Note: This is a contentious subject, and I have often shied away from it because it often erupts in food fights. However, Mr. Gill is making a good-faith effort here, and asks some relevant questions that I consider worth discussing. His original essay was sans graphics, and I’ve added two relevant graphics to aid in the discussion. – Anthony
Do Wien’s Law and Quantum Physics 101 prove CO2 can’t warm anything?
Guest essay by Rod Gill
WUWT has happily demonstrated many ways CO2 fails to produce measurable warming. I’ve thought of another way. It’s so simple I must have missed something, but I simply can’t work out what. It goes like this…
Experts suggest there is a net down welling 2W/m2 of long wave infra-red radiation (LWIR) that is causing global warming. I suggest the quality of that 2W of radiation is crucial to determining whether or not it causes any atmospheric warming at all. First a few key points which I think are facts and not open to dispute.
My understanding of Thermodynamics and Radiation from CO2 is as follows:
In Thermodynamics, Temperature is the average kinetic energy of the particles in a body (solid or gas).
The temperature of a volume of air has nothing to do with the amount of radiation (sometimes mislabelled as heat by scientists) passing through it. Unless that radiation is at a frequency that can be absorbed by the air, its temperature is completely unaffected by the radiation (ignoring any convectional heating).For example at the top of Mount Everest, there is a lot of solar energy (long and short wave radiation) there when the sun is out but the temperature is still cold.
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law). This linear variation is not evident in this kind of plot since the intensity increases with the fourth power of the temperature (Stefan- Boltzmann law). The nature of the peak wavelength change is made more evident by plotting the fourth root of the intensity. Source: http://hyperphysics.phy-astr.gsu.edu/hbase/wien.html
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Photons with not enough energy to raise the orbit of any of the electrons are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. If I gave you my references, which knowing the web may or may not be accurate, you might erroneously come to the same conclusions I have. However I have tried to limit my research to University papers and lecture notes hoping they are more reliable.
If I’ve got this right, CO2 caused global warming isn’t possible. If I haven’t got this right, then exactly how does LWIR radiated from CO2 warm anything?
Many thanks and please limit comments to specifics mentioned above. And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
1.) Molecules have one or more electrons circling them. blah blah blah
NO
That is classical physics junk. In QM the electrons have complex distribution clouds only S orbitals 1s,2s,3s are circular. The reason is that electrons have half-integer QM spin and subject to Pauli exclusion principle that says two or more identical QM spin particles can not occupy the same space at the same time. They will instead by subject to pairing creating a waveform between them.
The reason for the half spin can be seen by seen by watching the field movement
You will also note the momentum is in the field it is not a real spin like in the physical sense.
So Quantum spin can be thought of as momentum but it can not be equated to a physical object spinning.
2.) For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
PARTLY
That is the process of excitation and it creates an excited state it equates to temperature in classical physics. Outside the two ways listed you can also excite an atom via the electric or magnetic fields.
You need to be careful the temperature however is not always positive, it can be negative 🙂
3.) For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit. blah blah
NO
You need to break that into Photoexcitation which says a Photon must have a frequency that matches the one of the excitation energy level to be absorbed. You can view it as a resonate frequency with one of the excitation states of the molecule. Any energy absorbed in this manner departs from the equilibrium Boltzmann distribution as viewed by classical physics.
If the energy does not match those special resonant frequencies absorption of the photon takes place in accordance with Planck’s quantum theory.
4.) Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum blah blah
NO
Photoexcitation is an independant process and will occur at any temperature
Laser cooling illustrates this in the most extreme way. https://en.wikipedia.org/wiki/Laser_cooling
The energies that are outside the resonant frequencies behave in the more normal way as you are trying to describe.
5.) Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them. blah blah blah
NO
see item 4, temperature does not play a part in photoexcitation only photon energy match
Now, you need to stop following the thermal collision effects and deal with only the photoexcitation effect and key to understanding that is bring Nitrogen into the mix. Nitrogen exists mainly as a simple diatomic atom in the atmosphere and it’s excited states are metastable and relatively long-lived and they happen to overlap a couple of CO2 frequencies. Classical collisions between CO2 and N2 will exchange energy in the photoexcitation frequencies. So the normal thermal energy and the photoexcitation energies can interchange. Without the interplay the two sets of energy would remain isolated.
Martin, do all your posts provide zero value add? Someone takes the time to write a detailed rebuttal of the author’s points, and all you have to say is blah blah blah. Truly the hallmark of a lightweight.
Much to read here in the comments, and I WILL get to it all later.
My overall observation, though, is that the talk still seems to center on warming.
The atmosphere keeps Earth’s surface from getting to hot too. The atmosphere, therefore, BOTH cools the surface AND warms the surface at the same time, depending on your perspective, … depending on what you are referencing as the main focus.
I think that talk should shift away from speaking of either extreme (warming or cooling) as the main focus and, instead, shift to the concept of REGULATION, which must consider both, or neither, depending on how you look at it.
Regulation involves an exchange between two processes, rather than either of those processes alone. And so it seems that CO2, H2O, and other gases REGULATE Earth’s temperature withing the habitable range familiar to us.
I agree Robert. As I noted earlier, the ocean, the soil, and the GHG merely serve as heat sinks for the atmosphere. The aspect of CO2 is limited by the amount of IR available to be absorbed. The equilibrium equation heat sink capacity can only reach a certain maximum simply because there is no more IR in that spectrum to work with.
So, as you say, it plays a small role in stabilizing the atmospheric temperature, but it plays little role to n the overall stabilization process, simply because it is insignificant compared to the ocean (the overwhelming #1 heat sink), and water vapor, the primary heat sink in atmosphere.
CheshireRed
November 19, 2017 12:28 pm
One thing that posts like this definitely demonstrate is there is no consensus. When multiple highly-qualified professionals and well-informed amateurs cannot agree on something as fundamental to ‘global warming’ as a GHE or high sensitivity…then it’s obvious there’s still oodles of uncertainty.
The back radiation is not the only energy flux warming the surface. There is also the direct solar irradiation. Below is an energy balance sheet of the Earth. It is the only presentation showing the three sky conditions: first number is all-sky, the second number is clear sky, and the third underlined number is cloudy sky. The total energy flux warming the surface is Sd + Ed = 168,8 W/m2 +344,7 = 513,5 W/m2. And
by the way, the both energy fluxes are based on direct measurements. The energy balance of the Earth’s surface shows that the incoming energy fluxes and the outgoing energy fluxes are in balance. If they were not, the surface would cool or warm.
Robert W Turner
November 19, 2017 12:37 pm
The major misconception presented here is treating all molecular energy the same. There are two distinct types, translational kinetic energy that all molecules have, and quantized vibrational energy that dipole molecules can have.
The GHG theory treats the later energy type as if it is not quantized, in other words they conceptualize that a dipole molecule will always absorb a certain wavelength of energy even if it is already in the energized vibrational state, that is NOT correct. If the gas molecule is already in its excited energy state, it can not continue to absorb that wavelength of light, that light will be transparent, reflected, or in some instances it will stimulate that molecule to radiate that energy and drop back to the non energized state.
However, very small levels of energy (factions of 1 eV) can be lost or gained from the quantized energy and given to translational kinetic energy upon collisions with other gas molecules — that’s why the absorption is a band instead of an exact wavelength. These collisions theoretically occur every 10^-7 s at atmospheric pressure which is faster than CO2 molecules theoretically radiate heat. Therefore, we should expect some minor heating caused by an increase in CO2 molecules in the atmosphere from conversion of quantized energy into translational kinetic energy from their collisions with non dipole molecules.
If all GHG molecules in the atmosphere are already absorbing and reemitting all available spectra able to bump the molecules into the energized state, then the only mechanism for additional retardation of heat escaping from the atmosphere can occur from the collisions described above. But that’s only if the available infrared energy is the limiting factor whereas more heating would occur if it is the available number of dipole molecules is the limiting factor.
In my opinion, we have empirical data showing that it is indeed the available IR that is the limiting factor and therefore any additional dipole molecules in the atmosphere can only heat by losing some of their quantized energy to kinetic energy. That is the CERES and TERRA data that has shown that certain regions of the atmosphere lose more heat into space than they receive from the sun, one region in particular being the Sahara desert.
This data not only shows that it is water vapor dominating the GHG feedback, but to me it also suggests that there is something to the theory that the temperature of planet’s atmosphere is largely dependent on adiabatic and gravity pressure induced compression heating. The most heat leaving the planet happens to be where cooled dry air descends within the atmosphere and inducing high pressure.
Notice the outgoing longwave radiation coincident with the descending air associated with Hadley cells. Please, I invite anyone to explain this with the GHG planetary warming theory and how it is not explained by adiabatic and gravity driven heating.
No such thing really. Pressure does not cause heating or cooling, only changes in pressure. Rising air cools down, sinking air warms up. This is the basic reason for the lapse rate.
That there is more outgoing radiation in the descending part of the Hadley cell is because the air there has lost most of the water vapor (the really important GHG) and is therefore much more transparent to LWIR, so it comes from a lower and warmer layer.
What do you mean no such thing really? Very basic physics, really.
“work done by gravity = W = mgh (h = height lost by the object)
An alternate way of looking at this is to call this the gravitational potential energy. An object with potential energy has the potential to do work. In the case of gravitational potential energy, the object has the potential to do work because of where it is, at a certain height above the ground, or at least above something.”
The process is started by solar heating of the ground, air masses warm up on the ground, they rise and then fall in the Hadley Cells. Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.
tty is correct. Static pressure does not cause any energy transfer.
Robert’s equation of “work done by gravity = W = mgh (h = height lost by the object)” actually proves this.
“h” in this equation is zero for the atmosphere as a whole. The atmosphere has no height to lose in toto. Any downdrafts are exactly balanced by updrafts elsewhere.
In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.
“Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.”
However the effect would be very weak in an atmosphere without GHG since very little heat would be transferred from the ground to the atmosphere (though it is rather unlikely that such an atmosphere exists anywhere).
“In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.”
Very true. As a matter of fact this is the main mechanism regulating the surface temperature of the Earth. And it can not be modeled realistically, now or in the foreseeable future (it would require increasing computational capacity by something like 10^12).
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
You are confusing the one-time effect of dynamic compression — which can and does result in “heating” (and can be immense on a planetary scale) — with the ongoing effect of static pressure — which cannot and does not.
If you compress the air in a bicycle tire pump, it gets hot. If you leave the compressed air in the pump, it does not stay hot, but your analysis says that it would
The gas giant planets go incredibly hot in the dynamic compression of their formation. Some think that Jupiter’s core got almost hot enough to initiate fusion. These planets still emit significantly more energy than they receive from the sun, which means they are still cooling.
The earth, however, does not emit any significant amount more than it absorbs from the sun. Its geothermal flux is only about 0.1 W/m2 averaged over the surface.
In what world is the atmosphere static? The down draft limits the warming, is that what’s observed?
However the effect would be very weak in an atmosphere without GHG since very little heat would be transferred from the ground to the atmosphere (though it is rather unlikely that such an atmosphere exists anywhere).
Complete rubbish. The composition of the gas has absolutely nothing to do with convection.
The sum is not zero in this process. Solar radiation does the work to lift the air and gravity does the work to compress it. Energy is actually input into the system this way, and lo and behold, it’s actually what is observed.
Doesn’t descending air become exposed to greater pressure as it descends? Isn’t gravity doing work on it? Doesn’t it’s density increase, from less dense to more dense? — and it’s kinetic energy increase from less to more? And doesn’t gravity cause this descending, compressing, increasing kinetic energy in conjunction with the already warmer level into which it descends?
I confuse nothing. It only requires “a one time heating”
The Kinetic energy at the bottom of the atmosphere doesn’t dissipate to the top again. To do that it must work against gravity. Take a look at Neptune, where you have much less solar input to confuse you. It gets warmer as you descend into it, until it is thousands of degrees C.
Outside the fraudulent area of climate change on earth, this sort of thing is universally accepted.
It’s about time people on here looked up from their narcissistic intellectual self aggrandisement and checked with the rest of the scientific world
yes it can continue to absorb at that or nearly at that wavelength. you missed out that vibration in a molecule has harmonics so two photon absorption is possible. or a photon of double that energy can be absorbed.
just like a harp string will have a fundamental frequency it will have higher energy harmonic frequencies. a harpist will pluck the string at a different height to change the harmonics of the string.
it can continue to absorb at that or nearly at that wavelength. you missed out that vibration in a molecule has harmonics so two photon absorption is possible. or a photon of double that energy can be absorbed
So you’re saying that a photon can be absorbed that is double the energy level at which the molecule actually absorbs? And given an infinite number of photons in the 15-20 nm range, a CO2 molecule will continue to absorb that thermal radiation regardless of all three normal modes energized? And then can theoretically emit at a higher energy level than the radiation it absorbs?
To R turner at 9:27 pm.
1 carbon dioxide has 4 modes of vibration. Symmetrical stretch, asymmetrical stretch, and two bending modes of equal energy. The word for equal energy in spectroscopy is DEGENERATE. so they are a degenerate pair of benders!
the symmetrical mode does not absorb infra red since there is no change in dipole moment. the other 3 modes are infra red active. there are an awful lot of vibrational energy level present in each mode so each mode can sequentially absorb a lot of almost equal energy photons one after an other. ( due to asymmetry in the vibration the second photon energy will be slightly less energetic than the first and so on). The molecule can absorb photons equal to 2 of these energy bands (or even 3) this is analogous to a musical string vibrating at twice its frequency ie one octave above the fundamental note.
there are not an infinite number of energy levels so it cannot absorb an infinite number of photons. if it absorbs too many photons then the molecule will vibrate so strongly that the atoms will fly apart and it no longer exists it will be CO and O. Double strength photons can come off but has much lower probability than sequential single photons. I some how remember that some lasers can produce double strength photons.
indeed at any one time there will be a fraction of the population of CO2 with excited vibrational modes, and these will be absorbing photons as well.
This is how molecules break down in heat. infra red lasers can be used to heat single bonds in a molecule and break it. however the method is in its infancy and may just as well break a nearby bond.
Robert W Turner ,
Note that your NASA illustration (second one) has a range of reflected light (0–700 W/m^2) that is more than twice the commonly accepted value of incoming TOA radiation (342 W/m^2). How can something reflect more than it receives?
If CO2 did affect climate, than the increase in CO2 over the past 30 years should have caused at least a measureable change in the dry lapse rate in the troposphere, but such has not happened.
There are many lapse rates in the atmosphere. g/Cp defines the Dry Adiabatic Lapse Rate (DALR) which is about 9.8 K/km. The more typical troposphere lapse rate is the Environmental Lapse Rate (ELR) which is 6.5 K/km. There are many more important lapse rates and one or more of them *might* be affected by CO2. However, all of them, except the DALR, are affected by radiation to/from CO2.
Whu should the dry lapse rate change? The small increase in CO2 is not enough to measurably change the specific heat of the dry atmosphere. If the increased CO2 resulted in an increased amount of H2O in the atmosphere (known. by friends and admirers as “water feedback”), then the average lapse rate would go down, but as far as I know this has not been observed.
willhaas. You forget that there is a possibility that the CO2 effect is much less than IPCC reports. In that case the other cosmic warming effects have decreased and therfore the temperature has paused, even though the warming by CO2 has increased slightly.
According to the IPCC, the warming effect of CO2: dT = 0.5 * 5.35 *ln(CO2/280) resulting to the climate sensitivity of 1.8 C degrees. I have reproduced the calculation of the warming formula of CO2 and it is: dT =0.27 * 3.12 *ln(CO2/280) resulting to the climate sensitivity of 0.6 C degrees.
The warming effects of different main forces are in 2015: the Sun 0.35 C, GH gases 0.28 C, and astronomic harmonic resonances 0.13 C, together 0.75 C. Link: https://www.climatexam.com/cosmic-theory-caws
The system is a lot more complex than many (including we token ‘warmists’) think.
angech
November 19, 2017 1:14 pm
“Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um”
–
This statement needs a lot of modification.
We are talking about our atmosphere.
Temperature is the sum total of all the energy in all the molecules in the atmosphere in any particular place.
Individually molecules will have different energy levels,
LWIR is produced by both the sun and the earth surface and the atmosphere and clouds.
So the atmosphere certainly has all LWIR frequencies trying to pass through it.
I guess the CO2 molecule can pick up these other two frequencies if needed if it runs across them?
–
If a CO2 molecule has picked up a higher “hotter” frequency energy packet and it subsequently emits it this does not mean that the whole atmosphere has to be at say 120C does it?
Yet that CO2 molecule will be buzzing around giving energy [heat[temperature]] to a lot of other O2, H20,N and other molecules while it is charged up.
–
Something is wrong in the state of Denmark but I am not good enough yet to explain it. There must be lots of people who do understand radiative physics to simply knock this contention out.
Where are they, please.
–
Quite happy to be a skeptic for lots of reasons but not on the CO2 is not a GHG as technically it will raise temperatures according to the laws of physics.
Andrew Hamilton
November 19, 2017 1:21 pm
Just a minor comment.
Should 274 K be 273 K and 15 C be 288 K, not 289 K?
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit” I stopped there, too many misconceptions about quantum mechanics. The IR absorption spectrum has nothing to do with the electronic excitations, but with rotational-vibrational spectrum of the molecule (cannot be purely vibrational, because the photon has helicity). I’m going to have a post on my blog about this sometime, specifically for CO2 and H2O, calculate some frequencies and explain a few things…
MODTRAN answers the question. MODTRAN, whose accuracy has been verified by satellite measurement calculates heat flux either outward or downward at any point in the atmosphere. The difference between heat flux outward at 0 altitude and at the top of the the atmosphere is the heat deposited in the atmosphere. If the GHG concentration is anything above 0 the heat deposition is positive. If it is 0, then all of the IR heat from the earth escapes to outer space and we are left with a frigid, uninhabitable earth.
Excuse me, but all the IR ultimately does escape to outer space (including geothermal heat).
“If the GHG concentration is anything above 0 the heat deposition is positive.”
