Guest post by Bob Fernley-Jones by Bob Fernley-Jones AKA Bob_FJ
CAUTION: This is written in Anglo-Oz English.
Here is the diagram as extracted from their 2009 paper, it being an update of that in the IPCC report of 2007 (& also 2001):
The unusual aspect of this diagram is that instead of directly showing radiative Heat Transfer from the surface, it gives their depiction of the greenhouse effect in terms of radiation flux or Electro-Magnetic Radiation, (AKA; EMR and a number of other descriptions of conflict between applied scientists and physicists). EMR is a form of energy that is sometimes confused with HEAT. It will be explained later, that the 396 W/m^2 surface radiation depicted above has very different behaviour to HEAT. Furthermore, temperature change in matter can only take place when there is a HEAT transfer, regardless of how much EMR is whizzing around in the atmosphere.
A more popular schematic from various divisions around NASA and Wikipedia etc, is next, and it avoids the issue above:
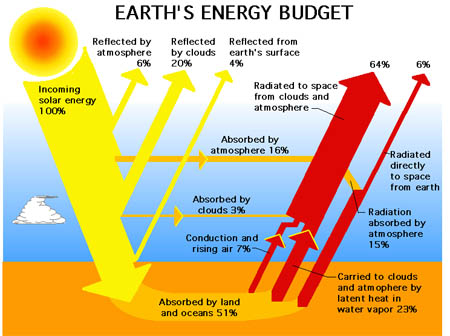
- Figure 2 NASA
Returning to the Trenberth et al paper, (link is in line 1 above), they give that the 396 W/m2 of EMR emitted from the surface in Fig.1 is calculated primarily by using the Stefan–Boltzmann law, and global year average conditions. Putting aside a few lesser but rather significant issues therein, it is useful to know that:
1) The Stefan-Boltzmann law (S-B) describes the total emission from a flat surface that is equally radiated in all directions, (is isotropic/hemispherical). Stefan found this via experimental measurement, and later his student Boltzmann derived it mathematically.
2) The validity of equally distributed hemispherical EMR is demonstrated quite well by observing the Sun. (with eye protection). It appears to be a flat disc of uniform brightness, but of course it is a sphere, and at its outer edge, the radiation towards Earth is tangential from its apparent surface, not vertical. It is not a perfect demonstration because of a phenomenon called limb darkening, due to the Sun not having a definable surface, but actually plasma with opacity effects. However, it is generally not apparent to the eye and the normally observed (shielded) eyeball observation is arguably adequate for purpose here.
3) Whilst reportedly the original Stefan lab test was for a small flat body radiating into a hemisphere, its conclusions can be extended to larger areas by simple addition of many small flat bodies of collectively flat configuration, because of the ability of EMR waves to pass through each other. This can be demonstrated by car driving at night, when approaching headlights do not change in brightness as a consequence of your own headlights opposing them. (not to be confused with any dazzling effects and fringe illumination)
4) My sketch below demonstrates how radiation is at its greatest concentration in the lateral directions. It applies to both the initial S-B hemispherical surface radiation and to subsequent spherical radiation from the atmosphere itself.
5) Expanding on the text in Figure 3: Air temperature decreases with altitude, (with lapse rate), but if we take any thin layer of air over a small region, and time interval, and with little turbulence, the temperature in the layer can be treated as constant. Yet, the most concentrated radiation within the layer is horizontal in all directions, but with a net heat transfer of zero. Where the radiation is not perfectly horizontal, adjacent layers will provide interception of it.
A more concise way of looking at it is with vectors, which put simply is a mathematical method for analysing parameters that  possess directional information. Figure 4, takes a random ray of EMR (C) at a modestly shallow angle, and analyses its vertical and horizontal vector components. The length of each vector is proportional to the power of the ray, in that direction, such that A + B = C. Of course this figure is only in 2D, and there are countless multi-directional rays in 3D, with the majority approaching the horizontal, through 360 planar degrees, where the vertical components also approach zero.
possess directional information. Figure 4, takes a random ray of EMR (C) at a modestly shallow angle, and analyses its vertical and horizontal vector components. The length of each vector is proportional to the power of the ray, in that direction, such that A + B = C. Of course this figure is only in 2D, and there are countless multi-directional rays in 3D, with the majority approaching the horizontal, through 360 planar degrees, where the vertical components also approach zero.
