Guest post by Reed Coray
The following example illustrates the issues I have with reasoning often used to argue that increasing the amount of CO2 in the Earth’s atmosphere will increase both the Earth’s surface temperature and the Earth’s atmosphere temperature. Immediately following is a direct quote from URL
http://www.school-for-champions.com/science/heat_transfer_earth.htm
“The present situation is that there has been an increase in infrared-absorbing gases in the atmosphere, such as carbon dioxide (CO2) and methane (CH4). Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere and spreading through convection currents. The average temperature of the atmosphere has increased 0.25 °C since 1980, mainly attributed to an increase in infrared-absorbing gases in the atmosphere.”
Although the above statement makes no direct reference to Earth surface temperature, I believe it carries the implication that greenhouse gases in the Earth’s atmosphere increase the Earth’s surface temperature.
I make two comments: the first is relevant only if the above implication is valid, the second is relevant independent of the validity of the implication. First, placing matter adjacent to a warm surface such that the matter is capable of absorbing/blocking radiation to space from the warm surface can lead to a decrease in the warm surface’s temperature. Second, increasing the amount of the absorbing/blocking matter can lower the temperature of the absorbing/blocking material.
Take for example an internal combustion engine whose metal surface is exposed to a vacuum. In addition to doing useful work, the engine produces thermal energy (heat). That thermal energy will produce a rise in the temperature of the engine’s surface such that in energy-rate equilibrium the rate energy is radiated to space from the engine’s surface is equal to the rate thermal energy is generated within the engine. By attaching radiating plates to the engine’s surface, some of the energy radiated to space from the engine’s original surface will be absorbed/blocked by the plates; but because thermal energy can be transferred from the engine to the plates via both radiation and conduction, the temperature of the engine’s original surface will be lowered. This is the principle of an air-cooled engine[1]: provide a means other than radiation of transferring heat from an engine to a large surface area from which heat can be removed via a combination of conduction, convection and radiation, and the engine’s surface temperature will be lowered.
If plates at a temperature lower than the original engine surface temperature are attached to the engine, it’s true that the temperature of the plates will increase to establish energy-rate equilibrium. Once energy-rate equilibrium is established, however, increasing the plate radiating area (adding additional matter that blocks more of the energy radiated from the original engine surface) will likely lower the plate temperature.
Thus, blocking the amount of surface radiation escaping to space does not necessarily increase the surface temperature; and increasing the amount of radiation blocking material does not necessarily increase the temperature of that material. In both cases (the Earth/Earth-atmosphere and the internal combustion engine in a vacuum), the heat eventually escapes to space–otherwise the temperature of the Earth’s surface and the engine would continue to rise indefinitely. The difference isn’t that the energy doesn’t eventually escape to space (it does in both cases), the difference is in the path the energy takes to reach space. The amount of generated thermal energy in conjunction with the path the thermal energy takes to get to space determines temperatures along the path; and adding more material may increase or decrease those temperatures. To say that “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere…” by itself is unwarranted; because an equivalent statement for the case of adding extra plate material to the engine would be “Energy that would normally escape to space from an engine with small attached plates is absorbed by additional plate material, thus heating the plates…” For air-cooled engines, this statement is not true—otherwise the plate surface area of air-cooled engines would be as small as possible.
It’s fairly easy to visualize why (a) adding thermally radiating plates to an air-cooled engine might decrease the engine’s surface temperature, and (b) increasing the area of the radiating plates might decrease the plate temperature. It’s not so easy to visualize, and may not be true, why (a) adding greenhouse gases to the Earth’s atmosphere decreases the Earth’s surface temperature; and (b) increasing the amount of atmospheric greenhouse gases lowers the temperature of the Earth’s atmosphere. I now present one possible argument. I do not claim that the argument is valid for greenhouse gases in the Earth’s atmosphere, but I do claim that the argument might be valid, and can only be refuted by an analysis more detailed than simply claiming “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere.”
If we assume that (a) matter cannot leave the Earth/Earth-atmosphere system, and (b) non-greenhouse gases radiate negligible energy to space, then for a non-greenhouse gas atmosphere the only way thermal energy can leave the Earth/Earth-atmosphere system to space is via radiation from the surface of the Earth. The rate radiation leaves the surface is in part a function of both the area and temperature of the surface. For a greenhouse gas atmosphere, energy can leave the Earth/Earth-atmosphere system to space both via radiation from the Earth’s surface and radiation from greenhouse gases in the atmosphere. Suppose it is true that the density of greenhouse gases near the Earth’s surface is such that radiation emitted from low-altitude greenhouse gases does not directly escape to space, but is in part directed towards the Earth’s surface and in part absorbed by other atmospheric greenhouse gases. As the atmospheric greenhouse gas density decreases with increasing altitude, radiation emitted from high-altitude greenhouse gases can directly escape to space.
