Guest post by Jim Steele

Most people are unaware that the greenhouse gases CO2 and H2O, both warm & cool our planet. When I mention that CO2 has a cooling effect, I’m amazed by the hateful tirades from paranoid people who dismiss scientific truth as “dangerous misinformation”.
However, discussions about temperature inversions have occasionally induced more respectful debate with critical thinkers. Most people have observed “frost fans” erected in orchards and vineyards, so are interested in why they work. Frost fans disrupt freezing layers of surface air that can develop at night during the spring, damaging flowers and fruits. Frost fans simply pull warmer layers of air from above down to the surface raising minimum temperatures. But why does that warmer layer of air exist?
During the day, earth’s surface absorbs both solar radiation and the downward infrared heat emitted from greenhouse gases. Absorbing that energy faster than it can emit infrared back towards space, the surface warms. However sunlight doesn’t heat the lower atmosphere (aka troposphere) directly. Nitrogen, oxygen and argon comprise ~ 99% percent of our atmosphere and is transparent to incoming solar energy. Furthermore, unlike greenhouse gases, those gases neither absorb nor emit infrared energy. The troposphere warms primarily by gaining energy via collisions with a heated earth surface. During the day, the warmest air layer lies closest to the heated surface. Rising warm air causes turbulent mixing and collisions with cooler air above that raises air temperatures there. However because air cools as it rises due to decreasing air pressure, warming is limited.
Without solar heating, earth’s surface cools by emitting more infrared heat than it absorbs from recycled heat emitted by greenhouse gases because greenhouse gases don’t intercept all emitted heat. “Atmospheric windows” allow about 23% of the surface heat to escape directly to space without being recycled. The air layer closest to the surface then cools by transferring heat to the colder surface. However, higher air layers can’t sink and collide with the surface again unless they lose their heat. But nitrogen, oxygen and argon can only shed that energy by colliding with cooler greenhouse gases which will absorb their energy and emit half back toward space.
Because the bulk of our atmosphere only cools by transferring heat to greenhouse gases, a small percentage of greenhouse gasses creates a “cooling chokepoint”. Consequently, the atmosphere sheds energy more slowly than the solid earth that more quickly loses energy via atmospheric windows. This difference in cooling rates creates a warmer layer of air above the cooler surface air and is called a temperature inversion. Now imagine a world without greenhouse gases. Without greenhouse gases nitrogen, oxygen and argon can’t lose enough heat back to space and the atmosphere would keep warming.
Outside the tropics, inversion layers more readily form in winter and spring. The earth’s surface holds less heat during winter’s reduced solar heating. Where people use fireplaces to stay warm, inversions layer are revealed by rising smoke that suddenly flattens when it encounters the warmer air above. Frost fans work by drawing down warmer air layers to mix with cooler surface layers, and thus protect crops from freezing. Similarly, months of “polar nighttime” cools Antarctica’s interior surfaces to as low as −89.2 °C (−128.6 °F), creating a continent‑wide inversion layer. When above average surface temperatures are periodically reported, it’s often the result of high winds that, like a frost fan, disrupted Antarctica’s inversion layer.

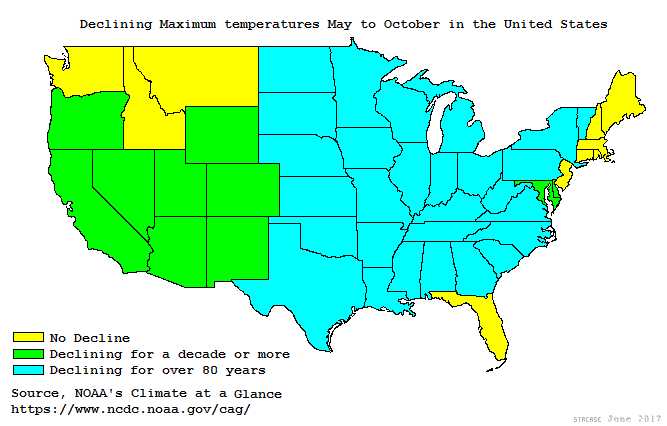
In the 1990s, climate scientists determined urban heat effects raised minimum temperatures several degrees but not maximum temperatures. Such areas weren’t warming but getting less cold. That suggests urbanization disrupted local inversion layers. Increasingly covering the land with heat retaining asphalt and concrete, reduces surface cooling. Removal of vegetation or wetness results in hotter surfaces that store more heat. Traffic, tall buildings or frost fans disrupt surface winds bringing warmer air to the surface. All those dynamics raise minimum temperatures, and thus average temperature. Various local disruptions of inversion layers may better explain why some US weather stations show warming trends while 36% show long term cooling.

Our atmosphere also has a global inversion layer. Above the troposphere, is the warmer stratosphere where temperatures increase with altitude due to absorbing solar UV. Because CO2 in a warmer stratosphere emits infrared faster than it absorbs it from the troposphere, more CO2 cools the stratosphere. (For similar reasons CO2 has a cooling effect in Antarctica.) Furthermore storm clouds bring the tremendous amounts of heat stored in water vapor to the stratosphere. Again we can see where the warm inversion begins as clouds stop rising and develop an anvil shape at the stratosphere. Because the stratosphere is nearly devoid of water, the wavelengths of infrared heat released as water vapor condenses to liquid and ice, mostly pass freely to outer space, without recycling it back to earth.
If these dynamics were better understood, people would more likely laugh at climate catastrophe narratives rather than succumb to paranoia.
Great post Jim. I often bring these issues up during my weather/climate talks down here in The Villages. I often get open-mouthed, silenced stares in response. There’s also hurricanes, which force tremendous amounts of water vapor into high levels for additional cooling … kind of like Mother Nature’s temperature relief values.
I agree, great post! ( Mr Steel has a tendency for great posts)
“Now imagine a world without greenhouse gases. Without greenhouse gases nitrogen, oxygen and argon can’t lose enough heat back to space and the atmosphere would keep warming.”
I often say, GHGs are cooling to conducted from the surface energy, and warming to some of the surface emitted LWIR. I only get blank states, or on blogs a non response.
Although atmospheric energy that is conducted from the surface is not as great as LWIR, it’s residence time in the atmosphere INCREASES as, or if, GHGs are reduced! Indeed, in an atmosphere void of GHGs how does atmospheric energy conducted from the surface leave the earth? Back conduction anyone! Energy can not be destroyed.
Residence time of energy is critical, especially with fairly steady state solar insolation.
So, in a world sans GHGs, the residence time of conducted energy would increase dramatically, and the percentage and total amount of conducted energy in the atmosphere would increase!
Would this increase ( over time) make up for the reduced LWIR energy in the atmosphere?
( Deeply appreciate any answers)
I don’t know? Steve McIntyre has long called for an engineering level break down of the GHE. All of this should be quantified yet AFAIK it is not.
It doesn´t matter if it´s Xenon or co2, if it´s colder than the heat source(surface) it cools. Back-conduction is not possible, neither is backradiation. A heat source can´t heat itself to higher temperature by recycling already emitted energy. If it could, then your pants would catch on fire when you piss in them, from the feedback loop of reabsorbed heat from the piss.
However, as evidenced by Mikey Mann, lying, and lying repeatedly does in fact create such a feedback loop in the pants, that said pants do indeed catch fire.
Dispite your “piss pour” analogy, it depends on the CURRENT temperature of the PREVIOUSLY emitting object.
That being said, I am looking for an explanation of how a non GHG atmosphere cools.
Everything radiates if it is above 0K, therefore non GHG atmospheres must also radiate, just not LWIR.
Mostly it is Microwave.
