By Andy May
18O is a rare isotope of oxygen. The ratio of 18O to the normal 16O in foraminifera fossils (“forams”) can be used to estimate paleo-ocean temperatures. Higher values mean lower temperatures. A recent article on geologypage.com (here) led me to Bernard, et al., 2017, which has experimental data that suggest 18O concentrations can be altered in fossils by solid-state diffusion after fossilization. This can corrupt the measurement and the resulting calculated temperature. According to Bernard and colleagues, the 18O concentration alteration is visually imperceptible, so one cannot tell the fossil has been altered by visual inspection. If their results are valid, how will this impact our view of climate history?
Fractionation of 18O and 16O takes place during evaporation and precipitation, so another problem for this paleothermometer is glacier and polar ice, which delay the return of excess 16O in water vapor to the ocean. Correlations of δ18O to temperature are for an ice-free Earth, the formation of polar ice caps and mountain glaciers have an additional effect on δ18O. In figure 1, the δ18O record and the δ13C record are shown for the Cenozoic (65 million years ago to the present day).

Figure 1 (source: Zachos, et al., 2001)
The red temperature scale at the bottom of figure 1 is only applicable when there is no significant permanent polar ice, Zachos, et al. call this the ice-free temperature. It is applicable prior to about 33 million years ago when Antarctica and the North Pole were ice-free, at least in their respective summer months. Once permanent ice caps appear, they remove 16O from the oceans indefinitely and lead to a large positive δ18O in the oceans and in forams. Both records shown in figure 1 are for global deep-sea pelagic fossils, water depths greater than 1,000 meters.
In the experiments, Bernard, et al. exposed foraminifera tests (shells) to elevated pressures and temperatures when submersed in pure H218O. The forams were then monitored with a micro-mass spectrometer and they observed oxygen-isotope changes. They then attempted to model the isotope diffusion process during sediment burial. Their models suggest that on a time scale of tens of millions of years, the isotopic diffusion could cause ice-free δ18O calculated ocean temperature estimates to be higher than reality. For this reason, estimated temperatures in the Paleogene and Cretaceous may have been lower than we estimate today. The error due to diffusion is sensitive to the initial foram temperature, so this is particularly significant for forams that grew in the deep ocean or at the poles.
Thus, the older paleotemperatures in figure 1 may be too high, if Bernard, et al. are correct. How much and how does this effect our view of ancient climate? First, let’s discuss how ancient climates are currently reconstructed. There are many indicators of ancient climate. A brief list of common paleotemperature indicators, other than δ18O concentration, follows. Remember that over time the continents have moved, relative to the equator and the poles. The following list of indicators assumes that this has been considered, as it is in figure 2 for the Upper Cretaceous.
Mg/Ca ratio: The proportion of magnesium in foram shells is proportional to ocean temperatures, this is considered an accurate thermometer in unaltered Holocene sediments.
Alkenone Proxies: UK’37 is an alkenone index that is temperature sensitive. TEX86 is a very widespread and easily identified alkenone that is commonly used as a temperature proxy as far back as the Jurassic (200 to 145 million years ago). Finally, there is a newer alkenone temperature proxy called LDI or the long chain diol index. These three proxies do not always agree and can reflect temperatures at different water depths and different seasons (Smith, et al., 2013)
Plant leaf analysis: Some plant leaves change their shape and size depending upon the air temperature. With some species these characteristics can be used to infer temperature. This proxy can be used for both Cenozoic and Cretaceous temperatures. See Wilf, 1997.
Pollen: Certain plants only occur in narrow latitude bands, by measuring the amount of their pollen in sediments, they can be used as a temperature proxy. See here.
