Readers may recall claims of 1000 year residence times for CO2. This essay suggests a much shorter interval. -Anthony
Guest essay by Leo Goldstein
Surplus CO2 is removed from the atmosphere by natural sinks at a rate proportional to the surplus CO2 concentration. The half-life of the surplus CO2 concentration is approximately 40 years. This is the conclusion of my research paper, published on defyccc.com today.
I am grateful to Prof. Fred Singer and Prof. William Happer for their help in writing this paper.
The correct (although approximate) formula for CO2 concentration leads to a number of conclusions of public interest:
- CO2 concentration in the atmosphere will increase much slower than has been claimed by the IPCC.
- A relatively small part of the anthropogenic CO2 in the atmosphere has been released by the US; a relatively large part of the anthropogenic CO2 has been released by China.
- If stabilizing or decreasing atmospheric CO2 content becomes desirable at some point in the future, that can be achieved by decreasing anthropogenic CO2 release at that time; no premature action is needed.
- The warming effect of anthropogenic CO2 is less than the warming effect of other gases and aerosols (according to IPCC calculations) in both the short and long term, so what are the motives behind this laser focus on CO2?
The topic of the CO2 removal rate has been discussed a number of times on WUWT (by Christopher Monckton of Brenchley, Docmartyn in comments on Dr. Lindzen’s article, Anthony Watts and others), and various opinions were expressed. Estimates of the half-life varied.
For some time, the subject was surrounded by confusion, created by sloppy definitions and evasive statements in IPCC assessment reports. There was a mix-up between the residence time of a CO2 molecule in the atmosphere and the rate of change of the surplus CO2 concentration. The residence time (~5 years) is of little interest, except as an indication of quick carbon turnaround. The true subject of interest is the rate of change of the surplus carbon concentration in the atmosphere. Another issue was the link between CO2 concentration and temperature. On the geological timescale, the rise in CO2 concentration tends to follow the temperature rise, concurring with a hypothesis that the latter causes the former. Nevertheless, such an effect is not significant on the multi-decadal scale. CO2 concentration in the atmosphere grows mostly because of anthropogenic release of CO2 through fossil fuels combustion and land use changes.
The paper’s full title is Simple Equation of Multi-Decadal Atmospheric Carbon Concentration Change. It is article-length (~5,000 words, not counting references), citable, and discoverable by search engines, including the Climate Sanity and Freedom Search. In a slight departure from a widely-used academic format, the paper contains a Summary (for busy readers). The abstract is as follows.
Surplus CO2 is removed from the atmosphere by natural sinks at rate, proportional to the surplus CO2 concentration. In other words, it undergoes exponential decay with a single decay constant. This conclusion is rigorously proven, using first principles and relatively recent observations of oceans. Historical data for CO2 concentrations and emissions from 1958–2013 are then used to calculate the half-life of the surplus concentration. This theoretically derived formula is found to be an excellent match to the historical CO2 concentrations over the measurement period. Furthermore, the “initial” CO2 concentration in the formula came out to be very close to the likely “pre-industrial” CO2 concentration. Based on the used datasets, the half-life of the surplus concentration of CO2 in the atmosphere is found to be approximately 40 years.
Discover more from Watts Up With That?
Subscribe to get the latest posts sent to your email.

If you work the numbers on IPCC AR5 Figure 6.1 you will discover that anthro C is partitioned 57/43 between natural sequestration and atmospheric retention. (555 – 240 = 315 PgC & 240/555) IMO this arbitrary partition was “assumed” in order to “prove” (i.e. make the numbers work) that anthro C was solely/90% responsible for the 112 ppmv atmos CO2 increase between 1750 – 2011. C is not CO2.
PgC * 3.67 = PgCO2 * 0.1291 = ppmv atmospheric CO2
IPCC AR5 Figure 6.1
…………………………………….PgC/y……ppmv/y
FF & Land Use Source…….8.9……….4.22
Ocean & Land Sink…………4.9……… 2.32
Net Source.……………….…..4.0……….1.90
If the anthro 8.9 Pg C/y (4.2 ppmv CO2/y) suddenly vanishes the natural cycle that remains would be a constant sink of 2.3 ppmv CO2/y. Reverse extrapolation (GCMs & RCPs apply forward extrapolation) calculates that 121 years in the past (278 ppmv CO2/2.3 ppmv CO2) or the year 1629 (1750-121) atmos CO2 would have been 0, zero, nadda, zip, nowhere to be found.
Oh, what a tangled web they wove!
The 8.9 Pg of anthro C simply vanishes in earth’s 45,000 plus Pg C cauldron of stores and fluxes. Mankind’s egoistic, egocentric, conceit means less than nothing to the earth, the solar system and the universe.
Slam-dunk, Nicholas Schroeder!
Schroeder 1
IPCC 0 (nada, zip!)
(times in brackets from below-posted video)
[36:34] Native Source of CO2 – 150 (96%) gigatons/yr — Human CO2 – 5 (4%) gtons/yr
(i.e., native = 2 orders of magnitude greater than human)
[37:01] Native Sinks Approximately* Balance Native Sources – net CO2
*Approximately = even a small imbalance can overwhelm any human CO2.
Source: Dr. Murry Salby, Hamburg, April, 2013
(youtube)
I’ve seen you post this 8 times now in various places……and I still agree with it..weird !!!
lol, Ha! So… you have not always been called “Marcus,” then, have you? Unless you were silent all those 2 years… . (smile) 8 times, eh? Well, what do you know…. Your memory is phenomenal!
Don’t worry, “Marcus,” I do not know who you are. There is someone I WISH you were, you sound like him to me, sometimes…… but, I will never say that name, in case remaining incognito is vitally important.
Janice, you could post that lecture once a week and I will still watch it; pure entertainment in the best possible way.
Salby’s employment at Macquarie was terminated in 2013; his return ticket from Paris was cancelled by Macquarie, stranding Salby in Europe. Macquarie University stated that he was not dismissed because of his views on climate change… Wikipedia no less
Love that quote of yours, Robert (of Ottawa)! lol — did ANYONE believe MacQ? Poseurs claimed to, but, for crying out loud, what BAD l1ars they were (and no doubt, are)! lololol (and then, remembering a very weary, betrayed, professor, I am no longer laughing….. what Dr. Salby endured–was–horrible)
Very interesting presentation by Prof. Murry Salby, thanks for the post! He says that CO2 emissions are due to the INTEGRAL of temperature and not to temperature itself. But I wonder how could CO2 levels ever sink then? The integral needs a dissipation factor, otherwise even at constant temperature, CO2 levels would continue to rise, indefinitely. Did I miss something? Maybe I have to watch it again…
StephanF:
are observed as being proportional to text
does NOT mean
are due to
Now you know that, it may benefit you to watch Salby’s lecture again.
Richard
Janice,
Repeating the same errors from Dr. Salby doesn’t make them true… He indeed integrates temperature (as Bartemus does) and then declares that all CO2 increase is from temperature. But by doing that he integrates both the variability and the slope. It can be proven that most of the variability is indeed the short time influence of temperature on CO2 releases from (tropical) vegetation, but vegetation is a proven sink for CO2 over periods longer than 3 years. Thus variability and increase rate are NOT from the same process. As both the biosphere and oceans are proven sinks for CO2, there is nothing important natural source left, only human emissions…
Stefan, the thinking is that eventually the ocean will simply run out of co2… (to put it more precisely, the ocean will eventually reach a new temperature equilibrium if SSTs stay flat). It’s the relative imbalance of temperature from it’s equilibrium state of hundreds of years ago that is causing the rise in co2:
http://www.woodfortrees.org/plot/esrl-co2/from:1958/mean:24/derivative/plot/hadsst3sh/from:1850/scale:0.22/offset:0.097
ferdinand, nice to “see” you… i really don’t like your deductive line of reasoning at times. I’ll give you an analogy. Say a man argues that life cannot exist without intelligent design. A “complex” life form (which even the simplest of life forms is) cannot begin to evolve until it is up and running. The chances of this life form happening by chance then is nil. (the analogy here is that radio signals from the far reaches of the universe will never randomly produce a signal that says “i want pizza”) So the man deduces that an intelligent designer (or God) created life. But, then he runs into the the same problem with the “complex” intelligent designer. Who created God? As a result of his deductive reasoning, he then comes to the only logical conclusion: life (or for that matter God) doesn’t exist…
StephanF,
You posed the right question. If Salby and Bartemis were right, a small temperature offset from a starting point at equilibrium would increase CO2 levels until infinity. In reality it is a transient response of CO2 from the oceans per Henry’s law: at about 16 ppmv/°C a new steady state is reached and CO2 doesn’t increase further:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/upwelling_temp.jpg
Temperature is responsible for almost all variability in the rate of change of CO2, but is only responsible for about 10 ppmv increase since the LIA. The 100 ppmv extra is from human emissions (at near 200 ppmv in the same period).
Ferdinand, I find you far too affirmative.
“variability and increase rate are NOT from the same process”. this is either nonsense or tautology.
Tautology if you mean that many processes are involved in carbon cycle. Each having its characteristic time (minutes, day, season, years and more) explains a part of variability and a (different) part of increase rate.
Nonsense if these processes are considered as a whole. Then obviously the whole process explain both variability and increase rate if it is to explain anything of the carbon cycle.
“As both the biosphere and oceans are proven sinks for CO2, there is nothing important natural source left, only human emissions”. I guess you mean “NOW, in current observed conditions”, because otherwise, if this were always true, CO2 level would had been zero before humans emissions began. Biosphere and ocean are sinks not per se, but dependent on conditions.
Besides, being a net sink do not mean you don’t contribute to an increase. Even being a stronger sink than before do not mean you don’t contribute to an increase (if, for instance, the growing sink replace an other more efficient sink, or if it stimulates a source). And kinetics can make things even more funny.
What would had been the increase without human emissions ? zero ? less than zero ? more than zero ? I am pretty sure we don’t know enough of carbon cycle to answer.
Richard, afonzarelli & Ferdinand, thanks for your input. This morning I thought this once more through, if Salby’s theory is correct, then we would have seen a remarkable increase of CO2 during other past warming events, but we haven’t (correct?). As we always comment on the warmist’s models that they can’t even ‘predict’ the past, we have to use the same standards here, too. I have to look for Salby’s research on the Internet, maybe he has applied his mathematical models to the past? My expertise lies in other areas (spectroscopy, atomic & plasma physics) and not in atmospheric physics, so I don’t know much of the scientific literature here. If the current CO2 emissions were mostly from natural sources, why did we not see this fast rise in the past? These were questions that bugged me when I listened to Salby’s presentation.
