By Steve Goreham
“In 2015, scientists at NASA predicted that the Ozone Hole would be half closed by 2020. That hasn’t happened. Other scientists have forecasted that the hole will not begin to disappear until 2040 or later. But the longer the hole persists, the greater the likelihood that the ozone layer is dominated by natural factors, not human CFC emissions.”
Another year has passed, and that stubborn Ozone Hole over Antarctica refuses to go away. Data from the National Aeronautics and Space Administration (NASA) shows that the area of the Ozone Hole remains about the same as it has been over the last 30 years. But will scientists admit that they didn’t save the ozone layer?
Background
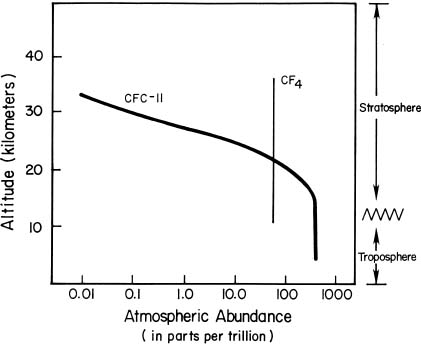
Ozone is a gas made up of three oxygen atoms (O3). Ninety percent of the ozone in the atmosphere is found in the stratosphere, a layer of atmosphere between about 10 and 50 kilometers in altitude. The amount of ozone in the atmosphere varies with time of year.
Dr. Mario Molina and Dr. Sherwood Rowland of the University of California published a paper in 1974 warning that industrial chlorofluorocarbon (CFC) pollution was destroying the ozone layer in Earth’s stratosphere. CFCs were gases used in hair spray, refrigerators, and insulating foams.
The theory of Molina and Rowland postulated that CFCs from human industry move upward through the atmosphere to the stratosphere, where ultraviolet radiation breaks down CFC molecules, releasing chlorine atoms. Chlorine then acts as a catalyst to break down ozone molecules into oxygen, reducing the ozone concentration. According to the theory, the more CFCs consumed, the greater the destruction of the ozone layer.
In 1983, researchers from the British Antarctic Survey discovered a thinning of the ozone layer over Antarctica which occurred during August, September, and October. This became known as the Ozone Hole. This appeared to confirm the theory of Molina and Rowland, who were awarded a Noble Prize in chemistry in 1995 for their work.
Montreal Protocol (1987)
The ozone layer blocks ultraviolet rays, shielding the surface of the Earth from high-energy radiation. According to scientists, degradation of the layer would increase rates of skin cancer and cataracts and cause immune system problems in humans. In Earth in the Balance (1992), Al Gore claimed that hunters reported finding blind rabbits in Patagonia and that fishermen were catching blind fish due to human destruction of the ozone layer, but this was not confirmed.
In 1987, 29 nations and the European Community signed executed the “Montreal Protocol on Substances that Deplete the Ozone Layer”. Over the next decade, signers of the treaty rose to over 180 nations, all agreeing to ban the use of CFCs.
Because of the Montreal Protocol ban, world consumption of ozone depleting substances (ODS), or chlorofluorocarbons, began falling in 1990. By 2005, ODS consumption was down 90 percent and is now down more than 99 percent, according to the European Environment Agency.
Result?
The Montreal Protocol was hailed as an example of international success of how nations could unite to resolve a major environmental issue. The Protocol has been praised as an example to follow for elimination of greenhouse gas emissions in the fight to halt global warming. But despite the elimination of CFCs, the Ozone Hole remains as large as ever.

NASA reported this fall that the mean ozone hole area for September 7 to October 13 again reached 23 million square kilometers, roughly the same level as in the last three decades stretching back to 1994–1995. The hole remains large, despite that fact that world ODS consumption has almost been eliminated.
In 2015, scientists at NASA predicted that the Ozone Hole would be half closed by 2020. That hasn’t happened. Other scientists have forecasted that the hole will not begin to disappear until 2040 or later. But the longer the hole persists, the greater the likelihood that the ozone layer is dominated by natural factors, not human CFC emissions.
___________________
Steve Goreham is a speaker on energy, the environment, and public policy and the author of the bestselling book Green Breakdown: The Coming Renewable Energy Failure. His recent Wall Street Journal op-ed, If Green Energy Is the Future, Bring a Fire Extinguisher, brought national attention to the lithium battery problem.
Be interesting to know if this ozone hole is a natural feature or not and how big it was say 200 years ago.?
Did this get blown up to let DuPont have a clear run with there second generation refrigiants on expiration of the patents on the first generation ?
If so was this the inspiration for the current generation climate scams ?
That’s just what I was wondering. How big was the hole before we knew how big it is? The assumption seems to be that it didn’t exist. The whole(hole?) Issue is just made up. Just like AGW.
Don’t forget, it’s CAGW. Every part of ‘Catastrophic Anthropogenic Global Warming’ must be valid for there to be any problem.
And of course it needs just one part to be false for the whole edifice to collapse.
Insert Einstein’s quote here.
“It doesn’t matter how beautiful your theory is, it doesn’t matter how smart you are. If it doesn’t agree with experiment, it’s wrong.” ― Richard P. Feynman
I made a comment here about the ozone hole in 1979 before I saw Mike’s comment below.
Even if there were no hole, how historically anomalous was that situation? Is that the normal case or was than an unusual case?
Well at least they only got a Noble Prize and not the prestigious Nobel Prize 🥸
Even better. The very earliest measurements are a lot smaller, but… If you read “the small print” (stuff that usually won’t be presented on the same pages as “first glance” graph), it turns out that the methods changed to more advanced when it went big, in either sense. So it looks like they just stitched together data from different methods of measurement, smoothed the line and then of course it was presented as evidence of actual increase.
If you look at Figure 2(a),(b) in The ozone hole and a phase change in lower stratospheric temperature, you will see that the continuous increase in CFCs from almost nothing in 1963 had no noticeable effect for 20 years. Note that CFCs are in reverse order scale. Although those promoting the Montreal Protocol said that there was no Antarctic ozone hole before 1979, and although they arbitrarily defined an ozone hole so that the holes began in 1979 (they thought), the data shows that there actually were Antarctic ozone holes in 1964, 1966, 1969, 1974 and 1977.
