who have made those posts include Ryan, jae, mkelly, jae43 (who is perhaps jae), Steve Richards, gbaikie, Allen B. Eltor, Will, ThePhysicsGuy, Greg House, and (if I understand him) johnmarshall.
8.
None of them has came back to me about my explanations to refute them or to ask for clarifications. Instead, they have continued – with no reasoning and/or evidence – to assert the points I had refuted.
9.
I fail to understand why these people are refusing to consider the explanations which I took the trouble to provide. (The post provided by MikeB (at February 8, 2013 at 3:17 pm) suggests they have a “conceptual” problem, but that does not explain their refusal to consider the explanations I provided.
Hence, I am bemused by the thread.
Richard
MiCro says: “MikeB, Here’s the equation …”
Micro, that is not “the SB equation”, but rather a “radiative heat transfer equation”. The equation you referenced is specifically for the transfer of heat between two specific black bodies. It includes “F” which deals with the specific geometry involved.
The SB equation is given just a little further down on that same wikipedia page as P = σ A T^4 (or the more general case general case when ε is not equal to 1: P = ε σ A T^4).
http://en.wikipedia.org/wiki/Thermal_radiation#Radiative_power
** SB gives the power radiated out from each surface.
** The other equations gives the net power (power out – power in).
davidmhoffer says:
February 9, 2013 at 6:34 am
“Your statement that back radiation exists but no thermalisation occurs is a violation of the Law of Conservation of Energy, First Law of Thermodynamics and Stefan-Boltzmann Law and to be true would require that both the joule and watt as fundamental units of physics be re-defined.”
I rest my case, you are clue-less.
Ice cannot melt ice because it cannot thermalise its own or lower flux density radiation. If it could there would be no ice.
dikranmarsupial says:
February 9, 2013 at 7:17 am
No further comment.
Alright Anthony! Take some photos for us!. To really put the theory to the test, in addition, consider surrounding the higher wattage bulb with 3 lower wattage bulbs, and then surround the whole experiment with tin foil to act as an IR reflector. (OK, maybe that is overkill)
richardscourtney says, February 9, 2013 at 8:59 am: “…Greg House explained why at February 7, 2013 at 6:30 pm. He asserted that there is no physical demonstration of a cooler object making a warmer object hotter.
…So, at February 8, 2013 at 3:37 am, I refuted that by saying my microwave oven IS a cooler object that makes a warmer object hotter.”
===========================================================
Let me give your another example of equal scientific value: your warm finger makes your warm house colder! (by pressing a button on the air condition). Of course, it goes also like that: your cold finger makes your house warmer!
What insulation is in contact with the planetary nuclear core ?How is this insulation affecting the temperature of the planetary nuclear core? If we put extra mass directly in contact with the nuclear core this would also increase the temperature of the core because heat is lost more slowly from the core.If instead of adding more mass to make a shell you took some of the mass close to the nuclear core and moved it out until it formed a shell as described would the nuclear core not cool down and the energy lost to space increase.
richardscourtney
I appreciate your problem. I gather, like me, you and David Hoffer are not a true believers in the Global Warming catastrophe, but, as I said earlier, we sometimes feel morally obliged to correct some of the more embarrasing misconceptions that would-be supporters of our case have, before they give all sceptics a bad name.
Coming from a scientific background, your initial expectation is that once you point someone in the right direction they might at least go and check it out. But that is wishful thinking. They carry on like those zombies who get knocked down but won’t admit to be dead. Makes for good films but very frustrating.
What I like to do is guess what they are going to come back with next (apart from just ignoring you). For instance when Micro talks about a non-existant T1-T2 term in the SB equation I know the mistake he has made and that he is going to come back quoting some other equation with aT1-T2 term in it. And that is exactly what he does. Thanks to tjfolkerts for pointing that out.
OK… maybe I have had too much wine after dinner.
I would call it the two body version of S-B, but if that’s not what it’s called, I’m fine with that.
Greg, it’s tested out in millions of things everyday, your protests are just plain wrong.
richardscourtney says:
February 9, 2013 at 8:59 am
>>>>>>>>>>
Richard,
Like you, I am bemused, but also frustrated. There is so much wrong with the CAGW meme that can be debunked with good solid science. How to get to that when the skeptic side is littered with people that cling to a belief system that can be debunked by the simplest of observational evidence? People who the warmists can point to in order to discredit skeptics? People who attempt to influence others to join them in their folly, forcing a tremendous amount of energy to be expended trying to set the record straight.
On setting the record straight, one wonders if we’re even being successful. The same people come back with the same tired arguments over and over again, debunked by the same simple examples over and over again, but what do the silent majority think? Are they influenced one way or the other? I don’t know.
But I am beginning to see a pattern. The fact is that the notion that cold things radiate energy to warm things is counter intuitive. But the notions that the earth is round and that the earth orbits the sun are also counter intuitive. Yet somehow most people get past those.
The examples are easy. Venus, despite being further from the sun than Mercury, is warmer than Mercury. Venus has an atmosphere, Mercury doesn’t. The Earth is warmer than the Moon, despite being (on average) the precise same distance from the sun. Earth has an atmosphere, the Moon doesn’t. Then we have every day experience to draw upon. Cloudy nights are warmer than clear nights. The clouds are much colder than earth surface. Deserts cool off rapidly at night due to lack of ghe effect form water vapour while jungles which have very high water vapour cool off at night very slowly. Are these simple observations so hard to understand?
But then out come the nay sayers with their version of science. Cold things can’t heat up warm things, because the Second Law of Thermodynamics says so. Point out that the 2nd Law refers to net energy flux and they’ll start quoting Clausius. Pointing out that the 2nd Law has been reworded several times since 1850 as our understanding of physics has improved, is meaningless to them. Pointing out that Clausius wording refers to heat transfer which is not the same as energy flux similarly falls on deaf ears. But it is at this point that I find the discussion gets truly bizarre.
At this point I sometimes link to ARM or a paper on the topic showing that we can measure downward LW in the middle of the night, with no moon, and get readings in the hundreds of w/m2. We know from instrumentation we have in orbit that downward LW above the atmosphere is on the order of 2.7 w/m2. So, at night, no moon, nothing to produce a couple hundred w/m2 of downward LW but the atmosphere itself, where does it come from?
That’s when the real lunacy begins. Oh, back radiation exists someone will shout, but it doesn’t “thermalize” anything. I find that those who scream the loudest about the 2nd Law become even more shrill (but unconvinced) when I point out that this would violate Conservation of Energy and the 1st Law. And Stefan-Boltzmann Law. And the very definitions of joules, watts, and temperature itself.
They start hollering that I am claiming that you can pressurize a tank with an inlet pressure that is lower. A difficult thing to debunk because it is true. It just has zero to do with how radiated energy works. In the meantime, through all this, millions of engineers around the world used the exact physics we’re trying to explain to design and build everything from the mundane kitchen stove to the environmental control systems for the space station. Tjfolkerts, mikeB, bart, MiCro and others are having a conversation amongst themselves that I for one have learned from. They are refining their collective knowledge and learning from each other rather than shouting belief systems at each other while ignoring valid points that someone else advances. That’s science in action and we need more of it. As for the nutters who want to selectively choose which laws of physics to use and which not…
Bemused? Yes.
Frustrated? Yes
Exasperated? Bingo.
David Hoffer says
“But then out come the nay sayers with their version of science. Cold things can’t heat up warm things, because the Second Law of Thermodynamics says so.”
David you do not have a clue about what the word ‘Heat’ means.
Heat is always capable of doing thermodynamic work.
Go find a textbook covering the Carnot Cycle.
This is the usual introduction to the second law.
Then you will not make such elementary mistakes.
tjfolkerts says, February 9, 2013 at 9:17 am: “** SB gives the power radiated out from each surface.
** The other equations gives the net power (power out – power in).”
=============================================================
The problem is that this “other equation” about that “net” thing has apparently never been confirmed experimentally. Therefore it can not be considered to be a scientific fact.
On the other hand, of course, anyone has the right to produce any equation. For example, I like this one: “2 oranges + 3 apples = 5 tomatoes”, why not, but the difference is that I do not claim it to be a scientific fact.
davidmhoffer says, February 9, 2013 at 10:30 am : “Cold things can’t heat up warm things, because the Second Law of Thermodynamics says so. Point out that the 2nd Law refers to net energy flux and they’ll start quoting Clausius. Pointing out that the 2nd Law has been reworded several times since 1850 as our understanding of physics has improved, is meaningless to them.”
==========================================================
The problem is that in linguistic terms your rewording (I mean the insertion of the word “net” into the 2nd Law) produces a formal contradiction to the 2nd Law. This is important to understand. The Clausius statement and your statement formally contradict each other. Both can not be true at the same time.
So, we have your statement versus Clausius statement. Two different things.
The Clausius statement means that something is not possible, but your statement declares it possible. Your statement is however not the 2nd Law, this is important to understand. If you change words you get formally another statement, however, it is still possible that those 2 different statements are physically equivalent. So, you need first either to prove the equivalence or just prove your statement scientifically, thus refuting the Clausius statement. I am looking forward to it.
….and as if on cue:
Bryan says:
February 9, 2013 at 10:47 am
David Hoffer says
“But then out come the nay sayers with their version of science. Cold things can’t heat up warm things, because the Second Law of Thermodynamics says so.”
David you do not have a clue about what the word ‘Heat’ means.
Heat is always capable of doing thermodynamic work.
Greg House – The “net transfer” is a consequence of viewing the transfer of heat as being mediated by the radiation and absorption of IR photons. Unless you can come up with a mechanism by which a cold body can know which directions it can safely emit a photon without it hitting a warmer body, or for a body to choose not to absorb photons from a cooler body (impossible as there is no way to determine the temperature of the body that emits an individual photon), then an exchange of photons (and hence energy) is inevitable. However, the net transfer is still from warmer to colder body, so there is no violation of the second law of thermodynamics.
davidmhoffer says, February 9, 2013 at 10:30 am: “That’s when the real lunacy begins. Oh, back radiation exists someone will shout, but it doesn’t “thermalize” anything. I find that those who scream the loudest about the 2nd Law become even more shrill”
==========================================================
The Wood experiment demonstrates how back radiation “thermalize” anything, see above.
Will wrote:
“The colder substance/object radiates IR with a lower flux density than the warmer object. The IR from the colder substance/object cannot be thermalised by the warmer substance/object and therefore is just scattered/re-emitted at light speed.”
The notion that matter cannot be thermalised by the infrared radiation from cooler objects seems to be the core concept for those who do not agree with the basis of Willis’s article
There are practical implications to this idea. One is that a vacuum flask would not work other than as a device to prevent heat loss by conduction and convection. So a vacuum flask would not work in space (or other vacuum) at all; the contents would lose heat by radiation at the same rate as if those contents were not in the flask.
This should be easy to demonstrate.
PJF, as far as I can see a vacuum flask essentially does only work by preventing heat loss from conduction and convection. They are often silvered to reflect heat back into the flask, but that is reflection, rather than back-radiation. If the flask were unsilvered and made of a material that did not absorb IR radiation, it would work on Earth, but not in space.
If an object cannot be “thermalised” (presumably this means “made warmer”) by IR radiation from cooler objects, the key question is how can the object know which IR photons come from cooler objects (and should not be absorbed) and which come from warmer objects (and should be absorbed). All objects emit photons with a distribution of wavelengths, the distribution depends on the effective temperature of the object, but that can only be determined by looking at the distribution of the wavelengths of photons it emits, not a single photon.
Well I’m rather glad that I exhausted my interest in this thread some time ago. Why David, and Richard are still here taking all this abuse, I cannot fathom.
I always learn some stuff here. I had no idea there were “Translational Temperatures” and “Rotational Temperatures”, but if Phil says there are, that is good enough for me. Evidently they relate to the internal resonance excitation modes of certain molecules, and hence belong in the realm of quantum mechanics of real materials.
I’ve been outside of academia for more than half a century, and left before developing quantum mechanics at my finger tips.
So I can’t actually cite any peer reviewed papers on experimental observations of isothermal bodies anywhere in the universe. So for all I know, there may not be ANY such thing as an isothermal object, anywhere in the universe. I also can’t cite any peer reviewed papers on experimental observations of 100 percent absorption of EM radiation by any physical body or bodies.
So I’d be happy to read such papers if those who know about them would cite references. I do still have inside connections at my alma mater, and can get papaers from behind paywalls, if I need them.
But based on what I have read, I would say that black body radiation theory, is a solution in search of a problem; since Planck’s black body radiation derivation explains no actual experimental observations anybody ever made, that I am aware of. It may involve matter consisting of “particles” which thermally interract (collide with each other) but it takes no acount of whether those particles comprise a solid, or a liquid or a gas; it doesn’t even involve any properties at all of any of those particles.
So we have a theoretical model of something that nobody has ever experimentally observed, since none of the ingredients exist anywhere.
The Stefan-Boltzmann Law is of course nothing but the definite integral of the Planck radiation law, over all frequencies or wavelengths. If I’m not mistaken, a form of S-B was derived from statistical mechanics, and ordinary classical thermodynamics; well just the T^4 part was.
The beauty of Planck’s formula, is that it derived a value for sigma, the S-B constant.
Actually the total radiated power density, is not sigma x T^4, as is most commonly stated.
The correct formula is Wtot = N^2.sigma T^4 Watts per metre squared, where of course N is the refractive index of the medium in which the total radiant emittance is measured or observed.
But this thread has lots of people bandying about the “S-B ;law” as if it is somehow connected to the so-called “greenhouse effect”; that being of course the atmospheric climatism involved greenhouse effect; not the fictitious greenhouse effect by which real greenhouses do not operate, and RW Woods experiment relating to that.
Some posters here clearly have English as a second language, or maybe a third language; Richard I’m sure is having a fit trying to translate some of the stuff here into English. Well David too, is as dumbstruck as I am reading some of the utterances.
As I have said before, I do not have QM at my fingertps where I can manipulate it for useful results, and I’m not a chemist, so I don’t have a lot of interest in the calculus of greenhouse absorptions.
It’s the non quantum mechanical thermal radiation that interests me most. So far as I know, there atre NO exceptions to its emission from thermally interracting particles at Temperatures above zero Kelvins.
Perhaps a single atom or molecule in free flight in space, does not emit thermal radiation; but that is not an exception, because such a non interractng isolated atom or molecule by definition is at zero Kelvins, so is not expected to radiate. And very sparse collections of molecules that collide less frequently, and at lower velocities, such as gases, would naturally be expected to emit (or absorb) less of the thermal radiation, than denser more frequently colliding collections of “particles”.
And those emissions are not related to atomic or molecular energy levels so they are not quantized; but they are emitted as “photons”, which may have any energy value. I’m not aware that Planck ever contemplated the exact physical cause of such radiant emissions. Heinrich Hertz and JC Maxwell simply derived it from accelerated electric charges; basic antenna theory, since a varying current is simply an accelerating electric charge.
I’ve already cited one paper on the “collision induced quadrupolar and hexadecapolar emissions in gases”. You can find it here at WUWT for yourselves. Dipole modes are not the onlt antenna radiation patterns.
Well material itself can only exist as atoms or molecules of a limited number of types or species; probably no more than 1,000 different types. Does that make chemistry quantum mechanics.
Photons of thermal radiation may have any energy from near zero eV to many GeV, with no missing values forbidden. That is NOT the case of the emissions that are involved in the “green house effect”, which may have only certain quantized values, and their broadened resonance lines.
And so long as we still have a 24 hour day on planet earth, this planet, will never be in thermal equilibrium.
davidmhoffer says
“But then out come the nay sayers with their version of science. Cold things can’t heat up warm things, because the Second Law of Thermodynamics says so.”
David
Why does every physics book and every physics department on the planet agree with Clausius.
There is no debate in physics about the direction of heat flow.
It ALWAYS flows spontaneously from a higher temperature to a lower temperature.
Why do advocates of the Greenhouse Theory find great difficulty with the thermodynamic meaning of HEAT?
dikranmarsupial wrote:
“PJF, as far as I can see a vacuum flask essentially does only work by preventing heat loss from conduction and convection. They are often silvered to reflect heat back into the flask, but that is reflection, rather than back-radiation.”
Curious. I chose the vacuum flask example for a couple of reasons. Willis’s steel shelled planet is essentially a vacuum flask in space. Willis also mentioned (later in the thread) multi-layered vacuum flasks that do benefit from increased back radiation to keep the contents warm for longer.
David
Why does every physics book and every physics department on the planet agree with Clausius.
There is no debate in physics about the direction of heat flow.
>>>>>>>>>>>>>>>>>
For the precise same reason that they agree with all the work that came after Clausius.
For the precise same reason that the 2nd Law has been reworded many times since Clausius.
For the precise same reason that they differentiate between heat flow and energy flux.
For the precise same reason that SB Law, Conservation of Energy and First Law can ONLY be reconciled with the 2nd Law by interpreting the 2nd Law as net flux. Either that, or we must choose which laws are right and which are wrong.
For the precise reason that 2nd Law interpreted as net flux means they are all right.
dikranmarsupial says, February 9, 2013 at 11:11 am: “Greg House – The “net transfer” is a consequence of viewing the transfer of heat as being mediated by the radiation and absorption of IR photons. Unless you can come up with a mechanism by which a cold body can know which directions it can safely emit a photon without it hitting a warmer body, or for a body to choose not to absorb photons from a cooler body (impossible as there is no way to determine the temperature of the body that emits an individual photon), then an exchange of photons (and hence energy) is inevitable.”
============================================================
No, the problem is that that your “consequence of viewing” is simply a conjecture. To become a scientific fact it has to be proven experimentally first.
Even Wikipedia says: “The modern photon concept was developed gradually by Albert Einstein to explain experimental observations that did not fit the classical wave model of light.” As you can see, experiments come first, then comes a term and knowledge about the properties of those “photons”. In other words, we know for sure about how “photons” behave in real world only from experiments. What is not known from experiments is just a conjecture or fiction.
And this “net” thing has apparently never been proven experimentally, hence it is not a scientific fact.
dikranmarsupial;
If an object cannot be “thermalised” (presumably this means “made warmer”) by IR radiation from cooler objects, the key question is how can the object know which IR photons come from cooler objects (and should not be absorbed) and which come from warmer objects (and should be absorbed).
>>>>>>>>>>>>>>>>>>>>>
A most excellent question!
May I offer a supplemental question?
If the photons from the cooler object are not “thermalised”, where does the energy associated with them go? Does it simply cease to exist, violating too many laws of physics to count by doing so? Does it rotate into another dimension, lurking there until a cold enough object to “thermalise” comes along at which point it leaps out and “thermalises” it? Perhaps it passes through a time warp and emerges at some point in the future as part of a Star Trek episode?
I’m certain Greg House must know the answer to both these questions, and that he derived same from an experiment with cardboard boxes.
MikeB says:
February 9, 2013 at 3:13 am
My math agrees. But, the ratio of the inner to the outer radius does matter.
This is the astounding thing about the formula: increasing the thickness of the shell while holding the mean radius constant reduces the surface temperature. This suggests that adding CO2 to the atmosphere does not necessarily result in an increase in surface temperature, and may even decrease it.
That leaves the GHE in place – the shell definitely heats the surface beyond what it would be without the shell. But, there is a limit, and increasing the depth of absorbing particles may actually have the opposite effect of incrementally decreasing the surface temperature.
There are at least two ways I see this claim potentially overreaching:
1) it could be too much of an idealization – as the shell becomes very thin, there is some point at which it does not intercept all the outgoing radiation from the planet
2) it does not account for any temperature gradient across the width of the shell
And, then there is my earlier objection that separating the shell by a vacuum does not really capture the dynamics on the Earth, where the atmosphere is in actual contact with the surface.
But, I think that at some level where the thickness is beyond critical yet the gradient is “small”, increasing thickness should decrease the surface temperature, at least for the idealized separation by a vacuum.
Things become even more complex as you add additional shells. One could represent an H2O shell, a CO2 shell, and a CH4 shell, for example. Then, one has to allow for transport of heat through conduction and convection. I do not believe anyone can reliably extrapolate the precise behavior of such a complicated system based on intuition and a very idealized back-of-the-envelope calculation for a grossly simplified analogous situation, which I strongly suspect is essentially what underlies the calculations of the climate establishment.
Words are imprecise, and intuition is fallible. Let’s have it mathematically.
Let’s not quibble and sidetrack the conversation from the meat on the table. You know what I mean.
tjfolkerts says:
February 9, 2013 at 6:41 am
Canceled out by neighboring emitters. The emission from one point on the shell to another is, on average, 180 degrees out of phase with that from another point at the same distance on a great circle passing through all three points. In the end, it all has to add up to the balance given. Not instantaneously, but time averaged, and in a very tight statistical band.
MiCro says:
February 9, 2013 at 8:11 am
No, it has a T1^4 – T2^4 term. You can factor that as (T1^2+T2^2)*(T1+T2)*(T1-T2), but T1 – T2 is not all there is.
davidmhoffer says:
February 9, 2013 at 10:30 am
That’s the problem with debating in words. Words are imprecise. Words can mask specious reasoning. Math is absolute.
Bryan says: “There is no debate in physics about the direction of heat flow.”
Just like there is no debate that “heat” is the net flow of energy due to a thermal gradient, and this this flow depends on the temperatures of BOTH objects involved.
Heat flow from a hot object to a cold object = “large”
Heat flow from a hot object to a not-quite-so-cold object = “not-quite-so-large”
So if you surround the earth by “not-quite-so cold” surroundings (ie the atmosphere), rather than “wickedly-cold” surroundings, then the heat flow from the earth’s surface will get reduced. “reduced heat flow out” of course means “more energy retained” which in turn means “the object warms up”.
The cool atmosphere does not “heat the surface” and no one (who is speaking in strict thermodynamic language) would say it does
The cool atmosphere DOES restrict the rate at which heat leaves the surface, which will lead to a warmer surface since the energy input from the sun remains fixed)
In other words, the atmosphere (in conjunction with the sun) “warms” the surface, but does not “heat” the surface.
Why do a̶d̶v̶o̶c̶a̶t̶e̶s̶ opponents of the Greenhouse Theory find great difficulty with the thermodynamic meaning of HEAT = net flow of energy?
PS. The “modern” interpretation of the 2nd law is something like “a system tends toward the most probable macrostate”. Entropy and the direction of net energy flow are a consequence of this. If this paragraph makes no sense to you, then you need to study statistical mechanics more before trying to teach others about the meaning of thermodynamics.
TJFOLKERS SAYS
“The cool atmosphere does not “heat the surface” and no one (who is speaking in strict thermodynamic language) would say it does”
Fine, you agree with me that David Hoffer made a mistake when he claimed that the second law allowed colder objects to ‘heat’ warmer objects.
But then you go and spoil it all by implying that statistical mechanics contradicts classical thermodynamics.
Of course it doesn’t!
Advocates of the Greenhouse Theory continue to muddy the water.
Despite 16 years of flat global temperatures and increasing atmospheric CO2.
Despite millions of years global temperatures leading and increasing atmospheric CO2 lagging.
No historical evidence backs this increasingly futile theory.
All this in spite of Woods historic hunch and subsequent experiment that the greenhouse theory was in error.
@Bryan
” There is no debate in physics about the direction of heat flow.”
Nor is there any debate that all objects above absolute zero radiate ir photons. And it’s photons that carry heat energy between objects in a vacuum.
Depending on the wave length, it is either absorbed and thermalized or re-emitted, reflected, or it doesn’t interact at all, based on the materials emissivity.
@MikeB, if I didn’t use the right terms, please let me know, and i’ll try to use them correctly the next time.
I’ve learned a lot about this stuff, but know I still have a lot to learn.
“based on the materials emissivity”
I think it should read emissivity and absorption spectrum.
“REPLY: Seems like an easy experiment to perform to find out. I think I’ll actually do this – Anthony”
Honestly, Anthony, you have better things to do. The true believers (TB) will ignore or “explain” away anything that goes against their religion, especially facts. Upthread, for example, Phil (February 8, 2013 at 11:26 am) cited an actual practical case, and was ignored. I pointed out another (February 8, 2013 at 7:09 am) and was–surprise!–ignored. A TB asks for textbooks, Willis cites chapter and verse, and the TB says, “See? You can’t deliver!” (I paraphrase.)
