Guest post by Reed Coray
The following example illustrates the issues I have with reasoning often used to argue that increasing the amount of CO2 in the Earth’s atmosphere will increase both the Earth’s surface temperature and the Earth’s atmosphere temperature. Immediately following is a direct quote from URL
http://www.school-for-champions.com/science/heat_transfer_earth.htm
“The present situation is that there has been an increase in infrared-absorbing gases in the atmosphere, such as carbon dioxide (CO2) and methane (CH4). Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere and spreading through convection currents. The average temperature of the atmosphere has increased 0.25 °C since 1980, mainly attributed to an increase in infrared-absorbing gases in the atmosphere.”
Although the above statement makes no direct reference to Earth surface temperature, I believe it carries the implication that greenhouse gases in the Earth’s atmosphere increase the Earth’s surface temperature.
I make two comments: the first is relevant only if the above implication is valid, the second is relevant independent of the validity of the implication. First, placing matter adjacent to a warm surface such that the matter is capable of absorbing/blocking radiation to space from the warm surface can lead to a decrease in the warm surface’s temperature. Second, increasing the amount of the absorbing/blocking matter can lower the temperature of the absorbing/blocking material.
Take for example an internal combustion engine whose metal surface is exposed to a vacuum. In addition to doing useful work, the engine produces thermal energy (heat). That thermal energy will produce a rise in the temperature of the engine’s surface such that in energy-rate equilibrium the rate energy is radiated to space from the engine’s surface is equal to the rate thermal energy is generated within the engine. By attaching radiating plates to the engine’s surface, some of the energy radiated to space from the engine’s original surface will be absorbed/blocked by the plates; but because thermal energy can be transferred from the engine to the plates via both radiation and conduction, the temperature of the engine’s original surface will be lowered. This is the principle of an air-cooled engine[1]: provide a means other than radiation of transferring heat from an engine to a large surface area from which heat can be removed via a combination of conduction, convection and radiation, and the engine’s surface temperature will be lowered.
If plates at a temperature lower than the original engine surface temperature are attached to the engine, it’s true that the temperature of the plates will increase to establish energy-rate equilibrium. Once energy-rate equilibrium is established, however, increasing the plate radiating area (adding additional matter that blocks more of the energy radiated from the original engine surface) will likely lower the plate temperature.
Thus, blocking the amount of surface radiation escaping to space does not necessarily increase the surface temperature; and increasing the amount of radiation blocking material does not necessarily increase the temperature of that material. In both cases (the Earth/Earth-atmosphere and the internal combustion engine in a vacuum), the heat eventually escapes to space–otherwise the temperature of the Earth’s surface and the engine would continue to rise indefinitely. The difference isn’t that the energy doesn’t eventually escape to space (it does in both cases), the difference is in the path the energy takes to reach space. The amount of generated thermal energy in conjunction with the path the thermal energy takes to get to space determines temperatures along the path; and adding more material may increase or decrease those temperatures. To say that “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere…” by itself is unwarranted; because an equivalent statement for the case of adding extra plate material to the engine would be “Energy that would normally escape to space from an engine with small attached plates is absorbed by additional plate material, thus heating the plates…” For air-cooled engines, this statement is not true—otherwise the plate surface area of air-cooled engines would be as small as possible.
It’s fairly easy to visualize why (a) adding thermally radiating plates to an air-cooled engine might decrease the engine’s surface temperature, and (b) increasing the area of the radiating plates might decrease the plate temperature. It’s not so easy to visualize, and may not be true, why (a) adding greenhouse gases to the Earth’s atmosphere decreases the Earth’s surface temperature; and (b) increasing the amount of atmospheric greenhouse gases lowers the temperature of the Earth’s atmosphere. I now present one possible argument. I do not claim that the argument is valid for greenhouse gases in the Earth’s atmosphere, but I do claim that the argument might be valid, and can only be refuted by an analysis more detailed than simply claiming “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere.”
