From the “Montreal Protocol success is not weather, unless it is” department and NASA Goddard:
Using measurements from NASA’s Aura satellite, scientists studied chlorine within the Antarctic ozone hole over the last several years, watching as the amount slowly decreased. This is the first definitive evidence of the success of the Montreal Protocol on Substances that Deplete the Ozone Layer, which was ratified in 1987.
The international treaty banned the use of chlorofluorocarbons and related compounds, which break down in the stratosphere and release chlorine molecules. This chlorine depletes the ozone layer and is responsible for creating the hole in the ozone layer over Antarctica. The ozone hole fluctuates each year, reaching an annual maximum at the end of southern winter, usually in September. The hole has been trending smaller for the last few years, but as temperature has an effect on ozone-depletion, this was not definitive evidence of the Montreal Protocol’s efficacy.
Although scientists have been measuring levels of chlorine near the ground for decades, this study is the first time anyone accurately measured chlorine levels inside the ozone hole, confirming that the Montreal Protocol is doing its job.
Here is the Press release from January 4th, 2018:
Measurements show that the decline in chlorine, resulting from an international ban on chlorine-containing manmade chemicals called chlorofluorocarbons (CFCs), has resulted in about 20 percent less ozone depletion during the Antarctic winter than there was in 2005 — the first year that measurements of chlorine and ozone during the Antarctic winter were made by NASA’s Aura satellite.
“We see very clearly that chlorine from CFCs is going down in the ozone hole, and that less ozone depletion is occurring because of it,” said lead author Susan Strahan, an atmospheric scientist from NASA’s Goddard Space Flight Center in Greenbelt, Maryland.
CFCs are long-lived chemical compounds that eventually rise into the stratosphere, where they are broken apart by the Sun’s ultraviolet radiation, releasing chlorine atoms that go on to destroy ozone molecules. Stratospheric ozone protects life on the planet by absorbing potentially harmful ultraviolet radiation that can cause skin cancer and cataracts, suppress immune systems and damage plant life.
Two years after the discovery of the Antarctic ozone hole in 1985, nations of the world signed the Montreal Protocol on Substances that Deplete the Ozone Layer, which regulated ozone-depleting compounds. Later amendments to the Montreal Protocol completely phased out production of CFCs.
Past studies have used statistical analyses of changes in the ozone hole’s size to argue that ozone depletion is decreasing. This study is the first to use measurements of the chemical composition inside the ozone hole to confirm that not only is ozone depletion decreasing, but that the decrease is caused by the decline in CFCs.
The study was published Jan. 4 in the journal Geophysical Research Letters.
The Antarctic ozone hole forms during September in the Southern Hemisphere’s winter as the returning sun’s rays catalyze ozone destruction cycles involving chlorine and bromine that come primarily from CFCs. To determine how ozone and other chemicals have changed year to year, scientists used data from the Microwave Limb Sounder (MLS) aboard the Aura satellite, which has been making measurements continuously around the globe since mid-2004. While many satellite instruments require sunlight to measure atmospheric trace gases, MLS measures microwave emissions and, as a result, can measure trace gases over Antarctica during the key time of year: the dark southern winter, when the stratospheric weather is quiet and temperatures are low and stable.
The change in ozone levels above Antarctica from the beginning to the end of southern winter — early July to mid-September — was computed daily from MLS measurements every year from 2005 to 2016. “During this period, Antarctic temperatures are always very low, so the rate of ozone destruction depends mostly on how much chlorine there is,” Strahan said. “This is when we want to measure ozone loss.”
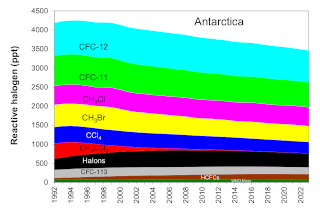
They found that ozone loss is decreasing, but they needed to know whether a decrease in CFCs was responsible. When ozone destruction is ongoing, chlorine is found in many molecular forms, most of which are not measured. But after chlorine has destroyed nearly all the available ozone, it reacts instead with methane to form hydrochloric acid, a gas measured by MLS. “By around mid-October, all the chlorine compounds are conveniently converted into one gas, so by measuring hydrochloric acid we have a good measurement of the total chlorine,” Strahan said.
Nitrous oxide is a long-lived gas that behaves just like CFCs in much of the stratosphere. The CFCs are declining at the surface but nitrous oxide is not. If CFCs in the stratosphere are decreasing, then over time, less chlorine should be measured for a given value of nitrous oxide. By comparing MLS measurements of hydrochloric acid and nitrous oxide each year, they determined that the total chlorine levels were declining on average by about 0.8 percent annually.
The 20 percent decrease in ozone depletion during the winter months from 2005 to 2016 as determined from MLS ozone measurements was expected. “This is very close to what our model predicts we should see for this amount of chlorine decline,” Strahan said. “This gives us confidence that the decrease in ozone depletion through mid-September shown by MLS data is due to declining levels of chlorine coming from CFCs. But we’re not yet seeing a clear decrease in the size of the ozone hole because that’s controlled mainly by temperature after mid-September, which varies a lot from year to year.”
Looking forward, the Antarctic ozone hole should continue to recover gradually as CFCs leave the atmosphere, but complete recovery will take decades. “CFCs have lifetimes from 50 to 100 years, so they linger in the atmosphere for a very long time,” said Anne Douglass, a fellow atmospheric scientist at Goddard and the study’s co-author. “As far as the ozone hole being gone, we’re looking at 2060 or 2080. And even then there might still be a small hole.”
To read the study, visit: http://onlinelibrary.wiley.com/doi/10.1002/2017GL074830/abstract
However, just a couple of months ago, NASA claimed the most recent shrinkage of the Antarctic Ozone Hole was due to a warm winter, not the Montreal protocol. Next year, I wonder what they’ll say if the hole is bigger than 2017. They wrote then:
NASA and NOAA scientists work together to study the ozone layer, monitoring the hole over Antarctica as it fluctuates with the seasons.
This year, the ozone hole’s annual maximum set a record — the smallest it’s been since 1988.
The hole in the ozone layer is caused each year as ozone molecules react with chlorofluorocarbons (CFCs) in the atmosphere. The reactions occur at cold temperatures, so the hole reaches a maximum size each year at the end of southern winter, and then heals during the warmer summer months.
Although CFCs have been banned since 1987 under the Montreal Protocol on Substances that Deplete the Ozone Layer, the compounds decay very slowly, and still remain in the atmosphere. This year, the small ozone hole was mostly caused by warmer temperatures, which slowed down the reactions between ozone and CFCs.
NASA also claims the ozone hole will be gone by 2040, we’ll see then if this was just a natural feature all along, or if CFC reduction really did have an impact:
Big Ozone Holes Headed For Extinction By 2040
The next three decades will see an end of the era of big ozone holes. In a new study, scientists from NASA Goddard Space Flight Center say that the ozone hole will be consistently smaller than 8 million square miles by the year 2040.
Ozone-depleting chemicals in the atmosphere cause an ozone hole to form over Antarctica during the winter months in the Southern Hemisphere. Since the Montreal Protocol agreement in 1987, emissions have been regulated and chemical levels have been declining. However, the ozone hole has still remained bigger than 8 million square miles since the early 1990s, with exact sizes varying from year to year.
The size of the ozone hole varies due to both temperature and levels of ozone-depleting chemicals in the atmosphere. In order to get a more accurate picture of the future size of the ozone hole, scientists used NASA’s AURA satellite to determine how much the levels of these chemicals in the atmosphere varied each year. With this new knowledge, scientists can confidently say that the ozone hole will be consistently smaller than 8 million square miles by the year 2040. Scientists will continue to use satellites to monitor the recovery of the ozone hole and they hope to see its full recovery before the end of the century.
Research: Inorganic chlorine variability in the Antarctic vortex and implications for ozone recovery.
Journal: Geophysical Research: Atmospheres, December 18, 2014.
Link to paper: http://onlinelibrary.wiley.com/doi/10.1002/2014JD022295/abstract.
I keep harping on about it, but if most CFCs are produced in the Northern hemisphere, why isn’t there a bigger hole in the north pole? And don’t harp on about some special cloud that only appears in the south pole.
