Guest post by Robert G. Brown
Duke University Physics Department
The Problem
In 2003 a paper was published in Energy & Environment by Hans Jelbring that asserted that a gravitationally bound, adiabatically isolated shell of ideal gas would exhibit a thermodynamically stable adiabatic lapse rate. No plausible explanation was offered for this state being thermodynamically stable – indeed, the explanation involved a moving air parcel:
An adiabatically moving air parcel has no energy loss or gain to the surroundings. For example, when an air parcel ascends the temperature has to decrease because of internal energy exchange due to the work against the gravity field.
This argument was not unique to Jelbring (in spite of his assertion otherwise):
The theoretically deducible influence of gravity on GE has rarely been acknowledged by climate change scientists for unknown reasons.
The adiabatic lapse rate was and is a standard feature in nearly every textbook on physical climatology. It is equally well known there that it is a dynamical consequence of the atmosphere being an open system. Those same textbooks carefully demonstrate that there is no lapse rate in an ideal gas in a gravitational field in thermal equilibrium because, as is well known, thermal equilibrium is an isothermal state; nothing as simple as gravity can function like a “Maxwell’s Demon” to cause the spontaneous stable equilibrium separation of gas molecules into hotter and colder reservoirs.
Spontaneous separation of a reservoir of gas into stable sub-reservoirs at different temperatures violates the second law of thermodynamics. It is a direct, literal violation of the refrigerator statement of the second law of thermodynamics as it causes and maintains such a separation without the input of external work. As is usually the case, violation of the refrigeration statement allows heat engines to be constructed that do nothing but convert heat into work – violating the “no perfectly efficient heat engine” statement as well.
The proposed adiabatic thermal lapse rate in EEJ is:

where g is the gravitational acceleration (presumed approximately constant throughout the spherical shell) and cp is the heat capacity per kilogram of the particular “ideal” gas at constant pressure. The details of the arguments for an adiabatic lapse rate in open systems is unimportant, nor does it matter what cp is as long as it is not zero or infinity.
What matters is that EEJ asserts that ![]() in stable thermodynamic equilibrium.
in stable thermodynamic equilibrium.
The purpose of this short paper is to demonstrate that such a system is not, in fact, in thermal equilibrium and that the correct static equilibrium distribution of gas in the system is the usual isothermal distribution.
The Failure of Equilibrium

In figure 1 above, an adiabatically isolated column of an ideal gas is illustrated. According to EEJ, this gas spontaneously equilibrates into a state where the temperature at the bottom of the column Tb is strictly greater than the temperature Tt at the top of the column. The magnitude of the difference, and the mechanism proposed for this separation are irrelevant, save to note that the internal conductivity of the ideal gas is completely neglected. It is assumed that the only mechanism for achieving equilibrium is physical (adiabatic) mixing of the air, mixing that in some fundamental sense does not allow for the fact that even an ideal gas conducts heat.
Note well the implication of stability. If additional heat is added to or removed from this container, it will always distribute itself in such a way as to maintain the lapse rate, which is a constant independent of absolute temperature. If the distribution of energy in the container is changed, then gravity will cause a flow of heat that will return the distribution of energy to one with Tb > Tt . For an ideal gas in an adiabatic container in a gravitational field, one will always observe the gas in this state once equilibrium is established, and while the time required to achieve equilibrium is not given in EEJ, it is presumably commensurate with convective mixing times of ordinary gases within the container and hence not terribly long.
Now imagine that the bottom of the container and top of the container are connected with a solid conductive material, e.g. a silver wire (adiabatically insulated except where it is in good thermal contact with the gas at the top and bottom of the container) of length L . Such a wire admits the thermally driven conduction of heat according to Fourier’s Law:

where λ is the thermal conductivity of silver, A is the cross-sectional area of the wire, and ΔT=Tb–Tt . This is an empirical law, and in no way depends on whether or not the wire is oriented horizontally or vertically (although there is a small correction for the bends in the wire above if one actually solves the heat equation for the particular geometry – this correction is completely irrelevant to the argument, however).
As one can see in figure 2, there can be no question that heat will flow in this silver wire. Its two ends are maintained at different temperatures. It will therefore systematically transfer heat energy from the bottom of the air column to the top via thermal conduction through the silver as long as the temperature difference is maintained.

One now has a choice:
- If EEJ is correct, the heat added to the top will redistribute itself to maintain the adiabatic lapse rate. How rapidly it does so compared to the rate of heat flow through the silver is irrelevant. The inescapable point is that in order to do so, there has to be net heat transfer from the top of the gas column to the bottom whenever the temperature of the top and bottom deviate from the adiabatic lapse rate if it is indeed a thermal equilibrium state.
- Otherwise, heat will flow from the bottom to the top until they are at the same temperature. At this point the top and the bottom are indeed in thermal equilibrium.
