Data suggests it is a non-problem
By Steve Goreham
For almost a month, the ongoing saga of the eruption of Hawaii’s Kilauea volcano has captured the attention of world media. Fountains of red-orange fire, lava flows, and ash-plume explosions destroyed dozens of homes and forced thousands of residents to flee the area. But media warnings about dangerous acid rain resurrected a long-believed myth of the environmental movement.
CBS and CNN ran with headlines listing acid rain as a danger from the Kilauea eruption. US News said, “Acid rain could be the next threat Hawaii residents face in the coming days…” CNN stated “if you do come in contact with acid rain, wash it off as soon as possible.”
The sulfur dioxide gas (SO2) emitted by Kilauea is a pollutant that can be harmful if inhaled. Inhalation of SO2 causes irritation of the nose and throat and can cause life-threatening accumulation of fluid in the lungs at high concentrations. In contrast, there is no evidence that anyone has ever been directly harmed by acid rain.
Scientists measure the acidity or alkalinity of solutions using a 14-point logarithmic scale, called the pH scale. Water is neutral with a pH of 7. Battery acid has a pH of about one. In contrast, lye, which is alkaline or basic, has a pH as high as 13. Rainwater is naturally acidic, along with milk and most of our foods.
Both nitrogen dioxide (NO2) and sulfur dioxide gases react in the atmosphere to form nitric and sulfuric acid. These gases dissolve in water droplets and can increase the acidity of rainfall. But acid rain is only mildly acidic with a pH of about 4, about 100 times less acidic than lemon juice.
In the early 1980s, acid rain caused by NO2 and SO2 emissions from industry became a major environmental concern. Acid from rain was blamed for acidifying lakes and damaging forests in Eastern Canada, the Northeastern United States, and Northern Europe. Magazines and newspapers showed images of dying trees, blaming industrial air pollution.
In Germany, the word “Waldsterben” (forest dieback) was coined, and acid raid was said to be destroying Germany’s Black Forest. In 1981, Professor Bernard Ulrich of the University of Göttingen, predicted, “The first great forests will die in the next five years. They are beyond redemption.”
The National Acid Precipitation Assessment Program (NAPAP), a $500 million multi-year research effort, was established in the US in 1982 to study the issue. The Geneva Convention on Long-Range Transboundary Air Pollution began efforts in Europe in 1983. Acidification of lakes and destruction of forests from air pollution became a widely held belief taught in universities across the world. Acid rain became a driver of US and European efforts to reduce SO2 and NO2 emissions.
But the actual impact of acid rain was much less than feared. The 1990 NAPAP report, titled “Acidic Deposition: State of Science and Technology,” concluded that “acidic deposition has not been shown to be a significant factor contributing to current forest health problems in North America,” with the possible exception of the high-elevation red spruce in the northern Appalachian Mountains. Another study found that damage to Appalachian red spruce forests was caused by the conifer swift moth, not acid rain.
The NAPAP study also found that only 4.2 percent of lakes in the Eastern US were acidic, and that acidic conditions for many of these lakes were due to natural factors or surface mining runoff, not acid rain. The NAPAP study also concluded that 1990 levels of pollution-caused acid rain were not harmful to agriculture or human health.
In Europe, subsequent analysis showed German forest dieback to be due to disease, weather, and other factors, with acid rain playing an insignificant role. The great forests of Europe remain with us today.
Over the last four decades, the United States and the nations of Europe have been remarkably successful reducing emissions of nitrogen dioxide, sulfur dioxide, and other dangerous pollutants. According to EPA data, US emissions of NO2 are down 61 percent and emissions of SO2 are down 87 percent from 1980 to 2016. Emissions in Europe have also been reduced to a small fraction of 1980 levels.
Possibly the greatest evidence against harmful effects of acid rain is the fact that acidic lakes have not “recovered” after most sulfur and nitrogen pollution was removed from the atmosphere. The 2011 NAPAP report to Congress stated that SO2 and NO2 emissions were down, that airborne concentrations were down, and that acid deposition from rainfall was down, but could not report that lake acidity was significantly reduced. The report states, “Scientists have observed delays in ecosystem recovery in the eastern United States despite decreases in emissions and deposition over the last 30 years.” In other words, the pollution was mostly eliminated, but the lakes are still acidic.
Unfortunately, both the news media and many colleges continue to proclaim the myth that acid rain is a dangerous problem. In any case, if you do come in contact with either lemon juice or acid rain, be sure to wash it off as soon as possible.
Originally published in Master Resource. Republished here at request of the author. Steve Goreham is a speaker on the environment, business, and public policy and author of the book Outside the Green Box: Rethinking Sustainable Development.


I used to have a ‘Stop Acid Rain’ umbrella. It stopped protecting me from rain after about one week of use, so I tossed it.
When red hot lava hits seawater, HCl gas (hydrochloric acid) is produced.
You need only get a whiff of that stuff to know it is bad.
This happens frequently enough in Hawaii, and so is a well understood hazard for people who know about the volcanoes. But people can be stupid, so a public information message is only prudent.
One should not live on a floodplain, whether the threat is water or lava.
Oh crap.
Relearn your first year chemistry.
There is no gas form of hydrochloric acid in my text books. It is present in your stomach but you can’t smell it there.
For others, sulphur dioxide plus water gives sulphurous acid, rather more mild than sulphuric acid.
All of these mineral acids become more dangerous when they are more concentrated.
Nature rarely produces them concentrated enough for danger. If Nature did produce them, adaptation over long times would plausibly have countered their danger. Geoff
HaHaHaHa.