6) Trenberth’s figure 1 gives that 65% of the HEAT loss from the surface is via thermals and evapo-transpiration. What is not elaborated is that as a consequence of this upward HEAT transfer, additional infrared radiation takes place in the air column by virtue of it being warmed. This initially starts as spherical emission and absorption, but as the air progressively thins upwards, absorption slows, and that radiation ultimately escapes directly to space. Thus, the infrared radiation observable from space has complex sources from various altitudes, but has no labels to say where it came from, making some of the attributions “difficult”.
DISCUSSION; So what to make of this?
The initial isotropic S-B surface emission, (Trenberth’s global 396 W/m2), would largely be absorbed by the greenhouse gases instantaneously near the surface. (ignoring some escaping directly to space through the so-called “atmospheric window”). However, a large proportion of the initial S-B 396 surface emission would be continuously lateral, at the Trenberth imposed constant conditions, without any heat transfer, and its horizontal vectors CANNOT be part of the alleged 396 vertical flux, because they are outside of the vertical field of view.
After the initial atmospheric absorptions, the S-B law, which applied initially to the surface, no longer applies to the air above. (although some clouds are sometimes considered to be not far-off from a black body). Most of the air’s initial absorption/emission is close to the surface, but the vertical distribution range is large, because of considerable variation in the photon free path lengths. These vary with many factors, a big one being the regional and more powerful GHG water vapour level range which varies globally between around ~0 to ~4%. (compared with CO2 at a somewhat constant ~0.04%). The total complexities in attempting to model/calculate what may be happening are huge and beyond the scope of this here, but the point is that every layer of air at ascending altitudes continuously possesses a great deal of lateral radiation that is partly driven by the S-B hemispherical 396, but cannot therefore be part of the vertical 396 claimed in Figure 1.
CONCLUSIONS:
The vertical radiative flux portrayed by Trenberth et al of 396 W/m^2 ascending from the surface to a high cloud level is not supported by first principle considerations. The S-B 396 W/m^2 is by definition isotropic as also is its ascending progeny, with always prevailing horizontal vector components that are not in the field of view of the vertical. The remaining vertical components of EMR from that source are thus less than 396 W/m^2.
It is apparent that HEAT loss from the surface via convective/evaporative processes must add to the real vertical EMR loss from the surface, and as observed from space. It may be that there is a resultant of similar order to 396 W/m^2, but that is NOT the S-B radiative process described by Trenberth.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
ADDENDUM FOR AFICIONADOS
I Seek your advice
In figure 5 below, note that the NIMBUS 4 satellite data on the left must be for ALL sources of radiation as seen from space, in this case, at some point over the tropical Pacific. The total emissions, amount to the integrated area under the curve, which unfortunately is not given. However, for comparison purposes, a MODTRAN calculator, looking down from 100 Km gives some interesting information for the figure, which is further elaborated in the tables below. Unfortunately the calculator does not give global data or average cloud/sky conditions, so we have apples and pears to compare, not only with Nimbus, but also with Trenberth. However, they all seem to be of somewhat similar order, and see the additional tabulations.
| Compare MODTRAN & “Trenberth”, looking down from 2 altitudes, plus Surface Temperature | ||||
| Location | Kelvin | 10 metres | 100 Km. | (Centigrade) |
| Tropical Atmosphere | 300K | 419 W/m^2 | 288 W/m^2 | (27C) |
| Mid-latitude Summer | 294K | 391 W/m^2 | 280 W/m^2 | (21C) |
| Mid-latitude Winter | 272K | 291 W/m^2 | 228 W/m^2 | (-1C) |
| Sub-Arctic Winter | 257K | 235 W/m^2 | 196 W/m^2 | (-16C) |
| Trenberth Global | 288K ? | 396 W/m^2 | 239 W/m^2 | (15C ?) |
| Compare MODTRAN & “Trenberth”, looking UP from 4 altitudes: W/m^2 | ||||
| Location | From 10 m | From 2 Km | From 4Km | From 6Km |
| Tropical Atmosphere | 348 | 252 | 181 | 125 |
| Mid-latitude Summer | 310 | 232 | 168 | 118 |
| Mid-latitude Winter | 206 | 161 | 115 | 75 |
| Sub-Arctic Winter | 162 | 132 | 94 | 58 |
| Trenberth Global | 333 Shown as coming from high cloud area (= BS according to MODTRAN) | |||
Discover more from Watts Up With That?
