Public Release: 20-Dec-2018
New threat to ozone recovery
Study finds chloroform emissions, on the rise in East Asia, could delay ozone recovery by up to 8 years
Massachusetts Institute of Technology
Earlier this year, the United Nations announced some much-needed, positive news about the environment: The ozone layer, which shields the Earth from the sun’s harmful ultraviolet radiation, and which was severely depleted by decades of human-derived, ozone-destroying chemicals, is on the road to recovery.
The dramatic turnaround is a direct result of regulations set by the 1987 Montreal Protocol, a global treaty under which nearly every country in the world, including the United States, successfully acted to ban the production of chlorofluorocarbons (CFCs), the main agents of ozone depletion. As a result of this sustained international effort, the United Nations projects that the ozone layer is likely to completely heal by around the middle of the century.
But a new MIT study, published in Nature Geoscience, identifies another threat to the ozone layer’s recovery: chloroform — a colorless, sweet-smelling compound that is primarily used in the manufacturing of products such as Teflon and various refrigerants. The researchers found that between 2010 and 2015, emissions and concentrations of chloroform in the global atmosphere have increased significantly.
They were able to trace the source of these emissions to East Asia, where it appears that production of products from chloroform is on the rise. If chloroform emissions continue to increase, the researchers predict that the recovery of the ozone layer could be delayed by four to eight years.
“[Ozone recovery] is not as fast as people were hoping, and we show that chloroform is going to slow it down further,” says co-author Ronald Prinn, the TEPCO Professor of Atmospheric Science at MIT. “We’re getting these little side stories now that say, just a minute, species are rising that shouldn’t be rising. And certainly a conclusion here is that this needs to be looked at.”
Xuekun Fang, a senior postdoc in Prinn’s group, is the lead author of the paper, which includes researchers from South Korea, Japan, England, Australia, and California.
Short stay, big rise
Chloroform is among a class of compounds called “very short-lived substances” (VSLS), for their relatively brief stay in the atmosphere (about five months for chloroform). If the chemical were to linger, it would be more likely to get lofted into the stratosphere, where it would, like CFCs, decompose into ozone-destroying chlorine. But because it is generally assumed that chloroform and other VSLSs are unlikely to do any real damage to ozone, the Montreal Protocol does not stipulate regulating the compounds.
“But now that we’re at the stage where emissions of the more long-lived compounds are going down, the further recovery of the ozone layer can be slowed down by relatively small sources, such as very short-lived species — and there are a lot of them,” Prinn says.
Prinn, Fang, and their colleagues monitor such compounds, along with other trace gases, with the Advanced Global Atmospheric Gases Experiment (AGAGE) — a network of coastal and mountain stations around the world that has been continuously measuring the composition of the global atmosphere since 1978.
There are 13 active stations scattered around the world, including in California, Europe, Asia, and Australia. At each station, air inlets atop typically 30-foot-tall towers pull in air about 20 times per day, and researchers use automated instruments to analyze the atmospheric concentrations of more than 50 greenhouse and ozone-depleting gases. With stations around the world monitoring gases at such a high frequency, AGAGE provides a highly accurate way to identify which emissions might be rising and where these emissions may originate.
When Fang began looking through AGAGE data, he noticed an increasing trend in the concentrations of chloroform around the world between 2010 and 2015. He also observed about three times the amount of atmospheric chloroform in the Northern Hemisphere compared to the Southern Hemisphere, suggesting that the source of these emissions stemmed somewhere in the Northern Hemisphere.
Using an atmospheric model, Fang’s collaborators on the paper estimated that between 2000 and 2010, global chloroform emissions remained at about 270 kilotons per year. However, this number began climbing after 2010, reaching a high of 324 kilotons per year in 2015. Fang observed that most stations in the AGAGE network did not measure substantial increases in the magnitude of spikes in chloroform, indicating negligible emission rises in their respective regions, including Europe, Australia, and the western United States. However, two stations in East Asia — one in Hateruma, Japan, and the other in Gosan, South Korea — showed dramatic increases in the frequency and magnitude of spikes in the ozone-depleting gas.
The rise in global chloroform emissions seemed, then, to come from East Asia. To investigate further, the team used two different three-dimensional atmospheric models that simulate the movement of gases and chemicals, given global circulation patterns. Each model can essentially trace the origins of a certain parcel of air. Fang and his colleagues fed AGAGE data from 2010 to 2015 into the two models and found that they both agreed on chloroform’s source: East Asia.
