Rate May Be Ten Times Faster, According to New Data

From Lamont-Doherty Earth Observatory: Some 56 million years ago, a massive pulse of carbon dioxide into the atmosphere sent global temperatures soaring. In the oceans, carbonate sediments dissolved, some organisms went extinct and others evolved.
Scientists have long suspected that ocean acidification caused the crisis—similar to today, as manmade CO2 combines with seawater to change its chemistry. Now, for the first time, scientists have quantified the extent of surface acidification from those ancient days, and the news is not good: the oceans are on track to acidify at least as much as they did then, only at a much faster rate.
In a study published in the latest issue of Paleoceanography, the scientists estimate that ocean acidity increased by about 100 percent in a few thousand years or more, and stayed that way for the next 70,000 years. In this radically changed environment, some creatures died out while others adapted and evolved. The study is the first to use the chemical composition of fossils to reconstruct surface ocean acidity at the Paleocene-Eocene Thermal Maximum (PETM), a period of intense warming on land and throughout the oceans due to high CO2.
“This could be the closest geological analog to modern ocean acidification,” said study coauthor Bärbel Hönisch, a paleoceanographer at Columbia University’s Lamont-Doherty Earth Observatory. “As massive as it was, it still happened about 10 times more slowly than what we are doing today.”
The oceans have absorbed about a third of the carbon humans have pumped into the air since industrialization, helping to keep earth’s thermostat lower than it would be otherwise. But that uptake of carbon has come at a price. Chemical reactions caused by that excess CO2 have made seawater grow more acidic, depleting it of the carbonate ions that corals, mollusks and calcifying plankton need to build their shells and skeletons.

In the last 150 years or so, the pH of the oceans has dropped substantially, from 8.2 to 8.1–equivalent to a 25 percent increase in acidity. By the end of the century, ocean pH is projected to fall another 0.3 pH units, to 7.8. While the researchers found a comparable pH drop during the PETM–0.3 units–the shift happened over a few thousand years.
“We are dumping carbon in the atmosphere and ocean at a much higher rate today—within centuries,” said study coauthor Richard Zeebe, a paleoceanographer at the University of Hawaii. “If we continue on the emissions path we are on right now, acidification of the surface ocean will be way more dramatic than during the PETM.”
The study confirms that the acidified conditions lasted for 70,000 years or more, consistent with previous model-based estimates. “It didn’t bounce back right away,” said Timothy Bralower, a researcher at Penn State who was not involved in the study. “It took tens of thousands of years to recover.”
From seafloor sediments drilled off Japan, the researchers analyzed the shells of plankton that lived at the surface of the ocean during the PETM. Two different methods for measuring ocean chemistry at the time—the ratio of boron isotopes in their shells, and the amount of boron –arrived at similar estimates of acidification. “It’s really showing us clear evidence of a change in pH for the first time,” said Bralower.
What caused the burst of carbon at the PETM is still unclear. One popular explanation is that an overall warming trend may have sent a pulse of methane from the seafloor into the air, setting off events that released more earth-warming gases into the air and oceans. Up to half of the tiny animals that live in mud on the seafloor—benthic foraminifera—died out during the PETM, possibly along with life further up the food chain.
Other species thrived in this changed environment and new ones evolved. In the oceans, dinoflagellates extended their range from the tropics to the Arctic, while on land, hoofed animals and primates appeared for the first time. Eventually, the oceans and atmosphere recovered as elements from eroded rocks washed into the sea and neutralized the acid.
Today, signs are already emerging that some marine life may be in trouble. In a recent study led by Nina Bednaršedk at the U.S. National Oceanic and Atmospheric Administration, more than half of the tiny planktic snails, or pteropods, that she and her team studied off the coast of Washington, Oregon and California showed badly dissolved shells. Ocean acidification has been linked to the widespread death of baby oysters off Washington and Oregon since 2005, and may also pose a threat to coral reefs, which are under additional pressure from pollution and warming ocean temperatures.
“Seawater carbonate chemistry is complex but the mechanism underlying ocean acidification is very simple,” said study lead author Donald Penman, a graduate student at University of California at Santa Cruz. “We can make accurate predictions about how carbonate chemistry will respond to increasing carbon dioxide levels. The real unknown is how individual organisms will respond and how that cascades through ecosystems.”
Other authors of the study, which was funded by the U.S. National Science Foundation: Ellen Thomas, Yale University; and James Zachos, UC Santa Cruz.
This cannot be : a recycle of all the previous catastrophic ocean acidification “garbage” fear mongering studies. Simply shuffle the positions of words such as “could”, “perhaps”, “possibly”, and, of course, “estimate”. The watermelons just keep recycling this trash, and the left of liberal media spoon feed it to the 47 percent too dumb, but allowed to vote anyway.
As I have posted before, this will never end, I see nothing but very bad, ahead.
You mean all the sea organisms today are the ones which survived the last event and are therefore capable of accepting similar levels?
You mean we wouldn’t be here at all if the last event had not happened?
” the pH of the oceans has dropped substantially, from 8.2 to 8.1–equivalent to a 25 percent increase in acidity”
Really???
“she and her team studied off the coast of Washington, Oregon and California showed badly dissolved shells.”
Probably right around spreading centers where a lot of acidic water is being pumped into the ocean.
A pH of 7.8… Uh, that’s still alkaline. Not acid until it goes below 7.0.
Where’s the news here?
“have made seawater grow more acidic”
Utter BS..
The seas ARE NOT acid and they WILL NEVER be acid.
Since they are not acidic, they cannot become more acidic.
They may have become a tiny bit LESS BASIC or LESS CAUSTIC
,
but that is not the same thing, and may I fact be a GOOD THING…..
just like everything else to do with increasing atmospheric CO2.
And I would love to see how they measured the PH of sea water to the nearest 0.1 , 150 years ago. !!
Just like in the recent past, the warming in the PECM preceded the increase in CO2 levels.
As usual, alarmists don’t like facts getting in the way of their theories.
http://adsabs.harvard.edu/abs/2007Natur.450.1218S
In the last 150 years or so, the pH of the oceans has dropped substantially, from 8.2 to 8.1–equivalent to a 25 percent increase in acidity. By the end of the century, ocean pH is projected to fall another 0.3 pH units, to 7.8.
Better check the promulgation and evolution of The Message.
http://ocean.nationalgeographic.com/ocean/critical-issues-ocean-acidification/
(no date, apparently dynamic page, subject to change)
So drop about fifty years from the dramatic rise of Ocean Neutralization for a more worrisome rate.
But the new work says the pH drop by 2100 has been drastically cut, down to 0.3 pH from 0.5.
There is hope! Human impact will not be as bad as was thought!
From 2008, article, shouldn’t change:
http://www.scientificamerican.com/article/rising-acidity-in-the-ocean/
So what they used to say we filthy humans did in 50 to 80 years, we perversions of the natural order now did in 150 to 200 years, we took three times longer.
The “facts” are getting less alarming, humans aren’t as badly the horrendous destroyers of innocent planets we used to be.
Things are really looking up!
Oh my God noooooo….It’s even worserer than we thought.
From A Basic History of Acid—From Aristotle to Arnold:
There is no global database of pH variability in the oceans. Dore et al. (2009) uses data from station ALOHA to document a “decreasing trend” of pH, “which is indistinguishable from the rate of acidification expected (emph. added) from equilibration with the atmosphere.”
From:
Dore et al. (2009) Physical and biogeochemical modulation of ocean acidification in the central North Pacific. PNAS July 28, 2009 vol. 106 no. 30:12235–12240
From (Reference 9 in quote above):
9. Takahashi T, et al. (2009) Climatological mean and decadal change in surface ocean
pCO2, and net sea-air CO2 flux over the global oceans. Deep-Sea Res II 56:554–577.
Seriously. Can someone explain to me how the warming oceans take up this carbon dioxide whilst at the same time giving it off??
http://www.newscientist.com/article/dn20413-warmer-oceans-release-co2-faster-than-thought.html#.U47SPdq9KSM
pH is a logarithmic scale, so to say that it is 25% more acidic because it has moved by 0.1 pH units is utter bs. It would be 25% more acidic if it moved by 3.5 pH units. True, there are 25% more hydrogen ions, but there are 25% more hydrogen ions for any 0.1 movement in pH. If we used another set of pHs for example, that water at a pH of 7.0 (that is, completely neutral) is 25% more acidic than water at pH 7.1 (barely alkaline) shows you the meaninglessness of their statement.
The benthic extinction at the PETM is a bit more complex than this study suggests. There was another step in the process of generating the CO2 which is overlooked. The most popular theory of the origin of the CO2 was a massive dissociation of methane hydrate on the continental shelves and perhaps deeper environments. The resultant methane would have quite rapidly (geologically) degraded into CO2. That was not the killer. When one liter of methane hydrate dissociates on the seabed, it produces 160 liters of methane and 800ml of pure water. Note that – pure water – not salt water. The result would have been a fresh water, or at least brackish water horizon at the seabed sediment/ seawater interface. This would have killed or severely depleted benthic faunas. The evidence of this can be seen in some areas where Paleocene/Eocene boundary sediments have manganese layers in sediments interpreted to have been deposited in relatively shallow waters – not the deep waters where manganese nodules are well known to be deposited. Manganese will only precipitate out of sea water in the presence of freshwater. The manganese rich layer at the P/E boundary across the North Sea, for instance, could not have been deposited due to fluvial influences around the basin as it is so widespread. It had to have been a synchronous major event such as a methane dissociation event.
A secondary effect of the release of methane into the oceans at that time would have been a reduction of buoyancy for marine planktic species from whales to foraminifera. The partial extinction of planktic foraminiferal species during the same event may be related to this as some species were more able to cope with the reduction in buoyancy due to their shape than others.
To say that CO2 was the killer is to simplify the extinction event and to focus the attention on a fashionable possibility.
It may be worth saying that the greenhouse earth that developed after this event was responsible for massive greening of the Eocene world which allowed the development of a variety of mammalian species and the rapid evolution of primates.
Any article that claims a pH of 8.1 is acidic is pure BS and it, together with the researchers, should be binned and banned.
Ocean pH has not been measured for long enough yet to make any assumptions about pH cycles. It has been known for years, longer than the CAGW crap, that ocean pH varies round the planet from 8.4 to 7.6 in surface waters. Waters surrounding thermal vents is acidic at pH4,5 or less.
This claim is total crap produced by morons.
‘From seafloor sediments drilled off Japan’. There is, off course, no tectonic activity there.
Timothy Bralower, a researcher at Penn State who was not involved in the study. “It took tens of thousands of years to recover.
I guess I can make a legitimate comment too. After all, I wasn’t involved in the study either.
My comment is ‘ Utter BS’
So, what was the pH of the ocean during the geological epochs when atmospheric CO2 levels were over 1500 ppm?
What is the error in the measurement of pH levels?
IMHO, there isn’t enough data to reliably calculate the average pH of today’s oceans, let alone the average pH 150 years ago.
Usually I don’t agree with with those who simply dismiss papers as ”garbage” but in this case I tend to agree.
“Some 56 million years ago, a massive pulse of carbon dioxide into the atmosphere sent global temperatures soaring.”
Actually the best profiles show the normal pattern – warming (and the biotic changes it caused) came first, the carbon isotope changes followed slightly later.
“In the last 150 years or so, the pH of the oceans has dropped substantially, from 8.2 to 8.1”
The pH scale wasn’t even invented until 1909, and consistently measuring pH differences of 0,1 units in the laboratory is challenging even today, so how do they know?
“ocean acidity increased by about 100 percent in a few thousand years”
And exactly what does 100% mean on a logarithmic scale?
“Up to half of the tiny animals that live in mud on the seafloor—benthic foraminifera—died out during the PETM, possibly along with life further up the food chain.”
One of the most embarrassing things with the PETM is that despite the very large and sudden warming practically no extinctions resulted. About the only exception is the benthic foraminifera (which by the way are very far from the only animals “that live in mud on the seafloor”). That extinction is usually ascribed to the fact that the temperature of the deep seas increased by about 10 degrees (centigrade) in just a few thousand years. There is no sign of any extinctions “further up the food chain”, nor in surface waters where this “terrible acidification” occurred.
To the contrary during the PETM life both on land and in the oceans flourished and dispersed as never since. The ancestors of a very large proportion of the animals alive today (including us) first show up during the PETM.
“more than half of the tiny planktic snails, or pteropods, that she and her team studied off the coast of Washington, Oregon and California showed badly dissolved shells. Ocean acidification has been linked to the widespread death of baby oysters off Washington and Oregon since 2005”
It should be pointed out that the “acidification” in this case is due to increased upwelling of deep water in the coastal areas. Deep ocean water contains less oxygen and more CO2 than surface water. Deep ocean water has a turnover time of c. 1000 years before it returns to the surface. During these 1000 years deep ocean life consumes a lot of the oxygen and turns it into CO2. Upwelling waters incidentally are also very rich in nutrients, so the fisheries on the West Coast are very dependent on these periodic upwellings, though it can be bad for oyster larvae. So – no this “acidification” has little or nothing to do with the modern increase of atmospheric CO2. Must be due to all those medieval SUV:s which caused the MWP.
From 2012, a WUWT repost of a great Jo Nova piece examining a new Scripps paper:
Many additional “acidification” links at Jo Nova, the WUWT version includes the full Matt Ridley piece. Both have informative graphs. Enjoy.
For those who are wondering:
pH=8.2 gives a H concentration of 6.3095E-9
pH=8.1 gives a H concentration of 7.9432E-9
7.9432-6.3095=1.6337
(1.6337 · 100)/6.3096 = 25.89%
So, let me see if I have this straight: human related emissions are adding CO2 to the atmosphere, resulting in warming. (Apparently Mosher says CO2 is a “control knob”, right?).
Warming causes the oceans to warm; when they do, they outgas CO2, correct?
How then do the oceans manage to absorb more CO2, resulting in a lowering of pH? (doesn’t matter whether anyone calls it acidifying, or being less alkaline: that’s what it comes down to).
Are the oceans capable of absorbing more CO2 than they outgas as they warm, because of higher concentrations in the atmosphere? Has anyone proven this using experiments?
This is a bit bizarre. Ocean ‘acidification’ is if anything causing the ocean to become less alkaline based on those pH values – as others have commented. But we have to assume surely that the authors of this work also know this. So what would they say is the reason why the ocean becoming more neutral is bad for shellfish? I’d they were here defending their work I mean? We can’t just assume they don’t know basic chemistry surely?
Caustic comment about acidic sea.
Fish swim and pee in the sea.
Bull Mer’d comes from land.
Thankyou Urederra- the first intelligent comment on this thread.
Siberian_husky says:
June 4, 2014 at 2:06 am
Thankyou Urederra- the first intelligent comment on this thread.
Wow! Really?
Siberian_husky said on June 4, 2014 at 2:06 am:
Thanks for the insult. The best to you too, bud.
So which of the two urederra comments was the first intelligent one? If it was the second, in what ways were the first one unintelligent? If the first, does the second also pass your threshold for Sign of Intelligence?
Offhand I’d think someone bright enough to absolutely determine which was the first intelligent comment, would have been smart enough to say which comment that was. But that could just be me.
Alex:
“Siberian_husky says:
June 4, 2014 at 2:06 am
Thankyou Urederra- the first intelligent comment on this thread.
Wow! Really?”
If you were thinking SH was referring to Urederra’s explanation of the 25% change, I expect it may actually have been Urederra’s first comment, regarding pH levels in other epochs; like in the Cambrian, for example, when corals evolved.
Acidification a nicely scary sounding word
Less alkene , boring sounding but more accurate word s.
You decide which one you will use when you’re looking for funding .
Meanwhile once again using ‘proxies ‘ we are told how things can be ‘measured ‘ from millions of years ago and they ‘compared’ with actual valid measurements from observations now , BS of the first order .
Dave
which one of those two is worthy of the Nobel Prize?
I’m not saying there is anything wrong with those comments. I’m just wondering why those 2 comments are considered ‘the first intelligent’. I saw many other comments that were intelligent too. I’m getting the feeling that there are ‘sock-puppets on this thread.
urederra says:
June 4, 2014 at 1:24 am
So, what was the pH of the ocean during the geological epochs when atmospheric CO2 levels were over 1500 ppm?
What is the error in the measurement of pH levels?
2nd comment
urederra says:
June 4, 2014 at 1:46 am
For those who are wondering:
pH=8.2 gives a H concentration of 6.3095E-9
pH=8.1 gives a H concentration of 7.9432E-9
7.9432-6.3095=1.6337
(1.6337 · 100)/6.3096 = 25.89%
David Schofield says: June 4, 2014 at 1:10 am
“Seriously. Can someone explain to me how the warming oceans take up this carbon dioxide whilst at the same time giving it off??”
CO2 flux goes both ways. And there’s a seasonal movement – loss during summer, gain during winter.
On balance, more CO2 goes into the sea if the CO2 ppmv in air rises; less if the sea is warmer. Since CO2 in air has risen, and the sea has warmed, these effects compete. So far, higher CO2 ppmv has prevailed, and the sea has absorbed CO2, but your link says, for the reasons given, this may change.
Jeff Szuhay says: June 4, 2014 at 12:34 am
“A pH of 7.8… Uh, that’s still alkaline. Not acid until it goes below 7.0.”
pH 7 is the neutral point of pure water. Marine organisms never encounter pure water, so that is irrelevant. They have evolved in a sea of pH>8.
Human blood has a pH of about 7.4. If it drops to 7.35, that is acidosis = bad news. Blood pH 7 is not compatible with life.
Nick
‘Since CO2 in air has risen, and the sea has warmed, these effects compete. So far, higher CO2 ppmv has prevailed, and the sea has absorbed CO2’
Can you point me towards some literature that can verify the last sentence?
This sort of stuff just has to stop as it’s all most unsettling for earth scientists-
http://blogs.news.com.au/dailytelegraph/timblair/index.php/dailytelegraph/comments/fault_lines/
Trenberth cannot even measure the temperature of the world ocean. What makes these people think they can measure the pH any more accurately?
There are also reasons to think increased CO2 will be beneficial:
https://www.researchgate.net/publication/229561219_ELEVATED_ATMOSPHERIC_CARBON_DIOXIDE_INCREASES_ORGANIC_CARBON_FIXATION_BY_EMILIANIA_HUXLEYI_%28HAPTOPHYTA%29_UNDER_NUTRIENTLIMITED_HIGHLIGHT_CONDITIONS1
The exoskeleton also grows thicker. Another study looked at genetic changes under elevated CO2 and found only decreased carbonic anhydrase expression. Again this suggests life becomes easier.
The physical evidence for the pH change is lacking. This study also does not offer any explanation why we should be worried about this derived alleged 0.1 reduction in pH in a system whose dynamic range is in reality much, much wider. Additionally, they offer no evidence that ocean absorption goes simply to physical chemistry of seawater and not to biological and geological processes.
Once again we see a marketing effort by the CO2 obsessed dressed up as science.
Alex says: June 4, 2014 at 3:00 am
“Can you point me towards some literature that can verify the last sentence?”
Yes. AR4 7.3.1 describes the carbon cycle. It gives many references. Key quote:
“Thus, the terrestrial biosphere and the oceans together have consistently removed 45% of fossil CO2 for the last 45 years, and the recent higher rate of atmospheric CO2 increase largely reflects increased fossil fuel emissions.”
michael hart says: June 4, 2014 at 3:05 am
“Trenberth cannot even measure the temperature of the world ocean. What makes these people think they can measure the pH any more accurately?”
pH is determined by equilibrium relations involving carbonates, which are much more abundant in the sea than H+ or CO2. Two quantities, dissolved inorganic carbon (DIC) and total alkalinity, are easy to measure, are fairly stable, and pH can be deduced when you know them.