Lithology suite: For very old rocks this is the most reliable temperature proxy. Certain rocks tend to occur only in warm climates and others only in cold climates. The map and legend in figure 1 are from Christopher Scotese’s monograph “Some thoughts on global climate change: The transition from Icehouse to hothouse,” see here. In figure 2 the continents are shown in their Upper Cretaceous (100 to 66 million years ago) locations. To see the location of the continents anytime in the last 1.5 billion years, see this Youtube video by Prof. Christopher Scotese. This is an age discussed in Bernard, et al. The Upper Cretaceous rocks, suggest that the warm temperate climate, which currently is seen in the southeastern United States and in Italy and Spain, occurs much farther north and south than it does today.

Figure 2 (source here)
Many environmental factors have been found to affect oxygen isotope ratios, some are discussed by Spero, et al., 1997. The alkalinity, carbon dioxide and carbonate problems discussed by Spero, et al. will also affect the accuracy of very old (pre-Cenozoic) δ18O and δ13C paleotemperature reconstructions.
Bernard, et al. discovered that elevated temperatures and pressure cause a slow process of solid state diffusion of oxygen isotopes that result in the calculation of higher temperatures than were present when the foram test (shell) was formed. The problem is much worse in forams that formed in colder water, either on the ocean floor (benthic) or in high latitudes. It is much less of a problem in surface dwelling (planktonic) forams or in the tropics. The result is that the use of oxygen isotope temperature proxies can make the ocean temperatures appear more divergent, from pole to equator and from sea floor to ocean surface, in the Cretaceous and early Cenozoic than they were. For sediments less than 10 million years old or from the tropics, this process will have little effect. To see the impact that Bernard, et al. computed, see figure 3.

Figure 3, source Bernard, et al., 2017
The left side of figure 3 shows the modeled effect of the temperature difference (center scale is the δ18O estimated temperature for each line) by age and typical sediment burial conditions. The temperature labels, for each line, are the actual foram formation temperature. The difference between the δ18O estimate temperature and the original foram temperature is large in colder polar waters and for benthic (bottom dwelling) forams than for sea-surface (planktonic) forams in the tropics. This can also be seen in the model results shown in figure 3b. Further, younger sediments (less than 10 million years old) are not significantly affected, especially if they are of planktonic origin.
Discussion
If the study is accurate and stands the test of time some paleo-climatological maps of the Cretaceous and early and middle Cenozoic may need to be revised. However, because many other temperature proxies are also used over these periods of time, it is unlikely to have a large effect on the overall picture. It will affect deep water ocean temperature estimates and estimates of the polar to equator temperature gradient. This work affects ocean paleotemperature estimates, it should not affect δ18O temperature estimates from ice cores. The ice core temperatures are too low for this sort of diffusion to take place. Figure 4 is a compilation of paleotemperatures from various sources over time (original author Dr. Goswami, 2011, PhD thesis):

Figure 4, average Pole to equator temperature gradient for the Cretaceous, source Christopher Scotese here.
There are many sources of paleotemperature data plotted in figure 4 and you can find them in the references in Scotese’s monograph. Some are oxygen isotope data, but not all. If what Bernard, et al. have discovered is true, the Cretaceous curve shown is probably closer to the faint gray line that shows the current temperature distribution. It cannot be the same, since we know there were no polar ice caps in the Cretaceous.
The Cretaceous and the Cenozoic up to 33 million years ago were much warmer than today, we can tell that because polar ice caps did not exist then and tropical ocean surface temperatures were higher. But, the paper by Bernard et al. will affect deep-ocean temperature estimates and polar-ocean temperature estimates. Bernard, et al. conclude:
“In conclusion, accounting for the diffusion-controlled burial-induced O isotope re-equilibration of fossilised benthic foraminifera removes the requirement for a strong, continuous and global cooling of the deep-ocean (on the order of 15 °C) during the late Cretaceous and the Paleogene. Furthermore, the present study suggests that the vertical and latitudinal temperature gradients of the late Cretaceous and Paleogene oceans were likely not very different from the current ones. Importantly, because O isotope re-equilibration through solid-state diffusion is a slow process, it likely had little impact on recent (<10 Ma) high frequency signals, such as the glacial to interglacial fluctuations (driven by oscillations of Earth’s orbit and mainly related to fluctuations of the seawater O isotope composition). However, these processes have potentially attenuated the relative amplitudes of older, transient signals, such as the Eocene-Oligocene transition or the Palaeocene-Eocene thermal maximum.”
