Guest lampooning by David Middleton

Hat tip to Latitude…
Human activities are dissolving the seafloor
by the Office of Communications
Nov. 5, 2018 2:07 p.m.With increasing carbon dioxide from human activities, more acidic water is reaching the deep sea, dissolving some calcite-based sediments, say an international team of researchers.
The seafloor has always played a crucial role in controlling the degree of ocean acidification. When a burst of acidic water from a natural source such as a volcanic eruption reaches the ocean floor, it dissolves some of the strongly alkaline calcite like pouring cola over an antacid tablet. This neutralizes the acidity of the incoming waters and in the process, prevents seawater from becoming too acidic. It can also help regulate atmospheric carbon dioxide levels over centuries to millennia.
As a result of human activities, the level of carbon dioxide in the water is high enough that the rate of calcite (CaCO3) dissolution is climbing, say the researchers. Their findings appear this week in the journal Proceedings of the National Academy of Sciences.
[…]
Human activities are dissolving the seafloor…
Where’s that Billy Madison clip? Here it is…
If the seafloor was actually dissolving, it would certainly fix that whole sea level rise thingy.
The paper, Sulpis et al., 2018, is pay-walled and probably not worth $10. Here’s the abstract:
Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2
Abstract
Oceanic uptake of anthropogenic CO2 leads to decreased pH, carbonate ion concentration, and saturation state with respect to CaCO3 minerals, causing increased dissolution of these minerals at the deep seafloor. This additional dissolution will figure prominently in the neutralization of man-made CO2. However, there has been no concerted assessment of the current extent of anthropogenic CaCO3 dissolution at the deep seafloor. Here, recent databases of bottom-water chemistry, benthic currents, and CaCO3 content of deep-sea sediments are combined with a rate model to derive the global distribution of benthic calcite dissolution rates and obtain primary confirmation of an anthropogenic component. By comparing preindustrial with present-day rates, we determine that significant anthropogenic dissolution now occurs in the western North Atlantic, amounting to 40–100% of the total seafloor dissolution at its most intense locations. At these locations, the calcite compensation depth has risen ∼300 m. Increased benthic dissolution was also revealed at various hot spots in the southern extent of the Atlantic, Indian, and Pacific Oceans. Our findings place constraints on future predictions of ocean acidification, are consequential to the fate of benthic calcifiers, and indicate that a by-product of human activities is currently altering the geological record of the deep sea.
Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2
So… What kind of CO2 was dissolving CaCO3 at the seafloor before GM invented the Suburban? Because CaCO3 did dissolve and it did get redeposited as carbonate rocks way back when atmospheric CO2 was a lot higher than it is today.
The Austin Chalk is contemporaneous to the White Cliffs of Dover. It can be more than 1,200′ thick. It outcrops in the Dallas, Texas area. In South-Central to South East Texas, it is buried to depths >7,000′ below sea level and is a significant hydrocarbon source and reservoir rock. It was deposited at a time when atmospheric CO2 concentrations were 800 to 1,000 ppmv.


Lower Cretaceous CO2 levels ranged from 1,000 to 2,000 ppmv and the seafloor not only didn’t dissolve, but 1,000’s of feet of carbonate rocks were deposited in the in northern Gulf of Mexico basins…


However, there has been no concerted assessment of the current extent of anthropogenic CaCO3 dissolution at the deep seafloor.
That’s because there’s not much CaCO3 at the deep seafloor. The notion that CO2 partial pressure influences the pH of seawater isn’t a new concept. We even studied it in college way back in the Pleistocene (1976-1980); however the phrase “ocean acidification” never appeared in any of my college textbooks.
