Guest essay by Clive Best
Summary
The currently held belief that we must decarbonise the world’s economy in order to to stop climate change is a dangerous illusion. It is a ‘hiding to nothing’. I have come to the conclusion that if emissions were held constant for 30 years then the airborne fraction of CO2 emissions would reduce to zero. In other words if the world can hold emissions constant at say 30GT CO2/y then sinks will increase to balance all annual emissions. Thereafter CO2 levels would remain at below 440ppm indefinitely, so long as emissions remain constant.
Introduction
I will argue below that in order to stop global warming all we really have to do is simply stabilise CO2 emissions, not reduce them to zero! This alone will stabilise CO2 levels within less than 30 years. The origin of the ‘myth’ as promoted by nearly all IPCC climate scientists is that we have to stop burning all fossil fuels i.e. we must ‘keep it in the ground’. This is a fallacy and I will try to explain why in this post.
The origin of this belief that we must stop burning any fossil fuels by ~2050 can be traced back to Figure 10 which appeared in the AR5 ‘Summary for Policy Makers’. Here it is again.
Figure 10 from SPM AR5
Figure 10 was intended to send a simple message to the world’s political leaders. Namely that there is a finite total amount of fossil fuel that mankind can ever safely burn, and that we have already burned half of it. Therefore unless all major industrialised countries stop burning fossil fuels altogether by 2050, the world will warm far above 2C (the red curve) causing a global disaster. This message worked, but I find that there is so much wrong with the hidden assumptions and even subterfuge used to produce Figure 10 that I wrote a post about it at the time.
The principal hidden assumptions, as I see it, in Fig 10. are as follows:
1. Carbon sinks are saturating (they are not)
2. ECS (Equilibrium Climate Sensitivity) is 3.5C (Very uncertain – and could even be as low as 1.5C)
3. The subtle replacement of logarithmic forcing of CO2 with a linear forcing.
4. The assumption that past emissions stay in the atmosphere essentially forever.
As a direct consequence of IPCC successful lobbying based essentially around Figure 10, the Paris treaty now proudly “sets the world on an irreversible trajectory on which all investment, all regulation and all industrial strategy must start to align with a zero carbon global economy“. Does anyone really believe that this is even feasible, let alone realistic? It simply is not going to happen because well before then, their citizens will revolt and kick them out. The best we can hope for in the short term is a stabilisation of global CO2 emissions. The minimum condition needed is that annual growth in emissions needs to be brought to zero.
Carbon Cycle
To understand the carbon cycle means understanding the difference between CO2 decay time and CO2 residence time. The decay time for an individual CO2 molecule emitted by man is only about 5-10 years (based on C14 measurements in both bomb tests and those produced by cosmic rays). Every CO2 molecule in the atmosphere is rather quickly absorbed either by photosynthesis or by the ocean. However on average most of them are simply replaced by another CO2 molecule entering the atmosphere through evaporation from the ocean surface or by biological respiration. The residence time however, is the e-folding time needed for a sudden net increase in CO2 to decay back to normal as the carbon cycle reacts. At equilibrium the total CO2 content of the atmosphere remains constant over centennial time scales. Currently though, as a result of our emissions, slightly more net CO2 molecules are being absorbed than are being returned to the atmosphere each year. As a consequence the atmosphere is not quite in equilibrium with the rest of ‘natural’ life and the oceans. We have given the carbon cycle a kick, and as a result it is reacting to return back into balance. The problem is that we have continued to kick a little harder each year so that balance is never reached.
If you sum up all the sources and sinks since 1960 then you find a remarkable fact, which was unexpected by climate scientists. About half of man-made emissions are being absorbed each year. This means that just one half of the net CO2 emitted by humans remains in the atmosphere each year. This is called the airborne fraction. The strange thing is that this airborne fraction hasn’t changed at all in 60 years, despite exponentially increasing human emissions.
AR4 plot: The fraction of Anthropogenic CO2 retained in the atmosphere (b) is unchanged in 50 years, despite increasing emissions (a). Note how the annual change stalled after the 70s oil crisis
Plot of Carbon content of air versus Cumulative Carbon emissions produced by Nick Stokes.
This means that today we are emitting about twice as much carbon dioxide as we did 30 years ago, yet still only half of it survives a full year. Or putting that another way, the equivalent of 100% of 1990 emissions are now absorbed each year. This ratio of 50% airborne fraction has been true for over 100 years while emissions have been forever increasing.
Comparison of Carbon emissions and that retained in the atmosphere. Increases in Emissions consistently remain about twice the levels of increases in CO2. Flat periods occur when growth stalls.
Natural carbon sinks are increasing dynamically to offset our emissions. The problem is that they don’t have enough time to catch up with the ever increasing rate of emission. The best they can do in a single year is offset half of them. Why is that the case and what does it really mean?
There is a ‘concentration effect’ acting on ocean sinks due to the increasing partial pressure of CO2 in the atmosphere. Similarly land biota (plants and soil) react to increased partial pressure by absorbing more CO2. While we are still increasing emissions then CO2 levels in the atmosphere will always continue to rise. If instead we can stabilise emissions at some fixed number of Gtons/year then CO2 levels would also stabilise, albeit at a slightly higher level than now and in the future. This is because the sinks will finally be able to catch up to balance our CO2 source. The atmospheric faction will decay to zero.
Dissolution/Absorption of CO2 at Ocean surfaces.
In stability there is a balance of CO2 Partial Pressures between the surface of the ocean and the atmosphere. At any given temperature the exchange of carbon dioxide molecules between the atmosphere and the ocean surface always reaches an equilibrium. This equilibrium is controlled by the partial pressure of CO2 in the atmosphere equalising to the partial pressure of CO2 in the surface of the ocean. Then the number of carbon dioxide molecules that escape from the sea surface is balanced by the number that enter the sea from the atmosphere.
