 By Christopher Monckton of Brenchley
By Christopher Monckton of Brenchley
The monthly satellite lower-troposphere temperature anomaly from Remote Sensing Systems, Inc., is now available.
Taking the least-squares linear-regression trend on this dataset (the bright blue horizontal line through the dark blue data), there has now been no global warming – at all – for 17 years 5 months.
Would readers like to make a projection of how many mainstream media outlets will report this surely not uninteresting fact?
It shows that the Hiatus hernia for true believers in the New Religion continues.
My own prediction is that the number of media reporting 17 years 5 months without any global warming will be approximately equal to the number of general-circulation models that predicted such a long Pause notwithstanding ever-rising CO2 concentration.
Print out the graph as a postcard and send it to the editor of a newspaper near you that has shut down democratic debate by announcing that it will refuse to print any letters at all from “climate deniers”.

How many times do I have to repeat, the temperature gradient evolves in all solids, liquids and gases. Thus it evolves in any wire or silver rod, and that stops any perpetual cycle of energy.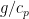 ? So the lapse rate for all materials is the same?
? So the lapse rate for all materials is the same?
Ah, so you are saying the lapse rate is not
Because if one has a lapse rate in an fluid in equilibrium, then if one alters the temperature of a surface in contact with it away from that equilibrium temperature, heat will flow and the material cannot come back into equilibrium at that lapse rate.
Once again, it is difficult to explain or understand how you could think that two different temperature profiles could both be “equilibrium” for a confined fluid. Either the temperatures of two neighboring parcels are such that no energy flows between them, or it is not.
rgb
It seems that the discussion should focus upon quasi-steady-state conditions in a non-equilibrium system. That is the actual geophysical problem.
FWIW:
A column of dry air in hydrostatic equilibrium is considered, bounded by two fixed values of the pressure, and the question is asked, what vertical temperature profile maximizes the total entropy of the column? Using an elementary variational calculation, it is shown how the result depends on what is kept fixed in the maximization process. If one assumes that there is no net heat exchange between the column and its surroundings—implying that the vertical integral of the absolute temperature remains constant—an isothermal profile is obtained in accordance with classical thermodynamics and the kinetic theory of gases. If instead the vertical integral of the potential temperature is kept fixed—as argued by several authors to be appropriate in the case of convective mixing—an isentropic profile results. It is argued that, if one wishes to apply the latter constraint, it should be used as an additional, rather than as an alternative, constraint. The variational problem with both constraints leads to a profile in between the isothermal and the isentropic extremes. This profile has the merit of reproducing very accurately the tropospheric part of the U.S. Standard Atmosphere, 1976.
This is Verkeley and Gerkema, 2004 (one of your favorites, right?). Note well that convective mixing is not an isostatic fluid at rest. If there is heat exchange, then sure then “equilibrium” won’t be isothermal. Nor will it be equilibrium!
Nobody is arguing that adiabatic lapse rates are not sustained in open atmospheric systems. However, those rates are not thermodynamic equilibrium, they are sustained by the flow of mass and heat as energy flows in and out of the system. Calling this “equilibrium” is as silly as calling the thermal gradient in matter connecting two thermal reservoirs “equilibrium”. It is a steady state, to be sure. It is not thermodynamic equilibrium, a phrase that has a very specific meaning.
Y’all are trying to twist something that you do not understand into a series of statements that are a) irrelevant; and b) taken at face value, clearly and unequivocally violate the laws of thermodynamics. I pointed this out (Trick) last time I read Verkeley — he was not then and is not now describing thermodynamic equilibrium, and he openly acknowledges that in the absence of convective mixing thermodynamic equilibrium is isothermal.
So you want to try again?
rgb
rgbatduke 4:39pm: “..Trick, explain to me how thermal equilibrium (defined as a state where heat does not flow) depends on the boundary conditions of a system, again?…5:30pm: Try again.”
Ok. Very astute question with maybe a minor terminology issue, congrat.s as this is informed & critical, shows you are making progress thinking this thru.
I will assume you mean thermodynamic equilibrium in these ideal columns as that is what I discussed and is more encompassing than just thermal equilibrium. If I am correct in this assumption, I will refer you to your linked Caballero text sec. 2.17 pp.35-37 “Thermodynamic equilibrium and heat conduction”. If not fill me in on what you mean exactly by writing “thermal equilibrium (defined as a state where heat does not flow)” with text book cites as regards entropy maximization theory in the adiabatic system of interest (the tall column of standard air).
