From the EPA might have something to say about that department…this press release from LNL suggests dumping tons of Calcium Bicarbonate as a byproduct of CO2 scrubbing into the oceans instead. Only one teeny little problem – most coal fired power plants (at least in the USA), aren’t anyway near the ocean.
Yeah, that’ll work. Ok I’ll give him a pass for saying it’s only applicable to plants near the ocean, but how many of those are there compared to the map above? Coal still rules.
| US Electric Power Industry Net Generation, 2009 |
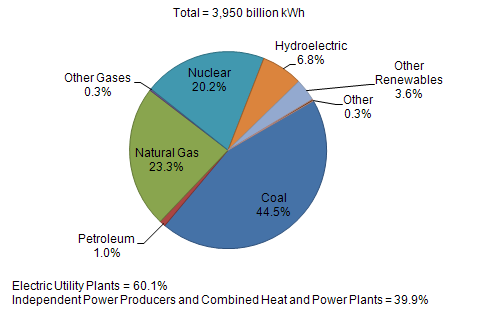 |
| Sources: U.S. Energy Information Administration, Form EIA-923, “Power Plant Operations Report.” |
================================================================
Speeding up Mother Nature’s very own CO2 mitigation process

LIVERMORE, Calif. — Using seawater and calcium to remove carbon dioxide (CO2) in a natural gas power plant’s flue stream, and then pumping the resulting calcium bicarbonate in the sea, could be beneficial to the oceans’ marine life.
Greg Rau, a senior scientist with the Institute of Marine Sciences at UC Santa Cruz and who also works in the Carbon Management Program at Lawrence Livermore National Laboratory, conducted a series of lab-scale experiments to find out if a seawater/mineral carbonate (limestone) gas scrubber would remove enough CO2 to be effective, and whether the resulting substance — dissolved calcium bicarbonate — could then be stored in the ocean where it might also benefit marine life.
In addition to global warming effects, when carbon dioxide is released into the atmosphere, a significant fraction is passively taken up by the ocean in a form that makes the ocean more acidic. This acidification has been shown to be harmful to marine life, especially corals and shellfish.
In his experiments, Rau found that the scrubber removed up to 97 percent of CO2 in a simulated flue gas stream, with a large fraction of the carbon ultimately converted to dissolved calcium bicarbonate.
At scale, the process would hydrate the carbon dioxide in power plant flue gas with water to produce a carbonic acid solution. This solution would react with limestone, neutralizing the carbon dioxide by converting it to calcium bicarbonate — and then would be released into the ocean. While this process occurs naturally (carbonate weathering), it is much less efficient, and is too slow paced to be effective.
“The experiment in effect mimics and speeds up nature’s own process,” said Rau. “Given enough time, carbonate mineral (limestone) weathering will naturally consume most anthropogenic CO2. Why not speed this up where it’s cost effective to do so?”
If the carbon dioxide reacted with crushed limestone and seawater, and the resulting solution was released to the ocean, this would not only sequester carbon from the atmosphere, but also would add ocean alkalinity that would help buffer and offset the effects of ongoing marine acidification. Again, this speeds up the natural CO2 consumption and buffering process offered by carbonate weathering.
Earlier research has shown that ocean acidification can cause exoskeletal components to decay, retard growth and reproduction, reduce activity and even kill marine life including coral reefs.
“This approach not only mitigates CO2, but also potentially treats the effects of ocean acidification,” Rau said. “Further research at larger scales and in more realistic settings is needed to prove these dual benefits.”
Rau said the process would be most applicable for CO2 mitigation at coastal, natural gas-fired power plants. Such plants frequently already use massive quantities of seawater for cooling, which could be cheaply reused for at least some of the CO2 mitigation process.
“This method allows a power plant to continue burning fossil fuel, but eliminates at least some of the carbon dioxide that is emitted, and in a way that in some locations should be less expensive and more environmentally friendly than other carbon dioxide sequestration methods,” he said.
The work, funded by the Energy Innovations Small Grant Program of the California Energy Commission and LLNL, appears in the journal Environmental Science & Technology.