It is hopefully clear that the first of these statements is impossible. Heat will flow in this system forever; it will never reach thermal equilibrium. Thermal equilibrium for the silver no longer means the same thing as thermal equilibrium for the gas – heat only fails to flow in the silver when it is isothermal, but heat only fails to flow in the gas when it exhibits an adiabatic lapse in temperature that leaves it explicitly not isothermal. The combined system can literally never reach thermal equilibrium.
Of course this is nonsense. Any such system would quickly reach thermal equilibrium – one where the top and bottom of the gas are at an equal temperature. Nor does one require a silver wire to accomplish this. The gas is perfectly capable of conducting heat from the bottom of the container to the top all by itself!
One is then left with an uncomfortable picture of the gas moving constantly – heat must be adiabatically convected downward to the bottom of the container in figure 1 in ongoing opposition to the upward directed flow of heat due to the fact that Fourier’s Law applies to the ideal gas in such a way that equilibrium is never reached!
Of course, this will not happen. The gas in the container will quickly reach equilibrium. What will that equilibrium look like? The answer is contained in almost any introductory physics textbook. Take an ideal gas in thermal equilibrium:
![]()
where N is the number of molecules in the volume V, k is Boltzmann’s constant, and T is the temperature in degrees Kelvin. n is the number of moles of gas in question and R is the ideal gas constant. If we assume a constant temperature in the adiabatically isolated container, one gets the following formula for the density of an ideal gas:

where M is the molar mass, the number of kilograms of the gas per mole.
The formula for that describes the static equilibrium of a fluid is unchanged by the compressibility (or lack thereof) of the fluid – for the fluid to be in force balance the variation of the pressure must be:
(so that the pressure decreases with height, assuming a non-negative density). If we multiply both sides by dz and integrate, now we get:

Exponentiating both sides of this expression, we get the usual exponential isothermal lapse in the pressure, and by extension the density:
![]()
where P0 is the pressure at z=0 (the bottom of the container).
This describes a gas that is manifestly:
- In static force equilibrium. There is no bulk transport of the gas as buoyancy and gravity are in perfect balance throughout.
- In thermal equilibrium. There is no thermal gradient in the gas to drive the conduction of heat.
If this system is perturbed away from equilibrium, it will quickly return to this combination of static and thermal equilibrium, as both are stable. Even in the case of a gas with an adiabatic lapse rate (e.g. the atmosphere) remarkably small deviations are observed from the predicted P(z) one gets treating the atmosphere as an ideal gas. An adiabatically isolated gas initially prepared in a state with an adiabatic lapse rate will thermally equilibrate due to the internal conduction of heat within the gas by all mechanisms and relax to precisely this state.
Conclusion
As we can see, it is an introductory physics textbook exercise to demonstrate that an adiabatically isolated column of gas in a gravitational field cannot have a thermal gradient maintained by gravity. The same can readily be demonstrated by correctly using thermodynamics at a higher level or by using statistical mechanics, but it is not really necessary. The elementary argument already suffices to show violation of both the zeroth and second laws of thermodynamics by the assertion itself.
In nature, the dry adiabatic lapse rate of air in the atmosphere is maintained because the system is differentially heated from below causing parcels of air to constantly move up and down. Reverse that to a cooling, like those observed during the winter in the air above Antarctica, and the lapse rate readily inverts. Follow the air column up above the troposphere and the lapse rate fails to be observed in the stratosphere, precisely where vertical convection stops dominating heat transport. The EEJ assertion, that the dry adiabatic lapse rate alone explains the bulk of so-called “greenhouse warming” of the atmosphere as a stable feature of a bulk equilibrium gas, is incorrect.
Bryan says: “The ‘known reserves’ of fossil fuels is often touted as being the actual reserves.”
Quite true. But wikipediasuggests that 5.5×10^24 J of solar energy hits the earth each year, but the total reserves of petroleum are 7.9×10^21 J. In other words, diverting 0.1% of the solar energy for one year straight into chemical energy would be enough to creates ALL of the known petroleum in the world. This amounts to 0.3 W/m^2 of absorbed energy, which would be a significant amount. But this requires a net creation and storage of energy equivalent to ALL the known petroleum in the world EACH YEAR. If instead we consider the production spread out over 100’s of millions of years, the sequestration rate would be microwatts per square meter — which counts as insignificant in anyone’s book.
Creation of fossil fuels is clearly not the answer. But there could be other answers. Can you find specific suggestions and specific numbers to show the significance?
Myrrh has some website that states that water is transparent, but instead of contacting that web site and asking whether they really mean ‘nearly transparent’ he has decided that everyone else has falsified physics. Seems backwards to me.
For anyone who is actually interested, here is the explanation of how water absorbs (weakly, but not zero!) in the visible.
http://www.lsbu.ac.uk/water/vibrat.html#ir
Note that amusingly (since Myrrh insists that only molecular vibrations are ‘heat’) that it is actually a vibrational (overtone) band that is responsible.
Robert Brown: “I’ve been working on a matlab/octave ODE program that directly compares the thermodynamics of an isothermal gas column and a DALR gas column.”