Good post Mike. I was wondering why there was no ozone hole in 1979. It looked rather arbitrary to me.
I recall an article stating that there were observations of higher ultraviolet levels in the Antarctic around the turn of the 20th century. That would indicate that the ozone hole has been around for a lot longer than assumed.
If I find the article, I’ll share it. If I recall, the observations were noticing higher UV not ozone level.
Speaking of which, ozone is used as a proxy for surficial UV, which can cause cataracts and melonoma. However, when was the last time you saw an article talking about measurements of the surficial UV flux, rather than the proxy? The situation is a lot more complex than is usually implied. For one, the sun is never directly above the so-called hole. The rays come in obliquely from outside the ‘hole,’ often passing through regions of anomalously high stratospheric ozone outside the Antarctic Circumpolar Vortex. (Note the orange and yellow colors in the lede ozone map at the top of the page.) Even in the situations where the high ozone concentrations are missing, the rays from the sun have a much longer slant-range than do rays entering vertically over the tropics, and thus experience much more absorption and scattering. This is another one of the potentially catastrophic problems created by Man, for which there is a lot of arm waving, and a lot of unanswered questions.
Wrong Mike there were no holes “in 1964, 1966, 1969, 1974 and 1977″. The minima prior to 1979 occurred in the winter due to the gradual decline in O3 due to the lack of UV light followed by a rapid regrowth to about 500DU when the sunlight returned in the spring. The new phenomenon observed from ’79 onwards was the sudden drop in O3 in the spring when formally it had grown, it is the low level of O3 in the spring that is defined as an Ozone hole.
Where is old data from?
Googling for an answer to my own question… “Marked decreases in column ozone in the Antarctic spring and early summer compared to the early 1970s and before have been observed using instruments such as the Total Ozone
Mapping Spectrometer (TOMS).”
then
“Nimbus 7; launched October 24, 1978. Operated until 1 August 1994. Carried TOMS instrument number 1.”
It sounds to me like someone was measuring by some proxy before 1978. Maybe they know what was happening and maybe they don’t. I’ve seen enough of less than 100-year data records being used to characterize systems that I’m told have been running for billions and billions of years.
It’s fun that people are working on it, I hope they get it right, but I cannot type enough “greats” to specify which grandchild would have access to sufficient measured data to make a plausible prediction.
There is radiosonde ozone data at the South Pole from 1961 onwards. There was a British Antarctic station in the 1950s that took ozone measurements, but I don’t know how or at what altitudes.
Dobson himself was one of the first people to take instrumental measurements from the ground, looking into the sun when it first came over the horizon in the Spring.
The ozone hole is defined as being column ozone below 220 Dobson units. Ozone measured at the South Pole fell below 220 Dobson units in 1964, 1966, 1969, 1974 and 1977. Read my paper.
In the spring not the winter.
From your paper..
“This suggests that on such a very short timescale it is ozone that drives temperature, or possibly of course that something else is driving both. But on longer timescales, as in the phase change described above, it appears that temperature has a major influence on ozone (or something else is driving both). In any case, the phase change in both temperature and ozone bears no relation to CFC concentration”.
Yes, something else is driving both.
The two are linked, but temperature does not control ozone levels.
The same as ozone does not control the circulating wing speed. That theory is as stupid as the CFC ozone dilution theory.
Regards
Martin
The Brewer-Dobson circulation moves ozone from the tropics to the poles. When the Antarctic Circumpolar Vortex forms, neither ozone or warmer air can make it across the barrier. Thus, metastable ozone decaying naturally (short half-life) and that destroyed photo-catalytically, cannot be replaced until the vortex breaks up in the late Spring.
I suspect that the asymmetry shown by the orange and yellow pseudocolors in the NASA ozone maps indicate where vortex turbulence and/or transient diffusion through the vortex reduces the anomalously high ozone outside and shifts the center of mass of the ‘Ozone Hole.” However, I’m not prepared to provide proof of the working hypothesis.
Mike
See the attached chart. The SH and NH ozone minimums occur at virtually the same time.
Martin
I am surprised at the claim based on everything I have read over that last 40 years. First off, the North Pole doesn’t experience a ‘Hole’ every year because the vortex isn’t as regular as in the Antarctic. I would expect a 6-month difference between the lows, which even the Arctic experiences because there is no sunlight to create new ozone and it has to depend on the Brewer-Dobson Circulation to replenish O3 that decays naturally. Something isn’t right here.
The biozone area is inverted, the minimums shown is actually the maximum.
The ozone hole was first observed in 1956 by Gordon Dobson (after whom Dobson Units are named) and its natural cause explanation was published by French scientists Rigaud and Leroy in 1959. But there was no environmental lunacy back then so it was only of interest to scientists who specialized in natural phenomena.
re: “with there second generation refrigiants on expiration of the patents on the first generation ?”
We went down this road before (here is mankind’s Achilles heel: we don’t learn very well as a ppl) with the patents on R12 and R22 maybe 10 years back now on this very website.
The patents on the original R12 and R22 had expired LONG before the mid 1970 time frame (sure, look it up! Like I did). What had been patented since the initial introduction of these Freons were improved methods and techniques to make those Freons … I suspect these ‘replacements’ are in the same category,, and I can say that because patents work/are enforceable for ONLY a fixed number of years, 17 or perhaps 20 (I don’t recall the exact number ATTM.)
For instance, regarding early Freon patents:
https://web.archive.org/web/20180326124508/http://www.imcool.com/articles/aircondition/refrigerant_history.htm
Interesting article! 40 years ago, I was installing & maintaining equipment used for low temperature sterilization of heat sensitive items. The machines used a mixture of 12% ethylene oxide and 88% Freon.
Yes, it was a successful test case
an unproven conjecture
immediate Nobel prize
scare of global doom
it is all our fault
poor penguins are dying from cancer!
but there is a solution, we just need to pay for it
global problem requires global bureaucracy to enforce
All repeated and amplified with CCCGW
but controlling CO2 is much more lucrative
potential global investments in CO2 control are mind boggling
everybody, every activity emits CO2
controlling CO2 is controlling everything
Has anyone checked the Emperor Penguin that recently showed up in Australia for cataracts? Maybe he couldn’t see where he was going. 🙂
Obviously it is natural. Since the holes are an inevitable result of conditions near the poles: poor incidence angle reduces production of ozone and cold accelerates its decay. Conversely, in tropics the situation is the opposite. And the stratospheric vortex that affects how air (including ozone) actually moves.