Subscribe to get the latest posts sent to your email.



Tim Folkerts says:
October 26, 2011 at 7:49 pm
“If 10^19 photons of energy 10^-19 J pass thru a 1 m^2 surface oriented perpendicular to the earth’s surface, then 1 J passes thru the surface independent of the direction that the photons are moving.”
Unh-uh. If that were true, we would not need to steer solar arrays to point at the sun to get maximum power. You have to integrate over the projected area, which is proportional to the cosine of the angle between the (-) surface normal and the direction of propagation.
davidmhoffer says:
October 27, 2011 at 1:32 pm
Myrrh;
Utter codswallop. Water is a transparent medium to visible light, visible light is transmitted through without being absorbed >>>
_______________________________________
I don’t have any proof to the unhindered transmission of light threw pure water. However the sea is not pure. Particles and dissolved organics will decrease penetration of light in the sea. How these effect temperature I don’t have a clue.
Myrrh says:
October 27, 2011 at 11:30 am
Utter codswallop. Water is a transparent medium to visible light
You have been shown several times that this is not true. Are you not embarrassed by displaying such ignorance? and repeatedly.
Not only did you get a reference to such a textbook, but also a condensed explanation that should be understandable to the general reader: “A water molecule consists of an oxygen atom with two hydrogen atoms sitting on ‘hydrogen bonds’ [like little springs sticking out from the oxygen atom]. The two springs form an angle of some 105 degrees. There are three main modes of vibrations: mode 1, where the hydrogen atoms vibrate in and out in unison [‘symmetric vibration’]; mode 2 where the bonds bend back and forth, changing the angle;and mode 3 where the hydrogen atoms vibrate in opposite directions [when one goes in, the other goes out – called ‘asymmetric vibration’]. Because of the dense packing in liquid water, mode 2 does not happen [there is not enough room], so in liquid water only mode 1 and 3 occur. As with any vibration, there is a fundamental frequency [which is activated by far infrared], but here are also overtones [or harmonics – this is what makes a note, like ‘A’, sound differently on a violin and a trumpet]. The overtones that combine mode 1 and 3 [combined to 6th and 5th harmonics] correspond to the visual frequencies of light of 511 nm [green] and 606 nm [yellow], so visual light can and does excite vibrations. Overtones are normally of much less amplitude [strength] than the fundamental vibrations, so the visual light absorption is up to million times smaller than that of far IR. That infrared is absorbed [and thus heats] in the first few millimeters of the water column, while it takes 100 meters or more of water to absorb [and be heated by] visual light. But the ocean is deep enough for that, so even visual light gets absorbed [otherwise the ocean floor would be bathed in light – which it is not] and thus heats the oceans.”
Myrrh says:
October 27, 2011 at 11:30 am
Water is a transparent medium to visible light
Here you can learn more about absorption of visible light by water:
http://www.lsbu.ac.uk/water/vibrat.html
” Water is very slightly blue in color [131]c as overtone and combination vibrational absorption bands (albeit far less intense, see above [130]) extend through the red part of the visible spectrum with a small peak at 739 nm and shoulder at 836 nm, both varying somewhat with temperature [268] plus a smaller fourth overtone of the v1:v3 stretch at 606 nm, and very small fifth overtone (at 514 nm) and combined overtone (at 660 nm) bands. This absorption spectrum of water (red light absorbs 100 times more than blue light), together with the five-times greater scattering of blue light over red light, contributes to the blue color of lake, river and ocean waters.”
MostlyHarmless @ur momisugly October 27, at 3:18 am
You seem to have misunderstood the article in two ways:
1) The problem is expressed in the absorptive spectra, which is why I wrote: (ignoring some [radiation] escaping directly to space)
2) The problem is concerning the S-B isotropic radiation when affected by an absorptive atmosphere. It is not chrystal clear to me what they did with their initial S-B calculation in 2009, but it doesn’t matter how the final number of 396 was derived, and the same argument would apply if they stayed with their 390 number of 1997. That is why I wrote: they give that the 396 W/m2 of EMR emitted from the surface in Fig.1 is calculated primarily by using the Stefan–Boltzmann law, and global year average conditions. And why I also put a question mark against the temperature in the tables
Nice Job Bob FJ,
A couple interesting points,
1. The 63 Wm-2 (396-333) is a good bit different than the NASA value of 83Wm-2 (21% at surface) and 59Wm-2 absorbed by the atmosphere versus only 23Wm-2 absorbed in the K&T.
