NOAA finds rising emissions of ozone-destroying chemical banned by Montreal Protocol
Emissions of one of the chemicals most responsible for the Antarctic ozone hole are on the rise, despite an international treaty that required an end to its production in 2010, a new NOAA study shows.
Trichlorofluoromethane, or CFC-11, is the second-most abundant ozone-depleting gas in the atmosphere and a member of the family of chemicals most responsible for the giant hole in the ozone layer that forms over Antarctica each September. Once widely used as a foaming agent, production of CFC-11 was phased out by the Montreal Protocol in 2010.
The new study, published today in Nature, documents an unexpected increase in emissions of this gas, likely from new, unreported production.
“We’re raising a flag to the global community to say, ‘This is what’s going on, and it is taking us away from timely recovery from ozone depletion,'” said NOAA scientist Stephen Montzka, lead author of the paper, which has co-authors from CIRES, the UK, and the Netherlands. “Further work is needed to figure out exactly why emissions of CFC-11 are increasing and if something can be done about it soon.”
CFCs were once widely used in the manufacture of aerosol sprays, as blowing agents for foams and packing materials, as solvents, and as refrigerants. Though production of CFCs was phased out by the Montreal Protocol, a large reservoir of CFC-11 exists today primarily contained in foam insulation in buildings, and appliances manufactured before the mid-1990s. A smaller amount of CFC-11 also exists today in chillers.
Because CFC-11 still accounts for one-quarter of all chlorine present in today’s stratosphere, expectations for the ozone hole to heal by mid-century depend on an accelerating decline of CFC-11 in the atmosphere as its emissions diminish– which should happen with no new CFC-11 production.

Despite the increase in CFC-11 emissions, its concentration in the atmosphere continues to decrease, but only about half as fast as the decline observed a few years ago, and at a substantially slower rate than expected. This means that the total concentration of ozone-depleting chemicals, overall, is still decreasing in the atmosphere. However, that decrease is significantly slower than it would be without the new CFC emissions.
Precise measurements of global atmospheric concentrations of CFC-11 made by NOAA and CIRES scientists at 12 remote sites around the globe show that CFC-11 concentrations declined at an accelerating rate prior to 2002 as expected. Then, surprisingly, the rate of decline hardly changed over the decade that followed. Even more unexpected was that the rate of decline slowed by 50 percent after 2012. After considering a number of possible causes, Montzka and his colleagues concluded that CFC emissions must have increased after 2012. This conclusion was confirmed by other changes recorded in NOAA’s measurements during the same period, such as a widening difference between CFC-11 concentrations in the northern and southern hemispheres – evidence that the new source was somewhere north of the equator.
Measurements from Hawaii indicate the sources of the increasing emissions are likely in eastern Asia. More work will be needed to narrow down the locations of these new emissions, Montzka said.

The Montreal Protocol has been effective in reducing ozone-depleting gases in the atmosphere because all countries in the world agreed to legally binding controls on the production of most human-produced gases known to destroy ozone. Under the treaty’s requirements, nations have reported less than 500 tons of new CFC-11 production per year since 2010. CFC-11 concentrations have declined by 15 percent from peak levels measured in 1993 as a result.
That has led scientists to predict that by mid- to late-century, the abundance of ozone-depleting gases would to fall to levels last seen before the Antarctic ozone hole began to appear in the early 1980s.
However, results from the new analysis of NOAA atmospheric measurements show that from 2014 to 2016, emissions of CFC-11 increased by more than 14,000 tons per year to about 65,000 tons per year, or 25 percent above average emissions during 2002 to 2012.
To put that in perspective, production of CFC-11, marketed under the trade name Freon, peaked at about 430,000 tons per year in the 1980s. Emissions of this CFC to the atmosphere reached about 386,000 tons per year at their peak later in the decade.
These findings represent the first time emissions of one of the three most abundant, long-lived CFCs have increased for a sustained period since production controls took effect in the late 1980s.
If the source of these emissions can be identified and mitigated soon, the damage to the ozone layer should be minor. If not remedied soon, however, substantial delays in ozone layer recovery could be expected, Montzka said.
David Fahey, director of NOAA”s Chemical Science Division and co-chair of the United Nations Environment Programme’s Ozone Secretariat ‘s Science Advisory Panel, said ongoing monitoring of the atmosphere will be key to ensuring that the goal of restoring the ozone layer is achieved.
“The analysis of these extremely precise and accurate atmospheric measurements is an excellent example of the vigilance needed to ensure continued compliance with provisions of the Montreal Protocol and protection of the Earth’s ozone layer,” Fahey said.
###
The paper: https://www.nature.com/articles/s41586-018-0106-2

OFF TOPIC
The Exxon hearing is on the 24th. In the meantime I have just reviewed the Amicus brief filed by Indiana and other states. It is devastating. Read it. I think dismissal is very much on the cards.
http://climatecasechart.com/case/people-state-california-v-bp-plc-oakland/
I so much wish that the defendants, cease all financial relationships with Oakland and San Francisco, but continue to use the loading/unloading facilities as defined by law.
But no product or support paid/given to Oakland and San Francisco. So what ever fossil fuels that they receive from the defendants should be shut down.
I so much wish that the defendants, cease all financial relationships with Oakland and San Francisco, but continue to use the loading/unloading facilities as defined by law.
But no product or support paid/given to Oakland and San Francisco. So what ever fossil fuels that they receive from the defendants should be shut down.
With all this hype of being ‘illegal’…..what exactly is the ‘punishment’?
That is up to the country that catches the illegal trade.
In USA the enforcement is under EPA law
https://www.epa.gov/ods-phaseout/enforcement-ods-regulations#main-content
In Australia it comes under Customs (Prohibited Imports) Regulations section 5K. Fines of up to $170,000 can be applied for each illegal import breach but the fine is under review to be increased..
I loved the neat political sidestep in this statement
Everyone knows the source you only have to look at CFC11 level studies in China. The UN has done countless reports into ODS smuggling involving CFC-11.
It is like with much in CAGW there seems to be this thing with green, eco groups in not wanting to call China to account.
The last large Communist dictatorship? Of course not. There is a very thin rind on those watermelons.
Just a technical question. As an official old fart I remember Freon 12 and 22. Where did CFC 11 come in?
It replaced Freon 12 & 22 and was known unsurprisingly as Freon 11 it’s advantage was it worked at a lower pressure.