So why do I join these pointless discussions? Certainly not because I expect to change anyone’s mind (that leads only to frustration), but because:
1) It helps clarify my own understanding of the subject. If I can’t explain why a TB is wrong on a particular point, I need to learn more. I’ll note here that the Scienceofdoom blog has some nice articles related to this discusson.
2) I’m fascinated by our (human) capacity to build our own realities. This is my opportunity to explore one such “reality” in some depth (yes, extreme fear of CAGW is another).
3) Let’s face it, these guys are a hoot!. You just can’t make this stuff up. Their mangling of the 2nd Law of Thermodynamics, for example, is exceeded only in certain creationist arguments.
Rather than do yet another pointless experiment, Anthony, why not put the onus on the TBs, where it belongs? “Convenional” physics works just fine despite the TB insistence that it can’t; the TBs are the ones with something to prove, and everything to gain by doing so. In these threads I endlessly suggest that TBs actually perform a definitive experiment themselves, e.g. Dr. Roy Spencer’s “Yes Virginia” thought experiment. Strangely, nobody accepts the challenge, but the mere fact of the refusal is to me far more telling than any argument.
Ryan says:
February 7, 2013 at 1:53 am (Edit)
Ummm … ummm … where to start.
This thought experiment of the “steel greenhouse” is an extremely simplified conceptual model. It is the simplest conceptual model I know of that could actually work and that functions using the poorly named “greenhouse effect”.
I use it because you could actually build a scale model of it, and it would actually work using the actual (although poorly named) greenhouse effect. As a result, it explains why the greenhouse effect works, and it makes it clear that the essential mechanism of the greenhouse effect has nothing to do with and does not require the presence of:
1. CO2 or other GHGs
2. An atmosphere
3. Lapse rates
4. Effective radiation heights
To me this is a crucial step, to establish just what it is that we are calling the “greenhouse effect”, and how it works at its simplest. Not how it works in its particular incarnation here on Earth, but how the greenhouse effect itself actually works.
Should we be concerned if “IT IS COMPLETELY WRONG FOR PLANET EARTH!”?
My friend, it’s not a model of the planet Earth, nor was it meant to be. It is a simplified system that likely doesn’t exist anywhere in the universe, a radioactive planet surrounded by a steel shell.
However, this kind of simplified physics model can teach us lots of things that are useful for understanding how a greenhouse effect really works, whether on Earth or anywhere. For example, it shows us that the shell needs to be thermally isolated from the surface, or the greenhouse effect doesn’t work at all … which makes it immediately relevant to the Woods experiment, for obvious reasons … which is why I brought it up.
w.
PJF Reflecting the IR is generally more eficient than absorbing and re-radiating it, as more than 50% can be reflected back. If it were constructed from steel, rather than glass, so that the walls did absorb IR radiation, then it would work as Willis mentions. They key point I was trying to make is that vacuum flasks do work principally by preventing conduction and convection, so whether it works in space very much depends on the materials it is made out of, rather than that it is vaccum flask per se. Sorry to have confused the issue.
And, will people stop arguing about “a colder object cannot heat a warmer one”? That is not what is happening. The flow is always from higher temperature to lower. The argument is not about flow of energy (Watts). It is about accumulation of energy (Joules).
It is exactly like the water analogy I described above. The colder object does not heat the warmer one, it slows down the cooling of the warmer one and, in the time it takes to equilibrate, the warmer object accumulates more energy.
It is so annoying, this confusion between the time rate of change of energy in Watts versus the storage of energy in Joules. The water barrier does not act as a source of water, either. But, it does cause the water to pool up behind it. That’s all that is going on. It is very simple.
johnmarshall says:
February 9, 2013 at 3:25 am
I have said many times that I will not have any truck with a scumball who calls me a liar and accuses me of trying to deceive people. I am not that man.
You are just the latest in a string of officious douchebags who wants to announce your entry into the discussion by starting off with a sleazy, uncited, untrue, and deeply offensive insult.
You can apologize, or you can get ignored from here on out.
Your choice.
w.
Greg House:
At February 9, 2013 at 9:58 am you say to me:
Aha! I am starting to understand your confusion! But you are starting to ‘get it’.
The finger doesn’t do it: the finger only triggers it.
The air conditioner moves heat from the cool air inside your house and pumps that heat to the warmer air outside of your house thus heating the warmer air outside the house.
Other than that, your example is good. Indeed, it is a form of refrigeration system and I used refrigeration systems as an example myself in my post at February 9, 2013 at 3:30 am
Richard
David Socrates says:
February 9, 2013 at 4:05 am
Perhaps … or it might relate to the fact that Tallbloke banned me from his site for saying that a perpetual motion machine wouldn’t work, and I haven’t been back since. He’s still welcome here and visits my threads, but I won’t go to a site where people are banned for their scientific views. He put himself in the same boat as RealClimate and Open Mind, I won’t increase either their or his page count by one, they censor scientific discussion, and that to me puts them beyond the pale.
YMMV, of course,
w.
Greg House – it has not been demonstrated experimentally that photons of IR emitted by a cooler object are never absorbed by a warmer object either, so that isn’t a scientific fact according to your criterion either. This cannot be determined by observing the temperature of macroscopic objects, as you would get the same result whether there was a directional transefer of heat proportional to (T1 – T2) or a bidirectional transfer proportional to T1 in one direction and T2 in the other. If you know of an experiment where the behaviour of the photons themselves is observable, then please provide the details.
Do you agree that heat energy is transmitted by photons, yes or no?
ThePhysicsGuy says:
February 9, 2013 at 7:33 am
First, PhysicsGuy, you fail to distinguish between the NET energy flow (called “heat”), and the two individual energy flows from A to B and B to A, which are not called heat.
For Anthony, let me propose one change in the experiment. Take the measurements as the bulbs cool. This avoids the issue of the changing resistance of the filament of the bulb with temperature, and changing electrical power draw and the like. So … three stages.
1. Put a 100w bulb in a socket, and let it reach equilibrium. Turn it off, and take the temperature every 30 seconds or so as it cools.
2. Repeat the experiment with a 60w bulb.
3. Repeat the experiment with two sockets and two bulbs. First, move the sockets far apart, and let the bulbs come to equilibrium. Turn both off, and immediately move the sockets near to each other. Then measure the cooling rates of the bulbs.
This setup avoids a lot of variables in the first one. The only remaining conflating variable is the fact that both are air-cooled … still, that shouldn’t make any appreciable difference.
I’m glad you’re talking about doing the experiment, Anthony. I was gonna do it, but I don’t have an IR thermometer …
w.
Bryan:
At February 9, 2013 at 12:33 pm you ask David Hoffer
Allow me to provide an answer.
They don’t: indeed, they do understand “the thermodynamic meaning of HEAT”.
The problem is that those who want to disbelieve in the real, physical and observed GHE have no understanding of electromagnetic (EM) energy and how that energy can be converted to heat.
EM radiation is quantised; i.e. it exists as discrete particles called photons. Each photon has an energy and carries a wavelength. If a photon is absorbed by something (e.g. the Earth’s surface) then its energy is absorbed by the something. That energy is added to the something and (unless it is a specific molecule which gains rotational or vibrational energy) that addition of energy raises the temperature of the something. And that happens whatever the temperature of the absorbing something.
Richard
richardscourtney
One of the problems in dealing with a two object heat exchange is that the hotter object is often in addition heated continuously by an external source.
This can cause confusion.
Lets simplify the situation and miss out further heating of the hotter object.
Picture an object at say 80C and another neighbouring object at 20C
If the only heat transfer is by radiation then this is what happens.
The hot object will radiate more intensivly than the cold.
Both hot and cold will absorb each others radiation.
But the hot object will lose internal energy and its temperature will drop.
The cold object will gain internal energy and its temperature will rise.
Its as simple as that.
The cold object does not increase the temperature of the warmer.
MiCro says:
February 8, 2013 at 12:17 pm
Phil. says:
February 8, 2013 at 10:56 am
“Its translational temperature will stay the same its rotational and vibrational temperatures will increase depending on which particular energy levels have just been populated. The excited state will now attempt to lose that excess energy by a combination of collisional deactivation and radiation. Which one is favored will depend on conditions, near the earth’s surface collisions are the major route, up near the tropopause it’s radiation.”
Thanks, if the photon is thermalized, will the molecule’s kinetic temperature increase proportional to the energy of the photon?
The process of thermalization is the collisional exchange of vibrational energy in the excited molecule with its neighbors eventually all the vibrational energy ends up as kinetic energy (translational) shared among all the molecules involved (the atmosphere). Near the Earth’s surface mean time between collisions is less than a nanosec, whereas the characteristic time for emission by CO2* molecules is several orders of magnitude greater.
Willis/Anthony I don’t think the experiment will work as it stands, because the 100W bulb won’t absorb much of the heat emitted by the 60W bulb (and vice versa), but will instead simply pass through the bulb or be reflected off it, so there won’t be much of a change in temperature. Metal spheres heated by e.g. the heating elements from soldering irons would be a better bet (painted black to minimise reflection/maximise absorption). Also, unless the experiment is performed in a vacuum, an objection may be that the transfer of heat is by conduction of heat through the air. I don’t mean to be awkward, its just I want the experiment to be successful and convincing.
Willis,
Yes there are two EM radiation flows from A to B and from B to A. But there is not a “net” heat flow. Heat only goes in one direction, from hot to cold per the 2nd law.
MiCro says:
February 9, 2013 at 8:29 am
Anthony, if the bulbs are say 6″ apart, measure between them, then 3″ on the outside of both, and with a say 60w and 100w bulbs you will get 3 different readings. Also note the filaments will both be similar temps, since they both output similar color spectrums (color temps).
I refer you to the dichroic halogen lamps which improve the efficiency of the lamps by selectively reflecting IR light from the envelope to the light source, thereby increasing its temperature and increasing the proportion of visible emitted. It’s a perfect illustration of the effect discussed in this thread, the product can be purchased in stores, yet every time I mention it it’s ignored and we still get the comments of the idiotic Greg House etc.
http://www.gelighting.com/LightingWeb/emea/images/Halogen_MR16_IR_Lamps_Data_sheet_EN_tcm181-12732.pdf
“Halogen IR Technology
Standard incandescent and halogen lamps lose approximately 76% of the input energy by radiating heat, and convert only 8% into useful light. The PreciseTM IR halogen capsule has multiple layers of very durable, thin, interference film which redirects heat, which would otherwise be wasted, back onto the lamp filament. This increases the filament temperature and allows it to give off more visible light for the same input power.
The increased burning efficiency provides the same light performance with a significantly reduced power input, alternatively allows a longer lamp operating life or a combination of both.”
I have never actually read the exact (German language) statement by Rudolph Clausius, of the Second Law of Thermodynamics; perhaps one of our German posters can dig it up and cite it for us.
I’m under the impression that it translates as:-
“No cyclic machine may have no other effect than to transport heat from a source at one Temperature, to a sink at a higher Temperature.”
Our German friends can verify (or not) that for us too.
Well I don’t know what cyclic machine translates to in German, or what it means in that language, but to me, it implies that heat may move in both directions; or else all bets are off.
And it implies that something other than nothing must happen in addition to heat going from cold to hot; like some work being done for instance.
In any case, Clausius, a Thermodynamicist of impeccable credentials, also used the second law to derive what we call “The Optical Sine Theorem”, which basically says that no optical system can create an image that has a higher radiance than the source object. It also says that no optical system can form an image that is brighter than the image formed by an “Aplanatic ” system; that being a system that is corrected for both spherical aberration and coma, the two simplest of the Seidel aberrations of optical images.
Clausius’ thermodynamic limit also applies to “non-imaging” optics, which is the main discipline behind illumination engineering.
In any case, a one way system, where energy or (heat at least) cannot flow both ways, would be outside the purview of Clausius’ statement.
ThePhysicsGuy:
At February 9, 2013 at 2:54 pm you say
That shows such an immense ignorance of heat, EM radiation and the Second Law that it is mindblowing.
There is no flow of heat: there is a flow of EM radiation in two opposing directions. The energy of the EM radiation is converted to heat when it is absorbed by the surfaces.
I provide a brief explanation of this in my post at February 9, 2013 at 2:38 pm. Please read it.
Richard
” donald penman says:
February 9, 2013 at 10:13 am
What insulation is in contact with the planetary nuclear core? How is this insulation affecting the temperature of the planetary nuclear core? If we put extra mass directly in contact with the nuclear core this would also increase the temperature of the core because heat is lost more slowly from the core.If instead of adding more mass to make a shell you took some of the mass close to the nuclear core and moved it out until it formed a shell as described would the nuclear core not cool down and the energy lost to space increase.”
On Earth:
” Away from tectonic plate boundaries, it is about 25°C per km of depth (1°F per 70 feet of depth) in most of the world.”
http://en.wikipedia.org/wiki/Geothermal_gradient
So if you added 1 km of dirt to earth it would have some effect upon our core temperature- not 25 C, but rather some immeasurable amount.
But bury a box 10′ underground, then put the 1 km dirt above it, and eventually the temperature in the box would rise by about 25 C. And 2 km of dirt- 50 C, etc.
You already have 50 km of rock insulating the mantle temperature and more than thousand km insulating the core. So maybe if added as much as 100 km of dirt it could make noticeable affect on the core temperature- but adding this same amount of mass to the core, should make more of a difference in increasing the core’s temperature.
Earth does not radiate 235 watts per square meter at the surface, instead it’s about 1/10th of a watt per square meter.
And we presently know of no planet [or Jupiter moon] which radiates this much energy.
But it conceivable that planet without plate tectonics and with earth’s level of geothermal energy at various times could emit this much energy.
Or some think so:
“Venus has no evidence of plate tectonics, so this theory states that the interior of the planet heats up (due to the decay of radioactive elements) until material in the mantle is hot enough to force its way to the surface. The subsequent resurfacing event covers most or all of the planet with lava, until the mantle is cool enough for the process to start over.”
http://en.wikipedia.org/wiki/Geology_of_Venus#Global_resurfacing_event
It’s also conceivable that Earth even with it’s plate tectonics did at some point in history emit this level of energy [for a geologically brief time period]- and certainly did during Earth’s planetary formation period- it emit more than this amount of energy- for hundreds of thousands of years.
So life being something like 3.8 billion year old- life may have experienced times when average geothermal energy of Earth for hundreds or thousands of years was this high.
But even this would not be so dire as Hansen’s idea of what could happen- Earth becoming like Venus.
And it should be obvious that such an event would heat Earth more than a greenhouse effect from CO2 [and methane] could reasonably be expected to do- though such dramatic increase geothermal heat would also in addition release vast amount of CO2 and methane.
But for a planet to constantly emit this much energy for billions of years would require it to be quite strange.
@dikranmarsupial
As an aside, my vacuum flask is actually made entirely of steel (stainless, but still a bit magnetic) and the inside is a satin finish rather than highly polished. It’s incredibly effective and informs me that heat loss from radiation is a lot less significant than heat loss from conduction and convection. Near boiling inside, barely more than ambient temp outside. Very handy when out with my telescope under the stars, wishing that greenhouse gas theory was just a bit more warming than imagined.
Bryan;
Its as simple as that.
The cold object does not increase the temperature of the warmer.
>>>>>>>>>>>>>>>>>>>>
If you remove the cold object and replace it with “nothing”, what will happen?
Hint: The temperature of “nothing” is -273 degrees C.
It is as simple as that.
“dikranmarsupial says:
February 9, 2013 at 7:17 am
Will, no, they are not equivalent. The system will reach thermal equilibrium whether or not the vacuum is maintained.
The nuclear core generates energy at a constant rate, the outer shell radiates heat at a rate proportional to the fourth power of its temperature. Thus as the temperature of the outer shell increases, there will come a point where it radiates as much energy as the core generates and thermal equilibrium is reached. This is true whether the energy is transmitted from the core to the shell by radiation, conduction or convection, so whether the internal vacuum is maintained is irrelevant.
Willis is correct, there is no paradox, there is no double counting of energy.
ThePhysicsGuy says:
February 9, 2013 at 7:33 am
Willis Eschenbach says:
February 6, 2013 at 5:01 pm
Here’s an example to consider. Mount a light bulb socket on a table. Insert a 60-watt bulb. Measure the temperature, we’ll say it’s 150°C. Try a 100-watt bulb, it’s hotter, say 200°C.
Now, mount another socket right next to the first one. Insert a 100-watt bulb in one and a 60-watt bulb in the other. What will the final temperatures of the bulbs be?
I say both of them will run hotter in that situation, because A warms B, and B warms A
You also stated that, “..of course it has been proven, many times over. It’s in every college thermo textbook”.. In 1850 Clausius stated, “Heat cannot of itself pass from a colder to a hotter body.” That statement (or similar version) of the Second Law of Thermodynamics has been in every college textbook since that time. Yet you indicate that the colder light bulb will warm the warmer light bulb. So, what will happen with your version of thermodynamics? The 150°C bulb will cause the 200°C. bulb to become warmer. But now the warmer 200°C+ bulb (B) will then warm bulb (A) even more, with the cycle going back and forth until both bulbs thankfully explode, before melting a hole to China.
Care to explain yourself?
REPLY: Seems like an easy experiment to perform to find out. I think I’ll actually do this – Anthony”
It might warm the lightbulbs. But will it warm the filaments?
The lightbulbs will mostly warm due lack of convection.
Having cooler air or simply using a fan will do wonders cooling
the lightbulbs.
But can 60 watts make the 100 watt brighter?
Can two 100 watts make both brighter?
The filaments can increase the bulb temperature.
So if had two filament- one 60 w and other 100 w
in same lightbulb the bulb temperature should increase.
Since the filaments in above example would further apart it
would have less effect. But main affect seems to the inhibition
of convection- which the main way they cool down.
Or light bulbs in a vacuum should be hotter- but not
burn brighter.
Bryan wrote:
“The hot object will radiate more intensivly than the cold.
Both hot and cold will absorb each others radiation.
But the hot object will lose internal energy and its temperature will drop.
The cold object will gain internal energy and its temperature will rise.
Its as simple as that.
The cold object does not increase the temperature of the warmer.”
You’re almost there, Bryan – but you’re just-not-quite-thinking-it-through.
In your thought experiment, in which you acknowledge the hot object will absorb the radiation from the cold object, add another scenario where an 80C object is in isolation. In that isolation situation the 80C object will cool faster than the 80C object next to the 20C object. Why is that? How can it be otherwise than that the 80C object is heated by the radiation from the 20C object?
Not convinced? Change your 20C object to one at 79.999999999C and think about it further.
Bryan:
I am copying all your post at February 9, 2013 at 3:17 pm so it is clear that I am answering all of it.
Your ‘simplification’ causes confusion of a simple problem. Forget it and consider the following when the energy source exists.
Firstly, in the considered cases of the hypothetical planet and the real Earth they have a source of energy input: i.e. the radioactive reaction heating of the hypothetical planet and the Solar energy heating of the Earth.
So they don’t cool from the radiation they emit. Any loss of “internal energy” is replenished by the energy source.
Secondly, you admit that “Both hot and cold will absorb each others radiation” so you admit that they each gain an additional energy input from each other.
The hotter object thus gets the energy input from the energy source AND the energy from the cooler object. So, the hotter object obtains an increase to its energy input (because its input from the energy source does not change but it also gets the energy from the cooler object). This additional energy increases its temperature until it gets hot enough to radiate away ALL the energy it is obtaining.
In other words, and using your phraseology, the cooler object heats the hotter one.
Richard
Anthony,
May I propose an additional twist to your light bulb experiment?
For those who insist that energy flux is a one way street, could you please get one of those 5,000 watt spot lights? If they are correct, you ought to be able to shut off the 60w and 100w light bulbs simply by pointing the 5,000 watt spot light at them.
Willis Eschenbach says:
February 9, 2013 at 2:37 pm
ThePhysicsGuy says:
February 9, 2013 at 7:33 am
Willis Eschenbach says:
February 6, 2013 at 5:01 pm
For Anthony, let me propose one change in the experiment. Take the measurements as the bulbs cool. This avoids the issue of the changing resistance of the filament of the bulb with temperature, and changing electrical power draw and the like. So … three stages.
Wiilis the best way to do the experiment might be to use dichroic halogen bulbs since they are designed to heat up the filaments with IR. It would depend on the exact orientation of the dichroic coating though.
Use two “Precise™ MR16 lamps” When they face each other they should each heat up and increase their respective light outputs. I’d suggest using a narrow angle lamp facing a wide angle one and the wide angle one should get brighter if I understand their tech drawings correctly. By varying the respective lamp powers you could get some good data.
Richardscourtney
Lighten up dude. No reason to be insulting. In using the term “flow”, I was restating what Willis said. “Flow” is just another term for “flux”, It is not uncommon to see the term “heat flux” used in radiation physics. Heat is transferred from the warmer body to the cold by thermal radiation, the radiation being absorbed by the the cooler body and converted to heat. I know the basics of heat transfer and EM radiation.
I am not the one who made the following statement:
“Greg house had claimed there was no “experiment” which shows a cooler object warming a hotter object. I pointed out that I own an apparatus – a microwave oven – which does exactly that by the action of a flow of energy providing electromagnetic radiation to the hotter object.”
The “experiment” is supposed to be in context of the 2nd Law. You can’t add an external source of “work” to it per the 2nd Law. So a microwave, oven, and refrigerator are incorrect examples.
Please go Wikipedia, look up Second Law of Thermodynamics, and read the portion under Clausius Statement for further clarification.
Phil. says, February 9, 2013 at 2:57 pm: “I refer you to the dichroic halogen lamps which improve the efficiency of the lamps by selectively reflecting IR light from the envelope to the light source, thereby increasing its temperature and increasing the proportion of visible emitted. It’s a perfect illustration of the effect discussed in this thread, the product can be purchased in stores,
http://www.gelighting.com/LightingWeb/emea/images/Halogen_MR16_IR_Lamps_Data_sheet_EN_tcm181-12732.pdf”
==============================================================
On this thread above we have the Wood experiment refuting the notion of reflected IR being capable of warming the source. The result in the Wood experiment was zero or negligible.
I can understand the manufacturer though, because an allegedly more efficient lamp can be better sold. I do not necessary think that the manufacturer intentionally lied, because this tale about “back radiation warming” has been around for many decades, so why not “use it”.
ThePhysicsGuy says:
February 9, 2013 at 4:26 pm
Richardscourtney
Lighten up dude. No reason to be insulting. In using the term “flow”, I was restating what Willis said. “Flow” is just another term for “flux”, It is not uncommon to see the term “heat flux” used in radiation physics. Heat is transferred from the warmer body to the cold by thermal radiation, the radiation being absorbed by the the cooler body and converted to heat. I know the basics of heat transfer and EM radiation.
In which case you will be aware that thermal radiation is also transferred from the cooler to the hotter, that radiation being absorbed by the the hotter body and converted to heat also. The only way around that being if the hotter ‘knows’ that a particular photon incident on it came from a colder source and ‘refused’ to absorb it!
Greg House says:
February 9, 2013 at 4:35 pm
Phil. says, February 9, 2013 at 2:57 pm: “I refer you to the dichroic halogen lamps which improve the efficiency of the lamps by selectively reflecting IR light from the envelope to the light source, thereby increasing its temperature and increasing the proportion of visible emitted. It’s a perfect illustration of the effect discussed in this thread, the product can be purchased in stores,
http://www.gelighting.com/LightingWeb/emea/images/Halogen_MR16_IR_Lamps_Data_sheet_EN_tcm181-12732.pdf”
==============================================================
On this thread above we have the Wood experiment refuting the notion of reflected IR being capable of warming the source. The result in the Wood experiment was zero or negligible.
Because of a poorly designed experiment which was incapable of determining that, as detailed above.
I can understand the manufacturer though, because an allegedly more efficient lamp can be better sold. I do not necessary think that the manufacturer intentionally lied, because this tale about “back radiation warming” has been around for many decades, so why not “use it”.
Mr House you’re an idiot and not worth wasting breath over! I guess the manufacturer managed to fool patent offices around the world too!
I note that you made no response concerning the definitive experiments with thermocouple shields, and the associated example calculations from heat transfer texts!
ThePhysicsGuy:
I am replying to your post at February 9, 2013 at 4:26 pm.
A factual statement is not an insult. And my post (at February 9, 2013 at 3:06 pm) did not object to your use of the word “flow”. As I stated, I was objecting to your use of the phrase “heat flow” (in your post at February 9, 2013 at 2:54 pm). As I replied
Clearly, you did not read it.