You will find in Caballero ref. that your isothermal hypothesis is attained ideal and exact if boundary condition constraints are “weak” and there is no external gravity field (Caballero Fig. 3). Verkley 2004 Fig. 1 showed these constraints exactly in math and added the external pervasive gravity field.
Caballero’s isothermal “weak” constraints were used by the thermo. grand masters as Verkley et. al. intro. discusses – boundary constraints were “weak” in that they allowed the column to do work on the columns above and below to attain the classic isothermal solution.
Strong boundary conditions as in an ideal adiabatic container in a gravity field allow no work on the column above and below, and attain the isentropic, non-isothermal solution as shown in Bohren 1998 sec. 4.4 and Verkley 2004. Both of which can be found on line along with Caballero text.
Understanding all this thoroughly is an entry into the science behind working on understanding the FLAT 17+ years in top post. Otherwise, w/o these pre-req.s accomplished, gibberish is the blogging result.
NB1: “Thermal equilibrium” is not discussed in Caballero on-line, that cite would not be informative.
NB2: Verkley is indeed discussing an atm. in thermodynamic equilibrium, you must have missed this under 2. Maximum Entropy Profiles: “For an atmosphere in local thermodynamic equilibrium…”. Indeed the very defn. of thermodynamic equilibrium is the max. entropy point profile.
The big universe will reach this point one day in 10^100 eons, no heat will flow anywhere at max. entropy, IF our universe is truly isolated and not closed by gravity.
Trick says @ur momisugly February 12, 2014 at 5:21 pm
A little further down, Trupp specifies Loschmidt’s “source”:
Sufficiently waterboarded, The 2nd Law agrees to cooperate.
Ted 6:47pm: Well, in so far as the vertical gas column heat engine wouldn’t be perpetual motion. Last only as long as the sun is the energy source. If Loschmidt wrote that, he simply meant the sun lasts a long time – not perpetually long.
Trick says @ur momisugly February 12, 2014 at 7:08 pm
Trupp concludes his article with a juicy Loschmidt quote:
Evidently, Loschmidt first tried to be a businessman, several times. Academia was his fall-back, and he only started at 33. He appears to have copped quite an attitude toward Capitalism.
NB1: “Thermal equilibrium” is not discussed in Caballero on-line, that cite would not be informative.
NB2: Verkley is indeed discussing an atm. in thermodynamic equilibrium, you must have missed this under 2. Maximum Entropy Profiles: “For an atmosphere in local thermodynamic equilibrium…”. Indeed the very defn. of thermodynamic equilibrium is the max. entropy point profile.
There are not two thermodynamic equilibria. As Caballero and everybody else who does atmospheric physics seems to understand, the Dry Adiabatic Lapse Rate is derived by assuming parcels of air that are in motion, uplifting and adiabatically expanding from a base temperature on the ground. They thus follow an adiabatic curve, which is of course not isothermal. Nor does it describe a state of global thermodynamic equilibrium — it is a quasi-static curve associated with net energy transfer. In the atmosphere, it is even fussier than it is when one considers fluid in cylinders in classical thermo, because what goes up one place must come down someplace else. It is actually interesting to note that there enough persistent vertical mixing in the atmosphere to maintain an approximately adiabatic lapse rate.
Take that same atmosphere and place it in an unheated insulating container and wait, and it will first stop convecting, as convection is driven by energy transfer from a hot to a cold reservoir. Its original profile might well have been a DALR, but once it has time to equilibrate it will be isothermal. Verkeley seems to understand this. Textbooks on atmospheric physics most definitely discuss this. If one solves the NON-equilibrium Navier-Stokes equations for non-turbulent convection one can derive this.
I repeat — what is at issue here is not that there is a lapse rate in a planetary atmosphere, any more than that was at issue back when I first discussed this with e.g. Jelbring. It is whether or not that atmosphere is in stable thermodynamic equilibrium, whether or not GRAVITY ACTS LIKE A MAXWELL DEMON to sort out hotter molecules with more average kinetic energy on the bottom AS A STABLE, ONGOING PROCESS rather than as a one-shot heating as e.g. an atmosphere collapses. It does not. That is a butchery of both stat mech and the concepts of thermodynamics. If molecules on the bottom of an atmosphere are intrinsically hotter because they have more gravitational potential energy in bulk, then one can build perpetual motion machines of the second kind. And one can’t.