Hard to believe the same folks who put melamine in children’s food would CHEAT on stuff, huh?
CFCs are measured in parts per TRILLION, so there isn’t even one molecule of CFC per billion molecules of air.
” one of the chemicals most responsible for the Antarctic ozone hole”
Any evidence of this in existence? I always thought the entire thing was a farce.
The ozone whole over Antartica waxes during the sunlit Austral summer and wanes in the dark polar winter. It appears to be a natural phenomenon unrelated to CFC-11.
The question never ever answered since the so-called hole was discovered was, “How do we know that it hasn’t always been there?”.
Alan the Brit,
The discovery wasn’t really that there was a hole, but that there had been large Ozone losses over time in this region, which can be said to have the appearance of “a hole” given its geographic properties. They had measurements going back to the 1950s and the measurements in the 1980s showed significant large declines in Ozone compared to then, increasingly so. There’s no question that such “a hole” had always been there. The measurements show it wasn’t there in the 1950s and 1960s.
You could perhaps argue that, maybe, there’s a chance it could be due to natural variability. However, more than thirty have now passed since the finding and the Montreal Protocol and there’s no sign of a clear divergence from the path of CFC concentration, which would be necessary to support a natural variability hypothesis.
@Alan the Brit on May 17, 2018 at 3:59 am
I was told in school in the late ‘60s or early ‘70s, before the satellite went up, that there was a South Pole ozone hole that varied with the seasons, but no one knew for sure if it had always been there, or if not when did it first appear.
2ndly, I’m still not convinced CFCs and the hole in the ozone are even related. I once read the Montreal Protocol was really nothing more than a dry run on an energy consumption reduction treaty.
Why is there no hole over the arctic ? I have no idea.
Har Old,
There is no ‘ozone hole’ over the Arctic because the Arctic lacks the strong circumpolar vortex that blocks tropical ozone in Antarctica.
Alan the B
Quite right to ask that. The article (and many others) takes the position that there should not be any ozone hole at all. This, in spite of plenty of evidence (measurements, theory) that the hole will appear every spring because of many natural influences.
Prof Lu of the University of Waterloo has taken a fresh look (meaning, ‘not that view’) starting about 10 years ago and shown multiple influences on the hole, plus a pretty well documented explanation of the influence of GCR’s on these chemicals. Further, he finds that bromine (which is not mentioned but is also controlled for the same reasons) has a significant influence on ozone destruction. It is from the oceans, mostly. In other words, absent all human emissions of everything, there would be an ozone hole caused by bromine and chlorines.
And even further, Prof Lu shows the mechanism by which ozone over Antarctica is a major heat ventilation control. I won’t bore anyone with how it works, but the global temperature is strongly affected by what happens to the ozone layer down south. Ozone has a massive GHG-heating effect. When and if the southern hole was ‘closed’ the Earth would warm considerably. Consider the implications of that!
The big deal is that even if we reduced our influence to nothing, the hole would be there and it would, in combination with cosmic rays, continue to modulate the heat flux. How about that.
To a certain extent, Prof Lu’s atmospheric chemistry group has shown that Svensmark’s mechanism is real and concentrated at the poles, strongly modulating the ozone concentration.
Wow it’s been twenty years since I thought about this one. The golden oldies do have a way of coming back, don’t they? Is Al Gore going on a “Best Of” tour or something?
Just my guess, but somebody finally decided that they needed to explain why the Montreal Protocol didn’t work, i.e. why there is still an ozone hole. And since the theology has to be right, the explanation has to be that somebody is cheating on the treaty. (Of course they almost certainly are cheating, but since the theory is a crock, that doesn’t matter any more than it matters how much extra CO2 we pump into the air).
“You could perhaps argue that, maybe, there’s a chance it could be due to natural variability.”
You certainly could, particularly chlorine and fluorine from Mount Erebus, the largest natural halogen source in the world…..
“However, more than thirty have now passed since the finding and the Montreal Protocol and there’s no sign of a clear divergence from the path of CFC concentration, which would be necessary to support a natural variability hypothesis.”
… which has been erupting continuously since 1971.
tty,
You certainly could, particularly chlorine and fluorine from Mount Erebus, the largest natural halogen source in the world….. … which has been erupting continuously since 1971.
Which shows the problem with your theory. The trend in Ozone Hole area changed dramatically from an increase up to mid-90s, to a flattening, and now looking like beginning to decline. This tracks extremely well with CFC concentrations.
Something which has been happening continuously since 1971 clearly can’t explain those trend changes.
Clyde, I have not seen those graphs but I understand that there is a local maximum outside the vortex and a local minimum inside it. I can buy that. As I explained in a comment below, ozone is a very unstable molecule, otherwise the atmosphere would be filled with ozone, and it is going to break down when it collides with water, ice, bromine or just about anything. Ozone moves down the vortex, collides and get destroyed, and that has been happening for the last few hundred million years.
Here the question is if the CFCs have a significant contribution on the ozone destruction or the natural ozone destruction process still trumps. I would say that CFCs don’t have a significant contribution because the maximum CFCs levels are in the equator, where temperatures are the highest and there is not a significant increase of ozone destruction in there.
Aside from the strong correlation between CFC concentration and Ozone Hole area? Sure. Direct understanding of the chemical and physical processes involved.
paulski0: If you wish to claim that there is a strong correlation between CFC concentration and Ozone Hole area, then it would be a good idea to provide evidence.
Mike Jonas,
Sure, here’s a time series of Ozone Hole area.
Here’s Ozone Hole area plotted along with combined concentrations of just the main two CFCs (11 and 12). Even with interannual variability the r^2 coefficient in annual averages is 0.64. For discrete 5-year averages the r^2 is 0.94.
The reason we are supposed to care about the ozone “hole” is that the increased UVAs and UVBs will cause more skin cancer, etc. Unfortunately for ozone alarmists, as far as I know, no data exist showing increased UVs reaching the earth’s surface, nor is the rate of skin cancer increasing, after adjustments for increased sun-based leisure time, better diagnoses, etc.
Dupont – 50 year patent expiring in latter 90’s; conveniently wound up with a new patent for the replacement CFC. How fortunate that the Ozone Hole ‘catastrophe’ just happened to be noticed shortly prior.