I am certainly no expert in chemistry and have no way of knowing if this study and their conclusions are correct or not. The procedures and analysis seem reasonable to me, but that is as far as I can go. Certainly, the work has implications in paleoclimatology. I would hope to see others, with access to the very expensive equipment needed for studies like this, would attempt to duplicate this work and study it further. It is an important study in a critical area.
.
Spero et al 1997 (as mentioned) found especially that dissolved CO2 had a major impact on d18O in foram shells. Now there is a very high correlation in late Pleistocene d18O in forams and CO2 in Antarctic ice cores (Vostok, EPICA DomeC). So what would be the conclusion there?
I mean if the ambient CO2 concentration is strongly correlated with forma 18O, what part thereof is a valid proxy for temperature/ice volume.
The same mechanism will occur for co² in ice. Measurements in ice cores of co² are almost certainly low.
Proxies in general are about as useful as tea leaves or Ouija boards or magic 8 balls.
Proxies are all we’ve got. Careful, detailed, extensive, and critical examination makes them useful because their interpretation is more grounded in fact than the devices you list.
+10
no, they are not … they are grounded in guesses not fact …
sure , but the point with proxies is you need several independant ones ,but well, temperatures of themometers in boxes…to measure temperature of “some” air that is not even in thermal equilibrium, it is all that s we had for a while
While a proxy obviously is some kind of quantification, I imaging that proxies are called proxies becasue they would have problems meeting definitions of measurements:
measurand: well-defined property that can be observed or quantified by a measurement
measure: quantify a measurand by establishing the ratio between that measurand and a reference that serves as a unit – and assign a number representing that ratio together with the associated unit to that measurand
calibration: comparison of a measurement with a reference having a known uncertainty
uncertainty: quantified accuracy
reference: a measurement device or procedure that has an unbroken chain of calibrations to the definition of the unit
I always wonder how accurate these proxies are, what they actually quantify, and how they were calibrated.
Still proxies can only give us a relative understanding of past temperatures regimes. What this oxygen isotope studies says it that they are basically inaccurate. Problem is that the news media and many in the world of “climate science” pretend that we have very precise measurements of past temperatures going back thousands and millions of years.
I guess the MWP and LIA never happened
The “scientist” speaks 😀
So you’d deny the actual accounts of the MWP and the LIA written at the time?
Some English Major you are!
“Bernard, et al. discovered that elevated temperatures and pressure cause a slow process of solid state diffusion of oxygen isotopes that result in the calculation of higher temperatures than were present when the foram test (shell) was formed.”
Do they?
Do they really?
It was an accepted fact back in the 80s when I was studying physics. The mechanism by which molecules or elemental particles could migrate out of a closed system was not fully proven but that it happens was.
Before elemental or isotopic ratios in any historic material can be used as evidence of the conditions that pertained at the time of its formation, or of the time elapsed since that formation, it must be demonstrated that the material has remained a closed system throughout its history.This is basic science but is seldom demonstrated. All too commonly it is assumed.
What appears to be demonstrated by Bernard et al. is that individual forams cannot be assumed to remain closed systems over geologic time. Their modelling of this result is quite another matter.
Good comment Dr. K.A., and the maintenance of a closed system, ie not altered even by difusión, is a remnant homogeneous color/texture/mineralogy. They are saying the formas appear uniform, ie, where is the evidence of natural-state O18 difusión? Exploration geologists are used to looking for these alteration/difusión fronts as they suggest the introduction of a potentially mineralizing fluid. It looks like proxy reconstructions of paleo-climate need to be multi-variate and unequivocal to be generally useful.
