CORVALLIS, Ore. – SAR11, the most abundant plankton in the world’s oceans, are pumping out massive amounts of two sulfur gases that play important roles in the Earth’s atmosphere, researchers announced today in the journal Nature Microbiology.
“Everyone knows these gases by their smells”, said Steve Giovannoni, a distinguished professor of microbiology in the College of Science at Oregon State University, and corresponding author of the study.
“One of these compounds – dimethylsulfide, or DMS – we recognize as the smell of the sea. The other gas – methanethiol – makes us think of leaking gas lines. In the atmosphere dimethylsulfide oxidizes to sulfuric acid, which some scientists think can seed cloud formation and alter heating of the Earth.”
What is most interesting, the scientists said, is that the newly discovered metabolic circuit is hardwired into cells. Normally, cells turn genes on and off, as they are needed, but the newly discovered circuits for sulfur gas production by SAR11 are on all the time.
“That doesn’t mean the cells are always producing the gases,” said Giovannoni. “But they are always ready if algae in the surrounding water make DMSP, a compound that the SAR11 cells harvest for energy, releasing sulfur gases as waste products.”
Many types of gaseous fumes emerge from the ocean, such as formaldehyde, acetone and methanol, Giovannoni said. However, researchers were very surprised that the cells produced both DMS and methanethiol. The DMS is made by a newly discovered gene, according to the study, and it was completely unexpected. And while the author’s knew the cells could make methanethiol internally, they did not expect it to be released in large quantities.
###
This work is part of the North Atlantic Aerosols and Marine Ecosystem Study, funded by NASA, and other agencies. Collaborators were from OSU, the University of East Anglia in the United Kingdom, Louisiana State University, the Plymouth Marine Laboratory in the United Kingdom, the Qingdao Aquarium in China, and the Pacific Northwest National Laboratory.

Yet more negative GHG feedbacks from the self-regulating planet of life and water.
(Sure, Bye, He knows what he’s doing ; )
Contrary to what Roy Spencer says, God has nothing to do with it.
SAR 11’s ancestors evolved this metabolism in response to increased oxygen in the air and water.
Bye Doom
Haven’t you’ve ever studied logic? Why beg the question?
Have you ever examined the probability resources over the life of the universe? If you dare, see mathematician William Dembski’s The Chance of the Gaps. There is no conceivable mathematical probability for an abiotic origin of life, let alone neoDarwinianistic development of the DNA required to for cell functioning or the SAR11 required to synthesize either DMS or methanethiol. Then to rise above elementary principles and address the real challenges, see the quantitative technical / mathematical proofs in Evolutionary Informatics Publications.
Dembski is a chemical and biological ignoramus. The fool imagines that life has to emerge from an elemental soup rather than from complex organic compounds, which abound in the universe. All the major components of life were delivered to earth on meteorites, to include possibly simple microbes.
Citing such creationist cant here on an ostensible science site ought to get you 86ed.
Abiotic origin of life is not only possible. It’s a fact, ie an observation.
As I’ve pointed out elsewhere on this blog, RNA self-assembles under a variety of conditions, including in the water pockets of ice. It is also able to conduct both metabolism and replication, the twin functions of life. At this basic level, the two processes are similar, one with amino acids (peptides to polypeptides, ie proteins) and the other with nucleotides (making a copy of itself).
Just last year, it was also observed producing peptide chains longer than itself.
No deep, dark mystery requiring a fantasy sky-father. Just chemistry and physics.
“Citing such creationist cant here on an ostensible science site ought to get you 86ed.”
Fortunately, or unfortunately in the eyes of some, Mr. Watts does not appear to be an Evo justice warrior type . .
“Abiotic origin of life is not only possible. It’s a fact, ie an observation.”
Some folks seem to consider their imagination to be a sort of magic crystal ball, through which they can “observe” the time space continuum, at any point in time or space ; )
So, Mr. Doom be sure to fill us in when you make your first cell or even explain how one is made.
There are more things on heaven and earth that will fit your imagination. I’m not religious, just hate arrogance.
