Guest essay by Michael Wallace, Hydrologist
The prevailing model of seasonal and long term global ozone depletion is grounded in a principal assertion that emissions of industrial refrigerants and propellants (also known as Ozone Destroying Substances, ODSs) are the primary causal agents.
I review that assertion from a perspective relating to my ongoing research of the global geostrophic circulation regime and its associated moisture. I often write that motions of mass and energy within the planet’s hydrosphere are routinely captured by geostrophic integration of kinetic and enthalpic signatures. The enthalpic signatures are known to scale according to routinely identified categories of temperature, vapor pressure, and the total air pressure of any parcel of the atmosphere. This holds in particular for the full atmospheric integration of the troposphere with the stratosphere and the mesosphere, up to an average altitude of 100 km above mean sea level (AMSL).
Vertical and horizontal profiles of the energy and momentum of these parcels with respect to any conceivable orientation can be examined for practically any time span. I’ve developed some profiles along these lines from easily available ERAI source material [1]. I have focused on parameters such as the “Evaporation minus Precipitation for the Full Atmosphere”, EP (mm/day), at posts such as this example on Geostrophic Waves.
Geostrophic maps of EP patterns are often interesting. In addition to Atmospheric Moisture Waves (AMW)s, they often align with easterly equatorial streamlines and sometimes with gyres. The equally important Geopotential Height Z (kg/m) contours don’t clearly express such waves, but are also interesting if only because they often align with the westerly streamlines of the middle latitudes and sometimes with gyres as well.
I compared some full atmosphere ozone (O3) maps to equivalent months, from ERAI for EP and Z as shown in Figure1 a and b. Much might be said of the resulting comparison, including that the patterns sometimes align with EP and /or Z patterns and sometimes align with gyres. The January plot of Figure 1b, with the associated ozone hole lobe in blue branching northeast from the Philipines, and the associated gyre shown by the streamlines of the adjacent plots, seems particularly compelling.
Those figures obviously suggest that ozone can correlate inversely with atmospheric moisture. From my perspective, it appears that the major gyres, such as the Equatorial Northern Hemisphere Pacific Gyre continually replenish their long lived atmospheric parcels with moisture as they rotate through the wet Equatorial Trough that encircles the planet along the equator. That might account for continual destruction of O3 via Reaction 1, leading to the blue contour bands in the central images column of that figure set.
Figure 1. 1.a. Comparison of three full atmosphere parameters for December 1982 and
1.b. January 1983. Sources [1], [2].
These apparent connections are supported by empirical industrial knowledge. Ozone is prized for its remarkable properties at purifying water when dissolved in it. Naturally ozone must first be synthesized before it is added to the water. Yet many water treatment textbooks and other resources can be found to detail the problems of industrial production of ozone in the presence of trace water. Such knowledge includes evidence that Ozone will readily react with water and itself be destroyed, sometimes according to the simple equation labeled below as REACTION 1.
H30 + O3 + EUV → OH + H20 + O2 (REACTION 1.)
Where:
H30 is the hydronium ion, whose concentration defines pH
O3 is ozone
EUV is the UV energy source that drives the reaction
OH is the hydroxide radical
H20 + O2 are water and oxygen respectively.
It follows that an increase in atmospheric moisture, such as a rise in vapor pressure of water, or relative humidity, might be expected to lead to a decrease in ozone concentration (which is equivalent to its partial pressure). This notion appears to be verified from randomly selected vertical profiles of concurrent measurements from sources for the following Figure 2 a and b.
Figure 2 2a. Vertical profile of RH and Ozone for 20 September 2011 over Boulder, CO 2b. Vertical profile of RH and Ozone for 3 April, 2012 over Boulder, CO. Source [3].
Confirmation of high and inverse correlations between water vapor and ozone therefore emerges from both lateral and vertical comparisons, including from exploration of individual research airplane flights that concurrently monitored O3 and H2O with altitude, as shown in Figure 2.
Figure 1 demonstrates that many aspects of these patterns appear to be captured by barotropic weighted environments. A quasigeostrophic (QG) conceptual model of ozone distribution therefore seems justified. For a QG model to be tested, the parameter that is measured must be integrated across the full atmosphere thickness, but allowed to vary laterally. Although Figure 1 is a good example of that, I’ve found that the NOAA resource for Figure 2 also contains some time series of ozone and moisture for the full atmosphere. From their data I’ve produced Figure 3, to cover the full time series of the moisture as a limiting case. The actual ozone record for this location goes even further back in time. Given the high correlations shown, it likely offers potential for reconstruction of the past moisture patterns in my view. This is yet another purely scientific reason that I find ozone so interesting.
Figure 3. Time series of ozone and water vapor for full atmosphere thickness over Boulder Colorado. Source listed in subtitle within image. Source [3].
In any case, once again this alternate conceptual model of water vapor concentrations inversely driving ozone concentration is demonstrated. As noted, the latest confirmation shown of three independent comparisons is from a full atmosphere thickness parcel series over several decades of measurements.
Profoundly drier statements about water vapor can be found, if water is even mentioned at all, in many comprehensive ozone reports including relatively recent publications by the World Meteorological Organization (WMO) (2014, 2005)[4,5]. In the most recent of those summary reports, the primary causal constituents cited for ozone destruction in the atmosphere remain chloroflourocarbons (CFCs) and Halons. Although rarely some low key sources will partially acknowledge the potential for ozone destruction by water vapor [6], the role of atmospheric moisture is typically carefully constrained [7] and segregated to a limited domain of intermittent high Antarctic stratospheric clouds that are asserted to incubate ODSs every Antarctic winter[4].
This Antarctic winter incubation and the subsequent Antarctic Spring release of ODSs over that continent, are key foundations of the entire ozone destruction argument. Readers are encouraged to explore those assertions deeply, where they will find that the very winds themselves are argued to be caused by this ODS incubation phenomenon. I could cite many for this strange assertion but for now I quote from the 2014 WMO report [4] which states on page 4.1 that “Stratospheric temperature changes due to Antarctic ozone depletion are very likely the dominant driver of the observed changes in Southern Hemisphere tropospheric circulation in summer over recent decades, with associated surface climate and ocean impacts.”
I’ve engaged in limited explorations of that prevailing Antarctic ODS incubation model, simply to confirm or falsify the associated claim that the peak in polar ozone depletion for the Southern Hemisphere is in its (austral) Spring and that the peak for polar ozone depletion in the Northern Hemisphere occurs in its (boreal) Spring. The WMO resource and others sources appear to be careful to leave a reader with this perception even as low key qualifying statements accompany that assertion.
