From the EPA might have something to say about that department…this press release from LNL suggests dumping tons of Calcium Bicarbonate as a byproduct of CO2 scrubbing into the oceans instead. Only one teeny little problem – most coal fired power plants (at least in the USA), aren’t anyway near the ocean.
Yeah, that’ll work. Ok I’ll give him a pass for saying it’s only applicable to plants near the ocean, but how many of those are there compared to the map above? Coal still rules.
| US Electric Power Industry Net Generation, 2009 |
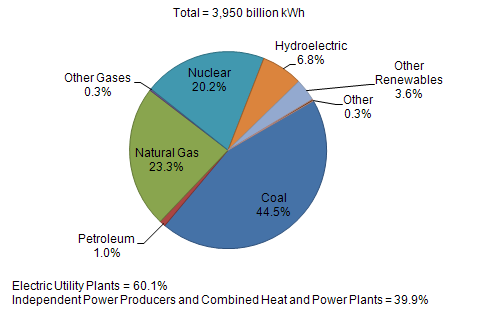 |
| Sources: U.S. Energy Information Administration, Form EIA-923, “Power Plant Operations Report.” |
================================================================
Speeding up Mother Nature’s very own CO2 mitigation process

LIVERMORE, Calif. — Using seawater and calcium to remove carbon dioxide (CO2) in a natural gas power plant’s flue stream, and then pumping the resulting calcium bicarbonate in the sea, could be beneficial to the oceans’ marine life.
Greg Rau, a senior scientist with the Institute of Marine Sciences at UC Santa Cruz and who also works in the Carbon Management Program at Lawrence Livermore National Laboratory, conducted a series of lab-scale experiments to find out if a seawater/mineral carbonate (limestone) gas scrubber would remove enough CO2 to be effective, and whether the resulting substance — dissolved calcium bicarbonate — could then be stored in the ocean where it might also benefit marine life.
In addition to global warming effects, when carbon dioxide is released into the atmosphere, a significant fraction is passively taken up by the ocean in a form that makes the ocean more acidic. This acidification has been shown to be harmful to marine life, especially corals and shellfish.
In his experiments, Rau found that the scrubber removed up to 97 percent of CO2 in a simulated flue gas stream, with a large fraction of the carbon ultimately converted to dissolved calcium bicarbonate.
At scale, the process would hydrate the carbon dioxide in power plant flue gas with water to produce a carbonic acid solution. This solution would react with limestone, neutralizing the carbon dioxide by converting it to calcium bicarbonate — and then would be released into the ocean. While this process occurs naturally (carbonate weathering), it is much less efficient, and is too slow paced to be effective.
“The experiment in effect mimics and speeds up nature’s own process,” said Rau. “Given enough time, carbonate mineral (limestone) weathering will naturally consume most anthropogenic CO2. Why not speed this up where it’s cost effective to do so?”
If the carbon dioxide reacted with crushed limestone and seawater, and the resulting solution was released to the ocean, this would not only sequester carbon from the atmosphere, but also would add ocean alkalinity that would help buffer and offset the effects of ongoing marine acidification. Again, this speeds up the natural CO2 consumption and buffering process offered by carbonate weathering.
Earlier research has shown that ocean acidification can cause exoskeletal components to decay, retard growth and reproduction, reduce activity and even kill marine life including coral reefs.
“This approach not only mitigates CO2, but also potentially treats the effects of ocean acidification,” Rau said. “Further research at larger scales and in more realistic settings is needed to prove these dual benefits.”
Rau said the process would be most applicable for CO2 mitigation at coastal, natural gas-fired power plants. Such plants frequently already use massive quantities of seawater for cooling, which could be cheaply reused for at least some of the CO2 mitigation process.
“This method allows a power plant to continue burning fossil fuel, but eliminates at least some of the carbon dioxide that is emitted, and in a way that in some locations should be less expensive and more environmentally friendly than other carbon dioxide sequestration methods,” he said.
The work, funded by the Energy Innovations Small Grant Program of the California Energy Commission and LLNL, appears in the journal Environmental Science & Technology.
