
Guest post by Kevin Kilty
Introduction
Within the past week or two we have read posts from Dr. Spencer
(6/7/2019), Nick Stokes (6/6/2019), Lord Monckton (6/8/2019), and Willis Eschenbach (6/8/2019) covering a variety of topics involving simple block models; and each one involving, in one way or another, climate feedback. I have had a few thoughts banging around in my mind for a long time which relate to these topics and build on each of these recent works in a series of postings. This one presents a simple block model illustrating the Earth as a thermal solar panel or solar collector. It will have a direct, independent and supportive bearing on Dr. Spencer’s post.
1. Basics of a Collector
Earth bound solar panels are constructed to collect solar irradiance, reduce parasitic heat losses from conduction and convection, and transfer the solar energy they have collected as heat into a working fluid. For the Earth as a whole the only heat loss is outgoing longwave radiation. This makes analyzing the Earth as a solar collector particularly simple. Let’s begin with two important balance equations from thermodynamics.
Energy balance is our primary analytical tool. Stated in words energy balance is:
(1)
Solar energy in = Longwave (IR) energy out + energy being stored
Or symbolically for the Earth this becomes:
(2) ![]()
Where Is represents solar irradiance, αs is solar absorptivity, r is Earth radius, σ is the Stefan-Boltzmann constant (5.67 × 10−8 in S.I. units), e is the effective infra-red (IR) emissivity of the Earth, T is absolute surface temperature, and C represents a capacity for thermal storage. Since the final term representing rate of new storage in Equation 1 is quite small compared to the others, we can ignore it for our purposes, and write energy balance in terms of surface temperature as
(3) 
I have separated out the ratio![]() because it is a common engineering figure of merit for solar collectors used to guide choice of materials.
because it is a common engineering figure of merit for solar collectors used to guide choice of materials.
The second balance equation, one I have mentioned in threads here before, is entropy balance.
(4) Entropy outgoing = Entropy incoming + Entropy generated
We have no particular use for this balance equation at present, but in terms of the operation of a real solar collector we could use it to calculate energy that might have been put to useful work but was wasted by parasitic losses instead. Be assured that global climate models and solar collectors alike have to adhere to both of these balance equations in order to be realistic and provide credible results.
2. Block Model of Our Collector
Let’s stipulate the following. The sun is a black body radiator with a surface temperature of 5900K, and solar irradiance of 1370W/m2 at the orbit of the Earth. Emissivity (e) is a parameter which we will determine from energy balance. Solar absorptivity (αs) equals (1 − A) where A is the Bond Albedo of Earth. We will use a value of 0.3 for A, recognizing that this is uncertain to a degree and varies with time.
Finally, we will use a temperature of 288K for the mean surface, recognizing that this temperature is of the atmosphere at about two meters above the surface. The surface must be, on average, different than 288K in order to allow heat transfer between the surface and the air, but using 288K for the mean surface temperature serves our purposes just fine.
A block model one might derive here is shown in Figure 1. It is much simpler than the block models with feedback loops. In engineering science such a model we refer to simply as a system or possibly as a black box. If this system is linear we can state its operation using an impulse response function, or a transfer function. If the system is non-linear we usually have to get down and dirty and specify the input/output relationship in detail. In control engineering the block is known as a plant, and represents the workings of a facility or machine. It makes good sense to think of Earth as a facility.

In this case of our solar collector the input to the system is solar irradiance, which is, in fact, the only driver of the climate system (CO2 and water vapor feedbacks are internal to the system). The diagram shows that mean temperature is our only observable at present although we could choose to measure others. We use mean surface temperature as a proxy for what is happening in the climate system most of which is hidden from view inside the block. The block appears simple, but may contain great complexity including feedback loops, time constants, delays, and even additional blocks. I plan to address such hidden detail in a subsequent post building on Nick Stokes’ contribution on feedback.
3. Calculating Apparent Emissivity
Let’s put what numbers we can into the model of Equation 3.

In order to make this an equality and produce energy balance emissivity (e) must have a value of about 0.61.
We now arrive at what seems like a paradox. All the materials making up the Earth‘s surface are very black at infrared wavelengths. Pavement, water, soil, plants, skin, snow and ice all have emissivity in the range of 0.9 to 0.96. Yet, energy balance reveals that the effective emissivity is one-third lower. This is a robust result. The resolution of the paradox is that all the dark stuff on the surface is covered by an atmosphere containing infrared active gases. Just as we apply thin coatings to materials to change their radiative properties, the thin coating of atmosphere does the same for the Earth. One cannot use measured temperatures, solar irradiance, and absorptivity, and at the same time balance energy without including the effect of our greenhouse gases.
4. A Note about the Figure of Merit
The ratio ![]() is known as the figure of merit for solar collectors. To make a solar collector that becomes very hot in sunlight, we choose to make it from materials in which
is known as the figure of merit for solar collectors. To make a solar collector that becomes very hot in sunlight, we choose to make it from materials in which ![]() is as large as possible. Think of a chrome alloy tool. Its figure of merit is approximately 6 – It lays in sunlight and…Ouch! On the other hand to fabricate a surface which stays cool in sunlight we seek materials with
is as large as possible. Think of a chrome alloy tool. Its figure of merit is approximately 6 – It lays in sunlight and…Ouch! On the other hand to fabricate a surface which stays cool in sunlight we seek materials with ![]() as small as possible. Some aerospace materials, like aluminum with a thin titanium dioxide coating have a ratio around 0.2. For our Earth solar collector the figure of merit is approximately one.
as small as possible. Some aerospace materials, like aluminum with a thin titanium dioxide coating have a ratio around 0.2. For our Earth solar collector the figure of merit is approximately one.
5. Including Disturbances in the Model
In this model of Earth as a facility we can modify the block diagram to include a separate input that allows for disturbances to the system (Figure 2). It alters the system parameters and changes how the system behaves. If we know enough about the function of the system we can


6. Conclusion
The simple model of Earth as a solar collector shows conclusively that greenhouse gases in the atmosphere lower the effective emissivity of the Earth, which in turn raises the mean temperature of the surface in order to achieve energy balance. We can‘t balance energy using measured values of irradiance, albedo and temperature without a substantial greenhouse effect–A conclusion backing up Dr. Spencer‘s simple diurnal temperature model.
The model of disturbances to the Earth facility presented here is an alternative to block models containing explicit feedback loops. What I find attractive about eliminating feedback loops and making use of disturbance inputs instead, is that we can dispense with the complications which arise from the distinction between top of atmosphere values, and surface values. It also allows us to avoid feedback as an external forcing, which suggests it as a separate source of driving energy, when it is no such thing.
7. Notes:
The discussion about solar collectors and the figure of merit is available from most modern engineering textbooks on heat transport, Heat Transfer by Alan Chapman, MacMillan, 3rd edition, 1974 is an example.
For some discussion about entropy balance, and the engineering calculation of entropy transport and entropy generation, consult any engineering thermodynamics text, even one as old as Obert’s famous text from 1948, Engineering Thermodynamics; or better yet, Zemansky’s Heat and Thermodynamics in any of its eight editions.
From the article:
“We can‘t balance energy using measured values of irradiance, albedo and temperature without a substantial greenhouse effect”
So, the Greenhouse Effect is derived, why isn’t it being measured like for irradiance, albedo, and temperature?
I am not deriving it, but rather demonstrating that without it we can’t simultaneously use measured values of irradiance, temperature, and albedo and balance energy. A related point is that we don’t measure emissivity; there is no emissivity meter.
The Greenhouse Effect can’t be measured, I understand that. The Greenhouse Effect has been introduced only because there is a claim of missing heat in other equations based on assumptions.
Celestial Spheres were introduced to explain planetary orbits under a Geocentric assumption. They couldn’t be measured either. Once the Geocentric assumption was replaced by a Heliocentric assumption there was no need for Celestial Spheres, they never actually existed.
Measuring the Greenhouse Effect would go a long way to confirming its existence.
Actually it could be measured in principle. We could tune a spectrometer to the bands that are very specifically involved in CO2 emission, and monitor them over time under circumstances of say, constant surface temperature, and what we would observe is that above the top of atmosphere these bands are depending–i.e. becoming less transparent.
Actually the difference between the emissivities of surface objects and the effective emissivity of the earth viewed at the top of atmosphere is a way of measuring the greenhouse effect. This is the point I was hoping to convey.
Most of any greenhouse effect is water. It is the presence of lots and lots of water in three states and switching between them that makes this a bit too complicated for block models and averages.
It would be impossible. Water overlaps with CO2 bands so any water present would absorb the same IR CO2 would ostensibly be absorbing. It would look indistinguishable from water vapor. You are still left with trying to guess how much of the absorbed IR was converted to heat by water and how much by CO2 – but at that point it does not really matter.
Kevin kilty
So the bottom line is that the Greenhouse Effect is an assumption. The wishful thinking of ‘we can not fit the math without it so it must be’.
All based on Entropy outgoing = Entropy incoming + Entropy generated, all without acknowledging life on this planet takes energy, and that resolving entropy for the planet should be over the entire time that this planet and its processes will last, not just the milliseconds or years that the energy balance assumes, and certainly not beholden to a minor atmospheric gas.
Consider this, the human population has grown from a mere 1 million at a the beginning of the 1800 to the present level of about 8 billion and still growing. That a 3 times doubling, and yet climate science has the temerity to say that this growth take no solar energy, no conversion of solar energy to chemical bonds in the living world.
These calculations are and will always be poppycock as long as life in all its form are ignored from the evaluation. Life thankfully ignores your short term ideas, and attempts to rages against short term entropy theories as it converts solar irradiance to chemical bonds, bonds that (in trees for example) can last many hundreds of years.
And if you look at coal, that is very old solar energy bound up in that substance’s chemical structure, and only released again by destructive decomposition. And those are not the only ones, there are many processes that use solar energy and form new biological chemistry and do NOT give back the all the solar energy they’ve use in the short term, they sequester it away for many thousands of years. And no they don’t have to live for all that time they just have to leave behind new chemical compounds (chemical structures) in the environment that were not there but for their life and solar energy.
Those mathematical figures fit only because that is what climate science wishes, not because it is so!
tim0mason
+1
And ~4% of CO2 is anthropogenic – or much ado about almost but not quite nothing.
So how is measuring a secular differentiated GHG CO2 component going to tell us anything useful? Especially given the accepted model ‘fudge-factor’ to deal with the unknowns is still likely about 3 times larger than observed effect (i.e. of 4% or not much, when compared to H2O). The instrumental error alone is probably larger, even if you could split the atmospheric-window’s blobbing-up of various GHG overlaps.