2. The 396Wm-2 from the surface is via S-B with emissivity = 1, perfect black body. If the 333 is assume as a real energy source, via S-B emissivity = 1, The clouds where the DWLR would be originating are at approximately 277 degrees K, I have always found that interesting.
3. The assumption of only up or down radiant energyflux is perfectly fine, until the tropopause roughly. Then the horizontal window should be considered.
The cartoon needs a proper funeral so things can move on.
Michel @ur momisugly October 27, at 2:58 am
What I wrote in full was: The initial isotropic S-B surface emission, (Trenberth’s global 396 W/m2), would largely be absorbed by the greenhouse gases instantaneously near the surface. (ignoring some escaping directly to space through the so-called “atmospheric window”).
The reason I claim this is that most of the radiation is in lateral directions, and even if the photon free path lengths are long, their initial absorption would be near the surface. Of course those streams of a more vertical aspect would mostly go much higher.
>>Tim Folkerts says: October 26, 2011 at 7:49 pm
>>“If 10^19 photons of energy 10^-19 J pass thru a 1 m^2 surface oriented
>>perpendicular to the earth’s surface, then 1 J passes thru the surface
>>independent of the direction that the photons are moving.”
>Bart says: October 27, 2011 at 1:43 pm “Unh-uh. If that were true,
>>we would not need to steer solar arrays to point at the sun to
>>get maximum power. You have to integrate over the projected area… ”
If you read carefully what I said, then we are not in disagreement. Whatever angle a photon hits a solar array, if that photon gets absorbed, it deposits all of its energy. It does not deposit Ecos(theta) or any such thing. This deposits E. Bob_FJ seems to be saying that only the ‘perpendicular component of the photon energy” gets deposited.
I fully agree that if you change the angle of the surface, then the number of photons hitting the surface will change. That is separate issue.
Well, It can be put this way:
According to Trenberth heat transfer by thermal radiation from surface to atmosphere is 23 W/m², while thermals + latent heat (related to phase transitions of water) carry off 97 W/m². As 40 W/m² is supposed to escape directly from surface to space through the atmospheric window by thermal radiation, it is only 14.4% of the entire heat flux leaving the surface that can be connected to the greenhouse effect.
More importantly in Table 2a of their paper they say global land area has an overall 15.6 W/m² heat flux deficit, that is, it radiates 5.57 PW more to space than it receives from the sun. This figure matches exactly the latent heat carried by the amount of water vapor carried by winds over land that is seen again as global river runoff to the ocean, glacier discharge added (slightly more than a million cubic meter per second).
Their value of 80 W/m² for the latent heat of evaporation also matches global average precipitation closely (as it should). However, water, once up in the atmosphere in the form of vapor, seldom returns to the surface upon first condensation. Most droplets and ice crystals, up to 90% of them evaporate again as they descend to lower layers, well before reaching the surface. That vapor is then raised again until it re-condenses, and so forth.
That is, even that 14% of heat flux that left the surface as thermal radiation and was re-absorbed by the atmosphere at some (pretty low) level, does not have to proceed painfully upwards as radiation, but is aided in its progress by the water cycle, until it makes up to a level where the air is clear & dry & cold enough to let radiation escape to space.
This level is at the cloud top, of course, and most of the radiation there comes from ice crystals. Emissivity of ice (and liquid water) in the thermal infrared is very close to one, that is, it behaves like a black body. This broad band radiation can hardly be intercepted any more by narrow absorption lines of dilute greenhouse gases at low pressure, which are not even subject to pressure broadening up there.
If the atmosphere gets a bit more opaque in the thermal infrared (due to GHGs), this intermediate water cycle simply gets more vigorous, making the overall average upward heat flux nearly constant.
Anyway, radiative transfer can’t give a serious contribution to heat flux between atmospheric layers as long as various forms of water are not involved, otherwise thermal conductivity of carbon dioxide could not be smaller than that of dry air (which, unlike carbon dioxide, neither emits nor absorbs em radiation in the thermal infrared band).