If we assume that (a) matter cannot leave the Earth/Earth-atmosphere system, and (b) non-greenhouse gases radiate negligible energy to space, then for a non-greenhouse gas atmosphere the only way thermal energy can leave the Earth/Earth-atmosphere system to space is via radiation from the surface of the Earth. The rate radiation leaves the surface is in part a function of both the area and temperature of the surface. For a greenhouse gas atmosphere, energy can leave the Earth/Earth-atmosphere system to space both via radiation from the Earth’s surface and radiation from greenhouse gases in the atmosphere. Suppose it is true that the density of greenhouse gases near the Earth’s surface is such that radiation emitted from low-altitude greenhouse gases does not directly escape to space, but is in part directed towards the Earth’s surface and in part absorbed by other atmospheric greenhouse gases. As the atmospheric greenhouse gas density decreases with increasing altitude, radiation emitted from high-altitude greenhouse gases can directly escape to space.
Now it’s not impossible that since (a) in addition to radiation, heat is transferred from the Earth’s surface to greenhouse gases via conduction, and (b) convection currents (i) circulate the heated greenhouse gases to higher altitudes where energy transfer to space can take place and (ii) return cooler greenhouse gases to the Earth’s surface, that the process of heat transfer away from the Earth’s surface via greenhouse gases is more efficient than simple radiation from the Earth’s surface. Many engines are cooled using this concept. Specifically, a coolant is brought into contact with a heated surface which raises the coolant’s temperature via conduction and radiation, and the coolant is moved to a location where thermal energy transfer away from the coolant to a heat sink is more efficient than direct thermal energy transfer from the heated surface to the heat sink.
One way to realize increased thermal transfer efficiency would be to use a coolant, such as greenhouse gases, that efficiently radiates energy in the IR band (i.e., radiates energy at temperatures around 500 K). Another way would be to spread the heated coolant over a large surface area. Since surface area increases with increasing altitude, thereby providing expanded “area” (in the case of a gas, expanded volume) from which radiation to space can occur, it’s not clear to me (one way or the other) that greenhouse gases won’t act as a “coolant” reducing both the temperatures of the Earth’s atmosphere and the Earth surface.
[1] It’s true that for most air-cooled engines the main transfer of heat from the engine plates is via a combination of (a) conduction of heat to the air near the plates, and (b) convection that replaces the warm air near the plates with cooler air. To aid this process, a fan is often employed, or the engine is located on a moving vehicle and the vehicle’s motion through an atmosphere provides the flow of air across the plates. Although conduction/convection may be the primary means of heat dissipation from the plates, radiative cooling also dissipates heat.
An interesting alternative take. And convection is certainly ignored in the “Standard Model”. However, I can’t see the likes of Trenberth even giving it a second glance, the science is “settled”.
Convection via thunderclouds – as Willis has noted – would fit alongside this. There is a lot of commonsense in this thinking and it deserves study. Intuitively it begins to show how part of earth’s ‘thermostat’ may function.
This ought to rattle some cages!
Getting close to a better theory.
Heat cannot be stored by these ‘so called’ GHG’s as 2nd law states that heat must be lost, an increase in entropy, but adsorbed SIR will increase the molecular kinetic energy, increasing the temperature, but this kinetic energy will be transferred to the other gasses not directly affected by the SIR. The GHG’s will radiate LIR but at a reduced energy level, the frequency change from short to long wave IR is the evidence that this happens. Also the 1st law dictates that it must. If it did not then we would be driving in cars using perpetual motion engines which violate both 2nd and 1st laws.
The reradiated heat is in fact a reduction of solar heat reaching the surface so it cannot raise the temperature more than that reached without the intervention of the GHG molecule. If the theory of reradiated heat were to be true then warm liquids placed into a vacuum flask, with its mirrored internal surfaces, would raise the liquid’s temperature by a considerable amount. We all know from experience that this does not happen but a vacuum flask only reduces heat loss slowing cooling. It insulates well but not perfectly.
