Guest post by Robert G. Brown
Duke University Physics Department
The Problem
In 2003 a paper was published in Energy & Environment by Hans Jelbring that asserted that a gravitationally bound, adiabatically isolated shell of ideal gas would exhibit a thermodynamically stable adiabatic lapse rate. No plausible explanation was offered for this state being thermodynamically stable – indeed, the explanation involved a moving air parcel:
An adiabatically moving air parcel has no energy loss or gain to the surroundings. For example, when an air parcel ascends the temperature has to decrease because of internal energy exchange due to the work against the gravity field.
This argument was not unique to Jelbring (in spite of his assertion otherwise):
The theoretically deducible influence of gravity on GE has rarely been acknowledged by climate change scientists for unknown reasons.
The adiabatic lapse rate was and is a standard feature in nearly every textbook on physical climatology. It is equally well known there that it is a dynamical consequence of the atmosphere being an open system. Those same textbooks carefully demonstrate that there is no lapse rate in an ideal gas in a gravitational field in thermal equilibrium because, as is well known, thermal equilibrium is an isothermal state; nothing as simple as gravity can function like a “Maxwell’s Demon” to cause the spontaneous stable equilibrium separation of gas molecules into hotter and colder reservoirs.
Spontaneous separation of a reservoir of gas into stable sub-reservoirs at different temperatures violates the second law of thermodynamics. It is a direct, literal violation of the refrigerator statement of the second law of thermodynamics as it causes and maintains such a separation without the input of external work. As is usually the case, violation of the refrigeration statement allows heat engines to be constructed that do nothing but convert heat into work – violating the “no perfectly efficient heat engine” statement as well.
The proposed adiabatic thermal lapse rate in EEJ is:

where g is the gravitational acceleration (presumed approximately constant throughout the spherical shell) and cp is the heat capacity per kilogram of the particular “ideal” gas at constant pressure. The details of the arguments for an adiabatic lapse rate in open systems is unimportant, nor does it matter what cp is as long as it is not zero or infinity.
What matters is that EEJ asserts that ![]() in stable thermodynamic equilibrium.
in stable thermodynamic equilibrium.
The purpose of this short paper is to demonstrate that such a system is not, in fact, in thermal equilibrium and that the correct static equilibrium distribution of gas in the system is the usual isothermal distribution.
The Failure of Equilibrium

In figure 1 above, an adiabatically isolated column of an ideal gas is illustrated. According to EEJ, this gas spontaneously equilibrates into a state where the temperature at the bottom of the column Tb is strictly greater than the temperature Tt at the top of the column. The magnitude of the difference, and the mechanism proposed for this separation are irrelevant, save to note that the internal conductivity of the ideal gas is completely neglected. It is assumed that the only mechanism for achieving equilibrium is physical (adiabatic) mixing of the air, mixing that in some fundamental sense does not allow for the fact that even an ideal gas conducts heat.
Note well the implication of stability. If additional heat is added to or removed from this container, it will always distribute itself in such a way as to maintain the lapse rate, which is a constant independent of absolute temperature. If the distribution of energy in the container is changed, then gravity will cause a flow of heat that will return the distribution of energy to one with Tb > Tt . For an ideal gas in an adiabatic container in a gravitational field, one will always observe the gas in this state once equilibrium is established, and while the time required to achieve equilibrium is not given in EEJ, it is presumably commensurate with convective mixing times of ordinary gases within the container and hence not terribly long.
Now imagine that the bottom of the container and top of the container are connected with a solid conductive material, e.g. a silver wire (adiabatically insulated except where it is in good thermal contact with the gas at the top and bottom of the container) of length L . Such a wire admits the thermally driven conduction of heat according to Fourier’s Law:

where λ is the thermal conductivity of silver, A is the cross-sectional area of the wire, and ΔT=Tb–Tt . This is an empirical law, and in no way depends on whether or not the wire is oriented horizontally or vertically (although there is a small correction for the bends in the wire above if one actually solves the heat equation for the particular geometry – this correction is completely irrelevant to the argument, however).
As one can see in figure 2, there can be no question that heat will flow in this silver wire. Its two ends are maintained at different temperatures. It will therefore systematically transfer heat energy from the bottom of the air column to the top via thermal conduction through the silver as long as the temperature difference is maintained.

One now has a choice:
- If EEJ is correct, the heat added to the top will redistribute itself to maintain the adiabatic lapse rate. How rapidly it does so compared to the rate of heat flow through the silver is irrelevant. The inescapable point is that in order to do so, there has to be net heat transfer from the top of the gas column to the bottom whenever the temperature of the top and bottom deviate from the adiabatic lapse rate if it is indeed a thermal equilibrium state.
- Otherwise, heat will flow from the bottom to the top until they are at the same temperature. At this point the top and the bottom are indeed in thermal equilibrium.
It is hopefully clear that the first of these statements is impossible. Heat will flow in this system forever; it will never reach thermal equilibrium. Thermal equilibrium for the silver no longer means the same thing as thermal equilibrium for the gas – heat only fails to flow in the silver when it is isothermal, but heat only fails to flow in the gas when it exhibits an adiabatic lapse in temperature that leaves it explicitly not isothermal. The combined system can literally never reach thermal equilibrium.
Of course this is nonsense. Any such system would quickly reach thermal equilibrium – one where the top and bottom of the gas are at an equal temperature. Nor does one require a silver wire to accomplish this. The gas is perfectly capable of conducting heat from the bottom of the container to the top all by itself!
