Guest post by Robert G. Brown
Duke University Physics Department
The Problem
In 2003 a paper was published in Energy & Environment by Hans Jelbring that asserted that a gravitationally bound, adiabatically isolated shell of ideal gas would exhibit a thermodynamically stable adiabatic lapse rate. No plausible explanation was offered for this state being thermodynamically stable – indeed, the explanation involved a moving air parcel:
An adiabatically moving air parcel has no energy loss or gain to the surroundings. For example, when an air parcel ascends the temperature has to decrease because of internal energy exchange due to the work against the gravity field.
This argument was not unique to Jelbring (in spite of his assertion otherwise):
The theoretically deducible influence of gravity on GE has rarely been acknowledged by climate change scientists for unknown reasons.
The adiabatic lapse rate was and is a standard feature in nearly every textbook on physical climatology. It is equally well known there that it is a dynamical consequence of the atmosphere being an open system. Those same textbooks carefully demonstrate that there is no lapse rate in an ideal gas in a gravitational field in thermal equilibrium because, as is well known, thermal equilibrium is an isothermal state; nothing as simple as gravity can function like a “Maxwell’s Demon” to cause the spontaneous stable equilibrium separation of gas molecules into hotter and colder reservoirs.
Spontaneous separation of a reservoir of gas into stable sub-reservoirs at different temperatures violates the second law of thermodynamics. It is a direct, literal violation of the refrigerator statement of the second law of thermodynamics as it causes and maintains such a separation without the input of external work. As is usually the case, violation of the refrigeration statement allows heat engines to be constructed that do nothing but convert heat into work – violating the “no perfectly efficient heat engine” statement as well.
The proposed adiabatic thermal lapse rate in EEJ is:

where g is the gravitational acceleration (presumed approximately constant throughout the spherical shell) and cp is the heat capacity per kilogram of the particular “ideal” gas at constant pressure. The details of the arguments for an adiabatic lapse rate in open systems is unimportant, nor does it matter what cp is as long as it is not zero or infinity.
What matters is that EEJ asserts that ![]() in stable thermodynamic equilibrium.
in stable thermodynamic equilibrium.
The purpose of this short paper is to demonstrate that such a system is not, in fact, in thermal equilibrium and that the correct static equilibrium distribution of gas in the system is the usual isothermal distribution.
The Failure of Equilibrium

In figure 1 above, an adiabatically isolated column of an ideal gas is illustrated. According to EEJ, this gas spontaneously equilibrates into a state where the temperature at the bottom of the column Tb is strictly greater than the temperature Tt at the top of the column. The magnitude of the difference, and the mechanism proposed for this separation are irrelevant, save to note that the internal conductivity of the ideal gas is completely neglected. It is assumed that the only mechanism for achieving equilibrium is physical (adiabatic) mixing of the air, mixing that in some fundamental sense does not allow for the fact that even an ideal gas conducts heat.
Note well the implication of stability. If additional heat is added to or removed from this container, it will always distribute itself in such a way as to maintain the lapse rate, which is a constant independent of absolute temperature. If the distribution of energy in the container is changed, then gravity will cause a flow of heat that will return the distribution of energy to one with Tb > Tt . For an ideal gas in an adiabatic container in a gravitational field, one will always observe the gas in this state once equilibrium is established, and while the time required to achieve equilibrium is not given in EEJ, it is presumably commensurate with convective mixing times of ordinary gases within the container and hence not terribly long.
Now imagine that the bottom of the container and top of the container are connected with a solid conductive material, e.g. a silver wire (adiabatically insulated except where it is in good thermal contact with the gas at the top and bottom of the container) of length L . Such a wire admits the thermally driven conduction of heat according to Fourier’s Law:

where λ is the thermal conductivity of silver, A is the cross-sectional area of the wire, and ΔT=Tb–Tt . This is an empirical law, and in no way depends on whether or not the wire is oriented horizontally or vertically (although there is a small correction for the bends in the wire above if one actually solves the heat equation for the particular geometry – this correction is completely irrelevant to the argument, however).
As one can see in figure 2, there can be no question that heat will flow in this silver wire. Its two ends are maintained at different temperatures. It will therefore systematically transfer heat energy from the bottom of the air column to the top via thermal conduction through the silver as long as the temperature difference is maintained.

One now has a choice:
- If EEJ is correct, the heat added to the top will redistribute itself to maintain the adiabatic lapse rate. How rapidly it does so compared to the rate of heat flow through the silver is irrelevant. The inescapable point is that in order to do so, there has to be net heat transfer from the top of the gas column to the bottom whenever the temperature of the top and bottom deviate from the adiabatic lapse rate if it is indeed a thermal equilibrium state.
- Otherwise, heat will flow from the bottom to the top until they are at the same temperature. At this point the top and the bottom are indeed in thermal equilibrium.
It is hopefully clear that the first of these statements is impossible. Heat will flow in this system forever; it will never reach thermal equilibrium. Thermal equilibrium for the silver no longer means the same thing as thermal equilibrium for the gas – heat only fails to flow in the silver when it is isothermal, but heat only fails to flow in the gas when it exhibits an adiabatic lapse in temperature that leaves it explicitly not isothermal. The combined system can literally never reach thermal equilibrium.
Of course this is nonsense. Any such system would quickly reach thermal equilibrium – one where the top and bottom of the gas are at an equal temperature. Nor does one require a silver wire to accomplish this. The gas is perfectly capable of conducting heat from the bottom of the container to the top all by itself!
One is then left with an uncomfortable picture of the gas moving constantly – heat must be adiabatically convected downward to the bottom of the container in figure 1 in ongoing opposition to the upward directed flow of heat due to the fact that Fourier’s Law applies to the ideal gas in such a way that equilibrium is never reached!
