By Andy May, Michael Connolly and Ronan Connolly
In this post, we will discuss the tropopause, atmospheric molar density and the lapse rate (the change in atmospheric temperature with altitude). The key points are:
- A change in the molar density versus pressure best-fit line is a change in the equation-of-state.
- The NOAA and WMO definitions of the tropopause are different and somewhat clumsy.
- Molar density plots are a better and more robust way to pick the tropopause.
The World Meteorological Organization (WMO) defines the tropopause as:
“The boundary between the troposphere and the stratosphere, where an abrupt change in lapse rate usually occurs. It is defined as the lowest level at which the lapse rate decreases to 2°C/km or less, provided that the average lapse rate between this level and all higher levels within 2 km does not exceed 2°C/km.“ (International meteorological vocabulary, as seen in Wikipedia)
The lapse rate is simply the change in temperature with height, it is often presented as positive when temperature decreases with height (as in the WMO quote above), but here we will present it as a negative number when temperature decreases with height. In the “standard atmosphere” it is assumed to be -6.5°C per kilometer below the tropopause. NOAA defines the tropopause as an abrupt change in the lapse rate to neutral (zero) or positive (temperature increasing with height). These definitions often don’t work well, so here we will discuss a new method of locating the tropopause using atmospheric molar density.
We have analyzed the IGRA weather balloon dataset of over 20 million balloons for lapse rate and other values. The dataset includes balloon flight records from the 1940’s to the present day. The stations in the dataset at four selected times are shown in figure 1.

Figure 1
Global coverage is sparse in the 1940’s, but it fills in by 1960. The data collected includes atmospheric pressure, temperature, and relative humidity. Data is collected up to the point where the balloon bursts, generally an altitude of 30 to 40 km. In figures 2 and 3 we present the Northern Hemisphere summer (June-July-August) and winter (December-January-February) lapse rates computed from the balloon data in 15° latitude bands around the Earth.

Figure 2, Summer Lapse Rates, for each latitude band the tropopause is the sudden reversal of the lapse rate in the noted region.

Figure 3, Winter Lapse Rates, for each latitude band the tropopause is the sudden reversal of the lapse rate in the noted region.
In both figures the Southern Hemisphere rates are shown in oranges and reds. The Northern Hemisphere rates are shown in blues and greens. The inset figure is the international standard atmosphere (ISA) temperature distribution. The ISA temperature distribution is also plotted in the main graph as a heavy black line, so it can be compared to the data. As we can see the ISA lapse rate is only a very rough estimate of the average measured lapse rate. If we used the tropopause definition preferred by NOAA (a zero-lapse rate crossing) we can see that the various latitude bands cross this threshold between 8 and 18 km. After the lapse rate crosses zero, it typically reaches a local maximum and reverses direction. After it reverses direction it sometimes stays positive at about the same positive value. Other times, after the reversal, it returns to negative and then crosses the zero point again. Surface temperature inversions sometimes result in positive values at the surface.
Most of the points on the graphs are averages of over 10,000 balloon records, and some points are averages of well over 100,000 records. However, at very high altitudes (> 35 km) there are few records, so we don’t include any points on either graph with fewer than 10 records.
The two most southern winter (Northern Hemisphere summer) latitude bands (south of 60°S) do not cross the zero-lapse rate line until well into the stratosphere, thus using the NOAA definition they do not have a tropopause. However, both have a noticeable lapse rate reversal (see figure 2) at about 10 km and this point was used as the base of the tropopause since it meets the WMO tropopause definition (> -2°C/km).
The altitude displayed in figures 2 and 3 is computed from temperature and pressure readings. It is usually accurate to within 600 meters or so, but above 11 km it loses accuracy quickly and is potentially off by more than a kilometer. Some possible reasons for this are discussed in Connolly and Connolly 2014, paper 1 (section 4.1) and here. The tropopause occurs between about 8 km and 18 km depending upon the latitude and the surface temperature. It is higher in the tropics and mid-latitudes and lower in the polar regions. It is lowest in Antarctica in the Antarctic summer.
The height of the tropopause in the NH winter and summer is shown in figure 4. This is where the lapse rate first goes below zero, ignoring places where it is below zero at the surface, like in the polar regions during the winter. As mentioned above, the two points south of 60S in the NH summer are lapse rate reversals between -2 and zero (see figure 2).

Figure 4, height of the tropopause
Molar Density versus Lapse Rate
The weather balloons provide enough measurements to compute the molar density of the atmosphere, if we assume the ideal gas law. This is described in detail in Connolly and Connolly, 2014, paper 1, but we will summarize the key points here. Molar density is the number of moles of the atmosphere per cubic meter. Molar density or “D” is then n/V, where “n” is the number of moles and “V” is the volume. As Connolly and Connolly then show, D is also equal to P/(RT), where R=8.314 (the universal gas constant), “P” is pressure and “T” is temperature. So, all you need to do is divide the P (Pressure) values by the corresponding temperature multiplied by 8.314. One would expect a slope of 1/T, if we plot the molar density versus pressure as we have done for the Northern Hemisphere winter and summer in figure 5.


Figure 5
In the top portion of each seasonal plot in figure 5 we have plotted the average molar density and lapse rate versus pressure for the latitude band from 30N to 45N. The lapse rate first crosses zero at 9,000 Pa and 11,000 Pa for winter and summer, respectively. The left-hand lower plots show the troposphere points, they have a slope of 0.0004 and a positive intercept greater than 3.3, with an R2 of 0.998 and 0.999, due to some minor curvature at the lower pressures (higher altitude). The remaining points are in the tropopause or stratosphere and they are plotted to the lower right for each season. The points have a slope very close 0.0006 and a very small intercept of -0.03 to -0.06. The tropopause here is roughly at 9,000 Pa or approximately 17 km in winter. In the summer it is roughly 11,000 Pa or 15.5 km. The troposphere and stratosphere lines are significantly different, suggesting a state-change in the gases. There is no change in gas composition at that altitude and temperature changes can’t account for the change in slope, since the slope is 1/T anyway.
The Connolly and Connolly, 2014, paper 2 suggests that the change taking place at the tropopause could be the formation of oxygen multimers. This is a possible explanation that fits the known data at this point. See the paper for more details.
The remarkable new information is that the slope changes at the tropopause. Figure 6 shows the same set of plots for the Southern Hemisphere latitude band from 30S to 45S in the Southern Hemisphere winter.

Figure 6
In figure 6 we see that the line describing the troposphere is distinctly different from the line describing the tropopause and stratosphere. Both lines have an R2 of 0.999.
The Tropopause
The tropopause usually occurs at a temperature between 195K and 225K as seen in figure 7.

Figure 7
The details of the conditions in the tropopause are discussed in Connolly and Connolly, 2014, paper 2. Plots of all the latitude bands are presented in their figure 4.
So far, we have used the conventional definitions of the tropopause. We’ve interrogated the atmospheric pressure, altitude and temperature profiles and interpreted a tropopause when we see a sudden reversal of the lapse rate from negative to “more positive.” The WMO chooses a -2°/km cutoff and NOAA chooses a zero-degree cutoff. In both cases, other reversals near the surface or in the stratosphere must be ignored. All these rules and exceptions make these definitions less than satisfying.
A simpler definition is possible using molar density. The molar density versus pressure line in the stratosphere intersects the molar density versus pressure line from the troposphere at the tropopause everywhere as far as we can tell. The lines fit the data so well that few points are required for an accurate result. Figure 8 is for the Antarctic region in winter. This is one of the previously mentioned latitude bands where the lapse rate does not cross zero as required by the NOAA definition. By using crossing molar density best fit lines we can establish a tropopause at the lapse rate reversal between -2 and zero; which is where we would put it using the WMO definition quoted at the beginning of the post. Solving the two best fit lines for their intersection we can show they cross at 22,576 Pa.

Figure 8
Discussion
The data presented here shows that the tropospheric lapse rate is not the constant -6.5°C/km often assumed. The lapse rate curves vary considerably around the world and are never straight lines. It also appears that the atmospheric equation-of-state changes for some reason, possibly the formation of oxygen multimers, at the base of the tropopause.
While the NOAA tropopause definition works most of the time, the requirement for a lapse rate crossover at zero can be problematic. In some records, especially in the Antarctic in winter, the zero crossover is not reached; although the shape of the lapse rate versus altitude curve suggests a tropopause is present. In these circumstances the WMO definition works better. Both definitions are somewhat imprecise, and we suggest that molar density versus pressure best fit lines might locate the tropopause more precisely and rigorously. These lines can be created with points deeply in the stratosphere and deeply in the troposphere and the intersection of the lines would be the tropopause. There always seems to be a change of state at the tropopause that causes the slope and intercept of the lines to change abruptly. In some areas there is some apparent curvature near the boundary, but by choosing the points used to establish the lines above the tropopause and some distance below it, corruption by this transitional curvature can be avoided.
The tropopause is lower in the polar regions and higher between 40N and 40S latitude. This might be partly due to surface temperature, but the shape of the curves in figure 4 suggests that factors other than surface temperature are involved. As can be seen in figure 7 and in table 1 from Connolly and Connolly, paper 2, the temperature and pressure conditions at the tropopause vary considerably around the world.
Molar density is not often discussed in the meteorological literature to the best of our knowledge, but it greatly simplifies some atmospheric calculations. Temperature varies vertically in complex patterns, pressure trends can also be complex, but less so. Molar density is composed of two fundamental straight lines, one for the troposphere and one for the stratosphere and tropopause. There is a slight curvature between the two lines and more deeply in the troposphere there can be some slight curvature due to humidity changes or precipitation events. However, for any given location and time the detection of the slope and intercept for a molar density versus pressure plot is easy to detect and the fits are very good. This would seem to be a useful tool in meteorological and climate modeling.
