Guest Post by Willis Eschenbach
Short answer? Of course not, that would violate the Second Law of Thermodynamics … BUT it can leave the hot object warmer than it would be if the cold object weren’t there. Let me explain why this is so.
Let me start by introducing the ideas of individual flows and net flows. Suppose I owe you twenty-five dollars. I run into you, but all I have is a hundred dollar bill. You say no problem, you have seventy-five in cash. I give you the hundred, you give me the seventy-five, and the debt is paid.
Now, there are two equally valid ways to describe that transaction. One way looks at both of the individual flows, and the other way just looks at the net flow. Here they are:

Figure 1. Net flows and individual flows. The individual flows are from me to you, $100, and from you to me, $75. The net flow is from me to you, $25.
What does this have to do with cold and warm objects? It points out a very important distinction, that of the difference between individual flows of energy and the net flow of energy, and it relates to the definition of heat.
Looking at Figure 1, instead of exchanging dollars, think of it as two bodies exchanging energy by means of radiation. This is what happens in the world around us all the time. Every solid object gives off its own individual flow of thermal radiation, just as in the upper half of Figure 1. We constantly radiate energy that is then being absorbed by everything around us, and in turn, we constantly absorb energy that is being radiated by the individual objects around us.
“Heat”, on the other hand, is not those individual flows of energy. Heat is the net flow of energy, as represented in the bottom half of Figure 1. Specifically, a heat flux is the net flow of energy that occurs spontaneously as a result of temperature differences.
Now, the Second Law of Thermodynamics is only about net flows. It states that the net flow of thermal energy which we call “heat” goes from hot to cold each and every time without exception. However, the Second Law says nothing about the individual flows of energy, only the net flow. Heat can’t flow from cold to hot, but radiated energy absolutely can.
When an object emits radiation, that radiation goes on until it hits something that absorbs it, whereupon it is converted to thermal energy. The individual temperatures of the emitting and absorbing objects are not significant because these are individual energy flows, and not the net energy flow called “heat”. So there is no violation of the Second Law.
Here’s the thing that keeps it all in balance. If I can see you, you can see me, so there are no one-way energy flows.
Which means that if I am absorbing radiation from you, then you are absorbing radiation from me. If you are warmer than me, then the net flow of energy will always be from you to me. But that says nothing about the individual flows of energy. Those individual flows only have to do with the temperature of the object that is radiating.
So how do we calculate this net energy flow that we call “heat”? Simple. Gains minus losses. Energy is conserved, which means we can add and subtract flows of energy in exactly the same way that we can add and subtract flows of dollars. So to figure out the net flow of energy, it’s the same as in Figure 1. It’s the larger flow minus the smaller flow.
With all of that as prologue, let me return to the question that involves thermal radiation. Can a cold object leave a warm object warmer than it would be without the cold object?
While the answer is generally no, it can do so in the special case when the cold object is hiding an even colder object from view.
For example, if a person walks between you and a small campfire, they hide the fire from you. As soon as the fire is hidden, you can feel the immediate loss of the radiated energy. At that moment, you are no longer absorbing the radiated energy of the fire. Instead, you are absorbing the radiated energy of the person between you and the fire.
And the same thing can happen with a cold object. If there is a block of wood between you and a block of ice, if you remove the wood, you’ll get colder because you will be absorbing less radiation from the ice than you were from the wood. You no longer have the wood to shield you from the ice.
Why is all of this important? Let me offer up another graphic, which shows a simple global energy budget.

Figure 2. Greatly simplified global energy budget, patterned after the Kiehl/Trenberth budget. Unlike the Kiehl/Trenberth budget, this one is balanced, with the same amount of energy entering and leaving the surface and each of the atmospheric layers. Note that the arrows show ENERGY flows and not HEAT flows.
These ideas of individual flows, net flows, and being shielded from radiation are important because people keep repeating over and over that a cold atmosphere cannot warm the earth … and they are right. The temperature and the radiation are related to each other by the Stefan-Boltzmann equation. When we apply the S-B equation to the 321 W/m2 of downwelling “back radiation” shown in the graphic above, it tells us that the effective radiating level is somewhere around freezing, much colder than the surface.
BUT a cold atmosphere can leave the earth warmer than it would be without the atmosphere because it is hiding something even colder from view, the cosmic microwave background radiation that is only a paltry 3 W/m2 …
And as a result, with the cold atmosphere shielding us from the nearly infinite heat sink of outer space, the earth ends up much warmer than it would be without the cold atmosphere.
To summarize …
• Heat cannot flow from cold to hot, but radiated energy sure can.
• A cold atmosphere radiates about 300-plus W/m2 of downwelling radiation measured at the surface. This 300-plus W/m2 of radiated energy leaves the surface warmer than it would be if we were exposed to the 3 W/m2 of outer space.
My best regards to all,
w.
My Usual Request: When you comment, please QUOTE THE EXACT WORDS THAT YOU ARE DISCUSSING, so that we can all understand the nature of your objections.
My Second Request: Please keep it civil. Speculation about the other person’s motives and cranial horsepower are greatly discouraged.
Further Reading: My post entitled “The Steel Greenhouse” looks at how the poorly-named “greenhouse effect” work, based on the principles discussed above.
Math Notes: There’s an excellent online calculator for net energy flow between two radiating bodies here. It also has the general equation used by the calculator, viz:

with the following variables:

and Q-dot (left-hand side of the equation) being the net flow.
Now, when the first object is totally enclosed by the second object, then area A2 is set to a very large number (I used a million) and the view factor F12 is set to 1. This is the condition of the earth completely surrounded by the atmosphere. For the general case, I’ve set area A1 to 1 square metre. Finally, I’ve made the usual simplifying assumption that thermal IR emissivity is 1.0 for the surface and the atmosphere. The emissivity values are greater than 0.9 in both cases, so the error is small. With those usual assumptions, the equation above simplifies as follows, courtesy of Mathematica:

But sigma T ^ 4 is simply the Stefan-Boltzmann radiation for the given temperature. That is why, in the energy budget above, we can simply add and subtract the energy flows to produce the budget and check to see if it is balanced.
Phil, such an atmosphere will be bleeding of energy in evaporative cooling of its own molecules raised to escape velocity. Happens now, here. At some stage, the idea that any gas can be non-radiative entirely will need proving too. Quantum Mechanics demands it…..
With hydrogen and helium maybe, not with argon.
Earth’s escape velocity is about 11km/sec, the probability of an argon atom exceeding 1km/sec at 300K is zero.
QM quite clearly demands that Ar does not emit at the conditions present in our atmosphere.
Except of course it would absorb all the High UV energy currently absorbed by Ozone and any that Ozone doesn’t currently stop, wouldnt it?
A C Osborn December 4, 2017 at 11:16 am
Except of course it would absorb all the High UV energy currently absorbed by Ozone and any that Ozone doesn’t currently stop, wouldnt it?
If it were the hypothetical pure Argon atmosphere then there would be no ozone, in any case it would only absorb the wavelengths given in the NIST table which are below even those absorbed by O2.
Repeated here in case it gets lost in the thread:
Willis:
i) Kinetic energy at the surface can either be conducted away or radiated away. Both cannot occur simultaneously. If one rises the other drops. So, if you have kinetic energy sufficient to produce a temperature of 288k as per the Earth’s measured surface temperature there is no physics that prevents 255k radiating to space and the other 33k being conducted to the atmosphere.
ii) If that 33k goes upwards from the surface in convective ascent (effectively disappearing from the radiative energy budget as PE) then it comes down in convective descent (reappearing within the radiative energy budget as KE at the surface) but at a later time. It is well established physics that adiabatic cooling in ascent and warming in descent is fully reversible.
iii) The original 33k taken from the surface by conduction in the formation of the atmosphere came from energy that would otherwise have radiated to space. That being the case it cannot have reduced surface temperature below 255k which was the surface temperature pre atmosphere as per S-B.
iv) If the surface temperature remained at 255k whilst the atmosphere was being formed then it MUST rise by 33k when the adiabatic loop closes.
v) Although the surface temperature is then 288k you still have ongoing conduction of 33k upward to maintain convective overturning PLUS 255k of radiation going out to space which keeps the combined radiative and adiabatic loops stable and the atmosphere in hydrostatic equilibrium indefinitely.
Your previous objection to such a scenario missed the issue of the process of creating the atmosphere in the first place when the first convective overturning cycle formed. That is where the non zero consequence of conduction and convection comes from so that there is no breach of the first law.
You can only counter that by suggesting that a surface at 288k can both radiate at 288k AND conduct at 33k simultaneously and THAT would be the true breach of the first law.
“You can only counter that by suggesting that a surface at 288k can both radiate at 288k AND conduct at 33k simultaneously and THAT would be the true breach of the first law.”
This wording makes no sense. An object radiates at 288K and conducts at 288K, if in equilibrium or transiently. Why would an object be at 288K and 33K at the same time (simultaneously)?
Heat (kinetic energy) is simply molecular motion. If a photon is emitted the motion reduces so that there is less conduction and if conduction occurs the motion reduces and there are less photons released.
If there is a constant energy supply immediately replacing what has been lost in both processes combined then the temperature doesn’t drop.
Once a blackbody reaches its S-B temperature thereby converting ALL incoming radiation to kinetic energy you can only reduce that kinetic energy if the outflow is faster than radiation.
Conduction and convection are slower than radiation. They inhibit the free flow of radiation but your proposal requires that they act as an accelerant.
Stephen, concur with that except for the last sentence: “They inhibit the free flow of radiation but your proposal requires that they act as an accelerant.”
I do not understand that I have a proposal for your “requires” that conduction and convection act as an accelerant to radiation. They are all independent processes producing entropy. If conduction and convection are minimized (as in deep space), radiation will dominate in energy transfer processes. Perhaps you can expand on your last sentence.
For example, many like to discuss farmer’s greenhouses. Consider one situated where it is very windy and without much insulation. Then consider one situated in calm conditions, same sun load but with much insulation. Different energy transfer eqn.s will need be considered for any design of supplemental HVAC to protect profits.
If an object receives 240W/m and radiates 240W/m and then you introduce some conduction of say 40 W/m then you say that the radiation out does not drop to 200 W/m ?
Stephen, you are not exactly clear enough. If I can guess your meaning correctly, an earth like planet in space is at equilibrium radiating 240 steady state in/out from TOA like earth as defined by CERES orbit (in thermo. this is termed a control volume).
Then an apparatus is added to independently conduct 40 to deep space sink. Ok, now an additional 40 out crosses orbit of CERES (and is not measured by CERES). Sure, there will be a transient condition existing while the newly conducted energy flowing away increases and as 240 radiated reduces. Eventually establishing new equilibrium 200 out radiated measured by CERES and 40 out conducted as measured by an inline instrument at orbit of CERES. Still in steady state with 240in.
If I get your meaning correctly, you have essentially added another window to space, now have one radiating window to space from L&O surface and one conducting window to space from L&O surface.
Willis, are you going to respond to my summary as to how conduction and convection cause a surface temperature enhancement?
I thought you were inviting something along those lines.
Stephen Wilde December 3, 2017 at 5:14 am
Thanks, Stephen. It appears that you still think that in a non-GHG atmosphere, conduction and convection will warm the surface.
However, IF that is the case, as I showed in my proof, the surface would continuously radiate more than it is absorbing … so your claim is physically impossible because it violates the First Law of Thermodynamics.
If you can explain why my proof is wrong, I’m all ears …
w.
Do you believe that the same unit of kinetic energy (molecular motion) can be radiated away as a photon simultaneously with it being conducted to another molecule?
Nice article.
However, they key to the Greenhouse effect is that the molecules are at a lower temperature than the surface. This means that more surface radiation is absorbed than emitted OUT TO SPACE by certain molecules like CO2 – BUT ONLY WHEN COOLER than the surface.
In contrast the article implies that the effect is because molecules are warmer than the background from space.
In fact, if a lapse rate mechanism could produce a atmospheric temperature lower than space – the planet would still warm. The reason this doesn’t happen is that you’d need an atmospheric heat pump.
Trick December 2, 2017 at 6:56 pm
And you can stuff your endless ad hominems where the solar constant is zero. I’m tired of your ceaseless whining.
Come back when you want to talk about the science instead of talking about me. If you want an answer, ask like a decent honest man does, instead of complaining about what you see as my faults. Your opinion about science might or might not be valuable.
Your opinion of me, on the other hand, is worthless, meaningless, useless, and usually unpleasant. Cut it out. It just makes you look like a jerk. I don’t think you actually are one, but you are sure doing a good imitation of one.
w.
“If you want an answer, ask like a decent honest man does, instead of complaining about what you see as my fault.”
I do not see this as a fault or even a complaint Willis, I’d say I see it as the human condition. We all prove we’re human at times. Ok, here’s my question (based on yours) asked like a decent honest man does, where does N&Z write that a non-GHG atmosphere, say argon, can permanently raise the temperature of a planet?
Trick December 3, 2017 at 12:01 am
If you don’t see calling a man “tempermental” as finding fault or a complaint, there’s little use talking to you.
And if you don’t understand that when N&Z say that pressure and solar input are solely responsible for temperature, that they are therefore saying that an argon atmosphere can permanently raise the planetary surface temperature, then you’re not paying attention.
I give up. Do what you want to do. Lots of people here, people who work in the field, people who teach in the field, have told you over and over that you are wrong on any number of your foolish claims, and exactly where you went off the rails.
Your response?
Nada. Nothing. Nil. Zip. Zero.
I give up. When a man is as determined to remain mired in ignorance and misunderstanding as you are, I gotta admit that you are far beyond my poor power to add or detract.
As I said, I used to believe what you believe and N&Z believe, that pressure alone can raise planetary temperatures. Heck, I even argued with Dr. Robert Brown about it …
But unlike you, I LISTENED TO HIM AND LEARNED FROM HIM … but noooo, not Trick, he knows everything already.
Spare me …
w.
“I used to believe what you believe and N&Z believe, that pressure alone can raise planetary temperatures.”
Total pressure is part of the equation for the opacity of an atm., not the partial pressures of each of its constituents so increasing total pressure alone is shown to increase atm. opacity by theory and confirmed by test. The mass mixing ratio and opacity of each absorber present are also part of the eqn. and tests/observations.
If you want me to spare you the details, I’ll stop there, per your request. However, you are the one raising the questions and seem to be interested in discussing the answers. Science appears to have at least partially answered them already. There are also some known unknowns and possibly unknown unknowns that are interesting to discuss in this field.
If it matters, I find little if anything novel in any of N&Z (V&R) work even in their latest (2017) paper.
Trick December 3, 2017 at 7:09 am
Thanks, Trick. I’m unclear about why you bring up atmospheric opacity. Are you saying that opacity can permanently increase the surface temperature?
Regards,
w.
”Are you saying that opacity can permanently increase the surface temperature?”
Yes.
For a completely transparent argon atm. of Earthian parameters the terrestrial LWIR 100% escapes to deep space and the atm. opacity eqn. is identically zero as there are 0.0 absorbers. If Ar has any nonzero absorptance of that LWIR at all, then global atm. opacity eqn. increases above 0.0 driving the surface energy balances and global median temperature increase above ~255K. Some could argue if the absorbance is not measurable then what’s the difference. I would agree no currently measurable difference is as good as none.
The answer to your original question hinges on this science.
Phil. comments 0.0 absorbency is the case for atomic Ar at normal temperatures in Earth lower atm. as the rotational 3-D spin of the atom is not quantized so absorbs no light quanta. Or at least it is not measurable. Thus, your answer is No. This differs from what is found in C. Bohren’s 2006 text on Atm. Radiation where the science answer is YES.
It is well recognized rotational energies associated with the spinning of atoms of finite extent about three mutually perpendicular axes do not enter into the determination of specific heats of atomic gases at normal temperatures. However, Ar not having quantized rotational energy levels is new for me. Argon does have a specific heat (about half that of air).
What is not well recognized so far as I know per Phil. is that atomic Ar spin energy levels are not quantized at normal temperatures. It could be well known in the specialist world though, since the NIST ref. is from 1973. This is interesting aspect for me to dig into, but it will take some time going through source material.
SkepticGoneWild December 3, 2017 at 1:47 am
I don’t think you’ve thought your example all the way through, SGW.
If you do that, the hairdryer is sure to get hotter … a perfect example of a cooler object (the mirror) making a warmer object hotter than it would be without the cooler object …
w.
You do realise the internet is like a permanent record of your comments?
Willis, I share your negative opinion of Trick but not your positive opinion of Robert Brown’s postings. When I dealt with him in another thread he appeared to have little knowledge of convection, lapse rates and adiabatic processes so I gave up on him.
Therefore, I would hope that you can look at those issues afresh in light of my post at 9.48 a m on the 2nd December.
Stephen Wilde December 3, 2017 at 5:21 am
If you “gave up” on Dr. Brown, then more fool you … regarding your posting.
Stephen Wilde December 2, 2017 at 9:48 am
Willis:
OK …
OK …
Well, we don’t know that. The energy might have come from vulcanism.
Me, I would say the extra 33K came from the sun, not the surface or from vulcanism. Here’s the accounting. Atmosphere forms, contains GHGs, sun warms the surface, GHGs intercept outgoing LW, send half of it back to the surface, surface warms up … what’s not to like?
However, you are missing something very important. If there is no atmosphere, there are no clouds … and with no clouds, the sun would put an additional 70 W/m2 into the system constantly. So after the atmosphere forms, the surface receives less solar energy …
This is why people are asking for a proper accounting … your method leaves things out.
No, because a) the 33k is a one-off change which will quickly equalize out, and 2) the system is now getting 70 W/m2 LESS because there are clouds.
I don’t understand why you think that if you add energy to the atmosphere or take energy out of the atmosphere, that will cause a permanent change in the energy balance. If the atmosphere contains GHGs, the excess energy will be radiated to space. If the atmosphere doesn’t contain GHGs it will be absorbed by the earth.
A one-time addition or subtraction of energy to or from the atmosphere cannot cause a persistent change to the energy flow in the system. If that were so, the energy lost due to every volcano would keep adding up, and we’d be freezing by now.
Say what? I don’t understand that last one at all. I counter your assertion that a one-time event four billion years ago affects today’s energy flows by pointing out that this is clearly not true for other such one-time events.
w.
i) The initial energy required to form an atmosphere does come from the sun.
ii) In order to ascertain the extent of that initial energy only the adiabatic component is relevant. Everything else is just variability around that initial addition of energy. For Earth we can see that the amount of that energy is enough to raise surface temperature by 33k and it is held in the atmosphere as potential energy that does not radiate and does not register as heat until it returns to the surface in adiabatic descent.
iii) If the atmosphere continues to be suspended against gravity by the upward pressure gradient force then that initial energy added during formation continues to be present. It cannot dissipate without collapsing the atmosphere.
iv) Energy added by vulcanism, ghgs or anything else is short term because it does dissipate via convective adjustments neutralising any internal radiative imbalances unlike the initial energy used to lift up the atmosphere.
v) I invite you to consider my workings showing how by using a stocktaking analogy you can start with a specific amount of material but by making a single change at the right time you then hold extra units in stock permanently. You can find that in my response to Paul after he first introduced such an analogy which was flawed. Conduction and convection cause a delay in the throughput of energy through a planetary body so that the amount of energy content is raised to a level that can support both radiative and adiabatic balance.
It really is as simple as that.
You have an up and down adiabatic loop running in parallel with the in and out radiative flow and you need enough molecular agitation at the surface (heat) to maintain both.
Everyone is hung up on unnecessary complexities.
Stephen, if I have a system with two 255 K rocks, with one moving with KE of 200 joules and the other stationery with 300 joules of PE, What is my system radiating?
Unfortunately, that is not a suitable analogy because it invites consideration only of radiative flows operating at the same speed.
One needs a gaseous body with internal movement within a gravitational field in contact with an irradiated solid body.
Your mind is still locked into radiation only scenarios.
What we have to consider is the interplay between radiative flows or fluxes in and out to one body which run at one speed and non radiative energy transformations between two types of energy one of which registers as heat whereas the other does not AND furthermore the two systems are in contact so that conduction can occur.
The most elegant and satisfactory solution is that which I supplied. Two independent energy ‘loops’ running in parallel after the gaseous part has stabilised at hydrostatic equilibrium.
Until you shed the radiative only scenario you won’t be able to grasp the concepts involved but I assure you that my description complies with basic physics.
I find your latest a bit vague and appreciate some clarificacation…..