If the temperature of the ocean rises then the kinetic energy of the carbon dioxide molecules in the seawater increases and more carbon dioxide molecules will leave the ocean than would enter the ocean. This continues until the partial pressure of carbon dioxide in the atmosphere increases to balance the new pressure at the sea surface.
If instead the ocean were to cool then the reverse of the above would happen, and CO2 levels would fall. Consequently carbon dioxide is more soluble in cold water than in warm water. This is Henry’s law. One consequence of this effect is that the oceans “inhale” carbon dioxide from the atmosphere into cold sea surfaces at high latitudes and “exhale” it from warm sea surfaces at low latitudes.
Increasing the carbon dioxide concentration of the atmosphere therefore causes the oceans to take up (inhale) more carbon dioxide. Because the oceans surface layer mixes slowly with the deep ocean (hundreds of years) the increased carbon dioxide content of the surface ocean will be mixed very slowly into much larger carbon reservoir of the deep ocean. The rate of our adding carbon dioxide to the atmosphere has been too fast for the deep ocean yet to be a significant reservoir. So as the carbon dioxide content of the atmosphere rises, so too does the concentration in the ocean surface, causing short term acidification of surface waters. If atmospheric carbon dioxide remains constant then a ph balance throughout the ocean volume can be reached.
I argue that by simply stabilising emissions, we can halt global warming because CO2 levels will stabilise as the sinks will then be able reach equilibrium with emissions. Clearly the lower total ‘stable’ emissions become then the cooler the planet will be, but even if we only managed to stabilise emissions at current values, then the net warming will still be <2C and CO2 levels will soon stop rising and stabilise at <440 ppm.
Atmospheric CO2 levels must always reach an equilibrium as the natural carbon sinks catch up to balance emissions. For the last 40 years about half of man-made emissions have been absorbed mainly into the oceans, but also into soils and biota. The reason why CO2 levels have been continuously increasing since 1970 is that we have been increasingemissions each year, so the sinks never get a chance to catch up. Sinks will rather quickly balance emissions and CO2 levels will stop rising once emissions stop increasing. This fact is obvious because run-away CO2 levels have never happened before in the earth’s long history. Such a balancing mechanism has always stabilised atmospheric CO2 over billions of years during intense periods of extreme volcanic activity, ocean spreading and periodic tectonic mountain building. Fossil fuels in this context are an insignificant fraction when compared to the buried carbon contained in sedimentary rocks.
Simple Model
CO2 levels rise when the rate of change of the sources – S exceeds the rate of change of sinks – K. Without human emissions then S = K, averaged over one year. However with ever increasing human emissions the situation becomes dynamic
If C is the yearly value of CO2, S the net sources of CO2 and K the net sinks, then at time t.
However it has been measured for at least the last 60 years that
Now let’s assume that the world manages to stabilise annual emissions at current rates of 34 Gtons CO2/year indefinitely. CO2 sinks currently absorb roughly half of that figure – 17 Gtons and have been increasing proportional to the increase in partial pressure of CO2 in the atmosphere – currently that of 400ppm. Stabilising emissions now results in a decreasing fractional uptake by carbon sinks as the partial pressure imbalance between the surface and atmosphere begins to fall. The simplest assumption is that the sink increase depends only on the partial pressure difference for a given year. Therefore if this pressure difference is reduced by half in one year then the next year it will be reduced by one quarter, then one eighth and so on. The same argument applies for the case that it takes longer to reduce pressure difference by a half.
Year 1: 50% Year 2: 25% Year 3: 12.5% Year 4: 6.25% etc. which is simply equal to the infinite sum
So in this simplest of models, CO2 levels in the atmosphere will taper off after just ~10 years to reach a new long term value equivalent to adding an additional one year of emissions 34 Gtons of CO2 to the atmosphere. The atmosphere currently contains 3.13 x 10^12 tons of CO2 so the net increase at equilibrium would in this simple model be just 1%. Therefore for the years following 2016 the resultant CO2 curve would look like the red curve below. If instead it takes say 4 years for the sinks to increase by ![]() then we get the blue curve. In this case it would take 30 years for CO2 levels to to stabilise and the increase would be 5 times larger.
then we get the blue curve. In this case it would take 30 years for CO2 levels to to stabilise and the increase would be 5 times larger.
CO2 stabilisation curves for different time constants. The red curve assumes sinks match half the imbalance in 1 year while the blue matches it in 4 years.
Currently there is also a good chance that the world will achieve a fixed level of annual emissions, but there is no chance that it will meet an impossible target of zero emissions this century. This does mean that CO2 levels will remain at ~410 ppm indefinitely, which is far higher than a planet without human beings, but it buys us time to replace fossil fuels with say new nuclear energy. If I am right then CO2 levels will begin to level off within the next 10-20 years. This would also save trillions of dollars by trying too soon now to replace all fossil fuels, and then probably failing.
Reducing emissions in the future will slowly cause such ‘stable’ CO2 levels to fall. In the long term we will have to develop non-carbon energy sources anyway, most likely nuclear, since fossil fuels must run short. However using remaining fossil fuels to control CO2 levels may one day have another advantage. It could mean that we can eventually use ‘enhanced global warming’ as a thermostat, thereby avoiding another devastating ice age otherwise due to begin within the next 5000 years.
Discover more from Watts Up With That?
Subscribe to get the latest posts sent to your email.





Once again I don’t do math all that well so let me get this straight. The atmosphere has 400ppm of CO2.