Whether you call our country a Fascist one or a Corporatism, Gov’t and our International Corporations work hand-in-glove to benefit each other.
And here is the zonal mean distribution of CFC-11 distribution measured by the satellite ENVISAT. Please notice that the zone of maximum CFC-11 concentration is at the equator, while the zone of minimal concentration are at the poles. If I apply the causation principle, I would say that CFC-11 protects the ozone layer, since the layer is thicker where the CFC-11 levels are the highest (at the equator).
http://digital.csic.es/bitstream/10261/133614/1/acp_12_11857_2012.pdf
http://oi64.tinypic.com/6jkqzc.jpg
Urederra,
While ozone is moderately high in the tropics, where UV is strongest, the highest values are reached just outside of the Antarctic circumpolar vortex in the late-Winter.
Clyde, I don´t understand what are you trying to say. Do you mean that the highest values of ozone are outside of the circumpolar vortex?
And what that has to do with the fact that CFC-11 levels are highest in the equator and minimal at the poles?
Paulski0 May 17, 2018 6:05 a.m.
Your links do not contain numerical data. We can compare the data about ozone hole over Southern Hemisphere from NASA Ozone Watch (table):
https://ozonewatch.gsfc.nasa.gov/statistics/annual_data.html
and values of CFC concentrations in Earth’s atmosphere:
https://www.esrl.noaa.gov./gmd/hats/graphs/graphs.html
Year 1979 1985 1988 1994 2004 2013
Minimum ozone (DU) 225 146 171 94 123.5 132.7
CFC-12 (ppt) ~250 ~370 ~400 ~530 ~530 ~520
CFC-11 (ppt) ~140 ~180 ~220 ~230 ~220 ~220
Can somebody see a “strong correlation” here? I could not.
Please note also that the direct evidence of CFC’s presence in the stratosphere OVER ANTARCTICA is absent.
Where exactly is the evidence of this strong correlation?
How exactly did they measure CFC concentrations in the ozone layer?
Paulski0, since the hole was first measured in 1954 can you assure me it did not exist in 1900?
Correlation does not equal causation. The chemistry is pretty dodgy, and the transport appears impossible.
Urederra,
Look at any of the NASA ozone maps for early-September and you will see anomalously high ozone concentrations outside of the circumpolar vortex.
It has nothing to do with the CFC levels because it takes extremely low stratospheric temperatures to make the crystals that participate in the catalytic destruction of ozone. Those temperatures are principally reached in the nether regions of the poles where the sun doesn’t shine for months.
Sorry, I posted this reply in the wrong thread:
Clyde, I have not seen those graphs but I understand that there is a local maximum outside the vortex and a local minimum inside it. I can buy that. As I explained in a comment below, ozone is a very unstable molecule, otherwise the atmosphere would be filled with ozone, and it is going to break down when it collides with water, ice, bromine or just about anything. Ozone moves down the vortex, collides and get destroyed, and that has been happening for the last few hundred million years.
Here the question is if the CFCs have a significant contribution on the ozone destruction or the natural ozone destruction process still trumps. I would say that CFCs don’t have a significant contribution because the maximum CFCs levels are in the equator, where temperatures are the highest and there is not a significant increase of ozone destruction in there.
“Dupont – 50 year patent expiring”
Patents are for 17 years. But don’t let facts get in the way of your wild conspiracy story.
“The reason we are supposed to care about the ozone “hole” is that the increased UVAs and UVBs will cause more skin cancer, etc.”
In Antarctica. Few people there. Most cover up real well due to the cold.
As far as I’m concerned, if it were the case that manmade emissions caused high UV exposure even in Antarctica where there are few people, then the Montreal Protocol would be justified. It’s not appropriate that our activities should harm wildlife.
I don’t think that the elaborate mechanism proposed to explain why the “problem” is CFC concentration, but is only applicable to Antarctica makes sense. But let’s not lose sight of the fact that this supposed stratospheric cloud theory is not the explanation given at the time of the Montreal Protocol. At the time, we were told that CFCs were an existential threat to human life. The precautionary principle demanded that we ban CFCs because although the “problem” was only seen at the South Pole, it would soon extend over the whole planet.
We also heard Al Gore declaring that the Montreal Protocol had been a glorious success because the expected advance of the ozone hole had been averted.
So, paulski0, and any others who are true believers, do you have the intellectual integrity to admit that the original justification for the Montreal Protocol was mistaken?
“It’s not appropriate that our activities should harm wildlife.”
In Antarctica? What wildlife is being harmed? What wildlife is even there?
It looks like you stopped reading after the first sentence Gamecock. As a result, you totally missed my point which was that if there is any effect of higher UV exposure in Antarctica, it is not being caused by human activity. There is wildlife in Antarctica of course, not just the well-known penguins. There are other bird species and seals. If we really were harming them, I’d say it was wrong, but we’re not.
Taking the attitude “who cares about the wildlife” is giving the alarmists ammunition for their argument that skeptics don’t care about the environment. At least for this skeptic, that’s not true at all.
@gamecock
no need of a conspiracy. here is a pretty neutral and factual story: Dupont just rode the wave, rather reluctantly, but, hey, business is business.
http://www.topsecretwriters.com/2015/06/the-dupont-freon-conspiracy-was-the-ban-delayed-for-profits/
Lots of Watts readers get confused. Some even think the earth isn’t warming.
yes. And and they are flat-Earthers, for instance using this figure of a flat, homogeneous, non-rotating Earth.
Oh Wait. That’s not Watts readers job. Never mind
And they believe in consiracy theory, about 9/11 for instance, like this one.
http://www.nydailynews.com/news/world/oscar-winner-marion-cotillard-dismisses-9-11-conspiracy-article-1.286439
Oh. wait. She’s an action-against-climate-change militant. Never mind.
Bravo sierra. We know the earth is warming. And we agree that man may have a minuscule part in it. We just aren’t convinced it is a disaster
Please stop spouting ignorant strawmen.
Koontz, to clear up your confusion, the earth does seem to be warming slightly, tho where I live summer highs have actually decreased (I’m not in a UHIE city). But anyway, the “climate” has hardly ever been better, at least since the medieval climate optimum.
That’s an improvement, Scott used to claim that none of believed that the Earth was warming.
Of course he still convinced that only people who agree with him are doing science.
