Dr. Roy Spencer writes on Facebook:
Wayne Rowley has asked me to explain how a cold atmosphere can warm the Earth’s surface (which is what happens in global warming theory), a question I’ve been asked many times in the last 20+ years.
First of all, the temperature of anything depends upon the rates of energy GAIN and energy LOSS. When those 2 are equal, temperature remains the same; if they are unequal, the temperature changes.
Everyone knows that increasing the rate of energy gain increases temperature: e.g. turn up the heat under a pot of water on the stove, or turn up the thermostat in your house in winter.
But you can also increase temperature by reducing the rate of energy LOSS: put a lid on the pot of water while keeping the flame under it constant, adding insulation to the walls of a heated house while keeping the rate of furnace heating the same.
Now, note that in these examples, the lid is *cooler* than the heated water, and the walls (in winter) are cooler than the heated home interior, yet they can make the warmer object even warmer still. Your clothes in winter (or summer) keep you warmer than if you had no clothes on, even though the clothes are cooler than your body temperature. The examples are literally endless.
So, for the atmosphere, the net flow of infrared radiation from the surface to the “cold” depths of outer space is greatly reduced by the atmosphere (the so-called “greenhouse effect”), keeping the surface warmer than if the atmosphere was not there, absorbing and emitting its own infrared radiation. (An interesting side effect is that while the greenhouse effect keeps the surface and lower layers of the atmosphere warmer, the upper atmosphere is actually made colder. The same happens if you add more and more insulation to the walls of a heated house.)

How does this apply to global warming? Adding CO2 to the atmosphere from fossil fuel burning slightly enhances the atmosphere’s ability to keep the surface warmer by reducing the rate of energy loss by the surface. The question is, by how much? The *direct* effect of a doubling of atmospheric CO2 is small, only about 1 deg. C. But indirect changes in the atmosphere resulting from that direct warming (“feedbacks”) can either amplify it or reduce it. I believe those feedbacks will limit the warming to considerably less that what we are being told by climate modelers.
Sorry, don’t get the analogy. All of the heating in the examples is independent of the insulation.
My question is; without internal heating would an insulated house be warmer than an uninsulated house?
The insulation does not heat the house, just as the atmosphere does not heat the surface. Instead, the insulation (and atmosphere) reduce the rate at which the heated interior (and surface) loses energy. This is an example of how imprecise wording (e.g. the atmosphere “heating” the surface) leads to confusion. It’s the relevant physical processes as quantified with the physical equations that matter… not imprecise explanations with words.
Dr. Spencer –> Yet the drawing shows “back radiation” heating the surface. Insulation doesn’t radiate heat back into the house. As you say it works as a resistance. The only way to heat the atmosphere further is for CO2 near the earth to not only absorb IR from below but also from above. How hot then would a CO2 molecule have to be to raise the entire atmosphere by 10° during the day?
“Insulation doesn’t radiate heat back into the house. ”
Yes it does.
No it doesn’t
Everything above absolute zero radiates. Unless you are arguing that the insulation is at absolute zero, then it too radiates. Basic, first year, thermodynamics.
Heat flows from hot to cold. That would make insulation hotter than the house in winter. Only if you’re talking about summer, would heat flow from the insulation to the house.
Radiation doesn’t give a crap about what the temperature of the two objects is.
Cold objects radiate, if the object that is receiving the radiation is warmer, so what?
Mark W
Your 2-object example is only accurate for an instant.
Your 2-objects will each exchange photons until equilibrium is reached (even then, the objects radiate but stay in equilibrium). Warmer object will always radiate many more & higher energy photons per unit of time that your cooler object.
The 2nd law of thermodynamics is not absolute; as your theoretical 2-object system points out, it does not prohibit a photon flowing from the cooler object to the warmer one. However, the 2nd law is statistical and applies over all domains. On average at all points (let’s stipulate above the quantum level), heat always flows from warmer to cooler objects.
Yes, in the situation where there is no source of incoming energy, a temperature equilibrium will eventually be reached.
However that is not the case for the climate, there is a source of energy, the sun. The sun continuously heats the ground. This prevents equilibrium from being reached.
The claim is that a cold atmosphere can’t warm a warmer surface. My 2 object example shows that this claim is completely false. That’s all it was ever intended to do.
No it does not.
Heat is the net flow of energy. The radiation leaving the house will always be such that the house is cooling. Heat is flowing out of it. That comes from the very definition of heat in physics.
Jim – You say: “Insulation doesn’t radiate heat back into the house.”
Actually it does, and if you are careful, you can detect it without instrumentation. I first noticed this many years ago when I was studying thermodynamics. On winter weekends, we would rent cheap ski cabins. Unfortunately, “cheap” meant poorly insulated.
In those cabins, you could actually feel the difference in radiating energy between the warm internal walls and the cold external walls in the same room (with well mixed air).
If you are in a nicely temperature-controlled room, the surfaces of the room are radiating about 400 W/m2 toward you. You are so used to this that it feels like nothing. But remember that your body is radiating about 500 W/m2 toward the room, so the NET of the radiative exchange is still from hot to cold.
Insulation, Ed, keeps warmed air within the house and away from contact with cooler/warmer. Curtains (thick) over windows helps also. The internal walls would be in contact with the warmed air inside, the outside walls not. You overplay ‘back radiation’ with ‘heaters’ attempting to use that concept/radiation disappearing rapidly from the market due to poor real-world performance.
Again, it depends upon how you interpret the word “heating”… does it mean that the atmosphere generates its own energy and transfers it to the surface? No. Instead, the atmosphere which has received most all of its thermal energy from the solar-heated surface (and somewhat less from direct solar absorption by water vapor) emits some of that energy downward toward the surface in the infrared. That’s the big downward-pointing arrow in the energy budget diagram, and it is only one component of the IR flow.
If in winter I go to my house in the mountains that has thick stone walls I first have to use an enormous amount of energy to warm the walls and the floor. As long as the walls are cold it will not be cosy inside. Of course the warmer the walls get the more energy will be radiated out at the outer layer of the wall. So if I have more CO2 molecules eventually more energy will be radiated out.
“But you can also increase temperature by reducing the rate of energy LOSS: put a lid on the pot of water while keeping the flame under it constant, adding insulation to the walls of a heated house while keeping the rate of furnace heating the same.”
This sentence is not quite accurate. You can increase the RATE of temperature rise by reducing the rate of energy loss. But it is the rate of energy input that actually causes the temperature to go up.
What about Venus, that does not have a solar heated surface? If I recall correctly the Russian landers found that there was less than 7 w/m^2 of solar irradiance reaching the surface?
What about mars that has on a numerical molecular basis more molecules of GHGs in its atmosphere than Earth has in Earth’s atmosphere, and yet within 2 vertical metres, the temperature can go from circa 20degC plus at the surface, to freezing at 2m above the surface? There are plenty of photos emitted from the Martian surface at 20degC, and plent of GHG molecules to interecept these and re-radiate them, but they appear unable to maintain any heat even over a distance of less than 2 metres.
Why can’t you cook a steak say 30cm below a BBQ, when you can cook it to a burnt crisp 30cm above a BBQ? There are plenty of photons radiating downwards from the hot coals/charcoal, but they will not raise the temperature of the steak sufficient to cook the steak.
Why, given that the Earth is never in radiative balance, what stops the runaway GHE? Don’t forget that we have seen conditions where the temperature of the planet was say 30degC so emitting plenty of photons from the surface, the atmosphere was 7,000 ppm of CO2 and the oceans also having a surface temperature of 30degC so plenty of GHGs, both CO2 and water vapour. So under these conditions why aren’t the oceans getting hotter and hotter thus releasing more and more CO2 and more and more water vapour, leading to more and more GHGs thereby increasing the temperature of the surface and the oceans, leading to yet more CO2 and water vapour etc. What ends that cycle?
Richard, try Uranus, 30 time further from the Sun than Earth.
It has storms at 1500C – 2000C in its atmosphere as identified by the Geck telescope.
The base of the troposphere on Uranus is 320K at 100 bars pressure, despite the planet only receiving 3.71 W/m2 energy from the Sun.
It’s not the photons that cook your meat, it’s the hot air rising from the coals. The photons help, but they are not the main agent of heat exchange.
In a convection oven, convection cooks the meat. In a MW oven, photons cook the meat.
Trick, in a convection oven ‘heat’ is conducted to the meat and warms it directly. The air in thee convection oven is heated and comes into contact with the meat which then tries to meet an equilibrium temperature.
A microwave oven projects photons of a suitable property to interact and be absorbed by water molecules present in the meat. The molecules kinetically react and this effort to move warms the surrounding materials.
The photons do not cook anything directly, the photon is ‘cold’ energy.
Peter, I did not use the word “directly” which would imply the photons have a thermometer temperature but they do not. Referring to photons as ‘cold’ has no physical meaning.
Trick “Referring to photons as ‘cold’ has no physical meaning.” …is incorrect for indeed a photon’s energy has itself no (kinetic) temperature. What you struggle to realize is the photon is not analogous to ‘heat’ (a property of matter) and thermodynamics is invalidly used in ‘climate science’. You (and others) are still attempting the same arguments seem for the previous 30 years whilst the environment has shown you all to be wrong. The ‘energy budget’ concept dates from the time of Jules Verne.
“Insulation doesn’t radiate heat back into the house. ”
Yes it does. This is simple stuff. Heat your oven to 300 F. Open the door and stand in front of it. Your body radiates heat into the oven. Your body radiates IR in all directions all the time. Placing it next to an open oven doesn’t stop that.
As your body gets warm from the oven, it now raidiates more IR into the oven. You are insulation.
Ragnaar
You are correct that opening & standing in front of a 300 degree oven will heat your body, which, in turn, causes your body to radiate more IR back to the oven. This will continue until the bodies reach thermal equilibrium (actually, once at equilibrium, the bodies continue to radiate, staying in equilibrium).
However, assuming you body is the “insulation” in this example, the re-radiation is de minimus compared to a 300-degree oven. Unlike insulation, your body continues to produce net-new heat from biological processes, also effecting the IR radiation.
Insulation is passive, continually losing heat to the cooler environment in an attempt to reach equilibrium. Insulation adds no new energy – it simply impedes heat loss.
Two bodies with an emissivity of 1, one at 2° and one at 3°.
Body 1 –> 2^4 = 8
Body 2 –> 3^4 = 81
If 8 w/m^2 hits the hot body let’s say it raises 1° to 5. It now radiates at 5^4 = 625. IOW, it reflects the incoming IR.
In order for Body 2 to actually absorb the heat, the incoming IR would need to find molecules at 2°. But then Body 2 wouldn’t be at 3° would it?
Jim Gorman:
“If 8 w/m^2 hits the hot body let’s say it raises 1° to 5. It now radiates at 5^4 = 625. IOW, it reflects the incoming IR.”
But the reflection is it’s temp. Temp equals emission. It was warmed. This applies to IR.
Ocean skin surface morass seems to be the same argument. Emission at the skin layer is temperature. It’s warmer as demonstrated by the increased emission.
…a photon is not ‘heat’ regardless of the portion of the Electromagnetic Spectrum it’s properties place it in. ‘Infrared’ photons are not ‘heat’ and do not obey thermodynamic principles. Do any of you even notice how improperly these discussions substitute concepts of ‘heat’ with ‘IR’?
I notice Peter 7:49pm, thx. The misuse of the heat term as you point out continues to spread unchecked. Attempts at reducing the misuse has been a theme of mine over the years. Photons (EMR) are absorbed and radiated (emitted), not heat.
Then understand also Trick that the discussion above has little to do with an actual (kinetic) temperature. The energy of those photons in the atmosphere remains separate to the materials of the atmosphere and does not behave in terms of thermodynamics.
PKA: Little? Subthread started with “Heat your oven to 300 F” kinetic temperature and continued with that theme.
Trick, did you ever use a magnifying glass to etch wood?
Not that I recall, but I understand the process ought to work.
To Roy Spencer:
We had a similar discussion with more than 500 comments on March 12, 2020:https://wattsupwiththat.com/2020/03/12/comments-on-dr-ollilas-claims-that-greenhouse-effect-calculations-violate-energy-conservation/
In this blog, Dr. Spencer judged my GH effect to be wrong and he obviously accepted the definition of the IPCC but I could find only a few comments supporting his claims.
The key point is now if the atmosphere can add energy or reradiate LW radiation to the surface. In this blog, Dr. Spencer writes that “the atmosphere does not heat the surface”. In the blog on March 12th, he wrote like this: “The atmosphere is not, strictly speaking, adding more energy to the surface. It is merely returning a portion of the atmosphere-absorbed solar, infrared, and convective transport energy back to the surface in the form of infrared energy.”
The last sentence is almost the same as what I have concluded to be the magnitude of the GH effect and it is LW absorption 155, latent heating 91 and sensible heating 24, totally 270 W/m2. According to the IPPC, the LW absorption 155 W/m2 is the magnitude of the GH effect. It is a huge difference. The result is that the contribution of CO2 is only 7.5 % in the GH effect and not 19 % as calculated by Schmidt et al.
Why it is 270 W/m2? The Earth receives net solar energy 240 W/m2. But then it comes the GH effect into the picture: the surface receives direct solar energy 165 W/m2 and 345 W/m2 LW radiation, totally 510 W/m2 as the energy balance figures show. This difference is 270 W/m2. The reradiation or LW radiation downwards is 270 +75 (=solar energy) = 345 W/m2.
Dr. Spencer writes that the atmosphere does not add more energy to the surface. If it does not add more energy to the surface, how on the Earth Dr. Spencer can explain that the surface radiates about 395 W/m2 corresponding the temperature about 15.8 C according to Planck’s law, because the direct solar insolation to the surface is only 165 W/m2. This difference of 395 – 165 = 230 W/m2 is energy. That is a fact. The energy cannot be created from the void. It looks like Dr. Spencer thinks that it can be done. In the same way, the IPCC says that the LW absorption energy of 155 W/m2 can create the reradiation flux 345 W/m2.
I do admit that the atmosphere returns back the energy it absorbed from the surface in the form of latent and sensible heating and LW absorption. But it is a mere fact that the atmosphere emits this energy in the form of radiation. It seems to be too difficult for some people to admit that this radiation really adds energy to the surface.
Something I find missing from models and analogies is an answer to the simple question of whether “overnight” is long enough for a day’s arriving solar energy to escape. The incoming and outgoing mechanisms are not symmetrical.
An unimpeded photon will carry IR energy from earth’s surface to top-of-atmosphere in microseconds. If the probability of the photon careening into an extra molecule of CO2 increases by a little bit… all but a few microseconds of the night remains available for that photon’s energy to somehow escape into space.
Extending Dr. Spencer’s pot on the stove analogy, suppose we have two identical pots that we heat up. Just as the sun sets we turn off the heat, and lay a tea-towel over one of them.
I would not dispute that their temperatures will decay at slightly different rates because of the added insulation surrounding one pot. However, I expect to see very little difference between their temperatures when the sun rises the following morning.
Isn’t that what actually matters… whether the added insulating effect is capable of making a noticeable difference to heat retention over the duration of the process being studied?
Compared to H2O it obviously does not, you only have to compare Tropics to High Arid Desert to see what the diurnal swing is like.
However that same H2O also cools during the day a lot more than it can hold the heat at night.
Hence the anti-correlation between cloud cover and temperatures from the 1970s to the 1990s.
H2O + Solar rule.
Your comment makes sense. Consider this as well – if the atmosphere gains energy from the earth and radiates part of it back then why doesn’t the earth itself re-radiate it back toward the atmosphere again? When that re-radiation happens from the earth then part of the re-radiation would escape to space and a part would go back toward the earth. And on and on and ….. What you have is a damped sinusoid that ultimately goes to zero when the last little bit of re-radiated heat escapes to space.
If the atmosphere adds enough “insulation” that the nighttime damped sinusoid gets cut off by sunrise before it reaches zero then you might wind up with some heat remaining in the earth. But it certainly won’t be entire amount of that first bit of heat that gets re-radiated from the atmosphere to the earth.
“…of whether “overnight” is long enough for a day’s arriving solar energy to escape.”
Ground temp is 15 C. How did it get to that in the first place? The GH effect. A million years the ground temp was 15 C, because of the GH effect. In the beginning, it was cold. The bottom temp rose because of the GH effect and stayed higher than normal. It is getting back to the risen temp each night.
When there are clouds during the night, it’s warmer. The speed of light of photons isn’t some useful answer. Delayed slightly is a near sky dragon argument. There isn’t some however or aha on that path.
CO2 and water vapor are GHGs. If it doesn’t work for CO2, it doesn’t work for water vapor.
But how much of the GH effect is nothing more than an air space? The effect of an air space is clearly shown in heat transfer from heated water pipes and a space.
Thank you Ragnaar
– this is along my line of thought. What is in my eyes omitted in the discussion here is that there is not only the long term average balance of radiation – which has to be balanced every moment, but also the effect of the energy stored in the, hm, earth system over night or for a longer time, that affects that balance for every moment.
I tried to elaborate my thoughts in a comment further below. I guess it may be a little off topic when I look at all these comments discussing the radiations, etc, when I tried to ask a simple question. I would be happy if you can give me a feedback on it (just look for AJN)
I can’t put much weight in explanations based on ground temp being 15 C a million years ago and also a few hours ago. There’s an entire continent–Antarctica–and vast ocean expanses where the “ground temp” is almost never as high as 15 C. Only 12,000 years ago the planet was in an ice age. Substantial departures from 15C is unexplained.
“If it doesn’t work for CO2, it doesn’t work for water vapor”…Atmospheric CO2 and atmospheric water are entirely different creatures. Water changes phase at the droplet of a hat (or a change of a degree) and CO2 strongly resists leaving its vapour state. As you point out when there are clouds during the night, it’s warmer. Putting that another way, the ability of atmospheric water in its non-vapour state–clouds–to retain heat at night swamps its vapour-state GHG ability to retain heat. And at nominally 1% concentration, water as vapour will swamp CO2’s ability to retain heat.
My thinking is that the diurnal swings that AC Osborne mentions–if measured in clear desert (cloud-free) areas free from any UHI effects, should be gradually diminishing if accumulating CO2 is having a measurable GH effect. If those diurnal swings are not diminishing, then the GHG theory is pretty but dumb. If those diurnal swings are diminishing rapidly enough to explain a substantial portion of observed post-1950 warming, then perhaps we have cause for concern.
Nick, here are the high/low temperatures in Degrees F for a station in the Nevada Desert for the 25th of January, I had to use 1959 & 1961 as 1960 it was obvious the Thermometer was not working properly.
The same for 2010, I had to use 2011, 2009 had the coldest temperature at 12 noon, so they go wrong quite a bit.
Year High Low & diff
1950 4 30 26
1959 30 47 17
1961 0 52 52
1970 28 50 22
1980 25 55 30
1990 21 55 34
2000 23 45 22
2011 27 57 30
2020 34 60 26
Note 1961 had a drop down from 25F to zero at 10am in the morning hence the large swing.
Well, can anyone see a reduction in diurnal swing in winter temperature in the last 70 years?
Out of interest I looked at 1997 & 1998 el nino years and 2015 & 2016 el nino years
1997 low 32 high 50 diff 28
1998 low 43 high 65 diff 22
2015 low 29 high 63 diff 34
2016 low 30 high 50 diff 20
Interestingly the Summer swing is higher than the winter swing ie
July 25th
1950 low 55 high 95 diff 40
1960 low 58 high 100 diff 42
1970 low 49 high 92 diff 43
1980 low 57 high 102 diff 45
1990 low 52 high 82 diff 30
1994 low 57 high 95 diff 38
1995 low 55 high 92 diff 37
1996 low 57 high 95 diff 38
1997 low 59 high 85 diff 26
1998 low 64 high 92 diff 27
2000 low 55 high 95 diff 40
2010 low 68 high 93 diff 25
2015 low 57 high 87 diff 30
2016 low 58 high 95 diff 37
2019 low 68 high 95 diff 27
Which does show a reduction in swing, particularly after the 1997/8 el nino.
The problem is that not only is the low temp higher, but the High temp is lower.
The 100 degree temps have disappeared.
Well that is weather for you.
As temperatures increase, the ratio of water vapour to CO2 molecules–both GHG’s–increases for a given relative humidity. The potentially most legitimate observations are likely occur where there’s dry air during cold temperatures. So I would put more weight on the winter in Nevada example than the summer example.
Dry (clear) and cold Antarctic conditions where CO2 molecules are not as vastly outnumbered by water vapour would likely be most favourable for analyzing diurnal swings.
Dr. Spencer: I think the more interesting question is about the reflected energy from the sun. If the atmosphere reflects back to Earth, won’t it also reflect more back to the Sun?
The Sunshine consists of mostly short wavelengths that CO2 is transparent to, whereas the ground emits mostly longer wavelengths, some of which are absorbed readily by CO2.
Sorry I am not Roy but YES, as the NASA diagram and others show. Reflection is the dominant control of insolation that maintains our narrow range of temperature in Space, and water vapour how it is varied.
Perhaps he should have said “I’m glad you asked me that”.
The variability of this reflection by ocean evaporation and hence clouds is the primary control of climate, by varying reflection of the incoming solar radiation. The weather on land is primarily a modified function of this oceanic activity, the oceans where most of the stored surface heat is, 1,000 times that of the atmosphere, in the volatile medium of water – which can change state and can exist naturally in all three states on Earth. Simples!
In W/m^2 insolation average the incoming is 340, of which 77 are reflected by atmosphere and clouds, roughly 50 of that 77 by clouds, and , finally another 23 are reflected by the surface oceans and land.
Another 77 is absorbed by the atmosphere on the way, warming it directly, so only 163 gets absorbed by the surface to be re radiated.
The strong natural control of temperature is the 50W/m^2 reflection, plus the transport of heat from the oceans into space via the smart lagging that is the atmosphere, which increases as the oceans are warmed, evaporate and hence cool the majority of the Earth’s surface, while also forming clouds as the vapour condenses and leaves its latent heat in the cooler atmosphere above on its way to space.
nb: It’s worth pointing out that the heat stored in the atmosphere and oceans is massive compared to the variation resulting from a Watt per square metre or two. The planet has warmed over a considerable period.
The clouds reflect increasing solar energy until the energy balance is restored and SST’s returned to whatever equilibrium level balances the system. That level is the long term temperature record. This powerful negative feedback centred mainly on the tropics has kept the current equilibrium stable since there were oceans.
This is a massive control that maintains the thermal equilibrium against all comers, asteroids, Super Volcanoes, through extinctions of many organic life forms, etc. At roughly 150W/m^2 currently.
I recall Roy Spencer has put its sensitivity at 2.6Watts/m^2 per degree SST change, per John Christy’s paper on the failure of IPCC models to predict reality. I have asked him to confirm a few times but got no answer… yet.
https://www.dropbox.com/s/5aw6rkcfgwpcprj/JohnChristy-Parliament.pdf?dl=0
POINT: This dominant oceanic control of solar insolation reflection from clouds formed by the transport of heat to space by evaporation and condensation of oceanic water vapour is a far more powerful effect than any tiny human effect, and increases exponentially as it gets hotter as does evaporation, whereas any tiny CO2 effect is decreasing logarithmically with concentration. There is no chance of runaway warming when we are well within the limits of this natural control, in the coldest warm maximum this interglacial, itself 4 degrees cooler than the last, which also went as normal under the combined control of the three MIlankovitch cycles.
nb: not just the 100Ka, which is why the cycles are only nominally100Ka duration and vary in scale and profile.
Play with this tool if unfamiliar. It’s a fun and very instructive visualisation
https://cimss.ssec.wisc.edu/wxfest/Milankovitch/earthorbit.html
All this is why nothing unusual is happening to the climate due to humans in fact. Our puny effects are dwarfed by the natural effects and controls. It’s all under natural control, and the short term variations humans see a part of one cycle of are very similar multiple cycles up and down 2 deg every 1,000 years in the record of the last 10,000 years.
Before that these short cycles may also be happening, but we are into the larger scale but slower rate of change of the ice age interglacial warming event. This is even more interesting as regards the reflective control.
Again, the water vapour control driven by SST also ends the relentless 7,000 year 8 degree average warming at the end of each ice age, almost flat lining it once the Tropical evaporative engine really kicks off.
More details on how interglacials probably happen here, but off topic http://dx.doi.org/10.2139/ssrn.3259379
Once Tropical ocean temperatures reach the level of around 30 deg SST the atmosphere becomes so saturated with water vapour the seasons are defined by rainfall with only a few degrees change annually, rather than the large seasonal temperature ranges that occur elsewhere on the planet. So getting much hotter is really hard, and effectively moves tropical climate towards the poles to increase the size of the evaporation engine. All to increase , planetary sweating, and the resulting solar reflection required to maintain equilibrium.
Hope that helps. I will post separately on the reality of the smart lagging that is a planetary atmosphere, across the solar system. Even more interesting, on reflection 😉
One minor detail, you said “The planet has warmed over a considerable period.”, but it has cooled for even longer.
This question seems to come up every year since I began reading about climate science
IN 1997.
That must mean the explanation is never clear.
The atmosphere causes the surface to be
warmer by disrupting earths ability to cool itself from the incoming solar energy absorbed uring the day.
When that gets blank stares, I resort to a simple story.
Think of a room with lots of windows that is cold for people near the windows.
If you hang thick curtains over the windows less heat escapes.
Think of that as 100 ppm CO2.
Think of four curtains over each window as 400 ppm CO2.
Do you think five curtains over each window would keep the room much warmer than four curtains over each window?
Climate alarmists say 500 ppm CO2 (five cutains over each window) would be a crisis.
That’s scaremongering, not science.
Note: If the listener is a leftist, I then smack him upside the head with a rolled up Sunday New York Times.
“That must mean the explanation is never clear.”
err no.
First time it was explained to me by an engineer at Northop I got it.
Masher:
Thank you for assuring us
that you were so brilliant
… a long time ago
… we had no idea !
… what happened since then ?
heh heh
My own climate science blog
Stay away Masher,
I just cleaned up there !
http://www.elOnionBloggle.Blogspot.com
Actually what you are describing is a change in the rate of heat loss. Insulation acts as as a resistance to heat flow. The more resistance you put in the path of heat flow the smaller the rate of heat flow, but ultimately the same amount heat will flow.
“but ultimately the same amount heat will flow” – indeed, but to get that same flow, the temperature differential has to increase, i.e. it gets warmer inside (or on the surface).
No, it doesn’t get warmer, it just cools less quickly.
Otherwise during the periods of very high (5000ppm+) CO2 values and denser atmosphere there really would have been a runaway greenhouse effect.
But there wasn’t, so it doesn’t.
CO2 doesn’t have a linear response, it’s a more or less logarithmic one.
Most of the energy that CO2 is capable of capturing is captured by the time you reach 500ppm. Increases beyond that are just fighting over the remaining scraps.
5000ppm will only capture a few percent more energy than 500ppm does.
Richard, a photon is not ‘heat’ and CO2 is not stopping the surface cooling, the surface has already cooled to release the photon that interacts with CO2. The confusion is that the question is invalidly describing what is a non-existing situation. The surface warms the atmosphere by direct contact and conduction, again the surface cools to warm the atmosphere’s mass. Again the question is invalidly presenting and of a non-existing situation. There is no ‘greenhouse’ effect, the ‘greenhouse’ hypothesis cannot produce consistent affect on temperature.
Roy Spencer says …
‘It’s the relevant physical processes as quantified with the physical equations that matter… not imprecise explanations with words.’
Then why did you include all the examples of lids on pots of water and house insulation etc? … ‘The examples are literally endless’
The examples you give are all misleading. A colder object can never INCREASE the temperature of a warmer object, it can only slow its cooling. If you wrap a 100 degree steel ball in a coat, its temperature does not increase one bit. Yet, if a human wears that coat, the temperature of the human WILL increase … because the human has an internal source of new heat that the steel ball does not have.
If you want to get to the bottom of this tiresome debate, is the earth a steel ball or a human with regard to the coat?
The Earth is a human. It receives shortwave radiation as its primary source of heat.
Insulation prevents absorption in surroundings. GHGs enhance it. Learn the basics Roy.
“Thermal insulation provides a region of insulation in which thermal conduction is reduced or thermal radiation is reflected rather than absorbed by the lower-temperature body.”
https://en.wikipedia.org/wiki/Thermal_insulation
Insulation does the opposite of what the atmosphere does.
Also, read up on Prevosts principle. You say:
“First of all, the temperature of anything depends upon the rates of energy GAIN and energy LOSS. ”
According to Planck you´re wrong. The question of surface temperature is really about surface emission. Emission depends on the internal state of the emitter, ONLY!
Page 8, The theory of heat radiation, https://www.gutenberg.org/files/40030/40030-pdf.pdf
“But the empirical law that the emission of any volume-element depends entirely on what takes
place inside of this element holds true in all cases (Prevost’s principle).”
This means that surface emission cannot depend on the atmosphere.
“This means that surface emission cannot depend on the atmosphere.”
Net surface emission is impacted by the atmosphere on the Earth. In Minnesota the vertical frost line sinks and rises with the seasons. It is not strictly solar dependent. It is the atmosphere’s temp along with shortwave solar (not in a permanent shadow) working together.
There is no grand mistake. It’s boring and simple.
In winter an unheated insulated house will be warmer, in summer an unheated insulated house will be cooler.
More accurate to say that inside temperatures lag changes in outside temperatures. A constant-temperature “soak” would reach equilibrium. As real-life temperatures are always changing, your statement is good enough.
House = closed system.
Atm = open system.
There is a difference.
This is one of the mo’ stupid threads I’ve seen on this site.
Meiggs
Nope, house is not a closed system, just a slow-moving one.
Left alone long enough, it will indeed reach equilibrium with the atmosphere.
Anybody want to bet on this? My 2nd law of thermodynamics against your $100 (we’ll send the winnings to Anthony’s tip jar).
Does the house have windows? Can the sun shine on the walls and roof? Can air leak in and out?
If you have a well sealed, insulated box protected from the sun, and filled with some kind of thermal mass, like bricks for instance, it will be warmer than outside about half the time and cooler than outside about half the time.
Since the 1970s, people have been able to build houses that need no extra heat. They can be heated by the occupants and their activities. Besides the cost though, there are other considerations.
General answer: No. The insulated and un-insulated houses would be the same temperature if there was no internal heat source.
Nuanced answer: The above assumes a constant outside temperature with which the inside of the houses would eventually reach equilibrium. Given that temperature varies between night and day (most of the time, most places), the un-insulated house would have a shorter lag time in response to outside temperature change, and greater daily temperature swings, than the insulated house.
MJB,
It doesn’t need to be an internal heat source, in the case of the earth, the sun is the external heat source that heats the surface during the day. Assuming that it gives a (rather) constant energy input to the surface, the absence or presence of greenhouse gases makes a lot of difference in heat loss and thus in average surface temperature…
Ferdinand Engelbeen,
I agree completely, I was merely addressing the specifics of the question posted by Boff Doff that referenced “internal heating”.