“We conclude that eastern China can explain almost all the global increase,” Fang says. “We also found that the major chloroform production factories and industrialized areas in China are spatially correlated with the emissions hotspots. And some industrial reports show that chloroform use has increased, though we are not fully clear about the relationship between chloroform production and use, and the increase in chloroform emissions.”
“An unfortunate coherence”
Last year, researchers from the United Kingdom reported on the potential threat to the ozone layer from another very short-lived substance, dichloromethane, which, like chloroform, is used as a feedstock to produce other industrial chemicals. Those researchers estimated how both ozone and chlorine levels in the stratosphere would change with increasing levels of dichloromethane in the atmosphere.
Fang and his colleagues used similar methods to gauge the effect of increasing chloroform levels on ozone recovery. They found that if concentrations remained steady at 2015 levels, the increase observed from 2010 to 2015 would delay ozone recovery by about five months. If, however, concentrations were to continue climbing as they have through 2050, this would set a complete healing of the ozone layer back by four to eight years.
The fact that the rise in chloroform stems from East Asia adds further urgency to the situation. This region is especially susceptible to monsoons, typhoons, and other extreme storms that could give chloroform and other short-lived species a boost into the stratosphere, where they would eventually decompose into the chlorine that eats away at ozone.
“There’s an unfortunate coherence between where chloroform is being emitted and where there are frequent storms that puncture the top of the troposphere and go into the stratosphere,” Prinn says. “So, a bigger fraction of what’s released in East Asia gets into the stratosphere than in other parts of the world.”
Fang and Prinn say that the study is a “heads-up” to scientists and regulators that the journey toward repairing the ozone layer is not yet over.
“Our paper found that chloroform in the atmosphere is increasing, and we identified the regions of this emission increase and the potential impacts on future ozone recovery,” Fang says. “So future regulations may need to be made for these short-lived species.”
“Now is the time to do it, when it’s sort of the beginning of this trend,” Prinn adds. “Otherwise, you will get more and more of these factories built, which is what happened with CFCs, where more and more end uses were found beyond refrigerants. For chloroform, people will surely find additional uses for it.”
###
This research was supported by NASA, the National Institute of Environmental Studies in Japan, the National Research Foundation of Korea, the U.K. Natural Environment Research Council, the Commonwealth Scientific and Industrial Research Organization of Australia, and other organizations.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
Discover more from Watts Up With That?
Subscribe to get the latest posts sent to your email.
The biggest threat to the ozone layer is bullshit, now, thanks to modern technology, available in ultra-pure form.
I state that the so-called “ozone hole” is totally natural, a consequency of the distribution of solar energy on the lanet. Prove me wrong b
As one would expect from publications of this kind, the message of an increase in chloroform concentration in the atmosphere over East Asia is not accompanied by data on changes in the concentration of stratospheric ozone in this region. Very similar to the situation in 1980-90, when CFCs were produced in Europe and North America. and the “ozone hole” was discovered over Antarctica. As before, there is no reliable data on the concentration of chloroform in the stratosphere.
It seems to me that, until now, due attention has not been paid to the fact that the C-Cl bond is capable of photochemical splitting already in the lower layers of the atmosphere. Bond energy for C-Cl is of 339 kJ/mol, therefore, the wavelength of the radiation capable of splitting this bond is less than 354 nm, and this radiation freely passes through ozone layer.
Chloroform emissions into the atmosphere are certainly harmful to human health and the environment, but this is unlikely to be related to stratospheric ozone.
That’s why chloroform is classified as a VSLS (very short lived) so it doesn’t have time to reach the stratosphere except in extreme conditions. The AGAGE data shows N hemisphere concentrations of chloroform gradually increasing but not yet in the S Hemisphere.
http://agage.eas.gatech.edu/data_archive/data_figures/monthly/png/CHCl3_mm.png
This confirms that chloroform has no effect on atmospheric ozone, and therefore, the statement “chloroform emissions could delay ozone recovery by up to 8 years” is not justified. By the way, the remark about the splitting of the C – Cl bond in the near UV region also applies to the well-known “long-lived” ozone destroyers CFC-11, CFC-12, and so on.