What I don’t understand is why during the last iceage (and previous ones) the ocean pH was just under 8.25, because then I assume the ocean was colder so would hold more CO2 per unit volume and hence make pH lower than the interglacial pH of 8.15, well before any anthropogenic influence. Similarly when sea-ice forms then CO2 is expelled so would tend to decrease pH as it is absorbed into the cooling ocean. see
http://www.nature.com/scitable/knowledge/library/ocean-acidification-25822734
http://onlinelibrary.wiley.com/doi/10.1111/j.1600-0889.2011.00571.x/abstract
I could imagine if the carbon cycle slowed significantly then chemical action with rocks could become the predominant factor in reducing disolved CO2, and hence increasing pH. Is this the explaination?
About CO2 uptake and release by the oceans:
– seawater in equilibrium with the atmosphere gives 17 ppmv higher levels for each 1°C increase in temperature. See:
http://www.ldeo.columbia.edu/res/pi/CO2/carbondioxide/text/LMG06_8_data_report.doc
That is a dynamic equilibrium: some 50 GtC as CO2 goes in and out the oceans over the seasons (mainly in the mid-latitudes) and some 40 GtC/year is released at the upwelling places near the equator (mainly off the coast of Chili) and taken away near the poles (mainly NE Atlantic). A temperature increase augments the releases and depresses the uptakes, which leads to an increase in the atmosphere, until the new equilibrium is reached:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/upwelling_temp.jpg
– humans have emitted some 370 GtC over the past 160 years. The atmospheric increase is over 200 GtC (100+ ppmv).
An increase of 1°C since the LIA is responsible for maximum 17 ppmv (ocean only) or historically 8 ppmv (ocean + land vegetation), far from the measured 100+ ppmv increase.
Humans currently emit ~9 GtC/year (4.5 ppmv/year). The extra pressure caused by 100+ ppmv CO2 above equilibrium (for the current temperature around 290 ppmv) gives a net sink of ~4.5 GtC/year of which ~1 GtC/year in land vegetation, ~0.5 GtC/year in the ocean surface layer and the rest of ~3 GtC/year in the deep oceans. Other possible sinks are either too small or too slow…
Nick
Thanks for that link, but unfortunately I consider IPCC information as suspect. I will fall back on my high school science.
I learned that solids dissolve in liquids and the amount of solid dissolved in said liquid was temperature dependent. Excess solid would not dissolve. I am excluding super saturation because that requires special conditions.
With gasses in fluids it is a reverse effect. That is, higher temperatures lower the solubility of gasses. The effect is temperature dependent and not dependent on availability/excess gas. In other words if you had 5000 ppm CO2 in the air it would not dissolve more CO2 into water unless you changed the temperature of the water. In actuality the water would have to be colder to absorb more gas.
So I guess I am saying that with higher sea temperatures you would have less dissolved CO2 and therefore higher pH. With colder seas you would have more dissolved CO2 and a lower pH. It makes no difference how much CO2 is in the atmosphere because solubility is temperature dependent
The problem with the “25% more acidic” statement is that it implies that acidification has progressed 25% of the way to neutrality, which isn’t so.
We have an expression here in Blighty for such a study, & this one sounds absolute “bollocks”! Apologies, bad hair day!
Please provide citations to non paywalled studies which include raw data that indicate our capability to estimate (i say estimate since only a fool would believe that we could actually measure it) the distribution of pH throughout the volume of the oceans. I would be very curious to see just what the spatial and temporal sampling of the data is along with reasons to believe that there is enough data to justify the estimated probable error bands. Would one sample from each thousand cubic miles be enough? If it would, you would only need a little over 320,000 samples to get spatial coverage. What about temporal coverage? How could you demonstrate that such sampling was adequate? My only experience in this area is running a plating shop where we had to control acid based plating baths. The biggest one was 1.3 cubic meters and we did not need to control it as close as 0.1 pH units but, after sitting overnight, it had to be thoroughly mixed before we could get a valid sample.
It would take quite a project to convince me that you could estimate the pH of San Francisco Bay to within 0.1 pH units. Then you would need to show that combining all of that data into one number was meaningful.
Alex says: June 4, 2014 at 3:54 am
“The effect is temperature dependent and not dependent on availability/excess gas. In other words if you had 5000 ppm CO2 in the air it would not dissolve more CO2 into water unless you changed the temperature of the water.”
No, that’s not true. Henry’s Law says that the amount dissolved is proportional to ppm. The constant depends on temperature.
Upthread, Ferdinand has said that ppmv CO2 in equilibrium with seawater rises about 17 for each °C rise in SST. That means that warming so far might raise ppmv CO2 by about 12. But our fossil fuel burning has raised air CO2 by about 120 ppmv. That dwarfs the warming effect and forces CO2 into the sea.
Nick Stokes Says:
Nick, comments of others asked how the average ocean pH was measured to an accuracy of 0.1 over 100 years ago, and how the global ocean average is known when only small parts of the worlds oceans are (and were) even measured. Both these issues make the claims absurd. Also it was pointed out that in the PETM, it appears the rising temperature preceded the Carbon pulse, which then caused a temperature spike. It appears there was a warming trend (at a level much warmer than present) that eventually resulted in a methane release at some point in the warming trend. Even after recovery from the effect of the Carbon induced pulse, the average temperature still continued to climb, to a level comparable to the peak of the pulse spike. It does not appear that you or I know what was going on, but it is fairly clear that Carbon is the main control knob is too simplistic. Yes is has an effect, and can cause heating, but other effects do also, and the sensitivity cannot be determined from the PETM data.
A good pH meter has .01pH accuracy. About 10X the noted change. So if it was done by measurement the numbers are valid +/-20% of the change. That is also dependent on calibration. Accuracy of temperature measurement. And possible confounding factors.
Alex wrote:
‘Thanks for that link, but unfortunately I consider IPCC information as suspect. I will fall back on my high school science. … With gasses in fluids it is a reverse effect. That is, higher temperatures lower the solubility of gasses. The effect is temperature dependent and not dependent on availability/excess gas. In other words if you had 5000 ppm CO2 in the air it would not dissolve more CO2 into water unless you changed the temperature of the water.’
Surely you studied Henry’s Law? Quoting wikipedia:
“At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.”
(http://en.wikipedia.org/wiki/Henry's_law – you can also find this on various school chemistry revision websites. Also e.g. Lubos Motl, a climate skeptic quotes the same definition – I don’t want to clutter this comment with too many links.)
Maybe I have this wrong. If so I would appreciate a link to an explanation.
From Nick Stokes on June 4, 2014 at 3:21 am:
pH has been accurately measured for quite some time now, using common laboratory things like precision litmus papers. But electronic pH meters are normally used. They need frequent calibration against reference solutions, for best accuracy you calibrate before every measurement. Don’t expect more than two decimal places of precision.
Out of the lab, there are pH meters suitable for processing equipment in industrial uses, where calibration is much less frequent, as well as inexpensive “home use” models for aquariums and hydroponics (example), +/-0.1 accuracy.
Where they are measuring, they can measure pH reasonably accurate with field-grade equipment.
But as with temperature, the issue is spatial coverage of the measurements. You don’t measure the pH of an ocean at one spot. Nor at twenty. You’ll need a heck of a lot more measurements, and we don’t have them, nor do we have long records of sufficient many measurements thus we lack temporal coverage for calculating changes and trends.
BTW, if anyone knows what Nick is chattering about and how that relates to measuring by simply sticking a probe into seawater, feel free to elucidate.
From my poem — Al Gore American Bloviator
If acid rain scared you think about this!
Oceans acidic and warmer than piss!
Eugene WR Gallun
Leonard Weinstein says: June 4, 2014 at 4:30 am
“how the average ocean pH was measured to an accuracy of 0.1 over 100 years ago”
Measurement would be no problem. As I said above, all that is needed is DIC and TA. DIC is gravimetric (precip with BaCl2) and TA is just titration (methyl orange). To get pH to .1, that conventional chemistry has to be 25% accurate.
As to how widely this was done, I don’t know.
“it is fairly clear that Carbon is the main control knob is too simplistic”
Yes, but who says it is? This paper says that one particular CO2 pulse caused warming. But they don’t say all warming is caused by CO2, and few do. In fact events forcing CO2 higher, causing subsequent warming are not common. This methane pulse (if so) may have been one, and our mining fossil fuel is another.
” more than half of the tiny planktic snails, or pteropods, that she and her team studied off the coast of Washington, Oregon and California showed badly dissolved shells.”
I’d like to know how a supersaturated solution such as surface seawater can dissolve shells. A decrease in pH merely decreases the amount of calcium carbonate (and alkalinity in general) that can be kept in solution without precipitation.
I think the post by MichaelHart implies that carbon dioxide in the sea fertilizes the growth of the shells of shelled organisms like it fertilizes the growth of plants. It seems that the oceans might be capable of sequestering an awful lot of excess CO2. Also, it seems the variability of the Carbonate Compensation Depth provides some reserve in the operation of the carbon cycle of the oceans. This reserve might be thought of as a buffering of the system.
Have any of these studies ever arrived at a result that would show that humans had a positive affect on the planet? Surely there must be one somewhere.
But but but…won’t all the melting ice caps dilute the oceans with fresh water?
This climate catastrophism makes head hurt!
pH of fresh water is 7. pH of ocean water is about 8. Maybe we should start referring to ocean acidification as ocean “freshening?”
kadaka (KD Knoebel) says: June 4, 2014 at 4:43 am
“BTW, if anyone knows what Nick is chattering about and how that relates to measuring by simply sticking a probe into seawater, feel free to elucidate.”
DIC here, with link to TA.
Here is how to calculate.
John West says: June 4, 2014 at 4:56 am
“I’d like to know how a supersaturated solution such as surface seawater can dissolve shells.”
Sea water is on average supersaturated wrt aragonite. But it varies seasonally and with upwelling. Shell CaCO3 once dissolved won’t reform.
” the pH of the oceans has dropped substantially, from 8.2 to 8.1–equivalent to a 25 percent increase in acidity”
Really???
Really. pH is a log unit. On a log scale, 0.3 corresponds to doubling, 0.2 to increasing by 50%, and 0.1 to increasing by 0.25%. Find a calculator and punch in log(1.25) (base 10, not base e ln()). 0.09691 \approx 0.1. Technically, this means that the hydrogen ion content (hydronium) has increased by 0.25%, but of course the ocean is still predominantly basic.
Personally I have to say that this sounds like good science. Doubling the atmospheric concentration of CO_2 in 150 years may or may not cause catastrophic warming (personally I doubt that it will). It might well cause “catastrophic” changes in ocean chemistry. I’ve been, and will remain for a while at least, neutral on the issue because it is difficult to find precise proxies and because of the overwhelming confirmation bias prevalent in the community, which makes it far too easy to make up a story and look for evidence the confirm it rather than look at the evidence and make up a story to explain it (the latter process works better, go figure) but the description of the science above is at least moderately compelling.
True, most ocean life will probably adapt, although evolution on the timescale of a century is pretty tough. Some species, especially species that are already stressed by e.g. pollution or other changes and that are not “prolific” (meaning, not as many rolls of the genetic dice in reproduction), may not make it. In my opinion, though, this is a genuine area of concern. Anybody who has tried to run an aquarium knows that fish species are not all equally, or particularly, “robust” to small changes in water chemistry. This is, indeed, worth keeping an eye on.
The issue of whether it is worth spending trillions of short-run dollars and millions of human lives on is another debate entirely. Clearly it is better not to cause even mini-extinctions because alterations in the ecology can have unintended, and serious, side effects (like the die off of some important food species or even alterations in the entire oceanic food chain). On the other hand, spending trillions of dollars and millions of lives is a “consequence” of action to prevent this as well — no path may have zero consequences, and we are still stuck doing cost-benefit with incomplete information, a.k.a. “gambling”, either way.
rgb
Sorry, can’t read this crap. The ocean is not is not becoming more acidic, it is not even acidic at all .
By the way, in case it isn’t obvious, today is “I agree with Nick Stokes” day, right down the line. If this means that most of you consider me a warmist for a day, so be it. The science is the science, and this measurement (which may or may not be accurate — that is a different issue entirely and resolvable only with more/different measurements — but taken in good faith) of past ocean chemistry and correlation with radiometrically dated extinction events is not in any way equivalent to the statistically unsupportable predictions of failing GCMs used as the primary basis of the entire catastrophic climate change discussion.
rgb
As Nick Stokes already said, one doesn’t need to measure pH – even if it is done – to know the pH. If you measure two variables of seawater like DIC (dissolved inorganic carbon) and TA (total alkalinity), one can calculate the other variables. Nick has even a seawater pH calculator on the web:
http://www.moyhu.blogspot.com.au/2013/09/active-ocean-acidification-calculator.html
There were a lot of ocean measurements during the geophysical years and a few stations like Bermuda and Hawaii which have continuous series of measurements. See:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf Fig. 5
and
http://www.pnas.org/content/106/30/12235.full.pdf Fig. 1
The cause of the rapid PETM transition still is controversional: a hughe outburst of methane is one of the many theories, but what caused the outburst? A big meteor in the Arctic Ocean is a possibility, as that wouldn’t leave much deposit of iridium, but would trigger such an outburst of methane.
Anyway, if the mass extinction was caused by more CO2 or a lower pH, remains questionable, as a lot of other variables changed too.
Nick Stokes:
In your post at June 4, 2014 at 4:52 am you assert
That is so wrong it is risible!
The ice cores show such “events” have been the norm throughout the holocene with the delay of CO2 after temperature being typically ~800 years.
What “methane pulse” (a “popular suggestion” is not a demonstrated reality)?
And I would welcome any evidence that “our mining fossil fuel” is an “event forcing C12 higher” because although it is possible it is extremely improbable.
Richard
Interesting
“From Lamont-Doherty Earth Observatory: Some 56 million years ago, a massive pulse of carbon dioxide into the atmosphere sent global temperatures soaring. In the oceans, carbonate sediments dissolved, some organisms went extinct and others evolved”
Huh an emotive massive pulse, is this the new framing terminology? It rang a bit of bell after I noted a certain Mr R. Gates explaining his new frame.
http://judithcurry.com/2014/06/01/global-warming-versus-climate-change/#comment-580167
“HCV is a quick acronym for Human Carbon Volcano, representing the massive transfer of carbon from lithosphere to atmosphere that human activity (mainly fossil fuel burning) has created, not unlike what a natural volcano does, only the HCV had been erupting much longer (centuries) and the eruption has grown more intense with each passing decade”.
Ah massive evil Carbon pulses, ocean acidification – Human Carbon Volcano,- scary Hydrogen Bomb values, and of course back to Global Warming as it is scarier, heavens – New age verbal upchuck!
From Nick Stokes on June 4, 2014 at 5:10 am:
Yeah, right.
From an informative page with an US EPA logo:
http://omp.gso.uri.edu/ompweb/doee/science/physical/chph2.htm
I reiterate, if anyone knows what Nick is chattering about and how that relates to MEASURING by simply sticking a probe into seawater, feel free to elucidate.
Nick:
Dissolved inorganic carbon. How is the world wide level of this known to the accuracy necessary to provide an estimate of pH accurate to 0.1 today? How do we estimate the level 100 years ago?
Total alkalinity. Same two questions.
Finally. How was the temperature measured at the sample point? pH estimates from DIC and TA require temperature.
You imply this is an easy thing. I can assure you that, as someone responsible for providing regulators with these numbers, obtaining them to the accuracy you imply is labour intensive and subject to a lot of experimental error. Your assertion that this is a method of estimating pH 100 years ago, accurate to 0.1, is poorly grounded. If you are not asserting it applies to the state of the ocean 100 years ago, what basis do you have for the assertion that ocean pH has changed?
kadaka: The estimation of pH using DIC and TA at a particular temperature, is a simple exercise in equilibrium chemistry. The estimation of DIC and TA is a complicated exercise in sampling with potential for substantial errors. It should not be used to report pH unless the temperature of the sample at time of sampling and time of measurement is reported as well.
Aragonite is supersaturated in the ocean. This is just one more example where the assumption of equilibrium is hopelessly naïve.
You can’t be serious about referring to Henry’s Law, which specifically refers to constant temperature. The changes in partial pressure of CO2 these days are laughable when you try to calculate the solubility of CO2 in water by using Henry’s law. An extra CO2 molecule dissolved in a litre of water is insignificant to the pH. The greatest effect is in the temperature.
Don’t take my word for it. Refer to data that shows that CO2 in the atmosphere increases after temperature rise
richardscourtney says: June 4, 2014 at 5:25 am
“The ice cores show such “events” have been the norm throughout the holocene with the delay of CO2 after temperature being typically ~800 years.”
No. Temperatures rose, but few attribute that, generally, to CO2. The later CO2 rise was forced by temp. And yes, that CO2 rise probably was then responsible for a little more warming.
But CO2 rise is not the usual cause of warming. Immediately you have to ask – what caused the CO2 to rise? Re the PETM, that question is being asked here. In modern times, it is burning fossil fuel.
John Eggert says: June 4, 2014 at 5:46 am
“Finally. How was the temperature measured at the sample point? pH estimates from DIC and TA require temperature.”
I don’t know how extensive were the readings 100 years ago. All I am saying is that the means for measuring accurately were available. You don’t even have to do it on the spot; just take the sample to the home lab.
DIC and TA actually don’t vary that much. Here is a map of alkalinity. It varies fairly smoothly and over a not very wide range. DIC is similar – there is a map with the Wiki link I gave above. They probably measured SST at the same time, but in any case we also have good alternative measures. SST climatology, at least, is well known.
rgbatduke says:
”Technically, this means that the hydrogen ion content (hydronium) has increased by 0.25%
I think you meant 25%, but how is “per 100” a reasonable way to communicate changes in pH / acidity?
pH = H+ mol/L
0 = 1
1 = 0.1
2 = 0.01
3 = 0.001
4 = 0.0001
5 = 0.00001
6 = 0.000001
7 = 0.0000001
8 = 0.00000001
9 = 0.000000001
10 = 0.0000000001
11 = 0.00000000001
12 = 0.000000000001
13 = 0.0000000000001
14 = 0.00000000000001
8.2 = 0.00000000630957
8.1 = 0.00000000794328
The “science” is not compelling to me for they’ve shown their motive is activism/advocacy through this entirely inappropriate manner of communicating changes in acidity.
International Union of Pure and Applied Chemistry (IUPAC), published in Pure & Applied Chemistry in 1988, “Recommendations for the Determination of pH in Sea Water and Estuarine Waters”:
http://media.iupac.org/publications/pac/1988/pdf/6006×0865.pdf
Back in 1988, most common method of measuring pH in seawater was by simply sticking in a probe. The main issue was distortion from the salinity, and if you worried about it then you could calibrate from synthetic seawater solutions (buffers). Then measure by simply sticking in a probe.
Nick: The entire point of the paper is that pH has changed. If you don’t know how extensive the readings were 100 years ago, you too agree that there is no basis for saying the pH has changed. You too are saying the conclusion of the paper is based on a poorly grounded premise. This paper says more about the inadequacy of peer review than it does about ocean chemistry. And wikipedia for a source? Really?
What makes all these century and more projections absurdly ridiculous are the implied assumptions that : 1) carbon emissions levels will not only increase, but continue at the same rate for the next 100 years, or more; 2) the less carbon, the better ( a VERY dangeous notion) ;
3) something drastic needs to be done, yesterday. Actually,the biggest danger, by far, is that decarbonization will be TOO successful and carbon levels retreat to pre industrial levels.