The article states, “In the experiments, Bernard, et al. exposed foraminifera tests (shells) to elevated pressures and temperatures when submersed in pure H218O.” Obviously, the situation was one of extreme disequilibrium. This might be justifiable if a relationship is known to be linear. Then, pegging one end of a series allows linear interpolation. However, I don’t think that a case was made for a linear relationship. In the real world, it isn’t a surprise that solid-state diffusion can take place. However, the question is to what extent, when there is only a small disequilibrium. They have demonstrated the obvious, but the usual step of performing several experiments changing the 18O in small increments was not carried out.
Clyde – Einstein in 1904 formulated the equation governing diffusion. Yes, the relationship is linear between chemical potential gradient and diffusion rate. It actually featured in my thesis research back in the 1960s, but if you asked me to elaborate further than that I’d have to claim memory loss.
Over time, the Oxygen18 isotope ratio in foraminifera fossils and other core methodologies gradually changes. The Oxygen18 slowly moves out of the fossils and other indicators which would normally indicate temperatures are warmer. This has been previously described as a diagenesis process but the article proposes a very good explantion for why it happens.
This takes millions of years and it is an extremely slow process. But once you get to 3 million years or so, you have to start taking this effect into account.
All of the “serious” reconstructions done have taken this impact into account. Typically, this is done by detrending the downward trend in the isotope ratio in a simple linear manner and, when this is done, it seems to match up very well with the temperature history that we know about in Earth’s history – ice ages – hot periods etc.
The nature study linked to in the post proposes why this happens and, in my mind, it seems to be a good explanation and it holds together.
https://www.nature.com/articles/s41467-017-01225-9.pdf
Now some climate science researchers have not carried out the adjustment needed. They are doing this for the simple reason that they can take advantage of it and strengthen the global warming narrative. The Blue line below is what happens when they don’t make the simple adjustment required (with the rational for why it is needed explained in the Nature article). I previously described it as “miscalibrated”. This had led some to say the Eocene was +12C or whatever, Wrong it was at most +6C

When the simple linear transformation is carried out, this is the temperature history we are left with over 750 million years (750 Mys just to catch the last two Snowball Earth episodes).

Could you tell us what the ratio between O16 and O18 is in today’s atmosphere?
There is actually an international UN convention on standards and how to measure isotopes in water called the Vienna Standard Mean Ocean Water (VSMOW). The Oxygen18 ratios in rain water varies by temperature (seasonal temperature), latitude, proximity to the ocean and altitude.
http://www-naweb.iaea.org/napc/ih/documents/userupdate/Waterloo/animations/global_oxygen.gif
Bill,
And I believe that humidity will also impact O-ratios because it will impact evaporation of falling raindrops.
Ratio of 016 to 018……… approximately 500
http://pages.uoregon.edu/rdorsey/geo334/O-isotopes.html
Thanks Bill Illis, excellent contribution to the discussion.
Bill I love reading your stuff ! Deep, dark and informative !
It never ceases to amaze me just how many ways can be found to misinterpret any analysis in favor of the CAGW narrative. There are so many examples of it, and the resulting catastrophism that goes along with it, that it surely can’t be long before every sane rational person laughs this one into history. I hope so anyway.
I became a skeptik when I realized the data manipulation caused by “homogenization” of the surface temp record only ever seemed to point one way, the CAGW way. That was not an outcome that I would expect, not every time, conclusion ? It was being rigged. And yes I am a Scientist with great respect for the principles of the Scientific Method. I go where the science leads me.
Thanks Bill – I was looking forward to your comment on this issue.
Bill, as I understand the blue line in your first graph represents ‘deep ocean’ temperatures and so does the brown line. It is the anomaly from present deep ocean temperatures. Sometimes the deep ocean is seen as ‘the total water column below the warm surface layer’, other times only is meant ‘the deepest ocean’ (bottom waters). It is not clear to me what the reference for the blue / brown lines is in the first graph: are they representing everything below the warm surface layer or the temperature of only the deepest ocean?