Myrrh @5:15 am
As noted earlier by Gail, there are a number of processes that can happen to a photon as it interacts with matter(adsorber). Partial interaction of the photon with an atom will lessen the energy of the photon thereby changing its frequency/wavelength and the photon goes on to interact the adsorber at some distant spot. With complete adsorbtion, the atom will gain all of the photon’s energy. Interaction with a particular atom depends on the frequency/wavelength of the incomng photon and the chances that the photon will interact with an electron or the nucleus of the adsorber. The chances of interacting depend on: how many adsorber atoms there are; how big is the nucleus of each atom; and how many electrons associated with the atom? If there is enough of the absorber (lots of atoms), all photons will be absorbed.
Water does not adsorb light photons very well, but it does adsorb some and it will adsorb all of it if there is enough water. Therefore all of the energy from the sun’s light spectrum (and the rest of the sun’s electromagnetic spectrum photons such as microwave and IR) will get transferred to water just because there is so much water in the oceans.
When a photon, such as a light photon, is partially or completely absorbed by an atom, the atom’s electrons gain extra energy. The atom is now excited and its vibrational state increases. This is not the state that the electrons want to be in, so eventually that extra energy is re-radiated away from the atom at a different wavelength or the energy may be transferred to another atom. For example in water, the excess energy gets shared among the three atoms, H-O-H, and the water molecule is now in an excited state, which we call a “heat” state. I’m using the term heat very loosely here. When we experience hot water, we are experiencing the release of excess energy from the water in the form of photons that transfer energy to our atoms or as a direct transfer of energy to our body’s atoms that are in direct contact with the water molecules.
Anything will act as a photon adsorber, but some things are better adsorbers for specific wavelengths/frequencies than other adsorbers. A black piece of paper will stop most light photons, but not all. This is easily demonstrated using low intensity (few photons) light and a high intensity light (lots of photons). Some light will go through the paper with the high intensity. However, if you put enough pieces of paper together, the high intensity light will get stopped because the black (carbon) molecules are quite good at adsorbing the wavelength/frequencies of the light spectrum. If it was an X-ray photon, at its different wavelength/frequency, the black paper is basically transparent. The same basics apply to water as an adsorber no matter what the frequency/wavelength/frequency.
What is happening to the energy being deposited in the paper? It is exciting the paper molecules and some of it will expressed as heat, much of which gets radiated/transferred to the surrounds. If the energy deposition is too great, the paper molecules may shift and break down (burning).
“”””” Kelvin Vaughan says:
October 27, 2011 at 1:16 am
Just a thought! anti phase sine waves of the same frequency cancel out. Does this happen at infra red wavelengths? “””””
Kevin, the question EXACTLY as you have phrased it has a very simple answer; YES.; well actually with one little detail you left out; those sine waves also have to be of the exact same amplitude, to completely cancel. And I bet you knew that too, if you had just thought about it.
So let’s just say that your sine waves are coming from some electronic oscillator circuit. You can of course switch the power to that circuit off and on; whenever you like.
Just what do you suppose is the likelihood, that if you switched the power off, and then on again, that the oscillator will come up in EXACTLY the same phase, that it might have had if you DIDN’T switch the power off; well not bloody likely as they say.
So ingeneral any oscillator goes on and off from time to time, either deliberately or accidently, so it may not remain PHASE CONTINUOUS all the time.
Well most light sources, and most Infra-red sources, are like that off and on sort of sources. They don’t remain phase continuous FOR VERY LONG; ACTUALLY THEY REMAIN CONTINUOUS FOR A VERY DAMN SHORT TIME.
The infra red radiation, may only travel two inches, and suddenly the phase jumps discontinuously to some other place in the cycle. We call it the COHERENCE LENGTH.
Ordinary light sources or IR sources, don’t normally cancel out, because they never remain exactly out of phase, because of theat coherence length.
The most obvious exception is the LASER. A laser, is not unlike an ordinary oscillator, with its phase remaining coherent for quite alarge distance (or time if you will).
As anybody knows, if you shine one of those little red laser pointers at a wall or something, you see this bright SPECKLE PATTERN apparently dancing in front of the wall, and as you move your eye the speckle patter seems to follow you around.
