UPDATE: Jeff provides his answer below
Guest post by Jeff Condon (reposted by request from The Air vent)
Derek has been in a war with ScienceofDoom over the what appears to be Planck radiation. I’m actually not sure of his position because it doesn’t make sense yet to me but he left a thought experiment on the thread which could make for some interesting discussion. Some will find it pretty easy, while I bet others will get all tied in knots over it. As a suggestion, taking a thought experiment to an extreme is often a good way to identify a preferred design path or to understand differences in similar situations. I will give the answers in the coming days, they are already written so I can’t back out but as you consider them I’ll warn that this post is not about the subtleties but rather about the bulk differences.
I’m going to paraphrase Derek’s experiment below and then add another of my own. If it’s not the exact same as his it doesn’t matter the idea is still interesting.
For our experiment assume we have a bolometric camera for measuring emitted thermal radiation as an image. IOW a cool toy which in this case happens to detect all EM wavelengths with perfect sensitivity. To be clear, the camera integrates to measure the radiative emission temperature of the object.
We have two plants, one is contained in a transparent box (greenhouse) the other in open air, both thermally stable (temperature isn’t changing). We take an image of the two plants in the early afternoon on our fancy camera, what do you find in the image?
Derek asserts that the greenhouse plant will be warmer and therefore brighter, but lets continue this experiment further.
For our second experiment we have two thermally stabilized earths, one which has today’s CO2 and one which has 2X today’s level. All other conditions are identical and for some quirk of Id-ian physics, they orbit one right behind the other around the sun such that we can observe them simultaneously on our fancy camera. Now the CO2 of the higher concentration planet will block some of the emitted radiation creating the AGW greenhouse effect so the planet has a 1C warmer surface temperature. (For this thought experiment basic physics are required, planets are stabilized and I’m going with a 1C estimate chosen at random). Since it’s my universe, I’m staying on the warm Earth with a functional economy (right side) and sending all the vegetable eating enviros over to the cold economically devastated one on the left. haha- too fun, I probably should stick to the science for this though.
Now from a distant point we observe our otherwise identical worlds using our amazingly fancy camera. What would our camera reveal?
I’ll give the answers to these with supporting explanations tomorrow or the next day depending on how much fun people are having.
============================================================
1/2/2011 Jeff’s answer:
Ok, so the point of this thought experiment was to engage the public in a consideration of the differences between the greenhouse effect and an actual greenhouse. Most here already know that the name itself is a misnomer, but by considering the physics of what is going on we can better understand the argument and better present our opinions on the subject. The majority of the answer below was emailed to Anthony yesterday before he ran the post at WUWT with the note- just to make sure I can’t back out!
I know everyone is wondering what my answers will be to the two greenhouse situations, we’ll see how many will be convinced to change their opinions – or insist that I change mine 😉 . It turns out that both problems are fairly straightforward when considered from an engineering standpoint. In the thermally stabilized systems of the example where temperature is not changing, energy into the system is equal to energy out. We’ll cover the greenhouse vs free air plant situation first. Both plants receive the same energy but the ability to remove heat from the system is limited in the greenhouse plant through convection and evaporation. So in the case of the free air plant, although it is receiving the same energy it has 3 methods of cooling: convection, evaporation and radiation. In the case of the greenhouse, we can consider evaporation and convection negligible so the only option to release the energy is through radiation. Since our camera is only measuring radiation, and since both plants must emit the same energy they receive, the free air plant radiation will sum like this:
Measured EM radiation = Energy in – convection energy – evaporation energy
the greenhouse will sum like this
Measured EM radiation = Energy in – zero convection energy – zero evaporation energy
Therefore the camera will show the greenhouse plant as warmer (brighter) than the free air plant. This holds true even if we include non-zero convection and evaporation for the greenhouse because they are still reduced values requiring a higher EM emission to balance the energy equations.
So now we have the situation where we have two planets, one with more CO2 than the other. We know that CO2 absorbs certain outgoing wavelengths of light. We also know energy in is equal to energy out for both planets. Although this is called the greenhouse effect, it is actually quite different. For both the high and low CO2 planets, the only available cooling mechanism is EM radiation. All the energy coming in has to escape by EM radiation to space, so the equations balance like this.
Measured EM radiation = Energy in
That’s it really. Both planets will measure exactly the same to our camera yet one has a higher surface temperature. The reason this works is that the average energy emission altitude has gone up, allowing a warmer surface yet the net flow is the same.
Caveats: Now I warned that some will get tied in knots over the nuance of this example. There are all kinds of subtleties of the situation which cause minute differences in the planet example. For instance, increasing CO2 will increase the albedo to incoming light, reducing reflected energy and we get a microscopically higher energy in and therefore were our camera of perfect accuracy we could measure a very slightly higher measured radiation from the warmer planet. If this is your explanation, we are in agreement. There are other details as well, but in bulk the answers are A – greenhouse plant is brighter, and B – both are the same.
I read several comments which got the right answer, Carrick was the first to write the correct answer in the comments at tAV, although he kept the answers subtle enough that people had to read it carefully. If you were one who got them both, congratulations. If you are unconvinced by my explanations, ask away and I’ll do my best.
Jeff
IN = OUT … or … BOOM.
A tweak to the question that explicitly states that we aren’t interested in the instantaneous changes as opposed to the relative values of the differing equilibrium states would seem helpful.
In the first example the plant is slightly cooler due to the glass absorbing some of the light including some IR, however the glass enclosure is slightly warmer as a result. The volume occupied would contain the same amount of energy as the identical volume for the unenclosed plant (assuming no air movement – perhaps a vacuum is required, but that sucks for the plants).
The two planets will show the same temperature signature as you are not adding any more energy to either planet – so where would any additional heat be coming from?
Don’t you mean ‘subtleties’, not ‘subtitles’?
[Fixed, thanks. ~dbs, mod.]
“… I’ll warn that this post is not about the subtitles but rather about the bulk differences.”
Subtitles or subtleties?
[Fixed, thanks. ~dbs]
The same amount of heat would be leaving each if they are each in equilibrium. But the distribution of EM frequencies would be slightly different. The CO2 planet would have the EM at slightly higher frequencies.
An interesting connection between Common Purpose and Climategate
http://www.stopcp.com/cpclimategate.php
http://www.stopcp.com/cpclimategate.php
Happy New Year.
Ok – had a quick read and tried to get a handle on the basic premise. A bit unfair for a Jan 1st post methinks!
for the first one – it bothers me that there is no mention of transmissivity of radiation through the glass into (or out of, but I’m assuming its irrelevent!) the greenhouse compared to the external plants. The term transparent would imply completely transparent to all radiation (in and out) ? Anyway, I deduce that the thought experiment is basically about thermal convection/conduction restriction within the transparent box? (which of course is the PRIMARY way a greenhouse gets warmer than surrounding (external) air)
On the assumption that transmissivity and reflectivity is meant to be the same for both plants but convection (and conduction) is restricted within the ‘box’ – the plant in the box should indeed appear ‘warmer’ or brighter.
The second experiment describes identical planets at thermal equilibrium but with differing CO2 – but because the second planet is 1C warmer it should reflect that within its EM spectra. Because they are stated as thermally stabilized, and in equal orbits, etc, the radiation in must equal the radiation out but the measured spectra would/should show the 1C surface temp difference. Ergo, I would guess the warmer planet seems ‘brighter’ but the actual temp difference has arisen due to the time lagged equilization of radiation in and out at some time BEFORE the measurement was made and NOT as a result of a difference in radiation in/out between the two planets at the given time of measurement. (in other words, if the measurement was made shortly after the 2x CO2 was introduced on the second planet, it would appear to radiating less (cooler) than the first planet as the atmosphere ‘adjusted’ to equilibrium conditions)
In both cases, radiation in/out equilibrium is supposedly achieved (though the greenhouse box will of course change when the sun goes down!) and this is the critical point. The ‘brightness’ is reflecting only actual temperature (BB radiation curves and all that jazz) differences – not differences in incoming versus outgoing radiation between the objects…
If I am wrong, or missed some subtle point, I am sure its because its Jan 1st and I am hungover! LOL
My question is: what would happen if on these twin earths the normal one had 1 atmosphere but the other had 50 times normal atmosphere?
Kev has missed the point by looking for detail, the two observed systems are the same. Internal temperature is not important to the question, it is a distraction. I think myself that since the systems are at equilibrium, it is not representative of a climate system.
If you are SURE of the answer, let’s turn things inside out.
Let us say that there is no sun – that the warmer planet is warmer due to internal radioactive decay and gravitational collapse. Which planet will be brighter?
Does it matter if the energy is coming externally or internally with respect to radiation from a warm body?
Sean Houlihane says:
January 1, 2011 at 3:16 pm
thanks Sean – I am not 100% sure, but took the clue from this sentence….
<>
from that I took it to mean that the camera is basically a thermal measuring device, which of course, is exactly what BB radiation is all about.
I agree with your comment regarding a climate system – though I presume that the premise is that so long as radiation in/out isn’t changing significantly, the climate system is irrelevant?
In the first example the greenhouse plant is of course the warmest, because the enclosed area is able to hold onto some heat, to the point where equilibrium is reached between incoming and outgoing energy. There is no “runaway” or “catastrophic” warming – just an initial temperature rise then stability at that warmer temperature. Once equilibrium has been reached, the appearance to the camera would be the same as the outdoor plant: energy in = energy out.
In the second example (assuming these are non-real-world earths, so forgetting about increased clouds and albedo and all that jazz, and assuming that CO2 levels are not rising) I’ll refer you to the same answer. It’s a bit warmer in the earth with the extra CO2, but the view from the camera shows the same brightness for both earths, as the energy out is equal to the energy in (which of course is the same for both earths).
You’re going to tell me I’m wrong, I can sense it…
In the greenhouse the temperature will be slightly higher but not because of greenhouse gases but because of suppression of convection. That’s how greenhouses warm
In the second planetary case there is no suppression of convection, just a very slight increase in nighttime temperatures due to a slight reduction in thermal losses.
Ergo, the difference due to greenhouse gases would be lost in the natural oscillation of the atmosphere.
Change my last sentence to. “Does it matter if the energy is coming externally or internally with respect to radiation from TWO EQUALLY WARM BODIES?” To see the paradox that some of you have created.
HankHenry says:
“My question is: what would happen if on these twin earths the normal one had 1 atmosphere but the other had 50 times normal atmosphere?”
Well, scattering would certainly be very different, since any transmission path would be through a much greater density of atmosphere.
Because it is ideal conditions, in=out. Therein lies the problem with ideal conditions.
This should be fun to follow. Personally I agree with John Robertson above. Glass is a physical barrier and it changes things. Think of glass 2 molecules thick versus a half meter thick. The planets, on the other hand, are exposed to space. It doesn’t matter if one is warmer than the other, they will balance as energy in = energy out.
I get A,B,B,A,C,D,D.A,A – I might be wrong though as i have a 31st hang over.
The interior of the greenhouse is warmer not because the glass or whatever traps the radiation, but it is warmer because you do not have convection. In free air, the warm air will rise and be replaced by cooler air from higher altitudes. You can perform this experiment easily with your car. Take your car (when its warm enough, right now it is not so in Boston) and roll up the windows. Leave it in the sun. Then measure the temperature of the interior using one of the remove sensing IR thermometers you can obtain for not a lot of money. You are measuring the quantity of outgoing IR, which is how those operate. Now do the same with the windows of the car open. You will measure a much lower temperature since the convection currents of air out of the car will carry the heat away and replace it with cooler air.
The number of joules of energy retained will reach some constant in all 4 examples.
Those constant’s will not be equal.
Once that constant has been achieved then the inbound/outbound radiation will be identical.
David,
I don’t think the camera would show equilibrium, because the warm greenhouse would still be transfering much of the excess heat by convection along the outside walls, and thermal cameras only record radiation, not conduction and convection.
This might be a flaw in the thought experiment, because such cameras would tell us that Venus is a fairly cool planet since they see cloud tops.
just reviewing some others comments makes me think I’m losing my marbles!
Yes – if the camera was just measuring in and out radiation – in any ‘equal’ system any two identical bodies would register the same on the camera. However, (and I may be thinking too deep here) I presumed the idea was to consider the actual temperatures of the objects in question – and – without doubt, the camera as described, must ‘see’ any differences in actual ‘body’ temperature (i.e. radiative emissions) via any differences in BB radiation.
The effective height from which thermal radiation leaves the higher concentration planet will a bit greater than that of the lower concentration planet, so its radiating surface will be somewhat greater. Therefore, in order to radiate equally, its brightness (radiating temperature) must be slightly less. Extrapolating downward to the earth’s surface from the effective height of radiation, at the adiabatic lapse rate, the surface temperature of the higher concentration planet will be somewhat higher because of the greater height through which adiabatic convection must extend. Presumably both planets will reflect sunlight equally at wavelengths that do not interact with the greenhouse gases.
We could make this hard, by throwing in things like a variation in the emissivity of the greenhouse glass because of accumulating pollen and plant sap.
But that would be cruel.
alcuin says:
January 1, 2011 at 4:27 pm
The effective height from which thermal radiation leaves the higher concentration planet will a bit greater than that of the lower concentration planet….
sorry, but why?
the concentration of CO2 in the atmosphere is described as doubled – but there is no mention of any increase in atmospheric depth (all other conditions are described as identical)?
On our earth, according to Trenbeth’s figures, we lose about 24 watts in convection. The greenhouse plant won’t be losing the convection heat that a non-greenhouse plant loses-it will warm up more during the course of the day. Conversely, it would cool faster each night.
A second factor is evaporation- Both the indoor and outdoor plant will cool thanks to transpiration, but the outdoor plant will cool more thanks to wind, which is blocked by the greenhouse- this factor operates both day and night. The average temp of the greenhouse plant should be higher, but the relative difference should increase up untill around the warmest part of the day, around 2:00 PM or so, then drop off until dawn.
As to the two planets, since they’re in balance with the sun, they should both be receiving and raiating away an average of 324 watts per square meter.
Let us consider the two planets, and just for simplification, suppose that both atmospheres are completely transparent at all wavelengths — except for the CO2 absorption lines. For this scenario, all of the green house effect is due to the CO2. Since both planets are in thermal equilibrium, each must radiate (or reflect back due to albedo) the same total energy out as is incoming. For two identical objects at differing temperatures, the warmer object will radiate more at every wavelength, and will also have a maximum at a slightly shorter wavelength. Of course, these two objects are not identical. One has more CO2 and is at a higher temperature — and yet we know that both radiate the same total energy out. Since we have posited all other, non CO2 gases as transparent, there is only one way to modify the radiation curve for the warmer object so that total area under the curve equals that of the cooler object. The radiation out from the warmer object must have a stronger dip at the wavelengths where CO2 absorbs. After all, the CO2 (in this scenario) is the only thing in the atmosphere which is not completely transparent.
Is this reasonable? Aren’t good absorbers also good emitters? Won’t the CO2 mixed into the upper atmosphere be radiating outward? Why don’t we see a spike in radiation at the CO2 lines? And if we did, then doesn’t that makes an energy imbalance. I know that the green house gas effect is well accepted by almost everyone, so will someone point out the flaw in my reasoning, at least as it pertains to this simplified model?
Assuming the glass is totally transparent (to all EM radiation) then the trapped air in the box and by consequence the plant itself will be at a higher temperature due to no cooling from convection.
Therefore the plant in the box must be hotter and therefore it must emit more blackbody thermal radiation which will be seen by the camera.
I at once suspected this “thought experiment” had everything to do with a mythical beast called “The Greenhouse Effect” and accordingly I therefore opted in both cases to vote where it said “The same”
If I am wrong then I shall, be very happy as no doubt I can then look forward to an explanation for “The Greenhouse Effect”. I mean an explanation that: a) makes sense, and b) Does not prove the complete opposite. (All explanations I have read so far, fall into the ‘No sensible explanation ‘)
I feel certain that if a “Greenhouse effect” natural, forced or otherwise exists then there MUST be somebody out there who can explain it, calmly and rationally.
The usual; “The greenhouse effect is natural, is well understood by scientists bla – bla -bla –” does not cut it and cannot be seen as scientific proof one way or the other. Nor can opinions of how it may be happening, nor do opinions of those who have more opinions than others.
By the way:
Why not make it a project for say 2011’s first couple of months: Write your own essay explaining “The Greenhouse Effect” and poste it on WUWT.
It could become interesting reading!
To all those considering convection, air rising etc etc i remind you of this sentence in the article..
To be clear, the camera integrates to measure the radiative emission temperature of the object.
If the camera is “seeing” the plant, then it is measuring the radiation from that plant even before the air around it has a chance to warm and rise.
Seen as we were not given a time frame, I assume an instant measurement was taken. So both will be the same.
For the planets, the 2xCo2 one is already 1c warmer, meaning it has reached equilibrium already, so radiation out will be same as radiation in. If it was brighter, it would be cooling. If it was dimmer, it would STILL be warming, but it’s not. It has reached equilibrium.
“We take an image of the two plants in the early afternoon on our fancy camera, what do you find in the image?”
Is the image of the plant taken from inside or outside the greenhouse?
Greenhouses work. I dont think there is any question about that…but at equilibrium they dont “manufacture energy” so the net energy leaving the ground where the sunlight hits must be the same in both cases.
In the first experiment, assuming they are in equilibrium to the environment as you stated above the one in the eclosure would show cooler (not in tempereature but emittted radiation, temperatures equal, emissivity different) than the one in the open for it would be have converted some of the co2 and have a lower concentration and since co2 in the atmosphere has greater absorptivity it also has higher emissivity and a lack of this causes the lower emissision though the temps are equal.
In the second experiment the planet with +1°C will show more emission. No brainer. Trouble is you cannot “block some of the emitted radiation”, emissivity disallows this unless you actually CHANGE the emissivity and therefore equally change the absorptivity. The two planets would actually be at the same temperature. Sorry, AGW is a sham.
As a founding member of People Against Acronyms and Abbreviations or PAA&A, I had no idea what IOW meant. My first thought was that IOW meant “I own won”. There was a paranthetical phrase with a missing comma as in “I own won, a cool toy which…” Then I find Id-ian physics. I am beflummoxed, my temper-ature is rising. Maybe it is a typo and should be Ind-ian physics. Well, I did look up IOW and determined a good translation is “In other words”. I did not get anywhere with Id-ian physics. Maybe it’s Idaho-ian physics. Or maybe better yet, Intelligent Design-ian physics.
Willis Eschenbach had a good discussion of CO2 and radiation in People Living in Glass Planets.
Some will find it pretty easy, while I bet others will get all tied in knots over it.
You can say that again. Still working on the first example. Without determining an answer, I think some interesting things can be inferred. Assuming that both plants start surrounded by a normal atmosphere, then at the time of the camera snapshot:
1. The experiment has been running for some time.
2. The greenhouse atmosphere is seriously depleted of CO2.
3. The plant is dead.
These follow from the stated condition of equilibrium. I reason as follows: A live plant is converting some portion of incoming radiation to stored chemical energy through photosynthesis. The emitted radiation from the greenhouse is therefore some function of of incoming radiation less chemical storage. This results in disquilibrium, since photosynthesis is slowing down due to CO2 depletion. Eventually the plant dies, a state of equilibrium is reached, and we can finally take our picture.
I suggest we simplify the experiment by eliminating the plant. Perhaps we can substitute a government bureaucrat, since it is well known that bureaucrats, like other inanimate objects, do not move until they are pushed.
As to the question, if the camera really captures the entire spectrum, and both setups are really in equilibrium, then I would suppose they would be of equal brightness. Except that the live plant is still diverting radiant energy to chemical….
Doesn’t everyone here get it?
I assume most if not all of you are sitting in a room. Objects in that room are all at the same temperature, black or white. All of the objects are in resonance radiantly with the walls and each object affecting each other object much as gravity. Some would propose (AGW proponents) that if your room was evacuated without convection and conduction that the black objects (think co2) would be warmer temperature wise than the white objects (low absorptivity and emissivity) but I for one don’t buy that unless someone can concretely prove it to me, by physics.