StephanF November 24, 2015 at 11:01 pm
“But I wonder how could CO2 levels ever sink then? The integral needs a dissipation factor, otherwise even at constant temperature, CO2 levels would continue to rise, indefinitely.”
But, that dissipation factor, or other feedback, can be small enough that it is essentially unobservable over the timeline of observation.
E.g., we know that, for the past 57 years, the data are very well described by
dCO2/dt = k*(T – T0)
where T0 is an equilibrium temperature, and k is a coupling factor. This is undoubtedly a linearized model of a more complicated, nonlinear relationship. There could be another term, e.g.,
dCO2/dt = k*(T – T0) – CO2/tau
where tau is long enough that CO2/tau is very small, and its effects unobservable over the interval of observation, yet this term would ultimately limit the level of CO2 attained.
Or, it could be that T0 itself is a function of the CO2 available from upwelling waters, and will naturally rise towards T as that store becomes depleted.
Or, there could be a more complicated linearized relationship, e.g.,
dT/dt = -a*T – b*CO2
dCO2/dt = k*(T – T0)
for positive constants a, and b. where temperatures actually decline due to an increase in CO2. This system is stable, also, and has a limit on how high CO2 can go.
None of this matters. We do not need to worry about ultimate limiting factors. We only need to know that, within the interval of observation, atmospheric CO2 is described to very high fidelity by
dCO2/dt = k*(T – T0)
It explains the entire observation interval. And, it leaves little room for human forcing to have a significant impact.
StephaF November 25, 2015 at 8:01 am
“…if Salby’s theory is correct, then we would have seen a remarkable increase of CO2 during other past warming events, but we haven’t (correct?).”
Maybe. Maybe not. This is not a stationary system. It can easily have one response at one time, and another at another.
I would also say we do not genuinely know the past. We definitely do not have the resolution needed to see short term spikes. Ferdinand can make a very detailed case claiming that the ice core records are definitive. However, the records cannot be confirmed for the long ago past by any independent means, and so remain speculative.
It is futile to speculate on things that cannot be known, but that should not turn one from accepting the evidence before one’s eyes. Astronomers of the 1500’s had no inkling of how the planets could move in ellipses about the Sun. Galileo saw that they did. Whatever theoretical objections the others might have had, his observations trumped them. The data are primary, theorizing secondary, when attempting to grasp reality. And, the data tell us that
dCO2/dt = k*(T – T0)
for the past 57 years. As this is the era of most of the rise observed in atmospheric CO2, it establishes that the rise is not primarily anthropogenic. You have to start there, and then come up with theories to explain it. Otherwise, you risk the pitfall described by one famous detective as follows:
paqyfelyc,
The opposite CO2 changes and 13C/12C ratio changes are uniquely caused by the biosphere. If that was from the oceans, the changes would parallel each other.
On the other side: the oxygen balance shows that the biosphere is a net sink for periods longer than 3 years.
Thus I may be pretty sure that variability and increase in the atmosphere are not caused by the same process. If the CO2 increase is temperature related or not is a different question…
Of course, that all is for the past 165 years or so, especially for the past 57 years of accurate measurements.
Per Henry’s law, the CO2 increase in the atmosphere due to increased (ocean) temperatures would be around 10 ppmv higher, the rest is from human emissions (~200 ppmv emissions, 110 ppmv increase…).
StephanF,
The past 800,000 years show a rather steady 16 ppmv/°C ratio between CO2 and temperature, where CO2 lags temperature with 800 +/- 600 years during a warming episode and several thousands of years during the onset of a new glacial period. A similar change can be seen between MWP and LIA.
The current increase is 120 ppmv above pre-industrial, of which some 10 ppmv from warming oceans. The rest is from human emissions (near 200 ppmv since 1850).
Bart’s formula doesn’t take into account the increase of CO2 in the atmosphere which counters the effect of higher temperatures: it is a transient process which ends at 16 ppmv/°C. It is impossible that the CO2 release remains constant for a fixed temperature step without influence of the increased CO2 pressure in the atmosphere. See my new contribution at WUWT about spurious correlations…
Well, I don’t agree with it.
Well, yeah, I agree that we only emit ~3% of CO2. But the other 97% circulates back and forth (Atmosphere to/from soils, etc., and Atmosphere to/from Oceans.
So that 3% we add (plus anything from vulcanism) is added from the outside, and accumulates. The rest just goes back and forth and does not accumulate.
Of the ~8 BMTC that we add to the atmosphere, about a bit under half winds up in the atmospheric sink (780 BMTC). Therefore, we add a bit under half a percent to the atmosphere per year.
Now, I don’t think this is any threat. In fact, I think the additional CO2 and the accompanying mild lukewarming we have seen thus far are hugely beneficial. And Furthermore, I think the demographics and the tech are on track for leaving CO2 rise in the dust within half a century, and if that’s so, we will peak out at maximum environmental and human benefit.
But to claim our CO2 input has not caused a significant accumulation in our atmosphere is just plain wrong, so far as I can tell.
[i]afonzarelli November 25, 2015 at 12:33 am
ferdinand, nice to “see” you… i really don’t like your deductive line of reasoning at times. I’ll give you an analogy. Say a man argues that life cannot exist without intelligent design. A “complex” life form (which even the simplest of life forms is) cannot begin to evolve until it is up and running. The chances of this life form happening by chance then is nil. (the analogy here is that radio signals from the far reaches of the universe will never randomly produce a signal that says “i want pizza”) So the man deduces that an intelligent designer (or God) created life. But, then he runs into the the same problem with the “complex” intelligent designer. Who created God? As a result of his deductive reasoning, he then comes to the only logical conclusion: life (or for that matter God) doesn’t exist…[/i]
Afonzarelli, it is obvious you don’t understand intelligent design arguments. They are based on exactly the same historical scientific methods used by Charles Lyell and Charles Darwin, inference to the best explanation, or the method of multiple competing hypotheses. Since we never observe highly complex, functional information systems (such as cells) arising from unguided natural processes, and in fact all such systems in our experience arise from intelligence, then the design inference is the strongest hypothesis.
No amount of rhetorical arm-waving about who or what caused the intelligence in the first place can get around the fact that we can and do use the inference to intelligent design all the time in forensic science, code-breaking, SETI, etc.
By the way, though ID does not attempt to identify the designer — the evidence does not reach that far — it is by no means true that theologians are stumped by the “Who created God?” conundrum. God is, by definition, eternal. Hence, he has no beginning and was never created.
Or to put it another way, the Kalam argument is convincing to me: Everything that begins to exist has a cause. God never began to exist. Hence, God has no cause.
atmos CO2 would have been 0, zero, nadda, zip, nowhere to be found.
And it wasn’t found then was it? Quad Errat Demonstrandum.
Seriously, we only “know” some of these things in the past from major extrapolations and assumptions. The most reliable knowledge of climate for the past three thousand years is the historical and archaeological record and from that we know of several periods warmer than today, indicating that natural variation is the order of the day and there is nothing abnormal about current conditions.
Ice Core Proxies … pretty reliable data….
Ice cores, cylinders of ice drilled out of glaciers and polar ice sheets, have played an important role in revealing what we know so far about the history of climate. Today, United States scientists are embarking on a new ice coring project in Greenland with a wide range of state of the art analyses in the hopes of resolving questions about how the climate system functions. Drilling for The Greenland Ice Sheet Project Two* (GISP2) began in 1989. When they reach the bottom of the ice sheet, 3000+ meters thick, in 1992 they will have recovered the longest, most detailed, continuous record of climate available from the northern hemisphere stretching back 200,000 years or more through two glacial/interglacial (cold/warm) cycles.
From this page of the Greenland Ice Sheet Project: http://www.gisp2.sr.unh.edu/MoreInfo/Ice_Cores_Past.html
Janice Moore:
Ice core records ar “pretty reliable data” of what?
Air obtained from glacier ice is not comparable to air collected in a sample bottle.
Richard
Awhile back it became useless to argue about events too far back with the IPCC. I just concentrate on events that are closer and have historical data associated with them. In this case the LIA and the MWP.
From what I’ve looked at, the sinks are increasing at a rate faster than the production. If the sinks keep icreasing, then the co2 rate ppm will become zero for man made co2. 1998 was the highest level of co2 ppm. Everything since then has been less in terms of increase ppm, and co2 production increased year over year, till recently 1 bmt has been added additionally each year for the last 6 or 7 years. I wonder if they will report a negative or a net loss in co2. Even 40 years is too long for the half life. However, I’m not going to rule out the amounts could be proportional.
I’ve thought that if we stopped producing co2, that the huge sink that now exist, the amount of decrease would be ^2 of the increase. How did I come up with ^2? A little bird told me.. no, .. currently at least half of man made co2 is being sunk, additionally natural occurring co2 is also being sunk. Since there is no new additional co2, and a growing sink to accommodate the additional co2 that would be expected, then a ^2 is not unreasonable.
Based on historical data, the real rate of increase ppm should be 7 to 9 ppm/year.
Hi, Richard Courtney,
Here is what Dr. Salby says about the damping part of the conservation equation v. a v. ice core proxies (and other remarks) in his above-linked Hamburg, 2013 lecture:
[14:40] CO2 levels in ice change over time (due to natural modification and to measurement error) – Conservation Equation (includes non-conservative factor, i.e., CO2 sinks).
[15:56] – illustrated by biomass [17:05] – The Conservation Equation includes the total or “effective” damping [23:30] from atmospheric damping (i.e., non-conservative influences) of CO2 in the firn (when ice at top) and damping in the ice as it descends.
[25:40] Changes in atmospheric CO2 are underestimated in the proxy record (and this underestimation increases radically over time [see graph at 26:11], i.e., the change in the atmosphere is much greater than the apparent change of CO2 in the ice.
[27:01] Over time 10,000 years, the ice proxy underestimates atmospheric CO2 by a factor of 2; over 100,000 years, under by factor of 15 [27:29].
[27:52] Observed changes in the 20th century are certainly not unprecedented.
**********************************
That is, to answer your question, by taking into account the atmospheric damping of CO2 in the ice over time, the ice core proxy data is considered a reliable “observation.”