We must also ask ourselves why the earth’s temperature is fairly even in that max. temperatures rarely exceed 50C nor do we get any lower than -85C. The moon, receiving the same solar insolation as earth but having zero atmosphere, achieves over 150C in sunlight and less than -150C in shadow. So much for an atmosphere increasing temperature by reradiation.
But has it really? If you look at the warming from 1900 to present, look particularly at the warming from about 1910 to about 1945. Temperatures then cooled until about 1975. Beginning in 1976, things began warming again but it took several years to warm back up to where temperatures were in the 1930’s and 1940’s. We can not really consider that period to really be overall “warming” in the context of recovery from the LIA, we can only consider that to be recovery from a spate of cooling after temperatures reached their peak in the late 30’s early 40’s. So we must wait until temperatures get back to that point before we consider that we would have any additional overall warming of the planet.
Whether or not we ever reached the levels of the 1940’s is a matter of some debate, but I would say that the period since 1980’s saw NO temperature rise attributed to CO2 and only a recovery of temperatures to NEAR what they were in the 1930’s and 1940’s. From my point of view, the second half of the 20th century actually saw no or very little “warming” at all in the context of additional recovery from the LIA. It only saw some natural variation where temperatures declined from the 1940’s through the 1970’s and then recovered.
Took a long way getting there, but I understand it in the end. Adding greenhouse gas may be like adding blades to an air-cooled engine.
If our concern is surface temperature then perhaps the “more blades” equivalent on Earth might be adding more mountains?
In a still environment you have conduction and radiation emission. The surface temperature at night will always be coolest in a vacuum won’t it? Any atmosphere, even non-greenhouse, will cause heat to linger around the surface – the buffer – which raises the average temperature at the surface. Greenhouse gases create a thicker buffer.
Just thinking of the mountain example above. Incoming radiation is constant and if we add more surface area the radiation is spread across a greater area. Each unit area absorbs less heat and hence doesn’t radiate as fast – thermodynamics says the higher the difference the faster the loss. With that in mind perhaps a flat Earth endures greater extremes than a bumpy Earth? Perhaps that applies even where more finer details are concerned e.g. trees add to the surface area.
Most heat transfer from the Earth’s surface is not directly to space, as is so often assumed in such models. You should consider clouds, which cover 70% of the Earth’s surface. Their temperature is set by the lapse rate.
I am extremely sceptical of CAGW but I have to strongly disagree with the above analysis. Adding cooling fins to a motor decreases its surface temperature because it increases the surafce area availabel to radiate that heat away, In the case of the earth the surface area is not increased. The point that energy can be lost to space from the surface at all thermal IR wavelengths and from the atmosphere but only at the GHG wavelengths is true in principle. However, because the GHG effect is so strong over the atmospheric column in effect the surface can only lose enegy at the non GHG wavelengths while the atmopshere can only lose energy to space at the GHG wavelengths. What increasing the GHG concentration does is to slightly increases the range of GHG wavelengths so the surface can lose energy over a slightly smaller range of wavelengths and the atmosphere over a slightly larger range of wavelengths. Sine the atmosphere is cooler than the surface it loses less energy than would the surface at the same wavelength. Thus the actio of the GHG increase is to slightly reduce the energy loss to space and to restore balance the temperature of the system must slightly increase.
However – what is at issue is how much. By how much does the temperature have to increase to compensate? A simple calculation shows doubling CO2 would lead to an increase of about 1C in the absence of feedbacks. That’s not serious so are the feedbacks positive or negative? This is the crux of the debate but its worth noting that every naturally stable system shows strong negative feedback and that means the rise will be less than 1C not more than it.
Where does the negative feedbakc come from here? Higher temperatures means more evaporation which must mean more rain but rain comes from low clouds so it must mean more low cloud either in density or in coverage. Both increase Earth’s albedo and reduce temperatures.