All one needs are a pair of eyes to see that current technological and energy trends all
irrevocably lead to fewer carbon emissions, irrespective of any concerns of negative effects those emissions may visit upon us. And our totally inept President could have so easily made all this
an academic issue, had he transformed but one of his trillion dollar giveaways as a loan to construct 200 nuclear plants, which would have produced roughly 75% emission free power,and the loan paid back to the U.S. Treasury over a 30 year period,or less. But no, instead he funded projects designed to buy votes. Now there is one stupid, useless ass.
I don’t think the continents were in their present position 56 million years ago so the ocean currents were not the same. Blaming anything on CO2 at 56 million years ago is very dubious.
rgbatduke says:
June 4, 2014 at 5:14 am
” the pH of the oceans has dropped substantially, from 8.2 to 8.1–equivalent to a 25 percent increase in acidity”
Really???
Really. pH is a log unit. On a log scale, 0.3 corresponds to doubling, 0.2 to increasing by 50%, and 0.1 to increasing by 0.25%. Find a calculator and punch in log(1.25) (base 10, not base e ln()). 0.09691 \approx 0.1. Technically, this means that the hydrogen ion content (hydronium) has increased by 0.25%, but of course the ocean is still predominantly basic.
________________________________________________________________________
Check your decimal places
pH = -log(10)[H3O+] if you don’t want to go through all that activity stuff
Any 0.1 pH unit decrease is an increase in acidity as [H3O+] by 25.89%
So, pH 13.9 is theoretically 25.89% more acidic than pH 14.
The “increased acidity” by some percentage seems like propaganda to me. Acidic oceans with pH’s above 7? If someone had tried claiming acidic at pH>7 down the road on Hillsborough Street, it wouldn’t have been all that successful. But that was back when the the periodic table was much smaller.
Some critters don’t do as well at lower (basic) pH’s, but that begs the question of how they have managed to survive.
Something changed in the climate that caused a huge CO2 spike. But it was the CO2 that did all the damage.
Riiiiiiight.
Nick Stokes:
I write to request explanation of your assertions to me which make no sense.
At June 4, 2014 at 6:08 am you write
“Temperatures rose, but few attribute that, generally, to CO2.”
Really? Why then did the many not object when e.g. Al Gore did make that attribution in his sci-fi movie?
And if CO2 did not cause the temperature rises in the PETM and at the other times then what was the cause of those temperature rises?
Importantly, if CO2 did not cause those temperature rises in the past then how and why is “burning fossil fuel” doing it “in modern times”?
Thanking you in anticipation of your explanations
Richard
When earning my chemistry degree, a failure to do proper error analysis and report it with your results would have resulted in a “D”. Show me the error analysis and how the resulting uncertainty in all the measurements (for past and current pH claims) does not invalidate all your conclusions.
I have waited in vain for an advocate of Global Lukewarming to publish research on the ideal climate so we can know if ours is trending towards or away from that metric. But alas, I can only find information about the interglacial period. Nobody seems interested in knowing the ideal. Why might that be? Maybe because there is no juice in that research.
Likewise, what is the ideal pH of the ocean?
“Men whose research is based on shared paradigms are committed to the same rules and standards for scientific practice. That commitment and the apparent consensus it produces are prerequisites for normal science, i.e., for the genesis and continuation of a particular research tradition.” — Thomas Kuhn.
rgbatduke says:
June 4, 2014 at 5:14 am
. It might well cause “catastrophic” changes in ocean chemistry.
====
Then ask yourself how is it possible to keep fish aquariums in closed houses and buildings..where CO2 levels can exceed 1000ppm….
The oceans can not change pH until they run out of buffer…
..if you think CO2 will change the pH of the oceans…you have to first believe the oceans will run out of buffer
Atmospheric CO2 levels are nothing compared to biological processes…..Biology in the oceans is producing magnitudes more acidic compounds right now…..it will laugh at higher CO2 levels…and it’s the biology that’s buffering the oceans
One good to come out of pointing out the many holes in this latest bit alarmist propaganda is an opportunity to review carbon sinks.
One very important carbon sink is that of the freshwater systems of the world.
Although tiny in relation to oceans, freshwater systems are huge in the carbon cycle. And humans have created lakes and resevoirs worldwide. Limnology is the science that studies this, and a review of the implications of how powerful a role the freshwater systems of Earth play in the carbon cycle is eye opening.
tty – well said regarding the upwelling CO2-rich deep waters on the West coast.
++++++++
From the article:
” Eventually, the oceans and atmosphere recovered as elements from eroded rocks washed into the sea and neutralized the acid.”
If it remained alkaline all the time how can they speak of ‘the acid’ being ‘neutralized’? That is pretty poor chemistry, if you ask me. The article makes hay out of common misunderstandings about hydrogen ions in water. It got published, but should be binned. It does not add to understanding. It rather profits from exploiting common misunderstandings.
Looks like a blip, not a spike, of CO2 on a descending slope had no effect on temperatures. Obviously the Aragonia velascoensis went extinct because the CO2 was too low, much lower than when it evolved.
http://i46.tinypic.com/2582sg6.jpg
From John Eggert on June 4, 2014 at 5:58 am:
I know, and I noticed there are nice equations for calculating them using pH. Which is easily read off a meter.
Which was the issue. There was a simple question about measuring a quantity with accuracy, and Nicky wanted to show off by whipping out his big shiny throbbing intellect instead, with a complicated response about easily (for a chem major) deducing it.
Someone wanted to know about measuring temperature, Nicky talked about easily calculating it from relative humidity and the maximum air speed of a Northwest-flying English sparrow.
Why he keeps harping on some obsolete method kept around as an academic exercise when the modern faster way is long established, well, maybe he just likes telling passerby about his favored elaborate routine for keeping his intellect stimulated.
For any journalists in here. I think the story to be told is the story of those that believe in catastrophe and doom against those holding with the Gaia hypothesis. Gaia posits that organisms interact with their inorganic surroundings on Earth to form a self-regulating, complex system protected from the ravages of a runaway greenhouse effect. On the one hand Gaia is a more complex description and doesn’t jibe with Occam’s razor that the simpler explanation is the better one. On the other hand, in general, there is nothing simple about Earth Systems, and models diverging from actual surface temperatures are probably in need of more factors and more complexity.
I rather thought that, in a basic buffered solution, adding carbonic acid depleted carbonate by forming bi-carbonate without an increase in hydronium ions and a concomitant lowering of pH.
People want to think that earth systems are as simple and bound in universality as the general laws of physics. In truth there is little appreciation of how complicated this brand of science is. The science can’t be settled because there’s still too much territory to be explored.
Rubbish.
mebbe “basic buffered solution” is that “basic” as in non-acidic?
catweazle……not to say laws of physics don’t apply. Just that analysis of earth systems is not a simple matter that can be carried out the way that things are done in a controlled laboratory environment.
Ferdinand Engelbeen says:
“Anyway, if the mass extinction was caused by more CO2 or a lower pH, remains questionable, as a lot of other variables changed too.”
It is not often one finds factual errors in FE:s posts, but the odd thing about the PETM was that there wasn’t any mass extinction.¨
Also it is by no means certain that the changes in carbon isotope ratios at the PETM was due to release of methane hydrates. At least seven alternative hypotheses have been suggested:
1. Massive peat fires (the total absence of any large terrestrial plant-eaters after the extinction of the dinosaurs would have facilitated accumulation of plant material)
2. Oxidation of organic bottom sediments desiccated by tectonic movements related to the opening of the North Atlantic.
3. Baking out of buried organics by the massive basalt eruptions related to the opening of the North Atlantic
4. Melting of large areas of permafrost in the interior of Antarctica
5. Cometary impact
6. Massive outgassing of CO2 from the oceans due to warming of oceanic bottom water.
7. Methane hydrate dissolution.
None of these is obviously absurd or impossible, and of course any combination of two or more mechanisms is also possible.
They use the problems that shell fish farms off the coast of Washington State are having as an example of the effects of change in pH, but there may be another mechanism in play.
Victoria BC is still dumping the equivalent of a supertanker full of raw sewage into the Puget Sound each week. 130 million liters per day. There are two sub-surface outfalls in the Strait of Juan de Fuca, across from Port Angeles. Because they are out of sight, not many know about it.
The decay of “organic material” in water acts to consume dissolved oxygen. Reducing dissolved oxygen also has the effect of reducing pH. The chemicals that are put down the drain, such as soaps, detergents, etc, and heavy metals also combine with dissolved oxygen and reduce pH.
I am aware of local divers giving anecdotal evidence of this as they have observed deeper dwelling creatures struggling to get enough oxygen. The diffusion rate of oxygen at deeper levels is much slower – it takes a while for surface oxygen to get down there, especially without mixing.
This water is drawn into the Puget Sound by tidal action, where is is concentrated, then back out again and driven down the coast by prevailing currents. This is why reports of acidification are so localized – in this case Washington and Oregon coastline. If CO2 levels were responsible for ocean acidification on the scale they are stating, it would be widespread, as CO2 levels do not vary much with map coordinates. But that is not the case.
Also, what about the period in earths history when CO2 levels were at least two times higher than they are now? What was ocean pH back then? Weren’t the shellfish in question establishing their place on the planet at that time? IF what they say is true, then pH must have been much lower, yet those shellfish are here today.
Alex says:
June 4, 2014 at 6:06 am
You can’t be serious about referring to Henry’s Law, which specifically refers to constant temperature. The changes in partial pressure of CO2 these days are laughable when you try to calculate the solubility of CO2 in water by using Henry’s law. An extra CO2 molecule dissolved in a litre of water is insignificant to the pH. The greatest effect is in the temperature.
Don’t take my word for it. Refer to data that shows that CO2 in the atmosphere increases after temperature rise
Yes it was serious and correct to refer to Henry’s law, unfortunately you continue to reveal your ignorance on the subject! The Henry’s Law ‘constant’ is usually defined at 298K, however the more accepted usage is the HL ‘coefficient’ which reflects the fact that it is a function of temperature. Typically for CO2 in seawater the equilibrium partial pressure will double with a 16K increase in temperature. The pCO2 has increased by ~25% since 1960, far more than the rise in SST necessary to cause such an increase.
HankHenry says:
June 4, 2014 at 7:22 am
mebbe “basic buffered solution” is that “basic” as in non-acidic?
————————————-
Yes. AFAIK, bicarbonate is amphoteric, taking H from carbonic acid and giving H to carbonate
The decay of “organic material” in water acts to consume dissolved oxygen.
====
Kurt, that’s a biological problem…..aerobic/suboxic bacteria consume the oxygen, and produce magnitudes of acid….it’s the bacteria reducing the pH
You’re right, CO2 has nothing to do with it
tty, you are right. Besides the benthic foraminifera, there was no mass extinction and lots of possible causes for the PETM.
Nick
Why do you have yourself, on your website, as a ‘Scientist’? I have only encountered ‘scientists’ in Science Fiction novels. You seem to have considerable knowledge in the climate sphere. I probably could be like you with documents and graphs that I could ‘cut and paste ‘ from too.
Unfortunately there is a big difference between having knowledge and having the ability to use it
mebbe says:
June 4, 2014 at 7:14 am
I rather thought that, in a basic buffered solution, adding carbonic acid depleted carbonate by forming bi-carbonate without an increase in hydronium ions and a concomitant lowering of pH.
I’m afraid not.
CO2 (aq) + H2O ⇆ H2CO3 ⇆ HCO3− + H+ ⇆ CO32− + 2 H+
See the associated Bjerrum plot:
http://www.nature.com/scitable/content/ne0000/ne0000/ne0000/ne0000/25853439/2-626w_1_2.jpg
hunter says:
June 4, 2014 at 7:01 am
One very important carbon sink is that of the freshwater systems of the world.
Although tiny in relation to oceans, freshwater systems are huge in the carbon cycle.
Fresh water dissolves very little CO2, because it has no buffer capacity at all and therefore is slightly acidic. That does dissolve carbonate rocks, but even that costs a lot of time to digg out the beautifull underground caves…
I think there is an elephant in the room that is not being accounted for.
First, let’s do a simple, sober game-developer’s top-down analysis:
We are adding ~3 BMTC per year to an ocean sink of ~1800 BMTC down to below biota level (and ~42,000 BMTC below that). Some of that is transported downward. ~2 BMTC per year is “lost” to the deep sea floor.
But then there is the elephant.
Acidification has been occurring since 1750, according to the literature. But CO2 did not even begin to increase until 1850, and did not exceed 300 ppm until a century after that. Thus stipulated, this tells me that other factors are in play.
So what might those factors be? Draining, dredging, and dumping spring to mind. Especially as pH increase (as logy as those measurements are) appears to have occurred primarily in and near the coastal areas. There is not only the direct acidifying effect of the decaying effluence, but also the fact that a a large chunk is converted by biota into carbonic acid, much the same as CO2.
That would account for the 1750 start date. What we need to do is quantify those non-CO2 effects — dumping, draining, dredging (and whatever other factors may or may not apply) — and determine how much of a relative impact they have on carbonic acid and H+ levels, and see how it adds up and compares with what we (sort of) know. What are those impacts? Quien sabe? But I’m willing to bet heavily they are not zero.
Any attempt at quantification will be complicated by the wildly varying local pH and the fact that it is, so far, poorly measured. But I think that is what we need to do is conduct studies on non-CO2 anthropogenic feed-ins to the biological mechanisms that produce carbonic acid.
Then we may actually begin to get a handle on all this.
Nick, even though most of us disagree with what you say at least you do it constructively. Keep it up. It’s good to get a different perspective.
Phil
16K increase? where has that happened? Must have been sleeping and missed it
It is evident that the authors of this study have not come to grips with cause and effect relationships quite yet.
In a Limnology course I took in college we found out how difficult it was to determine the actual pH of seawater. Starting with DI-H2O we measured the pH and got a value. After bubbling N2 through the water the pH changed slightly (removal of residual CO2?). Adding NaCl to the concentration of sea water we got a different result. Measure actual sea water and get a different result, again. Filter the sea water with a 0.2 um membrane and get another pH. Change the temperature and get yet another pH result. Seawater that was kept in the dark vs. kept in the light? Yep…different pH results. Probably relates to seawater that was taken at 25 feet below the surface to seawater taken at the surface…different pH’s, again. Seawater taken from a ‘calm’ sea surface vs. an ‘active’ sea surface? Yep different pH’s.
Not to say that with extreme sampling control you can’t get reliable figures, but it takes some doing and to get equivalence to anyone else measurements to be comparable.
Phil
Thanks for that info. Now tell me what that means in regard to pH. F@ck all
Fresh water dissolves very little CO2, because it has no buffer capacity at all and therefore is slightly acidic.
===
Ferd, he was talking about fresh water ‘systems’…..
ex… Lake Malawi ph range from 7.7 to 8.6, and for Lake Tanganyika pH from 7.3 to 8.8, etc
JJ, too. says:
June 4, 2014 at 8:14 am
I couldn’t agree more.
I worked in labs or with labs for over 20 years. Unlike some of the ‘experts’ on this site
A few years ago a study appeared in PNAS, in which the effect of seawater pH on intracellular pH of coral was investigated. When the investigators decreased the pH of seawater from 8.0 to 7.4, the coral maintained its intracellular pH at 7.3 (note: also at pH 8.0, the intracellular pH was 7.3). When the seawater pH was brought to 7.2, the intracellular pH dropped from 7.3 to 7.2. They also investigated the effect on the pH of the mineralization zone and found that a decrease in pH from 8.0 to 7.4 caused a pH-drop in the mineralization zone from 8.3 to approx 8.0.
And the effect on mineralization? Well, not very large: in their study the authors found NO difference in extent of calcification between corals in seawater of pH 8.0 and 7.4!
kadaka (KD Knoebel) says:
June 4, 2014 at 7:08 am
From John Eggert on June 4, 2014 at 5:58 am:
kadaka: The estimation of pH using DIC and TA at a particular temperature, is a simple exercise in equilibrium chemistry. The estimation of DIC and TA is a complicated exercise in sampling with potential for substantial errors. (…)
I know, and I noticed there are nice equations for calculating them using pH. Which is easily read off a meter.
Not with the required precision and accuracy it isn’t, which is why research facilities use either the method referred to by Nick, using DIC and TA, or spectrophotometric methods.
Which was the issue. There was a simple question about measuring a quantity with accuracy, and Nicky wanted to show off by whipping out his big shiny throbbing intellect instead, with a complicated response about easily (for a chem major) deducing it.
For best accuracy and precision you use spectrophotometric methods, (±0.001 or better) not electrodes.
Why he keeps harping on some obsolete method kept around as an academic exercise when the modern faster way is long established, well, maybe he just likes telling passerby about his favored elaborate routine for keeping his intellect stimulated.
It’s you who’s ‘harping on some obsolete method’, incidentally pH is not measured by any method, it is calculated from measurements of other quantities, the ion selective probe that you are talking about measures a voltage which depends on [H+] via the Nernst equation and is calibrated in terms of the pH.
Sorry Phil
pH is measured by sticking a probe into a solution. We actually used a meter and not some esoteric calculation in ‘production control’. It would have cost thousands of dollars, otherwise.
This has developed into a really stupid discussion. I have managed to insult some people that I really didn’t mean to insult. Good night all
“suspected…” “…could be…” “CO2 ha[s] made seawater grow more acidic…”
No.
According to real world data from the Monterey Bay Aquarium’s intake pipe, located far out in the ocean, there has been no measurable change in pH.
Granted, this data stops several years ago, but it covers a time when CO2 was strongly ramping up.
The article also omits any mention of buffering — a major factor in any discussion of ocean pH; probably the major factor. Why no mention of buffering?
This is just the next in a long series of wild-eyed scares from the same people who have gotten every alarmist prediction wrong. No exceptions. They have been wrong about everything, from Polar bears, to accelerating sea level rise, to increased extreme weather events, to global warming, to vanishing Arctic ice, etc. They have been wrong about everything.
When one group makes constant predictions, and every one of them turns out to be flat wrong, rational people will at least say, “Hold your horses! We need to study this more — a lot more — before tilting at this latest grant-fed windmill.”
Alex says:
June 4, 2014 at 8:48 am
Sorry Phil
pH is measured by sticking a probe into a solution. We actually used a meter and not some esoteric calculation in ‘production control’. It would have cost thousands of dollars, otherwise.
I’m sure you do and I’m sure it’s sufficiently accurate for your purposes, however for measuring changing pH of ocean water that is inadequate, which is why the methods Nick and I described are necessary. By the way just because you use a meter doesn’t mean that there’s not an esoteric calculation embedded within the calibration.
The spectrophotometric method does cost thousands of dollars which is one reason why there is a prize awarded for the creation of “pH sensor technology that will affordably, accurately and efficiently measure ocean chemistry”. Perhaps you should enter your method, should be an easy way to pick up a couple of million$.
http://oceanhealth.xprize.org/competition-details/overview
“JJ, too. says:
June 4, 2014 at 8:14 am ”
What you are saying is that there is no “normal” value with which we can derive an anomaly (A diviation from “normal”. Thusmeaning, IMO, we simply do not know what is the “normal” pH of the oceans is).
Some 56 million years ago, a massive pulse of carbon dioxide into the atmosphere sent global temperatures soaring. In the oceans, carbonate sediments dissolved, some organisms went extinct and others evolved.
Might be the old case of wrong cause / wronge case. A quick search on lava flows 56 million years ago pops up.
The North Atlantic Igneous Province (NAIP) is one of the largest such on earth and extends from Baffin Island and Greenland northwards into the Arctic, east across to Norway and southwards down to Denmark, Scotland and Northern Ireland.