If life is merely an ongoing chemical process, when will scientists create life in the lab to prove that it is possible?
South River Independent,
My guess is, just as soon as they take care of the . . pesky “show us your work” skeptic problem they have ; )
when will scientists create life in the lab to prove that it is possible?
It is also able to conduct both metabolism and replication, the twin functions of life.
===========
light a match. fire eats, converts this to energy and uses this energy to replicate. just like life.
Does that mean life is like pornography? You cannot really describe it but you know it when you see it?
Expat,
I guess you missed the part where I told you that science has already observed RNA self-assemble and conduct metabolism and replication without any magic intervention.
Cells arose after these two processes. First life occurred, as observed, in ice or on a mineral catalyst. Then the RNA conducting these operations got enclosed in a bilipid membrane. Bilipids also self-assemble.
As I said, no need for a supernatural creator. I suppose it’s still possible for the needy to believe that our universe was designed so that elements heavier than hydrogen could exist, stars and galaxies form, etc. But that’s as far as it goes. Elements spontaneously form complex organic compounds which then self-assemble into the constituent molecules of life and then into living things, under the right circumstances.
John,
Allowing creationist claptrap here only gives alarmists ammunition with which to attack skeptics as anti-scientific.
Our esteemed host and moderators are of course free to permit such outrages against reality, as it’s their site, but IMO if Slayers are banned, then the infinitely less scientific creationists should be, too. But of course only when preaching their religion, not when making valid points, as of course does Dr. Spencer with respect to climatology, despite his anti-scientific creationist beliefs.
Mr Doom, science is a discipline, not a person. Perhaps you can name the scientist who has created a living organism in a lab and cite the report of this accomplishment, as well as the person’s who have also created living organisms in the lab, corroborating the first experiment, and cite the reports documenting the corroborating evidence. Showing that some building blocks of life assemble under some conditions is not creating a living organism.
Coincidentally, right after I made my previous posts, I read chapter 29 of Sean Carroll’s The Big Picture. Carroll is a theoretical physicist and his book is an (unconvincing to me) attempt to use science to prove there is no God. He calls his philosophy poetical naturalism. Carroll says that nobody knows what life is; there is no agreed-upon definition. He says NASA defines a living organism as a “self-sustaining chemical system capable of Darwinian evolution.” Carroll says we will never have a “correct” definition of life, but notes that life-forms we are familiar with have a number of properties: they move, metabolize, interact, reproduce, and evolve, all in hierarchical, interconnected ways. He also states Erwin Schrodinger’s definition of life from Schrodinger’s book, What is Life:
“When is a piece of matter said to be alive? When it goes on “doing something,” exchanging material with its environment, and so forth, and that for a much longer period than we would expect an inanimate piece of matter to “keep going” under similar circumstances.”
Carroll cites the work of the Miller and Ury experiments in 1952 that produced some amino acids, but goes on to say that assembling proteins is more difficult. According to Carroll, “it has become clear that scientists are going to have to be a lot more clever if we are to understand how the steps proceed after that.”
So no one has created life in the lab. God has not yet been eliminated from “the big picture.”
Of additional interest is that Carroll talks about “free energy” from the sun, by which he means low entropy useful energy that is used by biological organisms and returned to the universe in a highly degraded form. He says that “for every one visible photon it receives from the sun, the Earth radiates approximately twenty infrared photons back into space, with approximately one-twentieth of the energy each. The Earth gives back the same amount of energy it gets, but we increase the entropy of the solar radiation by twenty times before returning it to the universe.”
But revealingly, Carroll goes on to say:
“The energy here on Earth is not exactly constant, of course. Since the Industrial Revolution, we have been polluting the atmosphere with gases that are opaque to infrared light, making it harder for energy to escape and thereby heating the planet. But that’s another story.”
Sounds like AGW to me.
Here’s a link to an interesting lecture on the origin of life by Professor Tour … one of the world’s leading synthetic organic chemists.