LIT your arguments about cold regions not being able to heat warm regions is flawed because you conflate conduction of heat ie “Back-conduction” with radiative transfer of heat. A cool object can emit infrared that is added to heat content of a warmer body and reduce its cooling, ie warming
Horse manure
Isn’t it hilarious watching people who don’t understand quantum physics trying to explain how they think radiation works? 🙂
“…Back-conduction is not possible, neither is backradiation. A heat source can´t heat itself to higher temperature by recycling already emitted energy.”
It is sad to see this error repeated on WUWT after so many corrections. Lit, conduction is totally different from radiation. It is correct that a cooler object cannot warm an object with which it has physical contact to a higher temperature. However radiation from <i>anything</i> will slow the heat loss from something at a higher temperature. That is just how radiated energy works.
Are you sure about that, Crispin, and Jim? It is not actually true to say “everything radiates”. Photons are not that simple. More accurately, everything above absolute 0 *could* radiate. But for an atom to radiate a photon, another atom has to be able to accept it, and that just about always means that the emitting atom has to be “hotter” (more energetic, more excited, lower entropy) than the receiving atom. It is extremely unlikely for entropy to go the other way, although nothing is quite impossible in quantum land. Would you like to rephrase your statement in light of this recent news from the world of quantum physics 100 years ago?
I should probably clarify slightly that the rate of radiation from a hotter object to a colder one does depend on how much colder the colder object is. But that’s not because the colder object is radiating back toward the hotter object. That’s not how radiated energy works…
Explain inversion layers.
I think you have it a bit back to front here John. It is the buoyancy of the water vapor/gas carrying with it the Latent Heat which create the hurricanes not the other way round. This buoyancy is very different from convection which requires a temperature differential; but rarely, if ever, gets a mention in the literature.
In the 1980s I had to interview candidates for thermal design positions for a big Silicon Valley company. I found that a good way to find those people who really understood heat transfer and thermodynamics was to ask them what the purpose of those fans in vineyards was. Some who had good grade point averages couldn’t figure it out even with a lot of prompting.
About a decade ago, I drove from the floor of California’s Central Valley to a ski resort in the high Sierras on a cold, clear, still late December evening. When I left the floor of the valley, it was 0C (32F). As I climbed the west side of the Sierras, the temperature steadily increased until it hit 8C (46F) at the top of the pass to the east side at about 2600m (8500ft) elevation. I had not realized until then that the whole Central Valley could be in inversion.
https://en.wikipedia.org/wiki/Tule_fog
This is common in the winter in the mountains in Norway. I have skied in Hemsedal when it was -27C in the bottom of the valley, -17C at the mid-station and -7C at the top of the lifts. We only risked frostbite by returning to the bottom at lunchtime and the end of the day!
Tim …. I see inversions fairly regularly through the winter at my ski hill – Fernie BC Canada. Often it is warmer up top by 5 C or so … I would guess every 4 to 6 weeks we see such an inversion. Never of the magnitude u see however.
I like your posts, Jim. Yet, there is something problematic with this statement.
In fact, air cools as it rises because it must do work against the surrounding air in order to expand, and that work has no source of energy except the internal energy of the air itself.
To say that it cools because the pressure declines is part of the misunderstanding that the ideal gas law controls temperature, and the gas law has temperature proportional to pressure. It looks superficially plausible, but it is the first law of thermodynamics which determines temperature, and the tendency of temperature change.
Also the air in total does not rise. Some rises and cools, balanced by air that descends and warms.
That’s amusing Nick. Where is it claimed the “air in total” rises.Why doesnt the phrase “Rising warm air causes turbulent mixing ” suggest to you there is rising and falling air. Perhaps this pic of a turbulent boundary layer will alleviate your manufactured concern
Your sentence as quoted by Kevin was
“However because air cools as it rises due to decreasing air pressure, warming is limited.”
That clearly conveys that warming is limited by the cooling of rising air.
Air has to cool because it does work as it rises.
True, yet in a local air mass of X many molecules, all vibrating at a certain energy level, that same air mass at a higher elevation will contain fewer molecules of energy, and thus have lower energy per sq-M.
Winter time cold air sinks and stays in the valley where I live. All the productive orchards are up on the plateau catching the warmer air.
Yes, true, but the point here was why air cools as it rises. The descending air has work done on it by the surrounding air, and per the fist law of thermodynamics, its internal energy will rise.
I sould point out that over the long term, one also has to cosider that heat may flow in or out of a parcel; but generally heat transfer is slow compared to rising or falling.
Only over a large area and an extended period of time.
Pressure at the surface decreases, and aloft it increases, as the Sun warms the surface and convection proceeds to warm the air aloft.
And so the two are not exactly in balance in the short term.
There are also zones of rising air from atmospheric low pressure cells, and of descending air from cells of high pressure.
These form partly from imbalances created during convection.
If one wants to get nit picky about it.
I suspect that there are other major problems with this article. Are the statements about nitrogen, oxygen and argon correct, for example (I know they aren’t GHGs, but surely they are able to shed energy other than by “colliding with cooler greenhouse gases”)? I suspect that CO2 does not have any net cooling effect, although there would be negative feedback.
I suspect Mike you are in a state of denial and fishing for “major problems” as you cannot provide even a small problem
Mike, how would negative feedback look in a dynamic, complex system? Look up Ideal Gas Law. Look up Joule-Kelvin effect. Look up Carnot, Stirling and Otto Cycles (clue, not two-wheeled vehicles).
The problem with feedback is that to really characterize it, or theorize it in a system, you’ve really got to be able to describe the rest of the system.
Gases Nitrogen, Oxygen, Argon, CO2, CO, O3, and all known states of H2O are all coolants, under the right NATURAL conditions. Propane, Acetylene, Methane are coolants, too, as are evaporation of gasoline, kerosene, and diesel.
Ethanol is a coolant, else my liver’d be in better shape, as that is what makes distillation possible.
Point here is that there are normal, natural, common circumstances where the cooling does occur and demonstrably affects climate.
Thermodynamics is another good study, but continues to give me a headache years after I gratefully gave it up.
My query addressed the statements about nitrogen, oxygen and argon not being able to shed energy other than by “colliding with cooler greenhouse gases”. I was being very cautious with my wording, because I wasn’t sure of the answer. However, I have now looked it up and In https://www.sciencedirect.com/topics/engineering/radiation-heat-transfer I find:”All physical substances in solid, liquid, or gaseous states can emit energy via a process of electromagnetic radiation because of vibrational and rotational movement of their molecules and atoms. The intensity of such energy flux depends upon the temperature of the body and the nature of its surface. The radiation occurs at all temperatures, with the rate of emission increasing with the temperature.”. So it appears that raising the issue was reasonable – nitrogen, oxygen and argon can indeed shed energy without involving GHGs. This, to my simple mind, negates one of the arguments in the article.
Moving on to “how would negative feedback look in a dynamic, complex system?” – well there are lots of ways in which negative feedback could occur in a dynamic, complex system, and they would have many different looks, but one answer would be in my https://wattsupwiththat.com/2020/06/05/cloud-feedback-if-there-is-any-is-negative/ – not that it seems all that relevent here, but since you asked …..
Please Mike, You were not being cautious, When you say you suspect “major problems” when clearly you are ignorant of the science, comes across more like malicious trolling.
I’ve attached a diagram showing the radiation spectrum from the sun and earth to aid your education. The diagram provides you with an illustration of the important atmospheric gases and the wavelengths where the atmospheric window exists
When you simply quote “radiation occurs at all temperatures” and pretend it supports your bad acting reveals your understanding has “major problems”. Different gas molecules do NOT emit “radiation at all temperatures”. Why would you quote such a misleading over generalization? Simple diatomic atoms like N2 and O2 have little ability to vibrate and rotate the way H2O and CO2 can. Nitrogen only absorbs and emits radiation in the extreme UV range and has no bearing on our climate and the context of this article. Oxygen and ozone react to a wider range of UV, and as I mentioned the stratosphere warms due to that UV absorption. But the issue is again how does tropospheric nitrogen and oxygen shed its energy that is has absorbed by collisions with the earth’s surface. Look it up
Jim,
 &ehk=elDCl2wEq2tG%2fu1zmBuWIZWafiFO4VUEAMEhx4kHARA%3d&risl=&pid=ImgRaw
&ehk=elDCl2wEq2tG%2fu1zmBuWIZWafiFO4VUEAMEhx4kHARA%3d&risl=&pid=ImgRaw
I hesitate to jump in here, but I know what Mike is talking about.
