Guest Post by Willis Eschenbach
The British tabloid “The Guardian” has a new scare story about what is wrongly called “ocean acidification”. It opens as follows:
Pacific Ocean’s rising acidity causes Dungeness crabs’ shells to dissolve
Acidity is making shells of crab larvae more vulnerable to predators and limiting effectiveness in supporting muscle growth
The Pacific Ocean is becoming so acidic it is starting to dissolve the shells of a key species of crab, according to a new US study.
Sounds like the end of times, right? So let me start with a simple fact. The ocean is NOT acidic. Nor will it ever become acidic, except in a few isolated locations. It is alkaline, also called “basic”. The level of acidity/alkalinity is expressed on the “pH” scale, where neutral is 7.0, alkaline is from 7 to 14, and acidic is from 0 to 7.

Figure 1. The pH scale, running from the most acid at the bottom, through neutral in the middle, and up to the most alkaline at the top.
From the chart, the ocean has a pH of around 8 (although as we’ll see, that conceals great variation).
And from my high school chemistry class in titration, I know that adding a small amount of an acid to a basic solution, or adding a small amount of a base to an acidic solution, is called “neutralization” for a simple reason. It moves the solution toward neutral.
When carbon dioxide (CO2) dissolves in rainwater or in the ocean, it makes a weak acid. And adding that weak acid to the ocean will slightly neutralize the ocean. How much? Well, by the year 2100, if you believe the models, it is supposed to move the pH of the ocean from around 8 all the way down to around … wait for it … a pH of 7.92. In other words, a slight neutralization.
So why is it called “ocean acidification” rather than “ocean neutralization”? Sadly, because “acidification” sounds scary. We see this in the story above, where the opening line is:
“The Pacific Ocean is becoming so acidic it is starting to dissolve the shells of a key species of crab, according to a new US study.”
Well, no, that’s not true at all. The ocean is not acidic in the slightest. It is slightly less alkaline. Using “acidification” rather than “neutralization” lets us convince people that impossible things are happening. Consider the following restatement of their opening sentence.
“The Pacific Ocean is becoming so neutral it is starting to dissolve the shells of a key species of crab, according to a new US study.”
Huh? The Pacific Ocean is becoming so neutral that it’s starting to dissolve things? Say what?
Alarmism run wild.
Here’s another important and counterintuitive fact about pH. Living creatures deal with acidic substances much better than we do with alkaline substances. Look at Figure 1 above. We regularly consume quite acidic things. Grapes and orange juice are at a pH of three. Lemon juice has a pH of two, very acidic, five pH units below neutral. And at six pH units below neutral, with a pH of just one is … our own stomach acid.
But we don’t eat many things that are more alkaline than a pH of about 10, things like cabbage, broccoli, and artichoke. And while our stomachs happily tolerate a pH of one, we are badly burned by bleach, at the opposite end of the pH scale.
Next, the required disclaimer. I have a personal stake and a personal passion regarding this subject. I live on the West Coast of the US in the very area they’re discussing, and I fished commercially in these waters for many years. So I know a few things about the local oceanic ecosystems.
With that as prologue, the new Guardian scare story is based on a scientific study called “Exoskeleton dissolution with mechanoreceptor damage in larval Dungeness crab related to severity of present-day ocean acidification vertical gradients“ … the “ocean acidification” BS strikes again. Heck, it gets its own cute little acronym, “OA”, as in the portion of the abstract below:
Abstract
Ocean acidification (OA) along the US West Coast is intensifying faster than observed in the global ocean. This is particularly true in nearshore regions (<200 m) that experience a lower buffering capacity while at the same time providing important habitats for ecologically and economically significant species.
Now, I can’t find any reference in the study for the idea that somehow the US West Coast is acidifying faster than the global ocean. In fact, we have very little pH data for the global ocean.
But we do have some data. One most informative graphic gives us a look at a slice of the ocean from top to bottom and from Hawaii to Alaska. Over a 15-year period, scientists traveled that route, periodically stopping and sampling the pH from the surface to the seafloor. I discussed that “transect” in my post “The Electric Oceanic Acid Test“. Here’s the ocean cross-section with its original caption.

Inset at lower left shows the area studied. Click to expand. Graphic Source
Now, there are several fascinating things about this graphic. The first is the wide range of pH in the ocean. We tend to think of it as all having about the same pH, but that’s far from true. Around Hawaii (top left of the chart), the pH is about 8.05. But at a couple of hundred metres under the surface off the coast of Alaska (top right), the ocean is at a pH of 7.25. This pH is what hysterical scientists and the Guardian would call “MUCH MORE ACIDIC!!”, but is properly called “approaching neutral”.
Next, where is the most sea life in this chart? Why, it’s off the coast of Alaska, my old fishing grounds, which is replete with plankton, herring, salmon, sharks, flounders, whales, and every kind of marine creature. They flourish in those “MUCH MORE ACIDIC”, aka “more neutral”, ocean waters.
Finally, sea life thrives at every pH in the graphic. There are fish and marine creatures of all kinds at every pH level and every area in the graphic, top to bottom and Hawaii to Alaska. They are not tied to some narrow band where they will die if the pH changes by a tenth of a pH unit over a hundred years.
So please, can we get past this idea that a slight, slow neutralization is going to kill every poor creature in the ocean? Alkalinity is a problem for sea creatures, not acidity. It’s why so many of them are covered by a coating of slime or mucus—to protect them from the alkaline seawater. Fun Fact—if you want to dissolve a fish (or a human), use lye (pH 14), not sulfuric acid (pH 1) … but I digress.