My Stratigraphy & Sedimentation (Spring Semester 1979) textbook, Principles of Sedimentology by Friedman (yes, that Friedman) and Sanders features an entire section on the relationship between CO2 and pH and how if affects calcium carbonate precipitation and dissolution…
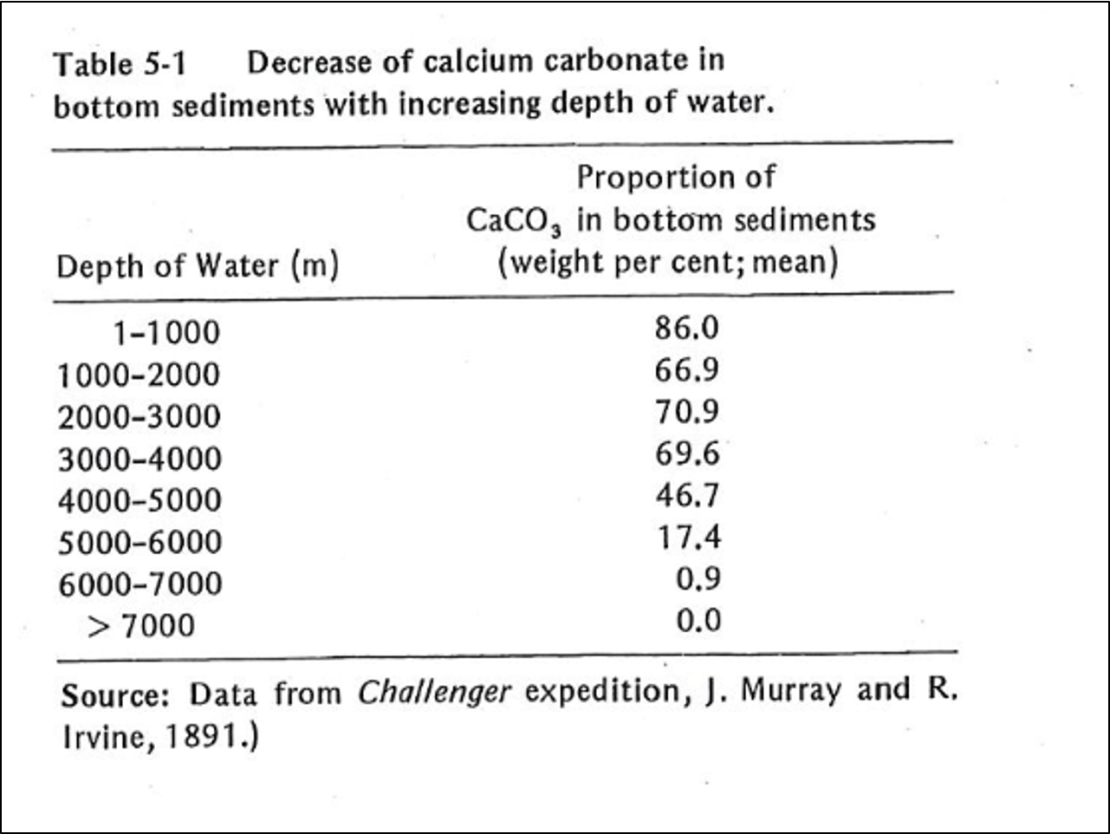
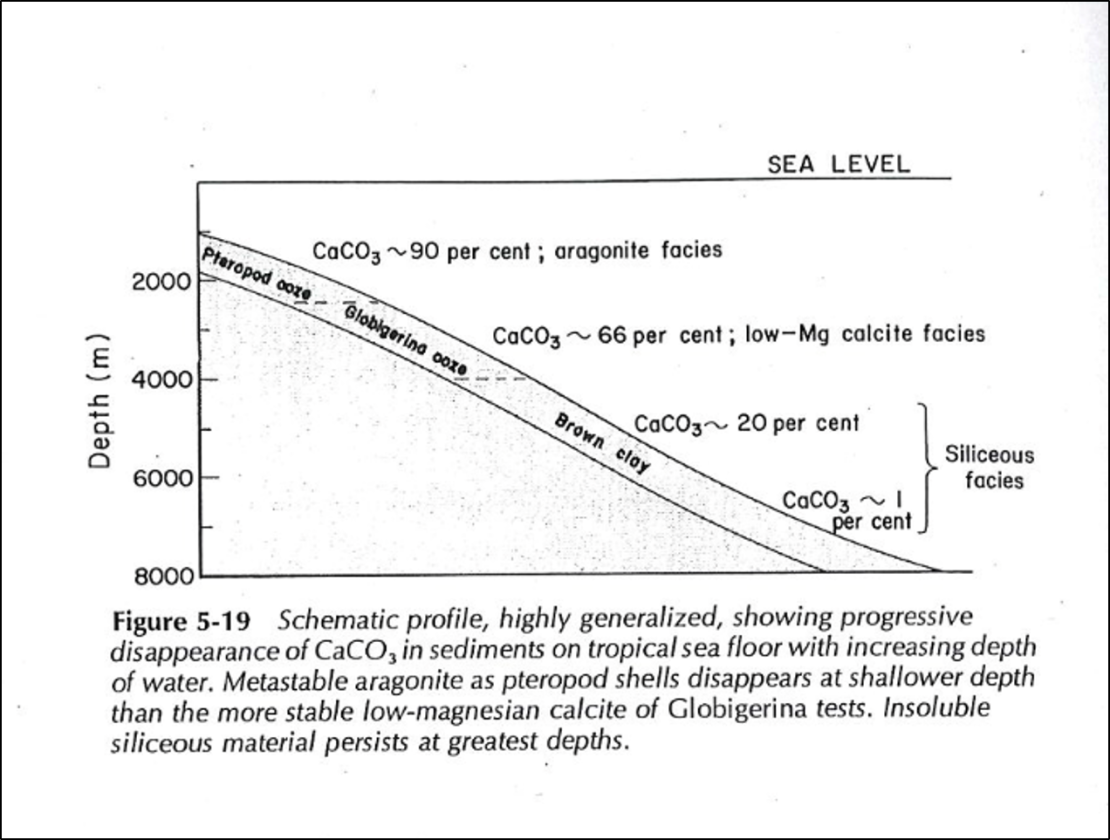
When the pH of seawater decreases, calcium carbonate dissolves. In warm, shallow seas, at a pH of about 8.3, dissolution of aragonite and calcite particles by inorganic processes is almost nonexistent. However, since the classical studies of the Challenger expedition, it has been known that the proportion of calcium-carbonate particles in seafloor sediments decreases as depth of water increases (Table 5-1). Such decrease is particularly rapid at depths between 4000 and 6000 m. Although the reasons for this decrease have been debated, the evidence suggests that calcium carbonate dissolves because the CO2 concentration increases with depth. The control on CO2 appears to be part biological; it results from biological oxidation of organic-carbon compounds. Also, the water masses at greater depth were derived from the polar region; their temperature is lower and the water contains more dissolved CO2. Increased concentration of CO2 is in turn reflected by lower pH, which leads to calcium carbonate dissolution. However, the increase of pressure with depth may also be involved; such increase affects the dissociation of carbonic acid (Eqs. 5-11 and 5-12). The depth at which the calcium-carbonate decreases most rapidly is known as the carbonate-compensation depth, defined as the depth at which the rate of dissolution of solid calcium carbonate equals the rate of supply.
Friedman and Sanders, pages 133-134.
A very thorough, easy to read, description of the relationship between CO2 and seawater pH… However, the phrase “ocean acidification” is notably absent from the entire 300+ pages. How is this possible?
The depth below which CaCO3 ceases to precipitate is call the Carbonate Compensation Depth (CCD). However, the seafloor does not dissolve below the CCD. Below the CCD, seafloor sediments consist of siliceous rather than carbonate muds.
By comparing preindustrial with present-day rates, we determine that significant anthropogenic dissolution now occurs in the western North Atlantic, amounting to 40–100% of the total seafloor dissolution at its most intense locations. At these locations, the calcite compensation depth has risen ∼300 m. Increased benthic dissolution was also revealed at various hot spots in the southern extent of the Atlantic, Indian, and Pacific Oceans.
First off… There’s no way to compare preindustrial with present-day rates. There are no measurements of preindustrial rates. Estimates of preindustrial rates are based on the rise in atmospheric CO2… Which they assume is unprecedented.
Scientific Reports volume 4, Article number: 5261 (2014)
Larger CO2 source at the equatorial Pacific during the last deglaciation
Kaoru Kubota, Yusuke Yokoyama, Tsuyoshi Ishikawa, Stephen Obrochta & Atsushi Suzuki
Abstract
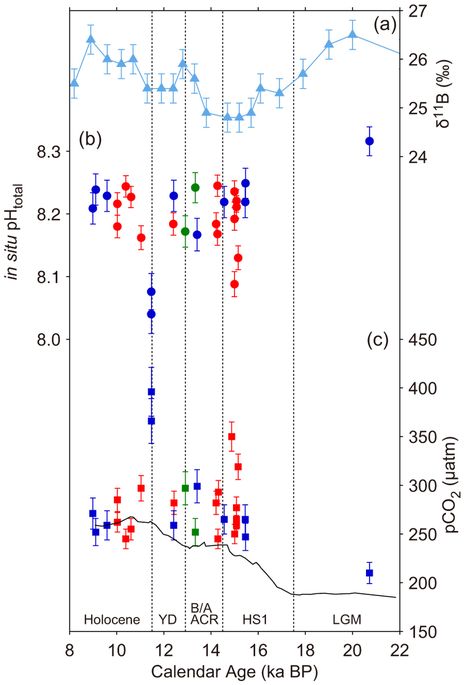
While biogeochemical and physical processes in the Southern Ocean are thought to be central to atmospheric CO2 rise during the last deglaciation, the role of the equatorial Pacific, where the largest CO2source exists at present, remains largely unconstrained. Here we present seawater pH and pCO2 variations from fossil Porites corals in the mid equatorial Pacific offshore Tahiti based on a newly calibrated boron isotope paleo-pH proxy. Our new data, together with recalibrated existing data, indicate that a significant pCO2 increase (pH decrease), accompanied by anomalously large marine 14C reservoir ages, occurred following not only the Younger Dryas, but also Heinrich Stadial 1. These findings indicate an expanded zone of equatorial upwelling and resultant CO2 emission, which may be derived from higher subsurface dissolved inorganic carbon concentration.
