Poste invité par David Middleton
Sacré bleu !
Trump Budget Attacks Montreal Protocol, Reagan’s Crown Jewel
May 24, 2017 David Doniger
The Trump FY18 budget proposal slashes funding to support compliance with the Montreal Protocol, Ronald Reagan’s treaty to save the ozone layer.
The cut—which appears to be on the order of 40 percent—welches on U.S. international commitments and will imperil the global phase-out of ozone-destroying chemicals.
The Montreal Protocol—widely considered the world’s most successful environmental treaty—was negotiated under President Ronald Reagan in 1987 and is his crowning environmental achievement. It has been strengthened repeatedly under both Republican and Democratic presidents.
[…]
This year will mark the 30th anniversary of the Montreal Protocol, the “treaty to save the ozone layer.” This treaty is often cited as the textbook example of a successful environmental treaty which literally saved the planet at a reasonable cost. In many ways it is a perfect model for global efforts to save the planet from climate change:
- Instrumental measurements of the phenomena do not date back far enough to establish natural variability.
- Measurable mitigation success won’t occur for decades.
- Unverifiable claims that things would be worse if we hadn’t acted are treated as evidence that the treaty was successful.
- The threat was hyped.
- The true economic costs have been blurred.
This excerpt from a Smithsonian Magazine article sums it up quite well:
[…]
Rumors of blind sheep—the increased radiation was thought to cause cataracts—and increased skin cancer stoked public fears. “It’s like AIDS from the sky,” a terrified environmentalist told Newsweek’s staff. Fueled in part by fears of the ozone hole worsening, 24 nations signed the Montreal Protocol limiting the use of CFCs in 1987.
These days, scientists understand a lot more about the ozone hole. They know that it’s a seasonal phenomenon that forms during Antarctica’s spring, when weather heats up and reactions between CFCs and ozone increase. As weather cools during Antarctic winter, the hole gradually recovers until next year. And the Antarctic ozone hole isn’t alone. A “mini-hole” was spotted over Tibet in 2003, and in 2005 scientists confirmed thinning over the Arctic so drastic it could be considered a hole.
Each year during ozone hole season, scientists from around the world track the depletion of the ozone above Antarctica using balloons, satellites and computer models. They have found that the ozone hole is actually getting smaller: Scientists estimate that if the Montreal Protocol had never been implemented, the hole would have grown by 40 percent by 2013. Instead, the hole is expected to completely heal by 2050.
Since the hole opens and closes and is subject to annual variances, air flow patterns and other atmospheric dynamics, it can be hard to keep in the public consciousness.
Bryan Johnson is a research chemist at the National Oceanic and Atmospheric Administration who helps monitor the ozone hole from year to year. He says public concern about the environment has shifted away from the hole to the ways in which carbon dioxide affects the environment. “There are three phases to atmospheric concerns,” he says. “First there was acid rain. Then it was the ozone hole. Now it’s greenhouse gases like CO2.”
It makes sense that as CFCs phase out of the atmosphere—a process that can take 50 to 100 years—concerns about their environmental impacts do, too. But there’s a downside to the hole’s lower profile: The success story could make the public more complacent about other atmospheric emergencies, like climate change.
[…]
Read more: http://www.smithsonianmag.com/science-nature/ozone-hole-was-super-scary-what-happened-it-180957775/#W5LRedAOT3ymcci1.99Give the gift of Smithsonian magazine for only $12! http://bit.ly/1cGUiGvFollow us: @SmithsonianMag on Twitter
Rumors of blind sheep!!! Drastic measures to eliminate CFC emissions!!! Thirty years on, the ozone hole has not significantly changed… Although it would have been worse without Montreal (wink, wink) and it will heal by 2050 (nudge, nudge). This is from NASA’s Ozone Hole Watch page:

The annual thinning of the ozone layer over Antarctica has occurred during every Antarctic spring in which anyone was actually trying to measure it and continuous records only date back to 1986.
Ozone in the upper atmosphere is created when UV radiation from the Sun strikes oxygen molecules. This leads to the creation of ozone. The ozone layer doesn’t work like sunscreen; it’s more like reactive armor.
Stratospheric ozone is created and destroyed primarily by ultraviolet (UV) radiation. The air in the stratosphere is bombarded continuously with UV radiation from the Sun.When high energy UV rays strike molecules of ordinary oxygen (O2), they split the molecule into two single oxygen atoms.The free oxygen atoms can then combine with oxygen molecules (O2) to form ozone (O3) molecules.
O2 + UV light → 2 O
O + O2 + M → O3 + M (where M indicates conservation of energy and momentum)
The same characteristic of ozone that makes it so valuable – its ability to absorb a range of UV radiation – also causes its destruction. When an ozone molecule is exposed to UV energy it may revert back into O2 and O. During dissociation, the atomic and molecular oxygens gain kinetic energy, which produces heat and causes an increase in atmospheric temperature.
During the Antarctic winter very little sunlight hits the upper atmosphere over Antarctica and the Antarctic polar vortex prevents much in the way of atmospheric mixing between the polar and higher lower latitude air masses. This leads to an annual depletion of Antarctic ozone from mid-August through mid-October (late winter to mid spring). As the Antarctic spring transitions to summer, there is more exposure to sunlight and the ozone layer is replenished.
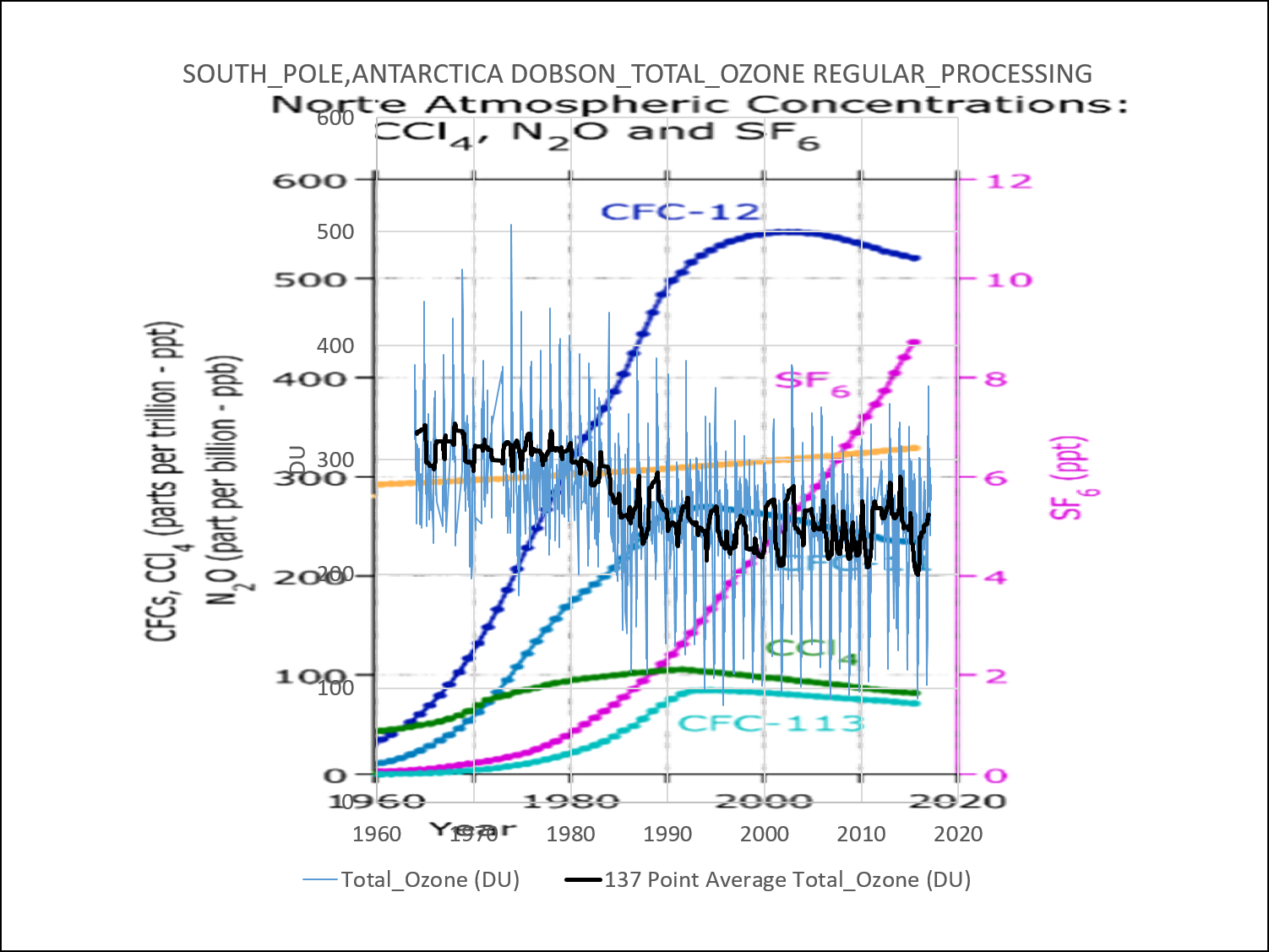
This process has occurred since the dawn of continuous ozone measurements in 1986. NOAA’s Earth System Research Laboratory / Global Monitoring Division used to feature a very disingenuous chart on their ozone page.

The image above has been replaced with the following:
The NOAA ESRL/GMD charts imply that the annual ozone hole did not exist during an earlier period of measurements from 1967-1971. This is wrong. The actual data from 1967-1971 clearly show that the annual ozone hole did exist. It may have been less pronounced at higher altitudes and it may have bottomed out in September rather than October; but it did exist. At low altitude (200 MB and 400 MB) it was nearly identical to the present-day…

There are a lot of reasons why earlier measurements differ from the modern data:
- The older data were sparsely sampled (1/4 the number of profiles) and the earlier ozonesonde balloons rarely, if ever, reached higher altitudes (40 MB and 25 MB).
- Natural climate oscillations. 1967-1971 was during a period of global cooling. 1986-1991 was during a period of global warming.
- Fluctuations in the polar vortex. It has been demonstrated that fluctuations in the polar vortex can influence Antarctic ozone observations (Hassler et al. 2010).
- Chlorofluorocarbons (CFC’s). It is possible that CFC’s did exaggerate the Antarctic ozone hole. However, the data clearly show that CFC’s did not create it.
Anthropogenic CFC emissions may very well have contributed to the area and depth of the annual Antarctic ozone hole. Reducing CFC emissions was a good thing. However, there clearly is no evidence that this was a crisis which required immediate, drastic, global action. CFC’s could have easily been replaced gradually with substances less hostile to the stratospheric ozone layer. The panic was so severe, that no cost analysis was even required for the Montreal Protocol.
No estimate of the global cost of replacing CFC-based technology has been made, but the Environmental Protection Agency estimated that the cost in the United States alone would be some $3 billion, mostly for replacing equipment made obsolete or unusable by the ban.
Unsurprisingly, the Competitive Enterprise Institute estimated that the cost in the United States would be more than ten-times the EPA estimate.
The CFC phaseout may well be the single most expensive environmental measure taken to date. During the policy debate, the costs were underemphasized to the point that they never became an important factor. The impact on consumers was scarcely considered. It may be too late to reverse course on the CFC phaseout, but it can serve as a lesson for the future.