Plots I’ve derived from the ozone researchers’ data such as this sample series Animation 1., and the plates of Figure 4., appear to falsify that claim. In other words as the images show, the ozone depletion doesn’t oscillate from one pole to another each year. Rather both hemispheres exhibit largely synchronous ozone trends. That’s quite interesting one might think. How many other aspects of the two poles oscillate in synchrony? I can only think of one myself, namely polar auroras.
Animation 1. Arctic Ozone Climatology
A generally accepted notion of ozone genesis, circulation, and extinction comes in part from studies of the Brewer – Dobson circulation [8] and of the well known vertical temperature lapse rate inflection at the tropopause. In this conception, UV forcings cause ozone to emerge into the middle stratosphere at its highest concentration levels, and to diffuse to a minimum at the tropopause[9]. Meanwhile tropospheric air parcels rich in water vapor, which cross the tropopause, are thermally altered by the loss (condensation) of that vapor.
I only bring this up to somewhat round out the limited treatment of ozone I cover in this post. I could use this as a segue to the implausible arguments found in [4] and others that a primary source of water vapor in the stratosphere is from oxidation of anthropogenic methane! How the researchers determined that only water vapor from such a singular source could violate the thermodynamic constraints of our stratosphere remains a mystery, perhaps to be explored in a future post.
I’m primarily interested in the tropopause because it may be a significant factor in the EP surfaces I’ve profiled in the past. Also I happen to wonder if the cause of the temperature inversion at the tropopause might also be related to the significant releases of latent heat which accompany the universal condensation of water vapor at that altitude.
It may be, given the correlations shown, that at some times, some aspects of ozone data will resemble the remarkable atmospheric wave approach. This remains to be seen. If this is true, and given the primary content of this post, then many aspects of the current narrative of ozone circulation and destroying might merit some reevaluation. In particular, I’m interested to know if ozone pattern analysis can improve hydrologic forecasting.
Others may be more interested in how the CFC-Halon ODS model has remained at the forefront of all scientific and media publications (see for example [10] and [11]) concerning ozone in our atmosphere, given what is clear now about moisture as the principal ODS. I hope many will consider requesting answers to such questions from any and all who work on ozone research or who impact ozone mitigation policy and funding.
As I said, from my limited perspective, I’d like to reconcile residence times that are published concerning atmospheric circulation. For example, CFC and Halon ODS values are listed in the WMO 2014 resource with typically long atmospheric lifetimes. These lifetimes, reaching close to 600 years in extreme cases, appear to be based on the same crude 1D eddy diffusion model from the 1974 Nobel Prize winning literature source for modern ozone science [12]. Without any disrespect intended, such a model appears to be extraordinarily deficient in addressing the rich patterns of atmospheric circulation that actually prevail across our planet.
Accordingly I believe that the addition of geostrophic circulation mapping to such models offers promise towards more rational residence time estimates. Currently it is known that gyres encompass the longest residence times of any subregion across the planetary atmosphere. Residence times of 600 years would be unusual however.
Could there be some greater consistency of estimates of residence times in the future? I think so, but this advancement would require a more candid and greater mindfulness of the fundamental roles in time, space, and in the chemistry of atmospheric moisture relating to ozone destruction. In approaching that reasonable goal, it would hopefully produce the added benefit of weaning researchers away from the myraid unproven, implausible, and expensive notions now associated with CFC and Halon based ODS models.
Michael’s blog is at http://www.abeqas.com
Figure 4. Ozone in this northern hemisphere location consistently reaches its lowest levels towards the boreal autumn (September and October) Adapted from source [2]
REFERENCES
[1] http://www.cgd.ucar.edu/cas/catalog/newbudgets/index.html#ERBEFs downloaded file named ‘ERAI.EP.1979-2014.nc ‘
[2] acdisc.gesdisc.eosdis.nasa.gov
[3] National Oceanic and Atmospheric Administration, United States Dept. of Commerce, Earth System Research Laboratory, Global Monitoring Division, Ozone and Water Vapor Research Group
[4] World Meteorological Organization (WMO), Scientific Assessment of Ozone Depletion: 2014, World Meteorological Organization, Global Ozone Research and Monitoring Project—Report No. 55, 416 pp., Geneva,Switzerland, 2014.
[5] WMO Global Ozone Research and Monitoring Project Report No. 49 An Overview of the 2005 Antarctic Ozone Hole Prepared by Geir O. Braathen WMO TD No. 1312 WORLD METEOROLOGICAL ORGANIZATION
[6] https://www.giss.nasa.gov/research/briefs/shindell_05/
[7] https://www.nasa.gov/centers/langley/news/factsheets/HALOE-Ozone.html
[8] Salby, M.L. and P.F. Callaghan, 2005, “Interaction between the Brewer-Dobson Circulation and the Hadley Circulation. Journal of Climate 18 (20)
[9] Sivakumar, V., Bencherif, H., Begue, N., and Thompson, A.M., 2011, “Tropopause Characteristics and Variability from 11 yr of SHADOZ Observations in the Southern Tropics and Subtropics” Journal of Applied Meteorology and Climatology 50, pp. 1403-1416
[10]http://www.bendbulletin.com/sciencetech/5409884-151/a-chemical-could-delay-ozone-healing. This contemporary article appears to support my current representations on CFC causation assertions.
[12] Molina, M.J. ,and F.S. Rowland, 1974, “Stratospheric sink for chlorofluoromethanes : chlorine atomc-atalyzed destruction of ozone”. Nature Vol 249 June 28, 1974







Have you considered that Ozone and RH may not be directly casually linked to each. Their anti-correlation may not necessarily be causation. But possibly to a seasonal insolation that independently drives each in their opposite direction to create the anti-correlation relationship.
I hope any with a notion will reflect on this information and come up with good ideas to explain. I think that moisture is the driver, and not ozone, in part because of the impetus to minimize humidity in industrial ozone production. All might agree that something orbital and extra planetary could make the whole planet change ozone dressings so quickly every August through October. Solar UV would be greatest in December. That is around the time that ozone concentrations return to typical highs.
Why is there longitudinal asymmetry in the NH ozone when the weather is averaged over a month?
Thanks for your comment Clyde. I don’t know the answer to that, but perhaps NASA does. I obtained those images from a NASA source. I have also requested the actual data and they sent me something but I haven’t been able to unpack that archive yet. The data seems authentic to me and I have examined daily variations as well.
Mike,
One sees similar asymmetry in the ozone maps for the SH. Because the ozone is generated primarily in theTropics, and migrates polewards until it hits the Antarctic Polar Vortex in the Winter, I have speculated that there are differences in the permeability of the ‘wall’ that retards the movement of ozone. But, I’m still a little surprised that the barrier persists in the same spot for so long. It might be an artifact of when the satellites pass over Antarctica.
