Guest post by Robert G. Brown
Duke University Physics Department
The Problem
In 2003 a paper was published in Energy & Environment by Hans Jelbring that asserted that a gravitationally bound, adiabatically isolated shell of ideal gas would exhibit a thermodynamically stable adiabatic lapse rate. No plausible explanation was offered for this state being thermodynamically stable – indeed, the explanation involved a moving air parcel:
An adiabatically moving air parcel has no energy loss or gain to the surroundings. For example, when an air parcel ascends the temperature has to decrease because of internal energy exchange due to the work against the gravity field.
This argument was not unique to Jelbring (in spite of his assertion otherwise):
The theoretically deducible influence of gravity on GE has rarely been acknowledged by climate change scientists for unknown reasons.
The adiabatic lapse rate was and is a standard feature in nearly every textbook on physical climatology. It is equally well known there that it is a dynamical consequence of the atmosphere being an open system. Those same textbooks carefully demonstrate that there is no lapse rate in an ideal gas in a gravitational field in thermal equilibrium because, as is well known, thermal equilibrium is an isothermal state; nothing as simple as gravity can function like a “Maxwell’s Demon” to cause the spontaneous stable equilibrium separation of gas molecules into hotter and colder reservoirs.
Spontaneous separation of a reservoir of gas into stable sub-reservoirs at different temperatures violates the second law of thermodynamics. It is a direct, literal violation of the refrigerator statement of the second law of thermodynamics as it causes and maintains such a separation without the input of external work. As is usually the case, violation of the refrigeration statement allows heat engines to be constructed that do nothing but convert heat into work – violating the “no perfectly efficient heat engine” statement as well.
The proposed adiabatic thermal lapse rate in EEJ is:

where g is the gravitational acceleration (presumed approximately constant throughout the spherical shell) and cp is the heat capacity per kilogram of the particular “ideal” gas at constant pressure. The details of the arguments for an adiabatic lapse rate in open systems is unimportant, nor does it matter what cp is as long as it is not zero or infinity.
What matters is that EEJ asserts that ![]() in stable thermodynamic equilibrium.
in stable thermodynamic equilibrium.
The purpose of this short paper is to demonstrate that such a system is not, in fact, in thermal equilibrium and that the correct static equilibrium distribution of gas in the system is the usual isothermal distribution.
The Failure of Equilibrium

In figure 1 above, an adiabatically isolated column of an ideal gas is illustrated. According to EEJ, this gas spontaneously equilibrates into a state where the temperature at the bottom of the column Tb is strictly greater than the temperature Tt at the top of the column. The magnitude of the difference, and the mechanism proposed for this separation are irrelevant, save to note that the internal conductivity of the ideal gas is completely neglected. It is assumed that the only mechanism for achieving equilibrium is physical (adiabatic) mixing of the air, mixing that in some fundamental sense does not allow for the fact that even an ideal gas conducts heat.
Note well the implication of stability. If additional heat is added to or removed from this container, it will always distribute itself in such a way as to maintain the lapse rate, which is a constant independent of absolute temperature. If the distribution of energy in the container is changed, then gravity will cause a flow of heat that will return the distribution of energy to one with Tb > Tt . For an ideal gas in an adiabatic container in a gravitational field, one will always observe the gas in this state once equilibrium is established, and while the time required to achieve equilibrium is not given in EEJ, it is presumably commensurate with convective mixing times of ordinary gases within the container and hence not terribly long.
Now imagine that the bottom of the container and top of the container are connected with a solid conductive material, e.g. a silver wire (adiabatically insulated except where it is in good thermal contact with the gas at the top and bottom of the container) of length L . Such a wire admits the thermally driven conduction of heat according to Fourier’s Law:

where λ is the thermal conductivity of silver, A is the cross-sectional area of the wire, and ΔT=Tb–Tt . This is an empirical law, and in no way depends on whether or not the wire is oriented horizontally or vertically (although there is a small correction for the bends in the wire above if one actually solves the heat equation for the particular geometry – this correction is completely irrelevant to the argument, however).
As one can see in figure 2, there can be no question that heat will flow in this silver wire. Its two ends are maintained at different temperatures. It will therefore systematically transfer heat energy from the bottom of the air column to the top via thermal conduction through the silver as long as the temperature difference is maintained.

One now has a choice:
- If EEJ is correct, the heat added to the top will redistribute itself to maintain the adiabatic lapse rate. How rapidly it does so compared to the rate of heat flow through the silver is irrelevant. The inescapable point is that in order to do so, there has to be net heat transfer from the top of the gas column to the bottom whenever the temperature of the top and bottom deviate from the adiabatic lapse rate if it is indeed a thermal equilibrium state.
- Otherwise, heat will flow from the bottom to the top until they are at the same temperature. At this point the top and the bottom are indeed in thermal equilibrium.
It is hopefully clear that the first of these statements is impossible. Heat will flow in this system forever; it will never reach thermal equilibrium. Thermal equilibrium for the silver no longer means the same thing as thermal equilibrium for the gas – heat only fails to flow in the silver when it is isothermal, but heat only fails to flow in the gas when it exhibits an adiabatic lapse in temperature that leaves it explicitly not isothermal. The combined system can literally never reach thermal equilibrium.