And even if an anthropogenic component of secular CO2 GHG could be measured and differentiated, how is that even related to ‘climate-change’™ when secular CO2 lags ∆T anyway?
The concept of a ‘solar collector’ added some lucidity though.
Bottom line though, it that the models are outputting rubbish because the ‘fudge-factor’ is rubbish, but no one really wants to admit it, as it’s all ‘settled’ in IPCC Land, Media Land and political Land. Only Science Land disagrees.
And no one cares about them, as this is not about science anyway, any more than it’s about climate™ (weather changes).
rhoda klapp, xenomoly
Your replies to Kevin Kilty contain typical misunderstandings.
1. CO2’s action indeed is nearly inexistent where water vapor is present, but unlike water vapor which nearly completely precipitates above the tropopause, CO2 is uniformly distributed in the atmosphere up to 50 km altitude.
Its activity, though exactly similar, happens above that of water vapor, both reducing the amount of longwave radiation (LR) outgoing directly from the surface.
Not only does this result in a part of the reemitted LR going lost in collisions with N2 / O2 / H2O / CO2 molecules; another factor is that the higher the altitude at which LR reaches outer space, the less energy is released because of the lower temperature of the reemitting molecules.
2. The devices analysing for example downwelling LR perform line-by-line analysis, and are perfectly able to distinguish what Is reemitted by H2O and what is by CO2, CH4, N2O etc etc.
3. Look at the HITRAN database using SpectralCalc, and consider the intensity of absorption/reemission of H2O and CO2 at 0, 10 and 40 km, under consideration of their respective atmospheric abundance:
0 km
https://drive.google.com/file/d/16Ly1OAnJPQuYnNh4J6PC0G6FUga9Ocao/view
10 km
https://drive.google.com/file/d/1xKg7debGXHrH83vIoXLGXqvgmMDjv_ng/view
40 km
https://drive.google.com/file/d/1oh2iUFd0yv8j7izd7bnh616RbDKO5rCF/view
Like me, you will have immediately observed that the intensity decreases by a factor of 100 from 0 up to 10 km, and from 10 to 40 km again.
But that is only a hint on how tiny CO2’s action is in comparison to H2O at the surface.
Rgds
J.-P. D.
rhoda klapp – in addition to the water phase change complexity, the block model output is (T) when energy is the input from the sun. (T) doesn’t capture the energy input, especially related to water phase changes.
tom0mason: You mean the population in 1800 was a mere billion – not million.
There is one way to separate out the H2O and CO2 greenhouse back radiation. Pick the driest desert in the world and measure the back radiation at nighttime only on the nights without any clouds. NASA refuses to do this and publish the results because their scientists have told them that the back radiation by CO2 alone is unmeasureable. In other words 0.0000000000 W/m^2
Bindidon:
“CO2’s action indeed is nearly inexistent where water vapor is present, but unlike water vapor which nearly completely precipitates above the tropopause, CO2 is uniformly distributed in the atmosphere up to 50 km altitude.”
“the higher the altitude at which LR reaches outer space, the less energy is released because of the lower temperature of the reemitting molecules.”
Apparently you didn’t notice that these two points contradict each other, because above the tropopause temperature increases with altitude, so more CO2 means that more energy is released, not less. This is easily seen in IR emission spectra from satellites as a marked “spike” in the middle of the CO2 absorption band where emission
altitude is above the tropopause. In winter in inland East Antarctica this happens all the way down to the surface.
“Pick the driest desert in the world and measure the back radiation at nighttime only on the nights without any clouds. ”
Even better inland Antarctica. IR observatories are now put there instead of in space, because of the low IR background.
http://find.spa.umn.edu/~pryke/southpolemeeting/washington_review_2011_burton.pdf
If you are going to comment at least understand how it is done, you can argue the results but stop with being stupid because you are too lazy to read
This is the kiddies explaination
https://ocov2.jpl.nasa.gov/observatory/instrument/
Here is the initial calibration
https://www.atmos-meas-tech.net/11/3111/2018/amt-11-3111-2018.pdf
There are also satellites from Japan and Europe doing slight variations.
Climate Science may have crazies but please stop trying to tell actual scientists what they can and can not do when what you know fits on a postage stamp.
Kevin kilty – June 12, 2019 at 1:38 pm
To wit: “ Emissivity is the measure of an object’s ability to emit infrared energy. ”
And an ……. “OH GOOD GRIEF” ……. because me thinks you are really talking silly.
Just because the emissivity (LWIR), ….. that is being radiated from the surface, ……. can be measured right at the surface, ……. DOESN’T MEAN THAT ……. the emissivity (LWIR) that you measured at the top of atmosphere (TOA) was absorbed and re-radiated by a CO2 molecule with a portion of said re-radiated LWIR being directed back toward the surface.
The surface emitted LWIR that you measured at the TOA might not have ever collided with a CO2 molecule on its journey up to the TOA, …… and/or, …… the hot surface, due to solar irradiance, could surely have CONDUCTED its thermal heat energy to an O2 or N2 molecule, which then rose up toward the TOA …… where it conducted said thermal energy to a CO2 molecule …… which then radiated it as LWIR with a portion directed toward the surface.
So, it is entirely possible for the near-surface GHG molecules (H20, CO2, etc) to radiate LWIR without ever having absorbed any that was radiated from the surface.
Kevin kilty, a simple physical experiment will prove that 400 to 800 ppm of CO2 has no, per se, “greenhouse effect” whatsoever ….. and that is exactly why no one will conduct such an experiment.
@ tty – June 13, 2019 at 3:18 am
Tty, …… I’m mighty curious, …… just what is the East Antarctica source of the LWIR that produced that …… marked “spike”?
@ Alan Tomalty – June 12, 2019 at 11:23 pm
Alan T, …… even with atmospheric CO2 at less than 600 ppm, I truly believe that measuring the back radiation (downswelling) in a desert environment during a cloudless mid-afternoon ….. would still not result in any significant or worthwhile (surface temperature affecting) amounts of LWIR.
maybe explain how drag is “measured”
keven kilty
Great start to an alternate explanation.
Suggest the next step of adding albedo difference between clouds and earth’s surface.
David
The inserted formulas-as-images are difficult to see, e.g., in my case they’re very grainy.
Yes, I too have difficulty reading the formulas.
Clyde,
That’s the point!
The equation that effectively describes the atmosphere is the same one that describes the insulated envelope of a house: Q = 1/R A dT = inverse thermal resistance of the atmosphere x the area x (Surface Temperature – ToA Temperature)
“R” thermal resistance includes: conduction + convection + advection (wind) + latent (evap & cond) + radiation. All the heat transfer forces and processes that resist energy flow.
To move current through an electrical resistance requires a voltage difference.
To move fluid through a hydraulic resistance requires a pressure difference.
To move energy (heat) through a thermal resistance requires a temperature difference.
Emissivity = radiation / (conduction + convection + advection (wind) + latent (evap & cond) + radiation) = 63/396 = 0.16 or 63/160 = 0.39
ToA is 32 km where the molecules disappear.
“R” is the same “R” printed all over in the insulation aisle at Home Depot.
No pseudo-science BB upwelling/downwelling LWIR GHG hocus pocus needed.
Besides which the atmosphere doesn’t warm the earth.
By reflecting 30% of the ISR the atmosphere cools the earth.
The atmosphere stabilises the earth temperature (reflect som insolation and also act as insulation)
Yes you are right.
This is really disturbing, because unlike text characters, these images can’t be effectively widened through the classical ‘crtl-+’ feature. Making pixels bigger isn’t the same as rendering Bezier splines at a higher size.
It took Charles and I a lot of effort to figure out what had happened. I consider it an MSWord deficiency that interacted badly with WordPress.
Gosh, the equations produced by reading a Latex document into MSWord, and then the transferrance to a post here, has not done well. I apologize. I don’t know if this can be repaired quickly, but I’ll never use this route again.
My fault, when preparing this post I thought the Latex had already been converted to images. I should be able to fix in the next half hour.
Updated now. Email me with any additional fixes that need to be made
Charles the Moderator: The first sentence following equation 3 still has a broken figure which should be a figure of merit–the alpha divided by epsilon. And the first paragraph above heading #6, conclusions, is mangled a bit–has some e’s in place of epsilon and a wrong partial derivative. I sent a revised file about 11:00 PDT. Could you just use the last paragraph of section #5 instead?
Thanks for your help.
Equation 1 is missing, and eqs 2 & 3 are unreadable
Actually Eq. 1 is there, it is written as text. I have submitted a new version to CTM which should fix the broken text. He also indicated he could repair soon. I am sorry.
Actually, the problem continues every time a term from the equations is mentioned throughout.
I am using Chrome on Windows 7
Earth may be a solar collector but it also recycles energy internally via atmospheric convective overturning.
You cannot simply ignore that and expect to have a useful model.
I am glad you have commented. The control surface for this model is top of atmosphere and therefore is independent of any specific processes within the atmosphere. However, some time back, on the post that Dr. Spencer made about his model, I answered a comment of yours that may not have seen. It explains why KE is negligible in comparison to changes in temperature, and that PE is already handled in lapse rates.
In general temperature in the atmosphere is dictated entirely by the First Law of Thermodynamics
changes in internal energy (temperature) = Heat in – Work out. Work includes KE and PE already.
Also the oceans are a huge storage module. The cycle of warm water, evaporation, thermal up draft moves heat into the upper atmosphere without intervention of the surface.
Since I agree with Stephen I will post this here. I just did this equation for Mars and found an emissivity of 0.997. Mars’ atmosphere contains a CO2 partial pressure of 575 pascals compared to Earth’s 40 pascals. The back radiation hypothesis claims CO2 is responsible for ~25% of the Earth’s “greenhouse” effect.
By claiming that the temperature of the Earth’s atmosphere at the surface has nothing to do with the mass of the gas itself is basically saying the 3.8X10^18 kg of nitrogen gas has no effect on temperature. Does this gas have mass? Does it have kinetic energy? Is the temperature of the atmosphere not literally a direct function of the average kinetic energy within the gas itself and does this kinetic energy not also transfer to surface?
http://www.feynmanlectures.caltech.edu/I_39.html
“The mean molecular kinetic energy is a property only of the “temperature.” Being a property of the “temperature,” and not of the gas, we can use it as a definition of the temperature. The mean kinetic energy of a molecule is thus some function of the temperature. But who is to tell us what scale to use for the temperature? We may arbitrarily define the scale of temperature so that the mean energy is linearly proportional to the temperature. The best way to do it would be to call the mean energy itself “the temperature.” That would be the simplest possible function. Unfortunately, the scale of temperature has been chosen differently, so instead of calling it temperature directly we use a constant conversion factor between the energy of a molecule and a degree of absolute temperature called a degree Kelvin. The constant of proportionality is k=1.38×10−23 joule for every degree Kelvin”
“ The mean kinetic energy of a molecule is thus some function of the temperature”.
