Guest Essay by Kip Hansen
 Every author I have ever queried about a quote attributed to them in a University Science Press Release has told me the same thing.
Every author I have ever queried about a quote attributed to them in a University Science Press Release has told me the same thing.
“That’s not exactly what I said, and not what I really meant,” they tell me. I can feel the shy grins on their faces as I read their replies. They are usually rather perplexed as to how the press release sent out to the media by their own academic institution came to contain such a questionable quote but almost always seem to shrug it off with good sense of humor. After all, what can they do?
This does not come as any surprise to those of us who follow science news — there are whole internet enterprises dedicated to nothing but gently re-writing University press releases on the science and medical research done on their campuses and promoting the latest journal articles published by their professors and students. In 2014, in the BMJ (formerly the British Medical Journal), Ben Goldacre wrote an editorial “Preventing bad reporting on health research” in which he insisted that “Academics should be made accountable for exaggerations in press releases about their own work”. The editorial accompanied a study (Sumner et al. 2014) that “found that much of the exaggeration in mainstream media coverage of health research — statements that went beyond findings in the academic paper — was already present in the press release sent out to journalists by the academic institution itself”. I’m not sure I agree fully with Goldacre that academics should be “made accountable” for the exaggerations of the folks in the University Media or PR department — but academics and corresponding authors should insist on pre-publication approval of any and all statements issued by the media office about their research.
We often see here at WUWT short posts about some Press Release regarding a new journal paper aghast at what some scientist has said — well, “said” according to the press release. We’ve had a recent example here:
Study: CO2 causes Starfish to Dissolve — a typical skeptical look at a university press release (from Heriot-Watt University in Edinburgh, Scotland) titled “Carbon dioxide ‘pulses’ threaten Scotland’s coralline algal reefs”.
In this press release, Dr. Heidi Burdett is quoted as saying;
“We found that there was a rapid, community-level shift to net dissolution, meaning that within that community, the skeletons of calcifying organisms like star fish and coralline algae were dissolving.” [emphasis added — kh ]
I can imagine the thoughts running through the minds of the men-and-women-on-the-street — “Oh, those poor starfish, writhing in pain as their skeletons dissolve! The horror of it.”
Our home team author, Eric Worral, quotes the abstract of “Community-level sensitivity of a calcifying ecosystem to acute in situ CO2 enrichment” in his post. The abstract says nothing about poor dissolving starfish. We would have been treated to a link to the full-study but unfortunately it was not only behind a fire-wall but also accidentally hidden by a broken link.
Bad and Poor Science Journalism is a particular interest of mine and this (the University Press Release, not Eric’s post) looked like a good example. Being denied a look at the full study by fire-walling and my usual work-around stymied by a bad DOI link, I took my ‘when-all-else-fails’ approach and emailed Dr. Burdett with a request for pdf copies of the study and its supplemental information, which she promptly sent along with a pleasant note.
We need to understand what the study is about and what its findings are before we revisit the press release.
What’s the study about?
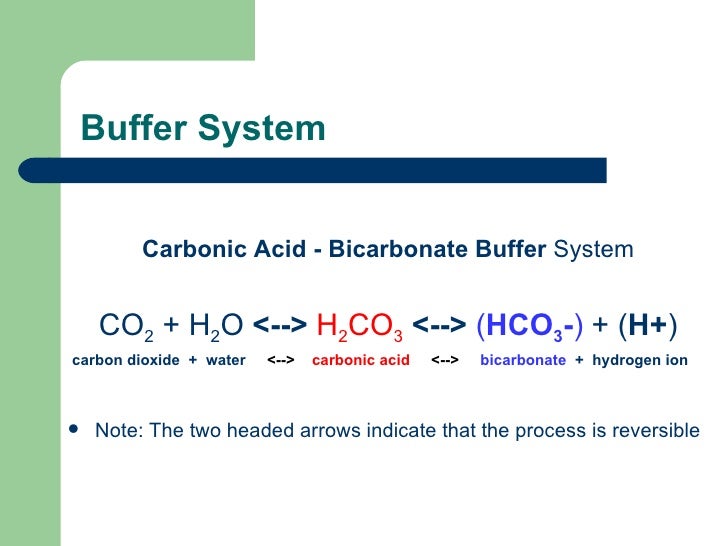
Dr. Burdett’s study is about “acute in situ CO2 enrichment” and its effects on the salt water chemistry in a “calcifying ecosystem”. That’s a mouthful. The field of study is known as “Ocean Acidification” [OA] — the fact that as CO2 concentrations increase in the atmosphere, sea water absorbs some of this CO2 at the surface and that absorption brings about this chemical reaction:

Sea water carbonate bio-chemistry is extremely complicated. So complicated that even scientists originally studying OA tended to get it wrong — so a field-wide effort was made to set this situation right (after a get deal of effort and money were mostly wasted) part of which I wrote about here at WUWT in “Ocean Acidification: Trying to Get the Science Right” and its follow-up “Dr. Christopher Cornwall Responds to “Ocean Acidification: Trying to Get the Science Right”. Magnificent detail is available in the 2011 250-page report “Guide to best practices for ocean acidification research and data reporting” published by the now-defunct EPOCA (European Project on OCean Acidification). Chapters 1 and 2 cover the basic chemistry and can be downloaded individually at the link above.
[UPDATE & CORRECTION 12 Mar 2018, 1100 hrs ET: Reader Kristi Silber correctly reports that the EPOCA web site no longer delivers the “Guide to Best Practices….” pdfs. Those interested in the Guide can download the entire report at this link:
https://www.iaea.org/ocean-acidification/act7/Guide%20best%20practices%20low%20res.pdf
Exactly why the International Atomic Energy Agency web site has a Climate Change section and hosts this document is a mystery to me (but I appreciate it!) — kh ]
If we over-simplify quite a bit, we can say that as CO2 mixes with sea water it tends to consume available carbonate ions forming bicarbonate ions and it is this aspect that interferes with (impedes) biological calcification. The mixed CO2 also lowers pH (the ‘acidification’ in OA). This represents pretty well understood sea water carbonate biological chemistry. The not-so-simple chemistry is complicated by the fact that some corals and other organisms have been found to manipulate the pH of the sea water in direct contact with themselves in a protective manner.
In this experiment, Dr. Burdett’s team studies CO2-enriched sea water and its effect on a “calcifying ecosystem” which looks something like this:

The pinkish nodules and little nubs are coralline algae and the pointy-wavy things are brittle stars (a relative of “starfish”) which “have five long, slender, flexible, whip-like arms…supported by an internal skeleton of calcium carbonate plates …” [Brittle stars come in all sizes and colors]. Coralline algae are red algae … characterized by a thallus that is hard because of calcareous [that is, composed of calcium carbonate] deposits contained within the cell walls. Obviously, calcium carbonate, and thus carbonate chemistry, is a major aspect of this ecosystem — a “calcifying ecosystem”.
The experiment is carried out in situ — meaning “in place”. Right out there in about 6 meters (18 feet) of water in Loch Sween, Scotland, UK. [Loch means “lake”, but of course this is really a “sea loch”, a seaside bay.] Tubes with a diameter of 38 cm (15 inches) and a height of about 25 cm (making up a volume of 28 liters or 7.3 gallons) were pushed down into the seabed enclosing some of that ecosystem pictured above and mounted with a lid with tubes and mixing paddles. Enclosed water was pumped to the surface where a mixing chamber bubbled in pure CO2 sufficient to lower pH by 0.2 pH units below ambient water pH, and the CO2-enriched water was returned to the experimental chamber on the sea floor. 4 such units were used.
Now comes the acute part. Acute means “of abrupt onset, of short duration, and is a measure of the time scale of a condition.” In this experiment, it means that after 15 hours, the researchers suddenly raised the CO2 concentration in the water (sufficient to lower pH by 0.2 units), kept it raised for 28 hours, and then shifted back to ambient conditions for 37 more hours. Water samples were taken at various times. All this underwater work was done with the aid of scuba gear.
So — what did they find when they did all this? They found that if they more than doubled the dissolved CO2 [p CO2 (µatm) from 821.6±343.4 to 1747.7±1403.33], then bicarbonate levels increased, along with dissolved inorganic carbon.
The conclusion drawn from these measurements:
“Under ambient CO2 conditions, the coralline algal community consistently exhibited a net calcification. During CO2 enrichment, a significant shift towards net dissolution was observed.”
[Note: there were several other conclusions about other technical points of sea water carbonate chemistry.]
A shift towards “net dissolution” — and what is that exactly when it puts on its best face to go out in the morning? I asked Dr. Burdett by email and received this reply:
“…what is meant by ‘net dissolution’ – this means that, at that point in the experiment, there was more dissolution of calcium carbonate than there was production. The loss may have come from live, calcified organisms such as coralline algae or starfish, dead carbonate skeletal remains or carbonate-rich non-biogenic sediment. Since we adopted a community-level approach, our measurements only give an indication of what is happening to the ecosystem as a whole – it is not possible to [identify] the individual components that are contributing to any differences we saw.”
Or, in other words, in the complex and complicated world of sea water carbonate chemistry, the sudden doubling of CO2 dissolved/mixed in the sea water in these chambers altered either (or both) the inorganic or the organic elements of carbonate chemistry inside the chambers — resulting in higher levels of both dissolved inorganic carbon and HCO3– (bicarbonate) — indicators that “there was more dissolution of calcium carbonate than there was production.”
This was an expected and interesting result.
SUMMARY:
What the Press release said: “the skeletons of calcifying organisms like star fish and coralline algae were dissolving.”
What the paper said: “a significant shift towards net dissolution was observed.”
What Dr. Burdett meant: “there was more dissolution of calcium carbonate than there was production. The loss may have come from live, calcified organisms such as coralline algae or starfish, dead carbonate skeletal remains or carbonate-rich non-biogenic sediment.”
There were no starfish writhing in agony as their skeletons dissolved, as might have been inferred by the general public reading the Press Release alone.
LESSON LEARNED: Never judge a study or its author by the contents of a University media release and never assume that the quotation marks around a statement from a study’s author signify words actually uttered or the meaning intended by the author.
# # # # #
End Note:
 There’s another recent example: the paper highlighted in Science last week regarding coral sediments dissolving was reproduced in many media outlets stating “coral reefs will dissolve”, which is completely different to the findings of the paper. The Science News article was “Ocean acidification is causing coral reefs to dissolve” commits the same offense as the media release in the main essay above but in reference to “Coral reefs will transition to net dissolving before end of century”. Even the title of the paper itself is very misleading – the study is about dissolving calcium carbonate (CaCO3) sands, not the reefs themselves, and deals with Aragonite Saturation State, one of the aspects of the complicated subject of sea water carbonate chemistry.
There’s another recent example: the paper highlighted in Science last week regarding coral sediments dissolving was reproduced in many media outlets stating “coral reefs will dissolve”, which is completely different to the findings of the paper. The Science News article was “Ocean acidification is causing coral reefs to dissolve” commits the same offense as the media release in the main essay above but in reference to “Coral reefs will transition to net dissolving before end of century”. Even the title of the paper itself is very misleading – the study is about dissolving calcium carbonate (CaCO3) sands, not the reefs themselves, and deals with Aragonite Saturation State, one of the aspects of the complicated subject of sea water carbonate chemistry.
# # # # #
Author’s Comment Policy:
I would love to read your examples of Press Release Science — science transmogrified at the hands of media relations departments in universities and institutions.
My thanks to Dr. Burdett for her suggestions on improving the paragraph directly below the image of the “calcifying ecosystem” to better agree with the science, all of which were gratefully incorporated.
This essay and the topic it covers is a lesson to those who would judge a researcher or his/her work by statements attributed to them by their institutional media relations departments — remember “It ain’t necessarily so!”
If you begin your comment with my first name, as “Kip…” I’ll be sure to see it and respond.
# # # # #
There is a further issue with this, which is that “acute” changes don’t give the creatures time to evolve and/or adapt to the new situation. In other words, this says NOTHING about a long-term slow slight reduction in alkalinity (falsely known as “acidification” although it is more properly called “neutralization” as it was in my high school chemistry class).
w.
+1
Ristvan and Bad Andrew: the rationale for evolution as explained by Darwin is mostly discredited since the 1950’s, and started down that path even earlier. That rationale was survival of the fittest random mutations. There are now thousands of excellent studies and books that discuss the newer, more rational theory. One good read that discusses these changes at a high level (with lots of substantiation) is “Evolution: a View from the 21st Century” by James A Shapiro, 2013. His doctorate is in genetics.
Of course, I suspect you were just referencing evolution in general. This book does not dispute evolution – just how and why it happens. Very interesting.
This posted in the wrong place – a little too high – and Kip specifically asked for a copy of the memo. It’s available from Amazon. A little long for a memo.
kalifornia ==> Darwinism is still the reigning narrative of the entirety of biology — albeit in a few hundred different denominations, variations on a theme, and interpretations.
Journal papers are still published every week that included Darwinist “Just So…” stories explaining things like How the Red Winged Blackbird Got Its Spots etc…..
Kip – I know I’m being picky, but assuming Darwinism describes evolutionary processes is as inaccurate as assuming the Bohr model of the atom accurately describes an atom. Well, the Bohr model is useful for a high school student, perhaps. “Survival of the Fittest” is just plain inaccurate in biology circles.
kalifornia ==> Well, biology is a field which has failed to even decide on a scientific definition of the word SPECIES — so a theory of of how species came to be is bound to be problematic for them.
Darwin and I (insignificantly) both agree.
I don’t think the pH is going to change one bit…..LOL
ristvan ==> My next piece about some guys trying convince us we need to un-Darwin Nature…..
Darwinism is dead. You guys apparently didn’t get the memo?
Andrew
Andrew ==> Guess not, can you send it along or post a copy?
w ==> Yes, that is correct. This is an intentional part of the experimental design, though. What Dr. Burdett was testing were the effects on sea water chemistry surrounding this type of calcifying ecosystem under conditions of acute changes in CO2 concentrations. Loch Sween has been found to undergo rapid shifts in pH (equivalent to the CO2 enrichment used) for similar periods.
The paper stretches a bit to consider that such acute CO2 shifts could take place when fish farms release water into the bay or if-and-when CO2 capture and storage plans accidentally release pulses of CO2 laden water (a scenario I didn’t consider very likely at all).
The release of fish-farm water would be “acutely” transient … the tides and currents would disperse the water and dilute it very rapidly, I guess that this would occur in a time frame that would mitigate any damage to “live, calcified organisms such as coralline algae or starfish, dead carbonate skeletal remains or carbonate-rich non-biogenic sediment.”
That’s the problem with academic research, it is “academic” and lacking connection to reality.
I agree entirely about “neutralisation” rather than mistaking the Ocean’s pH to be lower than 7. That annoys me greatly. It is not just technically wrong it is emotionally incontinent. The kind of pseudoscience you can find on SKS.
However, the complaint about “acute” is too harsh.
The study is looking at quick changes. Whether they are realistic or not is irrelevant. The paper is correctly titled; it doesn’t pretend to be talking about slow changes over decades. And it was done in situ – real science.
There are many silly papers in this field that ignore evolution in their assessment of changes over several generations. But this isn’t one of them.
The study is OK. The media “re-interpretation” is most definitely NOT.
But this isn’t one of them.
==================
OH! Where was the control? Did they use double blind experimental controls such that only some tubes had CO2 added, and the researchers didn’t know which tubes?
If not, then really this is junk science, because the experimenter would expect what was observed based on inorganic chemistry, and there is no way to determine if the observation was due to the “observer expectation effect” as documented many times in other scientific experiments.
quite simply human beings see what they expect to see, even when what they expect isn’t really there. for example, proof reading. the mind fills in worlds like ‘is’ and ‘it’ and you will see them on the page, even when they are missing.
ferdberple ==> AhHa! Quite right — the gold standard for FOCE is “Free-ocean CO2 enrichment (FOCE) systems: present status and future developments” — a control chamber is a requirement. I have no idea why it was omitted in this experiment — they used 4 FOCE chambers — one could have been set up as a control. Double-blinding the researchers in not required, but there MUST be a chamber that has all the features except the “treatment” (in this case, CO2-enrichment).
“the mind fills in worlds like ‘is’ and ‘it’”
Entire worlds then, is it?
Not sure if this was intentional irony.
You mean like the “get” in the article that should probably be a “great”?
Sorry to disagree but neutralization is changing pH to exactly 7.0 (under standard conditions) whereas ‘acidification’ refers to increasing the [H+] regardless of starting and end points.
Change in alkalinity is not the opposite of acidification.
“Seawater total alkalinity (TA) is commonly defined as “the excess base” in seawater, or the sum of excess proton acceptors, and its component ions are illustrated in light blue. Seawater TA slows down, or buffers, changes in ocean pH because it includes so many different acid-base pairs. TA stays constant even when CO2 is added to seawater because the charge balance of the solution stays the same, meaning that the number of positive ions generated equals the number of negative ions generated by these reactions.”