Now it’s not impossible that since (a) in addition to radiation, heat is transferred from the Earth’s surface to greenhouse gases via conduction, and (b) convection currents (i) circulate the heated greenhouse gases to higher altitudes where energy transfer to space can take place and (ii) return cooler greenhouse gases to the Earth’s surface, that the process of heat transfer away from the Earth’s surface via greenhouse gases is more efficient than simple radiation from the Earth’s surface. Many engines are cooled using this concept. Specifically, a coolant is brought into contact with a heated surface which raises the coolant’s temperature via conduction and radiation, and the coolant is moved to a location where thermal energy transfer away from the coolant to a heat sink is more efficient than direct thermal energy transfer from the heated surface to the heat sink.
One way to realize increased thermal transfer efficiency would be to use a coolant, such as greenhouse gases, that efficiently radiates energy in the IR band (i.e., radiates energy at temperatures around 500 K). Another way would be to spread the heated coolant over a large surface area. Since surface area increases with increasing altitude, thereby providing expanded “area” (in the case of a gas, expanded volume) from which radiation to space can occur, it’s not clear to me (one way or the other) that greenhouse gases won’t act as a “coolant” reducing both the temperatures of the Earth’s atmosphere and the Earth surface.
[1] It’s true that for most air-cooled engines the main transfer of heat from the engine plates is via a combination of (a) conduction of heat to the air near the plates, and (b) convection that replaces the warm air near the plates with cooler air. To aid this process, a fan is often employed, or the engine is located on a moving vehicle and the vehicle’s motion through an atmosphere provides the flow of air across the plates. Although conduction/convection may be the primary means of heat dissipation from the plates, radiative cooling also dissipates heat.
David Chamness
Note that “most of the atmosphere CAN absorb radiation from longwave IR” . . “the re-radiated IR CAN heat the atmosphere directly”, resulting in the average atmosphere being in effective thermal equilibrium at a given elevation. (a href=http://altmine.mie.uc.edu/nuclear/htmfile/atmcombXC.pdf>Essenhigh (2003, 2006) shows the fourth power of the reduced temperature is proportional to the reduced pressure.
Logically correct for internal combustion engines. Incorrect for Earth. To a very good first approximation, heat energy arises from incoming solar radiation (absorbed shortwave radiation). It escapes as outbound infrared (outbound longwave radiation). Again to a very good first order approximation, there is no convection or conduction of heat into a vacuum (space). There is only radiation (which is how vacuum thermos flasks work). Again to a very good first order approximation, the radiative ‘surface’ of the atmosphere, it’s TOA, does not change with temperature. Therefore it is the dynamics of ASR and OLR within the atmosphere that govern thermal change. AGW occurs (the degree, not the physics, is the question of interest) because as your chart from Wikipedia shows, GHG are less ‘transparent’ to OLR than ASR. A rise in GHG creates a temporary situation where ASR > OLR until temperature rise induces more OLR to restore equilibrium. What is interesting and provably wrong about the IPCC consensus is that they have gotten both the primary indirect feedback, rising water vapor (the most potent GHG), and the secondary indirect feedback, clouds, wrong. They provably have done so through classic selection bias in the meta-analysis that is IPCC AR4. And meta-analysis selection bias is by definiton deliberate. It therefore is tangible proof of agendas. Not for Mann and ‘hide the decline’. For the entire IPCC. I devote an 80 page chapter of a forthcoming book to irrefutable documenting this.
I think you make a good point. The distinction between fact and conjecture is sometimes blurred when the conjecture seems obvious. A case in point relayed to me by an Electrical Engineer once many years ago (yes, complete hearsay and relying on my memory from late 80’s to early 90’s). This Engineer worked for a large electronics manufacturing company in the capacitor division (60’s & 70’s). One day they (design / R&D team) were told that a customer wanted a particular capacitor model jacketed (in addition to the casing). The team of Engineers looked at each other all knowing this would increase the insulation value and potentially make the capacitor operate outside of design specifications and fail. But a customer is a customer, so they went to the lab and tried various jacket materials. Sure enough the jacketed capacitors ran hot and failed, EXCEPT ONE . They ran the experiment again and again, same results. So, they had happened upon a jacket material that wouldn’t increase the operating temperature of the capacitor. The EE told me he always wanted to go back and investigate why, but in industry answering such academic questions isn’t always (mostly not) the priority. He suspected the material increased the radiative heat loss more than it reduced the convective heat loss, but has trouble believing it considering how tiny the radiative heat loss from a capacitor is compared to the convective heat loss. The point being they all (experts in their field) thought it was a FACT that adding another layer would increase the temperature when actually it was a CONJECTURE and one that wasn’t always true.