I have not been able to find any recent estimates of the costs imposed on the U.S. economy by the Montreal Protocol. However, I know the costs were not insignificant and all of the benefits are either: 1) model-based assumptions and/or 2) 50 years in the future.
The ozone hole panic cost many people a lot of money. Refrigerating fluids, particularly in automobile air conditioners, had to be replaced. If you were the owner of a 1980’s motor vehicle or in need of air conditioner repairs in the 1990’s, you may as well have traded your vehicle in; because the cost of repairs became almost prohibitive due to new environmental regulations related to CFC’s (I owned a 1983 Chevy Camaro back then). If your home HVAC system was manufactured before the CFC ban, you faced a similar dilemma. The elimination of CFC’s even drove up the cost of asthma breathalyzers inhalers. The elimination of CFC’s may have evened worsened AGW!
The economic cost of this particular chapter of environmental junk science was minuscule in comparison to that of the current environmental swindle (anthropogenic global warming)… But this should serve as a clear reminder that citizen scientists have a duty to always check the work of government and academic scientists when they start Chicken Littling about the latest environmental crisis du jour.
References:
[1] Data Visualization >> South Pole Ozone Hole >> South Pole Total Column Ozone
[2] Hassler, B., G. E. Bodeker, S. Solomon, and P. J. Young. 2011. Changes in the polar vortex: Effects on Antarctic total ozone observations at various stations. GEOPHYSICAL RESEARCH LETTERS, VOL. 38, L01805, doi:10.1029/2010GL045542
[3] Oltmans, S. J., Hofmann, D. J., Komhyr, W. D., Lathrop, J. A. 1994. Ozone vertical profile changes over South Pole. NASA. Goddard Space Flight Center, Ozone in the Troposphere and Stratosphere, Part 2, p 578-581
Featured Image:

“Defeated” Paris?
That’s hardly le mot juste.
We came, we saw, we bombed the City of Love back to the Renaissance.
Now let’s bring our boys home. They’re going to need some solid R&R before shipping off to fight Trump’s War on The Planet Earth Itself. Let me tell you, that’s one oblate spheroid that can put up a fight. It’s no cheese-eating surrender-SandPerson, that’s fer sure.
Just ask George Carlin…
Well… if he was still alive, you could ask him about the resilience of the planet.
La guerre climatique is enough to get anyone down, so if the constant toll of body-bags has all become a bit depressing, check out these uplifting, moving letters from the parents of our servicemen and servicepeople on the ground in gay Paris (that’s the Paris which is not in Texas). Booya!
It’s enough to make one proud of one’s lapsed US passport.
Yes it can. It’s called “Nature”. It can reform Ozone, absorb and transform Man’s relatively tiny bit of CO2 into plants and food.
Man can’t control “Nature”, let alone Global Temperature, but some men will do anything to control the people on the globe.
(George Carlin got it right.)
I still don’t understand how vastly heavier than air gasses like CFC’s, known to be decomposed rapidly by soil bacteria, make it into the upper atmosphere.
Nor do I understand how millions of square kilometers of natural, dried powdered halide salts are overlooked as a possible cause for the ozone thinning.. especially when my understanding is beyond modelling, all that’s being sampled up their is halide ions
The CFCs are very stable in the lower atmosphere so they disperse until they effectively behave as single molecules.
Molecules are being bounced around all the time very violently by brownian motion, so whilst molecules heavier than Nitrogen will fall they fall extremely slowly. Think how much faster a rock falls than a speck of dust, and then think at least a billion times lighter.
This means that even a very gently upward breeze will move CFCs with it, ready to get broken down by UV light and start destroying Ozone.
The altitudes we are talking about can support ice crystals in clouds which are much heavier than a CFC molecule.
I was thinking more of this “CORRECTED: in the everyday world, gases do not expand to fill their containers.”
‘if you squirt some carbon dioxide out of a CO2 fire extinguisher, it will not instantly expand to fill the room. Instead it will pour downwards like an invisible fluid and form a pool on the floor. It behaves similarly to dense sugar-water which was injected into a tank of water: it pours downwards, and only after a very long time it will mix with the rest of the water. “Mixing” is very different from “expanding to fill!”
The “Perfect” treaty that does nothing? I guess in politics that is a perfect treaty.
Did lots for AC chemical producers and patronage jobs….
Just at the time patent was running out on CFCs IIRC 😉
Actually a perfect treaty is one that cost the USA more in as many ways as possible than the other signatories. It is one that makes us less competitive and less able to produce wealth while the others grow their economies in order to pay for social welfare programs.
Why is it nobody mentions the fact that the Dupont Chemical Co. funded researcher, on whose research the entire Montreal Protocol was based, has admitted to having made up the “science” of how CFCs supposedly react with ozone?
Now, we know that it is solar radiation and N2 gas in the atmosphere that breaks down ozone, not CFCs.
The Ozone Scare was a scam perpetrated by Dupont Chemical and jumped on by a bunch of politicians and environmentalists looking for a cause. Ignorance prevailed.
Will I get compensated for the expensive HVAC package unit that had much more expensive design and components and essentially-required maintenance contract for the higher failure rate of the higher pressure coil units? This was not in the “pennies” of cost claim of the advocates when it was forced through public policy. I got the real story from the HVAC technicians on the back end of the policy fraud.
As did I back in the 1990’s… Every time my 1980’s vintage AC units had to be recharged and/or repaired.
My parents had a CFC chest freezer bought back in the 1940s. It had a very simple design and never did leak its CFC. It was still running in 2011 when my parents died and we sold the freezer to someone for ten dollars. For all I know it’s still running!
Congress was leaning against the Montreal Protocol when NASA published a finding that they had discovered an north hemisphere ozone hole and it was impacting how much UV reached the ground in the US. This news caused a number of senators who had been wavering to start supporting the treaty.
About 6 months after congress voted to join the protocols, NASA admitted that they had made a mistake and there was no northern hemisphere ozone hole after all.
Sounds remarkably like NASA’s Palmdale Bulge.
Yes, and the debate over the bulge and satelite measured land elevation was measured in 100s of mm!
Yet we claim almost perfect elevation change accuracy on the ever moving ocean surface with ever changing multi decadal lunar cycles.
Just because a computer spits out a precise number, it does not make it accurate.
It was probably just a coincidence that the patent for CFC’s was about to run out.
Dupont made a bundle on replacing CFC’s.
… while claiming the new mantle of “Green Company”
Exactly what I was thinking! The phrase “The ozone hole panic cost many people a lot of money.”
should be much more accurately written as “The ozone hole panic MADE many people a lot of money.”
specifically, large international corps like DuPont and other chemical corps, who were about to be undercut on price by cheap 3rd world competition.
DuPont, like all businesses, is in business to make money. The fact that DuPont turned this to their advantage is a *good* thing. It’s the silver lining to the dark ozone hold cloud.
Money does not disappear. When it “costs” someone. it means someone else is receiving it.
OK, the two of you can cut it out right now! CFC’s were patented back in 1932 or so and have been long off patent. Now, if you want to ague that the replacement compounds offered higher profit margins, have at it, but be sure to look up when the base compounds were patented too.
DuPont had two choices: 1) Go out of business or 2) Figure out how to profit from the Montreal Protocol:
http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1099-0836(199711)6:5%3C276::AID-BSE123%3E3.0.CO;2-A/abstract
Unlike fossil fuels, CFC’s were relatively easy to replace and DuPont was well-positioned to market replacement chemicals.
DuPont held the patent for Freon until 1979, a year after it was banned in the U.S. …
https://en.wikipedia.org/wiki/Chlorofluorocarbon
DuPont didn’t just “figure out how to profit from the Montreal Protocol” – DuPont FUNDED the lawyers and activists who WROTE the Montreal Protocol, with the specific goal of creating something that would be intensely profitable for DuPont.
Nice try, David, but that’s a process patent. It’s a novel way to make existing compounds, not a new compound, and has nothing to do with anyone else making “Freon” by some other route. Thanks for playing.
I’m seeing a whole lot of banned in 1978, expires in 1979, on those documents.
Strange how suddenly when the eco fraud bubble is fixing to burst, now it’s Ronnie’s fault.
Reagan didn’t do this. More accurate to say they did this to him.
Here’s a view of the ozone hole from Mars.
http://btc.montana.edu/ceres/html/Polar/Images/marsnpc.jpg
Don’t get to see that angle a lot.
http://images.scienceworldreport.com/data/images/full/21458/martian-polar-ice-caps.jpg
here’s a cleaner pic. Notice how you can see dust storms right up to the edge of the hole.
We’ve been over this before. As far as I can tell DuPont’s patents had already expired. What did happen is that they had patents on replacements for CFCs that they could only sell if the government made CFCs illegal. Whichever way, the money trail ends at DuPont. WUWT
As many have pointed out R-12 (commonly called Freon) was well out of patent, the patents in question were process patents that DuPont held that likely helped them to make the compound better, faster, cheaper etc. But even with that they still would have been fine. Where their true advantage lie, however, was in not only the replacement but the replacement for the replacement. IIRC HFC-134A was the immediate replacement which was scheduled to be banned at a later date than R-12. At the time of passing the next generation refrigerants were still on the drawing board. That is where DuPont’s true advantage was. They had the resources and wherewithal to be on the leading edge of the replacement movement and could secure a majority stake in that market as it developed. The Montreal protocol took what was essentially a static market and put it back in play. The replacement chemicals still don’t have the thermal efficiency and thus the cooling capacity of the old R-12 systems. We get stuck with newer and better that is worse than what it is replacing. Which causes us to run our systems longer and use more electricity. That leads to burning more coal and voila, the cure to the last disease helped to cause the current one. Gotta love how that works. It’s the gift that keeps on giving.
The ozone hole was first measured by Dobson back in 1950’s. We have no idea how long it appeared before that. CFC’s have little if anything to do with hole. Volnaco’s in Antarctica spew chlorine into air.
Volnacos are covfefe, IMO.
Well that’s not a very nice thing to say about volcanoes. Or maybe it is a very nice thing to say about volcanoes. Please let me know which.
“BallBounces on June 5, 2017 at 7:16 am
Volnacos are covfefe, IMO”
It’s 5.30am here and I’ve just had a great laugh to start the day – thanks BallBounces!
The treaty was applied after 1988. This NASA poster only goes to 2012, but things haven’t changed much.
http://www.rockyhigh66.org/stuff/ozoneposter_front.jpg
http://nineplanets.org/news/wp-content/uploads/2014/12/cassinijupiter.jpg
Here’s the hole from yet another angle. THis time from the Cassini spacecraft. As you can clearly see the hole doesn’t change much due to distance.
papiertigre (june 5,2017 at 4:22) your picture looks strangely like Jupiter. Is it???
That’s Jupiter with a big old ozone hole. Well probably a helium methane hole, but why quibble?
There’s a hole up top all right, but it’s not in the ozone layer.
Yes we need plug the money hole.
Beijing next?
https://www.nytimes.com/2017/06/04/world/asia/china-media-climate-trump.html?_r=0
On the Paris exit, one of the most asinine excuses for staying put in the Paris Accord was that the commitment was “voluntary & non binding”.