Neutralization makes much more sense.
beng135 March 9, 2018 at 7:38 am
Neutralization makes much more sense.
Not to a chemist! Neutralization defines the endpoint, ‘acidification’ just tells the direction of the change.
You could have a lab instruction that told you to ‘acidify to a pH of 7.4’, which implies that the starting solution was more basic than 7.4 (say 8.0). If you were told to neutralize it you would add acid until the pH was 7.0 (assuming you were at standard temperature).
There is not one single dictionary ever published that defined the word “acidification” as anything other than “the process of becoming an acid”, or thereabouts.
None of them.
Anywhere.
Ever.
Acidification does not mean a lowering of pH.
That is made up nonsense.
You can say it if you want, like lots of people do.
You make up all sorts of stuff Philperiod, why not a few more on the heap?
Just to be sure I understand this Phil – Modern chemistry uses this approach “acidification refers to increasing the [H+] regardless of starting and end points”? So, changing the pH from 14 to 13.5 is acidification? They didn’t call it that when I was in school.
Just to make sure I understand, then, increasing the temperature of water from 1C to 4C would now be considered boiling the water?
This seems a lot more confusing than the nomenclature I was taught in the 70’s. But then I only took two years of chemistry. I was a physics major, and we didn’t use those words. In fact, I think it would have given the professors quite a chuckle – white they downgraded my papers.
kaliforniakook March 11, 2018 at 10:33 pm
Just to be sure I understand this Phil – Modern chemistry uses this approach “acidification refers to increasing the [H+] regardless of starting and end points”? So, changing the pH from 14 to 13.5 is acidification? They didn’t call it that when I was in school.
Sure the instructions in lab manuals frequently when describing methods without defining an acidic endpoint.
Just to make sure I understand, then, increasing the temperature of water from 1C to 4C would now be considered boiling the water?
No that would be ‘heating’ the water, those who favor the use of ‘neutralization’ would logically refer to this as ‘boiling’.
This seems a lot more confusing than the nomenclature I was taught in the 70’s.
What did you call adding an acid to a solution back then?
I’m sure in your physics classes you used the directional terminology ‘heating’ and ‘cooling’ rather than ‘boiling’ and ‘freezing’ when neither of those endpoints were reached?
The idea of the ‘acute’ and ‘short’ duration was associated with an industrial spill, massive uncontrolled runoff of limited duration. So these results are of very limited use.
Followup experiment. Go to the same Lock same area. Carefull vacumm up some sediment and sand debris, then run the tube experiment on a ‘bottom’ only ecosystem with no corals, stars etc… and see how much the situ ground yield up it carbonate. I would be it is almost exactly the increase they saw.
Timothy Sorenson ==> Read the original paper, its short. I didn’t find the justifications for this experiment convincing either….
“resulting in higher levels of both dissolved inorganic carbon and HCO3– (bicarbonate) — indicators that “there was more dissolution of calcium carbonate than there was production.”
Not only is adaptation time not allowed in these experiments, but they assume the rising carbonate concentration to be from dissolution. As photosynthesis is an alkalizing process, it would make sense that some (new) bicarbonate would be converted to carbonate, thus the increased concentration.
The disingenuous assumption that increased CO2 comes from dissolution is unfounded, but politically expedient. I would suggest C-14 labelling of the CO2 to follow carbon movement in this system. It only makes sense that increased carbonate concentrations would lead to more calcium carbonate formation, even in the cold waters of the UK, in which unprotected calcium carbonate (dead shells, etc.) will dissolve; at the very least, dissolution would be impaired by hight CO2, bicarbonate, and carbonate. This is unlike warmer tropical waters in which calcium carbonate is solution saturated and more CO2 fosters calcium carbonate formation.
In “layman’s terms” because the water in the oceans and lakes are below 14 PH there is an acidic dissolution of minerals and organics that are more basic than the water PH is. This dissolution increases the salinity of the water as water becomes more acidic, providing more of the carbonates in solution to the living organisms that build their inner skeletons and exoskeletons – depending upon the organisms – that increases their growth and offspring. Without this carbon cycle that uses the alkalinity to recycle the dead organisms and other minerals, there can be no increase in living organisms dependent upon those carbonates availability, that are co-dependent upon the acidification and photosynthesis.
Johchi,
Congrats…all of that and not one single word of truth or anything resembling a fact.
I suspect random word salad tossed together as a goof, but it is possible you are some ultra-confused and highly misinformed teen.
Its worse than that. The surface ocean is supersaturated in calcium carbonate. The deep oceans are under saturated in calcium carbonate (due to the higher pressure and lower temperature). As a result calcium carbonate is continuously precipitating in the surface ocean and dissolving in the deep ocean. This leads to a boundary called the calcium compensation depth or CCD which is the boundary between CaCo3 precipitating and dissolving. (have a look at “carbonate compensation depth” on the web). Currently this is between 4000 and 5000 meters depth in the oceans. Carbonate based exoskeletons in the surface ocean can hardly be dissolving if the carbonate is precipitating. So unless these organisms are living at a depth greater than 4000 meters I think the concern is misplaced. That’s of course before we consider the active calcium pumping means employed by living organisms which protects shells even if the environment was under saturated (which it isn’t).
The experiment mentioned is conducted in a sealed environment separate from the ocean. It is easy in such a small sealed environment to create conditions that cannot be duplicated in the whole ocean and thus do not represent the true situation. All she has shown is that if you increase the CO2 level there is a shift from carbonate to bicarbonate as defined by very well understood science – wow thats about like proving that gravity really does exist.
Thank you, Wills!!
A shift towards “net dissolution” — they were simply injecting CO2 faster than the buffers, carbonate, could keep up.
Obviously, they did not run out of buffer….there was faster dissolution than there was replacement.
Calcium reactors need a pH of around 5 to work effectively = fast enough
the researchers suddenly raised the CO2 concentration in the water (sufficient to lower pH by 0.2 units), kept it raised for 28 hours….
They could have saved themselves a lot of time and trouble…by simply putting the starfish in a bag, and shipping them FedEx….the pH would have been a lot lower
I’ve been all sides of this issue. Bleah!
There’s no easy solution. Probably a solution is to have the scientist (clue stick in hand) look over the writer’s shoulder. It would cost more and probably inflict undue suffering on both parties.
Here’s another maximum CO2 location that is perfectly healthy, so it upset the doomsayers when they went to see it. Time passes and they think we have forgotten?
http://jennifermarohasy.com/2008/07/ocean-acidification-photographs-from-bob-halstead-and-a-note-from-floor-anthoni/
I don’t recall starfish being mentioned but I have seen plenty of starfish along the ring of fire.
As it happens, there was a practice of chopping up Crown of Thorns starfish to stop them chewing on the GBR. Not wise – each piece grows into a new starfish. Wouldn’t the brittle stars do the same?
the activist ecosystem thrives on crisis. it’s lesson 1 of the activism degree program.
zort and stort is the mainstay of their negative sum game.
guilt doesn’t work nearly as well as gothic horror.
starfish can’t possibly be as effective as unborn dead babies, tho.
I agree with Willis. If you want to cause a massive die off in your salt water tank, change something drastically. However if you do it in small increments and let the tank recover as you go big changes are indeed possible. Changes in the environment may however not suit certain sea life but others will move in to take their place. It all just takes time and adaptation… something which the animals on this planet have been doing for a long long time.
Kip, thanks for the interesting ‘debunking’ of this reporting. However, as a layman that recently googled ‘CO2 and seawater’ and was gobsmacked by its complexity, can someone please write a scientifically accurate explanation of the key aspects of say the interaction of seawater with CO2 and carbonate buffers, that a laymen can understand? Thanks to all at Wattsupwiththat for so much useful and accessible science!
Boy ==> Well, the 250-page EPOCA booklet is probably more than you want. Try the link in the End Note for Aragonite Saturation.
Thanks, Kip. I just about understood it!
Kip: thank you for the link to the note on ‘Aragonite Saturation’. Some people might find confusing – or at least oversimplified – the statement “When Ω = 1, the seawater is exactly in equilibrium or saturation with respect to aragonite.” Aragonite is not a stable mineral at or near earth’s surface temperatures and pressures so its precipitation from seawater is not an equilibrium reaction. The stable form of CaCO3 in ocean water is calcite. In practice the surface waters of large areas of the oceans are oversaturated with respect to CaCO3, but calcite does not precipitate directly from normal sea water. This behaviour is attributable to ‘reaction kinetics’ – some component of sea water, possibly sulfate, stops the equilibrium reaction from proceeding. At high levels of oversaturation, as in the warm shallow water of the Bahamas, direct preciptation of CaCO3 can occur, but the mineral formed is not calcite but the metastable aragonite.
Marine animals such as corals and molluscs can grow CaCO3 skeleta (either calcite or aragonite, sometimes both) independently of the saturation state of the surrounding seawater. This is called the ‘vital’ effect.
coldish1 ==> Thanks for that clarification — sea water carbonate bio-chemistry is not for the timid.
A very simple reflection on a simple thing such as groundwater illustrates the point.
In the ground, water can become acidic and can dissolve limestone under certain conditions, leading to karst topography and large caves. But it can also precipitate out of that same water nearby under slightly different conditions, leading to stalactites and stalagmites.
But in the basic conditions of the ocean surface, calcium carbonate is insoluble.
I do not know where you are getting your information, but there is a lot of bad information flying around here.
The EPOCA FAQ is pretty good. The first Q is
“The ocean is not acidic, and model projections say the oceans won’t ever become acidic. So why call it ocean acidification?”
The A in EPOCA stands for Acidification.
I guess they don’t understand what acids and bases are.
Nick: I’m always encouraged when I find your comments on this and other blogs. You keep the sceptics up to the mark. Thank you for taking so much trouble.
Thank you also for the link to the EPOCA FAQ. Actually I’ve rarely found in an FAQ a question that I might have wanted to ask. The various authors in this case seem to be taking care not to suggest that anything positive could come from ‘ocean acidification’. As an example, in the section on photosynthesis, the third and final question and answer are as follows:
Q: “An increase of CO2 in seawater increases growth of photosynthetic algae – isn’t that a good thing?”
A: “The growth and photosynthesis of certain marine phytoplankton and plant species may increase with higher CO2 levels, but this is by no means a general rule. For other species, higher CO2 and rising acidity will have either negative or neutral effects on their physiology. Therefore some marine phytoplankton and plants will be “winners,” while others will be “losers.” This means that instead of benefiting all impartially, future acidification will instead probably cause major shifts in the species composition of ocean phytoplankton communities. Some of the experiments that have been done so far suggest that the likely new dominant phytoplankton species in the future acidified ocean may be less able to support the productive food chains that we presently rely on to support healthy ocean ecosystems and fisheries resources.” — David Hutchins, Professor of Marine Environmental Biology, University of Southern California, USA.
The author leaves the impression that ‘acidification’ will probably be a bad thing without actually making any definite statement or providing any relevant data. He woffles negatively. However he does make clear the equivalence of ‘higher CO2 levels’ and ‘acidification’.
As I’m sure you know, much of the surface waters of the open ocean are depleted (as compared with deeper levels of the oceans) with respect to total dissolved carbon dioxide in all its forms: dissolved CO2, H2CO3, HCO3(-), CO3(2-). An obvious contributory cause of this depletion is photosynthesis, another being skeletal and soft parts growth in marine animals, although without the photosynthesis to provide a primary food supply there would be no animals to grow skeleta.
Most of the carbon fixed by photosynthesis and animal growth ends up sinking to greater depths, where some of it is deposited as sediment (and thus lost to the ocean system) and some is dissolved and recycled via the oceanic conveyor belt. The proportion lost to the ocean system is replaced partly by emissions of CO2 from undersea volcanoes, and partly, when the water returns to the surface, by take-up of CO2 from the atmosphere. Without this CO2 replenishment (‘re-acidification’) from undersea volcanoes and from the atmosphere the oceans would die of, or at least suffer from, CO2 starvation. In pre-human times the CO2 content of the atmosphere itself was in turn ultimately and continuously reliant on aerial volcanoes for replenishment. Without volcanoes life on earth would have been tough, if it could survive at all.
Nowadays humans are helping the volcanoes along by supplementing their natural CO2 output through the burning of fossil fuels. Since there is no practical way to stop or even slow this supplementary supply of CO2 to the atmosphere and oceans (until the fossil fuels are depleted) all we as humans can do is to observe and where necessary adapt.
I confess to being more worried about the atmosphere and oceans running short of CO2 than about them suffering from an excess. That won’t happen as long as the volcanoes keep pumping out the stuff of life, but after another few thousand million years our successors on land and in the oceans may need all the CO2 they can lay their hands on.
Thanks again, Nick, for taking part in these discussions,
Coldish
,
I like this one…
You can’t even directly calculate modern pH just from the atmospheric concentration of CO2. This is another one of those “all other factors held equal” issues. All other factors held equal, a rise from 280-400 ppm atmospheric CO2 will lower the average pH of seawater a little. However, all other factors are never held equal.
pH is the ratio of dissolved inorganic carbon (DIC) and total alkalinity (TA). DIC can be reasonably calculated from atmospheric CO2; but TA cannot.
“pH is the ratio of dissolved inorganic carbon (DIC) and total alkalinity (TA).”
I agree it isn’t a good answer. pH isn’t that ratio, but it can be derived from TA and DIC. And I think that is the proper answer; there is much better historic data on those quantities than on pH.
The Argument from authority is a common form of argument which leads to a logical fallacy.
The appeal to authority relies on an argument of the form:
A is an authority on a particular topic
A says something about that topic
A is probably correct
How to monster* from authority:
*Monster, to show, to prove “de-monster-atively” (demonstratively). From the Latin, monstrare, meaning ‘to demonstrate’, and monere, ‘to warn’.
“appeal to authority”
I’m not the one who invoked EPOCA; in fact, I’m grateful to Kim for drawing my attention to it. Down-thread, Kip says that I should read EPOCA to diminish my ignorance. I did, and so I monster the results.
” In pre-human times the CO2 content of the atmosphere itself was in turn ultimately and continuously reliant on aerial volcanoes for replenishment. ”
Pre human times?
Do you mean the hundreds of millions of years of Earth history that CO2 was as much as 50 times as high as it is now?
Separately, and I do not even believe I have to point this out, pH is very simply defined as minus the log of the hydrogen ion concentration (although pretty much every chemist considers that the hydrogen ions do not actually exist as such, naked protons, but as hydronium ions).
The pH and pOH scale are based on the fact that water has a dissociation constant of 1.0×10 ^-14.
That is, water spontaneously ionizes to that very small degree, in the following equilibrium:
H2O + H2O ⇌ H3O+ + OH−
Equilibrium reactions are very important to understand precisely for anyone wishing to describe or predict what is happening in an aqueous solution, particularly and especially a multiply buffered solution such as seawater.
It is also handy to know some mineralogy, biology and a very good background in physical chemistry in general.
Because if one is not expert and well versed in these disciplines, there is no way to have any idea at all what is going on as things change, and therefore…to know what one is talking about.
The fact that these people start out using terms like “acidification” incorrectly, and refuse to use the proper words to refer to the lowering of the pH of a basic solution, is a good opening heads up to the general state of their knowledge of chemistry.
On this same subject, that of equilibrium reactions, it is completely obvious that whoever made this diagram:
has a woefully lacking background in chemistry.
Because that diagram shows a one way reaction proceeding to the right.
This is not at all the case, whatsoever.
This is what is happening:
These species all exist at once in an equilibrium, or more correctly still as several equilibria, and this is always, always, denoted by chemists with the two way arrows, not a single arrow which indicates a reaction proceeding in one direction only.
The Wikipedia page on the subject of equilibrium reactions looks decent enough, for anyone who does not but would like to begin to understand this in terms of proper chemistry.
Once in dynamic equilibrium, any addition or subtraction of any of the species in any of the various equilibria tends to cause the equilibrium to shift, in accordance with the various rate constants of each pair.
This is what makes this a buffer system…since there is always present both a weak acid and it’s conjugate base, the solution resists changes in pH. Added acid or base in neutralized.
And since this incredibly well know chemistry forms the basis of the primary buffering system in our own bloodstreams, it is a very good thing this is true.
Personally, I do not know how these people even have biology degrees, iffen they really do.
Where I went to school, you had to know basic chemistry, and know it well, to get any science degree, and certainly to get a degree in a biological science.
My guess this has something to do with the fact that, starting a decade or two ago, students were able to sue (and successfully!) instructors who gave them a poor grade.