Of course, this will not happen. The gas in the container will quickly reach equilibrium. What will that equilibrium look like? The answer is contained in almost any introductory physics textbook. Take an ideal gas in thermal equilibrium:
![]()
where N is the number of molecules in the volume V, k is Boltzmann’s constant, and T is the temperature in degrees Kelvin. n is the number of moles of gas in question and R is the ideal gas constant. If we assume a constant temperature in the adiabatically isolated container, one gets the following formula for the density of an ideal gas:

where M is the molar mass, the number of kilograms of the gas per mole.
The formula for that describes the static equilibrium of a fluid is unchanged by the compressibility (or lack thereof) of the fluid – for the fluid to be in force balance the variation of the pressure must be:
(so that the pressure decreases with height, assuming a non-negative density). If we multiply both sides by dz and integrate, now we get:

Exponentiating both sides of this expression, we get the usual exponential isothermal lapse in the pressure, and by extension the density:
![]()
where P0 is the pressure at z=0 (the bottom of the container).
This describes a gas that is manifestly:
- In static force equilibrium. There is no bulk transport of the gas as buoyancy and gravity are in perfect balance throughout.
- In thermal equilibrium. There is no thermal gradient in the gas to drive the conduction of heat.
If this system is perturbed away from equilibrium, it will quickly return to this combination of static and thermal equilibrium, as both are stable. Even in the case of a gas with an adiabatic lapse rate (e.g. the atmosphere) remarkably small deviations are observed from the predicted P(z) one gets treating the atmosphere as an ideal gas. An adiabatically isolated gas initially prepared in a state with an adiabatic lapse rate will thermally equilibrate due to the internal conduction of heat within the gas by all mechanisms and relax to precisely this state.
Conclusion
As we can see, it is an introductory physics textbook exercise to demonstrate that an adiabatically isolated column of gas in a gravitational field cannot have a thermal gradient maintained by gravity. The same can readily be demonstrated by correctly using thermodynamics at a higher level or by using statistical mechanics, but it is not really necessary. The elementary argument already suffices to show violation of both the zeroth and second laws of thermodynamics by the assertion itself.
In nature, the dry adiabatic lapse rate of air in the atmosphere is maintained because the system is differentially heated from below causing parcels of air to constantly move up and down. Reverse that to a cooling, like those observed during the winter in the air above Antarctica, and the lapse rate readily inverts. Follow the air column up above the troposphere and the lapse rate fails to be observed in the stratosphere, precisely where vertical convection stops dominating heat transport. The EEJ assertion, that the dry adiabatic lapse rate alone explains the bulk of so-called “greenhouse warming” of the atmosphere as a stable feature of a bulk equilibrium gas, is incorrect.
In this particular case, I am only questioning the validity of the test. I’m trying to choose my points carefully. This was a very narrow point.
There is no “Centrifugal Force”. That got pounded in to my head by a physics professor decades ago.
I do a fair bit of the pounding myself. But the point is that it still doesn’t matter. To a person riding in the frame, it feels like there is. The gas feels like there is too. Any differences if anything would be expected to enhance any steady state thermal separation.
Still, I have no problem with you not considering the Uranium centrifuges as not being conclusive evidence for isothermal equilibrium. They aren’t experiments designed to look at the question, for one thing, so there could be all sorts of confounding factors (although honestly I don’t think that coriolis force and rotational corrections to “true gravity” have a thing to do with it, any more than they do on Earth which is just such a rotating frame). They matter only as long as there is bulk “vertical” transport, but vanish as the gas reaches static equilibrium in the experimental chamber.
I do think that they can count as evidence for isothermal equilibrium — they certainly aren’t evidence against it — just perhaps not conclusive under the circumstances.
So now you have to weigh the preponderance of the evidence and the arguments.
The arguments are:
* A thermodynamically stable thermal lapse in any isolated system such that work can be done — even in principle — between different two different parts of the system at different temperatures violates the second law of thermodynamics. The possibility of doing work has to be “forbidden” by e.g. quantum barriers or the like, and even there there have to be no channels for transfer of energy (such as second order, two photon processes) that can make an end run around the quantum barrier.
* It can be and has been shown that an isothermal state in static force balance cannot do work and does not violate the second law of thermodynamics or any other laws of nature. In fact, it is a rather straightforward textbook example.
* It has been shown that the isothermal state has higher entropy than a temperature separated state, because it is capable of doing work. Maximum entropy states of isolated systems by definition and by demonstration cannot do work. Note how all of this ties together consistently. That’s why thermodynamics works. It is consistent, and empirically supported by a vast body of experimental evidence and everyday experience.
* It has been shown in a stat mech paper (that I still haven’t read) that equilibrium is isothermal.
* It can be shown with more detailed formal arguments that the isothermal state has maximum entropy.
* Such experimental evidence with extreme accelerations as exists fails to demonstrate a stable thermal lapse and appears to be isothermal. Whether or not it is “identical” to real gravity, the onus of proof is on you (or anyone) to demonstrate why such a lapse wouldn’t show up (especially when this is exactly the experiment proposed by a number of people who have wistfully hoped that it would confirm, not contradict, a lapse).
* The assumption that air is itself adiabatic — used in the derivation of the DALR in the first place — is false. The textbooks in which the derivation is given generally state that neither air nor any other gas really undergoes adiabatic expansion as it moves up and down, because air isn’t really adiabatically insulated by the air it expands into. Air is merely a relatively poor conductor and doesn’t have time to reach local thermal equilibrium as it convectively moves up and down, and it takes a long time to reach global thermal equilibrium across great distances via conduction alone.
The point of this last bullet is that real air on the real Earth is basically never in global thermal equilibrium. The air in my house is almost never in local thermal equilibrium. Turn the air conditioner on downstairs and see how long it takes to get cold upstairs! Air on the real Earth is always moving up, down and sideways as warmer air seeks cooler venues and cooler air circulates back to replace it and be warmed in turn. It is this mixing with poor conductivity that makes the differentially heated atmosphere exhibit a lapse rate that is often approximately (but almost never exactly) the classical DALR.