Fact: CO2 is a GHG.
Conjecture: Adding CO2 to the atmosphere will increase the temperature.
It seems pretty obvious, but is it true? Is it always true?
The problem I have with the “standard” explanation is the equation presented for increased down welling radiation from increased CO2 concentration [F=5.35Ln(CO2f/CO2i)] has no temperature variable and yet the outgoing radiation from CO2 to space is supposedly reduced due to being “colder” due to altitude. Is temperature a variable or isn’t it? Are GHG’s more like fluorescent bulbs (non-Stefan-Boltzmann applicable) or incandescent bulbs (Stefan-Boltzmann applicable)?
According to ModTran:
280 ppm CO2 20km upward: Iout, W / m2 = 289.351
380 ppm CO2 20km upward: Iout, W / m2 = 287.53
So, a 100 ppm increase in CO2 results in a 1.821 W/m2 decrease in outgoing radiation from TOA.
280 ppm CO2 0km downward: Iout, W / m2 = 347.598
380 ppm CO2 0km downward: Iout, W / m2 = 348.226
So, a 100 ppm increase in CO2 results in a 0.628 W/m2 increase in radiation to surface.
[Leaving all other variables at default values.]
Indeed, who can forget the satellite measureing increases in outgoing IR vs. the model outputs:
http://wattsupwiththat.files.wordpress.com/2012/02/image26.png
Perhaps this is the flaw in the slaw of post normal climate science. An obvious conjecture became a fact in their minds and perhaps it is true, perhaps just not always.
100 years ago just about every scientist would have agreed with the song lyrics: Time keeps on slipping into the future; but now most would say: well, not always.
David Chamness
PS The molecule’s motion transfers heat by conduction so radiation, convection (and conduction) provide heat transfer.
Reed Coray
PS for the impact of gravity versus convection within a room see:
Lucy Skywalker: Graeffs experiments and the second law of thermodynamics
Graeff (2011) demonstrated a negative temperature gradient after stopping convection an adiabatic gas by a fine glass powder that allowed gas diffusion. The consequence is “a negative gradient of T(Gr) = – 0.07 K/ m,” i.e., gas is hotter at the bottom and cooler at the top, NOT at constant temperature throughout the adiabatic chamber – even though the environment has a positive temperature gradient (hot air rises). (This appears to be the tradeoff between gravitational potential energy and kinetic energy (ie temperature) with conservation of energy.
Eli Rabett says:
July 21, 2012 at 5:39 am
“To maintain radiative balance (sun in, IR out) the entire Earth system warms until the temperature rises enough in the mid troposphere to restore the balance.”
Eli, always nice to have someone show up to remind us of IPCC dogma. Here’s a question for you. My house is insulated with an R value of 10. But I am a rugged individualist so I have no connection to electricity or source of fuel. I don’t even occupy the house. I Iive in a tent in the back yard. But I measure and record the temperature inside the housd every hour. At the end of the year I average all the readings. But an environmentalist has convinced me that if I remove all of the insulation the average temperature inside will go down, so I do. What do you think will happen to the average temperature?
Would a black body radiate into air that is the same temperature as it is?
Count me in with Jeremy.
When I first got interested in “global warming”, the first question that came to mind was how much energy at the frequencies that CO2 absorbs was left to absorb, ie; how much was escaping to space. When the answer came back as , not that much I realized someone was trying to con me.
michael hammer said
“However, because the GHG effect is so strong over the atmospheric column in effect the surface can only lose enegy at the non GHG wavelengths while the atmopshere can only lose energy to space at the GHG wavelengths.”
This statement is false. The earth does lose energy at GHG wavelengths, it is only the transit time from surface to space which changes, not the net energy flow. GHGs absorb LIR but radiate half toward space and have back to the surface. All LIR enerrgy from the surface eventually gets to space. If your statement were true, the atmosphere would continously warm during the day as it would be trapping LIR while the sun shines. We know that the atmosphere continues to warm for only about 2 hours after peak heating from the sun and then begins to cool. Therefore, the atmosphere is radiating the energy previously received and the time lag is only due to the inherent transit time of an atmosphere.
Only a step change in GHG’s will have a transient affect on the atmospheric temperature. Eventurally, the energy transit time will be re-established and the deltaT will dissapate at a log or hyperbolic rate. GHG’s do NOT absorb LIR, they delay its transit to space. The Earth’s surface atmospheric temperature can be completely determined by the Ideal Gas Law: PV=nrT. Venus is not warmer because of more CO2 in its atmosphere, it is warmer because it is 1/3rd closer to the SUN (2.25 more watts/m2) and its atmoshpere is significantly denser than Earth’s.
