I’ve been puzzling for a while about why the areas with the most power plants aren’t the areas with the worst levels of mercury pollution. Why aren’t the areas downwind from the power plants heavily polluted? I keep running across curious statements like “There was no obvious relationship between large-mouth bass or yellow perch fish tissue mercury concentrations and their locations relative to prevailing wind patterns and the incinerators” (source). In that regard I came across a critically important paper. The paper starts with what to me is a most surprising statement.
But before I get to that, a short digression. There are a couple kinds of mercury emitted by power plants, by forest fires, and by your automobile, for that matter. Well, actually three kinds, but there’s very little particulate mercury coming from any of those sources. The two kinds are “divalent” and “elemental”.
Elemental mercury (written as “Hg0”) means what you’d think, atoms of mercury vapor. Because it doesn’t bind with much and it is insoluble, it has a fairly long atmospheric half-life, on the order of a year or so. Elemental mercury is what forms the background mercury levels that are present everywhere in the atmosphere.

Figure 1. Areas in the US where fish have high levels of mercury. White areas have not been tested. EPA threshold as safe to eat is 0.30 ppm (two lightest shades of red). From the EPA’s Mercury Maps (PDF)
The other kind of mercury, divalent mercury (written as HgII), exists in the form of compounds like mercuric chloride (HgCL2). Because these compounds are both water-soluble and chemically reactive, they come out of the atmosphere quickly through deposition by precipitation. In addition, they come out slowly as elemental mercury is slowly changed into divalent mercury in the atmosphere. And as a result of all of these kinds of atmospheric mercury, plus mercury naturally in the soils, we end up with mercury in the fish.
To summarize: elemental mercury is added to the background mercury and doesn’t settle out near the power plant. Divalent mercury is reactive and water-soluble, so it rains and precipitates out near the power plant. And the problem is that analysis of the emissions from the smokestack of coal-fired power plants show on the order of 25% more divalent mercury than elemental mercury. Which sounds like bad news for those living downwind from our power plants.
With that as prologue, here’s the opening statement that I found so surprising, from a paper called “Modeling Mercury in Power Plant Plumes”.
First, the Mercury Deposition Network (MDN) data (1) along a west-to-east transect from Minnesota to Pennsylvania show no significant spatial gradient in annual mercury (Hg) concentrations in atmospheric precipitation although the Ohio Valley includes several large Hg emission sources located, under prevailing wind conditions, upwind of Pennsylvania.
Say what? No hot spots for mercury downwind of several large power plant mercury emission sources? How come I haven’t heard of that?
So I wandered off to the Mercury Deposition Network, where I found a couple more surprising maps.
 Figure 2. Total Mercury concentration in the atmosphere in 2010. Units are nanograms per litre. The red “hot spot” in the center of the US reflects the natural mercury coming from deserts and croplands, as I discussed in “The EPA’s Mercurial Madness” SOURCE
Figure 2. Total Mercury concentration in the atmosphere in 2010. Units are nanograms per litre. The red “hot spot” in the center of the US reflects the natural mercury coming from deserts and croplands, as I discussed in “The EPA’s Mercurial Madness” SOURCE
As an aside, the EPA and other scientists claim that much of the mercury in the atmosphere is “recycled” anthropogenic mercury. They say the natural emissions are in large part just man-made emissions being re-emitted. I certainly would hope that Figure 2 would put a stop to those claims. The main and overwhelming source of atmospheric mercury in the US is the natural mercury in the soils.
OK, another surprise for me. We’ve seen where the sources are and what’s in the air. Now, let’s see what gets deposited in the rain and snow. Figure 3 shows the wet deposition map for the US in 2010.
 Figure 3. Mercury wet deposition rates for the US. Units are micrograms per square metre of surface. SOURCE
Figure 3. Mercury wet deposition rates for the US. Units are micrograms per square metre of surface. SOURCE
Note that strangely, this is kind of a weather map. Why is it a weather map? Well, for example I live on the coast not far north of San Franciso Bay. I was amazed to see that I live in an area of relatively high mercury deposition. Why?
