
Science and media outlets claim ocean acidification is happening due to increased carbon dioxide in the atmosphere. But objective data show the ocean is far from acidic according to Dr. Caleb Rossiter, executive director of the CO2 Coalition and a statistician who has studied climate change closely.
Host Anthony Watts and Rossiter talk about how a pH of 7 is considered neutral, with anything below 7 considered acidic. Ocean pH averages 8.1, which is alkaline rather than acidic. Although climate models suggest the ocean’s surface pH may have dropped from pH 8.2 to 8.1 since 1750, that change was never actually measured.
The pH drop from 1850 is merely a modeled conjecture. The concept of pH was first introduced by in 1909, and agriculturalists first developed field instruments to measure pH in the 1930s.
Discover more from Watts Up With That?
Subscribe to get the latest posts sent to your email.
The ‘Elite’ who control the world have a Plan and junk science is an integral part. And they’re not about to let The Interloper derail them. How easy it was for The Virus to derail our economy and the murder of a petty thief to trigger violence and vandalism in his name to further derail and destabilize. According to Robert Welch it’s the playbook of the Illuminati, formed on May 1, 1776; the French Revolution kicked off their agenda to create a new world odor in their image and the demonization of carbon dioxide is the perfect vehicle to its completion.
So it’s curious to say the least that carbon is 6 protons, 6 neutrons & 6 electrons, because the human body is carbon-based and their admitted intention is to replace cash with a carbon chip, presumably in the hand. I know of nothing else that fits the dual meaning of 666 so perfectly and whether prophecy, playbook or both the threat is the same, and only ‘truth shall make us free’. How Perfect that real science exposes the monstrous fake science, but the media is apparently in their malignant control so the truth is not getting out; even James Delingpole won’t reveal the true motive, only that it’s about money from thin air. We need a new Pamphlet War !
Our hidden history sheds light on present events:
Listen: https://www.youtube.com/watch?v=e-TAegLQnO0&t=190s
The Truth in Time
Read: https://www.ourrepubliconline.com/Article/21
The Truth in Time
https://www.thenewamerican.com/world-news/asia/item/25404-at-world-government-summit-top-globalists-drop-the-mask
At “World Government Summit,” Top Globalists Drop The Mask
https://www.thenewamerican.com/world-news/asia/item/28339-creepy-world-government-summit-targets-america-freedom
Creepy “World Government Summit” Targets America, Freedom
The Plan: while the ‘elite’ feast on steak & lobster…
https://www.thenewamerican.com/tech/environment/item/15420-un-let-them-eat-bugs
UN: Let Them Eat Bugs!
https://www.pajiba.com/miscellaneous/psychologists-say-humans-could-adapt-to-cannibalism-which-remains-our-ultimate-taboo.php
Psychologists Say Humans ‘Could Adapt’ To Cannibalism. Wait, What?
The opening ceremony of the Gotthard Tunnel, the largest in the world, satanism on parade, in apparent anticipation of a third Clinton coronation, reportedly a black witch from way back. The goat head symbolizes Baphomet:
https://www.youtube.com/watch?v=ikDpJZRSqz0
Gotthard Tunnel Opening Ritual (Shocking)
Nothing to see here, folks:
https://ww w.bbc.com/news/world-europe-36423250
Gotthard tunnel: World’s longest and deepest rail tunnel opens in Switzerland
Even Avon is out of the closet; ding-dong Avon calling…
https://fellowshipoftheminds.com/avon-goes-to-the-devil-with-baphomet-catalog-cover
Avon goes to the Devil with Baphomet catalog cover
Don’t worry, it’s a good thing, a lesson in the First Amendment:
https://www.washingtonpost.com/local/education/an-after-school-satan-club-could-be-coming-to-your-kids-elementary-school/2016/07/30/63f485e6-5427-11e6-88eb-7dda4e2f2aec_story.html
An After School Satan Club could be coming to your kid’s elementary school
July 30, 2016
Microsoft chose satanist-to-the-stars Marina Abramovic to star in their new ad, then made it private when millions complained:
https://www.bitchute.com/video/qd2tQ463O6ko/
MARINA ABRAMOVIĆ PRESENTS “THE LIFE” IN MIXED REALITY
https://www.youtube.com/watch?v=g9ys-Lfu4Sc&feature=youtu.be
MARINA ABRAMOVIC SPIRIT COOKING
involving blood, urine, semen & breast milk
Oh my. I could say a lot, but my Mama tried to teach me, “if you don’t have anything nice to say, don’t say anything at all.”