While Andy May wrote this post, much of the technical work was done by Michael and Ronan Connolly.
Excel spreadsheets containing the data used to make the plots (and much more data) can be downloaded here.
As the Earth’s atmosphere is gaseous it is not very surprising that the important things we need to look at when trying to understand how it works are pressure,density and temperature variations with height. One must not fall into the trap of thinking this is a static system for the air is in a constant state of movement and there is a cyclical input of heat energy from the Sun.
The atmosphere has different sections/bands with different lapse rates. Each may need to be considered individually to understand the physical processes involved. From the point of view of understanding the relevance (or not) to the supposed theory of AGW/GHG it is likely to be the lowest band (troposphere) that is most significant. However these ideas about the tropopause are interesting and there is a lot of data to consider.
I would be interested in hearing explanations for the physical processes involved which result in the surface of the earth being as hot as it is.
I’ll link you to my description of the physical processes involved when I get back to my desktop pc in a couple of days.
Good to see more discussion of pressure and density at last.
For at least ten years I’ve been pointing out that the surface temperature enhancement over and above the S-B prediction where an atmosphere is present is a result of conduction and convection within a gravitational field as per the gas laws and nothing to do with ghgs at all.
Very simply, it’s that atmospheric clouds and GHG’s are not an infinite repository of heat and in the steady state, about half of the photon energy emitted by the surface and absorbed by GHG’s and clouds ends up being re-emitted by them into space while the remaining half is re-emitted back to the surface and added to incident solar energy making the surface warmer than it would be due to the Sun alone.
In effect, the energy returned to the surface making it warmer then it would be otherwise is a delayed version of earlier surface emissions which were temporarily stored by atmospheric GHG’s and clouds, some of which was eventually radiated back to the surface.
You suggest that instead of photons emitted from the surface finding their way directly and uninterrupted to TOA where they are then radiated to space, these photons bounce back and forth a bit.
And what is the time frame for all this back and forth transmission?
What is the delay time involved in a photon emitted from the Earth surface eventually finding its way to TOA?
The delay is on the order of seconds or less for photons captured by GHG’s and hours to days for photons absorbed by clouds.
Yet this ignores the screening effect of IR active gases (and suspended condensed particles) on said incoming radiation. So where does the extra energy implied come from?
@richard Verney: http://brindabella.id.au/climarc/dai/RadiativeDelay/RadiativeDelayInContext170828.pdf.
At standard temperature and pressure, the heat obtained by absorbing a photon is almost always shared by mechanical collision with adjacent atoms and molecules of gas. It is thus retained heat, but not much is actually radiated downward since only water vapor and CO2 can radiate anyway (and methane which contains carbon; water is a dipole molecule and can radiate and vibrate).
Michael2,
While some energy can be converted between the energy of translational motion and the state energy stored in electron shells consequential to absorbing a photon, this goes both ways in equal and opposite amounts. That is half the time, the molecule speeds up a bit and a the other half of the time it slows down a bit. The net exchange is approximately zero. The evidence of this is the fine structure on either side of the primary lines which represents energy added to or taken from a ‘rotational’ state.
If it behaved the way you claim, the Earth would be emitting a lot more than 240 W/m^2.
Michael2, Ashok,
About the only time there is any NET exchange between energy absorbed by a GHG and energy related to the translational motion of atmospheric gases is when an energized water vapor molecule condenses on a water droplet and another atmospheric gas molecule bounces off the water droplet.
The evidence that this is occurring is that at TOA, the emissions in the absorption bands are slightly less than half of what they would be without absorption. The emitted spectrum is also evidence of the 50/50 split, since in most saturated absorption lines, the emissions are half of what they would be without any absorption. Around where H2O lines overlap with CO2 lines, it is closer to a 45/65 split over a small band of wavelengths. The overall average is closer to 49/51 but this is close enough to 50/50 within the uncertainty of the data.
co2isnotevil
Your assumption that about half of the photon energy emitted by the surface and absorbed by GHG’s and clouds ends up being re-emitted by them into space while the remaining half is re-emitted back to the surface…. is incorrect. There are three axis x, y & z, however, you have just considered up/down. What about other directions ? Secondly, most of the energy absorbed by GHG thermalize to the surrounding atmospheric gases.
Ashok,
The other dimensions only add time to how long it eventually returns to the surface or is emitted into space. Photons will never travel exactly parallel to the surface.
See my earlier comment about ‘thermalization’. In a nut shell, it goes both ways.
” It is thus retained heat”
Rubbish.
Haven’t you ever heard of convection !.
CO2 is just another transfer mechanism for the COOLING of the atmosphere.
Andy,
Convection has nothing to do with the RADIATIVE balance or with the sensitivity. This red herring is the result of wiggle room added by Trenberth in order to support a sensitivity that is otherwise unsupportable.
Convection is a transfer of energy by matter and only the transfer of energy by photons has anything to do with the planets RADIATIVE balance.
Furthermore, the temperature of the atmosphere is mostly irrelevant to the balance and instead is a consequence of it.
co2isnotevil October 28, 2017 at 12:50 pm
OH GOOD GRIEF, ……. that is the same as one CLAIMING that the photons being emitted from an incandescent light bulb, …… dangling from a wire in the center of a room, ……. that half of said emitted photons will be directed toward the ceiling of the room directly above said lightbulb …… and the remaining half of those emitted photons striking the floor directly underneath said light bulb ……. leaving the remainder of the room in total darkness.
“DUH”, the direction of IR radiation being emitted from an airborne molecule (CO2) is not determined by the forces of gravity or anti-gravity.
co2isnotevil October 28, 2017 at 11:17 pm
“DUH”, here is a “thermal image” of the IR energy being emitted by a poorly insulated home.
So tell us, co2isnotevil. …….. are any of those “pictured” photons travelling exactly parallel to the surface? To wit, the “thermal image”:
http://www.hotel-r.net/im/hotel/hu/thermal-house-1.jpg
The influence on the surface is a function of the sine of the angle relative to it and that when integrated over all angles of photon emission, this becomes unity.
This kind of approximation is very common. For example, calculating the capacitance of a parallel plate capacitor.
The question for you is where do photons ultimately end up, if not into space or back to the surface, if they are not otherwise reabsorbed by another GHG molecule or the water in a cloud? Keep in mind that there are no walls in the atmosphere to intercept the photons.
Sam,
Regarding your thermal image, the Earth is not flat, nor does the Earth have enough gravity for photons to orbit. Those photons you perceive as parallel to the surface will eventually exit the atmosphere through either the top or bottom unless they are intercepted first.
cdquaries,
What extra energy? That’s been my point for decades. The IPCC requires extra energy to support their insanely high sensitivity. Such energy dosen’t exist. The power returned to the surface is not extra, but the energy of past surface emissions that didn’t get through the atmosphere the first time.
The IPCC got this non existent energy it needed by misapplying Bode’s feedback analysis to the climate. They ignored the fact that Bode requires an implicit, infinite source of Joules to power the gain. It’s this implicit power supply that enables both the extra energy they IPCC presumes as well as the possibility of a run away effect. This supply can not be the Sun as the Sun is already accounted for by the forcing input. If you connect the input pin and power supply of an amplifier to the same source, will you ever get more power out then you put in?
@co2isnotevil,
Got it and thanks.
How many individual molecules does the energy from a solar photon spend time in, before it is radiated back to space.
On average, 8,000,000,000 individual molecules. That should give you a better idea of what is really going on. It takes a lot of “time” for that energy to move around so much before it is emitted back to space.
Let’s look at some interesting Modtran radiation charts.
First looking UP (as in the infamous back-radiation) from the surface (at head-height) when all the GHGs are gone except water vapour and CO2. The Red line is when only CO2 exists. So we are getting back-radiation from the CO2 as if it were 10 metres high or so but just in the small spectrum of CO2. The Blue line is when we add normal levels of water vapour to the atmosphere. Now there is also all kinds of back-radiation from the troposphere and 10 metres above your head and much more than the CO2.

Now, we are going to add “clouds” to the mix looking UP again (as in back-radiation). The Blue line here. A perfect blackbody spectrum almost as if All of the heat is being held in by the clouds. Clouds act like a solid surface here although it is not quite a solid No CO2 lines or anything else, a perfect blackbody. The clouds have 20 times the power of CO2 and water vapour itself. And clouds are only present 65% of the time you know.

Now let’s go outside the atmosphere and look DOWN (as in energy being emitted to space). The Red line is CO2 by itself (no other GHGs, no water vapor and no clouds). Now energy goes directly to space, except in the CO2 absorption lines where CO2 is emitting energy to space in the stratosphere, 220K. CO2 actually cools off the Earth by emitting energy to space in the stratosphere. The Blue line, we add clouds and water vapour and now we have much more energy being emitted to space, from the troposphere and the lower stratosphere as well.

Well, this is a complicated mess. How are you going to be able to model this, especially when 8 billion molecules are holding the energy of one solar photon at different times over many hours (close to two days). AND, most of the energy is moving without being radiated at all. It is mainly collisional energy exchange and covection with N2 and O2 also playing a an extremely important role now. CO2 actually steals the energy back from N2 and O2 in the stratosphere and emits it to space.
Why have you not seen this before.
Bill,
Yes, clouds have a larger net warming influence on the surface than GHG’s.