Outpourings of its magma created the Scottish islands of Skye, Rhum, Eigg, Canna and the basalt columns of the Giant’s Causeway and Fingle’s Cave.
Flood basalts from this time are still widely exposed on the Faroe Islands, Greenland and Baffin Island whilst Iceland remains a volcanically active ‘hot spot’ to this day.
It is known that the NAIP was particularly active at two periods in the deep past; the first phase was between 62 – 58 million years ago, with a second phase at the time of the PETM, between 56 – 54 million years ago when the area began to be uplifted; the continental plate split apart and emitted large volumes of magma.
https://sites.google.com/site/thepaleoceneeocenethermalmaxim/5-molten-magma-the-north-atlantic-igneous-province
Or
During the birth of Mount McKinley 56 million years ago when molten magma solidified deep beneath central Alaska, volcanic activity (eruptions at the surface) was also occurring in the park, and produced red, yellow and brown basalts, rhyolites, and other volcanic rocks.
http://vulcan.wr.usgs.gov/LivingWith/VolcanicPast/Places/volcanic_past_alaska.html
How many tons of lava at 1500 F does it take to cover the north Atlantic and would this tend to heat things up to a greater or lesser extent than CO2.
rogerknights says:
The problem with the “25% more acidic” statement is that it implies that acidification has progressed 25% of the way to neutrality, which isn’t so.
Repeated for effect.
What caused the burst of carbon at the PETM is still unclear.
Probably the Kochasaurus.
In the last 150 years or so, the pH of the oceans has dropped substantially, from 8.2 to 8.1
What are the local highs and lows in pH? I find it hard to believe this matters beyond the margins. Also, what kind of measurement precision is that? The change is the same as the smallest increment, which suggests the error bound is 100% of the signal.
I did indeed, sorry. In a hurry to go teach. The answer to the second question is that many physical quantities are best understood in terms not of absolute values, but rather the log of the absolute values. Sound intensity, for example. Humans are exposed daily to sounds that range over at least ten to twelve orders of magnitude in intensity, with somewhat rarer exposures to another six or seven orders of magnitude (before the sounds become intense enough to not count as sounds any more, but as shock wave fronts that are likely to certain to be fatal). The human ear, amazingly, can render all of 16 or 17 orders of magnitude as perceptible sound, from the faintest sound we can hear to jet engines or guns being fired nearby. Somewhere between a 30-06 being fired near our heads and a nearby object generating a substantial sonic boom, sound stops being “sound” to our ears (and incidentally causes instant damage to our hearing) and become a blast wave from e.g. an explosion.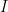 and one at intensity
and one at intensity 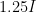 , you almost certainly could not differentiate the louder sound from the fainter one — that is an approximately 1 decibel increase, where sound intensity is rended into a log scale sound level relative to a reference intensity
, you almost certainly could not differentiate the louder sound from the fainter one — that is an approximately 1 decibel increase, where sound intensity is rended into a log scale sound level relative to a reference intensity 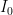 , the “threshold of hearing”, at
, the “threshold of hearing”, at  Watts/meter^2. In contrast, music at a rock concert 30 or 40 meters from the stage can easily be 1 Watt/meter^2! We can hear both easily as sound, the latter sound that is likely slowly damaging our hearing.
Watts/meter^2. In contrast, music at a rock concert 30 or 40 meters from the stage can easily be 1 Watt/meter^2! We can hear both easily as sound, the latter sound that is likely slowly damaging our hearing. etc, but that too involves a lot of nearly pointless writing. It is far easier to form $-\log_{10}(P_{H+})$, or more properly as $-\log_{10}(a_{H+})$ where
etc, but that too involves a lot of nearly pointless writing. It is far easier to form $-\log_{10}(P_{H+})$, or more properly as $-\log_{10}(a_{H+})$ where  is the activity of the hydrogen ion in a solution. The activity is defined relative to the chemical potential of a “standard” electrode, which can be directly mapped to a voltage generated on the electrode and measured by measuring the electrode voltage. This activity does directly involve
is the activity of the hydrogen ion in a solution. The activity is defined relative to the chemical potential of a “standard” electrode, which can be directly mapped to a voltage generated on the electrode and measured by measuring the electrode voltage. This activity does directly involve 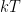 (or
(or  , if you are a chemist instead of a physicist) so one has to know the temperature at the electrodes to get anything like a precise measurement. I’m certain that there is other chemistry that can confound it, as well — the presence of other electrolytes or contaminants in the water, perhaps.
, if you are a chemist instead of a physicist) so one has to know the temperature at the electrodes to get anything like a precise measurement. I’m certain that there is other chemistry that can confound it, as well — the presence of other electrolytes or contaminants in the water, perhaps.
The ear is almost completely insensitive to much less than a doubling of sound intensity. If I presented you with two sounds one at intensity
pH is precisely such a quantity. The concentration of hydrogen ions in a water-based solution can vary by 14 orders of magnitude (although it is moderately difficult to reach the extreme ends of this range chemically). Sure, one can write it out with all of those zeros as you did above, but that is silly. One can use scientific notation, and write it as
Lots of other things vary by many orders of magnitude and are best described on a log scale in e.g. decibels rather than in absolute terms. Enough so that engineers (and physicists, and I’m sure chemists) have some simple rules of thumb. Doubling a quantity is an increase of 3 dB (decibels). Increasing it by an order of magnitude adds 10 dB. Halving a quantity subtracts 3 dB. The true beauty of log scales is that multiplication maps into addition — we don’t care what the original base is, doubling e.g. any sound intensity will increase its sound level in decibels by 3. Doubling the hydrogen ion activity in any solution will decrease the pH (from whatever original value it had) by 0.3.
rgb
The problem with the “25% more acidic” statement is that it implies that acidification has progressed 25% of the way to neutrality, which isn’t so.
Repeated for effect.
And I actually agree. This is very misleading. Acidity is generally measured, given, discussed in pH, and it makes this sound like a big change, rather than a tiny one (with various possible sources of error, which may or may not be significant).
rgb
Acidity is the quantitative measure of how much hydroxide ion can be titrated. Those who claim that the acidity of sea water has changed by 25% are incorrectly ignoring the presence of bicarbonate and other “acidic” ions that can neutralize OH-. Assuming that the models are correct, acidity has increased by a few percent.
Robert Brown says: June 4, 2014 at 10:58 am
“Lots of other things vary by many orders of magnitude and are best described on a log scale in e.g. decibels rather than in absolute terms.”
Actually, there is a more cogent reason here. The law of mass action, which provides equilibrium relations, is multiplicative. The conservation laws are additive, which often ends up meaning that when one concentration changes, equilibrium is maintained by changing just one other, and that changes in ratio. So here (in sea), for example, it’s a rough rule that a 25% rise in H+ requires a 25% rise in [H2CO3], even tho the latter is in much higher concentration. That’s how buffering works.
There is a typo in the first sentence.
“Some 56 million years ago, a massive pulse of carbon dioxide into the atmosphere sent global temperatures soaring.”
should read
“Some 56 million years ago, a massive pulse of global temperature sent carbon dioxide soaring.”
Ancient Koine greek has four “ifs”: if and it is, if and it isn’t, if and it might be or might not be, and if – I wish it was but it is not.
IF (Koine type 4) the scientific community really wanted to know whether CO2 in the atmosphere was “acidifying” the ocean or not, there is a straightforward way to find out.
Deep ocean water has been separate from the surface for years to centuries while surface water is in contact with air. Compare CO2 levels in ocean bottom water to surface water. What’s the problem?
How do universities get away with making press releases with such sensationalized garbage, half-truths, and flat-out lies? Papers I’ve read studying the PETM found that temperatures began to rise 60 m.a., 4 million years before the CO2 spike, and during this increase in temperature CO2 levels actually were falling. This early temperature rise was also about 3 times higher than the temperature rise following the CO2 spike.
richardscourtney says: June 4, 2014 at 6:48 am
“Really? Why then did the many not object when e.g. Al Gore did make that attribution in his sci-fi movie?”
The objection was made. It was item 4 on Judge Burton’s list of nine. The New Scientist blog commented:
“Historically, global warming events at the end of ice ages have not been triggered by rises in atmospheric CO2 concentrations. However, as explained in “Climate myths: Ice cores show CO2 increases lag behind temperature rises, disproving the link to global warming”, this does not disprove that CO2 warms the atmosphere and that rising CO2 emissions have cause warming since the 20th century.”
That’s the view of the non-few.
“And if CO2 did not cause the temperature rises in the PETM and at the other times then what was the cause of those temperature rises?
Importantly, if CO2 did not cause those temperature rises in the past then how and why is “burning fossil fuel” doing it “in modern times”?”
The cause of PETM etc warming is a research topic. I don’t know the answers. But for CO2 to cause warming, something has to be forcing CO2 into the atmosphere. In past times, there is mostly nothing obvious to do that. Now there is. It’s us.
John Eggert says: June 4, 2014 at 6:29 am
“Nick: The entire point of the paper is that pH has changed. If you don’t know how extensive the readings were 100 years ago, you too agree that there is no basis for saying the pH has changed.”
No, I’m just saying that I don’t know. I could find out. So could others here.
“And wikipedia for a source? “
Yes. It’s easily linked, and for matters like carbonate chemistry, it is fine. Did you find any errors?
Robert Brown says:
June 4, 2014 at 10:58 am
pH is precisely such a quantity. The concentration of hydrogen ions in a water-based solution can vary by 14 orders of magnitude (although it is moderately difficult to reach the extreme ends of this range chemically). Sure, one can write it out with all of those zeros as you did above, but that is silly. One can use scientific notation, and write it as 10^{-3}, 10^{-4}, 7.432 \times 10^{-5} … etc, but that too involves a lot of nearly pointless writing. It is far easier to form $-\log_{10}(P_{H+})$, or more properly as $-\log_{10}(a_{H+})$ where a_{H+} is the activity of the hydrogen ion in a solution. The activity is defined relative to the chemical potential of a “standard” electrode, which can be directly mapped to a voltage generated on the electrode and measured by measuring the electrode voltage. This activity does directly involve kT (or RT, if you are a chemist instead of a physicist) so one has to know the temperature at the electrodes to get anything like a precise measurement. I’m certain that there is other chemistry that can confound it, as well — the presence of other electrolytes or contaminants in the water, perhaps.
The pH notation is convenient for use with an electrode because the Nernst equation is linearly dependent on log[H+] so it’s very convenient to calibrate in terms of pH. Other methods such as titration are linearly dependent on [H+] so if you want to know how much acid it would take to neutralize a solution pH isn’t what you want. Back in the early 20th century when the pH scale was first devised, there were no computers or calculators so the log notation was a very convenient shorthand to describe [H+] and rendered the arithmetic simpler as Robert describes.
Lots of other things vary by many orders of magnitude and are best described on a log scale in e.g. decibels rather than in absolute terms. Enough so that engineers (and physicists, and I’m sure chemists) have some simple rules of thumb. Doubling a quantity is an increase of 3 dB (decibels). Increasing it by an order of magnitude adds 10 dB. Halving a quantity subtracts 3 dB. The true beauty of log scales is that multiplication maps into addition — we don’t care what the original base is, doubling e.g. any sound intensity will increase its sound level in decibels by 3. Doubling the hydrogen ion activity in any solution will decrease the pH (from whatever original value it had) by 0.3.
Yes so calculating pOH from pH in aqueous solution becomes pOH=14-pH for example.
rgbatduke says:
June 4, 2014 at 11:01 am
“The problem with the “25% more acidic” statement is that it implies that acidification has progressed 25% of the way to neutrality, which isn’t so.”
Repeated for effect.
And I actually agree. This is very misleading. Acidity is generally measured, given, discussed in pH, and it makes this sound like a big change, rather than a tiny one (with various possible sources of error, which may or may not be significant).
Not to anyone who understands chemistry it doesn’t. For example, blood pH is normally between 7.35 and 7.45, which makes it sound like a very narrow range whereas in fact [H+] varies by about 25%. A sustained drop in blood pH of 0.1 in pH doesn’t sound like much but is likely to prove fatal, saying that the Hydrogen ion concentration was 25% below the normal range would be more likely to convey the necessary degree of urgency!
If you want to evaluate the solubility product of aragonite for example:
it’s Ksp=[Ca++] *[CO3–]
but [CO3–] is given by DIC/(1+[H+]/K2 +[H+]^2/K1K2) (where logK1=-6.39 and logK2=-10.39
so the change in absolute [H+] is what’s relevant not the change in pH.
So in the context of dissolving shells it’s the relative change in [H+] that counts not the change in pH.
@rgb: Thanks. Could you craft a concise scientific response to the claim, “acidity has increased by 25%”?
John Eggert says:
June 4, 2014 at 6:29 am
And wikipedia for a source? Really?
Yes really, you’re given an accessible source so live with it, too many on here moan when given a source that’s ‘behind a paywall’. References to the original source is given on the page so quit moaning!
Robert Brown says: June 4, 2014 at 10:58 am
“The true beauty of log scales is that multiplication maps into addition”
It’s late in the thread so…
Noah said to the animals “Go forth and multiply!”.
But the snakes said “we can’t do that, we’re adders”.
So Noah was mighty wroth. He went ashore with an axe, into the dying forest.
He returned and dumped it on the deck.
“Here, I have made you a log table. Go forth and multiply!”.
Couple of additional problems;
1) They’ve basically used “Mike’s nature trick” again. This time splicing high resolution direct sampling data onto low resolution core proxy data. They cannot say the change is unprecedented because the proxy is incapable of revealing rapid change.
2) The notion that the oceans are becoming “more acidic” belies the fact that they are basic. Owing to the fact that they rest on a bed of basalt, they will remain so. The only thing that may occur is CACO3 may precipitate at a faster rate, causing . . . more limestone.
New cause celeb; Catastrophic Anthropogenic Global Shale Deposits!!!
Note the irony of that; Anthropogenic CO2 emissions result in faster growth of sea floor sedimentary limestone deposits, resulting in better geology for the trapping of hydrocarbons, eventually leading to more recoverable oil reserves.
There’s such poetry in nature.
Bob Kutz says: June 4, 2014 at 12:55 pm
“Anthropogenic CO2 emissions result in faster growth of sea floor sedimentary limestone deposits”
On the contrary, CO2 dissolves CaCO3. When you sort out the carbonate chemistry, and get past the role of intermediates like H+, the nett reaction is:
CaCO3 + CO2 + H20 -> Ca++ +2HCO3-
Each added CO2 dissolves a molecule of CaCO3. From somewhere, sometime.
After 4 years of studying Chemistry in university I have never seen a change in pH expressed as a percent change in “acidity”. That is, until we heard from the “ocean acidification” industry. You just don’t see it anywhere else because it is meaningless.
While it is correct to say that it reflects a ~25% increase in hydronium ions, it is grossly misleading and absurd to say it is 25% more acidic. The change is barely detectable on instruments and is widely eclipsed by the natural variations in ocean pH.
Try an experiment with your friends… have them taste test 2 beverages: Brand A at a pH of 7.0 and Brand B at 7.1 and see if they can tell the difference. Then tell them Brand A is 25% more acidic than Brand B and they should not buy Brand A.
This is nothing but propaganda.
This chart indicates no change in ocean pH. And going by the total and complete failure of the alarmist clique to either model, or predict the real world, the default position must be that they are wrong this time, too.
The Phanerozoic Carbon Cycle, CO2 and O2
Bob Berner, Prof. of Geology, Yale
pg 38, 2nd para; ” . . . The idea is simply that if atmospheric CO2 rises, the concentration in the ocean also rises, and there is enhanced uptake by marine basalt weathering. In this way the process serves as a negative feedback for stabilizing atmospheric CO2 . . . . Alt and Teagle (1999) stated that 70-100% of the calcium in CaCO3 found in altered submaring basalts is derived from seawater, not from the basaltic minerals. This meant that most of the basalt is not actually being weathered but is simply providing a place for the interstitial precipitation of CA++ and HCO3- from seawater. The neutral-to-alkaline interstitial seawater environment, due to hydrolysis of the basalt (Caldeira, 1995), may serve to raise pH and induce CaCO3 precipitation.”
Now, in all fairness, Berner does say that this may have been far more important in the period before 150 Ma than it is today, but he also says; “Global warming due to elevated CO2 brings about accelerated phosphate weathering and transport of P to the sea, leading to an increase in aqueous nutrient P. This in turn leads to greater organic carbon burial and greater CO2 consumption, with the overall process producing negative feedback.” – ibid, pg 43, 2nd para.
Wanna go some more?
Sorry, should have directed the prior comment at Nick Stokes, in re. his 1:13 comment directed at me.
dbstealey says:
June 4, 2014 at 9:07 am
“suspected…” “…could be…” “CO2 ha[s] made seawater grow more acidic…”
No.
According to real world data from the Monterey Bay Aquarium’s intake pipe, located far out in the ocean, there has been no measurable change in pH.
Unfortunately your link turned out to be a blank pdf.
However I was able to find a couple of publications by that group. Their pH meter really didn’t have the accuracy to measure the longterm change in pH, anyway not the best site to do that measurement anyway given the regular intrusions of hypoxic, low pH water into that area, which resulted in large variation in pH.
Granted, this data stops several years ago, but it covers a time when CO2 was strongly ramping up.
The article also omits any mention of buffering — a major factor in any discussion of ocean pH; probably the major factor. Why no mention of buffering?
Which buffer had you in mind?
[Reply: No problem here accessing the Monterey pH record. ~mod.]
Nick Stokes says: June 4, 2014 at 2:48 am
“pH 7 is the neutral point of pure water. Marine organisms never encounter pure water, so that is irrelevant. They have evolved in a sea of pH>8.
Human blood has a pH of about 7.4. If it drops to 7.35, that is acidosis = bad news. Blood pH 7 is not compatible with life.”
Nick, the world out side of your lab beakers is quite the dynamic place. To point out the obvious, human blood is encapsulated inside an epithelial container in a healthy human, isolated from the environment outside. (As are marine organisms.) Your body can tolerate immersion in water having a wide range of pH, from sea water to fresh water, hard water to soft water, alkaline to acidic, without compromising the blood pH balance maintained inside your skin.
From Scripps:
“A group of 19 scientists from five research organizations have conducted the broadest field study of ocean acidification to date using sensors developed at Scripps Institution of Oceanography, UC San Diego.”…
…”This study is important for identifying the complexity of the ocean acidification problem around the globe. Our data show such huge variability in seawater pH both within and across marine ecosystems making global predictions of the impacts of ocean acidification a big challenge. Some ecosystems such as coral reefs experience a daily range in pH that exceeds the predicted increase in pH over the next century.”…
Read more at: http://phys.org/news/2011-12-comprehensive-key-ocean-ph-variations.html#jCp
By the way, I understand that human emissions of CO2 account for 4% of the increase in atmospheric CO2. That would be 4% of the purported 25% drop in pH.
As usual, context is important.
rgbatduke says:June 4, 2014 at 5:14 am
See above.
Nick Stokes;
(final point)
To put this in terms you are possibly more familiar with;
silicate rock weathering;
CaSiO3+2CO2+H2O→2HCO3- +Ca2+ +SiO2 which eventually becomes;
silicate weathering + carb sedimentation;
CaSiO3+CO2→CaCO3+SiO2
While these are processes which take much much longer than the simple ‘ CO2 and CaCO3 produce bicarbonate and cationic calcium’, they are the ‘end state’ of the reaction in the presence of silicates rather than the intermediary ‘temporary state’.