Kubota et al, 2014 found…
pH and pCO2 reconstruction
Using our revised calibration, we reconstructed pH from our new δ11B measurements on Tahitian corals, as well as from previously reported data11 from both the Marquesas and Tahiti, and the overall result is consistent with the WEP foraminifer δ11B variations10 (Fig 3a and b). The oldest coral sample, dated to 20.7 ka BP during the last glacial maximum (LGM), exhibits a relatively high pH (8.26). From 15.5 to 9.0 ka BP, pH is generally constant within uncertainty (8.15–8.22) and consistent with the preindustrial value of 8.20. Four notable pH excursions are associated with HS1 and the YD. Two of our samples exhibit anomalously low pH at the end of HS1 (8.13 at 15.15 ka and 8.09 at 14.99 ka BP), in addition to those at end of the YD at the Marquesas11. The low pH following HS1 had been previously undetected at this location. Calculation of pCO2 (see Methods) reveals deglacial values significantly above those of the atmosphere (Figs. 3c and 4a). Conversely, ΔpCO2during last glacial and the early Holocene was nearly zero, suggesting air-sea CO2 equilibrium.
Periods of anomalously low pH and high pCO2 near the transitions from Heinrich Stadial 1 (8.08-8.15 & 300-350 μatm) to the Bølling/Allerød, during the Bølling/Allerød interstadial (8.15-8.20 & >300 μatm) and at the onset of the Holocene (8.02-8.10 & 350-420 μatm)… Sounds kind of precedented to me.
Our findings place constraints on future predictions of ocean acidification, are consequential to the fate of benthic calcifiers, and indicate that a by-product of human activities is currently altering the geological record of the deep sea.
We know what the fate of benthic calcifiers will be if the CCD shoals (becomes shallower)… They become extinct and then recover (they move).
Here’s a “funny thing” about the PETM benthic foraminifera “mass extinction”: The benthic foram’s rapidly recovered from their extinction (literally):
Palaeogeography, Palaeoclimatology, Palaeoecology 279 (2009) 186–200.
Extinction and recovery of benthic foraminifera across the Paleocene–Eocene Thermal Maximum at the Alamedilla section (Southern Spain)
L. Alegret , S. Ortiz E., Molina
A b s t r a c t
A complete succession of lower bathyal–upper abyssal sediments was deposited across the Paleocene–Eocene Thermal Maximum (PETM) at Alamedilla (Betic Cordillera, Southern Spain), where the benthic foraminiferal turnover and extinction event associated with the negative carbon isotope excursion (CIE) across the PETM have been investigated. Detailed quantitative analyses of benthic foraminifera allowed us to distinguish assemblages with paleoecological and paleoenvironmental significance: pre extinction fauna, extinction fauna, survival fauna (including disaster and opportunistic fauna) and recovery fauna. These assemblages have been associated with significant parts of the δ13C curve for which a relative chronology has been established. The correlation between the benthic turnover, the δ13C curve, the calcite and silicate mineral content, and sedimentation rates, allowed us to establish the sequence of events across the PETM. At Alamedilla, the benthic extinction event (BEE) affected ~37% of the species and it has been recorded over a 30-cm-thick interval that was deposited in c.a. 10 ky, suggesting a gradual but rapid pattern of extinction. The beginning of the BEE coincides with the onset of the CIE (+0 ky) and with an interval with abundant calcite, and it has been recorded under oxic conditions at the seafloor (as inferred from the benthic foraminiferal assemblages and the reddish colour of the sediments). We conclude that dissolution and dysoxia were not the cause of the extinctions, which were probably related to intense warming that occurred before the onset of the CIE.
The BEE is immediately overlain by a survival interval dominated by agglutinated species (the Glomospira Acme). The low calcite content recorded within the survival interval may result from the interaction between dilution of the carbonate compounds by silicicate minerals (as inferred from the increased sedimentation rates), and the effects of carbonate dissolution triggered by the shoaling of the CCD. We suggest that Glomospira species (disaster fauna) may have bloomed opportunistically in areas with methane dissociation, in and around the North Atlantic. The disaster fauna was rapidly replaced by opportunistic taxa, which point to oxic and, possibly, oligotrophic conditions at the seafloor. The CCD gradually dropped during this interval, and calcite preservation improved towards the recovery interval, during which the δ13C values and the calcite content recovered (c.a. +71.25 to 94.23 ky) and stabilized (N94.23 ky), coeval with a sharp decrease in sedimentation rates.
Benthic foram’s appear to have an even higher recovery rate from extinction than the Incilius genus of toads…
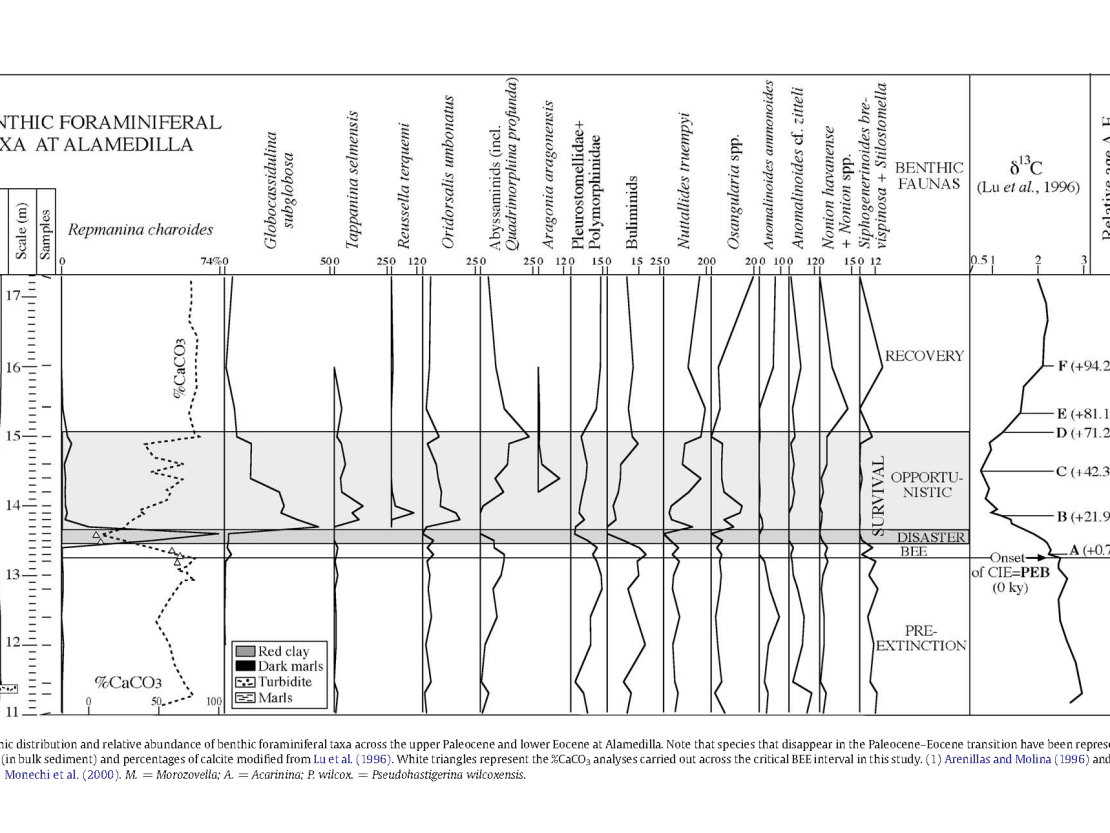
Thermal Maximum at the Alamedilla section (Southern Spain). Palaeogeography, Palaeoclimatology, Palaeoecology 279 (2009) 186–200
The shoaling of the lysocline during the PETM is represented by the 30 cm thick band of red clay from 13.4 to 13.7 m on the lithology column in figure 8. When the lysocline and carbonate compensation depth (CCD) briefly shoaled, the transition from calcareous to siliceous ooze moved shoreward. When the CCD dropped back down to its pre-PETM depth, the transition from calcareous to siliceous ooze moved seaward… Leaving a 30 cm thick layer of red clay in the middle of a thick marl sequence. Rising and falling sea level could have left a similar layer of red clay.