There is 200GT of the stuff added annually which goes away because the earth eats it one way or another.
Of the 200GT of CO2 put in every year maybe 5 to 10 of it is from human activity. Therefore of the 400ppm
lets call it 5 to10 ppm is from us and of that 5ppm(simplifying) only 2ppm (lots of uncertainty) remains annually to increase the total. After all the mathematical minutia.I think Alfred E. Newman has it right – what, me worry. AGW is total BS, enough said.
Why would you abet the know-nothings [to] create a true ecological catastrophe as the Plants eat out all atmospheric CO2 and then die from lack of CO2 and CO2 starvation?
Besides America produces less than ZERO net atmospheric CO2, as our useful bio- remediation provided by our farmers, already sequestrates more than North America produces. It already sequestrates a goodly amount that China and Japan create?
Under the IPCC toothless “treaties” China and India meets their obligation which is do NOTHING until 2035, and then think about maybe [consider] restricting its CO2 emissions! Lets promise to do the same !!!!
It is past time to Declare Victory in our 43 year war on Pollution and turn to a pure maintenance Policy of keeping our now clean Air and Water, clean.
All sinks saturates eventually. After all, the Earth is only finite, right? Finite sinks have to saturate sooner or later.
All sinks have to be smaller than the Earth and therefore have to be finite as well.
The question is not whether the sink saturate or not, they do. The question is whether the time to saturate is so long that the saturation may be irrelevant or not.
To the problem at hand; we know that the oceans and the biosphere are the main sinks. The oceans holds fifty times carbon than the atmosphere and will therefore not be saturated for some time, but the amount of carbon in the biosphere is comparable to the amount in the atmosphere. This means that the biosphere will saturate quite quickly.
/Jan
The concept of saturation is easily misconstrued. A growing organic biosphere will always be able to absorb more CO2 in the same way that an adult can eat more food than a baby. Thus it is better to consider rates of uptake, rather than imagined ultimate limits.
Now one could argue that an adult will “saturate” with food because they will reach a limit at which the adult cannot grow any more. This postulate is debatable in humans, but defensible as a way of illustrating a point. However, there is no evidence that the biosphere is currently behaving anything like this with regard to carbon dioxide, nor good reason to argue that it will at any clearly defined point in time. The rate of uptake of CO2 from the atmosphere is still rising commensurately with the rise in atmospheric CO2. Thus there is no evidence of sinks “saturating”, quickly or slowly, whatever you may suggest.
Thank Michael, I think this is a good parable to the carbon uptake in the biosphere. A human body will grow in weight as long as the uptake of carbon is larger than the rate of loss though respiration. The body can become huge, but not infinite.
Similar with the biosphere; the living biosphere will grow as long as the carbon uptake is larger than the loss though the rate of respiration in all living organisms. Exactly how big it can become is hard to say, but obviously there is a space limit for living organisms on the surface of the Earth.
This also holds for the carbon in dead plants and animals. We see no unlimited deposits of dead organic material around. The carbon is returned to the atmosphere when the organisms decay, and almost all is retuned within a few years.
/Jan
In the Carboniferous, CO2 levels fell from 4,131 ppm to 359 ppm over 60 million years due to the vigorous vegetation of the time. That is a sink of 8,034 billion tons Carbon over that time.
Between 32 to 24 million years ago, CO2 fell from 1,200 ppm to 280 ppm, most likely to the evolution of C4 grasses which now allowed plants to grow in all the dry and forest floor places where no plants could grow before. This increased the annual flows in the Carbon cycle and CO2 fell to 280 ppm for perhaps the very first time in Earth history. This is a sink of 2,000 tons Carbon over 8 million years.
Right now, the Oceans, plants and soils (don’t forget that one) are sinking about 4 billion tons Carbon per year and this continues to increase as the level of CO2 increases.
We are producing 9.5 billion tons Carbon per year today and the atmosphere has only increased by 265 billion tons Carbon since 1750.
The sinks have huge capacity over the long-run compared to our puny emissions but the annual sink rate is probably going to be smaller than our emissions for a long time out.
First, it needs to be proven that increased CO2 has caused any significant warming at all. I have yet to see anything more than a post-hoc argument for it.
Clive,
I have issues with some of the statements you make in your article.
1) “If atmospheric carbon dioxide remains constant then a ph [sic] balance throughout the ocean volume can be reached.”
I doubt that this statement is true. There is a constant rain of organic debris through the water column that oxidizes as it falls, producing CO2; this increases the pH such that up-welling water has a low pH compared to surface water. The deep water can probably take in considerably more CO2, but I doubt that an equilibrium will be reached unless there is a substantial increase in surface pH. That is true partly because CO2 is more soluble in cold water under high pressure. Therefore, the surface conditions are not as conducive to high pH as are deep water conditions.
2) “This fact is obvious because run-away CO2 levels have never happened before in the earth’s long history.”
Paleoclimatologists claim that there have been spikes in CO2 an order of magnitude greater than what is predicted for the worst-case scenario of anthropogenic CO2 emissions.
3) You claim that the rate of change of sequestration is approximately 1/2 the rate of change of emissions. What evidence do you have that the constant of proportionality (0.46) is actually constant? Might it actually change with the rate of change of emissions or the partial pressure? I would expect that as surface water approaches saturation with CO2 that the constant of proportionality would decline, perhaps even to zero.
4) “This does mean that CO2 levels will remain at ~410 ppm indefinitely, which is far higher than a planet without human beings,…”
As I commented in 2) above, paleoclimatologists dispute this claim. Indeed, high CO2 levels have persisted for geologically long periods of time, calling into question your prediction of rapid stabilization.