GHG’s only slow the transit of enery from the surface to space, they do not prevent that transit. Half of the LIR GHGs absorb are re-radiated toward space and half back to the surface. A step change in GHG’s temporarily change the transit rate until a new equilibrium is established. Steady state GHG concentrations have no net impact on the equilibrium.
Reed, not sure how this impacts your analysis, but we do not measure the temperature of the surface of the Earth, we measure the atmospheric temperature at some nnominal distance abouve the surface. Also, for the vast majority of the energy coming from the Earth, it is just the re-emmission of the energy received from the Sun. Your analogy of an engine, where the heat is generated internally, is a bit of a stretch.
We all know that the Earth would begin to cool with 8 minutes if the Sun suddely stopped shining (forget Novas for a moment). Our atmosphere would stop us feeling it immediately, but only for a short time as all the thermal energy in the atmosphere would quickly dissipate into space. The atmoshpere does have mass so it cann store thermal energy, briefly. We would become a cold, lifeless orb in minutes, GHGs or no.
Bill
JeffC says:
July 21, 2012 at 5:15 am
GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …
Other papers I have read use the term ‘scatter’ which is more correct.
The only way these molecules can heat the atmosphere is if they collide with a non-radiating molecule in the very short time between being hit by the IR photon and the retransmission of that photon’s energy.
David Chamness – ” So as far as I can tell, it’s impossible for the re-radiated IR to heat the atmosphere directly. It can bounce around from CO2 molecule to CO2 molecule for 10 years and never “heat” the nitrogen and oxygen that makes up 98% of the air.”
That doesn’t hold up, David.
I went and looked at the numbers for this some time back. An excited CO2 molecule takes on average 10^-6 seconds (1 millisecond) to emit an IR photon. However, at sea level each molecule collides with other gas molecules (including O2 and N2, I’ll point out) one billion (10^9) times per second. Gas molecules are very friendly that way 🙂
Therefore at sea level pressure an excited CO2 molecule has ~1000 collisions before it can emit IR. What this means is that CO2 will share its energy with collisions, transferring rotational, vibrational, and translation energy with the air around it, and that the air will be at the same temperature as the CO2 mixed with it.
—
Unfortunately, this entire thread fails to hold up. As Eli Rabett pointed out earlier, increased CO2 raises the altitude of emission to space for GHG frequencies in the atmosphere, and the lapse rate means that the emission is from lower temperature gas – less energy leaving. And hence the entire atmosphere warms until the amount of energy leaving the atmosphere to space can balance out what comes in. I would suggest reading the following:
http://scienceofdoom.com/roadmap/atmospheric-radiation-and-the-greenhouse-effect/
For example (to give an actual reference, so you can see that all of this was used in the very earliest papers by Hansen and other climate scientists as they embarked on what became a crusade — Hansen is actually not unreasonable in this early paper) — you can probably find: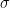 . In fact, he probably underestimates
. In fact, he probably underestimates 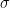 by a factor of two, at least, just from eyeballing the UAH series. This is consistent with what others (notably Koutsoyiannis) have determined analyzing the data — climate scientists for some reason consistently underestimate the variability natural or otherwise of the climate by at least a factor of two. The other is that if one concedes the starting point (which John Nielsen-Gammon pointed out to me is somewhat “cherry picked” not by intent on my part but because the paper in 1981 was predicting the behavior of the climate from 1980 on so that’s what I used) we are fairly clearly resolved at Hansen’s two sigma level for a feedback consistent with his lowest back-of-the-envelope climate sensitivity, the “no feedback” CO_2 only result.
by a factor of two, at least, just from eyeballing the UAH series. This is consistent with what others (notably Koutsoyiannis) have determined analyzing the data — climate scientists for some reason consistently underestimate the variability natural or otherwise of the climate by at least a factor of two. The other is that if one concedes the starting point (which John Nielsen-Gammon pointed out to me is somewhat “cherry picked” not by intent on my part but because the paper in 1981 was predicting the behavior of the climate from 1980 on so that’s what I used) we are fairly clearly resolved at Hansen’s two sigma level for a feedback consistent with his lowest back-of-the-envelope climate sensitivity, the “no feedback” CO_2 only result.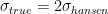 — there won’t even be a significant difference in
— there won’t even be a significant difference in  .
.