Here you say,
“What we have to consider is the interplay between radiative flows or fluxes in and out to one body which run at one speed”
Specify the nature of these flows and put same example numbers or Labels on them.
Then you say,
” and non radiative energy transformations between two types of energy one of which registers as heat whereas the other does not ”
Again, nature of flow and some numbers or labels.
Finally…..
“The most elegant and satisfactory solution is that which I supplied. Two independent energy ‘loops’ running in parallel after the gaseous part has stabilised at hydrostatic equilibrium.”
Describe these loops, nature of flow and sample numbers or labels.
Thanks. It would really help my understanding to have some common labeling to discuss further
I’ve already told you several times.
33k up and down adiabatically which is a non radiative process.
255k in and out which is a radiative process.
The two processes combine at the surface to give 288k.
The ‘extra’ 33k was acquired from convection and conduction when the atmosphere formed and is not removed until the atmosphere falls to the ground.
You can’t ignore the 33k coming down simply because it matches 33k going up because there is a time delay of one convective cycle. Once the atmosphere is in place the 33k going up is not taken from the simultaneous downward leg but from the upward leg one cycle previously so the current downward leg of 33k is always a net positive and must be added to the radiative 255k.
That is implicit in the stock control example that I gave you previously.
Correction:
You can’t ignore the 33k coming down simply because it matches 33k going up because there is a time delay of one convective cycle. Once the atmosphere is in place the 33k going up is not taken from the simultaneous downward leg but from the DOWNWARD leg one cycle previously so the current downward leg of 33k is always a net positive and must be added to the radiative 255k.
“Me, I would say the extra 33K came from the sun, not the surface or from vulcanism. Here’s the accounting. Atmosphere forms, contains GHGs, sun warms the surface, GHGs intercept outgoing LW, send half of it back to the surface, surface warms up … what’s not to like? ”
So how does the atmosphere form other than by conduction from the irradiated surface followed by convective ascent followed by convective descent.
GHGs play no part in it because they don’t appear until after the gases have lifted off.
That being the case the surface temperature enhancement comes from conduction and convection. If you then add another 33k from DWIR the surface temperature would be 321k not 288k
I made this comment upthread but it seems to have gotten lost in the stream. For the people who are in the school that says cooler sourced IR can not be absorbed by warmer targets……..
Without changing the subject, and without any need for models or esoteric physics nobody is talking about, answer this simple question…….
Here’s the shot:
Your claim is that IR from a 90 k source incident on a 91 k target, does nothing to the target. If true it has to then either pass through it (albedo of 0) or be reflected from it (albedo of 1). You further claim if that very same source hits the very same target but now the target has a temperature of 89 k it is absorbed, at least partially.
Here’s the chaser:
If you would grant me the liberty of putting that in an equation, it would be albedo= f(T). Do you agree that my equation correctly supports your claim?
Ben Wouters December 3, 2017 at 6:31 am
Obvious? That’s not “obvious” at all. If it were, the moon would be up somewhere near the Earth’s temperature. In fact, we get less net radiation than the moon. We get 340 W/m2 from the sun, of which about a hundred are reflected back to space. The remainder, 240 W/m2, would heat a blackbody to a maximum temperature of about -18°C.
So no, Ben, Stefan-Bolzmann is quite clear about this. It isnot physically possible for 240 W/m2 from the sun to bring the earth up to its current temperature without the poorly-named “greenhouse effect”.
w.
Willis Eschenbach December 3, 2017 at 11:27 am
Since we have only one sun, incoming solar illuminates only half a planet. The other half is “illuminated” by the Cosmic Background Radiation. A blackbody in RADIATIVE balance with these radiation flows (using albedo 0,11 and emissivity 1.0) would have a temperature of ~162K.
Actual average temperature of the moon is ~35K higher at ~197K due to geothermal heat plus some heat carried over from the dayside.
Do you really believe that our atmosphere is the reason the average surface temperature on Earth is ~288K,
over 90K higher than the moons?
Follow up question:
How did our deep (below 1km) oceans get a temperature of around 275K, already 20K above the 255K (-18C) you mentioned as the maximum temperature the sun can create on Earth?
Ben, do you know how many Watts/m2 that 20K would represent?
A C Osborn December 4, 2017 at 11:29 am
Sorry. Don’t understand your question. The ~275K represent the energy content of the deep oceans.
W/m^2 is an energy FLUX.
Ben, thank you for the response, I do realise the difference between Flux and temp.
I was trying to go back to first principles in the Sphere/Shell Example.
I have calculated the mass of a Steel sphere with a 1M surface Area to establish how many Joules it would take to warm it 1K.
But can the Sphere also radiate with a heat flux of 240W/m2 at 0.1K, or 0.001K?
Adding a steel shell even 1mm thick, which obviously is not very realistic, would very slightly increase the amount of Steel to be heated and as the heat source is fixed would this only slow down when it got to radiate 240W/m2 and would the overall temperature be lower?
Ben Wouters December 4, 2017 at 10:33 am Edit
Thanks, Ben. I’m generally careful about what I say. Note that I said that 240 W/m2 (which is a 24/7 average over the surface of the sphere) would heat, not the Earth, but a blackbody, to a MAXIMUM of -18°C. I stand by that statement.
The actual temperature of the earth if there were no GHGs will be lower than that, but how much lower depends on your assumptions. There’s an excellent post on the question here by Dr. Robert Brown, who teaches physics at Duke.
w.
Ok. Do you agree that the sun only illuminates half a planet and that no physical mechanism can distribute that radiation around an entire planet and then create RADIATIVE balance with the incoming radiation?
If yes a simplistic blackbody calculation could be:
1364 W/m^2 /2 = 682 W/m^2 average radiation on the day side giving ~ 331K radiative balance temperature.
(albedo 0,0, emissivity 1,0)
The other half of the blackbody would be at ~3K so the average temperature of a blackbody in radiative balance would be ~167K (actually a bit lower still, since solar isn’t distributed evenly over the day side)
A theoretical blackbody has no heat storage capacity, so rotation rate is irrelevant.
Going from this simple model to the actual moon is pretty straightforward.
Earth is way more complicated.
Thanks for the Dr. Brown post. He seems to agree with this (simplistic) approach.
One essential element is missing in his post, geothermal energy.
To answer my own question on how the deep oceans got their ~275K temperature:
by cooling down from much higher temperatures (350K or even higher)
See this video between ~4:30 and 12:50 for a possible/likely scenario:
https://www.youtube.com/watch?v=gTsJubN68WE&t=2263s
I’m pretty confident I have a mechanism that explains how the temperature of the deep oceans has been maintained over time, how the sun only increases the temperature of the upper layers of the oceans creating the observed surface temperatures, and why the role of the atmosphere is ONLY reduction of energy loss to space, no increasing of the surface temperatures necessary or possible.
So yes, without atmosphere the surface would cool down, and no, this does not mean that the atmosphere increases the surface temperatures.
Ben, thankyou yet again.
I have been arguing with Mr Eshenbach and others about the fact that the the long term Average Temperature is not a Steady State or Equilibrium if everything in the Atmosphere including inputs, outputs and temperatures are in a state of continuous change.
Someone invoked “Dynamic Equilibrium”, but there again the Flux is varying from night to day, year to year and for other periods.
Otherwise how could the Earth have cooled from it’s beginning and from normal to Ice Age and Ice Age back to normal.
Surely Time is a factor in all Flux calculations, ie The SI derived unit of heat rate is joule per second.
A C Osborn December 6, 2017 at 9:31 am
Biggest problem imo in discussing Earth is the adherence to RADIATIVE balance. Works more or less for the moon since it has a surface with very little heat storage capacity. Totally different on Earth, ~70% of the surface is water and several kilometers of it 😉
Incoming solar is ~1364 W/m^2 times the area of a disc with the same radius as Earth.
~30% is send back to space and lost for planet Earth.
~20% is thermalized in the atmosphere (O2, O3, H2O and CO2 mostly) and increases its temperature.
~50% is thermalized at the surface (day side) and increases the temperature of the upper ~10m of soil and ~200m of the oceans.
This energy is in the end lost again as radiation into space from all around the world, mostly via the atmosphere which slows this energy loss (some radiation comes directly from the surface via the atmospheric window).
If the in- and outbound fluxes carry the same energy we have a balanced ENERGY budget, but nothing like a RADIATIVE balance.
Willis,
(I think my original attempt at this reply showed up in the wrong place so I will repeat it here – apologies for any confusion)
Thanks for adding to my reply to Phil, but you did not actually reply to my original question to you which I will ask again:
Consider a white hot bar of steel weighing 1 Kg with a temperature of 1500 c. If an identical bar, also at 1500 c is placed next to it, the temperature of the first bar will not increase. In fact, if an infinite number of new bars, all at 1500 c, are added, the temperature of the first bar would still not increase.
Thus, despite an infinite increase in energy, there would be no temperature increase. This seems to contradict the energy budget approach to deriving temperatures in that no matter how much extra energy was added, the temperature would not increase.
I’m wondering how the Trenberth Energy Balance chart would cope with this scenario of extra energy with no temperature effect?
Best regards
Thanks for persevering, Bernard. I fear that your question is not specific enough.
Let’s further assume that the bar is placed in outer space, nothing around. As a result, it will start to cool at a certain rate. It is radiating a lot (about a half million W/m2), and receiving basically nothing from the environment. Remember, the cooling rate is dependent on what it is gaining less what it is losing.
Now, let’s repeat the experiment, but this time place another identical bar next to the first bar. Again, both bars will cool … but the important point is thatthe first bar will cool more slowly than a single bar. Why? Because in part of the view field of the first bar is no longer receiving ~0 W/m2 from outer space, it is now intercepting and absorbing a part of the half million W/m2 of radiation from the second bar. This perforce must slow the rate of cooling of the first bar.
As a result, and as I’ve said many times, the first bar will end up warmer than it would be without the presence of the second bar.
Is there a “temperature increase” as you ask? No. But then I didn’t say there would be. I said it would end up warmer than without the presence of the other object.
Hope that helps …
w.
Willis Eschenbach December 3, 2017 at 1:06 pm
Thanks for the reply Willis,
I do understand that you are saying that the key effect is to slow the cooling rather than increase the starting temperature. That is not my question.
My question was: In my example, why does adding infinite amounts of new energy to a bar of metal not increase the temperature of the bar?. That seems to contradict the Trenberth Energy Balance logic?
Best regards
Bernard, if you added “… infinite amounts of new energy to a bar of metal …” not only would there be no bar, there would be no universe.
Willis Eschenbach
December 3, 2017 at 1:06 pm: Adding another bar is to completely alter the experiment. It is no longer the same set of conditions when you add MATTER to thereference frame. There is more MASS.
I could do the same with gas. Such is the case for Venus-Earth comparisons, allowing for AU distance and gravities. But it is also true that the reason there are gas laws is that their matter phase is different than solid metals’. So their means of balancing energy flows are not identical, another story. However, the addition of mass may be completely explainable by you, of course. That is what makes physics interesting…..
Bernard Lodge December 3, 2017 at 3:15 pm
Regards, Bernard. It only seems that way because you haven’t specified your situation clearly enough. If your bar is floating in space, then it is cooling and putting another identical bar next to it will slow the cooling.
On the other hand, if it is in a 1500C oven, then it is at steady-state with the environment and is not cooling. It is receiving and emitting the same amount of energy. However, it is already receiving the 1500°C worth of energy from the walls … so putting another 1500°C bar in does nothing. It blocks out the amount of energy it is supplying, so there is no net gain.
The key is the idea “in place of what”? If you put a 1500°C bar next to something in space, it is replacing zero watts of radiation from space with half a million watts of radiation … so things will change.
But if you put a 1500°C bar next to something in a 1500°C oven, it is replacing half a million watts of radiation with half a million watts of radiation … so there’s no temperature change.
Hope this helps, if not, ask questions …
w.
Willis Eschenbach December 3, 2017 at 8:05 pm
“Bernard. It only seems that way because you haven’t specified your situation clearly enough. If your bar is floating in space, then it is cooling and putting another identical bar next to it will slow the cooling.”
+++++++++++++++++++++++
If one bar of metal at 1500 c is then joined by a million bars of metal all at 1500 c, it does not matter where the first bar is – it could be in an oven or a fridge or in outer space. My question is why the extra trillion watts of energy from the new bars does not increase the temperature of the first bar? A really basic question.
If I had added a second bar at 3000 c, the temperature of the first bar would have gone up. However, the energy in one bar at 3000 c is much less than the total energy from a million bars at 1500 c so why does the little bit of energy from the 3000 c bar increase the temperature of the first bar when a trillion watts from 1500 c bars could not?
In the Trenberth Energy Balance, all the energy flows are converted into temperature increases. Yet, in my example above, I introduced a trillion new watts of energy flow and the temperature of the first bar did not increase. It seems in real life that not all energy flows increase the temperature of an absorbing body. Trenberth’s energy budget assumes they do. On the face of it, the Trenberth Energy Budget looks to be incomplete, or maybe even wrong. Perhaps it needs to include the temperature of the source of each radiation flow, not just its energy content?
I hope my question is now clear.
Best regards
It is always best to keep your first steps baby steps. It helps you understand the basic premise before you expand to a million bars.
Most importantly don’t divert the discussion as a debate tactic. Very very common in these topics.
So keep it simple, very simple. Start with two thin plates with geometry such that radiation off their edges is irrelevant. Put them in Vacuum facing each other and start experiment with exactly same temp for each.
What happens? Each plate radiates in two directions. One direction, away from other plate. The other towards its mate.
In the space between the plates there is no NET radiation. Neither plate can change the energy of the other. Each is blasting the other with the same flow, like filling your pool while the fire dept. Pumps it out just as fast.
In the direction away from plates, each plate is radiating away half of the energy it would have radiated if it was alone.
The plates will cool half as fast as they would have had they been alone.
Now if some clown enters the thread and says, “yeah but they’re not rotating and there’s no convection, and the micro wedgies don’t have enough freznoodles to heat anything.” Remember these are clowns, in the arena dirt because they couldn’t get a ride on the bull.
Focus on the example. It’s really simple. You can add 999,998 plates later. Forget temperature it is irrelevant to understanding. Energy is the nut
Bernard Lodge December 4, 2017 at 4:43 pm
Willis Eschenbach December 3, 2017 at 8:05 pm
Thanks, Bernard. Your question is clear. It is your assertion that “it could be in an oven or a fridge or in outer space” that is wrong. Those lead to very different outcomes.
The part you appear to be missing is the part about what the next bar replaces. If we put a 1500°C bar next to another one in a 1500°C oven, there will be no change. Think about it from the point of view of the first bar: where before there was an oven wall radiating at half a million W/m2, now there is a second bar radiating at half a million W/m2 … so absolutely no change in the radiation absorbed by the first bar.
HOWEVER, if the first bar is floating in space, then you’ve replaced basically zero radiation from space with half a million watts of radiated energy … so yes, that changes the radiation absorbed by the bar.
Finally, you seem to think that surrounding a bar of metal at 1500°C with a bunch of metal at 1500°C will somehow raise the temperature of the first bar … but consider the condition of a metal bar in a 1500°C oven. It is totally surrounded by the oven walls at 1500°C.
Does it warm to something more than 1500°C??
Hope this helps, if not, ask more questions. On my planet, the only stupid question is the one I didn’t ask …
w.
Paul Bahlin December 4, 2017 at 5:28 pm
Paul, while you certainly have my thanks for a very clear exposition of the issues, as an erstwhile cowboy I must object to this comparison.
In bull riding the people who keep the riders safe haven’t been called “clowns” for some time now. They are now called “bullfighters”, and for a damn good reason. Their job is to fight the bull and keep him from turning on the fallen cowboy. The bull riders entrust their lives to the bullfighters because without them a lot of good men would have been stomped to death. And despite your claim that “they couldn’t get a ride on the bull”, some of them are former bull riders who couldn’t get the smell of the bulls and the cloying arena dust and the roaring of the crowds out of their systems …
Just saying … the bullfighters are not a group you’d want to diss in a bar around where I grew up.
Other than that?
Your comment was spot on.
w.
Yeah. I figured I would get heat if a rodeo guy read that. I have great respect for those guys. They probably have more stones than the riders.
Anyway, hope you got my drift. It really frosts me when trolls step on an honest attempt to learn
“Me, I would say the extra 33K came from the sun, not the surface or from vulcanism. Here’s the accounting. Atmosphere forms, contains GHGs, sun warms the surface, GHGs intercept outgoing LW, send half of it back to the surface, surface warms up … what’s not to like? ”
So how does the atmosphere form other than by conduction from the irradiated surface followed by convective ascent followed by convective descent.
GHGs play no part in it because they don’t appear until after the gases have lifted off.
That being the case the surface temperature enhancement comes from conduction and convection. If you then add another 33k from DWIR the surface temperature would be 321k not 288k
Stephen Wilde December 3, 2017 at 1:10 pm Edit
From the Smithsonian:
If the gases were indeed “spewed from volcanoes”, your theory that we needed to put energy into the atmosphere as it formed goes out the window. The atmosphere would have been hotter than the surface to start with … and that’s the opposite of your theory.
But that’s not the main objection to your theory. The main objection is that even if your claim is correct and energy was pumped into the atmosphere four billion years ago, the effects of that one-time event on the flow system we call the climate are long gone.
For example, yes, dumping a truckload of water into a creek will raise the level of the creek … but not for long. And dumping a bunch of excess energy into the atmosphere, say by a solar flare or something, will raise the atmospheric temperature …
… but not for long, and certainly not for four billion years.
w.
In the absence of solar heating at the surface being conducted to all the materials from volcanoes those materials would cool and fall to the ground so your objection is invalid.
Anyway, you are distracting from the initial premise of a simple argon atmosphere.
As for your ‘main’ objection please explain the continuing presence of a vast amount of non radiative non thermal PE still in the atmosphere if you say it has all dissipated away.
How exactly do you say that energy could have been removed or otherwise cancelled by some sort of unspecified setoff.
Stephen, you often write the atm. is by and large in hydrostatic equilibrium. This means there is very little available PE to convert to KE. Only when the atm. is out of this equilibrium does it transfer PE to KE. By simple logic.
Whilst the atmosphere is in hydrostatic equilibrium overall the fact is that all rising air and all falling air is in disequilibrium.
If you study meteorology (as I have done for 60 years) and look at the global pressure chart you will see that the entire planet is divided into low pressure cells that are rising air and high pressure cells that are falling air.
Accordingly the entire mass of the atmosphere is constantly engaged in converting surface KE to PE or back again.
The total of KE and PE is vast and it is that energy which provides the upward pressure gradient force that opposes the downward force of gravity.
Actually, first order consideration is atmosphere has mass, all of it taken together, that is held up by the surface
More correctly, it is held up by the force exerted by the surface which is equal and opposite to the force exerted by gravity. It takes no work to do so
Work is required to launch it off the surface in the first place and more work is required to move it upwards in convection. That work converts KE to PE as the molecules move apart along the declining pressure gradient.
The gas is held off the surface by the upward pressure gradient force which requires kinetic energy at the surface to create it. A gas held off a surface is a very different to a solid held above a surface by contact.
Gee whiz, never knew that Stephen.
Atmospheric pressure is the weight of the atmosphere pressing on the surface. It has nothing at all to do with the structure of that atmosphere. The earth exerts an equal and opposite force to it.
If you consider a column, it doesn’t matter if the column has each molecule stacked on one other in a row extending to Pluto, or mashed into the ground like a pancake, or arranged in a magnificent arrangement of layers provided by a pressure gradient. The mass is the same.
You are conflating that which gives it structure, with that which keeps it where it is.
Stated more succinctly….A box of rocks has mass. Shaking it does not change it
You said:
“More correctly, it is held up by the force exerted by the surface which is equal and opposite to the force exerted by gravity. It takes no work to do so”
That gave me the impression that it was you who had conflated that which gives the atmosphere structure with that which keeps it where it is.
To resolve your apparent confusion I pointed out that the declining pressure gradient with height combined with kinetic energy at the surface does do work against gravity to create the upward pressure gradient force.
So, actually, that is what gives the atmosphere structure and keeps it where it is and NOT the mere presence of a surface beneath it.
If you are still using solids such as rocks as an analogy then you have still not got your head around the behaviour of gases as compared to solids.
An increase in molecular motion at the base of a column of gas will cause an increase in the upward pressure gradient force and allow it to expand further upward against gravity.
No molecular motion at the base will cause the gases to fall to the surface.
So 60 years in meteorology and you don’t think atmospheric pressure exerts a force on the Surface?
