Guest Post by Bob Tisdale
UPDATE: Corrected the percentage of ocean heat loss though evaporation. Update 2: Added a link to a post by Willis Eschenbach at the end, and corrected a typo.
# # #
Ocean heat content and vertically averaged temperature data for the oceans have been the subjects of a couple of recent blog posts. As one might expect, the discussions on those threads tend to shift to the subject of whether or not the infrared (longwave) radiation from manmade greenhouse gases can cause any measureable ocean warming at the surface or at depth. According to the hypothesis of human-induced global warming, the warming of the global oceans to depth and the related ocean heat uptake are a function of the radiative imbalance caused by manmade greenhouse gases. There are a number of arguments for and against the hypothetical anthropogenic warming of the oceans.
So the topic of this post is ocean warming. I’ll present different opinions/arguments on anthropogenic ocean warming.
For a detailed overview of ocean heat content data, please see the post Is Ocean Heat Content Data All It’s Stacked Up to Be? And see the post AMAZING: The IPCC May Have Provided Realistic Presentations of Ocean Heat Content Source Data for another discussion by the IPCC.
INFRARED RADIATION CAN ONLY PENETRATE THE TOP FEW MILLIMETERS OF THE OCEAN SURFACE AND THAT’S WHERE EVAPORATION TAKES PLACE
It is often argued that infrared radiation from manmade greenhouse gases can only penetrate the top few millimeters of the ocean surface and that’s where evaporation occurs. That argument then continues that additional infrared radiation from anthropogenic greenhouse gases can only add to surface evaporation, and cannot heat the oceans. On the other hand, sunlight reaches into the oceans to depths of 100 meters or so, though most of it is absorbed in the top 10 meters. Even so, sunlight’s ability to warm the oceans is many orders of magnitude greater than infrared radiation. One of my earliest memories of this argument came from Robert E. Stevenson’s (Oceanographer Scripps) 2000 article Yes, the Ocean Has Warmed; No, It’s Not ‘Global Warming’. In April of this year, looking for solid answers on this topic, Roy Spencer presented the same arguments and a few counter arguments in his post, Can Infrared Radiation Warm a Water Body?
Field tests reported in the 2006 post Why greenhouse gases warm the oceans at RealClimate are often cited by those who believe infrared radiation is responsible for ocean warming. That guest post by Peter Minnett of the University of Miami includes:
However, some have insisted that there is a paradox here – how can a forcing driven by longwave absorption and emission impact the ocean below since the infrared radiation does not penetrate more than a few micrometers into the ocean?
So this argument was considered by climate scientists. The post then goes on to describe why it’s not an inconsistency and then to present the results of field tests. My Figure 1 is Figure 2 from that RealClimate post.
Figure 1 – The change in the skin temperature to bulk temperature difference as a function of the net longwave [infrared] radiation.
The summary text for the illustration at RealClimate reads:
There is an associated reduction in the difference between the 5 cm and the skin temperatures. The slope of the relationship is 0.002ºK (W/m2)-1. Of course the range of net infrared forcing caused by changing cloud conditions (~100W/m2) is much greater than that caused by increasing levels of greenhouse gases (e.g. doubling pre-industrial CO2 levels will increase the net forcing by ~4W/m2), but the objective of this exercise was to demonstrate a relationship.
That, however, creates a counter argument that has been discussed by others. See the HockeySchtick post RealClimate admits doubling CO2 could only heat the oceans 0.002ºC at most. Let me put this into more recent terms. According to the NOAA Annual Greenhouse Gas Index, infrared radiation has only increased about 1.2 watts/meter^2 from 1979 to 2013. Based on the findings at RealClimate, that rise in infrared radiation could only warm the sea surfaces by a little more than 0.002 deg C since 1979. Yet, looking at the global sea surface temperature data, Figure 2, the surfaces of the global oceans warmed more than 0.3 deg C from 1979 to 2013, leaving about 93% 99.3% of the ocean surface warming unexplained.
Figure 2
A continuation of the Minnett-field-test argument is that manmade greenhouse gases and ocean mixing will cause the warming of the mixed layer of the oceans. The HockeySchtick counter could be applicable here as well. The mixed layer ranges in depth from about 20 to 200 meters. Unfortunately, temperature data specifically for the mixed layer are not available in an easy-to-use format, so let’s assume that the NODC’s vertically averaged temperature data for the depths of 0-100 meters captures the vast majority of the mixed layer. As shown in Figure 2, the warming rate of the top 100 meters of the ocean is slightly less than the surface. In other words, the warming rate based on the field tests presented by RealClimate can’t explain the vast majority of the warming of the top 100 meters.
Further to the RealClimate post by Peter Minnett, see the very recent ClimateConversation post HotWhopper wrong on ocean heat. It includes links to a three part discussion titled “Anthropogenic Ocean Warming?” by Richard Cummings, which covers the Minnett findings and other proposed mechanisms of anthropogenic warming of the oceans:
- Part 1: Skeptical Science Offside
- Part 2: The Improbable IPCC Mechanism
- Part 3: Rahmstorf, Schmittner and Nuccitelli
“AIR-SEA FLUXES ARE THE PRIMARY MECHANISM BY WHICH THE OCEANS ARE EXPECTED TO RESPOND TO EXTERNALLY FORCED ANTHROPOGENIC AND NATURAL VOLCANIC INFLUENCES”
The quote in the heading is from Chapter 10 (WG1) of the IPCC’s 5th Assessment Report.
Richard Cummings comments from Part 2 of his series begins:
That’s it. 25 years and five assessment reports after its 1988 formation, the IPCC has not been able to firm up an anthropogenic ocean heating and thermal sea level rise mechanism. The one they have come up with is only “expected”, indicating that they are unable to cite studies of the real-world phenomenon of non-solar air => sea energy fluxes actually occurring on a scale that would explain 20th century ocean heat accumulation in the order of 18×10^22 J and subjugate a solar-only mechanism.
“…HEAT PENETRATES THE OCEANS FASTER IN A WARMER CLIMATE”
The heading is a quote from the concluding remarks by Stefan Rahmstorf in the RealClimate post Sea-level rise: Where we stand at the start of 2013 (my boldface).
My bottom line: The rate of sea-level rise was very low in the centuries preceding the 20th, very likely well below 1 mm/yr in the longer run. In the 20th Century the rate increased, but not linearly due to the non-linear time evolution of global temperature. The diagnosis is complicated by spurious variability due to undersampling, but in all 20th C time series that attempt to properly area-average, the most recent rates of rise are the highest on record. At the end of the 20th and beginning of the 21st Century the rate had reached 3 mm/year, a rather reliable rate measured by satellites. This increase in the rate of sea-level rise is a logical consequence of global warming, since ice melts faster and heat penetrates faster into the oceans in a warmer climate.
Is this a very simplified rewording of the argument that, although the atmosphere is cooler than the ocean surfaces, greenhouse gases will reduce the rate at which oceans can release heat to the atmosphere?
See Richard Cummings response in Part 3 of his series.
MECHANISMS FOR THE WARMING OF THE OCEANS
Donald Rapp presented a simple model to explain how manmade greenhouse gases could warm the oceans in his guest post at Judith Curry’s blog ClimateEtc, back in May 2014. See his post Mechanisms for the Warming of the Oceans. That post drew more than 400 comments. If you’re going to cut and paste one of your or someone else’s comments from that thread, please leave a hyperlink to it.
INFRARED RADIATION FROM MANMADE GREENHOUSE GASES HAS INCREASED SINCE 1979, WHILE TOTAL SOLAR IRRADIANCE HAS DECREASED. THEREFORE, INFRARED RADIATION CAUSED THE OCEAN WARMING.
This is one of the favorite arguments for anthropogenic warming of the oceans: Infrared radiation has increased since 1979 but total solar irradiance at the top of the atmosphere has decreased. Therefore, according to that ill-conceived argument, the sun can’t explain the warming.
Why is it ill-conceived? We’re interested in the amount of sunlight reaching the ocean surfaces and entering into them, not the amount of sunlight reaching the top of the atmosphere.
There is evidence the amount of sunlight reaching Earth’s surface increased from 1979 to 2013. It comes from a specialized climate model called a reanalysis, and the reanalysis being discussed is the NCEP-DOE R-2. Unlike the climate models used to hindcast and predict global warming, a reanalysis uses data (sea surface temperature data, cloud cover data, aerosol data, total solar irradiance data, and the like) as inputs and calculates variables that aren’t measured directly. It’s a climate model, so we still have to look at it with a skeptical eye, but even so, the sunlight reaching the surface of the Earth increased from 1979 to 2013, according to the NCEP-DOE R-2 reanalysis. See Figure 3.
Figure 3
I’ve added a note to the graph:
Above what value do the oceans accumulate heat?
That was to counter another ill-conceived argument. Someone might look at the graph and see that sunlight at the surface peaked around the year 2002 and has since dropped, expecting the oceans to lose heat during the decline. But that argument would fail to consider many things, including the one noted.
This also brings to mind something written by Carl-Gustaf Rossby in 1959. It is part of the opening chapter of the book The Atmosphere and Sea in Motion edited by Bert Bolin. That chapter is titled “Current problems in meteorology”. In it, Rossby made two suggestions while discussing ocean processes (my boldface):
a) The assumption that our planet as a whole stands in firm radiation balance with outer space cannot be accepted without reservations, even if periods of several decades are taken into account.
b) Anomalies in heat probably can be stored and temporarily isolated in the sea and after periods of the order of a few decades to a few centuries again influence the heat and water-vapour exchange with the atmosphere.
So, assuming the NCEP-DOE R2 reanalysis is correct, how long would the recent increase in the amount of sunlight entering the oceans impact climate? According to Rossby, it could be decades or centuries.
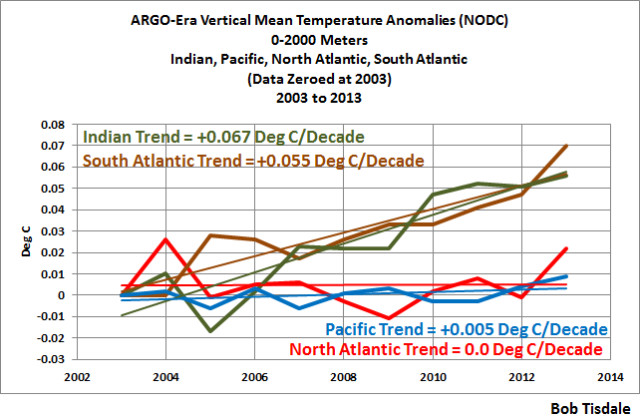
Something else to consider: according to the NODC’s vertically averaged temperature data to depths of 2000 meters, the North Atlantic and the Pacific Ocean show little to no warming since 2005. The other two ocean basins, the South Atlantic and Indian Oceans are showing warming, but they only cover about 1/3 of the ocean surface. See Figure 4.
Figure 4
That lack of warming to depths of 2000 meters for two ocean basins that cover 2/3 of the ocean surface (North Atlantic and Pacific) is hard to reconcile in a world where greenhouse gases are said to be well mixed, meaning they’re pretty well evenly distributed around the globe.
THE OCEANS HAVE THEIR OWN GREENHOUSE-LIKE EFFECT
In his post, The Deep Blue Sea, John L. Daly presented something that must be considered in every discussion of ocean warming: the oceans have their own greenhouse like effect (I’ve added a hyperlink to John Daly’s Figure 1):
A greenhouse effect, by definition, means that the medium through which radiation passes is more transparent at visible wavelengths, but more opaque at infra-red wavelengths, thus letting in visible energy but obstructing the escape of sufficient infra-red energy to maintain thermal equilibrium without a rise in temperature.
The oceans also behave this way.
Reference to fig. 1 shows that the oceans let in visible solar radiation right down to 100 metres depth. However, the oceans cannot radiate from such depths, as infra-red radiation can only take place from the top few millimetres of ocean. Thus, the oceans are also behaving in a greenhouse-like manner, taking in heat and then trapping some of it to cause a temperature rise.
Phrased differently, sunlight can warm the oceans to depths of 100 meters, but the oceans can only release heat at the surface. Now consider that the oceans release heat primarily through evaporation (if memory serves, somewhere in the neighborhood of 90% of the heat loss from the oceans is through evaporation). UPDATE: Sorry, in this instance my memory was off. Of the approximately 180+ watts/m^2 downward shortwave radiation reaching the ocean surface, about half (about 100 watts/m^2) is released through evaporation.
THERE ARE NATURALLY OCCURRING PROCESSES THAT CAN CAUSE THE LONG-TERM WARMING OF THE OCEANS TO DEPTH
The naturally occurring processes that can warm the oceans, of course, are not considered in the climate models used by the IPCC. Climate modelers’ force the warming of the oceans based on their assumptions of how the infrared radiation from manmade greenhouse gases warm the oceans.
We’re going to break the oceans down into ocean-basin subsets, because, for two of the subsets, climate scientists addressed those portions of the oceans in the studies linked to this post.
I’ve presented these discussions in previous posts using ocean heat content data. For a change of pace, I’m presenting the NODC depth-averaged temperature data for the depths of 0-700 meters.
THE WARMING OF THE NORTH ATLANTIC TO DEPTH
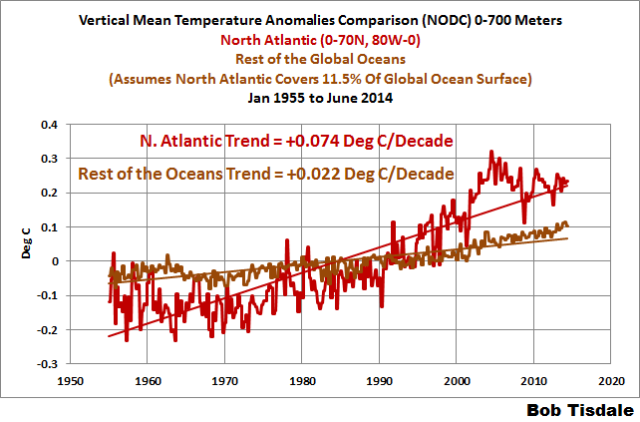
As a preface to our first discussion, Figure 5 presents the depth-averaged temperature anomalies (0-700 meters) for the North Atlantic and for the rest of the global oceans. To determine the depth-averaged temperature anomalies for the rest of the global oceans, I area-weighted the North Atlantic data (11.5%, see the NOAA webpage here) and subtracted it from the global data. The units are deg C.
Figure 5
It very obvious that the North Atlantic to depths of 700 meters warmed at a much faster rate than the rest of the oceans, about 3.3 times faster from 1955 to present. That ocean basin only covers 11.5% of the surface of the global oceans, yet it represents about 35% of the ocean warming to depths of 700 meters.
NOTE: It is unfortunate that the outputs of the climate model simulations of depth averaged temperature (or ocean heat content) are not available in an easy-to-use form so that the models can be compared to observations. We know climate models do not properly simulate the warming of ocean surfaces. They double the warming rate of the ocean surfaces over the past 33 years. See the model-data comparison graph here. Also see the posts here and here for additional discussions. It would be interesting to see how poorly the models simulate ocean warming to depth. [End note.]
Now consider what I wrote in that introductory portion from my upcoming book: It’s very obvious why the change in the ocean heat content is very important to the hypothesis of human-induced global warming. If the oceans could be shown to have warmed naturally, then the impacts of manmade greenhouse gases are much smaller than claimed by climate scientists.
And that’s exactly what a group of scientists did back in 2008. They determined the warming of the North Atlantic to 700 meters since 1955 was caused by naturally occurring processes, not by manmade greenhouse gases. We’ve discussed this paper a few times in recent years—in blog posts and in books. Here’s a portion of my ebook Who Turned on the Heat?
[START OF REPRINT FROM WHO TURNED ON THE HEAT?]
There is a study that provides an explanation for that additional warming. See Lozier et al (2008) The Spatial Pattern and Mechanisms of Heat-Content Change in the North Atlantic.
First, a quick introduction to one of the terms used in the following quotes: The North Atlantic Oscillation is an atmospheric climate phenomenon in the North Atlantic. Like the Southern Oscillation Index described in Chapter 4.3 ENSO Indices, the North Atlantic Oscillation is expressed as the sea level pressure difference between two points. The sea level pressures in Iceland, at the weather stations in Stykkisholmur or Reykjavik, can be used to calculate North Atlantic Oscillation Indices. Which Iceland location they elect to use as the high-latitude sea level pressure reference depends on the dataset supplier. The other point captures the sea level pressure at the mid-latitudes of the North Atlantic, and there are a number of locations that have been used for it: Lisbon, Portugal; Ponta Delgada, Azores; and Gibraltar. The North Atlantic Oscillation Index is primarily used for weather prediction. The direction and strength of the westerly winds in the North Atlantic are impacted by the sea level pressures in Iceland and the mid-latitudes of the North Atlantic, which, in turn, impact weather patterns in Europe and the East Coast of North America. If you live in those locations, you’ll often hear your weather person referring to the North Atlantic Oscillation. As will be discussed, winds in the North Atlantic can also impact Ocean Heat Content.
I’ll present two quotes from the Lozier et al (2008) paper. I’ll follow them with quotes from the press release that describes in layman terms how the North Atlantic Oscillation impacts the Ocean Heat Content of the North Atlantic. Back to Lozier et al (2008):
The abstract reads:
The total heat gained by the North Atlantic Ocean over the past 50 years is equivalent to a basinwide increase in the flux of heat across the ocean surface of 0.4 ± 0.05 watts per square meter. We show, however, that this basin has not warmed uniformly: Although the tropics and subtropics have warmed, the subpolar ocean has cooled. These regional differences require local surface heat flux changes (±4 watts per square meter) much larger than the basinwide average. Model investigations show that these regional differences can be explained by large-scale, decadal variability in wind and buoyancy forcing as measured by the North Atlantic Oscillation index. Whether the overall heat gain is due to anthropogenic warming is difficult to confirm because strong natural variability in this ocean basin is potentially masking such input at the present time.
In the paper, Lozier et al (2008) note, using NAO for North Atlantic Oscillation:
A comparison of the zonally integrated heat-content changes as a function of latitude (Fig. 4B) confirms that the NAO difference can largely account for the observed gyre specific heat-content changes over the past 50 years, although there are some notable differences in the latitudinal band from 35° to 45°N. Thus, we suggest that the large-scale, decadal changes in wind and buoyancy forcing associated with the NAO is primarily responsible for the ocean heat-content changes in the North Atlantic over the past 50 years.
Based on the wording of the two quotes, the paper appears to indicate that Lozier et al (2008) are describing the entire warming of ocean heat content in the North Atlantic. In other words, it seems that Lozier et al (2008) are not stating that the North Atlantic Oscillation is primarily responsible for the additional ocean heat-content changes in the North Atlantic, above and beyond the rest of the world, over the past 50 years; they’re saying it’s primarily responsible for all of the variability. The press release for the paper, on the other hand, leads you to believe the North Atlantic Oscillation is responsible for the North Atlantic warming above and beyond the global warming.
The Duke University press release for the paper is titled North Atlantic Warming Tied to Natural Variability. Though the other ocean basins weren’t studied by Lozier et al, the subtitle of the press release includes the obligatory reference to an assumed manmade warming in other basins: “But global warming may be at play elsewhere in the world’s oceans, scientists surmise”. To contradict that, we’ve found no evidence of an anthropogenic component in the warming of the other ocean basins.
The press release reads with respect to the North Atlantic Oscillation (NAO):
Winds that power the NAO are driven by atmospheric pressure differences between areas around Iceland and the Azores. “The winds have a tremendous impact on the underlying ocean,” said Susan Lozier, a professor of physical oceanography at Duke’s Nicholas School of the Environment and Earth Sciences who is the study’s first author.
Further to this, they write:
Her group’s analysis showed that water in the sub-polar ocean—roughly between 45 degrees North latitude and the Arctic Circle—became cooler as the water directly exchanged heat with the air above it.
By contrast, NAO-driven winds served to “pile up” sun-warmed waters in parts of the subtropical and tropical North Atlantic south of 45 degrees, Lozier said. That retained and distributed heat at the surface while pushing underlying cooler water further down.
The group’s computer model predicted warmer sea surfaces in the tropics and subtropics and colder readings within the sub-polar zone whenever the NAO is in an elevated state of activity. Such a high NAO has been the case during the years 1980 to 2000, the scientists reported.
“We suggest that the large-scale, decadal changes…associated with the NAO are primarily responsible for the ocean heat content changes in the North Atlantic over the past 50 years,” the authors concluded.
[END OF REPRINT FROM WHO TURNED ON THE HEAT?]
WHAT CAUSES THE WATER TO “PILE UP”, INCREASING OCEAN HEAT CONTENT?
Let’s discuss in more detail that “pile up” from the press release of Lozier et al. (2008). First, a few basics: The trade winds are a function of the temperature difference between the equator and higher latitudes. The warmer water near the equator causes warm air to rise there (convection). At the surface, winds blow from the mid latitudes toward the equator to make up for the deficit caused by the rising air, but the rotation of the Earth deflects that inrushing air to the west. Thus the trade winds blow from the northeast to the southwest in the Northern Hemisphere and from the southeast to the northwest in the Southern Hemisphere.
In the ocean basins, ocean circulation is driven primarily from the trade winds in the tropics blowing from east to west. That is, the trade winds push the surface waters from east to west in the tropics. Those westward-traveling waters warm under the tropical sun. They encounter a continental land mass and are directed toward the poles. In the North Atlantic, the poleward-flowing western boundary current is known as the Gulf Stream. It carries the warm tropical waters to the cooler high latitudes, where that water can release heat to the atmosphere more efficiently. At the mid-latitudes, those waters encounter the west to east winds known as westerlies and are blown eastward toward Europe and Africa. The eastern boundary current along Africa returns those cooler waters back toward the tropics, where they can be warmed again, completing the cycle. That ocean circulation loop is called a gyre.
Now for the “piling up”: Suppose the westerlies in the mid-latitudes slowed or reversed, while, at the same time, the trade winds were pushing the same amount of tropical water to the west and poleward. At mid-latitudes, the change in the strength or direction of the westerlies would resist the poleward transport of warm water from the tropics. That warm water would accumulate as a result. Here’s that quote from the press release again:
By contrast, NAO-driven winds served to “pile up” sun-warmed waters in parts of the subtropical and tropical North Atlantic south of 45 degrees, Lozier said. That retained and distributed heat at the surface while pushing underlying cooler water further down.
Presto. A naturally caused accumulation of heat in the North Atlantic.
Curiously, under the heading of “Beam Me Up, Scotty”, Stefan Rahmstorf of RealClimate presented a similar discussion in his post What ocean heating reveals about global warming. I, of course, commented on that in my post Comments on Stefan Rahmstorf’s Post at RealClimate “What ocean heating reveals about global warming”
Now suppose, at the same time, there were a series of strong El Niño events over a multidecadal period (1976 to the turn of the century for example), so that the tropical waters in the North Atlantic were naturally warmer than normal. Trenberth and Fasullo (2011) explain why some portions of the oceans remote to the tropical Pacific warm in response to an El Niño (my boldface):
But a major challenge is to be able to track the energy associated with such variations more thoroughly: Where did the heat for the 2009–2010 El Niño actually come from? Where did the heat suddenly disappear to during the La Niña? Past experience (Trenberth et al. 2002) suggests that global surface temperature rises at the end of and lagging El Niño, as heat comes out of the Pacific Ocean mainly in the form of moisture that is evaporated and which subsequently rains out, releasing the latent energy. Meanwhile, maximum warming of the Indian and Atlantic Oceans occurs about 5 months after the El Niño owing to sunny skies and lighter winds (less evaporative cooling), while the convective action is in the Pacific.
That additional sunlight during a period when El Niños dominated (1976 to the turn of the century) would add to the amount of accumulating warm water in the North Atlantic…and elsewhere.
And Trenberth now understands that the heat didn’t suddenly “disappear to during the La Niña”. It shows up as the “big jumps” in surface temperature in response to strong El Niño events. See the posts:
- Open Letter to the Royal Meteorological Society Regarding Dr. Trenberth’s Article “Has Global Warming Stalled?”
- The 2014/15 El Niño – Part 9 – Kevin Trenberth is Looking Forward to Another “Big Jump”
I also present those “big jumps” in the monthly sea surface temperature updates (November 2014 update is here). They stand out quite plainly in the sea surface temperature data for the South Atlantic, Indian and West Pacific Oceans. For a further discussion see the illustrated essay “The Manmade Global Warming Challenge” (42mb).
EXTRATROPICAL NORTH PACIFIC
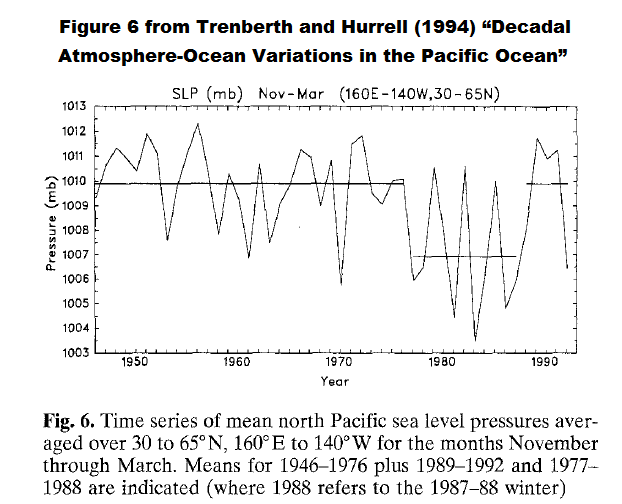
The next paper to be discussed is Trenberth and Hurrell (1994): Decadal Atmosphere-Ocean Variations in the Pacific. In it, Trenberth and Hurrell were using an index derived from the sea level pressures of the extratropical North Pacific (30N-65N, 160E-140W), called the North Pacific Index, to explain shifts in the sea surface temperatures of the North Pacific. Again, a sea level pressure index reflects changes in the wind patterns. My Figure 6 is Figure 6 from Trenberth and Hurrell (1994).
Figure 6
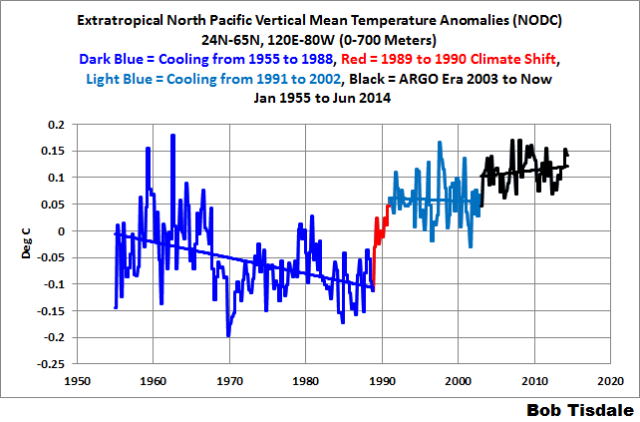
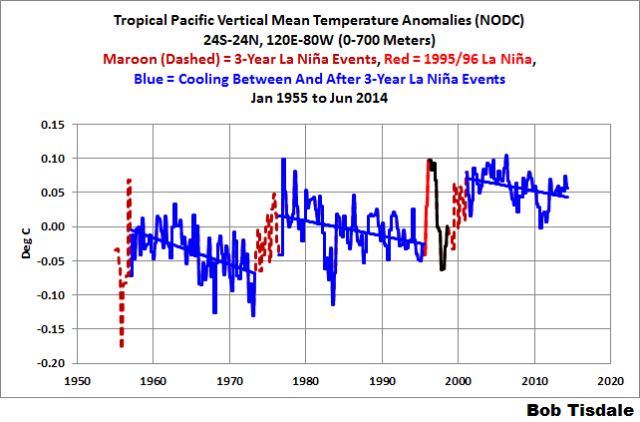
That same shift appears in the depth-averaged temperature data for the extratropical North Pacific (24N-65N, 120E-80W) for the depths of 0-700 meters. But the shifts are delayed a year in the subsurface temperature data. See Figure 7.
Figure 7
I’ve color-coded 4 periods on the graph. The first period from 1955 to 1988 (dark blue) includes the downward shift in 1978. As a result of that shift in 1978 (that should be related to the shift in the sea level pressures and wind patterns), the depth-averaged temperature data shows a cooling trend from 1955 to 1988. That is, the extratropical North Pacific to depths of 700 meters cooled (not warmed) for more than 3 decades. The second period (red) captures the upward shift in 1988 and 1989 that, once again, should be related to the shift in the sea level pressures and wind patterns. From 1991 to 2002 (light blue), the extratropical North Pacific cooled once again to depths of 700 meters. And since the ARGO floats were deployed (black), the extratropical Pacific shows a slight warming to depth.
It’s blatantly obvious the extratropical North Pacific to depths of 700 meters would show no warming from 1955 to present if it wasn’t for that upward shift in 1988 and 1989. It’s also obvious that the downward shift in 1978 that extends to 1988 also impacts the long-term trend. That is, without the naturally caused downward shift in the late-1970s the long-term warming rate would be less. Obviously, natural variability, not manmade greenhouse gases, dominates the variability and long-term warming of the extratropical Pacific to the depths of 700 meters.
TROPICAL PACIFIC
We isolate the vertically averaged temperature data to depths of 700 meters for the tropical Pacific because the tropical Pacific is where El Niño and La Niña events take place, and El Niño and La Niña events, collectively, are the dominant forms of natural variability on Earth. A further clarification: while El Niño and La Niña events are focused on the equatorial Pacific, they directly impact the entire tropical Pacific. See the animation here for an extreme example of the effects of an El Niño on the sea level residuals of the tropical Pacific.
Let’s start with two quotes from (again) Kevin Trenberth. According to Trenberth, El Niño events are fueled by sunlight, not manmade greenhouse gases. In the much-cited Trenberth et al. (2002) The evolution of ENSO and global atmospheric surface temperatures, they stated (my boldface and brackets):
The negative feedback between SST and surface fluxes can be interpreted as showing the importance of the discharge of heat during El Niño events and of the recharge of heat during La Niña events. Relatively clear skies in the central and eastern tropical Pacific [during a La Niña] allow solar radiation to enter the ocean, apparently offsetting the below normal SSTs, but the heat is carried away by Ekman drift, ocean currents, and adjustments through ocean Rossby and Kelvin waves, and the heat is stored in the western Pacific tropics. This is not simply a rearrangement of the ocean heat, but also a restoration of heat in the ocean. Similarly, during El Niño the loss of heat into the atmosphere, especially through evaporation, is a discharge of the heat content, and both contribute to the life cycle of ENSO.
NOTE: That’s the source of my standard description of ENSO as a chaotic, naturally occurring, sunlight-fueled, recharge-discharge oscillator…with El Niños acting as the discharge phase and La Niñas acting as the recharge phase. But La Niñas also help to redistribute the leftover warm waters from the El Niños. [End note.]
Also see Trenberth and Fasullo (2011). They confirm that ENSO is sunlight-fueled during La Niña events:
Typically prior to an El Niño, in La Niña conditions, the cold sea waters in the central and eastern tropical Pacific create high atmospheric pressure and clear skies, with plentiful sunshine heating the ocean waters. The ocean currents redistribute the ocean heat which builds up in the tropical western Pacific Warm Pool until an El Niño provides relief (Trenberth et al. 2002).
Figure 8 presents the vertically averaged temperature anomalies (0-700 meters) for the tropical Pacific. El Niño and La Niña events directly impact the top 300 meters, so this depth captures their direct impacts. I’ve highlighted in maroon the three 3-year La Niña events of 1954 to 1957, 1973 to 1976, and 1998 to 2001. After those 3-year La Niña events, the tropical Pacific shows cooling, not warming. That indicates that the shorter La Niñas that follow El Niños only recharge part of the warm water released from the tropical Pacific by the El Niños. Also, I’ve highlighted in red the 7-month period associated with the 1995/96 La Niña. (See the old version of the NOAA ONI index.) The 1995/96 La Niña created the warm water that fueled the 1997/98 El Niño, which is responsible for the sharp drop in temperature following the heat uptake of the 1995/96 La Niña. The “overcharge” from the 1995/96 La Niña and the recharge during the 1998-01 La Niña obviously caused an upward shift in the subsurface temperatures of the tropical Pacific.
Figure 8
What is also blatantly obvious is the warming of the tropical Pacific to depth is dependent on 4 La Niña events. And according to Trenberth et al. (2002) and Trenberth and Fasullo (2011), sunlight warms the tropical Pacific during La Niñas, not infrared radiation from manmade greenhouse gases. (In the real world, downwelling longwave radiation decreases during La Niña events.)
BOTTOM LINE ON OCEAN TEMPERATURE DATA FOR THE DEPTHS OF 0-700 METERS
Subsurface temperature data (and ocean heat content data) for the North Atlantic, the Extratropical North Pacific and the Tropical Pacific all indicate that naturally occurring coupled ocean-atmosphere processes are the primary causes of ocean warming to depth, not manmade greenhouse gases. In fact, the data for the tropical Pacific and extratropical North Pacific show those oceans can cool for decadal and multidecadal periods between short-term naturally caused warming episodes. Those decadal and multidecadal cooling periods further suggest that manmade greenhouse gases have no measureable impact on ocean warming to depth.
NOTE: Someone is bound to note that I’ve only presented subsurface ocean temperature data for the top 700 meters and only for the oceans of the Northern Hemisphere and the tropical Pacific. If I receive a comment to that effect on the thread, I will refer that blogger to the 2 posts linked in the introduction. Here they are again:
- Is Ocean Heat Content Data All It’s Stacked Up to Be?
- AMAZING: The IPCC May Have Provided Realistic Presentations of Ocean Heat Content Source Data
CLOSING
I’m sure I’ve missed a few arguments for and against the anthropogenic ocean warming. If you introduce others, please provide links where possible.
UPDATE 2: While preparing this post, I overlooked an excellent post by Willis Eschenbach Radiating The Ocean.



If GHG really have increased sea temps, even just down to 100m, the effect would be so small that we could not possibly measure it.
Oh my God, the sky dragons are back.
Infrared radiation can warm a metal bar. How far do you think it penetrates into that Bob?
Did you read the post, MikeB? Apparently, you didn’t understand it.
Bob,
In a post way too long, you’ve devoted far too much space to some very silly arguments. There is no issue about down IR penetrating sea water. The sea surface is warm and radiates upward more heat than it receives in sunlight. If it were not for down IR, it would cool rapidly. Down IR maintains heat flux balance at the surface. It does not need to penetrate. If down IR increases, the flux from below decreases, at the same temperature. The sea is warmed by that retained heat.
I have to agree with Nick. The effect of participating media is to decrease the rate of energy transfer. This is not a steady state affair. For the system to achieve steady state the hot side must get hotter. This then increases the rate of energy transfer and steady state is again achieved. On the other hand. Nicks argument allows for the principle of saturation. Given that combustion engineering models see saturation of CO2 it is odd that this is not seen in climate models.
Whether/how DLWR warms the oceans is key to whether or not we should be worried about increasing CO2 levels in the atmosphere, and Bobs post is important in giving this subject centre stage.
I have read of only one proposed mechanism by which DlLWR can contribute to ocean warming, and that is by heating of the ‘thin film’ surface layer, thus altering the temperature gradient across it and inhibiting heat loss by conduction through it. In other words DWLR generates an insulation effect at the surface.
Nick Stokes appears to be referring to this mechanism, but I have never seen any calculations that demonstrate quantitively that it is capable of leading to the increase in ocean heating which the alarmists happily describe in units of Hiroshima Bombs. Does any contributor know of any?
That post doesn’t make a bit of sense. Photons are not like (American) football players, blocking each other to prevent them from going downfield or, in this case, radiating away at the surface.
John Eggert says: “On the other hand. Nicks argument allows for the principle of saturation. Given that combustion engineering models see saturation of CO2 it is odd that this is not seen in climate models.”
Different problem. This issue was worked out in about the 1950s: What matters for the greenhouse effect is where the radiation is emitted such that it can successfully escape to space. It not just an issue of “Does the radiation emitted by the surface get absorbed at least once?” The role of greenhouse gases is to move this effective emission layer up to higher altitudes. Since there is a lapse rate in the troposphere, this means emission to space is occurring from colder regions and hence less radiation is emitted to space. This puts the Earth system out of radiative balance. (It is now emitting less to space than it is absorbing from the sun.) Hence, over time, it’s heat content increases and it warms until such a time that radiative balance is re-established.
Nick, thanks for commenting. First, my post is precisely as long as I wanted it to be. FYI, I trimmed 4000 words and 5 illustrations. Second, the silliness of an argument is in the eyes of the beholder. Those making the arguments didn’t think they were silly when they made them. And we also note you weren’t specific about which arguments were silly. One might suspect that your general characterization of the arguments (as silly) was simply your attempt to downplay everything but what you, personally, felt was important. Others may differ with you.
Additionally, this post was not about the background state of the oceans and atmosphere. It was about the additional impacts of manmade greenhouse gases, whether they had a MEASUREABLE impact on ocean warming.
We can discuss this for weeks, posturing about the possible impacts of a 1.2 watt/m^2 increase in infrared radiation against a background state, but that would be a waste of our times, Nick. I always have data to point to, and I will always point to data. According to the only reasonably reliable, much-tweaked, observations-based data, which is the ARGO-era data, the Pacific Ocean and the North Atlantic to depths of 2000 meters show little to no heat uptake for the last decade, and those two basins represent more than 2/3 of the surface of the oceans.
I do realize it’s a very short time period, but that’s all we’ve got.
Old’un
I agree with your brief and pithy comment. I understand Nick’s comment in the same way, and I think he would agree we understood it properly.
Missing from the description of that is happening is that the water vapour level immediately above the surface is saturated and as opaque to IR as the water surface. The effect CO2 exerts within 3 metres of the surface is vanishingly small. If the water vapour (absolute humidity) changed a few % because of the air temperature, or wind speed, it would swamp any putative warning effect of back radiation – and that Nick’s effect is rooted on that back radiation only. If there were no back radiation, there would be no GH effect, right? So if back radiation cannot produce a measurable effect at the ocean surface, claims it is a cause of a meaningful proportion of change in the ocean heat content is skating on thin ice.
There is a comment above about 4 watts/m^2 for a doubling of CO2. I think that includes water vapour feedback, not so? Well, that is an admission that water vapour which abounds at the ocean surface is more important that CO2. Include it in radiative arguments!
The effect of DWIR at the surface in the daytime is very different from night time. I don’t think this can be overlooked. Saturated air near the surface prevents a portion of IR reaching the water surface using the same mechanism that ‘prevents’ it getting into space. At night when the ocean is warmer than the air, there is a huge DWIR from water vapour near the surface that totally overwhelms anything from CO2, let alone AG CO2 – in other words a GHG effect involving water vapour near the surface. A tiny variation in water vapour concentration is bigger than a huge change in CO2. .As we all know, an increase in water vapour leads to rain which is, net, a cooling influence on the ocean as the process vents heat upwards well above the surface.
As you say, show me the numbers.
For 20 years we have had most scientists and thinkers saying, AGW is conceptually possible, let’s give them the benefit of doubt, after which skeptics tried to show that proposition was faulty at a lot of scales. Now, after 20 years of confusing signals from the real world, it is time to change this approach. It is time those who make AGW claims to start demonstrating the idea is valid, with the default position changing from ‘OK, maybe you are right’, to ‘No, it is not acceptable as a proposition until it is demonstrated’ – in other words when the null hypothesis has been falsified.
Bob, you are doing a great job at showing, in the face of ‘let’s assume it is correct’, that there is no case to answer. It is time for the AGW proponents to provide data and math that invalidate the null hypothesis.
IR heating and cooling at the surface of the oceans are dominated by water vapour. Conceptually, Nick’s model is going to work, but at what scale? I want to see the numbers. IR retransmissions is almost entirely from water vapour. CO2 is a tiny fraction of the total. AG CO2 changing the total CO2 by a tiny fraction over a decade causes a tiny change in the tiny fraction of IR exchanges attributable to CO2.
With the OHC changes barely quantifiable with modern instruments (see Willis’ discussions) we can’t assert a detectable influence from AG CO2 on ocean temperature without some mechanism, measurements and proof-of-concept calculations. And the calculations can’t ignore water vapour, for heaven’s sake.
Bob,
“And we also note you weren’t specific about which arguments were silly. “
I was specific about the IR can’t warm argument. But an even sillier one is the one where you say:
“Based on the findings at RealClimate, that rise in infrared radiation could only warm the sea surfaces by a little more than 0.002 deg C since 1979.”
That’s just completely wrong. The numbers given there (RC) relate to the difference between skin temp and temp at 5cm depth. They are a measure of flux, not of warmth. They are measuring the effect of down IR on flux in the water. And it’s substantial.
Nick Stokes: Down IR maintains heat flux balance at the surface. It does not need to penetrate. If down IR increases, the flux from below decreases, at the same temperature.
IR does not maintain heat flux “balance”, because most of the time there is not balance. If down IR increases, the flux from below is unaffected and the surface rate of evaporation increases, at least most of the time when the wind is not absolutely still.
Very few studies have looked at the change in the rate of evapotranspirative energy transport from the surface (evaporative in this case) in response to changes in CO2 or warming. The estimate of 0.002K/(Watt/m^2) change in surface temperature, measured in a study, is quite realistic considering the latent heat of evaporation of water. According to Trenberth et al, more heat is carried by wet thermals from surface to upper troposphere by evapotranspiration than by radiation.
Matthew Marler,
“IR does not maintain heat flux “balance”, because most of the time there is not balance. If down IR increases, the flux from below is unaffected and the surface rate of evaporation increases”
It maintains long term balance (over days). Heat is conserved and can’t accumulate at the surface without big temperature change.
If down IR increases, the flux from below is affected. That is exactly what the Tangaroa experiment is showing. Early morning, the nett LW comes almost into balance, and the temperature drop across the top 5 cm halves, relative to the other extreme. That drop measures flux from below (heat from sun).
Nick Stokes
December 9, 2014 at 6:16 am
////////////////////////////////////////////
” There is no issue about down IR penetrating sea water. The sea surface is warm and radiates upward more heat than it receives in sunlight. If it were not for down IR, it would cool rapidly. Down IR maintains heat flux balance at the surface.”
Nick, that is incorrect. You cannot use apparent emissivity measured within the Holorumn of the atmosphere to determine effective emissivity of water.
Any claim that incident LWIR can slow the cooling rate of the oceans can be checked by the simplest of experiments –
http://i42.tinypic.com/2h6rsoz.jpg
– fill both sample chambers with 40C water and record their cooling rate over 30min. You will note no significant difference between the samples under the weak and strong LWIR sources. Now repeat the experiment but put a couple of drops of baby oil on the surface of each water sample to prevent evaporation. Both sample can now only cool by conduction and radiation. Now the sample under the strong LWIR source cools slower.
The fact that incident LWIR cannot slow the cooling rate of water that is free to evaporatively cool raises the question – “what is keeping the oceans above theoretical blackbody temperature of 255K for an average of 240 w/m2 solar insolation?” The answer is painfully simple – The oceans are an extreme SW selective surface not a near blackbody.
Firstly for water SW absorptivity (~0.9 hemispherical) is asymmetric with IR effective emissivity (~0.7 hemispherical). Secondly water is SW translucent and solar radiation is absorbed at depth not at the surface. To water all watts are not equal, frequency matters. This simple experiment shows why –
http://oi61.tinypic.com/or5rv9.jpg
– Both blocks have equal ability to absorb SW. Both blocks have equal ability to radiate LWIR. The only difference is depth of SW absorption. Illuminate each block with 1000w/m2 of LWIR and they will both rise to the same temperature. Illuminate both blocks with 1000 w/m2 of SW and block A will run 20C hotter. This experiment is a clear demonstration of why S-B equations should never have been used on the oceans.
You can try it again with water that is free to convect –
http://oi62.tinypic.com/zn7a4y.jpg
– John Daly was correct. There is a greenhouse effect in the oceans. From experiments like those shown above conducted at differing scales the following five simple rules for SW translucent materials can be demonstrated –
http://i59.tinypic.com/10pdqur.jpg
– these rules apply no matter if the materials are radiatively, conductively or evaporatively cooled. These rules apply to our oceans. This incredibly basic physics is utterly missing form the “basic physics” of the “settled science”.
Climastrologists have gone and treated our deep convecting intermittently illuminated SW translucent oceans as if they were an opaque material constantly illuminated by 240w/m2. This leads to a fist-biting mistake of around 80K for “surface without atmosphere” temperature calculation for 71% of the planet’s surface. Our radiatively cooled atmosphere is not raising ocean temps from 255K, it is cooling them from around 312K. There is no net atmospheric radiative GHE on planet Ocean.
Nick Stokes: It maintains long term balance (over days). Heat is conserved and can’t accumulate at the surface without big temperature change.
If down IR increases, the flux from below is affected. That is exactly what the Tangaroa experiment is showing. Early morning, the nett LW comes almost into balance, and the temperature drop across the top 5 cm halves, relative to the other extreme. That drop measures flux from below (heat from sun).
first, balance is obtained over years, not necessarily days, and for certain not over short periods of time like sunrise and sunset and clouds. second, the Tangaroa experiment has at least two and maybe more explanations: the one that you describe, and the increase in vaporization caused by increased LWIR; where and when water is evaporating, a small increment of LWIR increases the evaporation rate without affecting the rate of heat diffusion from below.
Matthew Marler,
“first, balance is obtained over years, not necessarily days”
It depends on the length scale (thermal inertia). People speak of micron layers penetrable by IR; obviously they must balance in seconds or less. In the top 5 cm used for skin effect, a 1 W imbalance will raise the temperature 1°C in about six hours. etc. And so proportional to depth. Top meter, about 5 days. The Tangaroa shows response on a sub-daily scale.
“where and when water is evaporating, a small increment of LWIR increases the evaporation rate without affecting the rate of heat diffusion from below.”
There is no special linkage between fluxes. They interact through the temperature. Evaporation is determined by temperature, wind and humidity, not by some flux calculation. If LWIR increases it will increase surface temperature, which will simultaneously:
1. increase evaporation
2. reduce the temperature gradient in the water, reducing the flux from below
3. increase upward IR.
Each of these changes is determined by the temperature change, and tends to counter it.
Nick writes “If down IR increases, the flux from below is affected. That is exactly what the Tangaroa experiment is showing.”
No its not.
If down IR increases AND down SW decreases then the difference between the surface and bulk increases. And that could equally be because the bulk is cooling relative to the surface which will stay warmer until the residual warmth from the bulk has convected to the surface and been radiated/evaporated away. THATS what the Tangaroa experiment is showing.
Its a flawed experiment Nick.
Tim,
“And that could equally be because the bulk is cooling relative to the surface which will stay warmer until the residual warmth from the bulk has convected to the surface and been radiated/evaporated away. “
No, it can’t equally be that. Heat from SW is distributed over many metres of depth. Thermal inertia is huge. There is essentially no diurnal temperature variation over most of that range. But LWIR variations affect the surface on short time scales.
I have an early post on this. It shows the extent of diurnal variation with depth.
Nick writes “No, it can’t equally be that. Heat from SW is distributed over many metres of depth. Thermal inertia is huge.”
Not true. The moment SW decreases the energy deposited into the ocean slows and cooling immediately starts to take place. Minnett has provided no details as the how he made his measurements so your assumption that the effect is “immediately” measured is just that, an assumption.
Nick, you know the temperature profile of the top of the ocean is held at least in part due to the different wavelengths being absorbed at different depths. When that stops, the profile is bound to change.You made the observation yourself at SoD. I remember the thread…
“The moment SW decreases the energy deposited into the ocean slows and cooling immediately starts to take place”
It may immediately start. But it will take a long time to get anywhere. I said above to MM that it would take 6 days for 170W/m2 to change a 1m layer by 1°C. For 10 m, that is 60 days. And that is the full flux. A cooling flux could only be a fraction.
Nick writes “It may immediately start. But it will take a long time to get anywhere. I said above to MM that it would take 6 days for 170W/m2 to change a 1m layer by 1°C. For 10 m, that is 60 days.”
Irrelevent. Its 5cm not 1m and the profile at the 5cm and above changes quickly. Your own graphs (sourced from Wiki I expect) show that. Its only a 0.2C change or less for the vast majority of Minnett’s measurements.
Sorry Nick,
gallop around the race course as long as you like, you can never win. Turn the dial on “Flappy-hands” all the way to 11 (hummingbird) and you still can’t escape.
Climastrologists went and treated the oceans as a near blackbody instead of an extreme SW selective surface. You can’t turn back time. You can’t erase the shame. The reality is given 1 bar pressure, our radiatively cooled atmosphere is cooling the oceans from 312K to 288K. The climastrologists you are trying to defend are claiming that the oceans are SW opaque and given 1 bar pressure the net effect of our radiativley cooled atmosphere is to slow the cooling rate of the oceans. The engineers are all laughing at you.
Glue factory time for the racehorse 😉
Nick Stokes: If LWIR increases it will increase surface temperature,
That is the part that I am doubting. The effect could just as likely be to increase the excitation state of just “evaporated” water vapor.
Evaporation is determined by temperature, wind and humidity, not by some flux calculation.
Evaporation at a sustained rate requires a sustained influx, without which the temperature will decrease.
Would you happen to have a good reference on the exact changes occurring at the water surface as evaporation is occurring? It seems to me that a key to understanding the effects of CO2 on climate requires knowing more than I have found so far on the effect of downwelling LWIR on evaporation rate, especially by seasons and daytime. To me, too much of the discussion depends on assumptions of “balance” and equilibrium in systems that are never even in steady-state, much less equilibrium. Too much is expected of a 4W/m^2 increase in downwelling LWIR — and several widely cited papers assume a new equilibrium 1C higher than what we have now has already been obtained, and increased evaporative transfer of energy from surface to upper troposphere.
MM,
“Evaporation at a sustained rate requires a sustained influx, without which the temperature will decrease.”
How the temperature is maintained is a separate question. That’s the pattern of heat transfer analysis. The heat equation equates the local rate of temperature change to the flux imbalance (divergence). You calculate a temperature field, see how that changes the fluxes, see how that changes the temperatures etc.
To make an economic analogy; if steelmaking expands in China, it tends to contract in the US. The Chinese aren’t interfering with the operation of the US mills. Their production brings down the price. It’s the price drop that affects the US operation. flux~production, T~price.
“Would you happen to have a good reference on the exact changes occurring at the water surface as evaporation is occurring?”
It’s a critical issue for coupled AOGCMs. Heat and mass transfer at the surface. Here is one such. But I don’t think you’ll find much that directly links DWLWIR to evaporation. It’s a key plus of the analytic process that they operate separately, mediated by temperature.
Nick Stokes: How the temperature is maintained is a separate question. That’s the pattern of heat transfer analysis. The heat equation equates the local rate of temperature change to the flux imbalance (divergence). You calculate a temperature field, see how that changes the fluxes, see how that changes the temperatures etc.
That does away with incoming radiation absorbed by H2O and CO2 completely, does it not?
Doubling CO2 concentration is said to raise the downward llwir by 4 W/m^2. Is that enough power both to persistently warm the surface and increase the lightning flash rate by 12%? For that we need to know: (a) how much does the increased 2 W./m^2 increase the rate of evapotranspirational energy transfer from the surface and (b) how much does the rate of evapotranspirational energy transfer from the surface have to increase to produce a 12% increase in the lightning flash rate? I don’t think you can get those answers from the heat equation, but I would love to read if someone has done it.
joeldshore
You make a bizarre statement.
“Since there is a lapse rate in the troposphere, this means emission to space is occurring from colder regions and hence less radiation is emitted to space.”
Under what circumstances would you imagine heat transfer occurring WITHOUT a gradient of hot to cold? “Lapse Rate” is the term for this gradient in the atmosphere. The gradients in combustion engineering are much steeper than in climate science. At higher temperatures, >1000C, there are also a lot more spectral lines than at those seen at the temperatures in the atmosphere. The fact is, that “upward moving effective emission layer” gets to the top of the atmosphere at about 500 bar cm concentration, which on earth is about 800 ppm. Beyond that, there is so little increase in forcing, regardless of how much CO2 you add, that it can be approximated by 0. The methods of combustion engineering generate a forcing curve nearly identical to that found by Ramanathan and those who followed him. There is a relatively small divergence from 200 ppm to 800 ppm. It would nicely explain the divergence we are seeing in the modeled versus real temperatures.
Nick Stokes December 9, 2014 at 6:16 am
>’There is no issue about down IR penetrating sea water.”
No there isn’t. But what IR are you referring to? IR-A/B in the DSR spectrum or IR-C in the DLR spectrum. Big difference in penetration depth. Go way down thread to here (note the 2 subsequent corrections):
richardcfromnz December 11, 2014 at 2:50 pm
http://wattsupwiththat.com/2014/12/09/arguments-for-and-against-human-induced-ocean-warming/#comment-1812118
>”The sea surface is warm and radiates upward more heat than it receives in sunlight.”
It’s the energy budget that matters. In the in-situ example at the link SSN is 191.5 W.m-2, DLR – OLR (Rnl) is -57.1 W.m-2. Rnl + Hs + Hl is -168.1 W.m-2.
>”It does not need to penetrate. If down IR increases, the flux from below decreases, at the same temperature.”
Huh? “does not need to penetrate”? This is a new thermodynamic principle you’re introducing Nick, are you sure about this?. If no matter has been penetrated, the energy remains as radiation. But the speed of radiation delivery is the speed of light, that doesn’t suddenly stop at the AO interface i.e. it does need to penetrate, or to be reflected, or to be scattered. DSR (IR-A/B) penetrates water 1µm – 1m, DLR (IR-C) penetrates water (3µm – 100µm). See the linked comment above.
>”The sea is warmed by that retained heat.”
No not by “that” retained heat. In the tropical example linked above the ocean sub-surface gains heat because solar ingress is greater than radiative energy and sensible heat and evaporative heat egress from the surface. The excess heat is dissipated away from the tropics where thermal gradients allow.
Do metal bars evaporate?
“Do metal bars evaporate?”
Sure, depends on the metal, and the temperature. Isn’t that how (CO2?) lasers cut metal?
Mike,
Take a tank of water with the two most accurate thermometers you can find. Place one so it reads the first few millimeters of the water’s temperature (it will have to be an exceedingly small thermocouple device) and the other about a meter from the surface (or more). Insulate the tank from any external heat sources. Place an infrared light above the water shining onto the surface, but high enough not to allow convection from the air to build up. Measure this setup for the next 10 years.
You will find the tank surface temperature is high, but the thermometer one meter down has not changed in any appreciable way. IR does not penetrate beyond the skin of the water.
Repeat the experiment but this time use a broad spectrum visible light source of the same power as the IR source above. You won’t need 10 years to measure the temperature rise.
Repeat the experiment with the same power UV source – temperature rise may not happen if your tank is not deep enough as the higher the energy of the radiation source photons, the deeper the energy deposition peak is. This is well known in radiation physics, but the oceans however, are deep enough for this energy to be deposited well below the surface. If you use gamma rays to heat water, you need a very very deep tank, because their deposition curve places most of the energy very deep. (Of course they are not the significant radiation power intersecting the Earth!)
Now on your metal bar – shine that IR light on that metal bar with a number of thermocouples down its length. If only one end of the bar is in the light beam you will find that the metal does heat somewhat, but the end not in the light barely registers a change. We did that experiment in undergrad lab course and had to get something that would conduct the heat to the rod because the effect was too subtle for our equipment to detect with the heat lamp.
The thing not apparently understood – by some, although I think the head poster, Bob Tisdale, does understand – is the actual size of the oceans; over a hundred million square miles [the US is about 3 million, the UK les than 100,000].
And some two or slightly more – miles deep.
Over a billion cubic kilometres.
And the entire human race will fit into about one half a cubic kilometre (or a little less, my model doesn’t even use envelope-back precision).
7.5 billion, each at – say 60 Kg = 60 litres (roughly, I know!). Little allowance for infants, the morbidly obese [but – hey. Arm-waving numbers!] . . . .
450 billion litres.
450 million cubic metres.
A kilometre cubed is 1,000,000,000 m3 > so <half a kilometre cubed. ISH-ish.
Send me an envelope, please!
As has been noted – a lot of water to heat . . . .
Auto
Please answer a question. If NOAA’s Annual Greenhouse Gas Index says that the infrared has increased 1.2 watts from 1979 to 2013, and the Figure 3 graph shows an increase of 0.7 total, doesn’t that imply that at least 60% of the warming is easily attributable to the Sun?
Ron, Figure 3 presents downward shortwave radiation, not longwave (infrared) radiation.
Thanks. Missed that.
Ron, now consider the long term affect of that one 1.2 watts of S/W energy going into the oceans reading here, and here… http://wattsupwiththat.com/2014/12/09/arguments-for-and-against-human-induced-ocean-warming/#comment-1809882 and here… http://wattsupwiththat.com/2014/12/09/arguments-for-and-against-human-induced-ocean-warming/#comment-1809894
please note, it would be vey helpful to know what W/L composed that 1.2 watt increase at the surface, as well as the residence time of that increase in insolation.
The greenhouse effect is (just) a load of hot air (hotter than it would otherwise be). The ocean gets warmer (than it would otherwise be) simply because it sits beneath a blanket of warmer air, but it takes centuries for this to happen.
If you don’t believe that go outside naked now (winter) at night, and compare how it feels relative to a similar exposure on a summers night. The only difference is the temperature of the air. You and the ocean cool less under a blanket of warmer air than under a blanket of colder air.
One thing that gets my goat sometimes when seeing scientists arguing against the infra-red part of the global warming con, is how complicated they make it. Like this really is a discussion about science. It’s not. It’s a discussion about fraud. You don’t need a 10 page paper on back radiation with a dozen equations and graphs. You just need some basic common sense and some simple observations.
The heat is LEAVING the surface. It is going from hot to cold. It can’t be sent back and even if it could it’s not going to return to make the surface hotter than it was the second visit than it was originally. End of story. The maths and the absorbtion rates and all the rest should be called out for what it is: scientific bullshit!
That this concept was ever even considered by other scientists is a disgrace. Worse that it has been considered that 0.04% of the atmoshere could do it!
Just do a bit of basic thought here. The last 0.9C of warming has been attributed to a 0.012% increase in CO2. However you spin it every molecule of CO2 has been able to absorb enough energy from absorbing a fraction of the energy leaving the surface and redirecting half of it, enabling the heating of the surrounding 8333 molecules!
The post a few days back quoting from Mein Kampf wasn’t out of order. If you want to get away with fooling a large number of people you need to make a lie so outrageous that no one will believe it is possible for anyone to dare to say it if it wasn’t true!
This is such an outrageous lie. Committed by people with PhDs in physics. The king has no clothes on. It’s not “counter intuitive” it’s bullshit!
True in part, but if you think of the atmosphere as a large number of shells each at its own temperature, then the surface isn’t truly radiating to the 4K of space, but to the next layer of atmosphere at a temperature, which radiates not to the 4K of space but to the next layer up. This continues until a layer against that 4K of space radiates to it. To me the Greenhouse Effect is just the time constant of all those transmissions eventually to space. The thickness of each of those shells would be determined by how thick is required for a given level of opaqueness to the wavelength in question. That is my oversimplified explanation anyway.