This is a very baffling post Reed, it doesn’t make sense. An air cooled engine does not cool by blocking radiation. It cools by conducting heat away from the engine into the fins and, because they provide a larger surface area, more heat is subsequently radiated away (or convected away). You have to be very careful with analogies, and this one is too contrived to be useful. The atmosphere, clearly, doesn’t work like this.
.
As an ex-motorcyclist, first hand experience leads me to agree with much of what Reed Coray writes. As a layman in the sciences I must rely on educational web sites that explain heat transfere and IR radiation and such like. I am led to believe that gases in the atmosphere can absorb OR radiate specific radiation bands dependant on the local temperature. It cannot do both at the same time. Wein’s Law will give the peak temperature at any specific IR wavelength. Using Wein’s Law to look at CO2 I find that the 2.7 micron band peaks at ~800C, the 4.3 micron band at ~400C and the 25 micron band at about -80C!! I understand only limited areas of the Earth’s surface might radiate at up to 50C so the 2.7 and 4.3 micron bands will NEVER be exited enough to absorb any energy from the surface. They might absorb a very little from the sunlight but that is working as a coolant. The so called standard surface temperature of the Earth is said to be 15C, well above the the -80C temperature level of the 15 micron band for CO2. The problem now is most of the CO2 molecules in the atmosphere will be at a temperature comensurate with the adiabatic lapse rate starting at the surface. So assuming a drop of 10C per kilometre altitude air temperature should be down to -80C at about 9.5 kilometres altitude, almost the tropopause. Only then will the CO2 molecules be cool enough to absorb radiation at 15 microns.
BUT! There is indeed nothing to stop the CO2 radiating at 15 microns and some of that radiation reaching the surface. Now another BUT! The surface, except at possibly a small area at the south pole, is well above -80C!! Any element, black body or not, does not absorb radiative energy below its peak temperature.
A CO2 molecule IS a black body with rather specific characteristics. And so is any other gas molecule in the atmosphere.
Since I am completely unable to see any ‘greenhouse’ effect in the atmosphere I need more education. Please post links that will this layman.
What about the additional LH of Vapourization drawn from the surface by both any direct additional evaporation due to CO2 GH effect but also the increased transpiration from plants as their metabolism increases due to more CO2 (double -ve feedback)? H2O vapour up into the atmoshpere causes some more GH effect , true (+ve feedback) but then recondenses as clouds ( albedo => -ve feedback) and releasing the LHV at altitude where convection/cnduction takes it upwards and into space ( -ve feedback). What is the net feedback effect? Who knows but there is no hot spot at altitude so it cannot be much.
Is Trenberth’s approval a hurdle this has to clear?
Tcha!! Educate, educate, educate!
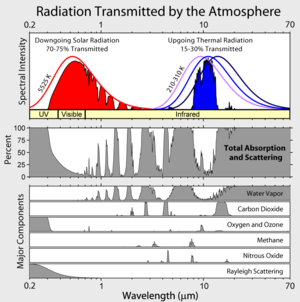
Those spectral intensity curves are wrong. The formula I = kT^4 gives the area under the curves (in W/m^2) (k = Stefan-Boltzmann constant).
Temperature Area under curve
210K 110 W/m^2
260K 260 W/m^2
310K 523 W/m^2
Solar 1367 W/m^2 at top of atmosphere.
The simple fact is that convection trumps radiation every time. Just hold you hand in front of a working ‘radiator’ and then above it. (This is why radiators are very often placed below windows, in fact).
There is no proof that I am aware of that more CO2 does not in fact cool the atmosphere by convection.
I keep looking at this: air and ground temperature recorded in the N. African desert during a solar eclipse.
http://www.shadowchaser.demon.co.uk/eclipse/2006/thermochron.gif
Could someone please show me how to use Plank’s Law of Radiation (I use Open Office) – as shown here:
http://pveducation.org/pvcdrom/properties-of-sunlight/blackbody-radiation
As I keep getting the “ultraviolet catastrophe”.