Why do you think we keep and use reagent Hydrochloric acid in the fume hood ????
The stuff reeks!
HCl is a gas.
Hydrochloric acid is HCl gas dissolved in water.
*sigh*
Geoff; you are wrong! As TonyL states correctly HCl is a gas and believe me it IS very corrosive to lungs. Try opening a drum of HCl (a 30% solution of HCl in water) as used for regulating swimming pool pH and see how fast you start to feel the effects. In a poorly ventilated area your lungs will feel sore for hours afterwards. I speak from experience.
“Geoff; you are wrong! As TonyL states correctly HCl is a gas”
He’s not wrong, just pedantic. Geoff doesn’t deny that HCl is a gas. He just says that then you should call it hydrogen chloride (see here). Hydrochloric acid in solution.
This is why I have chosen to never engage with Nick Stokes.
I have confirmed (on two occasions) when he was right, and others did not think so.
But these are rare events.
*sigh*
On the chance of getting eaten alive by other posters, HCL is a gas, but also colorless. If you can see “it” then it has combined with moisture in the air and is now an aerosol of Hydrochloric Acid. While not a gas per se, it certainly can drift around and do damage. Just because it has combined with water doesn’t mean it sits on the ground (or in the ocean) and behaves itself.
Geoff, you are right about the terms “hydrochloric acid” and “hydrogen chloride”, but in essence the formation of HCl under these conditions is possible. At high temperatures, SO2 dissolving in water rapidly oxidizes to H2SO4 which, in the presence of chloride ions in seawater, displaces the hydrogen choride into the gas phase. The reaction H2SO4 +2NaCl -> Na2SO4 + 2 HCl is well known.
‘but the lakes are still acidic’
As would be expected if the lakes were fed by streams with high organic content. Like leaves falling from trees.
‘The NAPAP study also found that only 4.2 percent of lakes in the Eastern US were acidic’
Frankly, I’m surprised the number is that low.
Not me. Acidic lakes are only found in areas where there is little or no limestone in the catchment areas, e. g. most of Canada and Scandinavia. There are very few such areas in the eastern US. There might be some up around Lake Superior.
In the acidification of the lakes in the NY Adirondacks is because of what you stated. The Adirondacks is a fairly young mountain range comprised of non-sedimentary rocks (i.e not made out of sandstone). The water that feeds the lakes primarily comes from surface water runoff vs. being spring fed (which tends to make water more alkaline).
The lake pH dropped when the forests recovered from past large scale logging, owing to an increase of rotting vegetation (leaves) on the forest floor.
Leave may contain humic, tannic and fulvic acids. Weak organic ones, I grant you, but still acids.
Leaves
I think it’s pretty clear that Acid Rain is much like Homelessness – a problem that only shows up when there is a Republican President, and which disappears from view as soon as a Democrat is elected.
Coca Cola has a pH of 2.5
Most soda’s contain citric acid (natural from fruit juices or added) to compensate for the lots of (added) sugar in the soda drink. All cola drinks use phosphoric acid, which is a far stronger acid than citric acid. The reason being that cola was originally a medicin for digestive problems, mixed by a pharmacist… Still it helps to digest because of its high acidity…
And it dissolves teeth.
Tom I tried that with Coke and a tooth mydaughter submitted. No dissolving apparent after a couple of weeks. I have the photos somewhere….
Coca cola is actually helpful if you get “Montezuma’s revenge”. I was told this by a peruvian physician many years ago. I was very skeptical, but I have had reason to try it a few times and it really seems to work.
tty- Everyone knows Tequila is the best remedy from Montezuma’s revenge.
Some claim that there’s enough H₃PO₄ in Diet Coke to dissolve kidney stones. “Phosphoric Acid” in cola recipes usually refers to the 85% strength acid.
Considerable experimentation was done by M. Henri Joulie regarding treatment of rheumatoid arthritis with dilute (~1000:1) phosphoric acid or its sodium salts in the early 20th Century. Papers on the subject were published by J. Charlton Briscoe, M.D. (BMJ, March 11, 1911) and by Frank Augustus Watkins, M.R.C.S. (Lancet, June 20, 1908). Drugstore “phosphates” are still available, last I heard, at a store by the El Capitan theater in Hollywood.
“Some claim that there’s enough H₃PO₄ in Diet Coke to dissolve kidney stones. ”
I can tell you from extensive experience that Diet Coke does NOT dissolve kidney stones in your kidneys. Maybe if you put kidney stones in dish and poured Coke on them… Several urologists have told me nothing taken orally will dissolve stones in your kidneys.
I researched acidic drinks and the potential for destroying teeth a few years ago. The data I found strongly found sugar to be at fault. Carbonated water had little effect on the teeth. As I recall, 20 years of Coke was the equivalent of… >250 years of carbonated water. If you expect to live that long, go light on the carbonated water, and eschew most of the sodas.
But then, most people seem aware of that, as Coca Cola, Pepsi, and others have been (successfully) searching for other revenue streams – teas, water, etc.
Back in my youth, I would head on down to the local apothecary, which had a soda fountain. There I would cajole the resident soda jerk to make my thirsty self a lime phosphate. With “lots” of phosphate. Cherry was also good. Maybe that explains my many tooth cavities. Oh well. Instant gratification.
One of my students had a problem with a steel bowl in the lab and asked me how to clean it, I suggested he use Coke. He thought I was joking but it cleaned up beautifully!