Is it any difference with the Earth just because it is only warmed on one side every 12 hours? If there was no sun but a diffuse radiation on every side of equal radiation (TSI) would that not be the same except for the diurnal up and down of the temperatures and seasonal variance? In that case it would be the same as your evacuated room, the same? Any thoughts at all or is this just politics on both sides, to hell with the actual physics and science?
Sorry, sometimes I think NObody is thinking. Just the same dribble.
The planets only have a radiative temperature because free space has an impedance. It’s about 300 ohm. (Any radio ham can tell you that.)
A planet heated from the inside is like a constant current device driving a load through a resistance. The voltage (temperature) drop across the resistor will be proportional to the impedance of that resistor (or thermal insulation). Once equilibrium is reached, all the generated current (heat) will pass into the load (free space) [according to Kirchoff].
The external radiative temperature therefore depends slightly on the insulating layer, but only if its impedance is similar in magnitude to the impedance of free space.
An object with “less escaping thermal radiation” is warmer, not cooler.
The image seen by the camera is cooler in that case because fewer photons are reaching the “film” or retina; but the camera’s film is not the object.
Without knowing the emissivity your camera can not give a temperature reading from the integrated intensity. In both cases, at equilibrium, the radiant energy in will be equal to the radiant energy out. I assume that we are ignoring convection and conductivity, although that may not be a safe assumption with the glass greenhouse. The two cases can be at different temperatures for the same radiant intensity, because they will have different emissivities. This is the essence of the Earth’s Greenhouse effect.
1) Thanks Jeff for posting this, you hit my sore point.
2) My dream for the day would be that by the end of this thread, even if 2000 comments long we would be able to answer the question — Does Kirchhoff’s law (emissivity and absorptivity, directly co2 related for it absorbs more) apply to Earth as other normal matter as view from afar like the moon. That would be an actual accomplishment scientifically here on wuwt. So many, maybe even myself, seem confused.
To me the increased emission equals the equally increased absorption and since in = out there is NO affect from co2. Maybe even getting back into Venus’s atmosphere later for it seems to show why this is so. Think lapse rate.
Both planets will reflect the same amount of energy. The hotter (CO2 planet) will emit more in the longer wavelengths.
Consider an alternative thought experiment. One planet covered with mirrors and the other covered with asphalt. They both reflect the same amount of energy but the mirror planet reflects mostly visible light and the asphalt planet reflects mostly IR radiation.
If tomorrow is clear, I will perform this experiment with my rustbucket Toyota Tercel and report back.
The two planets would have the same total, but if you looked at individual wavelengths, those from the surface would be brighter, and those from the top of the atmosphere would be dimmer, canceling out in the net.
For the first experiment none are correct.
If I assume the camera is outside the box then the camera will most likely see the box and not the plant inside.
Sigh. Jeff tells us that the 2XCO2 planet is 1 degree warmer. That’s a given, and it’s “true” regardless of differences with a glass greenhouse.
So, let’s simplify the question even further. In a world with half of it a given 1 degree warmer than the other half, would the warmer half show a brighter image? Unless you want to put a lot of people at FLIR and other imaging product companies out of business, the answer should be Yes (brighter).
The temperature of the plant is approximately a function of
(a) air temperature and
(b) transpiration.
All things being crudely equal, the plant in the greenhouse will register a higher temperature, since imposing limits on convection will increase both air temperature and humidity, reducing transpiration and the evaporative cooling effect this has on the plant, particularly its leaves.
Increasing wind speed and lowering humidity could reduce the temperature of the plant inside the greenhouse by increasing transpiration, despite a rise in air temperature.
Conversely, increasing the amount of CO2 available to the outdoor plant could increase its average temperature with no apparent increase in air temperature. This is because CO2-enriched plants don’t have to struggle so hard to breathe: stomata close in response to elevated CO2, reducing transpiration, meaning less evaporative cooling and water consumption.
If the first experiment, energy has to leave both planets as thermal EM radiation. One could assume that that the transparent box is high enough on planet 1 so that it is does not increase the atmospheric pressure and therefore planet 1 and planet 2 have exactly the same thermal emission temperature. Energy in = Energy Out.
In the second experiment, the two planets again have the same thermal emission temperature. In planet 1, with the doubled CO2, the layer that thermal emission temperature occurs is 460 metres higher than planet 2 and hence the surface temperature on planet 1 is 1.0C higher. Effectively, the lagtime in which the energy enters and then exits planet 1 is a few minutes longer than planet 2. 44.06 hours in planet 1 compared to 43.44 hours in planet 2.
“William Sears says:
January 1, 2011 at 6:10 pm
Without knowing the emissivity your camera can not give a temperature reading from the integrated intensity. In both cases, at equilibrium, the radiant energy in will be equal to the radiant energy out. I assume that we are ignoring convection and conductivity, although that may not be a safe assumption with the glass greenhouse. The two cases can be at different temperatures for the same radiant intensity, because they will have different emissivities. This is the essence of the Earth’s Greenhouse effect.”
I think you are exactly correct in the first statement but wrong in the second. Is this not why black objects in an IR camera show as bright and white objects (of anything with low absorption) show as dark. How can this be if their temperature is the same? It’s that the bright objects are absorbing more but equally emitting more, Kirchhoff’s law and the opposite for lighter colored objects or i.e. objects of less emissivity. Now apply that to co2 which absorbs more. To temperature it doesn’t matter, it emits more to space therefore preventing any warming, it has to.
In the second statement you are wrong, you said “The two cases can be at different temperatures for the same radiant intensity, because they will have different emissivities.”. If the have different emissivities they also have different absorptivities and the effects equate and the temperatures MUST BE EQUAL in a given radiative field (in the same room).
If speaking of inter-atmosphere effects instead of measurements from afar outside the object it gets more complicated.
At the moment the picture is taken I will use the same camera with midafternoon sun to photo the moon representing the plant out in the open and the earth representing the plant in the greenhouse . My hypothesis is the moon surface will read hotter than the earth surface because is reflects more energy back to space than an enclosed earth.
…now, where did I put the aspirin?
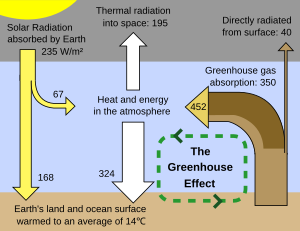
”earth’s land and ocean surface warmed to an average of 14c”…
the graphic seems to indicate that the sun warms the earth’s surface to an average of 14c?
those rescued chilean miners might beg to differ. it was not 90 degrees fahrenheit in that mine not because the sun warmed 390 ppm of CO2 and an inch of soil half a mile above their heads for a few hours a day. it was hot because they were closer to the source of heat.
i’m always amazed that scientists want to completely ignore the overwhelming mass of earth and the incomprehensible amount of btu’s it contains to entertain the thought that a trace gas in the comparatively negligent mass of our thin atmosphere is somehow going to overheat the entire planet with solar radiation that is normal to its surface for, at best, an hour a day.
have we all gone mad?
it’s not a coincidence that the mean annual temperature of air at the surface miraculously matches the temperature of the soil a few inches beneath the surface. if the temperature of our air is increasing, maybe we should first wonder what’s going on under our feet? and if it isn’t land use change, we shouldn’t be so quick to assume that a hair at the end of a dog’s tail is going to wag the whole dog.
maybe these global warming scientists should try warming their tea by heating the air above it? perhaps when they realize that they have to heat the same volume of air above their tea to 800 degrees or so in order to warm their tea by 1 degree, that then they will reconsider AGW?
we deforest, pave, build, convert matter to energy, and redistribute heat and energy all over our earth. we should be pleased that our thermometers tell us we’ve changed our environment by less than a degree, if at all.
michael mann should have been very curious when his tree rings told him things may actually be getting colder – he may have stumbled onto something that we should really be worried about.
Maybe the answers lies in the fact nobody is running around selling home climate systems based on indoor CO2 concentration levels. LOL
Argg! I don’t understand some of you people.
In the second ‘experiment’: a warmer body radiates more than a cooler body, period. Doesn’t matter where the warmer body’s temps come from; doesn’t matter what it’s atmosphere is; doesn’t matter if it’s receiving the same amount of energy as the cooler body; if it’s warmer, it radiates more energy (THAT’S THE ENERGY YOU ARE DETECTING WHEN YOU DETERMINE THAT IT’S WARMER THAN THE OTHER BODY!!!).
Lazy Teenager: that would depend of the optical properties of the greenhouse glass. What’s it’s optical depth, at what wavelengths does it emit? Even the best of glass will have different properties (emission, transmission, reflectivity) than a parcel of air. That being said though, I would have to say that this case assumes the plant can be imaged through the glass, else the question is moot.
(Pardon me if I seem hesitant…I am a new poster here, and it has been several decades since my Geography coursework, so I may sound somewhat foolish here and there)
I just want to add that I don’t believe 2X CO2 will actually add 1 degree. Most here probably already know my positions on CO2 which are – I don’t know. The 1 degree was used for the example only.
This fun post turned out to be more confusing than I thought it would so I’ll give another clue. The correct answers can be found through energy balance equations.
jeff: The correct answers can be found through energy balance equations.
Wrong. You have stated that the 2x planet is 1 C warmer at the surface. The thermal or blackbody radiation is therefore by definition higher or brighter.
Over time, steady state conditions may be assumed to exist and total energy in and out may balance but that does not change the fact that the spectrum will be different for these two examples with the hotter surface planet having greater thermal emissions than the cooler planet.
Kev-in-UK asks why the effective height of radiation is higher when the concentration of greenhouse gas is greater. The effective height of radiation from an atmosphere is conventionally taken to be that altitude at which the optical depth becomes unity, as integrated over height from outside the atmosphere. If the concentration of greenhouse gases are doubled, their opacities are also doubled at each level, so the integral becomes unity over a shorter distance down in the atmosphere, i.e., at a greater height. Of course the opacity = (1 – transmissivity) varies with wavelength, so a lot of averaging is involved, but the use of an effective height in this manner is the way that the radius of the solar photosphere is defined. Outward radiation from lower altitudes tends to be absorbed and reradiated at higher altitudes.
Jeff ID:
Then to take a stand on it – noticing you had helpfully posted both the Wiki energy balance graphic and said “it’s not the subtleties but about the bulk differences” – then I’d say the plant in the greenhouse will be imaged as thermally brighter than the plant outdoors.
The greenhouse emits more in the thermal, and that plant is sitting inside it, as opposed to a plant sitting outside in the open, unconstrained. Convection for the plant outside will draw away heat energy because there are no constraints (just parcels of air), whereas convection is unimportant inside the greenhouse, because all it does is just transport heat around inside of a sealed box. It will still emit it heavily in the thermal.
As I’d said it’s been many years since my coursework, but am I viewing this in the proper way?
This is the problem with talking about “global mean temperature”. Because there is day and night, each location has outgoing radiation that continuously varies. If the temperature in the greenhouse is warmer, it will radiate more. In the planet example, the high CO2 planet receives less radiation at the surface, as the sun’s radiation is about 45% in the infrared. In this example, it starts at dawn more than 1 degree warmer, and heats slower than the low CO2 planet, but after sunset, also cools slower than the low CO2 planet. On Earth, due to convection, peak surface temperature occurs around 2pm.
Net-net, the 2pm radiation from orbit should be about the same, as some of the higher surface temperature gets absorbed by the CO2; otherwise, the surface temperature wouldn’t be higher. At some times of day, radiation out observed will differ, unless the high CO2 planet keeps always 1 degree warmer than the low CO2 planet, in which case the radiation out would also always be the same. Lower radiation from the high CO2 planet from 2pm to 6am (cools slower). Higher radiation from 6am to 2pm (warms slower). Early afternoon, about the same.
The planets should maintain the same brightness, but I can’t help thinking there is something I don’t know about involving the rate of emission.
Many above are speaking, of the two planet experiment, maybe without knowing it, in blackbody terms (unrealistic) and forgetting the emissivity for the Stefan-Boltzmann realtion in a more realistic example. Blackbody is very theorlogical whereas a gray body is close to reality, especially if viewed as the sum across all frequencies. Black body uses E = σT^4 where as gray body includes the emissivity of the surfaces as E = εσT^4 with “ε” being the emissivity that you can lookup for a rough estimate for many subtances and surfaces. Just the fact that one planet is 1°C warmer does not immediately imply it emits more without taking into account the planets emmissivity as a whole.
However, in the example given above the warmer planet had more co2 which would increase its emmisivity AND equal absorptivity, and vise versa, in comparson to the other planet which would mean it would be radiating much more that the cooler planet that just the one degree difference implies but it equally would be absorbing more and of an equal amount. The 1 deg difference would not occur in reality, that is a great example of the AGW fallacy.
Like Dr. Feynman said of Q.E.D., it twists your mind a bit, sometime a lot, but that is the way physics is IN REALITY and there is nothing we can do but try to understand it properly and not to twist reality into something that isn’t.
Further, the plant inside the greenhouse will not (in general) reach equilibrium temperature at the same time as the outdoors plant. For example, on the moon, equilibrium temperature is not reached until late afternoon. So, due to lack of convection, the greenhouse plant would take a different amount of time to reach equilibrium. The same should hold, to a lesser degree, on a high CO2 planet. The planet that reaches equilibrium earlier (after noon) will have a higher energy in at that moment than the planet that reaches equilibrium later (sun lower in the sky) and so, the planet with a quicker equilibrium will show a higher temperature.
Note that this logic does not work on the moon, as peak temperature on the moon is much higher, but that is due to the two weeks of sunlight. Peak temperature rise per hour on the moon (late morning) is quite similar to that on Earth. If we had an airless moon that rotated every 24 hours, its peak would be late afternoon, at a lower temperature than Earth. Later equilibrium, lower peak temperature.
Oh, the high CO2 planet should hit equilibrium earlier, based on the statement that the temperature at equilibrium is higher.
“In the second experiment, the two planets again have the same thermal emission temperature. In planet 1, with the doubled CO2, the layer that thermal emission temperature occurs is 460 metres higher than planet 2 and hence the surface temperature on planet 1 is 1.0C higher. Effectively, the lagtime in which the energy enters and then exits planet 1 is a few minutes longer than planet 2. 44.06 hours in planet 1 compared to 43.44 hours in planet 2.”
nice bill.
Jeremy, I take it he’s assuming identical planets, and therefore identical emissivities, otherwise the emitted radiation could vary by a factor of 50 (the polished silver planet versus the carbon planet).
Or you could just change the temperature profile of the planet (hot side versus cold side, or hot regions (black) and cool regioins, while leaving the average temperature unchanged. That can generate huge errors in temperature estimation due to the fourth power in the Stefan-Boltzman law. For example, if you make half the planet absolute zero and double the temperature on the other half of the planet, leaving the average temperature exactly the same, the planet emits eight times more radiant energy. Or you could have a planet where the cities on the night side are lit with 6000K color-temperature LED’s, or one where everything’s been painted with US Navy low-IR emissivity paint, and of course you have Skull Island where there’s a super villain with a telescope aiming a laser at your camera.
Something like this formed the basis of the Gaiaia hypothesis, where the flowers bloom to regulate a planet’s reflectivity and emissivity to create the ideal environment for prancing unicorns.
There are so many complexities to accurate radiative temperature measurement that it boggles the mind. The usual solution is to ask what the temperature actually is and then adjust the sensor’s calibration to match.
For our second experiment we have two thermally stabilized earths, one which has today’s CO2 and one which has 2X today’s level. All other conditions are identical and for some quirk of Id-ian physics, they orbit one right behind the other around the sun such that we can observe them simultaneously on our fancy camera.
I say no change. Both will absorb the same level of upwelling LWIR from the surface. The 2X planet will achieve its quota at a slightly LOWER level. Both planets will experience the SAME quantity of energy being conducted into the atmosphere via molecular collisions. Both planets will handle the absorbed energy through the lapse rate up the atmosphere.
Areas of the tropics experience much more than a doubling of H2O without any dramatic rise in temperature. In fact seasonal temperature change over the tropic oceans is far more stable than seasonal temperature changes over desert regions.
The 3 options for the planet experiment are also all wrong.
The term brightness is too precise here.
The energy balance for both planets must be the same. The wavelength distribution of energy emitted by both planets is different. In other words the IR “colour” is different.
Kev-in-UK says:
January 1, 2011 at 4:44 pm
———
alcuin says:
January 1, 2011 at 4:27 pm
The effective height from which thermal radiation leaves the higher concentration planet will a bit greater than that of the lower concentration planet….
sorry, but why?
the concentration of CO2 in the atmosphere is described as doubled – but there is no mention of any increase in atmospheric depth (all other conditions are described as identical)?
———
The atmospheric depth has not increased but the optical depth at CO2 absorption wavelengths has.
Warning! I am using optical depth as absorption depth. Some specialities use a reversed definition of optical depth
O H Dahlsveen challenges
————
I feel certain that if a “Greenhouse effect” natural, forced or otherwise exists then there MUST be somebody out there who can explain it, calmly and rationally.
———–
There aren’t many out there and you would not be able to recognize one if you saw it, because there are too many junk explanations out there as well.
Jeffs is easy, but I voted on the first experiment on the basis of convection still being in play. Maybe this needs clarifying?
The term brightness is too precise here.
That should read IMprecise.
alcuin says:
January 1, 2011 at 9:47 pm
Sorry, – I meant, why in this case? – the planets are supposed to be identical in all aspects apart from the Co2 conc. This is presented as an ‘ideal’ situation with all other equal properties, so I am assuming the atmospheric ‘envelope’ is the same (density, mass, depth, etc).
40 years of climate science and there is a debate on this simple question?! Even amongst skeptics? I wonder how they would fare over at RC.
I hope we get more of this – we need to finally get the basics right.
Here is my shot at the planet question:
The image of the “warmer” (right) planet appears larger but dimmer. The overall received radiation (brightness) from the two planets nevertheless is identical.
My line of thought: The image of the “warmer” planet gets blurred, because more radiation comes from the absorption bands of CO2 in the (higher) atmosphere and less directly from the surface. If you would use a filter for just the CO2-bandwith, the “cooler” planet would look smaller but brighter while the “warmer” planet would look larger but dimmer.
Firstly Jeff, I strongly disagree wirh the diagram you show from Wikipedia. This shows 195 watts of emission to space from the atmosphere with about 40 watts/sqM from the surface. Have a look at some of the IRIS data from the Nimbus satellite, this shows the emission versus wavenumber (reciprocal of wavelength) as seen from space. These plots are normally shown with Plank black body curve overlays corresponding to different temperatures. The apparent black body temperature of the emission shows where the emission is coming from since it specifies the apparent temperature of the source. What these curves show exceptionally clearly is that the emission to space in the atmospheric window is far higher than 40 watts. Further, the atmospheric emission to space (at the GHG wavelengths) occurs from a region at about 220K (ie: the tropopause) and this is far to cold to emit 195 watts/sqM even if it was an iddeal black body emitting at all wavelengths. In fact the surface emission is more like 170 watts/sqM with the tropopause only emitting about 75 watts/sqM most of which comes from absorption of solar near infra red absorption by water vapour high up in the atmopshere (ie: near the tropopause).
With regard to your second thought experiment the answer is quite clear. You specified the two planets to both have stable temperatures which means they are both in thermal equilibrium. Thermal equilibrium means that energy in equals energy out so both will radiate exactly the same amount of energy HOWEVER, there is a difference. The warmer planet will be radiating slightly more energy per micron over a slightly smaller range of wavelengths. Put another way, the higher green house gas concentrations slightly widens the GHG absorption bands leaving a slightly smaller range of wavelengths over which the planet surface can radiate without impediment. If it radiates the same total amount of energy the energy density at those wavelegnths where it can radiate increases slightly.
In fact even this is a slight simplification. At the GHG wavelengths radiation is not entirely blocked. Surface radiation at those wavelengths is absorbed and replaced by radiation from the tropopause. Since the tropopause is much colder than the surface (except at the poles) the energy radiated at the GHG wavelegnths is considerably reduced. Non the less the overall impact is the same, slightly higher radiation at the non GHG wavelengths coupled with slightly wider GHG absorption bands.