May be, more correct is to say: “The temperature of a gas is a function of kinetic energy of its molecules according to Boltzmann’s equation E = mv^2/2 = 3kT/2”. With regard to the topic under discussion, it is crucial that this relationship is true for any gas, regardless of whether its molecules absorb IR radiation or not.
Heat energy reflected by earth and water is converted into kinetic energy. increasing the speed of gas molecules and. accordingly, raising the temperature of the gas. In other words, the “greenhouse effect” is created by the entire atmosphere, not “greenhouse gases”.
I agree with the generality that the the “greenhouse effect” is created by the entire atmosphere, not “greenhouse gases”, but there are some misunderstandings in your post that these people promoting the back radiative hypothesis keep making.
Heat radiated by Earth is not converted to kinetic energy, at least not anywhere near a 1:1 conversion. That’s one major and important misunderstanding they keep making. This misunderstanding seems to arise from not knowing that gases behave entirely different than solids and liquids when it comes to absorbing and emitting IR.
Photons have momentum just like the gas molecules they are interacting with. A photon being absorbed by a molecule which have opposite directions relative to each other actually decreases the kinetic energy of the molecule. This is unequivocally proven with laser cooling. This is also suggested by doppler broadening of the absorption and emission bands from gases because the absorption band is broadened evenly both above and below the peak band – collisions with other molecules both increases and decreases the energy level of the photon needed to interact with the internal modes of the molecule – in other words the “radiatively active” (GHGs) gases both donate energy and receive energy from “non-radiatively active” gases whereas the proponents of the BR theory often erroneously claim that the GHGs are only donating their “extra” energy to the non-GHGs.
The pseudoscience promoted by the BR theory is mind boggling that it is amazing so many people believe it. It’s quite simple, the temperature you feel on the surface of your skin right now is due to the average kinetic energy of the gas around you, it has nearly nothing to do with the IR emissions from that gas. If the air is cold, it takes energy away from you on collision, if the gas is hot it transfers energy to your skin and you need evaporative cooling to keep you from heatstroke. The surface of the Earth is no different than your skin, the kinetic energy retained within the atmosphere is also transferred to the surface if the gas is warmer, which it often is.
There is wisdom in the old saying “Test all things, hold fast to that which is good.”
Under Item 1, Equation 2 in the above article is this statement: “Since the final term representing rate of new storage in Equation 1 is quite small compared to the others, we can ignore it for our purposes.”
I have to question that.
For example, some portion (what percentage? I don’t know) of solar input energy is converted into biomass and thus is removed from thermal balance calculations. I believe a massive number of terrajoules has been bound up over the ages in the chemical bonds in shale and coal and oil and natural gas deposits. (And note this is separate from a dT/dt-based energy storage term).
And unless we have a very good idea of both the total rate of change of energy stored in Earth’s oceans and that involved in net global ice/water phase changes, I would ignore these massive energy sources/sinks only in interest seeing the mathematical exercise run to completion.
Earth is warming at a rate of about 1C per century. This amounts to new energy in storage that is truly minuscule compared to 952 W/m^2 of net solar isolation. I thus neglect it.
Well, not to be too sarcastic, but does it not then follow that we don’t need to concern ourselves over the truly minuscule amount of global warming energy associated with IPCC projection range of global warming (known to be erroneously high) of 1-3 C per century?
In turn, shouldn’t we then worry even less about that contribution portion of dT/dt that may be due d(CO2)/dt contributing to a change in total greenhouse effect?
And I don’t understand your reference to “952 W/m^2 of net solar insolation”. Following Trenberth, et.al, I thought the surface area and time averaged solar input was about 341 W/m^2 at TOA and only about 161 W/m^2 net absorbed at Earth surface.
It’s more than just GHG’s that lower the effective emissivity, clouds make an even larger contribution. The inverse of the effective emissivity, 1/0.61 = 1.64 represents the surface power gain of the system, where each W/m^2 of solar input produces 1.64 W/m^3 of surface emissions. The extra 640 mw/m^2 per W/m^2 of forcing represents the net result after all ‘feedback’, positive, negative, known and unknown have had their complete influence on the incident solar forcing. Since Joules are Joules, there’s no reason to believe that the next W/m^2 will not contribute the same 1.64 W/m^2 which results in a sensitivity factor less than the IPCC’s lower limit.
I’m starting to lean towards an effective emissivity of 1/g, or about 0.618, where g is the golden ratio of 1.618. I can’t prove it for the climate system yet, but the golden ratio frequently appears in the steady state solutions of chaotically self organized systems which certainly describes the climate system. This ratio and others derived from it often show up quantifying the values of the free variables whose variability manifests the chaos when equilibrium is possible for arbitrary values of those variables. For the climate, the free variables are the fractions of surface emissions absorbed by the atmospheric GHG’s and clouds, both of which are co-dependent on each other, yet both tend to converge to relatively constant values resulting in an even more constant effective emissivity. The effective emissivity is among the most tightly regulated ratio I’ve seen in any of the data and even monthly averages for each 2.5 degree slice of latitude from pole to pole converge to within about 20% of this ratio, while longer term averages converge to within a few percent. Considering that the stated ECS has about +/- 50% uncertainty and is considered ‘robust’, the extraordinarily tight regulation of the effective emissivity must be even more robust. Unfortunately, the consequences of acknowledging this tightly regulated metric is that it unavoidably leads to an ECS that’s lower than the lower limit currently considered by the IPCC/UNFCCC, thus falsifying their reason to exist.
This is 95% a political battle. Facts don’t matter to leftists. Facts sure won’t persuade any leftist. I’m certain that 99% +/- 1% of Leftist Climate Alarminists have insuffucient aptitude to understand (or care about) the actual science…about anything.
If the facts about the lack of increase in severe weather events could get out to the public, they might decide that they don’t want to pay 10’s of $Thousnds annually forever to keep events that are not increasing from increasing.
I’ve been informing everyone I can about severe weather statistics. Many are incredulous until I show them charts from NOAA and the IPCC confirming it. Most are convinced…though.some remain DENIERS.
Nobody I’m acquainted with.is living like or behaving like they believe that our civilization is threatened by Climate Change…let alone that our very existence is threatened. Almost nobody really believes it.
So, either the globe will start cooling soon(ish) or increasing taxes and energy expenses will intercede to smother this hoax.
co2isnotevil
Emissivity Feedback F ≠ 1/ε
(except when 1 + ε = 1.618, the Golden Mean)
Otherwise F = ε/(1-ε).
(This is the GHG amplification Factor.)
Derivation?
Feedback from infinite recirculation:
1) F = ε + ε^2 + ε^3 +…+ ε^∞
2) Fε = ε^2 + ε^3 +…+ ε^∞
1) – 2) => F(1-ε) = ε
∴ F = ε/(1-ε
blueice2hotsea,
The feedback amplifier gain equation is 1/Go = 1/g + f, where Go is the open loop gain (Go=1 for the zero feedback ideal BB), g is the closed loop gain and f is the fraction of the output returned to the input. For the equivalent gray body model, 1/ε = g is valid for all values of ε between 0 and 1 and all of the corresponding values of g. Solving for f = 1 – 1/g = 1 – ε. It just happens that the closed loop gain, g, is within the margin of error of being the golden ratio such that g = 1 + 1/g.
Your equation for F seems to calculate the closed loop gain, but only gets the correct value when the closed loop gain is the golden ratio and the open loop gain is assumed to be 1. Why do you think your equation is calculating a metric of feedback? It seems that you’re calculating the LWIR gain along the path from space to the surface while ε = 1/g characterizes the LWIR attenuation along the path from the surface to space. That being the case, the only value of g that will work where the input path gain is equal to the output path gain which is only the case when g is the golden ratio.
I went down this path once before looking for proof that the only effective emissivity that will work is when ε = 1/g and g is the golden ratio. The glitch I ran into is that the emissivity is not necessarily the same between the surface and space and between space and the surface since the incident energy and emitted energy are different wavelengths and absorbed differently by the atmosphere. It is interesting though that it does work out …
co2isnotevil
Whoops! You are using a 0-d model. Sorry I overlooked that. The effective surface emissivity looking skyward is different from a 1-d model emissivity looking downward and the gain formula is different as well.
BTW. If my analysis is OK, when the atmosphere is compressed down to the surface in a 0-d, it somewhat under-estimates the gain. Depending upon where and how the 3.7 Wm^-2 from 2xCO2 is introduced into the model also makes a difference.
Finally, I have no interest in the Golden Mean other than you find it interesting. Good luck.
blueice2hotsea,
The more I look at how and why the golden ratio seems to be emerging, the more interesting it gets, especially since it seems to be the most tightly regulated relationship among all climate variables.
Your 0-d characterization is misleading. The model is describing the relative behavior of the radiant energy density at the boundaries of the atmosphere as constrained by COE. This model can characterize the climate absolutely as,
Pi(t) = Po(t) + dE(t)/dt
where Pi is post albedo incident energy, Po is the power emitted by the planet, E is the energy density as stored by the planet manifesting its temperature and dE/dt is what’s usually referred to as the ‘forcing’ and like Pi and Po has the units of W/m^2 since E has units of Joules/m^2. The steady state is defined when the average dE/dt is zero.
T is linear to E and Po is demonstrably linear to Ps (surface emissions), thus Po is linearly proportional to (E)^.25 and not E as the pedantic climate model assumes.
Yes, I know the downward path is different, and that’s my point. Whatever it is, the chaos seems to be driving the net result so that it’s average effect is equivalent to when the LWIR emissivity of the atmosphere looking down is the same as the LWIR emissivity looking up while all the incident energy is in the same LWIR bands as are being emitted by the Earth.
As best as I can tell, 3.7 W/m^2 is the incremental absorption of surface emissions by the standard atmosphere including clouds upon instantly doubling CO2. So technically, it’s the instantaneous difference at TOT/TOA upon some change and is incorrectly claimed to be equivalent to an instantaneous post albedo increase in solar energy of the same 3.7 W/m^2. The difference being that all of the solar energy warms the surface, while about half of what the atmosphere absorbs does not and gets emitted into space.