One is then left with an uncomfortable picture of the gas moving constantly – heat must be adiabatically convected downward to the bottom of the container in figure 1 in ongoing opposition to the upward directed flow of heat due to the fact that Fourier’s Law applies to the ideal gas in such a way that equilibrium is never reached!
Of course, this will not happen. The gas in the container will quickly reach equilibrium. What will that equilibrium look like? The answer is contained in almost any introductory physics textbook. Take an ideal gas in thermal equilibrium:
![]()
where N is the number of molecules in the volume V, k is Boltzmann’s constant, and T is the temperature in degrees Kelvin. n is the number of moles of gas in question and R is the ideal gas constant. If we assume a constant temperature in the adiabatically isolated container, one gets the following formula for the density of an ideal gas:

where M is the molar mass, the number of kilograms of the gas per mole.
The formula for that describes the static equilibrium of a fluid is unchanged by the compressibility (or lack thereof) of the fluid – for the fluid to be in force balance the variation of the pressure must be:
(so that the pressure decreases with height, assuming a non-negative density). If we multiply both sides by dz and integrate, now we get:

Exponentiating both sides of this expression, we get the usual exponential isothermal lapse in the pressure, and by extension the density:
![]()
where P0 is the pressure at z=0 (the bottom of the container).
This describes a gas that is manifestly:
- In static force equilibrium. There is no bulk transport of the gas as buoyancy and gravity are in perfect balance throughout.
- In thermal equilibrium. There is no thermal gradient in the gas to drive the conduction of heat.
If this system is perturbed away from equilibrium, it will quickly return to this combination of static and thermal equilibrium, as both are stable. Even in the case of a gas with an adiabatic lapse rate (e.g. the atmosphere) remarkably small deviations are observed from the predicted P(z) one gets treating the atmosphere as an ideal gas. An adiabatically isolated gas initially prepared in a state with an adiabatic lapse rate will thermally equilibrate due to the internal conduction of heat within the gas by all mechanisms and relax to precisely this state.
Conclusion
As we can see, it is an introductory physics textbook exercise to demonstrate that an adiabatically isolated column of gas in a gravitational field cannot have a thermal gradient maintained by gravity. The same can readily be demonstrated by correctly using thermodynamics at a higher level or by using statistical mechanics, but it is not really necessary. The elementary argument already suffices to show violation of both the zeroth and second laws of thermodynamics by the assertion itself.
In nature, the dry adiabatic lapse rate of air in the atmosphere is maintained because the system is differentially heated from below causing parcels of air to constantly move up and down. Reverse that to a cooling, like those observed during the winter in the air above Antarctica, and the lapse rate readily inverts. Follow the air column up above the troposphere and the lapse rate fails to be observed in the stratosphere, precisely where vertical convection stops dominating heat transport. The EEJ assertion, that the dry adiabatic lapse rate alone explains the bulk of so-called “greenhouse warming” of the atmosphere as a stable feature of a bulk equilibrium gas, is incorrect.
Robert Brown wrote:
Well played, sir. One of my favorite movies too.
Thank you. The broader point there is that failure of human creativity, be it one or all humans, does not actually constrain reality. It only constrains our ability to work with reality and predict outcomes.
However, would you agree that the default position on this, the one that should have been taken by the referee of this paper, is “Ha ha ha, no.”, phrased more politely, perhaps accompanied by a strict requirement that all of the physics from the microscopic level to the macroscopic level be fully explained to show how the system does or doesn’t violate the second law before accepting it for publication?
This turns out to be a more interesting question than it first seems.
In theory, you are correct, and I accept that threshold for myself.
In practice, that threshold has proven to be beyond the reach of mankind since at least Theory of Heat. Please re-read my Two Gases model until it’s clear what I’m talking about. I have described a very real effect. At issue is whether or not there is a Gravitational Lapse Rate in temperature. If there is, then my model is explanatory of a real, readily observable effect.
The result is not in question. It happens, and has been observed by every single physicist. Only the explanation is in question. You’re welcome to dispute that, of course, but I don’t recommend doing so without first understanding the model.
If I am right, which I have not proven in this thread, then the two quotes (Eddington and Princess Bride) go a long way towards explaining how we came to reach our current state. The word ‘Orthodoxy’ comes to mind.
I’m more of a ‘Heterodoxy’ kind of guy, as it distributes eggs into more baskets, and helps reduce the probability of wagering everything on a single notion. This turns out to be more important than it seems, because the probabillity of an erroneous assumption in a theoretical structure approaches unity.
wayne says:
January 29, 2012 at 11:07 am
Wayne, you seem misinformed about what’s happening here. You don’t get to claim that Robert is somehow deficient because he doesn’t answer your question.
He has written a very interesting proof about the Jelbring hypothesis. This has absolutely nothing to do with the lapse rate on Venus. Nothing.
He is not under the slightest obligation to answer your question, and if he wants to keep the thread on track, I’d advise him not to do so.
So if he never does answer your question, well, try asking somewhere else. But don’t come claiming to us that Robert is shirking his obligations. He is not shirking anything, and in fact has done yeoman work here. He doesn’t have to answer anyone’s questions, particularly those that are wildly off-topic.
w.
@Pompous
C: We agree on some things and not others. I ignored your ad homs. Don’t worry.