This is the problem with so-called thought experiments: they try to substitute complex intertwined non-linear systems with simplistic ideas, loosing physical reality on the path.
In a word (or two), they are meaningless models, not experiments at all.
I noticed some comments referring to equivalent radiation altitude and the question as to whether it gets higher or lower as GHG concentration rises. The reason for the confusion is because the concept of an equivalent radiation altitude is nonsense and leads to paradoxes. The total energy radiated to space is the sum of the radiation at each wavelength. But the radiation at each wavelength can and does vary widely with wavelength. In the atmospheric window the surface radiates directly to space. At the GHG wavelengths surface energy is absorbed very rapidly by the atmopshere. Radiation at these wavelengths comes from the last 1 optical depth of the atmosphere. But one should remember that the total density of the atmospheric column is extremely high at the GHG wavelengths. For example at present CO2 concentrations at 14 microns the total optical depth is about 3000 abs. Thus only the top 1/3000 of the CO2 column can radiate to space. Where is this? For reasons I wont go into now (its a bit lengthy but simply look at the Nimbus experimental data for confirmation) its roughly at the tropopause or lower stratosphere.
There is no wavelength where the middle tropopause (the level of the so called equivalent radiation altitude) can radiate to space. No radiation emanates to space from the so called equivalent radiation altitude. What can change is not the radiation altitude but rather the proportion which comes from the surface and the proportion coming from the tropopause.
Here is another thought experiment:
I have three electric night storage heaters. The power setting is the same on each so the amount of energy consumed during the ON period ( from say 12:30 am to 7:30 am) is exactly the same for each heater. The observation period is 24 hours.
A) Is left as is.
B) I have removed all the thermal bricks. (No atmosphere)
C) I have added 4 extra bricks from B. (Increase in absorption-emmission of atmosphere)
Questions:
1. Does C) become warmer than A) because of the extra 4 bricks?
No, it just takes slightly longer to warm up and cool down in any 24 hour period. To increase the temperature you would need to increase the energy input.
2. Does B) become cooler than A) because of the lack of bricks?
No, it just becomes unbearably hot very quickly during the ON period and loses all of its heat in the first few minutes of the OFF period. (Like the Moon)
Conclusion:
The so called “Greenhouse Effect” is nothing more than a cheep parlour trick which utilises the complexities of atmospherics and fluid dynamics and most peoples inability to simultaneously consider all of the variables.
The TOA is the true heat emitting surface of the Earth, we are subsurface dwellers. Changing the composition of the atmosphere by trace amounts can do nothing to the over all temperature of the atmosphere. The true determining factor of atmospheric temperature is the mean temperature at equilibrium of its main components O2 and N2 @ 99%.
Adding a trace amount of a substance which can absorb and emit a narrow band of radiation so weak as to be practically undetectable to our human senses, cannot alter the mean temperature of 99% the atmosphere, O2 and N2 at equilibrium.
CO2 does not heat the atmosphere any more than water vapour does. For all the warming effects of water vapour there are equal numbers of cooling effects. The same is the case for CO2, That which absorbs, equally emits, AKA Kirchhoffs Law.
E in = E out.
The Sun drives the global mean temperature and therefore the climate on Earth.
http://www.spinonthat.com/CO2_files/The_Diurnal_Bulge_and_the_Fallacies_of_the_Greenhouse_Effect.html
Jeremy says:
January 1, 2011 at 5:01 pm
Assuming the glass is totally transparent (to all EM radiation) then the trapped air in the box and by consequence the plant itself will be at a higher temperature due to no cooling from convection.
Therefore the plant in the box must be hotter and therefore it must emit more blackbody thermal radiation which will be seen by the camera.
Ah, but would it actually be “seen by the camera?” Imagine two people sleeping on separate beds in a room. One is lying on top of the bed, the other is under a duvet. The one on top is comfortable and cool, the one under the duvet is sweating from the warmth. But which one do you think would look brightest to a heat-sensitive camera looking down on the two beds?
Answer: the head of the person who is sweating under the duvet.
Think about it. {/joking}
The answer to the plant question is simple. With the “perfect greenhouse” (lets through all radiation but doesn’t allow flow of air), the only way for energy to get in and out is via radiation (+ much less conduction which I’m ignoring here).
With the lack of the greenhouse, heat escapes via convection (hot air is taken away by the wind – assuming the wind is cooler!!!!)
The difference in apparent IR temperature is due to the heat flow via convection (hot air) and therefore the greenhouse plant must be warmer.
However, when you talk about two planets, they both have 100% perfect greenhouse enclosures, since no gas escapes via convection beyond the atmosphere. Discounting short term variations due to night/day, the average thermal IR in must equal the average therm IR out for both worlds, and therefore the apparent IR temperature must be the same.
From which we should draw the very useful insight that the world is a 100% perfect greenhouse irrespective of the amount of CO2 and therefore changing CO2 has absolutely no impact on the greenhouse function of the atmosphere!!!
Only two things can effect the energy content of any system in a radiative balance. Either a change in the input, or a change in the “residence time” of some aspect of those energies within the system.” The longer the “residence time” the greater the energy sink capacity. The greater the energy capacity, the longer it takes for any change to manifest. Convection shortens the residence time of energy within a system.
The observed radiation from both planets will be the same (effectively 255 K black-body radiation). However, the question is if the high-concentration planet has a 1 C (as you suggest ‘arbitrarily’) or 3 C (as climate scientists suggest).
Add: surface temperature change.
I dislike gedanken experiments.
Take your car in the sun, and an equivalent volume next to it. Leave a bottle of water in the shade on the seat and a bottle of water on the pavement in the shade next to it. Which bottle of water will be warmer? As cars easily reach 50C when the outside temperature is 30C the answer is obvious. The one inside the car will be reaching 50C and the one in the shade outside 30C. This is real facts.
Unless you mean to stress the fact that plants control their temperature. 😉 .
The answer would still be that the plant inside will be on the higher side of the controlled range than the one outside.
And for those who use the argument “energy in should be energy out”, note that radiant energy changes frequencies interacting with matter and the camera measures infrared only.
For the plant, the un-boxed plant will appear brighter, while the greenhoused plant will appear duller (cooler) through a hazy (indicating warmth) box.
For the planets, both will appear the same.
Jeff Condon. Sorry to say but as the Greenhouse effect is IMPOSSIBLE under the laws of physics- particularly thermodynamics. It is like expecting water to run uphill. Yes, with a pump (work put into the system) but not possible in the real world. Heat ALWAYS moves from a hot body to a cooler body and this also applies when radiation is the transmission medium as well. Those who try to claim otherwise either never studied a physical science degree or have forgotten the relevant lectures in their chemistry or physics degree.
Therefore your post and questions are totally irrelevant to the question of climate and climate change.
A very interesting thought experiment that is bound to create confusion. It creates confusion due to the confusing way that it is expressed.
I think that almost everyone agrees that the ‘energy in’ must equal the ‘energy out’. This is the radiation balance. It applies to all systems in equilibrium [Conservation of energy, 1st Law of Thermodynamics].
But is this the question being asked?
We are told that this magic camera measures something called “radiative emission temperature “. (Ironically, this confusion creator
is preceded by the words “To be clear”).
Now the plant within the greenhouse will be warmer (that’s what greenhouses do) – almost everyone agrees with that. So the plant’s temperature will be higher.
But the first question is “On our camera, will the greenhouse plant image be Brighter..” What does ‘brighter’ mean? Well we are told this means warmer. So, yes – it will be warmer. But then, to this option, is added the additional explanation – ‘More escaping radiation’. Well, no – the radiation out will still equal the radiation in. So the question is impossible to answer correctly! It depends on what the mythical camera is actually claimed to be measuring. Does it measure temperature or radiation energy? The temperature of the plant is higher but the energy escaping the greenhouse is the same as in the no-greenhouse case.
In these thought experiments, the language needs to be precise and unambiguous. This one, like most, is badly phrased.
Making a few assumptions. “Greenhouse” implies that the enclosure is differentially transparent to incoming and outgoing radiation. “Transparent” means it does not reflect all incoming radiation. For the plants I assume“Early afternoon” means the phenomenon is cyclical.
For the planets the problem is different, the camera would integrate, not just across all wavelengths but across all daylight hours because it would see all the daylight hours simultaneously, it is not cyclical.
Plants.
The camera is taking an instantaneous measurement during the course of a cycle.
If the phenomenon is cyclical then instantaneous equilibrium is never reached. Over a number of cycles cyclical equilibrium will eventually be reached – once the system has warmed. Part of the incoming radiation will cause a state change within the system and the energy used to achieve state change is latent, not directly apparent to the observer Whilst it’s warming the system emits less radiation than when it’s warmed because its storing some of it in the form of state change. During that part of the cycle it may appear dimmer in two senses – it’s light temperature is “cooler”, and the amount of outgoing radiation is less, but as energy is added to the system it warms up and the light temperature will brighten to a point where it it is the same as the other system,and then become brighter in terms of light temperature, however it will still be emit less less radiation until it reaches it peak brightness at equilibrium. I believe “brightness”here is intended in the quantitative, sense.
Since it is cyclical, of course, it never reaches instantaneous equilibrium. There is a shift in phase and amplitude (time and quantity) applied to the Greenhouse plant However, twice within each cycle it will achieve a faux equilibrium, in the way a stopped clock tells the right time twice a day. At first the faux equilibria will occur at different times for the greenhouse and non-greenhouse plants, but as the non-reversible state changes are completed by storage of incoming radiation the faux equilibria will be resynchronised. Whist they are in disequilibrium, as Derek says, in the early afternoon the greenhouse plant will be warmer and brighter. But necessarily, during the course of the early afternoon it will also have been dimmer and of equal brightness. It all depends on what you mean by “early” and also how long the experiment has been running.
Planets.
The camera is not taking an instantaneous picture, it is recording a total over all daylight hours. It’s given that the planets are “thermally stabilised” – there are no further irreversible state changes taking place – faux equilibria will be synchronous – and the radiative cycle for both will be in phase. The quantity of radiation from both at all times of day will be the same and will be equal to incoming radiation. The camera will see identical quantities of radiation from bodies which have different colour temperatures, they will appear equally bright.
I like thought experiments.
For 1) I’m going with the same. I’m assuming a decent greenhouse, ie double glazed so the plant will be warmer but the camera’s outside so would be sensing the greenhouse’s emissions, not the plant. I’m thinking the thermal images used by double glazing sales types demonstrate this.
For 2) I’m going with the same as well. But less sure about the camera. If it’s measuring all energy, then energy in should equal energy out but the spectrum would vary between planets.
How about this thought experiment for the two planets. Let them be two balloons.
Both balloons are inflated by a constant flow of air. They are leaky. At some point the inflow of air equals the outflow (equilibrium).
Now I put a few bits of tape on one of the balloons and make it slightly more airtight. (i.e. I add GHG to the second planet.) The air flows in at the same rate. The balloon inflates slightly further than before until once again inflow = outflow.
If the air inside each balloon is taken to represent energy then both balloons emit the same quantity at equilbrium. The slightly improved balloon has more energy in store (a greater volume of air) and thus a higher temperature.
There is something wrong with this picture, but I’m not sure what. Or how to extend it to the plant in the greenhouse.
Kev-in-UK seems to claim that the similarity of the two planets with respect to physical properties would preclude their having different optical depths. However, the postulated difference in the CO2 concentration by itself would result in a difference in the optical depth, as at each level there will be twice as many CO2 molecules interacting with the radiation.
Another commenter challenges the concept of effective height of radiation. The analysis can be done on a wavelength-by-wavelength basis. Of course energy will be somewhat differently distributed among wavelengths on the two planets, but for those wavelengths that interact with the greenhouse gases the planet with the greater concentration will tend to appear slightly larger and dimmer than will the planet with the lesser concentration, and if the energy emitted is integrated over all wavelengths, the energy from the planet with the more concentrated greenhouse gas will come from a somewhat larger, but dimmer atmosphere, while the total energy emitted will be the same.
anna v says:
January 2, 2011 at 3:55 am
…the camera measures infrared…
According to the question, the camera is a cool toy which in this case happens to detect all EM wavelengths with perfect sensitivity. To be clear, the camera integrates to measure the radiative emission temperature of the object.
Seems you have missed the point of the thought experiment, which is to avoid complicating the understanding of the class with real-world complexities. You may be correct in the real world, but you have not helped to clarify that energy balance is the first step necessary for understanding. Almost everyone is jumping in to this question at the wrong point…
For the Two Plant question, the answer is — not enough information. Because Energy is not Temperature, without knowing the energy exchange with the surroundings, you don’t know the Temperature. Example scenarios with different conduction, convection or radiant energy assumptions, including some intentionally absurd to make the point – thinking ‘outside the box’ (chuckle):
* Four plants, two in glass boxes (GB) and two Open. Place all 4 on my kitchen table .. after they come to equilibrium, temperatures are all the same
* Move one each, GB and Open … one to the Oven the other to the Refrigerator … Temperatures will be Different. and it doesn’t matter which one you put into the oven. You only said I had to measure the temperature in the afternoon, but I took some liberties with where.
* Take the remaining two, one Open & one GB, move them to the back yard on a sunny day .. one under a shade tree, the other in open sun. They will probably be different, but we are not sure because we have not specified other environmental conditions. Measuring in the afternoon makes it highly unlikely that the temperatures would be constant over any time period. What if it was cloudy or raining that day? Living plants transpire, so might humidity be a factor? Would it be different if we had 2 rocks?
* Two identical plants at the Florist .. one in the cooler case (meets the glass box requirement) and one displayed on the counter – temperatures different.
* Two aquatic plants at the bottom of a lake or pool … both reach equilibrium with surrounding water – so both same.
* Go to the desert, dig a pit and place the Glass Box plant at the shaded bottom of the pit, place the Open plant on the nearby surface. The Open plant is exposed to a constant breeze and is warmed or cooled to reflect that convective exchange with the air. The GB plant is not exposed to convection with the air because of the GB and the pit, and it is not heated directly by the sun, but it is radiating out to space and loses heat (the water in the pot may even freeze). When I measure the temperatures of the two, they will probably be different because of different energy exchange with their environments.
* Take two identical plants, one in a glass box and one open to the air … run the experiments and measure the temperatures.
“For our experiment assume we have a bolometric camera for measuring emitted thermal radiation as an image. IOW a cool toy which in this case happens to detect all EM wavelengths with perfect sensitivity.”
No other variables are provided or measured, therefore none can be assumed. Both images will look the same. “all EM wavelengths with perfect sensitivity” This is the null hypothesis. However if the images are not the same, then you have a basis for “grants for life”.
It’s always the same missunderstanding of the greenhouse effect:
A greenhouse warms up in the sun, because convection of the heated air is limited to the inside of the greenhouse. Hot air can’t leave the greenhouse. It has nothing to do with the blocking of IR-radiation by the glas of the greenhouse. So during sunshine (“early afternoon”) the greenhouse heats up, and of course you can measure increased thermal radiation with your camera.
The earth is totally different, it can’t be compared with a greenhouse! The heat from the surface is transported to the higher atmosphere through convection. The only thing that could increase the temperature at the surface of the earth is a more dense atmosphere. The composition of the atmosphere has nearly no effect! So the second question only makes sense, if the warmer earth has a more dense atmosphere. Of course in that case the incoming and the outgoing thermal energy will be the same than in the cooler earth (with the less dense atmosphere).
PS: Sorry for the poor english …
For clarity I will post my original simile that Jeff Id refers to.
It can be found in the comments on this thread at Jeff’s blog, the Air Vent.
http://noconsensus.wordpress.com/2010/12/19/fixing-the-basic-agw-calculations/#comment-44043
I wrote there,
” Question, have you ever looked at a thermal camera picture of a greenhouse and it’s surroundings. ?
The greenhouse will be warmer than it’s surroundings, so it will be a brighter image than it’s surroundings.
AGW “theory” says the greenhouse is warmer because it traps radiation,
YET the thermal image will clearly show beyond doubt that the greenhouse IS radiating MORE than it’s surroundings
(with the same solar input).
What does this show. ?
It shows whatever is cooling the surroundings is far more powerful than radiation, AND
that it is not radiation doing most of the cooling (otherwise the higher radiating greenhouse would be cooler than it’s surroundings).
If one opened the doors and windows in the greenhouse, especially on a breezy day,
the greenhouse would soon reduce it’s temperature to that of it’s surroundings.
Logically the temperature difference was removed from the greenhouse to the surroundings or aloft by air.
Air transporting sensible, and latent heat (of water vapourisation), I would suggest.
When the doors and windows are closed it logically follows that the increase in temperature inside the greenhouse is due to
the reduced transport of sensible and latent heat (of water vapourisation) from inside the greenhouse to the surroundings.
Obviously even with the doors and windows of the greenhouse are shut,
some conduction and convection at the greenhouse glass surfaces occurs, and
this explains why a greenhouse remains warmer than it’s surroundings for some time after sunset.
Hopefully this has illustrated that a greenhouse works by reducing conduction and convection,
NOT by “trapping” radiation, as AGW pseudo climate science, “physics”, and “theory” suggest. ”
The pdf that my simile came from can also be found at the GWS forum on this thread.
http://www.globalwarmingskeptics.info/forums/thread-1028.html
yours,
Derek.
Is your camera inside the box or outside the box?
I don’t know how Anthony wants to handle the answer. I can be added to the top post, added as a new post or just left here. I’ll leave it to the moderator of the morning to figure out what is best.
Ok, so the point of this thought experiment was to engage the public in a consideration of the differences between the greenhouse effect and an actual greenhouse. Most here already know that the name itself is a misnomer, but by considering the physics of what is going on we can better understand the argument and better present our opinions on the subject. The majority of the answer below was emailed to Anthony yesterday before he ran the post at WUWT with the note- just to make sure I can’t back out! I wrote the reply in terms of energy Joules, it would be more accurate to say power Joules/Sec or Watts but since the system is stabilized the answer works fine.
I know everyone is wondering what my answers will be to the two greenhouse situations, we’ll see how many will be convinced to change their opinions – or insist that I change mine 😉 . It turns out that both problems are fairly straightforward when considered from an engineering standpoint. In the thermally stabilized systems of the example where temperature is not changing, energy into the system is equal to energy out. We’ll cover the greenhouse vs free air plant situation first. Both plants receive the same energy but the ability to remove heat from the system is limited in the greenhouse plant through convection and evaporation. So in the case of the free air plant, although it is receiving the same energy it has 3 methods of cooling: convection, evaporation and radiation. In the case of the greenhouse, we can consider evaporation and convection negligible so the only option to release the energy is through radiation. Since our camera is only measuring radiation, and since both plants must emit the same energy they receive, the free air plant radiation will sum like this:
Measured EM radiation = Energy in – convection energy – evaporation energy
the greenhouse will sum like this
Measured EM radiation = Energy in – zero convection energy – zero evaporation energy
Therefore the camera will show the greenhouse plant as warmer (brighter) than the free air plant. This holds true even if we include non-zero convection and evaporation for the greenhouse because they are still reduced values requiring a higher EM emission to balance the energy equations.
So now we have the situation where we have two planets, one with more CO2 than the other. We know that CO2 absorbs certain outgoing wavelengths of light. We also know energy in is equal to energy out for both planets. Although this is called the greenhouse effect, it is actually quite different. For both the high and low CO2 planets, the only available cooling mechanism is EM radiation. All the energy coming in has to escape by EM radiation to space, so the equations balance like this.
Measured EM radiation = Energy in
That’s it really. Both planets will measure exactly the same to our camera yet one has a higher surface temperature. The reason this works is that the average energy emission altitude has gone up, allowing a warmer surface yet the net flow is the same.
Caveats: Now I warned that some will get tied in knots over the nuance of this example. There are all kinds of subtleties of the situation which cause minute differences in the planet example. For instance, increasing CO2 will increase the albedo to incoming light, reducing reflected energy and we get a microscopically higher energy in and therefore were our camera of perfect accuracy we could measure a very slightly higher measured radiation from the warmer planet. If this is your explanation, we are in agreement. There are other details as well, but in bulk the answers are A – greenhouse plant is brighter, and B – both are the same.