Since it has been above zero for the last 4 billion years or so (and mostly much higher than now) according to your physics the planet should have melted and boiled off the atmosphere long ago. As a matter of fact even if the “heat deposition” was only 0.000001 degree per year the Earth should be almost as bright as the sun by now.
Yes, all the IR energy does escape to outer space, but it does so at a higher temperature than it would have done without the IR absorption of the atmosphere. It does it via the Stefan-Boltzmann equation. Since it loses some of the (discrete) IR levels leaving the earth’s atmosphere it has to increase its temperature to make up with a continuous spectrum what it has lost from the discrete emissions. This is the mechanism of GHG heating. You can read about the details by googling “Nature Abhors a Positive Feedback” which is a 3-year old post to this site (Watts Up With That).
Problem is no one uses it right.
It changes just by altering air temp and not composition. So, unless you run it for each temp, you get the wrong answer.
Ned Nikolov
November 19, 2017 1:44 pm
I agree – the notion that a gas such as CO2 or water vapor can “trap” radiant heat in a free atmosphere is simply unphysical. Heat trapping by gases is only possible by preventing/obstructing convective heat exchange (as in a glass greenhouse) or by using of IR-reflective materials such as polished aluminum that have VERY low IR emissivity/absorptivity and a high IR reflectivity (these materials are called “radiant barriers”).
Our latest paper addresses this issue and demonstrates using NASA planetary data that the thermal effect of planetary atmospheres has nothing to due with trapping of outgoing thermal radiation, but instead is due to the force of atmospheric pressure, which adiabatically enhances the energy received from the Sun. In other words, the so-called “Greenhouse effect” (more accurately named Atmospheric Thermal Enhancement or ATE) is a thermodynamic (pressure-induced) phenomenon that is completely independent of atmospheric composition:
Nikolov N, Zeller K (2017) New Insights on the Physical Nature of the Atmospheric Greenhouse Effect Deduced from an Empirical Planetary Temperature Model. Environ Pollut Climate Change 1: 112. doi:10.4172/2573-458X.1000112
URL: https://tinyurl.com/ydxlfwn7
Did you take high school physics? If you had, you would understand that for a force (such as atmospheric pressure) to transfer any energy, it must act over a distance (Work = Force * Distance, or more precisely, Work = Integral of Force over distance).
If an object is actually falling in a gravitational field, gravity is doing work on that object, transferring energy to it. But the atmosphere is not falling — it has already fallen. No distance, no energy transfer. (Any downdrafts must be exactly matched by updrafts, so these fully cancel out.)
Any reasonably bright high school physics student can recognize your argument for the nonsense it is!
Take your 5kg barbell and hang it from the ceiling on a hook. No movement, no work. The hook and the ceiling have no power supply.
If you held up the weight with a motorized winch, the motor would expend some energy holding up the weight (as the body does in your example). But engage the brake on the winch and turn off the motor, no energy is expended to hold up the weight.
You would fail high school physics, and not get started in any higher level physics.
To those who think that all the work of gravity has already been done and that this somehow falsifies the premise that there should be higher temperatures at the surface than at the top of the troposphere because of gravity:
When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located? Is it located at the top of the atmosphere? Or is it located at the bottom of the atmosphere?
If you want to distribute that energy back to the top of the atmosphere, what force will you have to work against to get it there? Would it be gravity by any chance? If you do work what do you expend? Oh yes! Kinetic energy? Which is converted to potential energy once more!
So the natural equilibrium of a large planetary atmosphere, is colder near the top, hotter at the bottom. See Neptune, Uranus, Saturn, Titan, Jupiter, Earth and Venus for and use the simple formula T=Pn/Rp to understand that the temperatures of all of these planets and moon can be precisely calculated using nothing else.
You ask: “When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?”
I see that you have never done this type of analysis. The question is not even properly posed. When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.
All of the planets you cite have a radiatively absorptive atmosphere between a relatively warm surface and the incredible cold (3K) of deep space. Like a steel bar with one end in boiling water and the other end in ice water, there will be a temperature gradient between the hot and cold ends. If the gradient of the atmosphere exceeds the adiabatic lapse rate, as it is on all of these bodies, the lapse rate is unstable, and convection will start to bring it back toward adiabatic.
Yes, I took physics in HS and in college and in graduate school. How about you? Did you forget that kinetic energy measured in Joules = Pressure*Volume, and that gas temperature is proportional to the internal kinetic energy of a gas? Perhaps these Wiki articles might help your memory:
Oh, and make sure to retake the class about adiabatic processes to understand how pressure relates to temperature in standard thermodynamics. You can also read carefully our paper, where these things are explained on pp. 6 – 15, while noting that our accurate model (Eq. 10a) is based on actual VETTED OBSERVED data, hence, it’s real!
But the atmosphere is not falling — it has already fallen.
I’m throwing food again.
How so? Is the atmosphere just sitting there static? I thought that the atmosphere was ALWAYS falling, … AND rising, … AND falling, … all the time. Doesn’t this mean that the atmosphere is WORKING all the time?, … under the influence of gravity? — gaining potential energy on the rise?, “unleashing” that potential energy on the way down? … in a continuous cycle?
There is complete confusion here by multiple commenters between the concepts of force, energy, and power.
The hook holding up the 5kg weight exerts a (continuous) force on it of F = m*g = (5 kg * 9.8 m/s2) = 49 Newtons.
To hold it in place, the hook does work on the weight of W = F * d = 49 N * 0 m = 0 Joules. So the power required is 0 Watts (= 0 Joules / time).
What are your equations for force, energy (work), and power for this case?
Andy cannot get it through his head that a one time increase in strain energy is NOT an ongoing continuous power transfer, no matter how many times this basic and trivial point is explained to him.
Ned: I am absolutely appalled that with all your credentials, you do not understand the most basic points you should have gotten in high school physics.
Pressure/volume work requires a CHANGE in volume to do the work. It’s really p*dV work. This is the same thing fundamentally as force needing to create movement to do work. The gas in a piston chamber must move the piston head to do work on it.
The weight force of the atmosphere on the earth’s surface does not move the earth’s surface. (Even if an initial application of this weight force caused some compression, there is no further change.) So the pressure/volume work done is zero.
These are basic, basic points of high school physics, and you get them completely wrong!
Pressure/volume work requires a CHANGE in volume to do the work. It’s really p*dV work. This is the same thing fundamentally as force needing to create movement to do work. The gas in a piston chamber must move the piston head to do work on it.
Height of the atm changes daily. Goes Up and Down like a thermometer.
Ned: I am absolutely appalled that with all your credentials, you do not understand the most basic points you should have gotten in high school physics.
Ed, it’s worse than that. He’s had the basic points explained to him here on WUWT, over and over, and he STILL doesn’t get it.
But heck, if you want a good laugh, you can read about Ned Nikolov’s work here …
You say: “Height of the atm changes daily. Goes Up and Down like a thermometer.”
The claim of “Atmospheric Pressure Effect” (APE) enthusiasts like Ned Nikolov is that the static weight force (pressure) of the atmosphere does continual work on the planet’s surface. To do this, it must move the surface downward.
If the height of the atmosphere varies up and down a little bit, that does not mean anything to the surface.
If you don’t count the downwelling longwave infrared flux (back radiation), the surface energy balance is out of whack by about 250 W/m2, averaged over the surface. The APE enthusiasts claim that the weight force of the atmosphere can make up this imbalance. That’s 250 Joules of work every second for every square meter of the planet’s surface. With an atmospheric pressure of about 100,000 N/m2, the earth surface would need to be continually compressing at 250/100,000 m/s, or 2.5 millimeters every second. This means that the earth’s radius would need to shrink by over 75 kilometers each year! Seriously???
Facepalm. It’s really hard to deal with ingrained blind ingnorance.
Willis, how about you accept that Ned has a much better understanding of physics than you and that it would be a good idea to take that as a starting point to try and understand where your misconceptions lie, rather than try to keep proving that cold heats hot and work done equals energy no longer in existence? Truly the level of your ignorant stubbornness could teach my 5 year old grandson a thing or two!
To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?
Why is it that Ned and Karl can accurately model the temperatures of every planetary atmosphere in the solar system with the same mathematical formula, but greenhouse effect proponents can’t even get their mathematics for earth to agree with itself, much less even one other planet?
Facepalm. It’s really hard to deal with ingrained blind ingnorance.
Willis, how about you accept that Ned has a much better understanding of physics than you and that it would be a good idea to take that as a starting point to try and understand where your misconceptions lie, rather than try to keep proving that cold heats hot and work done equals energy no longer in existence? Truly the level of your ignorant stubbornness could teach my 5 year old grandson a thing or two!
If you wish to follow the “science” of a man using an ad-hoc equation with more tunable parameters than data points to explain, you are welcome to.
To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?
He is right. The kinetic energy is converted to thermal energy. You sure you understand how this “science” thing works?
Why is it that Ned and Karl can accurately model the temperatures of every planetary atmosphere in the solar system with the same mathematical formula, but greenhouse effect proponents can’t even get their mathematics for earth to agree with itself, much less even one other planet?
BECAUSE NED HAS MORE TUNABLE PARAMETERS IN HIS EQUATION THAN HE HAS DATA POINTS TO EXPLAIN, PLUS FREE CHOICE OF EQUATION WITH NO NEED THAT IT BE PHYSICALLY BASED!
Sheesh! Given more parameters than data points to explain, it would be surprising if Ned could NOT explain the datapoints … but then I guess you don’t know how that works. Let me offer you the following, come back once you’ve read it. It explains very clearly why Ned is just fooling the rubes …
You say: ‘To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?’
BTW, it’s “Mr. Numpty” to you 😉
I already answered your question above, when I said: “When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.”
Since I was obviously too fast for you, I’ll break it down for you ask I would a high school physics problem to a struggling student.
Let’s take Newton’s apple of 0.5 kg, hanging 3 m above the ground in earth’s gravitational field of 9.8 m/s^2. It has a gravitational potential energy relative to the ground of PE = mgh = 0.5 kg * 9.8 m/s^2 * 3 m = 14.7 Joules.
Now the stem breaks and the apple falls toward the ground. Just before it hits the ground, the gravitational potential energy of 14.7 J has all been converted to kinetic energy of KE = (1/2) * m * v^2.
We can calculate v = sqrt (2 * 14.7 / 0.5) = 7.67 m/s.
Next it hits the ground in an inelastic collision and stops moving. Such an inelastic collision converts all of the kinetic energy of 14.7 J to internal (thermal) energy. For simplicity, we’ll say that all of this added thermal energy is in the apple as it splats on the ground.
The apple is mostly water, so has a thermal capacitance of about 4 kJ/kg/K. This leads to a temperature increase in the apple of:
Do you think the falling air of the Chinook wind leaves a vacuum at the higher elevations?
No. Do you?
The most significant point about an unstable air mass is that if a parcel of air at a given level within the air mass is forced upwards it will continue to rise; but equally important is this: – if an identical parcel of air from the same starting level in the same unstable air mass is forced downwards, it will continue to fall. We can all see the effects of the rising air in an unstable air mass when it cools sufficiently for the water vapour to condense and form cumulus clouds. What we fail to see, and this is what makes them so dangerous for aircraft, is the descending unstable air, the cold invisible down draft located alongside the rising cell. It is a mistake to assume that this cold downdraft is the same air that rose inside the convection cell. It is not. The rising air inside the storm becomes separated from the water vapour that formed the storm cloud as the rain falls out of the cell and the lifted separated and now dry air remains aloft in the anvil cloud at the top of the storm.
It is this process of drying by physical separation of moisture that makes convection that produces rain an irreversible process. Descending moist air in a cloud can evaporate the surrounding water droplets from the cloud, dissipating it and so slowing the rate of adiabatic warming as the moist cloud containing air parcel descends. Dry air cannot be cooled by evaporation of surrounding cloud moisture because there is none available. Consequently in the descending limb of the Hadley cell dry air is warmed as it is forced down by the Coriolis Effect and ends up at the surface, just like the Chinook wind, at a higher temperature than its initial starting point. And the reason for this change in temperature is because the latent heat of condensation of water vapour that powered the initial rise to the top of the troposphere in the convection storms of the equatorial zone made the rising air cool more slowly.
Nikolov claimed that the weight force of the atmosphere provides an ongoing energy transfer to the earth’s surface that keeps the surface at a substantially higher temperature than it would be otherwise.
I showed him the very basic high school physics point that static pressure provides no energy transfer, and that any deviations from the static case due to updrafts and downdrafts must cancel out.
Note carefully that I am talking about the mechanical force due to the weight of the atmosphere. You are talking about something completely different.
Yes, the convection circulation of air is an irreversible cycle, both due to the evaporation at the surface and the condensation at altitude, and the reduced IR opacity of the atmosphere at altitude. But this does not affect the weight force of the atmosphere on the surface.
Besides, what you describe has a net cooling effect on the surface (even if there is localized warming at downdrafts). Nikolov was arguing for an atmospheric warming effect.
Why do we need to continuously explain basic physics…err an elementary concept?
Solar energy lifts air. Literal energy from an outside source does work on the atmosphere, it has warmed and lifted a parcel of air into the atmosphere. At this point, the d-nyers of basic physics suggest that the energy simply goes away. That’s not what happens. The air has potential energy at this point, it falls and energy is converted to kinetic energy in the lower atmosphere.
Let’s work backwards now, we have kinetic energy added to the lower atmosphere from compression, the work was done by gravity. How did that air get there, it was lifted from being heated. How was it heated, from an outside energy source or an internal energy source? Uhh, an external source, still with me? So is the net result zero energy input into the system, no, energy is retained within the system that was obtained from an EXTERNAL ENERGY SOURCE.
If we must explain that a parcel of air in the upper troposphere is actually moving a distance, h, when it falls then I don’t know how much further back in explanation we need to go. Was that parcel of air magically moved into the upper atmosphere? Was that air simply pulled into the upper troposphere because falling air left a void for it to replace? Errr, no. The entire process is started from energy being input from the sun.
Does this really need further explanation to people. Well, I guess it might to people saying
Ed Bo,
I am all for a dynamic atmosphere that moves both mass and energy, causes local surface heating (Chinook winds) and elsewhere local surface cooling (virtually every rainstorm I have ever experienced).
Sadly, the very meaning of the term “adiabatic” is forgotten here in arguing that gravitationally induced compression of a parcel of air cannot increase its temperature in accordance with the Ideal Gas Law. The compression factor PV/nRT need not be unity, as for an ideal gas, for the temperature to rise adiabatically.
This conversation is absurd. You need to define the system boundaries. This old engineer still remembers his statics. If a mass is not moving, then no work is being done. For an object to be static, all the forces must add to zero and all the moments must add to zero.
It’s a different problem if you add in how the forces are created. Holding a weight motionless does no work. However, there is energy being expended in the muscles of your arm to create that force.
One person is ignoring how the forces are created and the others are trying to include the force creating process. Neither side is discussing the same problem.
I agree – the notion that a gas such as CO2 or water vapor can “trap” radiant heat in a free atmosphere is simply unphysical.
This from Ned Nikolov, the man so ashamed of his own work that he published it under a false name … well, actually, he would have published it, but I pointed out his craven deception to the journal, and they pulled his article.
His “scientific theory” involves an equation with more tunable parameters than there are data points … and while that kind of mathturbation causes anyone who thinks about things to laugh, I fear he has found a following among the credulous.
Ah well … just saying, folks, he’s a man who is willing to lie about his own name …
Still works better than your greenhouse effect mathematics. It also is based on basic principles that cold doesn’t raise the temperature of hot without work being performed. Concepts you still struggle to comprehend.
I guess you must have missed the rest of this post because you never addressed it in your “take down” of the original Nikolav paper. And you pretend you have actually refuted anything
John Day
@ur momisugly Willis
> There is, of course, a technical term for what they have done,
> as there are no new mistakes under the sun. It is called “overfitting”.
I think you’re looking at this in the wrong way. You say ‘overfitting’, which suggests they are somehow dishonestly trying to ‘cook’ a formula to fit 8 examples.
I don’t think N&K (Ned&Karl) are dishonest. In fact, I think they are merely learning the relationships between pressure induced and radiative warming by trying to fit the set of parameters to a regression equation.
“Learning is compression” in the sense that they want to find the smallest set of parameters which fit the data. I.e Occam’s Razor: if two regressions, one with 5 parameters and another with 5000 parameters, both fit the data, which is better? Ans: keep it as simple as possible (but not too simple).
You’re also missing the main point:
“Pressure by itself is not a source of energy! Instead, it enhances (amplifies) the energy supplied by an external source such as the Sun through density-dependent rates of molecular collision. This relative enhancement only manifests as an actual energy in the presence of external heating. “
Note that the temperature T of a system in equilibrium can be computed from the just kinetic energy of the moving gas particles and their mutual collisions (density, implying pressure). We don’t need to know the radiative aspects of the system to compute the temperature! What part of the Ideal Gas Law do you not understand here?
So, having computed the temperature T we can then ask the question: where did the kinetic energy come from? Probably from solar heat energy absorbed by the surface.
But the point is we don’t need to know where the energy came from. Temperature T is soley dependent upon the internal kinetic energy of the gas and its density.
No change in pressure required. Yes, a pressure “gradient” necessarily exists on all planets with atmospheres, but that is accidental in the sense that even the gradient itself is not required to understand that at any point x,y,z the temperature is solely a function of kinetic energy and transfer of momentum by collisions.
N&K further make the claims that show no pressure change is needed
“In the case of an isobaric process, where pressure is constant and independent of temperature such as the one operating at the Earth surface, it is the physical force of atmospheric pressure that can only fully explain the observed near-surface thermal enhancement (NTE). “
Isobaric?
Yes, if you choose a long-enough time scale:
“the near-surface atmospheric dynamics can safely be assumed to be governed (over non-geological time scales) by nearly isobaric processes on average, i.e. operating under constant pressure. This isobaric nature of tropospheric thermodynamics implies that the average atmospheric volume varies in a fixed proportion to changes in the mean surface air temperature following the Charles/Gay-Lussac Law, i.e. Ts/V = const. “
Willis, please think about it some more before summarily rejecting it as nonsense.