Of course this is nonsense. Any such system would quickly reach thermal equilibrium – one where the top and bottom of the gas are at an equal temperature. Nor does one require a silver wire to accomplish this. The gas is perfectly capable of conducting heat from the bottom of the container to the top all by itself!
One is then left with an uncomfortable picture of the gas moving constantly – heat must be adiabatically convected downward to the bottom of the container in figure 1 in ongoing opposition to the upward directed flow of heat due to the fact that Fourier’s Law applies to the ideal gas in such a way that equilibrium is never reached!
Of course, this will not happen. The gas in the container will quickly reach equilibrium. What will that equilibrium look like? The answer is contained in almost any introductory physics textbook. Take an ideal gas in thermal equilibrium:
![]()
where N is the number of molecules in the volume V, k is Boltzmann’s constant, and T is the temperature in degrees Kelvin. n is the number of moles of gas in question and R is the ideal gas constant. If we assume a constant temperature in the adiabatically isolated container, one gets the following formula for the density of an ideal gas:

where M is the molar mass, the number of kilograms of the gas per mole.
The formula for that describes the static equilibrium of a fluid is unchanged by the compressibility (or lack thereof) of the fluid – for the fluid to be in force balance the variation of the pressure must be:
(so that the pressure decreases with height, assuming a non-negative density). If we multiply both sides by dz and integrate, now we get:

Exponentiating both sides of this expression, we get the usual exponential isothermal lapse in the pressure, and by extension the density:
![]()
where P0 is the pressure at z=0 (the bottom of the container).
This describes a gas that is manifestly:
- In static force equilibrium. There is no bulk transport of the gas as buoyancy and gravity are in perfect balance throughout.
- In thermal equilibrium. There is no thermal gradient in the gas to drive the conduction of heat.
If this system is perturbed away from equilibrium, it will quickly return to this combination of static and thermal equilibrium, as both are stable. Even in the case of a gas with an adiabatic lapse rate (e.g. the atmosphere) remarkably small deviations are observed from the predicted P(z) one gets treating the atmosphere as an ideal gas. An adiabatically isolated gas initially prepared in a state with an adiabatic lapse rate will thermally equilibrate due to the internal conduction of heat within the gas by all mechanisms and relax to precisely this state.
Conclusion
As we can see, it is an introductory physics textbook exercise to demonstrate that an adiabatically isolated column of gas in a gravitational field cannot have a thermal gradient maintained by gravity. The same can readily be demonstrated by correctly using thermodynamics at a higher level or by using statistical mechanics, but it is not really necessary. The elementary argument already suffices to show violation of both the zeroth and second laws of thermodynamics by the assertion itself.
In nature, the dry adiabatic lapse rate of air in the atmosphere is maintained because the system is differentially heated from below causing parcels of air to constantly move up and down. Reverse that to a cooling, like those observed during the winter in the air above Antarctica, and the lapse rate readily inverts. Follow the air column up above the troposphere and the lapse rate fails to be observed in the stratosphere, precisely where vertical convection stops dominating heat transport. The EEJ assertion, that the dry adiabatic lapse rate alone explains the bulk of so-called “greenhouse warming” of the atmosphere as a stable feature of a bulk equilibrium gas, is incorrect.
“”””” Steve Richards says:
January 26, 2012 at 6:15 am
Robert Brown:
The connection of a ‘shorting’ thermal conductor to your isolated column in Fig.2 could be viewed in a similar light to a shorting wire across an electrical capacitor.
When a capacitor is shorted, the voltage across its terminals drops to zero very quickly, but, due to dielectric absorption, when the shorting link is removed, the capacitor ‘self charges’, some times up to 10% of its originally charged value (a real problem in high voltage engineering).
The dielectric has ‘remembered’ a portion of the previously applied voltage. “””””
Dielectric “soak” is not so much of a mystery. Many types of good dielectric insulators, used in capacitors, are also piezo-electric. So the result of an electric field in a charged capacitor is a mechanical distortion of the molecules or atoms of the substance. Just the electric field itself will apply mechanical forces to the dielectric material. The effect of dielectric soak applies on charge, just as it does on discharge. Apply a charging VR circuit to a capacitor (current limited) and it will charge to the applied Voltage; 99% in five time constants, and 99.99% in ten time constants. If the charge circuit is opened, the capacitor Voltage will change. It could go either up or down. Same thing on discharge; 99.99% discharge in 10 time constants, but after removing the discharge circuit, the Voltage will again change (also up or down).
Somewhat related, but different, is a similar behavior in electrolytic capacitors; but the mechanism is different. Capacitors of electrolytic type such as “Tantalums” have huge surface areas formed from millions of tiny particles of metal connected to each other by nanoscopic small area contacts, that are high resistance. The dielectric is formed by a thin oxide or other insulating layer on the metal surface, and the liquid or gel electrolyte is the other plate of the capacitor.
So the “Capacitor”, is actually a very large network of RC interconnected nano sized capacitors.