The question is which factors (both natural or anthropogenic) do and which don’t add to natural decay enough to alter either overall layer thickness or hole size.
Specifically, strong response to these evil gases which only shows in trace amounts even at the sea level, yet somehow gets into stratosphere in non-negligible amounts despite being rather heavy, and there somehow withstands continuous bath of hard UV intact (while that ozone exists in the first place as a product of so much O₂ being broken by ionizing radiation and also failing to recombine properly, thus happy fun radiochemical furnace layer must have significant concentration of not only excited ozone but atomary oxygen)? Yes, this part increasingly seems to be not a thing that actually happens, or at least not enough to have noticeable effect. In a surprising twist.
Considering the “hole” area varies from year to year routinely by 1/5, and occasionally up to 1/3, anything that does not clearly show in covariance with a comparable effect likely is not important to it.
I don’t buy into the story that CFC causes an ozone hole. But let’s say it does for the sake of my question. Ozone consists of 3 oxygen atoms, CFC degrades into chlorine and other stuff. The chlorine separates the ozone into individual oxygen atoms. What prevents the oxygen atoms from reforming into ozone? The oxygen is still there it hasn’t been destroyed.
There have been various studies that conclude the reduced ozone concentration over Antarctica is indeed a natural phenomena, more do to upper atmosphere currents that mainly temporarily change the distribution the ozone layer rather than any major change in the amount of ozone. I don’t know if the widespread ignoring of those studies are due to them being poor quality studies or because there are powerful gatekeepers of a sacred (because so useful) story.
However, if the wild Chlorine atoms really do temporarily diminish the ozone density, they do it under very specific conditions that only occur during that time each year. Since the chlorine is not destroyed by the process, it can continue eating ozone as long as conditions are favorable (August, September, and October).
Valid points but also suggest a study to actually measure the concentration of Chlorine atoms in the stratosphere, globally, not just over the south pole.
I suspect there is a yet unidentified atmospheric mechanism that removes Chlorine. The atmosphere is amazing in what it does and we do not know 1% of it, yet.
Your last sentence is spot on
There is the issue of Mt. Erebus, an active volcano in Antarctica, that produces a lot of chlorine and fluorine, all within the circumpolar vortex. It conveniently gets overlooked in the haste to blame Man.
More importantly the CFCs float up to the top of the atmosphere and there breakdown story really is very dubious. Freon, the first, CFC is CCl₂F₂ which has a molar mass of 121 which is very heavy. Almost 3 times as heavy as CO2. How its gets to the stratosphere without a ticket on high altitude balloon is very unclear. Further, there is an almost infinite amount of chlorine available at the surface of the oceans why isn’t that sucked up by tropical thunderstorms and pumped into the stratosphere?
The Chlorine near the surface is very reactive and is rapidly removed, CFCs however only react as a result of strong UV radiation in the stratosphere. The atmosphere below ~100km is termed the homosphere because the composition does not change due to the molecular weight due to convective mixing of the atmosphere, above that height (the heterosphere) mixing is due to molecular diffusion which depends on molecular weight.
It’s not “unclear”…you’re just confused about that junior high school experiment where the teacher poured CO2 gas over a candle to extinguish it….with some bizarre assumption that a “puddle” of CO2 remains on the school floor that remained unexplained by your science teacher… After some small time period, heavy molecules are hammered by surrounding air molecules moving at about the speed of sound and become evenly mixed in the atmospheric column…. unless some condensation phenomenon causes droplets of them to fall to lower altitudes where they might re-vaporize or collect to become puddles, say like the Pacific Ocean….
It must be air currents and covections doing the mixing, not molecular hammering:
https://www.youtube.com/shorts/0AUkjTcrJtI
There is reputedly a problem of CO2 concentrating in depressions in the ground in the Ngorongoro Caldera in East Africa because of high emissions of CO2. It can be dispersed by the wind, but can also collect when the air is calm.
If there is a source that can supply new CO2 molecules that exceed the dispersion rate due to molecular kinetic energy, of course there will be a zone where CO2 concentration is tending toward source concentration. Especially in a partially enclosed situation as referred by “markx” at 3:01
It’s called reaction kinetics, the ClO radicals are being regenerated on PSCs at a much faster rate than the O3 is being formed so until the PSCs disappear as the spring temperature rises, O3 destruction wins.
UV light forms ozone as well as destroys it. The addition of a catalyst like chlorine or bromine upsets the equilibrium between the creation and destruction of ozone. During the lightless winter, ozone is consumed but not created so the concentration naturally drops. Then in the spring it is supposed to build back up until a quasi-steady state is reached. Then in the fall the concentration drops again.
What you describe is what used to happen the spring, however since 1979 the spring build up doesn’t occur, instead we get a rapid decline.
No. It separates into a regular O2 molecule and a monatomic oxygen ion, which goes off looking for another oxygen ion. The ions are in short supply. It takes energy in the form of UV to create an O3 molecule, which is metastable and has a short half-life.
If CFCs cause the ozone hole, why only in southern winter??
Why doesn’t the North Pole has a similar ozone hole, especially since it’s closer to the source of the CFCs??
It does, just not often.
I don’t doubt Redge’s answer, but the question was “why?”
I’m not sure I understand, the question I answered was “Why doesn’t….”
Arctic air doesn’t get as cold. Ozone is destroyed when the air is very cold, but sunlight is significant. That is why October is worst.
Brilliant! October is well known as the most intense for sunlight over the antarctic.
The main reason for the formation of the Ozone hole is the formation of Polar Stratospheric Clouds (PSC). These clouds form a surface upon which active chlorine containing radicals can form which catalytically destroy ozone, they form at temperatures below -78ºC. Once the temperature rises and the PSCs evaporate the destruction of Ozone stops and the increased UV during the summer replenishes it.
So now having established the oversimplification “cold destroys ozone”, would global warming help ozone’s cause? Or is there a difference between heat and UV radiation that ruins the oversimplification?