Interesting. It looks like he has actual testable hypotheses, but the evidence is a bit thin.
Is it as thin as the evidence that humanity is completely responsible for changes in the concentration of stratospheric ozone? Thin evidence appears to be irrelevant if the theory can make people a lot of money or build up their power over others.
All the models for ozone are based on inadequate evidence, and this one looks as plausible as the CFC proposition.
Thanks Tom. There is much more evidence that any are welcome to explore. I found the airplane flight databases easy to work with and so far the few I’ve randomly explored appear to reinforce. I hope more will explore the data base at an ftp page at National Oceanic and Atmospheric Administration, United States Dept. of Commerce, Earth System Research Laboratory, Global Monitoring Division, Ozone and Water Vapor Research Group
Interesting paper Mr. Wallace. The basic chemistry is pretty simple. In the stratosphere ozone, water, and oxygen can undergo free radical reactions very easily, along with chorofluoro carbons. At higher temperatures and pressures only results in persistent ozone under particular weather conditions such as temperature inversions where other pollutants also react an form ozone. Like CO2, CFC’s were chosen as the bad actors more for political reasons due to our still poor understanding of atmospheric chemistry.
It would be very helpful to clarify the technical terms of the trade. One doesn’t often run into “geostrophic”.
No, it is not. The inverse relationship between ozone and humidity is known and published. I have read about it. The causal relationship is unclear. One of them may cause the other, or both may be caused by something else. In one of the articles I read they seemed to propose that ozone dried the air.
OK. I found the article in question.
Kilifarska, N. A. (2015). Bi-decadal solar influence on climate, mediated by near tropopause ozone. Journal of Atmospheric and Solar-Terrestrial Physics, 136, 216-230.
http://www.sciencedirect.com/science/article/pii/S1364682615300316
You should know this article.
“The Sun’s contribution to climate variations was highly questioned recently. In this paper we show that bi-decadal variability of solar magnetic field, modulating the intensity of galactic cosmic ray (GCR) at the outer boundary of heliosphere, could be easily tracked down to the Earth’s surface. The mediator of this influence is the lower stratospheric ozone, while the mechanism of signal translation consists of: (i) GCR impact on the lower stratospheric ozone balance; (ii) modulation of temperature and humidity near the tropopause by the ozone variations; (iii) increase or decrease of the greenhouse effect, depending on the sign of the humidity changes. The efficiency of such a mechanism depends critically on the level of maximum secondary ionisation created by GCR (i.e. the Pfotzer maximum) – determined in turn by heterogeneous Earth’s magnetic field. Thus, the positioning of the Pfotzer max in the driest lowermost stratosphere favours autocatalytic ozone production in the extra-tropical Northern Hemisphere (NH), while in the SH– no suitable conditions for activation of this mechanism exist. Consequently, the geo- magnetic modulation of precipitating energetic particles – heterogeneously distributed over the globe – is imprinted on the relation between ozone and humidity in the lower stratosphere (LS). The applied test for causality reveals that during the examined period 1957–2012 there are two main centres of action in the winter NH, with tight and almost stationary winter ozone control on the near tropopause humidity. Being indirectly influenced by the solar protons, the variability of the SH lower stratospheric ozone, however, is much weaker. As a consequence, the causality test detects that the ozone dominates in the interplay with ULTS humidity only in the summer extra-tropics.
…
In this paper we show that energetic particles’ influence on the lower stratospheric ozone, followed by warming or cooling, and drying or moistening of the upper-troposphere-lower strato- spheric region (depending on the sign of the O3 change), is capable of explaining the surface T variability during the examined period 1957–2012.
Applying an additional test of causality for correlation between near tropopause ozone and humidity, we reveal an existence of “centres of action”, where lower stratospheric O3 constantly in- fluences the H2O vapour density near the tropopause. In the Northern Hemisphere there are two such centres – one of them is placed in North Atlantic, near the Azores islands (see Fig. 5, panel A). The other covers a broad area of the North Pacific Ocean in- cluding the Aleutian Islands and Eastern Asia. In the SH the winter lower stratospheric ozone does not influence the humidity near the tropopause (see Fig. 5, panel B). The dominant factor in ozone – humidity interplay in this season is the water vapour, and its influence on the surface T is detected as increased positive T anomalies in central and western Antarctica (see Fig. 5, panel B).”
Well, I also think he does not demonstrate what he thinks he demonstrates, but clearly he holds the opposite view, that changes in ozone drive changes in humidity. Although the relationship is clear, I remain skeptic of the cause in absence of more evidence. However since the Sun is an important driver of ozone changes a role for humidity in driving ozone changes should be secondary.
Javier,
The Kilifarska article is close to my proposition is it not ?
However, Kilifarska suggests cosmic ray variations to be the key whereas I suggest variations in the wavelengths and particles from the sun being the relevant variable in shifting the balance of ozone creation / destruction in the lower stratosphere.
What is your opinion ?
Stephen, I think Kalifarska is over-reaching in his conclusions. The relationship between ozone and humidity appears clear as shown in this WUWT article by Michael Wallace.
I don’t deny that GCR might have a climatic effect, but I have yet to see any evidence of that. GCR respond mostly to the geomagnetic field and what I see in the evidence is that climate responds to changes in the Sun, not in GCR.
I agree with you that changes in UV, and solar wind (particles and magnetic field) are better candidates, and that ozone is well placed to be an important mediator, but not the only one, in the climatic response to solar variability.
This figure from Kalifarska 2015 is important because it shows specific humidity lagging by 6 years changes in ozone.

Natalya Kilifarska is a female
(in some Slavic languages there is a surname gender distinction; male version is Kilifarski
Thanks Javier.
I focus on lower stratosphere ozone because that is the only variable with the power to raise and lower tropopause heights which is critical to the climate changes we observe, namely changes in jet stream patterns between zonal and meridional with consequent cloudiness changes.
What other variables do you have in mind that could affect global cloudiness without invoking GCRs.
I tend to doubt GCRs because there is no shortage of cloud seeding material in the atmosphere in any event.
Vukcevic, thank you for the info on her gender.
Stephen, solar effects not mediated by ozone have been proposed. The bottom up mechanism proposed by Meehl and van Loon produces changes in Equatorial Pacific SST that affect humidity and cloud cover directly at the troposphere. Solar magnetic changes transmitted by solar wind appear to affect surface pressure at the poles and perhaps high latitudes as in the Svalgaard-Mansurov effect. Changes in pressure translate into cloud and wind changes without a need for additional nucleation.