AGAIN, we must ask, what is it you are missing about the physical explanation for which mathematical models exist to explain and match the temperature of rocky planets with atmospheres?
I guess you must have missed the rest of this post because you never addressed it in your “take down” of the original Nikolav paper. And you pretend you have actually refuted anything
I guess you must have missed the fact that Nikolov is using more tunable parameters than the data points that he is fitting. Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
When you do that you are GUARANTEED a good fit … but of course, that fit is meaningless. It would be hard to not get a good fit with that procedure.
Or perhaps you don’t realize what it means when you have more tunable parameters than data points to fit. Once again let me recommend Freeman Dyson’s clear takedown of the bogus Nikolov style of analysis …
Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
But there is a physically meaningful mechanism for how this works. It has been described numerous times on here and no one has refuted it with anything but sophistry.
The descending cool dry air within the Hadley cells retains energy within the climate system. This is a basic fact that keeps being rejected for no reason aside from conventional wisdom. Where is this retention through gravitational potential energy in the oft cited energy balance cartoons that you see above and below? Those flat earth cartoons are so laughably flawed that it’s amazing anyone is still using them.
Others have cited this process in gas giants. It is the same concept, but in the case of gas giants the energy source to start the convection is internal and therefore there is net energy loss, but not nearly as much energy loss if there wasn’t a gravity driven compression by the returning downdraft. On Earth, the energy source to kick off the convection is external, so therefore any returned energy is a net addition into the system, it limits the energy lost via the thermals and release of latent heat. This is true regardless of the composition of the atmosphere, it just matters that there is an atmosphere at all for convection to take place.
And I already discussed the empirical observations demonstrating this effect. The latitude belts between 23-30 degrees emit more LWIR back into space than any other region, even losing far more heat to space than received from the sun in places like the Atacama. It even holds true for the atmosphere over the oceans at these latitudes despite 30 mm of precipitable water per cubic meter.
Notice the subtropical south Atlantic. Greenhouse gases are surely present there, spreading that notorious back radiation around, yet this is among the highest outgoing radiative regions of the atmosphere. What is the source of that heat? I would say it from the additional heat added to that part of the atmosphere from gravity driven compressional heating at the descending leg of the Hadley Cells. Yes, that heat came from a different part of the atmosphere, but that heat in turn came from the sun. That means the process is retaining heat in the climate system, ironically much like how a greenhouse actually works, but instead of glass roof it is the force of gravity.
Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
But there is a physically meaningful mechanism for how this works. It has been described numerous times on here and no one has refuted it with anything but sophistry.
Thanks, RW. First, whatever the mechanism that you are describing might be, it’s not described by his equation. Look at the equation! It has little to do with anything.
Second, if your proposed mechanism were true, it would allow a planet with an IR-transparent atmosphere (e.g. argon) to be warmer than the S-B temperature … but as I showed in A Matter Of Some Gravity, that violates conservation of energy.
Third, regardless of all of that, he has more tunable parameters than he has data points to fit. This is a common enough mistake that it has its own name, “over-fitting”, and his is the most egregious example of overfitting that I’ve ever seen.
So let us assume that we have the airless perfectly evenly heated blackbody planet that I spoke of above, evenly surrounded by a sphere of mini-suns. The temperature of this theoretical planet is, of course, the theoretical S-B temperature.
Now suppose we add an atmosphere to the planet, a transparent GHG-free atmosphere. If the theories of N&K and Jelbring are correct, the temperature of the planet will rise.
But when the temperature of a perfect blackbody planet rises … the surface radiation of that planet must rise as well.
I don’t think that’s what’s implied by the theory at all. You can’t take a temperature of something (atmosphere at the surface) that is not there, but the surface of the planet itself would not heat up, no.
If you added an atmosphere of pure argon, there would be absolutely no condensed or solid particulate matter in the sky to absorb incoming radiation, and let’s assume this atmosphere refracts no incoming light. The surface of that planet will receive the full brunt of the solar radiation.
But, molecules in the atmosphere at the surface will heat up and convection starts. This then cools the planet’s lower atmosphere as heated air carried to less dense areas of the atmosphere, the heated air decompresses, hot molecules bump into cool molecules, and heat is radiated into space.
This process cools the surface and is the complete climastrology certified version of planetary energy budget models regarding pure thermal convection. But what happens as that air falls down to the surface again due to gravity? Unequivocally, that air is compressed and heats up, that is if you believe in the work of William Henry. Latitudinal dependent heating takes place in the area of the Hadley Cells where the gravity driven compression warms the air, exactly what we see on Earth. Far less of the heat is lost to space than is modeled, it’s ironically similar to the argument that the back radiation heats the surface by slowing the radiation that leaves the planet.
So, if you believe that the inherent gravity driven “heat retention” of the atmosphere actually exists at all, and I hope for William Henry that you do, then how do you think that compares to the importance of the previously discussed 0.001-1.7 eV molecular vibrations of the inaptly named GHG molecules and there emittance of this energy within the atmosphere?
And let’s imagine a planet with the same gravity as Earth, circling the same sun in the same orbit, but with an atmosphere of pure argon. Would that planet be hotter or cooler than Earth? With an atmosphere of the same molar mass, I bet that planet’s total average atmosphere would be cooler than Earth’s with no latent heat, but probably a much hotter surface due to higher SWR. Convection would probably actually be more active due to the extremely hot surface and argon doesn’t freeze until until -189 C so the structure of the lower atmosphere would be quite different. So theoretically if that atmosphere was able to stay gravitationally attached to the planet, where the pressure in this imaginary atmosphere is 1 atm, it would probably be about the same temperature as Earth at the same latitude.
I think this qualifies as a great example of how hard it is to defeat erroneous conventional wisdom.
Researchers have literally had to infect themselves with diseases to defeat it. Petroleum geologists were busy controlling oil blowouts in Oklahoma while others were still claiming commercial oil would never be found west of the Mississippi. But eventually, the science progresses.
Ned Nikolov
November 19, 2017 1:50 pm
A key point in this discussion is the fact that an enhanced IR absorption by some gases does NOT imply an ability of such gases to trap heat in an open convective environment. IR absorption and IR trapping are two distinctly different processes with different control mechanisms that have erroneously been conflated since the time of Fourier and Tyndall in the 1800s …
Ned Nikolov
November 19, 2017 at 1:50 pm: IIRC, Fourier and Tyndall have been misquoted by all warmista. They do this everywhere, thinking we won’t check. Sadly, they have been correct in that, too much.
One can clearly see that Arrhenius simply took Fourier’s conjecture/assumption about “heat trapping” by an open atmosphere as a “physical truth” without any empirical verification. He further developed the idea by proposing a “model” (his Equations 3 and 4) that relates Earth’s surface temperature (T) to atmospheric CO2 (K expressed in relative units) via equating T^4 to a ratio of dimensionless quantities! By failing to match measurement units between the left- and right-hand side of his equation, Arrhenius violated a basic principle of dimensional analysis in physics, which of course renders his model nonsensical from a mathematical and physical standpoint of view .. And this nonsense has been touted by the mainstream climate science for decades as one of the greatest scientific achievements of the 19th century!! Simply amazing …
Experiments by Arrhenius’ colleague, physicist Knut Ångström, the results of which were published in his 1900 paper “Über die Bedeutung des Wasserdampfes und der Kohlensäure bei der Absorption der Erdatmosphäre,” (Annalen der Physik 308(12): 720-732.), showed that that CO2 is transparent to 90% of infrared radiation applicable to temperature variation; and that those infrared bands which CO2 readily obstructs are already almost totally blocked by atmospheric H2O. His finding, that the relationship between the concentration of CO2 in the atmosphere and its effect on back radiation is logarithmic, has been replicated by many subsequent experimenters; all of whom show that doubling of the present carbon dioxide content in the atmosphere would only increase the back radiation by about 3.6 W/m ², which would, in the absence of other factors, give rise to an increase in temperature of between 0.6 and 0.8 C°.
Some think that doubling might yield a temperature increase of up to 1.2 C°. The only way that Warmunistas can get a scary range of 1.5 to 4.5 C° is by assuming unphysical positive water vapor feedbacks not in evidence.
A key point in this discussion is the fact that an enhanced IR absorption by some gases does NOT imply an ability of such gases to trap heat in an open convective environment. IR absorption and IR trapping are two distinctly different processes with different control mechanisms that have erroneously been conflated since the time of Fourier and Tyndall in the 1800s …
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field. Despite that, you have not defined the term, so we have no idea what it is that you mean.
How about you give us a clear definition of what you mean by “IR trapping”, so we can follow your ideas? Because for all we know it’s just a strawman that you’ve erected to knock down …
Willis said, on November 21, 2017 at 9:42 pm, in reply to Ned Nikolov :
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field.
Odd, because, from what I have observed, this term IS a common term in the field:
From a NASA website: https://climate.nasa.gov/causes/ Most climate scientists agree the main cause of the current global warming trend is human expansion of the “greenhouse effect” — warming that results when the atmosphere traps heat radiating from Earth toward space.
Someone needs to tell NASA that a common conception of the “greenhouse effect” is not to “trap heat”. Oh, and why they are at it, someone also needs to remind NASA that heat does NOT radiate, and that by continuing to speak of it as such, they continue to confuse the two concepts of “heat” and “radiation”.
From a Harvard University website: http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html Some of this terrestrial radiation is trapped by greenhouse gases and radiated back to the Earth, resulting in the warming of the surface known as the greenhouse effect.
Well now, Harvard some folks seem to think that greenhouse gases TRAP radiation. They relish the word, “trap”, and they use it in a way to confuse people into associating “heat” DIRECTLY with radiation, as to suggest that the two terms might be interchangeable (wrong).
A Columbia University website: http://www.columbia.edu/~vjd1/greenhouse.htm While the dominant gases of the atmosphere (nitrogen and oxygen) are transparent to infrared, the so-called greenhouse gasses, primarily water vapor (H2O), CO2, and methane (CH4), absorb some of the infrared radiation. They collect this heat energy and hold it in the atmosphere, delaying its passage back out of the atmosphere.
Let’s look at definition #18 for the verb, “trap” at Dictionary.com: 18. to stop and hold by a trap, as air in a pipe. So, it sure looks to me like Columbia University is talking about TRAP — they just avoid the term and describe what the term means in other words. Sorry, but “holding” and “delaying” is TRAPPING.
Consequently, I’m afraid that straw men are overseeing NASA, Harvard, and Columbia University, at the least. I wonder whether they are also spineless, … as in too timid to alter the momentum of confusion that they have helped nurture.
Willis said, on November 21, 2017 at 9:42 pm, in reply to Ned Nikolov :
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field.
Odd, because, from what I have observed, this term IS a common term in the field:
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
And THAT’s the problem — this simplistically WRONG explanation, using a word that WRONGLY compares a familiar plant-house structure with a gaseous atmosphere, perpetuates the very use of a term that IS ill-defined. If this term is so rampantly used with the “general public”, then how is the “general public” ever to come to terms with any reality?
The person you criticized initially for using this term was merely using it as a reference point, as many organizations state it. Nikolov was, in effect, kowtowing to this use, letting it slide in its amorphousness — as it is spread amorphously without any clear definition already by all who use it, as if this is okay. You made his using the term an issue, which it should not have been, in my opinion, which seems like a distraction, rather than a call for a definition that was significant to Nikolov’s statements. It is this nebulosity of definition that tends to give alternate views some credibility, I would say.
Oh, and not only does the term appear in press releases, but also it appears in the context of professional scientific organization’s, as a reflection of professional identities, like the following:
The warming effect of CO2 and other heat-trapping gases is well established and can be demonstrated with simple science experiments and satellite observations.
Earth’s atmosphere does the same thing as the greenhouse. Gases in the atmosphere such as carbon dioxide do what the roof of a greenhouse does. During the day, the Sun shines through the atmosphere. Earth’s surface warms up in the sunlight. At night, Earth’s surface cools, releasing the heat back into the air. But some of the heat is trapped by the greenhouse gases in the atmosphere. That’s what keeps our Earth a warm and cozy 59 degrees Fahrenheit, on average.
NASA’s spineless straw men apparently want to start indoctrinating America’s (and the world’s) youth as soon as possible with the TRAPPING idea, AND setting up future confusions in adulthood with the “earth’s-atmosphere-is-like-a-greenhouse” idea. Tragic!
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
Why would you think that I think the term has any scientific meaning. The term is crap. I think Nikolov knows that it’s crap too, but he used it as a device to hold people’s attention a bit, without saying that it’s crap, while he put forth more info from his point of view. The straw man that I am seeing is raising an issue about the term at all in Nikolov’s context, since it was used merely to set the stage of discourse, rather than as a label for stating a belief via the term’s clear definition.
The “clear” definition of “trapping heat” that you might seek is purposefully veiled in the totality of faulty discourse defending the “greenhouse effect”. All the math, all the elaborate descriptions, all the minutia devoted to the “greenhouse effect” is shadowed by this convenient umbrella idea of “trapping heat” or “trapping radiation”.
Supposed educators use the term haphazardly, aimed at people from childhood to adulthood, conditioning their minds to have a sense of heat or radiation as some “stuff” that gets caught in a trap, and then, after all the years of childhood and early adulthood, they then get blasted for using a term as adults that they were NEVER taught any better prior to adulthood. Very convenient, I’d say — nurturing child-like minds, so that sophisticated minds can dissect them as faulty-thinking minds, while sophisticated WRONG arguments are peddled, based primarily on appeal to adult-mind authority.
Very scientific! [sarcasm intended]
Roderic Fabian
November 19, 2017 2:22 pm
Carbon dioxide lasers have been used in laboratories for a long time. They rely on the property of CO2 to absorb IR, hold on to it for a time, and then release it in the form of a coherent beam of IR collimated by the laser apparatus. You can feel the warmth of a beam of IR produced by a small CO2 laser. Carbon dioxide in the atmosphere does the same thing, but an atmospheric CO2 molecule releases the IR in a random direction, half of the time upward toward space and the other half down to the earth. So a portion of the IR headed toward space is re-directed back to earth. This does not warm the earth, but it keeps it from cooling as fast, all other things being equal. The earth’s surface will therefore equilibrate at a somewhat higher temperature. Hence the greenhouse effect.
Gill’s original post talked about CO2 emitted IR “bouncing around for a while”. Yes, that’s what causes atmospheric temperatures to equilibrate at a higher level than they would without CO2.
You can also feel the warmth of a beam of IR produced by a large CO2 laser but I don’t recommend it. Actually these are fantastic inventions, now due to careful engineering, design and lots of associated hardware gizmos it is possible to get efficiencies of around 10% from a commercial CO2 laser. Yes , pump 10kW into it (effectively thermal energy) and you can get a cutting beam of 1kW out. Of course there is 9kW of heat you need to throw away so much of the apparatus is hot hot hot and there is coolant, pumps, radiators, etc to get rid of it.
I think we done well ! Compared to the equivalent gaia design where you shove 10kW off the surface of the earth, through all that wispy gas (including 0.04% CO2) and get a downward beam of [REDACTED #1] kW making an efficiency of [REDACTED #2] %.
It’s like that jar at the fair with loads and loads of sweets in it and you are supposed to guess the number and write it down with your name.
The persons who said 5kW and 50% should now identify themselves..
This does not warm the earth, but it keeps it from cooling as fast, all other things being equal. The earth’s surface will therefore equilibrate at a somewhat higher temperature. Hence the greenhouse effect.
CO2 lasers do not work with the ‘cold’ radiation of 15 um but a much warmer one that was stated in the above post: “Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.” https://en.wikipedia.org/wiki/Carbon_dioxide_laser The CO2 laser produces a beam of infrared light with the principal wavelength bands centering on 9.4 and 10.6 micrometers (μm).
Val Ryland
November 19, 2017 2:38 pm
As the author expects, there are some errors in this post that are responsible for the erroneous conclusion. The most important are these:
1. Wien’s law refers to the _peak_ wavelength of emitted radiation, not the only one. The distribution of the spectral radiance is given by Planck’s law. If the spectral radiance peaks at 10 micrometers, you can be certain sure there’s a lot of radiation around 15 micrometers as well.
2. For an object close to room temperature, no matter if solid, liquid, or gas, the atomic/molecular energies are largely unimportant (it happen to be important for CO2, which is the whole shebang). This is because at any ordinary temperature the occupancy of any state other than the ground state is pretty much zero, because typical atomic energies are separated by several eV. In order to get a fraction of 1/e molecules at an energy 1 eV above the ground state, the temperature would need to be >10,000 K. The important degrees of freedom are translational, and, depending on the substance, rotational and vibrational. The latter two are typically quantized, but the former is continuous. In other words, even if you don’t have some atomic energy accessible, you can ALWAYS knock particles around a little bit. Thus, a hydrogen gas at room temperature can be treated for all practical purposes as a gas of featureless, structureless point particles.
3. There is no physical law that states that molecules in liquids or solids “need more energy” in order to be excited, and I have no idea where this assumption could’ve come from.
All in all, the basic greenhouse mechanism remains unchallenged.
Thanks for posting this Anthony, it includes most of the misconceptions and errors about radiative transfer and CO2, so gives a good opportunity to rebut them in one place. References to the science can be found in any college textbook on Physical Chemistry and Molecular Spectroscopy. https://books.google.com/books/about/Physical_Chemistry_5th_Edition.html?id=nIggbG9i8qEC
My understanding of Thermodynamics and Radiation from CO2 is as follows:
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law).
It’s a reciprocal relationship not linear.