Then there is the fact that there is no ‘hole’. It is simply an area of less dense ozone. The word ‘hole’ implies that there is no ozone over the Antarctic. In fact there is still ozone, just not as much.
Interesting graph form NASA (via Wikipedia)
Because idiotic “environmental” laws make it impossible to get rid of an appliance with the chemical, people just loosen the fitting and let it leak out. Then they can put the old refrigerator in the dumpster.
I have never understood why the alleged main effect of CFCs is at the furthest point from their release. Logic for me would be that there should be a massive hole at the NORTH pole, not the south.
That’s because CFCs probably are not responsible for the ozone decreases. The ‘hole’ is likely a natural feature that has existed long before commercial CFCs were produced in any quantity for refrigeration.
That was my conclusion too.
Also my conclusion.
The pulsing polar holes of the ozone are still regarded as evidence of its depletion from human activity and remains one of the chapters in the man-made global warming scary narrative nonsense.
We all know some industrial compounds (chlorofluorocarbons or CFC’s) chemically react with the O3 molecule of the ozone layer of the stratosphere thus “depleting” it. But there are other explanations.
The ozone layer is relatively thin (at 1 atm it would be less than 1/8 of an inch thick) and in a constant state of replenishment as well as depletion. 12 to 25 miles up high energy UV splits the O2 molecule into two atomic O1 molecules that then combine with O2 to form the unstable, temporary O3 ozone molecule which absorbs low energy UV. It is now understood, or should be understood the main reasons for the changing polar ozone hole sizes are natural and include the seasonal lack of light, the atmospheric fluid dynamics of the polar vortices, fluctuations with naturally occurring nitrous oxide and most importantly, the solar variances in UV radiation.
This whole Montreal protocol is just another huge scam. 1) The Ozone hole over the Antarctica has always been there. 2) The Antarctica is an extremely dry place and the Ozone hole only appears in the winter. when there is little sunlight. 3) The size varies tremendously from year to year. 4) How is anybody going to get skin cancer if they dont live in ANTARCTICA? 5) Even if they do live in Antarctica, there is little sunlight (sun isnt strong at that time of year) in the Antarctica winter. 6) CFCs are openly bought and sold in Argentina , China and many other countries. 7( There has been no change in worldwide skin cancer rates since the Montreal protocol
http://www.nature.com/news/2007/070924/full/449382a.html
The above article busts the Ozone hole theory. The Nobel prize should be retracted for the researchers and the Montreal protocol shold be dismantled.
Thanks Alan, once and awhile I receive proper comment regarding my comment….such is Anthony and WUWT. I wonder when, if ever, responsible journalists will “cover” this site. My guess….not in my lifetime.
Oh well, we do, as always, live in interesting times.
Manmade CO2 probably has the magic power to achieve this, like so many other things it can do.
Here’s an explanation of why you get this phenomenon at the South Pole and not elsewhere. But the point of release is largely irrelevant anyway given atmospheric mixing and the long atmospheric lifetime of these molecules. Measurements of CFC-11 and CFC-12 at the South Pole show concentrations which are very similar to those in the Northern Hemisphere.
But the concentration of CFC-11 are maximum at the equator and minimal at the poles.
And there is a problem in the explanation you provided. It works fine without the inclusion of chlorine. Once the author starts talking about chemical reaction involving chlorine and temperature the explanation stops making sense. Chemical reactions go faster as temperature rises, and not the opposite as the author claims.
Besides, ozone levels recover in late spring, because ozone is being formed. In fact, ozone has been being formed during the last few hundred of million years. If there was no natural mechanism of ozone destruction, there would be more ozone in the atmosphere than oxygen. There is very little ozone in the atmosphere because ozone is a highly unstable molecule and it is going to be destroyed anyway, no matter if it collides with chlorine or water.
I don´t buy any explanation that does not include the natural degradation of ozone. It is like trying to explain Earth climate solely with anthropogenic CO2 production and without taking into account any other natural factors that have been running for millions of years.
This link is not an explanation, it’s just a matter of reasoning, and there is no specific information about CFC in the stratosphere over Antarctica. There are real experimental data on HCl and ClO here:
https://www.researchgate.net/publication/307809073_HCl_and_ClO_profiles_inside_the_Antarctic_vortex_as_observed_by_SMILES_in_November_2009_comparisons_with_MLS_and_ACE-FTS_instruments
However, the authors did not tell anything about CFC as a source of these compounds. Most likely, HCl hits the stratosphere over Antarctica from the Erebus volcano:
https://www.deepdyve.com/lp/elsevier/the-antarctic-ozone-depletion-caused-by-erebus-volcano-gas-emissions-zUOrlpRELu
I would very much like to see a reference to the real measurements of CFC-11 and CFC-12 over the South Pole.
Urederra May 17, 2018 at 9:32 am
And there is a problem in the explanation you provided. It works fine without the inclusion of chlorine. Once the author starts talking about chemical reaction involving chlorine and temperature the explanation stops making sense. Chemical reactions go faster as temperature rises, and not the opposite as the author claims.
Not always, these are not single step reactions for which that would be true. There are chemical reaction mechanisms where there is a negative coefficient of rate, the reaction that leads to ‘knock’ in an spark ignition engine is one of them. In the case of ozone destruction over the poles it is associated with the formation of PSC which only occurs at low temperature, when those clouds warm up in the spring they release the sequestered reactants which cause the problem
Besides, ozone levels recover in late spring, because ozone is being formed. In fact, ozone has been being formed during the last few hundred of million years. If there was no natural mechanism of ozone destruction, there would be more ozone in the atmosphere than oxygen. There is very little ozone in the atmosphere because ozone is a highly unstable molecule and it is going to be destroyed anyway, no matter if it collides with chlorine or water.
There is a natural destruction mechanism which is part of the Chapman mechanism, it is photodissociation by UV light. In the absence of UV during the winter the O3 is stable because this mechanism can’t take place.
I don´t buy any explanation that does not include the natural degradation of ozone.
It is included, it’s just not capable of explaining the springtime degradation seen since the 70s.
I don’t have time right now to look it up, but my recollection is that Ozone CFC interaction is primarily a phenomenon that occurs where the stratosphere is extremely cold. Sufficiently cold temps only occur during the Antarctic Winter (June-Sep)? The Northern Hemisphere stratosphere doesn’t get as cold, so there’s no Arctic Ozone hole that will blind/kill zillions of people/caribou/people?