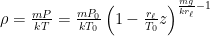

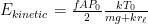 ,
, is lapse rate,
is lapse rate,  is the gas-column cross-sectional area, and
is the gas-column cross-sectional area, and  is the number of degrees of freedom. (Forgive any typographical infelicities; this is my maiden voyage with LaTeX.)
is the number of degrees of freedom. (Forgive any typographical infelicities; this is my maiden voyage with LaTeX.)
Here’s what I found when I attempted a pencil-and-paper solution:
and
where
Now, for me the math was a little taxing, so I’m not certain I got it right. If I did, though, the ratio of potential energy to total energy is independent of lapse rate: the center of mass wouldn’t rise if the lapse rate increased from zero to the dry adiabatic lapse rate.
Of course, Velasco et al. say a spontaneous lapse-rate rise of that size won’t happen at equilibrium, because the microstates that exhibit a nearly-zero temperature lapse rate are many times as numerous as those that exhibit temperature lapse rates of the same order of magnitude as the dry adiabatic lapse rate.
Tim Folkerts says:
February 9, 2012 at 4:53 pm
Quite true. But wikipediasuggests that 5.5×10^24 J of solar energy hits the earth each year, but the total reserves of petroleum are 7.9×10^21 J.
To be fair we would need to include the energy equivalent of the entire fossil carbon store, including that scattered through bulk sediments so thinly that it is generally not considered an extractable resource. That total is ~2.7×10^27J (OU S266 Block2 p16) or ~500 yr of solar energy. Assuming this has taken ~350,000,000 yr to form, the fraction of solar energy sequestered is ~1/700,000, amounting to ~0.5mW/sq.m. Surely Bryan could accept that this is negligible in the heat balance – where after all very little is known to better than say 1% accuracy, if that. Even geothermal heat conduction is two orders of magnitude greater at ~50mW/sq.m.
Yes the ‘everything being equal’ assumes there is no photosynthesis along with a dazzling set of pseudo scientific assumptions.
Photosynthesis is utterly irrelevant to the GHE.
All of the ways energy redistributes itself inside the atmosphere is irrelevant to the GHE.
The only thing that is relevant to the GHE is the outgoing radiation budget, which is the only way the Earth loses the heat it picks up from the Sun (with an extremely modest addition from all other sources). The Earth does move through hard vacuum, after all. No conduction away, no convection away, only radiation away.
If one looks at the radiation going away — the full top-of-atmosphere outgoing radiation spectrum — the GHE is clearly visible. Outgoing radiation from the IR band of CO_2 is emitted with an effective temperature (top of troposphere) that is much colder than the surface temperature. Colder means (much) less integrated outgoing power in these frequencies. One way or another radiation from the other available frequencies has to increases to compensate, because incoming solar radiation is what it is and to remain in balance ins ultimately must equal outs.
One way it the Earth compensates is by becoming warmer at the surface. This increases the rate of direct radiation from the surface in the water window so that the integrated outgoing power still equals the integrated incoming power, on average, for the entire Earth.
There isn’t really any question that this occurs. You can see it directly in the actual spectrographs any time you care to look. So make up any bizarre and complex tale you like for how heat moves around before it leaves — but at the end of the day the GHG GHE without question is the major contributor to the warming of the Earth above the mean temperature expected if there were no atmosphere at all or a GHG-free atmosphere. You can see it.
Of course, to see it you have to remove your head from the sand and look. I wish you well with that.
rgb
I believe that the atmosphere does lose some molecules to outer space, although I suspect that it is also modest.
Are there any numbers on this, since it would also transport some small amount of energy away.
kuhnkat says:
February 10, 2012 at 1:05 pm
Paul Birch, “The fact that the radiation is being absorbed means that its energy is being absorbed. Unless you imagine that the ocean is magically levitating off the ocean floor or chemically splitting into hydrogen gas and oxygen/chlorine, that energy can only appear as heat.”
Please do not get sloppy when trying to answer questions like this. I am certain you are aware of the photoelectric, pyrroelectric, and ferroelectric effects. They convert photons into movement of electrons, or, electric current. This is a very valid question for those of us who are less than experts. What does happen and where does it go is NOT something that many of us know and something that is too often assumed.
It doesn’t matter what weird and wonderful short-term effects might be happening! We have already seen how photosynthesis is insignificant in this context. So unless there is a permanent, drastic, progressive and one-way alteration in the chemical makeup of the oceans over geological epochs (which would entail the massive evolution of hydrogen gas and the production of oxygen, chlorine or hydrogen peroxide) or a similarly huge increase in its potential energy (levitating it off the ocean floor), the energy involved will still have to be dissipated as heat (there’s nowhere else for it to go, unless you get all science-fictiony and assume it vanishes into hyperspace or turns into neutrinos or something).
Robert Brown declaims,
“The only thing that is relevant to the GHE is the outgoing radiation budget, which is the only way the Earth loses the heat it picks up from the Sun (with an extremely modest addition from all other sources).”
Only you have no idea whether the Black Box of the earth system is receiving and emitting a balanced amount of energy over it history. Y’all wave your arms a lot, but, you simply DO NOT HAVE THE FACTS TO PROVE YOUR CASE!