“Assuming that it gives a (rather) constant energy input to the surface”
But it doesn’t does it? It gives a rather constant energy at TOA, but no where else.
Because the various temperature ranges are +50C/-50C that doesn’t sound rather constant to me.
A C Osborn,
I was assuming a rather overall constant heat input to the surface, averaged over all the surface and over a full year…
Even that is of course variable from year to year – which gives a few tenths of global warming or cooling from year to year – but that is not the point of discussion. The point is if GHGs make a difference in temperature of the surface for a given input of solar energy on that surface…
Boff, to all efects, Earth works like a house WITH internal heating, because the source of the heat, although external, passes through the atmosphere without (almost) any interaction with it, directly to the Earth surface. So it doesn’t matter that the source is external, if it heats the “inside” without heating the “cover”.
If you want a closer example, take a microwave oven that heats the water in a glass of water, without heating the glass. If the glass has a cover at the top, once the microwave stops heating (like earth at night), it will cool slower than without it. 5 minutes after the microwave stopped, a glass with a cover will contain hotter water than a glass without it. But it was not the cover that heated the glass, and the cover will be colder than the water. It only prevented the water from cooling faster.
This description ignores the fact that the “greenhouse” model they claim is in effect is centered around the upper tropical troposphere, which is supposed to be hotter than the surface (never observed to date) and thus heats the surface by IR radiation.
They have to show that their model works before we can adopt it. Sure, having at atmosphere keeps Earth’s surface warmer than it would be at night, decreasing the rate of energy loss, but during the day, the atmosphere serves to carry energy, by conduction and convection, away from the surface, serving to keep the surface cooler than with out atmosphere.
The atmosphere mediates the extremes of temperature we would otherwise experience.
The models assume strong positive feedbacks. That’s where the so called hotspot comes from.
The fact that the models are wrong does not discredit the claim that the greenhouse affect exists.
Charles — You misunderstand the issues around the “tropical hot spot”. Many models predict that the CHANGE in temperature of the upper tropical troposphere with increasing CO2 will be slightly greater than the CHANGE at the surface. ALL of them predict that the temperature LEVEL of the upper troposphere will remain substantially lower than that of the surface.
The issue in play in this argument is the transition between the dry adiabatic lapse rate and the moist (condensing) adiabatic lapse rate and how that might change. That is a much subtler issue.
Boff Doff
“Sorry, don’t get the analogy.”
Yet you can ask questions about insulation?
“All of the heating in the examples is independent of the insulation.”
Not true.
Two examples Roy gave.
“But you can also increase temperature by reducing the rate of energy LOSS: put a lid on the pot of water while keeping the flame under it constant, adding insulation to the walls of a heated house while keeping the rate of furnace heating the same.”
Here the insulation is the lid [extra insulation].
The heating is constant.
More insulation.
More heat in the pot and water.
Got it?
“My question is; without internal heating would an insulated house be warmer than an uninsulated house?”
Do you mean inside or outside, night or day.
Habited or not habited?
A house by definition is habited.
If no one ever lives in it, ever, it is just a building.
If someone is living in it, the purpose for building it in the first place, and it is insulated, to make it “warmer” then that house by definition, will always be warmer than an uninsulated house under the exact same conditions.
* Note the heat of the person living in it draining out less slowly is the reason the insulated house will always be warmer.
I would presume guilelessly that a person should not constitute of themselves internal heating even though they do produce heat.
How does a microwave heat water .. it isn’t hot?
Right. Neither is LWIR “hot”. The difference is microwaves are amplified to ~1000W over a 1/4 sq meter =~4000 W/m²! And the water molecules in food are good absorbers of microwaves. Thats what heats the food…. but not the cup or glass.
The earth is heated. By the sun. So it doesn’t matter what happens to an unheated house.
Your question seems beside the point, the earth has the sun heating its surface, and so there is an internal heat source.
The way i understand it insulated house would be warmer at night but cooler in the day.
So overall avg out the same.
As long as you don’t use internal heating.
Temperature gradient. Putting a warm atmosphere between a hot surfacer and a cold space reduces the temperature gradient. Reduces the rate of heat loss.
It’s more than that since that would still be a constant heat loss. The GHG theory says that CO2 and H2O will add more energy to the surface resulting in a temperature increase. In essence saying that a cold object (atmosphere) can raise the temperature of a hot object (surface).
If that works, we should all be filling our houses with 1000 – 2000 ppm of CO2 in the winter.
No, it isnt more than that at all. GH gasses insulate a hot body. They reduce the rate of heat loss, there by making it hotter.
If the Earth is staying at a nearly constant average surface temperature, even if it warmer than a previous time, the input solar energy would still have to be in balance with output radiation, your argument would not be valid. However if the average temperature is continually increasing enough, what you stated is true. Since the average temperature has been nearly constant for the last 18 years (with some small increase), this does not seem to be a fast enough change to result in the claimed cooling. The effect of the ocean sink in removing the increases energy associated with a higher but still slowly changing average surface temperature, may be the cause of the lower high altitude temperature.
Clear and simple analogies Roy. Nicely phrased and understandable.
Agreed.
Here’s another: Try sleeping outside without a blanket. Then try with a blanket. You will be warmer.
Evenso, the blanket does not direct net energy into you; the blanket merely slows your body’s loss of heat. The laws of thermodynamics are preserved.
Sometimes at WUWT, some writers seem to confuse net energy flows and absolute energy flows.
The Thermodynamic Law is that NET energy flow is always from higher temperature to lower temperature. Within any complex system, however, there may be absolute flow in multiple directions. A colder body yet emits some IR radiation which may be absorbed by a warmer body. At the same time, however, there is a greater flux from the warmer body to the colder body. There is “absolute” flow in both directions. The “net” flow is still from warmer to colder.
The complex system of the land, oceans, and atmosphere of the Earth is like this: a complex set of energy flows, but always net from warmer to colder.
And more complex yet because of secondary, or feedback, effects, especially related to the water cycle: cooling by evaporation, convection by updrafts, deposition of heat high in the atmosphere with condensation, cloud cover as a mixed screen blocking sunlight but also retaining heat (cooler cloudy days, but warmer cloudy nights), and so on.
Is it any wonder that climate models fail so regularly? The science on each of these effects is far from certain or settled. The unknown remains far in excess of the known
Meanwhile, the climate has been warming mildly for 200 years and the Earth recently greening. Rather nice.
Good explanation net vs absolute energy exchange. Q for you:
The e-balance chart shows a mere +0.6 W/m² net imbalance. Exactly what amount of CO2 ppm reduction from 415 would allow this paltry 0.6 to reach 0.0 so we could all stop fretting about GHG every damn day? All the way back to pre-industrial ~320 ppm would seem oer-kill imo.
Who cares about such a tiny imbalance. The “brightening” of the earth during the 80s and 90s produced much more warming. Now.. please tell me why, as I cannot explain it:
Source: Ramanathan et al., 1989, Wielicki et al., 2002
Satellite observations of decadal-scale cloud cover changes indicate that between the 1980s and 2000s about 3 to 6-7 Wm-2 of direct short wave forcing was additionally absorbed by the Earth’s oceans. This may account for the warming trend in recent decades.
+100000000000000000000000000000000000
Nice explanation. One little nitpick – “The science on each of these effects is far from certain or settled. ”
The science of the effects are certain and settled. What isn’t settled is the amount of each effect integrated over time and location. It’s kind of like the internal combustion engine. The science of how it works is pretty well settled, it’s impact in a Porsche and and Geo Metro are quite different however.
Again not a trace of an explanation why our deep oceans are so hot (~275K)
Their temperature is some 20K above what the sun is supposedly able to provide (the (in)famous 255K).
So how did the atmosphere increase the temperature of our ~4000m deep oceans by reducing their energy loss to space???
First thought is undersea geological processes?
Robert June 22, 2020 at 4:57 am
yes, just as continental crust is hot due to Earths hot interior, the oceans are hot because they “sit” on a hot oceanic crust. This in spite of the fluxes being low (65-100 mW/m^2)
Once one realizes that the heat content (~temperature) of the deep oceans is 100% from geothermal origin, it becomes possible for the ~50% of solar energy that actually reaches the surface to slightly increase the temperature of the upper 100-200 m of our oceans to the observed surface temperatures.
NOW the role of the atmosphere is to reduce the energy loss to space, the Insulation Effect.
The entire atmosphere is involved in this process, not just some greenhouse gasses.
“Once one realizes that the heat content (~temperature) of the deep oceans is 100% from geothermal origin”
This is not true. The deep oceans are also heated by water that has been warmed by the sun and then carried to the ocean deeps.
There is no storage of solar energy by non chemical processes.
Sun makes surface (to -200m) water molecules dance in real time. The sun goes down, they stop dancing. There is no storage of dancing for later. Assuming average year.
You don’t get to slow cook your turkey for 12 hours, turn it off for 12 hours, and repeat this ad infinitum and expect your turkey to be hotter each subsequent time.
Thermal action is in the here and now.
Zoe, so you are actually claiming that as soon as the sun goes down, everything goes to absolute zero?
That’s the only time molecules “stop dancing”, to use your phrase.
Zoe, I’m still waiting to find anything that you believe, that is actually true.
BTW, read up on the concept of thermal mass, and how it has been used in construction for thousands of years.
There are so many problems with your claims, that it’s hard to know where to start.
First off, the surface of the earth is warmer than it would be if there was no atmosphere. So your claims regarding what the sun alone is capable is utterly meaningless and can only serve as a point of confusion.
Secondly the ocean deeps are well below the average temperature of the surface.
This completely refutes your belief that the oceans can only be heated by geo-thermal heat.
Mark W
Ever been in a South African diamond mine – MUY CALIENTE! Ever watched a volcano (say, Mt St Helens) destroy thousands of sq miles in a matter of minutes?
All that heat comes from earth’s molten core and continental plates sliding around. Thermodynamics says all that heat will eventually radiate away thru earth’s cooler oceans & mantle. May take a couple billion years, but it’ll happen.
Javert, you need to learn the difference between temperature and heat flow.
Earth’s internal heat.
Obvious to you and me. see above.
Tiny compared to the energy from the sun.
MarkW June 22, 2020 at 8:26 am
Geothermal flux through continental is ~65 mW/m^2.
Are you saying that the increasing temperature with increasing depth (~25K/km) s from solar energy?????
And TSI is about 1361 W/m^2. 200 times greater. As I said, tiny compared to the energy from the sun.
“Are you saying that the increasing temperature with increasing depth”
Are you going out of your way to appear stupid?
But TSI does not go in to the water does it?
Add in the thermal capacity of the water and your 163 w/m squared aren’t going to achieve very much.
There are 1.4 × 10^21 kg of water in the oceans and it takes 419kj to raise 1 kg 1 degree C.
As there are 3.6kj per watt hour that would be 11 watt hours per kg.
So that looks like 1.4 x 10^21 x 11 watt hours + losses to evaporation etc. extra above and beyond what we have now to raise the temperature 1 degree C.
Please fell free to correct the numbers if they are wrong.
Why do you believe that TSI doesn’t go into water?
The vast majority of the energy in TSI is in short wave energy, that penetrates water down to over a hundred feet.
Really?
Dr Spencer’s diagram shows just 163 W/m2 getting to the surface, not yor 1361 at TSI.
The vast majority of the energy in TSI is in short wave energy, that penetrates water down to over a hundred feet.
The open ocean down to 600 feet absorbs more solar spectral energy from blue-green wavelengths and responds the most to daily peak insolation, not the average.
Oops, forgot about the mW, the sun’s energy is actually 200,000 times greater than the heat flow from the interior.
AC, even if true, 163W is still almost 25,000 times greater than 65mW.
MarkW June 22, 2020 at 12:37 pm
And yet the sun only warms the upper 10-20 m of the continental crust, the rest is heated by the tiny ~65 mW/m^2 geothermal flux.
A C Osborn June 22, 2020 at 1:49 pm
Better use the energy the sun delivers during a 24 hr period, in the tropics 20-30 MJ/m^2 maximum. This is just enough energy to warm the upper 5-10m 1K. Is lost again during the night.
Mark, you can’t compare energy to a heat flux. Heat flux is based on a differential of two energies.
All these profiles have same heat flux:
http://phzoe.com/2020/04/29/the-irrelevance-of-geothermal-heat-flux/
Heat flux carries energy.
Heat flux carries additional energy from greater energy to lesser energy.
SB Law of emission is based on energy available at the surface (Tcold), not the difference between Thot and Tcold.
There are ocean circulations, the water doesn’t stay at it’s place over time. Don’t forget the thermohaline circulations…
?? We have heat transport from the surface to the deep ocean via ocean currents and lots of heating from below from the Earth’s interior. Then throw in the time it takes for energy to flow through a 4000m column of water and why wouldn’t you expect to have hot and cold spots?
the question is always….why are the oceans heating
…it’s never why are the oceans not cooling off as much
Sun is heating surface water, nothing else, with different wavelenghts and in differen dephths.
Why is it so hot in a deep mine?
It amuses me that the ocean deeps are so cold.
Do you know the adiabate? The troposphere temperature follows the (wet) adiabate: the higher (uphill) you go, the cooler it is, and the deeper (down to the mine) you go, the warmer it is. About 1 deg C per 100 meter hight.
You go down a mine 1000 meters deep, you get 10 deg C warmer.
The water is nearly incomressible.
There is no “adiabate” in the ocean.
Water has its own equation of state and it leads to a very constant temperature of -2..+4 deg C at high pressures (deeper than 1500 meters).
Alex June 22, 2020 at 6:29 am
Not really. The Dry and Wet Adiabatic Lapse Rates are ONLY valid for the temperature change vs altitude of rising or sinking air.
The temperature profile of the atmosphere itself is NOT set by the DALR or WALR.
Actually there is one, although pretty small.
In the atmosphere, convection is complicated by humidity. In the oceans, convection is complicated by salinity. link
“The temperature profile of the atmosphere itself is NOT set by the DALR or WALR”
It IS.
That is why our part of the atmosphere is called “troposphere”: the air here is continuously mixing, establishing the adiabate on average.
The word troposphere is derived from the Greek tropos (meaning “turn, turn toward, change”) and sphere (as in the Earth), reflecting the fact that rotational turbulent mixing plays an important role in the troposphere’s structure and behaviour.
Alex June 22, 2020 at 10:15 am
Sorry, but you’re wrong.
Best explanation I’m aware of:
So what? Of course, I am right.
Just watch your video and UNDERSTAND it: it is the adiabate from land to tropopause.
ON AVERAGE.
Does not have to be locally.
commieBob June 22, 2020 at 8:24 am
Humidity (water vapor) is driving convection by the release of latent heat when the wv condenses. Without it convection wouldn’t reach very high.
‘ You go down a mine 1000 meters deep, you get 10 deg C warmer.’
Euhh, NO!
You have obviously never been down a mine, or inside a wine cellar/basement etc.
The average geothermal gradient is about 25 deg. C per 1000 meters, and can be a lot higher if the surrounding rock is a ‘good’ conductor or the earth’s crust is thinner.
And once you’re a couple of meters down into planet earth what happens on the surface is completely irrelevant.
That is why deep cellars are such good places to store things, due to the extremely stable temperature.
Stay sane,
Willem
Maybe you have not been down a deep mine. you are wrong. The same lapse rate applies underground. I have been more than 1000m down at the Mt Isa mine Australia. Air conditioning was require to give a healthy working temperature. With surface temperature about 35-40 C it could get 45-50C at the draw point of stopes. Air conditioning in maintenance areas was kept 25 to 30C. I was down a deep mine in Canada. Outside there was a blizzard with temperatures -30 to-35C. At the top of the mine over the main shaft it was close to zero. Some 2000m down it was around 15 to 20C in areas with still air but 0 to 10C in areas with ventilation because ventilation was drawn direct from the surface. air temperatures underground have little to do with rock temperature but everything to do with depth and ventilation.
How does CO2 back radiation warm air
underground?
The water has its highest density at 4 deg C.
That is why it is at the ocean bottom.
Anything warmer or cooler would buyout.
@Ben Klijn Wouters:
“Again not a trace of an explanation why our deep oceans are so hot”
What are you talking about? Do you mean the areas of thermovents? Cuz just a surprising short distance from the vents the water is freaking cold (about 4 degrees C on average). Actually, the temperature change in some places is quite dramatic and can be seen on video of samples taken at the vents. If the entire floor was that hot, how would we manage to get submarines down there for studies?
The ocean is a dynamic place, we don’t know much about all the midwater currents (most we know nothing about, but we can see effects of them), the deep water currents are also speculated, nobody has actually ridden them and mapped them to find out…they most likely move around a bit like the surface currents do…or they could have some other mechanism of movement we know absolutely nothing about. We know more about space than we do about the ocean.
Just Jenn June 22, 2020 at 5:40 am
I’m talking about the deep oceans being ~20K ABOVE the 255K the sun is supposedly providing. Anyone claiming that the atmosphere warms (“further heats” Lacis ea 2010) the surface (and ignores geothermal energy) is implicitly also claiming that the atmosphere warms our ~4000m deep oceans.
30 years they thought there was only a few undersea volcanoes and hydrothermal vents. Then 10 years later, hundreds. 10 years later thousands, 10 years later millions. Think about the amount of heat several million undersea volcanoes and hydrothermal vents are releasing. In November 2018, there was a large seismic “hum” heard around the world (you may remember the story). They eventually found out it was huge volcano that erupted off the coast of Africa. In six months of eruptions it rose 800 metres up and 5km wide. If that had happened on the surface, we would call it a supervolcano. And volcanoes this large probably erupt pretty regularly on ocean floor.
https://www.livescience.com/65545-largest-underwater-volcano-seismic-hum.html
The sun has been warming the earth for 4.5 billion years (or there abouts). That’s plenty of time for the sun’s heat to have made it’s way to the ocean depths.
The centre of the earth has been losing heat to the crust for 4.5 billion years, plenty of time to heat the water.
And the centre is still at 6000 degrees C.
There corrected it for you.
I have never denied that there is heat coming from the center of the earth. The only problem is that it is 3 to 4 orders of magnitude less than that coming from the sun.
Actually it’s over 5 orders of magnitude less.
MarkW June 22, 2020 at 7:44 am
Solar penetrates the oceans ~100m, direct warming only for the upper 5-10m.
Solar seasonal influence is noticeable down to max. ~500m.
On the planet I live on (rel.) warm water rises, (rel.) cold water sinks. How do you propose for solar heated water to reach the ocean floor? (and no, salinity does NOT do the trick)
Actually, salinity does do the trick.
In addition to salinity, there is also pure, old fashioned conduction.
MarkW June 22, 2020 at 2:18 pm
Well, the highest salinity in the oceans is NOT found near the ocean floor but near the surface.
Conduction works during spring and summer down to ~200m max. In autumn and winter that warmer water rises again to the now colder surface and loses its energy again to the atmosphere /space.
Yup, and those high salinities cause the surface waters to sink, mixing as they go.
That surface waters in the arctic sink all the way to the ocean floor has been known for decades. These waters then flow towards the equator where they once again rise to the surface.
MarkW June 23, 2020 at 4:53 pm
They even have a name: AntArctic Bottom Water (AABW), the coldest, saltiest water in our oceans. That water became so cold because it released lots of energy to the atmosphere (is called COOLING).
Perhaps you can explain how cold water at the ocean floor can move upward and penetrate a very warm surface layer near the equator.
This surely is an entirely new branch of physics
…and all you need to do to make your point is provide some non-trivial process for such massive heat transfer that isn’t laughably in conflict with 2nd law thermodynamics.
Hint: “Sun shining on oceans for 4.5 billion years” probably isn’t it. “Friction from 4.5 billion years of swimming fish” won’t work either.
Ocean over turning and salinity changes don’t violate the second law.
If it helps, 1.1×10^22 Joules pa enter the oceans from submarine volcanoes, 0.7W/m^2. They have 10 times greater average output than land based volcanoes and there are a lot more of them. Still small compared to the Solar input at 100W/m^2 reaching the ocean surface on average. And it takes 6×10^24 Joules to raise the entire ocean mass by 1degK
The magmatic heat is roughly 8 times the conducted internal hear of 1.5×10^21 Jpa or 0.09W/m^2.
Or 390TW of magma heat on top of the 50TW of conduction currently allowed for as a steady level of geothermal heat entering the oceans. Incorrectly. It’s much more and it also varies a lot from time to time, with Milankovitch cycles.
Far more magma enters the oceans than anyone allows for, because they use old consensual data that is wrong, of a few cubic kilometres pa when the true number is closer to 3,000 cubic kilometres pa than3 or 4. All easy to estimate and validate from the most recent observations of the last 20 years, also the science shows peak emissive events occurring at 100Ka, $41Ka and 23Ka, probably from gravitational forcings at the Milankovitch maximum, not the insolation effect the consensus is obsessed with, but it can’t be.
It’s underneath you!
http://dx.doi.org/10.2139/ssrn.3259379
Ben,
That is a strange thought. Our deep oceans are a couple of degrees above freezing, just a little warmer than the icemelt that supplies the water. Eventually getting warmed up to average surface temp of 15 C in about 500 to 1000 years time it takes to circulate (maybe) to the surface.
“The question is, by how much? ”
And: The question is benefits(plants/trees), versus costs(?)
I see no negatives in the data, just positives. If I am missing something, please post them.
If the climate sensitivity to CO2 is about 1.25 °C (fairly likely), there is a point at which the negative effects of the warming will outweigh the positive effects. Where is that point? I have no idea.
There is also the effect of CO2 on marine geochemistry (misleadingly called “ocean acidification”). Increasing the atmospheric content of CO2 will reduce the saturation state of calcite and aragonite in seawater. There is a point at which this could become a problem. That point is most likely above 1,000 ppm.
While the notion of a “climate crisis” and the need for rapid “decarbonization” are utter horst schist, there potentially is a long term problem that can be addressed over many decades in an economically sustainable manner.
At some point direct utilization of nuclear power will be more cost effective than the inefficient (but cheap) biologically/geologically stored indirect nuclear energy from the sun.
That point will come long before we need to worry about the quantity of CO2 in the atmosphere, or else we’ll have entered a new literal Dark Age.
Most likely.
Climate sensitivity at current levels of CO2 is based primarily on something called pressure broadening. Skeptics have pretty much accepted the value of ~1 C for CO2 alone because it does not create any problems. However, I question whether this number is valid. What is the pressure increase of adding a few gig-tons of carbon to an atmosphere that weighs 5.5 quadrillion tons? It is less than .01%.
I think the estimate is based on assuming CO2 is the only gas present. Once you add in water vapor and factor in the other gases it should change this number.
Has anyone ever seen a detailed analysis of how this works on our actual atmosphere? I haven’t and I really do wonder if it is valid.
Pressure broadening occurs regardless of what the other molecules in the atmosphere are.
Evaporation & precipitation keep temperature within a narrow band
http://www.vukcevic.co.uk/PNFb.htm
“Increasing the atmospheric content of CO2 will reduce the saturation state of calcite and aragonite in seawater. There is a point at which this could become a problem”
Already debunked. https://wattsupwiththat.com/2018/06/05/the-total-myth-of-ocean-acidification/
“there is a point at which the negative effects of the warming will outweigh the positive effects”
History says otherwise. Civilizations flourished in warmer times during the last 8000 years, and declined during the colder times.
So..where is the actual concrete evidence of harm in the current data, and historical and long term records?
If the world were to warm up by 100C, that would not be a positive outcome.
3 to 7 °C would be problematic.
Going to move the Earth a few million miles closer to the sun?
100C, try calculating the energy needed to do so.
Where did I say anything about expecting 100C of rise? The claim was that warming is always good. I gave an example of warming that wouldn’t be good.
Yes a stupidly impossible amount of warming.
AC, what is it about being proven wrong that makes you so cranky?
I was not proven wrong by you in any way.
You made a stupid remark, obviously thinking you were being clever and I called you on it.
What’s stupid about an extreme counter example?
What’s stupid is your fixation on trying to disagree with me whenever and where ever you can. I hope you don’t start charging rent for the space I’m occupying in your mind.
MarkW, “The claim was that warming is always good. I gave an example of warming that wouldn’t be good”.
And what has been requested, if anything, is an example of warming that WASN’T good. Not an imaginary example of something that would be or would not be. Real world data, not imagination.
I wrote that post. It absolutely doesn’t debunk basic marine geochemistry. The sequel to that post explains, in detail, the relationship between CO2 and CaCO3 saturation state.
However, The Total Myth of Ocean Acidification, basically explains the relationship between CO2 and CaCO3 saturation state.
These two images are from my 1978 Stratigraphy and Sedimentation textbook:
This nomogram relates CO2 and other variables to CaCO3 saturation state:

This shows how the seas have changed over the Phanerozoic Eon:
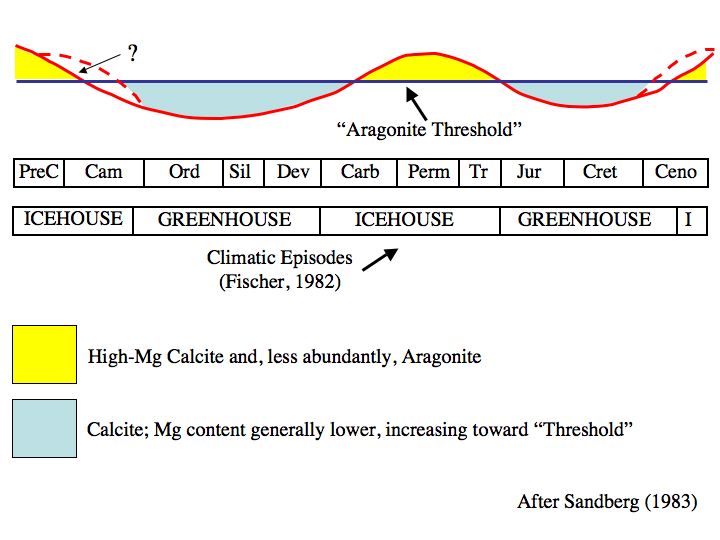
Many modern marine calcifiers have evolved in aragonite-saturated seas. If CO2 rises above the aragonite threshold, these species would have difficulty adapting, particularly if the rise occurred over a short time period.
History doesn’t say jack schist about how a 3-7 °C rise in average surface temperature would affect civilization. Civilization has thrived (more or less) in a band of about 2 °C total change in average temperature.
https://wattsupwiththat.com/2017/06/09/a-holocene-temperature-reconstruction-part-4-the-global-reconstruction/
The Paleogene was up to 7-8 °C warmer than it is today.
A doubling of the pre-industrial Late Holocene CO2 concentration from ~280 to 560 ppm is no big deal. It won’t take us out of the range of Late Quaternary interglacial temperatures.
A tripling or quadrupling… Could cause some serious problems.
The odds are we won’t burn enough fossil fuels to support much more than a doubling.
You still have not presented negatives. Hypothetical negatives don’t count.
Actual negative effects of our added CO2 must be visible.. We were warned about 350 ppm and now we have over 400 ppm.
What are the negatives, and can anyone help me find them?
Hypothetical negatives are all that count when you are looking at conditions that haven’t happened yet… Or haven’t happened in 100’s of thousands to millions of years.
A 1.5 ºC rise in temperature from the coldest part of the Holocene (The Little Ice Age) clearly was a good thing. To the extent AGW exists, so far it has only made it a little warmer than the 1970’s global cooling panic. An additional 0.5 to 1 ºC will probably not be a net negative.
3 ºC of warming (~1,120 ppm) would likely melt much of the Greenland Ice Sheet and West Antarctic Ice Sheet. Ten feet of sea level rise would be a negative.
The alarmists do have a huge “Boy Who Cried Wolf” problem. But that doesn’t mean wolves don’t exist.
“3 ºC of warming (~1,120 ppm) would likely melt much of the Greenland Ice Sheet and West Antarctic Ice Sheet. Ten feet of sea level rise would be a negative.”
Given that it will take a few thousand years to do that melting, and that our oil/coal/ng supplies will have been largely replaced by nuclear…
Finally, the earth is cooling slowly, and we will be in the next ice age before your ten feet of rise happens.
Where is the realistic quantification of your hypothetical wolf?
Can anyone help find these GW negatives? Anyone? All I see are CO2 fertilization positives. Should we not see the Vikings back in Greenland, growing grain by now? Are the alarmists BSing me?
The potential negatives are out beyond 1,000 ppm. The odds are that we will never burn enough fossil fuels to get there. That doesn’t mean that we shouldn’t do things that are economically viable to avoid getting there… Like:
1) More nuclear power.
2) More natural gas.
3) Employing carbon capture and utilization on large coal-fired and natural gas-fired plants where the captured CO2 can be used for enhanced oil recovery or other economic purposes.
Yes. the alarmists are BS’ing you.
How much energy does it take to convert 684,000 cubic miles of Ice in to water?
It lost 30-50% of its ice mass, relative to its current mass, during the Eemian interglacial, when global average temperatures were only about 2-3 °C (5-8 °C in Central Greenland) warmer than it is today.
Over how many millions of years?
At the current to rate, CO2 could rise above 1,000 ppm in 400 years.
David MiddletonJune 22, 2020 at 2:26 pm
3) Employing carbon capture and utilization on large coal-fired and natural gas-fired plants where the captured CO2 can be used for enhanced oil recovery or other economic purposes.
Why do you wan to make nergy more expensive, we need energy to be as cheap as possible, especially for the 3rd world.
It doesn’t make it more expensive when it leads to the recovery of otherwise unrecoverable oil in existing oil fields.
Furthermore, coal will be regulated out of existence in the US without carbon capture. President Trump has delayed this process (a good thing), but it’s only a temporary delay. The death of coal will make energy far more expensive than CCUS.
No one expects the Third or even Second World to do these sorts of things. Coal, along with local resources, will power these nations for decades to come.
The article was about whether it is possible for a cold atmosphere to heat a warm surface.
The question of costs isn’t relevant to this discussion.
But by 33ºC ?
I believe Roy stated in an old article that the number is closer to 58 C. The reason it is only 33 C is that the water cycle cools the planet by transporting much of that warmth to high altitudes where it is easily radiated to space.
I think this is the biggest part of the problem with climate models. We appear to already have this massive negative feedback going on. Why would any increased warming not be countered in the same way? The fact the models do not handle clouds and convection is a major problem.
In addition, besides the formation of clouds and heat transport to higher elevations, another negative feedback occurs due to a reduction in high altitude water vapor.
I would say definitely not. Roy didn’t mention anything about the gravito-thermal effect, which as far as I can tell is the main reason the surface is 33ºC warmer on average than the planet’s S-B temperature. IR radiation scattering may account for a few degrees, but I haven’t seen a good analysis of exactly how much.
Steve, “gravito-thermal effect”….that’s a very fancy and new name for “lapse rate”….