Dsystem — try this for answers to “Why has an “ozone hole” appeared over Antarctica when ozonedepleting substances are present throughout the stratosphere?”
https://www.esrl.noaa.gov/csd/assessments/ozone/2010/twentyquestions/Q10.pdf
this answers your question
http://www.abc.net.au/science/articles/2009/06/03/2588286.htm
Thanks Griff, that link had a nice explanation that is worth repeating:
So basically, the amount of CFCs is irrelevant, it is all about temperature.
I thought that it was heat, not cold, that increased chemical reactions.
Dsystem- Usually the Arctic isn’t as cold as the
Antarctic, and thus, doesn’t get an ozone “hole” (it’s
not really a hole but just a thinning. Hole is used
because it sounds a lot scarier- we’re all gonna die!)
IIRC, when the temperature gets down to -105°F in late
winter, the returning sun’s rays catalyze ozone
destruction using chlorine and bromine.
There was an ozone “hole” over the Arctic in 2012 that
peaked in March, six months from when the Antarctic
ozone “hole” peaks. That winter, the Arctic was greedy
& didn’t share it’s cold air with the rest of the NH
via the “dreaded” vortex & it stayed very cold there. We
had a very warm winter here in the States.
The Arctic’s First Ozone Hole | NOAA Climate.gov
https://www.climate.gov/news-features/understanding-climate/arctic%E2%80%99s-first-ozone-
hole
People have also looked for an ozone “hole” over the
Alps, but again, it isn’t cold enough there.
OOPS- The year the Arctic had an ozone “hole” was
2011, not 2012. We also had a warm winter here in
the States that year.
If you google “arctic ozone hole 2011”, there is a lot
of information on the 2011 Arctic ozone “hole”.
OOPS again- The actual temperature below which
the sun’s rays begin to catalyze ozone destruction
using chlorine and bromine.is -109°F (-78°C)-
http://www.theozonehole.com/ozonedestruction.htm
Urederra (9:19am)- as per
http://www.theozonehole.com/ozonedestruction.htm
it’s the polar stratospheric clouds (PSCs) that are
formed when winter minimum temperatures fall below
-109°F. Reactions on PSCs cause the highly reactive
chlorine gas ClO to be formed, which increases the
destruction of ozone. By mid-December, the
temperature’s warmed up (see graph in article).
Exactly. This is prima facie indication we are talking a natural phenomenon: the cold air having a more important contribution in destroying ozone, it being continuously produced through UV radiation and having a lifetime of weeks (?)
Even in the ’50s several measurements did found signs of an ‘ozone hole’ in Antarctica.
” By comparing MLS measurements of hydrochloric acid and nitrous oxide each year, they determined that the total chlorine levels were declining on average by about 0.8 percent annually.”
May be well within natural variations very much depending on the time frame. Unfortunately as a cynic I would think they drew conclusions from a too short time frame….
Btw, I wonder how much does the ozone production through UV vary over the years?
“Next year, I wonder what they’ll say if the hole is bigger than 2017”
Hey Dsystem, do you like irony? Here in Argentina freon is sold openly in all air conditioner shops, whether for home or auto. Too bad all of you northern hemisphere users have to settle for second best in refrigerants, but the rest of the world, even here in Argentina where crocodile tears were shed at the mention of freon up north, they use freon. Give me a break.
I thought there was a worldwide ban on CFCs.
Sheri, you must be thinking of the world-wide ban on robbing banks. That’s why we don’t have any more bank robberies.
I wonder how many other countries openly sell freon. I am becoming more and more convinced about the ozone scam so I shouldnt really care whether other countries defy the ban by not enforcing it. BUT the problem is that our consumer costs have gone up by having to use other chemicals instead. In the whole history of the UN its enforcement of any world wide ban has been a joke and will continue to be a joke.. What makes the CO2 ban even more laughable was there was no agreement on enforcement just vague promises to reduce as Chine merrily proceeds to build coal plants not only in China but in many countries in the rest of the world.
I see that there is no mention of the fact that the “scientist”, working for Dupont Chemical, who defined the CFC-ozone connection admitted 20 years later that he made up the data and the chemical reactions that our atmospheric scientists think they are studying. We know now that it is nitrogen gas and solar UV that breaks down ozone. It was the high UV activity of the Sun in the 80s and 90s that caused the ozone hole.
CFCs have nothing to do with ozone hole and have been demonized to force the adoption of more expensive, patented refrigerants. That was Dupont’s original reason for funding the fraud scientis,t lobbying Washington, and propagandizing the public about the Ozone Scare.
The fraud scientist waited 20 years as not the replacement refrigerant is out of patent as well. They are now trying to demonize HFCs, claiming they are also threatening the ozone layer; more junk science. I can be confident that Dupont has some new, more expensive refrigerant under patent to suggest.
Also notice the complete lack of mention regarding fluorine molecules.
It’s pure hand waving when they point to chlorine, yet ignore fluorine.
If the CDCs were actually involved, there would be fluorine molecules.
Chlorine and bromine sources are from the ocean. No CFCs needed, at all.
EXACTLY – In over 10 years of NASA Studies, Balloon Experiments, Radioactive CFC Releases, and later Satellite Studies, NOBODY ever found any traces of CFCs or CFC Byproducts reaching above the lowest layers of the atmosphere! Additionally, NOBODY ever proposed or proved a mechanism to transfer Northern Hemisphere CFCs to the Southern Hemisphere. Atmospheric Science 101 states that less than 2-3% mixing occurs at the equator between the Northern and Southern hemispheres and virtually NO mixing at high altitudes where Ozone depleting chemistry occurs. There IS, however, a direct correlation between the amount of storm produced lightning plus volcanic activity in the Southern Hemisphere and the state of the Naturally Occurring “Ozone Hole” over Antarctica! Both activities produce Free Chlorine which can assist in the destruction of Ozone at high altitudes.
HFCs were added to the Montreal Protocol about April 2017, I believe.
It’s also interesting to hear the `ozone hole is healing’ as Solar Activity slowly diminishes. NASA say it will `heal’ by 2040 … right about when the Sun is at a minimum. How interesting. How coincidental. When the sun was really cooking, so was the ozone hole…
I strongly suspect more Junk Science.
@sz939:
Just lolok here nad you see that you are spreading “alternative facts”.
http://journals.ametsoc.org/doi/pdf/10.1175/1520-0469(1977)034%3C1481%3ASMONCA%3E2.0.CO%3B2
ATheoK January 16, 2018 at 12:30 pm
Also notice the complete lack of mention regarding fluorine molecules.
It’s pure hand waving when they point to chlorine, yet ignore fluorine.
If the CDCs were actually involved, there would be fluorine molecules.
And there are fluorine containing molecules in the amounts expected as well as the expected intermediates.
Most of the fluorine ends up as HF, the total amount of HF in the stratosphere increased between 1978 and 1989 by a factor of 3 to 4. The stable intermediates COF2 and COFCl are measured in the middle stratosphere.
E.g. R. Zander, M.R. Gunson, J.C. Foster, C.P. Rinsland, and J. Namkung, “Stratospheric ClONO2, HCl, and HF concentration profiles derived from ATMOS/Spacelab 3 observations – an update”, J. Geophys. Res. 95, 20519, 1990.
There is less interest in the fluorine species because they aren’t involved in the O3 depletion, unlike Cl and Br.
Chemical reaction between O3 and Bromine and Chlorine takes place under colder temperatures. Formation of more frequently the circumpolar vortex in the south pole zone creates colder weather compared to north pole zone where the occurrence of circumpolar vortex takes place less frequent and the temperatures are not so cold and thus chemical reaction is less.
Because of the colder temperature character, the ozone layer’s thickness decreases from equator to poles and from summer to winter. These are natural conditions. General circulation patterns over different regions help mixing the CFCs with the upper air — southern and northern hemispheres.
Dr. S. Jeevananda Reddy
We know that when x reduces, causes a fall y.
Why, I just said it did. Surely i need say no more, look at my credentials.
My thoughts too. Correlation does not mean Causation.
Yes, I don’t see any claim that they have ruled out spurious correlation. Look at the anomalously high ozone concentrations in the animation, OUTSIDE the Antarctic circumpolar vortex. Those go away after the vortex breaks up in the Spring, and the polar ozone levels return to normal.