C: How do I deal with Robert who answered the question about the end of radiation thus:
++++
Robert Brown says:
January 29, 2012 at 6:42 am
In one of Climate Science’s favorite configurations you have two objects radiating against each other. When they finally equilibrate do they stop radiating??
++++
C: It was not exactly the same wording as the question posed, but close enough. I said no. Robert replied:
++++
R: “An excellent question. The answer is yes.”
C: So they stop radiating then? But they don’t, do they?
R: “…they will come into thermal equilibrium at the same temperature through a process called detailed balance.”
C: True, almost, but let’s keep our eye on the radiation:
R: “In the end, each body radiates exactly as much energy as it absorbs, with the entire cavity filled with BB radiation at the common temperature.”
C: Robert just said they will stop radiating then says they will continue to radiate as much energy as they absorb. Which which is it? Stopping or continuing? I thought that was covered in my physics courses and I do not recall flunking any of them.
I define the ‘continuing radiation’ as a transfer of energy, to wit, an energy flow. There is a continuous energy flow if Robert is right on his second opinion. That flow of energy is not necessarily a line-of-sight 1 for 1 exchange of quanta. From that there are implications relevant to the question at hand. Keep you eye on the energy flow ball: By definition a warmed molecule is never in a state where there is no ‘flow of energy’, which flow may be defined as ‘disequlibrium’. The contributions above are replete with examples of energy flow (at all) being described as taking place in a system that is still to reach equlibrium, still maximising entropy. It all depends on how closely you look.
Thermal equilibrium in a free floating gas atmosphere? Bulk, yes, detailed examination, no. (eternal energy flow)
In the presence of gravity? Bulk, unclear, detailed, no. (eternal energy transfer)
In the presence of extreme gravity? I have already cited Robert saying that with a black hole, no, in detail, no. (complicated eternal energy flow)
I bounced my question of simultanous isothermal state and equalised kinetic+potential energy at a common temperature for parcels of gas at different altitudes off my former intern Kyle this afternoon as we drove to the University of Guelph for a meeting. He agrees one cannot have it both ways. Either the total energy in the parcels are equal, relative to the surface, or the temperature is the same. Not both simultaneously. Which has the greater entropy? As there are some readers who want to hear only from the Lettered he is nearly finished his Science PhD at Waterloo U (you have heard of Waterloo U, right?) So maybe you will accept his opinion. Like Pompous, he also appreciated the CAGW=Consensus Physics and regaled me with stories about Consensus Medicine, a science of healing is apparently still in its infancy.
Tim Folkerts says:
January 29, 2012 at 12:27 pm
Thanks, Tim. If you like grains of truth, how about this one?
There’s a grain of truth in there as well. The moon’s bond albedo is about 0.11.
Does that grain make it a useful statement? Does it mean I should pay attention to it? Myrrh’s citation is new-age hogwash, lightly sprinkled with any kind of random scientific ideas in no particular order.
Here’s a sample from Myrrh’s citation, emphasis mine. The one thing I do have to say is that this guy Myrrh cited is the king of BS. Check this out, and if it doesn’t have you rolling on the floor, you don’t know electricity.
Don’t you hate it when the current “destroys the battery’s dipole”? Nothing an electrician fears more than that.
If there is a “grain of truth” in there, I don’t see it. The scary part is, Myrrh seems to believe it.
All the best,
w.
Willis wrote:
Myrrh’s citation is new-age hogwash, lightly sprinkled with any kind of random scientific ideas in no particular order.
I looked at Myrrh’s link. Speaking as someone who explicitly rejects the Second Law, I agree with Willis. Most of them appear to attempt to violate Conservation of Energy. The ideas do not reflect sufficient understanding of either Conservation of Energy or the Second Law to even begin to address success or failure in violating them. They’re just pieces plugged together randomly, in a manner. It appears to me to be a framework for obfuscation, for the purpose of concealing sleight of hand. Sorry, Myrrh.
If one considers the Second Law as part of “the straight and narrow”, then stepping off that course requires more care, not less. If you are going to stray from the path, you need to remember that the path is there for a reason, and reflects the collected experience of those who came before.
If you’re going to take up Doubt as your guide, then take it up wholeheartedly, applying it first and foremost to yourself.
ALL the atmospheres and all the solid bodies we know about are warmer at the bottom than they are at the top.
This includes objects which have no Sun/star to provide them with heating radiation.
So, nice theory, but the actual universe does not agree with you.
Just like in climate science, we should be trying to explain what really happens rather than what should happen based on some theory which is, obviously, incomplete according to way the real universe operates.
I again go back to the question of what force provides the extra thermal energy to mass in/falling into a gravity field. It is not outside radiation. It is internal to the atoms/molecules themselves or it is provided by the gravity field itself. The energy inside the atoms/molecules is being converted into thermal EM or the gravity field is imparted thermal EM to the atoms/molecules. Until someone can explain how that happens and how it cannot possibly happen in an equilibrium gravity situation, I cannot accept the theory. There is obviously a real phenomenon which is not being taken into account. There are untold numbers of joules in every object in the universe which are unaccounted for.
Willis,
The “grain of truth” is that energy DOES travel through space (not through the wires themselves) into a resistor (or similar device) by affecting the space around the resistor (as described by the Poynting vector & E & H fields).
But other than that isolated point (which is almost certainly not truly what Myrrh or the webpage are discussing), I agree that the rest of the discussion Myrrh points to is hogwash. I was just trying to point out that this is not THE worst physics I have ever seen (even if it is close). The webpage could have taken a bit of real physics (Poynting vectors) and then warped it to suit the author’s scheme.