Ozone is supposedly destroyed in stratosphere on PSC surface by a catalytic reaction requiring sunlight.
Formation of PSC requires cold stratosphere.
Than they say that global warming warms troposphere but cools stratosphere, causing more PSC formation and amplifying ozone destruction.
Remember, global warming makes everything worse. Always.
During warm years (typically El Nino years) the Circumpolar Vortex is typically weak or breaks up early. Those years have small ‘Holes.’
https://en.wikipedia.org/wiki/Brewer%E2%80%93Dobson_circulation
Dear Nick
What a load of garbage bundled up as a theory.
Its a theory, that does not automatically make it correct.
During an El Nino year, the so called “hole” goes right through to mid December, when the temperatures are higher. How do you explain that.
Regards
Martin
Can you provide a citation?
It may be well known to some, but it is news to me. Do you have a citation you can provide? It is like saying that sunlight is well-known to be intense at 7:00 in the morning.
That’s good news. We have a perfectly natural explanation for ozone depletion.
Yes as long as there are CFCs present.
Ozone depletion in the Antarctic was observed long before the introduction of CFC’s.
Slight depletion in the winter followed by regeneration in the spring, since 1979 we’ve had substantial depletion in the spring instead of regeneration. That’s the difference!
No, substantial depletion, big enough to be detectable even with the technology of the 1930’s.
Or Mt. Erebus.
If CFC presence was so relevant
than the Ozone Holes in the Antarctic during the 70ies /80ies would have been almost inexistent.
As almost all CFC ‘s were released during that period in the northern Hemisphere.
It takes about 5 years for the CFCs to reach the Antarctic stratosphere.
At what altitude? This is the stratosphere and air density is minute, therefore temperature is very low.
“Arctic air doesn’t get as cold.” I assume the sentence would continue with “as Antarctic air”.
Confirmed:
“Antarctica is colder than the Arctic, with the coldest air temperature ever recorded on Earth (-128°F).”
Then the question of why a (not really, but go with it) symmetric spherical shape would have different temperature at equally exposed poles? Ocean in the North changes temperature slower, continent in the South changes temperature faster.
So okay the Stokes answer works for me.
Thanks.
Land and ocean mass distributions and ocean currents are very different in N and S hemispheres. Antarctica is also very tall, about 2.5 km above see level on average, with temperatures dropping 6 deg C per km. North Atlantic also receives a lot of heat from Gulf Stream.
There are likely also orbital contributions. The current axial tilt of Earth and eccentricity of the orbit (where the aphelion occurs in early July, which is Southern hemisphere winter) would combine to also cause colder temperatures in Antarctica. Not sure that this would be a huge effect. Probably the elevation would be the largest effect.
I concur that these factors concur.
What a load of nonsense.
How long do we have to put up with BS ‘science’ before it gets walked back? 50 Years is long enough.
I see the opposite problem:
50 Years is long enough -> 50 thousand years might not be long enough
it expands and contracts in line with solar induced changes in the balance of the ozone creation/destruction balance in the stratosphere.
It was never our fault.
Nor is climate change since those stratospheric changes also alter the configuration of the global weather patterns.
Ozone thickness is measured in Dobson Units.
Named after the physicist who invented the device to measure ozone in 1924.
Oddly, though he’d never been to Antarctica, he predicted that there would be an ozone hole there based on how various frequencies of the Sun either create or destroy ozone and the balance between them would change with the seasons, making the hole grow and shrink every year.
Which is exactly what they found. Thinking quickly, they realized there were no research grants to be had to study something that was already known, so they claimed it was new and demanded research money to study it. Which in turn had to have some sort of result other than “we already knew this” so another cause had to be
foundinvented.I believe he actually observed ozone depletion in the Antarctic as early as 1935-6, decades before the introduction of CFC’s..
Why does anyone still think CFC’s are the cause?
Because the ozone depletion prior to CFCs was slight and occurred in the winter due to the lack of UV light (down to around 300DU). What was new from 1979 onwards was the formation of a much deeper hole in the spring (down to 90 DU), instead of the previous rapid growth (up to ~500DU),it is that hole that is caused by CFCs.
Good, we have established that ozone depletion occurs naturally.
How do you know there have never been deep ozone holes in the past?
Still waiting for you to answer my question.
For the same reason that alarmists blame CO2. Humans can be blamed and can be taxed to fix the problem they allegedly created.
I nominate davidmhoffer’s response to be perma-located at the top of the article.
What harm has it caused? Well, the CFC-containing refrigerants are much more efficient and effective at cooling. Air-conditioning systems have to be run longer and use more power to achieve the same result with CFC-free systems.
Anyone who owned a car in the 70s and 80s can attest to the fact that the AC could get so cold that you could get a literal snowstorm (with the right humidity) coming out of your vents.
Current automobile A/C, when working properly, can make inside conditions uncomfortably cold during very hot weather if run full on.
I remember the snow from the vents. Peugot 505, 1984’ish….
“The hole remains large, despite that fact that world ODS consumption has almost been eliminated.”
What counts is actual CFCs in the air. They are persistent. Here is a graph of concentrations and what is expected. Unfortunately, it is from 2019, but clearly shows that CFCs are only slowly declining.
“But the longer the hole persists, the greater the likelihood that the ozone layer is dominated by natural factors, not human CFC emissions.”
Measurements of thickness of the layer go back further. Here is a plot showing that October thickness reducing nearly by a factor of 3 in one location, then gradually recovering, and closely aligned with chlorine concentration (note that axis is inverted):
Yes and that is also consistent with the total column levels of CFC-11 by Cantos et al, https://orbi.uliege.be/bitstream/2268/295583/1/pardocantosetal_2022.pdf
“Consistent”?
Yes, that’s the scientific proof word.
But I must point out that the “column levels” and the Ozone hole measurements are also ” consistent” with the traditional explanation of bad weather. In other words, the activity of naughty witches.
Up to you which explanation to believe. No plausible evidence of causation, in either case.
“Best estimate.” An educated guess is not proof.
Measurement from one location only? OK…
The CFC concentrations are from multiple sites
The ozone concentrations are from only one site.
https://woudc.org/data/stations/
What does WOUDC have to say about the surficial UV flux? Ozone is only a proxy for the real concerns.