Is there an ozone hole over Syria?
More likely there are ozone alerts for Syria.
I wonder how much chlorine is liberated from the ocean into the upper atmoshere during a cyclone or a water spout?
RobK, sea weed, marine algae and soil microorganisms release very large amounts of chlorinated hydrocarbons into the atmosphere every year. Far more than the human emissions.
From J. A. Field, “Natural Production of Organohalide Compounds in the Environment” in L. Adrian and F.E. Löffler (eds.), Organohalide-Respiring Bacteria, Chapter 2: “The evidence presented in this chapter indicates an extensive scope of natural organohalogen production in the earth’s biosphere with over 5000 natural organohalogen compounds described and an estimated terrestrial storage of several million Gg of organochlorine. The vast majority of the identified organohalogen compounds were shown to be produced as natural metabolites by organisms such as fungi, algae, bacteria, and plants.”
Gg is a giga-gram = 1000 metric tons.
Examples from the Chapter. Chloromethane: “The median estimate of the global production of chloromethane and released to the atmosphere is 3000 Gg y^−1 (Keppler et al. 2005). The anthropogenic contribution is about 5 % (industrial, coal combustion, and incineration).”
Chloroform: “The global production of chloroform and released to the atmosphere is estimated at 700–820 Gg y^−1 (Laturnus et al. 2002; Gribble 2010). The known anthropogenic sources only account for 60–73 Gg y^−1, thus 90 % or more of the estimated annual chloroform production is natural. The most important natural sources are oceans, soil, termites, and microalgae.”
Field also surveys the complex chlorinated hydrocarbons produced in nature, including chlorinated phenylacetic acids and chlorinated dioxins.
So, detection of chlorine in the ozone hole region of the atmosphere is no indication of an anthropogenic source. Nature makes huge amounts of volatile chlorinated (and brominated) organics that will just as easily get lofted into the upper atmosphere.
Pat,
Many Thanks. My suspicion confirmed.
Pat Frank- 9:13 am
I found this online from the University of
Oregon (1994). It says that CFCs & not natural
chlorine & bromine were the major contributor to
ozone “hole” destruction because most of the
natural molecules don’t reach the stratosphere.
Most of the chlorine supposedly gets washed out
by water vapor in the troposphere as HCl or are
short-lived versus CFCs. How valid do you think
their arguments are?
http://zebu.uoregon.edu/text/ozone
The next article is a more current form of the
latest theory of ozone destruction & the website
was a winner of the Montreal Protocol 20th
Anniversary Public Awareness Award from the
UN in 2007. They do use the word “model” in the
article & I was wondering how valid this article
is, too.
http://www.theozonehole.com/ozonedestruction.htm
Attribution of shrinkage of the Antarctic ozone
“hole” due to the Montreal protocol versus warmer
temperatures sounds more like CYA than actual
science. NASA coming up with two totally different
“answers” in such a short period of time tells
me our understanding of the whole process isn’t
that complete yet, just like the recent cold spell
being more proof of “glo-BULL” warming versus a
disparate natural variation explanation that came
shortly thereafter!
As for me, as long as there are very cold
temperatures over either pole, there will be
ozone thinning, regardless of the sources of
chlorine & bromine because the ozone “hole” was
already discovered in 1956. Whether man’s
contribution to it was a problem that needed a
solution is another question. The Montreal
protocol was probably a result of environmentalism
already being a religion by then and common sense
was its first casualty!
Thanks.
OMW, the UO article is not right that only chloromethane (CH3Cl) is a natural source for ozone-depleting atmospheric chlorine.
A witches brew of chlorinated and brominated hydrocarbons are produced naturally, and released into the air. These include chloroform (CHCl3) and bromoform, and even iodoform.
Discussions include PD Nightingale, et al (1995) “Production of chloroform and other low-molecular-weight halocarbons by some species of macroalgae” Limnol. Oceanogr. 40, 680-689; abstrace here.
Weissflog, et al. (2005) “Sediments of salt lakes as a new source of volatile highly chlorinated C1/C2 hydrocarbons” GRL 32, L01401, abstract here report the production of methylchloroform (CH3CCl3) from archaic halobacteria in saline lakes. As there is a huge number of such lakes worldwide, global production of natural methylchloroform is unknown presently, but likely to be significant.
It appears the sources of atmospheric chloride needs up-dating.
Chlorine or Chlorid?
They are desperate to conclude that the variations in the ozone hole are anthropogenic rather than naturally induced by solar variability so I find this paper suspect.
Until they have a good understanding of ALL the multiple reactions that affect the ozone creation / destruction balance they cannot possibly justify their conclusion.
So, if they discovered it 1985, how do they know it wasnt there before and has to be man made?
Justify January 16, 2018 at 5:15 am
So, if they discovered it 1985, how do they know it wasnt there before and has to be man made?
Because they had been measuring it since 1957, the significant drop didn’t start until the late 70s.
http://www.realclimate.org/images/farman_et_al85_ozone.png
The drop was exactly at the time of the mid 70s climate shift when jet stream tracks became more zonal, warming began and global cloudiness fell.
It also coincided with the start of active solar cycle 21.
All of which suggests natural solar induced causation.
Especially since all those elements went into reverse around 2000 when weak cycle 24 came along and warming came to a virtual standstill.
The number of years of data required to establish a pattern and possible causality gets shorter and shorter. Next thing we know, two weeks of weather will prove climate warming and causality.
Phil,
And what can you say about ozone levels prior to the dozen years starting in 1957?
Seems to be at the same time as the 1970s cooling.
So, how do they know that the hole hasnt always been there?
Phil is right. But the ‘hole’ in the ozone layer discovered by Farman in the late 1970s to early 1980s was in Spring, (see the ‘October’ on Farman’s graph), not Winter. The winter anomoly was described and explained by Dobson in the early 1960s from data back to 1956. Why is this rarely mentioned? And why do scientists who should no better conflate the Winter anomoly with the spring hole?
I have not looked at the article but the video above shows data mostly before 22 September. The famous ‘smoking gun’ reverse correlation of ClH with Ozone was 16 September (1987 ?), and this new evidence, continues with data from around this time. Why?
According to the proposed mechanism, CFC action is about the amount of depletion and only in spring — because, according to chemistry proposed, that involves reactions on the PS Clouds as they break down in sunlight. Why does the video say that the CFC effect is best detected in Winter?
The correlation with temperature (colder give more depletion) is very strong (although there is small overall depletion trend in the Spring data).
The much discussed size of the hole could not be the indicator of CFC action, as that is dependent on the size of the containing vortex, which (with strength of the vortex) is related to the coldness of the winter.
In short there is so much distortion, distraction and inconsistency in the NASA public relations on this issue that at least one outside observer can’t help but worry that there is something to hide.
Ooops an error. I meant to say ClO not ClH. It should be: “The famous ‘smoking gun’ reverse correlation of ClO and Ozone concentrations was 16 September 1987.”
the ozone hole is cured thanks to Refrigerant Prohibition!
except you can’t see it…because…the hole isn’t getting smaller cuz of global warming!
you know- like sea level rise his hidden cuz of the oceans sinking.
Can someone help me out? I thought CFC’s were irreversibly dissociated in the stratosphere to chlorine atoms by short-wave UV. How can they be long-lived?
Graemethecat January 16, 2018 at 5:28 am
Can someone help me out? I thought CFC’s were irreversibly dissociated in the stratosphere to chlorine atoms by short-wave UV. How can they be long-lived?
The rate of photodissociation is very slow therefore the CFCs are long-lived, also it will only be dissociated when it is in the stratosphere and a large proportion of it is still in the troposphere.
Long-lived? Thirty years?!
OK, if so, the rate of photodissociation of the CFC’s must be very slow indeed, meaning the stationary concentration of Cl atoms must also be very low indeed. Enough to destroy ozone significantly?
If it takes 31 years before all of them have disassociated, then there aren’t enough disassociating in any one year to matter.
Graemethecat January 16, 2018 at 7:17 am
Long-lived? Thirty years?!