Take care,
Janice
http://www.tyndall.ac.uk/global-carbon-budget-2010
There are a lot of natural sources and sinks, mostly changing back-and-forth between source and sink with season, weather, or time of day. But the human contribution is fairly well known, and the amount gained by the atmosphere is very well known. Over each year since the Mauna Loa project started, atmospheric CO2 increased by less than human contributions, which means that nature has been removing CO2. I think the main natural sink of CO2 is the oceans, sinking CO2 over most of their area due to atmospheric CO2 having a concentration higher than being in equilibrium with the dissolved CO2 in ocean surface-level waters in most of the world’s area covered by oceans.
” IMO this arbitrary partition was “assumed” in order to “prove” (i.e. make the numbers work)”
It isn’t assumed; it is observed, and very simply. Here is a plot (from here) of observed Mass of C in the atmosphere (from ppm CO2) vs cumulative emissions (FF and land use). It’s remarkably linear, and the slope is .439 – just that “arbitrary partition”.
http://www.moyhu.org.s3.amazonaws.com/misc/ghg/m2.png
Nicholas Schroeder,
You do make an essential error. As Ari Halperin said: “by natural sinks at a rate proportional to the surplus CO2 concentration”. If all human emissions cease, the residual sink rate is NOT constant, but decreases with the difference between the CO2 level in the atmosphere and the equilibrium pressure at “steady state” for the current weighted average ocean temperature, which is ~290 ppmv. Thus if the CO2 level in the atmosphere drops to 290 ppmv, the sink rate is about zero…
Whilst I consider that there is merit in the general thrust of the point you are making, I do not consider it as simple as you suggest since neither the sources nor the sinks remain constant over time. In particular, we now know that greening of the planet is one response to the increasing levels of CO2 and this would appear, at least on a short time scale, to result in an increase in the available sinks.
Regretfully we do not have sufficient knowledge and data to properly evaluate and understand all the sources and all the sinks, including their capacity, and without full knowledge and understanding, we cannot get to the bottom of the Carbon Cycle. There is presently simply too much uncertainty in the underlying data, and it may well be that we have still to discover all CO2 sources and sinks. Hopefully, OCO-2 will lead us to some better insight and understanding of the processes involved and how CO2 is exchanged.
The above voiced opinion is absolutely correct.
But the proponents of CAGW will deny that factual accusation because they have undeniable positive provable evidence of this, to wit:
Surprise, surprise, …… the CAGW’ers can accurately measure the quantity of human emitted CO2 that resides in the atmosphere, to wit:
“The CAGW secret they don’t want you to know.
There is a nasty ole Anthropogenic Global Warming secret about CO2 that the proponents of CAGW are not telling you. So, surprise, surprise, there are actually two (2) different types of CO2.
There is both a naturally occurring CO2 molecule and a hybrid CO2 molecule that has a different physical property. The new hybrid CO2 molecule contains an H-pyron which permits the CAGW’ers to distinguish it from the naturally occurring CO2 molecules.
The H-pyron or Human-pyron is only attached to and/or can only be detected in CO2 molecules that have been created as a result of human activity. Said H-pyron has a Specific Heat Capacity of one (1) GWC or 1 Global Warming Calorie that is equal to 69 x 10 -37th kJ/kg K or something close to that or maybe farther away.
Thus, said H-pyron is very important to all Climate Scientists that are proponents of CO2 causing Anthropogenic Global Warming (CAGW) because it provides them a quasi-scientific “fact” that serves two (2) important functions: 1) it permits said climate scientists to calculate an estimated percentage of atmospheric CO2 that is “human caused” ……. and 2) it permits said climate scientists to calculate their desired “degree increase” in Average Global Temperatures that are directly attributed to human activity.
As an added note, oftentimes one may hear said climate scientists refer to those two (2) types of CO2 as “urban CO2” and ”rural CO2” because they can’t deny “it is always hotter in the city”.
And there you have it folks, the rest of the story, their secret scientific tool has been revealed to you.
Yours truly, Eritas Fubar”
Nicholas Schroeder wrote, “IPCC AR5 Figure 6.1 [indicates that] anthro C is partitioned… between [57%] natural sequestration and [43%] atmospheric retention.”
Other sources use somewhat different numbers. E.g., this presentation by Louisa Bradtmiller says that 55% (rather than 43%) of emitted CO2 remains in the atmosphere. She thinks that 30% goes into the oceans, and 15% goes to “greening” the planet (taken up by the plants):
http://slideplayer.com/slide/6027359/
My intuition says that all the numbers except the amount which remains in the atmosphere are very rough estimates, so nobody really knows whether the ratio is 60:40, 40:60, or 50:50. So I generally just say “about half” of the CO2 is sequestered by the oceans and biosphere, and the other half remains in the atmosphere.
The rate of uptake by the ocean & biosphere is mainly a function of the atmospheric CO2 level (not the rate of emissions), so if anthropogenic CO2 emissions suddenly went to zero then the rate of uptake by the oceans and biosphere wouldn’t instantly change: it would continue at the current rate, so the CO2 level in the atmosphere would immediately begin falling at a rate of about 2 ppmv/year (rather than rising at about that rate, as it currently is).
That rate of decline wouldn’t last, however. The CO2 uptake accounted for by “greening” goes into carbon stores such as wood and leaves which don’t retain it for very long: a few years, perhaps, on average. So, in the slightly longer term, as “greening” ceases, plant growth rates slow, and “browning” begins, most of the “excess” carbon sequestered in the biosphere would be released. That means the rate of CO2 level decline would decelerate, to perhaps 2/3 of that estimated 2 ppmv/year rate (if Bradtmiller’s 30:15 ocean:biosphere ratio is right). (The precise rates would also depend substantially on ocean temperature trends.)
It took about seventy years to add ~100 ppmv CO2 to the atmosphere. If anthropogenic CO2 emissions were to completely cease, and levels were to drop at 2/3 of the rates at which they increased over the last seventy years, then it would take about a century for atmospheric CO2 to approach the 300 ppmv level again.
Unfortunately, declines in agricultural yields would begin long before that. Mankind would miss the precious air fertilizer, a lot.
When fossil fuels run out (or we switch to something better), will our descendants express relief that the CO2 levels are decreasing, a cooler climate may be expected, and sea level rise may reverse; or will they be concerned about decreasing crop yields and colder winters? The latter seems more likely to me.
FYI, I emailed Dr. Bradtmiller, and asked her about the difference between her numbers (55% remains in atmosphere, 30% in ocean, 15% biosphere/greening), and the IPCC’s AR5 numbers (45% or 47% remains in atmosphere, 26% in ocean, 29% or 27% biosphere/greening). This was her reply:
“I was very surprised to see that presentation online since I didn’t upload it (or give permission), and don’t know the person who did- I wonder how they got it. It is a presentation I made as a student while studying with my friends for comprehensive exams. Anyway, most importantly for your question, it is very old (10+ years)! So, the difference between my values and the IPCC values can likely be chalked up mostly to out of date information. The differences between the two different sets of IPCC values you cite is probably due to the fact that they cover slightly different time periods. However, it is worth noting that those sets of numbers are effectively the same, since they are within the stated margins of error of each other. As you can see, it is much easier to measure the carbon accumulating in the atmosphere than in land or the ocean; this is reflected in the fact that the uncertainty on the atmospheric accumulation is very low as a percent of the total change (10/240=.041, or 4.1%), while the uncertainties on the ocean (30%) and land (46%) are much larger.”
StephanF November 24, 2015 at 11:01 pm
“But I wonder how could CO2 levels ever sink then? The integral needs a dissipation factor, otherwise even at constant temperature, CO2 levels would continue to rise, indefinitely.”
But, that dissipation factor, or other feedback, can be small enough that it is essentially unobservable over the timeline of observation.
E.g., we know that, for the past 57 years, the data are very well described by
dCO2/dt = k*(T – T0)
where T0 is an equilibrium temperature, and k is a coupling factor. This is undoubtedly a linearized model of a more complicated, nonlinear relationship. There could be another term, e.g.,
dCO2/dt = k*(T – T0) – CO2/tau
where tau is long enough that CO2/tau is very small, and its effects unobservable over the interval of observation, yet this term would ultimately limit the level of CO2 attained.
Or, it could be that T0 itself is a function of the CO2 available from upwelling waters, and will naturally rise towards T as that store becomes depleted.
Or, there could be a more complicated linearized relationship, e.g.,
dT/dt = -a*T – b*CO2
dCO2/dt = k*(T – T0)
for positive constants a, and b. where temperatures actually decline due to an increase in CO2. This system is stable, also, and has a limit on how high CO2 can go.
None of this matters. We do not need to worry about ultimate limiting factors. We only need to know that, within the interval of observation, atmospheric CO2 is described to very high fidelity by
dCO2/dt = k*(T – T0)
It explains the entire observation interval. And, it leaves little room for human forcing to have a significant impact.
I love it! So, actually, all of our ancestors starved in the 17th century, and none of us are here at all…
This is the AR5 figure 6.1 to which you’re referring, right, Nicholas?
http://www.climatechange2013.org/images/figures/WGI_AR5_Fig6-1_errata.jpg
This is their caption for Fig. 6.1:
Figure 6.1: Simplified schematic of the global carbon cycle. Numbers represent reservoir sizes (in PgC), and carbon exchange fluxes (in PgC yr–1 4 ). Dotted arrow lines denote carbon fluxes between the fast and the slow carbon cycle domain (see text). Darkblue numbers and arrows indicate reservoir sizes and natural exchange fluxes estimated for the time prior to the Industrial Era. Red arrows and numbers indicate fluxes averaged over 2000–2009 time period resulting from the emissions of CO2 from fossil fuel combustion, cement production , and changes in land use, and their partitioning among atmosphere, ocean and terrestrial reservoirs (see Section 6.3). Red numbers in the reservoirs denote cumulative changes over the Industrial Period 1750–2011.
(From page 6-8 here: http://www.climatechange2013.org/images/report/WG1AR5_SOD_Ch06_All_Final.pdf )
Nicholas Schroeder wrote, “IPCC AR5 Figure 6.1 [indicates that] anthro C is partitioned… between [57%] natural sequestration and [43%] atmospheric retention. …
…………………………………….PgC/y……ppmv/y
FF & Land Use Source…….8.9……….4.22
Ocean & Land Sink…………4.9……… 2.32
Net Source.……………….…..4.0……….1.90
“
A nit: 4.9/8.9 = 55% (not 57%), and 4.0/8.9 = 45% (not 43%).