One can indeed have stable states in open thermodynamic systems. As I said, put a conducting rod between two heat sinks and it will settle down to a stable thermal profile. Heat a pan full of water on the bottom gently, and it will establish a more or less stable pattern of convection driven by water heating, rising, cooling, falling. One can (with some license) call these states “equilibria”, and in coarse-grained chunks of the material in question there can be enough molecules with similar enough temperature for the notion of a local temperature to apply (which is all “local thermodynamic equilibrium” means, that one can talk about a varying temperature field in a material), but they are not global thermodynamic equilibria because that is isothermal. In all cases heat is moving around in the material, carried in the bulk motion of parcels as they rise or fall or as it enters on one (hotter) side of a slice and exits on the other (cooler) side of a slice.
The Earth’s atmosphere is without question heated on the bottom and cooled on the top. Other atmospheres are also differentially heated. Differential heating/cooling and gravity are a sufficient condition for convection, and convection leads to a DALR as opposed to isothermal atmosphere as a more or less steady state. But it is not thermodynamic equilibrium, and one cannot build a perpetual motion machine from it because it is a HEAT ENGINE. The entire atmosphere is a heat engine, with its motion driven by temperature differences. Of course it can do work.
rgb
rgbatduke 6:11am: “Its original profile might well have been a DALR, but once it has time to equilibrate it will be isothermal. Verkeley seems to understand this.”
Agree initial condition environmental DALR, ref. Verkley Fig. 2. Verkley understands and Caballero teaches that boundary conditions matter for the tall ideal column of standard air.
“Weak” boundary conditions meaning adiabatic column allowed to do work on column above and below = classic isothermal constant T(z) solution at thermodynamic equilibrium max. entropy point dS/dt=0.
“Strong” boundary conditions meaning adiabatic column not allowed to do work on columns above and below = modern text book non-isothermal varying T(z) given by Bohren 1998 eqn. 4.149 Poisson eqn. at thermodynamic equilibrium max. entropy point dS/dt=0.
“Differential heating/cooling and gravity are a sufficient condition for convection..”
The sufficient condition for convection also includes higher temperature to be at the bottom of the fluid. Troposphere increased in temperature from bottom = convection & DALR, stratosphere increased in temperature from the top = little convection, no DALR for certain z range.
“The Earth’s atmosphere is without question heated on the bottom and cooled on the top.”
Majority of Earth system thermal energy source is the higher temperature sun except for the negligible geothermal component and the negligible CMB. Earth system radiates energy from the TOA and reflects it to sink of deep space, all in ~steady state.
Modulating atm. composition infrared-active gas ppm will affect global near surface mean temperature in opposition to global mean temperature at great height; science can’t yet establish the magnitude of the modulation. Nor explain FLAT 17+ years; I confidently predict science will progress. I also think blogs evidently increase the progress speed as the former print publishing of Einstein/Maxwell eras was much slower – measured in something like decades. Couple posts from them:
http://www.jstor.org/stable/108968
http://www.fourmilab.ch/etexts/einstein/E_mc2/e_mc2.pdf
“Weak” boundary conditions meaning adiabatic column allowed to do work on column above and below = classic isothermal constant T(z) solution at thermodynamic equilibrium max. entropy point dS/dt=0.
“Strong” boundary conditions meaning adiabatic column not allowed to do work on columns above and below = modern text book non-isothermal varying T(z) given by Bohren 1998 eqn. 4.149 Poisson eqn. at thermodynamic equilibrium max. entropy point dS/dt=0.
“Allowed to do work” isn’t the point. The atmosphere can only actually do work when it moves. And in equilibrium it is not in motion. The boundary condition involved isn’t pressure at the bottom and/or top of the column, it is the temperature of the top and bottom of the column, or more precisely, whether or not the column is connected to heat source/sinks at the top or the bottom.
The DALR does not, after all, extend to the top of the atmosphere, does it? It extends to the tropopause. Why is that, do you thin? Could it be because the tropopause is the break point between radiative cooling as GHGs become transparent and radiative warming of the stratosphere on up? There is no possible way one can consider the atmosphere of a planet to be like a column of air, but that does not mean its steady state atmosphere (to the extent that any such thing exists, especially on the Earth where it certainly does not) is in thermodynamic equilibrium.