Clyde,
1) You’re right. The statement is incorrect. What I should have said is that ever accelerating rate of CO2 emissions is causing a change in ph in surface layers. This will stabilise once emissions are held constant and CO2 levels stabilise.
2) Yes there have been varying levels of CO2 in the atmosphere through different epochs. What has never happened though is a run away greenhouse effect caused by ever increasing CO2 levels. The only spike in the data is the PETM event which released more CO2 than that contained in all known reserves of fossil fuels. This spike decayed back to normal within ~ 100,000 years.
3) Nick Stokes has a proof that the exponential growth in emissions since 1930 has forced the airborne fraction to be a constant value. However this will change gradually to 100% once emissions stabilise. I have been scratching my head as to why the current value is ~0.5 I have no asnwer as yet.
4) By that statement I really meant that 410ppm is larger than 280ppm which presumably would be the current value if we were still in the stone age. Of course over geological time scales CO2 will always vary. For example it will fall as low as 190ppm during the next ice age.
Clive,
With respect to items 2) and 4), if you go to the illustration in the comment by Bartemis December 16, 2016 at 10:14 am, or the following one by LarryD, it is hard to even find the PETM ‘spike.’ Yes, the fact that Earth didn’t acquire a climate like that of Venus argues against the possibility of any kind of irreversible “Tipping Point.” The phrase is yet another scare tactic to try to get political action on what isn’t settled science. It isn’t even clear why there was a CO2 increase during the PETM. The Oligocene, which followed the Eocene, had unusually high volcanism, releasing CO2, yet the climate cooled.
I would dispute that in the next few decades that global emissions can be stabilized at 30 GtCO2e. Currently they are about 55 GtCO2e and rising by 1-2% a year. The reason that they cannot be stabilized is that developing countries as the 1992 Rio Declaration recognized
This is fair enough to any reasonable person. However, in the period 1990-2012 global GHG emissions increased by just over 40%. Developing countries collectively accounted for all of this growth and by 2012 these developing countries accounted for nearly two-thirds of global emissions at 34.5 GtCO2e. This is slightly more than the level of global CO2 emissions Clive Best believes GHG levels will be stabilized.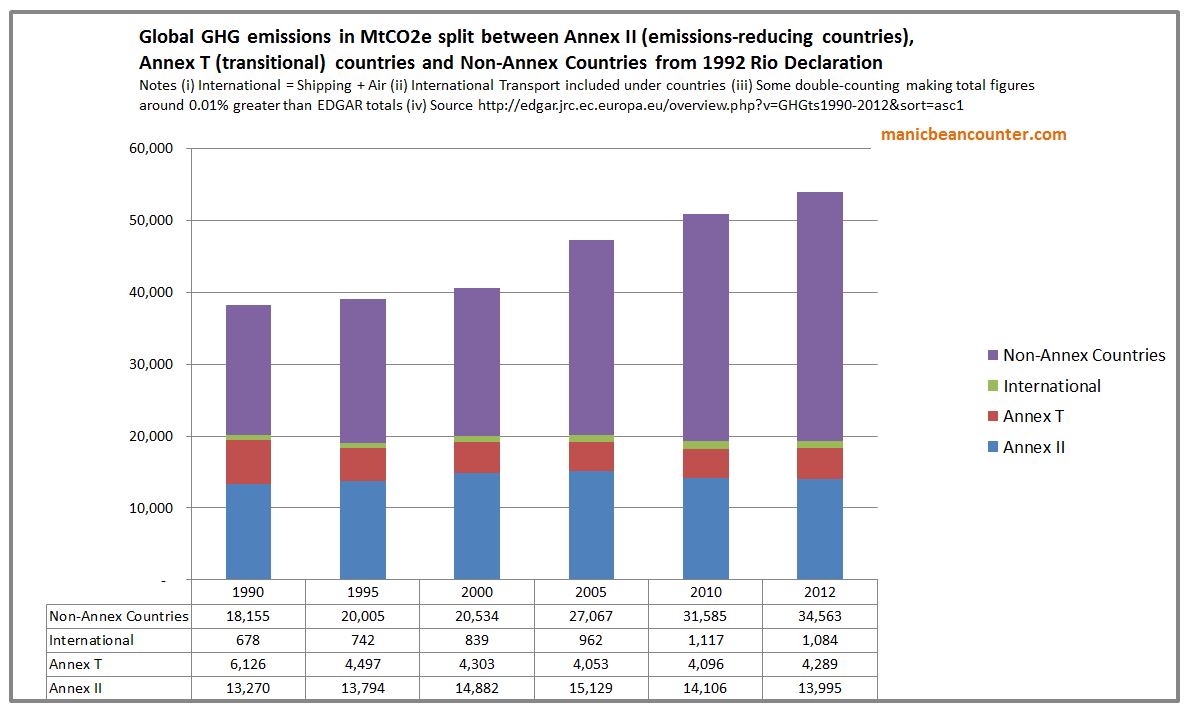 ?w=900
?w=900
There is a distinction to be made here. Following Robin Guenier’s notes of a year ago I looked at total GHG emissions, whilst Clive Best concentrates on CO2 emissions – two-thirds of the total. The near-saturation of Methane (CH4) means that stablization is achievable in the medium term if the developed countries make massive and fast emissions reductions. Using the policy proposals put forward for COP21 Paris 12 months ago this seems unlikely. But in the longer term the non-policy developing countries with >80% of the global population will, by their economic development, cause global CO2 emissions to rise well beyond 30 GtCO2e even if we assume that from 01/01/2017 emissions from developed countries are zero. This can be conclusion can easily be derived the historical trends in my recent post.