What’s the difference between
What is the pressure exerted on the surface by 1 kg of rocks? What’s the pressure exerted on the surface by a 1 kg column of air?
Not the point.
The point is that whatever the weight of an atmosphere the formation and maintenance of it depends on kinetic energy at the surface in excess of that predicted by the S-B equation and I’ve given you the same explanation multiple times in different formulations.
The atmospheric gases depart the surface without any input from GHGs so the conduction/convection explanation must be the correct one.
If one says that GHGs account for the surface temperature enhancement such that convection reduces the surface temperature from 255k to 222k and then returns it to 255k for a zero net effect and then GHGs radiate down to raise it to the observed temperature of 288k then you still have to account for yet another 33k worth of incoming radiation which was not being used by the system for the production of IR during the period of lowered surface temperature. What do you say happens to that further 33k?
It just won’t balance if GHGs are proposed as the cause because then you have to propose 321k instead of 288k.
Stephen the atmosphere is not ‘held up’by kinetic energy flowing in from the surface by conduction. It is ‘held up’, and I mean by that no net movement of mass, by the freaking surface.
The thermal energy fed to it by the surface can only heat air, causing differential density in the (fluid) atmosphere, which results in differential density, which results in buoyant forces, which results in air moving up. The mass in a convecting atmosphere is not changing. It is moving.
For every parcel moving up there is a parcel, somewhere, moving down, else we wouldn’t even be here. If you integrate these energies over a reasonable volume, say the planet, and time, say a million years, you must find that moving air and the lapse rate that develops from such movement does not, and can not effect the mass. It can not effect the net acceleration of that mass, which is 0. It effects the instantaneous structure, not the static properties of the entire system. Mass for instance.
I suspect this entire thread has been predicated on your assumption that the atmosphere needs energy to ‘stay up’ there. It doesn’t. It only needs a force to balance the force on it from gravity. Force is not energy. Potential energy is not energy. Energy requires mass to be displaced, net work to be done. The ‘atmosphere’ is not moving. Its parts are moving.
You seem to be describing a system kept in perpetual motion by events that occurred 4 billion years ago. All that PE that was put up there then is still there.
Finally, do a little thought experiment for me….
If there was suddenly no sun, What would keep the atmosphere ‘up there’ and what would the structure be? All that mass would still be above the surface and the temperature would be?????
Too many incorrect statements in there for me to have the will to respond.
Try them one at a time then…..
Does convection change the mass of the atmosphere?
Or this one. Does the atmosphere exert pressure on the surface of the planet?
Is potential-energy energy?
Does KE exchange energy by conduction?
Any one answer will do. You’ve made every one of these things into gobbly gook over the thread.
Stephen 4:43am, you just can’t have it both ways.
Either: “Accordingly the entire mass of the atmosphere is constantly engaged in converting surface KE to PE or back again.”
OR: “Whilst the atmosphere is in hydrostatic equilibrium overall”
If you study meteorology, then a paper written in the 1950s should have told you this. It is basic to the field. There is very little atm. PE available to transform into KE but you have to read the math to understand it and you will never do so. You will never understand this field of meteorology without mastering the math describing the changing processes. Simple logic.
Incorrect.
When energy up equals energy down both statements are compatible.
That doesn’t prevent lots of imbalances either side of the average.
When energy up equals energy down, energy net is zero.
Trick December 3, 2017 at 6:48 am Edit
Trick, once again I have to ask you to QUOTE THE EXACT WORDS THAT YOU ARE REFERRING TO!!!!
Maybe Bohren said something, maybe not. But until you QUOTE WHAT BOHREN SAID, it’s just your lips flapping …
w.
Bohren 2006 p. 4:
We often are told that when bodies are heated they radiate or that “hot” bodies radiate. True enough, but it is just as true that when bodies are cooled they radiate and that “cold” bodies radiate. All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times, although in varying amounts, possibly so small at some frequencies, for some materials, and at some temperatures as to be undetectable with today’s instruments (tomorrow’s, who knows?). Note that there is no hedging here: all means all. No exceptions. Never.
Bohren 1998 p. 119:
According to classical mechanics, a rotating object has rotational energy, which you can verify by trying tro stop a rotating bowling ball by your hand. According to quantum mechanics, the quantized levels of similarly spinning atoms are widely spaced relative to kT. This is why we obtained good agreement with measurement of (ratio of specific heats) for monatomic gases…
—–
Others:
Atomic and Molecular Processes edited by D.R. Bates
(Atomic) rotational quanta are much smaller than (molecular) vibrational quanta, and the probability of a translation-rotational energy transfer occurring in collision is correspondingly higher. Rotational relaxation times are therefore very small. Rotational relaxation phenomena thus play a comparatively unimportant role, and are difficult to measure. This, coupled with the fact that rotational energy is of less chemical significance than vibrational energy has led to rotational relaxation being given comparatively little attention.
Atoms By Jean Perrin
p. 73: Rotational energy does indeed vary by indivisible quanta like the atomic oscillation …dealing with rotations so rapid that each atom revolves more than a million times in one thousandth of a sec.
—–
There are more texts, papers I have yet to obtain & more leads from the 1973 NIST testing ref.s yet to be uncovered.
Thanks, Trick. The claim that
doesn’t even pass the laugh test. Take O3, for example. It only absorbs radiation in a few narrow bands. Kirchoff’s Law says that means it only emits radiation in a few narrow bands. I don’t know who Bohren is, but he doesn’t agree with lots of spectrographic results.
“All frequencies”, for example??? So every object we see is emitting radiation at every single frequency from one hertz to terahertz? Get real. That’s not happening.
I do love his CYA statement that although the most sensitive instruments known to man cannot detect such radiation, by gosh, he knows it is there … and you believe him? Really? HOW does he know it is there if nobody can either measure it or find it?
His are not scientific statements. They are statements of faith.
w.
“Take O3, for example. It only absorbs radiation in a few narrow bands.”
Now Willis is asserting (i.e. “it’s just your lips flapping”), please: “QUOTE THE EXACT WORDS THAT YOU ARE REFERRING TO!!!!”
“So every object we see is emitting radiation at every single frequency from one hertz to terahertz?”
Yes. And lower and higher freq.s. Plug any frequency and any temperature into the Planck Law and you will get an ideal nonzero radiance. This Law is what Bohren goes on to explain – found from actual testing! Maybe even Willis can learn from these discussions.
Trick December 4, 2017 at 4:20 am
“Take O3, for example. It only absorbs radiation in a few narrow bands.”
Now Willis is asserting (i.e. “it’s just your lips flapping”), please: “QUOTE THE EXACT WORDS THAT YOU ARE REFERRING TO!!!!”
“So every object we see is emitting radiation at every single frequency from one hertz to terahertz?”
Yes. And lower and higher freq.s. Plug any frequency and any temperature into the Planck Law and you will get an ideal nonzero radiance. This Law is what Bohren goes on to explain – found from actual testing! Maybe even Willis can learn from these discussions.
Planck’s Law applies to black bodies, gases are not black bodies and only emit at discrete wavelengths determined by quantum mechanics. Apparently Bohren doesn’t understand quantum mechanics. Planck’s Law gives an upper limit to emissions that’s all. Actual testing with gases bears this out, here’s one for Argon that I posted earlier:
Note it emits at discrete wavelengths not in a Planck continuum!
Phil, no fair; you are bringing data to a verbal gunfight.
Is there any recognised Scientist that MR Eshenbach is not prepared to rubbish other than Dr. Robert Brown and Leif, jesus what an EGO.
“They are statements of faith.” now who do we know that that description applies to?
A C Osborn December 4, 2017 at 11:39 am Edit
A C, if you assert as Bohren does that all object radiate at all frequencies at all times, and follow that with a statement that if we can’t find said radiation it’s because our instruments are not sensitive enough … sorry, but that is a statement of faith.
Remember Feynmann, he’s always worth listening to. He said:
w.
“Planck’s Law gives an upper limit to emissions that’s all.”
Yes, note the word ideal. Ideal radiance at all frequencies all temperatures all the time as Bohren notes.
To add to Willis’ comment: “Those of you who have heard the other two lectures will also find this lecture incomprehensible, but you know that that’s all right: as I explained in the first lecture, the way we have to describe Nature is generally incomprehensible to us.” Richard Feynman.
“Note it emits at discrete wavelengths not in a Planck continuum!”
What gas pressure? What power? Exposure time? To observe the continuum see results with much, much longer exposures.
The 1973 NIST data was produced at around 0.2-0.4torr and 1 to maybe 10 watts of power (assuming 120 volts), with such weak emissions some exposure times were for an hour. The apparatus used is not fully explained as it was in common use for a long time, they don’t even mention temperature just a 1.5L circulating water jacket with a thermostat. No setting given, just a ref. to earlier work to describe the apparatus maybe 20 years earlier (1953) that will be even harder to find.
Trick December 4, 2017 at 2:52 pm
“Planck’s Law gives an upper limit to emissions that’s all.”
Yes, note the word ideal. Ideal radiance at all frequencies all temperatures all the time as Bohren notes.
He doesn’t mention ‘ideal’.
Trick December 4, 2017 at 12:51 am
Bohren 2006 p. 4:
We often are told that when bodies are heated they radiate or that “hot” bodies radiate. True enough, but it is just as true that when bodies are cooled they radiate and that “cold” bodies radiate. All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times,
Not true this does not apply in the case of gases!
although in varying amounts, possibly so small at some frequencies, for some materials, and at some temperatures as to be undetectable with today’s instruments (tomorrow’s, who knows?). Note that there is no hedging here: all means all. No exceptions. Never.
The hedging is where he says that is might not be detectable!
Bohren 1998 p. 119:
According to classical mechanics, a rotating object has rotational energy, which you can verify by trying tro stop a rotating bowling ball by your hand. According to quantum mechanics, the quantized levels of similarly spinning atoms are widely spaced relative to kT. This is why we obtained good agreement with measurement of (ratio of specific heats) for monatomic gases…
There are no ‘quantized levels of similarly spinning atoms’ they are quantized levels of electronic excitation and as I’ve pointed out several times above their spacing far exceeds kT. It’s scary that someone can get a PhD in physics and publish books with such a fundamental misunderstanding of QM.
Trick December 4, 2017 at 12:51 am
Atomic and Molecular Processes edited by D.R. Bates
(Atomic) rotational quanta are much smaller than (molecular) vibrational quanta, and the probability of a translation-rotational energy transfer occurring in collision is correspondingly higher. Rotational relaxation times are therefore very small. Rotational relaxation phenomena thus play a comparatively unimportant role, and are difficult to measure. This, coupled with the fact that rotational energy is of less chemical significance than vibrational energy has led to rotational relaxation being given comparatively little attention.
Please note that the above ‘quotation’ from Bates is not accurate, the actual quotation is:
“Rotational quanta are much smaller than vibrational quanta, and the probability of a translation-rotational energy transfer occurring in collision is correspondingly higher. Rotational relaxation times are therefore very small. Rotational relaxation phenomena thus play a comparatively unimportant role, and are difficult to measure. This, coupled with the fact that rotational energy is of less chemical significance than vibrational energy has led to rotational relaxation being given comparatively little attention.”
The section it comes from is explicitly referring to Molecular processes, the addition of (Atomic) is deceptive!
The paragraph is comparing molecular rotational quanta with vibrational quanta, there are no atomic rotational or vibrational quanta. I don’t know where you got it from Trick but I would suggest treating that source with caution in future.
Atoms By Jean Perrin
p. 73: Rotational energy does indeed vary by indivisible quanta like the atomic oscillation …dealing with rotations so rapid that each atom revolves more than a million times in one thousandth of a sec.
This was published in 1913! He was explicitly talking about molecular rotations and citing the evidence for their quantization.
Just after Einstein and Bohr, before de Broglie, Heisenberg, Schrodinger, Born, Dirac, Pauli, Heitler and London to name just a few. The terms photon and quantum mechanics (quantenmechanik) didn’t exist yet!
Phil.: Thanks, these are just taken off the internet in quick scratch the surface searches to develop a reading list, Willis asked for what I had at the moment and I listed them (incomplete) for purpose – to attract a reply. I inserted the (words) as it seemed like that was the context reading the preceding page(s), so I differ with your comments. There is lot more work I need to do to complete dot connecting.
In 1913 I might point out the huge problems found between equipartition and specific heat testing were still a research subject from the late 19th century masters which wasn’t explained until QM. And this problem has been largely forgotten today. Would be interesting from a historical perspective to read Perrin.
“It’s scary that someone can get a PhD in physics and publish books with such a fundamental misunderstanding of QM.”
I would not recommend so hastily jumping to the classic internet futile tactic of attacking the person instead of the science. You need to read Bohren more thoroughly to appreciate his work in context, if interested enough. His source material includes a ref. to a delightful 1996 paper on this subject I would not have pulled had this discussion not occurred, for that I am grateful:
“Textbook treatments of specific heats of gases are discussed critically and thoroughly by Clayton A. Gearhart, 1996: Specific heats and the equipartition law in introductory textbooks. American Journal of Physics, Vol. 64, pp. 995-1000. Gearhart concludes that very few (of 27) physics textbooks treat specific heats correctly, yet another sad example of error propagation.”
Error propagation in textbooks is a recurring theme of Bohren’s as he repeatedly goes back to the source papers to compare current text book wording. Gearhart connects the dots from Perrin’s time to QM.
I’ve had 2or3 reads of the paper now and it will take more to absorb it all. For instance, the author explains why the 3 atomic rotational degrees of freedom are not needed for the heat capacity ratio that I mentioned earlier, only the translational DOF show up.
Plus this: “Atomic nuclei, of course, are no more rigid bodies than atoms or molecules. Nevertheless, collective “rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra.” A ref. to Cohen 1971 p. 147ff is given so I will now need to pull that work as this does not connect the dots to your writing. Then go back and try to connect the Gearhart dots to Bohren 1998 p. 119 treatment and your comments.
Dunno if my interest will persevere long enough (competing interests) but this task will likely outlive the life of this comment thread.
Ok Phil., I’m now convinced Gearhart 1996 adds enough to the answer that I’m satisfied dots are connected.
If still interested, what I find* re-reading Bohren, Gearhart is that the Ar atoms are indeed spinning and spectra from rotational base level to excited state has been measured for noble gases (see Cohen not NIST). Thus your “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.” is too simplistic an explanation for this very complex situation.
Classic physics calculates diatomic ratio of ideal gas specific heats 1.25 but all room temperature measurements showed closer to 1.4. This was a BIG problem at the time. Stumped all the late 1800s early 1900s physicists until QM was introduced. These guys at the time were offering odd ball solutions to protect classical physics similar to what you find in this comment thread et.al. .
Gearhart indicates when the QM was introduced it solved the problem of why certain DOF were excluded (from both monatomic and diatomic) in natural measurements but not by classical physics. Bohren writes this elimination of DOF guess had been eventually accurately made but no one knew why until QM explained it.
The quantum spacing of the rotational (monatomic spin and diatomic about the line joining the atoms) levels was too widely spaced relative to kT to matter at room temperatures where the specific heats/ratios were being measured, so some DOFs were ruled out by QM which then accounted for the measured 1.4 ratio. I am assuming for now this does go along with your base state comment iirc.
And it is consistent with the answer to Willis’ Ar atm. question being YES.
Note electronic excitation had nothing to do with the problem as they were much higher multiples of kT. NIST testing was a wild goose chase. It was the monatomic and diatomic QM rotational level DOF elimination considerations that explained the difference.
Gearhart explains this is now so uninteresting that it is being forgotten and written up wrongly in all but a few text books. He has a great story, must have spent a lot of time digging thru text books, something pissed him off to do so.
Says new authors are so hindered by the forgotten problem they seem afraid to rock the boat and just go along with other incorrect texts. Some word it incorrectly then put a small print footnote correction. Funny read.
*Recommend read the source material for the real stories first hand. I might pick up more on another re-re-read.
Trick December 4, 2017 at 6:57 pm
Ok Phil., I’m now convinced Gearhart 1996 adds enough to the answer that I’m satisfied dots are connected.
If still interested, what I find* re-reading Bohren, Gearhart is that the Ar atoms are indeed spinning and spectra from rotational base level to excited state has been measured for noble gases (see Cohen not NIST). Thus your “Argon is an atom not a molecule so it only has electronic states, no vibrational or rotational states.” is too simplistic an explanation for this very complex situation.
Is that the same Cohen referenced in Sandy Ashmore and Rog Donovan’s book? If so his work was on Argon hydrides not the same thing at all!
Gearhart mainly publishes on the history of quantum mechanics, however what I posted here is the current position of the subject, Argon does not have rotational or vibrational levels, your misreading and Gearhart’s misstatements notwithstanding.
Trick December 4, 2017 at 2:35 pm
Phil.: Thanks, these are just taken off the internet in quick scratch the surface searches to develop a reading list, Willis asked for what I had at the moment and I listed them (incomplete) for purpose – to attract a reply. I inserted the (words) as it seemed like that was the context reading the preceding page(s), so I differ with your comments. There is lot more work I need to do to complete dot connecting.
Yes I read the preceding page it refers to molecular spectra not atomic as you incorrectly stated. By the way when you claim to be quoting a reference it is important to clearly indicate any changes you made to it.
“It’s scary that someone can get a PhD in physics and publish books with such a fundamental misunderstanding of QM.”
I would not recommend so hastily jumping to the classic internet futile tactic of attacking the person instead of the science.
I’m not ‘attacking’ the author just expressing disappointment in his fundamental error!
”Is that the same Cohen referenced in Sandy Ashmore and Rog Donovan’s book?”
Dunno. I have the 1971 Cohen book on order, it is interesting that in 1996 Gearhart went all the way back to 1971 to find a ref. for noble gas spectra. But the NIST work goes all the way back to at least 1953 in their ref.s of the Ar apparatus used so there must have been some interest in Ar at those times.
”Argon does not have rotational…levels.”
Then your calculated ratio of Ar specific heats will be different than those you measure for Ar. You will have the same conundrum existing as before QM explained allowed atomic DOFs which Gearhart and Bohren explain.
”I’m not ‘attacking’ the author just expressing disappointment in his fundamental error!”
There is no error, experiments for the ratio of specific heats match calculations when the QM explanation for the atomic DOFs is understood to include quantum rotation.
Trick December 5, 2017 at 6:47 pm
”Is that the same Cohen referenced in Sandy Ashmore and Rog Donovan’s book?”
Dunno. I have the 1971 Cohen book on order, it is interesting that in 1996 Gearhart went all the way back to 1971 to find a ref. for noble gas spectra. But the NIST work goes all the way back to at least 1953 in their ref.s of the Ar apparatus used so there must have been some interest in Ar at those times.
If you can order the book surely you can give us the reference?
”Argon does not have rotational…levels.”
Then your calculated ratio of Ar specific heats will be different than those you measure for Ar. You will have the same conundrum existing as before QM explained allowed atomic DOFs which Gearhart and Bohren explain.
My calculated ratio is 1.66, that is also the measured ratio.
”I’m not ‘attacking’ the author just expressing disappointment in his fundamental error!”
There is no error, experiments for the ratio of specific heats match calculations when the QM explanation for the atomic DOFs is understood to include quantum rotation.
The error I referred to above was: “All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times,”.
However the ratio of specific heats for Argon does not allow for quantum rotation.
Trick December 4, 2017 at 2:35 pm
“Textbook treatments of specific heats of gases are discussed critically and thoroughly by Clayton A. Gearhart, 1996: Specific heats and the equipartition law in introductory textbooks. American Journal of Physics, Vol. 64, pp. 995-1000. Gearhart concludes that very few (of 27) physics textbooks treat specific heats correctly, yet another sad example of error propagation.”
Indeed, as he points out this is a failing of ‘Physics’ textbooks because they try to explain it without involving quantum mechanics, he also points out that ‘Physical Chemistry’ texts get it right because they involve quantum mechanics, specifically the one I used as an undergraduate, Fowler’s ‘Statistical Mechanics’.
He also says: “As it happens most if not all common monatomic gases have spherically symmetric nuclei , and hence cannot rotate.” (emphasis mine)
“:..spherically symmetric nuclei , and hence cannot rotate.”
The nuclei cannot rotate.
”If you can order the book surely you can give us the reference?”
See 2:35pm comment Gearhart ref. 21: ”ref. to Cohen 1971 p. 147ff..”
”My calculated ratio is 1.66, that is also the measured ratio.”