In the end, this entire thread has a single target and purpose. It seeks to convince the reader that they should not believe that the atmosphere intrinsically establishes a vertical thermal gradient as a spontaneous stable thermodynamic equilibrium state, one that would somehow “heat” the bottom relative to the top even if the whole thing were in a giant Dewar flask and one waited long enough for true equilibrium to be established. This static hypothesis is therefore not a candidate mechanism for explaining any part of “atmospheric warming”.
rgb
Joules quoted: “As a consequence, the heat transport is inhibited when the gas is heated from below.”
From “On the Influence of Gravity on the Thermal Conductivity”
http://arxiv.org/pdf/cond-mat/0002397v1.pdf
He was also so kind as to send the link to me offline. I therefore have looked it over. It (as I pointed out to J) confirms that equilibrium is isothermal. Joules just doesn’t understand their conclusion.
The paper examines a very dilute gas — so dilute that it is no longer properly speaking an ideal gas — in a container between two plates that are maintained at different temperatures. They assume that the gas does not convect. They assume that the system has “reasonable” dimensions for thermodynamics to work, e.g. that the mean free path is small compared to the size
Arrgh. Accidental hit post again while typing — continuing…
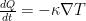
 to see if it remains symmetric for a thermal gradient in the direction of gravity and a thermal gradient in the opposite direction to gravity.
to see if it remains symmetric for a thermal gradient in the direction of gravity and a thermal gradient in the opposite direction to gravity. , so that:
, so that: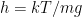 , is not (as it usually is) orders of magnitude larger than e.g. the mean free path of molecules in the gas. In the case where the gravitational scale height is (as usual) much larger than the mean free path and the characteristic length of secular changes in temperature (which goes to zero as the system approaches thermal equilibrium, which this paper does not study) the paper itself clearly states in the introduction that the usual symmetric Fourier law holds. Hence the need for a very dilute gas, with a long mean free path, a relatively large thermal gradient, and a large gravitational field compared to the mean temperature, none of which are true for the Earth’s troposphere. But no matter.
, is not (as it usually is) orders of magnitude larger than e.g. the mean free path of molecules in the gas. In the case where the gravitational scale height is (as usual) much larger than the mean free path and the characteristic length of secular changes in temperature (which goes to zero as the system approaches thermal equilibrium, which this paper does not study) the paper itself clearly states in the introduction that the usual symmetric Fourier law holds. Hence the need for a very dilute gas, with a long mean free path, a relatively large thermal gradient, and a large gravitational field compared to the mean temperature, none of which are true for the Earth’s troposphere. But no matter. becomes a spatial tensor form in Fourier’s law, so that heat conduction occurs faster in one direction than another, as long as
becomes a spatial tensor form in Fourier’s law, so that heat conduction occurs faster in one direction than another, as long as 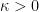 the equilibrium of the system is still established only by the vanishing of the thermal gradient!
the equilibrium of the system is still established only by the vanishing of the thermal gradient!
 if and only if
if and only if 
 vanishes (or is very small),
vanishes (or is very small),  takes on its usual Boltzmann value and conduction is actually symmetric. Again this is utterly expected, as in that limit the characteristic length of thermal fluctuations is again much smaller than
takes on its usual Boltzmann value and conduction is actually symmetric. Again this is utterly expected, as in that limit the characteristic length of thermal fluctuations is again much smaller than 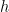 , although this seems to be true independent of the mean free path and hence is true even for the very dilute gas.
, although this seems to be true independent of the mean free path and hence is true even for the very dilute gas. . One has to be the usual Fourier term, unless you want the system to be even more insane than you are trying to make it (and not relax in response to a thermal gradient at all). The other has to somehow cause
. One has to be the usual Fourier term, unless you want the system to be even more insane than you are trying to make it (and not relax in response to a thermal gradient at all). The other has to somehow cause  to vanish when there is a nonzero thermal gradient.
to vanish when there is a nonzero thermal gradient. can vanish in Fourier’s Law except when the thermal gradient vanishes, given nonzero
can vanish in Fourier’s Law except when the thermal gradient vanishes, given nonzero  .
.
of the system and so on.
The entire purpose of the paper is to examine Fourier’s Law:
when there is a gravitational field in the direction of the thermal gradient. In particular, they seek to calculate
The paper finds — after a lot of hard work that I have no reason to doubt is correct — that under these particular conditions, there is a small asymmetry in
We observe that the heat flux increases with respect to its Navier-Stokes value when one heats from above, while the opposite happens when one heats from below.
What Joules did not observe is that the split vanishes precisely where the thermal gradient vanishes. Furthermore, it vanishes smoothly, at least in the Bhatnagar–Gross–Krook (Lattice Boltzmann) approach. Furthermore, the limit that this paper studies is one where the characteristic height associated with gravitational energy,
The thing that Joules somehow completely misses is that even if
The stationary state of even a very dilute gas at very low temperatures with a very strong field, in other words, is still isothermal.
Thanks, Joules, for helping to establish that even in this rather nonphysical limit — one not unlike that represented in the small molecular dynamical simulations one can find that turn on gravity for only a few particles bouncing around in a box so that particles visibly slow down between collisions — thermal equilibrium is still isothermal.
If one examines figure 1 in the paper, one notes that as the thermal gradient
Here’s a lesson, Joules. The entire point of Fourier’s Law is that it drives any system with conductivity towards isothermal equilibrium. What you are looking for is something that has at least two terms on the right hand side opposite
Oh, and don’t forget, the whole expression has to be derived from and related to the Navier-Stokes equation. That is, you can’t just make one up and claim that it is correct.
I wish you good luck with that.
If you disagree, feel free to present a counterargument, and show me any way that
rgb
Myrrh says:
January 26, 2012 at 6:50 pm
It’s the analogy to the passive solar water heating system. First lets get a few things clear on that.