I had the same thought whenever I hear that an atmosphere without triatomic species could not cool itself, because I get to thinking about the cold gas clouds I studied when I was really big into astronomy.
Typically these are studies of ionized gases, but neutral gasses also have emission lines.
I am also familiar with the chart you posted, and have posted it many times myself.
I think that both of you are correct.
Nitrogen is not a significant factor in climate studies of the atmosphere, and nitrogen does have an emissions spectrum:
And this:
“In dry air, the color of produced light (e.g. by lightning) is dominated by the emission lines of nitrogen, yielding the spectrum with primarily blue emission lines. The lines of neutral nitrogen (NI), neutral oxygen (OI), singly ionized nitrogen (NII) and singly ionized oxygen (OII) are the most prominent features of a lightning emission spectrum.[14]
Neutral nitrogen radiates primarily at one line in red part of the spectrum. Ionized nitrogen radiates primarily as a set of lines in blue part of the spectrum.[15] The strongest signals are the 443.3, 444.7, and 463.0 nm lines of singly ionized nitrogen.[16]“
Nicholas,
All this banter about N2 and O2 radiating heat offers nothing but red herrings. Of course everything above absolute zero is capable of radiating some wavelength. But the point of this article is how does our troposphere cool, and at the wavelengths between 4 and 100 microns with which the earth cools, there is no evidence of nitrogen or oxygen radiating away any significant heat whatsoever within those wavelengths.
Those who try to argue otherwise are either mindlessly trolling or completely ignorant of the dynamics that cool the earth.
Maybe this one is better:
Infrared electronic emission spectrum of nitrogen – PubMed (nih.gov)
Again, I do not think it is significant for purposes of climate studies, but just sayin’.
And then there is this, which I have no idea what to think about it:
Scientists: Oxygen & Nitrogen ‘Radiatively Important’ Greenhouse Gases With IR Absorption Temps Similar To CO2 (notrickszone.com)
Since there are people who have disagreements about this aspect, it maybe is not exactly fair to Mr. Jonas.
So then, non GHGs are in fact GHGs? As mentioned, it must be quantified.
What would the atmospheric residence time of conducted surface energy be, in an atmosphere equally dense to earths, assuming no GHGs?
Now add one GHG molecule. If that GHG molecule encounters surface conducted energy and radiates that energy to space, is it cooling instead of warming?
( Reducing the residence time of energy in the system)
If that single GHG molecule encounters surface radiated LWIR, and it redirects that energy toward the surface, is it now warming? ( Increasing the residence time of energy within the system)
So, if the premise of the article is correct, then, as mentioned, it needs an engineering level answer to quantify.
” Only two things can affect the energy content of a system in a radiative balance, either a change in the input, or a change in the residence time of energy within the system.”
“The residence time of energy within the system depends on the spectrum of energy entering it, and the materials encountered.”
At atmospheric temperatures you can neglect the radiation for O2 and N2 as insignificant.. To be precise, at these low temperatures they will emit some photons.
“not that it seems all that relevent here, but since you asked”
But you brought it up. Why, if it wasn’t relevant? I quite agree, which is, by the way, why I asked.
Yes, “colliding with greenhouse gases” is imprecise. Point is that characterizing one or two minor components in a dynamic mixture will not predict the behavior of the mixture. Yes, it was short hand. Yes you did miss the point. Yes, my statement was short hand and probably not precisely what was intended.
No, I do not think that the behavior of any individual component, or non GHGs in concert, negates the argument in the article. We can probably clarify it, but it doesn’t negate the point, at all.
I really think that what you are describing as negative feedback may indeed be the point the author was trying to make, it may be that you are using different language than I would for the same phenomena. So again, why did you bring it up if you didn’t think negative feedback was relevant?
dk_ – I’m trying to be very cautious while still asking the questions that arise. My suspicion is that CO2 does not generally actually cool, and that where the article claims cooling it is actually finding a negative feedback. This in turn suggests less warming than there might otherwise be, rather than a net cooling. It’s only a suspicion, which is why I ask a question rather than assert.
On the feedback topic: I am very satisfied that there are important negative feedbacks that have been ignored or misconstrued by the IPCC, so I am very open to the idea of negative feedbacks. I was asked what I thought a negative feedback looked like, in what I may have misconstrued as in a somewhat aggressive way, so I thought it best to give an example, but obviously there can be any number of other feedbacks.
Jim is correct here. If the “wings” of the absorption profiles of N2 and O2 were wide enough there would be some radiative transfer due to doppler and pressure broadening, but I am sure the wings are not nearly wide enough for this.
This topic of width of the wings of the absorption spectra is one of the major uncertainties in the amount of warming to expect for increasing CO2 — this is one of the points that Happer and Wijngaarten are trying to make; that the width of the Vogt profile is too wide to be realistic.
Mike,
I found this article that seems to include all of the pertinent details, starting from the observation that all substances emit radiation, and through consideration of each constituent of the atmosphere:
This:
“Radiation is energy transmitted by electromagnetic waves. All objects emit radiation.”
But also this:
“More generally, molecules that can acquire a charge asymmetry by stretching or flexing (CO2, H2O, N2O, O3, hydrocarbons…) are greenhouse gases; molecules that cannot acquire charge asymmetry by flexing or stretching (N2, O2, H2) are not greenhouse gases. Atomic gases such as the noble gases have no dipole moment and hence no greenhouse properties. Examining the composition of the Earth’s atmosphere.”
Note I also posted an article from No Tricks Zone about this subject.
One can readily find sources of info like this next article, but one also has to consider emission intensity:
Emission spectra from ArXe, ArKr, ArN2, ArCH4, ArCO2 and XeN2 gas scintillation proportional counters – ScienceDirect
CHAPTER 7. THE GREENHOUSE EFFECT (harvard.edu)
Nicholas – Thanks. I’m not trying to claim that N2 etc are GHGs. The part of the article that seemed wrong was “nitrogen, oxygen and argon can only shed that energy by colliding with cooler greenhouse gases”. It seemed to me that nitrogen etc could lose energy without a greenhouse gas being present, and therefore the cooling effect being claimed for greenhouse gases was, in this particular instance, dubious.
What is dubious is your sincerity oir intelligence. What needs to be shown to prove “nitrogen, oxygen and argon can only shed that energy by colliding with cooler greenhouse gases” is to show that nitrogen, oxygen and argon emit energy in the 4 to 20 microns which are the wavelength where the earth releases infrared.
Claiming N2 radiates energy at say 0.1 micron is a ridiculous strawman argument with no bearing on the earth’s climate
The question of whether greenhouse warming vs cooling results in net cooling or warming is an important one to evaluate. If you look at the energy budget from Stephens in the illustration here, they estimate there is a net addition of 0.6 W/m2. But if you look at the estimates of sensible and latent heat which is relevant to this article , you see that the uncertainty estimates are +/- 7 and 10 W/m2 which strongly indicates that the question of net warming vs cooling is far from settled
Jim – I agree that the question of net warming vs cooling is far from settled, and that the IPCC and others have tended to ignore the fact that the error bars are wider than their claimed warming.