Because you made a lucky guess when calculating this nature of an atom, this same guess will not work for diatomic molecules. As Bohren writes: “It is not enough to be content with getting right answers by making a lucky guess. Without understanding why your guess was right, your next guess (about diatomic molecules using this guess) might not be so lucky.”
And this is what Gearhart 1996 explains, your lucky guess was known to be useful to calculate the measured 1.66 (independent of temperature) but not understood until QM explained it.
It will be a few more days until the Cohen book arrives.
“However the ratio of specific heats for Argon does not allow for quantum rotation.”
Gearhart notes from his ref. 21 Cohen 1971: “Nevertheless, the collective “rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra.”
He then goes on to explain why they do not show up which I take to mean do not show up as in his earlier discussed specific heat 1.66 calculation which is independent of temperature.
I won’t be able comment more than this until the Cohen book arrives.
Trick December 6, 2017 at 9:34 am
“:..spherically symmetric nuclei , and hence cannot rotate.”
The nuclei cannot rotate.
Exactly that’s the whole point, that’s where the mass of the Argon atom resides. As the paper you referenced points out the rotation of the electrons are not an issue.
Trick December 6, 2017 at 10:02 am
”If you can order the book surely you can give us the reference?”
See 2:35pm comment Gearhart ref. 21: ”ref. to Cohen 1971 p. 147ff..”
”My calculated ratio is 1.66, that is also the measured ratio.”
Because you made a lucky guess when calculating this nature of an atom, this same guess will not work for diatomic molecules. As Bohren writes: “It is not enough to be content with getting right answers by making a lucky guess. Without understanding why your guess was right, your next guess (about diatomic molecules using this guess) might not be so lucky.”
And this is what Gearhart 1996 explains, your lucky guess was known to be useful to calculate the measured 1.66 (independent of temperature) but not understood until QM explained it.
As a physical chemist I apply quantum mechanics, I did not make a ‘lucky guess’. Gearhart is referring to the erroneous attempt by physics text books to explain the DOF using classical mechanics, he’s quite clear that the correct application of quantum mechanics yields the correct result (and that physical chemistry textbooks do it the right way using QM). My assessment is based on the science of 2017, not a century ago!
Trick December 6, 2017 at 10:15 am
“However the ratio of specific heats for Argon does not allow for quantum rotation.”
Gearhart notes from his ref. 21 Cohen 1971: “Nevertheless, the collective “rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra.”
If you erroneously try to apply classical mechanics but as he points out if you correctly apply QM they do not.
He then goes on to explain why they do not show up which I take to mean do not show up as in his earlier discussed specific heat 1.66 calculation which is independent of temperature.
Here is what he says about the application of quantum mechanics to the equation:
“In particular, a symmetric rotator model can describe the rotational states of a spherically or axially symmetric “rigid body”; but all allowable wave functions for such systems must correspond to zero angular momentum about a symmetry axis [J=0 in Eq. (4)]. Thus a spherical nucleus cannot rotate.”
(My emphasis)
”Exactly that’s the whole point.”
Phi., not exactly, you are not reading Gearhart words closely enough to get his meaning. NUCLEI! He specifically means the nuclei cannot rotate wrt to the electron shell. Once the electron shell is included he calls that the collective “rigid body” modes which can rotate and cites Cohen.
”I did not make a ‘lucky guess’.”
You guessed the rotation DOFs could be ignored for your 1.66 calculation and only included the translational DOFs. You guessed right for an atom but this guess is wrong for a diatomic molecule gamma. QM explains why the guess is wrong.
”If you erroneously try to apply classical mechanics..”
No, Gearhart just explained the QM “symmetrical rotator” which assumes masses of finite size which he is applying here.
”Thus a spherical nucleus cannot rotate.”
NUCLEUS!! The QM “symmetrical rotator” which assumes masses of finite size includes the electron shell; collective “rigid body” is MORE than just the atomic nucleus & collectively exhibit rotational spectra. Have to await Cohen to go deeper.
Trick December 7, 2017 at 10:20 am
Trick, I’m not following this. It appears you are saying that the argon atom can rotate, but the nucleus isn’t rotating. This sounds highly improbable, but my knowledge of QM approaches 0K … what am I missing here?
w.
Willis, the 1996 paper by Gearhart is explaining the whole atom (nucleus and electrons) as a “rigid body” can rotate and the structure absorb photon quanta per QM as shown in spectra by his ref. to Cohen. Gearhart explains since most of the mass of the “rigid body” is in the nucleus, it is NOT just the nucleus rotating inside the electron shell absorbing the photon but the whole atomic structure. The nucleus does not rotate wrt to the electron shell. Gearhart points to Cohen for the testing, so I can’t make more progress until I find out what Cohen wrote & tested. No doubt he will ref. somebody else and the trail will continue.
The paper is easy to find at a college library and summarizes the errors found in 22 out of 27 physics texts on the subject. If not even the specialists can get this right all the time, or even bat .300 it is easy to understand how difficult is the subject.
Trick December 7, 2017 at 10:20 am
”Exactly that’s the whole point.”
Phi., not exactly, you are not reading Gearhart words closely enough to get his meaning. NUCLEI! He specifically means the nuclei cannot rotate wrt to the electron shell. Once the electron shell is included he calls that the collective “rigid body” modes which can rotate and cites Cohen.
No I’m reading it correctly, QM quite explicitly states there are no quantized atomic rotations, period!
,em>”I did not make a ‘lucky guess’.”
You guessed the rotation DOFs could be ignored for your 1.66 calculation and only included the translational DOFs. You guessed right for an atom but this guess is wrong for a diatomic molecule gamma. QM explains why the guess is wrong.
No I correctly applied QM which states exactly why there are only 3 DOF for an atom and also why there are only 5 DOF (two rotational) for a a homonuclear diatomic.
”If you erroneously try to apply classical mechanics..”
No, Gearhart just explained the QM “symmetrical rotator” which assumes masses of finite size which he is applying here.
”Thus a spherical nucleus cannot rotate.”
NUCLEUS!! The QM “symmetrical rotator” which assumes masses of finite size includes the electron shell; collective “rigid body” is MORE than just the atomic nucleus & collectively exhibit rotational spectra. Have to await Cohen to go deeper.
You’re already out of your depth!
Trick December 7, 2017 at 12:40 pm
Willis, the 1996 paper by Gearhart is explaining the whole atom (nucleus and electrons) as a “rigid body” can rotate and the structure absorb photon quanta per QM as shown in spectra by his ref. to Cohen. Gearhart explains since most of the mass of the “rigid body” is in the nucleus, it is NOT just the nucleus rotating inside the electron shell absorbing the photon but the whole atomic structure. The nucleus does not rotate wrt to the electron shell. Gearhart points to Cohen for the testing, so I can’t make more progress until I find out what Cohen wrote & tested. No doubt he will ref. somebody else and the trail will continue.
The paper is easy to find at a college library and summarizes the errors found in 22 out of 27 physics texts on the subject. If not even the specialists can get this right all the time, or even bat .300 it is easy to understand how difficult is the subject.
Yes the Physics texts because they try to do it without reference to QM and so get it wrong, on the other hand he points out that Physical Chemistry texts use QM and get it right.
Your first paragraph is incorrect, he shows that application of the Born-Oppenheimer approximation specifically excludes the electrons and categorically states that: “Molecular rotations are thus due to nuclear motion.” (referring to Herzberg, one of the undergraduate texts I used).
”You’re already out of your depth!”
Everyone has an opinion Phil. Let’s see: I pointed out Bohren’s text discussion differed from that of Phil., and dug out the Gearhart paper supporting Bohren’s views. Phil. put up a link to incomplete NIST spectral line data that Phil. does not or did not know:
1) The source i.e. who, what, when, where the spectra were run
2) The pressures the spectra were run at
3) The exposure times
4) The apparatus used
5) The temperature
6) The power applied, the source of the illumination, so forth.
What is more Phil. then confused Gearhart’s discussion of the nucleus with that of the atom.
”(Gearhart) points out that Physical Chemistry texts use QM and get it right.”
Gearhart in footnotes: “I have not explicitly stated which books give incorrect or misleading explanations.”
So, I also have an opinion of Phil. but I’ll keep it to myself. I will not pre-judge what the existing collective “rigid body” modes testing will reveal about nuclear rotational spectra in Cohen’s 1971 book until I’ve read and understood that material which Gearhart cites.
Trick December 8, 2017 at 5:16 am
”You’re already out of your depth!”
Everyone has an opinion Phil. Let’s see: I pointed out Bohren’s text discussion differed from that of Phil., and dug out the Gearhart paper supporting Bohren’s views. Phil. put up a link to incomplete NIST spectral line data that Phil. does not or did not know:
On the contrary I put up a link which showed all the energy levels between the ground state and the first ionization level, it is complete. It also included the reference to the original paper which answered all the questions about how the spectra were acquired, which I had read, I had to explain to you that the spectra included on the NIST site were the result of an electronic discharge and even had to explain what an etalon was to you which apparently you didn’t understand.
What is more Phil. then confused Gearhart’s discussion of the nucleus with that of the atom.
No I did not, you are the one who is confused, Gearhart explicitly states that “It is apparent that the electrons cannot participate in any collective rotational mode corresponding to the “rigid body” rotation of the molecule as a whole about an axis of symmetry.” Based on this it is clear that he regards the rotation of the nucleus as the important factor in determining the rotation of an atom and all of his subsequent discussion focuses on the nucleus.
As Gearhart says:
“Now in a multielectron atom or molecule, it is in principle possible that a suitable approximation would turn up a collective mode that would appear as a rotational energy spectrum. However, any approximate separation of a Hamiltonian into single electronic states on the one hand, and collective rotational states on the other, would have to rely on something like a Born-Oppenheimer approximation, which requires that rotational energy levels be very different in magnitude from single electronic states. But a rough estimate of the moment of inertia of an orbital electron about the molecular axis of symmetry shows that the rotational energies are on the order of eV -about the same as typical electronic energy states in atoms and diatomic molecules. It therefore appears that one cannot interpret atomic or molecular electronic excited states in terms of collective “rigid body” rotation. (Note, of course, that these electronic states, however regarded, typically have energies >> kT at room temperature and so cannot contribute to the specific heat.)”
“Atomic nuclei, of course, are no more rigid bodies than are atoms or molecules. Nevertheless, collective ”rigid body” modes that lead to nuclear rotational spectra do exist, just as they do for molecular spectra. In principle, therefore, one might expect three rotational degrees of freedom for monatomic gas molecules such as mercury or the noble gases, corresponding to nuclear rotations about three mutually perpendicular axes through the center of the nucleus. These degrees of freedom do not show up for two reasons. First, as argued above, the lowest excited rotational energy state for such systems is on the order of 0.1 MeV, far greater than kT, and so the equipartition law could not apply.
A second, more fundamental argument, stemming from the symmetry of the wave function, provides yet another reason not to expect nuclear rotations about axes of symmetry. Consider a wave function rj, that is spherically symmetric. By a well-known theorem in quantum mechanics, such a state must have zero angular momentum. Similar considerations apply to systems that are axially symmetric. This theorem certainly applies to wave functions describing collective “rigid body” motion. Hence for spherically or axially symmetric nuclei, there can be no collective rotation about axes of symmetry. In particular, a symmetric rotator model can describe the rotational states of a spherically or axially symmetric “rigid body”; but all allowable wave functions for such systems must correspond to zero angular momentum about a symmetry axis [J=O in Eq. (4)]. Thus, a spherical nucleus cannot rotate.”
”(Gearhart) points out that Physical Chemistry texts use QM and get it right.”
Gearhart in footnotes: “I have not explicitly stated which books give incorrect or misleading explanations.”
That footnote is explicitly referring to the 27 Physics texts which he lists, not the physical chemistry texts he later references: “Since I am interested not in pointing fingers at any particular text, but in calling attention to a widespread problem, I have not explicitly stated which books give incorrect or misleading explanations. Readers will have little difficulty in making the identifications for themselves. ”
Gearhart is quite clear he takes issue with the physics text books’ attempt to explain atomic spectra using classical physics and not invoking QM and shows that their approach is wrong. He also points out that among the few that get it right: “It is noteworthy that two of the six texts that give correct explanations seem decidedly nervous about doing so. One states in the text that equipartition fails in diatomic molecules because the moment of inertia about the axis of symmetry is negligible, but then adds in small print in a footnote that ” A proper justification is provided by modern quantum mechanics.” A second book offers the same explanation with the observation that it was an “early attempt” which “leaves one feeling that the theory has been fudged,” and goes on to state that the “correct reason” is given by quantum mechanics. It is difficult to avoid the impression that both authors were a little intimidated by the weight of textbook tradition!”
He also comments that: “A full quantum mechanical treatment of atomic and mechanical rotations is neither trivial nor particularly well known to many physicists”
“By contrast, the quantum mechanical ”symmetric rotator,” which assumes masses of finite size, it is was not a standard textbook problem (although, interestingly, one of the earliest systems to receive a detailed quantum mechanical treatment). One typically sees it today in physical chemistry or molecular spectroscopy texts. One also sees it in older texts such as Pauling and Wilson’s Introduction to Quantum Mechanics and Fowler’s Statistical Mechanics.”
Texts which I used as an undergraduate, perhaps that is why I understand it better than ‘many physicists’?
So, I also have an opinion of Phil. but I’ll keep it to myself. I will not pre-judge what the existing collective “rigid body” modes testing will reveal about nuclear rotational spectra in Cohen’s 1971 book until I’ve read and understood that material which Gearhart cites.
Well Gearhart agrees with me that the only energy states that exist for Argon are the electronic levels indicated in the NIST report and that there are no rotational states, and therefore no absorption of IR under atmospheric conditions. Cohen’s book is about nuclear physics and may not give you the answers you seek.
”..even had to explain what an etalon was to you which apparently you didn’t understand.”
Ha, no Phil. that is just your opinion.
I’ll spend more time with your 9:07pm later if the thread doesn’t close, thanks for the major effort, but wanted to fill you in that Gearhart’s ref. Cohen does explain where the notion of “modern-physics courses” that write atoms do not spin comes from. I will let you find and enjoy that passage.
Cohen explains why atoms do spin and gives detailed measurements of photon absorption increasing the quanta of rotational energy for an atom. He lists (Fig. 6-17) the energies of excitation above the ground-state rotational band for some heavier atoms in a “low energy-level spectrum of this type.” Cites tests from a 1960 ref. So the trail for Ar goes cold in Cohen but possibly is discussed in the earlier text.
My interest will end there with 4 authors discussing atoms do have rotational energy level spectra as shown from experiment. These 4 authors (and possibly the 5 of 27) will vote with me that the answer to Willis’ challenge is YES.
SkepticGoneWild December 3, 2017 at 10:52 pm Edit
Oh, stop with the asinine snark. It makes you look ugly. The laws of thermo are just that, laws. Nobody can break them.
If the internal radioactivity of the sphere puts out 240 W/m2, then 240 W/m2 is emitted to space. Nothing is created.
NO. I’ll say it again using small words so you can grab it, because I’ve said it many times now and you’ve ignored it.
Heat can’t flow from cold to hot … but energy sure can.
The distinction between heat and energy still seems to be escaping you. Heat is is a special class of energy. It is the NET energy that that is moved by a temperature difference.
Radiation is a very different kind of energy. It just flows wherever it happens to flow. It can flow from cold to hot, as when my warm eyes can see cold bioluminescence in the ocean. According to you, that’s impossible. You’d claim that I’m saying that HEAT is flowing from the bioluminescence to my eyes. But it is not heat, it is energy. It flows from colder to hotter all the time. Put two objects near each other. Energy is radiated by the colder one and absorbed by the warmer one. At the same time, of course, MORE energy is also flowing from from the warmer one to the colder one. LOOK AT FIGURE 1. The flow goes both ways. We subtract one from the other, as I showed in the equation in the head post, to get the net flow. This net flow is called a “heat flux”, and as you would expect, it only flows from hot to cold.
As numerous people have noted, this is not complex. This is introductory thermodynamics. You take the larger energy flow, subtract the smaller energy flow, and the result is the heat flux. Standard stuff, well understood for a couple of centuries or so.
In the steel greenhouse, to pick a number, let’s say that in steady-state without a shell the sphere radiates 240 W/m2 to space. With a shell, the first law of thermo says it still must emit 240 W/m2 to space … but because a shell has two sides and a planet does not, the shell has twice the surface area of the planet.
So … we pop the shell around the planet. It is receiving 240 W/m2. But because it has twice the surface area, it only radiates 120 W/m2. Not only that, but half of it goes to space … and the other half is radiated inwards.
You seem to think that it cannot radiate inwards … but what is stopping it? If it radiates outwards it must radiate inwards. And what is absorbing that radiation?
The sphere. This makes the sphere heat up, and it continues to heat until 240 W/m2 is escaping to outer space from the shell. Then it is in steady-state, 240 W/m2 generated, 240 W/m2 emitted to space.
So. At steady-state, the shell MUST be radiating 240 W/m2 to space. This means it is also radiating 240 W/m2 inwards to the sphere. Now lets check first law.
The sphere is getting 480 W/m2, half from internal generation and half from the shell. It is radiating the exact same, 480 W/m2. No first law violation, no energy created.
The shell is receiving 480 W/m2. It is emitting 240 W/m2 through twice the area, which means it too is radiating exactly what it is receiving. No first law violation.
Now, let’s look at heat flow. The sphere is radiating 480 W/m2, and receiving 240 W/m2 from the shell. NET heat flow is outward to the shell, as we’d expect. It is a NET heat flow of 480 W/m2 emitted minus 240 W/m2 received from the shell, meaning the net heat flow is 240 W/m2 from the sphere to the shell … just as we would expect. Finally, the shell is emitting 240 W/m2 to space and receiving nothing in return. So it too has a net heat flow of 240 W/m2 to space.
Note that in all cases, heat is flowing from hot to cold—from the sphere to the shell, 240 W/m2, and from the shell to outer space, 240 W/m2. Everything balances, no thermodynamic laws broken.
What, am I your white knight? I’m supposed to defend your honor now? Sorry, pal, that’s on you. I’ll defend Ed Bo and many of the other commenters here, they don’t give me your nasty snark and personal attacks. I will not defend you, I view your actions as indefensible. That’s your job.
Truly, Skeptic, the first rule of holes applies here. Get a thermo text and stop making a public fool of yourself.
w.
Willis, you will receive no opposition from me.
Had you considered that, if there was a gap between the sphere and the shell, the shell would not be receiving 240 W/m^2?
Not sure what you mean, Dave. There is a gap between the sphere and the shell. It’s small, but it’s vacuum, so the exact dimensions don’t matter.
Well, if there is a gap, the interior area of the shell is greater than the area of the sphere. If the sphere is radiating at 240 W/m^2, the larger shell is receiving radiated energy over a larger area and must be receiving energy at a value less than 240 W/m^2. No?
This is a trivial matter, but illustrates the difference between per-unit values and absolute values.
Dave Fair December 4, 2017 at 2:32 pm
Thanks, Dave. Let’s put some numbers on it. The earth’s radius is about 6,378 km. The effective radiation altitude of the atmosphere is on the order of two km. The difference between the surface of the earth and the surface of a shell that is 2 km above the earth is a meaningless six hundredths of one measly percent (0.06%).
As such, it is customary to omit such trivial effects from the kind of first-order analysis I did in The Steel Greenhouse.
I suppose I should mention two other times I’ve explained this:
People Living in Glass Planets
and
The R. W. Wood Experiment
Perhaps those will clarify things for some people.
Regards,
w.
Dave Fair December 4, 2017 at 1:31 pm
Willis, you will receive no opposition from me.
Had you considered that, if there was a gap between the sphere and the shell, the shell would not be receiving 240 W/m^2?
That would be taken care of in the heat transfer equations by using the correct view factors, in the situation posited by Willis it will make a negligible change to the numbers.
Iterations to achieve 240 W/m2 out from Shell.
1. 240 out from sphere 60 out from shell 60 in to sphere at starting point
2. 300 out from sphere 75 out from shell 75 in to sphere
3. 375 out from sphere 93.7 out from shell 93.7 in to sphere
4. 468.75 out from sphere 117.1875 out from shell 117.1875 in to sphere
5. 585.9375 out from sphere 146.484375 out from shell 146.484375 in to sphere
6. 732.421875 out from sphere 183.1054688 out from shell 183.1054688 in to sphere
7. 915.5273438 out from sphere 228.8818359 out from shell 228.8818359 in to sphere
So we nearly have Your equilibrium, so to get to 240 out from shell we just need another 11.1 so that is doubled for the watts going in as well as out.