“There are untold numbers of joules in every object in the universe which are unaccounted for.”
Earth mass is: 5.97 10^24kg. Earth was formed from all this matter hitting itself at about 20 km/sec. So that equals 1.19 10^33 Joules.
“The Earth’s internal thermal energy flows to the surface by conduction at a rate of 44.2 terawatts (TW), and is replenished by radioactive decay of minerals at a rate of 30 TW.” wiki
30 TW is 3.0 10^13 joules per second. Per year it’s 9.4 10^20 joules. In 4 billion years it is:
3.7 10^30 joules.
So in earth’s history about 300 times more joules were created during formation of planet compare to heat created by radioactive decay of minerals.
One could assume that some time during and/or after earth formation, earth was much hotter than it is now. Not saying it kept any of this heat, just saying at some point earth completely molten ball of lava. And since life started around 3.8 billion years ago, it was before that.
One question is how hot could earth like planet’s core be? And I would guess Earth was probably near whatever that number is.
It seems to be that the cooler a planet is, the faster the heat loss of it’s internal heat, and hotter it is, the slower this loss.
And if talking about large atmosphere- Venus hits that threshold, whereas Earth does not, the contraction of atmosphere will heat the planet- or said differently as a planet cools it’s atmosphere and oceans and/or land will contract keeping heat temperature constant.
It is said:
“Despite this, Jupiter still radiates more heat than it receives from the Sun; the amount of heat produced inside the planet is similar to the total solar radiation it receives. This additional heat radiation is generated by the Kelvin–Helmholtz mechanism through adiabatic contraction. This process results in the planet shrinking by about 2 cm each year.When it was first formed, Jupiter was much hotter and was about twice its current diameter.” wiki
So this indicates that Jupiter is cooling and this cooling is causing contraction and this contraction is generating heat. So I assume Jupiter is still cooling from it’s planetary formation. And Jupiter and gas giant in general are thought to formed quickly at beginning solar system formation- so cooling for very long time. Therefore it seems the loss of the heat generated from planetary formation is much slower with large planets- large planets with big atmospheres.
Ever since “Venus Envy” I have tossed this around.
The ‘thermal energy’ is measured by thermometers and they are giving spurious readings in air at different densities. Pack those atoms/ molecules together at the bottom of a gravity field and the temperature reading will be higher than a reading higher at a lower density.
The temperature sensor at both ends of the column is indicating a difference that does not exist, it is simply not the same air.
Only temperature readings of the same air density can be compared.
Crispin in Waterloo said @ur momisugly January 29, 2012 at 5:07 pm
Pompous Gits do not worry 😉
You are correct that Robert’s initial explanation was in error. I seem to recall that he said he makes frequent errors in his lectures. When your smarter students catch you out, you know they are paying attention and learning. It’s only the very insecure pedagogs who never err.
I imagine you are correct about a residual amount of heat/motion. Even at 0K the atoms still continue to vibrate just a little. Exploitable? Maybe. Worth exploiting? I suspect it would make windmills look spectacularly efficient sources of “free” energy.
Now I want you to think very carefully about the following:
Aristotle claimed in his Physics that heavier weights fall faster than lighter weights. John Philoponus in the 6thC argued that this could not be the case. He asked us to consider that if we tied two differing weights together, then the lighter weight would retard the fall of the heavier weight, and the heavier weight would increase the rate of fall of the lighter weight. In addition, the combined weights would fall faster than either of the lighter weights. The only way to reconcile the contradictions was to realise that objects all fall at the same rate regardless of their weight.
A thousand years later, Galileo Galilei made an almost identical argument. The story goes that he dropped a small and a large cannonball from the tower at Pisa. The balls supposedly reached the ground simultaneously. There is no evidence for this. Galileo’s account of the experiment has his assistant dropping a wooden ball and an iron cannonball of identical size from a height some 300 feet greater thyan the height of the tower at Pisa. He recorded that initially, the wooden ball fell faster than the iron ball. The iron ball eventually overtook the wooden ball and reached the ground first.
So, who was correct? Aristotle, that fine marine biologist and philosopher, or Philoponus and Galileo (among others) who argued that objects all fall at the same rate? I have found much food for thought introspecting about the lessons here.
You argue for heterodoxy and I can only agree. That said, you can take heterodoxy too far. The Git is a great believer in the Middle Way. And a happy heretic 🙂
Tim Folkerts said @ur momisugly January 29, 2012 at 7:12 pm
So what was the worst physics you have come across? The Git is intrigued 🙂
Dear Professor Brown
You are, of course, entirely correct about isothermal atmospheres. I’ve had to restudy thermodynamics recently and it confirms my earlier impression: thermodynamics in equilibrium systems often confound people’s expectations of how they think the universe should work. They misunderstand temperature as a thermodynamic property that only exists at equilibrium and not as a number that appears on a thermometer and fail to appreciate that the Laws of Thermodynamics really are fundamental.
WUWT does have really good science on it, but it has occasional craziness from a minority of people who think that evolution is just a theory, the fossil record is a sham, that all liberals support state control of the commanding heights of the economy and the idea that the Earth’s climate is or has been in a state of unstable equilibrium from which a slight perturbation in the concentration of a trace gas causes the whole Earth to barrel into a terrible heat death.