That speckle pattern is nothing more that the laser beam travelling towards the wall, INTERFERING with the beam reflected off the wall, in the space above the wall, where they overlap, and the speckle is a pattern of dark spots, where the two beams cancel, and the bright spots are where they add together to make an even brighter spot.
So the short answer to you r question is yes they can cancel, but only for the time duration when they really are out of phase.
Non laser ordinary light or infrared sources, usually do not cancel each other because the coherence length is too short..
Now Michelson, measured the length of the standard Metre bar against some lines in the spectrum of Cadmium, which happen to be very narrow bandwidth atomic spectral lines, and in that case they have a much longer coherence length than other sources. The Cadmium Red line as measured by Michelson came out at 6438.4696 Angstrom units, or aqbout 643.84696 nano metres. He had to have his coherence length at least 10 cm long to do that measurment, otherwise he would have seen no interference fringes.
There is a green line in the Mercury 198 spectrum, which is even sharper, than the Cadmium Red line, and makes even nicer interference patterns. Trouble is Mercury 198 is not a natural isotope, or is extrremely rare; so you have to make it in a nuclear reactor out of gold; so 198Hg is far more valuable than gold. There are some even better ones, before you have to go to lasers.
Ordinary sources; specially thermal ones, are very noisy so have low coherence lengths, maybe not even mm in extent.
Tim Folkerts @ur momisugly October 27, at 12:05 pm
The problem with your argument is that Trenberth gives:
Sunlight absorbed by surface (161) = energy leaving surface + the controversial heat retained of 0.9.
161 = 17 + 80 + 63 + 1
The radiative heat transfer from the surface of 63 can also be obtained from the net of the alleged up-down radiation: 396 – 333 = 63
Thus the 396 & 333 is at the surface, but you argue that it is constant up to the high cloud level, including clear skies.
You earlier seem to admit that this can only be the case for an ideal transparent atmosphere, but clear skies are closer to this condition than are cloudy skies
davidmhoffer says:
October 27, 2011 at 1:26 pm
Tonyb;
Congrats on getting a response from R. Gates… well, sort of.
Me he continues to ignore entirely because the only answer he has left is to admit that he was totaly wrong and welched on his bet with me.
_____
Tony simply is far more polite than you and seems to have a better grasp of the the issues.
Tim Folkerts @ur momisugly October 27, at 3:22 pm & Bart @ur momisugly October 27 1:43 pm
Tim said: Bob_FJ seems to be saying that only the ‘perpendicular component of the photon energy” gets deposited.
Please see this simple analysis of Lambert’s law (or cosine law in optics, Lambertian or black body reflection)
http://escience.anu.edu.au/lecture/cg/Illumination/lambertCosineLaw.en.html
In the first figure, see the effect of incoming light angle variations. There are more detailed descriptions around, but you can see the effect of vectors in the simple diagrams.
In my previous post I neglected to mention the reflective property of water to light photons, which is relatively high compared to other matter. This is the result of partial interaction of the incoming photon without much energy deposition and is related to the unique structure of the water molecule, the frequency/wavelength of the incoming photon, and the incidence angle. Someone with a better theoretical physics background than I may be able to explain this phenomenon in laymen terms better than I. Obviously with reflected light photons, which do not enter into the water mass, this energy does not get adsorbed by the water. However, most of this light energy will be adsorbed by other matter, though a very small portion may escape into space.
Berényi Péter said “Anyway, radiative transfer can’t give a serious contribution to heat flux between atmospheric layers as long as various forms of water are not involved, otherwise thermal conductivity of carbon dioxide could not be smaller than that of dry air (which, unlike carbon dioxide, neither emits nor absorbs em radiation in the thermal infrared band).”
I don’t understand this paragraph, CO2’s thermal conductivity peaks at -20C and the Kinematic viscosity of air at -20 is very high relative 15C. Antarctica has a conductive/convective relationship that appears to negate most of the radiant impact of a change in CO2 forcing. The reason it is not warming as advertised.