A good passive solar system does not need a greenhouse for the collectors to operate well. Often they use just plain black piping. It works nearly as well as pipes in a greenhouse because the 1,000 plus watts of solar radiation far exceeds radiation losses without the greenhouse.
“Shouldn’t that be 240?”
No thats the “average”. If radiance were uniform it would be 240. the radiation averages 240. But “average” only matters if something cools at the same rate it warms.
Thus it requires 4 elements to exceed the average temperature 1) A variable heat source which is provided by a large object rotating in front of a more powerful source in which case the maximum you can change the storage system is limited to the maximum input; 2) you need heat storage capability (it doesn’t do any good to move heat for later use if you can’t store it, and 3) for the passive system a means of transferring heat that moves heat faster from the collectors to the storage than it does the storage to the collectors (you get that from convection), and 4)you need a storage system that cools slower than the collectors (in the case of the water system you insulate the storage tank and for the atmosphere you need an atmosphere that radiates more poorly than the surface giving you effective insulation of the heat in storage.).
You have all four elements in a planetary environment with an atmosphere
With no heat losses in the storage system (non-radiating atmosphere) the atmosphere GHE would be something north of 130K. Passive designers make these calculations all the time in passive system development. The upper limit of a perfectly insulated system is the upper limit of the fluctuating radiation. 1366 watts. That equates to 139K GHE.
Of course the common air in the atmosphere does radiate. It also conducts to the ground but the conduction is minor. At a 150k differential and 4ft to a Stevenson screen you only get about 3 watts/hour conduction. Thus over 12 hours you could lower the temperature about 12 degrees.
What the atmosphere does radiate is what lowers it to 33K GHE. If GHG make a difference its likely a cooling difference.
This is really basic stuff! Its demonstrated in passive solar heating systems all the time.
I told one gentleman that greenhousing the collectors doesn’t matter much. And for the most part it does not. The temperature of highly conductive collectors, or the ground in a greenhouse is not going to significantly change by putting it in a greenhouse.
Its going to be at roughly the max radiation temperature. What a greenhouse primarily changes is the atmosphere in a greenhouse not the surface. One can verify that by walking barefoot through a asphalt floored greenhouse to outside on a hot sunny day. What you will find in most cases is the ground is warmer outside the greenhouse and the air is warmer inside the greenhouse. The ground is warmer outside because nothing is completely transparent and all greenhouses block some of the light, particularly greenhouses used by growers which often do not have crystal clear glazing.
What we need to do with these academic climate scientists is take them outside and introduce them to the real world.
OK, so some of that latex didn’t come out so well, missed a $ in there someplace. Clearly, time for bed. I think the main point is clear, anyway. Not even in this extreme case is equilibrium anything but isothermal, and I’m certain the authors of the paper would be surprised and shocked to hear that someone was suggesting otherwise, given that the paper studies heat flow, based on underlying kinetic principles that make that impossible.
rgb
thepompousgit says:
January 26, 2012 at 11:52 am
“No numbers; just armwaving. You say “Greenhousing… only adds a few degrees to the system”. I say, if I don’t open the vents on my greenhouse early enough in the day, the crop will rapidly die. That’s not just “a few degrees”. But you remind me that I don’t have numbers. Time for some experiments so I can put some numbers on the greenhouse effect that is not The Greenhouse Effect (so to speak).”
You are looking at the “atmosphere” in the greenhouse. It isn’t much relevant to how much the surface warms in the greenhouse. The greenhouse atmosphere in an empty greenhouse cannot get warmer than the surface. Its usually much less. A greenhouse will have an atmosphere much closer to the surface temperature than the atmosphere outside of the greenhouse because the greenhouse prevent the heat from running away by convection.
Dr Brown,
Thank you for providing a forum on the subject of the physics of atmospheric temperature lapse.
I have been struggling to discover what might be causing so many to be confused enough to believe something like radiation is controlling the temperature distribution through the atmosphere, based on only faith, when it is so mind bogglingly obvious that the temperature lapse is the expected consequence of the pressurizing that occurs as gravity forces the air toward the surface.;
I have spent what seems like most of this year so far, reading through the many fallacies and contradictions that you and others have been offering, and even gloating over.
Yet, I struggled on. Then it hit me. The frame of reference has been skewed.
You started with this definition: “…because, as is well known, thermal equilibrium is an isothermal state” and try to make it the only steady state case. Nonsense! In reality there is no isothermal state, anywhere, and to use nonsense to frame examples leads to the same sort of results as when one substitutes “0” for “lim X as X –> 0”. Sometimes they are right..
The term “steady state” can be and is used to describe process conditions. Earth’s atmosphere responds to, and is continuously being processed by many forces, including gravity…
Robert Brown wrote:
A thermodynamically stable thermal lapse in any isolated system such that work can be done — even in principle — between different two different parts of the system at different temperatures violates the second law of thermodynamics. The possibility of doing work has to be “forbidden” by e.g. quantum barriers or the like, and even there there have to be no channels for transfer of energy (such as second order, two photon processes) that can make an end run around the quantum barrier.
I agree that such a system would contradict the second law.
It has been shown in a stat mech paper (that I still haven’t read) that equilibrium is isothermal.
I would be interested in reading that paper, unless you mean Coombs and Laue, in which case I I’ve read it. In my own analysis, if the Maxwell-Boltzmann Distribution retains spherical symmetry in a gravify field, the column is isothermal. If the Maxwell-Boltzmann distribution has different circular and vertical symmetries (but still symmetric about the origin), it is not isothermal. I believe the Velasco response reached the same conclusion. There are some words I’ve left out of that, but I’m not sure how to formulate it in a simple manner. My words in Tallbloke’s Loschmidt thread are probably more clear, and certainly more extensive.
