Can you keep an open mind on the cause of winds? Climate science needs your help!
by Anastassia Makarieva
Many of us who have become researchers have been attracted by the dynamic and constructive debate that lies at the heart of scientific progress. Every theory is provisional waiting to be improved or replaced by a more thorough understanding. In this perspective new ideas are the life-blood of progress and are welcomed and examined eagerly by all concerned. That’s what we believed and were inspired by. Is climate science a dynamic field of research that welcomes new ideas? We hope so – though our faith is currently being tested.
Five months have not been enough to find two representatives of the climate science community who would be willing to act as referees and publicly evaluate a new theory of winds. Of the ten experts requested to act as referees only one accepted. This slow and uncertain progress has caused the Editors to become concerned: recently they “indefinitely extended” the public discussion of the submitted manuscript. The review process is perhaps becoming the story.
Here the authors share their views and request help.
Background
On August 06 2010 our paper “Where do winds come from? A new theory on how water vapor condensation influences atmospheric pressure and dynamics” was submitted to the Atmospheric Chemistry and Physics Discussions (ACPD) journal of the European Geosciences Union. There we proposed a new mechanism for wind generation based on pressure gradients produced by the condensation of water vapor. ACPD ensures transparency during the review procedure: the submitted manuscripts and subsequent reviews are published online and available for public discussion. Authors can follow their submission through the process: they see when the Editor invites referees and whether they accept or decline.
Here are the standings as of 20 January 2011:

The Editor handling our paper has invited ten referees so far. Only one, Dr. Judith Curry, accepted. After 10 November 2010, in the record there have been no further attempts to find referees.
Normally ACPD’s discussion should take eight weeks. But in early January 2011, after twelve weeks in process, the status of the discussion of our manuscript was changed to “indefinitely extended”. In a recent letter to the authors, the Editor-in-Chief admitted that handling ‘a controversial paper’ is not easy, but assured us that the Journal is doing their best.
Discussion of our propositions secured over a thousand comments in the blogosphere within four weeks of publication indicating wide interest. Among the ACPD discussion participants two are active bloggers. Does blog culture outcompete formal peer review in evaluating novel concepts? It’s an open question. But let’s take a moment to focus on science.
Why condensation-induced dynamics is important
It would be generally useful to understand why the winds blow. It is sufficient to note that understanding the physical bases of atmospheric circulation is key for determining the climate sensitivity to changes in the amounts of atmospheric greenhouse substances, which is currently a highly controversial topic. The lack of current understanding may not be widely recognized outside the climate and meteorological community. But within the community moist processes in the atmosphere are admitted to be among the least understood and associated with greatest challenges. Not only theorists, but also modelers recognize their existence. For example, in a paper titled “The real holes in climate science” Schiermeier (2010) identified the inability to adequately explain precipitation patterns as one of such holes. In particular,
“a main weakness of the[ir] models is their limited ability to simulate vertical air movement, such as convection in the tropics that lifts humid air into the atmosphere.”
Any meteorological textbook will provide a discussion of buoyancy-based convection: how a warm air parcel ascends being lighter than the surrounding air. The convective instability of moist saturated air, so far neglected by the meteorological theory, is different. Any upward displacement of a saturated air volume, even a random fluctuation, leads to cooling. This causes the water vapour to condense. Condensation diminishes the total amount of gas and thus disrupts the hydrostatic distribution of moist air (if a hydrostatic equilibrium exists it is unstable to any such minor movements). The conclusion: moist saturated atmosphere in the gravitational field cannot be static.
Our analyses show that the current understanding of air movements being dominated by temperature and buoyancy is incomplete and flawed. Rather we find that the phase changes of water (condensation and evaporation) can play a much larger role than has previously been recognized. You can find out more if you see our paper. We would hope that a dynamic and advancing science would welcome new ideas.
Can the blogosphere help?
Perhaps we can help the Journal review our paper with your help. Are you an open minded climate scientist who would be ready and competent to discuss our ideas?
The ACP Chief-Executive Editor Dr. Ulrich Pöschl is aware that we are inviting your helps and asked that the following issues be noted (we quote):
1) ACPD is not a blog but a scientific discussion forum for the exchange of substantial scientific comments by scientific experts.
2) The open call for scientific experts who would be ready to act as potential referees would be a private initiative of the manuscript authors.
3) The list of potential referees compiled by the authors will be treated like the suggestions for potential referees regularly requested. The responsibility and authority for selecting and appointing referees rests exclusively with the editor.
If you have no conflict of interests and are willing to review our paper please contact the corresponding author (A. Makarieva) and we will forward your details to the Editor as a potential referee. For those who would like to remain anonymous please approach the ACP Chief-Executive Editor directly. We would be very grateful for your help – we have faith in you.
Anastassia Makarieva
on behalf of the authors:
A.M. Makarieva, V.G. Gorshkov, D. Sheil, A.D. Nobre, B.-L. Li
P.S. Thanks to Jeff for hosting our appeal on this blog. For a list of publications relevant to condensation-induced dynamics, please, see here.
An interesting approach from both angles.
Now this is how I have always believed “scientific inquiry” was supposed to be … form a theory, test it and if it seems to hold up, ask others to try and poke holes in it.
How refreshing!
Stimulating, unbiased and refreshing science. Censured of course…
I am becoming more and more impressed by Judith Curry. She is becoming a fine, courageous scientist. If only others would adopt her principled moxie, we might actually progress climate science. Kudos. GK
Thanks to Anthony for carrying this piece. I’ve closed the Air Vent blog down and am taking what will be at least an extended vacation from it to focus on other things. It is my hope that at least one more person will agree to review the paper in question above. It would certainly be another victory for the most popular climate science blog in the world.
I think Climategate and the revelation that “global warming” has been produced mainly by eliminating the coldest weather stations suggests very strongly that the peer review process needs a change anyway.
I have also been dismayed at appalling papers appearing in my field that were 20% science and 80% some clever idea of how some critter MIGHT be negatively impacted by warmer temperatures. Truth cannot be arrived at by such a process.
A controversial idea needs a hearing. Most prove rubbish–but this is breakthrough territory. So this delay is not a trivial matter.
Interesting that the only invitee with any courage was Judith Curry. What a lady!
I guess it’s hard to find a climate scientist who is willing to discuss anything that doesn’t involve CO2 induced climate destruction.
“a main weakness of the[ir] models is their limited ability to simulate vertical air movement, such as convection in the tropics that lifts humid air into the atmosphere.”
What, fossil fuel CO2 is going to cause the “destruction of creation” but it can’t even do that?
Re: “Our analyses show that the current understanding of air movements being dominated by temperature and buoyancy is incomplete and flawed. Rather we find that the phase changes of water (condensation and evaporation) can play a much larger role than has previously been recognized.”
The same obstructionism is happening within the space sciences as well. Well-meaning experts have come to view their educations as an unassailable foundation for their reasoning, rather than a starting point for an objective, skeptical investigation.
Condensation occurs as a result of the van der waals force. At any instant in time, electrons fail to electrically shield the positive nucleus of an atom. If the random movements of particles — the temperature — is sufficiently low, then like atoms will line up in a three-dimensional lattice structure …
hole-nucleus-electron-hole-nucleus-electron … etc.
In other words …
+ – + – + –
The molecule in question need not be bipolar in order for this resonance to occur, but bipolar molecules possess a stronger resonance — as with water. This is incredibly important to realize because bipolar molecules can be suspended within an electric field. Remember the JJ Thompson oil drop experiment?
In other words, the liquid state of matter is an electromagnetic resonance of molecules. Drop the temperature further, and you have yet fewer random collisions, and the electromagnetic resonance can enter into the solid state.
I urge all climate scientists to not just understand the effects of such processes, but also to understand how the electromagnetic resonance — the van der waals force — works on the microscopic scale. Were it not for this resonance, there would be no such thing as “capillary water” — a principle which serves as the foundation for the entire soil food web, and things like osmotic pressure — which plants require for feeding. Water’s inherent resonance is why it can be siphoned — in other words, why it appears to possess an apparent “tension”.
This is also the principle force which creates the sometimes-elaborate structures we see solids form into — like crystals and snowflakes.
Now, with regards to wind, I would urge people to consider another aspect of electricity: Look at the function of the ionic bladeless fan. When charged particles are acted upon with an electromagnetic field, this movement exerts a drag upon molecules as well. This is the same principle for why electromagnetic lifters can levitate. The movement of ions can induce a wind.
I would caution that so long as researchers barricade themselves into their own disciplines, and fail to consider outside theories for weather, that we will settle into long-term confusion and fail to make accurate predictions. The most complex questions out there — like weather and the Sun — require an interdisciplinary approach where theorists are eager to hear out all of the theories available to them. If you see evidence that the lines of communication have been broken, then don’t imagine that the underlying mechanisms for weather must be complex. The very act of silo’ing the disciplines, and then creating barriers to communications, creates the complexity which we struggle with.
And this gets to the very problem which you guys are facing: How can we force people like particle physicists, quantum physicists, cosmologists and climate scientists to listen to the objections and research of outsiders? Currently, there exists no check-and-balances to the current top-down approach. Our scientific institutions have become authoritarian. The research exists to protect the ideology, instead of serving mankind. We’ve made a real good mess of things!
But these are no accidents. These are all natural ramifications of what it means to be human. This is just human psychology. Left unrestrained by philosophy of science, science starts to take on the imprint of our personal preferences and prejudices — and our scientists come to imagine that their purpose is to prove that which they were taught in college.
Oops … That should read “dipolar” … Not “bipolar” …
And this …
“At any instant in time, electrons fail to electrically shield the positive nucleus of an atom.”
Should read …
“At any instant in time, electrons fail to electrically shield the positive nucleus of an atom ON ALL SIDES.”
Not enough coffee this morning … !
I don’t think it is true at all that this is ignored in meteorology. Here in Rochester, we get lots of lake effect snow and they are constantly talking about the instability produced by the cold air blowing over the warm lake waters. I used to think the role of the lake was just to make the air more unstable because it heated it and that another independent role of the lake was to provide the moisture that leads to the precipitation. However, I recently went through the module on lake effect snow available here http://www.meted.ucar.edu/ and understood for the first time that the addition of moisture to the air itself also increases instability because of the fact that a saturated air parcel cools less as it ascends (because the condensation of the vapor vapor releases latent heat). While this was a new understanding to me, I found no evidence that it is new to those who are actually formally trained in meteorology, atmospheric, or climate science (which I am not)…In fact, it seemed to be such old-hat to them that they basically took it as a given.
There seems to be much confusion between temperature change and quantity of heat change. When relevant to H2O vapor, cooling in temperature does not necessarily equate to loss of heat. Adiabatic cooling does not result in condensation of H2O vapor as there is no change in heat content. Heat has to be removed before H2O changes from its vapor state to its liquid state, i.e., condenses.
Well, golly gee, that leaves me out . . .
In a vapor steam heating plant, which functions at near atmospheric pressure, water expands 1,700 times into steam, which large expansion delivers the latent heat to radiators, where the saturated steam returns to the original vastly smaller volume of water. Moist air is not steam, but the great expansion and contraction of the water vapor portion of that air as it moves up from, say, ocean surface to condensing elevation, suggests that something might be learned from publication of the above paper.
Good to see Dr. Judith Curry willing to weigh in. But not surprising – she’s still a scientist.
Similar to heat transfer classes in University. Heat transfer is a hard enough class, once you change the topicto heat and mass transfer, all the nice dimensionless analysis and Fourier equations become less important and you’re slogging through steam tables.
anyone that knows anything knows it’s a hard problem and we probably can’t solve it for anything bigger than a teakettle.
I actually know someone who can possibly help. Anastasia, drop me a line. The person I know did atmospheric chemistry with NASA for several years.
To Jeff Id: Thanks for everything you did on the Air Vent, not least breaking Climategate! Many best wishes in what you do next.
Moisture-saturated warm air rises as water runs downhill, inducing a pronounced condensation/cooling effect which –ostensibly for reasons of interactive complexity– climate hysterics’ atrophied atmospheric models have purposefully ignored.
Anyone expecting the Green Gang of Briffa, Hansen, Jones, Mann, Trenberth et al. to entertain a serious research endeavor has been in hibernation since c. 1988. Death-eaters, Thanatists such as Paul “Population Bomb” Ehrlich, John “Seething Maggots” Holdren, latterly Keith “Down with Civilization” Farnish, will no more commit to objective or even rational inquiry than abjure homicidal “No Pressure” incitement to child-murder.
“Hot air rises, moisture condenses, rain doth fall”– what are you, some kinda kook?
“Heat has to be removed before H2O changes from its vapor state to its liquid state, i.e., condenses.”
Let me get this straight.. take a cubic meter of air(N, H2O vapour, CO2, O2) etc.. at some critical height where the lapse rate of T with Pressure causes condensation(rain).. thus water(rain)falls down out of the cube, warm air goes out the top.. that motion upwards of warm (dryer) air removes the incoming heat in the cube..
Well I haven’t read the paper yet (I will); but some immediate trivial questions come to mind.
#1 Some of the strongest winds on earth occur over the Antarctic plateau; which also is among the driest places on earth; so I don’t imagine a lot of water condensation going on there to start up winds ?
#2 How does the pressure created by molecular collisions required to keep water droplets buoyant, compare with the actual vapor pressure of the same amount of water before it condensed ?
Does this paper explain those things ?
But as to the approach (to review), seems like an interesting way to consider things.
Laurence M. Sheehan, PE says:
January 21, 2011 at 9:12 am
“When relevant to H2O vapor, cooling in temperature does not necessarily equate to loss of heat.”
Any vapor, actually. One cubic meter of gas that expands to three cubic meters of gas from a loss in pressure will have the same total energy content, but spread out over triple the volume. Temperature is average energy content per unit volume, so the temperature drops without any loss of energy by the mass as a whole.
“Adiabatic cooling does not result in condensation of H2O vapor as there is no change in heat content. Heat has to be removed before H2O changes from its vapor state to its liquid state, i.e., condenses.”
Meterologists disagree. Condensation will occur as soon as the dewpoint of the entire mass is reached. So although the total energy content of the mass remains the same, the drop in average energy content (temperature) determines the dewpoint.
http://en.wikipedia.org/wiki/Lapse_rate#Significance_in_meteorology
Martian wind speeds may average around 20-30 mph while bursting much higher during storms. Mars’ atmosphere is 0.03 % water vapor.
I’d say the phase change of water plays little role, if any, in the Martian winds, why should it play a significant role here on Earth?
I don’t believe temperature gradients explain winds on Earth either. I expect if the planet didn’t rotate, then there would be no wind, and only small mixing of atmospheric gases at the tropics.
My belief is that the mountain ranges and canyons stir up the atmosphere as the planet spins.
The average wind speed on Earth is about half of that on Mars (quick Internet search, not definitive). Mars has the largest mountains and canyons in the solar system, and a higher wind speed than Earth.
To me, this lends credence to the physical movement of air creating winds, not a temperature difference or water phase changing.
I would expect an easy experiment would be to spin a perfect sphere in a test chamber with several colored gases injected at different temperatures. I don’t think the gases would mix that fast. And once mixed, I don’t expect much wind would be observed.
Compare that to a spinning sphere with ridges and indentations to simulate mountains and canyons. I expect the gases would mix much faster and there would be a sustained wind observed.
In my vast scientific experience, I’ve come to the conclusion that the primary cause of wind has often been attributable to an overconsumption of beans.
“How does the pressure created by molecular collisions required to keep water droplets buoyant, compare with the actual vapor pressure of the same amount of water before it condensed ?”
Strange, I would have thought that water droplets migrate downwards,(as allowed to by air friction moving up) until they reach less than saturated air, then volatize, thus creating an illusion of staying buoyant.
Just read the abstract… Uhm, Are you telling me that evaporation/condensation has really been overlooked by the atmospheric physics research? Seems rather obvious and hard to miss that a phase change from gas-to-liquid represents a net loss of pressure. How can it be that our understanding of high/low pressure dynamics on Earth overlooked this?
As I understand the paper (read not too carefully) and most comments, the main question is the relative importance of latent heat contributions as compared to a mass defect contribution. The latter is basis for the claim of something like a “new theory of wind”, while the mass defect in the view of most commentators is a relatively small correction to latent heat produced buoyancy (in my opinion, too). The latent heat is very large (of order 0.4 eV per H2O molecule) and unique among gases, as it is produced mainly by the hydrogen bond and not by, e.g.; van der Waals forces, as Chris Reeves claims.
If the paper is revised along the line “first time analytical treatment (if true) of the mass defect correction to latent heat buoyancy” I would see a chance for acceptance. I guess that is about the line which Judy Curry advocates.
Would not a fully generalized account of atmospheric circulation find application in characterizing extra-terrestrial atmospheres? The authors’ approach seems to me to offer a (better?) way forward to explaining the main features of circulation on the gas-giant planets, though it would probably require more data on the atmospheric columns of those planets than we have at present.
I will join with others in wishing Jeff a pleasant well deserved break! I would leave him with this quote; “Any activity becomes creative when the doer cares about doing it right, or better.”
Rest well.
I thought reviewers were to remain anonymous? I like the idea of having Dr Curry review it, but it really should be kept confidential (until after the review at the very least).
I’m surprised that the paper’s subject is something that is not heavily studied. This subject would be easy to observe and model, as long as the scale is kept very small (to start). I would love to work on this research!
Why isn’t this paper submitted to the Journal of Atmospheric Science or another journal with a long-established legacy of editors and reviewers that have sufficient experience and skill to understand the arguments?
Suggesting dr. Browning who used to visit ClimateAudit.
retired I’m sure. That works in his favor
We are very grateful to Mr. Anthony Watts for carrying our appeal at WUWT. We hope very much it will evoke a constructive reaction.
George E. Smith
January 21, 2011 at 9:47 am
The strong winds over the Antarctica are due to the peculiar relief of the continent, rapid radiational cooling of air due to a very low vapor and clouds (greenhouse substances) amounts and concentration of mechanical energy in a narrow area. The continent occupies less than 3% of total Earth’s area. Atmospheric dynamics in the Antarctic versus atmospheric dynamics in the tropics, where most kinetic energy is generated, have different mechanisms.
Water droplets are not maintained in the atmosphere by pressure exerted by molecular collisions (see here, Sections 2 and 3, p. C12009, for a more detailed discussion of this inconsistency as implemented in numerical models). Water droplets can only be maintained if there is an ascending air flow (i.e., a macroscopic air movement).
Michael D Smith
January 21, 2011 at 10:31 am
Please, note that Dr. Judith Curry publicly revealed her identity having posted an open review.
Ryan N. Maue
January 21, 2011 at 10:39 am
This is an interesting point. It is presumed that the Editors and Reviewers of ACP (a peer-reviewed journal with impact factor of4.88) , with the Advisory Board chaired by Noble Prize winner Dr. Paul J. Crutzen, do not have sufficient experience and skills to understand the arguments. Personally, I do not think so.
Another logical possibility is that the climate community is — on average — unprepared to an open debate of science. In this case, the extraordinary low proportion, <1/10, of which the referee table testifies, would mean that people are scary of publicly expressing their views, even anonymously. In the ACPD the authors are entitled to reply to any review and may expose errors, if any, in a referee's assessment of their work. In contrast, in the dominating closed peer-review system the exchange between authors and negative referees is not encouraged.
I find strange the idea that somewhere there is a secret and localized group of climate scientists who "understand the arguments". Nobody knows them, and even if somebody gets to know and approach them with a review request, they decline the invitation. Only the right Editors "with skills" may approach them and hope for a favorable outcome of their invitation.
Really, shouldn't an average community member be competent and responsible?
Remember the JJ Thompson oil drop experiment
We talk about Millikan’s oil drop experiment in the UK.
Just let me get this one thing straight . . . Scientist do say that part of the cause of wind “is caused by the fact that Earth hurls around the sun as a part of our solar system.” . . . . plus the fact that it spins once around on it’s axis in a 24 hour period of time . . . RIGHT????? I mean to ask . . . this is assumed and acounted for right?
I mean Bill Nye the Science Guy can speak in plain english . . .
“”””” Anastassia Makarieva says:
January 21, 2011 at 10:47 am
We are very grateful to Mr. Anthony Watts for carrying our appeal at WUWT. We hope very much it will evoke a constructive reaction.
George E. Smith
January 21, 2011 at 9:47 am
>…………………………………………….<
#2 How does the pressure created by molecular collisions required to keep water droplets buoyant, compare with the actual vapor pressure of the same amount of water before it condensed ?
Water droplets are not maintained in the atmosphere by pressure exerted by molecular collisions (see here, Sections 2 and 3, p. C12009, for a more detailed discussion of this inconsistency as implemented in numerical models). Water droplets can only be maintained if there is an ascending air flow (i.e., a macroscopic air movement). """""
Thanks for commenting so quickly Dr Makarieva. Those were serious questions by the way; but I suspected that the Antarctic winds involved a much greater expanse than just the Antarctic highlands.