DMacKenzie, no, gravito-thermal effect and “lapse rate” are not the same thing. One is a consequence of the other, and it’s important to realize which is which. In particular, the “radiationists” (people who don’t believe there is such a thing as a gravito-thermal effect) seem to be claiming that the lapse rate is solely a consequence of radiative energy transfer, which is false.
Pressure doesn’t heat anything. It can’t. Changes in pressure can cause changes in temperature. However in a stable atmosphere, there are no changes in pressure.
Just because you have invented a mythical form of energy does not create a need for anyone else to believe in it.
@MarkW
You forget entropy. Entropy constantly forces gas molecules to try to escape earth’s gravity into space. The equilibrium between gravity and entropy sets the gravito-thermal effect through pressure.
A sealed environment cannot replicate this cause entropy is limited where for a planet it is not.
Sorry Steve, your perception is incorrect, atmospheric adiabatic dry lapse rate of -9.8 C per thousand meters (notice thar number is the same as “g”), has nothing to do with radiation, all to do with parcels of gas rising and falling in a gravitational field, totally a thermodynamic energy conservation effect.The actual lapse rate as measured by weather balloons is about -6C per thousand meters due to water condensation, radiation to outer space, greenhouse effect, and so on….
Ron, 100% wrong.
Entropy doesn’t force anything. It’s not a source of energy.
@MarkW
That is incorrect.
https://iopscience.iop.org/article/10.1088/0143-0807/34/3/729
Ron, no it isn’t.
@MarkW
You confuse force and energy. These are related but not interchangeable.
No Ron, you do.
MarkW, you said “Pressure doesn’t heat anything. It can’t”
That is true. However, the gravito-thermal effect is not the result of work being done on the atmosphere, like a bicycle pump. It is simply a sorting of molecules in motion by their kinetic and potential energies, which does not require work to be done. The result is a temperature gradient (but not an energy gradient). Of course the atmosphere has to be heated by some other source (e.g. the sun) to a gaseous state. But once it’s in that state, under the influence of nothing more than gravity, it will settle into a thermal gradient. It’s a simple enough experiment to try, although you’ll need a pretty tall insulated column of nitrogen to be able to measure the effect. People have done that, and verified it. So I’m not sure why you’re continuing to argue against it.
I am not the one inventing mythical forms of energy. You are the one arguing from apparently total ignorance of thermodynamics, gravity, and potential and kinetic energy.
Yes.. but some people think the lapse rate has something to do with radiative gas theory, so to make it easy to understand, the term gravity and thermal is added. Its basic thermodynamics, no green house gases are needed.
The belief that gravity ALONE will create a negative lapse rate (what Steve and others call the “gravito-thermal effect”) quickly runs into the brick wall of violations of both the first and second laws of thermodynamics.
James Clerk Maxwell realized this about 150 years ago. Over 50 years ago, Feynman showed a very basic proof in hist famed Lectures on Physics:
https://www.feynmanlectures.caltech.edu/I_40.html
Because the earth’s atmosphere is more transparent to shortwave solar radiation than it is to longwave terrestrial radiation, the atmosphere generally gains thermal energy (“is heated”) at lower altitudes that it loses thermal energy (“cools”). This causes a temperatuare gradient in the atmosphere (“lapse rate”).
If the gradient is steep enough, it creates a lapse rate greater than adiabatic. This is known as an “unstable lapse rate”, and upward convection starts to drive the lapse rate back toward adiabatic. This is very common in the earth’s atmosphere.
But it is vital to understand that the negative lapse rate is CAUSED by the radiative properties of the atmosphere.
Another way to think about this is, what would happen to the atmosphere if the sun were to go away.
If the “gravito-thermal effect” were to be real, then there would be no change, since the source of heat isn’t the sun.
So you think there is no lapse rate in a planet with an atmosphere with 100% non radiative gases? I hardly think so. (eg, no H2O, etc)
That would violate the first law of thermodynamics.
The source of heat is sensible heat on the surface. Energized molecules transfer energy by collisions, all the way up. The lapse rate is then a direct function of the atmospheric density.
Yeah… both sensible and latent heat are of major importance. In fact if you look at just the pink boxes on this e-bal chart… without the SW solar or LWIR factored in… you get the same 0.6 W/m² imbalance!
https://pbs.twimg.com/media/EaMGjKRX0AA3ztc?format=jpg&name=small
EdB:
I missed your comment. You ask: “So you think there is no lapse rate in a planet with an atmosphere with 100% non radiative gases?”
A two stage answer:
First the steady-state case. (Yes, I know it’s imaginary, but it’s an important first step in analysis). Yes, there would be no lapse rate in such an atmosphere. Let’s take a thermal conduction example to illustrate the point.
You have a long metal rod with one end in boiling water and the other end in ice water, very well insulated along the length. Thermal energy is input to the rod at the boiling water end, and output from the rod at the ice water end.
In this case, there will be a temperature gradient in the rod from 100C to 0C.
Now remove the cold end from the ice water and insulate it very well so the only heat transfer is from the boiling water. What happens? There is no output of energy from the second end of the rod, and the entire rod reaches a temperature of 100C.
So going back to the atmosphere, you can only have a steady-state lapse rate if the upper end of the atmosphere can output energy. This requires radiative gases.
Now onto a rotating planet with a totally transparent atmosphere. When the day starts in an area, the surface gets hotter and a negative lapse rate is established. But when night starts, the surface gets colder because it can radiate energy directly to space, and the atmosphere cannot. So a positive lapse rate (temperature inversion) is established.
On such a planet, positive lapse rates would be as common as negative lapse rates. On earth, with its radiating gases outputting energy to space from high in the atmosphere, negative lapse rates are far more common than positive.
I don’t agree : the Lapse Rate is due to convection : the lapse rate stops where convection stops (e.g. tropopause on Earth).
So, the question is : can there be convection in an atmosphere which does not contain any GHG ?
From my point of view, yes : of course, the bottom atmosphere will not be heated by radiation, but it will be by conduction at the contact between ground surface and atmosphere.
Jacques:
No, the physics is very clear. Lapse rates cause convection.
More specifically, if a negative lapse rate becomes larger than the adiabatic lapse rate, convection starts. For this reason, it is called an “unstable lapse rate”. Convection acts to drive the lapse rate back towards adiabatic.
Sure of what you say ?
https://en.wikipedia.org/wiki/Lapse_rate :
“The temperature profile of the atmosphere is primarily a result of an interaction between thermal radiation, and natural convection.”
Yes, I’m sure! Thermal radiation creates the lapse rate, convection can moderate a large negative lapse rate.
The Wikipedia page you cite agrees with my argument.
BTW, the reason the sign of the lapse rate changes at the tropopause is due the the fact that the troposphere is gaining energy higher up from UV radiation.
The lapse rate only regulates how fast temperature changes with altitude. It has no impact on the temperature at the bottom of the column of air.
As proof of this, we have the same atmosphere over an antarctic winter as we do over an Amazonian summer.
No it is not, I suggest you do some research before making such statements.
Unless you are saying 6kmat the poles = 20Km at the equator?
Your statement makes no sense. Are you trying to claim that the atmosphere is thicker at the equator?
Are you saying that the lapse rate is different between the poles and the equator? (aside from humidity driven changes in rate).
So I agree with Mark.
The atmosphere is thicker at the equator than at the poles.
I guess that it is the consequence of temperature. From PV=nRT
=> V = nRT/P,
nR/P is the same at pole and equator, thus V = k T, volume must vary proportional to temperature. V = Ah, you can’t change area, that means you must change height. In other words h is proportional to T. Height of the atmosphere is proportional to temperature. Since the average temperature of the atmosphere over the poles is lower than the average temperature of the atmosphere over the equator, the height of the atmosphere must be smaller at the poles and greater at the equator.
https://earthscience.stackexchange.com/questions/668/why-is-the-troposphere-8km-higher-at-the-equator-than-the-poles
MarkW June 22, 2020 at 7:52 am
The lapse rate doesn’t regulate anything, it is just a measurement of temperatures at different heights.
Apparently you’re blissfully unaware of the hydrostatic equlibrium against gravity our atmosphere is in.
Seem to be some rather poorly considered statements here that are simply made up and wrong, but easy to check first. Unscientific and hurried belief, so pointless.
I’m not commenting on the lapse rate here, which is a separate matter, but basic science says the atmosphere is obviously different between those locations, 20km at the equator and 6km at the poles. AND the atmospheric composition as regards so called greenhouse gasses is quite different.
At the equator there is proportionately much less CO2 due to the basic gas law density effect and much more water vapour because the atmospheric vapour pressure of water increases exponentially with temperature. Here is a better. more detail explanation from Doug Lightfoot :
https://www.dropbox.com/s/ut92jtugjh4tv9c/A%20new%20look%20at%20current%20climate%20science%20Two%20page%20summary%20Sept%2014%202019.pdf?dl=0
KEY POINT: As elsewhere and above it seems to me as an engineer that most of the arguments above are off the point and spurious as they relates to actual climate change, SST change on a 30 year average which controls land temperatures. which is overtly on its natural track controlled by multiple natural cycles.
Because the dominant natural control of perturbations is from the massive , direct and NEGATIVE feedback effect of water vapour evaporation from the oceans in response to SST change, that regulates SST by both evaporative cooling and the resulting cloud albedo modifying solar insolation by many 10’s of W/m^2, that is wholly dominant over GHE, super volcanoes and asteroids. This oceanic evaporative response is what has kept the planet in a narrow range of equilibrium since there were oceans. The other effects such as GHE/lapse rate change are small and easily contained by this dominant feedback. How can it be otherwise?
So I don’t see why any of this GHE change matters, because the dominant control will adjust the actual effect of SST to compensate for whatever GHE/lapse rate does.
The dominant climate system control is negative feedback of evaporative cooling and cloud formation by water vapour, not CO2 or GHE. This control is driven by the primary reference of the SST that we care about, dominates planetary climate, and maintains the equilibrium at the surface within a few degrees, whatever small variation might happen due to CO2 or water vapour’s effect on GHE and hence the lapse rates in the troposphere to make it all balance out, etc..
WHY MAKE THIS HARDER THAN IT IS? To divert the argument?
Mainly to confuse the argument with pointless detail no one can really prove, and actually doesn’t matter, to distract the discussion from the obvious dominant control of surface temperatures. Water vapour controls the climate by cooling the oceans and forming clouds to reflect the sun. Which is why we only observe natural change, not what models predict by blaming CO2 in virtual reality.
If CO2 modifies the lapse rate a little that is automatically cancelled out at the surface by the dominant control of negative feedback from water vapour. What is wrong with this self evident truth?
PS I will address lapse rates separately, as it seems to me the gas composition is not in fact controlling, its mainly the pressure of whateveritis. I will watch Feynman’s talk first, but he didn’t have the planetary information we now have. James Clerk Maxwell also debunked the GHE ideas, for similar reasons of the heat transport by massive convective (= adiabatic temperature change?) air currents as I understand it. Coming soon.
Pablo, 33C, often stated, is total radiative gas effect, including water vapor and CO2, assuming “Albedo” of planet Earth is 0.3. At ground level, water vapor is many times the CO2 concentration, on the other hand, at top of troposphere, CO2 is a few times the water vapor concentration.
The amount of heat being radiated from one surface to another is
q/a= [k/(1/ehot+1/ecold-1] x (Thot^4-Tcold^4).
The ground is at Thot due to being warmed by sunshine,
If the atmosphere was only N2 and O2, it would be completelely transparent to Infrared. The “surface” the ground would radiate to is outer space at -270 C.
But CO2 and H2O readily absorb and reradiate IR. Because the H2O and CO2 are the same temperature in the atmosphere as the N2 and O2, the “surface” the ground radiates to is “the sky”, and the “sky” is much warmer than outer space. You can take an IR thermometer and typically read the temperature of clouds at about freezing and blue sky down to -80 (but $40 IR guns do not have proper emissivity settings for this job).
Anyway my point is that the ground temp will get warmer, if you do the calculation, in order to radiate the same amount of heat it receives from the sun, when there are radiating gases between the ground and outer space. This can also be interpreted as the surface receiving more IR photons from “the sky” as CO2 or H2O levels increase.
Yes, it is foolish to assume a constant Albedo of .3 to come up with the often stated 33 C number, when incoming radiation is so dependant on Albedo, which in turn is dependent on clouds and clouds are made of water, but people who make this generalization are only trying to show how the radiative gas effect works.
Yes, N2 and O2 are at the same temperature as CO2 at a particular height.
But which is the only one that can radiate that heat to space?
So which is Greenhouse and which is Refrigerant gas?
They all can, though at different frequencies.
Even CO2 can only radiate to space at the highest levels of the atmosphere. Everywhere else is just radiates to other molecules.
A C Osborn…. CO2 and H2O reradiate IR photons in all directions, while N2 and O2 are transparent and let them pass unhindered.
At top of troposphere, water vapor is less than 100 ppm, while CO2 is 400 ppm, so increasing CO2 from 280 to 400 ppm should have increased IR to outer space over the last century, right ? And in fact, IT HAS and the stratosphere is cooling.
Down here at ground level, the extra 120ppm of CO2 that both absorbs and reradiates, is trivial compared to IR absorption and re-radiation from the 16,800 ppm of water vapor (times whatever % the relative humidity at ground level is). The additional CO2 at ground level sends a relatively minor amount of additional IR photons back to the surface causing a minor temperature increase. How “minor” isn’t part of “settled science” yet. Somewhere between 1.2 C and 4.5 C per doubling of CO2, assuming convection remains the same and cloud cover remains constant….oh, so very unsettled…
DM, my point is that something warms the O2 & N2 up to the same temperature as the CO2 at any level and it must be kinetic.
Can you explain how 0.04% of the atmosphere can kinetically warm up 99% of the adjacent atmosphere and at the same time re-radiate photons to warm the earth.
But once heated it cannot radiate anything like CO2 as MarkW well knows, so therefore the only way that the atmosphere can lose energy at 10km+ is via CO2.
Otherwise the atmosphere would cool very quickly with 99% of it radiating the heat away.
And wouldn’t it also radiate towards the surface.
Sorry, I do not believe in the 1C to 4C of CO2 warming, as you say the water does all the heavy lifting.
Down here at ground level, a CO2 molecule that absorbs an IR photon, will collide with another molecule before it has a chance to re-radiate that photon.
In the upper atmosphere the opposite occurs, because of low density of molecules, energy that is gained from a photon will re-radiate before the CO2 molecule has a chance to collide with some other molecule. Likewise, energy gained from a collision with another molecule will be radiated away as IR before it can be transferred to another molecule through a collision.
That is how CO2 molecules warm in the lower atmsophere, while cooling in the upper atmosphere.
A C, I’ve already explained to you how one CO2 molecule can warm 10’s of thousands of non-CO2 molecules.
The CO2 molecule absorbs an IR photon, then transfers that energy to another molecule. It is capable of doing this millions of times per second.
As to your claims to being able to know what I know, until you can prove your psychic abilities, do try to rein in the ego.
Mark,
Learn heat capacity. You’re an embarassment.
CO2 would need to be radiated to ~1600K to raise the rest of the air molecules by 1K.
A C Osborn “How can .04 % ……”
At say, 25 C ocean surface temperature somewhere in the tropics, the air above the water is over 3% water vapour. Radiatively, the water vapour and minor CO2 heat the air above by a few watts per square meter, convection by about 20 to 25 watts/ sq., evaporating water cools the ocean by nearly 100 watts/ sq.m. which is released in the atmosphere as heat when it condenses to rain. I don’t know if that answers your question, but those are the radiative and kinetic processes you are seeking.
Yes they also radiate half downwards…to the surface….yes water is predominant at the surface, CO2 in the upper troposphere…..if convection increases a little bit due to warmer surface temperature, or a cooler upper troposphere produces more SW reflective clouds, the whole 1.2 to 4.5 C per doubling would calculate out to a lower number. I got 1.4 from putting what I thought were realistic numbers into ModTran, which is pretty good for calculating the radiative effect of variable water concentration at different altitudes, but very parameterized for convective or cloud effects, so still “unsettled”….
Zoe, Zoe, Zoe. Did you go to school to learn how to be so stupid?
You seem to suffering from the delusion that CO2 only acts once. It acts over and over and over again, thousands upon thousands of times a second.
Mark,
The molecules CO2 hits with its EM vibratory motion do not perpetually keep that energy. They dissipate it. The hit made a motion. The neighbor moved. Done.
You can start a thousand camp fires, and … ?
You need CO2 radiated to ~1600K to ALWAYS have its neighbors be 1K higher.
You don’t have that kind of energy.
Motion does not accumulate.
Learn heat capacity, idiot.
I don’t know which orifice you’ve been pulling those numbers out of, but none of them bear any resemblance to reality.
Speaking of acting like an idiot there you go again. I specifically said that the energy absorbed by the CO2 molecule is either radiated away or transferred via collision. Those are the ONLY two methods by which a molecule can lose energy. In the lower atmosphere, a collision will occur long before the molecule has a chance to radiate.
Heat capacity doesn’t matter in the slightest.
The reason is almost as simple as you are, because the CO2 atom does it over and over and over again. Heat capacity would only matter if the CO2 atom was only able to absorb and thermalize one photon.
288 K w – 255 K w/o = 33 C cooler is 100% trash.
The 288 K is pulled out of WMO’s butt. The K-T diagram uses 289 K, 16 C. UCLA Diviner uses 294 K, 23 C.
255 K is for 240 W/m^2 which assumes the naked earth keeps its .3 albedo.
This assumption is scientific if not criminal malfeasance.
The naked earth would be much like the moon, albedo 0.1, and with 20% more kJ/h, hotter not colder.
Nick, my reply to Pablo further up sums up why 33 C is the simplified “answer” often given. Criminal malfeasance is a little harsh…maybe overly PollyAnna….If the Earth was the Moon (no GHG) your numbers are right.
“in order to radiate the same amount of heat it receives from the sun”
Hot doesn’t get hotter in order to maintain a balance of heat flow.
There is no such thing as the conservation of heat flow.
Balancing math equations creates energy for the balance that doesn’t exist. Math is not physics.
Using enough GHG layers you can amplify 0.0000000000000001 W/m^2 into 390 W/m^2. Just another reason GH effect is junk science. Just add layers and claim they are real.
GH effect junk science can’t predict surface temperatures. It can only in a post-hoc way tell you the number of layers it takes to amplify sun-induced BB temperature into surface temperatures.
A theory that can postdict observations is a useless theory.
BTW: Using the knowledge that CO2 is ~1/2400th of the air, the main atmosphere is 11,000 meters tall, and the pigeonhole principle, there are is:
a complete layer every 180 meters.
there is 61 layers!
Too much. Better stick to 1 to make your funny math work. Oh, and use absorption parameters that haven’t been observed, but make your math work.
It’s lunacy.
“Hot doesn’t get hotter in order to maintain a balance of heat flow.”
If the amount coming in exceeds the amount going out, the object will get hotter in order to balance the heat flow.
Basic first year physics.
Source!
No climate “physics” allowed. Show another context.
Heat flow is not balanced. Q tends to 0. No such thing as conservation of heat flow.
Try any entry level physics book.
So you claim. It’s not in the book, that’s why you can’t quote from it.
Pathological lying is your game.
>>
Heat flow is not balanced. Q tends to 0. No such thing as conservation of heat flow.
<<
There are several forms for the first law. The differential version using the Clausius standard is:
This is an energy conservation equation, where U is internal energy, Q is heat and W is work. U is a system state variable. The squiggle d’s for Q and W are to remind us that they are path variable. The minus sign in front of the work term is due to this being the Clausius standard. Therefore, heat added TO a system is positive heat transfer; heat removed FROM a system is negative heat transfer; work done BY a system is positive work; and work done ON a system is negative work.
There’s another little restriction–this equation is only valid for closed and isolated systems.
Jim
Ugh, Jim, you name Clausius then misuse his definition of heat. Q is a rate, as you write can be both positive and negative, thus cannot be Clausius’ heat which is always a positive quantity.
Q and W are methods of thermodynamic internal energy transfer between massive objects. Q is the method involving temperature differences. Temperature is an average. This distinction is usually well understood, but around here it is important in the discussion of atmosphere energy balances in order to not further confuse the sky dragons and Zoe.
When Q is written as heat, inconsistent with Clausius defn. of heat, then sky dragons argue Q can thus only transfer hot to cold which is incorrect as temperature is an average.
Zoe is sort of right, Clausius’ heat is not conserved quantity as it can increase and decrease in a massive object, energy is conserved quantity, energy can only be transformed.
Ugh, Trick. I don’t know which is more full of nonsensical statements–your comment or one of Zoe Phin’s.
>>
you name Clausius then misuse his definition of heat.
<<
The definition of heat: energy the crosses a system boundary due to a temperature difference. They often add from warmer to colder, although that is restrictive–what do you call an energy transfer from a colder to warmer region?
Notice, that a system does not contain “heat.” Heat is a transitory phenomenon–it only appears at system boundaries. Work is also a boundary phenomenon.
>>
Q is a rate . . . .
<<
No, it’s an energy. The classical units for heat were BTU’s and calories. The modern SI unit for heat is the joule. We can convert BTU’s and calories to joules (and vice-versa): 1 BTU = 1055.05585 joules; 1 calorie = 4.184 joules.
The first law (using the Clausius standard):
This is not a rate equation. However, you can make it a rate equation by taking the time derivative:
The units change from energy units to power units; such as watts.
Another standard is:
where there’s a plus sign (not the Clausius standard). In this case, positive heat is heat added to the system and negative heat is heat removed from the system still. But the work terms reverse, id est, positive work is work done on the system and negative work is work done by the system.
>>
. . . as you write can be both positive and negative, thus cannot be Clausius’ heat which is always a positive quantity.
<<
Raspberries!
>>
Temperature is an average.
<<
Absolutely not! Temperature is an intensive thermodynamic property. You cannot average intensive properties, id est, temperatures, period! Even though Mr. Mosher tries to confuse the issue with some nonsense about averaging colors.
>>
When Q is written as heat, inconsistent with Clausius defn. of heat, then sky dragons argue Q can thus only transfer hot to cold which is incorrect as temperature is an average.
<<
Huh?
>>
Zoe is sort of right . . . .
<<
That’ll be the day.
>>
. . . Clausius’ heat is not conserved quantity as it can increase and decrease in a massive object, energy is conserved quantity, energy can only be transformed.
<<
More raspberries. Heat is energy. I can’t even decide what you’re talking about. Have you ever taken a thermodynamics course? If so, then you need to take another.
Jim
Ugh, typos. The statement:
“The definition of heat: energy the crosses a system boundary due to a temperature difference. ”
should read:
“The definition of heat: energy that crosses a system boundary due to a temperature difference.”
Jim
Jim, thanks for thinking this through.
”The definition of heat: energy the crosses a system boundary due to a temperature difference.”
No, strictly that is defn. of a method with symbol Q per unit time; takes unit time for thermodynamic internal energy to cross a boundary due to temperature, all processes occur over time. Clausius defn. of heat is the total KE of the constituent particles in a massive object. Compare all others to that gold standard, many have drifted.
You have written the first law exactly the same with and without dots, if you do dot a rate you get an acceleration.
”negative heat is heat removed from the system”
In modern day, no system contains heat to be removed from the system; per Clausius heat is just a sum of the KE of the particles and thus is always positive – number of raspberries in the bowl is always positive or zero.
Temperature is defined as the local avg. of KE of the constituent particles in a massive object. This does make temperature intensive as local KE of the constituent particles does not depend on the size of the object. Energies are extensive; energies can be added as the total energy in a system depends on system size.
For my thermodynamics course(s), the Prof. wrote the book; here is what he wrote verbatim concerning the def. of heat: “…a body never contains heat.” Just like a body never contains work. A massive body contains KE of particles summing to a total KE: heat, which is always positive.
Sky dragons is a term given to a group of people that write the atm. cannot heat the surface as the sky is normally colder than the surface. Around here, one has to use the correct gold standard terminology to convey proper meaning in thermodynamics or the sky dragons (the ones not banned, that still disagree with Dr. Spencer fairly vehemently) will appear as you can already see whenever a top post atm. energy balance diagram is discussed.
I’m simply amazed at how wrong you can be I see it’s a waste of time trying to correct your misconceptions. It’s obvious you’ve never balanced an energy equation. If heat can’t be removed from a system, then how does anything cool down? Gee, I wonder how my AC cools the house–where does that heat go?
Jim
According to modern texts & my thermo. Prof. a body never contains heat. Something that does not exist in an object cannot be removed from it.
Your A/C expanded and then compressed a fluid causing your house thermodynamic internal energy to reduce at the expense of some electricity & warming the local OAT. And maybe one of Anthony’s surveilled USHCN thermometer stations causing a ruckus in the global warming community.
Jim, I’ll try this to maybe spur discussion about Clausius’ thoughts on the nature of heat. This is from my HS 11th grade physics teacher, one Mr. Reddy. On a cold, grey December day, starting a chapter on thermodynamics, Mr. Reddy drew a lab glass of hot tap water. He held it up so the class could get a good view. He then pointed out the window to a frozen over nearby huge lake & asked: “Is there more heat in this glass of hot water or out in that lake?”
I realized my immediate guess was wrong, as I thought more about it. Boom, right then & there I learned temperature is not heat though I wouldn’t really gut level understand why, as good as Mr. Reddy was, until college and I met the guy that wrote the thermo. book.
One of the problems I have when I deal with nonsense, is that I get very sloppy with my terminology. So let me rephrase.
It’s possible for a system to perform work. Work, like heat, is a boundary phenomenon, that is, a system doesn’t contain work. So when a system performs work, and it doesn’t contain work, where does that work come from? One source is the internal energy of the system. Likewise, when work is being performed on a system, where does that energy go? One place is the internal energy of system.
Then there’s heat. Systems do not contain heat. When heat is added to a system, where does that energy go? Again, one place is the internal energy of the system. Likewise, when heat is removed from a system, where does that heat come from. One place is from the internal energy of the system. Heat, like work, can be positive or negative.
I also should look things up instead of using my memory. The previous rate equation for the first law wasn’t correct. I’ll not derive it, but the correct rate equation is:
And no, it’s not an acceleration.
Jim
Jim, thanks for sticking with the conversation.
dQ/dt = dU/dt + dW/dt is fine 1LOT. If you put a dot on one of those it’s an unintended acceleration. You could replace all the d/dt with a dot too.
Jim wrote earlier 11:19am: “Heat is energy.” If so, then substitution of energy for heat term & vice versa ought to work in Jim’s 6:29pm resulting in a conclusion hmmmm… heat is not exactly just energy:
Work, like energy, is a boundary phenomenon
One source is the internal heat of the system.
Then there’s energy. Systems do not contain energy.
Energy, like work, can be positive or negative.
I am sure Jim can rewrite his 6:29pm more appropriately without using the heat term. Start with dropping heat from first paragraph, start 2nd with: “Then there’s energy transfer by virtue of a temperature difference.” Go from there.
So what exactly IS heat? Clausius’ gold standard defn. of heat is: “total KE of the constituents of a massive object”.
This is from the Research & Education Association (REA) Problem Solvers for Thermodynamics, Chapter 4: Entropy and the Second Law of Thermodynamics
Problem 4-8 page 160
“A 34 kg steel casting at a temperature of 427°C is quenched in 136 kg of oil initially at 21°C. Assuming no heat losses and the steel casting and oil to have constant specific heats of 0.5024 and 2.5121 kJ/kg-K respectively, determine the change in entropy for a system consisting of the oil and casting.”
I’m not going to go over the entire solution in detail, but the first thing stated is:
and that total energy is zero. That means that one of the heat terms is negative.
The final temperature is 40.3 °C. The heat term is not calculated directly, but that would be easy enough. The change in entropy of the oil is 21.72 kJ/K. The change in entropy of the casting is -13.73 kJ/K. It’s another indication that the heat term is negative.
The total change in entropy then = 21.72 + (-13.73) = 7.99 kJ/K.
Jim
REA: “a body does not store heat” so they concur with modern day texts. Also REA writes 1LOT as
DeltaU = Q – W
so Q and W are (no dot) rates over the time for deltaU to process.
REA* defines “Heat is energy in transit” so they are one of the many that have drifted from the gold standard def. of heat and allowed confusion & paranormal activity into their thermo. writing. They acknowledge a body does not store heat yet although a 34 kg steel casting at 427C has no heat stored in it, its transiting thermodynamic internal energy flashes into heat at the boundary to the 136kg of oil, then that heat flashes back out of existence to be not stored IN the oil. This all strikes me as paranormal heat activity, not physics.
Anyway, your writing “that total energy is zero” stops me from trying to understand your 10:11 am problem, with a headache. Perhaps a link to the problem might help.
*Ref.:
https://www.amazon.com/Thermodynamics-Problem-Solver-Editors-REA-ebook/dp/B00B2S5CAC
>>
Anyway, your writing “that total energy is zero” stops me from trying to understand your 10:11 am problem, with a headache.
<<
I’m not a bit surprised. I was REA’s term–not mine.
Jim
Zoe, “Hot doesn’t get hotter to maintain a heat flow”….
Think about what you are saying….if there is an energy source within the object, the surface will certainly get hotter to radiate the additional heat away….Proved in engineering student heat transfer labs every semester…
Sorry Mark, you beat me to Zoe’s nonsense.
I enjoyed my day today, fighting heat transfer ignorance from my CoVid quarantine. I think Dr. Roy only puts these RGHE posts out there to crank up the cranks….and maybe educate a few interested folks…
Great post, very clear, will be referring a few people, thanks Roy.
It is disappointing to see the muddling of ‘heat’ , as in radiation. and hot as in temperature.
Unlike the cartoon picture, 70% or more of the Earth’s surface is water. Low level infrared radiation in the CO2 bands has not been shown to raise the temperature of a body of water. If water does get warmer its evaporation rate increases and heat is taken from the surface as latent heat. Humid air is lighter than dry air so will rise and cool adiabatically and eventually the water vapor condenses releasing the latent heat of condensation at height. The amount of energy involved in this huge and yet the cartoon shows ‘evapotranspiration’ as the smallest loss of heat from the surface: this seems to be making up numbers to get the right result rather than anything based on observation.
Exactly. Same with thermals.
IR doesn’t warm water, and nobody has claimed that it does.
What warms the water is long wave radiation.
The atmosphere helps to regulate how fast the energy put in by the sun, escapes.
MarkW – I believe you meant ‘short wave radition’
But IR is what is the ‘back radiation’ from the CO2 that is supposedly keeping the surface warmer but as you say it doesn’t as 70% (probably more) of the surface is water and then you have to add transpiring plants which use the evaporation to stay cool. The latent heat extracted from the surface and the plants is carried upward until it is released when the water condenses. What we visibly see is clouds forming as the latent heat is released higher in the atmosphere and these clouds increase the albedo reflecting the short wave radiation that would otherwise warm the water.