OK, if so, the rate of photodissociation of the CFC’s must be very slow indeed, meaning the stationary concentration of Cl atoms must also be very low indeed. Enough to destroy ozone significantly?
One Cl atom destroys over 100,000 O3 molecules, so yes even a slow dissociation rate is enough to significantly destroy ozone. The Cl atoms act as catalysts, that is they don’t get consumed by the O3 depletion reaction, eventually they get removed by other side reactions. We have about 4000ppb of reactive Cl in the stratosphere, 1% of that is 40ppb which could result in the removal of over 4,000,000 ppb, the concentration of O3 in the S Polar stratosphere is about 3000ppb at peak in the fall, in the spring that is reduced to 0ppb. So yes there is adequate Cl available to cause the hole even if only a few % of the CFCs are destroyed each year.
There is an infinite amount of Cl available at sea level. Why a tiny amount of CFC would make a difference in the upper atmosphere, especially when they are very heavy molecules, has never been adequately explained.
Why do these type articles almost always fail to mention that O3 is created by UV light. It is an unstable molecule that needs to constantly created to maintain a certain level. One would therefore expect that O3 would be significantly less during periods of winter darkness over Antarctica.
Tom in Florida January 16, 2018 at 5:30 am
Why do these type articles almost always fail to mention that O3 is created by UV light. It is an unstable molecule that needs to constantly created to maintain a certain level. One would therefore expect that O3 would be significantly less during periods of winter darkness over Antarctica.
And respondents like you always forget to mention that the destruction of O3 is caused by UV light, which is why it doesn’t significantly decrease during the winter. The decrease of O3 and creation of the ‘hole’ takes place in spring after the return of sunlight (end of August).
https://www.esrl.noaa.gov/gmd/dv/spo_oz/spototal.html
Thanks for the link but I really wasn’t getting into the entire process, just wondering why the basics of O3 formation is often ignored.
Tom in Florida January 16, 2018 at 6:21 am
Thanks for the link but I really wasn’t getting into the entire process, just wondering why the basics of O3 formation is often ignored.
It isn’t, but you went on to make the incorrect assertion that because there was no UV in the winter that the O3 concentration would drop. You ignored the other side of the equation, that destruction is also due to UV.
Therefore, it is a natural process.
If ozone destruction by CFC were significant, the hole would have formed over the equator, since it is over there where the largest CFC levels are found, and where the highest UV radiaion levels and temperatures are found.
Phil,
Most of the world’s ozone is produced in the tropics and moves pole-wards through diffusion and Brewer-Dobson Cell migration. Ozone is prevented from making its way over the South Pole while the circumpolar vortex is in place.
https://en.wikipedia.org/wiki/Brewer-Dobson_circulation
Urederra January 16, 2018 at 9:29 am
Therefore, it is a natural process.
If ozone destruction by CFC were significant, the hole would have formed over the equator, since it is over there where the largest CFC levels are found, and where the highest UV radiaion levels and temperatures are found.
But the process is favored by low temperatures, basically requires the formation of PSCs which for the most part is restricted to the wintertime over the antarctic.
How can you say ozone is created yet destroyed by UV radiation. The article that you link to says nothing in that respect. It just says that the ozone hole develops in the Spring. The UV-V band of UV radiation creates ozone. If there is no light there is no UV-V radiation hence no ozone creation. Hence ozone deficient areas at the poles, seasonally and always. The whole CFC debacle is just a prime example of crony capitalism.
Oddly…
When I look at the video, I’m struck by the fact that the ozone hole does not appear to have shrunken much at all. The first vid. Looks about the same, looks about the same purple color. Maybe these researchers are seeing blips on curves. I also see that the depth of the “ozone hole” is still far greater than before 1987 (or at least at 1987, the first satellite recording).
Maybe I’m blind. Blinkered.
GoatGuy
My impression as well.
Same here. It seems to wobble, but no shrinkin at all. WTF?
Dsystem… “I keep harpingon about it…”
I remember in Chemistry class being taught that Ozone (O3) was formed in the upper atmosphere by Cosmic Rays or Alpha Particles from the Sun, bumping into Oxygen molecules (O2) according to the equation 3O2⇋ 2O3, a reversible reaction.
Reactive and unstable, Ozone decays pretty soon, back into O2 or an oxide of Nitrogen (NOx), there being plenty of Nitrogen around up there.
So imagine my surprise when I learned that in America Ozone is believed to come from automobile exhaust pipes in places like Los Angeles.
When, in the ‘80s a sharp eyed New York Times reporter first spotted the ‘Ozone Hole’ lurking over Patagonia in late October, I was curious. When, every year thereafter, the ‘Ozone Hole’ re- appeared at the same time and place as reported in the NYT, I became suspicious.
Now it was common knowledge among my classmates that our schoolmasters were Neanderthals, nevertheless to avoid being caned we paid attention, A.D.D. having not yet been invented.
We also knew from paying attention that the Antarctic, being a continent was 30o C or more colder than the Arctic which is an ocean.
With no sunlight for six months there are no ‘Cosmic Rays’ to generate fresh ozone over Antarctica. In addition the cold dense polar air mass descends over the South Pole and heads North in every direction creating the hurricane force katabatic winds. The Earth’s rotation or Coriolis effect , take your pick, gives the Northbound wind an Easterly kick and voila! the South Polar vortex is born, giving rise to the roaring forties, or screaming fifties depending how far south you go. All of this sucks more of the remaining ozone out of the upper atmosphere.
When, in September, spring in the antipodes, the Sun pops its smiling face over the horizon to warm things up again, relatively speaking, the polar vortex weakens and the ozone depleted winter air mass spirals Northward to show up in Patagonia on cue for the annual October/November Ozone hole spotting!
To panic about the “ozone hole” in the ozone layer, our shield against cancer causing UV radiation, seems strange given that UV radiation by creating the Ozone layer is absorbed in the process.
The energy needed to create the highly reactive Ozone molecule from the standard O2 Oxygen molecule reduces the high energy UV to a lower energy state with a corresponding longer and less harmful wavelength according to the formula E= H/λ, where E is ‘Energy’, λ (lamda) is wavelength, and H is Plank’s constant.
Clearly our knowledge, or should I say access to information, has expanded exponentially since my chemistry class, alas not so our understanding which appears to be contracting… at least collectively!
Cheers
Bahamamike
that was fun.
They will have to find a way to prevent bromine from emerging from the ocean if they want to completely eliminate the ozone hole. Also, the will have to block all in-coming galactic cosmic rays.
The ozone hole causes a vast heat loss from the Earth to space. If they are successful at “closing it” the temperature of the planet will rise significantly. When that happens, it will of course be blamed on AG CO2.
Of course. The cure will kill the patient even faster.
Crispin (wherever he can be actually):
“The ozone hole causes a vast heat loss from the Earth to space.“
Sure, this is the opinion of IPCC. But the truth is that ozone depletion heavily warms climate system. For example ozone hole at Antarctic spring time warms ground about 8 W/m2, a value about 5 times higher than the alleged warming effect caused by the increased carbon dioxide level.
As a matter of fact, ozone depletion has caused global warming.
The important metric is not chlorine, it is the intensity of UVA and UVB rays, which I don’t believe has varied significantly over the last several decades.
” the intensity of UVA and UVB rays, which I don’t believe has varied significantly over the last several decades.”
Hm, if I correctly remember several years ago there was some noise in the climate community about Sun’s radiation variation which were significant exactly in the UV area
ok, found something:
http://www2.mps.mpg.de/dokumente/publikationen/solanki/j132.pdf
“Variations at UV and shorter wavelengths exceed those in the visible by orders of magnitude.”
Lars P. January 16, 2018 at 7:13 am
ok, found something:
http://www2.mps.mpg.de/dokumente/publikationen/solanki/j132.pdf
“Variations at UV and shorter wavelengths exceed those in the visible by orders of magnitude.”
Yes in the E-UV but as the paper you refer to says the variation in the near-UV, which is what counts in O3 creation/destruction, is about 0.1%.
Phil: Did you include all those feedbacks that MUST be there? It’s probably huge after that operation is performed. UV may have 10 times the effect we thought it did.