The answer is, because when the moist air sweeps in off the Pacific and hits the coastal mountains, it rains. And when it rains, I get showered with natural mercury from the ocean. Further inland on the east side of California, you can see the western slopes of the Sierra Nevada mountains painted in red. They get the moisture that doesn’t fall on the coastal ranges. And since they are much higher, they pretty much wring the moisture (and the mercury) out of the air, leaving Nevada with little mercury deposition.
The main flow of air in the US is from west to east. As a result, the hot spot over the southwestern US precipitates out in the central US. It is aided by moist air flowing in from the Gulf of Mexico during some months. This can be seen all along the Gulf Coast. Florida, like where I live, is another victim of oceanic mercury poisoning.
Finally, to return to the surprising statement I started with, in Figure 3 the blue arrow shows the prevailing winds blowing over the power plants in the Ohio River Valley towards Pennsylvania. If it is the case that the majority of the mercury emissions are divalent mercury, then why is there no trace of them raining out along the way as we’d expect?
The authors look at several different possibilities. Their final conclusion? (emphasis mine)
A sensitivity study of the impact of the Hg dry deposition velocity shows that a difference in dry deposition alone cannot explain the disparity. Similarly, a sensitivity study of the impact of cloud chemistry on results shows that the effect of clouds on Hg chemistry has only minimal impact. Possible explanations include HgII reduction to Hg0 in the plume, rapid reduction of HgII to Hg0 on ground surfaces, and/or an overestimation of the HgII fraction in the power plant emissions.
We propose that a chemical reaction not included in current models of atmospheric mercury reduces HgII to Hg0 in coal-fired power plant plumes.
This has large implications for the regulation of power plants. All the big power plants in the Ohio River Valley aren’t increasing the mercury deposition towards Pennsylvania. The mercury is being converted into elemental mercury along the way somewhere, so it’s added to the background mercury rather than raining out near the power plants.
In fact, where I live, on the pure clean Pacific coast with lots of sea breeze, I get more mercury pollution than they get around Pennsylvania despite all the coal-burning power stations just upwind from them.
And that, dear friends, was a very big surprise … when’s the EPA gonna step in to save me?
Does this mean mercury is not a poison? By no means, mercury is a bad thing, and it’s everywhere … it just shows the story has lots of tricks and turns.
Always more to learn,
w.
@ur momisugly Robert Brown.
Sir, you are almost there, but when you state “we should focus on mercury ores and natural earthbound mercury” are you suggesting we sent the EPA in haz-mat suits to scrub the natural environment? Join us in the intellectual shelter of rational natural science, and recognize that mercury ores and natural earth-bound mercury are part of the normal fabric of planet earth and that the inhabitants of this planet have over billions of years learned to adapt and exist with these natural chemical fluxes.
We should of course make sure our industrial processes do not impact on the natural process of Hg in the environment, but when we reach the point that our anthropogenic emissions (in the United States) are an insignificant contribution, then Sir it is time to focus on more pressing matters.
That was a great read. I never knew mercury could be so complex. It is bad stuff and its deposition along the coastal areas from the oceans was a complete surprise to me.
One of the many reasons I love this site. I learn so much that I never expected. One question concerning this statement:
“The mercury is being converted into elemental mercury along the way somewhere, so it’s added to the background mercury rather than raining out near the power plants.”
If it is being added to the background levels do we have any historical or proxy measures for mercury background levels? At what level is the background levels going to be an issue?
Arthur Dent says:
April 2, 2012 at 2:28 am
I fear that you are fighting a losing fight with that one, Arthur. A search of Google Scholar, not regular Google but science Google, turns up some 79,700 pages of scientific documents that us the term methylmercury. A search of Google Scholar shows another 14,600 pages with the variant “methyl mercury”. Ethylmercury brings up another 8.700 pages.