Fortunately on this forum I am not censored from saying it like it is.
Some people have actually changed their minds as a result of your post. This can be said to be a successful teaching lesson although there are always a few who will Never Learn.
For those of us who have studied thin isothermal atmospheres and scale heights, none of the post was a surprise, but it’s nice to revise these things.
This being WUWT people by and large have stuck to the issues considered without accusing you of being bought off by a gubmint conspiracy against free energy machines.
Jellbring was and is wrong. Willis has written stuff on WUWT which he acknowledges was wrong (i dare him to reread “the Steel Greenhouse”).
That which is peer reviewed in a scientific journal is not Gospel. Who knew?
John A
I have a very little time this morning before I have to go in and teach. I will try to answer one or two select questions in the meantime.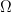 just outside either of the two surfaces, and computes the outgoing time average flux of the Poynting vector integrated over all frequencies:
just outside either of the two surfaces, and computes the outgoing time average flux of the Poynting vector integrated over all frequencies:
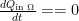
From gbaikie: Eventually it will reach thermal equilibrium, correct? At that time, in a gravitational field, the total energy of any upper molecule will be much greater than at the bottom (K+P), correct? Is that a state of maximum entropy?”
Thermal equilibrium is the state of maximum entropy. It is also the state where there is no free energy available to do work within the system. The picture in the top article above is one of a half dozen pictures that explicitly show that the an atmosphere with a thermal lapse in any direction you like is capable of doing work, because heat engines in the idealized world of thermodynamic arguments can run between any two thermal reservoirs. Or, as I’ve now shown what, four times? that one can use the simple, macroscopic definition of entropy to see that the entropy of a gas with a lapse rate strictly increases when moving heat irreversibly from the hot reservoir to the cold one so that a gas with a thermal lapse, gravity or not, is not in the state with maximum entropy or equilibrium. Conduction and radiation are two irreversible processes always available to the ideal gas, although Jelbring fails to acknowledge conduction and radiation is very, very slow for a GHG atmosphere. But then, he doesn’t actually do any physics in his “paper”, so it isn’t surprising that he fails to leave something important out.
If you want to talk about thermal equilibrium, perhaps you should learn what it really is and stop trying to do statistical mechanics in your head with some naive notion of “energy balance”. You might actually try to directly address the algebra instead of waving your hands with word arguments based on an incorrect heuristic that is leading you to accept a hypothesis that violates the second law of thermodynamics.
It seems to me you would cool the entire atmosphere.</i
Why? The wire and gas form a closed system in my picture above. The circular flow of heat is impossible, of course, but no net cooling occurs aside from tiny transfers of heat from the gas in or out of the wire as they come into a state of dynamic equilibrium.
I feel compelled to mention one talking about very small differences of heat, and therefore whatever engine you using will draw very little energy from the system.
Oh, so it is ok to violate the second law, as long as it is only violated a little bit, or slowly? It’s ok to build a perpetual motion machine that causes an isolated system to spontaneously cool to absolute zero and stores all of its heat energy reversibly in a battery or a spring as long as it happens at a rate that is slow compared to the rate of power production in a nuclear reactor?
I missed that lecture back when I took thermo.
I reiterate. Somewhere in your bookshelves or in a local library or on the web, you too can read authoritative, trustworthy descriptions of the laws of thermodynamics. You too can read about the second law of thermodynamics in particular. You can look at the pictures involved. It doesn’t say that you can’t build a perfect refrigerator that works slowly. It says that you can’t build it at all. It doesn’t say that a closed system can spontaneously decrease its entropy a little bit. It says that you can’t spontaneously decrease its entropy at all. The pictures used to analyze and prove the various statements are identical to the ones I utilize above. A system cannot be able to in principle violate the second law, even in the most idealized of circumstances, to be believable to most physicists, because most physicists don’t believe in magic and gross, stable, macroscopic violations of the second law are friggin’ magic.
Finally, you once again fail to understand that in a mind experiment like this how much energy is produced is a matter of the scale of the engines involved, which is under our control, so nothing stops us from making the actual amount of energy extracted as large as we like, as large as a nuclear plant. Even more amusingly, when you say “very little energy from the system” you are implicitly assuming on the one hand that the second law and its restrictions on the efficiency of heat engines is valid, so one is limited by the Carnot efficiency, while describing a macroscopic collective system that is 100% efficient at converting heat into work. Think about the consistency of such an argument.
So this silver wire would a refrigerator- a very poor and expensive refrigerator. Far, far worse than windmills and solar panels- in terms generating energy. And a windmill would be a cheaper and more efficient refrigerator.
No, the silver wire wouldn’t be a refrigerator, but putting a heat engine in its place that ran between the two reservoirs and (say) charged a battery with the work done would not only be a refrigerator, it would be a perfect refrigerator, one that decreased the entropy of the Universe and converted heat directly into reversible, storable work.
Second remark
Several people commented on my sloppiness in my short answer of “no” for the heat flow between two radiators in thermal equilibrium, because I failed to put the word “net” in front of flow. This is a pretty serious picking of nits — thermo textbooks regularly state that there is “no heat flow” between reservoirs in thermal equilibrium because there is no net macroscopic flow of heat between the two reservoirs, for the simple reason that thermodynamics averages over molecular (or in the case of radiation, single photon) scale local fluctuations. It wasn’t even unclear in the context of the discussion; later I talked explicitly about detailed balance at the microscopic scale.