I tutor physics. My first lesson is “common sense is your enemy”. The heat is indeed leaving the surface. In a simple, static system, the rate it leaves will be constant. The temperature required to achieve that constant depends on what is between hot and cold. If you put something in between that reduces the heat transfer at a particular temperature, the hot side gets hotter to achieve equilibrium. There are a number of mechanisms for heat transfer. Phase change, conduction, convection, radiative. All can be impacted by participating media. This debate is about one specific thing and its impact. It just happens to be the most complicated. Well. Convection can be pretty complicated too. Add in the fact that the system is never at steady state and you start to need many, many pages to describe an unperturbed state. Change something, like CO2 and now you have a peturbed, not steady state system. Common sense is not going to help you.
wickedwenchfan
I think you are confusing conduction of heat with radiation of energy. This is common in posts on this site. It is OK, we are here to help. This is a place of sharing science. Please read the following. This is what you wrote:
“The heat is LEAVING the surface. It is going from hot to cold. It can’t be sent back and even if it could it’s not going to return to make the surface hotter than it was the second visit than it was originally”
Let’s take that one part at a time:
“The heat is LEAVING the surface. It is going from hot to cold.”
Energy that is radiated is not ‘heat’ until it gets to an object. Temperature is a measure of how much energy is contained within some system (like a molecule). Energy can be absorbed, raising the temperature. It can re-radiate that energy and cool down again. The thing being transmitted is not heat, it is energy. Infra red wavelength radiation is just light at a lower frequency. All hot and cold objects radiate energy all the time: hot one to cold ones and cold ones to hot ones. If you raise the temperature of an iron bar to 900 C it will radiate energy in the visible spectrum. The net effect is that a hot object will radiate more energy to a cold one and the cold one will warm. But it is not true that the cold one is not radiating energy to the hot one. It does’ because it does not ‘know’ that the hot object is there. That being the case, if the cold one were even colder, there would be a net cooling of the hot one because it is no longer receiving as much radiation as it was initially from the ‘less cold’ object. The now-colder object in the second example is not ‘sucking’ heat from the hot one causing the hot one to cool faster, it is just not sending as much energy back. This is the ‘effective insulation’ effect Nick was talking about. Anything that interferes with the radiation getting to the cold object and going back to the hot one is ‘insulating’. At the ocean surface, the most important material doing this is water vapour.
The mechanism described by Nick way at the top is correct – the net effect should be insulative, however Old’un an I want to see the numbers. The effect is theoretically real, but we both believe it is it is very small.
>”It can’t be sent back and even if it could it’s not going to return to make the surface hotter than it was the second visit than it was originally.”
That is where you are thinking of a ‘heat conduction’ mechanism and applying it to a radiation case. It doesn’t hold. Heat does not conduct from the cold end to the hotter end of a metal bar, it is true. But there is no Infra Red radiation involved in that case. Step outside into the sunlight. Your body is radiating energy towards the sun and warming it every so slightly. That is just how things work.
Two suns rotating about a common point keep each other warm without any conduction, whatever their temperatures.
Stay well…
…and that is what I call a very clear explanation of a very common misconception.
“I think you are confusing conduction of heat with radiation of energy.”
That confusion is rife here.
If heat is conducted to the air from the surface it cannot also be radiated to space.
So, 255K from 288K radiates to space but the other 33K holds up the atmosphere via conduction / convection.
That extra 33K at the surface is a result of adiabatically warmed descending air in surface high pressure cells inhibiting convection so that incominmg solar energy can warm the surface above S-B.
The descending warmed air dissipates clouds and so is transparent and at the same time it reduces or reverses the lapse rate slope to reduce or prevent convection.
That is exactly how a greenhouse glass roof works.
The greenhouse effect is a result of the mass of warmed air descending towards the surface over 50% of the globe at any given moment.
As such, the description comparing it to a greenhouse is perfect as would have been known by the meteorologist who first coined it.
Absolute nonsense, take a temperature inversion where the air is above the surface temperature and CO2 is emitted at 15 micron and will be absorbed at the surface. In the case of a normal lapse rate the CO2 is cooler than the surface and still emits at 15 micron, that radiation will also be absorbed by the surface. Your bogus argument makes the assumption that the surface ‘knows’ the temperature of the emitter which is absurd.
And such an inversion can actually produce an anti-greenhouse effect where the CO2 emitting layer is warmer than the surface. Shown in the Nimbus downward-looking IR spectrum of Antarctica.
wickedwenchfan: You just need some basic common sense and some simple observations.
Nothing in nature operates according to human common sense.
Regarding…”Reference to fig. 1 shows that the oceans let in visible solar radiation right down to 100 metres depth. However, the oceans cannot radiate from such depths, as infra-red radiation can only take place from the top few millimetres of ocean. Thus, the oceans are also behaving in a greenhouse-like manner, taking in heat and then trapping some of it to cause a temperature rise.”
=======================================================
I just had this discussion with davidmhoffer, beginning here…http://wattsupwiththat.com/2014/12/05/friday-funny-over-a-centurys-worth-of-failed-eco-climate-quotes-and-disinformation/#comment-1808271
and continuing for several successive posts. I called the oceans a GHL (green house liquid) and elaborated a bit on the fact that the oceans are a three dimensional SW selective absorption surface.
Also the energy affected, SW radiation vs. the atmospheric CO2 affect on LWIR, has a very long residence time, thus an equal watt per square meter change can have a FAR greater impact on earth’s energy budget, due to the very long residence time of SW radiation.
BTW, the oceans receive sunlight up to 800′ in depth, and, due to the very long residence time of this energy, the oceans can accumulate far more energy then usually thought based on the long residence time, and decadal changes in insolation. (Just like a very well insulated pot under a very small flame can still reach a very high temperature, over time.)
David A: “the oceans receive sunlight up to 800′ in depth, and, due to the very long residence time of this energy, the oceans can accumulate far more energy then usually thought based on the long residence time, and decadal changes in insolation.”
So we should be able to measure that heat down to 800′. Can we? Is there a heat profile that we can look at and that conforms your theory?
Bernd, I do not have theory about the disphotic zone (disphotic means “poorly lit” in Greek) Actually I made a mistake and should have said it extends down to 800 meters, not feet in depth. The temperature is about 39 to 41 degrees, and it is a fairly simple Google search.
As to how much the energy balances of the oceans change over several decades of say weak solar cycles, changing to several decades of strong solar cycles, I do not know. but think, due to the very long residence time of some of this energy, it is worth considering. if I was a “climate scientist” with large grants, I would study such things as the disparate residence time of different W/L solar energy within the oceans.
The potential heating ability of an input is not so much its watt per sq. meter input, as its absolute temperature. In other words a large warmed rock, may put out as much energy as a very small hot flame of equal total energy. However that very small flame, when its input is fed into a very large well insulated pot can eventually bring the water to a far higher temperature. The large warm rock under the same pot, can never bring the water above it own temperature.
I consider it likely that changes in cloud cover amount, and in jet stream location and therefore cloud cover location, will have a greater impact on total ocean insolation. One other criticism of the experiments done regarding ocean T changes to LWIR is that water vapor (even in clear sky conditions) limits surface insolation due to the broad spectrum of absorption of W/V, and, to my knowledge, no measurement of water T change at deeper depth accompanied these experiments.
“I called the oceans a GHL (green house liquid)”
I’m stealing that one 😉
Konrad, you deserve it. I have a further post to you later tonight, so check back.
Konrad, I would like your thoughts on some of what I have expressed in this conversation here… http://wattsupwiththat.com/2014/12/05/friday-funny-over-a-centurys-worth-of-failed-eco-climate-quotes-and-disinformation/#comment-1808271
The fact that you have actually made some detailed effort to do these experiments is very commendable.
Please tell me your scientific background. I noticed Nick never answered your post detailing your experiments.
I do not have the scientific capacity to determine if your calculation of the overall affect of the oceans on the average T of the earth are correct. However I have long suspected that the ocean immense heat storage capacity, due to the long term residence time of energy entering it, relative to the shorter residence time of same energy being absorbed on the earths surface, brings in far more net energy into the system then would accrue without them. after all, the land cannot absorb the energy it receives at the tropics, and over long time scales move it to the polar regions to warm them. Of course like many natural processes, there is some unknown degree of Newtonian reactions (For every action there…) which Willis and others have detailed regarding cloud formation and SW energy absorption.
Konrad, or anyone for that matter, please help me better understand the process of SW absorption into the ocean, and conversion of that energy to heat.
I think the energy from the photon is supplied by a collision within the water, or any particulate within the water encountered, then said molecule is accelerated: the energy that was stored in the photon becomes kinetic energy in the ocean molecule. Is this close to correct?
David A
December 10, 2014 at 12:48 am
////////////////////////////////////////////////
David, sorry for the slow response, my time has been on the clock the last few days.
”I would like your thoughts on some of what I have expressed in this conversation here… “
Wake up and turn your olfactory attention to nearby caffeinated beverages. DMH is a “sleeper”. Ie: someone trying to pretend to be a sceptic trying to steer discussion back to the “lukewarmer” path.
”The fact that you have actually made some detailed effort to do these experiments is very commendable.”
The experiments have been run at a higher pay grade. What is presented is that which is needed to defeat high school teachers. Kids must be able to destroy the Fabian “long march through the institutions”. The experiments have been simplified so other may easily replicate.
”Please tell me your scientific background.”
Thankfully I don’t have a science background. My first job out of high school was computer programming. Then a decade in physical special effects for film and TV. (yes, I have my StarWars credit). But my university degree and current work is in design and engineering –
http://i57.tinypic.com/2q8n9k7.jpg
– welcome to my day job!
I have 3 collage certificates,
Explosives training from the army,
My SCUBA licence,
A pilots licence,
And an honours university degree in applied engineering.
”I noticed Nick never answered your post detailing your experiments.”
That would be because the “racehorse” has no answers 😉
”I do not have the scientific capacity to determine if your calculation of the overall affect of the oceans on the average T of the earth are correct.”
Ancient Chinese proverb –
“Tell me I’ll forget. Show me I’ll understand. Let me do it, I will know!”
David, I want you to know. I have run those experiments and I have data up the warzoo. So what? A few have replicated, but too few. You want to help David? Think empirical experiment. Remember, that’s what the host of this site did with surface stations. If I am in awe of Anthony’s surface stations crowd sourced project, where should you stand?
–Konrad, or anyone for that matter, please help me better understand the process of SW absorption into the ocean, and conversion of that energy to heat.
I think the energy from the photon is supplied by a collision within the water, or any particulate within the water encountered, then said molecule is accelerated: the energy that was stored in the photon becomes kinetic energy in the ocean molecule. Is this close to correct?–
Water has molecular structure so a water molecule is not going to accelerate.
I would say sunlight is heating the ocean like a microwave heats:
“In microwave cooking, the radio waves penetrate the food and excite water and fat molecules pretty much evenly throughout the food. No heat has to migrate toward the interior by conduction. There’s heat everywhere all at once because the molecules are all excited together. There are limits, of course. Microwaves penetrate unevenly in thick pieces of food (they don’t make it all the way to the middle), and there are also “hot spots” caused by wave interference, but you get the idea. The whole heating process is different because you are “exciting atoms” rather than “conducting heat.” ”
With microwave oven the microwaves are bounced around until are absorbed and a difference with the ocean it’s a “one pass thru” until there are absorbed or excited atoms.
So with ocean the “hot spots” would be within first 10 meters of the surface but like microwave also heats further down.
And if instead of meters water to warm the sunlight were to only heat say 1 cm thick layer [say a meter under the water, then you get warmer water fairly rapidly rising due to difference of density.
So the ocean is different then say a shallow pond because the sunlight heats at the bottom of pond which then heats water above it which would concentrate the heating and water would convect upward more rapidly.
So with ocean there is meters of depth being warmed fairly uniformly and this inhibits upward convection. Though there is certainly some upward convection.
Also the vast majority of the ocean surface is very clear and transparent water, when one gets closer to coast one gets less clear water and more plant life in the water- and in such water one would have a greater amount of heating nearer the surface [and have more convection of heat].
Let’s do some tests.
1) I take a bottle of beer out of the fridge to room temperature. My hypothesis is that it will get warmer.
2) I take a beer in a IR opaque bottle from the bridge to the room. My hypothesis is that it still gets warmer because someone would surely use this thermos bottle if it were working.
3) I use a radiotor to heat a big swimming pool room, pool full of water. My hypothesis is that the temperatures of the air and the water remain approximately the same.
Each of your tests rely on conduction of heat as the main gateway for temperature equilibrium.
I have been in many indoor pools where the water in the pool was 72 while the room was nearly 80 degrees, the question is: How long would it take for conduction to give you equilibrium in this setting? It is a great deal easier to heat air then water.
Sure. The temperatures depend on the heating power and volumes of air and water.
Coming back to climate, oceans decrease the temperature range in coastal areas: http://seawifs.gsfc.nasa.gov/OCEAN_PLANET/HTML/oceanography_currents_4.html . The question of this tread is “how the athmosphere heats the oceans”. We get an idea of durations of warmings, when we look at how lakes warm in springs. It takes months because there is not much energy in warm air to heat large amounts of water.
I forgot the sun, in my reply at 4:50 pm.
Minnett’s experiment is flawed. “Figure 1 – The change in the skin temperature to bulk temperature difference as a function of the net longwave [infrared] radiation.”
He is measuring the difference between skin temperature and the 5cm temperature but look at when the vast majority of his readings are taken. During the daytime when clouds cross over. So what happens is the bulk begins to cool as no SW radiation is warming it and relative to the surface the bulk cools so it appears the surface was warming relative to the bulk. But is that really the case? He doesn’t give his actual data so its impossible to know.
If the AGW theory were correct, it would happen at night as much as during the day but his measurements dont seem to show that.
Thanks Tim, a very important observation.
Exactly. Seeing what happens at night when low clouds pass over would be the ideal test for isolating the effects of increased DWIR.
Ulric, see “Cloud Radiation Forcing in the TAO Dataset“.
w.
Willis, I agree with, and learned from your link. However I did not see (perhaps I missed) how that link was relevant to the oceans, and the capacity of LWIR to heat below the surface. That it may is likewise not cogent. The question is to what degree, and to what degree does an equal watt per sq meter forcing of SW radiation have within the oceans, and on the surface.
In fact this…”This likely slightly overstates the radiation contribution of the clouds. This is because, although unraveling the effect on shortwave is simple, the effect on longwave is more complex.” I do not now agree with. The posts just below this are cogent to that question.
Thanks but that wasn’t pertinent. An instance of a jump in DWIR at night from incoming low cloud could provide useful measurements of skin and sub surface temperature changes.
My apologies, Ulric. My understanding was you wanted to see what happens to DWIR when clouds pass over. However, it seems that you wanted something else, which is fine.
However, no matter what you want to find out, the TAO dataset contains all of the information that you need. The buoys collect both surface temperature and air temperatures as well as DWIR, so you should definitely go and see what you can find out. Let us know the results, it should be very interesting.
All the best,
w.
I maintain not all watts are equal. The residence time of energy depends on both the materials encountered, and the WL of the watt under consideration. A law determines this…”“Only two things can change the energy content of a system in a radiative balance; either a change in input, or a change in residence time of some aspect of the energy within the system.”
I have, on the basis of residence time, questioned the veracity of Willis’s proposition that if the watt per square meter down welling LWIR due to clouds, is equal to the same watt per meter down welling SW , sans clouds, then they make the same contribution to earth’s energy budget. I do not think they do. I postulate that the SW radiation will enter the earths oceans to depth, having far longer residence time. I postulate that the LWIR will expend (I am open to how much?) its energy in accelerating the water cycle, reducing insolation to the surface, be lost in evaporation, and released at altitude, to eventually be liberated by GHG molecules, the more numerous, the more likely to be quickly liberated from our system.
Here is a simply analogy on residence time, and how it directly relates to heat capacity…
Numbers are simplified to a ten basis, for ease of math and communication. Picture the earths system (Land, ocean and atmosphere) as a one lane highway. Ten cars per hour enter, (TSI) and ten cars per hour exit (representing radiation to space.) The cars (representing one watt per square meter) are on the highway for one hour. So there are ten cars on the highway. (the earth’s energy budget)
Now let us say the ten cars instantly slow to a ten hour travel time. Over a ten hour period, the energy budget will increase from ten cars, to 100 cars, with no change of input. Let us say we move to a one hundred hour travel time. Then there will be, over a one hundred hour time period, an increase of 990 cars.
The longer the residence time on the highway, the more cars.
Of course the real earth has thousands of lanes traveling at different speeds, and via conduction, convection, radiation, evaporation and condension, albedo changes, GHGs, etc, etc, trillions of cars constantly changing lanes, with some on the highway for fractions of a second, and some for centuries, some slowing down the traffic on certain lanes (The CO2 affect at 15 microns) but at the same time limiting input to other slow traffic lanes, (W/V reducing surface insolation even in clear sky conditions.) Also The sun changes WL over its polarity cycles far more then it changes total TSI. Additionally the sun can apparently enter phases of more active, or less active cycles which last for many decades.
Such thoughts caused me to question the disparate contributions to earth’s total energy budget of SWR verses LWIR.
I disagree. A watt IS “A” watt. If I have 100 watts in a bag,I have 100 watts. It does not matter where they are in the bag, I have 100 watts.
That is how much energy there is in this “system”
The distribution does not change the total. And the total is what matters,verdad?
How big is your bag? If it’s a few microns across, you have a catastrophic amount of energy there. If the bag is a couple of thousand square miles in area and a couple of miles deep, your 100 watts doesn’t amount to much.
Watts are not energy, but power, energy over time, Btu/english hour or kJ/metric hour.
You can’t have Watts in a bag. Watts are a measure of power, not energy. Watt-hours in a bag, or joules in a bag might make some sense. But Watts would be a measure of how rapidly you let them out of the bag.
If you have Watts in a bag let him out he needs to run this blog. 🙂
Not true about a watt is a watt. If true the hottest place on earth would be at the bottom of the antenna of a 50000 watt radio station. But it is not because those watts cannot heat air etc.
Thanks N,n, N, and m. What I am saying is that the EFFECT of a watt on a particular object is dependent on many varibles, but if you are totaling the wattage in a system,a watt IS a watt.
Or do two skinny watts equal a fat watt? (;<)) Please, no fatwas….
David,
You are entirely correct, to our SW translucent oceans, all watts are not equal. For the empirical proof of this see “selective surface experiment 1” posted up thread.
SW radiation has a far greater heating effect in the oceans than can be shown by incorrectly applying S-B equations to translucent materials.
Thanks Konrad, I looked at experiment one. Again I note Nick was non responsive to your experiment. The normal in this discussion is to assume either A, LWIR cannot heat the oceans period, or B, LWIR does heat the oceans. (I always thought the pure black and white assertions unscientific. The question to be answered regarding A is how much of the LWIR energy goes into evaporation, relative to same energy input of disparate SW insolation. Your experiment begins to answer that. Think what you could do with several million dollars in funding available and with the massive astronaut pools to modify and experiment in. Through varying the translucence of the pools you could mimic the oceans varying temperature, placing hundreds of instruments to measure T and evaporation rates, subject the pool to disparate W/L mimicking solar cycle changes, and S/W vs LWIR, etc.
It is easy to estimate (as your experiment demonstrates) that the vast majority of LWIR goes into evaporation, and perhaps logical to assume that the closer the surface is to evaporating an ever higher percentage of the LWIR goes into more evaporation Did you quantify the evaporation rates in your experiments?
At any rate you are welcome to use the acronym GHL, instead of “deep convecting intermittently illuminated SW translucent oceans”, but you will likely have to explain the latter regardless.
Cheers
David A
Thanks Bob – I always value your work – and wish you could get a critical review paper published somewhere in the journals. When researching ‘Chill’ I came across two sets of data that easily showed that ‘global warming’ between 1980-2000 was not global….in terms of ocean heat content (and later redistribution) the warming was held primarily in the northern ocean gyres and by increasing the depth of warm water. What caused the oceans to warm was also clear: a 4% drop over two decades in low-level reflective cloud cover (data from International Satellite Cloud Climatology Project). This is confirmed by the NASA data for SW radiation reaching the global surface – which had an excess of 2-4 watts /square metre over the same period, compared to the 1 watt computed for LW radiation due to GHGs (which I think is computed for the Top of the Atmosphere). Cloud cover rebounded by 2% in 2001/2002, and of course, that is when global temperature rise ‘stalled’.
I would like to see someone gather the regional cloud data, surface flux and upper ocean heat content – this would readily show that GHGs were not the main driver and of course invalidate the pronouncements of the IPCC to that effect. I suppose that is why nobody with the resources to do so, has done so. I don’t have the resources.
Having shown that GHGs are unlikely to be responsible for the warming of the North Atlantic, Lozier comments (perhaps wryly)
‘The overall North Atlantic heat-content change, equivalent to an average increase in the surface heat flux of +0.4 W m–2, is the same sign yet slightly below the lower estimates of anthropogenic-induced radiative heating, ranging from +0.6 to +2.4 W m–2 since 1750 (19). Presumably, other parts of the global ocean and climate system have taken up the remainder of the excess heat input. ‘
Lozier’s paper shows a recent cooling of the sub-polar gyre – in terms of heat content. That means the heat has gone into the atmosphere thus warming northern regions (Arctic meltdown!) but once this heat source is run down, Arctic temperatures will fall and the ice-rebound continue – all except one Arctic temperature record (in Norway) show recent falls, for longer the further one gets from the Norwegian Sea current that brings warm water to the Arctic Ocean. If recent trends continue, the summer sea ice will be back within ‘normal’ bounds within five years. I think that is more likely than continued warming and ice-loss.
Hi Peter.
I am expecting a sharp increase in negative NAO through the next 10 years, which would give a renewed warming of the AMO, and likely more ice loss in some summers than 2007 or 2012. This would fit the natural pattern of the AMO being out of phase with solar cycles when in its warm mode:
http://www.woodfortrees.org/plot/esrl-amo/from:1880/mean:13/plot/sidc-ssn/from:1880/normalise
Electromagnetic radiation is a wave motion which transports energy by means of self-propagating electric and magnetic fields. This is true whether the radiation is infrared, visible light, X Rays, radio waves, microwaves etc.
When radiation strikes the surface of a material one of three things may happen.
1. It may be reflected, in which case it continues in a new direction with its energy conserved.
2. It may pass through the material, like light through a window, radio waves through the walls of a house or like X-rays through your body.
3. It may be absorbed by the material.
Often a combination of these effects apply.
In the third case, when radiation is absorbed, the energy it conveys is also absorbed. Energy must be conserved and, most commonly, the absorption of radiation causes the absorbing material to heat up. However, when radiation is absorbed by the ocean, some of the energy may be used evaporate water and in this case the energy supplies the latent heat of evaporation, instead of heating.
But there is a limit to this. The proportion of energy used for evaporation is governed by the temperature and the partial pressure of water vapour above the water (humidity).
If all the energy of ‘back-radiation’ (324 watts per square metre of ocean) were used for evaporation then there would be a hell of a lot of water in the atmosphere. What is more, water vapour in the atmosphere eventually falls back to earth as precipitation (rain, hail, snow) and we don’t get that much precipitation on this planet. We know how much precipitation we get globally throughout the year; it is equivalent to a latent heat input about 80 watts per square metre of Earth surface. So most of the infrared radiation must be warming the ocean, not evaporating it (this is without even accounting for the effects of direct solar radiation).
.
MikeB says: “If all the energy of ‘back-radiation’ (324 watts per square metre of ocean) were used for evaporation then there would be a hell of a lot of water in the atmosphere…”
Nice try, MikeB. Where in my post or in any of the documents linked in the post did anyone state, suggest or imply that all “the energy of ‘back-radiation’ (324 watts per square metre of ocean) were used for evaporation”? You wasted your breath.
Nice try, MikeB. Where in my post or in any of the documents linked in the post did anyone state, suggest or imply that all “the energy of ‘back-radiation’ (324 watts per square metre of ocean) were used for evaporation”?
Just a few posts above yours it was stated that the ‘vast majority of LWIR goes into evaporation’ and that is a common theme when this subject is discussed here. So I suggest you stop being so snarky and address that point.
David A December 10, 2014 at 1:57 am
It is easy to estimate (as your experiment demonstrates) that the vast majority of LWIR goes into evaporation, and perhaps logical to assume that the closer the surface is to evaporating an ever higher percentage of the LWIR goes into more evaporation Did you quantify the evaporation rates in your experiments?
That 80 watt/m2 is for about 1m of precipitation averaged over the globe for the year. An extra half a watt because of a warmer atmosphere equates to about an extra 7-8 millimeters of rain on average. How do you know that didn’t happen?
Bob,
On average all heating energy of the oceans is due to absorbed sunlight (neglecting the small heating from below the ground). The loss of this energy occurs three ways: conduction to the air (followed by convection), evaporation, and radiation (some direct to space, and some to be absorbed by the atmosphere). All of the loss processes occur at the surface. While there are cases (especially night and higher latitudes) where the sea is colder than the air, on average (long term and globally), the sea is warmer than the air. The only effect of back radiation is to add some energy to the surface, which reduces the net radiation loss. There never is an increase in absorbed net energy, only solar energy is the cause of net energy absorbed. It is the outgoing energy balance that is modified. This reduction in net radiation then requires that more conduction and evaporation occur to maintain the long term balance, which also results in an increase in the average altitude of final radiation loss to space. This increase in altitude in conjunction with the lapse rate is the cause of any increase in temperature. Obviously long term storage and currents make local and shorter term energy levels go out of balance, but I am only referring to long term overall balance.
If the primary result of increased GHGs is increased evaporation, it would seem to me that the change in cloud cover (and direct heating from SWR) resulting from this would trump any warming caused from the returned LWR for long term overall balance.
There is no returned NET energy from back radiation. There is energy, but it results in a reduction in NET radiation out, not a NET gain in. It is in effect radiation heat transfer resistance. Since total energy out has to equal input on average, this results in a required increase in evaporation and conduction/convection. You have to consider NET HEAT TRANSFER not individual sources of energy transfer. The equation for radiation heat transfer has both an outgoing and incoming term, but the net is always from hotter to less hot, and the net radiation out is always reduced (on average) by back radiation.
Leonard W. Says: “…the net radiation out is always reduced (on average) by back radiation.”
I disagree. The net “heat” transfer is reduced, but the amount of radiation leaving the hotter object stays the same depending on its temperature.
MKELLY,
Do you not understand the word NET. It is the radiation out minus the absorbed radiation.
Leonard what stated is true and what you stated is not. The radiative heat transfer equation shows that if two objects are the same temperature then ZERO heat is transferred but both continue to radiated as before based on their temperature.
The oceans are of course a greenhouse material in their own right and largely control the temperature of the air above:
http://www.newclimatemodel.com/the-hot-water-bottle-effect/
Bob’s findings sit squarely within the proposed sequence of events set out in my New Climate Model:
1) Solar activity increases, reducing ozone amounts above the tropopause especially above the poles.
2) The stratosphere cools. The number of chemical reactions in the upper atmosphere increases due to the increased solar effects with faster destruction of ozone.
3) The tropopause rises, especially above the poles altering the equator to pole height gradient.
4) The polar high pressure cells shrink and weaken accompanied by increasingly positive Arctic and Antarctic Oscillations.
5) The air circulation systems in both hemispheres move poleward and the ITCZ moves further north of the equator as the speed of the hydrological cycle increases due to the cooler stratosphere increasing the temperature differential between stratosphere and surface.
6) The main cloud bands move more poleward to regions where solar insolation is less intense and total global albedo declines via a reduction in global cloud cover due to shorter lines of air mass mixing.
7) More solar energy reaches the surface and in particular the oceans as the subtropical high pressure cells expand.
8) Less rain falls on ocean surfaces allowing them to warm more.
9) Solar energy input to the oceans increases but not all is returned to the air. A portion enters the thermohaline circulation to embark on a journey of 1000 to 1500 years. A pulse of slightly warmer water has entered the ocean circulation.
10) The strength of warming El Nino events increases relative to cooling La Nina events and the atmosphere warms.
11) Solar activity passes its peak and starts to decline.
and the process reverses when the sun is quiet.
http://www.newclimatemodel.com/new-climate-model/
Stephen, here is the link for your point 2:
http://www.science.uwaterloo.ca/~qblu/qblu_website/Welcome.html
See the graphic at the top right.
I hope those looking for a solar-temperature correlation read the papers below (some are dead links).
His prediction (well, the prediction based on the mechanism) is holding up well on an annual basis. Bottom line: 50-70 years of cooling ahead.
Thanks Crispin.
Thanks Bob, very interesting. A lot of things to consider.
Figure 4 and this statement:
That’s saying a lot. It may not be the focus of the post but it deserves more attention.
If CO2 is not well-mixed then we know nothing about it’s effect on the climate – our understanding and even measurements would be just wrong.
If CO2 is well-mixed then it has no effect one the climate relative to natural variation – because the ocean’s are big.
Can we have a further post focussing on this disparity?
CO2 is known to be well mixed. There are both temporal and spatial variances in temperature that completely dwarf any effect that CO2 might have in the short term or in a particular location. The typical temperature variation between day and night, or between winter and summer daytime temperatures is vastly greater than the most alarming projections of future climate change.
But the existence of such short term and local effects does not falsify the idea that increasing GHG concentrations could cause a gradual, long-term rise in overall surface temperatures. Because of the large diurnal, annual, chaotic and localized temperature fluctuations that are a natural part of the earth’s system, any such gradual, long-term temperature increase could only be detected by looking at long-term, globally averaged temperatures.
Why globally averaged temperatures?
If the oceans are behaving independently then the global average is meaningless.
That was my point. Either CO2 concentration is being mis-measured or the oceans are… or CO2 is pretty much irrelevant.
But is it well mixed? It was only last month that we were all entertained by NASA Goddard’s simulation run of daily CO2 (natural and man-made) changes around the globe (for a 2005/7 period).
http://wattsupwiththat.com/2014/11/18/who-needs-an-orbiting-carbon-observatory-when-you-can-model-of-carbon-dioxide/
It attracted a lively discussion and some not wholly respectful comments, but also gave rise to the suggestion that short term CO2 differences in quantity and persistence definitely exist between the NH and SH and therefore the corresponding ocean basins. People were , I got the impression , waiting for the new satellite data to resolve that question. Has that appeared?
CO2 is not well mixed at the local level. I have measures >1100 ppm in cities.
Leonard Weinstein’s – if the effect you are describing were perfectly offset by increased evaporation, meaning, I believe, the oceans would experience no greenhouse effect at all, would the heat content of the oceans be significantly different? If so, in what direction and by approximately how much?
The heat content of the ocean is continually increasing slowly as a recovery from the long colder glacial period ending 11,000 or so years ago. There are some up and down trends, but on average it is slowly up.
Thanks Bob. I trust you as much as anyone concerning this. The issue doesn’t concern the total GHG effect, but it very much concerns the issue of how much GHG warming can “hide” in the ocean & hugely affects transient & equilibrium climate responses to forcing.
Mods: I’m beng but had to change my name ’cause my email changed.
I would like an answer to the following.
First though, here is what the UN IPCC keeps telling us; Global warming/climate change is human induced by the release of CO2 into the atmosphere from burning fossil fuels.
OK. Here is the amount of CO2 in the atmosphere from man burning fossil fuels.
Amount of CO2 in the atmosphere = 0.04% which = 0.0004 of the atmosphere.
Man-made CO2 is 3% of that which = 0.0004×0.03 = 0.000012.
Burning fossil fuels is about 50% of that.
Therefore: The amount of man-made CO2 from burning fossil fuels is about 0.000006 of the atmosphere.
How does this amount of CO2 override natural climate change?
AND.. that; the Sun, Milankovitch cycles; continental drift; volcanism and earthquakes are irrelevant,
“Amount of CO2 in the atmosphere = 0.04% which = 0.0004 of the atmosphere.
Man-made CO2 is 3% of that which = 0.0004×0.03 = 0.000012.”
The 3% figure is nonsense. 0.04% of the atmosphere corresponds to about 800 Gtons carbon. We’ve burnt about 400 Gtons.
Hi Bob, another interesting post. You ask whether you have missed any other factors that can impact the distribution of ocean heat and I can think of at least three:
First the impact of changes in solar activity on the site of formation and warming of ozone in the stratosphere and its effect on surface pressure, largely through changes at 65 S in the Southern Ocean where due to the trajectory of Earth around the Sun some 90+ W/m2 are input in the southern summer than the northern
http://solarphysics.livingreviews.org/open?pubNo=lrsp-2007-2&page=articlesu11.html
http://www.space.dtu.dk/upload/institutter/space/forskning/06_projekter/isac/wp501b.pdf
http://climatechange1.wordpress.com/2011/09/19/climate-disaster-declining-rainfall-rising-sea-levels/
http://joannenova.com.au/2013/10/solar-effects-seem-to-shift-wind-and-rainfall-patterns-over-last-3000-years-in-chile/#
http://hockeyschtick.blogspot.com/2014/03/new-paper-finds-another-solar.html
Ozone depletion trumps greenhouse gas increase in jet-stream shift
Second the Lunar cycles, especially the Saros cylce that affect the flow of ocean and atmospheric tides with each 18 some year cycle linked to the time it takes for this southern ocean heat to reach the northern latitudes where it has the effect you describe with a probable link to ENSO cycles.
LOD and Climate Cycles Geophys. J. R. aslr. SOC(.1 976) 46,555-573
http://tallbloke.wordpress.com/2014/11/15/evidence-that-strong-el-nino-events-are-triggered-by-the-moon/#more-19477
http://hockeyschtick.blogspot.fi/2014/10/new-paper-finds-lunar-tide-cycle.html
Third, is also the effect of solar activity on the solar insolation at the surface.
http://hockeyschtick.blogspot.com/2014/05/new-paper-finds-large-increase-of-solar.html
http://hockeyschtick.blogspot.fi/2014/06/new-paper-finds-solar-control-of-clouds.html
This is just a sampling of papers in these areas. Perhaps you or others could comment on the relative importance of these factors to that of changes in the Trade Winds due to convective effects.
“Based on the findings at RealClimate, that rise in infrared radiation could only warm the sea surfaces by a little more than 0.002 deg C since 1979. Yet, looking at the global sea surface temperature data, Figure 2, the surfaces of the global oceans warmed more than 0.3 deg C from 1979 to 2013, leaving about 93% of the ocean surface warming unexplained.”
Just using those two sentences, it calculates at 99.3%, not 93%. Am I missing something? Anyone?
The back radiation does not directly result in ocean heating. Only the absorbed solar energy heats the ocean. Since the oceans cooled during the last glacial period, they are slowly warming this interglacial.
Is it 0.002C a year?
No chance, not enough energy.
David in Texas,
“Just using those two sentences, it calculates at 99.3%, not 93%”
The two sentences are nonsense. The RC graph shows the difference between surface and 5cm depth, not SST. SST can warm without changing that difference.
Bob
“INFRARED RADIATION CAN ONLY PENETRATE THE TOP FEW MILLIMETERS OF THE OCEAN SURFACE AND THAT’S WHERE EVAPORATION TAKES PLACE”
///////////////.
It is important to get it right. It is microns, not millimetres. That is a substantial difference.
SEE: http://scienceofdoom.files.wordpress.com/2010/10/dlr-absorption-ocean-matlab.png
Whilst the above plot suggest that approximately 60% of LWIR is absorbed within 3 microns, one has to bear in mind that that is perpendicular depth, ie., LWIR, interacting on a perpendicular basis, whereas DWLWIR is omni-directional such that as regards DWLWIR bearing in mind that much of DWLWIR will have a small grazing angle, without doing the maths, it is easy to see that we are talking about approximately 80% of all DWLWIR is fully absorbed within just 3 microns of ocean!
Even on K & T’s average figures for DWLWIR of 333 watts per sq.m, that is a heck of a lot of energy.
As I have been commenting on this site for many years, if DWLWIR is capable of sensible work and is absorbed in accordance with the optical absorption characteristics of LWIR in water there is so much energy being absorbed in the first few microns that it would result in more than 15 metres of rainfall annually (which is not happening) UNLESS the energy being absorbed in those few microns is dissipated to depth at a speed greater than that which would drive evaporation.
The problem is what is the mechanism,by which the energy is dissipated, and at what rate is it dissipated?
The energy cannot be dissipated to depth by conduction, since the energy flow/flux is upwards at the very top of the ocean, and unless we are wrong in our understanding as to how conduction works, the energy absorbed in the first few microns cannot swim against the tide of the upward energy flux. SEE: http://disc.sci.gsfc.nasa.gov/oceans/additional/science-focus/modis/MODIS_and_AIRS_SST_comp_fig2.i.jpg .Thus conduction cannot be the mechanism..
Two other mechanisms are put forward:-
First wind, waves and swell are said to mix the energy, but there are problems with this. (i) It is a slow mechanical process, (ii) there are periods/conditions where there is all but no wind, and waves etc to perform the mixing, eg BF3 and below.. (iii) in very severe conditions (BF8 and above) the top of the ocean becomes a divorced layer of wind swept spray and spume which would act like a LWIR block and energy absorbed in the water droplets that form this wind swept spray/spume would power evaporation and power the storm clouds raging above the ocean well before the divorced layer reunites with the ocean.
Second there is ocean overturning but this is not only a slow mechanical process, it appears to be a diurnal process. It is difficult to see how this process can dissipate the energy absorbed within the first few microns at a rate fast enough to prevent rapid and copious evaporation from the very top of the ocean.
Life would not be possible on planet Earth if the absorption charcteristics of solar in water were similar to that of LWIR. We are fortunate that sunlight penetrates to depth, with muuch of the solar energy being absorbed at a depth of 50cm and below such that the energy being received from the sun is dissipated (and thereby diluted) over a substantial volume of water so that it can slowly heat the ocean.
Something which cannot go on forever, won’t. It seems you are ultimately focusing only on heating mechanisms. But, at some point, that energy has to be released back again, or the oceans would boil. It cannot simply all, or mostly, go into the depths somehow.
I have not thought about this as deeply as you, but my initial reaction would be that perhaps surface wind carries most of the energy away.
I am definitely in favor of the idea of human-induced ocean warming, as it could help stave off the coming cold period, and maybe even postpone the next ice age indefinitely. But it would seem enormously expensive so doesn’t look to practical at this time.
Bob,
In your section “WHAT CAUSES THE WATER TO “PILE UP”, INCREASING OCEAN HEAT CONTENT?” you describe another process similar to the one that causes El Nino. This indicates to me that there may be a number of charge-discharge oscillator processes that can cause sudden changes in atmospheric temperature. There may even be areas where cold water is sequestered and is suddenly discharged to create a drop in temperature. (I know, cold water sinks, but humor me).
Thanks, Bob. An excellent post, lots to learn.
The NAO index can be seen at the KNMI:
http://climexp.knmi.nl/data/icpc_nao.png
“Rising” OHC “recorded” by Argo should be called the “Josh Willis” warming.
Incredible that the world’s best ocean temperature data is in the hands of a PhD student with a talent for data manipulation.
Well for the record, I am against human induced ocean warming. Seems like a sheer waste of energy to me, and we don’t have energy to spare just to warm the ocean.
Those who want ocean warming should move to a Caribbean island. The waters of St John,V.I. are my favorite ocean warming spot.
Bob, there is repeated here and there the thought that there are only two mechanisms for ocean cooling: radiation and evaporation. The discussion proceeds as if 90% is lost by evaporation therefore 10% goes out by radiation. Not so fast…
There are four ocean surface cooling mechanisms. Three are common to all objects in contact with an atmosphere: conduction (through still air), radiation and convection (which is a form of conduction but treated separately as ‘mass transfer’). Oceans also cool by evaporation. That makes 4.
If 90% of heat is transferred away from the ocean by evaporation, there is still conduction into the air (very small as air is a good insulator with poor conduction characteristics that vary with humidity). There is radiation which tends to be a small percentage of heat lost from an object. And finally there is convection whereby a mass of air is moved against a warm(er) object and energy is transferred to that air mass making it warmer.
I don’t see conduction and convection covered in the discussion. Having done these calculations I am willing to ignore the conduction portion, but I insist that convection of heat from the surface be given a little credit. Yes a moving air mass evaporates water, but after saturating, a cold, wet air mass can still pick up huge amounts of heat by mass transfer (which is called convective heat transfer).
This energy is transported high into air where it is released by the usual cloud and rain mechanisms. I don’t think the IR transfer rate (net) from the surface is as high as people are saying it is. For one thing, there is a heck of an IR absorber just above the surface in the form of a very wet (GHG) layer of air (with a tiny amount of CO2 in it, of course).
Crispin in Waterloo – Speaking to Bob Tisdale.
(Addressed also to Willis E.)
OK, then.
Let us get the actual (well, calculated numbers for all four at least): I think you will find that the “easy answer” that “everybody uses” is correct, but only for limited times and under very, very limited circumstances.
So: Calculate the 4 heat losses for four situations:
1. Tropic ocean: warm water (25 deg C), warm air (25-30 deg C), medium to high humidity air (65-85% RH), modest winds, scattered clouds with irregular completely clear skies. Very high solar angles, very low air mass, very high insolation.
2A. Gulf Stream or mid and south Atlantic, Mid latitudes: Modestly warm water (15 – 20 deg C), cooler air that will be both hotter or colder than the water temperatures, lower humidity air (45-60 % RH) , modest winds, again clear skies (No clouds, little haze). Medium solar angles, medium air mass => medium insolation values.
2B. Gulf Stream or mid and south Atlantic, Mid latitudes: Modestly warm water (15 – 20 deg C), cooler air that will be both hotter or colder than the water temperatures, lower humidity air (45-60 % RH) , modest winds, but now cloudy skies (Fully overcast clouds, mid-altitude haze). Still medium solar angles, medium air mass => medium insolation values but ALL diffuse radiation.
Less incoming IR. Outgoing LW radiation now ???. Much less radiation loss. Less evaporation loss.
3. Arctic/Antarctic (non-stormy): Cold water (2-4 deg C), Cold air (10 to -10 deg C), low humidity (10 – 25% RH), low winds, very clear skies, very low solar angles = very high water albedos, very high air masses => very low insolation values.
Just for consistency: Assume 2-4 m/sec winds for all cases.
Give sources for your assumed coefficients of convection and conduction heat transfer coefficients.
Show your work.
8<)
RACook
I was well into a reply and lost it (argh).
I have been thinking of things much closer to the surface so I had better explain myself better. There is a supersaturated layer of air just above the water with water vapour condensing and water evaporating continuously. The amount depends on the temperature. When this layer is blown away, evaporation is much more efficient. AS a heat conducting layer, it is quite efficient, far better than dry air.
The swimming pool people have a good set of formulas that are relevant to evaporation involving wind and as evaporation from a pool is ‘a cooling’ (evaporative cooling) it would be helpful when setting up this calculation (which I will not attempt).
There are three methods here http://cwanamaker.hubpages.com/hub/Determine-Evaporation-Rate-for-Swimming-Pool and a table is needed for the saturation vapour pressure http://cwanamaker.hubpages.com/hub/The-Amazing-and-Remarkable-Properties-of-Water about halfway down.
My approach was going to be very different. We are looking for a bounded range for the convective transfer portion (to get an idea of scale).
An alternative is to consider Prandtl’s approach of considering the thin layer at the water surface as ‘different’ and considering the free space above as infinite. This means we might be able to treat wind speed as a temperature change, not ‘cooling by impacting the surface’. Not sure but in the latter case we could treat the first 0.1 or 1 mm of air as saturated, conductive and passing heat to the atmosphere. All convected heat has to pass into the air through a ‘thin’ boundary layer, at least in simplified thinking which is adequate for an otherwise unsolvable problem (Bejan 2005).
How’s this as a start:
Wind speed, 0 m/sec
Water temperature, 18 C
Air temperature 16 C
Emissivity 0.98
Convection coefficient for gases 20 W/m2·K (I am not sure of this value for wet air)
SB Constant 0.0000000567
Convection = (Water T-Air T)*Conv Coeff
Radiation = SB*Emissivity*((Water T+273)^4-(Air T+273)^4)
Loss by radiation 11 watts/m2 = 21.3%
Loss by convection 40 watts/m2 = 78.7%
So it would appear that the convective heat transfer is very important even with only a 2 degree difference in water/air temperature and no wind. If evaporation is 90% (claimed above) and 4/5 of what is left is convection, there is only 1/5 of 10% leaving by radiation – 2% of the total. Wow.
I think the 90% is highly variable. But at a very modest 2 degree difference, convection dominates the non-evaporative portion.
Changing the Delta T to 3 degrees gives a 50% increase in both to a total of 76 Watts/m2
16 degree air over a 21 degree ocean (night) loses 127 W/m2 and the ratio is nearly the same.
-20 C air over a 4 deg ocean transfers 579 W/m2 and the radiation component drops to 17% of the total.
Conclusion: Assuming non-evaporative cooling is 100% lost by radiation is probably not representative of what is taking place. Generally speaking, increasing the wind speed increases the cooling rate. The formula for ‘wind chill factor’ might be incorporated into this. Above a certain speed at the surface, energy transfer would be limited by heat conduction vertically through the water.
Caution: This may be completely wrong – I was unable to locate a fully representative energy transfer function representing large scale air flow over open water that incorporated evaporation and surface cooling.
Crispin in Waterloo & RACookPE1978, I corrected a mistake in my post. It looked very odd to me. I added a correction to the post:
Of the approximately 180+ watts/m^2 downward shortwave radiation reaching the ocean surface, about half (about 100 watts/m^2) is released through evaporation.
Bob,
Ooh, that is interesting. Are you saying that the 100 watts is directly turned into evaporation at the surface?
If so there is 80 W left to ‘get back’.
If 90% is also from evaporation (possible?) then that is 72 W
4/5 of the remainder is convection = 6.4 W
1/5 of remainder is radiation = 1.6 W
That may seem very low, but it should be remembered that there is a gigantic GHG effect in the first few mm of the atmosphere because the air is saturated/super saturated with water vapour. I can’t see CO2 any difference larger that a spit in the ocean.
If the 100 W is evaporation after entering the water to some depth, the adjust the numbers above accordingly.
Given my example above, if I set the water to 16 C and the air to 12.8 the loss is 80 watts, with radiation being 21% of it or 16.8 W.
Interestingly, leaving the water at 16 and raising the air temp to 21, the convective transfer to the water is 100 W/m2 assuming there is not evaporation (saturated air, as in a rain storm).
I found another calculator for swimming pools at http://www.engineeringtoolbox.com/evaporation-water-surface-d_690.html
It says that with 25 deg water and 25 deg air, and the air at 50% humidity, wind at 2 m/sec it will cool evaporatively at 405 W/m2. Dang.
Bob T
I remain worried that the IR from the water vapour at the surface is not being considered. Also the recondensation of water vapour at the surface is a way to increase the IR up without increasing evaporation. I think the 100 watts you have there is ‘net’, correct? The actual amount of energy involved in what amounts to a very short heat pipe mechanism is larger, and increases the IR from the ‘region of the surface’.
This impacts my straw man calculation but I didn’t think deeply about how.
Per my comment to Willis on the ‘average’ evaporation, I get 80 watts global average for 1 metre of evaporation so his 70 is believable with is lower precip number. I read once evaporation is 2 metres but if rainfall is only 1 that can’t be right. Two metres of evaporation average is 160 watts. Your 100 watts is 1.25m evaporation. Still in the ballpark. I don’t like this ‘average’ business. What is DWIR in the major tropical evaporation zones, and what is the evaporation?
The warmer the average T is, the higher the percentage of any increase in DLWIR will go into evaporation. The relationship cannot be linear correct?
David A December 11, 2014 at 5:01 am Edit
It’s not linear, as you suspect it rises faster at higher temperatures. See my post, “Marginal Parasitic Loss Rates“.
w.
lots of theories but no definitive experiments in those studies … if IR can’t penetrate then IR CAN’T heat what it hasn’t penetrated … yes the water that is heated by IR can then heat the next layer … but that dies out pretty fast … its amazing to me that this is being debated when it should be possible to experiment and turn theories into facts one way or another …
Excellent post Bob! There should be a “start here” link to this for anyone who wants to discuss ocean warming.
For people who are still stuck or decoyed by the ignorati think of it this way. (where is Konrad by the way?)
The surface temperature of the oceans is, on average, about 14 degree C. I do not know the exact value, Bob probably does. But I do know that the ocean surface approximates to a blackbody in the infrared region. We see its emission from space and it accords with a blackbody. According to Kirchoff’s Law, this means it will also absorb as a blackbody.
A body at 14 degree C will emit at about 390 watts per square metre (another Law established in the 19th century but seemingly unknown to many here). If it loses heat at the rate of 390 watts per square metre, then how does it maintain its temperature? It can only do that by absorbing at least 390 watts per square metre from somewhere else (or is that to hard?)
The solar radiation absorbed at the surface is about 170 watts per square metre. Obviously, that is not enough to replace the energy lost by radiation alone, is it? Never mind convection and evaporation losses.
OK, there is some heat input from the hot planetary core. This amounts to a staggering 0.08 watts per square metre. Let’s agree this is insignificant.
So how does the ocean maintain its temperature? What is the other heat source? There is only one contender.
Have you worked it out yet?
MIkeB, You are in appropriately clinging to a radiative balance hypothesis and totally ignoring the well known physics of heat sequestration in the ocean that are the important dynamics here. To understand those dynamics, the analyses must be local as each ocean basing, each gyre will store and ventilate heat differently. The saltier Atlantic stores more heat at depth than anywhere else, and that can not be explained by CO2.
Solar heat is absorbed in the tropics to excess, and there solar radiation is more in the range of 500 to 800 W/m2, or more in cloudless regions. Evaporation causes more dense saline warmer water to sink below the surface and thus the heat is now stored, and that heat may remain stored at depth for decades. Tropical rains and summer surface warming stratifies the oceans. Mode waters are warm subsurface waters that are typically ventilated during the winter, when the upper strata cools and sinks, allowing mode waters to reach the surface and ventilate some heat. Winter storms help ventilate that heat but it takes at least 1-3 years to ventilate the initially stored heat. Climate outside the tropics depends on how that stored ocean heat is transported poleward via ocean and atmospheric currents as illustrated here http://landscapesandcycles.net/image/91464625_scaled_495x288.png
As Bob has pointed out. The ocean is virtually opaque to infrared. Once heat is stored below the surface, it is not radiating infrared back t space. So who are you slandering by calling them the “ignorant”? Your blackbox concept is not appropriate in this discussion.
Kirchoff’s law, is only valid for systems that are in thermal equilibrium. And when that is the situation, the absorbed and transmitted radiation must match at every single wavelength. There cannot be a send / receive unbalance at ANY wavelength.
And I don’t believe it applies to any radiation; just thermal (BB like) radiation. It is a thermodynamic macro property, and doesn’t apply to atomic and molecular line spectra, which depend on atomic structure.
Most things that are not actually changing temperature can be considered to be in thermal equilibrium as long as the thermal inputs on them are in a steady state. The ‘thermal equilibrium’ rule allows Kirchhoff’s Law to be proven by a simple mind experiment, logic alone, with no need for experimentation. I think there is such a proof in Wikipedia somewhere.
Even where this is not the case, once it is established that an object can radiate at a particular wavelength in thermal equilibrium it will be able to radiate at that wavelength when not in equilibrium. Or is that a step to far for you?
Kirchhoff’s Law applies to all electromagnetic radiation. It is universal. It also also applies to line spectra.
Here is an image showing absorption and emission spectra in the visible spectrum. See how the emission lines and absorption lines match. ( I don’t know what the intervening gas is for this diagram but the yellow line looks like sodium).
http://nptel.ac.in/courses/105104100/lectureD_19/images/2.jpg
MikeB @ December 9, 2014 at 11:05 am
I would quibble with a few of your comments, e.g., “We see its emission from space…” – we don’t, because a big divot is taken out by the atmosphere. But, near surface measures of emissivity typically put it in the range of at least 85%, and generally closer to 95%, across the frequency range. Not, however, from all aspect angles, so there is some reduction from there, too.
But, I agree with the larger point – there is an energy imbalance which cannot be reconciled without considering atmospheric absorption.
This does not, however, address the question of whether IR radiation specifically heats the ocean to depth.
It is also the case that,while atmospheric IR absorption undoubtedly heats the planet beyond what it otherwise would be, it does not necessarily follow that an incremental increase in IR gases will always produce an incremental increase in surface temperatures within the local neighborhood of a particular climate state.
We see its emission from space through the ‘atmospheric window’.. From that we can detrmine its temperature.
You are correcdt we cannot see the surface through the greenhouse gas absorption bands
.
MikeB, not sodium that would have a yellow doublet, without wavelength calibration it’s difficult but it looks most like helium.
http://pmm.nasa.gov/education/sites/default/files/article_images/components2.gif
The heat source is the absorbed solar energy (by the surface, which is ~51% of the incoming at TOA, which is ~340 W/m2). The surface loses heat by evaporation (23%), convection (7%), LWIR to the atmosphere (15%) and LWIR directly to space (6%). These are global averages (land and ocean) and are not very accurate. Oceans only is similar.
Right here laughing at you Mike 😉
The oceans are a SW selective surface not a near blackbody. The sun alone would drive them to 335K were it not for atmospheric cooling. Now how does the atmosphere cool?
Have you worked it out yet?
So why do you think “the ocean surface approximates to a blackbody in the infrared region” refers to SW radiation?
The ocean has evaporation before the additional infrared radiation. Let us call that “Ev1”. The additional infrared radiation causes an additional evaporation which we can call “Ev2”.
This Ev2 causes an additional humidity in the air just above the surface which is leading to a decrease in Ev1. This reduction in Ev1 leads to an increase in the ocean temperature.
It is quite easy to set up an experiment showing that an increase in infrared radiation can increase the temperature in a bucket of water. I hope some of the contributors here can do it. I am more than willing to help in the setup.
/Jan
Then you do it and come back and tell us how you did heating the bucket of water with your hair dryer.
Better yet, try heating that bucket of water using a real near BB radiator that is emitting LWIR about like the atmosphere; such as a 16 [ounce] bottle of water chilled to about 14 deg C. That is what is “beating down” on the oceans like a infrared blow torch to warm the oceans.
Hair dryer give warm wind, not IR.
But an electric terrace heater would do.
Two equal buckets or large glasses with water should be used and one of them should be shielded from the IR.
Like this:
The “downward” LWIR that is supposed to be warming the surface, including the ocean has an equivalent black body Temperature of around 288 K emitting about 390 W/m^2 at a wavelength peaking at about 10.1 microns.
The experiment sources you propose operate at a good fraction of the sun’s surface Temperature and have radiances that are thousands of times higher than the atmosphere.
I don’t know what sort of hair drier you use, but mine has a fan I can turn off when I don’t want hot air, and then it emits near IR radiation. (much higher radiance than the atmosphere, and much higher photon energy).
To do a realistic LWIR heating experiment, you have to use a radiation source that is at 288 K and emitting about 390 W/m^2 at a 10 micron peak spectral wavelength.
To do otherwise is scientific fraud.
As George points out, one has to do one’s best to recreate the 288K IR heat source.
Jan, I would suggest that rather than usuing your electric IR heater, you should use a 1.4m by 70cm flat panel water radiator (ie., one that has an area of about 1 sq.m) which should be painted black and filled with water at 14degC. If you like you should insulate one side of the radiator so as to slow heat loss, leaving exposed only the flat side that is painted black. This panel will then be radiating at 288k such that it will be replicating the equivalent atmospheric DWLWIR.
Get two identical buckets of water at 20degC, one being placed underneath the radiator but with sufficient gap so as not to impede convection, and note the time each takes to cool.
Yes, and to do it even more realistic we should also use a 2000 meter water column instead of the glass and let the experiment go on for three decades.
But that is not very practical. The experiment I sketched is easy to do, and it will answer the question of whether it is possible to heat water with infrared radiation to the surface. If the answer is “No” there is no reason to go further.
If the answer is “Yes”, one can set up a more refined experiment to see whether there is a cutoff on some temperature or frequency or whether there is a linear or non-linear decrease with the temperature of the source.
/Jan
Jan, you would be warming the glass sides, which would be conducting into the water, vastly different then the oceans. Have you looked at Konrad’s experiments?
Thanks David, you are absolutely right.
The setup should be modified to shield the [glass] sides. Tgis could be done with [for] instance a cardboard with hole for the water surface on top of each glass. The reason for having an identical plate on the glass already shadowed by the plywood is to let the glasses be as identical as possible.
first you have to start out with a temperature increase…that’s smaller than what can be measured
..how precious
RE”
————————————————–
Nick Stokes
December 9, 2014 at 6:16 am
Bob,
In a post way too long, you’ve devoted far too much space to some very silly arguments. There is no issue about down IR penetrating sea water. The sea surface is warm and radiates upward more heat than it receives in sunlight. If it were not for down IR, it would cool rapidly. Down IR maintains heat flux balance at the surface. It does not need to penetrate. If down IR increases, the flux from below decreases, at the same temperature. The sea is warmed by that retained heat.
—————————————————-
Unless Nick can provide information from a competent Oceanographer such as Dr. Robert Stevenson to back up his musings, I suggest that they are similar to his pea moving motions he has been exhibiting at CA in the last week. For more information read: http://www.21stcenturysciencetech.com/articles/ocean.html.
The statement “The sea surface is warm and radiates upward more heat than it receives in sunlight” is bunk. The reason land surfaces and sea surfaces increase in temperature is they receive more energy from the sun than they radiate. This can happen when daylight is longer than darkness so there is a net gain during the day which is also cumulative over a summer season when days are longer. When days become shorter there is a net loss. We call that winter. So the northern oceans cool in the winter when they lose sunlight. The amount of sunlight received is decided by the time of year and amount of cloud. Down IR does not prevent the ocean from cooling as it cal only go down millimetres or microns. Oceans close to the tropics do not change much in temperature as the days do not change much in length. The oceans and lakes cool slowly because they radiate IR from a depth of a hundred or so metres. They CANNOT cool rapidly. By the same token as the shortwave radiation has to penetrate a hundred metres or so, they will also warm slowly in the spring and summer. Land surfaces only radiate from the surface so can cool rapidly in one night as well as containing much less energy in those few centimetres of surface. The ground then cools downward by conduction. Similarly land surfaces warm rapidly during the day because the heat is not transferred rapidly downward as the ground is a good insulator.
Gerald, that was a good reply to the nonsense, “The sea surface is warm and radiates upward more heat than it receives in sunlight”. I couldn’t believe that Nick would say something like that.
You couldn’t believe it. Wow!
It’s back to the drawing board then for Science.
Bob,
Another good post. Worth the read. About this coefficient 0.002K/(Watt/m^2) change in surface temperature with increased LWIR — has that been published in a refereed journal. It would be good to have a citation other than a blog.