Instead, you chose to again display your ignorance of what you are trying – but failing – to talk about and wrote
I made that correct statement in response to Greg House claiming there is no demonstration of a cooler object heating a warmer one. A microwave oven does that.
And those of us who understand the subject ARE discussing situations which include “an external source of “work””. The examples are the hypothetical planet with radioactivity providing the heating, the Earth with the Sun providing solar energy, the proposed light bulb (or metal spheres) experiment with electricity providing the heating.
A microwave oven and refrigeration systems are good examples of how energy can be moved from a cooler object to a hotter one and then converted to heat with resulting heating of the hotter one. Indeed, the microwave oven is a very apt example because – like the GHE – it conducts the energy transfer by a flow of EM radiation.
Clearly, ThePhysicsGuy, your alias is a misnomer. You know no physics. You don’t understand the Thermodynamic Laws. And you think wicki. is all the information you need to hide your ignorance: it is not.
Several people on this thread have been explaining the realities to you. It seems your ignorance is so profound that you are incapable of understanding what they have written. Either that or you have chosen not to read the posts which explain your errors.
Richard
Greg House;
I can understand the manufacturer though, because an allegedly more efficient lamp can be better sold. I do not necessary think that the manufacturer intentionally lied, because this tale about “back radiation warming” has been around for many decades, so why not “use it”.
>>>>>>>>>>>
Well Greg, the manufacturer has to provide specifications for their products including the watts consumed and the lumins produced. There’s no room for lying because their competitors would sue them, various consumer groups would sue them, various regulatory bodies would slap them with fines and OEM customers would sue them.
Your marriage to your belief system in the face of compelling contrary evidence is beyond aggravating. I’m very sorry I spent a single moment of my life trying to honestly assist you in understanding the physics, and I consider it a mistake on Anthony’s part to continue allowing you to spout your drivel.
richardscourtney says, February 9, 2013 at 4:58 pm: “And those of us who understand the subject ARE discussing situations which include “an external source of “work””. The examples are the hypothetical planet with radioactivity providing the heating, the Earth with the Sun providing solar energy,”
=============================================================
Of course, you are entitled to discuss “situations which include “an external source of “work””, or the Sun providing solar energy and heating the mother Earth, absolutely. But I’d like to remind you that the trapped/back radiation allegedly warming the surface (according to the IPCC story) and refusing to do that in the real Wood experiment is a different thing. As the Wood experiment demonstrates, the trapped/back radiation won’t work. Other things work, wonderful, but this one does not.
dikranmarsupial says:
February 9, 2013 at 2:34 pm
He is wrong, but you are not right. Yes, a cool object can emit photons which are absorbed by the warmer object. But the warmer object sends back more photons, so overall, the warmer object always heats the cooler one.
But, that is not the dynamic playing out here. See comment above. It isn’t about the flows, it is about the delay in establishing equilibrium which holds back some of the flow to be stored behind the “barrier”. It is about Joules, not Watts.
Does anyone here plan to do any actual math, as I have, or do you just want to shout your opinions at each other? Never mind. It’s the latter. So much heat and so little light on this thread.
davidmhoffer says, February 9, 2013 at 5:31 pm: “Well Greg, the manufacturer has to provide specifications for their products including the watts consumed and the lumins produced. There’s no room for lying because their competitors would sue them, various consumer groups would sue them, various regulatory bodies would slap them with fines and OEM customers would sue them.”
===========================================================
By the way, I have not found these specifications in their data sheet: http://www.gelighting.com/LightingWeb/emea/images/Halogen_MR16_IR_Lamps_Data_sheet_EN_tcm181-12732.pdf. I assume, their reflector might redirect light and one can get a certain spot brighter, but not because of alleged higher temperature of the filament caused by trapped/back radiation. How trapped/back radiation works you can see above (the Wood experiment).
But I like your logic. So, because, let us say, the accused knew that he would be caught, prosecuted and put in jail, we can conclude that he could not have possibly committed the crime. Acquitted!
Of course, the same goes for climate scientists. Because they knew that they would be fired, disrespected and possibly sued they could not have possibly revived the old debunked notion about trapped/back radiation… (see above)! That trapped/back radiation warming must be true!
Bart;
Does anyone here plan to do any actual math,
>>>>>>
I’ve done the math for these bone heads in the past. It changes nothing. Thanks for yours though, I didn’t comment because I thought it stood on its own. to the cardboard box crowd though it is just a bunch of symbols. cardboard defeats science.
I assume, their reflector might redirect light and one can get a certain spot brighter, but not because of alleged higher temperature of the filament caused by trapped/back radiation.
>>>>>>>>>>>>>>>>>
Find me another type of light bulb that produces 6000 cd from 35 watts. with a reflector, without a reflector, with bunny rabbits stapled to it, I don’t give a shit. You’re only comeback is that the manufactuerer must have lied because your cardboard boxes say so. When you resort to reasoning that stupid, this ceases to be a science blog.
[Reply: Are you proposing censorship? — mod.]
Time for me to leave this conversation. As typically happens in these conversations, there is lots of wrong information (which is not getting any better), and lots of slightly wrong information (which is not worth nit-picking), and a little bit of correct information.
Thermodynamics is NOT easy, and statistical mechanics is tougher. “Soundbite Science” will not suffice for grappling with these issues. But at least the right information is in there, so that people might indeed be able to head in the right direction.
[Reply: Are you proposing censorship? — mod.]
I’m stating an opinion. What you guys do about it (or don’t do about it) is entirely up to you.
davidmhoffer says:
February 9, 2013 at 6:31 pm
Thanks. I hope you noted my point above though that, even though the GHE does raise the surface temperature above what it would be without the GHGs, there is no guarantee that an incremental increase in GHGs will lead to an incremental increase in surface temperature, i.e., the local sensitivity is not necessarily positive, much less significantly so.
davidmhoffer says, February 9, 2013 at 6:50 pm: “Find me another type of light bulb that produces 6000 cd from 35 watts. with a reflector, without a reflector, with bunny rabbits stapled to it, I don’t give a shit. You’re only comeback is that the manufactuerer must have lied because your cardboard boxes say so.
============================================================
I said specifically: “I do not necessary think that the manufacturer intentionally lied, because this tale about “back radiation warming” has been around for many decades, so why not “use it”.
A lot of people just believe things, especially when they are told that those things are scientific facts.
As for this allegedly “more efficient lamp”, as I said, their effect seems to be a redirection of light due to the reflector, that is all. You will get one certain spot brighter and the area around it darker than without the reflector.
And please, it was not just “my cardboard box” in the Wood experiment, it was an actual temperature measurement. Or can there be warming without an increase in temperature?
Phil. says:
February 9, 2013 at 2:57 pm
“…yet every time I mention it it’s ignored and we still get the comments of the idiotic Greg House etc.”
Aw, Phil, don’t you think you’re being a little hard on Greg? Rather let’s just say that his intelligence has never been proven experimentally.
@ tjfolkerts
I understand, I also appreciated your feedback. I don’t remember if you had any referenced doc’s above, but if you have some recommendations I’d love to read them.
Bart says:
February 9, 2013 at 7:09 pm
davidmhoffer says:
February 9, 2013 at 6:31 pm
Thanks. I hope you noted my point above though that, even though the GHE does raise the surface temperature above what it would be without the GHGs, there is no guarantee that an incremental increase in GHGs will lead to an incremental increase in surface temperature, i.e., the local sensitivity is not necessarily positive, much less significantly so.
>>>>>>>>>>>>>>>>>>
100% agreement. The system as a whole is ugly complex, the data itself increasingly shows sensitivity is low. Not that we can trust the data all that much given that it is a trend comprised of anomalies averaged together despite coming from completely different temperature regimes. When I ask the warmists to justify averaging an anomaly with a baseline of -30 with an anomaly from a baseline of +30, all I get is crickets chirping. Well, except for Joel Shore who evades the question by claiming that there’s no good single metric so its OK to use a crappy one.
I also challenge the notion that an increase in CO2 increases the mean radiating level and results in a linear temperature response down to earth surface. The first part makes sense, absent feedback mechanisms that change the result, the mean radiating level should be higher. But a linear response down to earth surface? The atmospheric air column is not uniform. Particularly in the tropics there is a band of water vapour as high as 40,000 ppm, but it is mostly close to earth surface. The bulk of LW re-directed doward by CO2 has to originate above this layer. The water vapour being a ghg must resist LW in BOTH directions, in part negating CO2’s effects.
Beyond that you have all the feedbacks which aren’t even close to being understood. The IPCC is even flip flopping now, suggesting that maybe solar cycles are significant after all, and not only that, they think they may have got the sign of the effect wrong. I don’t have the answers, but neither me nor anyone else is gong to get any closer to the right answers with cardboard box experiments done with crude apparatus and a methodology so stupid that even woods himself expressed reservations about it.
I had said:
To which he replied:
ThePhysicsGuy says:
February 9, 2013 at 2:54 pm
Your reading is worse than your comprehension of physics. I did not say anything about a net heat flow. I said:
Your comment about the “net heat flow” clearly establishes that I was right about your lack of comprehension. You desperately need to change your screen name before you get sued for false advertising … It wouldn’t be so bad if your weren’t so puffed up and arrogant about your misunderstandings.
w.
davidmhoffer says:
February 9, 2013 at 8:01 pm
As you are aware, I don’t even think there is valid support for the proposition that humans are significantly responsible for the uptick in atmospheric CO2. The whole thing is a scientific fiasco of the first order, and will be used as a cautionary tale against scientific hubris for generations to come.
Bart;
Can you walk me through what you mean by “integrated 0.2(LOTI+.4)”?
It is very disappointing to read the responses to the original article and to the comments generated therefrom. Surely most readers are familiar with the Latin phrase “reductio ad absurdum”. One does not need a knowledge of physics to realise that the original proposition is absurd especially once it was pointed out that adding more steel shells increased the radiant energy emitted by the sphere without any additional energy being generated by the nuclear source within that sphere. If this was the case then we would long ago have started running blast furnaces with the heat generated by a 5 Watt torch bulb.
Added to this absurdity is the claim that a colder source will increase the temperature of an already hotter body. We all know from personal experience that a hot source will increase the temperature of its colder surroundings. We have never experienced a cold source increasing the temperature of a hotter source for the very reason that to do so would mean that all objects in the Universe would be causing an increased temperature for all other objects, thereby increasing the temperature of the whole of the Universe without any addition of energy. If true, the cosmic microwave back-ground radiation throughout the life span of the Universe would long ago have caused the asteroids and planets to vaporise.
Nature is the final arbiter of what happens in reality, not our individual interpretations of the words/formulae in our physics text books.
As for the Greenhouse Effect, the radiation from a source is calculated by the Stefan-Boltzmann law. This requires a perfect black body, that is, complete absorption of incoming radiation and equally complete emission of the same amount of radiation. It also depends on the body being at thermal equilibrium meaning no change in temperature of the source. The resulting calculated radiation is then proposed as causing an increase in the temperature of the source whose temperature is required to be static. Yet another absurdity.
MattS says, February 7, 2013 at 9:00 pm: “Greg House,
“The original official IPCC version has been broken since it came into existence around 1860″
This statement is simply absurd. The IPCC was created in 1988. Therefore the official IPCC version of GHE or anything else for that matter did not exist before 1988.
================================================================
Nothing absurd here. The official IPCC version of the “greenhouse effect” (as presented here: http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-1-3.html) is much older than the IPCC itself. As far as I know, it all started in 1860, after Tyndall’s experiments with gases. It had been around for 49 years, before professor Wood found a few free hours to deal with the issue.
Hey Willis,
I am not the one who suggested the sun could be warmed by a candle. My statement regarding heat and EM radiation are correct. I thought we were saying the same thing, but I don’t think so since you believe a cooler body can warm a warmer body. Heat flows in one direction per the 2nd Law; from the warmer to the cooler.
Peace, brother.
Bart says: @ February 9, 2013 at 9:24 pm
…I don’t even think there is valid support for the proposition that humans are significantly responsible for the uptick in atmospheric CO2. The whole thing is a scientific fiasco of the first order, and will be used as a cautionary tale against scientific hubris for generations to come.
>>>>>>>>>>>>>>>>>>>>>>>>>>>.
That is only if the eco-luddites don’t manage to send us back to the stone age first. This seems to be the primary goal of the rank and file. A world dictatorship is the goal of those orchestrating the mess and the winner gets to writes history.
Its difficult to know whether to laugh or to cry when reading someof the comments.
Carnot, Clausius and Maxwell might as well have never been born.
The Hoffer tendency use to word ‘heat’ without defining it.
The major error for some of this group is to say infra red radiation is heat – wrong.
For two objects coupled by radiative exchange the net flux is the HEAT flow.
It always flows spontaneously from the higher to the lower temperature.
Another aspect of HEAT is that it is always capable of doing thermodynamic work.
if a heat engine or transducer is placed in the heat flow it will do work (such as drive the piston of a steam engine).
Can we get the piston to work if we take energy from a cold temperature reservoir source and dump the unused part at a higher temperature sink
No we cannot
Can we get the piston to work if we take energy from a high temperature reservoir source and dump the unused part at a lower temperature sink.
Yes we can.
I said previously that there is no debate in physics about the direction of spontaneous heat flow.
It is always from higher to lower temperatures.
Anyone who doubts this needs only to look at any physics text book
tjfolkerts agrees
Joel Shore agrees
Anyone who attended a physics thermodynamics class and remembers what went on agrees.
gbaikie says:
February 9, 2013 at 3:51 pm
“But can 60 watts make the 100 watt brighter?
Can two 100 watts make both brighter?
The filaments can increase the bulb temperature.
So if had two filament- one 60 w and other 100 w
in same light bulb the bulb temperature should increase.
Since the filaments in above example would further apart it
would have less effect. But main affect seems to the inhibition
of convection- which the main way they cool down.
Or light bulbs in a vacuum should be hotter- but not
burn brighter.”
Wired in series
When a 100-watt bulb and a 60-watt bulb are wired in series a 60-watt bulb will glow brighter when the current is turned on. because they share the same current. Since the 100-watt bulb has a thicker filament, which has less resistance, it will glow dimmer than the 60-watt bulb which has a thinner, higher resistance filament.
Wired in parallel
When a 100-watt bulb and a 60-watt bulb are wired in parallel the 100-watt bulb will glow brighter when the current is turned on. This is because In parallel, they share the same voltage. Since the 100-watt bulb has a thicker filament, which has less resistance, it will draw more current and glow brighter than the 60-watt bulb which has a thinner, higher resistance filament.
The rating on a bulb specifies its “room temperature” resistance. Thus a 100-watt bulb rated for 120 volts would have an ideal resistance of
P = IV
P = (V/R)V
P = V2/R
rearranging for R
R = V2/P
R = 1202/100
R = 144 ohms
while a 60-watt bulb rated for 120 volts would have an ideal resistance of
R = V2/P
R = 1202/60
R = 240 ohms
ThePhysicsGuy says, February 9, 2013 at 6:26 am: David Socrates, I took a look at Tallbloke’s “The Great Debate”, and the ground rules establish essentially the “alarmist” GHG model as developed by Trenberth as the starting point. So if you don’t agree with the basic concepts of the model as set forth, too bad, that is not up for debate. No thanks. Sounds more like an indoctrination session.
Well just shows how wrong you can be. I am an arch skeptic. Judging by your generally illogical and scientifically faulty responses here, you don’t get Willis’s thought experiment either . Your loss is Willis’s undoubted gain.
He is right and you, my friend, have a lot to learn about physics.
davidmhoffer says:
February 9, 2013 at 9:46 pm
LOTI refers to the GISTEMP LOTI global mean temperature metric at the WoodForTrees site. It is observed that it is affinely related by those parameters to the derivative of CO2 to a high degree of fidelity. Thus, to get CO2, we initialize at the beginning value in the record, and integrate the affinely mapped temperature relationship from there. The integration was done numerically with a simple rectangular formula (Euler integration).
As you can see, the relationship is pretty darned good, and that was with no optimization for selecting the affine parameters, just pure eyeballing.
Bart says:
February 9, 2013 at 1:28 pm
tjfolkerts says:
February 9, 2013 at 6:41 am
“Suffice it to say that not all the power emitted by the inner surface of the shell hits the planet.”
Canceled out by neighboring emitters.
My explanation here was not very satisfying. I should have replied with a link to Huygen’s Principle. That illustrates how the radiation from the inner shell converges spherically onto the planet’s surface.
Bevan says (February 9, 2013 at 9:56 pm): “Added to this absurdity is the claim that a colder source will increase the temperature of an already hotter body.”
Let’s get specific. Assume somebody carries out Dr. Spencer’s “Yes, Virginia” thought experiment for real:
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
What’s your prediction? In the presence of the cooler bar, the electrically heated bar is
a) warmer
b) cooler
c) the same temp
as it is in the absence of the cooler bar?
Gary Hladik
‘Warm’ as opposed to ‘heat’
The words are similar but not the same.
If you put on a blanket it will help keep you warm.
This assumes you have an internal source of energy.
A blanket round a warm statue will help it from losing heat at a faster rate but will never heat it up.
Likewise the Roy Spencers Virginia post.
What an atmosphere does is similar to a blanket .
It keeps the Earth surface warmer at night but also keeps it cooler by day.
Some have generalised the Yes Virginia ‘warm’ comment to mean the near presence of a cold object will always keep the higher temperature object warmer than it would otherwise be.
This is not correct.
Rearranging the Willis set up above.
The steel hollow sphere is in deep space (-273C) with an initial internal vacuum.
Its heated by an external variable power supply carefully monitored to maintain the temperature at 50C.
The voltage and current are noted, say as V1 and I1, to get power P1
Now suddenly insert an object inside the sphere at -40C ( I can because its a thought experiment! ).
What happens next?
Does the real radiation from the colder object warm the steel sphere when absorbed?
I think not.
To restore the steel sphere to 50C the supplied power (P2) would have to increase for a time.
So P2 > P1
Point being that in these apparent two object problems there is always a third (often ignored )temperature …..the surroundings.
In the example above the cold object at -40C is separated from the surrounding deep space by the steel sphere.
So no Virginia, colder objects don’t always ‘warm’ objects at a higher temperature.
Bryan says:
February 10, 2013 at 2:46 am
“Its difficult to know whether to laugh or to cry when reading someof the comments.”
Let me propose a simple hypothetical to see if I can bridge some of the misunderstandings here.
Let’s say it is -20C outside and I throw my parka outside and let it cool to the ambient temperature. I then walk outside in my shirtsleeves and let my skin cool to 10C. Then I put my parka on and keep it on until my skin temperature goes back up to 20C.
Now, since I’m pretty sure that you agree that I did not get warmer by taking heat from my colder(-20C coat) to my warmer skin :how would you describe what is happening in this process?
Fundamentally, IMO you will probably be better at making sense of the mainstream view of the GHE if you view it in the same terms as the above rather than as what you *think* others are saying.
Cheers, 🙂
Re Gary Hladik says: February 10, 2013 at 6:23 am
My comment said nothing about an energy source such as an electrically heated bar. A hot cup of coffee does not get even hotter if an ice block is placed beside it. However a cup of water at an intermediate temperature, placed between the two, would slow the rate of cooling of the hot cup of coffee.
george e. smith
You are correct to say that in practice true blackbodies may not exist.
A blackbody is a hypothetical object which has a value for absorptivity and emissivity of one, for all wavelengths. Such an object would therefore absorb all electromagnetic radiation which fell on it and would also emit radiation over all wavelengths in a spectrum conforming precisely to Planck’s distribution formula.
In practice, no substance is a perfect blackbody but some materials are a good approximation, especially over a restricted band of wavelengths (e.g. soot).
Although real-world materials are not perfect blackbodies, the concept of an ideal blackbody is useful in physics for determining the theoretical limits of emission by real objects.
Consequently, do you understand that in the steel shell model it does not matter if it the shell is a real blackbody or not? If it is not then the maths gets a bit more complicated but the principle is exactly the same.
Then you say
But Planck’s formula was produced to explain experimental observations? Let’s all agree that it was a big improvement on the ultra-violet catastrophe. If anything it was the theoretical derivation of Planck’s Law which was suspect. In order to derive this formula Planck had to make an unwarranted assumption. He assumed that energy was not infinitely divisible but came in ‘packets’ rather like atoms in matter. He offered no justification for this assumption, except that it gave the desired answer. Planck and everyone else at the time and for years afterwards regarded this as nothing more than a mathematical device, a trick needed to get an answer that matched observation. We now know that in fact Planck had laid the foundations for quantum mechanics
But Stefan’s Law (based on observation) and the Stefan-Boltzmann Law (reinforced with a theoretical derivation) pre-dated Planck and so this cannot possibly explain the derivation of Stefan-Boltzmann.
But of course you know too much not to know this.
Bart,
When I direct you to my fist post it is because it is easier to follow as it uses real numbers and lots of people find screeds of maths difficult to grasp.
However, if you want maths, do it like this….
The core is generating X W/sq.m at the planet surface.
We are told this 235W/sq.m but let’s use X and let’s also say that the shell is emitting Y W/sq.m to space.
In equilibrium, the shell must emit at the same rate as the core is generating, so
Y = X * Area of Planet/Outer Area of Shell
The shell also emits Y W/sq.m back to the planet, but the planet is smaller than the shell so it receives back from the shell (in W/sq.m)
Y * (Inner Area of Shell/ Area of Planet)
The planet also receives X W/sq.m from the core and so the Planet receives in total….
X + Y * (Inner Area of Shell/ Area of Planet) …….W/Sq.m
Substituting for Y, the planet receives (W/sq.m)
X + X * (Area of Planet/outer Area of Shell)* (Inner Area of Shell/ Area of Planet)
=X + X* (outer Area of Shell/inner Area of shell)
For a thin shell this approximates to 2X, that is the planet is now receiving and emitting twice as much radiation as it was without the shell. Its temperature is of course determined by how much it emits.
And so you see, the thicker the shell – the hotter the planet surface becomes. This is the opposite of your conclusion.
I suggest you do some dimensional analysis on your working.
Bevan says (February 10, 2013 at 7:24 am): “My comment said nothing about an energy source such as an electrically heated bar.”
Actually, in the same comment you wrote: “One does not need a knowledge of physics to realise that the original proposition is absurd especially once it was pointed out that adding more steel shells increased the radiant energy emitted by the sphere without any additional energy being generated by the nuclear source within that sphere.”
Since Willis’s “original proposition” explicitly included an energy source, I was just wondering what you’d predict in a similar but potentially testable situation also involving an energy source. But if you don’t want to answer my question, that’s OK.
” However a cup of water at an intermediate temperature, placed between the two, would slow the rate of cooling of the hot cup of coffee.”
This is how the Earth is, space is about 3K, the atm over my house on a 35F (274K) clear sky day was 233K. The atm doesn’t warm my house, but it does keep it warmer than if there wasn’t an atm.
Bryan says:
February 9, 2013 at 3:17 pm
Quite true, you got something right!
This is called the 2 body problem. But the 2-body body problem is not the real world because there are lots of bodies in it.
To see how a cold object makes a warmer on hotter you need to see the 3-body problem.
http://scienceofdoom.com/2010/11/05/the-three-body-problem/
I did point this out earlier but, as Richard Courtney says,
Bryan says (February 10, 2013 at 7:00 am): “Likewise the Roy Spencers Virginia post.
[snip]
So no Virginia, colder objects don’t always ‘warm’ objects at a higher temperature.”
Agreed. So what’s your prediction for the “Yes, Virginia” experiment?
According to the Greek philosopher Plato, we can only see because our eyes emit rays that strike the objects in our field of vision and then bounce back to our eyes so that we can see the object. This was proven in several experiments.
For example, look at an object a few feet away. Now insert your hand between your eyes and the object. Instantly, you can no longer see the object. This is because your hand has blocked the path of the rays from your eyes to the object. It doesn’t matter how many times you perform this experiment, you will get the exact same result. In fact, if you simply close your eyes, you are instantly unable to see anything at all because the rays from your eyes are completely blocked, proving that the rays from your eyes is how you see.