When a discharge circuit is closed, the charge stored on the capacitor “plates” has to flow through all those resistor interconnected particle bridges to get to the terminals and exit the capacitor. Not all of the charge successfully makes the journey before the discharge circuit is removed. The remaining trapped charge, then redistributes, untill all of the nano capacitors have the same Voltage, which registers at the open circuit terminals.
In this case, the residual Voltage is always of the same sign as the original Voltage, as not all of the original charge was removed. Yes it is always possible to use a discharge circuit that overdrives the discharge, and stores charge of the opposite sign.
Companies that make analog type oscilloscopes have to deal with the bane of dielcetric soak in capacitors all the time, since fidelity of transient signal shape is the cornerstone of their business; well at least it used to matter to companies like Tektronix.
kwik says:
January 26, 2012 at 4:25 am
I’m sorry to say, you do misunderstand it. It is the capacity per kilo of mass. Doubling the mass doesn’t change the specific heat at all.
w.
Joe Born says:
January 26, 2012 at 11:19 am
IIRC, I was among the first to “engage substantively” with you about Velasco. I pointed out to you that Velasco clearly says several times that no matter the circumstances, the column will be isothermal.
Since this didn’t agree with your preconceptions, for my heresy I was ignored.
Now other folks have looked at it. AFAIK, all of them say the same thing, that Velasco agrees with Dr. Brown that the column will be isothermal.
Only you are left alone, plaintively repeating your incorrect claim, that Velasco says it won’t be isothermal.
So someone here has not “engaged substantively” with Velasco, but it’s not me and the other commenters. Grab a mirror, my friend …
w.
Albert Stienstra’s (January 26, 2012 at 4:44 am) relativity argument is actually the best argument I have heard in favor of a permanent lapse rate. I had thought about that same idea a day or two ago.
A tube in gravity can be treated the same as a tube continually accelerating at 9.8 m^2 (eg a very long tube mounted an a spaceship). One end is continuously pressing in on the gas while the other is continuously expanding. I am not convinced, however, that this would create a permanent gradient.
Certainly if the rocket engines were suddenly turned on, gas would compress in the “back” of the tube, and would warm up there. Work would be done on the 1 m^3 of air at the back of the piston But eventually the gas would stop piling up at the back because a pressure gradient would be established. With no further compression (and no net work being done), I don’t see how the forces could continue to maintain a temperature gradient.
But it is a fascinating idea.
“”””” Doug Cotton says:
January 26, 2012 at 1:59 am
George, Robert G Brown (and others)
Please be sure to read the first linked item in the paper I linked above, namely http://www.nada.kth.se/~cgjoh/atmothermo.pdf
Of course I realise that this is leading to a very different result, but it is one which agrees quantitatively with reality and, in this case, I go with Professor Claes Johnson’s computations and obviously much more comprehensive coverage of the various processes in the atmosphere. “””””
Doug, you are obviously an expert on the subject; and admittedly, I am NOT.
You have cited this expert tome by this Professor Claes Johnson several times to counter the very specific system that Professor Robert G.Brown, of the Physics Department of Duke University, about which I plead total ignorance; the Duke U Physics dept. that is.
So I just finished reading your definitive text on the subject, from Prof Johnson, a computer scientist, I see.
I wonder if Professor Robert G. Brown knows anything at all about computer science !
Well wait a minute, this thread is about Thermodynamics, not computer science.
Johnson cites Arnold Sommerfeld as being confused about Thermodynamics; well given the time he did his important Physics,h e can be forgiven for that; his contributions to Atomic spectra and atomic Physics are landmark results.
But Prof Johnson goes on to say that he too is confused about Thermodynamics; well also understandable since he is a computer scientist; so he is forgiven for not understanding Thermodynamics.
Well specifically he stated that he cannot understand Clausius’ statement of the Second Law of Thermodynamics. Wow, what an admission, because if anybody understood Thermodynamics, and the second law, it was Clausius. He is also credited with applying the second law to first derive the Optical Sine Theorem, which in its paraxial form simplifies to the Lagrange Invariant, that applies to ALL optical systems, whether imaging or non-imaging.
Well nowhere in Professor Johnson’s admirable paper, did I find any reference to the closed system that Professor Robert G. Brown of the Duke University Department of Physics expounded on.
So Doug, you are the local expert on Prof Claes Johnson, so why don’t you cut and paste the portion of his paper that is specific to the closed system that Professor Brown analysed. Any court in the world would defend such a cut and paste as a legitimate application of the “fair use” doctrine of Copyrights, especially, since you have already made such use by linking to Professor Johnson’s paper.
And it seems to me that Johnson’s reliance on “Navier-Stokes equations of fluid dynamics” in his paper, removes it from relevence, since Brown describes a Thermal equilibrium system, and one thing about thermal equilibrium, is that NO FLUID DYNAMICS can be found in such a system, in fact nothing at all dynamic.
I’m sure that Professor Robert G. Brown of the Duke U Physics department, is perfectly capable of explaining Clausius’ statement of the second law of themodynamics to both you and Professor Claes Johnson.