The benthic foram’s above and below the red clay horizon ceased to exist at that location for about 70,000 to 220,000 years. However, the fact that at least some of them returned to that location after the PETM might indicate that the benthic foram “mass extinction” was more of a benthic foram depositional “mass relocation,” rather than a true extinction. I’ll let “Farmer Fran” explain how the benthic foram’s recovered from extinction:
Some authors actually have seriously referred to the PETM benthic foram extinction as a “mass extinction”… And they still expect to be taken seriously?
Excessive carbonate undersaturation of the deep ocean would likely impede calcification by marine organisms and therefore is a potential contributing factor to the mass extinction of benthic foraminifera at the P-E boundary. Although most plankton species survived, carbonate ion changes in the surface ocean might have contributed to the brief appearance of weakly calcified planktonic foraminifera (6) and the dominance of heavily calcified forms of calcareous algae (37). What, if any, implications might this have for the future? If combustion of the entire fossil fuel reservoir (~4500 GtC) is assumed, the impacts on deep-sea pH and biota will likely be similar to those in the PETM. However, because the anthropogenic carbon input will occur within just 300 years, which is less than the mixing time of the ocean (38), the impacts on surface ocean pH and biota will probably be more severe.
Good fracking grief!!! WTF is “the entire fossil fuel *reservoir*”? Last I checked, most fossil fuel (coal) isn’t in a reservoir (coal bed methane isn’t a coal reservoir) and the fossil fuels that do occupy reservoirs (petroleum and natural gas) occupy many thousands thousands of different reservoirs.
Accuracy not withstanding… So, if we burned all of the fossil fuels that Zachos et al think exist, the effects on deep-sea biota might be as bad as the PETM and the surface effects might be worse… if we burn all of those fossil fuels within 300 years. And? What’s the problem here? Is the field of speculative non sequitur reasoning now an academic discipline of science? Oh wait… Yes it is. It’s what climate science has become since 1988.
Chicken Little of the Sea will pretty well only be an issue, where it is already an issue… mostly in areas of strong upwelling.
Conclusion
The Earth isn’t behaving any differently over the past 150 years than it has behaved over the past [fill in the blank] years. Same as it ever was…
References
Dennen, Kristin O. and Paul C. Hackley Definition of Greater Gulf Basin Lower Cretaceous Shale Gas Assessment Unit, United States Gulf of Mexico Basin Onshore and State Waters. Search and Discovery Article #10429 (2012)
Adapted from oral presentation at AAPG Annual Convention and Exhibition, Houston, Texas, April 10-13, 2011
Sulpis, Olivier, Bernard P. Boudreau, Alfonso Mucci, Chris Jenkins, David S. Trossman, Brian K. Arbic, Robert M. Key. Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2. Proceedings of the National Academy of Sciences Oct 2018, 201804250; DOI: 10.1073/pnas.1804250115
See these two posts for detailed Chicken Little of the Sea references:
The Total Myth of Ocean Acidification
The Total Myth of Ocean Acidification, Part Deux: The Scientific Basis
The roof is leaking in Hillary’s future apartment.
As a social contract, wouldn’t it make more sense to simply pay these people their salaries but tell them they shouldn’t feel obliged to do any research and can just stay at home if they prefer? They’d be happier (money for nothing) and society’d be happier (not having to read their joke science).
Sinecures are sometimes the most humane arrangement.
No. This kind of ‘research’ should be defunded. If they want to do it, they should do it on their own dime. Unfortunately there is a lot of garbage that passes as science nowadays.
I am increasingly of the belief that the main benefit of higher education to society is that it keeps the unemployable from cluttering up the school leaver job market.
“Sinecures are sometimes the most humane arrangement.”
Relief from the ratio of the opposite side to the hypotenuse is…humane? I just don’t get it.
I have become convinced that either the global warming “advocates” are ignorant or believe everyone but themselves are ignorant. It reminds me of some of our politicians who stand in front of a camera and say stuff then deny they ever said it a month later in spite of video evidence.
And yes they are warming “advocates” because I have also become convince they actually want to see their gloom and doom computer games come true.
Don’t rule out “both”!
They are either scientists, or global warming advocates. There is no both !
As Ronald Reagan said, ” … it’s just that they know so much that ain’t ! “
Since very few people can actually see the sea floor it is an excellent subject to spread misinformation about. It only has to seem slightly plausible.
The same thought crossed my mind this am while reading other cc drivel. It seems like all of these so-called catastrophic events that might,could,possibly,chance of, happen are in very remote places that the average person has no way to go see for themselves. Antarctica,Greenland,North Pole,Svalbard,the Barrier Reef,Mt Everest,Poly Bears & Penguins,ozone hole & now today we have the ocean floor is going to hell.The average working person does not have the time to spend nor the money it would cost to go to these places to see what’s real & what’s not.So the blowhard sensationalists have a free rein to pretty much embellish the alarm any way they feel like doing.Take photos,selectively crop & edit said photos to make it seem catastrophic,write a few alarmist paragraphs & get it published.No one knows if it is a steaming load of horsehocky or if it’s for real.
I’m just an average high school grad (no college) with limited scientific knowledge,but my bullshit meter has been pegged in the red for the last 25 years. The over the top claims that don’t happen,the refusal to debate or to prove their ‘research’ or show how they got their answer,not to mention hiding &/or changing data & the climate gate email scandals the biased media & politicians that protects them pretty much did me in as far as believing anything they say… anything.
I don’t make it a habit to comment very much but this crapola really irritates me. I hate liars & nefarious people who will deliberately mislead others just because they can get away with it.
I wouldn’t trust these bas.., fatherless people any farther than I can spit.
Paul
Well said.
From someone else with a modest education.
Hi HotScot, thanks
I’ve been a lurker on this site for several years now& hardly ever comment,but every now & then I get incensed… & I don’t give a toss about ‘politically correct’.