More Information
“CO2 Mitigation via Capture and Chemical Conversion in Seawater,” Environmental Science & Technology
“Strengthening Our Understanding of Climate Change,” Science & Technology Review, December 2010
“Locked in Rock: Sequestering Carbon Dixoide Underground,” Science & Technology Review, May 2005
“The Siren Call of the Seas: Sequestering Carbon Dioxide,” Science & Technology Review, May 2004


This is a bad idea that is trying to solve a problem resulting from a bad idea. Nature efficiently sucks up the CO2. The half-life of CO2 in a power plant plum is a matter of hours. Clouds, trees, lakes, rivers, ponds, limestone, concrete, moist soil, etc, all soak up CO2. Power plant plumes of CO2 rapidly vanish into natural background levels. Very little CO2 coming from power plants in the eastern U. S. ever reaches the atlantic ocean. What little that gets that far will tend to equilibrate there.
Didn’t these guys ever watch Star Trek?
Anonymoose
That’s probably a good idea to make this scheme economical as long as you don’t over do it. That is raise the ph by more than half a point. It probably wouldn’t have a major negative impact on the rivers and might even be beneficial for some marginally acidic ecosystems.I can see the cost being way lower than some of the current ways of sequestration being discussed and already experimented with eg in Saskatchewan.
HaroldW, you might try to read the paper. Yes, Ca(HCO2)2 technically can be produced, and yes, it contains more CO2 than CaCO2. But the reaction is reversible, and the endproduct can be retained ONLY IN ISOLATION FROM ATMOSPHERE. And I quote the paper here. You can push more CO2 into lime, sure, but only if you retain high partial pressure. As soon as you release it, CO2 goes right back to the air.
Yes, the paper then starts going on a tangent how Ca(HCO2)2 can be utilized by marine animals. Which is all hunky-dandy, with only one tiny problem: they do it in the form of CaCO2, releasing excess CO2. Oops. Apparently, they do not mention it in the paper, I wonder why.
In any case, even IF there was a way of doing so (say, by utilization some other source of cations to tie up carbonates in insoluble form), the advisability of the process is highly debatable. Limestone accumulation irreversibly binds carbon in miles-thick depositions that already captured most of Earth carbon pool, and eventually will bind pretty much all of it. On geological scale of time, of course, but I do not see why hurry and speed it up.
Let’s start with a truism.
The farther one gets from Gillette, Wyoming the more expensive coal is.
The Pacific Ocean is 1,000 miles away and the Atlantic is 2,000 miles away.
There is no point is discussing coal fired electricity with carbon capture on either coast. The costs are just to high to be competitive.
Well, I posted above w/o reading the article, so my bad. The process chemically does eliminate the CO2-acidity issue.
It’s still a massive-scale “solution” to an imaginary problem.
Actually for the first few decades they wont even have to dump it into the rivers. There are thousands of farmers who buy limestone to de-acidify their soil I’m sure they would gladly replace that with an alternative if the price was low enough.Oh wait isn’t calcium carbonate the technical term for quicklime? That might cause a problem. The government outlawed its use for agriculture many years ago because bad people where using it to make their problems disappear. How ironic!
This is not a new idea. The USGS had something ( called AWL- Accelerated Weathering of Limestone ) out on this a couple of years ago but as some have said…distance from coastal areas is the main cost factor. The limestone is readily available and is some instances a waste product from limestone quarries. That’s where this push comes from, the USGS trying to utilize existing limestone quarry excess material. More here at:
http://crustal.usgs.gov/projects/CO2_sequestration/limestone2.html
Folks, we already have this process in place. It’s called a “scrubber” in the power plants. We grind limestone and dissolve it in water that’s cycled up to high hardness by the addition of limestone and all the flue gas nasties + evaporation. The goal is the production of calcium sulfite and calcium sulfate. However, this brings to mind the idea that I may need to check on the sodium, carbonate, and bicarbonate concentration in the recycle water. This may lead to some interesting thoughts and possibly the notion that we are already scrubbing CO2 at coal plants with scrubbers.
Then again, as it was alluded to earlier…. do you want less alkaline oceans, or acid rain? Sulfate cooling or CO2 warming?
I don’t see the problem with this idea.
the washing process would also remove actual pollutants, and if it is not too wildly expensive it could help fend off the waste of windmills and other junk energy.
CRS, Dr.P.H. says:
January 19, 2011 at 9:06 pm
Sorry, Greg, but it’s already a commercial process:
http://calera.com/
Producing construction materials such as aggregates for concrete is not a bad idea, actually…..much better than pumping the stuff deep underground!