I read several comments which got the right answer, Carrick was the first to write the correct answer in the comments at tAV, although he kept the answers subtle enough that people had to read it carefully. If you were one who got them both, congratulations. If you are unconvinced by my explanations, ask away and I’ll do my best.
Jeff
[Left in queue for Anthony’s review/editing/comments. Robt]
Sean Houlihane says:
January 2, 2011 at 5:35 am
According to the question, the camera is a cool toy which in this case happens to detect all EM wavelengths with perfect sensitivity.
Please note the exact description
of the camera just before introducing the thought experiment:
For our experiment assume we have a bolometric camera for measuring emitted thermal radiation as an image. IOW a cool toy which in this case happens to detect all EM wavelengths with perfect sensitivity. To be clear, the camera integrates to measure the radiative emission temperature of the object.
Thermal radiation is infrared by definition. So is the “radiative emission” in outside temperatures (0C to 60C).
So saying “energy in equals energy out” ignores the reflected non infrared energies (and energy taken by convection too, which btw is the major contributor in the temperature difference )and cannot be used as an argument.
Since the greenhouse effect clearly works for greenhouses ( nothing to do with C02 of course) the plant in the greenhouse will be hotter so if ( big if) this clever camera is measuring plant temperature it will measure it as hotter by detecting a different spectrum of energy and more energy. However I doubt this is the real thought experiment proposed.. please clarify Jeff.
Back to the question. The two planet problem is a different case since we are now dealing with, I presume a “top of the atmosphere question” where heat in must equal heat out at equilibrium so the fact that the surface( I presume) is 1c warmer is irrelevant.
In the greenhouse case the greenhouse will have a warmer surface than the air at the same level in the open air so even if the clever instrument is measuring this it will still be measuring more radiation and a shorter wavelength spectrum. Of course if the instrument measures the radiation from the greenhouse/ plant for the top of the atmosphere then the scattering effect of the atmosphere will all but dissipate this plant/greenhouse surface difference.
Jeff,
This was a poorly worded thought experiment – you describe your bolometric camera as measuring “thermal” radiation rather than the entire spectrum of energy.
Those who took your words at face value and assumed you were talking about measurement of emitted “thermal energy” will have a different answer from your final answer above.
These parameters are so vague there can be no answer. “thermally stable” is not a measurement but the description of a condition. It says nothing about the comparative conditions of the two plants.
One plant could be in an environment of thermally stability at 100C the other at 30C. Why even mention what time of day it is since apparently both plants live in a permanently stable environment?
You are posing a junk question and so it does not require an answer.
“This was a poorly worded thought experiment ” Sorry, I did my best. Can you explain how your answer differed?
Jeremy says:
January 2, 2011 at 8:11 am
“This was a poorly worded thought experiment ”
Sorry, I did my best. Can you explain how your answer differed and why?
Uhh… Guys?
If done correctly these two experiments would show that denser materials conduct heat better than lighter ones..
Comparable real world experiments were done back in about 1909 by R.W. Wood in England (I think). He found that plants in the closed, CO2 enriched, environment grew better but were not warmer than comparable plants in comparable closed green houses without the additional CO2. Kind of proving the obvious, but nevertheless a real result obtained by doing real work.
Jeff,
In my case it’s not a “thought” experiment because I own an actual greenhouse. The plants are warmer inside than outside, no doubt about it. That’s why I built my greenhouse — to keep plants warm — and it works.
It is stunning to me that the “warmer in the greenhouse” answer in poll question #1 did not get 100% agreement. Maybe I read the question wrong, but I have reread it numerous times.
For your next poll you should ask this crowd whether the Pope is Catholic or not, or where do they think bears defecate.
John of Kent says:
January 2, 2011 at 4:30 am
“Heat ALWAYS moves from a hot body to a cooler body and this also applies when radiation is the transmission medium as well”
This is the ‘simplified’ explanation which works for most things and is taught quite widely.
At the molecular level heat is radiated equally in all directions.
The reason heat moves from hot to cold is that colder objects are radiating less heat in all directions then the warmer objects.
As a simple thought experiment
Put two rotary sprinklers on either end of your yard.
Attach a fire hose to one and a garden hose to the other.
Both rotary sprinklers are pumping out water equally in all directions.
Which way does the water flow?
Observing the flow of mud we’ve just created in our back yard, the water(heat) is flowing from the fire hose side to the garden hose side.
But a close examination of the garden hose sprinkler shows it is spraying water equally in all directions and some of that water is ending up on the fire hose side.
Of course CO2 molecules aren’t connected to the water mains, so to further our thought experiment we replace the sprinkler on the garden hose side with a baby pool.
Eventually the baby pool fills up with water and the water overflows the sides of the pool equally in all directions, some of it flowing back towards the fire hose sprinkler and some flowing out into our neighbors yard.
So called ‘green house gases’ are basically baby pools. They absorb IR energy and then overflow in all directions equally.
Ok, I didn’t consider the experiment correctly, because I answered for both cases that the measured radiation would be the same (not thinking about convection, for example). Of course in a real, practical application, you would adjust the calibration of the bolometer to take into account atmospheric transmission and emissivity, which must be different between the greenhouse and the open plant. I wonder how much of a difference though?
Wayne says:
Jan 1, 2011 at 7:23 pm
“Is this not why black objects in an IR camera show as bright and white objects (of anything with low absorption) show as dark. How can this be if their temperature is the same?”
I don’t know what this refers to since I never made any such statement. If anything, it is the exact opposite to what I said. Also, an infrared camera will only measure a fraction of the e/m spectrum. It is not the perfect device given in the thought experiment and is therefore sensitive to spectral shifts caused by either temperature changes or the wavelength dependence of the emissivity.
“In the second statement you are wrong, you said “The two cases can be at different temperatures for the same radiant intensity, because they will have different emissivities.”. If the have different emissivities they also have different absorptivities and the effects equate and the temperatures MUST BE EQUAL in a given radiative field (in the same room).”
Again, this does not address my statement, which refers to the thought experiment given. In a closed room, at thermal equilibrium, you are correct. But, in the present case the sun is the source of illumination and the objects are in equilibrium with all their surroundings, not just the sun’s surface. However, I thank you for your comments. I could have expressed myself more clearly.
Jeff Id says:
January 2, 2011 at 7:14 am
“We know that CO2 absorbs certain outgoing wavelengths of light. “
Should read, “absorbs and emits“ as do all substances including O2 and N2. So what?
“Both planets will measure exactly the same to our camera yet one has a higher surface temperature. The reason this works is that the average energy emission altitude has gone up, allowing a warmer surface yet the net flow is the same.”
Only in your thought experiment!
Jeff Id, the tail does not wag the dog.
If the emission altitude increases it will be because the incoming energy has increased. Not because there is more CO2 in the atmosphere. See my earlier post which is a proper thought experiment because it contains no ambiguous, arbitrary or false statements.
http://wattsupwiththat.com/2011/01/01/greenhouse-thought-experiment/#comment-564125
Will,
“If the emission altitude increases it will be because the incoming energy has increased.”
Increasing/decreasing incoming energy by itself shouldn’t change the emission altitude. The resident time in the atmosphere for the incoming energy won’t change so the altitude won’t change. If you change the absorptivity of the atmosphere (particularly in the long wave regions of the spectrum) the energy stays resident in the lower atmosphere for a longer time on average before re-emission. This results in the average emission altitude increasing yet the same amount of energy being emitted from the body.
Jeff, wish you would have mentioned that evaporation would also play into your thought experiment. My explanation above no longer apply per se.
Might be fair to rewind YOUR explanation excluding evaporation from the experiments and see if you then find many answers above are correct in that light.
Jeff, you can’t throw excluded constraints in at the explanation phase and have a consistent response. Unknowns that impact the results should be in the list of constraints of the experiment in the beginning, not in the answer you just happen to select.
Pretty lame if you ask me (and one of the reasons I didn’t bother with this “thought experiment–I got to the end and had a sense that all was not complete.)
I think Jeff ID’s answer regarding the planets has an inconsistencey, possibly just a typo. He says:
“Both planets will measure exactly the same to our camera yet one has a higher surface temperature. The reason this works is that the average energy emission altitude has gone up, allowing a warmer surface yet the net flow is the same.”
If the average energy emission altitude increases, the radiating spherical surface increases in area, and if the energy radiated is to be kept the same, the surface must have a slightly lower radiation temperature. The temperature difference would not be large, but the difference would be necessary if the energy is to be balanced.
Wayne,
Certainly if the greenhouse didn’t stop evaporation or convection you would have the same situation as with free air. If it stops convection but not evaporation, the answer is unchanged. I specified the windows as transparent, although you could get into all kinds of creative thinking about which wavelengths it was transparent to.
The problem was simply intended to illustrate that the greenhouse effect doesn’t have a lot in common to do with an actual greenhouse.
Will:
Here’s another illustration of people with basic problems with the correct physical quantities, and the results of that semantic confusion….
Solar irradiance is measured in units of Joules/second (power not energy). It need not change in order to have the surface temperature rise:
Increasing the CO2 in the atmosphere results in more stored thermal energy via an increased surface temperature.
More CO2 causes more thermal energy to be stored. So what you claimed was flat wrong.
RockyRoad:
It’s always great to see people respond to fun little exercises in the spirit of comity in which they were posed. 😉
One correction to my comment to Will, irradiance is measured in power per unit area. No edit button and no preview makes it hard to fix these little warts once you notice them.
alcuin,
DeWitt estimated the effect you describe at tAV. It is one of those minor things I mentioned above.
“Nope. The luminosity is the same. For 2x CO2 the total power will be spread out over a slightly larger surface area so it will be slightly dimmer, not brighter. And an increase in effective altitude of 150 m (Tsurf + 1 C at a lapse rate of ~6.5 K/km) changes the surface area by 0.005%. If planet 1 radiates 240 W/m2 then planet 2 radiates 239.9889235 W/m2 and Teff is lower by 0.003 K. Good luck measuring that.”
RockyRoad
“Jeff, you can’t throw excluded constraints in at the explanation phase and have a consistent response. ”
No idea what you are saying. Quite a few people got the point of the thought experiment as it was, several didn’t. I’ll take full responsibility for anyone’s error who can correctly explain why their assumptions led to correct answers for their assumptions but different from my intent.
“Increasing/decreasing incoming energy by itself shouldn’t change the emission altitude.”
Wrong! Tell that to the thermosphere or rather Diurnal atmospheric bulge which tracks the solar point around the Earth bulging up to an altitude of 600 km at noon and disappearing back below the mesosphere at night.
“The resident time in the atmosphere for the incoming energy won’t change so the altitude won’t change.”
So if I boil a kettle for long enough the water will exceed 100º C will it?
Really Jeff that is sloppy stuff.
“If you change the absorptivity of the atmosphere (particularly in the long wave regions of the spectrum)”
You will also increase the emission. For every action there is a reaction. I have already pointed this out but as with all proponents of the “Greenhouse Effect” you appear to suffer from selective reading.
“the energy stays resident in the lower atmosphere for a longer time on average before re-emission. “
Not if the emission increases with the absorption, which it does according to Kirchhoffs Law. We are talking about radiation at light speed here Jeff.
“This results in the average emission altitude increasing yet the same amount of energy being emitted from the body.”
Does it? How?
Jeff; as I stated before (see my posts timed 2:13 am and 2:30 am) I strongly disagree with your claim the emission altitude increases. If your view is correct, how could one correctly determine from space which was the hotter planet? If it was simply a case of a higher emission altitude then the hotter planet would seem to be slightly larger in diameter and since we both agree the total emission is the same the emission per unit area would appear to be lower leading one to conclude the hotter planet was in fact cooler!
Yes, I agree with DeWitt’s evaluation of the situation. With regard to the practical problem of observing the difference, such considerations did not seem to affect other aspects of the proposed camera; the goal, as I understood it, was to tie down the principles involved. The point is that the radiating surface at the TOA of the 2x CO2 planet would be cooler, not warmer.
Will,
“Wrong! Tell that to the thermosphere or rather Diurnal atmospheric bulge which tracks the solar point around the Earth bulging up to an altitude of 600 km at noon and disappearing back below the mesosphere at night.”
You are correct that warming the air will make it less dense and increase the altitude. This is why emission altitude is sometimes referred to as a pressure rather than distance. We’re talking about an entirely different effect here.
“So if I boil a kettle for long enough the water will exceed 100º C will it?
Really Jeff that is sloppy stuff.”
I can’t follow your point but disparaging remarks won’t help you get answers from me.
“You will also increase the emission. For every action there is a reaction. I have already pointed this out but as with all proponents of the “Greenhouse Effect” you appear to suffer from selective reading.”
Not really. You see we’re discussing the long wave in this case which comes from the planetary surface and atmosphere, very little comes from the sunlight directly. I explained this nuance in my answer above – see caveats. Power in equals power out.
“This results in the average emission altitude increasing yet the same amount of energy being emitted from the body.”
Does it? How?”
What is going on is that the long wave radiation emitted from the atmosphere and surface gets absorbed and re-emitted over and over as the energy travels from ground to space. By adding more absorbing material the probability of striking an absorbing molecule goes up, shortening the mean path length of each photon. This also increases the probability that a photon will be absorbed in the upper atmosphere before escaping, increasing the mean height of emission.
For those interested I would like to give an example of why I get hot and bothered about over simplified hypothetical situations. This is a real actual question from an end of year 12 physics exam (we used to call it matriculation) here in Melbourne (some years ago). (this was the exam that determined entry to university!).
A space craft is in a stable circular orbit around the earth. It fires its rocket for a short time so as to increase its speed. After this firing the space craft is in a new circular orbit. Is the new orbit (1) of greater radius (2) the same radius (3) smaller radius.
The answer marked correct was (3). The reasoning given by the science teachers association (who set the exam) was that the space craft speed had been increased and higher speed corresponds to a smaller orbit.
Alternative point of view, increasing the speed increases the total energy of the space craft (same potential energy but more kinetic energy) therefore after the burn it must be in a higher energy orbit and that means a larger circular orbital radius.
That would seem to be a paradox. The true answer is that the question makes an invalid assumption/claim. After the burn the space craft is at the perigee of an elliptical orbit not a circular one. At the perigee the speed is higher than for a circular orbit of the same radius but the overall energy of the orbit is greater. The answer marked as correct was in fact wrong and worse, based on a fundamental misunderstaning of what is going on.
So did it matter? If you were a student wanting to get into you first choice course at university yes it certainly did. In this case, understanding why “equivalent radiation altitude” is wrong and what the true picture is, is fundamental to an understanding of the action of GHG in the atmosphere. If one wants to understand what is really going on and thus be able to make correct decisions it matters even more now.
michael hammer says:
January 2, 2011 at 12:20 pm
I left an explanation of why it happens for will. The mean path length and probability of photons being absorbed changes. The probability of escape for each emitted photon in a higher CO2 atmosphere goes down causing the mean emission altitude to shift to a higher position in the atmosphere. The whole warming effect is just this delay in the power flow from absorption to emission causing an energy buildup.
I don’t really enjoy talking much about it because people then assume I’m a big supporter of AGW extremism. I am not, but the basics are real.
alcuin,
“The point is that the radiating surface at the TOA of the 2x CO2 planet would be cooler, not warmer.”
I avoided these minuscule effects because there are several. In the caveats above, would a 2X C02 planet absorb enough extra incoming sunlight to offset the change in emission area? The effects are both small but of opposite sign.
Guess the little polar bear cub was spot on, wasn’t he?
But I’m not sure about the reasoning:
I followed the AGW narrative of radiation and “back radiation” or rather “absorption of longwave radiation from earth’s surface by way too many CO2 molecules in the atmosphere leads to dangerous back emission of heat towards the earth – thus killing cute little polar bears”. I’m still not sure about the exact physical processes involved. Can we really say absorption = emission, as proposed above? Which I also had in mind when I wrote:
Shouldn’t a picture in the CO2 absorption bandwidth be black?
Jeff, you said:
But what would the actual image from your camera look like? Brighter at the surface area due to 1 degree higher surface temp and on top a little larger due to higher emission altitude? This would indicate an increase in the net flow. Which can’t be happening. (And that’s why I said the image must be dimmer when it is larger.)
So maybe the surface is not warming but cooling when the radiating surface area is increased? Or when the surface IS warming than the (higher) atmosphere must be cooling? What then about back radiation?
Something needs clarification here, don’t you think?
As I said before: we need to finally get the basics right. Thanks for bringing it up.
Jeff Id says:
January 2, 2011 at 12:03 pm
alcuin,
DeWitt estimated the effect you describe at tAV. It is one of those minor things I mentioned above.
“Nope. The luminosity is the same. For 2x CO2 the total power will be spread out over a slightly larger surface area so it will be slightly dimmer, not brighter. And an increase in effective altitude of 150 m (Tsurf + 1 C at a lapse rate of ~6.5 K/km) changes the surface area by 0.005%. If planet 1 radiates 240 W/m2 then planet 2 radiates 239.9889235 W/m2 and Teff is lower by 0.003 K. Good luck measuring that.”
I was not aware that gases formed and presented two dimensional heat emitting surfaces around objects such as planets.
A 150 m increase in effective emission altitude would be 150 cubic meters not square meters. Looks like someone should take another look at their numbers.
Gases emit in three dimensions not two.
Jeff Id says:
January 2, 2011 at 11:35 am
Wayne,
Certainly if the greenhouse didn’t stop evaporation or convection you would have the same situation as with free air. If it stops convection but not evaporation, the answer is unchanged. I specified the windows as transparent, although you could get into all kinds of creative thinking about which wavelengths it was transparent to.
The problem was simply intended to illustrate that the greenhouse effect doesn’t have a lot in common to do with an actual greenhouse.
Jeff,
Thanks for the reply. I see your point clearly now that you point to the convection/evaporation of the open plant to the one in the enclosure. I agree. Water absorbs a great amount of energy in that process. Touché.
However, still don’t think the 1ºC difference due to co2 is possible as viewed and measured by the TOTAL E/M bolometric camera. That’s not the physics I know for you then would have to get into exactly where vertically you measured that 1ºC difference and at any point you pick will have the problem of what exactly is happening to the additional (1ºC worth) or energy that is being emitted, therefore cooling. A folly it seems to me.
Jeff, your views make sense
michael hammer says:
January 2, 2011 at 12:20 pm
Given the description, we can see the surface of the planet (sometimes) in visible light. Recall that the two planets were just Earth with different amounts of CO2 and economic vitality.
So, build a satellite (thought experiment – you can afford the satellite) with both a radar/lidar rangefinder that measures the height above the planet. Add a high resolution IR sensitive LWIR sensor that determines the angle of the IR horizon (heck, toss in one that measures the visible horizon too). From those you can figure out the emission altitude and some (much?) of the profile of where the emissions really come from.
I don’t recall when or how we figured out that Venus’ surface was hot. Why I was a kid people were hoping it would be somewhat warmer and truly Earth’s sister planet. That wound up being the case at a atmospheric pressure of about 1 bar, but sadly that was still awfully high above the surface.
OTOH, landing on and leaving the Moon, Mars, and many other interesting places is pretty easy. Well, accepting that we can’t make a manned round trip to the Moon any more. Pity.
Michael, why should someone be able to correctly determine from space the surface temperature of any planet that has surface? I’ll grant you it is a desirable feature in a well configured planet that is inexpensive to explore, but there’s no astronomical law that requires it.
Mike D says, January 2, 2011 at 9:14 am:
“It is stunning to me that the “warmer in the greenhouse” answer in poll question #1 did not get 100% agreement. Maybe I read the question wrong, but I have reread it numerous times.
For your next poll you should ask this crowd whether the Pope is Catholic or not, or where do they think bears defecate.”
======================================
The info I read was as follows:
“We have two plants, one is contained in a transparent box (greenhouse) the other in open air, both thermally stable (temperature isn’t changing). We take an image of the two plants in the early afternoon on our fancy camera, what do you find in the image?”