Tim Folkerts says:
October 27, 2011 at 3:22 pm
“Whatever angle a photon hits a solar array, if that photon gets absorbed, it deposits all of its energy. “
Nobody is interested in individual photons. The question is, what percentage of photons in an incident plane wave are being absorbed. That is very much dependent on the angle of incidence.
climatereason says:
October 27, 2011 at 1:29 pm
R gates
Thanks for your answer. So the observed 400 year warming trend only became a warming trend influneced by humans in the last 20 years or so? So for 380 years it was natural and then it switched to being man made. Is that correct?
tonyb
____
There was some recovery from the lower temps of the LIA, but it was hardly as strong as the temperature increases we’ve seen since 1980. Temperatures were wavering in the 19th century…up a little then back down before starting a pretty constant rise in the 20th century, becoming really pronounced after about 1980. Of course warming (or cooling) doesn’t switch from one cause to another, and I know you’re being facetious here, but climate is always a combination of factors, some reinforcing and some competing with each other. Human forcings on climate are a mixed bag, with some causing cooling and others causing warming. Over the several centuries since the industrial revolution, humans have been putting increasingly large amounts of carbon into the atmosphere and oceans. As this contribution has grown, and as the accumulated effect begins to show, you would expect the human forcing signal to begin to dominate over the natural background forcings at some point. Those other forcings are still there, but the anthropogenic signal would begin to dominate, with the signal getting easier and easier to see, even though the other forcings are still there. The next 10-30 years or so will be very interesting to watch, as if we have a quiet sun and cooler oceans, we will get to test first hand the relative strength of the human contribution. If you think this was similar to the Maunder Minimum period, we certainly know what happened then, so we can compare today’s climate response directly.
R. Gates;
Tony simply is far more polite than you and seems to have a better grasp of the the issues.>>>
Then by all means, answer his follow up question, it was politely worded, was it not?
As for the notion that you don’t answer me because I’m not polite…in one of my comments I suggested you were a girl and you instantly jumped all over me. Seems to me when I’m totaly rude you respond right away. But to factual questions you either go silent or, as in your response above, come up with some inane excuse that is patently and obviously not true.
I’ve asked if you had some sort of relationship with Trenberth on many occassions. You finaly answered by claiming you had no relationship other than you lived in the same state. I repeat my follow up question:
Did you or did you not arrange for Anthony Watts and 20 guests from the WUWT forum to meet with Kevin Trenberth? You claimed very proudly in another thread that you did. How did you accomplish this without having any contact with Kevin Trenberth other than living in the same state as him?
As for our bet, you welched. You can fail to respond to me, or come up with excuses, but the record is there for all to see. You accepted the wager, and asked me how much I would like to bet. Then you tried to redefine the experiment. Then you tried to claim that Anthony’s results showed only that Anchor Hawking glass absorbs IR. That wasn’t the bet. The bet was that if the experiment were done as illustrated, it would not show the results illustrated. You said they would, and they didn’t.
Of course when it became totaly obvious that you’d lost the bet, you turned from defending Al Gore’s “illustration” to throwing the guy under the bus as if we’d forget what you said in his defense only a few weeks before.
Frankly R. Gates, I’m being perfectly factual, and while you might accuse me of being blunt, there is nothing impolite about anything I said or the questions I asked. If you are uncomfortable with answering the questions, might I suggest that your discomfort originates from another cause?
Ooops. I just did. How impolite of me.
I do agree that every photon incident on the Earth will get processed in some way. But, the total percentage is 1/2 of the area of incidence (pi*r^2, being the projected area, rather than 2*pi*r^2, being the total area facing the Sun).
If what I am saying is not germane to the argument, nevermind. I haven’t read through the article carefully yet, I was just commenting on what appeared to be an erroneous argument which caught my eye.
Modtran results looking up from the surface in the tropics (looking at the back-radiation, the red line) when it is Clear.
http://img171.imageshack.us/img171/4308/rad12081141.gif
Modtran results looking up from the surface in the tropics when there is low cloud cover. A perfect black-body radiating at 20C (and it is cloudy 65% of the time).
http://img171.imageshack.us/img171/7268/tropicalsurfacelookingu.gif
Where is this described in Trenberth’s diagram?
It is an illustration which gives everyone a false impression of how the real atmosphere works, and how it really works over a 24 hour period.
Another good example, in the middle of the day in clear conditions, the solar irradiance coming in can be as high as 1200.000 joules/m2/second. But the surface energy level in the same conditions is only increasing by 0.008 joules/m2/second. It is flying out as fast as it is coming in. Where is that shown is Trenberth’s diagram?