Since the bicarbonate is itself a buffer in water, I’m not sure you have any place left to stand in the ‘ocean acidification’ argument.
As was my original point; CO2 induced ocean acidification simply doesn’t stand up to scrutiny. The very bed in which the oceans lay deny any chance of it.
Given the immense amount of ocean pH data which is contradictory to what the authors of this study claim, I’m not sure you really want to stand with this particular bogeyman.
Nick Stokes says:June 4, 2014 at 5:10 am
” Shell CaCO3 once dissolved won’t reform.”
Calcareous shells form under the mantle of the shellfish, isolated from the open aqueous environment. The rate of deposition exceeds the rate of dissolution, resulting in growth.
Your skin is constantly exfoliating as new skin grows underneath. Has your skin worn out yet? I sincerely hope not.
These processes have been going on a long time. Enjoy your life. There is no steering wheel on this clown car.
Nick Stokes says:
June 4, 2014 at 2:48 am
pH 7 is the neutral point of pure water. Marine organisms never encounter pure water, so that is irrelevant. They have evolved in a sea of pH>8.
====
nope…..Nick there are plenty of places that have much lower pH…and plenty of times when they are exposed to rain…here’s a shot of the barrier at low tide….and yes…it can rain at low tide
http://media-1.web.britannica.com/eb-media/00/65300-004-C334446B.jpg
If you look at the Mauna Loa co2 yearly rate of increase since 1960, then it can be seen that the rate increased with every El Nino and decreased with every La Nina. In 1987/88 co2 has 2 record peaks close to 2.0 ppm, and then the rate drops in the preceding several years until 1992 when it drops to 0.43 ppm. In 1998, El Nino, co2 has it,s highest peak at 2.94 ppm. Then in the following year, La Nina, co2 drops to 0.93 ppm. Co2 output by man has risen every year, so it is more than obvious that nature is adding and subtracting co2 into the atmosphere according to it,s internal changes. This raises a very important question ” How much of the atmospheric co2 increase is driven by the oceans vs being cause by human output? Perhaps all atmospheric co2 is driven by changes in the ocean temps. How would we know if we are unable to separate how much either one contributes?
Stipulating all the alarmist measurements, if CO2 is the sole culprit, how is it that acidification began in 1750? Ist bin Elefantenland.
From Phil. on June 4, 2014 at 2:19 pm (link added back into a quoted section):
Sorry, Phil-dot, but it loaded just fine for me. Check your software.
goldminor says:
June 4, 2014 at 4:36 pm
How much of the atmospheric co2 increase is driven by the oceans vs being cause by human output?
The increase is near entirely human: the oceans and vegetation are net sinks for CO2, not sources. Human input is about twice the increase of CO2 in the atmosphere, thus nature is a sink for halve of the human input (as mass, not original molecules):
http://www.ferdinand-engelbeen.be/klimaat/klim_img/dco2_em2.jpg
The variability you see in increase rate is a variability in sink rate and mainly caused by vegetation, not by the oceans, as reaction to temperature/precipitation changes mainly in the tropical rain forests. That can be deduced from the 13C/12C ratio variations which are opposite to the CO2 rate of change variations:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/temp_dco2_d13C_mlo.jpg
If the oceans were the cause, the 13C/12C ratio changes would be the same direction as the CO2 changes.
Richard G says:
June 4, 2014 at 2:32 pm
By the way, I understand that human emissions of CO2 account for 4% of the increase in atmospheric CO2. That would be 4% of the purported 25% drop in pH.
Bad argument… human emissions are 4% of the total emissions, but 0% of the total sinks, thus 100% of the increase (in fact a little less, the increase in temperature also has a small contribution). The natural emissions are an estimated 150 GtC/year, the natural sinks are some 4.5 GtC higher. Human emissions are around 9 GtC/year.
Yet another paper which cannot distinguish between sea water and surface sea water!
Why is that distinction important? Because the statement
“In the last 150 years or so, the pH of the oceans has dropped substantially, from 8.2 to 8.1”
is clearly wrong!
http://wattsupwiththat.com/2010/06/19/the-electric-oceanic-acid-test/
This pH change of sea water takes only place in about 1% of the sea water!
=> It speaks loudly of the professional level of the reviewers to let such a plunder pass repeatedly over the last decades.
Any time some one is talking about the sea is a net sink for CO2, ask them how the amount (about 200GtC) compares to the accuracy which which we know the total amount of CO2 in the oceans (about 1% of 38000GtC).
I claim that we dont know enough about the CO2-circuit to make such statements.
Laws of Nature says:
June 5, 2014 at 1:23 am
The distribution of CO2 between the ocean “mixed layer”, that is the upper 100-300 meters of the oceans is quite rapid (half life ~1 year) and the carbon content is comparable to the atmosphere (~1000 GtC mixed layer, ~800 GtC atmosphere). The distribution between atmosphere / mixed layer and the deep oceans is a lot slower (~50 years half life time), thus it takes time to get a new equilibrium, which indeed is at 1% rise of CO2 in the atmosphere and total ocean carbon content. But that also gives that the mixed layer pH is following the atmospheric CO2 content. In the mixed layer most of the ocean biolife is at work…
One doesn’t need to measure the increase in the deep oceans, a simple mass balance gives you the answer: sinks in the biosphere are known from the oxygen balance, in the ocean surface layer are a matter of buffer capacity (about 10% change for a 100% change in the atmosphere) and the rest is in the deep oceans.
Nick Stokes:
Thankyou for the clarification you provide to me at June 4, 2014 at 12:12 pm where you reply to my having asked
By saying
OK. I get that. You are saying
1.
Nobody knows the “cause of PETM etc warming” but it is not CO2.
2.
Atmospheric CO2 has no natural variability and only changes because “something has to be forcing CO2 into the atmosphere”.
3.
We are forcing CO2 into the atmosphere because otherwise “there is mostly nothing obvious to do that”.
Thankyou for your clarification of your assertions. But I like scientific assessments and I prefer them to superstitious assertions of the kind you have provided.
Richard
Ferdinand Engelbeen:
I have no intention of yet again repeating our arguments on WUWT. I write to explain to any who may not know that for many years you, I and Bart have been arguing the cause of the recent rise in atmospheric CO2 concentration.
Bart claims the rise has an entirely natural cause, you claim the rise has an entirely anthropogenic (i.e. human-induced) cause, and I don’t know if the rise has a natural cause, or an anthropogenic cause, or some combination of natural and anthropogenic causes.
Your post in this thread at June 4, 2014 at 11:35 pm says
Your reply is plain wrong.
At issue is what the increase in atmospheric CO2 would have been if there were no anthropogenic emission. Your answer assumes the anthropogenic emission is the total cause of the rise because the sinks cannot sequester all of the total emission.
Your assumption is not true.
I have repeatedly explained to you that the dynamics of the seasonal cycle demonstrate your assumption is plain wrong.
This is the CO2 data from Mauna Loa
http://www.esrl.noaa.gov/gmd/ccgg/trends/
The seasonal variation in each year is a slow rise indicating increase to atmospheric CO2 that is followed by a steep fall as sequestration of CO2 is greater than CO2 emission which is followed by a rapid reversal. There is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
The annual rise of any year is the residual of the seasonal variation of that year.
The dynamics of the seasonal change is consistent with the carbon cycle adjusting to a new equilibrium:
(a) adjustment of mechanisms with long rate constants provides the annual rise
while
(b) adjustment of the mechanisms with very short rate constants provides the seasonal variation.
The recent rise in atmospheric CO2 concentration is consistent with adjustment of the carbon cycle to a new equilibrium but is NOT consistent with the CO2 sinks lacking ability to sequester all the CO2 emissions. The anthropogenic CO2 emission may be the cause of a changed carbon cycle equilibrium but other causes are more likely.
Richard
“Nobody knows the “cause of PETM etc warming” but it is not CO2.”
I said very clearly that I do not know. I did not say nobody knows, nor that it is not CO2. You made that up.
“Atmospheric CO2 has no natural variability and only changes because “something has to be forcing CO2 into the atmosphere”.”
I said nothing of the sort. I said that if you want to say CO2 rise is forcing temperature rise, you have to ask what is forcing CO2 rise. Of course CO2 has natural variability. It responds to temperature and bio activity. It has a seasonal cycle.
“We are forcing CO2 into the atmosphere because otherwise “there is mostly nothing obvious to do that”.”
We are forcing CO2 rise in emitting about 30 Gt/year. This stuff isn’t difficult. Well, maybe it is if you don’t believe we’re emitting CO2.
Nick Stokes:
I object to your semantic drivel in your post addressed to me at June 5, 2014 at 5:32 am.
I did NOT “make up” anything. I quoted your words verbatim and stated the only understanding of those words which I could make. If you know people who know the cause of the PETM and what it was then say because I would like to know.
You say
No! We are emitting only about 30 Gt/year of CO2 and this is a trivial addition to the natural emission which is two orders of magnitude larger. Importantly, this tiny amount of anthropogenic emission is NOT sufficient to be “forcing CO2 rise” (as is explained in my above post to Ferdinand which is here).
This stuff isn’t difficult. Well, maybe it is if you superstitiously believe the trivial anthropogenic CO2 emission is a threat to the world.
Richard
richardscourtney says: June 5, 2014 at 5:45 am
“I did NOT “make up” anything. I quoted your words verbatim and stated the only understanding of those words which I could make.”
Yes, you quoted my words.
“The cause of PETM etc warming is a research topic. I don’t know the answers.”
Your understanding:
“Nobody knows the “cause of PETM etc warming” but it is not CO2.”
That constitutes making up.
“In the oceans, dinoflagellates extended their range from the tropics to the Arctic, while on land, hoofed animals and primates appeared for the first time.” Well put. Nothing special about primates.
kadaka (KD Knoebel) says:
June 4, 2014 at 10:23 pm
From Phil. on June 4, 2014 at 2:19 pm (link added back into a quoted section):
“Unfortunately your link turned out to be a blank pdf.”
Sorry, Phil-dot, but it loaded just fine for me. Check your software.
Yeah it loaded fine today, go figure.
Bob Kutz says:June 4, 2014 at 2:11 pm
The Phanerozoic Carbon Cycle, CO2 and O2
Bob Berner, Prof. of Geology, Yale
I live on the west slope of the Rockies. We have lots of basalt at 6-9000 feet with a lovely coating of calcium carbonate.
richardscourtney says:
June 5, 2014 at 5:07 am
Richard, the discussion with Bart was that an increase in the natural cycle (probably from the oceans) might be as good the cause of the increase as the human contribution.
Bart’s reasoning was based on the supposition that the cause of the natural variability in rate of change and the cause of the decadal increase in rate of change were from the same process. That point is resolved by plotting both the rate of change of CO2 and the rate of change of δ13C, which proves beyond doubt that the short term variability (both seasonal and year-by-year) is caused by vegetation. But the decadal trend in rate of change by vegetation is an increasing sink for CO2 as can be derived from the oxygen balance. Thus the short term and long term effects/processes are opposite to each other.
That also applies to your supposition that the seasonal uptake/release is large enough to absorb all human emissions. It can’t, because the sinks are saturated and only slower processes that incorporate more carbon in longer lasting parts of the biosphere make a difference. But that is a much slower process than what causes the rapid decrease of CO2 in NH spring and the rapid rise in NH fall.
I have seen a few people asking how warming oceans which give off CO2 can absorb CO2. I had originally addressed this in a few WUWT essays. First your talking about flux, when they give off CO2 that is a gross flux. When people are discussing the absorption of CO2 that’s generally a net flux. The problem is we don’t have enough information to measure the net flux into the ocean. So in general the hypothesis is that nature balances out, we are the change, and the difference in the atmospheric CO2 between projections and measurements is in flux to the oceans.
Its a gross exaggeration of legitimacy for prognostications built on assumption without sufficient measurement data. It could be true but the difference between theory and reality is measurement.
Nick Stokes:
Having been shown to be wrong on your main point, you continue to obfuscate at June 5, 2014 at 6:14 am. But I will not ‘let you off the hook’ on your side-issue because it emphasises your behaviour.
You wrote
You now claim that does NOT mean “Nobody knows the “cause of PETM etc warming” but it is not CO2.”
OK, what did you intend it to mean?
Richard
Ferdinand Engelbeen:
In reply to my post at June 5, 2014 at 5:07 am which is here and explained WITH CLEAR EVIDENCE
at June 5, 2014 at 7:46 am you say
NO!!!
I made no “supposition”. I provided clear evidence saying
You are making an assertion which is plainly and clearly wrong.
THE SINKS ARE NOT SATURATED.
Richard
Steven Burnett says:
June 5, 2014 at 8:12 am
the difference between theory and reality is measurement.
Well, we have a few measurements which show what happens: the mass balance, the δ13C balance and the oxygen balance.
To begin with the mass balance:
humans emit ~9 GtC/year as CO2. The increase in the atmosphere is ~4.5 GtC/year. That makes a nice balance:
increase in the atmosphere = human emissions + natural releases – natural sinks.
4.5 GtC/yr = 9 GtC/yr + X – Y
where
X – Y = -4.5 GtC/yr
In every year since 1959, natural sinks were larger than natural sources.
We know the uptake by the biosphere from the oxygen balance: since 1990 ~1 GtC/year and increasing.
We know the uptake from the ocean surface layer which is ~0.5 GtC/yr due to the buffer/Revelle factor.
The rest is going into the deep oceans, as all other possible sinks are either too small or too slow.
The exact height of X and Y is of little interest, except if there was a huge increase in turnover, but there is not the slightest sign for such an increase, to the contrary.
richardscourtney says:
June 5, 2014 at 9:18 am
Nick Stokes:
Having been shown to be wrong on your main point, you continue to obfuscate at June 5, 2014 at 6:14 am. But I will not ‘let you off the hook’ on your side-issue because it emphasises your behavior.
Actually it exemplifies your behavior Richard, I believe Anthony described it as your habit of getting into ‘food fights’ on here.
You wrote
“The cause of PETM etc warming is a research topic. I don’t know the answers. But for CO2 to cause warming, something has to be forcing CO2 into the atmosphere. In past times, there is mostly nothing obvious to do that. Now there is. It’s us.”
You now claim that does NOT mean “Nobody knows the “cause of PETM etc warming” but it is not CO2.”
Which it clearly does not to anyone with a passing knowledge of the english language!
You also claimed that Nick said:
2.
Atmospheric CO2 has no natural variability and only changes because “something has to be forcing CO2 into the atmosphere”.
Clearly Nick did not say that!
3.
We are forcing CO2 into the atmosphere because otherwise “there is mostly nothing obvious to do that”.
Again a warped interpretation of what Nick said which was in that: “In past times, there is mostly nothing obvious to do that. Now there is. It’s us”, did you miss the “Now there is”?
OK, what did you intend it to mean?
It’s rather obvious what was meant, clearly not your garbled version.
How many Giga-Tera Tonnes of exposed calcium in all its forms exists in the oceans? The calcium content of the oceans will neutralize any PH that CO2 may effect.
The so-called dramatic change claimed in the article is questionable at best.
The known dynamic range of ocean pH is far wider than the amount of change. And the claim of the change is not in actual measurements but is derived by a modelingprocess full of assumptions.
Life in the ocean is not showing the impacts of the claimed changes.
The interesting thing in the ocean acidification claims is the recursive nature of it. The climate obsessed put these alarmist talking points out, they fall apart under scrutiny, but a small group of people don’t realize that the cliams were bunk. It is like ratchet effect of bogosity, never increasing actual real knowledge. Instead the public square is incrementally cluttered with bogus alarmist garbage that is never completely cleared away.
The science is settled. I thought there was still a debate about the cause[s]
Let’s get more detail and they seem to have skimmed this bit.
Phil.:
At June 5, 2014 at 10:11 am you say to me
OK. Nick seems unwilling to say what he did mean, and you say it is “rather obvious ” to you, so perhaps you can say what you think is “rather obvious”. Perhaps Nick may agree with your unstated understanding of what he meant.
Richard
richardscourtney says:
June 5, 2014 at 9:30 am
There is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
Richard, the monthly data at Mauna Loa show that the seasonal uptake is saturating in September (which is a few months later than at ground level). If they were unlimited, the CO2 levels would go down until the CO2 level was back to equilibrium. From September on, the decay rate is larger than the uptake rate and CO2 levels go up again. Thus the sinks really fill up as they are limited in capacity, be it a huge capacity of ~60 GtC/season in and out.
The multi-year uptake is a much slower process and does take away ~1 GtC/year over the growing season.
hunter:
I write to support your post at June 5, 2014 at 11:42 am which says
Yes. And as you say it is important that
“The interesting thing in the ocean acidification claims is the recursive nature of it. The climate obsessed put these alarmist talking points out, they fall apart under scrutiny, but a small group of people don’t realize that the cliams were bunk. It is like ratchet effect of bogosity, never increasing actual real knowledge. Instead the public square is incrementally cluttered with bogus alarmist garbage that is never completely cleared away.”
Additionally, I point out that when called on their claims – as this thread shows – they retreat into semantic arguments about their claims.
Richard
Ferdinand Engelbeen:
re your post addressed to me at June 5, 2014 at 12:54 pm.
Sorry, but your repeatedly asserting that the sinks fill does not alter the fact that they don’t.
I yet again ask you to address the following information which I have repeatedly put to you (including twice in this thread).
This is the CO2 data from Mauna Loa
http://www.esrl.noaa.gov/gmd/ccgg/trends/
The seasonal variation in each year is a slow rise indicating increase to atmospheric CO2 that is followed by a steep fall as sequestration of CO2 is greater than CO2 emission which is followed by a rapid reversal. There is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
The annual rise of any year is the residual of the seasonal variation of that year.
The dynamics of the seasonal change is consistent with the carbon cycle adjusting to a new equilibrium:
(a) adjustment of mechanisms with long rate constants provides the annual rise
while
(b) adjustment of the mechanisms with very short rate constants provides the seasonal variation.
The recent rise in atmospheric CO2 concentration is consistent with adjustment of the carbon cycle to a new equilibrium but is NOT consistent with the CO2 sinks lacking ability to sequester all the CO2 emissions. The anthropogenic CO2 emission may be the cause of a changed carbon cycle equilibrium but other causes are more likely.
Richard
I don’t know how many sea creatures can keep up with all this ‘acid’, but here is something interesting regarding creatures not being able to keep up.
richardscourtney says:
June 5, 2014 at 1:06 pm
(a) adjustment of mechanisms with long rate constants provides the annual rise
while
(b) adjustment of the mechanisms with very short rate constants provides the seasonal variation.
Richard, the point that I tried to make is that (b) is clearly caused by vegetation as the 13C/12C ratio and the oxygen levels show, while (a) is clearly NOT caused by vegetation, as that is a net sink for CO2 over time, as derived from the oxygen balance. Neither is (a) caused by the oceans, as the difference in mass balance must go somewhere. Nor is an increase in turnover the cause of the increase in the atmosphere as there is no observed increase in turnover…
richardscourtney says:
June 5, 2014 at 1:06 pm
Ferdinand Engelbeen:
re your post addressed to me at June 5, 2014 at 12:54 pm.
Sorry, but your repeatedly asserting that the sinks fill does not alter the fact that they don’t.
I yet again ask you to address the following information which I have repeatedly put to you (including twice in this thread).
This is the CO2 data from Mauna Loa
http://www.esrl.noaa.gov/gmd/ccgg/trends/
The seasonal variation in each year is a slow rise indicating increase to atmospheric CO2 that is followed by a steep fall as sequestration of CO2 is greater than CO2 emission which is followed by a rapid reversal. There is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
You can’t be serious, “there is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill.”