Phil,
From the article, “As a result, solar UV radiation below 300 nm accounts for up to 14% of the total variation while contributing less than 1% to the value of total irradiance (Lean et al., 1997; Solanki and Unruh,
1998) only.” The article says further, ” Most prominent is a 0.1% increase of TOTAL solar irradiance in phase with the solar magnetic activity cycle.”
The paper does NOT say, “… the variation in the near-UV, which is what counts in O3 creation/destruction, is about 0.1%.”, as you claim.
Clyde Spencer January 16, 2018 at 11:06 am
Phil,
From the article, “As a result, solar UV radiation below 300 nm accounts for up to 14% of the total variation while contributing less than 1% to the value of total irradiance (Lean et al., 1997; Solanki and Unruh,
1998) only.” The article says further, ” Most prominent is a 0.1% increase of TOTAL solar irradiance in phase with the solar magnetic activity cycle.”
The paper does NOT say, “… the variation in the near-UV, which is what counts in O3 creation/destruction, is about 0.1%.”, as you claim.
Really? It does say the following:
“Relative irradiance variations near UV, visible and infrared wavelengths are on the order of 0.1% and less.”
Phil.
January 16, 2018 at 11:16 am
…. Really? It does say the following:
“Relative irradiance variations near UV, visible and infrared wavelengths are on the order of 0.1% and less.”
That is UV, visible and infrared combined…
Here is another post (from WUWT) that hopefully clarifies:
” The finding is notable, since climate scientists dismiss the role of the Sun in climate change by only looking at the tiny 0.1% variations in total solar irradiance [TSI] over solar cycles, ignoring the large variations in solar UV of up to 100% over solar cycles”
https://wattsupwiththat.com/2014/04/22/new-paper-finds-solar-uv-b-output-is-correlated-to-global-mean-temperature/
with link to the paper
http://hockeyschtick.blogspot.co.at/2013/10/new-paper-finds-solar-uv-varies-up-to.html
Has anyone ever seen a correlation between solar UV output and Antarctic ozone concentrations? I can’t recall ever seeing anyone attempt to construct one.
Seems to me that, given the solar UV output varies much more than other areas of the spectrum, the Antarctic ozone concentrations would fall during times of low solar UV output, albeit a lagged correlation. Obviously, the converse may also be true unless other factors dominate.
You have obviously spotted the major issue. Now, why can’t “credentialed” scientists understand this? Could it be that it is difficult to make someone whose income depends on a certain narrative understand anything that opposes that narrative?
NavarreAggie January 16, 2018 at 6:01 am
Has anyone ever seen a correlation between solar UV output and Antarctic ozone concentrations? I can’t recall ever seeing anyone attempt to construct one.
Seems to me that, given the solar UV output varies much more than other areas of the spectrum, the Antarctic ozone concentrations would fall during times of low solar UV output, albeit a lagged correlation.
Not clear that low solar UV would lead to low O3 since both creation and destruction depend on UV, just as at night O3 concentration does not decrease.
Phil, what do you think would happen if there were no UV in the solar spectrum?
Phil,
All one has to do is correlate TOMS data with surface UV, then the question is answered. Unfortunately, there is no worldwide, historical database of surface UV flux.
You claimed, “… at night O3 concentration does not decrease.” Can you provide a citation to back up your claim?
You overlook the fact that in the tropics, a balance is achieved between creation and destruction, with the net result being a surplus of ozone. It is only in the extremely cold, isolated Antarctic stratosphere that the equilibrium equation goes the other way, largely because new ozone from the tropics is unable to replace the destroyed ozone.
Clyde Spencer January 16, 2018 at 11:14 am
You claimed, “… at night O3 concentration does not decrease.” Can you provide a citation to back up your claim?
Referring to Antarctic ozone concentrations, I linked to a graph at: Phil. January 16, 2018 at 5:57 am
You overlook the fact that in the tropics, a balance is achieved between creation and destruction, with the net result being a surplus of ozone. It is only in the extremely cold, isolated Antarctic stratosphere that the equilibrium equation goes the other way, largely because new ozone from the tropics is unable to replace the destroyed ozone.
I haven’t overlooked that, ozone has been depleted elsewhere just not as dramatically as over the Antarctic.
Phil,
Your linked graph at 5:57 isn’t responsive to my request. If we define “night” as being a period of no sunlight in Antarctica, your linked graph DOES show a slow decline in O3 from July through August. However, we don’t really get the whole picture because the graph only goes from July through December.
Clyde Spencer January 16, 2018 at 5:20 pm
Phil,
Your linked graph at 5:57 isn’t responsive to my request. If we define “night” as being a period of no sunlight in Antarctica, your linked graph DOES show a slow decline in O3 from July through August. However, we don’t really get the whole picture because the graph only goes from July through December.
Statistically there’s no significant decline, if you look at the daily ozonesonde data you’ll see from the end of May through 1st September you’ll get ~240 DU +/- 10, then through September it drops to ~150 and in early October into the 140s
Not sure how you extrapolate rate of change of something by using the “First Ever” measurement data? Perhaps I got bored too soon and missed something?
So, do we believe NASA that the cause is the efforts of the Montreal Protocol or do we believe NASA that the cause is warmer weather?
Or, do we believe NASA simply doesn’t know but feels compelled to say something because, you know, “climate change”?
JohnWho January 16, 2018 at 6:24 am
So, do we believe NASA that the cause is the efforts of the Montreal Protocol or do we believe NASA that the cause is warmer weather?
As NASA says the mechanism that forms the ozone hole is destruction of O3 by the degradation products of CFCs in the stratosphere. This mechanism depends on CFC concentration and the formation of PSCs (which strongly depends on temperature). The CFC concentration is slowly decreasing so we would expect the destruction of O3 to gradually decrease from year to year, however fluctuations in winter temperature will cause fluctuations in that destruction rate, over time though the ozone hole will decrease.
So it’s both? Or NASA is making up the global warming effect on it? There are confounding factors and the effect of each interacting is not well understood but we banned CFCs anyway because “why not”?
Sheri January 16, 2018 at 9:12 am
So it’s both? Or NASA is making up the global warming effect on it?
It’s the effect of CFCs which is modulated by temperature.
My problem with the Montreal Protocol was not whether CFC’s were increasing the Antarctic ozone hole, but whether or not it was a problem. As I understand it, a business as usual scenario would be equivalent to moving about 100 miles closer to the equator over a 100 year period, which is ironically the same impact of 100 years of continued CO2 emissions. In other words, not a problem.
As Phil has pointed out, the reaction depends very much on temperature, and the Antarctic is the only place where the stratosphere gets that cold. Outside of that area, ozone reduction from CFCs is relatively inconsequential.
All reactions depend on temperature. The higher the temperature, the faster the reaction. That is why we store our perishables in the frigde.
All of them? Well, apparently ozone is stable at high temperatures and unstable at low temperatures. You’ll better store your ozone in the oven.
/Sarcasm
Uredda, you did not get the fact that Temperature might change reaaction mechanism:
-50°C: gaseous reaction
-75°C Reaction of gas on particle surface of PSC.
This makes a bigger difference than the temperature dependency of the reaction constants.
Sounds complicated, but is not if you once were in a chemistry 200 or above.
Urederra,
The models I built, starting in the late ’80s, suggested that surface UV saw a slight increase over time in the Winters. However, the light level was negligible because of the long slant range and low sun altitude. By the time that the sun got high enough on the horizon in the Antarctic for there to be a potential concern, the ozone levels were back up to where they needed to be. That is, much less surface UV than organisms on Earth had evolved to cope with at lower latitudes.
Urederra,
Sorry, my remarks were intended for jclarke341.
Urederra January 16, 2018 at 9:42 am
All reactions depend on temperature. The higher the temperature, the faster the reaction. That is why we store our perishables in the frigde.
Not true, many complex reactions have regions of negative temperature rate coefficient.
All of them? Well, apparently ozone is stable at high temperatures and unstable at low temperatures. You’ll better store your ozone in the oven.
The mechanism depends on the presence of the solid phase of nitric acid which requires a temperature below -79ºC.