I see. So it’s definitely obvious that CFCs are causing Climate Change, then?
Along those lines, we should stop Nick Cage making films.
A perfect correlation. Cage MUST be responsible!
So Cage caused the Ozone holes and is responsible for AGW:
Then global warming is indeed Mann Made.
By a single Mann.
So, what you are saying is that the CFC ban was brought in with an incorrect understanding of the atmospheric chemistry.
The lack of understanding of the atmospheric chemistry meant the effects of the ban were not understood.
Atmospheric CFCs did not decline.
Thus, because the basic science was wrong, the ban didn’t work.
This is a different way of saying the same thing. Poor science led to poor policies.
No. CFC were rising rapidly. The ban stopped the rise. But CFCs are persistent, so will take a long time to go away. That is exactly as expected.
But without a ban, the ozone layer would by now be full of holes.
What did Fred Singer say: without CR (cosmic rays) no ozone hole.
Do cosmic rays destroy the ozone layer?
A newer research
Also interesting:
https://www.americanthinker.com/articles/2016/08/is_the_antarctic_ozone_hole_aoh_really_mending.html
By Fred Singer.
Like your assumptions
Harsh!
Valid, though…
Too many replies breaks joke… AS calls back NS “full of holes.“
the ozone layer would by now be full of holes.
How big was the ozone layer hole in 1800? 1700? What is its long-term historical normal condition? How do we know that?
We have a few decades of data for something that has been around for millenia. What possible reason would we have to conclude that a decade or two of correlation actually means anything?
As was known at the time the CFCs in the atmosphere had a long lifetime, measurements at the time of Montreal showed that almost all the CFCs that had been produced were still in the atmosphere. CFC 112 has a lifetime of ~100 years and CFC 111 a lifetime of ~50 years. If we’d continued to produce CFCs at the expected rate the concentration in the atmosphere would be 5 times what it was at the maximum and as Nick says more and bigger holes.
CFC’s are inert and must be decomposed to atomic chlorine before ozone destruction can occur. Therefore, if CFC’s have a long half-life the amount of atomic chlorine they produce per unit time must be very small.
But each Cl radical produced destroys ~100,000 O3 molecules and each CFC-112 molecule produces 2 Cl radicals
How do you know the chlorine isn’t coming from the chloromethane emitted by marine organisms (ca. 4 million tonnes per annum)?
More FYI for other readers – I wanted more info around “persistent”.
“Despite their phaseout, it’s important to understand the warming impact of CFCs, because those we’ve already manufactured will be with us for a long time. The molecules can persist in the atmosphere for between 55 and 140 years,4 and the chemicals are present in old machinery that’s still being disposed of.”
You seem to have a problem differentiating between what can be proven with measurements and what is predicted by theory. I think that your crystal ball is full of holes.
Also there has been illicit production (hence emission) of CFC11 pinpointed to China.
The concentration of CFC11 in 2018 was barely less than that of of ~ 2002.
“Global CFC-11 emissions based on atmospheric measurements compared with the expected decline of this compound in the atmosphere if compliance with the Montreal Protocol was adhered to. CSIRO/AGAGE”
https://theconversation.com/eastern-china-pinpointed-as-source-of-rogue-ozone-depleting-emissions-117505
The Chinese are thought to have increased their CFC production to obtain credits when they stop doing it.
I read that they get money for destroying CFCs even though they just manufactured it.
My recollection is that the manufacture of alternative refrigerants produced some of the banned ones as by-products, and the credits were given to destroy those by-products. It then became profitable to arrange things so as to maximise formation of the by-products. I don’t think China was the only country where that went on.
Came back to check for updates and was struck by the phrase “pinpointed to China”
Somewhere in the worlds 3rd largest geographical area?
Not arguing the point, just quibbling over what pinpoint means.
Satellite measurements showed where local increases were taking place.
Ozone has been measured since the 1920’s. The 1957 Geophysical Survey went to Antarctica and measured it. I think Dobson was there.
https://gml.noaa.gov/ozwv/dobson/
Molina and Rowland put forth the idea of PSC’s interacting with ozone causing destruction. But no one accounted for the chlorine coming from volcanoes or the ocean.
China blackmailed the world by continuing to make Freon then getting paid to destroy it. No one knows if they really destroyed it.
CFCs are very heavy molecules and should settle near the surface as time goes on. Getting them in the stratosphere is difficult. Freon 12 is around 120 g/mole.
“…falls within the range of 100 to 200 grams per mole”
That’s not how the atmosphere works we have these things called winds, thunderstorms and hurricanes! Up to 100km the atmosphere[here is known as the homosphere because the gas composition is independent of molecular mass, above that is called the heterosphere where diffusion is the dominant means of distribution and depends on molecular weight.
Thanks Phil. I said difficult not impossible. As explained to me gravity does affect heavier molecules more over time and will bring them down near the surface.
Well whoever explained that to you was wrong, show them this paper:
https://uk-air.defra.gov.uk/research/ozone-uv/moreinfo?view=cfc-stratosphere#:~:text=CFC%2D11%20is%20unreactive%20in,high%2Denergy%20solar%20ultraviolet%20radiation.
How was CFC measured in the stratosphere prior to satellites?
O3 was measured using instruments tethered to balloons
O3 and CFC’s
IIRC, Dobson developed an instrument based on a Fraunhofer Line Discriminator. However, in the low light conditions of Spring in Antarctica, it had to be pointed directly at the sun on the horizon.
Here’s the more up to date graph Nick

(ppt) = parts per trillion? I wish the units were more wieldy. I also wish South America and Africa measured things more often – so much data is Northern Hemisphere Only.
Your graph has inadvertently demonstrated that the CFC’s are most unlikely to be responsible for the production of atomic chlorine since they are so stable.
Not at all, they are not stable in the stratosphere which is where the production of Cl radicals takes place, NASA measurements over Antarctica show high concentrations of the reactive species ClO which takes part in the destructive catalytic cycle.
CFC’s are clearly stable in the stratosphere as they have half-lives measured in decades. See my remark above concerning chloromethane.
Chloromethane has a half-life in the atmosphere between 6months and a couple of years so doesn’t make it to the stratosphere. CFCs are stable in the troposphere once they get to the stratosphere they rapidly decompose due to UV.