More Information
“CO2 Mitigation via Capture and Chemical Conversion in Seawater,” Environmental Science & Technology
“Strengthening Our Understanding of Climate Change,” Science & Technology Review, December 2010
“Locked in Rock: Sequestering Carbon Dixoide Underground,” Science & Technology Review, May 2005
“The Siren Call of the Seas: Sequestering Carbon Dioxide,” Science & Technology Review, May 2004


Why not just plant more trees and shrubberies around the power plants? You can expand the zone of plantings to suit the absorption rate. I wonder what kind of plants are most hungry for CO2?
Hahahahahahaha
Gary Hladik says:
January 19, 2011 at 8:07 pm
So the solution to our CO2 “problem” is baking soda?
The idea sounds pretty half-baked to me.
——-
You are confusing sodium bicarbonate with calcium bicarbonate.
The basic equation is CaCO3 +H2O + CO2 => Ca++ +2HCO3- . Check it out; it balances.
Here’s the problem – they instituted controls on sulphur and now they want to put it back (at government expense of course) Can anyone predict what will happen 20 – 30 years down the road with this?
Dave Dodd says:
January 19, 2011 at 8:42 pm
OK, will someone please explain for this old duffer who must have slept through this part in HS Chemistry: How does making something LESS alkaline, make it “MORE” acidic? I thought we had to measure a pH of <7.0 (by definition) to become acidic in the first place. These people have a serious negative effect on my sleep cycles!
———-
There is a subtle distinction between being acid/acidic and being more or less acidic.
The former is represented numerically as a pH < 7.
The latter represents progress to a lower pH, irrespective if the current pH.
The same apples to alkaline/basic versus higher/ lower basicity.
This is common usage and reflects the practice of making something more acidic, aka adjusting the pH, by adding small amounts of acid to a solution.
Some fields have standardized on a common usage, some refer consistently to basic and some to acidic, to streamline communication and avoid confusion.
I know you guys have tried to concoct a propaganda story out if these subtleties' but any one who deals with these things professionally wonders what you're on about.
Sorry, Dave Kyffin, the reaction does happen.
BUT –
What amount of CO2 is emitted to the air by the mining process that gathers the CaCO3?
The limestone to bicarbonate reaction is water based and its equilibrium is moderated by temperature and pH. The proposal would be easier to evaluate if these operating parameters were known.
The process is feasible on paper, but it’s so obvious that it would be widespread by now if it was economic. However, if used on a large scale, it introduces the same objections (though in the reverse direction) as letting foreign CO2 from air be absorbed by ocean water. Either way, one is altering the inorganic chemistry conditions under which much shell material grows. (How natural organics influence the reaction does not seem to be well researched).
Dave Kyffin says:
January 19, 2011 at 9:25 pm
The only way to absorb CO2 from a gas is to use lime (CaO or Ca(OH)2) which is produced by “burning” estone, i.e. driving off the CO2, like for making cement. Seems to me there’s some basic chemistry gone
———-
Errr no. You were asleep in secondary school Chem class apparently.
It’s the good old CO2 plus limewater reaction. Every kid has seen it!!!!
You start off with a slaked lime solution.
Add CO2. The result is a precipitate of CaCO3.
Add more CO2. The precipitate dissolves to produce Ca(HCO3)2.
Maybe they should try in in an aquarium first to see what happens when the water is saturated in calcium carbonate. But that would not be representative since calcium carbonate’s solubility increases with decreasing temperature and increasing pressure. Those conditions are met in deep oceans. Also, you can be certain that it is not pure calcium carbonate but it will also contain lots of other toxic pollutants as well, like mercury for example.
I am dubious about the practicality of the suggestion because of the tonnages of CaCO3 involved. It is some multiple of the tonnages of coal burned.
That’s a lot if limestone!!!
Exercise for the student: calculate the mass ratio of coal to limestone.