Where does the energy storage by water’s latent heat come into this analysis? It has to be much greater the GHG effect, surely
A good point John.
The latent Heat is trapped in the vapor which rises up through the atmosphere and to space for dissipation due to its molecular buoyancy. A wonderful cooling mechanism for the planet.
Everywhere you look here on earth you see water being used to cool things. The Mosoons cool India, we all sweat to keep cool, the oceans rarely get above 35C; but deserts get a lot hotter, water cools our engines. I could go on.
The scientists appear oblivious of these things. Pesky GHE doesn’t get a look in.
For every kilogram of water evaporated some 680 WattHrs gets pumped up into the atmosphere and beyond for dissipation and returns to earth without that energy.
True, but on the other hand, the latent heat can be stored in the atmosphere, then returned to Earth as rain. Or the cloud temperature isolates the lower atmosphere from a convection of heat upwards.
I have benn outside in winter with lots of clouds overhead and felt quite warm. I have never been outside in winter with clear skies and felt a toasty radiation from CO2.
Where is water in all this so-called analysis?
Please can you expand on the conclusions for those of us who do not intend to follow the calculations in detail. Apart from confirming that greenhouse gases are essential to achieve energy balance, what are the implications of your conclusions, 2nd paragraph concerning feedbacks?
You have captured the essential point I had intended to convey. Since energy balance alone can tell us nothing about the details of feedbacks, I can do nothing quantitative. However, you will note that the sensitivity of temperature to effective emissivity is -1.2C or so for each 0.01 increase. As adding CO2 and H2O to the atmosphere in their present amounts has decreased the effective emissivity from 0.96 to 0.61 or thereabouts, we ought to believe adding more will decrease emissivity further and warm the planet. I don’t consider this a bad thing.
A point lost on many is there may not be any real classical feedbacks, there is nothing to say there has to be it’s just classical physics mumbo jumbo on a quantum problem.
So if you look at a lab based experiment with photothermal catalysis for example
https://phys.org/news/2019-06-absorber-photothermal-catalysis.html
So that is generating 288°C with weak solar irradiation .. now try and work out what limits the temperature 🙂
If I am allowed to put quantum squeezing on the light I am pretty sure we could at least double that, the challenge is can you do the squeezing with a meta-material for next to zero power.
None of your classical laws can describe what is happening because they are not applicable.
Kevin ==> The total energy of the Earth system is not the same as Tmean.
What happens to the Energy In is wickedly complex and it most certainly is not simplistically converted to surface temperature (mean or otherwise).
There is no question that the Earth System has been retaining energy (forever?) and that “energy in minus energy out = retained energy” — but what it does not equal is temperature.
My control surface is at the top of atmosphere. Whatever goes on inside the atmosphere or oceans or cryosphere has no pertinence whatsoever to the energy balance as I have written it.
No, your control surface is not at the top of the atmosphere. It is just 2 m above the surface .
What makes you insist you know where it is?
You say “Finally, we will use a temperature of 288K for the mean surface, recognizing that this temperature is of the atmosphere at about two meters above the surface. ”
Your computations were using 288K in Equation 3, which tells us that you were not on the top of the atmosphere and were not blinded to events happening on the surface.
You simply cannot have it both ways.
Chris B ‘s comment destroys your whole argument. Emissivity as defined in the literature doesn’t change. It is a constant for each materiel. Also see my comment regarding testing for back radiation.
Kip – “what it does not equal is temperature” – may be correct in the short term, because Earth’s is such a noisy system, but in the long term surely it does relate to the ocean temperature?
Yes, this issue comes up every time. It’s essentially question begging to use the equation in this way.
What numbers do you need to pick to win the lottery? Well, start by assuming that the lottery numbers are 11, 20, 30, 45, 60 and 65. Then pick those numbers.
Easy.
Sorry this was supposed to go as reply to Kip Hansen’s comment:
“What happens to the Energy In is wickedly complex and it most certainly is not simplistically converted to surface temperature (mean or otherwise).”
Hi Kip,
I am curious to know where you think the stored energy might be hiding if it is not in the
temperature. At the end of the day the temperature is just a measure of the average kinetic
energy of the molecules and the second law of thermodynamics says that an increase in temperature
is going to be the state with the highest entropy.
Alternatives might be in directed ocean currents or winds but there is no evidence that such flows are
getting stronger. Unless you want to violate the second law of thermodynamics then the stored energy is going to end up as random motion of atoms and molecules i.e. temperature.
We can also be fairly certain that the earth has not been retaining energy forever. Clearly if it had been doing so
for any considerable length of time then the temperature of the earth would be far in excess of what it currently is.
Also the existence of ice ages 10 thousand years ago would also strongly imply that there was less stored energy then
so at most the earth has been retaining energy for the last 10 thousand years.
Simply looking at temperatures will not reliably allow you to estimate energy. Especially not when there is water involved.
The answer to your question is potential energy. A bucket of water at sea level has less potential energy as the same bucket 2 kilometers up, regardless of the temperature. That potential energy comes from somewhere.
Similarly, water at 0 degrees C has more energy than ice at 0 degrees, and the energy to drive that change in 1kg is greater than that required to change the temperature by almost 80 degrees.
Fossil fuels are simply solar energy energy retained in the form of chemical potential energy.
Beeze,
Potential energy is not the answer. Where in the earth are there large and increasing
amounts of potential energy? Kip is claiming that the earth is retaining energy but that
energy is not going into random thermal motion of the atmosphere and oceans.
Photosensythesis is at most 2% efficient and the amount of plants has not been increasing
significantly so we can discount that as a substantial store of energy. So if you want to
claim that the earth is storing solar energy as potential energy you need to saw where and how.
Izak: “Photosensythesis is at most 2% efficient and the amount of plants has not been increasing
significantly so we can discount that as a substantial store of energy.”
Huh? It is pretty widely accepted that the green area on Earth as gone up somewhere between 10% and 13% since 1980, based on satellite photos.
Since it is mostly plants that contribute to the green area on the Earth that is a pretty significant increase!
Izaak, you commented: “Unless you want to violate the second law of thermodynamics then the stored energy is going to end up as random motion of atoms and molecules i.e. temperature.”
This is incorrect. Compare the stored internal energy of 1 kg of steam at 100 C at a given pressure to the stored internal energy of 1 kg of liquid water at 100 C at the same pressure. Same temperature, huge energy difference . . . no violation of any thermodynamic law.
Gordon,
The second law of thermodynamics says that entropy increases. So it is to do with the
time evolution of systems not how much stored energy they have at a particular time.
Putting water under pressure and heating it up requires a lot of energy but it is not a
stable state. Leave it alone after some time and it will cool back down to room temperature which is the second law in action.
Energy can neither be created nor destroyed. This is the first law of thermodynamics. An isolated won’t cool down or change state.
The only reason your example will change is because thermal equilibrium is being sought, but thermal equilibrium is just one small aspect of energy equilibrium that entropy describes.
Entropy is not a function of time. Time is a function of entropy. A system where only temperature exists, where there are no flows to or from parts of a larger system not at equilibrium, and that is itself at equilibrium, would be timeless.
Fossil fuels are not in equilibrium with the surrounding rocks, and the ocean is not in equilibrium within itself or the atmosphere. A stick of dynamite may be at thermal equilibrium with its surroundings for a considerable time. Still best not to play fetch with it.
Izzak, in gedanken experiments like the ones in this thread, one has to be very careful in defining control volumes and cycle-states when discussing both the First and Second Laws of thermodynamics. My reference to the additional storage of internal energy resulting from a given mass quantity undergoing phase change (water steam going from quality = 0 to quality = 1) at constant temperature, says nothing about the change of entropy between those two states, nor does it need to. The point was to give a simple example where sensible temperature does not equal “stored” (i.e, internal) energy.
Your response about entropy references a (closed) system having a thermodynamic CYCLE wherein heat is exchanged with some undefined external source (“heating it up”, “it will cool back down”).
Beeze is correct: if the 1 kg of 100% quality steam that I referenced were to be “isolated” from everything else in this thought experiment (via a theoretical perfect insulator), it indeed would never cool down and this fact would not violate the Second Law of Thermodynamics.
By the way, the Second Law does permit a portion of a system within a control volume (one having no energy/momentum exchange across the boundary) to experience DECREASING entropy, just so long as over time the net entropy within the control volume is constant of increases. The most common example of this is a refrigerator operating on earth.
T cannot represent the surface temperature. It is the effective temperature of the Earth. Most of the thermal radiation to space comes from the atmosphere and the clouds.
My thought, also, is that T was rather arbitrarily chosen as 288K to calculate the emissivity in equation 3. To use the calculated e of 0.6 as a significant determinant of atmospheric physics, instead of the measured values mentioned (0.9-0.96), without at least a discussion of de/dT seems to be rather begging the question.
Thanks for presenting this very interesting approach.
Climate science as we need it.
Rgds
J.-P. D.
From me too
Solar energy in = Longwave (IR) energy out + energy being stored
Laymen’s question. What about evaporation? It takes energy to evaporate water. And, as a side effect, the process makes the surface temperature cool.
Latent energy is a form of stored energy, but over the long term the latent energy in the atmosphere, oceans and cryosphere is changing very much less than the flows of energy in and out. We can come very close to balancing energy without considering energy in storage by simply looking at solar insolation and thermal IR at the top of atmosphere.
That latent heat in storage (in water vapor) isn’t just sitting around. Much of it is accelerated upwards (very rapidly in storm cells) to altitudes high enough…where the atmospheric GHG’s help to radiate much of that energy out into space…bypassing most of the atmosphere’s GHG’s in the lower troposphere. There is a huge HEAT ESCAPE VALVE that involves this “stored heat”.
Storm cells are like worm holes for energy leaving the earth’s system.
Kevin K says, “We can come very close to balancing energy without considering energy in storage by simply looking at solar insolation and thermal IR at the top of atmosphere.”
Perhaps, yet one must understand all the factors that affect the residence time of said insolation to determine claimed balance. As 1000 times more energy is in the oceans separated from the atmosphere, then determining a radiatiative balance is difficult. There are only two ways to affect the total energy in a defined system (Defined system here is the atmosphere, land and oceans) in a radiative balance, either a change in input, or a change in the residence time of some aspect of the energy paths within the system – oceans atmosphere land.