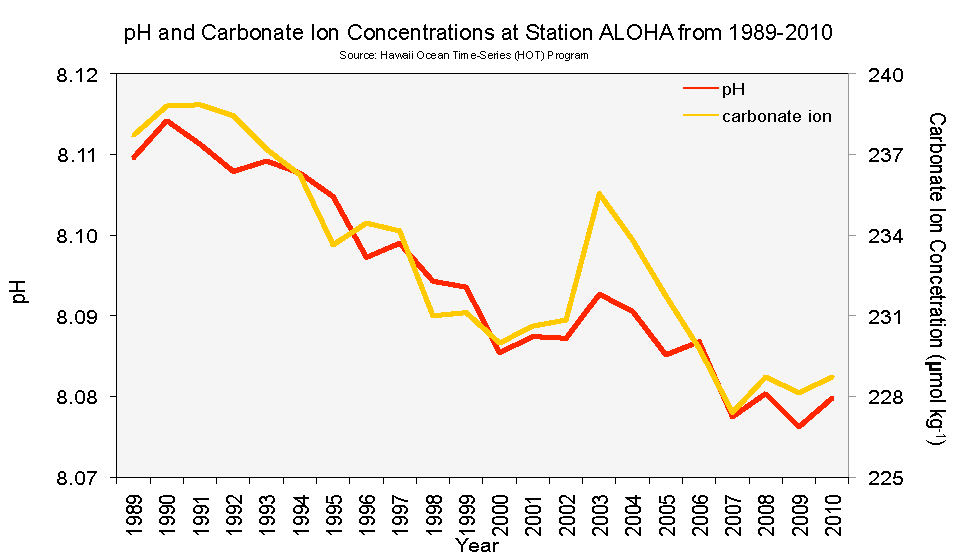
Moving on, I wrote before about the pH measurements at the intake pipe of the Monterey Bay Aquarium in a post entitled “A Neutral View of Oceanic pH“. In that post, it was obvious that the long-term trend in pH at the Monterey Bay Aquarium was smaller than the trend at the “H.O.T.” deepwater location off of Hawaii. Here’s the graph from that post showing the difference:

Figure 2. Surface pH measurements from HOT open ocean and Monterey Bay upwelling coastline. The Hawaii data shows both measured pH (black) and pH calculated from other measurements, e.g. dissolved inorganic carbon (DIC), total alkalinity, and salinity. You can see the higher pH around Hawaii that was visible in the previous Figure.
Sadly, the web page containing the Monterey Bay pH dataset has become some kind of unknown Japanese web-page. Fortunately, I kept the data. And I was also able to find further pH data which starts just after my old data, although it appears that the calibration of the pH sensors is slightly changed in the new set. In any case, I’ve put both datasets in one graph, with separate linear trendlines for the two datasets.

Figure 3. Twenty-five years of monthly average pH measurements at the inlet pipe that delivers 2.5 million gallons (9.5 million liters) of seawater per day to the Monterey Bay Aquarium. Two separate datasets were used. The entrance of the pipe is at a depth of 50 feet (15 metres). The size of the projected pH drop by the year 2100 using RCP6.0 is shown by the top-to-bottom size of the “whiskers” in white at the upper right.
The neutral pH of 7.0 is down at the bottom, a ways below the data. Note that the long-term trend of the average pH value of the water is about the same in both datasets, and that the trend is quite small compared to the projected slight neutralization by the year 2100.
And more to the point, that projected pH decrease by 2100 of 0.08 pH units is dwarfed by the daily change in the pH. Heck, it’s smaller than the size of the monthly change in the pH. The standard deviation of the daily change in pH is 0.6 pH units, and the standard deviation of the monthly change is 0.1 pH units.
Why is the pH changing so fast on the West Coast of the US? It all has to do with coastal upwelling. Varying winds along the coast cause deep, cold, CO2-rich, more neutral water to come to the surface in varying amounts, changing the pH literally overnight.

Figure 4. The mechanical action of the winds blowing southward along the West Coast of the US causes the upwelling of CO2-rich more neutral water from the ocean depths. Image Source NOAA
And that constantly-changing pH is why I find these claims about oceanic creatures here on the West Coast of the US being killed off or badly injured by some trivially small slow change in pH to be totally unbelievable. Every living being in the ocean along this coast undergoes much, much larger pH changes from one day to the next than they will see over the next century.
There’s one more dataset that I have to add to this before turning to the study itself. The study actually takes place up in the area near Seattle. So what is the oceanic pH up there doing?
Turns out it is very hard to find long-term pH measurements in that area. The best that I’ve been able to find are an intermittent series of measurements from an offshore buoy on the coast of Washington near the Strait of Juan de Fuca, a lovely part of the planet that I battled through a while back. Here’s where the La Push buoy is located:

Figure 5. The yellow square shows the location of the “La Push” offshore buoy. The Strait of Juan De Fuca is the blue channel leading into the land. Seattle and Tacoma, Washington are below the inner end of the Strait. Vancouver Island, Canada, is on the north side of the Strait.
It appears that the buoy is brought in when the weather gets very rough, because there is a gap in the data each winter. Here’s the La Push buoy data, to the same scale as the Monterey data above.

Figure 6. Daily surface pH records at the La Push, Washington offshore buoy. The background is an offshore island near La Push.
Once again, we see the same situation. The pH changes are much larger than the size of the projected change between now and the year 2100. And while I wouldn’t put much weight on the trend line because of the gaps in the data, it’s quite possible that the trend is actually becoming slightly more alkaline.
How can it become more alkaline? Remember that along this coast, the swings in the pH, and the average pH itself, are not direct functions of CO2 levels. Instead, they are determined by the instantaneous and average strength of the wind. If there is more wind, more of the deeper, more neutral waters come to the surface to lower the surface pH, and vice versa.
And lest you think that such swings in pH are limited to this coast, here’s some data from around the planet.