But as to question #2.
So I'm a drop of water in the atmosphere, let's assume I'm uncharged; so we don't have to get into a Millikan suspension situation. but I am fully subject to the gravitational force, which IS pulling me down towards the ground.
BUT; I am not being held up by collisions from air molecules ; so just what Physical force is it that I am being subjected to that overcomes gravity ?? The weak force, and the strong force don't have enough range to affect me; and I elected to not be subject to the electromagnetic force; by staying electrically neutral.
If the air was stationary (statistically), then yes I would expect the molecular collisions to balance out; well leaving a micro "Brownian" motion or random walk effect if you will, so that gravity should win, and I should sink.
If as you say, the air is ascending (mass flow); then the mean molecular momentum around me is no longer zero but has (at least) a positive z axis value . But those rising air molecules can only communicate with me via collisions; and thereby deliver the required rate of change of momentum to counterract the gravity force. So I maintain, that I am indeed being held aloft (and driven aloft) by just those molecular collisions; albeit from a set of molecules that do not have a net zero z-axis momentum.
And Newton's Laws would require that those colliding molecules, are required to be made aware of my weight, via the reaction on their own momentum; so my weight must in fact result in a pressure change in the form of an increased downward force on those air molecules caused by my gravitational weight (well me and my buddies together).
Note I am not disagreeing with your statement that the droplet buoyancy can only be maintained by mass air flow (upwards); I'm just ponting out that providing that buoyancy, must result in a net pressure increase due to the weight of those water droplets; and I'm curious as to how that pressure compares directly to the H2O vapor pressure that would exist sans the condensation.
And yes we are civilised here and happy to engage; sometimes we are norty; but we try not to get mean.
And yes I am going to read your paper; but I am a working stiff; whose computer does all my thinking for me; which gives me moments to dash in here, and learn from folks like you.
George
And for the legal disclaimer; since you may be a recent visitor; NO I am not now, and never have been, the 2009 Nobel Physics Prize Winner.
At last a true crusader in the cause of blog science. Denial Depot has been fighting for just this much maligned cause for…well…some time:
“We are not afraid to be called climate “deniers”. In fact we embrace it as medal of honor bestowed on us by our alarmist foes. Galileo was a Denier. It is not an insult. I call this blog “Denier Depot” for that reason.
Welcome to my climate science blog.
I believe that one day all science will be done on blogs because we bloggers are natural skeptics, disbelieving the mainstream and accepting the possibility of any alternative idea.
We stand unimpressed by “textbooks”, “peer review journals” and so-called “facts”. There are no facts, just dissenting opinion. We are infinitely small compared to nature and can’t grasp anything as certain as a fact.
Nothing is settled and we should question everything. The debate is NOT over Gore! When so-called “experts” in their “peer reviewed journals” say one thing, we dare the impossible and find imaginative ways to believe something else entirely.”
http://denialdepot.blogspot.com/
Here, fellow deniers, you can read ground breaking stories every bit as imaginative as the “Where do winds come from?” yarn!! Such as this online comments reviewed piece proving that Arctic ice cover is, as us hard core deniers have known all along, actually INCREASING!!
http://denialdepot.blogspot.com/2010/09/arctic-news-and-global-cooling-update.html
Yes, blogosphere scientists “we have faith in you”
#
Chris Reeve says:
I would caution that so long as researchers barricade themselves into their own disciplines, and fail to consider outside theories for weather, that we will settle into long-term confusion and fail to make accurate predictions. The most complex questions out there — like weather and the Sun — require an interdisciplinary approach where theorists are eager to hear out all of the theories available to them. If you see evidence that the lines of communication have been broken, then don’t imagine that the underlying mechanisms for weather must be complex. The very act of silo’ing the disciplines, and then creating barriers to communications, creates the complexity which we struggle with.
And this gets to the very problem which you guys are facing: How can we force people like particle physicists, quantum physicists, cosmologists and climate scientists to listen to the objections and research of outsiders? Currently, there exists no check-and-balances to the current top-down approach. Our scientific institutions have become authoritarian. The research exists to protect the ideology, instead of serving mankind. We’ve made a real good mess of things!
But these are no accidents. These are all natural ramifications of what it means to be human. This is just human psychology. Left unrestrained by philosophy of science, science starts to take on the imprint of our personal preferences and prejudices — and our scientists come to imagine that their purpose is to prove that which they were taught in college.
#
Chris, well said and worth acknowledging!
I apologize profusely for not yet being in a position to provide assistance.
Alas, my conflict of interest is not yet resolved.
My old economics lecturer, when dealing with the reality that some people confuse cause and effect used to quote Damon Runyan who amusingly quipped that “wind is caused by trees waving their branches!”
Temperature increases and CO2 increases seem to cause a similar misunderstanding in Al Gore et al.
Sailing in the tropics you might expect you are safe when a thunderstorm is downwind of you. However, they have a nasty habit of acting like a giant vacuum cleaner, sucking up the wind ahead of them as they travel up wind to clobber you with torrential rain and hurricane force winds. After they pass you get a tremendous downwash of very cold, dry air – a wind from directly above.
I always thought it was a given that the energy that drives these storms comes from the condensation of water vapor, not simply the rising of warm air. As the vertical windflow increases, they suck in more and more moisture laden air, and the storm increases in intensity.
Approaching the ICZ in a small boat on the open ocean can be quite something. It is awe inspiring and more than a little intimidating.
A.M. wrote:
“This is an interesting point. It is presumed that the Editors and Reviewers of ACP (a peer-reviewed journal with impact factor of4.88) , with the Advisory Board chaired by Noble Prize winner Dr. Paul J. Crutzen, do not have sufficient experience and skills to understand the arguments. Personally, I do not think so. ”
You reflexively responded. I have no opinion on the open-review process nor ACP. I just wondered why JAS isn’t an appropriate venue. The climate community is a separate matter altogether.
That so many nominated reviewers declined comes as no surprise. The thesis that Maharieva et al propose goes to the very absics of physics at a microscopic level with conclusions drawn at the macroscopic. For starters, few, if any, in climate climate science have mastered the intricacies of rigorous physics well enough to negotiate that leap. And the majority of them feel threatened by a thesis that stands to upset the whole apple cart of funding based on pseudo-thermodynamic arguments that do not survive rigorous physical scrutiny. My suggestion would have been to submit a theoretical paper first to a pure physics journal, and only then–armed with conclusive experimental data from field measurements–undertake the daunting task of revealing the implications to climate scientists. After all, the Soviet Union was not toppled overnight and the collapse would not have occurred with Stalin’s hand on the helm.
Condensation aloft is indeed not a simple process, despite what many texts imply. Water vapor needs condensation nuclei as well sub-dewpoint temperatures. Instead of being “released” diectly to the air, the latent heat is actually recaptured as ssensible heat in the condensate, which has a greatly higher thermal capacity as well as greatly smaller volume than the vapor. That is how enthalpy balance is maintained.
Publication of Makarieva et al would make a vital addition to meteorological understanding. In factors that affect climate, however, the thesis is more a scientific footnote than a chronicle of a revolution, because the winds and currents that circulate heat poleward from the tropics are the product of macro-scale horizontal pressure gradients and planetary rotation, rather than the intricacies of meso-scale moist convection and condensation.
I notice the main stream still has no knowledge of ionic charges or static charge suspension of droplets against gravity, that keeps the droplets from falling or condensing with each other (being force separated by mutual static repulsion).
Nor of the lunar declinational tides in the atmosphere that drives the meridional flows that maintain the positions of the jet streams, nor their interactions with the phase related tides that drive the Trade winds.
Totally unknowing of the charge and discharge of the homopolar generator effects that drive tornado production in the spring, Hail outbreaks in mid summer and hurricanes post mid summer till fall.
Mainstream progress is like tree root growth through solid rock, root hairs dissolving the mineral base ahead of itself. Electromagnetic and static charge gradients when considered produce rapid growth like corn and Pumpkins in compost.
Tidal interactions work like an irrigation system keyed to the plants needs, and drives the global circulation patterns by a combination of static, tidal, and moisture with latent heat transfers, into the soil and out of the atmosphere, to balance the ebb and flow of the changes in the background charge levels. While stabilizing the pole to equator heat energy and ionic charge budgets.
They’re getting rather confrontational over at the Nation (having lost the science debate, basically):
http://www.thenation.com/article/157903/confronting-climate-cranks
The Roman Gods of the winds, the Venti, in their baroque splendour.
http://iactaaleaest.files.wordpress.com/2008/07/tower-of-the-winds-imr.gif
If only in real life it was as easy to predict which of the Roman Gods would next exhale their breath onto us mere mortals, as the animation suggests…
Wow Richard Holle, that was some poetry.
I’ve recently found out about winds on Venus: near the surface they are apparently reasonably quiet, but higher up they are hyperventilating: blowing faster than the planetary rotation. Some 200 kph – with the net effect that high up Venus’ temperatures are pretty well mixed despite its extraordinarily long night. But hey, how come winds can blow that fast?
Right now I’m soaking up clues regarding electrical and magnetic influences which I think scientists have barely started to work on properly. It seems to me that the higher up one goes in the atmosphere, the more we see effects dominated by the plasma state of matter, rather than the gas state of matter. And it seems to me that electrical charges can sure have huge influences, far more than are generally realized.
Richard Holle reminds us about electrostatic forces in effect enabling clouds to exist at all, if I’ve understood correct. That sets off a huge flash of light for me. The hypothesis here is, with the formation of clouds, do we essentially have a plasma formation really low down in the atmosphere? and are we looking to ionized plasma laws for some (not all) of the clues as to behaviour?
This theory may be indirectly attacking one of the planks of global warming.
For instance, it is always presented that
– a) temperatures increase
– b) Because temperatures increase, there will be more extreme events.
Yet we know heat will be expelled from the surface by either radiation resulting from an increased temperature, or an increase in convection. All wind is either directly or indirectly caused by convection. It is an “or” situation, not an “and” situation. That heat which is carried away by enhanced convection will not increase temperature to be radiated away.
So if extra long-wave radiation merely makes it one percent windier, as is likely to occur if this phenomena is modeled, that will take away all the GHG threat, with strong implications for research funding.
The proponents need to understand. Only theories that will enhance the anthropomorphic threat will be considered.
Anna, have you looked at Erl Happ’s work that looks at winds relating to sea level pressure differentials? I found it very thought-provoking, in particular I’d like to know why there is an extraordinary permanent latitudinal dip in pressure at 60 degrees South. Now is this evidence for your hypothesis? At the physical level, 60 degrees S +- 5 degrees is the ONLY place on the globe where winds can blow East-West continually over the oceans without interruption from land.
ge0050 and Fred Harwood are on the right track.
It’s amazing how many comments above keep bringing in hot air as the driver, not moist air.
I had the opportunity years ago to experience sailplanes for a few years. When you get up in the atmosphere and that is your only ‘engine’ you get a totally different view of this discussion. This paper needs some deep, deep consideration. With that experience I view it always from the moisture side, not whether it is warm or cool. The moisture is the real driver, the others are secondary effects. You will never get a really, really good day just because it is hot, it has to be moist and hot. On those days you can fly for hundreds of miles.
Read closely what ge0050 and Fred Harwood brought into the discussion above. The expansion and contraction due to the moisture differential is where it’s all at. It has always amazed me how meteorologists tend to speak in pressure fronts and temperature differences without bringing into their daily presentation the moisture (latent heat) aspect for it’s that which creates the former, not totally, but a greater degree.
This paper does need to be reviewed!
peter_ga says:
January 21, 2011 at 3:08 pm
This theory may be indirectly attacking one of the planks of global warming.
——-
You hit it on the nose there!
Anastassia Makarieva
on behalf of the authors:
A.M. Makarieva, V.G. Gorshkov, D. Sheil, A.D. Nobre, B.-L. Li
I think this is a brilliant article and I congratulate you. In time it will result in a new paradigm of how the great ITCZ thunderstorm belt sets a big piece of the atmosphere in motion (temperature differentials and coriolis effects be damned). This may be the meteorological equivalent of “plate tectonics”.
Willis, here is the thermodynamical basis for your great “heat engine/temperature regulator” theory. The Makarieva paper could use some graphical figures illustrating how what they describe actually works in the real world. The graphs 1a-1c mean little to 99% of the world.
Thank God for water, truly “manna from heaven”.
Here’s our lovely author…
http://thd.pnpi.spb.ru/~makariev/
Any upward displacement of a saturated air volume, even a random fluctuation, leads to cooling. This causes the water vapour to condense. Condensation diminishes the total amount of gas and thus disrupts the hydrostatic distribution of moist air (if a hydrostatic equilibrium exists it is unstable to any such minor movements). The conclusion: moist saturated atmosphere in the gravitational field cannot be static.
As an HVAC engineer we well acquainted with evaporative cooling in cooling towers. I find that scientists trip themselves up making the assumption that processes are uniform in nature. They aren’t. Just as in a cooling tower only some (5%) of the water is evaporated carrying away heat to drop the remaining liquid water (95%) to 15 degrees F below ambient. I would bet on a molecular level there are low (cold) and high (hot) pressure air pockets which would account for the expansion and rise of warm moist air irregardless of the condensation. This is a matter of proportion between hot moist air versus cold water, that’s if you accept water expands a 1000 times in volume when heated to steam.
@sky says:
January 21, 2011 at 12:22 pm
“…Publication of Makarieva et al would make a vital addition to meteorological understanding. In factors that affect climate, however, the thesis is more a scientific footnote than a chronicle of a revolution, because the winds and currents that circulate heat poleward from the tropics are the product of macro-scale horizontal pressure gradients and planetary rotation, rather than the intricacies of meso-scale moist convection and condensation….”
I think you should go read the paper. Their thesis is that moist convection systems in fact produce a global horizontal pressure differential, sort of, you know, like that observed in nature.
Actually, each thunderstorm is a micro or meso (I don’t know your scale) affair. But the ITCZ is a global/macro thing operating 24/7. In the same vein, volcanoes are micro affairs, forming here and there above a subducting plate that stretches in some cases for thousands of miles. But they mean a heck of a lot to us people.
On page 24030 her paper gets into the suction aspect I mentioned locally for sailplane pilots though this same affect happens over large areas at the regional scale. I didn’t mean to imply that meteorologists are unaware of these factors just that you do not ever see such graphics on the nightly news even though that is really what is going on and that leaves the genear public with the wrong intuition of the atmosphere.
“”””” stephen richards says:
January 21, 2011 at 11:40 am
Remember the JJ Thompson oil drop experiment
We talk about Millikan’s oil drop experiment in the UK. “””””
Well I’ve never heard of JJ Thompson’s OD experiment; which doesn’t mean he never did one; just that nobody told me or asked me to come and watch. Adn Milliken used an oil droplet, because earlier attempts with water, failed, becasue the droplet evaporated too rapidly, so he changed to a low vapor pressure oil instead. Of course that experiment was to measure the electron charge; and that is why in my question to Dr Makariev specifically called for zero charge conditions so my water droplet, would be influenced only by gravitation, kinetic collisions with anything else in the enighborhood.
Even a single neutral atom can fall freely under gravity, in a good enough vaccuum; and of course can be caught in an “Optical Trap” and suspended, as was done by Steven Chu to win his Nobel Physics Prize. Those who made optical traps years before him, and taught him how to make them, did not receive any Nobel Prize; nor did any of Chu’s co-workers. But of course the Nobel Prizes are NOT Political; unlike the Peace Prize; which IS totally political.
All one needs to do is put some warm tap water in the bottom of a plastic jug or soda bottle, cap it tightly and watch. How the warm=mongers could discount the effects of condensation on winds speaks volumes about their understanding of the basics.
JimF says:
January 21, 2011 at 4:42 pm
“I think you should go read the paper. ”
I read the paper some time ago and am repeating here my comments (on tAV) on the “danger of over-reaching” on the spatial scale of the applicability of their thesis. Condensation, after all, is nowhere near as ubiqutous as the winds are.
Well. As the point of this was to find reviewers. Has Ferenc Miskolczi been considered?
He has the requisite qualifications.
DaveE.
“”””” Ed_B says:
January 21, 2011 at 10:00 am
“How does the pressure created by molecular collisions required to keep water droplets buoyant, compare with the actual vapor pressure of the same amount of water before it condensed ?”
Strange, I would have thought that water droplets migrate downwards,(as allowed to by air friction moving up) until they reach less than saturated air, then volatize, thus creating an illusion of staying buoyant. “””””
Well Ed; well if you read my response to our lead author, you should come to look at thw water droplet from the point of view od some kind of Maxwell’s demon riding along on our droplet. I specifically excluded a charged droplet, so w could consider the droplet to be under the influence only of gravity, and whatever influence it’s environment has. Gravity and the EM force both have infinite range; and the strong and weak forces are both too short range to affect our droplet.
So you talk about “air friction moving up”; Dr Makarieva talked about an air mass flow upwards. But HOW do those manifest themselves to our Maxwellian pilot and his chariot ? Well I’ve excluded all the “action at a distance” forces other than gravity, which will cause the droplet to fall to earth; so that leaves actual mechanical contact with other material objects, as the only means of counterracting gravity. And assuming clean air, that can only be collisions with air moelcules; well we’ll excuse the occasional Cosmic ray coillision shall we.
Both you and the good Dr. are correct, that the neighboring air mass, must have a net upward flow (z-axis), because the net result of all the collisions is to not move the droplet, relative to the centre of mass of the local air; except as dictated by gravity.
I’m simply saying that in the centre-of-mass space of the local environment (which is also subject to gravity) the average effect of all the molecular collisions must be zero, but they must impart a net upward rate of change of momentum in Laboratory space to keep the droplet moving upward with them, and that means the weight of the droplet results in a reaction on those neighboring molecules which is equivalent to a net downward pressure on those local air molecules, so the droplet’s weight does result in an increase in the local pressure as seen by the molecules; the same thing would happen if they were colliding in their upward journey; with an immovable object.
So I’m arguing that the weight of the liquid droplet(s) DOES contribute a non-zero increase in the local air pressure; and I’m simply curious as to whether Anastassia, and her colleagues has compared that pressure to the corresponding vapor pressure (as a partial pressure constituent) in the case, where that same mass of water is actually in the vapor phase.
We all understand the concept that if a mass of water vapor condenses to droplets, that the occupied voume (by those H2O molecules) drops precipitously, and given that that previously occupied space is now available for the non-condensing species; the gas law equation of State pressure should drop; but even here you have to be careful, because those equations of state also contain the number of gas molecules; which will also change with the condensation of the water.
Without actually reading the complete paper thoroughly, I am not going to speculate further; I just wanted to raise that little idea of who’s supporting the water droplets.
I don’t know why Dr Curry chose to put out an open public review; frankly I don’t care much either; I’ll assume she had her reasons, and it is bettwer to start with at least one review whether confidential or open, and frankly I don’t know whay referees would be so hesitant to give these authors a fair reading. If you are that shaky about your ability to read this paper and give it a proper critique; lest you be found to be approving another cold fusion paper; then maybe your knowledge is not sufficient that you should be reviewing any papers on this subject.
If the paper was total BS, or a cold fusion look-alike; well surely a competent reviewer would be able to declare it so, without fear.
Folks who like to hide behind pseudo-nyms (as distinct from “handles) know what their stuff is really worth; or they would be proud to put their own name to it.
Dr Makarieva is apaprently not shy to put her own name on her paper; too bad that “the experts” are too chicken to even have their name near it even in a confidential review.
So we amateurs should fill in while the experts circle their wagons; or gather some cajones. I’m certainly a neophyte when it comes to cloud chemistry; well any kind of chemistry; but I’m not too shabby when it comes to Physics, and Mathematics; which I see is Dr Makarieva’s forte; but I’m well short of her level.
An interestingg day Anthony; you too Chasmod.
And I see from the short bio that we are mispelling Anastasia’s name; so you have a headline error there Anthony; I thought the double ss was a little unusual.
@Mike Mangan says:
January 21, 2011 at 4:23 pm
Thanks for that. She is pretty (and scary smart)!
G.E. Smith, again thanx for your participation here. Reading your posts reminds me of my favourite wrassler, Gentleman Gene Kiniski, who’s interviews I always looked forward to. Such as I look forward to your contributions here.
Mike Mangan, WOW! 16 years old when she went to the Polytechnical, and just 26 when she recieved her PhD. No wonder there are few takers to referee her groups’ paper, they are probably afraid that they won’t understand half of it!