Where are global warming and polar amplification when we need them?
I’m wondering how the CFC’s find their way to Antarctica.
Since the Ozone Hole is over Antarctica that must mean that only sunbathers there have to worry about the extra UV.
I thought the ozone hole was healed after the Montreal Protocol
banned CFC which was depleting the ozone layer, which, at the
time, was blamed for causing global warming (and skin cancer).
But global warming ( and skin cancer) continued. So the depletion
of the ozone layer does not cause global warming.
Therefore, DOES IT MATTER?
I suspect that, particularly here in the UK, was the sudden modern fashion of young men stripping to the waist every time the sun came out from the mid to late 60s onwards! In young women, it was fashions that allowed more & more flesh to be on display, all unprotected from UV rays!
Then everybody panicked and started slathering themselves and their kids with SPF bazillion sunscreens, and now rickets and other symptoms of vitamin D deficiency are on the rise again. Face meet palm.
I don’t accept the premise that CFC-11 and other refrigerants are responsible for the ozone “hole”.
If these emissions are in the Northern hemisphere, why is the Ozone hole above Antarctica?
The asymmetry between northern and southern hemisphere, is it the first time it is seen, or the first time it was looked for and seen?
To blame the Chinese may be a bit premature. After all, they want to sell their fridges to the West. And sources like fridges and old foams have a long lead time anyway; so what was the economy of China, say 40 years ago? Let me consider the possibility that the source or sources are natural. What would that do to the credibility of Montreal?
See paulski0 post above, including https://createarcticscience.wordpress.com/2016/04/15/is-there-an-arctic-ozone-hole/
Sallie Baliunas had debunked the ozone hole since 2000
This is worth a read
https://kisslibrary.com/book/F2D2C3C2BECF9D2163D8?utm_source=b-dl-1901-4&utm_medium=banner&utm_campaign=newtraf&search=The+Holes+in+the+Ozone+Scare%3A+The+Scientific+Evidence+That+the+Sky+Isn%27t+Falling&x=453813
My first reaction was that satellite measurements should be able to tell us where the CFCs are coming from. I was surprised that I couldn’t find a map showing ground level CFC concentrations in the atmosphere. WUWT?
As some above have noted, the concentrations involved are ppt. Measuring something at the parts per million level is hard enough. All things being equal, measuring something at ppt is a million times more challenging.
If I were a cynic, I would suggest that this claim of increasing CFC’s in the atmosphere has been proposed soley to give an explanation of why the Ozone hole has not yet disappeared, despite the banning of the CFC’s decades ago by a consensus of nations. But I’m not a cynic, so I believe every word they say (do I need the sarc?).
There isn’t a claim of increasing CFC-11 in the atmosphere. What has been observed is that the decline in atmospheric CFC-11 has slowed, and this is inconsistent with international claims that emissions are practically zero.
There has never been any suggestion that the Ozone Hole should have disappeared by now, due to the long atmospheric residence time of CFCs. The typical proposed finishing line for recovery to 1980 levels has long been ~2050.
I’m curious (as always) what has the been the extent of the ozone layer over the last hundred years or so?
Haven’t read the whole story yet, but is this a work of fiction? They say there are emissions, but don’t know who is doing it. Sounds to me their readings rose and the rest is assumption, not proven.
Is it true that CFCs are heavier than air? If so then has it been explained how it gets up to the ozone layer?
If you pee in one end of a pool, why does the yellow color disappear?
Sounds like your saying that the weight can be ignored. I’ll try using that on my wife next dinner time when I want seconds.
There probably is a relationship between weight and how slowly one leaves the dining table. In any case, heavier molecules certainly diffuse more slowly.
Diffusion, and Henry´s law: Actually, the physics are similar to water. Water is also heavier than air, but air can “hold” certain amount of water, it is called humidity = the amount of water vapor present in the air.
The amount of water that can be present in the air varies with temperature, Hot air can hold more water vapour than cold air. Same happens with CFCs. If you look at the graph I have been posting here you will notice that there is more CFC-11 at the equator than at the poles. That is because the air over the equator is warmer and can hold more CFC-11 vapours, and more water vapour than the air over the poles.
It is not much CFC-11 anyway, it is measured in parts per trillion (be volume: pptv) Also, CFC-11 is one of the smallest CFCs around, with a molecular weight of 137.4 g/mol The concentration of heavier CFCs should be even lower.
thanks – that is well explained
Oh, dear! You’d better read that again. The water that is heavier than air is not the water that constitutes humidity. That water is “lighter than air”.
If Wikipedia is trusted Ted is right – water vapor is lighter than air. I guess I’m back to asking how CFCs from ground level gets up to the ozone layer.
Analogous to salt or sugar being soluble in water, CFCs diffuse into air. Salt and sugar are “heavier” than water (that is, in their pure isolated form, they have a higher density). They sink to the bottom, but once they dissolve into water, they diffuse throughout the solution until the concentration is uniform throughout.
The same thing applies with gases. An isolated volume of CFC such as a balloon would not float in the air, but popping the balloon it will all diffuse into the room. When you open a window, it will eventually all diffuse out into the atmosphere as a whole.
Once again we have a case where the scientific principle is sound but the effect is not significant. Don’t attack the claim by trying to d-ny diffusion. Debunk the idea that concentrations in parts per TRILLION are sufficient to significantly affect the natural ozone process. Ask why the mechanism isn’t happening outside of Antarctica as the advocates of the Montreal Protocol claimed would be a problem.
I’m on board with your attack that the concentration isn’t enough to have the effect that they believe it has.
It was just intellectual curiosity that I wanted to understand the weight issue.
Yes, Jouatt, CFC-11 and CFC-12 are 4 – 4.5 times heavier than air. Also, boiling point of CFC-11 is +23.8 degrees Celsius, so even in summer at altitudes 2-3 km it condenses in air. M.Molina and F.Rowland (1974) explain getting CFC into stratosphere by “vertical diffusion” that does not depend on molar mass. However, driving force of vertical diffusion is the temperature gradient that should be about zero in the tropopause. So, may be the proponents of “CFCs are ODS” theory know how to explain these facts. I do not.
aleks May 17, 2018 at 3:46 pm
Yes, Jouatt, CFC-11 and CFC-12 are 4 – 4.5 times heavier than air. Also, boiling point of CFC-11 is +23.8 degrees Celsius, so even in summer at altitudes 2-3 km it condenses in air.