“This is what is happening:”
No, it’s only part of it. You can write that equilibrium as
CO₂+H₂O ⇌ HCO₃⁻+H⁺
But at around pH 8, any H⁺ is immediately reacted with the strongest base around, which is carbonate
CO₃⁻⁻+H⁺ ⇌ HCO₃⁻
Since H⁺ is constrained to be at very low concentration, it can be eliminated by adding
CO₂+H₂O+CO₃⁻⁻ ⇌ 2HCO₃⁻
That is their reaction. It is an equilibrium, but they have written the forward reaction to emphasise how added CO₂ removes carbonate.
You are not being clear.
By adding what?
These are not reactions, they are equilibria.
CO3 is a -2 ion, and is on the far right of the series, in equilibrium with bicarbonate, HCO3-.
Adding CO2 (on the left) forces the whole series of equilibria over to the right>
I think you ought to understand forcing.
CO2 reacts with water to form carbonic acid, which partially dissociates into bicarbonate, so adding CO2 causes bicarbonate to rise. Bicarbonate partially dissociates into and is in equilibrium with carbonate, CO3-2.
So adding CO2 causes more bicarbonate, which causes more carbonate.
You do not understand what you are talking about Nick.
Carbonate is already present, in equilibrium with bicarbonate. Adding pushes the whole series to the right, towards carbonate.
This is a buffer system, which is why the pH is around 8.
Adding CO2 means you get more bicarbonate and more carbonate.
Not less. Does not matter what way you write it.
If you write it incorrectly, or correctly, the chemistry is the same.
It is very simple and an indisputable fact of chemistry…adding reactants on the left pushes the equilibrium to the right. Le Chatelier’s principle;
Bedrock science.
CO2 causes calcium carbonate to be more soluble. But it hardly matters, when calcium is present, the carbonate is removed because calcium carbonate is insoluble and precipitates out at the conditions in the ocean anywhere near the surface.
At lower temps and pressures, calcium carbonate becomes, oddly enough, more soluble, as pointed out above by someone. 4000 to 5000 meters is the depth that it begins to dissolve.
“By adding what?”
By adding CO₂. It shifts the equilibrium CO₂+H₂O+CO₃⁻⁻ ⇌ 2HCO₃⁻ to the right. The mechanism is that some of the added CO₂ reacts with CO₃⁻⁻ and removes it (just the reaction they showed). [CO₃⁻⁻] goes down (unless replenished by dissolving CaCO₃).
Here is a diagram of the whole series:
http://slideplayer.com/slide/3400196/12/images/1/Solubility+of+CO2+and+Carbonate+Equilibrium.jpg
“CO2 causes calcium carbonate to be more soluble. But it hardly matters, when calcium is present, the carbonate is removed because calcium carbonate is insoluble”
Something is not quite right there. In fact, the first sentence is correct. To a reasonable approximation, adding one molecule of CO₂ dissolves one molecule of CaCO₃, if available.
“By adding CO₂. It shifts the equilibrium CO₂+H₂O+CO₃⁻⁻ ⇌ 2HCO₃⁻ to the right. The mechanism is that some of the added CO₂ reacts with CO₃⁻⁻ and removes it (just the reaction they showed). [CO₃⁻⁻] goes down (unless replenished by dissolving CaCO₃).”
This is wrong.
You are incorrect.
Just as you were incorrect when you stated that seawater is on the acid side of neutral.
You oughta quit while you are in a shallow hole.
But keep digging if you want.
Me, I have a degree in chemistry.
You?
http://slideplayer.com/slide/2558773/9/images/4/pH+Scale.jpg
“In fact, the first sentence is correct. To a reasonable approximation, adding one molecule of CO₂ dissolves one molecule of CaCO₃, if available.”
There is a lot of CO2 in the sea.
But at the surface, under nearly every condition except near places where low pH conditions are introduced, calcium carbonate is for all practical purposes insoluble.
Adding CO2 does not dissolve an insoluble mineral.
You are now waxing into laughable wrongnesses.
“You?”
A science degree with chemistry as a sub-major. And forty years as a research scientist in CSIRO, the government research organisation.
Sorry, this was meant to go here:
A very simple reflection on a simple thing such as groundwater illustrates the point.
In the ground, water can become acidic and can dissolve limestone under certain conditions, leading to karst topography and large caves. But it can also precipitate out of that same water nearby under slightly different conditions, leading to stalactites and stalagmites.
But in the basic conditions of the ocean surface, calcium carbonate is insoluble.
I do not know where you are getting your information, but there is a lot of bad information flying around here.
Then you ought to know better.
I think your ideology has warped your ability to reason and keep things straight.
“calcium carbonate is for all practical purposes insoluble.”
Just not true. Here (from here) is a table of the dissolved ions in sea water. I’ve ringed CO₃⁻⁻ and Ca⁺⁺. If CaCO₃ were insoluble, they could not both be present.
Nick, you must know that nearly everything can dissolve in water to some degree. The term insoluble for not mean none of it is present in the dissolute state.
I leave it to you to look it up and see exactly what the limits are of the terms soluble, slightly soluble and insoluble.
There is gold dissolved in seawater…do you think that means gold is considered soluble under conditions which prevail in the oceans?
Although I cannot imagine anyone who spends any time at all actively discussing these issues could possible be unaware of any of this.
Besides I said practically insoluble.
You have said so many wrong things i am just going to leave it here.
I have stuff to do, and trying to make you understand anything just seems pointless and a waste of time.
I remind you that you stated every molecule of CO2 added to seawater will dissolve one molecule of calcium carbonate.
Well, first of all, the vast majority of CO2 is in the form of a dissolved gas, not as a species combined with water as carbonic acid.
The ratio is well known, it is slightly higher for sea water than fresh. It is about 1.2 out of every thousand molecules of CO2 present.
Of these one in about one thousand that are combined with a water molecule to form the weak acid called carbonic acid, only a very tiny amount is dissociated into bicarbonate. In fact, as you must know, this is precisely how a weak acid is defined…it only dissociates partially in water, and the ratio of this dissociation is called the Ka. Since Carbonic acid is diprotic, it has two Ka values. The first is 2.5 x10^4.
So two and a half of every ten thousand molecules are dissociated into bicarbonate. But, let us not forget that only 1.2 of every thousand CO2 molecules are in the form of H2CO3…the rest are all just CO2 floating as gas in the water.
So the amount of bicarbonate as a proportion of the amount of CO2 is 4.47 times ten to the minus 7th power: 4.47 x 10^-7
Four and a half molecules of bicarbonate for every ten million.
For carbonate, it is far lower than this. The conjugate base of a weak acid is a strong base, so bicarbonate is a strong base, by itself. It has a much lower Ka for the second hydrogen that carbonic acid did for the first one.
The second Ka is given as :
Ka2 = 4.69×10−11
Wow!
Ten to the minus eleventh.
Bicarbonate is a VERY weak acid!
And the conjugate base of a weak acid is a strong base.
So, when carbonate finds a calcium ion…it hangs on very tightly.
I did not just take some classes…I understand chemistry, and also the scale of the numbers here.
It is not clear at all that you understand either.
Kip Hansen March 10, 2018 at 6:51 am
Allan MacCrae ==> Sorry Allan, I was traveling all day yesterday.
Point 1: Nominally true — atmospheric CO2 reduces total alkalinity of the ocean surface water, incrementally, and slowly as concentrations increase and has a magnified effect on carbonate chemistry — important to many organisms.
Not true, CO2 has no effect on the total alkalinity of sea water.
http://www.whoi.edu/OCB-OA/page.do?pid=112136
Phil ==> If you prefer that reference, keep reading, it eventually tells you that the entire CO2 dissolution process results in excess H+ ions == acidification.
menicholas March 10, 2018 at 4:50 am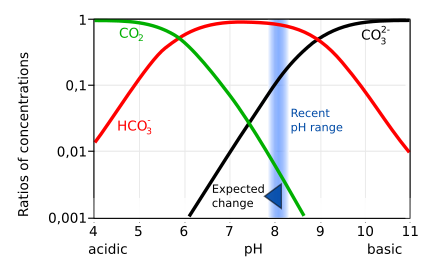
Well, first of all, the vast majority of CO2 is in the form of a dissolved gas, not as a species combined with water as carbonic acid.
The ratio is well known, it is slightly higher for sea water than fresh. It is about 1.2 out of every thousand molecules of CO2 present.
Of these one in about one thousand that are combined with a water molecule to form the weak acid called carbonic acid, only a very tiny amount is dissociated into bicarbonate. In fact, as you must know, this is precisely how a weak acid is defined…it only dissociates partially in water, and the ratio of this dissociation is called the Ka. Since Carbonic acid is diprotic, it has two Ka values. The first is 2.5 x10^4.
So two and a half of every ten thousand molecules are dissociated into bicarbonate. But, let us not forget that only 1.2 of every thousand CO2 molecules are in the form of H2CO3…the rest are all just CO2 floating as gas in the water.
So the amount of bicarbonate as a proportion of the amount of CO2 is 4.47 times ten to the minus 7th power: 4.47 x 10^-7
Four and a half molecules of bicarbonate for every ten million.
You have made a mistake in your equilibrium calculations somewhere. Here’s the Bjerrum plot for seawater.
For the sake of ease of reading let’s take a pH of 8.0, in that case [CO3–] will be about 0.1 of [HCO3-] and [CO2] will be less than 0.01. Your error is in your assumption only a small amount of carbonic acid is dissociated into bicarbonate in fact the concentration of carbonic acid is negligible and in the pH range 6-9 bicarbonate is the dominant species. Regarding the equilibria remember that if bicarbonate dissociates to carbonate a proton is produced thereby lowering the pH and pushing the equilibria the other way.
See here:
https://wikivisually.com/wiki/Bjerrum_plot
Kip Hansen March 10, 2018 at 12:12 pm
Phil ==> If you prefer that reference, keep reading, it eventually tells you that the entire CO2 dissolution process results in excess H+ ions == acidification.
Yes but that has no effect on Total Alkalinity which is constant, I can only assume you don’t know what TA is?
Here’s a definition for you: “Seawater total alkalinity (TA) is commonly defined as “the excess base” in seawater, or the sum of excess proton acceptors, and its component ions are illustrated in light blue. Seawater TA slows down, or buffers, changes in ocean pH because it includes so many different acid-base pairs. TA stays constant even when CO2 is added to seawater because the charge balance of the solution stays the same, meaning that the number of positive ions generated equals the number of negative ions generated by these reactions.”
Phil ==> You may be definitionally correct — nonetheless, even if I mispoke — the excess of H+ ions results from dissolution of atmospheric CO2 and results in what is called acidification in this case.
“Yes but that has no effect on Total Alkalinity which is constant,”
Yes. There are two ways of thinking about that, both leading to the same result:
1. TA is just charge balance of all the ions that can react. Adding CO₂ does not alter charge balance
2. The definition of TA is the number of moles/vol of H⁺ (strong acid) required in titration (methyl orange indicator) to reduce the pH to about 4.2. That is enough to convert any added and reacted CO₂ back to free CO₂ . So any acid reactions it engaged in would be undone without affecting the titration.
Phil,
I have not made a mistake, except that the entire chemistry of seawater is far more complicated owing to the presence of a great many other species in solution.
But you are omitting an important detail.
I do not have the time of inclination to find an open link to the original inorganic chemistry texts, but see here, from Wikipedia:
” At every pH, the concentration of carbonic acid is assumed to be negligible compared to the concentration of CO2, and so is often omitted from Bjerrum plots.”
And here:
“Carbon dioxide is soluble in water, in which it reversibly forms H
2CO
3 (carbonic acid), which is a weak acid since its ionization in water is incomplete.
CO
2 + H
2O ⇌ H
2CO
3
The hydration equilibrium constant of carbonic acid is K h = [ H 2 C O 3 ] [ C O 2 ( a q ) ] = 1.70 × 10 − 3 {\displaystyle K_{\mathrm {h} }={\frac {\rm {[H_{2}CO_{3}]}}{\rm {[CO_{2}(aq)]}}}=1.70\times 10^{-3}} K_{\mathrm {h} }={\frac {\rm {[H_{2}CO_{3}]}}{\rm {[CO_{2}(aq)]}}}=1.70\times 10^{-3} (at 25 °C). Hence, the majority of the carbon dioxide is not converted into carbonic acid, but remains as CO2 molecules, not affecting the pH.
The relative concentrations of CO
2, H
2CO
3, and the deprotonated forms HCO−
3 (bicarbonate) and CO2−
3(carbonate) depend on the pH. As shown in a Bjerrum plot, in neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater. In very alkaline water (pH > 10.4), the predominant (>50%) form is carbonate. The oceans, being mildly alkaline with typical pH = 8.2–8.5, contain about 120 mg of bicarbonate per liter.
Being diprotic, carbonic acid has two acid dissociation constants, the first one for the dissociation into the bicarbonate (also called hydrogen carbonate) ion (HCO3−):
H2CO3 ⇌ HCO3− + H+Ka1 = 2.5×10−4 mol/L; pKa1 = 3.6 at 25 °C.[19]
This is the true first acid dissociation constant, defined as K a 1 = [ H C O 3 − ] [ H + ] [ H 2 C O 3 ] {\displaystyle K_{a1}={\frac {\rm {[HCO_{3}^{-}][H^{+}]}}{\rm {[H_{2}CO_{3}]}}}} K_{a1}={\frac {\rm {[HCO_{3}^{-}][H^{+}]}}{\rm {[H_{2}CO_{3}]}}}, where the denominator includes only covalently bound H2CO3 and does not include hydrated CO2(aq). The much smaller and often-quoted value near 4.16×10−7 is an apparent value calculated on the (incorrect) assumption that all dissolved CO2 is present as carbonic acid, so that K a 1 ( a p p a r e n t ) = [ H C O 3 − ] [ H + ] [ H 2 C O 3 ] + [ C O 2 ( a q ) ] {\displaystyle K_{\mathrm {a1} }{\rm {(apparent)}}={\frac {\rm {[HCO_{3}^{-}][H^{+}]}}{\rm {[H_{2}CO_{3}]+[CO_{2}(aq)]}}}} K_{\mathrm {a1} }{\rm {(apparent)}}={\frac {\rm {[HCO_{3}^{-}][H^{+}]}}{\rm {[H_{2}CO_{3}]+[CO_{2}(aq)]}}}. Since most of the dissolved CO2 remains as CO2 molecules, Ka1(apparent) has a much larger denominator and a much smaller value than the true Ka1.[21]”
Sorry, the text does not copy well on this format.
Here are the articles:
https://en.wikipedia.org/wiki/Carbon_dioxide#cite_ref-21
https://en.wikipedia.org/wiki/Bjerrum_plot
Again, the salient parts:
“The much smaller and often-quoted value near 4.16×10−7 is an apparent value calculated on the (incorrect) assumption that all dissolved CO2 is present as carbonic acid, ”
“At every pH, the concentration of carbonic acid is assumed to be negligible compared to the concentration of CO2, and so is often omitted from Bjerrum plots. These plots are typically used in ocean chemistry to track the response of an ocean to changes in both pH and of inputs in carbonate and CO
2.[2]”
The vast majority of carbon dioxide in the ocean is in the form of gaseous CO2, but this is ignored, incorrectly.
The important thing to know, if one does not wish to be a panic stricken fearmongering and misinformed person, is that the entire combination or species present forms what is known as a buffered system.
Yes, adding CO2 will change the pH, and it will go down, meaning that there is less OH- and more H+, but it will not go down by much…because it is BUFFERED against changes in pH. And there are other buffering systems in the ocean as well.
How can you all ignore the fact that in the long history of the earth, all of these creatures not only survived but thrived in conditions of vastly greater atmospheric CO2, which necessarily cause a proportionately greater amount of CO2 to be present in the ocean, and so had the equilibrium pushed very much harder to the right?
I do not know the answer, but you must have some reason…likely the same reason you are all completely willing, collectively, to ignore the evidence from multiple disciplines of the long term stability of the Earth in terms of ability to sustain life.
In fact, we are at a low ebb of the ability of the Earth to continue to support life, given the historically low temps and frightfully low CO2 concentrations.
Y’all act like it would be a disaster if life once again thrived and prospered from pole to pole.
It would not be…the disaster was when this ceased to be the case when the current glaciation began, and killed entire continents full of living creatures.
“I have not made a mistake”
There is clearly a mistake here:
“So the amount of bicarbonate as a proportion of the amount of CO2 is 4.47 times ten to the minus 7th power”
In the listing I gave above, the concentrations are [CO₂]=10μM, [HCO₃⁻]=1770μM, [CO₃⁻²]=260μM
bicarb is not a tiny proportion. It is dominant.
Whether CO₂ is hydrated is, in terms of equilibrium, no more important than whether H⁺ is hydrated.
“The vast majority of carbon dioxide in the ocean is in the form of gaseous CO2, but this is ignored, incorrectly.”