But here, I’ll be more explicit. If one places a closed surface
where the $latex $ pair indicate a double integral average over frequency and coarse grained time. In other words the answer is no! — after a long time, the two radiators are in thermal equilibrium, neither gaining nor losing heat to each other or their environment. In particular, the total energy content inside any surface that completely contains either object is, on average, zero.
I’m not going to review the various arguments that indicate that this is indeed the equilibrium — they are straightforward consideration of the integrals over the blackbody spectra from the two bodies that shows that the hotter one loses heat (on average) and the colder one gains heat (on average) until they are at the same temperature and have identical spectra, where the (time/frequency averaged integral of the) flux of the Poynting vector vanishes within microscopic thermal fluctuations of the sort that are routinely ignored in thermodynamics. I’m not going to show the fact that this final state is maximum entropy — an elementary estimate based on heat transfer from the hot reservoir to the cold one by irreversible means suffices to do that.
gbaikie also goes on about DALR being affected by heating and humidity. Sure. Irrelevant to Jelbring, though.
myrrh said some things about You haven’t even read his explanation for writing it the way he did as your diabribe against him showed.
That’s because I don’t care why he wrote it badly. You show me one single original contribution in this article! It isn’t even a valid review article. I reiterate: He says “I’m going to prove that there is a stable thermal lapse in thermodynamic equilibrium”. He quotes a textbook that derives the DALR in a section on climate dynamics, atmospheric flow. He states “this is thermal equilibrium”. He concludes “I’ve proven that the DALR is thermal equilibrium and will heat an absolutely static, stable, isolated atmosphere with a fixed total energy content differentially after all thermalizing processes are finished.”
No he hasn’t, no it doesn’t. He hasn’t proven anything at all — he just begs the question by restating his assertion as his conclusion with a “QED”, and the conclusion he asserts violates the second law of thermodynamics.
I think you should begin again, Take II, and stick to his thought experiment and not your strawman silver wire deflection.
By all means, let’s. That way we won’t be able to trivially prove that his assertion that the system is in equilibrium is false.
The silver wire is hardly a “straw man”, of course. It is just a proxy for heat conduction, something that he seems to have left out of consideration when he listed the agents responsible for establishing thermal equilibrium in his “thought experiment”. Unless you can show that no irreversible transport of heat energy is possible — even in fluctuations — within this gas, which is prima facie absurd, it is trivial to show that moving heat from the bottom to the top increases the entropy of the closed system. I’ve done so several times above.
If you want to publish a paper for lay people to help them understand something, it helps to explain how the result doesn’t violate the second law of thermodynamics when obviously it does. Even for lay people. I mean, all it takes is a single course in introductory physics to see how his assertion fails. You don’t need a physics Ph.D, you just need to stop burying your head firmly in the sand because you don’t want to face the fact that a thermodynamically stable, DALR undriven by a thermal differential maintained by other means is bullshit magic.
rgb
But here, I’ll be more explicit. If one places a closed surface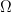 just outside either of the two surfaces, and computes the outgoing time average flux of the Poynting vector integrated over all frequencies:
just outside either of the two surfaces, and computes the outgoing time average flux of the Poynting vector integrated over all frequencies: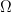 equals the time and frequency averaged outward direct flux of the Poynting vector integrated over that surface”. Since
equals the time and frequency averaged outward direct flux of the Poynting vector integrated over that surface”. Since  for either system, and since only radiation is a possible mechanism for the change, the net radiation through the surface vanishes.
for either system, and since only radiation is a possible mechanism for the change, the net radiation through the surface vanishes.
OK, so once again my efforts to put in a latex equation barfed. Sorry. It should be the algebra for “net rate of heat flow out of
I don’t give a damn if you want to view this as the detailed balance between outgoing and incoming flux, any more than I care about the fact that microscopically the total number of molecules inside a subvolume of an ideal gas in thermal equilibrium isn’t really a constant, they are always flowing in and out of the system. This is because in equilibrium, the scale of those thermal fluctuations are irrelevant by many, many orders of magnitude until you get down to very, very cold systems, where the fluctuations themselves are order of kT (and things like quantum mechanics start to matter).
Sorry about the algebra goof. The conclusion stands, though. By far the best answer is “no”; the yes answer is as irrelevant to radiation as the molecular flow is to density or the kinetic flow is to heat. Important, perhaps for establishing the conditions for detailed balance and equilibrium, but equilibrium is the state where balance is achieved, no net flow.
rgb
Robert Brown says: “Several people commented on my sloppiness in my short answer of “no” for the heat flow between two radiators in thermal equilibrium, because I failed to put the word “net” in front of flow. This is a pretty serious picking of nits … ”
Forewarned is forearmed.
The only reason I brought it up is that people here DO commonly make that assertion, and it will grow and spread if not continuously pruned. There is a vocal community within the skeptics that is SURE that IR from the cold atmosphere to the warm ground violates the 2nd Law. They are SURE that the word “net” is not implied in the 2nd Law but rather that NO energy can go from a hotter area to a colder area.
As I said, it is silly, but it is very much present.
Tim Folkerts says:
January 30, 2012 at 8:12 am
Robert Brown says: “Several people commented on my sloppiness in my short answer of “no” for the heat flow between two radiators in thermal equilibrium, because I failed to put the word “net” in front of flow. This is a pretty serious picking of nits … ”
Forewarned is forearmed.