No it does not condense in air, for that to happen its partial pressure would have to be ~1atm, as noted it’s only present at ppt levels
M.Molina and F.Rowland (1974) explain getting CFC into stratosphere by “vertical diffusion” that does not depend on molar mass. However, driving force of vertical diffusion is the temperature gradient that should be about zero in the tropopause. So, may be the proponents of “CFCs are ODS” theory know how to explain these facts. I do not.
Diffusion depends on concentration gradients, also winds and tropical thunderstorms rapidly mix the atmosphere.
The obvious solution is to raise a CFC tax in the countries that do comply.
That is pointless lets give you the the simple equivalent put a tax on illegal drugs and how much money do you raise???
The answer is next to zero because all the trade is concealed because it is illegal 🙂
/sarc
Another obvious solution is to raise the ban on CFCs.
Each level of state sponsored junk science reinforces the next forward claim; DDT ban, Keynesian economics, Climate change, Ozone hole are rooted in authority politics not science or logic.
The hole was already there when first measurements were made by Dobson, after whom the units of ozone were named.
And Gary, Dobson made the discovery in the 1950s during the International Geophysical Year (July 1, 1957, to December 31, 1958).
Dan Kurt
Gary,
I have read this claim many times, but have been unable to find a source for it. Do you by chance have a citation that you can provide?
Are these the same people who measure imaginary “back” radiation?
I’m Shocked I tell you Shocked that someone in Asia is cheating on an international treaty.
…would to fall to levels last seen before the Antarctic ozone hole began to appear in the early 1980s….
My! I didn’t know that Medieval scientists had been documenting the absence of an ozone hole in the 1200s. Perhaps they wen up in a chariot pulled by swans?
Chariots were naturally air conditioned then, didn’t have to worry about it.
.. but, unless you had the horses at the back, pushing, you would occasionally suffer quite an unpleasant atmosphere for a short time….
Mt Erebus is an active stratovolcano in Antarctica that has been spewing carbon dioxide, hydrochloric acid and hydrofluoric acid into the stratosphere since the year dot (~1.2 my). Among the elements in these gasses are: carbon, chlorine and fluorine.
http://cfc.geologist-1011.net
Carbon dioxide and hydrogen fluoride (it’s acid in solution, not in gas phase) do not oxidize by the ozone, and C=O bond energy is high enough, so CO2 and HF do not affect on ozone layer. Only HCl in this case is important.
Is that the same agent that was used in the foam insulation on the space shuttle main fuel tank prior to when the foam started falling off of it during lift off?
Shuttle external tank spray-on foam insulation (SOFI) initially used freon. It was banned, though Michoud got an extension thru 1997 to use it. Once the ETs with the new foam formulation started flying, more chunks of SOFI started evolving off during launch, gouging out more divots in the thermal protection tiles on the belly of the orbiter. I have read some reports that the number of divots increased by a factor of 10.
http://www.foxnews.com/story/2003/02/07/did-pc-science-cause-shuttle-disaster.html
The chunk of SOFI that took out Columbia came from the left bipod ramp. It was a pretty large chunk. NASA management decided not to take a look at the suspected impact either via USAF ground assets or via EVA and the rest is history.
EPA asbestos ban was blamed for the Challenger, as asbestos does not react to low temps like the rubberized replacement does (rubberized stuff got brittle). There are those of us that believe EPA bans contributed to both vehicle losses (along with a bunch of dumb operational decisions – launching a big solid when it is really, really cold and not taking a look after you think you have a problem).
My personal theory is that the launch crews were used to chunks of ice falling off the boosters during the early stages of flight. You can see this all the way back to early Atlas launches (the LV is stainless steel, the white you see is ice), perhaps as far back as Redstone. If you are used to seeing stuff fall off the booster and see it every single launch, you don’t pick up on a problem as quickly as you should. Cheers –
No, we’d almost lost at least one shuttle before Columbia due to foam strikes on the heat shield. I seem to remember it was the one launched after Challenger broke up, or maybe the one after that? If I remember correctly, they just got lucky and the tiles were lost in an area that used extra-thick metal that didn’t burn through.
Even Columbia’s first flight had damage from lost tiles, though I’m not sure whether they just fell off or were knocked off.
Either way, the problem and risks of foam strikes were well-known and never supposed to happen in the original design. As with the SRB leaks, NASA just preferred to cross their fingers instead of fixing the problems.
The Montreal protocols were very effective in banning a substance whose patent was about to expire so that it could be replaced with a less efficient one that had recently been patented.
Which patent was about to expire, ‘chapter and verse’ please?
“…one of the chemicals most responsible for the Antarctic ozone hole”
——————————————–
Bullpucky! That hole is a natural phenomenon that was discovered in 1956 long before (significant amounts of human CFC emissions) and scientifically explained in 1959.
Art
Do you have a link or reference.
Thanks
You could google G.M.B. Dobson who invented the instrument to measure ozone and discovered the antarctic “hole” in 1956. At first he thought it was an anomaly but when it reoccurred in 1957 he concluded it was a natural annual event.
In 1958, two French scientists determined that the hole was of natural origin (south polar vortex and lack of sunlight) and published their findings. When all the panic erupted after NASA noticed the hole, they reviewed their findings and republished them in 1990.
(Rigaud, P. and B. Leroy, 1990,”Presumptive Evidence of a Low Value of Total Ozone Content Above Antarctica in September, 1958″, Anales de Geophysique, Vol. 8 (11), pp. 791-94.)
Thanks Art.
Yes it is a natural occurring event from my research.
Look at the near continuous small “hole” for the past two months. No sunshine and teperatures above required level.
Seems no one really looks at the data.
Regards
found it
Annales Geophysicae Volume 8, Issue 11, 1990, Pages 791-794
Presumptive evidence for a low value of the total ozone content above Antarctica in September, 1958(Article)
Rigaud, P., Leroy, B.