Jeff Alberts,
Your claim is not acceptable because you are using a generic term (kidney stones) for materials that have variable composition. There are oxalate, citrate, carbonate, and other types of stones. Apparently ingesting a lot of food or drink that contains the formative anion can result in the formation of a stone. Eliminating that anion source should at least stop the growth of the stone(s). Ingesting sufficient quantities of a liquid that will either change the salinity or pH of the liquids reaching the kidney may reduce the stones, although, as a practical matter, that may not be possible. However, the pH of one’s urine can be changed by what is eaten or drunk, and the changing color indicates that the concentration of dissolved chemicals can vary also. Given enough time, water will dissolve almost anything, So, just eliminating the source of the stones and significantly increasing one’s water intake will probably be helpful. However, one needs to be careful that the water intake isn’t so high as to significantly imbalance the electrolytes in one’s system.
I tried coke once, but the bubbles got up my nose
I’ll get my coat
That’s why it’s not worth drinking
“In Germany, the word “Waldsterben” (forest dieback) was coined, and acid raid “………
Back in 2010, I downloaded all of the annual data from the National Atmospheric Deposition Program and calculated an annual average rainwater pH for the USA…
http://nadp.slh.wisc.edu/NTN/ntnData.aspx
Rainwater was not becoming more acidic prior to the initiation of the EPA’s Acid Rain Program in 1990. The pH of rainwater was actually rising (becoming less acidic) prior to the EPA’s efforts to fight acid rain. The really crazy thing is that the pH has been rising more slowly since the EPA started to fight acid rain!
Rain is supposed to be acidic. The pH of rain in a pristine environment, free from pollution (including volcanoes) is normally about 5.6. Most of the lakes which were showcased as acid rain victims were naturally acidic and had been acidic since well before mankind ever burned his first lump of coal.
Rather than being a global problem, anthropogenic acid rain was a localized problem in parts of Northern Europe which was relatively easily fixed.
The costs of reducing SO2 and NOx emissions haven’t been that awful… And the reductions did lead to some beneficial incremental environmental effects… But… No acid rain crisis ever existed.
David
My belief is that European acid rain was another myth. We Brits were accused of depleting Swedish forest’s with our emissions. The Swede’s, on the other hand, knew nothing about it and I believe laugh about it today.
Doubtless a dry run at climate change, as with vanishing ozone, water shortages in the UK (they never give up, it’s in the news again) and polluted air (we note, only in cities, but represented as nationwide).
Had we not evolved as a species with a skin tougher than Rhino’s and Elephants, we would have died out long ago. And when a natural disaster like a volcano is happening somewhere, an equal, and opposite phenomenon is happening elsewhere on the planet.
Exactly what I was going to post! I remember the collective guilt we were made to endure because of the harm to Sweden’s forests. Fortunately we’ve outsourced most industrial production to the developing world.
I’m amazed Russia never made a fuss about Germany’s similar impact on the Eastern bloc!
I saw a demise of forests in Ore Mountains on the border between former East Germany and Czechoslovakia. Once, hitchhiking there, I got a ride from a forest guard. I mentioned that there was a lot of alarm in the press about dying forests, and we were driving through a good looking forest. He said, when this spruce grows a new needle, it expects it to last 5 years. Today it lasts only two years. The trees are exhausting themselves. And in two years the forests went dead. There was a lot of smoke from heavy industry on both sides of the border.
Curious George
Smoke isn’t acid rain.
David Middleton May 23, 2018 at 11:02 am
Back in 2010, I downloaded all of the annual data from the National Atmospheric Deposition Program and calculated an annual average rainwater pH for the USA…
http://nadp.slh.wisc.edu/NTN/ntnData.aspx
Rainwater was not becoming more acidic prior to the initiation of the EPA’s Acid Rain Program in 1990. The pH of rainwater was actually rising (becoming less acidic) prior to the EPA’s efforts to fight acid rain. The really crazy thing is that the pH has been rising more slowly since the EPA started to fight acid rain!
Shows how effective the 1970 Clean Air Act was I guess, strange you forgot to mention that?
I didn’t mention it because it is irrelevant to the the irrelevance of the EPA’s war on pH levels of rainwater.
I did mention the CAA extensively in this post:
https://wattsupwiththat.com/2018/05/17/putting-the-clean-air-act-on-ice/
In the case of almost every reeal pollutant, the CAA and preceding measures rapidly reduced atmospheric concentrations nearly down to geological background levels. Yet EPA bureaucrats continue to push for further reductions at exponentially increasing unit costs for abatement.
David Middleton May 24, 2018 at 7:30 am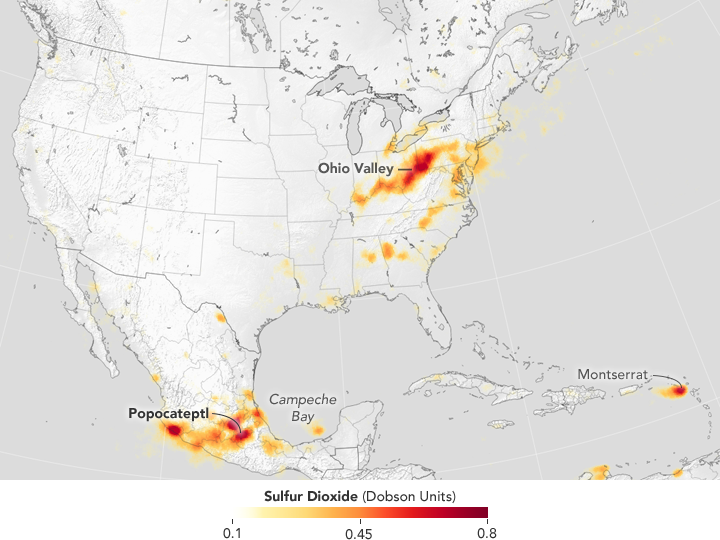
I didn’t mention it because it is irrelevant to the the irrelevance of the EPA’s war on pH levels of rainwater.