Figure 7. pH values and variations from different oceanic ecosystems. Horizontal black “whiskers” show the range of the pH values. The size of the expected slight neutralization by the year 2100 according to RCP6.0 is shown by the red whiskers at the top. Ischia South Zone, the site that goes the lowest in pH, is on the side of a volcano that is constantly bubbling CO2 through the water. DATA
Let me close by looking at the study itself, at least as much as I can bear. I’ll discuss a few quotes. The first line of their “Highlights” says:
Coastal habitats with the steepest [vertical] ocean acidification gradients are most detrimental for larval Dungeness crabs.
There’s no such thing as a “vertical ocean acidification gradient”. There is a vertical pH gradient, as you would expect with upwelling deeper CO2-rich water hitting the more alkaline surface waters with less CO2. But this is a natural condition that has existed forever and has nothing to do with “OA”. And they present no evidence to show that the gradient will change significantly in the future.
Next, in their conclusions they say:
Like dissolution in pteropods, larval dissolution observed in Dungeness crab is clear evidence that marine invertebrates are damaged by extended exposure to strong present-day OA-related vertical gradients in their natural environment.
However, they present no evidence that past “OA”, or mild oceanic neutralization, has had any effect on the “vertical gradients in the natural environment”. The vertical gradients in pH off of the coast are a function of the upwelling, which in turn is a function of the wind, which is constantly changing. They don’t have long-term data for the vertical pH gradient. Instead, they went on a two-month cruise, took some samples, and extrapolated heavily. We don’t even know if they’d have found the exact same “dissolution” a hundred, fifty, or twenty-five years ago. Or perhaps the dissolution was particularly bad during that particular two-month period in that particular small location. This should not surprise us. One reason that so many marine creatures spawn hundreds of thousands of larvae is that many, perhaps most, of them will drift into inhospitable conditions and die for any one of a host of reasons—problems with salinity, turbidity, pH, predators, temperature, the list is long.
Finally, this paper does prove one thing—that Neptune, the trident-wielding god of the ocean, definitely has a sense of humor. Here’s the ultimate irony.
They couldn’t see the parts of the crab larvae that they wanted to examine because those parts are covered by the “epicuticle”, the outer layer of the hard carapace that surrounds the larva. So they first had to dissolve the epicuticle in order to get access to what they wanted to study. Here’s their description of the problem and the solution. (The “megalopa” are a stage of the larval form of the crabs).
The carapace epicuticle, which otherwise overlies the crystalline layer and makes dissolution observations impossible, was removed from each megalopa prior to analysis. This was accomplished using sodium hypochlorite, which efficiently removes the epicuticle but does not damage the crystalline layers underneath, even at high concentrations.
Care to take a guess at the pH of the 6% solution of sodium hypochlorite, which is what they used to dissolve the carapace epicuticle?
It has a pH of 11 or more, almost at the very top of the scale in Figure 1, very strongly alkaline.
So it no wonder that Neptune is laughing—they’re all up in arms about “acidification” dissolving the crab carapaces … but in the event, they’re using an alkaline solution to actually dissolve the crab carapaces.
Ain’t science wonderful?
It’s clear today, and from my house perched high up on a hill six miles (ten km) from the coast, I can see a small bit of the very part of the ocean that we’re discussing. It’s foggy down there and it’s clear up here, as is often the case. And right out there, millions of marine creatures are happily going about their lives as the pH gyrates up and down every hour, every day, and every month.
If a slight oceanic neutralization were going to injure them as we are franticosolemnly assured at every opportunity by the bad boffin boys and the popular press, those oceanic inhabitants would all have died long ago.
My very best to everyone on a sunny winter day,
w.
PS: After early years of having to point out that “No, I didn’t say that, I said nothing like that”, I’ve taken to asking those who comment to quote someone’s exact words that you are going to discuss. This avoids endless misunderstandings and arguments.
The dishonesty of reporting about the so-called acidifying of the oceans as part of the climate hysteria has constantly annoyed me.
In agriculture, soils are classified strictly according to their pH levels and identified in accordance with that. When steps are taken to change the pH levels it is described as raising or lowering the pH levels or as reducing the acidity or the alkalinity of the soil in accordance with the desired outcome.
All I can put the difference in approach down to is that with the soil, the information is being provided to those who work with the soil and have a practical understanding of it, whereas the information about the oceans is structured to appeal to the climate alarmists who on the whole have little or zero understanding of the subject and only respond blindly to whatever climate propaganda is prepared for their consumption.
That was something that annoyed me when we visited the Baltimore aquarium some years ago. There was some young woman droning on about ocean acidification and my wife asked, “The ocean is getting acidic?” I said no, it’s getting less basic, maybe. I said there’s no way for it to become acidic unless runoff from ALL the continents somehow stopped all at once, and even then I wasn’t too sure.
These people who spout this nonsense aren’t scientific at all. It’s really annoying.
Thanks Willis for another great science class. A slight, possible Neutralization versus a scary, false Acidification was a great ah-ha moment for me. Thanks for the lesson on ocean pH.
And thanks to you, Drew. Inter alia, it’s comments like yours that encourage me to keep writing up my research.
w.
All of this hype is rather silly since we know that during the past, the planet was some 10, and perhaps at times, as much as 17 degC warmer than today, and at times had CO2 levels exceeding 7,000 ppm and ife on this planet, and in the oceans thrived. Indeed, the great explosi0n of life was during the Cambrian period when CO2 was circa 7,000ppm.
During the Devonian period, CO2 was around 4,500 ppm and the oceans were around 30 degC. This era (some 420 to 350 million years ago) wqas known as the age of the fish. The oceans teamed with life and the largest fish ever to swim the oceans swam during this era.
Further, ammonoids swam the oceans when CO2 levels were high, and they were rather big. See for example:

Given that CO2 was some 10 times that seen of today, and there was no adverse impact causing ocean acidification, we know that modest levels of CO2, even if they were to double to say 800ppm will cause no significant adverse impact.
wOw! Great image, Richard.