Please note that the two plants are both thermally stable (temperature isn’t changing). The best place I can think of where a plant can be thermally stable with unchanging temperature late in the afternoon whilst inside a green house in the Northern Hemisphere is north of the polar circle, say at this time of the year. Both the plant inside and the one outside will be frozen stiff of course but they will still radiate equally (the same) in the IR spectrum. The curious thing here is that there is no sunshine providing shortwave radiation. But as we all know anything with a temperature above absolute zero (0 °K) must radiate –etcetera, etcetera.
As for answers to what seems to interrest you at the moment:
It is great possibility that the pope is Catholic, but he could of course be an atheist. Only he knows. Where bears defecate depends greatly on where they are at the time but generally brown- and grizzly bears do it mostly in the forest whilst polar bears may decide to fertilize a couple of plants whether inside or outside of any green house erected in their Arctic habitat.
Now then, which type of bear did you have in mind?
Well, happily it looks like nobody has so far asserted that the greenhouse temperature is a function of the thermostat in the greenhouse, and that the owners are either trying to grow rare arctic flowers or mesquite bushes from the Mojave desert.
I didn’t read all the comments, so forgive me if this has been coveremy comments are repetitive.
The thought experiments make perfect sense, given the assumptions; i.e., that other variables, like clouds and increased evaporation, aren’t involved. The problem with the examples is that they have nothing to do with the real world.
This recent post does a good job explaining the problem with thought experiments that don’t deal with all the relevant variables: http://wattsupwiththat.com/2010/12/28/simple-physics-in-reality-my-feather-blew-up-into-a-tree/#more-30345
Willis Eschenbach has some great comments sabout non-linearity somewhere on this site which also speak to this issue.
Jeff,
Thanks for the reply. I explained my reasoning in my original posts. I am sorry but i find your logic is rather wooly and the whole thought experiment just sloppily worded. There are references to “thermal” radiation throughout your original thought experiment and yet you suddenly jump to discussion of total energy balance and total radiation in your answer. I get the impression that I am not the only physicist who found this wooly. I get the distinct impression that many here do not understand the theory of radiative physics and I’d recommend Liou’s excellent textbook – An Introduction to Atmospheric Radiation.
Ok Jeff Id, I know the happenings in the “Greenhouse theory” are different to what happens in a real green house. If the “Greenhouse theory” is a misnomer then that is because it has gone through some modernizations and alterations (as the science has changed) since it was first thought up and named way back in the early 19th century. What I would like to know is:
1) How can 99% of the Atmosphere be warmed by long wave “back radiation” from a trace gas when Nitrogen, Oxygen and argon are all transparent to the stuff?
2) Is it not more likely that since the “bottom” of the Atmosphere and the “top” of the Earth’s surface are in contact with each other – at all points – heat transfer is taking place first by conduction then convection (evaporation) rather than radiation?
3) Why is it warmer over the top of – than it is below a flame? If heat is radiated should the temperature not be distributed equally in all directions? If it is warmer above the flame does that not point to the fact that convection is a much better form of energy transport than radiation?
4) Why is “back radiation” always depicted as being “one directional” only? (The full force, say 333 W/m² is always pointed towards the surface but there must also be 333 W/m² emitted in all other directions i.e. towards space)
5) How do “Greenhouse gases” stop or delay convection or radiation in order to warm the Atmosphere or as it is called “trap heat”?
6) And lastly – for now – How can radiation which is an energy transport system warm the planet any more than conduction or convection can?
Let the “Greenhouse Theory” go and consider instead the fact that air movements on this planet are mainly horizontal and for that reason alone adiabatic cooling (and warming) take a lot longer than if they were vertical.
Mike D says, January 2, 2011 at 9:14 am:
“It is stunning to me that the “warmer in the greenhouse” answer in poll question #1 did not get 100% agreement. Maybe I read the question wrong, but I have reread it numerous times.
For your next poll you should ask this crowd whether the Pope is Catholic or not, or where do they think bears defecate.”
Because the measurement is being taken outside the box and is measuring ALL radiation energy, absorbed or transmitted or reflected and including all frequencies within it’s view, that is, you are more measuring the box than the plant inside the box. At equal stable temperatures with the same radiation falling on both cases the outgoing radiation measured *will* be identical, Kirchhoff’s law. (but not if evaporation / transpiration / convection is also in play as Jeff used in his answer, then the two cases are not really stable and identical.)
You are more thinking of a *thermometer within* the box and not what the thought experiment laid out. Yes, the thermometer inside would show warmer, duh.
Derek’s assertion doesn’t follow the thought experiment either for he speaks of the brightness of the plant in the image where as the experiment was using a total E/M bolometric camera integrated perfectly to a single temperature, not simply a portion of the image.
BTW: the crude remarks to marginalize many here are not needed from your shallow and incorrect viewpoint.
You appear to forget biotic mineralization on your two planets. Plants use photons to fix carbon and excrete IR. The end of plant life is either recycling or mineralization. Mineralization is also there as plants enter the soil and are converted to coal.methane or in the oceans becoming oil/methane.
The amount of mineralization, or ‘bottled sunshine’, is proportional to biotic productivity, which is dependent on plant growth conditions. All thing being equal, plants grown in high CO2 will grow faster, grow in more marginal niches and be a better source of mineralization. So the planet with with more CO2 will have more plant life, have more complex biomaterial (which is just another form of informatio) and so will emit less energy.
in response to Jeff at 12:49 and Rick Werne at 12:20;
Jeff I still have to disagree with the reason you give. Sure if the concentration of CO2 increases the top 1 absorbance will be at a higher altitude. But that only means the 14 micron emission comes from a slightly higher altitude. It does not mean there is a mean emission altitude for the Earth which goes up. In fact the Nimbus data shows clearly that there are 3 main emission regions. In the atmospheric window the emission is from the surface. At the CO2 line around 14 micron it is from a region at 220K which means the tropopause (no other point in the atmosphere is as cold as that). For the ozone emission at 10 microns it is coming from high in the stratosphere at a temperature somewhat higher than 220K. For the water bands below 8 microns the emission is again from a region at 220K which is again the tropopause. The picture for the region above 16 microns is a bit more complex. There are a large numberof narrow lines in this region and the interferometer on Nimbus did not have the resolution to separate them. The result is that it gives a smeared picture intermediate between the surface and the tropopause but with a very large amount of noise (which is how we can be sure it is not emission from some intermediate temperature).
Thus ignoring for the moment the ozone emission band, emission to space comes from the surface and the tropopause with a neglegible amount from anywhere inbetween. Changing green hosue gas concentration changes the line widths and thus changes slightly the fraction from the surface and the fraction from the tropopause. This is absolutely different from saying there is an equivalent emission altitiude which moves up or down. Claiming an equivalent emission altitude suggests that GHG mediation of emission to space is far greater than it actually is and this leads to an overestimation of the impact of rising GHG concentrations.
Because the concentration of CO2 in absorbance is so high, doubling it makes neglegible change even to the 14 micron emission altitude. I realise you will probably diagree with this statement and I would love to debate it with you and show you my data backing up this claim but it is too lengthy to go into here. By the way, paraxodically, one could argue that if the CO2 emission altitude did rise appreciably it would move into the stratosphere where the temperature is higher so emission to space at 14 microns would increase not decrease reducing the green house effect. Actually, more likely it would simply mean the region at 220K at around the tropopause got a bit bigger.
Rick, in answer to your comment – of course there is no law stating we must be able to measure the temperature of a planet from space but the reality is that we can do so and the concept of an equivalent emission altitude would mean that should not be possible. The way we measure it is to look at a wavelength where the surface can radiate to space and measure the emission intensity at that wavelength. Again, look at the Nimbus data (I tried to incude it here but the system does not allow incusion of images try looking for “CDFS lecture 3” on the web a power point file look at slide 4) and one can clearly see the difference in surface emission between a view over the Sahara, the Medditeranean and the south pole yet the CO2 emission temperature is constant at 220K. Incidentally, the south pole plot shows that the emission at the CO2 line is greater than it would be from the surface which means at the pole, the impact of GHG’s is to cool the surface not warm it. A minor issue but interesting non the less.
Jeff Id says:
January 2, 2011 at 12:49 pm
michael hammer says:
January 2, 2011 at 12:20 pm
I left an explanation of why it happens for will. The mean path length and probability of photons being absorbed changes. The probability of escape for each emitted photon in a higher CO2 atmosphere goes down causing the mean emission altitude to shift to a higher position in the atmosphere. The whole warming effect is just this delay in the power flow from absorption to emission causing an energy buildup.
I don’t really enjoy talking much about it because people then assume I’m a big supporter of AGW extremism. I am not, but the basics are real.
##########
jeff the thing that astounds me is this. people have a choice.
1. accept the basics and focus on all the uncertainty WITHIN climate science.
2. deny the basics and get pwnd in any technical discussion of the basics.
ourson polaire,
The image would be larger and slightly dimmer so no physics are violated. As DeWitt showed above though it is actually within very small fractions of the being perfectly equal.
Will,
“I was not aware that gases formed and presented two dimensional heat emitting surfaces around objects such as planets.”
You should read up on basic greenhouse theory. The atmosphere has a non zero mean emission altitude, again not controversial physics. Due to the random emission direction, the intensity is uniform as though it were a disk facing you. Look up Lambertian emitter for that. You can however think of it as a shell of slightly larger diameter than the planet.
“A 150 m increase in effective emission altitude would be 150 cubic meters not square meters. Looks like someone should take another look at their numbers.”
Will that really just doesn’t make any sense.
Jeremy,
I’m sorry if you found the wording difficult. My day job is an optical engineer so the concepts seemed clear to me but perhaps I’m too familiar. It would have been a complete surprise though if some didn’t find problems with the wording. It is the nature of blogs.
There is no need for thought experiments here, we already have detailed pressure and temperature data for two real planets with greatly differing concentrations of atmospheric carbon dioxide: Venus and Earth. And that data shows that, over the range of pressures in the Earth atmosphere, there is no difference due to their difference in carbon dioxide. There is no “greenhouse effect” at all, in either atmosphere:
Venus: No Greenhouse Effect
Jeff; I note your commment your day job is as an optical engineer. I do research for a multinational spectroscopy company so we should share the lingo – makes things much simpler. A very interesting starting point for an analysis of GHG issues:- it is abundantly documented that the temperature falls with increasing altitude throughput the troposphere reaching a minimum of about 220K at the tropopause. Thereafter the temperature rises again with increasing altitude. This is the case not just in one region of Earth but all over the planet and over the full year.
So the tropopause is a cold region completely surrounded by warmer regions. How is this possible (given the 2nd law of thermodynamics) and what are the implications for the theory of global warming. The starting point is incontrovertible and the implications are profound.
Sorry, that link has a missing colon; the proper link is:
Venus: No Greenhouse Effect
Michael Hammer
“Because the concentration of CO2 in absorbance is so high, doubling it makes neglegible change even to the 14 micron emission altitude. I realise you will probably diagree with this statement and I would love to debate it with you and show you my data backing up this claim but it is too lengthy to go into here.”
Send it by email and I’ll post it at tAV, I hold no opinion as to whether the difference is negligible as that science is still contestable. The only point I make is that the effect exists.
Judith Curry did a post on that recently where they used the 3.7 watts/meter^2 forcing number. I expressed doubts about the accuracy of that number and asked for explanations. Turns out that most of it was pretty weak. The 3.7 W/m^2 is required for the 1.2C. There are a few basic assumptions which sound straightforward enough that one can accept the number but I’m not sold yet. IMO, the jury is still out.
Glass blocks IR so the camera won’t see the plant at all inside the greenhouse but rather it will only image the glass walls and ceiling like a glowing red building with opaque walls. That half of the though experiment is meaningless.
The two planets are more interesting. They will appear to be identical to an IR camera that integrates all thermal radiation. However if you look at the IR spectrum you’ll see a difference in energy distribution by frequency. The planet with more CO2 will have a deficit of radiation at CO2 characteristic absorption wavelengths and that missing energy in those bands will show up as increased energy in all the other bands.
The glass of the greenhouse and the CO2 in the atmosphere are equivalent to lens filters. The camera only sees what the filter allows it to see.
Michael Hammer,
My previous comment was designed around doubling of CO2.
you wrote:
“But that only means the 14 micron emission comes from a slightly higher altitude. It does not mean there is a mean emission altitude for the Earth which goes up.”
Of course any increase in height for any band, increases the average emission height.
also:
“Thereafter the temperature rises again with increasing altitude. ”
I find this line of thought interesting as well but often it runs into more complex discussions that are unsolvable. A similar situation occurs in geologic borehole proxies where heat is apparently removed from subsurface levels.
“jeff the thing that astounds me is this. people have a choice.
1. accept the basics and focus on all the uncertainty WITHIN climate science.
2. deny the basics and get pwnd in any technical discussion of the basics.”
Can you please, once and for all, tell us all just why you have the “truth” here? Is it your background? Is it your religion? What, Oracle?
Will one of the oracles please answer Huffman?
Actually, Oracle Mosher should probably respond to Huffman, since he has apparently assumed the position of the final arbitrer for the physics of AGW. God help us!
Jeff; re your 6:20 posting, I would love to send you the analysis by email but I don’t have an email address. Anthony you have my email address from these posts. Could you please send it to Jeff so he can give me a return address to write to.
Thanks
By definition anything that is warmer will transmit more EM energy. A greenhouse causes the inside to be warmer so it will transmit more EM energy.
A planet that is 1C warmer (regardless of the cause) will transmit more energy. If the planet loses more energy than it receives, the excess energy loss will result in cooling until the equilibrium is reached.
Therefore the warmer planet will show as warmer until such a time that it is not warmer. Since the Earth does not instantly reach equilibrium it can lose more energy than it receives for a while, then it will cool down and likely then receive more energy than it gains until it warms up.
Any system with a lag will have such overshoot unless the PID’s are finely tuned.
John Kehr
The Inconvenient Skeptic
Burned by the lack of preview…
Since the Earth does not instantly reach equilibrium it can lose more energy than it receives for a while, then it will cool down and likely then receive more energy than it loses until it warms up.
For a tongue in cheek explanation in plain English, both the physics and the economics are explained (in complete agreement with Jeff) here. I am not responsible for coffee, beer, or anything else that gets sprayed on keyboards at the punch line:
http://knowledgedrift.wordpress.com/climate-humour-page-the-climatologist-and-follow-the-money-series/the-physicist-and-the-climatologist-follow-the-money/
On a more serious note, not only is Jeff’s explanation accurate, consider the transition phase from doubling CO2 instantly to a new equilibrium. The physics would suggest the camera would see a ripple, or shimmer, followed by a new steady state identical to before doubling. Jeff and the physics are backed up by the data. See the paper by Beenstock and Reingewertz that looks at the climate data and concludes, yup, that’s what happens:
http://wattsupwiththat.com/2010/02/14/new-paper-on/
jae.
if you’re looking for oracles, seek no further than mr Huffman.
http://stores.lulu.com/hdhsciences
if you want to understand the greenhouse effect on Venus, I’ll suggest other sources
http://www.spacetoday.org/SolSys/Venus/VenusGreenhouse.html
http://www.astronomynotes.com/solarsys/s9.htm
and even carl sagan was wrong above venus. so I guess its ok for you to be wrong as well.
http://www.aip.org/history/climate/Venus.htm
You or anyone else is welcomed to take the facts as we know them about venusian atmopshere and predict what the temperature at the surface will be. If your solution ignores every bit of radiative physics we know to be true AND you get the right answer, that would be something to write home about.
So, Jae, fire up Your physics, and given the constituients of the venusian atmosphere
predict the temperature you would see?
this might help
http://www.imcce.fr/vt2004/en/fiches/fiche_n13_eng.html
Jeff Id says:
January 2, 2011 at 5:18 pm
Will,
“I was not aware that gases formed and presented two dimensional heat emitting surfaces around objects such as planets.”
You should read up on basic greenhouse theory. The atmosphere has a non zero mean emission altitude, again not controversial physics. Due to the random emission direction, the intensity is uniform as though it were a disk facing you. Look up Lambertian emitter for that. You can however think of it as a shell of slightly larger diameter than the planet.
I well aware of what the basic greenhouse theory states Jeff, thank you. That is why I am here pointing out the fallacies in that theory. Points that many of which you have had to completely ignore in order to continue on with your false assertions.
You say: “The atmosphere has a non zero mean emission altitude, again not controversial physics.”
Meaning that the altitude at which the atmosphere emits radiation is not zero. Well a child could make that point so I guess you are right, well done to you. And yes that surely is not controversial physics is it, right again Jeff!
Actually the atmosphere begins to emit IR to space to any appreciable degree, in the troposphere at cloud level, around 500 meters. Which is why clouds generally begin to form at that hight. That is not controversial physics either. The atmosphere continues to emit IR all the way up to the point at which it no longer exists at all, at between 600 and 1000 kilometres.
So you see the Earths atmosphere is not a Stefan/Boltzmann two dimensional black body Lambertian radiating disk. Not even close.
That is why some of us are saying the “Greenhouse Effect” is not basic physics, it is bogus physics.
So the effective emission altitude is merely an incorrect calculation which falsely depicts the atmosphere as a two dimensional black body disk. But the Earth is neither a Lamertian scatterer nor is it a Lambertian emitter. A Lambertian surface is by definition a two dimensional black body disc. Spheres are not Lambertian surfaces. Particularly spheres that are strongly light from one side by bright stars. And particularly Spheres which posses thick gaseous atmospheres which above a few hundred meters, emit radiation in three dimensions (four if you include time).
You appear to be confusing/mixing emission with albido with regards to Lambertian surfaces. The Atmosphere most certainly does not have a uniform emission intensity. That is why it has a 600 km high bulge on one side the equivalent of 25% of the Earths surface area which tracks the solar point around the Earth and disappears below the Mesosphere at 100 km on the dark side. You know Jeff, its called night and day. Its not controversial physics.
See here: http://www.spinonthat.com/CO2_files/The_Diurnal_Bulge_and_the_Fallacies_of_the_Greenhouse_Effect.html
and here:
http://articles.adsabs.harvard.edu/cgi-bin/nph-iarticle_query?bibcode=1966SAOSR.207…..J&db_key=AST&page_ind=0&plate_select=NO&data_type=GIF&type=SCREEN_GIF&classic=YES
This is why the Earth is not a Stefan/Boltzmann two dimensional black body radiator nor a Lamertian emitting (or scattering) surface.
The so called “Greenhouse Effect” is based on arbitrary calculations, ambiguous assumptions and false science. Is it really any wonder that it can only exist in half baked thought experiments and cannot be physically demonstrated in reality?
Physics that cannot be demonstrated in reality by experiment, is nothing more than statistics.
Statistically speaking, “the tail wags the dog” is a valid statement.
A thought experiment is useful if and only if, it transposes into reality.
Derek’s original experiment is very useful because it clearly can be tested and shown to be correct.
My thought experiment likewise, can be tested in the real world and also shown to be correct. http://wattsupwiththat.com/2011/01/01/greenhouse-thought-experiment/#comment-564125
These thought experiments are useful because they demonstrate physical reality without the need to physically carry out the experiments.
Jeff’s “thought experiment” however can never be show to be correct. Which is why, I suspect, he has tagged it on to Derek’s very valid, legitimate thought experiment.
A thought experiment which can never be tested and verified in reality is utterly useless and can only ever have one purpose. To deceive.
Doubling CO2 does not double the greenhbouse effect. Heinz Hug shows that it
only increases 0.17 %.
Will,
I cannot follow any of what you have written other than you are not happy. Typically, I can follow a science discussion fairly well so hmm…. I’ve also taken a few minutes to check your internet work linked above and can’t follow that either. Weird statements about photons all followed by declarations of certainty that AGW is impossible.
If you have a question, I’m happy to try and answer but this discussion isn’t going anywhere.
Will is correct, IMHO. Heat Storage is the key. Rocks radiate back and forth, too, but we don’t call that a “greenhouse effect.”
It’s interesting watching people DEMAND that they are correct on issues that are as clear as mud!
Jae,
“Will is correct”
You’re kidding right? His posts are unintelligible.