So I tell you what, Arthur. How about you take your crusade for proper nomenclature to the blogs AFTER you’ve gotten the other 100,000 of your fellow chemists and scientists, writing in journals like the Journal of Applied Toxicology and the British Journal of Industrial Medicine and the Journal of Neurochemistry and the Journal of Chromatography, to agree that you are right and to stop using the term.
You do that, and I assure you, the blogs will fall right in line … because when I see the term “methylmercury” used in places from the journal Environmental Toxicology and Chemistry to Nature magazine, I gotta assume that the agreement on the terminology is by no means as solid as you seem to think.
Thanks,
w.
PS—I don’t even find that your claim about the nomenclature is correct. You say there is “no such chemical as … methylmercury”. But in “Advances in Molecular Toxicology“, they point to a completely different issue:
So in contrast to your claim, they say there IS a chemical called “methylmercury” …
federico says:
April 2, 2012 at 2:04 am
Thanks, federico. I had written Hg0 with a zero, not HgO with the letter O, and I’m aware of the difference. WordPress doesn’t do superscripts except in Latex,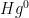 , but I try to avoid Latex because it prints small for some reason and is hard to read.
, but I try to avoid Latex because it prints small for some reason and is hard to read.
w.
jmrsudbury says:
April 2, 2012 at 3:25 am
Thanks, John, but it’s unclear what you are saying. The post only has one author, me. I live on the coast. Anthony lives in Chico, in the Central Valley. Not sure why you think he’s involved.
w.
izen says:
April 2, 2012 at 3:27 am
Thanks, izen. You’ll have to come up with a citation for both those claims. My previous post cited a paper which clearly shows that some 5,200 tonnes of mercury come from natural sources, and some 2,250 tonnes come from anthropogenic sources. So you’ve got the stick by the wrong end. Natural emissions are twice anthropogenic emissions, not the other way around.
And regarding pre-industrial beluga tusks … well, I find nothing.
Take a deep breath, my friend, you’re going to hyperventilate. The point of this article is not that we should not regulate mercury emissions. I did not say that, nor do I believe that.
What I do believe is that the regulations we have in place now are sufficient, and that the recently promulgated new EPA regulations are overkill.
Finally, I believe the findings that I highlight here, that the mercury from powerplant stacks is added to the background rather than depositing out near the power plants, means we have to look at HOW we regulate mercury. It says nothing about WHETHER we should regulate mercury, most reasonable people agree on that question, and the answer is yes.
w.
Deanster says:
April 2, 2012 at 6:27 am
That doesn’t make sense. Why would divalent mercury be found in areas “upstream from water bodies”? Who says it’s found there anyways?
Here I’ve lost you completely. No one here or elsewhere mistakes elemental mercury for natural mercury. I pointed out in the head post that elemental mercury is emitted from power plants, so that’s all being identified anthropogenic mercury, not natural mercury.
w.
Willis;
try Alt-248(numeric keypad). Hg°. Should work.
Greylar says:
April 2, 2012 at 7:00 am
Sorry for the lack of clarity. The dots are sampling stations, not power plants.
w.
Mercury in the Pacific coast ares makes perfect sense.
Where do you find Hg?
Tuna.
And where are tuna? In the Pacific.
I think I will apply for a grant.
Robert Brown says:
April 2, 2012 at 7:25 am
Thanks, Robert. A couple of comments. First, I put a map upstream which shows a host of cinnabar mines in the coastal ranges.
However, they are not the source for the hotspot of mercury raining down over my head for a couple reasons. First, they’re downwind of the coast. Second, in Fig. 3 you see the oceanic mercury all along the northernwestern coast of the US. Third, what is being measured in Fig. 3 is atmospheric deposition, not ground levels.
I have no problem with reasonable limitations of mercury from smokestack emissions. And in some countries it’s obviously necessary. The US generates 25% of the worlds electricity, and burns 21% of the world’s coal, but only puts out 8% of the global mercury emissions from power plants. And for overall emissions its even worse, we only emit about 6% of the world’s mercury.