The formation of clouds continues at night of course and the rising Sun has to evaporate the clouds before its heat will reach the surface. The latent heat of evaporation is then carried higher and released when the water condenses as higher cloud.
But this is difficult to model so all that is shown is dry land and arrows — clouds?
Hogwash. CO2 does not ‘heat’ the earth. But IF it did, the warmer earth would radiate at a higher rate (4X) and virtually instantly remove that ‘additional’ heat. It happens every single day.
I’ve had that same argument with folks about a cold object “heating” a hot object via radiation. Can a cold atmosphere really heat a hot surface? An object radiates proportional to T^4 (not 4X). The net is always more than what the cold object can supply. In essence you would need “cold” molecules in the hot object to do this. The laws of thermodynamics hold.
Your microwave oven heats and it isn’t hot?
Lasers can cool things.
What can we say layman think heat is one dimensional and they understand it and they don’t.
So you would hold the operating magnetron in your bare hands?
What you have made clear is you really don’t understand how it works.
Lest show you a datasheet for a very common 500W unit at the centre of many domestic microwaves.
https://www.ampleon.com/documents/data-sheet/BLC2425M10LS500P.PDF
See Section 5 … Typically it runs at 75degree C while outputting 500W
Okay probably a bit warm to hold it as such but use a pair of welding gloves and you would be fine.
So now explain how the 75degree will boil water which requires 100 degree in case you don’t know?
LdB so you admit what I said was correct you would not want to hold one in your hand. And by extension what you said about not being hot was not valid.
Jim Gorman,
The laws of thermodynamics hold, as long as you don’t have an internal (filament) or external (the sun) supply of energy. With an extra source of energy, that energy must be distributed via any means and radiation is one of them.
In the case of all gases, the wavelengths follow Wien’s law with a peak length and distribution directly related to the absolute temperature. That are wavelengths related to the whole molecule vibration.
For greenhouse gases, the wavelengths are very specific for the excitation of the internal structure of some molecules, which gets bending or stretching, completely independent of the whole molecule’s vibration. When going back to the ground state, a photon with exact the same wavelength (and thus energy) is emitted, no matter the vibration (“temperature”) of the whole molecule, whether that is 173 K of 373 K…
See: http://www.barrettbellamyclimate.com/page12.htm
Take the case of a CO2 laser. Maximum temperature 100ºC (cooled), gives a beam of around 10 μm, that is the “peak radiation” for a cold object at -40ºC or so (I haven’t calculated it…). Despite the “undercooled” energy beam, it can melt steel at over 1000ºC…
https://sciencing.com/co-lasers-work-4899566.html
+42×1042
Just like “cold” microwaves from an oven amplified to 1000W per 1/4 sq meter (4000 W/m²) can melt ice cream… cold LWIR if a similar intensity could heat the earth surface. But at a mere 333 W/m² back radiation intensity it can’t even warm up the black hood of your car.
Solar SW (UV+Visible+Near-IR) at over 1000 W/m² daytime certainly can however. Enuf to fry an egg.
UV meter,
Agreed, the point is that the cooling of the earth at night would be a lot faster without these 333 W/m2 back radiation with the same incoming solar energy during the day, thus less average temperature…
…realize that the ~333W/m2 of ‘back radiation’ is energy that has already left the surface, the surface has already cooled. The photons within the atmosphere cannot slow continued cooling by the planet’s surface by production of further energy into released photons.
The continued cooling is independent of ‘back radiation’ which can only, at best, re-warm partially and inconsistently as a warmer surface will shed energy faster and the warming will depend on the surface material involved.
The effort to skirt detail ‘hypothetically’ has ‘climate science’, and its models, fabricating an alternate reality where ‘crisis’ looms perpetually whilst the Environment persists little change… ( https://wattsupwiththat.com/2020/06/23/climate-change-temperature-hits-100-degrees-above-arctic-circle-just-like-100-years-ago/ ) …and so perhaps it needs to be more directly questioned if thermodynamics is even validly applicable as it is.
Peter KEITH Anderson,
The amount of energy emitted by the surface is independent of back radiation and only depends of its temperature and emissivity. The absorbed energy from back radiation is independent of the temperature of the surface, only depends of its absorbance, which is equal to emissivity for the same IR frequencies.
That makes that without the back radiation the energy loss would be much higher than with back radiation and thus the surface would cool much faster.
The back radiation is not new energy, indeed it is recycled energy and just like in a thermos flask, the energy is simply kept within the surface-atmosphere system by constraining the loss to the surroundings (space in this case).
Peter Keith,
The claim is that if you don’t allow photons to exit for several seconds, those photons will perpetually make the system warmer.
I developed a computer program to view their mental illness, here:
http://phzoe.com/2020/03/04/dumbest-math-theory-ever/
Zoe,
“The claim is that if you don’t allow photons to exit for several seconds, those photons will perpetually make the system warmer.”
Nice try, but I don’t know of anybody who makes such a claim, except you and even if it was the case, without emission of a photon there is no change in energy of a molecule…
Ferdinand, you seem to struggle to avoid noticing that the surface, as I mention, has already COOLED in releasing those photons. The ‘greenhouse’ hypothesis, and its ‘back radiation’, cannot stop the COOLING with a small ‘hypothetical’ rewarming by the very energy already released.
The ‘greenhouse’ effect is nonsense trying to be scientific. Then is ‘the science’ confusing a ‘vibrational’ shape change with a kinetic motion. The incident photon’s cold energy interacts with the internal bonding of the CO2 molecule. The atomic centres (+) are moved as the internal bonds flex.
This is not a kinetic movement of the molecular unit, it is not a temperature alteration.
Also, as velocity is a vectored unit and altering direction is an acceleration, such interactions have a greater chance of presenting a lower overall kinetic velocity i.e. excitation has a great chance of leaving the CO2 molecule COOLER.
Thus, in terms of seeking of a thermal equilibrium, CO2 will remain cooler than the surrounding atmosphere but upon being warmed is more likely to release a photon. Thus, CO2 will persistently cool by photonic release and persistently scavenge kinetic velocities (temperature) away.
Increase of atmospheric CO2 is, in this way, a cooling process leading to a ‘spectral’ brightness within the atmosphere as kinetic velocities are converted into photons.
Peter Anderson,
Take the hot coffee in a thermos flask.
According to your reasoning, that already cooled by radiating energy to the outside mirrors, which is true, before a lot of that energy is re-radiated by the mirrors and absorbed by the coffee again. The net effect is that there is a lot less loss of energy from the coffee to the surroundings and the coffee stays longer hot.
Take the earth without any GHGs (thus no water, no clouds,…). During the day a hothouse, except for some heat distribution by wind from hotter to cooler places. During the night a deep freezing world. Average temperature = S-B equation.
Add some GHG to the atmosphere that only recycles 1% of the outgoing LW energy back to the surface. That will increase the incoming SW + LW energy with 1% and decrease the outgoing LW energy with 1%. Thus an energetic unbalance as well as on the surface as on TOA. That will increase the temperature of the surface, until the balance is restored at some 2% extra back radiation and thus a higher average temperature, especially at night…
The incident photon’s cold energy interacts with the internal bonding of the CO2 molecule.
The energy of a photon is not cold or hot and a photon of exactly the same wavelength, thus energy content, may have an origin in the general temperature of any object or from the internal vibration of a CO2 molecule. There is no difference at all. The same for the capturing of specific wavelengths by CO2: the photon captured and emitted a fraction of a second later has exactly the same energy. Thus energy gain and loss of the CO2 molecule by photons are near the same.
Only at near zero K there seems some tricks to get even lower temperatures by repeatedly bombarding molecules with specific wavelengths that gives a small energy loss of the whole molecule for each repeated gain/loss of a photon. But that is about milliK changes…
What happens in the atmosphere is that collisions between excited CO2 and other (inert) molecules like O2 and N2 transfers the internal vibration energy to total molecule vibration of both molecules, without emitting a photon. That rises the temperature of both. What gets first, emission or collision is a matter of gas density.
What you fail to mention is that when the laser photon frequency is below the frequency of the Atom it hits the Atom gets cooler not hotter.
Which is how laser super cooling works.
A C Osborn,
Have found a peak wave calculator for Wiens Law. A CO2 laser emits at 10.6 μm. If that was the peak wavelength of a black body, that would be just around freezing (273 K).
So for steel at ambient temperature about half the iron atoms are “warmer”, half are “colder” than the IR beam at the start of the heating. As the iron gets hotter and hotter, melting at about 1700 K (peak wavelength at 1.6 μm, part of the spectrum in visible light), maybe 20% still “colder” than the CO2 laser beam.
Despite that, the speed of heating and melting remains practically the same, as the emissivity (and thus the absorbance) of steel may start low (0.07 polished to 0.69 rusted), but molten metal has an about 100% absorbance…
The atmosphere is a lot warmer than space.
The atmosphere doesn’t technically “warm” the surface, what it does do is slow down the rate at which energy leaves the warm surface. The end result being a warmer surface.
Using Dr. Spencer’s example. Insulation is colder than the inside of a house, but it results in a warmer house.
Mark W
Insulation is entirely passive. It can only impede to flow of energy from warm to cold, but it certainly cannot stop the flow, or more ridiculously, warm the room.
Nobody has ever claimed that insulation creates heat, IE makes things warmer.
The claim has always been that insulation reduces the rate of heat flow, resulting in the insulated object being warmer than it otherwise would have been.
If you have to lie about the other sides arguments in order to support your case, then you might as well admit that even know you can’t win on the facts.
It’s more than that since that would still be a constant heat loss. The GHG theory says that CO2 and H2O will add more energy to the surface resulting in a temperature increase. In essence saying that a cold object (atmosphere) can raise the temperature of a hot object (surface).
If that works, we should all be filling our houses with 1000 – 2000 ppm of CO2 in the winter.
Now, don’t get me wrong GHG’s could radiate toward the earth and warm it AT NIGHT when the surface loses its heat source. Two things though. One, that would mean the length if time that GHG’s “trap” heat would be fairly long. Two, night time temps would rise which is a good thing.
Jim Gorman,
Two things:
1. There a a constant loss at night, as good as there is a constant gain during the day.
2. The amount of energy within the back radiation is simply recycled energy from what the earth’s surface is sending out. That is at very specific wavelengths for each type of GHG. That is completely independent of the “temperature” (rotation) of the individual molecule that re-emits that radiation or of the average temperature of the surrounding atmosphere. Nothing to do with the physics of “cold” or “hot” objects…
3. If there is less heat loss to space, night temperatures remain higher
4. In wet areas the clear sky night temperatures are a lot higher than in dry deserts…
But that doesn’t make the surface warmer. It still loses heat just at a slower rate. If you were including time, it would radiate at 10 W/sec rather than 11 W/ sec.
GHG theory treats “back radiation” as new, additional radiation that can increase temperature.
A photon in the atmosphere can slow the surface’s release of further photons how?
Where did you get that nonsense from?
Once again, if you can’t understand someone else’s argument, ask questions. Don’t go around making stuff up, it just makes you look stupid.
…MarkW, did you notice the ‘?’ …no, you try to ignore that a question was asked and if you do not have a polite answer please ‘sod off’. The fact is that a photon in the atmosphere cannot prevent the surface further cooling by release of further energy as photons and it seems this point you avoid. Otherwise rising CO2 seems to have done little… ( https://wattsupwiththat.com/2020/06/23/climate-change-temperature-hits-100-degrees-above-arctic-circle-just-like-100-years-ago/ ) …and perhaps, after 30 years of ‘hypothesis’, it needs to be more directly questioned if thermodynamics is even validly applicable.
Peter Anderson,
A photon is energy, no matter if it is absorbed or emitted by some object.
In the case of the earth’s surface, the outgoing photon energy is in direct relationship to its temperature, no matter how much photons are coming in from the sky.
On the other side, (near) all photons in the IR range which come in from the sky (recycled photons from the outgoing LW energy) are absorbed by the surface, whatever its temperature or the amount of emitted photons. That is completely independent of each other.
That means that the net energy balance gives less loss over time than without the incoming extra energy besides solar. Thus the earth’s surface remains warmer (cools less fast) than without GHGs…
Jim Gorman,
The colder object emits IR photons back at the warmer object. The hotter object absorbs those photons (assuming it is a black body). The hotter object emits a lot more photons at any given temperature/wavelength than the the colder body.
But the hotter body only emits a certain number of photons at any given temperature/wavelength. As far as the hotter body is concerned photons received from the colder object do not change the amount of photons it emits at any given temperature/wavelength.
But if an energy source is supplying energy to the hotter object, that source can reduce the amount of energy it needs to supply by the amount of energy in the photons that the hotter object received from the colder object. If the energy source that produces the IR photons in the hotter object continues to force energy into the hotter object, the hotter object’s temperature will increase. Like the Sun heating the ground.
I’m really sorry if this explanation is confusing, but its the best I can come up with for a blog reply comment, and ignores that a black body absorbs all photons that strike it to start with, and jumps to a BB with an internal heat source too quickly.
It is often a good mental construct that IR consists of photons, and EM radiation isn’t “heat” until it is absorbed by matter. Like a microwave oven.
DMc, if only those who think they understand, actually realised EMF is a vector Force that cannot affect equal or stronger ie warmer Heat Fluxes. Quantum effects and oscilators forbid it or we would go up in smoke. Brett Keane, NZ
How then does a laser make atoms colder when the atom absorbs the photon which is at a lower frequency than the atom, hence a lower energy state?
The atom slows down and gets colder not hotter.
That lasers are used to cool atoms is not in doubt. Look it up. I’ve never understood how exactly it works either. I’m not arrogant enough to assume that everything that I don’t understand must be wrong.
I already have studied it, I didn’t ask the question beacuse I didn’t know.
It does so by using a Photon frequency just below that of the Atom, which when moving in the opposite direction to the atom will be absorbed by the Atom.
But instead of increasing the state of the atom it decreases it when the ato throws off the photon.
If the atom is moving away from the photon it Does Not Get Absorbed because the frequency is wrong.
Something for you to think about.
How petty, but then I’ve come to expect that from you.
Yep Jim Gorman,
Just the same way as the radiant energy from forest fires on Earth heats up the sun.
🙂
John Shotsky well said!
Dr Spencer does not understand nor has real experience with the engineering subject of heat and mass transfer. He might start by reading chapters 4 (thermodynamics) and chapter 5 (Heat and Mass transfer) in Perry’s Chemical Engineers’ Handbook and then look at some more detailed Engineering Books. He might also need to get a handle on some basic mathematics such as dimensional analysis (which can also be found in Perry’s CEH)
cementafriend….umm….there is nothing wrong with Dr. Roy’s interpretation, I can say from ample experience in heat transfer engineering….
Sorry, I can’t see any arguements in your response. What did you not understand from the meanpost?
cementafriend: As another engineer with long experience in thermal engineering (I would lose my job if I got things wrong), I concur with DMackenzie that there is nothing wrong with Roy’s analysis. It is in line with all of the engineering texts that I have seen.
Yup.
and now you know the dividing line between skeptics ( who question the amount of warming)
and deniers who deny working engineering
In the flow diagram, the back radiation from the atmosphere is 340W/m2 while the total outgoing radiation is 240W/m2. Assuming an emissivity of 1, then the effective temperature of the atmosphere radiating (580 w/m2) with Stefan Bolzman should be 45 degrees Celcius.
So what’s wrong here.
For an alternative way of how the greenhouse effect works:
https://www.sciencetalks.nl/the-real-greenhouse-effect/
Andre…
Radiation from ground….398.2 watts/ sq. m works out to 16.4 C, about the average temperature of the planet
From sky to ground…..340.3 watts/sq. m works out to 5.3 C, about the temperature of the atmosphere a mile up.
From sky to outer space….240 watts/ sq.m about -18 C, about the temperature half way up the troposphere. Temperature of top of troposphere, about -55 C
Not sure why you double up to get 580……
I dislike the “trapping heat” argument because it is confusing. The increased downward radiation from the sky argument is more direct, and does not rely on any trapping of heat. The atmosphere can get warm just by convection or condensation, there is no need for it to “trap” heat via radiation, though of course it does do that as well.
Yes, the phrase “trapping heat” is nonsense and is used because it fools so many non-technical people. However, keep in mind that without GHGs the atmosphere has no way to cool itself. As a result there would be not much convection to higher altitudes.
Convection exists, even without GHGs. The presence of water increases the rate of convection, not because it is a GHG, but because of it’s ability to change phases at the temperatures found in our atmosphere.
Insulation in a house or a coat doesn’t work thru radiation. It works thru conduction. Insulation is heat resistance just like electrical resistance. It slows the movement of energy (current).
The anthropogenic CO2 effect is sparse, and the alt-“greenhouse effect” is leaky.
It’s Q = 1/R A dT, same thang that allows colder walls to make your house warmer in winter. R is all those bundles of fiberglass “warming” “heat transfer “slowing” bundles stacked in the insulation aisle at HD.
To move current through an electrical resistance requires a voltage difference.
To move fluid through a hydraulic resistance requires a pressure difference.
To move energy through a thermal resistance requires a temperature difference.
BTW the “cold” depths of outer space are 394 K, 121 C, 250 F. UCLA Diviner knows that, the ISS HVAC engineer knows that, Nikolov and Kramm know that.
The heat (power flux) balance diagram has a major bookkeeping error. (Legitimate heat balances are conducted in Btu/ng h or kJ/metric h.)
According to the NASA heat balance computer model graphic (attached and/or linked) 163.3 W/m^2 make it to the surface.
18.4 W/m^2 upwell from the surface through non-radiative processes, i.e. conduction and convection.
86.4 W/m^2 upwell from the surface through latent processes, i.e. evaporation and condensation.
The balance upwells 163.3-18.4-86.4-0.6 = 57.9 W/m^2 as LWIR.
That’s it!
The energy balance is closed!
Fini!!
But what about this!?
LWIR: 398.2 total upwelling – 57.9 from balance – 0.6 absorbed = 340.3??
An “extra” 340.3 W/m^2 have just appeared out of thin air!!!???
So where does this 398.2 W/m^2 upwelling “extra” energy come from?
Well, actually the 398.2 W/m^2 is a theoretical “what if” S-B heat radiation calculation for an ideal, 1.0 emissivity, Black Body with a surface temperature of 289 K or 16 C.
The SINGLE amount of LWIR energy leaving the surface has just been calculated by TWO different methods!! and then combined to effectively double the amount!!!! much like entering your paycheck twice in your checking account register.
398.2 is THEORETICAL!!!!!
340.3 is NOT REAL!!!
340.3 VIOLATES conservation of energy!!!!!
And, no, it is NOT measured except by amateurs who don’t understand how IR instruments work or emissivity and assume 1.0 when emissivity is in theoretical fact 57.9/398.2=0.145 or in actual fact 57.9/163.3=0.355
There is no 398.2 upwelling “extra” energy, there is no 340.3 “trapping” and “back” radiating “extra” energy, no RGHE, no GHG warming and no CAGW.
https://en.wikipedia.org/wiki/Earth%27s_energy_budget
As demonstrated by experiment, the gold standard of classical science:
https://www.linkedin.com/posts/nicholas-schroeder-55934820_climatechange-globalwarming-carbondioxide-activity-6655639704802852864-_5jW
Nick,
The Stefan-Boltzmann equation between two parallel surfaces, a simplification of “ground” and “sky” in this case, is
q/a= [k/(1/ehot+1/ecold-1] x (Thot^4-Tcold^4)
The 398.2 is the “Thot” part of the equation , and the 340.3 is the “Tcold” part. There is no violation of any laws of thermodynamics, heat flows from Thot to Tcold.
And Q=1/R A dT is inappropriate for radiative heat transfer….maybe you missed that couple of weeks of lectures those many years of engineering school ago ?
The total thermal resistance (R or 1/U) of the atmosphere is in effect a complex combination of (cond+conv+advec+latent+rad) = 1.0 aka all of it.
This applies up to 32 km where the molecules end.
Emissivity = rad/all of it.
As I demonstrated in my experiment BB upwelling is not possible.
A BB only works into a vacuum, i.e. no contiguous participating media.
This doesn’t change the incorrect double entry “extra” energy appearing out of thin air.
“sky” is not a legitimate surface.
Stand near a red hot vat of iron slag, and then tell me that a Black Body only applies in a vacuum….. and my use of a “surface” to describe the “sky” is only to simplify the problem to avoid details concerning emissivities and atmospheric optical depths that would turn my comment/description into a graduate level heat transfer course, which I once took.
Stand next to the same size block of Ice, can you feel the radiation and back radiation warming your body?
Or step inside a walk in freezer, feel all that radiation heat reflected off the walls?
No I didn’t think so.
That red hot vat is losing energy to the surrounding molecules though conduction and convection.
If I set a fan to blow on it, advection.
And if I throw a bucket of water on it, latent.
I demonstrated this by the experiment linked in my original post.
Because of these non-radiative processes the vat cannot radiate as a BB which requires the vat to radiate ALL of it.
(cond+conv+adv+lat+rad) = 1.0 aka all of it.
Emissivity = rad/all of it.
Because of the contiguous molecules the earth’s surface cannot radiate BB = no 396 upwelling, no 333 downwelling & looping.
1) By reflecting 30% of the ISR the atmosphere cools the earth.
2) There is no “extra” BB upwelling energy.
3) There is no “extra” energy for the GHGs to “trap” and “back” radiate.
1+2+3 = Zero RGHE + Zero GHG warming + Zero CAGW.
Stand near a block of dry ice, then insert a sheet of water ice between you and the dry ice, and then tell me if you feel warmer or not.
” the “cold” depths of outer space are 394K”
Say what? You are missing a decimal point in there.
Cold & hot don’t mean anything in space – no molecules.
Temperature is the relative measurement of the kinetic energy of molecules.
Same reason there is no sound.
Did I say there are no molecules?
Well, that, too, is wrong.
Lots of molecules.
Moon.
ISS.
Space walking astronaut (with an HVAC unit in the MMU)
Earth.
And the ISR heats those molecules to 394 K, 121 C, 250F.
The background radiation is space is just a couple of degrees Kelvin. The fact that there are some things in space that are heated by the sun doesn’t change that.
The earth isn’t surrounded by background radiation.
That’s why the ISS has redundant ammonia refrigerant chillers.
And why the earth sans .3 albedo gets hotter not colder.
Background radiation is everywhere. That’s why it’s called background.
So yes, the earth is surrounded by it. ISS has redundant chillers because life would get uncomfortable fast if they only had one and it broke.
Nick Schroeder,
There are a lot of stations on earth that measure incoming and outgoing SW (sunlight) and incoming and outgoing LW (IR) radiation to from the surface for several decades.
The average around 300 W/m2 LW backradiation is measured in lots of stations, that are real measurements, not theory or modelling:
https://scienceofdoom.com/2010/07/17/the-amazing-case-of-back-radiation/
Still there is conservation of energy, as the backradiated energy simply is recycled energy from the solar input, transformed to LW energy by the surface that is partly captured and send back to the surface. No new energy is created…
Those real stations assume 1.0 emissivity and
that
is
wrong!
It is also obvious from USCRN data that the IR readings are “tweaked” to match the 1.5 m sensible surface readings so they are not independent measurements.
Those stations are not measuring downwelling IR. They are measuring upwelling-from-the-instrument IR.
Solar energy is not converted to longwave. The longwave is geothermal.
There is no material that converts shortwave to longwave – especially Earth’s solids, liquids, gases.
Only recently (2019) have scientists created a CHEMICAL method for doing the conversion.
Everything you know is wrong. Your theory is based on unphysically assumptions and gap-filling.
Really now, then what happens to that short wave radiation. For hundreds of years scientists thought it heated up the ground. Since it didn’t, where did it go. Did it just vanish, did the leprechauns steal it? Where is it?
“There is no material that converts shortwave to longwave ”
As usual, in Zoe’s mind, she has completely over turned all branches of science and mathematics. It’s just that she’s the only one who’s smart enough to understand what she’s talking about.
MarkW<
Indeed Zoe seems to live in an alternative world with its own physics.
After a few attempts to really understand what she is up to, I did give up…
Mark, the idiot,
Sun’ UV, Light, and Infrared directly heat the surface.
Sun’s spectrum is NOT converted to Earth’s spectrum.
Why you so stupid!
Once again Zoe demonstrates that not only does she not understand basic physics, she is so full of herself that she can’t accept the possibility that she could be wrong.
The sun’s spectrum, is determined by it’s temperature.
The earth’s spectrum is determined by it’s temperature.
If the earth warms up, then it’s spectrum will change and the amount of energy it radiates will increase.
Since you have admitted that the sun warm’s the earths surface, then you have admitted to the rest of it.
The only problem is that your pride won’t let your brain understand reality.
Mark,
“The earth’s spectrum is determined by it’s temperature.”
Yes, Indeed, “It’s” !
“Since you have admitted that the sun warm’s the earths surface”
Yes, in the daytime with its UV, Light, and Infrared
“then you have admitted to the rest of it.”
Non sequitur.
You need to prove the sun’s spectrum is converted to Earth’s spectrum.
Zoe, if you were half as smart as you believe you are, then you would realize that I just finished proving that.
Zoe,
When I was a young boy, already interested in a lot of scientific things, I once experimented with magnifying glasses and sunlight to get some paper on fire (and the only time I remember that my father was mad on me, as I tried that out near the stock of wood for the wood stove). Seems that the SW energy is quite easily converted to heat and maybe heat can be converted to LW energy? Or is that wrong too in your alternative world?
“Seems that the SW energy is quite easily converted to heat”
Yes
“and maybe heat can be converted to LW energy?”
Non sequitur! Prove it!
If something is warm, it emits radiation. If the temperature is in the right range, then that energy is LW.
This is really basic science. Perhaps if you would drop the ego and take a freshmen level, or even high school level physics course, you would learn these things.
As you repeatedly whine, there is nothing that directly, in a single step converts SW to LW. The mechanism has always been, SW warms an object, then that object emits LW.
Mark,
“The mechanism has always been, SW warms an object, then that object emits LW”
Prove it!
You can’t use geothermal denial as evidence.
Don’t forget: ”
There is no material that converts shortwave to longwave – especially Earth’s solids, liquids, gases.
Only recently (2019) have scientists created a CHEMICAL method for doing the conversion.”
Now why would scientists need a material like this when climate “scientists” claim that ordinary water, land, gases, etc (everything) already does this conversion?
Zoe,
1. You do agree that a material like the earth’s surface absorbs SW energy.
2. You do agree that the absorbed energy is warming the surface.
3. I hope that you agree that any material with a temperature above 0 K emits EM radiation.
4. I hope that you agree that according to Wien’s law, the whole frequency spectrum and the peak frequency of any body above 0 K depends of its absolute temperature and also the total energy emitted is exactly known for a lot of materials, depending of the S-B equation and the specific emissivity of that material:
https://www.engineeringtoolbox.com/emissivity-coefficients-d_447.html
5. Both the incoming and outgoing energy is measured in W/m2 in a lot of places and even the incoming and outgoing spectra are measured and can be compared to the theoretical one’s for the specific temperatures of the surface. That shows that both are more or less in balance for the W/m2 in and out, but totally differ in wave spectrum.
If you don’t believe that the outgoing LW energy is in fact the converted incoming SW energy, then:
6. Where does the incoming energy go?
7. Where does the outgoing energy come from?
And don’t mention the earth’s geothermal energy, which is just peanuts: 0.087 W/m2 compared to about 160 W/m2 solar energy directly absorbed by the surface…
Ferdinand,
Until you fully absorb the following material, your comments look like sophistry.
http://phzoe.com/2020/05/22/equating-perpendicular-planes-is-plain-nonsense/
http://phzoe.com/2020/04/29/the-irrelevance-of-geothermal-heat-flux/
Ferdinand, dump all the books you studied in school. Zoe has over thrown physics, the only accurate texts are those found on her site.
Don’t believe me, just ask her. She’ll tell you what a genius she is. Over, and over, and over again.
MarkW,
Indeed, I did give up some time ago, but again was surprised with her new inventions… Like her over 300 W/m2 geo energy:
Geothermal Emission = Upwelling Longwave Radiation – (Downwelling Shortwave Radiation – Upwelling Shortwave) + Latent Heat Flux + Sensible Heat Flux
Of course, if you completely ignore the over 300 W/m2 Downwelling Longwave Radiation, then it must come from the geothermal source…
No matter measurements of both DLR and geo…
Measurements are there only to confirm your theory. If they don’t agree, ignore (or “adjust”) the measurements…
Ferdinand,
“Of course, if you completely ignore the over 300 W/m2 Downwelling Longwave Radiation, then it must come from the geothermal source”
Of course if you ignore geothermal, then it must be DLR!
“No matter measurements of both DLR”
DLR is not measured. What is measured is upwelling-from-the-instrument IR.
http://phzoe.com/2019/11/11/why-up-is-not-down/
Someday they will make a measurement instrument capable of with standing lava. When put in a lava flow the measured “DLR” will be so outrages that it will become obvious.
P.S. Measurement instruments actually measure Net IR, and then you cranks derive “D”LR.
When put in a lava flow the measured “DLR” will be so outrages (sic) that it will become obvious? No, funny, but actually, as long as the instrument is constructed so as not to melt, it will read the proper DWIR.
Zoe then writes: you cranks. I dunno, that’s also quite funny if you ask me. Mercury thermometers actually measure the expansion of a column of mercury. Then the same cranks calibrate it to readout temperature. Seems to be a meaningful method just like Zoe’s DWIR outrages instrument.
Zoe,
You are extremely good in finding the few people on earth that support your world view…
I have met Claes Johnson several years ago when he was in Belgium for a lecture and then was already not easy with what he was telling. But let’s see what he says about the DLR measurements:
We see that a pyrgeometer does not measure DLR directly but invents it from the formula
E_in = E_net + E_out,
which is supposed to result from E_net = E_in – E_out expressing a Stefan-Boltzmann law of the form
E_net = sigma Ta^4 – sigma Te^4,
where Ta and Te are the temperatures of atmosphere and Earth surface. But Stefan-Boltzmann’s law is not described this way in physics literature, where it instead takes the form
E_net = sigma (Ta^4 – Te^4),
which does not allow extracting DLR as sigma Ta^4.
To begin with, Te is not the temperature of the earth’s surface but the temperature of the receiving (black body) plate of the instrument. No matter if you measure above glowing hot lava or above the ice at the South Pole.
Johnson says that he used the definition of Wikipedia:
E_out – Long-wave radiation emitted by the earth surface [W/m²]
But have a look at Wiki:
https://en.wikipedia.org/wiki/Pyrgeometer
E_out – Long-wave radiation emitted by the sensor surface [W/m2]
That is a hell of a difference!