SRI,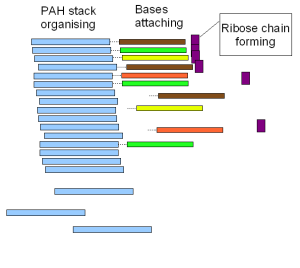
As I said, RNA all on its own can conduct both metabolism and replication. That’s life.
As for making a protocell, that’s easy in the lab. Just put RNA inside a two-layer lipid membrane. You could say it’s cheating to inject the RNA rather than waiting however long it takes to occur naturally, but there is no physical or chemical barrier to that happening. As noted, fatty bubbles with a hydrophilic and hydrophobic layer form spontaneously from lipid molecules in water.
The literature on origin of life research is large and growing. Here are some links:
About the discovery that RNA can act like an enzyme to catalyze protein synthesis:
http://exploringorigins.org/ribozymes.html
About spontaneous RNA formation in ice:
https://www.newscientist.com/article/mg22029413-600-earths-first-life-may-have-sprung-up-in-ice/
Enzymatic activity in ice:
http://www.nature.com/nchem/journal/v5/n12/full/nchem.1781.html
Other substrates besides ice also catalyze RNA formation. As noted previously, PAHs, ubiquitous in the universe, do so, as do mineral formations around hydrothermal vents and even clay.
No deities, spirits or jinns need apply.
BruceG
May 17, 2016 at 3:29 am
The good doctor blew it at the very start by showing what he called a simple cell. That was a model eukaryote, almost as complex as a single cell can get. A protocell is far smaller and simpler. Orders of magnitude so. Even a prokaryotic cell is much more complex than a protocell, ie RNA inside a semipermeable lipid membrane. Lots of evolution in biochemistry, structure and mechanism has to occur just to get to a prokaryote.
A protocell is akin to a retrovirus, except that it’s capable of both metabolism and replication. When the lipid bubble gets too full of reagents and products, it bursts or splits.
We’re getting close on autonomous protocell division:
http://exploringorigins.org/protocells.html
Thanks for the link, BruceG. Apparently Mr Doom does not understand the problem. Again I say, if it is so easy to create life from scratch, why have scientists been unable to do so? Professor Tour explains why creation of a simpler cell is also not possible, yet Mr Doom persists in saying life spontaneously generated out of the primordial ooze. I remember learning when I was young that life did not originate from spontaneous generation; however now the atheists claim that it does. As I said regarding Sean Carroll’s poetical naturalism, not very convincing.
SRI,
The self-assembly of the constituent components of living things, followed via chemical evolution by simply living things, is not the same as the spontaneous generation of frogs, flies and mice, as shown false in previous centuries.
Self-assembly is an observed fact, not a conjecture. The universe is awash in the basic components of living things, which arrived on earth on meteorites and comets and have also formed here by chemical means.
The good doctor is not talking about the simple beginnings of life at all. He discusses chemical reactions and processes in highly advanced cells, while ignoring the sort of bottom up research in which I’m active. He’s looking top down. Apparently you didn’t bother to read the papers I cited. Afraid of the inconvenient truths you’ll find?
If your faith relies on a creator having made the first living things, then your belief rests upon the shifting sands.
As noted, in the past decade or so, we have already observed RNA self-assembling and conducting metabolism, ie making peptides (and just last year polypeptides) and replicating itself. The next step, doing so within a self-assembled, two layer fatty acid membrane is but a small step. Even if it isn’t achieved in the next five or ten years, it’s such a trivial stage that it could not not have happened in nature over 3.5 billion years ago.
To give you an idea of how close we’re getting:
http://www.popsci.com/researchers-make-artificial-cells-that-can-replicate-themselves
Even this important breakthrough from September of last year has already been superseded.
David,
Yes. P = 1.
Now think before you speak.
One advantage of studying the origin of life over just throwing up your arms and asserting on blind faith that a “creator genie did it!”, is that basic scientific research in this field has led to important practical applications.