As I said, we are quibbling about an irrelevant point. We seem to be in agreement that Visiting Physicist is wrong in his belief that he alone understands the temperature profile of planets, and I assume that we would likely agree that Nikolov and Zeller are similarly wrong, especially since whatever they used for planetary and moon surface temperatures in their plot, they weren’t the accepted published values and when plotted correctly not even their multiparameter fit equation actually fit the data.
I would guess that we agree that the atmosphere of any planet warmed by a sun will have a troposphere (whether or not it is warmed from above or below). We agree that the troposphere will have a lapse rate, because any differentially warmed atmosphere will have a convective turnover and hence its steady state (driven) atmospheric thermal profile will tend to be adiabatic, not thermal equilibrium isothermal. Basically, conduction will be too slow to equilibrate the quasi-static, approximately adiabatic parcels as they turn over, carrying heat from source to sink. We disagree on whether or not an adiabatic lapse can be called “thermal equilibrium” or “thermodynamic equilibrium” — to me the question is absolutely clear but if you want to make up terminology that considers systems with active heat transport to be in equilibrium that’s fine with me as long as you recognize that you will have a hard time communicating with your own made up language. Sure, the parcels are in local equilibrium (have a well defined coarse grain average temperature and meaningful local properties like coarse grain average density and pressure) but they do not fit the thermodynamic equilibrium profile and net energy is being transported in order for the state to exist at all. But I can’t/won’t argue with a private language, as long as it isn’t used to communicate things or reason out things by using a term two inconsistent ways that are not true.
The one remaining point to clear up is the second law, which is precisely where your private language is getting you into trouble. The thing that makes it very clear that your nomenclature is not standard is that you are asserting that when I put a silver rod into an atmosphere connecting the top and the bottom, the atmosphere was in equilibrium before but now changes to a new equilibrium. You assert that the silver itself maintains some sort of “lapse rate”, and (most disturbing) that there exists a state where the top and bottom of the air column are at different temperatures and no heat is flowing through the silver!
This last thing is an absolutely direct violation of the second law, as I hope you recognize. No matter how you orient a silver bar in a gravitational field, the entropy of the system will increase if heat is transferred from the hot side to the cold side, and this transfer will occur as long as the two ends of the rod are at different temperatures. The precise same thing is true of the gas column without the silver, of course — it has a finite (poor) conductivity and given time and no external disturbance or heat sources or sinks it will eventually equilibrate isothermal. But with a good conductor connecting the two ends, no matter what state of stress you imagine the silver to be in as a function of gravitational height, it is pretty easy to see that the entropy gradient for any two adjacent slices at different heights is towards equal temperatures in the slices. Even if the silver does some work expanding or contracting as it reaches equilibrium, even if this work is differential along its length, this work is not cyclic! As it relaxes, the entropy gradient is monotonic towards isothermal, period, with heat always being conducted from hot to cold until it is attained.
The same exact thing is true in the atmosphere, of course. In a static air column, it might well expand or contract towards an adiabatic temperature profile if released from an arbitrary state as long as there is sufficient motion and freedom to allow the gas parcels to expand (do adiabatic work) or contract (have adiabatic work done on them), but this expansion is not cyclic and once the adiabatic lapse profile is attained, it is entropy-unstable. Parcel by parcel from the top to the bottom, one can always gain entropy by equalizing their temperatures. Nick pointed this out up above as well. The consequences of connecting the top and bottom of the air column with a good conductor of heat simply makes this consequence impossible to ignore.
To conclude, if one prepares a closed system in some way and then permits that system to relax for an infinite amount of time, there are not two possible states for that system to be found in, there is only one. That state is thermodynamic equilibrium, and it is isothermal as long as the different components of the system are thermally connected at all. If it were any other state, we could always use this system to build a perpetual motion machine of the second kind, build an engine whose sole effect is to take energy from a hot reservoir to a colder reservoir, build a heat engine whose efficiency exceeds the efficiency of a Carnot cycle (transforming heat directly into work) etc. The proofs of this are given in any intro thermo textbook. Even if you want to propose a true-equilibrium lapse rate, it would have to be the same true-equilibrium lapse rate for all materials in a gravitational field or one can always build a second law violating engine or refrigerator by tapping into the energy in flow when the two systems are connected, turning some of it into work, and then removing it. Wait, and the original air column has to return to a lapsed profile. Reconnect it, and it does still more work. Disconnect it and wait for it to come to a new lapsed profile. Reconnect it and it does more work.