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
Wrong, those wavelengths exist in solar radiation and in surface emissions, so they are absorbed by CO2, just not in large amounts because those wavelengths are in the tails of the respective spectra.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is wrong and meaningless. Wien’s Law tells you the location of the peak wavelength, as the graphs added by Anthony shows wavelengths above and below that exist in the spectrum. More relevant to the IR emissions is the following:
As you can see the CO2 absorption band at 15micron is present at all the temperatures shown (dashed lines). Even though the maximum shifts with temperature so does the radiance increase as temperature increases. Consequently we see the following:
A blackbody at 300K emits 26.8522 W/m2/sr between 13 and 17 microns
at 270K it emits 18.4933 W/m2/sr
at 223K (-50ºC) it emits 8.53892 W/m2/sr
at 193K (-80ºC) it emits 4.32413 W/m2/sr
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
This is more like atomic structure and doesn’t apply to molecules (it’s more complex, bonding and non-bonding orbitals etc.)
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
No, that refers to part of the internal energy, the temperature is the kinetic energy of the molecule.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
No, it must receive a photon of exactly the right energy to excite the transition. However electronic transfers in molecules are excited by UV light. The transitions which we are talking about in the IR are excitations of the vibrational and rotational modes. In the case of CO2 absorption at 15micron it is the vibrational mode that is excited, specifically the bending of the CO2 molecule (with associated rotations).
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Again the photons must have exactly the right energy to reach the excited vibrational state. The molecule can emit the radiation but it’s far from immediate it will undergo millions of collisions with surrounding molecules during that time and in the lower atmosphere is more likely to transfer the energy to the atmosphere (thermalizes)
Photons with not enough energy to raise the orbit of any of the electrons excite the vibration are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.
The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Won’t be scattered or reflected.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
In the band from 13-17micron, as you can see from the diagram above it’s significant part of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
No, it depends on the molecule, and again it’s not electron excitation it’s bond vibration.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
As explained above this is not true for several reasons.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
No, the earth’s surface is very close to a black body and will readily absorb a photon emitted by CO2, or it could be absorbed by another CO2 molecule, or thermalize.
So am I right?
Mostly not I’m afraid, but thanks for the opportunity to correct these misconceptions which as you suggest are frequently encountered on the web.
And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
As pointed out the concept of CO2 molecules at -80ºC is a major misunderstanding of Wien’s law (it also makes more sense to use the form of that law which relates peak frequency to temperature since that is proportional to energy).
Go to MODTRAN and test this for yourself. You will see that CO2 doesn’t have any impact what so ever until you get up to about 3 to 5 km when H2O starts dropping out of the atmosphere. If you then take a look at the temperature of the atmosphere at those heights where CO2’s signature is present, you will see that it is about -80°C. What you are seeing is the thermalization of CO2/15µ, and it results in a temperature floor, not an increase in the ceiling. CO2 only warms the upper atmosphere, not the lower troposphere.
What a fascinating collection of comments. I never would have thought there would/could be so many different ways to visualize radiation, temperature, and heat flow.
Well worth reading in their entirety several times I think.
More simply, radiation from a cooler object cannot naturally raise the temperature of a warmer object. This is so basic and so obvious that WUWT should hang its head in shame over the amount of articles it has posted with convoluted explanations to the contrary.
The whole premise of the Greenhouse Effect has nothing to do with how much Radiation CO2 can absorb, but how much the radiation, emitted by the CO2 in the atmosphere, can warm the surface of the earth. The answer, of course, is zero! Radiation from a cooler source cannot increase the temperature of a warmer source.
End of story. You don’t need to invoke quantum physics to understand it. You just need to stop holding sophists with PhDs in awe and use some common sense and basic observations.
radio waves can be absorbed by a receiving antena that is warmer than the emitter and be warmed up such that it glows red hot. try putting an electrical conductor in a microwave oven
You are right – a cold object can not warm a warmer object.
However, the temperature of the atmosphere is between the maximum surface temperature and the minimum surface temperature. Therefore, the CO2 in the atmosphere can increase the *average* surface temperature by keeping the nigh time minimum warmer than it would be if there was no atmosphere.
Lars P.
November 19, 2017 3:30 pm
“This is a contentious subject, and I have often shied away from it because it often erupts in food fights. ”
Well yes this opens a can of worms.
Here my 2 worms, er, cents 😉
1) Basically what increasing CO2 in the atmosphere does is shortening the path of the 15 um radiation, from let’s say 10 meters to 9.
One could argue that, in the whole air column, the heat transfer through radiation made with 9 meter steps, instead of 10 meter steps, would increase the lapse rate and thus increase the temperature of the surface. However this is dependant on the percentage of energy that flows through the 15um from the total which I guess is in the order of less then 1/1000.
=> effect cannot be measured
2) Personally I think the whole greenhouse effect is being built on a wrong basis as the elephant in the room is excluded: the oceans.
It is not the atmosphere that has the greatest greenhouse effect, but the ocean, water is practically opaque to infrared radiation, not transparent as the atmosphere is. Light penetrates the oceans to depth up to 100 meters and warms it, however the ocean loses heat only at the surface. The puny effect of CO2 ‘backradiation’ (and all other atmosphere including water vapor) is dwarfed by a 0.5 meter water strata at the surface of the oceans.
The idea that the oceans would freeze without the atmosphere’s backradiation – as the warmists maintain is nonsense. The oceans receive a 1300 W/m2 at the Equator. That heat is absorbed, stored and keep the oceans warm in the night too. No danger of frozen waters.
Of course the atmosphere has its contribution too, but the oceans cannot be completely dismissed as it is done.
Geology proves that the ocean currents define the climate, not CO2 variations, but hey, maybe it is still too early to consider this…
Clyde Spencer
November 19, 2017 3:53 pm
For the geologists and mineral collectors here (and those of you old enough to have ever bellied up to the bar in a discotheque), you are probably familiar with the effect of shining a ‘Black Light’ on solid (e.g. scheelite) and liquid substances (e.g. hydrocarbons). Short-wavelength lights will invariably give different ‘glowing’ colors and intensities than long-wavelength lights. In general, the observed fluorescence and phosphorescence is of a shorter wavelength than the stimulating electromagnetic energy. As a rule of thumb, the fluorescence is of a longer wavelength than the stimulating wavelength, and is variable in duration with different materials. The fluorescence is usually confined to one or two narrow wavelengths, which distinguishes it from thermal emissions. That is to say, if a substance (including individual gas molecules) receives EM radiation of greater energy than an electronic orbital transition, some or all of the energy may be absorbed, and then re-emitted when, after a highly variable period of time, the electron(s) falls back to the ground state. So, strictly speaking, narrow wavelength emissions from CO2 should be considered fluorescence, and should be occurring at wavelengths longer than the absorption bands.
[ https://micro.magnet.fsu.edu/primer/techniques/fluorescence/fluorescenceintro.html ]
On the other hand, the molecules of solids, liquids, and gasses possess kinetic energy in the form of the velocity of individual molecules, straining in the chemical molecular bonds, and in the case of solids, vibration in the crystal lattice. All of these sources of kinetic energy have different energies; the integrated energy is related to the average velocity of molecules, and the average vibrational bonding energy. At high temperatures, Doppler shift will cause the apparent emission wavelengths to also be different. At certain energy levels, bonds can be broken, and a change in physical state will take place, Thus, the distribution of emitted thermal energy with respect to wavelength will approximate the classic Black Body emission.
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption. That is to say, the presence or absence of water vapor will strongly effect whether the atmosphere retains the CO2 emissions. The thermal emission should correspond to the average temperature of the gas molecules.
Once again, the situation is more complex than climatologists are either aware of, or want to admit.
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption.
You’re mistaken because you’re extrapolating from electronic transitions with vibrational fine structure to vibrational transitions with rotational fine structure where the ‘red shift’ is far less pronounced and you don’t have to worry about the Frank-Condon effect.
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
You are simply demonstrating your complete scientific illiteracy.
You see an equation, like the one for Wien’s Displacement Law. It expresses the peak radiative flux wavelength of a blackbody as a function of its temperature.
If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.
But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.
This is, simply put, complete nonsense!
Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target. Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
“If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.”
Uhhh, just what do you think I was saying? Just what is a black body to you? Yes, you warm up a body, and that body emits a spectrum. Just what do you think I was talking about?
“But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
Uhhh, no I don’t, and you can verify everything I say and write by simply going to MODTRAN or SpectralCalc. The calculators don’t lie.
“Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target.”
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Ed bo says : Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
If absolutely true why do the rings on my stove never show RED when the stove is off? Or why do we know that Blue stars have a higher temperature than brown dwarfs?
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
AndyHce November 20, 2017 at 3:04 am
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
The individual photons carry no information about the temperature of their source so an absorber can not distinguish the difference between a 15micron photon emitted at -80ºC and one emitted at 100ºC and will absorb them both.
Temperature measurements are made by looking at the distribution of photons (the spectrum) which is a function of temperature.
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. In fact plotting it versus wavelength distorts the distribution, it makes more sense to plot vs wavenumber, which is proportional to energy. If you do that you will see that the radiance peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution, by contrast water absorbs between 1500-2000 cm-1 (radiance ~0.02 W/m2/sr/cm-1).
The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such. http://www.azimuthproject.org/azimuth/files/earth_and_sun_emission.jpg
“The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.”
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm. There are plenty of creative ways for a scientist to isolate this effect. The fact that they don’t even attempt to demonstrate the basics through experimentation proves they aren’t looking for the truth, and why they rely on computer models.
co2islife November 20, 2017 at 9:19 am
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such.
No, I’m disagreeing with the statement you made, as shown in your graph the earth emits between 3 and 60µm with peak radiance at 10µm.
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm.
It certainly will!
All the 15µm light will be absorbed in a 10 cm path length if you put 1%CO2 in N2 in your container.
I said: “So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
You responded: “Uhhh, no I don’t”
But then you followed up with: “Absorbing a small fraction of that [earth’s IR spectrum] can’t warm the atmosphere above the 10µ temperature.”
So you just calculated a temperature AS A FUNCTION of wavelength. You don’t even understand that you don’t understand anything about this subject!
As to the (irrelevant) point you were clumsily trying to make, that the atmosphere’s absorption of some of earth’s IR emissions cannot increase the atmosphere’s temperature past that of the surface, no one is claiming that it does. The atmospheric greenhouse theory REQUIRES the atmosphere to be colder than the surface.
co2islife November 19, 2017 at 3:53 pm
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
co2islife November 19, 2017 at 3:53 pm
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is false.
co2islife November 20, 2017 at 9:11 am
That point isn’t debatable. It comes from the SpectralCalc Black Body Calculator. You are claiming the math is wrong.
No, I’m claiming that the guy looking at the graph doesn’t know what he’s talking about!
Run Spectralcalc Gas cell simulator with 0.0004 VMR of CO2 at 296K and plot absorption between 600cm-1 and 700cm-1. You’ll see that it absorbs just fine.
co2islife,
You said, “The quantum physics are well known and defined. I’ve pointed out almost all the points highlighted in this article. The physics are the physics, you can’t change that.” I wish it were that simple!
The impression I get from reading the comments is that most posting here are reasonably well-educated, but have varying backgrounds or disciplines. Yet, there is disagreement on what the “physics” says, even amongst those who share similar views about the validity of AGW. In part that may be the result of how an individual interprets the ‘facts.’ Often times the ease with which a problem can be solved depends on how it is expressed or framed. That area probably could use some work. Overall, I’d say that one of the problems is really a failure to communicate with one’s peers. Beyond that, there are some here with a vested interest in a particular viewpoint, and they have trouble looking at the problem from a different viewpoint. Few outside the area of geology are familiar with Chamberlain’s Method of Multiple Working Hypotheses.
If those of us, who are skeptical about the claims made by AGW alarmists, could get our act together and speak with a unified voice, I think that we would have a better chance of pointing out the errors of alarmists. But, with infighting, we are little more than an intellectual rabble.
It doesn’t help when the first comment is by a d@nier of EM radiation and photons.
John
November 19, 2017 3:59 pm
Well, I can fully understand why this topic is so debatable. I’m an Engineer myself, and I’m still having trouble with deciphering so many viewpoints. I only started becoming interested in the topic around 6 months ago. Probably the only real thing I’ve discovered is that it is much more complex than I had envisioned. Perhaps only time is the ultimate arbiter.
No it’s not complex, it’s second year thermo and heat transfer.
One popular RGHE theory power flux balance (“Atmospheric Moisture…. Trenberth et al 2011jcli24 Figure 10) has a spontaneous perpetual loop (333 W/m^2) flowing from cold to hot violating three fundamental thermodynamic laws. (1. Spontaneous energy out of nowhere, 2. perpetual loop w/o work, 3. cold to hot w/o work, 4. doesn’t matter because what’s in the system stays in the system)
“So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. ”
Almost everything discussed in this article can be found is article previously written on this topic. This Blog has all the resources to validate the points made in this article. The physics are the physics, they aren’t up for debate and Modtran and any Black Body calculator will demonstrate that. https://co2islife.wordpress.com/
Your Quantum facts are almost to a tee wrong
1.) Molecules have one or more electrons circling them. blah blah blah
NO
That is classical physics junk. In QM the electrons have complex distribution clouds only S orbitals 1s,2s,3s are circular. The reason is that electrons have half-integer QM spin and subject to Pauli exclusion principle that says two or more identical QM spin particles can not occupy the same space at the same time. They will instead by subject to pairing creating a waveform between them.
The reason for the half spin can be seen by seen by watching the field movement#/media/File:Spin_One-Half_(Slow).gif)
You will also note the momentum is in the field it is not a real spin like in the physical sense.
So Quantum spin can be thought of as momentum but it can not be equated to a physical object spinning.
2.) For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
PARTLY
That is the process of excitation and it creates an excited state it equates to temperature in classical physics. Outside the two ways listed you can also excite an atom via the electric or magnetic fields.
You need to be careful the temperature however is not always positive, it can be negative 🙂
3.) For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit. blah blah
NO
You need to break that into Photoexcitation which says a Photon must have a frequency that matches the one of the excitation energy level to be absorbed. You can view it as a resonate frequency with one of the excitation states of the molecule. Any energy absorbed in this manner departs from the equilibrium Boltzmann distribution as viewed by classical physics.
If the energy does not match those special resonant frequencies absorption of the photon takes place in accordance with Planck’s quantum theory.
4.) Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum blah blah
NO
Photoexcitation is an independant process and will occur at any temperature
Laser cooling illustrates this in the most extreme way.
https://en.wikipedia.org/wiki/Laser_cooling
The energies that are outside the resonant frequencies behave in the more normal way as you are trying to describe.
5.) Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them. blah blah blah
NO
see item 4, temperature does not play a part in photoexcitation only photon energy match
Now, you need to stop following the thermal collision effects and deal with only the photoexcitation effect and key to understanding that is bring Nitrogen into the mix. Nitrogen exists mainly as a simple diatomic atom in the atmosphere and it’s excited states are metastable and relatively long-lived and they happen to overlap a couple of CO2 frequencies. Classical collisions between CO2 and N2 will exchange energy in the photoexcitation frequencies. So the normal thermal energy and the photoexcitation energies can interchange. Without the interplay the two sets of energy would remain isolated.
blah blah blah.
Martin, do all your posts provide zero value add? Someone takes the time to write a detailed rebuttal of the author’s points, and all you have to say is blah blah blah. Truly the hallmark of a lightweight.
Much to read here in the comments, and I WILL get to it all later.
My overall observation, though, is that the talk still seems to center on warming.
The atmosphere keeps Earth’s surface from getting to hot too. The atmosphere, therefore, BOTH cools the surface AND warms the surface at the same time, depending on your perspective, … depending on what you are referencing as the main focus.
I think that talk should shift away from speaking of either extreme (warming or cooling) as the main focus and, instead, shift to the concept of REGULATION, which must consider both, or neither, depending on how you look at it.
Regulation involves an exchange between two processes, rather than either of those processes alone. And so it seems that CO2, H2O, and other gases REGULATE Earth’s temperature withing the habitable range familiar to us.
Damn typos above that I cannot fix. Live with them and groan.
I agree Robert. As I noted earlier, the ocean, the soil, and the GHG merely serve as heat sinks for the atmosphere. The aspect of CO2 is limited by the amount of IR available to be absorbed. The equilibrium equation heat sink capacity can only reach a certain maximum simply because there is no more IR in that spectrum to work with.
So, as you say, it plays a small role in stabilizing the atmospheric temperature, but it plays little role to n the overall stabilization process, simply because it is insignificant compared to the ocean (the overwhelming #1 heat sink), and water vapor, the primary heat sink in atmosphere.
One thing that posts like this definitely demonstrate is there is no consensus. When multiple highly-qualified professionals and well-informed amateurs cannot agree on something as fundamental to ‘global warming’ as a GHE or high sensitivity…then it’s obvious there’s still oodles of uncertainty.
The back radiation is not the only energy flux warming the surface. There is also the direct solar irradiation. Below is an energy balance sheet of the Earth. It is the only presentation showing the three sky conditions: first number is all-sky, the second number is clear sky, and the third underlined number is cloudy sky. The total energy flux warming the surface is Sd + Ed = 168,8 W/m2 +344,7 = 513,5 W/m2. And

by the way, the both energy fluxes are based on direct measurements. The energy balance of the Earth’s surface shows that the incoming energy fluxes and the outgoing energy fluxes are in balance. If they were not, the surface would cool or warm.
The major misconception presented here is treating all molecular energy the same. There are two distinct types, translational kinetic energy that all molecules have, and quantized vibrational energy that dipole molecules can have.
The GHG theory treats the later energy type as if it is not quantized, in other words they conceptualize that a dipole molecule will always absorb a certain wavelength of energy even if it is already in the energized vibrational state, that is NOT correct. If the gas molecule is already in its excited energy state, it can not continue to absorb that wavelength of light, that light will be transparent, reflected, or in some instances it will stimulate that molecule to radiate that energy and drop back to the non energized state.
However, very small levels of energy (factions of 1 eV) can be lost or gained from the quantized energy and given to translational kinetic energy upon collisions with other gas molecules — that’s why the absorption is a band instead of an exact wavelength. These collisions theoretically occur every 10^-7 s at atmospheric pressure which is faster than CO2 molecules theoretically radiate heat. Therefore, we should expect some minor heating caused by an increase in CO2 molecules in the atmosphere from conversion of quantized energy into translational kinetic energy from their collisions with non dipole molecules.