Solving the engineering problems in making artificial life has led to major advances in understanding such issues as how cells divide. This opens up new avenues of attack against pathogenic microbes. The creation of artificial cell membranes has improved drug delivery systems. To name but two such applications.
http://phys.org/news/2016-01-closer-artificial-cell-divisionby.html
We’ve only just learned how to make liposomes thin enough to divide on their own, using the simplest version of the proteins that unzip natural cells.
Producing artificial, autonomous cells requires solving problems, but we learn a lot about basic biochemistry and molecular biology en route to engineering solutions.
Venter’s synthetic biology lab is approaching such problems from the top down, by stripping DNA down to the absolute minimum required for metabolism and replication.
http://www.nature.com/news/minimal-cell-raises-stakes-in-race-to-harness-synthetic-life-1.19633
His team still needs to figure out what about a third of the genes do in the simplest cell he has assembled yet. When science has discovered what each of the minimal genes does, it will be easier to make a more advanced prokaryotic cell, ie one which relies upon DNA rather than RNA, as in a primitive, simpler protocell.
This is why science is superior to religion in seeking and producing answers about the natural world. To the extent that religious belief has any uses, they lie outside science. Unless you consider science the pursuit of the understanding of God’s mind.
Bruce,
I couldn’t find a recent overall lecture on advances in origin of life research, but this three-parter by Harvard’s Jack Szostak from 2013 comes close. He’s not only a world-renowned organic chemist but a Nobel Prize-winning geneticist.
Some of the problems he mentions have been solved in the past three years by his lab and other workers. Part 2 on vesicles is relevant to the membrane discussion here.
BD,
To me. you are like a small child thinking that because they grasp the idea of a nut fitting onto a bolt, then building a fighter jet is pretty much understood by them. Just a few details to be worked out . .
John,
To me you are like a pre-schooler who believes in Santa Claus and the Tooth Fairy because your mommy told you so, completely in the absence of evidence.
I tend to be neutral on the subject. But those who assert thre’s no Creator sound no different to me than those who assert there is. Both are a matter of faith, not science.
DB,
I didn’t say there is no creator. Quite the opposite. I said that it’s possible to imagine such a being designing the universe.
What I do say is that there is no need to assume a creator to explain the origin and history of life on earth. You can suppose such a being if you want, but there is no scientific basis for such a belief. Indeed, faith in such a being short circuits science.
ID advocate Behe says that flagella are “irreducibly complex”, so there is no reason to try to understand their evolution. This pathogens get off free in his world. In my world, we try to find out how to interrupt the chemistry that produces bacterial flagella.
John thinks that scientists can’t solve the origin of life because we’re like children before a jet. But aeronautical designers reverse engineer opponents’ aircraft all the time. That’s what I do in my lab to create new drug delivery systems, environmental remediation microbes and a host of other applications from observing natural systems. It’s called synthetic biology and directed evolution.
John is afraid to educate himself about the reality of great strides in origin of life research since the 1980s, and especially in this century. Simplistic, blind faith requires fear, I guess.
DB,
“John is afraid to educate himself about the reality of great strides in origin of life research since the 1980s, and especially in this century. Simplistic, blind faith requires fear, I guess.”
What you imagine, apparently becomes reality so easily to you, that you speak as though you can actually “see” into my life, my mind, my heart (ie; fear, etc.), with nothing more needed than your power to imagine as an investigative “tool”. This is impossible (unless you are yourself a “heart knower”) . . but that does seem to dawn on you.
I do not have “blind faith”, for I never believed in God until I witnessed things that would require a God. You weren’t there, so, naturally, I do not ask your opinion of what I witnessed/experienced. Your imagination is simply not relevant.
But I find this notion of faith do to fear interesting. What is it I fear(ed), does your imagination tell you? Being a non-believer, in my own world/circle of friends and so on, was far “safer” in terms of being accepted, thought smart, not thought crazy, or foolish or gullible, etc. I was far more “afraid” to be a “believer”, than to continue being a non-believer . . that was easy and comfy to me.
Fear of death? Why? If there is no God/afterlife, then there is absolutely nothing fearful about death . . for one would experience precisely nothing at all when dead. One would never even know they were right/wrong about these matters, never suffer, or feel remorse or regret, nothing.