Javier, I’ve noted such other possibilities but find that they do not fit observations as neatly as my hypothesis which offers a complete chain of causation accounting for all observed climate variations.
Still, it is early days and I do not expect to convince at this point. I just want my hypothesis borne in mind as the various proposals are whittled down over time and I’ll try to keep involved in the whittling down process here and on other blogs.
Different solar mechanisms are not necessarily exclusive and attribution to them might be very difficult to quantify. In fact the high degree of signal amplification suggests more than one pathway coexists.
That’s interesting and thanks. I’m still learning myself but I’ve mentioned in this post that I wondered about auroras and the link you sent relates to that. I’ve seen some occasional intriguing correspondences myself between some polar vortex plots and auroras and ozone all for the same time period, but I don’t see that every time I’ve looked.
An interesting article which focuses on the ozone/ tropopause height aspect of climate change which I discussed in some detail here:
http://joannenova.com.au/2015/01/is-the-sun-driving-ozone-and-changing-the-climate/
The first question I would put to Michael before considering his submissions further would be why he considers the water vapour relationship with ozone quantities in the stratosphere to be indicative of water vapour causation when water vapour does not get past the tropopause in any significant quantity.
Is it not more likely that the ozone changes are solar induced and that the water vapour changes are a consequence rather than a cause ?
My compliments to Michael for focusing on the area of study that I see as most significant for both climate variations and the ozone hole panic.
Thanks! I took a brief look and promise to take a longer one. I’ve been active in my dissertation work which is focused on solar based forecasting of moisture in the atmosphere. and on land. If I’m not mistaken, your theory is based in part on a regression between sun spot number and/or TSI cycles and ozone patterns. I would like to see a time series plot for solar and some of those ozone series. I’ll also look into making some of my own with data I have, but it will take a little time.
I must say I was always somewhat skeptical of the “so called” hole in the ozone being caused exclusively by the use of CFC. So I’m heartened to read I’m not the only one!!
That makes two of us!
> The enthalpic signatures are known to scale according to routinely identified categories of temperature, vapor pressure, and the total air pressure of any parcel of the atmosphere.
Sorry, I’m going to have to call BS.
Fellow WUWT fans, feel free to correct me if you think I’m wrong. Just post, “You’re wrong, Pearson, that sentence means a lot to me.”
That sentence means a lot to me. 😀
Again, what process creates Ozone? There are lots of chemical analysis of how Ozone is changed to something else. Where are the graphs of the process that creates Ozone?
If Ozone is created and destroyed by Solar EUV (UV), where is the relationship of Solar Energy to Ozone creation? Does more Solar EUV(UV) created more Ozone? If not, what creates the Ozone?
Jerome Lurtz,
It is definitely solar UV that creates ozone. What isn’t commonly realized is that most of the ozone is created in the tropics and then moved poleward by the Brewster-Dobson cells, and isn’t created significantly in the poles, as we are commonly led to believe. The so-called Ozone Hole breaks up when the Polar Vortex collapses and the ozone is able to diffuse into the low ozone region.
Here is a good explanation of the Brewster-Dobson circulation-
http://www.ccpo.odu.edu/SEES/ozone/class/Chap_6/6_3.htm
Here’s the whole class on stratospheric ozone
http://www.ccpo.odu.edu/SEES/ozone/oz_class.htm
Clyde
It isn’t only UV that creates ozone. It is also created by electrical discharges in the atmosphere (lightning) and these are presumably more intense in the tropics. I have no idea what percentage of O3 is generated by each mechanism but just wanted to highlight that UV is not the only natural generator of ozone.
Phil,
Yes, you are right that lightning creates ozone. But, it does so in the troposphere, not the stratosphere. We were discussing the stratospheric ozone loss. Los Angeles has no shortage of ozone even though it rarely experiences thunder storms.
Thanks for the clarification, Clyde……much appreciated. Out of curiosity, would phenomena like “sprites” (the electrical discharges that occur in the stratosphere above thunderstorms following a conventional lightning strike) be capable of producing any ozone? I understand these can cover large areas (10s to 100s of square miles) so just wondered whether they are energetic enough to take part in ozone formation (or any other atmospheric chemical processes). Any response welcome. Thanks!
sounds good to me
I remember many, many, years ago . . .
when one of the first technical reports on the Ozone Hole was published in Scientific American (1970`s ?), by the guys who did the study, there was a wonderful paragraph near the end of the article. Although their belief was theory was good and should be accepted they said they could not account for the change in ozone levels over such large areas so rapidly, so rapidly that they could not put it down to mixing of air masses loaded with HFCFC`c / CFC.
clouds on the other hand dont require a change in air mass to create
Thank you for this post. I knew generally about the ozone CFC kerfuffle, but virtually nothing about atmospheric ozone or its water interactions. BTW, my Fort Lauderdale pool complex is ozone rather than chlorine purified. Much nicer swimming.
Thank You. I’m curious what you like best about an ozone purified pool.
UV as as tiny part of the TSI and Cosmic rays (re. Svensmark) are often dismissed ‘out of hand’ as insignificant factors in the climate’s change natural variability.
However it may be that there is more to learn
…………
Solar-Driven Variation in the Atmosphere of Uranus
“The changing brightness of the planet (Uranus) shows that something is happening to the clouds. We have found that the change is caused by two processes.
“One is chemical, caused as fluctuating levels of UV sunlight alters the colour of particles in the atmosphere.
The other is due to high-speed particles from outside the solar system, known as galactic cosmic rays, bombarding the atmosphere and influencing the formation of clouds.”
The scientists used data from telescopes on Earth, as well as cosmic rays measured by the Voyager 2 spacecraft, to make their assessment.
Professor Harrison said: “The sun has a magnetic field, diverting cosmic rays away from the solar system, including here on Earth. This protection is reduced when solar activity is at its lowest every 11 years, meaning more cosmic radiation gets through.
“The atmosphere of Uranus is, like Neptune’s, effectively a giant ‘cloud chamber,” able to respond to the incoming energetic particles. It is amazing that the effects are visible even from Earth, more than a billion miles away.”
https://phys.org/news/2017-12-sun-remote-planet-uranus-brightness.html
http://onlinelibrary.wiley.com/doi/10.1002/2017GL075374/abstract
Dr. Svalgaard’s comments would be appreciated.
Thank you for bringing this article to our attention. It is available at arxiv:
https://arxiv.org/ftp/arxiv/papers/1712/1712.07103.pdf
It is interesting that astronomers are telling us about the importance of solar variability on climate.
Noctilucent clouds are only seen during hemispherical summers. Wow.
Those are clouds in the mesosphere.