So the final temperature of the Sphere in now 937.5 W/m2 to obtain an output to space of 240 W/m2.
So unless you can point out where I went wrong does that look like a sphere at 480 = shell out at 240 to you Mr Eshenbach?
But we have a small problem here as the Sphere is now at 937.5 w/m2 and the shell is also exporting 240 W/m2 so although the 240 source heat to the Sphere is equal to the 240 out to space from the shell, the sphere is still getting 240 watt/m2 from the source and the shell is now putting 240 watt/m2 inwards.
Where can that be 240 W/m2 be going, OH I know someone said it must be being absorbed by the Sphere, after all what stops it from being absorbed.
So now the sphere is at 1177.5 and output from the shell is 394 W/m2.
Houston we have a problem.
Please explain what is wrong with my arithmetic as I have obviously gone badly wrong here.
Glad to, A C. For some reason, you seem to think that the output of the shell is 1/4 of the output of the sphere. It is not. The shell absorbs all of the radiation from the sphere. But because it has twice the surface area of the sphere, it emits at half the rate, not a quarter of the rate. So the iterations would be something like:
• 240 out from sphere 120 out from shell 120 in to sphere at starting point.
• 380 out from sphere 190 out from shell 190 in to sphere.
• 480 out from sphere 240 out from shell 240 in to sphere.
At that point the system is at steady state. The total amount generated in the sphere (240 W/m2) is being radiated to space.
w.
Also, A C, note that what the shell sends to the sphere is added to what the internal radioactivity sends to the sphere (240 W/m2). It is NOT added to whatever the sphere is radiating at the moment.
w.
I think you need to rethink that explanation.
I QUOTE YOUR EXACT WORDS.
“But because it has twice the surface area, it only radiates 120 W/m2. Not only that, but half of it goes to space … and the other half is radiated inwards.”
120/2 = 60 in the arithmatic they taught me at school.
OK, you say that it only adds to the 240 W/m2 and not the actual energy coming from the sphere.
The only problem left is how does the sphere increase it’s output without increasing it’s Temperature?
Willis,
A small remark, which doesn’t change the essence of the thought experiment:
When you insert the shell, its radiation in both directions only depends of its own temperature, it is not half the energy of the sphere in both directions as the surface is double that of the sphere. It may start at 3 K or room temperature. If it had the same initial temperature as the sphere, it would already send 240 W back to the sphere and 240 W to space.
But that is only for the initial temperature, once in equilibrium, the temperature of the shell would be such that the energy leaving it in both directions is always half that of the outgoing energy of the sphere. Or from the other viewpoint, the temperature of the sphere goes up until its outgoing energy doubles, thanks to the backradiation of the shell…
A C Osborn December 4, 2017 at 1:47 pm Edit
Thanks for the quote. I see the confusion. The shell is ALL radiating at half of the rate of the sphere, or 120 W/m2. The fact of where it radiates it doesn’t change how much it radiates. In other words, because half of the surface of the shell faces outer space, half of the 120 W/m2 radiation is directed to space … but that doesn’t cut the radiationper square metre in half.
If this is still not clear, please let me know.
A C Osborn December 4, 2017 at 1:43 pm Edit
Heck, I didn’t even realize that the Kardashians had a Scale 2 Civilization …
Best to you,
w.
The shell is ALL radiating at half of the rate of the sphere, or 120 W/m2. The fact of where it radiates it doesn’t change how much it radiates. In other words, because half of the surface of the shell faces outer space, half of the 120 W/m2 radiation is directed to space … but that doesn’t cut the radiationper square metre in half.
So let me get this right when you say half it doesn’t really mean half it actually means 120 W/m2 in BOTH directions.
Sorry my bad for reading the words wrong.
You made a little addition error on iteration 1. It doesn’t change your point at all. It still converges on the correct answer.
For anyone wanting a more formal approach here is why it works…..
If you assign b as the percentage of energy returned for any inbound value to the shell, E for the constant energy fed to the sphere and B as the total inbound from the shell you get inbound iterations from the sphere that sum to:
B=Eb+EB^2+Eb^3+…..Eb^n I want to add an E and take away an E for later trickiness so now I have…
B=E+Eb+EB^2+Eb^3+…..Eb^n -E then i factor to get B=E[( 1+b+B^2+b^3+…..b^n)-1]
Where n is the number of iterations and b is restricted to 0=<b=<1
The first term in the parenthetic expression is a power series equivalent to Taylor series expansion of 1/(1-b). So then B is simply B=E(1/(1-b)-1)
So for b=0, B= 0, transparent barrier. For b=1 B = infinity, no energy ever it gets out, exploding planet. And for everything in between the equation holds. Willis example is a b of 1/2. B=E, so planet receives 2E, shell receives 480, shell emits 240.
If you buy the argument that inbound energy from shell is absorbed and re-emitted, and you should, then this is the math that explains why it looks like something is being created from nothing. Remember that iterations can be thought of as little slices of time, each acting independent of the other. If you let the time slices approach zero, then n approaches infinity and this is the steady state math that represents it.
Paul Bahlin December 7, 2017 at 10:54 am
If you buy the argument that inbound energy from shell is absorbed and re-emitted, and you should, then this is the math that explains why it looks like something is being created from nothing.
No I shouldn’t without the proof that I keep asking for from Reputable Scientific Sites.
Let me ask you.
How many Joules per second per sq m is the heat source supplying to the Sphere, how many joules per second per sq m are leaving the Sphere and how many joules per second per sq m are leaving the Shell at iteration 0?
Upthread you will find a very detailed set of equations that you can plug all the numbers in. How do you learn anything if I do your arithmetic?
If you can’t follow what I wrote there is no shame in that. Maybe I wrote it crummy. If you have equation question fire away. What I won’t do is your arithmetic. Get straight on the math before you worry about the science. The math works for any system with feedback; money, yields on assembly lines, electronics….
Sorry, make that iteration1.
You are still not getting the actual situation.
You had the feedback from your Wife, it was something that you COULD NOT USE.
Paul, let me explain a bit further.
You are obviously a very talented Mathematician and have a passion for it.
In Thermodynamic Science there appears to be a written rule that Radiation from Cold can’t make hotter even hotter, I am trying to establish where reputable Scientific Physics establishments or Physics Scientists says it can.
So in Maths would you try and use a Trigonometry Formula for a Triangle to solve a problem for Circle?
Of course not as it would not be appropriate.
So why do you insist on using Positve Feedback where the Feedback is negative?
To go back to your Bank Analogy, assume that your Bank had a rule that it only accepted $s as depositing Currency and your Wife did actually manage to pay you some of your $100 dollars back but in Euros, You CAN’T put it in your Bank can you?
You could go elsewhere to spend it that does Accept Euros as you can with Radiation, if the Receiving object is cooler it will accept and Thermalise the Photon Energy.
A.C. Read carefully.
I come to this site to learn and I engage here when I think I have something to offer. I presume that everyone is here for similar reasons. But I am also not naive. Some come here with laser pointers to agonize the cats.
You are either a troll (laser) or you want to learn but are incapable of understanding how science works. My banking example was a freaking analogy. It doesn’t explain anything more than the math that would result for the hypothetical system I proposed.
Willis did the exact same thing for a hypothetical, closer in its definition to a, planet in space. The people who comment on that hypothetical with stupid shit like “come on, we don’t know how to build that” are assholes. Plain and simple!
I did the banking analogy in the hope that it would help you by deriving the math with a more tangible hypothetical than radiation. You have come back with a critique that changes the hypothetical and you never answered whether you agree with the math or not. You may be one that really wants to learn but when you play like that, It’s a laser and I won’t be your cat. It is very troll like.
So decide what it is that you are for. If you’re here to learn, answer the question. If not go away.
FYI, The question is do you agree with the math? Yes or no
So now let me pose my previous question as a Mathematical Formula.
Which one is correct bearing in mind X is a fixed Value and there is NO extra Energy in the system
X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = X (joules/second/M2 out of shell)
or
X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = 2 x X (joules/second/M2 out of shell)
Simplified
Output from Energy Source = Output from Shell
or
Output from Shell = 2 x Output from Energy Source
The Energy could just as easily be a dozen Apples being passed down a chain of people, there are no magic Apples allowed,
so 12 apples from person A to person B, 12 apples from person B to people C & D, 6 each, 12 apples eaten by people C & D (space)
Where do the extra dozen apples come from in this Steel Greenhouse Version.
12 apples from person A to person B, 12 apples from person B to people C & D, 6 each, 12 apples eaten by person C and 12 apples eaten by person D.
Or if you like you can use your $100 instead, as you seem to be cash orientated LOL.
AC, you are either breathtakingly ignorant or a particularly bad Troll.
I’ll say it only one more time: the sphere has an independent power source. The same as the earth’s solar input.
Do you deny that the earth’s surface is radiating at a rate greater than the sun’s input?
A.c. says:
“X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = X (joules/second/M2 out of shell)”
STOP RIGHT HERE….
Yes it is X out of she’ll but it is X/2 back to sphere and X/2 out to space.
“or
X (joules/second/M2 in to sphere) = X (joules/second/M2 out of sphere) = X (joules/second/M2 in to shell) = 2 x X (joules/second/M2 out of shell)”
This part is wrong. It has the shell creating energy..
It is a recursive process the next things to hit shell are:
X/2 which splits X/4 in X/4 out, then
X/4 which splits X/8 in X/8 out, then on and on all the way to X/infinity in, X/infinity out.
Converges on a steady state solution. X in to system, X out of system. Try it with 100 apples a day.
Paul, read carefully.
I am not a troll and I am trying to understand how Mr Eshenbach is at odds with all the Physics that I can find, which is why I keep asking for examples of his physics.
And also how the mechanics of Global Warming works, not just the physics.
It is now obvious why both your Physics and Maths are wrong.
The power Source is supplying sufficient Watts the sphere to emitt 240 for every one of the 1000 sphere’s Square metres, there is none to spare.
The Sphere has a surface area of 1000M2 and has 240 Watts coming out of each one which would equate to 240,000 Watts.
The Shell has a Surface Area of 2000M2 so it Can only recieve 120 Watts for each of it’s square metres, so that would also equate to 240,000 Watts as well.
Mr Eshenbach says the Sphere is emitting 120W per M2 to space, so 2000 x 120 also equates to 240,000 Watts.
The shell’s input and output is halved because it’s Surface area is twice the size, not because half goes in and half goes out.
Now according to Mr Eshenbach he can add the inward 120 (against all the current rules) to the Sphere until the Shell is outputting 240 W/M2, so 240 x 2000 equates to 480,000 Watts, so where have the extra 240,000 watts come from if those from power source have gone out to space, the Shell cannot generate them?
Paul, to show you how earnest I am on this subject, I have been following AGW for about 10 years now) I purchased a twin probe high quality Digital Thermometer and I have been conducting experiments to prove or disprove some of the quotes being made on here about how photons don’t know where they come from or are going to and are absorbed and therefore must be thermalised even by hotter objects.
I think today’s is the 4th one, I have posted the others on here and no one comments about how they are proving or disproving what they think. The only comments I have had are from Mr Eshenbarch criticising my lack of sketches etc and my designattempts at trying to prove the claims.
I will now be posting today’s one in a moment.
They take quite a long time to do as you have to wait for Equilibrium to reached by each object at each stage, on top of that my wife of 48 years is giving me real grief about both “Communicating” with people on the various forums I frequent and doing these stupid experiments.
I will not stop experimenting until I have satisfied myself that I can or cannot find these special conditions where cold makes hot hotter.
Man, AC. Instead of trying to recreate hundreds of years of physics on your own, why not just buy a recent radiative physics text?
Correction
This sentence
Mr Eshenbach says the Sphere is emitting 120W per M2 to space, so 2000 x 120 also equates to 240,000 Watts.
Should be
Mr Eshenbach says the Shell is emitting 120W per M2 to space, so 2000 x 120 also equates to 240,000 Watts.
Dave Fair, if you can tell me which ones describe how Cold things make hot things even hotter by radiation alone I am quite happy to buy one.
As long as it is NOT written by a Climate Scientist like Trenberth or Mann.
The reason that I am doing it myself is because that is my nature, I have an enquiring mind, but like see things for myself.
AC, I have never claimed that a colder object will warm a hotter object by radiation from the colder object alone.
Is it possible for one to see things for oneself through reading a college physics textbook, AC? And take a class and attend labs?
Dave, I did so 50 years ago, Thermodynamics and Electrical & Eletronics, I am now 70, 71 in January and I do not feel like going back to School.
I also do not need to, there is this thing which we are comminicating on call the Internet, where if you want to know something you just ask, sometimes you have to ask multiple questions phrased differently and then follow up questions as new “terms” are given in reply.
Plus of course I also enjoy actually designing and doing the Experiments, even if my wife does give me grief for doing them.
A.C…..
I’ve read your last few comments, and all kidding aside, I think I get you now. Your real passion is the experimentation. So let me suggest a new experiment.
Sometimes it is very difficult to prove something affirmatively but relatively easy to prove an opposite reality to disprove the first. So let me suggest an alternative proof…..
The essence of adherents to the theory that green house warming is not real is that warm objects are unaffected by ‘cold’ radiation. So Let’s prove that.
So what you need is a constant source of ‘cold’ radiation and a way to trap it. If the theory is correct, the trapped energy will build to a readily measurable value. Indeed the experiment should blow up at some point😁
The source of cold radiation is the earth and we want to be relatively unaffected by moisture changes and the sun so i would pick an area of Sandy soil in a shady spot.
You need to cover this soil with a well insulated box with an open bottom. Let it sit for a week or so and graph the temperature in the box say every 2 hours.
Since the theory says warm bodies reject cold radiation, it will enter the box and get trapped by the relatively warmer exterior ( you need to either heat the outside or do it when ambient never goes below 60 or so). The radiation can’t reenter the surface because now it is back radiation, by the same reasoning.
You should see the temperature go up to a value and settle into some day-night oscillation with a RMS value you can calculate.
Next, take a polished aluminum plate and insert under the box, shutting off the earth’s radiation. Run your measurements again.
Compare your RMS values. Your first run should be considerably higher than second. I would love to see your results.
Of course it won’t blow up but if your insulation is good, the radiated box should get warmer than the non-radiated one. Conduction will be your nemesis so give that some thought.
It would be valuable data to concurrently record outside ambient (shaded).
While living in Alaska, I needed to pound dog tethering stakes in the frozen ground. I took a bucket and light bulb on an extension cord. The bucket reflected the light’s energy and interfered with conduction, which melted the soil to depths sufficient to pound in the needed stakes.
Depends on where in the Internet you look and the questions you ask.
Radiative physics is well-documented for anyone to follow.
I, too, took the courses you listed in the early 1970’s. I was astonished recently at the level of ignorance exhibited by nominally educated people.
Forgot to add….
Shutting off the radiation will be tricky. I would put it on sticks so it doesn’t touch the surface.
that way the surface still is the radiator but when radiation hits the plate it will be rejected per the premise of the experiment. You don’t want the plate picking up earth temp via conduction, else it becomes the
.
Dave Fair, I missed this insulting comment from you ie
“AC, you are either breathtakingly ignorant or a particularly bad Troll.
I’ll say it only one more time: the sphere has an independent power source. The same as the earth’s solar input.
Do you deny that the earth’s surface is radiating at a rate greater than the sun’s input?
”
If you bothered to read my experiments carefully you will see that in many cases the emitting objects also had independent fixed power sources and at no time has any of them risen in Temperature without Insulation or volume of air and surface being involved.
First of all the Sun is not a Constant Source of Energy, only It’s Average Energy is roughly constant
Day/Night it is the equivelent of turning off the Energy Source in the Sphere for 12 hours out of 24.
Which means it would lose all it’s heat (and the shell’s) to space and when the energy source is turned back on it has to heat the sphere back up from 3.5K to the temperature where it can emit 240W/M2, just as the sun does every morning.
The Earth’s Energy Input, Temperature and Thermal Output is at no time Constant.
And as to your last question, along with many Scientists I do not believe The Thermo Balance Chart is correct, due to the BB equations being incorrectly applied, as the Earth’s Surface is nothing like a Black Body, is not in a Vacuum and due to the Energy Types (which seem to be Totally ignored) and where they end up.
Consider this, 70% of the high Energy and White Light and the low level from the Sun end up in the Oceans, which they heat to a depth of many metres, the water has a much bigger energy store to give up at night compared to the rest of the Surface. The remainder of the radiation heats the top few feet at most of the Earthern Surface of all different Albedo and Conductivity levels which is quickly lost to space in comparison.
The Radiation leaving the Surface however is lower power only LWIR, therefore when it is thermalised it’s energy output and the number of Photons emitted is far below Sunlight, that is any photons that can be emitted after all the collisions with all the other molecules that have removed the energy from the CO2 Molecules before the photon can be emitted.
Add to that, the area in the Atmosphere where the LWIR is thermalised by CO2 is a long way above the surface, half goes to space and the other half has somehow to get past the much more densely packed Molecules and getting denser the closer to the surface between it and the Surface.
There is also the question of “Balance” does Atmospheric H2O and especially Cloud increase or decrease the Surface Temperature over a 24 hour, Month, Year, decade period etc.
Well there is Emperical evidence that Clouds Cool the Surface overall, even though it warms it at night.
Add to that the Psuedo Warming by “Adjustments” and mis-use of Equipment give me absolutely no confidence in CO2 having any affect whatsoever on Temperatures, even with them there is no correlation.
What I do believe is that the Insulation affect of the whole Atmosphere, could be the “Equivalent” of adding x amount of watts to the system, just as it does with a house, a furnace etc.
The important word is Equivalent. For me Downward Back Radiation does not come into it.
AC, I’m done here. You just can’t seem to believe theoretical physics, backed up by empirical physical evidence.
That doesn’t mean that anthropogenic CO2 has any meaningful impact on average terrestrial temperatures. That has yet to be proven by observations. There are too many other things going on in our atmosphere and oceans that have the ability to overwhelm anthropogenic CO2’s feeble forcings.
You also realise that a Kardashev Scale 2 Civilisation could not build a Dyson Sphere as it would fry every living thing inside sphere.
A C Osborn,
The increase in radiation is not cummulative, it is always the 240 W heat source input + what is back radiated the moment before. That makes:
1. Sphere: 240 W out; shell: 60 W back, 60 W out.
2. Sphere: 300 W (240 W + 60 W) out; shell: 75 W back, 75 W out.
3. Sphere: 315 W (240 W + 75 W) out; shell 93.7 W back, 93.7 W out.
4. Sphere: 313.7 W out; shell 117.2 W back, 117.2 W out.
5. Sphere: 357.2 W out; shell 146.5 W back, 146.5 W out.
6. Sphere: 386.5 W out; shell 183.1 W back, 183.1 W out.
7. Sphere: 423.1 W out; shell 228.9 W back, 228.9 W out.
—
N. Sphere: 480 W out; shell 240 W back, 240 W out.
Seems fine to me…
Well apart from your arithmetic that is 315/ 4 = is less than 80 not 93.7.
357/4 less than 90 not 146.5 etc.
However I had already accepted that the incoming is only added to th base 240.
As I said the only problem is how the Sphere absorbs the energy and adds it the base without increasing the actual temperature of the sphere.
Perhaps you can explain it to me?
A C Osborn December 4, 2017 at 2:17 pm
Huh? Who said the temperature of the sphere did not increase? It has to increase, because instead of getting 240 W/m2 from the interior plus zero from outer space, at final steady-state it is receiving an additional 240 W/m2 radiated back from the shell.
Regards,
w.
Ferdinand, you’re making the same mistake that A C made. The shell radiates half the energy back, not a quarter. Set the numbers aside for a moment. Of whatever the shell receives, half of that is then radiated by the inside of the shell and half by the outside. Same is true in our atmosphere. Whatever the GHGs absorb, half goes up, half goes down (with a minuscule correction for depression of the horizon).
w.
A C Osborn,
Should have refreshed the discussion some time earlier, as I see that Willis had already responded…
Of course the actual sphere increases in temperature: as long as more energy is coming in than is going out, that energy increases the temperature of the sphere, or its outgoing energy can’t go up: the incoming radiation is absorbed, not reflected.
See what happens with the temperature of the original plate after I have inserted a second one in my own calculations above. That is as good the case for a sphere + shell as for two plates. As long as you have a continuous source of energy, the temperature of the heated part goes up with adding any hindrance to the outside world until the outside loss of energy is the same as the energy supply.