Nick Stokes December 9, 2014 at 6:16 am
There is no issue about down IR penetrating sea water. The sea surface is warm and radiates upward more heat than it receives in sunlight.
>>>>>>>>>>>>>
Where? And when?
If everything was an average, then the above would be true. But everything isn’t> and average, and the relationship between T and P isn’t linear, which makes averaging an even worse idea.
The oceans in the tropics absorb more energy than they radiate. In the polar regions, the opposite is true. The part in between is fuzzy depending on season and time of day. So Nick’s blanket statement above may be true on some level, but you’ll have trouble finding any specific spot on earth that follows that model.
David,
Poleward heat fluxes are small compared to the radiative fluxes (100s W/m2) described here. Fig 2.17 here puts it at 2 PW max. Divide that by ocean area and it’s about 7 W/m2. OK, some areas will be more affected, but again, it’s 2 PW max. It’s a small and fairly steady imbalance.
Its hard to trust your link in figure 2.17 that argues deep water formation in the North Atlantic is the driver of poleward heat transport. The authors have confuse cause and effect. That poleward transport is wind driven and pushes more mass poleward. Mass balance requires a return but not the depth of that return. It doesn’t matter of there is deep water convection or mid-level feeding the return. The winds will continue to determine the poleward transport, and the winds are driven by solar heating. The paleoclimate evidence is the opposite of what your link suggests.There was higher poleward heat transport during the Roman and Medieval Warm periods and higher insolation . And during low insolation during the Little Ice Age, there was a 10% reduction in poleward heat. http://www.nature.com/nature/journal/v444/n7119/full/nature05277.html
Well here’s a different figure. Oceans absorbing 90 w/m2 more than they radiate in the tropics, and radiating 100+ more than they absorb in the arctic regions.
http://eos.atmos.washington.edu/cgi-bin/erbe/disp.pl?net.ann.
And you don’t divide by the ocean AREA to get the flux in W/m2, you divide it by the CROSS SECTION.
But you still get the same answer. The radiative equilibrium you describe doesn’t exist in any body of water anywhere in the world. Oh some temperate loctions may achieve this for a brief period of time once in the spring and once in the fall…. for a few hours.
Nick,
I looked at your Fig 2.17 some more and thought to myself, assuming this figure is correct, what conclusions could I draw from it? The oddity is that the atmosphere’s pole ward energy transport is so much bigger than the oceans. How could that be? The oceans cover 2/3 of the earth surface, they absorb S/W to hundreds of meters down, they have a heat capacity 1200 times that of the atmosphere, how could energy transport in the oceans be so much less than the atmosphere?
Well here’s a thought. All that LW energy coming down from the GHE hits the ocean surface which responds by saying h*ll no, you ain’t comin’s in here, you’re going right back into the atmosphere and I’m sending some water vapour to escort you out. Poof, the majority of the LW then HAS to be transported poleward via atmospheric processes because the oceans just won’t let the LW in directly.
Note, I said directly. There’s an awful lot else going on of course, its the indirect effects that we don’t fully understand.
David,
“And you don’t divide by the ocean AREA to get the flux in W/m2, you divide it by the CROSS SECTION.”
No, we’re looking for the imbalance the flux creates for each m2 of ocean surface. In fact, poleward transport is a small effect compared with, say evaporation.
I tried your link; it gave six choices of format, but said they were all unavailable.
As to why the atmosphere carries more – well, that’s what the measurements say. I guess winds blow faster and in more organised convection cells (Hadley).
Nick Stokes;
I tried your link; it gave six choices of format, but said they were all unavailable.
>>>>>>>>>>>>>>
Try this one:
http://eos.atmos.washington.edu/erbe/
Then click on net radiation, annual, 2nd from the top, left hand side.
Nick Stokes:
No, we’re looking for the imbalance the flux creates for each m2 of ocean surface. In fact, poleward transport is a small effect compared with, say evaporation.
>>>>>>>>>>>>>>>
Such evaporation would be primarily driven by….?
But that’s beside the point. Your claim was that the oceans are in radiative balance, and that may be true on an annual basis across all the ocean area, but at any given point in space or time that is almost NEVER true. It doesn’t matter if evaporation is bigger or not, it still isn’t true, and treating it like an average is just misleading as there is so much else going on.
jim Steele
December 9, 2014 at 4:32 pm
“The paleoclimate evidence is the opposite of what your link suggests.There was higher poleward heat transport during the Roman and Medieval Warm periods and higher insolation.”
GISP cools in the 1st to 4th centuries, that’s the RWP, GISP then warms ~390-540, that’s the Dark Ages cold period, it then cools in the 8th and 9th centuries, which was some of the warmest of the MWP period for Europe. A number of mid latitude regions show a sharp cooling-drying in the late 10th and parts of the 11th centuries, that’s the next warm spike in GISP. The Minoan [Warm] Period on GISP (1350-1150 BC) is easily verified as a very cool-dry period for the mid latitudes.
I have been trying to explain that to Willis on many occassions, when we have argued the pros and cons of the gross and net energy budget. As you no doubt recall Willis adopts the view that DWLWIR must be heating the oceans otherwise they would freeze, but comes to such conclusion by setting up a circular argument on gross energy flows. The relaitywould appear to be that the oceans are losing the net energy out. .
You cannot deal with averages. The equatorial and tropical oceans receive a huge amount of solar energy, far more energy than they lose, and this excess energy is distributed predominantly polewards keeping high latitudes warm and polar oceans largely ice free in the summer when the poles additionally receive sufficient solar energy. When the poles do not receive sufficient of the additional solar energy, the polar oceans begin to freeze.
What the co2 driven global warming advocates don’t discuss is that if the ocean has started eating global warming since the trade winds changed during the negative phase of the ocean’s ~60 year multi-decadal cycles, they also emitted excess energy during their positive phase from 1975-2005. The implication is that the oceans are capable of storing energy on long timescales, and releasing it on long timescales too. And they store a lot of energy. The top two metres alone contain as much energy as the entire atmosphere above.
We know that the oceans keep the air temperature up over night as the release some of the energy the Sun poured into them during the day. We also know that there is a lag of a couple of months between the longest day of the year and the peak in surface air temperatures near coasts. This is thermal inertia and heat capacity at work. On longer timescales, we have recently confirmed that runs of El Nino events which release a lot of energy from the oceans are initiated on the falling side of the solar cycle, never on the upswing.
So we can go a stretch further and combine what we know. When solar activity falls, energy comes out of the ocean, not just over the period of the decline of a single 11 year solar cycle, but if the Sun stays low in activity terms, for many years. An integration of the sunspot number shows us that the ocean heat content rose all the way from 1934 to 2003. This is the real cause of ‘global warming’. A lot of excess energy is still retained in the upper ocean. We can expect the effect of a couple of low solar cycles to be softened by a proportion of that excess heat returning to space via the atmosphere warming it on the way.
In developing my understanding of the Earth’s systems, I developed a couple of very simple models to help me fathom the way the surface temperature stays fairly constant as the solar cycles wax and wane. Back in 2009, by analysing the data, I found that the global average sea surface temperature, the SST, stays fairly constant when the Sun is averaging around 40 sunspots per month. By calculating the running total departing from this figure in a simple integration I found that combined with the ~60 oceanic cycles (also solar influenced), I could reproduce the temperature history of the last 150 years quite accurately. By adding in a nominal forcing for co2 (or an allowance for the infamous ‘adjustments’ to the data), I was able to get a match to monthly data which has a Pearson R^2 value of 0.9.
The above is part of an article ROG TALKBLOKE wrote from his web-site talkblokes talkshop.
I think this article presents a strong case for solar/ocean connections.
Nick Stokes Dec 9 at 1:58
Nick, it is true the sea is warm enough to radiate up more than the absorbed sunlight, but you have cause and effect backward. The NET radiation up is well less than the absorbed sunlight, so conduction/convection and evapotransporation are needed to balance the outgo with input. The downward IR is absorbed at the surface where the upward radiation emits from, and the effect is exactly like putting a layer of selective insulation that only blocks part of the radiation. If you put a layer of regular insulation on a heater, resulting in the heater running hotter for a fixed power level, you would not call this heating by the insulation. The net radiation is the only quantity important to determine the amount of evaporation and conduction/convection that is needed. It is movement up of the level of radiation out of the atmosphere, and the lapse rate that caused the atmospheric greenhouse heating.
Sorry, that is Nick at 6:16 am, not 1:58
Leonard Weinstein
“but you have cause and effect backward”
Actually, here I’m not concerned about cause, just effect. If the nett flux in the water is upward, there is no need for DWLWIR to penetrate; it just makes up the surface balance (along with, as you say, evaporation and convection).
Nick
Just do the calculation.
According to K&T the average DWLWIR is 333 watts per sq.m. The optical absorption of LWIR in water is such that 60% is fully absorbed in 3 microns. SEE: http://scienceofdoom.files.wordpress.com/2010/10/dlr-absorption-ocean-matlab.png
Due to the omni-direction of DWLWIR it is more like 80% of DWLWIR that will be absorbed in just 3 microns.
One need not take into account the solar energy, since virtually no solar is absorbed within the first 3 microns, so for present purposes it can be ignored..
Accordingly, we have somewhere between ~200 watts per sq.m (ie., 60% of DWLWIR) to ~266 watts per sq.m (ie., 80% of DWLWIR) being absorbed in just 3 microns of ocean.
So what happens to the 200 to 266 watts per sq.m of energy which is absorbed in the first 3 microns?
What are the physical proceeses involved, and the speed/rate at which they occur, which prevent the ocean being boiled off from the top down?
I suggest to you that when you stop and consider what is going on, it is difficult to envisage that DWLWIR (if it possesses sensible energy in the environ in which it finds itself) is being absorbed by the oceans as you contend. May be there is some form of photonic exchange, but absorption of energy (other than solar at depth) there does not appear to be.
As I noted in my previous comment, we are lucky that all but no solar is absorbed in the top dozen microns of the ocean, and that the energy received from solar is dissipated and thereby diluted over a volume at least 10,000 times that in which LWIR is absorbed.
richard verney December 9, 2014 at 4:33 pm
“Just do the calculation.”
No need. K&T have done it. Yes 333W/m2 down, globally, but near enough for ocean. 396 up IR, 161 absorbed solar, 80 evap, 17 convection. 494 down, 493 up. All balanced at that 3μ surface layer. It’s balanced for land too, which really is opaque.
The only difference for ocean is that the 161 W/m2 is absorbed at depth, and returns to the surface via turbulent advection, with a skin effect T differential. Incidentally, that proves that down IR could be absorbed; it’s just a reverse pathway. But it isn’t. There’s nowhere for the heat to go.
Yeah Nick, do the calculation.
I put up a straw man calculation above. Have a look and offer an alternative that drives a different conclusion.
Crispin in Waterloo
Crispin,
Your calc is dependent on 2 dubious numbers:
1. Convection coefficient for gases 20 W/m2?K
I don’t believe there is any single number that would define convection over a wavy surface. And I’ve no idea where you got that one from.
2.Radiation = SB*Emissivity*((Water T+273)^4-(Air T+273)^4
That assumes the same emissivity for air and water. Far from true. Water is fairly black to IR, but air has, apart from anything else, the atmospheric window. K&T set this flux to 396-333. You can’t just assume that away.
As a sanity check, your calc should allow for the known precipitation (evap) of about 950 mm globally.
The alternative is K&T.
Nick, you criticized the straw man but you didn’t show a calculation. Are you going to sit on the sidelines?
Nick re the emissivity of water and air:
The air is supersaturated in the region of interest. The water vapour and or condensed droplets will have an emissivity similar to that of water. I think that is a reasonable approximation. Using the emissivity of dry air would be very misleading.
Nick
I guess that none of us are surprised that you chickened out, and did not do the calculation, and that you were unable to answer the question, and instead sought to duck it..
All you have done is state the gross energy flow. The gross energy flow proves nothing other than if you add ‘X’ to both sides of an equation, the equation still balances.
The heat transfer/heat loss from the ocean is governed by the net energy flow (not the gross energy flow). The reality is that the ocean only wants to give up a very little of its energy because the atmoshere above the ocean is at very nearly the same temperature as the ocean itself.
My question is simple, if approximately 250 watts per sq.m (from DWLWIR) is truly being absorbed in the top 3 microns of the ocean, what happens to that energy?
How much evaporation would that amount of energy drive, unless that energy was dissipated/sequestered to depth, and thereby diluted by volume, at a rate quicker than the energy would drive evaporation?
What are the physical processes and mechanisms involved in dissipating/sequesting that energy to depth, and at what rate do those processes operate?
I await your answers to the actual questions raised.
Richard,
“My question is simple, if approximately 250 watts per sq.m (from DWLWIR) is truly being absorbed in the top 3 microns of the ocean, what happens to that energy?”
The answer is simple and it comes from K&T. And I said it. The top three microns absorbs in total 494 W/m2 from down fluxes and gives out 493 W/m2 in up fluxes (the discrepancy is probably rounding). That 250 W/m2 is part of the 494 and is balanced by other fluxes. That is what happened to it.
There is a wrinkle in that the down solar flux is not absorbed directly in that layer, but lower, from whence the heat returns through the water, mostly by turbulent transport. But it’s the same flux (the heat was conserved), so the layer still balances.
It’s a thin layer, so any imbalance leads to rapid heating or cooling, which brings the fluxes back into balance. Comes a cloud – more DWLWIR. Temp rises, more evap, more emission, and critically, the steep T gradient at the top of the water lessens, reducing flux from below. These negative feedbacks quickly control the temperature rise and restore the flux balance. It’s the same mechanism that enforces Kirchhoff’s rule, say, in circuit theory.
“Phrased differently, sunlight can warm the oceans to depths of 100 meters, but the oceans can only release heat at the surface. ”
So the ocean is not a gas- not a greenhouse gas. So if assume ocean are part of Earth greenhouse effect then the theory of greenhouse effect is wrong:
“The surface temperature of this hypothetical planet is 33 °C below Earth’s actual surface temperature of approximately 14 °C.The mechanism that produces this difference between the actual surface temperature and the effective temperature is due to the atmosphere and is known as the greenhouse effect”
http://en.wikipedia.org/wiki/Greenhouse_effect
Though one modify the Greenhouse effect theory to include oceans as well as the atmosphere. But if so, then one should ask how much of the 33 C is warmed by the ocean greenhouse effect.
As rough guess it seems the ocean works better as greenhouse effect as compared to the atmosphere, so in terms of how much, it could 50% or more of the 33 C added,
And then if consider that water vapor is most of greenhouse effect of atmosphere this is only allowing a small amount warming from all other greenhouse gases.
It also seems to me that much of pseudo science of climate science say water vapor is not a Forcing agent, but the ocean has as much effect as the atmosphere on earth greenhouse effect
then water vapor become a major “forcing agent”.
Anyhow, it seems once you acknowledge the world’s Ocean is also greenhouse effect, one has thrown a huge monkey wrench into Greenhouse effect theory, requiring major overhaul or a simple rejection of the theory.
gbaikie
There is no need to ‘reject’ the hypothesis. It is just incomplete. The 33 degrees is probably not correct. No big deal. We are not endangered by that error. This idea that water vapour ‘is not a GHG forcing’ is a blatant error. If there were no CO2 at all in the atmosphere we would not be 33 degrees colder because even very cold air sublimates water vapour from ice and it would accumulate, creating an H2O vapour Greenhouse effect. But it is O3 (ozone) that would play a very big role (people forget).
Because the atmosphere is strongly self-regulating within temperature limits, probably by adjusting clouds, there is no reason to suppose that removing all CO2 would create snowball earth (which existed in the presence of CO2 at one point). It would kill almost all life on earth, of course were it to disappear.
I remember when water vapour was being touted as ‘only a feedback’. It was always a ridiculous proposition made by people desperate to shore up the untenable hypothesis that CO2 dominates everything to do with earthly temperature.
–There is no need to ‘reject’ the hypothesis. It is just incomplete. The 33 degrees is probably not correct. No big deal. We are not endangered by that error. This idea that water vapour ‘is not a GHG forcing’ is a blatant error. If there were no CO2 at all in the atmosphere we would not be 33 degrees colder because even very cold air sublimates water vapour from ice and it would accumulate, creating an H2O vapour Greenhouse effect. But it is O3 (ozone) that would play a very big role (people forget).–
In terms general understanding, maybe not big deal. But in terms of science, or terms of predicting
the future [which what science does] it is a huge deal. The idea of predicting century in the future is perhaps unwise in any circumstances, but if say trying to predict 10 years it’s a big deal.
Yes I agree that CO2 should not b interpret as controlling the entire 33 C of greenhouse effect and that one has to have some water vapor even at very low temperature.
But there are experts of climate science who do believe everything hinges on the forcing affect of CO2. Which is not too surprising and is related to another false idea that CO2 can cause a runaway effect in warming
Or if believe in potential of runaway warming effect from CO2, it follows “in this logic” that it also runs away in the opposite direction with the lack of CO2.
This shows heat uptake over the past 40 some years and I believe it is from IPCC AR5:
http://wwwf.imperial.ac.uk/blog/climate-at-imperial/files/2014/09/Figure-1-heat-taken-up.jpg
Roughly put, it is says that the CO2 caused the atmosphere to acquire 2.5 ZJs. At the same time the oceans acquired 250s ZJ. That 100 times the effect on the ocean surfaces, as compared to the TOA seems difficult to believe. If the case was the oceans had stayed at the same heat content, then that 250 ZJs likely would’ve passed through the TOA, and if it did not, it would be quite warm. I don’t get how CO2 can trap so much more heat in the oceans than it does in the atmosphere? I think the more likely answer is a decreased albedo and/or a recovery from a cooler time. Thanks Bob Tisdale for this post.
>”INFRARED RADIATION FROM MANMADE GREENHOUSE GASES HAS INCREASED SINCE 1979,”
Yes but only by about 0.3 W.m-2/decade lately (CO2). DLR is about 333 W.m-2 global average according to K&T. There is no consistent rise globally, can be up and down regionally (see BSRN, SurfRad), but about 2 W.m-2 global average increase last 2 decades 1990 – 2010 (Wild – see below).
DLR (global average 333) components from Wang & Liang (2009) – (see below):
Major
1) Air temperature
2) Clouds (liquid H2O), Water Vapour (gaseous H2O)
Minor
1) CO2 (6 W.m-2 @ 1976 US Standard Atmosphere)
2) The rest of the GHGs.
DLR rise from GHGs is negligible in the context of total DLR.
Solar Surface Radiation (SSR) change is much greater (Wild – see below) than DLR due to Dimming/Brightening.
>”WHILE TOTAL SOLAR IRRADIANCE HAS DECREASED. THEREFORE, INFRARED RADIATION CAUSED THE OCEAN WARMING”
Incredibly ignorant argument thermodynamically. Yes TSI peaked 1986 but the subsequent decrease is minimal i.e. the ocean (now only the Indian due to currents) is still gaining solar-sourced heat (not so much Pacific and Atlantic) and will continue to until solar levels fall appreciably, say post 2020. And the atmospheric response is not instantaneous. X.H. Zhao and X.S. Feng (2014) find the multi-millennial solar-temperature lag to be 30–40 years. Therefore a millennial solar peak at 1986 should be followed 30 – 40 years later by an atmospheric temperature peak around 2016 – 2026 once oceanic lag (mostly heat transport away from tropical warming) is accounted for and ignoring oceanic oscillations. Once the 40 year lag has elapsed, no more atmospheric warming from the 1986 solar peak, and no GHG warming of the atmosphere either (thermodynamically impossible – a perpetuum mobile otherwise).
Credible solar predictions for 1986 – 2050 in view of current observations vary as follows
W.m-2
-1.26 Krivova et al., (2007)
-2.55 Lean (2000)
-5.70 Abdussamatov (2012), mirrors Shapiro et al (2011) historical.
Much more on all of this in comments at Robin Pittwood’s Kiwi Thinker blog post:
‘An Empirical Look at Recent Trends in the Greenhouse Effect’
http://www.kiwithinker.com/2014/10/an-empirical-look-at-recent-trends-in-the-greenhouse-effect/
Many, many, papers linked including those dealing with the above. Also an in-situ study of west Pacific tropical heat budget (‘Warm-layer, cool-skin’, Fairall et al, 1996). Also citations supporting only 10 micron DLR penetration of water.
Those that venture there will notice the solar scenario at the bottom. Turns out Steinhilber and Beer’s (2013) prognosis is not credible. Neither is the IPCC’s AR5 scenario of course. I’ve put it all together and sent it to Jo Nova. Maybe she will make something of it – watch that space.
This subject seems to be not agreed upon and I wish it could be resolved. Attributing the last 40 years of OHC gains to CO2 means that the next 40 years will be about the same as the CO2 effect is not going to diminish. The problem solves itself or pushes itself quite a distance into the future. We can now avoid 250 ZJs of heat over the next 40 years by doing nothing with CO2 mitigation. CO2 saves us by keeping the heat where it really doesn’t matter for I’d guess a few centuries.
Bob, re:
“AIR-SEA FLUXES ARE THE PRIMARY MECHANISM BY WHICH THE OCEANS ARE EXPECTED TO RESPOND TO EXTERNALLY FORCED ANTHROPOGENIC AND NATURAL VOLCANIC INFLUENCES”
This quote is from the Chapter 10 SOD leaked by Alec Rawls, and which was what I had access to at the time of writing. This passage did not make it to FINAL draft that I can find, now there’s nothing explicit. You have wade through the waffle to even get the slimmest grasp of what they are on about, viz,:
Anthropogenic and Natural Radiative Forcing:
http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter08_FINAL.pdf
Page 712 pdf :
8.7.1.3 The Global Temperature change Potential Concept
“By accounting for the climate sensitivity and the exchange of heat
between the atmosphere and the ocean, the GTP includes physical processes
that the GWP does not. The GTP accounts for the slow response
of the (deep) ocean, thereby prolonging the response to emissions
beyond what is controlled by the decay time of the atmospheric concentration.
Thus the GTP includes both the atmospheric adjustment
time scale of the component considered and the response time scale
of the climate system.”
But,
“The GTP values can be significantly
affected by assumptions about the climate sensitivity and heat uptake
by the ocean. Thus, the relative uncertainty ranges are wider for the
GTP compared to GWP (see Section 8.7.1.4). The additional uncertainty
is a typical trade-off when moving along the cause–effect chain to an
effect of greater societal relevance (Figure 8.27). The formulation of the
ocean response in the GTP has a substantial effect on the values; thus
its characterization also represents a trade-off between simplicity and
accuracy.”
Firstly, in their narrative there is only an implicit link between CS and ocean heat but on which they don’t elaborate (no science or citation). Even so they are simply stating “exchange of heat between the atmosphere and the ocean”. That is not an insulation effect because the inference is that if there is anthropogenic forcing of ocean heat it is simply an air-to-sea energy transfer ([problematic even for the IPCC – see Chapter 3 below).
Secondly, their implicit CS-ocean heating link is only based on their “assumptions” anyway.
Chapter 3, is more explicit on page 274 pdf:
Observations: Ocean
http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter03_FINAL.pdf
3.4 Changes in Ocean Surface Fluxes
3.4.1 Introduction
“The net air–sea heat flux is the sum of two turbulent (latent and sensible)
and two radiative (shortwave and longwave) components. Ocean
heat gain from the atmosphere is defined to be positive according to
the sign convention employed here.”
Except ocean heat gain is by the heating agent – solar shortwave radiation (DSR) change modulated by cloudiness change – not the atmosphere. Downwelling longwave radiation (DLR) is not the ocean heating agent. DLR enhances evaporation which is the major oceanic heat loss mechanism (i.e. a cooling effect). The IPCC makes the respective major gains and losses clear – solar gain, evaporative loss:
3.4.2 Air–Sea Heat Fluxes
3.4.2.1 Turbulent Heat Fluxes and Evaporation
“The latent and sensible heat fluxes have a strong regional dependence,
with typical values varying in the annual mean from close to zero to
–220 W m–2 and –70 W m–2 respectively over strong heat loss sites”
3.4.2.2 Surface Fluxes of Shortwave and Longwave Radiation
“The surface shortwave flux has a strong latitudinal dependence with
typical annual mean values of 250 W m–2 in the tropics. The annual mean
surface net longwave flux ranges from –30 to –70 W m–2.”
This is all conventional and non-contentious. The problem(s) for the IPCC is that it is impossible to detect a net air-sea flux change – let alone a net air-to-sea flux (an anthropogenic fingerprint):
3.4.6 Conclusions
“Uncertainties in air–sea heat flux data sets are too large to allow detection
of the change in global mean net air–sea heat flux, on the order
of 0.5 W m–2 since 1971, required for consistency with the observed
ocean heat content increase. The accuracy of reanalysis and satellite
observation based freshwater flux products is limited by changing data
sources. Consequently, the products cannot yet be reliably used to
directly identify trends in the regional or global distribution of evaporation
or precipitation over the oceans on the time scale of the observed
salinity changes since 1950.”
# # #
In other words, as I interpret, simply a long-winded way of saying – no anthropogenic ocean heating signal detected.
>”This passage did not make it to FINAL draft that I can find, now there’s nothing explicit”
It did make it. I was looking in the wrong Chapters. See:
Chapter 10, Detection and Attribution of Climate Change: from Global to Regional
http://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter10_FINAL.pdf
Page 901,
10.4.1 Ocean Temperature and Heat Content
Despite the evidence for anthropogenic warming of the ocean, the
level of confidence in the conclusions of the AR4 report—that the
warming of the upper several hundred meters of the ocean during the
second half of the 20th century was likely to be due to anthropogenic
forcing—reflected the level of uncertainties at that time. The major
uncertainty was an apparently large decadal variability (warming in
the 1970s and cooling in the early 1980s) in the observational estimates
that was not simulated by climate models
[There was no “evidence” in AR4]
Gleckler et al. (2012) examined the detection and attribution of upper ocean
warming in the context of uncertainties in the underlying
observational data sets, models and methods. Using three bias-corrected
observational estimates of upper-ocean temperature changes
(Domingues et al., 2008; Ishii and Kimoto, 2009; Levitus et al., 2009)
and models from the CMIP3 multi-model archive, they found that multi-
decadal trends in the observations were best understood by including
contributions from both natural and anthropogenic forcings. The
anthropogenic fingerprint in observed upper-ocean warming, driven by
global mean and basin-scale pattern changes, was also detected.
[This is a wild assertion – model “forcing” is not empirical or physical proof]
‘Human-induced global ocean warming on multidecadal timescales’
Gleckler et al. (2012)
http://www.atmos.washington.edu/~caldwep/nobackup/research/papers/Gleckler_et12_nat.pdf
[The alternative solar integral is neglected]
Page 902,
An analysis of upper-ocean (0 to 700 m) temperature changes for
1955–2004, using bias-corrected observations and 20 global climate
models from CMIP5 (Pierce et al., 2012) builds on previous detection
and attribution studies of ocean temperature (Barnett et al., 2001,
2005; Pierce et al., 2006). This analysis found that observed temperature
changes during the above period are inconsistent with the effects
of natural climate variability. That is signal strengths are separated
from zero at the 5% significance level, and the probability that the
null hypothesis of observed changes being consistent with natural variability
is less than 0.05 from variability either internal to the climate
system alone, or externally forced by solar fluctuations and volcanic
eruptions. However, the observed ocean changes are consistent with
those expected from anthropogenically induced atmospheric changes
from GHGs and aerosol concentrations.
[“Consistent with” is not proof. OHC is consistent with the solar integral too]
Page 903,
Attribution to anthropogenic warming from recent detection and attribution
studies (Gleckler et al., 2012; Pierce et al., 2012) have made use
of new bias-corrected observations and have systematically explored
methodological uncertainties, yielding more confidence in the results.
With greater consistency and agreement across observational data
sets and resolution of structural issues, the major uncertainties at the
time of AR4 have now largely been resolved. The high levels of confidence
and the increased understanding of the contributions from both
natural and anthropogenic sources across the many studies mean that
it is very likely that the increase in global ocean heat content observed
in the upper 700 m since the 1970s has a substantial contribution from
anthropogenic forcing.
Although there is high confidence in understanding the causes of global
heat content increases, attribution of regional heat content changes
are less certain. Earlier regional studies used a fixed depth data and
only considered basin-scale averages (Barnett et al., 2005). At regional
scales, however, changes in advection of ocean heat are important and
need to be isolated from changes due to air–sea heat fluxes (Palmer
et al., 2009; Grist et al., 2010). Their fixed isotherm (rather than fixed
depth) approach to optimal detection analysis, in addition to being
largely insensitive to observational biases, is designed to separate the
ocean’s response to air–sea flux changes from advective changes.
Air–sea fluxes are the primary mechanism by which the oceans are expected
to respond to externally forced anthropogenic and natural volcanic
influences.
[There’s the quote – but still no elaboration on an actual mechanism]
The finer temporal resolution of the analysis allowed Palmer
et al. (2009) to attribute distinct short-lived cooling episodes to major
volcanic eruptions while, at multi-decadal time scales, a more spatially
uniform near-surface (~ upper 200 m) warming pattern was detected
across all ocean basins (except in high latitudes where the isotherm
approach has limitations due to outcropping of isotherms at the ocean
surface) and attributed to anthropogenic causes at the 5% significance
level. Considering that individual ocean basins are affected by different
observational and modelling uncertainties and that internal variability
is larger at smaller scales, detection of significant anthropogenic
forcing through space and time studies (Palmer et al., 2009; Pierce et
al., 2012) provides more compelling evidence of human influence at
regional scales of near-surface ocean warming observed during the
second half of the 20th century.
[Back to “forcing” again but how does that work? What are the details and quantification of whatever mechanism is operating. Just “forcing” is inadequte]
# # #
There has been no real advance since AR4. There is still no proof, no mechanism, no quantification, no empirical basis, no evidence beyond the inadequate “forcing” of “air-sea fluxes” conjecture.
richardcfromnz says: “In other words, as I interpret, simply a long-winded way of saying – no anthropogenic ocean heating signal detected.”
Bravo for finding that. I’ll have to take a closer look. Thanks.
Cheers.
Can we agree that down IR from resonating gasses, water and a pittance of CO2, is emitted by the atmosphere? If so there is simply no argument that they warm the oceans because the oceans, on average, are ALWAYS warmer than the atmosphere.
So perhaps you wish to imply as Nick does that the one molecule layer of the ocean surface that supposedly donates the enthalpy of vaporization becomes cool enough to receive atmospheric radiation. This is conceivable, but to the best of my knowledge it has never been measured and evaporation is limited by atmospheric humidity and temperature. In order to make this a serious argument one would need to show the distribution of relative temperature and humidity between the atmosphere and the ocean over the entire world ocean surface. Call me when you get this done.
Even granting this shaky proposition, if it is an equilibrium argument, then equilibrium with a one molecule layer should be accomplished in 18 years, no? Resonating gasses must warm the atmosphere BEFORE they can warm the oceans.
Hi gymnos. and others,
I wonder how much human impact might have happened on that mono layer..
Be it due to losses in the mining of crude oil (an oil film would likely decrease evaporation) or nano plastic waste (increased surface might lead to increased evaporation)
Are there any studies about this out there!?
Cheers,
LoN
Can we agree that down IR from resonating gasses, water and a pittance of CO2, is emitted by the atmosphere? If so there is simply no argument that they warm the oceans because the oceans, on average, are ALWAYS warmer than the atmosphere.
No we can not, IR from the atmosphere will be absorbed by water molecules in the surface of the ocean (about 1000 molecules thick), regardless of the temperature of the emitter. The absorbed energy will cause these molecules to increase their temperature i.e warming. For example a photon in the 15 micron band can be emitted by CO2 at temperatures of 35ºC or 0ºC, a water surface has no idea about the temperature of the CO2 which emitted the photon, it gets absorbed regardless.
I disagree. You are defining the 1000 molecule layer as the “surface”. That is the depth IR can penetrate, but relevant question is where does the energy for vaporization come from. I actually doubt the ocean contributes it all. Regardless, in an evaporating condition there must be a gradient through your 1000 molecules such that the ones closer to the top are more able to receive radiation from the atmosphere. Sure, an individual photon can buck the trend, but the net effect is always energy transfer from the warmer to the colder body. This is not my opinion. This is basic physics.
gymnosperm December 10, 2014 at 7:19 am
I disagree. You are defining the 1000 molecule layer as the “surface”.
No I didn’t, others stated the IR was absorbed in about 3 microns and I agreed pointing out that it was ~1000 molecules thick because someone had described it as a monolayer.
That is the depth IR can penetrate, but relevant question is where does the energy for vaporization come from. I actually doubt the ocean contributes it all. Regardless, in an evaporating condition there must be a gradient through your 1000 molecules such that the ones closer to the top are more able to receive radiation from the atmosphere.
The IR is absorbed in this layer, most in the top of the layer until after 3 microns it’s all gone. In a particular section of ocean that could be ~300W/m^2, if that ocean surface is at ~300K then it will emit ~450W/m^2 from the surface itself giving rise to a steep gradient within the first few microns. Thus the heat loss due to radiation will be made good by conduction/convection from the deeper layers.
Surface profile:
http://ghrsst-pp.metoffice.com/pages/sst_definitions/sst_definitions.png
Once again, allow me to invite people to consider the following arguments and questions that I posed in my post Radiating The Ocean, viz:
Argument 1. People claim that because the DLR is absorbed in the first mm of water, it can’t heat the mass of the ocean. But the same is true of the land. DLR is absorbed in the first mm of rock or soil. Yet the same people who claim that DLR can’t heat the ocean (because it’s absorbed in the first mm) still believe that DLR can heat the land (despite the fact that it’s absorbed in the first mm).
And this is in spite of the fact that the ocean can circulate the heat downwards through turbulence, while there is no such circulation in the land … but still people claim the ocean can’t heat from DLR but the land can. Logical contradiction, no cookies.
Argument 2. If the DLR isn’t heating the water, where is it going? It can’t be heating the air, because the atmosphere has far too little thermal mass. If DLR were heating the air we’d all be on fire.
Nor can it be going to evaporation as many claim, because the numbers are way too large. Evaporation is known to be on the order of 70 w/m2, while average downwelling longwave radiation is more than four times that amount … and some of the evaporation is surely coming from the heating from the visible light.
So if the DLR is not heating the ocean, and we know that a maximum of less than a quarter of the energy of the DLR might be going into evaporation, and the DLR is not heating the air … then where is it going?
Rumor has it that energy can’t be created or destroyed, so where is the energy from the DLR going after it is absorbed by the ocean, and what is it heating?
Argument 3. The claim is often made that warming the top millimetre can’t affect the heat of the bulk ocean. But in addition to the wind-driven turbulence of the topmost layer mixing the DLR energy downwards into lower layers, heating the surface affects the entire upper bulk temperature of the ocean every night when the ocean is overturning. At night the top layer of the ocean naturally overturns, driven by the temperature differences between surface and deeper waters (see the diagrams here). DLR heating of the top mm of the ocean reduces those differences and thus delays the onset of that oceanic overturning by slowing the night-time cooling of the topmost layer, and it also slows the speed of the overturning once it is established. This reduces the heat flow from the body of the upper ocean, and leaves the entire mass warmer than it would have been had the DLR not slowed the overturning.
Argument 4. Without the heating from the DLR, there’s not enough heating to explain the current liquid state of the ocean. The DLR is about two-thirds of the total downwelling radiation (solar plus DLR). Given the known heat losses of the ocean, it would be an ice-cube if it weren’t being warmed by the DLR. We know the radiative losses of the ocean, which depend only on its temperature, and are about 390 w/m2. In addition there are losses of sensible heat (~ 30 w/m2) and evaporative losses (~ 70 w/m2). That’s a total loss of 390 + 30 + 70 = 490 w/m2.
But the average solar input to the surface is only about 170 watts/square metre.
So if the DLR isn’t heating the ocean, with heat gains of only the solar 170 w/m2 and losses of 390 w/m2 … then why isn’t the ocean an ice-cube?
Note that each of these arguments against the idea that DLR can’t warm the ocean stands on its own. None of them depends on any of the others to be valid. So if you still think DLR can’t warm the ocean, you have to refute not one, but all four of those arguments.
My best to all,
w.
–Argument 1. People claim that because the DLR is absorbed in the first mm of water, it can’t heat the mass of the ocean. But the same is true of the land. DLR is absorbed in the first mm of rock or soil. Yet the same people who claim that DLR can’t heat the ocean (because it’s absorbed in the first mm) still believe that DLR can heat the land (despite the fact that it’s absorbed in the first mm).–
Obviously DLR can’t heat the land either. And the difference of land heating and ocean heating
is land surface can become 70 C with sunlight and if there is large temperature difference one get higher conduction of heat below 1mm on the land. Therefore a few inches below the surface of dirt can warm up considerable during warmer seasons of a year- without this occuring most crops in the world could not be grown. Or soil temperature is related to when one plants crops.
–Argument 2. If the DLR isn’t heating the water, where is it going? It can’t be heating the air, because the atmosphere has far too little thermal mass. If DLR were heating the air we’d all be on fire.–
Don’t why you claim the atmosphere has far too little thermal mass as each square meter has 10 tons of air above it. It’s true that water has 4 times the heat capacity per ton or the ocean have an enormous thermal mass or terms of joules of heat- similar to Venus’ massive atmosphere.
So in comparison to the ocean the atmosphere is thermal mass is puny, but compared to say the ground [not including the water in it] it’s equal to about 10 tons per square meter of the ground.
So the air in terms the amount the temperature changes per day is massive compared to the ground. And also in terms of daily changes in temperature the atmosphere is larger than the ocean. Or ocean is all about long term storage of heat, whereas the atmosphere is mostly about storing thermal heat for a few days. Or Ocean is climate and atmosphere is weather.
gbaikie December 10, 2014 at 12:24 am
Thanks, gbaikie. Yes, but if it’s not going into the ocean it’s going into the bottom 100 metres or so of the atmosphere … which doesn’t weigh ten tonnes.
And in any case, the specific heat of the air is 1 joule/gram/°C, which is 1MJ/tonne/°C. Downwelling IR is about 340W/m2. This means that in a day you’d get about 29MJ, or enough to warm the total air column by about 3°C per day … doesn’t take many days at that rate to get the air really hot.
And there is a further problem—if you choose “the IR warms the air”, then what keeps the ocean from freezing?
Regards,
w.
-Thanks, gbaikie. Yes, but if it’s not going into the ocean it’s going into the bottom 100 metres or so of the atmosphere … which doesn’t weigh ten tonnes.-
Each day the entire troposphere [80-90% of the atmosphere] changes in temperature- warms during day and cools during night. The changes in daily temperature is greater than say 6″ below the top 1 cm of the soil or at say, below 1 meter under the surface of bodies of water.
And the changing temperature indicate it’s being warmed up and it’s being cooling down.
–And in any case, the specific heat of the air is 1 joule/gram/°C, which is 1MJ/tonne/°C. —
yup.
–Downwelling IR is about 340W/m2. This means that in a day you’d get about 29MJ, or enough to warm the total air column by about 3°C per day … doesn’t take many days at that rate to get the air really hot.–
Since the air warms when the sun is out and cools when it’s night, it’s safe to say direct sunlight is warming the air. And mostly this has to do with sunlight warming clouds or the surface. Clouds are comprised of small droplets of water which scatter sunlight, but overcast cloudy day has warmer air temperature during the day time.
Whereas with DLR one should not have have large differences of day time and night time, assuming it warm anything. But you do indicate a problem with DLR if is does warming what governs the rate of it’s warming. In terms sunlight warming the surface, there a limit that direct sunlight could warm the surface.
For instance on the Moon the 1360 watts of direct sunlight can only warm the surface to about
120 C. With the earth with lower amount of direct sunlight the highest temperature it can warm the
ground is about 70 C. And in turn air above the ground never warms as hot as the surface- it tends to be about 20 C cooler than the hottest the ground surface gets.
And if the sun does get high in the sky [as in the winter or say near the poles [at summer] the sun must go thru more atmosphere and therefore as less direct sunlight. Or anywhere around 5 pm
one has less direct sunlight. So with less direct sunlight the surface can not get a hot as 70 C.
So clear day in winter with say sun never getting above 30 degrees above horizon the sun has less direct solar energy, and is limited to the temperature it can warm any surface.
So we can characterize what the limits of what the powerful sun could do in terms max temperature, but in same sense what can we say about DLR?
Plus of course the sunlight actually does actual work- it can almost fry an egg on sidewalk:)
–And there is a further problem—if you choose “the IR warms the air”, then what keeps the ocean from freezing?–
The guy wrote this article [Bob Tisdale] gives a clue:
“THE OCEANS HAVE THEIR OWN GREENHOUSE-LIKE EFFECT
In his post, The Deep Blue Sea, John L. Daly presented something that must be considered in every discussion of ocean warming: the oceans have their own greenhouse like effect (I’ve added a hyperlink to John Daly’s Figure 1):”
Willis writes “People claim that because the DLR is absorbed in the first mm of water, it can’t heat the mass of the ocean.”
Some people might claim that and you seem to hang on to it but the reality is it adds energy to the top 10um of the ocean. Big difference when you consider the cool skin is warming with increasing depth in that region.
Willis writes “If the DLR isn’t heating the water, where is it going?”
The age old problem you and I have… cold things dont heat warm things. Adding DLR cant make the ocean warmer than it was, all it can do is slow the cooling. Hence the proposition by Minnett for example that the skin temperature increases directly because of increased DLR that is warming it must be wrong.
Yes, DLR energy is absorbed into the top 10um of the ocean. Therefore some proportion of it must increase evaporation. And the rest must be radiated because it cant convect down. Yes, there is an argument that DLR could warm the ocean by reducing the ocean’s rate of cooling but its not a given because increased evaporation is also a cooling effect. Which wins? And under what conditions? Increased DLR may warm the ocean but nobody has ever proven that. Feynman would have been dismayed at the certainty of claims made with no experimental proof.
For what it’s worth, I agree with you Willis, but many here are not up to speed on the basics and don’t like it.
And conversely some of us have looked deeply into the issue and aren’t convinced. I’m quite sure you dont like the fact that without ocean warming AGW has no teeth and the ocean warming principle hangs on a blog post over at RC.
TimTheTool
On the contrary, I think the concept of heat suddenly deciding to hide in the oceans instead of warming the atmosphere as it used to is, in short, a load of tosh.
I am also sceptical that global warming or whatever we call it now poses any problem for the future. But I don’t want to deny science as you do. I think that is counter-productive.
On this topic the science is clear to those who understand it. To argue that IR cannot warm the oceans only plays in to the hands of those who wish to prove that sceptics are science denying fools.
Don’t help them.
MikeB “To argue that IR cannot warm the oceans only plays in to the hands of those who wish to prove that sceptics are science denying fools.”
I didn’t argue that IR cannot warm the oceans. I argued that it might not based an actual scientific argument. If you have a response to that argument I’d like to hear it but suggesting I dont understand it and calling me names is pretty counter productive and amounts to an argument from authority.
Argument 1.
The land is colder than the atmosphere (before the sun hits it) but the ocean is warmer than the atmosphere. You are conflating the entire solar spectrum with atmospheric IR.
Argument 2.
It is never absorbed by the ocean, on average. It is warming the atmosphere, contributing to evaporation near the air water boundary, driving the atmospheric heat engine, and wantonly dissipating to space.
Argument 3.
I agree. Basic “greenhouse” effect, but remember that energy is not created either. In order to continue “warming” the ocean in this fashion, the atmosphere must continue to warm. Ain’t seen much o’ that lately.
Argument 4.
See argument 3.
If Kevin’s energy budget is correct more energy cycles between resonating gasses and the surface than the earth receives from the sun in a photon food fight. This is hard to describe. Both the surface and the atmosphere are warmer for the presence of resonating gasses, but these gasses are not insulation, not a blanket nor a piece of glass. The photon food fight is like a plasma or whirling dervish, but it equilibrates very fast.
This keeps the oceans from freezing.
Willis,
You’re absolutely right! For all the people who believe that there is an actual, separate, thermodynamically working flux/transfer of radiative energy from the atmosphere down to the surface (more than twice as intense as the solar heat flux), your four arguments should be unassailable.
You’re right. In this case, there is no other way for the surface to get from 232K (-41C) (highest possible BB emission temp for a surface absorbing a radiative flux of 165 W/m^2, like the solar one) to 289K (+16C), the actual, measured (averaged) global surface temp, than to absorb an additional radiative flux of 345 W/m^2 coming in from the atmosphere:
(Derived from Stephens et al. 2012.)
Pure solar radiative equilibrium: 165 W/m^2 IN, 165 W/m^2 OUT; temp 232K.
With radiatively active atmosphere added: 165 W/m^2 + 345 W/m^2 = 510 W/m^2 IN, 398 W/m^2 + 112 W/m^2 (combined conductive/evaporative loss) OUT; temp 289K.
An increase of [289-232=] 57K strictly as a result of the additional 345 W/m^2 IN (minus the non-radiative 112 W/m^2 OUT) giving an equilibrated radiative output of 398 W/m^2.
Problem is, a spontaneous transfer of energy from a cooler thermodynamic system to a warmer thermodynamic system, where this transfer of energy ALONE* raises the absolute temperature of the warmer system (in this specific case, from 232 to 289K), constitutes a direct violation of the 2nd Law of Thermodynamics. It can’t and won’t happen in nature.
*It gets no help from the original incoming solar heat flux. It stays at 165 W/m^2. It gets no help from the outgoing radiative flux (your UWLWIR). It is never in any way reduced during warming. It increases during warming, forced to grow for each (re)cycle up to steady state. Thus, the warming is ONLY and wholly accomplished by the absorption of the additional incoming flux from the atmosphere, as if this were directly equivalent to the solar heat flux.
If your explanation of some real-world effect ends up directly violating the 2nd Law of Thermodynamics, you KNOW that there’s something wrong with your explanation. Simple as that. Back to the drawing board.
I’ll leave it to you (and the rest of the people here on this thread) to ponder exactly where and how the “extra surface warming by back radiation” explanation fails …
Willis,
On average (where water is warmer than the air) DRL does not heat the water, it slows net radiation loss up and this requires evaporation and conduction/convection to make up the difference in the balance between net absorbed solar radiation and release of this energy back up to space. There can be short term periods, or local cases where the air is warmer than the water, the DRL can radiate down more than radiation up, so there can be some local net energy absorption at the surface. This results in increased evaporation, or some conduction and convective mixing of the excess energy to a modest depth. Saying the DRL heats the water in general just because it is absorbed energy is exactly the same as saying a layer of insulation on a fixed power resistor heats the resistor. It makes it hotter, but by slowing loss from the resistor, and no heat is transferred from the insulation to the resistor. Also the DRL is always smaller than URL for the sea warmer than the air, and heat transfer (unlike individual energy transfers) can only go from warm to less warm.
Thank, Willis. I was hoping you’d contribute.
Cheers.
Energy Flux is omni-directional at all points. The ocean does not magically want to give up its heat to the air. At the surface film, 180 degrees of flux energy will aim skyward. The other 180 degrees is abyssal. The physics of boundary layers permit confinement and compartmentalization of ocean heat until disrupted by meridional and thermohaline circulations.When conditions favor a well-mixed ocean environment, a more idealized entropic distribution of heat will occur. Even strong boundary or inversion conditions can only aggregate a small portion of ocean heat content. When these conditions weaken, dissipation is accelerated, of which the 180 degree skyward portion will transfer some excess heat into the air via evaporation and radiation. The remainder scatters into the vast heat sink, until such time as their entropic meanderings are once again deferred by another boundary encounter.
Above what value do the oceans accumulate heat?
Above a sunspot number of 40.
Willis:
Argument 1. People claim that because the DLR is absorbed in the first mm of water, it can’t heat the mass of the ocean. But the same is true of the land. DLR is absorbed in the first mm of rock or soil. Yet the same people who claim that DLR can’t heat the ocean (because it’s absorbed in the first mm) still believe that DLR can heat the land (despite the fact that it’s absorbed in the first mm).
Well the ocean evaporates. Do rocks evaporate? Now soil has moisture. So that will have an effect.
The trouble with all this is that it is very complicated. Teasing out cause and effect is difficult.
But you do make a good point. Maybe the effect of LWIR is not as significant on land as is currently thought.
Argument 2. If the DLR isn’t heating the water, where is it going?
Too easy. Into the energy of motion.i.e. it drives convection. Which cools the Earth.
Argument 3. The claim is often made that warming the top millimetre can’t affect the heat of the bulk ocean. But in addition to the wind-driven turbulence
Wind driven turbulence affects evaporation. Probably a cooling effect. So even if the wind causes mixing it is also causing cooling which will cool the ocean.
Why is the debate 100 percent one way or the other? Is it possible that some of the DLWIR actually warms the ocean, but not nearly as much as if it was an equal flux of SWR?
Given the known heat losses of the ocean, it would be an ice-cube if it weren’t being warmed by the DLR.
It is an ice cube at the poles. And rather warm at the equator.
AND very cold at depth.
It is wrong to consider that the oceans have an average temperature of about 17 degC (ie., the avergage surface temperature). The oceans more realistically have an average temperature of about 3 deg C, and this is why there are ice ages.
If the oceans truly had an average temperature of about 17 degrees there would be so much stored latent heat that ice ages would not be extensive.
After more than 4 billion years of solar plus DWLWIR (for those that consider DWLWIR has any significant role to play) and such geothermal energy that is inputted from below, the oceans have only reached an average temperature of about 3 deg C.
It’s 105 F air temperature in Phoenix. If air heats water, how come if I don’t run the heater my swimming pool goes to 70 F? Evaporating towards ambient wet bulb.
Air sensible heat capacity = 0.24 Btu/lb -F
Water sensible heat capacity = 1.0 Btu/lb-F
Water latent heat of evaporation/condensation = about 1,000 Btu/lb w/ no temperature change
1 watt = 3.412 Btu/h
Raises one pound of air 14.2 F/h
Raises one pound of water 3.412 F/h
Evaporated into 0.003412 lb/h of water with no change in temperature.
Bob T
“Based on the findings at RealClimate, that rise in infrared radiation could only warm the sea surfaces by a little more than 0.002 deg C since 1979. Yet, looking at the global sea surface temperature data, Figure 2, the surfaces of the global oceans warmed more than 0.3 deg C from 1979 to 2013, leaving about 93% of the ocean surface warming unexplained.”
Where does the 93% come from?
0.002/0.3 = 0.00666 so 99.3% is missing. Was it a typo?
Early morning, no coffee… I am agog! That was supposed to read “Bob T”.
[Fixed. w.]
Crispin in Waterloo, regarding my typo. I’ll use the same excuse. Early morning, no coffee… I am agog!
Thanks. I’d better fix it.
I believe its geothermal heat flux through the ocean floor. No one knows what happening below 2,000 meters. A geothermal hot spot was discovered under that Antarctic ice sheet that floated away a year ago and there was a string of underwater volcanic vents recently discovered. Besides just because 99.3% is unaccounted for doesn’t excuse just making stuff up.
Willis, I liked your list but this has a couple of leaks, of not holes:
“Argument 2. If the DLR isn’t heating the water, where is it going? It can’t be heating the air, because the atmosphere has far too little thermal mass. If DLR were heating the air we’d all be on fire.”
There may be a slight exaggeration there. Vertical transport of moist air can easily handle that much.
This is more to my point:
>Nor can it be going to evaporation as many claim, because the numbers are way too large. Evaporation is known to be on the order of 70 w/m2, while average downwelling longwave radiation is more than four times that amount … and some of the evaporation is surely coming from the heating from the visible light.
The average evaporation – I just worked it out and got a very similar number for the average of all oceans for the year. That is probably not legitimate, meaning it would be better to look at a region where there is ‘that much’ incoming DWIR and see how much of it is turned into evaporation. Perhaps it is a factor of 10 higher. I didn’t check.
I see a problem using high IR in a tropical region then applying a global average evaporation rate saying it doesn’t add up. Well, it shouldn’t.
There is another mechanism which is often overlooked which is that heat can leave the surface by evaporation, but not evaporate water on a net basis.
This is best represented by water boiling in a pot with a lid on it. If you calculate the heat lost from the pot by measuring the mass of evaporated water only, it under-reports the total. There is heat leaving from the lid by convection, conduction and radiation that is not counted if the mass of water evaporated is the only yardstick.
Water evaporates from the bulk and the vapour condenses on the underside of the lid which conducts heat through to the top and it is lost from the lid. The overall effect is that heat lost to evaporation thermalises the air and the water drops back into the pot.
In the ocean this also happens but the ‘lid’ is the supersaturated layer of air just above the surface where the back-and-forth exchange is taking place. This region thermalizes the air and the water drops back into the ocean. Also, water vapour transports heat from the water to the air without itself ‘moving on’. Perhaps the best demonstration is to see what happens if that layer is blown away in the wind: The net evaporation rate increases dramatically and there is net (powerful) cooling far beyond the DWIR.
This layer can be seen by raising a lidless pot of water to the boiling point with low heat in a windless room. An undulating thin layer of white fog forms just above the water surface. That is a supersaturated layer convecting heat (and some water vapour) into the air above the pot, while dropping recondensed water back into the pot – without a lid. If you check the mass loss you will find that the heat lost to evaporation is much less than the total heat lost. Some of it is radiation, but there is still a missing quantity of energy. That is ultimately why mass of water evaporated is a poor measure when calculating the heat transfer efficiency to a pot.
The point repeatedly made is that DWIR can’t heat the water directly, which is true, but evaporated water can heat the bulk by mass transfer if there is some wind (and there usually is). This is a separate process as described well above this message. Warm moist air blowing over a pool of water can indeed warm it, but the mechanism is not DWIR, it is convection heat transfer (or convective, if you prefer). Cold dry air blowing over water can cool it, by evaporation AND ‘mass transfer’, something I have not seen emphasized during these discussions. People are distracted by the evaporation and radiation and built incomplete theoretical worlds. The real world has wind and water moving energy by multiple paths both up and down.
Crispin in Waterloo December 10, 2014 at 8:16 am Edit
I grow weary of handwaving. You need to demonstrate using NUMBERS that a) all of the DWIR goes into the air, and b) that this is totally used up in vertical transport of moist air.
I don’t underatand what you are saying. The global average DWIR is on the order of 340 W/m2, and the energy used in evaporation is about 80 W/m2 … you can wave your hands all you want, but it can’t all be going to evaporation. Period.
Again, that’s a lovely theory. But unless you think there is a metal lid over the ocean, I fail to see the relevance. There is a layer above the ocean that is saturated. There is not a layer that is “supersaturated.
But that layer is very thin. IF all of the DWIR were absorbed in that layer it would become very hot. I know of no one who has demonstrated that there is a very hot layer of air immediately above the ocean.
No, it’s not “supersaturated”, it is condensing … and having spent lots of time on the ocean, I can assure you that in general there is no such condensation immediately above the surface.
Cite? I grow weary of your uncited claims. I know of no scientific studies that show that “DWIR can’t heat the water directly.” It is absorbed by the water … what do you think happens when that absorption occurs? Does it turn into fairy dust? No … it turns into heat.
More handwaving. Provide citations, provide numbers if you want to be taken seriously. Anyone can spin a tale … but the natural world pays not attention, it just does what it does.
Look, Crispin, you are ignoring the following facts. The emissivity of water in the IR region has been measured hundreds of times. By Kirschoff’s Law, emissivity equals absorptivity. So we know for a fact that a) water absorbs IR, and b) it is turned into heat in the process.
Now, how that plays out in the ocean is a good question … but claiming without citation that “DWIR can’t heat the water directly” is just foolishness.
w.
PS—I’ll likely be sorry I asked, but what on earth is cooling by “mass transfer” which you refer to above?
Not to worry Willis; that’s code for “Convection”.
See how that works ?
I think they mean that ocean currents mass convey heat from the tropics to the polar regions, where it is so damn cold that the radiative cooling rate is much lower than it would have been if they had left all that heat down there in the tropics, where they really know how to radiate and cool efficiently.
I never get ice on my car’s radiator.
Willis
This is getting complicated because you did not follow the plot – probably because I am not explaining it well, though I think on rereading I did OK.
Maybe RACook can pitch in. You have several things wrong and I will try to do this efficiently.
>>There may be a slight exaggeration there. Vertical transport of moist air can easily handle that much.
>I grow weary of handwaving.
I grow weary of you calling anything you are not familiar with ‘handwaving’. To me handwaving has another more valuable meaning.
You are asking questions about a field that is not very ‘specialist’ so I had some expectation of you taking what I and probably others consider ‘obvious’ at face value. Thunderstorms can convect massive amounts of energy upwards. That is my reference for vertical transport.
>You need to demonstrate using NUMBERS that a) all of the DWIR goes into the air, and b) that this is totally used up in vertical transport of moist air.
That is not what I said so of course what I wrote is not the answer to that question. I was for an the ability of the atmosphere to dump the heat vertically if there was a mechanism shown for getting it from DWIR into thermalised air/water vapour. You said earlier that 340 watts/m2 was going to heat the air to ‘a high temperature’. We will calculate that in a moment.
>“The global average DWIR is on the order of 340 W/m2, and the energy used in evaporation is about 80 W/m2 … you can wave your hands all you want, but it can’t all be going to evaporation. Period.”
Well, that is not a complete description of what is happening. Sea water and the water vapour in the air immediately above it also radiate IR upwards. That 340 is not a net transfer into the sea surface, it is value of the DWIR. There is UWIR as well. Water and water vapour are powerful emitters of IR. That is why there is DW in the first place – most from water vapour in the troposphere and some from CO2.
Bob T and many others have described how SW radiation reaches the water and reflects off again. So…the same thing is happening with some of the IR which is that it is absorbed and re-emitted. It is not all showing up as an increase in temperature. Your question is expressed in a way that assumes there is a net 340 down. Like… “It is 680 down and 340 up so there is 340 to find and explain.” Not so. It is 340 down and X up and Y thermalised in the air and water vapour and Z transferred by convective heat transfer to the water.
We have a number for Down = 340
You have a number for evaporation = 80
You have no number for IR Up
You have no number for heating the water vapour after evaporation
You have no number for mass transfer to the water
You have no number for mass transfer from water to the air
I provided some numbers in a straw man calculation above to get people thinking about the missing element of energy flow – ‘mass transfer’ from the sea to the air or air to the sea depending on which was the warmer.
You asked what mass transfer was. http://cat.inist.fr/?aModele=afficheN&cpsidt=14751137 is an abstract (you need no more) indicating that ‘a mass of something with heat capacity per unit mass’ can gain heat and physically take it away. Cooling towers are good examples. They do not rely on radiation at all. It is the term used to describe convection of heat away from an object like a warm radiator. The ‘mass’ is the moving air. The heat capacity of air is dependent on the amount of water vapour in it. The shape of the object affects the efficiency of heat transfer by convection.
>>There is another mechanism which is often overlooked which is that heat can leave the surface by evaporation, but not evaporate water on a net basis….
>Again, that’s a lovely theory.
Thank you. I agree. And it is a realistic description of reality. I am not going to complicate the calculation below with it, however. No need to make my point.
>But unless you think there is a metal lid over the ocean, I fail to see the relevance.