According to the palio record 150 million years ago the CO2 content of the atmosphere was 2,500ppm.
The planet was lush (very green) and the critters were “yuge”. What’s not to like?
Best concludes: “there is also a good chance that the world will achieve a fixed level of annual emissions, ….If I am right then CO2 levels will begin to level off within the next 10-20 years.”
I may misunderstand Best’s argument, BUT it seems to ignore simple chemical-physical equilibrium.
IF an additional 2 ppm of CO2 is added to the atmosphere each year, and IF half of that is removed yearly into the oceans, plants, etc., then 1 ppm is added each year to the atmosphere. This is the constant increase rate Best favors. That will continue to increase atmospheric CO2, and warming will increase in proportion to whatever the CO2 climate sensitivity happens to be. Warming will not stop simple because the atmospheric addition rate is constant. Considerations of equilibrium CO2 climate sensitivity versus equilibrium CO2 sensitivity does not change this.
No the argument is that by holding emissions constant the sinks will begin to increase the fraction of emissions they remove until eventually they remove all of it. How long this takes will depends on the ‘half life’ of the sinks as they act to equalise CO2 partial pressure in Ocean, Soils and Biosphere with the atmosphere. A half life of just 1 year would achieve this in 10 years, whereas a half life of 4 years would achieve this in 30 years.
Thereafter CO2 levels stabilise at say 440ppm and reduce slowly as and when we cut back on emissions.
If anyone is interested here is post on my blog explaining why the increase in atmospheric CO2 is probably natural and temperature-induced (updated from a 2013 post): https://chipstero7.blogspot.co.uk/2016/12/the-revelle-factor-vs-henrys-law.html
Chipstero7,
Where you write in your blog:
Probably the most incredible thing about the Revelle Factor is that it implies that the world’s oceans are absorbing naturogenic CO2 molecules at a vastly greater rate than anthropogenic ones preferentially.
I have the feeling that you don’t have understood what the Revelle factor implies: It implies that for natural and human releases alike, any increase in the atmosphere is met with a an increase in the ocean surface of about 10% of the atmospheric change. That still is 10 times the amount that gets absorbed by fresh water at the same temperature for the same change in the atmosphere.
The Revelle factor and Henry’s law work perfectly together: All what Henry’s law says is that for a 100% change in the atmosphere, there is a 100% change in what gets absorbed by the oceams. But that only counts for CO2 as gas in solution, not for C in the form of bicarbonates and carbonates, as these give zero CO2 pressure in the surface waters.
A 100% change in CO2 pressure in the atmosphere will give a 100% change in free CO2 in solution for fresh and seawater alike, but as in fresh water 99% of all C is free CO2, there it stops. In seawater, free CO2 is only 1% of all inorganic carbon species and as that doubles to 2%, the reaction chain increases also bicarbonates and carbonates, leading to a much larger uptake by seawater than by fresh water for the same change in the atmosphere. That is the Revelle factor…
Further, you are still using the arguments of the late Dr. Jaworowski: completely wrong on several points and ciompletely outdated for other points. See:
http://www.ferdinand-engelbeen.be/klimaat/jaworowski.html
No Ferdinand, it is you who does not understand and have not taken the time to seriously consider my comment. I think that is because your mind has rejected it before you have had a chance to assimilate it and accommodate it in your thinking. In other words, you have put up a mental block to it – a wall of denial that prevents it from reaching your intelligence and being processed by it. The presence of the block in your mind is clear to me because you have made it explicit in saying: “The Revelle factor and Henry’s law work perfectly together” and that “It implies that for natural and human releases alike any increase in the atmosphere is met with an increase in the ocean surface of about 10% of the atmospheric change”. When you have embraced the scientific method (and basic logic) then we will be able to discuss the science. But until you have done that I have no interest in going round in circles with you ad nauseam, as Bart does.
chipstero7,
My background is chemistry and later process automation but I am interested in all kinds of science. I do look at facts and simply accept them as base of any science, until proven wrong.
Henry’s law is for the ratio between CO2 as gas in the atmosphere and CO2 as gas in solution. A 100% change of CO2 in the atmosphere gives a 100% change of free CO2 in water. No matter if that is fresh water or seawater. Nothing to do with the Revelle factor and completely independent of that factor.
In fresh water almost all inorganic carbon present is free CO2, thus there the solubility stops.
In seawater free CO2 is only 1% of all inorganic carbon present,the rest are bicarbonates and carbonates. These three species can be transferred into each other in either direction: if the CO2 pressure in the atmosphere changes (thus free CO2 in the ocean surface changes) or if the pH of the water changes.
With a 100% increase in the atmosphere, the amount of free CO2 in the ocean surface doubles, but the other species (bicarbonates and carbonates) don’t double, as the chain reaction with more free CO2 gives also more H+, thus the pH lowers, and that pushes the reaction chain back towards free CO2.
At the new equilibrium, there still is 100% more free CO2, but the ultimate increase in all C species together is ~10% of the change in the atmosphere. That is what the Revelle factor says.
If you have a different explanation of what the Revelle factor means, please show me the chemistry…
That is not only theory. It is actually measured in several places over time, here for Bermuda which has the longest series. See Fig. 5:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf
CO2 in the atmosphere increased with ~15% in the period 1984-2012.
DIC (total dissolved inorganic carbon) increased with ~1.7% in the same period…
When you have embraced the scientific method (and basic logic) then we will be able to discuss the science.
Wow, what do you know about ocean chemistry?
“No it doesn’t, it applies only to the surfac”I do look at facts and simply accept them as base of any science, until proven wrong”.