This is a direct rebuttal to Stephen Wilde’s assertion that water vapor doesn’t traverse the stratosphere. Mean free path diffusion of a low molecular weight species increases with altitude.
I didn’t say that NO water vapour gets past the tropopause, only that the quantities are insignificant for the ozone creation / destruction process relative to other factors.
“As the mesosphere contains very little moisture, approximately one hundred millionth that of air from the Sahara desert,[12] and is extremely thin, the ice crystals can form only at temperatures below about −120 °C (−184 °F)”
from here:
https://en.wikipedia.org/wiki/Noctilucent_cloud
So the mesosphere cools in the summer?
Yes thanks for that. I had incorrectly stated those incubating clouds were in the upper stratosphere.
Thanks Stephen, and for others, I will reply today but only a little later. I only noted a few things about the tropopause here but nothing regarding causality of moisture.. although I did detour slightly to speak to the Ozone research community’s interest in anthropogenic methane as some sort of causal factor for water vapor in the stratosphere. Most might agree that water vapor is barely recognizable in the stratosphere. I only feel that this water enters and exits the stratosphere through normal Hadley circulation patterns. I noted the Brewer Dobson circulation also in this post but ultimately to me that circulation seems like a subset of Hadley.
Mike,
To help you focus your response to my comment perhaps I should mention that I propose that a solar induced shift in favour of less ozone above the poles when the sun is more active allows the tropopause above the poles to rise so that the climate zones can expand poleward beneath the tropopause, the jet stream tracks become more zonal and global cloudiness reduce with warming of the oceans and increased global humidity.
I need to know what makes you think that the ozone reduction is caused by the water vapour increase rather than the other way around.
See here for humidity changes since 1970:
https://www.climate.gov/news-features/understanding-climate/2013-state-climate-humidity
One can see that specific humidity increased whilst the sun was more active (a period of decreasing ozone in the stratosphere) with zonal jets but since around 2000 when the sun became less active and the jets more meridional specific humidity has stabilised whilst stratospheric ozone appears now to be recovering.
So there one can see the inverse relationship varying with solar activity.
I have some interesting solar time series plots that compare to some interesting atmospheric moisture and additional hydrologic features. The comparisons are somewhat overdue for a decision in a journal as it has been about a year. If they ever get published, you might find them interesting also. I took a quick look at your link and it seems interesting again. What I think might help in those papers are comparison time series. You could plot ozone and moisture and sun together to support and/or test some of the rationales.
This post misses the obvious point – the ozone hole is caused by a decrease in ozone over a period of years. This post only concerns natural processes but they are presumably in equilibrium with ozone formation and so will not lead to the a decrease in ozone concentration.
Ozone is highly reactive and unstable so there are any number of reactions that lead to its destruction. What is interesting is whether the rates of any of these reactions are increasing and increasing faster than the creation of ozone. Only then is there a possible causative mechanism for the ozone hole.
Geranium,
You said, “… the ozone hole is caused by a decrease in ozone over a period of years.” You have it backwards. The ‘Ozone Hole’ occurs annually and dissipates annually. The Summer levels may have been getting worse over a period of years, but I doubt it. I say that because ozone has a relatively short half-life and the model I built years ago to predict surface UV only showed an increase in UV in the Winters. By Summer, the UV levels were back to where they should be.
The causative mechanisms for the ozone depletion and accretion regions that appear briefly over Antarctica each year (just as flowers bloom and die each spring), which you call a “hole” (ignoring the surrounding mountains, for some reason!), are very easy to explain without you imagining a “decrease in ozone over a period of years”.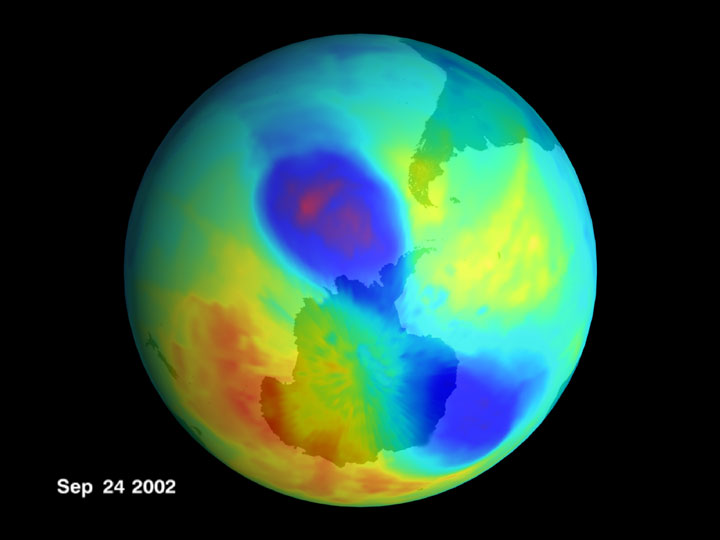
In fact, the very short collection of records we have shows the size of the region changing randomly from year to year in accordance with the behavior of the polar vortex, being the real cause of the depletion/accretion pattern we see:
Here’s the NASA explanation, annotated for clarity:
https://wattsupwiththat.com/2014/09/12/is-the-atmospheric-ozone-recovery-real-or-just-for-scoring-political-points/#comment-1735455
Why presumably?
Why not cyclical or quasi-cyclical. There is so much that we don’t know here. The alleged “ozone hole” is only recently discovered and we know nothing of historical ozone before the National Geophysical Year and the alleged “hole” was only discovered in 1985. Understanding a few chemical reactions does not we understand the phenomenon.
We are too impatient to observe natural discoveries long enough (several generations) before drawing hard conclusions about their properties and behaviors. We want answers NOW. That is the way we were indoctrinated to be by Boob Tube.
Progressive scientologists spin their initial analyses from the assumption that humans cause all variations from the hastily established “norm”, with no mention of the absence of any observations previous to the moment the phenomenon was discovered.
“H30 + O3 + EUV → OH + H20 + O2 (REACTION 1.)”
0 ≢ O
This reaction makes no sense, the charges don’t balance.
Correct: there is a single positive charge (the H3O+ ion) and a single negative charge on the right (the hydroxide ion). As written, this equation is nonsense.
Agree. Seems more likely along the route:
H2O + O3 => 2OH* +O2
via H2O2 formation (O3 => O* +O2) and splitting with UV
(the * indicates free radicals with OH* being very reactive)
When it comes to humidity in the air and climate change where l suspect its important will be. Is where a jet stream / weather pattern set up persistently pushes warm moist air from over the oceans up into the Arctic. But then drives cold and more humid air down across the landmass of N America and Eurasia where it leads to increases in snowfall amounts.