Jim Masterson wrote;
“These diagrams are “steady-state.” That means the transients have had time to damp out or stabilize.”
Thank you, I fully understand the concept of “steady-state”.
Unfortunately, the complex climate system of the Earth is NEVER in a “steady-state” condition.
My point is if you model/analyze a complex chaotic system as if it is a simple “steady-state” problem you are VERY LIKELY to get the wrong answer, no matter how well you analyze the “steady-state” condition.
For example, an airplane flying towards the surface of the Earth at 500 mph is in a “steady-state” condition. Once the plane and the Earth’s surface intersect the “steady-state” becomes chaotic very quickly.
Cheers, Kevin.
George E. Smith; says:
October 26, 2011 at 11:11 pm
“”””” CRISP says:
You CANNOT transfer heat from a colder body (the upper atmosphere) to a hotter body (the lower atmosphere and Earth surface) without doing work. The 2nd Law of Thermodymanics avbsolutely forbids it. “””””
EM radiation knows absolutely nothing about either “heat” or “Temperature.”, and it can go wherever it darn well pleases .
—————————————————–
George I hope you don’t want to imply that the 2nd Law doesn’t hold for radiation, Planck and Einstein surely build on it when they dug into the BB-radiation problem.
Anyways, I notice that many people think the entropy in k*ln*W has to do with the probability of jiggling atoms. It does not. It has to do with possible energy microstates, entropy is about the dispersal of energy itself. W stands for “Ways of energy distribution”, so in the case of atoms the energy distribution among all possible translation, rotation and vibrational states.
Why doesn’t the earth surface heat up infinitely by radiation? Because after absorption from a SWR photon the energy can leave dispersed as 20 diffuse LWR photons.
Backradiation may be real but it is clear that it cannot warm up the surface further because it can’t be dispersed any further than it was when it left the surface in the first place, it can only do this if it would be radiated away at an even LOWER surface temperature.
The ‘heat from hot to cold’ phrase that is often used, should be: Energy tends to flow from being concentrated in one place to becoming diffused and spread out and therefore less concentrated. This more concrete formulation with respect to the 2nd Law is well known in chemistry.
So EM radiation cannot transfer (heat)energy from a colder to a hotter body because the photon energy cannot be dispersed by the hotter body. Energy is still more concentrated here.
For an excellent website about the 2nd Law look here.
Bob_FJ says: “Thus the 396 & 333 is at the surface, but you argue that it is constant up to the high cloud level, including clear skies.”
That was not what I said (or at the very least, not what I meant). This seems to be a combination of two different ideas.
1) The 333 W/m^2 downward would be the net result (on average, of course) of all the atmosphere. The higher up you go, the less the you would see looking up. This is because 1) there is less atmosphere above you and 2) the atmosphere is getting cooler.
The value must depend on the GHGs and clouds, since in an IR transparent atmosphere, it would be 0.00 W/m^2 of IR from the 3 K background of space.
2) Conversely, the 396 W/m^2 upward IR from the earth’s surface would continue upward in a transparent atmosphere. THIS is the number I claim would be constant at any altitude. (The curvature of the earth would make a small correction. By the time you are 64 km high, you are 1% of the earth’s radius up, and there would be a 2% correction to the IR — it would decrease by ~ 8 W/m^2 to 388 W/m^2.)
In a real atmosphere, this value will decrease. Some of photons from the warm surface will get absorbed. They will partially be replaced by the photons from the cooler GHGs/clouds higher up. This is exactly the “bites” taken out of the observed and calculated spectra you posted.
Hans;
George I hope you don’t want to imply that the 2nd Law doesn’t hold for radiation, Planck and Einstein surely build on it when they dug into the BB-radiation problem.>>>
That’s not what George said. He said that all bodies radiate energy as per Stefan-Boltzmann and they do so in a spectrum as defined by Planck. The photons emitted by a body at any temperature don’t care WHAT the temperature of what they are aimed at is. They either get absorbed or they don’t depending on the properties of whatever they run into. SB, Planck and Einstein all require this to be the case. The 2nd Law holds up just fine if you define the NET exchange of radiation. So, a cold body does in fact send energy to a warm body, but not as much as it GETS from the warm body. That said, if you insert a cold body in between a warm body and a SUPER cold body (outer space for example) the warm body still cools, just not as fast as it would without the cold body in between.