My perception is that the ‘raw’ ECS of CO2 is about 1, and that both cloud feedback (as per my WUWT article which I have cited here) and water vapour feedback are quite likely to be negative. This would end up giving an ECS of somewhat less than 1. My understanding also is that negative feedbacks beyond -1 are extremely unusual, and are therefore unlikely in this case. So my expectation was that while ECS could be a lot less than as claimed by the IPCC, and probably below 1, nevertheless it would still be positive. I therefore read your article with great interest, but I felt that your case for a negative ECS (CO2 net cooling) did not feel quite right. As I read it, you are not actually claiming high negative feedback, but that there is cooling from GHGs that outweigh their warming effect. I’m happy to keep an open mind – thank goodness for WUWT and the way it supports open minds – but I am yet to be persuaded.
MIke, You misread me saying “As I read it, you are not actually claiming high negative feedback, but that there is cooling from GHGs that outweigh their warming effect.”
All Ive shown is greenhouse gases have a cooling effect. Ive said nothing about the level of negative feedbacks, nor claimed the cooling effects outweigh the warming effects. I only argue that by overlooking the cooling effect, alarmists are misled to believing there aree grave warming dangers, and that it is possible that cooling will offset warming, but that conclusin is definitely unsettled
Hmmm. Kevin are you overturning the gas laws? That’s impressive since scientific laws become laws after rigorous testing.
Indeed expanding gases do work dependent on internal energy, but that doe not negate the gas law, just describes its effect in different terms.
I am not negating the gas law, Jim, but only pointing out that you cannot determine temperature from it alone because the specific volume, or the density of the gas is also involved in the ideal gas law. Thus you need one additional law to determine this third variable, and that law usually concerns how heat enters or leaves the gas…if no heat leaves or enters, then the process is called adiabatic and the “other” relationship is PV to the gamma power = constant. Between the two you can now determine temperature.
There is such a thing as isothermal compression. Heat leaves the gas in exactly the amount that work enters it during compression, leaving the temperature unchanged. If the ideal gas law alone were all there is to it, then how could you explain this?
The point I am trying to make is that there are three variables in the ideal gas law, P, T, V; the gas law is a relationship among them, but you must have at least two relationships to pin all three down. Part of the problem is the law is usually written as an “implicit” function. Since P and T are the only ways we have of influencing the relationship, it would be better to treat them as the independent variables and write it as V=nR(T/P) I suppose, but this is not the tradition.
I taught engineering thermodynamics either as an adjunct or as a professor for 25 years, and the number one misconception among students is that the ideal gas law is the most important concept in thermodynamics, and that one could always use it to find T or P or V. They will even employ it on a liquid system. Most carry this misconception after graduation unless they have to work with fluid/thermal systems.
Kevin, I understand and agree with the details you present. My statement that the air cools as it rises due to lower pressure was a simplistic statement to address any layperson’s question of why is it colder at higher altitudes. For the point of this article, which I limited to about 800 words for a newspaper, I deliberately avoided going deep into the weeds of the gas laws. It was beside the point being made and would likely overwhelm most laypeople.
Here at WUWT I expect knowledgeable people to add those details and welcome it. But I object to your introduction of those details by saying “there is something problematic with this statement” as if I was misleading the reader, when the statement “air cools as it rises due to lower pressure” remains valid albeit superficial.
Well, I am sorry that it appeared I was challenging you, because I think your articles are great. Yet there is a lot of misunderstanding of the limitations of PV=nRT here (think about the N&Z paper), and they are the same as I observed teaching thermo. So, I keep trying to find a way of explaining this.
Kevin, the fact that gasses cool as they expand and warm as they are compressed is an observational fact and went into the development of the laws of thermodynamics. The ideal gas law is used in conjunction with the first law to calculate how much warming and cooling will be observed in an adiabatic system. We could use a real gas law (a more accurate one) to calculate this but the atmosphere is very close to an ideal gas, so PV=nRT works well enough for anything less than launching a rocket. Thermodynamics gives you the integral to evaluate, the gas law you choose gives you the relationship between T, P, and V that goes into the integral. For the ideal gas relationship, from this integral we get a very simple equation PV^gamma = constant where gamma is the ratio of the heat capacity of the gas at constant pressure and the heat capacity of the gas at constant volume. Wikipedia has a very good article explaining the process and setting up the integral, and then solving it for the ideal gas assumption. The authors of various other equations of state usually provide the same in their papers, or leave that to us poor thermo guys to work out.
PV to the gamma = constant power law comes from the ideal gas law plus the assumption of an isentropic process (adiabatic process). You cannot derive it from the ideal gas law, or even the real gas law, alone.
If you read what I said, I stated that. The first law of thermodynamics specifies the process and the differential equation to be solved. The ideal gas law supplies the relationship between P, T, and V.
Not the first law, but the second, Loren.
I live in the mountains. Our potato chip chip bags are swollen pillows due to reduced air pressure. Air cools as it rises because the heat contained in a volume of air doesn’t increase but the area or volume it inhabits does. The same amount of heat in a larger volume of air results in a lower temperature. The opposite happens when air is compressed, that is why air compressors have cooling fins.
I live in the mountains too. In fact yesterday a bunch of gradeschoolers sent a ballon to 100,000 feet. They attached a cheetos bag to the balloon. It swelled so much that the bag exploded and sent Cheetos over a large area below.
The air being compressed by a mechanical compressor becomes warm because the compressor is doing work on the air far faster than heat flow can flow out of the air to lower its the temperature. Thus we put fins on the compressor to conduct heat into the environment faster. This results in less mechanical work needed to reach a target pressure.
Wait a second…fins cool the compressor, which results in cooler compressed air.
I think those fins are there to keep the compressor from overheating.
Because if the air being compressed is heated and compressed, it will be under even more pressure.
That compressed air will cool and then be under less pressure as it moves through hoses or pipes away from the compressor, true.
So it is both. You need to keep the machine from melting.
Molecules of air moving faster are exerting more force on the container they are in.
It does matter how the compressor works, and whether the heat will prevent as much air from entering the compressor on each cycle to begin with.
Not all compressor designs have this limitation though.
But even compressors operatizing at relatively low pressures will become extremely hot if there is no way for them to dissipate heat, and mostly machines do not last as long when they become extremely hot.
Most especially electric motors, which many compressors have.
My Dad who was a specialist in liquifying helium showed quite clearly how it works.
You first compress the helium, and cool it down (like a fridge does thru a big heat sink) as you put it through the compressor, then you feed it thru a “reverse compressor” so that the pressurised gas does work on the pistons of the compressor.
Hey presto after a good deal of extra work being done by the gas, you drop to nearly absolute zero kelvin and it sloshes around all over the inside of the container.
(superfluidity takes over).
Do work on something you expand it and it gets cooler.
Lots of people just don’t get the connection of doing work with a gas makes it cooler and/or expand.
That is also how force air cooling of F1 car’s exhausts works, which is why they are never taken out of the forced air cooling airstream.
(It delivers more and more power the faster it goes, because the temperature gradient changes)..CQFD
Next time, instead of Cheetos, they should attach a law chair with a department store manakin sitting in it, holding a six pack of beer.
Keep from wasting a whole bag of perfectly good snack food.
“In fact, air cools as it rises because it must do work against the surrounding air…”
When a parcel of air rises, it expands because it moves to an environment with less pressure surrounding it.
It does not really matter how one describes it.
The end result is the same: The parcel that has expanded is now occupying a larger volume, and yet it has the same amount of internal energy. So it will be cooler.
Another way of understanding it is regarding the density. An expanded parcel of air has less molecules per cubic meter, and so if they are all still moving at the same velocities they had before they rose and expanded, the measured temperature will be lower, because
“The temperature in kelvins can be defined as the pressure in pascals of one mole of gas in a container of one cubic meter, divided by the gas constant.”
It is all different ways of saying the same thing.
If one has a gas in a container, there will be more molecules per square inch impinging on the walls of the container if you put more molecules into the container, or if you decrease the size of the container. In either case, one will measure the container as having become hotter. The reverse is also true, obviously.