By simply connecting and disconnecting a system with a different “equilibrium” lapse rate and hence different thermal profile supposedly incapable of doing work, one can turn the heat content of the original gas into work outside of the pair of systems altogether with no other change being made in the Universe. Oops. Imagine a simple ideal electrical thermocouple, for example, connected to the top and bottom of your lapsed fluid with thermally superconducting wires and running a small electrical motor outside of your isolated air column. It will run as long as there is a thermal lapse, turning heat in the air column into work. By alternately heating the gas to maintain its total energy content and then letting the thermocouple do work across the supposed spontaneous, stable lapse rate that results, one turns heat directly into work indefinitely.
All of this is well-known, as Ted pointed out above. It is old news! So old that it is nineteenth century news, in fact. Note that I do not care a whit what you imagine the “boundary conditions” on the gas to be — if you can ever prepare such a gas and it ever spontaneously develops a true thermodynamic equilibrium lapse rate, I will show you how to build a perpetual motion machine and violate all of the statements of the second law with it, because I will not need a cold reservoir into which to reject any added heat. I just have to wait a bit, and your gravitational Maxwell’s Demon will make a cold reservoir for me.
All of this is so very elementary. It is literally kiddy-physics textbook stuff. A system with an equilibrium thermal gradient can always be connected by a Carnot cycle engine across that gradient and the second law is finished. Hence thermal equilibrium is isothermal, or it would permit the violation of the second law. This is provable with big, block diagrams — it needs nothing fancy. You literally have to postulate that equilibrium temperature in a medium is an absolute function of gravitational field strength — something that is utterly indefensible and easily proven false and indeed contradicted by the actual formula for the DALR gradient — in order to avoid this failure, and I’m not certain one could avoid it even then.
rgb
Modulating atm. composition infrared-active gas ppm will affect global near surface mean temperature in opposition to global mean temperature at great height; science can’t yet establish the magnitude of the modulation. Nor explain FLAT 17+ years; I confidently predict science will progress.
And here, we do not necessarily disagree. Indeed, this doesn’t disagree with the GCM predictions. Increasing GHGs are naively expected to increase surface temperatures and may or may not have much effect at the top of the troposphere. I do not find the arguments there convincing, nor do I find them well supported even by books like Petty’s book on atmospheric radiation. Band spreading is a matter of absolute pressure, not partial pressure, so it is difficult to see how it will be measurably modulated by increasing a trace gas in an atmosphere with 5% modulations in surface pressure due to mere weather happening all the time. The troposphere itself is long since opaque, and it is well empirically established that even planets with no meaningful GHG concentrations develop troposphere and stratosphere — the top of the troposphere may be weakly modulated by GHG concentrations, but the dominant determinant of tropospheric height is very likely convective turnover. Also, the actual atmosphere’s effective radiation height is horrendously nonlinear, horrendously varying function of water vapor content, cloud cover and type, frequency, time of day, latitude, temperature, GHG concentrations, aerosol concentrations, particulate concentrations, and whether or not there is a kitchen sink floating around.
But there is literally no point in asserting that a lapse rate is stable thermodynamic equilibrium. It is sufficient to note that planetary atmospheres empirically establish a troposphere with a lapse rate, that the lapse rate can be understood by considering the thermal profile of approximately adiabatic parcels of atmosphere as they rise and fall in atmospheric convection (maintained by temperature differential between heating and cooling mechanisms), and that this is one important component of the mechanism responsible for atmospheric warming misnamed “the greenhouse effect” even though it is vastly more complex than single slab greenhouse models admit. In addition to being incorrect (equilibrium is isothermal, or the second law can easily be violated) it just gives warmists yet another opportunity to correctly point out that some skeptical arguments are crazy wrong. And what’s the good in that?
rgb
rgbatduke 8:38 & 8:54am: You continue to let conduction (and enthalpy) in solids cloud your thinking about ideal mathematical thermodynamic equilibrium (TE) in a gas and I fail to see why you think TE is my private term when I ref.d TE in Caballero for you. TE is ideal defined where dS/dt=0 at the max. point, look it up in any other thermo. text. (S=entropy value.)