Such experimental evidence with extreme accelerations as exists fails to demonstrate a stable thermal lapse and appears to be isothermal. Whether or not it is “identical” to real gravity, the onus of proof is on you (or anyone) to demonstrate why such a lapse wouldn’t show up (especially when this is exactly the experiment proposed by a number of people who have wistfully hoped that it would confirm, not contradict, a lapse).
I accept that the onus on is on me. I offer a brief sketch (not complete) here. “Not conclusive” may be the best agreement we can reach.
For this, the frame of reference is Oak Ridge. Consider that from time to time, one of the molecules will come to approximately 0 velocity relative to Oak Ridge. What are the forces acting on that molecule at that time? I believe that, until it is disturbed by collision with another molecule, the sum total of forces acting on it will be one g. The molecule has no information that it is in a centrifuge spinning near Mach 2. The only force it can ‘feel’ is gravity.
In the Gravity Lapse Rate theory, what matters is that the molecules experience acceleration between collisions. Since molecules in a centrifuge experience only 1 g between collisions, I see no basis for enhancement of the GLR beyond the factor of ~12 from the molecular mass.
The assumption that air is itself adiabatic — used in the derivation of the DALR in the first place — is false. The textbooks in which the derivation is given generally state that neither air nor any other gas really undergoes adiabatic expansion as it moves up and down, because air isn’t really adiabatically insulated by the air it expands into. Air is merely a relatively poor conductor and doesn’t have time to reach local thermal equilibrium as it convectively moves up and down, and it takes a long time to reach global thermal equilibrium across great distances via conduction alone.
I suspect this is incorrect, but I am not currently equipped to present a convincing case. I do understand the textbooks in this regard.
As for my sanity, that is rarely questioned by people who know me (fish, barrel).
Robert Brown’s refutation is contrived and irrelevant.
Any column of any substance from the top to the bottom of the cylinder is subject to the same gravity field with gravitational constant g as the gas in the cylinder. Or, what is the same according to the general theory of relativity, the cylinder with gas as well as the conductor (whatever material it is made of) are subject to the same constant acceleration (vector) of g m/s2. Therefore, the same temperature gradient will appear in the conductor and or along the walls of the cylinder as exists in the gas. The bottom of the cylinder pushes against the gas mass and any other top-to-bottom material like an adiabatic piston, raising the temperature at the bottom, whereas the top of the cylinder pulls like a similar piston, decreasing the temperature at the top.
As long as the acceleration is maintained, the temperature gradient in the direction of the acceleration vector will be maintained. If heat is added to the system by radiation from outside, the average temperature will increase, but as long as the acceleration continues, a temperature gradient between top and bottom of the system will be maintained.
Bravo, Albert! Bold effort! It doesn’t matter what it is, or where it is, everything has a higher temperature at the bottom than at the top. That will stop the damn heat from flowing! Good job proving it by calling my refutation “contrived and irrelevant”, to. That way you don’t have to actually address any of the — err — details. That would require confronting an inconvenient truth or two, such as the fact that the last time you actually took a course in real math (I don’t just mean wussie old intro calculus, either, I mean real math) or physics was — never? Real men don’t need to actually study and work — they just sort of “know things”.
But your best gambit by far is tying it to the general theory of relativity — a theory I would be amazed if you know anything about whatsoever except how to spell it correctly — you have made it more or less impossible for anybody on this list to challenge you! Hell, I teach graduate E&M and special relativity theory, and yet my heart quakes whenever I encounter GR because all of that nasty tensorial calculus gives me gas. I’m just not smart enough, or patient enough, and I’ve only learned a smattering of it along the way. Of course I’m almost as allergic to hard-core stat-mech even though I spent years working in it. It’s insanely difficult. And you my good man, have clearly mastered them both! Together!
So you win. I concede. Well played, sir!
Just don’t make me go wade through the GR texts to prove that no, you silly beanie you, a silver wire hanging vertically doesn’t have a stationary thermal lapse due to — pardon me while I compose myself — GR thermodynamics, not one you could measure with the most sensitive of instruments here on wimpy-gravity Earth (how could you measure it, damn thermometers suffer from exactly the same GR lapse!). Gads! It’s there even if we can’t see it! Heinous!
Far better it is to suffer the slings and arrows of outrageous misfortune and your justly earned ridicule than to try to compete with you, sir, on stuff even Steven Hawking got wrong. Hell, even Jelbring proposes that the lapse rate isn’t the same for two different kinds of gases! You’ve trumped even him!
Now, weeping softly to myself (tears of laughter) it really, truly is — off to bed.
rgb
Stephen Rasey says:
January 25, 2012 at 9:38 pm
“@Bill Hunter. Thank you for that example where the diurnal day-night pumping is so essential for a passive solar hot-water system. ”
Thanks for noticing! For a week and 3 of these threads I have been saying this with nary a response. I don’t think anybody read it even.
Most solar hot water systems are active. Thats because they take the passive system and turn it on its head. People like the collectors on the roof and the storage in the basement.
So to do that you need a circulatory pump that turns on when the collectors are warmer than the storage and shuts off when they are not to simulate convection but have it run backwards. You also need a value that closes when the collectors are colder than the storage so natural convection doesn’t send all your heat to the roof to radiate away.
There is no question this accounts for some of the greenhouse effect, I am favoring all of it but can’t be sure. At any rate it has to be an important part of it and it sure appears to me that as you increase the ability of the atmosphere to radiate you are going to lose heat seemingly as sure as stripping insulation off your storage tank. Just that adding .0002 of a gas about 8 times less efficient than the surface isn’t hardly going to do anything. Now water that no doubt does the heavy lifting, it cuts the lapse rate in half and it covers more than half the sky with clouds. Add in some emissivity for common air and you can trim it back pretty fast. Having well insulated storage can make up for having short days. Thats what passive solar design is all about!
ZP says:
January 26, 2012 at 7:55 pm
I actually built a vortex tube some years ago, when I was running a shipyard and machine shop on a remote South Pacific atoll.