Tim Folkerts says:
January 26, 2012 at 12:38 pm
So you think the Stienstra argument in favor of a permanent lapse rate is the best you’ve heard … but you just can’t figure out how it works?
Sometimes I think I’ve wandered through the looking glass. People keep coming out foursquare in favor of some cockamamie theory, saying its “the best argument that I have heard” and the like, and then they admit the DON’T KNOW HOW THE THEORY WORKS THAT THEY ARE SUPPORTING.
Tim, if you can’t explain how Stienstra’s idea will continue to maintain the gradient, how on earth can it be the best idea you’ve heard?
Please, folks, if you can’t give the elevator speech explaining how some theory works in clear and concise language, DON’T CLAIM THAT YOU SUPPORT IT. That just makes you look foolish, declaring that you support something you don’t even understand.
w.
Willis Eschenbach: “IRC, I was among the first to “engage substantively” with you about Velasco. I pointed out to you that Velasco clearly says several times that no matter the circumstances, the column will be isothermal.”
No offense meant, Willis. When I wrote that I was thinking of those who really dealt with the statistical mechanics at issue. Didn’t mean to slight you.
Willis,
Maybe I should have said it was one of the most difficult to refute rather than “best”. My point was that the relativistic argument is subtle, and provides an interesting perspective. Much like the “rising particles lose KE so it must more colder higher up” argument. Both are non-trivial and require a deep understanding of physics. If someone wants to really understand this issue, they should be able to understand (and ultimately refure) this argument.
It is rather like saying “this is the best argument I have heard for supporting Newt Gingrich, (even thought I really prefer Mitt Romney)”.
Willis says “People keep coming out foursquare in favor of some cockamamie theory… “
To paraphrase you, Willis, QUOTE MY WORDS! Where did I ever “come out foursquare in favor of” this theory like you are claiming? In fact, I specifically said “I don’t see how the forces could continue to maintain a temperature gradient.”
I actually understand (with ~ 95% confidence) this argument and I think both are ultimately wrong. After a little more thinking, I could give the “elevator speech” that refutes this argument.
I’m on your side on this issue, Willis 🙂
Joe Born says:
January 26, 2012 at 11:19 am
“Actually, with the exception of whether in the posed hypothetical the gas is allowed to exchange heat with the walls that confine it–you think so, I don’t–I believe we agree on the ultimate facts. It’s just that to me the most basic definition of temperature is indeed mean molecular translational kinetic energy; it avoids imprecise qualifications like “for ensembles large enough to apply said thermodynamics to.””
But that is just where you part company with Velasco et al. They explicitly state: “In … a finite system … the conclusion in statement (2b) is wrong …”, where statement (2b) is “Temperature is proportional to the average molecular kinetic energy”.
So your error – according to Velasco et al, as well as myself, DeWitt Payne and probably Robert Brown too – is in taking that marginal height dependence of average molecular kinetic energy for small N as a temperature lapse rate. It just isn’t.
The reason, in heuristic terms, is that this microcanonical ensemble is a combination of two essentially different things; a ballistic projectile (the first particle) and a thermodynamic gas (the rest). If we multiply Eq. 8 by N and separate out the terms in the brackets, we can see that the total kinetic energy basically comprises the ordinary thermal energy of the gas (f/2)(N-1)kT, independent of height, plus a term (-mgz) for the N=1 projectile, close enough. Of course, one can’t actually identify one particular particle as the projectile and the others as the gas, but that’s still probably a useful way of looking at it.
As for heat exchange outside the strict microcanonical ensemble, this is essential if we want to do anything at all with the gas – even if it’s only measuring its temperature, density or pressure.
Joe: “I personally think there is a sound when a tree falls in the forest even if no one’s around to hear it”
In scientific terms there is indeed no sound unless this sound can somehow be detected or measured, at least in principle. How one performs an observation, and what effect that has on the system being observed, is a crucial part of the science. Pretty much the whole of quantum physics depends on this.
Tim Folkerts says:
January 26, 2012 at 1:17 pm
Tim, my thanks, and I’m not trying to single you out. Unfortunately, your last line in the quote above is incorrect. It’s actually like saying “this is the best argument I have heard for supporting Newt Gingrich, even though I don’t have a clue what the argument means.”
I still don’t get it, Tim. You admit that you don’t understand the argument, but you think it’s the best argument you’ve heard. Read through that last sentence a few times.
Or let me put that in the way you would prefer—you admit that you don’t understand the argument, but you think it’s the most difficult argument to refute that you’ve heard. Read through that last sentence a few times.
Well, yes, it is hard to refute something you just admitted you don’t understand … so?
Tim, is your name “People”? Because I see nowhere in my statement that I said one damned thing about your words, so how could I quote words I don’t refer to? However, you seem to think I did, which should give you some cause for concern.
Tim, either you understand the guy’s theory or you don’t. At the point where you wrote and said that you thought it was one of the best theories you’d seen, you yourself admitted that you didn’t understand it.