J Clarke, Actually the problem for the scare was that the CFC modelling from 1974 (Rowland) to 1985 (Farman, the hole discovered) predicted a general global depletion and it did not (could not) predict the Antarctic Springtime hole. During this period, the European critics of US enthusiasm (where a ban was announced in 1977) talked down the problem with statements like: even a 10% depletion would only be the equivalent of moving from North to South England, which no one would surely complain about. By 1984, with the release of the latest NAS report on the matter, the prediction was down to 3% for our grandchildren–equivalent of creeping a few miles down the south road over 3 generation. Even in the US the newspapers proclaimed that the scare looked to be over.
Ozone layer action is supposed to be the model for AGW but there is one important lesson of the ozone scare that it has been convenient to forget. For a decade the scare was about a general depletion. The US unilateral action was based on the prediction of a general depletion. This did not happen. It could not happen for the reasons stated at the time by sceptics–because of negative feedbacks and circulation. When the complex series of reactions were proposed as the cause of the Antarctica springtime hole, it was clear that these could not happen anywhere else at any other time. They could not happen in lower latitudes because they involved a heterogeneous reaction on the solid surfaces of the polar clouds. The Arctic sometimes has these clouds but not a strong vortex and so the lesser chemical effect is swamped by circulating air. After 1985 the claim of general depletion fell away, although there was this pretense that the hole might expand beyond the Antarctic vortex or form over the Arctic. This was unscientific rubbish successfully promoted by scientists such as Ralph Cicerone and Bob Watson. Watson was also involved in a major distortion of evidence and obscuring of evidence to promote a public claim of general depletion (in the exec summary of a report released 3 years before the full report was quietly released).
Sceptics of the scare over general depletion (Lovelock, Scorer, Singer) never argued against CFC regulation. Instead, they focused their concern on the corrupting effect on science in the rush to produce science to support it. No one seemed to listen and the failure of the central prediction behind the scare (the prediction of a general depletion) has been entirely overlook in the celebration of the greatest multilateral treaty success in UN history.
Freon ™ patents were expiring, and lo, behold, DuPont just happened to have patents on new, more expensive, less efficient, freon-cousin just when Freon WAS to be banned because, you know, ozone hole.
Well, this I call a success. At least as far as DuPont profit are concerned.
Now, the fact is, Montreal protocol doesn’t cost me more than a few bucks. There are much higher stakes in other issues.
Correlation, right? If it’s good enough for global warming, why not for the banning of CFCs when a more expensive alternative was suddenly available? Kind of like those CFL bulbs that were hazardous waste, but mandated for no apparent reason exept to increase sales of the bulbs. Millions wasted. Had the government stayed out of it, LEDs would have come in on their own as energy savers. There is nothing more messed up than government intervention in the name of profit for their donors.
[QUOTE FROM ARTICLE]”They found that ozone loss is decreasing, but they needed to know whether a decrease in CFCs was responsible. When ozone destruction is ongoing, chlorine is found in many molecular forms, most of which are not measured. But after chlorine has destroyed nearly all the available ozone, it reacts instead with methane to form hydrochloric acid, a gas measured by MLS. “By around mid-October, all the chlorine compounds are conveniently converted into one gas, so by measuring hydrochloric acid we have a good measurement of the total chlorine,” Strahan said.”
Where does the methane come from in winter in Antarctica? Penguin flatulence? Or can methane produced by swamp gas or human-caused natural-gas leaks in warmer climates be blown clear down to Antarctica?
Methane concentrations in more temperate climates has been measured at about 1.8 ppm, and are probably lower in Antarctica in winter, since there are very few biological or human sources there. But if the chlorine runs out of ozone to react with, and starts reacting with methane, there’s probably not much ozone to begin with.
Penguin flatulance sounds suspiciously likely as another cause. Another mad alternative was the amusement in an office which I used to work in in the ‘eighties where one of the ladies maintained an elaborate beehive hairdo by applying staggering quantities of hairspray leading to an office joke about causing the ozone hole all on her own-the nickname “concrete head” soon followed.
Perhaps she has moved to the Southern Hemisphere?
Moderately Cross of East Anglia: “…an elaborate beehive hairdo by applying staggering quantities of hairspray…”
Aha! Final Net Superhold in the purple can: the preferred propellant for PVC potato(e) guns
Bahamamike
It has always puzzled me as to why the so-called CFC-induced ozone hole is in the southern hemisphere when most of the world’s population and industry is in the northern hemisphere.
It has always puzzled me as to why the so-called CFC-induced ozone hole is in the southern hemisphere when most of the world’s population and industry is in the northern hemisphere.
“A projected 10 percent UV increase from a worst-case global ozone depletion is the equivalent of moving just 60 miles closer to the equator….New Yorkers moving to Florida experience a more than 200 percent increase in UV because of the change of latitude.” – Professor Fred Singer (1995)
“Two years after the discovery of the Antarctic ozone hole in 1985,…”
Bullpuckey! The Antarctic ozone hole was discovered in 1956 by British scientist Gordon Dobson. It’s cause was explained in 1959 by French scientists Rigaud & Leroy. It’s natural, and it will always be with us.
I have always wondered about this. Is there any evidence that the ozone “hole” has not always been there? I find it hard to understand, as others seem to, why it is at the South pole where there is little CFC output and not at the north pole which is closer to the industrial centre of the world. As O3 has such a short half life it does not seem to add up that it persists at the antarctic pole. My guess (scientific!!) is that it has always been there.
go back to start and when you cross
https://wattsupwiththat.com/2018/01/16/nasa-claim-definitive-evidence-of-the-montreal-protocols-success-on-ozone-hole-but-may-be-premature/comment-page-1/#comment-2718535
do not ignore it.
This is the first time they have used the Aura satellite to measure the amount of ozone depletion in the stratosphere rather than at the surface – is a 12 year cycle of a fluctuating phenomenum enough to derive a comprehensive trend line?…..I doubt it. And how typical that this has then been used to produce a trend line and an end date to the ozone hole that noone knew existed 50 years ago and is more than likley to be an annual feature over the Antarctic land mass – at least since it moved in to that position on the globe. More bad science or at the very least jumpng the gun and shooting ones mouth off…..more money please
sorry 61 ears ago (should have looked it up)
Ok, so they seem confident that the HCl is what the CFCs make when Ozone is depleted. So, as always, confounding studies have to be brought in by sceptics because the climate worriers never look to hard at unhelpful literature. My instant thought was that we are a chlorine salt water planet! It seems that ehat is “acidifying” the ocean is hydrochloric acid,wadyaknow!
https://www.google.com/amp/s/amp.livescience.com/2032-seawater-treatment-plants-combat-climate-change.html
And natural coastal aerosols (I’ve seen the faint haze from the sea spray in a wind with the waves pounding the shore) result in chlorine emissions into the atmosphere reactions of salt with the atmosphere and folks, i don’t have to look up the fact that chlorine dissolved in water makes HCl acid.
http://pubs.acs.org/doi/abs//es025793z
Now how many readers here, scientists and high school grads would conduct an experiment involving Cl and HCl in the atmosphere above a large basin of saltwater that isn’t listed as a part of the experiment?
I would love to wager that the ozone hole will be about the same size +or- the variance of the past 30 yrs. Indeed, a way to save the great grandchildren would be to set up a fund to bet against all the predicted disasters due to man’s activities. All Universities, doomer institutions should be required to contribute to the betting fund -at least in their minds it should be a no-brainer endowment for their institutions. I would even offer to the institutions that they can decide on the odds for each bet. Fair? We ate talking settled science here. The way they would do this is by giving a probability of their being correcct within shown error bars as part of their published works. E.g. a a 67% probability would be a payout of 2:1 to the winner. Contributions would be funded by each side stsrting now. I leave the detais to experts in rhe gambling field for paying in, setting up a trust and rules to make it work asit should.
More dubious information from the team who brought us the Apollo Moon Landing simulations and the Mars Rover on Devon Island. Let’s all have a very close examination of this new presentation first.
Yeah, I saw the video of the yearly changes of the ozone hole. OK, it looked like it decreased slightly last couple yrs.
Eco-nut taking an inch and making a mile: OH!! OH!! LOOK!! LOOK!! The Montreal Thingy is working!!! SEE!! TOLD YOU!!!
(note the accretion region surrounding the depletion region – try to account for it in your explanation)
======
Scientists from NASA and the National Oceanic and Atmospheric Administration (NOAA) have confirmed the ozone hole over the Antarctic this September is not only much smaller than it was in 2000 and 2001, but has split into two separate “holes.”