I disagree. I think you selectively gather evidence to support your assertion that the increase in CO2 is man-made and do you best to dismiss all evidence suggesting otherwise. I find it hard to believe that you have a background in chemistry and I am even more amazed that people here still regard you as a top-expert on this issue. It baffles my mind. Truly, it does. There is so much confusion surrounding the Revelle Factor that I groan inwardly whenever I see it come up in public discussion. Everyone who has heard about it seems to be an authority on it yet most who profess to understand it don’t and unwittingly peddle illusions about it that just add to the general confusion. The reason why the Revelle Factor contradicts Henry’s law is explained in the article, and also by Segalstad (1998). But I shall explain it briefly here again, just so you can respond by saying “Sorry, you misunderstand”. The Revelle Factor sets a fixed equilibrium partitioning ratio for CO2 between air and water of around 1:10 respectively at the current oceanic DIC relationship, meaning the surface-ocean can only absorb around 10% of our emissions at equilibrium (according to the IPCC the ocean is currently absorbing around 30% of our emissions (2.2Gts) and this is because 1.6Gt of anthropogenic CO2 is diffused to the deep-oceans every year, which is not in immediate equilibrium with atmospheric CO2. This diffused CO2 essentially acts to free-up space in the surface-ocean. Without it, the oceans would only be absorbing 0.6Gts. The Revelle Factor applies at equilibrium and because it is chemically-generated it should apply to any water, not just the oceans). It suggests that the reason oceanic water cannot efficiently absorb anthropogenic CO2 is due to the fact that CO2(g) exists in equilibrium with CO2(aq) which only comprises a small percentage of total oceanic DIC. This means that as the partial pressure of CO2 increases, CO2(aq) will decrease (relative to CO32 and HCO3) and the oceans’ ability to absorb atmospheric CO2 will diminish (albeit only down to an alkalinity of about 7.5). However the solubility of CO2 is unaffected by changes to the relative concentrations of DIC, as the Revelle Factor implies. If this were the case then Henry’s coefficient for CO2 would change as the partial pressure of CO2 changed, and it does not. This is explained in more detail in the article. The solubility of CO2 is unaffected by changes to the relative concentrations of CO2, as can be appreciated by a Bjerrum plot, where the relative concentrations of DIC change in proportion to each other, leading to net-change in total DIC, irrespective of pH, and this is what gives the plot its characteristic mirror-image appearance. As the Handbook of Chemistry says: “Solubilities for gases which react with water, namely ozone, nitrogen, oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide, and sulfur dioxide, are recorded as bulk solubilities; i.e. all chemical species of the gas and its reaction products with water are included”. Hence there is no such mechanism that constrains water from absorbing CO2 based on changes to the relative concentrations of DIC. It does not exist. Furthermore your pH “bubble-bomb” example (which you have regularly used as “proof” of the Revelle Factor) is not valid, as has been explained to you exhaustively by Jeff Glassman. But of course, you ignored it, and just pretended that he was the one who was misunderstanding everything.
The first thing that struck me about the math is this article was the assumed instantaneous response of the removal of CO2 to the increase in CO2. While this may be a good approximation for ocean systems, my understanding of the more permanent removal of CO2 is mostly through chemical reaction with erosion products (>2/3) and bio-remains (<1/3) such as ocean floor deposits. More likely there is a time delay factor to the rate response, which I think has been hinted at in the comments but not explicitly. There are probably several of these factors but they could be possibly approximated by a single term in the form of a function of the CO2 level at t – C where C is the effective time response. If this is found then the response to making the CO2 levels entering the atmosphere a constant could be any of three responses: 1) an exponential drop in the levels, 2) a decaying oscillation like a ringing bell, leveling out such as in the results above, or 3) a varying but ongoing oscillation such as observed in rabbit/coyote populations. The later could be around a constant, a decreasing or increasing average value. The exponential decrease is the one to be avoided as we don't want to over do it.
My thinking has been along this line for some time. Why was the CO2 level so low in the atmosphere in the recent past? Because the biosphere (and limestone production) scavenged it out of the system until it hit starvation levels. No matter what rate CO2 is added, as soon as that rate becomes constant, the biosphere will increase until CO2 is reduced back to starvation levels.
YOU WROTE:
“I will argue below that in order to stop global warming all we really have to do is simply stabilise CO2 emissions, not reduce them to zero!”
MY COMMENT:
Why would anyone in their right mind want to stop global warming?
After 150 years of warming, the climate in 2016 is wonderful !
Plants are growing faster, and the Earth is greening.
The warming measured in the weather satellite age (the only measurements accurate enough to discuss) is mainly at night in the northern half of the Northern Hemisphere — I can’t imagine the few people living in those cold climates are unhappy about slightly less cold nights !
In addition, there is no scientific proof that CO2 is more than a minor factor in determining the average temperature.
In the past 75 years alone, we have had three different relationships of CO2 and average temperature:
1940 to 1975:
CO2 up / Temp. down
1975 to early 2000s:
CO2 up / Temp up.
Early 2000s to 2015:
CO2 up / Temp. flat trend
Other evidence that CO2 is not very important:
— No warming of Antarctica in satellite age — which means the warming is not really global
— No tropical troposphere hot spot ever found
In summary, Mr. Best:
(1) By starting with, and apparently believing, the assumption that CO2 is causing all the warming, you demonstrate that you are not very bright on the subject of climate science, and should not be listened to.
(2) Even if it was true that CO2 since 1975 has controlled the average temperature (which it has never done in the past 4.5 billion years), you would have us believe that you are smarter than all the scientists supporting the IPCC, and have a much ‘easier’ way to stop global warming.