And that goes for the casual attribution of fear as a motivation for belief in the supernatural throughout history, to me, logically speaking. I hear imagination worshiping atheists speak of frightened people finding comfort in believing powerful entities existed that could do virtually anything they wished to them, and I wonder what these guys have been smoking ; )
Oh, I see I got my d and b reversed . . prolly kinda obvious, and the use of BD involves the assumption those are not BD’s actual initials, name portions . .
Mr stealey, you are correct regarding faith. Some naturalists do not understand that their belief in naturalism is based on faith. In his book, Sean Carroll says that naturalism is different than religion, failing to see that they are both part of the same continuum, with fundamental religion on one end, fundamental naturalism at the other end, with variations of belief running between the two extremes. Naturalism should not be confused with science. They are two different things. Owen Gingerich has written some books that explain that there is room for a creator in our understanding of the physical universe. See his God’s Planet and God’s Universe. Gingerich is an astronomer and science historian.
It is interesting to note that everything that lives has come from something that lives. It seems that only life can pass on life through the act of creation. I don’t think it will ever be possible for man to animate inanimate matter because such knowledge will always be beyond our abilities.
The post reminds me of the general issue of biological volatile organic compounds, which very much affected my former business as the California Air Resources Board set allowable standards below that produced by trees and shrubs. The Great Smoky Mountains are not that way because of man-made air pollution.
A remarkable observation/conclusion. These sulphur compounds would be classed as dangerous poisons if released by humans. H2S is very nasty I know, and would expect DMS to be just as nasty. It’s the mountains for my next holiday (vacation)!
Don’t go to the Smokies!
The dose is the toxin.
Yes, the dose makes the toxin. Even water can be lethal. However, Jethro Bodine from the “Beverly Hillbillies” laughs at the typical LDL 50. One organism’s waste product is another organism’s food. It all depends on metabolic pathways. So, I will sit here and enjoy my glass of wine and some Camembert on leavened baguette. If you are in the area please stop by and we can test the dose limits together.
@ Richmond May 16, 2016 at 5:00 pm Is it a young or properly ripened Camenbert and should I a medium bodied burgundy or an older champagne?
Ditto ozone.
Please give credence to posts by supplying links.
http://www.eurekalert.org/pub_releases/2016-05/osu-oba051316.php
For those with knowledge of these things: Are there fresh water plankton that produce these gases? Are the background levels of these gases strongly influenced by these microbes?
John,
“SAR11 (is) the most abundant plankton in the world’s oceans.”
SAR 11 apparently are also found in freshwater;
http://www.sciencedirect.com/science/article/pii/S0723202014001398
Yes, periodically in lakes.
Yes, the hydrogen sulfide gas (H2S) is produced under anoxic conditions, whwn organic substances rot (by bacterial activity). It happens both in the ocean and in lakes. The gas is ok if you can smell it. However it becomes lethal if the concentration is above a certain, very low, threshold. Then you are not able to smell it any more, and you are in great danger. You see, the gas is so reactive that it takes over the liquids of your nose, mouth, and lungs, and you die, instantly
This may seem to be off topic, but Dr James Lovelock, father of the GAIA hypothesis studied the atmosphere in the far remote corners of the earth and found an enormous amount of DMS, totally unexpected. This is my take on how it ties in with the sin of the world and the lie.
https://lenbilen.com/2014/12/22/on-the-sin-of-the-world-and-the-lie-what-does-that-mean/
Where is the S coming from?
If the algae are making DMSP then they (the algae) must be getting sulfur from the water. Whether it is elemental sulfur or some other compound(s) is not stated and perhaps not known. There has to be a lot of it though!
Thanks to the sulfur cycle, the amount of mobile sulfur has been continuously increasing through volcanic activity and weathering of earth’s crust under the oxygenated atmosphere which evolved on our planet. Earth’s main sulfur sink is the oceans SO4 (2-), where it is the major oxidizing agent.