The weather pattern set up over N America during the next few days is going to test to see if my ideas are right. There will be a flow of warm moist air from the Mid eastern Pacific been pushed up into the Arctic, while cold air is been driven down across eastern N America. With this pattern in place there will be a short term climate set up like there was during the last ice.age. Where there was a warm Mid Pacific but cold N America landmass. Now what l suspect will happen with this set up it that it will lead to a noticeable increase in snow cover over N America as like what happened during the ice age. lf am right then it could show how the formation of a ice age from a warm climate takes place should this weather patterning become persistent.
silent spring, acid rain, the ozone hole, man-made global warming… the beat goes on.
you forgot the “nuclear winter” hoax (in between the acid rain bamboozle and the CFC-ozone hole hustle)
😎
I saw ‘Mad Max”. There won’t be a “nuclear winter”. There will be a “nuclear desert” where everyone is hunting for fossil fuel.
(Of course, none of that will happen until the day after “The Day After Tomorrow”.)
It’s not the physics we have to worry about, but the metaphysics!
……. And the pseudo-physics. !!
The troposphere contains both moisture and oxides of Nitrogen (NOx), both potent destroyers of ozone. In planets where there is no life in the soil the partial pressure of ozone increases all the way to the surface. The higher summer troposphere in the northern than the southern hemisphere is probably due to the relative abundance of land in the northern hemisphere.
The ozone hole in Antarctica is a product of the local circulation. It is most severe at 50 hPa where the origin and nature of the air in the circulation changes as part of the annual cycle. In Antarctic spring descent of very cold mesospheric air is curtailed and ozone poor NOx rich troposphere air. replaces the cold air over the polar cap. The process is gradual, takes place over several months as the troposphere air on the outside of the vortex contacts like the aperture on a camera. This can be verified by comparing the distribution of ozone with that of NOx here: http://macc.aeronomie.be/4_NRT_products/5_Browse_plots/1_Snapshot_maps/index.php?src=MACC_o-suite&l=TC
The ozone hole manifests, naturally. It was first observed using a Dobson Spectrometer in the 1950s, long before the emission of CFCs used in refrigeration became an issue.
Cool.
Glad to see you weigh in here Erl.
Reaction 1 chemical equation and the description is somewhat ambiguous. If it is the hydronium ion, H3O+, in the reactants, then the product would have to contain OH+, not the hydroxyl radical •OH, (which is neutral and contains an unpaired electron). “Hydroxide” is normally taken as referring to the anion OH−
That makes sense. I’ll try to ensure I don’t repeat that same mistake in the future.
I didn’t want to appear too critical, but I’m more interested in the origins of the equation.
I’m sure there are multiple ways for ozone to decompose in the presence of water: Mechanisms involving ionic species, and mechanisms involving free-radicals (frequently the ones promoted by UV irradiation). As written/described, equation 1 appears to suggest both, which is possible, but I wondered if it might be conflating two or more different reactions.
Michael & Mike….
I’m not sure about that so would ask others to comment please. Why do we need to invoke the hydronium ion in the first place? I thought the complete reaction was just H2O + O3 + UV —> O2 + 2OH~ (hydroxyl radicals). I may be out of touch but I thought the ozone absorbs the short wavelength UV (providing protection for the Earth) while being split into normal diatomic oxygen plus a reactive oxygen atom (“nascent oxygen”) which then reacts with water to produce 2 hydroxyl radicals. I would welcome some clarification to sort me out on this please. Thanks!
I noted that Figure 2 is plotted versus relative humidity, rather than absolute humidity. The difference between relative and absolute humidity is very large as the altitude increases, owing to the drop in temperature with increasing altitude. The relative humidity is just the ratio of the absolute humidity divided by the saturated humidity. The saturated humidity is closely approximated by the vapor pressure of water at the temperature of interest divided by the total pressure. The vapor pressure of water is an exponential function of temperature, so as the atmosphere cools, the holding capacity for water drops exponentially. 50% RH is not even close to the same absolute humidity at sea level and 5000 meters. As chemical reactions are dependent on absolute concentration, this figure should be plotted against absolute humidity to demonstrate any correlation.
I appreciate your comment Loren but I feel that it is premature. I explore correlations across a wide spectrum of climate variables in my research and work and I routinely standardize variables when exploring correlations. In the case of RH, the standardization is already in a sense done for me.
If I were attempting to calculate the absolute amount of water vapor required to destroy ## moles of ozone, then along the lines of your comment, it would be necessary to at some point derive the absolute water vapor concentration, or at least to directly calculate the associated moles of water vapor from the RH value. That’s obviously not the point of my essay.
It is interesting to think about that prospect however. If after finally recognizing the significant impact of moisture, the ozone-policing World Meteorological Organization (WMO) were to do such a calculation, then they could make demands on all of our governments to reduce the amount of water in the atmosphere. They could quantify precisely how much water we are expected to eliminate from the atmosphere. I see many high profile, important, and expensive programs and meetings to follow.
Splendid Post!
I remember in Chemistry class being taught that Ozone (O3) was formed in the upper atmosphere by Cosmic Rays, Ultraviolet Light, or Alpha Particles from the Sun, bumping into Oxygen molecules (O2) according to the equation 3O2⇋ 2O3, a reversible reaction.
Reactive and unstable, Ozone decays pretty soon, back into O2 or an oxide of Nitrogen (NOx), there being plenty of Nitrogen around up there.
So imagine my surprise when I learned that in America Ozone is believed to come from automobile exhaust pipes in places like Los Angeles.
When, in the ‘80s a sharp eyed New York Times reporter first spotted the ‘Ozone Hole’ lurking over Patagonia in late October, I was curious. When, every year thereafter, the ‘Ozone Hole’ reappeared at the same time and place as reported in the NYT, I became suspicious.
Now it was common knowledge among my classmates that our schoolmasters were Neanderthals, nevertheless to avoid being caned we paid attention, (A.D.D. having not yet been invented). We also knew from paying attention that the Antarctic, being a continent, was 30o C, or more, colder than the Arctic which is an ocean.
With no sunlight for six months there are no ‘Cosmic Rays’ to generate fresh ozone over Antarctica. In addition the cold dense polar air mass descends over the South Pole and heads North in every direction creating the hurricane force katabatic winds. The Earth’s rotation or Coriolis effect, take your pick, gives the Northbound wind an Easterly kick and voila! the South Polar vortex is born, giving rise to the roaring forties, or screaming fifties depending how far south you go. All of this sucks more of the remaining ozone out of the upper atmosphere.
When in September, spring in the antipodes, the Sun pops its smiling face over the horizon to warm things up again, relatively speaking, the polar vortex weakens and the ozone depleted winter air mass spirals Northward to show up in Patagonia on cue for the annual October/November Ozone Hole spotting!