You TOTALLY ignore the heating effects of the solar wind, birkeland currents, magnetic and gravitic fields. Yeah, when you pick and choose the physic you will discuss you can prove pretty much anything you want to prove.
jimmi_the_dalek says:
February 9, 2012 at 7:33 pm
Myrrh has some website that states that water is transparent, but instead of contacting that web site and asking whether they really mean ‘nearly transparent’ he has decided that everyone else has falsified physics. Seems backwards to me.
For anyone who is actually interested, here is the explanation of how water absorbs (weakly, but not zero!) in the visible.
http://www.lsbu.ac.uk/water/vibrat.html#ir
Note that amusingly (since Myrrh insists that only molecular vibrations are ‘heat’) that it is actually a vibrational (overtone) band that is responsible.
—
So you’re of the Folkerts school of science? I point out that the energy budget cartoon you work to claims that shortwave from the Sun is the only source of directly heating Earth’s land and oceans for it to radiate out the amount of thermal infrared, heat, claimed, and you come back with ‘it’s not completely zero’ and the idiocy of Tim’s so called experiment to prove shash might be there at the cold depths..?
Water is transparent to Visible light, the graph shows that. It shows that water doesn’t for any practical real world outside of shash insignificance absorb any visible light. That’s what transparent means. It means water doesn’t absorb visible light. Visible light cannot be heating all the vast amount of water in our great ocean which is the major part of our Earth because Water does not absorb visible light, it transmits it through without absorbing it. That’s what water does with visible light, it doesn’t absorb it. Yet you claim Visible light is this great super high energy peak output from the Sun heating Earth’s land and oceans!
Not only can you not see how absurd that claim, you also believe, because you’ve been told, that the Sun’s heat, thermal infrared, doesn’t reach the surface!
How on earth can you possible expect me to take you seriously as ‘scientists’?
You see, I can understand joe public being unaware of the manipulation here, I can even understand scientists in different fields not being aware of it, but what I can’t understand is anyone calling themselves a scientist not being able to understand what I’m saying and continuing to defend this fiction, and especially those vaunting their great credentials as proof of their own brilliance.
Here, some real world information about it:
NASA original page teaching previously traditional real world physics to children: http://science.hq.nasa.gov/kids/imagers/ems/infrared.html
““Near infrared” light is closest in wavelength to visible light and “far infrared” is closer to the microwave region of the electromagnetic spectrum. The longer, far infrared wavelengths are about the size of a pin head and the shorter, near infrared ones are the size of cells, or are microscopic.
Far infrared waves are thermal. In other words, we experience this type of infrared radiation every day in the form of heat! The heat that we feel from sunlight, a fire, a radiator or a warm sidewalk is infrared.
Shorter, near infrared waves are not hot at all – in fact you cannot even feel them. These shorter wavelengths are the ones used by your TV’s remote control.
Infrared light is even used to heat food sometimes – special lamps that emit thermal infrared waves are often used in fast food restaurants! “
Do you understand what this is saying?
It is saying that thethermal infrared direct from the Sun reaches the Earth’s surface.
We feel its heat.
This means the KT97 and variations is junk science, because it has excluded the real heating mechanism of Earth’s land and oceans.
There’s no getting around that…
There’s some useful information on this wiki page, scroll down to the difference between electronic transitions and thermal infrared, it gives the four possible outcomes of visible meeting matter. The second, refection/scattering when an electron absorbs the energy, is what happens in our atmosphere when the electrons of the molecules of nitrogen and oxygen briefly absorb visible and then send it back out again, so our blue sky. The third is visible in a transparent medium, like water, where it is not absorbed but transmitted through.
http://en.wikipedia.org/wiki/Transparency_and_translucency
I’d found something earlier which gives a bit of the picture of what’s happening when visible gets transmitted through a transparent medium:
I once read a great description of this, likening it to a dance with the visible trying to get into to join in but being rejected and moved on!
I believe that the atmosphere does lose some molecules to outer space, although I suspect that it is also modest.
Are there any numbers on this, since it would also transport some small amount of energy away.
You are quite correct — this is a form of “evaporative cooling” that occurs as a few molecules from the tail of the Maxwell-Boltzmann distribution end up with escape energy and happen to have the right direction to escape without suffering another momentum-energy transferring collision. However, this process is enormously slow, especially for the heavier molecules. It is described in most intro physics textbooks — not my own yet as I haven’t written the thermo chapters — but IIRC you can find a good description on wikipedia.
The rate of loss, while slow, is fast enough to have depleted parts of Earth’s atmosphere over 4 billion years, but our atmosphere is fairly steadily being replenished by outgassing from the crust.
The energy outflow in this form is even more negligible than the energy inflow from the Earth losing its internal heat, though — in any event the two have the opposite sign and are both even more negligible together than either one alone.