For 8 billion molecules and a mean path length of 1 meter between molecular absorption (it’s actually far less), the total length the energy travels is 8E9 meters. At 3E8 m/sec, this is only minutes including the time between individual absorption and re-emission events and not days. At a more reasonable mean path length of the emitted photons before re-absorption, it’s no more than seconds.
Clouds on the other hand retain energy for a much longer period of time. One of the persistent errors I’ve seen is that the effects of clouds are conflated with the effects of GHG’s making the FINITE influence of GHG’s seem much larger than it actually is.
Relative to the role of N2, O2 and collisions, these are largely irrelevant to the radiative balance and all they do is re-distribute the energy temporarily stored in the atmosphere.
There also seems to be far too much emphasis on the kinetic temperature of the atmosphere (i.e. the temperature manifested by molecules in motion). This is a consequence of the surface temperature and not the cause of it. Moreover; the emissivity of N2/O2 is approximately zero.
co2isnotevil October 29, 2017 at 8:53 am
What sort of gibberish were you spouting in your above comment, co2isnotevil, ….. or were you just hallucinating for the fun of it?
Citing such an example of similarity between a photon and an electron is asinine.
co2isnotevil October 29, 2017 at 8:53 am
Who the ell cares where the photons in question ….. ultimately end up? It has nothing to do with or explains your silly arsed claim that …… 50% of all the photons radiated from the earth’s surface will be re-radiated back to the surface ….. and the remaining 50% of said photons will be radiated or re-radiated into outer space.
And you are correct, the earth’s atmosphere has no walls or ceilings, ….. but physical greenhouses do, ……. so why are you calling a few of the gases in the atmosphere ….. “greenhouse gases”?
“DUH” the same/similar gases (all of them) that are “trapped” within the confines (walls and ceiling) of an actual greenhouse are exactly the same/similar as all of the unconfined, non-trapped, free-ranging gases in earth’s atmosphere. Thus it is utterly asinine and idiotic to imply, suggest, infer or claim that the heat-trapping potential of the “non-confined” atmospheric gasses is identical to the heat-trapping potential of the “confined” gasses in an actual greenhouse.
co2isnotevil October 29, 2017 at 9:06 am
co2isnotevil, … you are still “talking trash”.
We are talking about the trajectory path of a photon being radiated from a “point source” on the surface of the earth and/or re-radiated from the “point source” of a per se “Greenhouse” gas molecule in the atmosphere. And the number of feet altitude above sea level of the aforesaid radiation “point source” location is the determining factor of how many of said re-radiated photons return to the surface.
The higher the altitude, ……. the lesser the chances of the re-emitted photon striking the surface. (Except in deep valleys [aka: The Grand Canyon] with mountains all around the perimeter.)
Sam,
“Citing such an example of similarity between a photon and an electron is asinine.”
First of all, I never said they were the same. Nonetheless, all of the photons involved in the radiative balance originate from or are absorbed by an electron shell. Do you understand the significance of the fine structure constant as it relates to tightly connecting photons and electrons?
Do you understand the vernacular of climate science? While I agree that CO2 operates far differently than a greenhouse as it affects the temperature and I have NEVER stated or implied otherwise, CO2 is called a GHG and calling it anything else is silly and counterproductive.
You also seemed to misinterpret my comment of a point source which referred to the Sun, relative to the Earth.
You need to brush up on calculus so you can see the math behind integration that make the approximation I talk about a valid and is in fact widely used elsewhere.
You seem to be among those who think CO2 has no effect. It has a finite, but small effect and while relative to the skeptical cause, this difference is not particularly important, relative to the reality of the laws of physics, it’s crucial.
co2isnotevil October 30, 2017 at 10:00 am
And what about all the zillions of photons being emitted from the earth’s surface ….. that are not absorbed by the electron shell of a per se atmospheric GHG?
“DUH”, apparently you think that every photon being radiated from the surface will make contact with an atmospheric GHG molecule before it escapes into outer space.
GEEEEZUS, iffen the satellite sensors detect an IR emitted photon at the high altitude it is orbiting at, it really doesn’t have a clue as to whether that photon was radiated directly from the surface or was re-radiated from an H2O or CO2 molecule located somewhere in the atmosphere, ….. to wit:

“Yes”, and I can detect weazelworded “junk science” agitprop in half a heartbeat, so best you cease with your attempts at “blowing smoke” at my person.
You are absolutely right, …….. calling it anything other than a GHG would be silly and counterproductive to the “fearmongering” tactics of the avid proponents of CAGW in their quest to scare the BEJESUS out of miseducated and gullible populace.
co2isnotevil, ….. now that was one brilliantly worded CYA iffen I ever read one.
“HA”, a ….. “finite, but small effect ….. that is not particularly important”, ….. HUH?
So, that “finite effect” is like whale feces, ….. right, ….. something one doesn’t have to worry about.
Give it up, …… that “dog” you are chasing ……… won’t hunt.
“And what about all the zillions of photons being emitted from the earth’s surface”
Did you see the word ‘or’ in my statement? The ones that are not absorbed by another electron shell either exit into space contributing to the balance or are returned to the surface making it warmer than it would be as a result of the Sun alone.
You keep making statements that do not represent anything I said as you fail to comprehend what I did say. This isn’t particularly useful, so I’m done here.
Geometrically, the photon emmision rate into space has to be more than 50% except at or very close to ground level. There is a slight dependence on terrain with mineral surface capable of intercepting more that 50% of LWIR photons in mountainous areas until around 3,000 to 5,000 meters. But, this condition is local and for most of the planet, once a photon reaches even a meter or two above the surface, the interception rate is less than 50%. The interception rate begins dropping geometrically with altitude.
Duster,
The geometry consideration is the ratio between the area over which the energy is absorbed by the atmosphere and the area over which the energy is emitted, which is the origin of the factor of 2. In this case, the atmosphere is modelled as a single entity placed between the surface and space.
Where the photons originate doesn’t matter relative to the emergent fraction which is a bulk property of the atmosphere and varies by a couple of percent on either side of 50/50. When the fraction returned to the surface is more than half, the surface gets a little warmer than average and when it drops below half, the surface cools to a little below average. What seems to modulate this fraction is the ratio between cloud height and cloud area and if there’s any ‘control valve’ in the system, this is it.
Duster – October 30, 2017 at 8:58 pm
Thank you, Duster.
It utterly amazes me the number of per se ….. edumacated individuals …… that have a nurtured “mental image” and fanatical religious belief …… that the per se “GHG” gasses in earth’s atmosphere actually constitutes an “atmospheric brick wall” that intercepts any and all IR energy being radiated from the surface, ….. and that is EXACTLY WHY …… co2isnotevil was touting his/her “50% UP and 50% DOWN” piffle n’ tripe.
“DUH”, it ought to be plainly obvious to even the most sever of learning disabled persons that iffen astronauts, etc., can see and/or photograph the earth from far out in space, then that is literal proof that there is one hell of a lot of “re-emitted” Solar energy originating from the earth’s surface. To wit:
Duster,
Don’t be confused by Sam who is clearly misinterpreting crucial facts. For example, he said,
“co2isnotevil was touting his 50% UP and 50%”
Which Sam obviously doesn’t understand as this applies to the fraction of surface emissions absorbed by the atmosphere which is clearly not 100%. About 1/3 of those surface emissions are not absorbed by either GHG’s or clouds and go directly into space. Based on his comment about re-emitted solar energy, he also seem to confusing reflection of visible photons due to albedo from LWIR emissions consequential to temperature.
He also seems to think that once a photon is absorbed by a GHG or cloud, that energy stays in the atmosphere forever when in LTE, the energy flux entering the atmosphere is equal to the flux leaving the atmosphere. It’s just that the flux leaves over twice the area over which it arrives.
It’s people like Sam who express unscientific views that gives the alarmists ammunition for using the D word.
The proper perspective is not that the effect is 0, but that it’s finite and too small to be obsessing about and definitely nowhere near large enough to justify spending a dime to mitigate, much less many trillions of dimes.
Samuel,
It is a shame that you have posted such an obviously fake picture of the Earth touching the horizon of the Moon. The picture purports to show the Earth lit in its waning gibbous phase, with a view of North America and the Arctic polar ice on the right, while in the foreground is an Apollo moon buggy. A few hints:-
1 The antenna on the lunar rover is transmitting up into the sky and is pointing directly back to Houston and is clearly not pointing at the Earth in the picture.
2. Remember that on the surface of the Moon, when you look up at the Earth it literally hangs above you in the sky almost motionless, so the concept of Earthrise is meaningless except for an observer in lunar orbit.
3. Most importantly the only place on the Moon where a photograph even remotely like this could have been taken is on the limb of the Moon at a lunar longitude of 90 degrees east. But that location is impossible because the furthest east any Apollo landing made was the Apollo 17 at the Tautus-Littrow site with Landing Site Coordinates 20.19080° N latitude, 30.77168° E longitude.
If you want to publish proper pictures of the Earth from the Apollo catalogue, then I suggest you look here in NASA’s Index of Mirrors for the official images.
Here is a nice example from Apollo 17:-
Perhaps however you just wanted to publish a fake picture to go along with the fake science of AGW?
And here are the details of the original picture from the Apollo 16 mission
The input from the sun is practically constant, varying with only a few watts/m² over the year.