As opposed to what? A binding agreement under threat of enforcement by the UN or world court?
Was the US $uppo$ed to follow through voluntarily while naively expecting China, Russia and India would so the same when their time came many years later?
two statements
Of course the Chinese are against us leaving the accord. `1) we aren’t shooting ourselves in the manufacturing foot and 2) they won’t get more US money.
The ozone hole is not the issue. The issue is ozone depletion but without evidence of depletion.
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2748016
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2612810
My original home air conditioning condenser unit installed in 1985 was replaced a few years ago with a comparable unit that’s easily three times the size thanks to the Ozone Hole BS.
CFCs may be released by volcanoes in significant quantities: http://cfc.geologist-1011.net/
Also, there is a bit of a deafening silence about CFC impacts on ozone in the recent literature, would anybody dare to (or be able to) publish sceptical science on this issue?
Yep…
https://pubs.usgs.gov/of/1997/of97-262/of97-262.html
Well now that’s something I never knew. Thanks for that!
Won’t be long before he says “auf wiedersehen” to Bonn. Better take cover. Greenie greengoo head-explosion could be hazardous to your health.
At 2 – 8 ppm ozone must be more powerful than CO2 at 400 ppm or water vapor at 2,500 ppm.
Nicholas-San:
Water vapor is at around 30,000ppm, not 2,500ppm…
Vapor phase water over the tropical ocean surface is around 30000 ppm based on the saturation curve for tropical temperatures.
Any meteorology text shows total precipitable water is about 2.5 cm liquid water, about 2.5 kilograms per square meter.
2.5 kg divided by the total column weight of 10000 kilograms is about 0.00025 or 0.25 percent which is the same as 2500 ppm by weight.
Satellite measured total water vapor on a global basis averages 2500 ppm
http://www.climate4you.com/GreenhouseGasses.htm
That’s why some models use the 2500 ppm global average even though actual water vapor in any one location varies radically over and under that number.
Typical office building maintains 25C with 50 percent relative humidity which mean there is nearly 1 percent water vapor by volume, thats 10000 ppm wv.
Once upon a time, early in the game I was sent to question a climate modeler in public who was part of a dog and pony show traveling from state to state meeting elected officials and holding public workshops. After listening to his presentation and what our politicians were planning they ask if there were any questions. Note most of the people, several hundred, that were there were from organization environmental groups. There were only a handful of us that were not. I asked several questions and we spent an hour or longer discussing the whole topic. Two of my questions were as follows, (1) since water, especially oceans cover over 75% of the Earth, how it the interface being the ocean surface and the gaseous atmosphere used in the models? He explained that at the time basically all that was considered in the models was ocean surface and divided cubes of atmosphere directly above it. That led to a second question (2) So how is water vapor being considered in the models? The scientist laughed. His answer was “we are working on it.” Which led to a third question (3) Why is water vapor a significant component of the atmosphere not being modeled. He laughed again and said, “well for now considering all the ways water vapors presents itself in the environment, it is just too complex to model with any certainty that we would be close to right.”
China Receives US$95 Million Grant to End Production of Ozone-Depleting Global-Warming Gases to help Meet its Targets under the Montreal Protocol
http://www.worldbank.org/en/news/press-release/2013/05/02/china-receives-us-95-million-grant-to-end-production-of-ozone-depleting-global-warming-gases
…must be nice
The Chinese understand the art of the deal.
US negotiator: We want you to make the same costly change we are making.
Chinese negotiator: We won’t make the change unless you pay us first.
US negotiator: Make the change and we will pay you.
Chinese negotiator: You are the one wanting the change, pay first and we will change
US negotiator: OK, Here is the money
Chinese negotiator: Thanks, we will make change
US negotiator: When?
Chinese negotiator: We never agreed when, only to make change.
US negotiator: We need change by 2020
Chinese negotiator: That will cost extra
US negotiator: OK, Here is the money
Chinese negotiator: Thanks, we will make change 2020
2020:
US negotiator: Where is change?
Chinese negotiator: We made change
US negotiator: Nothing has changed
Chinese negotiator: We replaced old with new
US negotiator: New has same problem.
Chinese negotiator: Money was to fix old, not fix new
US negotiator: We want you to make the same costly change to new as well.
Chinese negotiator: We won’t make the change unless you pay us first.
US negotiator: Make the change and we will pay you.
Chinese negotiator: You are the one wanting the change, pay first and we will change
US negotiator: OK, Here is the money
Chinese negotiator: Thanks, we will make change
US negotiator: When?
Chinese negotiator: We never agreed when, only to make change.
Oh, this is a thing of beauty, and absolutely true!!!
The Chinese always get the better of those foolish Foreign Devils that think they can control what the Chinese do.
I can’t recall the source, but the phrase was priceless:
“A fool lies here who tried to hustle the East.”
Magnificent.
“The Chinese always get the better of those foolish Foreign Devils that think they can control what the Chinese do”
We got em hooked on opium.
Yes the British, not the USA, got them hooked on opium. The USA was there at the time but played a very minor role, we were yet a major power. The reason it could be done is that China was going through a period of somewhat disarray and confusion at their highest levels. Some of their leadership running the show was corrupt. However, the Chinese rose up and started a couple of wars because of it all. They lost but the Chinese have long, very long memories, so today it is all part of their “DNA” when negotiating with the West.
It took the English Empire to do it.
It was Kipling, “The Naulahka.”
Nah.
Obama could never talk so long without talking about Obama.
Which brings up a thought. Some culture place great store on proper bargaining.
Don’t or won’t bargain properly and you’re just not worth anything.
The biggest damage done by the treaty was the requirement to phase out CFC fire suppressants on ships. There is no suitable fire suppressant available which is A safe and B either takes up little space or is of low mass. Carbon dioxide is unsafe and has killed many engine room staff, even on naval vessels – it is NOT toxic, but the amount released into the engine room has to be so large to put the fire out that there is then insufficient oxygen for life. It cannot be released unless all personnel have evacuated the ER. Elsewise those left are dead. Steam used to be used but it has a similar effect – it drives out the air from the ER.
The only ‘safe’ system is believe to be that using Inergen. From the Monroe Extinguisher blurb: https://www.monroeextinguisher.com/inergen-clean-agent-fire-suppression-system/
INERGEN Agent – INERGEN agent is a mixture of three inerting (oxygen diluting) gases: 52% nitrogen, 40% argon, and 8% carbon dioxide. INERGEN gas extinguishes fire by lowering the oxygen content below the level that supports combustion. When INERGEN agent is discharged into a room, it introduces the proper mixture of gases that still allow a person to breathe in a reduced oxygen atmosphere. It actually enhances the body’s ability to assimilate oxygen. The normal atmosphere in a room contains 21% oxygen and less than 1% carbon dioxide. If the oxygen content is reduced below 15%, most ordinary combustibles will cease to burn. INERGEN agent will reduce the oxygen content to approximately 12.5% while increasing the carbon dioxide content to about 3%. The increase in the carbon dioxide content increases a person’s respiration rate and the body’s ability to absorb oxygen. Simply stated, the human body is stimulated by the carbon dioxide to breathe more deeply and rapidly to compensate for the lower oxygen content of the atmosphere.
The major problem with the system for a ship is that the cylinders are extremely large and heavy. This is because of the very large volume required to be inerted. The CFC system used far less gas, and was safe.
Dive support vessels use to use Halon systems and according to the online dictionary
halon
ˈheɪlɒn/Submit
noun
any of a number of unreactive gaseous compounds of carbon with bromine and other halogens, used in fire extinguishers, but now known to damage the ozone layer.
In the mid-1970s, Chrysler Corporation discovered that Halon was the only effective fire suppressant for use in their very popular Dodge vans – hence the great popularity, in the 70s and 80s, of Van Halon.
Don’t inhale the Halon, Michael.
Edward Lodewijk Van Halen is a Dutch-American musician, songwriter and producer. He is best known as the lead guitarist, occasional keyboardist and co-founder of the American hard rock band Van Halen.
Some designers use localised water mist systems.
See, for example, this Investigation report: –
https://www.ntsb.gov/investigations/AccidentReports/Reports/MAB1624.pdf
But it is crucial that these are triggered early, but working fire-detection systems, properly located, and big enough, to get enough water onto the location of the fire, should ensure that.
And because the mist is comparatively localised, people can survive elsewhere in the same compartment.
Auto
I would like a knowledgeable explanation of just how CFCs are supposed to reach the ozone in the first place. The ozone is high in the atmosphere, and forms from Oxygen. I can see oxygen and nitrogen being high in the atmosphere, the make up, what, 95+% of it, and CFCs are profoundly heavier molecules, and a miniscule concentration. This has never made sense to me.
Sand and dust particles are heavier than air and much heavier than CFC’s; yet they can be driven into the air and across the globe by winds. Wind and atmospheric circulation can lift lots of heavier-than-air particles into the upper atmosphere.
Diffuse gasses mix. The stratosphere also exchanges with lower atmosphere across the tropopause. There is also diffusion. https://water.usgs.gov/lab/chlorofluorocarbons/background/
Even solids can mix and diffuse into gasses. Clouds are massive, but don’t fall out of the sky. Whe tobacco is burned, the smoke particles are heavy, but don’t fall to the ground.
Thunderstorms can lift parachutists upwards, and snap the wings of unwary aircraft that fly through them. It’s called convection and its way more powerful at the molecular scale than little old gravity. Thunder clouds regularly punch up into the lower stratosphere.
Smaller scale uplift by convection means air is pretty well mixed and gravitational fractionation is trvial by comparison.
Did I not read a study not long ago that the Cl and F required for the O3 “destruction” equation could easily come from ocean evaporation?
“… come from ocean evaporation?”
Sort of. A rough ocean surface (spray) lifts the mixture of water and other compounds into the air. As water evaporates the other compounds, including Halogens, will remain to be lofted into the atmosphere. (It has been awhile since I read about these things.)
See David’s comment at 7:45.
I began reading WUWT in September 2008.
A post about that time — October 25, 2008 — is, I think, the earliest of the many you will find if you search for ‘ ozone ‘ via the box provided along the right side of the page, above. I’m not going to re-read all of them, plus all the other things about ozone I found during those earlier years.
proves that if there is a solution, you secondly have to create a problem. thirdly convince the gov’t to throw
money at unending and perpetual amount of research to pay for a house, kids through college and retirement. The bureaucratic’s American Dream.
It is interesting to not that the Ozone scare was, again, only based on model assertions and lab chemistry, and not conclusively proven at all.
The dangers of smoking are stated to be based on a strong statistical correlation, but it is surprisingly hard to find find any academic critical examination of this, and no one has yet provided a mechanism for the link between cancer and cigarettes.
It is surely the mark of flourishing science when early findings are constantly rechecked, and moribund science when they are never revisited, Kuhn points out that progress from an obsolete paradigm to a better one are driven by the re-examination of discrepancies in the pre-existing theories which are usually ignored as ‘exceptions’ by the vast bulk of regular scientists….
The problem with tobacco is that you can’t perform controlled experiments on people. You have to rely on statistical evidence.