To panic about the disappearing ‘ozone hole’, our shield against cancer causing UV radiation, seems strange given that UV radiation is absorbed in the process by creating the Ozone layer. The energy needed to create the highly reactive Ozone molecule from the standard O2 Oxygen molecule reduces the high energy UV to a lower energy state with a corresponding longer and less harmful wavelength.
(according to the formula E= H/λ, where E is ‘Energy’, λ is wavelength and H is Plank’s constant).
Thank you Mike Wallace for confirming my long held suspicions!
Merry Christmas
Mike
Interesting and thanks! I appreciate your perspective since my interest in ozone first emerged as I studied the Antarctic vortex a few years ago and was intrigued by the similarities of that “hole” to the surrounding atmospheric circulation patterns, which you mention. From those I first got caught up in the idea that marine aerosols from the Southern Ocean were upwelling into the stratosphere. I thought maybe these sea salts were the source of chlorine that is the purported orthodox culprit in long term ozone destruction. But after going through many hoops, it became fairly evident to me that although chlorine may be a nice catalyst for ozone destruction, there’s plenty of perfectly effective H2O to do the job. The correlations profiled here seem to corroborate.
I’m not sure that one gets significant upwelling into the stratosphere over the poles.
The thing is that the polar vortices in the stratosphere over the poles are columns of descending air which would tend to suppress any upwelling from the troposphere below.
The term ‘polar vortex’ is used indiscriminately to refer both to those columns of descending air in the stratosphere AND the ring of winds around the poles in the troposphere but they are very different phenomena.
That is why I focused on the ozone creation/destruction balance in the mesosphere as a starting point for my solar induced hypothesis.
Dear Mike,
Cosmic rays come form all directions not just the sun. Indeed they are guided in by the earth’s magnetic field towards the poles, Hence flights ove the poles give a higher dose to aircraft passengers even at night compared to flights at lower latitude where the magnetic fields protect the passengers, and the pilot!
You can tell when there is a lot of cosmic rays coming in the sky glows at night – the auroras
Michael.
In equation 1 you show H3O and call it hydronium ion. However hydronium ion is more exactly H3O+, a charged species whose concentration in both the troposphere and stratospheric is so tiny as to be considered negligible. This point needs correction or at least explanation
Yes thanks, that is related to the previous comment by Michael Hart. I’d add that the concentration of hydronium ion is equivalent to the pH scale. As pH drops, the concentration of hydronium ions rises. That’s perhaps negligible in alkaline environments but not so in the environment of a raindrop for example.
In the liquid phase yes but that’s not what’s being talked about here. As I pointed out above the reaction 1 is impossible in any case even if we were talking about reactions in solution.
Phil Rae, Thanks for bringing this up. I encourage the dialogue and at this point note that the industrial chemists likely have good answers that are applicable. I have gone through a great deal of literature on ozone chemistry and find that much of it appears to be highly speculative. The overall mechanism favored by the establishment appears to be first, migrate the heavy CFC and Halon molecules up into the stratosphere via crude eddy diffusion principles. Second, keep those heavy molecules high up there for decades so the sun can ultimately break out the chlorine and bromine. Third, keep the chlorine and bromine up there, hovering over the polar noctilucent clouds until just the right moment every polar spring. Fourth invoke all of the other players, hydroxides, protons, hydrochloric acids, nitric acids, and a number of organics. Fifth, mix until the ozone is destroyed. Finally, repeat as needed until everyone is fully indoctrinated. 😀 The chemical diagrams can make one’s head spin. There doesn’t seem to be much in the way of laboratory verification, or even basic oxidation state work, even through the CFC pathway for ozone destruction won a Nobel Prize.
https://undsci.berkeley.edu/article/0_0_0/ozone_depletion_01
Mike this appears to give an account of the saga and points ut to a lot of evidence, from satellites, ground based laser spectrometers, laboratory studies, and aircraft flights to measure those elusive compounds.
I’ll give you my bit of personal experience of the first step in the sequence, if you work with a strong UV source you can smell ozone.
Does it explain the accretion region that forms around the depletion region, Gerontius?
How do you account for the pattern of depletion and accretion that occurred in spring of 2002?
Khwarizmi and Gerontius thanks for adding to this discussion. I read through that link. It’s obviously well intended but it ignores moisture, as all of references it cites do, all the way down to the Molina & Rowland Nobel Prize paper and all the way up to current times. The elimination of water from the discussion of ozone is the primary topic of my post. I don’t try to argue that chlorine, flourine and bromine cannot catalyze ozone destruction. Ozone is very reactive in any case and can be “destroyed” by a large number of candidates. Water needs to be in the discussion. Then all of the Rube Goldberg arguments be “destroyed” accordingly, or hopefully at least.
mike, read towards the end of the series. It states that ice acts as a heterogeneous catalyst for the destruction of ozone.
Without high humidity you will not get ice!
Your description of the mechanism of O3 breakdown over the antarctic is seriously flawed although given your lack of understanding of chemical kinetics I’m not surprised. Water is not ignored in the mechanism, polar stratospheric clouds require water for their formation.
Type I clouds contain water, nitric acid and/or sulfuric acid and they are a source of polar ozone depletion.
Type Ia clouds consist of large, aspherical particles, consisting of nitric acid trihydrate. (Note that trihydrate means that it contains 3 H2O molecules for each HNO3 molecule)
Type Ib clouds contain small, spherical particles, of a liquid supercooled ternary solution of sulfuric acid, nitric acid and water. (formed by water and HNO3 condensing on sulfuric acid aerosol particles)
Type Ic clouds consist of metastable water-rich nitric acid in a solid phase.
Phil thanks again I welcome the debate. But sorry to say you appear to fail to recognize the correlations that are featured in this post. Accordingly, your concerns appear to be more of a deflection than a debate. I’ve demonstrated that the correlations simply between water vapor and ozone are distinctive across many time scales and over many regions. These correlations don’t need to invoke any of the acids you dwell on, nor do they require an icy incubation in a faraway place. As I point out, all of that peer reviewed and gray literature about antarctic high atmosphere incubation of ozone killing substances appears marvelously complicated. But data should trump marvelous theories, so where are the global supercooled-ternary-solution-acid-trihydrate-aerosol correlations to compare with the simple and verifiable ones I have featured?
If you want to disregard the vast and synchronous changes in ozone every time and everywhere in the atmosphere that water vapor varies, it’s your prerogative to do so. But it’s no longer your prerogative, or the WMO’s to do so on our dime.