As much Limestone as there is on land, the ocean has a lot more. That is a lot of surface area. Saying that limestone in the ocean won’t effectively react with the increased carbonic acid doesn’t make it so. If the carbonates are beneficial from the scrubbers than the limestone dissolving in the ocean is also beneficial and that was probably a mistake for them to admit that. How could acidification from CO2 cause something beneficial. They sound like a bunch of deniers to me. /sarc
It seems unlikely that there would be much change yet in any reaction rate with calcium carbonate in the ocean, if they even looked, since the amount pH change hasn’t been significant, and probably never would be considering the amount of buffers in the ocean even without the limestone reacting. I suppose, in their minds, as long as they get the money, it doesn’t matter if it is complete waste of resources. It still gives them the research money because they are essentially pushing the ocean acidification agenda. That alone would be enough for the left to fund that boondoggle if nobody stops them. They did it with ethanol on a massive scale with no consideration that it wasn’t actually helping even their imaginary problem of excess CO2 in the atmosphere. I wouldn’t put anything beyond them as far as wasteful government spending so long as it pushes an agenda or if it empowers politicians.
For every 12 grams/ounces/tons/etc. of coal burned, 100 grams/ounces/tons/etc. of limestone.
This concept would require hundreds of billions of dollars of expenditure in the piping industry, so I’m all for it. Since it would undoubtedly be government-funded, robbing Peter to pay Paul wouldn’t be a problem for me, as my name is Paul.
Piping Design Central Site Owner and Recipient of Big Oil Payoffs,
Paul
http://www.pipingdesign.com
This isn’t original, but I love it because it confuses Warmists. I’ve embellished it a bit.
CO2, ubiquitous waste material from combustion therefore cheap and plentiful. (not including the cost of capture).
CO2, a substance that has the remarkable ablity to ‘trap’, ‘absorb’, ‘amplify’ infra red energy.
We know this because a mere 400ppm has sufficient heat trapping capacity to warm the rest of the atmosphere ie the other 999 600 parts per million…and if this tiny amount manages to heat an open chaotic system with convection and radiation in play…just imagine the heat trapping/amplification capability of a 100 % pure body of CO2 at around atmospheric pressure I mean it must be awesome…right?
Well given these two ‘facts’, you’d have to ask yourself how long can it be before scientists and engineers develop a way to harness the heat amplifying/capturing properties of this abundant gas?
Surely if we could pipe it to desert/sunny areas for instance and feed it through solar arrays, wouldn’t it capture and amplify heat from the sun? This green heat could be used to drive steam turbines to generate electricity…couldn’t it?
It gets better…the energy generated through this CO2 solar array, is totally sustainable, in fact if we harness the power of enough CO2 eventually there should come a point where other forms of energy generation simply fall by the wayside because they have become un-economical! And we’re ‘scrubbing’ the atmosphere!
These people are usually right up on all the latest sustainable/renewable energy technology, so at this point I ask them if they have ever heard of such a scheme?
That’s usually when they get ‘the look’.
I don’t believe in man made global warming but I am interested in this proposal.
In Australia we are in danger of being saddled with a carbon tax. The greens are in alliance with labour.
I don’t think we can stop it. So lets make it as painless as possible.
1. CO2 is toxic in concentrations of over 10%. It is heavier than air, so it could get concentrated in a valley.
2. Current proposals to pump CO2 gas underground and store it under pressure could have lethal consequences. If the seals burst a lot of people could actually die from CO2 narcosis.
3. We could propose, in Australia a carbon tax on all new power plants which use coal or natural gas.
This tax would be levied on the power companies and re-couped in higher power bills. Pensioners and paupers could have the additional cost to the refunded by government.
It would be 2% in year one for coal fired power stations, increasing by 2% each year.
It would be 1% for gas fired power stations, increasing by 1% each year.
For coal fired stations, for every 3% of carbon dioxide recovered, 2% of tax will be returned.
For gas fired power stations, for every 1.5% of CO2 recovered, 1% of tax can be reclaimed.
For example a coal fired power station could be situated next to a mile deep abandoned gold mine. 500 metres of this mine will be filled with a solution of calcium hydroxide. The power could be transmitted (say) 1000 miles to the grid using high voltage DC transmission with the loss of only about 10% of the electricity.