A controlled experiment would take a group of identical twins. Have one twin from each pair start smoking at a specific age and the other would never inhale tobacco smoke. Keep all other environmental factors identical. Then measure the differences in respiratory, heart and cancer ailments. The odds are that the smoker twins would have much higher cancer, hear and lung disease rates… But you can’t perform this sort of experiment on people.
This is the only manner in which AGW and tobacco are similar. There is no “Planet B” to use as an experimental control.
… Have one twin from each pair start smoking at a specific age and the other would never inhale tobacco smoke. Keep all other environmental factors identical….
This is complete nonsense. There is no known way to keep all ‘other’ environmental factors identical – especially when you have those ‘environmental’ factors might be. Identical twins develop differently in all sorts of different ways – I have a pair myself, and have watched them.
You don’t find out anything new by random ‘controlled’ experiments unless it’s by chance – that was the sort of ‘scientific’ mistake that the ‘curio collectors’ of the 18th century made, and it’s also the well-known debating fallacy that environmental activists like to present as an argument for suppressing all opposition to their demands. You need to FIRST find a mechanism, and THEN test it using experimental controls.
Without a mechanism, statistical correlation remains an interesting curiosity, and one which, as we have seen from the AGW fraud, can readily be manufactured whenever more grants are needed.
There is a “known way to keep all ‘other’ environmental factors identical.” There just isn’t a practical, legal or ethical “way to keep all ‘other’ environmental factors identical.”
Science doesn’t start with the mechanism. It identifies the mechanism via the scientific method.
Smoking beagles .. “the failure of many investigators to induce experimental cancers, except in a handful of cases, during fifty years of trying, casts serious doubt on the validity of the cigarette-lung cancer theory.”
Of course, Richard Doll’s affable doctors study, weakly ‘researched’ was taken as proof positive over any other research, even when he was now known to be on the payroll of Monsanto and other companies, and even today he’s defended by scientists as an authority figure despite undeclared earning as high as 70 times the UK weekly income for being a ‘consultant’.. in secret.
Doll also said dioxin, 24D, Agent Orange and other nasties were all perfectly safe.>/a>
The dangers of smoking are stated to be based on a strong statistical correlation
======================
rural non-smoker – 0.1 % lung cancer
rural smoker – 1% lung cancer
urban non-smoker – 1% lung cancer
urban smoker – 7% lung cancer
What is interesting is that smoking is about as bad as living in a city as far as lung cancer goes. With all the hype about banning smoking, if we are really concerned about lung cancer the solution is to ban cities as well.
..The dangers of smoking are stated to be based on a strong statistical correlation..
It’s the ‘stated’ bit that I have problems with. I would like to see that investigated, rather than simply being treated as doctrine…
Lung X-rays show this graphically. Non-smoker city dwellers have lungs that resemble those of light smokers. Rural non-smokers have clear lungs. I’m sure that micro-inspection of alveoli in lung tissue from autopsy or biopsy shows the same.
Dodgy Geezer,
Same with the ‘saturated fats are bad for you’ mantra originally created with the 1970 Seven Countries Report (think I got that right). Diet doctors made a fortune with diets replacing everything with olive oil and avocado oil, etc. The Mediterranean Diet. The this diet. The that diet. Fat doctors making wild claims but millions with books that recommended high carb diets instead of meat, cheese, and butter (which the skinny French eat). I bought the whole enchilada.
[Disclaimer: I used to know the details of this whole thing cold three years ago, but I’m working off a sloppy memory that wasn’t interested in keeping these facts uppermost. So if I upset anyone by skewing minor details, my apologies. Feel free to correct me.]
A geeky researcher at the National Institutes of Health (NIH) about a decade ago looked up the original data claimed about saturated fats being bad for you just for s**ts and grins and decided to replicate it. He did not have the slightest intention of disproving the theory. No suspicions. It never entered his mind. It was just something that he liked to do during his off-hours. Replicate and verify medical theories. Check them out for the helluvit. Literally for the joy of research and nerdy stuff.
The long and short of it is that he discovered 80% of the data was falsified, and in 2014 the original doctor who made these claims had his papers removed from all medical journals and databases and his credentials revoked by the institutions that issued them. Disgraced. The story was so embarrassing to the NYC doctors who made a fortune for over four decades screaming about the evils of butter, pork fat, and beef tallow that the NYTimes ran only one complete story on it. (I have a copy on my storage drive, but no interest in searching for it right now.)
As my doctor said to me–he is an eat-your-saturated-fats guy now–you ever see skinny black women who have lived to be 108? What have they been cooking with all their lives? From chicken to collard greens. Pork lard. What about Jewish grandmas who are still charging up and down the stairs at 100, he said, going concerns who still boss me around like I’m 10? What have they been cooking with all their lives? Chicken fat. And he told me that the only real thing that protects your brain as your age, particularly after the age of 65, is fat. And his claim is that it has to be saturated animal fats. Butter. Butter. Butter. Pork and beef lard.
Your appetite is satiated with animal fats and you eat less, btw. Ask the French. I now buy duck fat from dartagnan.com in NJ because you can freeze it and unfreeze it and re-freeze it, and reuse it after cooking over and over and over again, and it is a true luxury in the taste department. Not to mention that it protects your heart.
Olive and avocado oils are full of saturated fats, and the Mediterranean diet is also a (mostly) saturated fat diet (and can easily be made low carb). So if these things were in vogue when sat fats were considered bad, and are in vogue now when trans fats have been discovered to be the enemy, they must be doing something right with their marketing.
DG, not completely true. There are a number of chemicals in cigarrette smoke that are known carcinogens. Granted, animal studies and statistics. But the carcinogenic pathways for these carcinogens are quite well known.
Ristvan, I did look into this and OHS guidelines for all of those chemicals were shown to be nowhere near the levels smokers experience – that is to say they’re thousands to tens of thousands of times lower.
The main carcinogen was benzene, and again filling your fuel tank at a gas station was measurable exposing people to 10,000 times the benzene smokers got in a day. Benzene’s also supposed to induce leukemia – and smoker leukemia rates are unremarkable.
I know we’re having chemical names hurled at us to induce fear, but never quantities ‘cigarette smoke contains ammonia!’ – yeah, about 100,000 times less than we exhale an hour. ‘Cyanide’ yup, a pack a day smoker would take in as much cyanide from cigarettes as they’d get from eating one whole apple every 3 weeks. It’s all rather silly considering many plants we eat contain carcinogens and toxins.
The funding cut “slashes funding to support compliance with the Montreal Protocol”. So what? If we have successfully phased out most CFC usage in the US, would there not be a reduced need for compliance checking?
They now want to phase out HFC’s and other CFC replacements.
More legislative obsolescence masquerading as enviro protection.
President Trump needs to take on the Green Mafia:
Funniest. Ad. EVAH!
+++ Thanks!
David–thanks for the charts. I’ve spent decades trying to explain that when O2 is in the shade, it can’t be ionized to O3.
Then there is the thickness part. High solar activity (ultra-violet changes the most) ionizes more O2, which makes the ozone layer thicker. Less active sun reduces ionization and the thickness. The modern industrial world has little to do with it.
David,
Back in the early ’80s I was struck by the observation that all the articles in the Media talked about how ozone was declining and UV “might” or “could” increase and cause problems for the biosphere. It was all speculation without any hard evidence showing that there was any trend in UV matching the trend in O3. Yet, academics were jumping on the bandwagon with research papers on such things as how increasing UV might be responsible for the decline in amphibian populations, or some such things.
Therefore, about 1984 I used my then-new Amiga computer to write a simulation in BASIC to predict what the surface UV might actually be. I based the simulation on Total Ozone Mapping Spectrometer ozone levels. I took into account the variation in TOA UV with sunspot cycles and also the eccentricity of the Earth’s orbit. I modeled the change in sunlight intensity at a given latitude with the seasons, taking into account both the longer path length and increased footprint for low sun angles.
What I found was that there was an apparent slow upward drift in UV intensity during the Winter over a period of years, when levels were so low as to be inconsequential. However, during the Summer (when we get our sunburns) the UV flux was flat. That is, those organisms that had evolved protective mechanisms against UV were adequately prepared to deal with the highest levels, which were essentially static.
Incidentally, David, if you look at the typical September TOMS ozone maps, just before the ozone ‘hole’ breaks up in the Spring, there are anomalously high concentrations of ozone just outside the circumpolar vortex because most ozone is created in the tropics and migrates polewards. So, when the polar vortex does break up, that ozone-rich air is able to mix with the ozone-depleted air and rapidly restore the ‘missing’ ozone.
This whole AGW alarm is strangely familiar, like a rerun of the old ozone science fiction movie.
Al Gore was also involved in ozone-hole hype.
I seem to remember that the Nobel Prize issued for the Ozone Problem/Solution said something about saving the earth sort of thing. Even if saving us was true, this was not science.
Montreal was the blueprint for Kyoto, Paris, etc, etc. Basically place all or most of the burden on the USA because we are the biggest economy. Liberals, here and abroad, believe that is only fair since they truly believe we became wealthy by exploiting all other nations. The whole ozone fiasco and media furor was also a blueprint for AGW religion. Yes, there are PR professionals that have studied both and how to feed the public. One a different note: Once upon a time when my staff measured something, collected data, they had never measured before and decided somehow “it” was the cause of a problem I would ask them how do we know before people traveled to the Antarctica and we put satellites in orbit that there were not holes in the ozone layer? Some would all look around the room bewildered. Some would get angry because “you are changing the subject.” The smarter and wiser ones would say “we didn’t know.” If we never measured it before then it is unknowable. We might develop methods to make it at least more knowable but if we didn’t measure it in the past we cannot know. Proxy data is OK but again it is difficult to impossible to know cause and effect in the past.
Ed,
I totally agree with you. The same problem occurs in medicine. We have fabulous new technological tools that can detect physical and chemical properties of our bodies that could never previously be observed. It is curious how many of these new features are deemed to be pathologies in need of expensive drug therapies. Biology not medicine should guide our understanding of heath and biology shows us how ignorant we actually are.
When tallying up the costs, don’t forget its not just Freon that got banned. TriChlorEthane was widely used in the electronics industry as a circuit board cleaner. After the soldering operation, boards were run thru the TriChlor tank and came out spotless. After TriChlor was banned by Montreal, we had to switch to water based cleaning. This added a few steps to the manufacturing process, since not all parts could be washed, so had to be installed post assembly and wash, and then cleaned with an alchohol scrub. There were a couple of years of learning to do this correctly where a huge amount of scrap was created. Corrison problems from the H20 would show up months after shipping product. There were also a lot of parts that had to be changed since they could not be washed in water. This led to perfectly working boards being re-designed and inventory being scrapped. I would be very suprised if this did not cost the industry billions of dollars, and it continues to add to the cost to this day, since it requires a more expensive manufcturing process.
Also, Kodak used to make a very effective film cleaner based on TriChlor which is unavailable today. There is a substitute available, but it costs much more than the Kodak cleaner did.
Used Triclo hot bath for cleaning mechanical parts for Gardena in Germany in the 80’s . No protection at all for “die Gastarbeitern”, so I used to hold my breathe and working 1 min in , on min out.