By implying that you know more than the IPCC, which is very unlikely, you again demonstrate you are not very bright on the subject of climate change, and should not be listened to.
This article is a complete waste of bandwidth.
PS: I am in a good mood today, in case you thought otherwise
I forgot to mention almost no one will know what “a hiding to nothing” means, so it should not be used as a title of an article, or anywhere else, if lear communication is your goal.
The writing style is also tedious and hard to follow.
It should go without saying that I favor more CO2 in the air to further green the Earth.
If that additional CO2 caused warming too, that would be even better news !
I have observed the climate history in the past 150 years, and even if I assume ALL the warming was caused by man made CO2 (a more radical position than the IPCC), then I would still say: GIVE US MORE OF THAT !
I wasn’t going to comment on your closing remarks, but I changed my mind:
YOU WROTE:
“Using remaining fossil fuels to control CO2 levels may one day have another advantage. It could mean that we can eventually use ‘enhanced global warming’ as a thermostat, thereby avoiding another devastating ice age otherwise due to begin within the next 5000 years.”
MY COMMENT:
That is a fantasyland statement no more useful than the fantasyland claim that manmade CO2 will cause runaway warming.
You must have an advanced degree in something because you learned some math and science — and both have replaced common sense.
No one knows when the next peak glaciation will happen.
And no one knows when the 1950 modern warming will end and a cooling trend will begin.
A multi-hundred year global cooling trend could have already started – no one knows yet.
The flat average temperature trend between the 1998 El Nino and 2015/ 2016 El Nino temperature peaks is very suspicious, given how much CO2 was added to the atmosphere between those natural climate events.
That’s 1850 Modern Warming, not 1950 modern warming
IMO, based upon prior warm periods during the Holocene, it’s too soon for the Modern WP to end. But who knows?
On average, it’s close to a millennium between temperature peaks and troughs, or about 500 years between a peak and a trough. For example, peak heat of the Medieval WP occurred around AD 1200 and the low of the LIA cold period shortly before 1700. The trough of the Dark Ages CP was circa 700. So, a rough estimate of the Modern WP peak would be near 2200.
How many centennial-scale cold periods remain before descent into the next glacial interval is anybody’s guess. Based upon its orbital mechanics, the Holocene could be a super interglacial, lasting longer than 20,000 years.
The airborne fraction is a merely an assumption based on a misuse of the mass-conservation law and it disregards Henry’s law. Until Clive (and the people making these sort of posts) decide to wake up one day and ever take into account Henry’s law (as it properly should be taken into account) and the fast equilibrium for CO2 (as evidenced by the bomb spike data) then their equations are utterly meaningless and have no relationship to reality. Read Segalstad 1998 (which is the only decent paper on this subject, together with Salby and Humlum), comprehend the evidence and arguments into your understanding, and then come back with something better.
chipstero7,
Sorry, I did and all three persons you name were wrong – be it for different reasons:
– Segalstad because he uses the residence time which is of zero interest to know what the decay rate is for an extra shot CO2 into the atmosphere. The residence tim for any CO2 molecule, whatever its origin, is about 5 years. The e-fold decay rate of an extra shot CO2, whatever its origin, above equilibrium is ~51 years. a factor 10.
– Humlum used the 14C decay rate, but didn’t take into account that besides (more or less) the same mass ratio 14C/12C does sink in the deep oceans, a much smaller mass ratio is upwelling from the deep: what sinks is the isotopic composition of today, what is upwelling is the composition of ~1000 years ago.
That makes that the decay rate of the 14C bomb spike is at least 3 times faster than for a 12CO2 spike…
– I don’t remember if Salby was wrong on the residence time – e-fold decay rate, but he was certainly wrong on several other points…
“The airborne fraction is a merely an assumption”
It’s not an assumption. It’s a measuremnt.
Leaving the science of CO2 sinks aside …. in order to keep atmospheric CO2 from human sources constant that means no economic growth in either the wealthy first world nations or the poor third world nations. In addition population growth is going to make that more difficult to accomplish. I suppose greater efficiencies in use of fossil fuels might help, but can the rate of increase in efficiencies keep up with the rate of population growth? In addition, “alternate sources of energy” have not been shown to be a viable alternative. So basically the argument is either 1) rich nations stay rich and poor nations stay poor or 2) rich nations become less rich so that their fuels can then be used by the poorer nations, and since poor nations are, by definition, poor, the wealthy nations will have to make gifts of their energy to the poor nations. Does anyone see option 2) happening? Is option 1) going to be a popular choice?
Clive Best writes “I accept that the simplistic ‘half life’ reduction model is incorrect, but I kind of knew that anyway. ”
https://andthentheresphysics.wordpress.com/2016/12/17/no-stabilising-emissions-will-not-stabilise-concentrations/#comment-89395
Clive Best writes “I didn’t claim it was correct. I just said it was the simplest model you could take”
https://andthentheresphysics.wordpress.com/2016/12/17/no-stabilising-emissions-will-not-stabilise-concentrations/#comment-89456
Drawing conclusions based on a model you know to be incorrect? Good to see skepticism at work ;o)
“The strange thing is that this airborne fraction hasn’t changed at all in 60 years, despite exponentially increasing human emissions.”
Ironically, the reason that the airborne fraction hasn’t changed much in 60 years is because of exponentially increasing human emissions. Drive a first order linear dynamical system with an exponential signal and the response will be exponential signal with the same time constant, so if you take the ratio of the two you get a constant. This is not exactly rocket science. Of course the carbon cycle (on short timescales) is only approximately described by a first order linear DE, and anthropogenic emissions are only rising approximately exponentially, but then again the airborne fraction is only approximately constant.