The only reason I brought it up is that people here DO commonly make that assertion, and it will grow and spread if not continuously pruned. There is a vocal community within the skeptics that is SURE that IR from the cold atmosphere to the warm ground violates the 2nd Law. They are SURE that the word “net” is not implied in the 2nd Law but rather that NO energy can go from a hotter area to a colder area.
As I said, it is silly, but it is very much present.
Hear, hear!
If there is anyone left who is thinking about the exchange of KE for PE, as particles rise:
Some clever programmer (litdev) has written a bouncing ball simulation in Microsoft’s educational Basic; ‘SmallBasic’ (5.7Mb) it has a ‘Program ID’ of PMT149 . Watching the prog run and fiddling with the gravity one can see roughly what might be going on [ the same proportion of slow and fast particles at all heights??] Rather than downloading smallbasic you can run the sim (if Silverlight is lurking somewhere on your computer) at
http://smallbasic.com/program/?PMT149
If you would like to see some numbers I have made an ugly stab at printing them out in : XKT572
http://smallbasic.com/program/?XKT572
-The sim needs to be run for about a minute for the numbers to settle down. Try it without gravity first and then with ~0.02; Despite the KE/area ( pressure ratio?) of the two halves being ~3.5:1 the KE’s per particle in the two halves are generally within +-20% .( the temperature ratio?)
-To reset the cumulative Time Averaging, after things are changed, click on the ‘Reset Av’ text at the bottom.
-The sim seems to run a bit more smoothly and faster in Smallbasic than it does in the silverlight viewer. Both sims seem to lose KE slowly over time under gravity, unfortunately.
Chas
Good post.
It would be interesting to see the equations used for the calculations.
For instance where have they included the gravity term.
In the equations for the kinetic theory or in the Maxwell Boltzmann distribution or some other method.
@Tim
” There is a vocal community within the skeptics that is SURE that IR from the cold atmosphere to the warm ground violates the 2nd Law. They are SURE that the word “net” is not implied in the 2nd Law but rather that NO energy can go from a hotter area to a colder area.”
I have sseen that repeated to my dismay uncountable times. There seems to be (somewhere else) a website where they discuss how a hot surface ‘can’t receive’ radiation from a cooler one. The problem with the ‘not radiating’ misstep is that it supports the notion that heat somehow ‘knows’ whether or not it is aiming towards a hotter surface, then concludes it will not leave because it is hotter at the other end. I can understand a novice living with that thought so I agree, it needs to be watched.
Everything we see and touch is radiating all the time omnidirectionally. I have to deal with people who insist that radiation is ‘more efficient’ than convection or conduction. Arrgh.
The same radiation misunderstanding gives rise to the meme that some gases can, and some gases can’t be insulators. Actually a vacuum is a pretty good insulator too, which is no gas at all so relative to a gas, a vacuum is a ‘better insulator’.
Anyway, I have greatly enjoyed exploring the putative (and slightly silly) world of Planet Jelbring. As several have said above, it is really good to review the basics and apply them to unreal worlds. If Jelbring’s paper is a demonstrated dud, does this mean TallBloke will let the banned blogger (and by extension, Willis) back into his virtual fold?
Time to sit back with a nice cuppa and
relaxgo isothermal for a while.Paul Birch:
I largely remain of the opinion I gave previously. But a point you raised gnawed at me, and I tentatively reached a result that is an argument for your point of view: if you start with our atmospheric pressure at ground level, the difference in kinetic energy Velasco et al. specify for an altitude difference of, say, 10 km would not be measurable with a time uncertainty less than a second even in principle unless the gas-column width is less than something on the order of 100 nitrogen-molecule diameters across. I say “tentatively” because I made the calculation in haste and have not double-checked it. I mention it without having double-checked because I’ll likely be unable to return to these diversions in much less than a week.
rgb
Tim Folkerts says:
January 30, 2012 at 8:12 am
Robert Brown says: “Several people commented on my sloppiness in my short answer of “no” for the heat flow between two radiators in thermal equilibrium, because I failed to put the word “net” in front of flow. This is a pretty serious picking of nits … ”
Forewarned is forearmed.
The only reason I brought it up is that people here DO commonly make that assertion, and it will grow and spread if not continuously pruned. There is a vocal community within the skeptics that is SURE that IR from the cold atmosphere to the warm ground violates the 2nd Law. They are SURE that the word “net” is not implied in the 2nd Law but rather that NO energy can go from a hotter area to a colder area.
As I said, it is silly, but it is very much present.
===
What’s silly is postulating a process exists because hey, it’s statistics, it’s all so very modern milly maths therefore it has to exist”! Wally thinking. Until you can show that this is what is actually happening on that scale, that HEAT flows from colder to hotter, then all your “net” is, is statistical garbage in. And moreover, you now also have to show just how that “net hotter to colder” appears as you claim it doesn’t violate the 2nd Law, what is the mechanism, the physical mechanism, which does this? Surely there’s a Nobbly prize in it for anyone who can show what that is??
Because until you do so provide, you have no way of showing that it will obey the 2nd Law, you have no way of stopping the colder heating the hotter.
Chas says:
January 30, 2012 at 12:20 pm
“If there is anyone left who is thinking about the exchange of KE for PE, as particles rise:”
— — —
Sure Chas, great start of an analysis! Can’t resist such a challenge so I downloaded the code and will re-write it ground up in some flavor of c so we can have more like 10,000 balls using a proper sympletic high-order integrator to handle the vectors when gravity is present and allowing time reversal.