So I’ll just offer this: Linda, I think you have confused “carbon chip” with “silicon chip.” The most common isotope of silicon has 14 protons, 14 neutrons, and 14 electrons per atom.
Linda,
Carbon is any atom with 6 protons.
The number of electrons and neutrons can and does vary, but if an atom has 6 protons, it is nonetheless carbon.
On the Earth, there are three common isotopes of carbon that can be found.
Besides for all of that…are you “pulling our leg” here?
Carbon fits the definition of 666?
What does that even mean?
The death molecule of alarmist fiction is carbon dioxide, CO2. References to it as simply “carbon” is just more jackass shorthand from that crowd.
And here I thought it was just a reference to that odd little birthmark on Damian’s scalp.
Adding CO2 to the oceans’ H2O leads to the creation of H2CO3. Correct?
H2CO3 is a weak acid. Correct?
Then it would follow that adding acid to an ocean is a process which adds acid to water (acidification) no matter what the pH levels are or were. This descent into language pedantry does not further the scientific education of the masses of illiterate.
The simple process of the sun rising, immediately takes the sting out of any added acid.
Here locally, ocean water pH was too low to allow hatching shellfish to form shells, so they added ash. It was quick and easy. They didn’t want to wait the THREE HOURS for the sunlight to help the algae convert enough CO2 to raise the pH to optimum levels.
Bullcrap.
Which part?
Okay.
So everything I stated above is factual, and observed information from our local shellfish hatchery, with exception of the ‘definition of terms’ having to do with ‘acidification.’
I realize there is a vested interest in demonizing the word ‘acidification’ and that is fine. The Left does it all the time demonizing all kinds of words and then promoting all kinds of redefined words to mean the opposite of the original meaning. (Case in point ‘Liberal.’ The Left calls themselves Liberal, but the stand against everything that Liberalism once stood for.)
I realize that our inshore ocean waters are not the norm. With 10 to 15 feet of rainfall annually our cold waters tend to have large amounts of dissolved CO2. The rain is such that I have to add baking soda to my cistern to keep the pH levels higher. With out it the rainwater acts on my copper pipes and turns the bathtubs green.
The discussion is regarding the deliberate corruption of language, something the left is famous for.
As such it is a sematic argument at one level, but that is not the same as it being pedantic.
Pedantry and semantics are two different things.
At the root of the matter is climate alarmism, and that is the only reason anyone here is bothering to take the time to comment on the subject.
As for the rudimentary explanations of the relevant chemistry you describe, that would be true if one was starting out with pure water to begin with. But this is far from the actual case.
No one is saying that CO2 plus water does not form a weak solution of the weak acid called carbonic acid.
The discussion is very far from what you are saying here.
Hence…bullcrap.
The oceans are not and never will be acidic, and therefore describing a somewhat higher concentration of the trace gas CO2 in the air as causing “ocean acidification” is wrong…and it is furthermore a deliberate obfuscation.
Not to mention that just like on land…in water, CO2 is what every living thing is made of, including the shells.
Haverwilde asked “Adding CO2 to the oceans’ H2O leads to the creation of H2CO3. Correct?”
Partly yes, and mostly no. Carbonic acid (H2CO3) is created as gaseous CO2 initially dissolves into seawater (or rainwater). However, H2CO3 is not stable and very quickly decays into bicarbonate and carbonate negative ions, with accompanying formation of hydronium positive ions. The hydronium ions very quickly react with OH- ions (abundant in water with a pH greater that 7.0) to produce neutral pH water, or they react with other oxidizing ions, and thus they are rapidly removed as potential reactants. It is the formation of bicarbonate and carbonate ions in seawater that causes the world’s oceans to be a highly stable, strongly buffered solution with an average pH in the range of 8.1-8.2.