It is important to remember that the atmosphere, at average -18C-ish, is heated by the earth’s internalism heat. According to the inverse square law, emission of heat at 249W/m² close to the boundary of a sphere, needs a source power of at least 960W/m². The surface needs a source at about 1550W/m². Since the atmosphere doesn’t absorb sunlight very well, and TSI isn’t enough as a source of surface emission, there is only the red hot magma left. TSI should be balanced to source power, not emission above the surface. Try this: TSI(1360.8)-960=390W/m².
The sb-equation for transfer rules them all.
The greenhouse effect is a dead horse, stop beating it.
Crappy phone-writing, 249W should be 240W
lifeisthermal. The input solar energy varies by 88 W/m2 over one year. See figure 1 here:
https://globalwarmingsolved.com/data_files/SCC2015_preprint.pdf
Yes, and compared to the source power needed for the observed emission(s), it is a few watts. But since the climate debate is focused on the magical “forcing” by co2, which adds energy from nowhere at a couple watts, I get your point.
Isn’t it weird in the gh-theory, how MORE molecules sharing a constant heat flow, somehow means more kinetic energy per molecule? There must be unicorns involved.
“The input from the sun is practically constant”
Sorry, but NO it is not.
There are large variations in frequency content that effect a myriad of things.
Particularly the changes in UV strength and frequency, which allow much greater penetration into the equatorial ocean.
Where in the sb-equation for heat transfer do you find frequency?
Sorry, but there is only T⁴ in there. Remember, always, that temperature and heat is a matter of power in watts, or even better, T⁴.
co2isnotevil
What stops photon to travel in all the three dimensions ?
Does the Sunlight travels only to earth or in all the three dimensions ?
Wow.
There is an endless supply of stupid after all.
I’ve got more stupid if you want. Heat and gravity is equal in the equation for an electric field in a hollow sphere with a ball at the center. TSI/(4/3)=4/3*8g².
Light/heat seems to behave as a fluid, since the reduced equation of state for a van der Waals fluid works: 8/3*sbT⁴(TSI)=4g²+4/3*g².
g²=95.7Nm²(stress, pressure, thermal resistance)
4/3*g²=128Nm²=
128W/m²=218K=average tropopause temp.
4g²=383Nm²=383W/m²=287K
Ashok,
“What stops photon to travel in all the three dimensions ?”
Nothing. The point is that no matter what direction an emitted or re-emitted photon goes, it eventually either leaves the planet, returns to the surface or is absorbed by something else.
It does turn out that energy from the Sun is almost exactly perpendicular to the surface, but this is because the Sun is far away and acts as a point source..
Asok.
I meant perpendicular to the equator. Obviously, sin theta owing to the curvature of the surface comes into play.
The variation in the Sun may be small , but the variation from peri- to ap- helion amounts to ~ 4.6K , much larger than the total perhaps 0.8K variation in estimated global mean this debacle is about .
A general note : gravitational energy cannot be left out of equations of total energy balance . And , as Alan Guth and others have persuasively pointed out , computes as a negative allowing the balance of temperature.pressure vs gravity .
“A general note : gravitational energy cannot be left out of equations of total energy balance . And , as Alan Guth and others have persuasively pointed out , computes as a negative allowing the balance of temperature.pressure vs gravity ”
I have been pointing this out in other, non climate related, forums.
Gravity is exactly balanced by Temperature, Density (mixed gas/liquid), Pressure (mixed gas/liquid).
You mean like this?:
dU(TSI)=W(4g²)+Q(4*0.0000000567*256⁴)
Solar radiation on a hemisphere, absorbed in a double shell spherical volume, result in that temperature.
1/2*TSI/(4/3)²
There it is.
The Earth rotates, and is not flat. News to anyone?
Rev Badger
As promised this is the explanation for the physical processes involved:
https://tallbloke.wordpress.com/2017/06/15/stephen-wilde-how-conduction-and-convection-cause-a-greenhouse-effect-arising-from-atmospheric-mass/
In winter the tropopause at the pole becomes invisible.
http://pics.tinypic.pl/i/00943/wgz7a47265tl.gif
And then there are thunderstorms in the tropics and inversions at the poles. Where is the TOA as defined by OLR?
The method you suggest would characterise something. But is it reliably what they normal definition is trying to get at – the approximate zero of the lapse rate?
Could the apparent change in molecular weight just be the reduction in water vapor component?
Nick, good question. I beat that drum for quite a while. But, the balloon records show the absolute humidity (AH) dropping to less than 0.1 g/m3 well below the tropopause and I don’t trust any measurements below that, call it zero. I have some plots and work already prepared, I may do a second post on that topic.
Take our band 0-15S, the AH is 0.13 at 9.5 km. The lapse rate reversal is 15 km.
Band 79-90S: AH is .15 at 5 km and we picked the tropopause at 11 km.
So, while it seems logical that it is related to AH, the data does not support this.
Gotta have better eyes than mine to see that.
Can we get a larger image, or one that can be embiggened?
Agreed, I can’t read that graph either.
Your article explains a lot of the questions I have had. I have always wondered why the speed of sound follows the temperature profile cure of the atmosphere as you increase in height. Still not explained is why the Speed Sound and temperature curve are almost identical. It seems to me with the proper change in scale for either temperature or speed of sound the two would be identical. These transitions would be perfect for creating a “cap” like barrier that would slowdown or even hold down the flow of energy from one layer to the next. Just like a smoke can rise straight up on a calm day and then flatten out when it meets these transition barrier/layer. Same thing happens in water and the Navy has used this to hide subs from surface ships looking for them. In water these layer transitions can even cause a refraction of the sonar signal. The layers in the atmosphere cause a refraction of radio signals. Obviously these layers cause a refraction of IR waves also. This same phenomenon will/should, also affect the temperature of the land/water below these layers.
Graphic
http://projectthunderstruck.org/wp-content/uploads/2014/10/Altitude-vs-temperature-pressure-density.jpg
Temperature for our purposes is the kinetic energy of the air molecules, which boils down to their speed. The higher the temperature, the higher their speed. Sound waves propagate through the air by one molecule bumping into the next molecule, which then bumps into the next and so on. The faster they are moving, the less time it takes to cover the distance to the next molecule, and the faster the sound wave travels.
A more simple explanation is that the speed of sound in a gas depends only on the temperature, and not the pressure (or density). But your explanation does cover it.
Measuring temperature by speed of sound:
https://www.edn.com/design/analog/4369636/An-introduction-to-acoustic-thermometry
Excellent article. Connects the dots on a few things I have been thinking about.
See they are still printing EDN. Subscribed to it years ago. Always had great articles. Glad it is still around. Have bookmarked its home page.
Tropopause above the equator is wide, and average temperature drops to -80 degrees C.
http://www.cpc.ncep.noaa.gov/products/stratosphere/strat-trop/gif_files/time_pres_TEMP_MEAN_ALL_EQ_2017.png
That is what we found as well. Our average temperature at the base of the tropopause, over the equator, is 196K or -77C. This is at 16 km and a computed average absolute humidity of 0.0006 g/m3. The pressure is 11,400 Pa. Like I said previously, any absolute humidity below 0.1 is essentially zero.
Common 0.1 bar tropopause in thick atmospheres
set by pressure-dependent infrared transparency
T. D. Robinson1,2* and D. C. Catling2,3,4
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.465.8585&rep=rep1&type=pdf
The absorption of solar energy by ozone in the lower stratosphere causes heating which expands the surrounding air and reduces molar density.
No need to invoke the concept of oxygen multimeters unless ozone can so be considered.
Meanwhile, the density gradient In the tropopause is governed primarily by the reducing weight of the air above as one moves upward. As that weight reduces the molecules are able to move further apart at any given temperature.
The intersection between the two effects can indeed occur at variable heights within stratosphere or troposphere and the tropopause can correctly be defined as being reached at that intersection.
It is the ever changing heights of that intersection around the globe that regulates the radiative energy flow to space rather than the presence or otherwise of ghgs.
If ghgs increase then we just get a minuscule convective adjustment that keeps the atmosphere in hydrostatic equilibrium with no need for any surface temperature enhancement.
The surface temperature enhancement above the S-B expectation that we do observe is entirely a result of atmospheric mass conducting and convecting within a gravitational field in observance of the gas laws.
Are temperature differences with height a secondary effect of the e-m radiation flows up and down or are they a primary effect of gravity (or something else)? How would we examine this? At night there is no downward e-m from the sun, would we expect (or are there) any differences in the temperature profiles of the atmosphere day to night?
Does temperature of the atmosphere where it is very thin and contains very little matter (fewer molecules) have any real meaning? Can we consider the energy content in Joules for each cubic metre at different heights and deduce anything interesting? If the energy in joules is many magnitudes lower in the upper atmosphere does it have any relevance at all to the way the atmosphere works as the e-m radiation up/down will overwhelmingly pass through it with virtually no effect?
Lapse rate down where we are is primarily a gravity effect. Temperature is the kinetic energy (speed) of the air molecules, and when they move upward against gravity, they lose speed (temperature), and when they move downward, they gain speed (temperature). The constant movement and bumping together of the air molecules tends to shuffle the warmth downward. The lapse rate is small and easily overcome, but it is always there trying to restore itself.
The key question is, at what altitude does the atmosphere cease to be opaque to 15-micron outgoing IR? Increase in CO2 raises this altitude, thus causing the atmosphere to radiate to space at a lower temperature, thus retaining more heat in the atmosphere. Thing is, this effect is well nigh impossible to calculate, as just how this happens is different at each frequency of IR and each density of CO2 molecules.
Modtran offers an approach.