Fred Harwood says:
January 21, 2011 at 9:23 am
In a vapor steam heating plant, which functions at near atmospheric pressure, water expands 1,700 times into steam, which large expansion delivers the latent heat to radiators, where the saturated steam returns to the original vastly smaller volume of water.
wayne says:
January 21, 2011 at 3:47 pm
ge0050 and Fred Harwood are on the right track.
It’s amazing how many comments above keep bringing in hot air as the driver, not moist air.
I had the opportunity years ago to experience sailplanes for a few years. When you get up in the atmosphere and that is your only ‘engine’ you get a totally different view
dscott says:
January 21, 2011 at 4:34 pm As an HVAC engineer we well acquainted with
evaporative cooling in cooling towers.
to; Anastassia Makarieva
on behalf of the authors:
A.M. Makarieva, V.G. Gorshkov, D. Sheil, A.D. Nobre, B.-L. Li;
It would appear to me that the people above are as well qualified to review your paper as any in “climate science”. I have had a foot in each of these fields and can attest that success in these fields require a very good grasp of the fundamentals of atmospherics. Following BS (bad science) can get you dead. pg
Chris Reeve says
——–
In other words, the liquid state of matter is an electromagnetic resonance of molecules.
——–
No it’s not.
Note that she references a piece of work in her paper authored jointly by K. Trenberth and J. Christy: Trenberth, K. E., Christy, J. R., and Olson, J. G.: Global atmospheric mass, surface pressure,
and water vapor variations, J. Geophys. Res., 92, 14815–14826, 1987. 24018
Surely between the disparate crowds these fellows run with there ought to be nine willing reviewers. Where is Diogenes when you need him?
A couple of suggestions for reviewers: Lubos Motl of motls.blogspot.com and Nir Shaviv of Hebrew University.
While Engineers deal with HVAC concerns (which go a long way to understanding the heat transfer), there are others in the free atmosphere which are outside the bulk of Engineering experience.
The total energy of a given parcel (mass) of moist air is the sum of heat “stored” in the molecules, kinetic energy due to the velocity and the potential energy due to the mass under gravity at altitude. Upon condensation, the heat of evaporation must be dispersed which can be by radiation or thermo-kinetic heat transfer to other molecules resulting in an expansion of the volume occupied by the mass of dry air in the parcel. The volume occupied previously occupied by the condensed water becomes available with condensation but the nett change is small (<5%). There will only be a few 10's of grams of water vapour per kg of air parcel. The density of water vapour is about a third lower than that of dry air, so per gram, it occupies a larger volume.
Where else can the heat go? Well, there's the liquid water droplet itself. Liquid water is an excellent radiator but the droplet surface is small.
Now, all the things that we (Engineers) like to hold as constant, such as pressure and the latent heat of evaporation, aren't for a parcel of buoyant air rising, unconstrained under free convection. Usually, in e.g. HVAC, that isn't important; but for combustion Engineers, it is very significant because the ranges of temperature and pressure are significant. And so it may well be for that parcel of air as it rises thousands of metres; with a steadily-reducing temperature and pressure.
Changes in those little variable “constants” add up. The energy equations are no longer straight-forward but become intractible; especially when considering the lack of necessary knowledge about how the droplets form; and where the heat released really goes.
I would like to nominate two possible reviewers. How about G. G. Anagnostopoulosa or D. Koutsoyiannisa? They are hydrologists with an interest in climate and might have an interest in this new theory.
George E. Smith
January 21, 2011 at 5:51 pm
Thank you for your comments. The atmosphere is brought to motion by non-equilibrium gradients of air pressure. Droplets that are falling at terminal velocity exert a drag force on the moving air that is equal to the weight of the droplet. Likewise, the ascending air exerts a force on the droplets that could support them at a stationary height in the atmospheric column. So the primary question is where the pressure gradients come from that make the air ascend.
An essential point is that the vertical distribution of water vapor is compressed severalfold compared to that of other air gases. In Fig. 1A of our paper one can see that the scale height hv (the height where partial pressure of the gas would decrease e-fold) is about 4 km for saturated water vapor at 20 C compared to over h = 8 km for other gases. The global mean scale height of water vapor at 15 C is around 2 km.
This difference is due to the Clausius-Clapeyron law: the colder the air becomes with increasing height, the less saturated water vapor it can bear.
The difference f = pv(1/hv – 1/h), where pv is saturated vapor pressure, describes the maximum magnitude of the upward-directed force (acting per unit air volume) that is theoretically available to accelerate air and/or sustain hanging droplets. If there are no droplets (no friction), the vertical acceleration and velocity are higher than in the presence of droplets. But this does not affect the air pressure gradient that is formed upon condensation.
One can calculate the maximum amount of liquid that could be sustained by this condensational pressure gradient force in the atmospheric column of height h: f*h/g, where g is acceleration of gravity. It turns out to be of the order of several hundred kilogram liquid H2O per square meter (taking pv = 2×10^3 Pa, hv = 2 km, h = 8 km). Meanwhile the observed mean liquid water content in the tropical atmosphere is of the order of 50 gram (!) per square meter. This indicates that the condensational pressure gradient force is apparently doing something else in the atmosphere rather than just supporting the droplets. In our paper we argue that it is driving winds.
Note: it is not about latent heat.
sky
January 21, 2011 at 5:23 pm
Thank you for your continued interest. In a nutshell, the physical mechanism that is proposed: the area where condensation occurs becomes a low pressure area due to continuous removal of gas from the gas phase. The air from the neighborhood streams to that area and ascends there sustaining condensation. Deprived of moisture, it returns to ‘the neighborhood’ in the upper atmosphere and, as matter conservation prescribes, descends there. There is no condensation in the descending air. So, every circulation pattern driven by condensation will necessarily have a ‘descending’ part with no condensation.
What are the grounds for a statement that condensation is a micro- or mesoscale process? What does such a statement mean at all? Molecular collisions are ‘microscale’ as well, but this does not prevent the ideal gas law from being globally applicable to the atmosphere.
If we look globally, clouds cover over 50% of the planetary surface. This means that the area that are permanently affected by condensation IS globally significant. Such a pattern also indicates that the ascending and descending regions of condensation-induced circulation patterns are, on average, of approximately the same size.
In the post we provided a link for those interested in what and where has been recently published on this topic. We are not aiming to get a particular paper published. Our goal is to generate interest and provoke a discussion in the climate community.
Anastassia Makarieva says: (January 22, 2011 at 12:21 am)
Our goal is to generate interest and provoke a discussion in the climate community.
I can only offer interest, M’am; and you have certainly generated that in this reader.
Mike Mangan says:
January 21, 2011 at 4:23 pm
Here’s our lovely author…
http://thd.pnpi.spb.ru/~makariev/
Thanks Mike, never argue with beauty.
I second Lucy’s call to try to integrate this work with Erl Happ’s.
==================
So meteorologists don’t comprehend thermodynamics AND fundamentals of chemistry. What else is new?
For separation of tiny droplets of liquid H2O, preventing raindrops coalescing into large enough drops to fall, try static electricity. Pith balls in a Leyden jar. Clouds have extremely large negative electrical charges, doth causes the lightning to discharge huge numbers of electrons from clouds to ground (earth) in lightning strokes, allowing the finely divided H2O particles to come together, and fall as raindrops to the ground.
In case no one noticed, the larger and faster the number of lightning strokes, the faster and larger the raindrops fall and become. Simple observation.
Ms. Anastassia Makarieva,
Are you saying the “consensus” science does not think that the pressure loss or “vacuum” obtained by condensation of water vapor in the atmosphere is a very significant cause of winds? If yes, then they are missing a lot and your paper is very interesting and important. Bravo!
Bernd Felsche
January 21, 2011 at 10:46 pm
Thank you forthis comment. This is how things are conventionally represented in models. In reality, in the presence of condensation, partial pressure pv of the condensable gas — water vapor — represents another store of potential energy. Namely this energy (not latent heat) has been so far neglected. When an air volume containing saturated water vapor is adiabatically displaced upwards and some vapor condenses, there appears an upward-directed pressure gradient force that is available to accelerate air.
This force is related to the decrease in the amount of gas, not to the amount of heat released. Just appreciate that these two processes are physically different and governed by different physical constants: theoretically, we can have a chemical reaction in the atmosphere that would not change the amount of gas molecules but lead to either release or uptake of heat. On the other hand, we could have a reaction that changes the amount of gas molecules but does not lead to any appreciable heat release or uptake. All emphasis in meteorological theory has been the effects of latent heat release. The formation of pressure gradient force (the dynamic aspect of condensation) due to the change of the amount of gas has not received the needed consideration.
Readers interested in specific details on how the potential energy from condensation was neglected in a numerical model designed to described a moist atmosphere (while latent heat release taken into account), please, read here starting from Section 2 on the bottom of p. C12009. Warning: this may demand the acquaintance with the other materials in the discussion.
The ten reviewers who refused to review the publication in a sense say “I do not believe it, but I am not confident in my physics knowledge enough to refute it with a QED at the end”.
I will nominate three other possible reviewers. Hopefully they have not already been asked. Richard Lindzen of MIT, Petr Chylek of Los Alamos National Laboratory or Stephen Schwartz of Brookhaven National Laboratory. Surely one of those guys would be interested, although they might not have the time.
Quick question. I just read Judith Curry’s review of the paper. She concludes that if a few issues with the paper were corrected, the paper could be published. Has a corrected version of the paper been completed and submitted? Or are the authors convinced they are right and Curry is wrong?
Anastassia,
Perhaps you can justify the assumption of an adiabatic process. It’s probably close to valid for gases which don’t radiate well, but significant water vapour and the formation of liquid condensate facilitates radiative heat losses. i.e. they can cool without necessarily experiencing an expansion or collision with cooler molecules.
I admit that I’ve only briefly scanned your papers and not worked through them to gain a full appreciation of how you think.
Ron Cram:
January 22, 2011 at 7:11 am
Normally the review procedure in the journal should run as follows. There are eight weeks for public discussion. Then the discussion is closed. Two referees should have submitted their comments by that time. Then the authors are given four weeks to publicly reply to all comments, including those of the referees, and then submit a revised version.
Since the process entered a stage when the discussion is indefinitely extended and there are not enough referees, we are unable to submit a revised version. We are now working on our response to Dr. Curry as well as to other commentators and will post them as soon as we are ready. I would prefer not to reply here in the right/wrong terms. Needless to say that we highly value the interest of Dr. Curry in our work and respect her opinions irrespective of whether we agree with them or not. We also appreciate contributions from all the discussion participants.
Thank you very much for your suggestion of referees. The point is that people should be interested in our work. We chose this form of the general appeal because we would like to avoid contacting knowledgeable persons whom we personally do not know and imposing our work on them. This is what the Editors have presumably done with little success. We have also provided a list of several potential referees at the time of submission.
Please, if you know people who would be interested, be so kind to pass this information to them (if you find this appropriate) such that they could contact me (ammakarieva at gmail dot com) or the ACP Chief-Executive Editor (address here).
Anastassia Makarieva:
How about one of these fellows:
Ralf D. Tscheuschner
Gerhard Gerlich
Their paper: Falsification Of The Atmospheric CO2 Greenhouse Effects Within The Frame Of Physics is a heavy-duty treatise in thermodynamics.
Bernd
True they can cool by radiation. But the matter is they do cool by expansion which necessarily occurs as the air ascends. The adiabatic assumption is not critical: if, as the moist air ascends and condensation occurs, some part of latent heat released is lost to radiation, the vertical temperature gradient will be steeper than the moist adiabatic one while the condensational pressure gradient force will be larger.
In order condensation to occur in the ascending air and the pressure gradient force to arise, the vertical temperature lapse rate (whether adiabatic or not, does not matter) must exceed a certain critical value, which is determined from the equation hv = h. (Scale height hv of saturated vapor, dictated by the lapse rate, coincides with the hydrostatic scale height h = RT/Mg). In this case water vapor is saturated everywhere in the column, but no condensation happens as the moist air ascends. On Earth the critical G is about 1.9 K/km. This is a small gradient compared to the mean tropospheric lapse rate of 6.5 K/km.
Bernd
Consider the simplest case: a pure vapor atmosphere over a flat isothermal Earth. Let us introduce a sufficiently large vertical temperature gradient. In this case there is much saturated vapor above the warm oceanic surface and very little in the upper cold atmosphere. The vertical pressure gradient of saturated vapor is highly non-equilibrium (i.e., hv is much smaller than h) due to large temperature gradient.
Governed by this upward pressure gradient force, there will appear a unidirectional upward motion of vapor that will condense in the upper atmosphere and return to the surface as liquid drops. (Note that in order this to be possible, all latent heat that is released in the upper atmosphere should be disposed to space via radiation. But this condition is implicitly accounted for after we have specified the temperature lapse rate.)
If we now add non-condensable gases the ‘circulation’ can no longer be 1-D imensional, because, unlike vapor which turns to liquid, dry air has nowhere to go as it ascends but to go downward somewhere else. We will witness an appearance of circulation cells that include both horizontal and vertical parts. This is what the condensation-induced dynamics is about.
Anastasia Makarieva wrote:
Consider the simplest case: a pure vapor atmosphere over a flat isothermal Earth.
Another simple case is a Cloud Chamber.
There appears to be a similar process at work on Titan, with Nitrogen and Methane.
Anastassia Makarieva says:
January 22, 2011 at 12:21 am
“What are the grounds for a statement that condensation is a micro- or mesoscale process? What does such a statement mean at all?”
The grounds are direct physical observation. Certainly the condensation of individual cloud droplets occurs on a microscopic scale and in the case of radiation fog is a microclimatic phenomenon–one not accompanied by any visible movement of the air mass. Thermally driven popcorn clouds are likewise microclimatic in scale and their formation create at best meso–scale horizontal pressure gradients, such as drive shallow, gentle sea breezes that never extend more than tens of kilometers inland. Hurricanes, of course, produce great winds and rain, but they are likewise meso-scale. Monsoons are perhaps the best bet for demonstrating your mechanism on a larger scale. Do you have any conclusive evidence from field measurements at that scale? And while clouds may cover roughly half the global surface at any time, that does not mean that they are being PRODUCED over a comparable area. As global satellite views clearly show, they tend to spread widely from the tropics into zones of westerly winds. Neither those winds, nor the “haboobs” that sweep sporadically across the Sahara (let alone the hemispheric dust storms on Mars) can be convincingly attributed to condensation.
I do applaud your work in elucidating a mechanism that has been sorely neglected in meteorology. But I would urge you temper your enthusiasm for its explanatory power with mature consideration of other dynamic mechanisms and the evidence from direct measurements. Great scientists have long understood that the latter can destroy beautiful theories.
Wasn’t it anna v who best explicated that ultimately it will be data that vindicates you or not, and that such data retrieval and analysis is beyond your scope at present? It seemed that she best placed the problematic aspects of eq. 34 in perspective, too. So, I nominate her.
=========
Without getting into the deep scientific arguments, lets just look at what happens in the real world from a glider piot’s point of view. (Guess what my online name means).
A glider pilot is losing altitude at his minimum sink rate of 150 feet per minute (fpm). As he approaches a developing thermal, that sink rate may increase to 300 fpm or more down, and as his altitude drops the poilot may even start to look for somewhere to land. Then Eureka, at 1000′ above ground he hits a weak thermal and starts to climb. His climb may initialy be 200 fpm which means the air around him is actually climbing at 350 fpm. For that air mass to be rising, there must be wind on the ground driving in to replace that rising air.
The glider pilot looks up, and somewhere above him he sees a cumulus cloud with a nice dark base. Now as he climbs slowly up towards that cloud, his rate of climb increases; maybe to 500 fpm. So where is the energy coming from to cause that air mass to be moving faster. Obviously there is a pressure difference as he approaches the cloud and air is being drawn in the side of the thermal. The hot ground may have been the kicker, but it is what is happening inside the cloud that is now providing the lift. At cloud base it is common for glider pilots to have to open their air brakes to prevent being sucked up into the cloud. Yes, I do mean “sucked up” as it is no longer the thermal energy from the ground that is the driving force.
So here is a practical example of condensation within the cloud causing localised winds at altitude. Maybe it is relevent to this paper and hence worthwhile looking at the subject on a broader scale. Or is a localised phenonenum not revelevent to macro meteorology?
Steve says:
This is wrong. First of all, the thermal energy of a gas that expands will decrease by the 1st Law of Thermodynamics because the gas does work on the environment. (Not true for so-called “free expansion” but that really isn’t relevant here.) This is in fact the reason why gases cool as they expand.
Second of all, the temperature of an ideal gas is proportional to the energy per particle, not the energy per unit volume. In particle, for a monotonic gas, the thermal energy is (3/2)N*k_B*T where N is the number of atoms, k_B is Boltzmann’s constant and T is the absolute temperature.
Yeah…For one thing, Sheehan is confused but what “adiabatic cooling” means. It means there is no exchange of energy with the surroundings via heat…However, there is still the exchange of energy via work. Also, when one talks of a parcel of air in the atmosphere undergoing adiabatic expansion when it rises, that means it does not exchange heat with its environment. It does not necessarily mean that there can’t be heat exchange within the system, e.g., between the water and the non-condensable gas.
An analogy would be if you put an ice cube in hot water inside a calorimeter (which a styrofoam cup can serve as a fair approximation of in a pinch). To the extent that the calorimeter is perfect, there is no heat exchange with the surroundings but there is still heat exchange between the ice cube and the water.
(I just gave my introductory physics students a test on thermodynamics, which is why I am in a bit didactic on this subject.)
Jim F says:
If you want two physicists who have shown a profound misunderstanding of basic principles of thermodynamics, then that might be the way to go. Otherwise, probably not such a good idea.
I think my question got lost in spam because I inadvertently first posted in another discussion.
Lucy Skywalker
January 21, 2011 at 3:36 pm
These are complex questions. As I said above, any condensation-induced circulation pattern has a low pressure zone where condensation and ascending motion occur and a high pressure zone where the air descends. One such low pressure zone is located near the equator. In our paper we show that the condensation-induced pressure gradients coincide with those observed in the Hadley cell (trade winds).
In principle, the high pressure zone could have been located somewhere at the poles, such that the Hadley circulation extended over the entire hemisphere. The existence of an additional low pressure zone to which your referred is due to the fact that Hadley cell is smaller than that. Can we estimate the size of Hadley cell (not only pressure gradients, but also the size) using our theory? In other words, is there a maximum size for a condensation-induced circulation? It looks like there is and we can estimate it. This work is in progress.
If there is a maximum cell size and it is smaller than the distance between the equator and the pole, this will cause several cells to co-exist in each hemisphere and lead to formation of at least one more low pressure zone, like the one you referred to. This is what actually happens: there are Hadley, Ferrel and polar cells.
The second question is why the intermediate low pressure zones are not symmetrical between the southern and northern hemispheres. This apparently has to do with the continental masses and distribution of vegetation. In the Northern hemisphere we have vast Siberian forests (plus Canadian forests) where evaporation patterns are different from evaporation from the oceanic surface. This is true both in winter (when trees are covered with snow providing extra evaporative surfaces) and in summer where there is active transpiration of plants. This causes the low pressure zone to become more diffused and spread over the continent rather than being concentrated over the ocean. This may have to do with the fact that in summer the number of Arctic cyclones (low pressure systems) decreases compared to winter.
I can almost hear them….
“Silly English man, we will taunt you!!!”… “Run Away…. ”
Were I able to be counted as a reviewer, I’d sign up in a heart beat. It looks like a very interesting question, and paper.
IMHO, we are in a transition. In the beginning, publishing was very rare, costly, and hard, but was done subtanitally ad. hoc. and folks like Einstein were “reviewed” by folks they talked with more or less frequently. Then it became a ‘business’ and “journals” found money in monopoly of “review process”. Lately some folks found power in control of the “review process” and climategate was born….
Now, perhaps, it is time to return to that point where ‘review’ happens under a bright carbon arc spot light… “Publishing” literally costs nothing. ( I run a blog with thousands of daily readers at zero cost, for example) So perhaps it is a good time for “peer review” to happen more quickly, and more in the light of day (or carbon arc 😉 and with less money changing hands ( i.e. exactly WHY is publicly funded research behine a paywall? Hmmm? )
So Kudos to these brave souls with a sound idea and a courageous heart. Let them go forth and contend with the old dragons… And may the best non-dragon win…
@chris Reeve in: http://wattsupwiththat.com/2011/01/21/an-appeal-to-the-climate-science-blogosphere/#comment-579727
Are ye a Scottsman lad? Or perhaps a Celt of some other sort? I ca’ ne read yoor writings but that I hear the lilt in what ye say… ‘N ken it I do…
I think you’ve got it rrrright…
Clausius-Capeyron law:the colder the air becomes with increasing height, the less saturated water vapor it can bear.
I don’t understand this. I thought that for water vapour to condense the air had to be at least 100% humidity, and more, supersaturated and isn’t this the same to form snowflakes? Doesn’t most rain even in summer start off as ice crystals?