I’ve enjoyed reading your comments for some time as well as all the others who comment. I kinda get a kick out of a certain few of the warmistias who keep coming back preaching their religion only to see them get stomped into the ground time after time. Kinda reminds me of the punch drunk boxer who keeps going back to center ring to get beat on some more cause he’s too stupid to quit. Anyway, I’ve got a really good education here by just reading the articles & especially reading the comments following. My science knowledge has improved quite a bit,although a lot of the deep stuff is over my head & I’m not too proud to admit it.
Another thing I like is that there are a lot of people on this site that have a lot more schooling than I do & there are numerous times someone will use a word I don’t know. I’ll highlight & copy the word into my search engine & find out what it means. Really have increased my word vocabulary quite a bit too. I especially like the British slang words,they amuse me
I like to think of those warmistas as batting practice pitchers.
Paul
Your experiences and learning from this site echo mine. One of the nice things about WUWT is if you ask a question, no matter how stupid or challenging, someone will pitch up with an answer.
Try that on an alarmist blog and you’ll get abused for being an idiot. I started my ‘climate journey’ (ugh, terrible expression) on alarmists sites because I did believe the MSM and Al Gore hype, I just had a few things to clarify.
About the last site I tried was WUWT, and I expected the same treatment, only worse, because it was a ‘sceptic’ site……………wooooooooooo!
Lo and behold, more answers than I could shake a stick at, and they made sense, assuming I let people know I’m a dunce beforehand.
Now I’m tolerated because everyone knows I’m thick. The class fool if you like. 🙂
I was the same, Paul / Hot Scot with a similar level of education (so please excuse my grammer!).
When my spider senses started registering the carp around CAGW, I started reading a site which gave a voice to both true believers and sensible people (can’t remember the name of the website). Whilst it offered an opinion, it was well balanced and I learnt a lot.
Then I came across ClimateAudit and WUWT.
Over at the BBC, I started to get involved on Richard Black’s blog. At first I kept falling for the strawman argument, but then fought back with links to the peer reviewed literature.
Several posters told me to read articles on SKS, which I did, so I tried to engage with the posters there. What a waste of time. I was used to be disparaged at the BBC, but SKS was just ridiculous. I posted a simple question and the first response was essentially that I was an odious little rat sent by Big Oil to spy on them (not exactly that, but you get the drift).
So I gave up with SKS, although I did get a dishonourable mention (troll inhabiting the hallowed pages of the BBC) in the leaked SKS files!
Paul November 6, 2018 at 10:58 am
there are numerous times someone will use a word I don’t know. I’ll highlight & copy the word into my search engine & find out what it means.
I’m in that boat, Brad Keyes used this one up thread:
sinecure
Got it from context but looked it up just to be sure and the pronunciation
Paul November 6, 2018 at 10:03 am
not to mention hiding &/or changing data
Like this?
When a solution is referenced to a neutral 7.0 pH – values above are alkaline/basic and become more or less alkaline/basic, values below are acidic and become more or less acidic.
The ocean’s pH is about 8.1. That’s alkaline. Variations are more or less alkaline, not more or less acidic. The obvious reason for incorrectly using the term “ocean acidification” is a propaganda gambit to scare the gullible and uninformed who associate acid with bad, like alien blood and spit.
Highly alkaline compounds such as caustic soda can be just as dangerous as acidic compounds, e.g. concentrated bleach, sodium hypochlorite, pH 9 to 13. On the other hand: rain has a pH of 4.5, lemon juice has a pH of 2.0, tomatoes a pH of 4.5, and vinegar a pH of 2.2. If they get on your hands the flesh doesn’t melt and they don’t burn a hole in the kitchen counter. (Might etch that granite, though.)
A solution goes from pH 0.0, dangerous acidity, to pH 7.0, neutral/safe, to pH 14.0, dangerous alkalinity. pH is chemical shorthand for the negative logarithm of H+ ion concentration.
pH = -log[H+] (1)
A pH of 9 represents 10^-9 or 1 part per billion H+ ions. A pH of 8 represents 10^-8 or 10 parts per billion H+ ions. A change from pH 8.2 (6.31 ppb M/l) to pH 8.1 (7.49 ppb M/l) is a (-1.18 ppb M/l) change in the direction of lower alkalinity, not more acidity. -1.18/7.49 = -16%. ((8.1-8.2)/8.2 = -.1/8.2 = 1.2%)
With a log function every whole number change is by a power of 10, a factor of 10. A change in pH from 9 to 8 means the H+ concentration increases by a power/factor of 10 or 1,000%!!!!!!! Makes the -16% look pretty trivial – which anything in ppb is. 0 pH to 14 pH is 14,000 %!!!!
Applying percentages to a logarithmic scale/function is very dicey, but that’s what you get when food and life style editors write science articles.
So, pH 8.1 is moving a YUGE 1 ppb in the direction of slightly more neutrality from pH 8.2 which is not much to begin with.
Improperly using the term ocean “acidification” to scare the public over bogus CAGW is a disgrace to science. Spit out the Kool-Aid and grow a backbone.
You could add the common ion effect to your arguments. The solubility product of [Ca++]{CO3–] should be a constant. More dissolved CO2 means more CO3– and since the product should be constant, formation of Calcium Carbonate results and [Ca++] drops . I worked in the sugar industry for years where carbonation was the addition of CO2 to juices mixed with Ca(OH)2 to form CaCO3. The residual [Ca++] would rise or fall as a result of the other ions present, not carbonate. It is simplistic to say pH will dissolve CaCO3. In reality, more CO2 forms CaCO3.
Thank you David! That concept started kicking around in my head near the beginning of the article and I was desperately trying to figure out why I was wrong. I couldn’t find any reason why adding CO2, forming HCO3- plus H3O+ and CO3– plus 2 H3O+ would push the equilibrium in the direction of the dissolution of CaCO3. It’s not like AgCl, where adding Cl-forms AgCl2- and AgCl3– (driving the silver back into solution). I don’t believe there is a soluble ion in the form of CaCO3.HCO3- or CaCO3.CO3–. And since there is only 1ea. H3O+ formed when CO2 combines with water to form HCO3- it seems to me like a “zero sum game” is afoot. The extra H3O+ can react with a CaCO3 to solubilize the CaCO3 but there’s an extra HCO3- lurking out there to trade away it’s other hydrogen for a Calcium. And there is plenty of ionized Calcium for it to meet up with. I’d love to find someone who can show me where I went wrong there.
Jeff: I am not a expert on Ca++ but there are a couple things to point out. The solubility of CaCO3 decreases as temperature rises. That would suggest that if the seas are warming, the uptake of CO2 would likely increase. You can see this effect as the amount of CaCO3 in sediment as a function of depth decreases. The bicarbonate ion does play a role but that does not change the issue with the solubility product. As an example, when I was in the sugar industry, I developed a strategy to vent non-condensable gases from the evaporator train (4 stage) and made the mistake of venting all to the main barometric condenser which used well water. The gases contained ammonia as well as CO2 and that put a high level of CO3– in the water as ammonium carbonate and began to deposit calcium carbonate on the leg of the condenser, almost shutting down the factory. The problem was that the ammonia allowed a vary high carbonate concentration and calcium had to leave by precipitation, in that case on the pipe wall. If we are going to say calcium is being dissolved, there must be some other acidic component being introduced to the sea water. It cannot be CO2 and it cannot be from warming. I am not an expert in ocean chemistry but a claim that CO2 forces dissolution needs to be better explained.