The rate of sequestration clearly becomes zero and then negative!
What school of mathematics/science did you attend?
The sinks are incapable of sequestering all of the CO2 entering the atmosphere during the year as every year over the last several decades the pCO2 has increased year over year!
Nick Stokes wrote:”On the contrary, CO2 dissolves CaCO3. When you sort out the carbonate chemistry, and get past the role of intermediates like H+, the nett reaction is:
CaCO3 + CO2 + H20 -> Ca++ +2HCO3-”
Net reactions don’t tell anything about reaction mechanisms. The increase in H+ (a result of increase pCO2) is the primary cause of the increased solubility of CaCO3, NOT (as you seem to suggest) the increase in CO2 concentration by itself.
So:
– if at elevated pCO2, pH is kept constant by addition of OH- ions, CaCO3 will not dissolve.
– if at constant pCO2, H+ is added, solubility of CaCO3 will increase.
Solubility of CaCO3 depends on Ca++ and CO3– concentrations:
Increase in H+ concentration causes a decrease in CO3– ( CO3– + H+ —>HCO3-) Decrease in CO3– causes a decrease in CaCO3– (equilibrium CaCO3 Ca++ + CO3– shifts to the right). The fact that in this case CO2 is causing the pH change, AND is a reactant, complicates things a bit, but still the basic rules of chemical equilibrium apply.
Allchemistry says: June 5, 2014 at 11:16 pm
“The increase in H+ (a result of increase pCO2) is the primary cause of the increased solubility of CaCO3, NOT (as you seem to suggest) the increase in CO2 concentration by itself.”
No, the primary cause is the addition of a Lewis acid, in this case CO2. That is what is actually added. Acidity is fungible. The nett result is adding acid dissolves CaCO3.
As FE kindly mentioned, I have an active calculator here. You can play around with adding H+, CO2 etc and see how everything is connected.
What continually amuses me is how climate alarmists manage to find the most miniscule changes, in this case from pH 8.2 = 0.00000000630957 to pH 8.1 = 0.00000000794328, and turn it into a scary number (25%) when in fact the actual change, when measured by it’s effects in the real world, is negligible, insignificant.
Reminds me of them claiming atmospheric CO2 levels have changed 40%, from almost nothing to next to nothing, with a resulting small COOLING of global temperatures, if any actual change, when they predicted catastrophic warming.
Doesn’t exactly lead one to have much confidence in so-called climate ‘scientists’ (apologies to the actual honest, non-alarmists ones out there, who I do not include in the ‘so-called’ category).
And, in case you were wondering:
The adjective minuscule is etymologically related to minus, but associations with mini- have produced the spelling variant miniscule. This variant dates to the end of the 19th century, and it now occurs commonly in published writing, but it continues to be widely regarded as an error.
I use the variant, for what I hope are obvious reasons.
Ferdinand Engelbeen says:June 4, 2014 at 11:35 pm
Bad argument vs your spaghetti logic. (I hope that does not constitute a food fight?/sarc)
If “human emissions are 4% of the total emissions, but 0% of the total sinks”,
then by extension natural emissions are 96% of the total emissions and, being emissions, are also 0% of the sinks. (SINKS by definition are not EMISSIONS. They are the opposite of emissions. You conflate emissions with sinks.)
Thus together they make up 100% of the increase.
“The natural emissions are an estimated 150 GtC/year, Human emissions are around 9 GtC/year.” On this we are in agreement.
Later you state:
“increase in the atmosphere = human emissions + natural releases – natural sinks.”
What, you don’t think there are human sinks? How many GtC/year of raw human bodies from population growth? Humans make great carbon sinks, 19% by weight. How about Increased biomass from modern human caused agricultural output? I guess these don’t fit in your world narrative.
You need clarity about what we know vs what we think we know.
*We Think* “We know the uptake by the biosphere from the oxygen balance: since 1990 ~1 GtC/year and increasing.”
*We Think* “We know the uptake from the ocean surface layer which is ~0.5 GtC/yr due to the buffer/Revelle factor.”
*We Think* “The rest is going into the deep oceans, as all other possible sinks are either too small or too slow.”
It is a mighty complex world out there. I think it is safe to say that your numbers are derived from calculations, not from measurements. In a word, estimates.
You are fixated on fixation. Sequestration as a goal is foolish. An increasing biosphere is a good thing. Lighten up and enjoy life. Life is good.
More CO2 = MORE SUGAR!
Unless the text book has changed from my University days of hard Chemistry???
pH is NOT the negative logarithm of the hydrogen ion concentration.
It is the negative logarithm of the hydrogen ion ACTIVITY.
Activity is not the same as concentration. They converge only at vanishingly low total ionic strength.
So bow out all those with a fail so far.
There are only a few papers on oceans in which I have seen the correct definition. This is rather important, because ocean waters have precisely the type of chemistry that requires the use of activity and complicated equations such as these
http://en.wikipedia.org/wiki/Debye%E2%80%93H%C3%BCckel_equation
Nick Stokes, how about a small essay on the method of coping with activity coefficients when deriving pH the way you state, from indirect indicators thus – “pH is determined by equilibrium relations involving carbonates, which are much more abundant in the sea than H+ or CO2. Two quantities, dissolved inorganic carbon (DIC) and total alkalinity, are easy to measure, are fairly stable, and pH can be deduced when you know them.”
Note, even filtering sea water (which would commonly be done before chemical analysis) has the capacity to change pH. Having done tens of thousands of pH measurements personally on high ionic strength solutions/suspensions, I can vouch for the difficulty in getting reproducibility to better than 0.1 pH in other than very dilute, filtered, synthetic solutions. Even the presence of suspended organic matter like seaweed fragments, bits of jelly fish tentacles and so can have an effect. Anything in the colloidal size range has to be studied for effect as well.
Regarding the paper cited for this thread, the comments must surely apply to the near-surface layer of oceans. I know of no reliable measurements deeper than a few metres from the comparison period 150 years ago and have grave doubts that anything valid can be stated about ocean pH then. Sure the mixing is good down to a few hundred meters or so, but how good? +/- 0.1 pH good? Prove it.
It ain’t easy, folks.
Phil.:
I am replying to your innumerate post at June 5, 2014 at 3:29 pm.
Even by your standards your post is daft and your silly comment about MY mathematical ability adds to the amusement it provides. I write to try to help you gain some understanding of the issue.
This is the CO2 data from Mauna Loa which I have repeatedly posted in this thread
http://www.esrl.noaa.gov/gmd/ccgg/trends/
As anybody who peruses the graph in the link can see, the seasonal variation in each year is a slow rise indicating increase to atmospheric CO2 that is followed by a steep fall as sequestration of CO2 is greater than CO2 emission which is followed by a rapid reversal.
And anybody can see that – as I said – there is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
A sudden reversal of a linear trend in the seasonal variation of atmospheric CO2 concentration is NOT a “reduction as the sinks fill”. The only way it could be a “reduction as the sinks fill” would be if all sinks had large capacity then (almost) all became completely full at the same time.
Of course, I would not expect people with your mathematical ability to understand this for themselves but I hope this explanation helps you to grasp it.
This is a link to one of my above posts which describes what really happens; i.e.
Richard
PS I am now scheduled to go in for heart surgery and will not be able to respond to any reply until I have recovered from the anaesthetic (probably tomorrow).
Richard G says:
June 6, 2014 at 12:43 am
For some reason, some skeptics never mention sinks. The point is that the natural sources are more than compensated by natural sinks and that there are very few human sinks.
Human bodies and the rest of the non-vegetation biosphere only consume the CO2 that was fixed a few months to a few years before bij plants out of the atmosphere: they are part of the natural cycle. They exhale CO2 back to atmosphere. The burning of fossil fuels is not part of that cycle.
Take as an example a fountain with a huge bassin where a lot of water, say 1,000 l/min is pumped over the fountain flowing back into the bassin. Some worker opens the supply valve to fill the bassin with 1 l/min. Even if it is 1/1,000th of the supply to the fountain, it will be the cause of the increase and eventually the overflow if he doesn’t close the valve on time…
the uptake by the biosphere from the oxygen balance
See: http://www.bowdoin.edu/~mbattle/papers_posters_and_talks/BenderGBC2005.pdf
the uptake from the ocean surface layer which is ~0.5 GtC/yr due to the buffer/Revelle factor
http://www.eng.warwick.ac.uk/staff/gpk/Teaching-undergrad/es427/Exam%200405%20Revision/Ocean-chemistry.pdf
and further the increase in DIC of the ocean surface layer to be compared to the increase in the atmosphere for the same time frame:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf and
http://www.pnas.org/content/106/30/12235.full.pdf
All based on measurements… There are indications that the difference is going into the deep oceans (as the change in 13C/12C ratio at the sink places shows) and ocean wide pCO2 measurements give an average pCO2 difference where pCO2 of the atmosphere is ~7 microatm higher than of the oceans. See Feely e.a.:
http://www.pmel.noaa.gov/pubs/outstand/feel2331/exchange.shtml and following pages.
I have no problems with more CO2, I have only problems with bad arguments, which is the case for a non-human increase of CO2 over the past 160 years. That is a lost battle and only discredits other real valid arguments one can have against (C)AGW…
richardscourtney says:
June 6, 2014 at 2:19 am
there is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
Richard, there wouldn’t be a reversal at all if the sinks weren’t saturating: the seasonal drop would go on continuously until zero CO2 or until a new equilibrium was reached between uptake and release.
Further, your other point:
The annual rise of any year is the residual of the seasonal variation of that year.
is mathematically right, but is the result of two different processes: the seasonal variation is entirely caused by vegetation, the residual is entirely NOT caused by vegetation. That is proven from the 13C/12C and O2/N2 balances.
The increase in the atmosphere and the seasonal variation have nothing to do with each other.
Anway: good luck with the surgery: I hope all get well and that you will have a fast recovery.
The CO2 level during PETM appear to have rose from a base of 1,000 ppm to 1,700 ppm compared to current levels of 400 ppm. Also, there appears to be no consistent consensus about the CO2 levels were during the PETM and CO2 levels appear to based on guestimates. Is anyone able to confirm the CO2 levels during the PETM? Current concerns about acidification appears to be exaggerated.
Geoff Sherrington says: June 6, 2014 at 1:45 am
“Nick Stokes, how about a small essay on the method of coping with activity coefficients when deriving pH the way you state, from indirect indicators thus “
Geoff,
how about some information on how much the activity coefficients differ from 1 at the ionic strength of sea water?
Here is a chapter from a standard chemical oceanography text on how it is done, just as I described. They do distinguish between fugacity and partial pressure for CO2, but Zeebe says it is good to 99%.
richardscourtney says:
June 6, 2014 at 2:19 am
Phil.:
I am replying to your innumerate post at June 5, 2014 at 3:29 pm.
Even by your standards your post is daft and your silly comment about MY mathematical ability adds to the amusement it provides. I write to try to help you gain some understanding of the issue.
Your posts attempting to argue math and science with your mathematical and scientific ‘betters’ (pace Monckton) are always amusing, your use of terms which you clearly do not understand make this post no exception.
This is the CO2 data from Mauna Loa which I have repeatedly posted in this thread
http://www.esrl.noaa.gov/gmd/ccgg/trends/
As anybody who peruses the graph in the link can see, the seasonal variation in each year is a slow rise indicating increase to atmospheric CO2 that is followed by a steep fall as sequestration of CO2 is greater than CO2 emission which is followed by a rapid reversal.
And anybody can see that – as I said – there is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
As anyone with a passing knowledge of calculus/science would know the ‘rate of sequestration’ is given by the slope of that graph, specifically -d[CO2]/dt. Therefore as I pointed out above your statement that: “there is no reduction to the rate of sequestration” is clearly false since in the course of three months it goes from being strongly positive, to approximately zero, to strongly negative!
A sudden reversal of a linear trend in the seasonal variation of atmospheric CO2 concentration is NOT a “reduction as the sinks fill”. The only way it could be a “reduction as the sinks fill” would be if all sinks had large capacity then (almost) all became completely full at the same time.
As an editor I’m sure you’re aware that inclusion of quotation marks (“……..”) means that you are quoting someone, since this post is addressed to me the unwary might assume that you were quoting me. Since you’re not quoting me who exactly are you quoting?
Of course, I would not expect people with your mathematical ability to understand this for themselves but I hope this explanation helps you to grasp it.
Your complete ignorance of the science/math is amusing, your hubris in believing that you can explain it even more so.
Since you would appear to like some numbers:
Beginning of september ~0.2ppm/wk, october ~0ppm/wk, november ~-0.1ppm/wk.
http://www.esrl.noaa.gov/gmd/ccgg/trends/weekly.html
Good luck with your surgery.
richardscourtney said @ June 6, 2014 at 2:19 am
Get well soon, Richard.
Ferdinand Engelbeen and Phil.:
I am replying to your posts which display innumeracy at June 6, 2014 at 6:54 am and June 6, 2014 at 2:40 am, respectively.
At June 6, 2014 at 2:19 am I again pointed out concerning the seasonal variation in atmospheric CO2 concentration which anybody can see here.
That is clearly, and unarguably true because – as I said in trying to educate Phil. – there is only one way that could be true; i.e.
But the two of you refuse to understand the blatantly obvious because you insist on applying a static model to a dynamic system.
Ferdinand, you mistakenly say
NO!!
A container does NOT stop emptying only when its drain fills.
The content of the container changes in response to the difference between the rate of flow into its drain (i.e. sinks) and the rate of flow from its input. A lowering content may become an increasing content because of
a change to the rate of flow into the container,
and/or
a change to the rate of flow out of the container,
and/or
changes to the rates of both flows.
Phil., you demonstrate your inability (refusal?) to understand this when you write
NO!
As anybody with an understanding of calculus/science knows the slope of that graph indicates the difference between the ‘rate of emission’ and the ‘rate of sequestration’.
I yet again repeat
The annual rise of any year is the residual of the seasonal variation of that year.
The dynamics of the seasonal change is consistent with the carbon cycle adjusting to a new equilibrium:
(a) adjustment of mechanisms with long rate constants provides the annual rise
while
(b) adjustment of the mechanisms with very short rate constants provides the seasonal variation.
The recent rise in atmospheric CO2 concentration is consistent with adjustment of the carbon cycle to a new equilibrium but is NOT consistent with the CO2 sinks lacking ability to sequester all the CO2.
Richard
Ooops.
I typed
“That is clearly, and unarguably true because – as I said in trying to educate Phil. – there is only one way that could be true; i.e.”
I intended to write
“That is clearly, and unarguably true because – as I said in trying to educate Phil. – there is only one way that could be untrue; i.e.”
Sorry.
Richard
richardscourtney says:
June 7, 2014 at 2:50 am
Ferdinand Engelbeen and Phil.:
I am replying to your posts which display innumeracy at June 6, 2014 at 6:54 am and June 6, 2014 at 2:40 am, respectively.
At June 6, 2014 at 2:19 am I again pointed out concerning the seasonal variation in atmospheric CO2 concentration which anybody can see here.
there is no reduction to the rate of sequestration as the sequestering ‘sinks’ fill. Clearly, the sinks do not fill.
That is clearly, and unarguably true because – as I said in trying to educate Phil. – there is only one way that could be (un)true; i.e.
A sudden reversal of a linear trend in the seasonal variation of atmospheric CO2 concentration is NOT a “reduction as the sinks fill”. The only way it could be a “reduction as the sinks fill” would be if all sinks had large capacity then (almost) all became completely full at the same time.
But the two of you refuse to understand the blatantly obvious because you insist on applying a static model to a dynamic system.
More of your nonsense Richard why do you keep persisting in it?
We are not applying a static model, the equation we use is a first order differential equation as shown below:
d[CO2]= Sources(t,T, etc)- Sinks(t,T, etc)
Ask some of the chemical engineers who published in your magazine.
There is no “sudden reversal of a linear trend in the seasonal variation of atmospheric CO2 concentration”, see the data:
http://www.esrl.noaa.gov/gmd/ccgg/trends/weekly.html
It’s more like a sine wave.
Over the months of Sept-Nov the rate of sequestration fairly smoothly (allowing for daily fluctuations, ‘noise’) varies from positive to negative. Photosynthetic production is dominated by the Northern hemisphere so it’s not surprising that CO2 reaches its minimum in the NH fall.
Ferdinand, you mistakenly say
Richard, there wouldn’t be a reversal at all if the sinks weren’t saturating: the seasonal drop would go on continuously until zero CO2 or until a new equilibrium was reached between uptake and release.
NO!!
A container does NOT stop emptying only when its drain fills.
The content of the container changes in response to the difference between the rate of flow into its drain (i.e. sinks) and the rate of flow from its input. A lowering content may become an increasing content because of
a change to the rate of flow into the container,
and/or
a change to the rate of flow out of the container,
and/or
changes to the rates of both flows.
As described by the equation above, when sinks are greater than sources sequestration occurs.
Phil., you demonstrate your inability (refusal?) to understand this when you write
As anyone with a passing knowledge of calculus/science would know the ‘rate of sequestration’ is given by the slope of that graph, specifically -d[CO2]/dt. Therefore as I pointed out above your statement that: “there is no reduction to the rate of sequestration” is clearly false since in the course of three months it goes from being strongly positive, to approximately zero, to strongly negative!
NO!
As anybody with an understanding of calculus/science knows the slope of that graph indicates the difference between the ‘rate of emission’ and the ‘rate of sequestration’.
No, as indicated above it indicates the difference between the flow into the atmosphere (sources) and the flow out (sinks), when it is negative sequestration is taking place, therefore the rate of sequestration is -d[CO2}/dt as I’ve told you before.
I yet again repeat
The annual rise of any year is the residual of the seasonal variation of that year.
The dynamics of the seasonal change is consistent with the carbon cycle adjusting to a new equilibrium:
(a) adjustment of mechanisms with long rate constants provides the annual rise
while
(b) adjustment of the mechanisms with very short rate constants provides the seasonal variation.
The recent rise in atmospheric CO2 concentration is consistent with adjustment of the carbon cycle to a new equilibrium but is NOT consistent with the CO2 sinks lacking ability to sequester all the CO2.
That’s exactly what’s happening, there is insufficient CO2 sink capability to keep up with the new CO2 being added to the atmosphere every year, hence pCO2 increases every year.
I.e. the integral of d[CO2]/dt over the year is always positive.
Phil.:
Your twaddle at June 7, 2014 at 8:14 am fails to hide that you were plain wrong when you wrote
The ‘rate of sequestration’ is NOT given by the slope of that graph, specifically -d[CO2]/dt.
A loss or gain occurs because of difference between INPUT AND OUTPUT.
It is NOT only induced by variation to output.
(It seems you have never operated a bank account).
You were wrong to say otherwise and your bluster does not disguise that error: it also does not hide your complete ignorance of what you are waffling about.
Richard
richardscourtney says:
June 7, 2014 at 9:46 am
Phil.:
Your twaddle at June 7, 2014 at 8:14 am fails to hide that you were plain wrong when you wrote
As anyone with a passing knowledge of calculus/science would know the ‘rate of sequestration’ is given by the slope of that graph, specifically -d[CO2]/dt.
The ‘rate of sequestration’ is NOT given by the slope of that graph, specifically -d[CO2]/dt.
A loss or gain occurs because of difference between INPUT AND OUTPUT.
It is NOT only induced by variation to output.
(It seems you have never operated a bank account).
You were wrong to say otherwise and your bluster does not disguise that error: it also does not hide your complete ignorance of what you are waffling about.
Your ignorance Richard, you don’t know what sequestration is!
CO2 is sequestered from the atmosphere when the outgoing flux is greater than the incoming flux.