It’s worth the click, y’all.
I love pickled onions. Anybody ever tried onions in a solution of Sodium Carbonate, or even just bicarb?
Calling a lowering of alkalinity to be an increase in acidity is like referring to someone who is paying off a large debt “richer” after each monthly payment. They are not “richer” as they still have put away no money (wealth). They are slightly less in debt – that is all.
Scare tactics are NEVER acceptable in hiding a truth.
Climate “Science” (i.e. pseudo-science) is built upon a foundation of scare tactics. They violate every ethical boundary in science – tamper with data, hide data, hide process and procedure, exaggerate, and yes, make stuff up.
It is one thing to believe in a hypothesis and be found wrong, and an entirely another to lie about the results in fear of being found wrong. These people are no longer scientists – they have become politicians.
Make stuff up is exactly correct.
The term “ocean acidification was invented, made up from whole clothe, specifically as an alarmist tactic.
Ever since it was, it is amazing how many people have glommed on to the hoax and come around insisting, INSISTING, that it is a correct usage of the word “acidification”.
There are zero dictionaries in the world that define the word as meaning a lowering of pH.
Because it never meant that.
Dave Middleton explains ( I think it was Dave) the whole sordid story in several articles right here.
Lemme grab one.
Here we go, took me all of 26 seconds.
There are others, but here is a small snippet quoted from the article and a link:
“The phrase “ocean acidification” was literally invented out of thin air in 2003 by Ken Caldiera to enable liberal arts majors to sound sciencey when scaring the bejesus out of the scientifically illiterate masses. The geochemical process has been well-understood for about 100 years… But didn’t get a crisis-monger nickname until 2003.”
https://wattsupwiththat.com/2019/07/25/the-total-myth-of-ocean-acidification-science-edition/
The Guardian….
– there are a few exceptions to the overwhelmingly dismal level of technical understanding of folk who write for them – I reckon though, that whoever dreamed up the Di-Hydrogen Monoxide prank scare was familiar with a lot of GMG output …
Braying donkeys are in comparison positively cerebral.
Here in the UK we also have the treasured BBC as the other cheek of the lefty alarmist ass – I expect some salaried producer / presenter combo/team has already reheated this bit of “OA” for inclusion in daytime TV, local programs, soaps, kids TV , Dr. Who and sports commentary.
Propagada-ish papers like this one appear to get published easily.
Science funding channels are administered by entrenched socialists within bureaucracies (bureaucrats are 95+% Socialist…the remaining 4-5% are Communists). Only Activist groups get funding and all that’s left after 30 years of that are Activist Scientists.
This is where massive defunding and restructuring has to occur during Trump’s 2nd and 3rd presidential terms.
3rd?
Good post, WE. There has been borderline to actual scientific misconduct about ‘ocean acidification’ for many years, this paper being just another example. I explained the underlying seawater pH science (even used your Exkman transport diagram to explain Pacific coast upwelling) then documented two clear examples of scientific misconduct (Milne Bay corals in PNG and Netarts Bay oyster Hatchery in Oregon) in essay Shell Games in ebook Blowing Smoke. The oyster part was previously posted at Judiths under the same title.Cliff Mass picked it up and reblogged with his own take and followup research on the Seattle Times fake ’OA’ news on his U. W. weather blog.
Thanks, Rud, always good to hear from you. Someone upthread claimed that “acidification” was destroying oyster farms in the Pacific NW. I asked them for a cite … nothing to date.
For those interested in Rud’s post on Judith Curry’s most excellent blog, it’s here.
w.
WE, ty. You still have more ‘fire’ for this than I, after 11 years and at least parts of 3 ebooks. CtM, with whom I now have the privilege of lunching about monthly on our SF Atlantic beaches and InterCoastal, agrees.
Highest regards to a long time climate science warrior.
Thanks, Rud. I’ve been beating this drum for almost two decades now, kinda getting used to the beat.
Please pass on my best regards to CtM when you see him, he’s done an outstanding job with the blog. I hope to get down your way and see you guys at some point, don’t know when.
Best to you and yours,
w.
When you do, my treat.
Thanks Willis.
I always enjoy your articles and learn things. I also enjoy the postscripts about your home conditions when you post an article here.
We’re the same age, and my thought about this one is that in the years I have left in this life, I hope that I am never seated opposite a person at a dinner party who might want to pontificate about –
Exoskeleton dissolution with mechanoreceptor damage in larval Dungeness crab related to severity of present-day ocean acidification vertical gradients
They never did mention in so many words that is, and certainly not upfront, that they were actually studying the mechanoreceptor of larval Dungeness crabs who had been severely injured by being soaked in a concentrated bleach solution until their outer cuticle dissolved clean off!
Exactly how certain are they that concentrated bleach has no effect on them thar mechanoreceptors, I wonder?
There’s a difference between “alkalinity” and “alkaline”.
“Akaline” is a pH above 7.
Water with a pH below 7 can still have “alkalinity” that continues to resist a change to a lower or higher pH.
Small point. The words are similar and related but don’t quite mean the same thing.
I’ve just added a pinch of salt to my glass of CocaCola. I now have a savoury drink!
Denier, unprecedented, emergency, extreme, doomsday….now acid(ificiation) goes into the “Dictionary for Warmists.”
As an environmental scientist and geologist, I now sob daily for the increasing destruction of scientific principle – led by ignorant journalists who live by only one mantra these days “if it bleeds, it leads”. We live in an era of unprecedented knowledge and access to information – yet society in general has never been as dumb (in terms of the ability to think critically) as since before the Industrial Revolution.
If you ‘believe’ in “ocean acidification” than you also must ‘believe’ that life in the oceans was impossible until Earth entered the ongoing Ice Age 3.5 million years ago. CO2 levels were just too high.