However, if EPA declares coal ash as a hazardous material (likely), this plan will be all goofed up as the flyash carries over into the aggregate.
——–
The concrete industry would probably give the EPA hell, as fly ash is a very useful admixture for concrete.
Retrofitting the Existing Coal Fleet with Carbon Capture Technology
http://www.fossil.energy.gov/programs/powersystems/pollutioncontrols/Retrofitting_Existing_Plants.html
While carbon capture is relatively new to power generation, it is not an uncommon industrial practice. CO2 is routinely separated and captured as a useful by-product from industrial processes such as synthetic ammonia production, hydrogen production, and limestone calcination.
In order to achieve the IEP program goal of 90 percent CO2 capture at no more than a 35 percent increase in the cost of electricity, a number of technical challenges need to be addressed.
Say What?
“35 percent increase in the cost of electricity” and parasitic loss of 20 to 30 percent of the power generated — used to capture and compress the CO2.
It’s time again to remember:
CO2 follows temperature, not the other way. Open a coke and you?ll see it: The more you have it in your warm hand the more gas will go out when you open it.
CO2 is the transparent gas we all exhale (and Not SUV: That dark is SOOT=Carbon dust) and plants breath with delight, to give us back what they exhale instead= The Oxygen we breath in.
CO2 is a TRACE GAS in the atmosphere, it is the 0.038% of it.
There is no such a thing as Greenhouse Effect: greenhouse gases are gases IN a greenhouse, where heated gases are trapped and relatively isolated not to lose its heat so rapidly. If greenhouse effect were to be true, as Svante Arrhenius figured it out: CO2 like the window panes in a greenhouse, but the trouble is that those panes would be only 3.8 panes out of 10000, there would be 9996.2 HOLES.
See:
http://www.scribd.com/doc/28018819/Greenhouse-Niels-Bohr
The atmosphere, the air can not hold heat, its volumetric heat capacity, per cubic centimeter is 0.00192 joules, while water is 4.186, i.e., 3227 times.
This is the reason why people used hot water bottles to warm their feet and not hot air bottles
CO2 is a gas essential to life. All carbohydrates are made of it. The sugar you eat, the bread you have eaten in your breakfast this morning, even the pants and underwear you wear (these are made from 100% cotton, a polymer of glucose, are made of CO2. You didn’t know it, did you?)
You and I, we are made of CARBON and WATER. So…baby: No Carbon dioxide=No YOU. Do you love yourself?…then you must love CO2.
From Hoser on January 20, 2011 at 7:23 am:
I know, I know…
Read my comments long enough, you should realize my default setting is a smirk on my face as I write. Really, (C)AGW and related nonsense deserves to be laughed at. Thankfully it’s all self-contradicting and self-conflicting enough that it’s easy to find the humor.
Which is why I talk about FREE!! solar energy. ☺
what about the coral and ocean acidification???? Have they forgotten about that wild claim?
Problems with seawater, Part 2. (Sorry for the delay, life stepped in …)
2) Seawater fouls things up at several levels. Seawater is not beautiful, clean, clear and sparkling blue (except in the holiday ads).
Seawater contains large non-living things – like timber, plastic bottles, pieces of neting.
It also contains organic things like fish, seals, dolphins , kelp and other seaweed, shells, jellyfish, faeces (human and other).
In addition, there is abrasive sand, deteriorated plastics and other small solid material.
Last-but-one, there are the lavae of many things but notably molluscs. Just ask about zebra mussels and the fouling they cause. Now, “That won’t happen here – zebra mussels grow in frshwater”. True, but unfortunately there are mussels that grow in seawater and are a major problem in the UK – you gave us zebra mussels – we may export proper blue mussels back to you in tonnes. They grow really well in the ends of tubes where there is a fresh supply of water flowing through – until they block it and die leaving their shells in place.
Finally, there are chemicals in seawater which can cause fouling such as manganese.
Add all of this together and you have a high potential for fouling of the system. That means chemical dosing (like chlorination …), coarse and fine screening, continuous cleaning of the screens to prevent total blockage and plant failure etc. etc. etc.
Now all of this is soluble (i.e. capable of being solved) but every step adds a further problem and extra cost with greater risk of it all going pear-shaped (do you have that expression in the US?).