Water vapour in higher colder air expands and become less dense when it is doing this, displacing the air, but anyway, I thought water vapour wasn’t bothered by how cold air was, if it could get to it it could saturate it.
Myrrh wrote:
I thought water vapour wasn’t bothered by how cold air was, if it could get to it it could saturate it.
If you take a well-mixed column of air (with a uniform fraction of water), if it’s saturated at sea level, it will be supersaturated at all points higher.
anna v wrote:
The ten reviewers who refused to review the publication in a sense say “I do not believe it, but I am not confident in my physics knowledge enough to refute it with a QED at the end”.
Here’s your sign.
I suspect you’re right.
I suspect the lack of confidence is well-founded.
Just because “I believe it” doesn’t mean it is. ‘Truthiness’ may work in soft sciences, but it’s not Physics.
I believe the difficulties in getting this paper reviewed say far more about the peer-review process than the paper.
BigWaveDave @ January 21, 2011 at 5:20 pm
All one needs to do is put some warm tap water in the bottom of a plastic jug or soda bottle, cap it tightly and watch.
Sometimes the simplest observations are the most profound.
Colonial @ January 21, 2011 at 10:31 pm
Suggested reviewer Nir Shaviv of Hebrew University
Here is a post from Nir on his blog ScienceBits on the subject of Open convection cells over the Negev?
Hopefully this will clear up a couple of points above where there was a bit of confusion
There are three v. d. Waals forces, 1. hydrogen bonding (water, ammonia) which is a particularly strong version of the second, 2. dipole-dipole interactions and 3. dispersion aka London forces. Dispersion occurs because of fluctuation in the charge distribution of molecules/atoms that do not have a permanent dipole moment.
Thompson measured the ratio of charge to mass of the electron by passing a beam of electrons (e/m) through a combination of magnetic and electric fields. Millikan measured the charge by measuring how fast an oil drop fell when it had a charge on it from an electron attaching. You can “do” the Thompson experiment using this applet(scroll down and select the e/m one), and the Millikan experiment using this applet.
Myrrh says:
That is 100% relative humidity, which is basically just tautology. I.e., relative humidity is defined as the ratio of the amount of water vapor divided by the amount of water vapor for saturation at that temperature. The Clausius-Clapeyron relation tells us that the saturation vapor pressure rises rapidly with increasing temperature. See, for example, this graph: http://kkd.ou.edu/METR%202603/satvp.jpg
Without going through all the comments, I still believe that winds are associated with uneven heating of the surface. Does water vapor play into that uneven heating? Maybe. At issue then is what causes uneven water vapor? Maybe it is the constant Sun hitting a somewhat chaotic oscillating cold versus warm, and choppy versus calm ocean surface. But that leads us always back to wind. So it seems to me that the cause may not be determinable, as the primary issue is a self-perpetuated system that is recharged via the Sun due to the leaky nature of the Earth’s atmospheric system.
http://www.areco.org/pdf/Global%20Winds_Jan07%20update.pdf
The refusal by the nine contains the eensiest weensiest little soupcon of racism. (Look at the nationalities of the authors.) AGW is at its core an anglosaxon club, with mascots like Pachauri for window dressing.
Thank you Dishman and Joel, I’ve done some more reading on it and I think I’m just getting a grasp of it.
Another novice question, re the paper. If winds are caused by this water induced play with pressures, has this been tracked to, say, give an explanation of winds across the Sahara?
Myrrh says:
January 24, 2011 at 5:51 am
Another novice question, re the paper. If winds are caused by this water induced play with pressures, has this been tracked to, say, give an explanation of winds across the Sahara?
The paper gives a cause for generation of winds in the particular situation of a storm forming, not all winds in general and exclusively.
Tall chimneys generate winds even without a fire.
I used to live in the first floor of a tall apartment house whose windows faced east on one side and west on the other. On windless days, an enormous wind would go through the apartment if both sides were open and the sun hit the east side.
Fires cause winds.
The hot / cold mechanism of wind generation has been observed and studied for ages.
So dr Makarieva tells us that also condensation causes winds, and should be taken into account as dominant in certain situations, of course not all.
sky
January 22, 2011 at 12:25 pm
Once again, thank you for your comments. I suggest that we should separate emotions (‘enthusiasm’) from scientific arguments. My colleagues and I are convinced that condensation-induced dynamics is dominant on Earth (we have not studied Mars or Venus yet) not out of enthusiasm, but because of quantitative evidence, which can be briefly summarized as follows (see the paper for details):
1) Consideration of mechanical power release associated with condensation on a global scale coincides in the order of magnitude with the observed power of global atmospheric circulation.
2) Theoretically estimated pressure gradients produced by condensation coincide with observations in both mesoscale circulation patterns as hurricanes and global scale circulation patterns as Hadley cell.
This makes us certain that future research will confirm the dominance of the mechanism we propose in generating winds on Earth. Neither wind bursts in Sahara nor breezes are global scale winds. Monsoons are, and they are accompanied by intense condensation.
Now then, when somebody puts forward a quantitative claim that condensation is unimportant, we go into details to show why such a conclusion is incorrect. Here is but one example. This demands time and involvement and we have done and are doing considerable work on this. So, when I say that I am convinced that condensation-induced dynamics is dominant, I mean all this work and all these arguments. If someone proves them wrong, I will accept that it is not dominant.
As for the human dimension — ‘enthusiasm’ — if you admit that there are two cents worth of good sense in what we are doing, you should realize that none of our findings would have never seen the light of the day had it not been for our radical ‘enthusiasm’. So, it is in the interest of all who think there is at least something worthy in what we are doing that our ‘enthusiasm’ persists rather than be tempered.
I’ll review your paper.
In Equation 1 you present an energy balance for air that remains saturated with water vapor. In Equation 3 you quote the Clausius-Clapeyron equation. This equation tells us the slope of the line that separates vapor and liquid in a p-T graph for water. You combine 1 and 3 and end up with Equation 11. Equation 11 tells us that when we drop the pressure of our air without removing any heat, the saturation pressure of water vapor in the gas decreases.
In Section 2.2 you say, “Our previous result refutes the proposition that adiabatic condensation can lead to a pressure rise due to the release of latent heat.” Your Equation 11 shows that we must drop the pressure to cause water to condense. It does not prove that the drop in pressure is caused by condensation, nor does it prove that the drop in pressure is increased by condensation. Indeed, simple calculations show that quite the opposite will be the case.
http://homeclimateanalysis.blogspot.com/2010/11/condensation-and-convection.html
You spend several lines deriving Equation 16, which shows “condensation cannot occur adiabatically at constant volume.” This conclusion is equivalent to the definition of saturation: water will not condense because it is stable in solution. So there is no need for the proof.
In Section 2.3, you show that condensation occurs only when pressure drops. Indeed, that is the case. But this does not imply that water vapor increases the pressure drop.
In 3.3 you argue that condensation causes a drop in surface pressure. You say that condensation of water vapor removes mass from the column, which causes its weight to drop, and therefore the surface pressure to drop. I think you are saying that when it rains, the column of air above gets lighter, so surface pressure decreases. That’s interesting.
The rest of your paper proceeds from the rain-induced pressure argument. Your earlier argument that condensation is always accompanied by pressure drop is irrelevant, as it would have to be, because the conclusion says nothing about what condensation does to the surrounding gas.
So, I would cut the many unnecessary initial argument from your paper and start with your calculation that rain reaching the earth causes the pressure to drop. From there you can make your horizontal pressure gradient argument, which is fascinating.
Yours, Kevan
Kevan Hashemi says:
January 24, 2011 at 8:26 am
“In Section 2.3, you show that condensation occurs only when pressure drops. Indeed, that is the case. But this does not imply that water vapor increases the pressure drop.”
If a vapor condenses, doesn’t the resulting condensation exert less pressure on the surrounding system than the original vapor? I would think that the act of condensation would exert a negative pressure, actually, so for total pressure to remain approximately the same the remaining dry air would have to expand in order to make up for the pressure loss of water vapor condensation.
Steve
January 24, 2011 at 9:24 am
Exactly. This is why the cloud sucks in the surrounding air, as Jantar said above. Since condensation occurs as the air moves upwards and cools, the resulting pressure gradient force is also upward directed.
There are two effects: contraction due to water vapor turning into liquid and heating due to water vapor giving up its latent heat of evaporation. The heat causes the air to expand. According to my calculation, the expansion due to heating is eight times greater than the contraction due to vapor becoming liquid.
Dear Kevan,
Thank you for your comments. We are compiling a list of potential reviewers to submit to the Editors. In the meantime, every one registered can post a comment to the ACPD discussion. The comments are most welcome. Perhaps if there is sufficient feedback from the scientific community, the Editors may decide that one referee (Dr. Judith Curry) plus the Editor’s own evaluation would suffice to make a decision.
In your post at http://homeclimateanalysis.blogspot.com/2010/11/condensation-and-convection.html you calculate the effect of condensation on buoyancy taking into account latent heat release. You conclude that condensation causes a net increase in pressure because of latent heat release.
The question is: increase compared to what? If there is air ascending without condensation nearby starting from the same surface temperature and if all latent heat remains in the volume where condensation takes place (nothing is radiated to the atmosphere), then the pressure in the column where condensation takes place can be higher — if if the surface pressure is the same. The comparison taking into account all these ‘ifs’ can be seen in Fig. 1c of our paper.
Let me explain why the effects of gas mass removal and latent heat release on pressure are physically different. Suppose that you gradually move upward a dry air volume in hydrostatic equilibrium. You can do it infinitely slowly without disturbing the equilibrium. If you look at the column of a given area where the dry air ascends infinitely slowly, you will always be able to identify and follow the original air volume — it will retain its ‘identity’ — it will expand, but it will be the same air volume (as if it were a balloon) (we neglect diffusional mixing).
To cancel out the effect of latent heat, suppose that we raise the dry air diabatically (warming it a little bit as it ascends) precisely such that the vertical temperature gradient is moist adiabatic (as if latent heat were being released).
Something different happens when you attempt to raise a saturated air volume in hydrostatic equilibrium. Before vapor condenses, it does not know that it will and behaves as non-condensable gases. Suppose you gradually move the moist air parcel upward to occupy the imaginary volume it should have occupied if it were dry. Now let the vapor ‘recall’ that it is condensable and condense. Air pressure drops immediately (remember: latent heat has been accounted for). Droplets do not make a contribution to the ideal gas pressure, so the air pressure where condensation occurs drops immediately — not after the rain falls out.
So, immediately upon condensation, there is disturbance of hydrostatic equilibrium: the air below our parcel has an uncompensated pressure to push the air parcel upward. This uncompensated pressure gradient force (N/m^3) is equal to pv(1/hv – 1/h), as I said above, where pv is partial pressure of vapor and hv is its scale height. Since the atmosphere is much wider than high, this force is redistributed, via hydrostatic adjustment, in the horizontal plane. If you look at typical horizontal pressure gradients observed in the atmosphere, you will find that pv(1/hv – 1/h) describes them very, very well. Yet you will not find a mention of this interesting fact anywhere in the meteorological literature. Moreover, when the facts are laid out, it appears very difficult to find two climate scientists willing to discuss it.
The buoyancy related force arises when the air nearby has not warmed — as you mentioned in your post. If the air is uniformly warm, if there is intense turbulent mixing, nothing happens. In contrast, the pressure gradient force due to removal of vapor from the gas phase arises whenever moist saturated air ascends — irrespective of the presence or absence of horizontal gradients of temperature and buoyancy.
You say, “Now let the vapor ‘recall’ that it is condensable and condense. Air pressure drops immediately (remember: latent heat has been accounted for).”
If you mean that we have accounted for the latent heat by adding it before the vapor condenses, then your statement describes the contraction in volume that occurs when a vapor condenses. But if I imagine the balloon of air going up as you describe, and the vapor suddenly condensing, as in a bubble chamber hit by a cosmic ray, then the latent heat will pass into the air and warm it up, causing the gas to expand. So I’m not sure how your thought experiment refutes the effect of latent heat that I calculate in my example.
You say, “So, immediately upon condensation, there is disturbance of hydrostatic equilibrium: the air below our parcel has an uncompensated pressure to push the air parcel upward.”
If, indeed, the air contracted when condensation occurs, then it would become more dense, and it would tend to sink. You appear to be saying that that contraction in volume requires an in-rush of air, and so causes wind. But the contraction is of order 0.1% (in my calculation), and can be accommodated by a 0.1% expansion of nearby air. Once that’s done, the contracted air in your thought experiment would be more dense, and would fall.
But that’s not what happens. Instead, the air in which water condenses appears to rise. According to my calculations, it rises because it is less dense, and it is less dense because it has expanded after receiving the latent heat of the water vapor. It begins to rise, and it keeps rising, and that causes wind.
“”””” Eli Rabett says:
January 23, 2011 at 5:37 am
Hopefully this will clear up a couple of points above where there was a bit of confusion
>…………………………………..<
Thompson measured the ratio of charge to mass of the electron by passing a beam of electrons (e/m) through a combination of magnetic and electric fields. Millikan measured the charge by measuring how fast an oil drop fell when it had a charge on it from an electron attaching. You can “do” the Thompson experiment using this applet(scroll down and select the e/m one), and the Millikan experiment using this applet. """""
Well this is the first time I've heard of using my cell phone to do Physics experiments with. Actually I don't own a cell phone; with or without a bite out of it.
When I went to a University; one that actually taught Physics, it was fashionable to have the sudents do Milliken's oil drop experiment using an actual oil drop, and a parallel plate capacitor to provide a controllable electric field. We actually observed the droplet under a microscope, so we could adjust the field to stop the drop from falling, and turn off the field to let the drop fall so we could determine its mass and size from the terminal velocity, and the properties of the oil. I believe we did that experiment during our freshman year at the University; which for me, would be my sixth year of Physics; having done it for five years in high school. (I still have all my grades; well actual exam marks for the High school physics).
But I can see why they would now do Physics on a cell phone; some of these fancy video games claim that their graphics follows real Physics laws.
That would prepare the modern Physicist to study climate on a super computer.
I notice that the "mathematician" who solved Fermat's Last Theorem, did that on a super computer. It's a fairly safe, free beer bet, that that mathematician, did NOT discover Fermat's proof of Fermat's last theorem.
I didn’t mean to ignore Eli Rabett’s introduction of Van der Waals forces to the intermolecular force kit. I had deliberately removed the charged droplet case to simplify the situation so that cloud Electric fields, didn’t get into the water droplet support. We used to fly Hydrogen inflated weather radiosonde balloons up into boomer storm clouds with custom apparatus on it, to actually monitor the electric fields under storm clouds. This was back when graduate students were cheap and naive; and indestructible.
But Eli of course is correct that VdW forces of the several species can also supply support to a raindrop. That doesn’t really change anything though. As a result of providing the levitation; be it through collision and rebound or VdW forces, the result is a downward reaction on the atmospheric molecules; which must manifest itself at the boundary (ground) as an increase in pressure; that being the necessary pressure to sustain the total mass (and weight) of the water droplets. So it is still not clear to me that mere condensation of water vapor into a droplet will drop the atmospheric pressure (or increase it). And of course as Anastassia points out the latent heat is a different issue. The condensation takes place as a consequence of the loss of that latent heat energy, so it seems to me that in the act of “condensing”, there isn’t a sudden heat surge. But any net change in the energy can be accommodated by the ordinary gas law equations of state.
The mass of the water molecules must be supported by the atmosphere whether as individual free molecules or some condensed state; they still have to be supported, and must produce a pressure increase (due to their presence).
The whole process may be fully explained by Dr Makarieva’s team or not. I just don’t have the gist of it yet; but am not arguing against their thesis.
Mr. Smith,
Your description of electrostatic force being able to support water droplets is fascinating. I will have to think about it more, because I don’t yet understand it, but it certainly adds a new dimension, because that means that the charge will keep building up where the water is condensing, as the air rises through the cloud-forming layer.
I may be missing something about the latent heat, and hope to be enlightened if I am, but it seems clear to me that the latent heat is not lost, but merely transformed into the kinetic energy of air molecules, causing them to expand. That makes the air lighter, which causes it to rise. If it fell instead, we would not see air rushing up into clouds. The fact that the cloud appears to stay still does not mean that the air within the cloud is still, so far as I can tell. There is that phenomenon of a stationary cloud on the top of a mountain when the air blows over the top.
Kevan Hashemi says:
January 24, 2011 at 11:21 am
“If, indeed, the air contracted when condensation occurs, then it would become more dense, and it would tend to sink. You appear to be saying that that contraction in volume requires an in-rush of air, and so causes wind. But the contraction is of order 0.1% (in my calculation), and can be accommodated by a 0.1% expansion of nearby air. Once that’s done, the contracted air in your thought experiment would be more dense, and would fall.”
It should be easy enough to test in a home refrigerator. Blow up a few balloons (warm, moist air) and stick them in. If water condensation does indeed result in a net pressure increase then you should see the balloons gradually deflate as they cool off, expand when the air inside the balloons hits the dew point, then deflate again as cooling proceeds. With breath near 100% humidity you won’t have to wait long for the expansion event (if any) – the air in the balloons only has to drop a couple of degrees before it hits the dew point (at sea level).
Nice idea with the balloons. But I don’t think anyone is claiming that condensation reverses a pressure drop. The balloons in the refrigerator will not start to expand again. Nevertheless, we might learn something from how much a dry nitrogen balloon contracts compared to a wet air balloon. I’ll have to think about it.
My calculation, which agrees with many others, shows that you must expand moist air by a greater extent in order to achieve the same pressure drop. If we double the volume of a cylinder containing air, and do so without allowing heat in or out, we calculate the pressure at the end with pV^1.4 = constant. But if it’s moist air, we get a smaller pressure drop. The heat liberated by condensation causes the pressure to rise above the pV^1.4 value, despite the contraction caused by the condensation. But we always have pressure decreasing as volume decreases.
If we compare a dry cell of air rising beside a wet cell, all other things being equal, once condensation starts, the wet cell will have to expand more in order to remain at the same pressure as the dry air beside it. Because it expands more, it will be lighter, and will tend to rise above the dry air.
Certainly, I don’t see anything in the paper that proves the opposite case, as I tried to show in my review above.
Correction: We always have pressure decreasing as volume INcreases.
Anastassia Makarieva says:
January 24, 2011 at 7:55 am
“My colleagues and I are convinced that condensation-induced dynamics is dominant on Earth (we have not studied Mars or Venus yet) not out of enthusiasm, but because of quantitative evidence, which can be briefly summarized as follows (see the paper for details):
1) Consideration of mechanical power release associated with condensation on a global scale coincides in the order of magnitude with the observed power of global atmospheric circulation.
2) Theoretically estimated pressure gradients produced by condensation coincide with observations in both mesoscale circulation patterns as hurricanes and global scale circulation patterns as Hadley cell.
This makes us certain that future research will confirm the dominance of the mechanism we propose in generating winds on Earth. Neither wind bursts in Sahara nor breezes are global scale winds. Monsoons are, and they are accompanied by intense condensation.”
Having spent many decades in scientifically testing theoretical expectations in geophysics against data gathered in carefully conducted field experiments, I cannot share your certainty about the results of future research on the generation of global winds.
My mention of haboobs and sea breezes was not to suggests that they are global winds. They are simply common examples of winds generated by differential heating rather than condensation . Mars clearly has global winds without any condensation. On Earth, it is the westerlies, in which the Coriolis effect plays an important dynamical role, that are global, along with the major convective cells. But there countless un-named cells that develop in a self-organizing manner on a much smaller scale that militate against coherent pressure changes over areas larger than where condensation is actively taking place at a given time . You might find order–of-magnitude agreement between your theoretical expectations and coarse estimates of the global wind power, but that is scarcely a basis for discounting all other factors. There’s simply too much data that informs us otherwise.
To repeat, I applaud your contribution to theoretical understanding of the mechanical effects of moist convection. Many times here at WUWT I found myself stressing its dominance from a thermal-energy-transfer standpoint to radiation-centered audience. But that is not the same as claiming it to be the dominant MECHANICAL player on the globe. Since I ‘m prohibited by contractual terms from revealing data and analysis results, and my attempts to be otherwise helpful violate your sense of “scientific argument,” I can only leave you to your academic pursuits.
Anastassia Makarieva.
I’d have liked to have responded earlier to you post, but social disciplines have prevented this.
I’ve spent ~4 hrs reading your paper and responses here (I don’t ‘speed read’ too well when it comes to reading tech stuff), and one thing becomes ‘blatantly’ apparent! I don’t see any inclusion of ‘Earth spin’ energy in your paper (or in ‘climate models’ either).
OK, so I’m just an engineer with an interest in climate change, but it becomes apparent to me that you are ignoring the effect of Earth’s rotational slow down following the ‘alleged collision’ with Thea that both accelerated Earth’s rotation and formed ‘the Moon’ way back.