I machined the vortex tube out of a block of solid aluminum. They are quite amazing, able to deliver very cold air once they are tuned and adjusted. I used some plans that I’d photocopied out of the “Amateur Scientist” column in the late lamented Scientific American. I mean the old Scientific American, back when they actually reported on science. I’m told they use vortex tubes these days to cool the drivers in the race cars during the pit stops.
As one of the few people who have personally built, tested, modified, measured, played around with and experimented with the Ranque-Hilsch vortex tube, I can say from personal experience that they don’t shed any light on the various “gravito-thermal” hypotheses, because the vortex tubes operate on very different principles than those claimed for the gravity theories.
w.
You started with this definition: “…because, as is well known, thermal equilibrium is an isothermal state” and try to make it the only steady state case. Nonsense! In reality there is no isothermal state, anywhere, and to use nonsense to frame examples leads to the same sort of results as when one substitutes “0″ for “lim X as X –> 0″. Sometimes they are right..
The term “steady state” can be and is used to describe process conditions. Earth’s atmosphere responds to, and is continuously being processed by many forces, including gravity…
No, I started (read the article at the beginning) trying to prove that the idealized adiabatic stationary atmosphere discussed in a specific paper by Jelbring (EEJ) is isothermal, not one with the DALR Jelbring asserted without real proof. I did so because Jelbring’s paper is cited over and over again on WUWT and tallbloke’s blog (tallbloke himself invited me to look at it) and when I did, it was obviously in violation of the second law of thermodynamics, which I have now proven several ways and times in the following discussion.
If you concede this one point — that Jelbring’s paper is nonsense — and promise never to cite it to support any of your future ramblings about how the atmosphere causes the ground to be warmer than the upper atmosphere — then I have succeeded in my aim. I am not at this time addressing all of the other abuses of the hapless laws of thermodynamics and physics in general that occur in on-list discussion because I’m just one person, and it has now taken me at a guess over a hundred posts over three days to mostly convince at least some of the people who believed that it was true and correct that they were mistaken. Others, I’m certain, will never be convinced, or will immediately try to pretend that “gravitational heating” occurs some other way.
Some of the stuff proposed in this regard is not at first glance objectionable, BTW. I’m not taking a position on it until I understand what is being proposed. Is that so unreasonable? I seek to avoid saying silly things like “Jelbring is wrong, so the DALR must be wrong too — of course it isn’t, it is just a non-equilibrium state, one that requires (I am almost certain) heating the atmosphere primarily from the bottom to maintain, as you assert as if you think that I think otherwise. Never mind that I’ve said that myself repeatedly in this and other threads discussing the subject. Straw men are the easiest ones to wrestle, I always say.
Other stuff is just as absurd. N&Z, for example, in their “unified climate theory” claim that their “miracle equation” predicts the temperature as a function of surface pressure nearly perfectly. The fact that it has an extreme nonphysical form with characteristic pressures of 54,000 bar and 202 bar in it and is the product of two exponentials used to independently fit three planets on one end, two on the other, with a four-parameter set is conveniently hidden, until one looks for it. Then it becomes clear that their “miracle” is utterly meaningless and nonphysical. That kind of thing I will eventually get around to whacking, as I have the time.
I must say, though, that it is really amusing to see the lengths people will go to to try to deny that CO_2 plays any role in warming the Earth. Why bother? Look at the IR spectrum of the earth as photographed from orbit! Seeing is believing. Hard to deny the evidence of our own eyes, don’t you think, even if those eyes are electronic ones.
But don’t let me stop you! Play right on through.
rgb
As one of the few people who have personally built, tested, modified, measured, played around with and experimented with the Ranque-Hilsch vortex tube, I can say from personal experience that they don’t shed any light on the various “gravito-thermal” hypotheses, because the vortex tubes operate on very different principles than those claimed for the gravity theories.
For one thing, for example, they require the input of work to operate. They don’t just sit there and spontaneously heat one place and cool another.
No second law violation there. But they are very nifty, I agree. Not that efficient, though, as air conditioners go.
rgb
Greg Elliott says:
January 24, 2012 at 10:15 am
OK, no force as complex as gravity can function like a “Maxwell’s Demon”.
Your move,
w.
There is no question this accounts for some of the greenhouse effect, I am favoring all of it but can’t be sure. At any rate it has to be an important part of it and it sure appears to me that as you increase the ability of the atmosphere to radiate you are going to lose heat seemingly as sure as stripping insulation off your storage tank. Just that adding .0002 of a gas about 8 times less efficient than the surface isn’t hardly going to do anything.
Bill, you sound reasonably smart and practical. I agree that the specific heat and storage capacity of things like the oceans (especially) play a role in regulating the Earth, but you have to bear in mind that from the point of view of outer space, the Earth is a system with precisely one channel. Radiative heat in, radiative heat out. Experience with heating and cooling stuff on the surface is a matter of balancing conduction, convection and so on. Balancing the Earth itself is pure radiation.
With that in mind, take a look at the actual IR power spectra taken from orbit. In them, you will very clearly see that the CO_2 band radiates power at a much lower temperature than the surface of the earth. That is, part of the actual active band of outflow radiation is losing heat much more slowly than it would be without the CO_2. It’s not the only place where this happens — Ozone plays a role, and water is both the major transparent window through which outgoing radiation DOES escape and itself something that drops the outflow over part of the spectrum.
I know you’re probably confused and cynical about the usual descriptions of the GHE, with their upwelling and downwelling radiation and flow diagrams, but all of that is crap. You don’t need it to understand the idea. The idea you can understand with nothing but a garden hose. The faucet is the sun, it pumps energy into the hose, which represents the earth. The other end of the hose is outer space. The hose has no leaks — there is nowhere but in one side, out the other for the water to go.
Put your thumb over the outflow end and obstruct a bit of the flow and what happens? The pressure INSIDE the hose goes up a bit to compensate until the water flow out of the remaining part of the outflow end matches the inflow.