Nick Schroeder,
+1
This always reminds me of Alice in Wonderland at the mad tea party when she is asked if she would like “more tea”. She replies, ” well, I haven’t had any tea yet, so I couldn’t very well have more tea.” Just substitute acid for tea.
Hoyt Clagwell,
+42!
Acid/Base chemistry eludes you.
Even at pH14, there are still [H] hydronium ions around. Just not very many compared to [OH] hydroxide ions.
Nick Schroeder
+14,000%!
Brilliant, this is a keeper.
Nick,
Your logarithmic math needs some serious polishing up. 14,000% is not even close.
To go from pH 0 to pH14 is a 10^14 increase in [OH]-, the hydroxide ion, and the corresponding decrease in the hydronium ion [H]+. Or, 1E+14 = 100,000,000,000,000 or 100 Trillion fold increase if you prefer.
To go from pH 14 to pH 0 is of course the inverse of that, or 1E-14 decrease in [OH] and a 1E+14 increase in the [H]+ ion.
Joel O’Bryan
So that should have been +100,000,000,000,000?
Better and better. 🙂
Joel,
Just highlights how dumb it is to apply a percent to a log scale.
Thnx.
“The depth below which CaCO3 ceases to precipitate is call (ed) the Carbonate Compensation ” ?
Another great essay, thanks…
Remind me to let you proof-read my posts in the future… 😉
Anytime..absolutely free !!!
( That means I get to be the first to read it !! ) LOL
uh no…..second….LOL
D’OH !
What is puzzling me about this suggested outcome is how increasing CO2 concentrations can do what they say. When I studied chemistry, the solubility product was discussed and the common ion effect explained. What chemistry says about solubility is that the product of the concentration of the ions of any particular set of ions is a constant when in equilibrium with the solid. Basically [Ca++][CO3–]=KSP. When the concentration of CO2 rises and creates its equilibrium concentration of CO3–, the calcium ion concentration will reduce by the formation of Calcium Carbonate and the product will remain constant. I have seen this occur in industrial processes. Unless chemistry has changed, a lowering of pH by CO2 adsorption should lead to the formation of more CaCO3. If some other anion is causing the pH to drop, then the dissolution can occur. This paper needs to explain why the common ion effect does not occur or what the other cause of pH drop has resulted in the proposed dissolution.
It’s not so much the lowering of pH as it is the fact that increasing CO2 saturation in water causes more calcium carbonate to become soluble calcium bicarbonate.
CaCO3 + CO2 + H2O → Ca(HCO3)2
A lot of factors affect this process.
The dissolution of CO2 in deep ocean waters is due to increasing pressure and lower water temperatures, period. How does one make a carbonated beverage? A cold coke , anyone?
Acidic? Sounds like a professional writer not a scientist.
The above paragraph sounds like a description of buffering.
Actually the water from volcanic/hydrothermal vents “smokers” is the only acidic water to be found anywhere in the ocean. The areas around these vents are famous for the rich “vent fauna” living there in contrast to the otherwise virtually lifeless deep ocean floor.
http://faculty.wwu.edu/~shulld/ESCI%20432/ramirez_et_al2007.pdf
A bit of research into “black smokers” and Bob Ballard’s “Lost Cities” vents probably wouldn’t go astray.
One of the vital functions of CO2 is rock weathering, the source of calcium for life in the oceans. Enough said?
Yep.
Geology + humans still equals geology.
So what I’m hearing here is the Earth has a way of countering Ocean acidification, or did I miss something?
You have that correct. Look up the term “buffering” of à solution.
Interesting post, David. Working in the Devonian overthrust zone in Nevada you see a lot of barite deposits around the paleo-basins where overthrust slices slid into basins and over-pressured them, quickly producing a change in the calcite compensation level, quickly recrystallizing aragonite to calcite and expelling barium. The barium combines with sulfate and you get barite deposits on the sea floor, especially near basin rims. By the way, David, you possibly should stop listening to that rock and roll before it is too late, just saying.
Ron, I fear it’s too late for Dave. He’s got it bad.
Well some contend that rock and roll
Is bad for the body and bad for the soul
Bad for the heart, bad for the mind
Bad for the deaf, bad for the blind
Makes some men crazy
And then they talk like fools
Makes some men crazy
And then they start to drool
Unscrupulous operators could confuse
Could exploit and deceive
The conditional reflex theories
And change the probabilities
I said it’s a crass and raucous crackass place
With pavlov on the human race
It’s a terrible illness, it’s a terrible case
And usually permanent when it takes place
It’s a teenage nervous breakdown
It’s a nervous teenage breakdown
It’s a tenenerve nervetene
It’s a tennenervenerna
Whoa!
Thanks to the late genius Lowell George
You’d think they’d be happy. Wouldn’t it prevent sea level rise if the floor is dissolving :0)
It may dissolve all the way through to the other side and cause sea level rise on the far side of the planet!
It would extinguish the core!!! Or vaporize the Earth in one massive phreatic eruption… 😉
Now that is funny. The problem is some warmista is going to take it and run with it.
The Gruniad and the Beeb have already run the story
We should all put limestone roofs on our houses. Increase the albedo effect And neutralize the acid….
“If the seafloor was actually dissolving, it would certainly fix that whole sea level rise thingy.”
Like” the missing heat is hiding in the deep oceans”, … this whole dissolving seafloor thingy is where the SLR went to; CO2 is doing everything they said was going to do. You just handed Percy one more piece of logic for his confirmation puzzle.
I was thinking Ivan.
“With increasing carbon dioxide from human activities, more acidic water is reaching the deep sea,…”
Do the Princeton communicators mean that water reaching the deep sea is ‘more acidic’ than formerly, or that there is more water reaching the deep sea that incidentally happens to be acidic, or both?
I would have expected better from Princeton!
More “acidic” water will eventually reach the deep sea. The residence time for oceanic bottom water is about 1,000 years.
Kristi Silber,
Now is your chance to jump in here and correct Middleton’s summary of ocean carbonate-geochemistry and explain exactly how “ocean acidification” works and how it ‘proves’ anthropogenic warming. Please hurry. I will be holding my breath until you do.
There actually was a number of extinctions of benthic forams during the PETM, but it was more likely due to warmer deep oceans than changes in the CCD.
However these few extinctions of benthic microorganisms have got to be blown up into a “mass extinction”, because they are the only extinctions that can be linked to the PETM. All other organisms diversified and spread during the PETM like never since.
If foram’s never went extinct, we wouldn’t be able to tell the difference between the Pleistocene, Pliocene and Miocene in the Gulf of Mexico… 😉
And note what is said in your citation:
“the extinctions, which were probably related to intense warming that occurred before the onset of the CIE.”