I did mention the CAA extensively in this post:
What have we learned here? We’ve learned the the Clean Air Act has been wildly successful. The EPA deserves a pat on the back for reducing key air pollutants down to nearly irreducible levels. So, why has the EPA been continuously pushing to further reduce these pollutants further below the current National Ambient Air Quality Standards (NAAQS) and often pushing to lower the NAAQS levels?
https://wattsupwiththat.com/2018/05/17/putting-the-clean-air-act-on-ice/
In the case of almost every reeal pollutant, the CAA and preceding measures rapidly reduced atmospheric concentrations nearly down to geological background levels.
Really?
“Total emissions of SO2 increased from 9 million metric tons (9.9 million short tons) in 1900 to a peak of 28.8 million metric tons (31.7 million short tons) in 1973, of which 60% was from electric utilities (USEPA 2000). By 1998, total annual SO2 emissions for the United States had declined to 17.8 million metric tons (19.6 million short tons). From 1970 to 1998, SO2 emissions from electric utilities decreased by 24%, largely as a result of the 1970 and 1990 amendments to the Clean Air Act. Emissions of NOx have increased from about 2.4 million metric tons (2.6 million short tons) in 1900 to 21.8 million metric tons (24 million short tons) in 1990. They have remained fairly constant since then.”
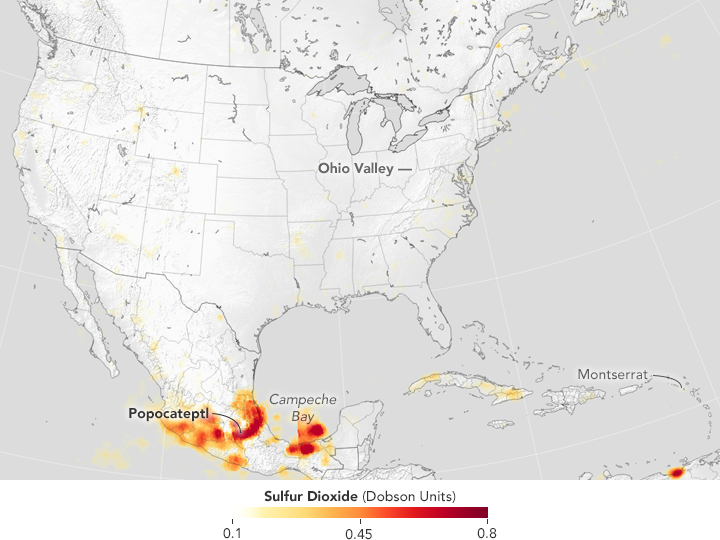
Since then the OMI results have shown a reduction in SO2 emissions of 80% (2005-2016) mainly from power stations in the Ohio valley.
2005
2016
Displaying the two linear trends on your graph of pH was a bit disingenuous since you’d expect more of an asymptotic approach to the CO2 value of 5.6.
We are told that… ” Both nitrogen dioxide (NO2) and sulfur dioxide gases react in the atmosphere to form nitric and sulfuric acid.”. This is not true – at least not initially. They form sulphurous and nitrous acid both of which are much weaker acids than nitric and sulphuric acids. The distinction is important.
Sulphjurous and nitrous acid don’t last long. They quickly do convert into sulfuric and nitric acids which are much stronger.
“The distinction is important”
The statement was correct. Merely hydrating NO₂ produces both HNO₂ and HNO₃ (nitric). And H₂SO₃ is easily oxidised to H₂SO₄ (sulphuric). So the distinction is unimportant.
Nick,
The nitrous/nitric oxide reactions creating acids in water are reversible reactions where the temperature and concentrations are important. Hand waving about the potential for creating strong nitric acid is alarmism. In order to say anything definitive about the probability of the direction of the reaction, the concentration and temperature have to be specified. The half-lives of the species are measured in hours, giving plenty of time to disperse and dissipate, unless one is climbing in a caldera,
https://en.wikipedia.org/wiki/Nitrous_acid
Partly true only, Nick.
The usual reactions to make concentrated sulphuric acid are strongly endothermic, with Nature normally too puny to force them through to completion.
Yes, for very dilute forms you are correct, sulphurous can produce sulphuric but then there is far less threat in Nature from such diluted acids at low concentrations, as others have noted re the risk of Coca-Cola etc. It is a matter of energetics and equilibria, modelled over a century ago.
In the many measurements I’ve personally done on natural materials like waters, I have yet to find evidence of significant quantities of concentrated strong acids anywhere. But, I have not studied the lava/seawater reaction specifically, so I cannot comment on that specifically.
“The usual reactions to make concentrated sulphuric acid are strongly endothermic”
Not so, Geoff. The process was the industrial lead chamber process:
“Sulfur dioxide is introduced with steam and nitrogen dioxide into large chambers lined with sheet lead where the gases are sprayed down with water and chamber acid (62–70% Sulfuric acid). The sulfur dioxide and nitrogen dioxide dissolve and over a period of approximately 30 minutes the sulfur dioxide is oxidized to sulfuric acid. The presence of nitrogen dioxide is necessary for the reaction to proceed at a reasonable rate. The process is highly exothermic, and a major consideration of the design of the chambers was to provide a way to dissipate the heat formed in the reactions.”
The NO₂ acts as a catalyst, oxidising the SO₂ and being reduced to NO, which is then oxidised back to NO₂. That doesn’t change the thermo.