Then, as far as I know,
sigma * (Ta^4 – Te^4)
is exactly the same as
sigma * Ta^4 – sigma * Te^4
but Johnson may be more familiar with modern mathematics than I am…
Not only that, all you need is Te to calculate the outgoing energy in W/m2 and what you measure is the incoming W/m2 minus the outgoing W/m2 in the form of an increase in voltage or whatever physics they use. That gives you the incoming DLR. That can easily transformed into Ta if you need that at all, as DLR is what one is interested in…
Maybe you should look for more reliable sources?
I’d post this PPT slide direct if I knew how.
So here’s a link to my LinkedIn page.
https://www.linkedin.com/posts/nicholas-schroeder-55934820_im-posting-this-so-i-can-link-to-it-on-wuwt-activity-6680911910633783296-l2vx
So now insulators have higher emissivities than non insulators, gotchya.
Your ability to refute what was never said is astounding.
The atmosphere does not warm the earth.
By reflecting away 30% of the ISR the atmosphere/albedo cool the earth.
The atmosphere doesn’t warm the surface, in the same way that insulation doesn’t warm a house and clothes don’t warm a body.
What they do is slow the rate at which heat escapes.
Q = (1/R or U) A (hot – cold)
If I increase R, Q goes down OR dT goes up or a combination.
W/o a thermostat to reduce the furnace firing doubling R doubles dT.
For a constant Q:
R of 6, 70 inside, 30 outside, dT = 40.
R of 12, 110 inside, 30 outside, dT = 80.
If the albedo increases Q decreases, dT decreases and the earth cools.
And vice versa.
All five heat transfer processes impact the thermal resistance of the atmosphere.
Change that “U” change the dT.
Radiation does not function separately from the non-radiative.
The huge amount of conduction, convection advection and latent process above the ocean’s surface make a 0.9 emissivity impossible.
If you paint a window some light will get through the thin sections of the paint.
If you paint it again, some light might still get through some still thin points.
If you paint it a third time you might only get a couple pin points of light that can get through.
By the time you paint it a 4th time, it should be completely opaque.
A 5th and all subsequent painting will in effect do nothing.
When you add some green house gas to the atmosphere, the atmosphere can capture some radiation and send it back at the Earth, keeping the surface warmer.
If you double the amount of gas, you can capture twice as much radiation, keeping the Earth warmer still.
If you double it again and and again and again you eventually get to the point where the atmosphere captures 100% of the available radiation. Our atmosphere has had the ability to capture 100% of the radiation since about 1 ppm of CO2 was in it.
The numbers below are made up, but give you an idea of what happens…
But there is action after getting to that saturation level. At 1 PPM it takes hundreds of feet of atmosphere to capture all the radiation. Doubling the greenhouse gas now lowers the level at which the radiation available gets fully absorbed. 2 ppm 256 feet, 4 ppm 128 feet, 8 ppm 64 feet, 16 ppm 32 feet 32 ppm 16 feet 64 ppm 8 feet 128 ppm 4 feet, 256 ppm 2 feet 512 ppm 1 foot
We are at a point where all radiation is absorbed before it gets past your ankle.
Now then, how does the green house effect work?
It takes that radiation and shoots it back out in a random direction. You need to be miles in the air for there to be any meaningful difference in what direction it shoots it to matter. As large as the Earth is, being feet off the ground closely means that 50% gets shot towards space and 50% gets shot back at the Earth.
So, while, yes, your physical clothes, the pot lid and so forth warm the pot, adding a second lid will not increase the heat gain in the pot. Once you have a certain R value of clothes or house insulation on, it becomes effectively meaningless to add any more. Unless you are talking about hundreds or thousands of degrees temperature difference between you and outside temperature.
An R value change from 1 to 2 saves you half the energy, a big savings! Going from 2 to 4 saves you 25%, 4 to 8 12.5%, 8 to 16 6.25%, 16 to 32 3.125%, 32 to 64 1.5625% … from 4096 to 8192 0.0122 … 8388608 to 16777216 .000006% . At what point does it make any real difference?
With CO2, the only meaningful differences happened in the sub 100 ppm range and everything after that is like adding a 1 R panel over top of an R16777216 panel.
The other thing that CO2 does is spread the warmth around. And send it to space.
The real work being done that keeps Earth warmer than what a black body analysis would indicate is from the Oceans and the thick atmosphere we have. The Ocean holds tremendous amounts of energy within. And the Atmosphere recirculates heat up and down its depth.
Even if out atmosphere was 100% CO2, it would still not be able to capture 100% of outgoing radiation.
I don’t know where you got the nonsense that 1ppm can capture 100% of outgoing radiation, but it isn’t true.
If I remember the numbers correctly, 50ppm is able to capture about 50% of outgoing radiation. Each doubling captures about 1/2 of the remaining radiation.
Look up saturation of CO2 and radiation. You will find quite a few that indicate that it is between a few inches of the ground and at most 10 meters off the ground at which every single available photon in the proper frequencies is absorbed.
Their argument for how it warms the atmosphere is that the photon has to make more jumps before making it out to space. Hence warmer.
When you look at water and CO2 you will note that CO2 really only has a single area of spectrum it can absorb. The 400 to 480 um. Water, which is in the air by orders of magnitude higher concentrations gets everything else. And CO2 re-radiates this energy out at a rate of about 90% in wavelengths outside of this band.
And if you think that water is not competition in general, even in deserts it is…
From Sciencing dot com
“The Mojave Desert is a land of temperature extremes and very little precipitation. Forty-degree temperature changes are typical in a single day, with peaks near 120 degrees Fahrenheit and lows significantly below freezing. The California Desert Studies Center has recorded humidity and temperature at the dry Soda Springs site down California’s famed Zzyzx Road since the 1980s. According to these measurements, typical summer afternoon humidity is 10 percent and winter afternoon humidity is 30 percent, with humidity highs most winter nights of 50 percent or greater.
The colder the air, the less water vapor it can hold, so similar amounts of water vapor can account for both the low summer relative humidity and higher winter measurements. For example, the Mojave’s mean winter temperature is 50 degrees Fahrenheit. At this temperature, the maximum possible humidity is 7.6 grams of water per kilogram of air. Its summer mean temperature is 90 degrees Fahrenheit, with a maximum humidity of almost 30 grams of water per kilogram of air. So winter 30 percent humidity is 2.28 grams of water per kilogram of air, while summer 10 percent humidity translates to 3 grams of water per kilogram of air.”
At 3 grams per KG that is 0.3% which is about 7.5 times as much water in the air as there is CO2…
Anyways. The work that CO2 can do to warm the planet has been accomplished. There is no new CO2 caused warming.
They try to claim that each doubling does 1C or so warming because of it being a logarithmic function. But I would argue that it is more in line with each doubling effectively does 1/2 of what the previous did, much like insulation. And it has been doubled down to the point of a doubling being capable of increasing the temperature 0.000000001C or less.
Dr. Spencer could maybe explain a conundrum. Looking at UCARS their radiation budget diagram shows greenhouse gases “back radiating” 333 w/m^2. That means 333 w/m^2 must be going up also since they radiate equally in all directions. Where does this 666 w/m^2 come from when the sun only has 341 w/m^2 incoming? Let me add.that the diagram only shows 239 w/m^2 total outgoing.
After being told on Twitter multiple times that O2/N2 are impervious to IR just what temperature must greenhouse molecules reach to achieve this level if IR radiation.
Lastly I believe conduction is vastly underrated. Each CO2 molecule is surrounded by 2500 other molecules and each H2O molecule is surrounded by a minimum of 25 other molecules. Most warmistas admit that collisions are more likely than an emission of IR. That’s a lot of energy yet UCARS only shows 17 w/m^2 in thermals.
I’m fairly sure that 333 W/m^2 IR “back-radiation” is fictional. I seem to recall that people have tried to measure it (how accurately, I don’t know, but there seem to be questions about that too) but didn’t find anything like that number, certainly not on a clear night. Heavy cloud cover *could* reflect quite a bit, but I suspect even that is a lot less than 333 W/m^2. That’s a huge number. I suspect what they’ve done here is to invent this number in order to make the radiation energy budget balance with the S-B temperature, because they haven’t taken the gravito-thermal effect into account first. If the radiation budget was measured at the S-B altitude of several kilometers, that “back-radiation” number would be a lot smaller.
In addition to leaving out gravito-thermal effects, they’ve also left out much of the role of convective heat transfer, as you noted. So I think this diagram is largely misleading rather than clarifying.
The “extra” 333 MUST be there so the instruments are fabricated, calibrated, tweaked and applied with 1.0 emissivity to confirm that MUST. Recall cold fusion?
BECAUSE – If there is no “extra” downwelling 333 what happens to the “extra” upwelling 333.
The “extra” 333 upwelling MUST NOT reach ToA ’cause that would truly screw the balance.
OR
there is no “extra” 333 upwelling – which is the actual fact, Jack!!
As I have demonstrated by experiment, the gold standard of science.
https://www.linkedin.com/posts/nicholas-schroeder-55934820_climatechange-globalwarming-carbondioxide-activity-6655639704802852864-_5jW
Radiative heat transfer does not function separately from the non-radiative processes. They are interconnected and co-dependent.
Go ask a heat exchanger engineer why they add fins, fans and sometimes water sprays.
More non-radiative means less radiative. The five are joined and comingled at the surface.
Go ask Eppley, Kipp-Zonen and Apogee about measuring downwelling IR and the proper use of emissivity.
Nick Schroeder,
I don’t see how an instrument that simply measures the heat caused by what of IR falls on a black plate can be manipulated to equal 1.0 emissivity, as that isn’t even in the equation: they measure W/m2, whatever the emissivity of the surface.
If that was not the case, how can they give a reasonable body temperature by pointing such a device to your body: 33ºC for a device of a few dollars worth (*)? Or now outside pointing downward +20ºC, pointing upward -20ºC (clear sky after sunset). The latter sends 232 W/m2 back to earth, the first emits 417 W/m2 upward for an emissivity of 1. All “tweaked” figures?
(*) Was an extra gift with the purchase of some electronics:
https://www.conrad.com/p/voltcraft-mini-ir-10-ir-thermometer-display-thermometer-11-33-up-to-500-c-pyrometer-1367583
Not exactly the same model, but similar with a smaller range an probably a lot cheaper…
Sorry, an instrument that measures heat is not measuring W/m2, the W/m2 come from calculations.
I have a couple of IR instruments. All they measure from the sky is erratic noise.
IR instruments are based on thermocouples which have temperature/mv relationships.
They are calibrated based on incorrectly assumed BB sources.
The temperature is converted to W/m^2 w/ S-B again assuming BB.
With surrounding air molecules moving heat BB is an invalid assumption.
Nick, IR thermometers are now in use to take body temperatures to reliably check for fevers. They also register 32F for glass of ice water and 212F for boiling water. Their calibration is accurate enough, it is not based on incorrectly assumed BB sources.
Trick, where is the range finder on the IR themometer?
On inexpensive ones (~$30) from the hardware store, the instrumental range for solid objects is defined in the instructions. They do come with a nice little laser dot indicating the center of field of view on the object – like a forehead.
So you are saying that when pointed at the sky the IR thermometer can measure a certain distance to some gas molecules and tell you their temperature?
And then repeat the process for any distance?
For a gas such as the atm., there is no surface, photons enter from all distances. The emissivity setting has to be adjusted to read the thermometer temperature at each distance. Inexpensive IR thermometers have a set emissivity so they read the thermometer temperature at the distance where the emissivity is matched to the setting.
AC Osborn,
These IR thermometers just measure the incoming LW radiation that is all. No matter from what height or distance that LW radiation comes from. You can call it the average temperature of the sky, but as most of that radiation is in specific wavelengths from GHGs, and thus not the general broad spectrum from a black body, that is a virtual temperature, not a real one…
Doesn’t matter at all as all you need is the W/m2.
Ferdinand, IR thermometers read out an avg. gas brightness temperature. The avg. brightness temperature read out would equal the avg. thermometer readings over the air column observed if the column avg. emissivity is equal the emissivity setting.
For an equatorial sky brightness reading an emissivity setting of 0.95 should be reasonably equal the thermometer avg.
For a dry polar atm. column, the IR thermometer setting nearer 0.65 to 0.7 would be used.
“The emissivity setting has to be adjusted to read the thermometer temperature at each distance.”
Emissivity has exactly zero to do with distance.
“Tweaking” to confirm your bias.
In other words they can return any value for a gas or moisture, unless there are clouds with a surface where they can actually get a measurement.
A C, for the hardware store IR thermometer, the setting for emissivity is fixed.
Usually at 0.95. So, any gas for which you measure brightness temperature with one of those, if the gas emissivity though the distance of the gas actually is 0.95, the brioghtness T readout will be the thermometer temperature for the gas. If that emissivity through the distance of the gas is not 0.95, then the readout will not be the thermometer temperature.
trick
About this child’s forehead.
You are inches away, the forehead is a defined surface, the thermometer was designed, fabricated and calibrated in air for this specific purpose.
Don’t try it on clouds and air molecules at 15,000 feet where that application was not included.
And don’t infer W/m^2 leaving that forehead.
As you noted these devices are usually set at 0.95 % emissivity.
The assumes that 95% of ALL the energy leaving the forehead is by radiation.
But that is incorrect.
Energy is leaving that forehead by both non-radiative (Conduction, convection, advection (fan), latent (sweating) and radiative processes.
I have a Klein IR thermometer with adjustable emissivity.
I point my Klein at the heating element in my experiment.
Say it reads 450 F.
Now the heating element is surrounded by air with conduction and convection and the small computer fan is blowing, all of those are removing energy and cooling that element thereby reducing the amount of LWIR.
So, let’s say half of the energy is leaving by non and half by radiation.
Emissivity is 50%.
I enter 50% in the instrument.
The temperature rockets upwards.
Why?
I have just asked the instrument how hot that element would have to be if it had to radiate all 100%.
ε=0.95
450 F
232.22 C
505.22 K
3,694.14 W/m^2
That’s half.
ε=0.50
7,388.27 W/m^2
600.81 K
327.81 C
622.06 F
That’s how hot the surface would need to be to radiate ALL energy as a BB.
Leaving the 16 C surface:
17 W/m^2 sensible, 80 W/m^2 latent, 63 W/m^2 LWIR
Emissivity: 63/160=.39.
Saying the surface upwells/radiates 396 as a 16 C BB assumes that ALL energy transfer is by LWIR.
That is obviously not so.
”Emissivity is 50%.”
Nick, the heating element emissivity didn’t change from about 0.95. The heating element brightness temperature changed when you starting blowing air on it because it cooled off. So did the heating element thermometer temperature. Better way to conduct your experiment is to install a thermometer. Compare the two. Industrial Klein IR thermometers are very useful, under your assumptions they would be useless.
A lab glass of ice water has conduction, convection, advection (fan), latent (sweating) and radiative processes going on, despite all those your Klein will readout 32F to start with adjustable emissivity set at about 0.95. Drop in a thermometer, move up the fan speed, record what happens to the two temperatures readouts without changing the emissivity setting.
Trick
I had a type K clamped to the heating element and a wattmeter measuring input.
When the fan or water spray cools the element, radiation goes down as does emissivity.
Emissivity = radiation / (conduction+convection+advection+latent+radiation)
Radiative heat transfer does not function separately from the others.
Nick, emissivity is not radiation. Emissivity of the element does NOT go down, it’s very stable. Radiation has very different independent physics than convection, conduction so they superpose. What you need is to clamp a thermometer to the element and chart its temperature vs. the fixed emissivity Klein brightness T readout as you vary the forced convection.
Trick – It’s no use.
Nick keeps using a completely incorrect definition of emissivity that screws up all of his following calculations.
I’ve shown him correct definitions, and he ignores them.
I’ve challenged him to provide a reference that supports the definition he uses, and he never does.
So it’s a hopeless case.
Nick, in my heat transfer book there is an equation for calculating the available power between two wavelengths. If I remember it depends on the temperature of the input in this case surface temperature. At 288 K there is no way a part of the total can be darn near equal to the whole as we are talking about one wavelength,15 micro.
Beside if IR could cause CO2 to warm why is it not mentioned in specific heat tables, or Shomate equation, or the NIST data sheet?
“Beside if IR could cause CO2 to warm why is it not mentioned…”
Because it does not do so.
Remember the tenants of RGHE.
1) The atmosphere makes the earth warmer: 288 K w – 255 K w/o = 33 C cooler. Remove the atmosphere and the earth becomes a -430 F ball of ice. (NOAA)
What is the mechanism?
2) GHGs magically “trap” and “back” radiate LWIR energy. Since the “trapped” amount would no longer reach ToA there would be an imbalance. There must be a mysterious source.
Where do the GHGs get this “extra” energy?
3) The surface radiating as an ideal BB at 289 K, 396 W/m^2 generates the “extra” energy.
1) Wrong. The atmosphere cools the earth. Remove it and the 0.3 albedo goes with it and the earth resemble the moon, a barren, dusty, dirt ball, hot^3 on the lit side, cold^3 on the dark. Nikolov, Kramm (U of AK) and UCLA Diviner all know this.
2) “Extra” energy is prohibited by conservation of energy.
3) Because of the non-radiative heat transfer properties of the contiguous participating atmospheric molecules BB LWIR radiating from the surface is not possible as demonstrated by experiment, the gold standard of science.
From all of the above comments, very few in agreement with each other, the ‘settled science’ argument should be dead and buried.
Maybe it’s like the never-ending royals – The settled science is dead. Long live the settled science. Then it never dies and the CAGW crowd can keep it at the forefront of their argument.
John: The argument Roy makes about the existence of the greenhouse effect is based on well over a century of repeatable and verifiable laboratory and field measurements.
The concepts and principles he uses have been at the core of successful engineering designs over this time period. So yes, to that extent, you can consider this “settled”. The people here arguing against it would not make it through an introductory engineering thermodynamics or heat transfer class.
This is a very different issue from predicting how much a slight change in the radiative properties of the atmosphere will change things. We have no century-long record of successful predictions or laboratory experiments in that case.
Photons are not ‘heat’ and thoughts such as your, Ed, have still not reached a definite solution after decades of discussion such as you’d attempt. Thermodynamics would seem incapable of properly and validly describing the situation that’s involving a cascade of Photons (and not just a bland ‘energy’).
doesn’t seem much has changed… ( https://wattsupwiththat.com/2020/06/23/climate-change-temperature-hits-100-degrees-above-arctic-circle-just-like-100-years-ago/ ) …perhaps it needs to be more directly questioned if thermodynamics is even validly applicable…
Peter:
Photons carry a very well-understood amount of energy (e = h * v). When a photon is absorbed, the energy in the photon goes to increasing the energy of the absorbing object by this precise amount.
This “definite solution” has been well understood for a century now.
Yes they do, except they replace one or more photons at a much higher energy level, so the overall energy level of the object is reduced, it may slow down the cooling of the body but does not “warm” it.
Taking it to it’s logical conclusion if you swapped all the molecules producing the high energy photons with the low level photons the object would be as cold as where the photons came from, would they not?
How many CO2 molecules are there, compared to the water molecules in the atmosphere and on the surface of the earth which are sharing these low energy level photons?
AC: I regularly use the low-energy photons of longwave infrared (10.6 um wavelength) to melt steel. It is an everyday industrial process.
You claim it is not possible, but somehow I manage it…
Wrong Ed, the energy of the Photon is ‘cold’ and the increase in ->temperature<- of the 'absorbing object' is not made directly in amount as the energy delivered of the incident photon. This is well realized. You cannot cite the energy of the Photon as 'heat' and this is the LIE of 'climate warmist science'. There is no Environmental indication of 'anthropogenic' affect as the 'warmist science' overstates the temperature changes.
Ed, the energy of the photon is ‘cold’ and without temperature. The steel you melt is taking the cold energy and using it to warm. You’d seem to confuse the delivery with the result. This is the flaw of ‘climate science’, its effort to consider a photon to be ‘heat’ by labeling as ‘long wave IR’ which is a term of Radio.
The photons are given Extra, the Amplification” in the word Laser of many megawatts, that is what does the work.
If you send a stream of unhenanced LWIR photons from a -50C source at a plate of steel they will do absolutely nothing.
The photons are given Extra Energy, the “Amplification” in the word Laser of many megawatts, that is what does the work.
If you send a stream of unhenanced LWIR photons from a -50C source at a steel plate they will do absolutely nothing, because they are not carrying enough energy, which is also the reason they do not penetrate water (70% of the surface) to any depth.
Think of a fairly cold bullet at 1000fps, whatever it hits will get get quite hot at the point of impact.
Think Freezing cold Asteroid travelling at 25,000,mph, when it hits the earth it will create a great deal of heat, depending on it’s mass.
AC:
A 10.6um photon from a laser is IDENTICAL to a 10.6um photon thermally emitted from a cold body (or even a warm body). It carries EXACTLY the same e=h*v energy, and has NO information as to the mechanism of its emssion.
The laser-emitted 10.6um has exactly the same probability of being absorbed as the thermally emitted 10.6um photon. In both cases, the thermal energy of the absorbing body is increased by EXACTLY the e=h*v energy carried by the photon.
This is very basic radiative physics, well understood for a century now.
The difference is that the laser emits a far higher DENSITY of these photons than the cold body thermally emitting them. But there is NO difference in the individual photons.
Do you have any idea what power rating of the Laser means?
It is the energy put in to the flow of photons, the more power put in the more heat is generated when the photon strike the target.
No extra energy in to the laser no extra heat to burn through steel.
Even water cuts through steel, were you even aware of that?
AC:
I work all the time on the design of both laser cutting and waterjet cutting machines. I am writing this comment as I am taking a short break from working on improving the control algorithms for these machines.
You still do not understand the the “amplification” of lasers increases the number of photons emitted, but each photon has EXACTLY the same energy as a thermally emitted proton of the same wavelength (e=h*v)
This is completely standard radiative physics at the most introductory level. Not controversial at all, and long since “reduced” to engineering. Have you ever taken such a course?
“The [Climate] Science” is defintely not settled.
Roy
here is the warming trend over the last 40 years during which CO2 rose by ca 50 ppm ( = 0.005% of the atmosphere):
https://woodfortrees.org/plot/hadsst3gl/from:1979/to:2021/trend/plot/uah6/from:1979/to:2021/trend
Are you actually saying that that 0.005% extra CO2 in the atmosphere caused the observed warming of all of that mass of the oceans (>10^21 kg) and atmosphere (> 10^18 kg)?
Is it not much more likely to assume that more heat entered into the oceans which subsequently also warmed the atmosphere?
(and I think I can give you a very plausible reason as to why more heat entered the oceans)
Why is it that some people never bother to try and actually understand an article before they seek to refute it?
Dr. Spencer specifically mentioned that there are feedbacks in the atmosphere.
MarkW
I still don’t understand. How exactly did 50 ppms CO2 extra in the atmosphere heat all the oceans of the earth by exactly the same amount as what the atmosphere got heated up? And then again, when I measured the minimum temperatures here, around myself, I could not find any heating caused by the extra CO2 in the air. How is that possible?
Click on my name to read my report.
Anyone?
Where did you get the crazy idea that the oceans have warmed up by exactly the same amount as the atmosphere? The claim is that the oceans have warmed up by around 0.01C. (Of course that’s nonsense because we don’t have instruments that can measure with that kind of accuracy.)
How do you know that there was no warming caused by CO2? Does your thermometer differentiate heat based on source?
The earth has warmed over the last 150 years. That is not in doubt. How much of that warming was from CO2 and how much was from other sources? That is the debate. The fact that warming caused by CO2 is too small to pick out in the noisy signal from the real world is not evidence that it is not there.
MarkW
Try reading my comments
Including the references to my summary of heat into the oceans & atm
They don’t say what you want them to say.
You are the only person who has claimed that the atmosphere have warmed up by the exact same amount.
If the climate sensitivity to CO2 is 1.25 °C per doubling (no one exactly knows what the sensitivity is), 0.6 °C would be expected for a rise from 280 to 400 ppm.
Analogies can be helpful, and this explanation is fine as far as it goes. It describes a static concept as experienced at the surface at any particular place, point in time, and set of conditions. However, the emphasis on the static concept is misleading in my view, as the atmosphere is not physically static, and certainly not so in respect to the longwave radiative coupling with the surface. To illustrate this point, consider the graph at this link. I generated a scatter plot of the downward longwave radiation (i.e. from the atmosphere) received at the surface, vs total column water (which includes vapor, liquid droplets, and ice crystals) for each hour of 2019. 8760 data points. This is from the ERA5 reanalysis product, by the ECMWF (European Centre for Medium-Range Weather Forecasts). It is for one gridpoint near where I live. A gridpoint near the poles would look a bit different. So also for the tropics, or a mountaintop. But the point is that the radiative greenhouse effect is not a static thing. It is dynamic by nature, and most powerfully related to water vapor and clouds. The climate, whether evaluated locally, regionally, or globally, is the composite result of a huge number of high-power energy transformations. The second and third links are the time-series plots of downward longwave and of total column water.
This is not meant as criticism, as I regard Dr. Spencer as a thoughtful and effective voice against climate alarm. My comment here is offered as a supplement to those WUWT readers who can see the limitations of the radiative insulating blanket analogy. Think of a heat engine feeding energy and mass to a variable emitter/reflector much higher than the surface.
Bleh. How can anyone stand this retardation?
http://phzoe.com/2020/03/04/dumbest-math-theory-ever/
Man that is an own goal 🙂
Thank you.
Deniers are still in denial, though.
Zoe darlin, that wasn’t a compliment. Sheesh.
‘So, for the atmosphere, the net flow of infrared radiation from the surface to the “cold” depths of outer space’
Sheer nonsense. There is no flow of EM radiation from matter to nothing. EM radiation only flows from matter to matter.
Dude, how do we converse with spacecraft outside of the atmosphere? How do we see stars? This is all EM radiation thru a vacuum!
Spacecraft and stars are matter. There is EM between matter AND there’s a dilution by inverse square law.
But if you believe matter transfers heat to space, then there is no dilution by inverse square law, and space becomes the ultimate heat sink! But experiments show it to be the best insulator!
It’s dudette, not dude. Thank you
Sorry dudette. EM waves don’t need aether to move thru. They will propagate thru a vacuum until intercepted. The power available at any point does follow the inverse square law. The fact that they are never intercepted doesn’t really matter.
Zoe 8:10am writes: ”There is EM between matter” so dudette Zoe reverses course from earlier 6:56am now writes there really is ”flow of EM radiation from matter to nothing.”
No reversal.
EM travels from matter to matter. That there is space in between is not the issue. There is no EM between matter and nothing, with space in between (not that space in between would mean anything in this context).
“There is no EM between matter and nothing”
What happened to the EM?
“What happened to the EM?”
Corpuscular Theory of Light is NOT true.
Zoe,
Some patent clerk called Einstein dared to propose that light (EM) has a mass, how could he say such a thing…
Except that several years later he was proven right when during an eclipse stars were visible that were just behind the sun: the enormous gravity of the sun deflected the EM waves…
Ferdinand,
Dr. Dowdye of NASA has proved that the only aberation of light by the sun happens due to corona
plasma effects, and not due to gravity. He has stated that no bending of light occurs outside a stars corona. There is no bending of light by gravity that has been observed.
Zoe,
See the comment on Dr. Dowdye’s theory here:
https://www.quora.com/Do-Edward-Dowdyes-findings-on-gravitational-lensing-debunk-GR-theory
Besides that the website you refer to promotes the Flat Earth Society (really…), although that doesn’t prove that Dr. Dowdye is wrong but as the above response shows, there is no real discussion of that theory anywhere on the Internet to find…
Congratulations, Ferdinand, you’ve completely discredited yourself by linking to some gibberish on Quora.
What flat earth site? Dowdye has been featured in many places. Where he is promoted has nothing to do with his observations.
He has plenty of PEER-REVIEWED articles.
Your link is a stupid and digusting “Nuh uh” denier nonsense.
If you have evidence that light bending has been observed outside of a star’s atmosphere please do present it.
Ferdinand, how dare you link to anyone other than Zoe. Don’t you know that she’s the world’s greatest expert on everything?
>>
EM travels from matter to matter. That there is space in between is not the issue. There is no EM between matter and nothing . . . .
<<
A star that is a hundred, thousand. million, or a billion light-years away knows that I’m going to look at it tonight. How did it know that? Obviously, that means there’s some form of instantaneous communication going on. It’s too bad–you just didn’t think that one out–did you?
Jim
“knows that I’m going to look at it tonight. How did it know that?”
Jim, that star has had an EM tether to Earth since the big bang.
Right? Don’t you believe in the big bang?
Everything was close and connected together. Then space drove it apart, and less connections were needed. So now you get 0.00000xxx W/m^2 from it.
Zoe,
I did not find one peer reviewed article by Dr. Dowdye, but if you have some references…
I agree, the website where his theory is promoted doesn’t matter, but neither does the web site where the response is published.
From Viktor T. Toth, IT pro, part-time physicist:
“More importantly though, starlight near the Sun is not our only evidence of gravitational effects on the trajectory of light. Precision radio navigation of deep space probes would not be possible without a full understanding and accounting for the closely related Shapiro effect, a delay of radio signals near any massive body. The perihelion advance of Mercury is a profoundly relativistic effect, one that cannot be understood without taking the nonlinearity of gravity (how gravity acts on itself) into account. And as for lensing, forget the Sun… we have plenty of lensing data from very far objects, such as distant galaxies being lensed by nearby objects, where the light in question never passes through any plasma worth mentioning.”
Seems as valid as what Dr. Dowdye says…
Further, see Dr. Dwodye’s own references:
http://www.scienceinthebible.net/author.htm
Where he seems to be determined to proof that Newton was right and Einstein wrong:
“Dr. Dowdye is an independent researcher and is Founder of Pure Classical Physics Research where he focuses in depth on the Truth and the Profound Fundamentals and Pure Laws of Nature, all first set in motion by the Devine Creator, the Almighty Lord God. Dr. Dowdye is a recognized and leading expert on the theories of both General and Special Relativity, Electromagnetism and Gravitation.”
No matter his beliefs, if his theory can be proven by independent research, that would be very interesting and real science…
>>
Zoe Phin
June 24, 2020 at 1:52 am
Right? Don’t you believe in the big bang?
<<
If you believe in it, then I may have to rethink my position on it.
Jim
Jim Masterton,
That is the utmost best reply I have seen these days on Zoe’s masterpiece of the
“Unified Theory of the Universal Laws of Zoe”…
“I did not find one peer reviewed article by Dr. Dowdye”
That’s because you didn’t want to. You’re a reality denier.
https://scholar.google.com/scholar?q=eh+dowdye+jr
Zoe,
My deepest apologies…
So Dr. Dowdye wrote a few works, but most of them were published within his own organisations or could be found by general search engines.
Thus I wonder who did the peer review, but nevertheless, I would like to see a good discussion on his findings anywhere on the net. It seems to me that his knowledge simply is ignored by the main stream science of astronomy and physics.