This confirms that chloroform has no effect on atmospheric ozone, and therefore, the statement “chloroform emissions could delay ozone recovery by up to 8 years” is not justified.
Read the paper, “The fact that the rise in chloroform stems from East Asia adds further urgency to the situation. This region is especially susceptible to monsoons, typhoons, and other extreme storms that could give chloroform and other short-lived species a boost into the stratosphere, where they would eventually decompose into the chlorine that eats away at ozone.”
If it weren’t for that I would agree with you.
By the way, the remark about the splitting of the C – Cl bond in the near UV region also applies to the well-known “long-lived” ozone destroyers CFC-11, CFC-12, and so on.
Actually it takes wavelengths less than 290nm to break the C-Cl bond.
“Actually it takes wavelengths less than 290 nm to break the the C-Cl bond”.
According to Bond Dissociation Energies data, bond energies in CFC-11 and CFC-12 are even less: 305 kJ/mol for Cl-CCl2F and 318 kJ/mol for Cl-CCl2F2 https://labs.chem.ucsb.edu/zakarian/armen/11—bonddissociationenergy.pdf Corresponding maximum wavelengths are 392 and 376 nm. M.Molina and F.S.Rowland (1974) claimed that “stratospheric photolysis will occur mainly in the “window” at 1,750 – 2,200 A (175 – 220 nm)”, https://unep.ch/ozone/pdf/stratopheric.pdf though at wavelengths less than 240 nm the O=O bond already splits and ozone itself is formed. So, I’d like to know who and how found this “actual value” 290 nm (link, please).
According to Bond Dissociation Energies data, bond energies in CFC-11 and CFC-12 are even less: 305 kJ/mol for Cl-CCl2F and 318 kJ/mol for Cl-CCl2F2 https://labs.chem.ucsb.edu/zakarian/armen/11—bonddissociationenergy.pdf Corresponding maximum wavelengths are 392 and 376 nm.
That’s your mistake, assuming that a wavelength corresponding to the bond dissociation is what is needed to break the bond. First of all the molecule needs to be able to absorb the wavelength, and that depends on the electronic structure of the molecule, subsequent distribution of the energy to the vibration of the various bonds can cause dissociation of the bond. Generally it’s necessary to absorb more energy in total that required for dissociation. In the case of CFCs they do not absorb in the wavelengths that you give but actually at much shorter wavelengths.
Atmos. Chem. Phys., 16, 8043–8052, 2016 http://www.atmos-chem-phys.net/16/8043/2016/ doi:10.5194/acp-16-8043-2016
M.Molina and F.S.Rowland (1974) claimed that “stratospheric photolysis will occur mainly in the “window” at 1,750 – 2,200 A (175 – 220 nm)”, https://unep.ch/ozone/pdf/stratopheric.pdf though at wavelengths less than 240 nm the O=O bond already splits and ozone itself is formed. So, I’d like to know who and how found this “actual value” 290 nm (link, please).
The ‘window’ is where the CFCs absorb and where the paper I cite measures the absorption cross-section.
Regarding Hawai being a accurate way to measure CO2, you must be joking. The station is surrounded vents puffing out CO2.
Best place would be Tasmania, the Mountains to their West. A vast area of Ocean too their West to accurately measure.
Re. the so called Ozone Hole, Du Pont did very well out of that farce, and no doubt when their present patient expires expect something similar to happen.
I recall the Fuss over Indian Hemp, because it competed against Nylon back in the 1930 tees. Du Pont was again involved.
MJE
Exactly, Cape Grim, where they also measure CO2, currently ~405ppm, less seasonal variation as is typical in the S hemisphere.
“What our country needs is tax reform and land reform. Instead, all we get is chloroform! Shall we continue to remain asleep?”
Moe Howard, Three Dark Horses, 1952
It’s about time they stopped beating this dead horse. The ozone “hole” has long been determined to be natural.
It would be great if there were some figures given fore the annual manufacture of chloroform, reliable ones.
Many references say it is used in the production of other chemicals, so that would often destroy the chloroform.
It is a heavy molecule, beats me how it makes it to these atmospheric heights.
As a chemist, I am not impressed by the supposed dangers of chloroform. Geoff
And also how they all migrate over the south pole.
It would be great if there were some figures given fore the annual manufacture of chloroform, reliable ones.
Many references say it is used in the production of other chemicals, so that would often destroy the chloroform.