Well, it isn’t gaseous, just not hydrated. But who ignores that? Not Wiki, in those Bjerrum plots. Not me or Phil.. Not Dr Burdett, in the equation you originally objected to.
If it is not hydrated, it is gaseous, by definition.
Just like O2 dissolved in water…it is a dissolved gas.
That is exactly why cold water can hold more of it.
Cold water dissolves more gas, but less of nearly every solid
Heat up some seawater, and the CO2 is forced out of solution.
What happens to the pH? Does it shoot up, since if you think nearly all of the CO2 is present as bicarbonate, obviously that is what would have to happen.
Those quotes in wiki are from an inorganic chemistry text.
I did not write that article, but it is accurate.
I restated some of it, and worded it somewhat differently before going to look it up in order to respond in a precise way.
My objection to showing the reaction of CO2 and H2O with CO3– leading to 2 HCO3-, is that this incorrectly diagrams the equilibrium reactions.
It is not what is happening.
What is happening is the way it is correctly diagramed, with CO2 on the left, and CO3 on the right.
It has to be written that way because that is the basis of the physical situation that results in what we see.
BTW…in your chart, it shows a tiny amount of CO2, with an asterisk. What is the asterisk denoting?
Because that cannot be right. I think what it is leaving out is exactly what I pointed out.
As for objecting to the diagrams, you stated that adding one molecule of CO2 to the solution (the sea?) would cause one molecule of CaCO3 to dissolve.
Malarkey Nick.
You or anyone else can do a kitchen experiment in which calcium carbonate is dissolved. Pour some vinegar on it. But at a pH of 8.2, it is not dissolving.
Some of each ion is in solution, yes…good thing.
Again, soluble and insoluble are relative terms in chemistry, not absolutes.
Everything has some dissociation constant. Water will solvate just about everything .
Water and oil do not mix, but try to get oil out of water sufficient to make it potable. Cannot be done.
Personally, i can still recall chem labs where we derived the solubility charts by actual measurements.
In those classes, the grade you go was determined by how well you followed the procedures and measured everything.
If CO2 was not a dissolved gas in seawater, Henry’s law would not apply.
That is a fact.
Honestly though, I have no idea what you are disputing or agreeing with.
Everything I have said comes out of chemistry textbooks.
Look it up…google the question is calcium carbonate soluble in water?
It is insoluble.
In acid, it does not exactly dissolve…it is decomposed, releasing CO2.
So…why and how does water dissolve calcium carbonate, only to have it precipitate out at some other point under the Earth?
Simples…the water must be saturated in CO2.
Saturated…reached the upper limit of solubility.
Most things that are soluble are only partially so. Something with infinite solubility has another word…those things are called miscible.
I am sure it is coming back to you when I mention it, which is no doubt why you dropped it when I pointed out that insoluble does not mean 100% insoluble.
But one can only wonder how it can be that someone with a science degree who worked his whole life doing science, and has chemistry credentials…does not know that one of the most common ROCKS on earth is not soluble in water, especially basic water?
I really would like to know.
And hey…how easy is it to just LOOK IT UP!
“BTW…in your chart, it shows a tiny amount of CO2, with an asterisk. What is the asterisk denoting?”
Standard meaning is combined concentration of all forms – ie dissolved CO₂ and H₂CO₃
“google the question is calcium carbonate soluble in water?”
So what is hard water?
A more relevant question is, what is the solubility product of CaCO₃. And the answer is:
for aragonite 6.0e-9 for calcite 3.36e-9 (units M^2)
Kip gets excited about the EPOCA section on supersaturation of aragonite (although it doesn’t go beyond the definitions). How can an insoluble substance be supersaturated?
In fact, calcifying organisms take calcium carbonate out of solution (where else) to build shells etc. If it came from solution, it can always go back. That’s the problem.
menicholas March 10, 2018 at 6:17 pm
Phil,
I have not made a mistake, except that the entire chemistry of seawater is far more complicated owing to the presence of a great many other species in solution.
But you are omitting an important detail.
I do not have the time of inclination to find an open link to the original inorganic chemistry texts, but see here, from Wikipedia:
” At every pH, the concentration of carbonic acid is assumed to be negligible compared to the concentration of CO2, and so is often omitted from Bjerrum plots.”
The error you have made is to assume that a certain proportion of the CO2 is hydrated to carbonic acid and that is the end of it. You can’t ignore the other equilibria.
The carbonic acid produced is very rapidly converted to bicarbonate thus CO2 is no longer in equilibrium with carbonic acid and following Le Chatelier’s principle more CO2 is hydrated, this will continue until all the equilibria are satisfied. The net result is that at the pH prevalent in the ocean (~8.2) bicarbonate is the dominant form at about 90% of the total, about 10% carbonate and ~1% dissolved CO2, carbonic acid is far less than the CO2 (being basically a transitional species it is usually omitted in the plots).
Here is a quote from you where you make that error:
“Well, first of all, the vast majority of CO2 is in the form of a dissolved gas, not as a species combined with water as carbonic acid.
The ratio is well known, it is slightly higher for sea water than fresh. It is about 1.2 out of every thousand molecules of CO2 present.
Of these one in about one thousand that are combined with a water molecule to form the weak acid called carbonic acid, only a very tiny amount is dissociated into bicarbonate.
If we were taking about unbuffered fresh water you would be closer to the truth because in equilibrium the pH would be ~5.6.
For example at pCO2 of 3.5×10-4:
pH= 5.65, CO2= 1.18×10-5 mol/L, H2CO3= 2×10-8, HCO3- = 2.23×10-6, carbonate negligible.
As to your statement about the insolubility of Calcium Carbonate you also appear to be thinking of a solution in pure water. In the case of sea water we have a buffered system with many other ions present, notably Magnesium which is ~5 times more concentrated than Calcium.
As Nick pointed out the solubility product of calcium carbonate is for aragonite 6.0×10-9 so you’d expect it to precipitate out, however Magnesium carbonate has a solubility product of 6.8×10-6 and among other things that allows calcium carbonate not to precipitate (magnesium provides a barrier to nucleation for example)
menicholas March 10, 2018 at 3:31 am
“In fact, the first sentence is correct. To a reasonable approximation, adding one molecule of CO₂ dissolves one molecule of CaCO₃, if available.”
There is a lot of CO2 in the sea.
But at the surface, under nearly every condition except near places where low pH conditions are introduced, calcium carbonate is for all practical purposes insoluble.
Adding CO2 does not dissolve an insoluble mineral.
You are now waxing into laughable wrongnesses.
Sorry that would be you.
I guess you don’t remember the high school chemistry experiment where you bubble CO2 into lime water (a suspension of Ca(OH)2). When you first bubble the CO2 you form a suspension of Calcium carbonate, as you continue to bubble CO2 the calcium carbonate dissolves into calcium bicarbonate solution. So, yes, adding CO2 does dissolve the otherwise ‘insoluble’ mineral.
“Bad and Poor Science Journalism” is there any other kind ?
Well, yes, but there used to be less bad journalism all around. The problem is made worse by the fact that we have three languages being spoken: sciencese, jounalese, and everybodyelsese. Kudos to Dr. Burdett for providing a translation from sciencese to everyday English.
Press release writers at universities tend to be generalists with little understanding of the topics they write about. It’s doubtful many of them have much of a background in science, engineering, and technology and so are likely to have difficulty comprehending the detail and nuance of their subject. Scientists, on the other hand, have been trained to write in a particular style that’s dense with fact and jargon that goes over the heads of those who read press releases. Few people possess the skills to bridge the gap so we get bad communication. I doubt that pre-publication approval will fly since it’s a lot of work for little or no gain.
” Few people possess the skills to bridge the gap so we get bad communication.”
I disagree.
This site and nearly every one I have frequented over the years is replete with people who are very easily able to communicate these concepts, and to do so clearly and concisely, and to do so in styles that are entertaining and engaging.
When people who are supposed to be communications professionals get important things wrong when mass communicating on topics of wide interest and of vital concern, we should not let them off the hook by saying it is hard, and few can do it.
It is not hard, lots of people can do it, and it is important, and with big ramifications from the aspect of making policy, informing public opinion so if policy makers are getting it wrong, voters can fix the situation by voting them out.
Besides, I am not convince in the slightest way that these are merely poorly communicated due to incompetence. I think they know very well how to make things sound bad, and have no real interest in accuracy…their job, whether self appointed or paid to do so, is to beat the drum of global warming alarmism, facts be damned.
Willis eschenbach. In other words, this says NOTHING about a long-term slow slight reduction in alkalinity (falsely known as “acidification” although it is more properly called “neutralization” as it was in my high school chemistry class).
,…………………………..
Oh come on Willis. Neutralisation tells you nothing of what is happening. You could be adding acid to alkaline solution Or adding alkali to an acidic solution. Making a solution more acidic tells you that the pH is being reduced. I.e. an acid is being added to the solution. The solution could be an acidic or an alkaline solution
“Neutralisation tells you nothing of what is happening.”
Indeed. Neutral means the state where there are equal molarities of the acid and alkali component. For pure water, and titrations of strong acids and alkalis, treated in high school, that is pH 7. For buffered solutions, it is something else.
For the carbonate/bicarbonate system, relevant to calcium carbonate dissolution, sea water is well on the acid side of neutral.
W and Nick ==> Read the referenced chapters of the EPOCA booklet — then you’ll have a better idea of what you are talking about. The Aragonite Saturation State link is short enough to read a couple of minutes — carbonate sea water chemistry is not about acidification – necessarily. They are, however, related values when calculations are done.
Kip,
I do know what I am talking about. You might like to play with the Ocean Acidification Calculator. Or just look at the (active) Bjerrum plots included.
The pKa for the HCO₃⁻/CO₃⁻⁻ system is 9.13. You can tell sea water is on the acid side of neutral by the fact that the ratio [HCO₃⁻]/[CO₃⁻⁻] is about 11.
The saturation state of aragonite is a different question, relating to the separate equilibrium between CO₃⁻⁻ and Ca⁺⁺. The possibility of supersaturation does not affect the acid/base issue.
I see that the EPOCA link, which is supposed to correct my ignorance, starts out
“Ocean acidification is an undisputed fact.”
Nothing about “neutralisation” there.
more pretzel logic from the anti mind jihadi… crazy af
“For the carbonate/bicarbonate system, relevant to calcium carbonate dissolution, sea water is well on the acid side of neutral.”
Exactly backwards Mr. Stokes.
I am not sure at this point if you are wrongly informed or merely misspoke.
“Exactly backwards Mr. Stokes”
Care to substantiate that? I’ll say it again:
“The pKa for the HCO₃⁻/CO₃⁻⁻ system is 9.13. You can tell sea water is on the acid side of neutral by the fact that the ratio [HCO₃⁻]/[CO₃⁻⁻] is about 11.”
Neutral is the point where the concentrations are equal.
The pH of seawater is well known to be on the basic side of neutral Nick, at around 8 or so, which is why acidification of the ocean is not a thing.
It cannot become acidic. It is multiply buffered at the value it has today.
Regarding pH and acid base chemistry and aqueous solutions, neutral is a pH of 7. This is where pH and pOH are equal.
Anything above 7 is basic.
Anything below is acidic.
You studies chemistry?
This is the most appalling abuse of terminology I have ever seen:
OMG!
What the hell are you talking about? Are you getting a bonus for absurdity?
“What the hell are you talking about?”
OK, let me say it yet again. A buffer is an equilibrium of the kind
B⁻⁻+H⁺ ⇌ HB
where HB is a weak acid, B⁻⁻ the conjugate base. Its neutral point is where [B⁻⁻] = [HB]. The corresponding pH=-log([H⁺]) is called pKa.
There are several buffers in sea water, but the one that involved CO₃⁻², which in turn determines CaCO₃ dissolution, is where B is CO₃⁻²
CO₃⁻²+H⁺ ⇌ HCO₃⁻
For that, pKa is high (basic); Zeebe gives it as 9.13. And seawater is on the acid side of neutral for that buffer, since the pH is about 8.1. The acid part of the buffer, HCO₃⁻, is at far higher concentration than its conjugate base.
Or, as I said above,
“The pKa for the HCO₃⁻/CO₃⁻⁻ system is 9.13. You can tell sea water is on the acid side of neutral by the fact that the ratio [HCO₃⁻]/[CO₃⁻⁻] is about 11.”
menicholas March 10, 2018 at 1:29 am
Ghalfrunt,
Say whatever you want, but do not claim that you are speaking from authority of are using words according to their actual definitions.
By incorrect usage of language, you mark yourself not as authoritative, but as an ignoramus.
The term ‘acidify’ is widely used in chemistry to refer to the increase in the [H+] regardless of the starting point, the end result does not have to be an acid solution. Here’s an example taken at random:
“The form of P fertilizer added to soil can affect soil acidity, principally through the release or gain of H+ ions by the phosphate molecule depending on soil pH (Figure 2). If phosphoric acid (PA) is added to soil, the molecule will always acidify soil as H+ ions will be released – one H+ ion if the soil pH is less than ~6.2 and two H+ ions is the soil pH is above 8.2. Monoammonium phosphate (MAP), single superphosphate (SSP) and triple superphosphate (TSP) all add P to soil in the form of the H2PO4- ion, which can acidify soil with a pH greater than 7.2 but has no effect on soil pH in acidic soils.
I do not know about widely.
Misuse of language is not widespread among chemists doing chemistry.
Yes, the terminology is misused by people who are not chemists when they talk about chemistry.
So what?
The oceans are not and never will be acidic. Being well schooled in chemistry, the terms acid and acidic and acidification hold no special emotional value for me. I know what they refer to.
Many laypeople have no such emotional detachment based on understanding the subject.
We regularly ingest substance that are highly acidic, and many very healthy food and beverages are several orders of magnitude more acidic than neutral water.
Look up above at the chart of common substances of various pH values.
Do you eat pickles?
Use vinegar on salad?
Eat oranges or drink orange juice?
Squeeze a lemon on your seafood?
Eat tomato sauce, or things which contain it?
There is nothing scary about the pH scale.
Our stomachs contain highly concentrated hydrochloric acid, so concentrated it harms us when our pyloric valve fails us.
Just past the point where the stomach empties into the duodenum, bile salts neutralize this acid to facilitate absorption in the ileum and jejunum, because absorption requires a basic environment.
Everything is chemicals, and every substance containing water has a pH of one value or another.
Large and healthy freshwater biomes exist that are completely acidic, and in these, shelled organisms live and thrive and do not dissolve.
Why is it that warmistas are always arguing against logic and reason…against common sense and the scientific method, while claiming to do the opposite?
Altered data is not data anymore.
Lowering of a pH of a basic solution does not result in an acid until and unless the pH falls below 7, even thought the distinction between solutions of pH 6.5 and pH 7.5 are hardly even detectable without careful analysis.
Both are considered neutral for any practical purpose.
Milk of magnesia has a pH of over 10, over a thousand times more basic than a glass of water that is 7.1…and lemon juice and vinegar are over ten thousand times more acidic than a glass of water at pH 6.9, sitting below 3 on the scale.
But anyone can guzzle a tankard of these substances without ill effect.
Ever check out the pH of the water around a black smoker on the ocean floor, which are surrounded by an literal explosion of life forms?
menicholas March 10, 2018 at 7:11 pm
I do not know about widely.
Misuse of language is not widespread among chemists doing chemistry.
Yes, the terminology is misused by people who are not chemists when they talk about chemistry.
So what?
The oceans are not and never will be acidic. Being well schooled in chemistry, the terms acid and acidic and acidification hold no special emotional value for me. I know what they refer to.
Nor me, alkali can be just as dangerous. However certain disciplines have ‘terms of art’ and acidify is used in that way by chemists, basically to add acid to something (increase [H+]). It’s not synonymous with ‘neutralize’, and ‘alkalinity’ has a quite well defined meaning so to try to use it the way some have here is also wrong. You’ll frequently see instructions in chem. manuals such as ‘acidify until color change occurs’ for example. Acidify a solution containing phenolphthalein until it changes from purple to clear means you adjusted the pH to somewhere between 10 and 8.
I will try this once more!
Seawater is not “well on the acid side of neutral” despite what any chemist might say!
The important political issue today, is what the laymen hears when they know nothing of the intricate debate that surrounds this “problem.”
The term “acidification” in relation to the ocean, is a misnomer. No matter how prevalent it is in the discipline of chemistry or in science generally. Why? Because pure water* is smack dab in the middle of the scale.
H20 is the measure upon which the entire scale is derived. It has the pH of 7 and it is in truth, neither acidic nor caustic (basic).
What the “political scientists” – the radical left warm-mongers – need to tell the layman is that the fresh water that falls out of the sky is more acidic. And the purest lake on Earth – that might have existed in the Garden of Eden – is even more so! Do they know that? Are you going out of your way to explain the reality? No, it is sensationalist and misleading language that is factually meaningless, in the end!