The scheme will operate for 5 years and would then be reviewed.
Would it work?
You see the Australian government has to do something. The point is to make it as cheap and as painless (and as futile) as possible
The greenies will not be happy unless all power generated is used to scrub the flue gases.
To them that’s 100% efficiency.
Powering pumps to squirt CO2 into the ground, or the ocean is a crock.
People get paid of this behavior.
Power engineers in China (and the world over)are laughing their a$$es off.
The secret to a profitable scam is to first identify a gullible target. Enjoy….
“Fraudsters are targeting climate scientists with fake conferences in a bid to make cash and obtain details.”
http://www.bbc.co.uk/news/science-environment-12219472
As Dave Dardinger points out, the chemistry balances. The principle is well known and the reaction works both in nature and in the lab. Dr Rau hasn’t discovered anything new there. Bit of a cheek putting such elementary stuff behind a paywall.
The post doesn’t seem to discuss the key issue, which is the energy (i.e. fuel) cost of transporting and crushing the limestone. Without crushing the rock to increase the surface area the reaction rate will be too slow to be effective. Crushing rock is an energy intensive process. Seems a neat way of increasing fuel usage with no clear economic or environmental benefit. As a conservationist I’d rather economise on un-necessary use of fossil fuels. If that’s typical of Lawrence Livermore output, I’m unimpressed.
There is a mature technology to remove CO2 from a feedstock gas.
Depending on the source, raw natural gas has significant amounts of CO2 content that has to be removed before it can be piped and used as a commercial product which has to have <4% CO2.
Amines, membranes and moleculer sieves are all commercial systems:-
http://www.moleculargate.com/nitrogen-rejection-N2-removal/Coal-Bed-Methane-Upgrading.html
http://www.newpointgas.com/amine_treating.php
To achieve effective rates of removal usually requires high pressure and/or heating so is energy intensive. The removed CO2 is generally released to the atmosphere. in regeneration of the removal media. I am not sure that this CO2 is included in calculations of the carbon footprint of natural gas energy generation.
Since the real problem is the CO2 famine, just the baking of limestone to release all that poor, trapped gas is the only sensible course.
For a warmist this isn’t too bad of an idea. It would cost a lot and wouldn’t have any benefit at all, but it wouldn’t actually harm anything. So other than wasting money…
What makes me crazy is the 20.2% from nuclear comes from 120 or so plants. Just adding 100 nuclear plants would get rid of 1000 coal plants and could get nuclear up to 50. If they really, truly cared about carbon they would push nuclear.
The basic equation is CaCO3 +H2O + CO2 => Ca++ +2HCO3- . Check it out; it balances.
But it’s an equilibrium, isn’t it? So it will just move back again.
The earth contains large amounts of calcium carbonate, but very little calcium bicarbonate. I would suggest that means calcium carbonate is the more stable form, and that bicarbonate will break down to form it in the sea.
It might be “plant food” but the net effect is to be transformed back into the carbonate and the carbon dioxide be released, one way or another.
If ocean acidification does prove to be a problem, then it might be worth doing for that reason, but that’s a long way into the future. As a way to reduce CO2 in the atmosphere, not so good.
Bill Sticker:
“I foresee the next environmental scare already; ‘Hard’ water pollution from power station carbon emissions causes……..(Insert own wild unsubstantiated claim here)”
… scale on fish?
More seriously is there any evidence that increased CO2 dissolved in the oceans is feeding plankton? Presumably having cycled a few times and up the food chain the carbon should sink into the anoxic mud and be stuck.
I find this amusing. You don’t need limestone to scrub CO2! Just straight seawater works quite well. Actually any “hard ” water will work. 20 years ago I invented and patented a fume scrubber that does this quite well. Too well actually. It would make concrete with the CO2 in the air and normal city water. Just how in the heck do you think god cleans the atmosphere. Breath mints! IT is the OCEAN that creates the atmosphere we enjoy. pg
Nature does this FREE OF CHARGE!!!
Another crass idea from a straw grabbing scientist trying to gain extra grant money. It will also cost billions!