The calculations of how fast the sphere and shell get in equilibrium is a matter of mass of sphere and shell.
I only used your own figures for convenience, but it is in fact a factor 2, not a factor 4, at light speed if the sphere and shell had no mass and/or zero specific heat…
Willis,
Indeed it is a factor 2, not 4, but only used his own figures to show that it goes (slower) to the right equilibrium… In fact a matter of initial temperature of the shell and also a matter of mass and specific heat of sphere and shell…
Stephen, Bernard, Phil etc.:We now seem to be deep in the parallel world of the Feynmannian “eternal sidestep of hypothesis” again. Seems endemic in certain circles. Circles being apt as of now…..
From my ‘First Metric’ edition of ‘Mechanical Engineering Science’ by Hannah and Hillier, p.275; 14.15 Radiation: “The transfer of heat by radiation is usually important at high temperatures or when conduction and convection are negligible.” I also note that air has greater heat or thermal capacity (Cp 1:0.85) than CO2. Once gasified by increased Kinetic Energy (of which radiation is just a byproduct, feeble at STP), convection dominates to the emission height. Pure argon may not remain for aeons, as Mr Wilde pointed out years ago in relation to non-ghgs. On Earth, water’s LH uplift supercharges cooling, so CO2 is superfluous and can get on with making life possible. Of course, no life on a waterless planet either. But Willis posits an argon body, and I have said it can be both gaseously covered and also cooled in that state.
Some seem to be unable to see that solids must be phase-changed by application of energy ie work done or heat, if they are to become gases eg an atmosphere. Conduction does that here, and would do it for argon. Not for the first time, the cart needs to be behind the horse or Neddy gets very unhappy. I note that Bull(s) are better at pushing, also Billygoats. Lest we get too serious about this debate among people hopefully looking in the same direction……
Brett Keane December 4, 2017 at 2:57 pm
I posted above a quote from the Smithsonian. It said that the initial atmosphere was vented from volcanoes, so no need to involve “conduction” …
w.
And I told you why that is insufficient on its own.
Brett Keane December 4, 2017 at 2:57 pm
I also note that air has greater heat or thermal capacity (Cp 1:0.85) than CO2.
Actually CO2 has a higher heat capacity than air.
Does that “Air” actually mean the rewst of the Atmosphere on Earth where the “Air” has so much Water Vapour in it?
Well, after having read most of the 1,582 comments so far, I still have not seen anyone come up with a viable, rational objection to Willis’s basic contention that a cool object really can warm a warmer object. I have seen numerous digressions into interesting but irrelevant side issues, some unintelligible expositions of alternative theories of planetary warming involving convection, KE and PE, and the usual quota of unnecessary personal criticisms and cynical ad homs, but no solid, coherent scientific arguments that decisively refute his proposition. I’m left with the impression that his detractors don’t really have one.
I don’t understand why they seem to be making such a complicated issue out of something that is basically very simple. We have an earth’s surface that is radiating somewhere in the region of 390 W/m² of power on average (corresponding with a global mean temperature of about 14°C) but which is receiving only about 240 W/m² of power continuously from the sun (corresponding with a global mean temperature of about –19°C). So where is the roughly 150 W/m² of power coming from to make up the shortfall and keep the surface radiating at 390 W/m²?
Since the surface can only receive power-inputs from two directions (i.e. above and below) and the amount of power emanating constantly and continuously from below the surface is negligible (discounting temporary power-surges from storage in oceans, etc.), the shortfall has to be made up by power coming from above, i.e. from the direction of the atmosphere and outer space beyond it. But the only significant power-source in outer space is the sun and we have already taken that into account (i.e. as ~240 W/m² of insolation at the surface), so we are left with the atmosphere itself as the only possible source of the ~150 W/m² that is required to make up the shortfall at the surface.
Now the question arises as to where the atmosphere is getting its ~150 W/m² from to give continuously to the surface. The obvious answer to that question is that it is getting it from the surface radiance, so that the atmosphere is effectively recycling back to the surface ~150 W/m² out of the ~390 W/m² which the surface is continuously radiating into it.
This answer is confirmed when we consider that the amount of power radiating out of the topo of the atmosphere is, again, ~240 W/m². This situation implies that although the atmosphere is receiving ~390 W/m² of power from the surface in the form of IR-radiation, it is passing only ~240 W/m² of it to outer space, whereby it has acquired a power-surplus of ~150 W/m² which it must output somewhere to maintain its internal thermodynamic equilibrium of the system. But, again, there are only two possible directions in which it can output that power-surplus, namely above into outer space and below into the planet’s surface. And since we already know that it is only outputting ~240 W/m² to outer space, the only direction left for it to be outputting the surplus ~150 W/m² is back into the surface.
This, then, is the unavoidable conclusion to which this analysis of power inputs and outputs in the climate system brings us: that the atmosphere must be continuously recycling some of the power of surface radiance (~150 W/m²) back to the surface in order to maintain the surface radiance at the level at which we find it (~390 W/m²). Applying the Stefan-Boltzmann law to these power-ratings we find that this recycling of surface radiant power maintains the average surface temperature at ~33°C above the ~-19°C that insolation of ~240 W/m² could support by itself. Thus, we can say that the atmosphere really does warm the planetary surface and keep it warmed well above the temperature that it would otherwise have if it was warmed by incoming solar radiation alone.
But the average temperature of the atmosphere is well below that of the surface. Thus, the atmosphere demonstrates existentially and continuously how a cold object really can warm a warmer one, just as Willis has proposed.
Dang, Cassio, yer bringing that “logic” stuff into the discussion …
Thanks very much for your clear and compelling comment. As you point out, if it is not the downwelling radiation that is keeping the oceans from freezing solid, then what is keeping the surface warm? I’ve never seen anyone even try to answer that except N&Z and Jelbring and the like, who insist that the energy comes from gravity acting on the atmosphere … not.
w.
I really don’t understand why it is so confusing to propose adiabatic descent plus a time delay as the source of the energy being returned to the surface.
Because putting money in the bank on monday and taking it out on thursday doesn’t leave you richer.
Stephen Wilde December 5, 2017 at 12:48 am
I really don’t understand why it is so confusing to propose adiabatic descent plus a time delay as the source of the energy being returned to the surface.
It’s some positive feedback not a source, your explanation of it makes it seem as if there’s one concerted cycle as opposed to the many out of phase cycles which actually take place.
It’s worse tha what you think.
From what I can piece together from his word generator, his theory is thus:
The early atmosphere accumulated potential energy. This potential energy is what is supplying the eternal net positive flow from atmosphere to surface today. Convection is a component in a ke-pe dance fueled by a net zero flow via conduction with the surface. The atmosphere is not a fluid kept in place in the gravity field by the surface, rather it is ‘held up’ by convection. And finally, his newest wordsmithing is that LW from the earth system interacts with SW from the sun to form standing wave that keeps the PE there.
Haven’t seen any equations for his theory. I am working on it though. The model is for the PE of a 4 billion year old rock on a mountain top, raining down energy to keep the mountain warmer than it would be if the rock fell down.
Making progress but so far, can’t seem to get the units right.
All out of phase cycles within the atmosphere net out to leave the atmosphere in hydrostatic equilibrium.
Willis Eschenbach December 4, 2017 at 5:13 pm
Thanks for those kind remarks, Willis. I’m glad you found it so.
As you say – “not”. I don’t buy that theory either for two reasons, viz.:
1: It seems to be in conflict with the basic laws of mechanics to me. Gravity can certainly produce a force, but a force is not the same thing as energy, nor is it the same thing as energy-flux, a.k.a. “power”. I think gravity can produce energy only when it causes a net displacement of matter (as per the fundamental definition of work as Force X Distance moved in the direction of the force). But in an atmosphere in hydrostatic equilibrium there is no net displacement of matter taking place.
2: It proposes a new, superfluous, unconventional theoretical principle to explain a phenomenon (i.e. the surface temperature elevations of planets) that is already explicable with the existing conventional principles of radiative physics, as shown in my previous comment above. Thus, it apparently falls foul of Occam’s Razor.
I wish N&K et al would wise up to these fundamental objections to their theory from conventional physics and address them honestly in public so that we can all move on, but I confess that I have little hope of them doing so in the foreseeable future as they seem to me too preoccupied with proving it to be capable of hearing how it might actually be wrong at present.
“Applying the Stefan-Boltzmann law to these power-ratings we find that this recycling of surface radiant power maintains the average surface temperature at ~33°C above the ~-19°C that insolation of ~240 W/m² could support by itself.”
We don’t need to apply the Stefan-Boltzmann law to the fluxes either, that’s the great thing. The surface temperature at approx. 288 K, and the temperature at the mass-weighted mean temperature of the atmosphere of 255 K (see page 3, here: http://paoc.mit.edu/labweb/notes/chap3.pdf) are both directly measured. So we know that this surplus ~150 W/m2 we’re all familiar with must equate to a 33 K difference. Without the need for any conversion of fluxes to temperatures.
Tony, December 4, 2017 at 6:06 pm
Thanks for that useful reference, Tony. OK, so the average temperatures of the surface and the atmosphere have been measured and confirm that the surface is 33°K/C warmer than the atmosphere. Now, what about their radiances, which we also need to know in order to track the power-flows through the system?
But if we’ve only measured their temperatures, how do we know about the ~150 W/m² difference between the radiances of the atmosphere and the surface without using Stephan-Boltzmann? OK, satellites can measure the radiance coming out of the top of the atmosphere, but last I heard they still cannot measure the surface radiance with current state-of-art technology and there aren’t enough ground stations to do it either. So, humbling as it might be to admit the truly primitive state of modern day “climate science”, I think the Stephan-Boltzmann law is indispensable for the time being.
True, Cassio, we don’t know whether that flux difference of ~150 W/m2 will be correct. Whatever that flux difference is, though, in reality; we know it cannot account for a temperature difference greater than 33 K. Since, as you agree, the temperature difference of 33 K has been measured and confirmed. It may well be that the flux difference is something other than ~150 W/m2, we’ll just have to wait and see, at such time as the surface radiance can be measured.
I believe what you say but am lost. The earth receives 240 w/m2 and 240 w/m2 leaves into space. where does the 150 w/m2 at the surface come from? there are zero watts available to reradiate is 240 come in and 240 leave. what warms the surface?
@cjw,
Yes, cjw, I can see why you’re lost…you’ve got all these watts/sq.m. rattling around in your head in great confusion. Cassio and Willis have put you wrong because they both subscribe to the Earth Energy Budget diagrams of Trenberth, which shows that there is insufficient solar radiation arriving at the Earth’s surface…and using the Stephan Boltzmann equation with an emissivity just rounded at 1….they get the surface average temperature showing an unreal frozen minus 18deg C.
The reason is because these EEB diagrams only show about 161 , 163, 168.. and this latest one of Willis’s…169 w/sq.m solar radiation at the Earth’s surface ( they are both rabbiting on about 240w/sq.m. at the moment…increasing all the time.. 🙂 )
In reality , the TSI of about 1360w/sq.m is what arrives at the Top of the Atmosphere (TOA). It’s real and measured by the satellites. It’s a yearly global average…a bulk load at the TOA which is not to be divided down by 4 . …at the TOA. The sun never sets in space, and space is right there at the TOA. The sun just sits there 24/7. It’s 1360 w/sq.m at the TOA…and this is sufficient solar radiation to keep Earth’s surface temperatures at what they really are.
If you take this 1360w/sq.m. ,you can then geometrically divide it down by 4 to account for the night and day geometric attenuation, to get your 340w/sq.m, but now that is at the EARTH’S SURFACE…not at the TOA.
Taking this 340 w/sq.m solar flux at the Earth’s surface, and an emissivity of 0.82 (courtesy of Nasif Nahle) we can slot those numbers into the Stephan Boltzmann equation and you come up with a surface temperature of about 19deg C….this is a bit high.
They say the real surface temperature is measured at about 15 deg.C …so there would be less than that average of 340w/sq.m at the Earth’s surface…caused by the atmospheric attenuation…clouds,etc.
Geometric attenuation plus atmospheric attenuation on the real ,measured 1360w/sq.m using the SB equation will give you the real global average temperature, with no such thing as a “greenhouse effect” from the atmosphere. End of story.
Mack December 5, 2017 at 1:37 am Edit
Man, I don’t know if I can explain all the ways that is wrong. Let me just say what is true, it might be shorter.
• TOA solar radiation is about 1360 W/m2
• The earth intercepts an amount of sun equal to the cross-sectional area of the planet.
• To average it over the surface of the planet, you divide by four. However, this is NOT what makes it to the surface. It is the 24/7 average of the 1360 W/m2 instantaneous radiation at the TOA. Not the surface. The TOA
• Of this 340 W/m2 at the TOA, about 100 W/m2 is reflected by the surface and the clouds. How do we know this? It is measured from satellites. This leaves 240 W/m2 making it past the reflections.
• Of this 240 W/m2 making it past the clouds and reflections, about 70 W/m2 is absorbed in the atmosphere before hitting the surface. The atmosphere itself absorbs some, and some is absorbed by various small particles, airborne chemicals, clouds, and aerosols. This leaves about 170 W/m2 hitting the surface.
Note that this all is either measured or estimated from measurements and cross-checked in a number of fashions. Your claim that you just divide the TOA sunlight by four and that 340 W/m2 is what hits the surface ignores cloud reflections, ground reflections, and atmospheric absorption.
However, the errors don’t stop there. You say:
Don’t know who Nasif Nahle is, but the satellites say that he is wrong about an emissivity of 0.82. That’s far too low. Only a small part of earth’s surface (deserts) have an emissivity of less than 0.85, and most of it is up around 0.95 or higher. From the Terra Satellite folks who measure the emissivity from space:
Bearing in mind that 72% of earth is covered with water, and that the rest (except deserts and arid areas) is covered with either vegetation or ice, it is clear why the average emissivity of the planet is around 0.95.
Sigh …
w.
@CJW
I mean no disrespect by this response. This is not simple and if it was then there would be no controversy and no endless discussion. Since it is not clear where you are baffled and I do not want to be the millionth explanation, permit me to speculate and offer a simple attempt to unconfuse you, and hopefully others.
Since you say you ‘believe’ Willis’ explanations, I’ll make a giant leap here and speculate that you either can’t or won’t do the math yourself and consequently are relying on the argumentation and kind of sense of the players. There is no shame in that and again please don’t think I am taking a poke at you.
There are tons of topics on this blog that are so far beyond my own abilities that sometimes I feel very small from reading. I read those and keep my mouth shut so I don’t embarrass myself. This topic is not one of those so here is a possible way out of your dilemma.
This topic is confusing because it is both in and out of our ‘scale’. Humans have innate skill at understanding the things in our ‘scale’: temperature, the distance to the next city, the time to dinner, etc. Conversely we are not good at things out of our ‘scale’: the distances to galaxies, the age of the earth, massive numbers, tiny numbers, energy, radiation, etc.
This radiation problem conflates temperature (very familiar to us and our scale), and radiant energy flow ( one of the most mysterious and furthest from our scale).
You are very reasonably conflicted by a system that claims to raise temperate by depositing nothing. It’s like getting rich by depositing money in the bank while your spouse takes it out the back door.
Let me suggest this as a solution. Stop thinking about temperature. It is just a proxy for what counts, energy. Think of radiation energy as water flowing into a swimming pool. When the pool is full it overflows and the water out = water in. That is the planet with no atmosphere.
Now add an atmosphere. It is like raising the sides of the pool. After a while the new pool will overflow too but now it has more water in it.
And oh, by the way, more water means more energy which will have a higher temperature.
Now I will be attacked for this post because the pool isn’t rotating , and you can’t put sides on a kardashian atmosphere, and the microdoodles don’t allow it, and you haven’t considered the spiral arms of the galaxies. Just remember that half of what you read in comments is bullshit spewed by people who should be keeping their mouths shut.
Energy is the nut. Temperature is distraction
cjw, December 4, 2017 at 10:40 pm:
Please don’t believe anything I say that you don’t understand. If you do that you will surely get hopelessly lost.
I can only repeat what I already said in my comment above to which you are replying: it has to come from the atmosphere.
There are substances residing in the atmosphere (i.e. gases, clouds, aerosols and particulates) which capture some of the outgoing surface radiance on its way through to outer space and which redirect some of that captured energy (~150 W/m² altogether) back to the surface, thereby supplementing the energy which the surface receives from the sun (~240 W/m²). This is what I think warms the surface above the temperature which the solar input of 240 W/m² could sustain by itself.
“There are substances residing in the atmosphere (i.e. gases, clouds, aerosols and particulates) which capture some of the outgoing surface radiance on its way through to outer space and which redirect some of that captured energy (~150 W/m² altogether) back to the surface, thereby supplementing the energy which the surface receives from the sun (~240 W/m²). This is what I think warms the surface above the temperature which the solar input of 240 W/m² could sustain by itself.“
Cassio, given what we discussed earlier, I would assume you agree that this captured energy of ~150 W/m2, could not possibly be warming the surface by an amount greater than 33 K?
Tony, December 6, 2017 at 3:49 pm:
I do agree, Tony, so long as you’re treating the 33 K figure as approximate and you’re not wanting to change any of the other parameters of the situation like albedo and insolation. The ~150 W/m² recycled power only produces a surface temperature elevation of ~33°K under very particular circumstances, you see.
The main factor here, though, is usually the 4th power relationship between radiant intensity and absolute temperature in the Stefan-Boltzmann formula, which we’re using to convert temperatures into radiances and vice versa. Because of this relationship, a ~150 W/m² increase at the lower end of the radiance-scale equates to a larger temperature-elevation than it does higher up the scale. So the ~150 W/m² of extra surface radiance that is boosting the surface temperature by ~33°K is specific to the effective insolation temperature of ~–19°C and the (estimated) actual surface temperature of ~14°C (give or take a degree here or there to allow for the large uncertainties that we’re having to deal with here).
Sounds about right. All seems pretty straight-forward to me. It’s the funniest thing, but I had a heck of a job trying to explain that to someone else, in an earlier conversation. They were doing absolutely anything they could to avoid agreeing to that point.
Here’s a link to an important part of that conversation:
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2680092
Tony, you obviously believe that Back Radiation is warming the Surface, so can I ask.
Do you believe that this Results from the Radiation actually striking, being absorbed and also thermalised by the Surface?
No, AC, I don’t think that.
Tony, if your answer to my question is yes, what part of the atmosphere do believe that the Radiation is coming from.
Also which Molecules are responsible CO2,Water Vapour or Clouds?
And to be clear, my “no” is in response to:
“Tony, you obviously believe that Back Radiation is warming the Surface”
Tont, thanks for the response, I obviously misread your chat with Cassio, sorry about that.
Tony it would help if I could accurately hit the Y and not the T.
Ha ha, no worries A C!
https://wattsupwiththat.com/2017/11/24/can-a-cold-object-warm-a-hot-object/comment-page-1/#comment-2688993
Cassio
December 4, 2017 at 3:46 pm: The Moon’s surface is indeed that of its mass. That of Earth is higher, and gas properties render it higher in temperature than the gas above ie the lapse rate. The lapse rate is an effect of gravity.
Willis, thanks for your reply. The atmosphere we have now is very much not the one we started with from initial outgassing. Whether or not there was enough insolation at a critical point after collisions lessened enough for geothermal heat not to float an atmosphere, the fact remains that every night on average the daily increase is lost with time to spare. And total freezing would soon ensue if the sun didn’t rise. As the PE went unreplaced.
So, instead of a steel shell, try this thought expt with no gravity…..:)
You’r one of those automated story generators aren’t you?
For those of you interested in thermodynamics, let me recommend that if you have not heard of it you read about the Constructal Law. It is a fundamental thermodynamic law first enunciated by Adrian Bejan in 1996, so if you studied thermo before that, you might have missed it. Here are a couple of posts of mine on the subject:
The Constructal Law of Flow Systems 2010-11-15
One of the most fundamental and far-reaching discoveries in modern thermodynamics is the Constructal Law (see the wiki entry as well). It was first formulated by Adrian Bejan in 1996. In one of his descriptions, the Constructal Law is: For a finite-size (flow) system to persist in time (to live),…
Constructal GDP 2010-11-16
Encouraged by the response to my post on Adrian Bejan and the Constructal Law, which achieved what might be termed unprecedented levels of tepidity, I persevere. Here’s a lovely look at the energy use of the United States: Figure 1. US 2002 Energy production and consumption by sector. There are…
My regards to everyone,
w.