Depending on how you classify H an O in H2O, sulphur is the 4-6th most abundant element in sea water (roughly 900 PPM). Quick Google turns up a “Sulphur cycle” – https://en.wikipedia.org/wiki/Sulfur_cycle
Thanks. Had no idea! Interesting that with all that S ocean ph is still about 8.1, and we are only concerned about acidity from co2 and not from rising S content. So much to know and understand.
“SAR11, the most abundant plankton in the world’s oceans, are pumping out massive amounts of two sulfur gases that play important roles in the Earth’s atmosphere, researchers announced today in the journal Nature Microbiology.”
Odd apparent claim of priority. DMS and climate is an old story.
This 2006 paper starts its abstract with:
“Flux of dimethylsulfide (DMS) from ocean surface waters is the predominant natural source of sulfur to
the atmosphere and influences climate by aerosol formation.”
And it also says:
“Marine bacterioplankton are known to degrade DMSP by a pathway that first converts DMSP to methylmercaptopropionate (MMPA) in a demethylation reaction, and subsequently to methanethiol (MeSH) (8) or mercaptopropionate (MPA) (fig. S1) (9).”
Stokes,
Old news and yet NASA and other organizations funded the study. And NM published the results. It would appear that people are not doing their homework.
I think the faults are in the press release, which gives the wrong impression of new information about emissions and climate effects. As Owen in GA says below, it seems the real novelty is the doscovery of the gene and how it is expressed, which would justify its place in Nature Microbiology.
Now that this new SAR11 DMS synthesis pathway has been discovered, we need to be vigilant as to how it is going to impact climate.
One can assume that this pathway did not operate before we discovered it. Now that it has been found however, those bugs are going to get busy pumping worrisome volumes of DMS into our atmosphere.
Seawater is going to start smelling of seawater for the first time. Since this has been discovered by government scientists it follows that it can’t be good.
BTW you probably noticed that I am a firm believer in the Steven Mosher school of logic and epistemology.
LOL. I thought it looked familiar.
It seems that Steve Giovannoni, distinguished professor of microbiology in the College of Science at Oregon State University, has rediscovered the wheel. It may be that the influence of SAR11 is newly discerned, but the impact of marine dimethysulfide on atmospheric sulfate has been known for at least 40 years. Check it out.
A quick search in Google scholar found T.S.Bates, et al. (1992) “Sulfur emissions to the atmosphere from natural sources” J. Atmospheric Chemistry 14(1), 315-337; abstract here.
Under Section 2.1.1. Oceanic concentrations of volatile sulfur gases. I’ve bolded the parts referring to dimethylsulfide (DMS).
“Several volatile sulfur gases are produced biologically (CH3SCH3, CS2, CH3SH, CH3SSCH3) and/or photochemically (H2S, OCS) in the surface waters of the ocean (Andreae, 1986). Of these compounds, dimethyl-sulfide (CH3SCH3 or DMS) is the most abundant and is the only compound which contributes significantly to the atmospheric burden of NSS [Non-Sea-Salt] sulfate (Cline and Bates, 1983; Nguyen et al., 1983; Andreae, 1986). Haas first identified DMS emissions from the marine algae Polysiphonia fastigiata in 1935. The precursor of DMS, dimethyl-sulfoniopropionate (DMSP), was isolated from this species by Challenger and Simpson (1948) and the biosynthesis of DMSP from methionine has been demonstrated by Greene (1962).
“The occurrence of DMSP in marine phytoplankton was first documented by Ackman and coworkers (1966). The DMSP concentration within phytoplankton cells is extremely species specific (Keller et al., 1989) and has been shown to contribute significantly to the intracellular osmotic pressure (Reed, 1983; Vairavamurthy et al., 1985), thus serving to maintain the osmotic balance required for cell growth.