The second graph speaks about the temperature anomaly for ‘global temperature estimates’ which I interpret as ‘surface temperatures’. The lowest atmosphere above the oceans adapts to actual ocean surface (water) temperatures, so there might be a relation with average ocean surface temperatures as oceans cover 71% of the surface.
Depending on the behaviour of the oceans, the relation between the temperatures of the deepest ocean and the surface temperatures above may differ substantially. Less mixing of the surface layers and less deep ocean upwelling enhances very rapidly ocean surface temperatures and also atmospheric temperatures, but in the meanwhile deep ocean temperatures may stay nearly unchanged.
So I am a bit confused when I try to understand what the graphs show me: do they show surface atmospheric temperatures, ocean surface temperatures, deep ocean (= below surface layer) temperatures or bottom ocean temperatures?
And if they show ‘below surface layer deep ocean temperatures’: is in the second graph a 1:1 relationship of that deep ocean temperatures with atmospheric surface temperatures assumed? Independent of oceanic behaviour in the constellation of that geological period?
I know Bill, a lot of questions, but I hope you can give some extra information about what we are looking at.
I think you are on the right track with your temperature graphs and already was years ago. That makes your answers about the exact interpretation important.
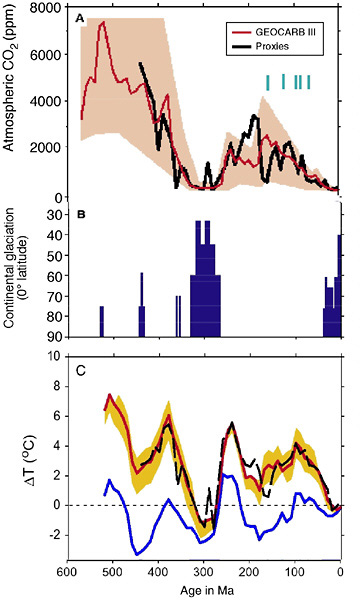
I realized I have never put out a chart of what the Raw dO18 isotopes actually look like and why this transform is required. The Nature article goes back 120 million years but the actual issue is FAR more clear when you go back even farther.
Here back to 600 million years ago. Now it is very clear that something happens to the buried cores and the impact continues the farther back in time one goes. There are literally 17,000 individual datapoints in this chart.

The Raw data says the Earth never had any ice ages (except in the last – well it would say one big long-lasting ice age for the past 50 million years, nothing before that). Snowball Earth periods happened when temperatures were +25C from today).
When the trend is removed, we get various estimates of the PaleoClimate. Veizer did one using a 50 million year smoothing cycle (I don’t have his actual numbers so not shown but it would be right in the mix here). Royer and Berner used Veizer’s estimates and corrected them for a small ph factor, again a 50 million year smooth. Robert Rohde of Global Warming Art (and Berekely Earth’s main data analyst now) used Veizer’s data with a 3 million year smoothing cycle. We have all seen Scotese’ estimates but this is more hand-drawn let’s say based on the geologic data of ice ages etc. I used Veizer’s and Zachos’ dO18 isotopes and used a 1 million year Gaussian smooth to produce a much higher resolution version (which actually matches some of the issues we know about in climate history that simply do not show up in a 50 million year smooth or a 3 million year one or a hand-drawn attempt).
This is hard to see but these are all the serious attempts at the history of the climate and all detrended the isotope database. Note: that there is no Hansen in these numbers because he has never carried out the detrending that is required.

Now if you want to see how a climate scientist will take advantage of the data and distort it so that it matches the narrative, this is a version of the chart that is now on Wikipedia. Done by Glen Fergus and posted on RealClimate a few years ago.