But no-one is suggesting that concentrated sulphuric acid is ever produced in the air. They talk of pH 4 or so. And in fact, any of these acids is sufficient to create that. The pH itself isn’t very significant, because there is very little actual acid, of whatever strength, so it doesn’t have much instantaneous effect on the buffered environments that we care about.
Sulphurous acid doesn’t really exist in aqueous solution, solutions of SO2 rapidly transform to sulphuric acid (a bit like carbonic acid). The effect of ‘acid rain’ on buildings is well documented:
http://chemistry.elmhurst.edu/vchembook/images2/196sculpture.gif
Prior to the clean air act in the UK the leading cause of referrals to doctors was bronchitis (and leading cause of sick days from work). In Sheffield in the evenings an acid ‘fog’ used to form over the hills to the west in the evenings and roll into the city, after the clean air changes (notably eliminating domestic coal fires) this stopped. In one of the lakes near Sheffield which was used for trout fishing the acidic water runoff from the moors used to stunt the fishes growth. This was fixed by putting limestone in the inlet streams and the fishing significantly improved.
The killer London smog in 1952 has been diagnosed as resulting from sulphuric acid aerosols.
https://www.cbsnews.com/news/londons-1952-killer-fog-cause-revealed/
Nick Stokes,
You initially said, “And H₂SO₃ is easily oxidised to H₂SO₄ (sulphuric). So the distinction is unimportant.” Than ten hours later you said, “The pH itself isn’t very significant, because there is very little actual acid, of whatever strength, so it doesn’t have much instantaneous effect on the buffered environments that we care about.” This sounds to me like you are back pedaling. Which is it? Are sulfuric and nitric acids something we should be concerned about, or not?
Nick Stokes,
You claim, “And H₂SO₃ is easily oxidised to H₂SO₄ (sulphuric).” You then go on to educate us about the Lead Chamber Process (unlike anything that happens in nature), which requires optimal NO2 catalysis. abundant water, and proceeds at elevated temperatures and concentrations that are not found in the free atmosphere. So, I wouldn’t call what happens to SO2 after it leaves a power plant ‘easy oxidation.’ Would you care to back pedal on this initial claim as well?
Clyde,
“This sounds to me like you are back pedaling.”
From what? I simply said that SO₂ would oxidise. I didn’t say that would be harmful. When it comes to buffered environments, all acids are equivalent, as long as they are stronger than the acid part of he buffer. Acid rain can have long term effects on trees, marble etc. But it won’t hurt to get caught in the rain.
The thing about the lead chamber process is that it shows that the reaction is exothermic, and under these conditions, at least, fast. In fact, Roebuck started it in 1746 without deliberate catalysis – it was an accidental consequence of using saltpeter to help burn the sulphur. It it can’t get very hot, since the sulphuric solution has to condense on the walls.
Just checking to see of anyone else commented on this:
“sulfur dioxide gases react in the atmosphere to form nitric and sulfuric acid.”
The distinction is important to those for whom it is important.
“Hydrochloric acid is HCl gas dissolved in water”
No. HCl without water is a gas known as hydrogen chloride.
Terminological inexactitude had to be corrected.
Now, can you write down a plausible reaction for hot lava and sea water to generate hydrogen chloride?
Geoff
So if you dissolve HCl in water, what do you get?
Geoff,
The release of HCl from seawater is a natural process, even with sunlight. HCl is a gas and any evaporation of water takes some HCl with it, leaving NaOH behind. The total amount worldwide – if I remember well – is around 400,000 tons/year – all natural. Diluted in lots of water, so not that important, except for dissolving carbonate rocks on very long term, together with dissolved CO2 in rainwater.
In the case of lava the heat makes that process much faster and thus lots of HCl are taken with the water vapor / fog. If the magma is highly acidic (as is the case in Iceland for deep magma, maybe the same for Hawaii), that adds to the evaporation of HCl, including HF, SO2 and other acids already present in the lava… HF is the main toxicant: the outbreak of the Laki fissure in Iceland a few centuries ago caused the death of a lot of livestock downwind…
Having worked as a coating chemist in the 80’s, I can say that acidified rain was a major concern with metal roof manufacturers. It was causing premature
failure of screw heads.
Research produced a way to engage in accelerated testing of a coating’s ability to resist acidified moisture. It was very difficult to achieve a 20 year certification.
I suspect that between the reduction in NO2,SO2 and the improvement in coatings the problem resolved itself.
Nonetheless, acidified rain dissolves heavy metals found in soils every where which are very harmful and permanent .
Maybe that is why lakes are very slow to recover.
I might suspect crevice corrosion and poor coating procedures was more of an issue. I would also suggest choosing materials closer together on the galvanic chart.
Right. The only coating I know of that works well as a corrosion preventive is hot-dipped zinc. Other coatings, unless they are absolutely free of pinholes, will fail rapidly at any tiny hole or scratch.
Was this the normal acid nature of rain or the additional acidity caused by industrial emissions? Maybe the screw heads weren’t fit for purpose in the first place?
RW,
Yes, it sounds to me like the problem was one of electrolysis. That is to say, any dissimilar metals immersed in a conductive liquid will experience electron exchange and therefore oxidation.
MAYBE he should have used Tef-Gel or Duralac ?
JDS,
Why the awful assumption that “heavy metals found in soils every where which are very harmful and permanent”?
There are metallic nutrients for plants, like Fe, Cu, Mo, Zn that have to be dissolved to some degree from their usual mineral state, to get into the plants via root solutions to nourish them. Otherwise the plants die. Who is the arbiter of what is a “nice” amount of dissolution, beyond which you want society to believe in “harmful and permanent”?