The ozone layer is self-healing. There was never any danger.
The chemical reactions that form the ozone layer have a positive pressure coefficient.
http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap10.html
As long as molecular oxygen exists in the atmosphere of Earth, ozone will form from solar UV.
The atmosphere is of biological origin. See atmospheric evolution.
Huge hole on Uranus.
http://a2.att.hudong.com/01/46/20300000241358132132464569431.jpg
My Brother, Dr. Dale Hugoson, reduced the CFC emissions by the greatest amount of anyone, except those that replaced CFCs in Styrofoam cups, with CO2. He worked EHS for Motorola in the ’90s. By 1992 they had STOPPED using CFC’s (liquid, which evaporated right after usage) to remove the left over resins on the wave soldered circuit boards, corporate wise. THIS WAS THOUSANDS OF TONS…combine that with ALL such operations being replaced with TURPINES, across the world, the CFC emissions went to a FRACTION.
http://img.clipartall.com/yay-cathay-pacific-clipart-yay-clipart-500_320.jpg
It still had no effect on the ozone hole.
Another thing that was destroying ozone in 1991-1994 was the SO2 from Mt Pinatubo. That stopped afterwards too.
Did the temperature of the stratosphere respond the the laudable efforts of Motorola?
I am surprised that you can do an Ozone hole story without once mentioning chlorine. The process by which Ozone was believed to be depleted was that chlorofluorocarbons (CFC’s) did not degrade in the low atmosphere at all so they became a vehicle to introduce chorine to the stratosphere with the UV breakdown of CFC’s at very high altitudes. The chlorine radicals from the decomposition of the CFC’s provided a much faster path for decomposition of ozone.
Its been a long time since I looked at this but I also don’t know how long the normal residence time is for ozone in the stratosphere but it is dynamic and dependent upon sunlight. So there should be no ozone over the poles during winter but it should reform in the spring when the polar atmosphere is out of the earth’s shadow. If there is a lot of chorine sources over the poles, it can delay the ozone layer formation in the spring. The question is what is the source of the chlorine and why does a hole form mostly over Antarctica. The latter question is likely answered by the stability of the polar vortex in the southern hemisphere vs. the northern one but you have to assume that the chorine sources that build in the atmosphere are from CFC’s. The fact that Antarctica is surrounded by ocean and winds circling the poles likely has a lot of ice crystals entrained, there is obviously a natural source of chlorine at these latitudes that needs to be accounted for. Has anyone tried to do sort out the CFC vs. the natural portion of chlorine at these latitudes?
FFS! The Smithsonian has made a freudian mistake due to their dogmatic pejoration of anything “warm” and angelizing anything “cold” as it relates to Earth science. The ozone hole forms at the beginning of spring because of COLD temperatures which make PSCs possible that are still extant as sunlight returns, and then disappears when the atmosphere becomes too warm for PSCs. The cult has truly diminished science to a state where anything warm must be bad and anything cold is good. The cult is basically the dark side.
They use the statements: ” solar uv both creates and destroys ozone”.
I defy anyone to find a graphical analysis showing the relationship between solar uv and ozone creation!;
also, find a graphical analysis of the relationship of solar uv and the destruction of ozone!
I’m stating that, if the relationship exist, they are not published. They chemical analysis is well stated. BUT, if Solar uv is, say, 200 sfu at Penticton, what is the amount of ozone created?
Is this just like the Climate Change scientific junk?
The equations balance and the Chapman Reactions explain the distribution of stratospheric ozone:
https://disc.gsfc.nasa.gov/ozone/additional/science-focus/about-ozone/ozone_cycle.shtml
https://www.ucar.edu/communications/gcip/m1sod/m1pdfc2.pdf
http://www.cchem.berkeley.edu/molsim/teaching/fall2008/ozone/Ozone%20website_files/Page603.htm
Thanks, useful stuff.
Looking at these transformations, I would guess that this is saying oxygen absorbs the incoming UV and converts it into kinetic energy instead of allowing it to pass through? That’s what it looks like to me, at least, but I am not a chemist. If UV is changing the O2 to 2 O, then this is absorbing the UV energy. When the UV finds O3 and splits it to O2 and O, then this, too, absorbs UV energy. So either way, whether you destroy O3 with a compound or not, incoming UV will be absorbed by Oxygen. How is this something that can get out of balance in the first place, unless you change the amount of UV? What am I missing?
And meanwhile the people who drive the economy think somewhat differently.
https://thinkprogress.org/kentucky-coal-mine-solar-farm-a5d10d6526bb
John Podesta (Think Progress) drives the economy?
I can’t find any information about a coal company called “Berkeley Energy Group.” Their partner EDF Renewable Energy seems to be in the business of harvesting taxpayer-funded subsidies.
https://www.usnews.com/news/best-states/kentucky/articles/2017-04-18/what-to-do-with-a-former-coal-mine-make-it-a-solar-farm
The Berkeley Energy Group executive doesn’t even know what the capacity will be or how much it will cost.
Drive the economy? That’s hilarious! Virtually all growth for the past 8 years has been produced from borrowed and or printed money. Hey Gareth! How much do you need to borrow to get completely out of debt!
Fungi are thought to produce about 160,000 tons of chloromethane annually.
There is a lot we do not know about ozone. It is reputed to be the cause of the lapse rate inversion in the stratosphere, but the stratosphere continues to warm well above the altitudes of high ozone concentration.
The initial stalling of the lapse rate may actually be due to CO2.
Take on geoengineering please.
Too easy.
Great post, and good discussion. Next stop, acid rain?
Rain is always acid due to CO2 dissolution, but the “acid rain” of the 70s was real and caused by SO2 from burning coal. Developed countries now install scrubbers to prevent this. China not so much on a lot of their older plant.
It was a regional/local problem that was easy to fix, before the EPA got involved.
Back when acid rain was the all the rage, I remember reading a geoengineering-type proposal to dump lime sludge into streams in the Northeast of the US to neutralize it.
So many environmental scams, so little time.
A perfect treaty according to the outgoing Kofi Annan , patting himself on the back and trying to use it justification for the war on “carbon” ( which of course means life ).
https://climategrog.wordpress.com/uah_tls_365d/
It was ozone depletion which was behind most of the stratospheric cooling which occurred at the end of the last century. But this was not a steady slide, it was two step changes which were the result of the two major stratospheric eruptions.
This was little or nothing to do with CFCs , the UN bogeyman of the day. This conclusion is only reached by drawing a straight line through the data and ignore the real form and information which it contains.
Since there have been no major volcanic eruptions since ozone has flattened out and slightly recovered. This is being falsely claimed as massively successful Montreal Protocol because the initial cause was falsely attributed.
The stratospheric cooling coincides with the late 20th c. warming in the troposphere : the drop in ozone was letting more sunlight into the lower climate system.
Both Paris and Montreal were false solutions for incorrectly attributed problems.
Pretty good correlation: ?w=680
?w=680
So what does a 40 percent budget reduction in compliance mean? Black market CFCs are going to take off in the US? Or just smaller payments to other countries to encourage them to comply?
The NRDC article is incorrect on HCFCs.
HCFCs were introduced as replacements for CFCs. CFCs have large OZONE DEPLETING POTENTIALS, HCFCs do not.
However, HCFCs have large WARMING POTENTIALS, hence the continued negotiations of also phasing out of HCFCs for other constituents that neither have ozone depleting potentials nor large warming potentials. And plenty of such alternatives have been developed over the years. In that sense, replacing HCFCs is not a ‘biggie’.
This is what this is all about – the warming effect of HCFCs and whether they should be phased out. It is NOT about ozone depletion. NRDC got it completely wrong by suggesting that this is about the ozone layer. But hey, never waste a good story – even is it is not true.
http://www.un.org/en/events/ozoneday/substances.shtml
The replacement for HCFCs in automobiles is called 1234YF, it costs over 1000 dollars for 10 pounds. Mercedes says it is not safe and are refusing to use it, they are developing a CO2 based system. If you buy a new car there is a decent chance that it is using 1234YF.
I hope I’m not repeating anyone – I don’t have time to read all the comments right now. But you missed the biggest cost of all: the catalytic converter. Cost is probably in the trillions with the added irony of tremendous toxicity released by mining for all the platinum.
Off- piste –
Imagine the hell if this was used in Fracking-
“Furthermore, the photovoltaic industry is one of the fastest growing emitters of hexafluoroethane (C2F6), nitrogen trifluoride (NF3), and sulfur hexafluoride (SF6), three greenhouse gasses that have a global warming potential 10,000 to 24,000 times higher than CO2 according to the Intergovernmental Panel on Climate Change. (The industry uses these gasses for cleaning sensitive manufacturing equipment.) In 2011, the CEO of Praxair, a major supplier of industrial gasses, opened the company’s annual report with a letter that highlighted the photovoltaic industry as one of its most impressive growth sectors”
Why DO the French say “Holy blue!” anyway?
It’s an archaic rhyming slang for sacre dieu which was considered taking the name of god in vain. It’s like saying “gosh” or “darn”.
WUWT has always been a great place to learn about climate science, but now it is also a great place to learn about foreign expressions!
Thanks! I never knew that.
I read in a book that because of ozone depletion that we got warming since the two coincide with each other each time there was a warming trend. If this is true then Trump should leave the Protocol alone. The book is called: In Praise of Carbon: How We’ve Been Misled Into Believing that Carbon Dioxide Causes Climate Change by David Bennett Laing
This is a good, clear article. It certainly resolves some very germane questions concerning CFC’s, the ban, and the costs.
I have suggestions for future articles, with similar attention to actual science and the true costs of doing unscientific things to address nonexistent problems.
Number one: Ethanol from corn produces lower fuel efficiency in cars and other internal combustion engines. Can we get rid of it, please?
Number two: Whirly-swirly light bulbs that are far more expensive than incandescents, don’t last all that much longer, and are apparently a deadly chemical hazard if they break. Get rid of them, please.
Number three: Replacing paper with internet transactions: Of all commodities on earth, paper is clearly one of the cheapest and most easily renewable or recyclable; and the internet adds greatly to people’s risks.
Was it George Carlin or P.J.O’Rourke who said the only thing you need to know about Climate Change ( and ozone depletion?) is there is nothing we can do about it.
Or did I just make that up? I’m not sure.
David
Interesting and timely main post as usual from yourself. The ozone hole season is near.
I note there is no real comment above about what the possible alternatives are as to what is causing, or is responsible for the seasonal reduction in ozone during the months of August through to December.above Antarctica. Or for the months preceeding Antarctic spring where values go below the magic 220DU value.
All I see is discussion about chemical destrution. All ozone reduction appears to be related to chemical destruction, without any real evidence.
What do others think is occuring above Antarctica ???
Winter.
Looks like the Pied Piper is leading the rats to the sea, i.e. UN “Special Envoy for Cities and Climate Change” is not only paying the UN Green Climate Fund for his “Specialness” (Church Lady says, “Well Isn’t That Special!”) and now is leading the “Rats” as in 5-particular States Governors and Sanctuary Cities to the sea, Mr. Michael Rubens Bloomberg. Au revoir duckies. Ha ha
https://www.youtube.com/watch?v=xQW_4kowyZ4
I only skimmed the comments, so forgive me if this has been addressed, but:
Why are we still spending money on the Montreal Protocol? Where is that money going?