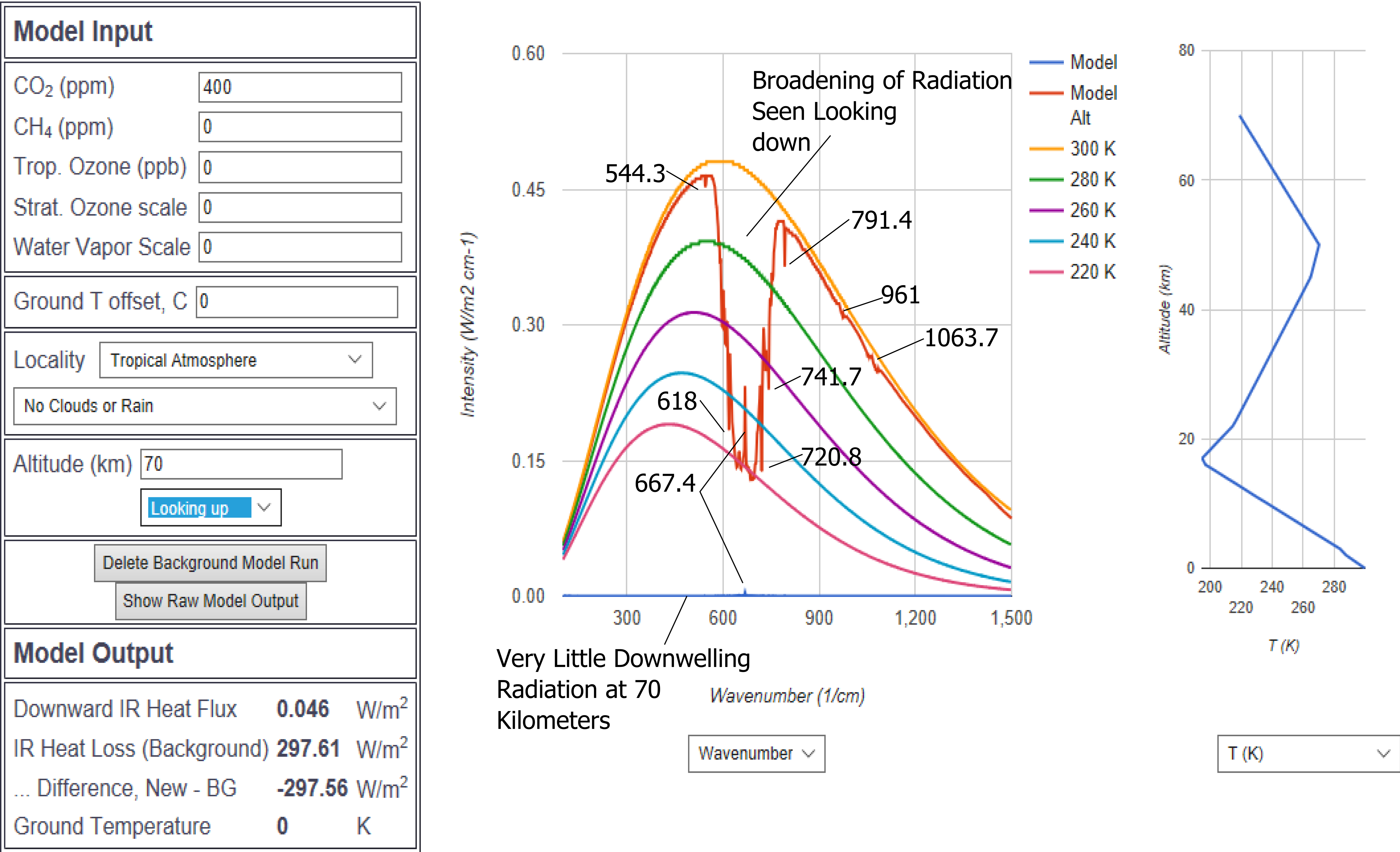
From 70km the 15 micron/667.4 WN band is seen radiating at a temperature of ~250K. This corresponds to 8km at the tropical lapse rate.
We know that 667.4 light is 98% absorbed within a meter at 400ppm CO2. We also know that CO2 is a lousy blackbody that re emits only a small fraction of what it absorbs as radiation. CO2 much prefers to vibrate rather than spit photons.
The conventional view is that the radiometer sees from 70km the the few photons CO2 has grudgingly emitted, at an effective altitude where they can escape.
But whathat is so special about 8km? There is still lots of CO2 above. Being well mixed, CO2 is thought to be at 400ppmv all the way to 70km.

Superimposing Modtran on the LBLRTM and setting tropopause equal, you get the above. It can be seen that a satellite is looking down through a lot of CO2 radiance to get that signal at 250K for the fundamental bend and 220K for the rotational sidekicks…
One must be aware that the temperature in the stratosphere and mesosphere depends on the ozone production.
http://www.cpc.ncep.noaa.gov/products/stratosphere/strat_a_f/gif_files/gfs_t01_nh_f00.png
This is the so-called “Chapman Mechanism” for forming ozone. Warmth and ozone (in the stratosphere) is then supposedly transported to the poles in the polar winter when there is very little sunlight. The various problems with this hypothesis are discussed in Connolly and Connolly’s paper 2, there are some links to this paper in the post. This idea that ozone causes the warming in the stratosphere and tropopause is only weakly supported and it has several significant problems. See section 3.3 in their paper 2.
Ozone can only be formed in the presence of a gas particle. Then ozone transmits the energy to this particle. Most ozone is produced on the border of the stratosphere and mesosphere. It’s not about the amount of ozone, but about the speed at which ozone particles are produced.
http://www.cpc.ncep.noaa.gov/products/stratosphere/strat-trop/gif_files/time_pres_TEMP_MEAN_ALL_NH_2017.png
Mechanism of Ozone Formation and Ultraviolet Absorption
I would say the other way around. Ozone production (and destruction) depends on temperature,
Urederra,
Ozone creation and destruction in the stratosphere involves a multitude of constantly varying factors. One of those factors is the proportion of solar energy that arrives in UV wavelengths. That is far more variable than total TSI.
Atmospheric chemistry for the studious.
http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap10.html
Basics of upper atmosphere chemistry, including the Chapman mechanism.
Chemical reactions involved in the formation and destruction of Ozone in the upper atmosphere have both pressure dependent and temperature dependent coefficients.
Ozone formation reactions have a definite positive pressure coefficient.
The new ozone then shields (blocks solar UV) from reaching lower altitudes, otherwise Ozone would keep being produced. There is always a dynamic balance between formation and destruction.
The first and second derivative of the molar density would be a good way to analyze the slope change. It should provide a step change in first derivative, and an obvious bump in second derivative.
A question ?
Do balloons measure CO2 as they float upwards .As CO2 is heavier than air do the concentrations vary into the stratosphere .?
CO2 is not measured, it is a very small component at 0.04% of the atmosphere and difficult to measure even on the surface.
Well, oxygen is also ‘heavier’ than air; but no one that I am aware of thinks that the partial pressure is less at altitude than it is at the surface. Sure, the absolute mass is less; but the percentage isn’t. At surface conditions, the translational velocities of the molecules comprising air are on the order of 1km/sec. Because the densities are low and the velocities are high, gases mix completely unless prevented from doing so by other causes.
Why don’t we just let climate models tell us? Lol
Hahahaha
Andy,
Great post thanks. You say:-
Your plots of the level of the tropopause in the tropics (Fig. 4) define the latitudinal reach of the Hadley cell. I suggest that it is time to introduce into this discussion the modelling work of Hunt, B.G. (1979) and Del Genio, A.D. & R. J. Suozzo (1987) that shows that for terrestrial planets the latitudinal reach of the Hadley cell towards the poles is determined by the daily rotational speed of the planet.
From the abstract of Hunt, B.G. 1979: The Influence of the Earth’s Rotation Rate on the General Circulation of the Atmosphere. Journal of the Atmospheric Sciences, Vol. 36 (8), 1392-1408.
From the abstract of Del Genio, A.D. & R. J. Suozzo 1987: A Comparative Study of Rapidly and Slowly Rotating Dynamical Regimes in a Terrestrial General Circulation Model. Journal of the Atmospheric Sciences, Vol. 44 (6), 973-986.
For the rapidly rotating Earth the effect of the Coriolis force on air lifted to the tropopause by the convective storms of the ITCZ, results in a forced return to the surface in the mid latitudes. This forced return of tropical air to the surface in the Horse latitude high pressure zones of the Hadley cell means that the descending air heats by adiabatic auto-compression and this non-radiative process helps to maintain the high surface temperature of our planet.
Thanks Philip Mulholland. These are good points and I’m inclined to agree with you. I suspect my co-authors may not agree and I would like to see their reply when they can get to it.
I remember an old definition of troposphere as a layer where heat is transported by convection. I assume that there is little convection in polar regions, therefore a troposphere may be completely absent there. It also explains different slopes of a molar density-pressure graph in the troposphere and the atmosphere.
.. and the stratosphere, of course, damn all those ..spheres.
Adiabatic auto compression within descending columns of air (half the atmosphere at any given moment) is the cause of the observed surface temperature enhancement above the S-B prediction and NOT downward radiation from radiatively active material.
The latter is already included in the thermal structure of the atmosphere via a contribution to the lapse rate slope but the essential point is that below the tropopause that slope is set primarily by conduction and convection within the density gradient set by the gravitational field.
The simplest conceptual device to assist in envisaging the process is to consider KE and PE as being interchangeable as one moves up or down the density/pressure gradient.
Thus descending air delivers KE back to the surface and that KE must be added to the continuing KE from insolation in prefer to derive the value of the surface temperature enhancement.