If pCO2 is decreasing then sequestration is occurring, sequestration can be increased by increasing the outgoing flux, e.g. by increasing photosynthesis or decreasing SST.
Phil.:
re your twaddle at June 7, 2014 at 10:39 am.
Please, please stop displaying your ignorance. You are wasting space on the thread.
You wrongly assert
NO!! You don’t know the difference between net sequestration and sequestration.
Anything which is collecting CO2 from the atmosphere is conducting sequestration.
And
Anything which is releasing CO2 into the atmosphere is conducting emission.
If total sequestration is greater than total emission then there is net sequestration.
And
If total sequestration is less than total emission then there is net emission.
You cannot determine whether sequestration is changing, or if emission is changing, or if both are changing solely by observing if there is a change from net emission to net sequestration. This is because there are two unknowns.
Richard
Many posts on here make claims that humans are the cause of the increase in CO2. This is just a theory with 1 piece of evidence for it (we produce CO2) and lots of evidence against it. Even the bit about CO2 warming a planet with an active hydrosphere and active biosphere is not so clear.
All of the chemistry formulas being posted that claim to demonstrate ocean acidification due to atmospheric CO2 don’t apply to seawater. Seawater is an extremely complex mixture of chemicals with many competing equilibrium equations, a very active biosphere.
http://buythetruth.wordpress.com/2009/03/19/toxic-seawater-fraud/
http://wattsupwiththat.com/2014/04/27/ocean-acidification-expansion/
One of the many reasons alarmists are so easily discounted is they call a lot of things facts which clearly are not. Then they make their claims based on these facts. It is so clearly obvious that we cannot possibly measure the ph of the entire ocean (yet). Therefore it is not possible to calculate a trend. Period. We don’t even know what the error bars should look like.
Stop wasting time defending the indefensible. Relax and enjoy the process of discovery.
richardscourtney says:
June 7, 2014 at 2:50 am
You cannot determine whether sequestration is changing, or if emission is changing, or if both are changing solely by observing if there is a change from net emission to net sequestration. This is because there are two unknowns.
Richard, you haven’t reacted on a previous point I made: it is proven from the oxygen balance that the seasonal variation is entirely from (mainly NH) vegetation and it is proven that the year-by-year increase is NOT from vegetation: vegetation is a net, small, but increasing sink for CO2 over the years, at least since 1990 when sufficient accurate oxygen measurements became available.
The seasonal uptake and release of CO2 and its accompanying O2 and 13C/12C changes clearly show that. Here for the CO2 and 13C/12C balance averaged over 13 seasons:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/seasonal_CO2_d13C_MLO_BRW.jpg
where the start of the seasons is zeroed in January.
Thus the vegetation sink IS largely saturating and reverses at the end of the growing season. Ocean 13C/12C and CO2 changes go in parallel, while vegetation 13C/12C and CO2 changes go countercurrent. Anyway, the residual increase after a full seasonal cycle is not from vegetation, thus a different process is at work which causes the year by year increase of CO2 in the atmosphere. That is not vegetation and not the oceans (as the latter should increase the 13C/12C ratio). Thus, what can it be?
JohnnyCrash says:
June 7, 2014 at 12:06 pm
Many posts on here make claims that humans are the cause of the increase in CO2. This is just a theory with 1 piece of evidence for it
Johnny, your claims make me sad. There is a lot of evidence that humans are the cause of the increase of CO2 in the atmosphere. It fits all known observations, while all alternative theories I have heard of fail one or more observations. Ocean heating fails Henry’s Law and the 13C/12C ratio decrease. Vegetation is a proven net sink for CO2, not a source. And that our emissions are about double the increase in the atmosphere simply shows that nature as a whole is a net sink for CO2… See further:
http://www.ferdinand-engelbeen.be/klimaat/co2_measurements.html#The_mass_balance
Further, the chemistry of seawater is well known, including its buffer capacity, for over 50 years. pH measurements are sporadic done, but one can calculate the pH from other measurements which are done far more regularly all over the oceans. That and a few longer continuous series (Bermuda and Hawaii) show that the pH is going down.
Further, your first link is quite accurate, but has one error:
an increasing concentration of CO2 in the water improves the efficiency of photosynthesis in the oceans
That is not the case, as CO2 is not the limiting factor for photosynthesis in the oceans, the lack of certain elements (like iron) is the main problem. Summer levels of total carbon (DIC) only change a few % compared to winter DIC.
And please read my many comments at your second link…
richardscourtney says:
June 7, 2014 at 11:14 am
Phil.:
re your twaddle at June 7, 2014 at 10:39 am.
Please, please stop displaying your ignorance. You are wasting space on the thread.
You wrongly assert
“CO2 is sequestered from the atmosphere when the outgoing flux is greater than the incoming flux.
If pCO2 is decreasing then sequestration is occurring, sequestration can be increased by increasing the outgoing flux, e.g. by increasing photosynthesis or decreasing SST.”
NO!! You don’t know the difference between net sequestration and sequestration.
Anything which is collecting CO2 from the atmosphere is conducting sequestration.
And
Anything which is releasing CO2 into the atmosphere is conducting emission.
If total sequestration is greater than total emission then there is net sequestration.
And
If total sequestration is less than total emission then there is net emission.
Much as it must irk you Richard you don’t get to redefine the terminology, sequestration is the net removal of CO2 from the atmosphere!
You cannot determine whether sequestration is changing, or if emission is changing, or if both are changing solely by observing if there is a change from net emission to net sequestration. This is because there are two unknowns.
There is one unknown, [CO2], and the two grouped terms in the balance equation:
d[CO2]= Sources(t,T, etc)- Sinks(t,T, etc)
All that matters as far as CO2 is concerned is the relative magnitude of the Sources and Sinks, averaged annually over the globe for the past several decades Sources are bigger than Sinks by a value approximately equal to half of the production by fossil fuel combustion. On a monthly basis Sinks exceed Sources from May to September. Most of the fluctuation occurs in the NH, there is little variation in the S Pole or Baring Head data just steady growth .
Ferdinand Engelbeen:
At June 7, 2014 at 12:29 pm you rightly chastise me when you write
Sorry, Ferdinand, but replying to blog posts has not been my major priority over the past few days. Clearly, your informed and sensible disagreements with me are much more important than the ridiculous idiocy from the anonymous troll posting as ‘Phil.’, but I do need to defend myself from the falsehoods from the troll.
As to your point, the oxygen measurements can be interpreted in many ways. One interpretation is consistent with your assertions. However, something being consistent with a hypothesis is not evidence the hypothesis is true.
Something being inconsistent with a hypothesis IS evidence the hypothesis is NOT true. And the saw-tooth form of the seasonal variation is inconsistent with your assumption that the sinks fill.
And your claim that the isotope ratio changes support your hypothesis is not true. The direction of the change is correct but there is a 50:50 chance it would be in that or the other direction. Importantly, the magnitude of the isotope change is wrong by a factor of ~3 for it to be consistent with the rise in atmospheric CO2 being accumulation of the anthropogenic emission.
Richard
Phil.:
I have lost patience with your idiocy.
I do NOT use my own definitions when I refute your misunderstanding that net sequestration is sequestration. I use the IPCC definition which is here
http://www.climatechange2013.org/images/report/WG1AR5_AnnexIII_FINAL.pdf
It defines
And
Emission is not specifically defined but ‘emission scenario’ is defined as follows
From context, this use of “emissions” in the IPCC Special Report on Emission Scenarios is as I stated when I wrote
In this thread you have provided several posts that consist solely of falsehoods. I shall ignore any additional ignorant and/or deliberately untrue assertions from you.
Richard
richardscourtney says:
June 8, 2014 at 6:18 am
Phil.:
I have lost patience with your idiocy.
I do NOT use my own definitions when I refute your misunderstanding that net sequestration is sequestration. I use the IPCC definition which is here
http://www.climatechange2013.org/images/report/WG1AR5_AnnexIII_FINAL.pdf
It defines
Sequestration bold See Uptake.
And
Uptake The addition of a substance of concern to a reservoir. The uptake of carbon containing substances, in particular carbon dioxide, is often called (carbon) sequestration.
Exactly, addition of substance to a reservoir! Exchange with a reservoir in equal quantities is not sequestration. For example in the case of a dam on a river with a reservoir behind it you can properly describe it as sequestration if the reservoir is not full, when it is full however, and water is flowing over the dam you are not sequestering water, you are not adding water to the reservoir.
In this thread you have provided several posts that consist solely of falsehoods. I shall ignore any additional ignorant and/or deliberately untrue assertions from you.
I challenge you to find one falsehood and support it with evidence.
Phil.:
re the “challenge” in your post at June 8, 2014 at 7:05 am.
Compare the IPCC definition to your falsehood of a definition in your post which sets the “challenge”.
Now, I will reciprocate: I challenge you to show you can tell the truth.
Richard
richardscourtney says:
June 8, 2014 at 5:47 am
Ferdinand Engelbeen:
At June 7, 2014 at 12:29 pm you rightly chastise me when you write
Richard, you haven’t reacted on a previous point I made: it is proven from the oxygen balance that the seasonal variation is entirely from (mainly NH) vegetation and it is proven that the year-by-year increase is NOT from vegetation: vegetation is a net, small, but increasing sink for CO2 over the years, at least since 1990 when sufficient accurate oxygen measurements became available.
Sorry, Ferdinand, but replying to blog posts has not been my major priority over the past few days. Clearly, your informed and sensible disagreements with me are much more important than the ridiculous idiocy from the anonymous troll posting as ‘Phil.’, but I do need to defend myself from the falsehoods from the troll.
You share with Monckton the disagreeable trait of referring to anyone who doesn’t agree with you and whose arguments you are unable to refute as a ‘Troll’ and their arguments as ‘Falsehoods’.
As to your point, the oxygen measurements can be interpreted in many ways. One interpretation is consistent with your assertions. However, something being consistent with a hypothesis is not evidence the hypothesis is true.
Something being inconsistent with a hypothesis IS evidence the hypothesis is NOT true. And the saw-tooth form of the seasonal variation is inconsistent with your assumption that the sinks fill.
It is only a sawtooth if you present it with insufficient resolution to show its true form, I have posted the daily data on here twice and you have studiously ignored it!
And your claim that the isotope ratio changes support your hypothesis is not true. The direction of the change is correct but there is a 50:50 chance it would be in that or the other direction. Importantly, the magnitude of the isotope change is wrong by a factor of ~3 for it to be consistent with the rise in atmospheric CO2 being accumulation of the anthropogenic emission.
Lets see some evidence in support of your assertion, either references or show us the equations.
Troll posting as Phil.:
I wrote to Ferdinand. He knows and understands the subject on which he and I strongly disagree.
I will reply to any points made by Ferdinand.
Please stop wasting space in the thread with your ignorant and untrue nonsense addressed to me.
Richard
richardscourtney says:
June 8, 2014 at 7:20 am
Troll posting as Phil.:
I wrote to Ferdinand. He knows and understands the subject on which he and I strongly disagree.
I will reply to any points made by Ferdinand.
Please stop wasting space in the thread with your ignorant and untrue nonsense addressed to me.
You are the one wasting space by your refusal to answer reasonable questions. As usual you come on here and make assertions you are unable to back up.
richardscourtney says:
June 8, 2014 at 7:15 am
Phil.:
re the “challenge” in your post at June 8, 2014 at 7:05 am.
Compare the IPCC definition to your
falsehood of adefinition in your post which sets the “challenge”.I did, they agree with me, what part of “addition of substance to a reservoir” don’t you understand?
Now, I will reciprocate: I challenge you to show you can tell the truth.
Done, you have yet to meet the challenge however.
richardscourtney says:
June 8, 2014 at 5:47 am
As to your point, the oxygen measurements can be interpreted in many ways.
Sorry. Richard, but oxygen measurements can only be interpretated in one way after substracting the change caused by human use of fossil fuels, which is rather accurately known: if there is a decrease, then the biosphere as a whole (land and sea plants, insects, molds, bacteria, animals) is more O2 sink than source. If there is an increase, then the biosphere as a whole is a net producer of oxygen. The latter is the case, which means that the biosphere as a whole is a net absorber of CO2 and preferential 12CO2, leaving relative more 13CO2 in the atmosphere. That is what you see in the seasonal NH variation.
Temperature changes do affect the CO2 solubility in seawater, but hardly affect O2 changes in/from the oceans (except for a small change in solubility). The main change there also is from the biosphere.
As the longer term trend of oxygen shows a small increase over time (after correcting for fossil fuel use), thus a small but increasing CO2 uptake, the increase over a full seasonal cycle is certainly not from the biosphere. Thus the biosphere largely reduces its uptake at the end of the growing season and the biomass decay takes over in carbon transfer the other way out.
And the saw-tooth form of the seasonal variation is inconsistent with your assumption that the sinks fill.
I don’t think that there is much uptake left when lots of trees in the NH have no leaves anymore…
The direction of the change is correct but there is a 50:50 chance it would be in that or the other direction.
Richard, this has nothing to do with chance. The seasonal change is clear and follows what happens in the biosphere. After a full cycle it increases the 13C/12C ratio of the atmosphere. Any substantial release of CO2 from the oceans also will increase the 13C/12C ratio of the atmosphere. Only human emissions reduce the 13C/12C ratio of the atmosphere and the oceans mixed layer. That the reduction of the 13C/12C ratio in the atmosphere is only 1/3rd of what can be expected from human releases is either a matter of dilution or addition by CO2 from the other reservoirs. But as the increase in the atmosphere is only halve the human addition, the total balance is only dilution and a net sink by the other reservoirs…
Ferdinand:
Given two measurements of “assumed globally equivalent average CO2 levels” (ppmv 1 and ppmv 2) what are the latest
(1) delta T for those two ppmv differences
(2) delta pH for those two ppmv values.
RACookPE1978 says:
June 8, 2014 at 10:27 am
(1) delta T for those two ppmv differences
Assuming the current 400 ppmv as 110 ppmv above the pre-Industrial equilibrium:
Ocean-atmosphere equilibrium increases with ~17 ppmv/°C. To increase the atmospheric CO2 content with 100 ppmv, you need to heat the ocean surface with ~6.5°C. But as the biosphere in general increases its uptake (plant growth…) with higher temperatures, the real change over the past 800,000 years was 8 ppmv/°C. With that figure, you need 13.8°C sea surface temperature increase to reach the 110 ppmv CO2 increase in the atmosphere.
(2) delta pH for those two ppmv values.
According to the Revelle factor, the increase of DIC in the mixed layer would be ~10% of the increase in the atmosphere. Which can be seen in the increase of DIC at e.g. Bemuda:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf Fig.5.
The increase in the atmosphere was 50 ppmv in the same period or 12.8% of total CO2, while DIC increased 30 microml/kg or 1.4% of total DIC, which is quite what the buffer/Revelle factor says.
According to Fig. 6 in the same work, that leads to a drop of ~0.06 pH points.
Phil.:
re your post at June 8, 2014 at 8:49 am
You ask me
I and the IPCC (who wrote it) understand it completely. But you lie about it.
“Addition to a reservoir” means uptake of a reservoir: it does NOT mean – as you assert – the net of uptake and output of the reservoir. This is clear from the actual definition which I quoted for you. viz.
The definition makes no mention of output of the reservoir or of uptake less any output of the reservoir. It says, “The uptake of carbon containing substances, in particular carbon dioxide, is often called (carbon) sequestration.”
Furthermore, this correct definition is used in all proper assessments of sources and sinks because otherwise it would not be possible to determine if and when a reservoir were acting as a net source or a net sink. Importantly, the discussion was of sources and sinks prior to your trolling by inventing your daft and incorrect definition of sequestration as being net sequestration.
Obviously, you have demonstrated that your sole purpose is to troll, and you place no value on truth. (I ponder if your name is Gleick.)
Richard
Ferdinand:
Thankyou for your post addressed to me at June 8, 2014 at 9:15 am.
This is a brief note to apologise for my tardiness of my reply which I hope to provide later today. Sorry.
Richard
richardscourtney says:
June 9, 2014 at 12:57 am
Phil.:
re your post at June 8, 2014 at 8:49 am
You ask me
what part of “addition of substance to a reservoir” don’t you understand?
I and the IPCC (who wrote it) understand it completely.
But you lie about it.Clearly you do not understand it!
“Addition to a reservoir” means uptake of a reservoir: it does NOT mean – as you assert – the net of uptake and output of the reservoir. This is clear from the actual definition which I quoted for you. viz.
Sequestration See Uptake.
And
Uptake The addition of a substance of concern to a reservoir. The uptake of carbon containing substances, in particular carbon dioxide, is often called (carbon) sequestration.
The definition makes no mention of output of the reservoir or of uptake less any output of the reservoir. It says, “The uptake of carbon containing substances, in particular carbon dioxide, is often called (carbon) sequestration.”
More of your tiresome nonsense Richard. It appears that you do not even know what a reservoir is!
None of my colleagues who work in the area of Carbon Sequestration use the term to mean other than ‘the process of capture and long-term storage of atmospheric carbon dioxide’.
In Roger Sedjo and Brent Sohngen (2012). “Carbon Sequestration in Forests and Soils”. Annual Review of Resource Economics (Annual Reviews) 4: 127–144.
“Carbon sequestration is the process of capture (through photosynthesis) and long-term storage of atmospheric carbon dioxide (CO2). Sequestration is possible through a range of processes, including those occurring naturally in plants and soils.”
and:
“Forests and soils sequester atmospheric CO 2 within their biomass or in organic matter that is stored in the ground. Oceans store most of the world’s carbon, but forests and soils store most of the carbon sequestered within land.”
Note the use of the word ‘store’.
also:
” However, both tilling soil and adding fertilizer significantly decrease the amount of carbon sequestered within soil. By soil tilling, microbes come in contact with previously untouched humus that is quickly decomposed, and carbon is released.”
Another reference:
“Ocean Sequestration of Crop Residue Carbon: Recycling Fossil Fuel Carbon Back to Deep Sediments”
Stuart E. Strand and Gregory Benford
Environ. Sci. Technol., 2009, 43 (4), pp 1000–1007
“For significant impact any method to remove CO2 from the atmosphere must process large amounts of carbon efficiently, be repeatable, sequester carbon for thousands of years”
“Significant removal at global scales requires that methods: (1) deal with very large quantities of carbon, (2) sequester the carbon efficiently, and (3) are repeatable over centuries.”
“For a successful carbon removal technology to have a significant impact on that rate of increase, the technology must remove and sequester at least 0.5 Pg per year (half a “stabilization wedge” (1)) over many years, at least as long as fossil fuel carbon is released to the atmosphere.”
“Because there are only three forms in which carbon can be sequestered (as CO2, carbonate minerals, and as reduced organic carbon), only a few methods for removing carbon from the atmosphere have been proposed:
sequestration of crop residue carbon in agricultural soils,
sequestration in growing forests,”
Clearly the definition of ‘sequestration’ used in the field is not yours.
The UNFCC uses:
“Carbon sequestration
The process of removing carbon from the atmosphere and depositing it in a reservoir.”
By now you will have looked up ‘reservoir’ in a dictionary and discovered that it is where one stores material, in this case carbon.
Obviously, you have demonstrated that your sole purpose is to troll, and you place no value on truth. (I ponder if your name is Gleick.)
Now that you are aware of what the correct definition of ‘Sequestrate’ is, hopefully you will apologise for your mis-statements and accusations of lying. If not, of course we will know who the troll is and who is lying. However I expect that we will continue to see your usual trolling and prevarication, but perhaps you will surprise me.
Ferdinand Engelbeen:
Ferdinand, this is my reply to your post at June 8, 2014 at 9:15 am which is here.