Indeed, I recall back in the 60s chloroform was used in labs as a ‘universal organic solvent’ to cleanup glassware etc., the labs always smelled of it (as did my lab coat!). I suspect that a similar laxity in its use may be the reason for the increase now.
It is a heavy molecule, beats me how it makes it to these atmospheric heights.
As a chemist, I am not impressed by the supposed dangers of chloroform. Geoff
Simple the lower part of our atmosphere is referred to as the homosphere, that is constant composition for long lived stable gases (up to 100km), heaviness has nothing to do with it.
SF6 is another ‘heavy’ gas which because of its inertness and IR signature is used as a tracer of atmospheric transport.
For example:
“SF6 provides a useful tracer of atmospheric transport in both the troposphere and stratosphere. Rates for transport of pollutants into, within and out of the stratosphere are important parameters that regulate stratospheric composition. The basic characteristics of the stratospheric Brewer–Dobson circulation (BDC) are known from observations of trace gases such as SF6: air enters the stratosphere at the tropical tropopause, rises at tropical latitudes, and descends at middle and high latitudes to return to the troposphere. Understanding the rate of this transport on a global scale is cru- cial in order to predict the response of stratospheric ozone to climatic or chemical change. SF6 is essentially inert in the troposphere to middle stratosphere and is removed by electron attachment and photolysis in the upper stratosphere and mesosphere (Ravishankara et al., 1993). This tracer therefore provides an ideal probe of transport on timescales of importance in the stratospheric circulation and quantitative information on mean air mass age for the lower and middle stratosphere.”
https://core.ac.uk/download/pdf/74236447.pdf
It takes something like 5-10 years for a release in one place to spread around the globe and into the stratosphere.
Phil,
While gaseous chloroform has a molecular weight of about 120 to compared with N2 of about 28 (and SF6 about 140), I am surprised by claims of atmospheric homogenization. After all, climate research makes frequent use of partitioning of gas isotopes like oxygen and the carbon in CO2, which are very much closer together in weight and size.
So, why do isotopes partition, when chloroform and nitrogen in the air are claimed not to? Geoff
Partioning of isotpes is taking place during phase-changes(liquid-gas) not weithin one phase.
BR
MFKBoulder
MFKB,
So why does the light gas helium escape to space, while you say that the heavy gases SF6 and CHCl3 can’t sink down to earth? And does not chloroform exist in nature as both liquid and gas?
Geoff.
Hi Geoff,
vapor of chloroform of SF6 druing conditions of laminar flow will gather at the bottom of a given space. As soon as the flow is not laminar (as in the turbulent atmosphere) the mixing with the other gases will occur. “Demixing” under normal (atmospheric) conditions is not observed: Otherwise only giraffe cpuld suvive stickng their head above the 3m CO2 covering the earth surfcae.
Yes choloform exists under “normal conditions” as gas and liquid. Is there an issue I did not see?
So why does the light gas helium escape to space, while you say that the heavy gases SF6 and CHCl3 can’t sink down to earth? And does not chloroform exist in nature as both liquid and gas?
The kinetic energy of Helium in the atmosphere is such that individual molecules can have an escape velocity so in the upper atmosphere if they don’t strike another molecule they will continue uninterrupted into space. Heavier molecules aren’t able to reach escape velocities. Heavier gases constantly collide with other gases and maintain their mixing, there’s no mechanism to segregate them. At 10ppt concentrations in the atmosphere chloroform is only capable of being a gas, its vapor pressure at -80ºC is ~0.1kPa (way above 10ppt).
Covering their arses — if ozone doesn’t recover as advertised, new “excuses” will be manufactured.
Stratospheric ozone hit it’s lowest (known) level in 1962. It hit a high in ’69-’70 and then dropped again and started rising in 1986. But cherry picking natural fluctuations is great for inducing panic and mass hysteria.
Hi Art,
can you supply any references?
Ozone generally is measured as a total Ozone coulumn at Halley, Antarctica:
The Earth is a whole lot bigger than Halley, Antarctica. That info comes from satellite data which covers the globe much more completely and is in the citations below in my response to Clyde.
Which satellite was measuring stratospheric ozone in 1962, just 5 years after Sputnik 1 was launched?
The satellite data refers to “hit a high in ’69-’70 and then dropped again and started rising in 1986.”