The energy required to evaporate the water is released when that water condenses as rain/snow/frost. Zero-sum.
No, it isn’t zero sum at all.
Convection caries A LOT of this latent heat high into the atmosphere where (after condensation and freezing) a lot of the latent heat gets radiated into outer space unimpeded by most of the GHG’s in the lower atmosphere.
It matters a lot WHERE water is thawed and evaporated and WHERE it is condensed and frozen.
HUNDREDS of times more energy can be transported into space by way of this well-known transport mechanism in a region than CO2 can “trap” in that same region.
This. 👍🏼. Rainfall has increased by 1.5 Mm pa. Thus cooling has also increased.
Even IPCC admits that more heat is transported away from the surface by convection than by radiation.
“Unfortunately our simple model doesn‘t specify how e depends on pCO2; so we cannot calculate climate sensitivity from it.”
Does this mean we can not use it to answer the question: “How much warming to expect from a doubling of CO2 since pre-industrial times?” If so, do you endorse the IPCC’s estimates? Or, do you accept Moncton’s argument that you have to calculate the feedback factor by relating the amount of feedback to the totality of long wave energy impacting the atmospheric water vapor, which yields a much lower estimated warming than does the IPCC’s approach?
No the model is too simple for such a purpose. I produced it only to demonstrate that energy balance at the top of atmosphere, using measured values, cannot be made to work without a greenhouse effect. The estimates of how much the addition of CO2 impacts effective emissivity including all feedbacks and at steady state is, in my mind, an open question because the current estimates run over a very broad range, and more recently have been revised downward.
I am still digesting Lord Monckton’s posts in order to address them in the near future.
Unfortunately, you may have your work cut out for you; Lord Monckton’s treatment of his theory is rather impressionistic and extends over something like ten head posts on this site as well as one on Roy Spencer’s and a couple of YouTube videos. But I may be able to boil it down for you; morbid fascination compelled me to review it all.
Instead of the usual equilibrium feedback equation T = (F + b T)g, where T is temperature, F is forcing, b is feedback coefficient, and g is open-loop gain, Lord Monckton writes E = R + fE, where E = T, R = gF, and f= bg.
He then observes that the ratio of equilibrium surface temperature E to the value R it would have if there were no feedback exceeds unity only slightly: for the greenhouse-gas concentrations that prevailed in 1850, E/R = 287.5 K / 265 K, or 1.085. That is, feedback is responsible for less than 8% of the total temperature.
The temperature increase that doubling CO2 concentration would cause, he concludes from that observation, similarly can’t exceed what the increase would be without feedback by much more than 8%.
But alarmists think it should exceed the without-feedback increase by 225%, he says, because they’ve made the “grave error” of instead going by only that portion of the temperature that exceeds the emission temperature 255 K. Greenhouse gases have caused a 287.5 K – 255 K = 32.5 K increase, whereas without feedback the increase would have been only 265 K – 255 K = 10 K, implying to official climatology that dE/dR = 3.25.
Climatology has thus failed, he says, to recognize the basic control-systems tenet that the loop gain f multiplies the entire response value E, not just some portion of it. When you instead recognize that feedback operates on the entire E value, he says, then you see that official climatology’s E/R = 3.25 would imply a sizzling 861 K surface temperature. That our real-world observations contradict that implication demonstrates conclusively that climate sensitivity can’t be what they claim. Not to put too fine a point on it, his co-author built a “test rig” to demonstrate that in feedback circuits the feedback does indeed respond to the entire output.
Of course, many commenters have responded that climatology could indeed be applying f to the entire E value. It’s just that climatology may believe that the loop gain f is not a constant but rather a function f(E) of E. E as a function of R would then be nonlinear, so the function’s local slope dE/dR could be high even though its average slope E/R isn’t.
True, such commenters might say, if there had been no greenhouse gases at all when the sun was dimmer, then feedback at those low temperatures to the temperature increase resulting from its brightening could have occurred in, say, the form of albedo reduction: there could well have been, as Lord Monckton says, feedback to that portion of the temperature below the emission temperature. But climatology could nonetheless believe that such feedback at those low temperatures would be represented by f(E) values much lower than those for a warmer earth, where a lot more evaporation occurs. So E(R) could be quite nonlinear.
(As far as “test rigs” go, they might add, it would be a trivial matter to design one that exhibits nonlinear feedback.)
Lord Monckton replies variously to such arguments:
First, as the “end of the global-warming scam in a single slide” at https://wattsupwiththat.com/2018/08/15/climatologys-startling-error-of-physics-answers-to-comments/ demonstrates, E/R has changed only negligibly since 1850. Lord Monckton thinks this implies that E(R) can’t be very nonlinear.
Second, according to him “official climatology” has said that the system parameters are “near invariant.” So dE/dR can’t change much, because one such parameter is the “feedback fraction” f = 1 – R/E (known in some circles as loop gain, as distinguished from open-loop and closed-loop gain, which in this case are respectively unity and E/R).
Third, the IPCC’s definition of the relevant parameters in terms of “perturbations” shows the climatology has ignored below-emission-temperature feedback.
Fourth, the near-exponential Clausius-Clapeyron relationship of evaporation to temperature—and thus that relationship of with-feedback temperature E to without-feedback temperature R—is nearly linearized by the near-logarithmic relationship of forcing to greenhouse-gas concentration.
Of course, you’ll want to read him for yourself to decide whether I’ve represented his position fairly; I’m among those who don’t think much of his theory. But the foregoing should make your review faster.
Energy into the system is measured in joules. Energy out of the system needs to be measured in joules as well. Temperature is not a proxy for energy. Temperature is the average kinetic energy of the molecules in the subject being measured. Heat, i.e. thermal energy, is the kinetic energy of each molecule multiplied by the number of molecules in the subject being measured. Heat, i.e. thermal energy, is an extensive property, the change in temperature from heat being transferred to the subject at test is based on how many molecules are in the subject at test. If you can’t enumerate the number of molecules heat is being transferred to then you simply cannot calculate the temperature change from heat energy being introduced. Temperature is, therefore, not truly a reliable proxy for the black box system you have described.
I agree that we ought to be a bit careful about the difference between rates and quantities. But for the energy balance I am using rate values, and I am doing the same also for entropy balance. If I have mixed units anywhere, please point it out.
Temperature is a proxy for energy, internal energy more correctly, as changes in temperature, when multiplied by heat capacity, become changes in internal energy. This is basic engineering thermodynamics. Now there are some places, such as change of phase, where the connection between temperature and energy is muddled, but as I have explained in several places in this thread over the course from year to year the material held in various phases is nearly constant.
Temperature is not a proxy for energy … even when you multiply it by heat capacity.
For example, a 1 kg ball of steel at a temperature 1000 degrees placed next to another 1 kg ball of steel at 1000 degrees will not increase the temperature of the second ball, despite doubling the energy. Even if the first ball ways 100 tonnes at 1000 degrees, it still will not change the temperature of the second ball. You could change the heat capacity (size) of the first ball to infinity and the temperature of the second ball would still not change.
Conclusion: Temperature is not a proxy for energy so any conclusions based on this false premise are suspect. In fact, adding more energy will not increase the temperature at all unless the source of the new energy has a higher temperature than that of the receiving body.
Are you confusing heat capacity with specific heat? It seems you are. Heat capacity is specific heat times mass. Specific heat is an intensive property; heat capacity is extensive. Please refer to chapter 4 in Zemansky.
Temperature cannot be a proxy for thermal energy, not unless you know the corresponding heat capacity and mass functions for the media being measured. The heat capacity of the earth is not location or time independent.
Temperature is an intensive property. Thermal energy is an extensive property. Trying to use an average of an intensive property as a proxy for an extensive property is destined to be misleading if not incorrect.
This isn’t an issue of “rate” vs “absolute”.
One obtains an extensive property from and intensive one by scaling using an appropriate quantity of material or volume or something of this nature. Heat capacity is just such a scaling factor. As long as this factor is constant temperature is a perfectly good proxy for internal energy.
The heat capacity of the thermal system known as Earth is not constant either in location or time. Thus temperature is simply not a good proxy for internal energy. Especially for a system as large and as varied as the Earth.
You are basically trying to make the same argument as those who say that the average temperature of the earth, as measured by numerous devices at numerous locations at various times, is representative of something because of the theory of large numbers. It’s a false argument. It always will be.
Please refer to the comment I made to Mr. Lodge’s comment just above yours.
Kevin – re “solar irradiance [is] the only driver of the climate system (CO2 and water vapor feedbacks are internal to the system)“: GCRs are external to the system, and it is known that they have an effect. You would probably think of them as no more than a disturbance, since they are not a direct w/m2, but it does seem that in the long term they are a very real driver of the system.
But in terms of the energy balance they, themselves, have negligible effect whatever effect they may have on energy flows within the atmosphere.
How does this compare to Dr Miskolczi’s empirical work?
CO2 cannot do any more heating since it is saturated already?
https://rclutz.wordpress.com/2017/05/17/the-curious-case-of-dr-miskolczi/
Dr Miskolczi did no empirical work.
He says he did.
Then what was it?
ClimateTruth: You used empirical data, rather than models, to arrive at your conclusion. How was that done?
Dr. Miskolczi: The computations are relatively simple. I collected a large number of radiosonde observations from around the globe and computed the global average infrared absorption. I performed these computations using observations from two large, publicly available datasets known as the TIGR2 and NOAA. The computations involved the processing of 300 radiosonde observations, using a state-of-the-art, line-by-line radiative transfer code. In both datasets, the global average infrared optical thickness turned out to be 1.87, agreeing with theoretical expectations.
That is amusing … you get its a standard quantum absorption and heat doesn’t exist in quantum mechanics it doesn’t even know what that is. So please explain what the heat which is just molecule speeds has to do with anything. Things in the quantum processes aren’t hot or cold, you can call them both or neither but not one. Things in the quantum domain only become evident as heat as a result of there own interaction they really don’t give a rats about standard classical laws.
So try putting your saturation on this kids experiment, we fire a red laser beam thru a red ballon and pop the blue ballon inside it. The laser is cold to the red ballon it is hot to the blue ballon that is how QM rolls. Come up with a classical physics formula that describes the behaviour of the heat 🙂
“using 288K for the mean surface temperature serves our purposes just fine”
What I don’t get is why it wouldn’t be just as fine to specify a distance of two meters below the ocean surface as the place to measure earth’s so called “surface temperature.” If one did this I don’t think 288K would be at all accurate as the earth’s surface temperature, yet it would seem even more justifiable. Data for two meters above the terrestrial surface is just what we happen to have. I prefer to argue that even simple models are too difficult to be of use.