Firstly, I ask you to accept my apology for taking so long to reply: my time is mostly not under my control at present.
Secondly, I draw attention to your statement saying
Sorry, Ferdinand, but your assertion is a demonstration of the profound difference between our views, and your assertion only demonstrates that difference.
I would never say anything “can only be interpreted in one way” because it is never true.
I may say I only know one possible interpretation of the available data, or that I only know of one published interpretation of the data. But it would require deific omniscience to know there is only one interpretation. And in the specific case I am writing to provide an alternative view.
The difference between our language usages is very, very important. It indicates the certainty you ascribe to your ideas and the doubt I ascribe to both my and your ideas.
Turning to your substantive point, you say
I will try to isolate each point I understand you to be making in that argument. And I will address each point as I have understood it in turn.
1.
You say that excluding anthropogenic effects on atmospheric O2 concentration, any change – increase or decrease – in atmospheric O2 concentration is induced by biota.
I say, not so if only because variations in natural fires consume O2 and generate CO2: the magnitude of such variations is not known.
2.
You say that after adopting your assumptions concerning anthropogenic effects then “there is an increase” in atmospheric CO2 and, you say, this indicates “the biosphere as a whole is a net producer of oxygen.”
I say, So what? The issue is whether or not the change to oxygen in the atmosphere indicates the cause of the rise in atmospheric CO2 concentration. And the oxygen change is so small that the choice of adopted assumptions defines the result: in other words, your argument is circular.
3.
You say the carbon isotope ratio change is in the direction expected from the biosphere being a net absorber of CO2 during the NH seasonal variation.
I agree but I point out that the magnitude of the annual carbon isotope ratio change is 300% wrong for it to agree with your assertion which indicates that your assertions and/or your assumptions are wrong.
4.
You say that because O2 solubility varies little with temperature it must be that variations in O2 must be from the biosphere.
Although I think the variation is probably biogenic, I say you provide the logical fallacy of a false dichotomy: the change could be from something you have not mentioned (e.g. soil).
5.
You claim that the seasonal O2 variation is net uptake but “the longer term trend of oxygen shows a small increase over time (after correcting for fossil fuel use)” so – you assert – this indicates “a small but increasing CO2 uptake“.
I agree that land-based biomass almost certainly does provide “a small but increasing CO2 uptake“ but I know of no data which could indicate whether or not that is true of the larger ocean-based biota. And I say your point is merely another example of your circular argument which indicates “fossil fuel use” as a cause by adopting assumptions which allow for “correcting for fossil fuel use”.
6.
You conclude, “Thus the biosphere largely reduces its uptake at the end of the growing season and the biomass decay takes over in carbon transfer the other way out.”
I conclude that your assertions are not valid conclusions because they are based on logical fallacies.
Moving on, in response to my statement
You have replied
Good one! I did laugh. Thankyou.
But, more seriously, we both know that leaves on trees are not the only – or the main – seasonal CO2 sink. The oceans alone are a larger sink.
And if you want to claim that leaves are causing the long-term rise in atmospheric CO2 concentration then you have to demonstrate it. Good luck with that!
Your concluding paragraph says
Sorry, Ferdinand, a change to the ratio is either up or down and that is a 50:50 chance.
The change is in the direction of your favoured opinion as to cause. That means the direction of the change does not falsify your favoured opinion. It means nothing more than that.
As you admit, the magnitude of the change is only a third of what your favoured opinion indicates it would be. That is evidence AGAINST your favoured opinion. However, it is not conclusive evidence that your favoured opinion is completely wrong because – as you say – it is possible to explain the discrepancy as being “dilution”.
In summation, you claim the recent rise in atmospheric CO2 concentration has an entirely natural cause but I do not know if it has an entirely natural cause, or an entirely anthropogenic cause, or some combination of natural and anthropogenic causes. I want to know the cause and, therefore, I regret that your arguments are not cogent.
Richard
Troll posting as Phil.:
The only thing you get right in your post atJune 9, 2014 at 5:57 am is that the definition I have cited and quoted is not mine. It is the definition in the IPCC AR5 glossary. That you choose to lie about it only informs about you.
Richard
richardscourtney says:
June 9, 2014 at 9:20 am
Troll posting as Phil.:
The only thing you get right in your post atJune 9, 2014 at 5:57 am is that the definition I have cited and quoted is not mine. It is the definition in the IPCC AR5 glossary. That you choose to lie about it only informs about you.
As anticipated Richard the Troll returns and continues his prevarication.
To repeat, the UNIPCC definition is correct but you continue to misrepresent it. All the authors who publish on the subject are using the wrong definition according to Richard, or perhaps you think I’m lying about the definitions they’ve used in their published work? That appears to be your opinion since you claim that I didn’t get the quotes right in my post, surely that would be easy to prove?
Disappointing, I’d expect a minister to have more integrity.
Ferdinand Engelbeen says:
June 8, 2014 at 3:13 pm
Thank you for your professional and polite response.
More later, but – IF (big “if” there! – I understand your basic point, the measured rise in seawater temperature cannot explain the global rise in CO2 concentration – WITHOUT adding an assumed (or calculated, or projected, or whatever) man-released CO2 component into the atmosphere, right?
Or, going the other way, if you add all of man’s measured coal, oil, and wood burned (paid for/supposedly accurate) masses of carbon evacuated/pumped/cut since 1800, you get “too much CO2” to account for the actual measured increase in CO2 ppmv levels —- unless you compensate with an assumed vegetation/plant mass/sea water absorb/ chlorophyll-used ratio, right?
So, the final “CO2-measured-today” values cannot work unless both assumed CO2 released and CO2 absorbed/used/put-into-plants values/rates/ratios/change-in-absorbed/change-in-release are estimated ?
Regardless of all of the above, it does appear that the simplistic “Hotter water releases more CO2” excuse is NOT sufficient to explain the measured increases in CO2 between 1850 and 2014, right?
RACookPE1978 says:
June 9, 2014 at 8:58 pm nad June 9, 2014 at 9:01 pm
Right!
Although for the latter, there is an escape possible: if there is an oceanic (*) process at work where an increase of C density / ocean upwelling combined with a temperature increase gives a lot of extra CO2. But that gives a lot of problems:
– timing of the increase in C flux must exactly mimic human emissions
– because the sinks don’t make any differentiation between human and natural CO2 (except a small one in isotopes), the threefold increase in human emissions over the past 50 years should be dwarfed by a threefold increase of natural emissions in the same time frame. That would be visible in a threefold shortening of the residence time. But that only lengthened somewhat, in line with little variation in the natural carbon cycle for an increased carbon content of the atmosphere.
– any natural increase of the release or increase in C cycle from the oceans would increase the 13C/12C ratio of the atmosphere, but we only see a rapid decrease…
(*) oceanic, as the biosphere is a proven net absorber of CO2 and the extra mass of CO2 in the atmosphere is comparable to burning 1/3rd of all land vegetation, but the earth as a whole is greening…
RACookPE1978:
I write to say that I, too, agree your two posts at June 9, 2014 at 8:58 pm and June 9, 2014 at 9:01 pm.
The difference between the views of Ferdinand and me is our different conclusions from the fact you state which is
Ferdinand claims the assumptions used to obtain the estimates MUST be true and, therefore, the observed recent rise in atmospheric CO2 concentration IS anthropogenic.
I say the assumptions used to obtain the estimates MAY be true and, therefore, the observed recent rise in atmospheric CO2 concentration COULD BE – but probably is not – anthropogenic.
Additionally, I draw attention to your stated recognition of the polite and honourable response you say you have obtained from Ferdinand. This is typical of discussions with Ferdinand, and it is why I think it is useful to discuss disagreements with him. Contrast that with the behaviour of the troll posting as ‘Phil.’ and you can see why I refuse to engage with him/her/them/it because such engagement has no value when it is with an egregious propagandist who posts falsehoods.
Richard
richardscourtney says:
June 11, 2014 at 3:21 am
RACookPE1978:
I write to say that I, too, agree your two posts at June 9, 2014 at 8:58 pm and June 9, 2014 at 9:01 pm.
The difference between the views of Ferdinand and me is our different conclusions from the fact you state which is
So, the final “CO2-measured-today” values cannot work unless both assumed CO2 released and CO2 absorbed/used/put-into-plants values/rates/ratios/change-in-absorbed/change-in-release are estimated ?
Ferdinand claims the assumptions used to obtain the estimates MUST be true and, therefore, the observed recent rise in atmospheric CO2 concentration IS anthropogenic.
I say the assumptions used to obtain the estimates MAY be true and, therefore, the observed recent rise in atmospheric CO2 concentration COULD BE – but probably is not – anthropogenic.
No Ferdinand provides facts and figures in support of his statements, you do not, you just waffle.
Additionally, I draw attention to your stated recognition of the polite and honourable response you say you have obtained from Ferdinand. This is typical of discussions with Ferdinand, and it is why I think it is useful to discuss disagreements with him. Contrast that with the behaviour of the troll posting as ‘Phil.’ and you can see why I refuse to engage with him/her/them/it because such engagement has no value when it is with an egregious propagandist who posts falsehoods.
More of your trolling Richard, I have been polite in responding to you in spite of your insulting remarks, however, I’ve decided to take a leaf from your book and henceforth address you for what you are, ‘Richard the Troll’. You bring nothing substantive to any discussion just spout your own opinions and when challenged become insulting and provocative and never address the questions or facts. Your usual MO is to refer to ‘falsehoods’ without ever identifying one! I provide chapter and verse regarding your misuse of the term ‘Sequestration’ and you call them lies!
Typical Troll.
RACookPE1978 says:
June 9, 2014 at 8:58 pm
Ferdinand Engelbeen says:
June 8, 2014 at 3:13 pm
Thank you for your professional and polite response.
More later, but – IF (big “if” there! – I understand your basic point, the measured rise in seawater temperature cannot explain the global rise in CO2 concentration – WITHOUT adding
an assumed (or calculated, or projected, or whatever)the measured man-released CO2 component into the atmosphere, right?Or, going the other way, if you add all of man’s measured coal, oil, and wood burned (paid for/supposedly accurate) masses of carbon evacuated/pumped/cut since 1800, you get “too much CO2″ to account for the actual measured increase in CO2 ppmv levels —- unless you compensate with
an assumedthe measured vegetation/plant mass/sea water absorb/ chlorophyll-used ratio, right?So, the final “CO2-measured-today” values cannot work unless both
assumedmeasured CO2 released and CO2 absorbed/used/put-into-plants values/rates/ratios/change-in-absorbed/change-in-release areestimatedaccounted for. ?Troll posting as Phil.:
Enough of your rubbish. I post link, citation and quotation of the IPCC AR5 definition of sequestration and you try to pretend the definition is other than I cite then you post purportedly to me
The only “waffle” has been your blatant falsehoods.
These falsehoods include that I have insulted you. Please be assured that I would have insulted you if that were possible. And try to understand that I have no intention of diverting my priorities to the unpleasant business of interacting with you especially when I have important personal matters to address.
Richard
richardscourtney says:
June 11, 2014 at 6:40 am
Troll posting as Phil.:
Enough of your rubbish. I post link, citation and quotation of the IPCC AR5 definition of sequestration and you try to pretend the definition is other than I cite then you post purportedly to me
No that is a lie, I agreed that the IPCC definition was correct and point out that you misunderstand/misrepresent it, and gave references to papers that show that the scientific usage of the term is not what you are claiming.
In Roger Sedjo and Brent Sohngen (2012). “Carbon Sequestration in Forests and Soils”. Annual Review of Resource Economics (Annual Reviews) 4: 127–144.
“Carbon sequestration is the process of capture (through photosynthesis) and long-term storage of atmospheric carbon dioxide”
“Ocean Sequestration of Crop Residue Carbon: Recycling Fossil Fuel Carbon Back to Deep Sediments”
Stuart E. Strand and Gregory Benford
Environ. Sci. Technol., 2009, 43 (4), pp 1000–1007
“For significant impact any method to remove CO2 from the atmosphere must process large amounts of carbon efficiently, be repeatable, sequester carbon for thousands of years”
The UNFCC uses:
“Carbon sequestration
The process of removing carbon from the atmosphere and depositing it in a reservoir.”
All of these you claim to be falsehoods!
Either you don’t understand plain english or you’re a propagandizing troll, the latter seems more likely .
Troll posting as Phil.:
The UNFCC definition you quote at June 11, 2014 at 6:59 am is correct and you again lie when you assert that I have claimed it is a falsehood.
The UNFCC definition means what it says. It does not mean what you say. Contrary to your lie the UNFCC definition does NOT mention emissions. The definition also does not mention the Golden Gate Bridge and for the same reason; i.e. it is not relevant.
Your silly lies are annoying.
Richard
richardscourtney says:
June 11, 2014 at 8:38 am
Troll posting as Phil.:
The UNFCC definition you quote at June 11, 2014 at 6:59 am is correct and you again lie when you assert that I have claimed it is a falsehood.
Bang on cue Dicky the Troll returns to try to lie his way out of the hole he has dug for himself.
To refresh your memory, I posted the UNFCC definition along with quotations from other references,
You said “The only thing you get right in your post at June 9, 2014 at 5:57 am is that the definition I have cited and quoted is not mine., so you’re saying that the UNFCC and other quotes are wrong.
The UNFCC definition means what it says. It does not mean what you say.
Yes it does, and is so used by everyone in the field.
“Carbon sequestration
The process of removing carbon from the atmosphere and depositing it in a reservoir.”
Contrary to your lie the UNFCC definition does NOT mention emissions.
I did not say that it did, that’s another lie by you!
You are like ‘Steve Goddard’, you make false statements and when challenged vehemently refuse to admit that you are wrong, as Anthony mentioned the other day that’s what got him banned from here. He claimed that solid CO2 was precipitated at the S Pole, when I and others pointed out his error he refused to back down despite all the evidence to the contrary.
Your
silly liesfacts are annoying.richardscourtney says:
June 9, 2014 at 9:17 am
Richard, sorry for the late reply. They are installing a new CV installation here, because one of these stupid safety rules which forbid the use of gas stoves without CO safety device…
I don’t know where to begin. Your last reply looks more like word playing than a real answer to my remarks… But let’s start with a little history of the earth…
For a very long time there was no free oxygen on earth. All oxygen was in bounded form: metal oxides, SiO2 and in the atmosphere: CO2. At a certain moment the first plants using solar energy and chlorophyll evolved and could use the carbon from that CO2 for their benefit as energy and structural building blocks. The byproduct was oxygen. Thanks to oxygen animals could evolve, by burning the energy contained in carbohydrates and fats from plants. Some of the plants were buried over time and that is what we use today as coal, oil and gas (I know of several stories of “inorganic” oil and gas, but that is not at stake now).
There are several points that make a distinction between organic and inorganic carbon: practically all inorganic carbon in the oceans, sediments, carbonate rocks, volcanic vents,… has a near neutral 13C/12C ratio, that is around zero per mil δ13C. Near all organic carbon (fossil and recent) has a much lower 13C/12C ratio: -15 per mil δ13C (C4 plants), -24 per mil δ13C (C3 plants, coal), -26 per mil δ13C (oil) and further down to -40 per mil δ13C and beyond (natural gas). The atmosphere is a mix of all the CO2 exchanges with the other reservoirs and was -6.4 +/- 0.2 per mil δ13C (pre-Industrial) going rapidly down to currently below -8 per mil. Further recent organics have incorporated some 14C produced in the atmosphere from cosmic rays, which can be used for carbon dating, while fossil organics don’t contain any measurable 14C anymore.
Further, indeed only plant growing and decaying/eating/burning makes a difference for the oxygen balance. Nothing else. That is a blunt statement, but based on all scientific evidence from the far past and current knowledge. Thus your statement:
1. I say, not so if only because variations in natural fires consume O2 and generate CO2: the magnitude of such variations is not known.
is not right, as any forest fire, uncontrolled coal seams fires, drying out of the rainforests (as is the case during an El Niño) is part of the natural carbon/oxygen cycle. The first two aren’t measurable in the year-by-year CO2/O2 cycle variations, the latter is measurable as a peak CO2 / low O2 and low δ13C around the trends.
2. So what? The issue is whether or not the change to oxygen in the atmosphere indicates the cause of the rise in atmospheric CO2 concentration. And the oxygen change is so small that the choice of adopted assumptions defines the result: in other words, your argument is circular.
The change in oxygen does indicate that the cause of the rise in atmosphere is NOT the biosphere. The biosphere is a net producer of oxygen, thus a net absorber of CO2. The change in O2 is stoichiometric for a change in CO2 in any direction in the biosphere, The problem is not the O2 variation itself but the necessary analytical accuracy: 1 part in 200,000 parts. But that is mainly a matter of sample handling.
3. I agree but I point out that the magnitude of the annual carbon isotope ratio change is 300% wrong for it to agree with your assertion which indicates that your assertions and/or your assumptions are wrong.
Sorry, but we have discussed that before: the change in the atmosphere is 30% of what one can expect if 100% of all human CO2 still resided in the atmosphere. But as you certainly know, some 20% of all CO2 in the atmosphere is exchanged each year with CO2 from other reservoirs, where the deep oceans have emissions which are app. 1000 years old with the isotopic composition of the deep oceans, which is a lot richer in 13C. My assumption is completely right if you take into account this “dilution” of the anthro 13C/12C contribution.
4. Although I think the variation is probably biogenic, I say you provide the logical fallacy of a false dichotomy: the change could be from something you have not mentioned (e.g. soil).
The O2 variations are all biogenic, that includes all plants (land and sea) as source, plant decay, (soil) microbes, fungi, insects, animals,… and all other O2 sinks, mentioned or not. Only a very small part is caused by de/outgassing of the oceans, mainly over the seasons.
5. I know of no data which could indicate whether or not that is true of the larger ocean-based biota. And I say your point is merely another example of your circular argument which indicates “fossil fuel use” as a cause by adopting assumptions which allow for “correcting for fossil fuel use”.
The ocean-based biota produce or use oxygen based on the balance between production and decay (direct or indirect via the food chain). These are included in the overall oxygen balance. I don’t think there is much change in the oceans, as CO2 is not the limiting factor in the oceans. The “assumptions” of oxygen use by fossil fuel burning have a reasonable accuracy: they are based on fossil fuel sales and oxygen use for each type of fuel. Worst case it may be underestimated because of under-the-counter sales, but that only means that the biosphere sink is larger than calculated…
6. I conclude that your assertions are not valid conclusions because they are based on logical fallacies.
Even without any assumptions, that is what both the O2 and δ13C measurements show: the biosphere as a whole reverses from a net source of oxygen (sink of CO2) to a net sink of oxygen (source of CO2) at the end of the growing season, while the long term trend (the integral of the residuals) shows that the biosphere is a net, but small source of O2 (sink for CO2). Which proves beyond doubt that the biosphere is limited in its short-term capacity as CO2 sink.
Sorry, Ferdinand, a change to the ratio is either up or down and that is a 50:50 chance.
Nothing to do with chance. The ice core measurements over glacial – interglacial periods give not more than 0.2 per mil δ13C for an enormous change in biomass and sea surface temperatures. The natural variability over the Holocene was not more than +/- 0.2 per mil δ13C in ice cores and the ocean surface layer (coralline sponges, resolution 2-4 years over the past 600 years). Since the Industrial revolution, the drop in δ13C level is over 1.6 per mil. 8 times more than over a glacial-interglacial transition. You may call that by chance, I don’t think so…
I want to know the cause and, therefore, I regret that your arguments are not cogent.
All my arguments are based on measurements and follow straightforward logic…