Again which satellite data, it isn’t cited in the reference you gave and just asserts it in vague terms?

Here’s some real satellite data:
Art
Could you please provide citations for specific information like this that is not widely known? I’m dubious about your claims about lowest and subsequent high ozone levels in the ’60s because the Total Ozone Mapping Spectrometer didn’t start providing world-wide coverage until late-1978.
I’m quoting from Dr. Dixy Lee Ray who cites:
‘Two Environmental Issues: 1. Ozone, 2. The Greenhouse Problem,” a report to the World Affairs Council, Pittsburgh, George C. Marshall Institute, Washington DC, “Ozone,” Dec. 1991, pp 1 – 7.
Ellsaesser, Hugh W., 1991 “The Holes in the Ozone Hole II,” Cato Institute Conference, Washington DC, June 5-6, 1991
A quote of a quote of a quote
During my studies this was a “NoQuote”.
So that disqualifies any info contained therein? I could have just quoted her sources and you wouldn’t have known the difference, but then you’d have judged them valid? Sounds like you’re just looking for an out.
Well in the Cato Institute paper the author says: “We know that ozone was at a lower level in 1962 than it is today.”
That’s it, no source, no justification for that assertion.
Some additional remarks about the photodissociation of C-Cl bond.
I think it’s important in this thread, because if this bond can split in the lower troposphere, this calls into question the theory that organochlorine compounds rise into the stratosphere and, by cleaving chlorine, destroy the ozone layer.
Objecting to me, Phil wrote (December 26, 8:41 a.m.):” That’s your mistake, assuming that a wavelength corresponding to the bond dissociation is what is needed to break the bond. First of all the molecule needs to be able to absorb the wavelength, and that depends on the electronic structure of the molecule, subsequent distribution of the energy to the vibration of the various bonds can cause dissociation of the bond. Generally it’s necessary to absorb more energy in total that required for dissociation. In the case of CFCs they do not absorb in the wavelengths that you give but actually at much shorter wavelengths.
Atmos. Chem. Phys., 16, 8043–8052, 2016 http://www.atmos-chem-phys.net/16/8043/2016/ doi:10.5194/acp-16-8043-2016 “
It’s not my mistake. I am ready to admit this as a mistake if, for example, the authors of this textbook agree with this: Th.L. Brown a.o. Chemistry. The Central Science. Pearson. 2009, p.771 (Calculation the wavelength requiring to break a bond). You can also recall that in this discussion, arguing with Anthony Barton (December 26, 9:10 a.m.) Phil have written:” The source is due to the absorption of UV radiation by oxygen molecules:
O2 + hν→ O+O (less than 240 nm)
O2 + O + M→ O3+M ”
So, the calculation of the maximum wavelength by the binding energy is correct for O = O bond and is incorrect f C – Cl bond (rhetoric question). And about the link given by Phil (I’ve read the full text): https://www.cfa.harvard.edu/atmosphere/publications/28.OrphalChance-491-504.pdf
This article talks about the UV spectra of some CFCs in the range of 190-235 nm. In this interval, photodissociation of C – Cl, C – F and O = O bonds occurs, so this study is not related to the question under consideration. The question is what the wavelength is necessary for splitting the C-Cl bond, and the article does not contain information on this issue.
Finally, a link to one important publication. G.Crecentini and F.Bruner (1980) using gas chromatography with mass-spectrometric detector found the presence of CFC-21 (CHCl2F) in the place that it was not used. https://www.sciencedirect.com/science/article/abs/pii/S0022113900841226 Authors’ explanation is: CFC-21 is a product of decompositon of CFC-11 (CCl3F) after photochemical splitting of one C-Cl bond and further substitution of Cl by hydrogen atom. It is important that this process takes place in the lower troposphere and can be considered as experimental evidence of the photolytic decomposition of the C – Cl bond under these conditions.
“That’s your mistake, assuming that a wavelength corresponding to the bond dissociation is what is needed to break the bond. First of all the molecule needs to be able to absorb the wavelength, and that depends on the electronic structure of the molecule, subsequent distribution of the energy to the vibration of the various bonds can cause dissociation of the bond. Generally it’s necessary to absorb more energy in total that required for dissociation. In the case of CFCs they do not absorb in the wavelengths that you give but actually at much shorter wavelengths.”