1
Sorry WUWT didn’t like that link for some reason.
Try this one.
Chloromethane (CH3Cl) is the most important natural input of reactive chlorine to the stratosphere, contributing about 16 % to stratospheric ozone depletion.
https://acp.copernicus.org/articles/19/1703/2019/
CFC’s have half-lives measured in decades, so are extremely STABLE, not unstable.
Exactly, which is exactly why they do not decompose in the troposphere and circulate into the stratosphere where they decompose in the presence of UV around ~20km to form Chlorine radicals which catalytically destroy the O3. It is their half-life in the troposphere which is measured in decades, not in the stratosphere!
RE: CSIRO chart of Total Ozone vs time
How was dat gotten for 1950 and was the same measurement method used for the whole chart?
It’s the thought that counts
/sarc
Steve, it’s worse than you thought. I spent my career, 30+ years, as a professional electrical engineer. I performed countless energy studies for clients.
In the mid 1990s, at a very large factory, the chief mechanical engineer one day told me “we’re going to convert the (1200 ton 4160V) chillers to R134 15 years early, to show how green the company is.” I asked “What will this do to their efficiency?” He said “it will drop about 5%.” So I pulled the data and calculated the cost: about $500,000. At the then price of oil, that was about 50,000 barrels. So I emailed the boss. He killed the project. A few years later, on the sly, the same ME converted one of the chillers. THEN after the fact told me. Sure enough, when I measured it’s power consumption, it was 5% more. To me, that was brown, not green.
Several years ago, as the CO2 craze intensified, I wondered what the cost, in CO2, of the last global rush to judgement was. Using conservative assumptions, the 5% loss, and DOE data, I estimated the annual US energy cost as being about like a coal train 1000 miles long. (On the efficiency loss, I checked the literature; some studies had the penalty at up to 15% – so the 5% looked reasonable, esp in view of my measurements).
Machine designs – as can be expected – keep getting better. So, someone might point out that current refrigeration machine efficiencies are higher today than when we used CFC. But that is misleading, because the efficiency loss of the new refrigerants is at the thermodynamic level – meaning that if current machines were designed for the old CFC refrigerants, they’d be that much more efficient. Ie, the efficiency loss is “off the top.”
The upshot: Not only is the CFC Treaty wasting untold sums of energy (if it’s ozone claims are wrong), but it is also causing vast amounts more CO2 (plant food) to be emitted. Thus, one global rush to judgement is fighting another global rush to judgement. Who knew.
Good comment, thanks Rod. Unfortunately this is an inconvenient truth that will not likely make it to prime time.
Unfortunately. Regarding the CFC Treaty, for years, I’ve been a voice crying in the wilderness to little avail. Someday, truth being the stubborn thing it is, it will WILL out.
Good they should ignore you and anyone else saying that a chemical which destroys stratospheric ozone and has a lifetime of a century should be released into the atmosphere in ever increasing quantities. But for Montreal we would have about five times the previous maximum of CFCs in the atmosphere. That is the truth.
One common replacement for CFCs in domestic refrigerators has been Butane.
The results of refrigerant leakage can get a lot more exciting, a lot quicker, than CFCs.
I know a guy that converted his old heat pump to propane. It was lot cheaper than CFCs but a lot more exciting. Oh, and he could use standard natural gas leak detection equipment.
The recent Arctic and Antarctic ozone situation, with history/reasons mentioned.
https://earthobservatory.nasa.gov/images/153363/arctic-ozone-hits-record-high
https://earthobservatory.nasa.gov/images/153523/ozone-hole-continues-healing-in-2024
Note that the color palette used in your links is the opposite of what NASA has used for years. It makes it difficult to compare historical ozone-concentration maps, and suppresses the information about the anomalous ozone highs outside of the circumpolar vortex. More game playing!
The ozone “hole” hysteria is pseudoscientific nonsense. Ozone on the ground is pollution. It’s regulated in the US by the EPA. It’s a big problem in large cities surrounded by mountains like Los Angeles and Beijing, especially in winter when atmospheric temperature inversions trap air pollutants on the ground. But ozone in the upper atmosphere is good because it blocks harmful UV radiation (just like it does on the ground). But you don’t want to breathe a lot of it. So far I haven’t seen an explanation why ground level ozone doesn’t eventually migrate to high altitudes. But apparently ozone-eating CFCs do. It’s magic.
In contrast, it happens that ozone subsides from higher level to grond levels.
They also neglect to mention that ozone forms when UV light ionises molecular oxygen, which is why the antarctic hole widens during the austral winter and reaches its maximum near the end of September or the beginning of October, when it begins to shrink again as the light returns. During the winter no sunlight reaches the majority of the antarctic region, while the circumpolar jetstream impedes mixing between higher and lower latitudes. Not prevents, because that’s impossible, but it does retard the mixing enough to make a difference.
The same effect is less pronounced in the north because all the land gets in the way and forces air mixing.
The other point about the circumpolar jetstream is that it creates a still region to its south where wind does not shear the blocks of air.
This means that lesser effects can work their magnetism. O3 is diamagnetic and so is repelled from the poles.
So long as the wind is held away, the hole will form.
This hypothesis also explains why Ozone concentrations are higher around the hole than in the regions just beyond.
NASA Ozone Watch: Antarctic ozone maps for September
Ground forming ozone often measured in late afternoon and evening in and around cities even in forests is based on NO and hydrocarbons and an intensive sunshine.
O3 molecules are heavier than O2 molecules and also CO2 molecules.
The point is, an assumption that O3 up there stays up there defies logic.
CO2 molecules are heavier that H2O, yet CO2 molecules are found at high altitudes.
Seems the blender is always running.
It would seem their ‘science’ is not very good.
.
Perhaps as bad as the SARS-CoV-2 / Covid-19 mRNA fiasco we are just now getting a perspective on … “Excess deaths published”: https://www.youtube.com/watch?v=jndykWR9O-c
.
Maybe the ‘govt’ should not be so, adamant, or demagogic with their ‘science’, and resultant imposition of their will upon their
subjects-er- people?Politicians are not scientists. They are merely mouthpieces for whatever scientists catch their ears.