I see people doing this all the time, just read through this thread. People are all on about some theory, saying things like that they ‘believe it has great promise’ or that ‘it resonates’ with them or their ‘gut feeling is that H&N are correct’ and the like, and they haven’t a clue what the theory says.
That’s what I was talking about, and if you’re not doing that, then you have my apologies.
w.
Joe Born says:
January 26, 2012 at 1:15 pm (Edit)
Thanks for the note, Joe, much appreciated.
My point was that Velasco dealt with the statistical mechanics at issue, and he came down strongly for isothermal, in agreement with Dr. Brown.
w.
The top of Robert Brown’s tube and the bottom of the tube are connected by the free flowing gas. For the two jars to be the same as the tube, the jars have to be connected by the wire and a free flowing gas. An air hose would do. The top of Brown’s tube has a different pressure than the bottom of the tube. Brown’s jars would have the same pressure.
kuhnkat says:
January 26, 2012 at 1:17 pm
kuhnkat, I don’t understand why operating a centrifuge at high speed on earth (as many people have proposed) is not a good test of the H&N theory.
OK, as is my habit, I’ve forsaken guessing so I just ran the numbers. The alloy tubes in a uranium centrifuge have an ID of 74.4 mm and they turn at about 100,000 rpm. This gives by my calculations a force of about 415,000 g’s at the walls.
The normal dry adiabatic lapse rate is proportional to “g’, the force of gravity, and inversely proportional to Cp, the specific heat. On earth it is 9.8°C per kilometer of elevation. The specific heat (Cp) of uranium hexafluoride gas is 370 J/kg °K, compared to 1004 for air.
With a force of 415000 g’s, that should give a lapse rate of something like eleven degrees per millimetre at the wall, decreasing to zero at the center, with an average of about 5.5°/mm. This would give a final difference over the diameter of the tubes of 200°C from the axis to the inside wall of the tube. This would be easily detected and is sure to be noticed, since (as A Physicist noted above) the UF6 would solidify in the cold temperatures near the axis of the tubes.
Since no one has ever come forwards to say “why are these centrifuges permanently staying so hot at the outside and cold on the inside, why is UF6 condensing and solidifying near the centre of the tubes”, I’m gonna say the question is settled experimentally as well as theoretically.
You can continue to believe that for some reason this is not a valid test, that the 1 g of natural gravity directed downwards somehow will invalidate the 415,000 g’s horizontally. Unless you can come up with some cogent explanation of why this is not a good test, I’m convinced.
w.
Willis Eschenbach,
You always have at least 1 G affecting the gas in the centrifuge no matter what orientation it is in if it is on the earh. The idea the there would be a lack of pressure at one end is not possible unless the centrifuge is out of the earth’snear surface gravitational field. The fact that you have a huge range of pressure does not change the 1g at the low end at right angles to the centrifuge’s action .
There is no zero pressure zone due to the earth’s gravitational field. You do accept just plain old basic gravity don’t you????
Willis Eschenbach,
I was being dense on my last post. I am not trying to claim that the whole experiment is overturned by the earth’s gravitational field. i am only pointing out that there is no zero force area in the centrifuge unless you know of some way of blocking gravity. This would be why there wouldn’t be frozen gas in the axis. Y’all can dance on all the pin heads you want, but, the gravity field is there affecting the gas. For that matter, since you are computing, what is the density of the gas toward the center when spinning?? I would think that much force could create a pretty good vacuum!!!
Willis,
I agree that many people jump onto an idea because it seems plausible given their current understanding. (Or worse, jump onto an idea because it agrees with their ideology). This is a definite problem in this thread. The fact is, thermodynamics & statistical mechanics is not easy even for those who have studied it. These are not topics that you can learn reading a few blogs.
>>”Tim, either you understand the guy’s theory or you don’t. ”
I disagree. Only a few days ago we were both pretty sure that the lapse rate should indeed to be a standard feature in a gravitational field (without the need for GHG cooling at the top of the atmosphere), because we both fell for the “particles lose KE as they rise, so it must be colder higher up.” We both understood part of the idea, but we both made a “rookie mistake”. So understanding is not a black-or-white proposition.
>>At the point where you wrote and said that
>>you thought it was one of the best theories you’d seen,
>>you yourself admitted that you didn’t understand it.
I don’t see it that way. I even described in some detail the theory and some of the reasoning behind it. I even gave a bit of the “elevator speech” that a proponent would use, while saying I didn’t accept his conclusions (in large part because the conclusions contradicted other, more convincing arguments). I did admit I wasn’t completely sure of all the implications, but that is different from not understanding at all. I’m still not sure, but I am mostly convinced that I know why it is wrong. (And if anyone really wants, I can post the elevator speech to refute this argument.)
>>That’s what I was talking about, and if you’re not doing that,
>>then you have my apologies.
Apology accepted. And I apologize for not being a little clearer about my thoughts. In the end, it is clear we are pretty much completely on the same page in this discussion.
There may be few engaging on Velasco paper b/c it is hard math. I’ll try to engage but it is hard. Admit approaching from unproven view the ideal gas column is non-isothermal in the presence of gravity.