This is inline with the real null hypothesis that I posted before.
We read all over internet that the black body temperature of the Earth would have been -18C, but the actual average temperature is +15C; consequently this 33 degrees difference is supposed to be the greenhouse effect. But is this true?
Is the blackbody situation the “null hypothesis”? I don’t think so. The black body calculation assumes a sphere with a constant flux of light energy, uniformely distributed over the surface, using the Stefan Boltzman equation to derive it’s temperature like this.
But the earth is nowhere near a blackbody and if we want to really look at the null hypothesis, we would have to look at an earth without greenhouse effect, but still with an (inert) atmosphere and still rotating in 24 hrs, with seasons and all.
Now instead of using an average steady state solar radiation, we need to realize that we have the diurnal cycle with max insolation radiation at noon and no radiation incoming when the sun is below the horizon. So during daytime the earth surface warms up and much more than the according the average radiation. Equilibrium temperature at the equator in a steady state with the sun in zenith, using the full incoming 1365 w/m2 (albedo 30%) would be 360K or 87C. This follows from applying the Stephan Boltzman equation for the spot directly under the sun, instead of a uniformely distributed radiation.
So this much higher temperature of the earth surface is transmitted via conduction to the lowermost boundary layer of the atmosphere. This heated air gets is less dense, and it becomes buoyant so it rises up; Convection, the very basics of meteorology. So at daytime the atmosphere receives thermal energy of the earth. How can it lose this energy again? Remember we are in the null hypothesis, no radiation, no greenhouse effect, so the inert atmosphere cannot lose the energy by radiation.
Now, at night time the Earth does not receive radiation energy from the sun but it radiates energy out and cools quickly, obviously much more quickly in the null hypothesis even than with the greenhouse effect, which would have directed (“reflects”) some radiation back to earth. Now the cooler earth also cools the boundary layer of the atmosphere by conduction again, however there is no negative convection as the cool air gets more dense and tends to stay put; the inversion; also very basic meteorology. So despite the cooling of the earth, the missing radiation from the atmosphere prevents it from cooling at night and the next day more conducted energy is convected into the atmosphere, that stays there again.
Obviously we have an unbalance. And equilibrium can only be reached, maybe after thousands of years, when the convection at daytime has reduced so much to balance heat loss at night time via conduction back to the surface. For that the lower level atmosphere needs to be at the same temperature / density than the boundary layer would reach due to the conduction of heat from the surface.
Conclusion, in the null hypothesis, without greenhouse effect, the average temperature of the lower atmosphere would be considerably higher than the black body temperature of the surface. How much I don’t know. But the main point is that a certain portian of the temperature difference between black body and actual atmospheric temperature is not due to greenhouse effect but to the inability of the inert atmosphere to cool down by radiation.
Pretty much fits my previous contention that when molecules in the atmosphere absorb more energy then the circulation changes so as to accelerate energy to space faster.
The effect on the energy content of the system being at or near zero but the price to be paid is that circulatory change.
Then the only question is whether the circulation change from human emissions is measurable as compared to the natural changes caused by sun and oceans which gave us the MWP, LIA and current warm period.
You do realise that this quote you dug up is from an educational website aimed at schoolchildren, right? And that more detailed analyses have been being published in the scientific literature since Victorian times? Your argument is “not even wrong”. Presumably you don’t have any idea why the Moon is colder than the Earth.
If it was, then the predicted surface temperature would be about 45°C. It’s one thing to be ignorant of the science, most people are ignorant of the science. Believing in your own ignorance is another thing.
I think Reed Coray is on the ball.
