From the EPA might have something to say about that department…this press release from LNL suggests dumping tons of Calcium Bicarbonate as a byproduct of CO2 scrubbing into the oceans instead. Only one teeny little problem – most coal fired power plants (at least in the USA), aren’t anyway near the ocean.
Yeah, that’ll work. Ok I’ll give him a pass for saying it’s only applicable to plants near the ocean, but how many of those are there compared to the map above? Coal still rules.
| US Electric Power Industry Net Generation, 2009 |
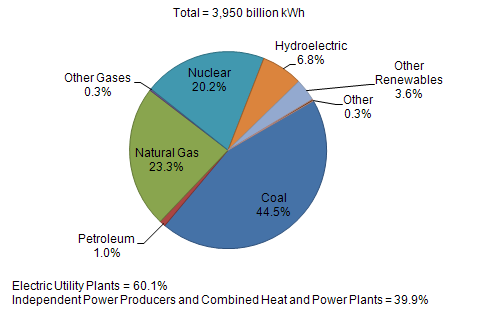 |
| Sources: U.S. Energy Information Administration, Form EIA-923, “Power Plant Operations Report.” |
================================================================
Speeding up Mother Nature’s very own CO2 mitigation process

LIVERMORE, Calif. — Using seawater and calcium to remove carbon dioxide (CO2) in a natural gas power plant’s flue stream, and then pumping the resulting calcium bicarbonate in the sea, could be beneficial to the oceans’ marine life.
Greg Rau, a senior scientist with the Institute of Marine Sciences at UC Santa Cruz and who also works in the Carbon Management Program at Lawrence Livermore National Laboratory, conducted a series of lab-scale experiments to find out if a seawater/mineral carbonate (limestone) gas scrubber would remove enough CO2 to be effective, and whether the resulting substance — dissolved calcium bicarbonate — could then be stored in the ocean where it might also benefit marine life.
In addition to global warming effects, when carbon dioxide is released into the atmosphere, a significant fraction is passively taken up by the ocean in a form that makes the ocean more acidic. This acidification has been shown to be harmful to marine life, especially corals and shellfish.
In his experiments, Rau found that the scrubber removed up to 97 percent of CO2 in a simulated flue gas stream, with a large fraction of the carbon ultimately converted to dissolved calcium bicarbonate.
At scale, the process would hydrate the carbon dioxide in power plant flue gas with water to produce a carbonic acid solution. This solution would react with limestone, neutralizing the carbon dioxide by converting it to calcium bicarbonate — and then would be released into the ocean. While this process occurs naturally (carbonate weathering), it is much less efficient, and is too slow paced to be effective.
“The experiment in effect mimics and speeds up nature’s own process,” said Rau. “Given enough time, carbonate mineral (limestone) weathering will naturally consume most anthropogenic CO2. Why not speed this up where it’s cost effective to do so?”
If the carbon dioxide reacted with crushed limestone and seawater, and the resulting solution was released to the ocean, this would not only sequester carbon from the atmosphere, but also would add ocean alkalinity that would help buffer and offset the effects of ongoing marine acidification. Again, this speeds up the natural CO2 consumption and buffering process offered by carbonate weathering.
Earlier research has shown that ocean acidification can cause exoskeletal components to decay, retard growth and reproduction, reduce activity and even kill marine life including coral reefs.
“This approach not only mitigates CO2, but also potentially treats the effects of ocean acidification,” Rau said. “Further research at larger scales and in more realistic settings is needed to prove these dual benefits.”
Rau said the process would be most applicable for CO2 mitigation at coastal, natural gas-fired power plants. Such plants frequently already use massive quantities of seawater for cooling, which could be cheaply reused for at least some of the CO2 mitigation process.
“This method allows a power plant to continue burning fossil fuel, but eliminates at least some of the carbon dioxide that is emitted, and in a way that in some locations should be less expensive and more environmentally friendly than other carbon dioxide sequestration methods,” he said.
The work, funded by the Energy Innovations Small Grant Program of the California Energy Commission and LLNL, appears in the journal Environmental Science & Technology.