If all GHG molecules in the atmosphere are already absorbing and reemitting all available spectra able to bump the molecules into the energized state, then the only mechanism for additional retardation of heat escaping from the atmosphere can occur from the collisions described above. But that’s only if the available infrared energy is the limiting factor whereas more heating would occur if it is the available number of dipole molecules is the limiting factor.
In my opinion, we have empirical data showing that it is indeed the available IR that is the limiting factor and therefore any additional dipole molecules in the atmosphere can only heat by losing some of their quantized energy to kinetic energy. That is the CERES and TERRA data that has shown that certain regions of the atmosphere lose more heat into space than they receive from the sun, one region in particular being the Sahara desert.
This data not only shows that it is water vapor dominating the GHG feedback, but to me it also suggests that there is something to the theory that the temperature of planet’s atmosphere is largely dependent on adiabatic and gravity pressure induced compression heating. The most heat leaving the planet happens to be where cooled dry air descends within the atmosphere and inducing high pressure.
http://pages.mtu.edu/~scarn/teaching/GE4250/absorption_lecture_slides.pdf
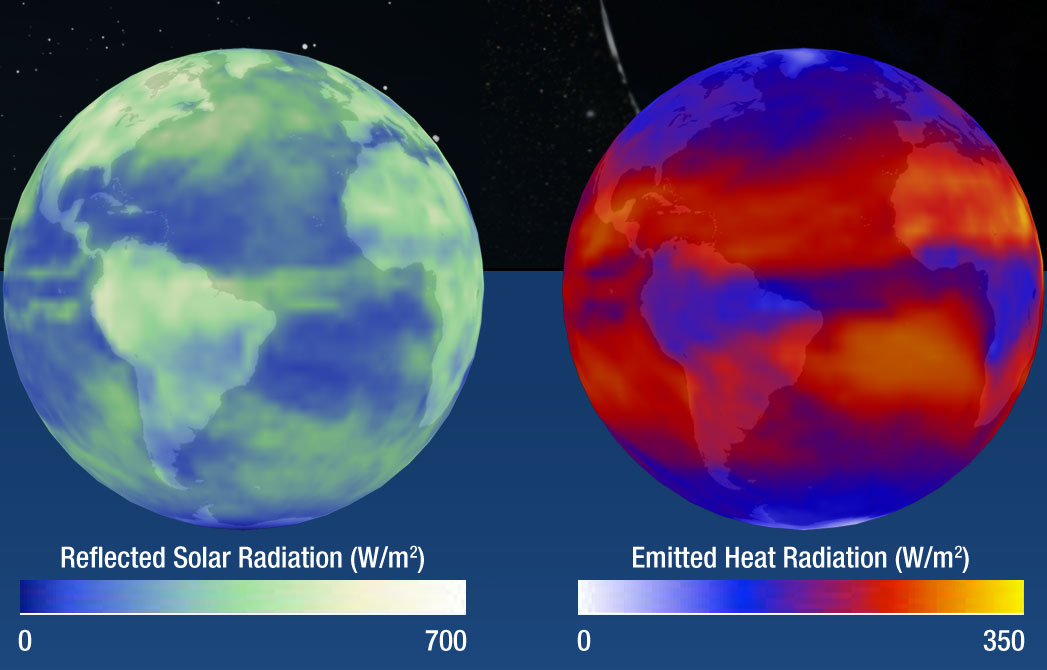

https://www.researchgate.net/publication/317570648_New_Insights_on_the_Physical_Nature_of_the_Atmospheric_Greenhouse_Effect_Deduced_from_an_Empirical_Planetary_Temperature_Model
Notice the outgoing longwave radiation coincident with the descending air associated with Hadley cells. Please, I invite anyone to explain this with the GHG planetary warming theory and how it is not explained by adiabatic and gravity driven heating.
“gravity pressure induced compression heating.”
No such thing really. Pressure does not cause heating or cooling, only changes in pressure. Rising air cools down, sinking air warms up. This is the basic reason for the lapse rate.
That there is more outgoing radiation in the descending part of the Hadley cell is because the air there has lost most of the water vapor (the really important GHG) and is therefore much more transparent to LWIR, so it comes from a lower and warmer layer.
What do you mean no such thing really? Very basic physics, really.
“work done by gravity = W = mgh (h = height lost by the object)
An alternate way of looking at this is to call this the gravitational potential energy. An object with potential energy has the potential to do work. In the case of gravitational potential energy, the object has the potential to do work because of where it is, at a certain height above the ground, or at least above something.”
http://physics.bu.edu/~duffy/py105/Energy.html
The process is started by solar heating of the ground, air masses warm up on the ground, they rise and then fall in the Hadley Cells. Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.
tty is correct. Static pressure does not cause any energy transfer.
Robert’s equation of “work done by gravity = W = mgh (h = height lost by the object)” actually proves this.
“h” in this equation is zero for the atmosphere as a whole. The atmosphere has no height to lose in toto. Any downdrafts are exactly balanced by updrafts elsewhere.
In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.
“Without an atmosphere this process would not occur, but since there is an atmosphere it does indeed happen and it depends on the density of the atmosphere nearly irrespective of atmospheric composition.”
However the effect would be very weak in an atmosphere without GHG since very little heat would be transferred from the ground to the atmosphere (though it is rather unlikely that such an atmosphere exists anywhere).
“In a radiatively active atmosphere (one with “greenhouse” gases), the updraft with water vapor, condensing at high altitude where it can more easily radiate to space, then the downdraft of dry air, actually serves to limit the warming due to the infrared opacity of these greenhouse gases.”
Very true. As a matter of fact this is the main mechanism regulating the surface temperature of the Earth. And it can not be modeled realistically, now or in the foreseeable future (it would require increasing computational capacity by something like 10^12).
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
It’s not a real thing eh? Well you had better go and inform the thousands of astronomy websites that all the stuff they’ve written about the internal temperatures of the gas giants (which they ALL attribute the gravitational compression heating) that what they’ve been saying for decades is all bunk.
That T=Pn/Rp (have used small p for rho) is pseudoscience and should be thrown out.
WWF:
You are confusing the one-time effect of dynamic compression — which can and does result in “heating” (and can be immense on a planetary scale) — with the ongoing effect of static pressure — which cannot and does not.
If you compress the air in a bicycle tire pump, it gets hot. If you leave the compressed air in the pump, it does not stay hot, but your analysis says that it would
The gas giant planets go incredibly hot in the dynamic compression of their formation. Some think that Jupiter’s core got almost hot enough to initiate fusion. These planets still emit significantly more energy than they receive from the sun, which means they are still cooling.
The earth, however, does not emit any significant amount more than it absorbs from the sun. Its geothermal flux is only about 0.1 W/m2 averaged over the surface.
For a clearer understanding of adiabatic heating/cooling in the atmosphere, see: https://www.cengage.com/resource_uploads/downloads/0495555061_137425.pdf
Earth pressure gradient allows it maintain a temperature gradient.
This is immediately and obviously MEASURABLE.
Unlike any mythical warming by CO2.
never been measured…. a fantasy !!
In what world is the atmosphere static? The down draft limits the warming, is that what’s observed?
Complete rubbish. The composition of the gas has absolutely nothing to do with convection.
The sum is not zero in this process. Solar radiation does the work to lift the air and gravity does the work to compress it. Energy is actually input into the system this way, and lo and behold, it’s actually what is observed.
Doesn’t descending air become exposed to greater pressure as it descends? Isn’t gravity doing work on it? Doesn’t it’s density increase, from less dense to more dense? — and it’s kinetic energy increase from less to more? And doesn’t gravity cause this descending, compressing, increasing kinetic energy in conjunction with the already warmer level into which it descends?
I confuse nothing. It only requires “a one time heating”
The Kinetic energy at the bottom of the atmosphere doesn’t dissipate to the top again. To do that it must work against gravity. Take a look at Neptune, where you have much less solar input to confuse you. It gets warmer as you descend into it, until it is thousands of degrees C.
Outside the fraudulent area of climate change on earth, this sort of thing is universally accepted.
It’s about time people on here looked up from their narcissistic intellectual self aggrandisement and checked with the rest of the scientific world
yes it can continue to absorb at that or nearly at that wavelength. you missed out that vibration in a molecule has harmonics so two photon absorption is possible. or a photon of double that energy can be absorbed.
just like a harp string will have a fundamental frequency it will have higher energy harmonic frequencies. a harpist will pluck the string at a different height to change the harmonics of the string.
So you’re saying that a photon can be absorbed that is double the energy level at which the molecule actually absorbs? And given an infinite number of photons in the 15-20 nm range, a CO2 molecule will continue to absorb that thermal radiation regardless of all three normal modes energized? And then can theoretically emit at a higher energy level than the radiation it absorbs?
To R turner at 9:27 pm.
1 carbon dioxide has 4 modes of vibration. Symmetrical stretch, asymmetrical stretch, and two bending modes of equal energy. The word for equal energy in spectroscopy is DEGENERATE. so they are a degenerate pair of benders!
the symmetrical mode does not absorb infra red since there is no change in dipole moment. the other 3 modes are infra red active. there are an awful lot of vibrational energy level present in each mode so each mode can sequentially absorb a lot of almost equal energy photons one after an other. ( due to asymmetry in the vibration the second photon energy will be slightly less energetic than the first and so on). The molecule can absorb photons equal to 2 of these energy bands (or even 3) this is analogous to a musical string vibrating at twice its frequency ie one octave above the fundamental note.
there are not an infinite number of energy levels so it cannot absorb an infinite number of photons. if it absorbs too many photons then the molecule will vibrate so strongly that the atoms will fly apart and it no longer exists it will be CO and O. Double strength photons can come off but has much lower probability than sequential single photons. I some how remember that some lasers can produce double strength photons.
indeed at any one time there will be a fraction of the population of CO2 with excited vibrational modes, and these will be absorbing photons as well.
This is how molecules break down in heat. infra red lasers can be used to heat single bonds in a molecule and break it. however the method is in its infancy and may just as well break a nearby bond.
Robert W Turner ,
Note that your NASA illustration (second one) has a range of reflected light (0–700 W/m^2) that is more than twice the commonly accepted value of incoming TOA radiation (342 W/m^2). How can something reflect more than it receives?
That particular image is for the reflected SWR.
If CO2 did affect climate, than the increase in CO2 over the past 30 years should have caused at least a measureable change in the dry lapse rate in the troposphere, but such has not happened.
Will, CO2 radiation can’t change the lapse rate, it’s dependent only on g/Cp
… and the slight change in aCO2 decreases Cp by a tiny amount , hence increases lapse rate.. immeasurable with so much H2O about.
There are many lapse rates in the atmosphere. g/Cp defines the Dry Adiabatic Lapse Rate (DALR) which is about 9.8 K/km. The more typical troposphere lapse rate is the Environmental Lapse Rate (ELR) which is 6.5 K/km. There are many more important lapse rates and one or more of them *might* be affected by CO2. However, all of them, except the DALR, are affected by radiation to/from CO2.
Only atmospheric warming has come from releases of energy from the oceans via El Ninos.
Nothing to do with CO2 or human anything.
Fixed it 🙂
Whu should the dry lapse rate change? The small increase in CO2 is not enough to measurably change the specific heat of the dry atmosphere. If the increased CO2 resulted in an increased amount of H2O in the atmosphere (known. by friends and admirers as “water feedback”), then the average lapse rate would go down, but as far as I know this has not been observed.
willhaas. You forget that there is a possibility that the CO2 effect is much less than IPCC reports. In that case the other cosmic warming effects have decreased and therfore the temperature has paused, even though the warming by CO2 has increased slightly.
According to the IPCC, the warming effect of CO2: dT = 0.5 * 5.35 *ln(CO2/280) resulting to the climate sensitivity of 1.8 C degrees. I have reproduced the calculation of the warming formula of CO2 and it is: dT =0.27 * 3.12 *ln(CO2/280) resulting to the climate sensitivity of 0.6 C degrees.
Link: https://wattsupwiththat.com/2017/03/17/on-the-reproducibility-of-the-ipccs-climate-sensitivity/
The warming effects of different main forces are in 2015: the Sun 0.35 C, GH gases 0.28 C, and astronomic harmonic resonances 0.13 C, together 0.75 C. Link: https://www.climatexam.com/cosmic-theory-caws
Very interesting comments on the greenhouse effect from Ray Pierrehumbert here: https://www.youtube.com/watch?v=slPMD5i5Phg
The system is a lot more complex than many (including we token ‘warmists’) think.
“Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um”
–
This statement needs a lot of modification.
We are talking about our atmosphere.
Temperature is the sum total of all the energy in all the molecules in the atmosphere in any particular place.
Individually molecules will have different energy levels,
LWIR is produced by both the sun and the earth surface and the atmosphere and clouds.
So the atmosphere certainly has all LWIR frequencies trying to pass through it.
I guess the CO2 molecule can pick up these other two frequencies if needed if it runs across them?
–
If a CO2 molecule has picked up a higher “hotter” frequency energy packet and it subsequently emits it this does not mean that the whole atmosphere has to be at say 120C does it?
Yet that CO2 molecule will be buzzing around giving energy [heat[temperature]] to a lot of other O2, H20,N and other molecules while it is charged up.
–
Something is wrong in the state of Denmark but I am not good enough yet to explain it. There must be lots of people who do understand radiative physics to simply knock this contention out.
Where are they, please.
–
Quite happy to be a skeptic for lots of reasons but not on the CO2 is not a GHG as technically it will raise temperatures according to the laws of physics.
Just a minor comment.
Should 274 K be 273 K and 15 C be 288 K, not 289 K?
Andrew Hamilton,
Yes
“For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit” I stopped there, too many misconceptions about quantum mechanics. The IR absorption spectrum has nothing to do with the electronic excitations, but with rotational-vibrational spectrum of the molecule (cannot be purely vibrational, because the photon has helicity). I’m going to have a post on my blog about this sometime, specifically for CO2 and H2O, calculate some frequencies and explain a few things…
Yes he hasn’t got there are two totally different things and they behave differently.
MODTRAN answers the question. MODTRAN, whose accuracy has been verified by satellite measurement calculates heat flux either outward or downward at any point in the atmosphere. The difference between heat flux outward at 0 altitude and at the top of the the atmosphere is the heat deposited in the atmosphere. If the GHG concentration is anything above 0 the heat deposition is positive. If it is 0, then all of the IR heat from the earth escapes to outer space and we are left with a frigid, uninhabitable earth.
Excuse me, but all the IR ultimately does escape to outer space (including geothermal heat).
“If the GHG concentration is anything above 0 the heat deposition is positive.”
Since it has been above zero for the last 4 billion years or so (and mostly much higher than now) according to your physics the planet should have melted and boiled off the atmosphere long ago. As a matter of fact even if the “heat deposition” was only 0.000001 degree per year the Earth should be almost as bright as the sun by now.
Yes, all the IR energy does escape to outer space, but it does so at a higher temperature than it would have done without the IR absorption of the atmosphere. It does it via the Stefan-Boltzmann equation. Since it loses some of the (discrete) IR levels leaving the earth’s atmosphere it has to increase its temperature to make up with a continuous spectrum what it has lost from the discrete emissions. This is the mechanism of GHG heating. You can read about the details by googling “Nature Abhors a Positive Feedback” which is a 3-year old post to this site (Watts Up With That).
” It does it via the Stefan-Boltzmann equation” The atmosphere is not a black body. Ex falso, quodlibet.
Problem is no one uses it right.
It changes just by altering air temp and not composition. So, unless you run it for each temp, you get the wrong answer.
I agree – the notion that a gas such as CO2 or water vapor can “trap” radiant heat in a free atmosphere is simply unphysical. Heat trapping by gases is only possible by preventing/obstructing convective heat exchange (as in a glass greenhouse) or by using of IR-reflective materials such as polished aluminum that have VERY low IR emissivity/absorptivity and a high IR reflectivity (these materials are called “radiant barriers”).
Our latest paper addresses this issue and demonstrates using NASA planetary data that the thermal effect of planetary atmospheres has nothing to due with trapping of outgoing thermal radiation, but instead is due to the force of atmospheric pressure, which adiabatically enhances the energy received from the Sun. In other words, the so-called “Greenhouse effect” (more accurately named Atmospheric Thermal Enhancement or ATE) is a thermodynamic (pressure-induced) phenomenon that is completely independent of atmospheric composition:
Nikolov N, Zeller K (2017) New Insights on the Physical Nature of the Atmospheric Greenhouse Effect Deduced from an Empirical Planetary Temperature Model. Environ Pollut Climate Change 1: 112. doi:10.4172/2573-458X.1000112
URL: https://tinyurl.com/ydxlfwn7
Ned:
Did you take high school physics? If you had, you would understand that for a force (such as atmospheric pressure) to transfer any energy, it must act over a distance (Work = Force * Distance, or more precisely, Work = Integral of Force over distance).
If an object is actually falling in a gravitational field, gravity is doing work on that object, transferring energy to it. But the atmosphere is not falling — it has already fallen. No distance, no energy transfer. (Any downdrafts must be exactly matched by updrafts, so these fully cancel out.)
Any reasonably bright high school physics student can recognize your argument for the nonsense it is!
Take a 5kg barbell, hold it with your arm horizontal out in front of you.
No movement.
so, no work involved….. Right Ed
That’s the trouble with your high school physics, Eb.
ITS “LIMITED”. !!
Take your 5kg barbell and hang it from the ceiling on a hook. No movement, no work. The hook and the ceiling have no power supply.
If you held up the weight with a motorized winch, the motor would expend some energy holding up the weight (as the body does in your example). But engage the brake on the winch and turn off the motor, no energy is expended to hold up the weight.
You would fail high school physics, and not get started in any higher level physics.
To those who think that all the work of gravity has already been done and that this somehow falsifies the premise that there should be higher temperatures at the surface than at the top of the troposphere because of gravity:
When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located? Is it located at the top of the atmosphere? Or is it located at the bottom of the atmosphere?