“DMSP is released from phytoplankton cells both during senescence (Nguyen et aL, 1988; Leck et al., 1990) and during zooplankton grazing (Dacey and Wakeham, 1986; Leck et al., 1990) and is then cleaved enzymatically to yield DMS and acrylic acid (Cantoni and Anderson, 1956; Dacey and Blough, 1987). DMS was reported first in oceanic waters by Lovelock and coworkers (1972) and has subsequently been measured throughout the Pacific (Andreae and Raemdonck, 1983; Cline and Bates, 1983; Barnard et al., 1984; Andreae, 1985; Bates and Cline, 1985; Bates et al., 1987), Atlantic (Barnard et al., 1982; Andreae and Barnard, 1984; Turner and Liss, 1985; Turner et al., 1988; Iverson et aL, 1989), and Southern Oceans (Berresheim, 1987; Berresheim et al., 1990). DMS concentrations vary substantially on a regional and seasonal basis, and have been recently summarized by Bates and coworkers (1987) and Cooper and Matrai (1989).
“Open ocean surface seawater DMS concentrations generally range from 0.5 to 5 nmol/L, and are lowest during the winter months in high latitudes and highest during the summer months at these same latitudes (Bates et al., 1987). On a regional and seasonal basis, the average natural variability of DMS concentrations in the North Pacific Ocean is approximately 30% (Bates et al., 1987). This includes the analytical uncertainty of approximately 10%. The natural variability in DMS concentrations can be much higher in coastal regions where concentrations can vary by an order of magnitude within a two-week period in a given area (Bates and Cline, 1985; Turner et al., 1989; Leck et al., 1990). The lifetime of DMS in surface waters is on the order of one day (Kiene and Bates, 1990) as it is degraded both microbially (Suylen et al., 1986; Taylor and Kiene, 1989) and photochemically (Brimblecombe and Shooter, 1986) and lost to the atmosphere via air-sea exchange.“
Yes and DMS is a major actor in Svensmark’s theory relating galactic cosmic rays (GCRS) to climate.
Trace carbonyl sulfide (COS) in the atmosphere is also very interesting. http://escholarship.org/uc/item/17j6m436#page-1
I think the novelty of the report is in the microbiology and not in the atmospheric gases portion. The exact gene involved in the synthesis and the fact the gene is always active within the organism is the key to this report. All mentions of the effects of the resultant gases can be looked upon as a statement of “send more money” to the climate money bags.
My memory leaks, but weren’t such sea sulfurous compounds a controversial point of conversation in the ozone depletion discussions? The consensus being an unimportant source?
You’re probably right, Fred. I don’t remember either. But you can be sure of one thing: if they couldn’t prove it was caused by man’s infidelities then it didn’t fit their narrative and it would therefore become inconsequential to their cause.
It’s a good thing the science is settled and we can ignore the denial-ist plankton.
/snark
“Many types of gaseous fumes emerge from the ocean, such as formaldehyde, acetone and methanol”
OMG, nobody tell the EPA, otherwise they’ll want to shut the oceans down
No way! They just want to keep you and me away from the elite’s beaches.
The abundant marine bacterium Pelagibacter simultaneously catabolizes dimethylsulfoniopropionate to the gases dimethyl sulfide and methanethiol
http://www.nature.com/articles/nmicrobiol201665
Gee are they telling me that the smell I sensed as a youngster coming off the salt meadows along the Jersey Shore was actually from sulfur gas?
That smell is gone since most of the meadows were filled in. So man just cleaned up the mess?
Ocean bacteria altering the climate? Those are the same slimy organisms that polluted Earth’s methane, ammonia, and CO2 atmosphere replacing it with nitrogen and that flammable poison, oxygen. They forever altered the climate already, and destroyed our pristine Earth. They must be stopped.
Can’t blame oceanic microbes for breaking ammonia down into nitrogen. Rays from the sun did that, acting on ammonia released by volcanoes.
But those nasty polluters the cyanobacteria are to blame for the catastrophic poisoning of the air with oxygen, allowing the rise of those evil despoilers of the pristine earth, animals, the worst of which of course are rapacious humans.
Beer brewing creates dimethyl-sulfide & imparts an aromatic note of cooked corn. Home brewers have to learn how forcefully to boil most of it off & then quick cool that wort since warmth lets more dimethyl-sulfide conversion occur. Novices have easier times with darker brews than lagers since have less dimethyl-sulfide.