Centre National de la Recherche Scientifique, 1, place A.-Briand, 92195 Meudon, France
Abstract
Ozone spectrographic measurements, using stars, moon or blue sky as light sources, have been performed in 1958 at Dumont d’Urville (66°40′S). Re-examination of the data shows that a strong minimum of the total ozone content has been observed that year in the Austral spring time. This suggests a natural phenomenon to explain the Antarctic “ozone hole’. -Authors
https://www.scopus.com/record/display.uri?eid=2-s2.0-0025586819&origin=inward&txGid=8ab7ca9d9d1d5d28706864f98d7c1420
ozonebust May 18, 2018 at 12:47 pm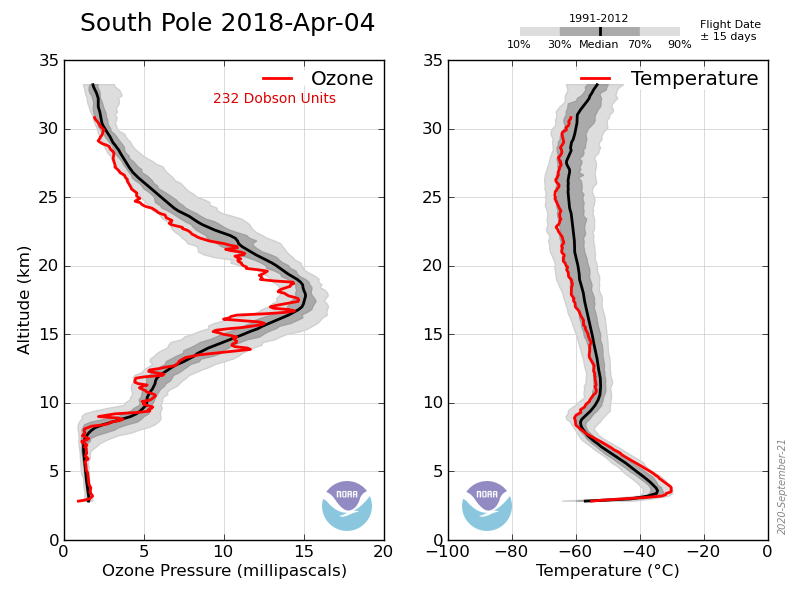
Look at the near continuous small “hole” for the past two months. No sunshine and teperatures above required level.
As it usually is at this time of year, the ‘hole’ doesn’t start to form until the austral spring in september.
Not true, that antarctic ozone levels were somewhat lower in the spring was detected, this is not the ‘Ozone hole’ however. Spring levels were still in the normal range (above 300DU) it wasn’t until the mid-70s that the levels started to drop rapidly, the ‘Ozone hole’ is usually defined as the region where column ozone drops below 300DU (not observed before 1980).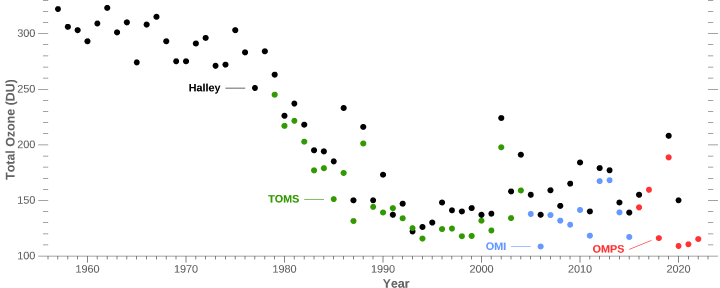
Here’s the data from the late 50’s:
Instruments on the ground (at Halley) and high above Antarctica (the Total Ozone Mapping Spectrometer [TOMS] and Ozone Monitoring Instrument [OMI]) measured an acute drop in total atmospheric ozone during October in the early and middle 1980s. (Halley data supplied by J. D. Shanklin, British Antarctic Survey ).
Phil
It’s below 220DU.
It coincides with increased ocean heat release over the same period from the equatorial region.
Regards
Thanks that was a typo I meant 200DU.
I HATE this kind of figure where the zero line is not zero. Not always made to decieve, but always deceptive nonetheless
paqyfelyc May 20, 2018 at 4:54 am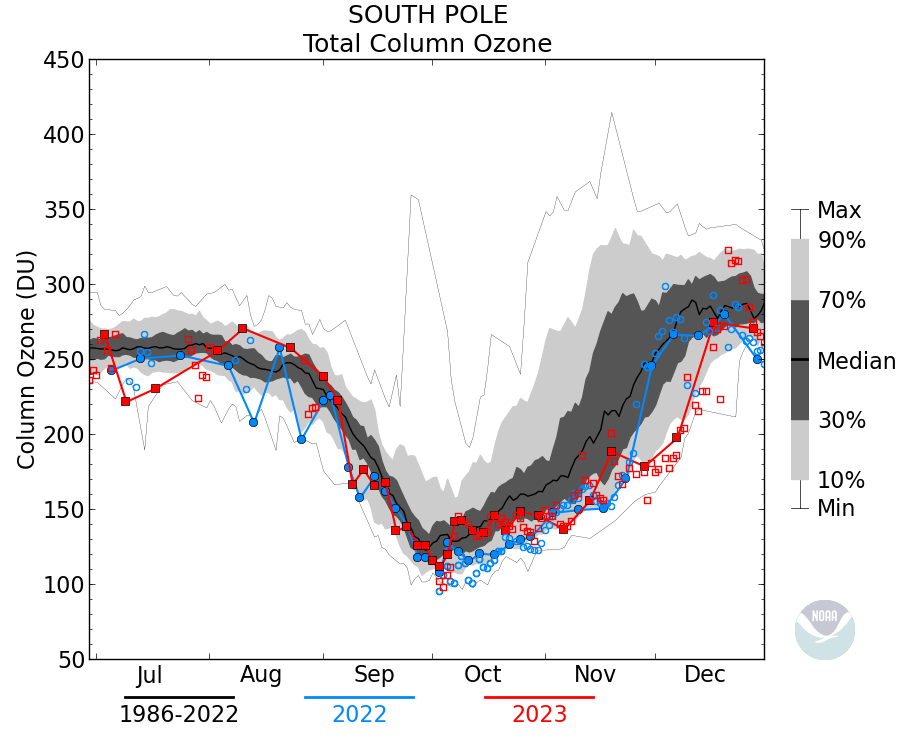

I HATE this kind of figure where the zero line is not zero. Not always made to decieve, but always deceptive nonetheless
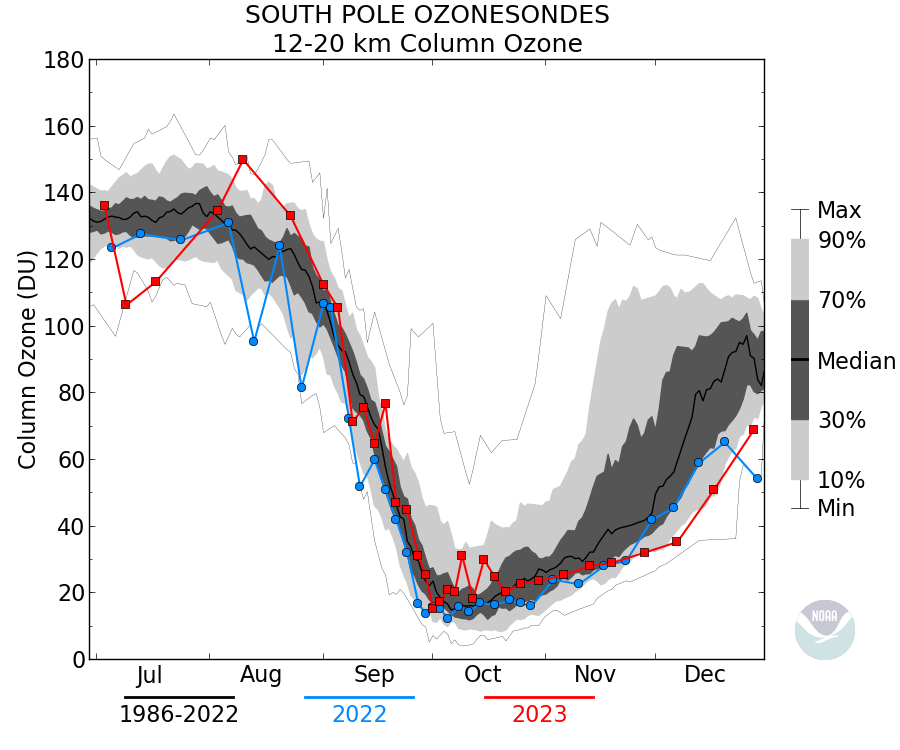
Actually in this case it makes sense since the ozone ‘hole’ occurs between ~12km and 20km where the conditions favor the depletion mechanism (PSC), thus the minimum value of Total column ozone is about 100DU. The data is sometimes plotted as the ozone between those heights rather than Total column:
Here’s the sonde data showing the profile corresponding to one of the lowest recorded value:
Some data on halogens from Mount Erebus:
https://s3.amazonaws.com/Antarctica/AJUS/AJUSvXXVIIn5/AJUSvXXVIIn5p271.pdf
https://www.researchgate.net/publication/283802406_The_Antarctic_ozone_depletion_caused_by_Erebus_volcano_gas_emissions
Maybe “scientists” can find one or more additional useful chemicals to ban.
underway. All of them chemicals.
“family of chemicals most responsible for the giant hole in the ozone layer that forms over Antarctica each September”
I thought the jury was still out on this. Do we in fact know that there was no seasonal ozone hole 50 or 100 years ago? When global ozone concentration mapping started, was there no seasonal ozone hole, then it appeared and got bigger year by year?
Just asking.
We know it was there in 1956 and was explained as a natural occurrence a couple years later. See my comment a few posts above.