Also, I wonder how things would change if one could increase and decrease the amount of gas in the atmosphere. I don’t see this kind of variable in any of the terms of the equations presented. This kind of analysis is really just meant for idealized bodies with no attached gaseous atmosphere or liquid ocean to confound measurement. Remember that the newest claim of the alarmists is that missing heat lurks in oceans and shifts around in mysterious ways.
Two meters below the ocean surface cannot radiate in the infrared to the atmosphere, any more than two meters below the soil or two meters below the ice could. The condensed materials transparent to infrared are specialty materials like germanium, zinc selenide, or silicon.
What I am pondering is all the radiant energy coming from the sun that’s not stopped by land. I would guess the majority of all radiation from the sun winds up being stopped in the top 100 or so feet of the ocean…. since the earth is 71 percent ocean that means a majority. To me the surface that the sunlight actually hits and warms up is the surface temperature we should be looking at. Sure, air temp 2 meters above ground is close, but I still think water temperatures should be in the mix. If the 288K number comes from averaged and weighted thermometer readings at weather stations, I feel something is being missed.
Has anyone done the experiment comparing air temperature over pavement versus over lawn? The UHI seems to be acknowledged as a real phenomenon. If it is real wouldn’t it be a demonstration of the shortcomings of air temperature as a proxy for surface temperature?
Ok …. I’ve been meaning to write this response for a while, in response to the other post.
I view the earth system a little differently than all these radiative models …. I think … or maybe not. Everyone wants to treat the earth as a black body radiator, but to me the earth acts more like a capacitor. Energy coming into the system stored, and released chaotically. Solar energy is stored in lots of things, some longer term some short term. The ocean and biomass would tend to be longer term storage items, whereas, solid surfaces and gases are shorter term storage items. Given the fact that the global temperature follows the SST almost to a tee, I exclude everything but the ocean and atmosphere.
So …. playing around with a Stefan-Boltzmann calculator, using an average SST of 17C, e of .9, we find that the ocean itself contributes approximately 360W/m2 to the atmosphere at the surface. The atmosphere itself absorbers approximately 78W/m2 directly, half of which is directed down, the other half goes back out to space. In the end, 360+39 = 399W/m2 at the near surface, which is awfully dang close to the 390W/m2 required for an atmospheric temperature of 288K that everyone always says the earth is, and these are just approximations.
I think the flaw in thinking is that none of the models account for the storage, movement, and release of energy in the system. The energy escaping the ocean today could have arrived at earth a thousand years ago given that only 50% of the solar energy hitting the ocean is absorbed in the top 10m (assuming it is only the top 10 meters that interacts meaningfully with the atmosphere). Also, given that LW rad has little to no impact on ocean heating, the contribution of the atmosphere to the surface layer temp is purely transient, as none of it gets absorbed by the ocean.
The storage capacity of the system is enormous and, as such, we r only going to observe small deviations over long periods of time. A little 2-3W/m2 change in the system is not a meaningful change in a system that is holding multiple orders of magnitude greater potential than the change. Further the stimulus leading the change has no set time table, thus it will be chaotic, as we see in the observations, and more likely driven by release of stored energy or absorption of energy from and into the ocean.
There is a reason that nobody has made a perfect model of the earths atmosphere. IMO, it’s because they are trying to make the earth behave as something it is not. You can’t model chaotic release of energy from the ocean. … and the sun shines 24/7, and the little changes from this or that are just not sufficient significantly impact the system.
Puts my foil hat on and braces for the onslaught. 🙂
That’s a lot of simplification, assumption, and averaging to find an unknown. It’s also based on a snapshot in time though the planet’s oceans contain most of the heat on the planet and distribute it unevenly through time. If it were that simple then the ECS to CO2 would have been found a long time ago rather than continuing to prove too high.
I’m not certain that there is an ECS for CO2. Frankly, nobody has proven there is outside of theoretical mathematical calculations based on black body info.
I tend to believe the data, …. there has never been a set of data that shows the earths temperature responding to changes in CO2. As such, if there is an ECS, it’s so dang small that it gets swamped by the rest of the internal system.
Like I said, the earths temperature ALWAYS follows the SST … not so much for CO2.
Kevin:Application of the First Law requires careful specification of a system or control volume.Could you please supply this missing information to allow a proper discussion of your model.Thanks.
Thank you. The control surface (enclosing the control volume) is that shell enclosing the Earth at or above the top of atmosphere. This enables me to use a simple balance of incoming solar radiation and outgoing longwave radiation to form an energy balance, because no other energy transfers are present.
You should also account for out-going SW: ie reflection.
Making albedo a fixed know constant seems to skew section 3 in that is makes determining the balance uniquely a case of choosing emissivity. It seems what you are arguing is more about determining the “value of merit” of the outer layer, not just emmisivity.
Simple but interesting, anyway.
Thank you Kevin, to be honest I don’t understand the Maths, but I found
it to be a interesting read.
MJE VK5ELL
As I have written before, please look at empirical data for some facts before creating hypotheses.
Satellite lower troposphere temperature data for the past 40 years is freely available from Dr Roy Spencer’s UAH data site. Atmospheric CO2 concentration data is available from hundreds of sites across the Globe. Mathematical analysis of some of this data has shown that the temperature is independent of the CO2 concentration, that is, there is no such thing as a “climate sensitivity” involving CO2. It is a meaningless metric.
Further, the data analysis shows that climate determines the rate of generation of atmospheric CO2. The Fourier spectra of both the temperature and the rate of change of CO2 are essentially identical, showing periodicities that are most likely related to the interaction of the bodies within the Solar System. Even the synodic period of the Moon is evident in the spectra. Input from an astronomer in identifying the sources of the spectral maxima would be greatly appreciated. There is nothing to show an anthropogenic effect.
For detail see https://www.climateauditor.com
Bevan Dockery
As far as I can remember, Mr Kilty’s guest post has to do with surface temperatures.
While there is (surprisingly good) agreement between land temperature anomaly trends and those of the lower troposphere above land (UAH6.0 LT: 0.18 °C /decade; GHCN daily: 0.19; GISS land: 0.22), this is not valid for a comparison of sea surfaces with the LT above.
I don’t have HadSST3, ERSST5 or JMA’s COBE-SST2 at hand right now (UTC+2), but this should easily be verifiable.
Moreover, everybody knows that the oceans store astronomic quantities of both heat and CO2, and release them asynchronously at own will. The residuals contained in the atmosphere at a given moment give no hint on anything.
Bindidon
May I suggest that Mr Kilty’s “6. Conclusion” may be reworded as :
“The simple model of Earth as a solar collector shows conclusively that the atmosphere lowers the effective emissivity of the Earth, …..”
In other words, the old fashioned Universal Gas Laws explain the Earth’s surface temperature.
We know full well that there is plenty of energy stored in the atmosphere. Presumably this is the factor “+ C.dT/dt” in the initial equation so there is no reason to invoke a greenhouse effect from CO2.
Thanks Kevin
proving once again that engineers who have to build things that work, understand that there is a green house effect
Steven, who doubts a green house effect exists? Practically every post has comments talking about various aspects of the over-powering GH-effect of H2O, and its variability.
CO2 is a GHG, it has a very low secular concentration and about 4% of the CO2 is from anthropogenic sources, but its NET effect is frankly going to be almost insignificant (as is the case because the real thermal and WX changes we notice come from El-Ninos that are related to ocean heat content, and its distribution with time). Those claiming CO2 is or will become catastrophic really need a 20mg of Valium dose 3 times a day, and to get an education and a sense of proportion.
“Steven, who doubts a green house effect exists?”
And
“……4% of the CO2 is from anthropogenic sources”
So, ~280ppm pre-industrial to ~410ppm now is 96% natural?
And only 4% anthro?
So how does that work, when ice-core data shows that it should take many thousands of years to change by that much.
Just a coincidence that since mankind started burning fossil in quantity it’s only taken ~130 years then?
What natural change in the biosphere is the cause, such that we have not noticed what this ~100x Delta from pre-industrial carbon cycle change from a natural (quasi) balance?
It is not the lack of acceptance of the GHE per se here (although there are some notable adherents) – it is that of the GHE of CO2 (+ WV feedback), and in this case the overall magnitude of the change in it’s atmospheric concentration that is denied here.
Please provide some evidence of your above claim (the 4%).
WUWT post: EPA document supports ~3% of atmospheric carbon dioxide is attributable to human sources – Anthony Watts / July 29, 2014
https://wattsupwiththat.com/2014/07/29/epa-document-supports-3-of-atmospheric-carbon-dioxide-is-attributable-to-human-sources/
Referencing this article:
THE HOCKEY SCHTICK : If you can’t explain the ‘pause’, you can’t explain the cause… Thursday, July 17, 2014 – New paper finds only ~3.75% of atmospheric CO2 is man-made from burning of fossil fuels
http://hockeyschtick.blogspot.com/2014/07/new-paper-finds-only-375-of-atmospheric.html
Citing this paper:
Simulating the integrated summertime Δ14CO2 signature from anthropogenic emissions over Western Europe – D. Bozhinova1, M. K. van der Molen1, I. R. van der Velde1, M. C. Krol1,2, S. van der Laan3, H. A. J. Meijer3, and W. Peters1
https://www.atmos-chem-phys.net/14/7273/2014/acp-14-7273-2014.html
I think you might be misunderstanding the ~4% figure. In fact this is exactly what we’d expect to happen and it is in no way inconsistent with the assertion that CO2 from fossil fuel burning is the reason for the increase in atmospheric CO2 since the 19th century.
Basically, as human produced CO2 increases in the atmosphere then more of it will be sequestered during the natural carbon cycle. This will be a lower proportion initially but eventually as the human proportion in the atmosphere approaches the human proportion of annual emissions the atmospheric concentration will stabilise at that proportion (i.e. around 4%).
Think about it. It does not mean that human CO2 is not the reason for the ~40% increase in he atmosphere since 1850. It just means that ‘some’ human CO2 rather than natural CO2 is being sequestered during the annual carbon cycle.
WXC – these references are of little use, the original claim was made on a misunderstanding of the data previously presented (possibly because of ambiguity in the review) and the retractions and subsequent corrections are, to be frank, a bit of an embarrassment.
Wxcycles:
Hi – I did not see your post re this ….