Mike, the current theory states that ice crystals provide a surface for the reaction to occur. I see nothing to stop a mechanism in which water droplets can act similarly by dissolving the reactants. The reactions here are fairly well established ozone is used to purify water. can you distinguish from ozone depletion where ice forms or where water forms? If you go into reactions with water vapour you will need to get to grips with free radial reaction kinetics.
Gerontius it looks to me as if no ice is required, but that in the presence of ice the destruction of ozone may be possible at least as long as some vapor phase remains. The ozone theorists only point to ice as an incubator for chlorine and other halides. For what it is worth, I feel that my main contribution is not in the simple chemistry that I’ve dug up, but that the ozone and water vapor distributions are inversely related whether one looks at a full atmosphere or a layer within the atmosphere. The connections are sustained whether one looks vertically or areally. The connections persist whether one looks at a day or at decades. Moreover, I’ve drawn attention to the ozone catastrophe industry’s longstanding assertions of those high ice clouds that act as some sort of ozone killing incubator awaiting spring thaws. For that assertion to “hold water”, that episode must occur at each pole at that pole’s respective spring. This is a critical leg of their assertions. Yet that is not the pattern shown by the data. I also note they tread lightly around that part of the claim because the data doesn’t support it.. and yet, they decline to make this easy for everyone to see. I am a student and continue to learn and welcome that, But after decades of fear mongering and millions if not billions of dollars spent towards the ozone fear, I feel that the ozone research community needs to get to grips with the high inverse correlations to water vapor and the poor match of their noctilucent ice incubation theory to reality. They need to acknowledge those and more and deal with the fallout transparently and candidly.
Perhaps water vapor explains the relationship between the ENSO and ozone variance heretofore mysterious?
That sounds intriguing. I hadn’t heard of that.
Mike, it’s in the stratospheric ozone class lecture materials at Old Dominion U’s CCPO site. I tried upthread to post the link but the spam filter got it twice. I’ll try again in a separate comment following.
Mike, see my link above in this comment:
https://wattsupwiththat.com/2017/12/23/the-profound-and-dominant-impact-of-atmospheric-water-vapor-upon-ozone-destruction/comment-page-1/#comment-2700782
Look at chapter 2, section 4.2.4 and 4.2.5. we might have a correlation here.
Ozone has an interesting property rarely discussed. It is diamagnetic, or essentially repelled by magnetic fields, whereas oxygen is paramagnetic-attracted to magnetic fields.
The “returning” magnetic field of the earth is focussed on the polar regions.
What impact may this have on ozone regionality?
bubbagyro this is very interesting. I don’t know the answer, but I have noticed that ozone patterns around Antarctica often resemble aurora patterns around the same location. Coupled with the circulation aspects, I wouldn’t be surprised to learn that moisture, ozone, and auroras have some deeper connections, perhaps related to the diamagnetic feature you brought up.
Mike, have you looked for possible relationship between ocean surface pollution and droplet formation? I have a vague memory of a problem with small droplets growing big enough to fall out: if droplets are not raining out then presumably the RH goes up. The oceans are certainly polluted enough for some effect to be happening. Wolf number change IIRC.
I’ll spare you my guess that polluted ocean surfaces are a contributor to warming.
JF
Hi Julian, I have looked into that a little bit. I often get detoured in moisture investigations by the well known principal of cloud condensation nucleii. I also have put some time in to study literature on upwelling of marine aerosols. In fact, my first notion on those was that they might be supplying the major supply of the chlorine that catalyzes ozone destruction. The ozone research community exclusively attributes such chlorine to CFCs and disregards marine aerosols. Yet the mass of marine aerosols and the associated chlorides from the sea salts, that are lifted into the atmosphere, dwarfs the mass of CFCs. However more recently as this post shows, I’ve come to realize that moisture in and of itself can do all or most of the heavy lifting in ozone destruction.
I’m wandering around the answer to your question, but there is a lot still unknown, I think when it comes to condensation of droplets from water vapor. Your “vague memory” is essentially correct though.. for any given dewpoint temperature, as the RH reaches 100%, condensation of droplets occurs. The less known part in my view is the notion that aerosols or other tiny particles, including pollutants, are an essential ingredient for moisture condensation for the “nucleation” of the raindrop. I’m still learning myself and note that water vapor can definitely condense to a liquid or to a solid without any need for nucleation around a foreign particle. It only takes somewhat colder temperatures to happen.
Much more could be written and has been by many others of course. Perhaps if researchers can come to terms with the moisture – ozone correlations I’ve profiled here, they can advance some of these other questions in a more productive way.
Pop, many thanks. I took a short look but I’m mainly going by your excerpt. I study the SOI for my own moisture forecasting research. It certainly is within the ENSO compedium of indexes and in fact covers the “SO” in ENSO. It’s historically grounded in the concept of those pressure differences between Tahiti and Darwin. However what I have found most useful is that the Tahiti portion directly underlies a limb (I would call it) of the so called Atmospheric Wet Pool (AWP) (Zhang and Chen 2008). The AWP constitutes the greatest concentration of atmospheric moisture on the planet. The high atmospheric moisture limb of the AWP is known as the Southern Pacific Convergence Zone (SPCZ) (Kiladis et al., 1989). That limb passes over Tahiti.
So I think you have pointed to another area that could support this moisture ozone connection and it’s interesting and helpful that prior researchers have studied it, even if they were more focused on pressure correlation than moisture (and the two can be closely related in any case). I somewhat feel at this point that the negative correlation between ozone and moisture can be found wherever one might look, so long as care is taken to ensure that the same atmospheric parcels are examined. But this one is particularly interesting to me at least because of the association with the globally high moisture mass. Over time, I’ll try to examine that more closely myself. The mixed message of negative and positive correlations in the excerpt you provided will require careful attention to exactly what regions are evaluated. Or I (or anyone else with the interest of course) could just focus on the SPCZ itself and bypass the traditional SOI.
Thanks again, and I hope to keep in touch.
Kiladis, G.N., H. von Storch, and H. van Loon, 1989. Origin of the South Pacific Convergence
Zone. Journal of Climate 2(10), 1185-1189.
Zhang, C. and Chen, G. 2008. The atmospheric wet pool: Definition and comparison with the
oceanic warm pool. Chinese Journal of Oceanology and Limnology, 26, 440–449.
I’m just beginning my self-education on this subject so I sure appreciate the references, Mike. I will be cramming on this for a while but I’ll watch for your next article. This is the best place I know to stimulate my mind in retirement.
Thanks! There will be plenty to keep you occupied.. 😀
This is all unnecessarily complex: ozone is naturally unstable. It has a half life, which, at the polar winter temperatures is about 3 months. This is the cause of the ‘ozone hole’