Tell them that the ocean’s “Alkalinity” is not a measure of how alkaline it is!
No don’t bother, it is too close to the truth, they might start to actually think for themselves, and perceive the deception.
Acid or caustic, tell the average person what actually happens when you add more of the “acid” H20 to sea water! If you add an acid to any solution in the continuum it will become more acidic…Yes? This is your argument or at least, the argument you make to the vast unwashed**
Now tell them the truth; that it is not the case!
These notions are triadic and all most of us are ever exposed too, taught or ever need to know, is the reasoning of dichotomies, dualities and binary oppositions.
The average person could not be expected to understand the “duplicity” inherent in marketing of this new scare tactic.
And that is why it is so very important to be particular in the use of terminology for fear that ‘communication’ might become jargon***.
*H20, pure distilled water.
**Average person, non chemist, non scientist or non specialist.
***Barbarous, in the archaic sense.
Ghalfrunt,
Say whatever you want, but do not claim that you are speaking from authority of are using words according to their actual definitions.
By incorrect usage of language, you mark yourself not as authoritative, but as an ignoramus.
In your specific attempt to use sophistry to redefine language to suit the alarmist warmistas goal of making everything sound scary and awful, if as you say, you do not know the details of a reaction, it is more important, not less so, to use precise language.
We have an epidemic of people who insist that words mean whatever they say they mean, and endless sophistry supports their redefinition of the language of science.
Find me a dictionary with your definition of the word acidification.
Personally, I have looked at at least twenty of them, everything from regular Oxford or Webster, to legal dictionaries, medical dictionaries, scientific dictionaries, all of them.
The word acidification means one thing: Something becoming an acid.
Not less basic.
Not lower in pH.
Not anything except what is obvious from anyone who knows English and what suffixes like -ification even mean to begin with. Even if that was all you knew.
“a·cid·i·fy.
[əˈsidəˌfī]
VERB
.
acidifies (third person present) · acidified (past tense) · acidified (past participle) · acidifying (present participle)
make or become acid:
“pollutants can acidify surface water” ·
“the paper was acidifying”
Powered by Oxford Dictionaries ”
”
acidify
/əˈsɪdɪˌfaɪ/
verb -fies, -fying, -fied
1. to convert into or become acid
Derived Forms
acidifiable, adjective
acidification, noun
acidifier, noun
Collins English Dictionary”
“a-cid-i-fy
[uh-sid-uh-fahy]
Spell Syllables
Examples
Word Origin
verb (used with or without object), acidified, acidifying.
1. to make or become acid; convert into an acid.
2. to make or become sour.
Dictionary.com”
” Noun 1. acidification – the process of becoming acid or being converted into an acid
acidification – the process of becoming acid or being converted into an acid ”
I had in mind quoting them all, but there is no point. They all same the same thing because the word means only one thing…the process of becoming an acid.
“Neutralisation tells you nothing of what is happening. You could be adding acid to alkaline solution Or adding alkali to an acidic solution. Making a solution more acidic tells you that the pH is being reduced.”
Pure bunkum.
It says no such thing…saying acidification tells you an acid has been created.
Sophistry is really an amazing thing, just make up or say anything that pops into your head and pretend it is true and or logical.
You are actually attempting here to redefine language in use for at least two hundred years because you think one word ought to be able to tell you everything you need to know about “what is going on”?
How about instead, you find out what “is happening”?
Instead of making some crap up?
Huh?
Did you ever think of that?
Thank you menicholas, for the time an effort you have given to this post. I am not a Chemist but am well read and I believe that the points raised deserve a post in their own right. I could add a paragraph or two on the topic of semantics!! 😉
Kip In the mining/exploration industry In Canada, a company must retain an independent ‘Qualified Person, QP, typically an experienced geologist or engineer who has to OK the technical details of Press releases, feasibility studies, prospectuses or presentations to protect the investing or stakeholder public from overly promotional or ambiguous reporting. Reporting itself has to be timely and complete. A public company must report negative or disappointing results of studies, assays, along with favorable material. You can’t be selective and you can’t unreasonably delay reporting appraisal results, environmental issues, etc.
In a long career I can’t count the number of times I’ve said, “You can’t say it that way.” “You can’t use the word confirmed unless you have an in situ sample or drill intersection in bedrock.”
I’ve commented a number of times that it’s time for scientists to have to adhere to a formal code of ethics. Engineers, geologists, lawyers, doctors, accountants, pharmacists…can’t practice outside of a licencing association legislated by statute with disciplinary powers up to and including suspension or permanent removal from practice for negligence, misrepresentation, malpractice or breaking the law.
The goodwill long accorded scientists is no longer justified. Nowadays, the public clearly needs protection from unfettered abuse of trust from the highly influential, and evermore corrupted fields of science. The unholy alliance of climate science and politics is the most outstanding example. Accountability and consequences for unethical work need to be front and centre.
Gary ==> we hold the researchers accountable for what they write in their formal papers, and for what they say when interviewed.
What we can’t hold them responsible for is what others say they said….
If they would insist on signing off on press releases about their work — officially — then we could hold them responsible for the contents.
In my view, Scientists should be made accountable for the content of their publication, including the press-release. With regard to current ‘press-releases’ they are plainly motivated by the interests of the corporation and not of the science. As we see all the time, there is a clear conflict of interest and many scientific press-releases are ‘Junk-Science’
I won’t trust a licencing association in science. Such association would soon be colonized by people with an agenda using the licensing power to police researchers into complying.
And, anyway, scientist are cats, it takes extraordinary circumstances and leaders to herd them into some sort of Manhattan Project, and the team dissolves as soon as possible.
Even USSR (inside) and KGB (outside) had trouble herding scientists for their goal, even when appalling to “peace” urge.
I find it completely fair that science stay completely powerless in itself, and, hence, irresponsible.
It just must be known that scientists are untrustworthy NUTS, all of them, including geniuses (possibly even more them geniuses… ).
I never trust a scientist. NEVER. Not even Einstein. Edison (reckless man, this one, tried to discredit alternative current by inventing the electrical chair), Tesla, Newton (alchemist, astrologist, biblical chronologist,… )
I only trust technicians and companies who somehow succeed in delivering results out of the science.
paq: unethical behavior in research doesn’t mean making a mistake or trying some way-out experiment. It means knowingly misrepresenting findings, throwing out data that doesnt fit preconceived pet theories, lying to support an agenda. The reason for regulation is not to stifle new ideas. Indeed look at the marvel of engineering creativity over the past half a century. It is why engineers tend to be sceptics and why their research has been so innovative.
My point is, sure, leave everybody alone except for those whose work can be a risk to a public that is obliged to trust their work. Driving over a bridge that doesn’t fall down, flying safely from place to place, undergoing medical procedures, etc.
The licence is at least a guarantee that the practitioner has passed critical courses and been been grilled and tested in ethical practice and case histories of disasters caused by negligence. A structural engineer has even more stringent codes and all engineers have to keep a record of their efforts annually to update skills and new procedures. You are notified of courses available to help you do this. I can tell you that, at 80, it is tough to do, but that’s the game it is.
If you say “QP” quickly, it brings to mind “kewpie doll.” Any connection?
Things have gotten to the point where I believe nothing reported by print or broadcast journalists unless I have direct corroborating first hand knowledge.
Many decades ago I got into the habit of listening to National Public Radio (or, as I now call it, National Propaganda Radio). It is, unfortunately, a rare day when I hear a report that I consider accurate, balanced, unbiased and non-partisan.
Where the subject is climate, NPR along with PBS, Pravda (a/k/a The New York Times) and the WaPo are completely and utterly bent.
Sadly, my exact experience as well.
“What the Press release said: “the skeletons of calcifying organisms like star fish and coralline algae were dissolving.”
What the paper said: “a significant shift towards net dissolution was observed.””
No. What the press release said was:
“We found that there was a rapid, community-level shift to net dissolution, meaning that within that community, the skeletons of calcifying organisms like star fish and coralline algae were dissolving.”
which is a lot closer to what Dr Burdett said in email. The only real difference is that she added the possibility that dissolved calcium could also have come from sediments as well as skeletons.
Actually NIck, what the press release says is ” the skeletons of calcifying organisms like star fish and coralline algae were dissolving.” This implies that the skeletons of organisms were in fact dissolving – the precursor does nothing to alter that impression.
You have an amazing ability to explain how people actually said (meant) something other than what they did say, but in this case you really do need to give up, they said what he said they said. Period
“the precursor does nothing to alter that “
It isn’t the precursor. It is the main statement, which matches what is quoted as “What the paper said”. The rest is exemplifying what that might mean, as it explicitly says.
So if I say:
“Nick Stokes is consistent, meaning that every word he says has to checked”
It should be considered an endorsement?
“The rest is exemplifying what that might mean”
“When I use a word,” Humpty Dumpty said, in rather a scornful tone, “it means just what I choose it to mean—neither more nor less.” “The question is,” said Alice, “whether you can make words mean so many different things.”
Nick ==> Try to be real. The average person reading the press release gets “the skeletons of calcifying organisms like star fish and coralline algae were dissolving.” They have no idea what “net dissolution” might might.
Other news outlets get the same “the skeletons of calcifying organisms like star fish and coralline algae were dissolving.” — and write nonsense like “starfish are dissolving”. The example in the end Note is a similar example.
The author of the original paper, Dr. Heidi Burdett, approved this test of this essay before publication.
They have no idea what “net dissolution” might mean.
“They have no idea what “net dissolution” might mean”
No. But it’s the language you quoted from the paper, as a contrast. Ad it is the language that the press release leads with too. Then they try to explain what it might mean. In quite similar terms to those Dr Burdett used.
Nick is obfuscating the point of the post, which is that the schools press release exaggerates the study results. In turn, the media takes the press release and hypes it further. Just do a duckduckgo search of starfish acidification. You get pages of links with headlines about starfish dissolving before your very eyes.
LOL – spell check tells me acidification isn’t a real word.
Greg61 ==> You have to add it to your spell checker dictionary….it ain’t going away.
Those who comment about everything except the point of the essay, even after being corrected several times, are engaging in the same behaviors as your everyday “angry ‘tween-aged troll” except they use more sciencey language.
would be interesting to know the control of the volume of enriched water pumped back into the tubes. they may have have inadvertently disturbed the sediment by over pressurising the tubes or during the process of removing the initial volume of water.
should have added that is why a control chamber should have been used.
bitchilly ==> I have linked the full study. The methods are in the study, including the detail you seek. It;s only a few pages long.
thanks for the reply kip. i have looked (twice now) and am obviously missing something right before my eyes. i read the entire post, clicked on various links including the doi link but cannot actually see the paper in any of them ?
bitchilly ==> I’m sorry, this is my error — I have only linked to the abstract, and not the full study. Email me at my first name at the domain i4 decimal net and I will send you a copy of the study and SI.
You are right, of course, a control chamber should have been used — but, inexplicably, was not.
thank you kip, much appreciated .
bitchilly ==> That said, I have not yet received an email requesting the pdfs.
just sent kip, i had a table booked at a local restaurant for mothers day . not long back and e-mail sent. once again thanks, much appreciated.
Have a look at media reporting of some topic upon which you are an expert, relative to the general populace. On most subjects with which I qualify as expert, I can spot numerous flaws. So why would I believe they are not making similar errors in other subjects? Like AGW, or economics or politics? I wish they’d just report facts…
Thanks for doing the work on this.
I think in journalism classes folks are told to NOT allow the subject-person to read and make corrections in what is written. Years ago, I told the university PR person I did not approve of the concept for the story they wanted to write it. I was told it would be their way or not at all. That was the end of that. No story.
My second point is about the word acid.
Our U. S. society has made this word caustic. (How about that for a pun?)
Meanwhile, the word basic has a gentle disposition, even gracious.
Consider, it can be said of someone she or he is a basic salt-of-the-Earth person; a representative of the best or noblest.
When the language is against you, winning an argument is doubly difficult.
First of all, let’s give these folks some credit for “acidifying” with CO2 rather than Hydrochloric Acid. And second let’s cut them a little slack with regard to experiment design. I suspect that bubbling controlled amounts of CO2 into an ecosystem in the presence of real waves, currents, etc is a lot harder than it sounds and that maintaining a high dissolved CO2 concentration for extended periods of time is not easy..
WRT to ocean acidification in general, I think that there’s a major problem in that it is generally assumed that atmospheric CO2 and water temperature are the only two relevant variables. Willis wrote a couple of interesting articles https://wattsupwiththat.com/2015/01/02/a-neutral-view-of-oceanic-ph/ and https://wattsupwiththat.com/2014/12/30/ph-sampling-density/. In Figure 2 in the second link, Willis plots pH geographically. It seems pretty clear that water temperature probably is NOT a major control on pH. There are low pHs along the coast of Antarctica, but also along the Caribbean coast of South America and High pH in the tropics but also around Greenland. And we know from Keeling’s CO2 measurement program and the OCO2 satellite that atmospheric CO2 is pretty well mixed with small seasonal variations and a slight decrease in high latitudes. It’s likely not driving local pH variations.
All things considered, it looks to me like atmospheric CO2 concentration probably is not a major driver of ocean pH.
Tangentially, brittlestars (Ophiuroidea) are clearly closely related to starfish (Asteroidea)
. But they don’t act much like starfish. They can move quite rapidly and the few I saw in my youth playing in tide-pools in California were doing exactly that — scuttling under cover within seconds of being exposed.
i>”All things considered, it looks to me like atmospheric CO2 concentration… ”
There are of course local variations in shallow water. But this paper is not about atmospheric CO2. The sub-heading says
“Scotland’s marine ecosystems may be more sensitive to carbon dioxide than previously thought, and could be damaged irreparably by the CO2 ‘pulses’ created by industrial activities, land run off or natural tidal processes. “
OK, Nick. We agree. This isn’t about atmospheric CO2. And I’m not sure about your “pulses of CO2” either, not because they aren’t conceptually possible. I just have a bit of trouble imagining an industrial process that discards vast amounts of carbonated sea water. Not that I doubt the reality of industrial pollution. But dissolved Carbon Dioxide is way down on my list of potential pollutants. Somewhere well below metabolism products from common medications.
Unfortunately, this thing has got me thinking about carbonate chemistry — which is bad because it is probably too complex for me to understand when I was young and smart. I’m no longer either. My first thought. I put distilled water in a flask and measure bicarbonate and DIC. I add some CO2 and a stopper. I shake the flask to mix some CO2 into the water, then measure DIC and HCO3. Surely, both have increased? By the logic of the paper that indicates Calcium Carbonate is dissolving. Except … I forgot to put any CaCO3 into the flask. Ooops.
I think I shall go pour a cup of coffee and watch the snow outside gently falling while I think about this a bit. Not much point in going out and shoveling snow until it stops snowing.
Nick and Don K ==> This particular experiment was to test the idea of damage from “CO2 pulses” — not the slow increase in atmospheric CO2. There is something wonky about the part of the study you quote Nick — “and could be damaged irreparably by the CO2 ‘pulses’ created by industrial activities, land run off or natural tidal processes. “ There is no CCS in Scotland (one of the given justifications for the research), land run off already happens and can be tested where it happens to see if coralline algae has been “damaged irrepairably”, and if natural tidal processes would cause such damage, then we’d already see it.
There are other oddities in this study which I didn’t cover — I was just doing the Press Release Science issue.
Besides the issue above, there was no control FOCES chamber used, even though researchers has four chambers available — a control chamber is required by best OA practices. The results from the four chambers are not broken out in the paper or in the SI — but rather all lumped together — so details and variances are obscured. Net Dissolution (lumped) is of small magnitude, and with SDs extends into Net Calcification range — this is not discussed in the paper or SI.
Further, the pH measurements of the ambient and the CO2-enriched conditions overlap one another, at one SD: 7.9±0.2 or 8.1 to 7.7 vs. 7.7±0.39 or 8.1 to 7.4 . The CO2-enriched treatment results include the entire spread of “ambient +/- one SD.” Again, no discussion, no breakout.
The time line and diel factors are not dicussed nor is the data made available — no way to tell how much the calcification/dissolution changed when despite the well known and acknowledged fact that all this is highly dependent on biological activity. Dr. Burdett emailed this “Within such a complex coastal ecosystem, there are many processes that affect the pH of the seawater (biological, chemical and physical) and thus an inherently high variability at hourly, monthly and seasonal scales is to be expected and is reflected in the pH record. As you note, the balance between photosynthesis (which takes up CO2) and respiration (which releases CO2) can lead to daily cycles in seawater carbonate chemistry (which in turn affects pH).” But we don’t see this in the paper and the data is not shown — though it was measured and recorded.
So, a critique of the paper as published would be far different than a discussion of the Press Release language. Dr. Burdett did not approve the text of the press release but she did approve the text of this essay.