When did you last hear of credible human deaths from metal poisoning in normal human environments? Where are the bodies, in sufficient numbers to raise concern?
Try chemistry instead of bogey man comments, eh? Geoff.
Partly true only, Nick.
The usual reactions to make concentrated sulphuric acid are strongly endothermic, with Nature normally too puny to force them through to completion.
Yes, for very dilute forms you are correct, sulphurous can produce sulphuric but then there is far less threat in Nature from such diluted acids at low concentrations, as others have noted re the risk of Coca-Cola etc. It is a matter of energetics and equilibria, modelled over a century ago.
In the many measurements I’ve personally done on natural materials like waters, I have yet to find evidence of significant quantities of concentrated strong acids anywhere. But, I have not studied the lava/seawater reaction specifically, so I cannot comment on that specifically.
Heh, should I remind everyone what pH stomach acid is? Stomach acid is 0.1N HCl. The pH is 1. True enough, it is only about 20 cc and diluted by food, such that pepsin can work doing its thing. Optimal pH for pepsin is 4.
Be sure to wash your stomach off after exposure. Oh, wait. Oops.
We’re 11 miles from the current eruption, and usually upwind. My catchment input water is pH 3.7-3.9. Keeps the car looking clean. 4 pounds per month of sodium bicarb in an 8500 gallon tank brings it up to 5.9-6.1 at the tap. We start feeling itchy whenever the vog blows this way, rain or shine. Acid rain is way down the worry list for us, but it’s a real problem leeward of the eruptions. It has affected crops, forest, and fencing in Ka’u.
Just a few thoughts among my sympathies for your problems in the beautiful islands of Hawaii.
I believe the carbon dioxide monitoring system is based there. What is it reading now – more than 400 ppm ?
Also I read a stupid article wondering if hydraulic fracturing for geo thermal heating could/ had caused this eruption.
Any observations on this?
They’re having some issues, but take advantage of convective currents for measurement periods.
https://www.esrl.noaa.gov/gmd/ccgg/trends/monthly.html
The Geothermal power plant was built about 25 years ago. The Geothermal wells are also that old. Geothermal well don’t need periodic hydraulic fracturing. The acidic nature of geothermal water slowly widens the cracks in the rock.
The power plants is built on a fault. The lava has for well over a 100 years has periodically flowed through the fault and erupted in that area. Long before the power plant was built. An eruption in that spot was inevitable.
In that case the lava should have come up at the frac’ing site, not in the crater.
Theoretically it might happen. I remember a case in Iceland where lava actually came up through an (unfracked) drill hole for geothermal energy. They had drilled rather too close to an active volcanic vent (Krafla). Very little damage except for a hole plugged by lava.
Initially, the main reason for hyperventilation over the geothermal plant was the presence of 60,000 gallons of pentane. The pentane has been moved to a safe location, but now the concern seems to be the possible release of H2S if the wells are damaged. I’m not sure if this is a valid concern or not. I don’t know why the H2S concentration at the geothermal wells would be any higher than the gasses released by the eruptions themselves, but I am not an expert. On the other hand, I don’t have to be an expert to know that the geothermal wells themselves could not have possibly caused the volcano to erupt.
Here in Kona, about 80 miles northwest of the eruptions, the problem is “vog”, the volcanic fog made up of water vapor, and particulate residue formed from the breakdown of SO2. The CO2 observatory is near the summit of Mauna Loa 40 or 50 miles from the eruptions, and several thousand feet higher. The normal trade winds move the volcanic gasses away from Mauna Loa.
Terry,
Thanks for the updates!
Good luck and stay safe.
The author of this article is conflated two very different phenomenon. Acid rain from coal-fired power plants was largely, if not entirely, overblown depending on perspective (whether you lived in the plume or not).
The issue of high levels of sulfuric acid droplets in the steam clouds from those volcanic fissures dumping into the cold ocean is real if you happen to be in the downwind pattern. Add in the common rainfall that would wash it out, and pH < 1.5 is entirely possible, with burned corneas and potentially even skin and lung damage. It is just this phenomenon is highly localized. No one on the northern half of the Big Island (like Kona) should be worried about this unless they plan to travel on sightseeing trip to south eastern the lava flows. Hilo might have a worry about getting an acid rain bath though if activity ramps up and the winds are just right.
In a past life as an analytical geochemist I had to clean PTFE reaction vessels in warm 1.5M Nitric acid, followed by cold 6M Hydrochloric.
6M HCl has a negative pH. It’s possible to tolerate it on unbroken skin for a few seconds, but you need to rinse it off PDQ. If you don’t, it will sting, especially on soft un-calloused skin.
I once got some in one eye (my fault), and it’s seriously nasty, ‘sting’ doesn’t quite describe it.
Lots of rinsing, and no lasting damage thankfully.
A volcanic Hydrochloric/Sulphuric acid mist or rain is something I’d give a very wide berth to.
Also be careful about cameras, spectacles etc exposed to volcanic steam. There is often hydrofluoric acid as well which etches glass.
‘Data suggests it is a non-problem’
But it just SOUNDS so scary – like in ‘Alien’ – when that critter’s blood ate right through the floor.
Although, just to show you where Hollywoods’s at these days, according the latest Alien movie, THAT was created by humans too.
In the latest sequel wasn’t it an AI (robot) who created them? A modern version of “Frankenstein’s Monster”?
rocketscientist
Spoiler alert!
Pack it in guys, I didn’t know the movie was out yet never mind glean the plot!
Frankenstein and his monster were an artist’s invention. They tell us something about how artists think, not about what scientists do.