I. e. the warming and extinctions came first, the CIE (Carbon Isotope Excursion), interpreted as an increase in pCO2 came later. Same old, same old…
Not to mention the fact that CIE occurred in response to warming long before they became a “fingerprint” for anthropogenic CO2.
They aren’t smart enough to figure it out for themselves, but they have discovered what the rest of us have been talking about for years.
Buffering.
The reason why the seas will never become acidic is because of buffering.
Yes, the ocean floor is dissolving, and in 100 billion years, when the amount that gets dissolved is enough to matter, we will start to get concerned.
Oddly enough the problem is not CO2 or pH, it’s temperature. When water is cold, calcium and magnesium bi-carbonate are stable. When it gets too warm, the bicarbonate decomposes to C02, water and CaCO3 or MgCO3. That’s why we all learned in grammar school that limestone was formed in warm shallow seas. The shallow is important as it keeps the temperature high enough to cause the bicarbonate to be unstable.
If the sea floor did dissolve a bit, wouldn’t that lower the sea floor making room for all the supposed melt water from the glaciers and polar ice caps negating the supposed rise in sea levels?
I think we take the wrong approach to these garbage papers. We should be naming and shaming the peer reviewers. Without them, this crap can’t get published. We should make concerted effort to call them out for failing to provide even the most basic of critique.
Dr. Willie Soon sent me a copy of the paper… It’s actually quite good… if you’re into models being used as if they were actual observations.
…actual observations say the white cliffs of Dover formed on the sea floor….when CO2 levels were ~3000 ppm
The seas were relatively shallow where the Austin and Dover chalk formations were deposited…
https://pubs.usgs.gov/sir/2012/5159/SIR12-5159.pdf
http://www.sepmstrata.org/Terminology.aspx?id=chalk
Higher CO2 levels just cause the carbonate compensation depth to be shallower. They don’t dissolve the seafloor or prevent the deposition of carbonate rocks.
The vast quantities of sulfur being emitted by ocean vents on the sea floor for hundreds of millions of years seems not to come close to a rise of 100ppm in atmospheric CO2
PSEUDO SCIENCE
Am I wrong in my assumption that none of this has been observed by actually going there or by actual measurements at the sea floor? That this is simply a “model” of how someone thinks things are happening. My goodness, if this all comes from models, have they been verified and validated through real, physical measurements?
Seafloor sediments have been “observed” since 1891…
They haven’t changed much.
Everything else is based on assumptions and models.
So as you transition from shallow to deep water the sediments transition from calcareous Globigerina ooze to silica radiolarian ooze at the Carbonate Compensation Depth (CCD) because of different solubility.
“The oozes are subdivided first into calcareous oozes (containing skeletons made of calcium carbonate) and siliceous oozes (containing skeletons made of silica) and then are divided again according to the predominant skeleton type.” Read more at:
https://www.britannica.com/science/ooze#ref59833
The silica is impervious to the water *acidified* by CO2. (Think of Coke Bottles full of acidic but tasty carbonated beverages.) Excuse me, but the sea bed is dissolving? **face palm**
There is an important process which has not been considered (please forgive me if I miss someone’s post). This is serpentinization of oceanic crust at spreading centers.
When basaltic magma meets oceanic water, some is altered to serpentine. Water that works down into new crust continues this serpentinization process. Overall this is a basic chemical reaction. Considering that this process occurs along all spreading centers, it is a major chemical process. Yet, there has been little research on the impact of ocean chemistry due to this process.
Hence, ocean chemistry cannot be understood until all geologic-chemical processes are studied.
KEITH PEREGRINE,
And there is an associated volume increase, which should result in causing sea level to rise. There is SO MUCH that gets overlooked!
There might be something to this…
Most of that Cretaceous sea-level rise must be due to ridge expansion. All the ice in the world is at the most equivalent to about 60 meter sea level rise, and probably less depending on isostatic effects on the seafloor. Thermosteric rise might contribute a few tens of meter, no more.
However reconstructing a “global sea level” that far in the past is tricky. For example at the same time in the Cretaceous as sea-level goes up everywhere else in the World it goes markedly down in Australia, probably more than 100 meters. Before this time Australia was mostly shallow seas, since then it has been almost completely dry land. Something odd must have happened down in the Mantle.
Keith writes: “ This is serpentinization of oceanic crust at spreading centers.”
I’ve not actually visited a spreading center, but I can show you Serpentinite in the mountains northwest of where I live. Search Google Earth for “Jolly Mountain, WA.”
Some of the bare slopes are from very active erosion but there are numerous outcroppings of serpentine rocks. Plants on the derived soils are scarce as major nutrients are poor and others are toxic to most plants.
See: https://en.wikipedia.org/wiki/Serpentine_soil#Botany
The area has numerous trails, so the differentiation of plant communities (wild flowers) is often of interest to the hikers.
Some might wonder how the rocks from oceanic spreading centers came to be at 6,000 feet in the mountains. That’s an interesting tale, also.
Anyway, thanks to you folks giving the chemistry lesson. My first college class was chemistry, in summer 1961.
Serpentine soils have a very unusual chemistry and often a very odd and specialized flora. New Caledonia is the prime example, but the effect is obvious almost anywhere with serpentine deposits.
tty,
And even some strange fauna. I remember being shown a picture by Salem Rice (formerly of the Calif. Div of Mines) of a green ant living in a serpentinite area of northern California. Presumably, the green color was a result of the high chromium content of the area.
National Academy of Science is big on us turning the oceans acid, but their numbers are pretty small and ain’t acid. I was just reading that paper. Look at this link at the bottom under “We recommend”
http://www.pnas.org/content/112/32/9950.figures-only?utm_source=TrendMD&utm_medium=cpc&utm_campaign=Proc_Natl_Acad_Sci_U_S_A_TrendMD_0
“Tipping point at 450ppm” DUCK!
More research conducted in isolation from common sense by using a self satisfaction model.
Then releasing the preferred results immediately to the gullible press.
More wasted research funds producing garbage.
At the current average ocean pH of ~8.1 (pH varies regionally), bicarbonate (HCO3), not carbonate (CO3), is the dominant carbon species. As pH decreases, CO3 decreases, and HCO3 remains relatively constant. Dissolved CO2 gas increases as pH decreases.
I haven’t read everything here and I’m no scientists but I do know a little about buffering capacity and I would presume that there is not enough co2 in the atmosphere to make the slightest difference to ocean pH. More carbonate dissolves and you are quickly back to square one. Any observed localized variations in pH would seem to be meaningless. At the very least the term ”ocean acidification” is contemptible.
Many calcifying organisms are able to grow in unfavourable ‘under-saturated’ conditions because they exert local cellular control of the conditions necessary for calcite deposition/crystallization.
Life wasn’t born yesterday, and has many tricks up its sleeve.
”Life wasn’t born yesterday, and has many tricks up its sleeve.”
Exactly. Why do modern researchers always find something wrong whenever they look at something natural?