Did you notice why it keeps swapping between the top and bottom? You’ve got the entire column ‘bouncing’ because the initial states are assuming even pressure at the start up. Try allowing a ‘reset’ without actually resetting the position vectors. If you can hit the reset at the correct moment, the bouncing should quite down or even stop. Better would be to calculate the pressure and density gradient depending on the gravity setting and initialize the balls in the proper density per some small delta z slices. Way to go Chas… there’s a scientific mind! Prove it or not!
Try a printout of the data to file and run the statistics easily on excel. Graph it over time with any trends. Rough but should say something.
Because until you do so provide, you have no way of showing that it will obey the 2nd Law, you have no way of stopping the colder heating the hotter.
Or, you could learn about detailed balance, which is how one — not me per se, but anyone who does a stat mech computation — shows both that it obeys the second law and is what stops the colder heating the hotter and all that. Detailed balance is an explicit assumption of thermal equilibrium.
But hey, don’t let me interfere with your rugged insistence that you have nothing to learn here, and that “energy balance” (undefined) is meaningful but “detailed balance” (carefully defined) is not, and so on.
rgb
The temperature sensor at both ends of the column is indicating a difference that does not exist, it is simply not the same air.
Only temperature readings of the same air density can be compared.
Well, or you could be completely incorrect and what you assert could be experimentally rejected a thousand times over, something you can test instantly by picking up a can of compressed air at many atmospheres that somehow, stubbornly, appears to be at room temperature. Or (if you put it in a freezer) much colder than room temperature.
Read the zeroth law of thermodynamics, because it is the only macroscopic definition of temperature you are going to get.
Microscopically, it is a scale multiplier associated with probability and detailed balance, where it appears as a scale factor that makes energy dimensionless. In both cases temperature is defined by being “what you measure with a suitably designed thermometer”. Understand this and you’ll understand why you are wrong, and why the zeroth law is important.
rgb
There is obviously a real phenomenon which is not being taken into account. There are untold numbers of joules in every object in the universe which are unaccounted for.
Well, or they are all perfectly well accounted for, but you don’t want to look at the bookkeeping.
Look the thermodynamics/molecular dynamics of gravitational collapse isn’t really computationally all that difficult. There are large scale simulations that let people pretty much completely understand where all those “untold joules” come from and how they get turned into heat and how the heat is radiated away and all of that. The bookkeeping is necessarily sloppy, because how things evolve in time depends on a lot of details, but it isn’t like it isn’t well understood.
The beauty of Jelbring’s model is that he got rid of all of this, as did I in following him. No gravitational collapse. Steady state. The only way gravitational potential energy can get turned into heat is to change its gravitational potential energy, sort of like dropping big rocks from orbit down to the ground converts (most of) their PE to KE to heat. But then, you see, the impact crater cools, as the heat is radiated, convected, and conducted away. Wait long enough, and there is none of that heat left.
That’s where your statement is categorically incorrect. For a large body — a brown dwarf sized body, maybe even a Jupiter sized body, they can give off measurable leftover energy from gravitational collapse for billions of years. For a small body, not so much. In the case of any sort of body, talking about true equilibrium in the light of, well, the Sun — is a bit difficult, as no body sitting in sunlight is in static thermal equilibrium.
Look, there are dynamic models of how the atmosphere can act as a refrigerator (in a sense). In particular, they suggest that the atmosphere can self-organize to increase the rate of heat loss in response to differential increases in thermal forcing (basically overall negative feedback). Some of the speculative models I’ve looked over (proposed by readers of this list) aren’t overtly wrong — they seem plausible to me, at least so far. And they don’t violate the laws of thermodynamics — quite the contrary.
It might be good to understand gravity moderately well, and understand thermodynamics moderately well, before stating There Are Things Man Was Not Meant to Know about gravity, or asserting that we have no idea how to track gravity in statistical ensembles. I don’t think either one is remotely true, at least not until you hit the extreme limits of GR and quantum gravity and stat mech all mixed up together.
rgb
Robert Brown says:
January 30, 2012 at 3:02 pm
Because until you do so provide, you have no way of showing that it will obey the 2nd Law, you have no way of stopping the colder heating the hotter.
Or, you could learn about detailed balance, which is how one — not me per se, but anyone who does a stat mech computation — shows both that it obeys the second law and is what stops the colder heating the hotter and all that. Detailed balance is an explicit assumption of thermal equilibrium.
But hey, don’t let me interfere with your rugged insistence that you have nothing to learn here, and that “energy balance” (undefined) is meaningful but “detailed balance” (carefully defined) is not, and so on.
That you input that as a stop in a stat mech computation is being given as proof that cold flows to hotter?
What stops the colder heating the hotter? What???
Oh I certainly don’t think I have nothing to learn here.
Until you can show that this is what is actually happening on that scale, that HEAT flows from colder to hotter, then all your “net” is, is statistical garbage in, [and you cannot claim that just because you input it as a limitation in stat mech comps that it proves that such a critter as heat flowing from colder to hotter exists, duh] . And moreover, you now also have to show just how that “net hotter to colder” appears as you claim it doesn’t violate the 2nd Law, what is the mechanism, the physical mechanism, which does this?
Surely there’s a Nobbly prize in it for anyone who can show what that is??
Because until you do so provide, you have no way of showing that it will obey the 2nd Law, you have no way of stopping the colder heating the hotter.