He takes the isotopes up to about 120 million years ago produced in Hansen 2013 (Zachos 2001 database, ice cores, Raymo) and doesn’t make the detrending adjustment required. One can see in this version of the chart, that he doesn’t cut-off the isotopes until 120 million years ago and they continue to sky-rocket off the chart of course. (Must have thought about why? you would think, so should have Hansen).
Then he takes Royer and Berner’s (2004) numbers and just doubles them (takes a +7C and makes it a +14C). Of course so it matches up with the other too high numbers.
http://gergs.net/wp-content/uploads/2015/06/All_palaeotemps.png
Royer and Berner’s (2004) actual temperature chart from their actual study in the bottom panel here. Top panel is CO2 with ice ages in blue.
In the comments at RealClimate, Royer even notes the “drift” in the isotopes needs to be corrected and a few posters tried to set Fergus straight but this is climate science.
All I can think of is to see how different proxies correlate over time. Flora and fauna fossils would seem independent of isotope changes, so if one has broad-leaf trees at a certain latitude and time, one can infer what the climate was like then.
“It cannot be the same, since we know there were no polar ice caps in the Cretaceous.”
Maybe, maybe not.
“… Either continental ice sheets paced sea-level changes during the Late Cretaceous, or our understanding of causal mechanisms for global sea-level change is fundamentally flawed. Comparison of our eustatic history with published ice-sheet models and Milankovitch predictions suggests that small (5-10 ?? 106 km3), ephemeral, and areally restricted Antarctic ice sheets paced the Late Cretaceous global sea-level change….”
“https://pubs.er.usgs.gov/publication/70027515”
I don’t know (or care) much about this, But cores drilled in New Jersey a few years ago seem to show fairly rapid 25 meter changes in sea level during the Upper Cretaceous. The folks that identified them believe the changes were due to polar ice. More unsettled science perhaps?
I may have overstated it. Small areally restricted polar ice caps may have existed at times. But, it seems unlikely that they were anything like today’s polar ice caps. Especially given the Cretaceous fossils seen in the Cretaceous polar rocks. Wikipedia notes that “In West Antarctica conifer forests dominated through the entire Cretaceous period (146–65 Ma), though Southern beech began to take over at the end of this period.”
Also: “During the Cretaceous, temperate forests thrived at polar latitudes,[2] as there was a notable difference from current conditions at high latitudes during the Cretaceous polar seasons.”
The cooler periods would probably have been in the early Cretaceous though. I suspect the late Cretaceous saw very little ice in the polar regions, except at the very end.
I don’t disagree Andy. I suspect that the North Polar climate in the Cretaceous was much milder than today because there was likely serious circulation of ocean water through the Arctic region whereas today, the Arctic Ocean is pretty much cut off from the rest of the oceans except for the narrow openings to the North Atlantic.
But I also think that geologists and (especially) paleontologists are prone to build huge speculative structures on scanty and incomplete data — scanty and incomplete data being all they have to work with most of the time. I’d suggest that ALL proxy paleo data should be viewed with skepticism. I’m not saying, don’t use it. But I’m suggesting keeping in mind that it’s quite possibly not all that great and may be really bad/misleading at times.
Don K, you are correct, of course. The data we have to work with prior to the Holocene is very thin. The data we have for the Cretaceous is even less. But, climate is a very long term thing. How far will we get if we restrict ourselves to the instrument period, circa 1880 to today? Not very far. Paleoclimatology is very important, the more we know about the proxies, the better we will be at interpreting them. The huge hole in our knowledge is the magnitude of the natural “forcings.” To borrow a somewhat ugly term from the modelers.
In Andy’s article: “In figure 1 the continents are shown in their Upper Cretaceous (100 to 66 million years ago) locations.” That should be “figure 2.”
Fixed, thanks
Can anyone tell me the correlation co-efficient for the ratio of O18 to O16 versus sea water temperature.
I am guessing it is another dandy item from the tooth fairy (a la CO2 correlations).