So, adding gaseous CO2 to seawater is not, in fact comparable to adding a stable acid to an unbuffered solution in a manner that would otherwise cause a significant lowering of the solution’s pH.
Anyone asserting that adding gaseous CO2 to the world’s buffered oceans is equivalent to “ocean acidification” is quite simply incorrect on a fundamental scientific basis.
One might read your explanation and conclude that when CO2 reacts with H2O, the resultant carbonic acid quickly dissociates.

In fact, carbonic acid is by definition a weak acid, which means that only a part of it is dissociated at any given time. In an otherwise neutral solution of pure water, only a tiny amount of the carbonic acid will actually be in the form of bicarbonate and a far more miniscule amount of the bicarbonate will dissociate further into carbonate.
Anyone who understands what a buffer system is knows that the conjugate base of a weak acid is a strong base. Bicarbonate is the conjugate base of the weak acid called carbonic acid. As such it is a strong base, and will pull protons out of the solution very strongly. Hence bicarbonate is an even weaker base than carbonic acid, which is itself one of the weakest of acids.
And on top of that, hardly any of the CO2 in an aqueous solution is even combined with H2O at any given time. Less that 2 tenths of 1% of it, as previously noted.
The situation is of course far more complicated in seawater, regarding the relative amounts of carbonic acid, bicarbonate and carbonate in solution at any given time.
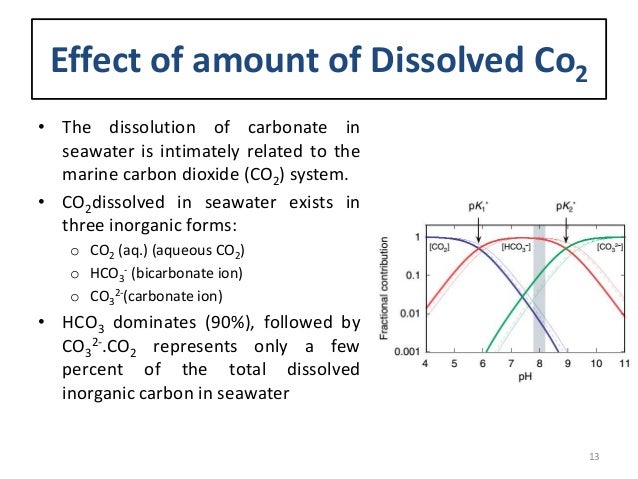
There is in fact a handy chart which shows the relative proportion of the various species present in water of various pH values.
Here it is:
Note that this is a logarithmic form of this graph.
The important takeaway is that the relative amounts of the various species present is highly dependent on the pH of the water.
Also important to note is that what is called CO2 here is actually CO2 in aqueous form, IOW that which has combined with water to form carbonic acid (theoretically). It does not include and should not be confused with the CO2 present as a dissolved gas…with is hundreds of times higher in concentration than any of these three species…because less than 1% of CO2 in water actually is combined with a water molecule at any given time.
Since the graph is logarithmic, it is not clear how strongly bicarbonate dominates at the pH of normal surface seawater…it is the vast majority, which is not what would be seen in a solution of plain water with some CO2 dissolved in it…about like in clean rainwater (although even rainwater is nothing like pure water with some CO2).
Given that, here is another version which is not logarithmic:
http://braukaiser.com/wiki/images/d/d1/Carbonic_acid_dissociation.gif
In this version, it can readily be seen that at pH just above 8 or so, bicarbonate is will over 90% of the aqueous CO2 in the water, with carbonate being the smallest fraction by far.
 ?cb=1415228041
?cb=1415228041
Here is yet another version, with some explanation inscribed:
And here is yet another form of the graph (called a Bjerrun plot BTW) showing the actual log concentrations of each species in seawater:
http://www.personal.psu.edu/u0y/nanoreef/examples/dissolved.inorganic.carbon.png
Correction: Instead of, “Hence bicarbonate is an even weaker base…”, I meant to say, “Hence bicarbonate is an even weaker acid…”
IOW…bicarbonate is a strong base, and carbonate is an extremely strong base. These two will thus grab up free hydrogen/hydronium ions.