The data from the “La Push” offshore buoy reminded me about a long blog post by Cliff Mass …
Ocean Acidification and Northwest Shellfish: Did the Seattle Times Get the Story Right?
“Other regional factors affecting ocean acidification in Washington include runoff of
nutrients and organic carbon (such as plants and freshwater algae) from land, and local
emissions of carbon dioxide, nitrogen oxides, and sulfur oxides, which are absorbed by
seawater from the atmosphere. The relative importance of these local drivers varies by
location. For example, acidification along the outer coast of Washington and Puget Sound
is strongly influenced by coastal upwelling while acidification in shallow estuaries,
including those in Puget Sound, may be particularly influenced by inflows of fresh water
(which is naturally lower in pH than seawater) carrying nutrients and organic carbon
from human and natural sources. The added organic carbon, as well as nutrients that
stimulate excessive algal growth, can make seawater more acidic when algae and other
organic matter decompose.”
https://cliffmass.blogspot.com/2013/10/ocean-acidification-and-northwest.html
To test if it is really becoming acidic, we should dunk authors’ heads in the ocean and ask “is it sour, is it sour?”
Chris B, are you avocating acid boarding?
I’m not good at chemistry, but from the first graph a correlation is obvious: apparently the alkalinity correlates with temperature — the deeper you go, the colder the water is, the lower the alkalinity is. The farther from equator you go, the colder the water is, the lower the alkalinity is. So, could it be that the alkalinity is a better measurement for the average temperature than using thermometers at too few points and trying to average their readings?
Interesting question, Luchezar. Actually, it’s much more complex than that. pH is a small part of the carbonate chemistry of the ocean. Here’s a glimpse of the various factors involved in pH.
ALK — ALK, total alkalinity (mol/kg)
Tinsi — In situ temperature in degrees Celsius
Tlab — Measurement temperature in degrees Celsius
Pinsi — In situ hydrostatic pressure in bar (surface = 0)
S — Salinity
Pt — value of the concentration of total phosphate in mol/kg
Sit — the value of the total silicate in mol/kg
k1k2 — “l” for using K1 and K2 from Lueker et al. (2000), “m06” from Millero et al.
(2006), “m10” from Millero (2010), “w14” from Waters et al. (2014), and “r”
from Roy et al. (1993). “x” is the default flag; the default value is then “l”,
except if T is outside the range 2 to 35oC and/or S is outside the range 19 to 43.
In these cases, the default value is “w14”.
kf — “pf” for using Kf from Perez and Fraga (1987) and “dg” for using Kf from Dickson and Riley (1979 in Dickson and Goyet, 1994). “x” is the default flag; the
default value is then “pf”, except if T is outside the range 9 to 33oC and/or S is
outside the range 10 to 40. In these cases, the default is “dg”.
ks “d” for using Ks from Dickon (1990), “k” for using Ks from Khoo et al. (1977),
default is “d”
I’m sure you can see the problems with using the pH to estimate the temperature.
w.
Think I have found the source of the problem:
“This work was supported by the NOAA’s Ocean Acidification Program”.
Hilarious, thanks. The US Government scientists clearly know where the fame and the grants are located, and it’s not where good science resides.
w.
Great post Willis.
The pH issue is a total cannard. The only thing that really matters is the aragonite saturation state. Adding CO2 lowers the saturation state, raising water temperature increases it. There’s no evidence at all that CO2 levels below 1,000 ppm pose any hazard to modern marine calcifiers… even above that, the hazard is speculative.
“The pH issue is a total canard. The only thing that really matters is the aragonite saturation state.”
Exactly so. And so pH 7 is a canard too. The fact is that adding CO₂ shifts an acid-base equilibrium so that CO₃⁻⁻ is converted (molecule for CO₂ molecule) to HCO₃⁻. That reduces the aragonite saturation state, since the equilibrium is
CaCO₃(aragonite) ⇌ Ca⁺⁺+CO₃⁻⁻.
Don’t forget that the Ocean waters already have 99% of the free CO2 in it, not much will change from the piddling it gets from the atmosphere.
Sure, Nick, carbonate becomes bicarbonate and pH is buffered, but within calcifying organisms, bicarbonate is transformed into carbonate, protons are pumped (and re-absorbed), aragonite and calcite are formed. Carbonate is not typically the raw ingredient for shell construction, rather, bicarbonate is.
Furthermore, the mineral structure is covered by the periostracum, a protein layer that isolates the shell from the surrounding water and its hydronium ions. The shells of living organisms are not degraded as long as they are producing chitin.
If we feel that there’s not enough carbonate in the ocean, how should we go about increasing it?
Interested in learning more about the particulars of shell formation, and your comment about the raw ingredient more commonly being bicarbonate than carbonate makes a lot of sense to me, because we can see many places where the pH is very low and yet shell forming life forms are not uncommon and often exist in profusion in such places.
Can you supply a reference?
Here’s a paper showing that foramanifera, amongst others, have known about Le Chatelier since long before he was born!
https://www.nature.com/articles/ncomms14145
Here’s a paper that shows that foraminifera, amongst others, have known about Le Chatelier since before he was born!
https://www.nature.com/articles/ncomms14145
Nick,
Le Chatelier’s Principle states that in an equilibrium reaction, adding more of one component of the equilibrium reaction will push the equilibrium away from the species being added.
This is obvious.
If a certain percentage of dissolved CO2 gas reacts with water to form carbonic acid, and a certain percentage of the carbonic acid dissociates into bicarbonate and hydronium ions, and a certain percentage of the bicarbonate dissociates into carbonate and hydronium (each of which percentages is extremely small as percentages go, but regardless), obviously more CO2 will result in more, not less, carbonate in solution as an ion.
“Changing the concentration of a chemical will shift the equilibrium to the side that would reduce that change in concentration. The chemical system will attempt to partly oppose the change affected to the original state of equilibrium. In turn, the rate of reaction, extent, and yield of products will be altered corresponding to the impact on the system.