If you want to distribute that energy back to the top of the atmosphere, what force will you have to work against to get it there? Would it be gravity by any chance? If you do work what do you expend? Oh yes! Kinetic energy? Which is converted to potential energy once more!
So the natural equilibrium of a large planetary atmosphere, is colder near the top, hotter at the bottom. See Neptune, Uranus, Saturn, Titan, Jupiter, Earth and Venus for and use the simple formula T=Pn/Rp to understand that the temperatures of all of these planets and moon can be precisely calculated using nothing else.
The greenhouse effect is falsified.
WWF:
You ask: “When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?When an object falls it gains kinetic energy as is loses potential energy. Energy is conserved. Once it has stopped falling, where is the kinetic energy located?”
I see that you have never done this type of analysis. The question is not even properly posed. When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.
All of the planets you cite have a radiatively absorptive atmosphere between a relatively warm surface and the incredible cold (3K) of deep space. Like a steel bar with one end in boiling water and the other end in ice water, there will be a temperature gradient between the hot and cold ends. If the gradient of the atmosphere exceeds the adiabatic lapse rate, as it is on all of these bodies, the lapse rate is unstable, and convection will start to bring it back toward adiabatic.
Poor Ed, you really don’t have the faintest clue past junior high level, do you?
Keep expounding your ignorance of how materials deflect and absorb strain..
Its funny to watch 🙂
“No movement, no work.”
Your base-level ignorance is exposed in that one statement.
“no energy is expended to hold up the weight.”
Again.. a junior high understanding of materials.
As expected.
“Take a 5kg barbell, hold it with your arm horizontal out in front of you.
No movement.
so, no work involved….. Right Ed”
It is noted that you immediately run away from the answer, try and distract, and in doing so highlight your ignorance of basic materials.
Ed says there is no work involved in holding a 5kg barbell horizontally in front him.
He must be superman or something. !
Yes, I took physics in HS and in college and in graduate school. How about you? Did you forget that kinetic energy measured in Joules = Pressure*Volume, and that gas temperature is proportional to the internal kinetic energy of a gas? Perhaps these Wiki articles might help your memory:
https://en.wikipedia.org/wiki/Joule
https://en.wikipedia.org/wiki/Ideal_gas_law
Oh, and make sure to retake the class about adiabatic processes to understand how pressure relates to temperature in standard thermodynamics. You can also read carefully our paper, where these things are explained on pp. 6 – 15, while noting that our accurate model (Eq. 10a) is based on actual VETTED OBSERVED data, hence, it’s real!
And just for Ed’s edumacation
Strain Energy within materials is also measured in Joules.
Ed writes,
“Take your 5kg barbell and hang it from the ceiling on a hook. No movement, no work. The hook and the ceiling have no power supply.”
There is still work in it,Ed or it would have IMMEDIATELY fallen to the floor. What is holding it to the surface of the ceiling,Ed?
I can stand still therefore I don’t float away from the surface?
As I said, Sunset…..
….poor ed is stuck with a junior high school understanding of structures and how they work.
Ed Bo
Have you never heard of the Chinook wind and seen what it does to snow on the ground?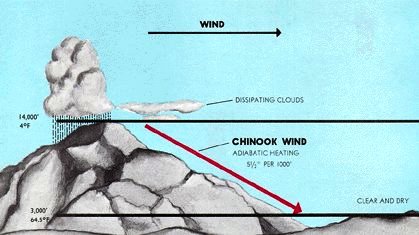
I’m throwing food again.
How so? Is the atmosphere just sitting there static? I thought that the atmosphere was ALWAYS falling, … AND rising, … AND falling, … all the time. Doesn’t this mean that the atmosphere is WORKING all the time?, … under the influence of gravity? — gaining potential energy on the rise?, “unleashing” that potential energy on the way down? … in a continuous cycle?
How is this merely “already fallen”?
There is complete confusion here by multiple commenters between the concepts of force, energy, and power.
The hook holding up the 5kg weight exerts a (continuous) force on it of F = m*g = (5 kg * 9.8 m/s2) = 49 Newtons.
To hold it in place, the hook does work on the weight of W = F * d = 49 N * 0 m = 0 Joules. So the power required is 0 Watts (= 0 Joules / time).
What are your equations for force, energy (work), and power for this case?
Andy cannot get it through his head that a one time increase in strain energy is NOT an ongoing continuous power transfer, no matter how many times this basic and trivial point is explained to him.
Ned: I am absolutely appalled that with all your credentials, you do not understand the most basic points you should have gotten in high school physics.
Pressure/volume work requires a CHANGE in volume to do the work. It’s really p*dV work. This is the same thing fundamentally as force needing to create movement to do work. The gas in a piston chamber must move the piston head to do work on it.
The weight force of the atmosphere on the earth’s surface does not move the earth’s surface. (Even if an initial application of this weight force caused some compression, there is no further change.) So the pressure/volume work done is zero.
These are basic, basic points of high school physics, and you get them completely wrong!
Height of the atm changes daily. Goes Up and Down like a thermometer.
Ed Bo November 20, 2017 at 9:33 am
Ed, it’s worse than that. He’s had the basic points explained to him here on WUWT, over and over, and he STILL doesn’t get it.
But heck, if you want a good laugh, you can read about Ned Nikolov’s work here …
w.
micro6500:
You say: “Height of the atm changes daily. Goes Up and Down like a thermometer.”
The claim of “Atmospheric Pressure Effect” (APE) enthusiasts like Ned Nikolov is that the static weight force (pressure) of the atmosphere does continual work on the planet’s surface. To do this, it must move the surface downward.
If the height of the atmosphere varies up and down a little bit, that does not mean anything to the surface.
If you don’t count the downwelling longwave infrared flux (back radiation), the surface energy balance is out of whack by about 250 W/m2, averaged over the surface. The APE enthusiasts claim that the weight force of the atmosphere can make up this imbalance. That’s 250 Joules of work every second for every square meter of the planet’s surface. With an atmospheric pressure of about 100,000 N/m2, the earth surface would need to be continually compressing at 250/100,000 m/s, or 2.5 millimeters every second. This means that the earth’s radius would need to shrink by over 75 kilometers each year! Seriously???
Philip:
You say: “Have you never heard of the Chinook wind and seen what it does to snow on the ground?”
Do you think the falling air of the Chinook wind leaves a vacuum at the higher elevations?
Facepalm. It’s really hard to deal with ingrained blind ingnorance.
Willis, how about you accept that Ned has a much better understanding of physics than you and that it would be a good idea to take that as a starting point to try and understand where your misconceptions lie, rather than try to keep proving that cold heats hot and work done equals energy no longer in existence? Truly the level of your ignorant stubbornness could teach my 5 year old grandson a thing or two!
To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?
Why is it that Ned and Karl can accurately model the temperatures of every planetary atmosphere in the solar system with the same mathematical formula, but greenhouse effect proponents can’t even get their mathematics for earth to agree with itself, much less even one other planet?
wickedwenchfan November 20, 2017 at 3:36 pm
If you wish to follow the “science” of a man using an ad-hoc equation with more tunable parameters than data points to explain, you are welcome to.
He is right. The kinetic energy is converted to thermal energy. You sure you understand how this “science” thing works?
BECAUSE NED HAS MORE TUNABLE PARAMETERS IN HIS EQUATION THAN HE HAS DATA POINTS TO EXPLAIN, PLUS FREE CHOICE OF EQUATION WITH NO NEED THAT IT BE PHYSICALLY BASED!
Sheesh! Given more parameters than data points to explain, it would be surprising if Ned could NOT explain the datapoints … but then I guess you don’t know how that works. Let me offer you the following, come back once you’ve read it. It explains very clearly why Ned is just fooling the rubes …
w.
WWF:
You say: ‘To the other numpty on this thread: “once an object has stopped falling there is no more kinetic energy” (or something similar. I paraphrase the ignorance). Really? Where did it go? Is it hiding? Did it disappear in a puff of smoke?’
BTW, it’s “Mr. Numpty” to you 😉
I already answered your question above, when I said: “When it has stopped falling, there is no more kinetic energy. But where is the (converted) energy located?
It is located in the thermal energy of the object and the surface that stopped it from falling. In an inelastic collision, the kinetic energy is completely converted to thermal energy. And yes, when this happens at the bottom of the atmosphere, this thermal energy is at the bottom of the atmosphere.”
Since I was obviously too fast for you, I’ll break it down for you ask I would a high school physics problem to a struggling student.
Let’s take Newton’s apple of 0.5 kg, hanging 3 m above the ground in earth’s gravitational field of 9.8 m/s^2. It has a gravitational potential energy relative to the ground of PE = mgh = 0.5 kg * 9.8 m/s^2 * 3 m = 14.7 Joules.
Now the stem breaks and the apple falls toward the ground. Just before it hits the ground, the gravitational potential energy of 14.7 J has all been converted to kinetic energy of KE = (1/2) * m * v^2.
We can calculate v = sqrt (2 * 14.7 / 0.5) = 7.67 m/s.
Next it hits the ground in an inelastic collision and stops moving. Such an inelastic collision converts all of the kinetic energy of 14.7 J to internal (thermal) energy. For simplicity, we’ll say that all of this added thermal energy is in the apple as it splats on the ground.
The apple is mostly water, so has a thermal capacitance of about 4 kJ/kg/K. This leads to a temperature increase in the apple of:
DeltaT = 0.0147 kJ / 0.5 kg / 4 [kJ/kg/K] = 0.00735K
This is a trivial high school physics problem. But neither you nor Ned has enough of a grasp of the basic concepts to get it.
Ed Bo is simply explaining standard, correct physics. He (and Willlis) are absolutely right.
Ed Bo (Again)
So you think that convectional overturn is a zero sum process?
You have forgotten about the water vapour that got left behind and fell out from the rising limb.
Here is a picture of a condensed greenhouse gas falling under gravity. This is clearly not the result of a zero sum process.
http://1.bp.blogspot.com/-NeGDYGjuBI8/UdFRSl28cbI/AAAAAAAAWrw/tJNkpRp4QxI/s1024/Waterfalls+Scenery+Wallpapers+%25281%2529.jpg
Ed Bo,
No. Do you?
The most significant point about an unstable air mass is that if a parcel of air at a given level within the air mass is forced upwards it will continue to rise; but equally important is this: – if an identical parcel of air from the same starting level in the same unstable air mass is forced downwards, it will continue to fall. We can all see the effects of the rising air in an unstable air mass when it cools sufficiently for the water vapour to condense and form cumulus clouds. What we fail to see, and this is what makes them so dangerous for aircraft, is the descending unstable air, the cold invisible down draft located alongside the rising cell. It is a mistake to assume that this cold downdraft is the same air that rose inside the convection cell. It is not. The rising air inside the storm becomes separated from the water vapour that formed the storm cloud as the rain falls out of the cell and the lifted separated and now dry air remains aloft in the anvil cloud at the top of the storm.
It is this process of drying by physical separation of moisture that makes convection that produces rain an irreversible process. Descending moist air in a cloud can evaporate the surrounding water droplets from the cloud, dissipating it and so slowing the rate of adiabatic warming as the moist cloud containing air parcel descends. Dry air cannot be cooled by evaporation of surrounding cloud moisture because there is none available. Consequently in the descending limb of the Hadley cell dry air is warmed as it is forced down by the Coriolis Effect and ends up at the surface, just like the Chinook wind, at a higher temperature than its initial starting point. And the reason for this change in temperature is because the latent heat of condensation of water vapour that powered the initial rise to the top of the troposphere in the convection storms of the equatorial zone made the rising air cool more slowly.
Philip:
You are confusing very separate issues here.
Nikolov claimed that the weight force of the atmosphere provides an ongoing energy transfer to the earth’s surface that keeps the surface at a substantially higher temperature than it would be otherwise.
I showed him the very basic high school physics point that static pressure provides no energy transfer, and that any deviations from the static case due to updrafts and downdrafts must cancel out.
Note carefully that I am talking about the mechanical force due to the weight of the atmosphere. You are talking about something completely different.
Yes, the convection circulation of air is an irreversible cycle, both due to the evaporation at the surface and the condensation at altitude, and the reduced IR opacity of the atmosphere at altitude. But this does not affect the weight force of the atmosphere on the surface.
Besides, what you describe has a net cooling effect on the surface (even if there is localized warming at downdrafts). Nikolov was arguing for an atmospheric warming effect.
Why do we need to continuously explain basic physics…err an elementary concept?
Solar energy lifts air. Literal energy from an outside source does work on the atmosphere, it has warmed and lifted a parcel of air into the atmosphere. At this point, the d-nyers of basic physics suggest that the energy simply goes away. That’s not what happens. The air has potential energy at this point, it falls and energy is converted to kinetic energy in the lower atmosphere.
Let’s work backwards now, we have kinetic energy added to the lower atmosphere from compression, the work was done by gravity. How did that air get there, it was lifted from being heated. How was it heated, from an outside energy source or an internal energy source? Uhh, an external source, still with me? So is the net result zero energy input into the system, no, energy is retained within the system that was obtained from an EXTERNAL ENERGY SOURCE.
If we must explain that a parcel of air in the upper troposphere is actually moving a distance, h, when it falls then I don’t know how much further back in explanation we need to go. Was that parcel of air magically moved into the upper atmosphere? Was that air simply pulled into the upper troposphere because falling air left a void for it to replace? Errr, no. The entire process is started from energy being input from the sun.
Does this really need further explanation to people. Well, I guess it might to people saying
Ed Bo,
I am all for a dynamic atmosphere that moves both mass and energy, causes local surface heating (Chinook winds) and elsewhere local surface cooling (virtually every rainstorm I have ever experienced).
Sadly, the very meaning of the term “adiabatic” is forgotten here in arguing that gravitationally induced compression of a parcel of air cannot increase its temperature in accordance with the Ideal Gas Law. The compression factor PV/nRT need not be unity, as for an ideal gas, for the temperature to rise adiabatically.
This conversation is absurd. You need to define the system boundaries. This old engineer still remembers his statics. If a mass is not moving, then no work is being done. For an object to be static, all the forces must add to zero and all the moments must add to zero.
It’s a different problem if you add in how the forces are created. Holding a weight motionless does no work. However, there is energy being expended in the muscles of your arm to create that force.
One person is ignoring how the forces are created and the others are trying to include the force creating process. Neither side is discussing the same problem.
Jim
Absurd!!! This site needs an editing feature.
Jim
Jim Masterson November 21, 2017 at 9:24 pm
That “editing feature” would be me … your error is fixed, I hate typos. Unfortunately, WordPress doesn’t offer editing.
w.
Thanks Willis–you are a great editor.
Jim
Ned Nikolov November 19, 2017 at 1:44 pm
This from Ned Nikolov, the man so ashamed of his own work that he published it under a false name … well, actually, he would have published it, but I pointed out his craven deception to the journal, and they pulled his article.
His “scientific theory” involves an equation with more tunable parameters than there are data points … and while that kind of mathturbation causes anyone who thinks about things to laugh, I fear he has found a following among the credulous.
Ah well … just saying, folks, he’s a man who is willing to lie about his own name …
w.
Still works better than your greenhouse effect mathematics. It also is based on basic principles that cold doesn’t raise the temperature of hot without work being performed. Concepts you still struggle to comprehend.
I guess you must have missed the rest of this post because you never addressed it in your “take down” of the original Nikolav paper. And you pretend you have actually refuted anything
AGAIN, we must ask, what is it you are missing about the physical explanation for which mathematical models exist to explain and match the temperature of rocky planets with atmospheres?
RWturner November 21, 2017 at 10:18 am
I guess you must have missed the fact that Nikolov is using more tunable parameters than the data points that he is fitting. Plus he has given himself free choice of equation, with no consideration of whether it is physically meaningful.
When you do that you are GUARANTEED a good fit … but of course, that fit is meaningless. It would be hard to not get a good fit with that procedure.
Or perhaps you don’t realize what it means when you have more tunable parameters than data points to fit. Once again let me recommend Freeman Dyson’s clear takedown of the bogus Nikolov style of analysis …
Regards,
w.
But there is a physically meaningful mechanism for how this works. It has been described numerous times on here and no one has refuted it with anything but sophistry.
The descending cool dry air within the Hadley cells retains energy within the climate system. This is a basic fact that keeps being rejected for no reason aside from conventional wisdom. Where is this retention through gravitational potential energy in the oft cited energy balance cartoons that you see above and below? Those flat earth cartoons are so laughably flawed that it’s amazing anyone is still using them.
Others have cited this process in gas giants. It is the same concept, but in the case of gas giants the energy source to start the convection is internal and therefore there is net energy loss, but not nearly as much energy loss if there wasn’t a gravity driven compression by the returning downdraft. On Earth, the energy source to kick off the convection is external, so therefore any returned energy is a net addition into the system, it limits the energy lost via the thermals and release of latent heat. This is true regardless of the composition of the atmosphere, it just matters that there is an atmosphere at all for convection to take place.
And I already discussed the empirical observations demonstrating this effect. The latitude belts between 23-30 degrees emit more LWIR back into space than any other region, even losing far more heat to space than received from the sun in places like the Atacama. It even holds true for the atmosphere over the oceans at these latitudes despite 30 mm of precipitable water per cubic meter.

Notice the subtropical south Atlantic. Greenhouse gases are surely present there, spreading that notorious back radiation around, yet this is among the highest outgoing radiative regions of the atmosphere. What is the source of that heat? I would say it from the additional heat added to that part of the atmosphere from gravity driven compressional heating at the descending leg of the Hadley Cells. Yes, that heat came from a different part of the atmosphere, but that heat in turn came from the sun. That means the process is retaining heat in the climate system, ironically much like how a greenhouse actually works, but instead of glass roof it is the force of gravity.
P.S. You forgot to add the link to Dyson’s takedown of Ned’s models.
RWturner November 21, 2017 at 2:11 pm
Thanks, RW. It’s fixed now, I’ll put the link here as well.
w.