Also important to realize all of these reactions are equilibrium reactions…they are constantly going in both directions, trying to reach a stable equilibrium. Adding more of any one thing will push the equilibrium in the opposite direction.
Nicholas, it is interesting that, after all of your arm waving, the most important fact is contained in the label of the Bjerrum plot for CO2 dissolution into seawater given in the last link that you provided. Quoting it verbatim:
“At the pH of most ocean waters, less than 1% exists as ([CO2(aq)] + [H2CO3]), and the H2CO3 concentration is only about 0.2% that of CO2(aq).”
I do believe this is at odds with your above statement “In fact, carbonic acid is by definition a weak acid, which means that only a part of it is dissociated at any given time.”
Have the prices of oysters, lobster, mussels, clams, and any other shellfish risen due to CO2? Why, NO! So, the shellfish are doing fine. All this talk of the pH of the oceans ignores the fact that records of the pH of the oceans are not comprehensive. “Acid” and “acidification” are scary words.
There really is no more to this story.
Oysters are a great source of zinc. There should be a run on them.
Zinc pills are an even better source. In fact there are lots of sources of dietary zinc, and many of them are not filter feeding shellfish which concentrate any toxins or bacterias or viruses which might be in the water.
I personally would not eat a filter feeder if you paid me.
Ever wonder how many people get hepatitis every year from eating things like oysters?
I just want to note that the audio podcast presented in the head posting *does* get really interesting as to the biology of ocean uptake of CO2 right around the halfway mark, 10 minutes ‘in’ or so.
So I’m obviously a ‘fan’ of *some* PhD’s, such as Dr. Rossiter, say.
Well, my beef with this whole topic is not about the definition of how to call a pH reduction which happens above a number of 7, apparently there are different definitions or different school of knowledge and people went in cycles for year now, completely missing the point IMHO:
!!! They all talk about a surface effect !!!
There is absolutely no direct knowledge how the pH of the oceans changed more then let´s say 50years ago, rather than saying with a Trumpian voice “but the number is above 7” any time someone talks about “ocean acidification” politely point out that this topic does not describe anything measured of the 90%+ volume this term seems to imply and politely insist, that the word “surface” is added to the discussion!
Sorry, but all poster in this thread seem to have found a tree, but miss the forest ..
Sorry I’m a bit late to the discussion, but I just read the quote on the NOAA website posted above, which is either ignorant or disingenuous, and am genuinely appalled by it. The analogy with temperature is completely spurious, as any half-competent scientist should know. Temperature has an absolute scale, absolute zero being the lower extreme below which it is impossible to go. Warm and cold are subjective terms in everyday use and relative terms scientifically. Zero Centigrade is a cold day around here but quite warm for the North Pole. The pH scale on the other hand has a real and physical mid-point, pH=7, where the concentration of H+ ions is equal to the concentration of OH- ions, 10exp-7 moles per litre. This is so because water naturally dissociates to a small extent and in the abcence of any other source generates one hydrogen ion for each hydroxide ion. Below pH=7 there is a net excess of H+ ions and above pH=7 a net excess of OH-. It is worth pointing out that pH is strictly only relevant to aqueous solutions.
“When CO2 is added to seawater, it reacts with water to form carbonic acid (H2CO3); hence acid is being added to seawater, thereby acidifying it…” This is a blatant sleight of hand – the first statement is true, CO2 does indeed acidify water, but then they change the subject to seawater which is not just water but a carbonate/bicorbonate buffered system, one is pH=7 and the other pH=8.1, one is being moved away from neutrality by CO2 and the other toward it. I really hope that this was written by an enthusiastic press officer and not a NOAA scientist, otherwise it can only be considered to be sophistry.
Also only a tiny fraction of CO2 in water actually reacts with water to form carbonic acid. The vast majority, over 99.8%, exists as dissolved CO2 gas.
And even that part that does react with water forms bicarbonate for over 95% of the amount. And bicarbonate is a strong base.
Hence the solution is buffered against changes in pH.
Adding CO2 means there is more carbonate, not less, because the pH hardly budges but the actual molar amount of all inorganic carbon species increases much faster.
You say toe-may-toe, I say toe-mah-toe. Let’s call the whole thing off.