Anthony,
Thanks for the opportunity on this post, I hope that some enjoyed it. It was my hope that people would understand this as the simple experiment it is and perhaps be better able to express the true problems with AGW theory – of which there are many.
Everyone,
The true AGW discussion going on in publication revolves around the magnitude rather than these simple points. Keep in mind that with all that said, the magnitude of warming by CO2 can still be undetectably small and whatever magnitude is very possibly below natural variability. I’ve seen no proof that it isn’t and the more I read the closer my position drifts to Lindzen. In my opinion, it makes for a better discussion when people grasp the basics but as has been clearly pointed out, there will always be those who don’t/won’t get it.
Jeff Id says:
January 3, 2011 at 7:20 am
Will,
I cannot follow any of what you have written other than you are not happy. Typically, I can follow a science discussion fairly well so hmm…. I’ve also taken a few minutes to check your internet work linked above and can’t follow that either. Weird statements about photons all followed by declarations of certainty that AGW is impossible.
If you have a question, I’m happy to try and answer but this discussion isn’t going anywhere.
Jeff,
I have given a clear example of a thought experiment which can be readily reproduced. and proves that the so called “Greenhouse Effect” is a false perpetual motion machine that does not occur in the real world.
Once again here: http://wattsupwiththat.com/2011/01/01/greenhouse-thought-experiment/#comment-564125
Simply, adding more bricks to a night storage heater does not increase the temperature, in fact the overall effect is a reduction in temperature because the energy is more widely transmitted and the “negative feedbacks” dominate. Still you don’t have to take me at my word you can do the experiment in the real world and see for yourself.
My Diurnal Bulge paper has been reviewed by thousands of individuals with scientific backgrounds and some have even stated that it makes more sense to them than anything they have ever read on the subject. It got two wow’s from a member of Mensa, though not being a name dropper I won’t mention it here.
One very well known IPCC reviewer (again no names) called it “another attack on the Steffan/Boltzmann law”, but I corrected him as per my above posts on that subject and he quickly fell silent on the issue.
And finally I have reproducible real world experimental evidence which back up the fact that increasing CO2 does not cause an increases in temperature.
It is no surprise to me that you feel this discussion is going nowhere Jeff!
As far as any questions I may want to ask you Jeff, I can’t think of anything in particular but thanks all the same.
Jeff: I agree with everything in your thought experiments, EXCEPT that I don’t think the temperature goes up significantly. Which you almost said in your last post. The energized CO2 almost instantaneously transfers energy to other air molecules, warming them. They then rise. More CO2/more radiation , more rising molecules. PLUS all of Lindzen’s concepts.
I’ve bucked many a hay bale on the Great Plains (Eastern CO, to be exact), and I’m very familiar with the weather patterns out there. The cool dry artic air plays tag with the humid air from the Gulf all year long, sometimes creating violent storms. But often the humid air just gradually replaces the dry air without even causing clouds. So some clear July days have high humidity and some have low humidity, even when the soil is as dry as toast. But despite the wide variations in humidity on these clear days, temperatures are NOT hotter on the humid days. Water vapor is the strongest greenhouse gas. If the greenhouse effect were all that important, it seems to me that one should see hotter weather on humid days.
“EXCEPT that I don’t think the temperature goes up significantly. Which you almost said in your last post.”
I certainly wouldn’t imply that on purpose. I’ve got no idea if the models are right but from my limited experience in reading the equations and code, they look like magic to me.
wayne says: Mike D. … [your] crude remarks to marginalize many here are not needed from your shallow and incorrect viewpoint.
Whoops. My bad. Sorry, your worship. Far be it for me to disturb majestic thinking.
The “thought experiment” proposed to measure (or sense) the warmth (escaping thermal radiation) of two plants, one in a greenhouse and one “in open air”. The assumption is that both plants are “thermally stable (temperature isn’t changing)”.
Pardon my ignorance, doctor, but if an object is thermically stable, doesn’t that mean it radiates no energy at all? Other than fissioning (matter become energy) atoms, what undisturbed matter radiates energy but doesn’t change temperature?
Of course, I am not a physicist, so I have no clue. I do know that greenhouses trap heat, but that’s just my empirical finding, based on my lying eyes, and not on theoretical physics about which I know less than nothing. My tiny brain can’t grasp the problem.
I see that Mr. Id has now given his answer, “In … thermally stabilized systems … energy into the system is equal to energy out.” Pretty much what I said above. He goes on, “Therefore the camera will show the greenhouse plant as warmer (brighter) than the free air plant.”
Stunning. The plant in the greenhouse will be warmer. Who’d a thunk it? Besides me, I mean, and 29.8% of the poll takers. The other 70.2% think the plant in the greenhouse would be the same temperature or cooler. And I’m the shallow and incorrect one?
Mea culpa. I bow down to the giants of theoretical physics. I’m just a hick from the sticks who happens to own and operate a greenhouse. I think it’s warmer in there, but what do I know?
Will, how is your thought experiment different from the following (which mostly uses your words) …
I have three IDENTICAL HOMES. The FURNACE is the same on each so the amount of energy consumed during the ON period ( from say 12:30 am to 7:30 am) is exactly the same for each FURNACE. The observation period is 24 hours.
A) Is left as is.
B) I have removed all the INSULATION
C) I have added EXTRA INSULATION.
1. Does C) become warmer than A) because of the extra INSULATION?
You seem to answer “no”, but I know *I* would rather live in (C). 24/7 it will be warmer than (A) which will be warmer than (B).
“It got two wow’s from a member of Mensa, though not being a name dropper I won’t mention it here. “
And your point is … ? Mensa members are “from 2 to more than 100 … preschoolers to high school dropouts to people with multiple doctorates … professors and truck drivers, scientists and firefighters, computer programmers and farmers, artists, military people, musicians, laborers, police officers, glassblowers….”
High IQ does not in any way correlate to an understanding of GHGs.
Tim
You have simply exchanged a transmitting substance for a non-transmitting/insulating substance. And then try to imply that it is the same experiment.
Am I not supposed to notice that “clever” slight of hand?
Or are you one of those people who believe CO2 is a heat “trapping gas”?
“High IQ does not in any way correlate to an understanding of GHGs.”
Interestingly enough, believe it or not, neither does a low IQ.
FYO the Mensa guy was a chemical analyst but my only point was simply that Jeff was not very convincing when he claimed that he didn’t understand my posts or that they are quote: “unintelligible”
There are many who would disagree.
As another explanation of the greenhouse effect I’m not sure what this article is supposed to be explaining.
In the first example between the two plants, the measurement of radiation is taken off of the surface of the plants within their respective systems. In the second example, the measurement of radiation is taken off of the surface of the entire system (top of atmosphere) after each has achieved equilibrium. The second example isn’t truly analogous because the measurement isn’t taken off of a plant within the system, but as a distributed average of measurements taken across the entire system’s edge.
Is that what the article is trying to explain – that although any given object may be warmer in the stronger greenhouse, each system as a whole must radiate the same amount of energy that it receives (eventually, at equilibrium)? If we measure less heat coming off of the system than going in, that system is heating up internally – which would be the case as the morning sun first hits the greenhouse. If we measure more heat radiating out than going in, the system is cooling down – a greenhouse just after sunset. The system with the higher heat capacity will take longer to swing back and forth from equilibrium, but the net flow of energy into/out of each system will eventually balance to zero, and since the flow of energy into both systems are equal, the flows out must eventually become equal.
Of course no physics are violated. I just don’t understand exactly how and why. I’m aware of the fact that you don’t want to get too much into detail, but if we don’t think it through to the end it’s not a thought experiment but rather an illustration of a preconceived concept. And we have too much of this already. Maybe someone else can help?
Following the arguments brought up by Michael Hammer above, Jeff’s (and my) statement that the image of the 2x CO2 planet is “larger and dimmer” (however slightly) is not correct. The outmost layers of the atmosphere, the stratosphere and above, are not affected by a doubling of CO2, therefore the size of the image of our “warmer” planet would not be different from the “cooler” planet. (The proposed higher emission altitude due to doubling of CO2 would just affect the brightness in that part of the image, not the overall size.)
I would restate: The images of both planets have the same size and overall brightness, but the distribution of thermal radiation might be slightly different.
The surface of the 2xCO2 planet will be a little brighter (because Jeff said so, basic physics) therefore some part of the atmosphere must appear cooler.
But how exactly does the troposphere or the tropopause get cooler when the surface warms up? That’s what I still don’t understand.
I understand the tropopause is emitting energy in the CO2 absorption bandwidth because there is not much CO2 in the tropopause to block it. The tropopause of our 2xCO2 planet gets the same energy form above (the sun and the stratosphere) and a little less energy from below in the CO2 absorption bandwidth. But isn’t this counteracted by more radiation at all other frequencies? Therefore I would say the tropopause on either planet is emitting the same amount of energy but maybe at slightly different frequencies. (Our camera won’t notice.)
In the troposphere we have most of the CO2 effect. As far as I understand it, it is like this: CO2, like any other gas, is absorbing all kinds of frequencies from above and below and is emitting the same amount of energy at slightly different frequencies because it is not emitting in its absorption bandwidth. We said before: if the surface is warmer now, some part of the atmosphere must be cooler. And CO2 is responsible. Is CO2 cooling the troposphere?
Most likely some of my reasoning is totally wrong, because if not, CO2 would help to transform the “dangerous” long wave radiation form the surface to less dangerous frequencies and may be even cooling parts of the atmosphere. What am I missing?
I am sure all the politicians understand how the climate works by now. Could somebody please help an economist understand it – in the end, it will land on our desks.
Thank you all.
Jeff Id says on January 3, 2011 at 9:04 am:
“The true AGW discussion going on in publication revolves around the magnitude rather than these simple points. Keep in mind that with all that said, the magnitude of warming by CO2 can still be undetectably small and whatever magnitude is very possibly below natural variability. I’ve seen no proof that it isn’t and the more I read the closer my position drifts to Lindzen. In my opinion, it makes for a better discussion when people grasp the basics but as has been clearly pointed out, there will always be those who don’t/won’t get it.”
I must confess Jeff that I am one of those dumb-sculls who don’t get it. That is not exactly the same as, in the same breath, to say that I won’t get it. I shall of course get it (I hope) when a few things as to where my thinking is going wrong is explained to me.
Hope You can do that, please read my thoughts below first:
One of the main reasons why I cannot grasp the “Greenhouse Theory” is this: I see Radiation, Conduction and Convection/ Evaporation as “Energy Transportation Systems” (ETS). It is therefore implausible, to my mind, that any of the above ETSs can increase the global temperature any more than the passenger railway system can increase the world’s human population – Nor any more than you can increase the temperature of water in a glass by pouring it into another glass and back again -.
I have seen and read many so called explanations for the “Greenhouse Theory” and as Dr. Roy Spencer is one man who knows a thing or two about what is happening I thought he might have a good explanation, but no, though he has had many attempts.
In his blog under the title Global Warming 101 he writes:
“As we add more CO2, more infrared energy is trapped, strengthing the Earth’s greenhouse effect. This causes a warming tendency in the lower atmosphere and at the surface. As of 2008, it is believed that we have enhanced the Earth’s natural greenhouse effect by about 1%.”
He has no plausible explanation for this enhancement, but adds: “Global warming theory says that the lower atmosphere must then respond to this energy imbalance (less IR radiation being lost than solar energy being absorbed) by causing an increase in temperature (which causes an increase in the IR escaping to space) until the emitted IR radiation once again equals the amount of absorbed sunlight. That is, the Earth must increase its temperature until global energy balance is once again restored. This is the basic explanation of global warming theory.”
Ok, so now I know the basic explanation of global warming theory. Fine but I want to know how the lower atmosphere can perform this trick of responding to this energy imbalance by causing an increase in temperature (which causes an increase in the IR escaping to space) until the emitted IR radiation once again equals the amount of absorbed sunlight. That is, how does the Earth increase its temperature until global energy balance is once again restored?
And then Dr Spencer adds an explanation: “(The same energy balance concept applies to a pot of water on a stove set on “low”. The water warms until the rate of energy loss through evaporation, convective air currents, and infrared radiation equals the rate of energy gain from the stove, at which point the water remains at a constant temperature. If you turn the heat up a tiny bit more, the temperature of the water will rise again until the extra amount of energy lost by the pot once again equals the energy gained from the stove, at which point a new, warmer equilibrium temperature is reached.)”
So then according to Dr. Spencer the answer is simple; “The concept is, just turn the heat up a tiny bit more”
He probably instinctively knows that back radiation from the rising steam or from the pot itself in general is not enough.
He could instead have increased the pots temperature by putting a lid on it or swapped the pot for a pressure cooker and thus stopped convection. But that would only have proved my point that when compared to convection, radiation (which every fan of the “Greenhouse Theory” is quoting as being “the master”at every opportunity) happens to be just a minor player.
Having said all that Dr. Roy Spencer is still a hero of mine and he has a good article on his blog today. You should all read it!
I have no idea what the point was, but maybe the hidden question was: Why is this irrelevant to AGW? Answer, because total emitted radiation is trivially equal to total absorbed radiation, and has little to do with conditions on the planet.
Dude, you are boring.
OH:
“But that would only have proved my point that when compared to convection, radiation (which every fan of the “Greenhouse Theory” is quoting as being “the master”at every opportunity) happens to be just a minor player.”
On behalf of us heretical skeptics, I think you are correct. These radiation freaks can see nothing else! It’s like a video game to them, perhaps?
Will,
I misunderstood the term “electric night storage heater” — I had never run across that phrase before and was thinking simply “electric heater”. After googling it, I have a better understanding of your example.
But even now I still don’t think it is a particularly apt analogy. You seem to be considering the atmosphere as the “thermal bricks” when in fact the heat capacity of the atmosphere is relatively small compared to the land and the oceans. The earth would be the thermal bricks and the atmosphere would be more analogous to the container around the bricks.
The storage heater gains energy from the electric heater; much like the earth gains energy from the sun. The storage heater loses energy as well, by convection, conduction and radiation to the cooler surrounding room (~20 C) thru the container. The earth similarly loses energy by radiation to the cooler outer space (~2.7K) thru the atmosphere. (There is no convection or conduction to outer space)
It seems the analogy you have is “there are three storage heaters, one that is inside a poorly insulated container, one that is inside a well insulated container, and one that is inside no container.” Then, yes, the well insulated storage heater will get warmer than the other, and will stay warmer longer.
Going farther, since the earth does not cool off to the temperature of its surroundings at night (ie 2.7 K of space), we should postulate that the storage heater also does not cool off to the temperature of its surroundings (ie 20 C in the house). So a the end of the first day of use, heater A might be still 22 C inside. Heater B might have cooled to 20.5 C. Heater C might be 25 C. When you then turn on the storage heaters the second night, they will reach a higher temp than the first night, and at the end of the day, they might be 20.6 C, 23 C, and 27 C. Eventually the increased heat loss from the hotter storage heaters will limit the temperatures, but indeed the temperatures will be different at maximum and on average.
“That which absorbs, equally emits, AKA Kirchhoffs Law. “
Yes, indeed. Specifically, the emissivity applies equally to absorption of photons and emission of photons. But this refers to efficiency, not amount.
If a given volume of atmosphere absorbs 10% of the 1.2 um photons coming into it (ie 10% as many photons as a perfect blackbody would absorb), it will also emit 10% as many 1.2 um photons as a perfect blackbody would. But since a chunk of the atmosphere is cooler than the surface, it will be absorbing 10% of a large number of photons (from the warmer surface) and will be emitting 10% of a smaller number of photons (from the cooler atmosphere). There will therefore be a net transfer of energy from ground to the atmosphere = warming of the atmosphere from IR radiation.
“CO2 does not heat the atmosphere any more than water vapour does.”
Quite true. H20 is much more prevalent and hence is a more effective greenhouse gas and hence heats the atmosphere more via IR radiation than CO2 does. But both do indeed heat the atmosphere.
Here is another thought experiment. This time its a thought experiment with a slight twist. Unlike Jeff Id’s thought experiment which can never be conducted and tested in the real world, this one can be reproduced by everyone. So its not really a thought experiment at all, its a real experiment but it also works as a thought experiment.
Here goes:
We all wear clothes to keep warm in the winter. The reason we do so is because several layers of clothes trap Air which is then warmed by our body heat via all three modes of heat transmission, conduction, convection and radiation.
So exhale some of that nasty CO2 from your evil old lungs into your under garments.
Your evil “Greenhouse Gas” breath will typically be warmer than the trapped air in your clothing so it will already have a head start in the heat trapping/IR warming stakes right?
So after five or six large exhalations of 40,000 ppm CO2, turning the air content of your string vest into an EPA environmental disaster zone, what happens?
A) Does it get warmer and warmer until you are forced to strip off to quell the vicious feedback loop now occurring with the rivers sweat evaporating from your fat hairy belly?
B) Does it remain at the temperature of your foul smelling human CO2 exhaust, a few degrees above the natural temperature of the air (99% O2 and N2) that was readily warmed conductively, convectively and by your bodies IR emissions?
Or
C) Does all that dangerous “heat absorbing” CO2 just simply cool back down to your bodies and clothes equilibrium temperature within just a few seconds, as you already knew it would?
Regardless of what the Jeff Id’s or Roy Spencer’s of this world try and convince us of with their impossible to replicate “Greenhouse Effect””thought experiments”, the temperature of the atmosphere is determined by the equilibrium temperature of its main constituents,
79% Nitrogen and 20% Oxygen
and not by its trace gases such as CO2 at 0.0385%.
As can be seen from the above example, even if you heat high concentrations of CO2 above ambient air temperatures, it will quickly, within just a few seconds, be forced into equilibrium with the ambient air inside that environment, be it trapped in your underwear, enclosed inside a greenhouse or trapped by gravity around a planet.
The so called “Greenhouse Effect” requires that you suspend all knowledge gleaned from your five senses and believe that a concentration of just 0.0385% CO2 heated by just a few hundred W/m2 can force the entire atmosphere of O2 and N2 into equilibrium with itself.
But the most bizarre thing of all is that millions of gullible fools fell for it.
The biggest and most brazen fraud in the history of science.
Tim
Mum said I mustn’t feed the Troll’s!
For Jeff ID :
I for one would like to thank you for conceiving this thought experiment. And thank you for clarifying the basic physics that underwrite the theory of GHG warming, even in the face of intimidating remarks against you by people who do not understand the physics, or seem to be distracted by irrelevant details or a political agenda. Thank you for standing up to such remarks and you have increased my confidence that there are really skeptics out there that confirm basic physics, and are willing to speak up for science in the face of political opinions.
ourson polaire
“The surface of the 2xCO2 planet will be a little brighter (because Jeff said so, basic physics) therefore some part of the atmosphere must appear cooler.
But how exactly does the troposphere or the tropopause get cooler when the surface warms up? That’s what I still don’t understand. ”
I don’t really understand why the atmosphere must appear cooler. The tropopause simply emits from a higher cooler layer.
O H Dahlsveen,
“one of the main reasons why I cannot grasp the “Greenhouse Theory” is this: I see Radiation, Conduction and Convection/ Evaporation as “Energy Transportation Systems” (ETS). It is therefore implausible, to my mind, that any of the above ETSs can increase the global temperature any more than the passenger railway system can increase the world’s human population – Nor any more than you can increase the temperature of water in a glass by pouring it into another glass and back again -. ”
The missing point, I think, is the difference between power and energy. Power is energy per second. In the case of outgoing energy, it flows from the surface to space. If you place a bottleneck in the flow, the energy crunches together tighter so the power can keep flowing at the same rate. Higher density energy, is heat. So you create a region of heat without affecting the net flow — the planets appear the same.
Don’t know if that helps.
Will:
January 3, 2011 at 8:26 pm
I can’t understand you again. You have heat capacity mixed with absorption mixed with mashed potatoes again.
How many apples does it take to make an orange?
Rob,
Thanks, it’s a little frustrating sometimes, but hopefully a worthwhile effort for some.
I have got a few more simple questions listed below:
Why, I wonder do “climate Scientists” seem obsessed with sending all the incoming solar radiation back into space?