‘In the latest sequel wasn’t it an AI (robot) who created them?’
Well, an android created by humans – so, still our fault.
Sorry for the spoilers, although, frankly, I’d recommend passing on the flick anyway.
Yes, between Disneyfication and self loathing there are not many modern movies worth paying money to see.
SHHHHHHH! Don’t tell people it’s not a problem! We’re planning to travel to Hawaii in August and I’m hoping airfares will be depressed by all the scaremongering.
The travel industry are reporting that hotel bookings are down.
Alaska Airlines is offering some great deals from Seattle.
Maybe volcanos are less daunting than Seattle’s Leftist taxation.
The problem I had with the acid rain myth was the fact that they were always worried about pine, conifers, etc. And always in areas with large old pine/conifer forests. It is my understanding that these needles make the soil acidic. My mother always planted the acid loving plants under or near them. Thus, obviously, they like acidic soil else they would never sprout from seeds.
I have to add sulfur to acidify the soil under my pines since the dirt is naturally alkaline around here.
In regards to the geothermal power plant their. THe news keeps repeating the issue that should the geothermal well heads be damaged toxic gases could be released. Never do they mention those toxic gases are the same ones being produced by the lava. If all the wells were breached the increase in toxic gases would not be significant.
+ 10
The same toxic gasses CNN shows on continuous feed spewing from all the other fissures. Somehow these toxic gasses must be different enough to merit mention. I might suspect that the lava will simply cool and plug the wells. They might geyser for awhile, that would be interesting.
Thanks for clearing that up. It was not clear what toxic gasses they were ranting Geothermal systems don’t use toxic gasses for coolants typically.
But then again, legacy media never lets facts get in the way of a story.
Back in the 1980s when the “acid rain” hysteria was popular, I had fished some lakes in the southern interior of British Columbia. At some 5000 feet elevation they are surrounded by fir trees, some of which had fallen into the small lakes. With all the acids from the trees, the lakes were naturally acidic. The water looked like tea. Of course, the acidity is natural. But try to explain that to liberals freaked out about acid rain!
However, not all are acidic. The Kentucky-Alleyne pair of lakes near Aspen Grove has remarkably clear water. So clear that we saw a loon chasing a fish some 8 feet down.
I asked a friend who headed up the salmonid enhancement program at UBC for an explanation. The lakes were scooped out by ice from a limestone host.
When the water warms up in the summer it changes the PH from the acidic “tea”. And the water gets clear.
Bob Hoye
Well its all because of climate change and it’s Trumps fault. /sarc
NO2 and SO2 dissolve in water to make nitrous and sulfurous acid–not nitric and sulfuric acid. Now SO3 in air would dissolve readily in water droplets to H2SO4, but the oxidation of SO2 to SO3 in air is very very slow and proceeds at a reasonable rate only at high temperature. However, the conversion of SO2 to Sulfuric Acid can be catalyzed by nuclei of metal salts in air. The process is one of SO2 dissolving in a water aerosol, then diffusing into the droplet and there encountering a chloride or sulfate of Mn or Cu to catalyze the oxidation. Perhaps NaCl could also perform the catalysis, and ocean air has a high concentration of NaCl aerosol obviously.
“NO2 and SO2 dissolve in water to make nitrous and sulfurous acid–not nitric and sulfuric acid.”
Not true for NO₂. The reaction is
N₂O₄ + H₂O → HNO₂ + HNO₃ (nitrous + nitric)
And oxidation of H₂SO₃ is fast on an atmospheric time scale.
Nick,
What does “an atmospheric time scale” mean? Does that mean enough time to be dispersed by wind and diluted by rain?
Oxidation of nitrous acid HNO2 in water by dissolved oxygen is very fast:
https://www.sciencedirect.com/science/article/pii/S1352231007000842
Oxidation of SO2 also proceeds mainly in water solutions. In seawater this process may be catalyzed also by magnesium ions:
https://nepis.epa.gov/Exe/ZyNET.exe/9101R0ZV.txt?ZyActionD=ZyDocument&Client=EPA&Index=1976%20Thru%201980&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C76THRU80%5CTXT%5C00000034%5C9101R0ZV.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=4&slide
I recall reading a study of “acid rain” that was undertaken because some of the worst “acid rain” areas were downwind of nothing but forest, with no industry for hundreds or even thousands of miles upwind. Turned out the worst emitters of sulphur dioxide were… trees, and absent trees, you don’t get significant “acid rain”. (You can sometimes see this on realtime SOx maps, as a plume downwind of America’s northeastern forests.)
Could they be taking the sulphur reduction thing a bit too far?
Sulphur is a major component of Keratin in hair and feathers and a component of the melanin in insects. For marine phytoplankton dimethylsufoniopropionate (DMSP) is an osmotic pressure balancing substance.
Some studies have indicated that insects are more numerous around sulphur emitting manufacturing plants (than they are further away, in more pristine conditions). It has also been suggested that birds who feed their chicks insects do so because insects are a better source of sulphur than plant matter.
After 50 years of good sulphur availabilty aren’t creatures going to find life a lot more difficult?
Data suggests it’s ALL a non-problem.
No reason to be concerned about this until they finally get around to regulating purchase of hydroxyl acid, available in almost all stores!
We can no longer get Hydrox cookies at the local store, so hydroxyl (or hydroxylic) acid is probably next to go.
Jorge,
From Wiki,
Hydroxylic acid – “One of several systematic acid names for water, H2O.”
https://en.wiktionary.org/wiki/hydroxylic_acid
Come on you guys, take care commenting about Chemistry unless you are a Chemist. (My Science major was in Chemistry).