On p. 6-3 AR5 gives somewhat different numbers:
“During 2002–2011, atmospheric CO2 concentration increased at a rate of 2.0 ± 0.1 ppm yr–1 (equivalent to 4.3 ± 0.2 PgC yr–1 54 ); the ocean and the natural terrestrial ecosystems also increased at a rate of 2.4 ± 0.7 PgC yr–1 and 2.5 ± 1.3 PgC yr–1 55, respectively.”
That would work out to:
4.3 / (4.3+2.4+2.5) = 47% remained in the atmosphere
2.4 / (4.3+2.4+2.5) = 26% went into the ocean
2.5 / (4.3+2.4+2.5) = 27% went into the biosphere
Right.
A relatively small part of the anthropogenic CO2 in the atmosphere has been released by the US; a relatively large part of the anthropogenic CO2 has been released by China.
That won’t please the developing countries that are trying to get the West to cough up for their evil ways.
Actually CO2 and H2O are both PERMANENT components of the atmosphere, so if one molecule goes it gets replaced by another; so there is always both CO2 and H2O in the atmosphere, and always more H2O than CO2.
And, fortunately, way more free oxygen than CO2.
@g.smith:
Nothing is /permanent/ …
Earth’s entire atmosphere could easily be stripped away by the solar wind if not for the its internal magnetic field generating dynamo.
Life on Earth /has/ changed the atmosphere.
The claims of the CAGW camp /were/ not entirely unfounded. However, the science done in the last 30 or so years shows that the alarmist position is likely well over played.
I, for one, am very grateful for that… that CAGW does not appear to be a correct hypothesis or assumption — depending on your view of the testability of the individual variables.
unknown…
permanent doesn’t mean eternal; more like “abiding”
(a permanent job doesn’t necessarily last till you die)
OK. There was no CO2 in the atmosphere before the big bang.
So I’ll limit my time scale to the period since Lucy’s ancestor climbed down out of the fig tree and first tasted grass fire cooked meat.
The only thing that is relevant to next month’s Paris decisions, is a planet with the current orbit and sun and the continental land masses about where they currently are.
Anything that might have changed in Geologic time is of no interest next month.
g
@g:
>Anything that might have changed in Geologic time is of no interest next month.
Actually this is precisely the topic. The present day versus geologic time, and the differences, if any.
please read tis classic paper by Peter Dietze
http://www.john-daly.com/carbon.htm
Thank you for sharing that EXCELLENT paper, Hans Erren!
(Source, above-linked article, “Little Warming with New Global Carbon Cycle Model” by Peter Dietze (1997))
The linked paper supports the quoted statement with well-reasoned, solidly data-backed, argument.
Worth reading.
**********************
About Peter Dietze: “Peter Dietze studied electrical and control engineering. Professional work was in software development for power system control. Special interest in natural sciences, energy and energy politics led to over ten years of intensive private (non-sponsored) work in global warming science with focus on carbon models. Results were published in a dozen of articles and several presentations.”
BUT…He’s not a ” Climate Scientist ” so he must be paid by Big Oil, just ask any liberal !!! sarc !!
Amazing
“CO2 concentration in the atmosphere grows mostly because of anthropogenic release of CO2 through fossil fuels combustion and land use changes.”
Actually, no. CO2 concentration in the atmosphere grows because of natural influx exceeding natural outflux, which is temperature driven.
http://i1136.photobucket.com/albums/n488/Bartemis/temp-CO2_zpsnp6z3jnq.jpg
Human inputs are essentially superfluous to this relationship, having negligible impact.
Yes! And Dr. Murry Salby backs you up on this: http://wattsupwiththat.com/2015/11/24/co2-residence-time-said-to-be-40-years-not-1000-as-noaa-claims/comment-page-1/#comment-2079112
I hope that is you, O Truth in Science Warrior, “Bart!”
#(:))
It would be good to know that you are still here to fight the Ferdinand (et. al.) with your resoundingly unanswerable arguments! (yes, F. is a nice fellow)
Here’s a more detailed analysis from Salby’s recent talk in London.
http://antigreen.blogspot.com.au/2015/04/new-video-from-prof.html
Same message follows from a more detailed analysis in Salby’s recent talk in London.
http://antigreen.blogspot.com.au/2015/04/new-video-from-prof.html
Oh, Janice Moore, I thought it was Dennis Moore
These plots are very complicated, Ferdinand, and too little information is given to understand exactly what you have done.
I am wary that, with the different time constants, you are applying sink activity differently for anthropogenic and natural CO2. These inputs must be treated exactly the same in terms of their removal from the atmosphere.
Give your exact equations, not just graphs.
Also, you should show the match all the way back to 1958, using the SH data.
Unfortunately, you have once again caught me at a time when I must travel. It is the Thanksgiving holiday weekend here in the US. I will be gone until Sunday. Will try to check in later, but can give no guarantees.
Bartemis,
I used no different sink rate for natural sinks, only a different transient response time of CO2 from the biosphere and oceans to temperature changes.
Any extra CO2 above the (temperature controlled) steady state level is threated the same way.
The background and calculations are in my guest contribution at WUWT of WUWT of today.
A pity you have to leave, we will need to wait till Sunday…
Atmospheric CO2 has grown less than humans contributed – nature is actually removing CO2 from the atmosphere:
http://www.tyndall.ac.uk/global-carbon-budget-2010
So what? This is the hopelessly misguided and jejune pseudo-mass balance argument. It has no impact on the discussion.
Bartemis,
Almost all variability is caused by temperature, almost all increase is from human emissions:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/rss_co2_emiss_deriv.jpg
That graph shows that both temperature alone or the sum of human emissions minus the half life time of ~40 years + the transient response from nature to temperature changes do largely match the increase of CO2 in the atmosphere.
The difference: temperature is increasing linearly, which is also the case for the transient response of the natural sinks and sources. In the derivatives that gives near zero slope. Human emissions increase slightly quadratic, which gives a linear slope in the derivatives.
Further, the temperature “match” violates all known observations, not at least Henry’s law for the solubility of CO2 in seawater, while the human cause fits all observations.
The match of temperature with variability and slope is entirely spurious…
The transient response of nature does 100% match the variability of temperature in the derivative for timing and form. Here enlarged for the period 1987-2002, which encompasses the two largest temperature changes: the 1991 Pinatubo and the 1998 El Niño:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/rss_co2_emiss_deriv_1987-2002.jpg
I have sent a new version of the complete story about the transient response to WUWT and hope that it will be published soon, so that this discussion can be finished for once and for good…
The “temperature increase” is “anthropogenic” only in the sense that it has been falsified by a few men. It’s time to drop these scientific-looking graphs, they are fraudulent.
Alexander Feht,
I used the RSS satellite temperature trend, as that is more reliable than ground thermometer readings…
I am in a hurry, and the nesting here is complicated, so this is a repeat of the above comment.
These plots are very complicated, Ferdinand, and too little information is given to understand exactly what you have done.
I am wary that, with the different time constants, you are applying sink activity differently for anthropogenic and natural CO2. These inputs must be treated exactly the same in terms of their removal from the atmosphere. (I very much suspect you haven’t ensured this.)
Give your exact equations, not just graphs.
Also, you should show the match all the way back to 1958, using the SH data.
Unfortunately, you have once again caught me at a time when I must travel. It is the Thanksgiving holiday weekend here in the US. I will be gone until Sunday. Will try to check in later, but can give no guarantees.
We have measurements of atmospheric CO2 concentrations going back many decades. How should these measurements behave in the future under the two competing scenarios of 1000 year and 40 year residence times? When should we be able to detect which hypothesis is correct?
Answer: in either case, emissions are accelerating, so atmospheric concentration should be accelerating.
But it isn’t.
The rate of change of atmospheric concentration instead leveled off to a near constant value at the onset of the “pause”
http://i1136.photobucket.com/albums/n488/Bartemis/temp-CO2-long.jpg_zpsszsfkb5h.png
It seems to me that the strongest driver of CO2 levels is temperature. It comes as no surprise that temperature is the dominant player in tow,
Unfortunately, we do not have the necessary data covering the ~1860 to ~1880, the ~1920 to~1940 warming episodes, and above all, the ~1940 to ~ 1970 cooling.
If we had that evidence, particularly detailed CO2 levels for the period ~1940 to 1960 which we could compare with pristine temperature data, we would be able to shed much light on the point that you are making.
As usual, in Climate Science we are faced with a lack of quality data, or data that covers too short a period from which to properly understand matters.
http://woodfortrees.org/plot/esrl-co2/from:1958/mean:24/derivative/plot/hadcrut4sh/from:1958/scale:0.204/offset:0.096
Richard, the southern hemisphere data is also a good fit and goes all the way back to the inception of MLO (and, of course beyond). One would think that over half a century of data is enough to come to the obvious conclusion that temperature is in lock step with the carbon growth rate…
The answer is now, or last April, for showing which of these figures is closer to the truth. It looks like that with oversimplifying natural removal of injections of CO2 into the atmosphere as going by the rules of exponential decay, the half life of an injection of CO2 into the atmosphere is 41 years.
http://wattsupwiththat.com/2015/04/19/the-secret-life-of-half-life/
Rabbit,
For the moment it is impossible to know who is right. The IPCC uses the Bern model, which is a mix of several decay rates, depending of the sink process, but includes a sink limit. That is right for the fastest sink; the ocean surface, which is saturated at about 10% of the increase in the atmosphere. They imply that to the deep oceans too, for which is not the slightest sign of saturation in the foreseeable future (that gives the ~40 years half life time) and they apply that to vegetation, which has no uptake limit at all, but is slower in uptake rate into more permanent storage (humus, peat, browncoal,…).
Hans Erren has somewhere a nice graph of the difference between the observed current decay rate, if sustained in the future, and the Bern model, but I have no link.
Anyway, it will take a few decades to be sure who is right or wrong in this case…
Well assuming that we now have 400 ppmm of CO2 and the stable base amount is 280 ppmm, then the excess CO2 is 120 ppmm.
At Mauna Loa where they have no oceanic ice melting, the CO2 drops by 6 ppmm in 5 months, every year.
At the north pole, and over all the Artic ocean that amount is 18-20 ppmm drop in 5 months. So at that rate the whole lot would be gone in 6 to 6.66 times five months or about 33 months.
So that is the decay time constant.
So 99% would be gone in 5 time constants, or about 14 years
So if we stopped adding any more CO2, the CO2 would drop to 280 ppmm in about 14 years.
g
Well if you want to take the ML rate as the global average, then 99% would be gone in about 42 years.