Good points Phillip.
This touches on an ignored aspect of the atmosphere: it’s a low density fluid.
Gaia rotates, and it’s surface topography will effect the fluid. Then we have a nearby gravity well, Luna, making her rounds (atmospheric tides). Importantly, Sol pumping energy into the fluid. Lastly… There’s no lastly there’s many other factors. Composition of the fluid, internal heat, geomagnetism.
Will incorporate your info, thanks.
So where exactly is the ‘top of the atmosphere’?
Ha ha! From my reading, pick a spot, any spot. For some it is the tropopause, sometimes it is the height where the temperature equals the S-B (blackbody) temperature of the Earth. I’ve seen other heights also. It’s wherever you need it to be to make whatever point you want to make.
The Karman line serves for most purposes, above it a vehicle cannot get enough lift from aerodynamics to stay aloft without exceeding orbital velocity. So it is the demarcation between aeronautics and astronautics. Gas atoms and molecules still captured by Earth’s gravity can extend higher, though.
Valid demarcation.
Stop confusing yourselves!
This is how to calculate the average near-surface atmospheric temperatures of planetary bodies very easily, using only 3 gas parameters;
1000Frolly
Good to see you getting around Frolly 🙂
No “Greenhouse Effect”!
Frolly,
Your equations are perfectly correct.
As to why they are correct please see here:
http://www.newclimatemodel.com/the-gas-constant-as-the-global-thermostat/
Stephen you have an excellent website there. I think what you are saying is basically what I am saying and so we are in agreement. What this does is reduce the climate sensitivity virtually to zero, eliminating any measurable warming effect in the troposphere from extra CO2.
I knew my formula was perfectly correct when It gave me the correct surface temperature for Venus, Earth and Titan by using just three gas parameters; density, pressure and molar mass. Nothing else is needed!
Thanks frolly.
Maybe you could also link to my site when you push forward with your video?
There are a number of persons who have long agreed with us and many others who are starting to ‘get it’ but I think my work is the only source of a conceptual narrative that explains why the equation works.
Yes, will put a link to your site on my video description.
I’m sure most of my 16 thousand subscribers are sure we are on the right track here!
Frolly,
The equation you have presented is so important that I believe it requires further clarification.
Because, as you say, it is based on the works of Loschmidt in the 1860s and his use of a derivation of Maxwell’s Ideal gas law, you will forgive me if I use the variation of your analysis presented elsewhere by AfroPhysics. T = PW/GD
Where
T is the temperature in kelvin
P is Pressure (Force per unit Area) in kPascals
W is the Avg. Molar Weight of Air in g/mol
G is the Universal Gas Constant in Joules per mol per kelvin
D is the Air Density (Mass per unit Volume) in kg per cubic metre
What is so important about this is that we can now see that the whole farrago of the greenhouse gas in the troposphere – providing the fictitious concept of back radiation from low temperature air aloft that heats the ground below in an aphysical process that traps heat –itself an impossibility because heat is not temperature, heat is difference in temperature, for what it truly is – Junk science.
We have two layers in our atmosphere the troposphere (the planetary weather layer) where pressures are greater than 10 kPa and temperature is governed by an equation of state derived from Maxwell’s ideal gas law. Above that we have a second layer, the stratosphere (the planetary climate layer) where pressures are less than 10 kPa and temperature is governed by an equation of state, the Stefan–Boltzmann law that describes the power radiated from a black body in terms of its temperature.
To misapply the Stefan–Boltzmann equation from the stratosphere to the troposphere in a curve fitting exercise of schoolchild extrapolation and then find that the -33C error this junk analysis creates can be explained by the magic of back radiation is truly terrible.
Philip good post; I think you have clarified it very well. Many folk do not get how important this is immediately.
About the rearrangement by AfroPhysics; there is nothing wrong with a rearrangement as such, but the correct, commonly-used gas law terms and engineering terms should still be used; i.e.;
T = Pn/Rρ
T = average near-surface atmospheric temperature in Kelvin
P = average near-surface atmospheric pressure in kPa
R = gas constant for; m³, kelvin⁻¹, moles⁻¹, kilograms = 8.314
ρ = average near-surface atmospheric density in kg/m³
n = average near-surface atmospheric mean molecular weight
Thanks Frolly
Frolly, Stephen, thank you for the data.
Looks good, Andy.
Kudos to Connolly and Connolly!
I thought NOAA has conducted some gas chemistry sweeps in the atmosphere as part of their ozone hole belief.
Perhaps whether NOAA captured some oxygen multimers might show up in their lists. Not that NOAA is particularly trustworthy.
How do the GCM’s handle the lapse rates by latitude? Are the internal GCM lapse rates at initialization even close to observation?
We are not sure, but I think I read that at least some of them start with the standard atmosphere profile.
Although ozone is the primary determinant of tropopause height for the earth it is not essential for the creation of a tropopause. Any radiatively active material in an atmosphere will create a tropopause which is why all planets with atmospheres have one.
The reason is that the tropopause marks the height at which radiative energy transmission takes over from energy transmission by conduction and convection.
Since the two processes work at different rates they produce different lapse rate slopes and the tropopause represents that disjunction.
It has been noted that the tropopause occurs at much the same pressure whatever the composition of an atmosphere.
The reason is a result of the ability of matter to interfere with transmission of infra red wavelengths. At a specific molar density of gases that interference becomes sufficient to allow conduction and convection to take over as the dominant method of energy transmission.
Once that density is achieved the resulting lapse rate through all lower levels is what determines any surface temperature enhancement and NOT radiation from radiatively active material within those lower levels.
Note that one must consider averages in connection with lapse rate slopes because in realty the internal motion of atmospheres causes a high degree of variability of the actual lapse rate slope from place to place in three dimensions and over time.
Any imbalances caused by radiative materials are corrected for by convective adjustments so as to maintain hydrostatic equilibrium for the atmosphere as a whole and that involves maintenance of surface temperature at the level set by insolation, atmospheric mass and the strength of the gravitational field and nothing else.
wildeco2014, This is the standard tropopause story. But, it is an oversimplification. The processes you describe may very well be part of the process, personally I think convection affects the height of the tropopause between 40S and 40N to some extent. But the convective/radiative story can’t be all of it. The average pressure at the tropopause from 45S to 45N is about 0.13 Bar, close to the claimed 0.1 Bar in Robinson, T. D., and Catling, D. C., Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency, Nature Geoscience 7, 12-15. But, at the poles the pressure is much higher, 0.25 to 0.33 Bar. Why? Also, review the critique of the Chapman mechanism in the Connolly’s paper 2, it has holes as well.
Pressure is higher at the poles because the air over the poles is colder and denser. That is why you usually see large anticyclonic cells containing descending air at the poles.
The truth really is as simple as I have described it.
I an unclear why R = 8.31 here, as in PV = nRT, R = 0.0821 atmL/molK. The units of R – 8.31 includes joules which makes no sense in PV = nRT.
Ref https://en.wikipedia.org/wiki/Gas_constant
You can use the unit of energy, J, which may represent the work needed to pressurize some gas. Or you can use standard Pa, N, m³, K etc. Or you can use convenient units where P = 1 in 1 atm. Whatever, as long as you know your units. All physics books don’t teach all unit systems, but there are plenty of even when skipping acrefathoms as a unit of volume and F as a unit of temperature.
I just read about energy and saw kilowatt, kW, KW, KiloWatt, MW, megawatt, Mega Watt, mega Watt Hour, MWh, MWH, and a god amount of mistaken use of power instead of energy and the other way around. It is too much to require greenies to get the units right when engineers may fail there as well. But anyway, any freaking idiot PV user who tells he got 50 mega Watts/year hasn’t passed the basic test and should not take part in discussing the viability of PV.
Once again Dimensional Analysis is your friend.
The Ideal Gas Law PV = nRT relates the product of Pressure (force per unit area M.L^-1.T^-2) with Volume (cubic metres L^3) to give us on the left of the equation the dimensions of energy M.L^2.T^-2
On the right of the equation we have the product nRT. That is the number of moles n (to be strictly clear moles n are a unit of mass measured in atomic mass units, but it helps to see the logic when we keep n as just mol and not show it in its correct form of M); combined with the universal gas constant R which is 8.31441 Joules per kelvin per mole [or to be absolutely clear Joules per (kelvin dot mole)] and has the dimensions:-
M.L^2.T^-2.K^-1.mol^-1
So for my Dimensional Analysis of the product nRT (now here T is temperature in kelvins so I will use K to distinguish it from T for Time) we have mol.M.L^2.T^-2.K^-1.mol^-1.K
We can now cancel the molar (mass) and kelvin (temperature) terms which gives us:-
M.L^2.T^-2 which is the quantity of Joules that equates with the product of Pressure with Volume as the Ideal Gas Law states.
Dimensional analysis is a way to prove your unit works. But then, PV = nRT is elementary. If you need to prove it, your audience has not (or should not) been admitted to the university.
Having said that, that’s what we do here.
It is generally recognised that the absorption of long wave radiation from the Earth itself by Water Vapour, Carbon Dioxide and Ozone results in atmospheric heating. There is very little water vapour in the stratosphere.
Ozone is present throughout the atmosphere, including the troposphere. But, unlike carbon dioxide it is not well mixed. It’s prime source is via the photolysis of oxygen that occurs in the upper stratosphere and mesosphere. It mixes downward into the lower stratosphere where it escapes photolysis at low sun angles, where day length is short, when there is no sun at all as in high latitudes of the winter hemisphere (where ozone partial pressure peaks) and in general due to the relative scarcity of photolyzing radiation in the lower stratosphere/upper troposphere.