There are a variety of mechanisms for energy coming both in and out that are effectively ignored in the energy budget, because they correspond to <0.1% variations. For a while I thought that direct magnetic inductive heating of the Earth might be associated with the increased warming observed during periods of high solar activity. Inductive heating occurs when a conductor (like the Earth) moves through magnetic flux (e.g. rotates and revolves around the Sun) or when the flux through a stationary conductor changes for other reasons. At one point in time the Sun's magnetic field was presumably strong enough to have caused at least some of the planetesimals in orbit around the Sun to fuse, explaining how they form solid bodies in spite of never being gravitationally heated enough to melt. However, when I did a Fermi estimate of the heating, while the total energy involved was suitably impressive, the power per square meter of surface was utterly ignorable. Many of the mechanisms are like that — the tides, the direct inductive heating, the heating caused by the days influx of falling meteorites — which incidentally is far greater than the rate of heat loss through outgassing, as meteoric dust and matter infalls at an average rate of at least millimeters per decade, from my own direct measurements — they have “impressively” large amounts of annual energy associated with them, right up to where you divide by the surface area of the earth and the number of seconds in a year.
In the end, only Solar radiation matters for incoming power, only radiation matters as outgoing power, at least as far as I know, that is to say, barring new physics (dark matter/energy forming a mysterious unaccounted for energy source, stuff like that). It’s not that there aren’t perturbations from other sources, it is that those sources or sinks are orders of magnitude less important and are themselves nearly steady state phenomena, forming a nearly constant background correction, not something that contributes to active average temperature changes in climate.
rgb
Water is transparent to Visible light, the graph shows that. It shows that water doesn’t for any practical real world outside of shash insignificance absorb any visible light. That’s what transparent means. It means water doesn’t absorb visible light. Visible light cannot be heating all the vast amount of water in our great ocean which is the major part of our Earth because Water does not absorb visible light, it transmits it through without absorbing it. That’s what water does with visible light, it doesn’t absorb it. Yet you claim Visible light is this great super high energy peak output from the Sun heating Earth’s land and oceans!
No, it doesn’t transmit it without absorbing it because it isn’t perfectly transparent. You keep asserting that water is transparent as if transparency is a binary property of matter — either it is perfectly transparent or it is perfectly absorbing. It is not.
Even if water were perfectly transparent, there would be the question of where it is transmitting all of that visible light to. That would be the ocean bottom, right, where it would heat the bottom and then warm the ocean anyway? Only — where’s the light down there? Oooo, it’s gone, isn’t it. Invisible fairies strike again!
Demons Out!
(I know, it’s not working, I need to hire a holy person experienced with exorcising Maxwell Demons, but in the meantime all I can do is keep trying.)
rgb
kuhnkat says:
February 10, 2012 at 3:17 pm
Only you have no idea whether the Black Box of the earth system is receiving and emitting a balanced amount of energy over it history. Y’all wave your arms a lot, but, you simply DO NOT HAVE THE FACTS TO PROVE YOUR CASE!
Do the sums! The energy in and energy out has to be closely balanced over the long term because there’s nowhere else for it to go!. There’s a residual ~50mW/sq.m out from the Earth’s internal heat. There is up to ~0.5mW/sq.m retained due to photosynthesis. Everything else will be even smaller. For example, suppose the air was at one time completely motionless, and suppose as little as 1% of the solar input went to creating wind; in just one day the global windspeed would reach 8m/s; and that’s it, the wind store is full. Or suppose we put the energy into ocean currents; again, just about a day gets the entire ocean moving at 1 knot. Even to heat the entire ocean would take only ~ 1 year’s solar input per degree rise.
Now, we’ve all been ignoring the influx of moonlight and starlight. Why? Because they are quantitatively negligible compared to solar radiation. We’ve ignored the solar wind and solar magnetic field. Why? Because when they reach the Earth they carry very little power per unit area. We’ve ignored cosmic rays. Ditto. As far as the overall heat balance is concerned, these are all negligible (some of them may of course have other effects – such as on cloud formation – that have a significant indirect impact on the heat balance).
In the short term – day to day or even year to year – there are of course imbalances. No one is denying it. Even then, those imbalances are quite minor – a small fraction of the power absorbed and re-radiated.
which indeed shows that in – \left(\frac{Mg}{R}\right) \frac{P}{T} you have eliminated density from consideration, and are in essence treating the air as a solid.
Au contraire, BWD, I simply used the known density of an ideal gas as a function of its pressure and temperature. Which, if you were not completely incompetent in physics and (apparently) unable to read, you would know.
This is not “treating the air as a solid”. It is “treating the air as an ideal gas”. Which I do because the entire article and discussion is about an isolated ideal gas. Pretty interesting how that works out, isn’t it!
rgb
Only you have no idea whether the Black Box of the earth system is receiving and emitting a balanced amount of energy over it history. Y’all wave your arms a lot, but, you simply DO NOT HAVE THE FACTS TO PROVE YOUR CASE!
You TOTALLY ignore the heating effects of the solar wind, birkeland currents, magnetic and gravitic fields. Yeah, when you pick and choose the physic you will discuss you can prove pretty much anything you want to prove.