Don K,
” Surely, both have increased?”
Yes. But if the added CO₂ is dissolving CaCO₃, then increase in DIC could be twice the added CO₂.
I worked in estuarine waters where the pH ranged from at least 6.9 to 9.0, sometimes looking unsuccessfully for clues to compare. Higher pH is more common in higher salinity, especially hypersalinites, lowest in very low salinities. A graph from a Texas study shows pH going from 8.2 to 8.9 in l2 hours, another barely moved in 24hrs with others in between.
One conclusion (based in part on alkalinity) was “It is evident that calcium carbonate precipitation is not actively involved in the diurnal changes in the carbonate ion concentration, even though the fluctuation in the carbonate ion concentration is considerable.”
One wonders if these researchers even know the scale is logarithmic and present arithmetic averages. It has happened. There are so many factors involved here there was little interest in pH except for extremes until the ‘acid’ scare. I was told that about century ago there was a lot which got nowhere, probably for a number of reasons.
HDHoese ==> OA research was hopeless prior to 2011 or so — and has only slowly improved. Cornwall and Hurd tried to correct this in 2014 (see my earlier essays) and have been somewhat ostracized since for “criticizing the work of others”.
It is getting better — but very slowly.
I think the word “debunked” in this case is inappropriate. The article stands as written. The science by press release, not so much.
Fundamentally, what happened was that the terms were more clearly defined through investigation and the implications better explained.
It boils down to “a little knowledge is a dangerous thing”. A “little misdirection” is even more dangerous.
…They are usually rather perplexed as to how the press release sent out to the media by their own academic institution came to contain such a questionable quote but almost always seem to shrug it off with good sense of humor. After all, what can they do?
This does not come as any surprise to those of us who follow science news…
I wonder if people think this only happens in the scientific world. because it happens all over academia. And further afield – politicians are famous for exaggerating things. Indeed, ALL news items nowadays are exaggerated way beyond any semblance of reality in a vain attempt to get viewing figures up…..
You have been reading the Daily Express ‘science’ section again, haven’t you?
https://www.express.co.uk/news/science
Check out the amazing ROBOWOLF!
The ‘sweetly-replying’ Heidi knew what she was doing. Using a mixed up expt supposedly designed for general situation-testing, she reported the dissolution of dead shells doing what they normally do, so they could/would be reported to support the propaganda meme.
Kip, as a newspaper reporter for 31 years who used to have to grapple fairly regularly with scientific (and financial) reports I can say with confidence that scientists do themselves no favours by their apparent inability to write in anything approaching “normal” English.
Their reports often read like a foreign language to the layman – and that includes most reporters and PR types. PR people have to turn a highly technical study into something sufficiently sexy to attract journalists and the latter, in turn, have to make it understandable for the readership.
Nowadays, staff cuts means that reporters are running around like headless chickens, under pressure to file whatever stories they’re working on a produce another few before lunch. They often don’t have the time to read the whole study. In short, it’s not so much the reporters who are at fault as the PR staff. But, the scientists could do much to help by writing clearly in the first place and if they say it’s not that easy then how the hell are reporters supposed to be able to do it?
What I do not understand on the subject of dissolution of carbonate shells is that both of the organisms mentioned here have organic material covering their carbonate shells and these protect the shells from dissolution. A short period with lower pH should therefore mean that it is impossible for the increase in bicarbonate concentration in the sea-water to have come from living organisms. I would have thought, if my understanding of this is correct, that a qualified marine biologist would have been aware of the fact too. Or of course, I could be totally misinformed….
Paul ==> Some organisms, among them is coralline algae, can have their bio-protective mechanisms damaged/impaired by rapid shifts in sea water chemistry — such as sudden shifts in pH on the scale used in this experiment. Other organisms experience these types of shifts on a daily or tidal cycle basis and are not adversely affected at all.
I feel actual pity for anyone who takes seriously the notion that a one part in ten thousand increase in CO2 is going to cause coral, or any of these other shelled creatures to dissolve.
If only because it is very well known that many of these species have freshwater cousins that thrive quite merrily under conditions that are quite acidic, not merely a tiny tad less basic.
But mostly because it is well known that these things have evolved, survived and thrived, and spread all over the world, as CO2 levels have regularly fluctuated over long and short scales of time, and that for the vast majority of earth history, levels of CO2 were vastly higher than anything that is conceivably possible from burning fossil fuels.
And what was the results of vastly higher CO2?
We see the evidence in the rocks all around the world, from the white cliffs of Dover, incredibly vast and deep limestone of the Kaibab Plateau, to name two of literally hundreds of such vast formations.
More CO2 does for shelled organisms what it does for plants…it gives them more of what they need to grow.
They evolved under conditions in which it was hugely higher.
But some simpleton snot nose who must know zero earth history and scant biology thinks it is dissolving living creatures shells, because of a poorly designed experiment in which she even admits she did not even verify the results of?
Seriously, it is pitiable.
But I cannot bring myself to believe that any truly educated person believes this nonsense.
So doubling CO2 concentration in seawater reduces PH by 0.2. That’s a change similar to the global variation of oceanic PH. An increase in atmospheric concentration from 0.04% to, say, 0.1% will not change the dissolved CO2 anywhere near doubling.
Ed Zuiderwijk ==> Lock Sween experiences shifts in pH on that scale as shown in the SI of the paper in question:
“So doubling CO2 concentration in seawater reduces PH by 0.2”
0.3? The base 10 log of 2 is 0.30103.
“An increase in atmospheric concentration from 0.04% to, say, 0.1% will not change the dissolved CO2 anywhere near doubling.”
Assuming no other factors like temperature changes (CO2 is a bit less soluble in warm water than cold) 0.4 to 1.0 is a bit more than doubling. 0.1/.04 = 2.5. But I agree, it’s likely not going to change ocean pH very much. Note that marine life thrived in the Cretaceous when atmospheric CO2 levels were thought to be 2000 ppm or higher.
That is a calculation for the equilibrium situation. Just suppose that atmospheric concentration of CO2 jumped overnight to 0.1% and stayed there ad infinitum. The time it takes to work that change at the boundary through the whole body of water is tens of thousands of years. Apart from the fact that there is not enough CO2 to do so, which a simple caculation shows: at a concentration of 0.4% the amount of CO2 per cm^2 is about 0.5 gram, of which at most 0.2 gram was added by us over the last 150 years or so. A similar amount, 0.2 gram was also added by us but subsequently disappeaed in the carbon sinks, of which the oceans are probably the most important. So per square cm we have added 0.2 gram of CO2. Per square cm there is about 280-300 kilogram, that is 300000 gram, of water on the planet. the idea that such a triffle of added CO2 will substantially alter the pH of the oceans is simply risible.
Sorry, 0.04%.
Australia’s Tim Flannery has had considerable difficulty with the chemistry of CO2 in the ocean.
“At the Tata Business Excellence Convention 2012 on ‘Reversing people induced climate change’ the best-selling author of The Future Eaters and The Weather Makers stressed the need to take immediate action in this critical decade to prevent further environmental damage and to leave a more acceptable inheritance for our children.”
‘Climate change is a threat to our civilisation’
http://www.tata.com/article/inside/tut2YR29NNU=/TLYVr3YPkMU=
verbatim from the article:
…we are responsible for changing conditions on the surface of the earth. However, much of the science is self-evident. One example is the issue of coral reefs. In Australia we have conducted a series of experiments where we have taken sea water, reduced its acidity and temperature to create conditions as they were 200 years ago and then put coral in that; the coral thrives in that water. We can take sea water as it is today and coral does not grow so well.
We can put coral into water with conditions as they will be in 10 years’ time and we will see that the coral is stunted and dying.
We can see that this will happen — carbon dioxide in the atmosphere produces carbolic acid in the ocean, the ocean becomes acidic and things die.
This was not a typo, because he said the same thing in one of his books, would need to look it up again, but a reviewer pointed it out also.
It seems EPOCA suffered from the same problem, the links are still live, the error still there:
http://epoca-project.eu/index.php/who-are-we.html
“The EU FP7 Integrated Project EPOCA (European Project on OCean Acidification) was launched in June 2008 with the overall goal to advance our understanding of the biological, ecological, biogeochemical, and societal implications of ocean acidification. The EPOCA consortium brings together more than 100 researchers from 27 institutes and 10 European countries.”
There is a blog linked directly to the project site:
http://oceanacidification.wordpress.com/about-2/
“This blog was started in July 2006 as a “one man” effort. It is a product of EPOCA, the European Project on Ocean Acidification since May 2008 and it is sponsored by the IMBER and SOLAS projects since January 2010.”
“This blog is coordinated by:
Jean-Pierre Gattuso, CNRS Senior Research Scientist
CNRS-Université Pierre et Marie Curie Paris 6, France”
The list of authors and review editors of IPCC Working Group II, 5th Assessment Report included Jean-Pierre Gattuso from EPOCA.
If this blog was co-ordinated by Gattuso, a lead author on AR5 WGII, you would assume he would read what is written there.
http://oceanacidification.wordpress.com/2010/09/16/ocean-acidification-audio/
“Too much carbon is flooding the ocean with carbolic acid, with devestating (sic) effects on life in the sea.” (no wonder!)
Check out Tony Thomas’s article for Quadrant, “The Fishy Science of Ocean Acidification” https://quadrant.org.au/opinion/qed/2016/01/fishy-science-ocean-acidification/
carbolic acid????
Really.
When I was at school, many years ago, my homework often required me to précis a paragraph with a single sentence of my own, to express the thrust, gist, essence, whatever, of what the paragraph was saying.
I gather from my grandchildren that this is no longer done.
To me the process is a fundamental educational tool and it’s apparent absence is now quite clear in the plethora of gobblegook that besets most of what we read today.
I suggest this educational deficit is responsible for much of the poor journalism and general comprehension we now witness as this example demonstrates.
What a pointless, useless and taxpayers’ money wasting “study”! It tells nothing, we learn nothing from it, it adds nothing to the corpus of scientific knowledge. Without the Goebels warming hysteria, such stupid academic exercise would never have been funded. And without the alarmist spin aided and abetted by the author, such drivel would never have been published by the climate change propaganda media.
Every sane taxpayer should be mad to know his hard earned money has been spent to finance such pseudo-science.
“This was an expected and interesting result.”
Well, for me it is either expected, or interesting result, but not both.
And it was definitely expected, not interesting, as this is classic limewater experiment done by pupils (is it still true?)
Ca(OH)2(aq) + CO2 → CaCO3 (solid) +H2O result in a milky limewater solution
with more CO2
CaCO3 (solid) + CO2 → Ca(HCO3)2 (water soluble) the solution turns transparent again
And you can easily calculate the loss of CaCO3 : 1.67g per 1g CO2 in excess.
Another instance of junk science, using tremendous resources to yield a result already known to 7th graders.
Besides, quite obviously, living being control their pH and pCO2 while dead one don’t, meaning, Only dead bodies contribute to the dissolution of CaCO3.
AND this dissolution is ridiculously small.
AND this dissolution will not last long, as Ca(HCO3)2 will soon be turned into live matter
Excellent summary. Why is Nick Stokes still exercised by this non-problem?
paqyfelyc ==> When all this occurs in sea water, a complicated solution, even without all the biological activity, it is much more complicated. See the EPOCA booklet for way-too-much information, or the single page Aragonite Saturation State link in the End Note.
Early OA researchers though the 7th grade version applied to sea water and wasted a great deal of effort and resources producing mostly-useless results.
See my earlier liked essays on OA research.
Kip
I wonder what the result is when several inches of acidic rainwater (with a pH as low as 4.5 on Americas East coast) is added to shallow coastal sea water? Then there is the increased runoff, as well. Is this not a natural experiment that only needs a little study?
Rockyredneck ==> I thought they could find some locations where the conditions occur naturally often enough to test their hypothesis….but they are in Scotland…….
I don’t doubt real life is vastly more complicated than a 7th grader lab.
I just don’t see any new result about CaCO3 / Ca(HCO3)2 in this study worthy of highlighting.
Actually, i just wonder about one sentence in the abstract : “Smaller changes from net respiration to net photosynthesis were also observed”.
I am surprised that the habitat was net respiring beforehand (I expect “globally ubiquitous, ecologically and economically important habitat” to be net photosynthetic, not net respiring…),
I am not surprised that the habitat increase photosynthesis, as algae use HCO3-, not CO3– nor CO2. Meaning the habitat was actually starved of CO2 (could had been otherwise, if the habitat was starved of some other nutrient). But, since this implies that CO2 is a boost in life of the habitat, not a threat, I am surprised that they conclude that it “raises concerns over the capacity for the system to ‘bounce back’ “.
Obviously, they are not afraid to toe the line even if their result suggest the very opposite.
Junk science.
Rocky–Decades ago I saw something like this in a Louisiana flood that showed me the great buffering capacity of sea water, even after several dilutions. Getting it below 7 with some sea water left requires mixing with reduced sediments, usually anoxic. There is a fair amount of study on the not really dead zone bottom water, usually ending in the complexity epithet.
Kip Hansen March 9, 2018 at 2:54 pm
Rockyredneck ==> I thought they could find some locations where the conditions occur naturally often enough to test their hypothesis….but they are in Scotland…….
Heavy rain runoff over peaty soil into the loch would certainly spike the CO2.
Phil ==> Dr. Burdett, however, didn’t mention that in the paper or in personal communication, though I suppose it is possible.
The paper’s SI has a graph of ambient pH (no indication of whether pH was measured at the surface (first 1 meter) or at the biologically active zone at 6 meters) showing there are naturally occuring pH shifts of the 0.2 pH point scale
Kip Hansen March 10, 2018 at 6:06 pm
Phil ==> Dr. Burdett, however, didn’t mention that in the paper or in personal communication, though I suppose it is possible.
Yes, I was thinking of a lake I used to fish for trout in northern England which was fed by streams from peat deposits, after storms the pH would be between 3 and 4! We were able to counteract this by dumping limestone in the streams, this greatly improved the pH and the trout got bigger. 🙂
Ben Goldacre wrote an editorial “Preventing bad reporting on health research” in which he insisted that “Academics should be made accountable for exaggerations in press releases about their own work”.
That was the same Ben Goldacre that made it very clear in the Guardian when he said what is unacceptable in other areas , was fine in climate ‘science ‘ not be any means a trusted source given his willingness to turn a blind eye to poor practice when it suits him.
BG is a hypocrite who knows only too well not to upset the Guardian.
I’ve frequently told my children that nothing is ever a waste of time, as long as you remember what went wrong and not to repeat the mistake, an expensive mistake should reinforce the memory. The problem is that rather than remembering what went wrong most climate scientists repeat the mistake because their theory cannot possibly be wrong despite the evidence of their own lying eyes.
Kip – thank you for a very interesting and informative article.
As I understand it:
1. Modern increases in atmospheric CO2 could make the oceans SLIGHTLY LESS ALKALINE, NOT ACIDIC, and this reduction in alkalinity causes no significant threat to aquatic life.
and
2. Atmospheric CO2 concentrations have been much higher in Earth’s history, and caused no significant threat to aquatic life.
Kindly agree or modify.
Best, Allan
ALLAN MACRAE March 9, 2018 at 3:30 am
Kip – thank you for a very interesting and informative article.
As I understand it:
1. Modern increases in atmospheric CO2 could make the oceans SLIGHTLY LESS ALKALINE, NOT ACIDIC, and this reduction in alkalinity causes no significant threat to aquatic life.
You are mixing up the terminology, Alkalinity is the sum of excess proton acceptors. TA stays constant even when CO2 is added to seawater because the charge balance of the solution stays the same, meaning that the number of positive ions generated equals the number of negative ions generated by these reactions.
and
2. Atmospheric CO2 concentrations have been much higher in Earth’s history, and caused no significant threat to aquatic life.
On the contrary during the Permian extinction 96% of all marine species became extinct (the increase in CO2 was about 2000ppm).
Kindly agree or modify.
Modified
“during the Permian extinction 96% of all marine species became extinct (the increase in CO2 was about 2000ppm).”
Even if the proxy evidence could be accurate enough to determine that these were concurrent events, is cause and effect established? Are there other instances of marine die-offs correlating with high atmospheric CO2 as corroborating evidence?
I have seen it proposed that high CO2 led to high temperatures and that they may have jointly caused the Permian extinction but I was not aware it was a generally accepted explanation.
There seems to be considerable uncertainty as to the source of the CO2 which increases the uncertainty as to cause and effect.
Could you expand your thinking on this?
Phil – really???
There are multiple theories about the cause of the Permian Extinction – not surprising that one of them is the “demon molecule that knows all, sees all, does all”, CO2.
Are you saying or implying that 2000ppm CO2 in the atmosphere:
1. Was the concentration of CO2 in the atmosphere during the Permian extinction?
.AND.