In the US, I’d argue we’re up against diminishing returns in terms of stack emissions, particularly when you include the most recent regulations. As a result, we should focus (as you suggest) on the other sources. As a reminder, from my previous post, here’s what those other sources are:
Total US mercury emissions are less than the mercury emissions from the burning of the rest of world’s trash …
w.
Deanster says:
April 2, 2012 at 10:00 am
Not true. Anthropogenic emissions are about half natural emissions, which is smaller but by no means “trivial” when you are talking about a poison.
w.
Brian H says:
April 2, 2012 at 12:14 pm
Of course, the degree symbol, Hg°. On my Mac that’s shift-option-8, never thought of using that.
w.
@- richardscourtney says: April 2, 2012 at 6:59 am
“Your post at April 2, 2012 at 3:27 am consists solely of unjustified assertions that are denied by data in the above article. If you have contrary data then please state it. Otherwise, please withdraw your comment.
The important point is that Hg emissions are trivial when compared to natural sources. This is clearly demonstrated in the above article where it says; …”
If the above article did ‘clearly demonstrate’ that HG emissions are trivial when compared to natural sources it would not only deny my assertions but overturn all the contary data that I will be happy to present. I think you might have nis-read the above article. It does not claim that anthropogenic Hg is trivial when compared to natural sources. Just that the airbourne emissions from coal-fired plants are not detected (with the given sampling system and Hg/bioconversion cycle) locally.
ftp://info.mcs.anl.gov/pub/tech_reports/reports/CGC-003.pdf
Atmospheric Mercury – Global Biogeochemical
Cycles, Sources, and Sinks
Richard D. Doctor, 1 John A. Taylor, 2 and Jack D. Shannon3
1. Overview
Measurements of atmospheric mercury (Hg) at remote locations, such as the North Atlantic, confirm that the levels of mercury in the global atmosphere are increasing. In spite of considerable effort, estimating the global movement of mercury through the environment is difficult. However, anthropogenic emissions of mercury are at least double those from natural sources and are of such magnitude that they cannot be attributed to natural sources alone [Fitzgerald et al. 1998].
http://www.mercurynetwork.org.uk/wp-content/uploads/2011/10/IKIMP_Workshop_Report_Mercury_Cycle_2011.pdf
While the magnitude of the natural mercury flux (e.g., volcanic, evasion from oceans) is uncertain, it is clear from many studies of historical mercury accumulations in peat bogs, lake sediments, ice cores and biological samples that the global atmospheric mercury burden has increased on average by a factor of 3 ± 1 since the industrial revolution[1]. In some regions localised Hg pollution in soils has increased 10-fold. The impact of this increase on biota is considerable: Fig. 2 shows the steep rise in mercury in animal tissues from the Arctic since 1900.
http://www.agu.org/pubs/crossref/2008/2007GB003040.shtml
The spatial distribution of soil concentrations is derived from local steady state between land emission and deposition in the preindustrial simulation, augmented for the present day by a 15% increase in the soil reservoir distributed following the pattern of anthropogenic deposition. Mercury deposition and hence emission are predicted to be highest in the subtropics. Our atmospheric lifetime of mercury against deposition (0.50 year) is shorter than past estimates because of our accounting of Hg(0) dry deposition, but recycling from surface reservoirs results in an effective lifetime of 1.6 years against transfer to long-lived reservoirs in the soil and deep ocean. Present-day anthropogenic enrichment of mercury deposition exceeds a factor of 5 in continental source regions. We estimate that 68% of the deposition over the United States is anthropogenic, including 20% from North American emissions (20% primary and <1% recycled through surface reservoirs), 31% from emissions outside North America (22% primary and 9% recycled), and 16% from the legacy of anthropogenic mercury accumulated in soils and the deep ocean.
———————————–
Hope that clarifies why there is certainty that anthropogenic sources of Hg are known to exceed natural ones at present Richard.
Note that this may be why there is not an obvious local deposition pattern around power plants. local soil deposition may result in a re-release as bioconversion occurs and allows the Hg0 to spread globally.