I am always perplexed how heavier than air molecules produced in the northern hemisphere ended up high in the stratosphere above the south pole.
You call that tiny seasonal fluctuation a hole? Check this out.
http://www.extremetech.com/wp-content/uploads/2014/07/cassini-3.jpg
Saturn’s North pole. Now that’s a hole.
When it comes to environmental scares the CFCs hole in the ozone layer is classic when looking at AGW. As a none scare by creating the fear when that fear never eventuates rather than say it was never a problem they say look how our policies prevented a global economic disaster. It’s only a matter of time before AGW proponents rather than argue whether the temperature is increasing or not will explain that the lack of warming is due to measures to already taken to slow the rate of temperature rises. They will then argue that more money needs to be spent otherwise these gains will be reversed.
Even on moons with thickish atmospheres polar regions come equipped with low pressure “holes” RELATIVE to the standard air.
Like on Titan for instance.
http://www.clarksvilleonline.com/wp-content/uploads/2014/10/NASAs-Cassini-spacecraft-discovers-Methane-Ice-Cloud-in-Stratosphere-of-Saturns-moon-Titan.jpg
The leftists saw something “new” in Antarctica, took advantage of the situation, propelled by the nigh inexhaustible lobbying funds of Dupont, to hoodwink the entire human race.
That is a crime. Dupont deserves to suffer for that. Seems to me Brussels is always keen to crack the whip on a Microsoft or Budweiser.
What’s justice for them should be twice as just for a criminal corp like DuPont, which actually deserves it.
The reactive armor analogy is a bit off in that dissociation of ozone doesn’t block UV. Ablative effect is more precise analogy.
Still, not much correlation between ground level UV and total ozone. It is indeed oxygen which shields, explaining why sunburn is rapid at Mt Everest while nigh impossible at the Dead Sea.
David,
I am interested to know what evidence you would accept as compelling that CFCs were destroying the
ozone layer? There is evidence that they act as a catalyst in the lab, evidence that the ozone concentrations in the atmosphere is decreasing (albeit it is a slow decrease which is mostly hidden by seasonal fluctuations and large amounts of natural noise). As you stated there is no planet B on which a controlled experiment can be done.
In the meantime lower ozone concentrations mean larger instances of sun cancer so there is a real cost that is associated with low ozone concentrations. In addition plastic goods in the southern hemisphere degrade faster due to the increased UV concentrations. Thus you have to weigh the real costs associated with of a lack of ozone against real costs that come from banning CFCs. The question is then the costs outweigh the benefits? And again there is the selfish fact that using CFCs in the northern hemisphere kill people in the southern and how do you account for that?
Actual evidence would consist of decades of consistent measurements of stratospheric ozone starting well before the beginning of anthropogenic CFC emissions.
I stipulated in tbe post that CFC’s could have contributed to and enhanced the annual Antarctic ozone “hole” and that a gradual elimination of CFC’s would have been a good thing.
But no such measurements were made. So are you suggesting that people should die because the
ideal measurements were not made? Again the question is how do you weight the potential costs of continuing to use CFCs against the cost of implementing a ban? Would multiplying the cost of a
life lost by the probability that CFCs are the cause be acceptable? In which case again the case for a
ban would be quite strong.
There’s no evidence that any lives have been lost due to ozone depletion… None whatsoever. There is no clear evidence of a significant secular depletion of stratospheric ozone.

There is only the standard Chicken Littling that an x depletion of ozone would lead to a y% increase in melanoma deaths.
The gradual elimination of ozone-depleting emissions was a good thing. It just wasn’t a crisis which required drastic action.
Here are two plots of total stratospheric ozone over Bismarck, North Dakota, one with atmospheric CFC concentrations and the other with stratospheric temperature:
While CFC’s were skyrocketing from a few ppb to 100’s of ppb, total ozone rose from 1960-1966 and declined from 1966-1993. Since 1993 the CFC concentrations have declined slightly, while ozone has recovered to where it was in the late 1970’s and early 1980’s.
It is massively unlikely that a 500 ppb increase in CFC-12 (and comparable rise in other CFC’s) could instantaneously cause a 20 DU drop in ozone and a 40 ppm drop in CFC-12 (and comparable drop in other CFC’s) could instantaneously cause 10 DU increase in ozone.
Total ozone more or less tracks the stratospheric temperature over the entire time series, irrespective of CFC concentrations.
Ozone depletion can either cause or be caused by stratospheric cooling.
David,
the question of whether or not there is any “evidence that any lives have been lost due to ozone depletion” is a statistical one that will always rely on correlation. But there is extremely strong statistical evidence about how many melanomas are caused by UV radiation
(see How much melanoma is caused by sun exposure?. Armstrong, B. K.; Kricker, A.
Melanoma Research: November 1993 for example) evidence that the rate of skin cancer in Australia has risen dramatically since 1980, and evidence that CFCs deplete ozone causing UV levels to rise. Put it all together and there is a strong case that CFCs are causing people to die. Imagine it is a civil court case where things are decided on the balance of probabilities. What is the probability that people are dying due to CFCs?
Abject nonsense.
As moronic as the Precautionary Principle.
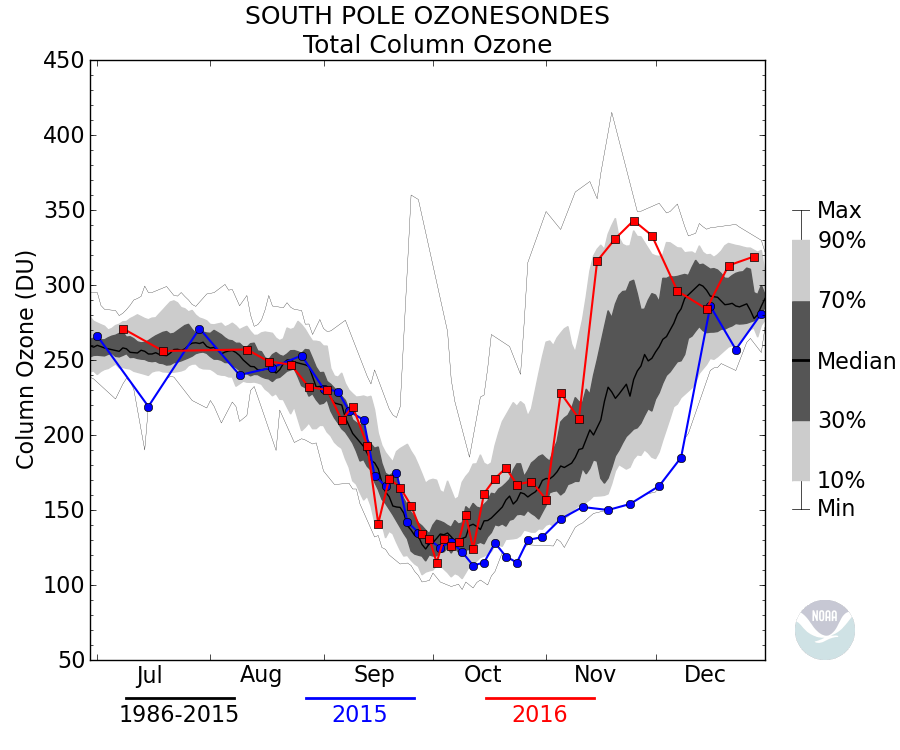
Like these?
Exactly not like those. The minimum occurred in September prior to the mid-1980’s and the ozone level recovered every summer.
The ozone column over Antarctica was stable from the early 1960’s through the mid 1980’s. It shifted then downward and stabilized in the early 1990’s…
The ozone column over Alaska has increaaed since the early 1970’s…
How did this happen if CFC’s were destroying the global ozone layer?
David Middleton June 7, 2017 at 1:55 am
Exactly not like those. The minimum occurred in September prior to the mid-1980’s and the ozone level recovered every summer.
You have evidence that the minimum in Total Ozone in the Antarctic occurred in September prior to the mid-1980s? What does the summer recovery have to do with whether the data from the BAS are a consistent dataset or not?
Try to follow along. This was the original question:
To which, I replied:
The question wasn’t about whether or not CFC’s might contribute to the annual Antarctic ozone thinning. The fact that the thinning recovers every summer pretty well destroys the notion that “CFCs were destroying the ozone layer.”
David Middleton June 7, 2017 at 5:32 am

The question wasn’t about whether or not CFC’s might contribute to the annual Antarctic ozone thinning. The fact that the thinning recovers every summer pretty well destroys the notion that “CFCs were destroying the ozone layer.”
No it does not, in the 70’s the recovery was to values between 400 and 450 DU, in the 90’s the recovery was to between 300 and 350 DU. So not only do CFCs cause a ‘hole’ in the ‘ozone layer’ at the S Pole between 15 and 20km but it also prevents its returning to the same levels as previously.
The Antarctic winter *causes* the seasonal ozone hole. CFC’s might have contributed to its area and depth.
Over the entire record of continuous measurements, the Ozone column starts out at 250-300 DU, drops to 100-175 DU and returns to 250-300 DU.
Total Ozone, South Pole Station, 1963-2017
 ?w=680
?w=680
https://www.esrl.noaa.gov/gmd/dv/iadv/graph.php?code=SPO&program=ozwv&type=ts
Ozone was stable from 1963-1983. from 1983-1993 it dropped from about 300 to 240 DU, where it has stabilized.
While CFC-12 was skyrocketing from 50 to 400 ppt, the ozone level remained constant. All of the drop in ozone occurred while CFC-12 was rising from 400-500 ppt. However, the ozone level remained stable while CFC-12 was rising to 540 and then dropping to 520 ppt.
Unsurprisingly, the ozone tracks the stratospheric temperature, irrespective of CFC concentrations…
David,
Back in the ’90s, CIESIN had a website with a sophisticated model of surface UV, that took into account clouds. One could look at the historical changes for various cities throughout the country. I could not see any evidence for a problem. That website is no longer available to the public.
David Middleton June 7, 2017 at 8:31 am
The Antarctic winter *causes* the seasonal ozone hole. CFC’s might have contributed to its area and depth.
No, it is the UV light from the sun after the Antarctic Spring sunrise that causes the seasonal ozone hole.
The formation of PSCs in the winter and their subsequent disappearance in the spring are the main ’cause’.
Over the entire record of continuous measurements, the Ozone column starts out at 250-300 DU, drops to 100-175 DU and returns to 250-300 DU.
No, the behavior in the late 50’s and 60’s was quite different than it is today. Typically in September and October the total Ozone was about 300-320 DU rising to about 350 in November and December then drifting back down to ~300 during the rest of the year.
Last year the value was 243 DU on July1, dropped to 114 DU on Oct 1 and rising back to 249 DU on Dec 31. Corresponding values in 1994 were: 277: 102: 304
David Middleton June 7, 2017 at 9:33 am
While CFC-12 was skyrocketing from 50 to 400 ppt, the ozone level remained constant. All of the drop in ozone occurred while CFC-12 was rising from 400-500 ppt. However, the ozone level remained stable while CFC-12 was rising to 540 and then dropping to 520 ppt.