Oddly the original article (Epstein, 1953) does not supply an R^2. The plot looks good and the data are in the article if you want to plot it up and compute one. Link to article: http://faculty.washington.edu/stn/ess_501/reading/Epstein_revised_isotopic_temps_GSAB_1953.pdf

It is my understanding that in coral and other salt water deposits, 18O is a proxy for salinity, not temperature.
There are many references, this is only the first I found.
https://link.springer.com/article/10.2307/1353206
Robert Clemenzi, It can only be used as a salinity proxy if the temperature stays the same or very close to the same.
In speleothems, ¹⁸O is usually a proxy for precipitation rather than temperatures. It all depends on what is causing more or less ¹⁸O appearing in the record.
“It is an important study in a critical area.”
It may be important, but I disagree on it being critical.
The Earth’s geography, continent locations, and climate of 66 Mya are nothing like today. 66 Mya climate will not recreate itself in today’s Pleistocene anytime within many millions of years. Thus it is not “critical.”
So many confounding factors….. It’s just a mess.
See essay Cause and Effect for more examples of paleoproxy confusion. The main point being a debunking of the Shakun 2012 nonsense.
This info = isotopic evidence in ice cores and deep sea sediments of foraminifera has been around for decades – hello!! – it supports Milankovitch cycle theory very well – since the 70 ‘s
please find evidence of this in this 1990 sci journal;
http://marineecology.wcp.muohio.edu/climate_projects_04/glacial_cycles/web/pdf/MilankovitchCycles.pdf
see page 15 – Imbrie – Shackelton and forams
whoops page 15
Wouldn’t a water molecule with the heavier oxygen isotope be a kind of ‘heavy water’ like a cousin of Deuterium? Being ‘heavier’ wouldn’t it be more likely to sink to the bottom of the ocean…therefore less likely to evaporate?
Settled science consensus faith foundations seem to rest on quicksand.
Yes. If solid-state diffusion corrupts fossil proxies, now wonder the ice core proxies excrete caca.
All proxies have problems.
Most proxies are useful.
Reconstructions are always tentative.
When multiple proxies agree, confidence in the reconstruction is higher.
An extreme example is borehole temperatures. Time-dependent temperature diffusion is known to erase even millennial resolution in just 1000 years, however DYE-3 and GRIP borehole temperatures support a MWP as warm or warmer than present in Greenland, and an Holocene Climate Optimum centered around 6000 years ago significantly warmer.
http://i.imgur.com/oWituvO.png
This result is also supported by other proxies that don’t present this problem, making our confidence in the result higher.
Mr. Layman here.
What forms O18?
¹⁸O is a naturally occurring stable isotope present at ~ 0.2% frequency with a very slightly different fractionation in different liquid or solid water reservoirs. It formed before the planet.
“Materials formed in the early Solar System generally exhibit a characteristic oxygen isotopic signature known as the non-mass-dependent oxygen isotope anomaly1, 2, the origins of which are unclear. The anomalies are thought to reflect isotopic fractionation in the chemical reaction that first formed solid material from the gaseous medium, but the proposed mechanism and environment of formation are the subject of debate3, 4, 5, 6. Here we analyse micrometre-sized grains of acid-insoluble organic matter from a carbonaceous chondritic meteorite recovered in Antarctica. We find that the organic matter has the highest 18O/16O and 17O/16O ratios known in planetary material, except for pre-solar grains7. The oxygen ratios are enhanced by up to 53±11% and the 13C/12C values by 29±5% relative to terrestrial values. We suggest that the coherent enrichments of 17O, 18O and 13C in the organic matter can best be explained by its formation being due to the photodissociation of carbon monoxides in a gas medium at temperatures of about 60 K or higher. These conditions are equivalent to those expected at the envelope of the proto-solar nebula, and we suggest the organic matter formed there.”