Now I know what some of you are thinking. If that’s true, then why can’t you see in the dark? Well Plato had that figured out too. You see the rays from your eyes don’t work unless they can interact with rays of light. This was also proven by Plato by experimentation also. Go into a dark room. You will notice right away that you can’t see anything. This is because the dark renders the rays from your eyes inert. Simply open a window in the day time (electric lights hadn’t been invented yet so Plato couldn’t just turn on a light) and instantly you can see again. The dark ceases to simply absorb the rays from your eyes, they become activated by the light. Once again though, even when an object is well lit, simply putting your hand between it and your eyes makes it impossible to see it because the rays from your eyes are blocked.
These experiments by Plato have stood up for 2500 years, and have been replicated thousands of times, proving that we see because of rays emitted by our eyes. If you read anything by any physicist that says otherwise, just remember that Plato proved them all wrong in the 5th century BC.
Bevan’
One does not need a knowledge of physics to realise that the original proposition is absurd
>>>>>>>>>>>>>>>>>>
Well now I have seen the light. Well not actually since you can’t see light, Plato proved that in the 5th centurt BC. But now I understand that there is no need to study physics in order to explain physics. The absurdity of the proposition is obvious. This is why people who have never had children can look down their noses and sneer at all the mistakes people who have children are making with their kids. Back seat drivers serve the exact same purpose. They exist so that people who have never driven a car can explain to the driver what the driver is doing wrong.
It is now just so obvious, I don’t know why I failed to grasp this before.
David Hoffer says
” But now I understand that there is no need to study physics in order to explain physics. ”
Well you certainly proved that on this thread.
In fact your explanations of thermodynamics are quite creative.
The second law can be discarded and cold bodies can heat warmer objects
Bart:
At February 9, 2013 at 1:28 pm you say
Sorry, but that ignores a fundamental principle of communication; viz. a transmitter intending to be received needs to transmit in a manner that the receiver can receive. I explain as follows.
Many people have a message they want to proclaim. Hence, their primary purpose is to be heard, and they have no interest in listening. There are many reasons for people wanting to proclaim a message, and these reasons include beliefs, emotional commitments, political values and religious values.
In this thread there are people trying to proclaim their message that all of radiative physics and quantum mechanics is wrong so energy cannot move from a cooler object to heat a warmer one. Why they want to proclaim that is not relevant to the fact that they do.
But, as David Hoffer said, their proclamations are so wrong that they are easily ridiculed with resulting discredit of all AGW-sceptics. Therefore, we need to get them to listen if we are to correct their misunderstanding and, thus, stop the discredit of AGW-scepticism which their proclamations provide.
A person with no interest in listening will not make an effort to listen. Only, somebody who wants to hear will attempt to ‘tune in’ to the language of the speaker. And a person interested in proclaiming a message is not interested in hearing – or allowing to be heard – those who point out that the message is wrong.
Somebody who wants to be heard will attempt to ‘tune in’ to the language of the listener (because a transmitter intending to be received needs to transmit in a manner that the receiver can receive).
The listener has to be engaged before details can be discussed. Clearly, I and others have failed to engage those proclaiming their ignorance as fact, although I and the others have each tried to talk the language of the proclaimers.
Mathematics is not the language of those trying to proclaim that energy cannot move from a cooler object to heat the hotter one. Clearly, anybody with knowledge of mathematics sufficient to discuss the natures of EM radiation and its interactions with matter will know that EM radiation energy is transmitted from a cooler object to an adjacent warmer one.
If we were to engage with the proclaimers then mathematics would be a useful tool for precise descriptions and discussions (which is why science uses it). But mathematics is not their language and we first need to talk to them in their language, not ours, if we are to achieve getting them to listen. We are nowhere near that. At present we listen to them and explain their errors while they ignore the explanations and iterate the errors.
Richard
Bryan;
In fact your explanations of thermodynamics are quite creative.
>>>>>>>>>>>>>>>>>>.
Well obviously. It took many years of study to come up with an explanation that was consistent with the oth, 1st, 2nd, and 3rd laws of thermodynamics, the law of conservation of energy, stefan-boltzmann law, wien’s displacement law, planck’s law plus the work of Einstein, Millicken and Bohr. All I has to do was ask someone who never studied any of it and I would have understood what a complete waste of my time that was. Why didn’t you say something before I wasted all that time?
I’d like to learn how to fly a plane. Are there any people out there who have never flown a plane who would volunteer to teach me how?
Richard,
Bart just might be right. Let’s produce the math showing that Willis’ model is actually a logarithmic function that approaches a limit.
Then we can get some people who have never studied math to explain what limits and logarithmic functions are to us. At that point we will see the light. Except we won’t be able to actually see the light because Plato proved that was impossible in the 5th century BC. Modern photography proves his theory btw, that’s what the “red eye” effect is, the camera captures the rays emerging from people’s eyes.
I’ve decided to learn how to swim. Are there any people out there who have never learned out to swim who can teach me?
davidmhoffer:
re your post to me at February 10, 2013 at 11:36 am.
Yes, of course you are right. I have been p**ing into the wind.
As you say rational argument cannot dispel lunacy. Perhaps confronting them with similar lunacy will reveal their own behaviour to them, but I doubt it. Anyway, I will observe their responses to your Platonic (pun intended) arguments.
Richard
richardscourtney;
Your last comment reminded me of a long since forgotten story that is germane to this thread and which I believe you in particular will get a chuckle out of.
Some decades ago I moved into a new neighbourhood. As it turned out, there were a large number of houses of worship within a few blocks, and mine was the first new family to have moved into the area in about 15 years. As you can imagine, there was considerable competition to recruit the new folks….
About the 10th knock on the door in the first month was by a young man from one of the nearby churches, inviting me to attend services. I politely declined. He asked why. I politely suggested that my reasons were personal. He asked me if I knew that the bible instructed me to do certain things. So, I decided to humour him. I asked him if he could show me where in the bible it said to do those specific things.
He responded that “everyone” knew that’s what the bible said. Really? I asked him what specific version of the bible he ascribed to. King James he said. So, I promptly produced my copy of King James, handed it to him, and asked him to show me where it said what he claimed.
After some furious leafing through the pages, he became more agitated, and repeated his claim that it was in there, and everyone knew that. Really? Have you actually read it, I asked him? I mean not just some here and some there, but actually read it, cover to cover. He admitted that he had not. I told him that I had, which was why I was confident that what he claimed was not true.
At this point he became even more agitated, and began shouting at me using words like “heathen” and “den*er”. I had forgotten the story until just now.
So Richard, I have a question about a passage in Exodus that has always intrigued me. Knowing how well versed you are in scripture, I of course must exclude you from the list of people I might ask to help explain it to me.
Is there anyone out there who has never read Exodus who can help me instead?
richardscourtney says:
February 10, 2013 at 10:42 am
“Mathematics is not the language of those trying to proclaim that energy cannot move from a cooler object to heat the hotter one.”
Net heat energy CANNOT “move” from a colder object to a hotter one. In this, they are correct. Whether radiated, conducted, or convected, the net flow IS always from the hotter object to the colder one. But, that is not the entire story.
In Willis’ setup, that requirement is fulfilled. The shell is colder than the planet, and heat is always moving outward.
But, that movement can be temporarily slowed, and it must respond by establishing a new equilibrium. When that new equilibrium is established, the energy which failed to get out before it could be reestablished is retained, and the planet is hotter than it otherwise would have been.
It is the problem of Watts versus Joules which I have been trying to explain. The Watts can rebalance and go to zero, but the integrated Watts over time, the Joules, do not in general go to zero, as well.
People are arguing past the true issue, and both sides are wrong because they are not viewing it from the proper physical perspective.
MikeB – hold tight. I am working through your write-up to find the flaw in one of our reasonings.
Bart:
In your post at February 10, 2013 at 12:56 pm you say to me
With respect, you are misunderstanding the issue.
They are denying the existence of “net flow” and claiming there is no flow of energy from the cooler to the hotter. Indeed, that is the basis of the disagreement.
Also, I doubt your equilibrium argument will convince them although I hope it will. My doubt is because their argument is not based on evidence and/or logic: their argument is a rationalisation of their desire to believe there is no atmospheric GHE.
Richard
David Hoffer says
” All I has to do was ask someone who never studied any of it and I would have understood what a complete waste of my time that was. Why didn’t you say something before I wasted all that time?”
You are implying that you have studied physics at a university level.
This I doubt from your garbled attempt on this thread.
My university thermodynamics textbooks were
Heat and Thermodynamics by Zemansky
Equilibrium Thermodynamics by Adkins.
I have them still if you want to page and book quote.
I have several others (maybe even one you claim to have).
Your errors are so gross that it is not a question that separates either side of the global warming debate.
Anyone who has successfully passed thermodynamics 101 knows heat does not spontaneously flow from a colder to warmer object.
TJ Folkers agrees
Joel Shore agrees
Both are well known ‘warmists’ who HAVE studied physics so you have no credible support for your erroneous conjecture.
To sum up
If two objects are coupled in a purely radiative exchange ;
Radiation transfer is a two way process
Energy transfer is a two way process
Heat transfer is a one way process always from the higher to a lower temperature object.
Infra Red radiation is not in itself heat.
Heat IS the net radiative flux and is capable of doing thermodynamic work.
All this is apparently above your comprehension but perhaps helps some who follow this thread.
Bart;
People are arguing past the true issue, and both sides are wrong because they are not viewing it from the proper physical perspective.
>>>>>>>>>>>>>>>
I don’t think so Bart. I think the people who understand the physics are using somewhat different terminology from one person to the next, and also explaining the exact same concepts but from different perspectives. tjfolkerts for example said a few things that the nay sayers immediately jumped on as being in disagreement with me. I didn’t see any disagreement at all. But I understood his explanation in the context of both his explanation and the physics, and agreed with it on all fronts.
Similarly, you used the words “temporarily slowed” in your comment. OK, I understand exactly what you meant by that, I even agree with it (as I do with your points about joules and watts btw). If I were to make a comment that the speed of light and hence the energy flux is immutable, but there is a temporary energy flux imbalance, you’d probably agree with that while thinking to yourself that you’d just said that. richardscourtney, mikeb, tjfolkerts, would all (i think) recognize that as two different explanations for the same thing. The nay sayers would jump on it as disagreement.
But at days end we have one group of people repeating over and over again that an experiment unrepresentative of the physical problem and conducted with an apparatus too primitive to measure the effect even if the experiment was representative of the problem, insisting that the results are conclusive. We have another group of people insisting that there is such a thing as “back radiation” but that it just doesn’t “do” anything. We have another group of people shout about the 2nd Law as worded by Clausius in 1850, and regard this as immutable in the face of all the physics that has come since.
I had a raging argument once with a physicist about the behaviour of magnetic flux lines in the classic bar and rail problem. I was using an engineering approach and he was using classic physics. We were both convinced the other was wrong. We formulated a problem to demonstrate….and wound up with the exact same answer. That’s what I see here. Several people with solid math and physics backgrounds but using slightly different terminology to explain the same thing. On the other “side” are people who have never studied math or physics, but are quite certain they know what the math and physics text books say. How to get through to them is beyond me.
I’ve decided to learn French. Is there anyone out there who doesn’t speak French that can help me?
Bryan;
Anyone who has successfully passed thermodynamics 101 knows heat does not spontaneously flow from a colder to warmer object.
TJ Folkers agrees
Joel Shore agrees
>>>>>>>>>>>>>>>>>
So do I.
MikeB says:
February 10, 2013 at 8:01 am
Your mistake is here:
It should have been
Let (Inner Area of Shell/Outer Area of shell) = (R_inner/R_outer)^2. Suppose, for example, that the inner radius stays the same so that R_outer = R_inner + d, where d is the thickness. Then the ratio R_inner/R_outer = R_inner/(R_inner + d). As d increases, the ratio decreases.
Suppose the outer radius stays constant, and the inner radius is given by R_inner = R_outer – d. Then R_inner/R_outer = 1 – d/R_outer. Again, increasing d decreases the ratio. I will leave it as an exercise for you to show that if the mean radius stays constant, the ratio also decreases with thickness. I believe the result probably holds for any radius within the shell held constant, but am not feeling motivated to verify it right now.
davidmhoffer says:
February 10, 2013 at 2:04 pm
Fair enough. It seems it is not possible to explain that they are right about the thermodynamic law, but that it is beside the point, as it does not close off avenues for the accumulation of energy.
David Socrates says:
February 10, 2013 at 1:53 am
Well just shows how wrong you can be. I am an arch skeptic. Judging by your generally illogical and scientifically faulty responses here, you don’t get Willis’s thought experiment either . Your loss is Willis’s undoubted gain.
He is right and you, my friend, have a lot to learn about physics.
___________________________________________________
Yes, David, I do have a lot to learn about physics. I need to go back to university, and learn about how, according to Willis, a lit candle on earth will warm the sun. Yes, I apparently was snoozing during my thermo lecture, and missed that bit. Wait a minute. I have a brilliant idea. On Earth Day, everyone should shut off all their energy sources. That way the sun will cool, and global warming will be a thing of the past!
And all these years I’ve been wasting needless energy in my house, because all I needed to do was place an IR reflecting plate next to my wallboard heater to make it even hotter (face palm).
David, you indicated my responses were “illogical” and “faulty”, yet you offered no specifics whatsoever. Have fun at “The Great Debate”, and don’t forget you tin foil hat.
davidmhoffer says (February 10, 2013 at 2:04 pm): “I had a raging argument once with a physicist about the behaviour of magnetic flux lines in the classic bar and rail problem. I was using an engineering approach and he was using classic physics. We were both convinced the other was wrong. We formulated a problem to demonstrate….and wound up with the exact same answer.”
That’s why I keep referring to Dr. Spencer’s “Yes, Virginia” thought experiment, a more down-to-earth version of so-called “back radiation” than Willis’s steel greenhouse (which I think is terrific, but tends to confuse some people)..
“I’ve decided to learn French. Is there anyone out there who doesn’t speak French that can help me?”
Your Plato, piloting, swimming, Exodus, etc examples are priceless. Thanks for adding more humor to an already amusing thread.
davidmhoffer says, February 9, 2013 at 10:30 am: There is so much wrong with the CAGW meme that can be debunked with good solid science. How to get to that when the skeptic side is littered with people that cling to a belief system that can be debunked by the simplest of observational evidence? People who the warmists can point to in order to discredit skeptics?
and: Tjfolkerts, mikeB, bart, MiCro and others are having a conversation amongst themselves that I for one have learned from. They are refining their collective knowledge and learning from each other rather than shouting belief systems at each other while ignoring valid points that someone else advances. That’s science in action and we need more of it.
David,
Well said. Over at the Talkshop we are currently carrying on that debate “refining collective knowledge and learning from each other” at:
http://tallbloke.wordpress.com/2013/02/04/david-cosserat-atmospheric-thermal-enhancement-part-i-the-great-debate-begins/comment-page-1/#comment-43430
Do drop by any time and help us out.
” davidmhoffer says:
February 10, 2013 at 2:04 pm
Bart;
People are arguing past the true issue, and both sides are wrong because they are not viewing it from the proper physical perspective.
>>>>>>>>>>>>>>>
I don’t think so Bart. I think the people who understand the physics are using somewhat different terminology from one person to the next, and also explaining the exact same concepts but from different perspectives.”
I think Bart is more correct.
“We have another group of people insisting that there is such a thing as “back radiation” but that it just doesn’t “do” anything. ”
Back radiation doesn’t warm the surface and/or doesn’t block a significant amount of heat from leaving earth. And that CO2 levels has even less impact in terms of warming the surface and/or preventing heat from leaving Earth.
Those who believe that “back radiation” is important can even agree that it has small effect, but that this small effect is amplified by “feedback”.
So, “back radiation” does not increase the skin surface temperature enough to be measured-
so we call that as being None. Nor is there a theory physics that says it should.
What causes the skin temperature to increase in temperature is sunlight and back radiation
does not make the skin temperature a higher temperature.
The earth’s atmosphere does not increase the highest surface temperature- the atmosphere
inhibits the sunlight from reaching the surface, because the sunlight is inhibited by the atmosphere the skin surface is heated less.
So the intensity of the sunlight determine skin temperature [greenhouse gases do not have any effect in this regard].
Next part is “back radiation” keeps surface warmer for longer time- it traps the heat. It acts like a blanket. This is also not true- it’s not measurable affect.
Rather than discussing skin surface temperature, some prefer to say back radiation keep the surface [skin and air temperature] warm. But let’s instead focus on skin temperature.
What affects the skin temperature is the heat capacity of the surface. A sidewalk “traps heat”.
And sidewalk traps more heat than sand, because the surface of the concrete heated by the sunlight, conducts the heat of skin temperature better than sand.
What the large affect concerning the surface skin temperature is the solar flux, how well the surface absorbs this energy and conduction of heat below the surface, and convection of heat of the skin to air above it, and evaporation of water.
So the temperature of pan of water in sunlight depends upon how much of the sunlight is absorbed [a dark color bottom of pan affected this] how much water is in the pan, how heat in convected by air, and most important how much water evaporated.
A larger amount of water will take longer to warm [has more capacity to store heat]. And larger volumes have less surface area- therefore the more water takes longer cooler to cool by radiation.
[And Earth’s ocean store vast amount of heat.]
So the “back radiation” blanket effect is suppose to slow the amount of energy being radiated
from the skin surface. And there is little evidence of back radiation inhibiting this radiation
as compared to a vacuum condition. A vacuum condition does not have convection loss [it could cool a lot from evaporation] and rather being seen as “cold” it works well retaining heat.
A jar of warm water in space works almost as well as a thermos bottle- you give the jar silver coating it’s as good or better than thermos bottles on Earth.
What does may a difference is having warm enough air- warmer air will effect how much heat is lost from the skin temperature.
So that’s means we have address whether back radiation keeps the the surface air warm.
And this generally gets us into discussion of lapse rates.
Greg House says:
February 9, 2013 at 6:28 pm
I assume, their reflector might redirect light and one can get a certain spot brighter, but not because of alleged higher temperature of the filament caused by trapped/back radiation.
I suggest you read it again, the white light which is emitted is not effected by the dichroic coating and carries on as if the coating wasn’t there, it is the IR which is reflected and returned to the filament.
“Is there anyone out there who has never read Exodus who can help me instead?”
I read it once.
So Egyptians with help of a smart Jew, developed a welfare state, the state stored
food for times bad harvest. The Jews went to Egypt and everything was wonderful,
but over time they became enslaved, and so they left Egypt.
Seems like good lesson in there.
Bart says:
February 10, 2013 at 2:20 pm
davidmhoffer says:
February 10, 2013 at 2:04 pm
You never realize how something might be read when you write it. I meant “It seems it is not possible to explain to them…” I wasn’t being snarky.
” MiCro says:
February 10, 2013 at 8:07 am
” However a cup of water at an intermediate temperature, placed between the two, would slow the rate of cooling of the hot cup of coffee.”
This is how the Earth is, space is about 3K, the atm over my house on a 35F (274K) clear sky day was 233K. The atm doesn’t warm my house, but it does keep it warmer than if there wasn’t an atm.”
Keeping a house warm is space is easy.
The problem with say the international space station [ISS] is getting rid of heat.
You won’t read any about the ISS having some exotic powerful heater, instead
the big component is the large radiators to shed heat. And designed not absorb
much heat- it isn’t black, it’s white and reflective.
Both cooling and heating are quite simple things to do in the space environment.
A refrigerator is dead easy- no motors required. And heating is as easy.
So in space your heating and cooling energy cost are minimal- would be 1/10th
of places of Europe.
The heating used to heat of house in the permanent dark crater of the Moon with
surface temperatures around 30 K, would be less than in the UK.
The only reason the Apollo 13 crew were cold, was because their vehicle was designed
to be cold in the space environment [because that is the hard part- keeping
it cool enough- heating it was easy [assuming you didn’t need to conserve all
the battery power]
gbaikie commented
That is a very good point!
It does reminds me of something that goes nicely with your explanation.
In the 80’s I was a Application Engineer for some electronic design tools, and one of my customers was NASA GSFC. One of the groups I worked with was redesigning circuit boards for the Space Shuttle, as many of the original components where no longer available. In many cases hundreds of chips would now fit into a single programmable array, while reducing power dissipation and heat generation at the same time. But while looking at one of the designs, I noticed a big resistor on a board from power to ground, and asked why, thinking saving power and heat would be a good thing. I was told that the Shuttle was design around a certain power and heat load, and changing either would require extensive analysis and a lot of re-engineering, it was simpler and a lot cheaper to just dissipate the power with a resistor.
Gary Hladik;
Your Plato, piloting, swimming, Exodus, etc examples are priceless. Thanks for adding more humor to an already amusing thread.
>>>>>>>>>>>>>
Well thanks! But I am re-thinking the whole enterprise. I mean, why should I take piloting lessons when I can teach them instead? I mean, isn’t that the saying? Them’s that can do, and them’s that can’t teach? Well I can’t, so I’m qualified!
Who wants to learn how to fly? How’s $150 an hour sound?
davidmhoffer says:
February 10, 2013 at 6:47 pm
Flying is dangerous, something you’d want the best teacher available for, and everyone knows getting the best costs more, so could I pay you $300/hr? I want the best teaching me!
davidmhoffer:
David, this is a reply to your anecdote at February 10, 2013 at 12:51 pm. It is somewhat off-topic and is not intended to initiate a religious discussion. Indeed, I have taken time to consider whether to make this response for fear that it could divert the thread. On reflection, I have decided to make the point but I will refuse to be side-tracked into religious discussion.
In this thread we have people who have convinced themselves that physical laws mean what they want the laws to say, and those laws don’t mean what the laws do say. Your anecdote was about people who have convinced themselves that the Bible says what they want it to say and not what it does. There are many people who demean the Bible by pretending it is all literal truth so their understanding of it alone must be accepted.
You say to me
I don’t know the passage you don’t want me to explain, but I think Exodus 34: 29-35 contains an example which is germane to understanding what is happening in this thread.
Those verses say Moses repeatedly met God in secret and this resulted in his face “shining” (whatever that means). So, he put a veil over his face so people would not be disturbed by seeing his face “shining”.
St Paul gives his opinion of Moses’ “shining” face in 2Corinthians 3: 12-13. Although some translations are not as clear about it as the original text, Paul suggests Moses’ veil was to hide when the “shining” had faded. In other words, Paul suggests that Moses pretended the “shining” effect at least some of the time so Moses was a fraudster.
Ignoring whether Moses was a real or mythical person, this poses a problem for those who claim all of the Bible is literal truth: the two passages cannot be equated within their claim. i.e.
Was Moses genuine so Paul was mistaken?
Or
Was Moses a trickster because Paul’s opinions are divinely inspired?
Moses and Paul can’t both be right if you wish to proclaim the Bible is literal truth.
The paradox (and many similar paradoxes between Biblical passages) only exists for people who want to belittle the Bible as containing only literal truth from which they can select to justify their prejudices. In reality, the Bible contains many truths of many kinds. But they want the Bible to say what they want it to say instead of what it does say. And when the paradox is pointed out they make all kinds of illogical rationalisations.
Similarly, those who want to misrepresent the Thermodynamic Laws on this thread are claiming those Laws say what they want to believe and they are rejecting what the Laws do say. But they cannot see the paradox that if what they are claiming were true then refrigerators, pulse pumps, microwave ovens and several other devices could not exist. And when the paradox is pointed out they make all kinds of illogical rationalisations.
Richard
richardscourtney says
“Similarly, those who want to misrepresent the Thermodynamic Laws on this thread are claiming those Laws say what they want to believe and they are rejecting what the Laws do say. But they cannot see the paradox that if what they are claiming were true then refrigerators, pulse pumps, microwave ovens and several other devices could not exist. And when the paradox is pointed out they make all kinds of illogical rationalisations.”
Right after the explanation of the Carnot Cycle in a thermodynamics textbook comes the explanation of how the refrigerator works.
A refrigerator does not spontaneously transfer heat from a colder to a higher temperature.
It has to be supplied with energy to do this.
So instead of the Carnot heat engine doing work the refrigerator requires work to be done on it.
You obviously have never studied thermodynamics or you would know this.
Still its never to late to learn.
Anyway David now agrees that heat cannot spontaneously go from a lower to a higher temperature.
Bryan says:
February 11, 2013 at 8:38 am
Bryan, as I admitted up the page I still have lots of things to learn, so maybe I don’t understand your point, but let me offer another explanation for the disagreement.