Geoff
See also
https://ssrn.com/abstract=2857442
I did a small study myself on SO2 emissions. Mt. Pinatubo put more sulfur dioxide into the atmosphere than all human activity combined, ever. Distributed SO2 measurements may wash this out, but humanity contributes little to at least the sulfurous/sulfuric acid rain.
The pH is measured wrongly claims this article and related studies http://sciencenordic.com/all-soil-more-acidic-we-thought
The recovery from acid rains is very, very, very long process. http://sciencenordic.com/acid-rain-still-affects-water-quality
Harri,
The articles conflict with each other.
The first points out that the consensus regarding pH of soil is wrong. Especially about eco sensitivity. That article offers numbers and fact. The second seems to avoid giving facts. It appears to be more focused in defending an expensive and unsuccessful program.
What is clear is that the program of liming is not working and is probably perpetuatung the eco damage to the lakes.
The biggest result of the reduction in sulphur deposition in agriculture in the UK has been to make the routine application of sulphur in fertilisers necessary. In the 1960s samples of kale and wheat showed satisfactory levels of sulphur uptake from the soil. However sulphate ions are leached from soil almost as readily as nitrate ions, so without the annual replenishment of sulphur from the air sulphur has to be added to the spring nitrogen top dressings.
As far as organic farming is concerned with their antipathy to ‘chemical’ fertilisers, a farmer friend in South Devon had considerable problems establishing clover in his ‘fertility building’ part of his rotation. The problem was traced to a shortage of sulphur, so a derogation from the organic monitoring organisation was needed to allow him to apply sulphur. Problem solved.
On the heavy metal front, the old lead mining district of the Mendip Hills in Somerset, which dated from Roman times, had high levels of lead, zinc, selenium and other heavy metals. Enough in some areas to cause yellowing of the grass growing on these fields.
Imperial College caused a bit of a panic in the 1970s with some routine analysis of the soils in the area, and suggested that people should not eat vegetables grown in their gardens. However it turned out the soils were mostly high in lime which reduced the availability of the heavy metals, and analysis of the vegetables showed a low risk in most cases.
It is useful to be reminded just how consistently deceptive and flat out wrong the green grifters really are.
I have been involved in reducing pollutants from coal fired power plants for over 35 years. In earlier posts, I could not find them, I talked about the fact we have reduced the SO2 emissions to such a point that farmers are buying gypsum generated by the removal of the SO2 from the environment to supplement the soil to add sulfur. Very costly an inefficient method to do this. Simple solution – allow the utilities to emit more SO2. That would not be allowed by the regulatory agencies.
At the beginning of the acid rain scare in the late 70s early 80s 2 videos were made. To quote the UPI “Dying trees in the Great Smoky Mountains are turning the pristine wilderness into a barren wasteland, a researcher said Thursday, and the Tennessee Valley Authority is stepping up a study of whether acid rain is to blame.” There was a massive die off of red spruces and Fraser firs. One showing the damage that acid rain was doing to the forests in the Tennessee mountains, it was the fault of acid rain. The other showing that aphids were the cause along with other environmental issues.
Guess which of those got the attention of the media. There you have the start of an environmental movement with limited facts.
As an engineer I presented both sides to management and our conclusion was that it needed more study and could not reach a conclusive reason for the die off. Of course the environmentals did not wait. The dramatic pictures made for good drama and promoted the acid rain story.
Years later the same area was used to show how NOx was killing more of the trees. The theory (I hope that I am recalling in correctly) was that the extra NOx was causing the trees to produce new growth too early in the growth season and freezing weather biting back the new growth retarding the growth of the trees.
I do not think that this theory had legs to stand on so the story was switched to NOx causes a type of smog that makes the sky appear with a grey haze. The Blue Ridge Mountains are known for their dramatic sunrises and sunsets with the blue haze adding dramatic effects. The blue haze is generated by the unique growth of the trees in the mountains. This theory was that the NOx was causing a different light refraction changing the blue to grey therefore ruining the natural blue of the mountains. It was a theory and required a lot of study. Never the less NOx controls were dictated on power plants.
This just shows that the environmentalists do not let facts get in their way. The mantra is that some issues that are too important to wait until the facts are in.
[Thanks for your thoughtful contribution Gary. I believe this is the post you referenced above: garywgrubbs_comment -mod]
Thank you Mod. That was the post that I could not find.
Not to be nitpicky… “Water is neutral with a pH of 7″… Distilled water has a pH of 7. Water from any other source will not be likely to be pH 7 due to naturally occurring minerals dissolved in it (TDS). Even filtered water is less than likely to be pH 7. Most plants on the planet Earth like slightly acidic soil and water. There are exceptions (potatoes and blueberries come to mind immediately, one loving acid, and the other loving base…) I see no problem with “scid rain” and blew it off as “chicken little hogwash” back in the ’80s when I first heard about it…
Very curious to get a link to the “subsequent analysis” about Waldsterben in Europe. Could you post it here? Thx
The acid of concern here is hydrochloric and is not from the volcano. When the hot lava enters the sea hydrochloric is produced from the dissociation of salt dissolved the seawater. The evolution of steam bearing HCl also carries particles of volcanic glass so dont breath the stuff.
tty May 23, 2018 at 1:19 pm
“Coca cola is actually helpful if you get “Montezuma’s revenge”. I was told this by a peruvian physician many years ago. I was very skeptical, but I have had reason to try it a few times and it really seems to work.”
I only drink Coca cola or Pepsi when eating KFC, the phosphoric acid is the only thing that will break up the fat and grease, the two neutralize each other perfectly.