That doesn’t imply that he is wrong anyway, but nothing shows that he is right either…
Nobody has made the claim that matter, or anything else, transfers heat to space.
No wonder you are so easily confused.
Zoe has invented her own branch of physics. The fact that it doesn’t make sense to you is just proof that you aren’t as smart as Zoe.
Feminist physics to go along with feminist glaciology.
Not a feminist. Conservative/Libertarian.
Certified Quant.
IQ >130
Cute and nerdy.
Some things are so stupid, that only intellectuals can believe in them.
Zoe has not only mastered all of them, she’s invented a few of her own.
BTW, last time I was measured, I hit the scale 140.
Mark W
Well, even stipulating the IQ point (not conceding, just stipulating), you’re certainly not demonstrating that in this thread.
How so. I’ve disproven everything that you have claimed.
Let’s follow Zoe’s logic, shall we? She says: “There is no flow of EM radiation from matter to nothing. EM radiation only flows from matter to matter.”
The earth absorbs about 240 W/m2 of solar radiation. That is matter-to-matter, so that is “real”. So that’s a power input of 240 * Aearth (surface area of the earth) watts. OK.
The earth “attempts” to radiate about 240 W/m2 * Aearth of longwave radiation outward. This is the only possible mechanism for power output from the earth.
But the OVERWHELMING majority of this — from the time of formation of the earth billions of years ago — has never reached any other matter.
So by Zoe’s logic, the earth must be gaining energy at a rate of 239.999… W/m2 * Aearth!
But even the most alarmist scientist doesn’t believe that the earth is gaining energy at a rate of over 1 W/m2 * Aearth.
So Zoe must be an “ultra-alarmist”!
‘The earth “attempts” to radiate about 240 W/m2 * Aearth of longwave radiation outward. This is the only possible mechanism for power output from the earth.’
Completely WRONG
Solar radiation makes molecules DANCE. This mechanical motion is the spending of the 240 W/m^2 received from the sun.
The Earth does NOT emit 240 W/m^2 to space, but it does emit 240 to a satellite (matter).
There is no gaining of 240. The 240 is spent in REAL TIME making molecules moves – kinetic energy – tempetature.
“The Earth does NOT emit 240 W/m^2 to space, but it does emit 240 to a satellite (matter).”
How does Earth know where the satellites are, or will be, when the EMR gets there?
Trick,
You’re still clinging to the presumption of corpuscular theory of light.
A wave only forms from matter to matter.
The photoelectric effect has been explained with waves as well. There is no need for the particle theory.
Light is not a particle. It is a wave – from matter to matter.
What evidence do you have that light is a particle and cares not where it goes to?
Why count the possible modes in a photon gas box, if all frequencies are possible?
How does your particle know that it can’t exist in a box with certain dimensions?
All matter radiates Zoe. All the time, at every frequency & every temperature. EMR formation intensity from the Planck function at a temperature & frequency has no dependency on where it will be absorbed, reflected or transmitted.
”The photoelectric effect has been explained with waves as well.”
Then do so. Realize you cannot use the word photon (or Newton’s corpuscle) in doing so.
Light does not have a brain, light cares not about anything. An electromagnetic wave is just as much a thing as a photon: both possess energy and momentum (linear and angular) but not, it seems, mass.
The photon language is indeed less useful when you think about all the inventions that came about using the wave language. Single photon counters are the only invention I can think of came about using the photon language. Yet photon language seems more useful in discussion as a particle that can be kicked and kick back. Best science can do is discuss light with the wave-particle duality.
Trick, as near as I can tell, Zoe is a believer in aether, despite that fact that real scientists disproved that theory 100 years ago.
EM does usually come from matter, however there is no requirement that it hit something before it is allowed to exist.
Actually there is a requirement: Planck’s Law.
The density of “photon gas in a box” is determined by the dimensions of the box.
Only certain photons can form and not others and this is determined by box dimensions. If ANY photon can form and fly from a wall, as you suggest, then Planck’s Law for DENSITY of photon gas in a box is INCORRECT. It is not incorrect, therefore you are.
http://physics.ucsc.edu/~drip/5D/photons/photons.pdf
Boltzmann derived SB Law precisely on the idea that EM WAVES only form between matter and geometry matters.
Your silly corpuscular theory of light is antiquated nonsense fully debunked by the fathers of EM and quantum mechanics.
Zoe 9:27am – per your source the equilibrium temperature T of the box must also be known not just the box dimensions, plus 3 constants of nature. All photons CAN “form” because your source integrates over all photon frequencies.
Also, Zoe the dudette, it is of interest to note: Photons do not interact with each other, so the equilibrium distribution of a photon gas can come about only because of interactions of photons with the walls of the container held at that equilibrium T.
‘All photons CAN “form” because your source integrates over all photon frequencies’
No they can not. The density equations relies on only some photons forming. Can’t you read?
If all photons form the density function is incorrect. Can’t you logic?
Oh I get it, you can’t admit error on your part.
Zoe, look at the integration of eqn 6. Do you not see (or understand) the integration over all frequencies at temperature T from f=0 to f=infinity to obtain the radiant energy density eqn. 7?
Uhm, hello, the integral is an ideation, an assumption of a continuous function.
Why can’t you READ the WHOLE thing?
“Counting the modes in the box”
Not all photons will be generated. Only those that pass this test:
“There are an infinite number of such modes, with frequencies ω = mxπc/L, where mx is a positive integer.”
The possible frequencies (and thus wavelengths) MUST pass this test. And part of this test is L … The dimension of the box.
How does a photon know the dimension of the box to know if it can exist?
It does. According to you it doesn’t. Thus, according to you, this test doesn’t need to be passed. If so, then the number of photons goes beyond what actual math and evidence shows.
Think!
Zoe, the phrase “MUST pass this test” or even just “test” is not found in your source. That is Zoe wording.
The source: “integrate Eq. 6 over all values of the frequency” of light to get Eqn. 7 which has extensive physical testing supporting, it’s not just an ideation.
Trick,
Pay attention:
“Our goal is to determine the energy U of the radiation in the box. We now know the mean
thermal energy per oscillation mode of the electromagnetic field, so if we know the number
of modes in some small frequency range dω, we can multiply that by the energy per mode,
and then integrate over the values of ω to find U.”
You’re integrating values that pass the standing wave test.
Why can’t you accept reality?
Zoe, “then integrate over the values of ω” means from 0 to infinity, all frequencies, as they show to get eqn. 7.
Trick,
You’re an idiot.
Only those photons that pass the test have any meaning.
This infinite set is smaller than the infinite set of all.
You didn’t know that infinity can be a subset of infinity?
As you already know for an object at 288K, there is no emission at 0.5 micron (UB). At 0.5 micron, your integral will add ZERO.
There will also be an add of ZERO at all those frequencies that don’t pass the test.
Scientists use purposefully designed materials to remove or filter wavelengths they don’t want. But according to you, the material’s geometries don’t matter … all photons must exist. But they don’t!
Guys/ girls
Remember we are in a public space like a lecture hall and we are students and teachers to each other.
No name calling. Remember what Jesus said if you call another person an idiot.
Zoe, the Planck distribution formula is never identically zero. Again, your source doesn’t even mention the word “test”.
As Zoe should know for an object at 288K, there is non-zero intensity of Planckian emission at 0.5 micron (UB). Any massive object emits non-zero intensity radiation at all wavelengths and all temperatures per the formula.
Trick,
The text doesn’t have to explitly use the word “test”. Look at what they are doing!
They are counting standing waves in a box!
Only those frequencies that pass the test are included. Look at what is being integrated!
You seem to think that just because you’re integrating 0 to infinity, this means you’re adding something at every value; you’re not!
Stop with the sophistry.
Zoe, all frequencies are included, there is no test to pass. Or show one frequency where you’re not adding something at that value. You can plug in any frequency and any T and U/V is non zero for eqn. 7. No frequencies are excluded.
Mark — There’s a new show on TV called “Zoe’s Extraordinary Playlist” where the title character keeps imagining the people around her breaking into big song and dance numbers.
Similarly, here we have “Zoe’s Extraordinary Physics”, where the title character keeps imagining the world around her doing things that would never really happen.
“You can plug in any frequency and any T and U/V is non zero for eqn. 7.”
Eqn 7 doesn’t give you the ability to input a frequency. Total fail.
You can see which frequencies are not possible in Eqn 6.
You’re better off understanding the main points of this paper:
“The problem was that the observed spectrum of the radiation
emitted from a “black body” could not be explained in terms of classical electromagnetic
theory. It was not a minor problem: Classical theory predicted an infinite energy of
radiation:
“We imagine that electromagnetic radiation inside
our box exists as patterns of standing waves, or
modes. A single mode is like a standing wave
on a guitar string, and is characterized by a
frequency. ”
Hmm standing waves from wall to wall! No depiction of a wave that doesn’t end in a wall, i.e. your imaginary free willy nilly photon.
Continuing…
“The energy of a mode is quantized: It was Planck’s hypothesis that
only certain energies of these oscillation modes are allowed.”
“There are an infinite number of such modes,
with frequencies ω = mxπc/L, where mx
is a positive integer. Since our box exists in
three dimensions, identical sets of modes exist
for electromagnetic standing waves polarized
perpendicular to the y- and z-directions,
corresponding to the sequences of integers ”
You do realize that allowing all frequencies of photons is a direct contradiction of the discrete photons theory of Planck and quantum mechanics in general?
No you don’t realize that. Now finish me off with your brilliant “Eqn 7 is an integral, therefore …”
”Eqn 7 doesn’t give you the ability to input a frequency.”
Because Eqn.7 is the result of integrating over ALL frequencies. You can plug in any frequency and any T in any range for eqn. 6 energy per unit volume (all frequencies are possible) and U/V is non zero for eqn. 7 since “There are an infinite number of such modes.”
No depiction of a wave that doesn’t end in a wall because the photons do not interact they (waves,particles) ALL reach the other wall. There are waves from top to bottom not depicted also.
”You do realize that allowing all frequencies of photons is a direct contradiction of the discrete photons theory of Planck and quantum mechanics in general?”
I do not realize that. What I realize is it was Planck’s hypothesis that only certain energies of these oscillation modes are allowed..each oscillation mode can exist in any one of an infinite number of energy states whose energies are equally separated by the energy = Planck’s constant*frequency/(2*pi).
Trick,
OK. I see where the confusion lay. Let me describe the problem in your own words:
“ALL reach the other wall.”
“only certain energies of these oscillation modes are allowed”
The main question was whether matter emits to nothing (with no matter in sight).
You admitted that Planck’s Law only allows certain energies per mode to exist between walls.
Now there is no other wall. Does that allow more energies per mode to exist?
Fine! Then this violates Planck’s Law of emission from one wall (matter).
QED
”Does that allow more energies per mode to exist?”
Each oscillation mode can still exist in any one of an infinite number of energy states with no other wall in sight. No violation.
Trick,
How without a wall?
You didn’t answer the question. What happens when you remove the “certain” energies per mode limitation imposed by geometry?
Without an opposite wall, there is just photon birth, no photon death at the opposite wall. The escaped waves/photons live on, keep on waving and photoning.
The only hit on “certain” is for energies En, there is no hit on remove so the article sheds no light (what a pun) on your question. If you mean to remove some energy quanta from the box, make the opposite wall a little transparent, let a photon out. Boom, gone. Less U/V.
Trick,
Why you such a liar?
You can see that Planck’s Law is completely derived ONLY from treating light as a WAVE from matter to matter.
You can see that the certain energies per oscillation mode is dependant on geometry. If you remove the opposite wall, those constraints are removed. So even switching to your light as a particle theory, there is now emission much greater than SB Law suggests.
Either way you’re wrong.
You can’t derive Planck’s or SB Law without treating light as a wave.
You also intuitively realize that things like “frequency” and “wavelength” don’t make sense to a particle.
No photons, all waves? Zoe, look at the title of your source. More than 20 hits found on “photon” search. I didn’t remove the wall, Zoe did. Nothing makes sense to a particle of light; it doesn’t have a brain.
Wow, is there nothing that you won’t work hard to misunderstand?
Mark, you have to admit that Zoe has the most creative imaginary physics of any of the pink unicorn brigade. She even out does old Mr. (Natural Fiber) with his heat creep and pseudo-scattering.
It’s actually kind of fun seeing what ridiculous concept she will invent next. I especially like her assertion in this thread that molecular vibration from absorption is the “spending” (loss) of energy rather than its storage.
I can’t decide whether that one is funnier than her idea that there is no radiation from an object unless and until it reaches another object, even if that object is light years away.
Which is your favorite?
Trying to have a logical scientific discussion with Zoe is like playing chess with a pigeon.
“Trying to have a logical scientific discussion with Zoe is like playing chess with a pigeon.”
Says a guy who plays chess with a pigeon.
My favorite Zoe? “Those stations are not measuring downwelling IR. They are measuring upwelling-from-the-instrument IR.”
until I read: “Everything you know is wrong. Your theory is based on unphysically assumptions and gap-filling.”
Gasp. Last one could be all time award winner right there. Yet the thread is young. The pigeon is sure it won.
I’m still trying to wrap my mind around here claim that you can pump energy into something, forever, and it won’t heat up at all.
Ed,
I suppose you think that when you move your furniture, you’re not expending energy, you’re actually storing it? LMAO
Ok Zoe, let’s use an example from high school physics, which you seem to have slept through.
We have a mass hanging from a spring statically in earth’s gravity. To keep things simple, it’s in a vacuum.
Now, I apply an upward force on the mass to lift it, say 10 cm. I have done work on the mass (force time distance) and increased its energy level (gravitational potential energy in this case).
Next, I let the mass go, and it starts oscillating up and down, 10 cm above and below its static height. It is converting energy between potential and kinetic energy, but its overall energy level has not changed since I let it go. So yes, it is storing the energy I added to it by lifting it.
Similarly, if a molecule like H2O or CO2 absorbs an IR photon, it excites an oscillatory mode in the molecule, which is a higher energy state than when it is static. As long as it is in this oscillatory mode, it is in the higher energy state. So as with the mass-spring system, it is truly storing the additional energy imparted to it by absorbing the photon.
This energy is stored until the molecule either collides with another molecule (most common), transferring energy to that molecule, or emits a photon.
(Your example of moving furniture is not all appropriate. If I push it horizontally, I am doing work on it, increasing its kinetic energy. If it stops by itself, the kinetic energy is converted to thermal energy by friction. If I stop it, I am doing work on it in the opposite direction. Neither of these is analogous to the vibrating molecule.)
This is basic, basic stuff, and you have no clue about any of it!
“Next, I let the mass go, and it starts oscillating up and down, 10 cm above and below its static height. It is converting energy between potential and kinetic energy, but its overall energy level has not changed since I let it go. So yes, it is storing the energy I added to it by lifting it.”
Simple Harmonic Motion? Hooke’s Law?
So it goes on oscillating 10 cm above and below its static height? Are you sure? So I go back in 10 years time and it is still oscillatting 10 cm above and below its static height?
“Similarly, if a molecule like H2O or CO2 absorbs an IR photon, it excites an oscillatory mode in the molecule, which is a higher energy state than when it is static. As long as it is in this oscillatory mode, it is in the higher energy state. So as with the mass-spring system, it is truly storing the additional energy imparted to it by absorbing the photon.
This energy is stored until the molecule either collides with another molecule (most common), transferring energy to that molecule, or emits a photon.”
Really?
In both cases energy is being released by the very nature of the situation. The energy is not “stored” and then released when another input is introduced. The energy will always decrease until it is zero. Do you believe in PMMs?
This is such a stupid comparison. Maybe you could drop yourself of a building and we’ll come back in 30 minutes to see how you are doing?
leitmotif:
I have worked professionally on flywheel energy storage systems, which are always in a vacuum. They are called “energy storage” systems for a very good reason.
On a macroscopic scale with motion, it is impossible to eliminate dissipative losses completely, but you can come pretty darn close: high vacuum and magnet bearings in the case of my flywheels.
How about this example? I have a simple mass, no spring, and I raise it from one shelf to another shelf 10 cm higher. I have increased its potential energy, and it can hold it indefinitely. It is not used up.
But the bigger problem for Zoe is that none of the dissipative mechanisms you are concerned about operate at the molecular level. It is absolutely true that an excited vibrating molecule will stay excited until it emits a photon or collides with another molecule. (I had a couple of professosrs who loved to catch people like you in errors confusing macroscopic dissipation with molecular cases just to make a point for the whole class.)
“I have increased its potential energy, and it can hold it indefinitely. It is not used up.”
When the sun comes up in the morning, the atmosphere indeed inflates. Indeed some vibratory energy is used to “defeat” gravity.
As the sun sets and disappears for the night, all of this is REVERSED.
For an annually AVERAGED day, there is NO storage of energy over 24 hours.
Of course this comment does not take into account external things like solar/cloud changes.
CO2 vibrates in accordance to an external source. As the source is removed the vibration slows down and ceases.
Interesting how you would specifically focus on 1/6th of all motions possible: anti-gravitational (upward) motion, and nothing else. And yet even in this case, you are wrong. Upward inflation does occur, and its subsequent downward deflation follows.
leitmotif,
They are so desperate. They have a small set of assertions (assumptions) they repeat ad nauseum.
Zoe:
Wow! Another thing you get completely wrong. The atmsophere cools (and so “deflates”) at night because the solar radiative input has stopped, but it is still radiating outward to space.
You claim this does not happen, but we have excellent satellite spectroscopic data for both magnitude and wavelength/frequency showing that it does.
It is the gas molecule radiating away a photon that lowers its energy level, which when happening in a whole section of the atmosphere results in lower temperatures. Conservation of energy operates at the molecular level as well. You seem completely oblivious to this fact.
Once again, this is completely introductory material for the topic, but you simply cannot grasp it!
In ZoePhysics, energy is created and destroyed whenever it needs to be, in order to make Zoe’s ideas work.
“leitmotif,
They are so desperate. They have a small set of assertions (assumptions) they repeat ad nauseum.”
Zoe, the applied maths given by Ed Bo about the spring and the ghg molecule’s ability to store energy are both totally ludicrous.
Now he is expending energy raising a weight on to a shelf.
Of course, back radiation boy, MarkW is never far away with this sophistry.
Usually, there are only two jokers in a pack of cards but I fear they are about to break that rule too.
Ed Bo is completely uneducable.
His evidence that molecules emit radiation to space is evidence that molecules radiate to a satellite (matter). What an epic fail.
“Conservation of energy operates at the molecular level as well. You seem completely oblivious to this fact.”
Yes, energy is conserved. The sun makes molecules dance in real time. Take away the sun, the molecules slow down.
Eat food and you can move furniture. Conservation of energy. Don’t eat food for a while and you can’t move furniture.
The sun forces mechanical motion in matter. That is its energy in action. There is no further emission to nothing, but a satellite, yes of course.
“Usually, there are only two jokers in a pack of cards but I fear they are about to break that rule too.”
haha that’s good
Mark,
Energy isn’t perpetual.
Even consensus science says the big bang gave us some fixed energy and eventually the universe will die a heat death.
When the sun runs out of its supposed nuclear fuel, it’s gone too! No more energy, cools down and “dies”.
Do tell me I’m wrong because a Joule is “perpetual”. lol
Zoe:
You are so confused you don’t realize that you contradict yourself all the time.
You say: “Yes, energy is conserved. The sun makes molecules dance in real time. Take away the sun, the molecules slow down.”
When “the molecules slow down”, their energy level is reduced. You claim they can do this without transferring energy away from themselves. That would mean that energy is NOT conserved.
Let’s consider my example of the radiative sensing satellite. Let’s say it is in geosynchronous orbit, 36000 km up. (The exact height does not matter.) It takes over 100 milliseconds for radiation from the surface to reach the satellite. During that time, the satellite will have moved about 2 km.
Is it your claim that radiation works like a start NFL quarterback, anticipating where the target will be once the speed-of-light delay is accounted for?
At the moment the satellite absorbs the radiation, how is it communicated backwards in time to the surface 100 msec before that this was an actual transfer?
leitmotif:
You have complete scorn for the concept of “back radiation”, labeling it “sophistry”.
Please take a look at the textbook MIT uses to teach heat transfer to engineering students. A free download can be found here:
https://ahtt.mit.edu/
The introductory 1st section is entitled “The General Problem of Heat EXCHANGE”. On book page 31 (file page 43), you see the heading “Radiant Heat EXCHANGE”, with the equation for “Qnet” expressing the bidirectional nature of radiation heat transfer.
For more detail, you can look at Section IV on “Thermal Radiation Heat Transfer”. The first heading in this section is entitled “The problem of radiative EXCHANGE” (book page 539, file page 551). It goes into more detail in equations for Qnet.
The treatment of radiation heat transfer in the text is ENTIRELY based on the concept of EXCHANGE — as in FORWARD and BACK.
This is the same way texts taught the subject when I studied thermodynamics at MIT many years ago. It is also how EVERY text on the subject I have seen since explain it.
My question to you is: Do you think MIT (and virtually every other engineering school) is teaching its engineering students fundamentally WRONG treatments of heat transfer (for the last century)?
And if so, are you concerned about engineering disasters resulting from this error? (Where are they?)
Ed … “This energy is stored until the molecule either collides with another molecule (most common), transferring energy to that molecule, or emits a photon.” is incorrect. You are confusing a ‘vibrational’ shape change with a kinetic motion. The incident photon’s cold energy interacts with the internal bonding of the (CO2, as example) molecule. The atomic centres (+) are moved as the internal bonds flex. This is not a kinetic movement of the molecular unit, it is not a temperature alteration. Also, as velocity is a vectored unit and altering direction is an acceleration, such interactions have a greater chance of presenting a lower overall kinetic velocity i.e. excitation has a great chance of leaving the CO2 molecule COOLER. Thus, in terms of seeking of a thermal equilibrium, CO2 will remain cooler than the surrounding atmosphere but upon being warmer is more likely to release a photon. Thus, CO2 will persistently cool by photonic release and persistently scavenge kinetic velocities (temperature) away. Increase of atmospheric CO2 is, in this way, a cooling process.
Peter:
If you understood the principle of conservation of energy at all, you would realize that a CO2 molecule that absorbs a photon must increase its energy level by the exact amount of the energy carried by the photon (e=h*v).
You make a big deal of the fact that the key excitation mode for CO2 absorbing in the 14-16um range is vibrational, but neglect to note that in the almost inevitable collision with another molecule (>99% chance of happening before a photon emission), it will translate this energy into translational motion of the molecules.
This well-known (to most people) process is called “thermalization”, and so we know that this two-step process does indeed increase the internal energy and therefore the temperature of the gas. As the temperature increases, the chance of CO2 molecule getting into the excited state that could emit an IR photon in this range increases.
Remember that we are comparing this to the case of “transparency”, where the photon, and the e=h*v energy it carries, pass directly on to space unimpeded.
I’m afraid your ideas are not compatible with conservation of energy. It is the FIRST thing you should check for.
>>
EM radiation only flows from matter to matter.
<<
Nonsense.
Jim
If a tree falls in a forest and there is no-one to hear it, then it doesn’t make a sound.
Not true, since sound is compression and rare faction of air.
Remove the air and there is indeed no sound.
“First of all, the temperature of anything depends upon the rates of energy GAIN and energy LOSS.”
NO! The temperature of anything depends upon the average translational kinetic energy in that spot.
If something colder appears down the line, there is no gain in kinetic energy for our spot.
Less kinetic energy elsewhere does not cause more kinetic energy here.
Just because you want to believe in conservation of heat flow doesn’t make it physics.
“Your clothes in winter (or summer) keep you warmer than if you had no clothes on, even though the clothes are cooler than your body temperature. ”
If the argument was that GHGs prevent the surface from cooling below what the sun could make it alone (-18C), that would be one thing, but that is not what is claimed. What is claimed is 33C above what the sun could make it.
You’re effectively saying, by analogy, that clothes make you hotter than what your body temperature can make it.
If that’s not what you’re saying, then your analogy is inapt and purposefully misleading.
http://phzoe.com/2020/04/08/do-blankets-warm-you/
Yes, the kinetic energy of a point is determined by the energy in that point at this particular instant in time. So what.
The rate of energy flow in, vs energy flow out determines how much kinetic energy is in that particular point at any particular time.
If the rate of energy out over time decreases while the rate of energy in stays constant, than your particular point will warm, until the balance does equal again.
Your understanding of the analogy is even worse than your understand of first year thermodynamics.
Mark,
Your acting as if a POINT has two doors. Can you please diagram that?
Let’s assume energy at a point is 273 J. Let’s assume mass and Cp = 1. Your point is obviously at 273 K.
Now you’ve decided to overcomplicate things with inflows and outflows to a POINT. How a point could have dimensions to allow that will be ignored for now.
1) You have 273 W flowing in and 0 W flowing out: What’s the Temperature?
2) You have 273 W flowing in and 273 W flowing out: What’s the Temperature
3) You have 273 W flowing in and 173 W flowing out: What’s the Temperature?
4) You have 273 W flowing in and 100 W flowing out: What’s the Temperature?
If you do not answer these, then you concede you have no idea what you’re talking about.
“Your acting as if a POINT has two doors.”
If that’s what you think, no wonder you are so confused.
1) Depends on what the initial temperature was and how long that situation has been in place, the temperature is rising all the time.
2) Depends on what the temperature was to begin with, the temperature is unchanging.
3) This is the same scenario as 2)
4) Depends on what the initial temperature was and how long that situation has been in place, the temperature is rising all the time.
The fact that you can ask such stupid questions, proves that you don’t know what you are talking about.
The correct answer to all 4 questions is 273 K.
Learn science. I espouse conservation of energy – real physics, and you espouse conservation of hear flow – junk science.
I can explain why an electric water heater will easentially heat all the water to the same temperature.
Meanwhile, your cranky self is calculating the surface area of the coils and comparing it to the surface area of the container – and getting a weird parabolic steep gradient as your steady state prediction.
No depends, Mark. You have all the information needed to compute the temperatures. You’re struggling because your conservation of heat flow is retarded nonsense.
You literally can not hold onto the idea that temperature is based on kinetic energy in a spot (real physics) AND a balance of energy in/out at the same time.
It’s impossible. The best you can do is say energy in a spot = energy in. What is further down the line makes no difference.
Look at the entropy law:
dS = Qrev / T
If T increases, entropy decreases.
Entropy must always increase!
Total fail.
“The correct answer to all 4 questions is 273 K.”
Zoe, given enough time the answer to 1) is infinite temperature. A singularity, so cannot happen in real thermo. experiment. You have posed an ill-defined question.
Wrong. Not infinite. Exactly 273K.
The 273 W is making molecules dance with 283 J every second. m = Cp = 1. T = 273 K.
The energy is USED UP in REAL TIME for molecular mechanical motion.
There is no accumulation of motion for further motion, as you suggest.
Sigh.
Quack mathemeticians lost sight of physics.
>>
Zoe Phin
June 22, 2020 at 2:47 pm
<<
Trying to deal with your nonsense is like playing Whack-a-Mole.
>>
Look at the entropy law:
dS = Qrev / T
If T increases, entropy decreases.
Entropy must always increase!
Total fail.
<<
Yes it’s a total fail alright. You don’t know what you are talking about. The definition of entropy is:
It’s defined as an equality only for reversible processes. For processes in general it’s:
>>
Entropy must always increase!
<<
No, not true–it’s more nonsense again. The second law only applies to isolated systems. In an isolated system (the Universe as a whole is considered to be an isolated system), the change in entropy is:
In an isolated system, the entropy will increase until the system comes to equilibrium, at which point the change in entropy will be zero–at equilibrium.
In an open or closed system, the entropy can increase or decrease, depending on the direction of heat transfer.
Jim
Incorrect as usual, Zoe old girl. If you pump energy into something, it heats up. Ask any microwave.
Zoe, the only quack here is you.
“The energy is USED UP in REAL TIME for molecular mechanical motion.”
Used up? Zoe, energy cannot be used up. Energy can be converted to another form. The conversion in form means the mechanical motion increases over time without bound in your 1).
Jim,
You’re a sophist.
You can’t get to an equilbrium because YOU want to conserve heat flow.
Our system is sufficiently isolated for our consideration. We do not get much interfering radiation outside our solar system to invalidate my MAIN point.
We ARE talking about a reversible process. That is what your crackpot backradiation theology is all about.
Your stupid criticism shows you lack integrity.
Mark, the idiot,
The microwave does not output more energy than the electricity you supply at the time.
The max temperature is the max temperature. There is no storing of motion by microwave contents to be used later for more intense motion.
The input controls the max temperature. The output can never control the input.
The contents will be radiated to the max, at which point thermal equilbrium is established.
Your conservation of heat flow makes equilbrium impossible. Ever.
Mark, Jim, et. al., to understand Zoe just ref. urban dictionary def. of Phin: The greatest person to ever exist in history who is vastly superior to every single person in existence.
Jim, according to some here, if entropy doesn’t increase, then work is involved and everything should be heating up.
According to Zoe, actual physics is just sophistry.
As to the micro-wave, your ability to go right past the point into yet another fantasy of your own invention remains unsurpassed. The point was that the micro-wave energy, in the form of photons, heats water. Something you have been claiming can’t happen.
“Something you have been claiming can’t happen.”
Lying scum
>>
NO! The temperature of anything depends upon the average translational kinetic energy in that spot.
<<
This is an incorrect statement from the kinetic theory of gases. Most here are talking about Thermodynamic temperature. The two temperatures (kinetic vs. Thermodynamic) are not always equal when referring to the same system.
Jim
Temperature, that is kinetic energy, is not exclusive to gases.
Your term “thermodynamic” sounds like a euphamism for the unscientific doctrine of conservation of heat flow.
So you claim to have overthrown the laws of thermodynamics?
Conservation of heat flow is not a law of thermodynamics.
Q tends to zero. It’s not a value you must conserve. It’s not conserved.
As usual, Zoe redefines everything she can’t figure out for herself.
>>
Temperature, that is kinetic energy, is not exclusive to gases.
<<
No, but your definition (though wrong) is.
I refer to volume I of “Fundamental Formulas of Physics” by Donald H. Menzel. In chapter 12 on Kinetic Theory of Gases, page 293, we have the following:
The kinetic theory temperature T is defined by
whether or not the gas be in thermodynamic equilibrium.
Where k is Boltzmann’s constant, T is the absolute temperature, m is the mass of a gas particle, and C is the random velocity of a gas particle; but here it’s the average random speed squared.
What’s interesting is that this expression can be derived from the Ideal Gas Law (who my professors warned only applied to ideal gases). The Ideal Gas Law is based on Charles’s and Boyle’s laws which in turn are using the thermodynamic definitions of temperature and pressure. However, further down there’s this statement: At thermodynamic equilibrium:
If we multiply by 3/2 we get
Anyone with some physics training will recognize the term on the right as the expression for kinetic energy. The term on the left is interesting–it’s the energy of a gas particle with exactly three degrees of freedom. A gas particle can have translational motion, rotational motion, and vibrational motion. The equipartition theorem assigns to each degree of freedom. Because we are dealing with basically a monatomic gas, an ideal gas particle only has three degrees of freedom.
to each degree of freedom. Because we are dealing with basically a monatomic gas, an ideal gas particle only has three degrees of freedom.