In order to break a bond it is necessary to increase the vibrational energy in that bond until it exceeds the bond dissociation energy. In order to absorb UV radiation the wavelength has to excite a resonance in the electron cloud of the molecule. In order to cause dissociation of a bond sufficient energy must then be transferred to the bond to be broken.
So, the calculation of the maximum wavelength by the binding energy is correct for O = O bond
So in order to break the bond the molecule has to be able to absorb a photon of sufficient energy, frequently that isn’t possible at the wavelength corresponding to the bond energy. In the case of a diatomic molecule there is only one bond so it is possible that all the energy absorbed can be transferred to that bond and break it. This is indicated in the case of oxygen.
However in the case of a polyatomic molecule even if it is able to absorb photons of the bond energy the energy is likely to be distributed among the different bonds not all to one. In the case of CFCs the molecule doesn’t absorb at the wavelength corresponding to the bond energy.
The question is what the wavelength is necessary for splitting the C-Cl bond
That is the wrong question, what you should ask is what wavelength that the molecule is capable of absorbing is able to break the bond.
I read the paper you referenced and see no evidence that CFC-21 is a photolysis product, it seems more likely to me that it is the result of a reaction between OH and CFC-11
OH + CCl3F → CHCl2F + ClO
Some bronchodilators actually contained a mixture of CFC-21 and CFC-11
What puzzles me is that the ‘hole’ in the ozone layer receives much comment, but the concomitant increase in the ozone concentration around the hole receives so little comment.
I should appreciate it if somebody would explain why, as the ‘hole’ develops, the periphery shows an increase. It looks much like a moving sideways of the ozone rather than ozone being destroyed.
Its a long read but this chapter will assist. https://reality348.wordpress.com/2016/05/14/23-the-dearly-beloved-antarctic-ozone-hole-a-function-of-atmospheric-dynamics/
Specifically, The troposphere is rich in NOX. That’s why it is almost devoid of ozone. NOx is emitted from soil. More is emitted in summer than winter. Consequently stratospheric ozone levels peak in winter.
Re the hole:
NOx charged air gradually occupies the entire space over the Antarctic continent that formerly exhibited high surface pressure, extreme cold and a very dry atmosphere with some ozone. This is the process that erodes ozone to produce the ‘ozone hole’. It proceeds by gradual replacement of one sort of air with another, the latter including a compound, namely NOx, that soaks up ozone. It closes from the perimeter like the iris in the aperture of a camera.
Plainly NOx rich air is progressively entrained into the core of the circulation over the continent as mesospheric air stalls in its descent. NOx rich air from below 50 hPa accumulates in the lower stratosphere as the formerly descending circulation withdraws. The hole is a function of atmospheric dynamics that are initiated in August on the margin of the ‘night zone’.
Suggest you read up on ‘denitrification’ of the polar stratosphere, extreme low levels of O3 are associated with air pockets which have extremely low levels of nitric acid, i.e. those which has experienced denitrification by PSCs. The NOx rich air is a myth.
https://pdfs.semanticscholar.org/5df5/8604ef57a30b225113c55e9be68965f93f27.pdf
It’s due to the Brewer-Dobson circulation. Brewer-Dobson Circulation, is a circulation pattern that sets up between equator and pole. First, air is lifted out of the tropics from the troposphere to the stratosphere, where it acquires a high ozone content in the photochemical source region of the tropical high stratosphere. Then this high-ozone air moves poleward and downward, descending into the lower stratosphere in the polar latitudes . It is the reason for the observed column ozone distribution: low in the tropics and high in the polar regions. A complication in the southern hemisphere is the strong polar vortex that forms in the austral winter and effective isolates the polar air (~60ºS). Consequently the B-D flow stops short of the pole and ozone accumulates to the north of the vortex. In the late spring (november) the vortex weakens and the flow can resume and the accumulated ozone can mix with the ozone depleted polar stratosphere.
The Brewer Dobson circulation is a product of the imagination of two guys who worked in the nineteen thirties when the notion of the stratosphere was very new. Today, some of us know a lot more about atmospheric processes.
But for the release of NOx by soil ozone would increase in concentration all the way to the surface of the planet.
Ozone concentration peaks in winter due to lower soil temperature and diminished release of NOx.
In the winter, the tropopause is lower in the northern hemisphere than in summer.