Extreme oxygen isotope anomaly with a solar origin detected in meteoritic organics
https://www.nature.com/ngeo/journal/v4/n3/full/ngeo1070.html
I don’t like the experiment using 100% O18 water (they added salt, etc to simulate seawater). CaCO3 has a very low solubility in alkaline water, but the nature of solubility activity is, even in an equilibrium state, for solution to take place from the surface of the solid but for ions from the water to replace them. When conditions for net dissolution are extant, simply, more ions dissolve than are coming back out of solution. For ions coming out of solution, the “selection between O18 and O16 is random and depends on the relative abundances. A pure O18 source in the medium ensures selection of O18.
They would have to know the time parameter of this solution-precipitation activity at all temperatures, ptessures and pH ranges and how the rate changes with the reduced mobility in burial. This is not a simple statistical exercise. Somehow, real life data is required. I don’t pooh pooh the idea but it’s quantification would require huge error bars and remember post norman’s don’t believe in errors – that’s why they only had O18 in their medium.
BTW Andy, how’s this for a real life sample from 53million years ago from a diamond mine st the Arctic Circle!! Redwood chunks at 300m depth in the ore.
https://www.livescience.com/23374-fossil-forest-redwood-diamond-mine.html
A california climate at the Arctic Circle! Now I would measure the O18 in that baby to see if land creatures also fractionation O18.
Minor quibble :”Once permanent ice caps appear, they remove 16 O from the oceans indefinitely and lead to a large positive δ18 O in the oceans and in forams”
Shouldn’t that read “… they preferentially remove 16 O from the oceans..,,.” or its equivalent?
From the Bernard et al article:
Seems the “ocean temperature paradoxes” are a (big?) part of the reason for this article.
When one believes that the temperature of the deep oceans can be increased by the atmosphere the mentioned paradoxes do exist. However when one accepts that the deep oceans are warmed from below by geothermal energy, the paradoxes cease to exist.
To explain eg the Creataceous Hothouse the Ontong Java Event alone is more than enough to explain the high temperatures some 80-90 mya.
https://en.wikipedia.org/wiki/Ontong_Java_Plateau
(80 million km^3 magma carries enough energy to increase the temperature of ALL ocean water ~80K)
Relevant to the original post imo is this publication:
http://onlinelibrary.wiley.com/doi/10.1029/2011JC007255/full
Very interested in comments on how this publication relates to other reconstructions, eg the one from Bill Illis.
Thank you.
It sounds like we have an accepted theory of how it was formed but no actual observations?
PS No, I was not laying a trap or anything like that.
When I was younger (much younger) Carbon 14 was taught as a reliable dating method because the ratio of C14 to C12 was constant. (Perhaps I was a poor student but that was the impression given in the 60’s and 70’s.) Now we know that super novas etc. form it.
I just wondered about what forms O18.
(supposed to be a reply to https://wattsupwiththat.com/2017/11/04/oxygen-18-stability-in-foraminifera-fossils-implications-in-paleoclimatology/comment-page-1/#comment-2655585 )
I agree completely. What I find missing in this and most other data plots in these climate papers and articles is calculated ERROR BARS!
If all these uncertainties were taken into account, the error bars on many (most?) of these temperature and anomoly graphs would blur the results into meaninglessness. You simply cannot infer differences or trends when your calculated error is many times greater than the trend you are reporting.
One needs to be very careful when extrapolating experimental data obtained at 300 degrees C back towards Earth surface temperatures. This presupposes one has a good handle on the processes involved. It’s even more difficult when it appears the authors have made a fundamental error in interpreting previous data.
The authors claim they have extrapolated their data back to Earth surface conditions using an activation energy between 85 and 90kJ per mol and cite Anderson 1969 as a reference for this activation energy. Of course Anderson does find an activation energy for oxygen self-diffusion in calcite of 88kcal per mol, or closer to 380kJ per mol. It seems the authors may have mistaken kJ per mol for kcal per mol. If they have then their findings are completely invalid.