More Information
“CO2 Mitigation via Capture and Chemical Conversion in Seawater,” Environmental Science & Technology
“Strengthening Our Understanding of Climate Change,” Science & Technology Review, December 2010
“Locked in Rock: Sequestering Carbon Dixoide Underground,” Science & Technology Review, May 2005
“The Siren Call of the Seas: Sequestering Carbon Dioxide,” Science & Technology Review, May 2004


Ummm, don’t these guys always cry over putting crap in the ocean? I guess when it’s their idea it’s ok??? WTF?!?
Next up from Livermore:
Future water shortages in the southwestern US can be eliminated by desalinating Atlantic Ocean water.
So the solution to our CO2 “problem” is baking soda?
The idea sounds pretty half-baked to me.
Maybe you should read this….. LOL
http://www.therecord.com/news/article/365912–farmer-says-co2-injected-underground-is-leaking-on-his-property
At last I see Obama,s brilliant make work plan, running millions of miles of sea water pipe lines to coal power plants and back to the oceans. about a zillion dollars should cover the cost nicely.
Or about selling the calcium bicarbonate by products to TUMS and ROLAIDS. Given the fact there is so much acid and reflux stomach created by these AGW clowns, that should take care of 99.95% of the problem?
You’ll be able to tell if this is a good idea by how quickly the greenies mount opposition to it.
If it’s good stuff, why not dump this hard water into the nearest river?
Would New Orleans need a large water softener?
Pump it into the botanical biosphere through vertically oriented atmospheric carbon gas emitters. Solar powered carbon fixers can then be employed to scavenge the carbon by combining it with hydrogen to form long carbon hydrogen chains. I feel a grant coming on. Maybe the USDA can actually pay people to do this.
If it works why not? Many of the hard core environmentalists want to harm the economy. If this process provides power companies a solution in dealing with EPA, more power to them…sorry bad pun.
Lines of people with buckets.
Richard G’s idea is perfect. In fact, I have a secret plan to extend that idea: it may be possible to use the outputs of those solar-powered carbon fixing devices for nutritional purposes, or to generate green construction materials!
Awwww. Poor witto ocean has a tummy ache. Here ya go. Nanny give you medicine.
buuuuurrrrrrrb
All better?
I’ll soon invest (buy some stock) in a pipeline MLP (Managed Limited Partnership). Still waiting for the long overdue correction so I can get into “Medium Oil” (and 6+% dividends).
The MLP has thousands of miles of oil, natural gas, and CO2 pipelines.
The CO2 is pumped TO the oilfields to significantly increase production.
I don’t know (yet) where it comes from or how it’s produced.
Oil and NG pipelines criss-cross the US for hundreds of thousands of miles.
CO2 pipelines from coal plants might be economically feasible if piped to older oilfields.
“…when carbon dioxide is released into the atmosphere, a significant fraction is passively taken up by the ocean in a form that makes the ocean more acidic.”
OK, will someone please explain for this old duffer who must have slept through this part in HS Chemistry: How does making something LESS alkaline, make it “MORE” acidic? I thought we had to measure a pH of <7.0 (by definition) to become acidic in the first place. These people have a serious negative effect on my sleep cycles!
Oh great. Now we’re going to pollute, but call it “green”.
Somehow we’re supposed to be sold on the idea that pumping something that has to be produced and transported, then managed…at an expense of more generated CO2, the very substance we’re trying to mitigate….then pumped into the sea is better than just letting the CO2 from the stacks go?
Do we have any ideas of the net CO2 numbers from this proposal? But in any case, this is just another geo-engineering fiasco in the making, with untold unintended consequences.
I think this idea could have some merit. Certainly enough to setup a demonstration project as long as the science was setup properly and the long-term effects measured adequately.
However, I would predicate its funding on the presumption that CO2 was actually proven to be more than an atmospheric fertilizer as it appears now. What other conceivable reason would we have to waste so much money on a unproven concept such as AGW?