The ‘centrifuge effect’ imparted by Earth’s rotation supplies most of the energy at the equatorial ICG to supply the Hadley Cell with all the energy that it needs for it’s circulation (I calculated this to be more than ‘3 inches per second squared’ in counterpoise to the gravitational constant). However, IMHO, I think that your paper may well describe the ‘extra’ energy that supports the Brewer-Dobson circulation to some degree.
But, hey. I’m just an engineer!
Best regards, Ray Dart.
Dear Anastassia et al.,
Consider your Equation 1. Substitute for dT using RdT = pdV + Vdp. Assume dQ = 0. I’ll use w for the mass of water dissolved in 1 kg of air, so R is 287 J/Kkg, C is around 1 kJ/kgK, and L is 2 MJ/kg.
dp = -(1+R/C)pdV/V – RLdw/VC
In dry air we have dw = 0, and we are left with the derivative form of the adiabatic expansion equation. When water condenses we have dw < 0. Thus dp will be less negative than it would be for dry air.
When 1 g of water condenses at 100 kPa and 300 K, its volume contracts from 1 liter to 1 milliliter. Your Equation 1 does not account for the contraction of the water vapor. Your Equation 11, derived from Equation 1 and Equation 3, does not account for the contraction of water vapor either. The apparent relationship between dp and dw in Equation 11 is the slope of the coexistence line on the p-T graph for water.
The contraction due to water turns out to be insignificant compared to the latent heat, as I have already shown.
Yours, Kevan
“”””” Kevan Hashemi says:
January 24, 2011 at 3:01 pm
Mr. Smith,
Your description of electrostatic force being able to support water droplets is fascinating. I will have to think about it more, because I don’t yet understand it, but it certainly adds a new dimension, because that means that the charge will keep building up where the water is condensing, as the air rises through the cloud-forming layer. “””””
Kevan, when I talked about “somebody” supporting the water droplets, I of course was assuming the non-precipitating case (it ain’t rainin yet), so I assumed that the water droplets were still small enough to be supported. Now Dr Makarieva pointed out that the droplets are supported by upward mass air flow. So that is a macro view, and as she said the droplets are actually falling through that upward air flow at the terminal velocity. So in the macro view you have to consider Reynold’s numbers and the like but for small enough drops I think it is safe to assume a non turbulent laminar flow around the droplet. Someone mentioned friction forces, and I suppose in a sense you could call the interface forces a friction. Eli points out that VdW forces are acting between molecules. Some of those result from the dipole nature of the water molecule; so even though the droplet may have a net charge of zero, so that it is not subject to any local electric fields perhaps due to charged clouds, there can still be local electric forces due to molecular dipoles. As I recall it is usual for molecules to repel each other somewhat, which is why you get the small pressure adder term (b) in the Van Der Waals equation of state.
All of these kinds of forces the surface “friction”, viscosity shear (in the atmosphere) as well as the microscopic molecular level collisions, are all acting in concert to suspend the water droplet or raise it through the air column, till it get heavy enough to precipitate.
My point is simply, that whatever forces of any nature (besides gravity) are acting to support the water droplets, those forces must result in a net downward force on the atmosphere that simply is the weight of the droplet (unless it is accelerating too) so the weight of the water must still provide a pressure adder; and it isn’t clear to me, why that would be different from that supporting the water as a vapor.
Now we can imagine a closed vessel containing air (even water) on the space station; so now we have removed gravity; and I doubt that anything much has changed inside that closed vessel. So of coursae the gas pressure remains as it was, due to the kinetic energy of the molecules, and that includes the partial pressure of the water vapor. Now in this case, if the water vapor condenses, then my gravitational factor doesn’t come into play.
Now I’m curious as to just what the Makarieva Effect would do in that case. So if we still have the good Doctor’s attention, she might comment, on what happens in zero g. Or is her result a consequence of gravity; so no show in zero g.
As to the suspension by electric fields; that of course is what Milliken did; but he used a low vapor pressure oil so he wouldn’t have his droplets evaporating. The droplets are sprayed into the gap between two capacitor plates, which can be viewed from the side through a long focus microscope. You let the droplets fall, with no electric field, and they reach terminal velocity which you can time over a trap distance; or today you could have more sophisticated speed reading. From the terminal velocity and the density of the oil, you can derive the mass and weight of the droplet. You then turn on the field, and if the droplet has collected any electrons, the electric field can support the droplet and hold it still. Uncharged droplets will simply drop out of the way. The experiemtn depends on the fact, that the droplets are so small, that you can find droplets with just a single electron extra. Of course Millikan obtained a number of different charge values based on the electric field strength necessary to levitate each droplet; and of course they turned out to be integer multiples of a single value. He did get droplets with a single electron charge; I did too when I did it in school. Lots of people tried refined versions of the experiment, hoping to find charges of -1/3 or -2/3 thinking they could detect a free quark. All of those experimenters are buried in the same cemetary, as the Optician who tried to polish out #80 grit pits with Jewellers rouge; old age it says on their death certificates.
Kevan Hashemi says:
January 24, 2011 at 6:51 pm
“Nice idea with the balloons. But I don’t think anyone is claiming that condensation reverses a pressure drop. The balloons in the refrigerator will not start to expand again.”
My misunderstanding. I thought that was your claim when you stated “There are two effects: contraction due to water vapor turning into liquid and heating due to water vapor giving up its latent heat of evaporation. The heat causes the air to expand. According to my calculation, the expansion due to heating is eight times greater than the contraction due to vapor becoming liquid.”
But I agree, I don’t think the balloons will expand when internal air hits the dew point. I think the “giving up of latent heat” is occurring the entire time, with heat leaving the edge of the balloon system (transferred into the surrounding refrigerator air) every second. Overall deflation will be slower, but there will not be an expansion event.
The atmospheric process of adiabatic condensation confuses me, though. Condensation with no transfer of latent heat? My understanding is that the act of expansion is work, and at some point when enough of this work is done the water vapor condenses into liquid water that has the same energy content of the original water vapor. No transfer of energy occurs between the water and surrounding air, yet there is condensation.
Dear Kevan
For some reason, I cannot see here your comment that has just arrived to my mail box. I reproduce it below:
Your conclusion that condensational mass removal is insignificant is based on consideration of the buoyancy of a given air parcel. Your point is that if we raise this parcel sufficiently high, it will be much warmer than the dry air parcel would have been — had we raised it to the same height. This is due to the well-known difference between the moist and dry adiabatic lapse rates.
Air density (kg/m^3) rho = NM = pM/RT, where N is molar density (mol/m^3), M (g/mol) is molar mass, p is air pressure, R = 8.3 J/mol/K, T is temperature. What you propose is that when you raise your air parcel to sufficient height h = 8 km, the difference between moist adiabatic lapse rate (about 4.5 K/km) and dry adiabatic lapse rate (9.8 K/km), will amount to over 40 K. At T ~ 250 K your point is that this makes a solid impact on density and, hence, buoyancy. If pressure is the same, a comparative increase in temperature of 40 K raises buoyancy by about 20%.
Now looking at rho = NM and recalling that the saturated Nv makes at best 4% of N at the surface, you conclude that the maximum impact of vapor removal on buoyancy can amount to a few per cent, which is much less than the dozens per cent that you calculated from temperature differences due to latent heat release.
These calculations of buoyancy are totally irrelevant though to condensation-induced dynamics. As I said above, in order that buoyancy effects to be significant, there must be a horizontal temperature gradient. There must be air with buoyancy smaller than that of moist air. If the vertical temperature gradient is spatially uniform, warm or cold, the air will not move.
So, let us forget about buoyancy and concentrate on the new physics: you raise moist air parcels over an infinite area: at any height the temperature, pressure and buoyancy are the same. But, due to condensation, partial pressure of vapor changes non-hydrostatically: dpv/dz = -pv/hv, where hv is much smaller than the hydrostatic equilibrium height h.
Hydrostatic equilibrium is disturbed. The pressure gradient force that arises is equal to -[dpv/dz – (1/pv)dp/dz] = pv (1/hv – 1/h). This force acts throughout the entire column where the moist saturated air ascends. It accelerates the air upwards. Due to the fact that the height is smaller than width, hydrostatic equilibrium is restored much quicker in the vertical plane — some air from the surface moves upwards to fill the gap left by condensed vapor. In the result, pressure is lowered at the surface where condensation takes place.
As I said above, the comparison of the maximum possible effect of latent heat release on pressure versus condensational mass removal effect on pressure is given in Fig. 1c of our paper. This figure grossly overestimates the effect of latent heat release, because it assumes that there is no heat exchange between the columns. In reality there is never such a huge difference between the areas where the air ascends and where it descends because of large turbulent mixing.
Eq. 1 does not and cannot say anything specific about condensation-induced dynamics, because it contains the amount of condensed vapor, dq, as an independent variable. This equation does not tell anything about buoyancy either. This is because it is the first law of thermodynamics. For a given dV, you are right, pressure will fall less when there is condensation. For a given dT, it is easy to see that the opposite is true. These thermodynamic considerations do not teach anything though about the dynamics: namely, the appearance of non-equilibrium pressure gradients that make the air move.
Anastassia Makarieva says:
January 25, 2011 at 10:22 am
“As I said above, the comparison of the maximum possible effect of latent heat release on pressure versus condensational mass removal effect on pressure is given in Fig. 1c of our paper. This figure grossly overestimates the effect of latent heat release, because it assumes that there is no heat exchange between the columns. In reality there is never such a huge difference between the areas where the air ascends and where it descends because of large turbulent mixing.”
By “maximum possible effect”, does the figure assume that 100% of atmospheric condensation is the result of latent heat release to the surrounding air? Wouldn’t the figure then be grossly, grossly overestimating the effect of latent heat release? I understand that convective and radiative heat transfer of some magnitute is always occuring, but isn’t the lion’s share of atmospheric condensation adiabatic?
Anastassia:
Thanks for your detailed response. I will read it a few times to make sure I understand it. Will answer in when I think I have something worth saying.
Steve:
You say, “No transfer of energy occurs between the water and surrounding air, yet there is condensation.” I think that’s incorrect. Whenever water condenses, we get back the heat we had to put in to make it vaporize. I think that’s why steam burns are so nasty when compared to hot-air burns. The steam, when it condenses, gives up 2 MJ/kg.
Kevan Hashemi says:
January 25, 2011 at 3:18 pm
“Steve: You say, “No transfer of energy occurs between the water and surrounding air, yet there is condensation.” I think that’s incorrect. Whenever water condenses, we get back the heat we had to put in to make it vaporize. I think that’s why steam burns are so nasty when compared to hot-air burns. The steam, when it condenses, gives up 2 MJ/kg.”
I’m getting there. I think there is some confusion because we are conflating “heat” and “energy”.
My understanding of adiabatic condensation is that the expanding water vapor does work on the environment. This work is energy transfer but not heat transfer (ideally, but some percentage of energy loss is always heat transfer). My statement, “No transfer of energy occurs between the water and surrounding air, yet there is condensation.” is incorrect, and should be, “Little to no transfer of heat occurs between the water and surrounding air, yet there is condensation.” Your statement, “Whenever water condenses, we get back the heat we had to put in to make it vaporize.” should read, “Whenever water condenses, we get back the energy we had to put in to make it vaporize.”
For example, if I lift and move a bowling ball 20 feet I’ve converted chemical energy into kinetic energy. Yes, the bowling ball will pick up some heat from the friction of being moved, but the temperature of the bowling ball will not increase to the extent had I simply held the bowling ball and transferred the same quantity of chemical energy entirely as heat.
Anastassia Makarieva says:
January 24, 2011 at 9:42 am
Steve
January 24, 2011 at 9:24 am
If a vapor condenses, doesn’t the resulting condensation exert less pressure on the surrounding system than the original vapor? I would think that the act of condensation would exert a negative pressure, actually, so for total pressure to remain approximately the same the remaining dry air would have to expand in order to make up for the pressure loss of water vapor condensation.
Exactly. This is why the cloud sucks in the surrounding air, as Jantar said above. Since condensation occurs as the air moves upwards and cools, the resulting pressure gradient force is also upward directed.
—
Anastassia, fine article. And Jantar, great to have another sailplane pilot here.
Jantar here gave a great description of that experience. Many times just below the cloud base your variometer needle will be pegged at 1200 ft/min upward and you start counting seconds compared to the spinning altimeter to try to gauge your rate of climb, sometimes 1800 to 2400 ft/min. You only have seconds to respond.
But the real experience of condensing water vapor is to stand on your own patio and watch a tornado form over your house. It’s always on very warm day with stifling humidity. You look up at the dark base and start to see some very thin angle hair white clouds some 500 feet lower, always white compared to the cloud above. You start to feel the warm moist wind at your back as these wisps appear and disappear and they look so innocent. As they to start to appear and stay longer you notice a very slow rotation, not fast, just 5 r.p.m. at best. Winds still getting stronger. This whole process is within a few minutes. You now see they are persistent with a definite rotation and its faster, more are now forming even lower, these what I call white whisps… Go get in the closet.
These angel hair clouds are condensation occurring lower and lower and closer to the ground and the suction is awesome. Just look at some pictures of the vortexes filmed during the California forest fires last year. Those are thermal, 1000 deg+, vortexes and though looking impressive with the fire they are but as tiny dust devils compared to tornadoes. It’s the moisture and condensation people, not the heat, heat has it’s effect and usually in the same direction but the force from condensation is much greater.
And I have to believe this same process happens over large regional areas also, frontals. So what is the cause and what is the effect when it comes to pressure variance? Seems this paper has it right.
Anastassia, keep up the work, I love science when it finally, over years of misinterpretation, ends up correct in the end. That is why I have always read so many scientific papers for many, many years.
wayne
January 25, 2011 at 6:56 pm
Thank you very much for this description of tornadoes. I would dream to see one one day (two years ago there was a tornado in Moscow, but I do not live there). The maximum wind speed recorded for tornado that we were able to find in scientific literature is 130 m/s (see here, Table 1, Wurman J, Weather & Forecasting 17 (2002) 473). We have estimated that the pressure gradient force associated with condensation is sufficient to produce such velocities, see here and here. This is a unified explanation for the formation of both tornado and hurricanes: based on release of potential energy accumulated in the atmosphere, not on heat extraction from the ocean.
That’s the point. Nobody questions the existence of thermally driven convection. But in order to yield velocities comparable to what is produced by condensational mass removal of vapor from the gaseous phase, both temperature and its gradient in the thermally driven cells should have been much, much larger than they are actually observed to be in most conspicuous atmospheric vortexes like tornadoes and hurricanes. In reality, as I several times commented, in the atmosphere the warm air very often descends rather than ascends.
An open-minded student of meteorology would be prevented from noticing this ‘odd’ air behavior. Instead of operating with absolute temperature, in meteorology people use the so-called potential temperature:
theta = T (p0/p)^(R/c_p)
As one can see, this “temperature” grows as pressure p decreases. So, even if the temperature in the region of ascent is lower than in the region of descent, but pressure p is lower as well (for unknown reasons), potential temperature can be higher in the region of ascent. And we all know from textbooks that when the temperature is higher (who cares: absolute or potential!), the air ascends. So it’s all right, the student is led to conclude, thermal convection works and forgets the issue. In reality it does not.
Anastassia
January 25, 2011 at 9:28 pm
Thank you for some good links. I’ll read them all. And i think you see that I really do understand your groups theory, I understand it not from the science and math side but because I have many times actually experienced it in the real world. That is where I say stay with it, it is real, whether science wants to currently listen or not. Thermal has been the standard answer for so long and it’s hard to change consensuses.
So you want to see a tornado huh, just come to Oklahoma in late spring for a week or two. I’ve even heard you can now get special storm-chaser tours, don’t wait for one to come to you, go chase one down!
However, there is one aspect I don’t understand yet and I haven’t been able to completely absorb the paper. In the summer here there are many days with clouds (condensation) floating across the sky, building and dissipating, day after day, never raining, and the moisture is just being recycled over and over. As I spoke of sailplanes there is tremendous convection happening under these large clouds always but that is all very local wind in nature. It seems just by intuitive logic that until it does actually rain and liquid water is removed from the system in large quantities that the effect stays local. But with heavy rain I can then see the horizontal aspect you spoke of, the paper thin atmosphere compared to the horizontal plane. Is that the only time when it then becomes regional (500-1000 km) in nature, when rain/ice/snow is removed?
Anastassia
January 25, 2011 at 9:28 pm
So you and et al. are M10 in JeffId’s post! I think after reading all of these links I will have the answer to my question above, as I read that thread is already getting into the regional and Hadley aspects that was central to my question. The details of the models is an area I have so far shied from so I best just be quiet and learn some.
Steve
January 25, 2011 at 11:47 am
When we speak of adiabatic condensation this means that the latent heat released upon condensation remains within the air parcel where condensaton occurred. Strictly speaking, liquid water should also remain there — when it is removed we have what one calls pseudoadiabatic process.
In our Fig. 1c two atmospheric columns are compared, both are in hydrostatic equilibrium. But in one column the lapse rate is moist adiabatic (column A), while in another one it is dry adiabatic (column B). Surface temperatures are the same, so at any given height the air in column A is always warmer than the air in column B.
The second difference is that the amount of vapor in column A is smaller than in column B by the amount that is necessary to make the lapse rate in A moist adiabatic (to do so, we do need to condense some vapor throughout the column). So surface pressure (and total amount of gas) in column A is smaller than in column B.
One can see from Fig. 1c that below the height zc where pA-pB = 0, the effect of mass removal dominates and the air pressure is lower in column A. Above zc, the temperature difference (the one Kevan was talking about) plays finally in, such that the warmer air in column A has higher pressure than in B above zc.
What is meant by ‘maximum effect’ of latent heat: this means that there is no heat conductivity between the column, such that the temperature difference between them amounts to over 40 K at a height of several kilometers. In reality this never happens: due to strong horizontal mixing, the ascent is not adiabatic. Latent heat is at least partially removed from the air parcel where condensation takes place. It is lost by radiation to space and by turbulent mixing to the descending column which is being warmed. Actually such circulation pumps the latent heat from the condensation area to the surface of the area where the air descends. This was happening for over two months this summer during the heat wave in Russia.
George E. Smith
January 25, 2011 at 10:11 am
George E. Smith
January 25, 2011 at 10:11 am
The difference lies in the fact that in order to support droplets the air must move upwards — if we are talking of a stationary pattern. In order for the air to move, there must be a non-equilibrium gradient of air pressure. Considering one dimensional motion, if the pressure gradient force is balanced by the downward force associated with droplet friction. That is, a microscopic pilot riding on the droplet will serve as a brake for the upward air flow in very much the same manner as windmills, for example, serve as a brake for the horizontal air flow. In its turn, the upwelling air will be pushing the droplets upward as the horizontal air flow is pushing the windmills.
In contrast, no air flow is needed to sustain vapor molecules in the atmosphere. The air is static.
I agree, it does not.
My colleagues and I very much value the readers’ attention. It is a pleasure to share our thoughts. Zero g is a very interesting case.
Condensation occurs because the temperature drops with height. When we have gravity, this happens any time when the air ascends because of expansion. Let us put g = 0 and consider an atmosphere in a closed container. A vertical temperature gradient can form due to the greenhouse effect. Also, we can just impose it externally on our atmosphere by some technological device. This will lead to the appearance of a non-hydrostatic pressure gradient and air motion.
The latter case is realized in practice in heat pipes. These are technological devices that employ the pressure gradient of the saturated vapour to effectively transfer heat. The rapid transport of heat along the pipe is due to the difference in partial pressures of saturated vapour within the pipe. One end of the pipe is warm (attached to the body that we have to cool), another is cold. Vapor pressure is high where it is warm and low where it is cold. This pressure gradient causes the vapour to flow very rapidly along the tube from the hot end to the cold. The theoretical limit of flow velocity is, obviously, the velocity of molecules. This makes heat pipes very efficient coolers.
However, in heat pipes one must artificially remove the latent heat from the colder end of the pipe (otherwise the temperature gradient responsible for the vapor motion will disappear). This is a limitation on the pipe performance. Likewise, in an atmosphere with g = 0 and a temperature gradient due to absorbers of thermal radiation, the limitation is that the released latent heat must be ultimately radiated to space. That is, if you want to have a larger air velocity, you must radiate more efficiently.