Whether one takes a thermodynamic approach to the situation, or a kinetic theory approach, one is describing he same thing.
It is not true to say one approach is wrong, because one chooses to look at the situation using the other approach.
In reality, the rising parcel has things going on that complicate the situation greatly. There is water vapor that at some point will condense and release the energy that was absorbed when the gas evaporated, which rather than sensible heat was in the form of latent energy, but becomes sensible heat when it is released.
And because the air is rising through layers that are necessarily colder than the rising parcel (or else it would stop rising! All else being equal of course, such as both the parcel and the environment it is rising into having the same dew point, which is probably not exactly true very often. Moister air is also less dense than dryer air at the same temperature.), it is losing energy to the surrounding air the whole time.
It is losing energy by radiation, and by conduction, and by mixing with surrounding air.
So, even though convection of air parcels is often described as an adiabatic process, it is actually not one, although it can be modelled fairly well by assuming the process to be approximately adiabatic.
But it is the fact that energy is being passed to the surrounding air that lets convection gradually warm the air in the convective zone as the day progresses.
Which is I think what Jim Steele was saying.
As always, wonderful authentic science Jim!
The US Cornbelt/Midwest has had cooling Summer temperatures the last couple of decades(especially with lower afternoon temperatures) because of the tightly packed rows of corn, now twice as dense as they were 30 years ago.
This has created a cooler, more humid microclimate over close to a dozen states during the 3 hottest months of the year. Mainly with regards to afternoon temperatures. Besides the cooling affects on the near ground level temperatures form the corn, once it reaches the vegetative state the billions of rapidly growing plants transpire massive amounts of moisture into the air. This can increase dew points by over 5 degrees F in some corn fields. That increasing low level moisture reduces afternoon temperatures and acts a source to increase rainfall substantially.
A positive moisture feedback sets up with the evapotranspiration causing more rains, after they were returned to the atmosphere from the increased evapotranspiration, which happened after they have been absorbed into the top soil/are taken up by the corn plants.
Interesting item to report not related to this affect. For years, I’ve been passing along the article below to people to show them how long the scary predictions of a climate apocalypse have been going on…….well over 3 decades.
Note…………no more title for this article below. They took off the title and date so that it would not show up in searches as easily. I suspect the article itself may soon disappear too.
I added the original headline and date that was at the top of the original article for decades below that…..the last time that it was on there back in February.
https://apnews.com/article/bd45c372caf118ec99964ea547880cd0
Re: Re: Re: 50 years of Climate Forecasts
By metmike – Feb. 21, 2021, 11:31 p.m.
U.N. Predicts Disaster if Global Warming Not CheckedPETER JAMES SPIELMANN June 29, 1989
https://www.marketforum.com/forum/topic/65828/#65870
The US Cornbelt/Midwest has had cooling Summer temperatures the last couple of decades(especially with lower afternoon temperatures) because of the tightly packed rows of corn, now twice as dense as they were 30 years ago.
Interesting. That may go a long way to explaining this graphic that I’ve slapped up here at WUWT over the years:
Here in Milwaukee summer afternoons are obviously cooler. There have been recent years where it never got above 90°F.

Steve,
Your graphic/data confirms this wonderful affect and suggests that this micro climate actually changes the air mass enough over a large enough area, so that it’s transport downstream to areas outside of high crop production.
IIRC in corn (maize) fields by late afternoon much of the CO2 in the immediate area of the field has been absorbed by the crop.
Would this be significant enough to have an effect on the temperature balance and the observed effect in the late afternoon?
Stephan,
i never thought about the affect from lower CO2 because of tens of millions of acres of tightly packed field corn. Good question. Maybe it contributes slightly? Other thoughts please. Not sure what the CO2 would plunge to in the afternoon in a cornfield. With certainty, the moisture dynamic described above is measured and well documted, along with the affects.
I think this effect of using up all the CO2 requires the air be still.
Usually there is wind and convection.
But, it just can’t be! If increasing CO2 had a significant cooling effect on anything in the atmosphere, the conventional followers of Arrhenius’ global greenhouse theory would just have that perfectly well ‘cased’, right? Stratosphere or no stratosphere, any such annoyingly minor cooling effect would be thoroughly worked out, nothing much to see here — or would it?
Well, seriously, this seems to be all of a piece with Willis Eschenbach’s recent non conventional treatment of ground warming effect vs assumed back radiation from any CO2 increase in the troposphere, as such. If, as the head poster says here, storm clouds connect with the troposphere and release heat into outer space from there, with both CO2 and water vapor causing cooling, that’s the same as what Eschenbach was saying in his post “Surface Response to Increased Forcing https://wattsupwiththat.com/2021/05/05/surface-response-to-increased-forcing/ ?
We end up globally with a predicted 1/3 of a degree C warming due to any assumed doubling of the CO2, such a small amount that who could ever really confirm it.
…. which is probably why it has never been confirmed.
David Blenkinsop – I think that what you refer to as “both CO2 and water vapor causing cooling” is not a net cooling but just a negative feedback to their warming:
An example of why the effect of the 40% increase in CO2 we have already experienced can not be seen or felt.
Some numerical values of the Earth’s energy balance are not up to date in this story. I refer to two energy balance representations that have almost identical energy flux numbers, namely Stephens et al. (2018) and to my own balance figures:
http://iacweb.ethz.ch/doc/publications/StephensLiWild_etal_NatureGeoscience.pdf
https://www.climatexam.com/single-post/the-six-definitions-of-the-greenhouse-effect
Quote: “Nitrogen, oxygen and argon comprise ~ 99% percent of our atmosphere and is transparent to incoming solar energy.” This is true but these gases do not absorb the outgoing longwave radiation either. It is a common claim that the atmosphere is transparent to incoming solar radiation, but it is not. The GH gases and clouds absorb 75 W/m2 which is 31% of incoming solar energy.
Quote: “Atmospheric windows” allow about 23% of the surface heat to escape directly to space without being recycled.”. The 23% would mean an LW flux of about 90 W/m2. This figure cannot be found in any energy balance presentation. According to Kiehl & Trenberth, this flux is 40 W/m2 but is an ad hoc number not based on any spectral calculations. The correct number can be found in the two referred studies and it is about 26-28 W/m2 meaning a 7 % portion.
Quote: “Because CO2 in a warmer stratosphere emits infrared faster than it absorbs it from the troposphere, more CO2 cools the stratosphere. (For similar reasons CO2 has a cooling effect in Antarctica.” Firstly, I do not know any research study showing that CO2 has a cooling effect in Antarctica, and therefore a proper reference is needed here. CO2 is a very strong absorber in its wavelength zone from 12 to 18 micrometers. It means that the absorption by CO2 is completed below 1 km altitude. It does not help that the CO2 concentration is almost constant up to 80 kilometers. When the emitted radiation has been absorbed, there is none to be absorbed anymore.
The stratosphere has really been cooling but the cooling has stopped around 2000 and now it is slightly warming, thanks to elevated ozone concentration: https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2018JD028901
The cooling effect of increased CO2 concentration is mainly due to the fact that CO2 absorption wavelength zone slightly expands leaving less energy to water vapor in the stratosphere and the result is a very insignificant cooling.
“The stratosphere has really been cooling but the cooling has stopped around 2000 and now it is slightly warming, thanks to elevated ozone concentration: “
UAH AMSU data does NOT support that statement.
anomalies from Dr Roy Spencer’s UAH data set.
year 2000: TLS -0.054 ºC
year 2020: TLS -1.62 ºC
A full degree cooler in 20 years.
I checked tha UAH data from here: https://www.nsstc.uah.edu/data/msu/v6.0/tls/uahncdc_ls_6.0.txt
I got the 2020 temperature to be -0.11 C and 2020 to be -0.135 C meaning a slight cooling of 0.025 C.