The IPCC assertion that nitrogen and oxygen are not GHGs appears to conflict with the findings of Tyndall’s physical experiments (1861) which showed that N2 and O2 neither absorb nor radiate heat in the longwave infrared radiation (LIR) spectrum, even though they are transparent to incoming solar shortwave radiation. Modern spectroscopy reveals a total absence of N2 in the LIR, and barely any O2 relative to the atmospheric H2O and C2O, which dominate the infrared spectrum. Yet H2O and CO2 comprise only about 1% of the atmosphere, as against the 99% consisting of N2 and O2. Thus if the former are blanketing the earth, that is an achievement when they comprise so little of the atmosphere – most of us prefer blankets that are close to 100% wool.
Tyndall’s physical laboratory experiments found no evidence for any significant absorption of heat by nitrogen and oxygen in the longwave spectrum, and that meant for him they could not radiate heat to space. His experiments showed that air comprising only [H2O] and [CO2] both absorbed and radiated 15 times as much as air consisting only of N2 and O2:
“Air without [water vapour and CO2] produced an absorption of about 1.
Air direct from the laboratory, containing therefore its carbonic acid [CO2] and aqueous
vapour, produced an absorption [and radiation] of 15”.
(Lecture 1861:28).
Comparison of the Brazilian rainforest and the N. African Desert.
http://en.wikipedia.org/wiki/Barcelos,_Amazonas
http://www.google.co.uk/search?q=Barcelos+amazonas&num=10&hl=en&site=imghp&tbm=isch&gs_l=img.3..0l2j0i24l8.2563.8069.0.10019.10.8.0.2.2.0.532.2460.1j2j1j0j3j1.8.0…0.0.l9hxb0L6cLs&oq=Barcelos+amazonas
http://www.wunderground.com/history/station/82113/2012/5/20/MonthlyHistory.html
http://www.climate-charts.com/Locations/b/BZ82113.php
http://en.wikipedia.org/wiki/Adrar,_Algeria
http://www.google.co.uk/search?num=10&hl=en&site=imghp&tbm=isch&source=hp&q=adrar+algeria&oq=Adrar&gs_l=img.1.1.0l2j0i24l8.1151020.1152708.0.1155877.5.5.0.0.0.0.462.1453.1j0j2j0j2.5.0…0.0.PUZtKMJOlKc
http://www.wunderground.com/history/airport/DAUA/2012/5/20/MonthlyHistory.html
http://www.climate-charts.com/Locations/a/AL60620.php
For May 2012, Barcelos, Brazil (Lat: 1 South)
Temp: monthly min 20C, monthly max 33C, monthly average 26C
Average humidity 90%
For May 2012, Adrar, Algeria (Lat: 27 North)
Temp: monthly min 9C monthly max 44C, monthly average 30C
Average humidity around 0%
GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …
If the energy input to the Earth system (from the Sun) remained unchanged, then the fingerprint of an enhanced GHE as the culprit of tropospheric warming would be a gradual reduction in OLR at TOA. This is not what we observe. We observe the opposite. This suggests rather that the Earth system is working towards balancing an INCREASED energy input. Which is also observed. But in this case, the increased energy IN (heat gain) is clearly what causes the warming. The increased energy OUT (heat loss) is Earth’s attempt to keep pace.
Where’s the evidence of a greenhouse gas-driven warming?
Very interesting post Reed, but I have my doubts (hey, it’s what we do here).
The additional CO2 added to the atmosphere does not significantly increase its volume. A thicker atmosphere would certainly have a greater greenhouse effect (see Venus).
Keeping the volume of the atmosphere constant and increasing the surface area exposed to space (i.e. a bigger earth with thinner atmosphere) would be a more appropriate comparison to adding more metal plates to an engine.
The physical properties of the atmosphere are the sum of its constituent parts. Adding CO2 changes those physical properties. Consequently, I think a better analogy might be: adding CO2 to the atmosphere is like changing the composition of the metal (i.e. an alloy) used in the engine plates. Which is a very different situation from adding more plates.