The adiabatic tall column of standard air allowing no work on external columns is proven in modern times non-isothermal T(z) by both Dr. Bohren in 1998 and Verkley in 2004 (confirmed by Akmaev 2006) all by letting the math do the work not their intuition. Why you object to their solid proofs is not apparent to me in your responses that confuse idealized and real atm. processes. You will not get a passing grade in this class until you accomplish the reading assignment Bohren 1998 sec. 4.4 and/or can show exactly where these three published authors are incorrect, misstep. If you really think differently and can prove it, is a paper forthcoming?
I can see I am not getting through to you to update your thinking into modern times and it is useless to continue. My office door remains open.
Hint: My aluminum flagpole out in the backyard is just like your silver wire. Observation shows my pole obeys the second law (no PPM – it won’t power my BBQ forever as much as I wish it would and you seem to think it should); it matches the air temperature mean over time. Just like any silver wire would do inserted in the Bohren and Verkley tall columns attaining Poisson eqn. T( z) 4.149 at TE without causing any sort of PPM. Of course the wire will alter the math more difficult but not cause PPM.
Your silver wire does not cause PPM either even though the relevant Poisson eqn. is the ideal column T field solution, you need to let your intuition yield to the math in sec. 4.4 of Bohren 1998 to discover the detailed physics. The mechanics are really elegant & non-intuitive, which is probably what attracted my attention to all this in the 1st place.
Modern understanding is necessary but not sufficient to understand FLAT in top post.
Trick and rgbatduke:
Not to stir the pot, but I can’t resist the temptation to mention my forlorn attempt here: http://wattsupwiththat.com/2012/01/24/refutation-of-stable-thermal-equilibrium-lapse-rates/#comment-874313 to obtain help with the Velasco et al. and Román et al. papers–which I interpreted as demonstrating that Dr. Brown’s proof was flawed.
Despite Dr. Brown’s characteristically admirable patience in that thread, I remain of the opinion that he did not effectively defend his proof there, either. (But I have to admit that the majority on that thread thought otherwise.)
They thought otherwise because this isn’t complex, it is quite simple, and most people clearly understand this. Trick continues to fail to explain why one cannot do work by putting a heat engine between the temperature differences “spontaneously and stably” created. If one can, under any circumstances, then the second law is egregiously violated. Even his agreement that the lapse rate will change if one connects the top and bottom of the air column with a wire that is a good thermal conductor (which could easily be insulated everywhere except at the top and bottom) is sufficient to show that the second law is violated.
What Trick seems quite incapable of grasping is that whether or not heat flows from the bottom to the top of the wire is determined by one thing only. Whether or not the bottom and top are at the same temperature. The wire has no knowledge of why they are at different temperatures, it only knows that when molecules bounding the wire at the bottom bounce against it, they do so with a certain average kinetic energy, and ditto at the top. If they are at different temperatures, heat will flow through the wire continuously until they are at the same temperature.
If the bottom and top of the gas column are at different temperatures, heat will flow up the wire and down through the gas forever. Note that these are distinct flows. Note also that one could break the silver wire and attach its two sides to an ideal Carnot cycle engine (isolated from the gas) anywhere in between and do work across the temperature difference. It doesn’t matter how small it is — there cannot be any temperature difference or else the second law could be violated by an ideal Carnot cycle engine placed in between the reservoirs.
But again, I’m getting a bit bored working through this yet again. If you (Trick) want to email Caballero and ask him if an atmosphere with a DALR is in true thermodynamic equilibrium or if true equilibrium is isothermal, perhaps you will believe him. His contact information is readily available. Or write Bohren — I don’t care. Just be sure to ask why one cannot do work with the lapse-rate maintained temperature, because if one can, even in principle one can build a perpetual motion machine.
In the words of Eddington, I think you should all just collapse in abject humiliation. Your assertion violates the second law, and there is no hope for it.
rgb
But, Dr. Brown, as I explained to you at the time, your “proof” begged the question.
And you never did directly answer the (quite simple) thought experiment I opened with.
My reading of Velasco et al. is that it is a demonstration, based on no assumptions other than the basic axioms of statistical mechanics, that at equilibrium there is in fact a non-zero (albeit, for any significant quantity of gas, quite small) translational-kinetic-energy gradient in the presence of a gravitational field. If you haven’t disproved that through statistical mechanics, your “refutation” is illusory. .