I read the referred article, which data they have used. They have used a large set of radiosonde data and MSU satellite data. Quote from the study: “We use MSU Channel 4 data from 1979 to 1998 and AMSU Channel
9 data from 1998 to 2015. These channels provide information on the temperature
of the lower stratosphere (TLS). Advanced Microwave Sounding Unit‐A (AMSUA) Channel 10 data from 1998 to 2015 are also used. While some regions also show slight warming trends in satellite TLS data during the period from 2000 to 2015, the satellite observations generally exhibit considerably less warming than the weighted radiosonde measurements.
Just an addition. The referred article of Philipona et al. has very convincing graphs that radiosonde data and satellite data show almost exactly the same pattern of temperature trends.
Your original statement of “slightly warming” of global stratosphere is not supported… no matter how one analyzes the AMSU data.
I was using the TLS (Temperature of Lower Stratosphere) not the whole stratosphere. The TLS is where the reverse GHGE should be most pronounced if the theory is correct.
It strongly supported by the scientific research article which I referred to.
So that means that radiosonde data does *not* show the same pattern as bogus Hockey Stick charts.
So which one is wrong? The radiosonde data, which matches the satellite data, or the Hockey Stick chart data that doesn’t match anything?
Here you go on one Antarctica publication:
https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/2015GL066749
This was originally published in a Ph.D. thesis a few years earlier, but it didn’t fit the sea level rise narrative, so it was suppressed by the climate liars.
…… but the high mountains of Antarctica are not in the stratosphere. Can someone Jim or Antero educate me on this point please? Also, there should be a parallel situation in the Himalayas, which I’ve never seen mentioned
At the South Pole, the Amundsen Station is at 9,300 feet elevation. Because the tropopause is latitude dependent in height, it is much closer to the surface there at 90S. Hence the reverse GHGE was measurable over time.
Mt Everest in the Himalayas is at 27.9881° N. The tropopause is always far above that point, and no one can stay there very long to do any science unlike Amundsen Station.
In polar winter nights, the stratosphere can basically come down to the surface. More so in the Antarctic, thanks to higher elevation.
Antarctica has extreme cold temperature for a few reasons such the blocking of poleward heat by the Antarctic Circumpolar Current as well as its elevation. The cooling by CO2 that I referred to, can be seen in the last graph from a paper Will Happer co-authored and attached here.
The red dashed line indicates the expected emitted infrared radiation for the stated surface temperature. The black “curves” represent emitted IR based back to space based on modeled satellite data. It can be seen that instead of CO2 absorbing and recycling infrared at the wavelengths it normally does in the Sahara or Mediterranean, CO2 helps radiate more heat back to space. This is because in a inversion layer the warmer upper layer emits infrared faster than it absorbs it from the colder surface. That warmth in the upper layer is, at least in part, from heat absorbed further north in the Southern Ocean.
Antero,
Why are you twisting my statements
You wrote “Quote: “Nitrogen, oxygen and argon comprise ~ 99% percent of our atmosphere and is transparent to incoming solar energy.” This is true but these gases do not absorb the outgoing longwave radiation either. It is a common claim that the atmosphere is transparent to incoming solar radiation, but it is not. The GH gases and clouds absorb 75 W/m2 which is 31% of incoming solar energy.”
My very next sentence states “Furthermore, unlike greenhouse gases, those gases neither absorb nor emit infrared energy.” but you act as if you are making a correction by stating “but these gases do not absorb the outgoing longwave radiation either”
I was stating nitrogen, oxygen and argon are transparent to incoming solar and that 99% of the atmosphere requires collisions to gain energy. Yes water vapor and clouds do absorb incoming solar, but that was beside the poind.
Jim,
I did not twist anything. I wanted to make it sure that somebody could misunderstand your wording.
“Nitrogen, oxygen and argon comprise ~ 99% percent of our atmosphere and is transparent to incoming solar energy.” This is true but these gases do not absorb the outgoing longwave radiation either.”
Not directly, yet they do receive it via collisions with GHGs. Also they receive energy via surface conducted energy, as Mr Steels post articulates.
With a surface emissivity ~0.91 both are pretty inaccurate. Surface emissions are neither 398, nor 395, but only about 355W/m2.
Is nitrogen really a bigger factor than co2?
Your question is not fully formed (I think), but maybe this will help:
From the text:
“Because the bulk of our atmosphere only cools by transferring heat to greenhouse gases, a small percentage of greenhouse gasses creates a “cooling chokepoint”. Consequently, the atmosphere sheds energy more slowly than . . .”
Nitrogen makes up 78.08 percent by volume in dry air, and that makes it the most common gas in the atmosphere. In the context of this post, Nitrogen is important in cooling.
Rightly or wrong, most folks think of CO2 as the warming gas.
And more importantly is water vapor. Which when it condenses to clouds has massive surface area and radiates heat to space.
You are correct that the question is incomplete. I was listening to a podcast that was talking about climate. They noted that a change in co2 affected nitrogen which affected oxygen levels.
I was hoping someone here would just say yes or no so I could file that tidbit away for now and do more research later.
Ask an elf.
How about that? My second Hobbit reference today.
Water vapour is a net cooling agent in the atmosphere. Albedo trumps absorption and re-emission. Attached shows how the outgoing energy, both shortwave reflected and long wave, vary with atmospheric water.
Water in the form of ice in the atmosphere has the most profound impact on the energy balance. It absorbs much of the OLR as you point out (all over tropical oceans) and re-emits at temperature from 273K down to about 220K in the very high cloud. The same cloud reflects a goos portion on the incoming insolation.
The attached charts shows the heat loss in 5mm bands of moisture vapour across the oceans for July 2020. The upward slope corresponds to an energy loss with atmospheric water of 4.2W/sq.m/cm.
The atmospheric water heats and cools. It has a surface regulating function because it actually warms the ocean surface during the cooler months. The AWCC goes negative for November, December and January when the ocean surface is at its coldest.
There is a lag in the regulating response of the atmosphere to the ocean surface by about 1 month. The surface temperature in the attached chart has been moved forward 1 month to give the alignment.
Over the year Aug 2019 to Jul 2020 the average cooling of atmospheric water was 1.4W/sq.m/cm with peak of 4.2 and minimum of -3.3.
How does water vapor phase versus various droplet sizes effect these values? Esp. at different altitudes/pressures.
It is ice above freezing that make the greatest difference. Just 1cm of water that is regularly above the level of freezing the forms dense reflective cloud during the cloudburst stage and whisky reflective cloud during the CAPE phase to build the next cloudburst cycle.
Then there is the high level cloud that persists particularly over the southern ocean as all the water catapulted above the freezing level gradually solidifies as the air moves to high latitudes. Air at the tropics holds 6cm of water but is down to just 1cm by the time it reaches the southern ocean. All the solidification is forming reflective cloud that reduces surface insolation.
For those not familiar with fans in orchards and vineyards, follow the link, then go to Chinook Wind Machines.
https://www.hfhauff.com/
I’ve no connection to any of this technology, but do pass by the facility on the way to a grocery store.
In them olden days, farmers would stack woodpiles in the orchard, and kept lookout on cold nights. The moment conditions for frost arose, they set fire to the piles, which would circulate air through the orchard. The understanding (where I come from) was that frost only occurs in still air, so any mechanism to get the air moving, helped. Of course, the orchard need to be designed for this, otherwise you hurt your trees.
For city folk, frost is not a daily occurrence, so these fires are not lit every night, relax.
I remember a situation in a city where I once lived where there would be heavy frost on the one side of a line of trees and none on the other side. There are explanations for what is observed. Clearly real observations and measurements indicate that there is far more to climate than the models are able to capture.