I used to be an industrial chemist supporting power stations in the UK. All but the smallest operated beside the sea. All had their own set of problems. All had to abide by strict environmental regulation. For example, if we took a single fish out of the screens we would have been prosecuted for acting as a fishing vessel without a quota). Chlorination was accepted but only if kept to the minimum that would work and only if produced by electrolysis of seawater which was particulalry prone to particulate and manganese fouling. And so it goes round and round.
Best of luck to anyone who thinks working with seawater is easy! Because it ain’t!!Especially on a 2000 MW power station!!!
Allow me to elaborate on my earlier grant proposal:
Pump the CO2 into the botanical biosphere through vertically oriented atmospheric CO2 gas emitters. Solar powered carbon fixing botanical units can then be employed to scavenge the carbon from the atmosphere by combining it with hydrogen to form long carbon hydrogen chains. The USDA in partnership with EPA can actually pay people to do this. The program will be called ‘Facilitated Atmospheric Recapture Mediated Environmental Restoration’, or F.A.R.M.E.R, utilizing botanical Specialist (BS) technicians, or F.A.R.M.E.R.S. The resulting carbon rich biomass can be used in a myriad of ways as a feed stock with many industrial applications. We could probably develop a market based exchange for this product that would eventually become self sustaining.
Every time that humans start playing God with nature, we usually screw things up royally. I leave it to those like Alan Bates to identify the risks and some of the unintended consequences.
Anthony Scalzi is on the right track. Calera indeed has a process that essentially makes CaCO3 (not the bicarbonate) out of the CO2 from a power plant to use as “green cement”. Another company called Skyonic
http://green.blorge.com/2010/06/skymine-turns-smokestack-co2-into-baking-soda/
has a similar process that makes sodium bicarbonate solid from CO2 emissions.
The chemistry in any of these processes involves making a source of alkalinity, NaOH in this case, and some calcium ions from seawater or brine. There is no burning off CO2 from limestone, which would make it silly from a carbon capture perspective. Is it economic? Time will tell. And good points about dumping anything in the ocean, even if it is already there and is occurring naturally.
Excuse me, my previous post should say “use”, not “sue”.
I recently heard that Germany had become an Uranium exporter due to its strict legislation imposed on exhausts from coal fired plants (as a consequence of the assumed forest dieback). Such materials certainly had to be scrubbed separately, before exhaust gases undergo any wet CO2 absorption, as it would likely accumulate in that phase.
Say Greg; mebbe I can help y’alls out with your problem there; that distance from the ocean thingy; I mean you’d have to use up tons of fule to truck that stuff to the ocean.
Try this on for size. Convert your calcium bicarbonate into CO2 gas, and just turn that loose in the atmosphere, and Mother Gaia, will move the whole kit and caboodle over to the ocean for you.
There see how easy that is.
So essentially US coasts will become saturated with CO2 because the bone head hippie believes the CO2 will just automagically be spread even on the seven seas.
How very brilliant these geo-engineer wannabes are. And to their capable hands the crazed climate hippies want to leave the future climate too.
Suddenly, the acidification of the oceans is of no concern.
Carbon Dioxide is the “Sarah Palin” trace atmospheric gas: libeled and slandered with no definitive proof of complicity.
Greenhouse effect? Why not the closed car effect?
You probably have observed that when your car is parked in an open area with direct sunlight you can feel the trap heat inside your car to the point where you can fry an egg. Same thing goes with the light and heat inside the greenhouse.
How about the limiting of heat transfer by convection, or lack of, in the greenhouse (closed car).
What atmospheric trace gas limits heat convection in the atmosphere other than water vapor in the form of clouds.
Note, to Algore: Anthropogenic Carbon Dioxide was not a co-conspirator in the recent shooting violence in Tucson, Arizona.
From Ed Scott on January 20, 2011 at 2:56 pm:
Correction: A. Carbon Dioxide is identified as an Un-indicted Co-conspirator, due to a lack of a conclusive link between A.C.D. and any demonstrated damages.
Note that A.C.D. has been known to be disguised and go by the alias Anthropogenic Methane. When doing so it is reportedly at least 12 times as dangerous. Some form of delusional mental state is suspected. We can only hope Algore does everything possible to avoid the release of A. Methane around innocent unsuspecting people.