Now we see that the statement: “temperature of anything depends upon the average translational kinetic energy” isn’t exactly correct. A more correct statement is: “temperature is proportional to the average (translational) kinetic energy of an ideal gas particle.” But that’s not really correct either. An even more correct statement would be: “temperature is proportional to the (translational) kinetic energy of the average speed of an ideal gas particle.
>>
Your term “thermodynamic” sounds like a euphamism for the unscientific doctrine of conservation of heat flow.
<<
I’m referring to thermodynamics as a branch of physics.
Jim
Using actual physics. If you are not careful, Zoe will accuse you of being a sophist again.
Okay Jim, you may (along with Zoe) be one of the few people here with enough physics knowledge to answer this question. What is the vertical temperature gradient of an isolated gas column in a gravitational field? Remember that we are talking about a real gas here, not an ideal one, so collisions between gas molecules are significant. Let’s say the gas is nitrogen for simplicity, and the temperature at the bottom of the column is room temperature (so the gas won’t condense, freeze, or do anything else inconvenient), and the gas has been allowed to reach equilibrium. I’m not looking for the numeric value of the gradient, just the sign of the gradient will do.
>>
Steve Keppel-Jones
June 24, 2020 at 12:47 pm
<<
As a retired Navy pilot, off the top of my head I would say it’s the dry adiabatic lapse rate. So looking it up, I get 3 degrees C per 1,000 feet. If we’re at roughly room temperature at the surface (about 20 degrees C–let’s make it 21 degrees C), then we get to the freezing level at about 7,000 feet.
However, it’s not that simple. The lapse rate only applies to the Troposphere. At the Tropopause, the temperature does not drop as you go higher. In the Stratosphere, the temperature increases with altitude. It’s not a matter of simple physics, and I’m not an atmospheric physicist.
True, dry air is not pure nitrogen, but it’s close enough for government work, 78%.
Jim
Jim, yes, the magnitude will be something along those lines in Earth gravity. But notice that I stipulated that this column of gas is isolated, i.e. there is no energy coming in or out. You are correct that at equilibrium it will exhibit a thermal (temperature) gradient (along with a pressure gradient, of course). Now see if you can explain why.
It would be nice if we had that edit feature back. That should read 7.000 feet AGL.
Jim
>>
Now see if you can explain why.
<<
I didn’t know it was a test. In that case, I will decline.
Jim
Jim, “I didn’t know it was a test. In that case, I will decline.”
Well, no one can force you to think. But you are not making a very good case that you are properly equipped to quibble with Zoe’s physics. If you do agree that an isolated column of non-IR-interacting gas at rest will exhibit a thermal gradient in a gravitational field, then you are basically agreeing with Zoe that the CO2 radiation greenhouse effect is a minor factor in the atmosphere temperature profile, at most.
Jim 1:40pm, Menzel has led you to the total U of a system of N noninteracting, structureless gas atoms, just multiply by N:
U=3/2 NkT
Heat capacities defined Cv=3/2Nk, Cp=5/2Nk and their ratio gamma 5/3 = 1.666. Measured gamma values for atomic gases He,Ne,Ar, krypton, and xenon are indeed about 1.66 which is comforting. Classical mechanics equipartition theory works in the lab.
Menzel then likely takes you to N2,O2 where gamma was measured 1.4 at the turn of last century. Uh-oh, diatomic molecules are different, they have structure. There you will find the equipartition theorem is true only to the extent classical mechanics is true. Classical mechanics & equipartition will be found to predict N2,O2 gamma at 1.25 but alas as I wrote experiments found N2,O2 gamma was about 1.4.
Here Zoe should pay attention, see her source. That was quite a huge conundrum beginning of 1900s lasting decades of hard experimental work. To make a very interesting, long, and largely forgotten, story short, the quantum spacing of the energy levels shown by Zoe’s source “mode is quantized” was the answer to why diatomic gas gamma was measuring 1.4.
For all who write about emission of photons in the atm., you know who you are, this story should be completely understood for accuracy in writing about AGW.
Steve: You ask: “What is the vertical temperature gradient of an isolated gas column in a gravitational field?”
The answer is that there is no temperature gradient – its value is zero. You can consult Richard Feynman’s CalTech Lectures on Physics, Vol 1, No. 40. He considers it trivial.
(I would post a link, but whenever I do, it does not seem to get through moderation. But it’s trivial to search for.)
The great physicist James Clerk Maxwell figured this out in the mid-1800s, realizing that any other result would lead to violations of the 2nd Law of Thermodynamics. Maxwell used a similar proof to Feynman, but with two columns of different gases with different Cp values. The two columns are thermally well connected at the bottom so they are at the same temperature.
If they have different lapse rates due to different Cp values (the adiabatic lapse rate is –g/Cp), then they would have different temperatures at the top, and a heat engine connected between the tops could continually create work. This would be a blatant violation of the 2nd Law of Thermodynamics.
This proof can be found in his work “Theory of Heat”, 1872.
Lapse rate or not, the idea that static atmospheric pressure can elevate the surface temperature is even easier to disprove. For it to elevate surface temperature and compensate for the increased surface power output, the atmosphere must continually transfer power to the surface by its pressure.
It’s a simple matter of basic high school physics, that the energy transfer from force (pressure time area) is the force times the distance of motion the force creates. (Work = Force * Distance).
The rate of power transfer is Force * Velocity. But here the distance and velocity are zero, because the surface is not dynamically compressing. This means that the work and rate of power transfer are zero.
That is all you need to know that the so-called “gravito-thermal” effect is nonsense.
Oh, and with Zoe, you are best off assuming that every assertion she makes is absolutely wrong. In my decades of practicing and teaching in these fields, I have NEVER run into anyone so fundamentally confused.
”The answer is that there is no temperature gradient – its value is zero.”
Ed Bo, later research, after Maxwell and Feynman, showed their reasoned zero-lapse solution to this particular air universe’s “heat death” actually has lower entropy than another lapse solution that was found so was not final equilibrium & not this universe’s “heat death”. That improved solution for T(p) was also proved to be the max. entropy possible so the rod connection at the top would cease to produce entropy thus no perpetual heating.
Trick:
Reference please. I still don’t see any other solution satisfying the 2nd Law.
I still see physics professors in the 21st Century present the zero-lapse case to students as a non-intuitive but definitely correct analysis.
Do any of your sources argue that the natural lapse rate occurs on a macroscopic scale, or that this lapse rate is anywhere near adiabatic?
The classical isothermal solution and 2004 updated non-isothermal profile & discussion is presented. Both are fairly close in total entropy, 2004 higher. As is the case for the GHE, source debates have been prolific. Can’t test with perfect insulation.
https://journals.ametsoc.org/jas/article/61/8/931/25722/On-Maximum-Entropy-Profiles
Trick:
Thanks for the paper. It looks like a good exposition to me on first reading. I will do a fuller reading later.
However, I see nothing that contradicts the idea that the equilibrium (maximum entropy) state of a thermodynamically isolated column of a gas in a gravitational field will be isothermal. The so-called “gravito-thermal” theory states that gravity ALONE will tend to cause the adiabatic lapse rate, and so requires that an isolated column tend toward this lapse rate.
I fully agree that when convection is in play, the atmosphere will tend toward the adiabatic. But external interactions are required to drive this convection.
Ed Bo, in the derivation of the DALR, temperature(z) is pulled outside the integral and assumed constant wrt to z height. This causes the DALR “gavito-thermal” stuff to be very poor discussion material for the maximum entropy profiles where the integration of temperature wrt to height z or pressure(z) is handled mathematically much better.
Ed, Feynman and Maxwell were both wrong on this point. (I think Feynman later figured out that his first explanation was wrong, but I’m not sure about that.) His counterexample fails on two obvious fronts: one, there is no way to transfer heat upwards outside your column of interest without converting some of it to potential energy; and two, if you have two side-by-side columns with different Cp and therefore different lapse rates, sure, you can extract some energy from the difference for a little while, but in doing so, the temperature differential between the columns will decrease, in accordance with the second law. Eventually one or both columns will stop being a gas, and you can no longer extract energy from the temperature differential. This stuff is really not that complicated.
Here’s a simple thought experiment to help you fix your faulty intuition. Imagine an empty column with one gas molecule in it. It is bouncing up and down. At the bottom of the column, it has a speed of a couple of hundred km/h, like a room temperature gas molecule. It can bounce a certain height upward, until it runs out of kinetic energy, and then it will fall back down again. What is the temperature of this “gas” at the bottom of the column, and what is the temperature at the top? Now slowly add more molecules, one by one, and think about the lapse rate as you go. At what point does the lapse rate suddenly disappear, according to your view, and why?
Always remember that temperature measures only kinetic energy, but total energy in a gravitational field includes both kinetic and potential energy. At different heights in the gas column, the mix of kinetic and potential energy varies, but the potential part is invisible to a thermometer. The column has an *energy* lapse rate of 0, but a *temperature* lapse rate that is not 0.
”Feynman and Maxwell were both wrong on this point.”
Not at all, they were rigorously correct as early as 1876. The debate has continued to this day arising from defining the problem detail constraints consistently inconsistently. Medium google fu should find you the required reading on maximum entropy profiles for a universe of one column of STP air.
Steve:
I’m afraid it’s obvious that you have never been exposed to statistical mechanics, or you wouldn’t make the foolish arguments you do. This scenario was actually a key factor in developing the whole science of statistical mechanics. Single ball/molecule examples are not relevant — it is the overall interacting population of molecules that matters.
This was key to developing a lot of modern science. Gibbs, Maxwell, and Boltzmann were all heavily involved. You do not get the Maxwell-Boltzmann distribution equations without it.
Please read the paper Trick linked above. It confirms (you can skip to the conclusion if you want) that the equilibrium (maximum entropy) state for an isolated column is isothermal.
You are not even following your own logic in analyzing Maxwell’s thought experiment. Your argument is that a column will always tend to the adiabatic lapse rate, so with the bottoms well tied together, the difference at the top would remain, even as the overall temperature levels reduce. So you would continually be extracting energy, in violation of the 2nd Law.
Here are Maxwell’s actual words:
“We find that if a vertical column of a gas were left to itself, till by the conduction of heat it had attained a condition of thermal equilibrium, the temperature would be the same throughout, or, in other words, gravity produces no effect in making the bottom of the column hotter or colder than the top.
This result is important in the theory of thermodynamics, for it proves that gravity has no influence in altering the conditions of thermal equilibrium in any substance, whether gaseous or not. For if two vertical columns of different substance stand of the same perfectly conducting horizontal plate, the temperature of the bottom of each column will be the same; and if each column is in thermal equilibrium of itself, the temperatures at all equal heights must be the same. In fact, if the temperatures of the tops of the two columns were different, we might drive an engine with this difference of temperature, and the refuse heat would pass down the colder column, through the conducting plate, and up the warmer column; and this would go on till all the heat was converted into work, contrary to the second law of thermodynamics.”
Steve:
If you don’t want to go through integrals of the Maxwell-Boltzmann distribution, here’s a simpler argument:
As you go up in height in the vertical column, the pressure goes down. In an isothermal case (whether or not it is the equilibrium state), the density is directly proportional to the pressure.
Consider two adjacent slices across this vertical column. While it is true that each of the molecules in the higher slice has higher potential energy and the same (average) kinetic energy, there are fewer of them. So if you work out the math, the volumetric energy density of the two slices is the same, and the net energy transfer across their common boundary is zero — equilibrium!
If you consider what happens at this boundary, the molecules falling from higher to lower will have a higher average velocity, and so kinetic energy, than those rising from lower to higher due to gravitational acceleration, but there are fewer of them, so the kinetic energy transfer is the same in both directions.
To use a toy example, you may have 100 molecules pass upward through the boundary with an average kinetic energy of 99, and 99 molecules pass downward through the boundary with an average kinetic energy of 100. So the net energy transfer is 9900 – 9900 = 0.
”(The linked paper) confirms (you can skip to the conclusion if you want) that the equilibrium (maximum entropy) state for an isolated column is isothermal.”
Up through Feynman that was true. After that, their methods were shown to deliver less entropy than the maximum entropy which is obtained with a non-isothermal T(p) profile. The problem with Maxwell and Feynman is they never computed the entropy of the column. Or found the maximum entropy possible was higher than with non-isothermal column.
Oops…Or found the maximum entropy possible was higher with a non-isothermal column solution at thermodynamic equilibrium universe “heat death”.
”Imagine an empty column with one gas molecule in it. It is bouncing up and down.”
Steve has not controlled for the PE, that 1 molecule may not make it to the column top. In a gas filled column slice of the real atm., some molecules WILL always hit the top boundary as entropy increases to a max. Feynman and Maxwell missed that point also, they did not control for PE. In the paper I linked, they do control for PE in the column making the non-isothermal T(p) a better, more profound solution than the earlier isothermal constant T(p).
Ed, statistical mechanics is actually not required for this thought experiment.
Temperature is not a phenomenon that only magically appears when you accumulate the “right” number of molecules. If you think that it is, how many molecules do you need?
My arguments may or may not be foolish, but they do respect the laws of conservation of energy. Maxwell’s and Feynman’s attempts to disprove a gravitational thermal gradient by means of heat engines are, on the other hand, invalid, because heat engines can only extract a limited amount of work from a temperature differential. As work is extracted, the temperatures (and the temperature differentials) decrease. This process can obviously not continue forever, and the second law is never violated in the meantime. (The second law doesn’t prevent you from extracting SOME work from a temperature differential, and physical limits will prevent you from extracting an infinitesimal amount of work from an infinitesimal temperature differential.) Feynman, meanwhile, seems to have invented a hypothetical heat transfer device that can transfer heat (kinetic energy) vertically, infinitely fast, without converting any of it to potential energy. That’s quite a neat device, and I’d sure like to get my hands on one. I guess he based it on Maxwell’s equally impossible heat transfer substance.
I’m not sure where you’re going with explanations of the isothermal state being at constant vertical energy density, because that’s not the case I’m talking about. It sounds like you’re assuming your conclusion in order to try to disprove my point.
Here’s a question for you: what do you think the (total, not average, in reasonably small slices) potential energy gradient looks like in a vertical column? And then what do you think the (total) kinetic energy gradient looks like?
(Trick, I’m not sure why you think I forgot to control for PE, because it doesn’t matter where the “top” is, and I don’t think it’s possible to define one rigorously. Wherever you think the top is, sooner or later a molecule will randomly pass through it, if you have enough molecules. In my single-molecule case, sure, but the kinetic and potential energy gradients are well-defined up to however high the molecule goes.)
The paper Trick linked says, among other things, “a common misconception was that gravity would change the nature of thermodynamic equilibrium so as to create a vertical temperature gradient”. Where is this disproved? Does gravity create a potential energy gradient, or not? And if it does, what does that mean for the kinetic energy gradient if total energy is to be equally distributed at equilibrium? And how exactly does everyone think temperature (actual temperature, not potential temperature) is related to that kinetic energy gradient?
”I’m not sure why you think I forgot to control for PE”
When your molecule doesn’t make it to the top, D(potential temperature)/Dt is infinite in the empty volume element above which is a singularity. You need to control for the integrated potential temperature to be constant. Can’t do that with one molecule.
Steve:
Here’s a pro tip: Think long and hard before claiming that two of the top physicists of all times were wrong on a very basic point of physics. It will save you from making a fool of yourself.
In the real world, if there were an initial temperature difference between the tops of the columns of Maxwell’s system, the heat engine could extract work while driving the difference to zero, and it would stop.
But in your imaginary world, gravity would automatically keep re-establishing the temperature differentials in the columns, allowing the process to continue extracting work from the two-column system without rejecting any heat from it. That is a blatant violation of the 2nd Law.
I am puzzled by your objection to Feynman’s example. Do you really think that if there were a temperature gradient in a vertical column, that a vertical metal member would NOT conduct heat to tend to reduce that gradient? Nowhere does he claim that it would be “infinitely fast”. Where is the potential energy in conducting heat in a solid vertically???
Your fundamental argument is that gravity creates a positive vertical potential-energy gradient that, in equilibrium, must be balanced by a negative kinetic-energy gradient, and so negative temperature gradient.
But, as I pointed out earlier, you are ignoring the pressure and density gradient here. So, while a molecule in the upper slice has a higher potential energy than a molecule in the lower slice, there are fewer molecules in the upper slice, so you do not actually have this potential-energy gradient between the slices. So there is no need for a kinetic-energy gradient to offset this.
”That is a blatant violation of the 2nd Law.”
It is not. The column is a universe all to itself. Entropy would increase to a maximum and no further entropy could be produced so universe “heat death” would occur and no energy could be extracted from the wire tops in Maxwell’s universe even with a non-isothermal column at max. entropy..
Feynman and Maxwell did not foresee that a higher entropy solution would be found with the non-isothermal column by invoking the 2LOT thus their solution was not their universe’ actual “heat death” point, the isothermal column can still make entropy.
That is found in Verkley’s T(p) solution discussion in 2b.
Trick:
The paper YOU linked states very clearly that the equilbrium state for an ISOLATED column in a gravitational field is isothermal. Have you actually really read it? Please (re)read the first paragraph of the conclusion.
That was Maxwell’s and Feynman’s claim. Both immediately emphasized that the claim did not hold for a non-isolated atmosphere such as our own. Verkley makes no claim to overturn Maxwell — he specifically acknowledges that “this indeed brings us to the broader framework discussed by Maxwell”. This broader framework refers to NON-ISOLATED cases like our own.
In the section of Maxwell’s work immediately after the one I quoted above, he goes into a great deal of detail about the convective case, so much so that I see many people forget his claims about the isolated case.
The only real reason that the isothermal case is important even though real atmospheres are non-isolated and non-isothermal is to be able to understand why they do not tend toward isothermality — gravity ALONE cannot do it.
On the Maxwell’s dual-column case, remember that the work produced by the heat engine acts on the world external to the columns (so it is NOT a “universe all to itself”, as you assert). Reread Maxwell’s argument carefully from the section I quoted above. He gets the 2nd LoT correct — you do not!
Did I read it? “This (column isothermal) is the established classical result” of Gibbs, Maxwell, Feynman. “Here, we have taken, following Ball (1956) and Bohren and Albrecht (1998)” in 2b which “together lead to a (non-isothermal ) temperature profile that corresponds remarkably well to the tropospheric part of the Standard Atmosphere.”
If on the Maxwell’s dual-column case, remember that the work produced by the heat engine acts on the world external to the columns then it acts like every other heat engine. Somewhere a heat engine will run until the actual universe heat death.
That was obviously NOT Maxwell’s intention to maximize entropy on a universal scale only the isolated column scale “one considers an ideal gas in a gravitational field and seeks the state of maximum entropy” of the column NOT the entire universe.
Trick:
The “Standard Atmosphere” is NOT ISOLATED!!!!!
Steve and many others like him believe that gravity ALONE will create a large negative lapse rate, which means that they think a thermodynamically ISOLATED column of gas in a gravitation field will have this lapse rate in equilibrium.
I point out that the great physicists Maxwell and Feynman argue that an ISOLATED column of gas will be ISOTHERMAL at equilibrium.
You point to a recent paper that also concludes that an ISOLATED column of gas will be ISOTHERMAL at equilibrium.
Then you point out that this same paper says that NON-ISOLATED columns will NOT be isothermal, somehow believing that this is a counter-argument.
You are just wasting everybody’s time due to your logical confusion.
”even though real atmospheres are non-isolated and non-isothermal”
Earth’s atm. is isothermal for about 9or10km above the midlatitude tropics tropopause. Does that mean that layer achieved max. entropy? No, the fluid beyond the tropopause for that distance became warmed from above so convection ~ceased.
Ed Bo, did you read the paper? Section 2a is the classical isothermal solution of Gibbs, Maxwell, Feynman.
Section 2b is the updated more advanced higher entropy non-isothermal solution of which Gibbs, Maxwell, and Feynman were unaware. The conclusion discusses 2b non-isothermal T(p) as the “remarkably” better solution of the two.
Trick:
No Trick, Section 2b is for a DIFFERENT CASE! The NON-ISOLATED case! (There cannot be ongoing convection in an isolated system.)
They say it “corresponds remarkably well to the tropospheric part of the Standard Atmosphere.” The Standard Atmosphere is NOT ISOLATED!!!
I don’t know how many times I have to say it — my argument was about the ISOLATED case, to demonstrate that gravity ALONE does not create a temperature gradient. Maxwell and Feynman both agree with this.
Both Maxwell and Feynman explicitly state that this is not the case for our own actual atmosphere, so your claim that there is a “more advanced higher entropy non-isothermal solution of which Gibbs, Maxwell, and Feynman were unaware” is simply garbage. (I’d use a stronger term, but I will respect the standards of this blog.)
You are repeatedly demonstrating that you are a person incapable of a logical train of thought. You do not have any kind of solid foundation to deal with these subjects.
Ed: Don’t worry, I have thought long and hard about this, and I don’t believe I am making a fool of myself. Smarter people than me came up with these ideas. I have great respect for both Feynman and Maxwell, but that doesn’t mean they’re infallible, nor indeed that they were wrong about a “basic point of physics”. Clearly this is not a “basic point”, or it wouldn’t have confused so many smart people for so long.
A couple of important points: 1) a heat engine cannot extract enough work to drive the temperature differential (any temperature differential) to 0. Carnot proved this. Not even a theoretically perfect Carnot heat engine could do that, and a real heat engine is always less efficient than that. Heat engine efficiency is always less than 1 – Tc/Th, and as Tc approaches Th, efficiency approaches 0 faster. So you can’t use a heat engine to disprove a gravitationally induced thermal gradient. Yes, you can extract *some* work from a gravitationally induced thermal gradient. No, you can’t extract *all* of it. (Maybe you should check with the cold-temperature physicists. They spend a lot of effort cooling things down, and they don’t make an energy profit in the process. They certainly would if they could.)
2) It is true that Feynman did not claim infinite rates of heat transfer, but you can tell he was thinking it. Regardless, you should think very carefully about how heat (more accurately, temperature) in a solid material is transmitted upwards. Temperature in a solid material is a measure of the vibrational kinetic energy of individual molecules. How do you think an individual atom transmits kinetic energy to an atom above it? Do you think the entire amount of kinetic energy can be transmitted upwards without converting any of it to potential energy? If so, you need to study kinetic energy, potential energy, and gravitational fields a bit more closely.
3) Yes, my fundamental argument (it is not originally mine of course!) is that a vertical column of gas in a gravitational field will have a potential energy gradient. This is true for any type of matter, not just columns of gas. So, since you have now claimed that it won’t, we are getting somewhere! Wonderful! You have claimed this based on the hypothesis that the reduction in density (and pressure) exactly cancels the increase in potential energy (relative to, say, Earth’s surface) due to height. In order for this to be true, according to my math, the relationship between density and height (above surface) would have to be d=1/h. (Potential energy of a slice of the column is m*g*h*n, where n is number of molecules, if we take g to be more or less constant over the altitude of interest – if you want to fiddle slightly to account for the variation in g, go ahead. Of course m as the mass of a molecule of gas is also constant.) So, if you double h, you have to cut n in (approximately) half to retain a constant potential energy. Do you think the density profile of either our atmosphere or my notional GHG-free isolated column is 1/h?
The temperature of matter is linearly dependent on the amount of Joules stored by it. Solids store that energy as mechanical vibrations of the atoms comprising it. Gasses are different where the stored energy becomes that of translational motion. Gases don’t emit Planck spectrums, even at cosmological scales, and only emit and absorb photons with very specific energies. They loose energy by collisions with colder matter, rather than by radiating it away as photons since only those atoms not already in the ground state can radiate photons returning them back to the ground state.
If the energy of the mechanical vibrations of individual atoms in a solid responsible for its BB emissions is quantized, it’s at the Planck scale and for all intents and purposes can be considered a continuous range. The quantization of energy absorbed and emitted by gas molecules is at a far, far larger scale that can not be ignored.
You can even conceptualize the Planck spectrums emitted by matter to arise in the limit of infinite collisional broadening of the absorption/emission lines of the gaseous form of that matter.
”Gases don’t emit Planck spectrums”
Sure they do. There is no identifier for state of matter in the Planck function so it applies to all states of matter. The Planck function is worthy of great respect, if not awe. Three constants of nature!
Trick,
What’s the origin of the energy in the emitted photons? All of the thermal energy in a gas in the ground state is in the form of translational motion. How do you convert this energy into a photon? The distribution of translational energies is about the same as the distribution of the required photon energies, so in that respect, a Planck distribution of energy is still present.
If a molocules translational energy was converted into a photon, its velocity would drop to zero. In order to produce the highest energy photons, the translational energy of more than one molecule would need to be converted. In a liquid or solid, the photons are emitted by the electron shells by the same mechanism that produces line spctra, except that collisional broadening is for all intents and purposes infinite and the discrete line spectrum morphs into a continuum.
So, if you get a gas hot enough where collisions have enough energy to energize gas molecules and collisional broadening becomes a very large effect, the line emissions will converge towards a Planck distribution. The Earth’s atmosphere is no where near energetic enough to emit anything but the discrete line spectra of gas molecules not in the ground state.
The bottom line is that for Earth, the N2 and O2 in the atmosphere are irrelevant to the radiant emissions of the planet.
The Earth’s atmosphere is nowhere near energetic enough to emit anything but the discrete line spectra of gas molecules not in the ground state with very short film/ccd exposure times. Lengthen the exposure time and use a faster film or ccd to observe Planck distribution earth atm. at surface STP.
N2,O2 have measurable reductions in OLR, on global average under clear-sky conditions the OLR is reduced due to O2 by about 0.11 W/m^2 and due to N2 by about 0.17 W/m^2. Near as irrelevant as geothermal.
Trick,
The exposure time doesn’t matter. It’s not even something that you can measure with high certainty owing to the Planck spectrum being emitted by the surface overlapping the line spectra emitted by atmospheric GHG’s returning to the ground state.
When you count the emissions by clouds, which technically are part of the atmosphere, then you will observe a Planck distribution of LWIR photons, subject to GHG absorption between the cloud tops and space. But these emissions are from the liquid and solid water in clouds, not atmospheric gases.
The values you reported for OLR reduction by O2 and N2 are modeled incremental GHG absorption based on absorption lines far from where most of the energy is. None the less, geometry dictates that half of what was intercepted is re-mitted into space, while only the other half is re-mitted back to the surface.
”The exposure time doesn’t matter.”
Then you are no student of photography & spectroscopy. Of course, exposure time matters in spectroscopy e.g. Hubble stares at objects for really long exposure times to pick up enough photons across the spectrum.
Far from IR bands? No. The collision-induced fundamental vibration-rotation band at 6.4micron is the major absorption signature of O2 in the thermal infrared. N2 has two major bands influencing the infrared radiation: the collision-induced rotovibrational fundamental band at 2400 cm^-1 and the collision-induced rototranslational band at 100 cm^-1. Due to the atmospheric concentration of atmospheric N2,O2 even their weak infrared absorption can be radiatively quantified in the OLR.
Trick,
You still haven’t identified the mechanism for the Earth’s atmospheric gases to produce anything but a line spctra. Yes, collisional broadening morphs line spectra into Planck spectra, for example in the extreme case of a plasma. So yes, under the right extreme conditions, you can get a gas to emit a Planck spectrum, rather than a line spectrum, but the necessary conditions are only be found in the plasma of a lightning bolt. My point is that the N2 and O2 in the atmosphere are largely bystanders to the radiant balance. They may moderate highs and lows, but the averages, when measured in W/m^2 of emissions or as being proportional to T^4, would remain largely unchanged.
It’s the distribution of air molecule energies (v^2) that’s very similar to the Planck distribution of photon energies emitted by a BB at the same temperature.
Translation is quantized, but at the Planck level, however; the emissions of photons by molecules is quantized at a much coarser level and that must be honored.
Also, air includes all gases and most of what the atmospheric gases emits at TOA is in the absorption bands. In fact, the amount in each absorption band emitted into TOA is roughly half of the energy in that band that was absorbed by GHG’s. I can find no reference to an emissivity for N2. Only GHG’s like H2O and CO2 have an emissivity, which is actually temperature dependent.
Look here at lecture 38, especially slide 24.
https://my.eng.utah.edu/~whitty/chen3453/
”You still haven’t identified the mechanism for the Earth’s atmospheric gases to produce anything but a line sp(e)ctra”
Because I greatly fear the heavy lifting involved. And your link didn’t work.
So, spinach time, you have to eat some from time to time in field of spectroscopy. Realize whenever I get asked to do this, I refer y’all to the standard treatise on atomic spectra, by Condon and Shortley, fills 432 pages of text. Herzberg’s treatises fill 581 pages for diatomic molecules, 538 pages for polyatomic molecules. All that spinach & more is available for consumption but you can still live a good life without it. Basically, it all boils down to:
All the different lines of ALL the air constituents are separate but not completely: their tails overlap, and the sum of all this overlap gives a continuum background on which is superimposed a series of sharp lines.
—–
Some hints to incent picking up those tomes, it’s ok if most (all?) readers skip this, but I was asked:
Around sea level STP an appreciable fraction of air molecules may be in excited rotational energy states producing photons. A line-by-line radiative transfer code has to have the atm. spectrum divided into such small intervals (e.g., wavenumber intervals 0.001 cm^−1 or less) in order to capture the finest spectroscopic details.
The energy levels of a molecule depend on its structure and on the forces exerted on it by its neighbors (no molecule lives alone) and these forces depend on the everchanging distances between molecules. These interaction forces, often called collisions, result in perturbations of the energy levels of the isolated molecule hence the tails.
Carbon dioxide is a linear, symmetric molecule. By linear is meant that its bonds lie on a straight line. By symmetric is meant that it is composed of a carbon atom flanked on either side by oxygen atoms. This symmetry implies that the carbon dioxide molecule does not have a permanent dipole moment, which precludes this molecule from having what is called a rotational absorption band: an absorption band associated with rotation of the molecule unaccompanied by vibrations. But the carbon dioxide molecule can vibrate in such a way that it has a changing dipole moment. This in turn implies that this molecule, when vibrating in one of its vibrational modes, also can rotate to give a changing dipole moment. So carbon dioxide has vibration-rotational bands.
The different energy states are also states of different angular momentum. The photon carries quantized angular momentum. Thus, when a photon is absorbed by a molecule, and it undergoes a transition to a higher energy level, the transition must be such that angular momentum is conserved. If one unit of angular momentum is annihilated (so to speak) when a photon is absorbed, the molecule must increase its angular momentum, and hence energy state, by the same amount. And similarly, for emission: when a photon is emitted, the one unit of angular momentum created upon the birth of the photon must be compensated for by the same decrease in the angular momentum of the molecule.