Many thanks to Jim Steele for a very interesting and informative overview of a topic carefully hidden or ignored by the Alarmists. Together with Willis Eschenbach’s work on Emergent phenomena like thunderstorms and on clouds, it paints a very different picture of how the atmosphere works to the ‘Settled Science”..
Once again
Explained so dummies can understand
Like me
Thanks much
Always learning, the day I don’t is the day I fear is my last
It’s a bad day when you don’t learn something new
You should see the shocked looks when you tell them about R744.
Right! Actually some of the news releases seem to be supressed — 404 on the web. I shouldn’t wonder.
Some very good points but also an error inherited from the alarmists who fail to realise that even for a fully transparent atmosphere there will inevitably be convective overturning leading to cooling with height without any greenhouse gases at all due to the conversion of KE to PE as height is gained.
Such overturning can never be prevented due to uneven surface heating leading to density variations in the horizontal plane.
Furthermore what goes up in one place forces descent in another place and descent leads to warming again which reduces the net amount of radiative cooling of the surface to space beneath descending columns.
There is even a slow large scale overturning in the stratosphere and likely also higher up but the air is so thin in the mesosphere that we have been unable to discern it thus far.
What error are you referring to?
The assumption that there can be no cooling with height in the absence of radiative gases i.e. no lapse rate.
If the atmosphere were to remain static it would be behaving like a solid and would indeed become isothermal via conduction alone.
It cannot remain static due to uneven surface heating creating density variations in the horizontal plane and will therefore obey the gas laws instead so a lapse rate will form as a result of convective overturning even with no GHGs at all.
Potential energy in a gas is stored as temperature and pressure. As the air rises, temperature drops and pressure drops. Where’s the potential energy in that?
As air falls, temperature rises and pressure increases so any KE converted to PE in uplift is returned to KE in descent.
Objects in a gravity field possess what is called gravitational potential energy.
It may be the case that many people do not commonly think of the buoyancy effect of objects immersed in air.
But air is a fluid, and so all objects in the atmosphere are experiencing the effects of buoyancy all the time.
IOW, the apparent weight of all objects is decreased by the weight of the air they displace.
For objects in water, the same is true but the amount of buoyancy is calculated by the product of the volume of the object and the weight of that volume of water.
Any object submerged in water has it’s weight reduced by the weight of the water it displaces.
Toss a brick into a swimming pool, then reach down and pick it up. It will be much easier to lift than when it was not in the water.
So, if an object is less dense than water, it will float right up to the surface.
Punch a hole in a boat to see the potential energy released that it gained when it floated up to the top.
CO2 has only three IR absorption/emission bands that are equivalent to temperatures of 800,400, and -80 deg C. As sunlight comes from a sun at 6000 deg C, sunlight can energize CO2, which then emits IR at these temperatures in all directions. Thus, during daytime, CO2 is saturated with incoming energy and actually waylays some incoming energy by emitting it back to space and thus slightly decreases heating of Earth’s surface. The same would be true of water vapor.
It is during night time that CO2 and water vapor, with no energy source other than the surrounding air, actively converts energy in the air into IR and radiates it in all directions. As the surface is always warmer than the air, downwelling IR is reflected and sent off into space. It all ends up in space. This is why the air chills so rapidly after sundown and breezes kick up so quickly in the moving shadows of scudding clouds on a sunny day—that’s how radiative these gases are.
Greenhouse gases do not exist, they are radiative gases. CO2 turns out to be a wonderful, cheap, and nontoxic refrigerant. Left alone, it is radiating IR at -80 deg C and is thus constantly trying to cool its environment.
Here’s a way to think about it: E = K * (Th^4 – Tc^4). Separate the terms:
E = KTh^4 – KTc^4. The net radiance is reduced with the green house gas. Yes, the radiance of the hot body is not effected by the green house gas, but it does receive thermal photons and the NET overall balance decreases. When the temperatures are the same, the NET radiance is zero. You also have to adjust for optical depth and the fact that the gas only radiates <50% to the surface due to geometry, which is where the Climate Alarmists get into trouble, especially with water vapor/clouds.
Expanding on lee’s post. In about 2013, German automakers combined to start developing CO2. Classed as a refrigerant, it is designated as R744. BMW was first to market with a system, if I remember correctly, in about 2015 model year.
Weirdly, although a search turns up links to news announcements on the deployment, many seem to be supressed, showing for my search as “Error 404”– meaning they were really there when the search engine indexed them, but are very recently removed. Hmmm.
Having had to use CO2 fire extinquishers, unfortunately several times, and having cooled mechanical devices and tools using compressed CO2, and frost burned unprotected skin in both cases, I’m still amazed at what a pressure-density change can do in a “hothouse” (Saint Svante, not me) gas.
You can do this with compressed air or compressed oxygen, or nitrogen, too. With industrial gasses, a user can easily harm themselves from the cold, as wells as from the high-velocity gas.
It also works with propane tanks. Google “Ideal Gas Law.” Fascinating. Apparently gas interactions over a range of pressures, velocity, and temperatures is very complex.
Okay, yes, I knew. It is elementary physics and covered in many fields of study, including engineering. But still complex.
CO2 is also a coolant, sometimes, and the science is very, very settled on this.
An expansion on Lee’s great note, above. R744 is CO2 as coolant. German car manufacturers developed it about 8 years ago, and started delivering it in new models about six years ago.
Do a wiki search on “ideal gas law.” Quite complex, fascinating stuff.
Apologies for sort of dupe entries and repetition, I was interrupted by a browser update, and thought the originals lost. Ah jist keep a hittin this here ‘puter, and stuff keeps ona hapnin.
CO2 refrigerant is older than 8 years.
Around 2003 or 2004, the first supermarkets got CO2 cooling in Germany, and under the respective parket places of the markets was the place for the liquifiers.
Linde Cooling, now Carrier, was the leading enterprise.
All valves compressors etc had to be redesigned. There was a longer test phase and a completely different and centralised spare parts distribution for these projects.
I didn’t know that. Good info Thanks.
There was an old comedy, set in WWII Pacific, that included a short gag where Bob Hope’s character uses a CO2 fire extinguisher to chill canned beer. I assure you that it works.
Wonderful post.
“Similarly, months of “polar nighttime” cools Antarctica’s interior surfaces to as low as −89.2 °C (−128.6 °F), creating a continent‑wide inversion layer.”
From wikipedia table properties of CO2
“Sublimation Conditions: −78.5 °C (−109.3 °F); 194.7 K (1 atm (0.10 MPa))”
Paging Tony Heller. You can come in now.
“Why do orange growers spray their crops with water [to prevent] freezing?“
Water has a higher heat capacity, thus water can provide more energy that helps prevent freezing.This has been a common practice but frost fans are becoming more popular
Yup. Follow the link. It is actually quite good, but I think you summarized it pretty closely.
A Great Uncle (yes he was a great person, and a war hero, but also the Father of the guy who married my Aunt) showed me this in his orange grove in a freezing April (?) in Southern Florida (?) in the 1970s.
Oranges will not be damaged unless the temp is below about 28° F for four hours or more.
Using water does several things.
First, water from the ground in Florida is 70-74° F.
And when water freezes, it releases the latent heat of fusion.
But most importantly, as long as the water stays on, it can be recalled from basic physics that a mixture of ice and water will always stay at 32° F, until either all the ice melts, or all the water freezes.
This is why it is so important to keep the water on in the morning when it warms up, until all the ice has melted.
Not just until the temp rises about freezing.
Because if the water is turned off, one thing that occurs is that the melting ice sucks thermal energy from the leaves and wood…the same heat of fusion it released when it froze.
Orange trees I should have said will generally not be damaged unless it is below 28 for more than four hours.
There can be some fruit damage, which is why growers pick as much as they can ahead of any freeze.
Also no one is ever sure exactly how cold it will get and for how long.
I know this is a touchy subject but here it is again in Roy Clark’s excellent book “The Dynamic Greenhouse Effect and the Climate averaging Paradox”, on the true solar flux.