Willis,
You accepted the first three points of my elevator speech in favour of the adiabatic hypothesis but balked on the other two which were:
“iv) If the surface temperature remained at 255k whilst the atmosphere was being formed then it MUST rise by 33k when the adiabatic loop closes.
and
v) Although the surface temperature is then 288k you still have ongoing conduction of 33k upward to maintain convective overturning PLUS 255k of radiation going out to space which keeps the combined radiative and adiabatic loops stable and the atmosphere in hydrostatic equilibrium indefinitely.
Your previous objection to such a scenario missed the issue of the process of creating the atmosphere in the first place when the first convective overturning cycle formed. That is where the non zero consequence of conduction and convection comes from so that there is no breach of the first law.”
Your response was:
“If the gases were indeed “spewed from volcanoes”, your theory that we needed to put energy into the atmosphere as it formed goes out the window. The atmosphere would have been hotter than the surface to start with … and that’s the opposite of your theory.
But that’s not the main objection to your theory. The main objection is that even if your claim is correct and energy was pumped into the atmosphere four billion years ago, the effects of that one-time event on the flow system we call the climate are long gone.
For example, yes, dumping a truckload of water into a creek will raise the level of the creek … but not for long. And dumping a bunch of excess energy into the atmosphere, say by a solar flare or something, will raise the atmospheric temperature …
… but not for long, and certainly not for four billion years.”
and I replied:
“In the absence of solar heating at the surface being conducted to all the materials from volcanoes those materials would cool and fall to the ground so your objection is invalid.
Anyway, you are distracting from the initial premise of a simple argon atmosphere.
As for your ‘main’ objection please explain the continuing presence of a vast amount of non radiative non thermal PE still in the atmosphere if you say it has all dissipated away.
How exactly do you say that energy could have been removed or otherwise cancelled by some sort of unspecified setoff?”
In addition the creek example doesn’t work because the surplus water can be washed away downstream and lost in the much larger body of the ocean. That can’t happen for an atmosphere because the extra energy plonked into it has no means of escape to space whist fresh insolation and further conduction continue. A more apt analogy would be a standing wave above an obstacle such as a stone in the creek.
I think we should pursue the discussion further since I am genuinely curious as to what your further response might be.
Radiated away into space … it’s the only way to remove energy from the planetary system.
w.
When and how did that happen if radiation to space is limited to radiation received from space?
and
If it were radiated to space why did the atmosphere not fall to the ground given that there is a one cycle energy store in the atmosphere that would then have disappeared.
During the course of such radiation to space the atmosphere would have to shrunk progressively until it were all gone.
You seem to think that inflation does not require any energy and inflation of the volume of denser surface materials into a less dense gaseous form is all that an atmosphere represents.
I think the standing wave / feedback loop proposition is as good as it could be.
I think I finally get it. It’s the PE doing the work, correct?
Almost, PE is a consequence of work.
Work is measured in Joules thus:
“One joule is equal to the energy used to accelerate a body with a mass of one kilogram using one newton of force over a distance of one meter”
so you need one joule to raise 1kg of atmospheric mass one metre in height and that means work is being done.
In the process of rising, the molecules move further apart due to declining pressure and become cooler as per the gas laws (greater volume).
The energy from that KE cannot be eliminated (conservation of energy) so we say it has become potential energy. The term ‘potential’ means it does not show up on sensors as heat.
Not being heat PE cannot be radiated to space so it is wholly recoverable on the subsequent descent. If the atmosphere cannot radiate then it is only going to appear as heat again when it gets back to the surface one convective cycle later than when it went up.
Due to that one cycle delay the surface is then being heated both by that KE returning to the surface PLUS continuing insolation so you get 288k instead of 255k at the surface.
You can’t offset it with the KE going to PE in the simultaneous ascent, as Willis suggests, because that energy is derived from the previous descent. Refer to the stock control analogy that I gave you.
Work done in uplift (provoked by density differentials at the surface) creates PE from KE as the rising molecules move up along the declining density gradient caused by gravity.
PE is a consequence of work done in uplift and being an adiabatic process (PE cannot radiate to space) is fully reversible in descent.
It works as long as there are density variations at the surface and applies whether there are GHGs or not since you cannot eliminate such variations on a sphere lit from a point source of light.
Stephen you are creating system where uplift is derived from returning air which fuels the next uplift while simultaneouly delivering that same energy to a surface feeding in even more energy that does nothing. The phase delay is meaningless over any integration interval.
For crying out load, stop writing and draw your first picture of it.
Think of the phase delay like this….
Every morning i put a million bucks in the bank. Every evening I take a million bucks out.
Every Monday morning my wife puts in a million bucks. Every sunday evening she takes a million bucks out. I’m a millionaire all week but if I add up all my monthly statements I got nothing.
And that is exactly the amount of net energy your theory will deliver to the surface.
Sad.
You are not contributing here in good faith.
You are quite wrong. I have posed many many questions to you that remain unanswered. I am trying very hard to get you to answer them.
Every time you can’t answer you change the subject. That is demonstrable bad faith
Well between you there is 2 million somewhere.
In my dreams😃. More like rubber checks going in and out too fast for the bankers to catch up with me.
A good analogy for other features of your personality then?
Anyway, you’ve given me good exercise in boiling my proposals down to the simplest possible form but I don’t need to spend any more time on that.
I just now need to await Willis’s further comments since they are what I am after here.
Remember that this is a place of public access and likely to be a permanent record unless closed down or interfered with and even then there is still the so called ‘wayback machine’.
Pursuing the ‘creek’ analogy, better to regard radiation through space as a vast river.
If you put a planet in the way then the flow is slowed down and a standing wave forms, due to the presence of matter, which manifests as the generation of IR from the background flow to give a surface temperature for the obstruction.
If you then add an atmosphere the extra mass increases the size of the standing wave so that there is more IR but, due to the atmosphere being gaseous and therefore able to conduct and convect, a feedback loop forms between the mass of the planet and the mass of the atmosphere with the additional energy unable to escape to space so that the planet’s surface warms up instead of the atmosphere’s energy radiating to space.
Hope that is easier for those confused by the various more involved forms of words that I used previously in response to various objections.
So then your river analogy is proposing a river of SW developing a standing wave via the interaction with LW in the stream which is increased by the new gasses that don’t radiate LW.
GOT IT!
YES, hallelujah !
Interaction with matter, though.
You’ve worked hard to get away from the radiative only scenario.
Now we need a few others to see it 🙂
You forgot the sarc tag, right?
I don’t do sarc.
Was your comment sarc ?
Actually it was loaded with it. Your radiation river has SW interacting with LW out in vacuum of space.
That would be a fabulous discovery. For now it is laughably absurd. Could be wrong I suppose but you’d have to cite it for me.
A solid object in space absorbs shortwave, converts it to heat and emits longwave.
That is clear from my post.
You summary is inaccurate and misleading.
I thought we were talking about radiation in radiation rivers. Where did the matter come from?
We might as well add some ancient Greek philosophy here–
“No man ever steps in the same river twice”–Heraclitus of Ephesus
and then–
According to Aristotle, Cratylus went a step beyond his master’s doctrine and proclaimed that it cannot even be done once (to step in the same river).
It’s about the only thing I remember from my engineering HSS classes.
Jim
Willis,
Firstly, thanks for a stimulating article. The accounting analogy is excellent… (and never mind the ever-present trolls!)
Way back on November 25th at 9:56am, I responded to a comment by Philip Mullholland as follows: Philip, You say: What I had never noticed in the Trenberth diagram, until you pointed it out just now, is that there is no downward return vector to balance the upward mass transport of air that occurred during precipitation. That is because you were [not] aware that the two non-radiative energy transfers from the surface, for sensible heat (22W/m^2) and for latent heat (76W/m^2), are the net flows of energy due to these two phenomena. This is explained in the original paper that accompanied the original Trenberth diagram (I don’t know where Willis got his diagram from but the figures are in more or less the same ballpark as Trenberth’s and are definitely net figures.)
Philip did not respond to me but, browsing just now I noticed that I had missed Stephen Wilde’s response to me which was: Please substantiate that. Even Willis accepts that the net effect of convective overturning is zero so you cannot assert that the Trenberth diagram represents a net outturn. Trenberth just erroneously includes the upward leg alone (leaving out the downward leg) and compensates for that omission by incorrectly asserting a surface warming effect from DWIR.
Two questions for you:
1. My assumption is that some non-zero proportion of the sensible and latent heat moving up the atmospheric column by atmospheric processes gets radiated by GHGs to space in the upper atmosphere. If this were not the case then those mechanisms would provide no contribution to the balancing long-term steady-state LW energy flow from surface to space!
Can you please confirm whether you agree with this, contrary to Stephen’s claim that you think the ‘net effect of convective overturning is zero’.
2. He then suggests that the figure given for sensible heat convection from the surface is the ‘upward component only’ and that the downward component is in some way hidden in the general atmosphere-surface downward radiation figure.
Do you agree that all the energy flux values in your energy balance diagram (and in the original Trenberth diagram) are net figures, except for the two opposing up-and-down LW radiation values at the surface-atmosphere interface, which automatically offset one another, leaving a small net upward radiative flow?
All the best,
David
David,
It is only the adiabatic portion that nets out to zero. We were discussing an Argon atmosphere not radiating to space at all.
In the real world there is radiative leakage to space from within the atmosphere.
The Trenberth figures for thermals and latent heat appear to be IN ADDITION TO the radiative leakage from within the atmosphere since that portion is accommodated within other radiative outflows. They are also shown as a different colour which places them outside the yellow incoming and the blue outgoing radiation figures.
I did discuss an earlier version of the diagram in 2014 here:
http://www.newclimatemodel.com/correcting-the-kiehl-trenberth-energy-budget/
The same principles apply to the new diagram but the figures would need reworking.
What is the ‘part’ that does not net out to 0?
The first convective cycle during which KE is going into PE through conduction and convection. During that time the planet as viewed from space would appear to get cooler because some of the outgoing radiation is going to convection instead and the surface stays at 255k.
Once the atmosphere is in place the view from space goes to 255k and the surface goes to 288k.
Stephen, how do you know in your 1st convective cycle that the atm. was cooler than the surface? Your imagined 1st convective cycle must have had the the atm. warmer than the surface (volcano source, super heated steam source, so forth). Thus in your imagined 1st cycle the lower atm. fluid was warmed from above in a gravity field and no convection would be possible except in your imagination.
Trick 8.06 am
Output from volcanos cools very fast both by radiation to space and by expansion into the, much lower pressure, surrounding atmosphere as per the gas laws. The higher they go the faster they cool.
Shortly after each injection of new material conduction from the constantly irradiated surface takes over again.
Nice imagination Stephen. I get it. If in the first cycle up the atm. was cooler than the surface, then the 33K we enjoy today comes from some sort of imaginary PE/KE transformation that happened way back then. If in the first cycle up the atm. was warmer than the surface, then the 33K we enjoy today comes from some sort of imaginary volcano puff and/or super-heated steam outgassing being retained from way back then.
The atmosphere as a whole can never become warmer than the average surface temperature even with vast amounts of local or regional volcanic activity because if it did then the combination of KE plus PE within molecules high up would drive mass from the atmosphere out to space due to their high total energy content.
In that scenario the upward pressure gradient force would substantially exceed the downward force of gravity.
This is all pretty basic science and I’m increasingly surprised at how little you know.
“..because if it did then the combination of KE plus PE within molecules high up would drive mass from the atmosphere out to space due to their high total energy content.”
PE does not contribute to escape velocity Stephen. Sure the H2 could get out as it has done, and some helium but escape velocity could not be attained by (much) N2, O2 and higher molecular mass constituents.
So, would Stephen care to fill me in on the escape velocity and temperature that is needed for your original atm. N2 (or whatever molecules you imagine it consisted of) that you’ve computed for each constituent escape to space in order to come to this conclusion, just because of how little science you claim I know.
Total energy content does contribute to escape velocity and PE is part of total energy content.
If a molecule of N2 has its full load of PE for its actual height then to keep it below escape velocity it needs a commensurately reduced load of KE.
If it has too much KE for its position along the lapse rate slope then it will keep rising and unless something else puts a stop to that rise such as the ozone induced temperature inversion at the tropopause or unless it dissipates surplus KE by conduction to adjoining molecules (which it usually does) then it will keep rising until it is lost to space.
So you don’t need the details. You just need to know how basic physics plays out (often counterintuitively) in a real atmosphere which is a matter for the specialised discipline of meteorology. Not many atmospheric physicists seem to be aware of basic meteorological principles since they rely on radiative energy exchanges and fail to consider non radiative energy exchanges.
“If a molecule of N2 has its full load of PE for its actual height then to keep it below escape velocity it needs a commensurately reduced load of KE.”
LOL, really. Stephen you hardly ever cease to amuse. And you do not seem to be aware of basic radiative details so have to imagine what actually happens in radiative energy transfer. Adv. math in thermo. is far beyond your capability. You don’t understand even basic atm. convection let alone the small amount of available atm. PE as understood from a foundational 1950s paper.
Evidence, please.
Evidence? Margules 1903, Haurwitz 1941 and Lorenz 1955: “Evidently the total potential energy is not a good measure of the amount of energy available for conversion into kinetic energy under adiabatic flow….Consider first an atmosphere whose density stratification is everywhere horizontal. In this case, although total potential energy is plentiful, none at all is available for conversion into kinetic energy. .” so forth.
More than likely Stephen will not look these up as he’d rather imagine the atm. physics, since it is easier, no work involved.
I’ve not read every comment here, but most have nothing to do with thermodynamics.
The first thing one must do is define the system and its boundaries. Without that step, you can’t even apply the laws. Heat is defined as the energy crossing a system boundary due to a temperature difference–hotter to colder. They usually add in the phrase, not due to work. Systems do not contain heat or work–they only appear at their boundaries. Heat can be transferred by conduction, convection, or radiation. I don’t understand the phrase: “heat is not radiation.” Yes it is.
The first law in differential form using the Clausius standard is:

where U is internal energy, Q is heat, and W is work. The squigglely D’s are to remind you that heat and work are path variables. Internal energy is a state variable. Positive heat is heat added to the system, negative heat is heat removed from the system, positive work is work done by the system, and negative work is work done on the system. This isn’t the only standard or form for the first law.
The statement “ENERGY is not HEAT” is a problem. Maybe all energy is not heat, but all heat is energy. The SI unit for heat is the joule. In the past, they used BTUs and calories. Both are easily converted to joules by simple conversion factors.
The second law only applies to isolated systems. Closed systems can lose heat and entropy.
For example, here’s an isolated system:
Object B is hotter than object A and is transferring heat Q from B to A. Objects A and B are subsystems (otherwise we couldn’t talk about heat transfers) and they are closed systems–we’re allowing energy to transfer across their system boundaries. The entropy for A is where
where  is the absolute temperature of object A. The entropy for B is
is the absolute temperature of object A. The entropy for B is 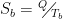 where
where 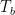 is the absolute temperature of object B.
is the absolute temperature of object B.
Now if , then
, then 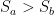 .
.
Using the second law we get:
 .
.
So this isolated system does not violate the second law. But notice that object B is losing entropy. The entropy lost by B is less than the entropy gained by A–for the same heat transfer. If a closed system couldn’t lose entropy, then nothing would ever cool down.
The internal energy of B is decreasing, while the internal energy of A is increasing. This will cause the temperature of A to increase, and the temperature of B to decrease. This will continue until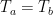 . Heat will stop being transferred, entropy will reach a maximum for this system, and
. Heat will stop being transferred, entropy will reach a maximum for this system, and  at equilibrium.
at equilibrium.
Jim (I hope this post works correctly.)
Jim, that is an excellent introduction to systems, entropy, heat, etc.
To use this approach to Willis’ scenario will take a bit more effort. We would need to consider a heat input (eg from the sun, an electric heater, or radioactive decay). We would need to consider a fixed temperature heat sink (eg outer space at 2.7 K or a room at 20 C). With these additions, the system of A+B (for example the earth + the atmosphere) is no longer isolated and A & B do not need to approach a common final temperature with Q=0.
Mr Folkerts, in your response to Jim Masterson you stated “To use this approach to Willis’ scenario will take a bit more effort. We would need to consider a heat input (eg from the sun, an electric heater, or radioactive decay). We would need to consider a fixed temperature heat sink (eg outer space at 2.7 K or a room at 20 C).”
Can you explain your logic here please?
It has already been admitted on here that 2 objects at the same temperature in a room cannot make each other warm, despite flooding each other with Photons, so how does one colder object increase the temperature of the hotter one as in “object A being Earth’s Surface and Object B being CO2 Molecules.”
I asked Paul Bahlin at
A C Osborn
December 3, 2017 at 10:10 am
what would be the result of one heated object A at equilibrium and 25 degrees C and an object B at 0 degrees C.
When I introduce Object B to within a few millimetres of Object A WHAT HAPPENS TO THE TEMPERATURE OF OBJECT A?
His answer
Paul Bahlin
December 3, 2017 at 10:33 am
Don’t know or care. In my house object B would be a beer and I would drink it before A radiation was allowed to touch it.
So perhaps you are prepared to offer a more “Scientific” answer to the question?
For clarification the “heat Sink” is my kitchen walls & ceiling, at 19 C.
Steven Wilde, perhaps an example will help. You seem to think that the atmosphere is kept warm by the 33K of warming from its original formation. If I understand you, the rest is just a constant exchange of PE and KE. Here’s a parallel.
Staying alive doesn’t require a static amount of energy. It requires a flow of energy in the form of money.
So suppose I give you $33,000 dollars. Then for fifty years, every year I give you another $33,000, and you give me the same amount, $33,000. And that’s all the money in the equation.
The question is, what are you going to live on?
Similarly, staying warm doesn’t require a static amount of energy. It requires a flow of energy in the form of heat.
So suppose I give the atmosphere some large amount of energy. Then for fifty years, I give the atmosphere another large amount of energy, and it gives me the same amount back. And that’s all the energy in the equation.
The question is, what will keep the atmosphere warm?
That’s why folks keep trying to point out that a one-time injection of either money or energy is not enough to keep you alive or to keep the atmosphere warm over an extended period of time. That requires a flow, a flow where more energy or more heat is required on a constant, ongoing basis.
Regards,
w.
Fair question, Willis, and the same as the one that Paul raised and which I dealt with in the stock control analogy.
I’ll deal with it afresh here for your convenience using your dollars instead of his cheeseballs.
i ) You give me 33000 dollars on 1st January 2000
ii) I give you 33000 dollars on 1st January 2001 and receive another 33000 dollars at the same time.
iii) I still have 33000 dollars do I not?
iv) If we then continue forever then I have 33000 dollars forever to do with as I will or as per your analogy ‘to live on’.
v) When I die you don’t pay me 33000 dollars on 1st January following my demise and my estate owes you 33000 dollars from which you are repaid (unless I’ve spent it on having fun – which these online exchanges are for me -it’s a neat pastime)
So, when the atmosphere falls to the ground it repays its PE but that won’t happen until the sun stops shining which is when the atmosphere dies.
What is happening is that there is a one cycle delay in refunding your 33000 dollars just as there is a one cycle delay for energy held in the atmosphere escaping to space.
Is there a flaw I haven’t thought of ?
How did Stephen live over the years without spending any of the 33000?
The atmosphere doesn’t have a constant energy demand as we do.
It simply needs the initial slug of energy to get it hoisted aloft. Once aloft it needs no further input so simple recycling of the available energy is sufficient.
Willis concedes that ongoing convection is a zero sum game so if it were to deplete that initial 33000 dollars by some means it would not be a zero sum game and the atmosphere would gradually deflate.
But we know that it does not.
“The atmosphere doesn’t have a constant energy demand as we do.”
It does Stephen. The atm. loses calories continously just as you do. You need to eat & the atm. needs the sun. A fuel needs to be burned to maintain your temperature & the atm. temperature near steady state.
The sun supplies fresh calories at a constant rate. That is sufficient to hold the mass of the atmosphere off the ground since the atmosphere has no metabolism to burn calories as we do.
Doe you think the atmosphere has something equivalent to a metabolism?
So, after a little diversion, how did Stephen live over the years without spending any of the 33000?
Huh? As others have noted, WHAT ARE YOU LIVING ON?
Your claim seems to be that you just heat something up by 33K and it stays at that temperature forever. But the atmosphere is constantly losing heat.
You’re still not accounting for the fact that it takes a continuous supply of energy to do continuous work, like keeping an object warm.
w.