Ozone in the stratosphere is not there due to photolysis of oxygen. That process is exhausted in the ionosphere. Wave lengths short enough to cleave oxygen are not present in the stratosphere and certainly completely unavailable over the poles in winter and springtime.
However, the longer wave lengths capable of cleaving ozone do reach the stratosphere, and in the absence of ozone the surface of the Earth. That said, the stratosphere is a relatively safe zone for ozone. Its free of attack by NOx from below or radiation from above……except in the polar regions during winter where there is a descent of NOx from the mesosphere. So, if ozone is transported from higher elevations into what we call the stratosphere, it can persist in concentrations sufficient to make the lapse rate positive rather than negative.
Its free of attack by NOx from below or radiation from above……except in the polar regions during winter where there is a descent of NOx from the mesosphere. So, if ozone is transported from higher elevations into what we call the stratosphere, it can persist in concentrations sufficient to make the lapse rate positive rather than negative.
Several observation refute your claim. Firstly the ozone peaks at ~16km over the S Pole during the winter. NOy in the form of nitric acid descends from the mesosphere but disappears from the gas phase as it crystallizes, those crystals fall through the lower stratosphere in a process referred to as denitrification, when it reaches the warmer atmosphere below the crystals sublime in the process of renitrification. During the spring the ozone around the peak starts to decline in the absence of HNO3, below ~16km and above ~22km the O3 is unaffected. This process continues until early october by which time the O3 concentration in the vicinity of 16-20km is zero, the O3 below ~16km and above ~22km is unchanged. By november the stratosphere above 22km has started to warm up significantly (about -40ºC at 25km vs -80º in winter), at the same time the O3 at 25km has increased to about 8mPa (Cf ~4mPa in winter) while the O3 in the hole is still ~0. By mid december the temperature at 25km has continued to warm and the O3 has increased to ~13mPa and this high O3 atmosphere has started to descend into the former hole and the low O3 minimum has descended to ~12km.
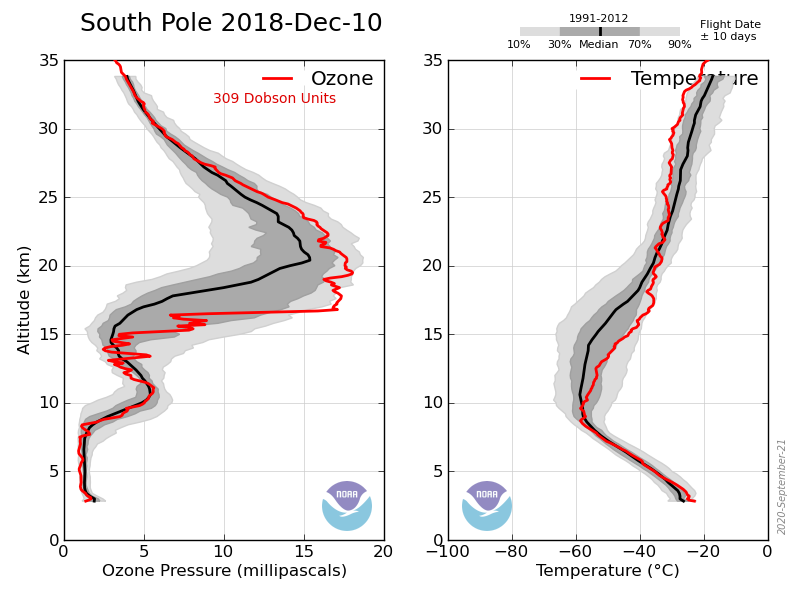
Your explanation: ” By November, gentle ascent is the rule and the circulation is clockwise rather than anticlockwise as it is in the winter. The tropospheric air is drawn in predominantly at the 50hPa level, and this creates the ‘hole’”, is contradicted by this data, the hole is being filled in november not created and the ozone appears to come from above not below.
Phil, Its important to grasp the fundamentals that condition the circulation of the air in high southern latitudes.
1. The zone of low surface pressure surrounding the Antarctic continent represents a planetary trough without comparison.
2. Low surface pressure is due to elevated total column ozone. Ozone absorbs infrared energy from the Earth. The upper portion of the atmospheric column is rarified. The entire column weighs less because there are fewer molecules stacked up at 60-70° south latitude.
3. Surface pressure has been in decline for seventy years across all high southern latitudes.
4. The temperature of the upper air in the entire southern hemisphere increased between 1948 and 1978 by about 10C. It has been in slow decline since that time but not in the ozone hole months when surface pressure falls to its annual minimum.