So I don’t believe the half life is as much as 40 years.
maybe about 7 times 33 months, so the half life is more like 19 years, rather than 40 years.
g
Rule of Thumb: 99% decay == 5 half lives.
42 / 5 = 8.4 years. Actually seems reasonable to me.
????
My estimate some ten years ago yielded a half life of 24 years for the virtual anthropogenic component of atmospheric carbon dioxide. I did not estimate the uncertainty of the result.
A more sensible approach than the ad hoc “half anthropogenic CO2 sequestered in the first year from emission, the rest over a thousand years”.
Actually; rule of logarithm calculator; 99% decays in 3.33 half lives.
Tony L is confusing half life with time constant. 99% decay is in 5 time constants; not 5 half lives.
2^3 = 8; 2^4 = 16; 2^5 = 32, NOT 10 !!
Well it seems that the adult version is a bit over the head of some WUWT readers.
So here’s the 4-H club version.
” Residence time ” is the time it would take to rid the atmosphere of EXCESS CO2, above the 280 ppm perfect base rate; IF ALL PROCESSES ADDING EXCESS CO2 CEASED.
Adding CO2 continuously and taking it away continuously has nothing to do with residence time.
Turning on both the hot and cold water taps into your bath tub, and then pulling the drain plug, is NOT how you determine how long it takes to EITHER fill, or empty your bath tub.
The ML CO2 amount drops 6 ppm in just five months, almost linearly when SOME of the excess CO2 processes shut off for that five months.
An EXPONENTIAL decay process has a starting decay rate, which if continued linearly, would reach ZERO in ONE time constant; that’s a common way of measuring time constants, by observing decay rate immediately after shutting off the process.
This discussion seems to have fooled a lot of WUWT readers; but it doesn’t fool Mother Gaia.
She can read the serial numbers on each and every CO2 molecule in the atmosphere, so she knows when your run one of them off the field and replace it with another one that you were pumping up with oxygen for the next play.
So she sees you sneaking NEW CO2 into the atmosphere to hide the fact that some perfectly natural process is sucking it out of the air big time.
You have to terminate ALL processes other than those that maintain the ” equilibrium ” 280 ppm amount that the chicken little crowd is cackling about.
g
sorry for the shouting and for the commas too
George, that Mauna Loa rate of decay does not continue for the entire year and at the north pole over the entire planet. In fact if rises at most other times for a small net annual gain
Another way to estimate it is as follows: If there were no excess absorption, CO2 would grow about 1 ppm / year from man made emissions. The actual rate is about one half of that. So net absorption is 0.5 ppm with a surplus of 400-180 or 220. That looks like a time constant of 440 years and a half life .69 X 440 or 305 years. However if a warming ocean emitted, say 2 ppm net, than net absorption would 2.5 ppm / year and half life drops to 305/2.5 or 122 years. In this case land and vegation must be a net absorber.
So what am I doing wrong given the above assumptions?
Major error in my above comment. The net absorption should be about 0.5% not 0.5 ppm. At 400 ppm net absorption is 2 ppm. So my estimates for half lives should be reduced by a factor of 4 giving about 76 years and 30 years for the two cases.
The annual 6 ppm drop at Mauna Loa is a seasonal one that is countered by its annual opposite seasonal event. Other parts of the world, especially in the northern hemisphere, show seasonal effects that cancel each other out over each whole year. As for specific cold ocean areas that are CO2 sinks, they sink CO2 at more than average for the world’s ocean sinking of CO2. For that matter, some tropical oceans source CO2 because their water was warmed by the sun after sinking CO2 previously when that water was colder.
If we ceased manmade net sourcing of CO2, the current 400 PPMV would likely go halfway to the pre-industrial 280 PPMV which is 340 PPMV in about 41 years.
So sorry, working too late.
I made another error in my calculations in my comment 2 above, Excess CO2 should be 400 – 280 or 120 ppm, not 220. With estimated man made emissions adding 1% per year, but CO2 as measured only growing about 0.5%, absorption is 0.5% or about 2 ppm per year. Since 2 is much less than the excess of 120, the time constant is close to 120/2 or 60 years. To get the half life multiply by – ln(0.5) or 0.69, which gives about 41 years. This is very close to the estimate in this paper.
It also means if we cut the emissions in half, there would be no more growth above 400 ppm. If we cut emissions by 25%, growth would to 0.25% per year increasing the doubling time from about 140 to 280 years.
@ur momisugly Richard Petschauer – November 25, 2015 at 12:51 pm
No, it would not.
If manmade net sourcing of CO2 stopped immediately there would be no detectable change in the bi-yearly (seasonal) cycling of atmospheric CO2 (avg. 6 ppm)…. or the annual increase of atmospheric CO2 (avg. 1-3 ppm).
As per the Keeling Curve graph and/or Mauna Loa record the aforesaid bi-yearly cycling and annual increase has been “steady & consistent” for the past 57 years …. regardless of what humanity was doing during those same 57 years
I compiled the following statistics via reliable sources, to wit:
(Note: I used December’s CO2 ppm count … but the yearly maximum CO2 ppm always occurs mid-May of each year.)
Increases in World Population & Atmospheric CO2 by Decade
year — world popul. – % incr. — Dec CO2 ppm – % incr. — avg increase/year
1940 – 2,300,000,000 est. ___ ____ 300 ppm est.
1950 – 2,556,000,053 – 11.1% ____ 310 ppm – 3.3% —— 1.0 ppm/year
1960 – 3,039,451,023 – 18.9% ____ 316 ppm – 1.9% —— 0.6 ppm/year
1970 – 3,706,618,163 – 21.9% ____ 325 ppm – 2.8% —— 0.9 ppm/year
1980 – 4,453,831,714 – 20.1% ____ 338 ppm – 4.0% —– 1.3 ppm/year
1990 – 5,278,639,789 – 18.5% ____ 354 ppm – 4.7% —– 1.6 ppm/year
2000 – 6,082,966,429 – 15.2% ____ 369 ppm – 4.2% —– 1.5 ppm/year
2010 – 6,809,972,000 – 11.9% ____ 389 ppm – 5.4% —– 2.0 ppm/year
2012 – 7,057,075,000 – 3.62% ____ 394 ppm – 1.3% —– 2.5 ppm/year
Source CO2 ppm: ftp://aftp.cmdl.noaa.gov/products/trends/co2/co2_mm_mlo.txt
Based on the above statistics, to wit:
Fact #1 – In 70 years – world population increased 207% – CO2 increased 31.3%
Fact #2 – Atmospheric CO2 has been steadily and consistently increasing at a rate of 1 to 2 ppm per year for the past 70 years, …… whereas human generated CO2 releases have been increasing exponentially every year for the past 70 years.
Fact #3 – Global Temperatures have been steadily and consistently increasing a few hundredths or tenths of a degree for the past 70 years, ……. whereas human created infrastructure, housing, vehicles, etc. (Heat Islands) have been increasing exponentially every year for the past 70 years.
Conclusions:
Given the above statistics, it appears to me to be quite obvious that for the past 70 years there is absolutely no direct association or correlation between:
Increases in atmospheric CO2 ppm and world population increases.
Increases in Average Global Temperature and world population increases.
Increases in Average Global Temperature and Heat Islands construction increases.
Increases in Average Global Temperature and atmospheric CO2 ppm increases.
But then of course, …… I am not looking through Rose Colored Glasses.
And ps: There is no “half life” associated with atmospheric CO2 …. anymore than there is a “half life” associated with the US money supply.
Some months back, I suggested to Ferdinand that the OCO-2 data suggested a CO2 residency time of about 15 years, or at any rate 20 years or less.
I am pleased to note that you too are of like opinion.
I consider the residency time claimed for CO2 is very much exaggerated. AND this is very important since if residency time is lower, it enables us to adopt a wait and see policy. It puts off the day when we need to go all out to mitigate CO2 emissions (not that I consider that we need to do that since I consider that observational evidence suggests that Climate Sensitivity, if any at all, is modest, and I consider that warming would be a net positive for life on planet Earth).
The residence time of carbon dioxide molecules, measured from isotopic concentrations, is confounded by diurnal two-way ocean-atmosphere exchanges.
George,
Different processes at work: the seasonal changes are 100% temperature controlled, where vegetation is leading. The sink rate of the extra CO2 in the atmosphere is 97% pressure (difference) controlled, 3% by temperature (as that influences the pCO2 if the oceans) where the oceans are leading…
If you were right, the remaining fraction of human CO2 in the atmosphere (as mass) should be much lower than around 50%…
What is this “extra CO2” you’re talking about ?
I fear the result depends much on your definition, and that’s no good.
If you mean “CO2 in excess of the equilibrium concentration according to Henry’s law”, well, the surprise would be if it were not “pressure controlled” and “oceans [leaded]” .
paqyfelyc,
Indeed the extra CO2 above steady state for the current weighted average ocean temperature per Henry’s law…
I see you know the difference, but some here insist that a fast response of vegetation to a temperature drop means that all sinks are not overloaded by human emissions…
All the talk about ‘equilibrium’ is ridiculous. The level of CO2 changes over time and has changed during the entire past history of the planet earth and it goes up and down and when we have Ice Ages which is the norm since the last roughly 2 million years, it is very low.
This is more like a ‘tick tock’ system going from one setting to the opposite like clockwork and we don’t fully understand how this works but we know that it is happening and the likelihood of it not tick tocking back to another Ice Age is wishful thinking.
Not 1,000, not 40, but probably less than 10 years. Jennifer Marohasy presented a convenient chart of peer-reviewed estimations/measurements of the CO2 residence time in 2009, here, with accompanying text:
“In order for increased human carbon dioxide emissions to cause accelerated global warming, the climate models need to assume that carbon dioxide remains in the atmosphere for a very long time, up to 100 or more years.
“Since the IPCC’s task is to prove any global warming is due to human CO2 emissions, they decided to proclaim that carbon dioxide was long-lived in the atmosphere — a fabricated assumption.