The researchers stressed the smaller hole is due to this year’s peculiar stratospheric weather patterns and that a single year’s unusual pattern does not make a long-term trend. Moreover, they said, the data are not conclusive that the ozone layer is recovering.

-NASA Earth Observatory, 2002
=====
(note the mountains surrounding the valley: try to account for the mountains in your explanation for the pattern)
Khwarizmi, I’ve been making this point for years but no one has shown much interest. I describe it like a turtleneck sweater rolled back and doubled over. There is no loss of ozone, it is simply pushed aside. Nasa’s simple coloured images always have this “roll neck” effect.
http://www.theozonehole.com
This is a great site but it has some weird blind spots. Oh well there are two of us who noticed this complete debunking of the human cause of the ozone hole sticking out of their own data. Eventually this could double sarc.
Gary, this winter anomaly was first described and explained in the early ’60s by Dobson himself. But it is not your fault that you missed that. The issue has become confused by the conflation of the ‘Spring hole’ (described by Farman, caused by CFCs) with the ‘Winter anomaly’ (cause by the vortex stopping Brewer-Dobson circulation). Now, with this video above explaining that the best time to measure the Chlorine (from CFCs) effect is in Winter, the issue is further confused. It’s hard to know what is going on both over Antarctica and in the interpretation of the evidence. No one who knows seems to have any interest in sorting out the confusions and distortions in the PR. I live in hope that one day some expert will provide a critique that will clarify the interpretation, the evidence.
Here is the original data from Halley Bay that lead Dobson to propose the anomaly and its cause:

berniel January 17, 2018 at 2:36 pm
Gary, this winter anomaly was first described and explained in the early ’60s by Dobson himself. But it is not your fault that you missed that. The issue has become confused by the conflation of the ‘Spring hole’ (described by Farman, caused by CFCs) with the ‘Winter anomaly’ (cause by the vortex stopping Brewer-Dobson circulation). Now, with this video above explaining that the best time to measure the Chlorine (from CFCs) effect is in Winter, the issue is further confused. It’s hard to know what is going on both over Antarctica and in the interpretation of the evidence. No one who knows seems to have any interest in sorting out the confusions and distortions in the PR. I live in hope that one day some expert will provide a critique that will clarify the interpretation, the evidence.
The top post refers to the paper which points out that during the spring O3 depletion event ultimately the Cl ends up as HCl, which is a stable Cl reservoir species. That is a good time to measure how much active Cl was involved in that spring
Basically during the winter the PSCs cause the reservoir species to react and form Cl2, once the UV hits the Cl2 the active O3 depleting molecules (Cl and ClO) are released and destroy the O3 and ultimately the reservoir species are reformed.
Here’s a graph showing the dramatic change in chemical composition as the vortex is entered.
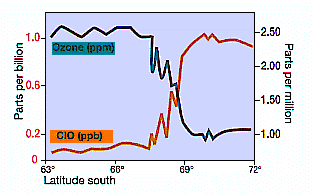
Measurements of ClO concentration and ozone from aircraft (NASA, 1987). Note the rapid incease of ClO as the aircraft enters the polar vortex and the Antactic ozone ‘hole’ (about 67ºS) and the inverse correlation of ClO with ozone decline in mid-September when the chemically unbalanced area in the vortex was sunlit.
Thanks Phil. I get the smoking gun reverse correlation of O3 and ClO in the spring sunlight. What remains confusing for me is when they say that the best time to measure Cl destruction of ozone is to measure ozone depletion in the winter dark. The top post says: During winter “Antarctic temperatures are always very low, so the rate of ozone destruction depends mostly on how much chlorine there is,” Strahan said. “This is when we want to measure ozone loss.” ” They want to measure then because they want to detect the Cl-from-CFCs effect. But the post also says “The Antarctic ozone hole forms during September in the Southern Hemisphere’s winter as the returning sun’s rays catalyze ozone destruction cycles involving chlorine and bromine that come primarily from CFCs.” Does the CFC effect require spring sunlight or is it an exacerbation of the winter anomaly? Or both? Are they playing hide-the-pea or have I just got distracted?
in a hundred years, if the human race hasn’t disappeared up its own backside due to the hubris of the well educated but terminally stupid ,this will be one of those paradigms that will be laughed at and used as an example of correlation not automatically mean causation.
oops, forgot the “ing” on the end of mean. oh well, how sad, never mind. obviously tired shovelling viner off the drive this evening. at least the children know what snow is this winter.
Perhaps the hurricane drought can be credited with the decrease in stratospheric chlorine more than the Montreal Accords. It would seem that oceanic chlorine and bromine would be a much more prolific source of stratospheric halides than would an industrial refrigerant. Just looking at an Atlantic hurricane, one has to conclude that quite a bit of salt has to be lofted into the upper atmosphere. I find a number of non-global warming and non-ozone depletion references on line regarding atmospheric halides. A real study of this would use isotopic tracers to determine where the chlorine comes from. But that is difficult, and doesn’t serve the narrative.
However the halides swept up from the ocean are water soluble and are precipitated out of the atmosphere in the form of rain (and snow). Measurements show that the HCl mixing ratio is less than 0.1 ppbv at
elevations above 7 km, and less than 0.04 ppbv at 13.7 km.
Stratospheric chlorine is ~80% from CFC’s and related manmade organic chlorine compounds,
such as carbon tetrachloride and methyl chloroform and ~15-20% from methyl chloride (CH3Cl), most of which is natural.
F. S. Rowland, “Stratospheric Ozone Depletion”, Ann. Rev. Phys. Chem. 42, 731, 1991.
Read this from the wikipedia site on ozone
“Computer modeling
Scientists have attributed ozone depletion to the increase of man-made (anthropogenic) halogen compounds from CFCs by combining observational data with computer models. These complex chemistry transport models (e.g. SLIMCAT, CLaMS—Chemical Lagrangian Model of the Stratosphere) work by combining measurements of chemicals and meteorological fields with chemical reaction rate constants. They identify key chemical reactions and transport processes that bring CFC photolysis products into contact with ozone.
“Ozone depletion and global warming[edit]
Main article: Ozone depletion and global warming
Among others, Robert Watson had a role in the science assessment and in the regulation efforts of ozone depletion and global warming.[31] Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a crucial role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment[31] to reach a consensus to provide the IPCC Summary for Policymakers.
There are various areas of linkage between ozone depletion and global warming science:
Radiative forcing from various greenhouse gases and other sources
The same CO
2 radiative forcing that produces global warming is expected to cool the stratosphere.[111] This cooling, in turn, is expected to produce a relative increase in ozone (O
3) depletion in polar area and the frequency of ozone holes.[112]
Conversely, ozone depletion represents a radiative forcing of the climate system. There are two opposing effects: Reduced ozone causes the stratosphere to absorb less solar radiation, thus cooling the stratosphere while warming the troposphere; the resulting colder stratosphere emits less long-wave radiation downward, thus cooling the troposphere. Overall, the cooling dominates; the IPCC concludes “observed stratospheric O
3 losses over the past two decades have caused a negative forcing of the surface-troposphere system”[14] of about −0.15 ± 0.10 watts per square meter (W/m2).[86]
One of the strongest predictions of the greenhouse effect is that the stratosphere will cool.[111] Although this cooling has been observed, it is not trivial to separate the effects of changes in the concentration of greenhouse gases and ozone depletion since both will lead to cooling. However, this can be done by numerical stratospheric modeling. Results from the National Oceanic and Atmospheric Administration’s Geophysical Fluid Dynamics Laboratory show that above 20 km (12 mi), the greenhouse gases dominate the cooling.[113]
As noted under ‘Public Policy’, ozone depleting chemicals are also often greenhouse gases. The increases in concentrations of these chemicals have produced 0.34 ± 0.03 W/m2 of radiative forcing, corresponding to about 14 percent of the total radiative forcing from increases in the concentrations of well-mixed greenhouse gases.[86]
The long term modeling of the process, its measurement, study, design of theories and testing take decades to document, gain wide acceptance, and ultimately become the dominant paradigm. Several theories about the destruction of ozone were hypothesized in the 1980s, published in the late 1990s, and are currently being investigated. Dr Drew Schindell, and Dr Paul Newman, NASA Goddard, proposed a theory in the late 1990s, using computational modeling methods to model ozone destruction, that accounted for 78 percent of the ozone destroyed. Further refinement of that model accounted for 89 percent of the ozone destroyed, but pushed back the estimated recovery of the ozone hole from 75 years to 150 years. (An important part of that model is the lack of stratospheric flight due to depletion of fossil fuels.)”