This can be illustrated by the equilibrium of carbon monoxide and hydrogen gas, reacting to form methanol.
CO + 2 H2 ⇌ CH3OH
Suppose we were to increase the concentration of CO in the system. Using Le Chatelier’s principle, we can predict that the amount of methanol will increase, decreasing the total change in CO. If we are to add a species to the overall reaction, the reaction will favor the side opposing the addition of the species. Likewise, the subtraction of a species would cause the reaction to “fill the gap” and favor the side where the species was reduced.”
Here is the equilibrium reaction of each of the species present when CO2 dissolves in water:
H2O + CO2 ⇌ H2CO3 ⇌ H+ + HCO3– ⇌ H+ + CO3⁻⁻
So obviously, when no interfering reactions are taking place (in seawater many other substances are present however), adding CO2 to the water will result in more carbonate.
How could it be otherwise?
And when carbonate is being removed by reacting with calcium ions to form calcium carbonate, it is necessarily the case that more bicarbonate will dissociate to oppose this removal.
Now, plenty of alarmists have posited exactly the opposite, as you do here, on the theory that since adding more CO2 also makes more hydronium ions exist in the solution, and that this will react with the carbonate to form bicarbonate, so carbonate will decrease.
But all of those species are in equilibrium prior to anything changing…except for the fact that other reactions are taking place simultaneously, and many of those are also equilibrium reactions, but some ARE NOT.
Given only what you have stated though…you have it exactly backwards, unfortunately.
People like Caldiera have been writing papers for years in which they postulate that adding CO2 to the oceans will result in less carbonate available and the result will be a decrease in such marine organisms as coccolithophores.
But he uses obtuse reasoning and modelled results.
The actual ocean says different, and more to the point, so do marine shell forming species such as coccolithophores…when they actually go look for them and do some counting!
This is the sort of thing that happens when you get stuff backwards and never bother to check if what your models and poor understanding of how things work for real, is actually true.
https://wattsupwiththat.com/2015/11/27/increased-carbon-dioxide-enhances-plankton-growth-opposite-of-what-was-expected/
Also, if you kept up with current events, you would know that all of the alarmist predictions about the ocean are being found to be incorrect and that the opposite is happening.
Shells are built of calcium carbonate, and to make that you need CO2.
It is just like on land, when alarmist scientists claim that higher CO2 will cause us all to starve to death when our crops fail…but hey instead it turns out that since life is built out of CO2, more means more life, not less!
Hooray…the end of the world is cancelled!
Good night, drive safely, thanks for playing…you have plenty to live for it turns out, and being an alarmist is not one of them.
“Here is the equilibrium reaction of each of the species present when CO2 dissolves in water”
But it isn’t dissolving in water. It is dissolving in seawater where a large amount of carbonate and bicarbonate is already present. Adding CO₂ does increase H⁺ (the pH does drop). So by Le Chatelier, in your last equilibrium, the change direction is that
HCO₃⁻ ⇐ H⁺ + CO₃⁻⁻
Le Chatelier could not be expected to operate in any circumstance where there was not already the various ions in equilibrium.
You want to ignore everything but the carbonate.
The first thing that happens is more CO2 is added to the water.
Then a little of it reacts with water to make carbonic acid.
Then, due to the existing pH, this mostly is transformed into bicarbonate and Hydronium.
But the solution is buffered, not just by bicarbonate.
Ignoring that for this part of the sequence, more bicarbonate means there will be more carbonate, because there is more CO2!
You cannot jump ahead and consider only the hydronium generated, and ignore that for every hydronium, there is also an additional ion of bicarbonate, which has a dissociation constant. This means that the two remain in a certain proportion relative to each other.
Carbonate is only there because there is CO2 to begin with, but by your logic, the more CO2 you put in, the less carbonate there will be.
That makes zero sense.
Because you are not looking at the entire picture.
And every time a calcium ion runs into an ion of carbonate, and/or they are absorbed, this is the other part of the principle…removing one species pulls the reaction towards the thing being taken away!
The Ka values are calculated from actual solutions. More bicarbonate means there will be more carbonate. The H+ generated is part of the calculation.
You cannot just pull them both over to one side!
If what you are saying was true, adding CO2 would cause more bicarbonate to combine with acid and make still more CO2!
This is exactly opposite of what happens.
Besides for everything else is the actual Earth.
There are a large number of biomes where the pH is below 7, and yet shell fish live in vast profusions.
Black smokers are highly acidic, and yet large numbers of various shelled organisms crowd around them.
Cold waters in the Arctic and Antarctic have very low pH compared to lower latitudes, and yet these places have huge populations of all sorts of shelled organisms.
Suwanee river and other so-called black water rivers have extremely low pH due to tannic acid and humid acid from decomposing leaves and such. Numerous freshwater mussels that live in these waters never got the memo that they could not live if the pH was low.
And then there is all of Earth history, when CO2 was almost always many times higher than now.
Neither chemistry or biophysics was different then.
” but by your logic, the more CO2 you put in, the less carbonate there will be.
That makes zero sense.”
It’s just chemistry. The overall reaction is
CO₃⁻⁻ + CO₂ + H₂O ⇌ 2HCO₃⁻
Adding CO₂ pushes the reaction to the right – twice as many moles of HCO₃⁻, with the excess at the expense of CO₃⁻⁻.
The problem with pointing to simplistic atomic equations is that the real world clearly does not reflect the theoretical problem.
Therefore the real problem is more complex, and the other effects are major or dominating, vs the “physics”.
Then there’s the entire problem of the evolution of the genus Mollusca – it occurred in the Cambrian era where CO2 levels were in the 3000 ppm order.