Science 213, #4511, p 957, 1981 “Climate Impact of Increasing Carbon Dioxide” by Hansen et. al.
on the internet (I did — a scanned version). Note well that he considers a variety of models from “straight CO_2, no feedback” which leads to 1.2 C increase in temperature upon a doubling of CO_2, through models that make the warming much worse when water vapor is included in certain ways (higher clouds, for example) and when he assumes all sorts of complicated macroscopic scale albedo feedbacks, e.g. melting ice caps and glaciers or dying off vegetation. Interestingly, in this early paper, his worst-case scenario warming was only 3.5 C. It’s also interesting to compare the “predictions” of his figure 7 — starting at any reasonable point in the late 70s through the early 80s — to the actual record. I actually did this, using the UAH lower troposphere data for the record in question, as I am deeply skeptical of the GISS or Hadley surface reconstructions — see http://www.phy.duke.edu/~rgb/uah-and-hansen81.jpg — and two things immediately pop out.
One is that Hansen horrendously, egregiously, underestimated
Of course that isn’t true if one shifts his curve down to look only at its slope, or equivalently starts it at different points. The trend in the curve could fit any of his proposed feedbacks if you start it or shift it or just compare the slopes, especially if one admits that
Once again, this is strong evidence that we are all, on both sides of this issues, looking for sheep in the clouds. One can look at this curve and “discover” whatever one wishes to discover. IMO the “best fit” with the different forcings Hansen examines, with complete freedom to shift the two curves vertically (but without doing the actual work as I lack his data and would have to construct some sort of numerical fit to get the curves themselves to compare) is almost certainly the 1.4C curve, but the three curves are narrowly resolved all the way out to 2010 with only 0.2C difference between the 5.8C and the 1.4C curve there! Compare this to a $\sigma_{real} \approx 0.2$ C! It is not possible to resolve this problem with 30 years of reliable data, I’m sorry.
Which is why I think that the only sensible thing to do is wait until it is possible to take any truly expensive measures to combat CO_2. There are reasons quite outside of this curve to think that the climate forcing is not Hansen’s extreme 5.6 C. Even the IPCC seems prepared to back off to 2.8 C (the middle curve) although again, resolving 2.8 from 1.4 from 1.2 from 1.0 is all but impossible on a 30 year (or even somewhat longer) base and allowing for the very real possibility that some unknown fraction of the warming and feedback comes from other causes than those considered in the models.
It might take fifty or sixty years of observations to resolve this issue, where we are only halfway there at best. It might take another ten, or twenty. It might take a full century of observations with modern instrumentation of the Sun and Earth to allow us to build a truly reliable model of the Earth and its climate, where by reliable I mean a model with predictive skill one whole decade in advance.
In the meantime, I personally do think that it is quite reasonable to take moderate public measures to minimize the production of CO_2. In particular, investing money to bring alternative energy technologies to maturity. This is not so much because I think that we are at horrible risk of Hansen’s 5+C catastrophe — I don’t. But even a 2+ C rise could have negative consequences that outweigh the benefits and besides, we need to try to establish a civilization that will last not just the next century but the next 10,000, or 100,000 years. A steady-state global civilization requires energy resources that don’t have to be dug, or pumped, out of the ground. The 21st century is clearly the century where we need to be working this out and transitioning entirely independent of the CO_2 issue! Fossil fuels of all sorts — including uranium and thorium — are good for at most 10ky (and arguably a lot less given exponentially increasing cost of recovery). How are we going to build a steady state civilization on that?
If we could only turn the public debate away from alarmism and panic (and the associated political grabs for money and power) to something like a genuine vision of a future global civilization, we might find that the entire “warmist” versus “denier” debate has been a smoke screen for the picking of our pockets and a diversion away from anything like a sober consideration of investments likely to have a good ROI over the next century and beyond. Some things are unavoidable — we are almost certainly going to reach 500-600 ppm CO_2 before it comes down — if the CO_2 cycle itself is being correctly modeled or described, which is open to debate, I agree, but either way the trend is boringly monotonous and upward at the moment so the default assumption is that this will continue until proven otherwise. IMO pure economics (plus advances in technology) will be the fundamental factor that eventually clips the rise. Depending on how a lot of unknown stuff works out, there will fairly likely be a warming that goes along with this. I doubt that it will be as large as the 2.8 IPCC AR5 estimate, and since AR-X estimates are on a decreasing trend, it seems likely that AR6 will more likely agree with me than with AR5.
Will a temperature increase of 1 C have no negative consequences at all? That’s sort of the boundary, isn’t it. A degree over a century is well within the Earth’s natural variability anyway. Even 2 C is within the range apparent in the proxy records, but that rapid a warming might have negative sequellae. It is the risk of greater warming that does, indeed, motivate at least cautious investments to ameliorate. Even if you think it is 99 to 1 against, the expectation value of the 1% risk is not zero, and deserves a nonzero investment to hedge the bets, especially when that investment is likely to have positive ROI anyway, to be a good idea quite aside from CO_2.