2. This 2000ppm CO2 in the atmosphere was the primary cause of the Permian Extinction?
Color me highly skeptical on point 2 – I mean, really really highly skeptical.
CO2 has been this high and higher during other eras when no major extinction occurred.
KIp – awaiting your response.
At other times in the Earth’s history, CO2 levels exceeded 5000ppm and life thrived.
Your desire to believe that 2000ppm killed of the life is contra-indicated by the evidence.
MarkW March 9, 2018 at 7:26 pm
At other times in the Earth’s history, CO2 levels exceeded 5000ppm and life thrived.
Your desire to believe that 2000ppm killed of the life is contra-indicated by the evidence.
The level of 5000ppm was before there was life on land, during the Devonian (400 Mya) plant life started on the land and the CO2 levels dropped precipitously and the O2 levels went up.
The era of CO2 above 5000ppm was the Cambrian (500 Mya) and life was certainly not what it is now, lots of arthropods! The periods in the earth’s history when there have been large changes in the CO2 levels (both ways) have been accompanied by major extinctions.
Phil – with no references provided and plenty of your arm-waving, I reject your responses as not credible.
There certainly have been MANY long periods where there were (relatively) high concentrations of atmospheric CO2 that do NOT correspond to major extinction events. In those cases where that coincidence did occur, there is no evidence that high CO2 was a cause of extinctions rather than one of many effects of the primary cause.
Blaming every catastrophe or imagined future disaster on high CO2 concentrations is a modern fantasy, indulged in by scoundrels and imbeciles. Don’t do it – this is not the company you want to keep.
ALLAN MACRAE March 10, 2018 at 9:10 pm
Phil – with no references provided and plenty of your arm-waving, I reject your responses as not credible.
Really, unlike your arm-waving which includes copious references!
There certainly have been MANY long periods where there were (relatively) high concentrations of atmospheric CO2 that do NOT correspond to major extinction events. In those cases where that coincidence did occur, there is no evidence that high CO2 was a cause of extinctions rather than one of many effects of the primary cause.
If you read what I wrote carefully you’ll notice that I didn’t refer to high CO2 being the cause of extinctions, I referred to the change in CO2 concentration. I also referred to both increases and decreases in pCO2 as being implicated in extinctions.
That’s how evolution works, for example in the Cambrian those species were adapted to High CO2, low O2 and lower T than now. When the conditions changed including CO2 dropping many species died out, some which had the ability to adapt to the new conditions did so and survived, new species derived from those survivors. Just because there are current descendant species from the genera that existed at that time does not mean that those species are able to survive a higher CO2 level because there have been so many changes since. Similarly for subsequent changes with rises in CO2 such as the Permian, again many species died out and another selection event occurred. The statement I was responding to: “At other times in the Earth’s history, CO2 levels exceeded 5000ppm and life thrived”, with the implication that an increase from our current levels would have no effect is incorrect. Under stable conditions live does thrive but when there are changes extinctions occur followed by new species developing to exploit those changes.
Blaming every catastrophe or imagined future disaster on high CO2 concentrations is a modern fantasy, indulged in by scoundrels and imbeciles. Don’t do it – this is not the company you want to keep.
Indeed which is why I don’t do it, I also don’t subscribe to the equally fallacious argument that ‘CO2 has been high before therefore a future increase will have no effect’.
Phil – now you are “bobbing and weaving” as you modify your argument, which is not credible either.
Your hypothesis is rank speculation, not supported by the literature, which states that there is no conclusive cause of the Permian extinction.
You are just blaming “the usual suspect” pursuant to the current fad – the “demon molecule” CO2. Nonsense!
.
ALLAN MACRAE March 11, 2018 at 9:11 am
Phil – now you are “bobbing and weaving” as you modify your argument, which is not credible either.
No I haven’t changed a thing, your reading comprehension is lacking, that’s all.
Your hypothesis is rank speculation, not supported by the literature, which states that there is no conclusive cause of the Permian extinction.
Not supported by the literature, really?
http://www.pnas.org/content/early/2011/12/12/1118675109
http://science.sciencemag.org/content/305/5683/506
http://www.cell.com/trends/ecology-evolution/fulltext/S0169-5347(07)00256-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS016953470700256X%3Fshowall%3Dtrue
Sorry Phil.
Your three references do NOT say what you said – yours is the rankest speculation, unsupported even by your OWN references.
You are wasting everyone’s time with your nonsense.
ALLAN MACRAE March 11, 2018 at 9:06 pm
Sorry Phil.
Your three references do NOT say what you said – yours is the rankest speculation, unsupported even by your OWN references.
Thus proving beyond all doubt your inability to understand what you read, it is you who always wastes everyone’s time with your unsupported polemics.
Here’s a part of one of the abstracts of those papers, which you claim isn’t discussing a rapid rise in pCO2:
“The temporal association of the extinction with the Siberia flood basalts at approximately 250 Ma is well known, and recent evidence suggests these flood basalts may have mobilized carbon in thick deposits of organic-rich sediments. Large isotopic excursions recorded in this period are potentially explained by rapid venting of coal-derived methane, which has primarily been attributed to metamorphism of coal by basaltic intrusion. However, recently discovered contemporaneous deposits of fly ash in northern Canada suggest large-scale combustion of coal as an additional mechanism for rapid release of carbon. This massive coal combustion may have resulted from explosive interaction with basalt sills of the Siberian Traps. Here we present physical analysis of explosive eruption of coal and basalt, demonstrating that it is a viable mechanism for global extinction. We describe and constrain the physics of this process including necessary magnitudes of basaltic intrusion, mixing and mobilization of coal and basalt, ascent to the surface, explosive combustion, and the atmospheric rise necessary for global distribution.”
‘Massive coal combustion’ and ‘rapid venting of coal derived methane’, and you don’t think the authors are talking about a rapid rise in pCO2!
For those who may be able to access it here is their conclusion:
“Our hypothesis attempts to explain the sudden release of approximately 1 trillion tonnes of carbon into the surface environment inferred from the carbon isotopic record (5) and to associate one of these events with the end-Permian mass extinction. The analysis presented here coupled with the depositional evidence of fly ash (17) strongly suggest that explosive interaction of basalt and coal in the Siberian traps may have played a role in the end-Permian mass extinction. This process may also be a contributing mechanism for other extinctions involving large carbon isotopic excursions.”
Where they even more explicitly refer to a rapid increase in pCO2 being associated with the end-Permian mass extinction. To put that into context 2ppm of CO2 in the atmosphere is a Gigatonne of carbon so 1 trillion tonnes of carbon would be ~500ppm CO2 increase and the paper refers to three such events which accounts for the increase of ~2000ppm referred to elsewhere.
One other work which references this study concludes: “Times of biological crisis in the past were times when pCO2 increased rapidly, not when pCO2 was high”.
I think that’s you were referring to when you said: “Your hypothesis is rank speculation, not supported by the literature”.
Phil – your long quote has NOTHING TO DO WITH ANYTHING HAPPENING TODAY. The slight increases in atmospheric CO2 from whatever cause are totally different in magnitude from what your quote describes.
You write such nonsense. Let’s end this waste of time..
ALLAN MACRAE March 12, 2018 at 8:02 am
Phil – your long quote has NOTHING TO DO WITH ANYTHING HAPPENING TODAY. The slight increases in atmospheric CO2 from whatever cause are totally different in magnitude from what your quote describes.
Really? Nearly a Gigatonne/ year net increase, no reason to believe that the Permian increases was any faster than taking place over less than 200 years.
You write such nonsense. Let’s end this waste of time..
Let’s, everything you have said so far has been shown to be incorrect.
Allan MacCrae ==> Sorry Allan, I was traveling all day yesterday.
Point 1: Nominally true — atmospheric CO2 reduces total alkalinity of the ocean surface water, incrementally, and slowly as concentrations increase and has a magnified effect on carbonate chemistry — important to many organisms. This change is minor compared to the changes in alkalinity experienced in daily biological and tidal cycles — but not something to be entirely ignored. Acute and high magnitude changes in CO2 concentrations, like all acute changes in sea water chemistry, have effects — mostly negative — on the associated biota.
Point 2: I’m not sure we have any reliable numerical data on past atmospheric CO2 concentrations (ice bubble data is a troubled data set). Qualitatively, it is generally believed that CO2 concentrations have been higher in the past. We have no reliable information on the effects of that difference on the level that is currently being investigated in the field of research called OA — where effects are in the possibilities of relative shifts in species, abundance ratios, calcification rates and sea water chemistry in surface waters.
I hope y’all know that starfish don’t do ‘pain’ or ‘agony.’
In most cases, the AGWs outrageous fear-mongering predictions and press releases only cause the majority of thoughtful Americans to roll their eyes. I think, in net, the loss of their credibility has driven global warming and climate change out of the “Top Ten Concerns” of Americans, and the more they do it, the more they loose credibility.
I’ve been watching the Alexa.com ranking of WattsUpWithThat, ClimateDepot, and looking at Google trends. I’m almost at the point where I can say that Kip, Lord Moncton, and the other “skeptics” have stripped the alarmists of their ability to capture public attention (despite their dying MSM allies), neutered their ability to create motivating fear, and most importantly influence policy. It may be time to take some credit and a bow.
Tom ==> not sure if it’s true, but it’s a nice thing to say. Credit really due to Anthony Watts.
Kip Hansen:
If you are looking for a solution to this problem, maybe give me an email — I may be able to help you. You can find me through my website (linked to my name).
I mean a solution not just for this paper.
Michael ==> Yes, I know.
Michael ==> Thank you — I use the ubiquitous “download a journal paper” solution used by “nearly everyone”. In this case, it didn’t work because there was something wrong with the DOI itself.
Yes, I’ve seen this first hand. You hand the press office the hard-won product and even a summary version and they re-write the summary with their own unique, uninformed slants. This changes the meaning of some of the key points in the process and you either take more time to fix it or watch it go out with alternate meanings. It can be a sad outcome to some long, hard work efforts unless the original work comes from a biased, sensational monger to begin with. In that case later case, it’s double trouble.
ResourceGuy ==> My suggested solution is that researchers, at least lead or corresponding authors, insist on approval-before-release.
I even had this essay approved by the lead author of the paper it is about — Dr. Heidi Burdett — to make sure she had no objections and that I had quoted her correctly.
Hi to Gary Pearse
I’m a “Geo” who has been in the financial markets for decades. An old one from the old Vancouver Stock Exchange sums it up elegantly:
“In the beginning, the promoter has the vision and the public has the money. At the end of the promotion, the public has the vision and the promoter has the money.”
The other observation is that governments will always promote the theory that enhances their wealth and power. In economics, it’s been, Keynes. In climate, it has been CAGW.
Both blatant promotions.
Bob Hoye
subtle2: I worked out of Vancouver 1968-71 In Yukon and northern BC in exploration. ‘Them were the day’s’. There were still a couple of old Klondike miners alive – one called Willie the Weasel in Dawson.
The shenanigans in the mining industry were legendary and led to a crackdown and controls on promotion and reporting of exploration results that I describe above. Climate Science needs a crackdown of a similar kind. Their promotions will impoverish everyone. At least in the mining game you had to be a player to get burned.
“LESSON LEARNED: Never judge a study or its author by the contents of a University media release and never assume that the quotation marks around a statement from a study’s author signify words actually uttered or the meaning intended by the author.”
Yep. I whinge from time to time in my comments here and elsewhere, about the low quality of press releases. I have no idea why scientists aren’t allowed to write their own press releases. Many scientists are quite articulate and even those who aren’t would have a hard time doing worse than “communication specialists”
I don’t expect the situation to improve any time soon.
Agreed. If they could write their own press release then that WOULD put the monkey squarely on their back.
eyesonu ==> eGads! I can’t imagine a press release written by a researcher….have you read many published journal papers? Man are simply gobbledegook to most English speakers.
Don K ==> It will only improve when researchers demand that they “approve-before-release” all media releases from their institutions.
Agreed. Anything that puts the monkey squarely on their back. No possible way to blame a third party when they get called out.
Good presentation by Kip Hansen. Nice to see condensed explanations putting things in perspective.
eyesonu ==> Thank you.
I should have add:
nice article by kip (however junk the reported article is)
Agreed
“The loss may have come from…”
There’s that MAY word again !
What does the author mean by ” inorganic carbon”? Organic chemistry is about the chemistry of carbon,relevant to all life on this Earth. What is inorganic carbon?
Roger ==> I believe the carbon in Carbon Dioxide is/can be inorganic carbon. Carbon compounds created by life-forms are organic carbon.
Since polyethylene is created by humans (a life form), does that makes it “organic carbon?”
C. Paul Pierett ==> Cute.
How about the CH4 in bovine flatulence?
For a chemist, if it has carbon, it is organic.
https://en.wikipedia.org/wiki/Organic_chemistry
To make a difference is vitalism: the belief that living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things. A belief belonging to the realm of religion rather than that of science
https://en.wikipedia.org/wiki/Vitalism
Roger March 9, 2018 at 10:32 am
What does the author mean by ” inorganic carbon”?
Carbon in organic molecules, which excludes CO, CO2, carbonates, bicarbonates, carbides, cyanides and maybe a more obscure one that I’ve missed.
Organic molecules usually contain at least one C-H covalent bond, a few exceptions come to mind: carbon tetrachloride and some other freons.
Kip,
didn’t you mean “sly grins on their faces”?
Robert Austin ==> Terrific! You get the “Reader of the Day” award for careful reading.
Robert ==> No, I meant “SHY” grins…slightly embarrassed grins….the “what-can-one-do?” grins….
Kip, I think most of the scientific world has this CO2/Ocean relationship on its head. There is 50x more CO2 stored in all the oceans than all the atmosphere. Henry’s Law states that solubility of CO2 will decrease with increasing ocean temperatures. This is most likely the source of the extra CO2 we see in the recent atmospheric records, not fossil fuels which is orders of magnitude smaller. Warming oceanic water moves CO2 from the ocean to the atmosphere and this will result in a HIGHER oceanic pH. This is all based on well known chemistry and physics. So far, I have heard a lot about ocean acidification but the only hard evidence I have seen is impossible to parse the contribution from waste water treatment, acid rain, and CO2 contribution. My gut feeling is the loss of oceanic CO2 signal is probably there but indiscernible through all the other noise. There is a false assumption that de novo increase in CO2 is from another source and then the ocean is reacting to that whereas this is a complex system in continuous equilibrium and that assumption cannot be supported by any empirical evidence.
Daniel, some of your assertions seem to be questionable. As you say about “50xmore CO2 stored in all the oceans…”, do you mean CO2(aq) or total inorganic carbon? Carbonate and bicarbonate ions will not be removed from seawater at higher temperatures.
Henry’s Law describes the CO2 solubulity dependence on the partial pressure, not on temperature.
The source of extra CO2 can not be “in the recent atmospheric records”, because the solubility at equlibrium is determined by the the gas partial pressure, temperature, and salinity. Moreover, extra CO2 is consumed by phytoplankton.
Also based on the temperature dependence of the Henry’s Law coefficient there has not been enough temperature rise to account for the increase in [CO2]. Also the atmospheric level is somewhat above the equilibrium value as I recall.
Agree with Aleks and *gasp* Phil.
When you are right you are right.
And not only has the ocean not warmed sufficiently to account for increasing CO2, even if it had, the bulk of the ocean is deep and cold and hundreds of years needs to pass for it to come into contact with the atmosphere and outgas any excess.
This is of course the likely reason for the lag of some 8 hundred years or so in the ice core records, where temperature leads CO2 on the way up and the way down.
Plus, the relationship between temperature water and the amount of gas it can cold is well known, as is the relationship between changing CO2 in the air and subsequent increases, due to the partial pressure, in water.
The too I have not heard anyone claim that the amount of CO2 from burning fossil fuels is insufficient to account for changes in the atmospheric concentration of CO2. In fact, it is the other way around…a lot of the emitted CO2 does not appear in the air…it is absorbed somewhere…likely a bunch of somewhere…soil, plants and trees, and in water. We do live on the planet Water, after all.
As a side note, recall the amount of warming of the ocean that was claimed to have been found by the IIRC, Argos buoys? It was such a tiny amount, to make it sound not just scary but even remotely plausible (even though it aint) the numbers were given in ergs of energy or some such miniscule unit (yes I know it was joules…a joke). Took me all of ten minutes with a calculator to determine that umpteen bazillion skazillion joules is still only a few hundredths of a degree, averaged over the who ocean volume.
And the buoys did not seem to have that resolution…
Hmmm…
Those were the good olds days!
Or, are these the good old days?
I’ll get back to you on that.
‘Loch’ does not mean ‘lake’.
‘Lake’ means ‘loch’.
photios ==> fine, have it your way….