That’s all the greenhouse effect is. It doesn’t matter HOW you imagine things happen in the atmosphere proper, or what you think might help store heat or moderate or modulate temperatures. The IR spectra show that part of the outgoing nozzle is partially blocked, radiating a lot less power than the surface temperature would by itself be expected to radiate. By any mechanism you like — this isn’t about mechanism, this is about conservation of flow — the temperature of the rest of the system must increase enough to drive up the flow in the unblocked part of the garden hose until dynamic equilibrium is once again reached.
This argument doesn’t mean that other stuff doesn’t happen, or that it isn’t interesting or important. It just indicates that there is no question that the GHE exists and is real, because you can photograph it in action from orbit.
The real skeptical argument isn’t — or shouldn’t be — “there is no such thing at the GHE (false)”, or “real greenhouses don’t work that way (possibly true, but irrelevant)”, or “it’s all due to the DALR (reversing cause and effect)” or worse “it’s all due to stationary atmospheric pressure heating (impossible)”. It should be things like “What are the feedbacks?” “What other mechanisms modulate the rate of heat loss, and what is their relative contribution to global temperature and climate?” , “How does the Earth’s climate system self-organize to cool more efficiently as it warms (see what are the feedbacks)”, “given optical saturation in the CO_2 band, what is the real marginal effect of more CO_2 (could be very small indeed)?”
Ideally, taking the time not to shoot from the hip with whatever arguments are advanced. This is a hard problem, and deserves more effort and attention than just throwing out and vigorously defending an idea that even a tiny bit of later reflection with an actual textbook or two in hand might make you reconsider.
Just my opinion, of course, but — take a look at the IR spectra. That might be enough to at least convince you that some of the warming is straight up due to the GHE and CO_2.
rgb
OK, no force as complex as gravity can function like a “Maxwell’s Demon”.
Can I play, Willis?
Heh, heh. Try this one: Except when the gravity in question is gravity on the edge of a neutron star and extreme general relativity holds. That’s when the invisible fairies are liberated, and they sort out all of the hot and cold molecules.
Yeah! Try to argue with that, suck-ah!
rgb
Robert Brown says:
January 26, 2012 at 11:13 pm
Not only do they require the input of work, they require the input of lots of work. I was shocked when I calculated the efficiency on the one that I built. Absent very special circumstances, it’s not cost-effective for cooling or for heating. (Unless you are getting your power from Stygian Gas and Electric, where they pay you to use their energy.)
In addition, since Murphy isn’t dead, it’s a tradeoff. You can tune a vortex tube for greater or lesser ∆T between the hot and cold ends. But the greater the ∆T, the lower the efficiency. Murphy never sleeps, parasitic loss always goes up faster than output.
Which is another point in favor of the overall feedback on increasing global temperature being strongly negative. Increasing the operating temperature of the climate engine will greatly increase parasitic losses. But I digress.
w.
In the Gravity Lapse Rate theory, what matters is that the molecules experience acceleration between collisions. Since molecules in a centrifuge experience only 1 g between collisions, I see no basis for enhancement of the GLR beyond the factor of ~12 from the molecular mass.
So I guess you don’t agree with Einstein, then. Oh, well.
I would have said that all that matters is their effective acceleration in the accelerating frame, since the equivalence principle says that the molecules don’t experience anything from gravity either way. It just affects the way they hit the things around them.
You want to stop “feeling gravity”? Jump. While you are in free fall, you can’t feel it. What you “feel” about gravity is the things opposing it. Jump out of a tall building and you feel fine. What matters — and all that matters to the molecules — is the violence with which they strike other molecules.
To put it yet another way, the average buoyant forces that have to be exerted, the density and pressure profile, and all of the thermodynamic properties of the gas in the centrifuge are pretty much determined by the horrendously large value of effective g in the centrifuge. Now you want to assert that adiabatic lapse a phenomena that every single derivation of it requires a) that the gas be adiabatic, that is, a perfect insulator, which real gases (even ideal ones) are not; and b) be uplifting and downfalling — it is the “adiabatic expansion” that occurs as air parcels lift and fall due to variations in buoyancy that establish the rate, after all — some how lapses vertically along “real g” intead of horizontally opposite to the enormous density gradient due to 10,000g that determines the actual direction of convection and buoyant force in the frame of the gas.
Nonsense. I don’t mean that in an insulting way — you seem like a smart guy, you can see the inconsistencies in that yourself. You’re trying to fit a camel through the eye of a needle, and I think that you know it. The camel doesn’t like it.
rgb
It is not the intention of the writer to demean, degrade, humiliate, despise, or disparage any god, godess, offspring of god, demiurge, inamorata, fetish or other supernatural being.
ROTFL. Even more than the GR induced universal thermal lapse. Remind me not to radiate any photons towards the sun!
rgb
Bill Hunter said @ur momisugly January 26, 2012 at 10:14 pm
I couldn’t find my digital thermometer, so I “borrowed” the very old max/min mercury/alcohol thermometer from the greenhouse to measure water temp.
Time 7 pm Eastern Summer Time (close up the greenhouse time)
Greenhouse air temp 80F
Max greenhouse temp reached 110F
Open air temp 69F*
Water from 150 yards long 2 inch black polypipe running exposed to sun on bone dry ground (no rain for 2 weeks) 72F
Maximum open air temp reached 81F*
Cloudless, still sunny day.
* Weatherstation 4 yards from greenhouse. Temp converted to degrees F from C.
FWIW I’m a semi-retired farmer and spend quite a lot of time outside.
If you put 2 bricks in the sun, and suppose after hour or so the highest
temperature they got was 140 F.
Then put bricks in oven and heat to 250 F. Put one brick in sunlight and other in shade.
How long would it take for sun lit brick and shaded brick to cool to 150 F?