If Carbonate starvation due to CO2 levels is really such a problem, or pH, or whatever panicmongering “acidification” nonsense is true – these animals would never have evolved to start with.
The ginormously thick shells of the early mollusks – cephalopod variety – argue that HCO3 = H+ + CO3 is not a serious issue.
For the two molecules, one the ionic carbonate and the other CO2, whether in solution or existing as the reaction product with water, carbonic acid, a certain proportionality exists.
Part of the Ka and what causes it to vary as conditions vary is the fact that one of each have to collide in order to react.
But for dissociation to occur, that is not the case.
At a pH above 7, carbonic acid is immediately and almost totally dissociated. The reaction is reversible because the two dissociation products can collide and then combine again…the Ka is a small number when this is thermodynamically favored, and gets larger when the thermodynamics changes to make it less favorable.
The predominant species at the pH of the ocean is bicarbonate…it is thermodynamically favored.
When one examines the tables regarding what happens under various conditions, some certain temperature and CO2 partial pressure is specified, and yet under the ocean the temperature and the pressure are at far different and varying levels.
The upshot is that it is extremely complex, which is why looking at some modelled result, even if the modeler had no inherent bias, must be compared to what is observed to occur in the actual ocean, in rivers, and lakes, near volcanic vents, etc.
What is actually observed is increasing numbers of coccolithophores in the ocean, vast teeming profusion of shelled organisms including molluscs crowding around black smokers which are expelling hot concentrated brine loaded with CO2.
Empirical evidence not gathered in ridiculous contrived aquarium tanks shows none of the effects predicted by alarmists, who have decided to rewrite whole textbook sections based on flawed reasoning, while tossing out and ignoring decades and centuries of accumulated knowledge and observations.
And worst of all ignoring what we can observe to be occurring at the present time.
Life evolved under real conditions, not calculated models, and has continued to evolve and prosper and survive as conditions have changed over time, in response to variations in the air above the ocean, the contents and conditions of the rivers dumping into the seas, the volcanoes and dep sea vents spewing and churning, upwelling of deep cold water of various mineral content…
It is well known it has been far warner than now, even in the recent past, that sea level 18,000 years ago was hundreds of feet lower and therefore it is factual that over a period of thousands of years as the water rose, warmed, and increased in CO2 content, all of the reefs we see today that are above 400′ feet deep have grown from nothing to now exists.
They survived the ice age and had no problem keeping up with huge changes in water level, salinity, temperature, pH, and CO2 concentration, over and over again…just in the recent geological past.
Carbon dioxide is what life is built from…life in the water, and on land.
It is only a political agenda that inspired the ideas now being promulgated by warmistas and alarmists of various stripe…the grifter, the opportunist, the politically power hungry, the control freaks, the gullible and credulous, the uneducated…
If the theory does not agree with what is observed, the theory is wrong.
It does not matter who says it, it does not matter how elegant it is.
It is wrong.
Period.
https://wattsupwiththat.com/?s=ocean+acidification
“Therefore the real problem is more complex, and the other effects are major or dominating, vs the “physics”.”
Physics, chemistry…these meet at a place called physical chemistry.
Ask any medical school student or doctor what the hardest undergrad class they took was, and most of them will tell you P. Chem, without hesitation.
These issues are where physical chemistry and biology intersect.
What happens as things change introduces other factors relating to various other disciplines.
It is impossible to separate what happens in the real world into separate disciplines and try to understand what happens while looking at only a small slice of reality, at some artificial division created in the minds of people.
It all interrelates.
Everything can and sometimes does affect everything else.
By nothing occurs that does not accord with the physical laws of nature.
How well people understand them and account for various factors, means that someone who thinks they can predict what will happen as something changes, can be and often is wrong.
Besides for ignoring what is actually occurring, people bitten by the warmista bug have some sense that they cannot be wrong, that they do not need to be guided by what is observed, that if data contradicts what they have decided is the case…best to ignore it or change it or just make stuff up.
We do not have to observe how these questions are argued by the same people who have shown themselves, in one area of science and argumentation after another, to be dishonest purveyors of junk science and propaganda and alarmism.
Nick, every time you make an assertion, you want to look at only on part of what is in reality a complex interactive series of equilibria.
In the ocean, the buffering of numerous species means that pH changes very gradually, even with large changes in the concentration of CO2 and the weak acid it forms when combined with water.
This means that whatever changes might take place as a result of changing pH, as described in the Bjerrum plot, are tiny compared to the changes forcing a new equilibrium with regard to Le Chatelier.
It is inane sophistry to attempt to understand or explain one part of a chemical equilibrium without taking account of all of the reactions and equilibria which are affected as one or more change(s) occurs.
Instead of honest investigation to resolve questions, the process has been short circuited by alarmist ideology.
Conclusions are now assume.
Questions are not honestly assessed, data is not objectively analyzed for purposes of verification.
Anything that might contradict alarmist mentality and dispel warmista mythology are not even investigated.
The mentality is exactly that which prevailed prior to the scientific revolution.
Back in those days, very few of the things people thought they knew about the natural world were correct.
“Phenomena in apparent contradiction to Le Chatelier’s principle can arise in systems of simultaneous equilibrium: see the article on the theory of response reactions. “
Stokes
You said, “And so pH 7 is a canard too.” But it brings into focus that statements such as “becoming more acidic” are disingenuous, being unsupported by the facts or commonly accepted chemistry definitions.
Willis,
Excellent summary on ocean acidification.
For general reading on the topic the following have excellent coverage and support your demolition of the feeble claims by the IPCC –
1. “Climate: the Counter Consensus” ,pp 102-111 by the late Professor Robert M. Carter a marine biologist and Paleoclimatologist. This is the best short coverage I have read.
2. “ Climate Change: The Facts”(2017), Ch. 2. ‘Ocean Acidification: Not yet a catastrophe for the Great Barrier Reef’ by Dr. John Abbott and Dr. Jennifer Marohasy.