PS – I will continue to use the name izen even if it does remind you of a toilet paper.
I expect you will continue to use yours, even if I am remineded of its diminutive – Dick.
[Moderator’s Note: Fine. Tit for tat. Now enough. -REP]
Gee, I guess there is some air dam that blocks mercury from reaching Mississippi and east TN when you get to the big muddy(?). Either that or the fishing is really bad there, or EPA staff balked at going there, or they forgot to air brush red onto that state.
Willis, RE the difference between Ocean and Anthropogenic emissions
There is a big difference between the 5,200 tonnes of mercury come from natural sources and some 2,250 tonnes come from anthropogenic sources. The natural sources constitute cycling in the system, the input into the system is the 90 tonnes from volcanoes and geothermal activity, the sink is the mineralizeation deep underground or at the bottom of the oceans in the sludge. At steady state, the natural input matches the sink rate, and the steady state concentration in the oceans, plants, estuaries, e.t.c. are stable. Adding mercury into this closed system increases the steady state level in all reservoirs.
So the rule of thumb is that pre-anthropogenic influx into the biosphere was 90 tonne per year and mineralization was 90 tonnes per year; adding 2,250 tonnes per year from anthropogenic sources will raise the steady states in the various reservoirs by ((2,250 +90)/90) or about 26 times, assuming the sink rate remains constant.
izen:
I agree, I did say;
“anthropogenic emissions are trivial when compared to the natural sources.”.
I meant;
“THE anthropogenic emissions FROM US POWER STATIONS are trivial when compared to the natural sources.”.
I thought it was obviousfrom context, but clearly not. I apologise for my lack of clarity.
Richard
DocMartyn says:
April 2, 2012 at 2:40 pm
So it is your claim that until humans hit the planet, there was no flow of mercury into and out of the atmosphere other than via volcanoes?
My friend, look at Figure 2. The red spot in the US Southwest is mercury entering the atmosphere. It will eventually end up in the ocean, as it has done for millions of years. Note that the mercury is NOT returned to the US Southwest, so it is NOT “cycling in the system”.
The idea that the only “input into the system” is volcanoes ignores a host of other land (and likely sea-floor) based sources of mercury.
w.
Also, DocMartyn, you discount the oceanic mercury as simple “cycling in the system” … but it is still the major source of the mercury that is raining down on my head. How is my body supposed to tell the difference in the local fish I eat?
Does the fact that the mercury in the fish is from “natural cycling” somehow make it irrelevant?
w.
Willis, we have influx, reservoirs and efflux; demineralization (mostly geothermal/vulcanoes by some chemolithotropic bacteria), reservoirs (oceans, atmosphere, dust, plants, fish, lakes, mud, e.t.c.) and efflux (mineralization).
You have to understand that transport from oceans is going to equal oceans in the steady state.
@Izen
I went to check your references in your last post and remain unconvinced.
You quote an abstract (doi:10.1029/2007GB003040) and go on to say “Hope that clarifies why there is certainty that anthropogenic sources of Hg are known to exceed natural ones at present Richard.”
If you read the paper, you will see there is less certainty than you claim. For example the paper states that they have rewritten a global ocean-atmospheric model for mercury. In their rewrites they have added to the anthropogenic tally 600 tonnes/yr from biomass burning and another 450 from artisanal mining. Think about that – do you really believe that every single volcano on earth (supposedly estimated at 500 tonnes/yr) puts out practically the same amount of Hg as shovel and sluice-box miners? Nobody else in the world prior to this thought so until the authors of this paper decided to Enron the Hg budget. Do you believe it reasonable that a forest fire should be classified as an anthropogenic source of Hg? Understand what the paper is about – the authors develop a model to explain observed Hg deposition rates with the model sources. They choose to define the far larger percentage of the global Hg budget to anthropogenic sources and hey presto their model proves an anthropogenic source in Hg deposition. If you were, say a young faculty member publishing this sort of science, probably it would help you with continuing funding from the EPA. Oh wait for it, the EPA did fund this research and one of the co-authors works for the EPA. Fancy that.