Thank you… 😉
Even though they are more like bays than lakes, eh?
Mr. Hansen,
Your article and the topic you discuss are of course an important part of the whole CAGW story.
If I did not comment on it directly, it is only because it is, IMO, so well known to be true.
It seems to me that the same discrepancy exists and has been long discussed regarding the IPCC reports, specifically the glaring variance between the summary for policy makers and the actual long version text of the reports.
Exaggeration is nothing new in media, indeed nothing new in human communication of any sort.
But when it involves maters of science, matters which are affecting weighty and damaging public policy changes, diversion of resources from more worthy causes and needs, and the miseducation of our children…it is in a special class of awfulness.
To the extent that is may be deliberate rather than simply mistaken or obfuscated by the need to make it understandable to the lay public, it is way beyond troubling, galling, and reprehensible.
It needs to stop,
menicholas ==> Yes — my approach has been to occasionally call out egregious examples — simultaneously involving the researcher/author of the original study by seeking their comments on the Press Release, asking for verification of a quote and then asking for pre-approval of my essay, as I did in this case. This is an appraoch that I hope drives the point home, author by author.
Starfish and most other calcified marine phyla evolved in the Cambrian era during which atmospheric concentration of CO2 was 20-50,000 ppm. They spent a hundred million years or so at these CO2 levels without dissolving. In what kind of reality do these same organisms now start dissolving at CO2 concentrations 1 or 2 orders of magnitude lower? And no – this is not about “getting ised to it” and rate of change – it’s basic inorganic chemistry.
erratum “getting used to it”
You’re high by a factor of 10 in your Cambrian CO2 levels, also note there was a mass extinction towards the end of the Cambrian when the CO2 level dropped.
Yes, and…?
Neither of which contradicts what Ptolemy correctly points out…even if the actual amount of CO2 was not quite as high as the number he gave…it was far higher than we will ever see even after all of the fossil fuels are dug up and pumped out and burned…an amount I believe to be far higher than most current estimates.
After all, the amounts estimated that are still in the ground have only ever gone up, even as we (well, not me) burn it faster and faster.
Kip, your observation of the discrepancy between the true opinion of the researchers and press releases is very interesting. I think, it’s also interesting to compare the statements and facts in the same source. You’ve mentioned “Guide to best practice for ocean acidification” edited by European Commission:
https://www.iaea.org/ocean-acidification/act7/Guide%20best%20practices%20low%20res.pdf
The preface of this book begins from the words: “The ocean acidification is an undisputed fact. The ocean takes up one-fourth of the carbon CO2 (?) emitted to the atmosphere from human activities. As this CO2 dissolves in the surface ocean, it reacts with seawater, increasing ocean acidity…” There is no direct evidence on any page of this book that atmospheric CO2 is the only source of seawater acidification. And what do we see when researchers begin to describe real facts?
Page 19. “About 90% of total dissolved inorganic carbon is present as bicarbonate ion, the proportion of carbonate ion is about a factor of 10 less (~10%) and that of unionized carbon dioxide yet another factor of 10 less (<1%)." Let's remember that only about 1/800 of CO2(aq) converts to H2CO3 (hydration constant) and carbonic acid is weak one. Eventually, only a very small fraction of carbonate ions can be converted by dissolved CO2 into bicarbonate ions. So, is this fact a proof of CO2 effect on ocean acidity?
Page 54, Table 3.1. Observed range of pH (present-day) 7.91 – 8.46 (strong spatial variation). One may compare this range with the change in pH by 0.1 for 20 years at one research station allegedly as a result of carbon dioxide exposure: https://skepticalscience.com/Mackie_OA_not_OK_post_12.html (Fig.6). Is it not enough to question the theory?
Aleks ==> I’m not sure which theory you are asking about — what theory you wish us to question.
The chemistry itself is not a theory — it is, however, complicated and complex.
For example, you state above:
“There is no direct evidence on any page of this book that atmospheric CO2 is the only source of seawater acidification. ”
Certainly there is no evidence for that — because no one in the field of OA thinks that is the case.
Another good article on the chemistry of CO2 in seawater is here:
https://www.soest.hawaii.edu/oceanography/faculty/zeebe_files/Publications/ZeebeWolfEnclp07.pdf
One might out of interest ask this question:
If ALL the CO2 in the atmosphere suddenly dissolved (equilibrated as HCO3- and H2CO3) – what would be the resultant change in pH of the ocean – assuming even dissolution in the entire ocean?
One could even go further and ask what would be the change if an atmosphere composed of 100% CO2 were to dissolve fully in the ocean.
The answer to both these questions would be a surprisingly small change.
Ocean acidification researchers appear unaware of the fact that there are more molecules of seawater than there are of air.
KIP
Your request for examples got sometimes got lost in the subject, but is a good observation. It is difficult to know where to start.
EVERYTHING IS ADVERTISING?
Check journal impact factors, university tours, sports, chamber of commerce type programs, curricula, etc. It is not just journalistic methods, but too often the paper itself, and I need always to read what used to be called ‘fine print.’ Now it is ‘hidden’ somewhere in the paper or addenda. One good thing is the movement to require raw data, something many of us have been long guilty of not saving.
This is not the best example, but the text does not confirm the headline and comes across as pandering for money.
https://www.nature.com/articles/d41586-018-00927-4
Quantity emphasis. No wonder they like consensus. Note that if you read the article it does not quite agree with the headline. Nature reminds me of National Enquirer, no offense, as I first learned about the rare Megamouth shark from a front page photo there. Another example that I found in a paper which maybe should have killed it, except for the need for negative results.
“There are three assumptions that are both critical to the results and can be considered highly uncertain.”
A suggestion along your line of thinking that might not work is to emphasize a discussion just on the methodology, not the subject. This might require (self?) censorship to limit comments not relevant to the question and it might take a long time to develop the discussion. With homework most readers should be able to find problems with the methodology regardless of the lack of familiarity with the subject.
Keep at it. Thanks for the OA links, still trying to understand it.
HD ==> Thanks for the example. sometimes I find that the actual study findings themselves are the “fine print” — hardly worth mentioning by the journalist with his own axe to grind.
So. As there pop social reporters do, science news reporters take out of context quotes and say they mean something that they don’t. Seems the cure for this is remedial journalism classes.
Even with science they inject their own bias. It’s almost like they have an agenda.
Kip,
Thanks for addressing this topic. It’s an interesting and important one.
(I tried downloading the EPOCA manual, and got messages saying “The file is not available on this server.”)
I completely agree with your thesis. The hype is helping no one. However, I don’t think it is always or entirely about agenda. Some alarmism is simply a byproduct of financial decisions. Drama sells. Potential catastrophe certainly sells. Scandal sells. However, climate change has become such a loaded topic that there is that aspect of salesmanship, too. There are many people extremely frustrated by the fact that climate science has so little power in American policy due to the opposing power held by deniers. It’s especially bad now, with an administration so averse to AGW. I think there is genuine fear about the future, especially among young people (naturally!), and it probably feels like inaction is bringing problems ever closer. This may also be why people react so strongly and irrationally to fires and extreme weather events.
Scientists are notoriously poor at communicating with the public. The terminology alone in some fields can be incomprehensible to a layman and difficult to translate. In “dumbing down” the results of research, it is very easy to get the main message wrong, and I think it’s a very good idea to have researchers review press releases, not only so they are responsible for the content, but to avoid censorship of their interpretation.
I think it’s a little ironic to talk about poor journalistic practices here. This may “just” be a blog and not claim to be a news source, but it certainly is a source of news/news-based editorials. You mention Eric Worall’s piece, “Study: CO2 causes Starfish to Dissolve”. The first sentence is, “Dr. Heidi Burdett has published a study which claims intense CO2 shocks cause starfish and coraline algae to dissolve.” This is even worse than “the skeletons of calcifying organisms like star fish and coralline algae were dissolving.” This is a very consistent pattern on WUWT: titles have shock value and/or ridicule, and articles from other sources are summarized, excerpted and quoted in ways that send messages other than what they do as a whole. Often there is a blurb of a couple sentences at the very end full of ridicule and rejection, making sure people know how to feel about what they’ve just read. But it’s a blog geared toward a group, so it’s to be expected, I suppose. This is a commercial enterprise, I imagine, just like a newspaper.
Then again, catering to the market is a poor excuse for lowering ethical standards. Are there ethical standards when it comes to blogs? What about propagandistic blogs? Is the fact that they exist on one side of the political spectrum an ethical justification for those on the opposite end? One factor to consider is their effect on society as a whole. They are helping to divide the country, perpetuating and adding to all kinds of erroneous ideas, increasing tribalism and decreasing understanding.
(Does anyone know of any sites that have a mission of bringing people of diverse political backgrounds together to talk respectfully? I would like to find one.)
……………………………………………
You mentioned the fact that the experiment had no control. This is a valid point, and a weakness in the design (although I’d like to read the paper before absolutely deciding that). However, I would like to suggest that this is in itself not a reason for throwing out all its results, as some suggested. Rather, it’s something to take into account in weighing the importance/significance of the results.
Scientific advancement is most often based on the combined input of research by multiple people. Each experiment or observation adds a piece of the puzzle – sometimes as new information, sometimes finding errors in or correcting older research, sometimes by introducing a new method. Each of the pieces has potential to contribute, but they do not do so equally. Some studies are groundbreaking, or have classic, excellent design and perfect analysis. Other studies have design limitations beyond researchers’ control, but the limitations are overcome in other ways. Some studies have less-than-optimal designs, have odd results or poor interpretation. Even the last group should not necessarily be ignored, but instead taken with reservation and caveats – it should have low weight compared to the first group when considering the Big Picture. A good scientist will discuss in the publication the limitations of the experiment.
…………………………………..
Krisiti ==> Sorry you had trouble with the EPOCA booklet download — the EPOCA site indeed seems to be refusing to deliver up the .pdfs.
Use this link for the entire booklet:
https://www.iaea.org/ocean-acidification/act7/Guide%20best%20practices%20low%20res.pdf
If you really want to read the entire study, you can 1) Do what I did and email Dr. Burdett for a copy or 2) email me at my first name at the domain i4 decimal net and I’ll send a copy to you.
This essay is not a criticism of Dr. Burdett’s study — but rather a an example of poorly written institutional Press Releases and how they subsequently spread misleading information — the result is inevitable to make “Science” seem foolish in the eyes of the general public when the misinformation is corrected down the line. The general effect is called “whipsawing” — “chocolate kills” today, “chocolate is good for the heart” tomorrow .
For an example, see the recently highlighted essay by Boisvert which will cause a lot of people to experience this whipsaw effect.
Kristi ==> Thanks to your alert on the failure of the EPOCA site to deliver up the Guide pdfs, I have added an Update and Correction to the main essay linking to the International Atomic Energy Agency, which inexplicably has a Climate Change section and hosts the document for download.
pH – Many have discussed this. I don’t know if it was resolved, or if this is helpful: It’s from a very nice page about ocean acidification, answering lots of questions and explicating some errors I’ve seen: http://www.epoca-project.eu/index.php/what-is-ocean-acidification/faq.html
For what it’s worth…
“[S]tep-by-step equilibrium equations describing the carbonate system in seawater do not capture the dynamic chemical environment of seawater. There are several reactions that can occur between carbon dioxide (CO2), water (H2O), carbonic acid (H2CO3), bicarbonate ion (HCO3-), and carbonate ion (CO32-). One of the possible reactions does create carbonate ions and lowers pH:
“CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3- ⇔ 2H+ + CO32-
“However, at the current ocean pH level, another reaction also occurs that consumes carbonate ions and does not change pH:
“CO2 + H2O + CO32- ⇔ 2HCO3-
“The second equation describes the reaction that occurs most often in the modern oceans, but the first reaction also occurs, so the resulting overall change is a decrease in carbonate and a decrease in pH.”
IIRC, the biological component of this system is also a major factor in the equilibrium states of the various components in biological calcification.
……………………………………..
Many marine organisms can adjust the pH of the fluid at the site of calcification. This is (in any of the literature I’ve read) within the organism – the fluid is not continuous with seawater. It takes energy to pump the H+ ions out into the surrounding sea water, and the lower the sea water pH, the greater the difference in H+ across the membrane, and the more energy it takes to pump the ions across the gradient. This could be a reason for the differences seen across studies. The movement of the water could affect the H+ gradient (something to consider in still lagoon areas).
Ample light of the right wavelength could affect energy balance of some corals that are symbiotic with algae. Continuous acidification might be more of a stress than periodic due to continuous extra energy requirements. And different species of coral are better able to control their pH than others.
…………………………………..
In addition to CO2 from the atmosphere, industrial pollution, acid rain and algal blooms are potential contributors. In the last case, algae near the surface growth into such massive populations (often due to inputs like fertilizer run-off) that they run out of resources and die off. The microorganisms that then break down the organic matter respire lots of CO2, acidifying the water.
…………………………………
IT IS ABSOLUTELY VITAL TO CONSIDER THE RATE ORGANISMS CAN EVOLVE AND ADAPT TO CHANGING CONDITIONS
I often see people talk about organisms being able to survive in past environments quite different from ours, using that to suggest that modern organisms will survive despite climate change. This completely disregards the fact that the current change is much more rapid than most of those in the past, or that rapid past changes often resulted in mass extinctions. Organisms that normally survive rapid, drastic short-term changes, such as those in tidal pools, may not even be able to cope with static conditions – or they may cope so well that they outcompete other species.
When environments change abruptly, some species may have physiological capability of acclimating or the genetic capacity to adapt, or the ability to migrate to better habitat. Some won’t. This may currently be one of the least predictable but most important effects of climate change: which organisms will thrive, which will survive but at a cost, and which will go extinct. There are some types of organisms that form the basis of whole food webs: plankton, for example. A collapse in plankton populations would do immense damage to marine life and those that depend on it, including humans. This is one reason OA is important. Coral reefs, too, are extremely important for marine productivity.
I don’t mean to pick on this poster, it just conveniently raises a few points.
“Starfish and most other calcified marine phyla evolved in the Cambrian era during which atmospheric concentration of CO2 was 20-50,000 ppm. They spent a hundred million years or so at these CO2 levels without dissolving. In what kind of reality do these same organisms now start dissolving at CO2 concentrations 1 or 2 orders of magnitude lower? And no – this is not about “getting ised to it” and rate of change – it’s basic inorganic chemistry.” [As another poster pointed out, there’s an extra zero on the CO2 content]
!. An atmospheric 2000 ppm CO2 is not directly indicative of ocean pH; water temp is also a major factor. It’s such a complex system, it’s hard to infer much that far back – but then, I’m no paleontologist.
2. Even if these phyla evolved in the Cambrian, they are not the same as today’s species. Species don’t evolve then remain that way for the next 100 million years.
3. CO2 during the last 800,000 years at least (some say 8 million years or more) has never gone above 300 ppm.
3. This is very much about rate of change, and if you all don’t accept that then you will never understand the potential problems of AGW, never know that whole story, and never be justified in judging whether it deserves action.
To ignore the rate of change factor in AGW, or to deny that it’s important, is a convenient way of being blind and deaf to potential costs. A similar one is ignoring the fact that species which would normally migrate due to changing conditions are often in remnant patches of habitat. Humans have completely changed the landscape, and organisms have different abilities to migrate. Tree species that take years to grow before producing seed and have short seed dispersal distances may take centuries to move a few hundred miles – if they have the habitat available. Species have such a vast range of variation in their needs and interactions I could think of countless ways rapid climate change might stress populations or exterminate species. I have no idea how much that will happen, though – not a clue. There is much we can not foresee about the effects of climate change, but it is a terrible error to therefore dismiss the risk. This is just the way things are, I’m not being an alarmist. I’m really genuinely trying to be pragmatic about this. I know transitioning away from fossil fuels is a sacrifice, at least in the short term. (Conserving them isn’t really a terrible idea, though, especially if deposits get more expensive to extract. And it’s good to have plenty of our own reserves on hand for security reasons.)
Just because something sounds alarmist is not an excuse to ignore it. One may just have to dig a little deeper to find out what the science really says. Unfortunately, this is often tough for the layman. A very good place to start exploring the current and potential effects of climate change is on the NOAA and NASA sites. This area is fun to explore: https://www.climate.gov/news-features/department/climate-tech Those with adequate hardware can download Science on a Sphere – SOSx Lite is the free, flat-screen version. I’m not sure how many datasets it contains. I got a private showing of SOS at NOAA in Boulder a few weeks ago, and it’s astounding all the capabilities is has; I’m sure the free one is pretty cool, too, but my computer doesn’t have the memory.
https://sos.noaa.gov/SOS_Explorer/
Kristi ==> Your desire to be pragmatic is admirable. Read the Boisvert essay for a pragmatic view of Climate Change from an alarmist — who sees that alarm is not appropriate.