RWturner November 21, 2017 at 2:10 pm
Thanks, RW. First, whatever the mechanism that you are describing might be, it’s not described by his equation. Look at the equation! It has little to do with anything.
Second, if your proposed mechanism were true, it would allow a planet with an IR-transparent atmosphere (e.g. argon) to be warmer than the S-B temperature … but as I showed in A Matter Of Some Gravity, that violates conservation of energy.
Third, regardless of all of that, he has more tunable parameters than he has data points to fit. This is a common enough mistake that it has its own name, “over-fitting”, and his is the most egregious example of overfitting that I’ve ever seen.
w.
I don’t think that’s what’s implied by the theory at all. You can’t take a temperature of something (atmosphere at the surface) that is not there, but the surface of the planet itself would not heat up, no.
If you added an atmosphere of pure argon, there would be absolutely no condensed or solid particulate matter in the sky to absorb incoming radiation, and let’s assume this atmosphere refracts no incoming light. The surface of that planet will receive the full brunt of the solar radiation.
But, molecules in the atmosphere at the surface will heat up and convection starts. This then cools the planet’s lower atmosphere as heated air carried to less dense areas of the atmosphere, the heated air decompresses, hot molecules bump into cool molecules, and heat is radiated into space.
This process cools the surface and is the complete climastrology certified version of planetary energy budget models regarding pure thermal convection. But what happens as that air falls down to the surface again due to gravity? Unequivocally, that air is compressed and heats up, that is if you believe in the work of William Henry. Latitudinal dependent heating takes place in the area of the Hadley Cells where the gravity driven compression warms the air, exactly what we see on Earth. Far less of the heat is lost to space than is modeled, it’s ironically similar to the argument that the back radiation heats the surface by slowing the radiation that leaves the planet.
So, if you believe that the inherent gravity driven “heat retention” of the atmosphere actually exists at all, and I hope for William Henry that you do, then how do you think that compares to the importance of the previously discussed 0.001-1.7 eV molecular vibrations of the inaptly named GHG molecules and there emittance of this energy within the atmosphere?
And let’s imagine a planet with the same gravity as Earth, circling the same sun in the same orbit, but with an atmosphere of pure argon. Would that planet be hotter or cooler than Earth? With an atmosphere of the same molar mass, I bet that planet’s total average atmosphere would be cooler than Earth’s with no latent heat, but probably a much hotter surface due to higher SWR. Convection would probably actually be more active due to the extremely hot surface and argon doesn’t freeze until until -189 C so the structure of the lower atmosphere would be quite different. So theoretically if that atmosphere was able to stay gravitationally attached to the planet, where the pressure in this imaginary atmosphere is 1 atm, it would probably be about the same temperature as Earth at the same latitude.
I think this qualifies as a great example of how hard it is to defeat erroneous conventional wisdom.
Researchers have literally had to infect themselves with diseases to defeat it. Petroleum geologists were busy controlling oil blowouts in Oklahoma while others were still claiming commercial oil would never be found west of the Mississippi. But eventually, the science progresses.
A key point in this discussion is the fact that an enhanced IR absorption by some gases does NOT imply an ability of such gases to trap heat in an open convective environment. IR absorption and IR trapping are two distinctly different processes with different control mechanisms that have erroneously been conflated since the time of Fourier and Tyndall in the 1800s …
Ned Nikolov
November 19, 2017 at 1:50 pm: IIRC, Fourier and Tyndall have been misquoted by all warmista. They do this everywhere, thinking we won’t check. Sadly, they have been correct in that, too much.
The classic paper by Arrhenius (1896) illustrates quite well the roots of confusion in the modern radiative “Greenhouse” theory:
“On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground”
http://blogs.dickinson.edu/cop20/files/2014/08/1896-publication.pdf
One can clearly see that Arrhenius simply took Fourier’s conjecture/assumption about “heat trapping” by an open atmosphere as a “physical truth” without any empirical verification. He further developed the idea by proposing a “model” (his Equations 3 and 4) that relates Earth’s surface temperature (T) to atmospheric CO2 (K expressed in relative units) via equating T^4 to a ratio of dimensionless quantities! By failing to match measurement units between the left- and right-hand side of his equation, Arrhenius violated a basic principle of dimensional analysis in physics, which of course renders his model nonsensical from a mathematical and physical standpoint of view .. And this nonsense has been touted by the mainstream climate science for decades as one of the greatest scientific achievements of the 19th century!! Simply amazing …
Experiments by Arrhenius’ colleague, physicist Knut Ångström, the results of which were published in his 1900 paper “Über die Bedeutung des Wasserdampfes und der Kohlensäure bei der Absorption der Erdatmosphäre,” (Annalen der Physik 308(12): 720-732.), showed that that CO2 is transparent to 90% of infrared radiation applicable to temperature variation; and that those infrared bands which CO2 readily obstructs are already almost totally blocked by atmospheric H2O. His finding, that the relationship between the concentration of CO2 in the atmosphere and its effect on back radiation is logarithmic, has been replicated by many subsequent experimenters; all of whom show that doubling of the present carbon dioxide content in the atmosphere would only increase the back radiation by about 3.6 W/m ², which would, in the absence of other factors, give rise to an increase in temperature of between 0.6 and 0.8 C°.
Some think that doubling might yield a temperature increase of up to 1.2 C°. The only way that Warmunistas can get a scary range of 1.5 to 4.5 C° is by assuming unphysical positive water vapor feedbacks not in evidence.
English translation of Ångström’s paper:
https://ozonedepletiontheory.info/Papers/Angstrom1900-English.pdf
Ned Nikolov November 19, 2017 at 1:50 pm
A key point in this discussion is that as near as I can tell you are the first person in this discussion to say that GH gases “trap heat”. Nor is that a common term in the field. Despite that, you have not defined the term, so we have no idea what it is that you mean.
How about you give us a clear definition of what you mean by “IR trapping”, so we can follow your ideas? Because for all we know it’s just a strawman that you’ve erected to knock down …
w.
Willis said, on November 21, 2017 at 9:42 pm, in reply to Ned Nikolov :
Odd, because, from what I have observed, this term IS a common term in the field:
From a NASA website:
https://climate.nasa.gov/causes/
Most climate scientists agree the main cause of the current global warming trend is human expansion of the “greenhouse effect” — warming that results when the atmosphere traps heat radiating from Earth toward space.
Someone needs to tell NASA that a common conception of the “greenhouse effect” is not to “trap heat”. Oh, and why they are at it, someone also needs to remind NASA that heat does NOT radiate, and that by continuing to speak of it as such, they continue to confuse the two concepts of “heat” and “radiation”.
From a Harvard University website:
http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html
Some of this terrestrial radiation is trapped by greenhouse gases and radiated back to the Earth, resulting in the warming of the surface known as the greenhouse effect.
Well now, Harvard some folks seem to think that greenhouse gases TRAP radiation. They relish the word, “trap”, and they use it in a way to confuse people into associating “heat” DIRECTLY with radiation, as to suggest that the two terms might be interchangeable (wrong).
A Columbia University website:
http://www.columbia.edu/~vjd1/greenhouse.htm
While the dominant gases of the atmosphere (nitrogen and oxygen) are transparent to infrared, the so-called greenhouse gasses, primarily water vapor (H2O), CO2, and methane (CH4), absorb some of the infrared radiation. They collect this heat energy and hold it in the atmosphere, delaying its passage back out of the atmosphere.
Let’s look at definition #18 for the verb, “trap” at Dictionary.com: 18. to stop and hold by a trap, as air in a pipe. So, it sure looks to me like Columbia University is talking about TRAP — they just avoid the term and describe what the term means in other words. Sorry, but “holding” and “delaying” is TRAPPING.
Consequently, I’m afraid that straw men are overseeing NASA, Harvard, and Columbia University, at the least. I wonder whether they are also spineless, … as in too timid to alter the momentum of confusion that they have helped nurture.
Robert Kernodle November 22, 2017 at 8:13 am Edit
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
Thanks in advance,
w.
Willis on November 22, 2017 at 11:12 am wrote:
I meant it was not common in scientific papers, because it is just a simplistic way of explaining the greenhouse effect. However, as you point out, it does appear in press releases aimed at the general public.
And THAT’s the problem — this simplistically WRONG explanation, using a word that WRONGLY compares a familiar plant-house structure with a gaseous atmosphere, perpetuates the very use of a term that IS ill-defined. If this term is so rampantly used with the “general public”, then how is the “general public” ever to come to terms with any reality?
The person you criticized initially for using this term was merely using it as a reference point, as many organizations state it. Nikolov was, in effect, kowtowing to this use, letting it slide in its amorphousness — as it is spread amorphously without any clear definition already by all who use it, as if this is okay. You made his using the term an issue, which it should not have been, in my opinion, which seems like a distraction, rather than a call for a definition that was significant to Nikolov’s statements. It is this nebulosity of definition that tends to give alternate views some credibility, I would say.
Oh, and not only does the term appear in press releases, but also it appears in the context of professional scientific organization’s, as a reflection of professional identities, like the following:
American Association for the Advancement of Science:
http://whatweknow.aaas.org/get-the-facts/
The warming effect of CO2 and other heat-trapping gases is well established and can be demonstrated with simple science experiments and satellite observations.
TRAPPING, right? — I didn’t misread that?
Australian Academy of Science:
https://www.science.org.au/curious/earth-environment/enhanced-greenhouse-effect
The result is that some of the sun’s energy becomes ‘trapped’—making the lower part of the atmosphere, and Earth, warmer than it would be otherwise.
TRAP, yes?
NASA website for childhood education:
https://climatekids.nasa.gov/greenhouse-effect/
Earth’s atmosphere does the same thing as the greenhouse. Gases in the atmosphere such as carbon dioxide do what the roof of a greenhouse does. During the day, the Sun shines through the atmosphere. Earth’s surface warms up in the sunlight. At night, Earth’s surface cools, releasing the heat back into the air. But some of the heat is trapped by the greenhouse gases in the atmosphere. That’s what keeps our Earth a warm and cozy 59 degrees Fahrenheit, on average.
NASA’s spineless straw men apparently want to start indoctrinating America’s (and the world’s) youth as soon as possible with the TRAPPING idea, AND setting up future confusions in adulthood with the “earth’s-atmosphere-is-like-a-greenhouse” idea. Tragic!
However, since you are here, and you think the term has a scientific meaning, perhaps you could give us a good clear definition of “trapping heat” in the atmosphere.
Why would you think that I think the term has any scientific meaning. The term is crap. I think Nikolov knows that it’s crap too, but he used it as a device to hold people’s attention a bit, without saying that it’s crap, while he put forth more info from his point of view. The straw man that I am seeing is raising an issue about the term at all in Nikolov’s context, since it was used merely to set the stage of discourse, rather than as a label for stating a belief via the term’s clear definition.
The “clear” definition of “trapping heat” that you might seek is purposefully veiled in the totality of faulty discourse defending the “greenhouse effect”. All the math, all the elaborate descriptions, all the minutia devoted to the “greenhouse effect” is shadowed by this convenient umbrella idea of “trapping heat” or “trapping radiation”.
Supposed educators use the term haphazardly, aimed at people from childhood to adulthood, conditioning their minds to have a sense of heat or radiation as some “stuff” that gets caught in a trap, and then, after all the years of childhood and early adulthood, they then get blasted for using a term as adults that they were NEVER taught any better prior to adulthood. Very convenient, I’d say — nurturing child-like minds, so that sophisticated minds can dissect them as faulty-thinking minds, while sophisticated WRONG arguments are peddled, based primarily on appeal to adult-mind authority.
Very scientific! [sarcasm intended]
Carbon dioxide lasers have been used in laboratories for a long time. They rely on the property of CO2 to absorb IR, hold on to it for a time, and then release it in the form of a coherent beam of IR collimated by the laser apparatus. You can feel the warmth of a beam of IR produced by a small CO2 laser. Carbon dioxide in the atmosphere does the same thing, but an atmospheric CO2 molecule releases the IR in a random direction, half of the time upward toward space and the other half down to the earth. So a portion of the IR headed toward space is re-directed back to earth. This does not warm the earth, but it keeps it from cooling as fast, all other things being equal. The earth’s surface will therefore equilibrate at a somewhat higher temperature. Hence the greenhouse effect.
Gill’s original post talked about CO2 emitted IR “bouncing around for a while”. Yes, that’s what causes atmospheric temperatures to equilibrate at a higher level than they would without CO2.
You can also feel the warmth of a beam of IR produced by a large CO2 laser but I don’t recommend it. Actually these are fantastic inventions, now due to careful engineering, design and lots of associated hardware gizmos it is possible to get efficiencies of around 10% from a commercial CO2 laser. Yes , pump 10kW into it (effectively thermal energy) and you can get a cutting beam of 1kW out. Of course there is 9kW of heat you need to throw away so much of the apparatus is hot hot hot and there is coolant, pumps, radiators, etc to get rid of it.
I think we done well ! Compared to the equivalent gaia design where you shove 10kW off the surface of the earth, through all that wispy gas (including 0.04% CO2) and get a downward beam of [REDACTED #1] kW making an efficiency of [REDACTED #2] %.
It’s like that jar at the fair with loads and loads of sweets in it and you are supposed to guess the number and write it down with your name.
The persons who said 5kW and 50% should now identify themselves..
AN ORDERLY QUEUE PLEASE, and stop pushing…
That’s just it, it doesn’t stay the same.
CO2 lasers do not work with the ‘cold’ radiation of 15 um but a much warmer one that was stated in the above post:
“Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.”
https://en.wikipedia.org/wiki/Carbon_dioxide_laser
The CO2 laser produces a beam of infrared light with the principal wavelength bands centering on 9.4 and 10.6 micrometers (μm).
As the author expects, there are some errors in this post that are responsible for the erroneous conclusion. The most important are these:
1. Wien’s law refers to the _peak_ wavelength of emitted radiation, not the only one. The distribution of the spectral radiance is given by Planck’s law. If the spectral radiance peaks at 10 micrometers, you can be certain sure there’s a lot of radiation around 15 micrometers as well.
2. For an object close to room temperature, no matter if solid, liquid, or gas, the atomic/molecular energies are largely unimportant (it happen to be important for CO2, which is the whole shebang). This is because at any ordinary temperature the occupancy of any state other than the ground state is pretty much zero, because typical atomic energies are separated by several eV. In order to get a fraction of 1/e molecules at an energy 1 eV above the ground state, the temperature would need to be >10,000 K. The important degrees of freedom are translational, and, depending on the substance, rotational and vibrational. The latter two are typically quantized, but the former is continuous. In other words, even if you don’t have some atomic energy accessible, you can ALWAYS knock particles around a little bit. Thus, a hydrogen gas at room temperature can be treated for all practical purposes as a gas of featureless, structureless point particles.
3. There is no physical law that states that molecules in liquids or solids “need more energy” in order to be excited, and I have no idea where this assumption could’ve come from.
All in all, the basic greenhouse mechanism remains unchallenged.
Thanks for posting this Anthony, it includes most of the misconceptions and errors about radiative transfer and CO2, so gives a good opportunity to rebut them in one place. References to the science can be found in any college textbook on Physical Chemistry and Molecular Spectroscopy.
https://books.google.com/books/about/Physical_Chemistry_5th_Edition.html?id=nIggbG9i8qEC
My understanding of Thermodynamics and Radiation from CO2 is as follows:
Different gases have different emission spectrums. For example Oxygen and Nitrogen do not absorb or emit Long Wave Infrared Radiation (LWIR) at all, so are not considered to be “Greenhouse” gases.
The temperature of a body (gas, liquid or solid) directly affects the wavelength of the radiation it emits and absorbs.
Wien’s Law defines the temperature – wave length relationship. The formula is Temperature (in degrees Kelvin) = 2898 / peak wave length in µm (micro metres). So for the average temperature of the Earth, lets call it 15C (=289 Kelvin), the wave length is 2898 / (15+274) = 2898 ÷ 289 = 10um.
The wavelength of the peak of the blackbody radiation curve decreases in a linear fashion as the temperature is increased (Wien’s displacement law).
It’s a reciprocal relationship not linear.
Carbon Dioxide’s absorption spectrum shows it absorbs LWIR at three different narrow wave lengths, sometimes called finger frequencies. Two of those wave lengths happen at temperatures too hot to exist in the atmosphere, the remaining wave length is 15um.
Wrong, those wavelengths exist in solar radiation and in surface emissions, so they are absorbed by CO2, just not in large amounts because those wavelengths are in the tails of the respective spectra.
15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is wrong and meaningless. Wien’s Law tells you the location of the peak wavelength, as the graphs added by Anthony shows wavelengths above and below that exist in the spectrum. More relevant to the IR emissions is the following:
http://lasp.colorado.edu/~bagenal/3720/CLASS5/EarthBB.jpg
As you can see the CO2 absorption band at 15micron is present at all the temperatures shown (dashed lines). Even though the maximum shifts with temperature so does the radiance increase as temperature increases. Consequently we see the following:
A blackbody at 300K emits 26.8522 W/m2/sr between 13 and 17 microns
at 270K it emits 18.4933 W/m2/sr
at 223K (-50ºC) it emits 8.53892 W/m2/sr
at 193K (-80ºC) it emits 4.32413 W/m2/sr
So now we need to examine the quality of that 15um radiation and its ability to heat the lower atmosphere. To do this we need to understand basic Quantum Physics as taught in 101 classes to Physics and Engineering students at University. Confession: I’m an Engineer, but trained before Quantum Physics was introduced to University courses so I’m self-taught, hence my need for a sanity check. Which, dear reader, is where you come in.
The key points in basic Quantum Physics, regarding radiative heat transfer, are:
Molecules have one or more electrons circling them. Their orbital height is not variable, But fixed. The electrons only orbit at set altitudes, the closer to the molecule the lower the kinetic energy of the molecule and so the lower the molecule’s temperature.
This is more like atomic structure and doesn’t apply to molecules (it’s more complex, bonding and non-bonding orbitals etc.)