Have they never heard of latent heat?
Don’t they know that all the solar energy is in space already, just like the Earth is?
AGW people may wish to shut down the world economy (and with all the good help they are getting from politicians at the moment, they may succeed) but it should be quite easy for scientists to deduce that if energy in equals’ energy out 24/7 then we would only be left with “geo-thermals” There would be no fossil fuels and nobody to burn them.. – The planet would be dead. The Earth itself and everything on it uses /transforms energy all the time. Where do these people think “Nuclear Energy” comes from? It comes from bits containing latent energy which when brought together react in a certain way to create heat so that we can raise steam with which we can run turbines to create electricity.
Now then, with the kind of radiation that nuclear creates we would maybe be better off if it would leave the Earth system. But it won’t and it can’t.
And lastly if energy was to leave the system then natural “Adiabatic Cooling” would still be possible but the opposite natural “Adiabatic Warming” would not as once convection stop at, or near to, the top of the troposphere the energy would be “radiated further through the different spheres and eventually out to space.
However the Foehn Winds proves that the energy is still there. These particular winds happen on the ‘lee side’ of mountains all over the world and the proof of that is that regionally, these winds are known by many different names. These include:
• Lyvas wind in Greece
• Zonda winds in Argentina
• Chinook winds east of the Rocky Mountains, in the United States and Canada, and north, east and west of the Chugach Mountains of Alaska, United States
• The Nor’wester in Hawkes Bay, Canterbury and Otago, New Zealand
• Föhn in Wollongong and South Coast, NSW Australia. Often associated with heavy orographic lifiting on the windward side of the escarpment
• Halny in the Carpathian Mountains, Central Europe
• Fogony in the Catalan Pyrenees
• Bergwind in South Africa
• Viento del Sur (Southern Wind) in the Cantabrian region (northern Spain)
• Terral in Málaga (southern Spain)
• Föhn in Austria, southern Germany, German-speaking regions of Switzerland and Northern Italy (even non German-speaking regions)
• Puelche wind in Chile.
• Favonio in Ticino and Italy
• The Helm wind, on the Pennines in the Eden Valley, Cumbria, England
• Garmoosh, Garmesh, Garmbaad (Warm Wind): (Persian: گرمباد, Gilaki: گرموش) in Gilan region, in the south of Caspian Sea in Iran
• Hnúkaþeyr in Icelandic.
• Vântul Mare in the Carpathian Mountains, Romania.
The Santa Ana winds of southern California are in some ways similar to the Föhn, but originate in dry deserts as a katabatic wind.
All these names have been collected and reprinted from “Wikipedia”
Only reading the first couple responses (so I couldn’t cheat), I’d say the 2 planets will have the same integrated “brightness”, but the one w/more CO2 will have its spectrum shifted to slightly lower frequencies (a cooler TOA). If your camera just measured bulk (integrated) radiation w/o considering frequency, it would show no difference in watts emitted.
Jeff Id
“I don’t really understand why the atmosphere must appear cooler. The tropopause simply emits from a higher cooler layer.”
If the surface of the 2xCO2 planet is 1 degree warmer it’s surface area will look brighter on the image of our fancy camera. Some part of the atmosphere must therefore be cooler. Otherwise the image of this planet would really be brighter than that of the other planet. This can not be the case in equilibrium.
Are you implying that all the energy that was absorbed by the column of CO2 molecules is released at the top of it? And with a doubling of CO2 the column extends higher into the tropopause where it is cooler? Releasing trapped energy in the absorption bandwidth of CO2 but at a lower temperature than it could have if the column would only reach up to lower and warmer strata of the atmosphere?
I have already described how I see it. Was it wrong?
Thank you again for bringing up and discussing the “simple” issues.
Yes, Jeff ID it would help if you could tell me where that “new Bottleneck” is. According to the IPCC’s own models a bottleneck (i.e. warming by greenhouse gases) would create a “Hot Spot” just below that bottleneck. IPCC models even pin pointed where that Hotspot should or could be found.
No “Hot Spot” has ever been found. And quite meekly we seem to agree with the AGW crowd “That it does not matter anyway.” Maybe that is because a missing hot spot proves there is no Natural Greenhouse effect either.
Power v. energy: All Energy Flow Plans use the same description for energy in as for energy out, i.e. W/m². They even say “energy out equals energy in”
O H Dahlsveen says:
January 4, 2011 at 9:38 am
“According to the IPCC’s own models a bottleneck (i.e. warming by greenhouse gases) would create a “Hot Spot” just below that bottleneck. IPCC models even pin pointed where that Hotspot should or could be found.”
Now that is a warming magnitude issue, and I think we’re in agreement that there are some serious problems there.
“Power v. energy: All Energy Flow Plans use the same description for energy in as for energy out, i.e. W/m². They even say “energy out equals energy in”
I know, it’s a bit of a pet peeve of mine – even though I messed it up above 😉 Watts is a unit of power of course, not energy. Whether you get it or not, people misunderstand that point all the time.
Jeff Id says:
January 4, 2011 at 7:22 am
“Will:
January 3, 2011 at 8:26 pm
I can’t understand you again. You have heat capacity mixed with absorption mixed with mashed potatoes again.”
CO2, O2 and N2
Thermal Conductivity – k – (W/mK)
Temperature (ºC)
Carbon dioxide (gas) 0.0146
Nitrogen 0.024
Oxygen 0.024
Specific Heat Capacity
cp cv
(kJ/kg K) (kJ/kg K)
Carbon dioxide CO2 0.844 0.655
Nitrogen N2 1.04 0.743
Oxygen O2 0.919 0.659
“The specific heat capacity represents the amount of energy required to raise 1 kg by 1oC, and can be thought of as the ability of a substance to absorb heat. Therefore the SI units of specific heat capacity are kJ/kg K (kJ/kg oC). Water has a very large specific heat capacity (4.19 kJ/kg ºC) compared with many fluids.”
Water is a good heat carrier!
From: http://www.engineeringtoolbox.com/heat-work-energy-d_292.html
So as you might expect, water has a much higher specific heat capacity than CO2. Well guess what? So does O2 and N2 as you can see above.
But wait, didn’t Jeff Id tell us in his amazing, impossible to reproduce, “thought experiment” that quote:
“We know that CO2 absorbs certain outgoing wavelengths of light.”
So surely that must mean that CO2’s ability to absorb a tiny portion of IR in the 15 mµ region of the IR spectrum means it is a really scary “Greenhouse Gas” and is so powerful that it determines the temperature of the entire atmosphere right?
Because we all know that absorption of tiny portions of the IR spectrum trumps the specific heat capacities of gases and their ability to absorb heat don’t we. You know “heat”, that stuff that melts ice and causes “global warming”.
Well lets try another thought experiment shall we:
Oopsie!
That was a REAL easily reproducible experiment, not an impossible to replicate and therefore impossible to validate post normal “thought experiment”.
Sorry, my bad!
When a CO2 molecule absorbs energy in the form of light/IR, the energy will cause the CO2 molecule to vibrate faster (become hotter). But if that CO2 molecule is at the same time being forced into temperature equilibrium by the higher specific heat capacities of both O2 and N2 in which it is immersed at a ratio of more than 1200/1, the only thing the CO2 molecule can do is re-emit (becoming cooler).
Therefore the dominant factor in the atmosphere with regard to energy and more importantly, temperature, is specific heat capacity not radiation. I back this up with real easily replicable experiments. NOT impossible to replicate post normal “thought experiments”.
Jeff you say above in reply to my: “Power v. energy: All Energy Flow Plans use the same description for energy in as for energy out, i.e. W/m². They even say “energy out equals energy in”
“I know, it’s a bit of a pet peeve of mine – even though I messed it up above 😉 Watts is a unit of power of course, not energy. Whether you get it or not, people misunderstand that point all the time.”
Why does it have to be people who misunderstand “that point” all the time? Is it “unthinkable that it could be something else” that is wrong all the time?
If I read W/m² I think Watts per square meter is what is meant by it.
Jeff, I would still like to know the scientific explanation/answer to my oft repeated question: How are gases like Nitrogen, Oxygen and Argon warmed if they cannot absorb IR radiation?
O H Dahlsveen says:
January 4, 2011 at 3:38 pm
“Jeff, I would still like to know the scientific explanation/answer to my oft repeated question: How are gases like Nitrogen, Oxygen and Argon warmed if they cannot absorb IR radiation?”
Well, who said they couldn’t? I see IR in the oxygen band! See fig 2. It sounds like you’ve been reading Will’s blog.
http://noconsensus.wordpress.com/2010/08/06/radiative-physics-simplified-ii/
Anthony carried that one as well. One of the things I’ve done at tAV is to try and make things as simple as possible so I’ve made a few posts like this. BTW, Figure 2 of that post should convince every person on the planet that CO2 warming is real. What it shouldn’t do is convince people that it warming is big, dangerous, bad, slightly measurable, significant, or in any way not good. Those points are separate issues.
“Why does it have to be people who misunderstand “that point” all the time? Is it “unthinkable that it could be something else” that is wrong all the time?”
Well, as an optical engineer I’ve worked this math often, and were you to even slightly consider chucking this simple stuff out (as you suggest), you should chuck every incandescent light out of your window. I mean right out the window b/c it turns out that if the math is bad, incandescent lights must not function.
The Plank spectrum is the exact same math that you use to calculate the output from filaments that light your house. In addition, the absorption spectrum of gasses are also used all the time. How do you think people identify CO2 on distant worlds? Ever hear of a gas chromatograph? Emission lines are calculated the same way.
“If I read W/m² I think Watts per square meter is what is meant by it.”
Watts are power not energy, the measure is Joules per second – energy flow. Joules are energy, Joules per second are power. So when you say energy in Watts/meter^2, the term “energy” is incorrect. The Watt unit is actually power.
Thank you Jeff for what you posted on January 4, 2011 at 4:53 pm It is going to take me longer than a few hours to soak it all up. But it is in the middle of the night here where I am just now (02:00) so I shall have to answer it at some other time. As I have got to “hit the sack” now.
Perhaps Will is hard to understand and a little cryptic, BUT:
“Therefore the dominant factor in the atmosphere with regard to energy and more importantly, temperature, is specific heat capacity not radiation. I back this up with real easily replicable experiments. NOT impossible to replicate post normal “thought experiments”.
Maybe I’m a nut, like many thing, but I think he has a point here. It’s all about heat storage, not radiation cartoons (which doesn’t necessarily mean the cartoons are wrong, just misleading…
““Jeff, I would still like to know the scientific explanation/answer to my oft repeated question: How are gases like Nitrogen, Oxygen and Argon warmed if they cannot absorb IR radiation?”
Well, who said they couldn’t? I see IR in the oxygen band! See fig 2. It sounds like you’ve been reading Will’s blog.”
WOW, now I’m gobsmacked again! Jeff, don’t you know ANYTHING about thermalization? ALL the atmosphere has to be warmed to preserve LTE, and for the non-IR active diatomic molecules, this happens via THERMALIZATION. Google it!
Jae,
Warmed relative to what? How warm?
The full quote should be:
“When a CO2 molecule absorbs energy in the form of light/IR, the energy will cause the CO2 molecule to vibrate faster (become hotter). But if that CO2 molecule is at the same time being forced into temperature equilibrium by the higher specific heat capacities of both O2 and N2 in which it is immersed at a ratio of more than 1200/1, the only thing the CO2 molecule can do is re-emit (becoming cooler).
Therefore the dominant factor in the atmosphere with regard to energy and more importantly, temperature, is specific heat capacity not radiation. I back this up with real easily replicable experiments. NOT impossible to replicate post normal “thought experiments”.
Another quote from my first post is also worth repeating here.
“The so called “Greenhouse Effect” is nothing more than a cheep parlour trick which utilises the complexities of atmospherics and fluid dynamics and most peoples inability to simultaneously consider all of the variables.”
That means all of us.
How many times have I read people saying in this thread (including Jeff Condon), well you have to accept the basic physics, if you can’t do that then blah blah blah?
Basic physics my arse!
Lets have a brief look at the basic physics of the “Greenhouse Effect” hypothesis.
Or rather lets look at just a fraction of the “basic physics” which the “Greenhouse Effect” hypothesis fails to incorporate.
Such as:
The ideal gas law
Specific heat capacity
All three laws of thermodynamics
Conduction
Convection
Solar variability
Solar magnetism
Solar ionisation
Temporary di-pole moments
The Diurnal Atmospheric Bulge, etc, etc.
Thats not to mention the lack of correlation between temperature and enormous levels of CO2 up to 7000 ppm in the historical record. Or the multitude of other climatic variables which are proven to effect global temperature yet have nothing whatsoever to do with any kind of “Greenhouse Effect”.
I know I certainly don’t have all the answers, not by any stretch of the imagination. It is perfectly obvious to anyone that I am not a scientist. But one thing I know for sure is that if we do not consider every parameter involved, we can never fully understand the “basic physics” let alone the climate.
Those who support and promote the “Greenhouse Effect” hypothesis could not possibly be further from understanding “basic physics”.
The “Greenhouse Effect” hypothesis is NOT basic physics. It’s half baked physics. It’s incomplete physics. It’s dumbed down physics. It’s retarded physics. Above all the “Greenhouse Effect” hypothesis is bullsh!t physics.
Of all those who think they understand the basic physics of the “Greenhouse Effect”, how many of you have actually read John Tyndall’s ” Contributions to Molecular Physics in the Domain of Radiant Heat”?
Not one I bet.
You will therefore be blissfully unaware that this work, from which the “basic physics” of the “Greenhouse Effect” hypothesis originates, contains the classic structure of the “basic” confidence trick. Using as it does, the “Straw Man” device in the form of Professor Magnus, to convince the reader that Tyndall is correct in all of his fallacious assertions such that, Oxygen and Nitrogen are quote: “practically transparent radiant heat”. and his implication that CO2 is a heat trapping gas, among many other fallacies.
Don’t talk to me about the basic physics.
If increasing CO2 content causes increasing air temperature show it, with REAL replicable experiments. How hard can it be? After all, its just basic physics right?
To imply or actually state as many fools have, that you would need another planet, identical to our world but with double the CO2 concentrations in order to demonstrate the “Greenhouse Effect”, perfectly characterises the level of dishonesty and moral bankruptcy of its proponents.
Will,
You are the Cliff Claven of WUWT. 🙂
Will says:
January 5, 2011 at 4:25 am
“To imply or actually state as many fools have, that you would need another planet, identical to our world but with double the CO2 concentrations in order to demonstrate the “Greenhouse Effect”, perfectly characterises the level of dishonesty and moral bankruptcy of its proponents.”
Good grief, of course you don’t! Much simpler experiments that anyone can carry out are described on the internet – Google “greenhouse effect demonstration”.
The simple “vinegar and baking soda in a jar” experiment is described here: http://www.rmets.org/activities/schools/greenhouse-effect.php
There’s a nice video of a more sophisticated setup here: http://www.youtube.com/watch?v=Ge0jhYDcazY
Jeff
I have presented a sound logical argument as to why the “Greenhouse Effect” is bogus. I have given data backed by real replicable experiments.
Im at a loss to know what more I can do!
You claim the “Greenhouse Effect” is indeed real and you present, as a head post no less, an impossible to reproduce thought experiment, then make an ambiguous meaningless claim about CO2 IR absorption, quote:
“We know that CO2 absorbs certain outgoing wavelengths of light.”
and when someone disagrees with you, rather than supporting your statements with facts and data or even dare I say it, a real experiment, all you can do is say, “well if you can’t except the basic physics blah blah blah” and then resort to name calling.
Forget the name calling, lets see you with a real experiment please.
Steve
With regards to the examples of experiments you claim prove the “Greenhouse Effect” all I have to say is this.
The first link is to a written description of an experiment using baking soda to produce the CO2. I have tried many versions of his experiment and yet I get no warming bias in the container of CO2. All my results using baking soda have been consistent with the results I get using carbonated water.
See here: http://www.spinonthat.com/CO2.html
As for the second link you have given it is to a short video produced by the BBC. Everyone knows the BBC are heavily invested in Carbon Credits. They have every motive to produce fraudulent science and this film is just a classic example of precisely that.
It is very interesting to me because this link you give is the remake of a News Night program that the BBC threw together in a knee-jerk reaction to my original experiment which I first uploaded on my website back on the 14 Dec 09.
The original is here: http://news.bbc.co.uk/1/hi/programmes/newsnight/8418356.stm
Notice the date, 17 Dec 09. The reason that they had to do a remake, the one in your link, is because even though in both they employ “the magic of TV”. In other words the cut the film and only show what they want you to see, in the first version that they hurriedly put together for this News Night program they actually committed fraud on national television.
If you watch the original and pay attention to the temperature of the air bottle you will see that it ends up being 1º C colder at the end of the test after apparently being heated for 10 minutes. So they had no choice but to make a second version which is one you have linked to, which incidentally still uses cuts in the footage and actually proves nothing.
So sorry, no cigar this time Steve, keep googling, you may just get lucky!
“I have presented a sound logical argument as to why the “Greenhouse Effect” is bogus. I have given data backed by real replicable experiments. ”
No you haven’t, you’ve used scientific words and concepts mixed together in a pattern which is so messed up,it’s completely indecipherable.
I’ll tell you what Will, who is so certain he’s proved science wrong. If you like, I’ll do a post on your spinonthat page linked above that got the approval of your friend. If I can’t find 10 distinct errors in the single post, I’ll apologize for comparing you to Cliff Claven.
This might be a stupid question, if it is an explanation of why would be welcome.
Is the IR in these models and thought experiments measured taking into account that not all IR is equal, that there is a range of temps in it?
Jeff Id says January 4, 2011 at 4:53 pm :
O H Dahlsveen says January 4, 2011 at 3:38 pm: “Jeff, I would still like to know the scientific explanation/answer to my oft repeated question: How are gases like Nitrogen, Oxygen and Argon warmed if they cannot absorb IR radiation?”
Jeff: “Well, who said they couldn’t? I see IR in the oxygen band! See fig 2. It sounds like you’ve been reading Will’s blog.” (Jeff sends me a link which I promised to look at later, as a quick look took up all the time I could spare right then.)
OHD: Well, I have for a long time been of the opinion that they can, as the few air-molecules present as high up as the Thermosphere are said to be N² and O² I have of course got no idea whether they are exited/warmed by short-wave IR, gamma or by x-ray radiation. But The IPCC and other “Greenhouse specialists” have given me the impression that they cannot be warmed by either incoming short wave IR or by out-going long wave IR.
So if 99% of the atmosphere (Nitrogen78%+Oxygen21%+Argon0.9%) cannot be warmed by radiation and at the same time heat exchange between the top of the surface and the bottom of the atmosphere by conduction is so unthinkable these days that it is never even mentioned, I feel I have got to ask: How is atmospheric heating accomplished? As to ignore conduction between the two is to ignore The Zeroth Law of Thermodynamics. (Unless there is a vacuum between the surface and the atmosphere that I don’t know about).
And yes, I have been reading Will’s comments but I did not know he had his own blog. So – no, I haven’t.
Jeff: “One of the things I’ve done at tAV is to try and make things as simple as possible so I’ve made a few posts like this. BTW, Figure 2 of that post should convince every person on the planet that CO2 warming is real. What it shouldn’t do is convince people that it warming is big, dangerous, bad, slightly measurable, significant, or in any way not good. Those points are separate issues.”
OHD: “We may be talking or writing at “cross purposes” here. – If you are not trying to convince me, and others, that warming (by CO²) is big, dangerous, bad, slightly measurable (or measurable at all), significant, or in any way not good. Then we are more or less in agreement as I have no problem with understanding that certain gases, especially Water vapour, has better heat retention capacities than others. And I do understand that the planet is “warmer over all” i.e. warmer on the night side and cooler on the day side than it otherwise would be. However only an atmosphere that can stop or delay both convection/evaporation and if you please radiation can hope to have a “greenhouse effect”
Jeff: Well, as an optical engineer I’ve worked this math often, and were you to even slightly consider chucking this simple stuff out (as you suggest), you should chuck every incandescent light out of your window. I mean right out the window b/c it turns out that if the math is bad, incandescent lights must not function.