The 2nd Law was developed prior to Quantum Mechanics, and while what you describe is true in Thermodynamics, QM demands the exchange of photons that have a defined energy, and therefore energy does transfer from the colder object to the warmer object, physics demands it. But the net energy flow as Thermodynamics demands does only flow from warmer to colder.
MiCro;
Hilarious!
Richard;
I knew you would “get it”.
Bryan;
I knew you would not.
MiCro says
“and therefore energy does transfer from the colder object to the warmer object, physics demands it. But the net energy flow as Thermodynamics demands does only flow from warmer to colder.”
That’s pretty much how I see it.
I would add the net energy flow is called heat and it is capable of doing thermodynamic work.
As I said above……
“To sum up
If two objects are coupled in a purely radiative exchange ;
Radiation transfer is a two way process
Energy transfer is a two way process
Heat transfer is a one way process always from the higher to a lower temperature object.
Infra Red radiation is not in itself heat.
Heat IS the net radiative flux and is capable of doing thermodynamic work.”
Bryan says:
February 11, 2013 at 11:23 am
So, let me ask this, If you have a pot of water, next to a roaring fire, that’s heated by IR, and the pot boils, is that not IR doing work? This may just be a terminology issue, where I’m not using the terms correctly.
I thought this picture of the James Webb Telescope was appropriate for this discussion.
http://upload.wikimedia.org/wikipedia/commons/1/1c/James_Webb_Telescope_DC.jpg
Bryan;
I would add the net energy flow is called heat and it is capable of doing thermodynamic work.
>>>>>>>>>>>>>>>>
The thing is Bryan, you are very close to getting it right. If you’d stop telling other people how stupid they are, you might actually learn something.
Heat cannot do thermodynamic work. Heat is the product of thermodynamic work. Or more correctly, one possible product. You are also using the word “flow” where you should be using the word “flux”. MiCro is on the right track.
David says
“Heat cannot do thermodynamic work. Heat is the product of thermodynamic work. Or more correctly, one possible product”.
More ‘creative’ thermodynamics here, which is unfortunately wrong.
MiCro
“If you have a pot of water, next to a roaring fire, that’s heated by IR, and the pot boils, is that not IR doing work? ”
The net flux of IR would be from fire to pot
If the pot was very shiny or if the pot was black would determine how much net IR was absorbed.
That’s one of the reasons why its important to stress that IR radiation is not in itself heat.
The usual situation is mostly absorption by the pot and conduction to the water.
This increases the temperature of the water and the water gains internal energy from the heat supplied .
If a heat engine were placed between fire and pot it would be capable of driving a piston or doing work.
However in this case the thermal energy from a high temperature is increasing the thermal energy of the water so strictly speaking no mechanical work is done
All in accordance with the laws of thermodynamics
Bryan says:
February 11, 2013 at 1:06 pm
It’s energy. But why does an IR lamp make you warm, where visible light which has more energy does not warm you?
Bryan
Replace the pot with a photo voltaic cell. Assume the PV cell is 100% efficient. Use the electricity to charge a battery.
Question:
Where is the “heat”?
Answer:
There is none. There never was. Since the work done by the energy flux went entirely into charging the battery, no heat was generated. If no heat was generated, there was none to flow in the first place. And don’t start whining that the battery would heat up from being charged or anything else stupid like that, you know very well that represents only a fraction of the energy transfer we’re talking about. Nor should you whine about a 100% efficient pv cell not existing, you know that’s not the point too.
And how about responding under your full name. What are you afraid of?
“””””…..MikeB says:
February 10, 2013 at 7:50 am
george e. smith
You are correct to say that in practice true blackbodies may not exist.
A blackbody is a hypothetical object which has a value for absorptivity and emissivity of one, for all wavelengths. Such an object would therefore absorb all electromagnetic radiation which fell on it and would also emit radiation over all wavelengths in a spectrum conforming precisely to Planck’s distribution formula.
.
Consequently, do you understand that in the steel shell model it does not matter if it the shell is a real blackbody or not? If it is not then the maths gets a bit more complicated but the principle is exactly the same.
Then you say
Planck’s black body radiation derivation explains no actual experimental observations anybody ever made, that I am aware of.
But Planck’s formula was produced to explain experimental observations? Let’s all agree that it was a big improvement on the ultra-violet catastrophe. If anything it was the theoretical derivation of Planck’s Law which was suspect. In order to derive this formula Planck had to make an unwarranted assumption. He assumed that energy was not infinitely divisible but came in ‘packets’ rather like atoms in matter. He offered no justification for this assumption, except that it gave the desired answer. Planck and everyone else at the time and for years afterwards regarded this as nothing more than a mathematical device, a trick needed to get an answer that matched observation. We now know that in fact Planck had laid the foundations for quantum mechanics……”””””
Well MikeB, most often in science, observers (experimentalists) make real world observations and/or measurements on some real world phenomena. Otherwise it does not belong in the purview of science.
That means there is some physical system, that is exhibiting the phenomenon. People do not obseve phenomena that are not exhibited by objects or systems that do not exist; and hence can exhibit nothing to obseve.
Black bodies do NOT exist, and I cited simply physics reasons why they cannot.
Now the real world is often too complicated for us to possibly know or see what is going on let alone explain it.
So we create “models” to which we assign certain properties and behaviors which we CAN study/compute/whatever, using mathematics; all of which is as fictional as our models. We compare our calculations of the expected performance of our model to the observed performance of the real system we are trying to explain. Any time we find a discrepancy between what we reliably observe, and what our models claim should occur, we then alter our description of our model, until it predicts better results that are closer to the real system.
We NEVER assign properties to our model, which we know a priori, the real system cannot possibly have; because that is a fatal difference between the reality we observe, and the modelled calculations prediction.
Nobody ever observed ANY object or system which can absorb any amount of EM radiation at any possible frequency or wavelength, which we believe covers all vallues except zero and infinity.
Nobody ever made observations of any real object or system emitting EM radiation at any possible frequency or wavelength.
Nobody ever made any observations of any object or system where everything in the system was at a single Temperature, with no heat able to move (net) in any direction in such an isothermal system. The sun is NOT isothermal and as it happens, it does not emit a Planck formula black body spectrum. A whole lot is known about what Temperatures various bits of the sun are and what that implies.
Purportedly, Planck’s actual derivation of the radiation law, requires an equally fictional object which also cannot exist; namely an isothermal cavity with perfectly reflecting conductive walls, that also do not conduct “heat”. Nothing can escape from this fictional object except radiation from a “small hole” in the otherwise perfectly reflecting walls.
Now in the real actual observable world where real observations and measurments can be made, most good reflectors of EM radiation, and especially over wide frequency ranges, turn out to be metals, and as it happens metals are also among the best conductors of “heat”
In particular, Silver, which has the highest known visible radiation reflectance among all metals, also has the highest electrical conductivity. Optically transparent media (in the bulk) typically are not electrically conductive.
A few exotic things, like Indium Tin Oxide, are both optically transparent, and electrically conductive (weakly) in thin films.
My point is the hypothetical black body, of Planck’s theory, is assigned properties which we knwo that nothing real can possess, and in his historic development of the Planck radiation formula he studies a cavity model, which also has properties no real cavity has been observed to have.
Hey let’s face it, the Planck et al theory of BB radiation is both remarkable and of great practical utility; and yes, I concede that it led to the development of quantum theory; but one thing it did NOT do is limit Planckian thermal radiation to only certain allowed frequenciesor wavelengths, as happens with atomic and molecula line/band spectra, that truly are the consequence of specific quantum numbers of real atoms and molecules.
Planck only said that the radiation must consist of packets (photons) whose packet energy was related to the frequency via E = hf. We can take this to mean that each cycle of the frequency of the photon wave packet, has a quantity of action (energy x time) of value h .
But since no restrictions are placed on the value of f, photons of any possible energy value are allowed. In that sense BB radiation is not quantized in the sense that atomic spectra radiations are, where only certain frequencies are permitted.
So the history suggests, that when researchers were unable to explain the results of real observations on real materials, and systems; they chose to construct a completely fictional ideal, and non existing object; a “Black Body” to which they ascribed properties no object has exhibited.
Yes it is useful in approximating what real objects seem to do.
I’m not aware that Planck’s derivation gives any insight into what happens when you don’t have an isothermal cavity , with perfectly reflecting perfectly conducting walls.
So I see no basis for asserting that Willis’s steels shell planet, which is not isothermal, and not in thermal equilibrium, emits anything like black body radiation.
I can’t see merit, in constructing mind exercise objects, which we know do not exist, such as steel shell planets; it teaches us nothing about real planets with real atmospheres that do permit thermal interractions.
Plancks surprise assertion of packets of energy E = hf came in 1900. Einstein’s similar assertion to explain the photoelectric effect came in 1905, and won him his Nobel Physics prize.
To this day, I am not aware of any classical explanation for the observations of the photo-electric effect.
Plancks 1900 declaration of energy packets (of all possible energy values), is no where near as arbitrary and unwarranted, as Nils Bohr’s quite irrational suspension of the Hertz/Maxwell observations that accelerated electric charges always radiate during acceleration.
It seems they still do despite Bohr, and Arnold Sommerfeld, who nevertheless gave us so much insight into observed atomic line spectra.
So quantum mechanics bailed them out by effectively dispensing with the orbital acceleration, rather than the requirement that radiation not happen sometimes.
The early chemists could be said to have quantized chemistry, by saying you could only get matter in certain sized chunks with only a limited number of sizes; maybe 92, later expanded to maybe 1000 or so different chunks. Planck placed no restrictions on the size of the chunks, only that it (energy) was related to the infinitely variable frequency f via h. so he didn’t really quantize thermal radiation in the sense that Bohr/Sommerfeld and other had for atomic line spectra, or the molecular spectr that haunt the GHG phenomenon.
MiCro;
But why does an IR lamp make you warm, where visible light which has more energy does not warm you?
>>>>>>>>>>>>>>
What do you mean by “more energy”? Per what? 10 w/m2 of IR and 10 w/m2 of visible light are each…rough math…approximation….round off….. hmmm…. 10 w/m2 😉
The visible light on the other hand tends to reflect off of you instead of being absorbed. Well, that’s what people like Bryan will try and tell you anyway. Everyone knows that Plato proved in 5th century BC that light only works by interacting with the rays from your eyes.
@ davidmhoffer
Per photon.
davidmhoffer
Here’s an equation.
Its from a thermodynamics book so you wont have seen it before.
Q = dU – W
Q= heat which flows into the system
dU = the change in internal energy of the system
W = work done by the system
So if heat is added to the system by water say absorbing IR radiation from a higher temperature emitter, then the water temperature will rise (dU) minus any work done by the system (W)
The above equation is a restatement of conservation of energy in a form suitable for discussing thermal processes.
Your post of February 11, 2013 at 2:20 pm contains so many mistakes I must assume its you having a deliberate ‘wind up’
On the other hand your head must be so full of new correct information as a result of this thread that it will take you some time to assimilate it.
Friends:
I am to leave on another of my frequent but irregular absences which take me out of communication. Hence, I cannot continue to participate in this discussion. I hope that leaves none of my points ‘hanging’ and if it does then I have genuinely overlooked them.
Richard
Here’s an equation
>>>>>>>>>>>>>>>>>>>>>
OOOOOOOOOOOOOOOOH!
Bryan has an equation!
No answer to my question, but he’s got an equation.
And still anonymous.
“”””””…..davidmhoffer says:
February 11, 2013 at 3:02 pm
MiCro;
But why does an IR lamp make you warm, where visible light which has more energy does not warm you?
>>>>>>>>>>>>>>
What do you mean by “more energy”? Per what? 10 w/m2 of IR and 10 w/m2 of visible light are each…rough math…approximation….round off….. hmmm…. 10 w/m2 😉
The visible light on the other hand tends to reflect off of you instead of being absorbed. Well, that’s what people like Bryan will try and tell you anyway. Everyone knows that Plato proved in 5th century BC that light only works by interacting with the rays from your eyes……”””””
The following is an exact quote from:- “The Science of Color.” published by The Committee on Colorimetry, of the Optical Society of America, which Society is one of the founding bodies of The American Institute of Physics.
Specifically it is from Chapter 7 titled “Psychophysics of Color.”.
Item #1 after a brief introduction is:- “Definition of Light.”
“””””…..The relations between radiant energy, light, and visual sensation can be defined in the following generalized and qualitative manner: Light is the aspect of radiant energy of which a human observer is aware through the visual sensations which arise from the stimulation of the retina of the eye……””””
It then goes on to say:- “Light as thus defined is a psychphysical concept. Light is not identified with either radiant energy or visual sensation. Light is one of many conceivable aspects of radiant energy.”
Ergo, Light by definition is visible; so there is NO ultra-violet light or NO infra-red light Both of those are invisible.
This distinction is fortified by the fact that we use completely different units for “light” from what are used for other EM radiation phenomena., and we use Photometry rather than Radiometry to talk about light quantities.
So “Luminous Energy” in Talbots replaces Joules in Radiometry.
Luminous flux in lumens replaces Watts
Luminous intensity in Candela replaces Watts per steradian
Illuminance in lux (Lumens per m^2) replaces irradiance ; and so on.
Which is why I have said, “We get no light from the sun; we conjure it all up in our eyes and brains from certain Radiant energy frequencies.”
Likewise, we get no “heat” from the sun; heat is just one possible form we can convert incoming solar radiant energy into.
Photons know nothing about Temperature. Any single photon arriving from the sun, which can be uniquely detected by certain photo-multiplier tubes, tells us absolutely nothing about the Temperature of the sun. Temperature is a macroscopic property of large assemblages of material particles which physically interract with each other (collide).
If we collect a very large number of single photons from the sun and determine their statistics, it then becomes possible to approximate the emitting surface Temperature of the sun, by assuming that Planck’s radiation formula works well enough; but since that surface is not isotropic, the actual spectrum differs from Planckian black body radiation.
Lots of people have gotten Radiation and heat so much identified as being the same thing, that we still teach students that radiation is a method of transporting heat. Well so is a grocery shopping cart; just fill it with some bags of coal or charcoal.
But then on a recent visit to my Alma Mater Physics Department, one of the top Physics Professors told me, that they still teach that E = I x R is Ohm’s law.
It isn’t of course; Ohm’s law says “R is a constant” E = IR is simply a definition of R in a linear system.
Most electrical circuits; and virtually all the ones in your computer, do not obey Ohm’s law
So we should reserve use of “light” to that psycho-physical aspect of visible EM radiation, and not confuse it with the radiation itself. Otherwise people will never know what we are talking about.
MiCro says:
February 11, 2013 at 5:36 pm
@ davidmhoffer
Per photon.
>>>>>>>>>>>>>>>
yikes!
No idea. I’d have to go research that.
george e smith? comment?
I suspect ratio of photons simply reflected vs absorbed would be a big part of the equation. Albedo varies with frequency.
george e smith says;
one of the top Physics Professors told me, that they still teach that E = I x R is Ohm’s law.
>>>>>>>>>>>>>>
I think most universities do. But even 30+ years ago they also made sure we understood that it was an approximation. Under a lot of circumstances it isn’t even a good approximation. An incandescent light bulb for example has an R of about 1 ohm at room temp, and 100 ohms at operating temp. Only a couple orders of magnitude….
Then we discovered there’s a small capacitance and inductance associated even with a bit of wire….
Then some moron came up with these silly semi-conductors where there’s holes in the darn things and the holes flow rather than the electrons… well sorta kinda… gotta start the explanation somewhere, that’s as good a place as any…. but E=IR is pretty much out the window by then.
george e. smith says (February 11, 2013 at 2:31 pm): “I can’t see merit, in constructing mind exercise objects, which we know do not exist, such as steel shell planets; it teaches us nothing about real planets with real atmospheres that do permit thermal interractions.”
How about something that should be constructible, like Dr. Spencer’s “Yes, Virginia” thought experiment?
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
If the experiment is ever performed, what do you predict the result will be? In the presence of the cooler bar, the electrically heated bar is
a) warmer
b) cooler
c) the same temp
as it is in the absence of the cooler bar?
davidmhoffer says:
February 11, 2013 at 2:20 pm
Ummm … no. Energy cannot be created or destroyed. However, it can be converted from one form to another. Light, for example is energy in the form of radiation. It can be converted into a variety of different forms. It doesn’t contain those forms of energy, but it can be converted into those forms.
For example, in a solar cell, part of the energy that strikes it (in the form of radiation) it is converted into another form, thermal energy. Another part of the radiation is converted into a second form, electricity.
Note, please, that “heat” is the NET flow of energy to and from an object. All objects are constantly losing energy and gaining energy. It is the only the NET energy that is called “heat”.
To summarize:
1. Light (or infrared) is energy in the form of radiation. It does not “contain heat”, that statement makes no sense.
2. Energy in the form of radiation can be converted into other forms of energy, including mechanical energy (e.g in a Crooke’s radioscopy), chemical energy (in a leaf), atomic bond energy (raising the energy levels the absorbing substance), or electrical energy (solar cell), or thermal energy.
3. Whenever radiation is absorbed by anything, it changes form, it is no longer energy in the form of radiation.
4. None of these energy conversions are perfectly efficient, so at least some of the energy is lost as thermal energy going into the objects or the environment.
w.
willis;
Ummm … no. Energy cannot be created or destroyed. However, it can be converted from one form to another.
>>>>>>>>>>>>
that was my point.
consider a line from say 1 million km from the sun (A) to 1 million km from earth (B) . Is there an energy flux from A to B? yes. Over 1300 w/m2 at B. Is there heat? No. There is no heat until the energy flux is absorbed by matter. At which point it might be turned into heat. Or electricity. or something else. But if it is turned into heat, then the heat is the product of the work being done. No work, no heat. From the path of A to B, no work is done, no heat is created, no heat flows.
” MiCro says:
February 11, 2013 at 1:32 pm
Bryan says:
February 11, 2013 at 1:06 pm
That’s one of the reasons why its important to stress that IR radiation is not in itself heat.
It’s energy. But why does an IR lamp make you warm, where visible light which has more energy does not warm you?”
The simple answer is IR lamp is designed to give heat. The difference between a once standard
100 watt incandescent bulb and IR lamp is filament would larger and not glow as bright, and use the same or less watts [power]. So if increased the volts to IR lamp it would glow brighter [probably burn out cause not designed to handle more power- and not be bright as light bulb].
IR lamps may have reflective backing [spotlight and other light bulbs can also have reflective backing to give directed light] if these are IR lamps you mean, having reflective backing is another reason it can heat thing better than light bulb.
But your typically spotlight also deliver a fair amount of heat to area they are pointed at.
Up the thread, there is the halogen reflector lamps:
http://www.gelighting.com/LightingWeb/emea/images/Halogen_MR16_IR_Lamps_Data_sheet_EN_tcm181-12732.pdf
A problem with “normal” halogen reflector lamps is they give a lot heat-
So above ad it says they reflect this heat back the bulb to give energy [so the vaporized
tungsten gets redeposited on filament “better”]. Which apparently allows one *design*
higher filament temperature than “normal” halogen reflector lamps [and reduces the
amount heat- which is problem they trying to minimize. The coating probably also reduces
UV- which could be problem for some uses- other uses may want the UV].
As for the shuttle using old specs and not changing them, this common with all
spacecraft- if not broken don’t fix- is strong tendency due high cost development
and where one mistake causes hundreds millions dollars in damage- and/or loss
of crew.
Some more on Apollo 13:
“There were 2,181 ampere hours in the LM batteries. Ground controllers carefully worked out a procedure where the CM batteries were charged with LM power. All noncritical systems were turned off and energy consumption was reduced to 1/5, which resulted in having 20 percent of LM electrical power left when Aquarius was jettisoned. ”
So car battery has 220 ampere hours- so power of about 10 car batteries. That is all power which was available.
“Water was the main consumable concern. It was estimated that the crew would run out of water about five hours before Earth re-entry, which was calculated at around 151 hours. ”
http://www.nasa.gov/mission_pages/apollo/missions/apollo13.html
So 2,181 ampere hours to last 151 hours plus had to charge the Command Module with this charge.
The say, above: The temperature dropped to 38 degrees Fahrenheit and condensation formed on all the walls.
Other sources give different number:
“Further examination of Biomedical Results of Apollo reveals that actual cabin temperatures dropped to a low of 43°F ”
http://history.nasa.gov/SP-4029/Apollo_18-39_CModule_Cabin_Temperature_History.htm
And:
“The temperature dropped to around 40 degrees by the end of the trip. ”
http://www.lpi.usra.edu/lunar/missions/apollo/apollo_13/return/
Wow, this thread is still going ?
Ok, here’s some more empirical evidence regarding two light bulbs in close proximity and the alleged “cross-heating” that some folks predict will happen.
You can purchase commercially an integrating sphere that will accept multiple light bulbs through ports in the exterior. By turning on or off different combinations of these light bulbs it is possible to create a range of radiance values leaving an exit port (radiance is the amount of light per unit of solid angle (how much light is in a cone leaving the exit port)). If you add a shutter between one of the lamps and the entrance to the sphere you can get a variable light source with a pretty constant spectra distribution. This is pretty standard instrumentation used to test digital camera sensor chips and radiometers.
Very high quality power supplies with highly regulated current levels are used to excite the lamps. So we are controlling exactly the amount of power entering the sphere (assuming the lamps are on all the time rather than in a pulsed mode).
So we have two hypothesis;
1) The lamps have no discernible effect on each other and the total light output is a simple linear sum of the individual output of each lamp. I.E. we can measure lamp #1’s output (with lamp #2 OFF) and likewise measure lamp #2’s output (with lamp #1 OFF) and add those spectral measurements together and get what the total will be when both lamps are ON.
OR
2) Lamp # 1 (when ON) heats lamp #2 (which is also ON) and the light output of lamp #2 goes up (it’s an incandescent lamp, it’s output is proportional to the temperature of the filament) and the total when both lamps are ON is somehow higher than the sum of the individual lamps when they are ON at separate times.
I can save you the time doing this experiment, within the accuracy of the measurements THERE IS NO DISCERNIBLE “cross heating” effect between the lamps, hypothesis #1 is correct. It is also in conformance with the laws of thermodynamics.
Now please note that I specifically mentioned running the lamps in a constant current mode with a fixed power supply. If you where to run them in constant voltage mode (i.e. just plug them into a wall outlet), then yes indeed one lamp will heat the other by a slight amount. If you hold your hand within a few inches of an incandescent light bulb your hand will warm by maybe 10-20 degrees F. This heating will reduce the resistance of the filament slightly (10 degrees F compared to about 3100 K color temp is not much). And the light bulb will output more light, BUT IT WILL ALSO REQUIRE MORE CURRENT (AND ENERGY) from your power supply. So the folks that believe hypothesis #2 are mistaking a system that consumes more energy with a system (hypothesis #1) that outputs no more light with a fixed energy supply.
If you do this experiment with an energy supply (a battery) instead of a power supply you might measure just a bit more light output but for a shorter time duration (the battery will run down faster) and have NO NET ENERGY GAIN.
You can probably find some more application notes on the web from the folks that sell integrating spheres with light bulbs inside. They have been around for decades.
Cheers, Kevin.
Willis,
The Second Law of Thermodynamics says nothing about a “net” flow regarding heat. The flow of heat is in one direction only, from warmer to colder. A cool body will simply not warm a warmer body under any circumstances without external work applied.
SkepticGoneWild says (February 11, 2013 at 8:27 pm): “The Second Law of Thermodynamics says nothing about a “net” flow regarding heat. The flow of heat is in one direction only, from warmer to colder. A cool body will simply not warm a warmer body under any circumstances without external work applied.”
Just so there’s no semantic confusion about your statement (we’ve seen plenty of that upthread), could you look at Dr. Spencer’s “Yes, Virginia” thought experiment and predict the result if the experiment is ever run for real?
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
There’s a number of post talking about Dr Spencer’s thought experiment.
Well, it isn’t just a thought experiment.
I should have included this quote from the above link
My IR thermometer testing (and my work with the NCDC data set) shows most of the IR is from water vapor. My testing @35F, the sky’s temp was less than -40F. In the NCDC data I found the average annual night time drop in temp is ~18F, almost an exact match to the ~18F increase during the day. I also found a small sample set where with low humidity, low wind speeds, the swing was a matching (daily rise/fall) 40F. I’ve also found that when looked at on a daily average basis, the difference changes with the length of day, as the day starts to get longer, rising temp is more than falling. This difference peaks about April where it starts to fall, crossing zero in August, with the maximum cooling (fall larger than rise) about November.