Another example where a natural phenomenon, when first discovered, was falsely deemed to be a man made disaster with a host of unnecessary consequences to the world’s economy.
Since club of rome was created they are searching for ways to blame&control humanity.
And they regularly appear : CFC’s,DDT ‘ sIce Age Scare,AGW,Covid,
forced experimental drug ihjection falsely labelled as vaccines.
And the most funny thing : CFC’s are being released at ground level.
Way heavier than than Air.
Then they travel thousands of miles high up in the air to wreck ozone havoc – at the poles.
And they do most damage where they are not released = southern hemisphere.
Most of CFC’s had been released in the northern hemisphere as the southern hemisphere was 97% 🙂 2nd,3rd world with low industry levels except for the few people in NZ and AUS.
To fix the ozone hole, all we need to do is nudge the Earth’s axis back toward the vertical so that it isn’t dark for months at a time in the winter down there. That way the high-energy UV keeps converting diatomic oxygen to ozone and no hole develops. Simple.
Either that or giant mirrors. Decisions, decisions.
Solved!
Research scientists have found that predictions of disaster are a great way to gain funding in a profession where funding is tight and highly competitive.
The ozone hole is an example of the parade leader effect. Find a group marching, walk to the front and pretend you are the leader. Carry a marching stick. To those watching it appears you are the leader and everyone else is marching in support.
Like the parade in Animal House. Yes.
I wasn’t paying much attention to this when it came around, but I know that we have a limited amount of historical data on the “ozone hole”.
Was there ever a time that we observed it to be “closed”? And does anyone know what it’s normal condition should be? (If so, how is that known?)
Reading through all the comments I see that the question about observing it “closed” was answered
They will just claim that without CFC ban it would have been much worse. Much bigger hole in the Antarctic, hole over Australia and Argentina, hole in the Arctic. Dead polar bears. We would be all doomed, but now there is hope.
Salts are added to pure water, or sea water is used, to aid in electrolysis for hydrogen production. Pure water is a horrible conductor, and electrolysis requires current flow. As a result, along with the H2 and O2 produced are small amounts of chlorine gas and sulphur dioxide.
How does the future ramp up of H2 production as fuel, via ‘free’ electrolysis from ‘renewables,’ dispose of these pollutants? How does the level of chlorine gas production from H2 electrolysis compare with the contribution from stratospheric HCls in the past? How much chlorine is produced by electrolysis per metric ton of H2?
Will future schemes to produce H2 violate or invalidate the Montreal treaty?
Nobody else in the comment chain touched the idea that NaCl in the oceans could become Cl in the air during electrolysis. I assume the Cl and Na will get back together at the end of the process, but it would be nice if someone verified it. I think the consensus in this forum is that H2 power is a bad idea, so they have not become fluent in H2 production technology.
Proponents all seem to come back to ‘free’ electricity (nuclear, renewable, hydro) can make ‘free’ H2, wrt any of the handling, production, or use costs and tradeoffs. Chlorine gas is even more of a problem if they start from chlorinated feed stream — city water supply or waste. Sulphur and nitrogen compounds much more a problem with untreated waste water.
If electricity is ‘free,’ there’s no reason to imagine it is limited or to put up with the small relative yeild from electrolysis: carbon arc and thermal decomposition work better at scale. Just don’t imagine that wind or pv solar will be able to work it. Or that it is without tradeoffs.
But all of them take more input than you get output. Concatenating compromises isn’t a way forward when there’s known, proven, cheap, clean, and better substances available.
The thing with CFCs is that they are inert, so can make the long drift to the stratosphere. Chlorine is far to reactive (and soluble) to get that far.
Not true. Halon puts out (even metal) fires, in air, by displacing oxygen. Enough of it will suffocate firefighters and bystanders. Degreasers and cleaners dissolve and remove other materials while not affecting the contaminated substrate or using water or other contaminants. Propellant HCLs carry other materials in a shaped and directed stream. All of the ones I’ve used are denser than air, and disperse/evaporate slowly, even when heated to greater than ambient. These are the qualities that make them useful. As a ‘carrier’ of chlorine to the upper atmosphere they actually leave a lot to be desired, and decomposition is more likely to happen at surface level.
The ‘theory’ depends on the ‘long drift’ which has never been observed or quantified. Leaving pseudoscientific agency flaks to contrive ‘evidence’ that the treaty is working.
None of what you describe involves chemical reaction with CFCs. They are inert.
From DEFRA
“CFC molecules are indeed several times heavier than air. Nevertheless, thousands of measurements from balloons, aircraft, and satellites demonstrate that the CFCs are actually present in the stratosphere. This is because winds and other air motions mix the atmosphere to altitudes far above the top of the stratosphere much faster than molecules can settle according to their weight. Gases such as CFCs that do not dissolve in water and that are relatively unreactive in the lower atmosphere are mixed relatively quickly and therefore reach the stratosphere regardless of their weight.”
None of what you describe tells how a dense, heavy material can loft one of its chemical components to the stratosphere, and only decompose there. Nor have you described how lighter chlorine gas at the surface can somehow be exempted from the same altitude change. Nor how only the chlorine component significantly interacts wiith ozone at greater rate than at the surface.
A couple comments:
re: “He said that a DuPont patent (apparently not on the chemical but a follow on patent)”
Note: The original Freon patents would have expired by 1950, at least, and what has been patented over the years with those same Freons were methods to make or produce those original Freons … See the comment earlier on with an embedded link for more information on this.
People, it seems, Dunning-Kruger this patent aspect all wrong all the time in the vein of an Old Wives’ Tale.
The sun-Earth geometry does complicate things, but is rarely taken into consideration. Nor is the anomalously high ozone concentration outside the Antarctic Circumpolar Vortex, delivered by the Brewer-Dobson Circulation, and its role in the depletion and restoration of ozone ever mentioned.
The ozone hole is a natural phenomenon – no sun in winter, it grows. Recently, an ozone hole has appeared over the Arctic – unusually cold stratospheric temperatures. It seems that CFCs are not effective alone, but require very low temperature and ice crystals in the stratosphere to be effective.
So, the Antarctic ozone hole persists, fluctuates, and now, we have one over the Arctic too. CFCs are just another Nobel idea gone awry, as are all climate interventions so far.