Way back when the panic over the discovery of the hole by NASA was in the news, I read that one of their scientist figured it couldn’t have just suddenly formed because of those chemicals, it would have gradually built up over the years as the concentration increased, so he looked into NASA’s satellite photo archives and saw it was there all along, ever since 1972 when they first started. Seems they had previously dismissed it as a refraction caused by the curvature of the atmosphere.
But don’t let scientific fact get in the way of a good doomsday scenario.
Quinn May 17, 2018 at 2:00 pm
“family of chemicals most responsible for the giant hole in the ozone layer that forms over Antarctica each September”
I thought the jury was still out on this. Do we in fact know that there was no seasonal ozone hole 50 or 100 years ago?
We know that there was no ‘hole’ from 1957 when measurements began until about 20 years later.
When global ozone concentration mapping started, was there no seasonal ozone hole, then it appeared and got bigger year by year?
Yes, that’s right.
“That has led scientists to predict that by mid- to late-century, the abundance of ozone-depleting gases would to fall to levels last seen before the Antarctic ozone hole began to appear in the early 1980s.”
It didn’t begin to appear in the early 80’s, it was always there and always has been. It was detected in the early 80’s.
Alarmist drivel!
Actually it was first detected in 1956.
Patrick MJD May 17, 2018 at 4:40 pm
“That has led scientists to predict that by mid- to late-century, the abundance of ozone-depleting gases would to fall to levels last seen before the Antarctic ozone hole began to appear in the early 1980s.”
It didn’t begin to appear in the early 80’s, it was always there and always has been. It was detected in the early 80’s.
Alarmist drivel!
No it’s the truth, see the results from the British Antarctic Survey since 1957:
https://wattsupwiththat.com/2018/05/17/ooops-despite-montreal-protocol-ozone-destroying-cfcs-on-the-rise/comment-page-1/#comment-2821111
Once upon a time I asked my research staff the question, “How did we know there was not a hole in the ozone over the Antarctic in the centuries prior to 1980 since most of the evidence that there is one is derived from satellite data?”
The first thing a Indian villager buys when his village gets electricity is a refrigerator. So gee I wonder who might be producing the illicit CFCs today.
Edwin May 18, 2018 at 8:23 am
Once upon a time I asked my research staff the question, “How did we know there was not a hole in the ozone over the Antarctic in the centuries prior to 1980 since most of the evidence that there is one is derived from satellite data?”
Hopefully one of them replied that ‘We know because the British Antarctic Survey results since 1957 show the development of the ‘Hole’ around 1978′.
Phil, you missed the point. The first expedition to actually land in the Antarctica was around the turn of the 20th and they were not looking up. The 1957 exploration was part of IGY which was the first real modern scientific exploration of Antartica. Before that it was primarily whaling ships in the area. Whether or not there was a hole prior to humans actually landing during the IGY years is unknowable. I know of no proxy for the lack or present of a ozone hole in the ice cores.
A critical review of the ozone chemistry assumptions of the Montreal Protocol
https://chaamjamal.wordpress.com/2018/04/01/ozone-depletion-and-ozone-holes/
Really, how can that be the case when you don’t mention PSCs?