From the EPA might have something to say about that department…this press release from LNL suggests dumping tons of Calcium Bicarbonate as a byproduct of CO2 scrubbing into the oceans instead. Only one teeny little problem – most coal fired power plants (at least in the USA), aren’t anyway near the ocean.
Yeah, that’ll work. Ok I’ll give him a pass for saying it’s only applicable to plants near the ocean, but how many of those are there compared to the map above? Coal still rules.
| US Electric Power Industry Net Generation, 2009 |
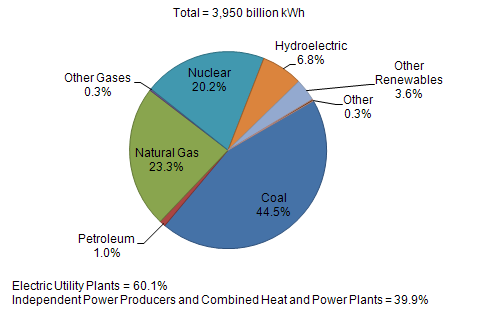 |
| Sources: U.S. Energy Information Administration, Form EIA-923, “Power Plant Operations Report.” |
================================================================
Speeding up Mother Nature’s very own CO2 mitigation process

LIVERMORE, Calif. — Using seawater and calcium to remove carbon dioxide (CO2) in a natural gas power plant’s flue stream, and then pumping the resulting calcium bicarbonate in the sea, could be beneficial to the oceans’ marine life.
Greg Rau, a senior scientist with the Institute of Marine Sciences at UC Santa Cruz and who also works in the Carbon Management Program at Lawrence Livermore National Laboratory, conducted a series of lab-scale experiments to find out if a seawater/mineral carbonate (limestone) gas scrubber would remove enough CO2 to be effective, and whether the resulting substance — dissolved calcium bicarbonate — could then be stored in the ocean where it might also benefit marine life.
In addition to global warming effects, when carbon dioxide is released into the atmosphere, a significant fraction is passively taken up by the ocean in a form that makes the ocean more acidic. This acidification has been shown to be harmful to marine life, especially corals and shellfish.
In his experiments, Rau found that the scrubber removed up to 97 percent of CO2 in a simulated flue gas stream, with a large fraction of the carbon ultimately converted to dissolved calcium bicarbonate.
At scale, the process would hydrate the carbon dioxide in power plant flue gas with water to produce a carbonic acid solution. This solution would react with limestone, neutralizing the carbon dioxide by converting it to calcium bicarbonate — and then would be released into the ocean. While this process occurs naturally (carbonate weathering), it is much less efficient, and is too slow paced to be effective.
“The experiment in effect mimics and speeds up nature’s own process,” said Rau. “Given enough time, carbonate mineral (limestone) weathering will naturally consume most anthropogenic CO2. Why not speed this up where it’s cost effective to do so?”
If the carbon dioxide reacted with crushed limestone and seawater, and the resulting solution was released to the ocean, this would not only sequester carbon from the atmosphere, but also would add ocean alkalinity that would help buffer and offset the effects of ongoing marine acidification. Again, this speeds up the natural CO2 consumption and buffering process offered by carbonate weathering.
Earlier research has shown that ocean acidification can cause exoskeletal components to decay, retard growth and reproduction, reduce activity and even kill marine life including coral reefs.
“This approach not only mitigates CO2, but also potentially treats the effects of ocean acidification,” Rau said. “Further research at larger scales and in more realistic settings is needed to prove these dual benefits.”
Rau said the process would be most applicable for CO2 mitigation at coastal, natural gas-fired power plants. Such plants frequently already use massive quantities of seawater for cooling, which could be cheaply reused for at least some of the CO2 mitigation process.
“This method allows a power plant to continue burning fossil fuel, but eliminates at least some of the carbon dioxide that is emitted, and in a way that in some locations should be less expensive and more environmentally friendly than other carbon dioxide sequestration methods,” he said.
The work, funded by the Energy Innovations Small Grant Program of the California Energy Commission and LLNL, appears in the journal Environmental Science & Technology.
More Information
“CO2 Mitigation via Capture and Chemical Conversion in Seawater,” Environmental Science & Technology
“Strengthening Our Understanding of Climate Change,” Science & Technology Review, December 2010
“Locked in Rock: Sequestering Carbon Dixoide Underground,” Science & Technology Review, May 2005
“The Siren Call of the Seas: Sequestering Carbon Dioxide,” Science & Technology Review, May 2004


Dave Kyffin says:
“The only way to absorb CO2 from a gas is to use lime (CaO or Ca(OH)2) which is produced by “burning” estone, i.e. driving off the CO2, like for making cement. Seems to me there’s some basic chemistry gone astray here.”