There seems to be widespread ignorance of the energy gathered by ozone and transmitted to the atmosphere that is due to ozone intercepting radiation from the surface of the Earth. There is as much heating from this source in the troposphere as in the stratosphere. Heating rates depend upon atmospheric density. So, with little ozone in the lower troposphere heating rates are still large due to the density factor.
The available energy from this surface radiative source peaks in the band where ozone intercepts at around 9.6um. Very large molecules like ozone absorb at relatively long wave lengths. This source of energy is available day and night.
I go into this in the post called “Why is the stratosphere warm”. This article was picked up here: https://notalotofpeopleknowthat.wordpress.com/2016/03/31/why-is-the-stratosphere-warm/
Heating of the atmosphere by radiation from the Earth and its atmosphere occurs wherever there is ozone….out into the mesosphere. This is due to the relative scarcity of the ozone molecule and the superabundance of radiation at 9.6um.
By comparison, the energy absorbed by the atmosphere from very short wave energy in the short wave (light) spectrum is tiny. That source of energy is virtually all used up above the stratosphere.
Others who are aware of the heating power of ozone include:
http://onlinelibrary.wiley.com/doi/10.1029/89JD01635/full
http://onlinelibrary.wiley.com/doi/10.1002/qj.49711046312/full
http://onlinelibrary.wiley.com/doi/10.1029/92GL01937/full
But, it’s fair to say that very few are informed.
There is another fundamental feature of the atmosphere that indicates the heating power of ozone. Low atmospheric pressure always occurs when there is more ozone in the atmospheric profile. In fact, it’s possible to infer atmospheric pressure from measurements of total column ozone. This, and the relatively lower tropopause (commonly 8km rather than 10km) where Total Column Ozone is elevated was noticed a century ago. The lower density (and smaller number of molecules in the atmospheric column) is due to the heating response.
Are the authors aware of these fundamentals? Apparently not.
But this is not unusual. Nobody is aware of the role of ozone in generating polar cyclones at 50-70° of latitude that have much enhanced power and geographical influence in the winter hemisphere. And that’s pretty basic stuff. It’s no wonder that pressure groups forcing the AGW argument have been so successful.
Erl,
Those links refer to ozone warmth above the tropopause.
Below the tropopause virtually all atmospheric heating is via conduction from the surface which,being uneven,results in convective overturning.
Water vapour,being lighter than air, facilitates such overturning.
I’ve never heard (apart from you) of ozone energy absorption below the tropopause being a significant contributor to convective overturning.
The response to ozone absorption of infrared in the troposphere is evident in radiosonde data. Ozone is entrained in down-draughts.
Erl, those links refer to ozone heating in the stratosphere. As far as I know you are the only person who thinks ozone heating is significant in the troposphere. The usual proposition is that convective overturning is caused by conduction from the unevenly heated surface and is enhanced by the fact that water vapour is lighter than air.
Well, O3 is ozone wherever it occurs and it has the same properties throughout. The limitation on its heating power is atmospheric density, determining the rate of conduction from one molecule to another. So, the smaller quantity of ozone in the troposphere (90% of the atmosphere) accomplishes as much heating as in the stratosphere where the molecules are further apart (stratosphere comprising only 10% of the atmosphere).
On Mars ozone peaks at the surface. On Earth oxides of nitrogen produced in soil (a product of life and decay) chemically destroy ozone. The Northern hemisphere has more soil than the southern and in consequence, a higher tropopause.
I think the problem is that you are ignoring the vast amount pf energy passing from the solar heated surface to the bulk atmosphere via conduction at ground level which is then passed upward in convection and converted to potential energy which does not register as heat. Since that process involves the entire mass of the largely non radiative atmospheric gases I must conclude that any heating effect in the troposphere from ozone’s absorption capability is insignificant.
At the tropopause convection ceases so the effect of that conduction at the surface drops to zero which leaves ozone above the tropopause as the primary source of heat and thus the cause of the reversal of the lapse rate slope.
Stephen, I agree with you that the sources of heating in the troposphere include many factors more influential than ozone. But, that doesn’t change my statement that there is as much heating due to ozone in the troposphere as there is in the stratosphere……..and its due to infrared rather than short wave absorption.
Erl,
There may well be more ozone heating in the troposphere than in the stratosphere but it is insignificant compared to conductive heating from the surface and convective uplift converts the whole lot to potential energy at the tropopause so that the ‘heat’ (whatever the source) is no longer present.
That leaves the much lower level of heat from ozone reactions in the stratosphere in complete control from the tropopause upwards.
Interesting. The ancient Earth’s atmospheric physics must have been very alien before the great oxygenation pulses a few billion years ago.
Yup, before it was the way it is now, it was different.
Common 0.1 bar Tropopause in Thick Atmospheres Set by Pressure-Dependent Infrared Transparency
Tyler D. Robinson, David C. Catling
(Submitted on 24 Dec 2013)
A minimum atmospheric temperature, or tropopause, occurs at a pressure of around 0.1 bar in the atmospheres of Earth, Titan, Jupiter, Saturn, Uranus and Neptune, despite great differences in atmospheric composition, gravity, internal heat and sunlight. In all these bodies, the tropopause separates a stratosphere with a temperature profile that is controlled by the absorption of shortwave solar radiation, from a region below characterised by convection, weather, and clouds.
https://arxiv.org/abs/1312.6859
In winter stratospheric polar vortex enters the troposphere over the polar circle.
http://ds.data.jma.go.jp/tcc/tcc/products/clisys/STRAT/gif/zu_nh.gif
What is importantly missing here is an unambiguous definition, and clear description, of “molar density”. Talking about using it, its results, it changes, etc. etc. to get a picture of something without making clear what is being discussed is quite unhelpful if bringing enlightenment on the article’s topic is the goal. The readership is quite varied, there is zero reason to believe that all or even a majority will know the term well enough to follow the discussion.
Certainly, one can search, and if one is willing to read enough search results and puzzle out the different usages, one might come up with a useful idea of what the heck is being discussed here, but the article would be much better without imagination and/or hard outside work being required before some comprehension can be obtained.
Always worth doing Dimensional Analysis to check the equation relationships:-
Now Equation 1:- D=n/V
Symbol Property Dimensions Metric
D Molar Density M.L^-3 moles/cubic metre
n moles M atomic mass units
V Volume L^-3 cubic metres
And Equation 2:- D=P/RT
Symbol Property Dimensions Metric
P Pressure M.L^-1.T^-2 Pascals
R Gas Constant M.L^2.T^-2.K^-1mol^-1 Joules/ (Kelvin mole)
(If you prefer you can read the Universal Gas Constant R as Joules per kelvin, per mole, but do watch out for what you mean with the placement of those tricky pers).
T Temperature K Kelvin
For the equation D=P/RT
For the part above the line we have pressure (force per unit area) with the dimensions:- M.L^-1.T^-2
For the part below the line we have RT with the dimensions:-
M.L^2.T^-2.K^-1.M^-1.K
For this line we can cancel out the M and the K terms giving us:-
L^2.T^-2
We can now cancel T above and below the line in equation 2 giving us in dimensional form:-
Molar Density (D) = M.L^-1/L^2
And so we have as expected Molar Density = M.L^-3
Correction:- V Volume L^3 cubic metres
If I spot anything else I’ll let you know 😉
AndyHce
I was rushing a bit back there, but please note that Andy clearly states in his text:-
The equation relationship used by Connolly & Connolly for this analysis clearly sound.
All you have to do is type into any search engine “molar volume definition”.
Right at the top it will give you the precise definition of the term, if you use Bing or Google.
If you want the definition of a word or phrase, just type that word or phrase along with “definition”.
Every word or phrase cannot be defined in every article.
That is what dictionaries and encyclopedias are for.
You have the right idea…anytime you are reading anything and you come across a word or phrase you are unfamiliar with, stop right there and get the definition before proceeding, or else everything you read after that point is a waste of your time…you have just lost the train of thought, the thread.
I think this is a main reason why people do not understand or have any particular knowledge of many things…they never bother to look stuff up, and so before long, nothing they are reading is able to be digested and integrated into their base of knowledge.
Arcane terminology is also a way that some knowledge is purposefully kept within a select group.
You will never understand what your auto mechanic, or plumber, or electrician, or anyone is talking about, if you do not know the meaning of the words they use. Then every little thing they do sounds complicated, and people pay for others to do stuff they could easily do themselves, and do in less time than it takes to call someone or take something to a shop for repair.
You gotta know the werdz.
I see my error but I don’t comprehend the reason. My first effort was to search for molar density in the article, looking for a definition. That search did not find the defining sentence nor find anywhere near as many references to the term as I get today but it did find molar density in a number of places from page top to bottom, then started over again at the top. Is that a common occurrence with Firefox in anyone else’s experience?
Including “definition” as part of the search string, as suggested by menicholas, does produce a different list than just putting in the term itself and the search results do depend on the search engine used. In my case a clear definition now emerges about 3/4 of the way down the search page (but is not what is defined near the top). It is my problem that I used a search engine not optimal to my needs of the moment but I still see a more reasonable composition as providing basic insight about the central concepts up front, in the opening statements.
I am not saying this is a poorly written article or that it has an uncommon fault. Many articles make no concessions at all to outsiders. Obviously, when my background covers enough of the topic for me to feel comfortable about what I’m reading I am unlikely to notice any lack but on this occasion I just felt frustrated enough to complain. I wanted to understand but kept seeing big blank spaces. We have perhaps all read comments from people who clearly have an idea about what is being discussed that differs so much from what the author intended that they are totally lost to the points at hand. Sometimes that is the author’s fault.