You have apparently difficulty in reading your own graph, you appear to have misread 300 as 400. Also taking Northern Hemisphere, tropospheric CFC-12 and comparing it with South Pole, stratospheric ozone is a bit of a stretch (~5 year lag, Antarctic value in 1980 ~250 DU). Also there was a slow decline in ozone starting in ~1970 which accelerated in 1980, the ozone minimum was reached in about 1994 by which time the CFC-12 was very close to maximum.
Once again, the original question was: “I am interested to know what evidence you would accept as compelling that CFCs were destroying the ozone layer?”
David Middleton June 7, 2017 at 8:31 am
The Antarctic winter *causes* the seasonal ozone hole. CFC’s might have contributed to its area and depth.
My original reply to this appears to have vanished so I’ll try again.
The ozone hole is not caused by the antarctic winter, it is caused by the return of sunlight over the pole in the spring.
Over the entire record of continuous measurements, the Ozone column starts out at 250-300 DU, drops to 100-175 DU and returns to 250-300 DU.
That’s not correct, in the late 50s and 60s Total Ozone in September/Oct was ~300 DU, it rose to ~350-400 DU in Nov, and then declined back to ~300 DU during the rest of the year.
Nowadays this is what we see:
A different profile altogether, if we go back to 1994 we see the following:
June 28, 291 DU, Sept 26, 102 DU, Dec 27, 304 DU
Geronimo, if CFC were the culprit, why has there not been a major step up in Ozone since the massive reduction in CFCs.
Instead Ozone worldwide has continued a steady decline, there is absolutely no correlation between the 2, just as there is no correlation between CO2 and temps.
Because CFCs stay in the atmosphere for decades.
The why did they drop so quickly after the Montreal Protocol went into effect?
http://cdiac.ornl.gov/oceans/images/nhemispherecfcs2.png
http://cdiac.ornl.gov/oceans/images/shemispherecfcs2.png
David Middleton June 6, 2017 at 1:38 pm
The why did they drop so quickly after the Montreal Protocol went into effect?
Less than 10% drop over ~2-3 decades, that’s not ‘so quickly’.
From 1990-2004, CFC-12 rose from 500-545 ppb (3.2 ppb/yr). From 2004-2016, it dropped from 545-520 ppb (2.1 ppb/yr). It dropped almost as fast as it was rising.
David Middleton June 6, 2017 at 3:36 pm
From 1990-2004, CFC-12 rose from 500-545 ppb (3.2 ppb/yr). From 2004-2016, it dropped from 545-520 ppb (2.1 ppb/yr). It dropped almost as fast as it was rising.
Well CFC-12 production flattened out in the early 70s and dropped precipitately from 1990, it was growing faster prior to 1990. Went up from 300- 480 ppt between 1980 and 1990, that’s 18 ppt/yr (sic).
Geronimo,
You state, “In the meantime lower ozone concentrations mean larger instances of sun cancer so there is a real cost that is associated with low ozone concentrations.” First off, it is an implied association, not one demonstrated by measurements of UV. It is my understanding that melanomas are most commonly found on the lower body, while people are more commonly exposed to direct sunlight on their upper bodies.
In the case of Antarctica, there is no sunlight at all for nominally 6 months. When the sun first comes up over the horizon in the Spring, the rays are not only passing through a longer path length of air than they will three months later, but the rays are also passing through an ozone enriched region outside the circumpolar vortex. (See the images above in this thread, provide by Mike McMillan.) The sun is not high enough for the rays to pass directly down through the so-called hole. By the time that the sun does get high on the horizon, the vortex has broken up and the ozone levels are back to their protective levels. An unanswered question is whether or not the break-up of the polar vortex allows low-ozone air to dilute air masses north of the Antarctic Circle. It is unanswered because all the researchers are focusing on ozone and not measuring UV. There is a possibility that in the absence of stratospheric ozone, UV will be absorbed by the continuous creation of ozone at lower altitudes. Again, I don’t think that anyone has looked into this.
You are parroting the MSM script on ozone and apparently haven’t done any original thinking on the subject. The amount of UV that reaches the surface is not controlled just by ozone, but also by the strength of sunlight, which varies with the solar altitude, meaning the seasons. UV is also affected by clouds. Anytime a natural process is simplified to a single parameter, such as ozone, one should be suspicious that it is no longer science driving the discussion.
It’s the standard EPA “bear in the woods” or “bogeyman” fallacy…
http://www.who.int/uv/faq/skincancer/en/index1.html
Note that there is no statistical evidence presented or even linked to support the claim that “the incidence of both non-melanoma and melanoma skin cancers has been increasing over the past decades.”
The connection to ozone depletion is in the future, if we don’t abide by Montreal.
Then, almost as an aside, they state, “the main factors that predispose to the development of melanoma seem to be connected with recreational exposure to the sun and a history of sunburn. These factors lie within each individual’s own responsibility.”
Clyde Spencer June 6, 2017 at 1:17 pm
In the case of Antarctica, there is no sunlight at all for nominally 6 months. When the sun first comes up over the horizon in the Spring, the rays are not only passing through a longer path length of air than they will three months later, but the rays are also passing through an ozone enriched region outside the circumpolar vortex. (See the images above in this thread, provide by Mike McMillan.) The sun is not high enough for the rays to pass directly down through the so-called hole.
There is no sunlight so the atmosphere gets so cold that polar stratospheric clouds (PSCs) form, chlorine nitrate, ClONO2, breaks down on the surface of the cloud particles forming Cl2. While in the dark this isn’t a problem but once the sun reaches the stratosphere and warms it up enough to dissipate the PSC and Cl2 is photolysed by UV light (not the same wavelength as absorbed by O3) and the Cl radicals formed catalytically destroy the O3. Passing ‘down’ through the hole is an irrelevancy since the ‘hole’ exists between 15 and 20km where the Cl reduces the O3 to ~0 (down from ~12mPa a month earlier).
Phil,
I don’t disagree with your assessment of the mechanism of ozone destruction. However, what you seem to be missing, or at least not mentioning, is that depleted ozone itself is NOT the mechanism by which the biosphere might be harmed. Rather, it is the UV that may reach the surface that could be a problem. You don’t seem to have a good grasp of the geometry of the sun’s rays that reach the surface. The rays that first reach the surface in Spring enter the atmosphere well outside the ozone-depleted region. They pass through a longer path than if the sun were higher in the sky. That means, there is greater scattering and absorption than if the sun were high in the sky, and the rays encounter normal if not anomalously high ozone levels outside the vortex! Also, the footprint of a bundle of rays is spread out at low sun altitudes, meaning the sunlight (including the UV) is weak. The point is, by the time that the sun gets high in the sky, and the atmospheric path length is shortened, and the footprint is shrunk, the ozone has recovered to the protective levels of the previous year. Watch the pea under the shell!
Clyde Spencer June 7, 2017 at 11:36 am
Phil,
I don’t disagree with your assessment of the mechanism of ozone destruction. However, what you seem to be missing, or at least not mentioning, is that depleted ozone itself is NOT the mechanism by which the biosphere might be harmed. Rather, it is the UV that may reach the surface that could be a problem. You don’t seem to have a good grasp of the geometry of the sun’s rays that reach the surface. The rays that first reach the surface in Spring enter the atmosphere well outside the ozone-depleted region. They pass through a longer path than if the sun were higher in the sky. That means, there is greater scattering and absorption than if the sun were high in the sky, and the rays encounter normal if not anomalously high ozone levels outside the vortex! Also, the footprint of a bundle of rays is spread out at low sun altitudes, meaning the sunlight (including the UV) is weak. The point is, by the time that the sun gets high in the sky, and the atmospheric path length is shortened, and the footprint is shrunk, the ozone has recovered to the protective levels of the previous year. Watch the pea under the shell!
On the contrary I have an excellent grasp of the geometry, we’re not just concerned about UV rays reaching the surface during the spring in Antarctic. Ultimately continued depletion of O3 at the S Pole leads to depletion elsewhere. The Ozone does not ‘recover to the protective levels of the previous year’, as pointed out to you before the summer levels over time have decayed.
Phil,
As I stated in an early post, my work based on TOMS data doesn’t support your claim of declining Antarctic ozone having a significant impact in Antarctica, let alone a worldwide impact. The Antarctic decline is the result of special circumstances of extremely low temperatures, and the circumpolar vortex preventing ozone-rich air from migrating into the region of the ‘hole’ until after the vortex breaks up. Can you cite any research showing increasing surface UV either in Antarctica or the Southern Hemisphere?
Clyde Spencer June 8, 2017 at 11:37 am
Phil,
As I stated in an early post, my work based on TOMS data doesn’t support your claim of declining Antarctic ozone having a significant impact in Antarctica, let alone a worldwide impact. The Antarctic decline is the result of special circumstances of extremely low temperatures, and the circumpolar vortex preventing ozone-rich air from migrating into the region of the ‘hole’ until after the vortex breaks up. Can you cite any research showing increasing surface UV either in Antarctica or the Southern Hemisphere?
Your model for how the ozone hole is formed is wrong as pointed out above. I take it you don’t know how a Dobsonmeter works, since in order to measure decreased Total Ozone by definition surface UVB must increase.
I recall reading about industrial plants in China and other locations that were paid to destroy CFC stocks while other plants upped their production to satisfy this new market. A bureaucratic black market win-win. Has that been sorted yet?
Peta from Cumbria, now Newark June 6, 2017 at 1:57 am
And where were all our educated population when Montreal was put together?
Did *nobody* have the where-with-all or the guts to use their basic school chemistry education to say that chemical reactions DO NOT start and accelerate as temperatures drop?
Takes a bit more than basic school chemistry to understand that it required the formation of PSCs at -78ºC to create the substrate for the heterogeneously catalysed reaction to take place, followed by a photolytic reaction.
Phil,
You say that my “model for how the ozone hole is formed is wrong as pointed out above.” I don’t know exactly what you are referring to. I acknowledged that your description was essentially correct. Are you questioning the Brewer-Dobson circulation and the role of the circumpolar vortex in interrupting it?
I have not used a Dobson, but I know that they depend on a ratio of UVB to UVA, and they don’t measure absolute values. According to https://en.wikipedia.org/wiki/Dobson_ozone_spectrophotometer,
“The Dobson method has its drawbacks. It is strongly affected by aerosols and pollutants in the atmosphere, because they also absorb some of the light at the same wavelength. Measurements are made over a small area in the direction of the sun.” Therefore, they don’t work in total darkness, and unless the unit is positioned inside the “hole” it doesn’t measure properly. Something that isn’t addressed is how the sunlight coming in at low angles, outside the depleted air mass, is compensated for in the measurement technique.
I asked if you could cite measurements demonstrating an increase in surface UV (particularly in the Summer), and you referenced Dobson Spectrophotometers. That was not responsive to my question. An apparent increase in UVB could be observed if UVA decreased for some reason such as scattering outside the instrument site, overhead clouds, or a shift in the spectral composition of sunlight with the sun spot cycle.
So, besides the confounding effects of aerosols in measurements, recent work has found that water vapor apparently can accelerate photolytic losses.