My thoughts exactly.
Presumably, the Chinese will produce vast quantities of lime (and CO2) and ship it to the US who will then soak up all their CO2 (at vast cost) and because China is an emerging market and needs to grow …. it’ll all be OK and help the planet.
If I remember going up from a ph value of 7 goes towards being caustic and going down, towards being acid. So you are more or less caustic or more or less acid.
Lazy Teenager:
“….Some fields have standardized on a common usage, some refer consistently to basic and some to acidic, to streamline communication and avoid confusion”….
and to those pedants amongst us, if you add acidic solution to an alkaline solution the latter either becomes less alkaline or dependant on the quantity/mole of the former an acidic solution. WRT seawater, less alkaline as it’s pH does not drop below 7.0. There is no streamlining of “communication” issues here. “streamlining communication” is what non-scientist scientists do to the sheeple. Sheeple are easily confused.
We need to build giant compressors and then ship the CO2 to the ocean in gigantic haulage trucks. It’s okay though, because the compressors and trucks can run on the hot air generated by cranky watermelons.
‘“The experiment in effect mimics and speeds up nature’s own process,” said Rau. “Given enough time, carbonate mineral (limestone) weathering will naturally consume most anthropogenic CO2. Why not speed this up where it’s cost effective to do so?”’
I would like to see the business case for this!
The costs would be interesting, but the monetary benefits, the profits to pay for it, would be even more interesting!
What would the criteria be to judge it “cost effective”?
I do this in the bath with methane quite often can I get a patent?
Spot on.
So, In ideal conditions, for every ton of coal used you need 8.33 tons of limestone to neutralize the CO2 produced during combustion.
Also in ideal conditions, that ton of coal could be converted into 2.5 tons of sugar by sugar canes or beets or whatever sugar producing plant you want to use.
One wonders what kind of chemistry and biology do these ecologists study.
There is a MUCH BETTER method already being worked on:
http://www.physorg.com/news135820173.html
With proper siting the proposed process can use FREE solar energy. Thus one major benefit is already noticeable. There are many remote places where solar power is abundantly available yet it isn’t used for electricity generation due to the problems of getting all that electricity to the proper markets (lack of transmission lines, losses in transmission). So use the FREE solar power to convert the limestone to calcium hydroxide, which is then dumped in the ocean. At the power plants, which are located where the electricity is needed, DO NOT bother them with the questionable “CO2 sequestration” methods like the potentially hazardous pumping of CO2 under the ground, which divert some of the power generated to do so thus leading to EVEN MORE fossil fuels being consumed to compensate.
For lovers of carbon offsets, this is FAR BETTER than the current wishy-washy schemes like the Chicago Climate Exchange (CCX) was running. Rather than buying carbon credits for essentially a promise that “somehow, somewhere, sometime” a project will generate an appropriate offset, this method offers conscientious buyers hard results. Release a ton of CO2 emissions, know that enough calcium hydroxide will be dumped to absorb a ton of CO2. Be certain that You Are Doing Your Part To Combat Global Warming.
There’s a website for it:
http://www.cquestrate.com/
It’s being developed as an open source project. Likely that will help keep those dirty capitalists from making obscene profits from a worthwhile project to Save The Planet, as they unfortunately do with wind and solar power.
If you can, stop on by, help them out. Just about everyone here can agree they do need help.
LazyTeenager says:
January 19, 2011 at 11:00 pm
And you slake limestone by firing it in a Lime Kiln, using Industrial Coke fuel.
This is the cute little circle the green doggie makes chasing his tail.
So, in order to satisfy LEPA Jackson, one must mine more coal, limestone and transport more product to the coal-fired plants.
Truly, I say to you, these people are idiots.
Some further points on the idea (I don’t think it has been covered yet but if it has, apologies) …
“Natural” seawater is nasty stuff in at least 2 ways:
1) It is surprisingly corrosive. Seawater makes many materials more susceptible to pitting, corrosion fatigue, stress corrosion cracking and many other forms, depending on the materials concerned. While materials resistant to seawater corrosion are available, they are expensive and must be chosen with care if sensible lifetimes are required. If not, you are into large scale inspection and maintenance programmes.
CO2 plant failures will presumably lead to outages for the coal fired plant and increase costs. A large coal fired plant can have outage costs of £1M per day – especially if outages occur in winter (for US, read “summer”) or whenever peak generation is needed. That is, assuming less efficient plant is available.
To ensure long term plant integrity is maintained, the plant will have to have built-in redundancy of key plant items. However, stagnant seawater in non-operational plant can also lead to crevice corrosion etc. etc.
All this is soluble but at an every-increasing cost.