Please don’t play games. It was an analogy intended for those who are not familiar with these physical processes.
>There is a layer above the ocean that is saturated. There is not a layer that is “supersaturated”.
Oh yes it is. The exchange between the water and the air is bidirectional because it is supersaturated. If it was saturated, it the exchange would stop in equilibrium. It doesn’t. The region above the surface, say 100mm, is usually saturated. Higher up, it is warmer (heated by DWIR).
>But that layer is very thin.
Correct. You understand the mechanism.
>IF all of the DWIR were absorbed in that layer it would become very hot.
1. You did not put a number on ‘very hot’
2. It is not entirely absorbed in that layer or the surface.
DWIR, the 340 watts, approaches the surface; not all of it makes it to the water. As the humidity increases near the surface the DWIR is captured and re-emitted in all directions. Like a ‘reverse GH effect’. It shades the surface and sends out UWIR. How much? We should find out. There is a great deal of water vapour near the water surface. It is impossible that 100% of the 340 watts reaches the ocean. It might be only 80 watts.
If some GHG alarmist complains that there is no such re-radiation mechanism from the water vapour, point out that it is exactly the same mechanism that is responsible for the DWIR. It is receiving DWIR and sending it back up (and to all sides including down). There is no such corresponding ‘layer’ in the troposphere of course. The near-ocean layer is unique.
>I know of no one who has demonstrated that there is a very hot layer of air immediately above the ocean.
That ‘very hot’ is of course the number you didn’t provide. Now I have to digress because of something else you didn’t understand.
The average of 80 watts to evaporation is not a reasonable approach. It is an average of 80 watts, but in hot places, it is far more. Convective cooling is not nearly the same everywhere, agreed? Thunderstorm hypothesis and all that. DWIR is not 340 everywhere. That is an average. That is why ‘they’ say there will be a tropospheric hot spot. The GHG effect is supposed to be concentrated in the tropics. The DWIR is also concentrated in that same region, and it is not 340 w/m2. It is much more. So you are not looking for a solution to an excess (340-80) you are looking for much bigger numbers in that zone. OK? It is not 340 and it is not 80. This whole process (see my straw man calc above) is highly dependent on temperature.
We should not work with average numbers – it should be a realistic TSI in an important zone, say 20 Deg N or something. All that aside, back to business:
>>This layer can be seen by raising a lidless pot of water to the boiling point with low heat in a windless room. An undulating thin layer of white fog forms just above the water surface. That is a supersaturated layer convecting heat (and some water vapour) into the air above the pot, while dropping recondensed water back into the pot – without a lid. If you check the mass loss you will find that the heat lost to evaporation is much less than the total heat lost. Some of it is radiation, but there is still a missing quantity of energy. That is ultimately why mass of water evaporated is a poor measure when calculating the heat transfer efficiency to a pot.
>No, it’s not “supersaturated”, it is condensing … and having spent lots of time on the ocean, I can assure you that in general there is no such condensation immediately above the surface.
When the humidity is above 70% there is water vapour condensation. You cannot see it. [I can measure it in the lab, however!] The surface layer is thin as you said, and it has a very high humidity. It is where the sea and air exchange H2O molecules. In a sunny, windless, miserably hot environment, say Jakarta, the humidity of the air near the water shoots up because of the evaporation of water. That humidity captures DWIR like the little upside down GH layer it is. How much reaches the surface? I don’t know – but sure as heck it isn’t all of it because water vapour is a GHG and it blocks a lot of it. I would not be at all surprised to find it is only the 80 W that goes into evaporation because what does reach the surface only heats the top few microns.
You didn’t comment on the mechanism. Summary: some of the DWIR never makes it to the surface in a real atmosphere so there is no point in looking for all of it. It is not AWOL, it was reassigned to outer space.
>>The point repeatedly made is that DWIR can’t heat the water directly, which is true, but evaporated water can heat the bulk by mass transfer if there is some wind (and there usually is).
>Cite? I grow weary of your uncited claims. I know of no scientific studies that show that “DWIR can’t heat the water directly.”
I thought there were some good examples but I will not be citing them, I was thinking of the very many claims made on WUWT that it can’t. That is another post.
Technically heating the top 10 microns is heating the water so the bald claim fails, but if that 10 micron heating almost all turns into evaporation and doesn’t heat the water below the, general claim is in practice, true. Given that ‘boiling’ (evaporation is slow boiling) has many problems with definitions and mechanisms http://www.ucl.ac.uk/sts/staff/chang/boiling/index.htm#5 ‘boiling’ by IR emission from above for all practical purposes is going to take place at the surface only.
>It is absorbed by the water … what do you think happens when that absorption occurs? Does it turn into fairy dust? No … it turns into heat.
Only what gets there turns into heated water. See above. Some of it turns into heated water vapour above the water. Some is turned into UWIR. Some of the heat in the water vapour is transferred to air (until they equilibrate). The temperature rise in the air (which contains the evaporated moisture) can be calculated.
http://www.engineeringtoolbox.com/heating-humid-air-d_693.html
The enthalpy (total energy) in the air can be calculated
http://www.engineeringtoolbox.com/psychrometric-chart-mollier-d_27.html
Scenario numbers:
Temperature of the evaporated water vapour = 15 C
Temperature of the heated water vapour by the time it moves vertically 2 metres = 25 C
Enthalpy change according to http://docs.engineeringtoolbox.com/documents/816/psychrometric_chart_29inHg.pdf (unit conversions required) = 14,775 Joules
In Jakarta it is more like 40 C than 25. In Bali, closer to 35. But it depends on the wind, doesn’t it? There is a missing number: the mass of air that is involved in the cooling and transport of the energy. Without that no one can say, ‘the air is going to be hot’.
>>This is a separate process as described well above this message. Warm moist air blowing over a pool of water can indeed warm it, but the mechanism is not DWIR, it is convection heat transfer (or convective, if you prefer). Cold dry air blowing over water can cool it, by evaporation AND ‘mass transfer’, something I have not seen emphasized during these discussions. People are distracted by the evaporation and radiation and built incomplete theoretical worlds. The real world has wind and water moving energy by multiple paths both up and down.
>More handwaving. Provide citations, provide numbers if you want to be taken seriously.
This was not a discussion of numbers it was a discussion of mechanisms. Citations for what? How the whole HVAC industry works? http://www.engineeringtoolbox.com
>Anyone can spin a tale … but the natural world pays not attention, it just does what it does.
Your hot air (literally) story is a tale and not a very good guess. If the evaporation was 80 watts and the rest went be some mechanism into the air and zero into the ocean, the mass transfer needed to collect the remaining 260 is:
Heat capacity: 1.2 kg air, heat capacity of 1252 Joules per m3 per degree C.
That is for saturated air at 15 C (leaving the ocean). As the temperature rises the moisture content remains the same and the relative humidity drops. If there are fog droplets they evaporate (providing cooling). Let’s assume that doesn’t happen and KIS.
http://www.engineeringtoolbox.com/enthalpy-moist-air-d_683.html see the worked example. There is also a worked example of saturated air containing fog. You can skip that.
Velocity: Wind speed 2m/sec, air assumed to be well mixed far above the surface because of the rough surface of the ocean. This gives is we have a time variable. Let’s multiply:
1252 x 2 cubic metres of air = an enthalpy change of 2504 Joules/degree C
Energy transfer capacity of 2 cubic metres of that wet air is 2504 Watts PER DEGREE. With our (25-15) = 10 degree worked example the change in enthalpy (Δ total heat content) is 25,040 Watts at that air flow.
A requisite condition in this example is that all that air interacts with the water surface on that square metre per second, which clearly it will not.
You are looking for 260 watts. Given the example above it would require, on average, 260/25,040 x 2000 litres = 20.7 litres of air to meet and leave the surface, on average, per second and rise to 25 C by 2 metres altitude. That is 2 cc/sq cm/second. That is how easily mass transfer can transport heat from IR to the upper atmosphere. This calculation shows that Little Miss Atmosphere can significantly cool Big Mr Ocean without getting ‘very hot’.
>Look, Crispin, you are ignoring the following facts.
Oh, come on.
>The emissivity of water in the IR region has been measured hundreds of times. By Kirschoff’s Law, emissivity equals absorptivity. So we know for a fact that a) water absorbs IR, and b) it is turned into heat in the process.
That heat is easily carried away in the warmed air and water vapour (much less important) and latent heat of evaporation (not as important as is often assumed – see the enthalpy calc ref above in detail). Air holds a lot of heat. Heat transport works and it is not hot. Most of the DWIR never reaches the ocean surface, that is my conclusion.
“””””…..… and some of the evaporation is surely coming from the heating from the visible light…..”””
First problem: LIGHT, by definition IS visible. (it’s all in your head)
Second problem: Ocean water is MOST highly transparent at the very solar spectrum wavelengths that carry most of the solar spectral energy. The ocean water absorption coefficient at the peak of the spectrum, is about 1E-4 cm^-1.
That means the 1/e absorption depth is 10^4 cm or 100 meters of sea water to absorb 37% of the highest spectral radiance solar energy. Now it is not that deep for all of the visible spectrum, but more than 90% of the solar spectrum energy goes at least 10 meters deep.
The evaporative surface “skin” (it’s NOT a skin) of the ocean is a few microns thick.
Water molecules do not escape from depths of more than a few microns, and I’m being very generous in allowing any escape from more than say a dozen molecular layers deep.
It is ONLY the highest energy tail of the Maxwell-Boltzmann molecular KE distribution that can escape from the surface in evaporation. It is absurd to talk of evaporation from more than a few molecular layers deep.
If as you say, downward LWIR radiation is about 280 W/m^2, while incident solar radiation is maybe 1,000 W/m^2 (when the sun shines), and that LWIR has a spectral peak wavelength of about 10 microns, well the absorption coefficient of sea water in that spectral region, is in excess of 1E3 cm^-1, and as high as 1E4 cm^-1 (at 3.0 microns), then absorption of LWIR in the surface layer is at least 1E6 times that for visible solar radiation and as much as 1E8 times as high.
So nyet on much evaporation due to incident (visible) solar energy, when compared to down LWIR.
I shouldn’t even have to say this, because it has to be rather obvious to anyone, but when the down LWIR gets absorbed all in the top 50 microns or less of the sea water (it does), you do get prompt evaporation from the very surface molecular layer, and local heating of that top 50 microns. Remember it is an exponential decay with depth, so 37% is absorbed in the top 10 microns or less, and 95% in the top 30 microns or less, so the layer of water getting heated is very thin, so its Temperature rise above the deeper water is substantial, so that surface layer expands, and gets less dense, so it tends to convect back to the surface, while at the same time conducting to the deeper layers (we’re talking deep compared to 50 microns).
So even though maybe only 70 out of the 280 W/m^2 is returned to the atmosphere as latent heat of evaporation, and though the remaining 210 W/m^2 is absorbed in maybe only 50 microns of water and maybe more like 30, whatever that surface “skin” (which isn’t a skin) Temperature rise is, the next one millimeter of sea water can not possibly rise in temperature by more than a tiny fraction of whatever the peak surface Temperature elevation is.
So for ALL practical purposes, there simply is no way that that 210 W/m^2 of energy can propagate BY CONDUCTION deeper than ONE mm of sea water.
And it CAN’T propagate deeper BY CONVECTION, because the convection density gradient is UPWARDS.
As for the evaporated water being fresh water, leaving a higher surface salinity behind it, which is denser, the very same sort of argument demonstrates that this too is a butterfly wing beat effect as well. Any salinity gradient due to evaporation is also confined to mere mm at most of surface water. No way is that going to convey by convection, to the ocean depths.
So yes; downward LWIR does heat the ocean; maybe a whopping one mm of it.
Please don’t ignore the CONDUCTIVE component of energy transfer from the heated ocean silly millimeter back up into the atmosphere. I can’t recall if Trenberth puts a number on that.
The oceans do have a cool-skin – the surface is almost always cooler then the centimeter of water below – therefore the net heat transfer goes from below to the surface .
There is no net heat transfer from the surface to the water below no matter how much stirring and mixing one does. This cool skin does not seem to be really mentioned in the above post and it does influence the energy transfer process.
The only way how the ocean could be warmed indirectly by infrared is when the surface would get warmer – thus slowing down the transfer of heat from below – but we do have a relative good measurement of the ocean’s surface temperature.
If backradiation does not provide a measurable effect on the surface, due to the cool skin it cannot warm the ocean. It has first to warm the surface.
Where does the added “backradiation” energy go?
Backradiation is part of the heat energy transfer, it is no net energy transfer. It does not exist without the “first” radiation.
“According to the NOAA Annual Greenhouse Gas Index, infrared radiation has only increased about 1.2 watts/meter^2 from 1979 to 2013.”
How did that affect the net heat transfer is the next question which is not answered. To consider it is a net added heat into the ocean is wrong.
“THE OCEANS HAVE THEIR OWN GREENHOUSE-LIKE EFFECT”
Yes, correct.
Is this greenhouse-like effect considered when the overall “greenhouse effect” is being calculated? (the much touted + xxx°C due to greenhouse)
No, only gases are considered there when calculating how warmer is the planet in comparison with an average rock planet.
“Based on the findings at RealClimate, that rise in infrared radiation could only warm the sea surfaces by a little more than 0.002 deg C since 1979. Yet, looking at the global sea surface temperature data, Figure 2, the surfaces of the global oceans warmed more than 0.3 deg C from 1979 to 2013, leaving about 93% of the ocean surface warming unexplained.”
Big Bear Observatory & Earthshine comes to mind (change in albedo which trumps CO2 greenhouse):
http://www.bbso.njit.edu/science_may28.html
Lars P
“The oceans do have a cool-skin – the surface is almost always cooler then the centimeter of water below – therefore the net heat transfer goes from below to the surface .
That applies only when the water is transferring energy to the atmosphere which is not always the case.
“There is no net heat transfer from the surface to the water below no matter how much stirring and mixing one does.”
Well, there is no net heat transfer BY RADIATION to the water below. In the real world what you wrote is only true if the air doesn’t touch the water, and it does.
“This cool skin does not seem to be really mentioned in the above post and it does influence the energy transfer process.”
Well it is mentioned. What was not mentioned is that ordinary ‘mass transfer’ which is convection heating or cooling, still takes place, even though there are alternative energy pathways. The diversion into discussions of radiative energy transfer only has done this whole argument a disservice because ‘ordinary’ heat transfers are taking place.
Hot wind blowing over a cold ocean definitely warms it, but not by radiation.
Hot wet wind blowing over a cold ocean warms it and wet air is dried by the cold surface condensing and removing water vapour. As it blows across the cold water, the air cools. This can only happen if there is heat transferred to the water. Yes, it is not by radiation, that points is correct. But it is not correct to conclude that because there is no net radiative transfer, there is no transfer by convection.
If there was no additional evaporation driven by wind and cold dry air (convective cooling because of surface contact with the water) there would be no lake-effect snow in Buffalo. There is.
Cold dry air warmed by Lake Erie transfers far more energy out of the lake than is possible by radiation OR evaporation in still air. That is why they got 8 feet of snow in a couple of days and Waterloo didn’t. Because some additional evaporation is attributable to the wind, it is proven that the mechanism is convection, not radiation. All the standard formulas apply.
Crispin said: “That applies only when the water is transferring energy to the atmosphere which is not always the case.”
http://researcher.most.gov.tw/public/tsuang/Data/722815193371.pdf
“[2] It is well known that temperatures at the sea surface are typically a few-tenths degrees Celsius cooler than the temperatures some tens of centimeters below [Saunders, 1967] ”
“The cool skin is recognized as an important feature of the ocean viscous layer as a result of new satellite remote sensing methodologies emerging for air-sea fluxes estimates Chou et al. , 2003].”
The cool skin is typically there, most of the time. Warmer winds warming the ocean is the exception. You are treating the exception as the general case.
Crispin: “What was not mentioned is that ordinary ‘mass transfer’ which is convection heating or cooling, still takes place, even though there are alternative energy pathways. ”
Net heat transfer goes from warm to cold. Stirring a colder surface with a warmer deeper level will transfer heat from depth to the surface and not the other way around.
Crispin: “Hot wind blowing over a cold ocean definitely warms it, but not by radiation.”
It may warm the surface layer, however as long as this surface layer is colder then the water below it will not tranfer net heat to the water below it, it may only slow down the net heat tranfer from the water below, if the surface is getting warmer. Please explain how will a colder surface warm a deeper warmer water layer? How do you implement that heat pump there?
Crispin: “But it is not correct to conclude that because there is no net radiative transfer, there is no transfer by convection.”
See above net heat transfer. Please explain how convection transfers net heat in the ocean from the cooler skin to the warmer deeper water layer.
Crispin: “If there was no additional evaporation driven by wind and cold dry air (convective cooling because of surface contact with the water) there would be no lake-effect snow in Buffalo. There is. ”
I agree, I understand evaporation is the reason for the cool skin feature.
Crispin: “Because some additional evaporation is attributable to the wind, it is proven that the mechanism is convection, not radiation. All the standard formulas apply.”
Radiation looks important if one looks at one factor, one side – which is false. It is the net heat transfer throughout the system which is the reality.
This study seems to point to one mechanism of IR/water energy exchange which they could measure. Not sure if their test included appropriate wavelengths or is applicable.
http://www.amolf.nl/news/news-archive/detailpage/artikel/water-molecules-as-efficient-infrared-antennae/
Fascinating study, DT. The study is paywalled but the full-size images are not, worth looking at. The wavelengths they used are about 2700 cm-1.
w.
This is an interesting subject.
Given we know that the Oceans have not warmed as much as models suggest, if this study could be extrapolated to co2 wavelengths and quantify the effect against ocean water, we could have increasing certainty of co2’s actual if limited impact.
This study, similar in nature is not pay walled.
http://scitation.aip.org/content/aip/journal/jcp/135/2/10.1063/1.3605657
Here’s the part I don’t get in the argument that downwelling infrared radiation (DWIR) can heat the land but not the ocean.
The earth receives (as a global 24/7 average) about a half-kilowatt per square metre of downwelling radiation. Of that, about 170 W/m2 is downwelling solar radiation, and about 340 W/m2 is DWIR.
Now IF the DWIR cannot warm the ocean as some folks claim … then
a) why is the ocean warmer than the land and
b) why is the ocean not frozen?
Regarding a), if the claim is correct then the land is absorbing about three times the radiation that the ocean is getting, and yet it is cooler … why?
And regarding b), we know that on average the ocean loses about a half a kilowatt per square metre, with about 390 W/m2 being radiated away and about 110 W/m2 being the combination of sensible and latent heat loss.
So where is that energy that is constantly being lost being replaced from, if not from DWIR?
If you say DWIR can’t heat the ocean, then you need to explain where the missing ~390 W/m2 is coming from. Geothermal is on the order of a tenth of a watt per square metre, so it’s not coming from that.
So for all of you that do think that DWIR can’t warm the ocean … where is the energy coming from to keep the ocean from freezing?
I gotta admit … I don’t see why what to me is a relatively unexceptional everyday claim, that DWIR warms whatever absorbs it, is so hard for folks to accept.
w.
Willis, I added a link to your post from a couple of years ago, in an update at the end of this one.
Cheers.
Willis
As i frequently point out to you, you cannot properly deal with averages.
The planet is driven by solar energy going into the eqitorial and tropical regions of the planet. This region just happens to be disprortioately populated by ocean. See: http://en.wikipedia.org/wiki/Tropics#mediaviewer/File:World_map_indicating_tropics_and_subtropics.png
That map clearly shows the distribiution of land and ocean. Eyeballing it, it is about 85% ocean to 15% land. And of these land masses, much of that land is either tropical rain forest or desert, or land where seasonal variance in temperature is dominated/controlled by monsoon,
Now the reason why there is such a large diurnal range between day and night temps on land is that the earth/land/ground is not heated to any great depth and retains little in the way of stored latent heat so that the earth/land/ground cannot replenish the heat being lost from the surface as the atmosphere cools and cannot thereby prevent the atmosphere from cooling as the night progresses.
However, the oceans, in contrast, possess (for practical purposes considering the length of a day) an unlimited capacity of stored latent heat and this enables the oceans to continually heat the atmosphere during the night. as the nightime atmosphere would naturally cool, it is being continuously warmed throughout the night by the ocean below. There is very little diurnal range in atmosphere temperature above the oceans.
In your series on ARGO, you suggested that the ocean temps predominantly cap out at 30degC. Now if you look at equitorial/tropical areas, and select those that are not tropical rain forest or desert, you will find many land areas that have a similar average temperature, ie., one which varies between about 28.5degC in the ‘off seasons’ to about 32degC in the ‘prime’ seasons. For example see Jizan (about 16N) that has an annual average temperature of 30degC. Now I accept that that is a cherry picked example, but one has to carry out some selection since deserts and tropical rain forest have different temperature profiles because they have their own micro climate because of what they are, rather than simply because of the amount of solar energy received.
Now the equitorial/tropical ocean receives sufficient solar to drive temperatures well above 30degC, probably more in the region of 40degC plus. In your ARGO post, you (initially) claimed that the reason that temperature caps at 30 degC is due to evaporation. Whilst evaporation certainly plays a part, it cannot be the limiting factor since there are many areas of the ocean with temps of 34 degC plus (I referred you to about half a dozen areas which were currently showing daytime highs/month highs/or yearly highs of 34degs and I have seen many hundreds of entires in ships logs recording temperatures of around 36degC and that would be water temperature drawn from about 8 to 10 metres below surface so surface temperature may well be even hotter).
The prime reason why the equitorial/tropical ocean caps out at 30degC is current, both in the form of ocean over turning where warm surface water is sequested to depth, and of cource the warm currents that distribute the equitorial/tropical heat polewards. It is these currents that carry the water away before it gets an opportunity to warm above 30degC. It is these currents that prevent the mid to high latitute oceans from freezing, and which keep the polar ocean from freezing as long as the polar oceans are additionally heated by Solar (when there is insufficient additional solar, the polar oceans freeze). You can see the effects of these currents for example areas of the Baltic freeze, whereas the sea around Iceland at the same latitude does not freeze.
You state; “So where is that energy that is constantly being lost being replaced from, if not from DWIR?” The crux of this question is how much energy is actually being lost from the ocean.
The ocean is only losing energy consisting of sensible heat of ~ 30 w/m2 and evaporative losses of ~ 70 w/m2 and radiative losses of .~ 70 w/m2. The total energy loss from the ocean is ~ 170 w/m2.
Whilst you may be able to detect a signal (ie., measure the temperature of the surface) of some 390w/m2 that does not mean that the ocean is losing this amount of energy; it is not. The ocean does not want to lose very much energy because the atmosphere above the ocean is nearly the same temperature as the ocean itself. It only wants to lose ~ 170 w/m2 of energy and that is why it gives up only that amount of energy, and no more..
Willis, we went through this on your radiating the ocean post. Most of the energy you are so worried about the ocean losing is going right back into the ocean. You are fixated on the output side, but thanks to resonating gasses most of the energy radiated out of the oceans is radiated right back. This does not “warm” the oceans to a higher energy state than they already were from absorbing solar shortwave to the bottom of the mixed layer. It limits the rate that they can cool, and the net effect is ALWAYS a net transfer of energy from the higher energy state ocean to the lower energy state atmosphere.
It is not that the oceans do not absorb lower energy state photons. They do, but these lower energy photons cannot increase an energy state that is already above their own level. It’s not like throwing another log on the fire.
The ocean is not always warmer than the land. The air over the ocean is usually warmer than the air over land after diurnal variation is anomalied out. The ability of “land” to absorb atmospheric LW depends on the individual properties of the widely ranging materials on the land surface. I frankly don’t know these properties except for ice and snow which oddly have very similar spectral properties to water.
Willis writes “If you say DWIR can’t heat the ocean, then you need to explain where the missing ~390 W/m2 is coming from.”
Its straightforward, it comes from the atmosphere exactly as you imagine it. But there’s a difference between warming and slowing cooling that it appears to me you cant quite grasp.
If a stone at 20C is surrounded by aluminium foil to give it very high DLR, how much warmer does it get?
Ocean warming/cooling is a balance of ULR from the surface, DLR from above and evaporation. Increase DLR and you increase evaporation and the resulting net energy balance over time is not obvious and certainly not scientifically quantified right now, its that simple.
TimTheToolMan December 11, 2014 at 2:34 am
TIm, I’ve been over this dozens of times, but since it appears you “can’t quite grasp” it, I’ll go over it again.
Does DWIR “warm” the ocean? Yes, in exactly the same sense that a coat “warms” you when you go outside in the cold. You are correct in that both of them are only “slowing the cooling” (by very different mechanisms to be sure).
But in common parlance, nobody says “Put on your coat, dear, it will slow your cooling”. We say a coat warms you up.
Now of course, a more accurate statement would be that a coat doesn’t actually “warm” you. Instead, it leaves you warmer than you would be without the coat. And in the same way, CO2 doesn’t actually “warm” the earth, it simply leaves the earth warmer than it would be without CO2.
Do I know that? Yes, and I’ve discussed it for years. Here, for example, is a quote from my 2011 post that is linked in the head post:
So yes, Tim, I do understand that, which you would have known if you were paying attention.
But SO WHAT? It’s just a semantic nit-pick. The end result is the same, it ends up warmer. If you don’t understand what is meant by “CO2 warms the oceans” you’re not following the story.
So … do you “quite grasp” it now?
Regards,
w.
PS—Could you leave the snark, e.g. your fantasies about what I “can’t quite grasp”, out of your future comments? They do nothing for your reputation, they just make you look petty and vindictive.
Willis writes “PS—Could you leave the snark, e.g. your fantasies about what I “can’t quite grasp”, out of your future comments?”
Practice what you preach.
Willis, put your coat on that rock. Does it get warmer?
Now I’m going to add a little heat pump that will cool the rock right past that coat but I’m not going to tell you what setting I’m putting it on. Now does the rock stay warmer?
TimTheToolMan December 11, 2014 at 10:25 pm
Tim, if I wanted to use a rock as an example I would have. We’re talking about objects (the earth, a person) that are WARMER than their surroundings. Put a coat on anything warmer than its surroundings, and it will be warmer than without the coat.
w.
TimTheToolMan December 11, 2014 at 10:18 pm
I’m working on it, Tim, I’m working on it. Your first comment to me in this thread was snarky … but I didn’t snark back. Instead, I quoted your own words back to you so you could see what it sounds like, and I made a polite request. Like I said, I’m working on it.
Now it’s up to you.
w.
Oh..and DSW, obviously.
OMG, I missed this before. Tim is absolutely right. You cannot grasp the second law of thermodynamics, and just went through this whole Al Gore “coat” thing with Stephen/Tony. Greenhouse gasses DO NOT insulate. Insulation is a blockage of conduction and convection, NOT radiation, except in unusual situations which do not apply here.
Willis: “a) why is the ocean warmer than the land and”
Due to the way how the ocean accumulates heat in depth and loses only at the surface
Willis: “b) why is the ocean not frozen?”
Ocean frozen = Falacy of averaging heat transfer.
Does the ocean radiate heat from under the ice? I would think that the ocean is not losing any heat at all in all those 10 million+ of square km from under the ice.
Could the ocean be frozen at the equator with 1000 W per sqm? Obviously not, as long as the sunlight comes down.
So we have a bulk of water that would be unfrozen and we have a bulk of water that is frozen and does not lose heat. Therefore the ocean reaches its own heat transfer equilibrium.
=> tropic is the heat engine
The fact that the oceans are warmer then the earth is telling also about the “ocean greenhouse” mentioned above.
Lars P. December 11, 2014 at 2:44 am Edit
Of course it does. Why would you think otherwise?
w.
Willis Eschenbach
December 11, 2014 at 2:57 pm
Lars P. December 11, 2014 at 2:44 am Edit
Does the ocean radiate heat from under the ice?
Of course it does. Why would you think otherwise?
w.
Why would I think otherwise? Because I thought ice and snow on ice makes a good isolation for an igloo, but what do I know? How much heat does the ocean lose through a 3 meters ice sheet? My guess is that there is a significant reduction in comparison with open sea…
Lars, sorry for the brevity of my answer. Snow is a pretty good insulator, because it contains air spaces. Ice, on the other hand, is not so good.
In fact, the conductivity of ice is about four times as large as that of water (2.18 vs 0.56 W/m-K). Of course, water also moves heat by convection, but even so, ice transfers heat quite well.
Snow, on the other hand, has a thermal conductivity of between 0.05-0.25 W/m-K.
Best regards,
w.
Willis writes “Snow, on the other hand, has a thermal conductivity of between 0.05-0.25 W/m-K.”
And “ice” is typically covered by a layer of snow which eventually compacts to become the “ice”. It is the snow that is at the top that radiates to the atmosphere. Snow/ice is indeed an insulator to the ocean.
TimTheToolMan December 12, 2014 at 1:47 pm Edit
Thanks for the comment, Tim. I fear you are conflating glacial ice and sea ice. Glacial ice is compacted snow. Sea ice is not made out of compacted snow, it’s made out of frozen ocean water.
And because the Arctic is so dry, there is typically very little snow on the sea ice. From the NSIDC:
All the best,
w.
Willis writes “I fear you are conflating glacial ice and sea ice. Glacial ice is compacted snow. Sea ice is not made out of compacted snow, it’s made out of frozen ocean water.”
From an actual paper, “Snow Depth on Arctic Sea Ice”
http://journals.ametsoc.org/doi/abs/10.1175/1520-0442%281999%29012%3C1814%3ASDOASI%3E2.0.CO%3B2
“The ice is mostly free of snow during August. Snow accumulates rapidly in September and October, moderately in November, very slowly in December and January, then moderately again from February to May. This pattern is exaggerated in the Greenland–Ellesmere sector, which shows almost no net accumulation from November to March. The Chukchi region shows a steadier accumulation throughout the autumn, winter, and spring. The average snow depth of the multiyear ice region reaches a maximum of 34 cm (11 g cm−2) in May. The deepest snow is just north of Greenland and Ellesmere Island, peaking in early June at more than 40 cm, when the snow is already melting north of Siberia and Alaska. The average snow density increases with time throughout the snow accumulation season, averaging 300 kg m−3, with little geographical variation.”
Willis writes “Tim, if I wanted to use a rock as an example I would have.”
I used the example in the post you responded to.
And went on to say “Put a coat on anything warmer than its surroundings, and it will be warmer than without the coat.”
It will cool more slowly. Putting a coat on a rock wont make it warmer than it was. Now I agree that there are mechanisms whereby the DLR could make the ocean warm but without trying to be snarky you dont appear to agree that an the cooling effect of evaporation can impact that. Perhaps there is only a very little warming. The point is that DLR might warm the ocean and then again for all intents and purposes it might not.
Bob has made good arguments that it doesn’t (much) simply by pointing out just how uneven the ocean warming is. That’s not the kind of effect you’d expect to see from a well mixed gas imparting the same forcing worldwide.
Willis wrote “Tim, if I wanted to use a rock as an example I would have. ”
I used the rock as an example.
And then used the analogy of a heat pump as the cooling mechanism and you didn’t address that at all. Firstly, do you agree that increased DLR results in increased evaporation or not?
This second post is more or less redundant. My first seemed to disappear…
TimTheToolMan December 12, 2014 at 3:15 am
The difference is that a rock has nothing which is constantly warming it, whereas both the earth and our bodies have a heat source constantly warming each one. As a result, your example was not relevant.
Anything that increases temperature (or wind) increases evaporation.
Regards,
w.
Willis writes “Anything that increases temperature (or wind) increases evaporation.”
Only temperature at the surface. How do you know the net effect if you accept additional energy is lost through evaporation (and radiation) at the higher SST? Energy that could potentially offset any AGW induced DLR increase?
TimTheToolMan December 12, 2014 at 7:45 pm Edit
Thanks, Tim. It’s true that it could work out as
Increased DWIR –> increased temperature –> increased evaporation and UWIR
and that at the end of the day the increased evaporation and radiation losses balance out the increase DWIR.
However … note that the first step in that chain is
Increased DWIR –> increased temperature
or in your words “higher SST”.
Which is what I’ve been saying all along. DWIR is absorbed by the ocean and leaves it warmer than in the absence of DWIR. What happens after that, as you point out, may be one of a number of outcomes … but that’s the start of the chain, “higher SST”.
All the best,
w.
Thank you, all, for your contributions on this thread.
–Now IF the DWIR cannot warm the ocean as some folks claim … then
a) why is the ocean warmer than the land and
b) why is the ocean not frozen?
Regarding a), if the claim is correct then the land is absorbing about three times the radiation that the ocean is getting, and yet it is cooler … why?–
Ocean is 70% of surface area of Earth.
In terms of the sun warming the Earth, the most important region is 40% of the surface of Earth which is called the tropical zone. Or 23 degrees north and south latitude.
In tropical zone the average temperature is well above 20 C, or 60% of rest of earth is below 20 C
in terms of average temperature.
More than 70% of surface area of tropics is ocean area- or somewhere around 10 to 15% is land.
So since we have a thick atmosphere and global oceans, the tropical zone warms warms the rest of the planet to much larger extent, than if we did not have the global oceans or the thick atmosphere.
So if agree with every climate scientist that tropics is the heat engine of earth and warms the rest of the Earth, and you agree that most tropics is ocean surface, then one has to conclude that earth’s oceans do much of the warming of Earth.
The warmth of the tropical ocean should rule out the possibility of a snowball earth ever happening, and believer of such ideas generally concede that the snowball earth’s may have ribbon of open water at the equator- but still want to call it a snowball earth.
It could possible that 10 degree north and south may be the only unfrozen but that is still an large area of earth not frozen. It vaguely possible one had only 10 degree north and south- it’s largely a slight possibility because the possible ways land mass might be configured in some distant past
and other factors- say sun goes very quiet, and etc.
From Lozier et al (2008):
“The group’s computer model predicted warmer sea surfaces in the tropics and subtropics and colder readingshttp://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/nao.timeseries.gif within the sub-polar zone whenever the NAO is in an elevated state of activity. Such a high NAO has been the case during the years 1980 to 2000, the scientists reported.”
The NAO shifted strongly negative in 1995-98, that accelerated the warming of the AMO and the Arctic.
http://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/nao.timeseries.gif
As increased forcing of the climate increases positive NAO, it’s a matter of checking which solar metric was reduced in those years:
http://snag.gy/ppB3v.jpg
richard verney December 10, 2014 at 4:46 pm
Thanks, richard. And as I just as frequently reply to you, averages are quite useful for some things, and totally useless for others.The trick is distinguishing between the two situations.
The radiative loss you cite, ~ 70 W/m2, is the NET radiative loss. By that I mean it is something like 390 W/m2 upwelling less something like ~320 W/m2 downwelling radiation, giving about seventy watts per square metre as the net surface loss.
(Different sources give slightly different numbers for these figures. CERES puts it at 345 W/m2 DWIR, 398 W/m2 UWIR, for a net surface loss of 53 W/m2. The Kiehl/Trenberth energy budget puts it at 396 UWIR, 333 DWIR, for a net surface loss of 63 W/m2. There are other values out there, such as your ~70 W/m2.)
In any case, from the fact that you are using the net figure which includes the DWIR, I have to assume that you agree that the DWIR actually IS absorbed by and warms the ocean … so it appears i have no disagreement with you in that regard.
Your comment, as always, is appreciated.
w.
PS—you say regarding the tropics:
Eyeballs are deceiving. The amount of ocean in the tropics (23.5°N/S) is 73.0%, not far from the global average of 68.6%.
Willis
Thanks for your response.
Averages are generally only used to simplify matters so that the human mind has less data to process. In taking such an approach some elements of the data are lost. For example if you take the average height of people consisting of both sexes in a room, you lose the fact that women are generally less tall than men. The one thing you can be fairly certain of is that in real life, the average is rarely ever encountered.
Precisely where on planet Earth, and at what times of the 24 hour day, are the energy flows as set out by K&T in their energy budget cartoon?
One of the problems in the climate debate is a failure to appreciate that climate is regional, not global. We only get the climate (Koppen etc classification for the various climatic zones) that we see today because planet Earth is not energised as depicted in the K&T energy budget cartoon.
I am talking about ‘real’ energy meaning energy capable of performing real work in the environ in which it finds itself. I am talking about the ‘real’ energy flows which are:
Input: 170 W/m2 from solar.
Output: ~ 30 W/m2 (sensible heat) PLUS ~ 70 W/m2 (evaporative losses) PLUS .~ 70 W/m2 (radiative losses).
You would refer to this as the net energy budget..
I agree that other figures are mentioned, from time to time. I merely used the first set of figures that I saw mentioned in your article on Radiating the Oceans. I have not checked, but I may well have used the lower ~63 W/m2 for radiative losss in my comments on your article (I certainly have used that lower figure many times in our discusssions).
You state: “In any case, from the fact that you are using the net figure which includes the DWIR, I have to assume that you agree that the DWIR actually IS absorbed by and warms the ocean … so it appears i have no disagreement with you in that regard”
With respect. that argument is patently disengenuous since you are well aware (i) that I am not accepting that DWLWIR warms the oceans, and (ii) that since I am suggesting that the figure for radiative loss out from the ocean is only 70 .W/m2, I am not accepting that the ~320 W/m2 of DWLWIR has been absorbed by the ocean and forms part of the ‘real’ energy budget.
~320 W/m2
I am sceptical of the claim that DWLWIR warms the oceans; I am not saying that it does not, but I do see major problems with the claim that it does. The fact that there are intelligent people arguing about this is because there is no empirical observational data (the results of real experiments) showing what is going on.
What no one will ever answer (and I have asked you, as well as others, number of times) is to set out precisely what is said to be going on in say the top 1 metre of the ocean on a micron by micron layer for at least the first centimetre or so (thereafter a courser resolution is probably acceptable) I want to know what each molecule in these layers is supposedly doing, what are the physical processes involved, and at what rate are they taking place.
Due to the absorption characteristics of solar in water, there is all but no solar energy being absorbed in the first few microns of the oceans. Those microns may contain the effects of solar that has been absorbed at depth and the energy from which has been conducted upwards to the surface. or brought up to the surface by currents, but they do not contain, on a real time basis, solar absorbed as and when it stikes the surface and penetrates through the very top and through the first few microns (because for practical purposes no such solar is absored).
The is means that the energy in top few microns consists only of upwelling solar energy that had previously been absorbed at depth, and the instaneous absorption of energy from DWLWIR. as you know, about 60% of LWIR is absorbed in just 3 microns. Since DWLWIR is omni-directional such that a significant component of this will have a low grazing angle, it may be that between 70 to 80% of all DWLWIR is fully absorbed within just the top 3 microns.
So what is happening to all that DWLWIR energy? Tell me Willis. I want to hear what you have to say.
60% of the 333 W/m2 of DWLWIR is some 200 W/m2 of energy absorbed into the top 3 microns. How is this dissipated before it would drive copious amounts of evaporation? I want to know the claimed physics of the excited molecules in the top microns and top millimetres and top centimetres of the ocean and what is said to be happening to these.molecules.
It would be intersting to see the results of an experiment verifying the ‘real’ energy of the energy that you claim to be flowing in both directions. For example, why not get 2 identical steel, or copper, or aluminium plates. Paint one side black and well insulate the other side and the edges.
Cool these plates to say about 10degC. Then on a warm 30degC sunny day in the tropics suspend these plates say 2 to 3 feet above the ocean which is also at 30degC; one with the black face pointing skywards and the other with the black face pointing towards the ocean and measure the time it takes for both plates to reach 30deg C.
Then check to see whether the plate that has the black face facing towards the ocean has been heated at the rate of 170 W/m2.[ie., ~ 30 W/m2 (sensible heat) PLUS ~ 70 W/m2 (evaporative losses) PLUS .~ 70 W/m2 (radiative losses)], or whether it has been heated at the rate of 490 W/m2 [ie., ~ 30 W/m2 (sensible heat) PLUS ~ 70 W/m2 (evaporative losses) PLUS .~ 390 W/m2 (upwelling radiative flow)].
Obviously, the figures will be slightly different (and will have to be measured) since we are not dealing with the average K&T energy budget figure. Of course, if you can find a place on planet Earth where the actual energy flows are those of the average energy flows set out in the K&T energy budget so much the better.
I want to see the claimed ~ 390 W/m2 upwelling radiative flow actually doing something, ie., performing some real work.
When one talks of convection for instance there is no “back-convection” in the energy transfer. The same for “back-radiation”.
If one changes the characteristics of the material for convection, adding isolating material, the result is not computed through an equivalent added heat transfer. The same for radiation. It is the net heat transfer that is the real factor.
Crispin in Waterloo December 10, 2014 at 4:51 pm Edit
Thanks for that, Crispin. So … if the DWIR is not reaching the ocean, what is providing the ~300+ W/m2 necessary to keep the ocean from freezing?
w.
Sorry it was so long. Introducing new ideas is not easy and would be best done in a dedicated article. If you see some calculations that do not include what any thermo engineer would have in the default spreadsheet, there is a problem. I’ll bet SolidWorks does a better job on ocean heat than do a lot of climate models.
I hope I haven’t missed too much above. Can’t read it all. I am applying for things 10% of the time while responding here 90%. 🙁
As the DWIR was never heating the oceans except by some heat from mass transfer (only when the air was warmer than the water of course) that leaves the rest of the non-IR DW: visible light, and a varying amount of UV depending on ozone. There is a small amount of EM heating.
We can use Bob T’s examples of how much energy gets into which latitude (did I mention what a genius I think he is?) which shows that there is much more heat entering -20 to +20, and that chart showing where the ‘neutral axis’ is in summer and winter was very useful (my analogies tend to be mechanical). The light shines into the water, the IR does very little, and the oceans go round. The best graphics of that are Bob’s.
The heat is transported up by convection, mostly, and radiates from the putative TOA, mostly by water vapour and the rest by CO2 and CFC’s. When CFC’s go up, ozone goes down and a lot more energy reaches the surface, simultaneously cooling the stratosphere, just like Prof Lu says. As it cools it gets dry just like the balloons say. All that is from measurements of course, with the CFC-ozone effect amplified by CR at the poles.
Do you remember the comment about AGW by Adrian Bejan, the author of so many textbooks on convection heat transfer? He said it is such a simple problem to solve it was “not even interesting”. That is what a real mech eng scientist who writes textbooks on heat transfer says.
I tried to find a worked example for you from his 2005 book (Wiley, 3rd edition) but would have had to stitch together multiple examples and the nomenclature would be very off-putting for this audience. He has wonderful examples showing how a plate heated from below with a ‘fluid’ above automatically breaks into circulation cells with very definite patterns. The math in the patterns is horrific.
I hope your enjoyed the show and I am happy to have given even a tiny morsel to Bob T in thanks for his dedicated and perspicacious efforts to alert and upraise us all.
Sorry to butt in
Nothing is needed since the oceans are only LOSING ~170 W/m2 of energy, consisting of ~ 30 W/m2 (sensible heat) PLUS ~ 70 W/m2 (evaporative losses) PLUS .~ 70 W/m2 (radiative losses).
And the sun is supplying all the energy (~170 W/m2) that is really being LOST by the oceans.
Cosequently, the oceans do not freeze, but the ocean is very cold (average somewhere between 3 to 4degC) notwithstanding some 4 billion (or so) years of Solar and geothermal energy from below (the latter might have been more significant in the early years).
The mysterious point is that if the oceans are being warmed by the claimed DWLWIR why are they not warmer after some 4 billion or so years of all this energy? Don’t forget what the warmists say about the rate at which the oceans are presently being warmed, and do not forget that in the past, Earth has had many periods when CO2 was at concentrations higher than today. .
richard verney December 11, 2014 at 2:36 am
As I said above, the NET ~70 W/m2 radiative loss you are using is the arithmetic sum of the individual flows of upwelling and downwelling radiation. If you use that figure you are agreeing that the ~320 W/m2 of downwelling radiation is absorbed by the surface.
Because if it’s not absorbed, then the NET radiative loss would be just the ~390 W/m2 of upwelling radiation … and you say it’s only 70 W/m2.
How do we know that the ocean emits upward radiation of ~ 390 W/m2? We have measured it, thousands of times in thousands of places, plus measuring it directly from satellites. And if the ocean were NOT absorbing the ~320 W/m2 of downwelling IR, that figure of ~ 390 W/m2 would be the NET radiation loss.
Alternatively, Richard, if you think that the DWIR is not a part of the ~ 70 W/m2 of net radiative loss, then perhaps you could explain why the ocean is radiating so little. Bear in mind that the temperature corresponding to 70 W/m2 of blackbody radiation emission is MINUS 85°C, while the average ocean temperature is ~ 17°C …
Or perhaps you think it’s because of low emissivity, despite the fact that the emissivity of the ocean has been measured thousands of times, and is known to be greater than 0.90 … for a body at 17°C to emit only 70 W/m2, the emissivity would have to be 0.17.
So tell us, richard, why in your formulation is the emissivity of a body of water at 17°C only 70 W/m2?
w.
Why is that the necessary amount? A small flame can heat a very large pot of water, if the residence time of the energy in the pot is very long. (If the pot is very well insulated)
“… if the DWIR is not reaching the ocean, what is providing the ~300+ W/m2 necessary to keep the ocean from freezing?”
So the assumption is ocean water which is one meter square if at 0 C radiates +300+ W/m2. Or in 24 hour period, at 300 watts per second and there is 86,400 second in 24 hours it’s 25.9 million watts seconds. Or in terms of kilowatt hours: 7.2 kW hours per day.
Now if earth had no atmosphere it would receive more than 7.2 kw hours per day at the equator.
At earth distance and always facing the sun: 1360 watts for 24 hours is 32.64 kW per 24 hours.
Whereas on the airless earth surface and pointing at sun on gets about 1/2 of 32.64 kW, or
about 16 kw hours. But because of Earth thick atmosphere you can’t get 16 kw hours of direct sunlight per day. A rather key aspect of this is the term “direct sunlight”- as the thick atmosphere of earth scatters fair amount of sunlight.
Or wiki says: “the value when the earth-sun distance is 1 astronomical unit) then the direct sunlight at the earth’s surface when the sun is at zenith is about 1050 W/m2, but the total amount (direct and indirect from the atmosphere) hitting the ground is around 1120 W/m2”
http://en.wikipedia.org/wiki/Sunlight
And key part of wiki statement is the term, zenith. Which means directly above above. Or the sun only spends about a hour per day at around zenith if at the equator. And if you outside the tropics
the sun is never precisely at zenith. If in say Kansas, at around noon and it’s summer time it
spends about an hour somewhere near zenith. Or if you take hour either side of it when then sun is at the highest point in the sky one get somewhere around 1000 watts per square meter of sunlight- assuming you aren’t living in Sweden or something- and it’s summer.
So when sun is at 90 degree it’s going thru the least amount of atmosphere, and when the sun is at 45 degree angle it’s going through 1.4 times as much atmosphere. So 1.4 times more troposphere and 1.4 times all the other types of atmospheres. Or assuming clouds the same thickness- 1.4 times more cloud.
So in terms of solar power generation, the sun moves 15 degree per hour- so 3 hours is 45 degrees and one gets about 3 hours either side of zenith [or near zenith] of the most amount of solar energy.
So roughly and as is well known one has about 6 hours of power hours with solar energy per average solar day. Or one can get little bit more solar energy if one uses solar panel that tracks the sun, but because one doesn’t get as much solar energy in the other +/- 6 hours of a day it’s generally not economic do this. Though if you have to point the sunlight at central location- you have to track the sun, ie, Power tower designs.
Example of amount sunlight:
CA, Inyokern 8.7 6.97 7.66 kw hours average per day
http://solardirect.com/pv/systems/gts/gts-sizing-sun-hours.html
And wiki: “Inyokern has the highest insolation of any locale on the North American continent, having over 355 days of sunshine each year.”
And without atmosphere Inyokern would instead get about 16 kw hour average per year of direct
sunlight. And in terms of indirect sunlight Inyokern gets significantly more than 7.66 kw hours average per day. But indirect sunlight is generally useless for solar power generation.
But in terms of making something above 0 C, indirect sunlight can’t be ignored.
Inyokern has enough direct sunlight to keep water above freezing, But Inyokern is outside the tropical zone. Or the tropical zone is region marked where sun is at zenith during it’s summer- 23 degree latitude north and south at summer has sun at zenith at noon. And coordinates of Inyokern is 35°38′49″N 117°48′45″W
So Inyokern will get more solar energy as compared to London per year, but tropics should get more solar energy than Inyokern, but instead on average Inyokern gets more direct sunlight than most of the area of the tropics [other than deserts]. And the tropics will tend to have higher average temperature than Inyokern
Or you flip this around and say if need just direct sunlight Inyokern does not need DWIR to keep water above freezing, yet apparently the tropics does?
I would say what Inyokern lacks is warm and moist atmosphere above it and I would say if tropics
had average temperature of 0 C, it too would have a less warm and moist atmosphere above it.
Or if the air was cold in the tropics, the tropics would get more direct sunlight than Inyokern- and Inyokern not a frozen waste land.
So in general keeping with idea of greenhouse effect is more water vapor, the warmer it gets. Rather warmer areas have more water vapor and make it colder.
One way to resolve paradox, is tropics has more water droplets than Inyokern. Or moist air in general has more water droplets.
Perhaps the droplets are scattering more sunlight than gas does.
Maybe the whole issue is related to poor measurement- not counting the energy of indirect sunlight.
I haven’t seen anyone cite actual in-situ observations and derived AO interface energy budget so here’s one
‘Cool-skin and warm-layer effects on sea surface temperature’
Fairall, Bradley, Godfrey, Wick, Edson, and Young (1996)
http://onlinelibrary.wiley.com/doi/10.1029/95JC03190/pdf
Rns 191.5 Fairall et al (1996) tropical west Pacific
-Q = Rnl – Hs – Hl
-168.1 = -57.1 -7.7 -103.3
191.5 – 168.1 = 23.4 W/m2 ocean heat gain (+Q) in the tropical west Pacific
Where:
RnS is solar radiation in the UV-A/B, visible, and IR-A/B spectrum i.e. DSR
-Q is heat loss from the ocean surface
Rnl is net radiation in the IR-C spectrum i.e. DLR – OLR
Hs is sensible heat
Hl is latent heat of evaporation
+Q is heat gain by the ocean sub-surface
In other words, the sun is the source of tropical ocean heat gain.
>Rns is solar radiation in the UV-A/B, visible, and IR-A/B spectrum i.e. DSR
Or SSR – Surface Solar/Shortwave Radiation i.e. measured at surface on average.
From Wild et al (2012) linked below:
Decadal changes in surface SW radiation [DSR]
[pages 42 – 48]
Observed changes at 23 BSRN sites since early 1990s:
23 longest BSRN records (totally 306 years) covering period 1993-2010:
20 stations with increase (11 significant)
3 stations with decrease (0 significant)
SW radiation [DSR]
Change: +2.7 Wm-2/decade
‘Surface radiative fluxes as observed in BSRN and simulated in IPCC-AR5/CMIP5 climate models’
Martin Wild, Doris Folini, Ellsworth G. Dutton
BSRN meeting Berlin, August 1-3, 2012
http://www.gewex.org/BSRN/BSRN-12_presentations/Wild_FriM.pdf
From Wild et al (2012):
http://www.gewex.org/BSRN/BSRN-12_presentations/Wild_FriM.pdf
Observed changes downward longwave [DLR page 37]
Observed changes at BSRN sites since early 1990s:
25 longest BSRN records (totally 353 years) covering period 1992-2011 [20 years]
• 19 stations (76%) with increase in LW down (9 significant)
• 6 stations (24%) with decrease in LW down (3 significant)
• Average change all sites: +2.0 Wm-2dec-1 [DLR]
From Wang and Liang (2008) linked below:
[28] Figure 6 shows that the increases in Ta and atmospheric water vapor concentration are the most important parameters controlling long-term variation of Ld [DLR]
[29] The dominant emitters of longwave radiation in the atmosphere are water vapor, and to a lesser extent, carbon dioxide. The water vapor effect is parameterized in this study, while the CO2 effect on Ld is not. The effect of CO2 can be accurately calculated with an atmosphere radiative transfer model given the concentration of atmospheric CO2. Prata [2008] showed that under the 1976 U.S. standard atmosphere, current atmospheric CO2 contributes about 6 W m-2 to Ld, and if atmospheric CO2 concentration increases at the current rate of +1.9 ppm yr-1 [Intergovernmental Panel on Climate Change, 2007], this will contribute to an increase of Ld by +0.3 W m-2 per decade. Therefore, the total variation rate in Ld is 2.2 W m-2 per decade
[32] We then applied these methods to globally available meteorological observations to estimate decadal variation in Ld. Long-term variation in global Ld under all-sky conditions are reported in this study at about 3200 stations from 1973 to 2008. We found that daily Ld increased at an average rate of 2.2 W m-2 per decade from 1973 to 2008. The increase in Ld is mainly due to the increase in air temperature, water vapor and CO2 concentration.
‘Global atmospheric downward longwave radiation over land surface under all-sky conditions from 1973 to 2008′
Wang and Liang (2009)
http://www.researchgate.net/publication/232784812_Global_atmospheric_downward_longwave_radiation_over_land_surface_under_all-sky_conditions_from_1973_to_2008
Wang and Liang (2008) is SurfRad
Willis, your arguments are convincing — any engineer/physicist knows you have to obey energy conservation (1st Law). I’m willing to concede that the “mixed layer” must be absorbing some/most of the DWLWIR. However, below the mixed layer, I don’t see it warming there — too little “heat” left over to be significant. The temps below the mixing layer that are below the avg local temps are evidence of that, plus other aspects like stratification. Maybe some heat could be “hiding” in the mixed layer, but not underneath.
beng1
>”I’m willing to concede that the “mixed layer” must be absorbing some/most of the DWLWIR.”
Where? And why?
I’ve demonstrated just a few comments above that the source of tropical west Pacific ocean heat gain (23.4 W/m2 1996) is solar radiation at the surface (SSN/DSR).
The reason for this is that solar radiation lays down energy over the “pathlength” (or just “length”) of radiation which is described here: http://en.wikipedia.org/wiki/Radiation_length
“In physics, the radiation length is a characteristic of a material, related to the energy loss of high energy, electromagnetic-interacting particles with it.”
Already much upthread on the “pathlength” of DLR but the tropical PENETRATION depth can be visualized by the simplified graph from Hale % Querry (1973):
http://omlc.org/spectra/water/gif/hale73.gif
The respective spectrum bands and the DSR vs DLR division are:
Solar (SSR/DSR), neglecting UV-A/B and Visible
IR-A: 0.7 µm – 1.4 µm
IR-B: 1.4 µm – 3 µm
Atmospheric air temperature, clouds, water vapour, and other GHGs (DLR)
IR-C: 3 µm – 16 µm [conventional]
Entire infrared C range
IR-C: 3 µm – 1000 µm
http://en.wikipedia.org/wiki/Infrared
The penetration depth of DSR is over the range 1µm – 1m (IR-A/B only)
The penetration depth of DLR is over the range 3µm – 100µm (IR-C)
In other words, the material (sea water) is been energized by the relatively higher energy solar radiation (SSR/DSR) over the 1m “pathlength” which in the tropics is vertical “penetration” depth and by radiation-material “tuning”. DSR will not energize above the level of energization already in the material due to SSR/DSR.
The IR-C/DLR action is confined to the top 100µm of the ocean “cool-skin” (when it actually exists – overwhelmed by solar at noon in the tropics). The net effect of DLR – OLR is -57.1 W.m-2 (Fairall et al, 1996) i.e. the net IR-C action is OLR. This is cooling, not heating.
In addition to OLR (net Rnl), the other heat loss actions at the surface are sensible heat (Hs) and latent heat of evaporation (Hl).
The total heat loss from the surface is by DLR-OLR, Hs, and Hl:
Fairall et al (1996) tropical west Pacific heat loss
Rnl – Hs – Hl = -Qsfc
-57.1 -7.7 -103.3 = -168.1 W.m-2
The total heat gain by the sub-surface is by the solar IR-A/B action after accounting for losses from the surface:
Fairall et al (1996) tropical west Pacific heat gain
DSR – (DLR – OLR) = +Qsub-sfc
191.5 – 168.1 = 23.4 W/m2
In short, the tropical ocean heating agent is SSR/DSR – not DLR.
Same for non-tropical ocean heat gain, see this North-South Pacific transect:
Figure 10. Vertical section of potential temperature (°C) along 150°W from data collected in 1991-1993 as part of the World Ocean Circulation Experiment. Data north of Hawaii were collected in 1984 (Talley et al., 1991). Potential temperature is the temperature a parcel of water would have if moved to the sea surface with no change in heat content, and is lower than measured temperature since temperature increases when water is compressed due to the high pressure in the ocean.
http://sam.ucsd.edu/junk/Fig10_p16theta.gif
Source
http://sam.ucsd.edu/papers/talley_tropical_pacific.html
Correction
The total heat gain by the sub-surface is by the solar IR-A/B action after accounting for losses from the surface:
Fairall et al (1996) tropical west Pacific heat gain
DSR – ((DLR – OLR) + Hs + Hl) = +Qsub-sfc
191.5 – 168.1 = 23.4 W/m2
Correction
[DLR] will not energize above the level of energization already in the material due to SSR/DSR.
richardcfromnz December 10, 2014 at 10:17 pm Edit
Thanks for your comment, richard. Unfortunately, you are making the same mistake made by richard verney above. You are confusing NET heat transfer with the individual energy flows. As you point out, Rnl is the NET radiation, which as the figures show is a loss of about 60 W/m2.
But we are not discussing NET radiation. We are discussing the individual flows. Your NET radiation is the net of the upwelling and the downwelling. Here are some rough numbers.
In tropical waters, call the temperature 25°C, the S-B upwelling radiation would be about 460 W/m2.
Downwelling IR to match your figures would then be about 400 W/m2, which is very near to the figures I gave in the link above.
As a result, your conclusion that “the sun is the source of tropical ocean heat gain” simply isn’t true. In fact, the sun is only providing ~ 190 W/m2, while the DWIR is providing ~ 410 W/m2.
All the best,
w.
>”Unfortunately, you are making the same mistake made by richard verney above. You are confusing NET heat transfer with the individual energy flows.”
Rubbish. No mistake. No confusion.
>”But we are not discussing NET radiation.”
Actually we are Willis. Moreover the entire energy budget including radiation both DSR and DLR, sensible heat, and latent heat. And we’re discussing the difference in effective radiation “length” DSR vs DLR.
>”Here are some rough numbers.”
I don’t care about your rough numbers. Address the in-situ example
>”As a result, your conclusion that “the sun is the source of tropical ocean heat gain” simply isn’t true.
It is true in the Fairall et al (1996) example.
>”In fact, the sun is only providing ~ 190 W/m2, while the DWIR is providing ~ 410 W/m2.”