For a molecule to “warm up” (have more kinetic energy) it needs its electrons to move to a higher, more energetic orbit. This can happen in one of two ways, get energy from a more energetic molecule via collision or receive energy via radiation.
No, that refers to part of the internal energy, the temperature is the kinetic energy of the molecule.
For an electron to move to a higher orbit from radiation it must receive a photon with sufficient energy for an electron to reach that higher orbit.
No, it must receive a photon of exactly the right energy to excite the transition. However electronic transfers in molecules are excited by UV light. The transitions which we are talking about in the IR are excitations of the vibrational and rotational modes. In the case of CO2 absorption at 15micron it is the vibrational mode that is excited, specifically the bending of the CO2 molecule (with associated rotations).
Photons with too much energy raise the electron to the higher orbit then the molecule immediately re-radiates surplus energy.
Again the photons must have exactly the right energy to reach the excited vibrational state. The molecule can emit the radiation but it’s far from immediate it will undergo millions of collisions with surrounding molecules during that time and in the lower atmosphere is more likely to transfer the energy to the atmosphere (thermalizes)
Photons with not enough energy to
raise the orbit of any of the electronsexcite the vibration are either scattered or immediately re-radiated (effectively reflecting or scattering them) with no change to the molecule’s kinetic energy, or temperature.The Photon must have a frequency that resonates with the molecule, otherwise the Photon is just scattered or reflected immediately with no temperature change to the molecule.
Won’t be scattered or reflected.
Carbon dioxide can only absorb Long Wave Infrared Radiation (LWIR) energy and radiate it at 15 micro metres, a fraction of the LWR spectrum.
In the band from 13-17micron, as you can see from the diagram above it’s significant part of the LWR spectrum.
Electrons orbiting molecules of a liquid or solid need more energy to boost an electron’s orbit than electrons in a gas, so require more energetic photons again to warm them.
No, it depends on the molecule, and again it’s not electron excitation it’s bond vibration.
Therefore it is my understanding that it is impossible for the LWIR emitted by a cold low energy CO2 molecule to have the energy required to warm any molecule in the atmosphere warmer than -80C and certainly no molecule in a liquid (EG water) or a solid body, as their electrons require even more energy.
As explained above this is not true for several reasons.
LWIR from CO2 simply bounces around the atmosphere until it escapes into space and it causes no warming of the lower atmosphere at all. The energy level of that 2W of LWIR is too poor to have any affect. It needs to be closer to 10um to be energetic enough to warm anything.
So the idea of CO2 trapping heat in the atmosphere is all wrong. Yes LWIR from CO2 is retained in the atmosphere longer, but it simply bounces around until it escapes into space without causing any warming.
No, the earth’s surface is very close to a black body and will readily absorb a photon emitted by CO2, or it could be absorbed by another CO2 molecule, or thermalize.
So am I right?
Mostly not I’m afraid, but thanks for the opportunity to correct these misconceptions which as you suggest are frequently encountered on the web.
And if you disagree with the science above, please explain which sentences you disagree with and exactly how, at the Quantum Physics level, photons from a CO2 molecule at -80C can warm anything.
As pointed out the concept of CO2 molecules at -80ºC is a major misunderstanding of Wien’s law (it also makes more sense to use the form of that law which relates peak frequency to temperature since that is proportional to energy).
Go to MODTRAN and test this for yourself. You will see that CO2 doesn’t have any impact what so ever until you get up to about 3 to 5 km when H2O starts dropping out of the atmosphere. If you then take a look at the temperature of the atmosphere at those heights where CO2’s signature is present, you will see that it is about -80°C. What you are seeing is the thermalization of CO2/15µ, and it results in a temperature floor, not an increase in the ceiling. CO2 only warms the upper atmosphere, not the lower troposphere.
http://lasp.colorado.edu/~bagenal/3720/CLASS5/EarthBB.jpg
What a fascinating collection of comments. I never would have thought there would/could be so many different ways to visualize radiation, temperature, and heat flow.
Well worth reading in their entirety several times I think.
More simply, radiation from a cooler object cannot naturally raise the temperature of a warmer object. This is so basic and so obvious that WUWT should hang its head in shame over the amount of articles it has posted with convoluted explanations to the contrary.
The whole premise of the Greenhouse Effect has nothing to do with how much Radiation CO2 can absorb, but how much the radiation, emitted by the CO2 in the atmosphere, can warm the surface of the earth. The answer, of course, is zero! Radiation from a cooler source cannot increase the temperature of a warmer source.
End of story. You don’t need to invoke quantum physics to understand it. You just need to stop holding sophists with PhDs in awe and use some common sense and basic observations.
radio waves can be absorbed by a receiving antena that is warmer than the emitter and be warmed up such that it glows red hot. try putting an electrical conductor in a microwave oven
It would work if it wasn’t for the fact that there are too many closed circuit loops that would generate heat and eddy currents and destroy it.
In fact many RF device send and receive on the same antenna all the time 🙂
Try a search on
“can two RF frequencies share the same antenna”
Or even lets try putting two full transmitters on the same antenna so try
“single antenna multiple radios”
It definitively answers your question 🙂
You are right – a cold object can not warm a warmer object.
However, the temperature of the atmosphere is between the maximum surface temperature and the minimum surface temperature. Therefore, the CO2 in the atmosphere can increase the *average* surface temperature by keeping the nigh time minimum warmer than it would be if there was no atmosphere.
“This is a contentious subject, and I have often shied away from it because it often erupts in food fights. ”
Well yes this opens a can of worms.
Here my 2 worms, er, cents 😉
1) Basically what increasing CO2 in the atmosphere does is shortening the path of the 15 um radiation, from let’s say 10 meters to 9.
One could argue that, in the whole air column, the heat transfer through radiation made with 9 meter steps, instead of 10 meter steps, would increase the lapse rate and thus increase the temperature of the surface. However this is dependant on the percentage of energy that flows through the 15um from the total which I guess is in the order of less then 1/1000.
=> effect cannot be measured
2) Personally I think the whole greenhouse effect is being built on a wrong basis as the elephant in the room is excluded: the oceans.
It is not the atmosphere that has the greatest greenhouse effect, but the ocean, water is practically opaque to infrared radiation, not transparent as the atmosphere is. Light penetrates the oceans to depth up to 100 meters and warms it, however the ocean loses heat only at the surface. The puny effect of CO2 ‘backradiation’ (and all other atmosphere including water vapor) is dwarfed by a 0.5 meter water strata at the surface of the oceans.
The idea that the oceans would freeze without the atmosphere’s backradiation – as the warmists maintain is nonsense. The oceans receive a 1300 W/m2 at the Equator. That heat is absorbed, stored and keep the oceans warm in the night too. No danger of frozen waters.
Of course the atmosphere has its contribution too, but the oceans cannot be completely dismissed as it is done.
Geology proves that the ocean currents define the climate, not CO2 variations, but hey, maybe it is still too early to consider this…
For the geologists and mineral collectors here (and those of you old enough to have ever bellied up to the bar in a discotheque), you are probably familiar with the effect of shining a ‘Black Light’ on solid (e.g. scheelite) and liquid substances (e.g. hydrocarbons). Short-wavelength lights will invariably give different ‘glowing’ colors and intensities than long-wavelength lights. In general, the observed fluorescence and phosphorescence is of a shorter wavelength than the stimulating electromagnetic energy. As a rule of thumb, the fluorescence is of a longer wavelength than the stimulating wavelength, and is variable in duration with different materials. The fluorescence is usually confined to one or two narrow wavelengths, which distinguishes it from thermal emissions. That is to say, if a substance (including individual gas molecules) receives EM radiation of greater energy than an electronic orbital transition, some or all of the energy may be absorbed, and then re-emitted when, after a highly variable period of time, the electron(s) falls back to the ground state. So, strictly speaking, narrow wavelength emissions from CO2 should be considered fluorescence, and should be occurring at wavelengths longer than the absorption bands.
[ https://micro.magnet.fsu.edu/primer/techniques/fluorescence/fluorescenceintro.html ]
On the other hand, the molecules of solids, liquids, and gasses possess kinetic energy in the form of the velocity of individual molecules, straining in the chemical molecular bonds, and in the case of solids, vibration in the crystal lattice. All of these sources of kinetic energy have different energies; the integrated energy is related to the average velocity of molecules, and the average vibrational bonding energy. At high temperatures, Doppler shift will cause the apparent emission wavelengths to also be different. At certain energy levels, bonds can be broken, and a change in physical state will take place, Thus, the distribution of emitted thermal energy with respect to wavelength will approximate the classic Black Body emission.
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption. That is to say, the presence or absence of water vapor will strongly effect whether the atmosphere retains the CO2 emissions. The thermal emission should correspond to the average temperature of the gas molecules.
Once again, the situation is more complex than climatologists are either aware of, or want to admit.
Clyde Spencer November 19, 2017 at 3:53 pm
Thus, if the major absorption feature for CO2 is around 15 microns, I would expect the fluorescence emission wavelengths to be out in the region of the water vapor absorption.
You’re mistaken because you’re extrapolating from electronic transitions with vibrational fine structure to vibrational transitions with rotational fine structure where the ‘red shift’ is far less pronounced and you don’t have to worry about the Frank-Condon effect.
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
Climate “Science” on Trial; CO2 is a Weak GHG, it has no Permanent Dipole
If anything CO2 helps to act as a temperature floor for the globe, as it’s main contribution is to thermalize energy consistent with a blackbody temperature of -80º C (-50º C to -110º C).
https://co2islife.wordpress.com/2017/01/30/climate-science-on-trial-co2-is-a-weak-ghg-it-has-no-dipole/
co2islife:
You are simply demonstrating your complete scientific illiteracy.
You see an equation, like the one for Wien’s Displacement Law. It expresses the peak radiative flux wavelength of a blackbody as a function of its temperature.
If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.
But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.
This is, simply put, complete nonsense!
Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target. Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
“If you had any real scientific understanding, you would realize the context of this equation, that it calculates a wavelength AS A FUNCTION OF temperature.”
Uhhh, just what do you think I was saying? Just what is a black body to you? Yes, you warm up a body, and that body emits a spectrum. Just what do you think I was talking about?
“But because you have no real scientific understanding, you simply see an equation relating wavelength and temperature. So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
Uhhh, no I don’t, and you can verify everything I say and write by simply going to MODTRAN or SpectralCalc. The calculators don’t lie.
“Radiation does not “have” a temperature. 15um radiation is thermally emitted from substances (including CO2) over a wide range of temperatures. As far as absorption of this radiation, it does not matter whether the source had a higher, lower, or same temperature as the target.”
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Ed bo says : Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.
If absolutely true why do the rings on my stove never show RED when the stove is off? Or why do we know that Blue stars have a higher temperature than brown dwarfs?
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
AndyHce November 20, 2017 at 3:04 am
“Radiation carries NO information as to the temperature of its source, or even whether it was thermally emitted or not.”
Why do Spenser and Christy believe they can obtain the temperature of the atmosphere by measuring microwave emissions of oxygen therein?
Why do astronomers believe they can determine the temperature of stars and nebula by analyzing their spectrum?
The individual photons carry no information about the temperature of their source so an absorber can not distinguish the difference between a 15micron photon emitted at -80ºC and one emitted at 100ºC and will absorb them both.
Temperature measurements are made by looking at the distribution of photons (the spectrum) which is a function of temperature.
co2islife November 19, 2017 at 4:41 pm
Uhhh, my point was that earth emits a full IR spectrum with a peak of 10µ. Absorbing a small fraction of that can’t warm the atmosphere above the 10µ temperature. Just what do you think a molecular spectrum is? CO2 doesn’t absorb or emit much IR, it absorbs at 2.7, 4.3 and 15µ Are you denying the CO2 molecular spectrum? Test it yourself, shine 15µ on a flask of CO2, it won’t warm.
Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. In fact plotting it versus wavelength distorts the distribution, it makes more sense to plot vs wavenumber, which is proportional to energy. If you do that you will see that the radiance peaks at 550cm-1 for 288K (peak radiance 0.136 W/m2/sr/cm-1) whereas the CO2 absorption band is centered at 667cm-1 (radiance ~0.132 W/m2/sr/cm-1). So CO2 absorbs near the maximum of the earth’s BB energy distribution, by contrast water absorbs between 1500-2000 cm-1 (radiance ~0.02 W/m2/sr/cm-1).
The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such.
http://www.azimuthproject.org/azimuth/files/earth_and_sun_emission.jpg
“The experiment you suggest would be rather difficult since the flask will be continuously bathed in 15µm radiation from its surroundings. You’d also have to surround it with N2 to avoid having all the 15µm absorbed by the air.”
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm. There are plenty of creative ways for a scientist to isolate this effect. The fact that they don’t even attempt to demonstrate the basics through experimentation proves they aren’t looking for the truth, and why they rely on computer models.
co2islife November 20, 2017 at 9:19 am
“Earth does not emit a full IR spectrum with a peak of 10µm, it emits a spectrum with a peak radiance at 10µm. ”
You are disagreeing with a calculator, and are wrong. Yes, the Earth may not be a perfect black body, but all calculations treat it as such.
No, I’m disagreeing with the statement you made, as shown in your graph the earth emits between 3 and 60µm with peak radiance at 10µm.
Yep, just like our atmosphere. The experiment would be adding additional energy to the system through the light. You could also put the flask in a -80°C container and shine the light on it. It won’t warm.
It certainly will!
All the 15µm light will be absorbed in a 10 cm path length if you put 1%CO2 in N2 in your container.
co2islife:
I said: “So you think you can calculate a temperature AS A FUNCTION OF wavelength.”
You responded: “Uhhh, no I don’t”
But then you followed up with: “Absorbing a small fraction of that [earth’s IR spectrum] can’t warm the atmosphere above the 10µ temperature.”
So you just calculated a temperature AS A FUNCTION of wavelength. You don’t even understand that you don’t understand anything about this subject!
As to the (irrelevant) point you were clumsily trying to make, that the atmosphere’s absorption of some of earth’s IR emissions cannot increase the atmosphere’s temperature past that of the surface, no one is claiming that it does. The atmospheric greenhouse theory REQUIRES the atmosphere to be colder than the surface.
co2islife November 19, 2017 at 3:53 pm
“15um equates to 2898 ÷ 15 = 193K = -80C or -122F. In the atmosphere this temperature only occurs about 90-100Km high in the atmosphere.
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.”
Thank you!!!! I’ve been making that point a million times.
And you’ve been wrong every time you’ve done so!
That point isn’t debatable. It comes from the SpectralCalc Black Body Calculator. You are claiming the math is wrong.
co2islife November 19, 2017 at 3:53 pm
Carbon Dioxide only emits and absorbs radiation at -80C from a narrow layer of atmosphere 90Km above the Earth’s surface.
This is false.
co2islife November 20, 2017 at 9:11 am
That point isn’t debatable. It comes from the SpectralCalc Black Body Calculator. You are claiming the math is wrong.
No, I’m claiming that the guy looking at the graph doesn’t know what he’s talking about!
Run Spectralcalc Gas cell simulator with 0.0004 VMR of CO2 at 296K and plot absorption between 600cm-1 and 700cm-1. You’ll see that it absorbs just fine.
“Note: This is a contentious subject, and I have often shied away from it because it often erupts in food fights.”
Mr Watts, why is this a contentious issue? The quantum physics are well known and defined. I’ve pointed out almost all the points highlighted in this article. The physics are the physics, you can’t change that.
How to Discuss Global Warming with a “Climate Alarmist.” Scientific Talking Points to Win the Debate.
https://co2islife.wordpress.com/2017/01/16/how-to-discuss-global-warming-with-a-climate-alarmist-scientific-talking-points-to-win-the-debate/
co2islife,
You said, “The quantum physics are well known and defined. I’ve pointed out almost all the points highlighted in this article. The physics are the physics, you can’t change that.” I wish it were that simple!
The impression I get from reading the comments is that most posting here are reasonably well-educated, but have varying backgrounds or disciplines. Yet, there is disagreement on what the “physics” says, even amongst those who share similar views about the validity of AGW. In part that may be the result of how an individual interprets the ‘facts.’ Often times the ease with which a problem can be solved depends on how it is expressed or framed. That area probably could use some work. Overall, I’d say that one of the problems is really a failure to communicate with one’s peers. Beyond that, there are some here with a vested interest in a particular viewpoint, and they have trouble looking at the problem from a different viewpoint. Few outside the area of geology are familiar with Chamberlain’s Method of Multiple Working Hypotheses.
If those of us, who are skeptical about the claims made by AGW alarmists, could get our act together and speak with a unified voice, I think that we would have a better chance of pointing out the errors of alarmists. But, with infighting, we are little more than an intellectual rabble.
Everyone of my comments can be verified by MODTRAN or SPECTRALCALC. I simply reading a calculator’s output. If the computer is wrong, then I am wrong.
It doesn’t help when the first comment is by a d@nier of EM radiation and photons.
Well, I can fully understand why this topic is so debatable. I’m an Engineer myself, and I’m still having trouble with deciphering so many viewpoints. I only started becoming interested in the topic around 6 months ago. Probably the only real thing I’ve discovered is that it is much more complex than I had envisioned. Perhaps only time is the ultimate arbiter.
John,
No it’s not complex, it’s second year thermo and heat transfer.
One popular RGHE theory power flux balance (“Atmospheric Moisture…. Trenberth et al 2011jcli24 Figure 10) has a spontaneous perpetual loop (333 W/m^2) flowing from cold to hot violating three fundamental thermodynamic laws. (1. Spontaneous energy out of nowhere, 2. perpetual loop w/o work, 3. cold to hot w/o work, 4. doesn’t matter because what’s in the system stays in the system)
“So am I right? I deliberately have not included any references because I want you to confirm or deny my understanding independently. ”
Almost everything discussed in this article can be found is article previously written on this topic. This Blog has all the resources to validate the points made in this article. The physics are the physics, they aren’t up for debate and Modtran and any Black Body calculator will demonstrate that.
https://co2islife.wordpress.com/