Robert Brown wrote:
You want to stop “feeling gravity”? Jump. While you are in free fall, you can’t feel it. What you “feel” about gravity is the things opposing it. Jump out of a tall building and you feel fine. What matters — and all that matters to the molecules — is the violence with which they strike other molecules.
I would still be experiencing gravity. I’ve gone several seconds without “feeling gravity”, but I was distinctly aware of it, and the “feeling” all caught up with me in the end. Done right, it’s quite enjoyable.
So I guess you don’t agree with Einstein, then. Oh, well.
In a great many ways. I learned to visualize multiple frames at once at a young age. The only particular thing I disagree with is, “God does not play dice with the Universe”. My terminology, however, is sometimes sloppy.
I would have said that all that matters is their effective acceleration in the accelerating frame, since the equivalence principle says that the molecules don’t experience anything from gravity either way. It just affects the way they hit the things around them.
The ‘acceleration’ is an artifact of the rotating frame, not an accelerating frame. That is, it results from how you set up your math. If you model the interior of the centrifuge from the accelerated but non-rotating frame of Oak Ridge, you get different answers. Particularly, consider the case of a single molecule that comes to a dead stop for an instant, and accelerates downwards under gravity. In the Oak Ridge frame, its motions are simple. In the rotating Centrifuge frame, one might conclude that the core of the centrifuge itself was exerting an enormous gravitational field.
If you get different results in different frames (after correcting for dilation), you’ve either made a mistake setting up one of your frames, or you botched your Lorenz Transform, or coordinate substitution.
Nonsense. I don’t mean that in an insulting way — you seem like a smart guy, you can see the inconsistencies in that yourself. You’re trying to fit a camel through the eye of a needle, and I think that you know it. The camel doesn’t like it.
Thank you. I respect you as well, though it may seem otherwise. I do know it, but not necessarily in the way you think.
Where I think a lot of people go astray, particularly on this subject, is that within their interpolated regions, the laws of physics are very solid. It’s only the extrapolations that are problematic. Newtonian mechanics are a good example of this.
I believe the Second Law holds except under very specific criteria. I believe Gravity meets those criteria, but centrifuges do not, at least not in a way that that breaks isothermal. I haven’t checked the math on Dalton’s Law, and won’t, particularly since I don’t have the Oak Ridge numbers.
ZP says:
January 26, 2012 at 7:55 pm
While reading about Maxwell’s demon, I came across an amazing invention that appears to shed light on the “gravitationally induced” temperature gradient idea. The Ranque-Hilsch Vortex Tube generates a temperature differential within a tube by injecting a compressed gas stream which then generates a vortex. The temperature along the walls is higher than the incoming gas, while the temperature within the center is colder than the incoming gas. The two temperature streams can be physically separated.
Here are some links, including a patent on an embodiment of the invention, if you are interested in learning more about this phenomenon:
http://www.filtan.de/ENGLISH/VTS_A.htm
http://www.me.berkeley.edu/~gtdevera/notes/vortextube.pdf
http://www.google.com/patents?hl=en&lr=&vid=USPAT3546891&id=KppYAAAAEBAJ&oi=fnd&dq=%22vortex+tube%22+gravity+%22temperature+gradient%22&printsec=abstract#v=onepage&q&f=false
=====
For interest:
http://www.arizonavortex.com/vortex-tube/
“Vortex Tube Information
A vortex tube is a tool that can take normal compressed air and convert into two air streams. One stream is hot air and the other stream is cold air. The beauty of the vortex tube is that it has no moving parts , which translates into no maintenance. The cold air can be adjusted down to -50 degrees Fahrenheit, and the hot side can be adjusted up to a temperature of 260 degrees Fahrenheit.”
http://www.lovesedona.com/01.htm
Bell Rock Vortex of the twisted juniper:
“Juniper trees respond to the vortex energy in a physical way that reveals where this energy is strongest. The stronger the energy, the more of an axial twist the Juniper trees have in their branches. Instead of going straight down the branch, the lines of growth follow a slow helical spiral along the length of the branch. This spiraling effect can sometimes even bend the branch itself.”
If masculine/feminine not your thing, the following page has more information on the streams within the vortexes/vortices
http://gosw.about.com/od/sedonaarizona/a/sedonavortex_2.htm
Which says this is a “pure upflow” vortex.
Q. Daniels, here is a celebrated scientific quote that is worth pondering:
Here Eddington’s point is that the Second Law is believed to hold universally (even under unusual or extreme circumstances), and therefore, “thought experiments” that predict Second Law violations are regarded as convincing evidence of a theory’s invalidity.
Dr.Brown,
When you say “I must say, though, that it is really amusing to see the lengths people will go to to try to deny that CO_2 plays any role in warming the Earth.
Why bother?”, you do realize that there is no proof that CO-2 plays any role in warming the Earth, don;’t you?
You continue:
“Look at the IR spectrum of the earth as photographed from orbit! Seeing is believing. Hard to deny the evidence of our own eyes, don’t you think, even if those eyes are electronic ones” Indeed, just look at all the places that are emitting heat but not receiving it.
I think you have intentionally distorted the claim that gravity is the cause of the temperature lapse to mean whatever you wish, so that you may claim that gravity is supplying heat, when all that is necessary is for gravity to redistribute heat..
Consider, for example, a centrifugal compressor running continuously under steady conditions. The compressor draws air from a temperature and humidity controlled space.at 1 bar, and discharges it into a process at 2 bar. .The entering air is steadily and continuously heated from ambient (Ti) of 15°C to an exit temperature, (To),which will be 78°C or higher,. It is a function of the ratio of the outlet pressure (Po) to the inlet pressure, (Pi) and the efficiency of the compressor; which can be close to, but will be less than one. To = Ti * (Po/Pi)^((R m eff)/Cp mean), R is the universal gas constant, and m is the molecular weight of the gas. Is it thermodynamically stable?. Why is the discharge hotter?
Willis,iIt is similar to the vortex tube. Face it, you are in denial.