Your 2011 Mercury Workshop reference was equally flabby. It contains statements like this “Current mercury emissions to the atmosphere are estimated to be equally distributed among anthropogenic, legacy and natural emissions (Fig. 1).” The trouble is that there are no quantifiable arguments as to how they estimated the various reservoirs. Read a little farther and legacy is defined as a mixture between natural and old anthropogenic sources, but they admit it is not possible to distinguish what percentage of legacy is natural and anthropogenic. Essentially what this does is acknowledge that they cannot prove anthropogenic sources are responsible for observed Hg values, but they can claim that “legacy” reservoirs are contributing anthropogenic Hg. How convenient.
Your words: “In some regions localized Hg pollution in soils has increased 10-fold. The impact of this increase on biota is considerable: Fig. 2 shows the steep rise in mercury in animal tissues from the Arctic since 1900.” What Figure 2 shows is a suspiciously “hockey-stick” shaped graph with not actual Hg concentrations but with Hg in “% of present day Hg concentrations”. Doesn’t that strike you as slightly misleading? Is this a verifiable, standard unit of measurement? Whatever happened to good old ppm or ppb Hg, you know something we can actually check. But it gets worse, notice the sample density variations. From 1550 to 1880 there are no samples, of any sort, and yet from 1880 to the present the researchers are able to find 15 Gyrfalcon and 7 Peregrine feathers from the Arctic. [One feather has a reported value of 0 “% of present day Hg concentrations”]. Similarly the 20th Century has 13 samples of polar bear fur and only one other cluster at year 1300. Notice how most of the “hockey-stick” blade is made up of falcon feathers and polar bear hair samples. There should be an equal number of samples from the same animal collected through time. I understand that it may be difficult getting ancient samples but notice how human hair and teeth are used to define the ancient baseline, but there are no modern human hair or teeth samples. Do you really think modern samples of human hair are hard to get? This figure is not the basis to make important scientific interpretations – don’t you think that if the case was so strong, then a more compelling figure could be produced?
Willis Eschenbach says:
April 2, 2012 at 12:47 pm
> Brian H says:
> April 2, 2012 at 12:14 pm
>> Willis;
>> try Alt-248(numeric keypad). Hg°. Should work.
> Of course, the degree symbol, Hg°. On my Mac that’s shift-option-8, never thought of using that.
You can (in WordPress’s HTML subset) up ⁰ for a superscript 0, I think: Hg⁰. The problem with substituting a degree sign is that it’s a circle, zeroes are not circles.
Ric,
On a Mac, opt + 0 [zero] gives: º
shift + opt + 8 = °
Hmm, what did I type there? I forgot that WordPress is more agressive about conversions than HTML is. What I meant to say was you can use ⁰ for a superscript 0.
Willis ….
Your losing me here. What the heck are you talking about???
Your very first line in the article says, “I’ve been puzzling for a while about why the areas with the most power plants aren’t the areas with the worst levels of mercury pollution.”
Then you have this quote from some mercury guys … “We propose that a chemical reaction not included in current models of atmospheric mercury reduces HgII to Hg0 in coal-fired power plant plumes”
OK .. so you’re puzzled, as to why mercury pollution is not “upstream” of the power plant. WELL .. between your article, and what I just gave you, you have your pathway. I see nothing puzzling about it at all.
Pathway — HgII emiitted from power plant –> converted to HgO by some reduction process –> atmospheric mixing over a years period of HgO residency in atmosphere –>results in ubiquitous deposition, including deposition in the oceans –>conversion to watersoluble organic mercury by Microorgansims —> evaporation into weather systems –> rains out in places upstream of water system .. like San Francisco and Florida.
Thus .. is probable that some of that HgII raining on your head from storms comming off the pacific originated as released Hg from a power plant in Ohio. Like I said, I have no idea if there’s been an increase in HgII precipitation in San Francisco .. but if there has been, it would not suprise me in the least. .. ie., nothing puzzling about it to me at all.