“WUWT post: EPA document supports ~3% of atmospheric carbon dioxide is attributable to human sources – Anthony Watts / July 29, 2014
https://wattsupwiththat.com/2014/07/29/epa-document-supports-3-of-atmospheric-carbon-dioxide-is-attributable-to-human-sources/”
However, I thought that might come up.
You need to read it more sceptically, at the very least more thoroughly…..
“UPDATE:
Anthony Watts July 29, 2014 at 7:42 am
Thanks to everyone who pointed out the difference in the chart and the issues.
I was offered this post by the author in WUWT Tips and Notes, here: http://wattsupwiththat.com/tips-and-notes/#comment-1696307 and reproduced below.
The chart refers to the annual increase in CO2, not the total amount. So it is misleading.
Since the original author had worked for the Tucson Citizen I made the mistake of assuming it was properly vetted.
The fault is mine for not checking further. But as “pokerguy” notes, it won’t disappear. Mistakes are just as valuable for learning. – Anthony Watts
wryheat2 says:
July 28, 2014 at 12:28 pm
Mr. Watts,
John Droz suggested I contact you.
On my blog, I commented on the reasearch by Denica Bozhinova on CO2 content due to fossil fuel burining. She apparently scared The Hockey Schtick into taking down his post on the matter. However, there is an older table from EIA which I reproduce on my post.
Denica Bozhinova has commented extensively, and frankly, I can’t understand her position since she seems to contradict what she wrote in the abstract to “Simulating the integrated summertime Ä14CO2 signature from anthropogenic emissions over Western Europe”
See my post here (you may reprint it if you wish):
http://wryheat.wordpress.com/2014/07/19/only-about-3-of-co2-in-atmosphere-due-to-burning-fossil-fuels/
Jonathan DuHamel
Tucson, AZ
So it turns out the “4%” is actually that of ANNUAL CO2 into the CC and not the TOTAL – which has now accumulated to greater than 40%.
So a fake fact re climate change science becomes a myth amongst (some anyway) adherents here, despite it’s prime source (NTZ and WUWT) quickly correcting the error.
So it turns out the “4%” is actually that of ANNUAL CO2 into the CC and not the TOTAL – which has now accumulated to greater than 40%.
—
Which is what I said below, so clearly I did understand it, and you’ve made no counterpoint except to smear what’s already accepted to be true, even by the US EPA.
PeterGB June 13, 2019 at 3:48 am
WXC – these references are of little use, the original claim was made on a misunderstanding of the data previously presented (possibly because of ambiguity in the review) and the retractions and subsequent corrections are, to be frank, a bit of an embarrassment.
>>
A <4% of total annual addition to CO2 being anthropogenic is generally accepted to be correct. If you think not you should clarify your view.
WXC: I agree with you that the annual anthropogenic contribution to atmospheric CO2 level is 4% or less, I hope my posting did not imply otherwise, if so I have phrased it poorly. My comment was to point out that the methodology utilised in the article/paper you referenced was wrong. I too had these originally bookmarked as important references, I applaud the authors for the speedy and thorough corrections at the time, but I have removed these items from my list.
I see that Anthony Barton has previously commented that (I was unaware of this additional doubt) Denica Bozhinova seems to have published self-contradictory work, but I can’t see myself having time to look deeply into that.
John Finn June 13, 2019 at 3:17 am
THE HOCKEY SCHTICK : If you can’t explain the ‘pause’, you can’t explain the cause… Thursday, July 17, 2014 – New paper finds only ~3.75% of atmospheric CO2 is man-made from burning of fossil fuels —
I think you might be misunderstanding the ~4% figure. In fact this is exactly what we’d expect to happen and it is in no way inconsistent with the assertion that CO2 from fossil fuel burning is the reason for the increase in atmospheric CO2 since the 19th century.
>>
I did not misunderstand it John, it is consistent with what I wrote.
“Which is what I said below, so clearly I did understand it, and you’ve made no counterpoint except to smear what’s already accepted to be true, even by the US EPA.”
I said in my OP …
“Please provide some evidence of your above claim (the 4%).”
In the context that plainly referred to total.
To which you responded with ..
“WUWT post: EPA document supports ~3% of atmospheric carbon dioxide is attributable to human sources – Anthony Watts / July 29, 2014”
Which was incorrect as Anthony said in an update below his OP.
(What does “below” mean) – I talk of your posts I responded – I didn’t see any other and addressed you by name on the ones responding to me.
Which clearly show that you did not “understand” the (3.75%) in that EPA report was an annual one … else why link to it when the post (without the correction) claimed was the total.
I note PeterGB also though the same as me.
The counterpoint I made is that you implied that anthro CO2 is only 4% of the total atmospheric concentration.
It is not.
It is 40+%.
Your doubling down on a post that said 4% (3.75) shows that you did indeed mean that.
Otherwise why do it, when the importance of it was that it was INCORRECT.
And quit with the “smear” ad hom.
The only “smear” here is the one you are trying to make of me.
And so, how about acknowledging that the 280 to 410ppm is indeed 40+% and of anthro origin?
WXcycles June 13, 2019 at 6:36 am
Ok – but are you misinterpreting the result. Just because the human produced CO2 is only 4% of total atmospheric CO2, it does’t mean that the human contribution to the increase since ~1850 is only 4%.
But perhaps you didn’t mean that either. If so -apologies.
The H20 GHG effect is significantly stronger than that of CO2. I survive a relative-humidity swing per annum of about 30% to 95% RH. Plus as much as a 50% swing within a single 24-hour period, with 35% swings in this powerful GHG, each day, being fairly typical. This daily change in H2O dwarfs the NET GHG effect of the entire rise in CO2 since 1850.
Yes, temperature varies considerably each day, and also each year, but it’s entirely manageable and not in the least bit ‘catastrophic’, sans the odd weather event’s extremes, every few decades. I open or close a window, I have a hot or cold shower, I turn on or else off a fan, have a salad for dinner, or else a curry, etc. Thus I struggle to see how an annual rise in CO2 since 130 years ago from 270ppm to 410, of which the annual rise in additional anthropogenic component is taken to be a bit less than 4%, is going to create a ‘crisis’ any time within the foreseeable future for humanity or wider biota.
Open or close a window, wear lighter clothing (I’m all in favor of girls in bikinis), but whatever method or action taken it’s manageable, and we will successfully adjust, with slight cultural and technological changes that are effectively imperceptible. Because an actual global climate-change trend is also imperceptible over the course of a human life, as it often takes several centuries to take place. And I’m yet to see any background climate-noise sequence or events that is out of the range of natural variation so I’m unimpressed by the EFFECT of the scale of the CO2 changes measured.
“The H20 GHG effect is significantly stronger than that of CO2.”
We know that.
Point is CO2 is non-condensing.
WV condnses and precipitates out.
Thus it’s atmospehric content (abs humidity) is dependent on temp – which s where CO2 comes in.
“swings in this powerful GHG, each day, being fairly typical”
That is irrelevant to the hydrological cycle on a planetary basis as above.
The GHE of CO” takes pce primarily in dry air (aloft and in the Arctic).
“This daily change in H2O dwarfs the NET GHG effect”
You don’t seem to understand the concept of “Global”, as in the whole atmosphere (wher of course natural variations in WV content take place).
” Thus I struggle to see how an annual rise in CO2 since 130 years ago from 270ppm to 410, of which the annual rise in additional anthropogenic component is taken to be a bit less than 4%, is going to create a ‘crisis’ any time within the foreseeable future for humanity or wider biota.”
Your appeal to your own incredulity is not a scintific basis to deny empirical scince.
WV is dependent on temp rise to increase its VP in the atmophere as it condenses out to keep atmospheric WV in quasi-equilibrium on a GLOBAl basis.
CO2 does not condense and it’s GHE effect can accumulate asits concentration rises.
“of which the annual rise in additional anthropogenic component is taken to be a bit less than 4%, is going to create a ‘crisis’ any time within the foreseeable future for humanity or wider biota.”
You’ve said it again without any reference to scientific substantiation,
Please?
Where has the additional ~40+% increase come from this last ~130 years if the anthro proportion is just 4%?
@ Anthony Banton – June 13, 2019 at 3:57 am
Rather than avoid the point, here’s the bit you need to take on board, but don’t seem inclined to.
“And I’m yet to see any background climate-noise sequence or events that is out of the range of natural variation so I’m unimpressed by the EFFECT of the scale of the CO2 changes measured.”
That’s the point, the EFFECT of this GLOBAL non-condescending GHG is next to nothing, even with am ~40% increase over 130 years.
It’s hard to get excited over approximately nothing.
Anthony Banton – June 13, 2019 at 3:57 am
” Thus I struggle to see how an annual rise in CO2 since 130 years ago from 270ppm to 410, of which the annual rise in additional anthropogenic component is taken to be a bit less than 4%, is going to create a ‘crisis’ any time within the foreseeable future for humanity or wider biota.”
Your appeal to your own incredulity is not a scintific basis to deny empirical scince.
>>
You mention “empirical science”, but fail to give any evidence to which I am apparently being accused of having somehow denied? It is evidence which is empirically gathered (not the discipline of Science itself), and I do not recall denying any evidence within my remarks, so I’ll have to discount your critique of my incredulity regarding NET CO2 GHG warming EFFECT, as garbled and mysterious.
You can clearly see from my text which you quoted that I am referring to a slightly <4% annual increase in CO2. And just to be clear, this conversely implies that more than 96% of the annual increase in CO2 doesn't come from Anthropogenic contributions.
Please don't choke on that Anthony.
Anthony
The 4% figure is about right. However, there is a great deal of misunderstanding over this issue.
The natural carbon cycle will, over time, ensure that the proportion of human produced CO2 in the atmosphere is the same as the proportion of annual human produced CO2 emissions. It’s not a mystery. It’s what we would expect to happen.
This doesn’t not mean that human CO2 is not responsible for the CO2 increase in the atmosphere. It just means that human produced CO2 is being sequestered rather than “natural” CO2 during the annual carbon cycle.
If you think about it – it makes complete sense. While there is very little ‘human’ CO2 in the atmosphere, a much bigger proportion of natural CO2 will be absorbed by the biosphere. As the proportion of ‘human’ CO2 increases a larger proportion of human CO2 is absorbed. Eventually the proportions will be exactly the same as the proportions of the annual emissions.
Joh- the natural carbon cycle is millions of years long, not an annual phenomenon.