“The static, average energy balance diagrams of the Earth’s radiation budget such as those published by the IPCC conceal the dynamic aspects of the energy transfer and imply a nonexistent climate state. The sun only heats the surface during the day. To illustrate the time dependent, dynamic nature of the energy transfer, the energy balance has to be separated into an average 12 hour convective cycle and an average 24 hour LWIR emission cycle. The 12 hour averages for the solar flux are just twice the static IPCC averages. The net average solar flux reaching the surface is 336 Wm2 in 12 hours. This heats the surface and drives the daytime moist convection. The convection then heats the two atmospheric thermal reservoirs. The lower reservoir, the first 1 to 2 km layer of the troposphere provides almost all of the downward LWIR flux at the surface. It acts as a ‘thermal blanket’ and is
not strongly coupled to the upper atmospheric thermal reservoir. It is the thermal storage in this layer that provides the ‘greenhouse effect’.
“Furthermore, unlike greenhouse gases, those gases neither absorb nor emit infrared energy”
Great post Jim but I’ll be a pedant and question the above sentence. Non radiative gases may be transparent to infrared, but they still radiate according to their temperature. A translucent sphere, filled with non radiative gas at room temperature and floating in outer space will radiate energy. If you’re standing next to it it will warm you, but 2 adjacent spheres won’t warm each other.
Gases are not black bodies and radiate at certain wavelengths. You admitted you were being pendantic, so yes, the sphere you describe will radiate at room temperature in certain bands. I believe the major band is UV, and at room temperature the radiance is minuscule.
I disagree James. I think the gas will emit radiation as a black body at room temperature. The kinetic energy of the molecules will decrease as the temperature within the sphere drops.
To argue gases emit radiation as a black body is to deny all observations that unequivocally show each gas species only absorbs and emits specific wavelengths.Changes in temperature only modify the intensity
Wouldn’t containing the gas in the translucent container create a black body?
Say the gas is Argon, that will not emit at any wavelength when at room temperature. The only way the gas can cool is by contact with the wall of the container, this material will emit as a black (or grey) body and so cool off thus cooling the gas by conduction.
<em> I think the gas will emit radiation as a black body at room temperature.</em>
No it will not, to emit radiation at around room temperature, the molecules would have to undergo either vibrational or rotational transitions. In order to do that they need a dipole, since N2 and O2 are linear molecules they don’t have one and therefore don’t emit.
Isn’t it a basic law of physics that everything radiates at its measured temperature? Wouldn’t the molecular collisions cause dipole movements ? It seems hard to believe that the gas would maintain it’s measured temperature.
Yes.
All gasses have characteristic emission spectra.
Nitrogen gas, N2, has a strong emission line in the red band of visible light.
All atoms and molecules emit radiation.
The relevant issue for the subject at hand is the relative intensity, and whether or not it is trivial at the relevant temperatures.
Even cold clouds of neutral gas in space have emission/absorption lines.
This is how we know what they are made of, after all.
Note the emission and absorption are at the same wavelengths.
And this is what Jim Steele is talking about in this article.
CO2 absorbs certain wavelengths, and also emits them.
Just as a CO2 molecule that absorbs a IR photon can thermalize this energy by a subsequent collision, prior to reemitting it, and thus warming the surround gas molecules, the reverse process also occurs. CO2 can be excited to a high energy state by collisions. All gasses at any temperature have some molecules moving slower than the average velocity, and some moving faster than the average velocity, all the time.
And so CO2 and other radiative gasses (what I prefer to call the so-called “greenhouse gasses”) can and are excited to a high energy state by collisions and emit IR radiation before the energy can be dissipated by subsequent collisions.
This is one of the ways air cools off at night.
Some other relevant facts are, gas molecules move very fast at Earthly temperatures (about 500 meters/second), there is not much space between them (~3.4 nm at STP)and so collisions are “frequent”(to put it mildly), and photons travel at the speed of light. At the speed of light (which is slower in air than in a vacuum but still very fast, about c – 90km/sec), a photon will travel from the surface of the Earth, to 100 miles up, and back again, about 930 times in one second.
BTW,
-500 meters per second is about 1000 miles per hour…twice as fast as a cruising passenger jet.
-100 miles up is an altitude generally regarded as outer space.
62 miles is the conventional altitude taken to be the start of outer space. 76 miles is the altitude NASA considers the “re-entry” altitude, also called the entry interface.
Now, 930 times in one second means roughly one millisecond, for a photon to go up to 100 miles and turn right around and come back to the surface.
It occurs to me this is a amount of time that most people have no intuitive grasp of, and so it might be helpful to try to think of some familiar process that takes a comparable amount of time.
It is listed that a strobe flash is about a millisecond.
The time for a housefly to flap it’s wings once is about 3 milliseconds, which is not especially helpful.
3.3 milliseconds is the time from initiation to detonation of C4 explosives.
Maybe the most helpful is that a human eye blink is about 150 milliseconds.
So, a photon makes the round trip 150 times in an eye blink.
Which is kind of right back where we started…to fast to fully appreciate.
A strobe flash is so fast, how long we perceive it for, is likely more to do with the limits of perception and thoughts, than the time it takes for the flash.
Here is one that helps: A standard shutter speed for a camera, 125, is 8 milliseconds.
It takes of the order of a millisecond for a vibrationally excited CO2 molecule to emit a photon and thus lose that energy. At atmospheric pressure that same molecule will have a collision with a neighboring molecule about every 100 picoseconds, so millions of collisions before it can emit a photon.
Yes, I am familiar with all the usual calculations that have been bandied about.
By atmospheric pressure I take you to be referring to sea level pressure, and of course as the pressure changes, so to does this calculated result. And yet, even at sea level, CO2 does seem to be emitting some IR.
It does not go far, but all these things happen over and over again very fast.
There are so many collisions, and things happening so fast, and so many molecules, that even rare events are occurring with head spinning frequency.
How does it all shake out?
We know it cools off very rapidly at night, when the sky is clear and the air is dry. When it is humid or cloudy, so so fast.
When both humid and cloudy, it hardly cools off at night at all.
Thanks for that Nicholas, things are becoming a little clearer for me. The translucent sphere will radiate at the temperature of the non radiative gas inside because conduction will raise the temperature of the container which then radiates to space. My mistake was thinking that the gas itself would be radiate directly to space.
A tiny quibble. You say:
“~” means approximately. That’s important because as much as 3% of the local atmosphere could be water depending on the temperature and the availability of water. And water is a greenhouse gas.
Of course, wet air is lighter than dry air. That means it will rise, lose heat in the upper atmosphere, condense and come down as precipitation. So that’s another way a greenhouse gas removes heat from the lower atmosphere.
I just love the way the warmists talk about non-condensing greenhouse gasses. I think it’s because they are trying to ignore the most important greenhouse gas, which is water.
Will this be the last wave of Arctic air over the northeastern US?
Growers will need to take extra precautions for their sensitive plants as the risk of a frost or even a freeze in some locations persists across the eastern U.S. for the next few days.
This does not appear to be all that unusual for this time of year.
In other news:
Sea ice remains higher than many recent years, and is close to long term averages.
It certainly is not decreasing.
A region that has produced a CME is now rotating into view, and may be in position to send a Earth directed CME our way soon.
Space Weather by SolarHam
Did the weather station survey collect enough data on the surrounding areas to determine if local wind patterns had been changing?
The thought experiment I read a while ago was this.
Fill a long tube, transparent at both ends, with 100% CO². Place a heat source at one and a thermometer (kinetic) at the other. If the temperature of the heat source is 50°C what temperature will the thermometer read ?
The short answer is probably ambient.
The long answer involves the refractive index of the tubing material at long wave infrared. Presumably you could create something like a waveguide and see some energy transmitted the length of the tube.