THE FOLLOWING IS IN CAPS SO THAT YOU KNOW IT IS MY COMMENT
NOW I KNOW WHY THIS WHOLE SITUATION ABOUT THE OZONE DEPLETING REGS AND LAWS AND AGW REGS AND LAWS AROUND THE WORLD ARE CONNECTED. THE CONNECTION IS THROUGH THE IPCC
AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAGH
i AM ASHAMED OF THE HUMAN RACE THAT ONE PANEL CAN CAUSE SO MUCH HARDSHIP. I AM NOT AS MUCH CONVINCED THAT THE OZONE HOLE IS A SCAM AS MUCH AS I AM CONVINCED THAT AGW IS A SCAM BUT AFTER READING THE WIKI ARTICLE MY SCAM DETECTOR IS GROWING ABOUT THE OZONE HOLES AS E WELL
I haven’t looked at it for a while but the BAS web site had data for 2009-10 and reported that:
“Ozone values dropped, to reach a minimum of around 125 DU (60% depletion) in late September, (Antarctic spring). The lowest daily value measured was 107 DU on October 1. This minimum value is similar to those recorded each October since the early 1990s.”
It was also similar to those in the spring of 1958 at the French Antarctic Observatory at Dumont d’Urville [opposite side of the South Pole from Halley Bay], when Rigaud and Leroy [quoted in Annales Geophysicae (November, 1990)] reported atmospheric ozone levels as low as 110 DU.
Don’t forget Mount Erebus, which started erupting in 1971 and has been active ever since. It is one of the largest, if not the largest, natural source of stratospheric halogens and situated right in the middle of the Antarctic ozone hole.
https://www.sciencedirect.com/science/article/pii/S1352231015304246
Every time I see an article on the Ozone Hole and the impact of CFC’s, I’m reminded of a sidebar in the 1969 Time Life Life Science Series book on Mathematics.
The side bar, A Topological Redhead, was accompanied by a photo of a boy showing his hair swirl and read,
“…most human heads have a fixed point, in the form of a whorl, from which all hair radiates. Topologically, it would be impossible to cover a sphere with hair — or with radiating lines — without at least ONE such fixed point. For the same reason, the wind cannot blow everywhere over the earth’s surface at once; there must be a point of calm.”
Just because the ozone hole was discovered in 1985 and CFC measurements were first obtained in 2005 as noted in this post, does not mean the hole, whatever we choose to call it, recently emerged. It, and potentially many more, have existed for ever.
Joe Armstrong January 17, 2018 at 8:30 am
Just because the ozone hole was discovered in 1985 and CFC measurements were first obtained in 2005 as noted in this post, does not mean the hole, whatever we choose to call it, recently emerged. It, and potentially many more, have existed for ever.
The stratospheric CFCs were measured starting at least in 1985.
I’ll try one more time! There is no depletion of ozone! it’s pushed out of the “hole” and banked in a ring around the hole,. Like shovelling snow there is no disappearance its just pushed aside. See the images from Nasa:
http://www.abc.net.au/reslib/200906/r380242_1771273.jpg
A chemical reaction can’t do this! The ozone has simply been redistributed. I ‘ve been trying to attract notice to this here in vain for several years. Im now joined by one other comenter who has an excellent 3D graphic of it.
https://wattsupwiththat.com/2018/01/16/nasa-claim-definitive-evidence-of-the-montreal-protocols-success-on-ozone-hole-but-may-be-premature/comment-page-1/#comment-2718899
Com’on people, this is science.Are there only two of us that get It?
Gary Pearse January 17, 2018 at 11:43 am
I’ll try one more time! There is no depletion of ozone! it’s pushed out of the “hole” and banked in a ring around the hole,. Like shovelling snow there is no disappearance its just pushed aside. See the images from Nasa:
Not true, the ozone is mainly generated in the tropics and flows towards the poles, when the S polar vortex forms it prevents further ozone entering the polar region and O3 accumulates outside the vortex. Meanwhile the previous ozone trapped inside the vortex remains at about 300DU until the sunlight returns and causes destruction of this ozone, thus forming the hole. As the atmosphere inside the vortex warms up the vortex breaks down and the ozone which has accumulated outside is able to mix with the depleted atmosphere inside.
A chemical reaction can’t do this! The ozone has simply been redistributed. I ‘ve been trying to attract notice to this here in vain for several years. Im now joined by one other comenter who has an excellent 3D graphic of it.
Com’on people, this is science.Are there only two of us that get It?
No the science is as I describe it above.
Gary Pearse January 17, 2018 at 11:43 am
I’ll try one more time! There is no depletion of ozone! it’s pushed out of the “hole” and banked in a ring around the hole,. Like shovelling snow there is no disappearance its just pushed aside. See the images from Nasa:
Not true, the ozone is mainly generated in the tropics and flows towards the poles, when the S polar vortex forms it prevents further ozone entering the polar region and O3 accumulates outside the vortex. Meanwhile the previous ozone trapped inside the vortex remains at about 300 DU until the sunlight returns and causes destruction of this ozone, thus forming the hole. As the atmosphere inside the vortex warms up the vortex breaks down and the ozone which has accumulated outside is able to mix with the depleted atmosphere inside.
A chemical reaction can’t do this! The ozone has simply been redistributed. I ‘ve been trying to attract notice to this here in vain for several years. Im now joined by one other comenter who has an excellent 3D graphic of it.
https://wattsupwiththat.com/2018/01/16/nasa-claim-definitive-evidence-of-the-montreal-protocols-success-on-ozone-hole-but-may-be-premature/comment-page-1/#comment-2718899
Com’on people, this is science.Are there only two of us that get It?
No the science is as I describe it above.
Mods, why is my reply to Gary Pearse being blocked?
[? Nothing in queue. .mod]
Just tried it for the fourth time, still no show!
No, you have this backwards, the ozone is mainly formed in the tropics and is transported towards the poles.
When the S Polar vortex forms the ozone is prevented from entering the polar region and accumulates outside the vortex. Meanwhile as a result of the heterogeneous reactions on the PSCs chlorine is released in the polar stratosphere and when the sunshine returns the ozone trapped inside the vortex is rapidly destroyed. As the atmosphere warms up the vortex breaks down and the ozone outside mixes with the ozone depleted atmosphere inside.
Clearly a chemical reaction can do it, the science is as I described it above.
Phil. January 18, 2018 at 9:09 am
Mods, why is my reply to Gary Pearse being blocked?
[? Nothing in queue. .mod]
Two versions predating this post by the Mod have just appeared so I guess they were in the queue!
Apparently Gary’s post contains a poison pill because whenever I tried to quote him it didn’t post!
I find it quite incomprehensible that a CFC thinned ozone layer is not considered,even by those critical of the dominant paradigm of CO2/warming, as the most probable cause of global warming instead of CO2. A thinned ozone layer allows greater irradiation of the atmosphere and Earth’s surface with ionizing solar UV-B radiation, which is near the top of Sun’s radiative spectrum, so a little goes a long way. Further, the record of temperature anomalies over the past 50 years correlates much better with total equivalent atmospheric chlorine concentration than it does with CO2 concentration. The latter has been steadily rising since records have been kept, but global temperature has leveled off following a strong spurt of global warming from 1975 to 1998, in the now two-decade-long, level trend of the so-called “global warming hiatus.” Since the Montreal Protocol took effect through the ’90s, the temperature slowdown is entirely consistent with a chlorine-thinned ozone layer being the true cause of global warming. Certainly, if ionizing UV-B radiation is capable of causing severe sunburn and genetic alteration, it is also capable of causing global warming.
More on this can be found by Googling “David Bennett Laing WUWT” and selecting any of the top four urls.