What bothers me is that so much of the climate hype depends on such a small amount of data. We have maybe 100+ years of local air temperatures collected over land with, 40 years of satellite data, and 10 years or so of Argo data, for the most part, a resolution of barely .5°C. More extensive but much less precise paleo temperature records go back some 800,000 years, and geological evidence for several millions of years. All the data shows that the surface temperature has stayed within a range of about 15°C despite huge changes in climates through many glacial eras and many with nearly tropical temperatures.
Assuming that small changes in CO2 in the atmosphere are or will make major changes in the climate in the face of 100% contradictory evidence seems rather silly.
“Steven, who doubts a green house effect exists?”
plenty of skeptics. One was featured at WND today
skydragons.
and guys up thread.
read more
OK Steven, I’ll watch for it.
There are also plenty of actual climate scientists that believe in it but don’t actually understand it, so don’t feel bad. Given acceptance of QM runs at around 20% in scientists that is actually pretty amazing. Around 45% off scientists embarrassingly believe you just tack QM onto classical physics as if it is some addon laws.
So probably lets not be too hard on layman, they are in good company with actual scientists.
Thanks mr. Kilty for an enlightening article.
Your model differs from the Kiehl & Trenberth (K&T) “energy” budget (actually a radiation flux calculation) in that in your budget the sun hits half the earth correctly and radiation out from the whole earth at any time, but K&T averages the incoming radiation over the whole earth all the time, which is unphysical. Thus they must “invent” back radiation in order for the calculation to add up.
You find that there must be an atmospheric effect (aka greenhouse effect) on the emissivity due to IR active elements in the atmosphere, mainly H2O, for your calculation. CO2 is only 400 ppm in the air and only 4 % of this from burning fossil fuels, so the real trillion $ question is; how much influence does this small amount of emission have on global temperature?
Measurements indicates very low influence (ECS).
PetterT
“but K&T averages the incoming radiation over the whole earth all the time, which is unphysical. ”
Could you please exactly show me where this appears in their model?
Check out Dr. Spencer’s article here:
On the Flat Earth Rants of Joe Postma
http://www.drroyspencer.com/2019/06/on-the-flat-earth-rants-of-joe-postma/
“Joe’s claim (as far as I can tell) is that that the solar flux value (often quoted to be around 342 W/m2) is unrealistic because it is for a flat Earth. But as an astrophysicist, he should recognize the division by 4 (“Fs(1-A)/4” and “S/4”) in the upper-left portion of both figures, which takes the solar constant at the distance of the Earth from the sun (about 1,370 W/m2) and spreads it over the spherical shape of the Earth. Thus, the 342 W/m2 value represents a spherical (not flat) Earth. ”
I think Mr. Kilty’s model; energy in over 1/2 earth = energy out over 1/1 earth is correct.
Solar energy in = Longwave (IR) energy out + energy being stored 0/10
You ignored the huge contribution of natural variation. At its simplest level this is the way El Nino causes the temperature to rise. That isn’t in your equation and as such your model does not work.
“That isn’t in your equation and as such your model does not work.”
Balances out to zero.
As NV is moving heat around internal to the climate system, have once been absorbed from the Sun.
That “heat being moved around” can also impact how much LWIR is emitted from the Earth at any point in time as some of that heat becomes available for generating radiation. It’s why a simplistic “energy in = energy out + energy stored” equation is almost useless when it is based on a long term average temperature. It’s why a long term average “global” temperature is useless. It can’t tell you whether days are getting warmer, nights are getting warmer, or if there is a combination of both. Warmer days and warmer nights have a hugely different impact on the environment known as Earth. The long term average is only useful for supporting a political agenda, nothing else.
+ + +
The article states proof that GHG’s lower the effective emissivity of the Earth. Surely there is no proof? The atmosphere lowers the effective emissivity, GHG’s may contribute to the effect but I see no proof that they are the effect.
Assuming a flat Earth (Is*pi*r-squared?)
I don’t think antone denies a greenhouse effect, but it is water-vapour that is responsible. CO2 is piddling.
I might add that we really do lack the precision necessary to detect any CO2 impact.
Hey Kevin,
Nice intro! My understanding is that discussion around subject of feedback was not about mere existence of the greenhouse effect but rather about magnitudes such feedback mechanisms operate. Lord Monckton and his co-authors are convinced that ‘official’ climatology overstates climate sensitivities by misunderstanding how actually feedback works in the context of control theory. I reckon your text does not address directly this issue – but looking forward for next parts!
Kevin Kilty – Thanks for your replies above.
One assumption that all of your calculations are based on is analogous to this argument:
sine(0) = 0
sine(90) = 1
therefore the average sine for 0 thru 90 is .5
But that is wrong. And, since all of your subsequent calculations are built on this wrong assumption how can you claim correctness?
I hope you’ll apply your analytical skills to correct this initial faulty assumption. When attempting to project an evenly distributed two dimensional disk over a hemisphere of the same radius with a gradient result, the equations of area need to be employed and equations of hemisphere segment areas require the use of sine:
________________________________________________________________________________________
Thus the area north of a line of latitude is
A = 2*pi*R^2(1-sin(lat))
The area between two lines of latitude is the difference between the
area north of one latitude and the area north of the other latitude:
A = |2*pi*R^2(1-sin(lat2)) – 2*pi*R^2(1-sin(lat1))|
= 2*pi*R^2 |sin(lat1) – sin(lat2)|
Thomas Homer
“One assumption that all of your calculations are based on is analogous to this argument:
sine(0) = 0
sine(90) = 1
therefore the average sine for 0 thru 90 is .5”
I don’t think that Kevin Kilty does this indeed wrong assumption.
The average incident solar radiation over the sunlit hemisphere is computed by using the square of the cosine of the incident angle (0 ° at the Equator, 90 ° at the Pole).
Integrating cos²(x) from 0 to 1 gives exactly 0.5, and the total incident is therefore obtained by using
pi * R² * flux
Rgds
J.-P. D.
>>
Integrating cos²(x) from 0 to 1 gives exactly 0.5 . . . .
<<
Really? I don’t think that’s right. I’m long past doing integrals from scratch by myself–I use integral tables. On page 420 of the CRC Standard Mathematical Tables is integral number 302 that fits the bill:
If we set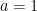 and
and 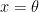 , we have our integral.
, we have our integral.
I’m not sure why you’re integrating from 0 to 1. You should be integrating along the horizontal axis. Instead of degrees, we need to use radians; therefor and
and  .
.
Now we can do the complete integrations:
Jim
Actually you’re right, but the integral should be from -pi/2 to +pi/2. That would give 1/4. But I don’t think cosine squared is the correct function.
Jim
Thanks
Sorry for butting in. I just saw a calculus question and without looking at the context thought I could save someone some time by whipping out the answer.
Obviously, your subsequent calculation below is correct. (Even more obviously, the usual approach dividing the projected circle’s area by the sphere’s area is easier.)
Opps, I left off the differentials. The two integrals should be:
and
Jim
OK
>>
The average incident solar radiation over the sunlit hemisphere is computed by using the square of the cosine of the incident angle (0 ° at the Equator, 90 ° at the Pole).
<<
I was thinking of this problem when I messed up the limits of my integration.
Say the Sun is directly over the Equator. Then the spot on the equator directly underneath the Sun has intensity I. As you move away from that spot or point, the intensity drops off. Here, we are talking about concentric rings centered on that central point. By the time you reach the terminator, the intensity is zero. The logical function describing that drop-off would be , where
, where 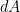 is a differential area that’s a function of
is a differential area that’s a function of 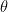 .
.
Now let’s solve for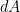 .
. 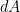 represents one of the concentric rings of infinitesimal width. The length of the ring is
represents one of the concentric rings of infinitesimal width. The length of the ring is  where
where  . We compute the width of the ring from the formula for the length of an arc:
. We compute the width of the ring from the formula for the length of an arc: 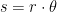 . Notice that when
. Notice that when 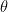 equals a full circle or
equals a full circle or  we have a circumference of a circle of radius
we have a circumference of a circle of radius  . The differential is:
. The differential is:  . Therefore the area of a ring is
. Therefore the area of a ring is  .
.
As a test, if we integrate from to
to  , we should get the area of a hemisphere.
, we should get the area of a hemisphere.
That seems to check, because is one-half the area of a sphere.
is one-half the area of a sphere.
It would be interesting to see what we’d get if we integrate the intensity of the entire hemisphere. That integral is:
If we divide by the area of a sphere, we get
It’s our old divide by 4 process that no one likes.
Jim
Hi Kevin,
You mentioned your reference surface as being TOA, but in equation 2 you used surface temperature. So your effective emissivity just becomes the ratio of OLR to upward surface flux, which is indeed around 0.6.
Your C.dT/dt is a very important number, in the long term it is what drives increased sea temperature, though its absolute instantaneous magnitude may seem small at less than 1 W /m2.
Cheers
“We now arrive at what seems like a paradox. All the materials making up the Earth‘s surface are very black at infrared wavelengths. Pavement, water, soil, plants, skin, snow and ice all have emissivity in the range of 0.9 to 0.96. Yet, energy balance reveals that the effective emissivity is one-third lower. This is a robust result.”
You have neglected the 6% Rayleigh scattering and the roughly 16% of solar shortwave that is absorbed by atmospheric water vapour.
This entire presentation constitutes an attempt to make an ill-founded paradigm fit a few known empirical facts. The standard of proof in rigorous science, however, is that no empirical facts contradict the theory. The clear contradiction here is the putative “effective emissivity” value of 0.61. But in the real world emissivity is almost always nearly equal to the coefficient of absorption, which is much higher in the IR range. As Kilty admits: “Pavement, water, soil, plants, skin, snow and ice all have emissivity in the range of 0.9 to 0.96. Yet, energy balance reveals that the effective emissivity is one-third lower. ”
Far from being “a robust result” readily explained by GHGs, as claimed, the stark discrepancy is due to the conflation of radiative intensity I of blackbody cavity radiation with actual transfer of heat Q by various known mechanisms, of which evaporation is the most important on Earth’s surface. Nowhere is this empirically established fact (see Bowen ratio from a plethora of careful experiments) adequately incorporated in Kilty’s “balance [of] energy,” who mentions entropy without ever dealing with enthalpy. In fact, the only reference to the thermodynamic Q found in this entire thread with well over a hundred comments is in the astute posting by Schroeder on June 12. Even little leaguers have better batting averages!
What exactly is the 30% albedo based upon? It cannot be 30% of the total incident solar irradiance, as around 49% of the solar heating effect is in the near infrared, of which lots will be absorbed rather than reflected.