Paul Birch already answered this, but I’ll chime in with my own observation that I don’t TOTALLY ignore these things at all. In fact, in a number of cases I’ve directly and personally estimated or computed them (specifically I’ve directly estimated gravitational/tidal and magnetic heating, heating and cooling due to outgassing and infalling micrometeorites, and the solar wind — all of these are negligible corrections. Birkeland currents are interesting, although they seem to be a possible correction to direct solar irradiance only at the poles and only in the ionosphere, which is already enormously hot — between 1500C and 2500C — but so tenuous that you wouldn’t feel heat if you stuck your arm out into the near vacuum of the ionosphere, you’d feel intense cooling as your blood started to boil and ordinary thermometers would radiate heat away faster than they would equilibrate (and hence would read very cold temperatures). You’d have to do some actual arithmetic to convince me that they are a major direct contributor to the Earth’s energy budget either way.
The interesting question is: Why are you reciting this list? If you are really interested in the answer to the question “What are the sources and sinks that are primarily responsible for maintaining the average temperature of the Earth?”, one would think that you would take the time to “do your homework” and actually work out the numbers and check them against the work of others to ensure that your answers were plausible. On the other hand, if you already have picked an answer, and are just interested in making it sound like your answer is plausible by invoking a lot of things that might be confounding to the accepted answer while deliberately not looking (because if you look you might learn that you are wrong), well then, how exactly can any “debate” or “discussion” proceed? Your mind is made up, your head is firmly jammed in the sand lest you be forced to face the possibility of error, end of story!
So please, by all means, provide me with quantitative estimates for the contribution to the overall heat budget of the planet of each of the things you cite. Be prepared to defend your estimates.
rgb
“One way or another radiation from the other available frequencies has to increases to compensate, because incoming solar radiation is what it is and to remain in balance ins ultimately must equal outs.”
The atmosphere is analogous to a flexible lens that is shaped by the density distribution of the gas molecules,of the atmosphere in the space between the sphere holding them, and space; Incoming heat gets collected in many ways and places,,primarily by intermittent solar radiation gets stored, in vast quantities, and slowly but also a barrage of mass and energy fluxes from all directions ; that are slowly transported great distances and to higher altitudes mostly by oceanic and atmospheric mass flows. The role played by radiation in the flow of heat through the atmosphere is minor.
But are ins ultimately equaling outs? Only for the planet as a whole,instantaneously at various intervals, provided you are looking from far enough away from Earth, (like maybe from mars), to homogenize the areas that are net absorbers with the areas that are net emitters.
But are ins ultimately equaling outs? Only for the planet as a whole,instantaneously at various intervals, provided you are looking from far enough away from Earth, (like maybe from mars), to homogenize the areas that are net absorbers with the areas that are net emitters. solid angle and all frequencies. No need to go to Mars.
solid angle and all frequencies. No need to go to Mars.
Yes, precisely, except that we in the trade like to just call this “on average over time”, and recognize that “far enough away” is just outside of Earth’s atmosphere, with outgoing radiated power integrated over the
As far as the time average is concerned, averaging over a year is probably pretty decent as far as detailed balance is concerned, although yes, there is heat stored and released in at least the ocean over much longer time scales. The atmosphere itself is pretty well mixed and dynamic, the ground beneath our feet is nearly irrelevant once you go a meter deep. I suppose glaciers and sea ice are also a factor as reservoirs, although the latter is just a part of the ocean.
Pretty much all of the rest of the Earth’s thermal buffering system would relax to temperatures well under 200K in a matter of weeks if the Sun disappeared overnight.
Empirically, I think there is good evidence that the overall climate relaxation time is order of 1-3 decades — that is, although there is good reason to think that the Earth had “started to cool” by the middle of the last solar cycle (about the time it stopped even thinking about getting overall warmer) it has taken a solid decade for actual cooling to think about revealing itself, and that is still masked by a lot of noise in the complex system.
Even this isn’t a really good “average”. The climate exhibits significant long term variability on the scale of centuries to millennia. We don’t completely understand what drives this variability. And then there is the predominant 100KY boreal oscillation, with interglacials rought 10KY long, where we have a lot of theory suggesting that it is due to this or to that — orbital variations, tilt, and more — but none of those theories fits old data or predicts new data terribly well or believably.
I agree that it is a complex problem. But do understand the role of the GHE in all of this. If it weren’t for the GHE, we would get much colder very, very quickly, precisely as if you went up to your attic and stripped away all of the insulation without turning up the furnace.
rgb
Dr. Brown,
What you seem to not be considering is that gas molecules are compressible, and that “compressibility” is a property of a gas that relates, pressure, and temperature to its unconfined volume.
Perhaps its apparent obscurity is an artifact of its importance in secret defense work during WWII, but this is not a new or unvetted concept. The derivation of this concept may be found presented as either lapse rate or potential temperature, in many reference works and text books about Physics, Thermodynamics, Fluid Mechanics, and Meteorology, and likely other fields as well. If you need specific references, I can list the books I have on hand, but all you really need to do is go to the library and look at some texts from the ’50s, ’60s or ’70’s.