Remove this, or stop that, and then nothing happens – except for prizes awarded.
Clearly, since CFCs are of human manufacture and release, it is possible to remove them from the atmosphere. They were declining, as a result of the Montreal Protocol, a singularly effective event, for a time. The ozone hole remained.
However, China has increased CFC production (see attached data), reversing the decline of CFC-11 and 12. Meanwhile, the West sticks slavishly to the the Montreal Protocol, a misguided piece of paper.
In 2000, arch-global warming advocate Hansen and other prominent modelers proposed (PNAS) that ALL global warming since 1960 could be accounted for by the potent greenhouse effect of CFCs. This was confirmed by Canadian modelers – so much for models, since BOTH CFCs and CO2 cannot be responsible for ONE effect. The replacements for CFCs: HFCs and HOFCs are also potent greenhouse gases, making it clear, once again, that the Earth’s temperature changes are due to much LARGER natural events than puny human efforts to alter the climate.
“The ozone hole is a natural phenomenon – no sun in winter, it grows”
No it’s not natural (as Clorine is not naturay present in the atmosphere).
And it doesn’t grow in winter ……
“Under normal atmospheric conditions, the two chemicals that store most atmospheric chlorine (hydrochloric acid, and chlorine nitrate) are stable. But in the long months of polar darkness over Antarctica in the winter, atmospheric conditions are unusual. An endlessly circling whirlpool of stratospheric winds called the polar vortex isolates the air in the center. Because it is completely dark, the air in the vortex gets so cold that clouds form, even though the Antarctic air is extremely thin and dry. Chemical reactions take place that could not take place anywhere else in the atmosphere. These unusual reactions can occur only on the surface of polar stratospheric cloud particles, which may be water, ice, or nitric acid, depending on the temperature.
These reactions convert the inactive chlorine reservoir chemicals into more active forms, especially chlorine gas (Cl2). When the sunlight returns to the South Pole in October, UV light rapidly breaks the bond between the two chlorine atoms, releasing free chlorine into the stratosphere, where it takes part in reactions that destroy ozone molecules while regenerating the chlorine (known as a catalytic reaction). A catalytic reaction allows a single chlorine atom to destroy thousands of ozone molecules. Bromine is involved in a second catalytic reaction with chlorine that contributes a large fraction of ozone loss. The ozone hole grows throughout the early spring until temperatures warm and the polar vortex weakens, ending the isolation of the air in the polar vortex. As air from the surrounding latitudes mixes into the polar region, the ozone-destroying forms of chlorine disperse. The ozone layer stabilizes until the following spring.”
https://ozonewatch.gsfc.nasa.gov/facts/hole_SH.html
How do you explain Dobson’s observations of ozone depletion in the Antarctic years before the introduction of CFC’s?
The depletion he observed was slight due to there being no UV to regenerate O3 during the winter and some natural depletion took place. In the Spring they observed a substantial regeneration as the sunlight returned. Following 1979 a different phenomenon was observed, substantial depletion despite the return of sunlight, that’s what CFCs do.
See my remarks above. Since ozone depletion occurs in the absence of CFC’s and the observation record is so short, your assertions are unjustified.
In the absence of CFCs ozone depletion only occurs in the winter in the absence of sunlight. In the presence of CFCs substantial depletion occurs in the spring in the presence of sunlight. My statements are justified by the observations over the last 70 years.
How do you know ozone depletion in sunlight never occurred before the introduction of CFC’s?
If atomic chlorine is responsible, how do you know it comes from CFC’s and not natural marine sources? CFC’s decompose so slowly the amount of chlorine produced per unit time must be tiny.
Not so. Mt. Erebus is a constant source of chlorine within the Antarctic circumpolar vortex, and after a long period of dormancy, became active again about the time that the ‘Hole’ became a concern.
“Mt. Erebus is a constant source of chlorine”
Elemental chlorine?
Evidence?
Ozone oxidises hydrogen chloride to water and elemental chlorine.
O3 + 2HCl -> O2 + H2O
Have often wondered about seawater being picked up into the clouds.
The idea that all halides precipitate out without reacting seems extreme.
There is an ocean’s worth of seawater. That’s a lot of NaCl.
It doesn’t take a large percentage to be diverted into other reaction cycles for the idea of “no natural atmospheric chlorides” to become laughable.
Volcanoes also emit vast quantities of hydrogen chloride.
No they both precipitate out and react and therefore don’t reach the stratosphere. CFCs are inert and not water soluble so they reach the stratosphere.
You might want to rebalance that reaction!
Indeed. I forgot the chlorine.
O3 + 2HCl -> O2 + H2O + Cl2
How do you know HCl precipitates out?
HCl doesn’t precipitate out. But it is extremely soluble in water.
Even a dilute solution will produce a small partial pressure of gaseous HCl.
I was at UC Irvine during that time and was forced to take PChem under Molina. He was by far the worst teacher I ever encountered. Little English skills and no ability to explain physical chemistry to other which in my mind means he had little understanding of PChem himself.
Sherry Rowland had a massive lab with much high vacuum glassware, so this is where he did his experiments. Thus all his results were in situ (in glass). Nothing he did was in vivo (in life or more appropriately the real world). Thus he could show that CFC’s can destroy ozone in glassware but it is an unsupported allegation that this happens in reality. AFAIK, there has never been a direct correlation established between CFC’s and ozone depletion in the South Pole.
Small World. I was at Irvine around then too, in Engineering (emphasis Mechanical, lol). While I was there they did away with Engineering Chemistry (Chem 61) and lumped everybody together in good ol Chem 1, to much rending of hair and gnashing of the teeth from Bio and Pre-Med types.
I recall those discussions at the time though. Basically he had identified a reaction, but nobody could figure out the transfer mechanism to get enough ground generated CFC’s up to the stratospheric south pole.
Bob-2, UCIPD-0 ?
Has anyone actually proven how large the ozone hole should be?
Isn’t it strange how the ozone hole in the Southern hemisphere corresponds almost exactly with the “hole” in the ice around the North polar cap? If there’s clear water around the Eurasian and North American coasts, and a little around Greenland, there’s less ozone over Antarctica. When those Arctic bodies of water start to ice up, the ozone coverage over the South increases.