I quote:
I agree this is very likely “wrong”. Ocean chemistry and dynamics are indeed complex, so please read the responses above before dismissing the basic argument that fixed sources and sinks must stabilise.
You are also relying on an elegant mathematical result of Nick Stokes when you state.
However what you write makes no sense. Neither nature itself or the Berne model could be considered a ” first order linear dynamical system”.
If AF decreases as emissions stabilise the Fig 10 above will be proved wrong.
Clive Best wrote “I agree this is very likely “wrong”.”
There is no reason for the quotes around wrong, it is just wrong. Apparently you “kind of knew that anyway.” but carried on promulgating the idea anyway. Not very “skeptical”.
Clive Best wrote “Ocean chemistry and dynamics are indeed complex, so please read the responses above before dismissing the basic argument that fixed sources and sinks must stabilise.”
Of course atmospheric CO2 will stabilise, just not on the sort of timescale you are suggesting and at a much higher level. Please read the responses on your own blog and at ATTPs that explain why.
Clive Best wrote “You are also relying on an elegant mathematical result of Nick Stokes when you state.”
No, you will find the same result obtained in a different way in my journal paper (note date):
Gavin C. Cawley, On the atmospheric residence time of anthropogenically sourced carbon dioxide, Energy & Fuels, volume 25, number 11, pages 5503–5513, September 2011. (preprint).
You will find it in the section “Modelling the Ariborne Fraction” (page 22, and the result is mentioned in the abstract). Now I pointed this paper out to you here on your own blog, but your comment above suggests you didn’t actually bother to read it (despite requesting me to read the “responses above”, again not very “skeptical”). I rather doubt I am the first person to derive this result, as I said, it is hardly rocket science.
The simple model in my paper would suggest that stabilisation would happen on the timescale of 70 years or so, but the paper also has a section on the limitations of this sort of simplistic model (which is still more realistic than yours), which means that is not actually the case and in reality it will take much longer if emissions were stabilised.
Clive Best wrote “However what you write makes no sense. Neither nature itself or the Berne model could be considered a ” first order linear dynamical system””
LOL, I can see you didn’t bother reading the whole paragraph, which ended:
“Of course the carbon cycle (on short timescales) is only approximately described by a first order linear DE, and anthropogenic emissions are only rising approximately exponentially, but then again the airborne fraction is only approximately constant.” [emphasis mine].
Again pretty ironic given that you requested me to read the “responses above” when you clearly didn’t bother (or did and chose to ignore the parts that didn’t suit you, which is arguably worse).
“The Revelle factor is about 10, which means that the fractional change in atmospheric CO2 will be about 10 times bigger than the fractional change in DIC. What this tells you straight away is that you can’t change the amount of CO2 in the oceans without also change the amount in the atmosphere; stabilising emissions will not stabilise concentrations.”
This is where ATTP is full of thermally enhanced gas (aka hot air)… Yes, he gave the Revelle factor as 10 – good. He mentions DIC, and that the fractional change in DIC will be lower than the fractional change in CO2. BUT HE FAILS TO MENTIONS THE AMOUNT OF DIC. You see, DIC is about 37,000 Gt C, almost 60 times the amount of CO2 in the atmosphere. So the fractional change would not be 25% but closer to 80-85%.
But there is a reason it is only 25% and not 80% absorbed and that is that the whole ocean, including the deep ocean, is not in direct contact with the atmosphere; the process of moving CO2 to the deep ocean is a slow one. Only the ocean surface and surface waters absorb CO2, which then has both chemical (carbonates) and biological processes that absorb the CO2, convert it into other DIC forms, and then send it to the deep ocean. slowly. Or at least at a slower rate than we are adding CO2 today.
Which further means that even though we changed atmospheric CO2 from 300ppm to 400ppm, the process of adjusting the deep ocean is catching up. So ATTP fails to mention is that DIC IN THE DEEP OCEAN IS STILL CLOSE TO THE 290 PPM EQUIVALENT IN THE ATMOSPHERE. In other words, DIC is both huge AND far from the equilibrium it would be at were the
That’s why ATTP’s rejoinder is wrong. He sets 20% as the “lower limit”, but that is a lower limit ONLY AFTER REACHING EQUILIBRIUM. Before then, the oceans capacity to absorb is based on the CO2 disequilibrium and the limits of ocean-surface-air interface.
One merely has to ask: “How much CO2 has to go into the oceans to get to equilibrium?” And it is about 6 times the CO2 we’ve emitted thus far. In other words, we could continue to emit at current levels of CO2 into the air for about 200 years before hitting ‘equilibrium’ in the oceans; we would hit CO2 equilibrium well before then, in fact, in about the next 30-40 years. In the intervening 200 years, we would be at stabilized CO2 levels, more or less.
Don’t believe in
“Reducing emissions in the future will slowly cause such ‘stable’ CO2 levels to fall.”
____________________________________________
– Do want heated highways, no snow no ice.
– What’s wrong with.
But isn’t there a limit to the effect that carbon dioxide can have on average climate temperature, most of which has already been realized?
Reason is basic physics of absorption and emission of energy from “greenhouse gas” molecules and the overlap of spectra of carbon dioxide and the vapour of dihydrogen monoxide (the most plentiful greenhouse gas).
Then note that runaway warming did not occur during the Medieval Warm Period when Vikings farmed southwest Greenland – i.e. climate was stable at temperatures warmer than today.
So why do you want to bother even limiting CO2 emissions by humans?
As for an individual’s weight plateauing at a higher level, some obese individuals have gotten to 600 pounds or beyond. Bad analogy.
Actually, obesity and anorexia may be self-limiting through death. 😮