“They did this despite the overwhelming majority of peer-reviewed studies (and corroborating empirical measurements) finding that CO2 in the atmosphere remained there a short time. Literally, a fabricated assumption, driven by political agenda, became a cornerstone of fraudulent climate model science. As a result, billions spent on climate models that are unable to predict climate with any accuracy…”
So NOAA’s claim was known to be fraudulent years ago.
http://wattsupwiththat.com/2015/04/19/the-secret-life-of-half-life/
An individual surplus molecule of CO2 will probably be removed from the atmosphere within 10 years, as shown by the “bomb test data”. But a CO2 molecule being absorbed from the atmosphere by the ocean makes the ocean more prone to gas-out an already-dissolved molecule of CO2. Willis Eschenbach showed in the article linked above that over a time period that counts, the half-life of CO2 in the atmosphere that is in excess of being in equilibrium with nature as a whole such as the oceans is 41 years.
I have only quickly rescanned the article by Willis, but I did not see that he had proved the half life to be 41 years. I note that he talks about recycling/turn over and suggests that that adds to the theoretical half life.
Perhaps you would like to quote the passage in which the period of 41 years is said to be proved.
“Jennifer Marohasy presented a convenient chart of peer-reviewed estimations/measurements of the CO2 residence time in 2009, here,”
The link is mangled; I think you mean here. As DK says, Willis shows what is wrong with that. It’s comparing different things. Mr Halperin says
“For some time, the subject was surrounded by confusion, created by sloppy definitions and evasive statements in IPCC assessment reports. There was a mix-up between the residence time of a CO2 molecule in the atmosphere and the rate of change of the surplus CO2 concentration.”
But there is no sloppiness in the IPCC reports on this. The confusion is created on the skeptic side, as in JM’s post. The chart shows a whole lot of residence times, contrasted with the IPCC estimate with the rate of change. They aren’t the same, as Willis says. Mr Halperin gets that right.
And note Jenny’s chart shows the IPCC estimate as 100 years, not 1000. But in fact, the IPCC notes that there are multiple time scales, and you can’t reliably quote a single half-life. Decay isn’t exponential. Box 6.1 in the AR5 sets this out very explicitly.
Nick Stokes November 24, 2015 at 11:02 pm
Here I am agreeing with Nick again, is this a sign of the end times? In any case, yes, the two things are often confused or worse, conflated.
One is the airborne residence time of an average CO2 molecule, which is under ten years. Determining this is pretty straightforward, with a couple lines of evidence.
The other is the “half-life” (or the “e-folding time”) of an increase in atmospheric CO2. This is the time it takes for a pulse of CO2 injected into the atmosphere to decay to 1/2 (or 1/e) of its original value. Determining this is much harder, for the reasons I give elsewhere in this thread.
w.
You people confuse yourselves and any passing readers with such pretentious drivel as “the half-life of CO2 in the atmosphere that is in excess of being in equilibrium with nature as a whole”. So many words for a critical definition implies vague thinking–as in, “in equilibrium with nature” sounds like a moving, and elusive, target. But your comments merely remind me that there is no consensus on the “basic physics”, and I really have no interest in feeding the fires of disagreement among all of the egos involved (including my own). Just be assured that none of us knows that which has not even been properly defined.
SO…… What happens if the natural contribution of CO2 to the atmosphere is actually several times the amount in this simple analysis. This is the satellite generated data of CO2 concentration. Looks to me like significant contributions from the active tectonic zones particularly the shield volcano on the southern end of the African Rift Valley. it also brings tears of laughter when compared to the CO2 Model on the same NASA VO2 Page. Environmentalism at its naked best.. http://www.scientificamerican.com/article/first-maps-from-carbon-monitoring-satellite-show-global-co2-levels/ Sorry couldn’t find the actual link to the NASA data.
The kinetics of CO2 have to be approach to equilibrium kinetics, not first order decay kinetics.
Make assertions about natural and anthropogenic sources, make an assertion about the source of “excess” CO2, then apply the wrong kinetics model. You will get answers for an initial value and a decay rate. The fits may even look good, but they will not be right.
The kinetics of removal of surplus CO2 from the atmosphere by nature (especially the oceans) has a non-constant time constant, as described by the Bern model. The Bern model can be fine-tuned according to climate sensitivity to CO2, but it shows that an injection of CO2 into the atmosphere starts with approaching equilibrium at a fast rate and possibly almost as fast as the !10 years cited by the “bomb test data”, but slowing as ocean at levels under the surface get CO2 gains from surface-level ocean water. As I have cited in other comments here, a “one size fits all” half life of manmade increase of CO2 was shown (by someone who showed his work) as 41 years.
So to what extent does CO2 generated by forest fires and volcanic eruptions contribute to atmospheric concentration?
Excellent point, Bob (in Castlemaine). That jumped out at me, too. Given that natural sources of CO2 (and sinks, roughly in balance) OUTWEIGH human by TWO ORDERS OF MAGNITUDE. See: http://wattsupwiththat.com/2015/11/24/co2-residence-time-said-to-be-40-years-not-1000-as-noaa-claims/comment-page-1/#comment-2079112
It appears that the author of this thread’s posted article is, for some reason, a bit timid/lukewarm.
Bob,
Volcanic emissions are estimated at 1% of human emissions, based on permanent monitoring of active volcanoes like the mount Etna in Sicily/Italy. Even the Pinatubo eruption, larger than all volcanic eruptions of the past century together, didn’t increase the CO2 rate of change, to the contrary.
Forest fires only recycle CO2 that was captured a few years to a few decades before out of the same atmosphere and do regrow quite fast, thus over longer periods don’t add to the atmospheric inventory.
Human emissions are from CO2 taken out of the atmosphere millions of years ago (at much higher levels) and do ad to the current CO2 level…
I suspect that the amount of naturally outgassing CO2 is very much under-estimated since we only monitor the larger volcanos.
But my main gripe is the point you make about Forest Fires. As I understand matters, one has an existing forest that is an existing carbon sink and has an ongoing sink capacity of X per year. This forest then burns down and this results in the immediate releases Y amount of CO2 emissions adding to the total level of CO2 in the atmosphere. The forest then regrows thereby once again resulting in a sink. But the material point is that there is little difference in the sink capacity of an existing forest (ie., the one that was in existence prior to the forest fire) and that of one comprised of youthful and growing trees (ie., the regrowing/regrown forest).
If there is no material difference between the capacity of the sink before the fire and after the fire, then every forest fire simply adds to the total CO2 levels in the atmosphere.
This is the accounting myth behind biomass. Biomass, because it has a lower calorific value than coal or gas, results in more CO2 per unit of energy produced by its burning (when compared to the amount of CO2 released by burning coal or gas). It is claimed that it is CO2 neutral. This claim would only be true if it is sourced from a carbon sink that was not in existence prior to the felling of the trees, or if a new carbon sink was created after the trees are felled.
It would have some merit if the capacity of the carbon sink was materially different between that of say old virgin forwest, and new sapling/growing forests, but studies have shown that there is little difference in the amount of CO2 sequestered by old mature forests and that sequestered by young immature forests.
That forest fires statement makes sense with a CO2 residence time of about 1000 years.
But does it apply for a period of 40 years?
Actually Richard, studies show that new growth certainly exceeds old growth in CO2 uptake, as common sense says it should.
except that there are more 2x4s laying around now
VikingExplorer November 25, 2015 at 6:20 am
Whilst one would intuitively consider that there would be a significant difference between the carbon sequestering of mature forests and young ones, there has been much research which suggests that the difference is modest There have been many papers published on this. No doubt several of which have been discussed on this site, if one were to research the issue.
The theory presented here is correct. The rate that natural processes are absorbing CO2 out of the atmosphere does depend on how much “extra” CO2 there is and that means CO2 does NOT stay in the atmosphere for thousands of years.
The numbers are probably off a little however since the rate has been about 1.7% of the excess above 280 ppm for the past 50 years or so. At that rate, it take 130 years or so to remove the excess, if we stopped emitting today.
The 1.7% rate does appear, however, to be rising slightly over time.
http://s1.postimg.org/8ori6003j/CO2_Absorb_above_Equil_280_ppm_1750_2014.png
But keep in mind, we are still adding CO2 to the atmosphere at a faster rate than the natural absorbers operate at.
If we want to stabilize CO2 at 450 ppm, for example, the infamous target they are trying to get at Paris or +2.0C equivalent, then our emissions would have to be lowered by about 35% compared to today’s level over the next 30 years. if we want to stabilize at 560 ppm, the doubling level, our emissions can go up by a further 6% and thats it.
In other words, even with the natural absorption rate being so high, we will still blow right by the 450 ppm level or the doubling level without significant restrictions over the next 40 years.
“The 1.7% rate does appear, however, to be rising slightly over time.”
An epicyclic claim. For those who may not get the reference, it is to the epicycles added to planetary motion for the Ptolemaic universe model to explain the discrepancies with observations.
You can always make ad hoc claims, and pile things higher and deeper to get agreement between your hypothesis and observations. But, when there is a simpler explanation available that fits the observations, such arbitrary reasoning should be abandoned.
How do we assume that even if the atmosphere was magically stable at a given CO2 concentration, that there is not a lag in the planets ability to absorb more, IE trees and foliage continue to increase, etc… until that balance is established.
Even NASA agrees the planet is greening. The natural absorbers are increasing and therefore CO2 is being removed from the atmosphere faster and faster.
Someone should get the average amount of CO2, H2O, and methane given off by a human every day; and multiply by 365 and then, say, 80, to estimate one human’s contribution to GHGs over a lifetime. Then multiply by the world population, 7.2 billion. In addition to that, do the same for all living, non-plant creatures. Use the resulting mega- or giga-tons to make the case that all such life be drastically cut back from the face of the earth.
I think that it is generally accepted that the amount of (so called) GHGs emitted by ants and termites exceeds the GHG emissions by man. Man is a bit player in the biosphere.
I once removed sod from a 10-acre field in Washington State. Everywhere the machine shaved off the top 2 inches of sod, there were thousands of the tiniest red ants per square foot on the underneath side. Each one was about twice the size of this comma ,
“But keep in mind, we are still adding co2 to the atmosphere at a faster rate than the natural absorbers operate at.”
Bill, we don’t actually know this. What you’re saying here is based on as yet unverified deeper ice cores (which show lower co2 levels than today). The mlo and human emissions data show that the anthropogenic equilibrium sink rate is at least 47% (since the inception of mlo). If as much as half of all emissions are known to be taken out, then there is no reason that closer to 100% couldn’t be taken out. The difference being made up by an imbalance in nature…
Bart, nice to see you… You may think that your marathon a while back with ferdinand was a waste of time (as it sure looked like it), but for those of us who stayed tuned in, it was a very worth while time. I myself learned alot, yes, even from ol’ ferdi and i’m sure it was great practice for you in articulating your positions. (i think it really brought out the best in you…)
It could be argued that the biosphere’s photosynthesis response as evidenced by the greening of satellite imaging is a solar powered exponentially increasing sink rate. Not a linear response to CO2 concentration.