In fact, in the Fairall et al example, the sun is providing 23.4 W/m2 ocean heat gain (+Q) in the tropical west Pacific.
richardcfromnz December 11, 2014 at 4:39 pm
First, richard, a simple denial means nothing. Saying “rubbish” means nothing. Claiming “no mistake” means nothing. Saying “actually we are” means nothing. Saying “no confusion” means nothing. That’s just a grade-school retort, “Am not! Am not! Am not!” …
If you wish to make a scientific argument you need facts. Logic. Examples. Math. Citations.
Here’s some logic. If we are discussing downwelling radiation, as we are, then we are NOT discussing net radiation. Net radiation at the surface is upwelling radiation minus downwelling radiation. And the question we’re discussing is, does DOWNWELLING radiation leave the surface warmer than it would be without downwelling radiation. The question has nothing to do with net radiation.
And because we are discussing whether the DOWNWELLING radiation can leave the ocean warmer than if it were not there, your citation showing NET radiation is meaningless.
If you wish to participate, you’ll have to up your game, richard, and actually deal with the issues. For example, consider this interchange:
You somehow seem to think that the +Q [net energy flow] of 23.4 is what the “sun is providing” … but your own citation says different. It says:
Which is why I said that the sun was providing about 190 W/m2 … because that’s what your own citation says.
Sorry, my friend, but the sad truth is, it seems that you can’t even understand your own citation …
But I doubt that you’ll listen to any of this. It appears that you are impervious to anyone’s ideas but your own.
Regretfully,
w.
Willis, leave your insults and condescending attitude out of this. We had enough of that during the solar N-D model series at JoNova and here at WUWT. Instead, spend the time actually reading and comprehending the literature presented.
>”If we are discussing downwelling radiation, as we are, then we are NOT discussing net radiation. Net radiation at the surface is upwelling radiation minus downwelling radiation. And the question we’re discussing is, does DOWNWELLING radiation leave the surface warmer than it would be without downwelling radiation. The question has nothing to do with net radiation.”
The “surface” under consideration in respect to DLR is only 10 microns deep. That question is daft because the condition never occurs; there is always both DLR and OLR in differing proportions which varies the size and sign of the long-wave radiative component of the energy budget. That question is also irrelevant to ocean heat gain from the sub-surface heating agent which is DSR, not DLR. Read Fairall et al word-for-word Willis. You will see that consideration for DLR/OLR ceases at 10 microns depth. The only interest is the net DLR/OLR effect which is -57.1 W.m-2 i.e. cooling at the surface in the tropics. This is long-wave radiative heat loss, not long-wave radiative heat gain.
The ocean sub-surface heating agent is DSR. Fairall et al state in 2.4. Solar Radiation Profile:
“Saunders (1967) estimated that 5% of the net solar flux will be absorbed in the nearest 1mm of the water surface and that this heat acts to reduce the cooling effects of Q”.
Then in 3.1. Warm-layer Backgrounds:
“On a clear day the sun deposits about 500 W.m-2 of heat into the ocean over the 12 daylight hours. Roughly half of this heat [50%] is absorbed in the upper 2 m. The details are dependent on water clarity………….Observations [Price et al., 1986; Federov and Ginsberg, 1992] show that the region of significant warming begins near the surface and propagtes downward as it intensifies with increasing solar intensity………Measurable warming occurs as deep as 20 m………….”
Then in 3.5. Morning Onset of the Warm Layer:
“………It is useful to introduce the concept of the “compensation depth” Dc [Woods and Barkman, 1986]. Dc is the depth at which the absorbed solar radiation exactly compensates the surface heat loss Q:
Dc is defined by f(Dc) Rns (t) = Q …………………(27)”
Table 3 gives the values for D at different wind speeds varying between 0.7m @ 1 m.s-1 and 19m @ 7 m.s-1.
Therefore, conceptually, ocean heat gain is solar radiation penetration beyond those depths in the respective conditions.
>”You somehow seem to think that the +Q [net energy flow] of 23.4 is what the “sun is providing” … but your own citation says different. It says:Rns 191.5 RnS is solar radiation in the UV-A/B, visible, and IR-A/B spectrum i.e. DSR [downwelling solar radiation]”
My own citation, if you actually deign to read it, states in Table 5. “Htot 23.4”. This is the solar energy in excess of the average breakeven of -168.1 W.m-2 (23.4 + 168.1 = 191.5) at the conceptual Dc above i.e. sub-surface heat gain. It (23.4) cannot be DLR given the 0.7 m – 19 m range of Dc, DLR is only effective to 10 microns (Fairall et al 2.1. Cool-Skin Background, Hale & Querry, Segelstein, Wieliczka et al, and a number of others particularly from medical laser physics)
In short, the sun provides the ocean heat gain in the tropics Willis.
Fairall et al (1996)
http://clouds.eos.ubc.ca/~phil/courses/atsc500/code/matlab/cor3_0/95JC03190.pdf
Nobody seems able to understand that the ocean can “absorb” lower energy state radiation without warming, but that is exactly what it does. Warming and “not cooling” are different animals.
Many commenters on this thread seem to be ignorant of (or ignoring) the IR absorbency metrics of water, that is, the depth of penetration of IR into water. In terms of practical effect, water is opaque to IR, which is absorbed at the surface. This absorbed energy does not mix below the surface because it cannot. The reason it cannot is because evaporation removes the absorbed energy within seconds. The surface tension of water creates a film effect that prevents mixing of the upper 100 microns, more or less, with molecules below, while IR is absorbed in the upper ten microns.
It matters not how much IR is incident on the water’s surface. The basic physical process is evaporation, not warming.
As far as conduction is concerned, it is nil.
Concerning IR emittance by the water, it is immeasurable, but it is a function of water temp., not air temp., as some would have us believe.
The great AGW fallacy is that IR somehow contributes to SST or to ocean heat content.
The absorbency character of water makes fools of all who embrace the AGW meme.
mpainter, if as you say IR cannot contribute to ocean heat, then why is the ocean not frozen?
And again, you claim that the IR merely evaporates water from the surface … if so, why is the total evaporation not 320 W/m2, but only 100 W/m2?
Finally, why on earth would you claim that the IR emitted by the water is “immeasurable”? It has been measured literally thousands of times. See for example here, here, and here for a tiny fraction of the scientific measurements of up- and down-welling longwave over the ocean.
Gotta say, the stuff people believe about this subject is mindboggling. Yes, there is both up- and down-welling radiation. And yes, these fluxes have been measured thousands of times.
So when I ask “If the downwelling IR isn’t absorbed by the ocean, where does it go?”, people try to answer by just claiming it’s reflected by the surface … if so, where are the measurements of this effect?
And when I ask “If IR can’t contribute to ocean heat, why is the ocean not frozen”, it is based on real measurements. We know that upwelling longwave is about 390 W/m2, and that the other losses (sensible and latent heat) are on the order of 110 W/m2. So we know for a fact that the ocean on average is losing something like half a kilowatt per square metre.
But we also know that downwelling solar at the surface is only about 170 W/m2, and the downwelling IR at the surface is about 330 W/m2. This totals about half a kilowatt per square metre, which is why in general the ocean is neither heading for freezing nor heading for boiling.
But IF the downwelling IR is not being absorbed by the ocean, then we know from our measurements that the ocean would be steadily losing about 330 W/m2 … which means that very soon it would freeze solid.
So my friend mpainter, or anyone else, I STILL HAVE NOT GOTTEN AN ANSWER TO MY QUESTION—where is the energy coming from to keep the ocean from freezing, if it’s not coming from DWIR? It’s losing half a kilowatt per square metre, and the sun only provides about 170 W/m2 … so where is the rest coming from?
And please, don’t give me anything involving NET radiation. We are talking about the physically separate fluxes of DWIR and UWIR, not net IR.
w.
Willis,
I am not onee who accepts the figures of DWIR and so forth that you cite. Air temp. over the ocean is due to water temp. That seems clear enough, and I regard as idiocy the claim that the atmosphere determines water temperature. This is the fallacy of AGW, as I have said, that the warmer waters of the ocean are heated by the cooler air. Those who accept unquestionably such figures as “390 watts of DWIR” will be forced into error. The theory is all error. The “plateau pause” is the proof.
Or perhaps it has to do with the fact that nothing ever freezes in the tropics.
But, really I fail to see how you imagine that the oceans could freeze.
I would urge all skeptics to disregard theory (and AGW theory will mislead you every time) and to look at the problem on a molecular basis, with particular regard to the absorbency characteristics of water regarding IR.
Given an evaporation rate of one cm/day, a typical rate for the tropics, this works out to about seven microns per minute or one micron/8.5 seconds.
Consider this and you will come to see that the energy of IR is a transient matter and can only translate to latent heat. If one would argue that the energy from IR mixes below the “skin” layer if some 100 microns (at least; some authorities say 500), then it must be shown how, keeping in mind that very little IR penetrates below 20 microns, with most caught within ten microns.
Regarding measurement of IR from the surface of water, there is no air/water interface, if you regard the matter on a molecular basis. Instead there is a water vapor/water interface and this varies according to conditions (wind, temp., etc. Therefore I adhere to the view that IR measurements of the water’s surface to be unreliable as to accuracy, because of this vapor”cloud” which itself is immeasurable. In other words, what exactly is the source of data, the IR reading? I could be wrong on this.
Also Willis, I would propose that net energy flow perforce is from the ocean to the atmosphere. This I would deem as first principle and that which does not tally with this principle is prima facie wrong.
I would also suggest that you consider that about half of the IR emitted at the surface is not subject to ghg capture, but makes it through the atm to space instantly, via the “window”.
I myself am very leery of calculations based on imperfectly understood or misapplied theory. There are too many dubious scientists who present plausible yet still dubious science, when closely examined.
Willis Eschenbach says, December 12, 2014 at 1:38 am:
“(…) why on earth would you claim that the IR emitted by the water is “immeasurable”? It has been measured literally thousands of times. See for example here, here, and here for a tiny fraction of the scientific measurements of up- and down-welling longwave over the ocean.
Gotta say, the stuff people believe about this subject is mindboggling. Yes, there is both up- and down-welling radiation. And yes, these fluxes have been measured thousands of times.”
Pretty ironic, considering how it’s Willis Eschenbach himself who continues to believe ‘mindboggling’ stuff about this subject.
His last source, for instance:
http://www.iag.usp.br/meteo/labmicro/publicacoes/Articles/Bacellar_etal_2008-Assessing_the_diurnal_evolution_surface_radiation_balance_over_the_Tropical_Atlantic_Ocean_using_in_situ_measurements_carried_out_during_the_FluTuA_Project.pdf.pdf
makes it very clear (through Eq. (4)) how the ‘gross LW flux’ from the sea surface is really ‘measured’ – the surface is simply assumed to be a pure emitter (as if in a purely radiative setting) and from this an apparent radiative ‘flux’ is directly calculated from its temperature through the Stefan-Boltzmann equation. This is no secret. The thing that’s actually measured (as in ‘detected’) in all these cases is only the radiative HEAT, what people like Willis call ‘the net radiation’, plus the surface TEMPERATURE. Everything else is merely and strictly inferred from various assumptions and computations based on these.
What about the DWLWIR? How is that ‘measured’? Well, it’s again stated plainly in the paper above, through Eq. (2). It is even more indirectly deduced. You take the actually detected radiative HEAT FLUX and you subtract the purely calculated UWLWIR. What comes out is – voilà! – an apparent ‘atmospheric LW flux’.
–mpainter, if as you say IR cannot contribute to ocean heat, then why is the ocean not frozen? —
You keep asking this question, so I will give you a different answer.
For Ice to form it must form at the top layer of water [it floats]
And DWIR can only heat the top level.
So, it’s DWIR that stops ice from forming
There is problem with that because ice does form on the ocean and other bodies of water in the world, so can you explain why DWIR doesn’t stop all ice from forming on the surface of water on Earth?
I would ask a different question, if DWLWIR heats the ocean, why is the deep ocean so cold?
Given that the oceans (unlike the atmoshere or for that matter the land) acts as a huge storage capacitor, where has all the DWLWIR energy that has been absorbed by the ocean actually gone?
Why after some 4 billion years of DWLWIR heating, has the deep ocean only reached a temperature of about 3 to 4 degC?
Warmists would have one believe (without proper data to back this up due to lack of data length and insufficient spacialm coverage) that the deep ocean is heating at a rate of about 0.02degC per decade. It is claimed that this is due to the claimed present imbalnce/increased levels of CO2. Just imagine what a smaller imbalance would achieve in 4 billion years plus. When considering that do not overlook that there are many periods when CO2 was far higher than today.
-I would ask a different question, if DWLWIR heats the ocean, why is the deep ocean so cold?-
Because it would require thousands of years.
Or if you poured Earth’s ocean into Venus, it would dramatically lower the temperature of Venus.
Or put Earth at Venus distance it would require thousands of years to increase it’s temperature
by any significant amount [such as +10 C}
–Given that the oceans (unlike the atmoshere or for that matter the land) acts as a huge storage capacitor, where has all the DWLWIR energy that has been absorbed by the ocean actually gone?
Why after some 4 billion years of DWLWIR heating, has the deep ocean only reached a temperature of about 3 to 4 degC?–
In last hundreds of millions of years the earth’s ocean have been over 10 C. Or you can’t have Earth without ice cap without having the Ocean significantly warmer- most of Earth’s last 500 million history has been a world without polar ice caps and/or any significant area with glaciers.
Or our cool oceans and large polar ice caps are the reasons why the age we live in is call an ice box climate.
gbaikie
December 12, 2014 at 4:14 am
“There is problem with that because ice does form on the ocean and other bodies of water in the world, so can you explain why DWIR doesn’t stop all ice from forming on the surface of water on Earth?”
//////////////////////////////
I consider the issue with ice to be an interesting question, and one that deserves study.
In high latitudes when ice forms, a number of factors are at play. I suspect that the most significant of these is the lack of solar energy.
As one leaves summer, and autumn onsets, the days become shorter. But not only that, the sun is low in the sky such that the grazing angle at which it intercepts the ocean is low. this means that more solar is reflected than would be the case with noon summer sun. So the amount of energy that the high latitude ioceans absorb is very limuted because the day time hours are less and when even when there is day, much of the solar energy is reflected due to the low grazing angle.
To add on top of that, it is probable that one gets colder winds, and probably more cloud; the latter further exacerbating the reduction ion solar energy being inputted into the high latitude oceans.
Adding on top of that is that the currents which transport heat from the equatorial/tropical oceans begin to run out of steam since mid latitude oceans are cooling and high latitude oceans are cooling soo that when they reach the polar oceans they are carrying insufficient energy to prevent the polar ocean freezing over.
Because the norm in this field of science is to only use the ‘average’ condition, I do not know how much DWLWIR one finds in the Arctic at different times of year day and night but o
bviously if it is absorbed (and most of it would be absorbed in the top few microns of the ocean) it does not provide sufficient energy to prevent the top of the ocean freezing.
–gbaikie
December 12, 2014 at 4:14 am
“There is problem with that because ice does form on the ocean and other bodies of water in the world, so can you explain why DWIR doesn’t stop all ice from forming on the surface of water on Earth?”
//////////////////////////////
I consider the issue with ice to be an interesting question, and one that deserves study.
In high latitudes when ice forms, a number of factors are at play. I suspect that the most significant of these is the lack of solar energy.–
Let’s leave this world and go to Cere [dwarf planet in our main asteroid belt]. We sending a spacecraft called Dawn to Ceres and it’s now arriving- and will in orbit in the new year.
Ceres is thought to have more fresh water in it’s planetary crust than all the fresh water on
Earth. So it’s possible there is water ice on it’s surface.
Ceres gets about 1/10 or solar energy which reaches Earth and is thought to have a thin atmosphere [we will find out soon]. This thin atmosphere is thought to be mainly water vapor.
And since it’s thin it’s not going to interfere with sunlight reaching the surface.. So 136 watts
divide by 4 is 34 watts per square meter of direct sunlight.
Now suppose we could add the DWIR we suppose to have on Earth to Ceres
Would that DWIR melt the ice?
I don’t really know how satellites measure the ocean surface temperature. They can measure the air immediately above it by pinging Oxygen with their microwaves and get close? Scanning IR from the surface through all the intervening stuff seems a real can of worms.
So let’s just say they’ve got it right and total energy loss (O) from the surface film is .5KW/M2. What a lovely hieroglyphic! If you must continue in hieroglyphics to be happy then you write atmospheric DWIR absorbed by the thousand molecule skin (Ilw) as .43KW/M2 (ratio from Trenberth). Doing this makes me feel like I’m stamping clay tablets or something, but we continue the theme and call the outgoing O and the incoming I (short wave and long wave as different terms) and various losses V and write the equation in the style of a chemical equilibrium reaction:
Isw+O(imagine arrows pointed opposite directions here)Ilw+V.
More seriously, the ocean does absorb both solar and atmospheric DWIR in the skin but it is simultaneously losing your .5KW. The atmospheric DWIR component can never EVER “warm” the ocean above its atmospheric emission temperature, but it can, and does where the air temperature is high enough, keep it from freezing.
This silly discussion has continued for years when basically everything argued is true. It is true that atmospheric IR does not “warm” the oceans. It is true that the skin absorbs atmospheric IR. Willis has “gotten an answer”.
More seriously, the ocean does absorb both solar and atmospheric DWIR in the skin
////////////////////////////////
That is not so. There is all but no absorption of solar in the skin. In the top few microns of the ocean, for practical purposes, only LWIR is absorbed. SWIR passes straight through without absorption.
If there is solar energy in the top microns of the ocean, it as a consequence of solar energy having been absorbed at depth and slowly over a long length of time the energy absorbed at depth migrated to the surface through conduction and convective circulatory currents and/or brought up by turbulent mixing by wind and ocean overturning.
Um, you seem to be unaware that somewhere around half of the solar spectrum is longwave. Some of that is absorbed by resonating gasses (1% CO2) in a “top down” “greenhouse” effect that for some reason is largely neglected in the discussion.
You can see that water dominates the absorption bands of the incoming solar spectra and CO2 is utterly marginalized. I apologize for forgetting to color in the water bands at the far right that appear to equal the entire CO2 absorption.
Since you have got me started, I am very interested in the two “black hole” water bands where no solar light reaches the surface. What’s up with those?
–Um, you seem to be unaware that somewhere around half of the solar spectrum is longwave.–
Half of solar spectrum is infrared.
Or more than half total solar energy is IR
And infrared spectrum is a longer wavelength than visible light.
But infrared is big spectrum.
That solar spectrum graph include all of what is called the Near-infrared part of IR spectrum: 750 nm to 1400 nm and most of shortwave infrared: 1400 nm to 3000 nm.
The longer wave infared are called: Mid-wavelength infrared, Long-wavelength infrared, and Far-infrared.
http://en.wikipedia.org/wiki/Infrared
So those three go from 3000 nm to 1,000,000 nm. Or 3 µm to 1000 µm
And it’s usually the 3000 nm or 3 µm and longer wavelengths which are called longwave.
Or things would have to be fairly hot [hotter then sunlight warms things on earth] to emit shorter
lengths than 3 µm. Or as I recall a person body heat emits somewhere around 8 to 15 µm.
Though suppose if one were talking about the Planet Mercury which has higher surface temperature heated by Sun the LW might be regarded as shorter than 3 µm.
Or as wiki says:
Shortwave radiation:
“Shortwave radiation (SW) is radiant energy with wavelengths in the visible (VIS), near-ultraviolet (UV), and near-infrared (NIR) spectra.
There is no standard cut-off for the near-infrared range; therefore, the shortwave radiation range is also variously defined.
It may be broadly defined to include all radiation with a wavelength between 0.1μm and 5.0μm or narrowly defined so as to include only radiation between 0.2μm and 3.0μm”
http://en.wikipedia.org/wiki/Shortwave_radiation
Or for climate, longwave generally refers that what emitted as result of being heated by sunlight- and other fields or uses classify it differently.
–Since you have got me started, I am very interested in the two “black hole” water bands where no solar light reaches the surface. What’s up with those?–
Well one of the “holes” is referred to in first link above:
“Water absorption increases significantly at 1,450 nm. The 1,530 to 1,560 nm range is the dominant spectral region for long-distance telecommunications.”
Or for telecommunications they use longer IR wavelength to avoid that “black hole”, whereas with Microwave oven one could use that wavelength to heat food [it’s heating the water in food].
Also the intensity of sunlight at those hole is not very powerful, so that could part reason
it’s completely blocked by atmosphere, or if you were at higher elevation with less water vapor between you and the sun it could block less.
I would imagine the spectrum is as measured from a sea level elevation [or it should be].
richard verney December 12, 2014 at 7:25 am
Thanks, Richard, that’s an interesting question. The deep ocean is cold because water just above freezing is constantly sinking at both poles. It slowly moves towards the tropics, where over centuries it rises to the surface, is warmed, and moves polewards to complete the cycle. This keeps the bottom water very cold.

As to where all the energy absorbed by the ocean has gone, it’s gone where it came from—upwards.
In order to clarify this, I decided to gather the relevant values for the various fluxes from the CERES data. Here’s what CERES says about the energy budget of the oceans:
On an ongoing basis, all of the energy that flows into the ocean flows back out again … which is why the ocean is neither running off towards boiling nor running off towards freezing.
However, IF (as many folks claim) the downwelling infrared is not absorbed by the ocean, then we have a very big question … since the ocean overall is gaining about 186 W/m2 from downwelling solar, and it is losing about 545 W/m2 from a combination of reflection, radiation, and sensible+latent heat loss, then why didn’t it freeze solid long ago?
Best regards to all,
w.
Willis, your problem is that you believe stuff like the Ceres thing. Down welling solar?
That sort of term does not tickle your feelers?
And 359 W for DWIR vs 186 W for solar? Do you buy that?
Willis
Many Thanks.
The Ceres data is interesting, but like so much of the data, there are (as you recently explained in one of your interesting articles) data issues with this, and corrections made so as to not contradict the consensus meme. That said, I shall take the data at face value.
I understand the position with the bottom ocean waters; this is where the cold water is sinking to as part of the deep thermohaline circulation, and which continuously keeps the bottom ocean very cold.
But when I was referring to the deep ocean (and this was my fault since I was not being accurate enough), I was referring to the ocean below about 1000 to 2000 metres
As you are aware, the drop off in temperature is quite significant at around 150 to 200m.
In the equitorial/tropical sea even at about 100m the ocean is quite warm, but below ~200m it quickly falls to ~ 5 to 6 degC, and in the 1000 to 2000m range it is about 4 degC and below 2000m, it is about 3 degC (where the deep thermohaline circulation deposits the cold polar water), with bottom water in some parts of the ocean being even colder still.
So my question is that given some 4 billion years of DWLWIR heating, why is the ocean so cold below about 200m? .
You say: “As to where all the energy absorbed by the ocean has gone, it’s gone where it came from—upwards.” and that might be right.
There are a number of issues/possibilities.
First, there may be no absorption whatsoever, merely some form of photonic exchange at the boundary layer, such that 359 W/m2 of DWLWIR is ‘exchanged’ at the boundary layer with 359 W/m2 of UWLWIR so that none of the DWLWIR effectively enters the oceans and none of it is absorbed by the ocean. This would leave the oceans to radiate upwards as UWLWIR a further ‘surplus’ 49 W/m2 and this and only this forms part of the ‘actual’ energy lost by the ocean (in addition there is the sensible and latent energy losses of 122 W/m2). In this scenario, DWLWIR does not heat the oceans at all (since it never enters it). No doubt you would suggest that in this scenario, DWLWIR is acting akin to an insulator and thereby slowing down heat loss. You might further argue, by slowing down heat loss, it explains why the oceans do not freeze. Leaving aside that argument, if DWLWIR never enters the oceans then the recent claims by the warmists that the recent rise in GHGs has resulted in heat going into the oceans (which is the subject matter of Bob’s article) is much more difficult to maintain, especially since the ocean below about 150 -200m never aoppears to be warmed by DWLWIR or the oceans at that depth ought after some 4 billion years be far warmer than they are…
Second, 359 W/m2 of DWLWIR actually penetrates through the boundary layer with about 70 to 80% of it fully absorbed in the first 3 microns of the ocean (due to the omni-directional nature of DWLWIR more than 60% will be absorbed since ~50% of the DWLWIR is striking the ocean at a grazing angle below 45 deg). This (>215 W/m2) is too much energy to simply drive the amount of evaporation that is observed, so that means that if this energy is actually absorbed, how is it sequestered and dissipated to depth (thereby diluting the energy) at a speed greater than that which would drive evaporation. All the processes that I have seen put (action of wind/swell/waves/ocean over turning) are slow mechanical processes, so this would seem to raise an issue.
But lets turn away from the oceans. Let us consider crater lakes (ie., lakes in calderas). I pick these for example since they are often surrounded on all sides by hills/mountains such that there is little wind, or swell, or waves on crater lakes. Whilst there are obviously some thermal circulatory currents, they are not subject to the same intensity as that of ocean over turning.
In a crater lake, the mechanical processes that are said to mix the DWLWIR which is absorbed in the top few microns of the ocean and thereby heating the ocean, are not present. That is useful. So the issue here is given that the same mechanisms of mixing are not present: why do crater lakes not freeze, or not boil off from the top diown?
I say boil off from the top down since if about 200 to 300 W/m2 of DWLWIR is absorbed within the top few microns of the crater lake and if there are no mechanical processes that would mix that energy and dissipated it to depth, gradually, micron by micron those lakes would boil off from the top down.
The next example to consider is dew. In winter, one can often see hollows where dew falls. Within 1/2 to an 1 hour of sun up, the dew on the sunny side of the hollow is burnt off, but the dew on the shady side of the hollow may linger all day not withstanding that it has during the course of the day been absorbing all the DWLWIR (if DWLWIR possesses sensible energy and is absorbed). The amound of energy that the dew is subjected to in the shady side of the hollow throughout the day, is far more than the energy that the dew on the sunny side of the hollow received within the first hour of sunrise. So why can the sun burn off the dew in 1/2 hour or 1 hour, but DWLWIR cannot even after 6 to 8 hours?
I look forward to your further explanation and comments
Konrad. December 9, 2014 at 2:22 pm
Konrad, that is an interesting description of an experimental setup. Do you have the actual data of the experiment, such as:
Temperature of the water, start and finish
Temperature of the air, start and finish.
Temperature of the “strong” and “weak” IR sources.
Air speed of the ventilation system
Calculated heat loss vs. measured heat loss
I certainly agree that evaporative loss CAN be greater than IR radiative gain, depending on the situation. However, I (and thousands of actual observations) disagree that this is the case in the ocean.
By “selective surface” I assume you mean that it has a very high emissivity and a very low absorptivity for some combination of IR frequencies. This, of course, brings up some questions:
1) The emissivity of water has been measured literally hundreds of times … do you have a citation for this most unusual claim?
2) Kirchoff’s Law says that absorptivity = emissivity at any given frequency … are you saying that this doesn’t apply to water?
3) If not, what is your exact claim, and which frequencies of IR does it apply to?
Thanks for your answers,
w.
Willis, nothing but hypothesized heat transfer and bald assertions from you so far. You show that you have not studied the IR absorbency of water. The IR is not “trapped in the first half millimeter or so”, but in the first 20 microns, except for a negligible amount that gets a little further. The energy from received IR is translated to latent energy. The coolness of the skin layer proves this. Wind increases evaporation which is simply conversion of IR to latent heat. You have failed to show how the top 20 or so microns retain the heat of incident IR long enough to transmit it to depth. Yet you claim that this micro layer transfers… how much heat to depth? Hundreds of W/m2?
Actually, mpainter, that was nothing but questions, viz:
and
I’ll wait for konrad’s answers, thanks.
As to whether the “energy from received IR is translated to latent energy”, the numbers are way wrong. Downwelling longwave infrared over the ocean is about 360 W/m2, latent energy is less than 100 W/m2 … where is the rest of it going? It certainly doesn’t stay in the skin layer, it would boil it … so where does it go?
Finally, if the heat is NOT mixed downwards, then what is providing the necessary 360 W/m2 to keep the ocean in thermal balance?
Regards,
w.
I wanted to comment on a popular misunderstanding. This is the idea that because on average the ocean skin temperature is typically about a half a degree cooler than the sub-skin temperature, this means that energy absorbed from LWIR, which is absorbed in the first mm or so at the surface, can never be moved downwards. The idea is that because heat will not flow from cold to warm, this energy is trapped in the skin layer.
What this claim overlooks is that the skin layer is not stable. Why? Because cold water is denser than warm water. As a result, the skin layer is constantly cooling, sinking, and being replaced by warmer water. It is overturning constantly.
Now, consider what would happen if there were no downwelling IR. The skin would be much cooler because it would not be getting the IR energy which is absorbed in the skin layer. Perhaps instead of being half a degree cooler than the sub-skin layers, it would be a full degree cooler. And as mentioned above, it would constantly be cooling, sinking, and mixing downwards.
Now consider the condition with the downwelling thermal IR. The skin is still not warmer than the sub-skin. But it is warmer than it would be without the DWIR, because it has absorbed the downwelling radiant energy … and still being cooler than the underlying water, the skin water sinks and mixes with the warmer sub-skin water, taking the absorbed DWIR energy downwards with it.
As a result, despite the fact that heat only flows from warm to cold, the DWIR energy is mixed downwards by constant overturning driven by the temperature difference between the cooler skin water and the underlying warmer sub-skin water.
Nor is this the only mechanism that mixes the surface layer downward. It is also mixed mechanically by the action of the wind. In anything but the weakest of winds, the skin layer is broken up both by the horizontal force of the wind, and by the mechanical action of the waves.
Finally, in some situations in the downwelling IR makes the skin warmer than the sub-skin layer. Remember that the claim that the skin is cooler than the sub-skin is only true on average. Whenever the combination of IR plus any downwelling solar is stronger at the skin level than the evaporation, the skin layer is actually warmer than the sub-skin. In those conditions, heat is conducted directly downwards.
And as a result, the idea that the DWIR energy absorbed by the ocean is somehow trapped in the first half millimetre or so is simply not true.
All the best,
w.
Have you read these comments and criticisms of water vapor and evaporation calc’c from Dame Judith Curry?
http://curry.eas.gatech.edu/currydoc/Curry_JC5.pdf
http://judithcurry.com/2011/09/24/water-vapor-feedback-evaporation/
I’m still working through the “theoretical” for LW radiation, evaporation and convection heat heat losses for Arctic conditions (email me, I’d like to discuss some detailed questions), but she does address several of the assumptions you make above.
Thanks, RA. As always, Dr. Curry’s insights are fascinating. I note that she agrees with me, viz:
However, the downside was that the majority of her comments were about model worlds, not the real world. The papers she discusses say (emphasis mine):
and
Can’t say I’m all that impressed … while computer models have their uses, the current generation of GCM have proven totally unreliable. Which drives me nuts, because while they may be right, they may also be completely wrong.
w.
In due course, please share your comments/views since many would be interested in them.
Please look at my message to Willis (richard verney December 12, 2014 at 6:46 pm). I suggest that one should look at Caldera Lakes, and at dew where one does not get the same amount of turbulence/mecganical mixing as one sees in the oceans,
Eg., on a still winter’s day when dew can hang around all day long in a shady side of a hollow but is burnt off on the sunny side of the same hollow in hours. In this scenario there is no wind, waves, swell, ocean overturning so nothing to mix the incioming DWLWIR . The dew drop may be only about 1/4 centrimetre but is subject to copious amounts of DWLWIR being absorbed in just a few microns, but these microns are not burnt off bit by bit throughout the day even though the energy much accumulate as the seconds go by since there is nowhere else for the energy to go.
The same applies to Crater/Caldera lakes. Usually (ie., for much of the year) there will be no wind, waves or swell on these lakes since being in a crater they are surrounded 360degrees by a wind shield. So the only mixing that takes place in these is convective/circulating currents caused by differening heat profiles throughout the depth of the lake. But this must be a very slow process incomparison with absorption of DWLWIR which comes in second by second and is over 70% of it is fully absorbed in the the top 3 microns.
As I often point out, the oceans would not be here on planet Earth if the absorption characteristics of SWIR was similar to that of LWIR. We are lucky that solar is being absorbed in a million times the volume such that the energy from solar is dissipated and diluted throughout a large volume, not just concentrated in just a few microns.
Odd impressions here:
I’m going to post two photo’s here from the Space Station; The clouds visible over the tropics (-23.5 to +23.5) are ONLY over sea, then ONLY over land! Evaporation and radiation an effect on these “weather” albedo’ s?
http://www.telegraph.co.uk/news/science/picture-galleries/11177032/You-Are-Here-by-Chris-Hadfield-Photos-from-the-International-Space-Station.html?frame=3079875
http://www.telegraph.co.uk/news/science/picture-galleries/11177032/You-Are-Here-by-Chris-Hadfield-Photos-from-the-International-Space-Station.html?frame=3079871
–What this claim overlooks is that the skin layer is not stable. Why? Because cold water is denser than warm water. As a result, the skin layer is constantly cooling, sinking, and being replaced by warmer water. It is overturning constantly.–
But if this was the case, cold water would not be colder. Or in such a small distance it would be mixing nearly instantaneously.
One possible factor is that water has surface tension, which defies gravity in the case of the skeeter bugs:
“Water striders are able to walk on top of water due to a combination of several factors. Water striders use the high surface tension of water and long, hydrophobic legs to help them stay above water. Water molecules are polar and this causes them to attract to each other. The attractive nature results in the formation of a film-like layer at the top of water. This top layer has gravity acting downward in addition to the water molecules below pulling down the upper molecules. This combination creates a touch surface tension.”
http://en.wikipedia.org/wiki/Gerridae
And from post below it:
–Thanks, RA. As always, Dr. Curry’s insights are fascinating. I note that she agrees with me, viz:
I know that skeptics have been talking about evaporation, I think I recall Tallbloke discussing how the shallow IR penetration depth couldn’t possibly warm the ocean, he argued that only the surface layer warms, which then increased evaporation. This is incorrect since turbulence does mix heat in the upper ocean, and the physics of the cool skin layer right at the surface does not preclude heat exchange between the skin layer and the ocean mixed layer.–
If Tallbloke meant it could not warm ocean, maybe that is wrong, but if instead he might have meant is, that it could not significantly warm the ocean. Wind can mix tens of meters of water. Also the ocean is filled with gases which interacting with air above it. And the wind surface might vaguely resemble the surface of the sun, but there is structure to the surface of the Sun.
Also as recall from somewhere molecule at the surface of the water are racing across it having the liquid sort of behaving as gas. Or lots things are happening, but if have structural clumps sticking together [something which allows a bug to defy gravity] in riot of activity, such structure could inhibit convection- delaying convection- and delays 50% or 75% of total of something by seconds, then that going to have large effect.
I also tend to think there structure above the surface- particularly in regards droplets of water.
But as I see it the main problem is the .LWIR is is only does work in models- as compared to any evidence of work done in the real world.
You state: “But as I see it the main problem is the .LWIR is is only does work in models- as compared to any evidence of work done in the real world.”
And that is indeed the point. Trillions of dollars has been spent on cAGW and the GHE theory, but if you were an engineer looking at the K&T energy budget cartoon, would you not home in on DWLWIR in preference to Solar, after all it has twice the energy and operates 24/7 come rain or shine. In high cloudy Northern climes, sureley DWLWIR would have far better potential as an energy source compared to that of Solar.
Now with trillions$, would you not think that some attempt would be made to tap into this useful energy source, if it was useful, ie., was a source of energy capable of performing sensible work in the environ in which it finds itself.
If the enegineer fails to get results, it probably proves that DWLWIR lacks the ability to perform sensible work in the environ in which it finds itself and hence we have nothing to fear. Lacking the ability to perform sensible work, it will be unable to do achieve the marvels claimed off it, eg., heat the oceans, heat the low atmosphere near the ground etc.
If the engineer succeeds and is able to extract power from this source, then he proves the potential of cAGW, but at the same time, he has achieved something that enables us to put aside our concerns, since he will have found a limitless renewable clean energy source that operates 24/7 at all latitudes and solves the Earth’s energy problems in away that will curb CO2 emissions.
So a win win, or a no lose scenario.
The problem is that all of this is just in models based upon assumed facts and processes. Where is the real world empircal observational/experimental data ascertaining the work potential.
We do not know what is happening in the column say 30metres above the ocean to 30metres below the ocean surface. We need to know in great deal what is happening in this column. Towards the boundary layer (say I metre above and 1 metre below), we need to have very high resolution, ie., on a mm by mm basis, and perhaps at the boundary say 10 cm above/below the surface the resolution needs to be on a micron level and preferably much less.
We need to know in great detail the energy of each of these microns, how energy is passing from one layer to the next, the fluid dynamics involved. What processes are going on and at what speed those processes are effected.
Until we know that, there is no prospect of modelling the oceans, and if we cannot model the oceans, there is no prospect of modelling the climate of planet Earth.
The model;s fail we do not know or understand what is going on in a column 30 metres above and below the surface of the oceans. It is all guess work. .
You state: “I also tend to think there structure above the surface- particularly in regards droplets of water.”
/////////////////
I have been pointing this out for years. Essentially, and in very general terms, there are 3 sea states on planet Earth.
First BF3 and below. In this sea stae there is litlle wind and little in the way of waves (and probably little in the way of swell, but that is not always the case since swell is the aftermath of storms).
In this sea state many of the mechanical processes that are said to mix DWLWIR do not take place. The only effective mixing is ocean overturning which is a slow mechanical process and may be a diurnal event at that! Can ocean overturning realistically mix DWLWIR that is being absorbed on a per second basis in the top few microns of the ocean at a rate quick enough to prevent copious evaporation from those teop few miocrons? can it effective dissipate and dilute the energy absorbed into those few microns at a fast enough rate?
Second above BF3 and below BF8. This is the ideal scenario for wind/wave mechanical mixing particular above BF4. It is this sea state where there is some merit in the argument that the action of the wind and the waves performs some mixing. It obviously does. However, whether this mechanical process effectively mixes the energry abssorbed per second at a rate fast enough to prevent rapid and copious evaporation taking place in the top microns is a different amtter. That said, may be it does.
Third above BF8 to BF12. In this scenario the top of the ocean becomes a divorced layer. The very top of the ocean is ravished by the very high winds and there is copious windswept spray and spume consisting of water droplets which are not just a few microns in diametre, but much more. In this scenario the top of the ocean is actually airbourne and these water droplets act as a DWLWIR block preventing the full amount of DWLWIR entering the ocean below. These water droplets absorb the DWLWIR and become energized many of which will be swept upwards and away from the ocean help powering the storm that ravages above. In this scenario it may well be the case that a significant componenet of the DWLWIR never reaches the oceans, it is absorbed in the atmoshere and remains in the atmosphere.
As I see matters, no one has yet put forward a convincing process whereby 200 to 300 W/m2 absorbed and concentrated in just 3 microns is effectively mixed and dissipated/sequestered to depth at a rate fast enough to prevent copius evaporation from the,top microns of the ocean.
Richard Verney,
And no one can formulate such a process because mixing does not occur under such transient states. The micro-layer receiving the IR is evaporated before mixing can occur. The hypothesized mixing is purely imaginary, like so much of climate science.
richard verney, December 13, 2014 at 1:50 am:
“If the enegineer fails to get results, it probably proves that DWLWIR lacks the ability to perform sensible work in the environ in which it finds itself (…)”
OR, it ‘probably proves’ that DWLWIR as a separate thermodynamic flux/transfer of energy is merely a figment of someone’s imagination; it isn’t really there at all. It, after all, is something that has never been physically detected, hence ‘observed’ to be a real physical phenomenon.
“The problem is that all of this is just in models based upon assumed facts and processes. Where is the real world empircal observational/experimental data ascertaining the work potential.”
There is no real-world empirical observational/experminental data ascertaining the EXISTENCE of a DWLWIR ‘flux’ in the first place.
The natural conclusion drawn from such an utter paucity of real observational confirmation of an assumed physical phenomenon should be that it isn’t there to begin with. But, alas, in the ‘climate world’, theory always seems to trump observation. So people will just go on assuming it’s there.
Well, it isn’t. It is but a mathematical construct, from a flawed interpretation of this particular formula:
P/A = εσ (Th^4 – Tc^4)
What you see here on the righthand side is NOT two real, separate, opposing fluxes/transfers of energy. What you see is only two opposing temperature potentials and ONE spontaneously resulting flux/transfer of energy (the lefthand side P/A, the Q, the ‘heat flux’). The righthand side of the equation is merely the mathematical operation needed to estimate the ACTUAL flux/transfer of energy by radiation between the hot and the cold object in question.
P/A (the heat flux) is always the (only) one that is actually detected directly by the instruments in a heat transfer situation and which is therefore ‘observed’ to be a real physical phenomenon. Everything else (except the temperature) is simply derived from various assumptions and computations. The starting point is always the actual flux detected, the heat.
This is exactly equivalent to two voltages (potentials) at different strengths generating ONE electric current between them, from high to low potential. There aren’t two opposing currents flowing in opposite directions, in between them making up a NET current. That’s not how the real world works. It is also equivalent to two air pressure regions at different strengths generating ONE flow of air between them, from high to low potential. There aren’t two opposing winds flowing in opposite directions, in between them making up a NET wind. That’s not how the real world works.
Why is this concept so hard to grasp when it comes to energy flux between hot and cold in a heat transfer?
Kristian 6:39am: “Why is this concept so hard to grasp when it comes to energy flux between hot and cold in a heat transfer?”
Energy transfer. The root cause is that photons don’t interact with each other.
Your macro electric current flows one way because electrons interact with each other, collide. Same for your winds, molecules collide. (On a micro level though there are vibrations&velocities both ways). You are mistaken for photons, they do not collide or otherwise interact so this eqn. for P/A you write is for two real macro photon streams not potentials.
Proof not potentials: when we look down we see the ground & deep water (UWIR real photon emittance absorbed in retina); when we look up we see the clear sky & clouds (DWIR photon emittance absorbed in retina). If our eyes aren’t placed in position, the two streams still exist in all directions we can look (a bath) – our eyes do not suddenly make the streams real. Physics does.
One thing to really be careful about is taking emissivity (epsilon) out of the parentheses as epsilon is not necessarily the same in the two objects. Your math needs to be reasonable assumption. Also, we can see the ground, water, sky whether less T or higher T than our retinas. The emittance photons are still absorbed in retina no matter the T of the emitting (radiating) object.
Photon emittance = emissivity * Planck function; for all real objects & that emittance (energy) is real, always nonzero, for every T and every wavelength.
Trick, December 13, 2014 at 10:05 am:
“The root cause is that photons don’t interact with each other.
Your macro electric current flows one way because electrons interact with each other, collide. Same for your winds, molecules collide. (On a micro level though there are vibrations&velocities both ways). You are mistaken for photons, they do not collide or otherwise interact so this eqn. for P/A you write is for two real macro photon streams not potentials.”
Trick, ‘photons’ aren’t ping-pong ball particles flying along separate highways through a radiation field. They are conceptual energy packets. You completely miss the point. The gist of my equivalence argument with the electric current and the wind was not to be found in the particles/molecules themselves, but in the difference in POTENTIAL between the two opposite ends of the transfer. There is a potential gradient through a radiation field between two surfaces at different temperatures just like there’s a potential gradient between two voltages or pressures at different strengths. That’s why the current flows. The energy in a heat transfer, be it conductive or radiative, by necessity travels DOWN the gradient, not some of it down and some of it up, only a bit more down. Same with objects (like water droplets in a water fall) in a gravity well. They by necessity travel DOWN the well, not a little bit up and a little bit down, only more down so that the NET ends up in that direction.
“Proof not potentials: when we look down we see the ground & deep water (UWIR real photon emittance absorbed in retina); when we look up we see the clear sky & clouds (DWIR photon emittance absorbed in retina).”
You know of course, Trick, that everything we SEE with our eyes is relected light originally from very hot sources. That is, if we don’t look at the sources themselves. So you think that what we see when we look at the sea or the sky is the IR photons they send out. Well, good for you. You keep believing that, Trick …
Kristian 10:52am: Concur photons aren’t ping-pong balls. However, photonic energy does travel both ways in the bath proven by looking up and down and yes, there is a NET energy transfer positive, zero, or negative. Your analogies don’t work for light as photons do not interact with each other. Very private entities while they are born (emitted), live (transmitted, scattered, reflected) and die (absorbed).
“…everything we SEE with our eyes is relected light…”
Relected? Not everything, Kristian. We see reflected, scattered and emitted light as long as not too feeble in visible. A cabbage reflects about 5% of incident light, 95% emitted. Your pupil looks dark in the mirror b/c that reflected light is blocked by your head. Looks red in a camera flash.
While you might argue some light entering our eyes has high degree polarization on clear sky days, some light is seen un-polarized also. Cloudy days are weakly polarized. I can look at then switch on an incandescent light bulb. Where is the filament photon being reflected? Photon is just emitted (born) maybe scattered a bit by the bulb material in life only to die absorbed in my retina.
Here’s another test. Turn on an electric oven range coil in darkened room. See it glow red? That’s emitted visible light. Now turn it off, wait for it to disappear from your direct sight & averted sight. Wait a few moments. Snap a picture (no flash) with your smart phone. What do you know? The range coil appears in your picture even though you cannot see it in real life. (Well, this used to work on certain film, find if still good example.) This is what I mean by feeble.
“I wanted to comment on a popular misunderstanding. This is the idea that because on average the ocean skin temperature is typically about a half a degree cooler than the sub-skin temperature, this means that energy absorbed from LWIR, which is absorbed in the first mm or so at the surface, can never be moved downwards. The idea is that because heat will not flow from cold to warm, this energy is trapped in the skin layer.
What this claim overlooks is that the skin layer is not stable. Why? Because cold water is denser than warm water. As a result, the skin layer is constantly cooling, sinking, and being replaced by warmer water. It is overturning constantly.
Now, consider what would happen if there were no downwelling IR. The skin would be much cooler because it would not be getting the IR energy which is absorbed in the skin layer. Perhaps instead of being half a degree cooler than the sub-skin layers, it would be a full degree cooler. And as mentioned above, it would constantly be cooling, sinking, and mixing downwards.
Now consider the condition with the downwelling thermal IR. The skin is still not warmer than the sub-skin. But it is warmer than it would be without the DWIR, because it has absorbed the downwelling radiant energy … and still being cooler than the underlying water, the skin water sinks and mixes with the warmer sub-skin water, taking the absorbed DWIR energy downwards with it.
I beg to dissagree.
To my understanding the above contains wrong idea heat flow. An virtual inexistent heat flow is put into ecuation.
Slowing down heat flow is not = with additional heat flow input.
It is not the heat from back-radiation flowing into the ocean, but it is the reduction of heat flow from the ocean to the surface that is mentioned in the above sentence.
This is a different process.
Also the skin is warmer than it would be without the DWIR
=> it can be easily measured and gives a good understanding of the impact on the heat flow – again through the reduction of heat flow from the ocean to the atmosphere, whenever the cool skin phenomenon is present.
As we do have a pretty good measurement of the sea surface temperature. How warmer is it getting?
As a result, despite the fact that heat only flows from warm to cold, the DWIR energy is mixed downwards by constant overturning driven by the temperature difference between the cooler skin water and the underlying warmer sub-skin water.
No DWIR energy is mixed and flows down. No heat flows against the first principle of thermodinamics. There is no heat pump at work.
It slows the heat from below to the surface.
This is the heat flow.
And as a result, the idea that the DWIR energy absorbed by the ocean is somehow trapped in the first half millimetre or so is simply not true.
It is the simple reality when looking at the diagram posted and thinking in net heat flows.
the real question is: what happens if the radiation flow is being reduced – changing the heat transfer parameters?
From where to where is that radiation net heat flow exchange that we talk about reduced? From the ocean’s surface to the next 1-2-4-5-9 meters?
Here Lars invokes the first principle of thermodynamics correctly. The heat is in the ocean, not the atmosphere. Willis has it backward because he swallows the AGW misinformation.
The misconception is yours, Willis. Your comment only shows that you forget that 20 microns is only 2/100 mm and that IR energy cannot accumulate in such a minute interval to any significant extent when such an interval is converted to water vapor and latent heat in a minute or so. But in fact, over half of the incident IR is caught in the upper TWO microns and this is converted to latent heat in seconds. The bottom line is there is no energy from incident IR left for mixing downward. The posit of such mixing is untenable in consideration of the actual physics and dimensions.
I have been asking Willis for some years to explain the physics of the top few microns of the ocean.
Unless the energy that is absorbed in the top few microns of the ocean can be sequestered to depth at a speed greater than the speed that that energy in the top few microns drives evaporation, there can be no heating, by DWLWIR, of the oceans, and yet Willis does not want to address the rate of speed at which sequestration to depth takes place..
I do think that many people overlook that we are talking about just a few microns, not many millimetres. Even in this set of exchanges, Willis (December 9, 2014 at 11:37 pm) has incorrectly referred to 1 mm without correcting matters that the absorption is in microns not 1 mm, and you point out, we are taling about a volume 1/20th or 1/30th so energy is extremely concentrated.
.
richard verney December 13, 2014 at 5:24 pm
I have been asking Willis for some years to explain the physics of the top few microns of the ocean.
Here it is for you. The downwelling IR is absorbed in the first few microns, say ~300W/m^2, simultaneously radiation is lost from the surface (~450W/m^2 for a surface at 300K) resulting in the surface being slightly cooler than the layer below it. Conduction/convection transfers heat to the surface.
http://ghrsst-pp.metoffice.com/pages/sst_definitions/sst_definitions.png
At 1 bar atmospheric pressure evaporation takes up 5 times as much energy in latent form as is required to induce it.
That being the case I cannot see how there can be any left over DWIR to warm the sea surface or reduce the rate at which energy leaves from ocean to air.
The water vapour produced from more evaporation is lighter than air and so is whisked away upward taking that additional latent heat away with it.
In order for DWIR to warm a water surface the latent heat of evaporation would need to be the same as or less than the energy required to induce that evaporation.
A cooler atmosphere can never warm a warmer ocean except when the atmosphere itself warms and reduces the ocean’s ability to cool. Get over it. Whenever you violate the second law of thermodynamics, you are wrong.
Thanks, gymnosperm. The second law of thermodynamics doesn’t come into it, because you are asking the wrong question. The question is not whether “a cooler atmosphere can warm a warmer ocean”.
The question is whether the ocean is warmer when it is exposed to the ~360 W/m2 of downwelling radiation from the atmosphere, or if it would be warmer if it were exposed to the ~ 3W/m2 of radiation from outer space … the answer to that is quite obvious, and doesn’t involve any violation of the second law.
As I mentioned above, when you come in from the outside in the winter, your coat is much colder than you are … and yet you wear it to keep warm. Does this mean that a cold object (the coat) is warming a warm object (the person)?
Absolutely not, that would be a violation of the second law. We wear a coat because it leaves us warmer than the alternative of no coat.
And similarly (although for very different physical reasons) the GHGs in the atmosphere leave the world warmer than the alternative of no GHGs. Doesn’t mean a cold object warms a warm object, that’s not possible. It just means it leaves it warmer than having no GHGs.
w.
–The question is whether the ocean is warmer when it is exposed to the ~360 W/m2 of downwelling radiation from the atmosphere, or if it would be warmer if it were exposed to the ~ 3W/m2 of radiation from outer space … the answer to that is quite obvious, and doesn’t involve any violation of the second law.–
I don’t think it’s obvious.
But if you looking out a window of ISS into the blackness of the universe, I don’t your face would feel any cooler as compared looking out window in a house on Earth. Nor if touched the glass in space would it be any colder than a window on Earth.
And water is gas or solid in the vacuum of space. Or water would rapidly freezing and will cool to about -150 C rather quickly- but that is cooling from evaporation.
But for water near 0 C it does not need a lot of pressure to remain liquid. Or a strong plastic bag could withstand the pressure. So if had a clear strong mylar [other kinds of plastics might also evaporate] bag and filled it with water which was 5 C it will not instantly freeze.
And I am not sure how long it would take to freeze. It could take hours.
Or another thing water will form into a sphere if not touch something in microgravity. So one have say mylar bag 1 meter in diameter and put a 10 cm diameter sphere of water in the middle of it.
Put it in environment with say 2 psi- so bag of air will have 2 psig in a vacuum and have water sphere is in to middle of it.
And what happens?
I would guess the air in the bag would have to get to 0 C before the water could freeze. And I would guess it would take more than 3000 seconds before any ice formed on the outside of the sphere. And it would form on the outside of the sphere, first.
Anyone want provide another guess?
Or maybe someone done something like this already.
Willis, you (&warmists) keep on replacing isolation with a source of heat.
These are 2 different things and behave differently.
It is possible to replace one with the other – it may give almost same result for limited term, small variations, but will run into errors on long term or higher variations.
Repeating over and over again that isolation does help keep things warm will not fix the fact isolation is not a source of heat itself.
[“Isolation” or “Insulation” or (as I suspect) “Insolation” ? .mod]
Willis Eschenbach, December 13, 2014 at 10:21 pm:
“The second law of thermodynamics doesn’t come into it, because you are asking the wrong question. The question is not whether “a cooler atmosphere can warm a warmer ocean”.
The question is whether the ocean is warmer when it is exposed to the ~360 W/m2 of downwelling radiation from the atmosphere, or if it would be warmer if it were exposed to the ~ 3W/m2 of radiation from outer space … the answer to that is quite obvious, and doesn’t involve any violation of the second law.”
This is saying the exact same thing, Willis. The warmer surface ends up having a higher temperature solely because of your 360 W/m^2 energy transfer from the cooler atmosphere. That means you transfer energy from a cool system to a warm system specifically to raise the temperature of the warm system. A 2nd Law violation could hardly be any more obvious than that …
“(…) when you come in from the outside in the winter, your coat is much colder than you are … and yet you wear it to keep warm. Does this mean that a cold object (the coat) is warming a warm object (the person)?
Absolutely not, that would be a violation of the second law. We wear a coat because it leaves us warmer than the alternative of no coat.”
Yes, because it insulates us, making LESS ENERGY LEAVE our body per unit of time, almost exclusively by impeding convective/evaporative loss.
“And similarly (although for very different physical reasons) the GHGs in the atmosphere leave the world warmer than the alternative of no GHGs. Doesn’t mean a cold object warms a warm object, that’s not possible. It just means it leaves it warmer than having no GHGs.”
No, this is not what’s being claimed, Willis. You claim that the extra energy INPUT from these gases in our cooler atmosphere to the warmer surface is what makes the surface temperature 289K (from [165+345-112=] 398 W/m^2) rather than 255K (from 239 W/m^2) or 232K (from 165 W/m^2). The direct transfer of energy from a cooler object to a warmer object alone makes the temperature of the warmer object rise in absolute terms. This is a definite violation of the 2nd Law.
Insulation doesn’t work by the cooler layer feeding the heated object with more energy to warm it. It works by reducing the energy going OUT from the heated object per unit of time.
There’s a very important distinction to be made between these two scenarios. For the surface of the Earth, if you want to claim that it warms beyond pure solar radiative equilibrium by virtue of the “back radiation” from IR-active gases in the atmosphere, then it is this ‘cool’ atmospheric energy that piles up at/below the surface. Because there is no obstruction at any point of the outgoing IR from the surface. It is always free to leave.
If you instead were to claim that the atmosphere forces the surface to warm some more simply from having a higher temperature potential than space, making the transfer of energy from the warmer surface to the cooler atmosphere smaller per unit of time, then it is the ‘hot’ incoming solar energy that piles up at/below the surface. (Which is what actually happens in the real world.) Because now the absorbed solar energy isn’t able to escape back out again as fast as before. This was never the case in the first scenario.
Conclusion: You can’t transfer ‘cool’ energy to a warm object for energy gain, meaning an absolute increase in the warmer object’s ‘internal energy’ [U] and thus temperature [T]. Such accumulation with such a direct result must (by the laws of thermodynamics) come from a source hotter than the warm object. Because this would constitute a ‘heat transfer’ …
So you see, the atmospheric warming EFFECT is real. It does insulate the heated surface. But the “back radiation” (DWLWIR) EXPLANATION of how this effect comes about is wrong. Because it clearly violates the laws of thermodynamics. The EFFECT itself doesn’t. The “back radiation” EXPLANATION of it does.
[“Isolation” or “Insulation” or (as I suspect) “Insolation” ? .mod]
Thanks mod, sorry for that – “thermal insulation” should have been there, not “isolation”
http://en.wikipedia.org/wiki/Thermal_insulation
Kristian December 14, 2014 at 6:04 am
Willis Eschenbach, December 13, 2014 at 10:21 pm:
Conclusion: You can’t transfer ‘cool’ energy to a warm object for energy gain, meaning an absolute increase in the warmer object’s ‘internal energy’ [U] and thus temperature [T]. Such accumulation with such a direct result must (by the laws of thermodynamics) come from a source hotter than the warm object. Because this would constitute a ‘heat transfer’ …
You can via radiation because radiation transport does not depend on a temperature gradient.
A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.
Kristian 6:04am: “Conclusion: You can’t transfer ‘cool’ energy to a warm object for energy gain..”
Yes, you can. So inaccurate conclusion. Physics allow this, no 2LOT violation as you say since our warmer eyes can see cooler ice cubes absorbing their emitted radiant energy. Our warmer retinas absorb the” ‘cool’ “energy from the ice just fine. Focused rays thru a lens of ice can ignite flammable substance. Energy transfers both ways; 2LOT: net energy is one way to always increase entropy in real objects.
Phil., December 14, 2014 at 7:23 pm:
“You can via radiation because radiation transport does not depend on a temperature gradient.”
Yes, I know that in you people’s magical, pink little bubble world, radiation is free to violate the Laws of Thermodynamics, that it can do wonders and miracles. Fine. But I’m afraid your magical, pink little bubble world isn’t the real one. Out here in the real world, radiative transfers and their resulting effects must comply with the Laws of Thermodynamics before anything else, just like conductive transfers and their resulting effects have to. In the real world, in nature, it is not allowed for an energy transfer from a cool place to a warm place to make the temperature of that warm place rise. It simply doesn’t happen. If you observe something where it might LOOK like it does, it is your interpretation of what happens that’s wrong. Case in point:
“A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Your last sentence here describes a perfect violation of the 2nd Law of Thermodynamics, Phil. I’m surprised (or not) that you don’t realise yourself. It is pretty obvious. It’s in the very words you use. If you transfer energy (in this case, by radiation) from a cooler object to a warmer one … to HEAT it, then you’ve transferred HEAT to it. This cannot happen in nature.
The EFFECT you describe above is indeed real enough. Your “heating by back radiation” EXPLANATION of it, however, is blatantly wrong. Because it demands a direct violation of the 2nd Law.
Trick says:
A very precise and elegant example Trick
It is easy to be confused by the second law of thermodynamics. A net transfer of energy from a cold object to a warm would be a violation, but energy flows in both directions, only more from hot to cold than the other way.
/Jan
Kristian December 15, 2014 at 3:00 am
Phil., December 14, 2014 at 7:23 pm:
“A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Your last sentence here describes a perfect violation of the 2nd Law of Thermodynamics, Phil. I’m surprised (or not) that you don’t realise yourself. It is pretty obvious. It’s in the very words you use. If you transfer energy (in this case, by radiation) from a cooler object to a warmer one … to HEAT it, then you’ve transferred HEAT to it. This cannot happen in nature.