Atmosphere with gravity is unique in that there is an adiabatic temperature gradient that persists in the region of ascent irrespective of how large the vertical velocity is. There is no problem of disposing heat (the process is adiabatic yet the temperature drops) to maintain the temperature gradient that is needed to sustain condensation. This allows for a positive feedback between velocity and condensation: the larger the vertical velocity, the larger the condensation rate S, the larger the pressure gradients associated with condensation (see Eq. 37 in M10), and so on. Such a positive feedback would be absent at g = 0.
wayne
This is a very interesting question. Note also that it resonates with the comment of Dr. Judith Curry (the only reviewer so far of our work):
When vapor condenses, the air pressure is lowered. When moisture evaporates, air pressure rises. If we see a constant amount of cloud water but no rain, this means that this process is localized in the atmosphere on a small scale: condensation occurs in the region of ascent where the cloud resides, evaporation (of condensed droplets) occurs in the region of descent, where relative humidity is less than unity. This may result in the formation of regular patterns like cloud streets.
When is this possible? It should be possible when the droplets that are formed are sufficiently small (have high surface to volume ratio and low terminal velocity) and the circulation velocity is sufficiently low. Only in this case the droplets will have time to evaporate before reaching the surface. One can hypothesize that such a regime is unstable: if vertical velocity increases and/or droplets get larger, they are removed more quickly from the atmosphere across a larger area. The balance evaporation – condensation = 0 in the atmosphere is then broken in this area and it becomes a large scale area of low pressure.
But note also that persistent clouds without rain may also represent a non-stationary accumulation of liquid water in the atmosphere: in this case, the entire area with clouds gradually becomes the low pressure area despite there is no rainfall.
It would be great if climate scientists, many of whom do find time to do blogging and may even happen to read this thread, would join the discussion of condensation-induced dynamics. There is much to discuss here. It is beyond doubt that having got acquainted with the main propositions, people will be able to use them to explain many weather and climate patterns of which we may have little or no knowledge at all.
Anastassia Makarieva says:
January 26, 2011 at 6:28 am
“When we speak of adiabatic condensation this means that the latent heat released upon condensation remains within the air parcel where condensaton occurred….What is meant by ‘maximum effect’ of latent heat: this means that there is no heat conductivity between the column, such that the temperature difference between them amounts to over 40 K at a height of several kilometers. In reality this never happens: due to strong horizontal mixing, the ascent is not adiabatic. Latent heat is at least partially removed from the air parcel where condensation takes place. It is lost by radiation to space and by turbulent mixing to the descending column which is being warmed. Actually such circulation pumps the latent heat from the condensation area to the surface of the area where the air descends. This was happening for over two months this summer during the heat wave in Russia.”
In your statements I don’t see where you explain that at least some energy in latent heat is expressed as work, not heat, on the surrounding air during an adiabatic cooling process. The temperature of the ascending air mass goes down without any increase in temperature to adjacent air masses, just energy expressed as work, but at the moment of condensation suddenly there has to be a heat transfer between the water vapor and the surrounding air? Every joule (or most) ends up as released heat right then and there at the moment of condensation? If so, I don’t think that I am ever going to understand this adiabatic atmospheric process.
It seems like you are treating “latent heat” as a special kind of energy that, during adiabatic atmospheric cooling, acts differently from all of the other energy in the air mass. The temperature of the water vapor goes down without any heat exchange between the water vapor and surrounging air mass, just an energy exchange in the form of work. But this work is not an adequate energy exchange to allow water to condense without a significant heat exchange? I don’t understand why the energy exchange needed to achieve condensation cannot also be expressed as work on the surrounding air instead of heat.
Anastassia Makarieva says:
January 26, 2011 at 10:19 am
Thank you for spending so much time sharing your work on the web, open to all scientist and layman alike to appreciate and discuss. It is, in my view, the future happening now. I looked at your web site on land use issues and the effects of deforestation etc and find it intresting and informative. Have you been in communication with Dr Pielke Sr and Jr, http://pielkeclimatesci.wordpress.com/
http://rogerpielkejr.blogspot.com/ and are you familiar with their work on land use issues and climate?
Steve:
“The temperature of the ascending air mass goes down without any increase in temperature to adjacent air masses,”
That’s not true: the adjacent air masses warm up. The ascending gas compresses the descending gas. The descending gas heats up.
“just energy expressed as work”
The work done by the expanding gas turns into heat and pressure energy in the adjacent gas.
“but at the moment of condensation suddenly there has to be a heat transfer between the water vapor and the surrounding air?”
Absolutely, at that very moment.
“Every joule (or most) ends up as released heat right then and there at the moment of condensation?”
Yes, every Joule.
“If so, I don’t think that I am ever going to understand this adiabatic atmospheric process.”
Indeed: if what you said was true, nothing would make sense. But I hope that what I say holds together.
“I don’t understand why the energy exchange needed to achieve condensation cannot also be expressed as work on the surrounding air instead of heat.”
If you start thinking it through again, I hope you won’t get to this question. There are several answers. To start again, you could try this simple explanation of convection:
http://homeclimateanalysis.blogspot.com/2010/04/convection.html
To see why some gas has the energy to compress other gas, and thus cause circulation, see the p-V diagram here:
http://homeclimateanalysis.blogspot.com/2010/04/work-by-convection.html
And after that you will be able to explain to me how a jet engine works.
Yours, Kevan
PS. I still have not been able to understand Anastassia’s recent explanation of the condensation-suction process. I am still trying.
Steve: I noticed another comment above from you. You say that when water condenses, some of the energy we put in to vaporize it can manifest itself as work. No, it can’t. That would violate the Second Law of Thermodynamics, which says that you cannot make a machine that is 100% efficient at converting heat into work. All it takes to vaporize water is heat, so if I can get work out of the act of condensation, I’d be able to start again by adding heat, and so build a cyclic machine that creates work.
Anastassia Makarieva says:
January 26, 2011 at 10:19 am
wayne
This is a very interesting question. Note also that it resonates with the comment of Dr. Judith Curry (the only reviewer so far of our work):
When vapor condenses, the air pressure is lowered. When moisture evaporates, air pressure rises. If we see a constant amount of cloud water but no rain, this means that this process is localized in the atmosphere on a small scale: condensation occurs in the region of ascent where the cloud resides, evaporation (of condensed droplets) occurs in the region of descent, where relative humidity is less than unity. This may result in the formation of regular patterns like cloud streets.
When is this possible? It should be possible when the droplets that are formed are sufficiently small (have high surface to volume ratio and low terminal velocity) and the circulation velocity is sufficiently low. Only in this case the droplets will have time to evaporate before reaching the surface. One can hypothesize that such a regime is unstable: if vertical velocity increases and/or droplets get larger, they are removed more quickly from the atmosphere across a larger area. The balance evaporation – condensation = 0 in the atmosphere is then broken in this area and it becomes a large scale area of low pressure.
But note also that persistent clouds without rain may also represent a non-stationary accumulation of liquid water in the atmosphere: in this case, the entire area with clouds gradually becomes the low pressure area despite there is no rainfall.
It would be great if climate scientists, many of whom do find time to do blogging and may even happen to read this thread, would join the discussion of condensation-induced dynamics. There is much to discuss here. It is beyond doubt that having got acquainted with the main propositions, people will be able to use them to explain many weather and climate patterns of which we may have little or no knowledge at all.
— —
You know, after asking that question of you I had some secondary doubts on my own question.
If there is any condensation, this effect of lowered pressure is going to happen regionally whether there may also be local effects or not. Stratus clouds may not even have a local effect. Cumulus always do due to the clumps. But you know, both local and regional is always happening at the same time, it is never really just local. When clouds form, like after a clear night, that IS the condensation even though the droplets are not large enough to fall in participation. Thinking of that it doesn’t seem to matter if 1 cm3 of water has condensed to form 21 raindrops or 10,000 tiny particles forming the mist from which clouds are made.
The condensation has already occurred.
And if you imagine yourself raised up high in the atmosphere and look down (as in a plane) you see condensation has occurred everywhere, maybe enough to cover an entire state or more. That suction would then be horizontally. You would see individual clouds dissipate but there would always be another to take it’s place and the over all effect would be lower pressure compared to the cloudless night a few hours before. Most importantly, that would seem to make the atmosphere as a whole pulse every diurnal period as the clouds of that type normally disappear every night, reform the next day. There is something I had never thought of in that way.
That made me reconsider my whole question, what exactly was I asking you. Sure didn’t want to infer something that would lead to a thought that wasn’t real, like: If it doesn’t fall to the ground as rain there is no effect for now I see it does. Seems if it does participate that just adds permanency to the effect. Kind of like a closure of a single event. When precipitating, the effect is no longer a one-time event but a continuous force laterally as long as the water is falling out.
I see it now. (if not, please clarify) Don’t you love it when you are able to think in an entirely different direction that just yesterday you thought was so perfectly logical and in reality was so wrong. That’s exactly why I follow science as a hobby!
Darn that auto spell checker, try precipitation, NOT participation.
Pounding my head– always re-read before hitting ‘submit’!
Kevan Hashemi says:
January 26, 2011 at 11:49 am
“Steve – The temperature of the ascending air mass goes down without any increase in temperature to adjacent air masses…Kevan That’s not true: the adjacent air masses warm up. The ascending gas compresses the descending gas. The descending gas heats up.”
So because the expanding, rising volume is doing work on adjacent air volumes (moving them by applying pressure), those adjacent volumes must be heating up from the pressure increase? Well, if the pressure is applied to a gas that remains in the same volume, yes I can see how the temperature must increase (ideal gas law). But if the pressure is applied and the gas simply moves (wind)? Are you saying that in adiabatic processes there really is a heat transfer, it’s just in the form of work that is immediately translated into heat?! That would be confusing. Why don’t they just call it a heat transfer?
“The work done by the expanding gas turns into heat and pressure energy in the adjacent gas….If you start thinking it through again, I hope you won’t get to this question. There are several answers. To start again, you could try this simple explanation of convection: http://homeclimateanalysis.blogspot.com/2010/04/convection.html”
Hmmm, the explanation at that link doesn’t really help me. In it you state,”In short, we assume that any heat the Volume loses or gains on the way up is negligible. The expansion of the Volume will be adiabatic. As a gas expands adiabatically, it cools down, even though it loses no heat.” That statement doesn’t make sense to me. It cools down but it loses no heat? It most certainly does lose heat or it would be the same temperature! What it doesn’t do is transfer heat – it transfers energy. I understand that at some point somewhere all that energy will end up as heat, but that could end up being far away in time and space. For example, a helium balloon that rises to great altitude and lands on a mountain will have some portion of it’s original internal energy stored as potential energy as long as the mass of that balloon remains on that mountain. Adiabatic expansion is completely new to me, so I have no idea how long it takes for the work of moving an air mass to be expressed as heat, and how far away from the expanding air mass this work will be expressed as heat.
Kevan Hashemi says:
January 26, 2011 at 11:56 am
“Steve: I noticed another comment above from you. You say that when water condenses, some of the energy we put in to vaporize it can manifest itself as work. No, it can’t. That would violate the Second Law of Thermodynamics, which says that you cannot make a machine that is 100% efficient at converting heat into work. All it takes to vaporize water is heat, so if I can get work out of the act of condensation, I’d be able to start again by adding heat, and so build a cyclic machine that creates work.”
As I said to Anastassia, it seems like you are treating latent heat as a special kind of energy. Which may be true, I’m just looking for the “how”! Adiabatically, the energy of the rising water vapor can be transferred to the surroundings as work, except for the energy of it’s latent heat?
Hmmm. You don’t have a problem getting heat out of condensation, but if I say “work” (same units, joules) suddenly I’ve invented a perpetual motion machine? I don’t expect a 100% return in the form of work, which is pretty clear since you quote me as claiming “some of the energy we put in to vaporize it can manifest itself as work.” According to you, 0% of the latent heat can be transferred as work (“No, it can’t.”). How is that?
I’m doing a poor job of answering your questions. I apologize. It may be that you and I are saying exactly the same thing, and our effort to do so without mathematics is making us go around in circles.
When I say “It gains no heat” I mean “No heat enters or leaves.”
You say, “It cools down but it loses no heat? It most certainly does lose heat or it would be the same temperature!” Well, the word “loss” in thermodynamics means something irreversible. In adiabatic expansion, internal heat is turned into work and work only. No heat is “lost”. When heat is converted into work, it is not “lost”. We can convert it back into heat any time we want. Only when heat flows out of our system do we say it is “lost”. Or at least that’s how I was taught to use the word.
But you have pointed out that these words are making my answers confusing and useless. So I will try harder.
Work can always be turned into heat, but heat cannot always be turned into work. Condensation results immediately in faster-moving molecules, which is heat. After that you can see about turning some of that heat into work, but you need some other components in the operation, such as a cold reservoir. Adiabatic compression, on the other hand, is in itself reversible. The gas will expand out again for you with no other component required.
If we say that condensation energy is converted directly into work, we are glossing over the fact that some other component is required to cooperate with the conversion of heat into work. In this case, what is that component?
I’m saying that air with moisture will be warmer as it rises, so it will expand and be less dense. Now it will rise above the dry air, like your balloon to the top of a mountain. That’s work. But we needed the dry air to make the conversion possible.
Do you agree with all that?
Retired physicists who might help:
Hal Lewis
Freeman Dyson
@ Steve says:
January 26, 2011 at 2:53 pm
As I said to Anastassia, it seems like you are treating latent heat as a special kind of energy.
—-
What you might do is ask yourself: Is latent heat warm, in fact, does latent heat have a temperature at all? Doesn’t temperature rely on kinetic energy and therefore able to perform work. That might be where your logic hits problems. That is: Can latent heat express itself at all except at condensation time?
To me it’s potential energy and right there you can get in trouble if kinetic and potential are mixed in equations. There .are. two forms of energy. Not trying to correct you but those seem good questions to ask yourself.
Kevan Hashemi says:
January 26, 2011 at 4:36 pm
“You say, “It cools down but it loses no heat? It most certainly does lose heat or it would be the same temperature!” Well, the word “loss” in thermodynamics means something irreversible. In adiabatic expansion, internal heat is turned into work and work only. No heat is “lost”. When heat is converted into work, it is not “lost”. We can convert it back into heat any time we want. Only when heat flows out of our system do we say it is “lost”. Or at least that’s how I was taught to use the word.”
Ahhhh, I didn’t know about “loss” terminology. An energy transfer within two components of the system is not referred to as a “loss” by either component of the system – got it.
“Condensation results immediately in faster-moving molecules, which is heat.”
As state changes from gas to liquid to solid, the molecules have less kinetic energy, not more. What are the faster moving molecules – the air adjacent to the condensation?
“If we say that condensation energy is converted directly into work, we are glossing over the fact that some other component is required to cooperate with the conversion of heat into work. In this case, what is that component?”
Again, I’m not following how the latent heat transfer (as work) is any different from all of the other energy transfer in an adiabatic process.
I’m a visual thinker, and I imagine a hornets nest of air molecules pushing out as their mass rises. This pushing, ascending in altitude, is work and lowers the kinetic energy of every molecule in the air mass (i.e., the temperature of the air mass drops). Considering that the potential energy of the mass is increasing as it lifts itself through the atmosphere, it isn’t surprising that something has to give. Those molecules can’t do work, get an increase in potential energy, and keep their original kinetic energy! Within this air mass are water molecules that are losing their kinetic energy while still colliding with each other. Eventually the collisions lack the energy necessary to overcome the attractive power between the water molecules and they stick together – we have atmospheric condensation. (That’s simple condensation – a cloud nuclei, such as a bit of dust, is more likely to serve as the condensation surface.)
I understand that some of the temperature drop in the air mass will be due to heat exchange with adjacent, colder air masses. Cooling will not be 100% adiabatic! But what adiabatic cooling there is, to my understanding, is work, not heat transfer. That air mass climbed a mountain! I don’t see how it can be assumed that condensation results in a temperature increase to adjacent air masses that is equivalent to 100% of the energy of latent heat.
“I’m saying that air with moisture will be warmer as it rises, so it will expand and be less dense. Now it will rise above the dry air, like your balloon to the top of a mountain. That’s work. But we needed the dry air to make the conversion possible.”
I understand that. My confusion commenced when you stated that the transfer of latent heat at condensation would cause expansion of adjacent air that was 8 times the magnitude of the compression experienced when the water vapor condenses. I took that to mean that as warm air rises you suddenly get a burst of positive pressure when the dewpoint is reached. That seemed off (I expected a negative pressure), so I had to research this adiabatic cooling process.
wayne & steve;
you’re both trying to imagine what happens, and failing. 100% of the latent heat is transferred to the surrounding molecules. A window pane that condenses water vapour is warmed by that process. Air temperature cannot drop below the “Dew point” because condensing the dew dumps heat into the air, until humidity is lowered to the point that the dew point also drops. And so on.
Real heat is required to evaporate water (which is why sweat succeeds in cooling your skin). And condensing it releases all that heat again.
Steve,
I agree with your description beginning “I’m a visual thinker.” Furthermore, I can perfectly see how “I took that to mean that as warm air rises you suddenly get a burst of positive pressure”.
But what I meant was that adiabatic expansion of dry air from V1 to V2 where V2 > V1 gives a greater pressure drop than adiabatic expansion of moist air. The latent heat causes the air molecules around the water droplets to buzz around more rapidly.
If we have a 1-km wide body of air, we can imagine that the heat transferred by mixing, conduction, and radiation across the boundaries of the body will be negligible compared to the work done by the gas in expanding and rising. I assume you agree with that. If so, then you’ll see that the ideal adiabatic expansion equation is useful in understanding how the gas cools as it rises.
We can arrive at the effect of latent heat upon the gas in several ways, and one of the simplest is to imagine the gas expanding adiabatically (no heat transferred by conduction, mixing, or radiation) to a certain volume and pressure, and then we calculate the effect of condensation upon the pressure, even though we know that this condensation would occur during the expansion, not at the end. The net effect is positive: condensation raises the pressure compared to that of dry air.
In Anastassia’s paper, it seems to me (and recall that I still don’t understand her most recent explanation, so I could just be ignorant) that the authors are confused by the fact that condensation occurs only when pressure drops. They take this to mean that condensation causes a pressure drop, when in fact condensation reduces the pressure drop.
Steve:
A water molecule condenses when random encounters with other vapor molecules leave it almost stationary and not vibrating, and it just happens to be next to another such stationary and not vibrating water molecule. It evaporates when its liquid neighbors just so happen, by chance, to kick it super-hard and it has the energy to escape the liquid. At a certain temperature, the chance of either happening is equal and the gas is saturated with liquid. At a lower temperature, the chance of condensation is greater than that of evaporation, so the liquid condenses. You will note that in order for the water molecule to come to a stand-still, another molecule, say a nitrogen molecule in the air, must receive all its vibrational and translational kinetic energy through the chance encounter.
In Thermodynamics, we say that the “latent heat” is passing into the gas. Meanwhile, the water has not cooled down. It has lost most of its heat, but its temperature remains constant. Thus we see that the relationship between “heat” and “temperature” is subtle. I claim that my use of the words “loss” and “gain” are unambiguous in the context of the Thermodynamic definitions of “heat” and “work” and “temperature”. Outside these definitions, where a rise in “temperature” occurs if an only if there is an addition of something mysterious called “heat”, things get fuzzy.
Also, you will see that condensation is a statistical process, and does not happen immediately. There is nothing wrong with us imagining the pressure of a gas dropping rapidly to below the condensation point as we expand the gas in a cylinder, and the condensation happening later. In that case, we will in fact see the pressure of the gas reach a minimum at the end of the expansion and rise after that during the condensation process. Indeed, this is how a cloud chamber works. The condensation occurs when a charged particle goes through the super-saturated vapor.
Kevan Hashemi says:
January 27, 2011 at 12:43 am
Steve,
I agree with your description beginning “I’m a visual thinker.” . . . .
Laurie says, . . . . .that’s why graphs and diagrams are good. They are worth a thousand words.
http://en.wikipedia.org/wiki/File:Water_cycle.png
Kevan Hashemi says:
January 27, 2011 at 12:43 am
“In Anastassia’s paper, it seems to me (and recall that I still don’t understand her most recent explanation, so I could just be ignorant) that the authors are confused by the fact that condensation occurs only when pressure drops. They take this to mean that condensation causes a pressure drop, when in fact condensation reduces the pressure drop.”
Brian H says:
January 27, 2011 at 12:18 am
“wayne & steve;
you’re both trying to imagine what happens, and failing. 100% of the latent heat is transferred to the surrounding molecules. A window pane that condenses water vapour is warmed by that process. Air temperature cannot drop below the “Dew point” because condensing the dew dumps heat into the air, until humidity is lowered to the point that the dew point also drops. And so on. Real heat is required to evaporate water (which is why sweat succeeds in cooling your skin). And condensing it releases all that heat again.”
Thanks for all comments in trying to explain to me what happens to the energy of latent heat. I must admit that I find the popular literature confusing. The basic statement among every meteorology text I have found can be summarized as “latent heat is transferred to the atmosphere which is why clouds make the local atmosphere so warm.” Can’t get much plainer than that!