Well, yes though it isn’t as bad as it is made out to be. If you were to graph human emissions since, say 1980, it would look like a “hockey stick” curve, particularly since the massive industrialization in China. But atmospheric CO2 increase has been nearly linear. This means that either the removal rate has increased with the increase in emissions or that human emissions are not the primary source of the increase. Or maybe some combination of both. For example, one complete ventilation cycle of the ocean takes about 800 years. We still have water today that is upwelling and exchanging gas with the atmosphere that last did so during the Little Ice Age when temperatures were colder than today. So it would not surprise me of there is a large net release of CO2 from the oceans as they slowly warm due to recovery from the LIA and that process might take hundreds of years.
I don’t believe that is going to matter much. Reason is that we are already more than 50% past the doubling point. We have only seen 1 to 1.5 degrees warming since the LIA. Much (most?) of that warming is due to natural recovery from the end of the LIA (temperatures had recovered to near today’s levels in the 1930’s, before human CO2 emissions were much of a factor). So if we pretend that 1/2 of the warming is due to CO2 (which is a stretch) then we would see 0.5 to 0.75 degrees of CO2 warming so far due to CO2. This would seem to indicate that climate sensitivity to CO2 increase has been grossly overstated. Having gone over halfway to “doubling” we have seen only a small amount of warming and that has been coincident with a natural warming cycle that began toward the second half of the 19th century. And it *still* isn’t as warm as it was 1000 years ago in Greenland which would imply there is potential for additional natural warming.
I’m not sure I understand your graph. Is “absorption” (before dividing by “excess” to get a percentage) the difference between a year’s anthropogenic emissions and a year’s increase in total atmospheric content? In other words, is the absorption percentage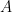 , i.e., your graph’s ordinate, given by
, i.e., your graph’s ordinate, given by  , where
, where 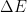 is a year’s worth of anthropogenic emissions,
is a year’s worth of anthropogenic emissions,  is a year’s change in atmospheric carbon-dioxide content,
is a year’s change in atmospheric carbon-dioxide content, 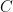 is the total content, and
is the total content, and 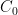 is the pre-industrial content?
is the pre-industrial content?
That’s not true Bill , that co2 is being produced faster than it can be absorbed. At current rates, the amount of ppm/ year will not only be zero, but may well be negative. The highest year on record for increased co2 was 1998 at 2.93 ppm. We are currently adding about 10 bmt/year more now than then. What’s being absorbed is all of the increase, and the sink is absorbing more of base amount each year. (Using 1998 as a base) . Of course that doesn’t mean that numbers can’t be adjusted at some later date. But then that presents another problem, more co2 would have produced a higher temperature. …. tsk, tsk,.. and as it is now, actual temperatures are well below any of the projections/predictions.
“That’s not true Bill , that co2 is being produced faster than it can be absorbed.”
To the extent you mean being produced by us, that is an hypothesis, not an evidence based deduction. It is an expression of faith. And, it fails to match the data because the increase is clearly temperature dependent, and human inputs are not.
You have to be very careful to sift through statements made on this topic, and separate out what is mere assertion to what is actually known.
What is actually known is: 1. The highest level of increase was in 1998 of 2.93 ppm of co2. 2. The amount of co2 produced by man has increased every year since then, and by a lot. 3. The current sink without a doubt overwhelms the total co2 produced in 1965. In fact the current sink would absorb all the carbon produced in 1965 and 1966 and half of 1967.
This is not an assertion, the numbers are from NOAA and there is a history to all of these numbers. . I have previously posted here and eleswhere those numbers. If you would care to do the math, you will see that the rate of increase of the sink is faster than our increase. How else are you going to get a billion metric ton each year increase and still have the co2 increase below 1998? Is all of that co2 hiding somewhere too?
The natural absorption rate has been lower than the human emission rate since about 1947.
CO2 actually fell in WWII, about 1.0 ppm in total from 1939 to 1946, but the last time that happened was the early 1850s and 1815-1820 before that.
Right now, human are producing about 4.6 ppm of CO2 per year and the natural absorption rate is only 2.0 to 2.5 ppm per year.
CO2 is going to keep going up and up until those two numbers are equal. Realistically, that is not going to happen until 30 to 50 years from now, if we also put deep restrictions on human emission growth rates.
By 2060 for example, we could keep our emissions to about 5.0 ppm per year and, by that time, the natural absorption rate would have risen to 5.0 ppm per year as well. Stabilization then at 560 ppm.
That is just the math.
I disagree, and the reason is as I have outlined or you missed. The sinks have expanded enormously. I can see one of 2 possibilities 1. The sinks continue to grow and overtake anthropogenic co2. 2. There is a proportionality.
The sinks are not finite, nor are they linear. If you do the math, there seems to be at least 10% more of the total co2 produced missing, some years 23%. Not accounted for in the atmosphere or in sinks.
“The key to science: on finding a new physical law: First, we guess. Then, we perform a calculation to see what are the implications of the guess. And then we compare the result with direct observations. If it disagrees, it’s wrong.”
Richard Feynman
Except in the case CAGW. Time stands still and any projection/prediction, whether it is true or not proves CAGW. There was no history before 1979, and it is still 2001. Any evidence that refutes CAGW is to ignored, covered up, or made to go away. In any case where it can’t, smear the person saying it as to disparage the data via the person.
The predictions/projections made in 2001 matter because of the math. Saying it doesn’t matter that the event will occur in 2100 or 2013 is not what CAGW stated to prove the science. They systematically ruled out everything that could cause the climate to change. In a chain of events these things have to happen in a given sequence and a given time. Stronger, more frequent hurricanes, extreme drought in the midwest us, an end to snow, warmer winters, ice free arctic, the Antarctic melting, global sea rise in meters not millimeters due to thermal expansion and glacier melt, global temperature at a 95% certainty at this date. None of that has happened.
Quite a number of studies have examined the half-life of CO2 in the atmosphere. The average of the half-lives is a little over 5 years. So, 40 years is a bit high.
I think there is a problem with the definition of residence time. In most chemical reactions the residence time relates to an individual molecule and the residence time is the average of the half lives of all the molecules emitted into that environment. The residence time can be a small number with the assumption that the particle does not return to the original environment.
The atmosphere is different in that than the same molecule can return back to the atmosphere depending on what environment the molecule where it was initially stored. What is the definition of residence time for the latter case?
The residence time for water in your bath tub; over and above what is normally in there (zero for the majority of bath tubs in most people’s houses) is how long it takes to empty the tub of say 99% of the total excess water (tub full) AFTER YOU PULL THE PLUG.
If you keep adding water from the faucets, then all bets are off. For residence time the taps have to be off.
g
Thanks, Ari Halperin. Good explanations on CO2 residency time.
“The half-life of the surplus CO2 concentration is approximately 40 years.” Interesting result pointing to a much more dynamic planet Earth.
Your website “Defy Climate Cult & Alarmism” will have links from my climate and meteorology pages.
Excess, What is excess? Plants love 1000 to 2000 ppm, roughly
This is why the paper says “surplus”, not “excess”. Plants love surplus CO2.
That is another nail in coffin for AGW, but wait to you all see what plan B is now that the Co2 thing isn’t going to plan. I’ve been waiting for this for some time know & they faithful did not let me down. The next bad gas is Nitrogen. Do you believe this crap? What universe are these people living in. And how stupid do they think the rest of us are? http://www.futurity.org/nitrogen-climate-models-908102/
Since N2 is about 78% of the atmosphere, any significant change to that would involve unimaginably huge processes, which simply aren’t happening. I notice the article you link to doesn’t give even a glimpse of the reasoning behind this. More “trust us, the sky is falling” lunacy. Thanks for bringing it to our attention – people who don’t have the background to be sure of their views on CO2 should have no trouble seeing through the nitrogen scam – and learning that the alarmists are simply shameless, shameful, liars.
It seems to me they are more concerned with NOx and nitrogen fertilizers than N2 in the air. And NOx was part of the issue with volkswagons.
Ah, so there is another Enviroprofiteer hu-stler lurking behind a rock…. someone with a product that wants contrived-market share (via government regulation) to eliminate a competitor with a more effective (and, according to REAL science, a safe) product that uses nitrogen, eh?
Just like the “organic” food sc@m….
Just like the freon-ozone hole sc@m…
Windmills…
Solar panels….
“Sustainability”….
and it never ends.
Truth wins, in the end, every time.
… then, the forces of pseudo-science-hu-stling come up with another money-grubbing angle.
And the SCIENCE GIANTS of the earth (like many of you on this thread) will knock them down….
And when the Enviroprofiteers and their Envirostalinist cronies in gov’t.
raise the game to “we must control the people of the world,”
that is when all the efforts of you WONDERFUL SCIENTISTS
AND ENGINEERS OF WUWT
make their most important difference, for
WUWT is mainly about: freedom.
******************************************
GO, ANTHONY, ET. AL.!! #(:))
******************************************
Agreed, and that is why I consider it a shame that some scientific topics are not aired on WUWT, although I fully understand the politics behind not airing these issues.
So I’m just a bit puzzled here. If we take the pre-industrial concentration of CO2 to be about 280ppm, then one would assume that at that level, the amount of CO2 entering and leaving the atmosphere must be nearly in balance. If there are no feedbacks, then adding extra CO2 will increase the rate of removal of CO2 from the atmosphere in proportion to its total concentration – not just the extra over 280ppm.
And is the assumption of no feedbacks correct? If increased CO2 concentrations lead to warmer oceans, that may reduce the rate at which oceans can take up extra CO2. So the simple exponential decay model may be a bit dodgy.
If I get time, I’ll look at how the warministas get their slow rate of decline of atmospheric CO2, and compare to the paper cited in this post.
Re: “the assumption of no feedbacks” — has to be. Otherwise, the world would have blown up (okay, okay, burnt to a crisp or whatever “planetary emergency” might possibly happen in Gore’s Fantasyland) long ago.
Here is an engineer’s take on it (I don’t advocate every sentence in his article, but it is a good one as to positive feedback):
Why wasn’t there thermal runaway in each, or any, prior interglacial (as is now feared for this interglacial)? We know that the Earth has never experienced thermal runaway (a tipping point). The likely answer is that the theory of pivotal positive-feedback CO2 may be just plain wrong.