KevinK says:
February 11, 2013 at 8:20 pm
I’m not sure this is actually what we want to test. It’s not that it would change the temp of the filament (though this might happen in a carefully constructed test), it’s that the air temp next to bulb 1, will be warmer when you turn bulb 2 on.
davidmhoffer says:
February 11, 2013 at 3:02 pm
So, @10W/m2 at 10u wavelength, takes 20x the flux as it would at .5u
Here’s the spectrum of the Sun and Earth in watt’s/M2/uM, note the Sun’s output is far higher than that of the Earths.
And the sky looking up is a lot colder than the earth.
MiCro says, February 12, 2013 at 7:04 am: “There’s a number of post talking about Dr Spencer’s thought experiment. Well, it isn’t just a thought experiment.”
==========================================================
This one is not a thought experiment, but has a problem with the thought.
If there are a few factors involved, you can not just choose one of them and declare it to be the cause. Instead, you need to create two states where the only (significant) difference between them is that very factor you intend to prove to be the cause. Like professor Wood did, see above.
Greg,
Can you provide any explanation for why the chamber is colder than the outside temperature other than the sky being colder than the surrounding air temp?
I found a page that provides a good example of what a IR thermometer will read when pointed at the sky.
MiCro says, February 12, 2013 at 11:21 am: “Greg,
Can you provide any explanation for why the chamber is colder than the outside temperature other than the sky being colder than the surrounding air temp?”
============================================================
MiCro, you do not wont to reverse the burden of proof, do you?
MiCro says, February 12, 2013 at 11:22 am: “I found a page that provides a good example of what a IR thermometer will read when pointed at the sky.”
======================================================
Right, thank you, this is another way to get rid of the so called “greenhouse effect”.
Just think of why they need the “focusing infrared light onto one side of the thermocouple using a specially designed lens” and can not just use a normal thermometer to measure the change in temperature without focusing. Or is “greenhouse effect” impossible without a lens?
Greg House says (February 12, 2013 at 11:49 am): “MiCro, you do not wont to reverse the burden of proof, do you?”
MiCro has a real experiment, you have only doubt. It’s up to you, now, to provide a better experiment or good reasons why MiCro’s (Dr. Spencer’s) experiment doesn’t show what he claims it does. Remember, experiment trumps theory every time.
You could start by naming some of the “few factors involved” that Dr. Spencer didn’t control for, and how failure to control for them would invalidate the experiment. Please be as specific as you can, so Dr. Spencer could presumably improve his apparatus.
Greg House says:
February 12, 2013 at 11:54 am
Dr Spencer’s experiment didn’t use a lens, and it shows a cooling effect due to the sky being colder than the air temp at the grounds surface.
My IR thermometer shows the sky being colder than the ground. This is why it gets colder at night, which I presume you want proof that it’s colder at night too?
As for a IR thermometer using a lens, camera’s use lenses to focus light and control the field of view, the same reason a IR thermometer would use a lens, since it’s focusing photons (that’s for you George!).
Greg, a lot of people have spent a lot of time trying to get you to see the light (sorry George). I am a firm believer that Co2 is not causing planetary warming beyond a slight non-worrisome amount. I’ve downloaded and worked on NCDC’s 120+ million station, CRU’s data, and BESTs data, what I’ve found is that there’s no loss of cooling at night. I am on the same side as you. But the sky isn’t -270C over my house, something is keeping it warmer than that, and I can measure IR coming from the sky, so did the link I posted, and so did Dr Spencer’s experiment, so have a number of other IR systems pointed at the sky.
I think most of the people here would rather we fight the hotheads, and make them realize that the temp of the sky over head is being controlled by water vapor, not Co2, because when there is no water, the temp at night drops a lot, quickly, because the sky is much colder than the surface air. We have multiple lines of proof. What we don’t have proof of is that the sky is the same temp as space, and Quantum physics demands that anything over absolute zero emits IR.
Gary Hladik says, February 12, 2013 at 12:41 pm: “You could start by naming some of the “few factors involved” that Dr. Spencer didn’t control for, and how failure to control for them would invalidate the experiment.”
=========================================================
This is obvious. Conditions inside the box and outside the box are essentially different. The factors are convection and possibly wind. And influence of the box material.
As I said before, you can compare temperatures in boxes, but you need to have only one (significant) difference between them, then you can conclude whether this difference plays any significant part or not. Two states with one difference, other factors equal. It is a basic stuff.
OK, let me give you an example, since you do not get it. Take the remote, point it to the TV set, say “abracadabra” and press the power button. You can watch TV now. Apparently, because you said “abracadabra”. But [let’s] check it, like climate scientists would do, I guess. Turn the TV set off again. Now, take the remote, point it to the opposite direction, do not say “abracadabra” this time and press the power button. You can not watch TV now. Apparently, because you did not say “abracadabra”. Scientists know it as the abracadabra effect.
MiCro says:
February 12, 2013 at 11:22 am
I found a page that provides a good example of what a IR thermometer will read when pointed at the sky.
MiCro I read the link you provided and it gives a good reason as to why CO2 is not doing anything. The graphic shows IR thermometers one pointed at a dry sky and the other at a “damp” sky. Showing that a “damp” shy has a higher temperature than the dry. But CO2 is well mixed the two shots have the same ppm so they are saying what many of us have said we live in a water world.
mkelly says:
February 12, 2013 at 1:54 pm
And pretty much every line of evidence agrees with this, except GCM’s which are specifically programmed not to.
If you follow the link in my name, I’ve blogged about the data I’ve mined from the NCDC data set, and it shows that on average night time temps fall as much as they go up during the day, with only slight differences, but no trend connected to raising co2.
But also let me make two additional notes, I suspect with careful measurements of dry air, we’d see an increase in the skies temps with increasing co2, but it is small, and dwarfed by the water vapor in the air, I’ve also noticed a high temp IR signal during the day time, that looks like it’s a reflection of the sun off the atm, if the reflection is from co2, an increase would cause an increase in warming, this could be part of the measured warming. But in all cases it’s water that controls cooling, and from what I can tell from my mining, it reduces cooling by half or more what the co2 alone cooling would be (~18F of cooling down from ~40F/night of cooling of dry air), and my IR reading of the sky on a cold day (which has low humidity) agrees with that (as the link does as well).
We just need to get this kind of proof out to the population so we can stop the politicians from their attempts to cure something that doesn’t exist.
Greg House:”This is obvious. Conditions inside the box and outside the box are essentially different. The factors are convection and possibly wind. And influence of the box material.
As I said before, you can compare temperatures in boxes, but you need to have only one (significant) difference between them, then you can conclude whether this difference plays any significant part or not. Two states with one difference, other factors equal. It is a basic stuff.”
Usually, in science, people who think that a particular result doesn’t demonstrate that a given hypothesis is true are expected to demonstrate why. Can you demonstrate how convection or wind can account for the *results* in Spencer’s experiment? All science is provisional so if you have a better explanation please offer it. Just because something else might account for a set of evidence doesn’t mean that it does.
Unfortunately, lots of folks think that all they have to do is raise some unquantifiable doubt but if you want to be taken seriously you need to quantify your objections somehow.
Cheers, 🙂
Greg House says (February 12, 2013 at 1:53 pm): “Conditions inside the box and outside the box are essentially different.”
Exactly. The whole point of the experiment is to make “inside” the box different from “outside” the box. That’s why there’s a temperature difference. Good, this is progress.
“The factors are convection and possibly wind. And influence of the box material.”
Good, good. But you haven’t explained how any of these factors invalidate the experiment. Verbal handwaving doesn’t challenge an experiment any more than “abracadabra” turns on a TV. But keep going, you’re doing great!
Gary Hladik says, February 12, 2013 at 6:43 pm: “Greg House says (February 12, 2013 at 1:53 pm): “Conditions inside the box and outside the box are essentially different.”
Exactly. The whole point of the experiment is to make “inside” the box different from “outside” the box. That’s why there’s a temperature difference.”
==========================================================
The fact that two things are different is not enough to conclude why exactly the temperatures are different. If there is only one significant difference, than you can conclude on it. If there a few significant differences, you can not just choose one of them and make it responsible. You need to take the influence of other factors into account or show that they are negligible and so on, what Roy Spencer failed to do in that particular experiment.
Please, read the abracadabra example again and make an effort this time.
Greg House says:
February 12, 2013 at 7:26 pm
I’m not sure what the complete list of references and test I provided doesn’t answer for you, but I also know I can bounce the IR from my remote control off the wall on the opposite side of the room and still turn off the tv.
There just changed the channel while point at the wall. No abracadabra required.
Greg House says (February 12, 2013 at 7:26 pm): “The fact that two things are different is not enough to conclude why exactly the temperatures are different. If there is only one significant difference, than you can conclude on it. If there a few significant differences, you can not just choose one of them and make it responsible.”
You still haven’t explained how even one of the factors you mentioned invalidates the experiment. You’re still handwaving. Why not start with wind? That’s one of the factors you mentioned before. How does wind invalidate the experiment?
Remember, MiCro has a real live experiment and so far you have nothing. Anybody who can tell what’s wrong wth the abracadabra experiment should be able to tell what’s wrong (if anything) with MiCro’s. Make an effort this time.
Well folks, I just did an experiment. I fixed a small surface thermometer to a piece of glass, which absorbs IR. I allowed the temperature to stabilize at 19.2 degrees C. I then pointed my TV remote control directly at the piece of glass and held it “on” for 15 minutes. At the end of the 15 minutes, the temperature was still 19.2 degrees C. I observed the temperature for another 15 minutes after that, and is remained exactly the same. I tested the remote control both before and after the experiment to ensure that it was working by turning the TV on and off. So if glass absorbs IR, why didn’t the temperature change? Ooooh, I see a whole bunch of people reaching for their keyboards….
But folks, please….
Let Greg answer this one.
Gary Hladik says, February 12, 2013 at 8:16 pm: “Anybody who can tell what’s wrong wth the abracadabra experiment should be able to tell what’s wrong (if anything) with MiCro’s.”
=========================================================
Well, go ahead then. Or are you still uncertain about the abracadabra effect? And why do you call it “MiCro’s”? I thought we were talking about Roy Spencers’.
davidmhoffer says, February 12, 2013 at 9:03 pm: “I fixed a small surface thermometer to a piece of glass, which absorbs IR. … I then pointed my TV remote control directly at the piece of glass and held it “on” for 15 minutes. … I tested the remote control… by turning the TV on and off. … Let Greg answer this one.”
==========================================================
OK, just do not forget to put your toys back in place after you have finished playing, be a good boy.
Greg House says (February 12, 2013 at 9:18 pm): Well, essentially nothing.
Greg, you still haven’t explained what’s wrong with MiCro’s (Dr. Spencer’s) experiment. Do you now accept its results?
Gary Hladik says, February 12, 2013 at 10:26 pm: “Greg House says (February 12, 2013 at 9:18 pm): Well, essentially nothing.”
=======================================================
I did not say “Well, essentially nothing.” Please, do not mislead the readers. Thank you.
Greg House;
OK, just do not forget to put your toys back in place after you have finished playing, be a good boy.
>>>>>>>>>>>>>>>>
In other words, either you can not or will not answer the question.
In other words, either you can not or will not answer the question.
>>>>>>>>>>
Mr Greg House, it seems you have a limited number of options:
1) Accept the results of my experiment, which proves that glass does NOT absorb IR as claimed in the Wood experiment, and that the Wood experiment is hence falsified.
2) Perform the experiment yourself and produce a different result. Good luck with that.
3) Provide a logical explanation for the result that does not invalidate Wood.
Or you can make jokes about toys and putting them away and similar attempts to distract everyone from the fact that you really don’t have the first clue as to what you are talking about.
(For all those that know the answer, please, let Greg answer)
davidmhoffer says (February 13, 2013 at 9:18 am): “(For all those that know the answer, please, let Greg answer)”
[through gritted teeth] Must…resist…temptation…urge…overwhelming…
I know! I know the answer! The glass stays cool because the Earth’s so-called “greenhouse” effect is zero or negligible just as R W Wood proved beyond question in 17447 when he pointed his TV remote at a salt shaker in a cardboard box and yelled “abracadabra!” and–look! a squirrel!
🙂
Gary Hladik;
R W Wood proved beyond question in 17447 when he pointed his TV remote at a salt shaker in a cardboard box and yelled “abracadabra!” and–look! a squirrel!
>>>>>>>>>>>>>>>>>>>>
Omigod! Omigod! Was it alive? Was it Schroedinger’s squirrel? Did the cat transform to a squirrel? Was the transformation before/after the squirrel was alive/dead?
Well it has been some hours since I posed my question to Greg House, and still no credible response. Shall we wait until the morning? I think we should give him until at least then. The answer of course is rather trivial. The question I guess is which of three options Greg will choose:
a) admit he doesn’t know and can’t figure it out
b) provide an answer that demonstrates his “grasp” of the physics
c) not answer at all pretending that after repeatedly shooting his mouth off, this issue isn’t important enough for him to bother.
OK, I tried another experiment.
I put the remote control for the TV in the deep freeze. For an hour. While it was cooling off, I got a hair dryer and heated up the sensor on the TV. I mean really heated it up. You know that smell plastic makes when it starts to break down? That hot.
Then I got the remote control out of the freezer. Now I’m convinced by Greg House et al that it is impossible for energy to flow in any way shape or form from cold things to hot things, so there is no way the remote control could work at this point.
Someone forgot to tell the remote control. Or the TV. Or both.
How does that work Greg?
(all you reaching for your keyboards whilst laughing to yourselves….. please. Let Greg explain)
So still no answer from Greg House to either of my experiments above. Does he think by not answering here that he’s off the hook? That I’ll just forget about these and fail to bring them up the next time he posts his wood experiment and claims it means something?
Tell you what Greg, I’ll even give you some hints.
Experiment1: Stefan-Boltzmann Law
Experiment2: Planck’s Constant
Surely with those hints to go by, someone with as firm a grasp of physics as you ought to be able to figure out how to explain the results?
Willis Eschenbach says:
February 9, 2013 at 2:18 pm
it might relate to the fact that Tallbloke banned me from his site for saying that a perpetual motion machine wouldn’t work, and I haven’t been back since.
This is a lie. I still have a copy of the comment Willis left on the suggestions thread at the talkshop saying that he would ban himself if I didn’t run my blog how he said I should and let Joel Shore run riot with his threadbombing tendencies. Nothing to do with his scientific views, which I devoted a thread of it’s own to. Needless to say I took him up on his offer.
The comment about perpetual motion machines here shows Willis still doesn’t understand how the higher heat capacity of the denser near surface air retains energy better than thin cold mountain top air at night, thus raising the near surface average temperature. I also have some more experimental evidence for publication demonstrating a temperature gradient in an enclosed tube, confirming Graeff’s work. Experimentum summas Judex – Albert Einstein.
He’s still welcome here and visits my threads, but I won’t go to a site where people are banned for their scientific views. He put himself in the same boat as RealClimate and Open Mind
People who know how to put forward scientific views and listen and respond to the criticism of others with courtesy and respect are always welcome at the talkshop. Bigmouths like Willis, and threadbombers like Joel Shore don’t get past the door however.
tallbloke says:
February 14, 2013 at 9:35 am
tallbloke, don’t ever call me a liar, you will always be wrong. I do many things. Lying is not one of them. Perhaps you call people liars routinely without evidence, I wouldn’t know. I do know that every time you make an unsubstantiated, false accusation that a man is a liar, your stock sinks in the marketplace. I don’t call a man a liar without serious solid facts to back it up, and your willingness to call me one without even a scrap of substantiation marks you as an unpleasant amateur. Here’s how it came down.
You banned Joel Shore for saying exactly what I had said, that N&Z’s hypothesis was junk because it violated thermodynamic laws. Despite your claim above that it was “nothing to do with his scientific views”, here’s what you said. Your exact words to Joel were:
tallbloke, you stand convicted by your own words. It was ALL about his scientific views, about whether N&Z violated the law of conservation of energy (they did), not the “threadbombing tendencies” you falsely try to blame above.
In protest, and because I HAD SAID EXACTLY THE SAME THING JOEL SAID, that N&Z was a load of a-scientific horse puckey, I said I wouldn’t go to your site. So you are correct that I banned myself … for doing exactly what you banned another man for.
Why did I do that? I banned myself to make a point, because you didn’t have the balls to ban me for saying exactly what Joel said. You knew you could get away with banning Joel, but not with banning me, so you flat out refused to ban me for saying exactly what Joel said … inconsistent much?
So to draw attention to what I saw and still see as a reprehensible act, banning a man for scientific views, I said screw your site and the horse you rode in on, I’m not going there no more.
However, contrary to your claim, that was just the opening moves. Subsequently, you most definitely banned me from your site, tallbloke, saying:
Yep. You banned me, and in accordance with your ban, I’ve stayed away to this day, and I strongly recommend that other people do the same. A man who bans people for their scientific views is bad enough. A man who bans an unpopular person for his scientific views, but refuses to ban a popular man for saying the exact same thing, is beneath contempt.
Now, unlike you, tb, I don’t charge a man with lying without solid evidence. So I don’t know if you are lying about banning me from the Talkshop, and in fact, I strongly suspect that you simply forgot that it is no longer a self-ban, that you converted it to a real ban.
Those still interested in the old debate between tallbloke and myself on the subject of bannings are invited to read first “A Matter of Some Gravity“, and then “Thanks and Apologies“, wherein tallbloke banned me. I know I’m not looking to reheat old wars, and I have done my best to be cordial with tallbloke since the rupture.
Your move, Rog … and considering how much ground you and your blog just lost by you coming here to make an ass out of yourself once again over a dead issue, I’d choose the move carefully. You don’t want to match wits with me, that’s a mug’s game, and calling me a liar just earns you people’s deserved contempt. If you were smart your next move would be to lift the ban on me and Joel and move on, it’s a blot on the old escutcheon, my friend …
w.
Ah, more bullying and blustering.
Willis said:
“Tallbloke banned me from his site for saying that a perpetual motion machine wouldn’t work”
This is a lie.
Actually it’s two lies.
Lie one: “Tallbloke banned me from his site”
No, you banned yourself from my site because I didn’t give in to your ultimatum telling me how i should run my website, AS YOU JUST ADMITTED ABOVE, by saying:
“I said I wouldn’t go to your site. So you are correct that I banned myself”.
Glad that one’s settled then, liar.
Lie two: ” for saying that a perpetual motion machine wouldn’t work”
No. You banned yourself, so how could I have banned you for anything?
You still don’t understand the difference between the various arguments about the effect of pressure on temperature either.
Your capability with logic is really on the slide.
Willis and TB, this tussle is old news, take it off-site.
Anthony Watts says:
February 14, 2013 at 11:16 am
Thanks, Anthony, you’re right, old news. Rog Tallbloke is a good guy, I have a lot of respect for him, and he seems to have the idee fixee on this one thing. I don’t have a clue why he shows up here to make these false accusations so many months after banning me from his site, viz:
I don’t go to his site, he banned me. I wish he’d return the favor and stay away from my threads, or at least set the disharmony aside when he’s here. I have made several overtures to him, I’d truly like to put all of this behind both of us.
Back to the real scientific issues,
w.
Sooooo, how about that steel greenhouse, eh?
Pretty nifty, huh?
Yeeeeep, pret-ty nif-ty…mmm hmm…
Anybody?
Gary Hladik says:
February 14, 2013 at 8:49 pm
“Sooooo, how about that steel greenhouse, eh?”
It’s done – see my posts above before things degenerated completely into a flame war. Verdict: the shell increases the planet’s surface temperature above what it would be without the shell, but the effect is maximized for a thin shell, and a thicker shell generally decreases the surface temperature below what it would be for a thinner shell.
I believe this happens because of the surface area for radiation. Increasing the thickness generally decreases the surface area of the shell facing the planet, while increasing it toward space. So, you get less radiated power going back to the planet, and more going out to space.
The implication for the climate debate is: adding CO2 to the atmosphere does not necessarily increase surface temperatures, and may even tend to decrease them. Certainly, the empirical evidence indicates that the CO2 effect on temperature is negligible compared to the temperature effect on CO2.
davidmhoffer says:
February 12, 2013 at 9:03 pm
Put your piece of glass in the freezer for a few minutes and your remote will stop working. The glass will absorb IR until it reaches equilibrium with the source of IR or ambient temps. Then the IR is re-emitted and the remote will work again. Of course glass absorbs IR. All substances absorb IR. There is no exception. You are still clueless.
Willis’ “shell game” thought experiment is a deception. A surface radiating 235 w/m2 cannot warm another surface radiating at 235 w/m2 any more than one ice-cube can warm another ice-cube. Not in the atmosphere and not in a vacuum.
Take a 1 ounce ice-cube. Now place a 1 ton ice-cube next to it. You have nearly 4000 times as much energy in the 1 ton ice-cube, yet no increase in temperature in the1 ounce ice-cube. That is because the 1 ounce ice-cube is radiating at the same temperature as the 1 ton ice-cube, 273K.
It is not the amount of energy but the flux density which determines temperature. In the vacuum of space there is limitless energy. It is called Zero Point energy.
Will says:
February 15, 2013 at 5:16 pm
“A surface radiating 235 w/m2 cannot warm another surface radiating at 235 w/m2 any more than one ice-cube can warm another ice-cube.”
The shell, to the degree that it is “thin”, is radiating at roughly twice that, because it has two sides, the inside and the outside, hence roughly twice the surface area.
An ice cube cannot warm another ice cube, but if you put them close together, the sides facing each other will melt slower.
That is exactly the situation in this thought experiment. It is about modulation of the flow of heat, not about one object serving as a heat source for another. The shell is not “heating” the planet. It is preventing it from shedding heat as fast as it otherwise would. And, with the continuous generation of heat from its core, that causes the surface temperature to rise from what it otherwise would have been.
This is how MLI blankets work. It is well established theory.
Bart says, February 15, 2013 at 8:28 pm: “This is how MLI blankets work. It is well established theory. http://en.wikipedia.org/wiki/Multi-layer_insulation”
=========================================================
Surprisingly, the Wikipedia article you referred to does not contain a single word about MLI blankets designed to protect satellites from excess solar heating. Not a single word. I wonder why. Because things can get very very hot in space if they are exposed to the solar radiation, and note, there is no air outside the satellite to cool it via convection. A satellite can easily reach temperatures over 100C and this is not necessarily what devices inside a satellite like.
Why would Wikipedia keep quite about it? I can not find any rational reason to do that other than mislead the readers.
Aha! I can foresee Willis’s next article now: “The MLI Greenhouse” 🙂
Davidmhoffer,
The remote control “experiment” really sucks. It is not the temperature of the remote control that determines its IR emission. The remote control has a battery, circuit board and an LED that sends a specific wave length. As long as the battery is functioning, it will emit IR. Secondly, very near infrared light is used in remote controls, and is in the 800-900nm range. This very short wavelength infrared energy can indeed go through glass.
______________________________
As Will stated above, a body cannot “warm” itself up. If that were the case, I could wrap my baseboard heater with a steel shell, and “abracadabra”, free heat. The light-bulb experiment Willis mentioned could be done with bulbs of the same wattage, since A warms B and B warms A. (according to Willis) According to physics, two bodies at the same temperature are in thermal equilibrium, and there is no exchange of heat, but according to Willis, they exchange heat, because A warms B and B warms A. When does the cycle end? What Willis has constructed is a device know in physics as “The Perpetual Motion of the Second Kind”. The Willis planet is indeed, as he states, “magical”, because it violates the Second Law of Thermodynamics.
If you read the MLI Wikipedia article, the original plate still radiates its original amount of 460 W and does not heat up as does the Willis model. MLI is just super-insulation that works great in the radiation spectrum of heat transfer, as opposed to convection and conduction. The Willis model bears no resemblance to the theory of MLI technology.
Bart says:
February 15, 2013 at 8:28 pm
“The shell, to the degree that it is “thin”, is radiating at roughly twice that, because it has two sides, the inside and the outside, hence roughly twice the surface area.”
It is still only radiating at 235 w/m2 in any given direction. You cannot sum the energy flow in different directions and apply it back to the other surface. A square ice-cube has 6 surfaces all radiating at 273K. Do we add those 6 surfaces together to get the total temperature of the ice?