Well, illusory except for the implicit violation of the second law. Because if a gas has a lapse rate and metal has a different one then you have perpetual motion. The only way to avoid a violation of the second law is for all material objects to come to the same thermal gradient in a gravitational field. I’m hoping you can see why this is not ever going to be the case.
This is also the way you can see why Velasco et al is almost certainly wrong in their conclusion. It is all about Maxwell Demon models. Microdynamics that preferentially sorts faster molecules spatially in equilibrium violates detailed balance and inevitably permits second law violations.
Note that the second law doesn’t care about how large the violation is — if it is macroscopically violated you can, in principle, work free-lunch magic.
Do you really think that this could happen, that there could be free lunch engine simply turning heat directly into work, a refrigerator that doesn’t require a power supply? Because those are direct consequences of a thermodynamic equilibrium lapse rate.
rgbatduke said @ur momisugly February 13, 2014 at 8:38 am
And not only was this all cut-and-dried science, but for several generations steam engines had been a veritable craze in 19th C society. While actual engineers were in charge at corporate design departments, “technicians” operated steam plants in vast numbers.
But whereas the main consequence of mishandling a modern gasoline, internal combustion engine is that it dies, the result of mishandling steam engines is all-too-likely that the operator and everyone nearby, dies.
Furthermore, steam engines were a large investment. Damage or premature wear could take the profit, quickly.
So actually … all steam plant operators were known as “engineers”, and were expected to have a functional command of the thermodynamics involved … which was basically the only way to remain alive, and productive.
Thus, not only was this all cut-and-dried in Academia, it was very real & intensely engaged, on the popular level. Like with the aviation that soon came along, there was no way to fake a command of heat engines, in the steam era.
NACA, the predecessor of NASA, was formed early the aviation era. It was institutionally modeled on the long-standing external combustion heat engine culture.
Joe Born said @ur momisugly February 13, 2014 at 1:40 pm
I glanced quickly at your linked comment, and see a specific misstep that will derail the effort.
You are posing a single molecule, and asking to consider it under manipulations.
However, the breakthrough of Maxwell & Boltzmann, was the realization that individual molecules cannot provide a way to treat thermodynamic matters.
Their contributions, was to put the effort on a statistical basic … considering masses of molecules, statistically. Individual or isolated molecules are not amenable to your goals … and that was central advance, back in the 19th C.
tc
Joe Born said @ur momisugly February 13, 2014 at 1:40 pm
The breakthrough of Maxwell & Boltzmann, was that we can’t do individual molecules.
Their key contribution, was to put the effort on a statistical basic … considering probabilities & parameter-distributions with masses of molecules, statistically. Individual or isolated molecules are not amenable to modeling or measuring … and that was the central advance, back in the 19th C.
Loschmidt (close associate of the above 2) wanted to stick with trying to say things about the isolated molecule, and that’s where he lost out, as the achievable future developed on a different track.
tc
rgbatduke 3:23pm: “If the bottom and top of the gas column are at different temperatures, heat will flow up the wire and down through the gas forever.”
No. It is your assertion violates the second law right there, and there is no hope for it. Your statement is incorrect. Entropy will increase, reach a maximum & heat will stop flowing. If what you write were possible, my aluminum flagpole out back would be a perpetual motion machine. It is not. Nor can it ever become one.
The 2nd law works; the silver wire in the ideal tall adiabatic column of standard air will come into thermodynamic equilibrium with the gas in the gravity field at the max entropy point if left alone long enough and heat will stop flowing as it must at dS/dt=0 with T( z) given by the Poisson eqn. Just like the heat death of our isolated big universe at max. entropy. Why you do not just look Dr. Bohren’s work up is beyond me. It has been in the literature now for 15 years, not disproved but backed and extended by further work from other authors.
There is nothing unphysical that happens dropping a silver wire into a tall adiabatic column of air with T( z) as a function of pressure & that was at thermodynamic equilibrium or even if it was at the standard lapse to begin. Left alone, there will be a new thermodynamic equilibrium established at dS/dt=0 T( z) non-constant in a gravity field, no flow of heat forever. The math proves it; check out Bohren 1998 sec. 4.4 where this is proven rigorously.