{emphasis mine}
Ronald D. Voisin, here: http://wattsupwiththat.com/2013/06/04/an-engineers-take-on-major-climate-change/
John,
The effect of temperature on CO2 levels at “steady state” is known by Henry’s law and is about 16 ppmv/°C. For the current average temperature, that means about 290 ppmv at steady state.
The opposite influence is about 1°C/2xCO2 (without other feedbacks) or +280 ppmv which gives about 0.5°C of the 0.8°C measured. Nothing to worry about and probably mostly non-CO2 + negative feedbacks (clouds).
The influence of 1°C increase in the CO2 uptake is less than 3% of the fluxes as the local pCO2 pressure of the oceans at the polar sink places is much lower than in the atmosphere (down to 150 μatm), thus a temperature change has less influence.
John the equilibrium 280 ppm of CO2 is busy already driving the removal system to keep the amount constant.
So if the amount changes to 281 ppm, there is only 1 ppm of CO2 that is not already working at getting CO2 removed; not 281 ppm.
g
It is my considered opinion that George E. Smith is considerably more accurate than the author of this post.
My estimation was int he neighborhood of 10 years, but I will defer to George this time.
Steve,
Different processes at work, not comparable…
Steve in SC, I agree that George E. Smith’s estimate is the most plausible. It also matches numerous other studies of this matter. However, because Ferdinand is a pure agenda driven fanatic, he will object strenuously.
Thanks VikingExplorer,
I have no other agenda than looking at the science, but some here can’t tolerate that even the slightest hint of AGW may be right, as in this case…
The residence time is around 5 years. That shows that any CO2 molecule in the atmosphere, whatever its origin, is exchanged with a CO2 molecule from another reservoir. Zero effect on the total amount of CO2 in the atmosphere.
The half life time of an extra injection of CO2, whatever its origin, is 41 years. That is the time needed to halve the extra amount above physical equilibrium (“steady state”) between the ocean surface and the atmosphere. The steady state for the current temperature is 290 ppmv per Henry’s law, not 400 ppmv.
Ferdinand, I’m not getting into another discussion with you, but I may have gone too far by calling you a fanatic. You could indicate that you aren’t by tolerating the slightest hint that AGW might be wrong.
Your “zero effect exchange” explanation sounds like pseudo-scientific doublespeak to this engineer’s ears. A central concept of science is Gibbs theorem which says that without Work Input, things tend toward lower energy states.
For example, when one body is warmer than another adjacent body, a delta-T exists which drives Heat (net energy transfer) from the warmer to the colder.
Similarly, Henry’s law implies that when the one gaseous body has a higher concentration (partial pressure) of a molecular gas than an adjacent liquid body, it will cause a net transfer of molecules into the liquid (dissolve). This decreases the amount in the gas.
You are claiming that a net transfer is taking place, but that it doesn’t result in any change. This is anti-science agenda speaking.
>> The steady state for the current temperature is 290 ppmv per Henry’s law, not 400 ppmv
Science doesn’t work on averages. The average concentration of the entire atmosphere is a non-physical construct. Studies have shown that when the wind is blowing, CO2 becomes well-mixed, greatly reducing the ppmv at the surface. Without wind, it settles, greatly increasing the ppmv at the surface. Plants only get to eat when it’s calm. Prevailing winds over oceans could explain the apparent non-equilibrium in what you wrote. It certainly makes no sense that Humanity could be causing (and maintaining) a large non-equilibrium imbalance, which doesn’t react to large changes in economic activity.
A little Fe in the oceans would see happy fisherman and the true CO2 control knob in place.
The Southern Oceans have a fertility surplus but shortage of Fe that has been variously calculated as being able to pull the global atmospheric CO2 level down by 77ppm and mega fish. Win win and no centrally governed world.
as an aside if what is reported above is valid then over doing the Fe could refrigerate a significant portion of planet earth.
It’s pretty easy to calculate an estimate of the capacity of the atmosphere to supply plants with plant food. The intake of CO2 by plants per year is about 1/20th the total atmospheric CO2 content. If no more CO2 entered, the air would be empty of plant food in 20 years, so 40 years for any particular CO2 impulse to be removed entirely seems perfectly reasonable.
CO2 residence times, many peer reviewed papers vs the IPCC:
http://c3headlines.typepad.com/.a/6a010536b58035970c0120a5e507c9970c-pi
Thanks Dave, It isn’t surprising to me that the more accurate studies you link to give a lower time, 10 years round about. In calling the 40 years estimate reasonable, I have done a “back of the envelope” estimate. Enrico Fermi popularised the idea that a simple, rough calculation could give you a feel for whether a complex detailed calculation is about right. In this case the 100 years of the IPCC is easily seen to be far too long.
Ron House,
I will always be grateful to you for your affirmation-by-re-blogging of my post about the little swift murdered by the wind turbine in 2013. I just checked and am very glad to see that Winged Hearts (LOVE that name) is still thriving (here: http://wingedhearts.net/notjustabird ). You are a rare one. Good to know people like you, with both a sharp mind and a deeply caring poet’s heart, exist.
Best wishes to you and Gitie.
Your sister creature-lover,
Janice
Please, don’t mix up residence time of a single molecule, whatever its source, which is around 5 years with the decay rate of an excess CO2 injection, whatever its source, to get back into equilibrium, which is over 50 years or 40 years half life time…
Residence time is the throughput of capital (via goods) through a factory
Decay rate is the gain (or loss) of that capital after passing the full chain.
Residence time says next to nothing about the decay rate…
Janice Moore,
Thanks so much for your kind comment. Those of us who care want the truth to get out. Building wind turbines and solar concentrators that kill flying creatures in the most horrific way, regardless of one’s opinion of their efficacy for any other reason, should offend every right-feeling person, I think you will agree. Why are so many prepared to bury the knowledge of what these devices are doing, this I don’t really understand.
I heard a profound remark that made a deep impression on me: “Truth is a seamless robe.” I think it is actually a new coinage by our own Christopher Monckton, as there are only four references to that exact quote in the search engines. (http://wattsupwiththat.com/2013/12/24/monckton-of-meteorology-and-morality/) I mention it because if more people trusted it, they would not make this kind of mistake. It is wrong to kill, main and torture animals, so we shouldn’t do it. Find another way. The other way, of course, is that the entire project is counter-productive as CO2 is plant food. We needn’t be concerned about increasing it, if we can better the world for ourselves and the animals we share our planet with.
Our very best wishes to you Janice, and to all who care for those who can’t speak for themselves.
Ron
Ferdinand Engelbeen November 25, 2015 at 1:11 am
“Please, don’t mix up residence time of a single molecule, whatever its source, which is around 5 years with the decay rate of an excess CO2 injection, whatever its source, to get back into equilibrium, which is over 50 years or 40 years half life time…”
Hi Ferdinand, I am not “mixing up” anything. I specifically said that I am doing a back of the envelope calculation – a rough and ready test to see if the answers are realistic. The worlds plants can entirely empty the atmosphere of CO2 in 20 years. And we know for a fact by many careful scientific experiments that they can respond to increased CO2 rapidly to pull it out and grow very much faster. So any answer from around 10 to around 40 years for restoration of equilibrium meets the BOTE credibility test. The IPCC’s 100 years simply doesn’t. They’re clearly wrong and they should have done this kind of simple test and then looked harder to find out where they made their error.
Ron House,
A lot of CO2 is circulating in the biosphere, but that is mainly two-way: what is absorbed from the atmosphere is released by bacteria, insects, animals,… What counts is what ultimately goes into more permanent storage like humus, peat, browncoal, coal… That is far more limited: some 1 GtC/year. Humans emit ~9 GtC/year in comparison:
http://www.sciencemag.org/content/287/5462/2467.short
and
http://www.bowdoin.edu/~mbattle/papers_posters_and_talks/BenderGBC2005.pdf
Thus still 40 years half life time…
dbstealey November 24, 2015 at 6:05 pm
CO2 residence times, many peer reviewed papers vs the IPCC:
That was hilarious !
Ari,
Thanks for a thoughtful paper. It is interesting to consider the land-use contribution as a non-industrial component and to simply ignore it.
If we use only the fossil fuel emissions, your model works just as well as before. I get a best-fit Do of 288ppm and a 32-y half life.
Other than small effects of a larger isotopic mass, C14 is just like any other carbon in CO2. The way it cleared from the atmosphere after the end of open air nuclear testing is completely consistent with a short clearance time for atmospheric CO2 and is shown clearly here: https://upload.wikimedia.org/wikipedia/commons/e/e2/Radiocarbon_bomb_spike.svg
bones,
In principle yes, but there is a confounding factor:
What goes into the deep oceans is the isotopic composition of today. What comes out the oceans is the composition of ~1000 years ago (minus the nuclear decay). That gives at the height of the 14C bomb spike for 100% sink of 12CO2 and 14CO2 (1960) some 97% of 12CO2 returns (as mass) but only 97% x 45% (as mass x concentration) of 14CO2 returns.
That makes that the 14CO2 decay rate is at least 3 times faster than a 12CO2 excess decay rate…
The C14 is just a marker.
It is showing us not simply what is happening to each individual C14 molecule, but rather to what is happening to the bulk in which the C14 has marked.
Richard,
14C is a marker if there is no return at all or the return is fast, as is the case for the biosphere and the ocean surface. It is problematic if some 40% returns, while from the bulk, 12C some 97% returns…
The same happens with the 13C/12C ratio: what goes into the deep oceans is the composition of today (minus the isotopic shift at the air-water border), what comes out is the composition of ~1000 years ago (minus the isotopic shift at the water-air border). That gives that the 13C/12C ratio caused by human emissions is diluted to about 33% of what it would be if all human CO2 remained in the atmosphere.
Both the 14C dilution and the 13C/12C ratio dilution show that about 40 GtC/year is passing the atmosphere from the equatorial upwelling to the polar sinks and return via the deep oceans.
Ferdinand, I agree, but 3 times a small number is still small. The clearance time is certainly not the many centuries that the warmistas often state.
Ron,
Another residency time chart (sorry, I lost the provenance):
http://s29.postimg.org/x7rjvexuv/CO2_Residency_Time_1750_2170.png
(looks like a Bill Illis chart)
Everybody, stop breathing. I have an experiment I want to try.
How much longer?
Thanks Dave. I think that one looks far too long, as plant have a huge capacity to increase intake of CO2.
😉
I believe this is exactly correct. It will be interesting to see your results.