5 The circulation of the upper air slows dramatically and reverses in November then back to West to East by February-March. Its the transition from descent to ascent over the pole that is associated with the tightening of a noose of high Nox air of tropospheric origin at 50hPa. That air is drawn into the ascending vortex created by polar cyclones that surround the continent as is the descending air over the continent itself. As atmospheric pressure falls away over the continent there is a natural decay of the descending circulation over the continent. Within the vortex there is ozone. But the tropospheric air has virtually none. The tropospheric air substitutes for the descending relatively ozone rich air as it ceases to descend. Meanwhile, ozone partial pressure is falling away north of 60° of latitude yielding a rise in surface pressure across all high southern latitudes. The flow of the atmosphere with its very different parcels of air, so far as ozone content is concerned is conditioned by surface pressure. These are the fundamentals of atmospheric dynamics that determine the air flows.
Change in the nature of the hole is due to change in the background circumstances that determine atmospheric dynamics.
There is no better place to study these phenomena than at http://macc.aeronomie.be/4_NRT_products/5_Browse_plots/1_Snapshot_maps/index.php?src=MACC_o-suite&l=TC
Thank you, Erl & Phil, much appreciated.
A lot to get my head around, particularly with your ongoing discussions…
Thank you again.
Dobson observed the hole from the British Antarctic base at Halley Bay before the advent of refrigeration.
In Philvs comments you find those Details, wjic are omitted by the folks wjo starte: Dobson found the ozone hole in 1957:
https://wattsupwiththat.com/2018/12/25/new-threat-to-ozone-recovery/#comment-2566912
BR
Sorry for the typos, not acquainted to MY smartphone…
No he didn’t, he observed that the springtime ozone was lower than observed in the Arctic (Spitzbergen), however it was still ~300DU well above today’s levels. What is known as the Ozone hole is the rapid drop in the springtime O3 to about 90DU which started in ~1979. Reasons for the levels being lower than the Arctic could include weaker Brewer-Dobson circulation, stronger polar vortex and lower temperatures leading to PSC formation. The rapid drop through the 80s required a new sink for the O3 involving Cl.
Phil, that’s all toss I am afraid.
Unlike the Arctic, the Antarctic experiences a global high in surface atmospheric pressure in winter creating a gently descending circulation inside the vortex of cyclones circulating on the margin of the continent. As winter gives way to spring this circulation weakens and tropospheric air, devoid of ozone takes the place of the ozone bearing air inside the vortex. As the descending circulation inside the vortex weakens, the zone of descent ascends. By November, gentle ascent is the rule and the circulation is clockwise rather than anticlockwise as it is in the winter. The tropospheric air is drawn in predominantly at the 50hPa level, and this creates the ‘hole’. Variation from year to year and across the decades is an entirely natural meteorological phenomenon due to changing surface pressure relations with the ‘hole’ peaking on the average a month later in recent decades by comparison with the decades prior to the 1970s.
The progressive appearance of the hole at the 50 hPa level can be traced at this site: http://macc.aeronomie.be/4_NRT_products/5_Browse_plots/1_Snapshot_maps/index.php?src=MACC_o-suite&l=TC
The northern hemisphere occasionally experiences the same phenomenon despite its very different geography and pressure relations.
The hole is a meteorological phenomenon.
As for the Brewer Dobson circulation hypothesised in the 1930s, the circulation of the atmosphere at high altitudes is away from, rather than towards the poles. That’s apparent if one looks at the distribution of ozone at the 10 hPa level and higher as seen here: http://www.cpc.ncep.noaa.gov/products/stratosphere/strat_a_f/
Dobson observed that low pressure cells had a rarified upper atmospheric column due to the enhanced ozone content. The tropopause in a low pressure cell is always low by several km by comparison with the tropopause of a high pressure cell. In fact, a map of total column ozone is as good for weather forecasting as a map of surface pressure. It is not strange therefore that a deficit of ozone at some particular elevation is related to meteorological phenomena and seasonal change in the same.
In similar news, a woman waved her hands around in the air in a farm barn at a fat cow and claims to have caused the spontaneous splitting of the cow into a little cow and one a little smaller than the original. People amazed and convinced her expert hand waving can create new life after watching the amazing video..