3.“Inconvenient Facts” by George Wrightson, Geologist. (Silver Crown 2017).
Inconvenient Fact 55. There is no historic correlation between CO2 and Ocean pH.
Inconvenient Fact 56.The oceans did not become acidic even with CO2 at 15 times modern levels.
4. Ian Plimer “ Climate Change Delusion and the great Electricity Rip-off.”- Ocean Acidification pp326-341. Plimer has an excellent coverage as a geologist and calls ocean acidification a ‘concocted non- issue,”and
“Mention of ocean acidification is a fraud.The oceans have been alkaline since the beginning if time (save very earliest oceans were probably acidic, Halevy 2017) and during times when the atmospheric CO2 was hundreds of times higher than now”.
Lastly from Steve Goreham’s book-
“Acid Oceans?
Ocean acidification is a grand whopper that is frequently delivered by climate scientists,the IPCC, and environmental groups like the NRDC.
… It is probably as difficult to develop a global average of ocean alkalinity as it is to develop a globalaverage surface temperature.
The pH of the ocean varies by depth, becoming less basic as one goes deeper . It varies by latitude as one moves from the equator to the poles.It varies by location, such as open ocean, coral reef , or kelp bed. Scientists still know little about the alkalinity of today’s ocean or the oceans of past centuries.
A December 2011 study by scientists at the Scripps Institution of Oceanography found large variations in ocean pH by month, week, and even time of day.Dr. Gretchen Hoffman led a team that measured pH at 15 locations in the Atlantic,Pacific and Antarctic oceans. They found that pH changes were large, from 0.1 to I.4 units over a 30 day-period. They also found that pH changed by as much as 0.35 units over a course of days!
The study concluded that ‘ climatology based forecasts consistently under estimate natural variability’ and that ocean residents ‘are already experiencing pH regimes that are not predicted until 2100’ by the climate models.”
The paper is Hoffman et al 2011.
“…the web page containing the Monterey Bay pH dataset has become some kind of unknown Japanese web-page. Fortunately, I kept the data.”
I see you’ve discovered the same thing I have, that the Internet changes too often for bookmarks to be useful. Whenever I find a good piece of information, I save it in PDF format, so I will always be able to go back to it later.
Willis,
Excellent overview in line with your body of work.
I would only add that calcium in the form of CaCO3 is the other line used by the panic mongers to scare.
And as far As I can see, there is no scarcity of Calcium in the ocean, period. In the southern ocean, because of the diatoms, there can be silicon scarcity but neither “dissolving” nor “insufficient calcium” is a teal thing.
There is over twice as much calcium ion in seawater as bicarbonate. Ca++ is usually given as about 400 ppm, and HCO₃⁻ as about 130-140 depending on the source.
Carbonate ion is roughly as basic as hydroxide ion…they are adjacent on tables of bases listed by increasing strength.
What this means is that there is very little of it to begin with, compared to other species it is in equilibrium with.
This is reflected in tiny Ka values for it’s conjugate acids.
4.7 x 10⁻¹¹ is the Ka of bicarbonate.
Carbonate is present in amounts so small it is typically disregarded in equations, and does not appear on most charts of the ions present in sea water.
One can find many assertions that carbonate is present at about 1/10th of the concentration of bicarbonate, but list after list from references do not even show it. Even ones that list major and minor components. I do not knw what is up with that…but the tables comport with what one might expect, and do not agree with assertions of as much as 14 ppm of carbonate.
https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1403%3A_General_Chemistry_2/Text/16%3A_Acids_and_Bases/16.3%3A_Equilibrium_Constants_for_Acids_and_Bases
Ok, here is one reference.
A graph from GSU.
sites.gsu.edu/geog1112/carbon-cycle-day-2-part-5/
228 micromoles per kilogram. 60 grams per mole is molecular weight, so that is about 13 milligrams per kilogram. Which if I am looking at this right is about one tenth of the bicarbonate concentration. So I wonder why none of the usual reference tables list it?
Is does not say if this is a measured or calculated result, although it would be worthless to just calculate it.
Forget the carbonic acid as EVs won’t save poor Nemo-
“This is dust and particulates that are emitted from our tyres constantly – and our brakes when we use them. This currently unregulated source of pollution contributes to particulates in the air, as well as microplastics in the ocean.”
https://www.msn.com/en-au/motoring/research/tyre-emissions-1000-times-worse-than-exhausts/ar-BBZvfoU
They’re gunna have to slash the weight of those EV batteries to comply with the new Regs for this dire threat to Gaia.
EV’s typically use regenerative braking, charging batteries as they do, when they brake so less wear on braking systems. EV’s typically have harder compound tyres too to reduce rolling resistance. But yeah, curb weight of a Tesla 3 is 1.9 tonnest give or take a few KGs. That’s a big vehicle to handle if not used to that mass. There will be some wear regardless.
Willis, I don’t know where your pH graphic originated but I’ll be concluding my day soaped down in the shower, though thereby fortunately not at all exposed to anything close to the alkalinity of the very lye used (and chemically defanged) in the making the soap from animal fats! Also sodium hypochlorite bleach is classically produced by passing chlorine gas through a lye (sodium hydroxide) solution, so the resulting pH is from the latter and not then neutralized with acid because that would destabilize the solution with chlorine gas release.
Consider the following restatement of their opening sentence.
“The Pacific Ocean is becoming so less caustic it is starting to dissolve the shells of a key species of crab, according to a new US study.”
The absurdity of “OA” language is made apparent when you consider that calling something acidic, that is on the alkaline side of neutral, is the equivalent of calling any substance on the acid side of neutral, caustic!
To restate, if you dilute an acid*, is it better described as (a) less acidic or (b) more caustic? /rhetorical
The stupid, it really burns! 😉
Great read thanks Willis