(Point 2, next post)
Another loonatic geoengineering brainstorm, proving that the “climate science” community is completely insane.
That´s what the current Landscheidt minimum will do, by increasing CO2 solubility in colder sea waters. 🙂
“Just wait in your front door….and you´ll see the corpse of your enemy passing by” (Confucius)
Hey Mr.Raw!. Did you know that for “Calcium” to capture CO2 you need TO BURN LIMESTONE to turn it into “Burnt limestone”?:
CaCO3+ Heat (with “fossil fuels”)= CaO + CO2
in order to make your reversed process:
CaO (milk of burnt limestone)+ CO2 = CaCO3
So, you will be just “Pouring the empty into the void”…..Congratulations, wise indeed!
Didn’t President Bush suggest using lime pits to absorb CO2, if it ever became a problem. As I recall, his idea got shot down for raising the spectre of Geoengineering, and distracting everyone from the politically correct solution of taxing the economy back to the stone age.
How the world turns… 🙂
I have already suggested to our dear Department of Energy and Climate Change (don’t get me started) that we surround any new ‘clean’ coal-fired power stations with market gardens, so that the CO2 could be pumped straight into their polytunnels, like tomato growers do anyway all the time (to concentrations of 800-1000ppm).
No response, of course… Bit too logical, I expect…
An issue about reacting with limestone is the need to crush it to increase the surface area. Another approach would be to cliff and mountain-sized chunks of limestone or chalk. Instead of building CO2 pipelines, we could use, umm, wind energy to transport the CO2.
This way, we can build minehead coal power plants and let the wind transport the CO2 to the white cliffs of Dover, which I hear are dangerously tall. I submit this as the most cost effective solution.
Hilarious! Worry about ocean-acidification from to much CO2, then recommend pumping CO2 into the ocean!
Dave —
I gather that the process involves passes CO2-laden gas through water, producing carbonic acid
CO2 + H2O => H2CO3
Calcium carbonate plus carbonic acid becomes calcium bicarbonate
CaCO3 + H2CO3 => Ca(HCO3)2
A little hard to see in this form, sorry — all the numerals should be subscripts.
Let’s see, is the coal choking all the birds too? But wait, there aren’t any birds left up there due to the wind turbines killing 100,000 of them per year.
“Earlier research has shown that ocean acidification can cause exoskeletal components to decay, retard growth and reproduction, reduce activity and even kill marine life including coral reefs.” Really? Show me the proof.
There are now hundreds of studies of how increases in ocean acidity affect marine life. This isn’t rocket science. Any high school kid with a salt-water aquarium and a bottle of hydrochloric acid can perform a credible experiment. As experiments are replicable, warmists and skeptics can give them a go and they have. And what, on the whole, do they show?
http://www.co2science.org/subject/c/summaries/calcificationother.php
http://www.co2science.org/subject/o/acidcorals.php
http://www.co2science.org/subject/o/subject_o.php
No model studies here. Just plain old experimental science that has thus far failed to support the notion of catastrophic acidification of the oceans.
kadaka says –
With proper siting the proposed process can use FREE solar energy.
There is no such thing as free solar energy. Nor is there free wind energy. Renewable energy sources cost at least 3x as much as natural gas power plants. Since Texas became the leader in wind energy, their power rates increased 50% in about 3 years. They have Rick Perry and Houston-based Enron to thank for that.
Green energy, the great con.
Just ship it to the coats in diesel or coal-powered trains. Duh.
It seems to me finding a cure for a problem not proven is futile because the cure will never work because it has nothing to work on. We could never know for sure if it worked if nothing happened but the claim could be made that because it did not happen it worked. It is a win win situation and only costs a lot of tax dollars.
There has got to be some good in scientific research on CO2 but the case should be proven before we jump off the deep end and insure disaster.
As long as CO2 is considered a pollutant, expensive schemes like this will be proposed to dispose of it.
Considered as a valuable resource, CO2 scrubbed from a power plant could be mixed with chicken poo in water and exposed to a little bit of algae and a lot of sunlight. The mass of resulting algae can be removed from the water by filter feeders such as clams.
Eat the clams with a cream sauce over linguini and use the shells with the sequestered CO2 to sweeten the soil where chicken feed is grown.
Nature is well designed.
I think I hit upon a process that actually scrubs CO2 from the atmosphere and converts it to a fairly inert polysaccharide, C6H10O5, that can then be buried in the ground. If buried deep enough ( 20 meters or so) its decomposition almost stops. Best of all, it is something we can do right now!
The process begins with an organism called trees. They produce the substance in abundance from Atmospheric CO2. The polysaccaride can then be used to make a wide variety of useful products, such as containers. After the product is no longer useful, it can be buried and covered with dirt.