No, it just shows your ignorance of the correct application of the Laws of Thermodynamics to radiational heat transfer, I suggest you read up on it, ‘Hottel and Sarofim’ would be a good start.
Two bodies in radiational equilibrium with each other are both radiating proportional to the fourth power of their surface temperature. Raise the temperature of one of the bodies and the temperature of the other will rise accordingly, this does not violate any law of thermodynamics! Thus in the thermocouple example replacing a surface at 300K by a surface at 1000K (the radiation shield) causes the ThC to be hotter and register a higher temperature.
The EFFECT you describe above is indeed real enough. Your “heating by back radiation” EXPLANATION of it, however, is blatantly wrong. Because it demands a direct violation of the 2nd Law.
There is no violation of the 2nd Law, since you think the effect is not the result of radiational exchange, which has long been the accepted reason, perhaps you could explain the phenomenon.
See here for example:
http://eyrie.shef.ac.uk/eee/cpe630/comfun2.html
Phil., December 15, 2014 at 8:36 am:
“No, it just shows your ignorance of the correct application of the Laws of Thermodynamics to radiational heat transfer, I suggest you read up on it, ‘Hottel and Sarofim’ would be a good start.”
Sorry. I cannot but laugh! Phil, there are no concessitons made in the Laws of Thermodynamics for ‘radiational heat transfer’. You wrote: “Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Again, there is nothing wrong with the EFFECT you describe. It is real enough. But you EXPLAIN it like this: “… the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
No, Phil. No. No! NO!!! Read your own words again. And again. Until you understand.
How is it even possible for a grown person to not see what’s wrong with this picture!? You’re explicitly stating a violation of the 2nd Law here. And you just go on to argue that I am ignorant of the ‘correct application’ of this law when it comes to ‘radiational heat transfer’. HEAT is HEAT whether it is transferred by conductive, convective or radiative means. And the transfer of HEAT from one system to another has a very distinct effect indeed. Do you know what it is, Phil? And this effect, in nature, is only allowed to result in ONE particular distributional pattern. Can you guess which one, Phil?
It makes no difference what you call it. If you transfer energy to a system to make it warmer than what it was before, you have transferred HEAT to it (if not ‘work’). And HEAT cannot spontaneously transfer from a cold to a hot place. Radiation or no radiation.
Look, if you postulate two opposing ‘fluxes’ in a radiative heat transfer between two surfaces, the vector sum of which make up the ‘net radiation’, the net flow or transfer of energy between them, then it is your NET flow (which is equal to the radiative HEAT) which is doing the heating and cooling. Nothing else. No other ‘flux’. This is what makes it the HEAT. Get it, Phil?
And the ‘heating’ invariably occurs in the cooler object (from energy GAIN), the ‘cooling’ invariably in the warmer object (from an equal energy LOSS). Because HEAT always spontaneously moves from hot to cold, never the opposite way.
This is the REAL, the ACTUAL transfer of energy between the two objects at different temperatures in a heat transfer.
In the real world, not in the conceptual world, the HEAT FLUX is indivisible. All there is. It is ONE flow, one transfer of energy. There is no way you can physically split it into two separate, oppositely flowing streams of energy. The HEAT itself is all you’ll ever register. Meaning, the individual ‘hemifluxes’ that conceptually (according to the archaic (caloric theory-derived) bidirectional principle) make up the ‘net flux’ (the HEAT), are not themselves real, separate fluxes/transfers of energy. They are purely mathematical constructs (see below).
If you claim (as you definitely seem to be doing) that both of these are real and BY THEMSELVES AND SEPARATELY would achieve the same result as the ‘net’ of the two (the HEAT), namely heating/cooling of the two systems, then you are effectively saying that ‘HEAT GOES BOTH WAYS AND HEATS IN BOTH DIRECTIONS’, only more goes from hot to cold than from cold to hot. Which is clearly absurd and at odds with reality. The Laws of Thermodynamics do not allow it.
What’s the point in your world in emphasizing the ‘net flux’ (the heat) as being the only one that matters to the Laws of Thermodynamics, if there is no difference between the result it gives and the result the two conceptual hemifluxes supposedly comprising it gives? Then they are all ‘heats’.
“Two bodies in radiational equilibrium with each other are both radiating proportional to the fourth power of their surface temperature. Raise the temperature of one of the bodies and the temperature of the other will rise accordingly, this does not violate any law of thermodynamics! Thus in the thermocouple example replacing a surface at 300K by a surface at 1000K (the radiation shield) causes the ThC to be hotter and register a higher temperature.”
Yup, because you raise the ‘temperature potential’ of the cooler opposing surface, thus creating a gentler potential gradient away from the hotter (and externally heated) object. That’s what you see here:
P/A = e s (Th^4 – Tc^4)
On the righthand side you see two opposing ‘temperature potentials’, the potential being the HEAT FLUX each object, thermally isolated from the other, would emit to a perfect vacuum at 0 K. On the lefthand side you see the ACTUAL flux/transfer of energy between the two, the HEAT FLUX, its intensity determined by the difference in the two temperature potentials. The righthand side of this equation simply shows the mathematical operation needed to estimate the actual flux in the heat transfer.
“… since you think the effect is not the result of radiational exchange, which has long been the accepted reason, perhaps you could explain the phenomenon.”
Explained above. There is a change in the potential gradient through the radiation field between the heated object and its surrounding layer. Therefore there will be less energy moving per unit of time from this hot object to the nearby cooler one. The transfer of energy still (always) goes only one way, from hot to cold, but when cold becomes not so cold as before, the transfer is reduced. Simple as that.
Again, if your EXPLANATION of an observed physical effect ends up violating the Laws of Thermodynamics, then it’s wrong. Your explanation does end up violating the Laws of Thermodynamics. Mine doesn’t.
Hehe, that novel term ‘concessitons’ was of course supposed to be ‘concessions’.
Kristian December 15, 2014 at 1:58 pm
Phil., December 15, 2014 at 8:36 am:
“No, it just shows your ignorance of the correct application of the Laws of Thermodynamics to radiational heat transfer, I suggest you read up on it, ‘Hottel and Sarofim’ would be a good start.”
Sorry. I cannot but laugh! Phil, there are no concessions made in the Laws of Thermodynamics for ‘radiational heat transfer’. You wrote: “Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
Again, there is nothing wrong with the EFFECT you describe. It is real enough. But you EXPLAIN it like this: “… the ThC has been heated to a higher temperature by radiation from an object cooler than itself.”
No, Phil. No. No! NO!!! Read your own words again. And again. Until you understand.
I do understand, unfortunately you continue to spout a load of pseudoscientific garbage which bears no resemblance to radiational heat transfer and the Second Law!
In the real world, not in the conceptual world, the HEAT FLUX is indivisible. All there is. It is ONE flow, one transfer of energy. There is no way you can physically split it into two separate, oppositely flowing streams of energy. The HEAT itself is all you’ll ever register. Meaning, the individual ‘hemifluxes’ that conceptually (according to the archaic (caloric theory-derived) bidirectional principle) make up the ‘net flux’ (the HEAT), are not themselves real, separate fluxes/transfers of energy. They are purely mathematical constructs (see below).
Far from being ‘mathematical constructs’ they are two separate flows, electromagnetic radiation flowing in both directions.
In the case of the thermocouple I cited you’ll see bright yellow light emitted in all directions which can be measured. Put the shield in place and it also emits yellow light in all directions, INCLUDING TOWARDS THE ThC, which can also be measured. Net energy flow as per the Second Law flows from hot to cold, the ThC will be slightly brighter than the shield. According to your weird theory the light emitted in the direction of the ThC somehow ceases to exist.
Phil., December 16, 2014 at 6:36 am:
“I do understand, unfortunately you continue to spout a load of pseudoscientific garbage which bears no resemblance to radiational heat transfer and the Second Law!”
This is a non-argument, Phil. It is just saying “DOES TOO”! You are not arguing. Why should the 2nd Law not apply to radiative heat transfer? Heat cannot move spontaneously from cold to hot. This is a thermodynamic absolute. If you know the thermodynamic definition of ‘heat’, then you know what it does. And you would know that a transfer of energy from cold to hot could never do in nature what you claim it does. Because it would constitute a transfer of HEAT.
You expect both of your ‘hemifluxes’ making up the NET, the ‘heat’, to give results as if they themselves were heat fluxes. Sorry. No go.
“Far from being ‘mathematical constructs’ they are two separate flows, electromagnetic radiation flowing in both directions.”
No, they are not. I too can play this game, Phil.
You just continuing to postulate it as truth doesn’t make it so.
<em"In the case of the thermocouple I cited you’ll see bright yellow light emitted in all directions which can be measured. Put the shield in place and it also emits yellow light in all directions, INCLUDING TOWARDS THE ThC, which can also be measured."
No, it can NOT be measured. It is CALCULATED. From the HEAT FLUX and TEMPERATURE. Are you being willfully obtuse?
“Net energy flow as per the Second Law flows from hot to cold, the ThC will be slightly brighter than the shield.”
Yes, you call it ‘net energy’. And your ‘net energy’ is all you’ll ever register, detect. It is the HEAT FLUX. Everything else you will have to CALCULATE based on your preconceived assumptions.
“According to your weird theory the light emitted in the direction of the ThC somehow ceases to exist.”
It is not a weird theory, Phil. It is reality. It is what we actually observe. The ‘weird theory’ is YOUR interpretation of this reality.
You are a person who truly believes that a transfer of energy from a cold to a hot place will raise the temperature of the hot place, not just in relative, but in absolute terms. This means you don’t understand the first thing about the basic Laws of Thermodynamics. And hence you are a person whose arguments cannot be taken seriously, Phil. I’m sorry.
Phil. says:
December 14, 2014 at 7:23 pm
You can via radiation because radiation transport does not depend on a temperature gradient.
A classic illustration of this in action is a thermocouple in a flame. Heat is transferred to the ThC from the flame by convection, heat is lost by radiative heat transfer, in an open flame ‘back radiation’ from the surroundings is received by the ThC resulting in a measured temperature about 100-200K below the flame temperature. Put a quartz radiation shield around the ThC and the ‘back radiation’ comes from a surface which is hotter than the original surroundings, the ThC temperature is now much closer to the flame temperature, the ThC has been heated to a higher temperature by radiation from an object cooler than itself.
Phil, you cannot transform insulation in a source of heat. This is why your ThC will never get warmer then the flame, no matter how much insulation you put around it.
If the insulation would be a source of heat as in your pink universe, then the ThC could get warmer then the flame.
It does not.
No matter what backradiation construct is done around it,, it will not get warmer then the source of heat = the flame. because insulation is not a source of heat, but a reduction in the heat flow.
Jeez.
Willis and Kristian.
I respect your efforts here and elsewhere and both of you are right in certain respects but nonetheless you both miss the essential issue.
Once convective ascent begins it requires an equal and opposite convective descent.
Convective ascent takes energy away from the surface that would otherwise have radiated to space and in the process converts kinetic energy (heat) to gravitational potential energy (not heat).
Convective descent the converts gravitational potential energy (not heat) to kinetic energy (heat) to the surface that can then be radiated to space.
Convective descent places adiabatically warmed air above the surface which reduces or eliminates (in an inversion) the lapse rate slope so that convection is in turn suppressed or eliminated.
Reducing or eliminating convection allows incoming solar radiation to raise surface temperature above that predicted by the S-B equation.
The mass of an atmosphere is the factor that allows convective ascent and descent NOT the radiative capability of that atmosphere.
Radiative fluxes between atmosphere and surface are a consequence of atmospheric mass subjected to external insolation and not a cause of the surface temperature.
The mass of an atmosphere acts exactly like a glass greenhouse roof in the descending convective phase.
The descent of adiabatically warmed air dissipates clouds so as to allow more solar radiation to reach the surface. That is equivalent to the transparency of a glass greenhouse roof.
The adiabatically warmed air above the surface reduces convection in exactly the same way as does a solid greenhouse roof.
The greenhouse effect was always an accurate description of how the mass of an atmosphere in adiabatic descent inhibited convection from the surface and thereby raised surface temperature above S-B.
The radiative theory is a complete dead end which offends basic thermodynamics.
That mass based description is consistent with Willis’s own thermostat hypothesis because it explains how the mass of an atmosphere uses the adiabatic warming of descending air in one location to trigger emergent convective phenomena in another location.
The observed global pattern of descending surface high pressure cells and ascending surface low pressure cells is the process in operation.
Change the proportion of GHGs in the atmosphere and all one does is change that circulation for a zero net change in surface temperature and since it is a matter of mass rather than the proportion of GHGs we could never measure the change from our emisssions..
–Stephen Wilde
December 14, 2014 at 10:00 am
Jeez.
Willis and Kristian.
I respect your efforts here and elsewhere and both of you are right in certain respects but nonetheless you both miss the essential issue.
Once convective ascent begins it requires an equal and opposite convective descent.
Convective ascent takes energy away from the surface that would otherwise have radiated to space and in the process converts kinetic energy (heat) to gravitational potential energy (not heat).
Convective descent the converts gravitational potential energy (not heat) to kinetic energy (heat) to the surface that can then be radiated to space.
Convective descent places adiabatically warmed air above the surface which reduces or eliminates (in an inversion) the lapse rate slope so that convection is in turn suppressed or eliminated.–
[Agree to all above.
And then I wonder about the wording which follows:]
–Reducing or eliminating convection allows incoming solar radiation to raise surface temperature above that predicted by the S-B equation.–
I would instead say the “Reducing or eliminating convection” is the point when S-B equation applies to the surface. Or the convection process dominates before this point is reached.
Or the atmosphere is joined to the surface.
Or surface is a VW bug and atmosphere is massive trailer it’s pulling. And/or one could say as analogy the S-B equation is measuring the weight of VW bug but not the weight of the trailer.
Or less of an analogy, the kinetic energy of VW bug and not the trailer
Or another way to say it, is only once one has “Reducing or eliminating convection” then can the S-B equation starts to appear to work. Or the surface radiate less energy to space until the point where one is at the point of “Reducing or eliminating convection”.
Or S-B equation measures or concerned about a surface and when there is an atmosphere “the surface” includes the atmosphere which warmed and cooled each day. And this “thick surface” radiate mostly at it’s bottom and not much at the top of the “surface” and convection process is going to make the top and the bottom the same energy [per molecule of gas- it has same kinetic energy at bottom as at the top- and difference temperature due to difference of air density].
Though perhaps not sure what you referring to by S-B equation, because basically if stop convection losses then one can get the temperature predicted by the S-B equation. Such as
with sealed boxes or solar ponds- which work by stopping convection of water heated by sunlight, and so thereby get the max temperature predicted- a couple feet under the water of the solar pond’s surface.
An irony of all this is that Willis and I were debating from exactly opposite sides of the first law not so very long ago when he was claiming adiabatic warming from the mass of the atmosphere was a violation thereof. The difference there was that adiabatic warming takes place outside the realm of radiation. There are probably “outside the realm of radiation” effects hiding in these radiation budgets, but lets put all the cards on the same table.
Can’t see how that formats until I press post, but assuming it’s readable you can see that there is little difference between the ocean budget and the planetary budget and if one is a bit generous they are basically all the same. We are talking about a huge accounting problem on the scale of one TSI, which happens to be what atmospheric back radiation amounts to.
To my mind it all boils down to what I’ve annotated as the photon food fight. The circularity of this process is also shown in Kristian’s graphic. It’s a bit of a stretch but this process can be thought of as a cold plasma. Photons flung frenetically. The one TSI is absorbed by the ocean skin, but it does not warm it except when latent losses cool the surface to below atmospheric emission temperature. It just keeps it from cooling below the very same atmospheric emission temperature.
In this sense the average emission temperature of IR resonating atmospheric gasses, with the same power as the sun, controls ocean temperature. Yet we come full circle because the atmosphere derives 75% of its energy from the ocean. Like I said, a plasma…
Let me try this again. Here’s a planet in space, heated by an internal nuclear reaction that delivers 235 W/m2 at the surface. We’ll assume it is a blackbody.


This planet is at equilibrium. The natural reactor in the core of the planet is generating energy that at the planet’s surface amounts to 235 W/m2. It is radiating the same amount, so it is neither warming nor cooling.
Now, imagine that without changing anything else, we put a blackbody steel shell around the planet. The next graphic shows that situation, with one side of the shell temporarily removed so we can look inside.
Note that for the sake of the graphic, I’ve greatly exaggerated the distance from the planet to the shell. In fact it’s lets say a metre above the surface, so there’s only about 0.0001% difference in the areas, which for the sake of this first principles analysis we can ignore.
Now, note that in the second graphic, there is additional radiation striking the surface of the planet, radiation that was NOT present there in the first graphic. This inward-bound radiation exists because the shell is warmed by the radiation striking it from the inside.
So my question is …
Does the new radiation being absorbed by the planet leave the planet a) warmer, b) cooler, or c) the same temperature as before we added the shell?
Please start by explicitly saying whether you think the answer is a), b), or c) … and then explain why.
Best to all,
w.
How about this?
..
http://www.principia-scientific.org/the-relentless-pseudo-science-of-wuwt.html
David Socrates December 15, 2014 at 4:35 pm
David, thanks for the link to the discussion of my ideas over at Principia Anti-Scientifia. I can do no better in answer to your (and their) questions about the Steel Greenhouse than to quote what Dr. Robert Brown said in response to their ascientific claims over at your link:
Also look at what Joel Shore (jshore) has to say at their post. Look, I’ve disagreed with Dr. Brown a few times in the past … and almost always I was wrong. He’s a very bright guy. And Joel Shore someone who I’ve disagreed with any number of times … but he’s also a very bright guy. And unlike me, they are both physicists, Joel (I think) in the private sector and Robert teaches physics at Duke.
So when we all agree, it’s not that often, and because we do in this case … well, you might profitably consider the arguments that all of us are making in favor of the Steel Greenhouse not violating any laws in any fashion.
My restatement and clarification is here.
All the best,
w.
You can wrap the core in as many shells as you wish, the amount of energy radiated will remain the same. That’s a result of the first law of thermodynamics.
..
However, if each shell is 1 meter in height, eventually the 235 w/m2 will drop, as the area of the shell’s outer surface increases.
David, was there a part of this that you didn’t understand?
w.
If you are looking from the outside, the net outflow is 235 w/m (unless the surface area is increasing due to the shells)
…
Where are you placing your thermometer?
…
What happens inside the “iron shell” doesn’t really matter, the overriding issue is the 1st law of thermodynamics as viewed from the OUTSIDE.
..
Remember, the Earth is a closed thermodynamic system when viewed from the TOS
..
http://www.bluffton.edu/~bergerd/nsc_111/thermo2.html
David Socrates December 15, 2014 at 5:16 pm
Thanks, David. The temperature is taken at the planetary surface.
The overriding issue is surface temperature of the planet … because that’s where we live.
w.
Then tell us….what are the properties of the “iron sphere”….does it allow the transfer of energy in either direction without impeding it, or does it have an insinuative value.
You see, the core of the sun is at muti-milions of degrees C, bu the surface is just roughly 5800K The problem you have with your “iron core” model is that temperature is not a reliable measure of the flow of energy. I suggest you re-phrase the question as……”Is the flow of energy at the surface of the planet less, more or the same as the flow at the outer surface of the iron sphere”
The problem you have is you are focusing on temperature and forgetting to model the flow of energy.
In other words, the question you ask is irrelevant.
–So my question is …
Does the new radiation being absorbed by the planet leave the planet a) warmer, b) cooler, or c) the same temperature as before we added the shell?–
Any such planet could be considered to have shells before it’s radiated thru the atmosphere to this blackbody shell.
This added shell will not heat anything on the surface. It will not melt ice of a puddle and it will increase evaporation of the water.
Without the blackbody shell one could radiate heat so you make something which is cooler. Or one insulate against the heat from the surface and radiate something into a 2 K universe, and thereby passively cool something to say 100 K. So shell stops you from doing this- or you would have to get on the other side of the shell to do this.
–Please start by explicitly saying whether you think the answer is a), b), or c) … and then explain why.–
C.
Though it’s eliminated the possibility of cooling things by passive cooling. Though active refrigeration is about heat lost to convection of air, and so doesn’t stop this.
How about if half the world had geothermal heat.
The blackbody shell would help heat the side of planet without the geothermal heat- but not by much. And atmosphere would probably add as much heat to cool side as the blackbody shell
would.
Noooooooooooooooooooooooooooooooo! Not the steel shell again! Lol. How many comments did that post get?
Let me try to explain where people seem to be getting stuck. It is true that heat only ever flows from warm to cold. However, heat is the NET of the individual flows. Here’s an example from another arena. Suppose that I owe you a hundred bucks, and you owe me seventy five dollars. We decide to settle the debt. Here’s two views of the situation:

Now you can see that both of the situations are the same. One shows the individual flows, and the other shows the net flows. Now, the net flow of money goes from me to you. But it is identical to the result of considering the individual flows.
Now, if you didn’t owe me the $75 then I’d end up seventy five bucks richer …
Here’s the thing. The situation regarding radiation heat flows is IDENTICAL to the flows of money. If we have two planets radiating in both directions, one radiating 100 W/m2 and the other radiating 75 W/m2, there is a net flow of 25 W/m2 from the warmer to the cooler.
And if there were no 75 W/m2 flow from the cooler to the warmer planet, it would end up warmer by that amount … and there’s no violation of the Second Law either way.
w.
Well, Willis, you ignore the physics of the sea surface and the IR absorbency characteristics of the same. The sea surface absorbs IR from CO2 in the top 3 microns.
This is where your attention should be focused but for some reason you can’t do that. You go off on a tangent.
mpainter, perhaps that’s what YOU should be focused on. I focused on that earlier in the post. At this point I’m trying to get past the common misconception that a poor person can’t make a rich person richer than they would be without the poor person.
Which is the same as saying that the Earth’s surface is warmer because of the existence of the GHGs in the cool atmosphere than it would be without those GHGs.
So no, it’s not a tangent. It’s a different part of the same discussion.
And yes, the sea surface absorbs the DWIR in the skin … and that ends up leaving the entire mixed layer warmer than it would be without the DWIR..
w.
There is no mixing of the micro layer, Willis. That is the whole point. Has to do with rheology and the surface characteristics of water. And there is no conduction downward because of the temp. profile of the sub skin layer (under insolation, that is. The profile alters at night).
This is where the rubber meets the road but you are up in the wild blue yonder.
Are the sentences preceded by an ellipses bass ackwards ?
“If we have two planets radiating in both directions, one radiating 100 W/m2 and the other radiating 75 W/m2, there is a net flow of 25 W/m2 from the warmer to the cooler.”
We know of no planets which do anything like this.
We have two notable binary planet-like bodies. Earth and the Moon. And Pluto and it’s largest moon.
We also have some very large planets- the gas giants. And they have comparatively little moons.
So on these comparatively little moons, which can be a big as our moon, their gas giant may occupy a large part of their sky. And doubtful such large visibly apparent bodies are radiating much energy at this moon and far less likely the moon as radiating anything significant at the gas giant.
Take Saturn and it’s moon, Titan.
Saturn is 58,232 km radius. Or 116,464 km diameter
Earth is 6,371 km radius. Or 12,742 km diameter
http://nssdc.gsfc.nasa.gov/planetary/factsheet/saturnfact.html
And our Moon is 3475 km in diameter or 3 to 4 times smaller than Earth..
A thumb held at arm reach blocks the Moon, though from the lunar surface
your thumb doesn’t block Earth.
And replaced Saturn with Earth, you need to hold up something like a basketball to block it.
But Titan is about 1.2 million km from Saturn [Our Moon is .38 million km from Earth].
Saturn from it’s moon, Titan, looks about 3 to 4 times bigger than Earth from
our moon.
[Or about 10 times larger than our Moon looks like from Earth.]
And from Saturn looking at Titan it’s about 1/3 smaller and a lot dimmer than
our Moon looks like as observed from the Earth.
And blackbody temperature of Saturn: 81.1 K
And Earth is 254 K.
http://nssdc.gsfc.nasa.gov/planetary/factsheet/saturnfact.html
Or Earth will warm our Moon more than any other body, and our Moon warms Earth more
than any moon warms [radiantly] any other planet.
And it’s not in the range of more than 10 watts per square meter [in either direction]
Here is what the rGHE/AGW proponents claim: You can just ADD the DWLWIR ‘flux’ as an extra INPUT of energy to the surface, as an addition to the original solar heat flux, thus directly creating extra warming (as if they were two of a kind, as if they were both heat fluxes); 165 W/m^2, 232K >> [165+345-112=] 398 W/m^2, 289K.
If I get 100$ from my bank and then hand them all to my friend, upon which he hands 90 of them straight back, then I end up with 90 dollars.
The rGHE “back radiation” argument then goes as follows: I get 190$ IN, but give away 100$, so end up with 90$. The 190 dollars IN are counted like this: 100$ from the bank + 90$ back from my friend; the 100 dollars OUT are simply the 100$ I hand over to my friend.
In this world view it would thus seem that there is 190$ in circulation. But we all know that there is only the 100$ originally from the bank available.
The rGHE “back radiation” hypothesis creates extra energy out of nothing. Just to make their preconceived radiative (Stefan-Boltzmann) numbers add up. There simply is no extra radiative INPUT of energy from the atmosphere to the surface. No more than there being a conductive or evaporative (latent) input of energy from the atmosphere to the surface.
Willis
I am very pleased to see that you have returned to this article and provided your further views. Many of these are a restated of the views that you have expressed before in the past on other articles that have raised similar issues.
However, your response is not particularly helpful, since I suspect that we all understand the theory, and what is claimed of the theory. But that is not the issue. To just state the theory does not in any way prove what is claimed of the theory is in practice, correct.
The question is, does all this DWLWIR have the ability to do real work in the environ in which it finds itself? Just repeating the theory, and what is claimed of it, does not answer this; what you say is theoretical, and theoretical only, whereas what is needed is empirical observational data of real work done.
As far as I am aware, no one is doubting the measurements (subject to errors factors), but rather questioning what does this do. Whilst not an exact analogy, I can measure 12 volts on a flat car batter, but as soon as I seek to draw off some load, the voltage may drop, in the limit, to nil. There is a signal of 12 Volts on the flat battery, but in practice it does not have the ability to do any work, not even to run the indicator.
I do not doubt for one momemet that we can measure DWLWIR, but that does not mean that it does work in the environ that it finds itself. It may be a signal incapable of performing sensible work, and this is why no engineer has sought to tap into this power source which would solve the world’s energy demands in a clean/green manner.
As I have been saying to you for years, what is going on in the top microns of the oceans? What are the physical processes involved and at what rate are they handling energy? I want to know the physics and how energy is moving around.
As I say, may be there is some form of photonic exchange at the boundary surface layer. This would therefore be at a molecular level and if this is what is happening DWLWIR never enters the ocean. If that is the case, it would appear that it does not heat the ocean, and the present claims by teh warmists (which is the subject of Bob’s article0 is a non starter. .
Alternatively, DWLWIR is absorbed in accordance with its optical absorption characteristics which means that at about 200 W/m2 to 300 W/m2 is absorbed in just 3 microns (and this is on average figures for DWLWIR, it will be gigher in the tropics). That is a heck of a lot of energy concentrated in a very small volume of water, and unless this energy is quickly dissipated to volume, it would drive copious evaporation (which we do not see). Now what happens to that energy?
In the past you have suggested it is mixed by the action of wind, the action of waves and ocean over turning, but these are slow mechanical processes, and ocean over turning may be diurnal at that. I have severe reservations that such slow mechanical processes can sequester to depth all this energy at a rate fast enough to prevent the rapid and copious evaporation which would occur from the top microns if there is some 200 to 300 W/m2 of energy, capable of doing real work, absorbed in these microns. See my comment upon sea states at richard verney December 13, 2014 at 2:12 am. which considers the problems caused by sea conditions of say BF3 and less, or for that matter BF 8 and above.
To try and assess how effective the sl;ow mechanical processes that you invoke for mixing the energy that you claim is going into the oceans and preventing the oceans from freezing (ie., the slow mechanical processes of wind, waves and ocean over turning), I suggested that you should look at crater lakes (ie., lakes in caldera) which are usually protected from wind such that there is little mixing of the top layer through the action of wind and waves.
I also suggested that you should consider dew that lingers all day in the shady side of a hollow om a still winters day, yet which is burnt off in just an hour or so on the sunny side of the same hollow. The sunny side and shady side of the hollow are exposed to the same environmental conditions save other than sunshine, yet solar energy can burn off dew in hours and yet DWLWIR cannot do so all day even though the total DWLWIR over the course of a day is greater than the energy that the sunny side of the hollow received in just an hour or so of sumn up.
On a still winters day, there is no mixing of DWLWIR that is absorbed by the dew by the slow mechanical actions of wind and waves since those processes are not present. See my comments at: richard verney December 12, 2014 at 6:46 pm and richard verney December 13, 2014 at 1:30 am
Why not add to the equation ice. In ice there is no mixing (by wind, waves, ocean overturning) of DWLWIR that is absorbed in the first few microns of the surface that is exposed to DWLWIR. So why does ice not quickly melt during the night on a still night when the air temperature is just below freezing. Why os there not a pool of liquid water in the centre of the ice slowly spreading outwards?
I await your comments on the real world that we inhabit.
richard 5:56pm: “The question is, does all this DWLWIR have the ability to do real work in the environ in which it finds itself?….So why does ice not quickly melt during the night on a still night when the air temperature is just below freezing….await…comments on the real world that we inhabit.”
Yes LWIR can do real work in this real world as is shown by real world experiment in the picture below – night frost (or your ice) formation can be governed by the differing sky & surrounding LW radiation absorbed in still air. Explained in an “Essay on dew” by Dr. Craig Bohren in this google clip:
https://books.google.com/books?id=gHTDAgAAQBAJ&pg=PT111&lpg=PT111&dq=fig+9.2+bohren&source=bl&ots=jwicXAdT-z&sig=UiWp2bMjK7zxLfig1MO6lK6HjXo&hl=en&sa=X&ei=a6mPVNmGDdKdyATH9IHACQ&ved=0CB4Q6AEwAA#v=onepage&q=fig%209.2%20bohren&f=false
Ever seen frost on top of a car left outside at night, but not on the sides? I have. (No, frost does not fall from the sky.) Bring the top of the post down to ground level and understand why frost can linger in your hollow and the shade of trees as the sun rises.
–Ever seen frost on top of a car left outside at night, but not on the sides? I have. (No, frost does not fall from the sky.) Bring the top of the post down to ground level and understand why frost can linger in your hollow and the shade of trees as the sun rises.–
Only frost I have seen fall from the sky is called snow.
But that frost/snow formed on top of the post. So question is why does water condense and form
ice or just sublimate from gas into ice.
When water forms into ice it has latent heat. So shiny or smooth surfaces do not radiate heat as well as rough surfaces. So for ice to form need something cold or something that radiates heat well.
So dew does settle from above it onto surfaces, but to form ice it need to shed heat- which reflective surface don’t do well.
So also every morning my car will be wet on the hood or roof- and not the sides of the car, but if it were cold enough the dew could form into ice.
richard verney December 15, 2014 at 5:56 pm
Dear heavens, man, do you not trust your own senses? Haven’t you been out on a bitter cold clear winter night, and then have a cloud come over and felt the warmth from the cloud? That is DWIR doing real work, warming your body in a perceptible manner. Of course the net heat flow is your body warming the cloud, but your body ends up warmer just the same.
And that is real, not theoretical, warming … which means that it is capable of doing real, not theoretical, work.
w.
Willis
Once again, you offer no answer to question put. Stop acting like a politician and start answering the question put, and not the question that you want to be put to you.
We have been discussing this for years. As I mentioned to you, what you have said in your posts of December 15, 2014 at 4:15 pm and December 15, 2014 at 4:27 pm are merely a restatement of comments that you have posted in the past. As you are aware, you are simply restating your views on the theory, and what you claim it does, without answering the question raised. I understand what is claimed of the theory, and how it is said to work. I do not need to see some drawings of personal finances to see what is claimed of the theory.
Your persistent failure, which has now gone on for several years, to deal with the question that I have raised ie., what is actually happening in the top few centimetres of the ocean on a micron by micron layer basis, and what are the physical processes involved and how and at what rate are they dealing with energy, confirms why modellers cannot model the ocean.
Until one properly understands what is going on in the atmosphere immediately above the ocean (say 50 metres above the ocean with resolution scaling down to microns as one approaches the boundary layer) and the top of the ocean itself (say the first 50 metres of ocean depth initially in sub micron resolution gradually extending out to micron resolution then centimetre resolution and then metre resolution) there is no prospect whatsoever of modelling oceans. Until we understand precisely what is going on, we cannot begin to model what is actually going on.
I know the reason why you do not answer my question. That is because you do not know the answer. I do not know the answer either, and that is why I am raising the question. I suspect no one knows the answer, and herein lies the problem. The devil of theories is in the detail. You claim that the oceans are being heated by the DWLWIR that they absorb, and I say let us assume that they are absorbing DWLWIR as claimed by you, then in which case how is that DWLWIR handled, and what problems arise in the handling of this DWLWIR?
Forgive my shouting: I DO NOT WANT TO HEAR ABOUT CLOUDS AND WHAT YOU CLAIM THAT CLOUDS DO. I WANT TO HEAR ABOUT ENERGY. IN PARTICULAR, I WANT TO HEAR ABOUT THE ENERGY IN THE TOP 3 MICRONS OF THE OCEANS, THE ENERGY IN THE TOP 3 MICRONS OF A CRATER LAKE, THE ENERGY IN THE TOP 3 MICRONS OF A DEW DROP.
The reason why I am interested in looking at crater lakes and dew droplets is that in the past you have claimed that the way the oceans are warmed is that the DWLWIR that is absorbed at the top of the ocean (without defining what is precisely meant) gets mixed with the bulk of the ocean, thereby warming the bulk, through various mechanical processes, namely by turbulence via wind, the action of waves and the process of ocean overturning.
I have my doubts that those mechanical processes are sufficient to mix the energy absorbed at the top of the ocean at a rate fast enough to dissipate and dilute that energy, because these are all slow mechanical processes. But it is possible to look at things where those processes are not seen. For the vast majority of the year, there is all but no wind on the surface of crater lakes, and no waves. On a still winter’s day there is no wind, no waves and no ocean over turning on dew drops. They do not encounter those processes, so we can eliminate them as a mechanism of distributing energy over a larger volume.
As you know due to the absorption characteristics of SWIR, there is all but no incoming solar energy absorbed in the top 3 microns of the oceans, or crater lakes. Fortunately for us (because life would be impossible in the form that we know it if this were not the case) Solar irradiance is absorbed at depth into a very large volume of water. It is not concentrated and thereby heats the bulk ocean/lake slowly and because it is absorbed over such a large volume and due to slow ocean currents which further distribute and the energy over an even greater volume, it heats the ocean only to a limited extent.
The absorption of LWIR is very different. 60% of it is absorbed in just 3 microns of perpendicular depth. Virtually no LWIR makes its way past 13 microns. Given that DWLWIR is omni-directional, with a significant proportion of DWLWIR having a grazing angle of say less than 40 degrees to the surface of the oceans/crater lake, more like 80% of all DWLWIR is absorbed within just 3 microns (on vertical basis) of the oceans/crater lake.
Accordingly, if DWLWIR is absorbed by the oceans, on average figures, we have about 200 W/m2 to 300 W/m2 of energy being absorbed and concentrated in just a 3 micron volume. In 1 hour that is upwards of 10,800,000 Watts of energy all concentrated in just 3 microns unless it has in some way been mixed into a larger volume of water. In a crater lake, or in a dew drop where there are no winds or waves to perform the mixing, this energy remains concentrated in just an extremely small volume.
The natural consequence of this is, IF the energy is not being mixed to volume at a speed greater than the speed that that energy would drive evaporation, evaporation will occur.
Now we know that copious evaporation is not occurring. This suggests one of two things. Either, the energy in some way is being mixed to volume at a speed which is fast enough to dissipate and dilute the energy before it drives evaporation. Alternatively, DWLWIR is incapable of performing sensible work in the environ in which it finds itself (alternatively, DWLWIR is never absorbed in the first place).
Let us consider the example of a dew drop on a cold winter day. Say sun up at 8am and on the sunny side of the hollow, the dew is burnt off (completely evaporated) by about 10am, yet on the shady side of the hollow, the dew lingers all day, it., it is not burnt off by 4 pm when the sun begins to set and the day begins to cool.
According to the K&T energy budget, the energy in is 168 W/m2 and DWLWIR of 324 W/m2 making a total energy in on the sunny side of the hollow of 492 W/m2, and the total energy in on the shady side of the hollow of just 324 W/m2.
Now if the dew drop is burnt off by 10 am, the dew drop has absorbed between 8 am and 10 am some 3, 542,400 W/m2 (492 x 3600 x 2). This amount of energy has fully burnt off the dew drop. By contrast on the shady side of the hollow, the de lingers all day and has not been burnt off by 4 pm. In this time, between 8am and 4 pm, the dew drop on the shady side of the hollow has absorbed DWLWIR of some 9, 331,200 W/m2 (324 x 3600 x 8).
Now of course, these are average figures and adjustment has to be made to reflect that we are dealing with winter in high latitudes, but the ratio of the calculation remains constant. Of course the K&T energy budget does not view solar as a day time phenomena but rather as a 24 hour constant. During the day there is more solar energy, but this fact will probably balanced by the fact that solar has relatively little energy at high latitudes between 8 am and 10 am in winter. Further, I have seen (many times) dew burnt off within an hour of sun up, but lingering all day in shady hollows.
Willis, PLEASE TELL ME
!. why DWLWIR cannot (and does not) burn off dew in the shady side of hollows.
2. how on a per second basis, the DWLWIR energy that is said to be absorbed in the top 3 microns of a crater lake (where there is no wind, no waves and if there is the equivalent of ocean over tunring this probably only occurs on a diurnal basis) get dissipated to depth at a rate quick enough to prevent copious evaoporation from the top few microns of the lake.
3. how on a per second basis, the DWLWIR energy that is said to be absorbed in the top 3 microns of the oceans in BF3 and less conditions gets dissipated to depth at a rate quick enough to prevent copious evaoporation from the top few microns of the lake.
I do not know what is going on. Please tell me what you think is going on.
Richard,
It’s very simple. Either the DWLWIR exists as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will HAVE TO be accounted for. Willis is absolutely correct. You can’t have it both ways. OR, the DWLWIR does NOT exist as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will NOT have to be accounted for.
Your argument that there IS indeed a DWLIWIR flux, but it cannot do ‘sensible work’ on the surface has no thermodynamic merit. If you transfer energy to a thermodynamic system and this system absorbs this energy, then this energy becomes part of (increases) the system’s internal energy [U], which corresponds to the system’s temperature [T]. There is no way around it.
You have to make up your mind.
–Let us consider the example of a dew drop on a cold winter day. Say sun up at 8am and on the sunny side of the hollow, the dew is burnt off (completely evaporated) by about 10am, yet on the shady side of the hollow, the dew lingers all day, it., it is not burnt off by 4 pm when the sun begins to set and the day begins to cool.
According to the K&T energy budget, the energy in is 168 W/m2 and DWLWIR of 324 W/m2 making a total energy in on the sunny side of the hollow of 492 W/m2, and the total energy in on the shady side of the hollow of just 324 W/m2.
Now if the dew drop is burnt off by 10 am, the dew drop has absorbed between 8 am and 10 am some 3, 542,400 W/m2 (492 x 3600 x 2). This amount of energy has fully burnt off the dew drop. By contrast on the shady side of the hollow, the de lingers all day and has not been burnt off by 4 pm. In this time, between 8am and 4 pm, the dew drop on the shady side of the hollow has absorbed DWLWIR of some 9, 331,200 W/m2 (324 x 3600 x 8).–
You got me thinking. A dew drop is massive compared other droplets in the air.
Or basically DWLWIR would control the size air droplets. And dew drop only has DWLWIR
coming at from 180 degrees, whereas droplet mid air would get from 360 degree.
Is there paper somewhere explaining the effect of DWLWIR effect upon water droplet size?
Apparently in a rain drop there is N = 4.716278×10^20 molecules of H20.
http://www.had2know.com/academics/how-many-molecules-drop-water.html
So instead of 10^20 one could much smaller on which has 10^10. Or drop with million or 1000.
Are some droplets too small to be affected by most of the LWIR?
Is a size of droplet which DWLWIR would evaporate instantaneous and therefore don’t exist?
Does DWLWIR eat up clouds and fog?
The absorption of LWIR is very different. 60% of it is absorbed in just 3 microns of perpendicular depth. Virtually no LWIR makes its way past 13 microns. Given that DWLWIR is omni-directional, with a significant proportion of DWLWIR having a grazing angle of say less than 40 degrees to the surface of the oceans/crater lake, more like 80% of all DWLWIR is absorbed within just 3 microns (on vertical basis) of the oceans/crater lake.
Accordingly, if DWLWIR is absorbed by the oceans, on average figures, we have about 200 W/m2 to 300 W/m2 of energy being absorbed and concentrated in just a 3 micron volume. In 1 hour that is upwards of 10,800,000 Watts of energy all concentrated in just 3 microns unless it has in some way been mixed into a larger volume of water. In a crater lake, or in a dew drop where there are no winds or waves to perform the mixing, this energy remains concentrated in just an extremely small volume.
The natural consequence of this is, IF the energy is not being mixed to volume at a speed greater than the speed that that energy would drive evaporation, evaporation will occur.
Now we know that copious evaporation is not occurring. This suggests one of two things. Either, the energy in some way is being mixed to volume at a speed which is fast enough to dissipate and dilute the energy before it drives evaporation. Alternatively, DWLWIR is incapable of performing sensible work in the environ in which it finds itself (alternatively, DWLWIR is never absorbed in the first place).
Or what actually happens, which is that the surface itself radiates back to the atmosphere/space. For ocean surface at 300K that’s about 450W/m^2 which is why the surface is cooler than the layer below.
http://ghrsst-pp.metoffice.com/pages/sst_definitions/sst_definitions.png
richard verney December 16, 2014 at 3:13 am
My unpleasant friend, here’s your exact “question put”, which I very clearly answered:
Now, you can claim I’m wrong in that answer.
But claiming that I’m not answering your questions is a damn lie, and I don’t take well to that. Come back when you can keep a civil tongue in your head and ask your question again, whatever it may be, and I may get around to answering in … but you just moved yourself to the end of the line with your slimy accusation. I answer more questions than any climate blogger I know of, and in more detail, and I’ll thank you to remember that.
You tell me to “stop acting like a politician and start answering the question”, when I JUST ANSWERED YOUR SPECIFIC QUESTION?
I answer, stop acting like a p***k.
w.
[I note that the mod has censored mpainter’s use of the above word, so I’ve censored my use of it here myself as well. Sauce for the goose, etc. -w.]
–Kristian
December 16, 2014 at 5:43 am
Richard,
It’s very simple. Either the DWLWIR exists as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will HAVE TO be accounted for. Willis is absolutely correct. You can’t have it both ways. OR, the DWLWIR does NOT exist as an extra, separate radiative flux/transfer of energy from atmosphere to surface, in which case it will NOT have to be accounted for.–
Well let’s go back to Willis steel shell. If ground is 235 Watts per square meter then roughly the outside of shell will radiate 235 watts per square meter. And for the shell to radiate a 235 watts
it must be at the temperature that allows it to radiate. And since energy coming from the inside of shell the inside must be about as warm as the outside.
The shell will transfer heat to any side which can be heated, and since universe is 2 K, it radiate
it’s energy to the universe. And one to make something cold enough inside the shell it would radiate heat to that which is cold enough.
Or if you had equal conditions on either side of material which was the same it radiate equally to either side.
Now with such a large sphere a section 1 meter square is essentially flat- to think of it as curved in some way is simply wrong [it’s as flat as any table ever made].
On one side is the 2 K universe and radiant energy can go in 180 degree arc and it’s “field of view” is about 1/2 of the universe.
On the other side, is a universe which is confined to the inside of the shell. Which is mostly other parts of the shell plus the planet itself and “seeing” a portion of the surface of the planet.
[It should be noted that ideal blackbody would radiate equally in all directions- but we suppose to deal with a material which actually exists in your world, which we loosely identify as steel. But could assume that such steel is manufactured with exotic arts by the best engineering available on Earth to suit whatever our various purposes.]
If on surface and one has 1 meter square slab of something very cold, what will normal heat it the most would be conduction of heat or air convection. But the plan is we isolate the cold slab so these things or not heating it. Simple thing to do is not have slab on the surface, but have next to the steel shell. As there is no or little air up there and hang it from the shell itself. And you have to do is block or account for the radiate energy coming from the planet below.
We will make it simple and just account for radiate energy coming from the planet below. So have 4 wires hanging from shell, have the wires 2 meter long. Hang 1 square steel which the same stuff as shell. and have this 1 square meter steel be 2 K. What happens.
Roughly what happens is 2 K steel warms most from heat from the surface, and it’s blocking heat from the surface reaching the a section of shell, that section of shell continues radiating half whatever heat capacity it retains to 2 K universe and other half to 2 K 1 meter square slab of steel. Thus proving that inside of shell can radiate heat.
And within a few days [or minutes or hours] , the 1 meter square hanging from the shell will be the same temperature as the section of the shell above it.
And hanging it further from the shell merely makes it more complicated but gives same result in a few days.
One could imagine the shell is making uniform temperature, but what making the uniform temperature is mostly the ground radiating 235 watts per square meter. Or you can remove the shell and it makes little difference people living on the surface- though they can now launch rockets without hitting the shell. And see the stars.
gbaikie commented on Arguments For and Against Human-Induced Ocean Warming.
in response to Willis Eschenbach:
My heavens, dear fellow, it’s called a “thought experiment”. It’s how we conceptualize about things.
There are also no planets with a steel shell around them … but it’s still a very valuable thought experiment. It shows that the poorly named “greenhouse effect” depends only on the fact that the shell has twice the area of the surface.
w.
“Haven’t you been out on a bitter cold clear winter night, and then have a cloud come over and felt the warmth from the cloud?”
That is a consequence of the cloud reducing upward radiation to space from your skin. Your skin loses energy less fast and so the internal warmth of your body which is constantly being renewed is enabled to warm your skin once more.
Meanwhile the ground temperature will stop falling but will not rise as a result of DWIR from the cloud. The surface temperature will only actually rise if warmer air is advected in horizontally beneath the cloud.
In most cases the arriva of cloud is a sign of warmer air advecting in horizontally at a higher level.
A better knowledge of meteorology would help in this discussion.
A cloud moving over above a water surface reduces convection so that warmed humid air is unable to rise which is why it can become warmer at the surface. It is not a matter of DWIR from the cloud warming the surface.
50% of the atmosphere is descending at any one moment and being warmed adiabatically in the process. That descending air suppresses convection and allows incoming solar energy to heat the surface above the temperature that would be achievable from a purely radiative energy exchange as per the S-B equation.
On average that raises the surface temperature of the entire globe by 33C.
Stephen Wilde December 16, 2014 at 12:34 am
The clouds make my skin lose energy less fast? How do they do that? Block up the pores? Perhaps you could expand on the physics involved in clouds a kilometer above me being able to reduce the radiation from my body WITHOUT involving the true explanation of downwelling radiation.
I love the different bizarre physical theories that folks hold. Some say that there is no such thing as downwelling radiation, despite the fact that it has been measured all over the world thousands of times.
Others say there is downwelling radiation, but it can’t leave the surface warmer than it would be without the radiation because the surface is warmer than the clouds, and somehow the photons know that so they veer away from the surface or something.
Others say that there is downwelling radiation and that it can leave the land warmer but not the ocean. And once again, here there are a variety of explanations.
Fascinating.
w.
–The clouds make my skin lose energy less fast? How do they do that? Block up the pores? Perhaps you could expand on the physics involved in clouds a kilometer above me being able to reduce the radiation from my body WITHOUT involving the true explanation of downwelling radiation.–
So you are saying you have felt the warmth of downwelling radiation?
–I love the different bizarre physical theories that folks hold. Some say that there is no such thing as downwelling radiation, despite the fact that it has been measured all over the world thousands of times.–
I have never measured downwelling radiation [nor can I say that I felt it].
From what I understand the measuring device for downwelling radiation doesn’t work indoors.
But Is there downwelling radiation indoors and one would need different equipment to measure it?
Can it be felt indoors?
I can tell feel a warmer or cooler room inside a house. But I have always assumed this is related to the air temperature.
And it is my understanding that a human body is not regulated by radiant energy- obviously a fire
or anything quite hot can warm someone with radiant energy, but generally the human body
controls heat by evaporation and convection and conduction of heat.
Or astronaut which would have about two square meter of surface area and does not lose much heat in space. And heater are not used in spacesuits, but rather instead a cooling system is used.
I am going to strongly disagree with you about your statement concerning “indoor” (downward, er, inbound) thermal radiation to a warm body. Spanish hotel and apartment bathrooms and bedrooms are an excellent test case for you: They are usually built of tile, smooth sheets of plastic and bare stone: ceiling, floor, walls, tub and bath enclosures, sinks, etc. Almost no surface is wooden, cloth, or cloth-coated. (Almost no surface has a low emissivity, almost no surface (lights excluded) has a surface temperature greater than the unclothed human body – winter or summer, spring or fall. Almost every surface will have that of the (unheated or poorly heated) room air. ) And, of course, I invite any of our Euro-speakin’ brethern to use their own test case and report their results who find themselves in similar locations.
Now, before a hot shower or cold bath water is run, yet while your body still dry, stand in free-space with no thermal insulation. (I will NOT request Willis provide evidence of this test condition!)
Assume your body is “normal” – you will be radiating thermal energy from every cm^2 skin surface because your skin is 37 deg C (98.6 F) = 310 K. The room walls and tiles and ceiling and floor will be receiving that thermal radiation, and will in turn, be radiating a much smaller amount of energy from their surface.
Stand still for a few minutes.
Cover the floor with a rug or bath towel. Cover the nearest wall with a towel or cotton sheet. Hang a sheet or large over the door. Again, stand still for a few minutes.
the difference in heat loss will be the “missing” LW energy from the cold tiles and floor being “shielded” from your body by the low emissivity cloth and wool and towels.
Now, wrap one of the large towels loosely about your legs, and, again, wait a few moments.
Move that large towel up higher (around your shoulders and neck) and, again, wait a few minutes.
The difference is the closeness of the wrap and the extra heat energy it has from your body to even more strongly shield you from the thermal radiant loss.
If no one is pounding on the bathroom door yet, wrap yourself in aluminum foil, and try the shielding again. (Do not re-use the foil for cooking later, or you will agian be subject to random poundings from the local cook.)
–Assume your body is “normal” – you will be radiating thermal energy from every cm^2 skin surface because your skin is 37 deg C (98.6 F) = 310 K. The room walls and tiles and ceiling and floor will be receiving that thermal radiation, and will in turn, be radiating a much smaller amount of energy from their surface. —
Since you not in spacesuit, your body has roughly about 1.6 square meter of area. How much is being radiated from your body and how much do think of being radiated from colder walls at your body.
Next is there anyway of increasing the surface area your body radiates and thereby be able cool down more?
Yes, but I will answer it by reversing the effect. Classically, a tall skinny person with very little body fat (such as myself) will lose much, much more heat by radiation and convection and better conduction (less insulation) per body mass than a shorter, rounder, fatter person who has much less surface area “per person” or “per pound”. Typically, you will see the example of the short Eskimo or Finn or northern Canadian native compared to a taller sub-Sahara African for example. lots of counter-examples of course, and diet and available food are more important. BUT – If “I” (the tall, handsome one with low body weight) have frozen to death early in life because “I” have lost more heat than my competitor for my future wife’s hand in marriage, then “I” do not reproduce. He (the short fat one with hairy chest, hairy face, and hairy legs and short arms) does reproduce.
Wow, a week has gone by and this topic is still being discussed. I’m glad I raised it once again. I’ll hold off on part 2 for another week.
No, Bob.
Willis has taken the discussion off on a tangent and invokes the ghe while ignoring
the physics of the sea surface.
He has yet to address sea surface effects except with a hand wave and some mumbles.
So Willis waxes forth on GHG and GHE while forgetting that it is the radiative properties of water that are the issue.
mpainter December 16, 2014 at 1:36 am
Gadzooks, sire, that’s nonsense. I’ve given the most detailed description I can of a variety of ways that the energy gets mixed downwards from the ocean skin. You can accuse me of being wrong … but saying I haven’t addressed the question is simply untrue.
w.
Willis
I consider that mpainter has a point. Not that you have taken the discussion off target, but rather that you have not addressed the important real world consequences of what is claimed of the theory.
You are correct that you have offered some explanation, namely you have suggested “…that the skin layer is not stable. Why? Because cold water is denser than warm water. As a result, the skin layer is constantly cooling, sinking, and being replaced by warmer water. It is overturning constantly.” and that “It is also mixed mechanically by the action of the wind. In anything but the weakest of winds, the skin layer is broken up both by the horizontal force of the wind, and by the mechanical action of the waves.”
But what you have not done is to address the speed at which these mechanisms take place, and to address whether they can realistically operate at a speed that is fast enough to disspiate the energy to depth/volume at a rate which the energy in the top 3 microns would otherwise drive evaporation.
Further, because of the explanation, you have put forward, I have raised examples where some of these processes are not present (crater lakes where there is no wind or wave action to mix the top to depth), dew drops and ice (where there is no wind or waves or ocean over turning to mix this energy to depth).
Further still, with dew drops even if there was the mixing that you suggest (cold layers sinking to depth) this would not prevent the total evaporation of the dew drop by DWLWIR.
What you need to consider is at what rate does ocean over turning mix DWLWIR, and what about dew and ice where this process is not present.
At what rate does wind and waves mix DWLWIR but what about crater lakes when this process does not takeplace, or for that matter the ocean when in BF3 or less conditions, and what about dew drops and ice where these processes are absent.
At what rate do you claim that cold water sinks? What about dew drops where if the top few microns sinks into the droplet it will gradually heat the droplet leading to the eventual evaporation of the droplet. Ditto ice.
You seem to overlook that somewhere as we exchange views there are large areas of the ocean encountering BF3 or less conditions. This is important since even if on average only 1% of DWLWIR is not mixed as you suggest (since on average some 1% or more of the ocean is experiencing BF3 conditions or less0 the gross energy budget claim fails because the energy being absorbed in the top of the ocean and mixed into the ocean is ever soo slightly less than that budget sets out such that it does not balnce and even a slight impbalance over some 4 billion years 9or so) causes problems.
I concur with Richard Verney.
I would add that the uppermost few microns of the skin is never involved in overturning because it is _always_ present, being due to evaporation which itself is incessant. Overturning does not mean the cessation of evaporation hence the cool layer is ever present. Does the wind have any effect? Yes, it increases the rate of evaporation and this maintains the cool layer, always present as the uppermost surface layer.
I should add that most of the IR is absorbed in this cool layer and that includes all of the IR emitted by CO2. This added energy is quickly transformed to latent heat as part of the dynamics of evaporative cooling.
I’ve addressed what actually happens at the surface but you studiously avoid discussing it!
Phil. December 16, 2014 at 7:54 am
Phil. December 14, 2014 at 7:42 pm
Phil. December 13, 2014 at 9:06 pm
I have to say the same thing to you as I did to richard verney, mpainter.
You cannot get anywhere with this “DWLWIR exists as an extra, separate radiative flux/transfer of energy to the surface’, but it cannot do anything except (in your case, it seems) cause evaporation” argument. If you agree with Willis that there is indeed a DWLWIR flux and it is absorbed by the surface, then this energy will HAVE TO be accounted for. Then a total of [165+345=] 510 W/m^2 is absorbed by the ocean surface and only 112 W/m^2 of this is lost again by way of conduction/evaporation. That leaves … 398 W/m^2. The potential emission flux of a black body surface at 289K.
You too have to make up your mind. I don’t get all these people that apparently agree completely with Willis that the DWLWIR flux is real, but who for some convoluted reason don’t agree with him that it helps keep the surface warmer than at pure solar radiative equilibrium, that it doesn’t do anything (other than provoke evaporation).
Kristian: you are picking bones. I do not buy the DWIR story, particularly where it is put at greater than insolation, as Willis does.
The issue is whether or not IR can heat water. Theory and experimental data both says no.
Kristian
I am sceptical as to whether DWLWIR is anything more than a signal, ie., it is something that can be measured, it is something that can tell us something about the temperature of an object, but whether it is something more than that, in particular capable of performing work, in the environ in which it finds itself, is moot. Accordingly, I am sceptical of its existance as an extra, separate radiative flux/transfer of energy from atmosphere to surface. My first post looks at the net flow position only. As i said, solar provides all the energy that the oceans need to prevent the oceans from freezing. It is solar, and only solar, that warms the oceans.
However, as far as my discussions with Willis are concerned, i am adopting the position; let us accept for the sake of argument that DWLWIR does exist as an energy flux, in which case how is it absorbed and how is it handled and what consequences flow from this? If it is a real energy flux (and goes towards warming the oceans) then I foresee problems with this due to the absorption characteristics of LWIR in water.
There is a heck of a lot of energy going into just 3 microns of water. This energy is highly concentrated and unless sequestered to depth at a rate faster than that energy would otherwise drive evaportation, there would be a heck of a lot of evaporation which we are not seeing. The annual rainfall would be far higher. We know the annual rainfall (within say a 100% error band) so we know the maximum evaporation from the oceans. This is far less than would occur given the amount of energy that DWLWIR would impart into the top 3 microns of the oceans unless that energy could quickly be diluted to volume, which means quickly sequestered to depth.
I want to explore what happens in the real world if the energy that Willis claims is absorbed by the oceans is truly so absorbed. I am testing that.
“The clouds make my skin lose energy less fast? How do they do that? Block up the pores? Perhaps you could expand on the physics involved in clouds a kilometer above me being able to reduce the radiation from my body WITHOUT involving the true explanation of downwelling radiation.”
Poor wording on my part, shouldn’t rush so much.
Clouds reduce convection and block upward radiation which reduces the rate of cooling for the ground surface and the air mass beneath the cloud. The air around your body therefore cools less quickly and draws heat from your bare skin less quickly.You will soon feel warmer than under a clear sky.
It isn’t DWIR from the cloud that makes you feel warmer.it is the reduced rate of cooling of the air around you relative to your own production of body heat.
Thanks, Stephen. Clouds don’t “reduce convection”, they have a variety of effects. In general, they simply move with the air.
And while they “block upward radiation”, this cannot directly “reduce the rate of cooling for the ground surface”. The ground radiates at the same amount whether or not there’s a cloud. The photons leave the ground at a rate determined by the TEMPERATURE, not by what may or may not be absorbing their radiation.
w.
Well, when one says that clouds reduce convection one should really say that warmer cloudy air carrying layer cloud reduces convection by reducing the rate of temperature decline with height.