This property of a gas results in a distribution of pressure and temperature for a gas held radially about a sphere by the effect of gravity, only. And, because it is a physical property of a gas, it isn’t proper to ignore it whilst postulating atmospheric conditions.
BigWaveDave,
What you seem not to be considering is that physicists actually understand the topics you are addressing.
* we know the air is compressible.
* we recognize there is a lapse rate in the actual atmosphere
* we understand the derivation of the DALR (eg http://en.wikipedia.org/wiki/Lapse_rate#Dry_adiabatic_lapse_rate)
* we are not confused by the concept of “potential temperature”
(One interesting application of “potential temperature” is that commercial jets draw in very cold air fresh air, but have to cool that air before circulating it in the cabins. The potential temperature of the atmosphere at 30,000 ft is typically well above room temperature even for this cold air. By the time the outside air is compressed enough for the cabin (equivalent to quickly bringing that parcel of air adiabatically to surface pressure), it is typically too warm to be comfortable.)
Go read one of those references you are suggesting and see the conditions assumed in the derivation of the DALR. Wikipedia starts with “The dry adiabatic lapse rate (DALR) is the rate of temperature decrease with height for a parcel of dry or unsaturated air rising under adiabatic conditions.”
So yes, when convection is strong (ie when the ground is being heated and the top of the atmosphere is being cooled), then the conditions will be close to adiabatic. The air is moving up and down too quickly for conduction to play a major role, so the adiabatic lapse rate is a good approximation to the actual temperature profile. But convection only happens when it is driven by a temperature difference — ie the bottom is being warmed and the top is being cooled — ie NOT the conditions of this thought experiment. For this thought experiment (where the atmosphere is not heated or cooled), there will be no convection. Now the slower, less effective thermal conduction becomes important, eventually leading to an isothermal situation.
Yeah, BWD, what he (Tim) said…
But don’t mind me, I just teach this stuff.
Incidentally, the “compressibility”, like the thermal conductivity, of an ideal gas is pretty straightforward, don’t you think? Derivable from first principles? Sort of built into the expression I wrote for the density in the top article, as I’ve pointed out several times now? And Jelbring — they did write their paper on an assumed ideal gas, didn’t they? You know, I think that they did!
Why is it so very difficult to accept that a) sure, real atmospheres have a DALR; and b) Jelbring’s paper asserting that this is a property of a static isolated atmosphere question-begging, law-of-thermodynamics violating nonsense? It certainly isn’t a proof that it is true — the very length and complexity of this discussion is ample evidence of that.
Note well that the actual physicists on the list are pretty much unanimous that the result is incorrect, and the only people on the thread who disagree are those that either don’t know the laws of thermodynamics and statistical mechanics well enough to (for example) derive the Ideal Gas law in the first place or do and defend the actual algebra required to support their assertions. They are reduced to quoting books they don’t really understand, usually out of context.
Why is that, do you suppose? Does that make the slightest bit of actual sense to you? Sure, it is heroic and everything but really, it is a discussion that is quite reminiscent of the papers I get from complete flakes that somehow find me on the Internet and try to get me to read their paper on how the electron can be understood from Mach’s principle, or from a diagram that they made up all by themselves, or from…
So instead of saying I neglect density or compressibility when the expression I give manifestly does neither one, why not try to construct an actual algebraic proof that I’m wrong, or if you can’t, perhaps grant me the possibility that my fairly complete and consistent algebraic argument could be, in fact, correct?
rgb
Tim Folkerts says:
February 1, 2012 at 8:00 pm
What? The DALR is generated by adiabatic expansion of a gas.
PV = nRT
Suppose a gas in a cylinder is allowed to expand through a nozzle into deep space. The random kinetic energy (related to T) will become highly directed kinetic energy. The steam of particles will no longer have any random kinetic energy. It will have no temperature. Also, since P goes to zero, T must also go to zero. Since V is the totality of free space, you might consider it to be infinity. The only way the temperature could remain constant would be if zero times infinity would be equal to the original product.
2/19 8:19pm:
“..try to construct an actual algebraic proof that I’m wrong..”
Done. Already shown the algebra proving you are wrong above at 2/5 12:27pm. No need to repeat the standing algebraic proof steps. The thought experiment of Fig. 1 in top post being isothermal at equilibrium is wrong & shown irrefutably (by many published atmospheric thermodynamic physicists cited) to be non-isothermal, isentropic in equilibrium by the correct algebraic steps to maximize entropy and reasonable experiments as posted above.
Those published atmospheric physicists agree with these steps. I see no poster in this thread has proven anything wrong with those algebraic proof steps especially original poster Robert Brown.
It stands that Fig. 1 in top post reaches non-isothermal, isentropic equilibrium at max. entropy with the random vigorous mixing of the GHG-free air molecules at reasonable earth pressures and temperatures (80% of atmosphere up through troposphere). Atmospheric physicists have known this since reporting their results at least as early as 1996. Independent atmospheric physicists verified Fig. 1 is non-isothermal, isentropic in equilibrium again in 1998, 2004 and 2010 by publishing their algebraic work steps and physical logic entirely consistent with all thermo laws that include confirming experiments.