You might find this link to be of interest: https://www.physicsforums.com/insights/software-never-perfect/ Might it be that the apparent change in the Antarctic ozone around 1980 is an artifact of a change in the way it was measured?
Clyde Spencer June 8, 2017 at 6:52 pm



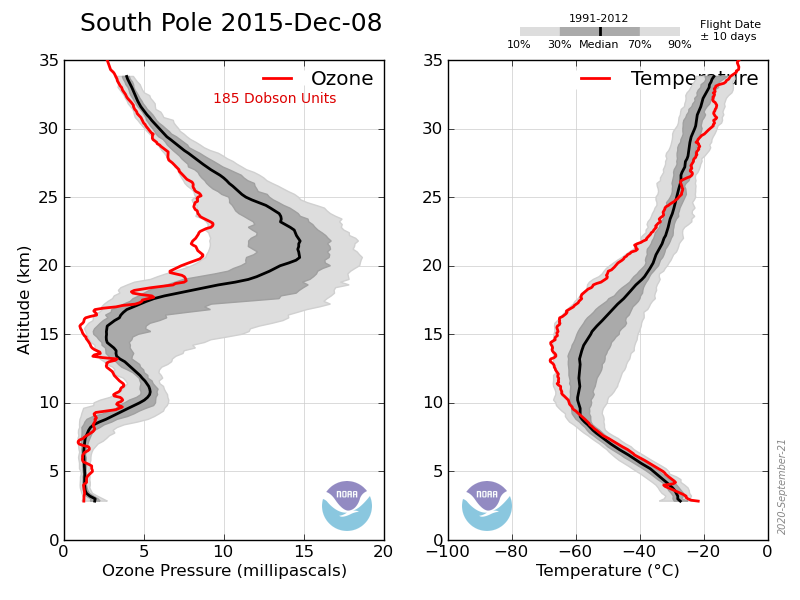

Phil,
You say that my “model for how the ozone hole is formed is wrong as pointed out above.” I don’t know exactly what you are referring to. I acknowledged that your description was essentially correct. Are you questioning the Brewer-Dobson circulation and the role of the circumpolar vortex in interrupting it?
Here’s the profile early in September:
Vortex seems to be forming well:
Note there is a robust ozone layer and the temperature between 10km and 25km is low enough to form PSCs.
Vortex seems to be forming well.
By early October the ozone layer between 13 and 20 km has gone and the temperature has risen and total ozone is down to 124 DU.
Vortex looking good:
By early December the Ozone layer is rebuilding as the temperature has risen and total ozone is up to 184 DU.
The vortex has weakened but not collapsed so the growth in ozone is not due to that.
Phil,
“The chemistry of the Antarctic polar vortex has created severe ozone depletion. The nitric acid in polar stratospheric clouds reacts with chlorofluorocarbons to form chlorine, which catalyzes the photochemical destruction of ozone.[39] Chlorine concentrations build up during the polar winter, and the consequent ozone destruction is greatest when the sunlight returns in spring… Since there is greater air exchange between the Arctic and the mid-latitudes, ozone depletion at the north pole is much less severe than at the south [pole].”
https://en.wikipedia.org/wiki/Polar_vortex
“Researchers face two crucial problems in ozone studies-finding a slow, long-term trend among a variety of short-term trends, and ascertaining how much of the change in global ozone is due to human activities and how much is attributable to natural atmospheric processes. In order to separate these factors, scientists must record data over at least a complete 11-year solar cycle.”
https://eospso.gsfc.nasa.gov/missions/total-ozone-mapping-spectrometer-earth-probe
I think that it would be more instructive to look at the TOMS ozone maps for the southern hemisphere, contrasting the high ozone values outside the polar vortex with the low values inside, instead of only looking at South Pole time series.
It appears to me that the Brewer-Dobson circulation goes a long ways in explaining the recovery of the depleted ozone in Antarctica.
Clyde Spencer June 9, 2017 at 7:12 am
Phil,
“The chemistry of the Antarctic polar vortex has created severe ozone depletion. The nitric acid in polar stratospheric clouds reacts with chlorofluorocarbons to form chlorine, which catalyzes the photochemical destruction of ozone.[39] Chlorine concentrations build up during the polar winter, and the consequent ozone destruction is greatest when the sunlight returns in spring…
Which is basically what I said.
I think that it would be more instructive to look at the TOMS ozone maps for the southern hemisphere, contrasting the high ozone values outside the polar vortex with the low values inside, instead of only looking at South Pole time series.
Which is exactly what I did, for some reason the png files of the relevant TOMS images don’t open (maybe you can find them by clicking on the little blue [?].
They were the ones for 1st Sept: https://ozonewatch.gsfc.nasa.gov/Scripts/big_image.php?date=2015-09-01&hem=S
6th Oct: https://ozonewatch.gsfc.nasa.gov/Scripts/big_image.php?date=2015-10-06&hem=S
8th Dec: https://ozonewatch.gsfc.nasa.gov/Scripts/big_image.php?date=2015-12-08&hem=S
And where were all our educated population when Montreal was put together?
Did *nobody* have the where-with-all or the guts to use their basic school chemistry education to say that chemical reactions DO NOT start and accelerate as temperatures drop?
Obviously not.
So, why did everybody wimp out, & pass the buck to the money grubbing ‘scientists’ employed by DuPont?
I say, because almost *everyone* is hitting them selves with a chemical cosh, 3 times per day and with endless snacks (all based around sugar) in between. Probably finishing their day with the ultimate legal chemical cosh, alcohol.
And so it takes someone who doesn’t drink to call this out for what it is. Here is someone with a clear head, self confidence, good memory, ability to speak without a teleprompter and all the things that are wrapped into the thing that is a ‘GSOH’
And since Ancell Keys. on his own personal preference, misused the scientific process to declare saturated fat as ‘bad’, what have we had if not an ever growing succession of panics – based upon 5hit science and spread/amplified by the very technology that is going to save us and propagated by politically correct people without the brains or guts to speak out.
Things like nuclear winters, silent springs, millennium bugs, mad cows, global cooling, avian flu, ebola, ozone, CO2 and what else?
But its *so* obvious, eating fat makes you fat and sugar gives you energy that you need. yeah, just like its obvious that CO2 traps heat and ozone stops the world being frazzled. Did no-one evn have the guts to ask where the fook the ozone came from?
Reduction in saturated fat consumption & the associated rise in sugar consumption. Look at the timescales.
Is that correlation or causation?
Is it one or the other that causes a tee-totaller to call BS on the current global scientific frauds – because everyone else is half asleep, morally gutless, entranced by the snake-oil salesmen, counting faeries and admiring the emperor’s new clothes.
Because of eating carbs (sugar)
The ozon hole is largely caused by emissions from the Mount Erebus volcano. It is one of the most active volcanoes on Earth and has been erupting continuomusly since 1981. And this has been known for decades
https://www.researchgate.net/publication/283802406_The_Antarctic_ozone_depletion_caused_by_Erebus_volcano_gas_emissions
http://onlinelibrary.wiley.com/doi/10.1029/93GL01879/abstract
Eric,
You reference an early Newsweek article that says “Rumors of blind sheep—the increased radiation was thought to cause cataracts— …”
Probably you know that Al Gore’s 1992 book “Earth in the Balance” has passages like these: “In Patagonia, hunters now report finding blind rabbits; fishermen catch blind salmon.” “We too are affected by extra UV radiation. The best-known consequences include skin cancer and cataracts, both of which are increasingly common, especially in areas of the Southern Hemisphere such as Australia, New Zealand, South Africa and Patagonia.”
I went looking for Australian data on cataracts of the eyes and found first “As in other developed countries, the most prevalent causes of blindness and vision loss in Australia are the age-related degenerative eye diseases, such as macular degeneration, glaucoma and cataract.” Ref: https://www.health.gov.au/internet/main/publishing.nsf/Content/D1A5409787D800F2CA257C73007F12F3/%24File/eyehlth.pdf
It is scientifically irresponsible to quote a change with time as if the stated variable was the only one. Gore implies UV is the main variable. The quote shows age is the main factor. Another possible confounding factor is the actual variation of UV over time, as intercepted by the eyes of the subjects. In this respect, in about 1988, I did a fair deal of research into natural UV abundance globally and locally. There was very little data in the public domain – a next to useless amount of data.
Unless Gore had access to unpublished data, he was simply making stuff up. There is no point to the development of his theory about UV and cataracts/blindness unless and until he can show a correlation between increases of both cataracts and UV and then account for the large effect of natural ageing.
However, so popular has his book been that we mere mortals will be abused for questioning its science.
Geoff.
Speaking of Al Gore…
http://www.crm114.com/algore/quiz.html
Time changed the meaning of a sun tan. The Victorian era looked down on a tan, it was a sign of a field worker. The 1960s changed this, sun exposure takes time. It just so happened to correlate with ozone dissociation due to ClO.
Chlorine monoxide does not prevent formation of ozone. It only catalyzes dissociation. Shifting the equation to the left.
This link might be of interest to readers: http://www.news-medical.net/news/20170606/Study-reveals-increased-risk-of-ozone-loss-over-the-central-United-States-during-summer-months.aspx
Note that after three decades the UV levels are still not being reported. Only focusing on ozone, and extrapolating possible implications. Some things don’t change.
I would recommend this The Holes In The Ozone Scare: The Scientific Evidence That The Sky Isn’t Falling By Rogelio A. Maduro;Ralf Schauerhammer. Available as a book on Amazon and, if you poke about, a free download as a pdf. A very comprehensive study of the ozone scam, and includes some interesting stuff by Dobson (DU = Dobson Units) among other things.
And while I’m here – Thank you David for some very useful information over the years. I use it in some presentations I give at the U3A in Cambridge. U.K. (Don’t know whether you have that organisation in the US). It’s swimming against the tide, but I live in hope.
MikeA
A promising site to poke about is http://b-ok.org/ (former bookz.com)
One of the talks at the NOAA Earth Sensing conference in Boulder
a couple of weeks ago , Stratospheric Ozone at South Pole Begins to Show Signs of Improvement in the Yearly Ozone Hole https://www.esrl.noaa.gov/gmd/annualconference/abs.php?refnum=112-170407-C , was far from convincing .
A related talk , The Continued Slowdown in the Decline of Atmospheric CFC-11 The Continued Slowdown in the Decline of Atmospheric CFC-11 , I felt raised questions whether there might be natural source for some of these compounds . They are talking parts per trillion on a lot of these measurements .
In any case , the banning of Primatene asthma inhalers for the couple of grams of CFC propellant is an obscene direct and in some cases almost surely deadly attack on human health .
I seem to remember reading that the space shuttle Columbia tragedy was a result of the CFC paranoia. A chunk of the foam, which covered the external fuel tank, came loose during liftoff and damaged the shuttle, which then burnt up during re-entry as a result of the damage. Seems that the foam manufacturing / application process was changed due to CFC “concerns”, and the resultant product did not adhere nearly as well. Perhaps someone with better knowledge of this could elaborate.
Some of the foam used on the shuttle was made using Freon free processes, however the piece that broke off was BX-250 foam which was still made using Freon so the change did not contribute to the accident.