I’m still trying to figure out the mechanism by which this heat is physically transferred to the surrounding air – conductive? radiative? What physically happens when those water molecules collide but do not repel each other? It isn’t a chemical bond, so I don’t expect a sudden radiative heat transfer due to electrons falling to a lower orbital. But is that what’s happening? Is it a pseudo-chemical bond in which radiative heat transfer must occur? Because as far as kinetic energy is concerned, all I see are water molecules getting gradually slower as they rise in altitude. I don’t understand why they could suddenly have a burst of kinetic energy, transferred to the surrounding air, when they finally stick together. I also keep finding references to “the potential energy gain due to altitude gain must be balanced by a loss of kinetic energy” (for adiabatic cooling).
If I boil water in my kitchen (near sea level) it will stop increasing in temperature at 100 C because it must go through a state change – heat of vaporization is required. Imagine that I can input precisely the heat needed to achieve vaporization. The steam that comes off is still 100 C, exactly the same temperature as the water. What do I gain by inputting all of that extra energy? Not temperature (average kinetic energy) but vapor pressure. In our atmosphere, the steam can now do something it wasn’t able to before – climb. There is the work done of pushing out, but even more obvious is that as it climbs it must be doing work.
So my 100 C rising steam is using some portion of it’s internal kinetic energy just to climb, not transfer heat. Before vaporization it was transferring energy to the environment as heat, and as steam it will continue to do so. But it seems like the latent heat goes into the climbing, not the heat radiation. We know that, eventually, the heat of vaporization put in must come back out as heat. Won’t that happen as the condensed water falls and ultimately hits the ground?
Basically, it’s a timing issue with me. I don’t understand why the energy of latent heat must be expressed as a heat transfer at the moment of condensation in the atmosphere, as opposed to a heat transfer spread throughout the entire up/down cycle.
Assuming that it must be expressed as a heat transfer at the moment of condensation, does adiabatic condensation follow an exact reverse process of the original vaporization? A LOT of heat has to be input to achieve that vaporization, with no change in temperature to the water. So does a LOT of work need to be done at condensation, with no change in temperature to the water vapor? At the dew point the air mass will rise and expand with no reduction in temperature, eventually BANG – condensation, and afterwards temperature can continue to drop? Or does the latent heat basically keep the air mass stuck at the same altitude until, via heat transfer to surrounding air, condensation occurs?
It’s been a busy week so I’ve not had time to follow this discussion in detail or to respond to Anastassia’s comments.
I left the discussion after she mentioned the simple model of a flat, isothermal Earth (to which I narrowly avoided quipping that that’s how many climate modellers see it). But I was reminded of the (classic) conditions for natural (free) convection in a compressible fluid (without phase change); and the heat transfer within. To date, this thread hasn’t mentioned Grashof, Prandtl or Nusselt.
Free convection is due to body forces; those due to gravity.
Forced convection is due to surface forces; those due to fluid pressure, exerted “mechanically” from “outside”.
The Grashof number is the ratio of buoyancy to viscous force.
The Prandtl number is the ratio of momentum to thermal diffusivity.
The Nusselt number is the ratio of convective to conductive heat transfer.
There is no free convection until the Rayleigh number (Gr.Pr) exceeds a threshold. All heat transfer is conductive below that threshold. (Radiative heating is insignificant near the surface when compared to conductive and subsequent convective.)
For free convection to occur, it is only necessary for the buouyant forces to overcome the viscous and gravitational ones; by thermal (conductive) expansion of the fluid.
It is not necessary for (part of) the fluid to undergo any phase change for free convection to occur. If it does; does warm air rise above a fog in calm conditions?
As a final short note: The Earth’s surface is far from an isothermal plane. It has vast thermal gradients that change with time.
Steve, you say, “I’m still trying to figure out the mechanism by which this heat is physically transferred to the surrounding air – conductive? radiative?”
I tried to answer that question in my second comment tonight. Did you see my description of how a water molecule becomes bound to others, as a result of statistical chance, and how it might escape. If you stick to your buzzing molecule picture, it will all work out.
Note that “potential energy” does not exist as a thing. It cannot be detected. We define it to help us do calculations more quickly.
Water molecules bind to one another when they make liquid, and it’s hard to get them apart. It’s the same as the sun and a planet: hard to pull them apart. But if you let go of the planet, it won’t hit the sun, it will zip past like a comet. To make a planet collide with the sun, you have to stop it moving very near the sun. All this will make sense if you don’t get distracted by the concept of “potential energy” and “heat” as they are commonly misunderstood in science fiction and school text books.
Brian H says:
January 27, 2011 at 12:18 am
wayne & steve;
you’re both trying to imagine what happens, and failing. 100% of the latent heat is transferred to the surrounding molecules. A window pane that condenses water vapour is warmed by that process. Air temperature cannot drop below the “Dew point” because condensing the dew dumps heat into the air, until humidity is lowered to the point that the dew point also drops. And so on.
———
Brian, you might re-read my comment giving Steve a few questions to think about. Latent heat until condensing begins is potential, or are you saying you disagree?
Kevan Hashemi says:
January 27, 2011 at 5:01 pm
…
Note that “potential energy” does not exist as a thing.
——
That potential energy does exist just as gravitaional potential energy exists but you are correct that it is invisible in normal settings until you devise the correct experiment to make it manifest itself, then you do see it is in fact real.
Kevan, I restated that for Steve might take your words so literal that once again it is out of reality though I understood your meaning.
Kevan Hashemi says:
January 27, 2011 at 5:01 pm
“Steve, you say, “I’m still trying to figure out the mechanism by which this heat is physically transferred to the surrounding air – conductive? radiative?”…… I tried to answer that question in my second comment tonight. Did you see my description of how a water molecule becomes bound to others, as a result of statistical chance, and how it might escape. If you stick to your buzzing molecule picture, it will all work out.”
I did see your explanation, but as soon as I read “A water molecule condenses when random encounters with other vapor molecules leave it almost stationary and not vibrating” I lost understanding. Water is still condensed as 99 C on my stove. That is a lot of kinetic energy – those condensed water molecules are most certainly moving and vibrating! Why must this be different for a gas? “Almost stationary and not vibrating” sounds like “almost absolute zero” to me, and the water vapor in a cloud is well above that temperature.
I did more of my own research and it looks like my question, “Is it a pseudo-chemical bond in which radiative heat transfer must occur?” points to the correct answer.
I know about hydrogen bonds but I didn’t realize they resulted in more stable electron configurations. They are pseudo-covalent bonds. Read this from 1999:
http://www.sciencedaily.com/releases/1999/01/990121074852.htm
At vaporization, energy breaks these hydrogen bonds, leaving the individual water molecules with all electrons in their “standard” covalent bonds. At condensation hydrogen bonds reform at an average of 2 – 3.6 hydrogen bonds per water molecule, depending on conditions (and experimentally there is some debate). As with any electron moving into a more stable covalent bond, some energy must be radiated immediately as a photon.
I think that kinda answers it for me. At condensation there will an immediate radiation of heat commensurate to the energy of the hydrogen bonds formed. So condensed air must indeed transfer latent heat into the atmosphere at the moment of condensation. Not during it’s ascent. Not when the water hits the ground. But the instant those electrons go to a lower energy state.
steve;
There’s a wee problem with your “rising steam at 100°C” image. TANSTAAFL. (No free lunches). At exactly 100°C, it can’t “use” its heat to expand (become less dense) and rise without cooling — which it can only do by condensing (some of it, enough to balance the energy budget). So rising steam always spawns lotsa visible mist droplets. (Unless it was live dry superheated steam, which has some energy to play with until it drops to 100°C., at which point the mist starts to form.)
Steve:
Yes, the reason liquid water molecules are hard to separate is because of hydrogen bonds. But you end with, “At condensation there will an immediate radiation of heat commensurate to the energy of the hydrogen bonds formed.” Absolutely not. Who said anything about radiation? You made that up yourself. Nothing is radiated. The reason the gas gets hot is because of the statistical reason I gave you: only when a water molecule is deprived of most of its kinetic energy by random collisions can it be bound to another water molecule. You can figure all all this out in terms of molecules bouncing around. It’s as simple as that. That’s Statistical Mechanics, and it works fine. There is no radiation involved. Why do you want to make it more complicated?
As to “water at 99C” being hot, well, that’s true. But what do you mean by “hot”? Two bodies are at the same temperature when they exchange no heat. That does not mean that they contain the same amount of heat per kilogram or per atom. Water contains far more heat per kilogram than iron at the same temperature. Steam contains far more heat per kilogram than water at the same temperature. What’s the problem with that?
wayne
I generally agree. But the diurnal changes of surface pressure are complex in nature and have also to do with temperature. In our paper we considered the stationary state with a continuous force laterally as long as the water is falling out. If we consider a hypothetical case when clouds are hanging in the air for a long time but no rainfall occurs, this means that condensation is compensated by evaporation on a small scale equal to the cloud size. In this case, obviously, there will be no average pressure reduction in the area covered by the clouds. The pressure gradient will be located within each particular cloud.
Kevan
Let me propose some additional food for thought. Let us take two adjacent boxes of equal size, 1 and 2, each containing 1 mol of gas. Box 2 is warmer than box 1 by T2-T1 and, hence, has a pressure access. If we let the partition between the boxes move under the action of the higher pressure, we will see an oscillating motion. It will continue until the temperatures and pressures equate. The partition returns to its central position.
However, the same thermodynamic equilibrium can set in differently: if we just allow for heat transfer between the boxes. If heat transfer is efficient, no motion will develop. The partition will not move at all.
Now consider the case when the pressure difference between the boxes is due to the difference in the amount of gas. That is, box 2 contains 2 mol of gas and box 1 contains 1 mol of gas. Temperatures are the same. In this case there is no way to reach the equilibrium other than to move the partition. Gas in box 2 will expand performing work on gas in box 1. When the resulting kinetic energy dissipates and the equilibrium sets in, the partition will no longer be in the center. It will be displaced towards box 1, such that molar densities on both sides of it coincide.
To summarize, in the first case work may or may not be performed while the equilibrium is being reached. In the second case, there is no way to reach the equilibrium other than by doing work (of the gas in box 2 on the gas in box 1).
In this sense it is misleading to quantitatively compare the “relative pressure rise” due to latent heat release (“warming”) with the pressure difference caused by the mass removal of the gas. Heat and work are not the same.
Anastassia:
Thank you for your further explanation, which I found thought-provoking.
You say, “If we let the partition between the boxes move under the action of the higher pressure, we will see an oscillating motion.” I suppose you mean the gas on each side will act as a spring, with the partition as the weight, so we get simple harmonic motion. If we allow the partition to conduct heat, I see that its average position will move to the center. Otherwise, the partition can remain away from the center forever.
You go on to say, “If the heat transfer is efficient, no motion will develop.” For this to be true, the mass of the partition has to be so large that the pressure difference causes only negligible acceleration during the time it takes for the temperature to equalize. I can’t think of any physical material that would meet that requirement. So you must be talking about fixing the partition in place with glue or a few nails.
Now you talk about a pressure difference and how, “In this case there is no way to reach the equilibrium other than to move the partition. ” Well, that’s true. If your partition is impermeable to pressure, the partition must move. But it’s also true that if your partition is impermeable to heat (insulating), the partition will have to move.
You say, “In the second case, there is no way to reach the equilibrium other than by doing work ” I disagree. You could make the partition vanish in an instant, and the two bodies of gas would mix freely without doing any work. We could make the partition out of thin latex and pop it. The mixing would be isenthalpic, but not isentropic. Or we could also put a throttle between the two, and let the gas go through the throttle without pushing anything around.
I agree that heat and work are different, but I don’t think your examples have pointed out the difference. Furthermore, the fact remains that moist air expands more than dry air when the two experience the same pressure drop. Moist air will end up less dense. It will float above any surrounding dry air.
Anastassia Makarieva says:
January 28, 2011 at 10:43 am
…
I generally agree. But the diurnal changes of surface pressure are complex in nature and have also to do with temperature.
—
You are right, I’m pulling this discussion into some rather small effects that overall might get lost. I had already come to the realization that there is also warming and cooling in the diurnal cycle and with that comes expansion and contraction in addition to the moisture aspect.
Have you tried Dr. Tom Vonk? I don’t know him personally but on the pure physics side and from what I have read in his posts and comments he seems to have a good background of proper physics. On the observation and logical side I see your paper has a big place in atmosphere physics, for sure. Jeff Id had it right, this aspect is important, very important. Can’t believe they left this out of the models, no wonder they are having problems mimicking reality.
I like your site. I’ve commented at wuwt before what I call myself, a conservationalist, so your site reverberates my concerns, especially about the forests. So many people look at all plant life here as generally the same without considering the depth of the roots, trees rule here, and that gets right back to your view on a moisture drivers in atmosphere.
It took me more than a month to decipher Dr. Ferenc Miskolczi’s paper, on line at a time, taking a copy of his simple budget chart at the top of his paper and using a paint program drawing arrows on it, all of the ties that the equations were saying. Finally I understood. It might take longer to absorb your paper. Tracing some of the equations with the help of the AMS dictionary this is much deeper than even his, but, this is what I like to do so I’ll give it a try.
I won’t comment back unless I have something to add so you might check back here from time to time, OR, post another article here at WUWT, clarifying some of the complex relationships. As myself, many here are not atmospheric scientists per se but can understand it all given the time to absorb the concepts and relationships. If you post an article on another site, drop a note here.
One more thing, it is the wind’s velocity, in general, that greatly dictates the evaporation rates removing the saturated air from the surface, temperature has it’s place but to a lesser degree. I don’t remember reading that recursive, self seeding, aspect coming into play in the equations but maybe I just skimmed over it. If you know the equation numbers where that interplay resides, just comment back the equation numbers. Later.
Kevan
For me it is a pleasure to discuss all this, so thank you for your further comments. We are looking for interest, that is why this unusual appeal.
In both cases in the box example we originally nail the partition such that it is, in your words, impermeable for pressure.
The important distinction is that if the partition is permeable for heat, in the first box the equilibrium sets in without generating any motion. In the second case, the disequilibrium will infinitely persists until the partition is allowed to move. If we remove the partition, as you point out, we will also see air motion in the second box.
The use of throttle will equally affect the motion in both boxes. This is just equivalent to introducing resistance for the flow.
This box example illustrates the well-known point that if you want to obtain work from heat, you must be concerned about the efficiency. That is, about the share of extra pressure that will leak as heat without performing any work.
You say
Several dozen comments ago I wrote:
In essence, we do not seem to be in disagreement. However, your general conclusion, about moist air floating above any surrounding dry air, neglects all those ifs and is equivalent to neglecting the efficiency of transformation of heat to work.
Let me also say that while discussing buoyancy is undoubtedly interesting per se, our paper is not about buoyancy and not about the impact of condensation on buoyancy. The condensational pressure gradient force can make the air rise even if it is negatively buoyant in comparison to the surrounding air. By the way, atmospheric updrafts characterised by negative buoyancy are pretty common (see, e.g., Folkins 2006).
wayne
Thank you for your comments. Surface evaporation is implicitly taken into account in the condition dNv/dx = 0. This means that despite air pressure falls along the x axis, the saturated concentration of water vapor is maintained constant over the considered isothermal surface by local evaporation. This is discussed in Section 4.5 “Evaporation and condensation”. We have neglected evaporation from droplets.
Regarding forests and their role, you might be interested in reading this paper. In particular, pay attention to Fig. 2c, which compares precipitation patterns in North America (forested) versus China (deforested) at one and the same latitude.
I am signed to receive comments from this thread, so please, if further questions arise, I will be delighted to discuss them as time permits. When we have new work published, I will certainly put a note here.
There are several well-described and empirically observable physical phenomena that can be called “cousins” of the condensational atmospheric dynamics. Contemplating these phenomena may help the reader appreciate what we are talking about. I talked above about one such phenomenon — the heat pipes. Let me here to mention another one — the osmosis. Indeed, condensation-induced atmospheric dynamics can be considered as a very peculiar case of osmosis.
In agreement with Dalton’s law, partial pressures of particular components of gas mixtures or liquid solutions tend to spatial homogeneity independently of each other. Consider two mixtures with different concentrations of various components that are separated by a semipermeable membrane, which impedes spatial propagation of one of the components and prevents it from reaching the equilibrium distribution. The resulting equilibrium distribution of partial pressures of all other components will be associated with a pressure gradient across the membrane. The trans-membrane pressure difference will be equal to the magnitude of deviation of the partial pressure of the considered non-equilibrium component from equilibrum. If now the membrane is removed, the dynamic fluxes of liquid or gas will follow governed by this pressure gradient until the mixture pressures and concentrations of all constituents in the two areas equate.
In the atmosphere, the role of semipermeable membrane of a unique nature is played by the vertical temperature gradient – it selectively removes, via condensation, one of the gases from the mixture (water vapor) and does not allow it to propagate to the upper colder atmosphere in quantities sufficient for the restoration of component equilibrium of water vapor in the gravitational field. At the same time, lacking material essence, this unusual “membrane”, unlike the conventional osmotic membrane, is penetrable to the dynamic flow of mixture as a whole, sustaining continuous air circulation. In the ordinary osmosis, the dynamic flow (that destroys the pressure difference) should be intermitted by periods of molecular diffusion via the semipermeable membrane, when the osmoitc pressure difference is regained. In the case of the condensational pressure gradient force, the dynamic flow itself sustains the “osmotic” pressure difference by bringing water vapor to the area of condensation.
It’s amazing Anastassia how different branches of science always tend to cross at some point. You bring in osmosis and my major was physiology and my final paper before graduation long ago was the osmotic transport of c14 tagged glucose across the semi permeable membrane in the intestines of a species of worms (pig gut worms actually, can’t recall the species). So, that last explanation is plain ‘English’ to me!
You must be speaking of what would visually show itself at the rolling tops of clouds, not the bottoms where condensation first appears, in the cumulus case the undefined silky gray bottoms. Of course I had never considered osmosis would have a hand in the replenishment of the water vapor. That is interesting.
Or, I guess some of that effect would occur at any stark edge of a cloud where clear air meets the saturated vapor, that in cumulus case, is the constant rolling at the distinct edges and tops and stratus type at mainly the tops. Is that close or way off?
You could be speaking more at the smaller molecular level and this occurring anytime condensation occurs which leaves that depleted parcel exposed with a osmotic gradient but there I don’t visualize an edge.
wayne
I am happy to have found a reader interested in this aspect. Personally I find it fascinating.
I presume that by ‘edge’ you mean the region occupied by the membrane, which separates solutions with different concentrations. In the conventional osmosis the concentrations change stepwise across the edge (membrane). If we take the thickness of the edge equal to zero, the non-equilibrium concentration gradient at this point is infinite.
In the atmosphere there is no edge. It would exist if the air temperature dropped stepwise at some height. It does not. It drops smoothly as the air ascends. The non-equilibrium gradient of vapor pressure is finite. In this sense all the atmospheric column where the moist saturated air ascends acts as a ‘membrane’. This ‘membrane’ removes a certain share of water vapor from each saturated air volume as the latter ascends by a certain height.
Here’s a complexification (note to Anna — that’s a made-up word! Don’t borrow it. 😉 ):
My understanding is that the bulk of precipitation begins its condensation/joining cycle not as “droplets” but as ice crystals (since the air temperature high in rain clouds tends to be well below 0°C), and then melt into raindrops on the way down. Hail, of course, goes through numerous fall-rise cycles into the cold zone to build up.
All of which introduces another layer or two of latent heat exchanges to be factored in.
Oops. I just checked, and it does indeed exist! I guess I should have used “complification”! 😉
Anastassia, I didn’t understand at first what you were trying to portray but it’s becoming clear. I had a problem grasping what exactly “atmospheric column where the moist saturated air ascends” statement meant in relation to the osmosis. Outside of that column you must mean air that has less humidity, as in a downdraft around that column. In that context I clearly see the virtual ‘membrane’ and the water vapor in the column would always be moving outward to mix with the less humid surroundings therefore losing a bit of humidity as it rose.
Re-reading your first comment over again, twice, I see you are trying to tell me the same thing I was trying to visualize. You were also describing the ‘rolling’ or ‘sustaining continuous air circulation’ as you put it and I went right by it. I’m not quite used to some of the proper atmospheric physics nomenclature, but I’m starting to get it down. I read the whole section four with no problem but section one, well, in general I understand but I would like to grasp each equation and that’s a bit slower. Thanks so much for opening my eyes to some new aspects. Spent a whole summer last year watching clouds float by but now I’ll never look at them quite the same. I need to read section 2 & 3.