This had me baffled for a long time but I think I’ve discovered the disconnect. Stephen, I know you’re pissed off at me and I have dished a lot of snark your way but I really am trying to understand your position.
Could it be that you think PE is Energy? You seem to be ‘living on It’ in Willis’ example because your daily money exchange is with money that’s never been touched. There’s no other money available.
Do you count the atmosphere’s PE in the PE-KE exchange of convecting parcels?
Willis, Stephen knows as should you, that after the initial input, the continuation of an atmosphere not an icefield depends on daily input until the sun returns to its original low or non-radiative state.. No need for confusion on something as basic and simple as this. But then again, I’m just a Kiwi cowboy and seaman…..who only got serious about physics late in life. Cheers, Brett
Thanks Brett.
If Willis can’t get past that then I’ll just have to give up here and revisit the issue in the next thread where it becomes relevant.
The thing is that Willis agrees my first three bullet points in support of the elevator description of the adiabatic effect on surface temperature.
He has only balked on points 4 and 5 but they follow naturally and logically from the previous points so he really has nowhere to go but doesn’t seem to realise that yet.
For example, he insists that an atmosphere has some sort of ‘burn’ rate which sheds adiabatic energy to space despite PE being non radiative yet at the same time he agrees that convection up equals convection down.
I’m only focusing on Willis now because the other critics seem to be beyond redemption.
Brett Keane December 6, 2017 at 12:08 am
Brett, I fear I have no idea what you are talking about. It appears you think I “should” know something, but what it is and why you think I don’t know it is a mystery. Also, you have no idea what Steven knows, and unless he’s appointed you his spokesdude, you have no right to speak for him as to what he knows.
As to “no need for confusion”, to avoid the confusion that you are adding to with this comment, please follow my polite request to QUOTE WHATEVER THE HELL IT IS YOU ARE BABBLING ABOUT.
I’m sorry to shout, but you and Stephen just toss out things and assume that I know what you are referring to. I don’t really understand Stephen’s claim, yet he keeps saying that I “agree with his first three bullet points” … and since there are 1800 comments in this thread and I have no idea how to find whatever bullet points he’s talking about, just how on earth am I supposed to answer?
People keep assuming that I’m having a one-on-one conversation with them, and that I can remember every detail of every claim they made and every response of mine … bad news. I’m holding like twenty conversations simultaneously, often with people using totally forgettable aliases, many of them making similar points.
Now, I know that you’re a cowboy and a seaman. But that’s no excuse for ignoring a polite request. I have no idea what Stephen knows or should know. I have no idea what you know or should know. So please, stop waving your hands and acting like it’s going somewhere. I’m tired of filtering through comment after comment making claims about what I said or they said or you said, WITHOUT ANY QUOTATION TO TELL ME WHAT IT IS THAT HAS THEIR KNICKERS IN A TWIST!
Why is following a simple polite request so damn hard? If you cowboy and go to sea in this slapdash fashion, god help your crew, your bunkmates, and your horse …
Sadly,
w.
Stephen Wilde December 6, 2017 at 1:21 am
“Get past” WHAT? WHICH “issue”? You and Brett think you are having a discussion, and your lips are moving, but the words make no sense.
Stephen, you have been asked many times, including just below (again) by TJF, to provide a coherent description of a) what it is that you are claiming, and b) EXACTLY how it works. In response we get bafflegab. My patience is run out.
So I will say, along with all the rest, again, show us the numbers!
Truly, at this point I don’t know what your basic claim is. Boil it down into an elevator speech about how it all works, and then PUT NUMBERS TO IT.
I’ve had it with the handwaving. What is your basic claim? What numbers support it? What is your ensuing claim assuming the first one is true? What numbers support it?
Exasperatedly,
w.
Mr Eschenbach is making ridiculous statements again.
“Can’t live on a single injection of cash”
Without any qualification of a single injection amount.
Give any reasonable person $1billion and they will live very nicely for the rest of their lives and so will their families.
Probably $10million would do it for most people.
A C Osborn December 6, 2017 at 12:33 pm
You do know that such snark just makes you look like you are worried about losing the debate, I hope …
You miss the point entirely, likely my fault for lack of clarity. Let me take another shot at it:
“Can’t live on a single injection of cash, you have to spend some to stay alive.”
In Steven’s explanation, he keeps the atmosphere warm indefinitely, but he’s not spending any energy to do so. That was what I was trying to point out with my example.
w.
But my comment is no where as “snarky” as many of yours.
And not only that, it was perfectly accurate, as was my previous correcting of your statement about “thermal energy” when you really meant IR energy.
It is not my fault you get carried away and make incorrect statements to make your points, it was yours.
So maybe if you cut out the rhetoric and just stuck to the facts I wouldn’t feel the need to set the record straight.
A C. .no matter how much time, effort and energy you expend setting the record straight, it will do nothing to convince Willis that he does not know everything, nor will it cure him of the problem of his over inflated ego.
I know, it is not for him it is for the lurkers, who may take what he says at face value.
Does that sound familiar?
Jesus, AC! Can’t you just address Willis’ science and avoid verbal coup-counting.
Dave, Mr Eshenbach does not need your.
Let me correct your statement for you.
Stop mimicking Willis just address Willis’ Fantasy science and avoid verbal coup-counting.
But as you are still asking questions.
Please show me any reputable (ie non Climate Science) sites that say that the radiation from a cold object makes a warmer one warmer and please do not quote a “Thought Experiment”.
That is all I am trying to Establish, what conditions that are currently not described by the Science allows photonic energy from whatever colder source to warm up a warmer object.
The definition of heat flow is the amount of Joules per second (Watts) per Metre2. Therefore at Equilibrum the amount of joules is a finite amount from the energy source without changing the energy source.
So that just leaves Emitting Surface Area as the variable.
Let’s assume that his sphere is spitting 2000 Joules per second per metre2 at Equilibrium, if we just take his sphere and expand it to the size of the Shell it’s Output will also drop by half as in the Steel Greenhouse Shell to 1000 Watts/M2 ie half the number of joules available per second per M2.
Please note that the System is still at Equilibrium putting out the same total of 2000 Joules per second when the total surface is considered, ie over twice as many square metres.
Also note that it is NOT at 4000 Joules per second, which are NOT available, totally impossible and are totally unnecessary for the sphere to obtain its original state of Equilibrium.
In fact I am not sure if it willactually be below 1000 joules per second as it takes twice as many joules to heat twice as much steel (Specific Heat) to the same Temperature, so am not sure if they can still be radiating the same.
As it would also take a little more to heat the extra Steel in the Shell.
I have been conducting REAL Experiments to try and find those magical conditions where the Colder surface Radiation heats the warmer surface and up till now I have failed.
As to the Light Bulb in a jar and foil which you so heartily endorsed, I am absolutely staggered that anyone believes that Back Radiation is directly involved in the warming of the Bulb.
I did not need to be shown that experiment as I had already done it for myself. Of course I used a Thermometer with 2 probes where it can provide not only the temperature from the probes, but also their difference in temp.
The extra heat comes from standard “Insulation” plus one other factor which comes after this one.
So what has changed, the addition of the Jar and the Foil have drastically reduced the heat loss via both Conduction and Convection. So what about the extra heat from adding the Foil, well that is where any back Radiation comes in.
Not only is the Foil doing the same to Conduction & Convection now the foil heats much quicker than the air between it and the bulb as it is a much better Conducter. The much hotter foil’s radiation excites the Air between the Foil and the Bulb increasing Temperature and thus slowing the cooling even more.
Experiment 1 with and without Foil box with a hot object
Without Box
Heated Object Temp (Facing Upwards) = 24.5C
Air above temp = 20.2C
Difference = 4.1C
With Box (with Foil) after 15 minutes
Heated Object Temp (Facing Upwards) = 26.0C
Air above temp = 24.0C
Difference = 2.0C
With Box (with Foil) after 20 minutes
Heated Object Temp (Facing Upwards) = 27.2C
Air above temp = 24.2C
Difference = 3.2C
With Box (with Foil) after 30 minutes
Heated Object Temp (Facing Upwards) = 27.5C
Air above temp = 24.4C
Difference = 3.2C
Foil at this point 22.5C
So do you see what happened, the Air heated most at 4.0C first due to the foil which in turn meant the heat sink was no longer as effective and the heated object warmed 2.0C.
But the air had started to stabilise at 24.2C and only rose another 0.2C while the object was still trying to reach stability.at 27.2C and then 27.5C.
However the Actual Heat Source which was a bulb around 25mm below the object could not be affected because all the heating was taking place above the Object, whereas the temperature to the side and below the object was only raised by 0.4C despite all that heat above neing reflected downwards by the Foil and By Conduction within the Air.
So now to the other factor involved, lets assume a warehouse with 20000 cubic metres of space. It has no heating so it remains at roughly outside ambient. We add X number of watts/m2 of Radiators with a large enough boiler to maintain a temp of say 30 degrees. Now if I put you in that Wharehouse with the Heating running at max you would be quite a bit hot, but not too uncomfortable. If I now take all thus radiators and stuff them into a room of 200 cubic metres with much better thermal Insulation how do you think you would feel.
Well you probably wouldn’t feel anything for very long as you would be Dead from heat exhaustion.
That is precisely what they did with the bulb in a box, took it out of a very spacous room and placed it in a very tiny one by comparison and added additional Thermal cladding..
Now tell me if you are surpised by the Air getting much hotter and thereby preventing the Bulb from cooling.
Perhaps they should fill the Box with CO2 at -180C to see how much it heats the bulb, I might try that with Cold air as I don’t have any CO2.
Experiment 2 with and without Foil box with 2 cold objects 13grms in weight at 12.0C and 12.7C in the box.
It is an established fact that a hotter object can warm quicker than a colder object so the one in the box may cool quicker. The Purpose of experiment is to demonstrate further that it insulation
After 0 minutes
Object out of box = 12.0C
Object in box = 12.7C
Difference = -.0.7C colder than outside
After 10 minutes
Object out of box 14.5C
Object in box = 14.9C
Difference = -0.5C
After 20 minutes
Object out of box = 17.3C
Object in box = 17,2C
Difference = +0.1C
After 30 minutes
Object out of box = 18.4C
Object in box = 18.1C
Difference = +0.3C
So there you have it A Foil Box also slows how much a cold object warms up, so it is insulating it from it’s surrounding, it did not add a single bit of back radiation to speed up the warming compared the one outside the box.
More Real Experiments to follow.
Well, AC, I was responding to a question about a steel sphere with an independent power source surrounded by a steel shell. It is a complete system suspended in outer space.
I assert that energy from the sphere is totally incident on the interior of the shell.
I assert that the shell radiates energy at both surfaces equally at all times; half of the shell’s energy is radiated outward to space, half radiated inward to the sphere.
I assert that all of the shell’s energy is supplied by the sphere.
I assert that the radiated energy of the interior of the shell is absorbed by the sphere, heating the sphere.
I assert that the heated sphere is emitting additional energy to the interior of the shell.
I assert that the process continues until the energy input to the sphere/shell system is at energy equilibrium with its surroundings; 1/2 of the energy supplied by the sphere is radiated by the outer shell, 1/2 back-radiated at the sphere.
Those are the only things I assert on this Thread.
You know, I think future generations (if they can be bothered) are going to look at some of my opponent’s comments with amusement as I do,so Trick and Paul, be more careful what you say.
As for Willis I currently believe him to be an honest broker.
Stephen, your theory all seems to hinge on the “initial uplift” of your “adiabatic loop” as the atmosphere is added. So let me ask you about that.
Suppose there is a planet with no atmosphere and a blackbody surface. I has a 240 W/m^2 power supply, which makes the surface 255 K. Now add an atmosphere (pure argon that doesn’t radiate noticeable thermal IR). I can imagine a few different options.
1) The atmosphere starts at 255 K. In this case, it is the same temperature as the surface and no convection is started. There is never energy lost from the surface to the atmosphere, never any ‘adiabatic loop’, and never any energy returned to the surface to warm it about 255 K.
2) the atmosphere starts at some colder temperature — say 200 K. The atmosphere will warm by contact with the ground and start to convect. However, that same contact must necessarily cool the surface below 255 K. That is how conduction work — the cool object warms and the warm object cools. The surface will continue to be held below 255 K and the uplifting air will also be below 255 K — until the “initial adiabatic loop” has been set up and the air returns. However, since the the loop is adiabatic, the air will return at the same temperature it left — somewhere below 255 K (having cooled on the way up and warmed again on the way down). Air below 255K returns to a surface below 255K, so that col air can’t warm surface above 255 K.
3) the atmosphere starts at a temperature about 255 K — say 288 K. Now the bottom of the atmosphere will cool by conduction, and the surface will warm above 255K. But no convection is started so there is no “adiabatic loop”. The surface — now warmer than 255 K will radiate more that 240W/m^2, so there is continuously energy lost and continuous over all cooling. The atmosphere above 255 K cools by conduction to the surface below 255 K. This continues (with no ‘adiabatic loop’) until everything equilibrates to 255 K.
None of these works at all to get the surface to 288K. Perhaps you can describe specifically your scenario for adding an atmosphere.
TJF: Yet again, still, after all this time, you act as if you do not know what makes a gas, any gas, different from other phases of matter. From above, you do not admit that a gas is matter. Or surely you would write different words. Hint: the effective surface is changed, and gravity is able to squeeze more matter with its energy into smaller spaces, lower down.
Enjoy.
Well, they can’t accept the effect of the gas laws can they?
If they do, it is game over.
Brett Keane December 6, 2017 at 12:36 am Edit
Could you be more unclear, please? There were times when I thought I could actually understand your point …
Setting the sarcasm aside, if you disagree with a man, QUOTE HIS EXACT WORDS, and then tell us exactly where they are wrong. Saying “From above, you do not admit that gas is matter” is a handwaving joke … where the hell did TJF say that?
Etcetera for your other points …
w.
Hmmmm … i said:
* gases warm when put in contact with warmer objects and cool when put in contact with cooler objects..
* gases expand when they get warm.
* warm, less dense gases will rise.
* rising gases in an atmosphere will expand and cool.
* sinking gases in an atmosphere will compress and warm.
* If a gas expends and then compresses back to the original volume adiabatically, it will return to its original temperature.
That all sounds exactly like the behavior of gases to me!
Paul Bahlin asked:
“Could it be that you think PE is Energy? You seem to be ‘living on It’ in Willis’ example because your daily money exchange is with money that’s never been touched. There’s no other money available.
Do you count the atmosphere’s PE in the PE-KE exchange of convecting parcels?”
Yes, of course it counts as energy. It just isn’t heat and doesn’t radiate.
Molecules of gases have electromagnetic forces between them.
If you move them closer together then those interactions become more intense because the same energy is focused within a smaller area and they become hotter.
If you move them further apart the interactions become less intense and the available energy becomes spread over a larger volume so they cool down.
That is the essence of the gas laws and the reason why the pressure gradient set up by gravity is so effective in creating a lapse rate with height.
The changing of the distance between molecules does not imply any loss of total energy (conservation of energy) but it does involve a change in the amount of sensible heat within the volume occupied by the gas.
And because an atmosphere is inanimate, with no metabolism, it has no energy burn rate once established and the PE content is unable to radiate to space so it just recycles constantly between surface and atmosphere.as long as the sun keeps shining.
That is a trivial and well known physical principle that you should all be well aware of, as Brett kindly pointed out.
The entire universe runs in a background of potential energy derived from the expansion of gases after the big bang but that is another story.
“And because an atmosphere is inanimate, with no metabolism…”
The atm. of course has no metabolism, YOU do though Stephen and you needed to spend some of the $33000 each year to support that metaboliosm which is the fatal flaw you hadn’t thought of in your 1:16pm comment. Likewise the sun/earth system also has to burn a fuel to support its energy loss.
Stephen Wilde December 6, 2017 at 1:47 am
Molecules of gases have electromagnetic forces between them.
If you move them closer together then those interactions become more intense because the same energy is focused within a smaller area and they become hotter.
If you move them further apart the interactions become less intense and the available energy becomes spread over a larger volume so they cool down.
That is the essence of the gas laws and the reason why the pressure gradient set up by gravity is so effective in creating a lapse rate with height.
The changing of the distance between molecules does not imply any loss of total energy (conservation of energy) but it does involve a change in the amount of sensible heat within the volume occupied by the gas.
You have a misunderstanding of the gas laws. The ideal gas law which applies under the conditions of our atmosphere is based on there being no attractions between the molecules. Under conditions of very low temperature and high pressure there are deviations from the ideal gas law and a different equation of state is used and attraction between molecules become significant (the a/V^2 term in the van der Waals equation for example)
No gas is ideal.
The concept of an ideal gas is just a construct upon which one then superimposes the characteristics of real gases in real atmospheres.
In real life the interactions between molecules are indeed significant hence the need for the gas laws. I think you are just thrashing about.
Do you really deny the relationship PV=nRT?
Stephen Wilde December 7, 2017 at 8:17 am
No gas is ideal.
The concept of an ideal gas is just a construct upon which one then superimposes the characteristics of real gases in real atmospheres.
In real life the interactions between molecules are indeed significant hence the need for the gas laws. I think you are just thrashing about.
Do you really deny the relationship PV=nRT?
No, that is the ideal gas law!
A good description is the one from Hyperphysics:
“An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in which there are no intermolecular attractive forces. One can visualize it as a collection of perfectly hard spheres which collide but which otherwise do not interact with each other. In such a gas, all the internal energy is in the form of kinetic energy and any change in internal energy is accompanied by a change in temperature.”
Under conditions when it’s necessary to take account of attraction between molecules and finite molecular size you need a different equation of state, a good one being the van de Waals equation:
(P+a(n/V)^2)(V/n-b)=RT
Hi Phil.
Thanks for that clarification of the appropriate formula in relation to real gases.
If the atmosphere can be treated as an ideal gas with no intermolecular forces and therefore no potential energy (all being kinetic energy) then why do we hear so much about potential energy in the atmosphere when one studies meteorology?
If one takes an ideal gas at a specific temperature and pressure and then expand it does the gas cool due to creation of PE at the expense of KE or not?
Stephen Wilde December 8, 2017 at 7:33 am
Hi Phil.
Thanks for that clarification of the appropriate formula in relation to real gases.
If the atmosphere can be treated as an ideal gas with no intermolecular forces and therefore no potential energy (all being kinetic energy) then why do we hear so much about potential energy in the atmosphere when one studies meteorology?
I don’t know I’ve not taught meteorology so I don’t know what the context is. I suspect it’s related to instability and buoyancy of an air parcel?
If one takes an ideal gas at a specific temperature and pressure and then expand it does the gas cool due to creation of PE at the expense of KE or not?
Assuming it’s done adiabatically then the gas cools because of the work done by expanding it.
Yes, the gas cools as a result of work done but work done in the reversible process of convective overturning doesn’t destroy energy. Instead it transforms KE to PE and if we are considering an adiabatic process then it is fully reversible.
An atmosphere is loaded with PE and KE in equal proportions and it is the interplay between them that creates weather and climate. Molecules closer together increases the interaction between molecules which then oscillate faster and the temperature rises.
So, reverting to my initial description, the intermolecular forces are important within a real atmosphere loaded with PE but as long as one adjusts for position along the lapse rate slope, which the standard atmosphere does, then you can use the ideal gas laws well enough for all practical purposes.
Themore refined equation you mentioned makes it even more accurate but that isn’t necessary in practice.
Stephen Wilde December 9, 2017 at 10:15 am
Yes, the gas cools as a result of work done but work done in the reversible process of convective overturning doesn’t destroy energy. Instead it transforms KE to PE and if we are considering an adiabatic process then it is fully reversible.
An atmosphere is loaded with PE and KE in equal proportions and it is the interplay between them that creates weather and climate. Molecules closer together increases the interaction between molecules which then oscillate faster and the temperature rises.
So, reverting to my initial description, the intermolecular forces are important within a real atmosphere loaded with PE but as long as one adjusts for position along the lapse rate slope, which the standard atmosphere does, then you can use the ideal gas laws well enough for all practical purposes.
Themore refined equation you mentioned makes it even more accurate but that isn’t necessary in practice.
The intermolecular forces are not important within our atmosphere, if they were the ideal gas laws would not accurately represent the atmosphere. Van de Waals equation isn’t used in gases at pressures of one atmosphere or less because the corrections are too small to be meaningful. Molecules in our atmosphere are about 10 diameters apart on average, if you look at the Lennard-Jones equation you’ll see that the attractive force falls off as r^-6, hence no meaningful attractive force.