If there is one thing that has been coming out recently, it is the fact that most climate scientists or earth scientists are not extremists or unreasonable or stupid or venal. A lot of them are just as “skeptical” of catastrophic warming as you or I might be on this blog, and the most honest of them admit fully that we cannot be certain even within a full degree C what the temperature is likely to be in the year 2100 assuming a full doubling of CO_2 to 600 ppm and beyond. Many of them would even agree that the warmer estimates are rather UNlikely, but not vanishingly so.
One very interesting question — perhaps even worth asking on this very blog with its many skeptics — is: What do you think the likely warming due to a doubling of CO_2 (from the current, say, 400 ppm to a presumed 600 ppm that is the “doubling” most people refer to compared to a fairly arbitrary 300 ppm base)? Express this as a probability distribution of possible answers, not just the mean answer — the tails are important! What do you think the real risks (expectation value of the costs) of this much warming will be, especially for outcomes in the high end tail (say, only 5% or 10% likely)? What do you think are reasonable investments — things that are likely to have a positive ROI in any event, for example — that could positively impact the projected costs should we end up in this tail region?
The need to answer questions like this in terms of a probability distribution is evident if you play poker or backgammon and have learned to evaluate expectation value. Backgammon is a perfect example. In the game of backgammon (played for money, of course) one can at any point double the stake on one’s opponent. When should you accept such a doubling of the bet?
Curiously, it isn’t when you think you have an even or better chance of winning. It is when you have to 3 to 1 or less chance of losing. If you play four games and lose 3 (doubled) and win 1 (doubled) your expected loss is 4. That is exactly equal to your certain loss turning down all four doubles. If you your chance of losing is less than 3 to 1 — say, only 2 to 1, you will lose 2 out of every three games played identically from this point on — you should accept the double as you will only lose 2 stakes rather than the 3 you would lose if you always turned down doubles at this risk.
This is the sort of risk analysis that one has to mentally perform when looking at “climate futures”. What kind of certain loss now is justified in terms of lowered long term expectation value of cost? The answer cannot be “zero”, not in any sort of sane analysis of the problem. Nor is it 30 trillion dollars.
Sobriety and objectivity are key to making the best decision here. Let go of your passions, your anger, your belief that the world is being manipulated towards the latter investment (even if true). Sure, religious people will always frame religious propositions in terms of Pascal’s Wager, and this only works if Hell is Hell, not if Hell is Heck, or just damn hot, sometimes, with no real damage done. But what is a reasonable assessment of the probable risks? Given a 10% risk of even moderately serious negative consequences, what really is a reasonable strategy of investment in the present, while we wait for sufficient data to improve our estimates?
I’m feeling kinda warmist today, just to balance out my more skeptical days. I think our knowledge is strongly insufficient to resolve the probable temperature question within a whole degree, but I do think that 2+C is not rejectable on the basis of the data so far, and that this much warming over a century and a half is at the outside edge of what naturally has occurred in the climate record. It wouldn’t be surprising if it had negative consequences, possibly balanced to some extent by positive ones, but probably not perfectly balanced. What is it reasonable to assume are the negative and positive consequences of 1, or 2, or even 3 C warming? What are the relative probabilities of each?
rgb
No! Radiation absorption and re-radiation, along with convection and evapotranporation and condensation transfer the heat absorbed by the Sun to a sufficient altitude to radiate to space. If the lapse rate and albedo do not change, the only way greenhouse gases increase the temperature is by raising the average altitude of outgoing radiation to space. It increases temperature at all altitudes in the Troposphere by shifting the entire temperature profile a small amount.
Yeah, like this. Although the lapse rate and albedo might well change along with the water content of the atmosphere. That’s what makes the problem complex instead of just “1.2 C on a doubling of CO_2 from 300 to 600 ppm”, which is interestingly the roughly 0.1C/decade we’ve observed over the last 30 plus years, except that it should be slower than linear because it is logarithmic and hence should slow down (as David Hoffer points out) from 400 ppm to 500 ppm compared to what was observed from 300 ppm to 400 ppm.
It’s those pesky feedbacks that are the problem. Water in the atmosphere changes lapse rates and albedo both. But how? And then there are (possible) longer term feedbacks — changes in ocean temperature, icepack melting, and so on. A complex problem…
rgb
michael hammer says:
July 21, 2012 at 3:52 am
I am extremely sceptical of CAGW but I have to strongly disagree with the above analysis. Adding cooling fins to a motor decreases its surface temperature because it increases the surafce area availabel to radiate that heat away, In the case of the earth the surface area is not increased
======
MikeB says:
July 21, 2012 at 3:52 am
This is a very baffling post Reed, it doesn’t make sense. An air cooled engine does not cool by blocking radiation. It cools by conducting heat away from the engine into the fins and, because they provide a larger surface area, more heat is subsequently radiated away (or convected away).