From your link, quote:
“The principle behind MLI is radiation balance. To see why it works, start with a concrete example – imagine a square meter of a surface in outer space, at 300 K, with an emissivity of 1, facing away from the sun or other heat sources. From the Stefan-Boltzmann law, this surface will radiate 460 watts. Now imagine we place a thin (but opaque) layer 1 cm away from the plate, thermally insulated from it, and also with an emissivity of 1. This new layer will cool until it is radiating 230 watts from each side, at which point everything is in balance. The new layer receives 460 watts from the original plate. 230 watts is radiated back to the original plate, and 230 watts to space. The original surface still radiates 460 watts, but gets 230 back from the new layers, for a net loss of 230 watts. So overall, the radiation losses have been reduced by half by adding the additional layer.”
The reality is the exact opposite of Willis’ “thought experiment” just as I said was the case further up the thread.
So the shell will actually only radiate 117.5 w/m2 in each direction. Like I said, Willis is double counting.
I love it when people don’t even bother to read or understand their own links.
Will,
“So the shell will actually only radiate 117.5 w/m2 in each direction.”
But the interior is still supplying 235W, so the surface temp goes up, until it stablizes at the temp it needs to radiate 235W. This cycle of inner surface temp increase, outer shell temp increase, continues until the outer shell is radiating the equivalent of the cores 235W/sq M.
SkepticGoneWild says:
February 16, 2013 at 12:38 am
What Willis has constructed is a device know in physics as “The Perpetual Motion of the Second Kind”. The Willis planet is indeed, as he states, “magical”, because it violates the Second Law of Thermodynamics.
I agree with SkepticGoneWild on this.
I think much of the trouble with the greenhouse hypothesis and such exercises is they try to calculate the energy budget based on independent calculated radiation from Stefan-Boltzmann’s law of the 2 bodies.
In my humble opinion, the way how to fix it is to calculate net-heat transfer throughout the system and take from it the resulting temperatures. See also:
http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/node137.html
This is why I like much more the following energy budget:
https://en.wikipedia.org/wiki/File:Breakdown_of_the_incoming_solar_energy.svg
Greg House says:
February 15, 2013 at 9:24 pm
“Surprisingly, the Wikipedia article you referred to does not contain a single word about MLI blankets designed to protect satellites from excess solar heating. Not a single word. I wonder why. Because things can get very very hot in space if they are exposed to the solar radiation, and note, there is no air outside the satellite to cool it via convection. A satellite can easily reach temperatures over 100C and this is not necessarily what devices inside a satellite like.”
They don’t say so explicitly but it is in there. From the article:”Clearly, increasing the number of layers and decreasing the emissivity both lower the heat transfer coefficient, which is equivalent to a higher insulation value.” If the heat transfer coefficient is lower the satellite will be protected from warmer temps (ie less warmth from the outside of the satellite to the inside).
Cheers, 🙂
MiCro says:
February 16, 2013 at 8:21 am
“But the interior is still supplying 235W, so the surface temp goes up, until it stablizes at the temp it needs to radiate 235W.”
No it doesn’t.
As I have previously explained, an object radiating at 235 W/m2 cannot raise the temperature of another object radiating at 235 W/m2. So how do you think an object radiating at half that, 117.5 W/m2 can do it?
Again, take one ice-cube radiating at 273K. Introduce another ice-cube radiating at 273K and what happens to the temperature of the first ice-cube? Nothing happens!
Now add another ice-cube, add another 100, 1000, or 1000,000 ice-cubes all radiating at 273K and what happens to the temperature of the first ice-cube? Again, exactly NOTHING!
Two words, FLUX DENSITY.
Even when you stack the deck with impossible thought experiments, it is impossible to show a “greenhouse effect”.
The Second Law, entropy, trumps the “greenhouse effect” fallacy.
Okay, I’m going to give you an analogy that everyone should be able to understand.
It’s cold, you’re cold, your feet are cold, and you jump into a cold bed, but in a little bit, your feet warm up, the bed is warm.
Second analogy, It’s warm, you jump in a cold bed, and in a little bit, you’re hot, the bed’s hot.
If you don’t understand this, don’t bother to ask me to explain it.
Greg House says:
February 15, 2013 at 9:24 pm
“Surprisingly, the Wikipedia article you referred to does not contain a single word about MLI blankets designed to protect satellites from excess solar heating.”
The purpose is to distribute the heat evenly about the spacecraft, otherwise, there would be huge gradients. It does this by preventing radiation from escaping in the wrong direction. And, then there is the question of surviving during eclipse, when there is no solar heating. The MLI keeps the heat from radiating away during that time. Typical operating temperatures are -10 C to +40 C. Without the MLI, it would be impossible to keep them in that range.
SkepticGoneWild says:
February 16, 2013 at 12:38 am
“If that were the case, I could wrap my baseboard heater with a steel shell, and “abracadabra”, free heat.”
The heater would get hotter. Not you.
” MLI is just super-insulation that works great in the radiation spectrum of heat transfer, as opposed to convection and conduction.”
And, the steel shell acts as an insulator to radiation in Willis’ example. It is the same.
Will says:
February 16, 2013 at 1:36 am
Read it again until you understand it.
SkepticGoneWild says:
February 16, 2013 at 12:38 am
“What Willis has constructed is a device know in physics as “The Perpetual Motion of the Second Kind”. The Willis planet is indeed, as he states, “magical”, because it violates the Second Law of Thermodynamics.”
No. The shell isn’t supplying the heat, the nuclear reaction in the planet’s core is. The flow of radiation is always outward. The shell settles out at a lower temperature than the planet’s surface, so everything is consistent with the laws of thermodynamics.
This is a continuous flow problem. If you impede a continuous flow, then the quantity flowing will backup behind the impediment. It is just like damming a river. The dam does not produce water, yet it causes water to accumulate behind the dam.
Bart says:
February 16, 2013 at 11:43 am
“The principle behind MLI is radiation balance. To see why it works, start with a concrete example – imagine a square meter of a surface in outer space, at 300 K, with an emissivity of 1, facing away from the sun or other heat sources. From the Stefan-Boltzmann law, this surface will radiate 460 watts. Now imagine we place a thin (but opaque) layer 1 cm away from the plate, thermally insulated from it, and also with an emissivity of 1. This new layer will cool until it is radiating 230 watts from each side, at which point everything is in balance. The new layer receives 460 watts from the original plate. 230 watts is radiated back to the original plate, and 230 watts to space. The original surface still radiates 460 watts, but gets 230 back from the new layers, for a net loss of 230 watts. So overall, the radiation losses have been reduced by half by adding the additional layer.”
This is a contradiction of your position, not mine. It is confirmation of my position right through this thread Bart.
You read it again until you understand that you are hopelessly in error.
The temperature is unchanged, a clue for you: “The original surface still radiates 460 watts”
Does that help at all Bart?
Micro,
<>
I think a better analogy is charging a capacitor. One way to charge one is using a constant voltage source and another way to charge is using a constant current source. Using a constant voltage source, the capacitor can only charge up to source voltage. Using a current source, it will charge up until it reaches the dielectric break down voltage or some other catastrophic failure. Think of energy transfer as analogous to current and temperature as anologous to voltage.
In the steel shell model, the energy transfer rate of the inner shell is fixed. The internal temperature of the planet is implicity adjusted to keep this number constant. This is analogous to a constant current source. So to maintain the 235 W/m^2 at the outside of the steel shell, the power generation of the planet would have to increase and this would increase the planet internal temperature.
However, the sun acts as a constant temperature source and so should the planet interior. The nuclear core should only generate enough energy to keep the interior at a constant temperature if the intention is to model the surface of a planet being illuminated by the sun since there is nothing that can be done on earth that can measurably affect the temperature of the sun.
Your body with a blanket works in a similiar fashion, with or without a blanket your core temp should be 98.6 – a constant, like the temperature of sun surface.
Shawnhet says, February 16, 2013 at 3:02 am: “They don’t say so explicitly but it is in there.”
=======================================================
It is not there.
To me, not to tell people in the article “Multi-layer insulation” that multi-layer insulation is designed to prevent the satellite from overheating because of solar radiation is a clear indication of the misleading nature of the article. Given the “explanation” in the article I can only suspect warmists.
MiCro says, February 16, 2013 at 10:19 am: “Okay, I’m going to give you an analogy that everyone should be able to understand.
It’s cold, you’re cold, your feet are cold, and you jump into a cold bed, but in a little bit, your feet warm up, the bed is warm.”
=========================================================
You are not cold. Your feet are not cold. Your skin might get cold even if it is very warm outside you, but it is a physiological phenomenon.
You have a “heating device” inside you.
Bart says, February 16, 2013 at 11:43 am: “The purpose is to distribute the heat evenly about the spacecraft, otherwise, there would be huge gradients. It does this by preventing radiation from escaping in the wrong direction. And, then there is the question of surviving during eclipse, when there is no solar heating. The MLI keeps the heat from radiating away during that time.”
==========================================================
I suggest you read the description of the R.W.Wood experiment again. It demonstrates that “trapped radiation” has no or only negligible effect on temperature of the source. The MLI article from Misleadingpedia simply repeats the same unproven claim. It is not any better scientifically than the MiCro’s analogy with cold feet.
The trick is apparently to find something ambiguous and just claim it is the back radiation warming.
@Greg House
” It is not any better scientifically than the MiCro’s analogy with cold feet.”
I expected you wouldn’t get it, and I expected Will to not get my posts either.
@Casual, they are both stuck in 19th century physics, and appear clueless with 20th century physics, I didn’t expect them to get electronics either. Cold feet, constant voltage, nuclear core constant current.
And I wouldn’t even care, except we get tared by the same brush.
Bart says:
February 16, 2013 at 11:50 am
“This is a continuous flow problem. If you impede a continuous flow, then the quantity flowing will backup behind the impediment. It is just like damming a river. The dam does not produce water, yet it causes water to accumulate behind the dam.”
There is NOTHING impeding the flow of energy in this “thought experiment” or in the so called “greenhouse effect”.
Such notions are purely a figment of your imagination Bart.
Will says (February 16, 2013 at 9:30 am): [snip]
Out of curiosity, Will, what’s your take on Roy Spencer’s “Yes, Virginia” thought experiment?
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
If the experiment is done for real, then with the second body present, do you predict the electrically heated body will be
a) warmer
b) cooler
c) the same temp
as it is with the second body absent?
Ok Will,
A word problem, with you and your families life at stake.
You’re all on the moon, there are two basketball court sized buildings, ones empty, the other is 3/4’s full of ice. You just lost building power, and it has just turned dark. You all have space suits that have power that will last 20 hrs. Help will be there in 40-45 hrs. And the empty shelter will cool to outside temp in less that 10 hrs. The suits would last 35 hrs at 0C.
What do you do and why? Remember everyone’s life is at stake.
I should have said the suits will last 20hrs on the dark surface of the moon.
Will says:
February 16, 2013 at 12:23 pm
I can explain it to you, but I cannot understand it for you. Read it again. Then, again. And, again. Until it gets through.
Greg House says:
February 16, 2013 at 1:39 pm
“The MLI article from Misleadingpedia simply repeats the same unproven claim.”
I see. So, all those satellites and space shuttles and space stations… all that stuff is bunk. Are you one of those guys who think pro wrestling is real, but the Moon landings were fake?
Will says:
February 16, 2013 at 2:02 pm
“There is NOTHING impeding the flow of energy in this “thought experiment” or in the so called “greenhouse effect”.”
Ooo-KAY. So, materials do not absorb or re-emit electromagnetic energy. Glad you cleared that up for us. Looks like the whole of 20th century science is a scam.
Guys, you are barking up the wrong tree. As I showed using math above, the “greenhouse effect” is real. There is no question that the Earth’s surface is warmer with its blanket of IR absorbing gases than it would be without. But, that does not mean that an incremental increase in those gases necessarily results in an additional rise in temperature.
If you want to have any hope of influencing anyone other than nitwits, you will adopt that position as your own. If you don’t, if you cling to this malignant fantasy that all of science is a great big scam, then you might as well move out to Montana and live in a shack, because none of the modern technologies actually work, and you are no better off for them. We are all blinded to the con by, I dunno… flouride in the water. Or, something. But, at any rate, my continued participation in this debate, if you can call it that, is clearly benefiting nobody. Sayonara.
Gary Hladik says:
February 16, 2013 at 2:40 pm
There is only one reason why people use “thought experiments” in place of real experiments. Go figure as they say.
To increase the temperature of a body requires radiation of a higher flux density than that which it is already emitting, as is clear from my real ice-cube example you referred to.
Gary Hladik says:
February 16, 2013 at 2:40 pm
“If the experiment is done for real, then with the second body present, do you predict the electrically heated body will be
a) warmer
b) cooler
c) the same temp
as it is with the second body absent?”
If the energy input is fixed (the energy sink, space, never varies) but mass is increased, obviously temperature will fall. This is the converse to the principle behind the filament of a lightbulb is it not?
Bart says, February 16, 2013 at 3:07 pm: “Greg House says: February 16, 2013 at 1:39 pm “The MLI article from Misleadingpedia simply repeats the same unproven claim.”
I see. So, all those satellites and space shuttles and space stations… all that stuff is bunk.”
========================================================
“Those satellites and space shuttles and space stations… all that stuff” is just fine. But the MLI article from Misleadingpedia is obviously a peace of crap. I hope you get the point now.
Bart says:
February 16, 2013 at 3:07 pm
“So, materials do not absorb or re-emit electromagnetic energy. Glad you cleared that up for us.”
Yes they do, they do both in equal measure as per Kirchhoff and it is called energy flow. I hope clears up your confusion Bart.
“I can explain it to you, but I cannot understand it for you. Read it again. Then, again. And, again. Until it gets through.”
Continuous repetition of fallacious argument cannot make it true.
“There is no question that the Earth’s surface is warmer with its blanket of IR absorbing gases than it would be without.”
The atmosphere “blanket” also has cooling effect. Care to discuss that at all Bart?
The so called “greenhouse effect” is provably a fallacy of logic.
Bart says:
February 16, 2013 at 3:07 pm
” As I showed using math above, the “greenhouse effect” is real.”
A Nobel prize for Bart! He has done what no man has ever been able to do, he has achieved the impossible and proven that the “greenhouse effect” is “real”.
Delusional!
Will says (February 16, 2013 at 3:16 pm): “There is only one reason why people use “thought experiments” in place of real experiments. Go figure as they say.”
It’s really too bad that you can’t appreciate the delicious irony of that paragraph, Will. Keep reading.
“To increase the temperature of a body requires radiation of a higher flux density than that which it is already emitting, as is clear from my real ice-cube example you referred to.”
Um, your ice cube “experiment” isn’t real. It’s just another thought experiment until you can point to an actual experiment with actual setup, actual measurements, etc. See the irony now?
I see also that you’re perfectly happy to comment on Willis’s thought experiment and point out at length why it won’t work. Yet you shy away from the more down-to-earth (and potentially performable) thought experiment of Roy Spencer. WUWT?
You’re not alone, either. Five times in this thread I’ve asked commenters to predict the result of this experiment done for real, and nobody has. It’s as if they’re afraid, or something. 🙂
BTW, one final comment: Dr. Spencer’s thought experiment uses two plates, presumably of metal, which are just the solid or “frozen” phase of something that’s liquid or even vapor at higher temperatures. So you’ll present a thought experiment using frozen water, but won’t go near one using, say, frozen aluminum? Again, WUWT?
MiCro says, February 16, 2013 at 4:00 pm : “Ok Will,
A word problem, with you and your families life at stake. […] What do you do and why? Remember everyone’s life is at stake.
=======================================================
I suggest Will call 911.
Gary Hladik says, February 16, 2013 at 4:16 pm: “Um, your ice cube “experiment” isn’t real. It’s just another thought experiment until you can point to an actual experiment with actual setup, actual measurements, etc.”
==============================================================
Gary, I am overwhelmed with all sorts of positive emotions now that you publicly recognized the value of real experiments, thank you.
MiCro says (February 16, 2013 at 4:00 pm): “A word problem, with you and your families life at stake.”
Well, I’d shoot the wife and kids, take their power packs, and use them to power my own suit, because I came to the moon to get away from them, dammit!
Sorry, sorry, everyone. Anthony still hasn’t explained the temp differences between the CRN and COOP networks, so I’m getting cranky. Sorry.
Gary Hladik says, February 16, 2013 at 4:16 pm : “Yet you shy away from the more down-to-earth (and potentially performable) thought experiment of Roy Spencer. WUWT?”
=======================================================
Gary, I suggest we wait till Roy Spencer has actually performed his potentially performable “thought experiment”, OK?
Greg House says (February 16, 2013 at 4:47 pm): ‘Gary, I suggest we wait till Roy Spencer has actually performed his potentially performable “thought experiment”, OK?’
Greg, it’s not a prediction unless it’s recorded before the experiment is performed.
And as I’ve explained before, I don’t expect Dr. Spencer to do the experiment, because he has nothing to gain. Disciples of the imaginary version of the 2nd Law of Thermodynamics (i2LT), on the other hand, have everything to gain by doing the experiment, since it would supposedly overturn mainstream physics and earn them both fame and fortune. In fact, I suspect one (or more) might be hard at work right now, which is why I want to get people’s predictions in early.
Greg House says:
February 16, 2013 at 3:42 pm
“I hope you get the point now.”
Do I ever.
Will says:
February 16, 2013 at 3:51 pm
“Continuous repetition of fallacious argument cannot make it true.”
Words of wisdom. If only you took them to heart.
“The atmosphere “blanket” also has cooling effect. Care to discuss that at all Bart?”
Sure. The atmospheric blanket cools the atmosphere as soon as a point is reached at which it radiates enough to balance all the equations. At that point, if the temperatures temporarily go higher, they will radiate away the heat to reestablish equilibrium. However, if temperatures temporarily go lower, they will prevent the energy from flowing out, until the equilibrium is reestablished. In this way, they act as a governor to maintain a particular surface temperature.
The question is, what happens if you make the “blanket” thicker? As I showed, it does not necessarily lead to higher surface temperature. But, whether or no, the GHE does exist. Without atmospheric gasses, the Earth would, on average, be colder than with them. Do you understand why the question of whether the GHE exists is uncoupled from the question of whether the relationship is linear or monotonic?
Will says:
February 16, 2013 at 4:10 pm
“A Nobel prize for Bart!”
Sadly, I thought I had a real winner when I showed that V = IR. Unfortunately, they had some quibbles about it having “been done before.” My response of “not by me!” didn’t seem to impress them.
Greg House says (February 16, 2013 at 4:40 pm): “Gary, I am overwhelmed with all sorts of positive emotions now that you publicly recognized the value of real experiments, thank you.”
Thanks, Greg, but you’re late to the party. I’ve linked to or discussed at least two real experiments in this thread alone. I respect real experiments so much, in fact, that I’ve repeatedly urged fringe physicists to perform a relatively simple real experiment (“Yes, Virginia”, or equivalent) that should (according to fringe physics) topple mainstream physics and free the world from the threat of Thermageddon. So far no takers, which I find baffling, given their supposed preference for “real” experimentation. I guess they like their imaginary version of the R W Wood experiment too much to try anything else. 🙂
So how’s your “Yes, Virginia” experiment coming, Greg? Got the vacuum chamber set up yet? Can you share any photos with us? Care to predict the result?
Gary,
All Greg needs to do is buy a ir thermometer measure the temp of the sky and explain it.
Will,
Did you make the right choice and save your family? All you had to do is use the heat of your ice cubes. But you only had an hour or so to figure it out, till the empty shelter dropped to freezing, then you had to get in the center of all of that ice.
Was it a teary good bye, or are you chilling out on ice waiting for your rescuers to show up?
Gary Hladik says, February 16, 2013 at 8:38 pm: “So how’s your “Yes, Virginia” experiment coming, Greg? Got the vacuum chamber set up yet? Can you share any photos with us? Care to predict the result?”
========================================================
My “Yes, Virginia”? (shock) I have nothing to do with that fictional story.
In science a fiction remains a fiction until proven real. I understand that you possibly prefer “a fiction should be considered real until proven to be a fiction”, it’s OK, no problem, but it is not science.
Greg House says:
February 16, 2013 at 1:23 pm
“Shawnhet says, February 16, 2013 at 3:02 am: “They don’t say so explicitly but it is in there.”
=======================================================
It is not there.
To me, not to tell people in the article “Multi-layer insulation” that multi-layer insulation is designed to prevent the satellite from overheating because of solar radiation is a clear indication of the misleading nature of the article. Given the “explanation” in the article I can only suspect warmists.”
No need to invoke a bizarre conspiracy theory just because they don’t phrase things exactly as you would like. If you look at the source pages from the wiki page you find this:
“Passive thermal control is obtained with multi-layer insulation, MLI, which is often the most visible part of a spacecraft. White or gold-colored thermal blankets reflect infrared, IR, helping to protect the spacecraft from excess solar heating. Gold is a very efficient IR reflector, and is used to shade critical components.”
http://www2.jpl.nasa.gov/basics/bsf11-4.php
Cheers, 🙂
I seem to have lost an earlier post into the ether. Sorry if this ends up duplicating.
“Greg House says:
February 16, 2013 at 1:23 pm
Shawnhet says, February 16, 2013 at 3:02 am: “They don’t say so explicitly but it is in there.”
=======================================================
It is not there.
To me, not to tell people in the article “Multi-layer insulation” that multi-layer insulation is designed to prevent the satellite from overheating because of solar radiation is a clear indication of the misleading nature of the article. Given the “explanation” in the article I can only suspect warmists.”
It’s not necessary to invoke a conspiracy to explain this. Even if one ignores the fact that something that acts as insulation will act to protect items from temperature extremes(that is what insulation does). The link the wiki page references says *exactly* what you seem to be requiring.
http://www2.jpl.nasa.gov/basics/bsf11-4.php
“Passive thermal control is obtained with multi-layer insulation, MLI, which is often the most visible part of a spacecraft. White or gold-colored thermal blankets reflect infrared, IR, helping to protect the spacecraft from excess solar heating. Gold is a very efficient IR reflector, and is used to shade critical components.”
Cheers, 🙂
Will,
Here is a black body power calculator http://www.calctool.org/CALC/phys/p_thermo/wien
You can use it to tell you how many watts are being exchanged between blocks of ice.
I’ve tried to look at this from a different point of view.
Let us assume both the core and the shell are perfect black bodies.
The temperature of the nuclear powered core emitting 235Wm2 would be ~254K.
Forgetting for a moment that the shell would be losing heat from 2 surfaces whilst gaining heat from one side only, this shell can only get to a maximum temperature of 254K
Yet it enables the core to increase its temperature to ~302K
Food for thought, but I’m yet to be convinced. There has to be an engineering application there where we can start with the smallest of energy sources and multiply it by just adding more and more shells until we have a unit capable of burning down whole cities or heating up swimming pools or running boilers for power or………..
MiCro says:
February 16, 2013 at 4:00 pm
You thought you were clever and you took your family into the ice building. By the time the rescuers arrived and discovered your shrivelled carcasses, the ice, like the building the ice was in had failen to almost 0K.
Yet the other building, which was designed to comfortably accommodate humans and therefore unlike the ice store, was fully thermally isolated and insulated from the luna extremes, was still a comfortable 288K
History is a litany of clever dead people.
@Will,
” You thought you were clever and you took your family into the ice building. By the time the rescuers arrived and discovered your shrivelled carcasses, the ice, like the building the ice was in had failen to almost 0K.
Yet the other building, which was designed to comfortably accommodate humans and therefore unlike the ice store, was fully thermally isolated and insulated from the luna extremes, was still a comfortable 288K
History is a litany of clever dead people.”
I thought saying they were the same other than one empty, and the other almost full of ice was the clear give away that they were identical otherwise, and without power the empty ones temp would quickly drop without power.
It is almost entertaining watching the mental gymnastics of you and Greg trying to somehow justify sticking to your incorrect versions of reality.
Did you go take a look at that link to the blackbody calculator? Or are you like Greg, everyone else is wrong, but you?