As regards photons leaving the ground I still consider that the conductive exchange between surface and atmosphere reduces the number of photons leaving the ground as radiation.
The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.
Your previous point about the conductive convective exchange having no effect on surface temperature because it is a net zero energy exchange is not correct IMHO.
Even a net zero energy exchange requires a fund of energy (heat) at the base of the convective column to sustain its height.
That fund of heat at the base cannot be radiated away otherwise the atmosphere would subside to the ground.
But I don’t see you ever accepting that.
“Some say that there is no such thing as downwelling radiation, despite the fact that it has been measured all over the world thousands of times.”
There is downward radiation from the molecules around the sensor and their temperature depends on their position along the lapse rate slope set up by mass, gravity and insolation.
The sensor can be designed to measure temperature at a remote location determined by optical depth but the temperature of that location is in turn determined by its position along the lapse rate slope.
Of course there are regional and local variations over time as the atmospheric gases move around but in the end the average lapse rate slope prevails.
Stephen Wilde December 16, 2014 at 12:07 pm
As regards photons leaving the ground I still consider that the conductive exchange between surface and atmosphere reduces the number of photons leaving the ground as radiation.
The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.
Not true, radiation depends only on the temperature, any conduction/convection is an additional loss/gain.
I said:
“The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.”
and Phil replied:
“Not true, radiation depends only on the temperature, any conduction/convection is an additional loss/gain.”
But what if conduction/convection is a zero sum energy exchange as it is at the surface ?
If it is a zero sum exchange it cannot amount to a loss/gain but it still requires heat at the surface to sustain the height of the convective column.
And that heat cannot be allowed to radiate away otherwise the convective column would have to dissipate.
So, it seems that a given temperature of surface will radiate at a level determined by surface temperature BUT a portion of that radiation is constantly moving in and out of the conductive/convective exchange and so cannot escape to space from the surface.
Radiative theory does not consider the effect of conduction/convection on the ability of a surface to lose energy via radiation. Quite simply, conduction and convection do reduce radiative loss from a surface by tying up a portion of the surface radiation within the ongoing conductive/convective exchange between surface and the mass of the atmosphere.
–Radiative theory does not consider the effect of conduction/convection on the ability of a surface to lose energy via radiation. Quite simply, conduction and convection do reduce radiative loss from a surface by tying up a portion of the surface radiation within the ongoing conductive/convective exchange between surface and the mass of the atmosphere.–
Hmm. Take pot of water and shine laser thru water to bottom of the bottom of the pot.
The reflection of the laser off the bottom is probably not effected by conduction/convection
of water.
And with laser which powerful enough, one make a hole in bottom of pot with the water in it.
Or laser has to be powerful enough to overcome the molecular bond or molecule bond dissipate
the energy. A 100 kw laser might punch thru and 100 1 kw laser shining on different spots does not.
So with 100 kw laser burning hole in the pot, the water in pot would stop the edges of hole to conduct heat to rest of the pot.
With sunlight conduction/convection is going to stop heat from going lower into the surface.
And if have an hour of say 600 watts per square meter of sunlight any evaporation and conduction/convection is going to remove a good portion of the 600 watts per square meter
so it does “have” or “could” balance with a hour of 600 watts per square meter of net energy
returning to space.
gbaikie December 16, 2014 at 5:30 pm Edit
Sure have. Anyone who has spent a good deal of time outdoors at night in the winter has felt it.
I didn’t say you had measured it. I said it has been measured repeatedly. You might try googling “downwelling longwave observations” … I immediately find this. Also, both the TAO buoy system and the SURFRAD stations measure downwelling radiation 24/7.
Regards,
w.
“I didn’t say you had measured it. I said it has been measured repeatedly.”
Right, thousands of times you said. I would say perhaps millions of individual readings, but I have not measured it, nor has seemed to me that there has been any need to measure it.
It suppose to be important, right?
The device I believe is called a Pyrgeometer.
Wiki:
“Pyrgeometers are frequently used in meteorology, climatology studies. The atmospheric long-wave downward radiation is of interest for research into long term climate changes.”
It would seem to me to be a waste of money to buy one, but I am open to idea that they could
useful for some reason. And one thing I would like to know is what be good ones to buy.
I check amazon be none are sold there- though they sell books about it.
Now I read some bad product reviews:
“DLR and backradiation is thus fiction invented from an ad hoc formula without physical reality, which is not described in the physics literature. Nevertheless there are companies selling pyrgeometers at price of 4.000 Euro, but of course selling fiction can also serve as a business idea. But is it legal to sell fiction as science? As science fiction?”
http://claesjohnson.blogspot.com/2011/08/how-to-fool-yourself-with-pyrgeometer.html
And I would like to more balance in the product review.
Is something as useful as say thermometer. What do use it for? And etc.
oh, there is this less harsh review, here:
https://tallbloke.wordpress.com/2013/04/26/pyrgeometers-untangled/
But it still says:
“These devices do not measure “downwelling Infra Red” and the figure is a computed number based on the actual outgoing IR and the temperature of the instrument body.
Put in another way, such a figure is a backwards reading with human added offset.”
And:
“I repeat, heat flow is from the ground upwards. (under very rare meteorological conditions a minor reverse flow happens, the former is overwhelmingly dominant)”
But I am interested in more positive reviews and why it worth spending any money on.
Willis
the impression which you give is that you are unable to answer my specific questions, and have instead resulted to ad homs. That says it all.
But just in case I am mistaken since you assert “You tell me to “stop acting like a politician and start answering the question”, when I JUST ANSWERED YOUR SPECIFIC QUESTION?, and you have in fact answered my questions, I will set out my questions on a numerical basis, and you can then answer each question number by number, if necessqary cutting and pasting your earlier answer so that we can see whether your claim that you have “JUST ANSWERED [MY] SPECIFIC QUESTION is correct.
1 Given the absorption characteristics of DWLWIR in water, how much DWLWIR is absorbed within the top 3 microns of the ocean?
2. Do you accept that unless there is a process (and/or processes) that dissipate/sequester that energy to depth at a rate faster than the energy absorbed into and contained within the top 3 micron layer would otherwise drive evaporation, the energy absorbed and not so sequestered to depth would drive evaporation? If not, why do you not accept this, and what instead do you contend takes place?
3. What processes are claimed, by you, to mix that energy downwards and dissipate it to depth AND at what rate does each indiviidual process move/sequester that energy to depth?
4. In the case of crater lakes (ie., lakes in caldera) do you accept that since these are in a basin, they are, for main part, effectively shielded from wind, and consequently, it is relatively rare to see much in the way of waves on crater lakes? No doubt you have seen the usual Postcard Photographs with views of these lakes as still as a millpond with the mountain range well reflected in the lake.
5 Do you accept that in the case of crater lakes when compared to the ocean, there is for the main part (by which I mean on average for the better part of the year) far less wind and/or waves and/or swell to mix the top 3 micron layer of crater lakes when compared to the sea?. At what rate do you claim that waves on crater lakes mix the energy absorbed in the first 3 microns? What rate to you claim that wind mixes the energy absorbed in the top 3 microns?
6 Do you claim that ocean overturning (or its equivalent) is a process that takes place in crater lakes? If yes, is this a diurnal process, or is it a process that on goes 24/7? At what speed/rate does this process (if it is claimed to take place) dissipate/sequester the energy in the top 3 micron layer to depth?
7. Please review my earlier comments on dew drops (where I mention how dew on the sunny side of a hollow can be burnt off within an hour or so of sun up on a winter’s day, but on the shady side of the same hollow the dew can linger all day) . What processes do you claim mix the DWLWIR that is absorbed within the top 3 microns of a dew drop on a still winters day (ie., one which has no significant wind), and at what rate does each process mix the energy absorbed in the top 3 microns?.
8. Further to the above dew drop point, if the energy absorbed in the top 3 microns is mixed in accordance with the processes that you claim to mix that energy, why does the dew drop not eventually warm and evaporate. After all there is little volume of liquid in a dew drop ( perhaps in the region of just 0.0648524 millilitres or a few times that amount) and 8 hours of DWLWIR (at average figures in the K&T energy budget) is 9, 331,200 W/m2 (324 x 3600 x 8).. Of course, i accept that the average figure for DWLWIR needs to be reduced, but the ratio between solar and DWLWIR would still remain the same such that the forcing from weak winter solar burns off a dew drop within an hour or so, but DWLWIR does not even after exposure for the entire day.
9. In conditions where the ambient air temperature is just a little below freezing, why can solar begin to melt ice after just an hour or so of exposure (eg., cloudless sunny day) but DWLWIR cannot melt ice on a cloudy day even though the ice is exposed to DWLWIR for many many hours? After all it is receiving the full benefit of those warming clouds you like
10, How much energy is absored in the first 3 microns of ice on a cloudy day, and what process do you claim distributes and dissipates that energy to depth at a rate faster than the surface would melt if that energy remained concentrated in the top 3 microns. What rate do each of these processes move/sequester/distribute the energy absorbed in the first 3 microns to depth?
11.When considering 1 to 3 above, what happens in the ocean when the prevailing conditions are BF3 or less, BF2 or less, BF1 or less? ie., what processes do you claim mix the energy absorbed in the top 3 microns of the oceans and at what rate does each process mix that energy to depth in these condfitions?
I look forward to receiving your answer to the specific questions detailed above.
Many thanks .
richard verney December 16, 2014 at 7:24 pm Edit
Richard, as I implied above, if you kept resorting to attacking me there was no sense in answering you. You have not answered my four questions in the slightest. I’ve answered yours, and you are still attacking me.
I give up. You win. I’m tired of your attacks. I’m done with your unpleasantness. Go bother someone else.
I can see that nothing I’ve said has made the slightest impression on you. Since you have claimed that your statement above “says it all”, I’ll take you at your word and let you go. You’ve said it all. I’ve said it all. Please address any further remarks to anyone but me.
w.
Willis
As you are aware, I have not attacked you once. At most I have suggested that your answering is like that of a politician. That is not an attack, that is an observation on an approach/style to dealing with issues/questions. It is not even a derogatory comment, since many politicians are quite intelligent.
When mpainter suggested/implied that you had given no answer as to mechanisms that you claim mix the DWLWIR into the oceans, I suggested that was not so and that you had given an indication of mechanisms, but what was missing was the speed at which these processes mix DWLWIR absorbed in the top 3 microns to depth, and hence dilute the energy to volume. So when you read the posts, you will see that I have even sprung to yoru defence and supported you.
On the other hand you have suggested that I am acticg like a prick. That is an attack on character, and a derogatory one at that. It is an ad hom.
The issue here is quite simple. The claim that DWLWIR is absorbed in the ocean leads to a number of issues. In particular, the energy absorbed is extremely concentrated somewhere betweem 60 to 80% of DWLWIR is concentrated in just 3 microns, and that is a heck of a lot of energy. That energy (if capable of performing sensible work in the environ in which it finds itself) would drive copious amounts of evaporation and/or carry the water vapour evaporated to great height, unless it is sequestered to depth (thereby dissipating and diluting the energy into a larger volume) at a rate faster than the rate that that energy would if not sequestered to depth, drive evaporation from the top microns. So the speed of the mixing proceeses that you cite is the central issue. You may be right that those proceeses exist and do mix energy at the very top microns of the ocean, but the issue is at what speed do they perform this mixing.
So that we can consider the effectiveness of those processes in more detail, I have sought to identify scenarios where those processes must be less effective, perhaps even no existent. Eg. in BF2 and blow there is no significant wind or waves, in crater lakes there is no significant wind or waves. On a still winter’s day dew drops are not subjected to significant wind or waves, nor ocean overturning. etc etc.
When comnsidering the oceans, it is claimed that the average wind conditions/sea state is globally on average just over BF4. If that is so, since this is an average figure, it must mean that at least 20% of the oceans on average experience average conditions of BF2 or less. This is material since if for 205 of the year there is no effective mixing of the top 3 micron layer by the action of wind and waves, how is ALL the DWLWIR effectively being mixed into the oceans. In these conditions only 2 mechanisms claimed by you can be operative, namely ocean overturning, and cold water sinking. Is ocean overturning operative 24/7 or is it only operative on a diurnal basis, and if a diurnal process what happens when it is not operative? What is the speed/rate at which ocean overturning mixes the top 3 micron layer to depth? What is the speed at which cold water sinking mixes the 3 micron layer to depth?
As I have said to you on a number of previous occassions, let us discuss the science. If you have an answer to the points I raise, then please detail it. If you do not know the answer,so be it, there is no discrace in that. I do not know the answers since, as far as I am aware, no one is carrying out the empirical testing and collecting impirical observational data on this.
That being the case, we are left to grasp with what may be objectively viewed as likely. I suggest that the action of wind and waves can not effectively mix DWLWIR absorbed in the top 3 microns of the oceans when the prevailing conditions are BF2 or less, ditto in crater lakes, ditto in dew drops on a still winter’s day.
I would suggest that ocean overturning is a slow mechanical process and appears to be a diurnal phenomena such that either way, it is not capable of mixing DWLWIR absorbed in the top 3 microns to depth at a rate fast enough to prevent DWLWIR absorbed in the 3 micron layer simply driving evaporation (including carrying it to height) and latent heat loss. In any event, ocean over turning cannot explain why a dew drop on a still winter’s day is not burnt off/evaportaed by DWLWIR.
The cold water sinking claim has a number of issues niot least that the top microns of the oceans are cooler only because of the effects of evaporation taking place at the very top of the ocean and the difference in buoyancy is such that cold water would be sinking at a ver slow rate and so any energy contained in that cold water is being seqestered to depth at a very slow rate not sufficient to prevent the DWLWIR absorbed in the top 3 microns from simply driving evaportion.
In would like to hear your views on the science since you coukd usefully contribute towards this debate if you were to address the specifics,
Richard Verney,
You see what happens when you try to force Willis to the issue: you get a sailor, not a scientist.
richard verney December 17, 2014 at 5:34 am
Richard, this is why I’ve given up dealing with you. As you know, you’ve lied about whether I answered you questions. That is assuredly an attack on my honesty.
w.
mpainter December 17, 2014 at 7:49 am
I see … lying about whether I’ve answered richard’s questions is now called “forcing” me to the issue.
I’ve got news for you, mpainter. Trying to “force” an honest man to do anything by lying about him gets you nowhere, whether the man is a sailor, a scientist, or both … but then if you hung out with honest men I wouldn’t have to explain this to you.
I answer more questions and in more detail than any other climate researcher you can name. I’ve answered your questions. I’ve made a good faith attempt to answer all honest questions.
And in particular, I’ve answered richard’s questions, over and over as nauseum, on this and previous threads. He just keeps asking the same questions in a new and different guise … and all the while he hasn’t answered my questions.
As a result, when he topped that off by claiming I’m not answering his questions, I pulled the plug on his endless whinging…. so sue me. Clearly you think that kind of passive-aggressive attack is fine. Your call.
w.
It seems if there isn’t back radiation then greenhouse gases do not warm Earth.
It seems most agree that clouds add warmth at night.
I think most agree that lower elevation [a big hole in the ground] would increase
air temperature. The hottest air temperature ever recorded was slightly below sea level and
it seems good rule that if you looking the highest air temperature one look at regions below
sea level.
It also well know that UHI increases mostly night time temperatures, but also seems to a some increase in daytime temperatures. And such thing as inversion layers also tend to increase daytime temperatures.
So in addition to below sea level, some kind UHI effect and inversion layers could cause the highest day time air temperature.
Ans I think a common aspect of all of the above can be related to having some kind of elevated surface. So clouds are elevated surface, as are inversion layers [sort of] and below sea level is the rest of world is an elevated surface, and cities have a bunch elevated surfaces in the form of buildings.
Though clouds are related to warming at night and I suspect that cloud are related to a general idea of back radiation. Or radiation from ground is intersecting a cloud which is a elevated
surface. And clouds at a higher elevation could be must cooler than the surface, so one violating colder things warming warmer things, instead something warmer is warming a colder thing.
So doesn’t seem too problematic that the ground could heat the clouds- the problem is related to
how could the cloud warm the ground. There seems to be many possible mechanisms, and perhaps there are more than one or two mechanism involved. For example clouds are water droplets which unlike gases reflect light. Another factor is clouds are where lots H2O gas still condensing into liquid, so creating heat.
Now regarding clouds, one can clouds at varying elevation. I don’t think fog [or clouds on the ground] make nights warmer, though perhaps it’s the dampness just make feel cooler and does
tend to keep it warmer. But do clouds cause more warming depending upon what elevation they
are at?
Stephen Wilde December 17, 2014 at 12:41 am
I said:
“The radiative rules as to the number of photons leaving at a given temperature only applies in a purely radiative scenario i.e. no conduction or convection.”
and Phil replied:
“Not true, radiation depends only on the temperature, any conduction/convection is an additional loss/gain.”
But what if conduction/convection is a zero sum energy exchange as it is at the surface ?
If it is a zero sum exchange it cannot amount to a loss/gain but it still requires heat at the surface to sustain the height of the convective column.
And that heat cannot be allowed to radiate away otherwise the convective column would have to dissipate.
Where do you get this fictitious ‘law’ from?
It’s about time you read up on heat transfer instead of making stuff up.
If you consider a control element at the surface, say a 1 micron cube, the top surface will be radiating at ɛ𝜎ATtop^4, the element will be absorbing some of the radiation incident on it, mostly IR. There will also be conductive and convective heat transfer at both the top and bottom surfaces, given by equations of the form Q=hA∆T. If the element is net losing heat it will cool down until a balance is achieved, in the case of the night-time ocean shown, by about 0.3K. The only way that conduction/convection will effect the radiation loss is due to their influence on the temperature, however the radiation is the dominant loss mechanism so their influence is small.
So, it seems that a given temperature of surface will radiate at a level determined by surface temperature BUT a portion of that radiation is constantly moving in and out of the conductive/convective exchange and so cannot escape to space from the surface.
This is gobbledygook do a proper analysis.
Radiative theory does not consider the effect of conduction/convection on the ability of a surface to lose energy via radiation. Quite simply, conduction and convection do reduce radiative loss from a surface by tying up a portion of the surface radiation within the ongoing conductive/convective exchange between surface and the mass of the atmosphere.
As stated above their only role is to reduce the surface temperature which has a negligibly small effect on radiation loss.
Conduction and convection reduce surface temperature on uplift and increase it on descent. The two balance in order to sustain atmospheric height.
The heating of the surface on descent is effected not by direct transfer of energy from air to ground but via adiabatically warmed descending air reducing the lapse rate slope and thereby inhibiting convection.
It is the inhibiting of convection globally beneath columns of adiabatically warmed descending air within surface high pressure cells that acts as a greenhouse effect.
Just like in a true greenhouse the warmed descending air becomes transparent (just like a greenhouse roof) through the dissipation of clouds and the layer of warmed air inhibits convection (just like a greenhouse roof).
That is why the mass induced greenhouse effect was so decribed by meteorologists from the very beginning.
The astrophysicists who took over climate science didn’t know much about meteorology.
This is just an observation I have found. Perhaps someone can explain it.
DWLWR shines both day and night, correct? And the figures I see batted about are roughly 320 w/m-2. So how come on clear night I don’t feel this 320 w/m-2 when I am out in the open? 320 w/m-2 is a lot of energy. That’s the equivalent of standing out in direct sunlight at about 9 to 10 in the morning. You can feel that heat on your face. But at night, I don’t feel a thing when walking out of a covered area into this 320 w/m-2 of intense radiation. I mean this is the equivalent of over three 100 watt light bulbs shining over a square meter of surface. I should feel the heat on my face. This should warm up exposed pavement or concrete, no? But the concrete under my car feels the same temperature as the concrete exposed to this DWLWR.
Forget the ocean warming business. Does DWLWR heat up the earth’s ground surface? Seems like an easier experiment to perform.
Thanks for the question, SkepticGW.
At about nine or ten AM, depending on location, you get about 320 W/m2 from the sun PLUS about the same amount from longwave radiation. This gives a total of about 640 W/m2 which is clearly perceptible.
Next, you say:
Nor should you … because you also get radiation from the covered area. Folks think that a roof shades you from the IR just like it shades you from the sun. And in fact it does … but while the roof is not radiating light, it’s radiating IR.
As a result there’s a big difference between what a roof does to visible light (cuts it off) and what it does to the IR (replaces DWIR with IR from the roof.)
Finally, you need to remember that 320 W/m2 is not “intense radiation”. It has the black-body temperature of about 0°C … so it has the same effect on your body as the radiation from a block of ice.
w.
–SkepticGoneWild
December 17, 2014 at 8:26 am
This is just an observation I have found. Perhaps someone can explain it.
DWLWR shines both day and night, correct? And the figures I see batted about are roughly 320 w/m-2. So how come on clear night I don’t feel this 320 w/m-2 when I am out in the open? 320 w/m-2 is a lot of energy. That’s the equivalent of standing out in direct sunlight at about 9 to 10 in the morning. You can feel that heat on your face. But at night, I don’t feel a thing when walking out of a covered area into this 320 w/m-2 of intense radiation. I mean this is the equivalent of over three 100 watt light bulbs shining over a square meter of surface. —
The 3 lightbulbs is more like the direct light of sunlight.
Or 3 100 watt halogen spotlights would be even more like sunlight.
DWLWR is not directed light.
Some people claim that DWLWR is like the infrared light of the walls of a dark cave or the IR of a ice cube.
Generally speaking DWLWR seems to me to be ill defined, but everyone would agree it’s not
direct light. Or wiki says about sunlight
“When the direct solar radiation is not blocked by clouds, it is experienced as sunshine, a combination of bright light and radiant heat. When it is blocked by the clouds or reflects off other objects, it is experienced as diffused light.”
And I would add that such diffused sunlight is more directed light than DWLWR.
Of course also everyone would agree that DWLWR is not the high energy of visible light.
So one point to the blue sky as something like DWLWR, except the blue sky is scattered blue visible light which very different species as compared to longwave IR.
Though if want to use visible light as analogy, photography deals with direct and indirect light:
“If you really think about it, there is no fundamental difference between direct light and indirect light. One is simply the overabundance of another. When you say the light is direct, you are saying a lot more about what isn’t happening than what is. Direct light is the absence of light on any other side except the side with a single light source.
Indirect light, by contrast, is light coming in from all sides. Technically speaking, there’s always some small amount of light bouncing around and hitting your subject on all sides too. The difference with indirect light is that it is more evenly balanced. In other words, the light reflecting onto the other side of your subject is nearly the same intensity as the source itself.
Most indirect light isn’t completely indirect either. The original source is usually a little brighter than the sources of reflected light. To see what I mean, just think of an overcast day. We would say this is an indirect lighting situation, but if you look at the sun, it’s still brighter than anything else around. The real difference, then, is the degree to which it is bright. On an overcast day, it’s relatively less bright than on a fully sunny day.”
– See more at: http://www.digital-photo-secrets.com/tip/2099/direct-and-indirect-light/#sthash.6APovvXd.dpuf
richard verney December 16, 2014 at 7:42 pm
Kristian
I am sceptical as to whether DWLWIR is anything more than a signal, ie., it is something that can be measured, it is something that can tell us something about the temperature of an object, but whether it is something more than that, in particular capable of performing work, in the environ in which it finds itself, is moot. Accordingly, I am sceptical of its existance as an extra, separate radiative flux/transfer of energy from atmosphere to surface. My first post looks at the net flow position only. As i said, solar provides all the energy that the oceans need to prevent the oceans from freezing. It is solar, and only solar, that warms the oceans.
However, as far as my discussions with Willis are concerned, i am adopting the position; let us accept for the sake of argument that DWLWIR does exist as an energy flux, in which case how is it absorbed and how is it handled and what consequences flow from this? If it is a real energy flux (and goes towards warming the oceans) then I foresee problems with this due to the absorption characteristics of LWIR in water.
There is a heck of a lot of energy going into just 3 microns of water. This energy is highly concentrated and unless sequestered to depth at a rate faster than that energy would otherwise drive evaportation, there would be a heck of a lot of evaporation which we are not seeing.
That’s because it’s radiating from the surface as I’ve pointed out to you several times but you choose to ignore! How do you think the SST is measured by satellites?
The annual rainfall would be far higher. We know the annual rainfall (within say a 100% error band) so we know the maximum evaporation from the oceans. This is far less than would occur given the amount of energy that DWLWIR would impart into the top 3 microns of the oceans unless that energy could quickly be diluted to volume, which means quickly sequestered to depth.
I want to explore what happens in the real world if the energy that Willis claims is absorbed by the oceans is truly so absorbed. I am testing that.
Except when it conflicts with your preconceived ideas, then you ignore it!
Either do a proper energy balance for the skin layer or stop wasting space here.
Phil., December 17, 2014 at 9:06 am:
“That’s because it’s radiating from the surface as I’ve pointed out to you several times but you choose to ignore! How do you think the SST is measured by satellites?“
Thanks for the good laugh, Phil 🙂 So, the satellite-borne instruments in space detect radiative ocean surface fluxes of 369 W/m^2 here, 424 W/m^2 there and 473 W/m^2 over there and from this they can easily calculate the SST in each place, courtesy of the Stefan-Boltzmann equation: 11, 21 and 29 degrees Celsius respectively. Is that it, Phil? Is that how they do it? Tell me that’s what you think …
richard verney December 17, 2014 at 5:34 am
Willis
As you are aware, I have not attacked you once. At most I have suggested that your answering is like that of a politician. That is not an attack, that is an observation on an approach/style to dealing with issues/questions. It is not even a derogatory comment, since many politicians are quite intelligent.
When mpainter suggested/implied that you had given no answer as to mechanisms that you claim mix the DWLWIR into the oceans, I suggested that was not so and that you had given an indication of mechanisms, but what was missing was the speed at which these processes mix DWLWIR absorbed in the top 3 microns to depth, and hence dilute the energy to volume.
But the predominant mechanism by which energy is lost from the surface is radiation upwards, not mixing to the depths, a fact you continue to ignore.
The issue here is quite simple. The claim that DWLWIR is absorbed in the ocean leads to a number of issues. In particular, the energy absorbed is extremely concentrated somewhere betweem 60 to 80% of DWLWIR is concentrated in just 3 microns, and that is a heck of a lot of energy. That energy (if capable of performing sensible work in the environ in which it finds itself) would drive copious amounts of evaporation and/or carry the water vapour evaporated to great height, unless it is sequestered to depth (thereby dissipating and diluting the energy into a larger volume) at a rate faster than the rate that that energy would if not sequestered to depth, drive evaporation from the top microns.
Or what actually happens, radiation from the surface, if you want to do heat transfer analysis you should include all the processes, not pick and choose.
–But the predominant mechanism by which energy is lost from the surface is radiation upwards, not mixing to the depths, a fact you continue to ignore.–
Energy is not lost “mixing to the depths”.
Mixing causes heat to be more uniform. Mixing is heating something which is colder- causing a increase of the overall amount of energy content- it’s heating something..
Though nearly all energy leaving Earth comes from some kind surface and things which can have a surface are liquids or solids- as compared to gases – a surface being comprised molecules in structural molecular bond.
Only a surface can reflect or direct radiant energy and only directed radiation can warm anything
by any significant amount. And only a surface can have a blackbody spectrum of light.
The surface [skin surface] of ocean and land is different, with the land most of the sunlight reaching the surface is radiated back into the universe and large amount is re-radiated in less than a second.
With ocean most of the energy of sunlight penetrates below couple feet under surface of the water.
Since most of Earth is covered with ocean, most of the energy of sunlight reaching Earth enter the ocean below it’s surface. And any heat under the water can not radiate up to the universe.
What can travel thru many meter of water is visible light, blue visible light can travel to furthest
thru to water and this is related to why the ocean is blue.
So for instance at 100 meter under water only blue light [and some UV ] can go thru so much
water as you go deeper than 100 meters under the surface eventually all visible light is stopped.
“Light may be detected as far as 1,000 meters down in the ocean,
but there is rarely any significant light beyond 200 meters.”
http://oceanservice.noaa.gov/facts/light_travel.html
Phil. December 17, 2014 at 9:22 am
Phil … now you’re starting to see why discussing things with richard goes nowhere. He’s not looking for an explanation. He’s looking to nit-pick, and he will happily ignore the facts and your answers, over and and over, until you get tired of endlessly answering.
And of course, at that point he’ll claim victory on the basis that, despite the fact that you’ve answered him over and over, you’re not answering …
w.
Hi Kristian, I see you’re back reprising your role of class clown. Interesting that accurate science amuses you so much.
If you’d have taken the trouble to look it up you would have found that the SST is indeed measured using IR radiometry, a slightly more sophisticated approach than you describe though. The Advanced Very High Resolution Radiometer (AVHRR) measures at 3.7, 11 and 12 microns as does MODIS, AMSR-E uses microwave in a similar manner in order to see through the clouds. So yes SST is measured using the very process that Verney and Painter exclude from their analysis of the ocean surface.
Avoiding the question, are we, Phil? Are the satellites estimating SST based on ‘flux intensity’ (like the rGHE is) or based on ‘frequency distribution’?
Avoiding the question, are we, Phil? Are the satellites estimating SST based on ‘flux intensity’ (like the rGHE is) or based on ‘frequency distribution’?
No I answered the question you asked. The SST is measured by satellites based on IR radiometry, tough to do if it doesn’t exist as Verney seems to think.
Phil. December 17, 2014 at 6:52 pm
“No I answered the question you asked. The SST is measured by satellites based on IR radiometry, tough to do if it doesn’t exist as Verney seems to think.”
No, you’re specifically avoiding what I’m getting at. You’re building a straw man to tear down. No one is claiming (not Verney either) that there is no IR being emitted from the surface. The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating. And even when confronted with it, you don’t fully want to scrap the idea. You state in your last answer: “(…) the SST is indeed measured using IR radiometry, a slightly more sophisticated approach than you describe though.”
Of course it’s using IR radiometry. That’s not the point. The point is: Do the satellite instruments estimate the SST based on detecting a BB ‘flux intensity’ from the surface of 395 W/m^2 (like what the rGHE hypothesis and its proponents would suggest, THAT’S the point) or do they do it based on a specific ‘frequency/wavelength distribution’ of the IR detected? If the latter rather than the former method is what’s used, then your original straw man argument has no bearing on the issue we’re discussing here at all.
It’s been shown
http://wattsupwiththat.com/2014/12/09/arguments-for-and-against-human-induced-ocean-warming/#comment-1812746
that the postulated BB emission flux from the surface is NOT measured in the sense of being ‘detected’. It is ONLY calculated by directly applying the Stefan-Boltzmann equation to the assumption that the surface is a thermally isolated, pure emitter (that is, in a purely radiative setting), which it is NOT. The 395 W/m^2 is not an actual flux/transfer of energy, no more so than the DWLWIR 340 W/m^2 is an actual flux/transfer of energy. Only the HEAT is. The two ‘hemifluxes’ are both merely mathematical constructs, conceptual, potential heat fluxes. The evidence is right there in the method descriptions of how they’re ‘found’, Phil.
Kristian December 17, 2014 at 11:04 pm
Phil. December 17, 2014 at 6:52 pm
“No I answered the question you asked. The SST is measured by satellites based on IR radiometry, tough to do if it doesn’t exist as Verney seems to think.”
No, you’re specifically avoiding what I’m getting at. </em.
No you keep changing what you're 'getting at' and it bears no relevance to what I said in any case.
You’re building a straw man to tear down. No one is claiming (not Verney either) that there is no IR being emitted from the surface.</em.
As pointed out above, by explicitly omitting the role of IR emission at the surface of the ocean in his analysis and refusing to even discuss it, that's exactly what he's doing.
The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating.
No I explicitly state that, it’s been measured many times, here’s an example:
http://www.azimuthproject.org/azimuth/files/emission.png
The measurement shows the radiance from the surface with absorption from atmospheric trace gases such CO2, H2O, O3 and CH4. Satellite measurements of the radiance are made in the region indicated as ‘Window’ to avoid interference by these species.
And even when confronted with it, you don’t fully want to scrap the idea. You state in your last answer: “(…) the SST is indeed measured using IR radiometry, a slightly more sophisticated approach than you describe though.”
Of course it’s using IR radiometry. That’s not the point. The point is: Do the satellite instruments estimate the SST based on detecting a BB ‘flux intensity’ from the surface of 395 W/m^2 (like what the rGHE hypothesis and its proponents would suggest, THAT’S the point) or do they do it based on a specific ‘frequency/wavelength distribution’ of the IR detected? If the latter rather than the former method is what’s used, then your original straw man argument has no bearing on the issue we’re discussing here at all.
As stated they do it by measuring the radiance in the ‘Window’ region so by your own admission I’m correct.
It’s been shown
No it hasn’t that’s just your repeating the same mantra.
that the postulated BB emission flux from the surface is NOT measured in the sense of being ‘detected’. It is ONLY calculated by directly applying the Stefan-Boltzmann equation to the assumption that the surface is a thermally isolated, pure emitter (that is, in a purely radiative setting), which it is NOT. The 395 W/m^2 is not an actual flux/transfer of energy, no more so than the DWLWIR 340 W/m^2 is an actual flux/transfer of energy. Only the HEAT is. The two ‘hemifluxes’ are both merely mathematical constructs, conceptual, potential heat fluxes.
No that’s your pet theory which no text on radiation heat transfer endorses, try ‘Hottell and Sarofim’ or ‘Holman’ for example.
Phil., December 18, 2014 at 6:25 am:
Me: “The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating.”
Phil: “No I explicitly state that, it’s been measured many times, here’s an example:
The measurement shows the radiance from the surface with absorption from atmospheric trace gases such CO2, H2O, O3 and CH4. Satellite measurements of the radiance are made in the region indicated as ‘Window’ to avoid interference by these species.”
For crying out loud, are you pulling my leg!? Why are you posting a ToA spectrum when arguing about surface emissions!? And how are these spectra actually generated? Any idea? Read up on it?
“As stated they do it by measuring the radiance in the ‘Window’ region so by your own admission I’m correct.”
*Sigh* Avoiding the issue ………..
“No it hasn’t [been shown] that’s just your repeating the same mantra.”
In the link provided it is shown, yes. It says so right in the text of the article discussed. Surface emission is NOT ‘measured’. It is CALCULATED using a specific S-B formula. It’s right there, Phil. You can deny it all you want. Read the bloody paper!!!
You’re looking more and more silly, Phil.
“No that’s your pet theory which no text on radiation heat transfer endorses (…)”
Heh, I’m sorry, Phil, but the physical discipline concerning itself with system/object temperature change from energy transfer is called … THERMODYNAMICS.
It is quite obvious that you have never opened even an introductory textbook on this particular subject.
I’ll repeat what I told you upthread: “Out here in the real world, radiative transfers and their resulting effects must comply with the Laws of Thermodynamics before anything else, just like conductive transfers and their resulting effects have to. In the real world, in nature, it is not allowed for an energy transfer from a cool place to a warm place to make the temperature of that warm place rise. It simply doesn’t happen. If you observe something where it might LOOK like it does, it is your interpretation of what happens that’s wrong.”
Yes Willis, I’m very patient though, I more interested in rebutting his nonsense so that other readers will realize he doesn’t have a clue about the subject and that he’s the one who doesn’t address the point.
Well then, proceed, Phil., rebut . I am interested.
I’ve already rebutted it, verney doesn’t answer.
Willis Eschenbach
December 17, 2014 at 9:51 am
/////
Willis
First you suggest that I am acting like a prick.
Now you suggest that I am lying.
Please quote my questions (together with time/date of posting) and please quote your answers thereo (together with time/date of posting)..
Lets see to what extent you have answered my questions.
As I mentioned above, richard, there is no end to your questions. I already answered this very question of yours above, and now you want me to answer it again.
I’m done with you.
w.
Are you done with me Willis? Because I’ve got some more questions for you but I’m afraid to ask lest I get called a p***k.
[language. .mod]
Thanks for the offer, mpainter, but I’ll pass. You wanted to jump in on richard’s behalf. There was no reason for you to be involved at all. As a result, I fear you have cancelled your vote on my planet.
Best regards,
w.
Goodness, gracious, moderator, you have some more work up thread.
“You better watch out,
You better not cry,
You better not pout,
I’m telling you why:
Santa Claus is coming to town!
He’s making a list,
And checking it twice,
Gonna find out who’s naughty or nice.
Santa Claus is coming to town!”
Read more: Christmas Song – Santa Claus Is Coming To Town Lyrics | MetroLyrics
mpainter December 17, 2014 at 6:21 pm
Not on my behalf he doesn’t … been there, done that several hours ago after reading your previous comment. You’re late to the party.
However, there may be someone other than me upthread, I haven’t read all the replies. I gave up on that a much earlier.
w.
Willis
It is a very serious allegation to call someone a liar, and I note that you have not substantiated that your derogatory allegation made against me is true.
As you are aware, I have not been asking you what is the energy budget of the oceans. Nor have I been asking you whether clouds warm, or slow the rate of cooling. I have not assked you rto restate your steel greenhouse etc. All of those issues may or may not be interesting, but they were not relevant to the specific questions that I was asking. To talk about them, is not to answer my questions..
My questions centre upon exactly what is going on in the top 3 microns of the oceans, and if DWLWIR is absorbed and capable of performing sensible work, whether there are processes that can mix the energy so absorbed (which is in the region of 200 to 300 W/sq.m) and sequester this energy to depth at a rate faster than the rate that the energy absorbed in the top 3 microns would drve evaporation if that energy remained concentrated in the top 3 microns and was not sequestered to depth and thereby diluted by volume. I have asked you about the physics of the top 3 microns and in particular the rate at which any physical process can effectively mix energy and sequester it to depth at a rate which is fast enough to evaporation from the very top of the ocean. .
I have not checked the above, but if I recall correctly, you have not mentioned to me (but rather to someone else) that the energy in the top microns is mixed by wind and waves and cold water sinking. You may or may not also have mentioned ocean over turning. I recall in the past you have mentioned that.
Accordingly, although I believe that those processes were never directed to me in a direct answer to my question, since those processes have been mentioned in the comment section of this Article, I desired to explore the rate at which these proceesses could effectively mix the energy in the top few microns of water.
I then suggested that one should look at crater lakes where there is little in the way of wind and waves, so that those processes could be ruled out. This would leave just ocean overturning, and I asked you whether that was a 24/7 process, or a diurnal process, and cold water sinking.
I asked you about the oceans in BF3 or less (particularly BF2 or less which given that the global average wind speed over the oceans is said to be a little over BF4, it follows that BF2 or less must be being experienced some 20% or so of the time), since again the action of wind and waves could largely be ignored. That again would leave just ocean overturning (to which whether it is a diurnal process would be relevant) and cold water sinking.
I asked you about a dew drop on a still winter’s day where there is no action of wind, waves or ocean overturning, so those processes could be completely ruled out. This would leave just your cold water sinking theory, and I pointed out that even if cold water sinks as you suggest this would go to mix energy into the very small volume of the dew drop and would still result in the dew drop being burnt off/evaporated if DWLWIR is being absorrbed by the dew drop is capable of sensible work and actually heats the dew drop.
You have not answered those questions.
Let us not waste time arguing whether you have or have not answered those questions. Instead, now set out your answers..Of course, you may cut and paste any previous answer that deals with the rate of mixing wwith respect to each process that you claim mixes the energy, and/or which specifically deals with crater lakes, with oceanss in BF3 or less, with dew drops.
I hope that you will now constructively respond taking the debate forward.
richard verney December 18, 2014 at 1:34 am
I then suggested that one should look at crater lakes where there is little in the way of wind and waves, so that those processes could be ruled out. This would leave just ocean overturning, and I asked you whether that was a 24/7 process, or a diurnal process, and cold water sinking.
I asked you about the oceans in BF3 or less (particularly BF2 or less which given that the global average wind speed over the oceans is said to be a little over BF4, it follows that BF2 or less must be being experienced some 20% or so of the time), since again the action of wind and waves could largely be ignored. That again would leave just ocean overturning (to which whether it is a diurnal process would be relevant) and cold water sinking.
I asked you about a dew drop on a still winter’s day where there is no action of wind, waves or ocean overturning, so those processes could be completely ruled out. This would leave just your cold water sinking theory, and I pointed out that even if cold water sinks as you suggest this would go to mix energy into the very small volume of the dew drop and would still result in the dew drop being burnt off/evaporated if DWLWIR is being absorrbed by the dew drop is capable of sensible work and actually heats the dew drop.
In all of those cases it leaves what you don’t wish to face up to, radiational loss from the surface, in the case of a dew drop at ~5ºC that would be ~330W/m^2.
Phil. ,
Thanks for the temp. profile of the sea surface that you provided in your comment above at 12-16, 7:45am. I have studied this before, but you have handily provided it for this dicussion.
This profile pretty well refutes the notion that energy from incident IR is mixed or conducted downward before it is converted to latent heat., as put by Willis, Judith Curry, and others. Specifically, the day temp. profile shows that there is no overturning of the water down to 10 meters. Nor can heat be conducted downward from this cooler interval to the water below. The night temp. profile, in comparison, shows that overturning, or convection, is the dominant process at the sea surface. Note that there is no temp .differential in night from aprox. 1 mm to 10 meters depth, and only about 0.2 C difference from 1 mm to the surface. This contrasts with the temperature “bump” seen in the day temp. profile which is the proof that no convection is occurring during the day, hence no mixing downward.
So far, no one has shown that IR has any effect on SST (or OHC), and indeed, the basic physics refutes the notion.
Clarification: “Nor can heat be conducted downward from this cooler interval..”, meaning the uppermost micro-layer where the IR photons are absorbed. Note the warming from this to the 1 mm point, hence no conduction of heat from incident IR is possible.
It shows quite clearly that heat is lost from the surface via radiation before any other mechanism, setting up a gradient of ~0.3ºC/mm over the first mm at both day and night, allowing conduction from below. During the day the layer from 1mm to 10m is warmed by solar insolation. The air at the air/water interface is saturated and at about 2m above the surface it’s typically 0.8RH so the amount of latent heat is controlled by mixing in this layer.
So far, no one has shown that IR has any effect on SST (or OHC), and indeed, the basic physics refutes the notion.
On the contrary the basic physics shows that it has a major effect.
Phil.,
Is this some kind of game?
You are not to be taken seriously. Once more, the issue is IR incident on the sea surface and its contribution to SST and OHC.
I gotta admit, I don’t understand this concern about the skin layer. A certain amount of the incident infrared energy is mixed downwards because the skin layer is not stable. This is because it is constantly cooling, which makes it denser than the underlying layers. A certain amount is mixed downwards by both waves and wind.


However, the majority of the downwelling infrared is simply re-radiated. Here’s a graphic showing the process:
The solar radiation plus some amount of the IR ends up in the mixed layer. From there, the energy returns to the surface where it is lost through the three processes (radiation, latent and sensible heat loss). It is important to notice that the amount of energy entering the mixed layer is equal to the amount leaving it.
Note that this downwelling IR keeps the entire mixed layer warmer than it would be without the IR. Suppose we could shut off the IR for an hour (or alternately that the DWIR is not absorbed by the ocean for an hour). Since the radiation and the latent and sensible heat loss would continue, the energy that is no longer supplied by the DWIR would be extracted from the mixed layer, which would leave it cooler than it is with the DWIR. Here is that situation:
Now, here’s the problem. The mixed layer is getting 186 W/m2 … but it’s losing 530 W/m2. Which is why I keep asking, if the DWIR is not absorbed by the ocean, why isn’t the ocean frozen?
As Phil points out above, this same process is true for dew … or for Crater Lake. Obviously in dew or in Crater Lake the amount of DWIR mixed downwards would be smaller than in the open ocean. But in both cases, the incoming IR is immediately lost in the form of radiation, evaporation, and conduction.
And in both cases, this DWIR leaves the underlying water warmer, despite the fact that the IR is absorbed in the skin layer.
w.
Agreed Willis, we must have been typing at the same time, I would have drawn basically the same flow diag as you. One aspect we both left out was the contribution of salinity to the convection term, important in the arctic.
–Now, here’s the problem. The mixed layer is getting 186 W/m2 … but it’s losing 530 W/m2. Which is why I keep asking, if the DWIR is not absorbed by the ocean, why isn’t the ocean frozen?–
So why isn’t the tropical ocean not frozen?
In this same regard, what is the difference between a cloud and a ocean.
Or what is the difference between the question, “why isn’t the ocean frozen?”
and the question “why isn’t the clouds frozen?”
One could answer by saying that there is a lot frozen water in clouds.
But cloud are at higher elevation and per 1000 meter elevation
it’s at least 5 C colder than the sea level surface, so first need the clouds
to freeze before lower elevation water freezes.
Or if at say 5 pm the sky is covered with clouds and it’s more than 5 C [45 F]
I would predict that it isn’t going to snow at nite. And/or bodies of water are not
going to freeze during the night. Whereas if instead it’s clear skies, I tend to think
it will freeze during the night. Of course it’s different if already freezing temperature
at 5 pm. Though if it’s windy and/or I know cold front sweeping down from northeast
then ir’s going to snow. Or if raining and around 40 F, the rain will become snow
during the night. One begins to get partial snow at about 38 F. Or at 40 F raining
at sea level, it’s starting to snow at 1000 ft elevation. Or in LA if 40 F and raining, in Big
Bear or Snow Summit it’s snowing or you will have snow if you go up the Grapevine
on I-5. So when raining in LA and it’s 40 F in clouds less than 1000 meters higher
the air is at freezing. And these cloud have been frozen- or must be a significant portion
of the cloud with ice.
So before ocean could have any chance of freeze any cloud 1000 feet higher above it must already be at least partially frozen. And though it does rain in LA when temperature are around 40 F [4 C] and ski mountains and grapevine gets snow. It very rare to have snow- even on the Hollywood Hills. And it would need to get even colder to freeze the Silverlake reservoir.
So rather than ocean freezing the first question could why aren’t the clouds frozen?
Or first clouds must freeze, if clouds aren’t frozen there is no way ocean can freeze-
unless there are no clouds in tropics.
Or if wondering about freezing at surface, first look freezing at cloud.
And:
“At an altitude of roughly 5-6 kilometers, the concentration of greenhouse gases in the overlying atmosphere is so small that heat can radiate freely to space.”
http://earthobservatory.nasa.gov/Features/EnergyBalance/page6.php
And clouds can be higher than 5-6 kilometers.
So say have region which is 1000 km by 1000 km square [on flat and uniform ocean area] and in this region one has various elevations of clouds which include a cloud 5 km high and clouds at 4 km, 3, 2, and 1 km elevation.
In order to get freezing temperature in this region, first the 5 km high cloud must freeze, then followed with 4, 3, 2, 1 km level clouds.
Then one can wonder about the zero elevation water freezing.
And what about clouds stop them from freezing. Why don’t clouds at same elevation as water
freeze long before water freezes. They have a vast amount surface area. It seems if you were to design water so freezes the quickest, you would make a cloud.
Well, Willis, such pretty pictures you have. They are wrong but Ill never convince you of that, Ugly fellow that I am.
My method is to study the physics of the sea surface. Nature instructs us.
In other words, you have no scientific arguments so you wave your hands.
Cute.
w.
Turn the crank on your DWIR zapper as fast as you may, you cannot overturn the physics of the IR absorbancy of water (water being opaque to IR).
Willis,
I must say, your energy budget diagrams are pretty freaky-looking. I mean, you appear to have no clue about how real-world objects warm and attain their temperature. If you do have a clue, you certainly don’t seem to put that knowledge to use. You seem to promote the idea that it’s all about fluxes. It’s not. Rather, it’s all about the total amount of stored energy, internal energy [U], up to the point of dynamic equilibrium (heat OUT [Q_out] finally balancing heat IN [Q_in]) and system ‘heat capacity’. The energy fluxes to and from an object in a steady state (dynamic equilibrium) are just there to balance Q_in with Q_out, to maintain the temperature already achieved. They are just the dynamic throughput. If they balance across a cycle, they don’t add to or subtract from the internal energy of the object (system) across that same cycle. And hence don’t affect mean temps in the least.
Only for a thermally isolated ‘pure emitter’ (an object/a surface in a purely radiative setting, all heat gain and heat loss through radiation only, radiating into surroundings at 0 K or at least very much colder than itself) will the final (steady-state) temperature correspond to the magnitude of the instantaneous radiative input flux [Q_in] and thus to the final magnitude of the instantaneous radiative output flux [Q_out]. In all other settings, this relationship is broken. With a massive atmosphere in place, all bets are off.
I’ll reiterate my money analogy from upthread:
“If I get 100$ from my bank and then hand them all to my friend, upon which he hands 90 of them straight back, then I end up with 90 dollars.
The rGHE “back radiation” argument then goes as follows: I get 190$ IN, but give away 100$, so end up with 90$. The 190 dollars IN are counted like this: 100$ from the bank + 90$ back from my friend; the 100 dollars OUT are simply the 100$ I hand over to my friend.
In this world view it would thus seem that there is 190$ in circulation. But we all know that there is only the 100$ originally from the bank available.”
This is directly equivalent to the warped rGHE “back radiation” argument for the surface of the Earth:
“You can just ADD the DWLWIR ‘flux’ as an extra INPUT of energy to the surface, as an addition to the original solar heat flux, thus directly creating extra warming (as if they were two of a kind, as if they were both heat fluxes); 165 W/m^2, 232K >> [165+345-112=] 398 W/m^2, 289K.”
– – –
There is only the 165 W/m^2 available (in from the bank, the Sun) as energy throughput for the surface system in a steady state at any one time. There is no ‘extra’ energy from the atmosphere! For the Earth system as a whole, the energy available increases to 240 W/m^2:
http://i1172.photobucket.com/albums/r565/Keyell/Varmesyklusen_zpsd9293c3e.png
Kristian 4:44am: Your diagram is faulty because you continue to become confused about basic physics invoking heat transfer when only energy transfers in nature. Your diagram indicates humans shouldn’t be able to see the atmosphere as your atm. white block doesn’t have an arrow for radiative energy transfer toward surface. Yet humans can see the sky and clouds thru natural radiative energy transfer. Back to the drawing board Kristian. Study Willis’ diagram 1:22pm to find faults in your diagram and physics of how humans can see clouds.
Also your banking analogy is faulty; banks can create money thru their lending practices; money supply is not conserved, energy is conserved.
In short, Willis, you appear to believe that the surface of the Earth needs to absorb a total energy flux of [165+345-112=] 398 W/m^2 (which is NOT a heat flux – it’s … something else, a mathematical construct) in order to be able to maintain its temperature of 289K. But the FLUXES are not the ones that generate the temperature. The temperature corresponds to and is ‘maintained’ by the INTERNAL ENERGY [U] (+ system ‘heat capacity’), the energy statically held within the system in question. The fluxes [Q_in and Q_out] (energy dynamically moving between (to and from) systems) are only there to balance the energy throughput (IN – OUT = 0). These are some of the most fundamental principles in Thermodynamics. And yet they’re summarily ignored.
Trick, it is thoroughly warped world views like the one you’re promoting here that make it possible for utterly absurd and backward hypotheses like the rGHE/AGW one to flourish in this modern age.
So with 165 watts going into the ocean, it’s like solar water heater getting 3960 watts hours per day.
So solar water heater can operate at about 60% efficiency. If solar water heater received 7200 watts hours per day of solar flux at .6 that is 4320 watts of storable heat. And there are places on earth where one can get 7.2 Kw per day. ‘
BUT ocean can more efficient than the human made solar water heater, because it has a “different” requirement. Because man made solar heater requires hot water, and ocean just need “air around temperature”.
So if just wanted slightly warmer water than tap water you could make a more efficient solar water heater [but other heating a pool, no one wants this].
And if the tropics were cooler than we have at moment, the Ocean could be even more efficient and/or get more watts of solar flux because there are less clouds.
And of opposite, if warmer than we have now, it would less efficient and less solar flux.
Or terms of efficiency solar ponds are consider to be cheap and very efficient. Oceans cost nothing and I would say are even more efficient [for Mother Earth’s purposes] than solar ponds.
And wind, evaporation, and waves [for Mother Earth’s purposes] make the ocean more efficient- in terms of warming earth].
So rather thinking mother nature can’t get 60% efficiency by accident [as not being as clever as humans] the ocean could be somewhere around +90 efficient [or 110 % efficient- because we too stupid to figure it out- like the bumble bee doing the impossible of flying].
Kristian 6:37am – I just looked outside. I can see the clouds today which is impossible in your faulty diagram.
Willis’ diagram explains per basic physics; your diagram is too confused to offer even a basic physical explanation. I suggest start with Planck’s original paper to find why you are incorrectly stating “With a massive atmosphere in place, all bets are off.” This situation is exactly addressed in that original paper and still offers the best explanation for why humans can see cooler clouds.
According to you Kristian: “The two ‘hemifluxes’ are both merely mathematical constructs, conceptual, potential heat fluxes”, so why do you treat the fluxes at the ToA differently than the fluxes at the surface? Are they not mere mathematical constructs? You also state that: “In the real world, not in the conceptual world, the HEAT FLUX is indivisible. All there is. It is ONE flow, one transfer of energy. There is no way you can physically split it into two separate, oppositely flowing streams of energy.” Surely that also applies to the sun/earth system? In that system they’re not even “oppositely flowing”.
Phil., December 19, 2014 at 7:39 am:
“According to you Kristian: “The two ‘hemifluxes’ are both merely mathematical constructs, conceptual, potential heat fluxes”, so why do you treat the fluxes at the ToA differently than the fluxes at the surface? Are they not mere mathematical constructs?”
Because Earth’s final radiative flux through the ToA to space is an actual, detectable flux/transfer of energy, that is, a HEAT FLUX, Phil. You need to start grasping the distinction.
The 240 W/m^2 mean radiative flux emitted by Earth to space is equivalent to the 240 W/m^2 mean radiative flux absorbed by the Earth system from the Sun. They are both thermodynamically working fluxes of energy between systems, i.e., HEAT FLUXES. Q_in and Q_out. They can do stuff. For the surface, the 240 W/m^2 fluxes are equivalent (directly comparable) to the incoming 165 W/m^2 solar radiative HEAT FLUX absorbed, AND to the outgoing 53 W/m^2 terrestrial radiative HEAT FLUX emitted. Those purely calculated 398 W/m^2 UWLWIR and 345 DWLWIR ‘fluxes’ of yours are nothing of the kind. Neither of them. THEY ARE NOT HEAT FLUXES, Phil. THEY ARE NOT ACTUAL, DETECTABLE FLUXES/TRANSFERS OF ENERGY AT ALL! They don’t do anything. Only the heat (what you call the ‘net’ of the two) is able to do thermodynamic work.
Yet you insist that we TREAT them as if they were heat fluxes. You expect everyone to agree that your calculated DWLWIR ‘fux’ will directly raise the temperature of the surface upon absorption. Then you effectively expect us to accept that a HEAT FLUX is transferred from the cool atmosphere to the warm surface. It doesn’t happen, Phil!
“You also state that: “In the real world, not in the conceptual world, the HEAT FLUX is indivisible. All there is. It is ONE flow, one transfer of energy. There is no way you can physically split it into two separate, oppositely flowing streams of energy.” Surely that also applies to the sun/earth system? In that system they’re not even “oppositely flowing”.”
Yes, of course. Hot Sun naturally heats cool Earth. Earth doesn’t heat the Sun. Just like the warm surface heats the cool atmosphere, NOT the other way around. There are TWO separate heat transfers going on here, if you’ve noticed …
Kristian 11:43am: “Those purely calculated 398 W/m^2 UWLWIR and 345 DWLWIR ‘fluxes’..”
More confusion shown by Kristian in using the word heat. The 345 DWLWIR flux is not “purely calculated”; humans can see part of the DWLWIR (clouds!) which can increase the thermometry measured temperature of ocean surface water night and day as shown in top post data. The DWLWIR flux is very physical Kristian; fix your confusion – drop heat from your confusing vocabulary and invoke use of energy transfer per Dr. Max Planck’s paper. Enter into modern times.
Suppose one had PV panels floating under the surface of the ocean.
So 1 meter under water and they were a meter square and they neutral buoyant so always had the 1 meter panel 1 meter under the surface and they were able to float at an angle so they were roughly facing the sun. Or point at the sun better than solar panel on someone’s roof.
And have them crowded into a 1 square km area ocean so that 1/2 of the total area was used and not shading each other. Or as efficient use of 1 km square area because one does need access them with needing space for roads. So 2 1 square km gives a 1 km square of area which receives solar energy.
So these things are going move with a wave just as boat or log moves with the waves.
So the upshot is these solar panel could get as much electrical power as solar panel on land.
Or the small amount solar flux blocked by the water isn’t a wavelength that solar panel get energy from, and keeping the solar panel cool increases it’s efficiency. So it’s even possible they work better at getting solar energy than solar panels on land.
Now let’s make big enough so one could imagine it could have some global affect on temperature. So a 1 by 2 km area has 1 million square meter of panels receiving sunlight. And 1000 by 2000 km has 1 trillion square meters.
Now where a solar map of ocean?
http://oceanworld.tamu.edu/resources/ocng_textbook/chapter05/chapter05_06.htm
http://oceanworld.tamu.edu/resources/ocng_textbook/chapter05/Images/Fig5-8A.htm
Apparent west Africa can get about 250 W/m2
So get 20% of 250 it’s 50 watts per square meter of electrical power. Though this is averaged
over a day and over a year.
Or at 250 W/m2 in 24 hours get 6000 watts. Or 6 Kw hours. And with 20 efficiency, 1.2 kw hour of electrical power per square meter per day. Or 1 trillion square meters get 1.2 trillion kw hour per day and 438 trillion kw hour per 365 days.
Now what is done with to electrical power depends on whether it’s warming or cooling earth- if used for lasers that sends a friendly message to the rest of galaxy or for lasers to burn a hole in the moon, then doing the most to cool Earth. If simply used to power the electrical grid, then probably having little effect in terms of net warming or cooling. If the electrical power it used to warm the bottom of the ocean [say pumping warm water to the bottom] then it’s basically storing heat at the bottom of the ocean- that be about most effective way to warm earth. Though human would see no significant amount of heating, and such heating would trivial to compared a underwater volcanic eruption or dead things decaying, hurricanes, whatever- or lost in the noise.
The other part of it, is what effect to regional environment would be caused drawing that energy from the region?
So before the solar panel are put in, one measures the surface of the water and 100 meter below the surface of the water, and do this for a couple years and make very precise measurements.
So 1 billion dollars spent doing the best job that could be done to measure the area and surrounding area. It’s going to the Moon type project to precisely measure it.
So after you got the solar installed and operating for a year, you go back and measure it again.
What would be the difference in average surface temperature and temperature at 100 meters.
So would the 100 meter be colder or warmer and how much. Would the surface be warmer or colder and how much?
It appears that you are playing some game instead of doing a proper energy balance. Since you claim to have read my post and seen the surface temperature profile it should be clear. You can’t just consider some of the processes and ignore other major ones as you and verney do, that’s nonsense.