They’ll find something toxic about this stuff too and I’ll no longer be able to have my plop plop fiz fiz the morning after Friday nights at the Lostine Tavern.
Sorry, Greg, but it’s already a commercial process:
http://calera.com/
Producing construction materials such as aggregates for concrete is not a bad idea, actually…..much better than pumping the stuff deep underground!
However, if EPA declares coal ash as a hazardous material (likely), this plan will be all goofed up as the flyash carries over into the aggregate.
From the pie chart, neither solar, wind, geothermal, or other renewable energy will replace any significant amount of oil. They won’t even make a dent in carbon dioxide emsissions.
Maybe the extremo’s have broken the code on this, and are turning their attention to the oceans as a place to dump CO2, or rather calcium bicarbonate.
So, either we get fizzy ocean water, or decreased acidity of ocean water with the CO2 being entombed in calcium bicarbonate.
Who is going to pay for China and India to convert all this carbon dioxide to carbonate form? They are the modern day ecological scofflaws. But, for some reason the whacko-eco-extremo’s expect the free world to pay, mostly America.
Actually, the material might be used as a fertilzer for certain crops. CO2 as bicarbonate can be taken up by roots. The growth of many plants is inhibited by even relatively small concentrations of bicarbonate, but cereals seem to do quite well. By picking the right crops, there may be a reasonable way to dispose of Ca(HCO3)2 on land.
It might be a great idea to dispose of CO2, if you ignore the fact it’s a huge waste of time, money, and energy.
I’m puzzled. Limestone is calcium carbonate and thus is “saturated” with CO2 – so how do you put more CO2 into it. Weathering is usually the breakdown of a rock, i.e. the release of CO2 from lime. And where, oh where, does calcium BIcarbonate come from? Since both (Ca and CO3) are divalent you can’t substitute a H into the equation – only happens with a monovalent metal like Na.
The only way to absorb CO2 from a gas is to use lime (CaO or Ca(OH)2) which is produced by “burning” estone, i.e. driving off the CO2, like for making cement. Seems to me there’s some basic chemistry gone astray here. Unfortunately I couldn’t get hold of the paper, it’s behind a paywall.
Dave
I am not one to look favorably on any of these geo-engineering schemes to do anything. I am as skeptical about this as AGW. If the customers of those power plants near oceans which to sustain the added cost I see nothing wrong with a few studies and maybe a little demonstration project. As long as what ever people do is based on empirical science and not stupid models, that is.
I am always skeptical of statements which contian the phrase ” ocean acidification” according to the IPCC report the oceans of the world range from a pH of 7.9 to 8.2 now for those who flunked chemistry this is alkali i.e. acid starts at 7.0 and LOWER so how can you increase the acidification of an alkali solution. I realize the US people especially the news media, have a total disregard for the english language, being an ex pat brit I find this illeteracy which is so common amongst the US people sad and inexcuasable, however, by definition, by my language at least, to increase acidification the liquids must be acid to start which according to the IPCC it is not. Thus the term “increased acidification” is pure propaganda. There is no scientific evidence that the world’s oceans pH are changing. Like all things locally one can cherry pick a natural variation and try to make a case out of it but overall there is no change.
Oh this sounds fantastic, led pump a bunch of seawater, mine a bunch of limestone, grind it up, and transport it to coal plants and mix it all up – this sound like a great way to make burning coal into a net loss of energy.
Here is a better plan for you greenies – give subsidies for coal plants to make MASSIVE greenhouses next to their plants to grow hemp or algae for biofuel. Oh wait, if we did something like that then people would realize that CO2 isn’t a pollutant.
Damn these people are idiots.
(Disclaimer: I wouldn’t actually do that since I’m a libertarian – that’s just a hypothetical suggestion if I believed in interfering with the free market. I wouldn’t be surprised if the coal produced more CO2 than the greenhouses could use but at least it would be more doable).
I foresee the next environmental scare already; ‘Hard’ water pollution from power station carbon emissions causes……..(Insert own wild unsubstantiated claim here)