You both appear to miss the point being made. The radiating surface area has been increased by the addition of radiating CO2 molecules that can be heated by sensible heat (conduction) both from the surface and also from N2 and O2 molecules that cannot radiate the sensible heat they have received.
This would be an extremely simple undergraduate experiment. Set up a chamber of IR transparent material with a heated base and with say 10 liters of a mixture of N2 80% and O2 20%. When the gases have been allowed to stabilize say at 80C by heating from the base release CO2 into the mixture to become 350ppm and see if there is an increase in IR radiation from the gas mixture.
An increase in radiation from the gas mixture would show that the GHG ‘increase the radiative surface’ of the Earth.
Not good to see such kind of postings on WUWT. The guy simply does not understand how the green house effect works.
May be, WUWT should put a scientific explanation of physics behind the climate. “skeptical science” does have a list of “transparent” explanations for the basic physic – but biased. I suggest, WUWT maintains a FAQ about GHG and how greenhouse works – truly scientific, showing what is basic and where are the problems.
Concerning this particular publication, the green house effect in its basic form is trivial. The average temperature at the Earth surface is defined not so much by radiation balance, but by the adiabate: the adiabate holds through the troposphere from the surface up to the tropopause. The adiabate defines the temperature lapse about 1 Grad per 100 meters here.
The visible sun radiation heats the Earth surface. The surface radiates in IR. Most of this IR radiation cannot leave directly to the space because of the GHGs. However, at some particular hight, the atmosphere becomes transparent to the IR radiation. Balancing the incoming sun radiation and the GHG radiation at this particular hight, we can find the atmosphere temperature at that hight. Then we start the adiabate from that height and calculate the temperature at the Earth suface.
Because the atmosphere density is decaying exponentially with the height, the “radiation height” depends logarithmically on the GHG concentration and so is the Earth surface temperature a logarithmic function on the CO2 concentration – we speak about temperature increase per doubling of CO2.
The above is only valid when the “radiative height” is below the tropopause. In the tropopause – there is no temperature lapse. Thus, if the CO2 concentration is so high that the radiative height is withing the tropopause (and for some bands it is already there) – we have the “saturation effect”. A further increase of CO2 levels does not lead to temperature increase at the surface.
Reed…invalid and incorrect on multiple levels. It would be a waste of time to refute all the errors in a comment section, but some info for objective readers to consider. The same “Radiation Transfered by Atmosphere” graph is on page 326 of Slaying the Sky Dragon, in the chapter by Dr Charles Anderson. One should note the top graph has no scale with 5525 K solar insolation and 210-310 K OLR presented side-by-side, implying equality. Therefore the absorbed incoming IR is 20 times the available absorbed outgoing. The absorption/emission cycle is billionth of a second. As the spectral lines indicate, water vapor and CO2 share the most active ~15 micron band and even in the driest locations, H2O molecules are 400x each CO2 molecule. Correct atmospheric physics theory and experimental proofs are posted at Principia-Scientific.org and it would be in the interest of complete scientific analysis for these ‘alternate’ views to be posted. Can there be a Slayers post at WUWT in the future ?
This is a tortured analysis. The analogy between the thought experiment and the earth breaks down because the only source of energy in the engine is internal whilst the primary source of energy for the earth is external.
Reed admits that increased greenhouse gas will absorb additional rising radiation and then emit it in a random direction. A portion of the increased rising radiation that was absorbed and emitted is now heading down and can be considered an addition to the earth’s energy budget.
Going back to the engine – consider it to be like adding an afterburner.
Lots of fun for an old volkswagen guy. It does ignore the lapse rate effect of radiating at higher altitude and therefore at lower temperature, but who knows how this balances against adding all that surface area?
Tim Curtin said:
“Tyndall’s physical laboratory experiments found no evidence for any significant absorption of heat by nitrogen and oxygen in the longwave spectrum, and that meant for him they could not radiate heat to space.”
http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1038788
This meant they cannot *stop* LW radiation from escaping to space, anymore than a vacuum could. This has all been explained to you at Deltoid, including quotes from Tyndall himself showing you are completely misrepresenting his work. Now you pretend you don’t know Tyndall said the following:
“No doubt, therefore, can exist of the extraordinary opacity of this substance to the rays of obscure heat: and particularly such rays as are emitted by the earth after it has been warmed by the sun. It is perfectly certain that more than 10 percent of the terrestrial radiation from the soil of England is stopped within 10 feet of the surface of the soil. This one fact is sufficient to show the immense influence which this newly-discovered property of aqueous vapour must exert on the phenomena of meteorology.