I have applied for a grant to determine whether drinking beer when out on a boat in satisfying because then our blood is more at one with the water. Anyone who wants to try and falsify this premise has to self-fund their test samples though.
Gringo, be careful about expressing ideas like this in a public forum. It could lead the EPA to declare beer a pollutant and lead to the closing of Coors and Anheuser Busch. Why it could even harm Sierra Nevada and Butte Creek breweries in Chico!
SHHHHHH!
Humans appear to have the gene for making ETHANOL turned on pretty often…
‘
One hopes they don’t learn too much about it. so as to release a giant hogweed of climate change.
James Lovelock wrote about DMS in his book Gaia: A New Look at Life on Earth back in 1979.
And in his “CLAW” paper in 1987:
http://www.jameslovelock.org/page35.html
This article…
http://phys.org/news/2016-05-ocean-bacteria-climate-gases.html
… says that Pelagibacterales (SAR11) bacteria produce increasing amounts of DMS (via an intermediate compound called DMSP), with increasing water temperature and/or increasing sunlight. That, in turn, increases sulfate aerosols in the air above and near the oceans, which act as cloud condensation nuclei. That increases cloud cover, which reduces the amount of sunlight reaching the ocean and thus reduces ocean temperature. That makes the process a negative (stabilizing) feedback mechanism called “CLAW Feedback” (named for the surnames of the authors of this 1987 paper).
Question: why is it ok for these organisms to pollute, but it’s not ok for us to emit CO2? Where’s the EPA when you need them (*Sarcasm*)
Was this mechanism not invoked by Lovelock for his Gaia hypothesis. In fact although Lovelock initially was an AGW supporter I believe he changed his position . Certainly his basic message was that gaia was maintained by negative feedback loops. I must say I found him rather compelling both at a scientific level and in a sort of spiritual way without getting too tree-huggy
So what this paper implies is that increased CO2 levels will increase phytoplankton biota from the CO2 fertilization effect, which will increase the level of atmospheric dimethyl-sulfide (a cloud seeding compound) leading to incread cloud cover, creating a higher albedo, and creating a natural negative feedback from any additional CO2 forcing…
Furthermore, any CO2 induced global warming will increase ocean evaporation, which also increases cloud cover, creating yet another negative feedback effect…
Oops…
No wonder CAGW projections are so hilariously devoid of reality… The “runaway CO2 positive feedback loops” baked into the CAGW computer models don’t exist, and all the negative CO2 feedbacks are likely not factored into the computer models at all…
CAGW has become such a joke.
What is the effect of SAR11 on ocean PH as atmospheric CO2 increases?
From NASA and UEA! They are covering their tracks!
When Sun’s Too Strong, Plankton Make Clouds
http://www.nasa.gov/vision/earth/environment/0702_planktoncloud.html
NASA-funded research confirms an old theory that plankton can indirectly create clouds that block some of the Sun’s harmful rays….DMS then filters from the ocean into the air, where it breaks down again to form tiny dust-like particles. These tiny particles are just the right size for water to condense on, which is the beginning of how clouds are formed. So, indirectly, plankton help create more clouds…
I think some may be confusing DMS with H2S. SAR11 produces DMS – that sea smell, but it is other bacteria which produce H2S, possibly even from DMS itself (though I’m not sure of that). The chemistry of the oceans is incredibly complex. Another product of SAR11 can be methane.
“South River Independent
May 16, 2016 at 7:44 pm
If life is merely an ongoing chemical process, when will scientists create life in the lab to prove that it is possible?”
Evolution [skeptic] : Professor Haldane, even given the billions of years that you say were available for evolution, I simply cannot believe it is possible to go from a single cell to a complicated human body, with its trillions of cells organized into bones and muscles and nerves, a heart that pumps without ceasing for decades, miles and miles of blood vessels and kidney tubules, and a brain capable of thinking and talking and feeling.
JBS: But madam, you did it yourself. And it only took nine months.
But where did the living cells that combined to form the fetus come from? Life begets life. That is not in dispute.