Joe Born says at 1/26 11:19am:
“you (Paul Birch)and DeWitt Payne are the only participants here who engaged substantively, at my request, on the Velasco et al. paper, and I do sincerely appreciate it.”
Willis’ says at 1/26 12:38pm:
“I was among the first to “engage substantively” with (Joe Born) about Velasco…”
A poster above said the 2nd to last concluding Velasco paragraph is obtuse. I’ll agree with that. It contains it least one double negative maybe more.
Repeated reading of it seems to me Velasco et. al. are saying the result of the prior paper – Coombes and Laue (C&L) arriving at answer 2 being wrong – is itself wrong or at least incomplete because C&L need to add a more full explanation to “discern between the cases of a finite system and an infinite system”.
Joe Born writes look at Velasco eqn. 8. I didn’t see anyone write above for N=1 (one molecule) this very neatly collapses to KE = TE – PE of the 1 molecule. The 1st Law! Constant 1 molecule total energy = KE + PE.
KE = TE – PE being a main reasoning in my view of a non-isothermal column. For Velasco to support paradox resolving to isothermal column, this equation would have collapsed to KE = TE meaning molecular velocities are isotropic in the presence of gravity thus isothermal.
Caballero supports isotropic velocities for isothermal T only in the no gravity ideal gas column case. Add gravity & Caballero in Sec. 2.3 supports gas column T is non-isothermal.
In my view, Velasco et. al. concludes a full explanation is not yet available as of 1996 and the column of ideal gas within a gravity field is more likely non-isothermal when a full explanation is available that also “must discern between the cases of a finite system and an infinite system.”
Silver Ralph January 26, 2012 at 6:00 am
Well maybe you should read up on the thermosphere – temperatures well above 100 deg.C and not many molecules.
The radiated energy in a vacuum flask passes directly from one wall to the other and is unlikely to affect the temperature of the few molecules of air that remain in the near perfect vacuum. The walls are silver to minimise radiation anyway.
Willis wrote:
Since no one has ever come forwards to say “why are these centrifuges permanently staying so hot at the outside and cold on the inside, why is UF6 condensing and solidifying near the centre of the tubes”, I’m gonna say the question is settled experimentally as well as theoretically.
You can continue to believe that for some reason this is not a valid test, that the 1 g of natural gravity directed downwards somehow will invalidate the 415,000 g’s horizontally. Unless you can come up with some cogent explanation of why this is not a good test, I’m convinced.
I’ve chewed on this for a while. I concluded that if it were a valid test, it would suffice for me. Unfortunately, it is not a valid test.
Part of the problem is your frame of reference. If you’re using a cyclindrical geometry, then the molecules follow curved paths. However, in a rectangular geometry, the gas molecules move in straight lines between collisions. It’s simply that their collisions often send them back into the interior of the chamber.
The equivalent of gravity in this experiment would be “Centrifugal Force”. There is no such thing as “Centrifugal Force”. There is only “Centripetal Acceleration”, which occurs only at the outer edge of the centrifuge.
Caballero supports isotropic velocities for isothermal T only in the no gravity ideal gas column case. Add gravity & Caballero in Sec. 2.3 supports gas column T is non-isothermal.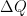 comes out of the system at some nonzero temperature T. The change of entropy in the system is thus
comes out of the system at some nonzero temperature T. The change of entropy in the system is thus 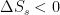 . It goes into a heat engine that (say) stores it reversibly by rolling up a string that lifts a mass.
. It goes into a heat engine that (say) stores it reversibly by rolling up a string that lifts a mass. 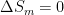 .
.

No, he doesn’t. Read 2.17, especially the exercise at the end. Or look at the exam question and solution I posted. Or learn the laws of thermodynamics. Sigh. I guess I’m going to have to post the maximum entropy argument as well.
Look, nothing — short of perhaps general relativity in extreme field conditions that measurably warp space — is going to permit a stable thermodynamic temperature gradient, because for one to exist you must not be able to use it to do work, not even in principle. There has to exist some exotic and nonlinear macroscopic constraint such that it is physically prohibited to do work off of the thermal difference. Otherwise as you do work with the thermal difference, you are reducing the heat content of the system (cooling it) and turning it into reversible work. This decreases the entropy of the universe! It’s a ten second, at a glance computation.
Finished. A system with a nonzero thermal gradient physically capable of doing work under any circumstances, including ideal ones simply cannot be in thermal equilibrium or at maximum entropy. It is an absolutely trivial homework exercise — not even a problem a conceptual exercise — to show this.
This eliminates 90% of the silly arguments that have been offered to “refute” my simple conclusions. No, an isolated gas with a thermal gradient is not at equilibrium, and does not have maximum entropy as it is capable of doing work. If the entropy of the gas does not increase while that work is being done, there is something wrong.
A very few people, perhaps intuitively realizing that one has to be incapable of doing work across the thermal gradient, have tried to argue that the thermal gradient involved cannot do work, but none of their arguments are convincing because there is nothing special about the gas at both ends. It’s an ordinary ideal gas! How could it not be capable of doing work across the thermal gradient. The only way would be if everything in the gravitaional field had exactly the same thermal lapse rate. But this is absurd on the face of it. No, they don’t. None of the proposed lapses have a universal form.