The distribution of frequencies, called a line shape, is narrow but its width is never identically zero. Because they are in motion, molecules are illuminated by radiation of frequency slighted shifted from that of the natural source, and emit radiation the frequency of which is also shifted. This Doppler broadening of the spectral line is equivalent to perturbing energy level differences but is a consequence of relative motion rather than inter-molecular forces. In the lower atmosphere Doppler broadening of spectral lines is usually less than that resulting from interactions, often called collisional broadening.
If all that does whet your appetite for spinach, now bear the physical strain of just lifting the nearly 3000 pages on spectroscopy which is as nothing compared to the mental strain of absorbing them all.
NB: Translation is indeed quantized at the Planck length level which is unobservable so doesn’t matter at all.
“and the sum of all this overlap gives a continuum background on which is superimposed a series of sharp lines.”
But this doesn’t add up to a Planck distribution. Indeed the tails of each line overlap down in the noise, but the strengths of these lines are not dependent on the temperature, as they would need to be in order to emit a Planck spectrum where the strengths pr wavelength are dependent on the temperature.
https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiX3qbS4J7qAhV1JTQIHTTDDNoQFjAAegQIARAB&url=https%3A%2F%2Fpubweb.eng.utah.edu%2F~whitty%2Fchen3453%2FLecture%252038%2520-%2520Radiation%2520Review.pdf&usg=AOvVaw2IRHBJxeZH7ahpxgcrEDE-
Try the above link. It’s a link to a pdf.
That link is so high level as to be not a source for what you need which is to go over to your local college library & spend a few hours up in the stacks looking at ageing tomes of 1920,1930s gas spectrums. Your librarians will be eager to ply their trade. Go through the detail discussion of the scene illumination method, the film emulsion used, the exposure time, the results in color.
At that time, the field was of big research interest due to the shiny brand-new solution of why gamma was 1.4 not the predicted 1.25 from equipartition for diatomic gas. Someone/anyone wanted to find an experimental reason disproving strange quantum mechanics and all they did was find experimental support for the new field. It was hard to take at first, you know, sort of like the GHE and continuum of gas spectrums. Took like 30 years for spectroscopy research to give up, lose interest, and realize the gas spectrum was continuous and totally supported quantum mechanics.
Ar, Ne were particular interesting as they had a hard time figuring out radiation from a featureless ball for which if it really was spinning they couldn’t tell. Boom, Las Vegas had neon lights.
“That link is so high level”
Really? It’s just lecture notes from a thermodynamics 101 class which is a basic prerequisite for understanding anything about how the climate system works. BTW, a continuum with strong emission lines is not a Planck spectrum, nor is a continuum arising from the overlapping tails of absorption lines. A Planck emission spectrum won’t arise until the emission peaks are so spread out by broadening that the emission peaks themselves overlap.
Why don’t you find a numeric reference for the emissivity of N2 or O2 at pressures less than about 10 bar and then explain why it matters relative to the Earth’s net emissivity relative to the surface temperature, the resulting RADIANT balance and sensitivity.
Far too many are overwhelmed by complexity as they obsess about things that are insignificant, irrelevant or demonstrably wrong. The bulk behavior of the planet to incremental forcing is predictable and quantifiable as the derivative of the SB Law. It really is that simple, moreover; this behavior is readily testable . Denying the bulk simplicity is what led to the IPCC and all that’s wrong with climate science.
>>
It’s just lecture notes from a thermodynamics 101 class . . . .
<<
Unfortunately, Trick doesn’t abide by the thermodynamics usually taught. He (and supposedly his professor) has a new fools-gold standard that supersedes everything. Things like heat can’t be negative, energy equations are rate equations, rate equations are acceleration equations, etc. If you present him with an actual problem in thermodynamics, he gets a headache.
Jim
Jim, I abide by thermo. as taught by Clausius, a grandmaster. To learn chess, I would also seek out a grandmaster. Present me with the whole original problem where total energy = 0 which doesn’t make sense. See what happens. To reiterate:
Q = a rate for a method of energy transfer between massive objects by virtue of a temperature difference, units of transferred energy per unit time.
Q dot is then an acceleration. Clausius taught heat is: total KE of the constituent particles in an object. Which is never identically zero nor negative.
—–
”The bulk behavior of the planet to incremental forcing is predictable..”
Yes, agreed, the increase in global temperature over a climate scale of time predicted in 1938 forced from added anthro. ppm CO2 actually occurred exactly (to the tenth of a degreeC) as of 2013. Go through the same exercise, for climate in 2095 & NO need for a GCM at all. You can do it on your TI BA II Plus battery powered calculator realistically to nearest 0.1C using observed data. Yes, I do wish the government funding institutions would realize this as the money could be better spent elsewhere.
”A Planck emission spectrum won’t arise until the emission peaks are so spread out by broadening that the emission peaks themselves overlap.”
To understand that, both the micro world and the macro world need to be connected in scale. The consequences of discrete energies are not negligible at the atomic scale but they are totally negligible at the macro level. According to quantum mechanics the energies of the harmonic oscillators are quantized, having only a discrete set of energies.
For example, oscillators at the macro level we live in might have natural frequencies of 100hz e.g. 60hz for an electrical grid, 15hz for your car’s independent suspension.
Move from macro to micro stepping down from a flea 1mm^3 volume on a dog so 10^-9 m^3 to its eyebrow at 100x smaller volume 10^-15 m^3. Suppose flea flesh is same density as water, get a mass of 10^-12 kg for the flea’s eyebrow 1m above ground on a great dane. The PE of the flea eyebrow above the ground is 10^-11 Joule.
As small as that PE is, it is still 10^21 times greater than the difference between adjacent energy levels of a macro harmonic oscillator with natural frequency 100hz.
Although the energies of macroscopic oscillators are quantized in principle, the spacing of energy levels, as they are called, is so small macroscopically that in practice the levels are continuous.
We are forced to come to grips with the discreteness of energy levels only when we consider systems with very high natural frequencies. All else being equal, natural frequencies increase with decreasing mass and hence the consequences of discrete energies are not negligible at the atomic scale where the forcing of CO2 molecules on atm. opacity originates.
You guys honestly believe that the sun/earth equilibria is in any way affected by less than 0.01% CO2 that was added by us to the atm when the lower part of the atmosphere contains ca. 4 -5 % water/ water vapor i.e clouds?
>>
Present me with the whole original problem where total energy = 0
<<
I stated the whole original problem. From the first paragraph of the REA solution:
“In this process, since there are no heat losses, whatever heat is rejected from the hotter medium is going to be added to the colder medium making the total energy of the oil and steel, zero.”
>>
To learn chess, blah, blah, blah.
<<
It’s unfortunate that some difficult concepts in physics, like thermodynamics, are completely confused by individuals like Zoe and Trick. I have several books on thermodynamics, and none of them state the nonsense of Trick (or Zoe). Repeating your fool-gold concepts and claiming Clausius said them is ludicrous.
Jim
Clausius’ first memoir “Mechanical Theory of Heat” p. 18: heat is the measure of the total KE of the particles within a body.
And yet, Trick, you still haven’t solved the problem i presented to you. It’s because you can’t with your fools-gold concept. I will help you out with the first step. From the first law:
This is one standard, but there’s also:
It really doesn’t matter which standard you use just as long as you remain consistent–meaning keep all the signs on the various terms correct. In this case the work term is zero, so we don’t need to worry about the different standards here.
The change in internal energy is zero, and we have the following:
To find the final temperature, we have the following equation:
You will notice that because the final temperature of the casting is less than the initial temperature, the casting heat term is negative. The final temperature of the oil is the same as the final temperature of the casting, so there is only one unknown. Plugging in the various values, we can solve for the final temperature.
Also, I don’t see a rate in this expression–just plain ol’ energy values. The rest of the problem should be easy for you.
Jim
Jim, thanks for the detail update, now you write for your defined universe “The change in internal energy is zero” where originally you wrote 10:11am: “that total energy is zero” without qualification.
Also note, deltaU = Q-W is the 1LOT sign convention in texts mostly used by engine engineers for systems that do work on their surroundings where Q and W are rates for the unit process time in doing the delta U (check your thermo. texts).
So, total universe thermodynamic internal energy Ut = Uoil+Ucasting which is a conserved quantity. Thus, your relevant system total energy is NOT zero as you originally wrote 10:11am, total thermodynamic internal energy is Ut nonzero.
—–
In your delta Ut=0 eqn. that is delta Ucast + delta Uoil stated multiplying out time during the process of delta U so Q can be added as shown.
Your “final temperature” eqn. is for a thermodynamic process of two unique masses in contact not in temperature equilibrium occurring over a certain time (Tf-Ti) in order to be added.
I see, Trick, you ascribe to the policy that “if you can’t dazzle them with brilliance, then baffle them with BS.”
>>
Thus, your relevant system total energy is NOT zero as you originally wrote . . . .
<<
What part of zero don’t you understand? The sum of the two heat transfers is zero. I guess thermodynamics is too difficult for you.
Maybe you need to try some elementary physics, like dropping masses. If we drop a mass from height h, then the total energy of this system is kinetic energy plus potential energy. At height h, the kinetic energy is zero (assuming the mass is not originally in motion), and the potential energy is a maximum at m*g*h, where m is the mass, g is the acceleration of gravity, and h is the height. When we are near the Earth’s surface, the acceleration of gravity can be considered a constant. Also for this problem, we’ll assume there is no friction from air drag.
As the mass falls, the potential energy decreases while the kinetic energy increases–keeping the total energy a constant. At the instant before impact with the surface, the potential energy is zero, and the kinetic energy equals m*g*h.
Now let’s say the mass is at rest on the surface. What’s the total energy of this system? The kinetic energy is zero, and the potential energy is zero–total energy is zero. But that isn’t true for all cases. If temperature of the mass is above absolute zero, then the internal energy of the mass is not zero. Does that matter in this case? It does, if you want to nitpick about nonsense. But that’s not all. The mass itself represents energy too–m-c squared. Are these additional energy terms required to solve our falling mass problem? If you’re wondering, the answer is no.
Now return to the mass at rest on the surface–total energy is zero. Next to the mass is a hole that is h deep. If we slide the mass over to the hole and drop it in–it will fall again. The kinetic energy of the mass at the instant before it hits the bottom of the hole is m*g*h. Where did that energy come from?
It turns out what we call zero potential energy is a matter of convenience. We can set the zero potential energy point anywhere. For a specific problem, the usable potential energy is the delta or difference between two potential energy points. For dropping a mass to the surface, it’s the difference of the potential at h above the surface and the surface. If we set the potential energy at the surface to zero (assuming the surface is the lowest point), then it simplifies these types of problems.
Or we can nitpick about what is meant by total energy–like you do.
Jim
”The sum of the two heat transfers is zero.”
There was never any heat in the casting to transfer out as no object contains heat in modern day thermodynamics. In modern thermodynamics, Q is a rate of energy transfer by virtue of a temperature difference & when over time multiplied by (Tf-Ti) becomes a quantity of thermodynamic internal energy U at process equilibrium when the two U are summed to Ut. Ut was always constant. The two U were changing during your process.
You cannot fundamentally sum U + (-U) and get zero total thermodynamic internal energy as you did at 10:11am.
In your example of falling mass: “the potential energy is a maximum at m*g*h” so you cannot dig a hole and get more PE, PE was already defined maximum by you!
It is not just about words, it is about proper thermodynamic and physical science fundamentals.
I have several books on thermodynamics–both old and recent. None of them do your version of thermodynamics. I picked that problem out of the REA Thermodynamics Problem Solver. It’s not MY problem, and it’s typical of many problems in thermodynamics. Since you are God’s gift to us on thermodynamics, you should be able to solve that problem easily. Instead, all we get is lots of talk and no action.
My undergraduate degree is EE. As an engineer, correct units are very important. When someone completely fumbles the units, I know they are blowing smoke. In a discipline (like physics in general and thermodynamics specifically) where solving problems are paramount, avoiding solving problems or stating wrong concepts are telling too.
I would simply warn everyone to not take any of your comments about thermodynamics as valid or truthful. No one does your brand of thermodynamics. And it’s obvious you’ve never solved a problem in physics.
Jim
”None of them do your version of thermodynamics.”
All the texts I’ve consulted do so. Can’t claim to have consulted every one. I’ve already cited Clausius def. of heat for you. I understand many other texts have drifted away from his principles since his writing.
Please provide at least one modern text book cite that claims heat exists in an object. The cite I’ll provide is Van Wylen & Sonntag “Fundamentals of Classical Thermodynamics”, 2nd ed., sec. 4.7, p. 73: “…a body never contains heat”. The REA folks have drifted from the experts & make the fundamental mistake to solve a problem as if heat is contained in an object.
In sec. 5.4 p. 90 also see “The First Law as a Rate Eqn.” :
“….basically classical thermodynamics deals with systems in equilibrium, and time is not a relevant parameter for systems that are in equilibrium….rate form of the first law finds extensive applications in thermodynamics.”
Your REA problem application should use the rate form 1LOT as I’ve pointed out since the problem is not in equilibrium during the process Ti to Tf.
Are we all agreed now that there is no man made warming by CO2?
Thx.
What’s surreal about this idiotic discussion, is that Trick seems to know the terms, but he (or she) doesn’t seem to draw the right conclusions from them.
>>
“…a body never contains heat”. The REA folks have drifted from the experts & make the fundamental mistake to solve a problem as if heat is contained in an object.
<<
Somewhere in this post, I believe Trick made one or two references to the conservation law of energy. Basically it’s just that energy cannot be created or destroyed, but it can change forms.
When heat is added to a system, it’s only defined as such when it’s crossing a system boundary. Once inside, it changes its name. Heat is energy–therefore it cannot be created or destroy, but it can change its name and add itself to the internal energy of the system. Likewise, some of the internal energy of a system can be removed. When it crosses the system boundary, its name changes to heat (in this case negative heat) or work or some other energy term–like chemical potential.
It’s true that a system doesn’t contain heat. However to then say you can’t extract heat from a system because there isn’t any heat (yet there’s plenty of internal energy) is a ludicrous statement.
Water at 0 °C can be changed into ice at 0 °C by removing heat. The term for this is latent heat of fusion. Latent means it’s hidden, that is, while the heat is being removed, a thermometer won’t show a temperature change. The fusion term applies to a phase change from liquid to solid or vice-versa. Where does this heat come from? it’s from the internal energy of the water.
>>
“….basically classical thermodynamics deals with systems in equilibrium, and time is not a relevant parameter for systems that are in equilibrium….”
<<
I have no argument there. Why the quote?
>>
. . . rate form of the first law finds extensive applications in thermodynamics.”
<<
And again, why the quote? Are you trying to claim that only the rate form of the first law is used? What you left off is the derivation of the rate form of the first law. I told you what it was, and for some silly reason you claim it’s an acceleration. That’s another ludicrous statement.
Jim
”It’s true that a system doesn’t contain heat…. Water at 0 °C can be changed into ice at 0 °C by removing heat.”
Jim, heat can’t be removed from water at 0C since as you correctly write heat was never contained in the water system to begin with. Sure, I’m being irreverent but helps make a point. Only dead fish go with the flow.
Yes, you write, as many do, the energy renaming at the border is a step, then another step to rename back to energy across the border. These are useless, confusing steps and your REA source has once again done its level best to put heat back in an object after all the effort to remove it over the last many years. Energy goes both ways across the border but heat does not, confusing a ton of writers by invoking heat as a vague, metaphysical, even paranormal entity jumping into/out of existence at the border. Grasp that and authors can write (and be read) way more clearly including the guys at REA.
The rate form 1LOT is not used in classical thermodynamics at equilibrium. In modern day, it is recognized all interesting, practical applications of thermodynamics occurs as a process over time that is not in equilibrium thus a rate 1LOT is used. The hard part is many authors still write on thermo. inexactly; for a good discussion of how this all began see Cliff Truesdell 1980 “The Tragicomical History of Thermodynamics 1822-1854.” There are some papers on the subject of misuse of heat term & at least one that cites maybe a dozen text book authors and pokes fun at them for the misuse.
>>
. . . heat can’t be removed from water at 0C . . . .
<<
It’s clear to me, Trick, that you haven’t got a clue as to how water turns into ice in your freezer or any where else for that matter. I say again, it’s called latent heat of fusion. It’s heat being removed from a substance to cause a phase change from liquid to solid. The same thing happens at the other phase change–latent heat of vaporization. It’s energy in the form of heat that is added or removed from a substance.
>>
Energy goes both ways across the border but heat does not . . . .
<<
Are you sure you’re not Zoe in disguise? That statement is complete balderdash. Does your stupid textbook discuss the Carnot cycle? Probably not, because you need negative heat.
>>
. . . one that cites maybe a dozen text book authors and pokes fun at them for the misuse.
<<
Only one? Ugh Trick. Ever think that maybe years of thermodynamics texts might be right, and your silly author doesn’t know what he’s talking about. You should try Thermodynamics for Dummies–it would be a start.
Jim
”I say again, it’s called latent heat of fusion.”
Jim, in modern thermo. that is the enthalpy of fusion.
The notion of heat in motion leads to perplexity. A chunk of ice at 0C melts when exposed to air at a higher temperature. While the ice is melting, the temperature of the ice and water mixture is observed to not change. The ice is being heated so we are supposed to believe heat is being transferred in across the border to change the ice state of motion*. But ice/water mix temperature does not change and hence we are supposed to believe that its state of motion does not change. That is perplexing.
”Ever think that maybe years of thermodynamics texts might be right…?”
You have yet to cite even one that disagrees with me, you just might find one, bring it on. Paranormal heat is conceived of as existing neither in body A or body B (as Jim wrote) but only on the journey between them. By hook or crook, a corporeal form of heat gets invented by REA in order to save appearances. This discussion is basic first course thermodynamics, it is made hard (perplexing) because of the reasons pointed out by Truesdell.
*See Clausius famous paper: “The Nature of the Motion which we call Heat”
>>
Jim, in modern thermo. that is the enthalpy of fusion.
<<
So? When you look up the term they say it is also the latent heat of fusion. The differential definition of enthalpy is
For a constant pressure process the change in enthalpy is exactly the change in heat–positive heat and negative heat–depending on the direction of transfer. The latent heat of fusion is usually defined at constant pressure–enthalpy is more general.
>>
That is perplexing.
<<
It’s only perplexing if your interpretations of these terms are flawed.
>>
You have yet to cite even one that disagrees with me, you just might find one, bring it on.
<<
Easy. Every text I’ve read disagrees with you. Let’s try “An Introduction to Statistical Thermodynamics” by Terrell L. Hill. From the very first sentence of the first chapter:
“The object of thermodynamics is to derive mathematical relations which connect different experimental properties of macroscopic systems in equilibrium–systems containing many molecules, of the order of, say, 10^20 or more. ”
And further down in the same paragraph:
“. . . the object of statistical mechanics is to provide the molecular theory or interpretation of equilibrium properties of macroscopic systems.”
I believe you don’t like the term equilibrium in thermodynamics–that means you don’t like the zeroth law of thermodynamics either.
You also ignored my comment about the Carnot Cycle. Every thermodynamics textbook discusses the Carnot Cycle. They should, because Carnot was the first person to state the second law of thermodynamics.
They develop two ideas which apparently are foreign to you–reversible processes and heat reservoirs. Neither can exist in your interpretation–how can you obtain heat from a heat reservoir if it doesn’t contain heat? (It doesn’t, of course, but it does provide it in any quantity required.) Thermodynamics textbooks usually spend a lot of written real estate on describing reversible processes. I won’t here, but reversible processes are a theoretical construct–they don’t actually exist. One property is that they are always in equilibrium and another is that they change states by infinitesimal steps. The slower a process changes, the closer to reversibility it becomes.
Basically a Carnot cycle is a reversible heat engine (usually called a Carnot heat engine) operating between two heat reservoirs–a high temperature reservoir at and a low temperature reservoir at
and a low temperature reservoir at  . It goes through four states. Starting out in state 1, it goes to state 2 by a reversible isothermal transition receiving heat
. It goes through four states. Starting out in state 1, it goes to state 2 by a reversible isothermal transition receiving heat  from the high temperature reservoir. It then goes to state 3 by a reversible adiabatic transition while creating work
from the high temperature reservoir. It then goes to state 3 by a reversible adiabatic transition while creating work  . The change to stae 4 is another reversible isothermal transition where heat
. The change to stae 4 is another reversible isothermal transition where heat  is rejected to the low temperature reservoir. The cycle completes by another reversible adiabatic transition back to state 1.
is rejected to the low temperature reservoir. The cycle completes by another reversible adiabatic transition back to state 1.
The first law expression for this process is:
As the cycle returns to state 1, then the term equals 0. We then have:
term equals 0. We then have:
Thermal efficiency of heat engines is one of the reasons thermodynamics was created. The thermal efficiency of this heat engine is:
The efficiency of a heat engine can’t be greater than 1 (or 100%), so the term must be negative. Again, every thermodynamics textbook then proves the following relation:
term must be negative. Again, every thermodynamics textbook then proves the following relation:
And we have:
It’s the maximum thermal efficiency possible for a heat engine operating between two thermal reservoirs. They also prove that no heat engine operating between two thermal reservoirs can be more efficient than a reversible heat engine operating between those same two reservoirs.
But according to your interpretation, no thermodynamics textbook ever discusses the Carnot Cycle. Did you know (apparently not) that the Clausius definition of entropy is:
Alas, there’s that reversible subscript.
Jim
Jim, nothing you clipped from Hill’s text disagrees with me. In fact “. . . the object of statistical mechanics is to provide the molecular theory or interpretation of equilibrium properties of macroscopic systems.” completely agrees with me using Clausius’ original def. of heat I already presented for you.
Here is the only definition of heat in Hill’s text that I could quickly find which is actually Clausius’ announced symbol for H which has become known as enthalpy:
p. 30: ”H is the heat content, E+pV”
Hill’s text (a body has “heat content”) thus disagrees with Van Wylen text (“…a body never contains heat”). You can learn from this that Hill has used heat content to really mean enthalpy. Clausius definitions clearly show there is a difference between heat and H (enthalpy). Wherever you read Hill writing “heat content”, Hill really means H, enthalpy which is fine but the reader ought not to have to do the translation. Truesdell’s text helps explain where this perplexity comes from.
Years ago, I read the conclusion that it is incredible writers and teachers are still referring to the “heat in a body” or “heat content” or “heat rises” or “heat bath” or even “radiant heat”. Water flows, electric current flows & when they enter a system they do not disappear, but when heat flows into a system: it disappears! Not conserved!
To put reality to this, and following enthalpy H=E+pV, consider a room temperature ideal gas put in contact with a large furnace (Hill’s “heat bath”) with the gas undergoing an isothermal expansion. Per Hill, heat goes in but the gas undergoes no change in temperature, since the work done by the gas compensates the inflow of heat. Heat continues to go in, but there is no change of Clausius’ heat IN the gas since there is no temperature change. Clausius def. wins. Clausius heat defn. is way better than Hill’s because Hill’s defn. heat is really enthalpy.
Thermodynamics is best, & more clearly discussed as heat and work are methods of relevant energy transfer. When all the energy flows are over (equilibrium) the words heat and work have no longer any usefulness or meaning.
I could write much more on Carnot cycle detailing this (on Q, a rate of energy flow due a T difference being path dependent) but will stop here.
>>
Jim, nothing you clipped from Hill’s text disagrees with me.
<<
We’re making progress then. You now agree that thermodynamics is the study of systems in equilibrium. You were denying that previously.
>>
p. 30: ”H is the heat content, E+pV”
<<
Very impressive. You were able to scan the text and find the term “heat content.” It’s a now obsolete term for enthalpy. Let’s see–it was developed in the 19th century by Clausius–among others.
You’re using an old lawyer trick, Trick–change the focus of the argument to something you can win. My argument has never been the definition of heat. Your demand that I find a different definition is silly. I’ve said over and over again, that heat added to a system is positive heat and heat removed from a system is negative heat. Every thermodynamics textbook says the same thing. This idea that because a system doesn’t contain heat, you then can’t extract heat is nonsense. You’re suffering from selective reading.
You’ve spent in incredible amount of comment space arguing about “heat content.” Go ahead–it’s an obsolete term and shouldn’t be used for enthalpy at present.
>l>
I could write much more on Carnot cycle detailing this (on Q, a rate of energy flow due a T difference being path dependent) but will stop here.
<<
But of course, let’s not stay on the the actual subject of positive-negative heat transfers. That might get you in difficulty with your previous statements.
Jim
”You now agree that thermodynamics is the study of systems in equilibrium.”
Strictly classical thermo. from which modern rate eqn.s were developed and used extensively today for practical application like in the top post. The term enthalpy is not obsolete or widely misused, Jim. Clausius term heat is widely misused as you demonstrate, not Clausius’ H (the first letter of heat).
”I’ve said over and over again, that heat added to a system…” wrongly as you admitted 12:35pm “It’s true that a system doesn’t contain heat.”
”heat added to a system is positive heat and heat removed from a system is negative heat. Every thermodynamics textbook says the same thing.”
Show where Hill writes that “same thing”. I will predict any passage you quote, Hill will have written about enthalpy as he defined his terms “heat content”. This idea that because a system doesn’t contain heat, you can then extract heat from the system is itself paranormal nonsense when you grasp Clausius def. of heat is correct. There is no such thing as the work in a body, just like there is no such thing as heat in a body to be extracted as a negative.
Carnot cycle adds no difficulty with anything I’ve written; Slater’s Introduction to Chemical Physics, “At first sight, it seems too bad that Q is not independent of path, for some such quantity would be useful. It would be pleasant to say (as does REA wrongly), in a given state of the system, that the system has so and so much heat energy….But the stubborn fact remains that we should get different answers if we heated it up in different ways….There is nothing to do about it.”
Think about the example of heating ice at 0C or the isothermal gas example, heating with no temperature change; it is the enthalpy that changed. No need to use heat term, ever, too many times leads to befuddlement (especially around here) as there are as many definitions of heat as bewildered readers of the term.
Clausius’ definition of heat is the gold standard, compare all subsequent defn.s to his. If you want to be clear, strictly use Clausius’ defn. Writers not desiring clarity, can use their own heat defn., picked from a host of sources that have drifted from Clausius.
I have never seen someone so confused about a simple concept as heat transfer. (Also trying to put words into my mouth–not to mention constantly changing the subject.)
From my college thermodynamics text (Fundamentals of Classical Thermodynamics by Wylen and Sonntag, page 74): “Further, heat transferred to a system is considered to be positive, and heat transferred from a system, negative.” (The statement is repeated at least once later in the book.)
From lecture 6 of The Great Courses: Thermodynamics: The Four Laws that Move the Universe, page 99 of the Transcript: “The sign conventions for this course are as follows: When heat is transferred into a system, Q is positive; when it transfers out of a system, Q is negative.”
On pages 13-14, Hill defines Q* and W as heat absorbed by the system and work done by the system–standard sign terminology following the IUPAP standard.
From Thermodynamics for Dummies, page 70: “Heat transfer to the fluid is a positive quantity; heat transfer from a fluid is a negative quantity.”
Even Wikipedia defines a positive and negative sign convention for the first law (depending on whether the IUPAP (also the Clausius standard) or IUPAC standards are used.
I guess I’ll have to ask again. If a high temperature heat reservoir is supplying heat to a Carnot heat engine, where does that heat come from? And just for grins, how long does it take a reversible process to make a finite step?
Jim
Trick,
Exposure time doesn’t matter relative to observing a Planck spectrum being emitted by the gasses in the atmosphere which is what you asserted would occur. All you will see are the emission lines getting brighter. The profile of the lines may appear to match a Planck spectrum, assuming absorption is the same in each line, but only because the power being absorbed was originally emitted as a Planck spectrum. What would the emitted spectrum of the gasses in the atmosphere look like if the atmosphere was heated by a 13u laser (where the deepest CO2/H2O absorption is) ? How about a 11u laser in the transparent portion of the atmosphere.
My point stands that the gasses in the atmosphere don’t emit a Planck spectrum according to their temperature and the SB Law, but emits a line spectra whose individual magnitudes are arbitrarily based on the incident energy and the specific line strength which has only a minor dependence on the temperature.
The majority of the power emitted by the planet is centered on 10-11u and the N2 line you reference is very weak. A reduction of 0.1 W/m^2 out of the 390 W/m^2 being emitted by the surface is in the noise and for all intents and purposes statistically insignificant relative to the radiant balance and the sensitivity.
Just to be clear, just about everything has a finite effect, but what’s important is not that an effect exists, but the magnitude of that effect. A failure to understand the importance of insignificant magnitudes is one of many failures in climate science, where a finite, but insignificant incremental GHG effect is magnified into a fake catastrophe.
As the exposure time lengthens you will eventually get a continuous spectrum photograph at surface STP for gasses in the atm. illuminated naturally. To write that atm. gases do not emit a Planck spectrum is to write an exception exists to Planck’s law. Not so, it really is a law, no exceptions have ever been found.
Spectroscopy is the science of details and you have not provided sufficient detail to support your view but you have obtained the view somewhere. Where is the somewhere?
Trick,
What is the specific mechanism that can turn translational energy into photons? I’ll give you a hint. Energetic enough collisions can boost a GHG molecule out of the ground state, after which it will return to the ground state, emitting a photon in its absorption/emission spectrum. So the real question is how can translational energy be converted into a photon that’s not in the absorption/emission spectrum?
BTW, a Planck distribution of kinetic energy exists among the molecules of a gas, it’s just not in the form of a distribution of emitted photons, but as a distribution of translational energy. Meanwhile, thermometrs can’t tell the difference. In principle, this is a manifestation of wave/particle duality, but in this case, photon emissions are actually waves, while particles in motion and colliding with other matter are not.
You need to take a step back from bulk behaviors like a Planck distribution and apply Quantum Mechanics to individual molecules. Like feedback, the requirements of Quantum Mechanics are esoteric and easily misinterpreted or ignored.
When we look into space and detect gas clouds from their IR emissions, what we are detecting are emissions from the dust in those clouds. We can only infer what gases are present by the absorption spectra.
Translation is not a quantized DOF. No photon emission from that air molecule DOF; translation doppler and other broadenings spread out the lines. Can’t write I’ve seen a Planck distribution of KE, you will have to fill me in. Perhaps you mean the Maxwell-Boltzmann probability distribution of air molecule velocities at STP, the source of doppler broadening.
Your point remains unsupported; air as a gas does exhibit Planck distribution at STP as there are no exceptions to that law. Air looking up emissivity at STP has not been shown to be identically zero at any frequency. The lowest measurement naturally illuminated air I am aware across the spectrum something like 130 W/m^2 in the dry polar regions.
The analogy proves the opposite. Place an ambient temperature lid on a boiling pot of water. What happens to the temperature of the lid?