Tim Folkerts says 2/17 at 9:09am:
“..convection only happens when it is driven by a temperature difference..”
Yeah, the general definition of convection implies heat flow and there is no longer any heat flow at equilibrium of Fig. 1. There is however vigorous random mixing of the molecules up and down the ideal gas column at equilibrium in a gravity field leading to non-zero pressure and non-zero temperature gradients at equilibrium proven by reasonable experiment and theory of published physicists.
“..the slower, less effective thermal conduction becomes important, eventually leading to an isothermal situation.”
No, not in a reasonable ideal gas in a gravity field where the maximum entropy will be eventually achieved and maintained w/temp. gradient at equilibrium even with conduction acting in this one system of Fig. 1 in top post. This max. entropy, conserving total enthalpy/energy equilibrium of Fig. 1 is irrefutably proven non-isothermal, isentropic by published atmospheric thermodynamic physicists as early as 1996, again in 1998, 2004 and 2010 including experiments validating their results. There is no convincing evidence found of an atmospheric physicist publishing a proof otherwise by experiment or theory since then.
Trick says:
February 20, 2012 at 6:07 am
For those who’d like to get a good laugh, take a look at the “algebra” that Trick claims to have “done” above at 12:27 pm.
Trick, for future reference, here’s a protip. You can recognize algebra because it has these things called “equations”. They are called “equations” because they have what is called an “equals sign” in the middle of them. Don’t know if you are aware of what an equals sign or an equation look like. I say that because what you claim to be “algebra” above doesn’t contain a single equation, as evidenced by a glaring lack of equals signs. For future reference, what we call an “equals sign” looks like this:
If they told you in high school that what you posted above is algebra, you should go and demand your money back. And whether they told you it was algebra or not, I’ve got bad news for you.
It’s not algebra. No way. No how. No shape. No form. Algebra has equations. Remember that. Algebra has equations.
Thanks for playing, though,
w.
Willis Eschenbach says 2/20 at 9:57am:
“You can recognize algebra because it has these things called “equations…with = signs….”.
Yes, I expected someone to call me out on that Willis. And I did just have a good laugh. Thanks.
As poster Robert Brown implies above, an atmospheric thermodynamic physicist can use 1st year college math to derive the proper “equations with =” proving Fig. 1 non-isothermal, isentropic given the proper outline algebra steps which I posted 2/5 12:27pm.
My inability to post the actual “equations with =” in the computer language used at WUWT is not proof that the Fig. 1 is isothermal at equilibrium. I’ll offer to send a word document following the steps with the “equations including =” proving Fig. 1 non-isothermal, isentropic at equilibrium to the mod.s with the experimental evidence if they have an interest to do the computer language translation work as they have done for others.
This “equations with =”algebra work is not new science, it has been published since at least 1996. There is no real need to write them again here. As I wrote at 2/20 6:07am above the 1996 paper has been followed up, verified and published more recently by several other independent atmospheric thermodynamic physicist authors proving Fig. 1 in the top post thought experiment to be non-isothermal, isentropic at equilibrium (they do include for Willis’ sake equations with = signs).
Poster Robert Brown complains 2/19 8:10pm above that citing these authors out of context is problematic. However, the whole of their work is freely available on the internet, links posted (way) above . If you want, I believe the “equations with =” work can be excerpted here with proper citation. I agree though with Robert in that better to read the original work in context.
I notice no poster on this thread has convincingly debated any of those outline steps I posted as being incorrect or wrong in any way. Maybe Willis could find something wrong with them? If so, I’m interested to blog along and not make the mod.s cry.
Tim Folkerts says: February 1, 2012 at 8:00 pm
>>Normally an adiabatic free expansion results in no cooling of an ideal gas.
Robert Clemenzi replies: February 19, 2012 at 11:59 pm
>What? The DALR is generated by adiabatic expansion of a gas.
“Free expansion” is a specific term where the gas expands into a previously empty region (for example, opening a valve between a full container and an empty container). “Free expansion” involves no work and does not cause temperature changes for ideal gases. For a while “free expansion” had been discussed in this thread, and I was specifically addressing “free expansion”
The expansion of a gas where the gas is NOT freely expanding but instead is doing some work (eg by expanding against the surrounding gas in the earth’s atmosphere) will indeed cause the sort of cooling that leads to a DALR.
Trick says:
> “When Fig. 1 in top post is at equilibrium it has reached max. entropy; no heat flow ….”
and
>”the general definition of convection implies heat flow”
Therefore you clearly conclude there is no convection. (And just to beat a dead horse, random molecular motion is NOT convection. Convection requires organized macroscopic motion.)
You then reference “Verkley et. al. part b” to support your position. But this favorite paper of yours specifically starts part b by saying
Can you not see the logical inconsistency in your argument? You are taking the paper out of context, exactly as Dr Brown concluded.
Part A of the paper corresponds to the situation here, and Part A clearly concludes that