“Kevan Hashemi says:
January 28, 2011 at 5:43 am
“Steve:
Yes, the reason liquid water molecules are hard to separate is because of hydrogen bonds. But you end with, “At condensation there will an immediate radiation of heat commensurate to the energy of the hydrogen bonds formed.” Absolutely not. Who said anything about radiation? You made that up yourself. Nothing is radiated. The reason the gas gets hot is because of the statistical reason I gave you: only when a water molecule is . You can figure all all this out in terms of molecules bouncing around. It’s as simple as that. That’s Statistical Mechanics, and it works fine. There is no radiation involved. Why do you want to make it more complicated?”
How does stating that the energy loss must be in the form of radiation make it more complicated?
By definition, during a phase transition there is no increase/decrease in average kinetic energy (temperature) of the molecules. Going in the heating direction, that means that at vaporization energy is transferred into a mass of liquid water with none of that energy showing up as an increase in kinetic energy. Water molecules are not gaining linear momentum for their collisions. For energy to go into this mass without a temperature change, SOMETHING must be gaining momentum. That ends up being the momentum of the electrons (angular momentum). Molecular bonds are broken, and the electrons end up in a higher (but less stable) energy state. Do you at least agree with this concept for the heating direction?
An equivalent process must occur in the cooling direction. At condensation, energy is transferred out of the mass of water vapor with no reduction in kinetic energy of water molecules. That energy had to come from somewhere! If not at the gross molecular level (temperature) it’s going to be lost on the atomic level. Somewhere an electron had to weasel it’s way back down into a lower energy state and radiate a photon.
Contrary to making the concept more complicated, I think it makes it easier to envision. If latent heat were a basic heat transfer through molecular collisions then rising water vapor would heat all adjacent gases equally. But as a radiant heat transfer the latent heat is statistically more likely to be absorbed by molecules with the same absorption/emission spectra – other water molecules. Latent heat released upon condensation in the upper atmosphere is more likely to be directly radiated to space. Latent heat released when water vapor condenses at the center of a thundercloud is more likely to heat water molecules (which can then transfer heat to the surrounding air mass by collision). Knowing that the energy transfer is expressed physically as radiation tells me a lot! Of course, someone needs to determine the emission frequencies of energy released by hydrogen bonds at condensation and check for overlaps with the absorption spectra of other atmospheric gases.
David
January 26, 2011 at 11:15 am
I contacted Professor Pielke Sr. several times starting from October 2008 asking for comments/criticisms regarding our work, both on the role of vegetation in driving the hydrological cycle, on the physical bases of condensation induced dynamics and on social issues associated with difficulties of challenging a consensus in climate science and the associated caveats of the peer-review process.
Regarding science, I received two comments from Prof. Pielke, which are as follows. First, Prof. Pielke does not “see how the effect of the changes partial pressure of water vapor can have a major effect relative to the much larger effect of the release of latent heat and other diabatic effects as they alter the vertical and horizontal pressure gradient”. Second, “if the atmosphere is in (or close to) hydrostatic balance, the mass remains unchanged overhead regardless of the phase the water is in”, such that condensation within the atmospheric column cannot instantaneously reduce local air pressure at the surface.
Prof. Pielke advised that we should submit our considerations to an AGU or AMS journal where they will be peer-reviewed. He also advised that we should consider submitting to physical journals as “the advantage of having physicists review your work is that they are not as likely to have some of the biases you report.” In response to our query whether he would find it possible to make a public comment on our work, Prof. Pielke clarified that he “receive(s) quite a few communications to comment on research, and prefer(s) not to generally do that”.
Posted with permission of Prof. Pielke Sr.
Prof. Pielke…“if the atmosphere is in (or close to) hydrostatic balance, the mass remains unchanged overhead regardless of the phase the water is in”, such that condensation within the atmospheric column cannot instantaneously reduce local air pressure at the surface.
Is he saying that the mass must remain unchanged because that is part of the definition of hydrostatic equilibrium? Or does it “just not happen that way” (which may be true)?
Looking at the force balance diagram for hydrostatic equilibrium(http://en.wikipedia.org/wiki/File:Hydrostatic_equilibrium.svg), I don’t see “mass remains constant” as a requirement. If the mass of the volume increases in that diagram then I can see two ways to maintain hydrostatic equilibrium. Either increase the upward force of gas from below, or reduce the downward force of gas from above.
Water condensation will lead to a loss of vapor pressure coupled with a large radiation of heat. That heat can accomplish two things – increase the vapor pressure of gases pushing up from below, and decrease the vapor pressure of gases pushing down from above.
Anastassia, does the system I described above elucidate a mechanism for the forces involved in tornadoes/hurricanes that you state are not accounted for in the current meteorological equations? When I asked “does it just not happen that way” I was not being facetious. I don’t know how the atmospheric dynamics play out. For mass to increase in the volume while maintaining hydrostatic equilibrium, I can imagine the dynamics but they are very tricky.
At condensation the water vapor itself loses vapor pressure, so there is a negative pressure coupled with latent heat. Is that negative pressure likely to pull in air from the same horizontal level? Is the air at that horizontal level likely to be at or near the same dew point at which condensation just occurred? In that case, the negative pressure of condensation can indeed pull in more air that condenses in the same volume, increasing the mass of that atmospheric column.
Coupled with the release of latent heat, you can get a growing mass of water condensate that remains in hydrostatic equilibrium if the energy of it’s own latent heat is increasing the vapor pressure from below and decreasing the vapor pressure from above. There is a large build up of pressure potential with the mass of water acting as a barrier in between. Take the water out of the way and you will experience a sudden large negative pressure at the surface. The atmosphere below is REALLY going to want to push through that cloud. It will eventually either: punch a hole through, spread out horizontally and seep up around the edges, or transfer its heat to the cloud by contact.
If a hole is punched, condensate will fall adjacent to warm, rising air. The condensate can then pick up the energy needed to vaporize again, allowing it to join the rising warm air. Depending on the scale of your suspended cloud layer and the size of the whole punched through it, I can imagine how this dynamic could create either a hurricane or a tornado.
Anastassia, just a thought, was reading your and Steve’s comments. I see a little of Dr. Pielke’s point of equal pressure viewpoint but pressure has only to do with the force/area and, with a static gravitational field at various altitudes, a constant weight/area whether a volume of water vapor is in a gas phase or a liquid phase. That view point does have to do with a static horizontal distribution.
However, the *density* (mass/volume) can change due to volume changes even though the pressure stays constant, vertically. That depends a lot on what scale you are speaking of. Air mass can only change over large areas so fast depending on the velocity of the winds. You might give a little deep thought on the density side. That is along the same lines of some prior investigations I was personally performing (just being curious) having to do with Venus’s atmosphere and lapse rate. Don’t leave density out of your considerations. Pressure is only on side of it. Both play into the dance in the sky so to speak. Mass on one side and volume on the other.
This is probably already well thought out but, just in case it wasn’t, I just had to raise that aspect.
Steve
February 2, 2011 at 5:09 pm
Thank you for your further comments.
You ask my view. Statements (1), (4) and (5) are correct. Statements (2) and (3) are not.
 (1)
(1)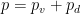 is total air pressure (vapor plus dry air),
is total air pressure (vapor plus dry air), 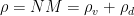 is total air density.
is total air density.
 , (2)
, (2)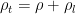 is the total amount of matter, including liquid, per unit volume. From this equation one concludes that surface pressure
is the total amount of matter, including liquid, per unit volume. From this equation one concludes that surface pressure 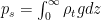 remains unchanged until the total mass of the column changes.
remains unchanged until the total mass of the column changes.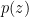 at an arbitrary height we have from (2)
at an arbitrary height we have from (2)
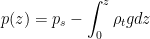 (3)
(3) . It satisfies both (1) and (2). Then we took an air volume at some height
. It satisfies both (1) and (2). Then we took an air volume at some height  and replaced it with an air volume with the same total density
and replaced it with an air volume with the same total density 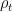 but containing liquid instead of vapor. That is to say, we first had
but containing liquid instead of vapor. That is to say, we first had  and then
and then  , with
, with  and
and  kept constant.
kept constant.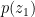 has not changed either. Dr. Gavin Schmidt referred to this as “condensation *per se* has no effect on air pressure”.
has not changed either. Dr. Gavin Schmidt referred to this as “condensation *per se* has no effect on air pressure”.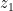 has dropped by the amount of partial pressure
has dropped by the amount of partial pressure  of vapor:
of vapor:  .
. . ) But it is not the other way round! The amount of liquid that suddenly appears in the atmospheric column has nothing to say about whether the hydrostatic equilibrium is maintained or not.
. ) But it is not the other way round! The amount of liquid that suddenly appears in the atmospheric column has nothing to say about whether the hydrostatic equilibrium is maintained or not.
The idea that nothing happens until the mass of condensate is removed represents a fundamental physical misunderstanding of the nature of hydrostatic equilibrium. This misunderstanding is spread widely, as illustrated, for example, by the quoted comment of Prof. Pielke above and earlier of Dr. Gavin Schmidt. The error is visualized with utmost transparency in the work of Bryan and Fritsch (2002) (see our critique here, p. C12011), where a static (motionless) atmosphere in equilibrium accomodates liquid droplets that neglect gravity by hanging around everywhere throughout the atmospheric column, while the air pressure is strongly non-hydrostatic (due to the rapid decline of vapor pressure with height).
The correct equation for hydrostatic equilibrium is
where
This fundamental equation is replaced in meteorology by the following one:
where
For pressure
Now let us see how (2) violates the ideal gas law. Suppose initially we had a static equilibrium atmosphere with no liquid,
Since neither the total amount of matter in the column nor total density distribution has been changed by such a procedure, from the modified “hydrostatic equilibrium” (2) and Eq. (3) we would have to conclude that pressure
But the ideal gas law teaches us that pressure (which is, by the way, a scalar) is proportional to the total number of particles per unit volume. By replacing vapor by liquid we have reduced the amount of particles by the number equal to the number of vapor molecules. In the result, pressure at
To summarize: liquid does not make a contribution to the ideal gas pressure, in contrast to what Eq. (2) supposes.
Immediately upon replacement of vapor by liquid, pressure has dropped and a non-equilibrium pressure gradient force appeared. By formulation, this force will be (and have been) ignored in all models where hydrostatic equilibrium is incorrectly represented by Eq. (2).
The deviation of the observed pressure distribution from (1) can tell us how much liquid falling at terminal velocity the upwelling flow could possibly sustain in the atmosphere. (Direct observations show, btw, that this amount is very minor,
Thanks Anastassia, you’ve given me a lot to chew on.
The meteorological terminology has been particularly confusing to me in respect to “water vapor” and “dry air”. Water condensate and water vapor are used interchangeably – but suspended water droplets, such as a mist, are not water vapor! (a mist is a colloid) Air is considered “dry air” until the dewpoint is reached, despite the fact that it could be nearly saturated with water molecules (a.k.a. water vapor). In meteorology, water vapor laden air is “dry air” and a mist of water is “water vapor”.
wayne
February 2, 2011 at 8:02 pm
Thank you for your further comments. One of our points is that the Archimedes force associated with buoyancy is minor in comparison with the condensational pressure gradient force associated with vapor removal from the gas phase.
Kevan provided several useful calculations which can be viewed as epitomising the concerns of Prof. Pielke and some other scientists about the “much larger effects of latent heat.” Let me overview them here once again.
The release of latent heat diminishes the rate at which temperature drops with height when the air ascends adiabatically. The moist adiabatic lapse rate depends on the amount of vapor and grows as the vapor is depleted: from about 4 K/km at the surface at T = 20 C. The dry adiabatic lapse rate is 9.8 K/km (you can see these lapse rate profiles in Fig. 2e here). At 10 km the cumulative temperature difference between the moist adiabatic and dry adiabatic columns amounts to about 30 K, or 10% of absolute temperature T ~ 300 K.
At one and the same pressure the air that is warmer by 10% will have approx. 10% lower density rho’ = pM/R(1.1T) than air at temperature T with rho = pM/RT . This estimates the net effect of buoyancy as (rho’ – rho)g as 0.1 rho g. This gives us 1 N/m^3 if we take rho = 1 kg/m^3 (in reality rho is lower at 10 km, but we neglect this). Kevan correctly estimated (see also my reply) that even complete removal of vapor by 10 km will make a minor correction to this estimate. (To which direction — depends on how you define the atmospheric columns that you are comparing. One can argue that removal of vapor with Mv = 18 g/mol makes the air heavier (reduces buoyancy) by increasing its molar mass M ~ 29 g/mol. Alternatively, one could conclude (as Kevan originally did, if I got him right) that vapor removal increases buoyancy by decreasing pressure p. But in either case the effect is minor due to the small ratio pv/p ~ 0.01.)
It is at this point where some people would stop having concluded that the condensational vapor sink is unimportant for atmospheric dynamics.
In our paper we are presenting a different force, not calculating a contribution of the vapor sink to buoyancy. This condensational pressure gradient force is estimated as dpv/dz = pv/hv ~ 1 N/m^3 in the lower atmosphere (pv ~ 2×10^3 Pa, hv = 2 km). As one can see, thus estimated, the two forces — buoyancy and condensational — are of comparable magnitude, 1 N/m^3. (This is another interpretation of the result shown in Fig. 1c of our paper, where the maximum pressure differences above and below zc are approximately the same).
But the buoyancy effect estimate is a gross overestimate. In reality 30 K temperature differences are practically never observed between the areas of ascent and descent. Horizontal mixing destroys this theoretical temperature difference, making the lapse rate close to the average value of 6.5 K/km everywhere. Moreover, the dry adiabatic descent increases surface temperature in the region of descent, further diminishing the theoretically possible temperature and density difference. There is also the problem of surface to volume ratio (a small warm balloon and a several hundred or thousand kilometers’ wide warm atmospheric volume will differently react to buoyancy differences).
In our paper we do not estimate the buoyancy effects (enormous work has been done on that). We show that the condensational pressure gradient force alone is sufficient to explain the observed circulation intensity on a variety of spatial scales. This is an independent argument that, in the same context, the buoyancy effects (whether due to latent heat release or differential heating) are minor in comparison. The continued neglect of the condensational pressure gradient force is scientifically unjustified.
Note that both the buoyancy-related (rho’-rho)g and the condensational pressure gradient pv/hv forces are vertical forces. Under the independent condition that the atmosphere is close to hydrostatic equilibrium, the action of both forces is manifested via formation of horizontal pressure gradients, like the one we estimate in Eq. 37 of our paper.
Note also the different spatial localization of the forces: as calculated above, the buoyancy related force depends on height and grows with increasing temperature difference caused by latent heat release. The condensational pressure gradient force, in contrast, dominates in the lower several kilometers of the atmosphere, i.e. where most weather phenomena that affect our life occur. The condensational pressure gradient force tends to zero in the upper drier atmosphere due to the decrease of pv.
Steve
February 3, 2011 at 4:51 pm
There is some confusion between daily life and meteorological terminology. By “dry air” in meteorology it is common to understand everything except H2O. That is, if you have moist air with pressure p, its pressure is a sum of partial pressures of “dry air” pd and vapor pv, p = pd + pv.
It is true, however, that sometimes in meteorology one speaks of “airborne moisture” thus treating both small droplets and vapor as a single constituent. In daily life, at least in Russia, by “vapor” people understand the small clouds that apppear, for example, when you breathe outdoors on a frosty day, or above a boiling kettle. This is, of course, incorrect.
Anastassia,
I can’t tell you how much I have enjoyed this conversation. You have really open my eyes to some very important aspects of water vapor. So I don’t overstay the welcome I’ll just add a few things that might let you know where I was coming from and a pointer that might make it easier for you to press your point while trying to get others to see deeply into your paper, but quickly.
I almost feel a bit of boyish guilty of hijacking this topic and dragging it into talking of single clouds while all of the time I did realize that your paper is speaking on a much, much larger scale, cloud/vapor systems at the global scale. Here’s why: I spent so many hours years ago in a sailplane trying to understand clouds. I was failing miserably. The manuals told of heat, thermals, condensation and what on the ground you were to look for to lead you to these invisible thermals. Of course it is heat, look for dark plowed fields below, large black parking lots, etc that is where you will find lift. It also pointed out to stay away from water, forests, and most anything green for that was always where the sink would be for these were always cooler.
Well, as it turned out that simple explanation in the manual was wrong about half of the time. A large lake with green around the edge usually, if there was a slight breeze, would have great thermals with clouds always on the leeward side and that made absolutely no since at the time. Here was two cool surfaces with great lift and the dry, dark plowed fields closer to the airport had terrible sink, we were then in a drought and it was bone dry. I was lost, moisture never crossed my mind. To the library for meteorology books. They said basically the same, it’s all in the heat.
I guess you see my absolute glee in your paper. You have answered a question I have pondered on for some twenty five years. I kick myself now, of course, it’s the condensation, dummy! See, I was trusting the authorities, the manual and meteorological books as the information source without ever questioning it. What enlightenment. Even though that sailplane experience only lasted for a few years and that was long ago I think I’ll pass this information on to some soaring clubs and maybe Schweizer sailplane manufacture to have them pass it along to any newbies. Every pilot when soaring should know this effect.
So much for that.
Now, on your paper, it was a tiny mention of someone above that mentioned that water vapor takes up 1000 times more volume that water. That is all it took to get my attention !! Otherwise, I might have skipped right by this whole post. Meteorology always interests me but seeing deep math sometimes I think, don’t have time right now and go on, super glad I didn’t.
I calculated that 1000x volume and came up with a much larger value, I hope I did it correct, some 23500 times the volume, water to water vapor. Really either if these values are big enough to work in letting anyone visualize immediately this huge differences involved, for without that mental view, it seems many just pass it something like this: it’s a big atmosphere so surely some water vapor is not going to make that much difference. Next time it rains 4 inches over this state I will know it is equal to some one and a half vertical miles of the atmosphere that just fell. Anyone with the least bit of imagination knows immediately that this results in huge horizontal pressure differences.
I wish all science papers were required to have a “plain English” lead in so a mental view could be formed before the heavy math came into play, but that’s just the way my mind works. Guess I’m suggesting that you might consider getting it visual up front. Sometimes pure equations can hide the huge scale until you get your mind deep into them and start applying some actual numbers. Just a thought.
Wish I could be more help but my sidetrack into planetary atmosphere physics (climate science ☺) started but a little over a year ago. My last thirty years of interest in physics has always been more in other areas, particle physics, astrophysics, gravity, etc.
wayne;
fascinating commentary. Thank you. But I don’t know where either of the figures come from! (1000, 23,500). What pressure, what temperature, what atmosphere?
Starting with a vacuum, one could “fill” the space with any amount of vapor desired. The conditions on Mars, e.g., are right at a “triple point” combination, where H2O can switch between vapor, liquid, solid with minute changes of conditions. In Earth’s air, there are various saturation points you could pick.
Having been ‘out of circulation’ in January I have only just found this. I am amazed how much I can understand of the discussion paper at first read and I fully intend to work my way though it and read all the comments again, in more detail. I had been aware of previous material at the Air Vent but not really engaged with it.
As wayne says 2 comments above:
“Meteorology always interests me but seeing deep math sometimes I think, don’t have time right now and go on,..”
However, I find the discussion paper quite accessable, so I just wanted to say thank you to Dr Makarieva, for such a clear exposition.
Also, Chris Reeve‘s comment above:
“…. the van der waals force — works on the microscopic scale. […] ” “This is also the principle force which creates the sometimes-elaborate structures we see solids form into — like crystals and snowflakes.”
Biology knows this well and exploits proteins to enable condensation / crystallisation reactions that chemistry tells us should not otherwise happen (under the gross conditions) – for example ice nucleating bacteria and carbonate shell formation.
Brian H says:
February 11, 2011 at 7:48 am
wayne;
fascinating commentary. Thank you. But I don’t know where either of the figures come from! (1000, 23,500). What pressure, what temperature, what atmosphere? ….
===============
Hi Bryan.
Thanks, but I do see a mistake in my thought.
Initially taken from an example from engineeringtoolbox site:
The specific humidity for saturated humid air at 20 ºC with water vapor partial pressure 2333 Pa at atmospheric pressure of 101325 Pa (1013 mbar, 760 mmHg) can be calculated as:
x = 0.62198 (2333 Pa) / ((101325 Pa) – (2333 Pa)) = 14.7 g water/kg air.
So how many times does that 14.7 g of water fit into one cubic meter of water, 55396 times. But one kg of air is not exactly one cubic meter so corrected for 1.23 kg/m3 of air = ~45000. Can’t find my original calculations but it was very similar to that. Can’t seem to reproduce the 23,500 figure.
But that is getting an answer to a wrong question, that is, how many cubic meters of air does it take to hold one cubic meter of water at that partial pressure when in vapor form, so step back to the 1000 figure which dscott used above because that is roughly the ratio of the densities. Whoops. But still even ~1000 times the volume leaves a certain visual impression that conflicts the thought that condensation effects are so small they can be totally ignored. That was my main point.