That’s why I hold out some hope for GR. That’s a difficult problem — if you warp space and time with a very large mass, you do have a bit of a problem defining temperature as energy itself gets a bit warped along with space and time as you go down into the well. However, in this case:
a) The effect is utterly negligible in any real gas for anything less massive than a star.
b) It probably cannot be measured. Your thermometers follow the same gradient as one goes up and down, and they would probably still read the same temperature as temperature itself would probably be following the curvature of spacetime.
c) It is completely irrelevant to the discussion at hand, involving a simple ideal gas in an ordinary vertical column with constant g.
This is as bad a case of wishful thinking as I have ever encountered. Most normal humans would have given up an argument once they learned that ordinary physics textbooks that are obviously uncorrupted by the IPCC completely contradict a stable thermal lapse in any closed system, not to mention common sense. Are you all really that desperate to find an alternative explanation to the Greenhouse Effect that you are willing to pretend that all of our conventional understanding of physics is wrong and Jelbring — the new Feynman, obviously — is right?
In a paper, I might add, where he does no actual work!
If you actually read the paper, he does nothing at all. There isn’t a single line of substantive algebra. He asserts his conclusion, says it is true, and stops!
A few papers have been put forth that at least try to do the work that shows that a thermal lapse might exist, but none of them are at all convincing, and none of them directly confront the obvious and egregious violation of the second law implied by the result that they are trying to prove.
Here’s the biggest hint in the world. If you ever do a whole ton of algebra and are led to a conclusion that violates the laws of thermodynamics in a context where they ought to apply, you had better be damn certain that you understand every single bit of the physics involved better than any other human alive, because you will have to justify every single step in your derivation and conclusion, and even then nobody — including you if you are wise — will believe it until and experiment directly verifies it.
rgb
Sigh, I meant decreases the entropy of the Universe in the previous reply. You know, the impossible one.
rgb
[I fixed it, thereby decreasing the local WUWT entropy, but increasing the entropy in the universe. -w.]
Bill Hunter,
A passive solar water heater and a greenhouse are not the same thing. A water heater is designed to generate hot water at some minimum temperature. So the object is to attain maximum efficiency. Each layer of glazing reduces the available power by a minimum of 4% from reflection at the front and back surfaces. And that assumes that the incident radiation is normal to the surface and neglects any absorption. It doesn’t take many layers of glazing to cut the incident power to inefficient levels, certainly no more than two. A greenhouse, by contrast, is designed to maintain a minimum temperature with the least amount of additional energy supplied by a heater. For much of the year, a greenhouse is entirely too effective and has to be ventilated and/or shaded during the day to keep the temperature from going too high.
What you really want is a wavelength selective low emissivity coating on your collector. That way the collector absorbs solar radiation with high efficiency but emits far less thermal radiation. Black chrome has an SW absorptivity to LW emissivity ratio of about 5. TiNOX, a synthetic coating, claims to have a ratio closer to 20.
Zac says:
January 25, 2012 at 3:10 pm
Hey Myrrh you are not stupid. Since last year I’ve been chasing up your statement that visible light does not create heat and believe me the info has not been easy to find and even when I find some it always conflicts with the one I found before.
Anyway I’ve come to the conclusion that you are correct. IR radiation is what warns the atmosphere and not visible radiation. Thank you.
Thanks for the vote of confidence, Zac.
I’ve been trying to find something for you, but haven’t yet..
The problem here is that this misinformation was deliberately introduced into the education system targetting primary school children first, their teachers non-specialists so could be themselves ‘educated’ in ‘science’. The classroom ‘demonstration’ opening a bottle of scent and then using erroneous physics to explain that as showing ‘carbon dioxide rapidly diffuses into the atmosphere’ is one such method. They’re the adults now who don’t have any particular interest in science but ‘remember the science from school’ and so unlikely to question whatever the AGW green agenda pushes, and even those in actual science fields where real knowledge of gas properties isn’t relelevant, but what I find astonishing though, is how many in actual science fields who come together to discuss AGW continue to not question something as basic as the difference between heat and light claims in the AGW energy budget which is well known still in the real science world. Obvious examples are light bulbs.. But there are others like this:
http://www.nytimes.com/2007/07/16/business/16thermal.html
It is now extremely difficult to find it mentioned that the direct power of heat from the Sun is what warms Earth’s land and oceans, even pages which begin their explanations by saying ‘heat and light from the Sun’ will end up garbling ‘solar’ heating the Earth, and solar refers to the shortwave of the junk energy budget, which claims only shortwave gets to the Earth’s surface to heat it, and thermal infrared doesn’t.
But you have to remember too, that sometimes they get too clever in their explanations and end up contradicting that..
http://earthobservatory.nasa.gov/Features/EnergyBalance/page6.php
..if the atmosphere is transparent to outgoing thermal infrared then it must also be transparent to incoming – claiming it doesn’t play any part in heating the Earth because it doesn’t reach the TOA let alone surface is just plain stoopid. Especially when industries all around still know the difference.