It is not that I can’t see it; you have to convince me where you see Dr. Bohren’s physical science work is incorrect – I don’t have to ask him, he already showed it and is further backed by work of Verkley and Akmaev. So far you have only incorrect, confused assertion & have not shown your own work to maximize entropy at dS/dt=0 of the adiabatic system of interest (with or without wire). Or provide a link as I have done.
******
Joe Born – I agree, Velasco eqn. 8 had it right also but in a limited circumstance; earlier backing for Dr. Bohren’s work that made T( z) field non-constant in gravity field & completely generalized to the system of interest.
I’ll say it again, even before this global warming fad started I knew my history. I knew there was a medieval warm period because historians, researching the historical records written by eye witnesses, said so. There was a Viking settlement in an area now covered by permafrost, therefore the area was warmer then than today. They had to import wine to England from France after the 14th century, (a little aside I found when reading A N Wilson’s biography of Elizabeth I) because climate change rendered the land incapable of growing wine. Etc Etc Etc. So what are my scientific credentials for repudiating the notion of AGW? None. And I don’t need them. The thought that only scientific knowledge is valid knowledge is absurd. Read some history. No. I am not rejecting science, for the science of those who know their business actually confirms the real historical record, surprise surprise. So I for one rejoice that I got my degree in the Arts. I don’t dispute that some know that AGW is nonsense through science. But I know it through history. And this is why I am incensed at the bare faced lies told by Mann and co to invent the hockey stick. I would trust a contemporary painting of an Ice Fair on a frozen Thames in the 17th century of thereabouts before I trust a “scientist” who would contradict an incontrovertible fact of history
Ted Clayton:
I agree with you that thermodynamics has little to say about an isolated particle (which was the subject of my thought experiment), but adding particles indefinitely to that experiment only reduces the gradient; it doesn’t eliminate it; the gradient approaches zero asymptotically but never reaches it.
As I interpret their work, that is what Velasco et al. say. (I’m not a physicist, and I’m open to being proved wrong. Indeed, I don’t profess to have comprehended all of Velasco et al. But I do recognize a reasoned argument, and, despite their credentials, none of the physicists who tried to do so has convincingly either (1) shown that Velasco et al. should be interpreted otherwise or (2) demonstrated that Velasco et al. were wrong.)
Trick: Yes, Velasco et al. did not attach the external system, whose attachment would cause the resultant composite system to reach a new equilibrium state, presumably one with an even smaller gradient than Velasco et al. define.
I doubt that I’ll invest in the Bohren book, but is the excerpt to which you refer available online somewhere?
Now, the gradient Velasco et al. specify is extremely small; its too small to be measured as a practical matter. Despite my surname, I’m no physicist, so I don’t know enough quantum mechanics to answer the further question: is it too small to be measured even in principle?
rgbatduke: “[I]f a gas has a lapse rate and metal has a different one then you have perpetual motion. The only way to avoid a violation of the second law is for all material objects to come to the same thermal gradient in a gravitational field. I’m hoping you can see why this is not ever going to be the case.”
As I said before, you’re begging the question. You’re assuming that at equilibrium the coupled system would have to exhibit a gradient the same as those the constituent systems do when they’re isolated. As I said before, I’m no physicist, just a retired lawyer. But it strikes me as extraordinary that physics would dictate such a relationship.
Joe Born 2:31am: No need to invest in the Bohren 1998 book, it is readily available for a loan thru a local college or even public library. I had thought rgbatduke would eventually ask where to find a copy, but shows no interest in learning the facts oppose his intuition from solids. Thermal equilibrium is for two bodies, zeroth law for 3 bodies where the tall column is one body. Thermodynamic equilibrium at dS/dt=0 applies to all single bodies like the tall column or the big universe.
Can also get the full text online here if this link works, you will have to know the pre-req.s to completely understand on the way to contributing to why the top post is FLAT but the authors do a good job explaining in the Queen’s English:
http://bib.convdocs.org/docs/7/6307/conv_1/file1.pdf
”..gradient Velasco et al. specify is extremely small…”
Agree, and I prefer to use Bohren’s p. 166 eqn. 4.149 to find the small percentage difference in mean T(p) at top of column from mean To (theta) at surface through the function of p(z) for an ideal column from say surface 1 bar to the pressure at earth tropopause – the salient fact being T( z) is non-isothermal when dS/dt=0 as the column comes into thermodynamic equilibrium with or without a silver wire (or my aluminum flagpole). How small exactly is a product of the physics and constants.