Perhaps a look at the operating principle of a Golay cell might be instructive. It is able to detect IR radiation through the radiative heating (or, ahem, “thermalizing”, if you will) and expansion of IR-absorbing gases(!), and though it operates at room temperature it can be used to determine temperatures of planetary surfaces by measuring the radiation they emit, including temperatures below that of the cell itself. It seems to me that if a cooler body cannot influence the temperature of a warmer body via a change in net radiative energy flow, a Golay cell would be unable to detect radiation from or measure temperatures of bodies cooler than itself. Whether one conceptualizes the cooler body “heating” the cell radiatively or conceptualizes the cooler body impeding the radiative losses of the cell, the net effect (we are the Knights Who Say… “NET!”) is the same, and reinforces my previous conclusion that Willis’ internally heated planet will warm to a higher equilibrium surface temperature if a shell is added as described, Wood experiment notwithstanding.
One can purchase a Golay cell with a credit card. That’s what I call an experiment. Atmospheric “greenhouse effect” objectors, please offer a plausible alternate explanation for the operating principle and pertinent implications of a Golay cell.
Don says:
February 17, 2013 at 1:10 am
“It is able to detect IR radiation through the radiative heating (or, ahem, “thermalizing”, if you will) and expansion of IR-absorbing gases(!),”
No, the gas does not “thermalize” the IR. The gas is warmed by a AC current through a metal plate.
This is an “optical microphone”.
http://gentec-eo.com/Content/uploads/downloads/5-THz_Detectors/AN_121D-201924_THz.pdf
Baa Humbug says:
February 16, 2013 at 11:14 pm
“Food for thought, but I’m yet to be convinced. There has to be an engineering application there where we can start with the smallest of energy sources and multiply it by just adding more and more shells until we have a unit capable of burning down whole cities or heating up swimming pools or running boilers for power or………..”
LOL! We already have this. It’s called insulation. It just doesn’t work in *exactly* the same way that you think it is supposed to.
Cheers, 🙂
MiCro says:
February 17, 2013 at 6:48 am
Yes, never mind the fact that you are so “clever” that you didn’t realise the ice would fall to 0K at practically the same rate as the building.
Mental gymnastics indeed!
“I thought saying they were the same other than one empty, and the other almost full of ice was the clear give away that they were identical otherwise, and without power the empty ones temp would quickly drop without power”
Here is what you said quote:
“Ok Will,
A word problem, with you and your families life at stake.
You’re all on the moon, there are two basketball court sized buildings, ones empty, the other is 3/4′s full of ice. You just lost building power, and it has just turned dark. You all have space suits that have power that will last 20 hrs. Help will be there in 40-45 hrs. And the empty shelter will cool to outside temp in less that 10 hrs. The suits would last 35 hrs at 0C.
What do you do and why? Remember everyone’s life is at stake.”
Nowhere do you stipulate that they in anyway the same except in size.
Mental gymnastics indeed!
” Nowhere do you stipulate that they in anyway the same except in size.”
No, I listed their only differences.
And you might want to check the thermal capacity of water vs air.
Though I suppose you’ll just make something else up proving you’re somehow on to something no other scientist in the 20th century has figured out.
The steel shell model does not apply. Forget it or be confused forever. Take a simple sphere with an internal heat source of 235 w/m^2. Solve the Boltzmann equation to find its blackbody temperature T1. Now add a layer of insulation and measure the equilibrium surface temperature. Keep adding insulation until T2 is 30C higher than T1. You now have a model of the earth with atmosphere, but it still does not apply, because the atmosphere is semi-transparent to infrared. So remove the insulation covering 1/6 of the area of the sphere to account for “window radiation” and add more insulation to the rest of the sphere to recover the 30C temperature difference. Now we are close to a valid model. What about greenhouse gas forcing, solar forcing, volcanos, etc. Here is where we must apply some negative feedback. If the temperature difference gets above 30C we must make the hole in the insulation bigger to recover the 30C, and if it gets below 30C we must make the hole smaller. That is what convection (which includes clouds) does for us automatically. Where does the 30C temperature difference come from? It is the difference between the radiating zone temperature and the effective surface temperature and it’s related to the atmospheric lapse rate g/Cp which depends only on the heat capacity of the atmosphere. The lapse rate together with the mass of the atmosphere (14.7 lb/in^2 based on surface area) and insolation which depends on latitude and season, determines surface temperature. One who thinks surface temperature is controlled by “Greenhouse Gases” is ignorant of the relevant physics.
http://en.wikipedia.org/wiki/Lapse_rate
Are there any other factors that can affect surface temperatures?
Yes. The above is an oversimplified picture.
MiCro says, February 16, 2013 at 10:01 pm: “Gary,
All Greg needs to do is buy a ir thermometer measure the temp of the sky and explain it.”
==========================================================
You already expressed the idea on this thread and referred to this: http://www.weatherquestions.com/A_backyard_greenhouse_effect_experiment.htm
Then I answered you using a quote from your link:
“Right, thank you, this is another way to get rid of the so called “greenhouse effect”.
Just think of why they need the “focusing infrared light onto one side of the thermocouple using a specially designed lens” and can not just use a normal thermometer to measure the change in temperature without focusing. Or is “greenhouse effect” impossible without a lens?”
Looks like focusing by a lens can help the IR from colder bodies penetrate a very small spot of the warmer sensor. This case is different from just “colder body – warmer body”. I suggest you remove the lens (the sensor will still be able to see the target) and please report us the result of the measurement of the natural effect. My guess is, without lens there would be no effect.
” I suggest you remove the lens (the sensor will still be able to see the target) and please report us the result of the measurement of the natural effect. My guess is, without lens there would be no effect.”
And if you removed the lens from your camera, you shouldn’t be surprised then your pictures suck.
Baa Humbug says:
February 16, 2013 at 11:14 pm
“There has to be an engineering application there where we can start with the smallest of energy sources and multiply it by just…”
This is the common misconception, confusing energy flow per unit of time with energy.
The nuclear core is producing some number of Joules of energy per second (a Joule per second is a Watt). So, if you can prevent those energy packets from escaping, then you are continually accumulating energy.
There is nothing magic about it. It is simple accounting. It is just like putting a dam across a river, such that the water starts accumulating behind it. Start with the smallest river or creek and, if your dam is big enough, you can make a lake. The lake is ultimately limited in size only by the rate of evaporation, just as the amount of energy held back by the shell is ultimately limited only by the rate of outward radiation.
Greg House says (February 16, 2013 at 9:45 pm): “My “Yes, Virginia”? (shock) I have nothing to do with that fictional story.”
Exactly. You and other disciples of the imaginary 2nd Law of Thermodynamics (Will, Bevan, Bryan, etc) won’t touch that experiment with a 10 foot pole, not to perform it, not even to predict the result, though you’re perfectly happy to debate endlessly over steel greenhouses. I’m beginning to suspect we’ve discovered the SooperFizzycyst’s Kryptonite. 🙂
“In science a fiction remains a fiction until proven real.”
Bingo. Yet none of the SooperFizzycysts will even attempt to prove their fiction. WUWT?
Gary Hladik says, February 17, 2013 at 12:21 pm: “You and other disciples of the imaginary 2nd Law of Thermodynamics (Will, Bevan, Bryan, etc) won’t touch that experiment with a 10 foot pole, not to perform it, not even to predict the result, though you’re perfectly happy to debate endlessly over steel greenhouses.”
============================================================
First, I did not debate over steel greenhouses. Second, what you call “that experiment” is not an experiment, it is a fictional story. On this thread you sometimes know that, then you do not know that, then you know that again, then you have forgotten that you knew that. I do not know, really. (lol)
Will says:
February 17, 2013 at 2:39 am
Don says:
February 17, 2013 at 1:10 am
“It is able to detect IR radiation through the radiative heating (or, ahem, “thermalizing”, if you will) and expansion of IR-absorbing gases(!),”
No, the gas does not “thermalize” the IR. The gas is warmed by a AC current through a metal plate.
This is an “optical microphone”.
http://gentec-eo.com/Content/uploads/downloads/5-THz_Detectors/AN_121D-201924_THz.pdf
****************************************
Will, you looked up Golay cells! Good for you. I hope other readers will do the same.
The link you provide says this:
“A Golay Cell is a “photo-acoustic” device that is sensitive, works at ambient temperatures and has a broad spectral response. The basic elements that make up a Golay Cell are: the 6 mm HDPE input window, a small fragile gas chamber that includes a thin, partially absorbing metallic film and what is called an “optical microphone section”. When IR or THz radiation is absorbed by the thin film in the gas cell, the gas is heated, causing it to expand and distort the mirrored back wall of the cell. This distortion (or movement) is monitored and measured by the combination of an LED, some optics, a grating and a photodiode. The output of the photodiode is proportional to the displacement of the mirrored wall of the gas cell. Its output is calibrated against a source of known power output in Volts/Watt.”
First, to be fair, let me say that some references such as the one above do not say the IR heats the gas directly; rather it heats a film which heats the gas which expands and contracts.
But at least one reference seems to speak of the radiation heating the gas directly, though this is an insignificant detail in the larger discussion. Per Rogalski (Infrared Detectors, Second Edition), “The Golay cell (Figure 8.1) is a thermal detector consisting of a hermetically sealed container filled with gas (usually Xenon for its low thermal conductivity) and arranged so that expansion of the gas under heating by a photon signal distorts a flexible membrane on which a mirror is mounted. The movement of the mirror is used to deflect a beam of light shining on a photocell and so producing a change in the photocell current as the output.” Either way (indirect or direct heating of the gas by radiation) it is radiation from the object under measurement that does the heating, which may be cooler than the Golay cell.
No reference on Golay cells that I have read, including the one you cite, says that the “gas is warmed by a AC current through a metal plate” as you claim. WUWT?
Greg House says (February 17, 2013 at 12:55 pm): “what you call “that experiment” is not an experiment, it is a fictional story.”
It’s an experimental setup that no SooperFizzycyst has ever used to turn his fiction into reality. WUWT?
Ok, this is getting farcical. For the most hard-headed of anti-CO2ers, I present the following:
http://www.flir.com/cs/emea/en/view/?id=41703
On this link, some scientists detail pictures that they believe show CO2 escaping from a champagne glass based on their understanding of the radiative properties of CO2. If CO2 does not have those radiative properties, then it stands to reason that this picture is capturing something else besides CO2 escaping that glass.
Honestly, I am all ears. If the understanding of CO2’s radiative properties is wrong, what do the pictures on this link show (ie what is the multicolored jet shown leaving the black champagne glass when the champagne is poured).
Cheers, 🙂
Don says:
February 17, 2013 at 1:17 pm
“The Golay cell (Figure 8.1) is a thermal detector consisting of a hermetically sealed container filled with gas (usually Xenon for its low thermal conductivity)”
And yet Xenon is apparently NOT a “greenhouse gas”.
http://www.medicalgasresearch.com/content/3/1/3#B8
Can’t seem to get your “greenhouse effect” story straight, any of you!
@Will
” a small fragile gas chamber that includes a thin, partially absorbing metallic film and what is called an “optical microphone section”. When IR or THz radiation is absorbed by the thin film in the gas cell”
“The Golay cell (Figure 8.1) is a thermal detector consisting of a hermetically sealed container filled with gas (usually Xenon for its low thermal conductivity)”
They’re not using the gas to absorb ir, so no It’s not a GHG, It’s not suppose to be.
pochas says:
February 17, 2013 at 10:35 am
The steel shell model does not apply. Forget it or be confused forever. Take a simple sphere with an internal heat source of 235 w/m^2. Solve the Boltzmann equation to find its blackbody temperature T1. Now add a layer of insulation and measure the equilibrium surface temperature. Keep adding insulation until T2 is 30C higher than T1. You now have a model of the earth with atmosphere, but it still does not apply, because the atmosphere is semi-transparent to infrared.
A terrible experiment, if you want to make it close to realistic you’d have to make the following changes:
Put it in a vacuum chamber with the walls cooled to iiq He temperature (cheap alternative liq N2),
surround it with a shield of germanium, vary the thickness of the shield or use concentric shields until T2 is 30ºC higher than T1. Poly IR 3 might make a cheaper substitute for the germanium.
Shawnhet says, February 17, 2013 at 2:06 pm : “For the most hard-headed of anti-CO2ers, I present the following: http://www.flir.com/cs/emea/en/view/?id=41703
On this link, some scientists detail pictures that they believe show CO2 escaping from a champagne glass based on their understanding of the radiative properties of CO2. If CO2 does not have those radiative properties, then it stands to reason that this picture is capturing something else besides CO2 escaping that glass.
=======================================================
It is not about CO2 just having radiative properties, it is about the “greenhouse effect” as presented by the IPCC not working.
Hey, Will, I corrected and qualified my original statement re the greenhouse gas in the cell. It’s a minor side point, doesn’t change the fact that if your position is right a Golay cell cannot work as advertised and in the way it has been used for decades. If I am wrong about this, there are many intelligent and helpful and better-qualified-than-I folks who monitor this site and can correct me in a reasonable and convincing manner, and I would happily learn from it.
Stupid me, I’ve been feeding a troll. ‘bye Will.
Greg House says:
February 17, 2013 at 3:11 pm
“It is not about CO2 just having radiative properties, it is about the “greenhouse effect” as presented by the IPCC not working.”
But, there are many ways in which their schema can “not work” without invalidating the basic concept. Here are a few:
1) As I have pointed out above, the effect of raising the surface temperature above where it would be without any GHGs at all does not establish that increasing the amount of GHGs will increase it further – you can have a positive function which does not have a positive partial derivative at all points. E.g., a little alcohol makes you feel good. A lot of alcohol does not generally make you feel better.
This is especially true for a spherical body – flat Earth approximations do not capture the effect of the increasing surface area radiating into space, and decreasing the surface area radiating back to the planet. The very significant value I see in Willis’ thought experiment is that it reveals this aspect of the problem in sharp relief. As I showed here, and MikeB independently corroborated here.
2) The GHGs are not separated from the planet’s surface by a vacuum – there are avenues for conduction and, especially, convection of heat upward which are more powerful than radiative transport.
3) On a planet which is 2/3 water, evaporative cooling plays a major role in transporting heat away from the surface.
4) Somewhat related to the above, evaporation produces clouds which block sunlight, which may nullify or even reverse any other warming effect.
So, you don’t have to go all conspiracy-minded and reject even the most well-established theory in order to maintain a healthy skepticism. The basic GHE, even if true (which it is), does not establish that the Earth in its current state will be heated further by additional GHGs (which, I believe the evidence shows, it isn’t).
Greg House says:
February 17, 2013 at 3:11 pm
“It is not about CO2 just having radiative properties, it is about the “greenhouse effect” as presented by the IPCC not working.”
Ok, maybe we’re getting somewhere here. If you agree that CO2 (and other greenhouse gases) has the capacity to absorb and emit radiation in some wavelengths( as demonstrated in my champagne link), doesn’t this necessarily imply that CO2 has some ability to insulate the Earth’s surface?
IOW, doesn’t heat from the surface take longer to reach space when it has to radiate through a medium with CO2 in it than through one without it (because it “bounces” off CO2 molecules on its way out)? If not, why not? Please be specific.
Cheers, 🙂
Bart says, February 17, 2013 at 4:41 pm: ““Greg House says, February 17, 2013 at 3:11 pm: “It is not about CO2 just having radiative properties, it is about the “greenhouse effect” as presented by the IPCC not working.”
But, there are many ways in which their schema can “not work” without invalidating the basic concept. Here are a few: …”
=============================================================
I mean, the Wood experiment demonstrates that the IPCC basic concept (the “greenhouse effect” as presented by the IPCC) does not work.
If you have another basic concept, then I suggest you find another name for it, just to avoid confusion. Call it e.g. “Bartgreenhouse effect”, no problem with that. Then, however, the question would arise, why should anyone be interested in discussing “Bartgreenhouse effect”. Because certain policy, treaties, taxes etc. are all based on the “greenhouse effect” as presented by the IPCC, not on the “Bartgreenhouse effect”.
Shawnhet says, February 17, 2013 at 4:43 pm: “…doesn’t this necessarily imply that CO2 has some ability … doesn’t heat from the surface … If not, why not? Please be specific.”
===========================================================
This stuff is 150 years old. Won’t work, see the R.W.Wood experiment above.
Greg House says:
February 17, 2013 at 5:15 pm
The Wood experiment does not really demonstrate anything. The signal being sought was very tiny relative to the overall heating, the apparatus and measurements were crude. There were conductive and convective effects which could nullify the effect. To proclaim the experiment demonstrated anything, you would need to repeat it under strictly controlled conditions, and pursue any possible alternative reason for the outcome failing to produce the expected result.
But, you don’t need to. As I have pointed out above, the experiment is performed every orbit on satellites in space with MLI blankets. The theory works. But, it only works under the specific conditions to which the theory applies. The IPCC is insisting it works even where those conditions do not specifically hold. That is the weak point in their argument, and where you should address your criticisms.
Bart says:
February 17, 2013 at 5:35 pm
Greg House says:
February 17, 2013 at 5:15 pm
The Wood experiment does not really demonstrate anything. …
==============================================================
I did not say that, these are your words. It would be nice, if you could avoid this kind of confusion in the future. Thanks.
Bart says, February 17, 2013 at 5:35 pm: “The Wood experiment does not really demonstrate anything. The signal being sought was very tiny relative to the overall heating, the apparatus and measurements were crude. There were conductive and convective effects which could nullify the effect. To proclaim the experiment demonstrated anything, you would need to repeat it under strictly controlled conditions, and pursue any possible alternative reason for the outcome failing to produce the expected result.”
=======================================================
Well, exactly, the “trapped radiation effect” was found to be less than 1C, if any, “even under the most favourable conditions”.
“The conductive and convective effects” could not have had any significant influence, since the boxes were well insulated. The difference in conductive properties of rock salt and glass results actually in a higher temperature in the glass covered box than in the other one, hence the effect was apparently zero or very much close to zero.
Bart says, February 17, 2013 at 5:35 pm: “As I have pointed out above, the experiment is performed every orbit on satellites in space with MLI blankets. The theory works.”
========================================================
I know, you referred to this article from Misleadingpedia: http://en.wikipedia.org/wiki/Multi-layer_insulation.
The article seems to be fraudulent to me, because it refers to the unproven claim about “back radiation warming” (not using this expression though) and does not mention the purpose of the multi-layer insulation blankets: to protect satellite from the excessive solar heating.
Greg House says:
February 17, 2013 at 5:23 pm
“This stuff is 150 years old. Won’t work, see the R.W.Wood experiment above.”
If you want to be taken seriously, you are going to have to explain your thinking. It is looking more and more like you can’t do this. I asked you some pretty simple questions that follow directly from an experiment that you apparently can’t find anything wrong with. Either you can answer them or you can’t.
Also, for the record, if you are going to quote me, please do so accurately or simply refer to the post. What you quoted from me made me look as though I was saying the opposite of what I was actually saying.
Shawnhet says, February 17, 2013 at 7:38 pm: “Also, for the record, if you are going to quote me, please do so accurately or simply refer to the post. What you quoted from me made me look as though I was saying the opposite of what I was actually saying.”
=========================================================
No, it was not a distortion. You mean this quote, I guess: “…doesn’t this necessarily imply that CO2 has some ability … doesn’t heat from the surface … If not, why not? Please be specific.”
Nobody can conclude anything from that, either what you were asking or what you were not asking. I made the quote this particular way to illustrate that your questions were irrelevant in my view and said that the Wood experiment addressed the main issue properly (the main issue is the effect of the back radiation on the temperature of the source).
Greg House says:
February 17, 2013 at 8:25 pm
Shawnhet says, February 17, 2013 at 7:38 pm: “Also, for the record, if you are going to quote me, please do so accurately or simply refer to the post. What you quoted from me made me look as though I was saying the opposite of what I was actually saying.”
=========================================================
No, it was not a distortion. You mean this quote, I guess: “…doesn’t this necessarily imply that CO2 has some ability … doesn’t heat from the surface … If not, why not? Please be specific.”
Nobody can conclude anything from that, either what you were asking or what you were not asking. I made the quote this particular way to illustrate that your questions were irrelevant in my view and said that the Wood experiment addressed the main issue properly (the main issue is the effect of the back radiation on the temperature of the source).”
No one can conclude anything from what you posted me as saying because *you butchered what I wrote*. Here is some of what I actually posted. Clearly what was saying was a lot more clear than you are pretending it was.
IOW, doesn’t heat from the surface take longer to reach space when it has to radiate through a medium with CO2 in it than through one without it (because it “bounces” off CO2 molecules on its way out)? If not, why not? Please be specific.
So how about it? Can you respond *intelligently* to the exceedingly simple question I pose above without garbling my question or otherwise dodging it? Again, please be specific.
By the way, just in case, you were unaware just because the surface doesn’t cool primarily due to the effect of radiation doesn’t mean that the atmosphere as a whole doesn’t. All energy entering the Earth’s system must ultimately leave as radiation, so the radiative properties of the atmosphere (which you apparently don’t disagree with) are still relevant regardless of what Wood’s experiment showed about surface temps.
Shawnhet says, February 17, 2013 at 9:29 pm: “Can you respond *intelligently* to the exceedingly simple question I pose above […] the radiative properties of the atmosphere (which you apparently don’t disagree with) are still relevant regardless of what Wood’s experiment showed about surface temps.”
==========================================================
To questions, there is no obligation to answer them. Besides, you can simply make your point and people will or will not comment on it.
To your point “the radiative properties of the atmosphere (which you apparently don’t disagree with) are still relevant regardless of what Wood’s experiment showed about surface temps”, I care primarily about “greenhouse effect” as presented by the IPCC, because it is politically relevant, and it is about the surface temperatures. Luckily, the Wood experiment debunked it.
If you want to talk about things irrelevant to the surface temperatures and thus also irrelevant to the “greenhouse effect” as presented by the IPCC, it is perfectly fine with me, but you can not really expect me to participate in that.
Greg House says:
February 17, 2013 at 10:58 pm
“To questions, there is no obligation to answer them. Besides, you can simply make your point and people will or will not comment on it.”
I agree with this. My problem was with your garbling my questions in an attempt to make it seem as though you have answered those questions.
“To your point “the radiative properties of the atmosphere (which you apparently don’t disagree with) are still relevant regardless of what Wood’s experiment showed about surface temps”, I care primarily about “greenhouse effect” as presented by the IPCC, because it is politically relevant, and it is about the surface temperatures. Luckily, the Wood experiment debunked it.
If you want to talk about things irrelevant to the surface temperatures and thus also irrelevant to the “greenhouse effect” as presented by the IPCC, it is perfectly fine with me, but you can not really expect me to participate in that.”
The trouble with your argument above is that you simply do not understand the issues involved well enough to draw the conclusions you do from it.
Richard Lindzen ( a prominent skeptic) states that “the surface of the earth does not cool primarily by thermal radiation” as part of his discussion of why the GH effect is real on this link. IOW, the fact that the surface cools differently from the atmosphere as a whole does not invalidate the GH effect.
http://heartland.org/policy-documents/taking-greenhouse-warming-seriously
The problem is not so much that you are wrong, but that simultaneously you are (quite arrogantly) convinced that you are right before studying these issues. My advice is to start with the radiative properties of CO2 and other gases, read Lindzen’s paper, think about it and then see where you stand at the end of the day. Your objections are what are irrelevant in this discussion but no one besides you can make you accept this.
Cheers, 🙂
Lindzen doen’t even understand the basics.
He seem to be under the impression that the atmospheric emission height can increase without any preceding temperature increase. Merely by the addition of GHG’s. Yet there are multiple sources of empirical data that show this is utter nonsense.
The radiosonde data for example clearly show that any increase in emission height is always preceded by an increase in temperature.
He also clings the false assumption that water vapour is a positive feedback mechanism. It isn’t, water vapour is strongly negative.
These are just two obvious points where he is so far from reality that it just beggars belief.
It is safe to say that anything Linzen has to say about the so called “greenhouse effect” is not of any consequence.
Will,
Don’t destroy your credibility by saying, “Lindzen doen’t even understand the basics.”
D.B. Stealey says:
February 18, 2013 at 12:52 pm
OK what I meant to say was, Lindzen doesn’t even understand the basics.
Typo, my bad!
Will,
I think Prof Lindzen has as much understanding as anyone in the field [scroll down].
(And my comment was about credibility, not about spelling.)
[Will – I’ve told you before, you are BANNED from this website for constantly trying (via thread bombing) to put your garbage science experiment on this website, doubly so today when you call Lindzen a liar. Be as upset as you wish, but don’t come back here – Anthony Watts]