OHD: The Governments are chucking the incandescent light bulbs out for us in any case Jeff. It is not the maths that is wrong. The maths get the same answer irrespective of what the atmosphere is made up of. Irrespective of whether the planet is the size of a pea or the sun, Whether it is completely dry or 100% water.
But as you mention the incandescent light bulb, the filament is maybe as close to a “blackbody” as a star is but what do you think cools the bulbous glass bit of the bulb? Why is the air above the bulb warmer than the air just below it when the bulb is lit?
When I was at primary & middle schools (1947 – 1954) we were told by our teacher that a “doubleglazed” light bulb had once been made, i.e. glass vacuum – glass vacuum – filament. When the bulb was lit, the inner glass soon melted. (This was done as an experiment to prove that in the atmosphere, conduction/convection was stronger than radiation.) – I have never found any references to this experiment, but then again I never thought I had to as by the time I was a mechanical engineer (1964) those principles had not changed. The interest in global warming (that had just stopped) was as high in those days as now, the difference was that then they were still looking for an explanation for “The Ice Ages” (At the time The Milankovitch Theory” was out of the window as summer in the Northern Hemisphere was thought to take place just as the earth was at its furthest point from the sun in its eliptical track. As long as the orbit stayed the same size the shape of it should not matter enough for a big freeze. (But that’s another story)
So to be fair, as a mechanical engineer I have never come across a thermo-dynamical problem that has been in need of Planck’s Law. Even when I briefly worked for the “Nuclear Power Industry” the only “extra thing” I had to learn about radiation was how to shield it, avoid it and de-contaminate myself. But then again, I was not designing the reactors.
Jeff: Watts are power not energy, the measure is Joules per second – energy flow. Joules are energy, Joules per second are power. So when you say energy in Watts/meter^2, the term “energy” is incorrect. The Watt unit is actually power.
OHD: I know, but tell that to the IPCC and Trenberth & al
“Jae,
Warmed relative to what? How warm?”
Well, as warm as the surrounding molecules. All the molecules in a given locale are in thermal equilibrium (Local Thermal Equilib. is the official phrase). We don’t have the situation where the HOH and OCO molecules are buzzing around at greater speeds than the N2 and O2 molecules, just because the latter don’t absorb IR. The GHGs don’t just absorb and fire photons, they COLLIDE with neighboring molecules and give them energy (as well as getting energy back from them). IIRC, there are thousands more collisions per microsecond than there are photons being “fired” at STP.
I think that it is all this energy (kinetic and potential) STORED in all these molecules that keeps the Earth “warmer than it should be,” not some “greenhouse effect.” Any greenhouse effects are probably overshadowed–or at least greatly weakened–by convection (lapse rate), just like they are in a real greenhouse when the doors and windows are opened.
It is no simple coincidence that atmospheres on other planets have about the same temperature at a pressure of 1 bar (sea level here) as our planet does. See this article: http://www.ilovemycarbondioxide.com/pdf/Rethinking_the_greenhouse_effect.pdf
OHD, My best guess is that CO2 has an immeasurably small effect on our climate, that doesn’t change the basics of this post.
“Why is the air above the bulb warmer than the air just below it when the bulb is lit?”
Because the glass envelope absorbs the IR and warms up. The air is heated by conduction. If the glass were perfectly transparent to IR a similar effect would occur but over a wider area because Air is more rarefied than glass so the energy would be diffused further from the source.
It’s too bad the government thinks badly of incandescent, they are nearly 100 percent efficient from October until May in my climate.
The point of the post is NOT to convince anyone that the microscopically tiny amounts of CO2 in the atmosphere are bad but rather that the basic effect is real and that it really is not correct to refer to it as a ‘greenhouse’ which actually behaves entirely differently.
My opinion is that if WUWT readers are very clear on understanding the basics, they can make their cases more clearly to the rest of the world. If we stomp around like Will, nobody will or should listen. When I discuss AGW with friends, I say yup it’s a real effect, but it is very much exaggerated, and anyone who tells you they know the answer to how much warming or how dangerous it is, you know you are speaking to an advocate. Nobody really knows.
I do believe that warming is not following mainstream predictions and that there is substantial and growing evidence that CO2 based AGW is definitely not a problem. I also believe that if it were a problem,there is still nothing we can do about it.
[SNIP]
Perhaps if there are things you did not grasp about what I have said, it might be a sensible approach to ask me to clarify them. But I would need you to a bit more specific than to simply accuse me of being “completely indecipherable”.
For someone who purports to be so knowledgeable on the subject, I am surprised that you seem to be completely unaware of the fact that there is no real scientific basis and absolutely zero real word evidence which supports the so called “Greenhouse Effect” hypothesis. On this matter you seem to be seriously lagging way behind the curve Jeff. As a matter of fact the only place the greenhouse effect actually exist is inside the UNIPPC and state funded computer models.
Computer models which in order to model the greenhouse effect, are fed with certain specific kinds of data. One of these, as you may or may not know, is a form of data called convective parameterisation.
What would be the chance of a computer model NOT producing a greenhouse effect if the convective parameterisation was underestimated I wonder ? What happens when you inhibit convection Jeff ? And finally what is the likelihood of the IPCC accurately estimating rather than underestimating the total global atmospheric convection, given the collective personification of a catalog of errors the IPCC have shown themselves to be ? Hmm…. that’s a tricky one!
As for an apology, save it. You are what you are Jeff, what good will apologies be?
[Reply] Sorry about the SNIP, but you should be able to make your points clearly without me needing to. Deep breath time. RT-mod]
JAE,
I agree with everything up to STP. Heat capacity is another matter entirely. It only determines how quickly the gas changes temperature. If the heat capacity of Air were 10x greater than we think it is, it would only affect the reaction time of warming — slightly.
Well Jeff ID I have by now read/studied your blog posting and must confess I am non the wiser.
I can go along with you even to the end of your thought experiment when you say: “So from a few simple concepts, two gasses at the same temp, one transparent the other black (at infrared wavelengths), we’ve demonstrated that different absorption gasses heat differently when exposed to an energy source.”
You have got the two gases (50% CO2-50% transparent) contained in a small canister. Whether one or both gases are heated by the laser is immaterial as they have no choice but to seek equilibrium whilst in the can and they do so just as you describe it.
The words you could have used are “by conduction”.
So let’s take your thought experiment a bit further. Why not ask ourselves what happens to the hot CO2 laden air when it exits the can and joins the rest of the atmosphere? One of the laws of nature (when made easy) says something like this: “Hot air is less dense than the adjacent air and experiences a buoyant force, just like a bubble of air in water”
Questions: How does CO2 get around that law? And if it doesn’t then why does it not form a band at a level above which the rest of the ever thinning atmosphere can no longer support it?
-Ozone (O3) does.-
When CO2 comes out of a chimney it rises straight up into the air with all the other hot gases. But then it cools off and comes down again. Or is blown around in the wind like it normally does. So even when pre-heated the rascal won’t behave any different than any other gas.
-Why is that?-
Jeff Id says on January 5, 2011 at 3:56 pm:
“OHD, My best guess is that CO2 has an immeasurably small effect on our climate, that doesn’t change the basics of this post.”
After reading the above and your further answers to my comments I have come to the conclusion that there are perhaps ‘not such a big difference’ between the ways we look at these things.
Jeff:
“If the heat capacity of Air were 10x greater than we think it is, it would only affect the reaction time of warming — slightly.”
I don’t understand this statement. If you put a bunch of hot rocks in your tent at night, you stay warmer longer. The temperature on Earth is NOT dictated by radiation alone. I hope you read the link I provided. Can you explain the observations presented there (data from NASA) with the GHE concept?
“The specific heat capacity represents the amount of energy required to raise 1 kg by 1ºC, and can be thought of as the ability of a substance to absorb heat. Therefore the SI units of specific heat capacity are kJ/kg K (kJ/kg ºC). Water has a very large specific heat capacity (4.19 kJ/kg ºC) compared with many fluids.”
From: http://www.engineeringtoolbox.com/heat-work-energy-d_292.html
Please see this link. Specific heat capacity is key to understanding atmospheric temperature, as is obvious from the information there.
Jeff says:
“Heat capacity is another matter entirely. It only determines how quickly the gas changes temperature. If the heat capacity of Air were 10x greater than we think it is, it would only affect the reaction time of warming — slightly.”
Well sorry but I don’t buy that, mostly because of what I have read on the subject and also based on that knowledge because it sounds to me like Jeff just made that up. Besides, reaction time of warming is not the point.
Specific heat capacity is the determining factor of the temperature at thermal equilibrium of all gas mixtures. So not only do the most abundant gases dominate but if they also have a higher specific heat capacity then there’s simply no arguing.
The point is can 385 parts per million CO2 determine the temperature of 999,615 parts per million O2 and N2 or is it more likely that 999,615 O2 and N2 molecules in every million will force the 385 CO2 to cool. Particularly considering that O2 and N2 both have higher specific heat capacities than CO2.
See here: http://www.engineeringtoolbox.com/spesific-heat-capacity-gases-d_159.html
I know Jeff is very convincing and all but it has to be said that it stretches credulity somewhat. But the clincher for me has to be the fact that there is zero evidence for Jeff’s claims. Jeff provides no evidence whatsoever and simply expects us all to take his word for it because he knows best.
Our everyday evidential experience tells us that a few hundred warm molecules will be cooled by almost a million cooler molecules, not the other way round.
Sorry if I seem pedantic but I need proof. I don’t have any religious tendencies.
To claim that the effect is so tiny that it probably can’t effect anything, about something that you can provide no evidence for in the first place, is pretty much the same as saying it doesn’t exist at all when all is said and done.
I mean if it’s that tiny of an effect would it not be just as valid to say that the O2 and N2 is cooling the CO2, rather than the CO2 is actually warming the O2 and N2. If it’s that close to call isn’t that what is referred to as semantics?
That is what it seems to have come down to with Jeff’s position here.
But with a concession to all those who have staked their reputation on the fraud, it kind of gets them off the hook, but it gives little comfort to those of us who’s governments seem intent on pushing ahead with Carbon taxes regardless, like here in the UK.
REPLY: take it over here please, you’ve been called out:
http://noconsensus.wordpress.com/2011/01/05/kicking-puppies/
This thread is closed to this argument. – Anthony
Will,
Here you go:
http://noconsensus.wordpress.com/2011/01/05/kicking-puppies/
JAE,
The temperature on earth is dictated by energy flow. The capacity to hold energy just determines the heating and cooling rates caused by changes in the flow.
OHD
“Questions: How does CO2 get around that law? And if it doesn’t then why does it not form a band at a level above which the rest of the ever thinning atmosphere can no longer support it?”
CO2 is very low mass per molecule. If you look at the average velocity of molecules in the atmosphere – speed of sound. It becomes more obvious why separation doesn’t occur. When you combine that with the miniscule 30ms (no reference) between collisions, it’s impossible for gasses to separate by IR absorption in Earths atmosphere.
jeffid:
“The temperature on earth is dictated by energy flow. The capacity to hold energy just determines the heating and cooling rates caused by changes in the flow.”
I hope you know that you are making no more sense than Will!
JeffID: You seem to be ignoring the important parts of my comments to you. Why? I am still waiting for your response to the link I provided above. WHY is the temperature on all the planets so close to ours when assessed at a pressure of 1 bar? Can you explain this with the “greenhouse theory?” Of course not! Somehow,most people cannot think beyond the “box of radiation cartoons!” It is truly wierd!
Jae,
I’m not intentionally ignoring anything. I read the link you gave. My first thought was – cool, my second was, is it true? Then I started considering the nuance.
Gas giants have substantial internal heating. Jupiter is nearly a star. Can we really look at is for an Earth proxy. Saturn has considerable radiation as well. I’m not sure what the meaning is WRT Venus or Mars. Mercury is gravitationally locked. Venus has a super dense atmosphere — consider how strange that is being close to the sun. Mars has a rarified atmosphere. I’ve been into astronomy since I was probably 8 when I accidentally discovered the moons of Jupiter with a spotting scope.
Why is it up to me to answer any of these questions? I wrote a post on the difference between a greenhouse and a greenhouse gas.
“Somehow,most people cannot think beyond the “box of radiation cartoons!”
My points here are no concession to the believers, they are the foundation by which the discussion can progress for all sides.
Jeff,
thank you for deconstructing Will’s “paper” over at tAV. And thank you for doing it so carefully. Otherwise some errors slip through and contaminate your reasoning when you read stuff like this and don’t know all the details.
Maybe you can now come back to my two questions? I think they are to the point of this thought experiment.
1. When the surface of the 2xCO2 planet is 1 degree warmer (and the larger CO2 column is also warmer) which other part of the atmosphere will be cooler and how? Because if not, the image of this planet will be brighter and possibly larger.
2. What happens at the tropopause on the 2xCO2 planet? Why is the tropopause so important?
Thanks again.
Jeff:
You evidently don’t understand the article at all! You, and nearly everyone else, are arguing that a “greenhouse effect” explains any heating that is above the BB temperature. Siddons is arguing that such an effect is not necessary to explain that extra heat, because of the gas law, T = PV/R. V and P depend ONLY upon stored energy (kinetic and potential). Therefore, T has to, also. The article backs up this fact by showing that all planets with atmospheric pressures above 1 bar have about the same T at one bar, REGARDLESS of how much GHGs are present in the atmospheres. IOW, it is impossible to have an atmosphere at a pressure of 1 bar without the temperature being raised above the BB temp (by about 33 C, in fact). He cites pretty powerful empirical evidence from NASA to back that up. There is NO empirical evidence for an atmospheric greenhouse effect! It is very likely, IMHO, that any warming caused by backradiation is immediatelly cancelled by convection–just like in a real greenhouse when the doors and windows are open.
ourson polaire says:
“1. When the surface of the 2xCO2 planet is 1 degree warmer (and the larger CO2 column is also warmer) which other part of the atmosphere will be cooler and how? Because if not, the image of this planet will be brighter and possibly larger.”
I think DeWitt’s answer is best for this one but it may be unsatisfying.
“The luminosity is the same. For 2x CO2 the total power will be spread out over a slightly larger surface area so it will be slightly dimmer, not brighter. And an increase in effective altitude of 150 m (Tsurf + 1 C at a lapse rate of ~6.5 K/km) changes the surface area by 0.005%. If planet 1 radiates 240 W/m2 then planet 2 radiates 239.9889235 W/m2 and Teff is lower by 0.003 K. Good luck measuring that.”
We know that the power out of the ‘stable’ planet must be equal to the power in. Energy must balance so if the 2xCO2 world had a larger emission surface, it must emit cooler or nothing balances. So with a warmer troposphere, how can that happen. The answer is in the lapse rate of 6.5 K/km, which DeWitt was pretty clever to think of this estimate for this calculation. If you increase your altitude by 150meters, you lose about 1C of temperature. By calculating the 1C difference in emission you can estimate how much cooler the emission must be in order to balance the power.
DeWitt found for 150 meters, a net area change of 0.005%, from the power balance he was able to determine the required watts per meter squared of emission such that the planet was stable. Because Watts emitted are are proportional to temp he was able to determine just how much temp change was required to maintain balance — 0.003C cooler.
But now we are talking about a 1C ground level temp increase and a 0.003C change in temp at the emission altitude. Basically the emission surface is microscopically larger than the first order calculations determined so you can think of it as a microscopically more effective radiator. At the average emission altitude of this example though, the atmosphere it is actually (1-0.003)C warmer than it was without the greenhouse gas.
Again, my prayer to the anti-AGW gods is that I don’t believe doubling of CO2 will cause 1C. I don’t know how much it would cause. This was just an example which happens to provide equal irritation to both crowds. The believer crowd finds my number of 1C denial low, others here find it believer high.
JAE.
“You evidently don’t understand the article at all! ”
I don’t see how it applies to this post.
“There is NO empirical evidence for an atmospheric greenhouse effect! It is very likely, IMHO, that any warming caused by backradiation is immediatelly cancelled by convection–just like in a real greenhouse when the doors and windows are open.”
I’m not convinced that there is NO evidence, but don’t disagree that changes in backradiation can be cancelled by convection/evaporation/cloud formation etc. It is just an entirely separate issue.
Jae,
New idea, you are a technical guy, why not do a post on it explaining the highlights for others to read. I’ll carry it at tAV.
jae says:
January 6, 2011 at 5:43 am
“You, and nearly everyone else, are arguing that a “greenhouse effect” explains any heating that is above the BB temperature. Siddons is arguing that such an effect is not necessary to explain that extra heat, because of the gas law, T = PV/R. … The article backs up this fact by showing that all planets with atmospheric pressures above 1 bar have about the same T at one bar, REGARDLESS of how much GHGs are present in the atmospheres.”
The study you reference simply shows that the atmospheres of all of the planets are behaving, approximately, as ideal gases. And you left out one variable – the ideal gas law is actually T=PV/nR, where n is moles (from which mass can be calculated).
For a given mass of gas in an enclosed container you can expect different temperatures to generate different pressures because the density (n/V) is a constant. The pressure must change as temperature changes because the volume available to that fixed number of heated molecules is constant.
What prevents an atmosphere from expanding as it heats up? What is your “container”? At a given pressure we fully expect atmospheres of similar makeup to achieve the same temperature – that’s what the ideal gas law shows. If the atmosphere at 1 bar of pressure on Venus were significantly hotter than Earth’s atmosphere at 1 bar of pressure, the question would be “That’s impossible – what is preventing Venus’ atmosphere from expanding? Where’s the invisible glass ceiling?!”
Think about it from sea level up. If we heated the Earth’s atmosphere a few degrees, the mass of the atmosphere wouldn’t change appreciably (with the mass of the increased energy calculated by m=E/c^2). So you get a hotter atmosphere with essentially the same mass, on a planet with the same surface area. With the same mass on the same area, the pressure of the atmosphere at sea level remains constant no matter what temperature you set your atmosphere to. Since the surface temperature is hotter, with the same pressure, the density of the atmosphere at sea level must decrease. And that expansion is going to happen all the way up.
Good point Steve. The key is that in the ideal gas law, the volume (of the atmosphere) is not fixed. It simply adjusts to the temperature. So the temperature of Earth (surface or any other point in the atmosphere) is not ‘fixed’ by the ideal gas law.
Jeff,
Thanks a lot elaborating on DeWitt’s calculations. Mathematically it definitely makes sense. IF you have 1C warmer surface temp THEN you must have a 150 m higher average emission altitude TO MAINTAIN the radiative balance.
But I’m still not sure about the underlying physics. How heat is transported, radiated and even stored due to GHGs: IF you have a doubling of CO2 THEN you have exactly WHAT?
Wouldn’t that be a nice thought experiment? 🙂
ourson:
There have been many different posts on the physics of the greenhous gas effect (and specifically related to CO2) on many different science blogs and science books, each targeting a particular audience. I found that many of the posts for general audience lack the scientific depth needed to understand the physics, and the scientific papers give full depth but not enough width to understand the bigger picture.
One of the best ‘compromise’ explanations I have ever seen is this post from Chris Colose. It’s got depth (explains the GHG effect in detail) and width (explains context, such as why water vapor has little influence over CO2 induced warming).
http://chriscolose.wordpress.com/2010/02/18/greenhouse-effect-revisited
Please check it out, and let me know if this at least addresses some of your questions regarding the physics of GHG theory.
“But I’m still not sure about the underlying physics. How heat is transported, radiated and even stored due to GHGs:”
Now again, I don’t know the magnitude of the effects but transport and radiation work the same for all gasses. Conduction, convection, phase change and radiation. The concept that heat is ‘stored’ in GHG differently than any other gas would be very strange indeed.
The concept of captured heat, has to do with energy but really the energy is not captured. Instead it is a buildup in a continuous flow of power. As energy flow from the surface to space faces a restriction, the energy concentrates a little before the restriction. Therefore the ‘energy’ need not be stored as new energy is continued to be supplied from the sun. If you shut the sun off, it wouldn’t make much difference which gas you had over your head.