Try again, for an ocean at 300K SST, consider a control volume 1m square and 3 micron thick, fluxes through the top surface are DWIR (+ve), IR thermal emission (-ve, ~450 W/m^2), conduction (-ve), evaporation (-ve) and convection (-ve); fluxes through the bottom surface are conduction (+ve), convection (+ve), DWIR (-ve, ~0). Most of the cooling at the surface is due to the thermal emission, both during the day and night. During the day the insolation penetrates and heats the layers below this thin surface layer, it has very little direct contribution to this control volume, flux in and out being virtually the same. Remove the DWIR absorbed in this volume and the surface temperature will drop significantly.
Kristian December 18, 2014 at 3:29 pm
Phil., December 18, 2014 at 6:25 am:
Me: “The issue here is that you imply that we know that the ocean surface emits a BB flux intensity of a full 395 W/m^2 according to its temperature because that’s how the ‘satellites measure the SST’. You don’t come out and say it, but that’s what you’re insinuating.”
Phil: “No I explicitly state that, it’s been measured many times, here’s an example:
The measurement shows the radiance from the surface with absorption from atmospheric trace gases such CO2, H2O, O3 and CH4. Satellite measurements of the radiance are made in the region indicated as ‘Window’ to avoid interference by these species.”
For crying out loud, are you pulling my leg!? Why are you posting a ToA spectrum when arguing about surface emissions!? And how are these spectra actually generated? Any idea? Read up on it?
More clowning around by Kristian, the subject under discussion was ‘satellite measurement of SST’, you had challenged my statement of how such measurements are made. So I presented a spectrum measured from a satellite, and then you protest that it’s made from space! Where do you think satellites make their measurements from?
“As stated they do it by measuring the radiance in the ‘Window’ region so by your own admission I’m correct.”
*Sigh* Avoiding the issue ………..
No, answering your question, you just don’t want to hear the answer.
“No it hasn’t [been shown] that’s just your repeating the same mantra.”
In the link provided it is shown, yes. It says so right in the text of the article discussed. Surface emission is NOT ‘measured’. It is CALCULATED using a specific S-B formula. It’s right there, Phil. You can deny it all you want. Read the bloody paper!!!
I did, it quite clearly says that the surface emission is measured using a Kipp-Zonen net radiometer and the results are presented in figure 7, where they describe it as “observed longwave radiation emitted by the ocean surface”. In their conclusions they state: “During 9 days, 5 min averaged measurements of (1) solar radiation fluxes (incoming and outgoing) and longwave radiation fluxes (atmospheric and surface emission), at 6 m above the sea level;………….were gathered continuously.”
So I’m not the one who’s denying anything.
You’re looking more and more silly, Phil.
No, that would be you.
“No that’s your pet theory which no text on radiation heat transfer endorses (…)”
Heh, I’m sorry, Phil, but the physical discipline concerning itself with system/object temperature change from energy transfer is called … THERMODYNAMICS.
And the discipline that concerns itself with how those transfers occur is HEAT TRANSFER. As Bejan says in his book on the subject: “Heat transfer has become not only a self-standing discipline, but an indispensable discipline at the interface with other pivotal and older disciplines. ………… Thermodynamics today is able to teach modeling, simulation and optimization of ‘realistic’ energy systems because of the great progress made in heat transfer.”
It is quite obvious that you have never opened even an introductory textbook on this particular subject.
Well it’s long been clear that what is obvious to you doesn’t relate to reality, this is another example since I’ve taught the subject at both the graduate and undergraduate level as well as written research papers which required its application.
I’ll repeat what I told you upthread: “Out here in the real world, radiative transfers and their resulting effects must comply with the Laws of Thermodynamics before anything else, just like conductive transfers and their resulting effects have to. In the real world, in nature, it is not allowed for an energy transfer from a cool place to a warm place to make the temperature of that warm place rise. It simply doesn’t happen. If you observe something where it might LOOK like it does, it is your interpretation of what happens that’s wrong.”
That depends on your having a correct view of the Laws of Thermodynamics which you do not. I’ll repeat the observation I made before: a thermocouple measuring 1000K in a open Bunsen burner flame is exchanging radiation with the surrounding surface at 300K, measurements of the energy flux in each direction can be made. Replace the surrounding surface by one at 600K and the measured temperature will go up, as will the measured energy flux in each direction, there is no violation of the Laws of Thermodynamics, net flow is still from hot to cold.
Phil., December 19, 2014 at 7:15 am:
“More clowning around by Kristian, the subject under discussion was ‘satellite measurement of SST’, you had challenged my statement of how such measurements are made. So I presented a spectrum measured from a satellite, and then you protest that it’s made from space! Where do you think satellites make their measurements from?”
Gee, who’s the clown here? Phil insists on ‘misunderstanding’ what I say so that he has something to argue against. I very clearly did not say that the spectrum was ‘made from space’. I said the spectrum presented was a ToA spectrum, not a sea surface spectrum. You cannot read SST from a ToA spectrum, Phil. And satellites cannot measure surface IR flux intensities. There’s an atmosphere in the way. This is well-known by everyone.
BTW, you continue to avoid the issue. Do you believe the satellites somehow do in fact estimate SSTs from surface IR flux intensity, or do you know they don’t? If you do know, why not just come out and admit it? In plain words. Why the constant evasion. Why the smoke and mirrors? What are you afraid of?
“I did [read the paper].”
You obviously didn’t. If you did, you would follow what I was saying in that previous comment I linked to. Take a look at Eq. (4). There’s how the sea surface ‘LW flux’ is ‘measured’, Phil. Not in any other way. All you basically need to measure is the surface temperature. Then you simply put this into the S-B equation. And voilà! This is how it’s always done. It’s no secret at all. You are simply apparently somehow prevented from getting it. It is based on an assumption of the sea surface as a ‘pure emitter’. (We all know it isn’t.)
The Kipp-Zonen net radiometer detect the NET RADIATION (that is, the HEAT FLUX), Phil, and then it has it built in to CALCULATE the DWLWIR ‘flux’ from this and the surface temperature. Simply subtract the computed UWLWIR from the measured heat flux (see Eq. (2)). And voilà! This is invariably how it’s done, Phil. It’s no secret at all. You’re just refusing to realise it, to take it in. Because then, apparently, your world view would crumble.
“(…) it’s long been clear that what is obvious to you doesn’t relate to reality, this is another example since I’ve taught the subject at both the graduate and undergraduate level as well as written research papers which required its application.”
You’re funny, Phil. I’m sure you have. It is still me who refer to reality and you who refer to a specific model of reality. What I discuss is what we actually observe out there in the real world, what physical phenomena we actually detect. What you do is assuming that your interpretation of real-world effects is fact. It’s not, Phil. It’s an assumption, a conceptual model attempt at explaining reality. The old masters inventing and refining this conceptual model knew this perfectly well. They pointed it out all the time. Today this knowledge seems to be lost. People take it for granted as fact. It’s not. It’s tradition.
When I say that what we actually physically detect in any heat transfer situation is the unidirectional HEAT FLUX from hot to cold only, the actual spontaneous transfer of energy between the objects/systems involved due to the difference in their temperatures, I’m telling the truth, Phil. I refer specifically to reality. The real situation. This IS the reality of things.
When you, however, say that in a heat transfer situation, there are in fact TWO opposite fluxes/transfers of energy, one from hot to cold and one from cold to hot, and that the unidirectional heat flux only somehow comes as a netted out end result of these two doing their work at each end, you are not referring to observed reality, Phil. You have to understand this. You are distinctly referring to your model, your interpretation of reality, the principle of bidirectional energy exchange in a heat transfer. This principle arose from the ideas of the archaic (and very much outdated) ‘caloric theory’ in the 18th and early 19th centuries, normally attributed to Pierre Prevost. It has indeed stuck. But even more than 200 years down the line, there is still no experimental evidence that such separate, opposite ‘hemifluxes’ inside a heat transfer really exist. It is still all a theory, an assumption, a conjecture. There are other models out there describing and interpreting the very same reality that DON’T end up violating the Laws of Thermodynamics. Just saying.
There’s a very simple reason why the postulated ‘hemifluxes’ inside a heat transfer have never been detected. They CAN’T be detected. Even if they did exist, they could never be physically isolated from one another to be separately observed. Inside the integrated radiation field through which the heat transfer moves. No matter what you do, the thing you end up with is ALWAYS the heat flux. It is the only thing that could ever possibly be physically detected within a radiative heat transfer. Everybody knows this, Phil. This is specifically WHY we apply the radiative formulas that we do to ‘extract’ our assumed ‘hemifluxes’. If we could simply go out and detect them just like that, we wouldn’t need to compute them. We know we can’t. It’s physically impossible. We DETECT only the heat and we measure the temps and from these we CALCULATE our ‘hemifluxes’. That’s all we can ever do. But you seem to be in total denial of this simple fact, Phil. Even though it was never a secret this is how it’s done. Always. Without exception. I’m sorry if this is too much reality for you to handle in one go. But I feel you ought to know …
“That depends on your having a correct view of the Laws of Thermodynamics which you do not.”
I’m afraid I do, Phil.
“I’ll repeat the observation I made before: a thermocouple measuring 1000K in a open Bunsen burner flame is exchanging radiation with the surrounding surface at 300K, measurements of the energy flux in each direction can be made.”
No. CALCULATIONS of the energy fluxes in each direction can be made, Phil. Major difference. The ‘is exchanging radiation’ part is YOUR assumption. That’s the Prevost bidirectional principle (his ‘Theory of exchanges’) dating from the times of the caloric theory right there.
“Replace the surrounding surface by one at 600K and the measured temperature will go up, as will the measured energy flux in each direction, there is no violation of the Laws of Thermodynamics, net flow is still from hot to cold.”
See, this is what you refuse to get, Phil. Yes, if you raise the surrounding temp from 300 to 600K, of course the measured temperature will go up. Because the TOTAL HEAT to the thermocouple will go up, for crying out loud! It’s not ‘evidence’ of bidirectional flow! No one has ever claimed that such an EFFECT would violate the Laws of Thermodynamics. For the nth time, Phil, it is your interpretation, your attempt at explaining how this effect comes about that violates the Laws of Thermodynamics. Not the effect itself.
There are no ‘measured’ energy fluxes in each direction. There are only CALCULATED energy fluxes in each direction, based on temperatures and the flux that is the one that’s ACTUALLY at all times being measured (as in detected): the HEAT FLUX.
Here is where and how your “back radiation” explanation violates the 2nd Law of Thermodynamics: You say that the energy flux from the cool atmosphere to the already warmer surface will directly make the surface even warmer, while maintaining that since the ‘net flow’ is still from hot to cold, then this is no problem, no violation of the Laws of Thermodynamics.
Er, it most certainly is! To see why, you need to understand what a HEAT FLUX is and what it does, plus what the 2nd Law says about the transfer of heat. You clearly don’t, Phil. If you transfer energy from one system to another and this transfer directly raises the temperature of this other system in absolute terms, then you have by definition transferred HEAT [Q] to it (disregarding ‘work’ [W]) to increase its internal energy [U]. That’s what heat does. It HEATS. And you have transferred heat from a cooler to a warmer system. Heat does not spontaneously transfer from cold to hot, Phil.
You seem utterly incapable of comprehending what I’m telling you. Or maybe just pigheadedly unwilling to. I’ve restated this so many times now:
You expect each of your two ‘hemifluxes’ to accomplish direct heating at each end of the heat transfer, as if they were both HEAT FLUXES in their own right. I’m sorry, but this is at odds with … reality, Phil. In any heat transfer, you can only ever have heating at ONE end. At the other end there will be cooling. The heat flux goes from hot to cold only, transferring energy from the hot object to the cold object, reducing the internal energy of hot and increasing the internal energy of cold. That’s how a heat transfer works. It doesn’t matter if the heat transfer is a radiative one. If the universe worked like you want it to, heating in all directions, there would be no universe for us to live in, complete anarchy. The laws of physics don’t allow it to happen. And thank goodness for that.
You confuse reduced cooling with increased heating. Insulation reduces cooling (less energy output per unit of time). An additional heat source increases heating (more energy input per unit of time). You imagine the atmosphere to be an additional, separate heat source to the surface. It’s not. How could it be!? The atmosphere is the recipient. It cannot be a source to its source. It cannot provide extra input of energy. To heat its own source.
Kristian 7:15am shows substantial confusion having not read & understood Planck’s original paper: “It is based on an assumption of the sea surface as a ‘pure emitter’.”
There is no such assumption in Dr. Max Planck’s original paper on the subject.
“What I discuss is what we actually observe..”
Not according to Dr. Max Planck’s original paper. Kristian discussion diagram 4:44am does not allow humans to observe clouds yet we do as Planck shows we should.
“When I say that what we actually physically detect in any heat transfer situation is the unidirectional HEAT FLUX…”
Not according to Dr. Max Planck’s paper on the subject where he uses the word “heat” correctly.
Heat does not exist in any object, heat has never been detected, only energy has physical existence in nature, our eyes detect what exists, visible energy flux. Energy flux was detected in the top post experiment 1st chart, heat flux has never been detected separate from energy flux.
“I’m telling the truth, Phil..”
Not according to Dr. Max Planck’s original paper.
“..there are in fact TWO opposite fluxes/transfers of energy, one from hot to cold and one from cold to hot, and that the unidirectional heat flux only somehow comes as a netted out end result.”
Correct according to Dr. Max Planck’s original paper quote verbatim below. And 2LOT. Except there is no physical heat flux detected separate from energy flux.
“There’s a very simple reason why the postulated ‘hemifluxes’ inside a heat transfer have never been detected. They CAN’T be detected.”
Correct, only energy flux exists to be detected, no separate heat flux has ever been detected.
“We DETECT only the heat…”
Not according to Dr. Max Planck’s original paper. Heat has never been detected separate from energy.
“There are no ‘measured’ energy fluxes in each direction.”
Not according to Dr. Max Planck’s original paper. His years of experiments are laid out with measured energy fluxes in both directions confirmed by much lab work of other experimenters:
“..body A at 100C emits toward a body B at 0C exactly the same amount of radiation as toward an equally large and similarly situated body B0 at 1000C. The fact that the body A is cooled by B and heated by B0 is due entirely to the fact that B is a weaker, B0 a stronger emitter than A.”
Read, study & understand Dr. Max Planck’s original paper Kristian, come into the modern world of energy transfer leave all the heat existing separately from energy confusion behind you:
http://www.gutenberg.org/ebooks/40030?msg=welcome_stranger
Trick, we’ve had this discussion many times before, so I’m surprised (or not) how you still manage to consistenly misrepresent what I’m saying. You say for instance: “Heat does not exist in any object”. Exactly right. That’s what I’m saying. As per the thermodynamic definition of ‘heat’. Heat is not something that is contained within a system. That notion is merely a remnant from the ‘caloric theory’. It is not a static quantity. It is a dynamic quantity. Energy i transit between systems or regions at different temperatures. This is the Q in the First Law of Thermodynamics: ΔU = Q – W. Ever heard of it? It would appear from the above that you haven’t. U is the system’s static fund of energy, its so-called ‘internal energy’, corresponding to its temperature, T. Q and W are the energy transfers to or from that system, changing its U (and thereby its T). Q is the ‘heat flux’ to/from. W is the ‘work done’ on/by. What about this basic thermodynamic setup is it that you don’t get? You state repeatedly: “heat flux has never been detected separate from energy flux”. And you show your confusion about the thermodynamic concept of heat. You simply don’t know what it is. And therefore you fail to see its worth.
A heat flux IS an energy flux, Trick? It is THE energy flux actually detected in a heat transfer. It IS simply the energy transferred from the hot to the cold system. What we observe. That’s why it’s called a ‘heat transfer’.
Also, I’m fully aware of the fact that both Planck and Maxwell and more of the good old fellas worked by the Prevost bidirectional principle, his theory of exchanges. That’s exactly what I told Phil above, wasn’t it? The point is, all these people were always fully aware (never trying to hide it, like you people do today) that it was and is just that: a principle, a theory, an assumption, an interpretation, a model of reality. It’s become tradition, convention. Part of the reason why is because of its simplicity and its usefulness in most situations. It works perfectly well as long as you deal with regular heat transfers where both (or all) objects involved actually COOL to various degrees. But as soon as you bring in a constant external heat source for ONE object and then try to analyse what happens when you radiatively insulate that heated object, THAT’S when cracks start to appear. Because then it all of a sudden creates a situation where the cooler insulating object or surface in fact ends up directly HEATING the warmer (heated) object. And such an analysis would evidently violate the 2nd Law of Thermodynamics. Because it would turn a cooler object into a HEAT source of a warmer one. The insulating layer would force the heated object to become warmer. The EFFECT is indeed real. But it would force it only indirectly. Not by directly heating it some more, by acting as a second heat source to its own heat source. Rather by itself warming from the absorbed heat flux from the warmer object, thus setting up a higher temperature ‘potential’ facing the heated object, consequently reducing the energy transferred per unit of time from the warmer heated object to the cooler insulating surface.
Kristian 8:09am: Still confused I see. No reading, study, comprehending of the Planck paper yet huh?
“Heat is not something that is contained within a system.”
Yes of course according to Planck paper p.72 verbatim for 1LOT: “the sum of the energy of radiation and the energy of the material bodies remains constant, we have dU + p dV – Q = 0.”
Planck invoking 1LOT eqn. precisely writes energy of radiation Q, not heat of radiation Q. Energy is conserved in 1LOT.
No heat in an object means heat cannot transfer from that object; since only energy is contained in an object only energy can transfer from/to that object – invoking 1LOT correctly as shown in Willis’ 1:22pm 1st chart.
“Q is the ‘heat flux’ to/from.”
Not according to Planck paper. In Planck’s precise 1LOT eqn., Q is the energy of radiation. When doing energy balance 1LOT, always invoke conserved energy transfer.
In fairness to Kristian, Planck and many authors do define & use the word “heat” interchangeably with “energy” correctly when they are not doing 1LOT. A problem arises when Kristian uses “heat” in a way that is not interchangeable with conserved “energy” the result is Kristian’s 4:44am faulty chart indicates humans cannot see clouds so 1LOT not invoked correctly. My advice continues to Kristian to end his 1LOT confusion and faulty charts, simply drop the word “heat” when invoking 1LOT as Planck does, as it adds nothing in modern times. Heat is a relic of ancient practices that can easily cause confusion as shown in Kristian’s faulty 4:44am chart.
Gee, who’s the clown here? Phil insists on ‘misunderstanding’ what I say so that he has something to argue against. I very clearly did not say that the spectrum was ‘made from space’. I said the spectrum presented was a ToA spectrum, not a sea surface spectrum. You cannot read SST from a ToA spectrum, Phil. And satellites cannot measure surface IR flux intensities. There’s an atmosphere in the way. This is well-known by everyone.
How do you think a ToA spectrum is made if not from space? Of course satellites can measure surface IR fluxes, they do so through the transparent ‘window’ region of the atmosphere which was clearly marked on the spectrum I presented, the AVHRR and MODIS both measure SST utilizing the 11 and 12 micron channels.
BTW, you continue to avoid the issue. Do you believe the satellites somehow do in fact estimate SSTs from surface IR flux intensity, or do you know they don’t? If you do know, why not just come out and admit it? In plain words. Why the constant evasion. Why the smoke and mirrors? What are you afraid of?
You seem to be delusional, I have repeatedly said that satellites measure SST by measuring the surface radiance, I’ve repeated it above.
“I did [read the paper].”
You obviously didn’t.
I did very carefully, you should do so too because your description of what they did is totally wrong!
Take a look at Eq. (4). There’s how the sea surface ‘LW flux’ is ‘measured’, Phil.
That is not how they measured the ‘LW flux’, as they state in the paper they used a radiometer, the data from which are plotted in fig 6: “observed longwave radiation emitted by the ocean surface without correction (grey solid circles)”, they also plot the data “with correction based on Pe ́rez and Alados-Arboledas (1999) (open black squares)”, this was necessary because the radiation sensor experienced some solar heating effects. I should point out that if as you assert they didn’t actually measure the flux but just calculated it such a correction wouldn’t be necessary! The measured upward longwave was consistently about 450 W/m^2, which agreed very well with the “estimated values of LWUP” which they made using Eq. (4).
Not in any other way. All you basically need to measure is the surface temperature. Then you simply put this into the S-B equation. And voilà! This is how it’s always done. It’s no secret at all. You are simply apparently somehow prevented from getting it. It is based on an assumption of the sea surface as a ‘pure emitter’. (We all know it isn’t.)
Clearly the paper which you cited refutes your argument, it clearly states that the upward flux was measured, describes exactly how it was done, and shows that the value estimated using the surface temperature (Eq. 4) agrees very well with those measurements.
The Kipp-Zonen net radiometer detect the NET RADIATION (that is, the HEAT FLUX), Phil, and then it has it built in to CALCULATE the DWLWIR ‘flux’ from this and the surface temperature. Simply subtract the computed UWLWIR from the measured heat flux (see Eq. (2)). And voilà! This is invariably how it’s done, Phil. It’s no secret at all. You’re just refusing to realise it, to take it in. Because then, apparently, your world view would crumble.
You have this completely backwards, the K-Z radiometer has both an upward and downward pointing sensor which independently measure the LWup and LWdown. This is how it is done not by the computational method which you have invented. It is you who is ‘refusing to realize it, to take it in’.
Your premise was that the fluxes couldn’t be independently measured and you cited this paper as supporting this view. Reading the paper shows that you have completely misunderstood it and in fact it shows that your premise is completely false. Hoist on your own petard!
Phil., December 20, 2014 at 9:00 pm:
“You have this completely backwards, the K-Z radiometer has both an upward and downward pointing sensor which independently measure the LWup and LWdown. This is how it is done not by the computational method which you have invented. It is you who is ‘refusing to realize it, to take it in’.”
Hahaha, so Phil here doesn’t even know how pyrgeometers work. That’s priceless. And still so cocksure. Like a true, clueless warmist. OF COURSE he believes, then, that a net radiometer would directly detect his imaginary UWLWIR and DWLWIR ‘fluxes’. No wonder. Newsflash, Phil. That’s exactly what they DON’T do. They COMPUTE them. From the ‘net radiation’ (the HEAT FLUX) to/from the sensor generating a voltage output, which is then put into the following equation:
U_emf/S + σT^4 = LW_dw
U_emf : thermopile output voltage [μV]; S : instrument sensitivity/calibration factor [μV/(W/m^2)]; T : sensor temperature [K]
The U_emf/S term gives the actual flux to/from the sensor surface, the HEAT FLUX (or ‘net radiation’). Normally it would be negative (heat flowing OUT OF the sensor to the cooler air above). The σT^4 term is the calculated emission ‘flux’ from the sensor, assumed to be a black body and a pure emitter. So if the sensor detects a heat flux going out/up of say 50 W/m^2 and we calculate the BB (UWLWIR) ‘flux’ from the sensor according to its temperature to be say 350 W/m^2, then we can derive a DWLWIR ‘flux’ from the atmosphere using the equation above and get 300 W/m^2.
This procedure is neatly explained here:
http://en.wikipedia.org/wiki/Pyrgeometer#Measurement_of_long_wave_downward_radiation
You could just go to Kipp & Zonen’s own website to check it out for yourself, Phil. This is invariably how it’s done. You’ll just have to live with it:
file:///C:/Users/Kristian/Downloads/KippZonen_InstructionSheet_Pyrgeometer_CGR4_V1001.pdf
http://www.kippzonen.com/Product/85/CNR-4-Net-Radiometer#.VJaeBV4AA
Do you want me to quote from these sources …? Or should we just put this topic to rest?
“..body A at 100C emits toward a body B at 0C exactly the same amount of radiation as toward an equally large and similarly situated body B0 at 1000C. The fact that the body A is cooled by B and heated by B0 is due entirely to the fact that B is a weaker, B0 a stronger emitter than A.”
I think what could be ignored is “stronger emitter” and “equally large [body]”
And atmosphere doesn’t seem to be an example of a body. One can call it a body of air, but body
in the above case is more like a chunk of metal or a brick. Or a larger or smaller body does not make much sense if one talking about gases [what could it mean larger atoms, volume, etc].
So body A could a brick and body B could be another brick [or chunk of steel] of the equal size.
As compared to difference size bricks. Or a brick [A} and some air [B] [somehow equally large].
And then we got “and similarly situated body B0 at 1000C”.
Or same distance and/or angle from A.
So with in regards to “DWLWIR flux ” all of the above is utterly ignored. It’s not a body. It’s not in any particular location, and no mention or clue as to how strong the emitter body is.
Let me state what seems to me very obvious, which is that sunlight has a stronger emitter than does the DWLWIR.
Or more precisely the Sun is vastly strong emitter [though at quite distant] as compared to the Earth sky.
And I go further and say the solar flux at Mars distance is much stronger emitter than Earth’s DWLWIR. And go even further and ask does the solar flux at Pluto distance have stronger emitter than Earth’s sky?
So can it be the solar flux at Pluto distance is a stronger emitter as compared to Earth
sky?
And I will leave it to any believer of back radiations to make their case why the Earth sky is a stronger emitter than the Sun at Pluto distance.
And if follow that with comparing it to our sunlight, that would be truly enlightening and wondrous.
According to Space Mission Analysis and Design [third edition] and on page 454, at Pluto
distance the solar flux is .9 watts per square meter.
The .9 watts per square meter solar flux should be mostly visible and Near IR light.
Less 1/2 visible light, so .3-.4 watts per square meter. Comparable [or more] visible light that we on Earth get from moonlight. So this sunlight is lighting millions of square mile of surface of Pluto at light level that roughly bright enough to read by.
Say 4 million square km of Pluto has this sunlight shining on it.
Which is 4 trillion square meter and is say it’s .3 watts, and so times 4 trillion, which totals 1.2 trillion watts of visible light.
Or in an hour that is 1.2 trillion watt hours of visible light.
Three Gorges Dam which is largest power plant in the world has 22,000 MW, though actual amount electrical power which is made: “generating 98.1 TWh in 2012”
And to generate 1.2 trillion watt hours of visible light in a year requires [at least- or assuming one had 100% efficient light bulbs] 10,512 TWh.
Or the Gorges Dam lacks enough energy generation capability to light such a large area.
Though entire world makes more than enough electrical power: “The production of electricity in 2009 was 20,053TWh. ”
http://en.wikipedia.org/wiki/Electricity_generation
Also with simple but perhaps quite large magnify glass, the sunlight Pluto could burn your finger.
The sun is strong emitter because it’s very hot and very large, but it’s also far away.
And the huge distance makes it a small apparent size.
As compared to CO2 molecule which has a weak and dim light, and is tiny [and a smaller apparent size 100 feet away from it]. And scattered in large volume of sky
One thing to add here. If steel greenhouse was source energy, rather medium the energy is conducted thru, it seems to me it would make a difference.
Or the body referred to above seems to assume, that the body has heat that it can radiate.
So the bodies A and B were heated up or it’s constantly having energy added to to the body it to keep it warm [whether 100 or 1000 C].
Phil., December 20, 2014 at 9:00 pm:
“How do you think a ToA spectrum is made if not from space?”
Yes. No one ever claimed otherwise. But a ToA spectrum is not the same as a surface spectrum. You do know that, right?
“Of course satellites can measure surface IR fluxes, they do so through the transparent ‘window’ region of the atmosphere which was clearly marked on the spectrum I presented, the AVHRR and MODIS both measure SST utilizing the 11 and 12 micron channels.”
You still don’t get it, do you? Yes, they have to use that IR emitted from the surface which goes straight through the atmosphere and out to space to be able to assess the SST. But this IR does not reach the satellite sensors with a flux intensity anywhere near your postulated pure surface BB emission of ~395 W/m^2. So there is no way you could ever use ‘flux’ in this way to estimate the surface temperature. Think it through, Phil. You understand this too. So what do you use? You assess the frequency/wavelength distribution of the surface IR you receive. This specific distribution corresponds directly to the surface temperature. And therefrom you can estimate SST.
To reiterate, the issue is whether or not DWIR contributes to SST and OHC.
The data we have regarding the IR absorbency of water and the temperature profile of the upper most sea surface resolves this issue conclusively:
IR can make no measurable contribution to SST or OHC.
Willis and I have shown energy analyses which show in some detail how it works, you have just handwaving assertions with no explanation which doesn’t carry any weight. Verney on the other hand just runs away and hides so I guess you have slightly more credibility than he does, however slightly more than nothing isn’t much.
If anyone is interested in the analysis of the physics of the sea surface which I presented in regard to IR, please see my comment 12/18 8:54am.
That’s hardly an analysis, it doesn’t even mention the absorption and emission of IR, it’s nonsense, do it right or stop wasting everyone’s time.
mpainter 8:55am: Your 12/18 8:54am is incorrect writing: “no one has shown that IR has any effect on SST”, disproven by the 1st chart in the top post. Chart shows measured SST effects by LWIR modulation day & night in real nature with real surface overturning. Shows LWIR can make precision instrument measurable contribution to SST.
Waste your time?
Then don’t read this. It is really simple.
Go read somewhere else.
Like, for example, a primer on radiative physics.
Trick December 19, 2014 at 7:09 am
Kristian 6:37am – I just looked outside. I can see the clouds today which is impossible in your faulty diagram.
That’s a good illustration. By the theory that photons from cold sources can’t do work on warmer surfaces we wouldn’t be able to see ice!
A photon from the ice at 0ºC hits a photoreceptor on the retina (at 37ºC) where it’s absorbed by a rhodopsin molecule causing a change in structure which ultimately leads to a cascade of electrons stimulating a signal in the brain.
That is correct for emitted photons, but most of the photons you “see” from ice are actually reflected. You don’t “see” the ice glowing on dark nights without a secondary source of photons.
Good, so what property of a photon is it that tells the absorbing entity what temperature source it originally came from?
None, but when you tell someone to “we wouldn’t be able to see ice” you are not talking about emission.
..
Just saying that most people’s eyes cannot “see” IR
David 9:40am – If we humans could see in the IR band, water snow would be black as opaque ice only reflects about 2-3% over the spectrum so we see water ice in daylight mostly by its emission in the visible, not very much by its reflection. Our eyesight is severely limited, sure, we cannot see ice glowing in IR. Nor the atm. glow in IR at night. Precision instrumentation is required to see ice et. al. glow at night. We need the moon, flashlights and such at night.
“Phil.
December 19, 2014 at 9:34 am
Good, so what property of a photon is it that tells the absorbing entity what temperature source it originally came from?”
Hot things emit more and higher energy photons. So ice always emits low amount longwave IR.
Hot water will emit more of same amount of longwave as ice, plus it emits shorter longwave IR than ice.
But ice can reflect or re-radiate or diffuse or transmit all kinds of wavelength. So expose ice to X-rays and it do all kinds of things and remain being ice. But when talking emitting one talking about the heat energy of a substance creating protons.
Or the kinetic energy of CO2 doesn’t create protons, rather it absorbs and re-emit a photons, or it’s re-radiating,
And could say the main difference between reflecting and re-radiating, is re-radiating sends the re-sends the photon in a random direction.
Reflection requires a surface [molecular structure as one has in all solids and liquids {not gases] and reflection can re-direct, direct light. Or reflecting light sends the light in a direction- and can reflect full spectrum of light as compared to just bits of it.
How ignorant can one get!? We don’t see ice because our eyes are bombarded by the IR photons emitted by the ice! Do you seriously believe this, Phil?
I know, but since we’ve established that photons don’t have a property which identifies their source temperature I was just trying to determine how the perverted second law theory was supposed to work.
Wiki:
Dissipation:
“Dissipation is the result of an irreversible process that takes place in inhomogeneous thermodynamic systems. A dissipative process is a process in which energy (internal, bulk flow kinetic, or system potential) is transformed from some initial form to some final form; the capacity of the final form to do mechanical work is less than that of the initial form.”
Second law of thermodynamics:
“The second law indicates increases in entropy due to dissipation of energy and to dispersal of matter and energy. It envisages a compound thermodynamic system that initially has interior walls that constrain transfers within it.”
http://en.wikipedia.org/wiki/Second_law_of_thermodynamics
That perverted second law?
No the one that Kristian expounds which says that light emitted from a colder body can’t be absorbed by a warmer body. Even though the light is the same frequency as light emitted from a hotter body which he maintains can be absorbed by the same body at the same temperature.
—No the one that Kristian expounds which says that light emitted from a colder body can’t be absorbed by a warmer body. Even though the light is the same frequency as light emitted from a hotter body which he maintains can be absorbed by the same body at the same temperature.—
Ok so take the lunar surface, it heats to around 120 C and hours of sunlight at 1360 watt per square meter does warm the surface to a higher temperature. There is a slight amount of heat
going below the surface but it’s dust act like very good insulation. So it amounts to a few watts per second per square meter. Or we shall ignore it as significant compare to the 1360 watts per second of sunlight.
Or one can say the temperature of the sun at lunar distance from it is around 120 C- at least in regard of material of lunar surface [other material can become hotter].
So lunar surface would not absorbing any more energy from the sunlight [other than a trickle of heat down further underground.
So it not a question that lunar surface does not warm up more, but rather it’s about the explanation of why it does not warm up more.
So 1360 watts of sunlight hit surface and +1300 watts is radiated from the surface.
So take some metal and put it on lunar surface and have sunlight shine on it for a couple days
and the temperature is same as lunar material- about 120 C.
Now have a lot of this metal and put it 1 meter above the ground and have cover football field area.
I would say in daylight the metal and the ground underneath it is 120 C.
So is that approx right or it somehow different.
And continuing, if put 1 meter cubes filled with water and these cubic can withstand 200 psi
and put them under the the metal. Then after days of daylight the top of the metal sheets could could less than 120 C and the water cube could less than 120 C and ground underneath it could less than 120 C. Or it may not reach 120 C and be absorbing a lot of sunlight energy. And then the sun goes down for 2 weeks, this things is not going to cool rapidly and would stay warmer
throughout the night than the ground normally is. And next day it’s going to absorb thousands times more energy than the ground. Or if replace those water cubes with solid blocks of copper it will be similar.
Kristian December 21, 2014 at 6:13 am
Phil., December 20, 2014 at 9:00 pm:
“How do you think a ToA spectrum is made if not from space?”
Yes. No one ever claimed otherwise. But a ToA spectrum is not the same as a surface spectrum. You do know that, right?
Yes, it’s the SST spectrum minus the portions absorbed by the atmosphere, in the ‘window’ region there is no absorption by the atmosphere, so in that region the ToA is the same as the surface.
“Of course satellites can measure surface IR fluxes, they do so through the transparent ‘window’ region of the atmosphere which was clearly marked on the spectrum I presented, the AVHRR and MODIS both measure SST utilizing the 11 and 12 micron channels.”
You still don’t get it, do you? Yes, they have to use that IR emitted from the surface which goes straight through the atmosphere and out to space to be able to assess the SST. But this IR does not reach the satellite sensors with a flux intensity anywhere near your postulated pure surface BB emission of ~395 W/m^2.
It certainly does, in the spectrum I showed at 900cm-1 the measured radiance is ~0.150W/m^2/sr/cm-1 which equals the radiance from a BB at 320K; for a surface at 300K it would be ~0.120W/m^2/sr/cm-1.
That’s the way it’s done, measure the radiance in the window region and compare it with the radiance from a grey body at the appropriate coefficient (~0.98) and the temperature at which they match is the surface temperature.
So there is no way you could ever use ‘flux’ in this way to estimate the surface temperature. Think it through, Phil. You understand this too. So what do you use?
See above.
Phil,
Read this carefully: The radiative flux from the surface straight to the satellite-borne instruments is as near a pure HEAT FLUX as you can get. This flux is NOT 395 W/m^2. You still manage studiously to avoid this fact. Why? The LWIR ‘atm window’ (heat) flux is (according to those infamous Earth energy budgets) anywhere from 20 to 40 W/m^2. Do you deny this?
You’re looking at a specific wavelength-radiance. Through this you get a picture of the wavelength/frequency distribution of the radiation actually escaping directly from surface to space. You do NOT get a picture of total surface BB flux intensity. So I wonder, why are you so hellbent at fighting this straw man? As if Verney is somehow denying the existence of any IR from the surface. If anything, Verney is questioning your 395 W/m^2 worth of IR from the surface. I say it can’t and doesn’t exist.
The only relevant thing here is the imaginary 395 W/m^2 surface ‘flux’ (and its supposedly opposing, but just as imaginary, 340 W/m^2 cousin down from the atmosphere). Not the fact that there is IR emitted by the surface and that some of it surely reaches directly into space. You come off as a clown still fighting this non-issue.
BTW, you already come off as a clown, Phil, denying and fighting the FACT that pyrgeometers (and hence net radiometers) do indeed always detect only the HEAT FLUX (‘net radiation’) to/from their sensor, from which they COMPUTE (using also their measured temp), using specified equations, the assumed UWLWIR and DWLWIR ‘flux’. (See my Kipp & Zonen-comment above.)
Read this carefully: The radiative flux from the surface straight to the satellite-borne instruments is as near a pure HEAT FLUX as you can get. This flux is NOT 395 W/m^2.
It leaves the earth’s surface as 395W/m^2 if the surface is at 289K, as I pointed out before some of it is absorbed by gases such as CO2, O3, CH4 etc.
You still manage studiously to avoid this fact. Why? The LWIR ‘atm window’ (heat) flux is (according to those infamous Earth energy budgets) anywhere from 20 to 40 W/m^2. Do you deny this?
I don’t know what you’ve been reading because I’ve been quite clear about this. If you measure the radiance over a particular wavelength range it will obviously be less than the total emitted. For the 8-12 micron band at 289K it will be ~32 W/m^2/sr, I have never denied this but it isn’t relevant to temperature measurement. You can determine the temperature of a BB emitter by measuring the radiance at any given wavelength, e.g. for a BB at 289K the radiance at 12microns will be 0.110 W/m^2/sr/cm^-1, so if you measure the radiance of a BB at 12 microns to be 0.110545 W/m^2/sr/cm^-1 then its temperature is 289K. If it’s a grey body like the earth’s surface you have to take into account 𝜺, (0.98 for earth).
When making measurements of the surface from space the radiance in the ‘window’ region is the same as was emitted from the surface (that’s why it’s called the ‘window’), so by measuring the radiance at 12 microns you can determine the temperature from the grey-body radiance.
You’re looking at a specific wavelength-radiance. Through this you get a picture of the wavelength/frequency distribution of the radiation actually escaping directly from surface to space. You do NOT get a picture of total surface BB flux intensity.
As explained above you’re wrong, that’s exactly what you do get.
So I wonder, why are you so hellbent at fighting this straw man?
Because it’s the truth, by the way you should check up what a straw man is.
As if Verney is somehow denying the existence of any IR from the surface.
By attempting an energy balance without including IR in either direction he is.
If anything, Verney is questioning your 395 W/m^2 worth of IR from the surface. I say it can’t and doesn’t exist.
You can say it as often as you like but you’re wrong, even the paper you cited gave measurements of it ~450 W/m^2 in that case (T ~299K).
BTW, you already come off as a clown, Phil, denying and fighting the FACT that pyrgeometers (and hence net radiometers) do indeed always detect only the HEAT FLUX (‘net radiation’) to/from their sensor, from which they COMPUTE (using also their measured temp), using specified equations, the assumed UWLWIR and DWLWIR ‘flux’.
As shown in the previous post you’ve been proved to be so wrong in multiple ways on pyrgeometers that I’d have thought that you’d be too embarrassed to bring it up. As stated before pyrgeometers measure the LW radiation incident on them, offset by the sensor temperature, by measuring the sensor temperature and calibration the incident LW is measured. The fluxes are real not assumed, if there were no incident flux the instrument would read zero!
Phil., December 22, 2014 at 11:42 am:
You don’t give up, do you, Phil?
You spout a constant stream of nonsense just to keep me busy it seems. Can’t possibly concede a single point, like a true dogma-driven warmist. Anyone would have realised long ago already that you simply don’t have a clue.
“It [the pure surface BB ‘flux’] leaves the earth’s surface as 395W/m^2 if the surface is at 289K, as I pointed out before some of it is absorbed by gases such as CO2, O3, CH4 etc.”
What you continue to ignore is that a ToA spectrum shows Earth’s final and total radiative heat flux to space, which is … 239 W/m^2. So your presented spectrum could NEVER add up to a 395 W/m^2 flux intensity. You seem not to comprehend at all what we’re discussing here. That doesn’t prevent you from babbling on and on as if you did, does it?
“I don’t know what you’ve been reading because I’ve been quite clear about this. If you measure the radiance over a particular wavelength range it will obviously be less than the total emitted. For the 8-12 micron band at 289K it will be ~32 W/m^2/sr, I have never denied this but it isn’t relevant to temperature measurement. You can determine the temperature of a BB emitter by measuring the radiance at any given wavelength, e.g. for a BB at 289K the radiance at 12microns will be 0.110 W/m^2/sr/cm^-1, so if you measure the radiance of a BB at 12 microns to be 0.110545 W/m^2/sr/cm^-1 then its temperature is 289K. If it’s a grey body like the earth’s surface you have to take into account 𝜺, (0.98 for earth).
When making measurements of the surface from space the radiance in the ‘window’ region is the same as was emitted from the surface (that’s why it’s called the ‘window’), so by measuring the radiance at 12 microns you can determine the temperature from the grey-body radiance.”
We have no quarrel on this, Phil. So why do you spend sooo much time on it? This is after all just you expanding on your straw man. No one has a problem with this. Neither does Verney, I’m sure. The point is, this whole thing is irrelevant to the discussion at hand: whether or not there IS actually an IR flux at 395 W/m^2 being emitted by a surface at 289K under a massive atmosphere like ours.
The real question is: Do you seriously believe, Phil, that if you add up the partial fluxes from all wavelengths within the atm window, then you end up with 395 W/m^2? From a temp of 289K? Even though we KNOW that the total from the ToA is 239 W/m^2.
“”You’re looking at a specific wavelength-radiance. Through this you get a picture of the wavelength/frequency distribution of the radiation actually escaping directly from surface to space. You do NOT get a picture of total surface BB flux intensity.”
As explained above you’re wrong, that’s exactly what you do get.”
As explained above you’re wrong. That’s exactly what you DON’T get. You get the ToA flux, Earth’s radiative heat flux to space of 239 W/m^2. The satellites CANNOT measure surface heat flux (not to mention your imaginary pure BB emission ‘flux’ of 395 W/m^2). Your continued obstinate denial of this simple point does not make you look particularly clever, I’m afraid. It is such a basic point, Phil.
“by the way you should check up what a straw man is.”
I know what a straw man is. That’s why I’m telling you you’ve constructed one. You claim that Verney deny the fact that there is IR coming off the sea surface. I’m pretty sure he doesn’t. But your convenient construction lets you go off on a tangent to divert from the real discussion.
“”As if Verney is somehow denying the existence of any IR from the surface.”
By attempting an energy balance without including IR in either direction he is.”
Shouldn’t you rather just ask him if this is what he believes? Rather than just assume that he does?
“As shown in the previous post you’ve been proved to be so wrong in multiple ways on pyrgeometers that I’d have thought that you’d be too embarrassed to bring it up. As stated before pyrgeometers measure the LW radiation incident on them, offset by the sensor temperature, by measuring the sensor temperature and calibration the incident LW is measured. The fluxes are real not assumed, if there were no incident flux the instrument would read zero!”
Are you being serious!? Instead of digging your hole ever deeper, you should really just read up on the subject. The incident LW to the sensor is specifically the ‘net radiation’ (equal to the HEAT FLUX). This is no secret. You can just go on with your lalalala tactic, Phil, but I’m afraid it won’t change this fact.
http://wattsupwiththat.com/2014/12/09/arguments-for-and-against-human-induced-ocean-warming/#comment-1818663
Just read what it says in the links provided. (Well, the pdf file you can find on the Kipp&Zonen website.)
I will quote from the Kipp & Zonen website. See if you manage to understand what is said:
“A pyrgeometer provides a voltage that is proportional to the radiation exchange between the instrument and the sky (or ground) in its field of view. The detector signal output can be positive or negative.
For example, if the sky is colder than the pyrgeometer, the instrument radiates energy to the sky and the output is negative.
In order to calculate the incoming or outgoing FIR it is necessary to know the temperature of the instrument housing close to the detector and the data must be recorded simultaneously with the detector signal.”
(You can find this quote in the CGR pyrgeometer brochure (PDF download) here: http://www.kippzonen.com/Product/17/CGR-4-Pyrgeometer#.VJiCNF4AA )
The equations to calculate the assumed ‘hemifluxes’ are freely provided in the pyrgeometer instruction manuals on the site.
And what is stated above (by the producer itself) agrees perfectly well with what is stated on the wikipedia page on pyrgeometers (to much to quote here and now):
http://en.wikipedia.org/wiki/Pyrgeometer#Measurement_of_long_wave_downward_radiation
Please read and report back, Phil. What does it say? Does the sensor DETECT either of the two ‘hemifluxes’? Or does it DETECT what is called the ‘net radiation’ (the IR ‘exchange’), but which really is simply the LW HEAT FLUX? And COMPUTES the assumed ‘hemifluxes’ from this and the sensor temperature? Based solely on the assumption of ‘energy exchange’/’bidirectional flow’.
Kristian December 21, 2014 at 2:37 am
Wow Kristian it’s really difficult keeping up with your changing stories!
First in reference to the paper you cited you said the following:
“Take a look at Eq. (4). There’s how the sea surface ‘LW flux’ is ‘measured’, Phil.Not in any other way. All you basically need to measure is the surface temperature. Then you simply put this into the S-B equation. And voilà! This is how it’s always done.
When I pointed out to you that that was not what they had done in the paper, they had in fact measured it using a radiometer you changed your tune. Then you said:
“The Kipp-Zonen net radiometer detect the NET RADIATION (that is, the HEAT FLUX), Phil, and then it has it built in to CALCULATE the DWLWIR ‘flux’ from this and the surface temperature. Simply subtract the computed UWLWIR from the measured heat flux (see Eq. (2)). And voilà! This is invariably how it’s done,
This of course is not how it’s done and Eq. (2) was in fact a means of estimating the LW emitted by the atmosphere and had nothing to do with the measurements made using the radiometer at all!
I then pointed out that you still had not gotten it right:
Phil., December 20, 2014 at 9:00 pm:
“You have this completely backwards, the K-Z radiometer has both an upward and downward pointing sensor which independently measure the LWup and LWdown. This is how it is done not by the computational method which you have invented. It is you who is ‘refusing to realize it, to take it in’.”
To which you replied:
Hahaha, so Phil here doesn’t even know how pyrgeometers work.
So you went from categorically asserting that it was always done by measuring the surface temperature and pyrometers weren’t used to incorrectly describing describing how pyrometers work!
Then this nonsense:
That’s priceless. And still so cocksure. Like a true, clueless warmist. OF COURSE he believes, then, that a net radiometer would directly detect his imaginary UWLWIR and DWLWIR ‘fluxes’. No wonder. Newsflash, Phil. That’s exactly what they DON’T do. They COMPUTE them.
Which is of course incorrect, pyrometers measure the incoming heat flux with an offset due to the temperature of the sensor which is also measured, in the case of this instrument there are as I said two pyrometers, one pointing up and one pointing down. Thus the LWup and LWdown are independently measured and calibrated. It’s no more a computation than the altimeter in a plane needing to be offset by the local surface pressure is a computation, they’re both measurements.
Then we get your usual :
“This is invariably how it’s done. You’ll just have to live with it:”
Third time’s a charm I guess, after being wrong the first two times and being forced by me to actually learn how the measurements you initially denied were being made. So yes you’ll have to live with the fact that the measurements of the LW fluxes are measured as opposed to your assertion last week that:
“It’s been shown that the postulated BB emission flux from the surface is NOT measured in the sense of being ‘detected’. It is ONLY calculated by directly applying the Stefan-Boltzmann equation to the assumption that the surface is a thermally isolated, pure emitter (that is, in a purely radiative setting), which it is NOT”
Which has now been confirmed to have been incorrect.
Fascinating.
And pyrometers are used in regard to measuring the surface of the ocean?
Again, Phil, you’re obviously completely clueless on how pyrgeometers work, how they ‘measure’ UWLWIR and DWLWIR.
Kipp & Zonen provided the net radiometer used by the research team. And if you then go their website and read HOW the net radiometer works – by way of pyranometers (for SW) pyrgeometers (for LW) – and then move on to read about how the pyrgeometers actually function, you will find exactly what I’ve stated all along: They DETECT the ‘net radiation’ (the radiative heat flux to or from the sensor) and measure the sensor temperature and from this they COMPUTE the ‘hemifluxes’ from equations like the ones provided in Eq. (2) and (4) (they are however slightly different, because they include reflected flux). Yes, the instruments do the calculations before presenting the results. So you THINK you get ‘real’ detected values. You don’t.
Yes, Phil, I know exactly what is stated in the paper. It makes no difference. It doesn’t make the pyrgeometers detect any UWLWIR or DWLWIR ‘fluxes’. They always detect the HEAT FLUX only. This is a trivial point, Phil. Which I can no longer be bothered to make. Read up. And have a happy holiday!
Hmm, well I typed ‘pyrgeometers’ so I guess the spell checker didn’t like it, well spotted! (It just did it again!)
So in the above post all references to ‘pyrometers’ should be ‘pyrgeometers’.
No such infernal nuisance as a spell checker.
Kristian December 22, 2014 at 1:03 pm
Phil., December 22, 2014 at 11:42 am:
You don’t give up, do you, Phil?
You spout a constant stream of nonsense just to keep me busy it seems. Can’t possibly concede a single point, like a true dogma-driven warmest.
I’d concede a point if you got one right and I was wrong, so far that hasn’t happened. You however were wrong about how the LWup was measured in the paper you cited, claiming it was always calculated from the surface temperature, you were proven wrong on that but refuse to concede the point, instead made one incorrect statement after another.
Anyone would have realised long ago already that you simply don’t have a clue.
“It [the pure surface BB ‘flux’] leaves the earth’s surface as 395W/m^2 if the surface is at 289K, as I pointed out before some of it is absorbed by gases such as CO2, O3, CH4 etc.”
What you continue to ignore is that a ToA spectrum shows Earth’s final and total radiative heat flux to space, which is … 239 W/m^2. So your presented spectrum could NEVER add up to a 395 W/m^2 flux intensity.
Why ever not? Do you have any facts to back that up or is it another of your ridiculous assertions.
Here’s an example for you:
MODTRAN run with tropical atmosphere, no clouds or rain, surface temperature 299.7K
with 400ppm CO2, 1.7ppm CH4, 28ppb O3, and a water scale of 1.0.
Upward IR heat flux to space – 287.84 W/m^2
0 CO2 318.4 W/m^2
0 H2O 386.53 W/m^2
0 CH4 389.99 W/m^2
0 O3 397.52 W/m^2
And there’s still some N2O left.
You seem not to comprehend at all what we’re discussing here. That doesn’t prevent you from babbling on and on as if you did, does it?
The one who doesn’t comprehend and keeps babbling on regardless is you.
Sorry, that last post should rather look like this:
Phil., December 22, 2014 at 5:58 pm:
“I’d concede a point if you got one right and I was wrong, so far that hasn’t happened.”
It has happened. You’re simply not willing to realise it, Phil. I can’t help you there. I’m afraid you will have to grapple with your obviously severe case of cognitive dissonance yourself.
Point 1: Satellites in space and their instruments could and do not in any way detect a pure BB 289K surface IR emission flux of 395 W/m^2. (This remains a mere mathematical construct.) If anything, they detect Earth’s total radiative heat flux of 239 W/m^2 through the ToA to space.
Point 2: Net radiometers (pyrgeometers) detect the HEAT FLUX and sensor TEMPERATURE and from these COMPUTE the assumed UWLWIR and DWLWIR ‘fluxes’. This is a trivial point that can easily be verified by anyone who cares to read through the instructions on the operational setup of these instruments provided by the people who actually make them (like Kipp & Zonen).
“You however were wrong about how the LWup was measured in the paper you cited, claiming it was always calculated from the surface temperature, you were proven wrong on that but refuse to concede the point, instead made one incorrect statement after another.”
What specifically in this description (straight from Kipp & Zonen themselves) is it that you don’t get, Phil?
“A pyrgeometer provides a voltage that is proportional to the radiation exchange between the instrument and the sky (or ground) in its field of view. The detector signal output can be positive or negative.
For example, if the sky is colder than the pyrgeometer, the instrument radiates energy to the sky and the output is negative.
In order to calculate the incoming or outgoing FIR it is necessary to know the temperature of the instrument housing close to the detector and the data must be recorded simultaneously with the detector signal.”
Or in this, from wikipedia:
“The pyrgeometer’s thermopile detects the net radiation balance between the incoming and outgoing long wave radiation flux and converts it to a voltage according to the equation below:
E_net = U_emf / S
[E_net = radiative heat flux to/from sensor] (…)
To derive the absolute downward long wave flux, the temperature of the pyrgeometer has to be taken into account. It is measured using a temperature sensor inside the instrument, near the cold junctions of the thermopile. The pyrgeometer is considered to approximate a black body. Due to this it emits long wave radiation according to:
E_out = σT^4
[E_out = ‘UWLWIR’ from sensor] (…)
From the calculations above the incoming long wave radiation can be derived. This is usually done by rearranging the equations above to yield the so-called pyrgeometer equation by Albrecht and Cox:
E_in = U_emf / S + σT^4
[E_in = ‘DWLWIR’ to sensor] (…)
As a result, the detected voltage and instrument temperature yield the total global long wave downward radiation.”
“”What you continue to ignore is that a ToA spectrum shows Earth’s final and total radiative heat flux to space, which is … 239 W/m^2. So your presented spectrum could NEVER add up to a 395 W/m^2 flux intensity.”
Why ever not? Do you have any facts to back that up or is it another of your ridiculous assertions.”
Why ever not!? Just read the quote, Phil. Read what it says. Don’t act as though you don’t understand. Like a recalcitrant child. Why are you playing obtuse? A ToA spectrum is the flux through the top of the atmosphere. You do know that Earth’s final radiative flux to space through the ToA is at 239 W/m^2, don’t you? How could a sensor in space ever detect a surface IR ‘flux’ of 395 W/m^2 that as per the rGHE hypothesis is never even meant to reach space?
Are you just trolling, Phil? I have to ask.
“Here’s an example for you:”
MODTRAN is a purely radiative model, Phil. Based on the same flawed, purely radiative assumptions about how the atmosphere works as the rGHE hypothesis. You cannot measure a 395 W/m^2 IR flux from space, Phil. No matter how you twist and turn. You can only ever at best measure Earth’s 239 W/m^2 final flux. Ask any warmist about this and you’ll get the same answer!
“The one who doesn’t comprehend and keeps babbling on regardless is you.”
I don’t know what more to say, Phil. There is no talking to a person who so completely refuses to acknowledge even the most unequivocal evidence presented to him, documented right there in front of him, who refuses to read, refuses to listen, who just go lalalalalala, meticulously blotting any inconvenient fact out from his view and thought, simply because his dogmatic beliefs tell him that’s what he needs to do to keep his cognitive dissonance at bay. You’re a hopeless case, Phil.
Phil., December 22, 2014 at 5:58 pm:
“I’d concede a point if you got one right and I was wrong, so far that hasn’t happened.”
It has happened. You’re simply not willing to realise it, Phil. I can’t help you there. I’m afraid you will have to grapple with your obviously severe case of cognitive dissonance yourself.
Point 1: Satellites in space and their instruments could and do not in any way detect a pure BB 289K surface IR emission flux of 395 W/m^2. (This remains a mere mathematical construct.) If anything, they detect Earth’s total radiative heat flux of 239 W/m^2 through the ToA to space.
Point 2: Net radiometers (pyrgeometers) detect the HEAT FLUX and sensor TEMPERATURE and from these COMPUTE the assumed UWLWIR and DWLWIR ‘fluxes’. This is a trivial point that can easily be verified by anyone who cares to read through the instructions on the operational setup of these instruments provided by the people who actually make them (like Kipp & Zonen).
“You however were wrong about how the LWup was measured in the paper you cited, claiming it was always calculated from the surface temperature, you were proven wrong on that but refuse to concede the point, instead made one incorrect statement after another.”
What specifically in this description (straight from Kipp & Zonen themselves) is it that you don’t get, Phil?
“A pyrgeometer provides a voltage that is proportional to the radiation exchange between the instrument and the sky (or ground) in its field of view. The detector signal output can be positive or negative.
For example, if the sky is colder than the pyrgeometer, the instrument radiates energy to the sky and the output is negative.
In order to calculate the incoming or outgoing FIR it is necessary to know the temperature of the instrument housing close to the detector and the data must be recorded simultaneously with the detector signal.”
Or in this, from wikipedia:
“The pyrgeometer’s thermopile detects the net radiation balance between the incoming and outgoing long wave radiation flux and converts it to a voltage according to the equation below:
E_net = U_emf / S
[E_net = radiative heat flux to/from sensor] (…)
To derive the absolute downward long wave flux, the temperature of the pyrgeometer has to be taken into account. It is measured using a temperature sensor inside the instrument, near the cold junctions of the thermopile. The pyrgeometer is considered to approximate a black body. Due to this it emits long wave radiation according to:
E_out = σT^4
[E_out = ‘UWLWIR’ from sensor] (…)
From the calculations above the incoming long wave radiation can be derived. This is usually done by rearranging the equations above to yield the so-called pyrgeometer equation by Albrecht and Cox:
E_in = U_emf / S + σT^4
[E_in = ‘DWLWIR’ to sensor] (…)
As a result, the detected voltage and instrument temperature yield the total global long wave downward radiation.”
“”What you continue to ignore is that a ToA spectrum shows Earth’s final and total radiative heat flux to space, which is … 239 W/m^2. So your presented spectrum could NEVER add up to a 395 W/m^2 flux intensity.”
Why ever not? Do you have any facts to back that up or is it another of your ridiculous assertions.”
Why ever not!? Just read the quote, Phil. Read what it says. Don’t act as though you don’t understand. Like a recalcitrant child. Why are you playing obtuse? A ToA spectrum is the flux through the top of the atmosphere. You do know that Earth’s final radiative flux to space through the ToA is at 239 W/m^2, don’t you? How could a sensor in space ever detect a surface IR ‘flux’ of 395 W/m^2 that as per the rGHE hypothesis is never even meant to reach space?
Are you just trolling, Phil? I have to ask.
“Here’s an example for you:”
MODTRAN is a purely radiative model, Phil. Based on the same flawed, purely radiative assumptions about how the atmosphere works as the rGHE hypothesis. You cannot measure a 395 W/m^2 IR flux from space, Phil. No matter how you twist and turn. You can only ever at best measure Earth’s 239 W/m^2 final flux. Ask any warmist about this and you’ll get the same answer!
“The one who doesn’t comprehend and keeps babbling on regardless is you.”
I don’t know what more to say, Phil. There is no talking to a person who so completely refuses to acknowledge even the most unequivocal evidence presented to him, documented right there in front of him, who refuses to read, refuses to listen, who just go lalalalalala, meticulously blotting any inconvenient fact out from his view and thought, simply because his dogmatic beliefs tell him that’s what he needs to do to keep his cognitive dissonance at bay. You’re a hopeless case, Phil.