Exactly. Why do modern researchers always find something wrong when they look at any natural system?
michael hart,
Yes, declining pH doesn’t make it impossible for calcifying organisms to live, they just have to expend a little more energy to produce their shells. However, it seems that different calcifiers have different ranges of optimal pH, probably depending on what conditions were like then they first evolved. However, additionally, most calcifiers have other tricks such as covering their calcareous shells with mucous and/chitin to protect them from transient changes, particularly frequent in upwelling zones.
Agreed, Clyde Spencer.
I wouldn’t contend that changing pH, from CO2, never has any affect on any organisms. But when it does, it need not necessarily be negative. There are DNA-analysis results from people looking at the response of the most common/voluminous (?) photosynthesizer, E.Huxlei, to such conditions. They found no significant change apart from a decrease in the expression of genes coding for carbonic anhydrase activity. And they grew somewhat bigger (a larger shell needs relatively less shell?)
In other words, these photosynthetic, CO2-fixing organisms had to spend less energy synthesizing the enzyme primarily responsible for accumulutaing CO2 when they going at full speed, and relatively less resources building their shell.
What is the total change when the cost-benefit equation is worked out?
I don’t know. And they don’t know either.
But the fact that life has flourished so much under previously much higher CO2 concentrations certainly suggests an answer.
I believe that this kind of analysis derives from an unrequited passion in climate science to apply PETM conditions to AGW but it doesn’t work and it can’t work because of certain fundamental differences between these two events. It is true that in the PETM there was a massive acidification, de-oxygenation, and a total poisoning of the ocean but it was more of a case of ocean suicide – the ocean was doing it to itself.
Please see
https://tambonthongchai.com/2018/10/28/petm/
” It is true that in the PETM there was a massive acidification, de-oxygenation, and a total poisoning of the ocean ”
In that case it is rather odd that there was no marine mass extinction. As a matter of fact there were no extinctions at all (except for benthic forams) and no OAE (Ocean Anoxic Event). CCD undoubtedly shallowed and the amount of oxygen in the deep decreased (an inevitable effect of warming deep waters) but the deep see didn’t become anoxic. There are no black shales. And hypoxia seems to have been mostly coastal and due to local eutrophication.
Apart from a few “bugs” that either recovered or were easily replaced, the PETM was a biodiversity jackpot… 😎
If a hole is dissolved in the sea floor, all the water will drain away.
Then we’ll be doomed.
Not to mention the water hitting the Earths core and the planet exploding. 🙂
With regards to the PETM, I’ve worked on the foraminiferal biostratigraphy that interval in the North Sea for some years and the extinction event is clear. It is associated with a purple/red layer and small concretions. When analysed, these concretions are found to be extremely high in manganese – some even have foraminifera as nuclei. I asked a sedimentologist how you could get manganese precipitated in a relatively shallow marine environment. That is, in an environment much shallower than those in which the familiar deep-sea manganese rich nodules are found. He immediately suggested that an influx of fresh water might do the trick. This is not an easy situation to imagine in the North Sea as the extinction/purple layer is rather widespread and river systems active at that time would probably not have been sufficient to develop this event. It is thought that the PETM was brought about by the dissociation of methane hydrate in the marine environment. If this dissociation is checked out, you find that “When melted, one litre of solid gas hydrate produces about 160 litres of methane gas and 0.8 litres of fresh water.” (Lovell et al, 2003). The (geologically) sudden influx of fresh water at the sediment water interface when the methane dissociated and which allowed the precipitation of the manganese may have been a factor in the extinction event. Not many fully marine foraminifera can live in a low salinity environment, even for a short time.
Lovell, M., Jackson, P., Long, D., Rees, J., 2003, Frozen Carbon Stores: environmental hazard or resource? Planet Earth Summer 2003, http://www.nerc.ac.uk. p.26-27
Methane hydrates are stable and can accumulate only at considerable depths, not in shallow waters. How deep was the North Sea at the time?
And there is another plausible large fresh water source. The Arctic Ocean was almost completely isolated and nearly fresh at the time (later in the Ypresian it even had huge blooms of the freshwater fern Azolla). It may well have drained on a large scale into the North Sea Basin at times. The Fram Strait area was a tectonically very unstable rift area and precipitation at high latitudes draining into the Arctic ocean must have been very high.
The foraminifera present at the P/E boundary in the North Sea would indicate a depth from within the bathyal environment according to King, 1989. From upper bathyal to lower lower bathyal is a very wide depth range, but can be from 200 – 2000m. In comparison to other similar microfaunas of approximately the same age (Paleocene/Eocene) by Charnock and Jones 1989, would suggest that depths in excess of 500m would be appropriate.
Biostratigraphically (palynology and microfaunas), the age of the manganese rich layer/extinction event coincides with the PETM. What actual biostratigraphic evidence is there for a freshwater influx from the Arctic into the North Sea at this time?
CHARNOCK, M.A., & JONES, R.W. 1989Agglutinated foraminifera from the Paleogene of the North Sea. In: Hemleben, C., et al. (eds.). Paleoecology , biostratigraphy, paleoceanography and taxonomy of agglutinated foraminifera. NATO ASI Series, Series C, 327, p139-244.
KING, C., 1989 Cenozoic of the North Sea. In: D.G. Jenkins & J.W. Murray (eds.) Stratigraphic Atlas of Fossil Foraminifera, 2nd edition, Ellis Horwood Ltd., Chichester. p372-417.
I don’t know about the North Sea but there is evidence for brackish condition during the PETM in Svalbard:
Dypvik, H., Riber, L., Burca, F., Rüther, D., Jargvoll, D., Nagy, J., & Jochmann, M. (2011). The Paleocene–Eocene thermal maximum (PETM) in Svalbard — clay mineral and geochemical signals. Palaeogeography, Palaeoclimatology, Palaeoecology, 302(3-4), 156–169.
Harding, I C et al. 2010. Sea-level and salinity fluctuations during the Palaeocene-Eocene thermal maximum Arctic Spitsbergen. Earth and Planetary Science Letters 303(1-2):97-107.
New Atlas just posted this nonsense. All four comments deride it if not mock it.
Uhh … maybe it’s in the paper, but just how did they measure “preindustrial” rates”?
Sure, today, “postindustrial”, we can reasonably estimate the rates, but “preindustrial”? No submarines. No bathyspheres. No Argos. No nothing to measure what was happening on the seafloor.
We can guess. We can theorize. We can’t measure the rate of changes that no one could have observed.
Exactly! I raise this over and over, how were pre-industrial levels of CO2 measured. Of course they could not have been. Just like a global sea land average in 1850. It’s all a WAG!
I guess Jules Verne never saw Steve McQueen in The Blob.
The secret to reaching the center of the Earth isn’t volcanoes. It’s CO2 fire extinguishers. Just blast them at the sea floor!
(Of course, they’d have to be really BIG ones to overcome Al’s millions of degree temperatures, but CO2 can do anything. Right?)
PS I looked for a video clip of Steve McQueen calling for them but couldn’t find one. 8-(
If seafloor is cecycled every 200m yrs, say 20 times in 4 b yrs, I cannot see why all this would matter……Brett