AndyHce, Here is a good explanation of molar mass and molar density. https://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/section/14.9/
It is also described fairly well in the Connolly’s paper 1.
Now people are beginning to accept that the lapse rate variable beast, I wonder what value climateers program there models with?
How do they account for the unknown lapse rate in 1 square km in the central atlantic or the swiss alps or the mid west plains, varying as it does from season to season etc etc.
I am sure they have programmed in a suitable value that is guaranteed to work /s
https://goldbook.iupac.org/html/M/M03971.html
“molar
Adjective before the name of an extensive quantity generally meaning division by amount of substance to make it intensive. Molar volume is volume divided by amount of substance. In a few special cases molar is used to denote division by amount concentration (e.g. molar absorption coefficient).”
A pet hate. Even wikipedia has an entry for molar concentration instead of amount concentration (amount = extensive property measured in moles) along with specific gravity
“specific
Attribute to a physical quantity obtained by division by mass. Specific volume is the volume of a sample divided by its mass.”
An elegant dissertation in climate science that, had it not appeared on WUWT, many of us would have been unlikely to have come across it. I say elegant because it uses classical science to explore a phenomenon and, in the process, makes a scientific discovery, pointing to cause for the shape of the lapse rate changes. PV=nRT, indeed! NOAA and WMO use a blindman’s understanding of what an elephant is by feel. https://wildequus.org/2014/05/07/sufi-story-blind-men-elephant/
(“It’s physics” indeed) and the boffins at NOAA and WMO don’t go to a physics equation that cries out to be employed in a tailor-made situation and miss out on cause, the Holy Grail of scientific inquiry. It is a change in the equation of state – whether it is oxygen monomerization, polymerisation or some other combination of the molecules of the atmosphere that reduces “n”. No wonder their models are patchwork quilts of guesses of blindmen experiencing the physical world.
Out of spite, the Philistines won’t change their definition in a hurry.
Gary, you incorrectly labeled this as a “dissertation.” It is not.
I think that the word, “dissertation”, has a general sense, as well as a more specific sense. Obviously, the article is not a PhD thesis, but I don’t think that this was the intended use of the word, “dissertation”, here.
This may seem like a dumb question, but if the place we call the tropopause is so indistinct and variable, what exactly is it’s significance?
Why do we need to know exactly where it is, or have a precise definition of it?
Is it an imaginary place, one that we are imposing but has no actual physical meaning?
I may be sticking my nose in where it doesn’t belong but …
The altitude and clear differentiation of the tropopause may differ from place to place but it seems there are significant differences between the troposphere and the stratosphere (such as the importance of water vapor and the fact that the temperature decreases with altitude in one yet increases with altitude in the other). At least in most parts of the world there seems to be some definite area that differs somewhat from both troposphere and stratosphere. The universe doesn’t always cooperate with our abstractions but having a term for that area, even if great precision is not possible, makes it easier to think about and easier to discuss.
The question we are addressing is that there is some sort of significant change taking place at the tropopause. We do not know what that change is, but it should be investigated.
Andy,
I don’t see your problem.
At the tropopause the lapse rate gradient changes.
The reason is that conduction driven convection comes up against warming from heat generated by radiative material directly intercepting incoming solar radiation.
Convection cannot go any higher because of the warming that takes place above the tropopause. Convection depends upon a falling density/temperature gradient with height and cannot overcome a reversal of the gradient. The tropopause is simply an inversion layer at great height.
Two separate means of energy transmission produce different lapse rate slopes and the tropopause is the point of contact.
The height of the tropopause is highly variable because of the cell based structure of convective overturning. Low pressure cells (low at the surface) push it up and high pressure cells (high at the surface) pull it down.
What are you seeing that makes that explanation hard to accept?
There are several valuable insights in this material. Although I am qualified in some aspects of the topic, that means not all aspects can escape my further study to do it justice to me. To help that further study and to expose my ignorance, I have a couple of questions.
First, I note the dilution of CO2 with altitude, such as in moles per cubic metre, plus repeated assurances from elsewhere that much radiation to space happens near the tropopause. This seems to impose additional energetic load on CO2. Such sparse disposition, plus the whole of that global warming to send to space. Is there no barrier to the energy that the molecule can hold before disposal by emission of energy? I have to study this because quantum state understanding I was taught in the 1960 era did suggest upper limits. To be fair, most spectroscopy theory then was in terms of atomic rather than molecular examples. But we are led to the old question of whether 400 ppm by volume at the surface is adequate to handle the energy, when one thinks instead of moles per cubic metre. Intuitively, a handful of molecules could not serve the load of the whole earth, so how many molecules per cubic metre do there have to be present before there is a measurable effect? From theory, not from inference, preferably.
The second question is whether the species above and below the tropopause, now defined by the break in slope, have equivalent emission properties. I am ignorant of oxygen multimers and their spectroscopy. Is there adequate literature about them? Have spectra been taken at places like the poles when seasons bring the tropopause to the surface?
Thanks. Geoff.
Geoff, very good questions. A lot happens at or near the tropopause that is not well understood, including a change in state. There is a lot of discussion of the changes here: http://oprj.net/articles/atmospheric-science/19. Spencer and Christy have noted that unusual microwave emissions come from the tropopause. While CO2 is a significant emitter to space from the tropopause, it is not the only emitter.
Thank you, Andy. Geoff.
I should deal here with a point made by Willis Eschenbach in the past.
His contention was that convective overturning is a zero sum process and so could not affect planetary surface temperatures. He proposed a cooling of the surface beneath rising columns of air as being exactly offset by a warming beneath descending columns.
His error arises from the fact that the S-B equation represents a MINIMUM surface temperature at a given level of insolation. In that situation energy can radiate out at the speed of light with no obstruction from an atmosphere.
To reduce the surface temperature below S-B at any given level of insolation, conduction and convection would have to operate FASTER than radiation in shedding energy to space but obviously it does not.
The truth is that the surface stays at the full S-B temperature throughout the first convective overturning cycle.
The descending columns then add the removed surface kinetic energy back to the surface in addition to continuing insolation so that it is not a zero sum process and the surface temperature must rise.
I urge folks to read this carefully:
https://tallbloke.wordpress.com/2017/06/15/stephen-wilde-how-conduction-and-convection-cause-a-greenhouse-effect-arising-from-atmospheric-mass/
As frolly’s video clearly points out, the radiative greenhouse theory is dead as the dodo in light of the gas laws.
Andy,
Your link says this:
“The air above the tropopause (i.e., in the tropopause/stratosphere) adopted a “heavy phase”, distinct from the conventional “light phase” found in the troposphere. This heavy phase was also found in the lower troposphere for cold, Arctic winter radiosondes.”
What do they mean by ‘heavy’ and ‘light’ in that context?
I suspect that they just mean that the tropopause is generally unstable and warmer parcels tend to rise whereas the stratosphere is generally stable and parcels of air resist upward movement.
There is nothing puzzling in that given the density differentials introduced by the temperature change when the lapse rate slope reverses.
I think they are getting terribly confused over simple non radiative fluid mechanics.
I have posed a second video relating to this subject that you guys may be interested in Regards 1000Frolly;
test
If the temperature of the earth is 255K + 33K, a result of solar insolation plus the gas laws (as stated earlier in the video) but on the other hand you derive surface temperature from surface pressure, surface density, and mean moles, then where does solar insolation come in? I’m confused.
frolly,
Nicely done.
Also see here:
http://www.newclimatemodel.com/greenhouse-confusion-resolved/
from 2008
http://www.newclimatemodel.com/the-ignoring-of-adiabatic-processes-big-mistake/
from 2012
http://www.newclimatemodel.com/why-the-radiative-capabilities-of-gases-do-not-contribute-to-the-greenhouse-effect/
from 2013
all of which support frolly’s proposition and other articles at my site are also of general relevance.
Don, good question.
The hypothesis I am proposing to explain Earth’s near-surface atmospheric temperature, is that solar insolation provides the first 255 Kelvin in accordance with the black body law, then the “other” 33 Kelvin comes from adiabatic auto-compression in the atmosphere, which occurs in any atmosphere which is >10kPa in pressure.
The key number to remember is 10kPa. Once the gas pressure passes this level, a temperature gradient is set up. See;
Robinson, T. D., & Catling, D. C. (2014). Common 0.1 [thinsp] bar tropopause in thick atmospheres set by pressure-dependent infrared transparency. Nature Geoscience, 7(1), 12-15.
First proposed by Loschmidt in the 1860’s by using a derivation of Maxwell’s Ideal gas law. See; Flamm, D. (1997). Four papers by Loschmidt on the state of thermal equilibrium Pioneering Ideas for the Physical and Chemical Sciences (pp. 199-202): Springer.
Now to your question; where is the input from the differing soar insolation levels on each planet?
The answer is that this is automatically “baked-in” to the gas parameter pie!
In other words, the gas pressure, density and even over time, the molar weight depend largely on the level of insolation at that planet’s location.
Note that other climate-related parameters are also automatically ‘baked in’, including albedo, cloud changes and yes, even any greenhouse gas effects.
frolly, thank you for your reply, it’s very helpful. I’ll have to think about all of that.
For those of us who weren’t science majors, can you elaborate on how solar insolation levels are baked into the gas pressure, density and molar weight of an atmosphere?
Nevermind, I’ve got it. Insolation affects pressure and density.