But I haven’t yet found any page which deals with the Earth’s energy budget accurately, some fudge trying to toe the line while attempting to get across that it’s the Sun’s heat and not light which heats the Earth, but even they’re few and far between, and I’ve yet to find that in any science pages specifically on the different energies from the Sun.
I must try and find it again, I found a page that went into great detail about the Sun and then simply avoided mentioning heat from it, but had a short sentence about light and that referred to chemical energy only not the AGWSF ‘converting to heat’. I found that desperately sad. It’s as if all but a few are under some hypnotic spell and those that aren’t have been forced into compromise to not rock the boat.
The equivalent of gravity in this experiment would be “Centrifugal Force”. There is no such thing as “Centrifugal Force”. There is only “Centripetal Acceleration”, which occurs only at the outer edge of the centrifuge. . The mass in the tube has to rearrange to provide this. The bottom provides the force to the things that touch it exactly as if the bottom was supporting an increased weight. The mass layers out where the force difference across any layer has to accelerate all that mass towards the center exactly as if it were supporting its weight. In the end, one gets a distribution that isn’t identical to the distribution one would get with uniform gravity or gravity that falls off like 1/r^2, but none of the differences matter to the question of thermal separation. If equilibrium is thermally separated in gravity, equilibrium would still be separated in the centrifuge.
. The mass in the tube has to rearrange to provide this. The bottom provides the force to the things that touch it exactly as if the bottom was supporting an increased weight. The mass layers out where the force difference across any layer has to accelerate all that mass towards the center exactly as if it were supporting its weight. In the end, one gets a distribution that isn’t identical to the distribution one would get with uniform gravity or gravity that falls off like 1/r^2, but none of the differences matter to the question of thermal separation. If equilibrium is thermally separated in gravity, equilibrium would still be separated in the centrifuge.
Piffle. The equivalence principle suggests that one cannot tell the difference. I agree that in a rotating frame the correction doesn’t have the same form as gravity, there is a coriolis force as well as additional pseudogravity, but this makes no difference in the argument.
Here’s the evidence. Go to your nearest grocery that gives away Helium balloons. Go over to the balloon corral and take one. Don’t be shy; if anyone asks you why a grown adult person is taking a balloon, tell them “for a physics experiment”.
Now, get back in your car to drive home. But tie the balloon to a small weight and place it so it floats freely up in the seat next you you.
When you accelerate forward, which way does the balloon swing? You get pulled back in your seat by the pseudogravity in your accelerating frame. The balloon goes forward, because all of the air almost instantly reacts to the new pseudogravity by making “down” back at an angle, rebuilds a gradient capable of accelerating the air along with the car, and the resulting buoyant force on the balloon pushes it forward to line up with the new “down”. Watch what happens, on the drive home when you round curves or brake. Uh huh.
The whole reason centrifuges work is because when you’re riding in them, the net force towards the center of the circle of motion of any piece of mass in the centrifuge has to be
But now, perhaps you could address the thermodynamic argument. Yes, such experimental evidence as there is refutes the assertion that there can be a stable thermal separation in any isolated system, including an ideal gas in a thermally isolated vessel like the Uranium centrifuges with their enormous g-forces. However, you should have given up even before discovering this — it is just the nail in a coffin built for something that died long ago.
You! Cannot! Violate! The! Second! Law! Of! Thermodynamics!
Really. No, you can’t. Neither can an ideal gas in an isolated container in gravity
rgb
Paul Potter says:
January 24, 2012 at 11:33 am
“For those who do not believe an warmer but still cold atmospheric layer cannot cause the surface to warm clearly do not understand the basic physics of radiative heat transfer”
Not sure if you meant the double negative here, but just to clarify matters, radiation from a cooler atmosphere is not converted to thermal energy when it meets a warmer surface because its frequency is below the cut-off. For more detail and links to computational proof see the Radiation page on my site http://climate-change-theory.com
If radiation from a cooler atmosphere could warm the oceans then it should be able to melt frost, but it doesn’t.
The discussion of centrifuges brings to mind another point from “Theory of Heat”. In this case, it’s on page 329 of the 10th edition. http://www.archive.org/details/theoryofheat00maxwrich
Any violation (ie displacement) of this would also violate the second law, as per the two-gas system described in my first posting.
The top of Robert Brown’s tube and the bottom of the tube are connected by the free flowing gas. For the two jars to be the same as the tube, the jars have to be connected by the wire and a free flowing gas. An air hose would do. The top of Brown’s tube has a different pressure than the bottom of the tube. Brown’s jars would have the same pressure.
No, they wouldn’t, because I filled and sealed the adiabatic jars at the top and the bottom. So the pressure inside of them remains constant as they are moved around. You can shoot a jar to the moon in a rocket ship and — aside from any teensy warming of the gas resulting from shaking it around, not from moving it around in a gravitational field per se — it will still have the same pressure, temperature, and density profile.
Now tell me why a gas that cannot be experimentally measured to be different in any way from the gas at the bottom and top of the adiabatic cylinder is capable of doing work and causing heat to flow, but the gas in the jars is not?
rgb