This aqueous vapour is a blanket more necessary to the vegetable life of England than clothing is to man. Remove for a single summer-night the aqueous vapour from the air which overspreads this country, and you would assuredly destroy every plant capable of being destroyed by a freezing temperature. The warmth of our fields and gardens would pour itself unrequited into space, and the sun would rise upon an island held fast in the iron grip of frost. The aqueous vapour constitutes a local dam, by which the temperature at the planet’s surface is deepened: the dam, however, finally overthrown, and we give to space all that we receive from the sun.
… Its presence would check the earth’s loss; its absence, without sensibly altering the transparency of the air, would open wide a door for the escape of the earth’s heat into infinitude.”
http://books.google.com/books?id=nO8OAAAAYAAJ&pg=PA421&source=gbs_toc_r&cad=4#v=onepage&q&f=false
You insist that Tyndall showed N2 and O2 to be the “real GHG’s”, when he explicitly said otherwise. You also claimed on that thread that A) photons are mostly fictitious B) vacuums don’t exist c) there is no vacuum between the Earth and the Sun. Have you no shame?
alex – “The above is only valid when the “radiative height” is below the tropopause. In the tropopause – there is no temperature lapse. Thus, if the CO2 concentration is so high that the radiative height is withing the tropopause (and for some bands it is already there) – we have the “saturation effect”.”
See Santer et al 2003 (http://www.sciencemag.org/content/301/5632/479.short) among others:
These so called “Thought-experiments” are steadily getting worse, –
“Take for example an internal combustion engine whose metal surface is exposed to a vacuum.”
I can only hope (I never suppose) you are referring to the said engine’s exterior surface.
If you are, then learn this: “All internal combustion engines are ultimately air cooled. Even a “water-cooled, say auto/car engine” gets its cooling water chilled in the radiator, or heat-exchanger, usually situated at the front end of the vehicle. Cooling by radiation even in a vacuum is impossible”
A thermos-flask cannot work if heat could radiate through a vacuum.
Why not do a proper experiment instead of one that only exists in your mind? In other words find a way of lighting an incandescent light bulb in a vacuum and then watch what happens to it.
I used a large glass container – one like the ones they use in kitchens all over the world. The jar had an opening large enough for an incandescent light bulb to be passed through (100 Watt is best – and brings a quick result) and a lid which was screwed down onto the top with a threaded metal ring. If the lid is made of glass (it usually is as mine was) then it is best to substitute that for a metal
one (plywood may be ok but I have never used wood before) because you need to drill two holes through the lid into each of which an engineering nipple (The type of nipple that has nuts and olives and is used by plumbers and on occasions by electricians) are to be fitted. Through one nipple an electrical lead is to be passed and a lamp holder can then be connected. The other nipple is to hold the pipe work necessary for a vacuum pump and ideally a vacuum gauge to be fitted. – Ok, this explanation is too short and maybe not easy to understand, but if you do thik up your own way of dangling a light-bulb in a vacuum then do so, in any case:
Put it all together and after adjusting the light bulb so that it is hanging free (not touching the sides or bottom of the jar, pump out the air so that the gauge, if fitted, shows a “slight vacuum”. Then light the bulb and observe. – If the bulb behaves normally then it is obvious that heat radiates away, if the bulb melts, then —
@ur momisugly Baa Humbug says:
July 21, 2012 at 5:57 am
Looks like the tech-know.eu site no longer exists. Is there and alternate
link for that pdf please?
Faux Science Slayer says:
July 21, 2012 at 8:40 am
Reed…invalid and incorrect on multiple levels. It would be a waste of time to refute all the errors in a comment section, but some info for objective readers to consider. The same “Radiation Transfered by Atmosphere” graph is on page 326 of Slaying the Sky Dragon
>>>>>>>>>>>>>>>
It would be a waste of time to refute this source and principia-scientific is as sketchy a source of information as the worst warmist sites. Just because it espouses a point of view that resonates with skeptics doesn’t mean it is credible.
The notion that GHG’s serve to cool the earth is absurd. The earth is far warmer than the moon, which gets the exact same amount of insolation, but has no atmosphere. Average temperatures on Venus, which has an atmosphere, are higher than even the peak temperatures on Mercury which gets much higher insolation than Venus, but has no atmosphere.
Re: Convection vs radiance
One happens at the speed of wind. The other happens at the speed of light. Yes major amounts of energy are moved around the system by convection, but the ONLY way that energy ENTERS the system is via radiance and the ONLY way that energy LEAVES the system is by radiance.
(the purists will jump up and down and shout about tidal friction and decay of radiative materials and such and while technicaly accurate, the amounts are insignificant in comparison to insolation)